- 1USDA/ARS Horticultural Research Laboratory, Fort Pierce, FL, United States
- 2Zhejiang A & F University, Hangzhou, China
- 3Southern Gardens Citrus Nursery, Clewiston, FL, United States
Florida orange trees have been affected by huanglongbing (HLB) for more than a decade. To alleviate disease-caused tree decline, maintain fruit productivity, and reduce disease transmission, enhanced foliar spray programs combining vector control and nutritional supplementation have been applied to healthy and diseased trees. The aim of this research was to discover if the various foliar sprays affect fruit peel oil chemical components. In this study, “Valencia” orange trees, with or without HLB (HLB±), were treated with the grower standard program (control, C) or one of four proprietary enhanced foliar spray programs (N1, N2, N3, and N4) over 16 months. Compared with HLB−, HLB+ samples had lower concentrations of typical peel oil components, including valencene, octanal, and decanal, and were abundant in oxidative/dehydrogenated terpenes, such as carvone and limonene oxide. However, limonene, the dominant component, was not affected by any treatment. Control and three out of four enhanced foliar spray programs, N2, N3, and N4, had very little influence on the chemical profiles of both HLB− and HLB+ samples, while N1 treatment greatly altered the chemical profile of HLB+ samples, resulting in peel oil similar to that of HLB− samples.
Introduction
Huanglongbing (HLB, or citrus greening), associated with the bacterium Candidatus Liberibacter asiaticus (CLas), is a very destructive citrus disease and is vector-transmitted by the Asian citrus psyllid (ACP; Diaphorina citri Kuwayama). Once infected, trees rapidly decline and eventually die (Bové, 2006; Gottwald et al., 2020). Before the severe decline, HLB-infected trees still produce fruits, however, with decreased yield and poor quality. Fruits are typically small, irregularly shaped, with a thick, pale peel that remains green at the stylar end (Baldwin et al., 2014; Chen et al., 2016; McCollum et al., 2016). Fruits and juice from HLB-diseased trees are also associated with low soluble sugar and ethyl butanoate, as well as high acid, limonoid, and flavonoid content, resulting in less sweet and fruity flavor with more sour and bitter taste (Dala-Paula et al., 2018).
Furthermore, HLB causes an increase in pre-harvest fruit drop, which contributes to the reduction in yield (Baldwin et al., 2014; McCollum et al., 2016). This premature fruit drop may be exacerbated by Lasiodiplodia theobromae (previously known as Diplodia natalensis), the causal organism of citrus stem end rot (SER), which infects citrus fruits under the calyx abscission zone (Zhao et al., 2015, 2016). HLB has spread over all major citrus growing regions of Florida and has been the primary cause of $7.8 billion in lost revenue and more than 7,500 jobs in the Florida citrus industry since 2006 (Hodges and Spreen, 2012). The orange production of Florida was estimated at 45 million boxes for the 2017–2018 season, which represents a decline of more than 80% from the state’s historic peak citrus production.
Previous research showed remission of citrus decline is more likely if trees remain vigorous by reducing stress caused by nutrient deficiencies, with or without application of plant growth regulators, to help host defense against HLB (Spann and Schumann, 2009; Stansly et al., 2014; Li et al., 2016). Several reports indicated that the application of mineral fertilizers, such as Zn, Cu, and Fe, alleviated the symptoms of HLB symptomatic trees and restored their productivity (Nwugo et al., 2013; Zhang et al., 2016). These favorable results were attributed to plant health maintenance and the promotion of root growth. In certain nutrient/systemic acquired resistance (SAR) programs, salicylic acid (SA) and/or its analogs were applied as foliar amendments to help the host defend against HLB by activating the SAR pathway. A combination of psyllid management and foliar nutrition/SAR sprays maintained tree health and productivity despite the presence of HLB (Rouse et al., 2012). However, any positive effects these treatments have on disease expression or fruit yield remain to be demonstrated (Stansly et al., 2014). In fact, no conclusive study has been conducted demonstrating how to control HLB by inducing plant defense (Li et al., 2016).
Citrus peel oil is the first byproduct obtained during the processing of citrus fruits and is widely used in foods, perfumery, and cosmetics (Dharmawan et al., 2009; Gonzalez-Mas et al., 2019). Citrus oil is also used as a cleaner, solvent, fungicide, and even aromatherapy material for humans (Dugo and Mondello, 2011). Citrus oil possesses strong insecticidal and biochemical activities (Oyedeji et al., 2020), as well as antimicrobial and antioxidant activities (Fancello et al., 2020; Oikeh et al., 2020). The essential oil from Mandarin revealed the inhibitory effects against Staphylococcus aureus (Song et al., 2020). A commercial citrus essential oil showed antimicrobial activity against Escherichia coli (Ambrosio et al., 2020). In addition, the Blanco peel essential oil exhibited potential for the treatment of skin acne (Hou et al., 2019). The volatile composition of citrus peel oil has also been extensively studied (Minh Tu et al., 2002; Njoroge et al., 2003; Dugo and Mondello, 2011; Liu et al., 2012; Lin et al., 2019). Aldehyde composition is the most important factor to evaluate citrus peel oil quality, and a better-quality oil usually has a higher concentration of aldehydes (Xu et al., 2017b). Aldehydes are known for their distinctly potent fragrances and are often main contributors to the overall flavor of an essential oil, and limonene is the major volatile compound in citrus peel oils (Spadaro et al., 2012). The quality of citrus oil is frequently affected by the climatic condition, disease, and harvest maturity (Vekiari et al., 2002; Zouaghi et al., 2019). However, there has been little research concerning the impact of HLB and nutritional/insecticide sprays on citrus peel oil volatiles. The objective of this study was to investigate the effect of enhanced foliar spray programs on volatile components of cold pressed oil from HLB+ and HLB− Valencia orange peel.
Materials and Methods
Chemicals
Standards were obtained from the following sources: octanal, nonanal, decanal, terpinyl aldehyde, (E,E)-2,4-decadienal, geranial, neral, octanol, linalool, citronellol, nerol, α-pinene, myrcene, and β-phellandrene were purchased from Aldrich (Milwaukee, WI, United States); valencene was bought from Bedoukian (Danbury, CT, United States); undecanal from Analabs (North Haven, CT, United States); sabinene was obtained from Treatt (Lakeland, FL, United States); δ-3-carene was from K&K (Royse City, TX, United States); terpinen-4-ol from Advanced Biotech (Totowa, NJ, United States); and α-humulene from Fluka (Buchs, Switzerland).
Field Management and Foliar Spray Programs
Experiments were carried out on a commercial block of Citrus sinensis (L.) Osbeck cv. Valencia orange on “Swingle” citrumelo (Citrus paradisi × Poncirus trifoliata) rootstock planted in 2,000 at 3.7 m between trees and 7.3 m between rows at the Southern Garden Citrus Nursery grove in Clewiston, FL (26°45′N; 80°56′W). The training system was round shaped, and average tree height was about 4.75 m. At least one border tree between two different foliar spay programs was provided. The block consisted of 99 trees, and about half of the trees were HLB affected based on a visual diagnose in 2010 by the leaf, fruits and canopy symptoms (Bové, 2006). The trees had received common cultural practices and the grower’s standard pest and disease management before the initiation of the trial in January 2012.
HLB+ and HLB− trees, 30 each, uniform in canopy size, were selected for the study by using a split-plot design with three replicates. Each plot consisted of two subplots, HLB+ and HLB−, and each subplot consisted of two trees. Each plot was treated with one of the following five foliar spray programs for over 16 months from the beginning of 2012 to May 2013, each program included multiple applications, and each application contained macro- (N, P, and K) and micro- (Ca, Mg, Zn, B, Fe, and Mo) nutrients and vector control agents (chlorpyrifos, imidacloprid, abamectin, fenpropathrin, malathion, ζ-cypermethrin, and mineral oil), independently or in combination. The control (C) was the grower standard (Table 1) with eight sprays from April 4, 2012 to May 14, 2013. Each foliar spray program (N1, N2, N3, and N4) was featured by recommendation of one or more nutrient suppliers or grower groups. The exact composition of each foliar spray is proprietary. The common enhancement was an increase in spray times from 8× in the control to 10× in N1 and N4, 11× in N3, and 12× in N2. All N1–N4 had a late dormant/spring flush in Feb 2012, in comparison with the earliest spray for control, which was April 4, 2012 (Table 1).
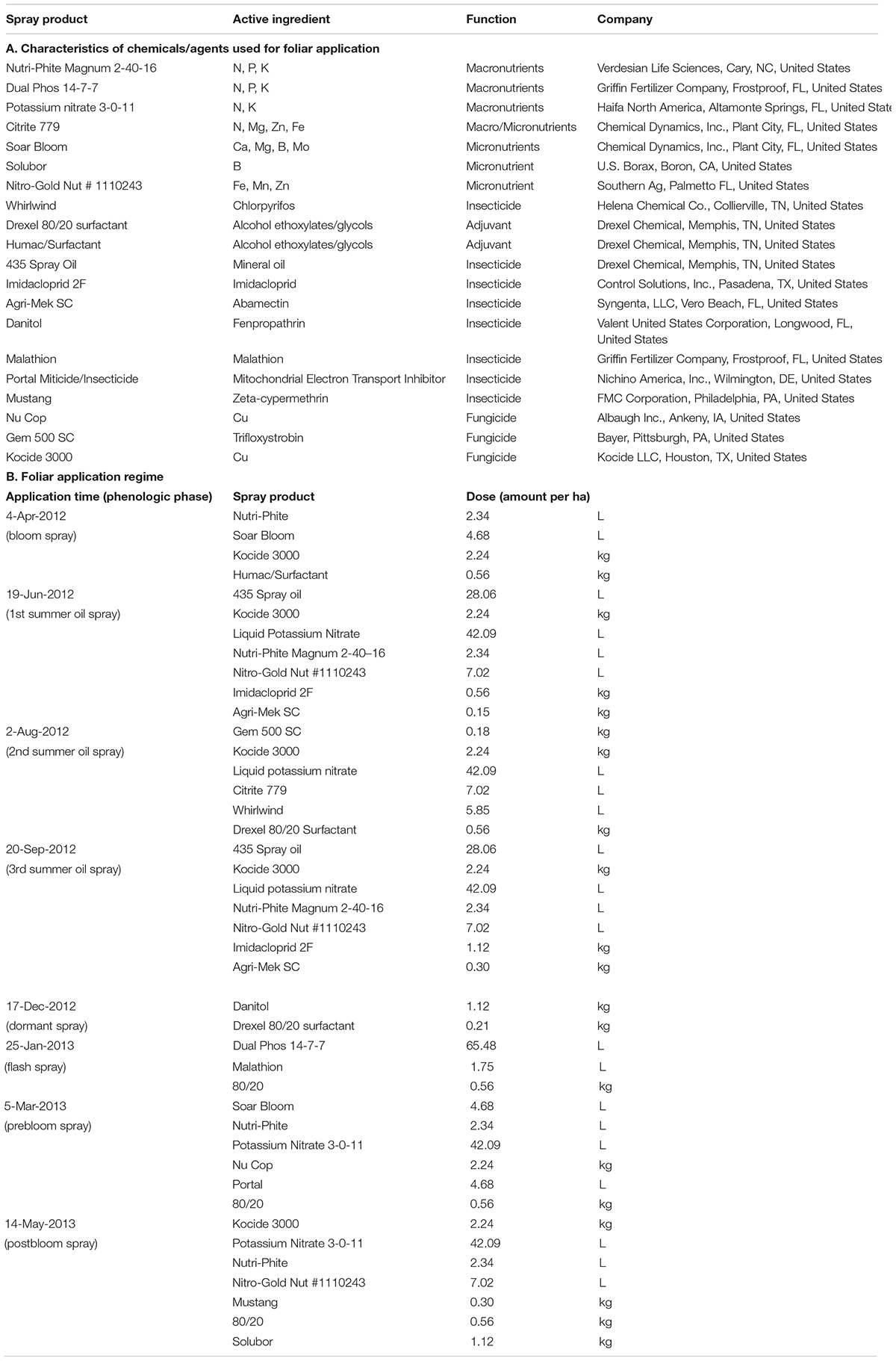
Table 1. Chemicals/agents used for the grower standard/control (C) foliar spray program and the application regime.
Fruit Harvest and Sample Processing
Fruits were harvested on April 28, 2014. For each replicate, approximately 38.5 kg (56 fruits) were harvested from the two trees. Fruits were washed and processed by using a standard processing method with a JBT extractor (JBT§ FoodTech, Lakeland, FL, United States) at premium setting (Bai et al., 2013). Juice samples were collected for CLas titer testing; and “frit” peel tissues, which are located in around of the stem end and are rich in peel oil, were also collected for peel oil extraction. Briefly, the frit peel tissues were cold pressed with a manual oil extractor, the collected emulsion allowed to settle for 30 min, and then the top oil layer was collected and centrifuged at 3,500 × g at 25°C for 15 min. Finally, the peel oil supernatant was collected and stored at −20°C for analysis. To protect the oil samples from oxidation, the headspace of the sample vial was flushed with nitrogen gas before air-tight capping.
DNA Extraction and qPCR Detection of Candidatus Liberibacter asiaticus in Leaves and Fruit Juice
For leaf samples, 10 leaves from each tree were randomly taken on the same day as fruit harvest, and DNA was extracted from 100 mg of the midrib tissues by following Li et al.’s (2006) procedures. Primers targeting CLas 16S rDNA (Li primers) were used (Li et al., 2006; Baldwin et al., 2018); and TaqMan qPCR was performed in a 7500 Real-Time PCR system (Applied Biosystems, Inc., Carlsbad, CA, United States).
For fruit juice samples, DNA was extracted from 500 μl of orange juice sample using a modified cetyl trimethylammonium bromide (CTAB) method (Zhao et al., 2018), and CLas level was quantified by qPCR as previously described (Zhao et al., 2018) using primers targeting CLas hyv1 (LJ primers) (Morgan et al., 2012); SYBR Green qPCR was performed in a 7,500 Real-Time PCR system (Applied Biosystems). The default melt curve (disassociation) stage was continued after the 40 cycles of PCR. Quantification cycle (Cq) values were analyzed using ABI 7,500 Software version 2.0.6 (Applied Biosystems) with a threshold setting of 0.02 and automated baseline settings.
Peel Oil Volatile Composition Analysis
The volatile composition analysis of the oil was carried out using a gas chromatography–mass spectrometry (GC-MS, 6890N GC and 5975 MS, Agilent Technologies, Santa Clara, CA, United States) system equipped with a split/splitless injector and a DB-5 capillary column (60-m length, 0.25-mm diameter, and 1-μm film thickness; J&W Scientific, Folsom, CA, United States). The injector and detector temperature was 260°C. The injection volume was 1 μl with a split ratio of 40:1. The oven conditions were 40°C (0.5 min) and then 4°C⋅min–1 to 225°C (held for 13.25 min) for a total run time of 60 min. Helium was used as carrier gas at flow rate of 1.5 ml⋅min–1. Inlet, ionizing source, and transfer line were kept at 250, 230, and 280°C, respectively. The mass spectrometry data were recorded in the scan mode from 40 to 400 m/z at 2 scans⋅s–1 with an ionization energy of 70 eV.
Identification of Volatile Compounds
Data were collected using the ChemStation G1701 AA data system (Hewlett-Packard, Palo Alto, CA, United States). A mixture of C-5 to C-18 n-alkanes was run at the beginning of each day to calculate retention indices (RIs) (Bai et al., 2014). The volatile components were identified by matching their spectra with those from NIST/EPA/NIH Mass Spectral Library (NIST 14) and authentic volatile compound standards, as well as by comparing their RIs with corresponding literature data (Lota et al., 2002; Deng et al., 2017). Quantification of major peel oil volatile components (limonene, hexanol, hexanal, linalool, etc.) was conducted by using a peak size vs. concentration curve built by a series of diluted standard solutions (Bai et al., 2002).
Statistical Analyses
Data presented were the mean values of three biological replicates. Statistical analysis was performed with JMP (version 11.2.2; SAS Institute, Cary, NC, United States). Differences were tested for significance by using a one-way analysis of variance (ANOVA), incorporating a split-plot design. Mean separations were examined by Tukey’s HSD tests with the significance level at 0.05. For both principal component analysis (PCA) and hierarchical cluster analysis, the complete dataset including all replicates was performed. To visualize quantitative results, a heatmap was designed to present all volatile compounds detected in 10 combinations of foliar spray programs × tree types with the relative amounts in each chemical compound.
Results
Candidatus Liberibacter asiaticus Infection Severity of Leaves and Juice
The qPCR analysis of leaves confirmed the CLas uninfected status of “healthy” trees (HLB−), with high Cq values (>38.29), while diseased trees (HLB+) had low Cq values (<21.76;Table 2). The cutoff Cq value between HLB+ and HLB− for Li primers has been determined to be 32 (Zhao et al., 2018).
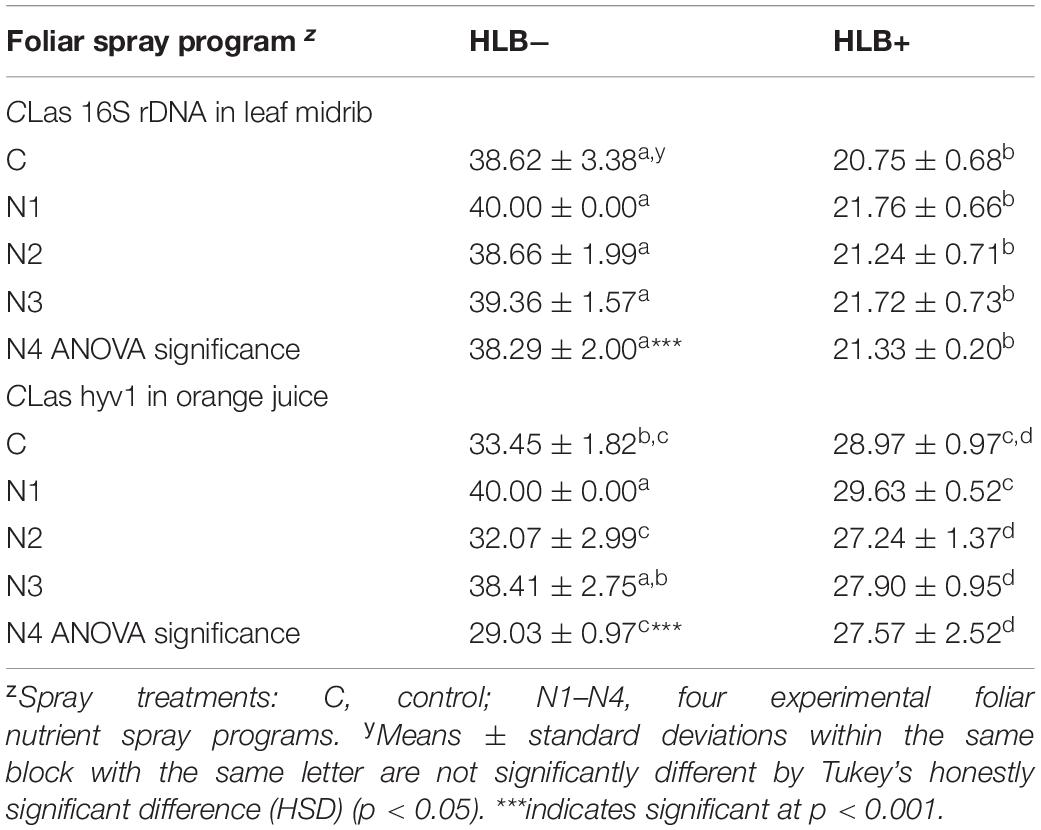
Table 2. Quantification cycle (Cq) values of CLas in “Valencia” orange leaf midrib and fruit tissue samples affected by Huanglongbing (HLB) and foliar spray programs.
Because the CLas titer in orange juice is much lower than in leaf tissue, we used another pair of primers targeting at CLas hyv1, which has more copies in the genome than that of rDNA (Morgan et al., 2012). With these primers, a cutoff Cq value of 29 was determined to differentiate between HLB+ and HLB− (Zhao et al., 2018). Similar to healthy leaf tissue, healthy juice had Cq values higher than the cutoff value, with N4 HLB− right at the cusp (Cq = 29.03) (Table 1). For HLB+ juice, N1 treatment resulted in a higher Cq value (29.63) than N2 (27.24) and N3 (27.90) treatments and slightly higher than cutoff value, indicating that N1 may have some effects on reducing CLas titer in juice (Table 2).
The Effect of Huanglongbing on the Volatile Profile of “Valencia” Citrus Peel Oil
A total of 53 volatile compounds were identified in peel oil samples (Table 3), including seven monoterpenes, 14 sesquiterpenes, 13 terpene oxides, 10 aldehydes, 8 alcohols, and 1 ketone. Monoterpenes were predominant in all the “Valencia” citrus peel oil samples with limonene accounting for the major constituent (89.74–90.48%, Table 3). In general, there were more differences among all the treatments within HLB+ fruits (23 volatile compounds total) than among all the treatment within HLB− fruits (hexanal only).
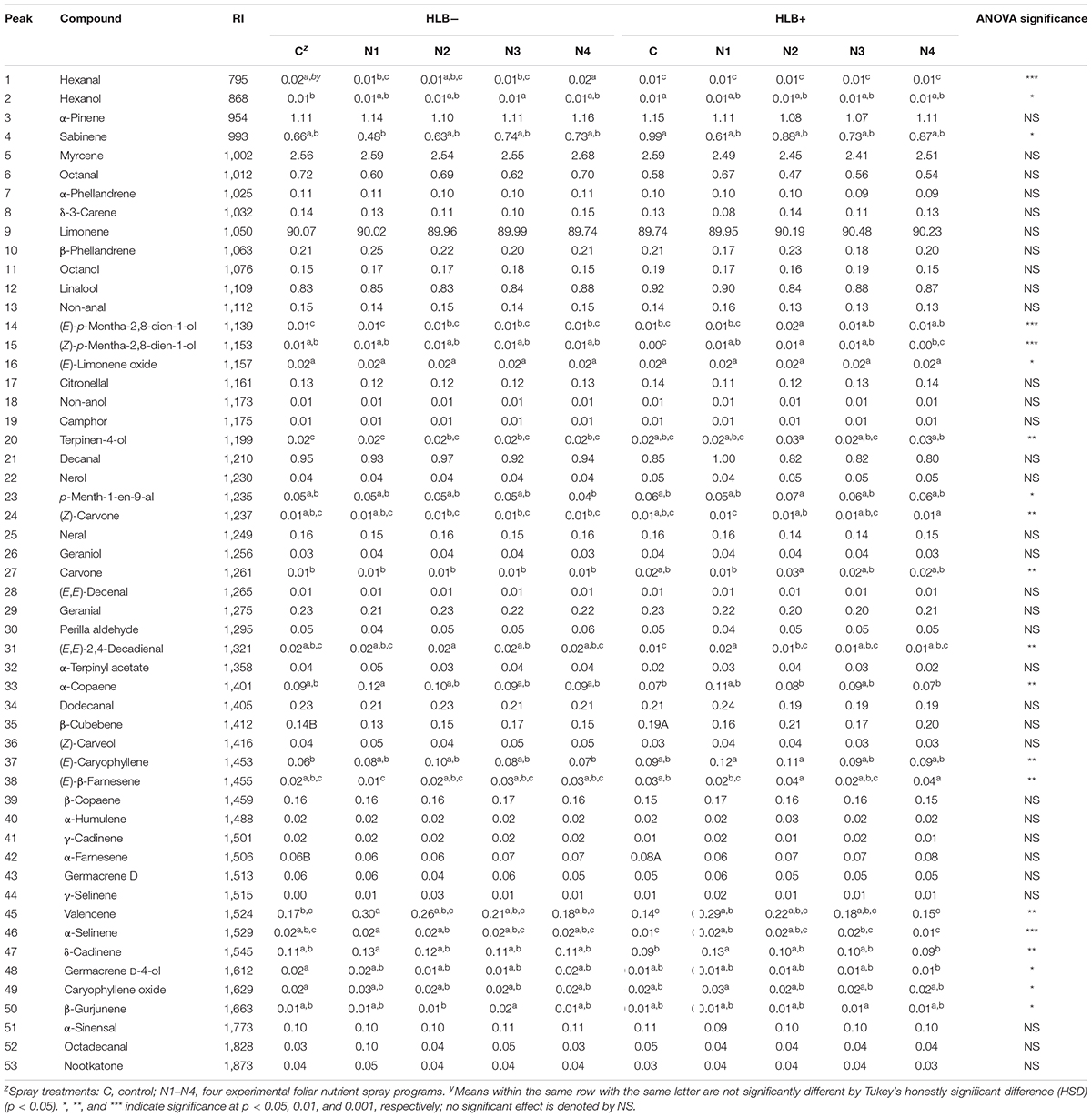
Table 3. Chemical compositions of peel oils of “Valencia” oranges affected by Huanglongbing (HLB) and foliar spray programs N1, N2, N3, and N4, with C as control fertilization program.
Huanglongbing disease dramatically affected the volatile profile of citrus fruits (Figures 1, 2). Cluster analysis (Figure 1) showed which compounds were more abundant in HLB− (in red) versus HLB+ (in green) and how the various treatments affected the profile. Hierarchical clustering shows that all the HLB+ N1 samples (dark pink/green) are clustered with the HLB− samples (red) (Figure 2) along with one replicate of HLB+ N4 sample (light purple/green). Analysis by PCA discriminated HLB+ from HLB− samples in both PC1 and PC2 (Figure 3B), explaining 52.8% of the variation in the first two components (Figure 3A). Most of peel oil volatiles from HLB+ samples were on the negative side of PC1 and positive side of PC2 (Figures 3A,B).
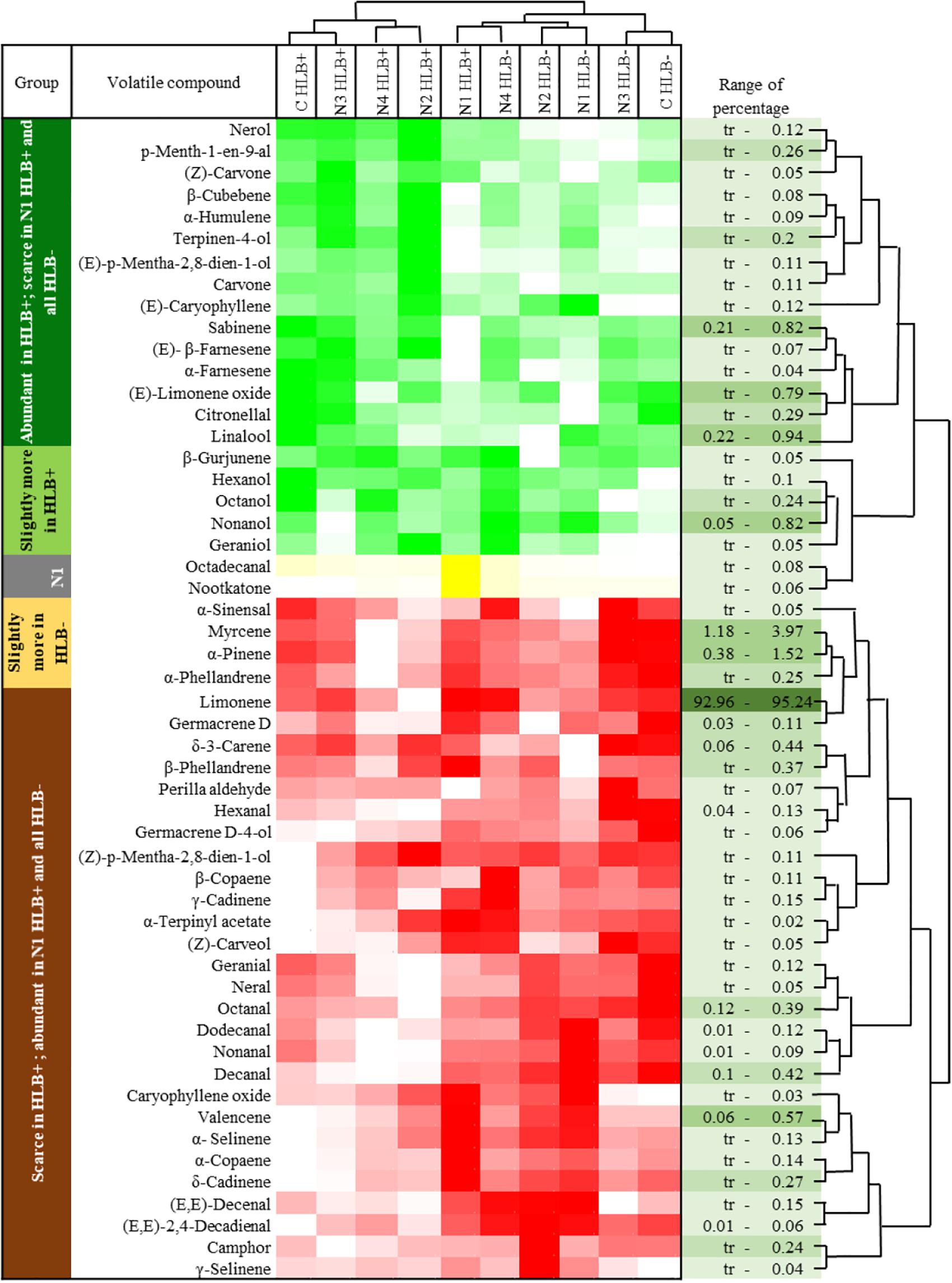
Figure 1. Chemical groups enhanced and suppressed by huanglongbing (HLB) determined by average concentration of volatiles in peel oils extracted from “Valencia” oranges affected by HLB and foliar spray programs (C, control; N1–N4, four different foliar nutrient spray programs). Green and red represent enhanced and suppressed volatiles, respectively. Yellow represents volatiles only enhanced by N1 HLB+ combination. Color density indicates the relative content.
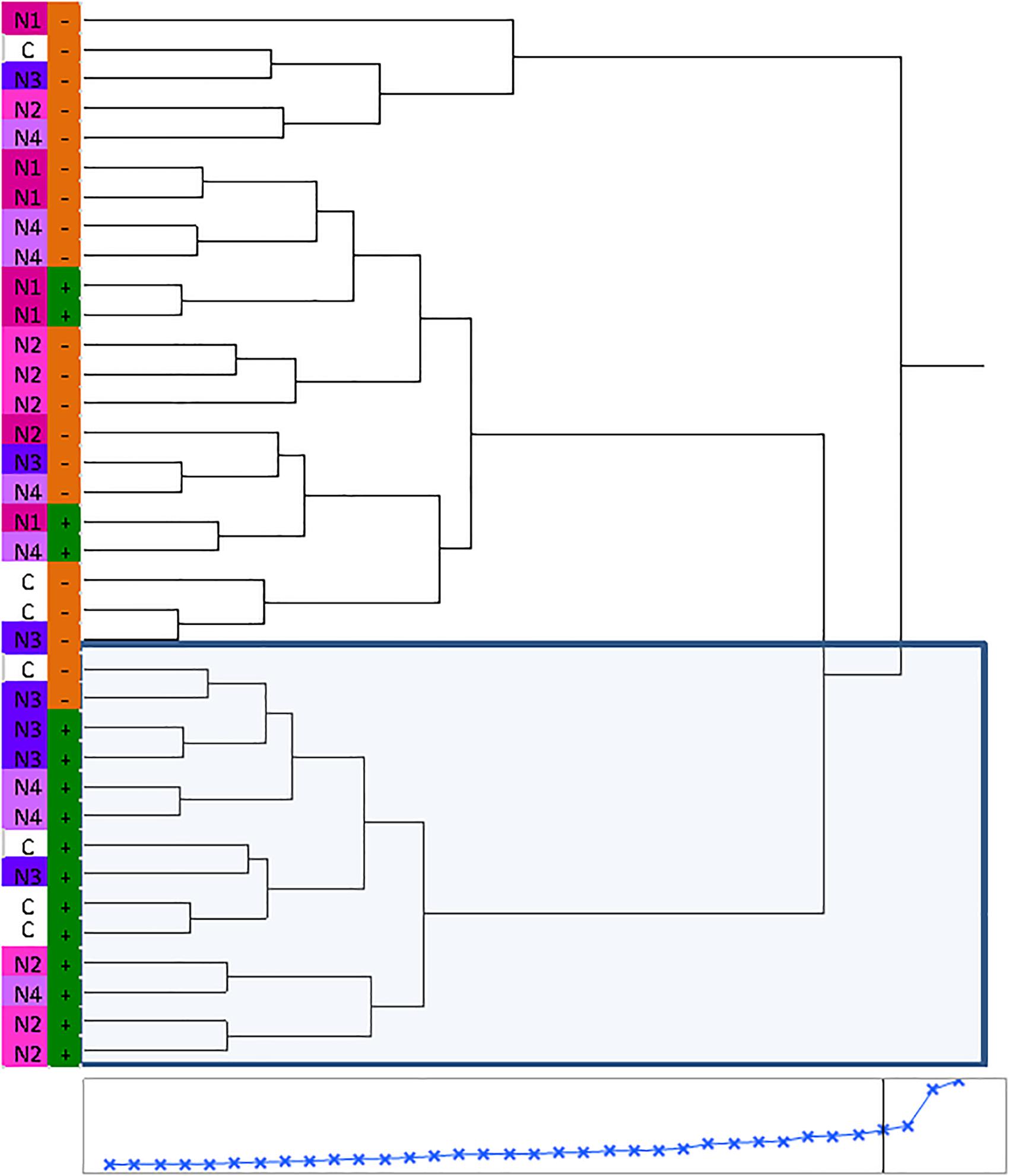
Figure 2. Hierarchical clustering of volatile chemicals in peel oils extracted from “Valencia” oranges affected by Huanglongbing (HLB) and foliar spray programs (C, control; N1–N4, four different foliar nutrient applications; + in green, HLB+; and – in orange, HLB–).
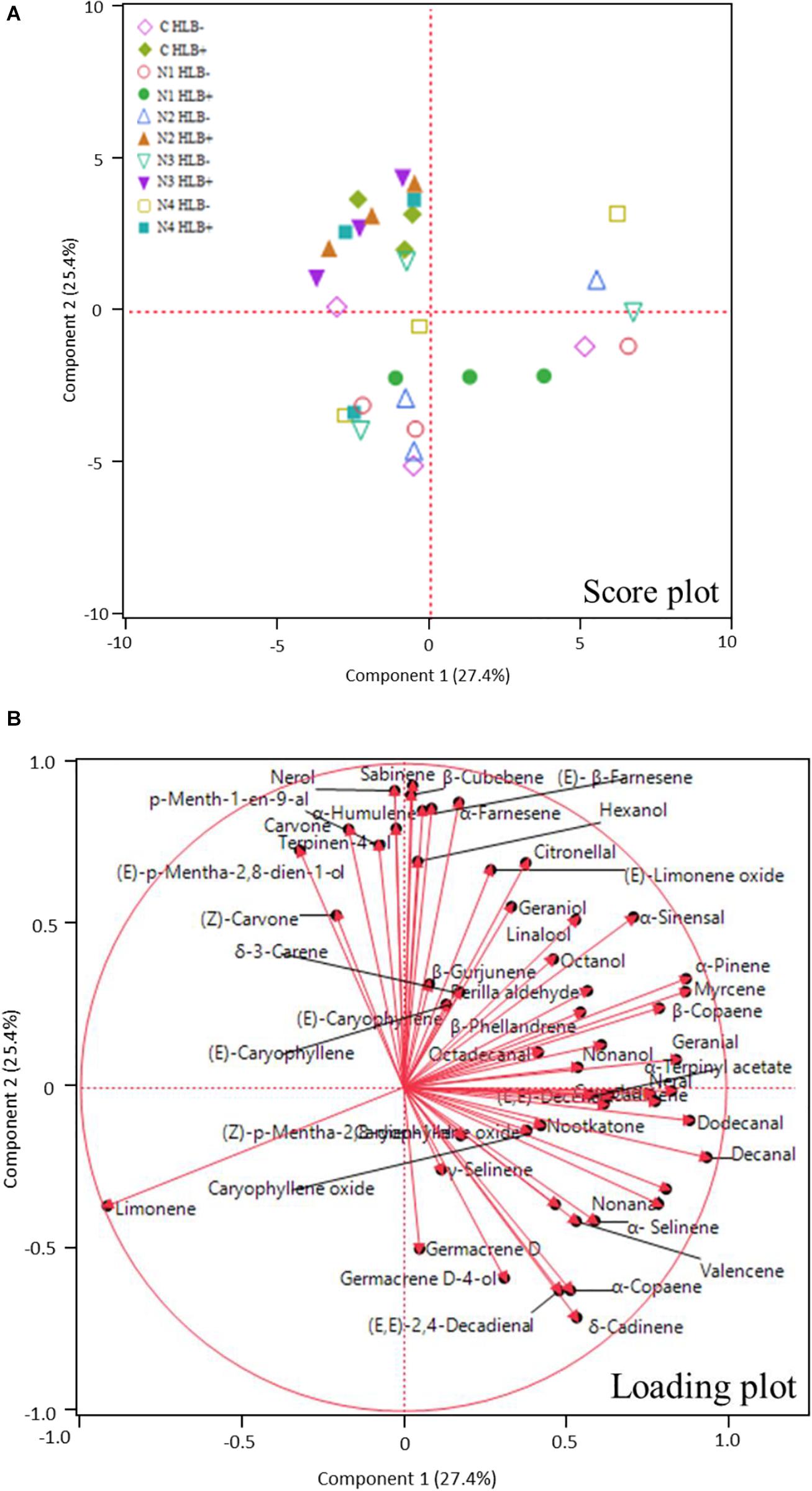
Figure 3. Principle component analysis (PCA) score plot (A) and loading plot (B) of volatile compounds in peel oils of “Valencia” oranges affected by Huanglongbing (HLB) and foliar spray programs (C, control; N1–N4, four different foliar nutritional sprays).
The Effect of Nutritional Spray Treatments on the Volatile Profile of “Valencia” Citrus Peel Oil
The treatment N1 showed the strongest impact on the volatile profile, especially for the HLB+ samples (Table 3 and Figures 1, 2). Treatment N1, for example, reduced (less green) compounds prevalent in HLB+ (e.g., sabinene) and enhanced (more red) some compounds prevalent in HLB− (e.g., α-terpinyl acetate), thus resulting in a profile more closely resembling peel oil from HLB− than oil from HLB+ trees subjected to other treatments. The N1 treatment, which had the highest fruit juice Cq values in both HLB+ and HLB− fruit tissue (Table 2), significantly reduced the concentration of hexanol, sabinene, δ-3-carene, (E)-limonene oxide, citronellal, p-menth-1-en-9-al, (Z)-carvone, and (E)-β-farnesene but significantly increased the concentration of (Z)-p-mentha-2,8-dien-1-ol, decanal, (E,E)-2,4-decadienal, α-terpinyl acetate, α-copaene, (E)-caryophyllene, valencene, α-selinene, δ-cadinene, and caryophyllene oxide (Table 3 and Figure 3B) for HLB+ samples compared with the HLB+ control. Comparing HLB+ with HLB− within N1 treatment showed widespread similarity, with the noticeable exceptions of octadecanal, δ-3-carene, and β-phellandrene, which were more abundant in HLB− than in HLB+ peel oil. All four replicate samples of HLB+ N1 and one replicate sample of HLB+ N4 were positioned on the negative side of PC2, together with most of HLB− samples (Figure 3A).
Discussion
Infection Severity of Fruit
A strict ACP control program had been thoroughly enforced since HLB was detected in the citrus orchard throughout the entire foliar spray research period. The qPCR results indicate all HLB− trees remained CLas-negative for the duration of the experiments (Table 2). All HLB+ trees remained CLas-positive (Table 2), indicating that the enhanced nutritional and ACP vector control applications (such as in Table 1) did not kill CLas. However, the qPCR tests using juice samples provided more complex results: in the HLB− trees with N4 foliar spray programs, the Cq value was 29.03 in the juice samples (close to the 29 cutoff value for juice samples determining CLas infection by LJ primers), while it was 38.29 in leaf midribs (well above the cutoff value of 32 for leaf CLas infection by Li primers) (Table 2). On the other hand, in the HLB+ N1 juice samples, a relatively high Ct value of 29.63 (just above the cutoff value for juice CLas infection by LJ primers) was determined, indicating the infection was less severe than in the other HLB+ samples (Table 2). The possible interpretations are as follows: although CLas is a phloem-restricted bacterium, and leaf midribs are rich in phloem vessels and thus high in CLas titers (Li et al., 2006; Bai et al., 2013), due to the uneven distribution of the pathogen organisms in the tree, leaf samples taken from a tree may test negative for CLas, even if some branches are already infected, especially at the early infection stages when only few branches are infected (Bai et al., 2013; McCollum et al., 2016). Although CLas titers in fruit juice are much lower than in leaves (Bai et al., 2013; Zhao et al., 2018), juice samples were from fruit harvested from the entire tree, including the infected branches, and thus represent the entire tree status better than a leaf sample. The difficulty of detecting CLas in juice samples was overcome by using a pair of primers with high copy numbers in the genome. It has been reported that qPCR using LJ primers targeting CLas hyv1 DNA improved the CLas detection accuracy in comparison with Li primers (Morgan et al., 2012), because there are more copies of hyv1 gene in the CLas genome than 16s rDNA, and therefore, the relative detectable threshold by LJ primers can be reduced by 7–11 cycles for leaf samples (Morgan et al., 2012) and 2–5 cycles for fruit juice samples (Zhao et al., 2018).
The Effect of Huanglongbing on the Volatile Profile of “Valencia” Citrus Peel Oil
The volatile profiles for both HLB− and HLB+ in this study are similar to what has been reported in previous research (Lota et al., 2002; Njoroge et al., 2003; Huang et al., 2017). Previous research showed that HLB resulted in a significant reduction in aldehydes, peel oil aroma volatiles formed during the normal ripening of HLB− fruits (Kiefl et al., 2018). In this research, the content of aldehydes hexanal, octanal, nonanal, decanal, and dodecanal was also significantly reduced in the HLB+ samples (Table 3 and Figure 1), confirming that HLB negatively affects peel oil quality. Furthermore, similar to previous research, citrus peel oil derived from HLB+ fruits had lower concentrations of citronellal and geranial than oil derived from HLB− samples (Table 3; Xu et al., 2017a). Additionally, some esters, such as α-terpinyl acetate, decreased in HLB+ compared with HLB− samples (Plotto et al., 2008; Baldwin et al., 2010). Conceivably, severe HLB infection could substantially inhibit host secondary metabolism and volatile formation (Xu et al., 2017a).
The oxidative/dihydrogen compounds typical of terpenes, such as carvone and limonene oxide, were significantly higher in HLB+ samples. It is possible that these compounds increased because of the stress induced by HLB. These compounds may have all increased due to oxidative stress, which occurred in trees with severe HLB symptoms, creating greater concentrations of these volatiles (Xu et al., 2017a).
The Effect of Nutritional/Insecticidal Spray Treatments on the Volatile Profile of “Valencia” Citrus Peel Oil
Florida growers have been using foliar nutritional spray products that often contain micro- and macro-nutrients to compensate for lack of nutrient assimilation due to the HLB disease and compounds that are believed to activate SAR pathways in plants to increase tree defense response (Masaoka et al., 2011; Gottwald et al., 2012). The results in this experiment showed that one of the experimental foliar spray programs, N1, substantially altered the chemical profiles in HLB+ peel oil, and interestingly, the chemical profile turned out to be similar to that of HLB− peel oil. In N1 sprayed HLB+ peel oil, the most significant changes were recovery of valencene and other sesquiterpenes, which were suppressed by HLB (Table 3 and Figure 3). Phylogenetic analysis of plant terpene synthase genes localized Cstps1 to the group of angiosperm sesquiterpene synthases (Sharon-Asa et al., 2003). Within this group, Cstps1 belongs to a subgroup of citrus sesquiterpene synthases (Sharon-Asa et al., 2003; Yu et al., 2019). Cstps1 was found to be developmentally regulated: transcripts were found to accumulate only during fruit maturation, which corresponds to the timing of valencene accumulation in fruits (Sharon-Asa et al., 2003). HLB causes deficiency of valencene and other sesquiterpenes most likely due to the delay of fruit maturation (Dala-Paula et al., 2018), and the recovery of valencene and other sesquiterpenes indicates that the N1 spray program improved tree defense response, through either nutritional improvement, vector control preventing ACP mediated exacerbation of HLB, or a combination of both (Stansly et al., 2014; Li et al., 2016). Table 2 clearly shows that the N1 spray program significantly reduced the CLas titer in orange juice sample. Further research is required to confirm if the presence of HLB results in a downregulation of Cstps1, and if a N1-like spray program can enhance the recovery.
As the experimental foliar sprays are of proprietary formulation, we do not know the exact difference between N2, N3, and N4. N1, which used two types of Zn and three types of Mg containing chemicals, was the only treatment that showed an improvement in the chemical profile of the peel oil (Table 3 and Figure 3).
Peel oil quality is determined by the proper chemical combination of volatile compounds. Several low−abundant sesquiterpenes, such as valencene, α−sinensal, and β−sinensal, stand out in citrus as important flavor and aroma compounds (Dugo and Mondello, 2011). The profile of terpenoid volatiles in various citrus species and their importance as aroma compounds have been studied in detail (Njoroge et al., 2003; Xu et al., 2017a; Lin et al., 2019), but much is still lacking in our understanding of the physiological, biochemical, and genetic regulation of their production. Proteomic approaches, such as SWATH-MS, could be used to facilitate functional analysis in plant research (Zhu et al., 2020). Proteogenomics combines proteomics, genomics, and transcriptomics and has considerably improved genome annotation in poorly investigated phylogenetic groups for which homology information is lacking and may be a fruitful approach for elucidating the host–pathogen relationship between citrus and HLB with respect to the biosynthesis of volatile components of peel oil (Blank-Landeshammer et al., 2019; Chen et al., 2020).
Conclusion
In this research, the influence of HLB disease and foliar spray programs on the chemical composition of “Valencia” orange peel oil was investigated. HLB disease altered the volatile profile of “Valencia” orange peel oil in that many terpene compounds were accumulated at a higher level in the HLB+ peel oil, indicating that disease stress up-regulated the terpenoid pathways. In contrast, some key aldehydes in peel oil were suppressed in the HLB+ samples, which may negatively impact peel oil quality. Of the four proprietary foliar spray programs tested in this research, only N1 shifted the chemical profile of HLB+ peel oil to resemble that of HLB− samples.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Author Contributions
EAB, MI, AP, and JB: conceptualization. JB, MI, WZ, XS, EAB, and AP: methodology. HY, WZ, EB, and XS: conducting experiments. XS, WZ, and JB: statistical analysis of the results. XS, JB, EAB, MI, and HY: writing—original draft preparation. XS, JB, EAB, WZ, AP, MI, HY, and EB: writing—review and editing. All authors approved the submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ambrosio, C. M. S., Contreras-Castillo, C. J., and Da Gloria, E. M. (2020). In vitro mechanism of antibacterial action of a citrus essential oil on an enterotoxigenic Escherichia coli and Lactobacillus rhamnosus. J. Appl. Microbiol. 129, 541–553. doi: 10.1111/jam.14660
Bai, J., Baldwin, E., Hearn, J., Driggers, R., and Stover, E. (2014). Volatile profile comparison of USDA sweet orange-like hybrids versus ‘Hamlin’ and ‘Ambersweet’. HortScience 49, 1262–1267. doi: 10.21273/HORTSCI.49.10.1262
Bai, J., Hagenmaier, R., and Baldwin, E. (2002). Volatile response of four apple varieties with different coatings during marketing at room temperature. J. Agricult. Food Chem. 50, 7660–7668. doi: 10.1021/jf020543n
Bai, J., Manthey, J. A., Ford, B. L., Luzio, G., Cameron, R. G., Narciso, J., et al. (2013). Effect of extraction, pasteurization and cold storage on flavonoids and other secondary metabolites in fresh orange juice. J. Sci. Food Agric. 93, 2771–2781. doi: 10.1002/jsfa.6097
Baldwin, E., Plotto, A., Bai, J., Manthey, J., Zhao, W., Raithore, S., et al. (2018). Effect of abscission zone formation on orange (Citrus sinensis) fruit/juice quality for trees affected by huanglongbing (HLB). J. Agric. Food Chem. 66, 2877–2890. doi: 10.1021/acs.jafc.7b05635
Baldwin, E., Plotto, A., Manthey, J., Mccollum, G., Bai, J. H., Irey, M., et al. (2010). Effect of liberibacter infection (Huanglongbing Disease) of citrus on orange fruit physiology and fruit/fruit juice quality: chemical and physical analyses. J. Agric. Food Chem. 58, 1247–1262. doi: 10.1021/jf9031958
Baldwin, E. A., Bai, J., Plotto, A., and Ritenour, M. A. (2014). Citrus fruit quality assessment; producer and consumer perspectives. Stewart Postharvest Rev. 2:7.
Blank-Landeshammer, B., Teichert, I., Marker, R., Nowrousian, M., Kuck, U., and Sickmann, A. (2019). Combination of proteogenomics with peptide de novo sequencing identifies new genes and hidden posttranscriptional modifications. mBio 10:e02367-19. doi: 10.1128/mBio.02367-19
Bové, J. M. (2006). Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 88, 7–37.
Chen, H., Mccollum, G., Baldwin, E., and Bai, J. (2016). Impacts of huanglongbing symptom severity on fruit detachment force and mechanical properties of sweet oranges (Citrus sinensis). HortScience 51, 356–361. doi: 10.21273/HORTSCI.51.4.356
Chen, M. X., Zhu, F. Y., Gao, B., Ma, K. L., Zhang, Y., Fernie, A. R., et al. (2020). Full-length transcript-based proteogenomics of rice improves its genome and proteome annotation. Plant Physiol. 182, 1510–1526. doi: 10.1104/pp.19.00430
Dala-Paula, B. M., Plotto, A., Bai, J., Manthey, J. A., Baldwin, E. A., Ferrarezi, R. S., et al. (2018). Effect of Huanglongbing or greening disease on orange juice quality, a review. Front. Plant Sci. 9:1976. doi: 10.3389/fpls.2018.01976
Deng, G., Craft, J. D., Steinberg, K. M., Li, P. L., Pokharel, S. K., and Setzer, W. N. (2017). Influence of different isolation methods on chemical composition and bioactivities of the fruit peel oil of Citrus medica L. var. sarcodactylis (Noot.) Swingle. Medicines (Basel) 4:1. doi: 10.3390/medicines4010001
Dharmawan, J., Kasapis, S., Sriramula, P., Lear, M. J., and Curran, P. (2009). Evaluation of aroma-active compounds in Pontianak orange peel oil (Citrus nobilis Lour. Var. microcarpa Hassk.) by gas chromatography-olfactometry, aroma reconstitution, and omission test. J. Agric. Food Chem. 57, 239–244. doi: 10.1021/jf801070r
Dugo, G., and Mondello, L. (2011). Citrus Oil: Composition, Advanced Analytical Techniques, Contaminants, and Biological Activity. Boca Raton, FL: CRC Press. doi: 10.1201/b10314
Fancello, F., Petretto, G. L., Marceddu, S., Venditti, T., Pintore, G., Zara, G., et al. (2020). Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 87:103386. doi: 10.1016/j.fm.2019.103386
Gonzalez-Mas, M. C., Rambla, J. L., Lopez-Gresa, M. P., Blazquez, M. A., and Granell, A. (2019). Volatile compounds in citrus essential oils: a comprehensive review. Front. Plant Sci. 10:12. doi: 10.3389/fpls.2019.00012
Gottwald, T., Poole, G., Mccollum, T., Hall, D., Hartung, J., Bai, J., et al. (2020). Canine olfactory detection of a vectored phytobacterial pathogen, Liberibacter asiaticus, and integration with disease control. Proc. Natl. Acad. Sci. U.S.A. 117, 3492–3501. doi: 10.1073/pnas.1914296117
Gottwald, T. R., Graham, J. H., Irey, M. S., Mccollum, T. G., and Wood, B. W. (2012). Inconsequential effect of nutritional treatments on huanglongbing control, fruit quality, bacterial titer and disease progress. Crop Protection 36, 73–82. doi: 10.1016/j.cropro.2012.01.004
Hodges, A. W., and Spreen, T. H. (2012). Economic Impacts of Citrus Greening (HLB) in Florida, 2006/07–2010/11. EDIS 2012. Available online at: https://journals.flvc.org/edis/article/view/119504
Hou, H. S., Bonku, E. M., Zhai, R., Zeng, R., Hou, Y. L., Yang, Z. H., et al. (2019). Extraction of essential oil from Citrus reticulate Blanco peel and its antibacterial activity against Cutibacterium acnes (formerly Propionibacterium acnes). Heliyon 5:e02947. doi: 10.1016/j.heliyon.2019.e02947
Huang, M., Valim, M. F., Shi, F., Reuss, L., Yao, L., Gmitter, F., et al. (2017). Characterization of the major aroma-active compounds in peel oil of an HLB-tolerant mandarin hybrid using aroma extraction dilution analysis and gas chromatography-mass spectrometry/olfactometry. Chemosensory Perception 10, 161–169. doi: 10.1007/s12078-017-9221-y
Kiefl, J., Kohlenberg, B., Hartmann, A., Obst, K., Paetz, S., Krammer, G., et al. (2018). Investigation on key molecules of huanglongbing (HLB)-induced orange juice off-flavor. J. Agric. Food Chem. 66, 2370–2377. doi: 10.1021/acs.jafc.7b00892
Li, J., Trivedi, P., and Wang, N. (2016). Field evaluation of plant defense inducers for the control of citrus huanglongbing. Phytopathology§ 106, 37–46. doi: 10.1094/PHYTO-08-15-0196-R
Li, W., Hartung, J. S., and Levy, L. (2006). Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 66, 104–115. doi: 10.1016/j.mimet.2005.10.018
Lin, L. Y., Chuang, C. H., Chen, H. C., and Yang, K. M. (2019). Lime (Citrus aurantifolia (Christm.) Swingle) essential oils: volatile compounds, antioxidant capacity, and hypolipidemic effect. Foods 8:398. doi: 10.3390/foods8090398
Liu, C., Cheng, Y., Zhang, H., Deng, X., Chen, F., and Xu, J. (2012). Volatile constituents of wild citrus Mangshanyegan (Citrus nobilis Lauriro) peel oil. J. Agric. Food Chem. 60, 2617–2628. doi: 10.1021/jf2039197
Lota, M. L., De Rocca Serra, D., Tomi, F., Jacquemond, C., and Casanova, J. (2002). Volatile components of peel and leaf oils of lemon and lime species. J. Agric. Food Chem. 50, 796–805. doi: 10.1021/jf010924l
Masaoka, Y., Pustika, A., Subandiyah, S., Okada, A., Hanundin, E., Purwanto, B., et al. (2011). Lower concentrations of microelements in leaves of citrus infected with ‘Candidatus Liberibacter asiaticus’. Jarq-Japan Agric. Res. Q. 45, 269–275. doi: 10.6090/jarq.45.269
McCollum, G., Baldwin, E., Gradziel, T. M., Mitchell, C. A., and Whipkey, A. L. (2016). Huanglongbing: devastating disease of citrus. Horticult. Rev. 44, 315–361. doi: 10.1002/9781119281269.ch7
Minh Tu, N. T., Onishi, Y., Choi, H. S., Kondo, Y., Bassore, S. M., Ukeda, H., et al. (2002). Characteristic odor components of Citrus sphaerocarpa Tanaka (Kabosu) cold-pressed peel oil. J. Agric. Food Chem. 50, 2908–2913. doi: 10.1021/jf011578a
Morgan, J. K., Zhou, L., Li, W., Shatters, R. G., Keremane, M., and Duan, Y. P. (2012). Improved real-time PCR detection of ‘Candidatus Liberibacter asiaticus’ from citrus and psyllid hosts by targeting the intragenic tandem-repeats of its prophage genes. Mol. Cell. Probes 26, 90–98. doi: 10.1016/j.mcp.2011.12.001
Njoroge, S. M., Ukeda, H., and Sawamura, M. (2003). Changes of the volatile profile and artifact formation in Daidai (Citrus aurantium) cold-pressed peel oil on storage. J. Agric. Food Chem. 51, 4029–4035. doi: 10.1021/jf021215q
Nwugo, C. C., Lin, H., Duan, Y., and Civerolo, E. L. (2013). The effect of ‘Candidatus Liberibacter asiaticus’ infection on the proteomic profiles and nutritional status of pre-symptomatic and symptomatic grapefruit (Citrus paradisi) plants. BMC Plant Biol. 13:59. doi: 10.1186/1471-2229-13-59
Oikeh, E. I., Ayevbuomwan, M., Irabor, F., Oikeh, A. O., Oviasogie, F. E., and Omoregie, E. S. (2020). Evaluation of the phenolic content, antioxidant and antimicrobial activities of oil and non-oil extracts of Citrus sinensis (L.) Osbeck Seeds. Prev. Nutr. Food Sci. 25, 280–285. doi: 10.3746/pnf.2020.25.3.280
Oyedeji, A. O., Okunowo, W. O., Osuntoki, A. A., Olabode, T. B., and Ayo-Folorunso, F. (2020). Insecticidal and biochemical activity of essential oil from Citrus sinensis peel and constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 168:104643. doi: 10.1016/j.pestbp.2020.104643
Plotto, A., Margaria, C. A., Goodner, K. L., and Baldwin, E. A. (2008). Odour and flavour thresholds for key aroma components in an orange juice matrix: esters and miscellaneous compounds. Flavour Fragrance J. 23, 398–406. doi: 10.1002/ffj.1888
Rouse, B., Irey, M., Gast, T., Boyd, M., and Willis, T. (2012). Fruit production in a southwest Florida citrus grove using the Boyd Nutrient/SAR foliar spray. Proc. Florida State Hortic. Soc. 125, 61–64.
Sharon-Asa, L., Shalit, M., Frydman, A., Bar, E., Holland, D., Or, E., et al. (2003). Citrus fruit flavor and aroma biosynthesis: isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound valencene. Plant J. 36, 664–674. doi: 10.1046/j.1365-313X.2003.01910.x
Song, X., Liu, T., Wang, L., Liu, L., Li, X., and Wu, X. (2020). Antibacterial effects and mechanism of mandarin (Citrus reticulata L.) essential oil against Staphylococcus aureus. Molecules 25:4956. doi: 10.3390/molecules25214956
Spadaro, F., Costa, R., Circosta, C., and Occhiuto, F. (2012). Volatile composition and biological activity of key lime Citrus aurantifolia essential oil. Nat. Prod. Commun. 7, 1523–1526. doi: 10.1177/1934578X1200701128
Spann, T. M., and Schumann, A. W. (2009). The role of plant nutrients in disease development with emphasis on citrus and huanglongbing. Proc. Fla. State Hort. Soc. 122, 169–171.
Stansly, P. A., Arevalo, H. A., Qureshi, J. A., Jones, M. M., Hendricks, K., Roberts, P. D., et al. (2014). Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by huanglongbing. Pest Manage. Sci. 70, 415–426. doi: 10.1002/ps.3577
Vekiari, S. A., Protopapadakis, E. E., Papadopoulou, P., Papanicolaou, D., Panou, C., and Vamvakias, M. (2002). Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J. Agric. Food Chem. 50, 147–153. doi: 10.1021/jf001369a
Xu, B. M., Baker, G. L., Sarnoski, P. J., and Goodrich-Schneider, R. M. (2017a). A comparison of the volatile components of cold pressed Hamlin and Valencia (Citrus sinensis (L.) Osbeck) orange oils affected by huanglongbing. J. Food Quality 2017, 1–20. doi: 10.1155/2017/6793986
Xu, B. M., Sims, C. A., Etxeberria, E., and Schneider, R. M. G. (2017b). Physicochemical and sensory properties of cold pressed oils from florida hamlin and valencia oranges affected by huanglongbing. J. Food Sci. 82, 2158–2166. doi: 10.1111/1750-3841.13814
Yu, Q., Huang, M., Jia, H., Yu, Y., Plotto, A., Baldwin, E. A., et al. (2019). Deficiency of valencene in mandarin hybrids is associated with a deletion in the promoter region of the valencene synthase gene. BMC Plant Biol. 19:101. doi: 10.1186/s12870-019-1701-6
Zhang, M. Q., Guo, Y., Powell, C. A., Doud, M. S., Yang, C. Y., Zhou, H., et al. (2016). Zinc treatment increases the titre of ‘Candidatus Liberibacter asiaticus’ in huanglongbing-affected citrus plants while affecting the bacterial microbiomes. J. Appl. Microbiol. 120, 1616–1628. doi: 10.1111/jam.13102
Zhao, W., Bai, J., Mccollum, G., and Baldwin, E. (2015). High incidence of preharvest colonization of huanglongbing-symptomatic citrus sinensis fruit by Lasiodiplodia theobromae (Diplodia natalensis) and exacerbation of postharvest fruit decay by that fungus. Appl. Environ. Microbiol. 81, 364–372. doi: 10.1128/AEM.02972-14
Zhao, W., Baldwin, E. A., Bai, J., Plotto, A., and Irey, M. S. (2018). Method for Assessing Juice/Cider Quality and/or Safety. Google Patents.
Zhao, W., Gottwald, T., Bai, J., Mccollum, G., Irey, M., Plotto, A., et al. (2016). Correlation of Diplodia (Lasiodiplodia theobromae) infection, huanglongbing, ethylene production, fruit removal force and pre-harvest fruit drop. Sci. Hortic. 212, 162–170. doi: 10.1016/j.scienta.2016.09.032
Zhu, F. Y., Song, Y. C., Zhang, K. L., Chen, X., and Chen, M. X. (2020). Quantifying plant dynamic proteomes by SWATH-based mass spectrometry. Trends Plant Sci. 25, 1171–1172. doi: 10.1016/j.tplants.2020.07.014
Zouaghi, G., Najar, A., Aydi, A., Claumann, C. A., Zibetti, A. W., Ben Mahmoud, K., et al. (2019). Essential oil components of Citrus cultivar ‘MALTAISE DEMI SANGUINE’(Citrus sinensis) as affected by the effects of rootstocks and viroid infection. Int. J. Food Properties 22, 438–448. doi: 10.1080/10942912.2019.1588296
Keywords: citrus greening disease, nutritional spray, insect vector control, cold pressed oil, volatile organic compounds
Citation: Sun X, Yang H, Zhao W, Bourcier E, Baldwin EA, Plotto A, Irey M and Bai J (2021) Huanglongbing and Foliar Spray Programs Affect the Chemical Profile of “Valencia” Orange Peel Oil. Front. Plant Sci. 12:611449. doi: 10.3389/fpls.2021.611449
Received: 16 October 2020; Accepted: 16 February 2021;
Published: 06 April 2021.
Edited by:
Zhi-Yan (Rock) Du, University of Hawaii at Manoa, United StatesReviewed by:
Riccardo Lo Bianco, University of Palermo, ItalyGeorgios Vidalakis, University of California, Riverside, United States
Mo-Xian Chen, Chinese Academy of Sciences (CAS), China
Copyright © 2021 Sun, Yang, Zhao, Bourcier, Baldwin, Plotto, Irey and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhe Bai, amluaGUuYmFpQHVzZGEuZ292
 Xiuxiu Sun1
Xiuxiu Sun1 Elise Bourcier
Elise Bourcier Jinhe Bai
Jinhe Bai