- 1Laboratory Center of Life Sciences, College of Life Sciences, Nanjing Agricultural University, Nanjing, China
- 2College of Sciences, Nanjing Agricultural University, Nanjing, China
- 3College of Life Sciences, Nanjing Normal University, Nanjing, China
Although previous results showed that β-cyclodextrin-hemin complex (β-CDH) could induce tomato lateral root (LR) formation, the corresponding downstream messengers are still not fully understood. In this report, similar to the inducing effects of exogenously applied hydrogen peroxide (H2O2), we discovered that β-CDH elicited RBOH1 transcript upregulation, endogenous H2O2 accumulation, and thereafter tomato LR development. Above responses were sensitive to dimethylthiourea (DMTU) and ascorbic acid (AsA), two membrane-permeable scavengers of H2O2, showing that accumulation of H2O2 and LR formation were significantly blocked. The test with diphenyleneiodonium (DPI; the inhibitor of NADPH oxidase) revealed that H2O2 mainly produced by NADPH oxidase, might be involved in LR formation triggered by β-CDH. qPCR combined with pharmacological and anatomical analyses showed that β-CDH-modulated several marker genes responsible for LR formation, such as CYCA3;1, CYCA2;1, CYCD3;1, and CDKA1 (four cell cycle regulatory genes), ARF7 and RSI-1 (two auxin signaling genes), LAX3 (an auxin influx carrier), IAA14 (encoding a member of the Aux/IAA protein family), PIN3 and PIN7 (two auxin efflux carriers), isocitrate dehydrogenase [NADP], NADH-cytochrome b5 reductase 1, and L-ascorbate oxidase homolog genes (two reactive oxygen species-associated genes and one LR formation-related gene), were causally related to above H2O2 signaling. Particularly, representative proteins related to H2O2 metabolism and lateral rooting, were specifically induced in β-CDH-treated tomato seedlings. Overall, the results clearly suggested a vital role of H2O2 in the β-CDH-induced tomato LR formation, and β-CDH-elicited H2O2-related target proteins responsible for LR formation might be, at least partially, regulated at transcriptional and translational levels.
Introduction
It was well known that lateral root (LR) not only acts as a physical support, but also enables plants to absorb and transport water and nutrients (Casimiro et al., 2003; Benková and Bielach, 2010). Since LR is a very important agronomic trait, the corresponding chemical inducers and corresponding mechanism of its formation have been widely studied (Fukaki and Tasaka, 2009). β-cyclodextrin-hemin (β-CDH), which combines hemin with β-cyclodextrin (β-CD), a cyclic oligosaccharide of seven α-(1,4) linked glucose units (Bodine et al., 2004), is previously discovered to be a novel inducer of LR formation in tomato seedlings (Li et al., 2015). Compared with hemin, the solubility of β-CDH in aqueous solution and its efficiency in inducing LR formation were significantly improved. Since the involvement of nitric oxide (NO), heme oxygenase-1 (HO-1), and glutathione (Li et al., 2015; Zhu et al., 2016) in above β-CDH response was respectively illustrated, the intricate signaling web triggered by β-CDH in LR formation is an excellent model. Corresponding mechanism may thus reveal that, how a vital agronomic trait elicited by an exogenous chemical is controlled by a complex array of signaling mechanisms.
Beside its toxic effects, ample evidence revealed that hydrogen peroxide (H2O2) can act as an important signaling molecule participating a series of physiological processes (Neill et al., 2002), including plant development (Li et al., 2011; Bai et al., 2012; Zhao et al., 2012), responses against abiotic and biotic stress (Levine et al., 1994; Alvarez et al., 1998; Desikan et al., 2004, 2006; Zhou et al., 2012, 2014; Rejeb et al., 2015), and even programmed cell death (Wu et al., 2011). In the downstream of H2O2 signal, proteomics changes as well as post-translational modifications are suggested as the important processes. For example, it was reported that protein expression and protein carbonylation were regulated in H2O2 signal response (Tanou et al., 2009, 2012; Lounifi et al., 2013). Although the role of respiratory burst oxidase homologs (RBOH)-mediated H2O2 as a second messenger in root organogenesis has been extensively illustrated (Li et al., 2007, 2009; Cao et al., 2014; Ma et al., 2014; Orman-Ligeza et al., 2016), it was not known whether H2O2 could act as an intermediate in β-CDH-induced LR formation. Meanwhile, several important downstream signaling components, including NO and HO-1 (Bai et al., 2012; Lin et al., 2012; Cao et al., 2014; Fang et al., 2014; Ma et al., 2014; Li et al., 2015), are shared in some aspects of β-CDH- and H2O2-induced root organogenesis. These results suggested the possibility that a linear pathway from β-CDH to H2O2 may exist in LR formation.
In this study, it was found that β-CDH elicited RBOH1 transcript upregulation, endogenous H2O2 accumulation, and thereafter tomato LR development, mimicking the responses of exogenously applied H2O2. By using pharmacological, anatomical, and molecular approaches, we further revealed that H2O2 operates downstream of β-CDH promoting LR development. Additionally, H2O2 metabolism related proteins or other target proteins responsible for LR formation might be regulated by β-CDH at transcriptional and translational levels. Combined with the inducing responses in adventitious root development elicited by β-CDH (Lin et al., 2012), our results thus provided a comprehensive window of the complex signaling transduction pathway in β-CDH-mediated root organogenesis.
Materials and Methods
Chemicals
Unless stated otherwise, all chemicals were purchased from Sigma (St Louis, MO, United States). According to previous reports (Li et al., 2015; Zhu et al., 2016), the preparation of β-CD-hemin (β-CDH) was carried out. Hemin (used as an inducer of HO-1) and β-CD with an appropriate molar ratio were mixed by grinding for at least 60 min after adding de-ionized water. After freeze-dried, the brown powder was regarded as β-CDH. Our pilot experiment confirmed that 1 nM β-CDH which contains 1 nM hemin and 500 nM β-CD, exhibited a maximal response in the induction of tomato LR (Li et al., 2015).
Hydrogen peroxide (H2O2), as a positive control, was applied at 100 μM. N,N′-dimethylthiourea (DMTU; Ma et al., 2014), a membrane-permeable scavenger of H2O2, was used at a final concentration of 500 μM. Ascorbic acid (AsA; another membrane-permeable scavenger of H2O2) purchased from Solarbio Life Sciences (Beijing, China), was used at 200 μM. Diphenyleneiodonium (DPI), a NADPH oxidase inhibitor (Xie et al., 2011), was used at 0.1 μM. According to our pilot experiments, the concentrations of above chemicals exhibiting the effective responses were selected.
Plant Material and Growth Conditions
Tomato (Solanum lycopersicum L.) seeds “baiguoqiangfeng” were obtained from Jiangsu Academy of Agricultural Sciences. Selected seeds were surface-sterilized with 2% NaClO for at least 10 min, and germinated in distilled water at 25 ± 1°C in the dark for 2 days. Afterward, tomato seedlings were transferred to an illuminating incubator (25 ± 1°C) with a light intensity of 200 μmol m-2 s-1 at 14-h photoperiod. After growing for 1 day, the selected identical seedlings were transferred to 4 ml solution containing the indicated chemicals for the indicated time points. Afterward, photographs were taken, and the number of emerged LRs (LRs; >1 mm) per seedling and the length of primary root (PR), as well as the emerged LR density (the number of LR per cm PR; LRs/cm) were determined with Image J software. LR primordia (LRP) per seedling were also observed by root squash preparations and quantified by a light microscope (model Stemi 2000-C; Carl Zeiss, Germany; Correa-Aragunde et al., 2006). In our test, at least three independent experiments were carried out for each treatment, and at least 15 seedlings were used for each.
For the subsequent biochemical, molecular and proteomics analyses, only the LR-inducible segments were used. Therefore, the root apical meristems of seedlings at the indicated time points were cut off, and the shoots were removed by cutting below the root-shoot junction (Zhu et al., 2016).
H2O2 Detection and Fluorescence Analysis
H2O2 signals were assessed by a laser confocal scanning microscopy (LCSM) using the ROS fluorescent probe 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) (Maffei et al., 2006; Li et al., 2011; Xie et al., 2011). Also, DMTU and AsA, two membrane-permeable scavengers of H2O2, were used to confirm its specificity. Roots were incubated in HEPES buffer (20 mM, pH 7.5) which contains 20 μM H2DCF-DA for 30 min in dark (25°C). Then the fresh HEPES buffer was used to wash three times. All images were visualized by using UltraVIEW VoX (Perkin Elmer, Waltham, MA, United States). Thereafter, photographs were representative of identical results obtained after the processing and analysis of seven samples for each condition in three independent experiments. Volocity Demo software was used to quantify the production of H2O2 in roots.
Real-time Quantitative RT-PCR (qPCR) Analysis
Total RNA was isolated using the Trizol reagent (Invitrogen, Gaithersburg, MD, United States) according to the manufacturer’s instructions. The RNA samples were treated with RNAase-free DNase (TaKaRa Bio Inc., Dalian, China) to eliminate traces of DNA, followed by the quantification by using the NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, United States). Afterward, total RNA (2 μg) was reverse-transcribed using an oligo(dT) primer and M-MLV reverse transcriptase (BioTeke, Beijing, China).
Real-time qPCR reactions were performed using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) with SYBR®Premix Ex TaqTM (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. The primer sequence information was listed in Supplementary Table S1. Relative expression levels of corresponding genes were presented as values relative to the control samples at the indicated time points, after normalization with Actin transcript levels (Zhu et al., 2016).
Proteomics Analysis
The total proteins in tomato root tissues were extracted by Plant Total Protein Extraction Kit (Sigma-Aldrich, St Louis, MO, United States). Protein samples (200 μg BSA equivalent) were digested using filter-aided sample preparation (FASP) method (Wiśniewski et al., 2009). The protein extraction was reduced with 10 mM dithiothreitol (DTT) for 1 h at 56°C, and then alkylated with 55 mM iodoacetamide (IAA) for 45 min at 25°C in darkness. Afterward, the protein samples were buffer-exchanged with 100 mM NH4HCO3 (pH 8.0–8.5) using 10 kDa molecular weight cut-off Amicon Spin Tube (Millipore, Billerica, MA, United States). Subsequently, 4 μg of sequencing-grade modified trypsin (Promega) was added to each sample, and digestion was carried out overnight at 37°C (trypsin: protein ration = 1: 50). Digested peptides were desalted by Ziptip C18 (Milipore) and quantified using a NanoDrop 2000 spectrophotometer (Wilmington, United States).
For LC-MS/MS conditions, a label-free quantitative method was used to detect the relative amount of proteins. Three biological replicates from three independent experiments (about 60 roots for each independent experiment) of β-CDH-treated and control groups were analyzed by nano LC system (Dionex, part of Thermo Fisher Scientific) on-line coupled to LTQ-Orbitrap mass spectrometer (Thermo Electron, Bremen, Germany). The resulting peptides (1.5 μg) were acidified with 0.1% formic acid (FA), and subsequently loaded onto the nano trap column (Acclaim PepMap100 C18, 75 μm × 2 cm, 3 μm, 100 Å, Thermo Scientific) at a flow rate of 4 μL⋅min-1 in loading buffer (2% acetonitrile, 0.1% FA in HPLC-grade water). Chromatographic separation was carried out on the analytical column (Acclaim PepMap®RSLC, C18, 75 μm × 15 cm, 3 μm, 100Å, Thermo Scientific) using a linear gradient of 3–55% buffer B (80% acetonitrile and 0.1% FA) at a flow rate of 0.25 μL⋅min-1 over 112 min. Due to loading and washing steps, the total time for an LC-MS/MS run was 160 min longer. For LTQ-Orbitrap analysis, one scan cycle included an MS1 scan (m/z 300–1800) at a resolution of 60,000, followed by 10 MS2 scans by LTQ, to fragment the 10 most abundant precursor ions at normalized collision energy of 35 eV. The lock mass calibration was activated, and dynamic exclusion time was set to 30 s.
Raw data were analyzed by MaxQuant (version 1.5.2.5) (Tyanova et al., 2016) using standard settings with the additional options match between runs, and LFQ selected. The generated ‘proteingroups.txt’ table was filtered for contaminants, reverse hits, and number of unique peptides (>0) in Perseus (from MaxQuant package).
Data Analysis
Where indicated, results were expressed as the mean values ± SE of at least three independent experiments (with at least three replicates for each) with similar results. Statistical analysis was performed using SPSS 17.0 software. For statistical analysis, one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (P < 0.05) was chosen.
Results
Endogenous H2O2 Production Is induced by β-CDH
First, tomato seedlings were loaded with reactive oxygen species (ROS)-specific fluorescent dye 2′,7′-dichlorofluorescein diacetate (H2DCF-DA), and LCSM was used to investigate changes in ROS-induced fluorescence. Since the DCF-dependent green fluorescence detected in 100 μM H2O2-treated tomato seedlings for 12 h, was obviously impaired following the addition of DMTU and AsA, two membrane-permeable scavengers of H2O2 (Figure 1), the visual signal can be mostly ascribed to endogenous H2O2 accumulation. Thus, the fluorescence was used to report endogenous H2O2 levels subsequently.
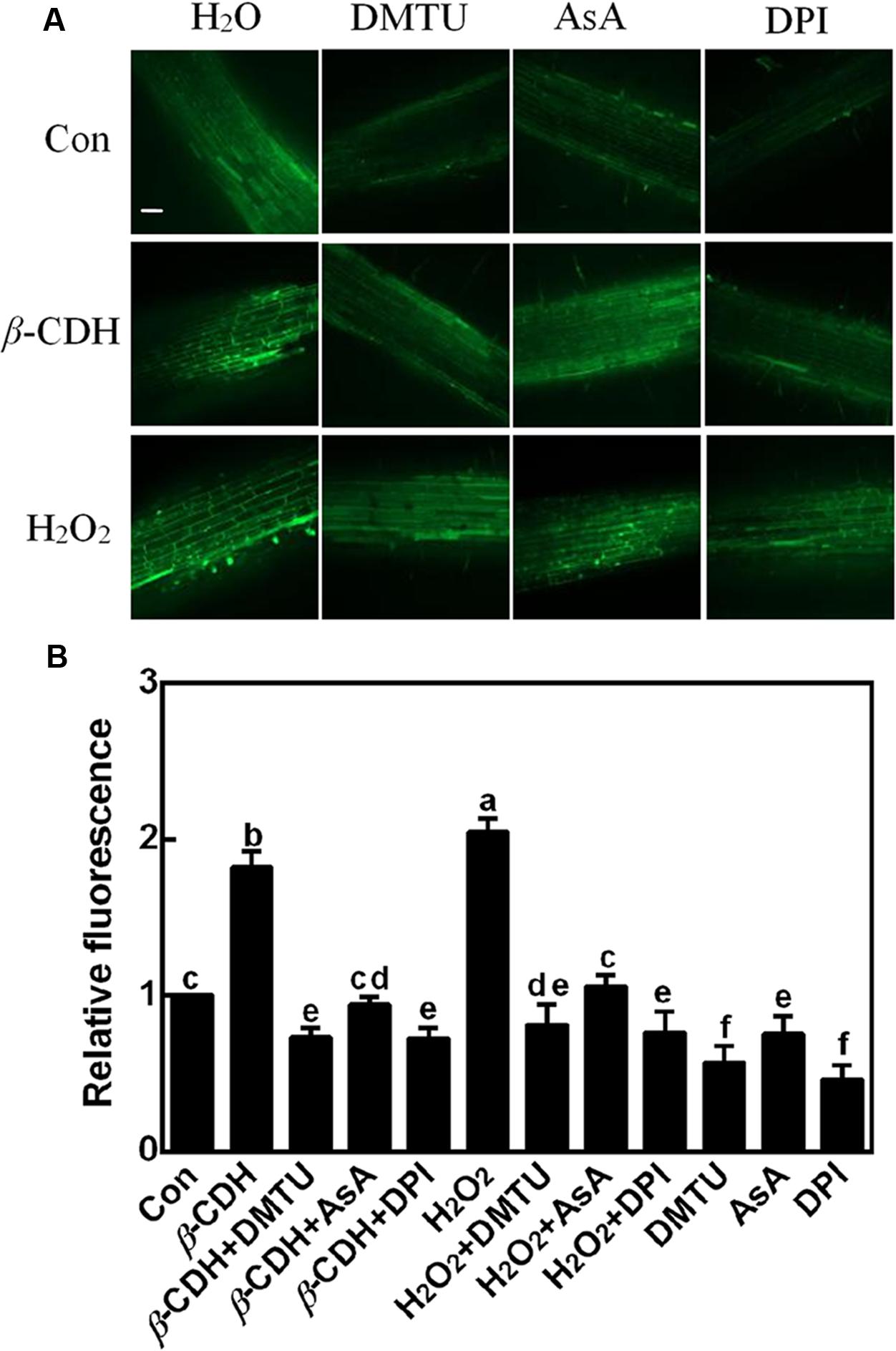
FIGURE 1. Changes of endogenous H2O2 production. Three-day-old tomato seedlings were treated with H2O (Con), 1 nM β-CDH, 100 μM H2O2, 500 μM DMTU, 200 μM AsA, and 0.1 μM DPI, alone or their combinations for 12 h. Corresponding confocal images of DCF-dependent fluorescence in seedling roots was shown in (A). Bar = 55 μm. Meanwhile, the relative fluorescence (B) was presented as values relative to control. Mean and SE values were calculated from at least three independent experiments with at least three replicates for each. Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Further result showed that, compared to the control sample, the addition of 1 nM β-CDH for 12 h was able to induce endogenous H2O2 production in tomato seedlings, mimicking the response of H2O2 when was exogenously applied. We also noticed that this time point of H2O2 production triggered by β-CDH and exogenous H2O2, apparently preceded LR formation, beginning at 48 h of treatments (Li et al., 2015). Above results indicated the possible link between endogenous H2O2 production and LR formation triggered by β-CDH.
The Removal of H2O2 Prevents β-CDH-Induced H2O2 Production and Thereafter LR Formation
In order to evaluate the possible role of endogenous H2O2 in β-CDH-induced LR development, DMTU and AsA were also used. Similar to the previous reports (Ma et al., 2014; Li et al., 2015; Zhu et al., 2016), both 1 nM β-CDH and 100 μM H2O2 increased tomato LR density and number (Figures 2A–C). Meanwhile, no significant difference in PR length was observed (Figure 2D). By contrast, the co-treatment with DMTU and AsA respectively not only blocked endogenous H2O2 production (Figure 1), but also arrested the thereafter induction of LR formation (Figure 2), triggered by exogenous β-CDH and H2O2. When applied alone, DMTU (in particularly) and AsA could inhibit LR formation respect to the chemical-free control plants. Meanwhile, endogenous H2O2 levels were also decreased (Figure 1).
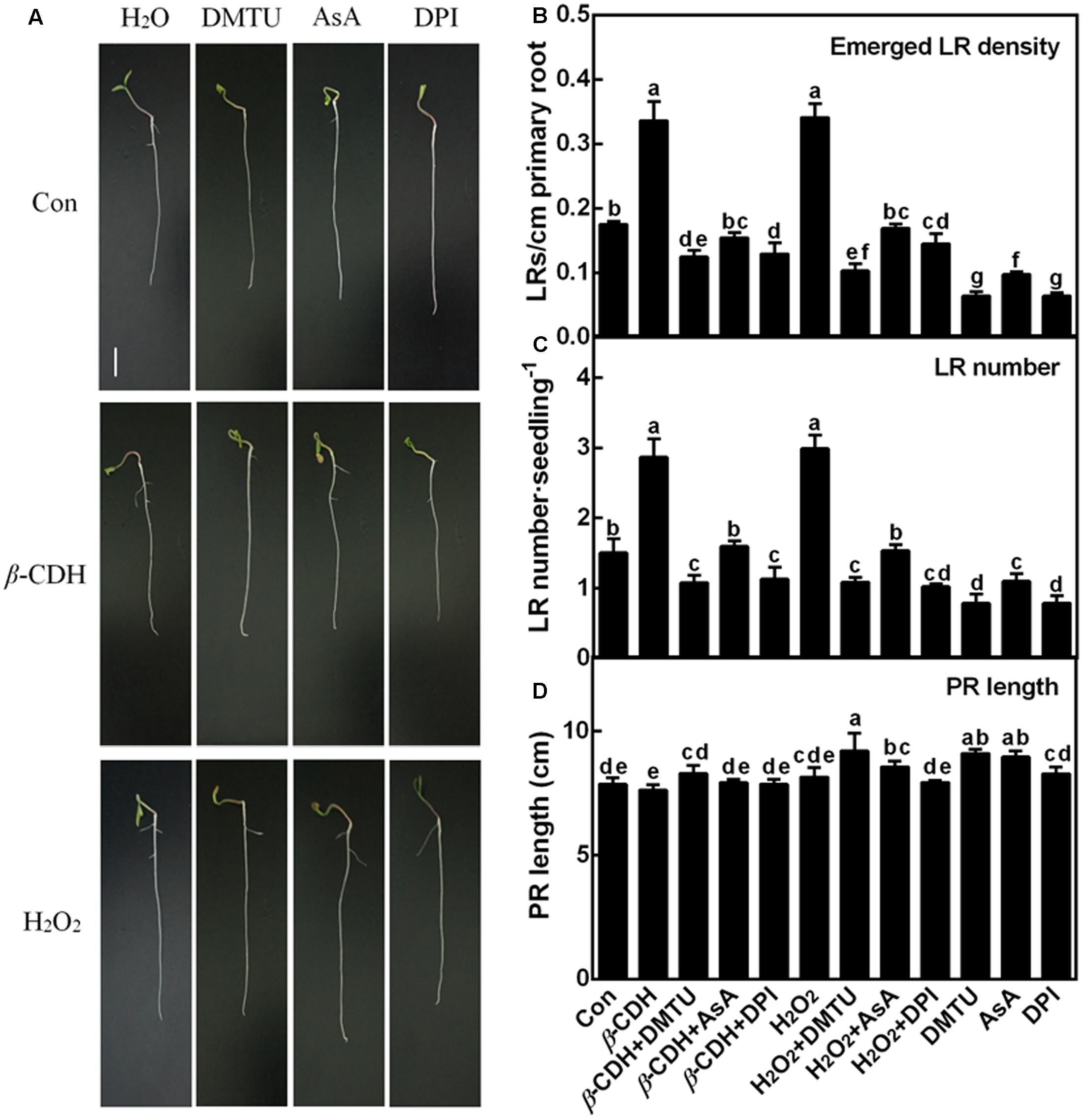
FIGURE 2. The lateral root formation triggered by β-CDH was blocked by H2O2 depletion. Three-day-old tomato seedlings were treated with H2O (Con), 1 nM β-CDH, 100 μM H2O2, 500 μM DMTU, 200 μM AsA, and 0.1 μM DPI, alone or their combinations for 4 days. Afterward, corresponding photographs were taken (A). Bar = 1 cm. Meanwhile, the emerged LR density (B), the number of emerged LRs (>1 mm) per seedling (C), and the primary root (PR) length (D) were determined. Mean and SE values were calculated from at least three independent experiments with at least three replicates for each. Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Generation of H2O2 and Induction of Lateral Rooting by β-CDH Are Mediated Partly by NADPH Oxidase
For the origin of endogenous H2O2, the plasma-membrane (PM) NADPH oxidase confers important roles in H2O2 signaling (Desikan et al., 2006; Xie et al., 2011). Since DPI is an inhibitor of NADPH oxidase responsible for endogenous H2O2 production during LR formation (Ma et al., 2014), this chemical was applied together with β-CDH. Similar to the inhibition responses of DMTU and AsA (Figures 1, 2), β-CDH-induced H2O2 and LR formation were respectively impaired by 0.1 μM DPI, suggesting the possible role of NADPH oxidase-dependent H2O2 in β-CDH action. When applied alone, DPI, similar to DMTU (in particular) and AsA, could inhibit LR formation, compared to the chemical-free control plants (Figure 2). Changes in endogenous H2O2 displayed the similar tendencies (Figure 1).
β-CDH- and H2O2-Triggered Lateral Root Primordial (LRP) Are Impaired by DMTU, DPI, and AsA
Further microscopical analysis showed that both β-CDH- and H2O2-triggered LR primordial (LRP; 3 days) exhibited a similar accelerated anatomic structure, both of which were individually impaired by the cotreatment with DMTU, DPI or AsA (Figure 3). When applied alone, DMTU, DPI, or AsA strongly inhibited the development of LRP. We also noticed that above results were comparable to the phenotypes in the LR formation (Figure 2).
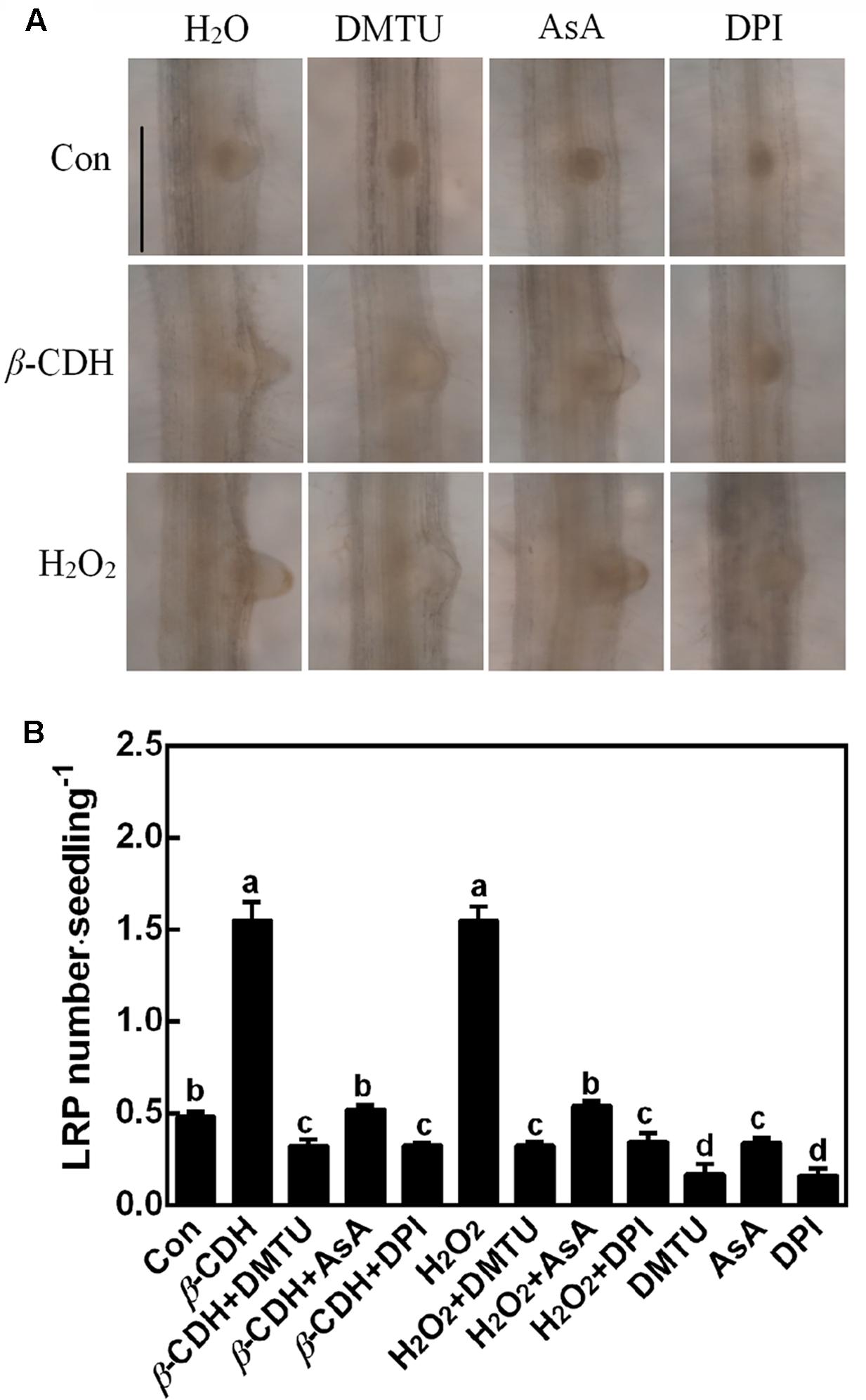
FIGURE 3. β-CDH-induced lateral root primordial (LRP) is sensitive to H2O2 depletion. Three-day-old tomato seedlings were treated with H2O (Con), 1 nM β-CDH, 100 μM H2O2, 500 μM DMTU, 200 μM AsA, and 0.1 μM DPI, alone or their combinations for 3 days. Afterward, photographs showing the representative morphology of LRP (about 75% of LRP at the shown stages) were taken (A). Bar = 0.25 mm. Meanwhile, the number of emerged LRP was also analyzed (B). Mean and SE values were calculated from at least three independent experiments with at least three replicates for each. Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Both β-CDH- and H2O2-Up-Regulated RBOH1 Are Sensitive to the Removal of H2O2
The inhibiting effect of DPI on β-CDH-elicited LR formation suggested the possible role of NADPH oxidase. The following experiments were carried out to test above hypothesis. As shown in Figure 4, the transcript of RBOH1 was rapidly increased after β-CDH or exogenous H2O2 treatments for 6 h. Meanwhile, the removal of H2O2 (Figure 1) by the scavengers of H2O2 (DMTU and AsA) and inhibitor of NADPH oxidase (DPI) completely blocked above responses. Similarly, DMTU, AsA and DPI alone also exhibited the inhibition in RBOH1 expression compared to control samples.
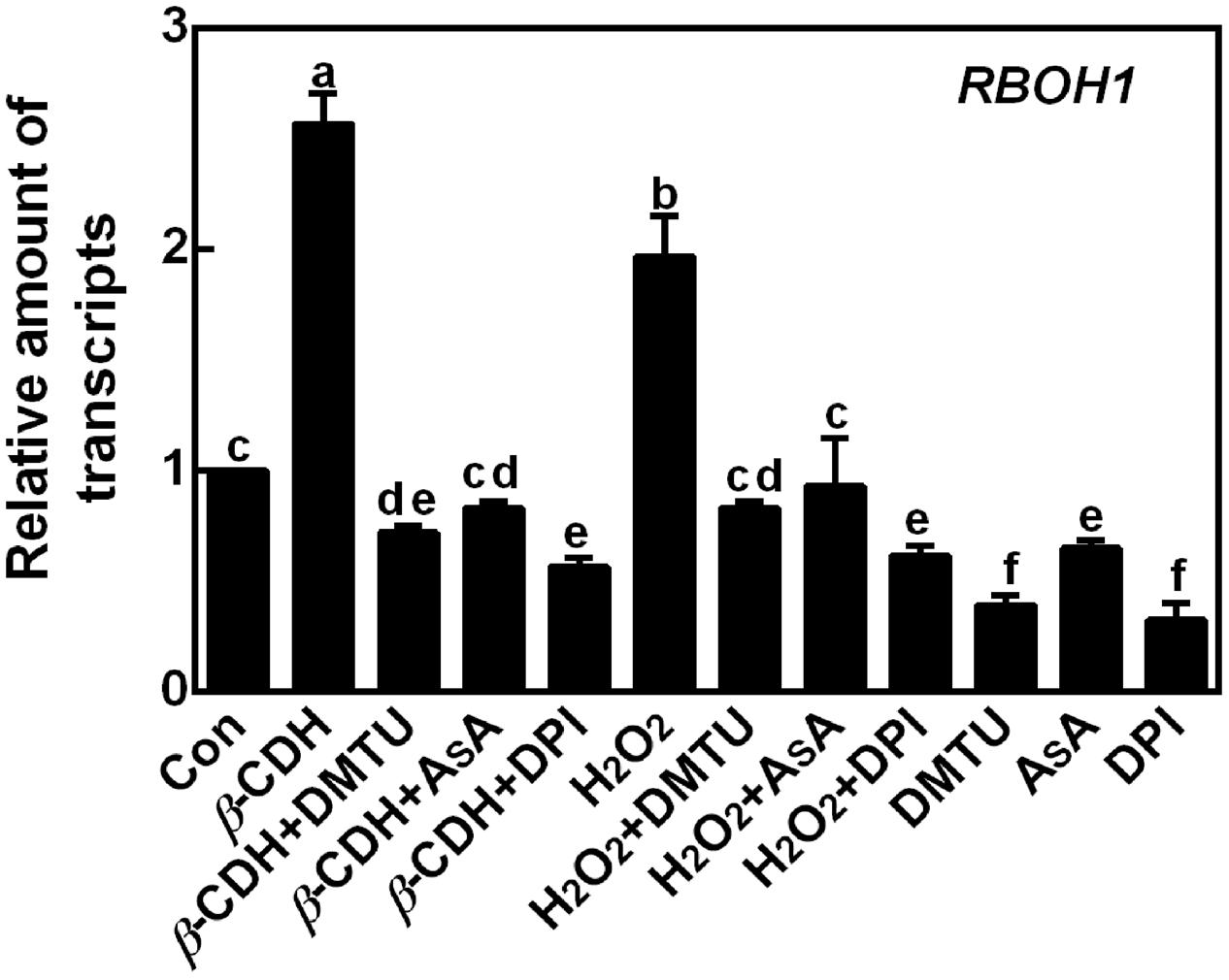
FIGURE 4. β-CDH-induced RBOH1 transcript is blocked by H2O2 depletion. Three-day-old tomato seedlings were treated with H2O (Con), 1 nM β-CDH, 100 μM H2O2, 500 μM DMTU, 200 μM AsA, and 0.1 μM DPI, alone or their combinations for 6 h. Afterward, the amounts of RBOH1 transcript were analyzed by qPCR, and presented relative to the control sample. Mean and SE values were calculated from at least three independent experiments with at least three replicates for each. Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
The Transcripts of Target Genes Are Regulated by β-CDH and H2O2
Furthermore, the transcripts of four cell cycle regulatory genes, CYCA3;1, CYCA2;1, CYCD3;1, and CDKA1, were analyzed by qPCR as molecular probes to further investigate the role of H2O2 in β-CDH-induced LR formation. After 12 h of β-CDH treatment, above transcripts were up-regulated (Figure 5). Similar results appeared in H2O2-treated seedlings. However, the addition with DMTU, AsA, or DPI could significantly prevent β-CDH- and H2O2-induced cell cycle regulatory gene expression, all of which were well matched with the LPR number, LR number and density (Figures 2, 3).
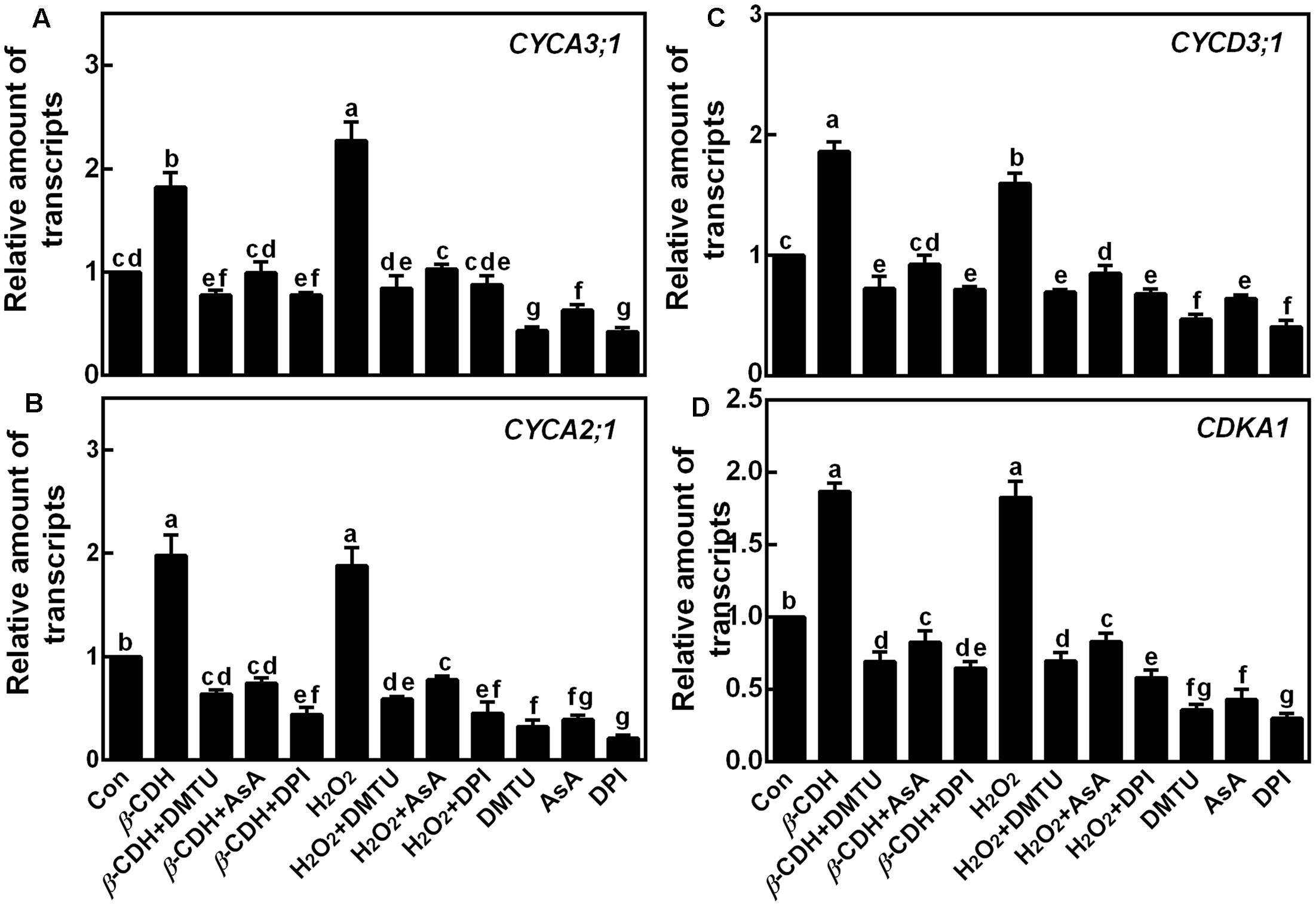
FIGURE 5. Changes of the transcripts of cell cycle genes. Three-day-old tomato seedlings were treated with H2O (Con), 1 nM β-CDH, 100 μM H2O2, 500 μM DMTU, 200 μM AsA, and 0.1 μM DPI, alone or their combinations for 12 h. Afterward, the amounts of CYCA3;1 (A), CYCA2;1 (B), CYCD3;1 (C), and CDKA1 (D) transcripts were analyzed by qPCR, and presented relative to the control sample. Mean and SE values were calculated from at least three independent experiments with at least three replicates for each. Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Subsequent experiment revealed that β-CDH and H2O2 were able to up-regulate the transcripts of auxin signaling genes (ARF1 and RSI-1), and an auxin influx carrier gene (LAX3), together with the down-regulation of IAA14 (encoding a member of the Aux/IAA protein family) and two auxin efflux carriers genes (PIN3 and PIN7; Figure 6). As we expected, the co-treatment with DMTU, AsA, or DPI differently blocked the above mentioned effects. Combined with corresponding endogenous H2O2 production (Figure 1) and phenotypes (Figures 2, 3), these findings suggested that above genes might be the targets of H2O2 signaling in β-CDH-induced tomato LR formation.
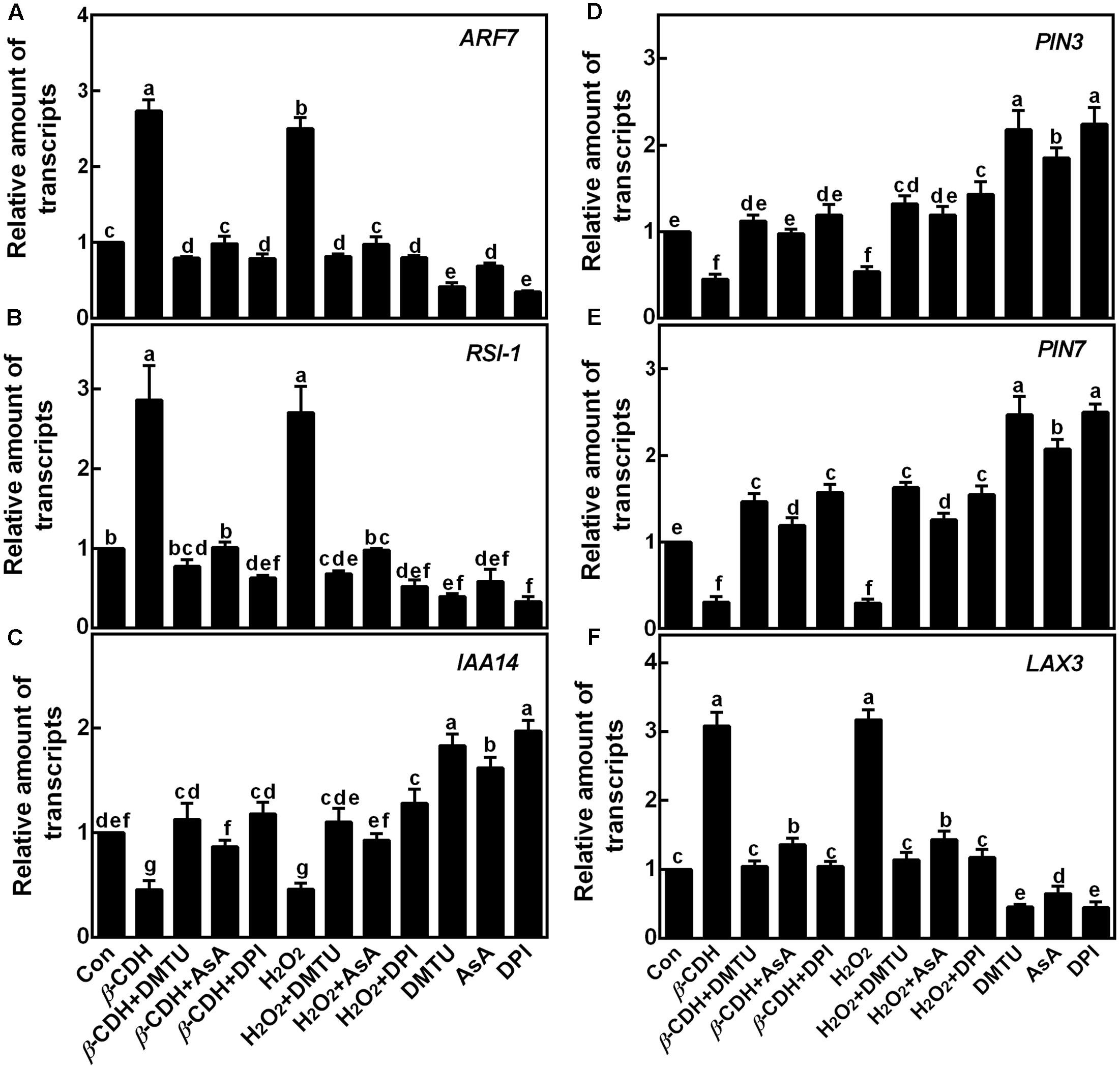
FIGURE 6. Changes of the transcripts of the target genes. Three-day-old tomato seedlings were treated with H2O (Con), 1 nM β-CDH, 100 μM H2O2, 500 μM DMTU, 200 μM AsA, and 0.1 μM DPI, alone or their combinations for 12 h. Afterward, the amounts of ARF7 (A) and RSI-1 (B; two auxin signaling genes), IAA14 (C; encoding a member of the Aux/IAA protein family), PIN3 (D) and PIN7 (E; two auxin efflux carriers), and LAX3 (F; an auxin influx carrier) transcripts were analyzed by qPCR, and presented relative to the control sample. Mean and SE values were calculated from at least three independent experiments with at least three replicates for each. Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Comparative Proteomic Analysis and Its Transcription Confirmation
To well address molecular mechanism of β-CDH-induced LR formation, comparative proteomic analysis from tomato seedling roots in the presence or absence of β-CDH was performed with LC-MS/MS. In this study, a total of 86 proteins were identified significantly regulated (fold change > 1.5 or < 0.667) after β-CDH treatment under P value < 0.05 (Supplementary Table S2). Some ROS metabolism related proteins were modulated by β-CDH treatment, such as Isocitrate dehydrogenase [NADP] (decreased), Catalase, Succinic semialdehyde reductase isofom1 (SSR1), NADH-cytochrome b5 reductase 1 (increased), etc (Table 1). Meanwhile, proteins related to LR formation, like L-ascorbate oxidase (increased), Protein ROOT HAIR DEFECTIVE 3, DWARF1/DIMINUTO, Phenylalanine ammonia-lyase, and Glycine rich RNA binding protein 1a, were found be regulated after β-CDH treatment. Furthermore, 20 proteins like Malic enzyme, Coatomer subunit alpha, PR10 protein, and 40S ribosomal protein S8, etc. were identified to be working in other biological process, such as metabolic process, intracellular transport, response to stress, and protein metabolic process, etc. Additionally, we also noticed that membrane-associated NADPH oxidase protein was not found in our experimental conditions.
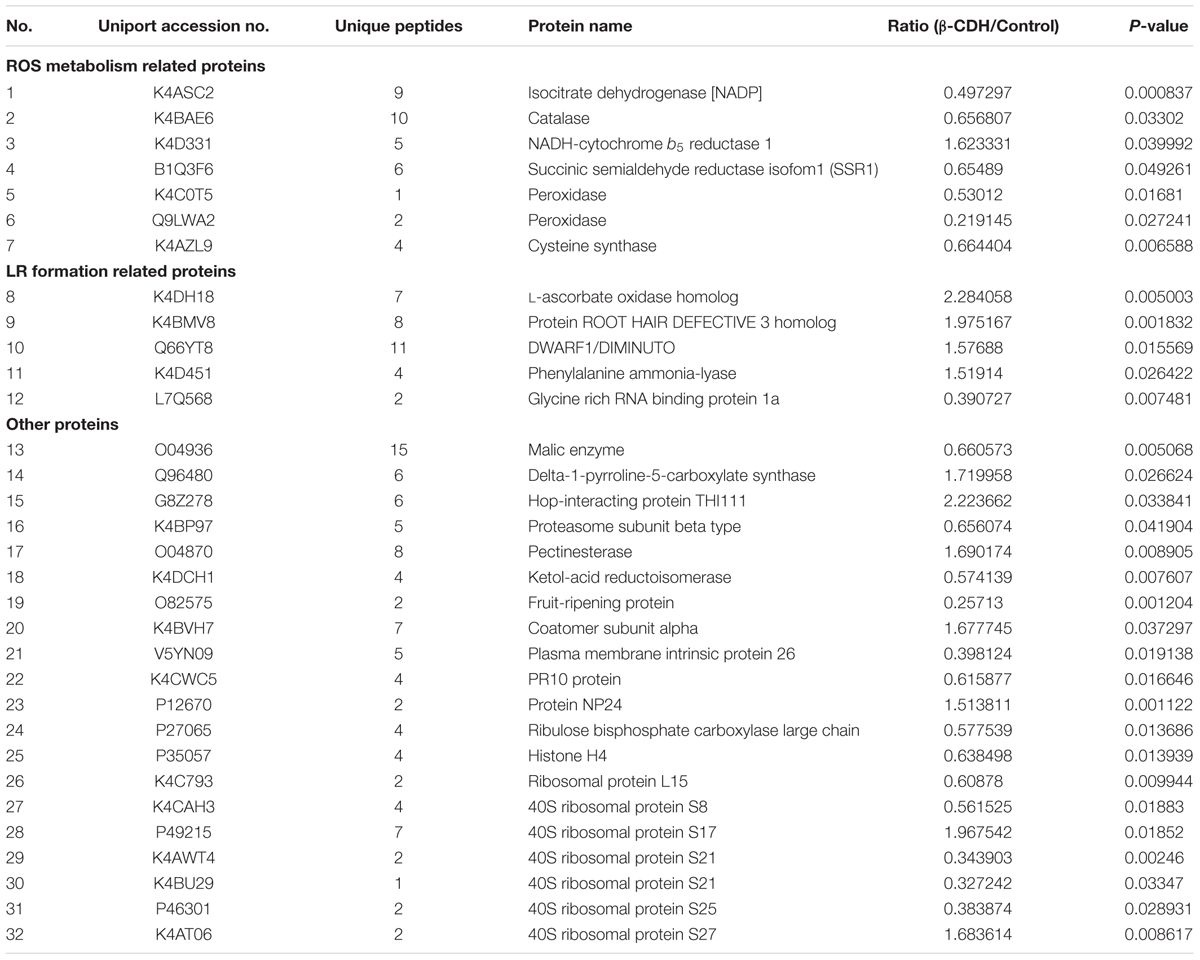
TABLE 1. Proteins in tomato seedling roots that differentially expressed greater than 1.5-fold or less than 0.667-fold after β-CDH treatment for 48 h using MaxQuant analysis.
To confirm above results, we further tested the effects of H2O2 scavengers and inhibitor on the transcripts of three representative genes (Table 1), isocitrate dehydrogenase [NADP], NADH-cytochrome b5 reductase, and L-ascorbate oxidase homolog (Figure 7). Results showed that, the added H2O2 scavengers (DMTU and AsA) and synthetic inhibitor (DPI) could effectively prevent the down-regulation of isocitrate dehydrogenase [NADP] gene expression elicited by β-CDH and H2O2. Whereas, the up-regulated NADH-cytochrome b5 reductase and L-ascorbate oxidase homolog (in particular) transcripts were blocked. These results could be well consistent with the data form LC-MS/MS (Table 1).
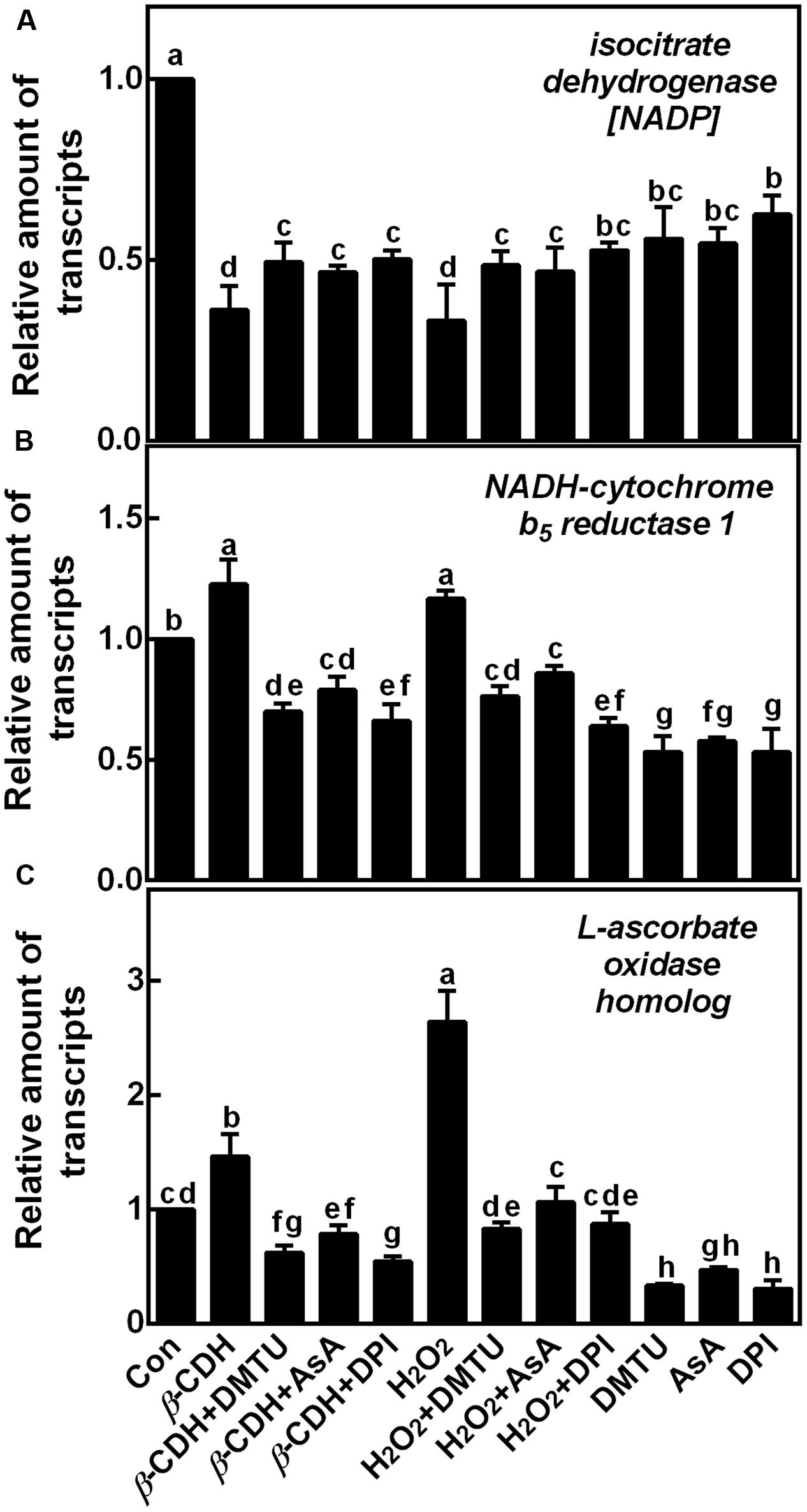
FIGURE 7. Changes of corresponding gene transcripts related to ROS metabolism and LR formation. Three-day-old tomato seedlings were treated with H2O (Con), 1 nM β-CDH, 100 μM H2O2, 500 μM DMTU, 200 μM AsA, and 0.1 μM DPI, alone or their combinations for 36 h. The relative amount of isocitrate dehydrogenase [NADP] (A), NADH-cytochrome b5 reductase 1 (B), and L-ascorbate oxidase homolog (C) transcripts were detected by qPCR, and presented relative to the control sample. Mean and SE values were calculated from three independent experiments with at least three replicates for each. Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple test.
Discussion
Although the induction of LR formation by β-CDH was reported in previous studies (Li et al., 2015; Zhu et al., 2016), the detailed molecular mechanism is still not fully elucidated. In this report, we further show that endogenous H2O2 is involved in β-CDH-mediated LR formation in tomato seedlings, and β-CDH-elicited H2O2-related target proteins responsible for LR formation might be, at least partially, regulated at transcriptional and translational levels.
H2O2 Is Involved in β-CDH-Induced LR Formation
It was well-known that H2O2 functions as a signaling molecule in regulating stress responses, development, and other cell processes (Neill et al., 2002; Cuypers et al., 2016; Niu and Liao, 2016). As expected, in our experimental condition, an increased endogenous H2O2 was induced by β-CDH in tomato seedlings (Figure 1). Evidences showed that, there are several enzymes that can produce H2O2 in plants, such as cell wall peroxidase, amine oxidase, flavin-containing enzymes, and NADPH oxidase in particularly (Cona et al., 2006; Xie et al., 2011; Francoz et al., 2015; Niu and Liao, 2016). We further identified that β-CDH-elicited H2O2 production resulted from the up-regulation of RBOH1 gene expression (Figure 4). The involvement of NADPH oxidase in β-CDH-triggered LR formation was further corroborated by the findings that NADPH oxidase inhibitor DPI (Bai et al., 2012; Ma et al., 2014) not only inhibited H2O2 production (Figure 1), but also caused a significant reduction of LR formation in β-CDH-treated seedlings (Figures 2, 3). Meanwhile, the removal of endogenous H2O2 by DMTU and AsA exhibited the similar blocking tendencies, further confirming that LR formation elicited by β-CDH is closely related to endogenous H2O2 concentration. Although we can not exclude the possibility that these chemical agents used in the present study may not specifically target H2O2, these results clearly revealed that β-CDH-stimulated H2O2-mediated LR formation was partly dependent on NADPH oxidase. Consistently, a recent genetic result revealed that RBOH-mediated ROS production facilitated LR emergence in Arabidopsis (Orman-Ligeza et al., 2016).
Cell cycle activation is an important event during LR formation (Himanen et al., 2002, 2004; Casimiro et al., 2003). In the pervious reports, cell cycle genes CYCA3;1, CYCA2;1, CYCD3;1, and CDKA1 were used as the molecular markers in tomato LR formation (Correa-Aragunde et al., 2006; Xu et al., 2011). Similar to the previous results (Li et al., 2015; Zhu et al., 2016), β-CDH treatment up-regulated CYCA3;1, CYCA2;1, CYCD3;1, and CDKA1 gene expression, and these responses mimicked the effects of H2O2 (Figure 5; Ma et al., 2014). By contrast, above β-CDH- and H2O2-induced expression of cell cycle regulatory genes were prevented or delayed when H2O2 was scavenged by DMTU or AsA, or its synthesis was inhibited with DPI (Figure 1). Combined with the impaired LRP and LR formation by the removal of H2O2 levels (Figures 1–3), our findings gave further evidence, suggesting that β-CDH-triggered H2O2 production was able to modulate the expression of cell cycle regulatory genes and this event is also required for LR formation in tomato seedlings.
Auxin controls cell cycle progression and asymmetric divisions during LR formation (Casimiro et al., 2001; Bhalerao et al., 2002; Lavenus et al., 2013). In our experimental conditions, it was further confirmed that, similar to the responses elicited by H2O2, β-CDH up-regulated two auxin signaling genes (ARF7 and RSI-1; Zhu et al., 2016) and an auxin influx carrier gene (LAX3), together with the down-regulation of IAA14 (encoding a member of the Aux/IAA protein family) and two auxin efflux carriers genes (PIN3 and PIN7; Figure 6). Comparatively, the removal of endogenous H2O2 drastically impaired corresponding changes conferred by β-CDH and H2O2. Previous results showed that slr-1, a gain-of-function mutant of IAA14 exhibited a crucial defect in LR formation in Arabidopsis (Fukaki et al., 2002). Three Arabidopsis thaliana mutants, lax3, pin3 and pin7, which are defective in auxin influx and efflux proteins, showed reduced or increased LR formation (Swarup et al., 2008; Lewis et al., 2011). Combined with previous genetic results, our molecular and pharmacologic evidence further indicated a possible link between β-CDH-induced H2O2-mediated LR formation and auxin signaling. This deduction should be investigated at genetic levels in the near future.
Proteomic Analysis Revealed the Target Proteins in the Process of β-CDH-Stimulated LR Formation
Proteomic analysis showed the presence of 86 proteins which were significantly regulated by β-CDH treatment for 48 h (Supplementary Table S2). Among these proteins, some were concerned with ROS signaling (Table 1). For example, Isocitrate dehydrogenase [NADP] (NADP-ICDH; EC 1.1.1.42; K4ASC2) catalyzes oxidative decarboxylation of isocitrate to 2-oxoglutarate using NADP+ to form NADPH, and the latter is an important cofactor in many biosynthesis pathways and important in cellular defense against oxidative damage (Lee et al., 2002). It was reported that, NADP-ICDH can be damaged by ROS, and the inactivation of ICDH may lead to the perturbation of the antioxidant defense system in many cell process (Lee S.M. et al., 2001). In our experimental conditions, the amount of NADP-ICDH protein was decreased after β-CDH treatment (Table 1), which was in line with the increased ROS in seedling roots (Figure 1). Similarly, the protein level of the major H2O2 scavenging enzyme, catalase (CAT; EC 1.11.1.6; K4BAE6), was also decreased by β-CDH. This was consistent with a higher concentration of H2O2 in tomato seedling roots after β-CDH treatment, because the altering of CAT level can modulate H2O2 levels in plant cells (Vandenabeele et al., 2004). NADH-cytochrome b5 reductase (K4D331) is found to play a key role in the NADH-dependent reduction of D-erythroascorbyl free radical, and can be active in the oxidative stress response of Saccharomyces cerevisiae (Lee J.S. et al., 2001). In HeLa cells, H2O2 -regulated expression of NADH-cytochrome b5 reductase was previously reported (Bello et al., 2003). In this study, the level of NADH-cytochrome b5 reductase protein was increased when β-CDH was supplied, also confirming that a rapid H2O2 production appeared in seedling roots (Figure 1).
Besides the changes of H2O2 and redox related proteins, the β-CDH could regulate some proteins related to LR formation, for example, L-ascorbate oxidase (AO; EC 1.10.3.3; K4DH18), Protein ROOT HAIR DEFECTIVE 3 homolog (RHD3; K4BMV8), DWARF1/DIMINUTO (Q66YT8), Phenylalanine ammonia-lyase (PAL; EC 4.3.1.5; K4D451), and Glycine rich RNA binding protein 1a (atRZ-1a; L7Q568). The activity and expression of AO are closely correlated with cell expansion, which implies a role in cell wall loosening, cell division, and cell elongation (Kato and Esaka, 1999; Sanmartin et al., 2003). In this study, the level of AO protein was induced by β-CDH (Table 1), and there might be a link between AO expression and β-CDH-induced lateral rooting. Protein ROOT HAIR DEFECTIVE 3 (RHD3; K4BMV8) encodes an 89 kD polypeptide with putative GTP-binding motifs, with a common function in eukaryotic cell enlargement (Wang et al., 1997). The rhd3 mutation alters the size of roots and root hairs. Here, our results showed that RHD3 homolog protein level was increased by β-CDH. DWARF1/DIMINUTO (Q66YT8) gene encodes a protein involved in steroid as well as brassinosteroid (BR) synthesis (Klahre et al., 1998). BRs are known interacting with auxin to promote LR development in Arabidopsis (Bao et al., 2004). Thus, the elevated DWARF1/DIMINUTO protein level can help to lateral rooting. Phenylalanine ammonia-lyase (PAL; EC 4.3.1.5; K4D451) is reported to be highly regulated during development and xylogenesis with the cell wall polymer lignin (Elkind et al., 1990). An increased PAL protein level was found during β-CDH-induced lateral rooting (Table 1). Glycine rich RNA binding protein 1a (atRZ-1a; L7Q568) over-expression plants showed delayed germination and seedling growth under salt and drought stresses (Kim et al., 2007). Since abiotic stress could induce LR formation, an important phenomenon of the stress-induced morphogenic response (SIMR) in plants (Potters et al., 2007), we further deduced that the decreased level in atRZ-1a protein by β-CDH might lead to a positive influence in LR formation. Additionally, changes in three representative genes related to ROS metabolism and LR formation, including isocitrate dehydrogenase [NADP] (A), NADH-cytochrome b5 reductase 1 (B), and L-ascorbate oxidase homolog (Figure 7), approximately matched with corresponding proteomic data.
In summary, our results showed a vital role of H2O2 in the β-CDH-induced tomato LR formation, and β-CDH-elicited H2O2-related target proteins might be, at least partially, regulated at transcriptional and translational levels.
Author Contributions
Conception and design of the study: WC, DZ, and LH. Acquisition of data for the study: WC, DZ, WenS, YM, DH, YS, YR, WeiS, QG, and DX. Analysis of data for the work: WC, DZ, and LH. Interpretation of data for the work: WC, DZ, WenS, WeiS, and LH. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31201617), the Natural Science Foundation of Jiangsu Province (BK20141361), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01445/full#supplementary-material
References
Alvarez, M. E., Pennell, R. I., Meijer, P. J., Ishikawa, A., Dixon, R. A., and Lamb, C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784. doi: 10.1016/S0092-8674(00)81405-1
Bai, X., Todd, C. D., Desikan, R., Yang, Y., and Hu, X. (2012). N-3-oxo-decanoyl-L-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide-and nitric oxide-dependent cyclic Gmp signaling in mung bean. Plant Physiol. 158, 725–736. doi: 10.1104/pp.111.185769
Bao, F., Shen, J., Brady, S. R., Muday, G. K., Asami, T., and Yang, Z. (2004). Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol. 134, 1624–1631. doi: 10.1104/pp.103.036897
Bello, R. I., Alcaín, F. J., Gómez-Díaz, C., López-Lluch, G., Navas, P., and Villalba, J. M. (2003). Hydrogen peroxide- and cell-density-regulated expression of NADH-cytochrome b5 reductase in HeLa cells. J. Bioenerg. Biomembr. 35, 169–179. doi: 10.1023/A:1023702321148
Benková, E., and Bielach, A. (2010). Lateral root organogenesis-from cell to organ. Curr. Opin. Plant Biol. 13, 677–683. doi: 10.1016/j.pbi.2010.09.006
Bhalerao, R. P., Eklöf, J., Ljung, K., Marchant, A., Bennett, M., and Sandberg, G. (2002). Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 29, 325–332. doi: 10.1046/j.0960-7412.2001.01217.x
Bodine, K. D., Gin, D. Y., and Gin, M. S. (2004). Synthesis of readily modifiable cyclodextrin analogues via cyclodimerization of an alkynyl-azido trisaccharide. J. Am. Chem. Soc. 126, 1638–1639. doi: 10.1021/ja039374t
Cao, Z., Fang, T., Chen, M., Li, J., Shen, W., and Huang, L. (2014). Involvement of haem oxygenase-1 in hydrogen peroxide-induced lateral root formation in tomato. Acta Physiol. Plant. 36, 931–943. doi: 10.1007/s11738-013-1472-x
Casimiro, I., Beeckman, T., Graham, N., Bhalerao, R., Zhang, H., Casero, P., et al. (2003). Dissecting Arabidopsis lateral root development. Trends Plant Sci. 8, 165–171. doi: 10.1016/S1360-1385(03)00051-7
Casimiro, I., Marchant, A., Bhalerao, R. P., Beeckman, T., Dhooge, S., Swarup, R., et al. (2001). Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13, 843–852. doi: 10.1105/tpc.13.4.843
Cona, A., Rea, G., Botta, M., Corelli, F., Federico, R., and Angelini, R. (2006). Flavin-containing polyamine oxidase is a hydrogen peroxide source in the oxidative response to the protein phosphatase inhibitor cantharidin in Zea mays L. J. Exp. Bot. 57, 2277–2289. doi: 10.1093/jxb/erj195
Correa-Aragunde, N., Graziano, M., Chevalier, C., and Lamattina, L. (2006). Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J. Exp. Bot. 57, 581–588. doi: 10.1093/jxb/erj045
Cuypers, A., Hendrix, S., dos Reis, R. A., De Smet, S., Deckers, J., Gielen, H., et al. (2016). Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front. Plant Sci. 7:470. doi: 10.3390/fpls.2016.00470
Desikan, R., Cheung, M. K., Bright, J., Henson, D., Hancock, J. T., and Neill, S. J. (2004). ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot. 55, 205–212. doi: 10.1093/jxb/erh033
Desikan, R., Last, K., Harrett-Williams, R., Tagliavia, C., Harter, K., Hooley, R., et al. (2006). Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 47, 907–916. doi: 10.1111/j.1365-313X.2006.02842.x
Elkind, Y., Edwards, R., Mavandad, M., Hedrick, S. A., Ribak, O., Dixon, R. A., et al. (1990). Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc. Natl. Acad. Sci. U.S.A. 87, 9057–9061. doi: 10.1073/pnas.87.22.9057
Fang, T., Li, J., Cao, Z., Chen, M., Shen, W., and Huang, L. (2014). Heme oxygenase-1 is involved in sodium hydrosulfide-induced lateral root formation in tomato seedlings. Plant Cell Rep. 33, 969–978. doi: 10.1007/s00299-014-1577-8
Francoz, E., Ranocha, P., Nguyen-Kim, H., Jamet, E., Burlat, V., and Dunand, C. (2015). Roles of cell wall peroxidases in plant development. Phytochemistry 112, 15–21. doi: 10.1016/j.phytochem.2014.07.020
Fukaki, H., Tameda, S., Masuda, H., and Tasaka, M. (2002). Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 29, 153–168. doi: 10.1046/j.0960-7412.2001.01201.x
Fukaki, H., and Tasaka, M. (2009). Hormone interactions during lateral root formation. Plant Mol. Biol. 69, 437–449. doi: 10.1007/s11103-008-9417-2
Himanen, K., Boucheron, E., Vanneste, S., de Almeida Engler, J., Inzé, D., and Beeckman, T. (2002). Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14, 2339–2351. doi: 10.1105/tpc.004960
Himanen, K., Vuylsteke, M., Vanneste, S., Vercruysse, S., Boucheron, E., Alard, P., et al. (2004). Transcript profiling of early lateral root initiation. Proc. Natl. Acad. Sci. U.S.A. 101, 5146–5151. doi: 10.1073/pnas.0308702101
Kato, N., and Esaka, M. (1999). Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol. Plant. 105, 321–329. doi: 10.1034/j.1399-3054.1999.105218.x
Kim, Y. O., Pan, S. O., Jung, C. H., and Kang, H. (2007). A zinc finger-containing glycine-rich RNA-binding protein, atRZ-1a, has a negative impact on seed germination and seedling growth of Arabidopsis thaliana under salt or drought stress conditions. Plant Cell Physiol. 48, 1170–1181. doi: 10.1093/pcp/pcm087
Klahre, U., Noguchi, T., Fujioka, S., Takatsuto, S., Yokota, T., Nomura, T., et al. (1998). The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell 10, 1677–1690. doi: 10.1105/tpc.10.10.1677
Lavenus, J., Goh, T., Roberts, I., Guyomaćh, S., Lucas, M., De Smet, I., et al. (2013). Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 18, 450–458. doi: 10.1016/j.tplants.2013.04.006
Lee, J. S., Huh, W. K., Lee, B. H., Baek, Y. U., Hwang, C. S., Kim, S. T., et al. (2001). Mitochondrial NADH-cytochrome b5 reductase plays a crucial role in the reduction of D-erythroascorbyl free radical in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1527, 31–38. doi: 10.1016/s0304-4165(01)00134-9
Lee, S. M., Huh, T. L., and Park, J. W. (2001). Inactivation of NADP+-dependent isocitrate dehydrogenase by reactive oxygen species. Biochimie 83, 1057–1065. doi: 10.1016/s0300-9084(01)01351-7
Lee, S. M., Koh, H. J., Park, D. C., Song, B. J., Huh, T. L., and Park, J. W. (2002). Cytosolic NADP+-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic. Biol. Med. 32, 1185–1196. doi: 10.1016/s0891-5849(02)00815-8
Levine, A., Tenhaken, R., Dixon, R., and Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79, 583–593. doi: 10.1016/0092-8674(94)90544-4
Lewis, D. R., Negi, S., Sukumar, P., and Muday, G. K. (2011). Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138, 3485–3495. doi: 10.1242/dev.065102
Li, J., Wang, X., Zhang, Y., Jia, H., and Bi, Y. (2011). cGMP regulates hydrogen peroxide accumulation in calcium-dependent salt resistance pathway in Arabidopsis thaliana roots. Planta 234, 709–722. doi: 10.1007/s00425-011-1439-3
Li, J., Zhu, D., Wang, R., Shen, W., Guo, Y., Ren, Y., et al. (2015). β-Cyclodextrin-hemin complex-induced lateral root formation in tomato: involvement of nitric oxide and heme oxygenase 1. Plant Cell Rep. 34, 381–393. doi: 10.1007/s00299-014-1716-2
Li, S., Xue, L., Xu, S., Feng, H., and An, L. (2007). Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul. 52, 173–180. doi: 10.1007/s10725-007-9188-9
Li, S. W., Xue, L., Xu, S., Feng, H., and An, L. (2009). Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ. Exp. Bot. 65, 63–71. doi: 10.1016/j.envexpbot.2008.06.004
Lin, Y., Li, M., Huang, L., Shen, W., and Ren, Y. (2012). Involvement of heme oxygenase-1 in β-cyclodextrin-hemin complex-induced cucumber adventitious rooting process. Plant Cell Rep. 31, 1563–1572. doi: 10.1007/s00299-012-1270-8
Lounifi, I., Arc, E., Molassiotis, A., Job, D., Rajjou, L., and Tanou, G. (2013). Interplay between protein carbonylation and nitrosylation in plants. Proteomics 13, 568–578. doi: 10.1002/pmic.201200304
Ma, F., Wang, L., Li, J., Samma, M. K., Xie, Y., Wang, R., et al. (2014). Interaction between HY1 and H2O2 in auxin-induced lateral root formation in Arabidopsis. Plant Mol. Biol. 85, 49–61. doi: 10.1007/s11103-013-0168-3
Maffei, M. E., Mithöfer, A., Arimura, G. I., Uchtenhagen, H., Bossi, S., Bertea, C. M., et al. (2006). Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 140, 1022–1035. doi: 10.1104/pp.105.071993
Neill, S., Desikan, R., and Hancock, J. (2002). Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 5, 388–395. doi: 10.1016/S1369-5266(02)00282-0
Niu, L., and Liao, W. (2016). Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front. Plant Sci. 7:230. doi: 10.3389/fpls.2016.00230
Orman-Ligeza, B., Parizot, B., de Rycke, R., Fernandez, A., Himschoot, E., Van Breusegem, F., et al. (2016). RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 143, 3328–3339. doi: 10.1242/dev.136465
Potters, G., Pasternak, T. P., Guisez, Y., Palme, K. J., and Jansen, M. A. K. (2007). Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 12, 98–105. doi: 10.1016/j.tplants.2007.01.004
Rejeb, K. B., Benzarti, M., Debez, A., Bailly, C., Savouré, A., and Abdelly, C. (2015). NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J. Plant Physiol. 174, 5–15. doi: 10.1016/j.jplph.2014.08.022
Sanmartin, M., Drogoudi, P. D., Lyons, T., Pateraki, I., Barnes, J., and Kanellis, A. K. (2003). Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 216, 918–928. doi: 10.1007/s00425-0020944-9
Swarup, K., Benková, E., Swarup, R., Casimiro, I., Péret, B., Yang, Y., et al. (2008). The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10, 946–954. doi: 10.1038/ncb1754
Tanou, G., Filippou, P., Belghazi, M., Job, D., Diamantidis, G., Fotopoulos, V., et al. (2012). Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 72, 585–599. doi: 10.1111/j.1365-313X.2012.05100.x
Tanou, G., Job, C., Rajjou, L., Arc, E., Belghazi, M., Diamantidis, G., et al. (2009). Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J. 60, 795–804. doi: 10.1111/j.1365-313X.2009.04000.x
Tyanova, S., Temu, T., and Cox, J. (2016). The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319. doi: 10.1038/nprot.2016.136
Vandenabeele, S., Vanderauwera, S., Vuylsteke, M., Rombauts, S., Langebartels, C., Seidlitz, H. K., et al. (2004). Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 39, 45–58. doi: 10.1111/j.1365-313X.2004.02105.x
Wang, H., Lockwood, S. K., Hoeltzel, M. F., and Schiefelbein, J. W. (1997). The ROOT HAIR DEFECTIVE3 gene encodes an evolutionarily conserved protein with GTP-binding motifs and is required for regulated cell enlargement in Arabidopsis. Genes Dev. 11, 799–811. doi: 10.1101/gad.11.6.799
Wiśniewski, J. R., Zougman, A., Nagaraj, N., and Mann, M. (2009). Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362. doi: 10.1038/NMETH.1322
Wu, M., Huang, J., Xu, S., Ling, T., Xie, Y., and Shen, W. (2011). Haem oxygenase delays programmed cell death in wheat aleurone layers by modulation of hydrogen peroxide metabolism. J. Exp. Bot. 62, 235–248. doi: 10.1093/jxb/erq261
Xie, Y. J., Xu, S., Han, B., Wu, M. Z., Yuan, X. X., Han, Y., et al. (2011). Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant J. 66, 280–292. doi: 10.1111/j.1365-313X.2011.04488.x
Xu, S., Zhang, B., Cao, Z., Ling, T., and Shen, W. (2011). Heme oxygenase is involved in cobalt chloride-induced lateral root development in tomato. Biometals 24, 181–191. doi: 10.1007/s10534-010-9386-1
Zhao, F. Y., Han, M. M., Zhang, S. Y., Wang, K., Zhang, C. R., Liu, T., et al. (2012). Hydrogen peroxide-mediated growth of the root system occurs via auxin signaling modification and variations in the expression of cell-cycle genes in rice seedlings exposed to cadmium stress. J. Integr. Plant Biol. 54, 991–1006. doi: 10.1111/j.1744-7909.2012.01170.x
Zhou, J., Wang, J., Shi, K., Xia, X. J., Zhou, Y. H., and Yu, J. Q. (2012). Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol. Biochem. 60, 141–149. doi: 10.1016/j.plaphy.2012.07.010
Zhou, J., Xia, X. J., Zhou, Y. H., Shi, K., Chen, Z., and Yu, J. Q. (2014). RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J. Exp. Bot. 65, 595–607. doi: 10.1093/jxb/ert404
Keywords: cell cycle regulatory gene, β-cyclodextrin-hemin complex (β-CDH), hydrogen peroxide, lateral root formation, Solanum lycopersicum
Citation: Cui W, Zhu D, Shen W, Mei Y, Hu D, Shi Y, Ren Y, Shen W, Gu Q, Xu D and Huang L (2017) Hydrogen Peroxide Is Involved in β-Cyclodextrin-hemin Complex-Induced Lateral Root Formation in Tomato Seedlings. Front. Plant Sci. 8:1445. doi: 10.3389/fpls.2017.01445
Received: 04 July 2017; Accepted: 03 August 2017;
Published: 18 August 2017.
Edited by:
Sergio J. Ochatt, INRA - UMR 1347 Agroécologie, FranceReviewed by:
Athanassios Molassiotis, Aristotle University of Thessaloniki, GreecePaola Leonetti, Consiglio Nazionale Delle Ricerche (CNR), Italy
Copyright © 2017 Cui, Zhu, Shen, Mei, Hu, Shi, Ren, Shen, Gu, Xu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqin Huang, bHFodWFuZ3NAbmphdS5lZHUuY24=
 Weiti Cui
Weiti Cui Dan Zhu1
Dan Zhu1 Wenbiao Shen
Wenbiao Shen Liqin Huang
Liqin Huang