Abstract
Plants respond to environmental pollutants such as heavy metal(s) by triggering the expression of genes that encode proteins involved in stress response. Toxic metal ions profoundly affect the cellular protein homeostasis by interfering with the folding process and aggregation of nascent or non-native proteins leading to decreased cell viability. However, plants possess a range of ubiquitous cellular surveillance systems that enable them to efficiently detoxify heavy metals toward enhanced tolerance to metal stress. As proteins constitute the major workhorses of living cells, the chelation of metal ions in cytosol with phytochelatins and metallothioneins followed by compartmentalization of metals in the vacuoles as well as the repair of stress-damaged proteins or removal and degradation of proteins that fail to achieve their native conformations are critical for plant tolerance to heavy metal stress. In this review, we provide a broad overview of recent advances in cellular protein research with regards to heavy metal tolerance in plants. We also discuss how plants maintain functional and healthy proteomes for survival under such capricious surroundings.
Introduction
Proteins are functionally versatile macromolecules that constitute the major workhorses of living cells. They function in cellular signaling, regulation, catalysis, intra and inter cellular movement of nutrients and other molecules, membrane fusion, structural support and protection (Amm et al., 2014). The function of a protein is basically determined by its structure, which is acquired following ribosomal synthesis of its amino acid chain. In addition, the conformation of a protein largely depends on the physical and chemical conditions of the protein environment as affected by extreme temperatures, reactive molecules, heavy metal (HM) ions and other stresses that not only disrupt the folding process of a newly synthesized protein, but also induce the mis-folding of already existing proteins (Goldberg, 2003; Amm et al., 2014; Zhou et al., 2016).
Over the last several decades, the emission of pollutants into the environment has been increased tremendously due to rapid industrialization, urbanization and excessive usage of agricultural amendments. Being sessile, plants are routinely confronted by a wide array of biotic and/or abiotic stresses including HM stress (Al-Whaibi, 2011). HMs are thought to obstruct the biological functions of a protein by altering the native conformation through binding on it (Hossain and Komatsu, 2013). For example, in yeast, methyl-mercury (MeHg) strongly inhibits L-glutamine: D-fructose-6-phosphate aminotransferase, and overexpression of this enzyme confers tolerance to MeHg (Naganuma et al., 2000). Similarly, cadmium (Cd) can inhibit the activity of thiol transferase leading to oxidative damage, possibly by binding to cysteine residues in its active sites. In Brassica juncea, Cd-dependent changes in beta carbonic anhydrase result in the enhancement of photorespiration which may protect photosystem from oxidation (D'Alessandro et al., 2013). The modifications caused by Cd disrupt the stabilizing interactions associated with changes in the tertiary structure and cause loss of promising functions of that protein (Chrestensen et al., 2000). Fallout dysfunction of protein stimulates the danger of protein aggregation.
The biosynthesis of metal binding cysteine rich peptides that function to immobilize, sequester and detoxify the metal ions is thought to be the central for detoxification of HMs (Clemens, 2006; Viehweger, 2014). Nonetheless, under extreme conditions, metal ions profoundly affect cellular protein homeostasis by interfering with their folding process and stimulate aggregation of nascent or non-native proteins, leading to the endoplasmic reticulum (ER) stress and a decreased cell viability. To restrict the aggregation as well as to mend them is-folded proteins, cells initiate different quality control systems that fine-tune protein homeostasis. In the center of the system, a typical set of proteins, called heat shock proteins (HSPs; Amm et al., 2014), function as surveillance mechanisms, which are preferentially expressed under stress to maintain functional and healthy proteomes. In contrast, the damaged proteins that fail to achieve their native conformations are subjected to degradation through the ubiquitinproteasome process (UPS), called as ER-associated degradation (ERAD) or through autophagy to minimize the accumulation of misfolded proteins in cells (Liu and Howell, 2016). Although a significant progress has been made in our understanding of protein quality control systems, information on plant system, especially pertaining to HMs stress still remain scanty. In this review, we aim to provide a better insight into the protein quality control system in plants with regards to heavy metal tolerance. We also discuss how plants try to ensure functional and healthy proteomes under HM stress.
Heavy metals (HMs) detoxification
Toxic metal ions at cellular level, evoke oxidative stress by generating reactive oxygen species (ROS; Li et al., 2016a). They promote DNA damage and/or impair DNA repair mechanisms, impede membrane functional integrity, nutrient homeostasis and perturb protein function and activity (Tamás et al., 2014). On the other hand, plant cells have evolved a myriad of adaptive mechanisms to manage excess metal ions and utilize detoxification mechanisms to prevent their participations in unwanted toxic reactions. In the first line of defense, plants utilize strategies that prevent or reduce uptake by restricting metal ions to the apoplast through binding them to the cell wall or to cellular exudates, or by inhibiting long distance transport (Manara, 2012; Hasan et al., 2015). In contrast, when present at elevated concentrations, cells activate a complex network of storage and detoxification strategies, such as chelation of metal ions with phytochelatins and metallothioneins in the cytosol, trafficking, and sequestration into the vacuole by vacuolar transporters (Figure 1; Zhao and Chengcai, 2011).
Figure 1
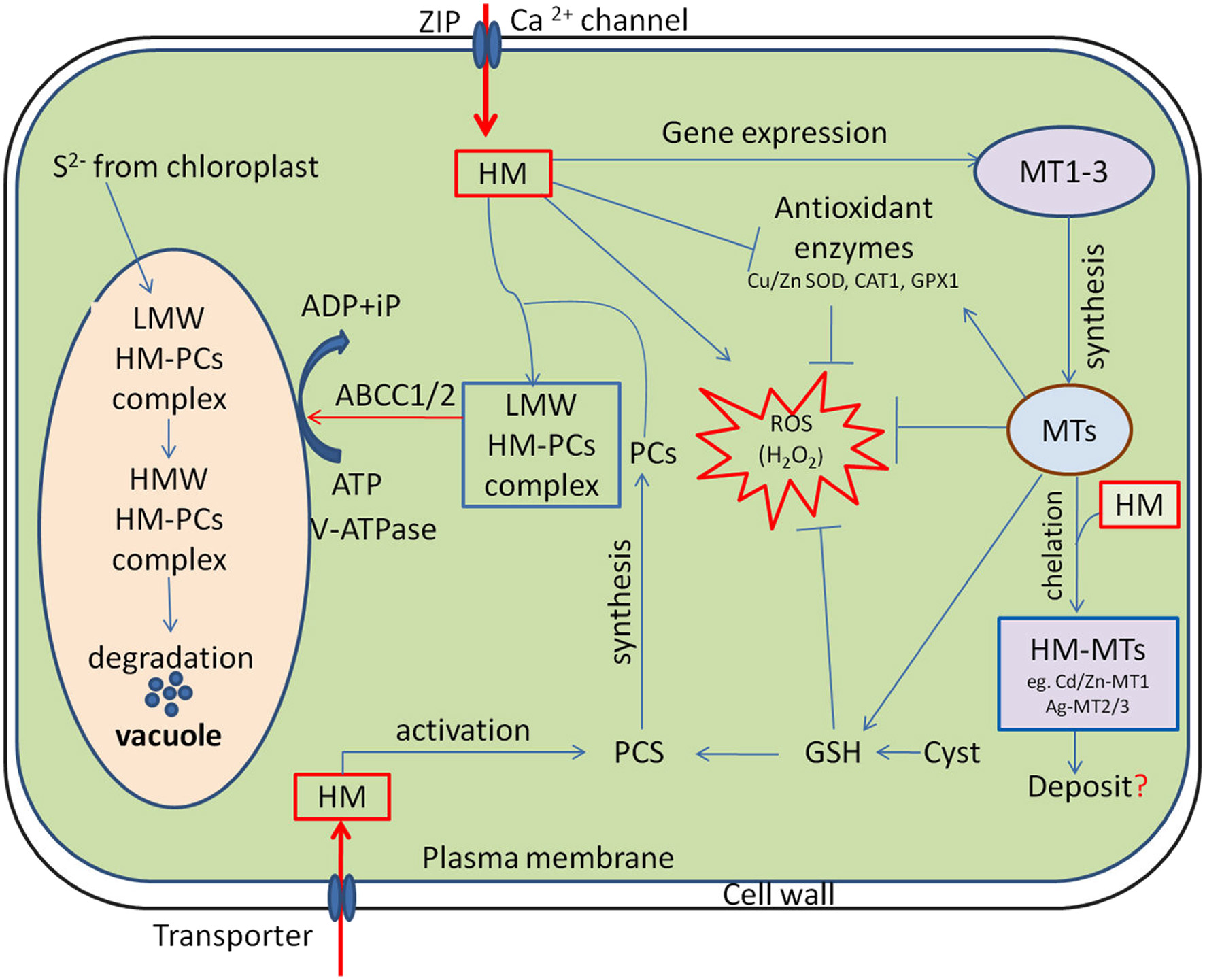
Cellular functions of phytochelatins (PCs) and metallothioneins (MTs) in heavy metal (HM) detoxification. HM activates phytochelatin synthase (PCS) and MTs expression, subsequently the low molecular weight (LMW) HM-PC and HM-MTs complexes are formed in the cytosol. The LMW HM-PCs complexes are consequently transported through tonoplast to vacuole by ATP-binding-cassette and V-ATPase transporter (ABCC1/2). Following compartmentalization, LMW complexes further integrate HM and sulfide (S2−, generated by the chloroplasts) to finally form high molecular weight (HMW) HM-PCs complexes. MTs regulates cellular redox homeostasis independently and also by stimulating antioxidant system and stabilizing relatively high cellular GSH concentrations. “→” indicates “Positive regulation” and “-|” represents “Inhibition”, whereas “?” is a “speculation.”
Phytochelatins: structure, regulation and function in heavy-metal stress tolerance
In order to reduce or prevent damage caused by HMs; plants synthesize small cysteine-rich oligomers, called Phytochelatins (PCs) at the very beginning of metal stress (Ashraf et al., 2010; Pochodylo and Aristilde, 2017). Notably, PC syntheses play the most crucial role in mediating plant tolerance to HMs (Clemens, 2006; Emamverdian et al., 2015). It has been well documented that the biosynthesis of PCs can be regulated at post-translational level by metal(oid)s in many plant species. However, the over-expression of phytochelatin synthase (PCS) gene in plants does not always result in an enhanced tolerance to HM stress. For instance, over expression of AtPCS1 in Arabidopsis, paradoxically shows hypersensitivity toward Cd and Zn; although, PCs production is increased by 2.1-folds, when compared with wild type plants (Lee et al., 2003). In reality, excess PCs levels in mutant plants accelerate accumulation of HMs like Cd without improving plant tolerance (Pomponi et al., 2006; Furini, 2012). This phenomenon possibly indicates some additional roles of PCs in plant cells, such as their involvement in essential metal ion homeostasis, antioxidant mechanisms, and sulfur metabolism (Furini, 2012). Therefore, prevention of the free circulation of toxic metal inside the cytosol exhibits a potential mechanism for dealing with HM-induced toxicity (Hasan et al., 2016).
The mechanism of HMs detoxification is not only limited to the chelation, but also involves accumulation and stabilization of HM in the vacuole through formation of high molecular weight (HMW) complexes with PCs (Figure 1; Jabeen et al., 2009; Furini, 2012). Generally, sequestration of metal ions is a strategy adopted by organisms to ameliorate toxicity. The arrested metal ions are transported from cytosol to the vacuole for sequestration via transporters. vacuolar sequestration is the vital mechanism to HM homeostasis in plants, which is directly driven by ATP-dependent vacuolar pumps (V-ATPase and V-PPase) and a set of tonoplast transporters (Sharma et al., 2016). RNA-Seq and de novo transcriptome analysis showed that different candidate genes that encode heavy metal ATPases (HMAs), ABC transporter, zinc iron permeases (ZIPs) and natural resistance-associated macrophage proteins (NRAMPs) are involved in metal transport and cellular detoxification (Xu et al., 2015; Sharma et al., 2016). A classic example of such protein in Cd uptake in A. thaliana is the Fe (II) transporter iron-regulated transporter 1 (IRT1) belonging to the ZIP family (Connolly et al., 2002). Furthermore, NRAMPs members such as NRAMP5 is recognized as an important transporter for Mn acquisition and major pathway of Cd entry into rice roots, which is localized at the distal side of exodermis and endodermis of the plasma membrane of cells (Clemens and Ma, 2016). Interestingly, another transporter HMA2 localized in the plasma membrane of pericycle cells is thought to transport Cd from the apoplast to the symplast to facilitate translocation via the phloem in rice, whereas HMA3 in the tonoplast sequesters Cd into vacuoles by serving as primary pump (Clemens and Ma, 2016; Sharma et al., 2016). The HM transporter 1 (HMT1) was first identified in 1995 in the yeast S. pombe, as a vacuolar PC transporter required for Cd tolerance (Mendoza-Cózatl et al., 2011). The HMT1 gene encodes ATP-binding cassette (ABC) membrane transport proteins; therefore, both HMT1 and ATP are required for the translocation of LMW PC-Cd complexes into the vacuole (Figure 1; Cobbett and Goldsbrough, 2002). In progression, two ABCC subfamily members of ABC transporters, ABCC1 and ABCC2 were also identified as additional vacuolar metal-PC complex transporter in S. pombe and Arabidopsis (Mendoza-Cózatl et al., 2010; Park et al., 2012). Using double mutants, Song et al. (2014) demonstrated that vacuolar sequestration by ABCC1 and ABCC2 is necessary for complete detoxification of Arsenic (As) and Cd in Arabidopsis. Interestingly, they also reported that the addition of necessary metal ions, such as zinc (Zn), copper (Cu), manganese (Mn) and iron(Fe) to the transport assay further enhances PC2 transport efficiency in barley vacuoles, suggesting that PCs might contribute to both the homeostasis of essential metals and detoxification of non-essential toxic metal(loid)s in plants (Song et al., 2014). Although the mechanism how the transporters regulate the sequestration of metal-PCs conjugates to vacuoles is not clear. Very recent, Zhang et al. (2017), for the first time provided evidence that phosphorylation-mediated regulation of ABCC1 activity is required for vacuolar sequestration of As. They found that Ser846 phosphorylation is required for the As resistance function of ABCC1 in Arabidopsis.
Metallothioneins (MTs): structure, regulation and functions in HM tolerance
Alike PCs, MTs are also naturally-occurring intracellular cysteine-rich major metal-binding proteins, which are used by cells to immobilize, sequester, and detoxify metal ions (Capdevila and Atrian, 2011). Although plant MTs have been discovered over last three decades, the precise physiological functions of MTs have not yet been fully elucidated (Liu et al., 2015a). The proposed roles of MTs include (a) participation in maintaining the homeostasis of essential transition metal ions, (b) sequestration of toxic HMs, and (c) protection against intracellular oxidative damage induced by stress (Hossain et al., 2012a).
Transition metals such as Cu, Fe, Mn and Zn are essential for all organisms because they play critical roles in a variety of physiological processes. For example, Cu is required for photosynthesis, respiration, ethylene perception, ROS metabolism and cell walls in plants (Burkhead et al., 2009; Peñarrubia et al., 2010). A number of studies suggested the involvement of plant MTs in the participation of metal ion homeostasis, especially for Cu, during both vegetative growth and senescence. For example, Benatti et al. (2014) demonstrated that the MTs deficient mutants accumulate 45% and 30% less Cu in shoot and root, compared to the WT, while there are no obvious differences in the life cycle between WT and quad-MT mutant plants under various growth conditions. Again, at early vegetative stage, there is no significant difference in Cu uptake in leaves of 4-week-old WT and MT-deficient mutants. However, the concentration of Cu remains twice in leaves of 12-week-old MT-deficient plants compared to leaves of WT. In contrast, the Cu concentration in seeds of MT-deficient plants was less than half compared to the seeds of WT (Benatti et al., 2014). All these results suggest that MTs are not required to complete life cycle, but are important for essential ions homeostasis and distribution in plants.
In general, sequestration of intracellular HMs in eukaryotes also involves binding of HMs with cytosolic cysteine-rich MTs peptides as well as compartmentalization (Sácký et al., 2014). The combination of low kinetic stability and high thermodynamic is the main features of metal-MT complexes, which bind the metals very firmly, while a part of the metal ions is easily exchanged for other proteins (Maret, 2000). Transgenic plants overexpressing MTs genes have been scored for enhanced metal tolerance and they demonstrate modified metal accumulation or distribution strategies (Gu et al., 2015; Liu et al., 2015a; Tomas et al., 2015). In plants, vacuole is considered as the final destination of detoxification of HMs. Although the chelation of metal ions by MTs is well documented, a little is known about the mechanisms of transport of metals-MT complex from the cytoplasm to the vacuole (Yang et al., 2011). Surprisingly, in ThMT3 (hispida metallothionein-like ThMT3) transgenic material, the expression of four genes (GLR1, GTT2, GSH1, and YCF1) which aid transport of HMs from the cytoplasm to the vacuole is not induced by Cd, Zn, or Cu stress (Yang et al., 2011). These results advocate that metals are not transported into vacuoles, and thatThMT3 may only regulate HMs accumulation in the cytoplasm.
The biologic functions of MTs have been a perplexing topic ever since their discovery. Many studies have suggested that in addition to the chelation or metal ion homeostasis, MTs play an important role in cellular redox homeostasis under diverse stress conditions (Kang, 2006). Abiotic stresses like HMs induce excessive accumulation of ROS in plants, and cause damages to the cellular macromolecules such as proteins, leading to metabolic and physiological disorders in cells or even cell death (Hasan et al., 2016). Interestingly, MTs have been proposed as an alternative tool by which plants protect themselves from stress-induced oxidative damage (Figure 1; Hassinen et al., 2011; Ansarypour and Shahpiri, 2017). Although many reports have indicated the roles of MTs in abiotic stress tolerance as ROS scavengers, the mechanisms through which MTs mediate ROS homeostasis remain unclear (Hassinen et al., 2011). It has been proposed that during ROS scavenging, metals are released from MTs and ROS moiety is bounded to the Cys residues of the same. A number of studies have also advocated that the released metals might be involved in the initiation of signaling cascade required for ROS scavenging (Hassinen et al., 2011). For example, normal cellular functioning requires Zn mobilization and its transfer from one location to another or from one Zn-binding site to another. The released Zn from MT mobilized by an oxidative reaction may either constitute a general pathway by which Zn is distributed in the cell or be restricted to conditions of oxidative stress, where Zn is essential for antioxidant defense systems (Kang, 2006), suggesting an important role of MTs in ROS homeostasis and protection of cellular macromolecules from stress-induced ROS. Additionally, the different classes of MTs have distinct tissue-specific expression patterns in plants. As example, GUS reporter constructs explored that MT1a and MT2b are expressed in the phloem, whereas MT2a and MT3 in the mesophyll cells of young leaves and in root tips (Hassinen et al., 2011). Likewise, Liu et al. (2015a) also demonstrated that OsMT2c gene encoding for type 2 MT expressed in the roots, leaf sheathes, and leaves of rice, whereas its weak expression was observed in seeds. Considering their diversified role and tissue specific expression, recently Irvine et al. (2017) showed an excellent effort to develop a low-cost MT-biosensor that can dramatically increase the signal associated with a metal of interest. Such a simple sensor technology could be potentially used in environmental monitoring specially in the areas with the metal contamination problems.
Repairing of damaged proteins
Proteins are the primary targets of HMs. They either form a complex with functional side chain groups of proteins or displace essential ions from metallo proteins, leading to impairment of physiological functions (Tamás et al., 2014). In addition, HMs interfere with the native confirmations of proteins by inhibiting folding process of nascent or non-native proteins that manifest both in a quantitative deficiency of the affected proteins and in the formation of proteotoxic aggregates (Bierkens, 2000; Tamás et al., 2014). Interestingly, plants inherently respond to stress by triggering the activation of the genes involved in cell survival and/or death in contaminated environments (Hossain et al., 2013). As a part of this plant response ubiquitously involves a set of genes, commonly termed as stress genes, are induced to synthesize a group of proteins called HSPs (Gupta et al., 2010). In stress conditions, the induced synthesis of HSPs plays a significant role in maintaining the cellular homeostasis by assisting accurate folding of nascent and stress accumulated misfolded proteins, preventing protein aggregation or by promoting selective degradation of misfolded or denatured proteins (Hüttner et al., 2012; Park and Seo, 2015).
In fact, HSPs functions as molecular chaperones; proteins which are involved in “house-keeping” inside the cell (Sørensen et al., 2003). Several classes of HSP have been identified in plants (Table 1) and the HSP proteins having molecular weights ranging from 10 to 200 KD are characterized as chaperones which participate in the induction of the signal in stress conditions (Schöffl et al., 1999). For example, in endoplasmic reticulum (ER) all the nascent polypeptides, firstly, are stabilized by chaperones (HSP40 and HSP70-like proteins) such as ERdj3 and binding protein (BiP) before they are properly modified and folded, which prevents aggregation and helps their proper folding (Figure 2; Howell, 2013).
Table 1
| HSP classes | Members | Plant species | Metals | References |
|---|---|---|---|---|
| HSPs70 | HSP70 | Populus trichocarpa, Lycopersicon peruvianum L., Glycine max, Arabidopsis thaliana, Populus tremula × P. alba, Populus tremula, Populus nigra | Cd | Lomaglio et al., 2015 Neumann et al., 1994 Hossain et al., 2012b Sarry et al., 2006 Durand et al., 2010 Kieffer et al., 2008 Lomaglio et al., 2015 |
| Elodea canadensis Michx | Cd Pb | Sergio et al., 2007 | ||
| Conocephalum conicum | Cd, Pb, Cu | Basile et al., 2013 | ||
| Lemna minor | Cu Cd, Pb, Cr, Zn | Basile et al., 2015 | ||
| Oriza sativa | As | Chakrabarty et al., 2009; Rai et al., 2015 | ||
| Suaeda salsa | Hg | Liu et al., 2013 | ||
| HSP 70,BiP | Populus alba L | Cu, Zn | Lingua et al., 2012 | |
| HSP70 | Oriza sativa, Suaeda salsa | Ag | Chen et al., 2012; Liu et al., 2013 | |
| HSP68 | Solanum lycopersicum | Cd | Rodríguez-Celma et al., 2010 | |
| BiP | Oriza sativa | Cu, | Ahsan et al., 2007a | |
| BiP | Oriza sativa | Cd | Ahsan et al., 2007b | |
| HSP 70 | Enteromorpha intestinalis | Cu | Lewis et al., 2001 | |
| HSPs 70A | Chlamydomonas acidophila | Fe, Zn | Spijkerman et al., 2007 | |
| HSP70 | Oryza sativa L. | Cr | Dubey et al., 2010 | |
| HSC70 | Phytolacca Americana | Cd | Zhao et al., 2011 | |
| HSC70-2 | Raphanus sativus | Cr | Xie et al., 2015 | |
| HSPs 60 | cpn602 | Oriza sativa | Hg | Chen et al., 2012 |
| HSP60, Cpn60-B | Solanum lycopersicum | Cd | Rodríguez-Celma et al., 2010 | |
| HSP60 | Arabidopsis thaliana | Cd | Sarry et al., 2006 | |
| HSP60 | Chlamydomonas acidophila | Fe, Zn | Spijkerman et al., 2007 | |
| HSPs 90 | HSP90-1 | Lemna gibba | Cu | Akhtar et al., 2005 |
| HSP90-1 | Arabidopsis thaliana | As | Haralampidis et al., 2002 | |
| HSP81-2 | Oryza sativa L. | Cu | Song et al., 2013 | |
| HSP82 | S. cerevisiae | As, Cu, Cd | Sanchez et al., 1992 | |
| HSP81.2, 81.3, 81.4, 88.1 & 89.1 | Arabidopsis thaliana | Cu, Cd, Pb, As | Milioni and Hatzopoulos, 1997 | |
| HSP90-1 | Solanum lycopersicum | Cr, As | Goupil et al., 2009 | |
| HSP82 | Oryza sativa | As | Chakrabarty et al., 2009 | |
| HSP81-1 | Oryza sativa | Cd | Oono et al., 2016 | |
| HSPs 100 | HSP104 | S. cerevisiae | As, Cu, Cd | Sanchez et al., 1992 |
| HSP101 | Oryza sativa L., | As | Agarwal et al., 2003 | |
| ClpB-C | Oryza sativa L. | Cans, Cu, Co | Singh et al., 2012 | |
| ClpB-C | Arabidopsis thaliana | As | Mishra and Grover, 2014 | |
| sHSPs | HSP17 | Lycopersicon peruvianum L, | Cd | Neumann et al., 1994 |
| HSP17 | Populus alba L | Cu, Zn | Lingua et al., 2012 | |
| HSP21 | Arabidopsis thaliana | Cd | Zhao et al., 2009 | |
| HSP20,HSP23p | Kandelia candel | Cd | Weng et al., 2013 | |
| HSP26.13p | Chenopodium album | Ni, Cd, Cu | Haq et al., 2013 | |
| HSP17 | Armeria maritime | Cu | Neumann et al., 1995 | |
| HSP17 | Silene vulgaris, Lycopersicon peruvianum | Hg, Cu, Cd, | Wollgiehn and Neumann, 1999 | |
| HSP24 | Capsicum annuum L | Zn | Zhu et al., 2013 | |
| HSP22 | Chlamydomonas acidophila | Cu | Spijkerman et al., 2007 | |
| HSP17.4 | Oryza sativa L. | Fe, Zn | Dubey et al., 2010 | |
| HSP20, HSP21, HSP22 | Raphanus sativus | Cr | Xie et al., 2015 | |
| HSP23 | Glycine max | Cr | Zhen et al., 2007 | |
| HSP23.9 | Oryza sativa | Al | Chakrabarty et al., 2009 | |
| HSP17.4, HSP17.5 | Tamarix hispida | As | Gao et al., 2012 | |
| HSP18.3 | Oryza sativa | Cu, Cd, Zn | Rai et al., 2015 |
Five major classes of heat shock proteins (HSPs) that are induced in response to heavy metal stress in plants.
Figure 2
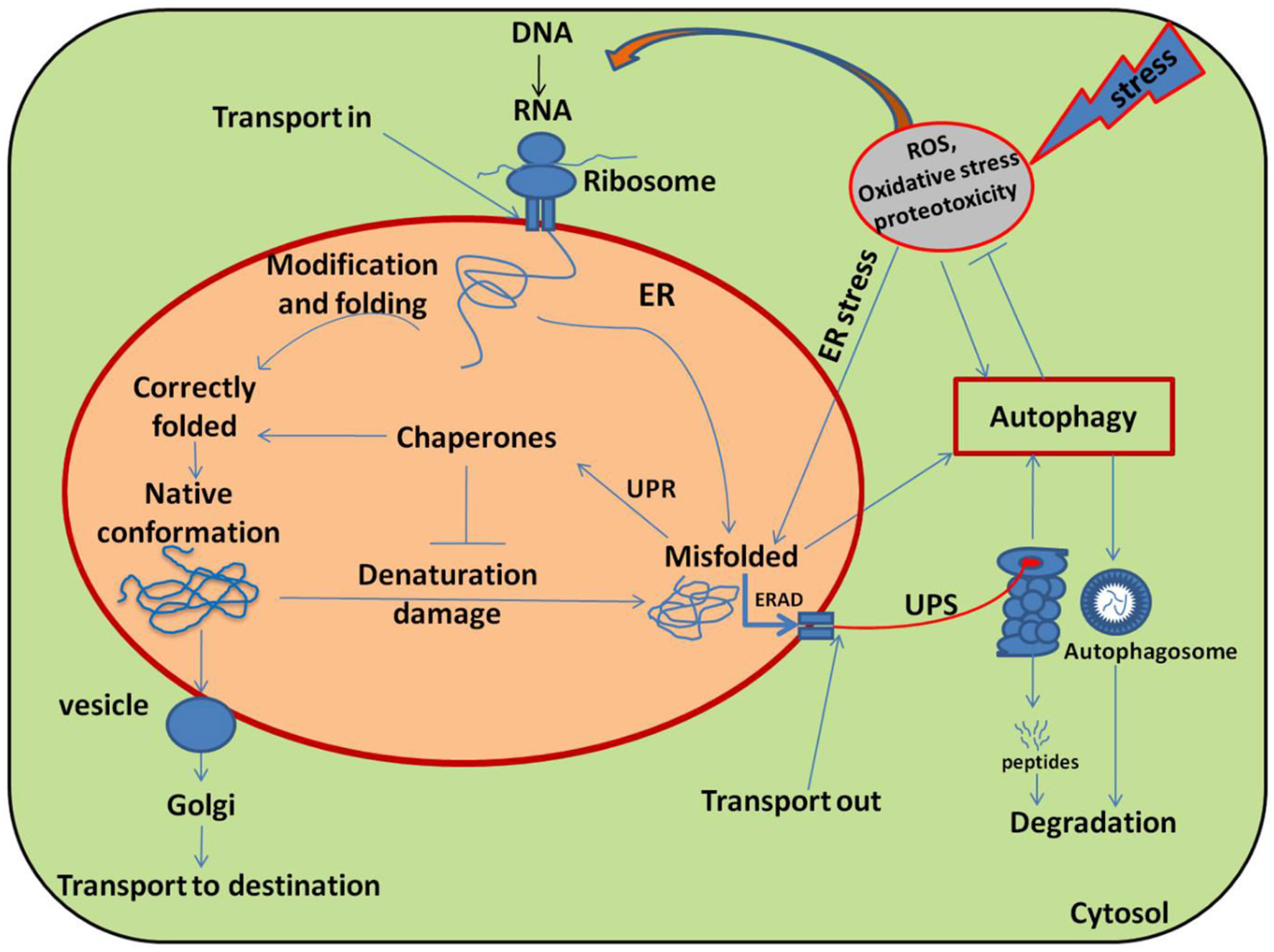
Schematic diagram illustrating the main pathways and regulation of protein folding and modification in the endoplasmic reticulum (ER). Many newly synthesized proteins are translocated into the ER, where proteins folded into their native three dimensional structures with the help of chaperones. The correctly folded proteins are then transported to the Golgi complex, followed by delivery to the destination where they eventually function. While exposure of plants to stress causes oxidative stress by generating over ride of ROS and stimulating the misfolding of proteins. The incorrectly folded proteins are then detected by quality control system, which stimulates another pathway called unfolded protein response (UPR). The terminally misfolded proteins are then eliminated through the endoplasmic reticulum associated degradation (ERAD) pathway, where they initially ubiquitinated and then degraded in the cytoplasm by proteasome system (UPS) or subjected to autophagy. Adopted from Dobson (2003) with modifications.
Role of HSPs in plant tolerance to HM stress
HM stress often causes disturbance to the cellular homeostasis by inactivating essential enzymes and by suppressing proteins functioning (Hossain et al., 2012b). Hence, the induction of HSPs proteins is thought-out as a critical protective, eco-physiologically adaptive and genetically conserved response of organisms to the environmental anxiety. Thus, they accomplish a key function in the hostility of stress by re-establishing normal protein conformation and cellular homeostasis (Rhee et al., 2009). Among the major categories of HSPs, HSP70 family members have extensively been studied. Functional characterization of HSP70 revealed that HSP70 is accumulated in response to environmental stressors in a wide range of plant species (Gupta et al., 2010). The specific members of this family are localized into the cytosol, mitochondria and endoplasmic reticulum(ER) and are constitutively expressed as well as regulated to maintain cellular homeostasis. An example to cite, the 70-KDa heat shock cognates(HSC70) are constitutively expressed in cells and often assist in the folding of de novo synthesized polypeptides and import or translocations of precursor proteins (Wang et al., 2004).
The recent advancements in proteomics research have enabled us to identify the functional genes or proteins involved in the responses of plants to HM stress at molecular levels (Ahsan et al., 2009). Transcript analysis in many plant species showed that HSP70 is highly expressed under a variety of metal stress (Table 1). Although many studies showed that the over-expression of HSP70 genes is positively correlated with the acquisition of tolerance to various stresses, including HMs, but the cellular mechanisms of HSP70 function under stress situation are not completely understood (Wang et al., 2004). HSP70 chaperones, together with their co-chaperones like DnaJ, make a set of prominent cellular machines to prevent accumulation of newly synthesized proteins as aggregates and ensure the proper folding of protein during their transfer to the destination (Al-Whaibi, 2011; Park and Seo, 2015). In transportation of precursor protein, the HSC70 is essential for cell-to-cell transport through interaction with the plasmodesmatal translocation pathway (Aoki et al., 2002). The induction of HSP70 not only limits the proteotoxic symptoms of metalions, but also helps the sequestration and detoxification of these ions by MTs (Haap et al., 2016). While the entire mechanism of HSPs-induced metal detoxification via MT has yet to be explored, only few studies pointed out that HSP60 might participate in protein folding and aggregation of many other proteins that are transported to organelles such as mitochondria and chloroplasts (Al-Whaibi, 2011). With our increasing understanding of the proteome, it is becoming clear that HSP60 is essential for cellular functions both at normal or stress environments, including metal stress (Table 1). Interestingly, proteomics analysis also revealed that the induction HSP60 chaperones prevents the denaturation of proteins even in the presence of metal ions in the cytoplasm (Sarry et al., 2006; Rodríguez-Celma et al., 2010). Similarly, a good number of studies showed the induction of HSP90 family proteins by different metals in many plant species (Table 1) which play a major role in protein folding and regulating signal-transduction networks, cell-cycle control, protein degradation and protein trafficking (Pratt and Toft, 2003; Al-Whaibi, 2011). Interestingly, they have also been found in association with several other intercellular proteins, including calmodulin, actin, tubulin and some other receptors and signaling kinases (Wang et al., 2004; Gupta et al., 2010; Park and Seo, 2015). The multiple sites of localization and high accumulation in combination with other intercellular proteins lead to the suggestion that these polypeptides perform a general mode of cellular activities (Prasad et al., 2010). This family of proteins might provide genetic buffering and contribute to the evolutionary adaptation of plant both in normal and stressful conditions (Wang et al., 2004). By contrast, there is no substantial evidence implicating HSP100/Clp proteins in HMs tolerance in plants (Agarwal et al., 2003). Recently, few studies reported that many members of this family are induced in response to metal treatments (Table 1), and they accomplish house keeping functions necessary for cellular homeostasis (Lee et al., 2006).
Most of the members of sHSPs are strongly inducible and some are also constitutively expressed under environmental stress conditions. One of the featured functions of this family of protein is the degradation of the proteins not suitable for folding (Gupta et al., 2010). Similar to other HSPs, sHSPs also function as molecular chaperones, however, the important characteristic that distinguishes sHsps from other chaperone classes, such as DnaK or ClpB/DnaK is that their activity is independent of ATP (Sun et al., 2002; Mogk and Bukau, 2017). sHSPs maintain denatured proteins in a folding-competent state and allow subsequent ATP-dependent disaggregation through the HSP70/90 chaperone system, thereby facilitating their subsequent refolding (Wang et al., 2004). Recently, it has been shown that BAG3 protein acts as a modulator, brings the chaperone families together into a complex and coordinates the potentiality of Hsp22 and Hsp70 to refold the denatured proteins (Rauch et al., 2017). Additionally, this family of HSPs is also involved in signaling similar to their counterparts, where they become phosphorylated by stress-kinases, and increase the amount of reduced glutathione in the cytoplasm (Arrigo, 1998). A number of studies suggest a strong correlation between sHSP accumulation and plant tolerance, particularly to HMs stress (Table 1; Wang et al., 2004). In Arabidopsis, 13 different sHsps have been identified in distinct cellular compartments, which probably function as mediators of molecular adaptation to stress conditions and are unique to plants. The differential regulation of chloroplastic i.e., Cp-sHSPs or HSP26.13p in C. album plays a dual role in protecting the plant from heat and metals like Ni, Cu, and Cd stress (Haq et al., 2013).
Notably, most of HSPs are activated early in the cascade of cellular events following toxic exposure even below the lethal dose and thus, considered as strong biomarkers for environmental pollution (Bierkens et al., 1998). The excessive accumulation of ROS at cellular level is a common consequence of abiotic stress such as HMs. Interestingly, the high HSP levels protect plants against abiotic stresses not only by preventing irreversible protein aggregation, but also by promoting cellular redox homeostasis through stimulating antioxidant systems (Mu et al., 2013). Recently, Cai et al. (2017) revealed that HSPs-induced metal tolerance in plants has a strong correlation with melatonin (N-acetyl-5-methoxy tryptamine) biosynthesis, which is regulated by heat-shock factor A1a (HsfA1a). Therefore, all the above presented results suggest that the inducible HSPs are important and beneficial for fitness in normal, as well as under capricious environment. As a part of protein quality control system, HSPs play a major role in maintaining the functionality of cellular machinery under environmental stress. Therefore, research on the functional and structural aspects, cross-talk with other chaperones and relationship between different HSP expressions along with the physiological stress response should be expanded to better understand their functions.
Heavy metals-induced ER stress
In cells, both recently synthesized and preexisting polypeptides are at constant risk of misfolding and agreegation. It has been estimated that one-third of recently synthesized proteins are misfolded under ambient conditions (Schubert et al., 2000). In addition, cells continuously face environmental challenges such as mutations, heat, active oxygen radicals and HM ions, which not only disrupt protein folding but also cause the misfolding of already folded protein, (Amm et al., 2014). The disruption of proper functioning of the ER is particularly relevant under stress conditions, whereas the demand for secreted proteins exceeds its working capacity and disrupts normal functioning of ER, termed as ER stress (Schröder and Kaufman, 2005). A number of studies have shown that HMs and metalloids inhibit refolding of chemically denatured proteins in vitro, obstruct protein folding in vivo and stimulate aggregation of nascent proteins in living cells (Sharma et al., 2011; Jacobson et al., 2012). For example, Cr has been shown to trigger oxidative protein damage and protein aggregation in yeast by enhancing mRNA mistranslation (Sumner et al., 2005; Holland et al., 2007). Likewise, Cd has also been shown to cause the widespread aggregation of nascent protein and ER stress in yeast (Gardarin et al., 2010), whilst the mechanistic details of protein misfolding in the ER and cytoplasm remain to be elucidated (Tamás et al., 2014). But somehow, this could be related to metal-induced structural alteration of ER. Recently, Karmous et al. (2015) demonstrated that Cu treatment in embryonic cells of Phaseolus vulgaris results in prevalently swollen cisternae of smooth endoplasmic reticulum and vesicles with electron-dense contents. Although this phenomenon is often not well recognizable, it robustly inhibits cellular homeostasis. While the toxicity of metals, like Cd, As, Pb, Hg, and Cr is unquestionable and their interference with protein folding in living cells is well-documented, the potency of accumulation of misfolded and aggregated proteins appears to be different (Tamás et al., 2014). In yeast cells, the accumulations of aggregated proteins occur in the order As>Cd>Cr upon exposure of equi-toxic concentrations of these metals (Jacobson et al., 2012). The in vivo potency of these environmental threats to prompt protein aggregation possibly depends on the efficiency of their cellular uptake/transport and their distinct modes of biological action (Tamás et al., 2014). Under such circumstances, a coordinated adaptive program called the unfolded protein response (UPR) is commonly initiated.
The UPR is a homeostatic response to alleviate ER stress through transcriptional and translational events. The induction of UPR has three aims; initially to restore the normal function of the cell by halting production of secreted and membrane proteins, removal of misfolded proteins through ER-associated degradation (ERAD) systems, and activation of the signaling pathways that lead to increased production of molecular chaperones involved in protein folding. If these cell-sparing activities are not achieved within a certain time span or the disruption is prolonged, then the UPR aims toward programmed cell death (PCD) which is called apoptosis (Deng et al., 2013; Liu and Howell, 2016). The UPR is a sensitive surveillance mechanism that monitors the ER loading capacity. If persistently misfolded proteins exceed the ER loading capacity, cellular communication between the ER and nucleus occurs for ER homeostasis, leading to the transcriptional activation that up-regulates the cellular chaperones (Brandizzi et al., 2014). Recently, it has been reported as two arm process in plants, where proteolytic processing of membrane-associated bZIP TFs and RNA splicing factor inositol-requiring enzyme-1 (IRE1) act as transducers in ER stress or UPR signaling pathway. Structural details of bZIP TFs, bZIP17, bZIP28 and IRE1/ bZIP60 and their underlying principles of mobilization in response to ER stress suggest that the UPR signaling pathway is assisted by these factors in order to protect plants from ER stress(Deng et al., 2013; Sun et al., 2013; Yang et al., 2014). This UPR transcriptional activation enhances the production of molecular chaperones such as binding protein (BiP) and glucose-regulated protein 94 (GRP94), involved in increased ER protein-folding potentiality (Yoshida et al., 1998). The molecular chaperones binding proteins (BiP) are the master regulatory elements of these quality control systems and a classical marker of UPR activation (Malhotra and Kaufman, 2007). For example, Xu et al. (2013) demonstrated that heterologous expression of AtBiP2 protein in BY-2 act as a negative regulator of Cd-induced ER stress and PCD. Recently, Guan et al. (2015) also showed that ER chaperone binding protein BiP acts as a positive regulator in Cd stress tolerance. To explore the mechanism, the author also examined the transcript level of GSH gene in LcBiP-overexpressed tobacco. Interestingly, the transcript levels of the GSH and the ER stress marker gene are not induced, meaning that BiP may enhance the folding capacity of GSH in the ER in plants. In contrast, proteins that fail to achieve their native conformation and aggregate in ER are eliminated through the ERAD pathway via a series of tightly coupled steps that include substrate recognition, targeting of terminally misfolded proteins, retro-translocations, and ubiquitin-dependent proteasomal destruction (Liu and Howell, 2016). Liu et al. (2015b) discover a plant-specific component of ERAD system in Arabidopsis. They uncovered that EBS7 (methanesulfonate-mutagenized brassinosteroid insensitive 1 suppressor 7) interacts with the ER membrane-anchored ubiquitin ligase AtHrd1a, one of the central components of the Arabidopsis ERAD machinery, whose mutation destabilizes AtHrd1a to reduce polyubiquitination. Similarly, very recently, Liu et al. (2016) demonstrated that α1, 6-linked mannose is necessary for luminal N-glycoproteins degradation via ERAD system in yeast where Htm1p-Pdi1p complex acts as a folding-sensitive mannosidase for catalyzing the first committed step. Thus, any defects in this sophisticated ERAD machinery system raise death and life issues of cells survival, especially under stressed environments. For instance, Van Hoewyk (2016) demonstrated that Arabidopsis HRD1 and SEL1L mutant plants showed decreased tolerance to selenate (Se) stress. As-Se toxicity causes both oxidative stress and protein misfolding due to the substitution of a cysteine to Se-cysteine (Van Hoewyk, 2013), whereas selenium enhances Cd tolerance in tomato plants (Li et al., 2016b). Actually, this is an alternative adaptive mechanism, involved in actively controlled and precise degradation of cellular components, and selective elimination of harmful, unwanted or damaged cells in eukaryotes (Tuzhikov et al., 2011; Brandizzi et al., 2014). Such selective suicide or PCD is paradoxically a crucial event which eventually provides survival benefits for the whole organism under extreme environmental conditions like HMs stress (Yakimova et al., 2007; Adamakis et al., 2011). However, the details of involved mechanisms are still obscure and remain to be further disclosed.
Degradation of metal-induced denatured proteins
Much of plant physiological processes, growth and development are controlled by the selective degradation of unwanted misfolded or damaged proteins in order to maintain cellular homeostasis. In plants, protein degradation or proteolysis occurs either by ubiquitin proteosome system (UPS) or by autophagosomes induction (Liu and Howell, 2016). The UPS is a fundamental, highly regulated multistep enzymatic cascade that tightly controls the cellular protein homeostasis. It is a very rapid and effective method for a precise degradation of an unwanted protein at a particular time, whereas most of the times a protein is degraded only in response to a specific cellular signal or event (Pines and Lindon, 2005). The recent progress in molecular genetics revealed that like a tip of iceberg, UPS components regulate critical processes in plants (Sadanandom et al., 2012). It has been found that over 6% of Arabidopsis protein-coding genes are dedicated to the UPS (Vierstra, 2009). In eukaryotic cells proteins that destined to degrade, firstly, labeled by ubiquitin and then the ubiquitylated protein digested to small peptides by the large proteolytic complex, the 26S proteasome (Goldberg, 2003). Ubiquitylation is an energy dependent key regulatory process, which is executed by three different E1-E2-E3 enzymes conjugation cascade (Maupin-Furlow, 2013). In the first step of this cascade, ubiquitin is covalently conjugated to ubiquitin-activating enzyme (E1) in an ATP-dependent reaction, and then this activated ubiqutin is transferred from E1 to E2 (or Ub-conjugating enzyme) by transesterification. Finally, E3 (ubiquitin ligase) catalyzes the transfer of the ubiquitin from the E2 enzyme to a lysine residue on the target protein. After initial polyubiquitination, this step is repeated to form polyubiquitinated chains on the target protein and designated for degradation by the 26S proteasome (Ruschak et al., 2011; Sadanandom et al., 2012). Upon delivery, the deubiquitinating enzymes remove the polyubiquitin chain before unfolding, import and proteolysis of targeted substrates (Hartmann-Petersen et al., 2003).
On the other hand, autophagy is a biological self-destruction process, by which eukaryotic cells maintain their cellular homeostasis by turning over damaged proteins or organelles into the vacuole during developmental transitions and under stress conditions (Liu and Bassham, 2012; Wen-Xing, 2012). Upon the induction of an autophagic pathway, the cytoplasmic components that designate to degradation, are surrounded by a double membrane structure, called autophagosomes (Figure 2). The autophagosome then delivers its cargo materials to the vacuole, where the outer membrane of the autophagosome initially fuses with the vacuole membrane, after which the cargo materials are degraded by vacuolar hydrolases in the vacuole and recycled (Yang et al., 2016). There are four different types of autophagy detailed out in eukaryotes, including microautophagy, macroautophagy, chaperone-mediated autophagy and organelle-specific autophagy (Liu and Bassham, 2012). Out of these, microautophagy and macroautophagy (hereafter referred as autophagy), have been shown to occur in plants (Han et al., 2011).
UPS-dependent proteasome activity and metals stress
Extreme environmental conditions such as HMs pollution often adversely affect proteins quality by increasing free radicals that encourage denaturation and damage, thus causing the misfolding of proteins. Cells under stress, need to prevent further damage by initiating defense machinery to repair the damaged proteins or remove them when irreparable. In such circumstances, the UPS plays a crucial role in plant response and adaptation to changing environmental conditions(Stone, 2014). The UPS, functions both in cytoplasm and nucleus, responsible to modulate the levels of regulatory proteins and to remove most abnormal peptides and short-lived cellular regulators which may accumulate following exposure to abiotic stress (Lyzenga and Stone, 2012). The significant role of the UPS in the cellular response to HM stress has been recognized few years ago and is evident to increased expression of polyubiquitin genes (Genschik et al., 1992; Jungmann et al., 1993). It is believed that the expression of polyubiquitin genes under stress conditions is one of the important indications that the UPS is involved in regulation of plant HMs stress tolerance (Sun and Callis, 1997; Chai and Zhang, 1998). The genome-wide transcription analysis of rice plants showed that low concentrations of Cd treatment induces polyubiqutin expression both in root and shoot (Oono et al., 2016). In contrast, elevated metal concentrations induce the disruption of proteasomal activity, resulting in the accumulation of abnormal proteins in the cytosol, which alter cellular protein homeostais and thereby activating apoptosis (Yu et al., 2011). For instance, proteome analysis in different plant species has shown that ubiquitin activity can significantly be reduced by Cd, Co, Cu, Cr, Hg, Ni and Pb at 100 μM but not by Al and Zn, whereas low concentrations can induce 26S proteosome activity (Aina et al., 2007; Pena et al., 2007, 2008). Although Co, Cu, Cr, Hg, Ni, and Pb induce accumulation of ubiquitin conjugated proteins, the abundance of 20S core protein in UPS system is not changed (Pena et al., 2008). In contrast, Karmous et al. (2014) showed that Cu treatment (200 μM) has a strong effect on UPS pathway and can inhibit about 88% of the 20S proteasome activity in the cotyledons of germinating bean seeds. This implies that the effect of HMs on proteolytic system can not be generalized; however, the functional impairment-induced decrease in proteases activities appears to be a common aspect of metal toxicity in plant.
In extreme environments, over-expression of genes involved in UPS cascade, enhances tolerance to multiple stresses without any adverse effects on growth and development in plants (Guo et al., 2008). For example, Lim et al. (2014) showed the E3 or ubiquitin ligase enzyme is an important regulator for the removal of aberrant proteins under metal stress. In addition, the heterogeneous expression of rice E3 ligase enzyme synthesis RING domain OsHIR1 gene in Arabidopsis has been found to be decreased with As and Cd accumulation in both root and shoot (Dametto et al., 2015). Although the mechanism is not clear, it could probably be regulated by its substrate protein, since the OsHIR1 protein positively interacts with the OsTIP4;1 related to As and Cd uptake. Therefore, the development of strategies to reduce metal uptake and translocation as well as to improve cellular protein homeostasis with the ubiquitin/proteasome 26S system in plants seems to be a promising approach to ensure crop yield and food safety.
Autophagy in metal stress responses
Autophagy has shown to be involved in the adaptation of plants to a wide range of drastic environmental stresses such as nutrient starvation, oxidative stress, heat stress, drought, salt, and pathogen invasion (Han et al., 2011; Wang et al., 2015; Xu et al., 2016; Yang et al., 2016). However, its pivotal roles in plants, particularly in HMs stress and adaptive responses, have perhaps not received the attention they deserve and thus remains elusive (Chiarelli and Roccheri, 2012; Pérez-Martín et al., 2015). Fascinatingly, in recent years scientists begun to explore, the involvement of autophagy in plant toward metal tolerance and the mechanism of adaptation is the same as in human and yeast. Firstly, Zhang and Chen (2010), afterwards, Zheng et al. (2012) demonstrated that autophagy is induced in metal treated plants. In progression, the expression profile analysis in tobacco seedlings after five different HMs (Cu, Ni, Zn, Cd and Mn) treatments showed that among the 30 ATGs genes, 18 ATGs genes are upregulated by more than two folds by at least one HM. They also explored that among the 18 ATGs, 11 ATGs are commonly up-regulated in seedlings by all five metals, and the expression is more sensitive to Zn treatment than others. Recently, Abd-Alla et al. (2016) for the first time demonstrated that Ag-NPs treatments result in the induction of autophagy in root nodules of Rhizobium leguminosarum as a mechanism of detoxification and surveillance. Taken together, recent studies explored the involvement of autophagy as a sophisticated regulator of surveillance under HMs stress, nevertheless, the mechanisms, especially how metals regulate autophagy, still remain to be elucidated in the future (Zhou et al., 2015).
Interestingly, the cellular sites of ROS production and signaling are thought to be primary targets of autophagy, which leads to either survival or death of cells (Scherz-Shouval and Elazar, 2007; Minibayeva et al., 2012). It has now been undoubtedly established that ROS can function as specific second messengers in signaling cascade. Environmental toxicants, like HMs are known to be strong inducers of oxidative stress due to excessive accumulation of ROS that alter the cellular homeostasis. In general excess accumulation of ROS causes many types of cellular injuries including damage to proteins, lipids and DNA, whereas some of which can result in apoptotic cell death and autophagy (Farah et al., 2016). Several lines of evidence suggest that metal-induced intracellular ROS production function in the signal transduction pathways, leading to induction of autophagy (Zhang and Chen, 2010; Pérez-Martín et al., 2015; Farah et al., 2016). Although an extensive number of studies showed that oxidative stress stimulates the autophagic induction to relieve the plants from oxidative damage, but the mechanistic information still remain limited. Very recently, Yang et al. (2016) put forward an excellent effort to open-up the mechanism for activation of autophagy. They demonstrated that unfolded proteins accumulation in the ER is a trigger for autophagy under conditions that cause ER stress. They showed that the reduction of unfolded proteins accumulation by PBA or TUDCA addition or BiP over-expression inhibits the autophagy in Arabidopsis. Whereas, the introduction of the constitutively unfolded proteins zeolin or CPY* activates the UPR and autophagy via IRE1b (inositol-requiring enzyme 1b) dependent manner. But how the ER stress activating these signaling cascades remains to be further revealed.
In addition to their core function, autophagic induction has also been shown to be involved in the regulation of metal uptake. For instance, Li et al. (2015) showed that induction of autophagy with mono-ubiquitination under Fe excess condition affects the functional activity and stability of exogenous Malus xiaojinensis iron-regulated transporter (MxIRT1) in yeast, thereby preventing Fe uptake via this root transporter. They also showed that in Fe led conditions, the transcript levels of ATG8 and ATG8-PE protein significantly increased, which resulted in enhanced MxIRT1 degradation, while the inhibition of autophagic initiation has opposite effects. Therefore, the development of strategies to regulate metal uptake by promoting autophagy under excess metal conditions could have potential implication in increased or even safe food production. However such kind of assumption in plant is still a matter of speculation and thus requires further extensive investigation.
Concluding remarks and future perspectives
The present review outlines the impact of HM stress on cellular protein homeostasis and illustrates the diverse mechanistic approach that operates inside cells to regulate quality control systems toward functional and healthy proteomes. Proteins are major workhorse of cells and directly involved in plant stress response. HMs can trigger the cellular pathways that are broadly classified as death and survival signals. As surveillance mechanism, the ubiquitous plants response to HM stress is the chelation of toxic ions in the cytosol by cysteine rich peptides such as PCs and MTs, compartmentalization of metals in the vacuole by tonoplast located transporters, and the process that involves repair of stress-damaged proteins. In-depth review of recent research works revealed that MTs are not only required to complete the plants life cycle, but also play significant roles in ionic homeostasis and distribution in plants as well as cleanup of ROS and sequestration of metals as that of PCs (Benatti et al., 2014; Tomas et al., 2016). Whilst in extreme conditions metals profoundly affect cellular protein homeostasis by interfering with the folding process, they also stimulate aggregation of nascent or non-native proteins leading to ER stress and decreased cell viability. However, there is a typical set of proteins, called stress proteins or HSPs proteins, which are preferentially expressed under stress. HSPs restrict aggregation of nascent or non-native proteins but trigger repair of misfolded proteins. In contrast, the damaged proteins which fail to achieve their native conformations, are removed from ER by the activation of ERAD machinery of ERQC system, leading to proteosomal (UPS) or autophagic degradation.
Recent advances in protein research, summarized herein, show that as core degradation process of misfolded or damaged polypeptides, the over-expression of E3 enzyme in UPS pathway and the autophagic induction with mono-ubiquitination prevent metal accumulation in plants (Dametto et al., 2015; Li et al., 2015). However, we are still far away from the complete understanding of the mechanism of subsequent signaling cascades that regulate metal accumulation. The development of strategies to reduce metal uptake and translocation by manipulating cellular protein quality control system in plants seems to be a promising approach that can potentially ensure increased yield as well as food safety.
Statements
Author contributions
MH and GA conceived the idea and designed the outlines of the article. MH, YC, MK and GA wrote the article. GA, XC, and ZQ revised the article.
Acknowledgments
Research in the authors' laboratories is supported by grants from the National Key Research and Development Program of China (2016YFD0201001), the Geological Exploration Foundation of Zhejiang Province, China (2014002-02) and the National Natural Science Foundation of China (31401877, 31550110201, 31772294), and the China Postdoctoral Science Foundation (517000-X91608, 2015M580515).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Abd-AllaM. H.NafadyN. A.KhalafD. M. (2016). Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: implications for induction of autophagy process in root nodule. Agri. Ecosys. Env.218, 163–177. 10.1016/j.agee.2015.11.022
2
AdamakisI. D. S.PanterisE.EleftheriouE. P. (2011). The fatal effect of tungsten on Pisum sativum L. root cells: indications for endoplasmic reticulum stress-induced programmed cell death. Planta234, 21–34. 10.1007/s00425-011-1372-5
3
AgarwalM.SahiC.Katiyar-AgarwalS.AgarwalS.YoungT.GallieD. R.et al. (2003). Molecular characterization of rice hsp101: complementation of yeast hsp104 mutation by disaggregation of protein granules and differential expression in indica and japonica rice types. Plant Mol. Biol.51, 543–553. 10.1023/A:1022324920316
4
AhsanN.LeeD. G.LeeS. H.KangK. Y.LeeJ. J.KimP. J.et al. (2007a). Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere67, 1182–1193. 10.1016/j.chemosphere.2006.10.075
5
AhsanN.LeeS. H.LeeD. G.LeeH.LeeS. W.BahkJ. D.et al. (2007b). Physiological and protein profiles alternation of germinating rice seedlings exposed to acute cadmium toxicity. C. R. Biol.330, 735–746. 10.1016/j.crvi.2007.08.001
6
AhsanN.RenautJ.KomatsuS. (2009). Recent developments in the application of proteomics to the analysis of plant responses to heavy metals. Proteom9, 2602–2621. 10.1002/pmic.200800935
7
AinaR.LabraM.FumagalliP.VanniniC.MarsoniM.CucchiU.et al. (2007). Thiol-peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots. Environ. Exp. Bot.59, 381–392. 10.1016/j.envexpbot.2006.04.010
8
AkhtarT. A.LampiM. A.GreenbergB. M. (2005). Identification of six differentially expressed genes in response to copper exposure in the aquatic plant Lemna gibba (duckweed). Environ. Toxicol. Chem.24, 1705–1715. 10.1897/04-352R.1
9
Al-WhaibiM. H. (2011). Plant heat-shock proteins: a mini review. J. King Saud Univ-Sci.23, 139–150. 10.1016/j.jksus.2010.06.022
10
AmmI.SommerT.WolfD. H. (2014). Protein quality control and elimination of protein waste: the role of the ubiquitin–proteasome system. Biochim. Biophys. AcaBBA. Molecul. Cell Res.1843, 182–196. 10.1016/j.bbamcr.2013.06.031
11
AnsarypourZ.ShahpiriA. (2017). Heterologous expression of a rice metallothionein isoform (OsMTI-1b) in Saccharomyces cerevisiae enhances cadmium, hydrogen peroxide and ethanol tolerance. Braz. J. Microbio.48, 537–543. 10.1016/j.bjm.2016.10.024
12
AokiK.KraglerF.Xoconostle-CázaresB.LucasW. J. (2002). A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata. Proc. Natl. Acad. Sci. U.S.A.99, 16342–16347. 10.1073/pnas.252427999
13
ArrigoA. P. (1998). Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed celldeath. Biol. Chem.379, 19–26.
14
AshrafM.QztürkM. A.AhmadM. S. A. (2010). Plant Adaptation and Phytoremediation. New York, NY: Springer.
15
BasileA.SorboS.CardiM.LentiniM.CastigliaD.CianciulloP.et al. (2015). Effects of heavy metals on ultrastructure and Hsp70 induction in Lemna minor L. exposed to water along the Sarno River, Italy. Ecotoxicol. Environ. Saf.114, 93–101. 10.1016/j.ecoenv.2015.01.009
16
BasileA.SorboS.ConteB.CardiM.EspositoS. (2013). Ultrastructural changes and Heat Shock Proteins 70 induced by atmospheric pollution are similar to the effects observed under in vitro heavy metals stress in Conocephalum conicum (Marchantiales–Bryophyta). Environ. Pollut.182, 209–216. 10.1016/j.envpol.2013.07.014
17
BenattiR. M.YookongkaewN.MeetamM.GuoW. J.PunyasukN.AbuQamarS.et al. (2014). Metallothionein deficiency impacts copper accumulation and redistribution in leaves and seeds of Arabidopsis. New Phyto.202, 940–951. 10.1111/nph.12718
18
BierkensJ. G. (2000). Applications and pitfalls of stress-proteins in biomonitoring. Toxicology153, 61–72. 10.1016/S0300-483X(00)00304-8
19
BierkensJ.MaesJ.Vander PlaetseF. (1998). Dose-dependent induction of heat shock protein 70 synthesis in Raphidocelis subcapitata following exposure to different classes of environmental pollutants. Environ. Pollut.101, 91–97. 10.1016/S0269-7491(98)00010-4
20
BrandizziF.FrigerioL.HowellS. H.SchäferP. (2014). Endoplasmic reticulum—shape and function in stress translation. Front. Plant Sci.5:425. 10.3389/fpls.2014.00425
21
BurkheadJ. L.Gogolin ReynoldsK. A.Abdel-GhanyS. E.CohuC. M.PilonM. (2009). Copper homeostasis. New Phyto.182, 799–816. 10.1111/j.1469-8137.2009.02846.x
22
CaiS. Y.ZhangY.XuY. P.QiZ. Y.LiM. Q.AhammedG. J.et al. (2017). HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal. Res.62:e12387. 10.1111/jpi.12387
23
CapdevilaM.AtrianS. (2011). Metallothionein protein evolution: a miniassay. J. Biol. Inorg. Chem.16, 977–989. 10.1007/s00775-011-0798-3
24
ChaiT.ZhangY. (1998). Expression analysis of polyubiquitin genes from bean in response to heavy metals. Acta Bota. Sini.41, 1052–1057.
25
ChakrabartyD.TrivediP. K.MisraP.TiwariM.ShriM.ShuklaD.et al. (2009). Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere74, 688–702. 10.1016/j.chemosphere.2008.09.082
26
ChenY. A.ChiW. C.HuangT. L.LinC. Y.Quynh NguyehT. T.HsiungY. C.et al. (2012). Mercury-induced biochemical and proteomic changes in rice roots. Plant Physiol. Biochem.55, 23–32. 10.1016/j.plaphy.2012.03.008
27
ChiarelliR.RoccheriM. C. (2012). Heavy metals and metalloids as autophagy inducing agents: focus on cadmium and arsenic. Cells1, 597–616. 10.3390/cells1030597
28
ChrestensenC. A.StarkeD. W.MieyalJ. J. (2000). Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J. Biol. Chem.275, 26556–26565. 10.1074/jbc.M004097200
29
ClemensS. (2006). Evolution and function of phytochelatin synthases. J. Plant Physiol.163, 319–332. 10.1016/j.jplph.2005.11.010
30
ClemensS.MaJ. F. (2016). Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol.67, 489–512. 10.1146/annurev-arplant-043015-112301
31
CobbettC.GoldsbroughP. (2002). Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Ann. Rev. Plant Biol.53, 159–182. 10.1146/annurev.arplant.53.100301.135154
32
ConnollyE. L.FettJ. P.GuerinotM. L. (2002). Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell14, 1347–1357. 10.1105/tpc.001263
33
D'AlessandroA.TaamalliM.GeviF.TimperioA. M.ZollaL.GhnayaT. (2013). Cadmium stress responses in Brassica juncea: hints from proteomics and metabolomics. J. Proteom. Res.12, 4979–4997. 10.1021/pr400793e
34
DamettoA.BuffonG.Dos Reis BlasiÉ. A.SperottoR. A. (2015). Ubiquitination pathway as a target to develop abiotic stress tolerance in rice. Plant Signal. Behav.10:e1057369. 10.1080/15592324.2015.1057369
35
DengY.SrivastavaR.HowellS. H. (2013). Endoplasmic reticulum (ER) stress response and its physiological roles in plants. Int. J. Mol. Sci.14, 8188–8212. 10.3390/ijms14048188
36
DobsonC. M. (2003). Protein folding and misfolding. Nature, 426, 884–890. 10.1038/nature02261
37
DubeyS.MisraP.DwivediS.ChatterjeeS.BagS. K.MantriS.et al. (2010). Transcriptomic and metabolomic shifts in rice roots in response to Cr (VI) stress. BMC Genom.11:648. 10.1186/1471-2164-11-648
38
DurandT. C.SergeantK.PlanchonS.CarpinS.LabelP.MorabitoD.et al. (2010). Acute metal stress in Populus tremula x P. alba (717-1B4 genotype): leaf and cambial proteome changes induced by cadmium2+. Proteomics10, 349–368. 10.1002/pmic.200900484
39
EmamverdianA.DingY.MokhberdoranF.XieY. (2015). Heavy metal stress and some mechanisms of plant defense response. Sci. World J.2015:756120. 10.1155/2015/756120
40
FarahM. A.AliM. A.ChenS. M.LiY.Al-HemaidF. M.Abou-TarboushF. M.et al. (2016). Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf. B. Biointerfaces141, 158–169. 10.1016/j.colsurfb.2016.01.027
41
FuriniA. (2012). Plants and Heavy Metals. Netherlands, Springer.
42
GaoC.JiangB.WangY.LiuG.YangC. (2012). Overexpression of a heat shock protein (ThHSP18. 3) from Tamarix hispida confers stress tolerance to yeast. Mol. Biol. Rep.39, 4889–4897. 10.1007/s11033-011-1284-2
43
GardarinA.ChédinS.LagnielG.AudeJ. C.GodatE.CattyP.et al. (2010). Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol. Microbial.76, 1034–1048. 10.1111/j.1365-2958.2010.07166.x
44
GenschikP.ParmentierY.DurrA.MarbachJ.CriquiM. C.JametE.et al. (1992). Ubiquitin genes are differentially regulated in protoplast-derived cultures of Nicotiana sylvestris and in response to various stresses. Plant Mol. Biol.20, 897–910. 10.1007/BF00027161
45
GoldbergA. L. (2003). Protein degradation and protection against misfolded or damaged proteins. Nature426, 895–899. 10.1038/nature02263
46
GoupilP.SouguirD.FerjaniE.FaureO.HitmiA.LedoigtG. (2009). Expression of stress-related genes in tomato plants exposed to arsenic and chromium in nutrient solution. J. Plant Physiol.166, 1446–1452. 10.1016/j.jplph.2009.01.015
47
GuC. S.LiuL. Q.DengY. M.ZhuX. D.HuangS. Z.LuX. Q. (2015). The heterologous expression of the Iris lactea var. chinensis type 2 metallothionein IlMT2b gene enhances copper tolerance in Arabidopsis thaliana. Bull. Environ. Contam. Toxicol.94, 247–253. 10.1007/s00128-014-1444-x
48
GuanC.JinC.JiJ.WangG.LiX. (2015). LcBiP, a endoplasmic reticulum chaperone binding protein gene from Lycium chinense, confers cadmium tolerance in transgenic tobacco. Biotech. Progre.31, 358–368. 10.1002/btpr.2046
49
GuoQ.ZhangJ.GaoQ.XingS.LiF.WangW. (2008). Drought tolerance through overexpression of monoubiquitin in transgenic tobacco. J. Plant Physiol.165, 1745–1755. 10.1016/j.jplph.2007.10.002
50
GuptaS. C.SharmaA.MishraM.MishraR. K.ChowdhuriD. K. (2010). Heat shock proteins in toxicology: how close and how far?Life Sci.86, 377–384. 10.1016/j.lfs.2009.12.015
51
HaapT.SchwarzS.KöhlerH. R. (2016). Metallothionein and Hsp70 trade-off against one another in Daphnia magna cross-tolerance to cadmium and heat stress. Aquat. Toxicol.170, 112–119. 10.1016/j.aquatox.2015.11.008
52
HanS.YuB.WangY.LiuY. (2011). Role of plant autophagy in stress response. Pro. Cell2, 784–791. 10.1007/s13238-011-1104-4
53
HaqN. U.RazaS.LutheD. S.HeckathornS. A.ShakeelS. N. (2013). A dual role for the chloroplast small heat shock protein of Chenopodium album including protection from both heat and metal stress. Plant Mol. Biol. Rep.31, 398–408. 10.1007/s11105-012-0516-5
54
HaralampidisK.MilioniD.RigasS.HatzopoulosP. (2002). Combinatorial Interaction of Cis Elements Specifies the Expression of the Arabidopsis AtHsp90-1Gene. Plant Physiol.129, 1138–1149. 10.1104/pp.004044
55
Hartmann-PetersenR.SeegerM.GordonC. (2003). Transferring substrates to the 26S proteasome. Trends Biochem. Sci.28, 26–31. 10.1016/S0968-0004(02)00002-6
56
HasanM.AhammedG. J.YinL.ShiK.XiaX.ZhouY.et al. (2015). Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci.6:601. 10.3389/fpls.2015.00601
57
HasanM. K.LiuC.WangF.AhammedG. J.ZhouJ.XuM. X.et al. (2016). Glutathione-mediated regulation of nitric oxide, S-nitrosothiol and redox homeostasis confers cadmium tolerance by inducing transcription factors and stress response genes in tomato. Chemosphere161, 536–545. 10.1016/j.chemosphere.2016.07.053
58
HassinenV. H.TervahautaA. I.SchatH.KärenlampiS. O. (2011). Plant metallothioneins–metal chelators with ROS scavenging activity?. Plant Biol.13, 225–232. 10.1111/j.1438-8677.2010.00398.x
59
HollandS.LodwigE.SideriT.ReaderT.ClarkeI.GkargkasK.et al. (2007). Application of the comprehensive set of heterozygous yeast deletion mutants to elucidate the molecular basis of cellular chromium toxicity. Genome Biol.8, R268. 10.1186/gb-2007-8-12-r268
60
HossainM. A.PiyatidaP.da SilvaJ. A. T.FujitaM. (2012a). Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot.2012:872875. 10.1155/2012/872875
61
HossainZ.KhatoonA.KomatsuS. (2013). Soybean proteomics for unraveling abiotic stress response mechanism. J. Proteom Res.12, 4670–4684. 10.1021/pr400604b
62
HossainZ.KomatsuS. (2013). Contribution of proteomic studies towards understanding plant heavy metal stress response. Front. Plant Sci. 3:310. 10.3389/fpls.2012.00310
63
HossainZ.MakinoT.KomatsuS. (2012b). Proteomic study of β-aminobutyric acid-mediated cadmium stress alleviation in soybean. J. Proteom.75, 4151–4164. 10.1016/j.jprot.2012.05.037
64
HowellS. H. (2013). Endoplasmic reticulum stress responses in plants. Ann. Rev. Plant Biol.64, 477–499. 10.1146/annurev-arplant-050312-120053
65
HüttnerS.VeitC.SchobererJ.GrassJ.StrasserR. (2012). Unraveling the function of Arabidopsis thaliana OS9 in the endoplasmic reticulum-associated degradation of glycoproteins. Plant. Mol. Biol.79, 21–33. 10.1007/s11103-012-9891-4
66
IrvineG. W.TanS. N.StillmanM. J. (2017). A simple metallothionein-based biosensor for enhanced detection of arsenic and mercury. Biosensors7:14. 10.3390/bios7010014
67
JabeenR.AhmadA.IqbalM. (2009). Phytoremediation of heavy metals: physiological and molecular mechanisms. Bot. Rev.75, 339–364. 10.1007/s12229-009-9036-x
68
JacobsonT.NavarreteC.SharmaS. K.SideriT. C.IbstedtS.PriyaS.et al. (2012). Arsenite interferes with protein folding and triggers formation of protein aggregates in yeast. J. Cell Sci.125, 5073–5083. 10.1242/jcs.107029
69
JungmannJ.ReinsH.SchobertC.JentschS. (1993). Resistance to cadmium mediated by ubiquitin dependent proteolysis. Nature361, 369–371. 10.1038/361369a0
70
KangY. J. (2006). Metallothionein redox cycle and function. Exp. Biol. Med.231, 1459–1467. 10.1177/153537020623100903
71
KarmousI.BellaniL. M.ChaouiA.El FerjaniE.MucciforaS. (2015). Effects of copper on reserve mobilization in embryo of Phaseolus vulgaris L. Environ. Sci. Poll. Res.22, 10159–10165. 10.1007/s11356-015-4208-1
72
KarmousI.ChaouiA.JaouaniK.SheehanD.El FerjaniE.ScocciantiV.et al. (2014). Role of the ubiquitin-proteasome pathway and some peptidases during seed germination and copper stress in bean cotyledons. Plant Physiol. Biochem.76, 77–85. 10.1016/j.plaphy.2013.12.025
73
KiefferP.DommesJ.HoffmannL.HausmanJ. F.RenautJ. (2008). Quantitative changes in protein expression of cadmium-exposed poplar plants. Proteomics8, 2514–2530. 10.1002/pmic.200701110
74
LeeS.MoonJ. S.KoT.-S.PetrosD.GoldsbroughP. B.KorbanS. S. (2003). Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol.131, 656–663. 10.1104/pp.014118
75
LeeU.RiofloridoI.HongS. W.LarkindaleJ.WatersE. R.VierlingE. (2006). The Arabidopsis ClpB/ Hsp100 family of proteins: chaperones for stress and chloroplast development. Plant J.49, 115–127. 10.1111/j.1365-313X.2006.02940.x
76
LewisS.DonkinM. E.DepledgeM. H. (2001). Hsp70 expression in Enteromorpha intestinalis (Chlorophyta) exposed to environmental stressors. Aquat. Toxicol.51, 277–291. 10.1016/S0166-445X(00)00119-3
77
LiM.AhammedG. J.LiC.BaoX.YuJ.HuangC.et al. (2016a). Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 7:615. 10.3389/fpls.2016.00615
78
LiM.-Q.HasanM. K.LiC.-X.AhammedG. J.XiaX.-J.ShiK.et al. (2016b). Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal. Res.61, 291–302. 10.1111/jpi.12346
79
LiS.ZhangX.ZhangX. Y.XiaoW.BerryJ. O.LiP.et al. (2015). Expression of Malus xiaojinensis IRT1 (MxIRT1) protein in transgenic yeast cells leads to degradation through autophagy in the presence of excessive iron. Yeast32, 499–517. 10.1002/yea.3075
80
LimS. D.HwangJ. G.HanA. R.ParkY. C.LeeC.OkY. S.et al. (2014). Positive regulation of rice RING E3 ligase OsHIR1 in arsenic and cadmium uptakes. Plant Mol. Biol.85, 365–379. 10.1007/s11103-014-0190-0
81
LinguaG.BonaE.TodeschiniV.CattaneoC.MarsanoF.BertaG.et al. (2012). Effects of heavy metals and arbuscular mycorrhiza on the leaf proteome of a selected poplar clone: a time course analysis. PLoS ONE7:e38662. 10.1371/journal.pone.0038662
82
LiuJ.ShiX.QianM.ZhengL.LianC.XiaY.et al. (2015a). Copper-induced hydrogen peroxide upregulation of a metallothionein gene, OsMT2c, from Oryza sativa L. confers copper tolerance in Arabidopsis thaliana. J. Hazard Mater.294, 99–108. 10.1016/j.jhazmat.2015.03.060
83
LiuJ. X.HowellS. H. (2016). Managing the protein folding demands in the endoplasmic reticulum of plants. New Phyto.211, 418–428. 10.1111/nph.13915
84
LiuX.WuH.JiC.WeiL.ZhaoJ.YuJ. (2013). An integrated proteomic and metabolomic study on the chronic effects of mercury in Suaeda salsa under an environmentally relevant salinity. PLoS ONE8:e64041. 10.1371/journal.pone.0064041
85
LiuY.BasshamD. C. (2012). Autophagy: pathways for self-eating in plant cells. Ann. Rev. Plant Biol.63, 215–237. 10.1146/annurev-arplant-042811-105441
86
LiuY. C.FujimoriD. G.WeissmanJ. S. (2016). Htm1p–Pdi1p is a folding-sensitive mannosidase that marks N-glycoproteins for ER-associated protein degradation. Proc. Natl. Acad. Sci. U.S.A.113, E4015–E4024. 10.1073/pnas.1608795113
87
LiuY.ZhangC.WangD.SuW.LiuL.WangM.et al. (2015b). EBS7 is a plant-specific component of a highly conserved endoplasmic reticulum-associated degradation system in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A.112, 12205–12210. 10.1073/pnas.1511724112
88
LomaglioT.RoccoM.TrupianoD.De ZioE.GrossoA.MarraM.et al. (2015). Effect of short-term cadmium stress on Populus nigra L. detached leaves. J. Plant Physiol.182, 40–48. 10.1016/j.jplph.2015.04.007
89
LyzengaW. J.StoneS. L. (2012). Abiotic stress tolerance mediated by protein ubiquitination. J. Exp. Bot.63, 599–616. 10.1093/jxb/err310
90
MalhotraJ. D.KaufmanR. J. (2007). Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword?. Antioxid. Redox Signal.9, 2277–2294. 10.1089/ars.2007.1782
91
ManaraA. (2012). Plant responses to heavy metal toxicity, in Plants and Heavy Metals, ed FuriniA. (Springer), 27–53.
92
MaretW. (2000). The function of zinc metallothionein: a link between cellular zinc and redox state. J. Nutr.130, 1455S–1458S.
93
Maupin-FurlowJ. A. (2013). Ubiquitin-like proteins and their roles in archaea. Trends Microbiol.21, 31–38. 10.1016/j.tim.2012.09.006
94
Mendoza-CózatlD. G.JobeT. O.HauserF.SchroederJ. I. (2011). Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol.14, 554–562. 10.1016/j.pbi.2011.07.004
95
Mendoza-CózatlD. G.ZhaiZ.JobeT. O.AkmakjianG. Z.SongW. Y.LimboO.et al. (2010). Tonoplast-localized Abc2 transporter mediates phytochelatin accumulation in vacuoles and confers cadmium tolerance. J. Biol. Chem.285, 40416–40426. 10.1074/jbc.M110.155408
96
MilioniD.HatzopoulosP. (1997). Genomic organization of hsp90 gene family in Arabidopsis. Plant Mol. Biol.35, 955–961. 10.1023/A:1005874521528
97
MinibayevaF.DmitrievaS.PonomarevaA.RyabovolV. (2012). Oxidative stress-induced autophagy in plants: the role of mitochondria. Plant Physiol. Biochem.59, 11–19. 10.1016/j.plaphy.2012.02.013
98
MishraR. C.GroverA. (2014). Intergenic sequence between Arabidopsis ClpB-C/Hsp100 and choline kinase genes functions as a heat inducible bidirectional promoter. Plant Physiol.166, 1646–1658. 10.1104/pp.114.250787
99
MogkA.BukauB. (2017). Role of sHsps in organizing cytosolic protein aggregation and disaggregation. Cell Stress Chaperones22, 493–502. 10.1007/s12192-017-0762-4
100
MuC.ZhangS.YuG.ChenN.LiX.LiuH. (2013). Overexpression of small heat shock protein LimHSP16. 45 in Arabidopsis enhances tolerance to abiotic stresses. PLoS ONE8:e82264. 10.1371/journal.pone.0082264
101
NaganumaA.MiuraN.KanekoS.MishinaT.HosoyaS.MiyairiS.et al. (2000). GFAT as a target molecule of methylmercury toxicity in Saccharomyces cerevisiae. FASEB J.14, 968–972.
102
NeumannD.LichtenbergerO.GüntherD.TschierschK.NoverL. (1994). Heat-shock proteins induce heavy-metal tolerance in higher plants. Planta194, 360–367. 10.1007/BF00197536
103
NeumannD.Zur NiedenU.LichtenbergerO.LeopoldI. (1995). How does Armeria maritima tolerate high heavy metal concentrations?J. Plant Physiol.146, 704–717. 10.1016/S0176-1617(11)81937-1
104
OonoY.YazawaT.KanamoriH.SasakiH.MoriS.HandaH.et al. (2016). Genome-wide transcriptome analysis of cadmium stress in rice. BioMed Res. Int.2016:9739505. 10.1155/2016/9739505
105
ParkC. J.SeoY. S. (2015). Heat Shock Proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J.31, 323–333. 10.5423/PPJ.RW.08.2015.0150
106
ParkJ.SongW. Y.KoD.EomY.HansenT. H.SchillerM.et al. (2012). The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J.69, 278–288. 10.1111/j.1365-313X.2011.04789.x
107
PenaL. B.PasquiniL. A.TomaroM. L.GallegoS. M. (2007). 20S proteasome and accumulation of oxidized and ubiquitinated proteins in maize leaves subjected to cadmium stress. Phytochemistry68, 1139–1146. 10.1016/j.phytochem.2007.02.022
108
PenaL. B.ZawoznikM. S.TomaroM. L.GallegoS. M. (2008). Heavy metals effects on proteolytic system in sunflower leaves. Chemosphere72, 741–746. 10.1016/j.chemosphere.2008.03.024
109
PeñarrubiaL.Andrés-ColásN.MorenoJ.PuigS. (2010). Regulation of copper transport in Arabidopsis thaliana: a biochemical oscillator?JBIC J. Biol. Inorg. Chem.15, 29.
110
Pérez-MartínM.Blaby-HaasC. E.Pérez-PérezM. E.Andrés-GarridoA.BlabyI. K.MerchantS. S.et al. (2015). Activation of autophagy by metals in Chlamydomonas reinhardtii. Eukaryot. Cell, 14, 964–973. 10.1128/EC.00081-15
111
PinesJ.LindonC. (2005). Proteolysis: anytime, anyplace, anywhere?Nature Cell Biol.7, 731–735. 10.1038/ncb0805-731
112
PochodyloA. L.AristildeL. (2017). Molecular dynamics of stability and structures in phytochelatin complexes with Zn, Cu, Fe, Mg, and Ca: Implications for metal detoxification. Environ. Chem. Lett. 1–6. 10.1007/s10311-017-0609-3
113
PomponiM.CensiV.Di GirolamoV.De PaolisA.Di ToppiL. S.AromoloR.et al. (2006). Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta223, 180–190. 10.1007/s00425-005-0073-3
114
PrasadB. D.GoelS.KrishnaP. (2010). In silico identification of carboxylate clamp type tetratricopeptide repeat proteins in Arabidopsis and rice as putative co-chaperones of Hsp90/Hsp70. PLoS ONE5:e12761. 10.1371/journal.pone.0012761
115
PrattW. B.ToftD. O. (2003). Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med.228, 111–133. 10.1177/153537020322800201
116
RaiA.BhardwajA.MisraP.BagS. K.AdhikariB.TripathiR. D.et al. (2015). Comparative transcriptional profiling of contrasting rice genotypes shows expression differences during arsenic stress. Plant Genome8:2. 10.3835/plantgenome2014.09.0054
117
RauchJ. N.TseE.FreilichR.MokS. A.MakleyL. N.SouthworthD. R.et al. (2017). BAG3 Is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock proteins. J. Mol. Biol.429, 128–141. 10.1016/j.jmb.2016.11.013
118
RheeJ. S.RaisuddinS.LeeK. W.SeoJ. S.KiJ. S.KimI. C.et al. (2009). Heat shock protein (Hsp) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants. Comp. Biochem. Physiol. C Toxicol. Pharmacol.149, 104–112. 10.1016/j.cbpc.2008.07.009
119
Rodríguez-CelmaJ.Rellán-ÁlvarezR.AbadíaA.AbadíaJ.López-MillánA.-F. (2010). Changes induced by two levels of cadmium toxicity in the 2-DE protein profile of tomatoroots. J. Proteom.73, 1694–1706. 10.1016/j.jprot.2010.05.001
120
RuschakA. M.SlassiM.KayL. E.SchimmerA. D. (2011). Novel proteasome inhibitors to overcome bortezomib resistance. J. Nat. Can. Inst.103, 1007–1017. 10.1093/jnci/djr160
121
SáckýJ.LeonhardtT.BorovičkaJ.GryndlerM.BriksíA.KotrbaP. (2014). Intracellular sequestration of zinc, cadmium and silver in Hebeloma mesophaeum and characterization of its metallothionein genes. Fungal Genet. Biol.67, 3–14. 10.1016/j.fgb.2014.03.003
122
SadanandomA.BaileyM.EwanR.LeeJ.NelisS. (2012). The ubiquitin–proteasome system: central modifier of plant signalling. New Phyto.196, 13–28. 10.1111/j.1469-8137.2012.04266.x
123
SanchezY.TaulienJ.BorkovichK. A.LindquistS. (1992). Hsp104 is required for tolerance to many forms of stress. EMBO J.11, 2357.
124
SarryJ. E.KuhnL.DucruixC.LafayeA.JunotC.HugouvieuxV.JourdainA.et al. (2006). The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics6, 2180–2198. 10.1002/pmic.200500543
125
Scherz-ShouvalR.ElazarZ. (2007). ROS, mitochondria and the regulation of autophagy. Trends Cell Biol.17, 422–427. 10.1016/j.tcb.2007.07.009
126
SchröderM.KaufmanR. J. (2005). ER stress and the unfolded protein response. Mutat. Res./Fund. Mol. Mechan. Mutagen.569, 29–63. 10.1016/j.mrfmmm.2004.06.056
127
SchubertU.AntonL. C.GibbsJ.NorburyC. C.YewdellJ. W.BenninkJ. R. (2000). Rapid degradation of a large fraction of newly synthesized proteins byproteasomes. Nature404, 770–774. 10.1038/35008096
128
SchöfflF.PrandlR.ReindlA. (1999). Molecular responses to heat stress, in Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants, eds ShinozakiK.Yamaguchi-ShinozakiK. R. G. (Austin, TX: Landes Co.,), 81–98.
129
SergioE.CobianchiR. C.SorboS.ConteB.BasileA. (2007). Ultrastructural alterations and HSP 70 induction in Elodea ca-nadensis Michx.exposed to heavy metals. Caryologia60, 115–120.
130
SharmaS. K.GoloubinoffP.ChristenP. (2011). Non-native Proteins as Newly-Identified Targets of Heavy Metals and Metalloids, in Cellular Effects of Heavy Metals, ed BanfalviG. (Springer), 263–274.
131
SharmaS. S.DietzK. J.MimuraT. (2016). Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ.39, 1112–1126. 10.1111/pce.12706
132
SinghA.MittalD.LavaniaD.AgarwalM.MishraR. C.GroverA. (2012). OsHsfA2c and OsHsfB4b are involved in the transcriptional regulation of cytoplasmic OsClpB (Hsp100) gene in rice (Oryza sativa L.). Cell Stress Chaperones17, 243–254. 10.1007/s12192-011-0303-5
133
SongW. Y.Mendoza-CozatlD. G.LeeY.SchroederJ. I.AhnS. N.LeeH. S.et al. (2014). Phytochelatin–metal (loid) transport into vacuoles shows different substrate preferences in barley and Arabidopsis. Plant Cell Env.37, 1192–1201. 10.1111/pce.12227
134
SongY.CuiJ.ZhangH.WangG.ZhaoF. J.ShenZ. (2013). Proteomic analysis of copper stress responses in the roots of two rice (Oryza sativa L.) varieties differing in Cu tolerance. Plant Soil366, 647–658.
135
SørensenJ. G.KristensenT. N.LoeschckeV. (2003). The evolutionary and ecological role of heat shock proteins. Ecol. Lett.6, 1025–1037. 10.1046/j.1461-0248.2003.00528.x
136
SpijkermanE.BaruaD.Gerloff-EliasA.KernJ.GaedkeU.HeckathornS. A. (2007). Stress responses and metal tolerance of Chlamydomonas acidophila in metal-enriched lake water and artificial medium. Extremophiles11, 551–562. 10.1007/s00792-007-0067-0
137
StoneS. L. (2014). The role of ubiquitin and the 26S proteasome in plant abiotic stress signaling. Front. Plant Sci.5:135. 10.3389/fpls.2014.00135
138
SumnerE. R.ShanmuganathanA.SideriT. C.WillettsS. A.HoughtonJ. E.AveryS. V. (2005). Oxidative protein damage causes chromium toxicity in yeast. Microbiology151, 1939–1948. 10.1099/mic.0.27945-0
139
SunC. W.CallisJ. (1997). Independent modulation of Arabidopsis thaliana polyubiquitin mRNAs in different organs and in response to environmental changes. Plant J.11, 1017–1027. 10.1046/j.1365-313X.1997.11051017.x
140
SunL.LuS. J.ZhangS. S.ZhouS. F.SunL.LiuJ. X. (2013). The lumen-facing domain is important for the biological function and organelle-to-organelle movement of bZIP28 during ER stress in Arabidopsis. Mol. Plant6, 1605–1161. 10.1093/mp/sst059
141
SunW.MotanguM. V.VerbruggenN. (2002). Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta1577, 1–9. 10.1016/S0167-4781(02)00417-7
142
TamásM. J.SharmaS. K.IbstedtS.JacobsonT.ChristenP. (2014). Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules4, 252–267. 10.3390/biom4010252
143
TomasM.PaganiM. A.AndreoC. S.CapdevilaM.AtrianS.BofillR. (2015). Sunflower metallothionein family characterisation. Study of the Zn (II)-and Cd (II)-binding abilities of the HaMT1 and HaMT2 isoforms. J. Iinorg. Biochem.148, 35–48. 10.1016/j.jinorgbio.2015.02.016
144
TomasM.TintiA.BofillR.CapdevilaM.AtrianS.TorreggianiA. (2016). Comparative Raman study of four plant metallothionein isoforms: insights into their Zn (II) clusters and protein conformations. J. Inorg. Biochem.156, 55–63. 10.1016/j.jinorgbio.2015.12.027
145
TuzhikovA. I.VartapetianB. B.VartapetianA. B.ChichkovaN. V. (2011). Abiotic stress-induced programmed cell death in plants: a phytaspase connection, in Biochemistry, Genetics, and Molecular Biology, Abiotic Stress Response in Plants - Physiological, Biochemical and Genetic Perspectives, eds ShankerA.VenkateswarluB. (InTech), 183–196. 10.5772/23785
146
Van HoewykD. (2013). A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann. Bot.112, 965–972. 10.1093/aob/mct163
147
Van HoewykD. (2016). Defects in endoplasmic reticulum-associated degradation (ERAD) increase selenate sensitivity in Arabidopsis. Plant Signal. Behav.10.1080/15592324.2016.1171451
148
ViehwegerK. (2014). How plants cope with heavy metals. Bot. Stud.55:35. 10.1186/1999-3110-55-35
149
VierstraR. D. (2009). The ubiquitin–26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol.10, 385–397. 10.1038/nrm2688
150
WangW.VinocurB.ShoseyovO.AltmanA. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci.9, 244–252. 10.1016/j.tplants.2004.03.006
151
WangY.CaiS. Y.YinL. L.ShiK.XiaX. J.ZhouY. H.et al. (2015). Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy11, 2033–2047. 10.1080/15548627.2015.1098798
152
WengZ. X.WangL. X.TanF. L.HuangL.XingJ. H.ChenS. P.et al. (2013). Proteomic and physiological analyses reveal detoxification and antioxidation induced by Cd stress in Kandelia candel roots. Trees27, 583–595. 10.1007/s00468-012-0811-7
153
Wen-XingD. (2012). Autophagy in toxicology: defense against xenobiotics. J. Drug. Metab. Toxicol.3:e108. 10.4172/2157-7609.1000e108
154
WollgiehnR.NeumannD. (1999). Metal stress response and tolerance of cultured cells from Silene vulgaris and Lycopersicon peruvianum: role of heat stress proteins. J. Plant Physiol.154, 547–553. 10.1016/S0176-1617(99)80296-X
155
XieY.YeS.WangY.XuL.ZhuX.YangJ.et al. (2015). Transcriptome-based gene profiling provides novel insights into the characteristics of radish root response to Cr stress with next-generation sequencing. Front. Plant Sci.6:202. 10.3389/fpls.2015.00202
156
XuH.XuW.XiH.MaW.HeZ.MaM. (2013). The ER luminal binding protein (BiP) alleviates Cd2+-induced programmed cell death through endoplasmic reticulum stress–cell death signaling pathway in tobacco cells. J. Physiol.170, 1434–1441. 10.1016/j.jplph.2013.05.017
157
XuL.WangY.LiuW.WangJ.ZhuX.ZhangK.et al. (2015). De novo sequencing of root transcriptome reveals complex cadmium-responsive regulatory networks in radish (Raphanus sativus L.). Plant. Sci.236, 313–323. 10.1016/j.plantsci.2015.04.015
158
XuW.CaiS. Y.ZhangY.WangY.AhammedG. J.XiaX. J.et al. (2016). Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal. Res.10.1111/jpi.12359
159
YakimovaE. T.Kapchina-TotevaV. M.WolteringE. J. (2007). Signal transduction events in aluminum-induced cell death in tomato suspension cells. J. Physiol.164, 702–708. 10.1016/j.jplph.2006.03.018
160
YangJ.WangY.LiuG.YangC.LiC. (2011). Tamarix hispida metallothionein-like ThMT3, a reactive oxygen species scavenger, increases tolerance against Cd2+, Zn2+, Cu2+, and NaCl in transgenic yeast. Molec. Biol. Rep.38, 567–1574. 10.1007/s11033-010-0265-1
161
YangX.SrivastavaR.HowellS. H.BasshamD. C. (2016). Activation of autophagy by unfolded proteins during endoplasmic reticulum stress. Plant J.85, 83–95. 10.1111/tpj.13091
162
YangZ. T.LuS. J.WangM. J.BiD. L.SunL.ZhouS. F.et al. (2014). A plasma membrane-tethered transcription factor, NAC062/ANAC062/NTL6, mediates the unfolded protein response in Arabidopsis. Plant J.79, 1033–1043. 10.1111/tpj.12604
163
YoshidaH.HazeK.YanagiH.YuraT.MoriK. (1998). Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins Involvement of basic leucine zipper transcription factors. J. Biol. Chem.273, 33741–33749. 10.1074/jbc.273.50.33741
164
YuX.PonceR. A.FaustmanE. M. (2011). Metals induced disruption of ubiquitin proteasome system, activation of stress signaling and apoptosis, in Cellular Effects of Heavy Metals, ed BanfalviG. (Springer), 291–311.
165
ZhangJ.HwangJ. U.SongW. Y.MartinoiaE.LeeY. (2017). Identification of amino acid residues important for the arsenic resistance function of Arabidopsis ABCC1. FEBS Lett.591, 656–666. 10.1002/1873-3468.12576
166
ZhangW.ChenW. (2010). Autophagy induction upon reactive oxygen species in Cd-stressed Arabidopsis thaliana, in Proceedings SPIE 7568, Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues VIII (San Francisco, CA). 10.1117/12.841394
167
ZhaoC. R.IkkaT.SawakiY.KobayashiY.SuzukiY.HibinoT.et al. (2009). Comparative transcriptomic characterization of aluminum, sodiumchloride, cadmiumand copper rhizotoxicities in Arabidopsis thaliana. BMC Plant Biol.9:32. 10.1186/1471-2229-9-32
168
ZhaoL.SunY. L.CuiS. X.ChenM.YangH. M.LiuH. M.et al. (2011). Cd-induced changes in leaf proteome of the hyperaccumulator plant Phytolacca americana. Chemosphere85, 56–66. 10.1016/j.chemosphere.2011.06.029
169
ZhaoY.ChengcaiC. (2011). Towards understanding plant response to heavy metal stress, in AbioticStress in Plants - Mechanisms and Adaptations, ed ShankerA. (Rijeka: Tech Europe), 59–78.
170
ZhenY.QiJ. L.WangS. S.SuJ.XuG. H.ZhangM. S.et al. (2007). Comparative proteome analysis of differentially expressed proteins induced by Al toxicity in soybean. Physiol. Planta.131, 542–554. 10.1111/j.1399-3054.2007.00979.x
171
ZhengL.PeerT.SeyboldV.Lütz-MeindlU. (2012). Pb-induced ultrastructural alterations and subcellular localization of Pb in two species of Lespedeza by TEM-coupled electron energy loss spectroscopy. Environ. Exp. Bot.77, 196–206. 10.1016/j.envexpbot.2011.11.018
172
ZhouS.OkekeogbuI.SangireddyS.YeZ.LiH.BhattiS.et al. (2016). Proteome modification in tomato plants upon long-term aluminum treatment. J. Proteom. Res.15, 1670–1684. 10.1021/acs.jproteome.6b00128
173
ZhouX. M.ZhaoP.WangW.ZouJ.ChengT. H.PengX. B.et al. (2015). A comprehensive, genome-wide analysis of autophagy-related genes identified in tobacco suggests a central role of autophagy in plant response to various environmental cues. DNA Res.22, 245–257. 10.1093/dnares/dsv012
174
ZhuW.LuM.GongZ.ChenR. (2013). Cloning and expression of a small heat shock protein gene CaHSP24 from pepper under abiotic stress. African J. Biotech.10, 4968–4976.
Summary
Keywords
heavy metals, phytochelatins, metallothioneins, protein quality control system, ubiquition proteasome system, autophagy
Citation
Hasan MK, Cheng Y, Kanwar MK, Chu X-Y, Ahammed GJ and Qi Z-Y (2017) Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 8:1492. doi: 10.3389/fpls.2017.01492
Received
30 May 2017
Accepted
11 August 2017
Published
05 September 2017
Volume
8 - 2017
Edited by
Minghui Lu, Northwest A&F University, China
Reviewed by
Shabir Hussain Wani, Michigan State University, United States; Liang Xu, Nanjing Agricultural University, China
Updates
Copyright
© 2017 Hasan, Cheng, Kanwar, Chu, Ahammed and Qi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Golam J. Ahammed ahammed@zju.edu.cn
This article was submitted to Plant Cell Biology, a section of the journal Frontiers in Plant Science
†These authors have contributed equally to this work.
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.