- 1Key Laboratory for Quality and Safety Control of Subtropical Fruits and Vegetables, Collaborative Innovation Center for Efficient and Green Production of Agriculture in Mountainous Areas of Zhejiang Province, Ministry of Agriculture and Rural Affairs, College of Horticulture Science, Zhejiang Agriculture and Forestry University, Hangzhou, China
- 2Engineering Laboratory of Genetic Improvement of Horticultural Crops of Shandong Province, College of Horticulture, Qingdao Agricultural University, Qingdao, China
- 3Hangzhou Lin’an District Agricultural and Rural Bureau, Hangzhou, China
- 4Yantai Institute of Agricultural Sciences, Yantai, China
Cucumber belongs to the family Cucurbitaceae (melon genus) and is an annual herbaceous vegetable crop. Cucumber is an important cash crop that is grown all over the world. From morphology to cytology, from canonical genetics to molecular biology, researchers have performed much research on sex differentiation and its regulatory mechanism in cucumber, mainly in terms of cucumber sex determination genes, environmental conditions, and the effects of plant hormones, revealing its genetic basis to improve the number of female flowers in cucumber, thus greatly improving the yield of cucumber. This paper reviews the research progress of sex differentiation in cucumber in recent years, mainly focusing on sex-determining genes, environmental conditions, and the influence of phytohormones in cucumber, and provides a theoretical basis and technical support for the realization of high and stable yield cultivation and molecular breeding of cucumber crop traits.
1 Introduction
The sexes of plants are diverse and have evolved over a long period of biological evolution through a process of sexual differentiation. Plant sex is generally defined in terms of unisexual flowers, i.e., “if a flower or plant contains only stamens, it is male, and if it contains only pistils, it is female,” so studies on plant sex determination have focused on the regulation of unisexual flower development (Bai, 2015). In the evolution of plant sex types, hermaphroditic plants are considered to be the original sex types of land plants, while monoecious and dioecious unisexual flowers may have originated from hermaphroditic plants and emerged only after several evolutionary processes (Tanurdzic and Banks, 2004). Most angiosperms are hermaphrodites, producing only complete flowers. Sex determination is a developmental evolutionary process in which the formation of unisexual flowers facilitates heterosis and promotes genetic diversity (Chen et al., 2016). Over the past few decades, there has been a concerted effort to identify the determinants that control plant sex and to explore the evolutionary forces that drive plant sex variation (Diggle et al., 2011). As research continued, it was discovered that cucurbits cover most of the sex types of angiosperms, and some species have even evolved sex chromosomes (Ming et al., 2011).
Cucumber is a cucurbit family and cucumber genus of annual sprawling herb crops that have been commonly cultivated worldwide for a long time. Cucumber has a unique flavor, a crisp texture, is rich in nutrients, has low heat, and is enjoyed by people from all over the world. Cucumber is one of the seven major vegetables in China; its production area ranks first in the world, and it has important economic value (Zhang et al., 2018; Li H. et al., 2022). Cucumber yield is importantly related to sex differentiation. The cucumber sex system is complex and diverse, and the mechanism of male and female flower differentiation in cucumber is also intricate and complex. Several studies have attempted to characterize the molecular aspects of sex determination in cucumbers. At the early stage of cucumber flower development, the flower primordium is bisexual, including the initial form of the anther and pistil. In cucumber development, sex determination requires selective cessation of male or female progenitors (Bai et al., 2004; Pawełkowicz M. et al., 2019; Li Z. et al., 2022).
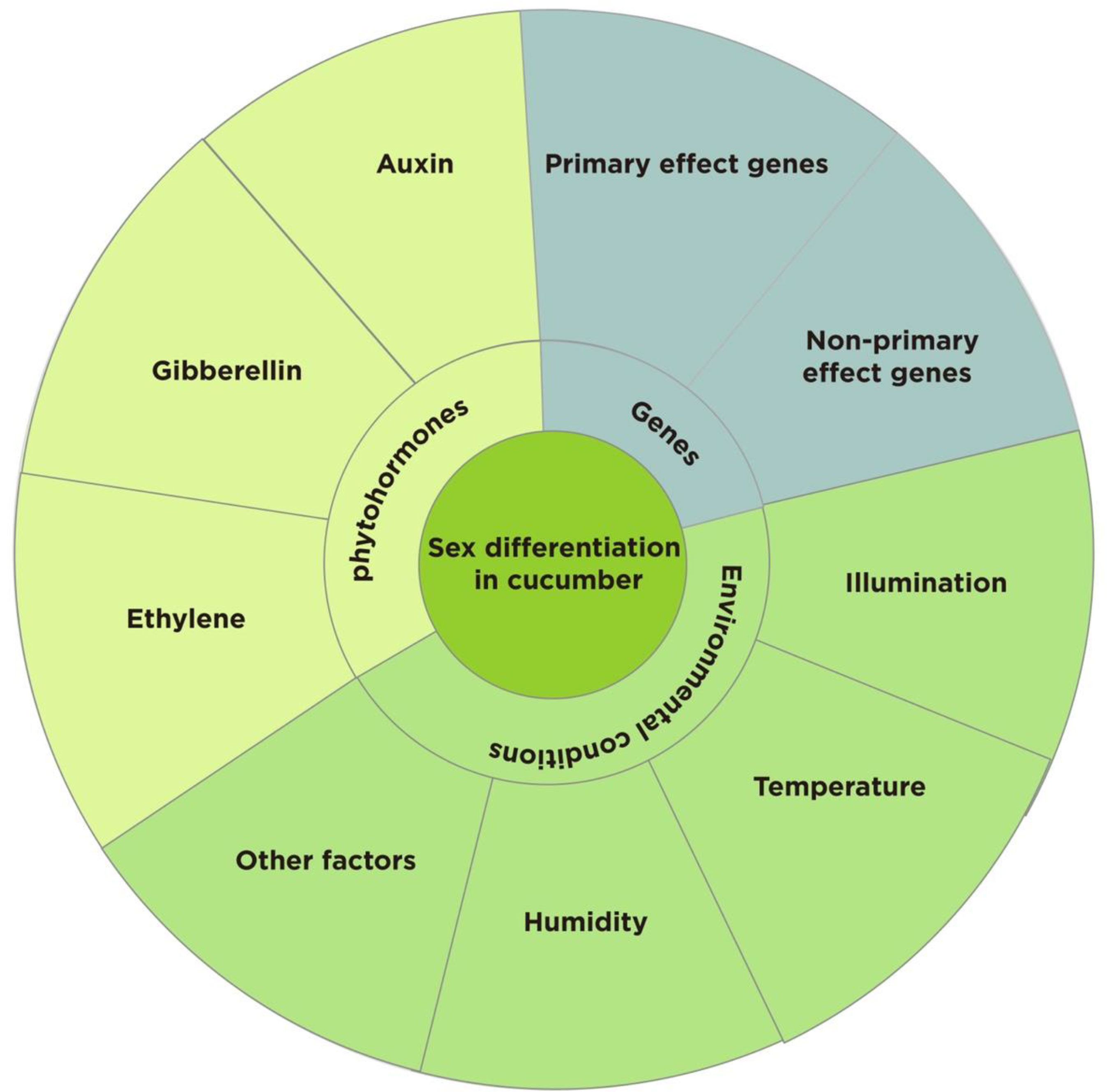
As a model plant for studying sex determination in plants (Malepszy and Niemirowicz-Szczytt, 1991; Pawełkowicz M. E. et al., 2019; Li Z. et al., 2022), several studies have been conducted on the morphological anatomy and genetic basis of sexual differentiation in cucumber, but the genetic background of cucumber is narrow and the expression of sex types is complex and diverse (Grumet et al., 2022). This study provides a comprehensive analysis of the effects of sex-determining genes, environmental conditions, and phytohormones on sex differentiation in cucumber with the aim of providing a reference for future in-depth studies on sex differentiation, cultivation regulation, and molecular breeding of sex types in cucumber and related crops (Figure 1).
2 Types and processes of sexual differentiation in cucumber
2.1 Floral organ development model and sex differentiation in cucumber
Developmental genetics suggests that the developmental process of an individual is the orderly expression of a series of genes that are activated or turned off in a certain spatial and temporal sequence. Homozygous mutants in the model plants Arabidopsis thaliana and Antirrhinum majus for floral organ development provide ideal material for studying the molecular mechanisms regulating floral organ development. On this basis, scientists have proposed models for the development of various flower organs, the most typical of which is the “ABC model” of flower organ development (Bowman et al., 1991; Coen and Meyerowitz, 1991; Zhang et al., 2019). The ABC model represents three decisive genes affecting floral organ differentiation: class A genes regulate the formation of sepals; class B genes and class A genes jointly control the growth and development of plant petals; and class C genes can regulate carpel formation both individually and in cooperation with class B genes to control stamen production and development. In addition, class A and C genes are functionally antagonistic, which explains the phenomenon of homozygous heterotypic transformation of floral organs (Theißen, 2001; Krizek and Fletcher, 2005; Bowman et al., 2012; Ali et al., 2019). From the ABC model, we can see that the flower of the plant consists of four rounds of concentric circles, from the outside to the inside: sepals, petals, stamens, and carpels (Yanofsky, 1995; Alvarez-Buylla et al., 2010). Subsequently, a gene that defines the formation and development of ovules was found in Petunia hybrida, which is defined as the D gene (Angenent and Colombo, 1996). SEEDSTICK (STK), SHATTERPROOF1 (SHP1), and SHP2 found in Arabidopsis have similar functions to those of D genes in petunias. In mutants of these three genes, the carpel of Arabidopsis substitutes for ovule formation (Pinyopich et al., 2003). In addition, class E genes were found and named in Arabidopsis, which can combine with other genes in the model to jointly maintain the development process of flower organs (Ditta et al., 2004). Sequencing and qualitative expression analysis of class A, class B, and class E MADS-box homologs in Australian shrubs and magnolia lilies added class D and class E genes to the ABC model, enriching and extending the model for controlling flower organ development to the “ABCDE” model (Kim et al., 2005; Shen and Wang, 2022).
As one of the most important classes of genes, MADS box genes regulate flower organ development in higher plants (Becker and Theißen, 2003). Class A, B, and C genes are homologous genes that can be transcribed into proteins, and the proteins encoded by these genes all contain a MADS cassette region. The STK and SEP genes belong to the MADS-box gene family. At present, several MADS box genes have been cloned from cucumbers, and the ERAF17 gene is one of them (Filipecki et al., 1997; Ando et al., 2001; Hu and Liu, 2012; Zhou et al., 2019). ERAF17 synthetic transcripts were induced in the growing points of cucumber plants of a monoecious variety and a purely female variety treated with ethylene glycol for 4 h. ERAF17 expression was found to be localized in the flower buds of purely female plants and persistently expressed during female flower development, indicating that female flower formation was induced by ethylene, probably regulated by ERAF17 expression in the apical flower buds of cucumber plants (Ando et al., 2001). Cucumis melo, a typical experimental material, has been found to be sexually differentiated by two loci, A and G. In melon, a gene encoding a C2H2-type zinc finger protein transcription factor, named CmWIPI, was found to control sex in melon together with another gene, CmACS-7, which controls ethylene synthesis in melon (Martin et al., 2009). In experiments with kiwifruit, a relative cytokinin regulatory gene, SyGI, was found on the Y chromosome, which suppressed pistil development in male kiwifruit flowers and is presumed to be a sex-determining gene in kiwifruit (Akagi et al., 2018).
2.2 Sex phenotype of cucumber
Cucumbers can produce female, male, and hermaphrodite flowers, and the three types of flowers are combined to produce a variety of sex systems. There are eight types: pure female plants, strong female plants, female whole plants, male and female whole plants, male and female plants, complete plants, male whole plants, and pure male plants (Table 1) (Wu et al., 2010). Cucumber plants have also been classified according to their floral phenotype into monoecious, strong female plants, all-female plants, all-male plants, intersexual flowering plants, male whole plants, female whole plants, trisexual flowering strains, and other sex system types (Che and Zhang, 2019). Early in cucumber bud development, both the androgynophores appear (Atsmon, 1960; Moraes, 2020); however, with the gradual growth and development of the plant, only one pistil primordium or stamen primordium can continue to develop, and the other gradually degenerates through apoptosis, thus forming a female or male flower (Perl-Treves, 2004). If the growth of both male and female flowers is not restricted, they will develop into bisexual flowers (Malepszy and Niemirowicz-Szczytt, 1991; Wang et al., 2022).
2.3 Sex differentiation process of cucumber
Through long-term research, scholars have found that the essence of sex differentiation in cucumbers is the differentiation of male and female flowers. The process of sexual differentiation in cucumbers is complex, as male and female flowers are initially hermaphroditic, and then selective abortion occurs when one of the male and female sex organs atrophies to form an unisexual male or female flower (Boualem et al., 2009). From a morphological point of view, the process of cucumber flower development can be divided into 12 stages and into two periods: the hermaphroditic stage and the differentiation stage (Bai et al., 2004; Wen et al., 2020). Stages 1–5 are when various organs in the flower, such as sepals, petals, stamen primordia, pistil primordia, and other floral meristematic tissues, begin to develop, and there is no obvious morphological difference between male and female flowers during this process, so it is called the hermaphroditic stage; in male flowers, the pistillate primordia are stagnant, anthers, and filaments start to develop at stage 6, anthers further expand at stage 7, locules differentiate at stage 8, microspore mother cells appear at stage 9, meiosis starts at stage 10, mononuclear pollen appears at stage 11, and finally mature pollen is formed at stage 12. In contrast, in female flowers, the carpel primordia begin to sprout and elongate at stage 6 and differentiate in the stigma and ovary at stage 7, followed by the elongation of the stigma, the development of ovules and beads at stage 8, the formation of macrospore cells at stage 9, meiosis at stage 10, the formation of embryo sacs at stage 11, and finally the maturation of all the appendage tissues at stage 12, with the stamen primordia lagging throughout the whole process (Hao et al., 2003; Zhang et al., 2021).
3 Genetic of sex differentiation in cucumber
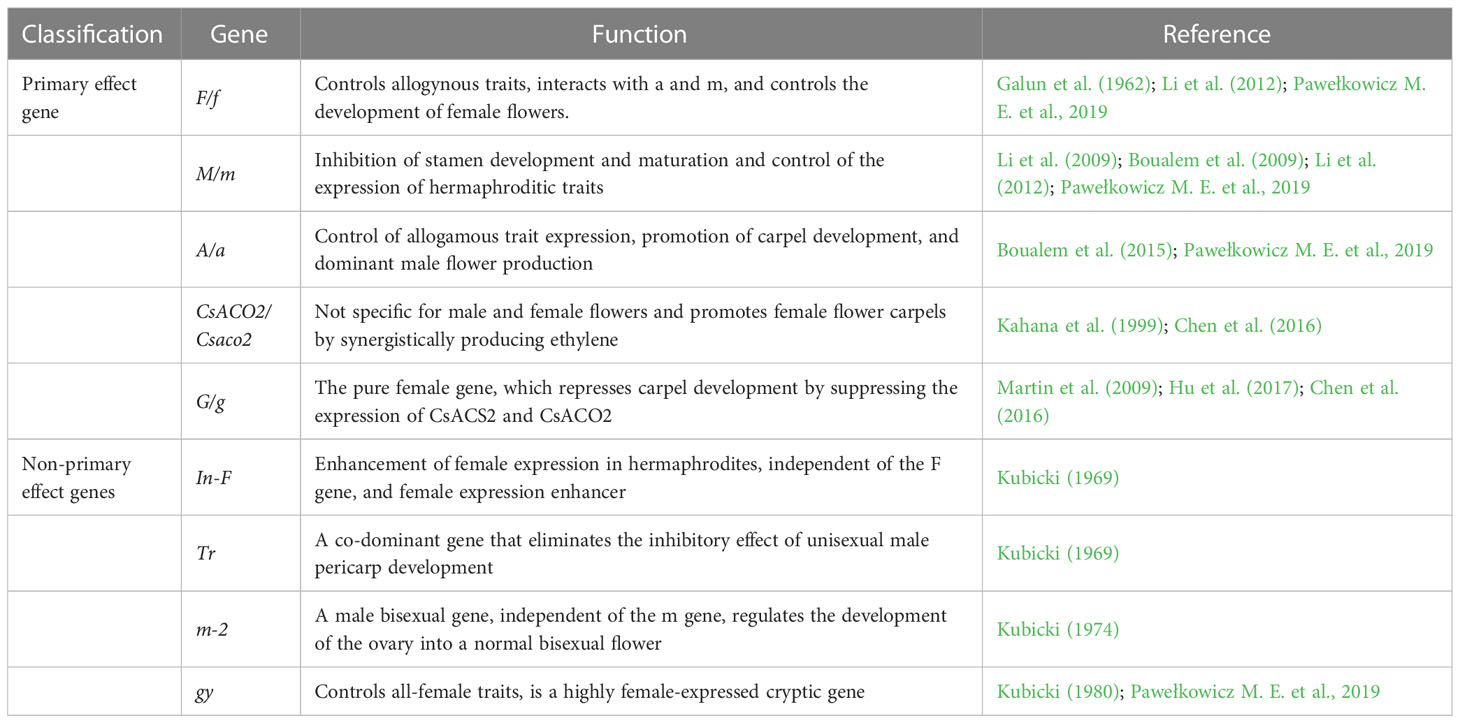
In the long-term study of sex differentiation in plants, it was discovered that sex differentiation in cucumbers is jointly determined by multiple genes, the primary effect genes being F/f, M/m, A/a, CsACO2/Csaco2, and G/g. The non-primary effect genes: In-F, Tr, m-2, and gy play different degrees of modifying roles (Table 2). Five of these sex-linked master effector genes include three genes encoding 1-aminocyclopropane-1-carboxylic acid synthetase genes F, M, and A, an ACC oxidase gene that converts ACC to ethylene (CsACO2), and a transcription factor G. The main function of CsACS2 is to inhibit stamen development; CsACS11 stimulates carpel development; CsWIP1 is inhibited by CsACS11 as a carpel inhibitor; and CsWIP1 can also inhibit the expression of CsACS2 and CsACO2 (Kubicki, 1969; Kubicki, 1974; Kubicki, 1980; Pawełkowicz M. et al., 2019). In genetic and regulatory research on sex differentiation, the master effect genes F/f, M/m, and A/a have been studied the most and are key genes in determining the direction of differentiation in cucumber. The F gene is partially dominant and controls female flower development and determines the female level of the plant; Ff is a strong female plant, FF is a full female plant; the M gene suppresses stamen development, while the m gene controls bisexual flower development invisibly; the A gene promotes carpel development, while the a gene controls invisibly. The sex type of cucumber is determined by the combination of three sex-determining genes: F_M_A/a is an all-female plant; F_mmA/a is a hermaphroditic plant; ffM_A_ is a dioecious plant; ffmmA_ is a male all-female plant; ffM_aa is a strong male plant; and ffmmaa is an all-male plant, where F has a dominant epistatic effect on A (Perl-Treves et al., 1998).
3.1 F/f gene
In 1928, scientists uncovered the master gene driving the all-female phenotype of cucumber plants through genetic experimentation, marking the first discovery of the F gene. This gene promotes the early development of female flowers and enhances feminization by controlling the all-female characteristic in an incompletely dominant way (Rosa, 1928). In 1997, a gene with one additional copy than CsACS1 and a complete linkage to the F gene was identified in the all-female material and named CsACS1G (Trebitsh et al., 1997). Subsequently, in 2000, the spatial expression of CsACS1/CsACS1G was examined by Northern blot, and the results showed that the gene was expressed in the apical meristem of all-female plants but not in dioecious plants (Kamachi et al., 2000).
In 2015, a breakthrough was made in the study of F/f genes. Using 115 cucumber germplasm resequencing data, the F region of cucumber was analyzed comparatively and was found to be a 30.2 kb repeat unit that occurs in one, two, or four copies in all-female plants. In this analysis, two new genes, A1 and B1, in addition to CsACS1G, were also identified for the first time in the F region (Zhang et al., 2015). In addition, in 2020, the phenomenon of “female loss” was found in no less than 0.11% of all-female cucumber plants, demonstrating the existence of some conserved and unstable CNP (copy number variant) variation structure of the F gene in cucumber (Li et al., 2020).
3.2 M/m gene
The M gene (CsACS2) encodes ACC synthase, a rate-limiting enzyme in ethylene biosynthesis. The earliest ACC synthase was not cloned for the first time until 1997, when the full-length sequence of CsACS2 was cloned and found to be expressed only in female flowers (Kamachi et al., 1997; Yuan et al., 2020); by 2001, it was found that the expression of the CsACS2 gene was positively correlated with ethylene content, and the expression of CsACS2 was higher in female plants than in monoecious and androgynous plants (Yamasaki et al., 2001); and in 2007, using in situ hybridization, it was found that CsACS2 was expressed only in the carpels of female flowers, while male flowers were in the carpels of female flowers but not in male flowers. This finding was positively correlated with the regulation of the M gene (Saito et al., 2007).
In 2009, the M gene was cloned, and a SNP site (G97T) linked to the M gene was found in the first exon of the CsACS2 gene, resulting in a heterozygous mutation of amino acid G33C (Li et al., 2009; Wang et al., 2022); in the same year, in other experiments, three types of heterozygous mutations of CsACS2 were obtained in cucumber germplasm: G33C, P209S, and S399L, and the results of enzyme activity assay showed that the enzyme activity of CsACS2 was reduced or even completely inactivated after the three types of mutations (Boualem et al., 2009). Finally, we concluded that CsACS2 is an M gene that encodes an ACC synthase. In 2012, the positive expression mechanism between the CsACS2 gene and ethylene was confirmed by ectopic expression of the CsACS2 gene in tobacco, and the promoter of the CsACS2 gene was activated by ethylene expression after transferring into tobacco (Li et al., 2012; Zhang et al., 2021).
3.3 A/a gene
Genetic research proved that A/a is entirely dominant and that a gene improves males. Genetic techniques revealed the existence of a recessive gene A regulating all-male features in plants as early as 1969 (Kubicki, 1969). Until 2015, a candidate gene for the A gene, CsACS11, also encoding ACC synthase, was cloned in cucumber using map cloning techniques. In all-male plants, a base deletion in exon 3 of CsACS11 resulted in the premature termination of CsACS11 translation. Two missense mutations, G39R and W58*, were identified by screening using the TILLING technique. After backcrossing with wild-type monoecious plants, CsACS11 pure plants were mutated to be all-male, and female flowers were produced by applying ethylene to CsACS11 mutants, thus further confirming that CsACS11 is an A gene. In situ hybridization revealed that CsACS11 was expressed in the 4-phase floral primordia of female flowers of monoecious plants and then in the inner and outer sides of the siliques of vascular bundles. CsACS11 was first expressed in the 4-phase floral primordia of female flowers of monoecious plants, and then in the inner and outer sides of the vascular siliques and in the sieve tube of the carpels (Boualem et al., 2015).
3.4 CsACO2/Csaco2 gene
The CsACO2 gene is capable of encoding an ACC oxidase. In 1999, in situ mRNA hybridization revealed that CsACO2 has a specific expression pattern in different tissues and stages of flower development, mainly in the ovary and stamens (Kahana et al., 1999). By 2016, an all-male plant mutant recessively controlled by the CsACO2 gene was identified using EMS mutagenesis, and this mutant trait. The population was constructed using a homozygous and all-male plant that encodes a rate-limiting enzyme in the ethylene synthesis pathway. In vitro activity assays showed that the mutant CsACO2 enzyme activity decreased and ethylene release was correspondingly reduced while the all-male mutant was treated with ACC. The all-male mutant produced no female flowers after treatment with ACC. After ACC treatment, the male-only mutant did not generate any female flowers. In contrast, when ethylene was applied to the all-male mutant, no female flowers were formed. When mutant plants were treated with ethylene, continuous female flower nodes formed. Experiments revealed that in the absence of CsACO2 enzyme activity, the plant was unable to catalyze the synthesis of sufficient ethylene by ACC and consequently was unable to stimulate the formation of female flowers. In situ hybridization showed that CsACO2 expression started in the second floral primordium and overlapped with CsACS11 expression at the carpels of the fourth floral primordium. The expression of CsACO2 itself is not specific for male and female flowers and promotes female flower carpels by synergistically producing ethylene with CsACS11 which is specifically expressed in female flowers (Chen et al., 2016).
3.5 G/g gene
The G/g gene was first identified in melon, which recessively controls the all-female phenotype of melon and encodes the C2H2 family of transcription factors. CmWIP1 is the only transcription factor among the sex-determining genes studied so far (Martin et al., 2009). The CsWIP1 mutant was created in 2017 using gene editing technology, and the mutant produces bisexual flowers in the lower node and continuous female flowers in the upper node (Hu et al., 2017). CsWIP1 is a pure female gene that can be repressed by endogenous ethylene. CsWIP1 can bind to the promoter of CsACO2 to repress its expression and can also directly repress the expression of CsACS2. This suggests that CsWIP1 plays a critical role in sex differentiation. This suggests that CsWIP1 plays a critical role in sex differentiation (Chen et al., 2016).
4 Environmental factors impact the sex differentiation of cucumber
Environmental conditions such as illumination, temperature, humidity, and mineral nutrients are important factors impacting the sex differentiation of cucumbers. Environmental stress regulates the sexual differentiation of flowers and influences both the quality and yield of cucumbers (Figure 2).
4.1 Illumination
Light is a very important environmental factor that plays a role in the growth and development of plants. Photoperiod, light intensity, and light quality all play different roles in plant bud differentiation (Chory and Wu, 2001; Sidhu et al., 2021). Research shows that most cucumbers easily form female flowers in short photoperiods but more male flowers in long photoperiods (Cho et al., 2005; Tian et al., 2021). Short- and long-day-sensitive cucumbers have increased photosynthesis efficiency and an increased number of female flowers under short- and long-light conditions, respectively (Takahashi et al., 1983; Yang et al., 2022). The number of female flowers of long-day-sensitive cucumbers increased under high light intensity, while the shading of short-day-sensitive cucumbers was more conducive to the formation of female flowers (Cantliffe, 1981; Roy and Saran, 2019). Under the induction of red light, the long-day-sensitive cucumber produced more female flowers than other color light sources, such as white light and green light, and the number of female flowers increased with the extension of light interval time (Matsuo and Fukushima, 1970; Roy and Saran, 2019).
4.2 Temperature
Temperature had a great influence on the sex differentiation of cucumber, mainly because it significantly affects the node position of female flowers and the ratio of male to female flowers. Low temperatures can promote female flower differentiation, while high temperatures can induce male flower formation. Under certain high temperatures, it can promote the development of cucumber flower organs and flowering in advance, but if it encounters continuous high temperatures for a long time, it will lead to male flower buds falling and not flowering (Chen L. et al., 2021). Under the action of high temperature, the percentage of female flower nodes in cucumber decreased, and the smaller the seedling age was, the greater the impact of high temperature. At 35°C, the pollen tube length of cucumber was significantly reduced, and at 40°C, the pollen germination rate was significantly lower than that at 28°C (Miao et al., 2000; Cheng et al., 2023).
4.3 Humidity
Humidity is closely related to the sex differentiation of cucumbers. Proper humidity will reduce the node position of the first female flower and increase the number of female flowers (Ito and Saito, 1960; Maeda and Ahn, 2021). Research shows that higher soil and air humidity are conducive to the female formation of plants. In a certain range, the higher the soil water content is, the more conducive it is to the formation of cucumber female flowers; the higher the air humidity is, the better the differentiation of cucumber female flowers (An et al., 2020). The development of female organs is easier than that of males in the presence of high air humidity. At the stage of bud differentiation, the increase in the number of flowers, especially the number of female flowers, is more facilitated by suitable air humidity.
4.4 Other factors
Different classes and levels of mineral nutrients also affect the outcome of sex differentiation in cucumber (Sarkar et al., 2019). Nitrogen nutrition is beneficial to promote the formation of female flowers. Under the condition of strong photosynthesis and good carbon nutrition, the supply of nitrogen can increase the number of female flowers on the main stem, but too much nitrogen is detrimental to the differentiation of female flowers; phosphorus fertilizer can promote the reproductive growth of cucumber plants and promote the differentiation of female flowers; potassium nutrition induces the formation of male flowers and is detrimental to the differentiation of female flowers. Nitrogen and phosphorus are applied separately to favor the formation of female flowers, but when potassium is applied separately, it favors the formation of male flowers instead (Vaudo et al., 2022). Increasing the amount of carbon dioxide in the air increases the photosynthetic efficiency of cucumber plants and promotes the differentiation of female flowers (Askary et al., 2020). After several experiments, the effect of short-time low temperature treatment (23°C during the day/15°C at night) on cucumber females was determined by whole-genome bisulfite sequencing (WGBS) of the stem tip. The effect of temperature on transposon-related RNA-directed DNA methylation was found by whole genome methylation sequencing, mRNA sequencing, and sRNA sequencing analysis of stem tip parts. The effect of temperature on transposon-associated RNA-directed DNA methylation mechanisms was found to be significant, resulting in substantial CHH-type cytosine demethylation (Lai et al., 2017). The results showed that blue light induced stronger induction of female flowers than other light substances, and the blue light-induced sex expression in female flowers was closely related to the blue light-induced changes in abscisic acid, growth hormone, gibberellin (GA), photosynthesis, starch, and sucrose metabolic pathways (Zhou et al., 2018).
5 Hormonal regulation of sex differentiation in cucumber
The most unique part of the study of sex determination in cucumbers is that the ratio of male to female flowers in the plant is influenced by phytohormones. These properties not only provide an efficient production method for cucumber cultivation but also provide a reliable way to understand the regulatory mechanisms of unisexual flower development. Among the plant growth hormones, ethylene, GA, and IAA have the greatest influence on the sex expression of cucumber. Among the plant growth hormones, ethylene, GA, and IAA have the greatest effect on sex expression in cucumbers. Among them, ethylene and IAA induce the growth and development of female flowers in cucumber, while GA promotes the development of male flowers and hinders the development of female flowers in cucumbers.
5.1 Ethylene
Ethylene is a phytohormone commonly used in cucumbers to promote the formation of female flowers (Zhang et al., 2017). It determines the sexual development of individual floral meristems of cucumber and promotes the formation of female flowers (Martínez and Jamilena, 2021; Cebrián et al., 2022). In a study by Garcia et al., two new ethylene-insensitive mutations (etr1a-1 and etr1b) were identified that blocked the female flowering transition in cucumber; this allowed the plant to produce male flowers (staminate flowers) indefinitely (García et al., 2020). Other ethylene biosynthesis genes of ACC oxidase, such as CsACO2 and CsACO3, are also involved in cucumber sexual expression (Chen et al., 2016); however, the transcript levels of CsACO2 and CsACO3 were negatively correlated with female flowering (Kahana et al., 1999). In addition, the ethylene receptor CsETR1 plays an important role in the inhibition of female cucumber flower stamens through the induction of DNA damage (Wang et al., 2010). The cucumber nuclease-encoding gene CsCaN induces DNA damage in the anther primordia in response to ethylene signaling, resulting in stalled development of the anther primordia and the formation of female flowers (Gu et al., 2011). Inhibition of acetylenic biosynthesis and acetylenic signaling results in a decrease in the number of female or bisexual flowers (Atsmon and Tabbak, 1979; Takahashi and Jaffe, 1984) and an increase in the number of male flowers (Ando et al., 2001). In the female flower experiment, ethylene signaling activated CsERF31 through CsEIN3, and then CsERF31 stimulated CsACS2, thus promoting the development of female flowers (Pan et al., 2021).
The “single hormone hypothesis” was widely accepted to explain the ethylene-mediated differentiation of unisexual flowers in cucumber, suggesting that ethylene in cucumber suppresses male flowers and induces female flowers by regulating the expression levels of F and M (Trebitsh et al., 1997). However, with the cloning of the M gene, this hypothesis was soon revised, and a positive feedback mechanism was proposed for the M-mediated regulation of ethylene (Li et al., 2008; Li et al., 2012). In 2018, it was discovered that CsERF31 directly binds to the promoter of M and promotes its expression, and on this basis, an “ethylene-CsERF31-M-ethylene” positive feedback mechanism was proposed: during female cucumber pistillate differentiation, F produces ethylene to promote pistil development, while CsERF31 responds to the ethylene signal and activates M, and M starts its positive feedback activation expression through CsERF31. Ethylene downregulates CsAP3 and CsPI by stimulating CsSUP, resulting in inhibition of CsETR1 expression and an inability to downregulate CsERF31. In this process, the development of stamen primordia is hindered and the production of female flowers is promoted. This model explains the development and evolution of cucumber unisexual flowers from the perspective of the developmental fate of pistil and stamen primordia, but it is not suitable for monoecious cucumbers because there is currently no evidence that M is activated by F in the predetermined female buds (Pan et al., 2018).
5.2 Gibberellin
Gibberellin (GA) is an important factor in cucumber sex differentiation that can promote male flower differentiation and hinder female flower differentiation (Staub and Bacher, 2023). In 1960, gibberellin was first discovered to induce male flowers in female line plants, thus solving the problem of female line selection and reproduction (Li et al., 2019). GA yield was higher in male hermaphroditic cucumber plants than in pure female and monoecious plants (Hemphill Jr et al., 1972; Kim et al., 2021). In subsequent experiments, it was determined that GA3 spraying increased the ratio of males to females in monoecious cucumber plants and induced the formation of male flowers in female plants (Pike and Peterson, 1969; Yadav et al., 2022). In Arabidopsis and rice, the GA signaling pathway is involved in the development of stamens and anthers in hermaphroditic plants (Aya et al., 2009; Song et al., 2013; Chen M. et al., 2021). GA3, GA4, GA7, and GA13 are all functional in the induction of male flowers in female cucumber plants (Wittwer and Bukovac, 1962; Clark and Kenney, 1969; Zhu et al., 2021); GA4 and GA7 were sprayed at 50 mg kg−1, while GA3 was sprayed at 100 mg kg−1, and the former produced more male flowers (Pike and Peterson, 1969).
5.3 Auxin
Auxin (IAA) is a key hormone that determines growth and developmental dominance and seriously affects cucumber sex differentiation (Qin et al., 2021). As early as the 1950s and 1960s, it was found that a certain concentration of IAA could increase the female/male flower ratio and promote female flower differentiation in cucumber under in vitro conditions, while the growth hormone content in all-female cucumber plants was higher than that in dioecious homozygous plants (Galun et al., 1965; Rudich et al., 1972; Shekari et al., 2019). In female cucumber flowers, the IAA content was higher than that in male flowers, and the IAA content determined the direction of sex differentiation in cucumber (Chen et al., 2002; Qin et al., 2021). It was shown that the male buds of cucumber could be converted into female flower buds by adjusting the IAA concentration (Galun et al., 1962; Cebrián et al., 2022). CsSPL in cucumber regulates anther and ovule development through interaction with the growth hormone-corresponding factor CsARF3 (Liu et al., 2018).
6 Perspectives
Sex differentiation in cucumbers is regulated by genes, environmental factors, and hormones, which is a very complex and long evolutionary process. The study of sex differentiation is important to guide the selection of stable female lines for breeding and production in cucumber. With the continuous improvement of genomics, molecular genetics, and gene mining technologies, the mechanism of sex differentiation in cucumber has made great progress, and the framework of the sex differentiation regulatory network has been gradually clarified, but the diversity and variability of cucumber sexes seem to be difficult to comprehensively describe by the changing pattern of a single metabolic pathway. In the future, by studying the transcriptional and expressional regulatory mechanisms of sex determination genes, we will clarify the specific relationship between sex determination and classical hermaphroditic flowers and thus improve the gene regulatory network of sex determination in cucurbits. At the same time, by integrating the latest multidisciplinary research results and technologies in genomics and establishing the internal and external multifactorial and multidirectional interaction regulatory network, it is expected that a more in-depth and comprehensive analysis of the molecular genetic mechanism of sex will provide an important theoretical basis and technical support for high-yielding and stable cultivation and phenotypic genetic breeding of cucumber.
Author contributions
HW, HL, and ZH conceived and designed the review. HL wrote the manuscript, and HW proposed revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Science & Technology Specific Projects in Agricultural High-tech Industrial Demonstration Area of the Yellow River Delta (Grant No: 2022SZX36), the Major Science and Technology Project of Plant Breeding in Zhejiang Province (Grant No: 2021C02065-2), the “Pioneer” and “Leading Goose” R&D Program of Zhejiang (Grant No: 2022C02051), the National Natural Science Foundation of China (Grant Nos. 31972221, 32002048, and 32172595), the Ministry of Agriculture, and the National Key Research and Development Program of China (2019YFD1000300).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akagi, T., Henry, I. M., Ohtani, H., Morimoto, T., Beppu, K., Kataoka, I., et al. (2018). A y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit. Plant Cell 30 (4), 780–795. doi: 10.1105/tpc.17.00787
Ali, Z., Raza, Q., Atif, R. M., Aslam, U., Ajmal, M., Chung, G. (2019). Genetic and molecular control of floral organ identity in cereals. Int. J. Mol. Sci. 20 (11), 2743. doi: 10.3390/ijms20112743
Alvarez-Buylla, E. R., Benítez, M., Corvera-Poiré, A., Cador, Á.C., de Folter, S., de Buen, A. G., et al. (2010). Flower development. Arabidopsis Book/American Soc. Plant Biologists 8, e0127. doi: 10.1199/tab.0127
An, S., Park, S. W., Kwack, Y. (2020). Growth of cucumber scions, rootstocks, and grafted seedlings as affected by different irrigation regimes during cultivation of ‘Joenbaekdadagi’and ‘Heukjong’seedlings in a plant factory with artificial lighting. Agronomy 10 (12), 1943. doi: 10.3390/agronomy10121943
Ando, S., Sato, Y., Kamachi, S., Sakai, S. (2001). Isolation of a MADS-box gene (ERAF17) and correlation of its expression with the induction of formation of female flowers by ethylene in cucumber plants (Cucumis sativus l.). Planta 213, 943–952. doi: 10.1007/s004250100571
Angenent, G. C., Colombo, L. (1996). Molecular control of ovule development. Trends Plant Sci. 1 (7), 228–232. doi: 10.1016/1360-1385(96)86900-7
Askary, S., Moradi, H., Dehestani, A., Ghasemi, K. (2020). The effect of nitrogen nutrition and ethephon spray on flowering and some photosynthetic characteristics cucumber victor. J. Plant Production Res. 27 (1), 129–142.
Atsmon, D. (1960). A morphogenetic study of staminate, pistillate and hermaphrodite flowers in cucumis sativus (L.). Phytomorphology 10, 110–115. doi: 10.22069/jopp.2020.15890.2425
Atsmon, D., Tabbak, C. (1979). Comparative effects of gibberellin, silver nitrate and aminoethoxyvinyl glycine on sexual tendency and ethylene evolution in the cucumber plant (Cucumis sativus l.). Plant Cell Physiol. 20 (8), 1547–1555. doi: 10.1093/oxfordjournals.pcp.a075957
Aya, K., Ueguchi-Tanaka, M., Kondo, M., Hamada, K., Yano, K., Nishimura, M., et al. (2009). Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 21 (5), 1453–1472. doi: 10.1105/tpc.108.062935
Bai, S.-N. (2015). The concept of the sexual reproduction cycle and its evolutionary significance. Front. Plant Sci. 6, 11. doi: 10.3389/fpls.2015.00011
Bai, S., Peng, Y., Cui, J., Gu, H., Xu, L., Li, Y., et al. (2004). Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus l.). Planta 220 (2), 230–240. doi: 10.1007/s00425-004-1342-2
Becker, A., Theißen, G. (2003). The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 29 (3), 464–489. doi: 10.1016/S1055-7903(03)00207-0
Boualem, A., Troadec, C., Camps, C., Lemhemdi, A., Morin, H., Sari, M.-A., et al. (2015). A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350 (6261), 688–691. doi: 10.1126/science.aac8370
Boualem, A., Troadec, C., Kovalski, I., Sari, M.-A., Perl-Treves, R., Bendahmane, A. (2009). A conserved ethylene biosynthesis enzyme leads to andromonoecy in two cucumis species. PloS One 4 (7), e6144. doi: 10.1371/journal.pone.0006144
Bowman, J. L., Smyth, D. R., Meyerowitz, E. M. (1991). Genetic interactions among floral homeotic genes of arabidopsis. Development 112 (1), 1–20. doi: 10.1242/dev.112.1.1
Bowman, J. L., Smyth, D. R., Meyerowitz, E. M. (2012). The ABC model of flower development: then and now. Development 139 (22), 4095–4098. doi: 10.1242/dev.083972
Cantliffe, D. J. (1981). Alteration of sex expression in cucumber due to changes in temperature, light intensity, and Photoperiod1. J. Am. Soc. Hortic. Sci. 106 (2), 133–136. doi: 10.21273/JASHS.106.2.133
Cebrián, G., Iglesias-Moya, J., Romero, J., Martínez, C., Garrido, D., Jamilena, M. (2022). The ethylene biosynthesis gene CpACO1A: a new player in the regulation of sex determination and female flower development in cucurbita pepo. Front. Plant Sci. 12, 817922. doi: 10.3389/fpls.2021.817922
Che, G., Zhang, X. (2019). Molecular basis of cucumber fruit domestication. Curr. Opin. Plant Biol. 47, 38–46. doi: 10.1016/j.pbi.2018.08.006
Chen, M., Nie, G., Yang, L., Zhang, Y., Cai, Y. (2021). Homeotic transformation from stamen to petal in lilium is associated with MADS-box genes and hormone signal transduction. Plant Growth Regul. 95 (1), 49–64. doi: 10.1007/s10725-021-00724-6
Chen, H., Sun, J., Li, S., Cui, Q., Zhang, H., Xin, F., et al. (2016). An ACC oxidase gene essential for cucumber carpel development. Mol. Plant 9 (9), 1315–1327. doi: 10.1016/j.molp.2016.06.018
Chen, L., Yun, M., Cao, Z., Liang, Z., Liu, W., Wang, M., et al. (2021). Phenotypic characteristics and transcriptome of cucumber male flower development under heat stress. Front. Plant Sci. 12, 758976. doi: 10.3389/fpls.2021.758976
Chen, X.-H., Zeng, G.-W., Cao, B.-S. (2002). Relationship between endogenous plant hormones and floral sex differentiation in cucumber (Cucumis sativus). Plant Physiol. Commun. 38 (4), 317–320. doi: 10.13592/j.cnki.ppj.2002.04.003
Cheng, Z., Liu, X., Yan, S., Liu, B., Zhong, Y., Song, W., et al. (2023). Pollen tube emergence is mediated by ovary-expressed ALCATRAZ in cucumber. Nat. Commun. 14 (1), 258. doi: 10.1038/s41467-023-35936-z
Cho, J., Koo, D.-H., Nam, Y.-W., Han, C.-T., Lim, H.-T., Bang, J.-W., et al. (2005). Isolation and characterization of cDNA clones expressed under male sex expression conditions in a monoecious cucumber plant (Cucumis sativus l. cv. winter long). Euphytica 146 (3), 271–281. doi: 10.1007/s10681-005-9023-1
Chory, J., Wu, D. (2001). Weaving the complex web of signal transduction. Plant Physiol. 125 (1), 77–80. doi: 10.1104/pp.125.1.77
Clark, R., Kenney, D. (1969). Comparison of staminate flower production on gynoecious strains of cucumbers, cucumis sativus l., by pure gibberellins (A3, A4, A7, A13) and Mixtures1. J. Am. Soc. Hortic. Sci. 94 (2), 131–132. doi: 10.21273/JASHS.94.2.131
Coen, E. S., Meyerowitz, E. M. (1991). The war of the whorls: genetic interactions controlling flower development. Nature 353 (6339), 31–37. doi: 10.1038/353031a0
Diggle, P. K., Di Stilio, V. S., Gschwend, A. R., Golenberg, E. M., Moore, R. C., Russell, J. R., et al. (2011). Multiple developmental processes underlie sex differentiation in angiosperms. Trends Genet. 27 (9), 368–376. doi: 10.1016/j.tig.2011.05.003
Ditta, G., Pinyopich, A., Robles, P., Pelaz, S., Yanofsky, M. F. (2004). The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr. Biol. 14 (21), 1935–1940. doi: 10.1016/j.cub.2004.10.028
Filipecki, M. K., Sommer, H., Malepszy, S. (1997). The MADS-box gene CUS1 is expressed during cucumber somatic embryogenesis. Plant Sci. 125 (1), 63–74. doi: 10.1016/S0168-9452(97)00056-3
Galun, E., Izhar, S., Atsmon, D. (1965). Determination of relative auxin content in hermaphrodite and andromonoecious cucumis sativus l. Plant Physiol. 40 (2), 321. doi: 10.1104/pp.40.2.321
Galun, E., Jung, Y., Lang, A. (1962). Culture and sex modification of male cucumber buds in vitro. Nature 194, 596–598. doi: 10.1038/194596a0
García, A., Aguado, E., Garrido, D., Martínez, C., Jamilena, M. (2020). Two androecious mutations reveal the crucial role of ethylene receptors in the initiation of female flower development in cucurbita pepo. Plant J. 103 (4), 1548–1560. doi: 10.1111/tpj.14846
Grumet, R., Lin, Y.-C., Rett-Cadman, S., Malik, A. (2022). Morphological and genetic diversity of cucumber (Cucumis sativus l.) fruit development. Plants 12 (1), 23. doi: 10.3390/plants12010023
Gu, H. T., Wang, D. H., Li, X., He, C. X., Xu, Z. H., Bai, S. N. (2011). Characterization of an ethylene-inducible, calcium-dependent nuclease that is differentially expressed in cucumber flower development. New Phytol. 192 (3), 590–600. doi: 10.1111/j.1469-8137.2011.03825.x
Hao, Y.-J., Wang, D.-H., Peng, Y.-B., Bai, S.-L., Xu, L.-Y., Li, Y.-Q., et al. (2003). DNA Damage in the early primordial anther is closely correlated with stamen arrest in the female flower of cucumber (Cucumis sativus l.). Planta 217, 888–895. doi: 10.1007/s00425-003-1064-x
Hemphill, D. D., Jr, Baker, L., Sell, H. (1972). Different sex phenotypes of cucumis sativus l. and c. melo l. and their endogenous gibberellin activity. Euphytica 21 (2), 285–291. doi: 10.1007/BF00036769
Hu, B., Li, D., Liu, X., Qi, J., Gao, D., Zhao, S., et al. (2017). Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol. Plant 10 (12), 1575–1578. doi: 10.1016/j.molp.2017.09.005
Hu, L., Liu, S. (2012). Genome-wide analysis of the MADS-box gene family in cucumber. Genome 55 (3), 245–256. doi: 10.1139/g2012-009
Ito, H., Saito, T. (1960). Factors responsible for the sex expression of the cucumber plant. XII. physiological factors associated with the sex expression of flowers. Tohoku J. Agric. Res. 11, 287–308. http://hdl.handle.net/10097/29324.
Kahana, A., Silberstein, L., Kessler, N., Goldstein, R. S., Perl-Treves, R. (1999). Expression of ACC oxidase genes differs among sex genotypes and sex phases in cucumber. Plant Mol. Biol. 41, 517–528. doi: 10.1023/A:1006343707567
Kamachi, S., Mizusawa, H., Matsuura, S., SAKAI, S. (2000). Expression of two 1-aminocyclopropane-1-carboxylate synthase genes, CS-ACS1 and CS-ACS2, correlated with sex phenotypes in cucumber plants (Cucumis sativus l.). Plant Biotechnol. 17 (1), 69–74. doi: 10.5511/plantbiotechnology.17.69
Kamachi, S., Sekimoto, H., Kondo, N., Sakai, S. (1997). Cloning of a cDNA for a 1-aminocyclopropane-1-carboxylate synthase that is expressed during development of female flowers at the apices of cucumis sativus l. Plant Cell Physiol. 38 (11), 1197–1206. doi: 10.1093/oxfordjournals.pcp.a029106
Kim, S., Koh, J., Ma, H., Hu, Y., Endress, P. K., Hauser, B. A., et al. (2005). Sequence and expression studies of a-, b-, and e-class MADS-box homologues in eupomatia (Eupomatiaceae): support for the bracteate origin of the calyptra. Int. J. Plant Sci. 166 (2), 185–198. doi: 10.1086/427479
Kim, R., Osako, Y., Yamane, H., Tao, R., Miyagawa, H. (2021). Quantitative analysis of auxin metabolites in lychee flowers. Bioscience Biotechnology Biochem. 85 (3), 467–475. doi: 10.1093/bbb/zbaa083
Krizek, B. A., Fletcher, J. C. (2005). Molecular mechanisms of flower development: an armchair guide. Nat. Rev. Genet. 6 (9), 688–698. doi: 10.1038/nrg1675
Kubicki, B. (1969). Investigations on sex determination in cucumber (Cucumis sativus l.). v. genes controlling intensity of femaleness. Genetica Polonica 10 (1-2), 23–68. https://eurekamag.com/research/014/535/014535712.php.
Kubicki, B. (1980). Investigations on sex determination in cucumbers cucumis sativus l. IX. induced mutant with the recessive character of gynoecism. Genetica Polonica 21 (4), 409–424.
Lai, Y.-S., Zhang, X., Zhang, W., Shen, D., Wang, H., Xia, Y., et al. (2017). The association of changes in DNA methylation with temperature-dependent sex determination in cucumber. J. Exp. Bot. 68 (11), 2899–2912. doi: 10.1093/jxb/erx144
Li, Z., Han, Y., Niu, H., Wang, Y., Jiang, B., Weng, Y. (2020). Gynoecy instability in cucumber (Cucumis sativus l.) is due to unequal crossover at the copy number variation-dependent femaleness (F) locus. Horticulture Res. 7(32). doi: 10.1038/s41438-020-0251-2
Li, Z., Huang, S., Liu, S., Pan, J., Zhang, Z., Tao, Q., et al. (2009). Molecular isolation of the m gene suggests that a conserved-residue conversion induces the formation of bisexual flowers in cucumber plants. Genetics 182 (4), 1381–1385. doi: 10.1534/genetics.109.104737
Li, Z., Niu, H., Guo, Y. (2022). Cucumber sex determination: aspects of gene interactions. Cucumber Genome, 145–157. doi: 10.1007/978-3-030-88647-9_11
Li, Z., Pan, J., Guan, Y., Tao, Q., He, H., Si, L., et al. (2008). Development and fine mapping of three co-dominant SCAR markers linked to the m/m gene in the cucumber plant (Cucumis sativus l.). Theor. Appl. Genet. 117, 1253–1260. doi: 10.1007/s00122-008-0859-3
Li, D., Sheng, Y., Niu, H., Li, Z. (2019). Gene interactions regulating sex determination in cucurbits. Front. Plant Sci. 10, 1231. doi: 10.3389/fpls.2019.01231
Li, H., Wang, S., Chai, S., Yang, Z., Zhang, Q., Xin, H., et al. (2022). Graph-based pan-genome reveals structural and sequence variations related to agronomic traits and domestication in cucumber. Nat. Commun. 13 (1), 682. doi: 10.1038/s41467-022-28362-0
Li, Z., Wang, S., Tao, Q., Pan, J., Si, L., Gong, Z., et al. (2012). A putative positive feedback regulation mechanism in CsACS2 expression suggests a modified model for sex determination in cucumber (Cucumis sativus l.). J. Exp. Bot. 63 (12), 4475–4484. doi: 10.1093/jxb/ers123
Liu, X., Ning, K., Che, G., Yan, S., Han, L., Gu, R., et al. (2018). Cs SPL functions as an adaptor between HD-ZIP III and Cs WUS transcription factors regulating anther and ovule development in cucumis sativus (cucumber). Plant J. 94 (3), 535–547. doi: 10.1111/tpj.13877
Maeda, K., Ahn, D.-H. (2021). A review of Japanese greenhouse cucumber research from the perspective of yield components. Horticulture J. 90 (3), 263–269. doi: 10.2503/hortj.UTD-R017
Malepszy, S., Niemirowicz-Szczytt, K. (1991). Sex determination in cucumber (Cucumis sativus) as a model system for molecular biology. Plant Sci. 80 (1-2), 39–47. doi: 10.1016/0168-9452(91)90271-9
Martin, A., Troadec, C., Boualem, A., Rajab, M., Fernandez, R., Morin, H., et al. (2009). A transposon-induced epigenetic change leads to sex determination in melon. Nature 461 (7267), 1135–1138. doi: 10.1038/nature08498
Martínez, C., Jamilena, M. (2021). To be a male or a female flower, a question of ethylene in cucurbits. Curr. Opin. Plant Biol. 59, 101981. doi: 10.1016/j.pbi.2020.101981
Matsuo, E., Fukushima, E. (1970). Studies on the photoperiodic sex differentiation in cucumber, cucumis sativus l. IV. effect of light-break. J. Japanese Soc. Hortic. Sci. 39 (2), 144–148. doi: 10.2503/jjshs.39.72
Miao, M., Li, Q., Cao, B., Chen, X. (2000). Effect of high temperature on reproductive growth and yield formation of cucumis sativus l. Acta Hortic. Sin. 27 (6), 412–417. doi: CNKI:SUN:YYXB.0.2000-06-004
Ming, R., Bendahmane, A., Renner, S. S. (2011). Sex chromosomes in land plants. Annu. Rev. Plant Biol. 62, 485–514. doi: 10.1146/annurev-arplant-042110-103914
Moraes, T. (2020). Unravelling the transition from vegetative to reproductive stages using passiflora organensis as a model plant (Universidade de São Paulo). doi: 10.11606/T.64.2020.tde-19052022-141124
Pan, J., Wang, G., Wen, H., Du, H., Lian, H., He, H., et al. (2018). Differential gene expression caused by the f and m loci provides insight into ethylene-mediated female flower differentiation in cucumber. Front. Plant Sci. 9, 1091. doi: 10.3389/fpls.2018.01091
Pan, J., Wen, H., Chen, G., Lin, W.-H., Du, H., Chen, Y., et al. (2021). A positive feedback loop mediated by CsERF31 initiates female cucumber flower development. Plant Physiol. 186 (2), 1088–1100. doi: 10.1093/plphys/kiab141
Pawełkowicz, M., Pryszcz, L., Skarzyńska, A., Wóycicki, R. K., Posyniak, K., Rymuszka, J., et al. (2019). Comparative transcriptome analysis reveals new molecular pathways for cucumber genes related to sex determination. Plant Reprod. 32, 193–216. doi: 10.1007/s00497-019-00362-z
Pawełkowicz, M. E., Skarzyńska, A., Pląder, W., Przybecki, Z. (2019). Genetic and molecular bases of cucumber (Cucumis sativus l.) sex determination. Mol. Breed. 39, 1–27. doi: 10.1007/s11032-019-0959-6
Perl-Treves, R. (2004). “Male To female conversion along the cucumber shoot: approaches to studying sex genes and floral development in cucumis sativus,” in Sex determination in plants (Garland Science), 193–221.
Perl-Treves, R., Kahana, A., Rosenman, N., Xiang, Y., Silberstein, L. (1998). Expression of multiple AGAMOUS-like genes in male and female flowers of cucumber (Cucumis sativus l.). Plant Cell Physiol. 39 (7), 701–710. doi: 10.1093/oxfordjournals.pcp.a029424
Pike, L., Peterson, C. (1969). Gibberellin A4/A7, for induction of staminate flowers on the gynoecious cucumber (Cucumis sativus l.). Euphytica 18 (1), 106–109. doi: 10.1007/BF00021988
Pinyopich, A., Ditta, G. S., Savidge, B., Liljegren, S. J., Baumann, E., Wisman, E., et al. (2003). Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424 (6944), 85–88. doi: 10.1038/nature01741
Qin, B., Lu, X., Sun, X., Cui, J., Deng, J., Zhang, L. (2021). Transcriptome-based analysis of the hormone regulation mechanism of gender differentiation in juglans mandshurica maxim. PeerJ 9, e12328. doi: 10.7717/peerj.12328
Rosa, J. (1928). The inheritance of flower types in cucumis and citrullus. Hilgardia 3 (9), 233–250. doi: 10.3733/hilg.v03n09p233
Rudich, J., Halevy, A., Kedar, N. (1972). Ethylene evolution from cucumber plants as related to sex expression. Plant Physiol. 49 (6), 998–999. doi: 10.1104/pp.49.6.998
Saito, S., Fujii, N., Miyazawa, Y., Yamasaki, S., Matsuura, S., Mizusawa, H., et al. (2007). Correlation between development of female flower buds and expression of the CS-ACS2 gene in cucumber plants. J. Exp. Bot. 58 (11), 2897–2907. doi: 10.1093/jxb/erm141
Sarkar, M. D., Moniruzzaman, M., ALAM, M. S., Rahman, M. J., Quamruzzaman, R. N. R., Subramaniam, S. (2019). Growth, sex expression and nutrient composition of cucumber (cucumis sativus) as influenced by maleic hydrazide. Pak. J. Bot. 51 (1), 117–123. doi: 10.30848/PJB2019-1(9)
Shekari, L., Aroiee, H., Mirshekari, A., Nemati, H. (2019). Protective role of selenium on cucumber (Cucumis sativus l.) exposed to cadmium and lead stress during reproductive stage role of selenium on heavy metals stress. J. Plant Nutr. 42 (5), 529–542. doi: 10.1080/01904167.2018.1554075
Shen, G., Wang, W.-L. (2022). Circlize package in r and analytic hierarchy process (AHP): contribution values of ABCDE and AGL6 genes in the context of floral organ development. PloS One 17 (1), e0261232. doi: 10.1371/journal.pone.0261232
Sidhu, V., Bernier-English, V., Lamontagne-Drolet, M., Gravel, V. (2021). Effect of light quality and extended photoperiod on flower bud induction during transplant production of day-neutral strawberry cultivars. Can. J. Plant Sci. 102 (2), 356–367. doi: 10.1139/cjps-2021-0081
Song, S., Qi, T., Huang, H., Xie, D. (2013). Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in arabidopsis. Mol. Plant 6 (4), 1065–1073. doi: 10.1093/mp/sst054
Staub, J., Bacher, J. (2023). “Cucumber as a processed vegetable,” in Processing vegetables (Routledge), 129–193.
Takahashi, H., Jaffe, M. (1984). Further studies of auxin and ACC induced feminization in the cucumber plant using ethylene inhibitors. Phyton 44 (1), 81–86. doi: 10.1016/0031-9422(84)83062-9
Takahashi, H., Saito, T., Suge, H. (1983). Separation of the effects of photoperiod and hormones on sex expression in cucumber. Plant Cell Physiol. 24 (2), 147–154. doi: 10.1093/pcp/24.2.147
Tanurdzic, M., Banks, J. A. (2004). Sex-determining mechanisms in land plants. Plant Cell 16 (suppl_1), S61–S71. doi: 10.1105/tpc.016667
Theißen, G. (2001). Development of floral organ identity: stories from the MADS house. Curr. Opin. Plant Biol. 4 (1), 75–85. doi: 10.1016/S1369-5266(00)00139-4
Tian, Z., Jahn, M., Qin, X., Obel, H. O., Yang, F., Li, J., et al. (2021). Genetic and transcriptomic analysis reveal the molecular basis of photoperiod-regulated flowering in xishuangbanna cucumber (Cucumis sativus l. var. xishuangbannesis qi et Yuan). Genes 12 (7), 1064. doi: 10.3390/genes12071064
Trebitsh, T., Staub, J. E., O'Neill, S. D. (1997). Identification of a 1-aminocyclopropane-1-carboxylic acid synthase gene linked to the female (F) locus that enhances female sex expression in cucumber. Plant Physiol. 113 (3), 987–995. doi: 10.1104/pp.113.3.987
Vaudo, A. D., Erickson, E., Patch, H. M., Grozinger, C. M., Mu, J. (2022). Impacts of soil nutrition on floral traits, pollinator attraction, and fitness in cucumbers (Cucumis sativus l.). Sci. Rep. 12 (1), 21802. doi: 10.1038/s41598-022-26164-4
Wang, D. H., Li, F., Duan, Q. H., Han, T., Xu, Z. H., Bai, S. N. (2010). Ethylene perception is involved in female cucumber flower development. Plant J. 61 (5), 862–872. doi: 10.1111/j.1365-313X.2009.04114.x
Wang, Z., Zhang, S., Yang, Y., Li, Z., Li, H., Yu, R., et al. (2022). Novel bisexual flower control gene regulates sex differentiation in melon (Cucumis melo l.). J. Agric. Food Chem. 70 (49), 15401–15414. doi: 10.1021/acs.jafc.2c05998
Wen, H., Chen, Y., Du, H., Zhang, L., Zhang, K., He, H., et al. (2020). Genome-wide identification and characterization of the TCP gene family in cucumber (Cucumis sativus l.) and their transcriptional responses to different treatments. Genes 11 (11), 1379. doi: 10.3390/genes11111379
Wittwer, S., Bukovac, M. (1962). Quantitative and qualitative differences in plant response to the gibberellins. Am. J. Bot. 49 (5), 524–529. doi: 10.1002/j.1537-2197.1962.tb14975.x
Wu, T., Qin, Z., Zhou, X., Feng, Z., Du, Y. (2010). Transcriptome profile analysis of floral sex determination in cucumber. J. Plant Physiol. 167 (11), 905–913. doi: 10.1016/j.jplph.2010.02.004
Yadav, N. P., Bahadur, V., Singh, G., Singh, N. V. (2022). Influence of foliar spray of brassinosteroids (BR), salicylic acid (SA) and gibberellic acid (GA3) on vegetative growth and flowering parameters of cucumber (Cucumis sativus l) cv. arpit. Int. J. Environ. Climate Change 12 (12), 607–615. doi: 10.9734/ijecc/2022/v12i121498
Yamasaki, S., Fujii, N., Matsuura, S., Mizusawa, H., Takahashi, H. (2001). The m locus and ethylene-controlled sex determination in andromonoecious cucumber plants. Plant Cell Physiol. 42 (6), 608–619. doi: 10.1093/pcp/pce076
Yang, A., Xu, Q., Hong, Z., Wang, X., Zeng, K., Yan, L., et al. (2022). Modified photoperiod response of CsFT promotes day neutrality and early flowering in cultivated cucumber. Theor. Appl. Genet. 135 (8), 2735–2746. doi: 10.1007/s00122-022-04146-4
Yanofsky, M. F. (1995). Floral meristems to floral organs: genes controlling early events in arabidopsis flower development. Annu. Rev. Plant Biol. 46 (1), 167–188. doi: 10.1146/annurev.pp.46.060195.001123
Yuan, H., Yue, P., Bu, H., Han, D., Wang, A. (2020). Genome-wide analysis of ACO and ACS genes in pear (Pyrus ussuriensis). In Vitro Cell. Dev. Biology-Plant 56, 193–199. doi: 10.1007/s11627-019-10009-3
Zhang, X., Lai, Y., Zhang, W., Ahmad, J., Qiu, Y., Zhang, X., et al. (2018). MicroRNAs and their targets in cucumber shoot apices in response to temperature and photoperiod. BMC Genomics 19, 1–15. doi: 10.1186/s12864-018-5204-x
Zhang, H., Li, S., Yang, L., Cai, G., Chen, H., Gao, D., et al. (2021). Gain-of-function of the 1-aminocyclopropane-1-carboxylate synthase gene ACS1G induces female flower development in cucumber gynoecy. Plant Cell 33 (2), 306–321. doi: 10.1093/plcell/koaa018
Zhang, Z., Mao, L., Chen, H., Bu, F., Li, G., Sun, J., et al. (2015). Genome-wide mapping of structural variations reveals a copy number variant that determines reproductive morphology in cucumber. Plant Cell 27 (6), 1595–1604. doi: 10.1105/tpc.114.135848
Zhang, Y., Zhao, G., Li, Y., Mo, N., Zhang, J., Liang, Y. (2017). Transcriptomic analysis implies that GA regulates sex expression via ethylene-dependent and ethylene-independent pathways in cucumber (Cucumis sativus l.). Front. Plant Sci. 8, 10. doi: 10.3389/fpls.2017.00010
Zhang, J., Zhao, Y., Liu, R., Zhou, C. (2019). AGAMOUS-LIKE FLOWER regulates flower and compound leaf development through different regulatory mechanisms in medicago truncatula. Plant Signaling Behav. 14 (7), 1612683. doi: 10.1080/15592324.2019.1612683
Zhou, Y., Ahammed, G. J., Wang, Q., Wu, C., Wan, C., Yang, Y. (2018). Transcriptomic insights into the blue light-induced female floral sex expression in cucumber (Cucumis sativus l.). Sci. Rep. 8 (1), 1–12. doi: 10.1038/s41598-018-32632-7
Zhou, Y., Hu, L., Ge, L., Li, G., He, P., Jiang, L., et al. (2019). Ectopic expression of CsMADS24, an AGAMOUS ortholog from cucumber, causes homeotic conversion of sepals into carpels in transgenic arabidopsis plants. Arch. Biol. Sci. 71 (1), 13–20. doi: 10.2298/ABS180528037Z
Keywords: cucumber, sex differentiation genes, environmental conditions, phytohormones, regulatory mechanism
Citation: Luo H, Zhang H and Wang H (2023) Advance in sex differentiation in cucumber. Front. Plant Sci. 14:1186904. doi: 10.3389/fpls.2023.1186904
Received: 15 March 2023; Accepted: 20 April 2023;
Published: 17 May 2023.
Edited by:
Weiwei Zhang, Shanghai Vocational College of Agriculture and Forestry, ChinaCopyright © 2023 Luo, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huasen Wang, d2hzeWNoNjZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Haiyan Luo
Haiyan Luo Huanchun Zhang4†
Huanchun Zhang4†