- 1Department of Agricultural Biotechnology, Crop Improvement Division, School of Agriculture, Gandhi University of Engineering and Technology (GIET) University, Rayagada, Odisha, India
- 2Department of Genetics and Plant Breeding, Crop Improvement Division, School of Agriculture, GIET University, Rayagada, Odisha, India
- 3Department of Agricultural Biotechnology, College of Agriculture, Odisha University of Agriculture and Technology, Bhubaneswar, Odisha, India
In recent times, the demand for food and feed for the ever-increasing population has achieved unparalleled importance, which cannot afford crop yield loss. Now-a-days, the unpleasant situation of abiotic stress triggers crop improvement by affecting the different metabolic pathways of yield and quality advances worldwide. Abiotic stress like drought, salinity, cold, heat, flood, etc. in plants diverts the energy required for growth to prevent the plant from shock and maintain regular homeostasis. Hence, the plant yield is drastically reduced as the energy is utilized for overcoming the stress in plants. The application of phytohormones like the classical auxins, cytokinins, ethylene, and gibberellins, as well as more recent members including brassinosteroids, jasmonic acids, etc., along with both macro and micronutrients, have enhanced significant attention in creating key benefits such as reduction of ionic toxicity, improving oxidative stress, maintaining water-related balance, and gaseous exchange modification during abiotic stress conditions. Majority of phytohormones maintain homeostasis inside the cell by detoxifying the ROS and enhancing the antioxidant enzyme activities which can enhance tolerance in plants. At the molecular level, phytohormones activate stress signaling pathways or genes regulated by abscisic acid (ABA), salicylic acid (SA), Jasmonic acid (JA), and ethylene. The various stresses primarily cause nutrient deficiency and reduce the nutrient uptake of plants. The application of plant nutrients like N, K, Ca, and Mg are also involved in ROS scavenging activities through elevating antioxidants properties and finally decreasing cell membrane leakage and increasing the photosynthetic ability by resynthesizing the chlorophyll pigment. This present review highlighted the alteration of metabolic activities caused by abiotic stress in various crops, the changes of vital functions through the application of exogenous phytohormones and nutrition, as well as their interaction.
1 Introduction
Feeding the global population rise which is soon to reach 2.3 billion by 2050 is a challenging task in every way, so a considerable increase in grain productivity to at least about 70% is the need to accomplish this global challenge efficiently (Tilman et al., 2011). However, the major drawback in achieving this objective is the frequent occurrence of abiotic stress which affects the plant’s metabolic activities and triggers the biosynthetic pathways ultimately reflected in the reduction in quality and yield loss. Plants show their own mechanism to overcome the period of abiotic stress, for which maximum of their energy synthesized by the plant becomes diverted towards creating resistance or tolerance to the stress condition. The abiotic stress includes drought, cold, salinity, heat, water logging, metallic stress, etc. in plants transferring the energy to prevent the plant from such stresses and maintain normal growth. In the current scenario, these abiotic stressors are the major factors affecting production and productivity. Amongst various abiotic stresses, high temperature, water scarcity, and salinity are the most widespread and significant ones (Wani et al., 2013).
Plant body is a complex of several biomolecules, among them phytohormones are the molecules produced in very low concentrations, however, they show their active participation in regulatory activities (Shabir et al., 2016). The cellular activities are mostly regulated by the chemical communication inside the plant body with low-volume phytohormones (Vob et al., 2014). Phytohormones are most important to regulate various signal transduction pathways during abiotic-stress response. They regulate external as well as internal stimuli (Kazan, 2015). Auxin, cytokinin (CK), gibberellic acid (GA), ethylene, abscisic acid, brassinosteroids, salicylic acid, jasmonates, and strigolactones are the major phytohormones that have the major network in plant growth and development as well as in alleviating abiotic stress in plants. Nutrients are another crucial component that can minimize the effect of abiotic stress in plants by maintaining the inner homeostasis of the cell. Plant nutrients are considered the available form of food for plants for their normal growth and development. The plant nutrients are grouped into primary nutrients like nitrogen (N), phosphorus (P), and potash (K); secondary nutrients like calcium (Ca), magnesium (Mg)and sulfur (S); micronutrients like boron(B), zinc (Zn), iron (Fe)conditions, copper (Cu); and other beneficial nutrients like cobalt (Co), selenium (Se), silicon (Si). Due to global climate change, plant suffers a lot from nutrient deficiency. It was also noted that nutrient deficiencies are the major cause of yield loss during abiotic stress. Hence, proper nutrient management can elevate abiotic stress conditions in plants to some extent. Plant nutrients can mitigate stress also by activating stress resistance genes, enhancing antioxidant enzyme activity, creating osmoprotectant in cells, synthesizing heat shock proteins and other proteins related to stress tolerance, decreasing ROS activities, creating membrane stability, repairing DNA, enhancing chlorophyll content in leaves, reducing the uptake of heavy metals in the plant.
2 Effects of abiotic stress on plants
Abiotic stresses cause disorders in plants like osmotic stress in cells, retardation in cell development, reduced photosynthetic activity, seed dormancy, and late reproduction, and eventually show a negative effect on yield (Table 1). Among different types of abiotic stresses, water-deficit stress is most frequent in nature and causes ample of damage to crop plants. The rigorous impact of water deficit stress is due to reduced plant relative water content which causes osmotic and oxidative stress (Diouf et al., 2018). This condition occurs in salinity stress also and further triggers the same effect as drought (Munns and Tester, 2008). Both the drought and salinity stress the most menacing global abiotic stresses, which force a series of morphological, physiological, and molecular changes in plants, and in order to survive they require osmotic adjustment, ROS detoxification stomata closure, and cellular signaling (Diouf et al., 2018). Among the other stressors, high temperature can impact plants’ hormone production, nutrient uptake, stomatal conductance, transpiration rate, photosynthetic activities, enzymatic activity, antioxidants level, membrane stability index and reactive oxygen species (ROS) production (Hussain et al., 2018). Similarly, chilling stress in plants can also affect by putting impacts on tissue water content, membrane fluidity, and chlorophyll content (Zhang et al., 2012).
2.1 Drought stress
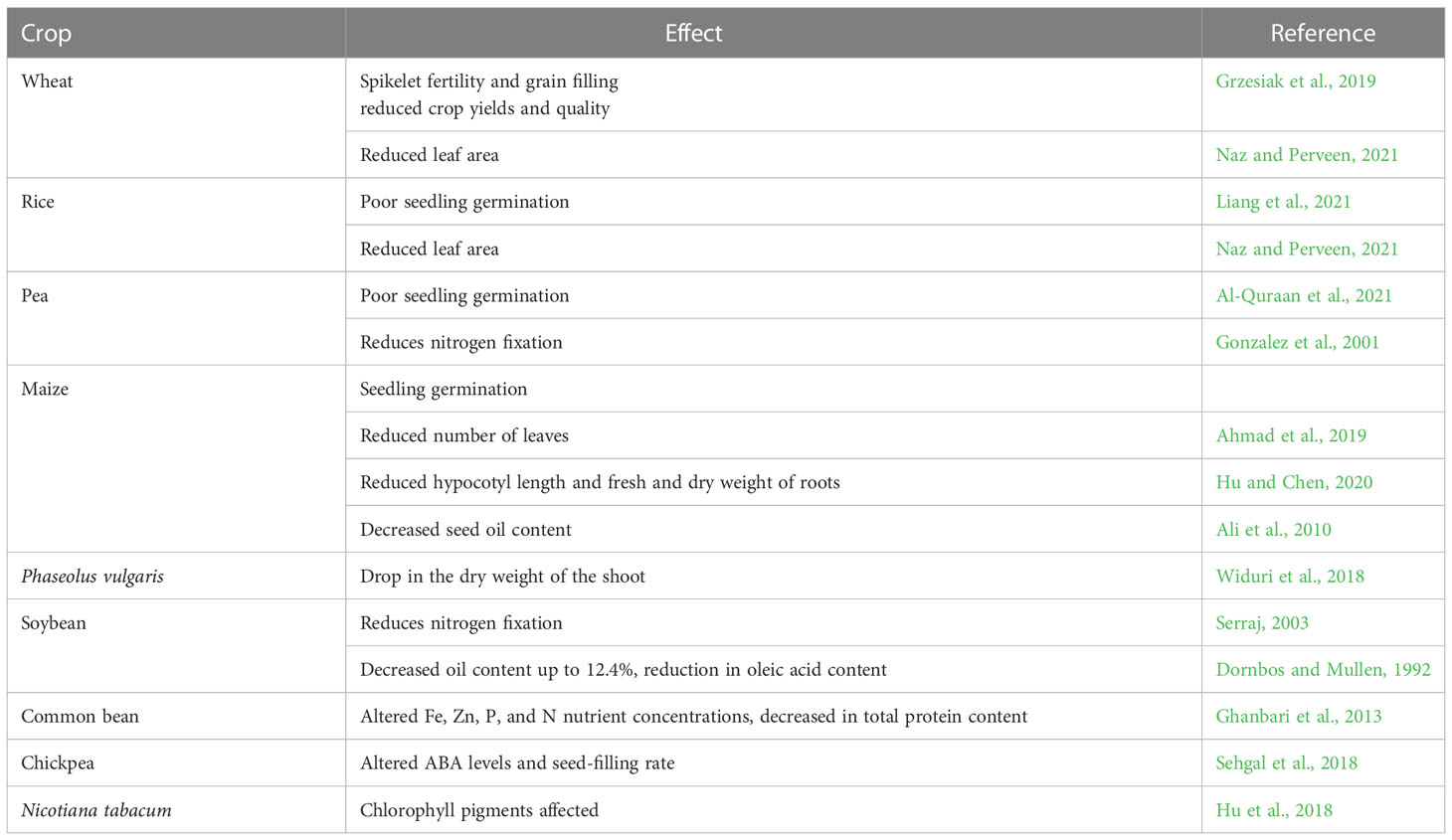
About half of the global arid and semi-arid regions are affected by drought stress. Under the conditions of drought stress, photosynthesis, growth, and physio-biochemical processes of plants are highly disrupted, which inhibits plant growth and development and results in yield loss. A significant loss in total biomass and productivity has resulted due to water stress conditions. Many researchers have reported that oxidative stress from excessive ROS i.e. superoxide, hydroxyl ions, nitric oxide, singlet oxygen production, and nutrition imbalance, altered cell membrane balance and biomolecules like DNA, proteins, and lipids, imbalanced photosynthetic efficiency reduced turgor pressure, and alterations in leaf gas exchange rates as some of the harsh impacts due to drought (Perveen and Hussain, 2020; Sofy et al., 2021; Alam et al., 2021; Zandi and Schnug, 2022). Numbers of morphological characteristics of plants, including seed germination, plant height, relative root length, root diameter, the total biomass of leaves and roots, number of leaves/plants, number of branches/plants, etc. are negatively impacted by drought stress (Table 2) which are more or less observed in every crops. Among the physiological impacts, crop plants experience partial stomatal closure and an increase in photorespiration due to an imbalance in carbon metabolism during water stress (Hu et al., 2019). Additionally, during stress, plants produce more reactive oxygen species (ROS), which harms chloroplasts through oxidation. All of these factors work together to limit photosynthates, which eventually lowers agricultural productivity. In response to the deadly impacts of water stress, plants activate their natural defense systems including various morphological, physiological, and biochemical adaptations, leaf rolling, altered leaf angle, deep root system, drought-resistant epigenetic phenotypic plasticity and gene activation, production of osmolytes, soluble proteins, proline, soluble sugars, and glycine betaine, etc. (Ozturk et al., 2021; Ghafar et al., 2021). While considering the effect of drought on phytohormones, the impact of stress depends on balancing of IAA and ABA content (Krishnan and Merewitz, 2014). Rapid ABA accumulation has also been observed under salinity and heat stress (Xiong et al., 2001). Experimental evidence regarding the exposure of moderate drought on Triticum aestivum and T. spelta showed initial increased accumulation of ABA and SA, decreased level of GA3 and IAA, alteration of CKs in roots and shoots (Kosakivska et al., 2022). ABA and ethylene significantly reduced gas exchange parameters, chlorophyll a and b content in cotton (Pandey et al., 2003).
2.2 Salinity stress
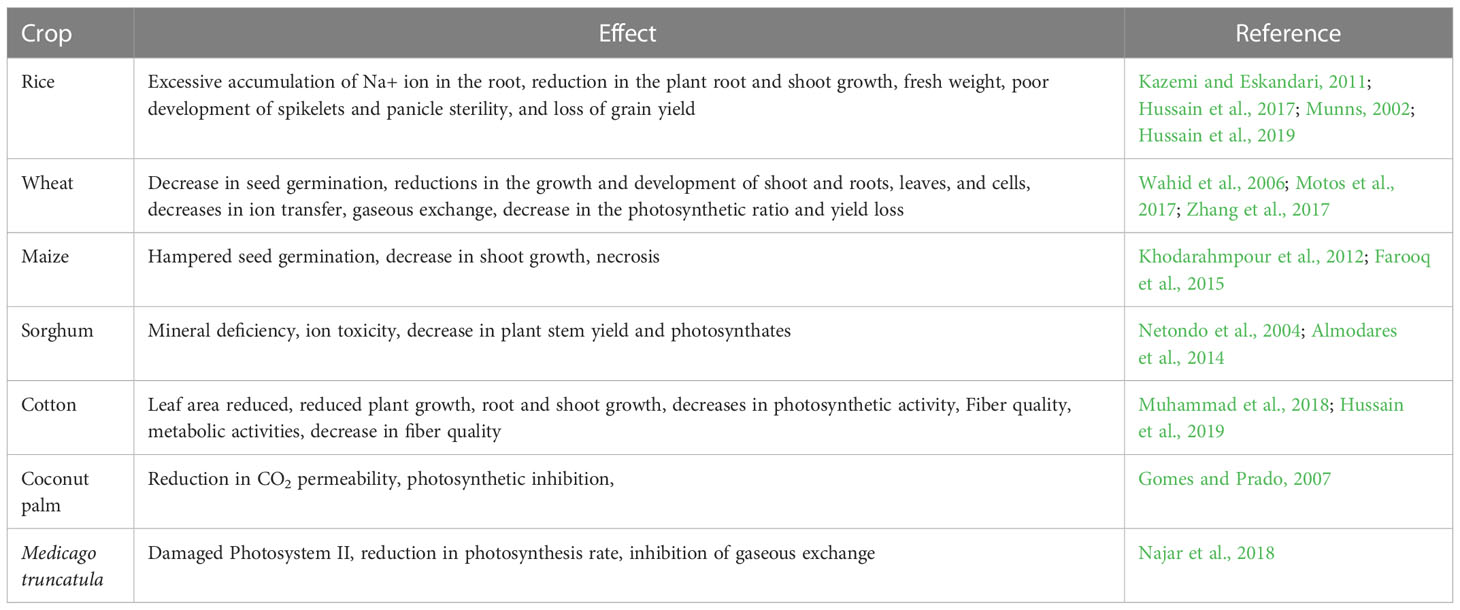
Saline soil having a high concentration of soluble salts with an ECe value of 4 dS/mL or higher in the soil. Salinity in the soil make it harder for roots to absorb water, and make it hazardous for plants. Salinity-resistant plants display morphological, biochemical, and physiological adaptations in an effort to maintain their life cycles. It’s estimated that 50% of cultivated agricultural lands will be under salt stress by 2050 (Shrivastava and Kumar, 2015; Salts of NaCl and Na2SO4 are the main reasons affecting the salinity of agricultural lands (Pessarakli and Szabolcs, 2010). Germination and early seedling stages are the most susceptible stages to soil salinity (Munns and Gilliham, 2015). By disrupting ionic and osmotic equilibrium, salinity creates stress, which ultimately causes physiological drought in plants. Salt stress causes a number of cellular and metabolic changes such as cellular growth and expansion disruption, plant membrane instability, ion toxicity, altering metabolism, inhibited seed germination, reduced photosynthesis, and reduced shoot, root, and leaf development in various crops (Table 3).
Along with the aforesaid effects, there is a fall observed in osmotic potential which ultimately reduced the uptake of nutrients and water by salinity stressed roots (Jose et al., 2017). Salinity induced stomata closure led to the inhibition of CO2 fixation and destruction of photosynthetic pigments (Qados, 2011), which adversely affected the photosynthetic processes, and electron carrier (Sudhir et al., 2005). Salinity stress has a negative impact on plants considering the hormonal level as well as nutrient level. It causes a hormonal imbalance of the ABA, and IAA levels in stressed plants as reported (Wu et al., 2005). Further, salts of NaCl increases concentrations of Na+ and Cl- ions which put forward the ionic stress by getting in to competition with essential nutrients such as K+, Ca2+, and Mg2+ leading a nutrient deficiency condition in plants (Botella et al., 2007). The aforesaid negative implications of NaCl salt will gradually lead to decreasing photosynthetic activity, generation of ROS, and programmed cell deaths (Serrano et al., 1999).
3 Response of phytohormones during abiotic stress
Low molecular weight phytohormones are considered to be the most important endogenous compounds having a crucial role in regulating physiological reactions of helps plants to heal in adverse environmental stress condition. (Khan et al., 2013). Reduced seed germination and plant growth have been linked to lower endogenous levels of phytohormones which can further be aggravated by various abiotic stresses (Iqbal et al., 2006). Stress can induce and activate various plant endogenous phytohormonal activities which further help in expression of various beneficial plant genes and proteins (Hamayun et al., 2010). Exogenous phytohormone application has also been proposed as a useful tactic to address various abiotic stresses, such salinity, drought, etc. (Iqbal et al., 2006), and also associated with several studies in reducing the negative impacts of abiotic stressors (Sharma et al., 2013; Iqbal and Ashraf 2013a, b; Amjad et al., 2014). The primary location for auxin production is in the apical meristem of shoots, immature leaves, and seeds. They contribute to phyllotaxis, apical dominance, root formation, embryogenesis, and reaction catalysis. In the molecular mechanism of auxin production, the TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA) family, YUCCA gene families are the most important contributors. Majorly YUC gene family from which YUC flavin monooxygenases (YUC1, YUC2, YUC4, and YUC6) play essential roles in its auxin biosynthesis and plant development (Cheng et al., 2006).
The main cytokinins found in higher plants are zeatin, isopentenyl adenine, and dihydrozeatin, however zeatin is the most common cytokinin (Kieber and Schaller, 2018). The inhibition of lateral root initiation (Bielach et al., 2012), differentiation of phloem and metaxylem in roots (Bishopp et al., 2011), differentiation of photomorphogenic cells in expanding leaves and shoots (Efroni et al., 2013), and inhibition of leaf senescence are just a few examples of the significant regulatory functions of cytokinins at the tissue and organ levels (Zwack and Rashotte, 2015). The phytohormone has a good control over cell division (Miller et al., 1955), cell homeostasis, and adaptation of plants to climate change (Landrein et al., 2018). ABA is also known as stress hormone as whilst under stress, plants build up ABA, which sets off a reaction to deal with the adverse environment (Mahajan and Tuteja, 2005). It is a signaling molecule for regulation of seed germination and plant growth and development and seed maturation (Yan and Chen, 2016). From seed germination until senescence, the physiological and developmental processes of plants are thought to be significantly regulated by ethylene (Pierik et al., 2006). It plays a part in the regulation of photosynthesis, the metabolism of nutrients and proline and the antioxidant defense mechanism that shields plants from environmental stressors. Numerous studies have shown both benefits as well as negative impacts of the phytohormone. while in corn, Arabidopsis, tomato, and grapevines, ethylene and its precursor ACC (1-aminocyclopropane-1-carboxylate) helps to tolerate environmental adversities; in Cucurbita pepo, tomato, Arabidopsis, and tobacco ethylene claimed its negative impact on plant growth (Lin et al., 2012; Yang et al., 2013; Freitas et al., 2017; Gharbi et al., 2017; Xu et al., 2019; Cebrián et al., 2021). The genetic basis unravels the APETALA 2/ethylene-responsive element binding factor (AP2/ERF) which is a plant specific transcription factor family is an important ethylene biosynthesis factor. It has four major subfamilies: DREB (Dehydration Responsive Element-Binding), ERF (Ethylene-Responsive-Element-Binding protein), AP2 (APETALA2) and RAV (Related to ABI3/VP), and Soloists (few unclassified factors). These subfamilies act as crucial regulators in a variety of biological and physiological processes, including signal transduction, regulator of plant morphogenesis, stress-response mechanisms, and metabolic activities (Li et al., 2020). Gibberellins (GA) are growth regulators that are particularly effective for seed germination, stem lengthening, enlarging fruit, and inducing blooming (Camara et al., 2018). Gibberellins’ main function is to promote cell elongation, which in turn promotes cell division, accelerating both the vegetative and reproductive stage of plant growth (Colebrook et al., 2014; Kang et al., 2014). Exogenous GA treatment has also several benefits like it promotes early and large number sprouting in potato tuber (Alexopoulos et al., 2017), further it can improve the amount of viable seeds and antioxidant enzyme activity, increases the weight of individual fruits (Zang et al., 2016). Among the other phytohormones, brassinosteroid (BR) which was initially discovered in pollen of Brassica napus (Saini et al., 2015) was reported to be involved in root extension, maintenance of meristem size, initiation of lateral roots, creation of root hairs, mycorrhiza, and nodule formation (Mc Guinness et al., 2019; Wei and Li, 2016). Further during stress condition, crops like maize, soybean and banana are benefitted from methyl jasmonic acid in terms of increasing photosynthetic rate, grain yield, and drought tolerance (Anjum et al., 2016; Yu et al., 2019) Stress responses are essentially driven differently by different phytohormones and their crosstalk, that leads to transcriptional reprogramming in plants’ response. The pivotal roles of phytohormones can be manipulated for mitigating the effect of the stressor.
4 Response of nutrients during abiotic stress
All the seventeen essential nutrients of plants are more or less responsible for abiotic stress alleviation in their own way. The most important plant nutrients, nitrogen (N), have an impact on physiology, growth, the reduction of biotic and abiotic stress, and structural integrity (Karim et al., 2016). However, it has a significant impact on crop plants’ ability to effectively use solar energy, increase photosynthetic activity, and synthesize chlorophyll (Waraich et al., 2011). Phosphorus not also improves root architecture and proliferation in the soil even in soil drying conditions, but also stimulates root volume and hydraulic conductivity (Tariq et al.,2017). The modulation of numerous morphological, physiological, and biochemical processes by phosphorous within the plant system helps them to withstand stress better. Plant growth and development under stress are strongly and positively correlated with the use of phosphoric fertilizers. Ge et al. (2012) reported that potassium is another crucial nutrient for many fundamental physiological and metabolic processes including photosynthesis, stomatal control, photosynthesizing, carbohydrate metabolism, preservation of cell turgidity, enzyme activations, etc. Potassium is also essential for improving crops’ tolerance to various abiotic stresses (Danial et al., 2010).
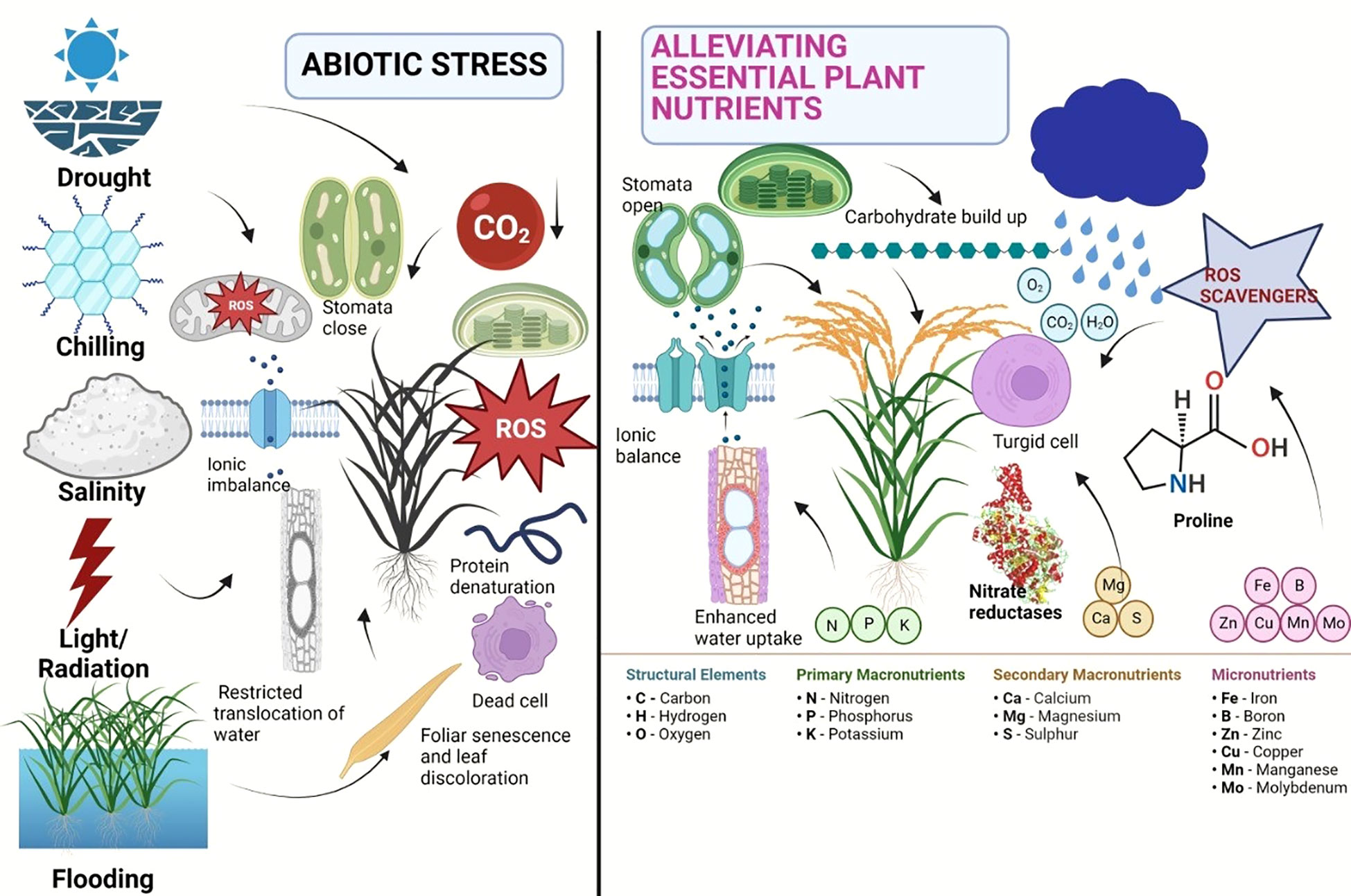
Calcium (Ca), an important secondary nutrient, acts as a signaling molecule in a number of physiological and biochemical processes that are necessary for a plant to develop stress tolerance (Ahmad et al., 2015). Magnesium (Mg) is essential for the conformational stabilization of macromolecules such as nucleic acids, proteins, cell membranes, and walls and is a structural component of the ribosome (Marschner, 2012). Its absence can have an impact on photosynthesis because it is a crucial element of the chloroplast, which controls photosynthetic activity. In the abiotic stress response, cellular acclimatization, and adaptability to challenging circumstances, sulfur performs protective roles (Cao et al., 2014). According to reports, an exogenous dose of sulfur increases crop productivity while maintaining regular metabolic processes that enable plants survive in harsh settings (Hasanuzzaman et al., 2013). The micronutrients like boron, zinc, iron, and copper reduce environmental stress through a variety of mechanisms, including glucose metabolism and transport, production of cellular integuments, preservation of membrane integrity, and activation of numerous enzymes. The structural role of selenium (Se) in the synthesis of glutathione peroxidase (GPX), which protects plants from the damaging effects of ROS, is also well documented (Lobanov et al., 2008). An adequate supply of Zn shields plants from the damaging effects of heat stress because it plays a significant role in maintaining membrane permeability (Peck and McDonald, 2010). The plant nutrients can be very much effective similar to the phytohormones for alleviation of various negative impacts of abiotic stresses. A brief account of abiotic stress alleviation using plant nutrients has been depicted in Figure 1. It is observed that in response to several abiotic stresses, major nutrients like N can enhance the photosynthesis of plant, phosphorus can be able to produce proliferate and strong root system, calcium can enhance the membrane stability and cellular integrity in plant, the micronutrients can able to regulate the cellular activity and mitigate abiotic stress by activating numerous enzyme and selenium can protect the plant from ROS activities.
5 Phytohormones and their effect on abiotic stress
During abiotic stress, it was observed that the phytohormones levels are altered; majorly ABA and ethylene level enhanced along with reduction of auxin and cytokinin are seen in a number of crops. The phytohormone works both in the response to stress as well as works for alleviation of stress. Both endogenous and exogenous level of phytohormones is showing equal importance in alleviation by regulating the internal and external stimuli in plants. The genes responsible for the phytohormone level regulation are activated and their upregulation can be helpful for enhancing stress tolerance in plant. When plants are affected by several stresses, especially water deficit, plant hormones play vital roles in their growth and development (Raza et al., 2021). Several plant growth regulators, including salicylic acid, gibberellins, auxins, cytokinin, and abscisic acid, have reacted to drought (Chen et al., 2019). Phytohormones regulate internal and external stimuli, as well as signal transduction pathways, in addition to stress responses. Water logging or flood is a major constraint in low land conditions, the use of phytohormones signaling pathway can lead to a better way to alleviate the stress and achieve higher yield. Cold stress is a major problem in tropical and subtropical crops, whereas heat stress in temperate crops hampers crop production and productivity. It was observed that endogenous phytohormones level like gibberellic acid, brassinosteroids, cytokinins, abscisic acid, salicylic acid, jasmonic acid, and, auxin modified and regulates plant growth. A number of genes are activated during the exogenous application of plant hormones as a result tolerance can be created in the plants. So, studies on gene regulation and translation mediated by phytohormones can unlock a new way to recover low-temperature stress in plants.
5.1 Effect of auxin on stress
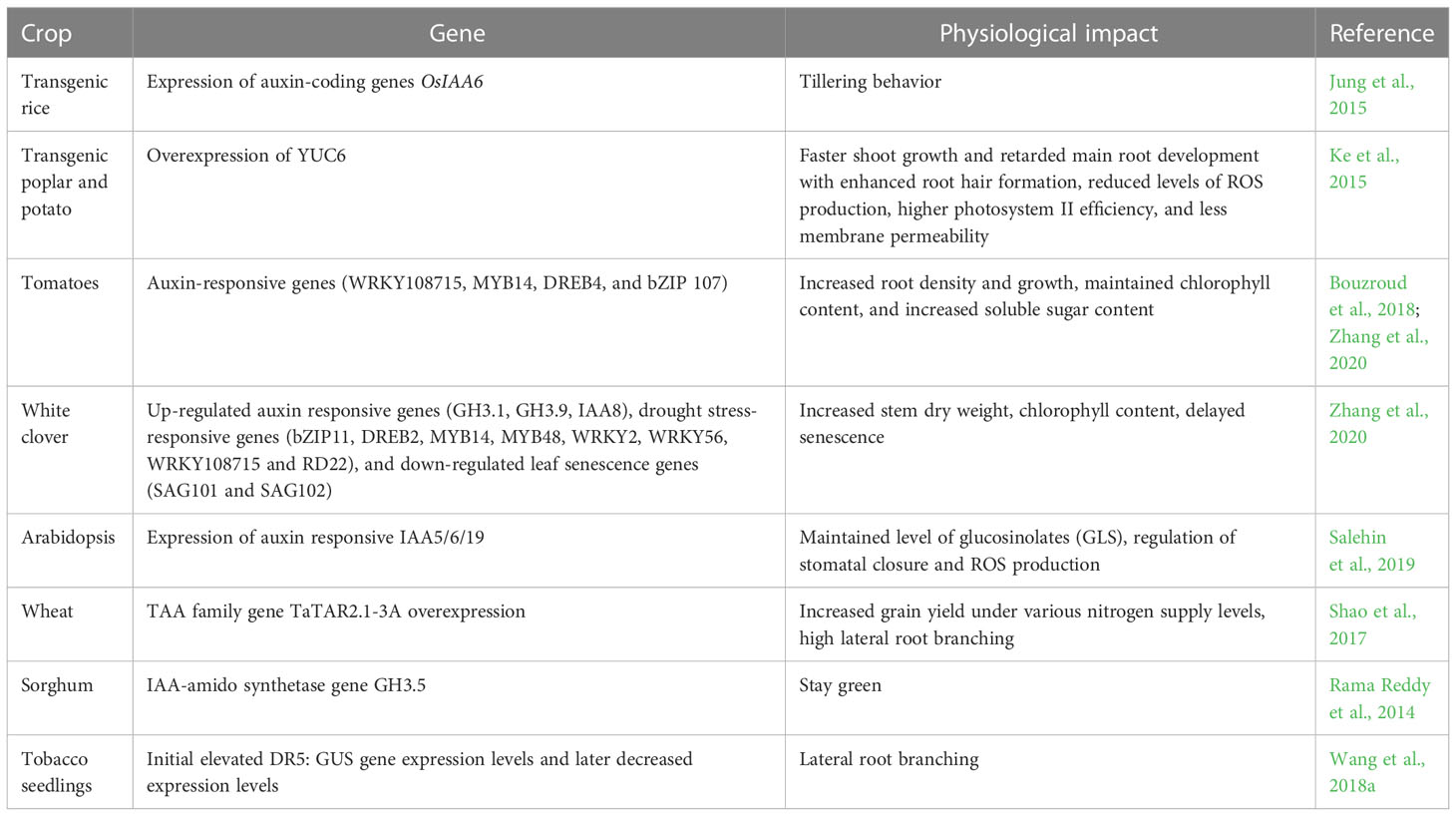
On exposure to drought, the plasticity of the plant root is affected that is regulated by the auxin. Auxin buildup in the root system reduces daytime and nocturnal water use and modifies hydraulic characteristics to allow the expression of water-saving features in wheat, maize, and sorghum yields during droughts. (Shao et al., 2017; Li et al, 2012; Rama Reddy, 2014). The exogenous application of auxins has shown to be effective in managing drought stress in plants. Indole-3-acetic acid (IAA) is the most common plant hormone of the auxin class and is mainly synthesized from the amino acid tryptophan (Trp). IAA triggers the activation of other stress-responsive hormones as well as the production of ROS. ROS production molds several physiological processes in a plant in response to water deficit stress. Discovery and characterization of numerous auxin-responsive genes in a number of plant species including rice, soybean, and Arabidopsis has paved the way for exploiting the genes to induce stress response (Hagen and Guilfoyle, 2002). A membrane-bound transcription factor NTM2 was used for auxin signaling controls for seed germination in salinity stress (Jung and Park 2011; Park et al.,2011). The number of genes like TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA) family, YUCCA gene family is the most important contributors to auxin biosynthesis (Cheng et al., 2006) that controls several metabolic activities in the drought-affected crop plants. Table 4 highlighted the impact of auxin-linked genes on the stress response.
Auxins have significant involvement in temperature-related stress. Shibasaki et al. (2009) performed a direct transport assay using an auxin-responsive marker (IAA2-GUS) on cold stress and concluded that the intracellular auxin efflux carriers are inhibited in plants due to cold stress. In high temperature, the plant mainly suffers due to reduction of pollen viability in major crop, affecting seed set and eventually reducing yield. In crops like wheat and barley, it was observed that the initial pollen development stage is majorly hampered due to the reduction of pollen auxin concentration at high temperatures (Tadashi et al., 2010). They concluded that tissue-specific auxin concentration reduction can lead to pollen abortion during high-temperature stress. An analogous study was reported by Zhang et al. (2018) through an experiment in rice by exposing the rice spikelet to high temperature which drastically reduced the spikelet fertility and mitigated its effect by the application of NAA (1-naphthaleneacetic acid). Through the application of NAA in rice crops, auxin concentration was increased and leads to the proper development of pollen tube growth, elongation in the pistil, stylar length of the flower, and ultimately the pollen behaves normally under high temperatures leading to proper pollination and fertilization. In wheat, the effect of the exogenous application of auxin was estimated under heat stress conditions and found that the application of 1 µM of IAA can enhance higher grain number and yield by 6% – 8% under heat stress conditions (Abeysingha et al., 2021).
5.2 Effect of abscisic acid (ABA) on stress
Abscisic acid is a signaling molecule in plants in responses to stress conditions and noted as second group of phytohormone. ABA is a 15-carbon atom compound that belongs to a group of metabolites, known as isoprenoids or terpenoids, which are synthesized in the plastids (Xiong and Zhu, 2003; De Ollas et al., 2013). Under optimal conditions, ABA is expressed at low concentrations in plants (Parveen et al., 2021) and the concentration increases with the signal of stress to plants. In drought conditions, ABA alteration of guard cell ion transport regulates stomatal opening that reduces water loss (Kim et al., 2010). Upon exposure to drought stress, ABA is synthesized in roots and translocated to leaves wherein mesophyll cells are the predominant location of ABA synthesis (McAdam and Brodribb, 2018). Biosynthesis of ABA triggers drought adaptation mechanisms in the plants such as growth retardation, stomata closure and activation of several drought-responsive genes (Qi et al., 2018; Wilkinson and Davies, 2010). ABA regulates turgor by decreasing transpiration as well as by increasing water influx into roots (. Root-specific ABA signaling helps in balanced root growth toward soil exploration which regulates the transpiration and increases water influx into roots (Glinka and Reinhold, 1971, Duan et al., 2013). Considering the molecular activities of the phytohormone, in drought tolerant transgenic Arabidopsis overexpression of IbARF5 gene up-regulates the ABA biosynthetic genes (IbZEP, IbNCED, and IbABA2) was reported (Kang et al., 2018). Various transcription factors such as DREB2A/2B, AREB1, RD22BP1, and MYC/MYB are known to be the regulator of the ABA-responsive gene expression (Tuteja, 2007). SAPK2 ((Stress-Activated Protein Kinase) of SnRK2s (Sucrose nonfermenting1–Related protein Kinase 2) family which is an important ABA regulator, upregulates the expression of several drought responsive genes, including OsLEA3, OsOREB1, OsRab16b, OsRab21, and OsbZIP23 (Lou et al., 2017). From the aforesaid reported studies, it has been clear about the roles of endogenous ABA regulator genes and their role in the drought alleviation. Similarly, the exogenous application of ABA during drought in maize seedlings can also play role in activating antioxidant enzymes which in molecular basis regulates expression of ASR1, and endogenous ABA level, as well as reduce oxidative damage (Yao et al., 2019).
Similar to drought, salt-responsive genes’ expression is also known to be regulated by both endogenous and exogenous ABA-mediated signaling when the soil is affected by salinity (Wang et al., 2001, Narusaka et al., 2003). Zhang et al. (2006) found a proportionate link between plants’ exposure to salinity and their ABA content. Both endogenous ABA and its exogenous application demonstrate a critical role in preserving ionic balance in plants, as shown by their ability to prevent chloride toxicity in citrus leaves, avoid Na+ and Cl-, and maintain K+/Na+ ratio response in rice, K+ and Ca + homeostasis (Gomez et al., 2002; Bohra et al., 1995; Gurmani et al., 2013). In addition to stomatal regulations, this phytohormone also aids in the osmoprotectants like proline (Iqbal et al., 2014) and dehydrins in response to ROS production during salinity-induced dehydration (Szabados and Savoure, 2009; Kim and Wang 2010; Hara 2010; Gurmani et al. 2013). Sripinyowanich et al. (2013) reported that, the expression of the OsP5CS1 gene increased proline accumulation and increased the survival rate (20%) of Indica rice seedlings by exogenous application of 100 M ABA. Shi and Zhu (2002) noted that ABA induced AtNHX1 expression in barley in response to salt stress. Keskin et al. (2010) stated that, ABA treatment caused quicker expression of MAPK4-like genes (TIP1 and GLP1) in wheat crops under salinity. Apart from drought and salinity, the exogenous application of abscisic acid (ABA) and ethylene also plays role in controlling other abiotic stresses. In green under greenhouse conditions the above hormones inhibit the suppression of photosynthesis in waterlogging by rejuvenating several factors like photosynthetic rate, transpiration rate, stomatal conductance, chlorophyll content and leaf water potential (Ahmed et al., 2002).
In case of cold stress, the effect of ABA application was studied on bermudagrass at 4 0C with application of 100 μM ABA which showed increased levels of chlorophyll content, maintained cell membrane stability, improved the performance of photosystem II, and altered expression of ABA or cold-related genes, including ABF1, CBF1, and LEA developing cold resistance in the grass (Huang et al., 2017). When wheat was exposed to low temperatures (0°C, −10°C, −20°C, and −25°C) application of exogenous ABA decreases the amount of H2O2 and relative conductivity (Jing et al., 2020). ABA was found to enhance cold tolerance in both leaves and rhizomes at −10°C and −20°C by increasing ROS production (Jing et al., 2020). In the same way, the ABA application was studied for its effect on heat tolerance in two rice germplasm lines. Li et al. (2020) used rice germplasm lines having flat leaves called wild type (WT) and others having rolling leaves with high-temperature sensitivity (hts) lines exposed to high temperature. The high-temperature lines showed a higher respiration rate with high tissue temperature and lower transpiration rate and stomatal conductivity but the WT line showed increased carbohydrate content, dry matter increased production of heat shock proteins (HSP71.1 and HSP24.5) under high-temperature stress. Through ABA application in these two lines, it was observed that thermo-tolerance was increased in the wild type but tolerance was reduced in hts plants (Li et al., 2020).
5.3 Effect of cytokinins on stress
Many drought-related processes are mediated by the stress hormone (ABA) as well as cytokinins (CKs). When plants are under drought stress, their CK content falls, and this increase in ABA responses causes the stomata to close and impede photosynthesis (Rivero et al., 2010). Stomatal conductance and transpiration are increased by CKs’ ability to keep the stomata open (Lechowski, 1997). These CKs and ABA alterations brought on by stress encourage early leaf senescence and hormonal adjustments that cause leaf abscission, which results in a smaller canopy and less water loss. Pospisilova et al. (2000) observed that the expression of a cytokinins biosynthetic gene isopentenyltransferase (IPT), catalyzes the rate-limiting step in CK synthesis. Overexpression of IPT enhances the antioxidant system activity and increases drought tolerance by improving root growth in plant (Xu et al., 2016). Hormones like cytokinin enhance primary root growth in Arabidopsis by giving positive signaling to plant (Naulin et al., 2020). I In transgenic barley (Hordeum vulgare) and in tobacco it was observed that root specific reduction of cytolinin led to the enlarged root system under stress situations (Werner et al., 2010; Pospíšilová et al., 2016). It has also been shown that the increased transcriptional level of CKX genes and/or CKX activity was due to the exogenous application of cytokinin. It was observed that due to oxidase/dehydrogenase (CKX) which catalyzes CK and the overexpression and breakdown of CKX in Arabidopsis carried out as a result endogenous CK contents is decreased in plant (Werner et al., 2010). The abnormal expression of CKX in barley via maize β-glucosidase, a mild root-specific promoter has also been found to alter root architecture leading to lignification of the root tissue as well as activation of flavonoids biosynthesis (Vojta et al., 2016). Plant shows a higher level of accumulation of CK in root tissues due to a decrease in the activity of CKX, during drought stress (Havlova et al., 2008). The plant growth rate was slow down and elevate the content of protective compounds due to overexpression of CKX, which finally gives rise to increased drought tolerance in Arabidopsis, tobacco, and barley (Macková et al., 2013; Nishiyama et al., 2011; Pospíšilová et al., 2016).
Salt-sensitive plants’ development was negatively impacted by salinity by lowering CK levels, indicating genotypic specificity (Kuiper et al., 1989). After being exposed to salinity, the amounts of CKs such as zeatin (Z), zeatin riboside (ZR), and isopentenyl adenine (iP) in the shoots and roots of barley cultivars drastically decreased (Kuiper et al., 1990). The negative effects of salt on plant growth are also known to be mitigated by CKs (Barciszewski et al., 2000; Fahad et al., 2014). Plant resistance to salt stress was reported to enhance with seed primingB with CKs (Iqbal et al., 2006). Iqbal et al. (2006) reported that CKs operate as ABA antagonists and IAA antagonists/synergists in a variety of plant processes and assist reduce salinity stress (Iqbal et al., 2014). Under exogenous application of CKs, it enhanced salt resistance via increased proline levels in brinjal (Wu et al., 2013). Plant hormones, particularly CKs, control the expression of a large number of stress-induced genes. Merchan et al. (2007) reported that the changes in osmotic circumstances also affect the expression of CKs receptor genes, showing that these receptors may have a similar function in the osmotic stress response despite the lack of a clear mechanism.
In waxy corn, exogenous application of 6-benzyl adenine (BA) in water logging conditions noted that not-treated plants showed chlorosis and necrosis in leaves, inhibiting growth and leading to the accumulation of O2, H2O2, and MDA-like reactive oxygen species (ROS) but in treated plants, the reduction of ROS accumulation and increase of enzyme activities like ascorbate peroxidase, glutathione reductase, dehydroascorbate reductase, and monodehydroascorbate reductase (Wang et al., 2021). Hence, the application of exogenous BA can alleviate water-logging-induced damage and improve water logging tolerance in waxy corn via the activation of the AsA-GSH cycle system and the elimination of ROS. The application of BA in waterlogged maize crops showed enhanced grain filling by improving grain weight and volume, which was beneficial to yield increase as compared to the untreated plant (Baizhao et al., 2019). It was recorded that the application of exogenous BA alleviated endogenous hormone levels of IAA, zeatin, and GA3, and at the same time, ABA content was decreased during grain-filling periods of waterlogged summer maize. The foliar application of CK and GA3 under waterlogged conditions revealed that growth and biomass were enhanced, which was associated with increased levels of photosynthetic rate and pigments in the plant (Islam et al., 2022). It was reported that the accumulation of ROS and malondialdehyde levels is reduced during the water logging condition by application of CK and GA3. Therefore, a better osmotic adjustment was carried out through proline and TSS level improvement in plants. Both CK and GA3 were effective in water-stressed plants, however, CK was considered more effective than GA3 (Islam et al., 2022). Prerostova et al. (2021) identified two genes which to be associated with cytokinin metabolism in plant, i.e., CK biosynthetic gene isopentenyl transferase (DEX: IPT) and CK degradation gene HvCKX2 (DEX: CKX). They observed that plants containing the DEX: IPT gene showed better stress tolerance with increased production of CK and SA levels in shoots and also auxin in the apex. At the same time plant containing the DEX: CKX gene and control plants showed weaker stress tolerance with lowered levels of CKs and auxins in cold conditions.
5.4 Effect of ethylene on stress
Ethylene (ET) has a significant role in fruit softening along with a vital role in mitigating the harmful impact of stress conditions due to abiotic factors (Pech et al., 2018; Wang et al., 2020). In diverse range of abiotic and biotic stress condition it was found that ET has a major role in nodule formation and nodule signaling (Khalid et al., 2017). Furthermore, it also enhances root emergence from nodal region which leads to retardation in development of nodal root and ultimately give rise to a negative effect on root-lodging resistance in Zea mays (Shi et al., 2019). Drought induces ethylene synthesis in shoots, by up-regulating the synthesis and xylem transport from roots to shoots of the ethylene precursor ACCs (Sobeih et al., 2004). It was found from research that adventitious root initiation sites in Arabidopsis hypocotyls are controlled by ethylene (Rasmussen et al., 2017). The overexpression of ethylene response factor such as GmERF3 of AP2/ERF gene family, leads to improvement in proline content, soluble sugar, and decreases in the accumulation of malondialdehyde to improve drought tolerance in the tobacco plant (Zhai et al., 2017). Further, SlERF5 of the aforesaid transcription family in over-expressing transgenic tomato plants resulted in high tolerance against drought (Pan et al., 2012). It was also found that gene 269 AP2/EREBP in cotton showed water stress response in plant (Liu and Zhang, 2017). Ethylene application response was also studied under water logging stress in soybean. It was noted that after the application of ETP (ETP; donor source of ethylene) in soyabean under water logging stress, the chlorophyll content significantly enhanced, and also cellular gibberellic acid is increased in the treated plant as compared to untreated plants (Yoonha et al., 2018). The amino acid content was also found appreciably higher in ETP-applied soybean plants than in the control. Several adventitious roots were induced in the plant after ETP application which enhance the root surface area and considerably amplified the expressions of glutathione transferases which that control ROS under water stress (Yoonha et al., 2018).
In the case of Arabidopsis thaliana, it was observed that freezing tolerance decreases by the introduction of the ethylene overproducer1 gene and by the application of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid but the freezing tolerance enhanced when ethylene biosynthesis inhibitor amino-ethoxyvinylglycine was applied (Shi et al., 2012). Shi et al. (2020) thus suggested from their research that ethylene can negatively regulates cold signaling through the direct transcriptional control of cold-regulated CBFs and type-A ARR genes. Sun et al. (2016) found a positive correlation between ethylene (ET) and cold stress was studied in grapevine. The treatment of exogenous 1-aminocyclopropane-1-carboxylate a form of ethylene was able to mitigate the cold stress in crops compared to the application of ET biosynthesis inhibitor amino-ethoxyvinylglycine which reduced the cold tolerance of grapevine. It was also observed that overexpression of gene ‘VaERF057’ enhances cold tolerance in Arabidopsis and ethylene is associated with the signaling of this gene. Thus, the research concluded that ET positively regulates cold tolerance in grapevine by regulating the expression of VaERF057 gene associated with cold tolerance (Sun et al., 2016). Wang et al. (2021) reported in case of apple seedlings, when treated with 1-aminocyclopropane-1-carboxylate (an ethylene precursor) and amino-ethoxyvinylglycine (an ethylene biosynthesis inhibitor), it was observed that the cold tolerance was increased and decreased respectively in the crop. They reported that during low-temperature treatment, ethylene level enhanced which leads to the over expression of MdERF1B significantly, increasing the cold tolerance of apple planting materials (seedlings and calli) as well as in Arabidopsis seedlings by mediating ethylene signaling pathway. Furthermore, molecular analysis proved that MdERF1B interacted with the promoters of two ethylene biosynthesis genes, i.e., MdACO1 and MdERF3. Wang et al. (2021) result thus concludes that MdERF1B–MdCIbHLH1 is a potential regulatory pathway that integrates the cold and ethylene signaling pathways in apples by up-regulating ethylene production under cold stress. While under high-temperature stress or heat stress ethylene is found to affect the pollen viability and sterility in plants similar to the auxins. In research conducted by Jegadeesan et al. (2018), it was observed that tomato pollen sterility can be overcome by the application of ethylene hormone (ethephon) during heat stress conditions. A protein analysis conducted during the study showed pollen development was hampered during heat stress due to the degradation of some proteins responsible for pollen development, pollen tube germination, and tube growth under the pistil surface. Jegadeesan et al. (2021) reported that ethylene hormone had a positive impact on pollen viability and germination, and the ability to increase the overproduction of heat tolerance genes like SlHSP17, SlHSP101, SlMBF1 in tomatoes, when applied exogenously reducing the harmful effects of heat stress in due course. Another study in wheat showed ethylene again plays a vital role by regulating the biosynthesis of proline and modifying the antioxidative mechanism under heat stress. Application of 200 µL of ethephon and 50mM of proline showed improved tolerance of wheat in heat stress by activation of defense mechanism and protecting the photosynthetic pigment by enhancing the photosynthetic gene expression in crops (Sehar et al., 2022).
5.5 Effect of gibberellins on stress
On drought stress conditions, down regulation of GA could be a major target in making drought-tolerant plants. Nir et al. (2014) reported that the transgenic plants with the lower GA level tend to produce high stomatal intensity, lower stomatal conductance, and smaller leaves, which reduces the transpiration rate in stress. Further, the overexpression of SlDREB of the AP2/ERF family down-regulates GA biosynthetic genes in tomatoes. In tomato internode elongation and leaf expansion is reduced as a result of lower GA level in plant which ultimately create drought tolerance mechanism in plant (Li et al., 2012). Further studies confirmed water deficiency leads to downregulation of GA biosynthesis genes GA20 oxidase1 (GA20ox1) and GA20ox2 and induce the GA deactivating gene GA2ox7 in guard cells and leaf tissue, resulting in reduced levels of bioactive Gas in tomato (Shohat et al., 2021). Moreover, the over-expression of another transcription factor PtGA2ox1 decreases the GA level in the roots, stems, and leaves of the tobacco plant to promote drought tolerance (Zhong et al., 2014). In addition to maintaining protein and RNA levels, higher water level was also credited with GA’s beneficial effects under salinity stress (Yamaguchi 2008). Maggio et al. (2010) reported that the application of GA to tomato plants reduced stomatal resistance and increased plant water usage effectiveness at lower salinity levels. Under salinity, the root and leaf cell nitrogen and magnesium are increased due to GA application (Tuna et al., 2008). Multiple factors, including an increase in reducing protein synthesis, activity of enzymatic antioxidants, sugars, and decreased activity of ribonuclease and polyphenol oxidase, contributed to GA3’s beneficial effects on salt-stressed mung bean seedlings (Mohammed, 2007). Modulation of ions absorption and partitioning (inside shoots and roots) as well as hormonal homeostasis brought on by GA3 priming under salinity (Fahad et al., 2014). Through changed GA levels, the seed germination rate is enhanced due to the salt-inducible DDF1 gene (dwarf and delayed flowering 1) in high saline stress condition.
The applications of gibberellins in soybean plants found to reduce chlorophyll damage and also enhance the endogenous level of GA1 and GA4, and jasmonic acid in the plant along with the reduced level of ABA under flooding conditions (Muhammad et al., 2018). The research reveals that exogenous application of GAs during short-term waterlogging could enhance the transcriptional pathways and biochemicals which are majorly needed for maintaining plant growth during stress. Calvin et al. (2019) reported that the application of GA3 (200 ppm) in combination with salicylic acid (150ppm) on the soybean plant provides better mitigation effects by improving the number of pod and seed, chlorophyll content in waterlogged conditions. Gibberellins were found to be extremely sensitive towards cold stress and several GA metabolic genes, GA3ox1, GA20ox1, and GA2ox1 were found to be activated during cold temperatures (Ding et al., 2015). GA3 treatment has also improved fruit storage under low temperatures by decreasing malondialdehyde content and electrolyte leakage, increasing proline content and improving antioxidant enzyme activities as compared to untreated conditions (Ding et al., 2015). Shashibhusan et al. (2021) observed that pre-treatment of plants with 1gm, 2gm, and 3 gm of GA3 promotes plant growth and other yield-attributing traits in cold stress conditions in rice. Whereas, GA3 was found to have no direct role in heat tolerance but rather be associated with cell expansion gene activation and also positively affect the test weight of the seed in wheat (Nagar et al., 2021). They also noted that the application of paclobutrazol showed a thermo-tolerance effect rather than GA3 biosynthesis inhibition in wheat. Guo et al. (2022) suggested that gibberellins can mitigate the effects of heat stress response in plants by providing evidence obtained in tomatoes. They concluded from the result that exogenous application of gibberellic acid (GA3) of 75 mg/L can mitigate heat stress by improving the plant growth, morphology, and physiological characteristics of tomatoes.
5.6 Effect of brassinosteroids on stress
Under stress condition, BRs increase Rubisco and the water usage efficiency of leaves hence improving CO2 assimilation and leaf water economy (Farooq et al., 2009). Several studies have also revealed that brassinosteroids s play a beneficial function in drought-stressed Brassica napus, wheat and Arabidopsis (Kagale et al., 2007). Exogenous 24-epibrassinolide treatment raises BRs content while lowering ABA and ROS levels, which further aids in increasing stomatal hole for water stress resistance (Nie et al., 2019; Tanveer et al., 2019). Unraveling the molecular basis of BRs control, three WRKY transcription factors—WRKY46, WRKY54, and WRKY70—have been identified as crucial signaling components that play oppositely positive and negative roles in BRs-regulated growth and drought responses (Chen et al., 2017). It was reported that the overexpression of a BRs biosynthetic gene AtDWF4, isolated from Arabidopsis in applied in transgenic Brassica napus results in improved drought tolerance (Sahni et al., 2016). BRs along with ABA showed a major role in drought stress in plants.
The negative effects of salt on plant growth performance are also known to be mitigated by BRs (Zhu 2002; Krishna 2003; Zhang et al., 2007; Kartal et al., 2009; Wang et al., 2011). By restoring pigment levels and elevating nitrate reductase activity, application of BRs through exogenous application reduced the negative effects of salt stress on root elongation, seed germination, and subsequent growth of rice (Anuradha and Rao 2001). Krishna (2003) found that barley leaf segments pre-incubating with BRs prior to exposure to salinity was successful in minimizing the cells’ ultra-structures, such as their nucleus and chloroplasts. Under salinity, treatment of seed with BL considerably improved the accumulation of dry matter and antioxidant enzyme activity in lucerne (Zhang et al., 2007). In rice, Arabidopsis, and brassica, treatment with 24-epibrassinolide significantly increased seed germination, seedling growth, antioxidant system, and proline content, while reducing lipid peroxidation under salinity stress (Ozdemir et al., 2004, Kagale et al., 2007, Divi et al., 2010).
5.7 Effect of jasmonate on stress
Jasmonic acid (JA) encourages plant water uptake and methyl JA encourages increased osmoprotectant and compatible solute accumulation to increase chlorophyll content, antioxidant activity, and leaf gas exchange to trigger stomatal closure and improved water usage efficiency (S’anchez-Romera et al., 2014). There were negative impacts during drought stress; it also modifies polyamine and endogenous phytohormones (Xiong et al., 2020). It has been shown that exogenous administration of 0.5 mM methyl JA can preserve wheat growth and output during water deficit stress (Anjum et al., 2016). The application of 10 M methyl JA to sugar beet decreases the negative impacts of severe drought (Fugate et al., 2018). Kang et al. (2005) reported the comparison of salt-sensitive and tolerant rice cultivars and observed that salt-tolerant rice cultivars have a much higher concentration of JA. A critical component of the barley response to salt was thought to be the induction of JA-responsive genes (Walia et al., 2006). Endogenous JA contents in barley leaf segments that were subjected to sorbitol or mannitol osmotic stress increased significantly (Kramell et al., 2000).
JA is considered to have a major role in alleviating heat and light stress damage in the plant. A study conducted in the Arabidopsis crop showed a combination of high light and high heat (HL+HS) stress causing major damage to photosynthetic pigments and reducing the D1 protein level in plants with the same time accumulation of jasmonic acid that may provide tolerance in plant (Balfagón et al., 2019). They found that the plant deficient in jasmonic acid is highly sensitive to heat and light stress. Convergent study was conducted in Ryegrass; a temperate grass is sensitive to high temperatures. In this study impact of jasmonic acid on ryegrass was studied, it was observed that methyl jasmonic acid (MeJA) has a positive effect on augmenting tolerance in plants to a high temperature by altering the antioxidant defense mechanism, decreasing chlorophyll loss due to heat, maintaining good water balance in plant and lowering electrolyte leakage in the crop (Su et al., 2021). Along with that also the plant oxidize activity was enhanced by exogenous MeJA treatment which can increase the scavenging ability of ROS produced during heat stress and leads to alleviating the oxidative damage caused by heat stress and production of more heat shock proteins may be expressed in the plant during heat stress condition. (Su et al., 2021).
5.8 Effect of salicylates on stress
A phytohormone called SA is produced by chloroplasts (Dempsey and Klessig, 2017). According to reports, SA treatments sustain the cell’s turgor pressure by increase the amount of osmolyte and proline in the root and shoot without affecting the other metabolic processes. Further, when SA is applied exogenously to canola, it increases the number of pods and seed output and is also involved in cell division and expansion (Keshavarz and Sanavy, 2018). Additionally, its use on marigolds under drought stress boosts bioproduction, enhances a number of physiological processes, and lessens the detrimental effects of water stress (Abbas et al., 2019). When crop plants under drought stress, such as wheat, saffron, and Brassica rapa, are exposed to it, SA activates nonenzymatic defensive mechanisms like sugar accumulation for energy saving and osmoregulation and lowers their malondialdehyde and free radical contents (Chavoushi et al., 2019; Ilyas et al., 2017). Through redox homeostasis and proline metabolism in agricultural plants, SA treatment increases drought-stress resistance (Chavoushi et al., 2019; Ilyas et al., 2017); La et al., 2019). By accumulating endogenous SA, the Arabidopsis loss of function lines cpr5 and acd6 demonstrated a drought tolerance mechanism (Miura et al., 2013). It has been revealed that in Arabidopsis, the SIZ1-mediated buildup of endogenous SA improves drought tolerance and encourages stomatal closure (Miura et al., 2013). It was observed that the osmolyte content in the vegetative phase of barley, safflower and corn has been increased by triggering multiple defense mechanisms, along with the antioxidant system through exogenous administration of SA which improve drought tolerance in those plants (Abdelaal et al., 2020; Chavoushi et al., 2019). Thus, a potential transgenic strategy for making plants resistant to drought would be to target genes involved in triggering the effect of drought resistance in response to the exogenous administration of SA.
SA’s salt-ameliorating effects have also been widely reported in various crops including bean (Azooz 2009), wheat (Sakhabutdinova et al., 2003), barley (El-Tayeb, 2005), and mung bean (Khan et al., 2010; Syeed et al., 2011). Another study indicated that SA treatment of salt-stressed maize and mustard increased their ability to tolerate salt by speeding their photosynthesis and carbohydrate metabolism (Khodary, 2004; Nazar et al., 2011). Bastam et al. (2013) applied an exogenous treatment of SA to enhance the salt tolerance of pistachio seedlings. Palma et al. (2009) reported that under salinity stress, SA activates the antioxidant systems and is also attributed to the buildup of suitable solutes like proline and glycine betaine (Nazar et al., 2011). In addition, plants treated with SA showed reduced levels of membrane permeability and lipid peroxidation, which were otherwise rather significant under salinity (Horvath et al., 2007). Salicylic acid (SA) application was studied under waterlogging conditions in wheat crops revealing that lateral roots development was enhanced along with the emergence of surface adventitious roots which originate from the basal stem nodes of wheat, but root elongation was hindered, leading to the development of a shallow root system able to survive in water logging condition. (E scholar encyclopedia, 2022). The effects of salicylic acid become more apparent in plants under stress conditions. In maize crops, application of 0.5mM of salicylic acid improves the growth rate of plant under hydroponic conditions under cold stress (Janda et al., 1997; Janda et al., 1999). It was observed that SA application reduced electrolyte leakage and improves CAT activity with a level of enhancement in the activities of glutathione reductase and guaiacol peroxidase. Application of SA in normal conditions may cause deleterious effects on plants but in stress conditions, it can act positively (Waraich et al., 2011). Likewise in the wheat crop that treatment with salicylic acid at the rate of 0.5 mM can mitigate heat stress damage by increasing the production of proline and reducing the activities of proline oxidase (PROX) which finally leads to maintaining osmotic potential and photosynthetic activities in the plant (Khan et al., 2013). From the result, it was observed that plant tolerance was created SA through interacting with proline activity and ethylene formation and eventually leads to alleviating the photosynthetic damage caused by heat stress in wheat.
6 Nutrients and their effect on abiotic stress
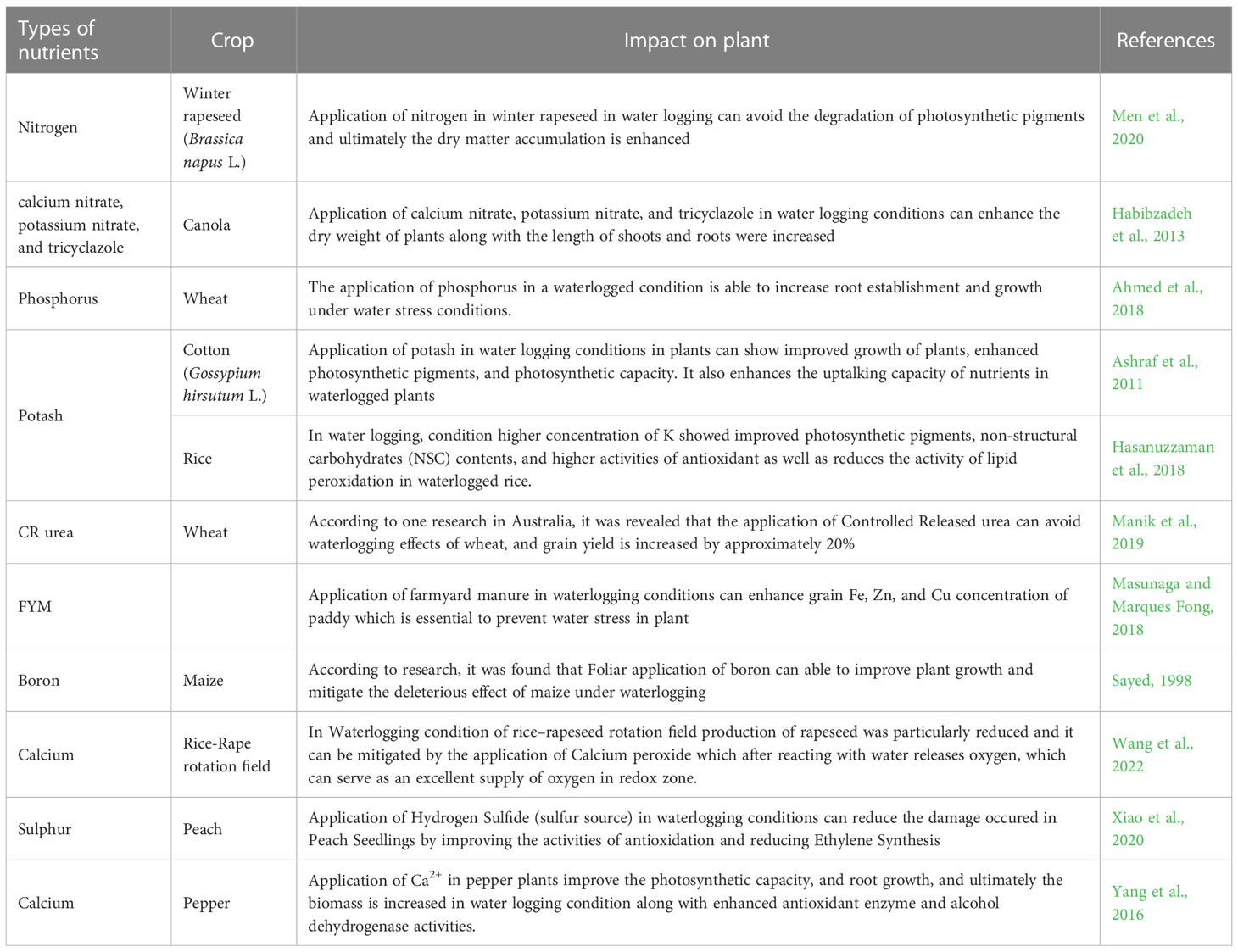
Nitrogen is a major component of all cellular and metabolic activities in crop plant as it is a major element of proteins, chlorophyll, nucleic acids, amino acids, plant hormones, enzymes, and osmolytes, all of which are involved in plant abiotic stress tolerance mechanisms through different pathways (Arghavani et al., 2017; Singh et al., 2019). The application of N enhances the plasticity and water extraction capacity of plant roots from the soil, which helps to maintain optimal relative leaf water content and increase water use efficiency in environments with limited moisture (Yang et al., 2012; Tran et al., 2014). Nitrogen supplementation was able in alleviating NaCl-induced toxicity in tomato seedlings which up-regulate the AsA–GSH cycle, K+, and K+/Na+ ratio, which resulted in better growth performance (Nazar et al., 2011). In Brassicas it was found that application of N may improves a lot of cellular activities and also prove to be mitigate the ill effects of salt stress in plant. Under the salinity stress condition application of N can improve growth attributes, physio-biochemical parameters, nutritional enrichment, and yield attributes in brassicas (Siddiqui et al., 2010). Application of nitrogen fertilizer to crops promotes antioxidative defense mechanisms and reduces leaf senescence. These processes include carbon partitioning, carbohydrate buildup, cellular membrane stability, and osmoregulation (Saneoka et al., 2004; Saud et al., 2017), cell synthesis and expansion of plant cells (Li et al, 2012), increased photosynthetic capacity (Gessler et al., 2017). N can boost the root system in crops including rice, wheat, rapeseed, and pearl millet as well as improve xylem transport, photosynthetic enzyme activity, antioxidant defense, delay cell senescence, control stomata, increase proline accumulation, and encourage profuse branching (Rostamza et al., 2011; Albert et al., 2012; Tran et al., 2014; Abid et al., 2016.). Under drought conditions, phosphorus promotes root architecture and proliferation in the soil, which increases root volume and hydraulic conductivity (Jin et al., 2015). Application of phosphorous during the early stages of the wheat crop boosted root growth and establishment (Ahmed et al., 2018). The application of P reduces the formation of ROS caused by drought by energizing enzymatic antioxidants as POD, CAT, APX, SOD, and monodehydroascorbate reductase (MDHAR), which consequently increases resistance to stress (Meng et al., 2021). Sardans and Penuelas (2012), P treatment has also been linked to the remodeling of nitrogenous compounds in terms of buildup and absorption of NH4 + and NO3 in water-stressed agricultural plants. Phosphorus fertilization significantly increased all growth parameters, chlorophyll content, nucleic acid content and minerals content of the common bean plants under salinity stress (Mohamed et al., 2021). Protective effect of potassium application on salt stress in two tomato genotypes (Nasir and Skyland-II) more dry biomass production, shoot K+ concentration, chlorophyll contents, stomatal conductance, and K+/Na+ ratio under saline condition (7.5 dSm−1) (Muhammad et al., 2020). Exogenous K fertilizer treatment of 160 kg/ha under water stress enhances grain yield, harvest index, and other physiological indicators in rice (Zain et al., 2014). K can increase the photosynthetic process and glucose metabolism in a stressed cotton crop (Zahoor et al., 2017). In order to reduce abiotic stress in plants, secondary nutrient like calcium is also necessary for food uptake, enzymatic and hormonal up-regulations, and stabilization of cell membranes (Rahman et al., 2015), improves the ability to preserve water (Shao et al., 2008). Ca2+ alters the plasma membrane’s level of hydration, which enhances the cohesiveness of the cell walls and raises the viscosity of the protoplasm, enhancing the resistance of cells to dehydration (Ma et al., 2009). Xu et al. (2013) reported that the application of 10 mM Ca in drought conditions caused the production of more root and shoot biomass and dry weight. Magnesium can produce photosynthetic pigments, accumulate higher proline content in mungbean, and encourage better root proliferation in rice (Thalooth and Tawfik Mohamed, 2006; Ding and Xu, 2011). Min et al. (2016) reported that Sulfur helps to nullify the oxidative stress produced due to drought stress by increasing the activities of ROS scavengers like CAT, SOD, and APX; higher H2S and soluble sugar contents along with reducing H2O2. Boron promotes the resistance of crop plants by improving hormone synthesis, lipid metabolism, pollen formation, sugar transport, photosynthetic efficiency, seed germination, flower retention, and seed yield during drought stress (Michael et al., 2016). Under water scarce conditions, B improved water uptake, and nutritional status from the rhizospheric soil by enhancing the growth of more root hairs and mycorrhizae, ROS detoxification process in chloroplasts preventing photooxidative damage hence establishes membrane integrity and improves drought tolerance in plants (Venugopalan et al., 2021). Zn as an important micronutrient has been observed to improve the synthesis of IAA and gibberellic acid (GA3) like plant hormones under moisture stress conditions and thereby improving plumule length and increase shoot dry weight under drought stress. Zn application also helps in a significant expansion in leaf surface area, stomatal conductance, relative leaf water content, and improvement in chlorophyll and accumulation of osmolyte, thus resulting in enhancing cellular growth, plant harvest and prevention the destructive impacts on leaf cell due to moisture deficiencies (Hassan et al., 2020). Spraying with Fe reduces oxidative stress by depleting H2O2 content along with breakdown of lipid peroxidation activities by accelerating the enzymatic antioxidant mechanisms (CAT, SOD, and GPX) under water scarce situations and also showed a major impact in triggering the quality and resistance of protein under drought stress (Baghizadeh and Shahbazi, 2013; Afshar et al., 2013). While going for role of copper under drought condition, Copper chlorophyllin (Cu-chl) has been proved to be an important modified water-soluble and semi-synthetic bio-stimulant that helps to improve the antioxidative capacity which leads to decreased oxidative stress in plant (Kamat et al., 2000). Cobalt imparts drought tolerance in plants by increasing water use efficiency by reducing the rate of transpiration, further it activates the antioxidant defense mechanisms in plants (Banerjee et al., 2021).
Among the other nutrients, there are several elucidations of the alleviation effects of Si in salt-induced osmotic stress (Zhu et al., 2015) and oxidative stress (Yin et al., 2019). Si-mediated up-regulation of aquaporin gene expression and osmotic adjustment play important roles in alleviating salinity-induced osmotic stress (Zhu et al., 2019). Further foliar application of micronutrients could be useful for improving the nutrient status, root features, and physiological performance of wheat plants (Fouly et al., 2011). Nutrients in combination with phytohormones, it was noted that many plant nutrients can also alleviate water-logging stress and temperature stress. For example, it is reported that application of boron can improves the activity of the antioxidant system significantly and which leads to nullify the toxic effects of ROS produced by heat stress (Waraich et al., 2011). Similarly, selenium (Se) is known for its major role in synthesis of glutathione peroxidase (GPX) and ultimately prevents the plants from the negative impact of ROS (Lobanov et al., 2008). Also, Zn micronutrients can be used to maintain the permeability of cellular membrane and the optimum dose of Zn can mitigate plants from the devastating impacts of heat stress (Peck et al., 2010). Tables 5–7 highlighted the nutrient application in the alleviation of abiotic stresses in plant systems.
7 Crosstalk with abiotic stress, phytohormones and nutrients
Crosstalk among and between the phytohormones and nutrients has been reported to have important role in abiotic stress alleviation. Auxin being an important phytohormone enhances drought resistance by interacting with other phytohormones. During drought stress, auxin regulates various members of the ACS (1-aminocyclopropane-1-carboxylate synthase) gene family, which is a rate-limiting enzyme in ethylene biosynthesis further increasing resistance against the stress in plants (Colebrook et al., 2014). It was reported that the exogenous application of IAA can enhance ABA and JA content and it can promote the up-regulation of over expression of drought stress-responsive genes (WRKY2, WRKY56, bZIP11, MYB14, DREB2, MYB48, WRKY108715, and RD22), auxin-responsive genes (GH3.9, GH3.1, IAA8) and down-regulation of leaf senescence genes (SAG101 and SAG102) and auxin responding genes (GH3.3, GH3.6, IAA27) which ultimately improves the plant tolerance towards drought stress in white clover (Zhang et al., 2020). Further, during drought ABA accumulation maintains maize primary root elongation by restricting the production of ethylene (Spollen et al., 2000). Furthermore, in drought stress endogenous CK level reduction in the roots also leads to higher concentrations of macro- and micro-elements, such as manganese (Mn), phosphorous (P), or zinc (Zn) (Ramireddy et al., 2018; Nehnevajova et al., 2019). ABA-activated type-A ARR5 magnifies the ABA-mediated response to stress e. Simultaneously, restricts plant growth by repressing CK signaling via a negative feedback loop in Arabidopsis (Huang et al., 2018). Further osmotic stress trigger synthesis of CK which down-regulate the genes of ABA synthesis and ABA-mediated responses, which reduces the damage caused by ROS and lipid peroxidation, reduce the senescence ability of leaves and thus improves the abiotic stress tolerance ability of plant and plant growth (Gujjar and Supaibulwatana, 2019). Further, ABI1 and ABI2 which negatively regulate ABA signaling interact with BIN2 and regulate BRs signaling, which ultimately shows stress responses in Arabidopsis (Wang et al., 2018a; Wang et al., 2018b).
The crosstalk is also having an important place in dealing with salinity resistance in plants. The effect of salt stress can be nullified by seed priming with IAA on wheat seed germination and growth via regulation the biosynthesis of free salicylic acid induced by auxin and maintaining ionic homeostasis in leaves (Iqbal and Ashraf 2007). Fahad and Bano (2012) observed that during salinity stress plant can produce significant amount of IAA and reduce the synthesis of ABA in maize plants; however, the application of salicylic acid can significantly increase the IAA. Application of auxin restricts the nodes of tiller in rice by biosynthesis of cytokinin in nodes along with down-regulating OsIPT expression (Liu et al., 2011) during salinity stress. CKs play an important role by acting as a bridge in showing the protective role of epibrassinolide and methyl jasmonate in wheat under salinity (Shakirova et al., 2010). Iqbal and Ashraf (2013a) reported a non-consistent effect of GA3 priming (150 mg L-1) on auxin concentration in wheat genotypes under salinity stress. GA improved the growth of soybean by regulating the level of other phytohormones under salinity (Hamayun et al., 2010), and increased levels of bioactive GA1 and GA4 showed a concurrent decrease in the level of ABA and SA. In brassica, the application of GA in conjunction with nitrogen was helpful in alleviating salinity stress (Siddiqui et al., 2010). Moreover, BRs-mediated stress tolerance in Arabidopsis was linked with ABA, SA, and ETHY pathways (Divi et al., 2010). The BRs act as synergists to GA and IAA during the hypocotyl elongation of Arabidopsis (Tanaka et al., 2003). ABA acts as an antagonist as it repressed the BR-enhanced expression (BEE1, BEE2, and BEE3) proteins (Friedrichsen et al., 2002). Exogenous application of jasmonates (JA) may change the endogenous ABA, which provides a significant hint for understanding the protection mechanisms against salt stress (Kang et al., 2005). Furthermore, foliar application of N fertilizers at the reproductive stage, particularly in leguminous crops, significantly slows the synthesis of abscisic acid with an enhance synthesis of cytokinin production, which promotes cell elongation, nodulation, shoot development, apical dominance, photosynthetic activity, and assimilates translocation to the sink organs under drought conditions (Vries et al., 2016). Likewise, the synergistic regulation of H2S with phytohormones such as abscisic acid, ethylene, and salicylic acid can able to regulate the plant stress response (Zhang et al., 2021). It was observed that a balanced application of nutrients can be useful to mitigate cold stress by protecting the cell against freeze-dry death for a limited period of time (Huixia et al., 2018). The plant supplemented with potassium and magnesium provides better protection during a cold injury in the plant. The application of potassium can regulate the closing of stomatal cells, improves water balance, and prevents uncontrolled water loss through the leaves (Danilova et al., 2016m). Also, Magnesium promotes root growth up to a deeper zone of soil and therefore helps ensure that plants can still absorb water from deeper soil layers via a well-developed root system, even when the soil is slightly frozen (Danilova et al., 2016m). Whereas, Auxin a plant growth that promotes its synthetic pathway can create thermo-tolerance in crops. During heat and moisture stress conditions, soil cobalt application combined with foliar K and B sprays manifested immense potential to achieve higher black gram production (Banerjee et al., 2021). A similar study was also carried out in Lathyrus sativus by the authors that showed the combined application of N, P, and K with Mo improved growth, physiological efficiency, nutrients uptake, and yield ameliorate heat and moisture stress (Banerjee et al., 2021). Combined application of Zn, B, and Si increased plant height, shoot dry weight, number of stems per plant, leaf relative water content, leaf photosynthetic rate, leaf stomatal conductance, chlorophyll content, and tuber yield in potato during salinity stress condition (Mahmoud et al., 2020). Co-application with other plant nutrients like N, P, K, Zn, Si, etc. can be proven beneficial in alleviating salinity, heat, and moisture stress in plants (Akeel et al., 2020). Application of nutrients like K and Ca improves root growth and improve the uptake of water which leads to regulating the stomatal cell and maintaining the plant body temperature during heat stress. The application of micronutrients like B, Mn, and Se can alter the physical, biochemical and metabolic processes in plants in a positive direction to alleviate the adverse effects of heat stress. Combine application of Selenium (Se) and Salicylic acid (SA) can improve tolerance in crops by activating antioxidant production which can eliminate the ROS and make the plant free from membrane damage (Kumari et al., 2022). It was concluded that hormonal balance and their cross-talk with themselves and the nutrients are critical regarding signal perception, transduction, and mediation of stress response in plants.
8 Conclusion
In this current review highlighted the comprehensive information on the response of phytohormones, nutrients application and their interaction in crops grown under various abiotic stress conditions. Majority of phytohormones control and sustain the homeostasis inside the cell by detoxifying the ROS and enhancing the antioxidant activities during varied abiotic stress and can enhance tolerance in plants. In drought condition, application of IAA can trigger the activation of other stress-responsive hormones as well as the production of ROS. Enhanced level of ABA in drought condition can alter the guard cell ion transport and stomatal opening which leads to reduced water loss. Cytokinin application increases transcriptional level of CKX genes leads to enhanced CKX activity in many plants. Proline activity is enhanced by applying CKs to create salt resistance in plant. It was also concluded that endogenous hormone levels of IAA, zeatin, and GA3 is enhanced by application of that exogenous application of BA. In water logging condition, the accumulation of ROS and malondialdehyde levels is reduced by application of CK and GA3. The overexpression of ethylene response factor such as GmERF3 of AP2/ERF gene family, leads to improvement in proline content, soluble sugar, and decreases in the accumulation of malondialdehyde to improve drought tolerance in plant. During heat stress, the pollen sterility is the major cause of yield loss, which can be overcome by application of application of ethylene hormone (ethephon) during heat stress conditions. In saline condition by altering the GA levels can enhance seed germination by overproduction of the salt-inducible DDF1 gene (dwarf and delayed flowering 1). It was estimated that application of GA3 (200 ppm) in combination with salicylic acid (150ppm) on the soybean plant provides better mitigation effects by improving the number of pod and seed, chlorophyll content in waterlogged conditions. Also, it was observed that methyl jasmonic acid (MeJA) has a positive effect on augmenting tolerance in plants to a high temperature by altering the antioxidant defense mechanism, decreasing chlorophyll loss due to heat, maintaining good water balance in plant and lowering electrolyte leakage in the crop. It was also revealed that application of 0.5mM of salicylic acid improves the growth rate of plant under hydroponic conditions under cold stress condition. Besides, the application of plant nutrients like N, K, Ca, and Mg are also found to reduce the ROS activities through elevating antioxidants quantity that can scavenge the ROS effect and finally leading to the reduction in cell membrane leakage and increase the photosynthetic ability in the plant by recuperating the chlorophyll cells. Hence, it is concluded that the crosstalk with phytohormones and nutrients can complement each other streamlining the antioxidant activities or ROS signaling pathway in cells and improving the tolerance of crop plants. More amalgamated and detailed research is needed with the combined application of hormones and nutrients to precisely understand the mechanism involved.
Author contributions
RS, writing and conceptualization of the manuscript. SS, drafting of the manuscript, preparing the table and figure. MB, drafting the manuscript, preparing the different tables. GR, editing and critical reviewing of the final version. All authors contributed to the article and approved the submitted drafted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, S. M., Ahmad, R., Waraich, E. A., Qasim, M. (2019). Exogenous application of salicylic acid at different plant growth stages improves physiological processes in marigold (Tagetes erecta l.). Pakistan J. Agric. Sci. 56 (3), 541–548. doi: 10.21162/PAKJAS/19.7276
Abdelaal, K. A., Attia, K. A., Alamery, S. F., El-Afry, M. M., Ghazy, A. I., Tantawy, D. S., et al. (2020). Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability. 12 (5), 1736. doi: 10.3390/su12051736
Abeysingha, D. N., Ozga, J. A., Strydhorst., S., Doyle, P., Iqbal, M., Yang, R. C., et al. (2021). The effect of auxins on amelioration of heat stress-induced wheat (Triticum aestivum l.) grain loss. J. Agron. Crop science. 207 (6), 970–983. doi: 10.1111/jac.12555
Abid, M., Tian, Z., Ata-Ul-Karim, S. T., Cui, Y., Liu, Y., Zahoor, R., et al. (2016). Nitrogen nutrition improves the potential of wheat (Triticum aestivum l.) to alleviate the effects of drought stress during vegetative growth periods. Front. Plant Sci. 7, 981. doi: 10.3389/fpls.2016.00981
Afshar, R. M., Hadi, H., Pirzad, A. (2013). Effect of nano-iron on the yield and yield component of cowpea (Vigna unguiculata) under end season water deficit. Int. J. Agric. Sci. 3, 27.
Ahmad, S., Kamran, M., Ding, R., Meng, X., Wang, H., Ahmad, I., et al. (2019). Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. Peer J., e7793. doi: 10.7717/peerj.7793
Ahmad, P., Sarwat, M., Bhat, N. A., Wani, M. R., Kazi, A. G., Tran, L. P. (2015). Alleviation of cadmium toxicity in Brassica juncea l. (Czern. & coss.) by calcium application involves various physiological and biochemical strategies. PloS One 10, e0114571. doi: 10.1371/journal.pone.0114571
Ahmed, M., Khan, S., Irfan, M., Aslam, M., Shabbir, G., Ahmad, S. (2018). “Effect of phosphorus on root signaling of wheat under different water regimes,” in Global wheat production: IntechOpen. Eds. Fahad, S., Basir, A., Adnan, A. doi: 10.5772/intechopen.75806
Akeel, A., Jahan, A., Naeem, M., Ansari, A., Gill, S. (2020). “Role of cobalt in plants: Its stress and alleviation,” in Contaminants in agriculture (Cham, Switzerland: Springer).
Alam, H., Khattak, J. Z., Ksiksi, T. S., Saleem, M. H., Fahad, S., Sohail, H., et al. (2021). Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei l. Physiol. Plant 172, 1336–1351. doi: 10.1111/ppl.13273
Albert, B., Le Cahérec, F., Niogret, M. F., Avice, J. C., Faes, P., Leport, L., et al. (2012). Nitrogen availability impacts oilseed rape (Brassica napus l.) plant water status and proline production efficiency under water-limited conditions. Planta. 236, 659. doi: 10.1007/s00425-012-1636-8
Alexopoulos, A., Aivalakis, G., Akoumianakis, K., Passam, H. (2007). The effect of foliar applications of gibberellic acid and daminozide on plant growth, tuberisation and carbohydrate accumulation within tubers grown from true potato seed (TPS). J. Hortic. Sci. Biotechnol. 82, 535–540. doi: 10.1080/14620316.2007.11512270
Ali, Q., Ashraf, M., Anwar, F. (2010). Seed composition and seed oil antioxidant activity of maize under water stress. J. Am. Oil Chem. Soc 87, 1179–1187. doi: 10.1007/s11746-010-1599-5
Almodares, A., Hadi, M. R., Kholdebarin, B., Samedani, B. (2014). Kharazian, Z.A. the response of sweet sorghum cultivars to salt stress and accumulation of na+, cl–, k+ ions in relation to salinity. J. Environ. Biol. 35 (4), 733–739.
Al-Quraan, N. A., Al-Ajlouni, Z. I., Qawasma, N. F. (2021). Physiological and biochemical characterization of the gaba shunt pathway in pea (Pisum sativum l.) seedlings under drought stress. Horticulturae 7, 125.
Andrianary, B. H., Tsujimoto, Y., Rakotonindrina, H., Zaw Oo, A., Rabenarivo, M., Ramifehiarivo, N., et al. (2021). Alleviation of temperature stress by nutrient management in crop plants: a review. J. Soil Sci. Plant Nutr. 12 (2), 221–244.
Amjad, M., Akhtar, J., Anwar-ul-Haq, M., Yang, A., Akhtar, S. C., Jacobsen, E. (2014). Integrating role of ethylene and ABA in tomato plants adaptation to salt stress. Sci. Hortic. 172, 109–116. doi: 10.1016/j.scienta.2014.03.024
Anjum, S. A., Tanveer, M., Hussain, S., Tung, S. A., Samad, R. A., Wang, L., et al. (2016). Exogenously applied methyl jasmonate improves the drought tolerance in wheat imposed at early and late developmental stages. Acta Physiologiae Plantarum 38 (1). doi: 10.1007/s11738-015-2047-9
Anuradha, S., Rao, S. R. (2001). Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice (Oryza sativa l.). Plant Growth Regulation. 33, 151–153. doi: 10.1023/A:1017590108484
Anza, M., Riga, P., Garbisu, C. (2005). Time course of antioxidant responses of capsicum annuum subjected to progressive magnesium deficiency. Ann. Appl. Biol. 146, 123–134. doi: 10.1111/j.1744-7348.2005.04023.x
Arghavani, M., Savadkoohi, S., Mortazavi, S. N. (2017). Salinity tolerance of Kentucky bluegrass as affected by salicylic acid. J. Ornam. Plants. 7, 237–245.
Ahmed, S., Higuchi, H., Nawata, E., Sakuratani, T. (2002). Effects of exogenous ABA and ethylene application and waterlogging on photosynthesis in mungbean (Vigna radiata (L.) wilczak). Jpn.J.Trop.Agr. 46(3), 166–174.
Ashraf, M.A., Ahmad, M.S.A., Ashraf, M., Al-Qurainy, F., Ashraf, M.Y. (2011). lleviation of waterlogging stress in upland cotton (Gossypium hirsutum L.) by exogenous application of potassium in soil and as a foliar spray. Crop Past. Sci. 6, 25–38.
Arora, S., Sharma, V. (2017). Reclamation and management of salt-affected soils for safeguarding agricultural productivity. J. Safe Agric. 1, 1–10.
Arora, S., Singh, Y. P., Vanza, M., Sahni, D. (2016). Bioremediation of saline and sodic soils through halophilic bacteria to enhance agricultural production. J. Soil Water Conserv. 15, 302–305. doi: 10.5958/2455-7145.2016.00027.8
Azooz, M. M. (2009). Salt stress mitigation by seed priming with salicylic acid in two faba bean genotypes differing in salt tolerance. Int. J. Agric. Biol. 11, 343–350.
Baghizadeh, A., Shahbazi, M. (2013). Effect of zn and fe foliar application on yield, yield components and some physiological traits of cumin (Cuminum cyminum) in dry farming. Int. J. Agron. Plant Prod. 4, 3231–3237. doi: 10.5829/idosi.aejaes.2016.16.5.12923
Baizhao, R., Juan, H., Jiwang, Z., Shuting, D., Liu, P., Zhao, B. (2019). Spraying exogenous synthetic cytokinin 6-benzyladenine following the waterlogging improves grain growth of waterlogged maize in the field. J. Agron. Crop Science. 205 (6), 616–624. doi: 10.1111/jac.12355
Balfagón, D., Sengupta, S., Gómez-Cadenas, A., Fritschi, F. B., Azad, R. K., Mittler, R., et al. (2019). Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol. 181 (4), 1668–1682. doi: 10.1104/pp.19.00956
Banerjee, P., Venugopalan, V. K., Nath, R., Althobaiti, Y. S., Gaber, A., Al-Yasi, H., et al. (2021). Physiology, growth and productivity of spring–summer black gram (Vigna mungo l. hepper) as influenced by heat and moisture stresses in different dates of sowing and nutrient management conditions. Agronomy 11, 2329.
Barciszewski, J., Siboska, G., Rattan, S. I. S., Clark, B. F. C. (2000). Occurrence, biosynthesis and properties of kinetin (N6-furfuryladenine). Plant Growth Regul. 32, 257–265. doi: 10.1023/A:1010772421545
Bastam, N., Baninasab, B., Ghobadi, C. (2013). Improving salt tolerance by exogenous application of salicylic acid in seedlings of pistachio. Plant Growth Regul. 69, 275–284. doi: 10.1007/s10725-012-9770-7
Bielach, A., Podlešáková, K., Marhavý, P., Duclercq, J., Cuesta, C., Müller, B., et al. (2012). Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell. 24, 3967–3981. doi: 10.1105/tpc.112.103044
Bishopp, A., Help, H., El-Showk, S., Weijers, D., Scheres, B., Friml, J., et al. (2011). A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 21, 917–926. doi: 10.1016/j.cub.2011.04.017
Bohra, J. S., Dorffling, H., Dorffling, K. (1995). Salinity tolerance of rice (Oryza sativa l.) with reference to endogenous and exogenous abscisic acid. J. Agron. Crop Sci. 174, 79–86. doi: 10.1111/j.1439-037X.1995.tb00197.x
Botella, M. A., Rosado, A., Bressan, R., Hasegawa, P. M. (2007). “Plant adaptive responses to salinity stress,” in Plant abiotic stress. Eds. Jenks, M. A., Hasegawa, P. M. (USA, Iowa: Blackwell Publishing), 37–70. doi: 10.1002/9780470988503.ch3
Bouzroud, S., Gouiaa, S., Hu, N., Bernadac, A., Mila, I., Bendaou, N., et al. (2018). Auxin response factors (ARFs) are potential mediators of auxin action in tomato response to biotic and abiotic stress (Solanum lycopersicum). PloS One 13 (2), e0193517. doi: 10.1371/journal.pone.0193517
Cakmak, I., Marschner, H. (1992). Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidise and glutathione reductase in bean leaves. Plant Physiol. 98, 1222–1227. doi: 10.1104/pp.98.4.1222
Calvin, Damanik, R. I., Siregar, L. A. M. (2019). “Growth and production of soybean (Glycine max l. merril) varieties in response to waterlogging at vegetative (V5) growth phase by application of gibberellic acid and salicylic acid,” in IOP conf. series: Earth and environmental science, vol. 260. . doi: 10.1088/1755-1315/260/1/0121431
Camara, M. C., Vandenberghe, L. P. S., Rodrigues, C., De Oliveira, J., Faulds, C., Bertrand, E. (2018). Current advances in gibberellic acid (GA3) production, patented technologies and potential applications. Planta. 248, 1049–1062. doi: 10.1007/s00425-018-2959-x
Cao, M. J., Wang, Z., Zhao, Q., Mao, J. L., Speiser, A., Wirtz, M., et al. (2014). Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J. 77, 604–615. doi: 10.1111/tpj.12407
Cebrián, G., Iglesias-Moya, J., García, A., Martínez, J., Romero, J., Regalado, J. J., et al. (2021). Involvement of ethylene receptors in the salt tolerance response of Cucurbita pepo. Hortic. Res. 8, 508z. doi: 10.1038/s41438-021-00508-z
Cheng, Y., Dai, X., Zhao, Y. (2006). Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in arabidopsis. Genes Dev. 20 (13), 1790–1799. doi: 10.1101/gad.1415106
Chavoushi, M., Najafi, F., Salimi, A., Angaji, S. A. (2019). Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Products. 134, 168–176. doi: 10.1016/j.indcrop.2019.03.071
Chen, K., Wang, Y., Zhang, R., Zhang, H., Gao, C. (2019). CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70, 667–697. doi: 10.1146/annurev-arplant-050718-100049
Chen, J., Nolan, T. M., Ye, H., Zhang, M., Tong, H., Xin, P., et al. (2017). Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell. 29 (6), 1425–1439. doi: 10.1105/tpc.17.00364
Colebrook, E. H., Thomas, S. G., Phillips, A. L., Hedden, P. (2014). The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 217 (1), 67–75. doi: 10.1242/jeb.089938
Danial, H. F., Ewees, M. S., Moussa, S. A. (2010). Significance of influence potassium on the tolerance to induce moisture stress and biological activity of some legume crops grown on a sandy soil Egypt. Egypt J. Soil. Sci. 43, 180–204.
Danilova, M. N., Kudryakova, N. V., Doroshenko, A. S., Zabrodin, D. A., Rakhmankulova, Z. F., Oelmüller, R., et al. (2016). Opposite roles of the arabidopsis cytokinin receptors AHK2 and AHK3 in the expression of plastid genes and genes for the plastid transcriptional machinery during senescence. Plant Mol. Biol. 4, 533–546.
Dempsey, D., Klessig, D. F. (2017). How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 15 (1), 1–11.
De Ollas, C., Hernando, B., Arbona, V., Gomez-Cadenas, A. (2013). Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant 147, 296–306. doi: 10.1111/j.1399-3054.2012.01659.x
De Vries, F. T., Brown, C., Stevens, C. J. (2016). Grassland species root response to drought: consequences for soil carbon and nitrogen availability. Plant Soil. 409 (1), 297–312. doi: 10.1007/s11104-016-2964-4
Diouf, I. ,. A., Derivot, L., Bitton, F., Pascual, L., Causse, M. (2018). Water deficit and salinity stress reveal many specific QTL for plant growth and fruit quality traits in tomato. Front. Plant Sci. 6 (9), 279. doi: 10.3389/fpls.2018.00279
Ding, Y., Sheng, J., Li a, S., Nie, Y., Zhao, J., Zhu, Z., et al. (2015). The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum l.) fruit. Postharvest Biol. Technology. 101. doi: 10.3389/fpls.2021.680376
Ding, Y., Xu, G. (2011). Low magnesium with high potassium supply changes sugar partitioning and root growth pattern prior to visible magnesium deficiency in leaves of rice (Oryza sativa l.). Am. J. Plant Sci. 2. doi: 10.3389/fpls.2019.00140
Efroni, I., Han, S. K., Kim, H. J., Wu, M. F., Steiner, E., Birnbaum, K. D., et al. (2013). Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev. Cell. 24, 438–445. doi: 10.1016/j.devcel.2013.01.019
Divi, U. K., Rahman, T., Krishna, P. (2010). Brassinosteroid-mediated stress tolerance in arabidopsis shows interaction with absicisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 10, 151–165. doi: 10.1186/1471-2229-10-151
Dornbos, D. L., Mullen, R. E. (1992). Soybean seed protein and oil contents and fatty acid composition adjustments by drought and temperature. J. Am. Oil Chem. Soc 69, 228–231. doi: 10.1007/BF02635891
Duan, L., Dietrich, D., Ng, C. H., Chan, P. M. Y., Bhalerao, R., Bennett, M. J., et al. (2013). Endodermal ABA signaling promotes lateral root quiescence during salt stress in arabidopsis seedlings. Plant Cell. 25, 324–341. doi: 10.1105/tpc.112.107227
E scholar encyclopedia (2022) Plant science. Available at: https://encyclopedia.pub/entry/history/show/48811.
El-Tayeb, M. A. (2005). Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul. 45, 215–225. doi: 10.1007/s10725-005-4928-1
Fahad, S., Bano, A. (2012). Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pakistan J. Botany. 44, 1433–1438.
Fahad, S., Hussain, S., Bano, A., Saud, S., Hassan, S., Shan, D., et al. (2014). Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ. Sci. pollut. Res. doi: 10.1007/s11356-014-3754-2
Farooq, M., Wahid, A., Basra, S. M. A. (2009). Improving water relations and gas exchange with brassinosteroids in rice under drought stress. J. Agron. Crop Science. 195 (4), 262–269. doi: 10.1111/j.1439-037X.2009.00368.x
Fouly, M., Abou El-Nour, E., Shaaban, S., Zeidan, M. (2012). Effect of different levels of NPK and micronutrients fertilization on yield and nutrient uptake of maize plants. J. Am. Sci. 8 (8), 209–214.
Farooq, M., Hussain, M., Wakeel, A., Siddique, K. H. M. (2015). Salt stress in maize: effects, resistance mechanisms, and management. a review. Agron. Sustain. Dev. 35 (2), 461–481. doi: 10.1007/s13593-015-0287-0
Freitas, V. S., de Souza Miranda, R., Costa, J. H., de Oliveira, D. F., de Oliveira Paula, S. (2017). Ethylene triggers salt tolerance in maize genotypes by modulating polyamine catabolism enzymes associated with H2O2 production. Environ. Exp. Bot. 145, 75–86. doi: 10.1016/j.envexpbot.2017.10.022
Friedrichsen, D. M., Nemhauser, J., Muramitsu, T., Maloof, J. N., Alonso, J., Ecker, J. R., et al. (2002). Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics. 162 (3), 1445–1456. doi: 10.1093/genetics/162.3.1445
Fugate, K. K., Lafta, A. M., Eide, J. D., Li, G., Lulai, E. C., Olson, L. L., et al. (2018). Methyl jasmonate alleviates drought stress in young sugar beet (Beta vulgaris l.) plants. J. Agron. Crop Science. 204 (6), 566–576. doi: 10.1111/jac.12286
Ge, T. D., Sun, N. B., Bai, L. P., Tong, C. L., Sui, F. G. (2012). Effects of drought stress on phosphorus and potassium uptake dynamics in summer maize (Zea mays) throughout the growth cycle. Act. Physiol. Plant 34, 2179–2186. doi: 10.1007/s11738-012-1018-7
Gessler, A., Schaub, M., McDowell, N. G. (2017). The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 214, 513–520. doi: 10.1111/nph.14340
Ghafar, M. A., Akram, N. A., Saleem, M. H., Wang, J., Wijaya, L., Alyemeni, M. N. (2021). Ecotypic morphological and physio-biochemical responses of two differentially adapted forage grasses, Cenchrus ciliaris l. and Cyperus arenarius retz. to drought stress. Sustainability. 13, 8069. doi: 10.3390/su13148069
Ghanbari, A., Mousa, S. H. V. I., Gorji, A. M., Rao, I. M. (2013). Effects of water stress on leaves and seeds of bean (Phaseolus vulgaris l.). Turkish J. Field Crops. 18, 73–77.
Gharbi, E., Martínez, J. P., Benahmed, H., Lepoint, G., Vanpee, B., Quinet, M., et al. (2017). Inhibition of ethylene synthesis reduces salt-tolerance in tomato wild relative species solanum chilense. Plant Physiol. 210, 24–37. doi: 10.1016/j.jplph.2016.12.001
Glinka, Z., Reinhold, L. (1971). Abscisic acid raises the permeability of plant cells to water. Plant Physiol. 48, 103. doi: 10.1104/pp.48.1.103
Gomes, F. P., Prado, C. H. B. A. (2007). Ecophysiology of coconut palm under water stress. Braz. J. Plant Physiol. 19 (4), 377–391. doi: 10.1590/s1677-04202007000400008
Gomez, C. A., Arbona, V., Jacas, J., Primomillo, E., Talon, M. (2002). Abscisic acid reduces leaf abscission and increases salt tolerance in citrus plants. J. Plant Growth Regul. 21, 234–240. doi: 10.1007/s00344-002-0013-4
Gonzalez, E. M., Galvez, L., Royuela, M., Aparicio-Tejo, P. M., Arrese-Igor, C. (2001). Insights into the regulation of nitrogen fixation in pea nodules: Lessons from drought, abscisic acid and increased photoassimilate availability. Agronomie 21 (6-7), 607–613. doi: 10.1051/agro:2001151
Grewal, J. S., Singh, S. N. (1980). Effect of potassium nutrition on frost damage and yield of potato plants on alluvial soils of the punjab (India). Plant Soil. 57, 105–110. doi: 10.1007/BF02139646
Grzesiak, M. T., Hordy ´nska, N., Maksymowicz, A., Grzesiak, S., Szechy ´nska-Hebda, M. (2019). Variation among spring wheat (Triticum aestivum l.) genotypes in response to the drought stress. ii–root system structure. Plants 8, 584.
Gujjar, R. S., Supaibulwatana, K. (2019). The mode of cytokinin functions assisting plant adaptations to osmotic stresses. Plants (Basel). 26 (8-12), 542. doi: 10.3390/plants8120542
Guo, T., Gull, S., Ali, M. M. (2022). Heat stress mitigation in tomato (Solanum lycopersicum l.) through foliar application of gibberellic acid. Sci. Rep. 12, 11324. doi: 10.1038/s41598-022-15590-z
Gurmani, A. R., Bano, A., Ullah, N., Khan, H., J-ahangir, M., Flowers, T. J. (2013). Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na?) transport and bypass flow in rice (Oryza sativa indica). Aust. J. Crop Sci. 7, 1219–1226.
Habibzadeh, F., Sorooshzadeh, A., Pirdashti, H., Modarres-Sanavy, S. A. M. (2013). Alleviation of waterlogging damage by foliar application of nitrogen compounds and tricyclazole in canola. Aust. J. Crop Science. 7, 401–406.
Hagen, G., Guilfoyle, T. (2002). Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol. 49, 373–385. doi: 10.1023/A:1015207114117
Hakerlerler, H., Oktay, M., Eryüce, N., Yagmur, B. (1997). “Effect of potassium sources on the chilling tolerance of some vegetable seedlings grown in hotbeds,” in Food security in the WANA region, the essential need for balanced fertilization. Ed. Johnston, A. E. (Basel: International Potash Institute), 317–327.
Hamayun, M., Khan, S. A., Khan, A. L., Tang, D. S., Hussain, J., Ahmad, B., et al. (2010). Growth promotion of cucumber by pure cultures of gibberellin-producing phoma sp. GAH7. World J. Microbiol. Biotechnol. doi: 10.1007/s11274-009-0248-3
Hara, M. (2010). The multifunctionality of dehydrins. Plant Signal Behav. 5, 503–508. doi: 10.4161/psb.11085
Hasanuzzaman, M., Borhannuddin Bhuyan, M. H. M., Kamrun Nahar, I. D., Hossain, M., Mahmud, J., Al Hossen, M., et al. (2018). Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy. 8 (31), 1–29. doi: 10.3390/agronomy8030031
Hasanuzzaman, M., Nahar, K., Mahabub, A., Roychowdhury, R., Masayuki, F. (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 14, 9643–9684. doi: 10.3390/ijms14059643
Hassan, U. M., Aamer, M., Umer Chattha, M., Haiying, T., Shahzad, B., Barbanti, L., et al. (2020). The critical role of zinc in plants facing the drought stress. Agriculture 10, 396.
Huang, X., Shi, H., Hu, Z., Liu, A., Amombo, E., Chen, L., et al. (2017). ABA is involved in regulation of cold stress response in bermudagrass. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01613
Huang, L., Ye, Z., Bell, R. W., Dell, B. (2005). Boron nutrition and chilling tolerance of warm climate crop species. Ann. Bot. 96 (5), 755–767. doi: 10.1093/aob/mci228
Havlov, A. M., Dobrev, P. I., Motyka, V., Storchova, H., Libus, J., Dobr, A. J., et al. (2008). The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environment. 31 (3), 341–353. doi: 10.1111/j.1365-3040.2007.01766.x
Horvath, E., Szalai, G., Janda, T. (2007). Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 26, 290–300. doi: 10.1007/s00344-007-9017-4
Hu, Y., Chen, B. (2020). Arbuscular mycorrhiza induced putrescine degradation into γ-aminobutyric acid, malic acid accumulation, and improvement of nitrogen assimilation in roots of water-stressed maize plants. Mycorrhiza 30, 329–339. doi: 10.1007/s00572-020-00952-0
Huixia, L, Chen, Z., Zhou, T., Liu, Y., Raza, S., Zhou, J. (2018). Effects of high potassium and low temperature on the growth and magnesium nutrition of different tomato cultivars. Hortscience 53 (5), 710–714. doi: 10.1007/s00572-020-00952-0
Hussain, S., Bai, Z., Huang, T., Cao, X., Zhu, L., Zhu, C., et al. (2019). 1-methylcyclopropene modulates physiological,biochemical and antioxidant responses of rice to different salt stress levels. J. Integr. Agriculture. 10 (124). doi: 10.3389/fpls.2019.00124
Hussain, H. A., Hussain, S., Khaliq, A., Ashraf, U., Anjum, S. A., Men, S., et al. (2018). Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00393
Hussain, S., Zhong, C., Bohr, J. A., Hu, J. J., Jin, Q., Cao, X. C., et al. (2017). Effects of salt stress on rice growth and development characteristics and the regulating ways: A review. J. Integr. Agriculture. 16 (11), 2357–2374. doi: 10.1016/S2095-3119(16)61608-8
Hu, W., Tian, S. B., Di, Q., Duan, S. H., Dai, K. (2018). Effects of exogenous calcium on mesophyll cell ultrastructure, gas exchange, and photosystem II in tobacco (Nicotiana tabacum linn.) under drought stress. Photosynthetica. 56, 1204–1211. doi: 10.1007/s11099-018-0822-8
Hu, B., Jiang, Z., Wang, W., Qiu, Y., Zhang, Z., Liu, Y., et al. (2019). Nitrate–NRT1.1B–SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants 5, 401–413. doi: 10.1038/s41477-019-0384-1
Ilyas, N., Gull, R., Mazhar, R., Saeed, M., Kanwal, S., Shabir, S., et al. (2017). Influence of salicylic acid and jasmonic acid on wheat under drought stress. Commun. Soil Sci. Plant Anal. 48 (22), 2715–2723. doi: 10.1080/00103624.2017.1418370
Iqbal, M., Ashraf, M. (2007). Seed treatment with auxins modulates growth and ion partitioning in salt-stressed wheat plants. J. Integr. Plant Biol. 49, 1003–1015. doi: 10.1111/j.1672-9072.2007.00488.x
Iqbal, M., Ashraf, M. (2013a). Gibberellic acid mediated induction of salt tolerance in wheat plants: growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 86, 76–85. doi: 10.1016/j.envexpbot.2010.06.002
Iqbal, M., Ashraf, M. (2013b). Salt tolerance and regulation of gas exchange and hormonal homeostasis by auxin-priming in wheat. Pesqui Agropecu Bras. 48, 1210–1219. doi: 10.1590/S0100-204X2013000900004
Iqbal, M., Ashraf, M., Jamil, A. (2006). Seed enhancement with cytokinins: changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 50, 29–39. doi: 10.1007/s10725-006-9123-5
Iqbal, N., Umar, S., Khan, N. A., Khan, M. I. R. (2014). A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ. Exp. Bot. 100, 34–42. doi: 10.1016/j.envexpbot.2013.12.006
Islam, M. R., Rahman, M. M., Mohi-Ud-Din, M., Akter, M., Zaman, E., Keya, S. S., et al. (2022). Cytokinin and gibberellic acid-mediated waterlogging tolerance of mungbean (Vigna radiata l. wilczek). Peer J. 10, e12862. doi: 10.7717/peerj.12862
Janda, T., Szalai, S., Antunovics, Z. S., Horvath, E., Paldi, E. (2000). Effect of benzoic acid and aspirin on chilling tolerance and photosynthesis in young maize plants. Maydica 45, 29–33.
Janda, T., Szalai, G., Tari, I., Paldi, E. (1997). “Exogenous salicylic acid has an effect on chilling symptoms in maize (Zea mays l.) plants,” in Crop development for cool and wet European climate. Eds. Sowinski, P., Zagdanska, B., Aniol, A., Klaus, P. (ECSP-EEC-EAEC, Brussels, Belgium), 179–187.
Janda, T., Szalai, G., Tari, I., Paldi, E. (1999). Hydroponic treatment with salicylic acid decreases the effect of chilling injury in maize (Zea mays l.) plants. Planta. 208, 175–180. doi: 10.1007/s004250050547
Jegadeesan, S., Etan, P., Avital, B., Singh, V., Peres, L., Shabtai, S., et al. (2021). An ethylene over-producing mutant of tomato (Solanum lycopersicum), epinastic, exhibits tolerance to high temperature conditions”. Am. J. Plant Sci. 12 (4), 1–16.
Jegadeesan, S., Palak, C., Arindam, G., Etan, P., Shimon, M., Adi, F., et al. (2018). Proteomics of heat-stress and ethylene-mediated thermotolerance mechanisms in tomato pollen grains. Front. Plant Science. 9. doi: 10.3389/fpls.2018.01558
Jiawei, P., Rahat, S., Xu, X., Xuehao, C. (2021). Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.627331
Jing, Y., Jing, C., Qiuwei, L., Bo, F., Qinghua, X., Weina, L., et al. (2020). ABA enhanced cold tolerance of wheat ‘dn1’ via increasing ROS scavenging system. Plant Signaling Behavior. 15 (8), 1–11. doi: 10.1080/15592324.2020.1780403
Jin, J., Lauricella, D., Armstrong, R., Sale, P., Tang, C. (2015). Phosphorus application and elevated CO2 enhance drought tolerance in field pea grown in a phosphorus-deficient vertisol. Ann. Bot. 116, 975–985. doi: 10.1093/aob/mcu209
Jose, R. A., Ortuno, M. F., Bernal-Vicente, A., Diaz-Vivancos, P., Sanchez-Blanco, M. J., Jose, H. A. (2017). Plant responses to salt stress: Adaptive mechanisms. Agronomy. 7 (18), 1–38.
Jung, H., Lee, D.-K., Choi, Y. D., Kim, J.-K. (2015). OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 236, 304–312. doi: 10.1016/j.plantsci.2015.04.018
Jung, J. H., Park, C. M. (2011). Auxin modulation of salt stress signaling in arabidopsis seed germination. Plant Signaling Behavior. 6 (8), 1198–1200. doi: 10.4161/psb.6.8.15792
Kafkafi, U. (1990). “Impact of potassium in relieving plants from climatic and soil-induced stresses,” in Food security in the WANA region, the essential need for balanced fertilization. international potash institute. Ed. Johnston, A. E.(Basel), 317–327.
Kagale, S., Divi, U. K., Krochko, J. E., Keller, W. A., Krishna, P. (2007). Brassinosteroid confers tolerance in arabidopsis thaliana and brassica napus to a range of abiotic stresses. Planta. 225 (2), 353–364.
Kamat, J. P., Boloor, K. K., Devasagayam, T. P. (2000). Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 1487, 113–127. doi: 10.1016/S1388-1981(00)00088-3
Kang, C., He, S., Zhai, H., Li, R., Zhao, N., Liu, Q. (2018). A sweet potato auxin response factor gene (IbARF5) is involved in carotenoid biosynthesis and salt and drought tolerance in transgenic arabidopsis. Front. Plant Sci. 9, 1307. doi: 10.3389/fpls.2018.01307
Kang, S., Radhakrishnan, R., Khan, A., Kim, M., Park, J., Kim, B., et al. (2014). Gibberellin secreting rhizobacterium, pseudomonas putida h-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 84, 115–124. doi: 10.1016/j.plaphy.2014.09.001
Kang, D. J., Seo, Y. J., Lee, J. D., Ishii, R., Kim, K. U., Shin, D. H., et al. (2005). Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 191 (4), 273–282. doi: 10.1111/j.1439-037X.2005.00153.x
Karim, A. U. S. T., Liu, X., Lu, Z., Yuan, Z., Zhu, Y., Cao, W. (2016). In-season estimation of rice grain yield using critical nitrogen dilution curve. Field Crop Res. 195, 1–8.
Kartal, G., Temel, A., Arican, E., Gozukirmizi, N. (2009). Effects of brassinosteroids on barley root growth, antioxidant system and cell division. Plant Growth Regul. 58, 261–267. doi: 10.1007/s10725-009-9374-z
Kazan, K. (2015). Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 20 (4), 219–229. doi: 10.1016/j.tplants.2015.02.001
Kazemi, K., Eskandari, H. (2011). Effects of salt stress on germination and early seedling growth of rice (Oryza sativa l.) cultivars in Iran. Afr. J. Biotechnol. 10 (77), 17789–17792. doi: 10.5897/AJB11.2219
Keshavarz, H., Sanavy, S. A. M. M. (2018). How salicylic acid modulate photosynthetic pigments, growth and yield of canola. J. Global Agric. Ecology. 8 (1), 45–53.
Keskin, B. C., Sarikaya, A. T., Yuksel, B., Memon, A. R. (2010). Abscisic acid regulated gene expression in bread wheat. Aust. J. Crop Sci. 4, 617–625.
Ke, Q., Wang, Z., Ji, C. Y., Jeong, J. C., Lee, H. S., Li, H., et al. (2015). Transgenic poplar expressing arabidopsis YUCCA6 exhibits auxin-overproduction phenotypes and increased tolerance to abiotic stress. Plant Physiol. Biochem. 94, 19–27. doi: 10.1016/j.plaphy.2015.05.003
Khalid, M., Bilal, M., Hassani, D., Iqbal, H., Wang, H., Huang, D. (2017). Mitigation of salt stress in white clover (Trifolium repens) by azospirillum brasilense and its inoculation effect. Botanical Stud. 58 (1), 1–7. doi: 10.1186/s40529-016-0160-8
Khan, N. A., Syeed, S., Masood, A., Nazar, R., Iqbal, N. (2010). Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int J Plant Biol. 1:e1.
Khan, M. I., Iqbal, N., Masood, A., Per, T. S., Khan, N. A. (2013). Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav. 8 (11). doi: 10.4161/psb.26374
Khodarahmpour, Z., Ifar, M., Motamedi, M. (2012). Effects of NaCl salinity on maize (Zea mays l.) at germination and early seedling stage. Afr. J. Biotechnol. 11 (2), 298–304. doi: 10.5897/AJB11.2624
Khodary, S. E. A. (2004). Effect of salicylic acid on growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 6, 5–8.
Kieber, J. J., Schaller, G. E. (2018). Cytokinin signaling in plant development. Development 145 (4), dev149344. doi: 10.1242/dev.149344
Kim, T. W., Wang, Z. Y. (2010). Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Biol. 61, 681–704. doi: 10.1146/annurev.arplant.043008.092057
Kosakivska, I. V., Vasyuk, V. A., Voytenko, L. V., et al. (2022). The effects of moderate soil drought on phytohormonal balance of triticum aestivum l. and triticum spelta l. Cereal Res. Commun. doi: 10.1007/s42976-022-00332-8
Kramell, R., Atzorn, R., Schneider, G., Miersch, O., Bruckner, C., Schmidt, J., et al. (2000). Occurrence and identification of jasmonic acid and its amino acid conjugates induced by osmotic stress in barley leaf tissue. J. Plant Growth Regul. 14, 29–36. doi: 10.1007/BF00212643
Krishna, P. (2003). Brassinosteroid-mediated stress responses. J. Plant Growth Regul. 22, 289–297. doi: 10.1007/s00344-003-0058-z
Krishnan, S., Merewitz, E. (2014). Drought stress and trinexapac-ethyl modify phytohormone content within Kentucky bluegrass leaves. J. Plant Growth Regul. doi: 10.1007/s00344-014-9434-0
Kuiper, D., Kuiper, P. J. C., Lambers, H., Schuit, J., Staal, M. (1989). Cytokinin concentration in relation to mineral nutrition and benzyladenine treatment in plantago major ssp. pleiosperma. Physiol. Plant 75, 511–517. doi: 10.1111/j.1399-3054.1989.tb05617.x
Kuiper, D., Schuit, J., Kuiper, P. J. C. (1990). Actual cytokinin concentrations in plant tissue as an indicator for salt resistance in cereals. Plant Soil. 123, 243–250. doi: 10.1007/BF00011276
Kumari, V. V., Banerjee, P., Verma, V. C., Sukumaran, S., Chandran, M. A. S., Gopinath, K. A., et al. (2022). Plant nutrition : An EffectiveWay to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 23, 8519. doi: 10.3390/ijms23158519
La, V. H., Lee, B. R., Zhang, Q., Park, S. H., Islam, M., Kim, T. H. (2019). Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in brassica rapa. Horticulture Environment Biotechnol. 60 (1), 31–40. doi: 10.1007/s13580-018-0099-7
Landrein, B., Formosa-Jordan, P., Malivert, A., et al. (2018). Nitrate modulates stem cell dynamics in arabidopsis shoot meristems through cytokinins. PNAS. 115 (6), 1382–1387. doi: 10.1073/pnas.1718670115
Lechowski, Z. (1997). Stomatal response to exogenous cytokinin treatment of the hemiparasite melampyrum arvense l. before and after attachment to the host. Biol. plantarum. 39 (1), 13–21.
Liang, Y., Tabien, R. E., Tarpley, L., Mohammed, A. R., Septiningsih, E. M. (2021). Transcriptome profiling of two rice genotypes under mild field drought stress during grain-filling stage. AoB Plants 13, plab043. doi: 10.1093/aobpla/plab043
Li, H., Chen, Z., Zhou, T., Liu, Y., Raza, S., ZhoU, J. (2018). Effects of high potassium and low temperature on the growth and magnesium nutrition of different tomato cultivars. Hortscience. 53 (5), 710–714. doi: 10.21273/HORTSCI12983-18
Li, H., Li, M., Luo, J., Cao, X. Q. L., Gai, Y., Jiang, X. N., et al. (2012). N-fertilization has different effects on the growth, carbon and nitrogen physiology, and wood properties of slow- and fast-growing populus species. J. Exp. Bot. 63, 695–709. doi: 10.1093/jxb/ers271
Lin, Y., Wang, J., Zu, Y., Tang, Z. (2012). Ethylene antagonizes the inhibition of germination in arabidopsis induced by salinity by modulating the concentration of hydrogen peroxide. Acta Physiol. Plant 34, 1895–1904. doi: 10.1007/s11738-012-0989-8
Li, G., Zhang, C., Zhang, G., Fu, W., Feng, B., Chen, T., et al. (2020). Abscisic acid negatively modulates heat tolerance in rolled leaf rice by increasing leaf temperature and regulating energy homeostasis. Rice. 13 (1), 18. doi: 10.1186/s12284-020-00379-3
Liu, Y., Xu, J., Ding, Y., Wang, Q., Li, G., Wang, S. (2011). Auxin inhibits the outgrowth of tiller buds in rice (Oryza sativa l.) by downregulating OsIPT expression and cytokinin biosynthesis in nodes. Aust. J. Crop Sci. 5 (2), 169–174.
Liu, C., Zhang, T. (2017). Expansion and stress responses of the AP2/EREBP superfamily in cotton. BMC Genomics 18 (1), 1–16. doi: 10.1186/s12864-017-3517-9
Lobanov, A. V., Hatfield, D. L., Gladyshev, V. N. (2008). Reduced reliance on the trace element selenium during evolution of mammals. Genome Biol. 9, R62. doi: 10.1186/gb-2008-9-3-r62
Lou, D., Wang, H., Liang, G., Yu, D. (2017). OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front. Plant science. 8. doi: 10.3389/fpls.2017.00993
Macková, H., Hronkova, M., Dobra, J., Tureckova, V., Novak, O., Lubovska, Z., et al. (2013). Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J. Exp. botany. 64 (10), 2805–2815. doi: 10.1093/jxb/ert131
Maggio, A., Barbieri, G., Raimondi, G., de Pascale, S. (2010). Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J. Plant Growth Regul. 29, 63–72. doi: 10.1007/s00344-009-9114-7
Mahajan, S., Tuteja, N. (2005). Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 444, 139–158. doi: 10.1016/j.abb.2005.10.018
Mahmoud, A. W. M., Abdeldaym, E. A., Abdelaziz, S. M., El-Sawy, M. B., Mottaleb, S. A. (2020). Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 10, 19.
Mandal, S., Raju, R., Kumar, A., Kumar, P., Sharma, P. C. (2018). Current status of research, technology response and policy needs of salt-affected soils in India – a review. Ind. Soc Coast. Agric. Res. 36, 40–53.
Manik, N. S. M., Pengilley, G., Dean, G., Field, B., S. and Zhou, M. (2019). Minimize the impact of waterlogging on crop productivity. Front. Plant Sci. 12.
Marschner, H. (2012). Marschner’s mineral nutrition of higher plants. 3rd (London, UK: Academic Press), 178–189.
Ma, Y., Song, W., Liu, Z., Zhang, H., Guo, H., Shao, H., et al. (2009). The dynamic changing of Ca2+ cellular localization in maize leaflets under drought stress. Comptes Rendus Biol. 332, 351–362. doi: 10.1016/j.crvi.2008.12.003
Masunaga, T., Marques Fong, J. D. (2018). “Chapter 11 - strategies for increasing micronutrient availability in soil for plant uptake,” in Plant micronutrient use efficiency. Eds. Hossain, M. A., Kamiya, T., Burritt, D. J., Phan Tran, L.-S., Fujiwara, T. (Cambridge, MA: Academic Press), 195–208.
Mayland, H. F., Cary, J. W. (1970). Frost and chilling injury to growing plants. Adv. Agronomy. 22, 203–234. doi: 10.1016/S0065-2113(08)60269-2
McAdam, S. A., Brodribb, T. J. (2018). Mesophyll cells are the main site of abscisic acid biosynthesis in water-stressed leaves. Plant Physiol. 177 (3), 911–917.
Mc Guiness, P. N., Reid, J. B., Foo, E. (2019). The role of gibberellins and brassinosteroids in nodulation and arbuscular mycorrhizal associations. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00269
Men, S., Chen, H., Chen, S., Zheng, S., Shen, X., Wang, C., et al. (2020). Effects of supplemental nitrogen application on physiological characteristics, dry matter and nitrogen accumulation of winter rapeseed (Brassica napus l.) under waterlogging stress. Sci. Rep. 10 (1), 401–406. doi: 10.1038/s41598-020-67260-7
Meng, X., Chen, W. W., Wang, Y. Y., Huang, Z. R., Ye, X., Chen, L. S., et al. (2021). Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PloS One 16, e0246944. doi: 10.1371/journal.pone.0246944
Mengutay, M., Ceylan, Y., Baris, U., Kutman and Cakmak, I. (2013). Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant Soil. 368, 57–72. doi: 10.1007/s11104-013-1761-6
Merchan, F., de Lorenzo, L., Gonzalez-Rizzo, S., Niebel, A., Meglias, M., Frugier, F., et al. (2007). Analysis of regulatory pathways involved in the reacquisition of root growth after salt stress in medicago truncatula. Plant J. 51, 1–17. doi: 10.1111/j.1365-313X.2007.03117.x
Michael, L. C. Y., Espada-Y Gil, F., Fuentes Ortiz, G., Santamaria, J. M., Gonzalez-Mendoza, D. (2016). Bioaccumulation and changes in the photosynthetic apparatus of prosopis juliflora exposed to copper. Botanical Sci. 94 (2), 323–330. doi: 10.17129/botsci.507
Miller, C. O., Skoog, F., Von Saltza, M. H., Strong, F. M. (1955). Kinetin, a cell division factor from deoxyribonucleic acid1. J. Am. Chem. Society. 77 (5), 1392–1392. doi: 10.1021/ja01610a105
Min, Y., Qin, B. P., Ma, X. L., Wang, P., Li, M. L., Chen, L. L., et al. (2016). Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum l.). J. Integr. Agric. 15, 2745–2758. doi: 10.1016/S2095-3119(16)61358-8
Miura, K., Okamoto, H., Okuma, E., Shiba, H., Kamada, H., Hasegawa, P. M., et al. (2013). SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in arabidopsis. Plant J. 73 (1), 91–104. doi: 10.1111/tpj.12014
Mohamed, H. I., El-Sayed, A. A., Rady, M. M., Caruso, G., Sekara, A., Abdelhamid, M. T. (2021). Coupling effects of phosphorus fertilization source and rate on growth and ion accumulation of common bean under salinity stress. PeerJ. 9, e11463. doi: 10.7717/peerj.11463
Mohammed, A. H. M. A. (2007). Physiological aspects of mungbean plant (Vigna radiata l. wilczek) in response to salt stress and gibberellic acid treatment. Res. J. Agr Biol. Sci. 3, 200–213.
Motos, J. A., Ortuño, M. F., Vivancos, P. D., Vicente, A. B., Hernandez, J. A., Sánchez, B. M. (2017). Plant responses to salt stress: Adaptive mechanisms. Agronomy 7 (1), 18. doi: 10.3390/agronomy7010018
Muhammad, S., Ditta, A., Iqbal, M. S., Hussain, S. B., Khan, M. I., Ramzan, M., et al. (2018). Effect of salinity stress on cotton growth and role of marker assisted breeding and agronomic practices (chemical,biological and physical) for salinity tolerance. Scholars Rep. 4 (1), 1–13.
Muhammad, J., Muhammad, A. H., Tanveer, H., Anser, A., Safdar, H., Muhammad, I. (2020). Protective effect of potassium application on NaCl induced stress in tomato (Lycopersicon esculentum l.) genotypes. J. Plant Nutr. 43 (13), 1988–1998. doi: 10.1080/01904167.2020.1766071
Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environment. 25 (2), 239–250. doi: 10.1046/j.0016-8025.2001.00808.x
Munns, R. (2005). Genes and salt tolerance: Bringing them together. New Phytologist. 167 (3), 645–663. doi: 10.1111/j.1469-8137.2005.01487.x
Munns, R., Gilliham, M. (2015). Salinity tolerance of crops- what is the cost? New Phytologist. 208 (3), 668–673. doi: 10.1111/nph.13519
Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nagar, S., Singh, V. P., Arora, A., Dhakar, R., Singh, N., Singh, G. P., et al. (2021). Understanding the role of gibberellic acid and paclobutrazol in terminal heat stress tolerance in wheat. Front. Plant Science. 12. doi: 10.3389/fpls.2021.692252
Najar, R., Aydi, S., Sassi-Aydi, S., Zarai, A., Abdelly, C. (2018). Effect of salt stress on photosynthesis and chlorophyll fluorescence in medicago truncatula. Plant Biosyst. - Int. J. Dealing All Aspects Plant Biol. 153 (1), 88–97. doi: 10.1080/11263504.2018.1461701
Narusaka, Y., Nakashima, K., Shinwari, Z. K., Sakuma, Y., Furihata, T., Abe, H., et al. (2003). Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 34 (2), 137–148. doi: 10.1046/j.1365-313X.2003.01708.x
Naulin, P. A., Armijo, G. I., Vega, A. S., Tamayo, K. P., Gras, D. E., de la Cruz, J., et al. (2020). Nitrate induction of primary root growth requires cytokinin signaling in arabidopsis thaliana. Plant Cell Physiol. 61 (2), 342–352. doi: 10.1093/pcp/pcz199
Naz, S., Perveen, S. (2021). Response of wheat (Triticum aestivum l. var. galaxy-2013) to pre-sowing seed treatment with thiourea under drought stress. Pakistan J. Bot. 53, 1209–1217.
Nazar, R., Iqbal, N., Syeed, S., Khan, N.A. (2011). Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol. 167, 807–815.
Nehnevajova, E., Ramireddy, E., Stolz, A., Gerdemann-Knörck, M., Novák, O., Strnad, M., et al (2019). Root enhancement in cytokinin-deficient oilseed rape causes leaf mineral enrichment, increases the chlorophyll concentration under nutrient limitation and enhances the phytoremediation capacity. BMC Plant Biol. 19, 83. doi: 10.1186/s12870-019-1657-6
Netondo, G. W., Onyango, J. C., Beck, E. (2004). Sorghum and salinity:I.response of growth,water relations,and ion accumulation to NaCl salinity. Crop Science. 44 (3), 797–805. doi: 10.2135/cropsci2004.0797
Nie, S., Huang, S., Wang, S., Mao, Y., Liu, J., Ma, R., et al. (2019). Enhanced brassinosteroid signaling intensity via SlBRI1 overexpression negatively regulates drought resistance in a manner opposite of that via exogenous BR application in tomato. Plant Physiol. Biochem. 138, 36–47. doi: 10.1016/j.plaphy.2019.02.014
Nir, I. D. O., Moshelion, M., Weiss, D. (2014). The arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell environment. 37 (1), 113–123. doi: 10.1111/pce.12135
Nishiyama, R., Watanabe, Y., Fujita, Y., Le, D. T., Kojima, M., Werner, T., et al. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell. 23 (6), 2169–2183. doi: 10.1105/tpc.111.087395
Ozdemir, F., Bor, M., Demiral, T., Turkan, I. (2004). Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa l.) under salinity stress. Plant Growth Regul. 42, 203–211. doi: 10.1023/B:GROW.0000026509.25995.13
Ozturk, M., Unal, B. T., García-Caparrós, P., Khursheed, A., Gul, A., Hasanuzzaman, M. (2021). Osmoregulation and its actions during the drought stress in plants. Physiol. Plant 172, 1321–1335. doi: 10.1111/ppl.13297
Palma, F., Lluch, C., Iribarne, C., Garcia-Garrida, J. M., Garcia, N. A. T. (2009). Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in phaseolus vulgaris. Plant Growth Regul. 58, 307–331. doi: 10.1007/s10725-009-9380-1
Pandey, D., Goswami, C., Kumar, B. (2003). Physiological effects of plant hormones in cotton under drought. Biol. Plantarum 47, 535–540. doi: 10.1023/B:BIOP.0000041058.65442.41
Pan, Y., Seymour, G. B., Lu, C., Hu, Z., Chen, X., Chen, G. (2012). An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 31 (2), 349–360. doi: 10.1007/s00299-011-1170-3
Park, J., Kim, Y. S., Kim, S. G., Jung, J. H., Woo, J. C., Park, C. M. (2011). Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in arabidopsis. Plant Physiol. 156 (2), 537–549. doi: 10.1104/pp.111.177071
Parveen, A., Ahmar, S., Kamran, M., Malik, Z., Ali, A., Riaz, M., et al. (2021). Abscisic acid signaling reduced transpiration flow, regulated na+ ion homeostasis and antioxidant enzyme activities to induce salinity tolerance in wheat (Triticum aestivum l.) seedlings. Environ. Technol. Innovation. 24, 101808. doi: 10.1016/j.eti.2021.101808
Pech, J. C., Bouzayen, M., Latche, A. (2008). Climacteric fruit ripening: Ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 175, 114–120. doi: 10.1016/j.plantsci.2008.01.003
Peck, A. W., McDonald, G. K. (2010). Adequate zinc nutrition alleviates the adverse effects of heat stress in bread wheat. Plant Soil. 337, 355–374. doi: 10.1007/s11104-010-0532-x
Perveen, S., Hussain, S. A. (2020). Methionine-induced changes in growth, glycinebetaine, ascorbic acid, total soluble proteins and anthocyanin contents of two Zea mays l. varieties under salt stress. J. Anim. Plant Sci. 31, 131–142. doi: 10.36899/JAPS.2021.1.0201
Pessarakli, M., Szabolcs, I. (2010). “Soil salinity and sodicity as particular Plant/Crop stress factors,” in Pessarakli m,editor. handbook of plant and crop stress, 4th (Routledge Handbooks Online), p.1–p19. doi: 10.1201/B10329-3
Pierik, R., Tholen, D., Poorter, H., Visser, E. J. W., Voesenek, L. A. C. J. (2006). The janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci. 11, 176–183. doi: 10.1016/j.tplants.2006.02.006
Pospíšilová, H., Synkova, H., Rulcova, J.. (2000). Cytokinnins and water. Biologia Plantarum 43 (3), 321–328.
Pospíšilová, H., Jiskrova, E., Vojta, P., Mrizova, K., Kokáš, F., Čudejková, M. M., et al. (2016). Transgenic barley overexpressing a cytokinin dehydrogenase gene shows greater tolerance to drought stress. New Biotechnol. 33, 692–705. doi: 10.1016/j.nbt.2015.12.005
Prerostova, S., Cerny, M., Dobrev, P. I., Motyka, V., Hluskova, L., Zupkova, B., et al. (2021). Light regulates the cytokinin-dependent cold stress responses in arabidopsis. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.60871
Qados, A. M. A. (2011). Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J. Saudi Soc. Agric. Sci. 10 (1), 7–15.
Qi, J., Song, C. P., Wang, B., Zhou, J., Kangasjarvi, J., Zhu, J. K., et al. (2018). Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 60 (9), 805–826. doi: 10.1111/jipb.12654
Rahman, A., Mostofa, M. G., Alam, M. M., Nahar, K., Hasanuzzaman, M., Fujita, M. (2015). Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. BioMed. Res. Int. 340812. doi: 10.1155/2015/340812
Rama Reddy, N. R., Ragimasalawada, M., Sabbavarapu, M. M., et al. (2014). Detection and validation of stay-green QTL in post-rainy sorghum involving widely adapted cultivar, M35-1 and a popular stay-green genotype B35. BMC Genomics 15, 909. doi: 10.1186/1471-2164-15-909
Ramireddy, E., Hosseini, S. A., Eggert, K., Gillandt, S., Gnad, H., von Wirén, N., et al. (2018). Root engineering in barley: Increasing cytokinin degradation produces a larger root system, mineral enrichment in the shoot and improved drought tolerance. Plant Physiol. 177, 1078–1095. doi: 10.1104/pp.18.00199
Rasmussen, A., Hu, Y., Depaepe, T., Vandenbussche, F., Boyer, F. D., van der Straeten, D., et al. (2017). Ethylene controls adventitious root initiation sites in arabidopsis hypocotyls independently of strigolactones. J. Plant Growth Regulation. 36 (4), 897–911. doi: 10.1007/s00344-017-9692-8
Raza, A., Charagh, S., Zahid, Z., Mubarik, M. S., Javed, R., Siddiqui, M. H., et al. (2021). Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 40, 1513–1541. doi: 10.1007/s00299-020-02614-z
Rivero, R. M., Gimeno, J., Van Deynze, A., Walia, H., Blumwald, E. (2010). Enhanced cytokinin synthesis in tobacco plants expressing PSARK: IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol. 51 (11), 1929–1941. doi: 10.1093/pcp/pcq143
Rivero, R. M., Ruiz, J. M., Romero, L. M. (2004). Importance of n source on heat stress tolerance due to the accumulation of proline and quaternary ammonium compounds in tomato plants. Plant Biol. (Stuttg). 6 (6), 702–707. doi: 10.1055/s-2004-821293
Rostamza, M., Chaichi, M. R., Jahansooz, M. R., Rahimian, M. H., Sharifi, H. R. (2011). Effects of water stress and nitrogen fertilizer on multi-cut pearl millet forage yield, nitrogen, and water use efficiency. Commun. Soil. Sci. Plant Anal. 42, 2427–2440. doi: 10.1080/00103624.2011.609252
Ruehr, N. K., Grote, R., Mayr, S., Arneth, A. (2019). Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol. 39, 1285–1299. doi: 10.1093/treephys/tpz032
Sahni, S., Prasad, B. D., Liu, Q., Grbic, V., Sharpe, A., Singh, S. P., et al. (2016). Overexpression of the brassinosteroid biosynthetic gene DWF4 in brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 6 (1), 1–14. doi: 10.1038/srep28298
Saini, S., Sharma, I., Pati, P. K. (2015). Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00950
Sakhabutdinova, A. R., Fatkhutdinova, D. R., Bezrukova, M. V., Shakirova, F. M. (2003). Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg J. Plant Physiol. 29, 314–319.
Salehin, M., Li, B., Tang, M., Katz, E., Song, L., Ecker, J. R., et al. (2019). Auxin-sensitive Aux/IAA proteins mediate drought tolerance in arabidopsis by regulating glucosinolate levels. Nat. Commun. 10 (1), 1–9. doi: 10.1038/s41467-019-12002-1
Salvi, P., Manna, M., Kaur, H., Thakur, T., Gandass, N., Bhatt, D., et al. (2021). Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. doi: 10.1007/s00299-021-02683-8
Sanchez-Romera, B. (2014). Regulation of root hydraulic properties by methyl jasmonate, reactive nitrogen species and arbuscular mycorrhizae Vol. 180 (Granada: Universidad de Granada). http://hdl.handle.net/10481/34487
Saneoka, H., Moghaieb, R. E. A., Premachandra, G. S., Fujita, K. (2004). And fujita, K.(2004). nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in agrostis palustris huds. Environ. Exp. Bot. 52, 131–138. doi: 10.1016/j.envexpbot.2004.01.011
Sardans, J., Penuelas, J. (2012). The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 160, 1741–1761. doi: 10.1104/pp.112.208785
Sarwar, M., Saleem, M., Ali, B., Hamzah Saleem, M., Rizwan, M., Usman, K., et al. (2022). Application of potassium, zinc and boron as potential plant growth modulators in gossypium hirsutum l. under heat stress. Turk Tarim ve Ormancilik Dergisi/Turkish J. Agric. Forestry. doi: 10.55730/tar-2201-63
Saud, S., Fahad, S., Yajun, C., Ihsan, M. Z., Hammad, H. M., Nasim, W., et al. (2017). Effects of nitrogen supply on water stress and recovery mechanisms in Kentucky bluegrass. Plants. Front. Plant Sci. 8, 983. doi: 10.3389/fpls.2017.00983
Sayed, S. A. (1998). Impacts of boron application on maize plants growing under flooded and unflooded conditions. Biol. Plant 41, 101–109. doi: 10.21203/rs.3.rs-1661201/v1
Sehar, Z., Gautam, H., Masood, A., Khan, N. (2022). Ethylene and proline-dependent regulation of antioxidant enzymes to mitigate heat stress and boost photosynthetic efficacy in wheat plants. Res. square. 1-25. doi: 10.1007/s00344-022-10737-8
Sehgal, A., Sita, K., Siddique, K. H., Kumar, R., Bhogireddy, S., Varshney, R. K., et al. (2018). Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01705
Serraj, R. (2003). Effects of drought stress on legume symbiotic nitrogen fixation: physiological mechanisms. Indian J. Exp. Biol. 41 (10), 1136–1141.
Serrano, R., Rios, G., Ros, R., Marquez, J. A., Proft, M., Mulet, J. M., et al. (1999). A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Botany. 50, 1023–1036. doi: 10.1093/jxb/50.Special_Issue.1023
Shabir, H.W., Vinay, K., Varsha, S., Saroj, K. S. (2016). Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 4 (3), 162. doi: 10.1016/j.cj.2016.01.010
Shakirova, F. M., Sakhabutdinova, A. R., Ishdavletova, L. O. V. (2010). Influence of pretreatment with methyl jasmonate on wheat resistance to salt stress. Agrochimiya. 7, 26–32.
Shao, A., Ma, W., Zhao, X., Hu, M., He, X., Teng, W., et al. (2017). The auxin biosynthetic TRYPTOPHAN AMINOTRANSFERASE RELATED TaTAR2. 1-3A increases grain yield of wheat. Plant Physiol. 174 (4), 2274–2288.
Shao, H. B., Chu, L. Y., Jaleel, C. A., Zhao, C. X. (2008). Water deficit stress-induced anatomical changes in higher plants. CR Biol. 331, 215–225. doi: 10.1016/j.crvi.2008.01.002
Sharma, I., Chin, I., Saini, S., Bhardwaj, R., Pati, P.K. (2013). Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem. 69, 17–26.
Sharma, D. K., Chaudhari, S. K., Singh, A. (2014a). CSSRI vision 2050 (Karnal, India: Central Soil Salinity Research Institute).
Shashibhusan, D., Reddy, A., Bhadru, D., Pradeep, T. (2021). Effect of gibberelic acid (GA3) on the yield attributing traits during cold period in rice. Int. J. Environ. Climate change. 10, 9734.
Shibasaki, K., Uemura, M., Tsurumi, S., Rahman, A. (2009). Auxin response in arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell. 21 (12), 3823–3838. doi: 10.1105/tpc.109.069906
Shi, F., Dong, Y., Wang, M. (2020). Transcriptomics analyses reveal that OsMIOX improves rice drought tolerance by regulating the expression of plant hormone and sugar related genes. Plant Biotechnol Rep. 14, 339–349. doi: 10.1007/s11816-020-00608-7
Shi, J., Drummond, B. J., Habben, J. E., Brugire, N., Weers, B. P., Hakimi, S. M., et al. (2019). Ectopic expression of ARGOS 8 reveals a role for ethylene in root-lodging resistance in maize. Plant J. 97 (2), 378–390. doi: 10.1111/tpj.14131
Shi, H. Z., Zhu, J. K. (2002). Regulation of expression of the vacuolar na?/ h? antiporter gene AtNHX1 by salt stress and abscisic acid. Plant Mol. Biol. 50, 543–550. doi: 10.1023/A:1019859319617
Shi, Y., Tian, S., Hou, L., Huang, X., Zhang, X., Guo, H., et al. (2012). Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-a ARR genes in arabidopsis. Plant Cell. 24 (6), 2578–2595. doi: 10.1105/tpc.112.098640
Shohat, H., Cheriker, H., Kilambi, H. V., Eliaz, I. N., Blum, S., Amsellem, Z., et al. (2021). Inhibition of gibberellin accumulation by water deficiency promotes fast and long-term ‘drought avoidance’ responses in tomato. New Phytol. 232 (5), 1985–1998. doi: 10.1111/nph.17709
Shrivastava, P., Kumar, R. (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2), 123–131. doi: 10.1016/j.sjbs.2014.12.001
Siddiqui, M. H., Mohammad, F., Khan, M. N., Al-Whaibi, M. H., Bahkali, A. H. A. (2010). Nitrogen in relation to photosynthetic capacity and accumulation of osmoprotectant and nutrients in brassica genotypes grown under salt stress. Agric. Sci. China. 9, 671–680. doi: 10.1016/S1671-2927(09)60142-5
Singh, M., Singh, V. P., Prasad, S. M. (2019). Nitrogen alleviates salinity toxicity in solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol. Biochem. 141, 466–476. doi: 10.1016/j.plaphy.2019.04.004
Sobeih, W. Y., Dodd, I. C., Bacon, M. A., Grierson, D., Davies, W. J. (2004). Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J. Exp. Botany. 55 (407), 2353–2363. doi: 10.1093/jxb/erh204
Sofy, M. R., Aboseidah, A. A., Heneidak, S. A., Ahmed, H. R. (2021). ACC deaminase containing endophytic bacteria ameliorate salt stress in pisum sativum through reduced oxidative damage and induction of antioxidative defense systems. Environ. Sci. pollut. Res. 28, 40971–40991. doi: 10.1007/s11356-021-13585-3
Spollen, W. G., LeNoble, M. E., Samuels, T. D., Bernstein, N., Sharp, R. E. (2000). Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 122 (3), 967–976. doi: 10.1104/pp.122.3.96
Sripinyowanich, S., Klomsakul, P., Boonburapong, B., Bangyeekhun, T., Asami, T., Gu, H., et al. (2013). Exogenous ABA induces salt tolerance in indica rice (Oryza sativa l.): the role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ. Exp. Bot. 86, 94–105. doi: 10.1016/j.envexpbot.2010.01.009
Su, Y., Huang, Y., Dong, X., Wang, R., Tang, M., Cai, J., et al. (2021). Exogenous methyl jasmonate improves heat tolerance of perennial ryegrass through alteration of osmotic adjustment, antioxidant defense, and expression of jasmonic acid-responsive genes. Front. Plant Science. 12. doi: 10.3389/fpls.2021.664519
Sudhir, P. R., Pogoryelov, D., Kovács, L., Garab, G., Murthy, S. D. (2005). The effects of salt stress on photosynthetic electron transport and thylakoid membrane proteins in the cyanobacterium spirulina platensis. BMB Rep. 38 (4), 481–485. doi: 10.5483/BMBRep.2005.38.4.481
Sun, X., Zhao, T., Gan, S., Ren, X., Fang, L., Karungo, S. K., et al. (2016). Ethylene positively regulates cold tolerance in grapevine by modulating the expression of ETHYLENE RESPONSE FACTOR 057. Sci. Rep. 6, 24066. doi: 10.1038/srep24066
Syeed, S., Anjum, N. A., Nazar, R., Iqbal, N., Masood, A., Khan, N. A. (2011). Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea l.) differing in salt tolerance. Acta Physiol. Plant 33, 877–886. doi: 10.1007/s11738-010-0614-7
Szabados, L., Savoure, A. (2009). Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. doi: 10.1016/j.tplants.2009.11.009
Tadashi, S., Takeshi, O., Shinya, M., Mari, T., Yuta, T., Nahoko, H., et al. (2010). Auxins reverse plant male sterility caused by high temperatures. Agric. Sci. 107 (19), 8569–8574.
Tanaka, K., Nakamura, Y., Asami, T. (2003). Physiological roles of brassinosteroids in early growth of arabidopsis: Brassinosteroids have a synergistic relationship with gibberellin as well as auxin in light-grown hypocotyl elongation. J. Plant Growth Regul. 22, 259–271. doi: 10.1007/s00344-003-0119-3
Tanveer, M., Shahzad, B., Sharma, A., Khan, E. A. (2019). 24-epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 135, 295–303. doi: 10.1016/j.plaphy.2018.12.013
Tariq, A., Pan, K., Olatunji, O. A., Graciano, C., Li, Z., Sun, F., et al. (2017). Phosphorous application improves drought tolerance of phoebe zhennan. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01561
Tewari, R. K., Kumar, P., Sharma, P. N. (2006). Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry. plants. Sci. Hortic. 108, 7–14. doi: 10.1016/j.scienta.2005.12.006
Tewari, R. K., Kumar, P., Tewari, N., Srivastava, S., Sharma, P. N. (2004). Macronutrient deficiencies and differential antioxidant responses-influence on the activity and expression of superoxide dismutase in maize. Plant Sci. 166, 687–694. doi: 10.1016/j.plantsci.2003.11.004
Thalooth, A. T., Tawfik Mohamed, H. M. (2006). A comparative study on the effect of foliar application of zinc, potassium and magnesium on growth, yield and some chemical constituents of mungbean plants grown under water stress conditions. World J. Agric. Sci. 2, 37–46.
Tilman, D., Balzer, C., Hill, J., Belfort, B. L. (2011). Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U. S. A. 108, 20260–20264. doi: 10.1073/pnas.1116437108
Tran, T. T., Kano-Nakata, M., Takeda, M., Menge, D., Mitsuya, S., Inukai, Y., et al. (2014). Nitrogen application enhanced the expression of developmental plasticity of root systems triggered by mild drought stress in rice. Plant Soil. 378, 139–152. doi: 10.1007/s11104-013-2013-5
Tuna, A. L., Kaya, C., Dikilitas, M., Higgs, D. (2008). The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 62, 1–9. doi: 10.1016/j.envexpbot.2007.06.007
Tuteja, N. (2007). Abscisic acid and abiotic stress signaling. Plant Signal Behav. 3), 135–138. doi: 10.4161/psb.2.3.4156
Venugopalan, V. K., Nath, R., Sengupta, K., Nalia, A., Banerjee, S., Sarath Chandran, M. A., et al. (2021). The response of lentil (Lens culinaris medik.) to soil moisture and heat stress under different dates of sowing and foliar application of micronutrients. Front. Plant Sci. 10, 679469.
Verhoeven, A. S., Demmig-Adams, B., Adams, W. W. (1997). Enhanced employment of the xanthophylls cycle and thermal energy dissipation in spinach exposed to high light and n stress. Plant Physiol. 113, 817–824. doi: 10.1104/pp.113.3.817
Vob, U., Bishopp, A., Farcot, E., Bennett, M. J. (2014). Modelling hormonal response and development. Trends Plant Sci. 19, 311–319. doi: 10.1016/j.tplants.2014.02.004
Vojta, P., Kokáš, F., Husičková, A., Grúz, J., Bergougnoux, V., Marchetti, C. F., et al. (2016). Whole transcriptome analysis of transgenic barley with altered cytokinin homeostasis and increased tolerance to drought stress. New Biotechnol. 33, 676–691. doi: 10.1016/j.nbt.2016.01.010
Vries, F. T., Brown, C., Stevens, C. J. (2016). Grassland species root response to drought: 1528 Consequences for soil carbon and nitrogen availability. FPlant Soil. 409, 297–312.
Wahid, A., Gelani, S., Basra, M. A., Perveen, M. (2006). Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J. Plant Physiol. 164 (3), 283–294. doi: 10.1016/j.jplph.2006.01.005
Walia, H., Wilson, C., Wahid, A., Condamine, P., Cui, X., Close, T. J. (2006). Expression analysis of barley (Hordeum vulgare l.) during salinity stress. Funct. Integr. Genomic. 6, 143–156. doi: 10.1007/s10142-005-0013-0
Wang, Z., Han, Y., Luo, S., Rong, X., Song, H., Jiang, N., et al. (2022). Calcium peroxide alleviates the waterlogging stress of rapeseed by improving the rhizosphere oxygen environment in a rice-rape rotation field. Res. square. doi: 10.21203/rs.3.rs-1373782/v1
Wang, Y., Yuan, M., Li, Z., Niu, Y., Jin, Q., Zhu, B., et al. (2020). Effects of ethylene biosynthesis and signaling on oxidative stress and antioxidant defense system in Nelumbo nucifera G. under cadmium exposure. Environ. Sci. Pollut. Res. 27, 40156–40170.
Wang, Y., Jiang, H., Zuolin Mao, Z., Wenjun Liu, W., Jiang, S., H. Xu, H., et al. (2021). Ethylene increases the cold tolerance of apple via the MdERF1B–MdCIbHLH1 regulatory module. Plant J. 106 (2), 379–393. doi: 10.1111/tpj.15170
Wang, J., Wang, D., Zhu, M., Li, F. (2018b). Exogenous 6-benzyladenine improves waterlogging tolerance in maize seedlings by mitigating oxidative stress and upregulating the ascorbate-glutathione cycle. Front. Plant Sci. 12, 1–16. doi: 10.3389/fpls.2021.680376
Wang, C., Zhao, Y., Gu, P., Zou, F., Meng, L., Song, W. (2018a). Auxin is involved in lateral root formation induced by drought stress in tobacco seedlings. J. Plant Growth Regulat. 37, 539–549. doi: 10.1111/ppl.12444
Wang, W. X., Vinocur, B., Shoseyov, O., Altman, A. (2001). Biotechnology of plant osmotic stress toelrance and physiological and moleucalr considerations. Acta Hortic. 560, 285–292. doi: 10.17660/ActaHortic.2001.560.54
Wang, B., Zhang, J., Xia, X., Zhang, W. H. (2011). Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regul. 65, 407–413. doi: 10.1007/s10725-011-9595-9
Wani, S. H., Singh, N. B., Haribhushan, A., Mir, J. A. (2013). Compatible solute engineering in plants for abiotic stress tolerance–the role of glycine betaine. Curr. Genomics 14, 157–165. doi: 10.2174/1389202911314030001
Waraich, E. A., Ahmad, R., Ashraf, M. Y., Saifullah, Ahmad, M. (2011). Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand. B Soil. Plant Sci. 61, 291–304.
Wei, Z., Li, J. (2016). Brassinosteroids regulate root growth, development, and symbiosis. Mol. Plant 9, 86–100. doi: 10.1016/j.molp.2015.12.003
Werner, T., Nehnevajova, E., Köllmer, I., Novák, O., Strnad, M., Krämer, U., et al. (2010). Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in arabidopsis and tobacco. Plant Cell. 22, 3905–3920. doi: 10.1105/tpc.109.072694
Widuri, L. I., Lakitan, B., Sodikin, E., Hasmeda, M., Meihana, M., Kartika, K., et al. (2018). Shoot and root growth in common bean (Phaseolus vulgaris l.) exposed to gradual drought stress. Agrivita 40, 442–452. doi: 10.17503/agrivita.v40i0.1716
Wilkinson, S., Davies, W. J. (2010). Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell environment. 33 (4), 510–525.
Wu, Q., Xia, R., Zou, Y. (2005). Reactive oxygen metabolism in mycorrhizal and nonmycorrhizal citrus (Poncirus trifoliate) seedlings subjected to water stress. J. Plant Physiol. 51, 437–447.
Wu, L., Liu, D., Wu, J., Zhang, R., Qin, Z., Liu, D., et al. (2013). Regulation of flowering locust by a MicroRNA in Brachypodium distachyon. Plant Cell. 25 (11), 4363–4377. doi: 10.1105/tpc.113.118620
Xiao, Y., Wu, X., Sun, M., Peng, F. (2020). Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00696
Xiong, L., Gong, Z., Rock, C. D., Subramanian, S., Guo, Y., Xu, W., et al. (2001). Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in arabidopsis. Dev. Cell. 1, 771–781. doi: 10.1016/S1534-5807(01)00087-9
Xiong, L., Zhu, J. K. (2003). Regulation of Abscisic Acid Biosynthesis. Plant Physiol 133 (1), 29–36. doi: 10.1104/pp.103.025395
Xiong, X. P., Sun, S. C., Zhang, X. Y., Li, Y. J., Liu, F., Zhu, Q. H., et al. (2020). GhWRKY70D13 regulates resistance to verticillium dahliae in cotton through the ethylene and jasmonic acid signaling pathways. Front. Plant science. 11, 69. doi: 10.3389/fpls.2020.00069
Xu, C., Li, X., Zhang, L. (2013). The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of zoysia japonica under drought conditions. PloS One 8, e68214. doi: 10.1371/journal.pone.0068214
Xu, L., Xiang, G., Sun, Q., Ni, Y., Jin, Z., Gao, S., et al. (2019). Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Hortic. Res. 6, 114. doi: 10.1038/s41438-019-0197-4
Xu, Y., Burgess, P., Zhang, X., Huang, B. (2016). Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in agrostis stolonifera. J. Exp. Botany. 67 (6), 1979–1992. doi: 10.1093/jxb/erw019
Yamaguchi, S. (2008). Gibberellin metabolism and its regulation. Ann. Rev. Plant Physiol. 59, 225–251. doi: 10.1146/annurev.arplant.59.032607.092804
Yan, A., Chen, Z. (2016). The pivotal role of abscisic acid signaling during transition from seed maturation to germination. Plant Cell Rep. 36 (5), 689–703. doi: 10.1007/s00299-016-2082-z
Yang, Y., Guo, J. Y., Wang, G. X., Yang, L. D., Yang, Y. (2012). Effects of drought and nitrogen addition on photosynthetic characteristics and resource allocation of abies fabri seedlings in eastern Tibetan plateau. New For. 43, 505–518. doi: 10.1007/s11056-011-9295-3
Yang, Y., Jiang, Y., Mi, X., Gan, L., Gu, T., Ding, J., et al. (2016). Identification and expression analysis of cytokinin response regulators in fragaria vesca. Acta Physiol. Plant 38, 198. doi: 10.1007/s11738-016-2213-8
Yang, L., Zu, Y. G., Tang, Z. H. (2013). Ethylene improves arabidopsis salt tolerance mainly via retaining k+ in shoots and roots rather than decreasing tissue na+ content. environ. Exp. Bot. 86, 60–69. doi: 10.1016/j.envexpbot.2010.08.006
Yao, C., Zhang, F., Sun, X., Shang, D., He, F., Li, X., et al. (2019). Effects of s-abscisic acid (S-ABA) on seed germination, seedling growth, and Asr1 gene expression under drought stress in maize. J. Plant Growth Regulation. 38 (4), 1300–1313. doi: 10.1007/s00344-019-09934-9
Yin, J., Jia, J., Lian, Z., Hu, Y., Guo, J., Huo, H., et al. (2019). Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotoxicol. Environ. Saf. 169, 8–17. doi: 10.1016/j.ecoenv.2018.10.105
Yoonha, K., Seo, C. W., Khan, A. F., Mun, B. G., Shahzad, R., K, J.W., Yun, B. W., et al. (2018). Exo-ethylene application mitigates waterlogging stress in soybean (Glycine max l.). BMC Plant Biol. 18, 254. doi: 10.1186/s12870-018-1457-4
Younis, M. E., EL-shahabya, O. A., Nemat alla, M. M., El-bastawisy, Z. M. (2003). Kinetin alleviates the influence of waterlogging and salinity on growth and affects the production of plant growth regulators in vigna sinensis and zea mays. Agronomie 23, 277–285. doi: 10.1051/agro:2003010
Yu, X., Fei, P., Xie, Z., Zhang, W., Zhao, Q., Zhang, X. (2019). Effects of methyl jasmonate on growth, antioxidants, and carbon and nitrogen metabolism of Glycyrrhiza uralensis under salt stress. Biol. Plantarum. 63 (1), 89–96. doi: 10.32615/bp.2019.011
Zahoor, R., Dong, H., Abid, M., Zhao, W., Wang, Y., Zhou, Z. (2017). Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 137, 73–83. doi: 10.1016/j.envexpbot.2017.02.002
Zain, N. A. M., Ismail, M. R., Puteh, A., Mahmood, M., Islam, M. R. (2014). Drought tolerance and ion accumulation of rice following application of additional potassium fertilizer. Commun. Soil. Sci. Plant Anal. 45, 2502–2514. doi: 10.1080/00103624.2014.932374
Zandi, P., Schnug, E. (2022). Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biol. (Basel) 11, 155. doi: 10.3390/biology11020155
Zang, Y. X., Ik, J. C., Zhang, L. L., Hong, S. B., Zheng, W. W., Xu, K. (2016). Effect of gibberellic acid application on plant growth attributes, return bloom, and fruit quality of rabbit eye blueberry. Scientia Horticulturae 200, 13–18.
Zhang, S., Hu, J., Zhang, Y., Xie, X. J., Knapp, A. (2007). Seed priming with brassinolide improves lucerne (Medicago sativa l.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust. J. Agric. Res. 58, 811–815. doi: 10.1071/AR06253
Zhang, Y., Li, Y., Hassan, M. J., Li, Z., Peng, Y. (2020). Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol. 20 (1), 1–12. doi: 10.1186/s12870-020-02354-y
Zhang, X., Fu, X., Liu, F., Wang, Y., Bi, H., Ai, X. (2021). Hydrogen sulfide improves the cold stress resistance through the CsARF5-CsDREB3 module in cucumber. Int. J. Mol. Sci. 22 (24), 13229. doi: 10.3390/ijms222413229
Zhang, C., Li, G., Chen, T., Feng, B., Fu, W., Yan, J., et al. Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice. 11 (14), 1–14. doi: 10.1186/s12284-018-0206-5
Zhang, C., Li, G., Chen, T., Feng, B., Fu, B., Yan, J., et al. (2018). Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils rice. Rice. 11 (14), 1–14. doi: 10.1186/s12284-018-0206-5
Zhang, X., Li, K., Liu, S., Zou, P., Chen, X., Qin, Y., et al. (2017). Relationship between the degree of polymerization of chitooligomers and their activity affecting the growth of wheat seedlings under salt stress. journal. Agric. Food Chem. 65 (2), 501–509. doi: 10.1021/acs.jafc.6b03665
Zhang, Q., Li, J. J., Zhang, W. J., Yan, S. N., Wang, R., Zhao, J. F., et al. (2012). The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 72, 805–816. doi: 10.1111/j.1365-313X.2012.05121.x
Zhang, Y. Y., Wang, L. L., Liu, Y. L., Zhang, Q., Wei, Q. P., Zhang, W. H. (2006). Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na1/H1 antiport in the tonoplast. Planta 224, 545–555. doi: 10.1007/s00425-006-0242-z
Zhu, Z., Gerendas, J., Bendixen, R., Schinner, K., Tabrizi, H., Sattelmacher, B., et al. (2000). Different tolerance to light stress in N03–and NH4+-grown phaseolus vulgaris l. Plant Biol. 2, 558–570. doi: 10.1055/s-2000-7498
Zhai, Y., Zhang, R., Yang, W., Yang, M. (2017). Effects of interphase on the dispersion of MWCNTs in ethylene-α-octene copolymers revealed by solid-state NMR spectroscopy. Polymer. 114, 44–53. doi: 10.1016/j.polymer.2017.02.076
Zhong, T., Zhang, L., Sun, S., Zeng, H., Han, L. (2014). Effect of localized reduction of gibberellins in different tobacco organs on drought stress tolerance and recovery. Plant Biotechnol. Rep. 8 (5), 399–408. doi: 10.1007/s11816-014-0330-7
Zhu, Y. X., Gong, H. J., Yin, J. L. (2019). Role of silicon in mediating salt tolerance in plants: A review. Plants (Basel) 8 (6), 147. doi: 10.3390/plants8060147
Zhu, J. K. (2002). Salt and drought stress signal transduction in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 53, 247–273.
Zhu, Y. X., Xu, X. B., Hu, Y. H., Han, W. H., Yin, J. L., Li, H. L., et al. (2015). Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus l. Plant Cell Rep. 34, 1629–1646. doi: 10.1007/s00299-015-1814-9
Keywords: abiotic stress, phytohormone, nutrient, signaling, antioxidant, gene expression
Citation: Swain R, Sahoo S, Behera M and Rout GR (2023) Instigating prevalent abiotic stress resilience in crop by exogenous application of phytohormones and nutrient. Front. Plant Sci. 14:1104874. doi: 10.3389/fpls.2023.1104874
Received: 22 November 2022; Accepted: 12 January 2023;
Published: 09 February 2023.
Edited by:
Muhammad Rizwan, Government College University, Faisalabad, PakistanReviewed by:
Mona F. A. Dawood, Assiut University, EgyptZahoor Ahmad, University of Central Punjab, Pakistan
Copyright © 2023 Swain, Sahoo, Behera and Rout. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gyana Ranjan Rout, Z3Jyb3V0QHJlZGlmZm1haWwuY29t
†These authors share first authorship
 Rinny Swain
Rinny Swain Smrutishree Sahoo2†
Smrutishree Sahoo2† Gyana Ranjan Rout
Gyana Ranjan Rout