- 1Institute of Biological Sciences, Universidad de Talca, Talca, Chile
- 2Chinese Academy of Agricultural Sciences, Beijing, China
- 3Department of Plant Breeding and Genetics, The University of Haripur, Haripur, Pakistan
- 4College of Plant Science and Technology, Huazhong Agriculture University, Wuhan, China
- 5Graduate School of Biotechnology & Crop Biotech Institute, Kyung Hee University, Yongin, South Korea
Agriculture is an important source of human food. However, current agricultural practices need modernizing and strengthening to fulfill the increasing food requirements of the growing worldwide population. Genome editing (GE) technology has been used to produce plants with improved yields and nutritional value as well as with higher resilience to herbicides, insects, and diseases. Several GE tools have been developed recently, including clustered regularly interspaced short palindromic repeats (CRISPR) with nucleases, a customizable and successful method. The main steps of the GE process involve introducing transgenes or CRISPR into plants via specific gene delivery systems. However, GE tools have certain limitations, including time-consuming and complicated protocols, potential tissue damage, DNA incorporation in the host genome, and low transformation efficiency. To overcome these issues, nanotechnology has emerged as a groundbreaking and modern technique. Nanoparticle-mediated gene delivery is superior to conventional biomolecular approaches because it enhances the transformation efficiency for both temporal (transient) and permanent (stable) genetic modifications in various plant species. However, with the discoveries of various advanced technologies, certain challenges in developing a short-term breeding strategy in plants remain. Thus, in this review, nanobased delivery systems and plant genetic engineering challenges are discussed in detail. Moreover, we have suggested an effective method to hasten crop improvement programs by combining current technologies, such as speed breeding and CRISPR/Cas, with nanotechnology. The overall aim of this review is to provide a detailed overview of nanotechnology-based CRISPR techniques for plant transformation and suggest applications for possible crop enhancement.
Introduction
Food safety has become a worldwide issue because of increasing food demand and reducing crop yields resulting from climate change, soil degradation, and crop disease proliferation (Shaheen and Abed, 2018). By 2050, the global population will reach an estimated 9.6 billion, with the demand for staple crops increasing by 60% (Bajželj et al., 2014). Current efforts are focused on sustainably increasing crop yields without the excessive use of pesticides and fertilizers. However, traditional strategies used for crop improvement are laborious, time-consuming, and challenging (Figure 1). Therefore, novel plant breeding technologies equipped with capabilities (gene knock out/in, epigenetic modifications, generation of heritable targeted mutations in specific genomic region) need to be urgently utilized to tackle the drawbacks of the classical plant breeding methods (Chen et al., 2019; Fiaz et al., 2021). In the last decade, there have been major developments in the field of biotechnology, e.g., the advent of third-generation genome editing (GE) techniques, genome sequencing, advancements in plant-based synthetic biology, and bioengineering (Altpeter et al., 2016; Wang et al., 2019; Zhang et al., 2019a). These techniques have been successfully employed to develop elite germplasm, ensuring grain yield, quality, and resistance against biotic and abiotic stresses as well as climate change (Cunningham et al., 2018; Fiaz et al., 2020).
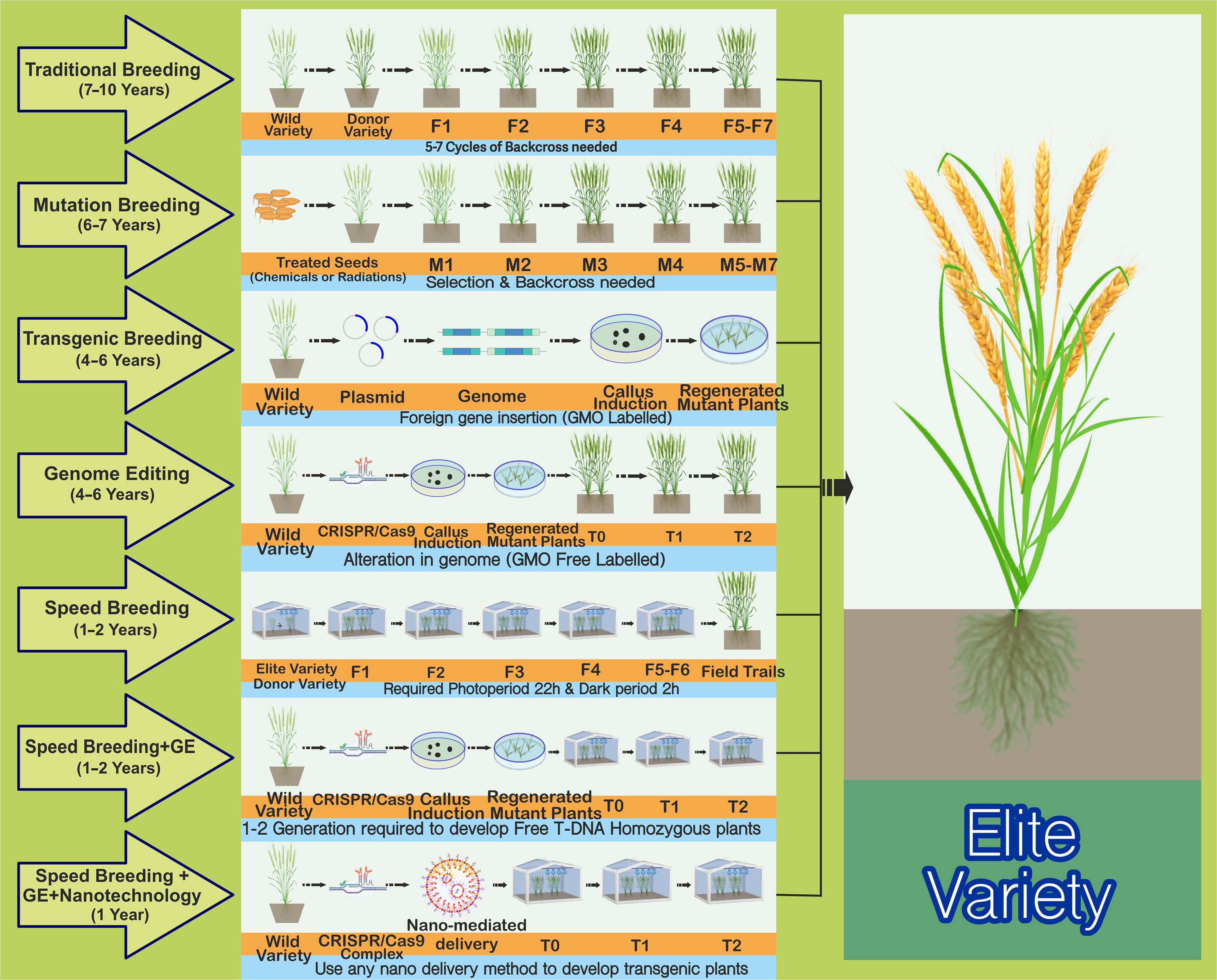
Figure 1. Comparison of the most commonly employed plant breeding mutagenic and time-saving strategies for crop improvement. Traditional plant breeding is used to enhance plant characteristics. The complex successive backcrossing and rigorous selection process of the elite recipient’s parent line with a donor line leads to the development of an outstanding progeny with desired traits. This is a time-consuming, laborious, and less-effective technique. Mutation breeding, also known as “variation breeding,” refers to seeds being treated with chemicals or radiation to produce mutants with suitable characteristics to develop elite cultivars. It would require 6–7 years to produce desirable outcomes and is also a time-consuming process. Random mutations in the genome are one of the critical drawbacks and disadvantages of this strategy. Transgenic breeding has been successfully utilized to improve various crops with different traits by importing a gene of interest from one plant genome to another. These are regarded as genetically modified organisms (GMOs) owing to the insertion of foreign DNA/elements into the genome, and one of the biggest problems with GMOs is their comparative lack of acceptance among the public and a large group of plant scientists worldwide. Genome editing (GE) methods, such as the CRISPR/Cas9 method for trait improvement, provide a cost-effective, stable, time-saving, and less laborious solution than other existing techniques. Moreover, these methods can also be used to evade the GMO law, labeling the products as “non-GMO” because of the absence of any foreign DNA. Speed breeding that extends the photoperiod (22 h with 2 h of darkness in a 24-h diurnal cycle) improves the flowering time compared with that under normal conditions, potentially achieving four to six generations per year rather than the single generation achieved under normal conditions. Regarding photoperiod, continuous light is another option, but the dark period slightly improves the plant health. The optimal temperature regime (maximum and minimum temperatures) should be applied for each crop. This presents the best strategy for developing elite organic varieties within 1–2 years. The GE technology could also be improved by using speed breeding to establish a transgene-free plant within 1–2 years rather than waiting for an entire season under average growth. Another strategy involving nanotechnology and a combination of speed breeding and GE is proving reliable for speedy crop improvement. Here, plants can be grown under speed breeding conditions, and NPs coated with DNA, RNA, or RNP can deliver CRISPR reagents into meristematic cells. Transgene-free edited plants are obtainable from the edited tissues, either sexually or asexually.
GE techniques have revolutionized biological sciences via precise modifications in the genome of both plants and animals. GE is broadly categorized into three generations: meganuclease (MegaN) and zinc finger nucleases (ZFNs) are first–generation tools, transcription activator-like effector nucleases (TALENs) are second-generation tools, and the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 Cas9) nuclease system is considered the third-generation tool. Third-generation GEs, e.g., CRISPR/Cas9, CRISPR–CRISPR from Prevotella and Francisella 1 (Cpf1), base editing, and prime editing, were shown to be powerful tools for the successful modification of genome sequence in a precise and straightforward manner (Puchta et al., 1993; Wright et al., 2005; Christian et al., 2010; Butler et al., 2016; Tang et al., 2017; Yin et al., 2017; Anzalone et al., 2019; Manghwar et al., 2019; Lin et al., 2020). A sequence-specific nuclease catalyzes double-strand breaks at a target region in the genome under consideration. MegaN, a naturally occurring endonuclease discovered in the late 1980s, requires enzymes specific to the targeted sequence and is costly and time-consuming (Townsend et al., 2009). ZFNs were demonstrated in 1996 for the first time as site-specific nucleases for cutting DNA at strictly defined sites. The design of ZFNs is complicated because of the complex interaction of ZFNs among themselves with the risk of off-target mutations (Sander et al., 2011). Furthermore, to increase efficiency, researchers have no other option but to utilize commercially produced ZFNs that are not budget-friendly (Ramirez et al., 2008). TALEN effectors for DNA targeting were realized in 2009. The construction of TALENs is relatively easier and popular than that of ZFNs; however, repetitive sequences in the composition of TALENs can increase the rate of homologous recombination. Both ZFNs and TALENs are the same at the structural and functional level because they harbor the restriction endonuclease Fokl (Boch et al., 2009). The classical first- and second-generation GE techniques have drawbacks, and researchers developed a third-generation GE system (Nekrasov et al., 2013). The CRISPR/Cas system is a powerful gene-editing tool that can be used with various model and non-model plant species. It has been used for improving major crops, including rice (Oryza sativa; Dong et al., 2020; Fayos et al., 2020), sorghum (Sorghum bicolor; Char et al., 2020), tobacco (Nicotiana tabacum; Tian et al., 2020), wheat (Triticum aestivum; Ferrie et al., 2020; Li et al., 2020), maize (Zea mays; Zhang et al., 2020), barley (Hordeum vulgare; Zeng et al., 2020), cotton (Gossypium hirsutum; Lei et al., 2020), tomatoes (Solanum lycopersicum; Santillán Martínez et al., 2020), soybeans (Glycine max; Wang L. et al., 2020), and rapeseed (Brassica napus; Zheng et al., 2020). However, the safe, efficient, and precise time-saving delivery of CRISPR components remains a challenge (Rui et al., 2020). Speed editing strategies have been proposed to address this challenge recently. A web tool has been developed by Hong et al. (2020) to accelerate speed editing strategies to achieve 2% genetic gain in crop productivity (2050 food demand challenge). The powerful GE technology CRISPR/Cas has facilitated functional genomic studies of several crops with simplicity and accuracy. However, genomic research has become congested because of functional redundancies in the genome, ultimately masking the phenotypes of knockout mutants by functional compensations and redundancies. To cope with this concern, an intuitive tool called CRISPR was applied to a functional redundancy inspector to accelerate functional genomics in rice (CRISPR Applicable Functional Redundancy Inspector [CAFRI]-Rice; cafri-rice.khu.ac.kr). The tool is based on a phylogenetic heatmap that can estimate the similarity between protein sequences and expression patterns. This CAFRI-Rice-based target selection for CRISPR/Cas9-mediated mutagenesis has accelerated functional genomic studies in rice; moreover, it can also be easily expanded to other plant species (Ahmar et al., 2020b; Hong et al., 2020).
Conventional biomolecule delivery methods in plants have critical drawbacks, such as low efficiency of gene transmission, narrow species range for applocation, limited cargo types, and tissue damage. Nanotechnology advancements have created opportunities to overcome limitations in conventional methods: nanoparticles (NPs) are promising for the species-independent passive delivery of DNA, RNA, and proteins (Cunningham et al., 2018). There are hundreds of transformation methods. Of them, the two primary genetic transformation methods used in plants are typically genotype-specific for gene delivery (Stewart et al., 2011). The first method, Agrobacterium-mediated transformation (AMT), is widely used for incorporating of target DNA to the nuclear genome and is available for a limited number of plant species. The AMT method leads to random DNA integration, disrupting endogenous plant genes and variation in gene expression arising from the inserted sites (Niazian et al., 2017). The second method, the biolistic delivery of DNA, involves a high-pressure gene gun that directly targets plant tissues, randomly integrating DNA into the chromosomal region across cell walls and membranes. This leads to the destruction of tissues and multiple insertions in random portions of the plant genome (Toda et al., 2019). Thus, plant transformation presents a major bottleneck for GE capacity. Therefore, the delivery method of the biomodifier-conjugated complex to plant cells remains a topic of study for many scientists to develop new strategies for transformation with ease, robustness, and significant efficiency.
Nanotechnology is modern science, and molecular biology has significantly benefited from research in this subject. The inclusion of nanotechnology in the development of genetically modified (GM) organisms (GMOs) represents a powerful tool involving the use of NPs as nanocarriers by producing a binding complex with biomodifier molecules (CRISPR/Cas system) and delivery into plant cells (Abd-Elsalam, 2020; Demirer et al., 2021). The implementation of nanotechnology in the existing molecular technologies could also create a forum for overcoming barriers to produce genetically engineered plants as well as for biotransformation (Cunningham et al., 2018; Gad et al., 2020). Nanomaterial (NM) engineering has emerged as a cutting-edge technology to develop crops for sustainable farming systems (Panpatte et al., 2016). The development of nanodevices and NMs can reduce the effect of significant stresses on food and energy production while maximizing the use of limited resources, including water or nutrients (Giraldo et al., 2019).
Nanobiotechnology techniques have improved the precision of plant breeding in generating exciting new possibilities for gene selection and transition, reducing the time required to remove unwanted genes and enabling the breeder to access essential genes from large plantations (Pérez-de-Luque, 2017). The magnetofection of the bioconjugated complex of transgene and NPs have successfully been reported in dicots (Zhang et al., 2019b). Here, nanotechnologies that enable the force-independent supply of DNA without integrating transgenes indicate that the transient expression of CRISPR techniques can be used in most countries for permanent GM-free GE (Figure 1; Li et al., 2018). The main steps in GE include introducing transgenes or CRISPR/Cas9 into plants via specific gene delivery systems (Hansen and Wright, 1999; Grunewald et al., 2013). Gene transformation delivery using nanotechnology is superior to traditional biomolecular approaches mainly because the former enhances the transformation efficiency for both temporal (transient) and permanent (stable) genetic modifications in various plant species (Serag et al., 2013; Vanhaeren et al., 2016; Novák et al., 2017). Hence, nanotechnology can reduce uncertainty and help coordinate the management strategies of agriculture using molecular production approaches as an alternative to conventional technologies (Figure 1).
NPs have begun to facilitate and enhance GE via an efficient and targeted delivery of plasmids, RNA, and ribonucleoproteins (RNPs). In mammalian cells, NPs are routinely used for the efficient, direct cytosolic/nuclear delivery of Cas–RNPs in many cell types (Mout et al., 2017). RNP delivery has been shown to greatly reduce off-target effects compared with plasmid-based CRISPR systems (Liu et al., 2017). However, in plants, the cell wall has hindered the development of an analogous system that can passively deliver GE cargo into mature plants. Thus, there remains much potential for designing NP carriers (DNA, RNA, and proteins) with diverse cargo-loading capabilities and optimal geometry/chemistry to efficiently bypass the cell wall and membranes in dense plant tissues without external aid. A previous work (Burlaka et al., 2015) showed that some NP formulations undergo passive internalization in plants with DNA, RNA, or protein cargo (Demirer et al., 2018).
With the discoveries of several advanced technologies, the need to develop short-term crops to feed the ever-increasing population daily remains pertinent. This review presents a concept to hasten the existing crop improvement technologies by combining them to produce efficiently and high-yield improved crop plants. Speedy crop improvement can be achieved using CRISPR under speed editing strategies via NMs combined with speed breeding (For speed breeding, see the detailed review by Watson et al. (2018). This review first describes the nanobased delivery methods and plant genetic engineering techniques along with NP-mediated genetic engineering challenges in addition to the speedy crop improvement concept. Further challenges and the future use of nanotechnology are also described in detail. The overall goal is to provide a comprehensive summary of plant-related CRISPR techniques that incorporate nanotechnology and consider future application prospects for crop improvement.
Conventional Plant Biomolecule Delivery Approaches and Their Limitations
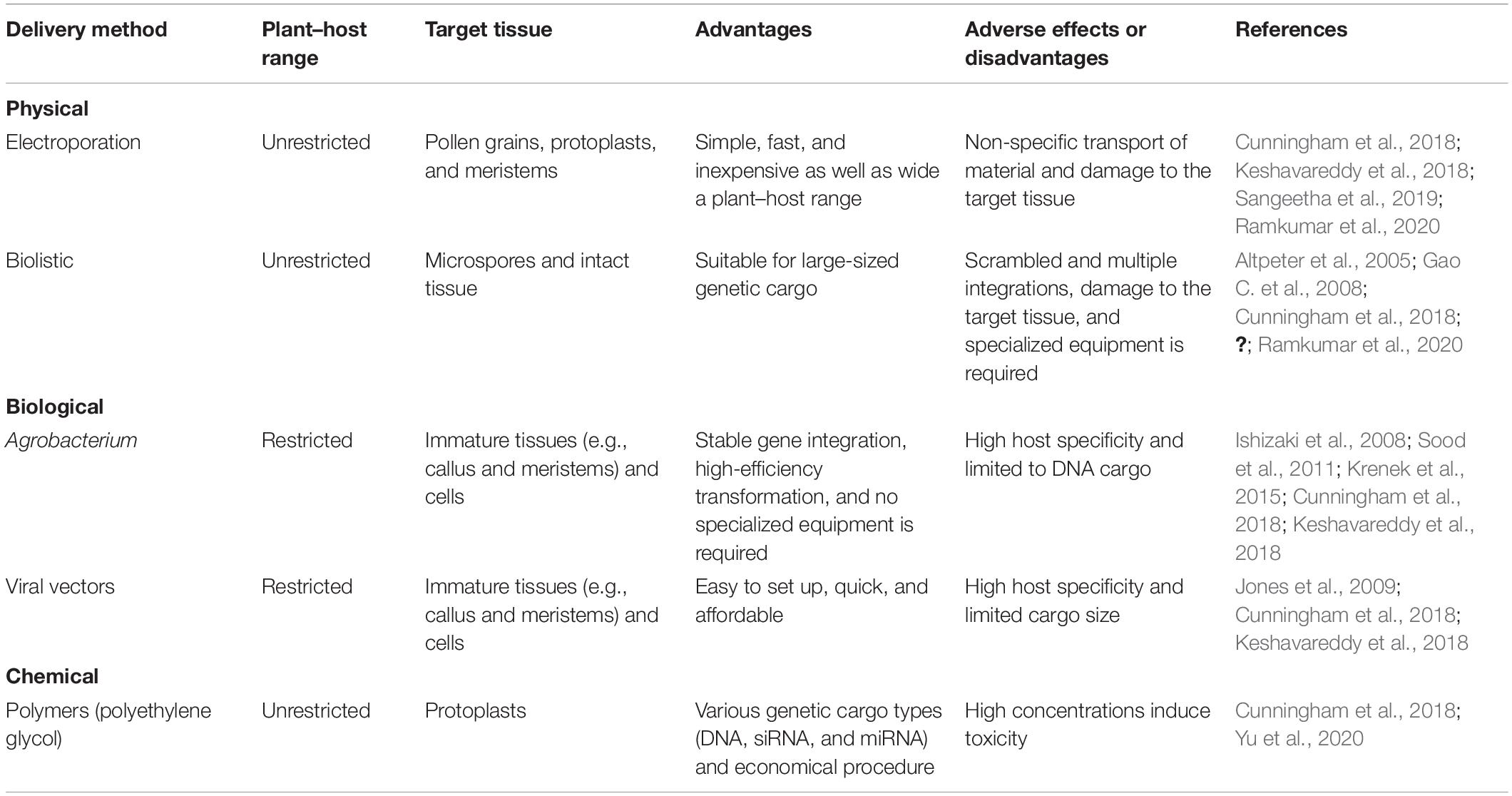
The genetic transformation of plants involves two main steps: genetic cargo delivery and the regeneration of transformed plants. Here, the regeneration capacity depends on the biomolecule delivery method employed and whether a stable transformation is desired (i.e., constitutive or transient; Cunningham et al., 2018). Various biological, chemical, and physical methods are available to deliver genetic materials into plant cells, including the aforementioned AMT, viral-mediated transformation, polymer-mediated delivery (e.g., polyethylene glycol [PEG)]), particle bombardment (gene gun-mediated or biolistic transformation), and electroporation (Ahmed et al., 2018; Mohammed et al., 2019; Shin et al., 2019; Tian et al., 2019; Imai et al., 2020; Ozyigit, 2020). The features of the conventional transformation methods are summarized in Table 1. Gene gun-mediated transformation and AMT are among the most efficient and commonly utilized gene delivery methods for plant-related genetic transformation. These methods have been adopted for various crops, including soybeans (Li et al., 2017; Zhao et al., 2019; de Melo et al., 2020), sorghum (Che et al., 2018; Liu G. et al., 2019; de Melo et al., 2020; Sharma et al., 2020), maize (Char et al., 2017; Anand et al., 2018; Raji et al., 2018), sugarcane (Mayavan et al., 2015; Wu et al., 2015; Dessoky et al., 2021), wheat (Liang et al., 2018; Kumar et al., 2019; Zhang et al., 2019c), and rice (Endo et al., 2015; Ling et al., 2016; Feng et al., 2017).
Despite more than three decades of development, plant transformation and regeneration remain a challenge in several crop plants. AMT has proven to be more efficient for dicots than monocots (Sood et al., 2011; Van Eck, 2018; Mohammed et al., 2019) and is limited to a specific plant–host range (Cunningham et al., 2018; Demirer et al., 2019a). For example, AMT efficiency tends to be highly variable (6–99%) depending on the variety and subspecies of rice (Mohammed et al., 2019). In general, monocots are considered recalcitrant for Agrobacterium tumefaciens-mediated transformation (Sood et al., 2011; Hiei et al., 2014; Hofmann, 2016; Mookkan et al., 2017). However, the AMT method has undergone several changes to optimize monocot genetic modification (Hiei et al., 2014; Singh and Prasad, 2016; Anand et al., 2018). For example, the use of hypervirulent strains with standard or superbinary vectors has been shown to improve the transformation efficiency of the AMT method (Shrawat and Lörz, 2006; Singh and Prasad, 2016). In addition, Anand et al. (2018) developed a ternary vector system that has a high transformation frequency in an elite maize inbred line.
One of the advantages of the biolistic method over AMT is related to the variety of species transformed by the former (Matsumoto and Gonsalves, 2012; Cunningham et al., 2018). In general, this method is preferred for rapid assays using transient expressions, such as protein localization, the functional analysis of promoters, and transcription factor characterization (Lenka et al., 2015, 2018; Wang et al., 2018). The particle bombardment method enables the delivery of DNA sequences > 150 kb, albeit with the possible compromise in DNA integrity (Chandrasekaran et al., 2020). Moreover, gene gun-mediated plant transformation can result in scrambled and multiple integrations (Altpeter et al., 2005; Gao C. et al., 2008). Viruses have been used as a vector to introduce foreign genes into various crops (Meziadi et al., 2017; Bouton et al., 2018). In general, viral vector systems are developed for transient expression analyses.
Of note, several viral vectors have been specifically developed to transform plants recalcitrant to AMT, such as monocots (Bouton et al., 2018). Meanwhile, virus-mediated transformation is limited by the virus’ host specificity (Jones et al., 2009). Electroporation is less frequently used than other plant transformation methods, whereas an efficient transformation has been achieved in terms of monocots and dicots (Barampuram and Zhang, 2011; Ozyigit, 2020). Similar to viral vector-mediated transformation, electroporation-mediated transformation has largely been used for transient analyses and the investigation of gene functions at the cellular level (Ramkumar et al., 2020).
Along with biolistic methods, PEG-mediated transformation is one of the most commonly used methods for introducing genetic cargo into chloroplasts (Yu et al., 2020). This method enables the carrying of several genetic cargo types, such as DNA and RNAs (small interfering RNA [siRNA] and miRNA; Cunningham et al., 2018). However, it requires regeneration from protoplasts, which is highly challenging because of the limited number of plant species amenable to protoplast regeneration.
Traditional biomolecule delivery methods have several drawbacks, including limited cargo type, narrow species range, low efficiency, and the potential for tissue damage. Novel strategies are therefore needed for efficient gene delivery in crop plants. Tissue culture and regeneration steps are the principal constraints in plant transformation. Clough and Bent (1998) developed the floral dip method that involves directly dripping flower buds into an Agrobacterium suspension (or an Agrobacterium inoculum is dropped onto the buds) while avoiding cell or calli culture. This plant transformation method has been adopted for several important crops, including maize (Mu et al., 2012), rice (Rod-In et al., 2014; Ratanasut et al., 2017), and rapeseed (Li et al., 2010). However, as noted by Imai et al. (2020), the existing protocols can involve low reproducibility.
Advanced Plant Biomolecule Delivery Approaches Via the Application of Nanobiotechnology
Nanotechnology-based methods have been proposed as inexpensive, easy, and robust techniques to transfer genes or other molecules into plants with high efficiency and low toxicity (Chandrasekaran et al., 2020). Nanotechnology has significantly impacted various research fields, including medicine, energy, and manufacturing. Nanotechnology-based methods have been used to deliver biomolecules and chemicals into cells in both plant and mammalian cell systems (Chang et al., 2013; Wang et al., 2014; Mahakham et al., 2017; Fortuni et al., 2019); however, compared with the mammalian cell delivery process, NP-mediated plant biomolecule delivery has proven to be more challenging because of the presence of the natural barrier provided by the cell wall (Mao et al., 2019). It has been suggested that the use of NPs enables an efficient plant transformation because NPs protect the genetic cargo from cellular enzymatic degradation (e.g., nucleases; Finiuk et al., 2017; Joldersma and Liu, 2018). NPs for gene delivery are classified according to the base material used and include carbon-based NPs, silicon-based NPs, metallic NPs, and polymer-based NPs. Each NP type delivers different genetic cargos. For example, carbon nanotubes (CNTs) can carry RNA and DNA (Bates and Kostarelos, 2013; Karimi et al., 2015), but metallic NPs can only deliver DNA as genetic cargo (Zhao et al., 2017). In addition, silicon-based NPs can carry DNA and proteins, whereas polymeric NPs (e.g., PEG and polyethyleneimine) can transfer encapsulated RNA, DNA, and proteins into cells (Silva et al., 2010; Moon et al., 2011; Su et al., 2011; Hasanzadeh Kafshgari et al., 2015; Zhou et al., 2018).
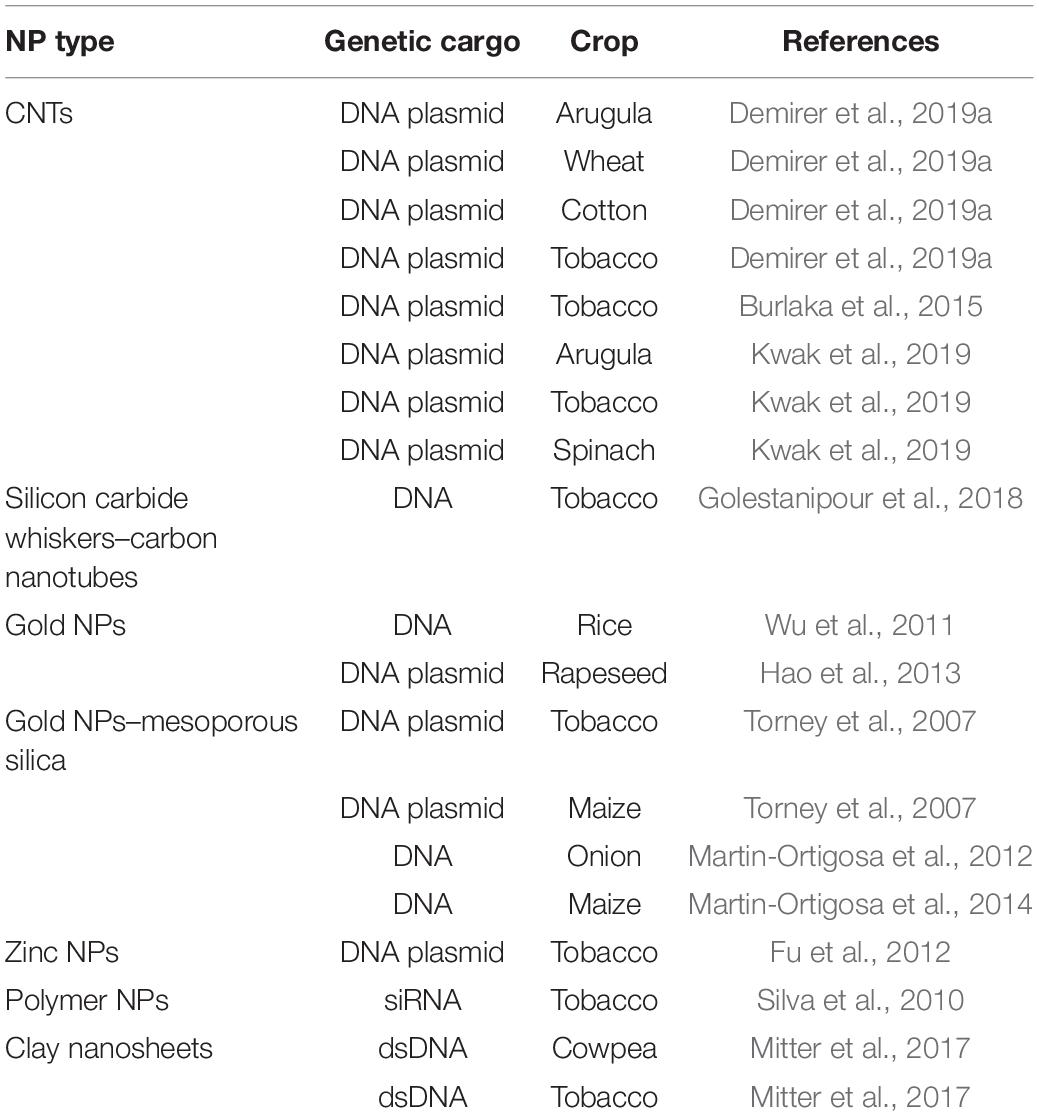
Overall, NPs should be capable of crossing the cell wall and localizing into organelles. Cationic NPs are preferred for plant gene delivery because this NP type can bind to the plant cell wall (negatively charged) and perform gene transfer (Albanese et al., 2012), whereas CNT NPs have been used to deliver plasmid DNA into various crops (Table 2 and Figure 2). However, NPs generally require additional physical methods (e.g., magnetoinfection and electroporation) for gene delivery into plant cells. By contrast, NPs, such as silicon carbide whiskers (SCW) and mesoporous silica NPs (MSN), have been effectively used to transfer genes into the plant without using other physical methods (Chang et al., 2013). Here, SCW-mediated transformation has been successfully used to transform tobacco (Golestanipour et al., 2018).
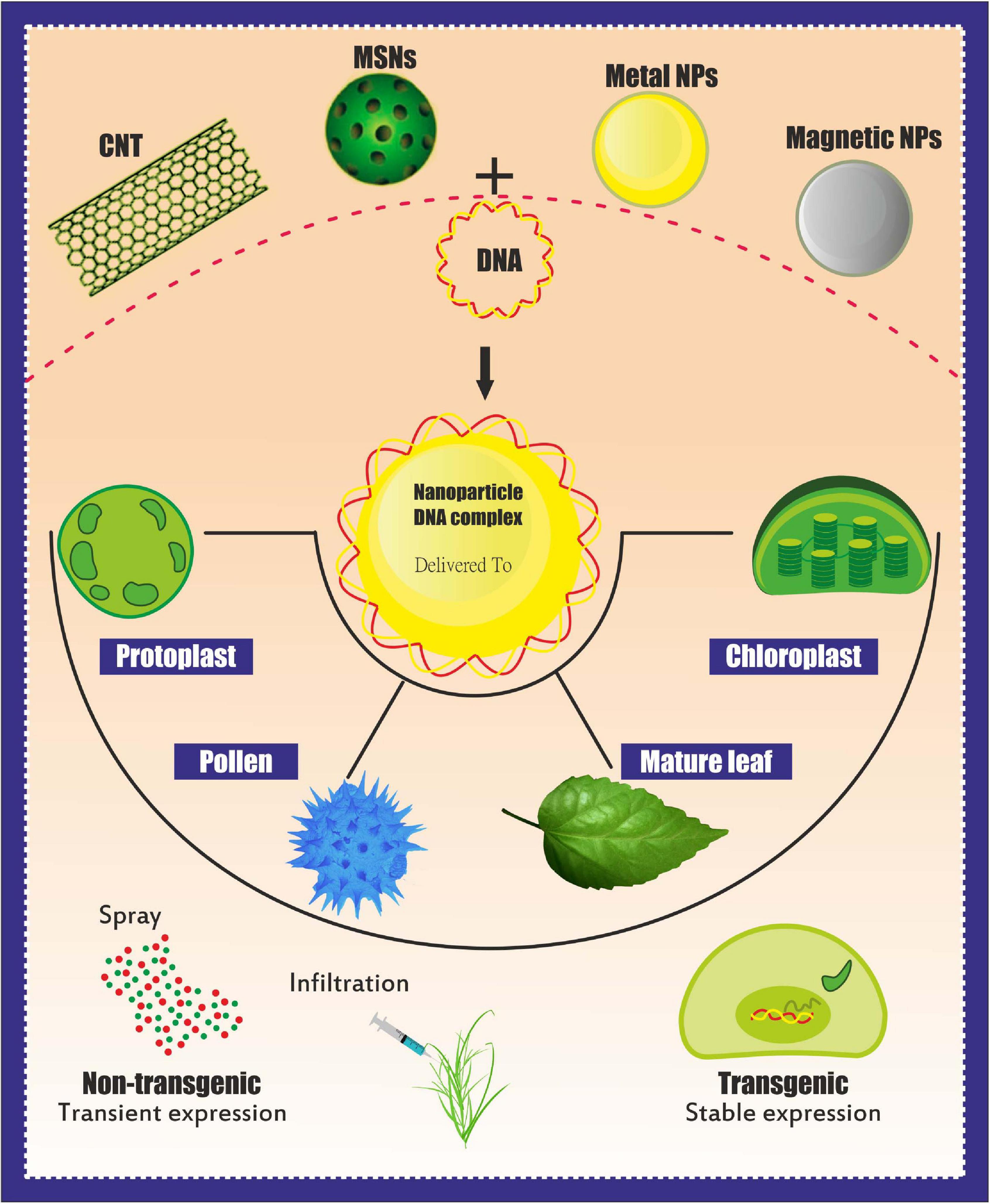
Figure 2. Different-shaped nanoparticles (NPs) for use as genetic cargo for genome editing. The shape and size can be engineered to bind specific biomolecules to produce the most stable bioconjugate complex. NPs can also be used in force-free delivery, i.e., using magnetic properties and electric field usage for penetration (Jat et al., 2020).
Nonetheless, in general, the SCW method has one disadvantage compared with other NP-mediated plant transformation in that an adequate protocol is required for plant regeneration from cell cultures. Polymer NPs can also deliver nucleic acids into plant cells. Of note, Silva et al. (2010) used polymer NPs to introduce siRNA into tobacco protoplasts, providing an alternative gene knockout mechanism in plant cells.
Several NPs can penetrate the cell wall (e.g., CNTs and mesoporous silica), whereas other NPs require chemical or physical pretreatments, such as gold NPs and magnetic NPs (MNPs), for genetic cargo delivery into the cells. Meanwhile, NP-mediated passive delivery has been reported with tobacco (Burlaka et al., 2015; Mitter et al., 2017; Golestanipour et al., 2018; Kwak et al., 2019), cowpea (Mitter et al., 2017), and arugula crops (Kwak et al., 2019). NPs and other new materials might serve as useful vehicles for editing systems (Gao, 2021). Working within this context, Hamada et al. (2017) proposed a method involving plant bombardment in which the shoot apical meristems of wheat were used as the target tissue (Imai et al., 2020). In this study, gold particles coated with the green fluorescent protein gene construct were delivered into the L2 cell layer of the shoot apical meristems of wheat. This approach provided a stable transformation in wheat without embryogenic callus culture and can be applied to other crops that have not been successfully transformed via the conventional methods.
Role of Nanotechnology in Agriculture
Current farming techniques, established during the green revolution, have proven to be largely untenable within the backdrop of the increasing population and climate change (Lowry et al., 2019). Nanotechnology presents reliable solutions for tenable farming, such as encompassing effective pest management and nutrient use, decreasing the impact of environment in food production, and alleviating the effect of climate change (Hofmann et al., 2020). Plant nanotechnology is a flourishing domain in which engineered NMs have been established for analyzing plant functions (Wang et al., 2016, 2019; Giraldo et al., 2019; Kah et al., 2019; Lowry et al., 2019). Meanwhile, NMs are becoming a convenient medium for introducing biomolecules in plants and can be modulated to direct their translocation and distribution in plant cells and organelles (Torney et al., 2007; Kwak et al., 2017, 2019; Demirer et al., 2019b).
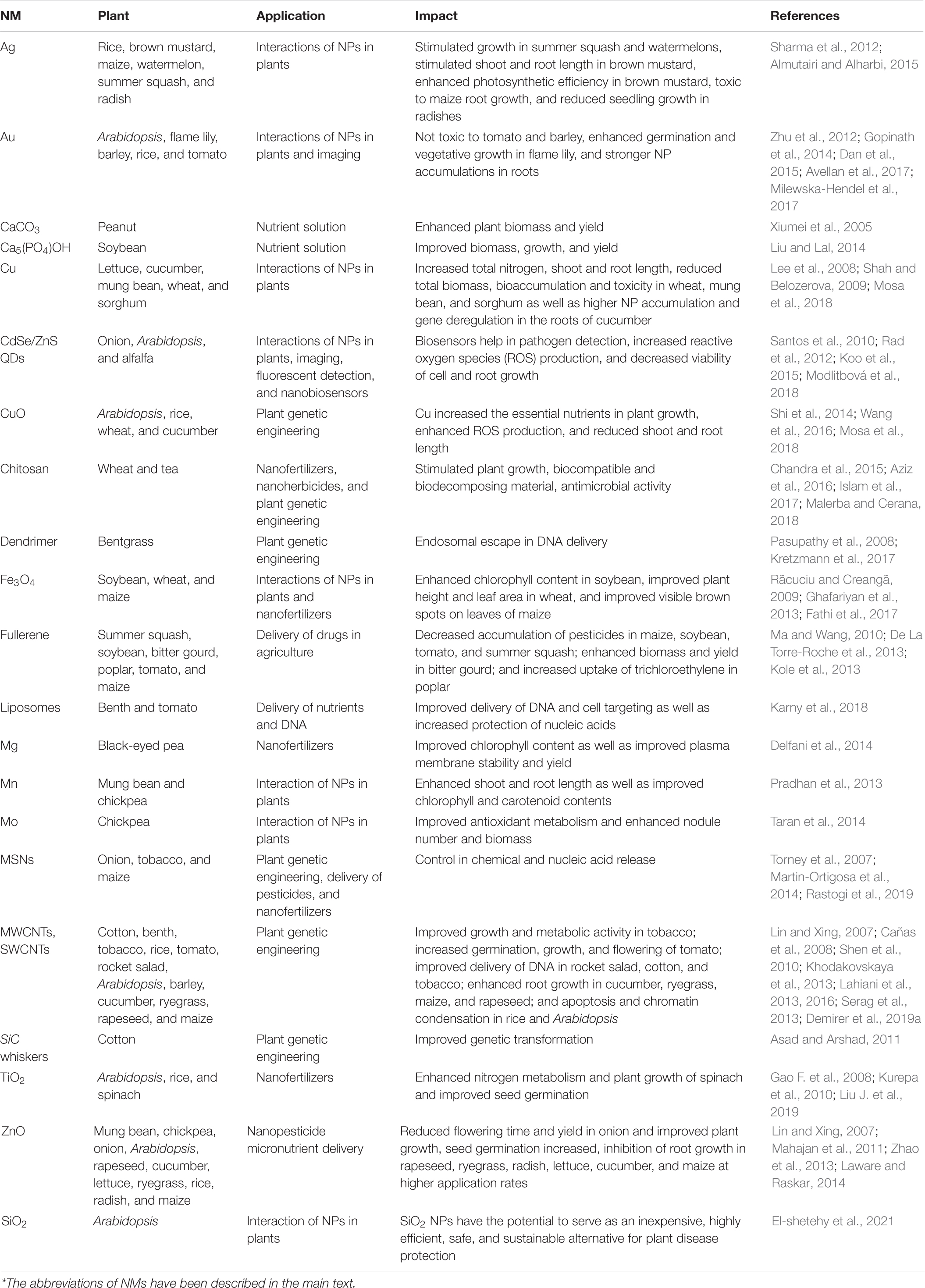
Thus far, various NMs have been assessed, including nanofertilizers (employing a thin coating of NMs on plant nutrients and delivering in the form of nanosized emulsions), nanopesticides (tiny molecules that are the only constituent of pest control derivatives and/or entraps the active constituent of pesticide into a protective nanocarrier), and nanobiosensors (nanobiosensor synthesized from the combination of nanotechnology and biosensors, equipped with immobilized bioreceptor probes; e.g., antibodies and enzyme substrate; Usman et al., 2020). Crop production and soil health can be enhanced using different types of NM. Nanofertilizers are regarded as micronutrients or macronutrients and act as transporters for added substances via the incorporation of minerals for the nutrients (Kah et al., 2018). DeRosa et al. (2010) stated that nanofertilizers are also effective in confining nutrients inside the NMs and Kah et al. (2018) have observed an 18%–29% increase in the efficiency of nanofertilizers compared with that of synthetic fertilizers. Iron, manganese, zinc, copper, molybdenum, and silver can be used to improve transportation systems, thereby enhancing the assimilation and efficiency of synthetic fertilizers (Liu and Lal, 2015). The different doses of silver NPs significantly enhance the rate of seed germination in maize, Citrullus lanatus (watermelon), and Cucurbita pepo L. (pumpkin) crops by having a small toxic impact leads to seed germination (Acharya et al., 2020; Wang F. et al., 2020). Meanwhile, Lactuca sativa (lettuce) germination is often improved via titanium dioxide NM electrospraying, although studies have revealed that NMs can remarkably decrease fertilizers’ application in the face of both soil and foliar application, thereby enhancing the efficacy and reducing discharge into the environment compared with synthetic formulations (Adisa et al., 2019).
Synthetic pesticides can be replaced with nanopesticides with a higher potential capacity. The gradual degradation and precise discharge of active components with appropriate NMs can enhance pest management efficacy over long periods (Chhipa, 2017). Therefore, nanopesticides are vital for the effective and tenable management of various pests and can reduce the usage of agrochemicals and, as such, mitigate the existing environmental hazards. These pesticides behave differently from synthetic pesticides, which enhance their efficiency (Kah et al., 2019). The dissolving power of active components could enhance the movement and degradation of soil-inhabiting microorganisms. Furthermore, NP-based pesticides improve the solubility of aluminum and are less hazardous to the environment than synthetic pesticides (Kah and Hofmann, 2014).
In general, nanopesticides bind water and energy as they are released to a small extent and less frequently than synthetic pesticides. They also improve pesticide efficacy and crop production because of greater yields and lower input costs by decreasing waste and labor costs. NPs also exhibit a well-organized antimicrobial activity against viruses and bacteria. Silver, copper, and aluminum are considered vital inorganic NPs with good pesticide properties (Gogos et al., 2012; Kim et al., 2012; Stadler et al., 2012). The efficacy of herbicides can be increased via nanoherbicides that generally anticipate biodegradable polymeric components. For example, poly(-caprolactone) is widely used to contain atrazine owing to its better physiochemical properties and greater bioaccessibility and biocompatibility (Abigail and Chidambaram, 2017). Synthetic chemicals can be introduced in hosts using a conveyer system based on CNTs (Raliya et al., 2013) after targeting a decrease in the number of chemicals discharged into the environment that may damage other plant cells (Hajirostamlo et al., 2015).
Nanobiosensors are more substantial and are associated with next-generation sensors that detect different elements at ultra-low concentrations via a physiochemical transducer (Scognamiglio, 2013). In short, nanobiosensors can enable plant protection by allowing plants to converse with farmers (Giraldo et al., 2019; Wang et al., 2019), which could help ensure timely decision-making to improve crop productivity via appropriate water, land, fertilizer, and pesticide management. Nanobiosensors have a longer shelf-life than older-generation sensors owing to their greater stability and sensitivity, fast electron kinetics, and higher surface-to-volume ratio (Scognamiglio, 2013). Different nanosensor types have been used in plants, including plasmonic, fluorescence resonance energy transfer-based, carbon-based electrochemical, nanowire, and antibody nanosensors. They can be used to detect substances, such as urea, glucose, and pesticides, monitor metabolites, and detect various microorganisms or pathogens (Rai et al., 2012). Using different NMs, the delay in plant nanotechnology could be controlled. This could be achieved by using smart NMs and NPs, which could ultimately revolutionize the farming industry (Table 3 and Figure 3).
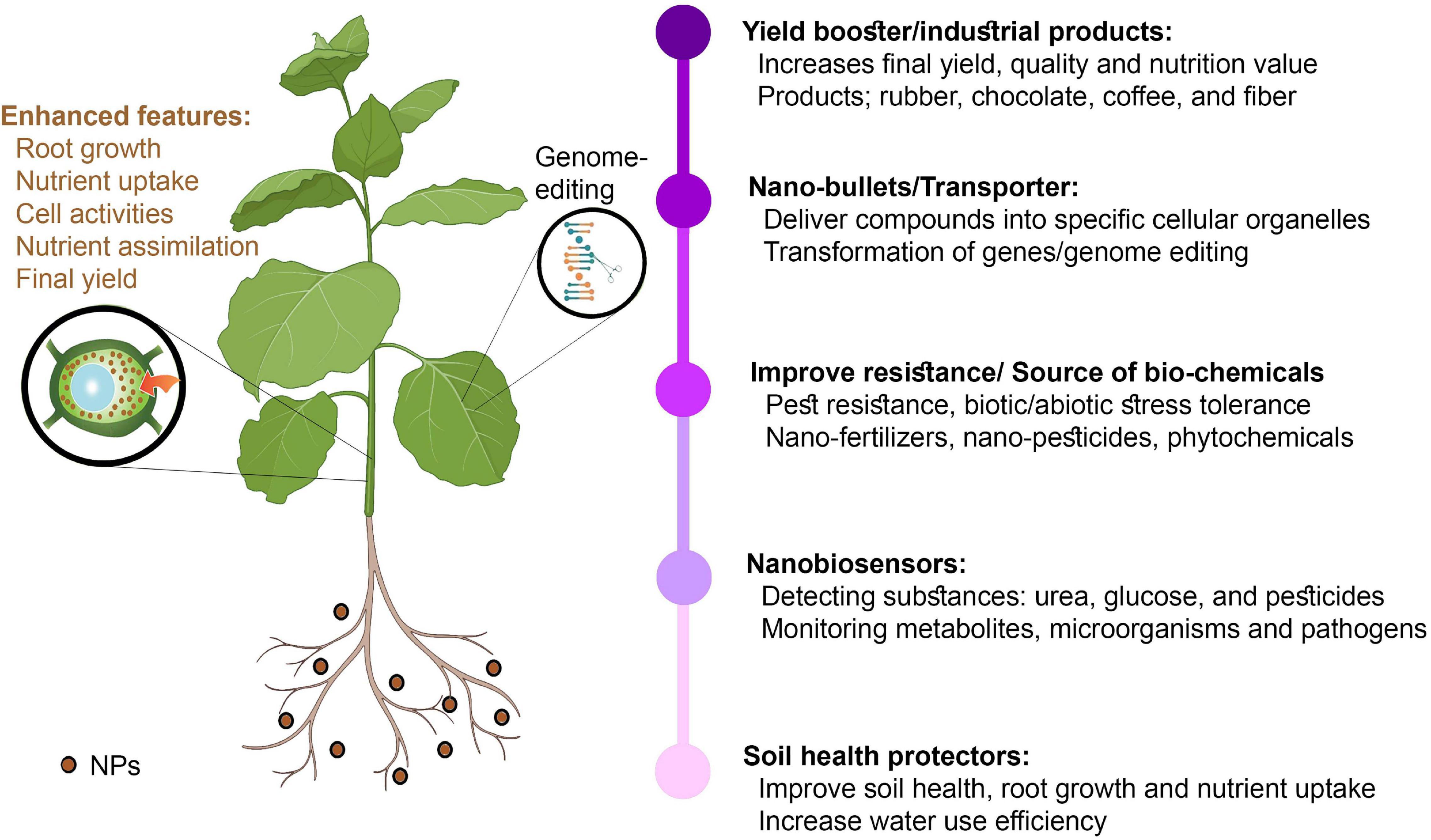
The focused NP distribution mechanisms of cellular organelles have been highly successful. However, other plant sections do not influence NP particles because they function as silent bullets to release compounds into specific cellular organelles (de Oliveira et al., 2014; Saranya et al., 2019). Several NPs can improve the photosynthetic system. The delivery of NMs is directed at either the plant roots or vegetative parts, whereas the primary focus is the leaves (Usman et al., 2020). The leaf lamina penetration approach could enhance NP infiltration in plant tissues (described for single-walled CNTs) and has proven to be applicable for gene delivery (Giraldo et al., 2014; Demirer et al., 2019b). Nanotechnology could help in developing faster manufacturing and industrial processes. The major tropical crop farmers, such as rubber, chocolate, coffee, and cotton farmers, will accomplish new goals that could lead to a new and enhanced nanoeconomy. GM crops will contribute to the new levels of sugar stopping, providing customers with many options. A programmed and centrally regulated industrial and agricultural sector can now be fulfilled via molecular sensors, automatic distribution systems, and low-cost technologies.
NPs have been used in various plants, including fruits, such as Avena sativa L. (Armendariz et al., 2004), blackberry (Nadagouda et al., 2014), Citrus sinensis L. (Sujitha and Kannan, 2013), olive (Khalil et al., 2012), and pear fruit (Ghodake et al., 2010). In particular, nanocalcium improves “Red Delicious” properties in apple fruit (Ranjbar et al., 2020). Recently, MNPs have been identified with antifungal properties that could be utilized in various fruit-bearing tree plants, including apples, pears, grapes, and citrus fruits, as well as other industrial crops. MNPs can also serve as biosensor particles to detect various biochemical disruptions in plants and humans (Thakur et al., 2020). The functional utilization of NPs is not limited to specific crops; moreover, NPs have a wider utilization and adoptability from medicinal and industrial crops to fruits and woody trees.
Challenges in GE and NP-Mediated GE in Plants
The GE technique modifies plant cell genomes, involving the efficient delivery of modifier biomolecules as genetic cargo to targeted plant cells (Demirer and Landry, 2017; Nandy et al., 2020). However, the available biomolecule cargo delivery techniques are non-efficient, causing a lag in genetic transformation. Moreover, these methods have several limitations that hinder robust GE because of non-specific site integration, damage to plant tissues, non-significant gene expression after integration, tissue specificity, and species specificity (Altpeter et al., 2016). These techniques are available for the narrow host range and cause postmodification regeneration and fertility problems in transgenic plants. Methods, such as AMT and gene gun-based transformation, have certain limitations of use, making them non-versatile for general use. However, they are now well-established and have produced numerous successes. With the development of multiplex GE using the CRISPR/Cas9 technique (Ma et al., 2015), research on GE has been significantly progressed. However, certain complications remain, which limit the robust delivery of genetic cargos. One of the main obstacles here relates to how plant cells have an additional cell wall compared with animal cells, which provides them with rigidity, definite shape, and growth potential while acting as a physical barrier from environmental conditions (Cosgrove, 2005). The delivery of biomolecules to plant cells for GE remains a bottleneck owing to the physicochemical properties of the cell wall (Azencott et al., 2007). A plant cell wall mainly contains complex polysaccharides with a pore size varying from 3.5 to 5.2 nm, which provides rigidity (Carpita et al., 1979). Because of this narrow pore size and rigid structure, many genetic cargos cannot pass through it. Although AMT is widely used for genetic transformation, its efficiency depends largely on the host species and leads to undesired DNA integration in the host genome (Baltes et al., 2017). In view of the abovementioned issues, NPs have emerged as the best genetic cargo material because of their ease of use and success in several cases (Cunningham et al., 2018; Wang et al., 2019).
The unmatched potential of the NP-based delivery of biomolecules to plant cells (Deng et al., 2019) has revolutionized the GE delivery process (Deng et al., 2019; Landry and Mitter, 2019). In this method, the NP-bound GE nuclease is efficiently transferred to plant cells without causing damage to the target tissue. The use of NP-based methods instead of the conventional methods of genetic cargo delivery has emerged as a part of a cutting-edge technology that provides new insights and a robust GE. The NP-mediated transfer of biological molecules to plant cells has abrogated all issues previously hindering the success of GE, and it thus presents a promising technique for enhancing the efficiency, robustness, and versatility of GE (Cunningham et al., 2018). Due to their small size, NPs can transverse the cell wall and overcome barriers to delivering biomolecules to plant cells.
Despite its significant importance, certain challenges are hindering the effective use of NPs in GE. The first relates to nanophytotoxicity (Cox et al., 2016). Nanophytotoxicity is defined as the negative effect of NMs on plant growth, causing damage to either the plant or the environment because of the subsequent release of NMs up to a toxic level (Figure 4). Various studies have demonstrated that the uptake of NPs by plants results in some phytotoxicity due to the blockage in the plant vascular system, resulting in structural damage to the plant’s DNA and inducing oxidative stress (Pachapur et al., 2016; Du et al., 2017; Rastogi et al., 2017). The reproductive growth of plants is also negatively regulated by the toxicity of silver NPs (Dutta Gupta et al., 2020). Nevertheless, increases in leaf and root growth as well as improved chloroplast production have been observed after NP-based transformation (Cox et al., 2016; Zuverza-Mena et al., 2017). the translocation, deposition, and culture of nanotoxic-free plants in subsequent generations need to be addressed. Here, although the amount of engineered NPs required as genetic cargo is significantly less than the toxic level in terms of both the environment and the plant, their deposition and dispersal to other plant cells after application require further research.
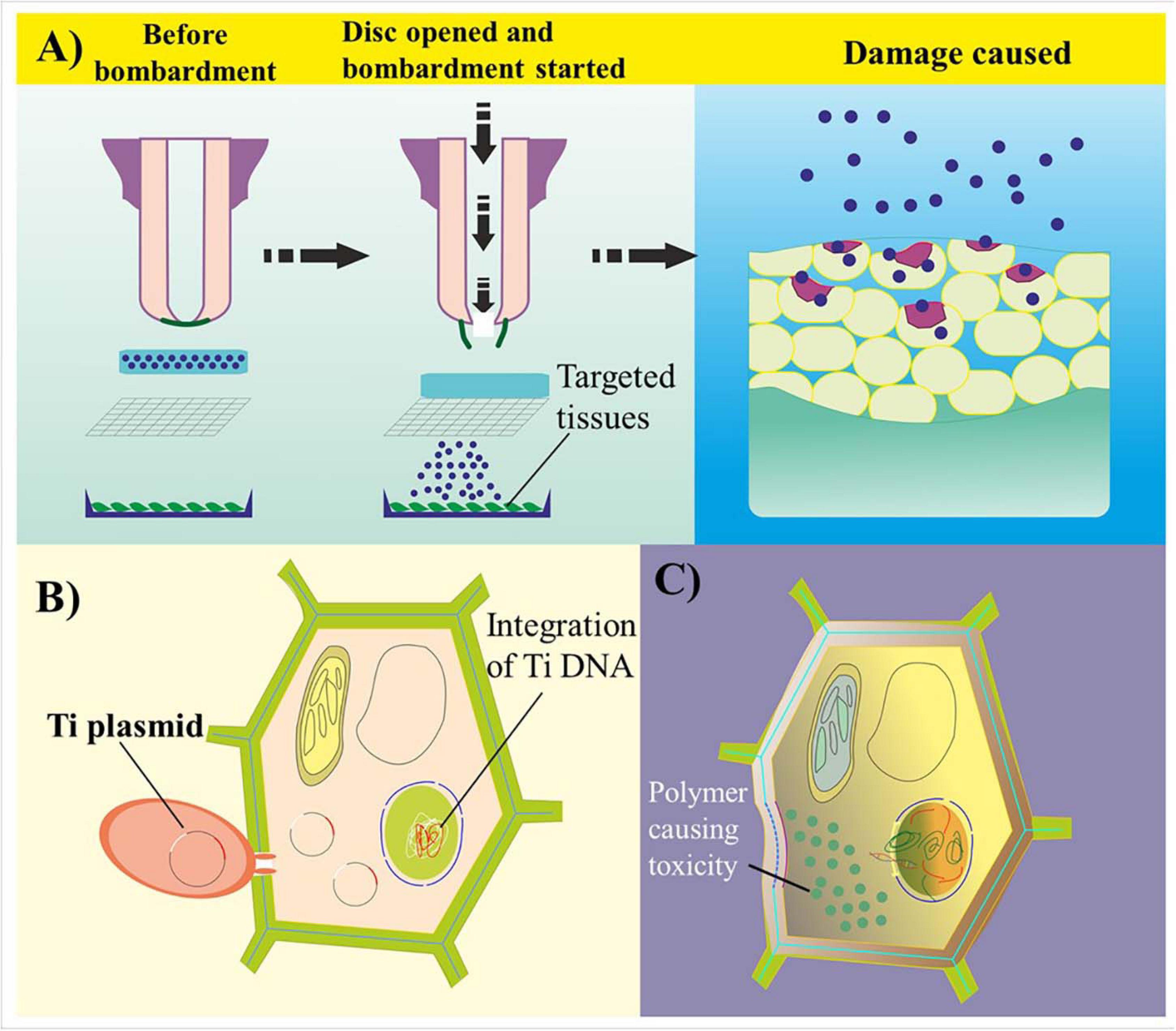
Figure 4. Common challenges in genome editing- and nanoparticle-mediated plant transformation. (A) Biolistic delivery of biomolecule-coated particles into targeted plant cell tissues. Because of the unavoidable high velocity of genetic cargo, the bombarded particles damage the cell wall through penetration and disrupt cell homeostasis. (B) Transformation of plant cells via Agrobacterium-mediated transformation. The T-DNA of Agrobacterium integrates within the host genome, causing a tumor or a change in the genetic information of the transformed cells. (C) Polymer-based transformation leads to cytotoxicity in plant cells because of the accumulation of high-density charged polymer-based genetic cargo. A reduction in charge leads to an impairment in the bioconjugated complex.
Different NPs can behave very differently in specific plant cells, which require optimizing their application for different plant species and their dose and spatiotemporal tuning. In plant cells, NM deposition results in extra reactivity, dynamic transfer to other plant parts, and instability (Lv et al., 2019). Several NPs have high oxidative properties, which lead to a disturbance in normal cell metabolism and interfere with the genetic regulation of plant cells, resulting in the oxidative rupture of the transformed cells (Hossain et al., 2015; Du et al., 2017). Their optimization for successful use as genetic cargo for the successful application in plant cells is crucial. At the same time, there should be no or, at least, minimum interference from NPs in cellular processes. Cell structural stability and metabolic pathway disturbance is another challenge that needs to be researched to improve the use of NPs as genetic cargo (Hossain et al., 2015; Lowry et al., 2019). However, studies have reported an efficient delivery of biomolecules for gene silencing in plants with no toxicity and no physiological disturbance or metabolic hindrance after CNT-mediated gene silencing and transient expression in mature plants (Demirer et al., 2018). Given that plant species and their tissues have different cell structures, the broad-spectrum application of NP-mediated delivery remains a challenge.
Another challenge for NP-mediated GE’s efficacy relates to the efficient binding of biomolecules to NPs and the disintegration of the binding complex in plant cells (Saptarshi et al., 2013; Fleischer and Payne, 2014). Different biomolecules have a different binding affinity with other NPs based on their structure, charge, chemical composition, and surface area, making them ideal for a bioconjugation complex. The interaction between EMNs and plants largely depends on intrinsic properties, such as chemical composition, spatiotemporal occurrence, shape, size, hydrophilic or hydrophobic nature, and crystalline structure (Nel et al., 2009; Dasgupta et al., 2014). NPs can also be charged, and their surface can be designed to bind with diversely shaped biomolecules and hence can be an excellent platform for delivering biomolecules (Dasgupta et al., 2014; Hu et al., 2020). Moreover, NPs can be engineered to mediate cargo delivery to any subcellular parts that AMT cannot target, such as mitochondrial or chloroplast DNA. They can also be used without the species- and tissue-specific limitations of the previously available biomolecule delivery methods. However, their optimization for binding specific biomolecules requires further research to enhance their versatility as genetic cargo.
For the promising future of NP-mediated GE, scientists have attempted to understand how an NP-biomolecule bioconjugated complex will be delivered in a force-independent manner (Busch et al., 2019). Such nanocarriers have been studied for their specific delivery to plant organelles without damaging the transformed cells and having the least residual effect on the daughter cells with no toxic impact on the plant or the environment (Hu et al., 2020). Plant nanobiotechnology is an emerging field and requires input from all scientific fields, including biochemistry, molecular biology, biophysics, and structural chemistry. After optimizing the dose, the delivery method, and the NP type, it will be possible to establish a complete revolution in delivering genetic cargo based on nanocarriers.
Speedy Crop Improvement Coupled With the Application of Nanobiotechnology
A speedy crop can be produced using CRISPR under speed editing strategies by incorporating NMs to combine with speed breeding. One significant issue of the CRISPR gene-editing technologies for agricultural applications is that transgenic plants must be free of target genetic alteration to preserve the stability of the traits and secure regulatory clearance for commercial development (He and Zhao, 2020). Nanotechnology can help distribute genetic materials to plants to enable genetic engineering and stabilize genetic materials, including improving their double-stranded RNA efficacy for plant improvement (Hofmann et al., 2020). Furthermore, NMs used for grafting can be leveraged, and relevant biomolecules can be subsequently delivered for GE via plant cells owing to the difficulties in transporting exogenous biomolecules across cell walls (Wang et al., 2019). Demirer et al. (2019b) devised a tool for the species-independent, targeted, and passive delivery of genetic materials into plant cells without transgene integration for diverse plant biotechnology applications. Here, the authors demonstrated the efficient diffusion-based biomolecule delivery of CNTs into several mature plant species with a suite of pristine and chemically functionalized high aspect ratio NMs. Efficient DNA delivery and strong transient protein expression were accomplished in mature Eruca sativa (arugula-dicot) and T. aestivum (wheat monocot) leaves and protoplasts. In addition, Demirer et al. (2019b) demonstrates a second NP-based strategy in which low interfering RNA (siRNA) is delivered to mature Nicotiana benthamiana leaves to silence a gene with 95% efficiency. The developments in plant transformation include the delivery of DNA using polyethyleneimine-coated iron oxide MNPs as carriers and the application of a magnetic force to direct the MNP–DNA complexes into the pollen of cotton before pollination (Zhao et al., 2017). NPs can potentially deliver gene-editing cargos to any plant cells, including meristematic cells (Mohamed and Kumar, 2019; Sanzari et al., 2019; Wang L. et al., 2020). The delivery of GE reagents via NPs into meristematic cells can potentially generate chimerically edited plants. Transgene-free and edited plants can be regenerated from the edited tissue via tissue culture or by propagating cuttings. Elsewhere, a recent exciting report indicated that plasmid-coated carbon dots could be delivered into plant cells via foliar application (spraying on). The Cas9/gRNAs produced via this method successfully edited target genes (Doyle et al., 2019). The development of nanobiotechnology has presented new ideas for transgenic approaches using NPs as the gene carriers. However, it remains challenging to establish GM crops quickly and easily. This obstacle can be overcome by using a combination of existing technologies. A speedy crop improvement has been proposed as the best strategy for addressing these challenges. However, it is unclear how this speedy crop improvement process will work. In fact, there are four steps to perform a speedy crop breeding. First, we can select the best candidate(s) using speed editing strategies (CAFRI-Rice)1 based on protein sequence similarity and coexpression trends among homologous candidate genes with functional redundancy to enable more efficient multiple GE. This online tool remains limited to rice but will soon be updated for other crop species, with the process requiring a maximum of a single day (Ahmar et al., 2020a; Hong et al., 2020). Second, after the selection of candidate genes, the delivery of genetic materials (CRISPR binary vector) can be performed using a different type of nanotube or a different type of delivery method according to the lab facilities. This will take a maximum of 2 weeks (Zhao et al., 2017; Giraldo et al., 2019; Zhang et al., 2019a; Chandrasekaran et al., 2020; Wu et al., 2020). Third, after delivering the genetic/CRISPR vector, the transgenic plant will be grown under a speed breeding protocol to obtain the T0-generation seed. In the final step, T0 should be grown for T1 under speed breeding conditions to achieve T2 generation or segregation to develop transgene-free plants (Figure 5). The time required for this step varies depending on the plant species. However, four to six generations per year can be achieved, and the seed and plant density can be increased to efficiently scale-up the plant numbers using the single-seed descent method (Chiurugwi et al., 2018; Watson et al., 2018). The speed breeding protocol optimizes the rapid growth of oat, various Brassica species, chickpea, pea, grass pea, quinoa, and Brachypodium distachyon crops (Hickey et al., 2017; Ghosh et al., 2018; Jähne et al., 2020). This new strategy can be potentially extended to other plants, thereby offering a simple, fast, and inexpensive method for editing plant genomes.
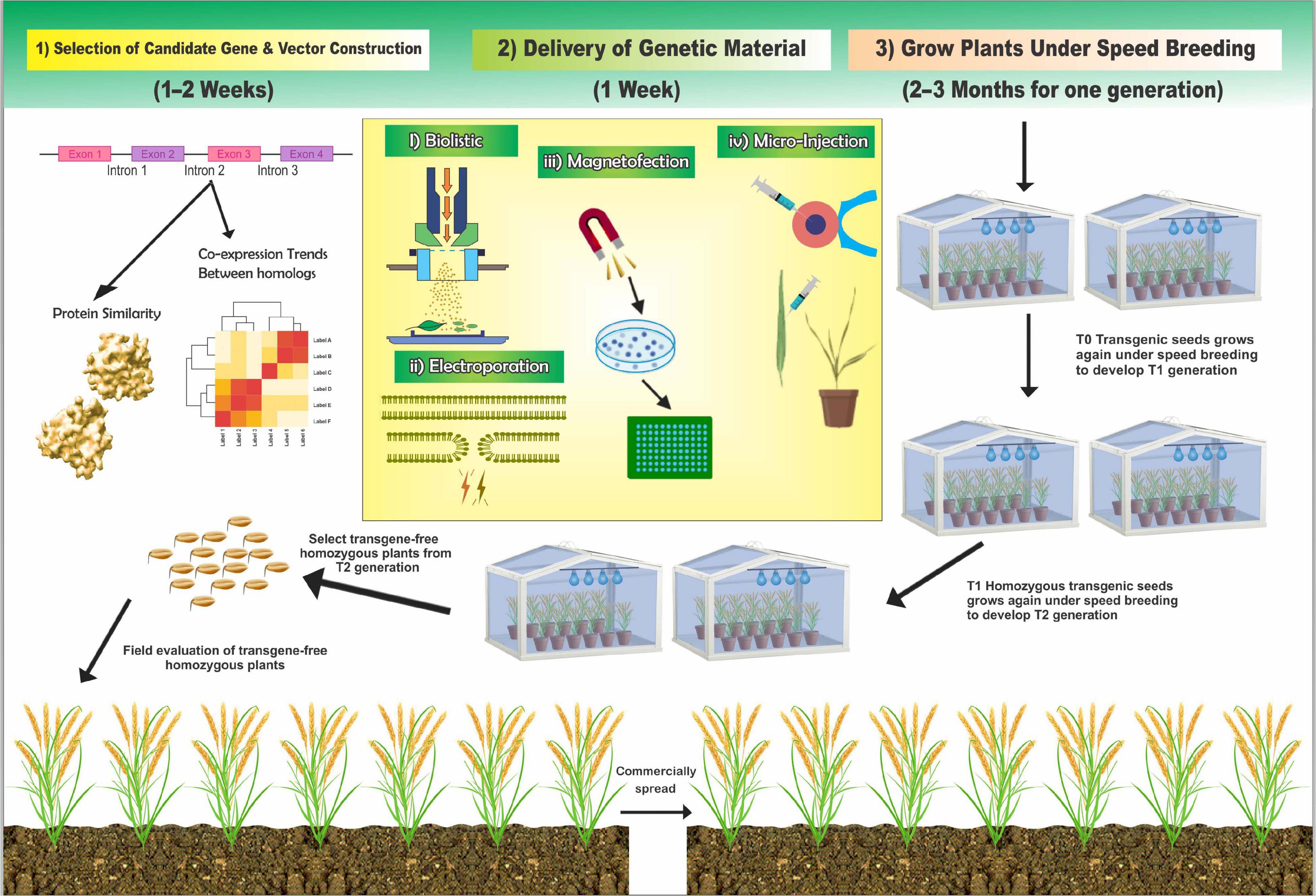
Figure 5. Major steps to efficiently improve the speedy crop process by combining the existing technologies.
Future Directions
A revolution is needed in agriculture science to ensure that the agricultural sector is more effective, robust, and sustainable, and nanotechnology will play a critical role in designing smart crop systems. These smart crop systems will help address the food storage issue, which is a major global challenge. NPs can be successful in transmitting micronutrients or providing insect or pathogen protection mechanisms by increasing the amount of enzyme and non-enzymatic compounds as well as genetic supplies. Moreover, NMs can help increase crop quality and performance by reducing production costs and postharvest losses via nanograms, nanofertilizers, nanopesticides, nanosensors, nanobags, and nanochips. Furthermore, NMs will reduce the amount of sprayed agrochemicals and increase their efficacy through the intelligent supply of active ingredients and the reduction of nutrient losses during the fertilization process.
The GE field is highly complex because of significant obstacles to effective genetic transformation, including the issue of DNA transport through the plant cell walls and subsequently via the nucleus. In addition, the use of site-directed techniques for GE, such as CRISPR/Cas9, tends to be unreliable in terms of plant improvement.
The CRISPR/Cas9 technique entails various issues, including the off-target effects, high costs, low security, and device delivery and editing inefficiencies, that must be resolved in the current system. The development of new nanovehicles serving as molecular transporters could become a core catalyst for the genetic transformation of plants. It could play a key role in determining the delivery methods and enhancing the efficiency of transformation. The different types of NPs, such as hybrid NPs, graphene oxide NPs, peptide-based NPs, and nanogels, could facilitate the GE process of the CRISPR/Cas9 system. A potential natural CRISPR carrier exists for specific inorganic NPs, such as gold NPs, CNTs, MSNs, and dense silicon NPs, that could be used for relevant applications, such as those discussed above.
Despite the exceptional ability of NPs to introduce CRISPR/Cas9, there remain significant challenges that need to be resolved, including scale-up problems, poor encapsulation, bioprotection, continuous expression, and low transfection rates. However, the attendant distribution in cells and the possibility of editing the genome in the cells’ nucleus are key issues that can affect the success or efficiency of plant transformation. In particular, although the application of NMs within the CRISPR/Cas9 device distribution is superior to the previous delivery strategies in all fields of crop science, further testing is needed to ensure that the CRISPR method is delivered powerfully and reliably and is utilized in the short term. The CRISPR/Cas9 system in combination with NPs will provide a breakthrough in plant genetics in terms of testing other biological systems. Furthermore, previously developed technologies, such as speed breeding and speed editing strategies, could be developed to speed up the GE process that incorporates NPs and rapidly establish speedy crop improvements with the desired traits. Rapidly advancing technologies are undoubtedly providing plant scientists with new directions for overcoming the challenges of agricultural food supply faced throughout the world. This will also help develop new varieties, playing an important role in developing transgene-free plants using GE.
The widespread use of NPs has drawn public interest given that the food supply could be polluted by metal-based NPs. Here, zinc oxide NPs are among the most widely researched metal-based NPs in human and ecosystem health as well as plant nanotoxicology (Chen et al., 2015; Van Aken, 2015; Zhang et al., 2015; Tripathi et al., 2017). In earlier studies, the different impacts of metal-based NPs have been observed in plants, including those related to robustness, potential harm, or a non-influence (Millán-Chiu et al., 2017). However, most of these research has discussed easily observed parameters, such as the rate of germination and development. Plant responses to metal-based NPs are based not only on dosage but also on the plant species (Ghosh et al., 2019). Therefore, it will be in the best interest of all to focus on potential strategic studies for improving a regulated NP synthesis via a greener process and to gain a detailed understanding of a large number of unidentified NPs formed by the fungi and endophytes of the roots, which could play an important role in plant productivity. In this context, various organizations with the necessary skills and facilities that conform to the NP biosafety evaluations could be established. These organizations could operate in an interconnected manner to appropriately track the experimental findings for chemical and biological research institutes. Meanwhile, the global food protection and standard authorities should strictly comply with the Food and Agriculture Organization/World Health Organization standards and specific recommendations for monitoring or assessing NP-based systems. Furthermore, all nanobased foods should be tested to address any safety concerns before their commercial introduction, whereas corresponding data from many samples should be collected. Meanwhile, the scientific community as a whole should be encouraged to use multiple digital programs related to the possibilities and functioning of nanotechnology. Focusing on the essential facets of plant physiology can, of course, be expanded to include various identified applications. The delay in the advancement of plant nanotechnology could be resolved by promoting multidisciplinary approaches to the intelligent design and synthesis of NMs. However, the improvement of NPs or microparticles and the distribution methods for biolistic gene transfer in different plants are still required to enhance seed growth and improve plant and crop protection.
Conclusion
This review critically examines the various NP-mediated transgenic delivery strategies and the existing method-congested field of plant biotechnology. Here, we propose that more exciting techniques could be incorporated in the processing of modifying crops, such as the CRISPR technique, alongside a combination of the several recently developed technologies. However, several major issues still need to be resolved. Most of these issues could be addressed by integrating different solutions for the effective delivery of different genomes, the design and fabrication of modern hybrid NMs, and the improvement of pollen magnetofection and CRISPR strategies. Overall, the food and farming sectors of the future should perhaps not be a concern because, while the nanotechnology applications may take some time to enter the field, the continued support and awareness of these issues will ensure that the field will continue to grow and develop.
Author Contributions
SA and K-HJ conceived and designed the article. FM-P, TM, MSS, SF and MSC contributed to the manuscript revision. SA and K-HJ, FM-P, TM, MSS, SF and MSC wrote the manuscript and supervision FM-P and K-HJ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the New Breeding Technology Center Program (PJ01492703 to K-HJ), the Rural Development Administration, Republic of Korea and the National Research Foundation (NRF), and Ministry of Education, Science and Technology (2021R1A2C2010448 to K-HJ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge Chilean National Fund for Scientific and Technological Development (FONDECYT) grant number 1201973.
Footnotes
References
Abd-Elsalam, K. A. (2020). Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems: A Note From the Editor. Amsterdam: Elsevier Inc.
Abigail, E. A., and Chidambaram, R. (2017). Nanotechnology in Herbicide Resistance. Nanostructured Materials: Fabrication to Applications. Rijeka: IntechOpen, 207–212.
Acharya, P., Jayaprakasha, G. K., Crosby, K. M., Jifon, J. L., and Patil, B. S. (2020). Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 10:5037. doi: 10.1038/s41598-020-61696-7
Adisa, I. O., Pullagurala, V. L. R., Peralta-Videa, J. R., Dimkpa, C. O., Elmer, W. H., Gardea-Torresdey, J. L., et al. (2019). Recent advances in nano-enabled fertilizers and pesticides: a critical review of mechanisms of action. Environ. Sci. Nano 6, 2002–2030.
Aghamiri, S., Talaei, S., Ghavidel, A. A., Zandsalimi, F., Masoumi, S., and Hafshejani, et al. (2020). Nanoparticles-mediated CRISPR/Cas9 delivery: Recent advances in cancer treatment. J. Drug Deliv. Sci. Technol. 56:101533. doi: 10.1016/j.jddst.2020.101533
Ahmar, S., Saeed, S., Khan, M. H. U., Khan, S. U., Mora-Poblete, F., Kamran, M., et al. (2020a). A revolution toward gene-editing technology and its application to crop improvement. Int. J. Mol. Sci. 21, 1–28. doi: 10.3390/ijms21165665
Ahmar, S., Saeed, S., Khan, M. H. U., Ullah Khan, S., Mora-Poblete, F., Kamran, M., et al. (2020b). A revolution toward gene-editing technology and its application to crop improvement. Int. J. Mol. Sci. 21:5665. doi: 10.3390/ijms21165665
Ahmed, R. I., Ding, A., Xie, M., and Kong, Y. (2018). Progress in optimization of agrobacterium-mediated transformation in sorghum (Sorghum bicolor). Int. J. Mol. Sci. 19:2983. doi: 10.3390/ijms19102983
Albanese, A., Tang, P. S., and Chan, W. C. W. (2012). The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 14, 1–16.
Almutairi, Z., and Alharbi, A. (2015). Effect of silver nanoparticles on seed germination of crop plants. J. Adv. Agric. 4, 280–285. doi: 10.24297/jaa.v4i1.4295
Altpeter, F., Baisakh, N., Beachy, R., Bock, R., Capell, T., Christou, P., et al. (2005). Particle bombardment and the genetic enhancement of crops: myths and realities. Mol. Breed. 15, 305–327. doi: 10.1007/s11032-004-8001-y
Altpeter, F., Springer, N. M., Bartley, L. E., Blechl, A. E., Brutnell, T. P., Citovsky, V., et al. (2016). Advancing crop transformation in the era of genome editing. Plant Cell 28, 1510–1520. doi: 10.1105/tpc.16.00196
Anand, A., Bass, S. H., Wu, E., Wang, N., McBride, K. E., Annaluru, N., et al. (2018). An improved ternary vector system for Agrobacterium-mediated rapid maize transformation. Plant Mol. Biol. 97, 187–200. doi: 10.1007/s11103-018-0732-y
Anzalone, A. V., Randolph, P. B., Davis, J. R., Sousa, A. A., Koblan, L. W., Levy, J. M., et al. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157.
Armendariz, V., Herrera, I., Peralta-Videa, J. R., Jose-Yacaman, M., Troiani, H., and Santiago, P. (2004). Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J. Nanopart. Res. 6:377.
Asad, S., and Arshad, M. (2011). Silicon Carbide Whisker-Mediated Plant Transformation. London: INTECH Open Access Publisher.
Avellan, A., Schwab, F., Masion, A., Chaurand, P., Borschneck, D., Vidal, V., et al. (2017). Nanoparticle uptake in plants: gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging. Environ. Sci. Technol. 51, 8682–8691.
Azencott, H. R., Peter, G. F., and Prausnitz, M. R. (2007). Influence of the cell wall on intracellular delivery to algal cells by electroporation and sonication. Ultrasound Med. Biol. 33, 1805–1817. doi: 10.1016/j.ultrasmedbio.2007.05.008
Aziz, H. M. M. A., Hasaneen, M. N. A., and Omer, A. M. (2016). Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Spanish J. Agric. Res. 14:17.
Bajželj, B., Richards, K. S., Allwood, J. M., Smith, P., Dennis, J. S., Curmi, E., et al. (2014). Importance of food-demand management for climate mitigation. Nat. Clim. Change 4, 924–929. doi: 10.1038/nclimate2353
Baltes, N. J., Gil-Humanes, J., and Voytas, D. F. (2017). Genome engineering and agriculture: opportunities and challenges. Prog. Mol. Biol. Transl. Sci. 149, 1–26. doi: 10.1016/bs.pmbts.2017.03.011
Barampuram, S., and Zhang, Z. J. (2011). Recent advances in plant transformation. Methods Mol. Biol. 701, 1–35. doi: 10.1007/978-1-61737-957-4_1
Bates, K., and Kostarelos, K. (2013). Carbon nanotubes as vectors for gene therapy: past achievements, present challenges and future goals. Adv. Drug Deliv. Rev. 65, 2023–2033. doi: 10.1016/j.addr.2013.10.003
Boch, J., Scholze, H., Schornack, S., Landgraf, A., Hahn, S., Kay, S., et al. (2009). Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512.
Bouton, C., King, R. C., Chen, H., Azhakanandam, K., Bieri, S., Hammond-Kosack, K. E., et al. (2018). Foxtail mosaic virus: a viral vector for protein expression in cereals. Plant Physiol. 177, 1352–1367. doi: 10.1104/pp.17.01679
Burlaka, O. M., Pirko, Y. V., Yemets, A. I., and Blume, Y. B. (2015). Plant genetic transformation using carbon nanotubes for DNA delivery. Cytol. Genet. 49, 3–12. doi: 10.3103/S009545271506002X
Busch, R. T., Karim, F., Weis, J., Sun, Y., Zhao, C., and Vasquez, E. S. (2019). Optimization and structural stability of gold nanoparticle-antibody bioconjugates. ACS Omega 4, 15269-15279. doi: 10.1021/acsomega.9b02276
Butler, N. M., Baltes, N. J., Voytas, D. F., and Douches, D. S. (2016). Geminivirus-mediated genome editing in potato (Solanum tuberosum l.) using sequence-specific nucleases. Front. Plant Sci. 7:1045. doi: 10.3389/fpls.2016.01045
Cañas, J. E., Long, M., Nations, S., Vadan, R., Dai, L., Luo, M., et al. (2008). Effects of functionalized and nonfunctionalized single−walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. Int. J. 27, 1922– 1931.
Carpita, N., Sabularse, D., Montezinos, D., and Delmer, D. P. (1979). Determination of the pore size of cell walls of living plant cells. Science 205, 1144–1147. doi: 10.1126/science.205.4411.1144
Chandra, S., Chakraborty, N., Dasgupta, A., Sarkar, J., Panda, K., and Acharya, K. (2015). Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci. Rep. 5:15195.
Chandrasekaran, R., Rajiv, P., and Abd-Elsalam, K. A. (2020). Carbon Nanotubes: Plant Gene Delivery and Genome Editing. Amsterdam: Elsevier Inc.
Chang, F. P., Kuang, L. Y., Huang, C. A., Jane, W. N., Hung, Y., Hsing, Y. I. C., et al. (2013). A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J. Mater. Chem. B 1, 5279–5287. doi: 10.1039/c3tb20529k
Char, S. N., Lee, H., and Yang, B. (2020). Use of CRISPR/Cas9 for targeted mutagenesis in Sorghum. Curr. Protoc. Plant Biol. 5:e20112.
Char, S. N., Neelakandan, A. K., Nahampun, H., Frame, B., Main, M., Spalding, M. H., et al. (2017). An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol. J. 15, 257–268. doi: 10.1111/pbi.12611
Che, P., Anand, A., Wu, E., Sander, J. D., Simon, M. K., Zhu, W., et al. (2018). Developing a flexible, high-efficiency Agrobacterium-mediated sorghum transformation system with broad application. Plant Biotechnol J. 16, 1388–1395. doi: 10.1111/pbi.12879
Chen, J., Liu, X., Wang, C., Yin, S. S., Li, X. L., Hu, W. J., et al. (2015). Nitric oxide ameliorates zinc oxide nanoparticles-induced phytotoxicity in rice seedlings. J. Hazard. Mater. 297, 173–182. doi: 10.1016/j.jhazmat.2015.04.077
Chen, K., Wang, Y., Zhang, R., Zhang, H., and Gao, C. (2019). CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70, 667–697. doi: 10.1146/annurev-arplant-050718-100049
Chhipa, H. (2017). Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 15, 15–22.
Chiurugwi, T., Kemp, S., Powell, W., Hickey, L. T., and Powell, W. (2018). Speed breeding orphan crops. Theoret. Appl. Genet. 132, 607–616. doi: 10.1007/s00122-018-3202-7
Christian, M., Cermak, T., Doyle, E. L., Schmidt, C., Zhang, F., Hummel, A., et al. (2010). Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186, 757–761. doi: 10.1534/genetics.110.120717
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313X.1998.00343.x
Cosgrove, D. J. (2005). Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6, 850–861. doi: 10.1038/nrm1746
Cox, A., Venkatachalam, P., Sahi, S., and Sharma, N. (2016). Silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol. Biochem. 107, 147–163. doi: 10.1016/j.plaphy.2016.05.022
Cunningham, F. J., Goh, N. S., Demirer, G. S., and Matos, J. L. (2018). Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 36, 882–897. doi: 10.1016/j.tibtech.2018.03.009
Dan, Y., Zhang, W., Xue, R., Ma, X., Stephan, C., and Shi, H. (2015). Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma–mass spectrometry analysis. Environ. Sci. Technol. 49, 3007–3014.
Dasgupta, S., Auth, T., and Gompper, G. (2014). Shape and orientation matter for the cellular uptake of nonspherical particles. Nano Lett. 14, 687–693. doi: 10.1021/nl403949h
De La Torre-Roche, R., Hawthorne, J., Deng, Y., Xing, B., Cai, W., Newman, L. A., et al. (2013). Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ. Sci. Technol. 47, 12539–12547.
de Melo, B. P., Lourenço-Tessutti, I. T., Morgante, C. V., Santos, N. C., Pinheiro, L. B., de Jesus Lins, C. B., et al. (2020). Soybean embryonic axis transformation: combining biolistic and Agrobacterium-mediated protocols to overcome typical complications of in vitro plant regeneration. Front. Plant Sci. 11:1228. doi: 10.3389/fpls.2020.01228
de Oliveira, J. L., Campos, E. V. R., Bakshi, M., Abhilash, P. C., and Fraceto, L. F. (2014). Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: prospects and promises. Biotechnol. Adv. 32, 1550–1561. doi: 10.1016/j.biotechadv.2014.10.010
Delfani, M., Baradarn Firouzabadi, M., Farrokhi, N., and Makarian, H. (2014). Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 45, 530–540.
Demirer, G. S., Chang, R., Zhang, H., Chio, L., and Landry, M. P. (2018). Nanoparticle-guided biomolecule delivery for transgene expression and gene silencing in mature plants. Biophys. J. 114:217a. doi: 10.1016/j.bpj.2017.11.1209
Demirer, G. S., Silva, T. N., Jackson, C. T., Thomas, J. B., Ehrhardt, D. W., Rhee, S. Y., et al. (2021). Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat. Nanotechnol. 12, 1–8.
Demirer, G. S., Zhang, H., Goh, N. S., González-grandío, E., and Landry, M. P. (2019a). Carbon nanotube – mediated DNA delivery without transgene integration in intact plants. Nat. Protoc. 14, 2954–2971. doi: 10.1038/s41596-019-0208-9
Demirer, G. S., Zhang, H., Matos, J. L., Goh, N. S., Cunningham, F. J., Sung, Y., et al. (2019b). High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 14, 456–464. doi: 10.1038/s41565-019-0382-5
Deng, H., Huang, W., and Zhang, Z. (2019). Nanotechnology based CRISPR/Cas9 system delivery for genome editing: progress and prospect. Nano Res. 12, 2437–2450. doi: 10.1007/s12274-019-2465-x
DeRosa, M. C., Monreal, C., Schnitzer, M., Walsh, R., and Sultan, Y. (2010). Nanotechnology in fertilizers. Nat. Nanotechnol. 5:91.
Dessoky, E. S., Ismail, R. M., Elarabi, N. I., Abdelhadi, A. A., and Abdallah, N. A. (2021). Improvement of sugarcane for borer resistance using Agrobacterium mediated transformation of cry1Ac gene. GM Crops Food 12, 47–56. doi: 10.1080/21645698.2020.1809318
Dong, O. X., Yu, S., Jain, R., Zhang, N., Duong, P. Q., Butler, C., et al. (2020). Marker-free carotenoid-enriched rice generated through targeted gene insertion using CRISPR-Cas9. Nat. Commun. 11:1178. doi: 10.1038/s41467-020-14981-y
Doyle, C., Higginbottom, K., Swift, T. A., Winfield, M., Bellas, C., Benito-Alifonso, D., et al. (2019). A simple method for spray-on gene editing in planta. bioRxiv doi: 10.1101/805036
Du, W., Tan, W., Peralta-Videa, J. R., Gardea-Torresdey, J. L., Ji, R., Yin, Y., et al. (2017). Interaction of metal oxide nanoparticles with higher terrestrial plants: physiological and biochemical aspects. Plant Physiol. Biochem. 110, 210–225. doi: 10.1016/j.plaphy.2016.04.024
Dutta Gupta, S., Saha, N., Agarwal, A., and Venkatesh, V. (2020). Silver nanoparticles (AgNPs) induced impairment of in vitro pollen performance of Peltophorum pterocarpum (DC.) K. Heyne. Ecotoxicology 29, 75–85. doi: 10.1007/s10646-019-02140-z
El-shetehy, M., Moradi, A., Maceroni, M., Reinhardt, D., Petri-fink, A., Rothen-rutishauser, B., et al. (2021). Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 16, 344–353. doi: 10.1038/s41565-020-00812-0
Endo, M., Kumagai, M., Motoyama, R., Sasaki-Yamagata, H., Mori-Hosokawa, S., Hamada, M., et al. (2015). Whole-genome analysis of herbicide-tolerant mutant rice generated by Agrobacterium-mediated gene targeting. Plant Cell Physiol. 56, 116–125.
Fathi, A., Zahedi, M., Torabian, S., and Khoshgoftar, A. (2017). Response of wheat genotypes to foliar spray of ZnO and Fe2O3 nanoparticles under salt stress. J. Plant Nutr. 40, 1376–1385.
Fayos, I., Meunier, A. C., Vernet, A., Sanz, S. N., Portefaix, M., Lartaud, M., et al. (2020). Assessment of the roles of OsSPO11-2 and OsSPO11-4 in rice meiosis using CRISPR/Cas9 mutagenesis. J. Exp. Bot. 71, 7046–7058. doi: 10.1093/jxb/eraa391
Feng, D., Wang, Y., Wu, J., Lu, T., and Zhang, Z. (2017). Development and drought tolerance assay of marker-free transgenic rice with OsAPX2 using biolistic particle-mediated co-transformation. Crop J. 5, 271–281. doi: 10.1016/j.cj.2017.04.001
Ferrie, A. M. R., Bhowmik, P., Rajagopalan, N., and Kagale, S. (2020). CRISPR/Cas9-mediated targeted mutagenesis in wheat doubled haploids. Methods Mol. Biol. 2072, 183–198. doi: 10.1007/978-1-4939-9865-4_15
Fiaz, S., Khan, S. A., Anis, G. B., Gaballah, M. M., and Riaz, A. (2021). “CRISPR/Cas techniques: a new method for RNA interference in cereals,” in CRISPR and RNAi Systems: Nanobiotechnology Approaches to Plant Breeding and Protection, 1st Edn, ed. C. Cockle (Amsterdam: Elsevier publisher), 233–252.
Fiaz, S., Xiukang, W., Afifa, Y., Alharthi, B., and Ali, H. (2020). Apomixis and stratgeies for induce apomixis to preserve hybrid seed vigor for multiple generations. GM Foods Crop 12, 57–70.
Finiuk, N., Buziashvili, A., Burlaka, O., Zaichenko, A., Mitina, N., Miagkota, O., et al. (2017). Investigation of novel oligoelectrolyte polymer carriers for their capacity of DNA delivery into plant cells. Plant Cell Tissue Organ Cult. 131, 27–39. doi: 10.1007/s11240-017-1259-7
Fleischer, C. C., and Payne, C. K. (2014). Nanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 47, 2651–2659. doi: 10.1021/ar500190q
Fortuni, B., Inose, T., Ricci, M., Fujita, Y., Van Zundert, I., Masuhara, A., et al. (2019). Polymeric engineering of nanoparticles for highly efficient multifunctional drug delivery systems. Sci. Rep. 9:2666. doi: 10.1038/s41598-019-39107-3
Fu, Y. Q., Li, L. H., Wang, P. W., Qu, J., Fu, Y. P., Wang, H., et al. (2012). Delivering DNA into plant cell by gene carriers of ZnS nanoparticles. Chem. Res. Chinese Univ. 28, 672–676.
Gad, M. A., Li, M., Ahmed, F. K., and Almoammar, H. (2020). Nanomaterials for Gene Delivery and Editing in Plants: Challenges and Future Perspective. Amsterdam: Elsevier Inc.
Gao, C. (2021). Genome engineering for crop improvement and future agriculture. Cell 184, 1621–1635.
Gao, C., Long, D., Lenk, I., and Nielsen, K. K. (2008). Comparative analysis of transgenic tall fescue (Festuca arundinacea Schreb.) plants obtained by Agrobacterium-mediated transformation and particle bombardment. Plant Cell Rep. 27, 1601–1609. doi: 10.1007/s00299-008-0578-x
Gao, F., Liu, C., Qu, C., Zheng, L., Yang, F., Su, M., et al. (2008). Was improvement of spinach growth by nano-TiO 2 treatment related to the changes of Rubisco activase? Biometals 21, 211–217.
Ghafariyan, M. H., Malakouti, M. J., Dadpour, M. R., Stroeve, P., and Mahmoudi, M. (2013). Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 47, 10645–10652.
Ghodake, G. S., Deshpande, N. G., Lee, Y. P., and Jin, E. S. (2010). Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Coll. Surf. B Biointerf. 75, 584–589.
Ghosh, M., Ghosh, I., Godderis, L., Hoet, P., and Mukherjee, A. (2019). Genotoxicity of engineered nanoparticles in higher plants. Mutat. Res. Genet. Toxicol. Environ. Mutagene. 842, 132–145. doi: 10.1016/j.mrgentox.2019.01.002
Ghosh, S., Watson, A., Gonzalez-Navarro, O. E., Ramirez-Gonzalez, R. H., Yanes, L., Mendoza-Suárez, M., et al. (2018). Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 13, 2944–2963. doi: 10.1038/s41596-018-0072-z
Giraldo, J. P., Landry, M. P., Faltermeier, S. M., McNicholas, T. P., Iverson, N. M., Boghossian, A. A., et al. (2014). Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 13, 400–408. doi: 10.1038/nmat3890
Giraldo, J. P., Wu, H., Newkirk, G. M., and Kruss, S. (2019). Nanobiotechnology approaches for engineering smart plant sensors. Nat.Nanotechnol. 14, 541–553. doi: 10.1038/s41565-019-0470-6
Gogos, A., Knauer, K., and Bucheli, T. D. (2012). Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J. Agric. Food Chem. 60, 9781–9792.
Golestanipour, A., Nikkhah, M., Aalami, A., and Hosseinkhani, S. (2018). Gene delivery to tobacco root cells with single-walled carbon nanotubes and cell-penetrating fusogenic peptides. Mol. Biotechnol. 60, 863–878. doi: 10.1007/s12033-018-0120-5
Gopinath, K., Gowri, S., Karthika, V., and Arumugam, A. (2014). Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna, for the enhanced seed germination activity of Gloriosa superba. J. Nanostruct. Chem. 4:115.
Hajirostamlo, B., Mirsaeedghazi, N., Arefnia, M., Shariati, M. A., and Fard, E. A. (2015). The role of research and development in agriculture and its dependent concepts in agriculture. Asian J. Appl. Sci. Eng. 4, 78–80.
Hamada, H., Linghu, Q., Nagira, Y., Miki, R., Taoka, N., and Imai, R. (2017). An in planta biolistic method for stable wheat transformation. Scientific Reports. 7:11443. doi: 10.1038/s41598-017-11936-0
Hansen, G., and Wright, M. S. (1999). Recent advances in the transformation of plants. Trends Plant Sci. 4, 226–231.
Hao, Y., Yang, X., Shi, Y., Song, S., Xing, J., Marowitch, J., et al. (2013). Magnetic gold nanoparticles as a vehicle for fluorescein isothiocyanate and DNA delivery into plant cells. Botany 91, 457–466. doi: 10.1139/cjb-2012-0281
Hasanzadeh Kafshgari, M., Alnakhli, M., Delalat, B., Apostolou, S., Harding, F. J., Mäkilä, E., et al. (2015). Small interfering RNA delivery by polyethylenimine-functionalised porous silicon nanoparticles. Biomater Sci. 3, 1555–1565. doi: 10.1039/c5bm00204d
He, Y., and Zhao, Y. (2020). Technological breakthroughs in generating transgene-free and genetically stable CRISPR-edited plants. aBIOTECH 1, 88–96. doi: 10.1007/s42994-019-00013-x
Hickey, L. T., Germán, S. E., Pereyra, S. A., Diaz, J. E., Ziems, L. A., Fowler, R. A., et al. (2017). Speed breeding for multiple disease resistance in barley. Euphytica 213:64. doi: 10.1007/s10681-016-1803-2
Hiei, Y., Ishida, Y., and Komari, T. (2014). Progress of cereal transformation technology mediated by Agrobacterium tumefaciens. Front. Plant Sci. 5:628. doi: 10.3389/fpls.2014.00628
Hofmann, N. R. (2016). A breakthrough in monocot transformation methods. Plant Cell 28:1989. doi: 10.1105/tpc.16.00696
Hofmann, T., Lowry, G. V., Ghoshal, S., Tufenkji, N., Brambilla, D., Dutcher, J. R., et al. (2020). Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 1, 416–425.
Hong, W. J., Kim, Y. J., Kim, E. J., Kumar Nalini, Chandran, A., Moon, S., et al. (2020). CAFRI-Rice: CRISPR applicable functional redundancy inspector to accelerate functional genomics in rice. Plant J. 104, 532–545. doi: 10.1111/tpj.14926
Hossain, Z., Mustafa, G., and Komatsu, S. (2015). Plant responses to nanoparticle stress. Int. J. Mol. Sci. 16, 26644–26653. doi: 10.3390/ijms161125980
Hu, P., An, J., Faulkner, M. M., Wu, H., Li, Z., Tian, X., et al. (2020). Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano 14, 7970–7986. doi: 10.1021/acsnano.9b09178
Imai, R., Hamada, H., Liu, Y., Linghu, Q., Kumagai, Y., Nagira, Y., et al. (2020). In planta particle bombardment (IPB): a new method for plant transformation and genome editing. Plant Biotechnol. 37, 171–176. doi: 10.5511/PLANTBIOTECHNOLOGY.20.0206A
Ishizaki, K., Chiyoda, S., Yamato, K. T., and Kohchi, T. (2008). Agrobacterium-mediated transformation of the haploid liverwort Marchantia polymorpha L., an emerging model for plant biology. Plant Cell Physiol. 49, 1084–1091. doi: 10.1093/pcp/pcn085
Islam, P., Water, J. J., Bohr, A., and Rantanen, J. (2017). Chitosan-based nano-embedded microparticles: impact of nanogel composition on physicochemical properties. Pharmaceutics 9:1.
Jähne, F., Hahn, V., Würschum, T., and Leiser, W. L. (2020). Speed breeding short-day crops by LED-controlled light schemes. Theoret. Appl. Genet. 133, 2335–2342. doi: 10.1007/s00122-020-03601-4
Jat, S. K., Bhattacharya, J., and Sharma, M. K. (2020). Nanomaterial based gene delivery: a promising method for plant genome engineering. J. Mater. Chem. B 8, 4165–4175.
Joldersma, D., and Liu, Z. (2018). Plant genetics enters the nano age? J. Integr. Plant Biol. 60, 446–447. doi: 10.1111/jipb.12646
Jones, H. D., Doherty, A., and Sparks, C. A. (2009). Transient transformation of plants. Methods Mol. Biol. 513, 131–152. doi: 10.1007/978-1-59745-427-8_8
Kah, M., and Hofmann, T. (2014). Nanopesticide research: current trends and future priorities. Environ. Int. 63, 224–235.
Kah, M., Kookana, R. S., Gogos, A., and Bucheli, T. D. (2018). A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 13, 677–684.
Kah, M., Tufenkji, N., and White, J. C. (2019). Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 14, 532–540.
Karimi, M., Solati, N., Ghasemi, A., Estiar, M. A., Hashemkhani, M., Kiani, P., et al. (2015). Carbon nanotubes part II: a remarkable carrier for drug and gene delivery. Expert Opin. Drug Deliv. 12, 1089–1105. doi: 10.1517/17425247.2015.1004309
Karny, A., Zinger, A., Kajal, A., Shainsky-Roitman, J., and Schroeder, A. (2018). Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 8:7589.
Keshavareddy, G., Kumar, A. R. V., and Ramu, S. V. (2018). Methods of plant transformation- a review. Int. J. Curr. Microbiol. Appl. Sci. 7, 2656–2668. doi: 10.20546/ijcmas.2018.707.312
Khalil, M. M. H., Ismail, E. H., and El-Magdoub, F. (2012). Microemulsion method: a novel route to synthesize organic and inorganic nanomaterials: 1st nano update. Arabian J. Chem. 5:431.
Khodakovskaya, M. V., Kim, B., Kim, J. N., Alimohammadi, M., Dervishi, E., Mustafa, T., et al. (2013). Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small 9, 115–123.
Kim, H.-J., Phenrat, T., Tilton, R. D., and Lowry, G. V. (2012). Effect of kaolinite, silica fines and pH on transport of polymer-modified zero valent iron nano-particles in heterogeneous porous media. J. Coll. Interf. Sci. 370, 1–10.
Kole, C., Kole, P., Randunu, K. M., Choudhary, P., Podila, R., Ke, P. C., et al. (2013). Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). Bmc Biotechnology 13:37. doi: 10.1186/1472-6750-13-37
Koo, Y., Wang, J., Zhang, Q., Zhu, H., Chehab, E. W., Colvin, V. L., et al. (2015). Fluorescence reports intact quantum dot uptake into roots and translocation to leaves of Arabidopsis thaliana and subsequent ingestion by insect herbivores. Environ. Sci. Technol. 49, 626–632.
Krenek, P., Samajova, O., Luptovciak, I., Doskocilova, A., Komis, G., and Samaj, J. (2015). Transient plant transformation mediated by Agrobacterium tumefaciens: principles, methods and applications. Biotechnol. Adv. 33, 1024–1042. doi: 10.1016/j.biotechadv.2015.03.012
Kretzmann, J. A., Ho, D., Evans, C. W., Plani-Lam, J. H. C., Garcia-Bloj, B., Mohamed, A. E., et al. (2017). Synthetically controlling dendrimer flexibility improves delivery of large plasmid DNA. Chem. Sci. 8, 2923–2930.
Kumar, R., Mamrutha, H. M., Kaur, A., Venkatesh, K., Sharma, D., and Singh, G. P. (2019). Optimization of Agrobacterium-mediated transformation in spring bread wheat using mature and immature embryos. Mol. Biol. Rep. 46, 1845–1853. doi: 10.1007/s11033-019-04637-6
Kurepa, J., Paunesku, T., Vogt, S., Arora, H., Rabatic, B. M., Lu, J., et al. (2010). Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 10, 2296–2302.
Kwak, S.-Y., Giraldo, J. P., Wong, M. H., Koman, V. B., Lew, T. T. S., Ell, J., et al. (2017). A nanobionic light-emitting plant. Nano Lett. 17, 7951–7961.
Kwak, S. Y., Lew, T. T. S., Sweeney, C. J., Koman, V. B., Wong, M. H., Bohmert-Tatarev, K., et al. (2019). Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 14, 447–455. doi: 10.1038/s41565-019-0375-4
Lacroix, B., and Citovsky, V. (2020). “Biolistic approach for transient gene expression studies in plants,” in Biolistic DNA Delivery in Plants. Methods in Molecular Biology, Vol. 2124, eds S. Rustgi and H. Luo (New York, NY: Humana), 125–139. doi: 10.1007/978-1-0716-0356-7_6
Lahiani, M. H., Dervishi, E., Chen, J., Nima, Z., Gaume, A., Biris, A. S., et al. (2013). Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interf. 5, 7965–7973.
Lahiani, M. H., Dervishi, E., Ivanov, I., Chen, J., and Khodakovskaya, M. (2016). Comparative study of plant responses to carbon-based nanomaterials with different morphologies. Nanotechnology 27:265102.
Landry, M. P., and Mitter, N. (2019). How nanocarriers delivering cargos in plants can change the GMO landscape. Nat. Nanotechnol. 14, 512–514. doi: 10.1038/s41565-019-0463-5
Laware, S. L., and Raskar, S. (2014). Influence of zinc oxide nanoparticles on growth, flowering and seed productivity in onion. Int. J. Curr. Microbiol. Sci. 3, 874–881.
Lee, W., An, Y., Yoon, H., and Kweon, H. (2008). Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water−insoluble nanoparticles. Environ. Toxicol. Chem. Int. J. 27, 1915–1921.
Lei, J., Dai, P., Li, J., Yang, M., Li, X., Zhang, W., et al. (2020). Tissue-specific CRISPR/Cas9 system of cotton pollen with GhPLIMP2b and GhMYB24 promoters. J. Plant Biol. 64, 13–21. doi: 10.1007/s12374-020-09272-4
Lenka, S. K., Ezekiel Nims, N., Vongpaseuth, K., Boshar, R. A., Roberts, S. C., and Walker, E. L. (2015). Jasmonate-responsive expression of paclitaxel biosynthesis genes in Taxus cuspidata cultured cells is negatively regulated by the bHLH transcription factors TcJAMYC1, TcJAMYC2, and TcJAMYC4. Front. Plant Sci. 6:115. doi: 10.3389/fpls.2015.00115
Lenka, S. K., Muthusamy, S. K., Chinnusamy, V., and Bansal, K. C. (2018). Ectopic expression of rice PYL3 enhances cold and drought tolerance in Arabidopsis thaliana. Mol. Biotechnol. 60, 350–361.
Li, J., Tan, X., Zhu, F., and Guo, J. (2010). A rapid and simple method for brassica napus floral-dip transformation and selection of transgenic plantlets. Int. J. Biol. 2, 127–131. doi: 10.5539/ijb.v2n1p127
Li, J., Wang, Z., He, G., Ma, L., and Deng, X. W. (2020). CRISPR/Cas9-mediated disruption of TaNP1 genes results in complete male sterility in bread wheat. J. Genet. Genomics 47, 263–272. doi: 10.1016/j.jgg.2020.05.004
Li, L., Hu, S., and Chen, X. (2018). Non-viral delivery systems for CRISPR/Cas9-based genome editing: challenges and opportunities. Biomaterials 171, 207–218. doi: 10.1016/j.biomaterials.2018.04.031
Li, S., Cong, Y., Liu, Y., Wang, T., Shuai, Q., Chen, N., et al. (2017). Optimization of agrobacterium-mediated transformation in soybean. Front. Plant Sci. 8:246. doi: 10.3389/fpls.2017.00246
Liang, Z., Chen, K., Zhang, Y., Liu, J., Yin, K., Qiu, J. L., et al. (2018). Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat. Protoc. 13, 413–430. doi: 10.1038/nprot.2017.145
Lin, D., and Xing, B. (2007). Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 150, 243–250.
Lin, Q., Zong, Y., Xue, C., Wang, S., Jin, S., Zhu, Z., et al. (2020). Prime genome editing in rice and wheat. Nat. Biotechnol. 38, 582–585. doi: 10.1038/s41587-020-0455-x
Ling, F., Zhou, F., Chen, H., and Lin, Y. (2016). Development of marker-free insect-resistant Indica rice by Agrobacterium tumefaciens-mediated co-transformation. Front. Plant Sci. 7:1608. doi: 10.3389/fpls.2016.01608
Liu, C., Zhang, L., Liu, H., and Cheng, K. (2017). Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J. Control Release 266, 17–26.
Liu, G., Li, J., and Godwin, I. D. (2019). Genome editing by CRISPR/Cas9 in sorghum through biolistic bombardment. Methods Mol. Biol. 1931, 169–183. doi: 10.1007/978-1-4939-9039-9_12
Liu, J., Williams, P. C., Goodson, B. M., Geisler-Lee, J., Fakharifar, M., and Gemeinhardt, M. E. (2019). TiO2 nanoparticles in irrigation water mitigate impacts of aged Ag nanoparticles on soil microorganisms, Arabidopsis thaliana plants, and Eisenia fetida earthworms. Environ. Res. 172, 202–215.
Liu, R., and Lal, R. (2014). Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 4:5686.
Liu, R., and Lal, R. (2015). Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 514, 131–139.
Lowry, G. V., Avellan, A., and Gilbertson, L. M. (2019). Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 14, 517–522. doi: 10.1038/s41565-019-0461-7
Lv, J., Christie, P., and Zhang, S. (2019). Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environ. Sci. Nano 6, 41–59. doi: 10.1039/C8EN00645H
Ma, X., and Wang, C. (2010). Fullerene nanoparticles affect the fate and uptake of trichloroethylene in phytoremediation systems. Environ. Eng. Sci. 27, 989–992.
Ma, X., Zhang, Q., Zhu, Q., Liu, W., Chen, Y., Qiu, R., et al. (2015). A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284. doi: 10.1016/j.molp.2015.04.007
Mahajan, P., Dhoke, S. K., and Khanna, A. S. (2011). Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 7, 1–7.
Mahakham, W., Sarmah, A. K., Maensiri, S., and Theerakulpisut, P. (2017). Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 7:8263. doi: 10.1038/s41598-017-08669-5
Malerba, M., and Cerana, R. (2018). Recent advances of chitosan applications in plants. Polymers 10:118.
Manghwar, H., Lindsey, K., Zhang, X., and Jin, S. (2019). CRISPR/Cas system: recent advances and future prospects for genome editing. Trends Plant Sci. 24, 1102–1125. doi: 10.1016/j.tplants.2019.09.006
Mao, Y., Botella, J. R., Liu, Y., and Zhu, J. K. (2019). Gene editing in plants: progress and challenges. Natl. Sci. Rev. 6, 421–437. doi: 10.1093/nsr/nwz005
Martin-Ortigosa, S., Peterson, D. J., Valenstein, J. S., Lin, V. S.-Y., Trewyn, B. G., Lyznik, L. A., et al. (2014). Mesoporous silica nanoparticle-mediated intracellular Cre protein delivery for maize genome editing via loxP site excision. Plant Physiol. 164, 537–547.
Martin-Ortigosa, S., Valenstein, J. S., Lin, V. S. Y., Trewyn, B. G., and Wang, K. (2012). Gold functionalized mesoporous silica nanoparticle mediated protein and DNA codelivery to plant cells via the biolistic method. Adv. Funct Mater. 22, 3576–3582. doi: 10.1002/adfm.201200359
Matsumoto, T. K., and Gonsalves, D. (2012). Biolistic and other non-Agrobacterium technologies of plant transformation. Plant Biotechnol. Agric 117–129. doi: 10.1016/B978-0-12-381466-1.00008-0
Mayavan, S., Subramanyam, K., Jaganath, B., Sathish, D., Manickavasagam, M., and Ganapathi, A. (2015). Agrobacterium-mediated in planta genetic transformation of sugarcane setts. Plant Cell Rep. 34, 1835–1848. doi: 10.1007/s00299-015-1831-8
Meziadi, C., Blanchet, S., Geffroy, V., and Pflieger, S. (2017). Virus-induced gene silencing (VIGS) and foreign gene expression in Pisum sativum L. using the ‘One-Step’ bean pod mottle virus (BPMV) viral vector. Methods Mol. Biol. 1654, 311–319. doi: 10.1007/978-1-4939-7231-9_23
Milewska-Hendel, A., Zubko, M., Karcz, J., Stróż, D., and Kurczyńska, E. (2017). Fate of neutral-charged gold nanoparticles in the roots of the Hordeum vulgare L. cultivar Karat. Sci. Rep. 7, 1–13.
Millán-Chiu, B. E., del Pilar Rodriguez-Torres, M., and Loske, A. M. (2020). “Nanotoxicology in plants,” in Green Nanoparticles. Nanotechnology in the Life Sciences, eds J. Patra, L. Fraceto, G. Das, and E. Campos (Cham: Springer), 43–76. doi: 10.1007/978-3-030-39246-8_3
Mitter, N., Worrall, E. A., Robinson, K. E., Li, P., Jain, R. G., Taochy, C., et al. (2017). Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 3, 1–10.
Modlitbová, P., Pořízka, P., Novotný, K., Drbohlavová, J., Chamradová, I., Farka, Z., et al. (2018). Short-term assessment of cadmium toxicity and uptake from different types of Cd-based quantum dots in the model plant Allium cepa L. Ecotoxicol. Environ. Saf. 153, 23–31.
Mohamed, M. S., and Kumar, D. S. (2019). “Application of nanotechnology in genetic improvement in crops,” in Nanoscience for Sustainable Agriculture, eds R. Pudake, N. Chauhan, and C. Kole (Cham: Springer), 3–24. doi: 10.1007/978-3-319-97852-9_1
Mohammed, S., Samad, A. A., and Rahmat, Z. (2019). Agrobacterium-mediated transformation of rice: constraints and possible solutions. Rice Sci. 26, 133–146. doi: 10.1016/j.rsci.2019.04.001
Mookkan, M., Nelson-Vasilchik, K., Hague, J., Zhang, Z. J., and Kausch, A. P. (2017). Selectable marker independent transformation of recalcitrant maize inbred B73 and sorghum P898012 mediated by morphogenic regulators BABY BOOM and WUSCHEL2. Plant Cell Rep. 36, 1477–1491. doi: 10.1007/s00299-017-2169-1
Moon, J. H., Mendez, E., Kim, Y., and Kaur, A. (2011). Conjugated polymer nanoparticles for small interfering RNA delivery. Chem. Commun. 10:291. doi: 10.1039/c1cc10991j
Mosa, K. A., El-Naggar, M., Ramamoorthy, K., Alawadhi, H., Elnaggar, A., Wartanian, S., et al. (2018). Copper nanoparticles induced genotoxicty, oxidative stress, and changes in Superoxide Dismutase (SOD) gene expression in cucumber (Cucumis sativus) plants. Front. Plant Sci. 9:872. doi: 10.3389/fpls.2018.00872
Mout, R., Ray, M., Yesilbag Tonga, G., Lee, Y. W., Tay, T., Sasaki, K., et al. (2017). Direct cytosolic delivery of CRISPR/Cas9- ribonucleoprotein for efficient gene editing. ACS Nano 11, 2452–2458.
Mu, G., Chang, N., Xiang, K., Sheng, Y., Zhang, Z., and Pan, G. (2012). Genetic transformation of maize female inflorescence following floral dip method mediated by Agrobacterium. Biotechnology 11, 178–183. doi: 10.3923/biotech.2012.178.183
Nadagouda, M. N., Iyanna, N., Lalley, J., Han, C. H., Dionysiou, D. D., and Varma, R. S. (2014). Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate, and turmeric extracts. ACS Sustain. Chem. Eng. 2:1717.
Nandy, D., Maity, A., and Mitra, A. K. (2020). Target-specific gene delivery in plant systems and their expression: insights into recent developments. J. Biosci. 45:30. doi: 10.1007/s12038-020-0008-y
Nekrasov, V., Staskawicz, B., Weigel, D., Jones, J. D. G., and Kamoun, S. (2013). Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 691–693. doi: 10.1038/nbt.2655
Nel, A. E., Mädler, L., Velegol, D., Xia, T., Hoek, E. M. V., Somasundaran, P., et al. (2009). Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557. doi: 10.1038/nmat2442
Niazian, M., Sadatnoori, S. A., Galuszka, P., and Mortazavian, S. M. M. (2017). Tissue culture-based Agrobacterium-mediated and in Planta transformation methods. Czech J. Genet. Plant Breed. 53, 133–143. doi: 10.17221/177/2016-CJGPB
Novák, O., Napier, R., and Ljung, K. (2017). Zooming in on plant hormone analysis: tissue- and cell-specific approaches. Annu. Rev. Plant Biol. 68, 323–348. doi: 10.1146/annurev-arplant-042916-040812
Ozyigit, I. I. (2020). Gene transfer to plants by electroporation: methods and applications. Mol. Biol. Rep. 0, 1–16.
Pachapur, V. L., Dalila Larios, A., Cledón, M., Brar, S. K., Verma, M., Surampalli, R. Y., et al. (2016). Interaction of metal oxide nanoparticles with higher terrestrial plants: physiological and biochemical aspects. Plant Physiol. Biochem. 110, 210–225.
Panpatte, D. G., Jhala, Y. G., Shelat, H. N., and Vyas, R. V. (2016). “Nanoparticles: the next generation technology for sustainable agriculture,” in Microbial Inoculants in Sustainable Agricultural Productivity: Functional Applications, Vol. 2, ed. D. P. Singh (Berlin: Springer).
Pasupathy, K., Lin, S., Hu, Q., Luo, H., and Ke, P. C. (2008). Direct plant gene delivery with a poly (amidoamine) dendrimer. Biotechnol. J. Healthcare Nutr. Technol. 3, 1078–1082.
Pérez-de-Luque, A. (2017). Interaction of nanomaterials with plants: what do we need for real applications in agriculture? Front. Environ. Sci. 5:12. doi: 10.3389/fenvs.2017.00012
Pradhan, S., Patra, P., Das, S., Chandra, S., Mitra, S., Dey, K. K., et al. (2013). Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular, biochemical, and biophysical study. Environ. Sci. Technol. 47, 13122–13131.
Puchta, H., Dujon, B., and Hohn, B. (1993). Homologous recombination in plant cells is enhanced by in vivo induction of double strand breaks into DNA by a site-specific endonuclease. Nucleic Acids Res. 21, 5034–5040. doi: 10.1093/nar/21.22.5034
Rãcuciu, M., and Creangã, D.-E. (2009). Cytogenetical changes induced by β-cyclodextrin coated nanoparticles in plant seeds. Rom. J. Phys. 54, 125–131.
Rad, F., Mohsenifar, A., Tabatabaei, M., Safarnejad, M. R., Shahryari, F., Safarpour, H., et al. (2012). Detection of candidatus phytoplasma aurantifolia with a quantum dots FRET-based biosensor. J. Plant Pathol. 94, 525–534.
Rai, V., Acharya, S., and Dey, N. (2012). Implications of nanobiosensors in agriculture. J. Biomater. Nanobiotechnol. 3, 315–324.
Raji, J. A., Frame, B., Little, D., Santoso, T. J., and Wang, K. (2018). Agrobacterium- and biolistic-mediated transformation of maize B104 inbred. Methods Mol. Biol. 1676, 15–40. doi: 10.1007/978-1-4939-7315-6_2
Raliya, R., Tarafdar, J. C., Gulecha, K., Choudhary, K., Rameshwar, R., Prakash, M., et al. (2013). Scope of nanoscience and nanotechnology in agriculture. J. Appl. Biol. Biotechnol. 1, 41–44.
Ramkumar, T. R., Lenka, S. K., Arya, S. S., and Bansal, K. C. (2020). A short history and perspectives on plant genetic transformation. Methods Mol. Biol. 2124, 39–68. doi: 10.1007/978-1-0716-0356-7_3
Ranjbar, S., Ramezanian, A., and Rahemi, M. (2020). Nano-calcium and its potential to improve ‘Red Delicious’ apple fruit characteristics. Hortic. Environ Biotechnol. 61, 23–30.
Rastogi, A., Tripathi, D. K., Yadav, S., Chauhan, D. K., Živčák, M., Ghorbanpour, M., et al. (2019). Application of silicon nanoparticles in agriculture. 3 Biotech 9:90.
Rastogi, A., Zivcak, M., Sytar, O., Kalaji, H. M., He, X., Mbarki, S., et al. (2017). Impact of metal and metal oxide nanoparticles on plant: a critical review. Front. Chem. 5:78. doi: 10.3389/fchem.2017.00078
Ratanasut, K., Rod-In, W., and Sujipuli, K. (2017). In planta agrobacterium-mediated transformation of rice. Rice Sci. 24, 181–186. doi: 10.1016/j.rsci.2016.11.001
Ramirez, C. L., Foley, J. E., Wright, D. A., Müller-Lerch, F., Rahman, S. H., Cornu, T. I., et al. (2008). Unexpected failure rates for modular assembly of engineered zinc fingers. Nat. Methods 5, 374–375.
Rod-In, W., Sujipuli, K., and Ratanasut, K. (2014). The floral-dip method for rice (Oryza sativa) transformation. J. Agric. Technol. 10, 467–474.
Rui, Y., Varanasi, M., Mendes, S., Yamagata, H. M., Wilson, D. R., and Green, J. J. (2020). Poly(Beta-Amino Ester) nanoparticles enable nonviral delivery of CRISPR-Cas9 plasmids for gene knockout and gene deletion. Mol. Ther. Nucleic Acids 20, 661–672. doi: 10.1016/j.omtn.2020.04.005
Sander, J. D., Dahlborg, E. J., Goodwin, M. J., Cade, L., Zhang, F., Cifuentes, D., et al. (2011). Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat. Methods 8, 67–69.
Sangeetha, J., Sarim, K. M., Thangadurai, D., Amrita Gupta, R., Mundaragi, A., Sheth, B. P., et al. (2019). “Nanoparticle-mediated plant gene transfer for precision farming and sustainable agriculture,” in Nanotechnology for Agriculture: Advances for Sustainable Agriculture, eds D. Panpatte and Y. Jhala (Singapore: Springer), 263–284. doi: 10.1007/978-981-32-9370-0_14
Santillán Martínez, M. I., Bracuto, V., Koseoglou, E., Appiano, M., Jacobsen, E., Visser, R. G. F., et al. (2020). CRISPR/Cas9-targeted mutagenesis of the tomato susceptibility gene PMR4 for resistance against powdery mildew. BMC Plant Biol. 20:284. doi: 10.1186/s12870-020-02497-y
Santos, A. R., Miguel, A. S., Tomaz, L., Malhó, R., Maycock, C., Patto, M. C. V., et al. (2010). The impact of CdSe/ZnS quantum dots in cells of Medicago sativa in suspension culture. J. Nanobiotechnol. 8, 1–14.
Sanzari, I., Leone, A., and Ambrosone, A. (2019). Nanotechnology in plant science: to make a long story short. Front. Bioeng. Biotechnol. 7:120. doi: 10.3389/fbioe.2019.00120
Saptarshi, S. R., Duschl, A., and Lopata, A. L. (2013). Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 11:26. doi: 10.1186/1477-3155-11-26
Saranya, S., Aswani, R., Remakanthan, A., and Radhakrishnan, E. K. (2019). “Nanotechnology in agriculture,” in Nanotechnology for Agriculture: Advances for Sustainable Agriculture, eds D. G. Panpatte and Y. K. Jhala (Singapore: Springer).
Scognamiglio, V. (2013). Nanotechnology in glucose monitoring: advances and challenges in the last 10 years. Biosens. Bioelectron. 47, 12–25.
Serag, M. F., Kaji, N., Habuchi, S., Bianco, A., and Baba, Y. (2013). Nanobiotechnology meets plant cell biology: carbon nanotubes as organelle targeting nanocarriers. RSC Adv. 3, 4856. doi: 10.1039/c2ra22766e
Shah, V., and Belozerova, I. (2009). Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 197, 143–148.
Shaheen, A., and Abed, Y. (2018). Knowledge, attitude, and practice among farmworkers applying pesticides in cultivated area of the Jericho district: a cross-sectional study. Lancet 391, Supplement 1, S3. doi: 10.1016/s0140-6736(18)30328-3
Sharma, P., Bhatt, D., Zaidi, M. G. H., Saradhi, P. P., Khanna, P. K., and Arora, S. (2012). Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 167, 2225–2233.
Sharma, R., Liang, Y., Lee, M. Y., Pidatala, V. R., Mortimer, J. C., and Scheller, H. V. (2020). Agrobacterium-mediated transient transformation of sorghum leaves for accelerating functional genomics and genome editing studies. BMC Res. Notes 13:116. doi: 10.1186/s13104-020-04968-9
Shen, C., Zhang, Q., Li, J., Bi, F., and Yao, N. (2010). Induction of programmed cell death in Arabidopsis and rice by single−wall carbon nanotubes. Am. J. Bot. 97, 1602–1609.
Shi, J., Peng, C., Yang, Y., Yang, J., Zhang, H., Yuan, X., et al. (2014). Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicology 8, 179–188.
Shin, J. H., Han, J. H., Park, H. H., Fu, T., and Kim, K. S. (2019). Optimization of polyethylene glycol-mediated transformation of the pepper anthracnose pathogen colletotrichum scovillei to develop an applied genomics approach. Plant Pathol. J. 35, 575–584. doi: 10.5423/PPJ.OA.06.2019.0171
Shrawat, A. K., and Lörz, H. (2006). Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnol. J. 4, 575–603. doi: 10.1111/j.1467-7652.2006.00209.x
Silva, A. T., Nguyen, A., Ye, C., Verchot, J., and Moon, J. H. (2010). Conjugated polymer nanoparticles for effective siRNA delivery to tobacco BY-2 protoplasts. BMC Plant Biol. 10:291. doi: 10.1186/1471-2229-10-291
Singh, R. K., and Prasad, M. (2016). Advances in Agrobacterium tumefaciens-mediated genetic transformation of graminaceous crops. Protoplasma 253, 691–707. doi: 10.1007/s00709-015-0905-3
Sood, P., Bhattacharya, A., and Sood, A. (2011). Problems and possibilities of monocot transformation. Biol. Plant 55, 1–15. doi: 10.1007/s10535-011-0001-2
Stadler, T., Buteler, M., Weaver, D. K., and Sofie, S. (2012). Comparative toxicity of nanostructured alumina and a commercial inert dust for Sitophilus oryzae (L.) and Rhyzopertha dominica (F.) at varying ambient humidity levels. J. Stored Prod. Res. 48, 81–90.
Stewart, C. N., Touraev, A., Citovsky, V., and Tzfira, T. (eds) (2011). Plant Transformation Technologies. Hoboken, NJ: John Wiley & Sons.
Su, X., Fricke, J., Kavanagh, D. G., and Irvine, D. J. (2011). In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharm. 8, 774–787. doi: 10.1021/mp100390w
Sujitha, M. V., and Kannan, S. (2013). Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 102, 15–23.
Tang, X., Lowder, L. G., Zhang, T., Malzahn, A. A., Zheng, X., Voytas, D. F., et al. (2017). A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 3:17018. doi: 10.1038/nplants.2017.18
Taran, N. Y., Gonchar, O. M., Lopatko, K. G., Batsmanova, L. M., Patyka, M. V., and Volkogon, M. V. (2014). The effect of colloidal solution of molybdenum nanoparticles on the microbial composition in rhizosphere of Cicer arietinum L. Nanoscale Res. Lett. 9:289.
Tian, B., Navia-Urrutia, M., Chen, Y., Brungardt, J., and Trick, H. N. (2019). “Biolistic transformation of wheat,” in Transgenic Plants. Methods in Molecular Biology, Vol. 1864, eds S. Kumar, P. Barone, and M. Smith (New York, NY: Humana Press), 141–152. doi: 10.1007/978-1-4939-8778-8_9
Thakur, A., Sharma, N., Bhatti, M., Sharma, M., Trukhanov, A. V., Trukhanov, S. V., et al. (2020). Synthesis of barium ferrite nanoparticles using rhizome extract of Acorus calamus: characterization and its efficacy against different plant phytopathogenic fungi. Nano Struct. Nano Objects 24:100599. doi: 10.1016/j.nanoso.2020.100599
Tian, Y., Chen, K., Li, X., Zheng, Y., and Chen, F. (2020). Design of high-oleic tobacco (Nicotiana tabacum L.) seed oil by CRISPR-Cas9-mediated knockout of NtFAD2-2. BMC Plant Biol. 20:233. doi: 10.1186/s12870-020-02441-0
Toda, E., Koiso, N., Takebayashi, A., Ichikawa, M., Kiba, T., Osakabe, K., et al. (2019). An efficient DNA- and selectable-marker-free genome-editing system using zygotes in rice. Nat. Plants 5, 1–6. doi: 10.1038/s41477-019-0386-z
Torney, F., Trewyn, B. G., Lin, V. S.-Y., and Wang, K. (2007). Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2, 295–300.
Townsend, J. A., Wright, D. A., Winfrey, R. J., Fu, F., Maeder, M. L., Joung, J. K., et al. (2009). High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 459, 442–445.
Tripathi, D. K., Mishra, R. K., Singh, S., Singh, S., Vishwakarma, K., Sharma, S., et al. (2017). Nitric oxide ameliorates zinc oxide nanoparticles phytotoxicity in wheat seedlings: Implication of the ascorbate–glutathione cycle. Front. Plant Sci. 8:1. doi: 10.3389/fpls.2017.00001
Usman, M., Farooq, M., Wakeel, A., Nawaz, A., Cheema, S. A., ur Rehman, H., et al. (2020). Nanotechnology in agriculture: current status, challenges and future opportunities. Sci. Total Environ 721, 137778.
Van Aken, B. (2015). Gene expression changes in plants and microorganisms exposed to nanomaterials. Curr. Opin. Biotechnol. 33, 206–219. doi: 10.1016/j.copbio.2015.03.005
Van Eck, J. (2018). The status of setaria viridis transformation: Agrobacterium-mediated to floral dip. Front. Plant Sci. 9:652. doi: 10.3389/fpls.2018.00652
Vanhaeren, H., Inzé, D., and Gonzalez, N. (2016). Plant growth beyond limits. Trends Plant Sci. 21, 102–109. doi: 10.1016/j.tplants.2015.11.012
Wang, F., Li, K., and Shi, Z. (2020). Phosphorus fertilization and mycorrhizal colonization change silver nanoparticle impacts on maize. Ecotoxicology 30, 118–129. doi: 10.1007/s10646-020-02298-x
Wang, H., Zhao, Q., Fu, J., Wang, X., and Jiang, L. (2018). Re-assessment of biolistic transient expression: an efficient and robust method for protein localization studies in seedling-lethal mutant and juvenile plants. Plant Sci. 274, 2–7. doi: 10.1016/j.plantsci.2018.03.032
Wang, J. W., Grandio, E. G., Newkirk, G. M., Demirer, G. S., Butrus, S., Giraldo, J. P., et al. (2019). Nanoparticle-mediated genetic engineering of plants. Mol. Plant 12, 1037–1040. doi: 10.1016/j.molp.2019.06.010
Wang, L., Sun, S., Wu, T., Liu, L., Sun, X., Cai, Y., et al. (2020). Natural variation and CRISPR/Cas9-mediated mutation in GmPRR37 affect photoperiodic flowering and contribute to regional adaptation of soybean. Plant Biotechnol. J. 18, 1869–1881. doi: 10.1111/pbi.13346
Wang, P., Lombi, E., Zhao, F. J., and Kopittke, P. M. (2016). Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci. 21, 699–712. doi: 10.1016/j.tplants.2016.04.005
Wang, Y., Cui, H., Li, K., Sun, C., Du, W., Cui, J., et al. (2014). A magnetic nanoparticle-based multiple-gene delivery system for transfection of porcine kidney cells. PLoS One 9:e102886. doi: 10.1371/journal.pone.0102886
Watson, A., Ghosh, S., Williams, M. J., Cuddy, W. S., Simmonds, J., Rey, M. D., et al. (2018). Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 4, 23–29. doi: 10.1038/s41477-017-0083-8
Wright, D. A., Townsend, J. A., Winfrey, R. J., Irwin, P. A., Rajagopal, J., Lonosky, P. M., et al. (2005). High-frequency homologous recombination in plants mediated by zinc-finger nucleases. Plant J. 44, 693–705. doi: 10.1111/j.1365-313X.2005.02551.x
Wu, H., Awan, F. S., Vilarinho, A., Zeng, Q., Kannan, B., Phipps, T., et al. (2015). Transgene integration complexity and expression stability following biolistic or Agrobacterium-mediated transformation of sugarcane. In Vitro Cell. Dev. Biol Plant 51, 603–611. doi: 10.1007/s11627-015-9710-0
Wu, H., Nißler, R., Morris, V., Herrmann, N., Hu, P., Jeon, S., et al. (2020). Monitoring plant health with near-infrared fluorescent H 2 O 2 nanosensors. Nano Lett. 20, 2432–2442. doi: 10.1021/acs.nanolett.9b05159
Wu, J., Du, H., Liao, X., Zhao, Y., Li, L., and Yang, L. (2011). An improved particle bombardment for the generation of transgenic plants by direct immobilization of relleasable Tn5 transposases onto gold particles. Plant Mol. Biol. 77, 117–127. doi: 10.1007/s11103-011-9798-5
Xiumei, L., Fudao, Z., Shuqing, Z., Xusheng, H., Rufang, W., Zhaobin, F., et al. (2005). Responses of peanut to nano-calcium carbonate. Plant Nutr. Fertit. Sci. 11, 385–389.
Yin, K., Gao, C., and Qiu, J. L. (2017). Progress and prospects in plant genome editing. Nat. Plants 3, 1–6.
Yu, Y., Yu, P.-C., Chang, W.-J., Yu, K., and Lin, C.-S. (2020). Plastid transformation: how does it work? Can it be applied to crops? What can it offer? Int. J. Mol. Sci. 21:4854.
Zeng, Z., Han, N., Liu, C., Buerte, B., Zhou, C., Chen, J., et al. (2020). Functional dissection of HGGT and HPT in barley vitamin E biosynthesis via CRISPR/Cas9-enabled genome editing. Ann. Bot. 126, 929–942. doi: 10.1093/aob/mcaa115
Zhang, H., Demirer, G. S., Zhang, H., Ye, T., Goh, N. S., Aditham, A. J., et al. (2019a). DNA nanostructures coordinate gene silencing in mature plants. Proc. Natl. Acad. Sci. U.S.A. 116, 7543–7548. doi: 10.1073/pnas.1818290116
Zhang, J., Zhang, X., Chen, R., Yang, L., Fan, K., Liu, Y., et al. (2020). Generation of transgene-free semidwarf maize plants by gene editing of gibberellin-oxidase20-3 using CRISPR/Cas9. Front. Plant Sci. 11:1048. doi: 10.3389/fpls.2020.01048
Zhang, R., Meng, Z., Abid, M. A., and Zhao, X. (2019c). Novel pollen magnetofection system for transformation of cotton plant with magnetic nanoparticles as gene carriers. Methods Mol. Biol. 1902, 47–54.
Zhang, R., Zhang, H., Tu, C., Hu, X., Li, L., Luo, Y., et al. (2015). Phytotoxicity of ZnO nanoparticles and the released Zn(II) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environ. Sci. Pollut. Res. 22, 11109–11117. doi: 10.1007/s11356-015-4325-x
Zhang, Z., Hua, L., Gupta, A., Tricoli, D., Edwards, K. J., Yang, B., et al. (2019b). Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol. J. 17, 1623–1635. doi: 10.1111/pbi.13088
Zhao, L., Sun, Y., Hernandez-Viezcas, J. A., Servin, A. D., Hong, J., Niu, G., et al. (2013). Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: a life cycle study. J. Agric. Food Chem. 61, 11945–11951.
Zhao, Q., Du, Y., Wang, H., Rogers, H. J., Yu, C., Liu, W., et al. (2019). 5-Azacytidine promotes shoot regeneration during Agrobacterium-mediated soybean transformation. Plant Physiol. Biochem. 141, 40–50.
Zhao, X., Meng, Z., Wang, Y., Chen, W., Sun, C., Cui, B., et al. (2017). Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat. Plants 3, 956–964. doi: 10.1038/s41477-017-0063-z
Zheng, M., Zhang, L., Tang, M., Liu, J., Liu, H., Yang, H., et al. (2020). Knockout of two BnaMAX1 homologs by CRISPR/Cas9-targeted mutagenesis improves plant architecture and increases yield in rapeseed (Brassica napus L.). Plant Biotechnol. J. 18, 644–654. doi: 10.1111/pbi.13228
Zhou, Y., Quan, G., Wu, Q., Zhang, X., Niu, B., Wu, B., et al. (2018). Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharmaceutica Sinica B. 8, 165–177. doi: 10.1016/j.apsb.2018.01.007
Zhu, Z.-J., Wang, H., Yan, B., Zheng, H., Jiang, Y., Miranda, O. R., et al. (2012). Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 46, 12391–12398.
Zuverza-Mena, N., Martínez-Fernández, D., Du, W., Hernandez-Viezcas, J. A., Bonilla-Bird, N., López-Moreno, M. L., et al. (2017). Exposure of engineered nanomaterials to plants: insights into the physiological and biochemical responses-a review. Plant Physiol. Biochem. 110, 236–264. doi: 10.1016/j.plaphy.2016.05.037
Keywords: CRISPR, genome editing, nanotechnology, nanoparticles, speedy crop improvement, crop enhancement
Citation: Ahmar S, Mahmood T, Fiaz S, Mora-Poblete F, Shafique MS, Chattha MS and Jung K-H (2021) Advantage of Nanotechnology-Based Genome Editing System and Its Application in Crop Improvement. Front. Plant Sci. 12:663849. doi: 10.3389/fpls.2021.663849
Received: 03 February 2021; Accepted: 26 April 2021;
Published: 28 May 2021.
Edited by:
Joachim Hermann Schiemann, Julius Kühn-Institut, GermanyReviewed by:
Gen Li, University of Maryland, United StatesJimmy R. Botella, The University of Queensland, Australia
Copyright © 2021 Ahmar, Mahmood, Fiaz, Mora-Poblete, Shafique, Chattha and Jung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ki-Hung Jung, a2hqdW5nMjAxMEBraHUuYWMua3I=; Freddy Mora-Poblete, bW9yYXBvYmxldGVAZ21haWwuY29t
 Sunny Ahmar
Sunny Ahmar Tahir Mahmood2
Tahir Mahmood2 Freddy Mora-Poblete
Freddy Mora-Poblete Muhammad Sohaib Shafique
Muhammad Sohaib Shafique Ki-Hung Jung
Ki-Hung Jung