- 1The Genetic Engineering International Cooperation Base of Chinese Ministry of Science and Technology, Key Laboratory of Molecular Biophysics of Chinese Ministry of Education, College of Life Science and Technology, Huazhong University of Science & Technology, Wuhan, China
- 2School of Chemistry and Chemical Engineering, Huazhong University of Science & Technology, Wuhan, China
Microelement contents and metabolism are vitally important for cereal plant growth and development as well as end-use properties. While minerals phytotoxicity harms plants, microelement deficiency also affects human health. Genetic engineering provides a promising way to solve these problems. As plants vary in abilities to uptake, transport, and accumulate minerals, and the key enzymes acting on that process is primarily presented in this review. Subsequently, microelement function and biosafety assessment of transgenic cereal plants have become a key issue to be addressed. Progress in genetic engineering of cereal plants has been made with the introduction of quality, high-yield, and resistant genes since the first transgenic rice, corn, and wheat were born in 1988, 1990, and 1992, respectively. As the biosafety issue of transgenic cereal plants has now risen to be a top concern, many studies on transgenic biosafety have been carried out. Transgenic cereal biosafety issues mainly include two subjects, environmental friendliness and end-use safety. Different levels of gene confirmation, genomics, proteomics, metabolomics and nutritiomics, absorption, metabolism, and function have been investigated. Also, the different levels of microelement contents have been measured in transgenic plants. Based on the motivation of the requested biosafety, systematic designs, and analysis of transgenic cereal are also presented in this review paper.
Introduction
Rice, corn, and wheat are the three main staples, providing 60 percent of the world's food energy intake. The United States Department of Agriculture (USDA) estimates that the 2017/2018 global production will be 481.04 million metric tons of rice, 1031.86 million metric tons of corn, and 753.09 million metric tons of wheat. The United Nations Food & Agriculture Program has declared that global production of food, feed and fiber might have to be approximately doubled due to the growing global population, and this is greatly challenging traditional agriculture. Modern agriculture would benefit tremendously from the application of genetic engineering technology to enhance food crop quality, to increase yield production and also to improve resistance.
Transgenic cereal crops, known as genetically modified (GM) crops, have gained attention worldwide since they have emerged. By introducing bioengineering technology into crop breeding issues, GM crops with improved quality, enhanced resistance to biotic or abiotic stresses, increased yield, or reduced harmful components were generated (Toriyama et al., 1988; Gordon-Kamm et al., 1990; Vasil et al., 1992). Compared with the conventional breeding approach, the molecular breeding technique showed more efficiency at directly improving crop quality. However, exogenous gene transformation and expression may cause unforeseen modifications of the genome, and the commercially grown and promoted GM crops have to be subjected to safety assessments. Therefore, reliable safety evaluations are vital for the commercialization and public acceptance of GM cereal crops as well as their products (Tabashnik et al., 2013; Jin et al., 2015; Pulla, 2016). In 2016, the USA National Academies of Sciences, Engineering, and Medicine reported on genetically engineered crops including experiences and prospects, and the results showed that transgenic cereals are safe (Gloud et al., 2016). In the same year, the UK Royal Society also made an announcement that currently available GM food is safe to eat as non-GM food, and more than 100 of the world's top scientists have taken a firm position in the controversy over genetically modified organisms (GMO) by publishing an open letter to the international Greenpeace organization as well as those who campaign against GM crop marketing. So far, several international authoritative organizations, including the Organization for Economic Cooperation and Development (OECD) and the World Health Organization (WHO), have set standards for safety assessments including GM crops and products (Kitta, 2013). The substantial equivalent principle, which was first described by the OECD and further optimized by the FAO/WHO, is the underlying principle in GM food safety assessment. It was formulated to comparatively analyze the nutrients, anti-nutrients, and other components in GM crops and their non-transgenic comparators (OECD, 2000).
Among the crop nutrients, trace minerals play important roles in human and animal health (Bhullar and Gruissem, 2013). However, trace mineral deficiencies are still common throughout the world. Three main strategies have been proposed to defeat micronutrient malnutrition: food fortification, traditional breeding, and genetic engineering (Lucca et al., 2006). Among them, the transgenic approach is the most efficient at producing micronutrient-fortified crops. Whether genetic modification techniques affect the mineral compositions in the GM crops is an essential part of the biosafety assessment. Depending on whether the transformed genes are directly microelements relevant or not, effects on microelements from GM crops can be divided into two kinds. Thereby, the relationship between particular trace minerals, related exogenous genes and crops' end uses will be discussed, as will the relationship between transgenic technology, main trace minerals, and biological safety.
Microelements and Their Genetic Regulation via Transgenic Approach
Selenium
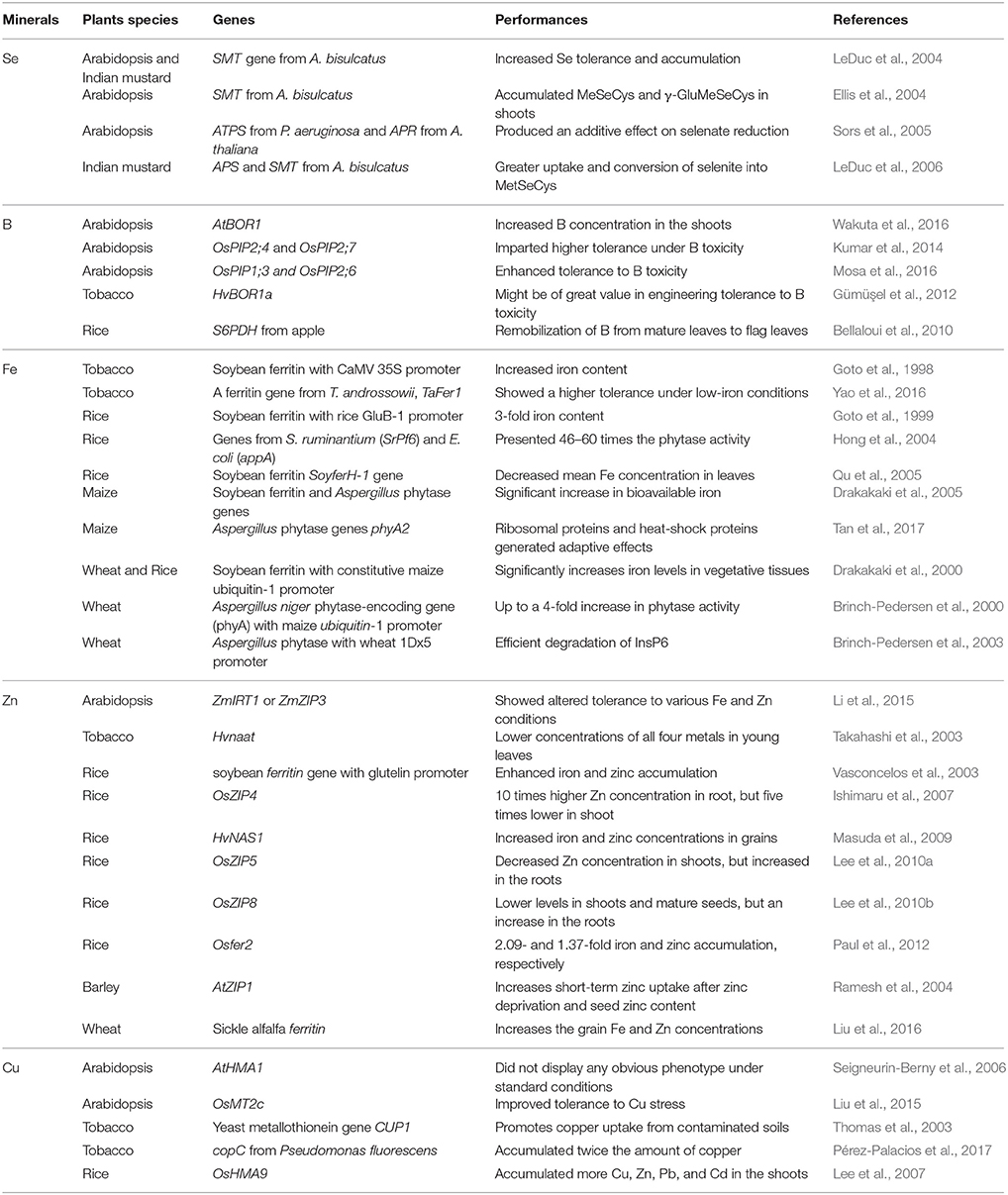
Interest in selenium (Se) has been growing since it was shown to reduce cancer risk in a landmark trial (Clark et al., 1997). Trace amounts of Se are essential to cellular functions of many organisms, while it is toxic at higher concentrations. Plants show variation in their characteristics of Se uptake, translocation, assimilation, and metabolism, as well as in their tolerance to excessive Se concentrations (White and Broadley, 2005; Gupta and Gupta, 2016). Se accumulators are those plants that can hyperaccumulate Se in their shoots while growing on seleniferous soils, many of which belong to the genus Astragalus. Se accumulators are able to accumulate Se from hundreds to several thousand mg kg−1 dry weight. However, most crop plants are Se non-accumulators, which accumulate no more than 100 mg Se kg−1 dry weight when grown on seleniferous soils and cannot tolerate higher tissue Se concentrations (Brown and Shrift, 1982; White, 2016). The Se uptake ability of agricultural crops largely depends on the plant species; cruciferae usually rank first, then rye grass, leguminosae, and cereals (Bisbjerg and Gissel-Nielsen, 1969). As for wheat, it was found to be more tolerant of Se than tobacco, soybeans or rice. It was exhibited that early root growth of wheat was inhibited when solution concentrations of Se were above 10 mg L−1. Interestingly, different forms of Se behave differently. While 70 mg L−1 Se concentration was found to inhibit germination for selenite, 150 mg L−1 Se concentration failed to repress seed germination for selenate. These results demonstrated the potential of wheat biofortification in Australia as Se phytotoxicity will not be observed in wheat (Lyons et al., 2005; Li H. F. et al., 2008; Wu et al., 2015).
Se shares similar metabolic pathways in plants with sulfur (S) because of their chemical and physical similarities (Shinmachi et al., 2010; Tian et al., 2017). There are two key enzymes involved in Se metabolism pathways: ATP sulfurylase (ATPS) and selenocysteine methyltransferase (SMT). ATPS respectively catalyzes ATP with either sulfate or selenate to form adenosine 5′-phosphosulfate or adenosine 5′-phosphoselenate (Sors et al., 2005; Schiavon et al., 2015). SMT is involved in the synthesis of Se-methylselenocysteine by methylating selenocysteine (SeCys; Neuhierl and Böck, 1996). Simultaneous overexpression of both the ATPS and SMT genes from the Se hyperaccumulator Astragalus bisulcatus in Indian mustard resulted in 4–9 times increased accumulation of Se in transgenic lines (LeDuc et al., 2004, 2006). There are many other enzymes involved in Se and S metabolic pathways in addition to APS and SMT. APS reductase is one of them. It participates in the metabolism of selenoamino acids and sulfur by acting on the reduction of APS to sulfite in plants (Suter et al., 2000). Heterologous expression of the APS reductase gene (PaAPR) in Arabidopsis thaliana resulted in enhanced sulfite reduction, which might indirectly affect Se metabolism (Tsakraklides et al., 2002). Furthermore, selenate reduction has been dramatically increased in transgenic A. thaliana by overexpressing both ATPS and APR; thus, the total Se concentration in shoots of transgenic lines was less than in wild type (Sors et al., 2005). However, the relation between APS reductase and APSe reduction remains unclear, and more studies need to be conducted. While these enzymes exhibit various abilities in Se accumulation of plants, which might be applied in Se biofortification, they also play roles in phytoremediation. Overexpression of SMT from A. bisulcatus in A. thaliana increased selenite tolerance and foliar Se accumulation, and this indicates that it is feasible to allow a Se non-accumulator to accumulate Se-methyl selenocysteine and γ-glutamylmethyl selenocysteine through genetic engineering techniques (Ellis et al., 2004). Therefore, transgenic methods provide a possible approach to generating Se-tolerant plants that could be applied to phytoremediation of Se-contaminated land, and a field trial of transgenic Indian Mustard with the adenosine triphosphate sulfurylase (APS), ç-glutamyl-cysteine synthetase (ECS), and glutathione synthetase (GS) genes overexpressed showed better tolerance to selenium-contaminated sediment (Bañuelos et al., 2005). Although transferring certain genes to plants has obvious effects on the Se accumulation or tolerance of plants, fertilization remains a promising strategy for Se biofortification of crops (White and Broadley, 2009; Wu et al., 2015).
Boron
Boron (B) is required by both plants and animals. B deficiency has been implicated in human osteoporosis (Nielsen, 1997). As for plants, small amounts of B play a vital role in the cell wall as a component of a B-dimeric rhamnogalacturonan II (RG-II) complex during normal plant growth, and B is also found to be toxic at high concentrations like some other minerals (Miwa and Fujiwara, 2010; Fang et al., 2016). While B deficiency problems can be easily settled by fertilization, high soil B is a major problem. Therefore, new varieties that are tolerant of high B concentrations need to be cultivated.
B uptake is conducted by transport proteins through plant roots. In Arabidopsis, there are two main types of transporters localizing in the plasma membrane: BORs and NIPs (nodulin26-like intrinsic proteins). BOR1 efficiently transports B into xylem, and the A. thaliana mutant bor1-1 was found to be more sensitive to B deficiency (Takano et al., 2001, 2002; Uraguchi et al., 2014). Furthermore, transgenic lines overexpressing AtBOR1 exhibited greater B accumulation in shoots than non-transformants (Wakuta et al., 2016). Unlike BOR1, BOR2 transports B from symplasts to apoplasts, which is necessary for effective cross linking of RG-II in the cell wall, as well as elongation of root cells under B-limited conditions (Miwa et al., 2013). BOR4, a BOR1 homolog, was found in relation to the B concentration in shoots (Miwa et al., 2007; Miwa and Fujiwara, 2011). NIP5:1, which is upregulated under B limitation, differing from BOR family acting in B transport, plays a crucial role in B absorption in plants (Takano et al., 2006), while NIP6:1 facilitates boric acid transfer at the nodal regions of shoots but not roots (Tanaka et al., 2008). These research studies that try to figure out in which way plants are able to tolerate B can be applied to improving crop yields on boron-contaminated soils. Based on the AtBOR gene sequences, four rice BOR-like genes have been identified, and the activity of GUS as a reporter in transgenic rice varies with cell-type specificity in response to the B content in the medium (Nakagawa et al., 2007). Likewise, genes expressing borate transporters in wheat as well as barley have been reported (Reid, 2007). Furthermore, overexpression of HvBor1a encoding a barley transporter in tobacco suggested that the HvBor1a gene might be a promising gene for enhancing the B tolerance of crop plants (Gümüşel et al., 2012). In addition to BORs and NIPs, members of the PIP subfamily also participate in B transport. PIPs are plasma membrane intrinsic proteins. Maize aquaporins ZmPIP1 and ZmPIP2 were shown to be involved in transporting B (Martinez-Ballesta et al., 2008). Two PIP genes in rice, namely OsPIP2;4 and OsPIP2;7, were characterized as being involved in B permeability and tolerance, overexpression of which in Arabidopsis resulted in higher tolerance under B toxicity (Kumar et al., 2014). Similar results appeared from other aquaporins members, Arabidopsis heterologously expressing OsPIP1;3 and OsPIP2;6 showed increased tolerance to B toxicity through both B influx and efflux transport (Mosa et al., 2016), making it useful to manipulate PIPs in crops in improving B tolerance in crops. In addition, transgenic rice with enhanced sorbitol synthesis was found to facilitate the remobilization of B in phloem (Bellaloui et al., 2010). However, it still remains a great concern to increase yield by engineering tolerance to B toxicity in crops by the transgenic approach (Reid, 2010).
Iron
Iron is an essential microelement for many organisms including plant species, particularly for humans. Iron deficiency anemia is considered the most common type of anemia, and it can impact work efficiency, impair thermoregulation, and cause immune dysfunction in adults (Clark, 2008). Iron deficiency can induce the inhibition of plant growth as well (Bocchini et al., 2015). Iron, when its excess is not great, presents predominantly in the form of a water-soluble iron protein called ferritin (Zielinska-Dawidziak, 2015). Ferritin is an iron-storage protein existing widely in animals, plants and bacteria that consists of 24 protein subunits and can store ~4,000 iron atoms as a hollow globular protein complex (Vigani et al., 2013). Ferritin is considered to play at least two major parts in plants: firstly, as a source of iron required by metal enzymes involved in photosynthesis and other respiratory processes, and secondly, as a defense component, protecting cells from the toxic effects of overloaded iron (Briat et al., 2010). After transferring soybean ferritin cDNA to tobacco via Agrobacterium tumefaciens, iron accumulation of transformant leaves was up to 30% higher than that of wild type, and these results provided evidence of breeding high iron content crops by transferring the ferritin gene (Goto et al., 1998). By introducing the soybean ferritin gene into rice, high iron content transgenic lines were generated. The iron content of self-pollinated T1 seeds was as much as triple that of the control line (Goto et al., 1999). Further study showed that transgenic wheat and rice constitutively expressing the soybean ferritin gene via particle bombardment had increased iron content in vegetative tissues instead of seeds, suggesting that in order to accomplish ferritin overexpression and iron accumulation in transgenic seeds, seed-specific promoters such as Gt-1 and Glu-B1 should be applied (Drakakaki et al., 2000). Through overexpression of the soybean ferritin SoyferH-1 gene with a strong endosperm-specific promoter, the storage ability of iron in rice seeds has been improved; nevertheless, an increase in Fe content failed to parallel the higher expression level of the foreign ferritin gene (Qu et al., 2005). Interestingly, increased expression of the endogenous NtFer1 gene was greatly correlated with that of an exogenous TaFer1 gene in transgenic tobacco, suggesting interactions between different ferritin subunits (Wang J. et al., 2015; Yao et al., 2016).
Phytic acid is among the main inhibitory factors of iron availability; in other words, iron absorption may be extremely low due to inhibitors (Hurrell et al., 2003). Transgenic wheat and rice that constitutively express heterogenous phytase have been generated (Brinch-Pedersen et al., 2000, 2003; Hong et al., 2004). Aspergillus phytase and soybean ferritin have been transferred to maize separately or together, with the hope of increasing the bioavailable iron content in the endosperm. The result demonstrated that the heterologous expression of ferritin and phytase in crops could contribute to an increase in iron bioavailability and absorption, especially in cereal-based diets that more or less lack some trace elements (Drakakaki et al., 2005). It is worth noting that proteomic analysis of phytase transgenic corn seeds showed that some ribosomal proteins and heat-shock proteins might present adaptive effects in response to the insertion of Aspergillus phytase phyA2 (Tan et al., 2017). Three ways have been proposed to increase the accumulation of iron in rice grains. The first is to insert a P. vulgaris ferritin gene into rice grains to enhance iron accumulation and an A. fumigatus heat-tolerant phytase into the rice endosperm to improve its bioavailability. Second, an endogenous gene encoding a cysteine-rich metallothionein-like protein was overexpressed to increase the absorption of iron. Finally, these different transgenic lines were hybridized to introduce new quality improvements, further substantially improving iron nutrition in daily diets of rice (Lucca et al., 2001).
Zinc
As an essential trace element for plants, humans, and microorganisms, zinc (Zn) participates in various biochemical pathways. Zn deficiency is one of the most severe problems threatening as much as one-quarter of the world's population. Nevertheless, excess Zn can also be toxic (Broadley et al., 2007). The ZIP family, as one of the Zn transporter families, can transport not only Zn but a variety of cations, including Ca, Fe, and Mg, and it is considered to be part of the primary uptake of Zn in plants (Guerinot, 2000). Several transporters of this family in rice have been characterized, such as OsZIP4, OsZIP5, and OsZIP8. Overexpression of the OsZIP4 gene in rice resulted in the 10-fold accumulation of Zn concentration compared with vector controls in roots, while it was five times lower in shoots and four times lower in seeds, indicating that overexpression of OsZIP4 disrupts Zn distribution in rice (Ishimaru et al., 2007). Overexpression of the OsZIP5 gene caused a decrease in shoot Zn concentration but an increase in the roots, indicating that OsZIP5 transporter might be involved in root to shoot translocation (Lee et al., 2010a). OsZIP8 is also a plasma membrane zinc transporter that participates in Zn uptake and translocation. Overexpression of the OsZIP8 gene resulted in a decrease in Zn levels in shoots as well as mature seeds, but the opposite was found in roots (Lee et al., 2010b). In addition, transgenic barley overexpressing Arabidopsis zinc transporter AtZIP1 revealed increases in both zinc and iron contents in seeds (Ramesh et al., 2004). ZmZIP3 was introduced into Arabidopsis, leading to increased Zn concentration in roots but a decrease in shoots (Li et al., 2015). Ferritin protein is also found to take part in the accumulation of Zn in addition to Fe (Vasconcelos et al., 2003; Paul et al., 2012; Liu et al., 2016). Heterologous expression of the soybean ferritin gene in transgenic rice grains results in high iron and zinc levels (Vasconcelos et al., 2003). Metal transporters might be of great value in increasing zinc and iron contents, to further improve the micronutrient content of crops. However, while the Fe and Zn concentrations increased in wheat grains heterologously expressing sickle alfalfa ferritin, the grain Cu and Cd concentrations decreased significantly (Liu et al., 2016). Additionally, phytosiderophores (MAs) and nicotianamine (NA), which are members of the mugineic acid family, also have important roles in the uptake and distribution of Zn and iron in plants (Ricachenevsky et al., 2013; Wang et al., 2013). Zn and iron concentrations have been decreased via expressing barley genes involved in the biosynthesis of Mas (Takahashi et al., 2003), and transgenic rice with the barley NA synthase gene HvNAS1 displayed increased endogenous NA and MA contents and subsequently increased iron and zinc concentrations in grain (Masuda et al., 2009).
Copper
Copper (Cu), another micronutrient necessary to plants and humans, is nonetheless toxic at elevated levels. The copper transporter (CTR) family is one type of high-affinity Cu transport protein that contains three predicted transmembrane segments (Burkhead et al., 2009). The COPT proteins are transporters of the CTR family, and five members (COPT1–5) have been identified in A. thaliana. In plants, mRNA levels of COPT1 and COPT2 were strongly reduced, while those of COPT3, COPT4, and COPT5 were apparently unaffected in response to Cu treatment of leaves (Sancenón et al., 2003). COPT1 antisense transgenic Arabidopsis showed reduced 64Cu transport rates in seedlings compared with the controls (Sancenón et al., 2004). Transgenic tobacco hairy roots expressing a bacterial copC gene could remove copper from aqueous solutions by rhizofiltration (Pérez-Palacios et al., 2017). Members of the heavy metal P1B-ATPase (HMA) superfamily function to remove Cu from the cytoplasm. AtHMA1, which belongs to the HMA family, affects Cu homeostasis in yeast expression experiments. Arabidopsis hma1 mutants exhibited reduced Cu content in cell chloroplasts and presented a photosensitivity phenotype under high light compared with the wild type (Seigneurin-Berny et al., 2006). OsHMA9 knockout rice exhibited increased Cu, Zn, Pb, and Cd uptake, while they were much more sensitive to excessive Cu, Zn, and Pb (Lee et al., 2007). In addition, plants and other organisms can also remove elevated Cu within the cell through a type of metal-binding protein called metallochaperones (MTs). Transgenic tobacco with the yeast metallothionein gene CUP1 exhibited increased copper uptake from contaminated soil (Thomas et al., 2003). Metallothioneins are also involved in ROS scavenging; Arabidopsis overexpressing OsMT2c exhibited enhanced tolerance to Cu stress due to increased ROS scavenging compared with the controls (Liu et al., 2015).
Particular minerals and transformation of certain genes have been discussed above, and these have been summarized and described in Table 1.
Transgenes and Their Impact on Minerals
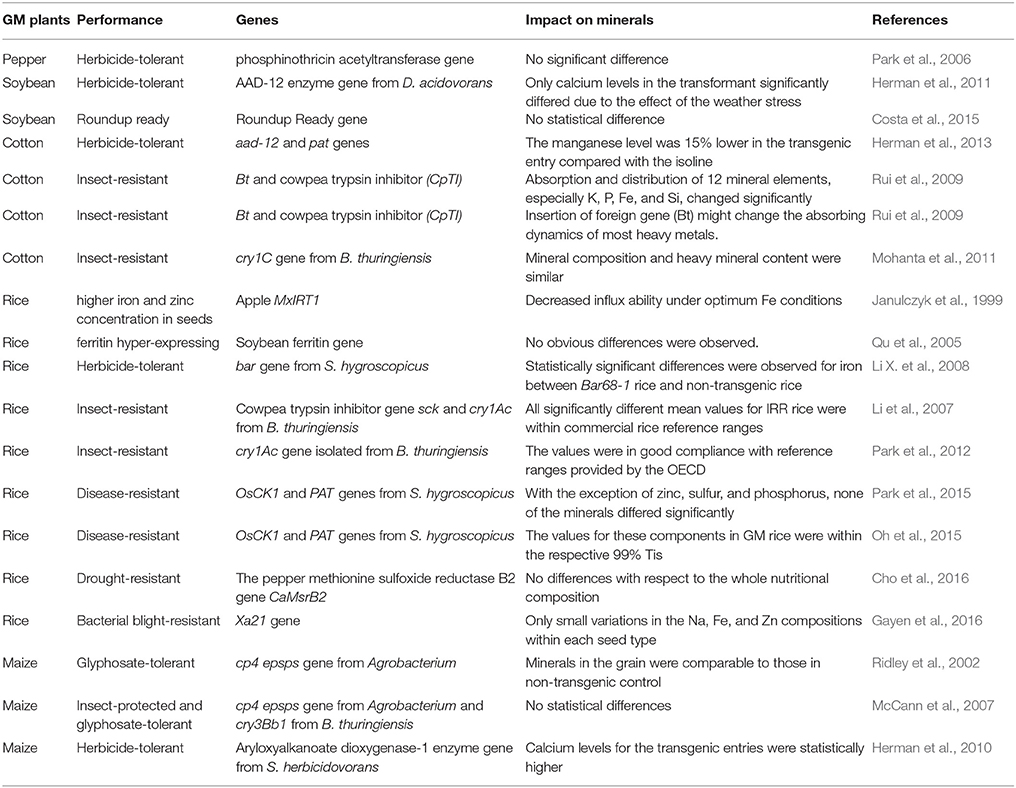
According to the 2016 report of the ISAAA, the global acreage of GM crops has increased 100-fold from the previous 1.7 million hectares in 1996 to 185.1 million hectares in 2016, indicating that biotech crops are the fastest growing and fastest adopted crop technology in recent years (http://www.isaaa.org/inbrief/default.asp). Meanwhile, lots of fundamental research is being conducted all over the world. As for wheat, many genes involved in good processing and nutritional quality have been transferred into wheat on a large scale, such as high-molecular-weight glutenin subunit (HMW-GS) genes 1Ax1, avenin-like b gene (Wang et al., 2010; Li et al., 2012; Ma et al., 2013; Ellis et al., 2014; Jin et al., 2015; Han et al., 2017). However, whether gene transformation and expression will cause unintended compositional changes while producing improved end-products in these GM wheat varieties remains unclear. These unintended compositional changes may include mineral changes and secondary metabolite changes. Take transgenic wheat with 1Ax1 overexpression for example (Wang et al., 2010), the excess accumulation of HMW-GS formed by intermolecular disulfide bond, might indirectly affect the selenium homeostasis due to the sophisticated interactions of molecules in cells of transgenic wheat, which was implied in Stanleya pinnata (El Mehdawi et al., 2018). It is worth mentioning that hybridization breeding efforts have also vastly enhanced HMW-GS content of wheat, yet whether one variable of single gene insertion or multivariate of vast genes on chromosomes has more effect on minerals content or functions is unknown. Therefore, food made from transgenic crops must undergo standard safety assessment to guarantee that no allergenicity or toxicity to humans or animals exists.
Many disease-resistant crops have been generated, with the OsCK1 GM rice among them. A choline kinase (CK1) gene isolated from Oryza sativa and the phosphinothricin acetyltransferase (PAT) gene from Streptomyces hygroscopicus have simultaneously been expressed in the OsCK1 GM rice (Lee et al., 2005). A comparative analysis of OsCK1 GM rice and its corresponding controls together with two commercial rice varieties was performed in two different locations in Korea (Park et al., 2015; Oh et al., 2016). In these results, significant differences between GM rice and its conventional counterpart were found for three minerals (Mg, P, and Na; Park et al., 2015). However, these did not go beyond the ranges of values formulated by the OECD. Another study that analyzed levels of phytic acid, which participates in mineral absorption, revealed that GM rice varied significantly from non-GM rice at the phytic acid level. However, these values in GM rice were still within the respective tolerance interval (TI) range (Oh et al., 2015). The application of TIs in statistical analysis was to assess the effects caused by environmental variants. Therefore, the mineral contents were impacted by the environmental factors rather than genetic modification. In view of substantial equivalent analysis, the mineral components of the GM rice should be equivalent to those of non-GM rice varieties (Oh et al., 2016). A similar analysis was performed to assess the safety of a drought-tolerant GM rice harboring the CaMsrB2 gene (Cho et al., 2016). The sodium content in the GM rice was 1.24-fold of that in the non-GM controls. Nevertheless, the statistical analysis proved that the difference was more likely caused by the regional environment (Gayen et al., 2016). Moreover, analysis of a transgenic rice variety overexpressing the disease resistance gene Xa21 showed that the contents of the main minerals in the GM rice were equal to those in the conventional rice controls (Gayen et al., 2016). The same assessment results were also found in other transgenic rice with exogenous genes (Li et al., 2007; Li X. et al., 2008; Park et al., 2012).
As substantial equivalence is the concept that is most widely adopted and used to evaluate the biosafety of GM crops, compositional safety has been investigated by comparing transgenic crops like soybean, pepper, cotton, and corn with non-transgenic counterparts (Ridley et al., 2002; Park et al., 2006; McCann et al., 2007; Rui et al., 2009; Herman et al., 2010, 2011, 2013; Mohanta et al., 2011; Costa et al., 2015). Most of these studies draw a similar conclusion that there were no statistical differences between GM plants and their controls. Even though the concentrations of exceptional microelements in GM cotton (Herman et al., 2013), rice (Li X. et al., 2008; Oh et al., 2015), and corn (Herman et al., 2010) changed remarkably, they were still within the respective 99% TIs. Nevertheless, the vast majority of these studies only focused on the composition of minerals when making comparative analysis of GM crops while paying little attention to the distribution changes in transgenic plants, because different tissues or organs of the same plant species differ in mineral absorption and accumulation. A study on the distribution pattern of 12 minerals including heavy metals in GM cotton (Bt+CpTI) revealed that the transgenic cotton accumulated less Cd and As than common cotton in all organs but lower Pb in the stem (Rui and Qu, 2009).
Nonetheless, there are many other concerns, such as mineral uptake and allocation acting reciprocally. One ABC transporter in S. pyogenes has affinity for three metal minerals: Zn, Fe, and Cu. Moreover, Zn and Cu were detected to competitively bind to the same site (Janulczyk et al., 1999). In previous studies, the Fe/Zn accumulation in rice endosperm was fortified by overexpressing genes participating in Fe uptake, translocation, and storage (Kobayashi and Nishizawa, 2012; Lee et al., 2012). Heteroexpression of a soybean ferritin gene gave rise to iron storage ability in rice seeds, while no obvious differences in other divalent-metal concentrations were displayed in the seeds comparing all transgenic lines and wild types (Qu et al., 2005). Safety concerns have arisen in molecular Fe/Zn fortification because the cadmium can be concurrently taken up through the Fe uptake system (Nakanishi et al., 2006). MxIRT1 has been introduced into rice to alleviate the Fe deficiency, and this transformation unexpectedly induced Cd uptake on trial. But it is worth noting that MxIRT1 takes up the lowest Cd in comparison with AtIRT1 and OsIRT1 in transgenic protoplasts (Tan et al., 2015). Another investigation on genetic Zn fortification grains reported that the Cd content was slightly higher in GM crops than in control crops, however this result was still under the toxic threshold (Zhang et al., 2012). Since the Cd uptake pathway in plants has been identified before (Uraguchi et al., 2009, 2011), genetic modification can be used to prevent excess Cd accumulation by depressing expression of genes functioning in Cd uptake (Ishikawa et al., 2012; Ishimaru et al., 2012).
In addition, the effects of genetic modification and growth environment have been evaluated simultaneously. While 20 to 22 protein levels differentially changed in transgenic rice seeds comparing to wild types, 21 proteins levels were found to be higher or lower as a consequence of growth environment. These results suggested that the impact of the single gene insertion into the genome on the nutrient quality of crops was no more than that of the growth environment (Wang et al., 2012). Another study also revealed that bacterial inoculation enhanced selenium and iron concentration in wheat (Yasin et al., 2015). As traditional fertilization is a simple and easy way to handle soil microelement deficiencies, effect of basic fertilizer on transgenic cereals has been assessed as well. A significant reduction in AMF (arbuscular mycorrhizal fungal) colonization in Bt11 maize roots emerged with limited fertilizer, while no evident difference was found between the Bt11 and the control with appropriate fertilizer (Cheeke et al., 2011). Similar research was seldom reported in mineral related transgenic crops, which might be due to the rare licensed and mineral relevant GM crops.
All the transgenic crops mentioned above have been listed and described in Table 2.
Discussion and Future Prospects
Transgenic technology has brought many promising GM plants with improved quality and/or enhanced stress tolerance and has also allowed microelement non-accumulators to accumulate trace minerals and common plants to hyperaccumulate them. Lots of work has been done to compare GM crops and their non-transgenic counterparts (Yang et al., 2013; Wang L. et al., 2015). These comparative analyses were mostly conducted by proteomics methods, which mainly use two-dimensional electrophoresis (2-DE) coupled with mass spectrometry (MS) to distinguish differential proteins or protein content between GM crops and their wild types (Barros et al., 2010; Brandão et al., 2010; Yang et al., 2013; Wang L. et al., 2015). Metabolomics techniques such as HPLC, NMR, and GC/MS have also been used to analyze differences between metabolites including carbohydrates, lipids, and amino acids (Brandão et al., 2010). In addition, other compositions containing amino acids, fatty acids, minerals, vitamins and anti-nutritive components have been analyzed to investigate the biosafety of GM crops (Li et al., 2007; Li X. et al., 2008; Barros et al., 2010; Wang et al., 2012; Gayen et al., 2013).
However, there are more details that should be considered and improved in these studies. When transforming microelement transport- or metabolism-related genes, not only the particular one but other minerals have to be analyzed. Even the distribution pattern of the microelement including the composition changes should be considered. Besides, microelements exist in various forms in plants, the bioavailable forms and biologically active precursors need to be analyzed separately. Apart from the analysis of samples, the treatment of plants could be diverse and correlated in parallel with the performance of GM plants, for instance, comparative analysis between drought-resistant GM rice and conventional rice should be taken from both common circumstances and drought stress treatment. Certainly, all of the GM plants should be demonstrated in field trials, but still, stress treatment should be taken into consideration during safety assessment. In addition to nutritional quality, growth environment and fertilization way should be taken into consideration in later studies on GM cereals. Therefore, various approaches including genetic engineering, fertilization, hybridization should be integrated and complemented each other to accomplish biofortification of plants.
With the aid of transgenic techniques, it is feasible to manipulate cereal plants to improve or tolerate concentrated minerals. However, we should guarantee that the transgenic cereals are safe in both environmental friendliness and end-use properties, especially in mineral metabolism respects. Based on the requested motivations of biosafety issues, systematic design and analysis of transgenic cereal should be investigated comprehensively at different levels of gene confirmation, genomics, proteomics, metabolomics, nutritiomics, and the absorption, metabolism, and function of minerals.
Author Contributions
XY, QL, KH collected materials; XY wrote the draft manuscript; GY and GH revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Genetically Modified New Varieties of Major Projects of China (2016ZX08010004-004) and the National Natural Science Foundation of China (No. 31771418).
References
Bañuelos, G., Terry, N., Leduc, D. L., Pilon-Smits, E. A. H., and MacKey, B. (2005). Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium-contaminated sediment. Environ. Sci. Technol. 39, 1771–1777. doi: 10.1021/es049035f
Barros, E., Lezar, S., Anttonen, M. J., van Dijk, J. P., Röhlig, R. M., Kok, E. J., et al. (2010). Comparison of two GM maize varieties with a near-isogenic non-GM variety using transcriptomics, proteomics and metabolomics. Plant Biotechnol. J. 8, 436–451. doi: 10.1111/j.1467-7652.2009.00487.x
Bellaloui, N., Yadavc, R. C., Chern, M., Hu, H., Gillen, A. M., Greve, C., et al. (2010). Transgenically enhanced sorbitol synthesis facilitates phloem-boron mobility in rice. Physiol. Plant. 117, 79–84. doi: 10.1034/j.1399-3054.2003.1170110.x
Bhullar, N. K., and Gruissem, W. (2013). Nutritional enhancement of rice for human health: the contribution of biotechnology. Biotechnol. Adv. 31, 50–57. doi: 10.1016/j.biotechadv.2012.02.001
Bisbjerg, B., and Gissel-Nielsen, G. (1969). The uptake of applied selenium by agricultural plants. Plant Soil 31, 287–298. doi: 10.1007/BF01373572
Bocchini, M., Bartucca, M. L., Ciancaleoni, S., Mimmo, T., Cesco, S., Pii, Y., et al. (2015). Iron deficiency in barley plants: phytosiderophore release, iron translocation, and DNA methylation. Front. Plant Sci. 6:514. doi: 10.3389/fpls.2015.00514
Brandão, A. R., Barbosa, H. S., and Arruda, M. A. Z. (2010). Image analysis of two-dimensional gel electrophoresis for comparative proteomics of transgenic and non-transgenic soybean seeds. J. Proteomics 73, 1433–1440. doi: 10.1016/j.jprot.2010.01.009
Briat, J. F., Duc, C., Ravet, K., and Gaymard, F. (2010). Ferritins and iron storage in plants. Biochim. Biophys. Acta 1800, 806–814. doi: 10.1016/j.bbagen.2009.12.003
Brinch-Pedersen, H., Hatzack, F., Sørensen, L. D., and Holm, P. B. (2003). Concerted action of endogenous and heterologous phytase on phytic acid degradation in seed of transgenic wheat (Triticum aestivum L.). Transgenic Res. 12, 649–659. doi: 10.1023/B:TRAG.0000005113.38002.e1
Brinch-Pedersen, H., Olesen, A., Rasmussen, S. K., and Holm, P. B. (2000). Generation of transgenic wheat (Triticum aestivum L.) for constitutive accumulation of an Aspergillus phytase. Mol. Breed. 6, 195–206. doi: 10.1023/A:1009690730620
Broadley, M. R., White, P. J., Hammond, J. P., Zelko, I., and Lux, A. (2007). Zinc in plants. New Phytol. 173, 677–702. doi: 10.1111/j.1469-8137.2007.01996.x
Brown, T. A., and Shrift, A. (1982). Selenium: toxicity and tolerance in higher plants. Biol. Rev. Camb. Philos. Soc. 57, 59–84. doi: 10.1111/j.1469-185X.1982.tb00364.x
Burkhead, J. L., Gogolin Reynolds, K. A., Abdel-Ghany, S. E., Cohu, C. M., and Pilon, M. (2009). Copper homeostasis. New Phytol. 182, 799–816. doi: 10.1111/j.1469-8137.2009.02846.x
Cheeke, T. E., Pace, B. A., Rosenstiel, T. N., and Cruzan, M. B. (2011). The influence of fertilizer level and spore density on arbuscular mycorrhizal colonization of transgenic Bt11 maize (Zea mays) in experimental microcosms. FEMS Microbiol. Ecol. 75, 304–312. doi: 10.1111/j.1574-6941.2010.01013.x
Cho, Y. H., Puligundla, P., Oh, S. D., Park, H. M., Kim, K. M., Lee, S. M., et al. (2016). Comparative evaluation of nutritional compositions between transgenic rice harboring the CaMsrB2 gene and the conventional counterpart. Food Sci. Biotechnol. 25, 49–54. doi: 10.1007/s10068-016-0007-9
Clark, L. C., Combs, G. F. J., Turnbull, B. W., Slate, E. H., Chalker, D. K., Chow, J., et al. (1997). Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. J. Am. Med. Assoc. 277, 1520–1520.
Clark, S. F. (2008). Iron deficiency anemia. Nutr. Clin. Pract. 23, 128–141. doi: 10.1177/0884533608314536
Costa, G. R., de Oliveira Couto e Silva, N., Mandarino, J. M. G., Leite, R. S., Guimarães, N. C., Junqueira, R. G. A., et al. (2015). Isoflavone and mineral content in conventional and transgenic soybean cultivars. Am. J. Plant Sci. 6, 2051–2059. doi: 10.4236/ajps.2015.613205
Drakakaki, G., Christou, P., and Stöger, E. (2000). Constitutive expression of soybean ferritin cDNA in transgenic wheat and rice results in increased iron levels in vegetative tissues but not in seeds. Trans. Res. 9, 445–452. doi: 10.1023/A:1026534009483
Drakakaki, G., Marcel, S., Glahn, R. P., Lund, E. K., Pariagh, S., Fischer, R., et al. (2005). Endosperm-specific co-expression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Mol. Biol. 59, 869–880. doi: 10.1007/s11103-005-1537-3
Ellis, D. R., Sors, T. G., Brunk, D. G., Albrecht, C., Orser, C., Lahner, B., et al. (2004). Production of Se-methylselenocysteine in transgenic plants expressing selenocysteine methyltransferase. BMC Plant Biol. 4:1. doi: 10.1186/1471-2229-4-1
Ellis, J. G., Lagudah, E. S., Spielmeyer, W., and Dodds, P. N. (2014). The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 5:641. doi: 10.3389/fpls.2014.00641
El Mehdawi, A. F., Jiang, Y., Guignardi, Z. S., Esmat, A., Pilon, M., Pilon-Smits, E. A. H., et al. (2018). Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 217, 194–205. doi: 10.1111/nph.14838
Fang, K., Zhang, W., Xing, Y., Zhang, Q., Yang, L., Cao, Q., et al. (2016). Boron toxicity causes multiple effects on Malus domestica pollen tube growth. Front. Plant Sci. 7, 208. doi: 10.3389/fpls.2016.00208
Gayen, D., Paul, S., Sarkar, S. N., Datta, S. K., and Datta, K. (2016). Comparative nutritional compositions and proteomics analysis of transgenic Xa21 rice seeds compared to conventional rice. Food Chem. 203, 301–307. doi: 10.1016/j.foodchem.2016.02.058
Gayen, D., Sarkar, S. N., Datta, S. K., and Datta, K. (2013). Comparative analysis of nutritional compositions of transgenic high iron rice with its non-transgenic counterpart. Food Chem. 138, 835–840. doi: 10.1016/j.foodchem.2012.11.065
Gloud, F., Amasino, R. M., Brossard, D., Buell, C. R., Dixon, R., Falck-Zepeda, J. B., et al. (2016). Genetically Engineered Crops: Experiences and Prospects. Washington, DC: The National Academies Press.
Gordon-Kamm, W. J., Spencer, T. M., Mangano, M. L., Adams, T. R., Daines, R. J., Start, W. G., et al. (1990). Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2, 603–618. doi: 10.1105/tpc.2.7.603
Goto, F., Yoshihara, T., and Saiki, H. (1998). Iron accumulation in tobacco plants expressing soyabean ferritin gene. Trans. Res. 7, 173–180. doi: 10.1023/A:1008836812714
Goto, F., Yoshihara, T., Shigemoto, N., Toki, S., and Takaiwa, F. (1999). Iron fortification of rice seed by the soybean ferritin gene. Nat. Biotechnol. 17, 282–286. doi: 10.1038/7029
Guerinot, M. L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta 1465, 190–198. doi: 10.1016/S0005-2736(00)00138-3
Gümüşel, D., Oz, M. T., Eyidogan, F., Yücel, M., Çiftçi, Y. Ö., and Öktem, H. A. (2012). Heterologous expression of a barley boron transporter gene in tobacco (Nicotiana tabaccum) and analysis of transgenic plants. New Biotechnol. 29:S128. doi: 10.1016/j.nbt.2012.08.357
Gupta, M., and Gupta, S. (2016). An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 7:2074. doi: 10.3389/fpls.2016.02074
Han, J., Yu, X., Chang, J., Yang, G., and He, G. (2017). “Overview of the wheat genetic transformation and breeding status in China,” in Wheat Biotechnology. Methods in Molecular Biology, eds P. L. Bhalla and M. B. Singh (Parkville: Humana Press).
Herman, R. A., Fast, B. J., Johnson, T. Y., Sabbatini, J., and Rudgers, G. W. (2013). Compositional safety of herbicide-tolerant DAS-81910-7 cotton. J. Agr. Food Chem. 61, 11683–11692. doi: 10.1021/jf404043y
Herman, R. A., Phillips, A. M., Lepping, M. D., Fast, B. J., and Sabbatini, J. (2010). Compositional safety of event DAS-40278-9 (AAD-1) herbicide-tolerant maize. GM Crop 1, 294–311. doi: 10.4161/gmcr.1.5.14285
Herman, R. A., Phillips, A. M., Lepping, M. D., and Sabbatini, J. (2011). Compositional Safety of DAS-68416-4 (AAD-12) Herbicide-Tolerant Soybean. Int. J. Food Sci. Nutr. 1:02. doi: 10.4172/2155-9600.1000103
Hong, C. Y., Cheng, K. J., Tseng, T. H., Wang, C. S., Liu, L. F., and Yu, S. M. (2004). Production of two highly active bacterial phytases with broad pH optima in germinated transgenic rice seeds. Trans. Res. 13, 29–39. doi: 10.1023/B:TRAG.0000017158.96765.67
Hurrell, R. F., Reddy, M. B., Juillerat, M.-A., and Cook, J. D. (2003). Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 77, 1213–1219. doi: 10.1093/ajcn/77.5.1213
Ishikawa, S., Ishimaru, Y., Igura, M., Kuramata, M., Abe, T., Senoura, T., et al. (2012). Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. U.S.A. 109, 19166–19171. doi: 10.1073/pnas.1211132109
Ishimaru, Y., Masuda, H., Suzuki, M., Bashir, K., Takahashi, M., Nakanishi, H., et al. (2007). Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants. J. Exp. Bot. 58, 2909–2915. doi: 10.1093/jxb/erm147
Ishimaru, Y., Takahashi, R., Bashir, K., Shimo, H., Senoura, T., Sugimoto, K., et al. (2012). Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2:286. doi: 10.1038/srep00286
Janulczyk, R., Pallon, J., and Björck, L. (1999). Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol. Microbiol. 34, 596–606. doi: 10.1046/j.1365-2958.1999.01626.x
Jin, L., Zhang, H., Lu, Y., Yang, Y., Wu, K., Tabashnik, B. E., et al. (2015). Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nat. Biotechnol. 33, 169–174. doi: 10.1038/nbt.3100
Kitta, K. (2013). Availability and utility of crop composition data. J. Agric. Food Chem. 61, 8304–8311. doi: 10.1021/jf400777v
Kobayashi, T., and Nishizawa, N. K. (2012). Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 63, 131–152. doi: 10.1146/annurev-arplant-042811-105522
Kumar, K., Mosa, K. A., Chhikara, S., Musante, C., White, J. C., and Dhankher, O. P. (2014). Two rice plasma membrane intrinsic proteins, OsPIP2;4 and OsPIP2;7, are involved in transport and providing tolerance to boron toxicity. Planta 239, 187–198. doi: 10.1007/s00425-013-1969-y
LeDuc, D. L., AbdelSamie, M., Móntes-Bayon, M., Wu, C. P., Reisinger, S. J., and Terry, N. (2006). Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances selenium phytoremediation traits in Indian mustard. Environ. Pollut. 144, 70–76. doi: 10.1016/j.envpol.2006.01.008
LeDuc, D. L., Tarun, A. S., Montes-Bayon, M., Meija, J., Malit, M. F., Wu, C. P., et al. (2004). Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiol. 135, 377–383. doi: 10.1104/pp.103.026989
Lee, J. S., Suh, S. C., Lee, Y. H., Kim, Y. H., and Heu, S. G. (2005). OsCK1 gene from Oryza sativa, expression vector comprising the gene, transformants transformed with the vector and production method of the transformants. Korea, KR20050027204. 2005-03-31.
Lee, S., Jeong, H. J., Kim, S. A., Lee, J., Guerinot, M. L., and An, G. (2010a). OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol. Biol. 73, 507–517. doi: 10.1007/s11103-010-9637-0
Lee, S., Jeon, J.-S., and An, G. (2012). Iron homeostasis and fortification in rice. J. Plant Biol. 55, 261–267. doi: 10.1007/s12374-011-0386-7
Lee, S., Kim, S. A., Lee, J., Guerinot, M. L., and An, G. (2010b). Zinc deficiency-inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol. Cells 29, 551–558. doi: 10.1007/s10059-010-0069-0
Lee, S., Kim, Y. Y., Lee, Y., and An, G. (2007). Rice P1B-type heavy-metal ATPase, OsHMA9, is a metal efflux protein. Plant Physiol. 145, 831–842. doi: 10.1104/pp.107.102236
Li, H. F., McGrath, S. P., and Zhao, F. J. (2008). Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 178, 92–102. doi: 10.1111/j.1469-8137.2007.02343.x
Li, S., Zhou, X., Li, H., Liu, Y., Zhu, L., Guo, J., et al. (2015). Overexpression of ZmIRT1 and ZmZIP3 enhances iron and zinc accumulation in transgenic arabidopsis. PLoS ONE 10:e0136647. doi: 10.1371/journal.pone.0136647
Li, X., He, X., Luo, Y., Xiao, G., Jiang, X., and Huang, K. (2008). Comparative analysis of nutritional composition between herbicide-tolerant rice with bar gene and its non-transgenic counterpart. J. Food Compos. Anal. 21, 535–539. doi: 10.1016/j.jfca.2008.06.001
Li, X., Huang, K., He, X., Zhu, B., Liang, Z., Li, H., et al. (2007). Comparison of nutritional quality between Chinese indica rice with sck and cry1Ac genes and its nontransgenic counterpart. J. Food Sci. 72, S420–S424. doi: 10.1111/j.1750-3841.2007.00416.x
Li, Y., Wang, Q., Li, X., Xiao, X., Sun, F., Wang, C., et al. (2012). Coexpression of the high molecular weight glutenin subunit 1Ax1 and puroindoline improves dough mixing properties in durum wheat (Triticum turgidum L. ssp. durum). PLoS ONE 7:e50057. doi: 10.1371/journal.pone.0050057
Liu, D., Wang, Y., Guo, C., Cong, Q., Gong, X., and Zhang, H. (2016). Enhanced iron and zinc accumulation in genetically engineered wheat plants using sickle alfalfa (Medicago falcata L.) ferritin gene. Cereal Res. Commun. 44, 1–11. doi: 10.1556/0806.43.2015.039
Liu, J., Shi, X., Qian, M., Zheng, L., Lian, C., Xia, Y., et al. (2015). Copper-induced hydrogen peroxide upregulation of a metallothionein gene, OsMT2c, from Oryza sativa L. confers copper tolerance in Arabidopsis thaliana. J. Hazard. Mater. 294, 99–108. doi: 10.1016/j.jhazmat.2015.03.060
Lucca, P., Hurrell, R., and Potrykus, I. (2001). Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains. Theor. Appl. Genet. 102, 392–397. doi: 10.1007/s001220051659
Lucca, P., Poletti, S., and Sautter, C. (2006). Genetic engineering approaches to enrich rice with iron and vitamin A. Physiol. Plant. 126, 291–303. doi: 10.1111/j.1399-3054.2006.00609.x
Lyons, G. H., Stangoulis, J. C. R., and Graham, R. D. (2005). Tolerance of wheat (Triticum aestivum L.) to high soil and solution selenium levels. Plant Soil 270, 179–188. doi: 10.1007/s11104-004-1390-1
Ma, F., Li, M., Li, T., Liu, W., Liu, Y., Li, Y., et al. (2013). Overexpression of avenin-like b proteins in bread wheat (Triticum aestivum L.) improves dough mixing properties by their incorporation into glutenin polymers. PLoS ONE 8:e66758. doi: 10.1371/journal.pone.0066758
Martinez-Ballesta, Mdel C., Bastías, E., Zhu, C., Schäffner, A. R., González-Moro, B., González-Murua, C., et al. (2008). Boric acid and salinity effects on maize roots. Response of aquaporins ZmPIP1 and ZmPIP2, and plasma membrane H+-ATPase, in relation to water and nutrient uptake. Physiol. Plant. 132, 479–490. doi: 10.1111/j.1399-3054.2007.01045.x
Masuda, H., Usuda, K., Kobayashi, T., Ishimaru, Y., Kakei, Y., Takahashi, M., et al. (2009). Overexpression of the barley nicotianamine synthase gene HvNAS1 increases iron and zinc concentrations in rice grains. Rice 2, 155–166. doi: 10.1007/s12284-009-9031-1
McCann, M. C., Trujillo, W. A., Riordan, S. G., Sorbet, R., Bogdanova, N. N., and Sidhu, R. S. (2007). Comparison of the forage and grain composition from insect-protected and glyphosate-tolerant MON 88017 corn to conventional corn (Zea mays L.). J. Agr. Food Chem. 55, 4034–4042. doi: 10.1021/jf063499a
Miwa, K., and Fujiwara, T. (2010). “Role of Boron in Plant Growth and its Transport Mechanisms,” in Cell Biology of Metals and Nutrients, eds R. Hell and R.-R. Mendel (Berlin; Heidelberg: Springer), 1–15.
Miwa, K., and Fujiwara, T. (2011). Role of overexpressed BOR4, a boron exporter, in tolerance to high level of boron in shoots. Soil Sci. Plant Nutr. 57, 558–565. doi: 10.1080/00380768.2011.596473
Miwa, K., Takano, J., Omori, H., Seki, M., Shinozaki, K., and Fujiwara, T. (2007). Plants tolerant of high boron levels. Science 318:1417. doi: 10.1126/science.1146634
Miwa, K., Wakuta, S., Takada, S., Ide, K., Takano, J., Naito, S., et al. (2013). Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiol. 163, 1699–1709. doi: 10.1104/pp.113.225995
Mohanta, R. K., Singhal, K. K., Ebrahimi, S. H., Rajput, Y. S., and Mohini, M. (2011). Comparative nutritional evaluation of transgenic cottonseeds containing Cry1C protein for ruminant feeding. Livest. Res. Rural Dev. 23:14.
Mosa, K. A., Kumar, K., Chhikara, S., Musante, C., White, J. C., and Dhankher, O. P. (2016). Enhanced boron tolerance in plants mediated by bidirectional transport through plasma membrane intrinsic proteins. Sci. Rep. 6:21640. doi: 10.1038/srep21640
Nakagawa, Y., Hanaoka, H., Kobayashi, M., Miyoshi, K., Miwa, K., and Fujiwara, T. (2007). Cell-type specificity of the expression of OsBOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 19, 2624–2635. doi: 10.1105/tpc.106.049015
Nakanishi, H., Ogawa, I., Ishimaru, Y., Mori, S., and Nishizawa, N. K. (2006). Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 52, 464–469. doi: 10.1111/j.1747-0765.2006.00055.x
Neuhierl, B., and Böck, A. (1996). On the mechanism of selenium tolerance in selenium-accumulating plants. Purification and characterization of a specific selenocysteine methyltransferase from cultured cells of Astragalus bisculatus. Eur. J. Biochem. 239, 235–238. doi: 10.1111/j.1432-1033.1996.0235u.x
Nielsen, F. H. (1997). Boron in human and animal nutrition. Plant Soil 193, 199–208. doi: 10.1023/A:1004276311956
Oh, S., Park, S. Y., Yeo, Y., Park, S. K., and Kim, H. Y. (2015). Comparative analysis of genetically modified brown rice with conventional rice varieties for the safety assessment. Int J. Food Sci. Technol. 50, 1244–1254. doi: 10.1111/ijfs.12742
Oh, S. W., Park, S. Y., Lee, S. M., Oh, S. D., Cho, H. S., Park, S. K., et al. (2016). Multivariate analysis for the safety assessment of genetically modified rices in the anti-nutrients and phenolic compounds. Int J. Food Sci. Technol. 51, 765–776. doi: 10.1111/ijfs.13017
Park, H., Lee, S., Jeong, H., Cho, S., Chun, H., Back, O., et al. (2006). The nutrient composition of the herbicide-tolerant green pepper is equivalent to that of the conventional green pepper. Nutr. Res. 26, 546–548. doi: 10.1016/j.nutres.2006.09.001
Park, S. Y., Kim, J. K., Jang, J. S., Lee, S. Y., Oh, S., Lee, S. M., et al. (2015). Comparative analysis of nutritional composition between the disease-resistant rice variety OsCK1 and conventional comparators. Food Sci. Biotechnol. 24, 225–231. doi: 10.1007/s10068-015-0030-2
Park, S. Y., Lee, S. M., Lee, J. H., Ko, H. S., Kweon, S. J., Suh, S. C., et al. (2012). Compositional comparative analysis between insect-resistant rice (Oryza sativa L.) with a synthetic cry1Ac gene and its non-transgenic counterpart. Plant Biotechnol. Rep. 6, 29–37. doi: 10.1007/s11816-011-0192-1
Paul, S., Ali, N., Gayen, D., Datta, S. K., and Datta, K. (2012). Molecular breeding of Osfer2 gene to increase iron nutrition in rice grain. GM Crop Food 3, 310–316. doi: 10.4161/gmcr.22104
Pérez-Palacios, P., Agostini, E., Ibáñez, S. G., Talano, M. A., Rodríguez-Llorente, I. D., Caviedes, M. A., et al. (2017). Removal of copper from aqueous solutions by rhizofiltration using genetically modified hairy roots expressing a bacterial Cu-binding protein. Environ.Technol. 38, 2877–2888. doi: 10.1080/09593330.2017.1281350
Pulla, P. (2016). India nears putting GM mustard on the table. Science 352:1043. doi: 10.1126/science.352.6289.1043
Qu, L. Q., Yoshihara, T., Ooyama, A., Goto, F., and Takaiwa, F. (2005). Iron accumulation does not parallel the high expression level of ferritin in transgenic rice seeds. Planta 222, 225–233. doi: 10.1007/s00425-005-1530-8
Ramesh, S. A., Choimes, S., and Schachtman, D. P. (2004). Over-expression of an Arabidopsis zinc transporter in Hordeum Vulgare increases short-term zinc uptake after zinc deprivation and seed zinc content. Plant Mol. Biol. 54, 373–385. doi: 10.1023/B:PLAN.0000036370.70912.34
Reid, R. (2007). Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol. 48, 1673–1678. doi: 10.1093/pcp/pcm159
Reid, R. (2010). Can we really increase yields by making crop plants tolerant to boron toxicity. Plant Sci. 178, 9–11. doi: 10.1016/j.plantsci.2009.10.006
Ricachenevsky, F. K., Menguer, P. K., and Sperotto, R. A. (2013). kNACking on heaven's door: how important are NAC transcription factors for leaf senescence and Fe/Zn remobilization to seeds? Front. Plant Sci. 4:226. doi: 10.3389/fpls.2013.00226
Ridley, W. P., Sidhu, R. S., Pyla, P. D., Nemeth, M. A., Breeze, M. L., and Astwood, J. D. (2002). Comparison of the nutritional profile of glyphosate-tolerant corn event NK603 with that of conventional corn (Zea mays L.). J. Agric. Food Chem. 50, 7235–7243. doi: 10.1021/jf0205662
Rui, Y., and Qu, G. (2009). Distribution pattern of heavy metals in transgenic(Bt+CpTI) cotton. Spectrosc. Spect. Anal. 29, 819–821. doi: 10.3964/j.issn.1000-0593(2009)03-0819-03
Rui, Y., Wang, W., Li, P., and Zhang, F. (2009). Mineral element distribution in organs of dual-toxin transgenic (Bt+CpTI) cotton seedling. Plant Biosyst. 143, 137–139. doi: 10.1080/11263500802709731
Sancenón, V., Puig, S., Mateu-Andrés, I., Dorcey, E., Thiele, D. J., and Peñarrubia, L. (2004). The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J. Biol. Chem. 279, 15348–15355. doi: 10.1074/jbc.M313321200
Sancenón, V., Puig, S., Mira, H., Thiele, D. J., and Peñarrubia, L. (2003). Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol.Biol. 51, 577–587. doi: 10.1023/A:1022345507112
Schiavon, M., Pilon, M., Malagoli, M., and Pilon-Smits, E. A. H. (2015). Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation-a comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front. Plant Sci. 6:2. doi: 10.3389/fpls.2015.00002
Seigneurin-Berny, D., Gravot, A., Auroy, P., Mazard, C., Kraut, A., Finazzi, G., et al. (2006). HMA1, a new Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. J. Biol. Chem. 281, 2882–2892. doi: 10.1074/jbc.M508333200
Shinmachi, F., Buchner, P., Stroud, J. L., Parmar, S., Zhao, F.-J., McGrath, S. P., et al. (2010). Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiol. 153, 327–336. doi: 10.1104/pp.110.153759
Sors, T. G., Ellis, D. R., Na, G. N., Lahner, B., Lee, S., Leustek, T., et al. (2005). Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J. 42, 785–797. doi: 10.1111/j.1365-313X.2005.02413.x
Suter, M., von Ballmoos, P., Kopriva, S., den Camp, R. O., Schaller, J., Kuhlemeier, C., et al. (2000). Adenosine 5'-phosphosulfate sulfotransferase and adenosine 5'-phosphosulfate reductase are identical enzymes. J. Biol. Chem. 275, 930–936. doi: 10.1074/jbc.275.2.930
Tabashnik, B. E., Brévault, T., and Carrière, Y. (2013). Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31:510. doi: 10.1038/nbt.2597
Takahashi, M., Terada, Y., Nakai, I., Nakanishi, H., Yoshimura, E., Mori, S., et al. (2003). Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15, 1263–1280.
Takano, J., Noguchi, K., Yasumori, M., Kobayashi, M., Gajdos, Z., Miwa, K., et al. (2002). Arabidopsis boron transporter for xylem loading. Nature 420, 337–340. doi: 10.1038/nature01139
Takano, J., Wada, M., Ludewig, U., Schaaf, G., von Wirén, N., and Fujiwara, T. (2006). The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18, 1498–1509. doi: 10.1105/tpc.106.041640
Takano, J., Yamagami, M., Noguchi, K., Hayashi, H., and Fujiwara, T. (2001). Preferential translocation of boron to young leaves in Arabidopsis thaliana Regulated by the BOR1 Gene. Soil Sci. Plant Nutr. 47, 345–357. doi: 10.1080/00380768.2001.10408398
Tan, S., Han, R., Li, P., Yang, G., Li, S., Zhang, P., et al. (2015). Over-expression of the MxIRT1 gene increases iron and zinc content in rice seeds. Trans. Res. 24, 109–122. doi: 10.1007/s11248-014-9822-z
Tan, Y., Tong, Z., Yang, Q., Sun, Y., Jin, X., Peng, C., et al. (2017). Proteomic analysis of phytase transgenic and non-transgenic maize seeds. Sci. Rep. 7:9246. doi: 10.1038/s41598-017-09557-8
Tanaka, M., Wallace, I. S., Takano, J., Roberts, D. M., and Fujiwara, T. (2008). NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20, 2860–2875. doi: 10.1105/tpc.108.058628
Thomas, J. C., Davies, E. C., Malick, F. K., Endreszl, C., Williams, C. R., Abbas, M., et al. (2003). Yeast metallothionein in transgenic tobacco promotes copper uptake from contaminated soils. Biotechnol. Progr. 19, 273–280. doi: 10.1021/bp025623q
Tian, M., Hui, M., Thannhauser, T. W., Pan, S., and Li, L. (2017). Selenium-induced toxicity is counteracted by sulfur in Broccoli (Brassica oleracea L. var. italica). Front. Plant Sci. 8:1425. doi: 10.3389/fpls.2017.01425
Toriyama, K., Youichi, A., and Hirofumi, U. H. K. (1988). Transgenic rice plants after direct gene transfer into protoplasts. Nat. Biotechnol. 6, 1072–1074. doi: 10.1038/nbt0988-1072
Tsakraklides, G., Martin, M., Chalam, R., Tarczynski, M. C., Schmidt, A., and Leustek, T. (2002). Sulfate reduction is increased in transgenic Arabidopsis thaliana expressing 5'-adenylylsulfate reductase from Pseudomonas aeruginosa. Plant J. 32, 879–889. doi: 10.1046/j.1365-313X.2002.01477.x
Uraguchi, S., Kamiya, T., Sakamoto, T., Kasai, K., Sato, Y., Nagamura, Y., et al. (2011). Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. U.S.A. 108, 20959–20964. doi: 10.1073/pnas.1116531109
Uraguchi, S., Kato, Y., Hanaoka, H., Miwa, K., and Fujiwara, T. (2014). Generation of boron-deficiency-tolerant tomato by overexpressing an Arabidopsis thaliana borate transporter AtBOR1. Front. Plant Sci. 5:125. doi: 10.3389/fpls.2014.00125
Uraguchi, S., Mori, S., Kuramata, M., Kawasaki, A., Arao, T., and Ishikawa, S. (2009). Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 60, 2677–2688. doi: 10.1093/jxb/erp119
Vasconcelos, M., Datta, K., Oliva, N., Khalekuzzaman, M., Torrizo, L., Krishnan, S., et al. (2003). Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci. 164, 371–378. doi: 10.1016/S0168-9452(02)00421-1
Vasil, V., Castillo, A. M., Fromm, M. E., and Vasil, I. K. (1992). Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Nat. Biotechnol. 10, 667–674. doi: 10.1038/nbt0692-667
Vigani, G., Tarantino, D., and Murgia, I. (2013). Mitochondrial ferritin is a functional iron-storage protein in cucumber (Cucumis sativus) roots. Front. Plant Sci. 4:316. doi: 10.3389/fpls.2013.00316
Wakuta, S., Fujikawa, T., Naito, S., and Takano, J. (2016). Tolerance to excess-boron conditions acquired by stabilization of a BOR1 variant with weak polarity in Arabidopsis. Front. Cell Dev. Biol. 4:4. doi: 10.3389/fcell.2016.00004
Wang, J., Meng, Y., Li, B., Ma, X., Lai, Y., Si, E., et al. (2015). Physiological and proteomic analyses of salt stress response in the halophyte Halogeton glomeratus. Plant Cell Environ. 38, 655–669. doi: 10.1111/pce.12428
Wang, L., Wang, X., Jin, X., Jia, R., Huang, Q., Tan, Y., et al. (2015). Comparative proteomics of Bt-transgenic and non-transgenic cotton leaves. Proteome Sci. 2, 13–15. doi: 10.1186/s12953-015-0071-8
Wang, M., Gruissem, W., and Bhullar, N. K. (2013). Nicotianamine synthase overexpression positively modulates iron homeostasis-related genes in high iron rice. Front. Plant Sci. 4:156. doi: 10.3389/fpls.2013.00156
Wang, Y., Li, Y., Zhang, L., Gao, X., Miao, Y., Wang, C., et al. (2010). Expression of the 1Ax1 transgene in an elite Chinese wheat variety and its effect on functional properties. J. Sci. Food Agric. 90, 106–111. doi: 10.1002/jsfa.3790
Wang, Y., Xu, W., Zhao, W., Hao, J., Luo, Y., Tang, X., et al. (2012). Comparative analysis of the proteomic and nutritional composition of transgenic rice seeds with Cry1ab/ac genes and their non-transgenic counterparts. J. Cereal Sci. 55, 226–233. doi: 10.1016/j.jcs.2011.12.004
White, P. J. (2016). Selenium accumulation by plants. Ann.Bot. 117, 217–235. doi: 10.1093/aob/mcv180
White, P. J., and Broadley, M. R. (2005). Biofortifying crops with essential mineral elements. Trends Plant Sci. 10, 586–593. doi: 10.1016/j.tplants.2005.10.001
White, P. J., and Broadley, M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182, 49–84. doi: 10.1111/j.1469-8137.2008.02738.x
Wu, Z., Bañuelos, G. S., Lin, Z.-Q., Liu, Y., Yuan, L., Yin, X., et al. (2015). Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 6:136. doi: 10.3389/fpls.2015.00136
Yang, Y., Dai, L., Xia, H., Zhu, K., Liu, X., and Chen, K. (2013). Comparative proteomic analysis of rice stripe virus (RSV)-resistant and -susceptible rice cultivars. Aust. J.Crop Sci. 7, 588–593.
Yao, W., Wang, S., Zhou, B., and Jiang, T. (2016). The Tamarix ferritin gene confers low-iron tolerance in transgenic tobacco. Plant Growth Regul. 80, 149–158. doi: 10.1007/s10725-016-0151-5
Yasin, M., El-Mehdawi, A. F., Anwar, A., Pilon-Smits, E. A. H., and Faisal, M. (2015). Microbial-enhanced selenium and iron biofortification of wheat (Triticum aestivum L.)–Applications in phytoremediation and biofortification. Int. J. Phytoremediat. 17, 341–347. doi: 10.1080/15226514.2014.922920
Zhang, Y., Xu, Y. H., Yi, H. Y., and Gong, J. M. (2012). Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 72, 400–410. doi: 10.1111/j.1365-313X.2012.05088.x
Keywords: biosafety, cereal, mineral, transgene, trace element
Citation: Yu X, Luo Q, Huang K, Yang G and He G (2018) Prospecting for Microelement Function and Biosafety Assessment of Transgenic Cereal Plants. Front. Plant Sci. 9:326. doi: 10.3389/fpls.2018.00326
Received: 14 November 2017; Accepted: 27 February 2018;
Published: 15 March 2018.
Edited by:
Junhua Peng, Center for Life Sci&Tech of China National Seed Group Co. Ltd., ChinaReviewed by:
Hong Luo, Clemson University, United StatesEva Stoger, University of Natural Resources and Life Sciences, Vienna, Austria
Liang Chen, University of Chinese Academy of Sciences (UCAS), China
Copyright © 2018 Yu, Luo, Huang, Yang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangyuan He, aGVneUBodXN0LmVkdS5jbg==
 Xiaofen Yu1
Xiaofen Yu1 Guangxiao Yang
Guangxiao Yang Guangyuan He
Guangyuan He