- Department of Cellular and Developmental Biology of Plants, University of Bielefeld, Bielefeld, Germany
Algae inhabit diverse environments with highly variable light conditions, making light sensing essential for survival and ecological success. This review explores the remarkable diversity of algal photoreceptors, enabling detection and response to a broad spectrum of sunlight, from ultraviolet to far-red wavelengths. Algae utilize various light-sensitive proteins—including flavin-based receptors (phototropins, cryptochromes, aureochromes, BLUF proteins), retinal-based rhodopsins, tetrapyrrole-based phytochromes, hybrid neochromes, and UV-B photoreceptors — to sense and integrate both light quality and quantity. The evolution of these photoreceptors has been shaped by endosymbiotic events, gene duplication, and domain fusion, equipping algae with robust mechanisms for environmental adaptation. Advances in genomics and transcriptomics have revealed many novel algal photoreceptors, some of which are being harnessed as optogenetic tools in biomedical research. Channelrhodopsins from green algae, for example, have revolutionized neuroscience by enabling precise, light-controlled manipulation of neuronal activity. The ongoing discovery and engineering of algal photoreceptors continue to expand the molecular toolkit for both basic research and practical applications. In summary, algal photoreceptors exemplify evolutionary innovation in adapting to diverse light environments and underpin numerous physiological processes critical for algal survival. Study and exploitation of these proteins offer profound insights into light perception, signaling, and technological applications, particularly in the rapidly growing field of optogenetics.
1 Introduction
The Sun, despite being 150 million kilometers from Earth, is the driving force behind all life. Its emitted continuous spectrum ranges from X-rays through ultraviolet, visible, and infrared, with peak intensity in the blue-green (approximately 500 nm) (Figure 1). Only part of the extraterrestrial solar radiation reaches Earth’s surface; the atmosphere absorbs much of the X-ray and ultraviolet radiation and modifies the spectrum with thousands of absorption lines (Fraunhofer lines), reflecting both solar and atmospheric composition. Solar radiation relevant for algae and plants can be divided into ultraviolet (UV, 100–380 nm), visible (approximately 380–780 nm), and infrared (IR, >780 nm). Photosynthetically active radiation (PAR) spans approximately 400–700 nm, largely overlapping with human-visible light. Blue-violet (400–500 nm), green (500–575 nm), yellow-orange (575–620 nm), red (620–700 nm), and far-red (700–780 nm) constitute the major color bands, with IR subdivided further (Endres and Breit, 2009). When sunlight enters water—the main habitat of algae—the spectrum changes significantly (Smith and Baker, 1981; Muaddi and Jamal, 1991; Kirk, 1994; Tedetti and Sempere, 2006; Simpson et al., 2014; Mat et al., 2024): UVB is strongly attenuated, UVA less so. At 50 cm water depth, 85% of UVA remains, whereas only 60% of UVB does. Visible light above 600 nm is rapidly attenuated with depth, and below 10 m, virtually no red light remains (Figure 2) (Kirk, 1994). Below 50 m, only violet-blue, blue, and blue-green light persist, and at 200 m only traces of violet-blue and blue light remain (Figure 2).
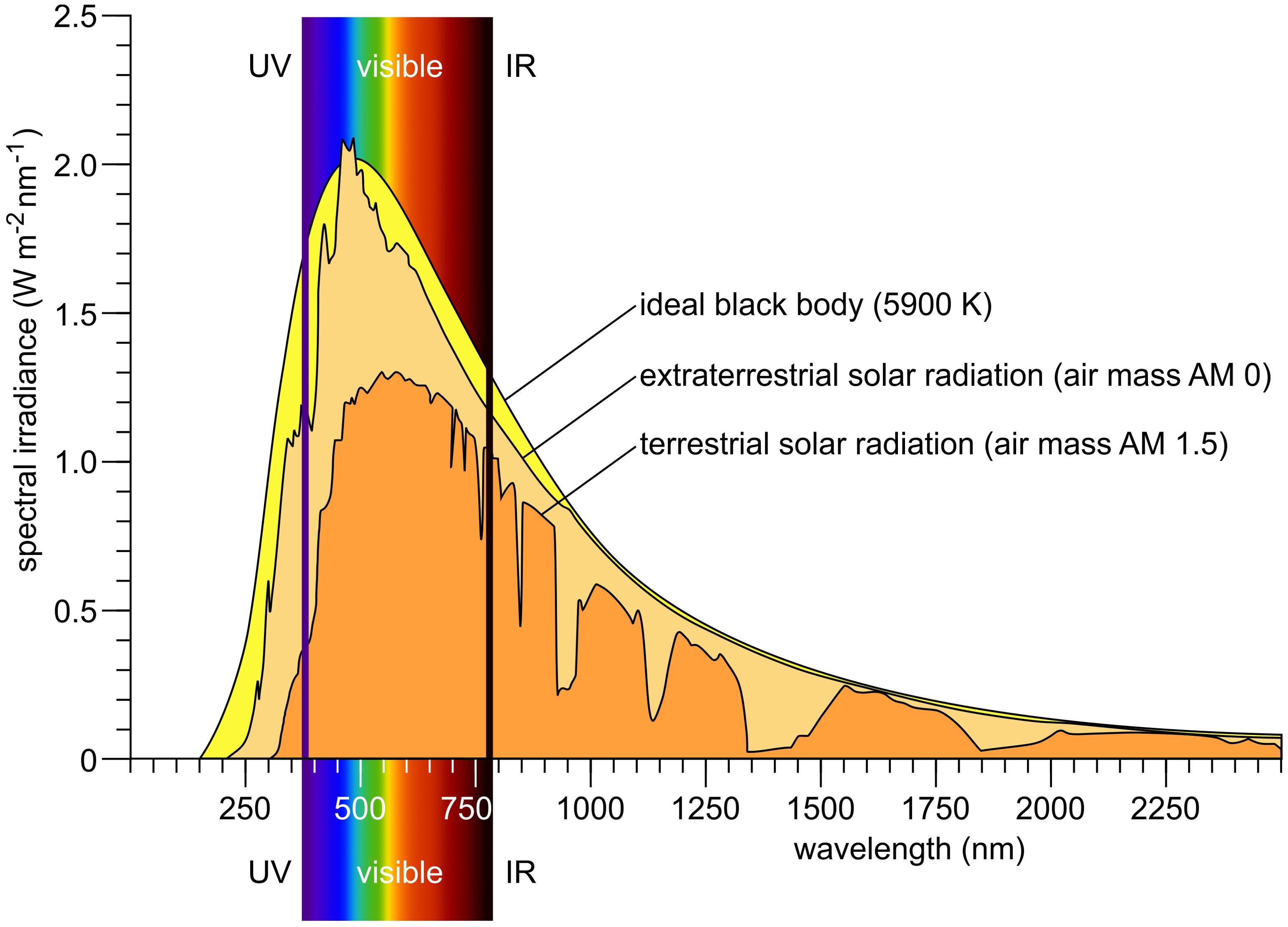
Figure 1. Solar irradiance spectrum. The surface temperature of the Sun is approximately 5900 K; therefore, a black-body radiation curve at this temperature serves as a theoretical model for the Sun’s emitted light. This model is compared to the solar irradiance spectrum measured outside Earth’s atmosphere (extraterrestrial solar radiation; air mass AM 0) and to the spectrum observed at Earth’s surface when the Sun is 48.2° above the horizon—typical for midday conditions at many locations (terrestrial solar radiation; air mass AM 1.5). Redrawn and modified after Iqbal (1983); Kirk (1994); Goswami et al. (1999); Wang et al. (2021); Leppäranta (2023).
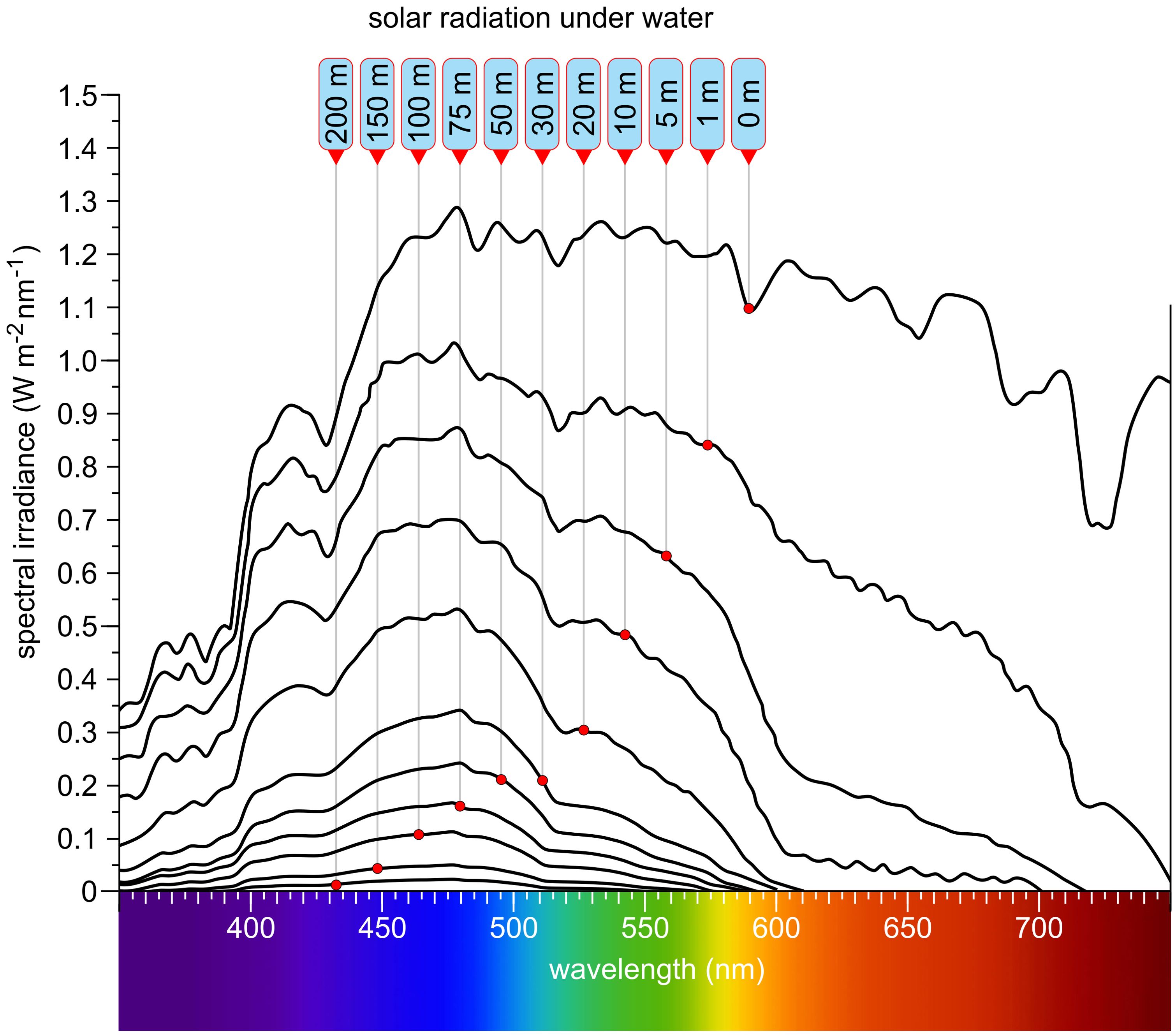
Figure 2. Solar radiation at different depths in clear water. Labels are arranged with the shallowest depth on the right and the greatest depth on the left. As depth increases, longer wavelengths are progressively attenuated, and the overall light intensity diminishes. Far-red and red wavelengths are absorbed first, followed by orange and yellow. Green light penetrates deeper, while blue and violet-blue wavelengths reach the greatest depths—up to approximately 200 meters. At this depth, typically only about 0.1% to 0.5% of the surface light intensity remains. Redrawn and modified after Smith and Baker (1981); Muaddi and Jamal (1991); Kirk (1994); Tedetti and Sempere (2006); Simpson et al. (2014).
Despite the wide variety of light conditions, algae have successfully colonized virtually all aquatic environments and are also found in many other settings. The habitats they occupy differ greatly not only in light availability, intensity, and spectral quality, but also in water properties such as salinity, pH, turbidity, depth (Figure 2), temperature, nutrient content, and other ecological factors. All these parameters together influence the growth and light absorption of the algae inhabiting these environments. In this review, the term ‘algae’ refers specifically to photosynthetic eukaryotic algae, excluding prokaryotic phototrophs such as cyanobacteria (often referred to as ‘blue-green algae’ in older literature). The diversity of eukaryotic algae encountered across habitats could hardly be greater: they differ in color, motility, cell number, phylogenetic position, and structural complexity, ranging from unicellular to colonial and multicellular forms (Graham and Wilcox, 2000; Barsanti and Gualtieri, 2014), with the smallest species (Ostreococcus tauri, Prasinodermophyta) under 1 µm (Courties et al., 1994) and the largest species (Macrocystis pyrifera, Phaeophyta) (Figure 3) exceeding 60 meters (Hallmann, 2007). Some multicellular algae consist exclusively of cell clusters with similar cells or simple filaments, while others are composed of tissues (Figure 3). In many species, the evolution of multicellularity has been accompanied by the differentiation of specialized cell types (Graham and Wilcox, 2000; Guiry, 2012; Barsanti and Gualtieri, 2014).
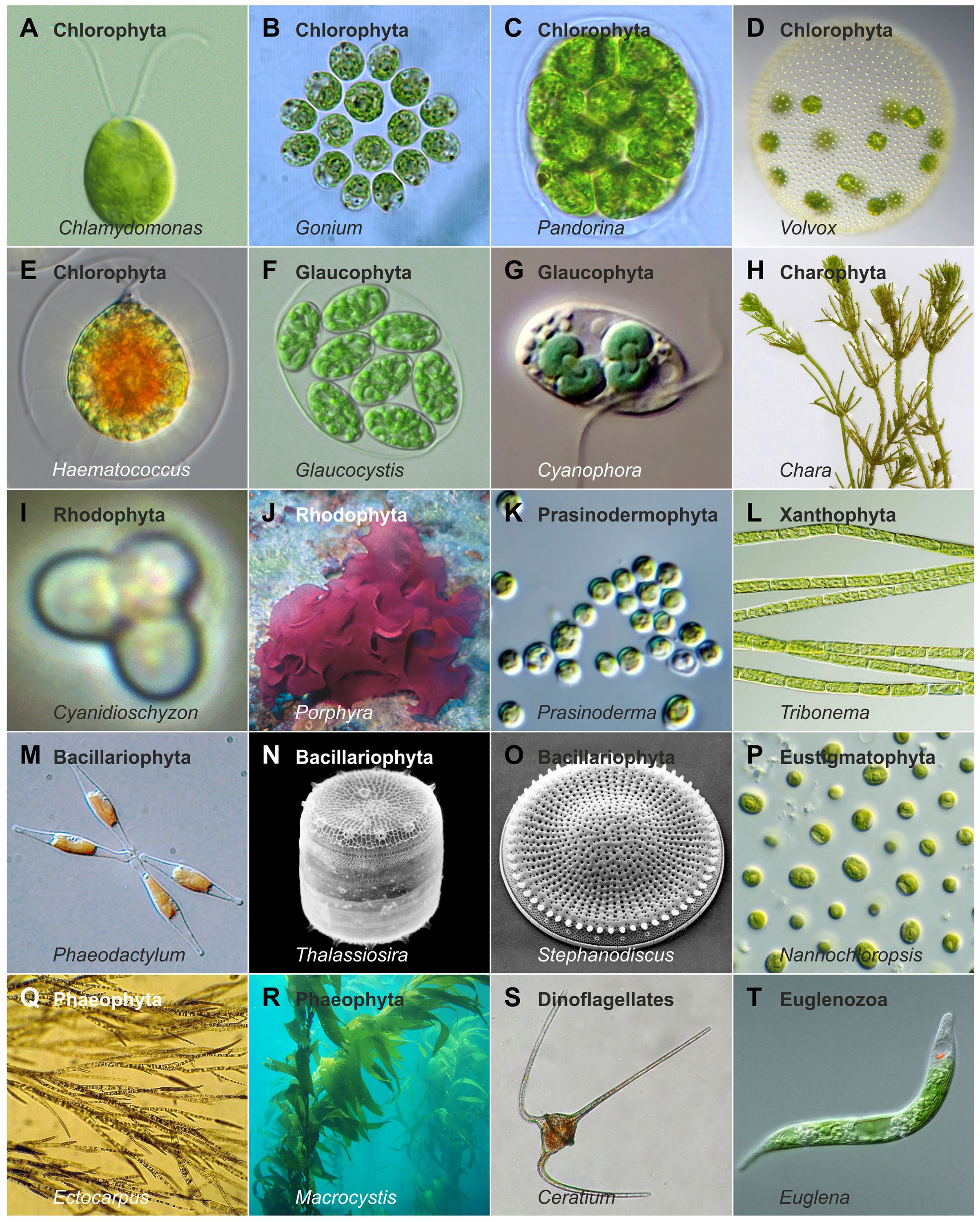
Figure 3. Example algal species illustrating morphological and ecological diversity. A selection of algal species illustrating diversity in cell number, structural complexity, size, motility or attachment strategies, pigmentation, habitat, and phylogenetic position. Photos by Alessandra de Martino and Chris Bowler (Phaeodactylum), Christian Fischer (Chara), Claire Fackler (Macrocystis), CSIRO (Nannochloropsis), djpmapfer (Euglena), Frank E. Round (Stephanodiscus), Keisotyo (Ceratium), Li and colleagues (Li et al., 2020) (Prasinoderma), Neon (Cyanidioschyzon), Nils Kröger (Thalassiosira), Sarka Martinez (Ectocarpus), Wiedehopf20 (Haematococcus), Wolfgang Bettighofer (Cyanophora), Y. Tsukii (Glaucocystis), and own work (Hallmann, 2011; 2015;Kianianmomeni and Hallmann, 2016; Hallmann, 2020, 2024).
Given the vast diversity of algae—currently about 177,000 documented species, with estimates reaching up to one million (Guiry, 2012; Raven and Giordano, 2014)—and their ecological importance, understanding algal (photo)biology has become a major focus of research. The principal algal groups and their phylogenetic relationships are illustrated in a simplified tree of life (Figure 4). To unravel fundamental molecular and cellular principles, a number of algal model species have been established, including Chlamydomonas reinhardtii (Chlorophyta) (Figure 3), Volvox carteri (Chlorophyta) (Figure 3), Cyanidioschyzon merolae (Rhodophyta) (Figure 3), Phaeodactylum tricornutum (Bacillariophyta) (Figure 3), Vaucheria frigida (Xanthophyta), Mougeotia scalaris (Charophyta), Ectocarpus siliculosus (Phaeophyta) (Figure 3) and others (Kianianmomeni and Hallmann, 2014).
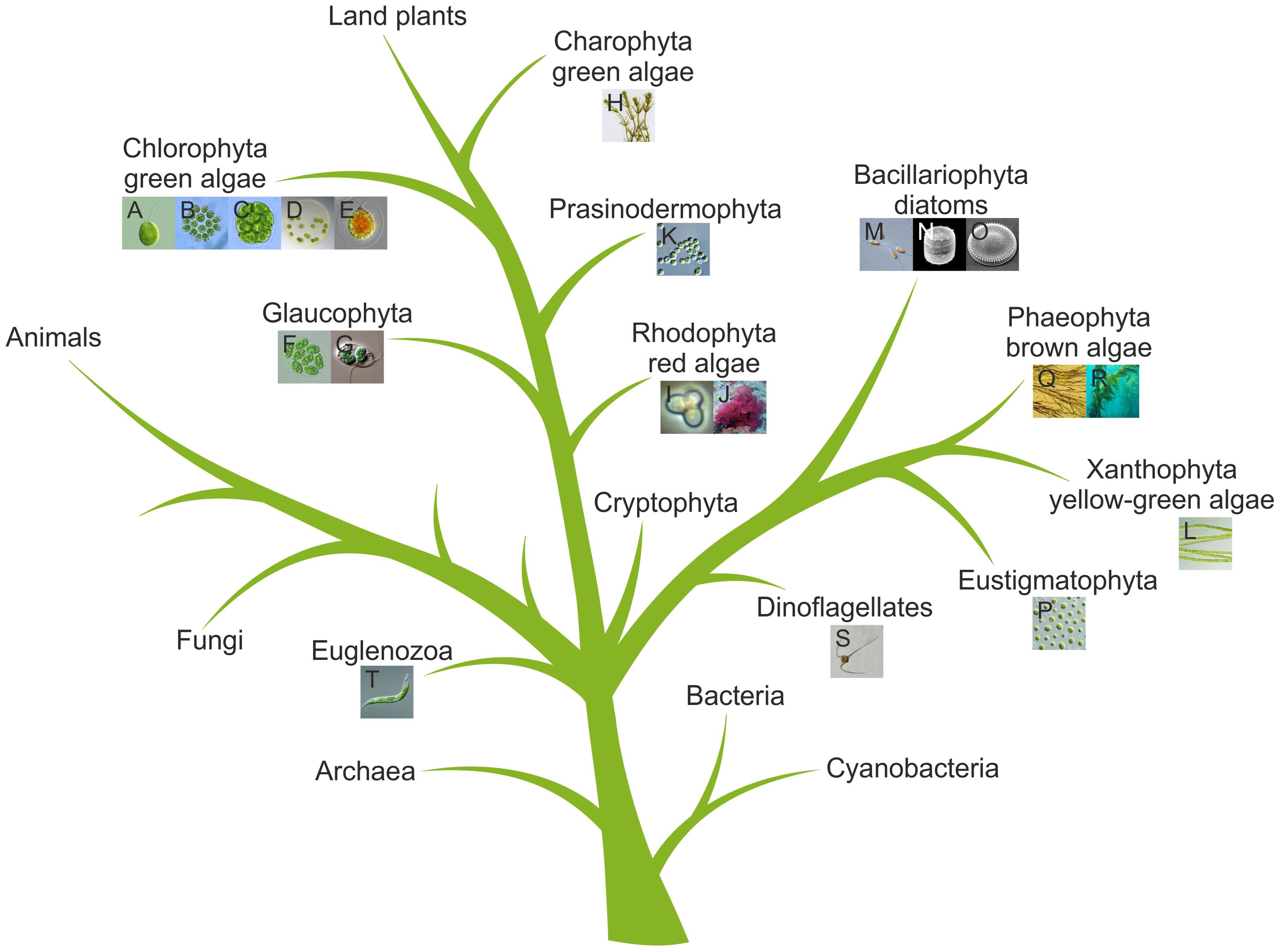
Figure 4. Simplified tree of life highlighting algae. The tree is based on previous phylogenetic analyses (Kranz et al., 1995; Baldauf, 2003; Maddison and Schulz, 2007; Prochnik et al., 2010; Hallmann, 2011; Kianianmomeni and Hallmann, 2014). Algal species from Figure 3 are shown as thumbnails and are assigned to their respective groups. The letters in the thumbnails correspond to those in Figure 3.
Previous reviews have addressed various aspects of photoreceptors (Hegemann et al., 2001; Kateriya et al., 2004; Hegemann, 2008; Kami et al., 2010; Möglich et al., 2010; Kianianmomeni and Hallmann, 2014; Parihar et al., 2016; Duanmu et al., 2017; Kottke et al., 2017, 2018; Sasso et al., 2018; Petersen et al., 2021; Shankar et al., 2022; Vierock and Hegemann, 2023). Building on these and further foundations, this review first outlines the diversity of light environments and habitats that algae occupy and describes the fundamental physiological processes in algae that are regulated by light. The evolutionary origins and habitat adaptations of algal photoreceptors are then discussed, followed by a brief overview of the scientific history of their discovery. The main classes of algal photoreceptors—including their molecular structures, chromophores, and mechanisms of action—are reviewed in detail. Recent advances in algal genomics and transcriptomics as sources for the discovery of new light-sensitive proteins are highlighted. Finally, current and emerging applications of algal photoreceptors in optogenetics are discussed, and an outlook on future directions in this rapidly evolving field is provided.
2 Light-dependent processes in algae
Light serves algae not only as an energy source for photosynthesis but also as an environmental cue regulating a multitude of physiological and developmental processes. Light-harvesting pigments and complexes capture photons to drive photosynthesis, which converts solar energy into chemical energy for growth and energy storage (Falkowski and Raven, 2007; Croce and Van Amerongen, 2014). However, light is also sensed through specialized photoreceptors, which detect changes in light intensity and quality.
Upon light detection, various signal transduction cascades are activated. Light-dependent ion channels initiate ion currents that alter membrane potential and activate downstream signaling pathways. Key second messenger systems include cyclic nucleotides (cGMP, cAMP), calcium ions, and reactive oxygen species (ROS). For example, changes in cGMP/cAMP modulate enzymes, rapid Ca²+ fluxes influence flagellar movement and gene expression, and high light intensities generate ROS, which act as signals for stress responses or the induction of sexual reproduction.
Light coordinates gene expression and protein turnover in algae, aligning photosynthetic capacity and metabolism with prevailing light conditions. It acts across all regulatory layers—from chromatin organization and transcription to translation, post-translational modifications, and metabolism. In doing so, light shapes numerous physiological responses, including:
– Photosynthesis and chloroplast adaptation: Algae adjust their photosynthetic machinery and pigment composition in response to light quality and quantity, optimizing energy capture while preventing photodamage (Kirk and Kirk, 1985; Grossman et al., 2004; Falkowski and Raven, 2007; Kupper et al., 2009; Ozawa et al., 2009; Roy et al., 2011; Frank and Cogdell, 2012; Nickelsen and Rengstl, 2013; Wada, 2013; Croce and Van Amerongen, 2014; Maltsev et al., 2021).
– Photoprotection: Protective mechanisms, including non-photochemical quenching, antioxidant production, and repair of photodamaged components, are activated by light (Demmig-Adams and Adams, 1996; Niyogi, 1999; Allorent and Petroutsos, 2017; Vecchi et al., 2020).
– Photobehavior and phototaxis: Algae can move toward or away from light sources, optimizing their position for growth or protection (Lenci and Colombetti, 1978; Foster and Smyth, 1980; Kreimer, 1994; Sgarbossa et al., 2002; Harris et al., 2009; Sasso et al., 2018; Wakabayashi et al., 2021; Seth et al., 2022; Leptos et al., 2023).
– Phototropism: Some filamentous and other multicellular algae reorient growth in response to directional light (Rico and Guiry, 1996; Takahashi and Mikami, 2016; 2019).
– Circadian rhythms: Endogenous biological clocks synchronized by light regulate daily cycles of cell division, metabolism, and behavior (Mittag, 2001; Werner, 2002; Mittag et al., 2005; Matsuo et al., 2008; Matsuo and Ishiura, 2010; Schulze et al., 2010; Matsuo and Ishiura, 2011; Bouget et al., 2014; Galvão and Fankhauser, 2015; Noordally and Millar, 2015; Kuhlman et al., 2018; Farre, 2020; Matsuo et al., 2020).
– Development and morphogenesis: Light influences developmental pathways, including multicellular differentiation and photomorphogenic responses (Dring and Lüning, 1983; Kirk and Kirk, 1985; Dring, 1988; Lüning, 1990; Agarwal et al., 2023).
– Cell cycle and sexual reproduction: Light modulates the timing of cell division, the induction of sexual cycles, and gamete formation (Craigie and Cavalier-Smith, 1982; Donnan and John, 1983; Weissig and Beck, 1991; Pan et al., 1997; Saito et al., 1998; Zachleder et al., 2002; Huang and Beck, 2003; Oldenhof et al., 2004a, 2004; Goodenough et al., 2007; Mouget et al., 2009; Bisova and Zachleder, 2014; Cross and Umen, 2015; Müller et al., 2017; Zou et al., 2017; Permann et al., 2022).
– Pigment production: Light regulates the synthesis of photosynthetic and protective pigments (Begum et al., 2016; Lee et al., 2018; Mutschlechner et al., 2022; Zarekarizi et al., 2023).
Most physiological responses involve the integration of multiple photoreceptors (Kami et al., 2010). Moreover, light signals are often integrated with other environmental factors such as temperature, pH, salinity, nutrient availability, and biotic interactions to trigger specific adaptive responses.
3 Evolutionary origins and habitat adaptations of algal photoreceptors
For light to affect algal cells, it must first be absorbed by biomolecules. Light-harvesting pigments collect photons for photosynthesis, while sensory photoreceptors provide information about the environment, enabling cells to regulate growth, development, and stress responses. The interplay between light perception and downstream physiological reactions is critical for survival (Kirk and Kirk, 1985; Huang and Beck, 2003; Grossman et al., 2004). The algal light-sensing system must detect not only light intensity, but also its spectral composition.
Photoreceptors are evolutionarily ancient. Light-sensitive proteins already existed in early prokaryotes, where microbial rhodopsins such as bacteriorhodopsin acted as light-driven proton pumps for energy generation (Spudich et al., 2000; Ernst et al., 2014; Gushchin and Gordeliy, 2018). True photoreceptors, in contrast, evolved as signaling proteins that couple chromophore absorption to downstream regulatory responses (Möglich et al., 2010; Losi and Gärtner, 2012). Plastids originated from the primary endosymbiosis with a cyanobacterium-like ancestor (Keeling, 2010; Archibald, 2015; Ponce-Toledo et al., 2017). This event not only established photosynthesis in the host cell but was also accompanied by extensive endosymbiotic gene transfer from the endosymbiont to the host nucleus (Timmis et al., 2004; Archibald, 2015). Subsequent secondary (e.g., brown algae, diatoms) and tertiary endosymbioses (e.g., in some dinoflagellates) introduced plastids into additional eukaryotic lineages and contributed further genes to their nuclear genomes (Keeling, 2013; Dorrell and Howe, 2015).
The evolutionary trajectories of individual photoreceptor families remain complex and in several cases unresolved. While some photoreceptor types may trace back to cyanobacterial genes, others appear to have arisen independently or to have been shaped by convergent evolution, horizontal gene transfer (HGT), or lineage-specific innovation (Losi and Gärtner, 2012; Li et al., 2015a, 2015; Li and Mathews, 2016; Rockwell and Lagarias, 2020). Thus, the algal photoreceptor repertoire reflects a mosaic evolutionary history rather than a single ancestral inheritance.
Today, eukaryotic algae occupy a vast array of habitats: freshwater (rivers, lakes, ponds), wetlands, marine (coastal, open ocean, deep-sea, estuaries), and terrestrial (soils, rocks, tree bark). Some algae are airborne, colonize extreme sites (e.g., snow, hot springs, hypersaline lakes), or inhabit artificial environments (e.g., irrigation systems, buildings) (Raven and Giordano, 2014).
Light availability and spectral quality vary dramatically between habitats, particularly with water depth (Figure 2). Shallow waters are rich in red and blue light, but at greater depths only blue and violet wavelengths remain, and light intensity drops sharply. Also hypersaline waters lead to an altered light spectrum. Terrestrial algae, especially those on snow or ice, must withstand high-intensity UV and blue light. Low-light environments, such as those found in deeper water columns or densely shaded habitats, pose a particular challenge. Moreover, incident light is frequently spectrally filtered by overlying vegetation (e.g., leaves) or other algal biomass.
Adaptation to such variable light conditions has driven diversification of both light-harvesting pigments and photoreceptors, enabling algae to exploit a wide range of ecological niches.
4 A brief look at the scientific history of algal photoreceptors
Although light perception and response mechanisms have existed in algae for millions of years, scientific understanding of these processes is relatively recent. In the 19th century, Andrei S. Faminzyn observed that unicellular algae like Chlamydomonas (Chlorophyta, Figures 3, 4) and Euglena (Euglenozoa, Figures 3, 4) could move toward or away from light sources (Faminzin, 1866)—a phenomenon now known as phototaxis and an early indication of light sensitivity in algae. Early studies also identified carotenoids as algal pigments, and foundational research by Jan Ingenhousz and Robert Emerson established that light functions not only as an energy source but also as a regulatory signal (Emerson, 1957; Govindjee, 1999).
The mid-20th century saw the elucidation of photosynthesis mechanisms by researchers like Hill, Calvin, Benson, and Bassham (Jahn, 2004; Eberhard et al., 2008). In the 1950s–1970s, the discovery of blue light sensitivity in algae led to the hypothesis of specialized blue light photoreceptors. This period also saw the identification of flavin-based blue light receptors in algae, and the association of the algal eyespot with light-sensing pigments such as carotenoids (Briggs, 2006; Kreimer, 2009; Losi and Gärtner, 2011).
Subsequent decades brought the discovery of cryptochromes and phototropins in both higher plants and algae (Liscum and Briggs, 1995; Briggs et al., 2001; Chory, 2010; Yu et al., 2010; Beel et al., 2013; Christie et al., 2015). In the 1970s, rhodopsins—originally identified as visual pigments in animals—were found as bacteriorhodopsin in microorganisms, i.e. in halobacteria (Archaea, Figure 4) (Oesterhelt and Stoeckenius, 1971; 1973; Kayushin and Skulachev, 1974). In the early 2000s, microbial-type rhodopsins were identified in green algae such as Chlamydomonas reinhardtii, where they function as light-gated ion channels (channelrhodopsins) (Nagel et al., 2002; Sineshchekov et al., 2002; Nagel et al., 2003; Suzuki et al., 2003). The unique ability of channelrhodopsins to induce cell depolarization by blue light was fundamental for the emergence of optogenetics (Nagel et al., 2002, 2003).
In the following years, rhodopsin domains from channelrhodopsins and other photosensitive domains were increasingly employed to control key intracellular processes such as protein secretion, nuclear import, chromatin targeting, and gene expression (Kianianmomeni, 2014; Schmidt and Cho, 2015). Additionally, photoswitchable enzymerhodopsins have emerged as promising tools for optogenetics. In algae, this group includes histidine kinase rhodopsins (HKRs) (Kateriya et al., 2004; Luck et al., 2012) and rhodopsin guanylyl cyclases (RGCs) (Tian et al., 2018a).
With the increase in sequenced algal genomes and transcriptomes, many new light-sensitive proteins have been found. It is now clear that many algae possess multiple photoreceptor classes that work together to precisely perceive environmental stimuli. For instance, cryptochromes and rhodopsins may act in concert to regulate development. Further newly discovered photoreceptors are expanding the molecular toolbox for optogenetics and providing new insights into light-regulated cellular processes.
Previous reviews have covered different aspects of algal (Hegemann et al., 2001; Hegemann, 2008; Kianianmomeni and Hallmann, 2014; Duanmu et al., 2017; Shankar et al., 2022; Vierock and Hegemann, 2023) and plant photoreceptors (Lariguet and Dunand, 2005; Galvão and Fankhauser, 2015; Kong and Okajima, 2016; Parihar et al., 2016; Kowalik and Chen, 2017; Kottke et al., 2018; Pierik and Ballare, 2021), especially their chromophore (Galvão and Fankhauser, 2015; Ziegler et al., 2016; Kowalik and Chen, 2017; Tian, 2018) and domain structures (Hegemann, 2008; Kianianmomeni and Hallmann, 2015b; Kong and Okajima, 2016; Parihar et al., 2016; Kottke et al., 2018) and their applications in optogenetics (Dugué et al., 2012; Hegemann and Nagel, 2013; Hegemann and Sigrist, 2013; Kianianmomeni and Hallmann, 2014; 2015a; 2016).
5 The different types of algal photoreceptors
Light-sensitive proteins are widespread throughout the tree of life, including in animals, plants, algae, fungi, protists, archaea, and bacteria. These proteins enable organisms to sense environmental light and to modulate their growth, development, behavior, and reproduction accordingly. Photoreceptors play key roles in regulating adaptive light responses such as phototaxis and phototropism. They are also essential for various developmental processes, including the coordination of flowering time, sexual development, and adaptation to light-dark cycles. Additionally, the regulation of gene expression at different hierarchical levels by light-responsive photoreceptors provides a complex mechanism for controlling the abundance and functionality of the respective proteins. As a result, light exerts indirect control over a wide range of cellular and physiological processes.
All photoreceptors contain a chromophore—an organic molecule that absorbs light—bound to a protein (apoprotein) that determines specificity and mediates signal transduction. The primary function of photoreceptor proteins is to facilitate the absorption of photons and, upon absorption, to trigger a biological response either directly or indirectly. The protein component is responsible for converting light signals into biochemical reactions or signal transduction pathways; it also determines the specificity of the photoreceptor and influences the absorption wavelength of the chromophore. In most cases, photoreceptor proteins are capable of detecting a relatively broad range of absorption wavelengths found in sunlight. Overall, they cover the entire spectrum from UV-B to far-red light (Figure 5).
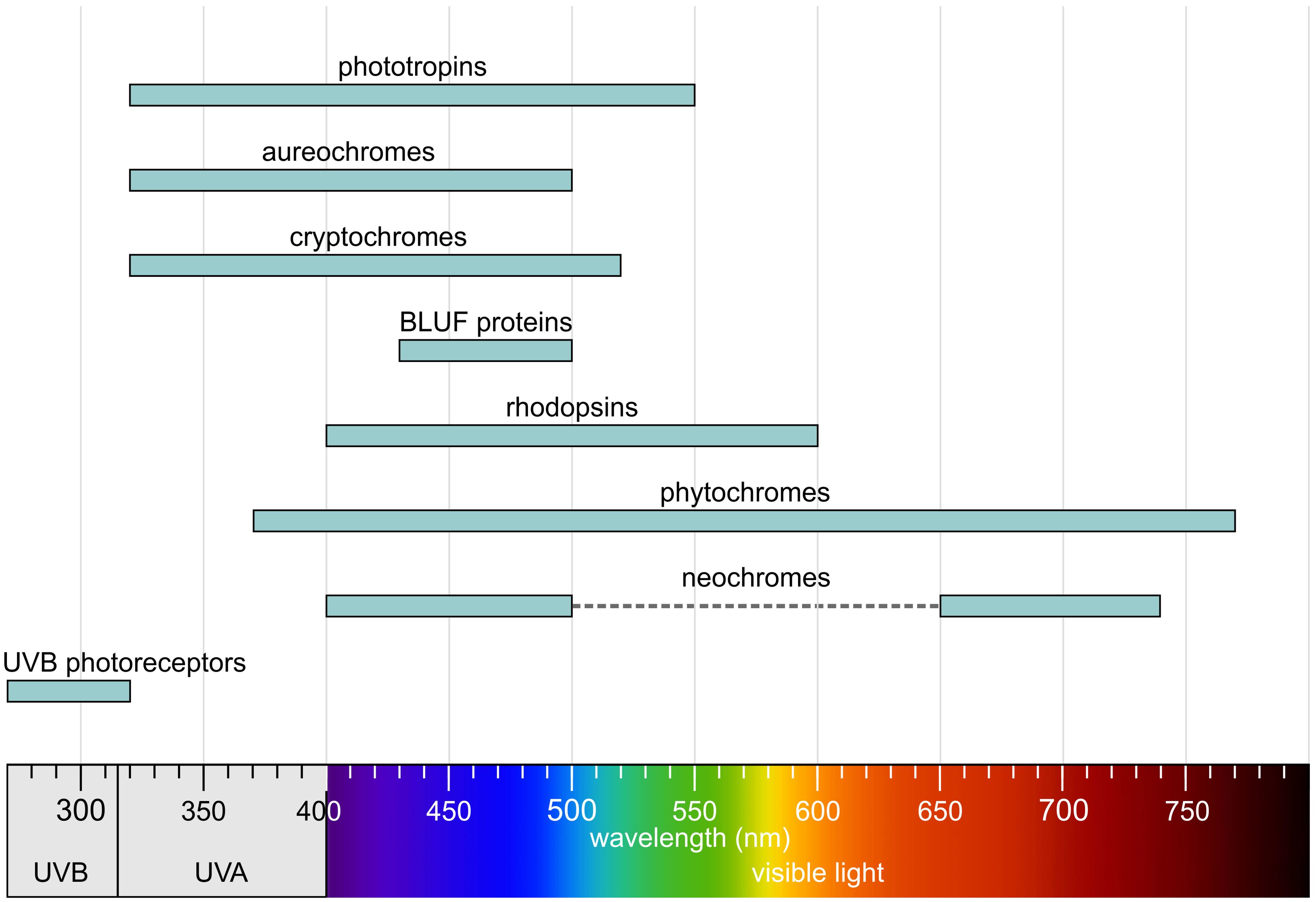
Figure 5. Absorption regions of photoreceptors. Redrawn and modified after Sager et al. (1988); Taiz and Zeiger (2002); Sullivan and Deng (2003); Lariguet and Dunand (2005); Rockwell et al. (2014); Galvão and Fankhauser (2015); Shankar et al. (2022), with additional information included.
There are various ways to categorize photoreceptors (Kottke et al., 2018), for example by the wavelength of light absorbed, their mode of operation, the signaling pathways they serve, their evolutionary history, or the type of chromophore they use. In this context, the focus is on algal photoreceptors, which are classified into five main groups based on their chromophore. This classification also reflects structural similarities and evolutionary relationships. The five major groups are:
– Flavin-based receptors (phototropins, aureochromes, cryptochromes, BLUF proteins).
– Retinal-based receptors – rhodopsins (type-1 – microbial rhodopsins, type-2 – animal rhodopsins).
– Tetrapyrrole-based receptors (phytochromes).
– Hybrid receptors (neochromes).
– Proteins with UV absorption (UV-B photoreceptors).
It is noteworthy that only the retinal-based receptors are membrane-bound, while all others are soluble photoreceptors.
5.1 Flavin-based receptors
5.1.1 Phototropins
Phototropins are flavin-based photoreceptors that are sensitive to blue light (Figure 5) and are widespread in both algae and plants (Losi and Gärtner, 2012; Suetsugu and Wada, 2013; Christie et al., 2015; Li et al., 2015b; Okajima, 2016; Hart and Gardner, 2021; Huq et al., 2024). They were first discovered in Arabidopsis thaliana in connection with blue light-dependent phosphorylation (Huala et al., 1997; Christie and Briggs, 2001; Christie, 2007). The basic mechanism of signal transduction is conserved in both algae and plants, even though the sequence identity is only 30–40% (Onodera et al., 2005; Kianianmomeni and Hallmann, 2014). Phototropins are involved in regulating various light-dependent processes such as phototaxis, chloroplast movement, and photomorphogenesis. In algae, phototropins are associated with evolutionary adaptation to light-dependent aquatic environmental conditions (Kami et al., 2010). Phototropins also influence the expression of genes encoding enzymes for chlorophyll and carotenoid biosynthesis in response to blue light (Im et al., 2006).
Only a single phototropin, PHOT, has been identified in the green algae Volvox carteri (Figure 3) and Chlamydomonas reinhardtii (Figure 3) (Huang et al., 2002; Prochnik et al., 2010). This single phototropin is involved in the regulation of photoprotection (Petroutsos et al., 2016), eyespot formation (Trippens et al., 2012), reproduction (Huang and Beck, 2003), and starch accumulation (Yuan et al., 2025). In Volvox, phototropin accumulation is higher in the small somatic cells than in the large reproductive cells, suggesting a link between PHOT expression and the mechanism controlling cell size during Volvox development (Kianianmomeni and Hallmann, 2014). Due to gene duplication, higher plants possess two phototropin isoforms, PHOT1 and PHOT2 (Li et al., 2015b; Hart and Gardner, 2021).
Phototropins have a modular structure; in algae, they consist of two N-terminal light, oxygen, or voltage (LOV) domains and a C-terminal serine/threonine kinase (S/T kinase) domain (Figure 6) (Crosson and Moffat, 2001; Huang and Beck, 2003; Kottke et al., 2006; Aihara et al., 2008; Losi and Gärtner, 2012; Christie et al., 2015). The LOV domains are responsible for blue light perception, with each binding a flavin mononucleotide (FMN) as a chromophore (Figure 6) (Crosson et al., 2003; Kottke et al., 2003; Kennis et al., 2004; Losi et al., 2004; Briggs et al., 2007; Losi, 2007; Hoffman et al., 2018; Losi et al., 2018; Flores-Ibarra et al., 2024; He et al., 2025a).
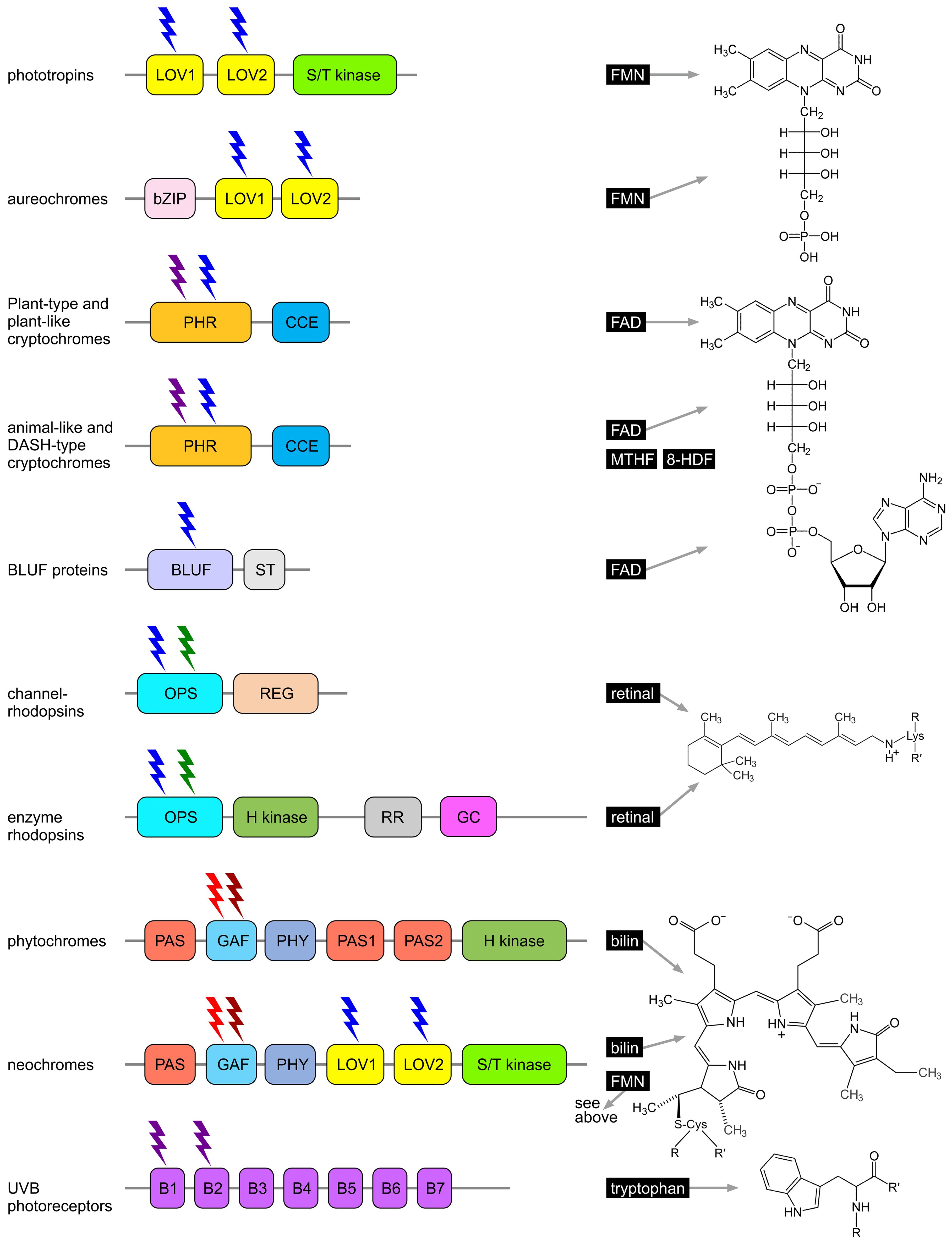
Figure 6. Algal photoreceptors and their chromophores. The domain structure of each photoreceptor protein is shown on the left, and the chemical structure of the associated chromophore is depicted on the right. Phototropins, aureochromes, channelrhodopsins, and cryptochromes primarily absorb blue light, while UVB receptors (UVR8) are sensitive to UVB light. Neochromes and animal-like cryptochromes respond to both red and blue light; enzyme rhodopsins are receptive to both blue and green light; and phytochromes respond to red and far-red light, with some also sensitive to orange, blue, and green light. 8-HDF, 8-hydroxy-7,8-didemethyl-5-deazariboflavin; B1-7, β-propeller domains; bilin, phycocyanobilin (PCB); BLUF, blue light using FAD; bZIP, basic region/leucine zipper; CCE, cryptochrome C-terminal extension (CCT, CTE); FAD, flavin adenine dinucleotide; FMN, flavin mononucleotide; GAF, cGMP phosphodiesterase/adenyl cyclase Fh1A; GC, guanylyl cyclase; H kinase, histidine kinase; LOV, light, oxygen, voltage; MTHF, 5,10-methenyltetrahydrofolate; OPS, opsin; PAS, PER-ARNT-SIM; PHR, photolyase homology region; PHY, phytochrome-specific; REG, regulator; retinal, all-trans-retinal; RR, response regulator receiver; ST, signal transduction; S/T kinase, serine/threonine kinase. Redrawn, modified, and supplemented from Galvão and Fankhauser (2015); Kong and Okajima (2016); Parihar et al. (2016); Shankar et al. (2022).
Upon absorption of blue light, a covalent bond forms between the FMN and a cysteine residue, triggering a conformational change in the protein. In particular, LOV1 plays a supportive role in stabilizing the protein and regulating its interactions with other molecules, while the conformational change in LOV2 upon light activation enables activation of the kinase domain. The S/T kinase domain catalyzes autophosphorylation and subsequently transmits the signal by phosphorylating target proteins at serine and threonine residues. This domain is crucial for propagating the light signal by activating downstream signaling cascades (Christie et al., 1998, 2002; Hart and Gardner, 2021; Flores-Ibarra et al., 2024).
The 3D structure of phototropins has only been partially elucidated, as the structure of the light-dependent S/T kinase domain has not yet been fully resolved. However, the structures of the LOV domains from phototropins in both plants and algae—under dark and light conditions—have been determined using crystallographic techniques (Christie et al., 1999; Crosson and Moffat, 2002; Fedorov et al., 2003; Halavaty and Moffat, 2007; Flores-Ibarra et al., 2024; Gotthard et al., 2024). The LOV1 and LOV2 domains belong to the same family as PER-ARNT-SIM (PAS) domains. LOV domains are composed of five antiparallel β-strands flanked by two α-helices, creating a specific binding pocket for the flavin chromophore (Crosson and Moffat, 2001; 2002;Nash et al., 2011; Zoltowski et al., 2013). The conformational change that occurs after formation of the light-induced covalent bond between the cysteine residue of the LOV domain and the FMN particularly affects the so-called Jα helix, which is associated with the LOV2 domain (Crosson et al., 2003; Harper et al., 2003, 2004; Halavaty and Moffat, 2007; Vaidya et al., 2011). The Jα helix becomes accessible, facilitating transmission of the signal to the S/T kinase domain. The S/T kinase domain belongs to the family of serine/threonine protein kinases and exhibits the typical bilobal structure of these enzymes. The catalytic core consists of a small N-terminal lobe, which typically features a β-sheet and an α-helix, and a larger C-terminal lobe that is predominantly α-helical. Activation of this core is triggered by light-induced activation of the LOV2 domain (Matsuoka and Tokutomi, 2005; Jones and Christie, 2008; Hart and Gardner, 2021).
5.1.2 Aureochromes
Aureochromes are flavin-based photoreceptors that are sensitive to blue light (Figure 5) (Suetsugu and Wada, 2013; Takahashi, 2016; Essen et al., 2017; Kroth et al., 2017; Matiiv and Chekunova, 2018; Coesel, 2024; Im et al., 2024). More specifically, they are light-controlled transcription factors that regulate genes important for algal adaptation to light, such as those involved in cell movement (phototaxis), chloroplast arrangement, or light-dependent differentiation. Aureochromes also contribute to optimizing photosynthesis under varying light conditions (Schellenberger Costa et al., 2013; Kroth et al., 2017; Matiiv and Chekunova, 2018).
Aureochromes possess a modular structure, combining sensor and regulatory functions within a single molecule (Mitra et al., 2012; Banerjee et al., 2016a; Heintz and Schlichting, 2016; Hepp et al., 2020). They consist of C-terminal light, oxygen, or voltage (LOV) domains and an N-terminal basic leucine zipper (bZIP) domain (Figure 6). The arrangement of the sensor and regulatory domains is therefore reversed compared to many other photoreceptors (Herman et al., 2013; Banerjee et al., 2016b; Kalvaitis et al., 2019; Coesel, 2024). The LOV domains are responsible for blue light perception, each binding a flavin mononucleotide (FMN) as a chromophore (Takahashi et al., 2007; Herman et al., 2013; Coesel, 2024). As in phototropins, LOV domains are composed of five antiparallel β-strands flanked by two α-helices (Mitra et al., 2012; Banerjee et al., 2016a; Heintz and Schlichting, 2016; Hepp et al., 2020), forming a specific binding pocket for the flavin chromophore. Upon absorption of blue light and formation of a light-induced covalent bond between a specific cysteine residue in the LOV domain and the FMN, a conformational change occurs, particularly affecting the Jα helix associated with the LOV domain. This activates the bZIP domain, which then binds DNA in a sequence-specific manner and functions as a transcription factor. The activated bZIP domain thus regulates the expression of target genes in a light-dependent manner (Mitra et al., 2012; Banerjee et al., 2016a; Heintz and Schlichting, 2016; Hepp et al., 2020).
Aureochromes were first identified in the alga Vaucheria frigida (Xanthophyta, Figure 4) (Takahashi et al., 2007; Matiiv and Chekunova, 2018), where they play an important role in the blue light-dependent regulation of sexual organ development and the induction of branching (Takahashi et al., 2007). Subsequently, aureochromes were also found in the diatoms (Figure 4) Phaeodactylum tricornutum (Figure 3), Thalassiosira pseudonana (Figure 3), and Thalassiosira oceanica; the eustigmatophyte (Figure 4) Nannochloropsis gaditana (Figure 3); the raphidophyte Chattonella antiqua; the golden-brown alga Ochromonas danica; and the brown algae (Figure 4) Ectocarpus siliculosus (Figure 3) and Fucus distichus, among others (Armbrust et al., 2004; Bowler et al., 2008; Ishikawa et al., 2009; Cock et al., 2010; Rayko et al., 2010; Lommer et al., 2012; Radakovits et al., 2012). A common feature of these organisms is that yellow-green algae (Xanthophyta), diatoms (Bacillariophyta), eustigmatophytes (Eustigmatophyta), raphidophytes (Raphidophyta), golden-brown algae (Chrysophyta), and brown algae (Phaeophyta) all belong to the stramenopiles. To date, aureochromes appear to be restricted to photosynthetic stramenopiles (ochrophytes) (Ishikawa et al., 2009; Takahashi, 2016; Kroth et al., 2017; Coesel, 2024; Im et al., 2024) and have not been detected in nonphotosynthetic stramenopile lineages such as oomycetes (e.g., Phytophthora), opalinids (e.g., Opalina), or bigyra (e.g., Blastocystis), nor in any other eukaryotic groups. This distribution suggests that aureochromes either evolved in the common ancestor of ochrophytes after secondary endosymbiosis with a red alga, or were lost early in nonphotosynthetic stramenopiles. The strict correlation with photosynthetic lineages is consistent with their role in regulating light-dependent morphogenetic and photosynthetic processes (Takahashi et al., 2007; Takahashi, 2016; Kroth et al., 2017; Madhuri et al., 2024; Zhang et al., 2024a).
5.1.3 Cryptochromes
Cryptochromes are flavin-based photoreceptors that primarily absorb in the UV-A and blue light ranges. They covalently bind flavin adenine dinucleotide (FAD) as a catalytic chromophore (Lin and Todo, 2005; Yu et al., 2010; Chaves et al., 2011; Losi and Gärtner, 2012; Essen et al., 2017; Kottke et al., 2017; Petersen et al., 2021; Deoliveira and Crane, 2024). However, depending on the redox state of the flavin, they can also absorb red light (Beel et al., 2012; Oldemeyer et al., 2019; Kiontke et al., 2020). Some cryptochromes are also capable of binding 5,10-methenyltetrahydrofolate (MTHF) or 8-hydroxy-7,8-didemethyl-5-deazariboflavin (8-HDF) as a second chromophore and antenna pigment (Sancar, 2003; Chaves et al., 2011). Cryptochromes, together with photolyases, form the cryptochrome/photolyase family (CPF), which includes cryptochrome photoreceptors as well as cyclobutane pyrimidine dimer (CPD) photolyases and 6–4 photolyases—light-dependent enzymes involved in the repair of UV-damaged DNA (Coesel et al., 2009; Heijde et al., 2010; Losi and Gärtner, 2012; Juhas et al., 2014; Franz et al., 2018; Yan et al., 2024). However, most cryptochromes lack DNA repair activity. Nevertheless, all cryptochromes possess a conserved photolyase homology region (PHR) and a C-terminal extension of varying length, known as the cry C-terminal extension (CCE) domain (Figure 6). The PHR domain binds the chromophore (Lin and Todo, 2005; Fraikin et al., 2023). Light absorption triggers a redox reaction in the chromophore (e.g., FAD), and the resulting conformational changes are transmitted to the CCE domain, which acts as the effector domain for signal transduction. The CCE domain interacts with specific target proteins and mediates cellular responses characteristic of the particular cryptochrome (Yu et al., 2010; Kondoh et al., 2011; Liu et al., 2016; Petersen et al., 2021). Cryptochromes are broadly categorized into plant-type, plant-like, animal-like, and DASH cryptochromes (Kottke et al., 2017; Rredhi et al., 2021; Shankar et al., 2022). The type and number of cryptochromes vary greatly among different algal species (Fortunato et al., 2015; Kottke et al., 2017).
Plant-type and plant-like cryptochromes, as the name suggests, closely resemble cryptochromes found in higher plants (Müller et al., 2017; Wang and Lin, 2025). Plant-type cryptochromes are primarily responsible for regulating growth processes and synchronizing circadian rhythms. They are also involved in the regulation of phototaxis and influence the expression of light-regulated genes. In Chlamydomonas, the blue light-regulated, plant-type cryptochrome pCRY (formerly designated CPH1) has been shown to regulate key aspects of the circadian clock and life cycle progression (Müller et al., 2017). Also noteworthy is a dual photoreceptor known as dualchrome, a chimeric protein that contains both a plant-type cryptochrome and a phytochrome domain (Makita et al., 2021). This orange/far-red and blue light photoreceptor originates from the marine picoplankton alga Pycnococcus provasolii.
Plant-like cryptochromes also show high similarity to plant cryptochromes but are distinguished by species-specific sequence characteristics. Plant-like cryptochromes are involved in entraining the circadian clock (Galvão and Fankhauser, 2015), synchronizing biological rhythms with day length and light availability to regulate processes such as cell division, photosynthesis, and growth. They may act directly or indirectly on the expression of genes associated with light-dependent processes. By upregulating or downregulating genes related to light utilization and protective mechanisms, such as during light stress, plant-like cryptochromes enable algae to adapt to changing light conditions. Moreover, plant-like cryptochromes can interact with other photoreceptors, such as phototropins and phytochromes, to allow responses to a broader spectrum of light (Figure 5) (Lin, 2002; Wang et al., 2014; D'amico-Damião and Carvalho, 2018; Wang and Lin, 2025).
Animal-like cryptochromes exhibit the highest sequence similarity—and thus the closest evolutionary relationship—to cryptochromes from animals (Zou et al., 2017; Mat et al., 2024). Animal-like cryptochromes are responsive to nearly the entire visible spectrum, including red light (Figure 5). These cryptochromes may activate signaling pathways that are particularly specific for the perception of light quality and light dynamics, whereas plant-like cryptochromes are more specialized for synchronization with the daylight cycle. Animal-like cryptochromes play a key role in the sexual life cycle and, in combination with plant cryptochromes, can serve as negative regulators of mating ability, in contrast to the function of phototropin (Zou et al., 2015). However, animal-like cryptochromes can positively regulate vegetative germination in algae. In Chlamydomonas, the animal-like cryptochrome aCRY modulates the light-dependent expression of various genes encoding proteins involved in chlorophyll and carotenoid biosynthesis, light-harvesting complexes, nitrogen metabolism, cell cycle control, and the circadian clock (Beel et al., 2012).
DASH-type cryptochromes (CRY-DASHs) are evolutionarily most closely related to 6–4 photolyases and animal cryptochromes, but their biological function remains uncertain. They have a maximal absorption peak in the UV-A range and use 5,10-methenyltetrahydrofolate (MTHF) as an antenna chromophore. In addition to their role as photoreceptors, they have been shown to repair photodamaged single-stranded and loop-structured double-stranded DNA in vitro (Selby and Sancar, 2006; Pokorny et al., 2008; Beel et al., 2012). As UV photoreceptors, they are also believed to serve an important function: DASH cryptochromes may act as UV-A sensors required to balance components of the photosynthetic machinery and support photoautotrophic growth (Rredhi et al., 2021). Since UV-A is not detected by the photochemical pigments of photosystem I and II, a central UV-A receptor in the chloroplast may be necessary to regulate the photosynthetic machinery independently of the chloroplast’s pigments, which mainly absorb in the blue and red regions of the visible spectrum (Rredhi et al., 2021).
5.1.4 BLUF proteins
BLUF (blue light using FAD) proteins are photoreceptors that primarily absorb light in the blue spectral range, typically at wavelengths around 430–500 nm (Figure 5). They non-covalently bind flavin adenine dinucleotide (FAD) as a catalytic chromophore (Gomelsky and Klug, 2002; Losi and Gärtner, 2012; Park and Tame, 2017; Losi et al., 2018; He et al., 2025a). BLUF proteins are found in certain algae, many bacteria, and some fungi. The blue light detected by BLUF proteins serves as a signal to control processes such as phototaxis, photomorphogenesis, and the regulation of cellular activity in response to light. These proteins may also be involved in regulating the expression of genes responsible for adaptation to light conditions, including genes for photosynthesis and protection against light stress (Park and Tame, 2017; Kaushik et al., 2019). A BLUF domain was first identified in the prokaryote Rhodobacter sphaeroides (Rhodobacterales), which belongs to the purple non-sulfur photosynthetic bacteria, where this domain regulates the expression of photosynthesis genes (Gomelsky and Kaplan, 1998; Gomelsky and Klug, 2002). In Euglena (Euglenozoa) (Figure 3, Figure 4), BLUF proteins are known to be involved in photophobic responses (Iseki et al., 2002). The central light-sensitive unit of BLUF proteins is the N-terminal BLUF domain, which contains FAD as the chromophore (Figure 6). The domain consists of a conserved ferredoxin-like β-α-β-β-α-β fold (Jung et al., 2005, 2006; Wu and Gardner, 2009; Kennis and Mathes, 2013), which is important for signal transduction (Fujisawa and Masuda, 2018). Absorption of blue light induces a conformational change in the BLUF domain (Mathes and Götze, 2015), characterized by a typical and reversible 10 nm red shift in the absorption spectrum, while the chromophore remains in the oxidized state. A C-terminal signal transduction (ST) domain serves as an effector domain for this conformational change and can function as an enzyme domain. The ST domain is responsible for interactions with other proteins (Mathes and Götze, 2015; Kaushik et al., 2019; Taguchi et al., 2024).
5.2 Retinal-based receptors
5.2.1 Rhodopsins
Rhodopsins, also known as retinylidene proteins, are retinal-based, membrane-bound photoreceptors that can be sensitive to wavelengths ranging from violet to orange light (Figure 5) (Nathans, 1992; Zhang et al., 2011; Nagata and Inoue, 2021; Rozenberg et al., 2021; De Grip and Ganapathy, 2022). However, the majority of algal rhodopsins absorb light in the 450–550 nm range (blue to green light) (Hegemann, 2008; Kato et al., 2015; Mcisaac et al., 2015). The absorption spectra of different rhodopsins vary due to subtle differences in the structure of the retinal chromophore or the surrounding protein environment. Rhodopsins are found in all three domains of life: bacteria, archaea, and eukarya. They are involved in a range of essential light-dependent processes, including phototaxis, circadian rhythms, regulation of the cell cycle, and stress responses. Rhodopsins can function as monomers, but they frequently also form dimers or oligomers. Retinal forms a covalent linkage via a protonated Schiff base with a conserved lysine located in transmembrane helix 7 (TM7). Based on their retinal configuration, rhodopsins are divided into two major groups: type 1 (microbial rhodopsins) and type 2 (animal rhodopsins). In type 1 rhodopsins, the protein environment is evolutionarily optimized for light-induced retinal isomerization from all-trans to 13-cis. In contrast, type 2 rhodopsins are optimized for isomerization from 11-cis to all-trans (Nakanishi, 1991; Spudich et al., 2000). The photon-absorbing retinal chromophore, which is the aldehyde form of vitamin A, is derived from cleaved β-carotene and is covalently linked to the opsin apoprotein (Spudich et al., 2000). When light strikes the retinal, photoisomerization occurs. This conformational change stores the energy of the photon and transfers it as mechanical energy to the opsin apoprotein, initiating downstream reactions. All rhodopsins share a common architecture of seven transmembrane α-helices, with the N-termini oriented outward and the C-termini facing inward (Soppa, 1994; Spudich et al., 2000; Pierce et al., 2002). The retinal chromophore is attached via a Schiff base linkage to the ϵ-amino group of a lysine residue located in the middle of the seventh transmembrane helix. Typically, the retinal Schiff base is protonated and exists in the 15-anti configuration. Changes in the protonation state are essential for the signaling or transport activities of rhodopsins. Despite these structural similarities, it is notable that type 1 and type 2 rhodopsins show no recognizable sequence similarity, as they are the result of convergent evolution (Soppa, 1994; Kojima and Sudo, 2023).
Type-2 rhodopsins (animal-type) are mentioned here only for the sake of completeness. They are characteristic of animals and have not been detected in any algal lineage, including dinoflagellates. These rhodopsins, which have been known the longest, belong to the superfamily of G-protein-coupled receptors (GPCRs) and were first identified as the visual pigment in the rod photoreceptor cells of vertebrate eyes (Spudich et al., 2000; Pierce et al., 2002; Palczewski, 2006; Rosenbaum et al., 2009; Mat et al., 2024). Unlike most GPCRs, which are activated by the binding of small ligands, rhodopsins are activated by the light-induced isomerization of their chromophore, which in turn initiates the G-protein-mediated signaling cascade. The membrane-bound rhodopsin thus serves as a sensor that activates transmembrane or soluble transducers. In addition to their role in visual phototransduction, type-2 rhodopsins are also involved in non-visual phototransduction, regulation of the circadian clock, and as enzymes that catalyze the isomerization of photopigments (photoisomerases) (Shichida and Matsuyama, 2009; Schmidt et al., 2011).
Type-1 rhodopsins (microbial-type) (Figure 7) are found in algae as well as in bacteria, fungi, archaea, protists (such as choanoflagellates), and even viruses (Ruiz-González and Marín, 2004; Grote et al., 2014; Govorunova et al., 2017; Zabelskii et al., 2020; Nagata and Inoue, 2021; Rozenberg et al., 2021; De Grip and Ganapathy, 2022; Govorunova et al., 2022; Govorunova and Sineshchekov, 2023; Vlasova et al., 2024). Because they were originally discovered in archaea (Halobacterium), they were formerly referred to as archaeal rhodopsins (Oesterhelt and Stoeckenius, 1971).
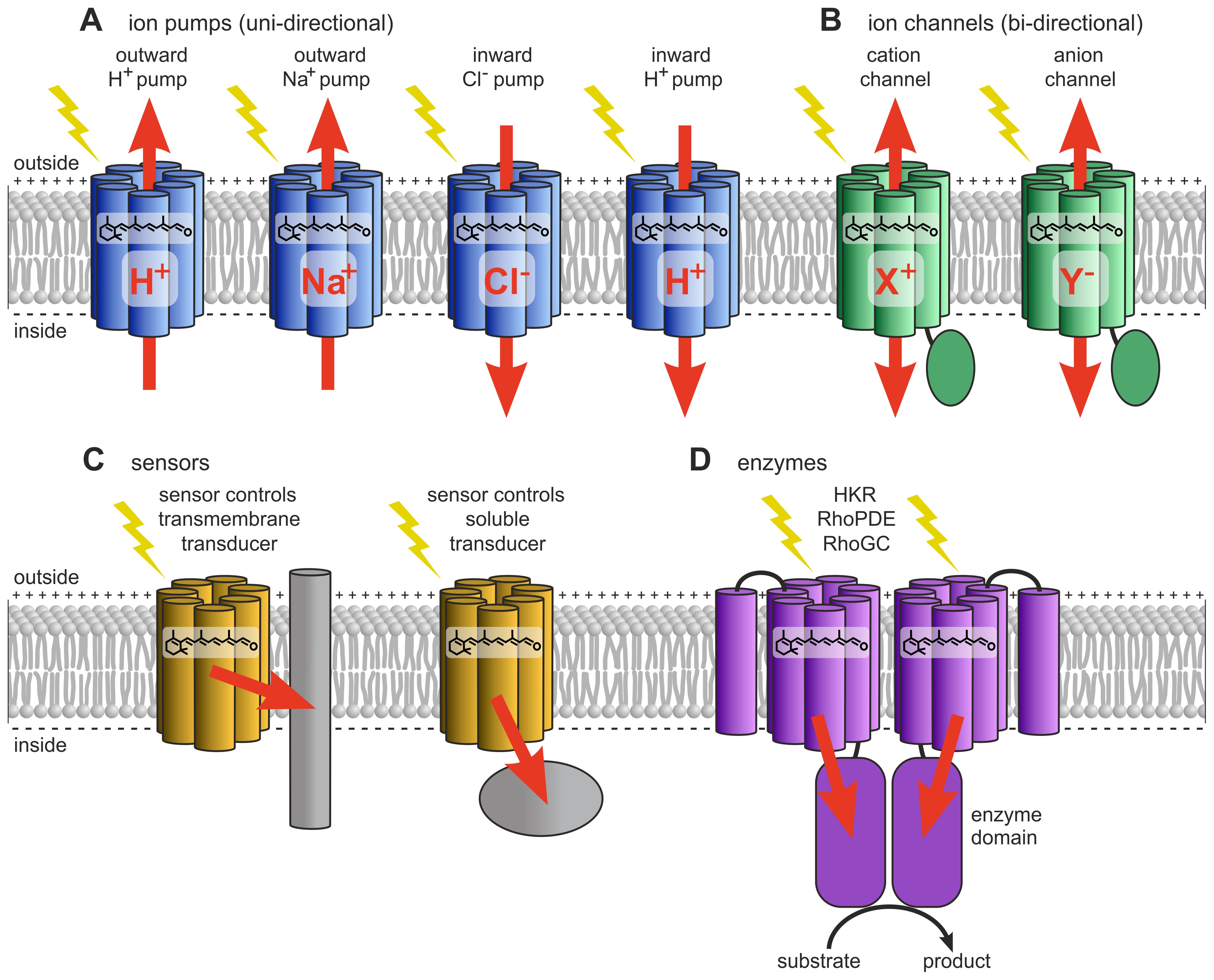
Figure 7. Type-1 rhodopsins (microbial type). (A) Ion-pumping rhodopsins include outward and inward-directed proton pumps, outward sodium ion pumps, and inward chloride ion pumps. (B) Ion-channeling rhodopsins comprise cation channelrhodopsins (CCRs) and anion channelrhodopsins (ACRs). (C) Sensory rhodopsins regulate transmembrane or soluble cytosolic transducers. (D) Enzyme rhodopsins include histidine kinase rhodopsins (HKRs), rhodopsin phosphodiesterases (RhoPDEs), and rhodopsin guanylyl cyclases (RhoGCs). Redrawn and modified after Zhang et al. (2011); Rozenberg et al. (2021); Tsunoda et al. (2021).
In algae, type-1 rhodopsins are widespread, and each algal species appears to possess a whole series of these rhodopsins. For example, at least seven rhodopsin-based photoreceptors have been identified in each of the green algae Chlamydomonas reinhardtii and Volvox carteri (Hegemann, 2008; Kianianmomeni and Hallmann, 2014; 2015b). Dinoflagellates likewise encode multiple type-1 rhodopsins (often termed proteorhodopsins or rhodopsin-like proteins), which are present in both photosynthetic (e.g., Prorocentrum) and heterotrophic (e.g., Oxyrrhis) species and are thought to function in light-driven proton pumping, sensory roles, and possibly energy supplementation (Ruiz-González and Marín, 2004; Slamovits et al., 2011; Shi et al., 2015).
Based on their biological function, type-1 rhodopsins can be classified as light-dependent outward and inward ion pumps, ion channels, sensory rhodopsins, and enzyme rhodopsins (Figure 7) (Klare et al., 2008; Ernst et al., 2014; Rozenberg et al., 2021).
Light-driven ion pumps transport ions in a single direction (Figure 7). They are subdivided into outward and inward-directed proton pumps, outward sodium ion pumps, and inward chloride ion pumps (Kandori, 2015).
Light-driven outward-directed proton pumps (Figure 7), a type of cation pump, are typically involved in energy generation (Oesterhelt and Stoeckenius, 1973; Inoue et al., 2016). In proton pumps such as bacteriorhodopsin (from Halobacterium) and proteorhodopsin (from marine bacteria), light energy is used to pump protons out of the cell, thereby creating an electrochemical proton gradient across the membrane. This gradient is then utilized by ATP synthase to produce ATP as the protons flow back into the cell.
Ion-transporting rhodopsins also include the less common inward-directed proton pumps. Schizorhodopsins (SzRs) are examples of such light-driven inward proton pumps (Inoue et al., 2020; Brown, 2022). They have been found in Schizochytrium (Thraustochytriaceae) and other members of the Thraustochytriaceae, which are unicellular heterotrophic marine protists of the Stramenopile group, often considered non-photosynthetic microalgae. These inward proton pumps are also present in Asgard archaea, a superphylum that appears to include the closest archaeal relatives of eukaryotes.
Additionally, there are rare xenorhodopsins (XeRs), which transport protons inward while simultaneously transporting anionic substrates (e.g., Cl-, NO3-, or other negative ions) outward, i.e., in the opposite direction (Shevchenko et al., 2017; Inoue et al., 2018; Weissbecker et al., 2021; Brown, 2022).
Light-driven outward-directed sodium ion pumps (Figure 7) (Inoue et al., 2013), another type of cation pump, generate a sodium gradient across the membrane. The resulting electrochemical gradient can be utilized for various physiological processes, such as ATP synthesis or osmotic adaptation under extreme environmental conditions. The characterization of the light-driven sodium pump from Krokinobacter eikastus, rhodopsin 2 (KR2), demonstrated for the first time that light-driven cation transport is not limited to protons (Inoue et al., 2013).
Light-driven inward-directed chloride ion pumps (Figure 7) (Mukohata and Kaji, 1981), a type of anion pump, are found, for example, in the halorhodopsins of halophilic archaea and bacteria. These pumps actively transport chloride ions across cell membranes, contributing to the maintenance of osmotic balance and also playing a role in energy production.
Light-regulated ion channels (Figure 7) enable cells to adapt to changing light conditions (Spalding, 2000). Typically, ion channels allow ions to passively flow down their concentration gradient across the membrane, meaning that ions can move in both directions. This group includes cation channelrhodopsins (CCRs), bacteriorhodopsin-like cation channelrhodopsins, and anion channelrhodopsins (ACRs).
Light-gated cation channelrhodopsins (CCRs) (Figure 7, Figure 6) (Nagel et al., 2002, 2003) selectively permit the passage of H+, Na+, K+, and Ca2+ ions in response to light. Illumination, particularly in the blue spectrum, induces a conformational change in the retinal cofactor within the protein. This change opens the channel, allowing cations to flow bidirectionally along their electrochemical gradient. In the chlorophyte Chlamydomonas, two plasma membrane-localized channelrhodopsins, ChR1 and ChR2, regulate positive and negative phototaxis as well as photophobic responses (Ridge, 2002; Sineshchekov et al., 2002; Berthold et al., 2008). ChR1 and ChR2 were the first channelrhodopsins to be discovered (Nagel et al., 2002, 2003). ChR1 has an absorption maximum at 480 nm (blue) and is selective for protons, whereas ChR2 has a similar absorption maximum at 470 nm (blue) but displays broader specificity, being conductive for monovalent and divalent cations such as Na+, K+, and Ca2+. These channelrhodopsins are localized in the plasma membrane above the eyespot (Foster and Smyth, 1980; Foster et al., 1984). Light-induced cation influx causes depolarization of the plasma membrane at the eyespot (Berthold et al., 2008). This depolarization is then transmitted to the peri-flagellar plasma membrane, where it activates voltage-gated calcium channels (VGCCs), resulting in an immediate calcium influx and an increase in intracellular Ca2+ concentration (Berthold et al., 2008). The local rise in Ca2+ ions in the peri-flagellar membrane region is crucial for regulating flagellar movement by modulating the beating frequency and angle of the flagella (Kamiya and Witman, 1984). This process enables directed movement of the alga toward a light source (positive phototaxis) or away from it (negative phototaxis) (Rüffer and Nultsch, 1998; Isogai et al., 2000; Wakabayashi et al., 2021). Photophobic responses (light avoidance behaviors) are triggered by sudden strong illumination or abrupt changes in light intensity, where a pronounced Ca2+ influx leads to a rapid alteration in flagellar activity, allowing the alga to quickly correct its movement (Hyams and Borisy, 1978; Bessen et al., 1980; Fujiu et al., 2009). The influx of cations, particularly protons, also lowers intracellular pH, thereby regulating enzymatic activities and other cellular responses.
Relatives of ChR1 and ChR2 from Chlamydomonas reinhardtii have been identified in several other chlorophytes (Figure 8), including VChR1 and VChR2 in Volvox carteri, Chrimson (also known as CnChR) in C. noctigama, Chronos (ShChR) in Stigeoclonium helveticum, CoChR in Chloromonas oogama, TsChR in Tetraselmis striata, TcChR in T. cordiformis, SdChR in Scherffelia dubia, MChR in Mesostigma viride, CsChR in Chloromonas subdivisa, AgChR in Asteromonas gracillis-B, NsChR in Neochlorosarcina sp., BsChR in Brachiomonas submarina, CbChR in C. bilatus-A, PsChR in Proteomonas sulcata, PsChR in Platymonas subcordiformis, HpChR1 in Haematococcus pluvialis, HdChR in H. droebakensis, CaChR1 in C. augustae, CyChR1 in C. yellowstonensis, and CraChR2 in C. raudensis (Kianianmomeni et al., 2009; Govorunova et al., 2011; Mattis et al., 2011; Govorunova et al., 2013; Chuong et al., 2014; Klapoetke et al., 2014; Venkatachalam and Cohen, 2014; Kim et al., 2015; Olofsson et al., 2015; Wietek and Prigge, 2016; Bi et al., 2022; Rindner and Lur, 2023; Vierock and Hegemann, 2023). There are a few reports in algae on cation channelrhodopsins with naturally increased selectivity for specific ions that would be of interest for optogenetic applications. One example is the enhanced sodium conductance of PsChR in Tetraselmis subcordiformis (Sineshchekov et al., 2013; Coli et al., 2024).
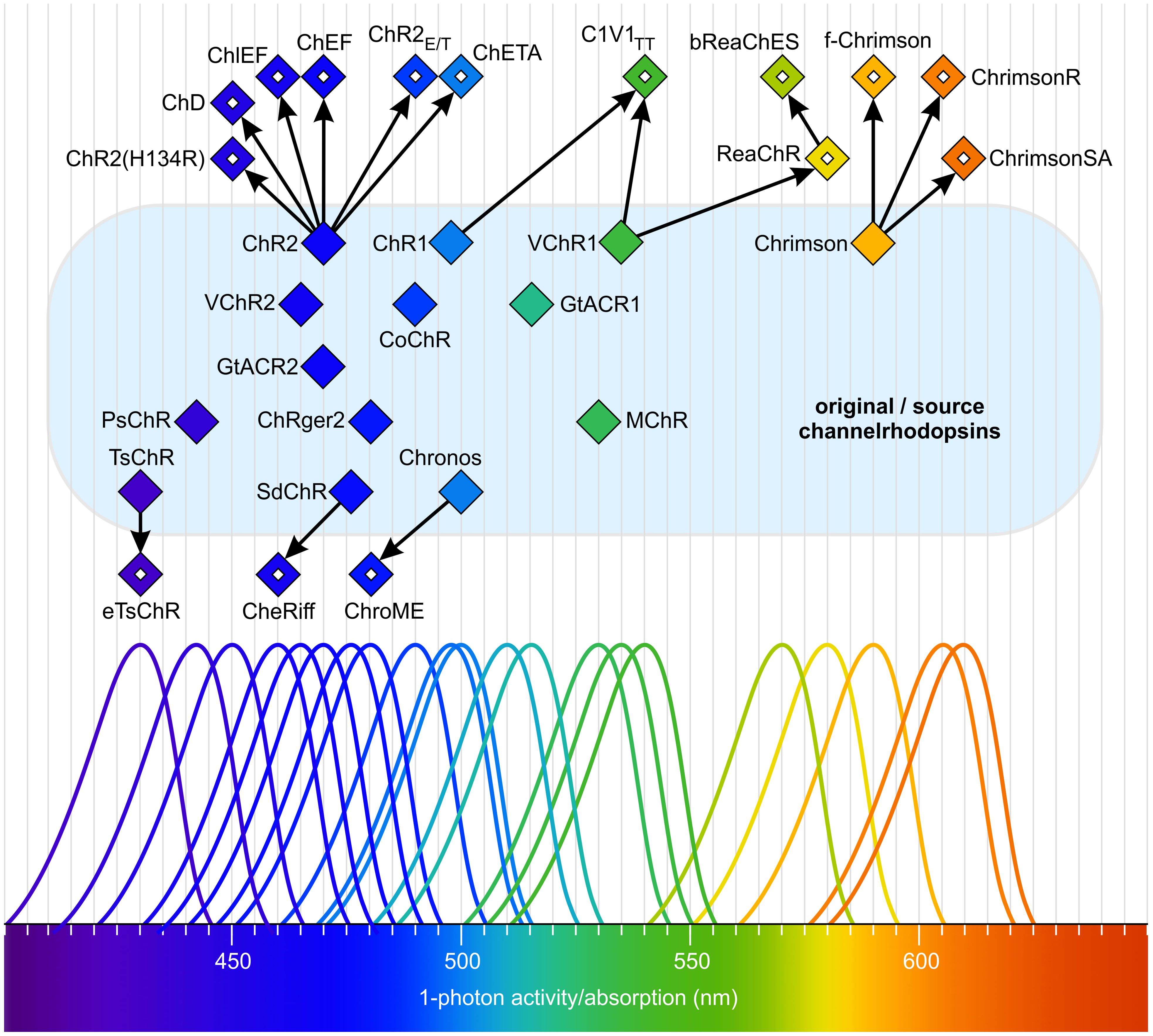
Figure 8. Natural and mutated channelrhodopsins span a wide range of the visible spectrum. Filled diamonds indicate the absorption maxima and single-photon activity of natural channelrhodopsins. Open diamonds represent the absorption maxima and single-photon activity of mutant channelrhodopsins derived from the original forms. All natural (original/source) channelrhodopsins are located on a light blue surface. Arrows indicate the relationships between origins. The schematic absorption spectra are intended to highlight the maxima and to illustrate the approximate portion of the total spectrum covered. Redrawn, modified, and supplemented after Mattis et al. (2011); Chuong et al. (2014); Klapoetke et al. (2014); Venkatachalam and Cohen (2014); Kim et al. (2015); Olofsson et al. (2015); Wietek and Prigge (2016); Rindner and Lur (2023); Vierock and Hegemann (2023).
Cation channelrhodopsins from cryptophyte algae (Cryptophyta, Figure 4) differ from the light-gated cation channelrhodopsins described in chlorophytes (Govorunova et al., 2016; Shigemura et al., 2019). These unique channelrhodopsins are more homologous to haloarchaeal rhodopsins, such as the proton-pumping bacteriorhodopsin, than to chlorophyte channelrhodopsins (Govorunova et al., 2016; Shigemura et al., 2019). Furthermore, chlorophyte and cryptophyte channelrhodopsins exhibit different structural features, indicating that cation channelrhodopsins have evolved independently through convergent evolution.
Light-gated anion channelrhodopsins (ACRs) (Figure 7) (Govorunova et al., 2015; Sineshchekov et al., 2015) allow the passage of NO3- and Cl- ions in response to light. These channels also exhibit some conductivity for Br- and I- ions. The best-studied anion channelrhodopsins are GtACR1 and GtACR2 from the marine alga Guillardia theta (Cryptophyta, Figure 4) (Govorunova et al., 2015), with absorption maxima in the blue region of the light spectrum (515 nm and 470 nm, respectively) (Figure 8). Both have been shown to mediate the gating of Cl- ions, and for GtACR1, the passage of NO3- appears to be its natural function (Ohki et al., 2023).
Type-1 rhodopsins also include sensory rhodopsins (Figure 7), which are not involved in ion transport across membranes. Instead, sensory rhodopsins function as photoreceptors, signaling via a specific transmembrane or soluble cytoplasmic transducer protein that is functionally and structurally distinct from the animal G-proteins associated with type-2 rhodopsins (Bogomolni and Spudich, 1982; Krah et al., 1994; Hoff et al., 1997; Spudich, 2013). Sensory rhodopsin I (SRI) was the first light sensor discovered in a microorganism, specifically in the archaeon Halobacterium (Bogomolni and Spudich, 1982). It acts as a phototaxis receptor, modulating swimming behavior in response to light intensity gradients (Bogomolni and Spudich, 1982; Spudich, 2013).
Enzyme rhodopsins (Figure 7, Figure 6) combine the properties of light-sensitive retinal-binding domains with enzymatic activity and are found in eukaryotes such as fungi, green algae, and choanoflagellates (Avelar et al., 2014; Lamarche et al., 2017; Yoshida et al., 2017; Scheib et al., 2018; Tian et al., 2018a, 2018; Luck et al., 2019; Mukherjee et al., 2019; Ikuta et al., 2020; Sugiura et al., 2020; Broser, 2021; Tsunoda et al., 2021; Tian et al., 2022). Dimerization appears to be necessary for enzyme rhodopsins, and they lack both pumping and channel activities. Unusually, enzyme rhodopsins possess not seven, but eight transmembrane helices, with the extra helix (TM0) located at the N-terminus of the rhodopsin domain. Upon photon absorption, the retinal undergoes a conformational change, initiating light-regulated intramolecular signaling that subsequently controls an associated enzyme, thereby triggering downstream biochemical processes. The evolutionary origin of enzyme rhodopsins is thought to involve gene fusion events, where an ancestral rhodopsin gene was combined with genes encoding various enzymatic domains. There are essentially three families of enzyme rhodopsins: histidine kinase rhodopsins (HKRs), rhodopsin phosphodiesterases (RhoPDEs), and rhodopsin guanylyl cyclases (RhoGCs) (Luck et al., 2012; Avelar et al., 2014; Govorunova et al., 2017; Yoshida et al., 2017). In rhodopsin guanylyl cyclases and rhodopsin phosphodiesterases, light activates the enzyme activity, whereas in histidine kinase rhodopsins, light inhibits activity. These enzyme rhodopsins regulate the concentration of cyclic nucleotides by catalyzing their synthesis or degradation (Avelar et al., 2014; Yoshida et al., 2017; Scheib et al., 2018; Mukherjee et al., 2019; Ikuta et al., 2020; Sugiura et al., 2020). Cyclic nucleotides can act as secondary messengers in gene expression by activating transcription factors (Mcdonough and Rodriguez, 2011), and they are frequently involved in the regulation of cell type-specific gene expression during developmental processes (Shaulsky and Huang, 2005). In the alga Ostreococcus (Prasinodermophyta, Figure 4), light-dependent changes in cAMP levels affect the biosynthesis of cyclin A, which in turn interacts with retinoblastoma protein (RB) to regulate the cell cycle (Moulager et al., 2010). In Chlamydomonas, a correlation has also been demonstrated between rhodopsin activation and cAMP levels (Boonyareth et al., 2009).
Histidine kinase rhodopsins (HKRs) are light-regulated, ATP-dependent hybrid histidine kinase systems resembling two-component signaling pathways, some of which contain a guanylyl cyclase effector domain (Tian et al., 2018a). Light is detected by the rhodopsin (opsin, OPS) (Figure 6), which subsequently inhibits the activity of the histidine kinase domain (H kinase). In the absence of this inhibition, the histidine kinase domain undergoes autophosphorylation using a phosphoryl group from ATP. Following autophosphorylation, the kinase transfers the phosphoryl group to a response regulator domain (RR). The response regulator domain can interact with either attached or independent effector domains. In HKRs that possess an attached guanylyl cyclase domain (GC), the response regulator intramolecularly activates the GC domain, leading to the production of cGMP from GTP (Figure 6). The cGMP then serves as an effector molecule, triggering various cellular processes. A variety of other output responses can also be controlled through the response regulator domains (Galperin, 2006).
Rhodopsin phosphodiesterases (RhoPDEs) catalyze the hydrolysis of cAMP and cGMP to AMP and GMP, respectively, in a light-dependent manner (Watari et al., 2019; Ikuta et al., 2020; Sugiura et al., 2020). The N-terminal rhodopsin domain is linked to a C-terminal phosphodiesterase domain. Light-dependent isomerization of retinal induces a conformational change in the rhodopsin, which activates the catalytic domain of the phosphodiesterase. In this way, the degradation—and thus the intracellular concentrations—of the secondary messengers cAMP and cGMP can be dynamically regulated in response to light (Watari et al., 2019; Ikuta et al., 2020; Sugiura et al., 2020).
In rhodopsin guanylyl cyclases (RhoGCs), the N-terminal rhodopsin domain is directly linked to a C-terminal guanylyl cyclase (GC) domain (Avelar et al., 2014; Scheib et al., 2018; Tian et al., 2018a; Fischer et al., 2021). The first enzyme rhodopsin with confirmed enzymatic activity was a RhoGC identified in the fungus Blastocladiella emersonii (Saranak and Foster, 1997). Light-induced isomerization of retinal triggers a conformational change in the rhodopsin, thereby activating the guanylyl cyclase to produce cGMP from GTP (Avelar et al., 2014; Scheib et al., 2018; Tian et al., 2018a; Fischer et al., 2021).
There are also additional subclasses of rhodopsins, including heliorhodopsins, xanthorhodopsins, fungal rhodopsins, and viral rhodopsins (Rozenberg et al., 2021), which will not be discussed further here. Figure 7 illustrates the main mechanisms of rhodopsins.
In algae, numerous light-controlled channels, pumps, and enzymes from the group of type-1 (microbial-type) rhodopsins have already been identified. Given the broad distribution of rhodopsins in algae and the large number of still poorly characterized algal species, it is likely that additional interesting rhodopsins will be discovered in the future.
5.3 Tetrapyrrole-based receptors
5.3.1 Phytochromes
Phytochromes are highly conserved and widely distributed multi-domain proteins (Figure 6). These photoreceptors are present not only in algae, but also in plants, fungi, and bacteria (Ulijasz and Vierstra, 2011; Duanmu et al., 2014; Rockwell et al., 2014; Anders and Essen, 2015; Li et al., 2015a; Hughes and Winkler, 2024). For a long time, phytochromes were known only as red light photoreceptors, which could be reversibly switched between active and inactive states by a change in color from red to far-red light (Chen and Chory, 2011). However, over time, a wide range of phytochromes with different absorption spectra has been identified (Figure 5). In algae, phytochromes collectively cover the entire visible light spectrum (Figure 9) (Rockwell et al., 2014; Rockwell and Lagarias, 2020). As with all photoreceptors, but especially for phytochromes, an important ecological consideration is that the further the absorption maximum is shifted into the long-wavelength (red/far-red) range, the closer to the water surface the respective alga must live for that wavelength to be available (Figure 2) and thus detectable.
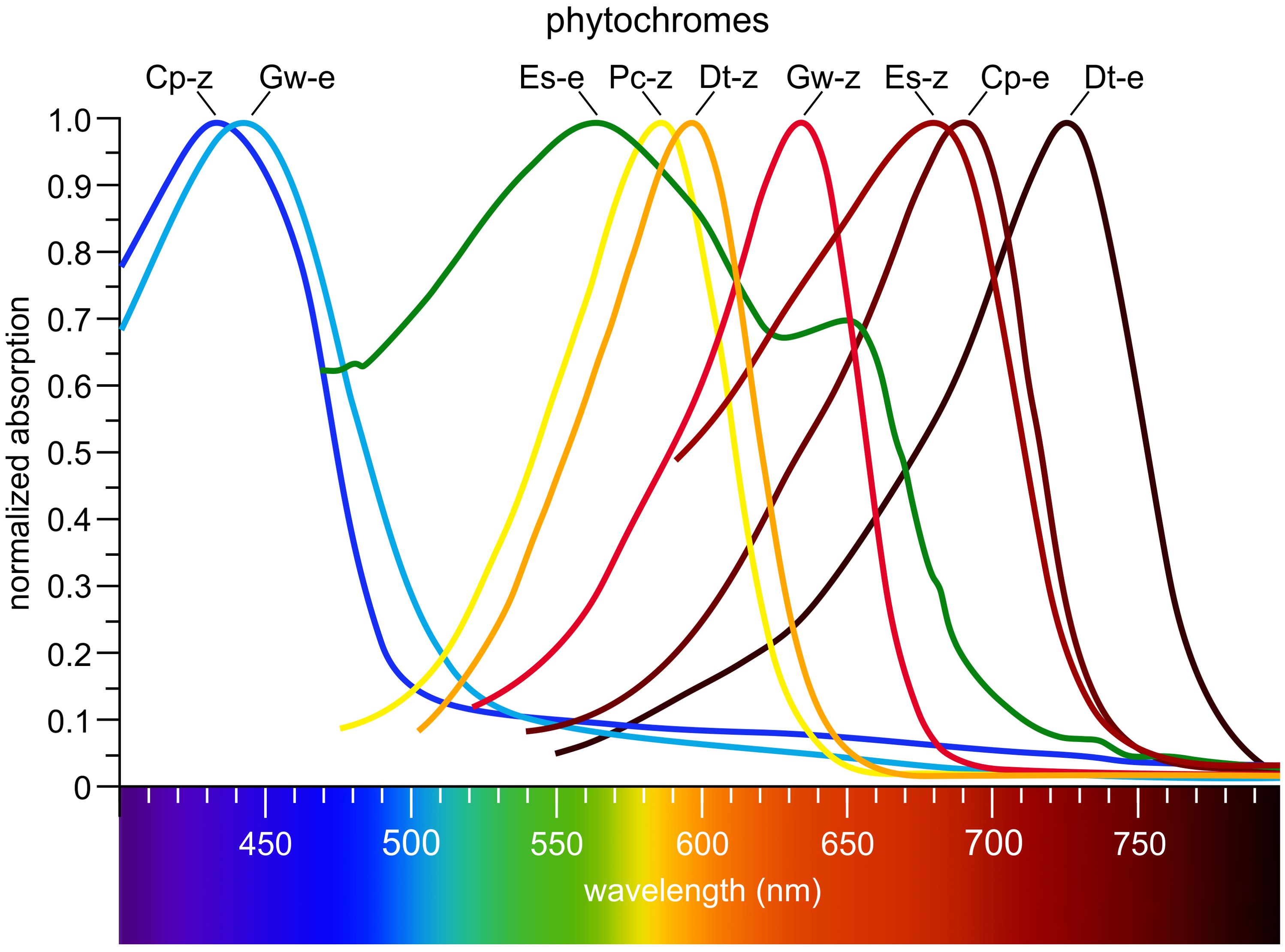
Figure 9. Algal phytochromes span the entire visible spectrum. Cp-z: Cyanophora paradoxa (Glaucophyta, Figure 4), 15Z CparGPS1; Gw-e: Gloeochaete wittrockiana (Glaucophyta), 15E GwitGPS1; Es-e: Ectocarpus siliculosus (Phaeophyta), 15E EsilPHL1; Pc-z: Prasinoderma coloniale (Prasinodermophyta), 15Z PcolPHY1; Dt-z: Dolichomastix tenuilepis (Chlorophyta), 15Z DtenPHY1; Gw-z: Gloeochaete wittrockiana (Glaucophyta), 15Z GwitGPS1; Es-z: Ectocarpus siliculosus (Phaeophyta), 15Z EsilPHL1; Cp-e: Cyanophora paradoxa (Glaucophyta), 15E CparGPS1; Dt-e: Dolichomastix tenuilepis (Chlorophyta), 15E DtenPHY1. Redrawn and modified after Rockwell et al. (2014). Phylogenetic positions are shown in Figure 4.
Phytochromes possess a characteristic cGMP phosphodiesterase/adenyl cyclase Fh1A (GAF) domain (Figure 6) that accommodates an open-chain tetrapyrrole chromophore. Unlike higher plants and mosses, which employ phytochromobilin (PΦB), green algal phytochromes utilize phycocyanobilin (PCB) (Figure 6) (Wu et al., 1997; Jorissen et al., 2002; Duanmu et al., 2013, 2017; Rockwell and Lagarias, 2017; Frascogna et al., 2023). The GAF domain mediates reversible photochromic transitions between two stable conformational states, most prominently the red light-absorbing Pr state and the far-red light-absorbing Pfr state. In most phytochromes, Pr represents the dark-adapted ground state, whereas Pfr is the photoactivated and often biologically active state (Franklin and Quail, 2010; Chen and Chory, 2011). This photoconversion enables organisms to discriminate between full sunlight and shaded conditions: in sunlight, phytochromes absorb red light and accumulate in the Pfr state, whereas in shade, where red light is depleted and far-red light is enriched, the spectral shift signals competition from neighboring photosynthetic organisms and promotes reversion to Pr either through far-red photoconversion or thermal relaxation in darkness. Beyond the red/far-red light pair, additional spectral pairs such as ultraviolet/blue, blue/green, or blue/orange have been described. A few phytochromes even adopt Pfr as the dark-adapted state, requiring far-red light for conversion back to Pr (Burgie and Vierstra, 2014; Rockwell and Lagarias, 2017). To date, however, no such Pfr-dark-adapted phytochromes (15E configuration) have been identified in algae. Classical plant and algal phytochromes instead adopt Pr (15Z configuration) in darkness and convert to Pfr upon red light absorption (Sharrock, 2008; Rockwell et al., 2014; Rockwell and Lagarias, 2020). Inverse dark-state configurations have thus far been reported only in certain bacterial phytochromes, most notably in the Pseudomonas phytochrome, which predominantly resides in Pfr in darkness (Yang et al., 2008; Rottwinkel et al., 2010; Rockwell and Lagarias, 2020; Huber et al., 2024).
In addition to the GAF domain, phytochromes contain several other domains (Figure 6) (Rockwell et al., 2006; Essen et al., 2008; Sharrock, 2008; Burgie and Vierstra, 2014). The phytochrome-specific PHY domain enhances the stability of chromophore binding, supports photoconversion by structurally stabilizing the GAF domain, and is essential for light perception and efficient switching between active and inactive states. A key structural feature of the PHY domain is the so-called tongue motif, a flexible loop that folds back onto the chromophore-binding pocket (Essen et al., 2008; Anders et al., 2013; Burgie and Vierstra, 2014). Upon photoconversion, the tongue undergoes pronounced secondary-structure transitions (typically from β-sheet to α-helix or vice versa), which represent one of the most prominent light-induced conformational changes in phytochromes. These rearrangements are thought to play a central role in transmitting the structural signal from the chromophore to the adjoining PHY and output domains, thereby linking local photochemistry to large-scale protein reorganization and signal propagation (Essen et al., 2008; Anders et al., 2013; Burgie and Vierstra, 2014). Together, these features establish the PHY domain as a central mediator between the chromophore-binding pocket and the downstream signaling machinery.
The PER-ARNT-SIM (PAS) domain, named after the proteins PER, ARNT, and SIM in which it was first described, is a versatile sensor and interaction module that occurs in many signaling proteins (Rockwell et al., 2006; Möglich et al., 2010; Burgie and Vierstra, 2014). In the N-terminal photosensor module of phytochromes, a PAS domain is tightly integrated into a compact, knotted architecture together with the GAF and PHY domains. This PAS–GAF–PHY unit harbors the bilin chromophore and mediates photoconversion (Rockwell et al., 2006; Burgie and Vierstra, 2014). By contrast, many eukaryotic phytochromes also possess one or more PAS repeats in their C-terminal region. These C-terminal PAS domains are structurally and functionally distinct from the N-terminal PAS domain and are thought to contribute primarily to dimerization, signal transmission, and interactions with downstream signaling partners (Essen et al., 2008; Sharrock, 2008; Burgie and Vierstra, 2014). The histidine kinase (H kinase) domain represents the signal transduction domain, often containing an autophosphorylating histidine residue, and ultimately transmits signals through phosphorylation to downstream targets such as transcription factors. Like other photoreceptors, phytochromes orchestrate a complex signaling network (Cheng et al., 2021; Han et al., 2024).
5.4 Hybrid receptors
5.4.1 Neochromes
Neochromes are chimeric photoreceptors composed of six domains organized into two larger units (Nozue et al., 1998; Suetsugu et al., 2005; Kagawa and Suetsugu, 2007; Kong and Okajima, 2016; Parihar et al., 2016). The first unit comprises the three N-terminal domains and is structurally similar to phytochromes. The second unit consists of the three C-terminal domains and resembles phototropins. The three most N-terminal domains form a sensory module (Figure 6): these are the PER-ARNT-SIM (PAS) domain, the cGMP phosphodiesterase/adenyl cyclase Fh1A (GAF) domain, and a phytochrome-specific (PHY) domain, all typically found in phytochromes. The PAS domain mediates protein-protein interactions, contributes to protein dimerization, stabilizes the structure, and acts as a sensor for environmental stimuli such as physical or chemical signals. The GAF domain binds a phycocyanobilin chromophore, as in phytochromes, enabling the detection of (red) light signals. The PHY domain stabilizes the GAF domain, enhances chromophore binding, and supports photoconversion and effective light perception (Suetsugu et al., 2005; Kong and Okajima, 2016; Parihar et al., 2016).
The three most C-terminal domains form a phototropic region (Figure 6): these are two LOV domains (LOV1 and LOV2), which use flavin mononucleotide (FMN) as their chromophore, and a serine/threonine kinase (S/T kinase) domain (Shankar et al., 2022). The LOV domains absorb blue light; LOV1 also modulates interactions with LOV2 and other domains. Upon light absorption, LOV2 undergoes conformational changes that transmit signals to other parts of the protein, such as the S/T kinase domain. The S/T kinase domain catalyzes the phosphorylation of target proteins at serine or threonine residues, thereby regulating interactions with target proteins and initiating specific cellular responses (Suetsugu et al., 2005; Shankar et al., 2022).
Overall, neochromes are sensitive to red, far-red, and blue light (Figure 5), and they exhibit red/far-red reversibility. Neochromes were first identified in the green filamentous zygnematophycean alga Mougeotia (Charophyta, Figure 4), where they play an important role in regulating chloroplast movement in response to blue light stimuli (Suetsugu et al., 2005; Li et al., 2015a). Neochromes are found in zygnematalean algae and, outside of algae, in ferns and hornworts (Suetsugu et al., 2005; Li et al., 2014, 2015). Phylogenetic evidence indicates multiple independent origins of neochromes, with domain fusion as the underlying mechanism and horizontal gene transfer (HGT) shaping their distribution across lineages (Li et al., 2014, 2015, 2015; Li and Mathews, 2016).
5.5 Proteins with UV absorption
5.5.1 UV-B photoreceptors
Ultraviolet-B (UV-B) radiation (280–315 nm) (Figure 5) is potentially harmful to all living organisms (Yin and Ulm, 2017). High doses of UV-B radiation can damage DNA and other macromolecules, induce the production of reactive oxygen species, and negatively affect cell viability (Brosché and Strid, 2003; Frohnmeyer and Staiger, 2003). However, UV-B radiation also serves as a signal that triggers various physiological responses. In algae, UV-B photoreceptors play a key role in sensing and responding to UV-B exposure. UVR8 (UV Resistance Locus 8) is the most important UV-B photoreceptor, found in both plants and algae (Rizzini et al., 2011; Tilbrook et al., 2013; Jenkins, 2014; Podolec et al., 2021; Depaepe et al., 2023). UVR8 exists as a homodimer that undergoes a conformational change upon UV-B exposure, resulting in dissociation into monomers, which in turn initiate downstream UV-B signaling events (Christie et al., 2012; Li et al., 2022; Depaepe et al., 2023). Before this conformational change, the homodimer is stabilized by interprotein interactions involving salt bridges, hydrogen bond networks, and hydrophobic clusters with entrapped water molecules (Li et al., 2022). The UV-B-dependent activation and dissociation of the dimer is unique because it does not require external chromophores (Figure 6); instead, tryptophan residues within the UVR8 polypeptide absorb the UV-B photons (Rai et al., 2019; Li et al., 2022). Upon activation, UVR8 monomers interact with signaling partners such as COP1 (CONSTITUTIVE PHOTOMORPHOGENIC 1), leading to the regulation of UV-B-responsive genes (Podolec et al., 2021). COP1, like UVR8, is highly conserved and is found not only in algae but also in plants and animals, including humans (Zhang et al., 2023). The UV-B-induced dissociation of the UVR8 dimer is reversible; the monomers spontaneously reassemble within hours, allowing the dimer to respond to UV-B again (Christie et al., 2012).
UV-B radiation detected by UVR8 also activates protective mechanisms, including the synthesis of UV-absorbing compounds such as carotenoids, mycosporine-like amino acids (MAAs), and antioxidant enzymes (Tilbrook et al., 2013; Singh et al., 2014; Ulm and Jenkins, 2015; Rosic, 2019; Groves and Franklin, 2025). In algae, these protective systems are often linked to the induction of genes required for the biosynthesis of these substances. DNA repair mechanisms can also be activated, such as photolyases, which repair DNA damage caused by UV radiation. Photolyases specifically catalyze the repair of UV-induced DNA lesions such as cyclobutane pyrimidine dimers (CPDs), which form when two adjacent pyrimidine bases (e.g., thymine or cytosine) in DNA become covalently linked. Pyrimidine-pyrimidone (6–4) photoproducts (6–4PPs), another type of UV-induced DNA damage involving covalent linkage between two pyrimidine bases, can also be repaired by photolyases. In addition to activating DNA repair systems, UV-B-activated photoreceptors mediate the protection of the photosynthetic machinery and regulate growth processes to prevent oxidative stress (Favory et al., 2009; Tilbrook et al., 2013; Jenkins, 2014; Müller-Xing et al., 2014; Allorent et al., 2016).
6 Algal genome and transcriptome data as sources of new light-sensitive proteins
Following the characterization of known algal light-sensing proteins, attention is now shifting toward the discovery of previously unrecognized photoreceptors, an endeavor greatly accelerated by the recent surge in algal genomics and transcriptomics. The primary resource for algal genomics is PhycoCosm (https://phycocosm.jgi.doe.gov), hosted by the Joint Genome Institute (JGI), and interconnected with plant genomes in JGI Phytozome (Goodstein et al., 2012) via the Embryophyta node. PhycoCosm contains over 200 algal genome projects from about 157 species, spanning the major algal lineages (Figure 4): Chlorophyta, Rhodophyta, Bacillariophyta, Charophyta, Haptophyta, Phaeophyta, Eustigmatophyta, and several smaller groups. This broad taxonomic coverage is crucial, as algae span highly diverse and evolutionarily complex—often bushy—branches of the tree of life.
Beyond genomics, PhycoCosm and related resources provide a wealth of multi-omics data, including transcriptomes, proteomes, and metabolomes. The expansion of such data has accelerated through initiatives such as the One Thousand Plant Transcriptomes Initiative (1KP) (Carpenter et al., 2019; One Thousand Plant Transcriptomes Initiative, 2019), the 10,000 Plant Genomes Project (10KP) (Cheng et al., 2018), and the Earth BioGenome Project (EBP), the latter of which aims to sequence and publicly release the genomes of all known eukaryotic species (Lewin et al., 2018; Lawniczak et al., 2022).
The availability of these comprehensive datasets allows for in silico identification of novel light-sensitive proteins and domains by sequence comparison and bioinformatics. This approach is not limited to algae but extends across photosynthetic and non-photosynthetic lineages. Newly identified genes can then be cloned, heterologously expressed, functionally characterized, and structurally modeled, revealing new properties and potential applications.
Earlier, de novo transcriptome sequencing of 127 algal species led to the identification of 61 channelrhodopsin homologs, which were subsequently expressed and physiologically characterized (Klapoetke et al., 2014). Among these were the well-known light-sensitive proteins Chronos and Chrimson. The ongoing exploration of these rapidly growing resources is expected to yield many more novel photoreceptors, further expanding the molecular toolkit for research and biotechnology.
7 Application of algal light-sensitive proteins in optogenetics
Optogenetics has revolutionized the ability to control the activity of genetically modified cells in living organisms, particularly within the field of neuroscience (Deisseroth, 2011; Zhang et al., 2011; Deisseroth, 2015; Hippler, 2017; Awasthi et al., 2020; Kojima et al., 2020; Emiliani et al., 2022; Tan et al., 2022; Zhang et al., 2022; Piatkevich and Boyden, 2023; Rindner and Lur, 2023; Busskamp et al., 2024; Levesque et al., 2024; Vlasova et al., 2024; Zhang et al., 2024b, 2024; He et al., 2025b; Pobozy et al., 2025; Zhou et al., 2025). This technique has been applied both for therapeutic purposes (Zarbin et al., 2013; Busskamp et al., 2024; Pobozy et al., 2025; Zhou et al., 2025) and to advance our understanding of brain circuitry (Deisseroth, 2011; Diester et al., 2011). Even optogenetic brain-computer interfaces are under development (Tang et al., 2024; Ahmed et al., 2025).
By employing light-sensitive proteins, researchers can manipulate neuronal activity with exceptional spatial and temporal precision. The fundamental principle behind optogenetic experiments is the use of genetically encoded optical actuators—light-controlled proteins that respond to specific wavelengths and intensities of light, transmitting signals to their targets and initiating precise biological responses.
The genes encoding these light-sensitive proteins originate from a diverse array of organisms, with microorganisms being the primary source. Among these, microalgae represent important contributors, but additional sources include archaea (e.g., halobacteria) that produce halorhodopsins and archaerhodopsins, as well as bacteria that contribute proteorhodopsins and various light-sensitive enzymes (Spudich et al., 2000; Zhang et al., 2011; Losi and Gärtner, 2012; Govorunova et al., 2017). Phytochromes and LOV domains are derived from cyanobacteria. The availability of a broad repertoire of light-controlled proteins and their respective genes is integral to the field of optogenetics.
While a wide range of light-sensitive proteins from different organisms have been adapted for optogenetics, the following section emphasizes channelrhodopsins. Among algal-derived photoreceptors, these have become the most widely adopted tools in optogenetics and remain the best-characterized in terms of structure, mechanism, and functional versatility.
A hallmark of optogenetic research is the use of channelrhodopsins (Bamann et al., 2010; Hou et al., 2011; Lin, 2011; Prigge et al., 2012; Hegemann and Nagel, 2013; Guru et al., 2015; Mcisaac et al., 2015; Schneider et al., 2015; Wietek and Prigge, 2016; Govorunova et al., 2017; Losi et al., 2018; Coli et al., 2024; Pobozy et al., 2025; Wang et al., 2025), a class of light-sensitive proteins from algae that play a pivotal role in manipulating neuronal activity. The discovery of channelrhodopsins ChR1 and ChR2 in Chlamydomonas reinhardtii marked the inception of the field of optogenetics (Nagel et al., 2002, 2003). Since then, numerous additional channelrhodopsins have been identified in various chlorophytes, thereby expanding the potential of optogenetic tools. The development of mutated channelrhodopsin variants has driven significant technological progress. These engineered variants possess altered functional properties, such as modified channel closing kinetics or spectral sensitivities shifted toward blue or red light, which are crucial for broadening the versatility of optogenetic applications (Berndt et al., 2009; Lin et al., 2009; Kleinlogel et al., 2011; Lin et al., 2013; Klapoetke et al., 2014). Notably, ChR2 from C. reinhardtii has become the gold standard in optogenetics, serving as the prototype for further variants designed to optimize photocurrent amplitude, response speed, and light sensitivity (Boyden et al., 2005; Nagel et al., 2005; Lin et al., 2009; Gunaydin et al., 2010; Berndt et al., 2011). Extensive color tuning, which modifies the spectral sensitivity of these proteins, has opened new avenues for experimental design (Figure 8) (Mattis et al., 2011; Chuong et al., 2014; Klapoetke et al., 2014; Olofsson et al., 2015; Wietek and Prigge, 2016).
The development of optogenetic tools typically follows a bottom-up approach, whereby novel channelrhodopsins are first identified and characterized before being engineered to meet specific experimental requirements. In neurobiology, for instance, the expression of channelrhodopsins under the control of cell type- and species-specific promoters allows for the selective activation of defined neuronal populations, ensuring precise spatial and temporal control (Boyden et al., 2005; Fenno et al., 2011; Zhang et al., 2011; Deisseroth, 2015). Optogenetics thus enables exceptionally accurate and specific manipulation of neuronal activity.
Numerous engineered channelrhodopsin variants have been developed to address a wide range of research needs (Figure 8). These include various ChR2 variants from C. reinhardtii, such as ChR2-H134R (Nagel et al., 2005; Mattis et al., 2011), ChR2(E123T/T159C) (Berndt et al., 2011), ChD, ChF, ChEF (Lin et al., 2009, 2013), ChIEF (Lin et al., 2013), ChR2E/T, and ChETA (Gunaydin et al., 2010). From a ChR2-homologous channelrhodopsin from Scherffelia dubia (Chlorodendrophyceae), SdChR, which produces larger photocurrents than ChR2, further valuable optogenetic tools have been developed. One such derivative is SdChR(E154A), known as CheRiff (Hochbaum et al., 2014; Venkatachalam and Cohen, 2014). Among all channelrhodopsins, the ChR2-homologous channelrhodopsin TsChR from Tetraselmis striata (Chlorodendrophyceae) is the most blue-shifted, absorbing at the shortest wavelength (~436 nm), but it generates only weak photocurrents (Klapoetke et al., 2014). The engineered variant eTsChR, based on TsChR, exhibits improved trafficking and robust neuronal spiking (Farhi et al., 2019). Another ChR2-homologous channelrhodopsin, CoChR from Chloromonas oogama (Chlamydomonadaceae), demonstrates high light-driven photocurrents but slow channel kinetics. Here, optimized variants with enhanced light sensitivity and maintained high photocurrent amplitudes have been engineered, including CoChR-LC (CoChR-L112C), CoChR-3M (CoChR-H94E/L112C/K264T), and CoChR(H94E/E103A) (Ganjawala et al., 2019; Bi et al., 2022).
New channelrhodopsin variants have also been created through the construction of fusions and chimeras from two or more sequences (Figure 8). Notably, chimeras derived from ChR1 (C. reinhardtii) and VChR1 (V. carteri)—which contain no ChR2 sequence—such as C1V1TT (Yizhar et al., 2011) and mVChR1 (Tomita et al., 2014), are sensitive to green light. The optimized variant ComV1(ex3mV1Co) (Tomita et al., 2014; Watanabe et al., 2021) exhibits enhanced sensitivity under daylight conditions. White-opsin is a fusion of three distinct opsins—ChR2, C1V1TT, and ReaChR—each responsive to different regions of the visible spectrum (blue, green, and red wavelengths, respectively) (Batabyal et al., 2015), thereby conferring broad-spectrum sensitivity.
Red-shifted channelrhodopsins are of particular importance for optogenetics because they respond to longer wavelengths of light, enabling deeper tissue penetration and minimizing phototoxicity (Figure 8). VChR1 from V. carteri, discovered after ChR2, was noteworthy for its more than 50-nm red shift compared to ChR2. Subsequently, an even more red-shifted channelrhodopsin was identified: CnChR from C. noctigama (Chlamydomonadaceae), commonly known as Chrimson (Klapoetke et al., 2014). Additional red-shifted variants, such as ReaChR (red-activatable channelrhodopsin), were engineered from VChR1. ReaChR’s activation spectrum peaks at ~530 nm with short light pulses, but longer pulses can further shift the peak to ~580 nm (Inagaki et al., 2014). The variant bReaChES is a faster red-shifted channelrhodopsin, generated by Glu123Ser substitution and replacement of the N-terminal residues in ReaChR with those of ChR2 (Kim et al., 2016). The yellow-orange-peaked Chrimson has served as the basis for a family of derivatives: CsChrimson, f-Chrimson (Chrimson Y261F/S267M), vf-Chrimson (Chrimson K176R/Y261F/S267M), ChrimsonR (Chrimson K176R), and ChrimsonSA (Klapoetke et al., 2014; Oda et al., 2018; Gupta et al., 2019).
Another highly sought-after property of channelrhodopsins is fast kinetics. The most notable example is ShChR from Stigeoclonium helveticum (Chaetophoraceae), usually referred to as Chronos (Klapoetke et al., 2014). While faster kinetics typically result in reduced light sensitivity, Chronos is the fastest channelrhodopsin identified and still maintains relatively high light sensitivity (Mardinly et al., 2018). Its absorption maximum lies in the blue-green range (~500 nm). Chronos derivatives include ChroME, ChroME2.0, ChroME2f, ChroME2s, ST-ChroME, and ChrMD (Sridharan et al., 2022). In neurobiology, Chronos and its mutants enable rapid neuronal activation with considerable light sensitivity. Combining Chronos and Chrimson enables two-color activation of neural spiking and downstream synaptic transmission in neurobiological experiments (Klapoetke et al., 2014).
Bistable channelrhodopsins, also known as step-function opsins (SFOs), represent a distinct functional class. They sustain prolonged photocurrent activation even after the light stimulus has ceased and exhibit sensitivities several orders of magnitude higher than those of fast channelrhodopsins (Berndt et al., 2009; Guru et al., 2015). ChR2(C128A) and ChR2(C128S) were the first SFOs, generated by single amino acid mutations at C128 in the ChR2 sequence, which extend the open-state lifetime to tens of seconds. ChR2-C128S can be turned on with blue light (~450 nm) and turned off with yellow light (~550 nm). The double mutant ChR2(C128S/D156A), known as stabilized step-function opsin (SSFO), maintains stable photocurrents for several minutes (Yizhar et al., 2011; Guru et al., 2015).
Another ChR2 mutant, ChR2(L132C), exhibits a sixfold increase in calcium ion permeability (Kleinlogel et al., 2011), and is termed CatCh (Calcium transporting Channelrhodopsin). In animal models, CatCh enables neuronal activation at much lower light intensities, reducing the risk of phototoxicity. Calcium affinity was further enhanced by targeted mutagenesis at various positions, generating CapChRs (Calcium-permeable Channelrhodopsins) that exhibit reduced sodium and proton conductance alongside markedly improved Ca2+ permeation at negative voltage and low extracellular Ca2+ concentrations (Fernandez Lahore et al., 2022).
Mutation of E90 in ChR2 to the positively charged amino acid arginine can even convert ChR2 into a light-gated chloride channel (ChloC) (Wietek et al., 2014). Ion selectivity has also been altered in the engineered channelrhodopsin PsCatCh2.0, derived from the highly blue-shifted Platymonas subcordiformis (Chlorodendraceae) PsChR (Figure 8) through an L115C mutation and further modifications (Govorunova et al., 2013; Chen et al., 2022; Pobozy et al., 2025). Due to its low proton permeability, which minimizes perturbations to cellular pH, this variant is well suited for clinical applications where acidification is undesirable.
Synthetic opsins such as MCO1 (Multi-Characteristic Opsin 1) and MCO-010 have also been developed (Wright et al., 2017a, 2017). These opsins exhibit broad spectral sensitivity, high light sensitivity, and fast kinetics, and are currently being tested for the treatment of retinal degenerative diseases such as retinitis pigmentosa and Stargardt disease (Mohanty et al., 2025). Additionally, machine learning-guided engineering approaches (Bedbrook et al., 2019) have resulted in new channelrhodopsins such as ChRger1, ChRger2, and ChRger3. AI-based methods are expected to produce further variants in the near future.
In the context of neurological disorders, optogenetic approaches are being explored for the treatment of Parkinson’s disease, epilepsy, and depression. Researchers use light to modulate specific neuron populations, potentially correcting aberrant neural activity (Deisseroth, 2015; Kianianmomeni and Hallmann, 2015a). Optogenetic strategies are also being investigated for vision restoration in conditions such as retinitis pigmentosa, by rendering retinal cells light-sensitive (Sahel et al., 2021; Pobozy et al., 2025).
Similarly, optogenetic strategies are under investigation for cochlear implants. Instead of electrical impulses, light stimulation could provide more precise targeting of auditory neurons, potentially improving sound resolution and speech recognition in noisy environments (Wrobel et al., 2018; Huet et al., 2024). There are also optogenetic applications in oral and craniofacial fields (Zhang et al., 2024c).
Furthermore, in cardiac medicine, optogenetics is being studied as a method to control heart rhythms by targeting cardiac cells with light, offering potential treatments for arrhythmias (Bruegmann et al., 2018). Optogenetic techniques are also being researched to modulate pain pathways and may offer alternatives to conventional painkillers (Iyer et al., 2014; Li and Ji, 2024). Moreover, pancreatic beta cells have been engineered using optogenetic techniques to regulate insulin release in response to light. This approach holds potential as a strategy for managing type 1 diabetes by enabling precise, non-invasive control of insulin secretion (Shao et al., 2017).
Overall, optogenetics remains a vital tool for investigating complex biological processes and is facilitating the development of novel medical therapies. Numerous studies and reviews provide detailed descriptions of this foundational research (Fenno et al., 2011; Hegemann and Nagel, 2013; Tischer and Weiner, 2014; Deisseroth, 2015; Gao, 2015; Guru et al., 2015; Kianianmomeni and Hallmann, 2015a).
Looking forward, the integration of different light-sensitive proteins for multicolor optogenetics is expected to become increasingly important. Addressing challenges such as spectral overlap, crosstalk, and interference between different opsins or light sources will be crucial. Optogenetic tools will also continue to aid in mapping entire neural circuits and exploring complex brain functions. Applications in non-neuronal cells (e.g., muscle cells, immune cells, or engineered tissues) should be further expanded. Light could be used to control cell growth, differentiation, or migration, potentially enabling tissue-specific therapies where cells are engineered for optogenetic control. Finally, there is also a need for improved light delivery systems and more precise control of light exposure.
8 Conclusion and perspectives
Algae have evolved a remarkable array of photoreceptors that allow them to sense and adapt to an extraordinary diversity of light environments. This diversity is not only a product of their evolutionary history—marked by endosymbiotic events, gene duplications, and domain fusions—but also a reflection of the tremendous variety of ecological niches they occupy, from sunlit surface waters to deep oceans, shaded terrestrial environments, and extreme habitats.
The main classes of algal photoreceptors—flavin-based (phototropins, cryptochromes, aureochromes, BLUF proteins), retinal-based (rhodopsins), tetrapyrrole-based (phytochromes), hybrid (neochromes), and UV-B receptors (UVR8)—collectively cover the entire spectrum from ultraviolet to far-red light. These receptors underpin a broad range of physiological processes, including photosynthesis, photoprotection, phototaxis, circadian rhythms, development, sexual cycles, and pigment synthesis. The interplay between different photoreceptors and signaling pathways enables algae to finely tune their responses to fluctuating light conditions and to integrate light signals with other environmental cues.
The rapid expansion of algal genomics and transcriptomics, supported by large-scale sequencing initiatives, is accelerating the discovery of new light-sensitive proteins. Bioinformatic and functional characterization of these photoreceptors is expanding our understanding of algal biology and providing a rich source of novel molecular tools.
A particularly transformative application of algal photoreceptors has been in optogenetics. Channelrhodopsins and other light-sensitive proteins from algae have revolutionized neuroscience by enabling precise, light-controlled manipulation of neuronal and other cell activities. The ongoing engineering of channelrhodopsins for altered spectral sensitivity, kinetics, and ion selectivity continues to enhance the versatility of optogenetic techniques.
Future directions include:
– Continued discovery and functional analysis of novel photoreceptors from diverse algal groups, facilitated by advances in sequencing, bioinformatics, and synthetic biology.
– Integration of multiple light-sensitive proteins for complex, multicolor optogenetic control of cellular processes.
– Expansion of optogenetic applications beyond neuroscience, including cardiac, endocrine, and immunological systems, as well as in plant and algal biotechnology.
– Development of improved light delivery systems and opsin variants for use in tissues with limited light penetration.
– Use of machine learning and protein engineering to design custom photoreceptors with tailored properties for specific research and therapeutic needs.
The study of algal photoreceptors not only enriches our understanding of how life adapts to light but also fuels technological innovation in biology and medicine. As the molecular diversity of algal photoreceptors continues to unfold, these proteins will remain at the forefront of both fundamental discovery and applied science.
Author contributions
AH: Writing – original draft, Writing – review & editing, Conceptualization, Visualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Bielefeld University. The article processing charge was covered by the German Research Foundation (DFG) and the Open Access Publication Fund of Bielefeld University.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, A., Levitan, O., Cruz De Carvalho, H., and Falkowski, P. G. (2023). Light-dependent signal transduction in the marine diatom Phaeodactylum tricornutum. Proc. Natl. Acad. Sci. U.S.A. 120, e2216286120. doi: 10.1073/pnas.2216286120
Ahmed, A., Alegret, N., Almeida, B., Alvarez-Puebla, R., Andrews, A. M., Ballerini, L., et al. (2025). Interfacing with the brain: How nanotechnology can contribute. ACS Nano 19, 10630–10717. doi: 10.1021/acsnano.4c10525
Aihara, Y., Tabata, R., Suzuki, T., Shimazaki, K., and Nagatani, A. (2008). Molecular basis of the functional specificities of phototropin 1 and 2. Plant J. 56, 364–375. doi: 10.1111/j.1365-313X.2008.03605.x
Allorent, G., Lefebvre-Legendre, L., Chappuis, R., Kuntz, M., Truong, T. B., Niyogi, K. K., et al. (2016). UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A. 113, 14864–14869. doi: 10.1073/pnas.1607695114
Allorent, G. and Petroutsos, D. (2017). Photoreceptor-dependent regulation of photoprotection. Curr. Opin. Plant Biol. 37, 102–108. doi: 10.1016/j.pbi.2017.03.016
Anders, K., Daminelli-Widany, G., Mroginski, M. A., Von Stetten, D., and Essen, L. O. (2013). Structure of the cyanobacterial phytochrome 2 photosensor implies a tryptophan switch for phytochrome signaling. J. Biol. Chem. 288, 35714–35725. doi: 10.1074/jbc.M113.510461
Anders, K. and Essen, L. O. (2015). The family of phytochrome-like photoreceptors: diverse, complex and multi-colored, but very useful. Curr. Opin. Struct. Biol. 35, 7–16. doi: 10.1016/j.sbi.2015.07.005
Archibald, J. M. (2015). Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 25, R911–R921. doi: 10.1016/j.cub.2015.07.055
Armbrust, E. V., Berges, J. A., Bowler, C., Green, B. R., Martinez, D., Putnam, N. H., et al. (2004). The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306, 79–86. doi: 10.1126/science.1101156
Avelar, G. M., Schumacher, R. I., Zaini, P. A., Leonard, G., Richards, T. A., and Gomes, S. L. (2014). A rhodopsin-guanylyl cyclase gene fusion functions in visual perception in a fungus. Curr. Biol. 24, 1234–1240. doi: 10.1016/j.cub.2014.04.009
Awasthi, M., Sushmita, K., Kaushik, M. S., Ranjan, P., and Kateriya, S. (2020). Novel modular rhodopsins from green algae hold great potential for cellular optogenetic modulation across the biological model systems. Life (Basel) 10, 259. doi: 10.3390/life10110259
Baldauf, S. L. (2003). The deep roots of eukaryotes. Science 300, 1703–1706. doi: 10.1126/science.1085544
Bamann, C., Nagel, G., and Bamberg, E. (2010). Microbial rhodopsins in the spotlight. Curr. Opin. Neurobiol. 20, 610–616. doi: 10.1016/j.conb.2010.07.003
Banerjee, A., Herman, E., Kottke, T., and Essen, L. O. (2016a). Structure of a native-like aureochrome 1a LOV domain dimer from Phaeodactylum tricornutum. Structure 24, 171–178. doi: 10.1016/j.str.2015.10.022
Banerjee, A., Herman, E., Serif, M., Maestre-Reyna, M., Hepp, S., Pokorny, R., et al. (2016b). Allosteric communication between DNA-binding and light-responsive domains of diatom class I aureochromes. Nucleic Acids Res. 44, 5957–5970. doi: 10.1093/nar/gkw420
Barsanti, L. and Gualtieri, P. (2014). Algae: anatomy, biochemistry and biotechnology (Boca Raton, FL, USA: CRC Press).
Batabyal, S., Cervenka, G., Ha, J. H., Kim, Y. T., and Mohanty, S. (2015). Broad-band activatable white-opsin. PloS One 10, e0136958. doi: 10.1371/journal.pone.0136958
Bedbrook, C. N., Yang, K. K., Robinson, J. E., Mackey, E. D., Gradinaru, V., and Arnold, F. H. (2019). Machine learning-guided channelrhodopsin engineering enables minimally invasive optogenetics. Nat. Methods 16, 1176–1184. doi: 10.1038/s41592-019-0583-8
Beel, B., Müller, N., Kottke, T., and Mittag, M. (2013). News about cryptochrome photoreceptors in algae. Plant Signal Behav. 8, e22870. doi: 10.4161/psb.22870
Beel, B., Prager, K., Spexard, M., Sasso, S., Weiss, D., Muller, N., et al. (2012). A flavin binding cryptochrome photoreceptor responds to both blue and red light in Chlamydomonas reinhardtii. Plant Cell 24, 2992–3008. doi: 10.1105/tpc.112.098947
Begum, H., Yusoff, F. M., Banerjee, S., Khatoon, H., and Shariff, M. (2016). Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci Nutr. 56, 2209–2222. doi: 10.1080/10408398.2013.764841
Berndt, A., Schoenenberger, P., Mattis, J., Tye, K. M., Deisseroth, K., Hegemann, P., et al. (2011). High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. U.S.A. 108, 7595–7600. doi: 10.1073/pnas.1017210108
Berndt, A., Yizhar, O., Gunaydin, L. A., Hegemann, P., and Deisseroth, K. (2009). Bi-stable neural state switches. Nat. Neurosci. 12, 229–234. doi: 10.1038/nn.2247
Berthold, P., Tsunoda, S. P., Ernst, O. P., Mages, W., Gradmann, D., and Hegemann, P. (2008). Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell 20, 1665–1677. doi: 10.1105/tpc.108.057919
Bessen, M., Fay, R. B., and Witman, G. B. (1980). Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J. Cell Biol. 86, 446–455. doi: 10.1083/jcb.86.2.446
Bi, X., Beck, C., and Gong, Y. (2022). A kinetic-optimized CoChR variant with enhanced high-frequency spiking fidelity. Biophys. J. 121, 4166–4178. doi: 10.1016/j.bpj.2022.09.024
Bisova, K. and Zachleder, V. (2014). Cell-cycle regulation in green algae dividing by multiple fission. J. Exp. Bot. 65, 2585–2602. doi: 10.1093/jxb/ert466
Bogomolni, R. A. and Spudich, J. L. (1982). Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc. Natl. Acad. Sci. U.S.A. 79, 6250–6254. doi: 10.1073/pnas.79.20.6250
Boonyareth, M., Saranak, J., Pinthong, D., Sanvarinda, Y., and Foster, K. W. (2009). Roles of cyclic AMP in regulation of phototaxis in Chlamydomonas reinhardtii. Biologia 64, 1058–1065. doi: 10.2478/s11756-009-0194-4
Bouget, F. Y., Lefranc, M., Thommen, Q., Pfeuty, B., Lozano, J. C., Schatt, P., et al. (2014). Transcriptional versus non-transcriptional clocks: a case study in Ostreococcus. Mar. Genomics 14, 17–22. doi: 10.1016/j.margen.2014.01.004
Bowler, C., Allen, A. E., Badger, J. H., Grimwood, J., Jabbari, K., Kuo, A., et al. (2008). The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456, 239–244. doi: 10.1038/nature07410
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., and Deisseroth, K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268. doi: 10.1038/nn1525
Briggs, W. R. (2006). “Blue/UV-A receptors: historical overview,” in Photomorphogenesis in plants and bacteria. Eds. Schäfer, E. and Nagy, F. (Springer Netherlands, Dordrecht), 171–197.
Briggs, W. R., Beck, C. F., Cashmore, A. R., Christie, J. M., Hughes, J., Jarillo, J. A., et al. (2001). The phototropin family of photoreceptors. Plant Cell 13, 993–997. doi: 10.1105/tpc.13.5.993
Briggs, W., Tseng, T.-S., Cho, H.-Y., Swartz, T., Sullivan, S., Bogolmoni, R., et al. (2007). Phototropins and their LOV domains: versatile plant blue-light receptors. J. Integr. Plant Biol. 49, 4–10. doi: 10.1111/j.1744-7909.2006.00406.x
Brosché, M. and Strid, A. (2003). Molecular events following perception of ultraviolet-B radiation by plants. Physiol. Plant 117, 1–10. doi: 10.1034/j.1399-3054.2003.1170101.x
Broser, M. (2021). Far-red absorbing rhodopsins, insights from heterodimeric rhodopsin-cyclases. Front. Mol. Biosci. 8, 806922. doi: 10.3389/fmolb.2021.806922
Brown, L. S. (2022). Light-driven proton transfers and proton transport by microbial rhodopsins - A biophysical perspective. Biochim. Biophys. Acta Biomembr. 1864, 183867. doi: 10.1016/j.bbamem.2022.183867
Bruegmann, T., Beiert, T., Vogt, C. C., Schrickel, J. W., and Sasse, P. (2018). Optogenetic termination of atrial fibrillation in mice. Cardiovasc. Res. 114, 713–723. doi: 10.1093/cvr/cvx250
Burgie, E. S. and Vierstra, R. D. (2014). Phytochromes: an atomic perspective on photoactivation and signaling. Plant Cell 26, 4568–4583. doi: 10.1105/tpc.114.131623
Busskamp, V., Roska, B., and Sahel, J. A. (2024). Optogenetic vision restoration. Cold Spring Harb. Perspect. Med. 14, a041660. doi: 10.1101/cshperspect.a041660
Carpenter, E. J., Matasci, N., Ayyampalayam, S., Wu, S., Sun, J., Yu, J., et al. (2019). Access to RNA-sequencing data from 1,173 plant species: The 1000 Plant transcriptomes initiative (1KP). Gigascience 8, giz126. K, K.U. doi: 10.1093/gigascience/giz126
Chaves, I., Pokorny, R., Byrdin, M., Hoang, N., Ritz, T., Brettel, K., et al. (2011). The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62, 335–364. doi: 10.1146/annurev-arplant-042110-103759
Chen, M. and Chory, J. (2011). Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 21, 664–671. doi: 10.1016/j.tcb.2011.07.002
Chen, F., Duan, X., Yu, Y., Yang, S., Chen, Y., Gee, C. E., et al. (2022). Visual function restoration with a highly sensitive and fast Channelrhodopsin in blind mice. Signal Transduct. Target Ther. 7, 104. doi: 10.1038/s41392-022-00935-x
Cheng, M. C., Kathare, P. K., Paik, I., and Huq, E. (2021). Phytochrome signaling networks. Annu. Rev. Plant Biol. 72, 217–244. doi: 10.1146/annurev-arplant-080620-024221
Cheng, S., Melkonian, M., Smith, S. A., Brockington, S., Archibald, J. M., Delaux, P. M., et al. (2018). 10KP: A phylodiverse genome sequencing plan. Gigascience 7, 1–9. doi: 10.1093/gigascience/giy013
Chory, J. (2010). Light signal transduction: an infinite spectrum of possibilities. Plant J. 61, 982–991. doi: 10.1111/j.1365-313X.2009.04105.x
Christie, J. M. (2007). Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58, 21–45. doi: 10.1146/annurev.arplant.58.032806.103951
Christie, J. M., Arvai, A. S., Baxter, K. J., Heilmann, M., Pratt, A. J., O'hara, A., et al. (2012). Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335, 1492–1496. doi: 10.1126/science.1218091
Christie, J. M., Blackwood, L., Petersen, J., and Sullivan, S. (2015). Plant flavoprotein photoreceptors. Plant Cell Physiol. 56, 401–413. doi: 10.1093/pcp/pcu196
Christie, J. M. and Briggs, W. R. (2001). Blue light sensing in higher plants. J. Biol. Chem. 276, 11457–11460. doi: 10.1074/jbc.R100004200
Christie, J. M., Reymond, P., Powell, G. K., Bernasconi, P., Raibekas, A. A., Liscum, E., et al. (1998). Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282, 1698–1701. doi: 10.1126/science.282.5394.1698
Christie, J. M., Salomon, M., Nozue, K., Wada, M., and Briggs, W. R. (1999). LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. U.S.A. 96, 8779–8783. doi: 10.1073/pnas.96.15.8779
Christie, J. M., Swartz, T. E., Bogomolni, R. A., and Briggs, W. R. (2002). Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J. 32, 205–219. doi: 10.1046/j.1365-313X.2002.01415.x
Chuong, A. S., Miri, M. L., Busskamp, V., Matthews, G. A., Acker, L. C., Sorensen, A. T., et al. (2014). Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129. doi: 10.1038/nn.3752
Cock, J. M., Sterck, L., Rouze, P., Scornet, D., Allen, A. E., Amoutzias, G., et al. (2010). The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465, 617–621. doi: 10.1038/nature09016
Coesel, S. N. (2024). More than a photoreceptor: aureochromes are intrinsic to the diatom light-regulated transcriptional network. J. Exp. Bot. 75, 1786–1790. doi: 10.1093/jxb/erae004
Coesel, S., Mangogna, M., Ishikawa, T., Heijde, M., Rogato, A., Finazzi, G., et al. (2009). Diatom PtCPF1 is a new cryptochrome/photolyase family member with DNA repair and transcription regulation activity. EMBO Rep. 10, 655–661. doi: 10.1038/embor.2009.59
Coli, A., Gao, S., and Kaestner, L. (2024). Sodium-selective channelrhodopsins. Cells 13, 1852. doi: 10.3390/cells13221852
Courties, C., Vaquer, A., Trousselier, M., Lautier, J., Chrétiennot-Dinet, M.-J., Neveux, J., et al. (1994). Smallest eukaryotic organism. Nature 370, 255. doi: 10.1038/370255a0
Craigie, R. A. and Cavalier-Smith, T. (1982). Cell volume and the control of the Chlamydomonas cell cycle. J. Cell Sci 54, 173–191. doi: 10.1242/jcs.54.1.173
Croce, R. and Van Amerongen, H. (2014). Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 10, 492–501. doi: 10.1038/nchembio.1555
Cross, F. R. and Umen, J. G. (2015). The Chlamydomonas cell cycle. Plant J. 82, 370–392. doi: 10.1111/tpj.2015.82.issue-3
Crosson, S. and Moffat, K. (2001). Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc. Natl. Acad. Sci. U.S.A. 98, 2995–3000. doi: 10.1073/pnas.051520298
Crosson, S. and Moffat, K. (2002). Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell 14, 1067–1075. doi: 10.1105/tpc.010475
Crosson, S., Rajagopal, S., and Moffat, K. (2003). The LOV domain family: photoresponsive signaling modules coupled to diverse output domains. Biochemistry 42, 2–10. doi: 10.1021/bi026978l
D'amico-Damião, V. and Carvalho, R. F. (2018). Cryptochrome-related abiotic stress responses in plants. Front. Plant Sci. 9, 1897. doi: 10.3389/fpls.2018.01897
De Grip, W. J. and Ganapathy, S. (2022). Rhodopsins: An excitingly versatile protein species for research, development and creative engineering. Front. Chem. 10, 879609. doi: 10.3389/fchem.2022.879609
Deisseroth, K. (2015). Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225. doi: 10.1038/nn.4091
Demmig-Adams, B. and Adams, W. W., III (1996). The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1, 21–26. doi: 10.1016/S1360-1385(96)80019-7
Deoliveira, C. C. and Crane, B. R. (2024). A structural decryption of cryptochromes. Front. Chem. 12, 1436322. doi: 10.3389/fchem.2024.1436322
Depaepe, T., Vanhaelewyn, L., and van der Straeten, D. (2023). UV-B responses in the spotlight: Dynamic photoreceptor interplay and cell-type specificity. Plant Cell Environ. 46, 3194–3205. doi: 10.1111/pce.v46.11
Diester, I., Kaufman, M. T., Mogri, M., Pashaie, R., Goo, W., Yizhar, O., et al. (2011). An optogenetic toolbox designed for primates. Nat. Neurosci. 14, 387–397. doi: 10.1038/nn.2749
Donnan, L. and John, P. C. (1983). Cell cycle control by timer and sizer in Chlamydomonas. Nature 304, 630–633. doi: 10.1038/304630a0
Dorrell, R. G. and Howe, C. J. (2015). Integration of plastids with their hosts: lessons learned from dinoflagellates. Proc. Natl. Acad. Sci. U.S.A. 112, 10247–10254. doi: 10.1073/pnas.1421380112
Dring, M. J. (1988). Photocontrol of development in algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 157–174. doi: 10.1146/annurev.pp.39.060188.001105
Dring, M. J. and Lüning, K. (1983). “Photomorphogenesis of marine macroalgae,” in Photomorphogenesis. Eds. Shropshire, W. and Mohr, H. (Springer Berlin Heidelberg, Berlin, Heidelberg), 545–568.
Duanmu, D., Bachy, C., Sudek, S., Wong, C. H., Jimenez, V., Rockwell, N. C., et al. (2014). Marine algae and land plants share conserved phytochrome signaling systems. Proc. Natl. Acad. Sci. U.S.A. 111, 15827–15832. doi: 10.1073/pnas.1416751111
Duanmu, D., Casero, D., Dent, R. M., Gallaher, S., Yang, W., Rockwell, N. C., et al. (2013). Retrograde bilin signaling enables Chlamydomonas greening and phototrophic survival. Proc. Natl. Acad. Sci. U.S.A. 110, 3621–3626. doi: 10.1073/pnas.1222375110
Duanmu, D., Rockwell, N. C., and Lagarias, J. C. (2017). Algal light sensing and photoacclimation in aquatic environments. Plant Cell Environ. 40, 2558–2570. doi: 10.1111/pce.v40.11
Dugué, G. P., Akemann, W., and Knöpfel, T. (2012). A comprehensive concept of optogenetics. Prog. Brain Res. 196, 1–28. doi: 10.1016/B978-0-444-59426-6.00001-X
Eberhard, S., Finazzi, G., and Wollman, F. A. (2008). The dynamics of photosynthesis. Annu. Rev. Genet. 42, 463–515. doi: 10.1146/annurev.genet.42.110807.091452
Emerson, R. (1957). Dependence of yield of photosynthesis in long-wave red on wavelength and intensity of supplementary light. Science 125, 746–746. doi: 10.1126/science.125.3251.746
Emiliani, V., Entcheva, E., Hedrich, R., Hegemann, P., Konrad, K. R., Luscher, C., et al. (2022). Optogenetics for light control of biological systems. Nat. Rev. Methods Primers 2, 55. doi: 10.1038/s43586-022-00136-4
Endres, L. and Breit, R. (2009). “UV radiation, irradiation, and dosimetry,” in Dermatological phototherapy and Photodiagnostic Methods. Eds. Hönigsmann, H., Elmets, C., and Krutmann, J. (New York, Berlin, Heidelberg), 3–59.
Ernst, O. P., Lodowski, D. T., Elstner, M., Hegemann, P., Brown, L. S., and Kandori, H. (2014). Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem. Rev. 114, 126–163. doi: 10.1021/cr4003769
Essen, L. O., Franz, S., and Banerjee, A. (2017). Structural and evolutionary aspects of algal blue light receptors of the cryptochrome and aureochrome type. J. Plant Physiol. 217, 27–37. doi: 10.1016/j.jplph.2017.07.005
Essen, L. O., Mailliet, J., and Hughes, J. (2008). The structure of a complete phytochrome sensory module in the Pr ground state. Proc. Natl. Acad. Sci. U.S.A. 105, 14709–14714. doi: 10.1073/pnas.0806477105
Falkowski, P. G. and Raven, J. A. (2007). Aquatic photosynthesis (Princeton, NJ, USA: Princeton University Press).
Faminzin, A. (1866). Die Wirkung des Lichtes auf die Bewegung der Chlamidomonas pulvisculus Ehr., Euglena viridis Ehr. und Oscillatoria insignis Tw. Mélanges Biologiques tirés du Bull. l’Académie Imperial Des. Sci. St-Petersbourg 6, 73–93.
Farhi, S. L., Parot, V. J., Grama, A., Yamagata, M., Abdelfattah, A. S., Adam, Y., et al. (2019). Wide-area all-optical neurophysiology in acute brain slices. J. Neurosci. 39, 4889–4908. doi: 10.1523/JNEUROSCI.0168-19.2019
Farre, E. M. (2020). The brown clock: circadian rhythms in stramenopiles. Physiol. Plant 169, 430–441. doi: 10.1111/ppl.v169.3
Favory, J. J., Stec, A., Gruber, H., Rizzini, L., Oravecz, A., Funk, M., et al. (2009). Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 28, 591–601. doi: 10.1038/emboj.2009.4
Fedorov, R., Schlichting, I., Hartmann, E., Domratcheva, T., Fuhrmann, M., and Hegemann, P. (2003). Crystal structures and molecular mechanism of a light-induced signaling switch: The Phot-LOV1 domain from Chlamydomonas reinhardtii. Biophys. J. 84, 2474–2482. doi: 10.1016/S0006-3495(03)75052-8
Fenno, L., Yizhar, O., and Deisseroth, K. (2011). The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412. doi: 10.1146/annurev-neuro-061010-113817
Fernandez Lahore, R. G., Pampaloni, N. P., Schiewer, E., Heim, M. M., Tillert, L., Vierock, J., et al. (2022). Calcium-permeable channelrhodopsins for the photocontrol of calcium signalling. Nat. Commun. 13, 7844. doi: 10.1038/s41467-022-35373-4
Fischer, P., Mukherjee, S., Schiewer, E., Broser, M., Bartl, F., and Hegemann, P. (2021). The inner mechanics of rhodopsin guanylyl cyclase during cGMP-formation revealed by real-time FTIR spectroscopy. Elife 10, e71384. doi: 10.7554/eLife.71384.sa2
Flores-Ibarra, A., Maia, R. N. A., Olasz, B., Church, J. R., Gotthard, G., Schapiro, I., et al. (2024). Light-oxygen-voltage (LOV)-sensing domains: Activation mechanism and optogenetic stimulation. J. Mol. Biol. 436, 168356. doi: 10.1016/j.jmb.2023.168356
Fortunato, A. E., Annunziata, R., Jaubert, M., Bouly, J. P., and Falciatore, A. (2015). Dealing with light: the widespread and multitasking cryptochrome/photolyase family in photosynthetic organisms. J. Plant Physiol. 172, 42–54. doi: 10.1016/j.jplph.2014.06.011
Foster, K. W., Saranak, J., Patel, N., Zarilli, G., Okabe, M., Kline, T., et al. (1984). A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature 311, 756–759. doi: 10.1038/311756a0
Foster, K. W. and Smyth, R. D. (1980). Light antennas in phototactic algae. Microbiol. Rev. 44, 572–630. doi: 10.1128/mr.44.4.572-630.1980
Fraikin, G. Y., Belenikina, N. S., and Rubin, A. B. (2023). Molecular bases of signaling processes regulated by cryptochrome sensory photoreceptors in plants. Biochem. (Mosc) 88, 770–782. doi: 10.1134/S0006297923060056
Frank, H. A. and Cogdell, R. J. (2012). “Light capture in photosynthesis,” in Comprehensive Biophysics. Ed. Egelman, E. H. (Elsevier, Amsterdam), 94–114.
Franklin, K. A. and Quail, P. H. (2010). Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61, 11–24. doi: 10.1093/jxb/erp304
Franz, S., Ignatz, E., Wenzel, S., Zielosko, H., Putu, E., Maestre-Reyna, M., et al. (2018). Structure of the bifunctional cryptochrome aCRY from Chlamydomonas reinhardtii. Nucleic Acids Res. 46, 8010–8022. doi: 10.1093/nar/gky621
Frascogna, F., Ledermann, B., Hartmann, J., Perez Patallo, E., Zeqiri, F., Hofmann, E., et al. (2023). On the evolution of the plant phytochrome chromophore biosynthesis. Plant Physiol. 193, 246–258. doi: 10.1093/plphys/kiad327
Frohnmeyer, H. and Staiger, D. (2003). Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 133, 1420–1428. doi: 10.1104/pp.103.030049
Fujisawa, T. and Masuda, S. (2018). Light-induced chromophore and protein responses and mechanical signal transduction of BLUF proteins. Biophys. Rev. 10, 327–337. doi: 10.1007/s12551-017-0355-6
Fujiu, K., Nakayama, Y., Yanagisawa, A., Sokabe, M., and Yoshimura, K. (2009). Chlamydomonas CAV2 encodes a voltage- dependent calcium channel required for the flagellar waveform conversion. Curr. Biol. 19, 133–139. doi: 10.1016/j.cub.2008.11.068
Galperin, M. Y. (2006). Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J. Bacteriol. 188, 4169–4182. doi: 10.1128/JB.01887-05
Galvão, V. C. and Fankhauser, C. (2015). Sensing the light environment in plants: photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 34, 46–53. doi: 10.1016/j.conb.2015.01.013
Ganjawala, T. H., Lu, Q., Fenner, M. D., Abrams, G. W., and Pan, Z. H. (2019). Improved CoChR variants restore visual acuity and contrast sensitivity in a mouse model of blindness under ambient light conditions. Mol. Ther. 27, 1195–1205. doi: 10.1016/j.ymthe.2019.04.002
Gao, S. (2015). Characterizing new photoreceptors to expand the optogenetic toolbox. Würzburg, Germany: Julius-Maximilians-Universität Würzburg.
Gomelsky, M. and Kaplan, S. (1998). AppA, a redox regulator of photosystem formation in Rhodobacter sphaeroides 2.4.1, is a flavoprotein. Identification of a novel fad binding domain. J. Biol. Chem. 273, 35319–35325. doi: 10.1074/jbc.273.52.35319
Gomelsky, M. and Klug, G. (2002). BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem. Sci. 27, 497–500. doi: 10.1016/S0968-0004(02)02181-3
Goodenough, U., Lin, H., and Lee, J. H. (2007). Sex determination in chlamydomonas. Semin. Cell Dev. Biol. 18, 350–361. doi: 10.1016/j.semcdb.2007.02.006
Goodstein, D. M., Shu, S., Howson, R., Neupane, R., Hayes, R. D., Fazo, J., et al. (2012). Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186. doi: 10.1093/nar/gkr944
Goswami, D. Y., Kreith, F., and Kreider, J. F. (1999). Principles of Solar Engineering (Philadelphia: Taylors & Francis Co).
Gotthard, G., Mous, S., Weinert, T., Maia, R. N. A., James, D., Dworkowski, F., et al. (2024). Capturing the blue-light activated state of the Phot-LOV1 domain from Chlamydomonas reinhardtii using time-resolved serial synchrotron crystallography. IUCrJ 11, 792–808. doi: 10.1107/S2052252524005608
Govindjee, G. (1999). “Carotenoids in photosynthesis: An historical perspective,” in The Photochemistry of Carotenoids. Eds. Frank, H. A., Young, A. J., Britton, G., and Cogdell, R. J. (Springer Netherlands, Dordrecht), 1–19.
Govorunova, E. G. and Sineshchekov, O. A. (2023). Channelrhodopsins: From phototaxis to optogenetics. Biochem. (Mosc) 88, 1555–1570. doi: 10.1134/S0006297923100115
Govorunova, E. G., Sineshchekov, O. A., Janz, R., Liu, X., and Spudich, J. L. (2015). Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science 349, 647–650. doi: 10.1126/science.aaa7484
Govorunova, E. G., Sineshchekov, O. A., Li, H., Janz, R., and Spudich, J. L. (2013). Characterization of a highly efficient blue-shifted channelrhodopsin from the marine alga Platymonas subcordiformis. J. Biol. Chem. 288, 29911–29922. doi: 10.1074/jbc.M113.505495
Govorunova, E. G., Sineshchekov, O. A., Li, H., and Spudich, J. L. (2017). Microbial rhodopsins: diversity, mechanisms, and optogenetic applications. Annu. Rev. Biochem. 86, 845–872. doi: 10.1146/annurev-biochem-101910-144233
Govorunova, E. G., Sineshchekov, O. A., and Spudich, J. L. (2016). Structurally distinct cation channelrhodopsins from cryptophyte algae. Biophys. J. 110, 2302–2304. doi: 10.1016/j.bpj.2016.05.001
Govorunova, E. G., Sineshchekov, O. A., and Spudich, J. L. (2022). Emerging diversity of channelrhodopsins and their structure-function relationships. Front. Cell. Neurosci. 15. doi: 10.3389/fncel.2021.800313
Govorunova, E. G., Spudich, E. N., Lane, C. E., Sineshchekov, O. A., and Spudich, J. L. (2011). New channelrhodopsin with a red-shifted spectrum and rapid kinetics from Mesostigma viride. MBio 2, e00115–11. doi: 10.1128/mBio.00115-11
Graham, L. E. and Wilcox, L. W. (2000). Algae. 1st Edition (Upper Saddle River, NJ, USA: Prentice Hall).
Grossman, A. R., Lohr, M., and Im, C. S. (2004). Chlamydomonas reinhardtii in the landscape of pigments. Annu. Rev. Genet. 38, 119–173. doi: 10.1146/annurev.genet.38.072902.092328
Grote, M., Engelhard, M., and Hegemann, P. (2014). Of ion pumps, sensors and channels - perspectives on microbial rhodopsins between science and history. Biochim. Biophys. Acta 1837, 533–545. doi: 10.1016/j.bbabio.2013.08.006
Groves, C. L. and Franklin, K. A. (2025). UV RESISTANCE LOCUS 8 signalling enhances photosynthetic resilience to herbicide-induced damage in Arabidopsis thaliana. New Phytol. 247, 1763–1776. doi: 10.1111/nph.v247.4
Guiry, M. D. (2012). How many species of algae are there? J. Phycol 48, 1057–1063. doi: 10.1111/j.1529-8817.2012.01222.x
Gunaydin, L. A., Yizhar, O., Berndt, A., Sohal, V. S., Deisseroth, K., and Hegemann, P. (2010). Ultrafast optogenetic control. Nat. Neurosci. 13, 387–392. doi: 10.1038/nn.2495
Gupta, N., Bansal, H., and Roy, S. (2019). Theoretical optimization of high-frequency optogenetic spiking of red-shifted very fast-Chrimson-expressing neurons. Neurophotonics 6, 025002. doi: 10.1117/1.NPh.6.2.025002
Guru, A., Post, R. J., Ho, Y. Y., and Warden, M. R. (2015). Making sense of optogenetics. Int. J. Neuropsychopharmacol. 18, pyv079. doi: 10.1093/ijnp/pyv079
Gushchin, I. and Gordeliy, V. (2018). Microbial rhodopsins. Subcell. Biochem. 87, 19–56. doi: 10.1007/978-981-10-7757-9_2
Halavaty, A. S. and Moffat, K. (2007). N- and C-terminal flanking regions modulate light-induced signal transduction in the LOV2 domain of the blue light sensor phototropin 1 from Avena sativa. Biochemistry 46, 14001–14009. doi: 10.1021/bi701543e
Hallmann, A. (2011). Evolution of reproductive development in the volvocine algae. Sex. Plant Reprod. 24, 97–112. doi: 10.1007/s00497-010-0158-4
Hallmann, A. (2015). Algae biotechnology – Green cell-factories on the rise. Curr. Biotechnol. 4, 389–415. doi: 10.2174/2211550105666151107001338
Hallmann, A. (2020). “Advances in genetic engineering of microalgae,” in Grand challenges in algae biotechnology. Eds. Hallmann, A. and Rampelotto, P. H. (Springer, Cham, Switzerland), 159–221.
Hallmann, A. (2024). Genetic Engineering von Mikroalgen für eine nachhaltige Zukunft. BIOspektrum 30, 61–65. doi: 10.1007/s12268-024-2110-4
Han, Y. J., Kim, S. H., and Kim, J. I. (2024). Phytochrome phosphorylation in plant light signaling. Front. Plant Sci. 15, 1259720. doi: 10.3389/fpls.2024.1259720
Harper, S. M., Christie, J. M., and Gardner, K. H. (2004). Disruption of the LOV-Jalpha helix interaction activates phototropin kinase activity. Biochemistry 43, 16184–16192. doi: 10.1021/bi048092i
Harper, S. M., Neil, L. C., and Gardner, K. H. (2003). Structural basis of a phototropin light switch. Science 301, 1541–1544. doi: 10.1126/science.1086810
Harris, E. H., Stern, D. B., and Witman, G. B. (2009). The Chlamydomonas sourcebook. 2nd edition (San Diego, CA: Academic Press).
Hart, J. E. and Gardner, K. H. (2021). Lighting the way: Recent insights into the structure and regulation of phototropin blue light receptors. J. Biol. Chem. 296, 100594. doi: 10.1016/j.jbc.2021.100594
He, Y., Barone, M., Meech, S. R., Lukacs, A., and Tonge, P. J. (2025a). Light-driven enzyme catalysis: Ultrafast mechanisms and biochemical implications. Biochemistry 64, 2491–2505. doi: 10.1021/acs.biochem.5c00039
He, Y., Wei, Z., Xu, J., Jin, F., Li, T., Qian, L., et al. (2025b). Genetics-based targeting strategies for precise neuromodulation. Adv. Sci. (Weinh.) 12, e13817. doi: 10.1002/advs.202413817
Hegemann, P. (2008). Algal sensory photoreceptors. Annu. Rev. Plant Biol. 59, 167–189. doi: 10.1146/annurev.arplant.59.032607.092847
Hegemann, P., Fuhrmann, M., and Kateriya, S. (2001). Algal sensory photoreceptors. J. Phycol. 37, 668–676. doi: 10.1046/j.1529-8817.2001.01095.x
Hegemann, P. and Nagel, G. (2013). From channelrhodopsins to optogenetics. EMBO Mol. Med. 5, 173–176. doi: 10.1002/emmm.201202387
Heijde, M., Zabulon, G., Corellou, F., Ishikawa, T., Brazard, J., Usman, A., et al. (2010). Characterization of two members of the cryptochrome/photolyase family from Ostreococcus tauri provides insights into the origin and evolution of cryptochromes. Plant Cell Environ. 33, 1614–1626. doi: 10.1111/j.1365-3040.2010.02168.x
Heintz, U. and Schlichting, I. (2016). Blue light-induced LOV domain dimerization enhances the affinity of Aureochrome 1a for its target DNA sequence. Elife 5, e11860. doi: 10.7554/eLife.11860
Hepp, S., Trauth, J., Hasenjager, S., Bezold, F., Essen, L. O., and Taxis, C. (2020). An optogenetic tool for induced protein stabilization based on the Phaeodactylum tricornutum Aureochrome 1a light-oxygen-voltage domain. J. Mol. Biol. 432, 1880–1900. doi: 10.1016/j.jmb.2020.02.019
Herman, E., Sachse, M., Kroth, P. G., and Kottke, T. (2013). Blue-light-induced unfolding of the Jα helix allows for the dimerization of aureochrome-LOV from the diatom Phaeodactylum tricornutum. Biochemistry 52, 3094–3101. doi: 10.1021/bi400197u
Hochbaum, D. R., Zhao, Y., Farhi, S. L., Klapoetke, N., Werley, C. A., Kapoor, V., et al. (2014). All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 11, 825–833. doi: 10.1038/nmeth.3000
Hoff, W. D., Jung, K. H., and Spudich, J. L. (1997). Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu. Rev. Biophys. Biomol Struct. 26, 223–258. doi: 10.1146/annurev.biophys.26.1.223
Hoffmann, M. D., Bubeck, F., Eils, R., and Niopek, D. (2018). Controlling cells with light and LOV. AD Biosys. 2, 1800098. doi: 10.1002/adbi.201800098
Hou, S. Y., Govorunova, E. G., Ntefidou, M., Lane, C. E., Spudich, E. N., Sineshchekov, O. A., et al. (2011). Diversity of Chlamydomonas channelrhodopsins. Photochem. Photobiol. 88, 119–128. doi: 10.1111/j.1751-1097.2011.01027.x
Huala, E., Oeller, P. W., Liscum, E., Han, I. S., Larsen, E., and Briggs, W. R. (1997). Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278, 2120–2123. doi: 10.1126/science.278.5346.2120
Huang, K. and Beck, C. F. (2003). Phototropin is the blue-light receptor that controls multiple steps in the sexual life cycle of the green alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A. 100, 6269–6274. doi: 10.1073/pnas.0931459100
Huang, K., Merkle, T., and Beck, C. F. (2002). Isolation and characterization of a Chlamydomonas gene that encodes a putative blue-light photoreceptor of the phototropin family. Physiol. Plant. 115, 613–622. doi: 10.1034/j.1399-3054.2002.1150416.x
Huber, C., Strack, M., Schultheiss, I., Pielage, J., Mechler, X., Hornbogen, J., et al. (2024). Darkness inhibits autokinase activity of bacterial bathy phytochromes. J. Biol. Chem. 300, 107148. doi: 10.1016/j.jbc.2024.107148
Huet, A., Mager, T., Gossler, C., and Moser, T. (2024). Toward optogenetic hearing restoration. Annu. Rev. Neurosci. 47, 103–121. doi: 10.1146/annurev-neuro-070623-103247
Hughes, J. and Winkler, A. (2024). New insight into phytochromes: connecting structure to function. Annu. Rev. Plant Biol. 75, 153–183. doi: 10.1146/annurev-arplant-070623-110636
Huq, E., Lin, C., and Quail, P. H. (2024). Light signaling in plants-a selective history. Plant Physiol. 195, 213–231. doi: 10.1093/plphys/kiae110
Hyams, J. S. and Borisy, G. G. (1978). Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J. Cell Sci. 33, 235–253. doi: 10.1242/jcs.33.1.235
Ikuta, T., Shihoya, W., Sugiura, M., Yoshida, K., Watari, M., Tokano, T., et al. (2020). Structural insights into the mechanism of rhodopsin phosphodiesterase. Nat. Commun. 11, 5605. doi: 10.1038/s41467-020-19376-7
Im, C. S., Eberhard, S., Huang, K., Beck, C. F., and Grossman, A. R. (2006). Phototropin involvement in the expression of genes encoding chlorophyll and carotenoid biosynthesis enzymes and LHC apoproteins in Chlamydomonas reinhardtii. Plant J. 48, 1–16. doi: 10.1111/j.1365-313X.2006.02852.x
Im, S. H., Lepetit, B., Mosesso, N., Shrestha, S., Weiss, L., Nymark, M., et al. (2024). Identification of promoter targets by Aureochrome 1a in the diatom Phaeodactylum tricornutum. J. Exp. Bot. 75, 1834–1851. doi: 10.1093/jxb/erad478
Inagaki, H. K., Jung, Y., Hoopfer, E. D., Wong, A. M., Mishra, N., Lin, J. Y., et al. (2014). Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 11, 325–332. doi: 10.1038/nmeth.2765
Inoue, K., Ito, S., Kato, Y., Nomura, Y., Shibata, M., Uchihashi, T., et al. (2016). A natural light-driven inward proton pump. Nat. Commun. 7, 13415. doi: 10.1038/ncomms13415
Inoue, K., Ono, H., Abe-Yoshizumi, R., Yoshizawa, S., Ito, H., Kogure, K., et al. (2013). A light-driven sodium ion pump in marine bacteria. Nat. Commun. 4, 1678. doi: 10.1038/ncomms2689
Inoue, K., Tahara, S., Kato, Y., Takeuchi, S., Tahara, T., and Kandori, H. (2018). Spectroscopic study of proton-transfer mechanism of inward proton-pump rhodopsin, Parvularcula oceani xenorhodopsin. J. Phys. Chem. B 122, 6453–6461. doi: 10.1021/acs.jpcb.8b01279
Inoue, K., Tsunoda, S. P., Singh, M., Tomida, S., Hososhima, S., Konno, M., et al. (2020). Schizorhodopsins: A family of rhodopsins from Asgard archaea that function as light-driven inward H+ pumps. Sci. Adv. 6, eaaz2441. doi: 10.1126/sciadv.aaz2441
Iseki, M., Matsunaga, S., Murakami, A., Ohno, K., Shiga, K., Yoshida, K., et al. (2002). A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 415, 1047–1051. doi: 10.1038/4151047a
Ishikawa, M., Takahashi, F., Nozaki, H., Nagasato, C., Motomura, T., and Kataoka, H. (2009). Distribution and phylogeny of the blue light receptors aureochromes in eukaryotes. Planta 230, 543–552. doi: 10.1007/s00425-009-0967-6
Isogai, N., Kamiya, R., and Yoshimura, K. (2000). Dominance between the two flagella during phototactic turning in Chlamydomonas. Zoolog. Sci. 17, 1261–1266. doi: 10.2108/zsj.17.1261
Iyer, S. M., Montgomery, K. L., Towne, C., Lee, S. Y., Ramakrishnan, C., Deisseroth, K., et al. (2014). Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat. Biotechnol. 32, 274–278. doi: 10.1038/nbt.2834
Jahn, I. (2004). Geschichte der biologie. Theorien, Methoden, Institutionen, Kurzbiografien (Hamburg: Nikol Verlagsges. mbH).
Jenkins, G. I. (2014). The UV-B photoreceptor UVR8: from structure to physiology. Plant Cell 26, 21–37. doi: 10.1105/tpc.113.119446
Jones, M. A. and Christie, J. M. (2008). Phototropin receptor kinase activation by blue light. Plant Signal Behav. 3, 44–46. doi: 10.4161/psb.3.1.4848
Jorissen, H. J., Braslavsky, S. E., Wagner, G., and Gartner, W. (2002). Heterologous expression and characterization of recombinant phytochrome from the green alga Mougeotia scalaris. Photochem. Photobiol. 76, 457–461. doi: 10.1562/0031-8655(2002)076<0457:HEACOR>2.0.CO;2
Juhas, M., Von Zadow, A., Spexard, M., Schmidt, M., Kottke, T., and Buchel, C. (2014). A novel cryptochrome in the diatom Phaeodactylum tricornutum influences the regulation of light-harvesting protein levels. FEBS J. 281, 2299–2311. doi: 10.1111/febs.2014.281.issue-9
Jung, A., Domratcheva, T., Tarutina, M., Wu, Q., Ko, W. H., Shoeman, R. L., et al. (2005). Structure of a bacterial BLUF photoreceptor: insights into blue light-mediated signal transduction. Proc. Natl. Acad. Sci. U.S.A. 102, 12350–12355. doi: 10.1073/pnas.0500722102
Jung, A., Reinstein, J., Domratcheva, T., Shoeman, R. L., and Schlichting, I. (2006). Crystal structures of the AppA BLUF domain photoreceptor provide insights into blue light-mediated signal transduction. J. Mol. Biol. 362, 717–732. doi: 10.1016/j.jmb.2006.07.024
Kagawa, T. and Suetsugu, N. (2007). Photometrical analysis with photosensory domains of photoreceptors in green algae. FEBS Lett. 581, 368–374. doi: 10.1016/j.febslet.2006.12.041
Kalvaitis, M. E., Johnson, L. A., Mart, R. J., Rizkallah, P., and Allemann, R. K. (2019). A noncanonical chromophore reveals structural rearrangements of the light-oxygen-voltage domain upon photoactivation. Biochemistry 58, 2608–2616. doi: 10.1021/acs.biochem.9b00255
Kami, C., Lorrain, S., Hornitschek, P., and Fankhauser, C. (2010). Light-regulated plant growth and development. Curr. Top. Dev. Biol. 91, 29–66. doi: 10.1016/S0070-2153(10)91002-8
Kamiya, R. and Witman, G. B. (1984). Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J. Cell Biol. 98, 97–107. doi: 10.1083/jcb.98.1.97
Kandori, H. (2015). Ion-pumping microbial rhodopsins. Front. Mol. Biosci. 2, 52. doi: 10.3389/fmolb.2015.00052
Kateriya, S., Nagel, G., Bamberg, E., and Hegemann, P. (2004). “Vision” in single-celled algae. News Physiol. Sci. 19, 133–137. doi: 10.1152/nips.01517.2004
Kato, H. E., Kamiya, M., Sugo, S., Ito, J., Taniguchi, R., Orito, A., et al. (2015). Atomistic design of microbial opsin-based blue-shifted optogenetics tools. Nat. Commun. 6, 7177. doi: 10.1038/ncomms8177
Kaushik, M. S., Sharma, R., Veetil, S. K., Srivastava, S. K., and Kateriya, S. (2019). Modular diversity of the BLUF proteins and their potential for the development of diverse optogenetic tools. Appl. Sci. 9, 3924. doi: 10.3390/app9183924
Kayushin, L. P. and Skulachev, V. P. (1974). Bacteriorhodopsin as an electrogenic proton pump: reconstitution of bacteriorhodopsin proteoliposomes generating delta psi and delta pH. FEBS Lett. 39, 39–42. doi: 10.1016/0014-5793(74)80011-6
Keeling, P. J. (2010). The endosymbiotic origin, diversification and fate of plastids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 729–748. doi: 10.1098/rstb.2009.0103
Keeling, P. J. (2013). The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu. Rev. Plant Biol. 64, 583–607. doi: 10.1146/annurev-arplant-050312-120144
Kennis, J. T. and Mathes, T. (2013). Molecular eyes: proteins that transform light into biological information. Interface Focus 3, 20130005. doi: 10.1098/rsfs.2013.0005
Kennis, J. T., Van Stokkum, I. H., Crosson, S., Gauden, M., Moffat, K., and Van Grondelle, R. (2004). The LOV2 domain of phototropin: a reversible photochromic switch. J. Am. Chem. Soc. 126, 4512–4513. doi: 10.1021/ja031840r
Kianianmomeni, A. (2014). More light behind gene expression. Trends Plant Sci. 19, 488–490. doi: 10.1016/j.tplants.2014.05.004
Kianianmomeni, A. and Hallmann, A. (2014). Algal photoreceptors: in vivo functions and potential applications. Planta 239, 1–26. doi: 10.1007/s00425-013-1962-5
Kianianmomeni, A. and Hallmann, A. (2015a). Spotlighted brains: Optogenetic activation and silencing of neurons. Trends Biochem. Sci. 40, 624–627. doi: 10.1016/j.tibs.2015.09.004
Kianianmomeni, A. and Hallmann, A. (2015b). Transcriptional analysis of Volvox photoreceptors suggests the existence of different cell-type specific light-signaling pathways. Curr. Genet. 61, 3–18. doi: 10.1007/s00294-014-0440-3
Kianianmomeni, A. and Hallmann, A. (2016). Algal photobiology: A rich source of unusual light sensitive proteins for synthetic biology and optogenetics. Methods Mol. Biol. 1408, 37–54. doi: 10.1007/978-1-4939-3512-3
Kianianmomeni, A., Stehfest, K., Nematollahi, G., Hegemann, P., and Hallmann, A. (2009). Channelrhodopsins of Volvox carteri are photochromic proteins that are specifically expressed in somatic cells under control of light, temperature, and the sex inducer. Plant Physiol. 151, 347–366. doi: 10.1104/pp.109.143297
Kim, S. S., Franconville, R., Turner-Evans, D., and Jayaraman, V. (2015). “Optogenetics in Drosophila melanogaster,” in New Techniques in Systems Neuroscience. Ed. Douglass, A. D. (Springer International Publishing, Cham), 147–176.
Kim, C. K., Yang, S. J., Pichamoorthy, N., Young, N. P., Kauvar, I., Jennings, J. H., et al. (2016). Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328. doi: 10.1038/nmeth.3770
Kiontke, S., Gobel, T., Brych, A., and Batschauer, A. (2020). DASH-type cryptochromes - solved and open questions. Biol. Chem. 401, 1487–1493. doi: 10.1515/hsz-2020-0182
Kirk, J. T. O. (1994). Light and photosynthesis in aquatic ecosystems (Cambridge: Cambridge University Press).
Kirk, M. M. and Kirk, D. L. (1985). Translational regulation of protein synthesis, in response to light, at a critical stage of Volvox development. Cell 41, 419–428. doi: 10.1016/S0092-8674(85)80015-5
Klapoetke, N. C., Murata, Y., Kim, S. S., Pulver, S. R., Birdsey-Benson, A., Cho, Y. K., et al. (2014). Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346. doi: 10.1038/nmeth.2836
Klare, J. P., Chizhov, I., and Engelhard, M. (2008). Microbial rhodopsins: scaffolds for ion pumps, channels, and sensors. Results Probl. Cell Differ. 45, 73–122. doi: 10.1007/400_2007_041
Kleinlogel, S., Feldbauer, K., Dempski, R. E., Fotis, H., Wood, P. G., Bamann, C., et al. (2011). Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 14, 513–518. doi: 10.1038/nn.2776
Kojima, K., Shibukawa, A., and Sudo, Y. (2020). The unlimited potential of microbial rhodopsins as optical tools. Biochemistry 59, 218–229. doi: 10.1021/acs.biochem.9b00768
Kojima, K. and Sudo, Y. (2023). Convergent evolution of animal and microbial rhodopsins. RSC Adv. 13, 5367–5381. doi: 10.1039/D2RA07073A
Kondoh, M., Shiraishi, C., Muller, P., Ahmad, M., Hitomi, K., Getzoff, E. D., et al. (2011). Light-induced conformational changes in full-length Arabidopsis thaliana cryptochrome. J. Mol. Biol. 413, 128–137. doi: 10.1016/j.jmb.2011.08.031
Kong, S. G. and Okajima, K. (2016). Diverse photoreceptors and light responses in plants. J. Plant Res. 129, 111–114. doi: 10.1007/s10265-016-0792-5
Kottke, T., Heberle, J., Hehn, D., Dick, B., and Hegemann, P. (2003). Phot-LOV1: photocycle of a blue-light receptor domain from the green alga Chlamydomonas reinhardtii. Biophys. J. 84, 1192–1201. doi: 10.1016/S0006-3495(03)74933-9
Kottke, T., Hegemann, P., Dick, B., and Heberle, J. (2006). The photochemistry of the light-, oxygen-, and voltage-sensitive domains in the algal blue light receptor phot. Biopolymers 82, 373–378. doi: 10.1002/bip.20510
Kottke, T., Oldemeyer, S., Wenzel, S., Zou, Y., and Mittag, M. (2017). Cryptochrome photoreceptors in green algae: Unexpected versatility of mechanisms and functions. J. Plant Physiol. 217, 4–14. doi: 10.1016/j.jplph.2017.05.021
Kottke, T., Xie, A., Larsen, D. S., and Hoff, W. D. (2018). Photoreceptors take charge: Emerging principles for light sensing. Annu. Rev. Biophys. 47, 291–313. doi: 10.1146/annurev-biophys-070317-033047
Kowalik, L. and Chen, J. K. (2017). Illuminating developmental biology through photochemistry. Nat. Chem. Biol. 13, 587–598. doi: 10.1038/nchembio.2369
Krah, M., Marwan, W., Vermeglio, A., and Oesterhelt, D. (1994). Phototaxis of Halobacterium salinarium requires a signalling complex of sensory rhodopsin I and its methyl-accepting transducer HtrI. EMBO J. 13, 2150–2155. doi: 10.1002/j.1460-2075.1994.tb06491.x
Kranz, H. D., Miks, D., Siegler, M. L., Capesius, I., Sensen, C. W., and Huss, V. A. (1995). The origin of land plants: phylogenetic relationships among charophytes, bryophytes, and vascular plants inferred from complete small-subunit ribosomal RNA gene sequences. J. Mol. Evol. 41, 74–84. doi: 10.1007/BF00174043
Kreimer, G. (1994). Cell biology of phototaxis in flagellate algae. Int. Rev. Cytol 148, 229–310. doi: 10.1016/S0074-7696(08)62409-2
Kreimer, G. (2009). The green algal eyespot apparatus: a primordial visual system and more? Curr. Genet. 55, 19–43. doi: 10.1007/s00294-008-0224-8
Kroth, P. G., Wilhelm, C., and Kottke, T. (2017). An update on aureochromes: Phylogeny - mechanism - function. J. Plant Physiol. 217, 20–26. doi: 10.1016/j.jplph.2017.06.010
Kuhlman, S. J., Craig, L. M., and Duffy, J. F. (2018). Introduction to chronobiology. Cold Spring Harb. Perspect. Biol. 10, a033613. doi: 10.1101/cshperspect.a033613
Kupper, H., Andresen, E., Wiegert, S., Simek, M., Leitenmaier, B., and Setlik, I. (2009). Reversible coupling of individual phycobiliprotein isoforms during state transitions in the cyanobacterium Trichodesmium analysed by single-cell fluorescence kinetic measurements. Biochim. Biophys. Acta 1787, 155–167. doi: 10.1016/j.bbabio.2009.01.001
Lamarche, L. B., Kumar, R. P., Trieu, M. M., Devine, E. L., Cohen-Abeles, L. E., Theobald, D. L., et al. (2017). Purification and characterization of RhoPDE, a retinylidene/phosphodiesterase fusion protein and potential optogenetic tool from the choanoflagellate Salpingoeca rosetta. Biochemistry 56, 5812–5822. doi: 10.1021/acs.biochem.7b00519
Lariguet, P. and Dunand, C. (2005). Plant photoreceptors: phylogenetic overview. J. Mol. Evol. 61, 559–569. doi: 10.1007/s00239-004-0294-2
Lawniczak, M. K. N., Durbin, R., Flicek, P., Lindblad-Toh, K., Wei, X., Archibald, J. M., et al. (2022). Standards recommendations for the earth bioGenome project. Proc. Natl. Acad. Sci. U.S.A. 119, e2115639118. doi: 10.1073/pnas.2115639118
Lee, C., Ahn, J. W., Kim, J. B., Kim, J. Y., and Choi, Y. E. (2018). Comparative transcriptome analysis of Haematococcus pluvialis on astaxanthin biosynthesis in response to irradiation with red or blue LED wavelength. World J. Microbiol. Biotechnol. 34, 96. doi: 10.1007/s11274-018-2459-y
Lenci, F. and Colombetti, G. (1978). Photobehavior of microorganisms: a biophysical approach. Annu. Rev. Biophys. Bioeng. 7, 341–361. doi: 10.1146/annurev.bb.07.060178.002013
Leppäranta, M. (2023). “Freezing of Lakes,” in Freezing of Lakes and the Evolution of Their Ice Cover. Ed. Leppäranta, M. (Springer International Publishing, Cham), 17–62.
Leptos, K. C., Chioccioli, M., Furlan, S., Pesci, A. I., and Goldstein, R. E. (2023). Phototaxis of Chlamydomonas arises from a tuned adaptive photoresponse shared with multicellular Volvocine green algae. Phys. Rev. E 107, 014404. doi: 10.1103/PhysRevE.107.014404
Levesque, M., Avoli, M., and Kokaia, M. (2024). “Optogenetic modulation of focal seizures,” in Jasper's Basic Mechanisms of the Epilepsies, 5th ed. Eds. Noebels, J. L., Avoli, M., Rogawski, M. A., Vezzani, A., and Delgado-Escueta, A. V. (New York, USA: Oxford University Press), 223–240.
Lewin, H. A., Robinson, G. E., Kress, W. J., Baker, W. J., Coddington, J., Crandall, K. A., et al. (2018). Earth BioGenome Project: Sequencing life for the future of life. Proc. Natl. Acad. Sci. U.S.A. 115, 4325–4333. doi: 10.1073/pnas.1720115115
Li, Y. Z. and Ji, R. R. (2024). Gene therapy for chronic pain management. Cell Rep. Med. 5, 101756. doi: 10.1016/j.xcrm.2024.101756
Li, X., Liu, Z., Ren, H., Kundu, M., Zhong, F. W., Wang, L., et al. (2022). Dynamics and mechanism of dimer dissociation of photoreceptor UVR8. Nat. Commun. 13, 93. doi: 10.1038/s41467-021-27756-w
Li, F. W. and Mathews, S. (2016). Evolutionary aspects of plant photoreceptors. J. Plant Res. 129, 115–122. doi: 10.1007/s10265-016-0785-4
Li, F. W., Melkonian, M., Rothfels, C. J., Villarreal, J. C., Stevenson, D. W., Graham, S. W., et al. (2015a). Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat. Commun. 6, 7852. doi: 10.1038/ncomms8852
Li, F. W., Rothfels, C. J., Melkonian, M., Villarreal, J. C., Stevenson, D. W., Graham, S. W., et al. (2015b). The origin and evolution of phototropins. Front. Plant Sci. 6, 637. doi: 10.3389/fpls.2015.00637
Li, F. W., Villarreal, J. C., Kelly, S., Rothfels, C. J., Melkonian, M., Frangedakis, E., et al. (2014). Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. Proc. Natl. Acad. Sci. U.S.A. 111, 6672–6677. doi: 10.1073/pnas.1319929111
Li, L., Wang, S., Wang, H., Sahu, S. K., Marin, B., Li, H., et al. (2020). The genome of Prasinoderma coloniale unveils the existence of a third phylum within green plants. Nat. Ecol. Evol. 4, 1220–1231. doi: 10.1038/s41559-020-1221-7
Lin, C. (2002). Blue light receptors and signal transduction. Plant Cell 14 Suppl. S207–S225. doi: 10.1105/tpc.000646
Lin, J. Y. (2011). A user's guide to channelrhodopsin variants: features, limitations and future developments. Exp. Physiol. 96, 19–25. doi: 10.1113/expphysiol.2009.051961
Lin, J. Y., Knutsen, P. M., Muller, A., Kleinfeld, D., and Tsien, R. Y. (2013). ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508. doi: 10.1038/nn.3502
Lin, J. Y., Lin, M. Z., Steinbach, P., and Tsien, R. Y. (2009). Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814. doi: 10.1016/j.bpj.2008.11.034
Liscum, E. and Briggs, W. R. (1995). Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7, 473–485. doi: 10.1105/tpc.7.4.473
Liu, B., Yang, Z., Gomez, A., Liu, B., Lin, C., and Oka, Y. (2016). Signaling mechanisms of plant cryptochromes in Arabidopsis thaliana. J. Plant Res. 129, 137–148. doi: 10.1007/s10265-015-0782-z
Lommer, M., Specht, M., Roy, A. S., Kraemer, L., Andreson, R., Gutowska, M. A., et al. (2012). Genome and low-iron response of an oceanic diatom adapted to chronic iron limitation. Genome Biol. 13, R66. doi: 10.1186/gb-2012-13-7-r66
Losi, A. (2007). Flavin-based Blue-Light photosensors: a photobiophysics update. Photochem. Photobiol. 83, 1283–1300. doi: 10.1111/j.1751-1097.2007.00196.x
Losi, A., Gardner, K. H., and Möglich, A. (2018). Blue-light receptors for optogenetics. Chem. Rev. 118, 10659–10709. doi: 10.1021/acs.chemrev.8b00163
Losi, A. and Gärtner, W. (2011). Old chromophores, new photoactivation paradigms, trendy applications: flavins in blue light-sensing photoreceptors. Photochem. Photobiol. 87, 491–510. doi: 10.1111/j.1751-1097.2011.00913.x
Losi, A. and Gärtner, W. (2012). The evolution of flavin-binding photoreceptors: an ancient chromophore serving trendy blue-light sensors. Annu. Rev. Plant Biol. 63, 49–72. doi: 10.1146/annurev-arplant-042811-105538
Losi, A., Kottke, T., and Hegemann, P. (2004). Recording of blue light-induced energy and volume changes within the wild-type and mutated phot-LOV1 domain from Chlamydomonas reinhardtii. Biophys. J. 86, 1051–1060. doi: 10.1016/S0006-3495(04)74180-6
Luck, M., Mathes, T., Bruun, S., Fudim, R., Hagedorn, R., Tran Nguyen, T. M., et al. (2012). A photochromic histidine kinase rhodopsin (HKR1) that is bimodally switched by ultraviolet and blue light. J. Biol. Chem. 287, 40083–40090. doi: 10.1074/jbc.M112.401604
Luck, M., Velazquez Escobar, F., Glass, K., Sabotke, M. I., Hagedorn, R., Corellou, F., et al. (2019). Photoreactions of the histidine kinase rhodopsin Ot-HKR from the marine picoalga Ostreococcus tauri. Biochemistry 58, 1878–1891. doi: 10.1021/acs.biochem.8b01200
Lüning, K. (1990). Seaweeds: Their environment, biogeography and ecophysiology (New York: Wiley-Interscience).
Maddison, D. R. and Schulz, K.-S. (2007). The tree of life web project. Available online at: http://tolweb.org. (Accessed September 29, 2025).
Madhuri, S., Lepetit, B., Fürst, A. H., and Kroth, P. G. (2024). A knockout of the photoreceptor PtAureo1a results in altered diel expression of diatom clock components. Plants (Basel) 13, 1465. doi: 10.3390/plants13111465
Makita, Y., Suzuki, S., Fushimi, K., Shimada, S., Suehisa, A., Hirata, M., et al. (2021). Identification of a dual orange/far-red and blue light photoreceptor from an oceanic green picoplankton. Nat. Commun. 12, 3593. doi: 10.1038/s41467-021-23741-5
Maltsev, Y., Maltseva, K., Kulikovskiy, M., and Maltseva, S. (2021). Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biol. (Basel) 10, 1060. doi: 10.3390/biology10101060
Mardinly, A. R., Oldenburg, I. A., Pegard, N. C., Sridharan, S., Lyall, E. H., Chesnov, K., et al. (2018). Precise multimodal optical control of neural ensemble activity. Nat. Neurosci. 21, 881–893. doi: 10.1038/s41593-018-0139-8
Mat, A., Vu, H. H., Wolf, E., and Tessmar-Raible, K. (2024). All light, everywhere? Photoreceptors at nonconventional sites. Physiol. (Bethesda) 39, 0. doi: 10.1152/physiol.00017.2023
Mathes, T. and Götze, J. P. (2015). A proposal for a dipole-generated BLUF domain mechanism. Front. Mol. Biosci. 2, 62. doi: 10.3389/fmolb.2015.00062
Matiiv, A. B. and Chekunova, E. M. (2018). Aureochromes - Blue light receptors. Biochemistry (Mosc.) 83, 662–673. doi: 10.1134/S0006297918060044
Matsuo, T., Iida, T., Ohmura, A., Gururaj, M., Kato, D., Mutoh, R., et al. (2020). The role of ROC75 as a daytime component of the circadian oscillator in Chlamydomonas reinhardtii. PloS Genet. 16, e1008814. doi: 10.1371/journal.pgen.1008814
Matsuo, T. and Ishiura, M. (2010). New insights into the circadian clock in Chlamydomonas. Int. Rev. Cell Mol. Biol. 280, 281–314. doi: 10.1016/S1937-6448(10)80006-1
Matsuo, T. and Ishiura, M. (2011). Chlamydomonas reinhardtii as a new model system for studying the molecular basis of the circadian clock. FEBS Lett. 585, 1495–1502. doi: 10.1016/j.febslet.2011.02.025
Matsuo, T., Okamoto, K., Onai, K., Niwa, Y., Shimogawara, K., and Ishiura, M. (2008). A systematic forward genetic analysis identified components of the Chlamydomonas circadian system. Genes Dev. 22, 918–930. doi: 10.1101/gad.1650408
Matsuoka, D. and Tokutomi, S. (2005). Blue light-regulated molecular switch of Ser/Thr kinase in phototropin. Proc. Natl. Acad. Sci. U.S.A. 102, 13337–13342. doi: 10.1073/pnas.0506402102
Mattis, J., Tye, K. M., Ferenczi, E. A., Ramakrishnan, C., O'shea, D. J., Prakash, R., et al. (2011). Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172. doi: 10.1038/nmeth.1808
Mcdonough, K. A. and Rodriguez, A. (2011). The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat. Rev. Microbiol. 10, 27–38. doi: 10.1038/nrmicro2688
Mcisaac, R. S., Bedbrook, C. N., and Arnold, F. H. (2015). Recent advances in engineering microbial rhodopsins for optogenetics. Curr. Opin. Struct. Biol. 33, 8–15. doi: 10.1016/j.sbi.2015.05.001
Mitra, D., Yang, X., and Moffat, K. (2012). Crystal structures of Aureochrome1 LOV suggest new design strategies for optogenetics. Structure 20, 698–706. doi: 10.1016/j.str.2012.02.016
Mittag, M. (2001). Circadian rhythms in microalgae. Int. Rev. Cytol. 206, 213–247. doi: 10.1016/S0074-7696(01)06023-5
Mittag, M., Kiaulehn, S., and Johnson, C. H. (2005). The circadian clock in Chlamydomonas reinhardtii. What is it for? What is it similar to? Plant Physiol. 137, 399–409. doi: 10.1104/pp.104.052415
Möglich, A., Yang, X., Ayers, R. A., and Moffat, K. (2010). Structure and function of plant photoreceptors. Annu. Rev. Plant Biol. 61, 21–47. doi: 10.1146/annurev-arplant-042809-112259
Mohanty, S. K., Mahapatra, S., Batabyal, S., Carlson, M., Kanungo, G., Ayyagari, A., et al. (2025). A synthetic opsin restores vision in patients with severe retinal degeneration. Mol. Ther. 33, 2279–2290. doi: 10.1016/j.ymthe.2025.03.031
Mouget, J. L., Gastineau, R., Davidovich, O., Gaudin, P., and Davidovich, N. A. (2009). Light is a key factor in triggering sexual reproduction in the pennate diatom Haslea ostrearia. FEMS Microbiol. Ecol. 69, 194–201. doi: 10.1111/j.1574-6941.2009.00700.x
Moulager, M., Corellou, F., Verge, V., Escande, M. L., and Bouget, F. Y. (2010). Integration of light signals by the retinoblastoma pathway in the control of S phase entry in the picophytoplanktonic cell Ostreococcus. PloS Genet. 6, e1000957. doi: 10.1371/journal.pgen.1000957
Muaddi, J. A. and Jamal, M. A. (1991). Solar spectrum at depth in water. Renewable Energy 1, 31–35. doi: 10.1016/0960-1481(91)90100-4
Mukherjee, S., Hegemann, P., and Broser, M. (2019). Enzymerhodopsins: novel photoregulated catalysts for optogenetics. Curr. Opin. Struct. Biol. 57, 118–126. doi: 10.1016/j.sbi.2019.02.003
Mukohata, Y. and Kaji, Y. (1981). Light-induced membrane-potential increase, ATP synthesis, and proton uptake in Halobacterium halobium, R1mR catalyzed by halorhodopsin: Effects of N,N'-dicyclohexylcarbodiimide, triphenyltin chloride, and 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile (SF6847). Arch. Biochem. Biophys. 206, 72–76. doi: 10.1016/0003-9861(81)90067-9
Müller, N., Wenzel, S., Zou, Y., Künzel, S., Sasso, S., Weiß, D., et al. (2017). A plant cryptochrome controls key features of the Chlamydomonas circadian clock and its life cycle. Plant Physiol. 174, 185–201. doi: 10.1104/pp.17.00349
Müller-Xing, R., Xing, Q., and Goodrich, J. (2014). Footprints of the sun: memory of UV and light stress in plants. Front. Plant Sci. 5, 474. doi: 10.3389/fpls.2014.00474
Mutschlechner, M., Walter, A., Colleselli, L., Griesbeck, C., and Schöbel, H. (2022). Enhancing carotenogenesis in terrestrial microalgae by UV-A light stress. J. Appl. Phycology 34, 1943–1955. doi: 10.1007/s10811-022-02772-5
Nagata, T. and Inoue, K. (2021). Rhodopsins at a glance. J. Cell Sci. 134, jcs258989. doi: 10.1242/jcs.258989
Nagel, G., Brauner, M., Liewald, J. F., Adeishvili, N., Bamberg, E., and Gottschalk, A. (2005). Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284. doi: 10.1016/j.cub.2005.11.032
Nagel, G., Ollig, D., Fuhrmann, M., Kateriya, S., Musti, A. M., Bamberg, E., et al. (2002). Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296, 2395–2398. doi: 10.1126/science.1072068
Nagel, G., Szellas, T., Huhn, W., Kateriya, S., Adeishvili, N., Berthold, P., et al. (2003). Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U.S.A. 100, 13940–13945. doi: 10.1073/pnas.1936192100
Nash, A. I., Mcnulty, R., Shillito, M. E., Swartz, T. E., Bogomolni, R. A., Luecke, H., et al. (2011). Structural basis of photosensitivity in a bacterial light-oxygen-voltage/helix-turn-helix (LOV-HTH) DNA-binding protein. Proc. Natl. Acad. Sci. U.S.A. 108, 9449–9454. doi: 10.1073/pnas.1100262108
Nathans, J. (1992). Rhodopsin: structure, function, and genetics. Biochemistry 31, 4923–4931. doi: 10.1021/bi00136a001
Nickelsen, J. and Rengstl, B. (2013). Photosystem II assembly: from cyanobacteria to plants. Annu. Rev. Plant Biol. 64, 609–635. doi: 10.1146/annurev-arplant-050312-120124
Niyogi, K. K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333–359. doi: 10.1146/annurev.arplant.50.1.333
Noordally, Z. B. and Millar, A. J. (2015). Clocks in algae. Biochemistry 54, 171–183. doi: 10.1021/bi501089x
Nozue, K., Kanegae, T., Imaizumi, T., Fukuda, S., Okamoto, H., Yeh, K. C., et al. (1998). A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc. Natl. Acad. Sci. U.S.A. 95, 15826–15830. doi: 10.1073/pnas.95.26.15826
Oda, K., Vierock, J., Oishi, S., Rodriguez-Rozada, S., Taniguchi, R., Yamashita, K., et al. (2018). Crystal structure of the red light-activated channelrhodopsin Chrimson. Nat. Commun. 9, 3949. doi: 10.1038/s41467-018-06421-9
Oesterhelt, D. and Stoeckenius, W. (1971). Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat. New Biol. 233, 149–152. doi: 10.1038/newbio233149a0
Oesterhelt, D. and Stoeckenius, W. (1973). Functions of a new photoreceptor membrane. Proc. Natl. Acad. Sci. U.S.A. 70, 2853–2857. doi: 10.1073/pnas.70.10.2853
Ohki, Y., Shinone, T., Inoko, S., Sudo, M., Demura, M., Kikukawa, T., et al. (2023). The preferential transport of NO3- by full-length Guillardia theta anion channelrhodopsin 1 is enhanced by its extended cytoplasmic domain. J. Biol. Chem. 299, 105305. doi: 10.1016/j.jbc.2023.105305
Okajima, K. (2016). Molecular mechanism of phototropin light signaling. J. Plant Res. 129, 149–157. doi: 10.1007/s10265-016-0783-6
Oldemeyer, S., Mittag, M., and Kottke, T. (2019). Time-resolved infrared and visible spectroscopy on cryptochrome aCRY: Basis for red light reception. Biophys. J. 117, 490–499. doi: 10.1016/j.bpj.2019.06.027
Oldenhof, H., Bisova, K., Van Den Ende, H., and Zachleder, V. (2004a). Effect of red and blue light on the timing of cyclin-dependent kinase activity and the timing of cell division in Chlamydomonas reinhardtii. Plant Physiol. Biochem. 42, 341–348. doi: 10.1016/j.plaphy.2004.02.002
Oldenhof, H., Zachleder, V., and Van Den Ende, H. (2004b). Blue light delays commitment to cell division in Chlamydomonas reinhardtii. Plant Biol. (Stuttg) 6, 689–695. doi: 10.1055/s-2004-821341
Olofsson, N., Lazaridis, I., Meletis, K., Carlén, M., Tingström, U., and Karlsson, H. (2015). Lasers, optics enhance optogenetics studies. BioPhotonics, 31–35.
One Thousand Plant Transcriptomes Initiative (2019). One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574, 679–685. doi: 10.1038/s41586-019-1693-2
Onodera, A., Kong, S. G., Doi, M., Shimazaki, K. I., Christie, J., Mochizuki, N., et al. (2005). Phototropin from Chlamydomonas reinhardtii is functional in Arabidopsis thaliana. Plant Cell Physiol. 46, 367–374. doi: 10.1093/pcp/pci037
Ozawa, S., Nield, J., Terao, A., Stauber, E. J., Hippler, M., Koike, H., et al. (2009). Biochemical and structural studies of the large Ycf4-photosystem I assembly complex of the green alga Chlamydomonas reinhardtii. Plant Cell 21, 2424–2442. doi: 10.1105/tpc.108.063313
Palczewski, K. (2006). G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767. doi: 10.1146/annurev.biochem.75.103004.142743
Pan, J. M., Haring, M. A., and Beck, C. F. (1997). Characterization of blue light signal transduction chains that control development and maintenance of sexual competence in Chlamydomonas reinhardtii. Plant Physiol. 115, 1241–1249. doi: 10.1104/pp.115.3.1241
Parihar, P., Singh, R., Singh, S., Tripathi, D. K., Chauhan, D. K., Singh, V. P., et al. (2016). Photoreceptors mapping from past history till date. J. Photochem. Photobiol. B 162, 223–231. doi: 10.1016/j.jphotobiol.2016.06.020
Park, S. Y. and Tame, J. R. H. (2017). Seeing the light with BLUF proteins. Biophys. Rev. 9, 169–176. doi: 10.1007/s12551-017-0258-6
Permann, C., Becker, B., and Holzinger, A. (2022). Temperature- and light stress adaptations in Zygnematophyceae: The challenges of a semi-terrestrial lifestyle. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.945394
Petersen, J., Rredhi, A., Szyttenholm, J., Oldemeyer, S., Kottke, T., and Mittag, M. (2021). The world of algae reveals a broad variety of cryptochrome properties and functions. Front. Plant Sci. 12, 766509. doi: 10.3389/fpls.2021.766509
Petroutsos, D., Tokutsu, R., Maruyama, S., Flori, S., Greiner, A., Magneschi, L., et al. (2016). A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature 537, 563–566. doi: 10.1038/nature19358
Piatkevich, K. D. and Boyden, E. S. (2023). Optogenetic control of neural activity: The biophysics of microbial rhodopsins in neuroscience. Q. Rev. Biophys. 57, e1. doi: 10.1017/S0033583523000033
Pierce, K. L., Premont, R. T., and Lefkowitz, R. J. (2002). Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650. doi: 10.1038/nrm908
Pierik, R. and Ballare, C. L. (2021). Control of plant growth and defense by photoreceptors: From mechanisms to opportunities in agriculture. Mol. Plant 14, 61–76. doi: 10.1016/j.molp.2020.11.021
Pobozy, K., Pobozy, T., Domanski, P., Derczynski, M., Konarski, W., and Domanska-Poboza, J. (2025). Evolution of light-sensitive proteins in optogenetic approaches for vision restoration: A comprehensive review. Biomedicines 13, 429. doi: 10.3390/biomedicines13020429
Podolec, R., Demarsy, E., and Ulm, R. (2021). Perception and signaling of ultraviolet-B radiation in plants. Annu. Rev. Plant Biol. 72, 793–822. doi: 10.1146/annurev-arplant-050718-095946
Pokorny, R., Klar, T., Hennecke, U., Carell, T., Batschauer, A., and Essen, L. O. (2008). Recognition and repair of UV lesions in loop structures of duplex DNA by DASH-type cryptochrome. Proc. Natl. Acad. Sci. U.S.A. 105, 21023–21027. doi: 10.1073/pnas.0805830106
Ponce-Toledo, R. I., Deschamps, P., Lopez-Garcia, P., Zivanovic, Y., Benzerara, K., and Moreira, D. (2017). An early-branching freshwater cyanobacterium at the origin of plastids. Curr. Biol. 27, 386–391. doi: 10.1016/j.cub.2016.11.056
Prigge, M., Schneider, F., Tsunoda, S. P., Shilyansky, C., Wietek, J., Deisseroth, K., et al. (2012). Color-tuned channelrhodopsins for multiwavelength optogenetics. J. Biol. Chem. 287, 31804–31812. doi: 10.1074/jbc.M112.391185
Prochnik, S. E., Umen, J., Nedelcu, A. M., Hallmann, A., Miller, S. M., Nishii, I., et al. (2010). Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science 329, 223–226. doi: 10.1126/science.1188800
Radakovits, R., Jinkerson, R. E., Fuerstenberg, S. I., Tae, H., Settlage, R. E., Boore, J. L., et al. (2012). Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropsis gaditana. Nat. Commun. 3, 686. doi: 10.1038/ncomms1688
Rai, N., Neugart, S., Yan, Y., Wang, F., Siipola, S. M., Lindfors, A. V., et al. (2019). How do cryptochromes and UVR8 interact in natural and simulated sunlight? J. Exp. Bot. 70, 4975–4990. doi: 10.1093/jxb/erz236
Raven, J. A. and Giordano, M. (2014). Algae. Curr. Biol. 24, R590–R595. doi: 10.1016/j.cub.2014.05.039
Rayko, E., Maumus, F., Maheswari, U., Jabbari, K., and Bowler, C. (2010). Transcription factor families inferred from genome sequences of photosynthetic stramenopiles. New Phytol. 188, 52–66. doi: 10.1111/j.1469-8137.2010.03371.x
Rico, J. M. and Guiry, M. D. (1996). Phototropism in seaweeds: A review. Scientia Marina 60, 273–281.
Ridge, K. D. (2002). Algal rhodopsins: phototaxis receptors found at last. Curr. Biol. 12, R588–R590. doi: 10.1016/S0960-9822(02)01099-0
Rindner, D. J. and Lur, G. (2023). Practical considerations in an era of multicolor optogenetics. Front. Cell Neurosci. 17, 1160245. doi: 10.3389/fncel.2023.1160245
Rizzini, L., Favory, J. J., Cloix, C., Faggionato, D., O'hara, A., Kaiserli, E., et al. (2011). Perception of UV-B by the arabidopsis UVR8 protein. Science 332, 103–106. doi: 10.1126/science.1200660
Rockwell, N. C., Duanmu, D., Martin, S. S., Bachy, C., Price, D. C., Bhattacharya, D., et al. (2014). Eukaryotic algal phytochromes span the visible spectrum. Proc. Natl. Acad. Sci. U.S.A. 111, 3871–3876. doi: 10.1073/pnas.1401871111
Rockwell, N. C. and Lagarias, J. C. (2017). Phytochrome diversification in cyanobacteria and eukaryotic algae. Curr. Opin. Plant Biol. 37, 87–93. doi: 10.1016/j.pbi.2017.04.003
Rockwell, N. C. and Lagarias, J. C. (2020). Phytochrome evolution in 3D: deletion, duplication, and diversification. New Phytol. 225, 2283–2300. doi: 10.1111/nph.v225.6
Rockwell, N. C., Su, Y. S., and Lagarias, J. C. (2006). Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 57, 837–858. doi: 10.1146/annurev.arplant.56.032604.144208
Rosenbaum, D. M., Rasmussen, S. G., and Kobilka, B. K. (2009). The structure and function of G-protein-coupled receptors. Nature 459, 356–363. doi: 10.1038/nature08144
Rosic, N. N. (2019). Mycosporine-like amino acids: making the foundation for organic personalised sunscreens. Mar. Drugs 17, 638. doi: 10.3390/md17110638
Rottwinkel, G., Oberpichler, I., and Lamparter, T. (2010). Bathy phytochromes in rhizobial soil bacteria. J. Bacteriol. 192, 5124–5133. doi: 10.1128/JB.00672-10
Roy, S., Llewellyn, C. A., Egeland, E. S., and Johnsen, G. (2011). Phytoplankton pigments: characterization, chemotaxonomy and applications in oceanography (Cambridge, UK: Cambridge University Press).
Rozenberg, A., Inoue, K., Kandori, H., and Beja, O. (2021). Microbial rhodopsins: The last two decades. Annu. Rev. Microbiol. 75, 427–447. doi: 10.1146/annurev-micro-031721-020452
Rredhi, A., Petersen, J., Schubert, M., Li, W., Oldemeyer, S., Li, W., et al. (2021). DASH cryptochrome 1, a UV-A receptor, balances the photosynthetic machinery of Chlamydomonas reinhardtii. New Phytol. 232, 610–624. doi: 10.1111/nph.v232.2
Rüffer, U. and Nultsch, W. (1998). Flagellar coordination in Chlamydomonas cells held on micropipettes. Cell Motil. Cytoskeleton 41, 297–307. doi: 10.1002/(SICI)1097-0169(1998)41:4<297::AID-CM3>3.0.CO;2-Y
Ruiz-González, M. X. and Marín, I. (2004). New insights into the evolutionary history of type 1 rhodopsins. J. Mol. Evol. 58, 348–358. doi: 10.1007/s00239-003-2557-8
Sager, J. C., Smith, W. O., Edwards, J. L., and Cyr, K. L. (1988). Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASAE 31, 1882–1889. doi: 10.13031/2013.30952
Sahel, J. A., Boulanger-Scemama, E., Pagot, C., Arleo, A., Galluppi, F., Martel, J. N., et al. (2021). Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 27, 1223–1229. doi: 10.1038/s41591-021-01351-4
Saito, T., Inoue, M., Yamada, M., and Matsuda, Y. (1998). Control of gametic differentiation and activity by light in Chlamydomonas reinhardtii. Plant Cell Physiol. 39, 8–15. doi: 10.1093/oxfordjournals.pcp.a029292
Sancar, A. (2003). Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 103, 2203–2237. doi: 10.1021/cr0204348
Saranak, J. and Foster, K. W. (1997). Rhodopsin guides fungal phototaxis. Nature 387, 465–466. doi: 10.1038/387465a0
Sasso, S., Stibor, H., Mittag, M., and Grossman, A. R. (2018). The natural history of model organisms: From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. Elife 7, e39233. doi: 10.7554/eLife.39233.011
Scheib, U., Broser, M., Constantin, O. M., Yang, S., Gao, S., Mukherjee, S., et al. (2018). Rhodopsin-cyclases for photocontrol of cGMP/cAMP and 2.3 A structure of the adenylyl cyclase domain. Nat. Commun. 9, 2046. doi: 10.1038/s41467-018-04428-w
Schellenberger Costa, B., Sachse, M., Jungandreas, A., Bartulos, C. R., Gruber, A., Jakob, T., et al. (2013). Aureochrome 1a is involved in the photoacclimation of the diatom Phaeodactylum tricornutum. PloS One 8, e74451. doi: 10.1371/journal.pone.0074451
Schmidt, T. M., Chen, S. K., and Hattar, S. (2011). Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 34, 572–580. doi: 10.1016/j.tins.2011.07.001
Schmidt, D. and Cho, Y. K. (2015). Natural photoreceptors and their application to synthetic biology. Trends Biotechnol. 33, 80–91. doi: 10.1016/j.tibtech.2014.10.007
Schneider, F., Grimm, C., and Hegemann, P. (2015). Biophysics of channelrhodopsin. Annu. Rev. Biophys. 44, 167–186. doi: 10.1146/annurev-biophys-060414-034014
Schulze, T., Prager, K., Dathe, H., Kelm, J., Kiessling, P., and Mittag, M. (2010). How the green alga Chlamydomonas reinhardtii keeps time. Protoplasma 244, 3–14. doi: 10.1007/s00709-010-0113-0
Selby, C. P. and Sancar, A. (2006). A cryptochrome/photolyase class of enzymes with single-stranded DNA-specific photolyase activity. Proc. Natl. Acad. Sci. U.S.A. 103, 17696–17700. doi: 10.1073/pnas.0607993103
Seth, K., Kumawat, G., Vyas, P., and Harish (2022). The structure and functional mechanism of eyespot in Chlamydomonas. J. Basic Microbiol. 62, 1169–1178. doi: 10.1002/jobm.202200249
Sgarbossa, A., Checcucci, G., and Lenci, F. (2002). Photoreception and photomovements of microorganisms. Photochem. Photobiol. Sci. 1, 459–467. doi: 10.1039/b110629e
Shankar, U., Lenka, S. K., Ackland, M. L., and Callahan, D. L. (2022). Review of the structures and functions of algal photoreceptors to optimize bioproduct production with novel bioreactor designs for strain improvement. Biotechnol. Bioeng. 119, 2031–2045. doi: 10.1002/bit.v119.8
Shao, J., Xue, S., Yu, G., Yu, Y., Yang, X., Bai, Y., et al. (2017). Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Sci. Transl. Med. 9, eaal2298. doi: 10.1126/scitranslmed.aal2298
Sharrock, R. A. (2008). The phytochrome red/far-red photoreceptor superfamily. Genome Biol. 9, 230. doi: 10.1186/gb-2008-9-8-230
Shaulsky, G. and Huang, E. (2005). “Components of the Dictyostelium gene expression regulatory machinery,” in Dictyostelium genomics. Eds. Loomis, W. F. and Kuspa, A. (Horizon Scientific Press, Norwich), 83–101.
Shevchenko, V., Mager, T., Kovalev, K., Polovinkin, V., Alekseev, A., Juettner, J., et al. (2017). Inward H+ pump xenorhodopsin: Mechanism and alternative optogenetic approach. Sci. Adv. 3, e1603187. doi: 10.1126/sciadv.1603187
Shi, X., Li, L., Guo, C., Lin, X., Li, M., and Lin, S. (2015). Rhodopsin gene expression regulated by the light dark cycle, light spectrum and light intensity in the dinoflagellate Prorocentrum. Front. Microbiol. 6, 555. doi: 10.3389/fmicb.2015.00555
Shichida, Y. and Matsuyama, T. (2009). Evolution of opsins and phototransduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2881–2895. doi: 10.1098/rstb.2009.0051
Shigemura, S., Hososhima, S., Kandori, H., and Tsunoda, S. P. (2019). Ion channel properties of a cation channelrhodopsin, Gt_CCR4. Appl. Sci. (Basel) 9, 3440. doi: 10.3390/app9173440
Simpson, A. S., Ludu, A., Cho, H. J., and Liu, H. (2014). Experimental and theoretical studies on visible light attenuation in water. arXiv: Atmospheric Oceanic Physics. 1408.3883. doi: 10.48550/arXiv.1408.3883
Sineshchekov, O. A., Govorunova, E. G., Li, H., and Spudich, J. L. (2015). Gating mechanisms of a natural anion channelrhodopsin. Proc. Natl. Acad. Sci. U.S.A. 112, 14236–14241. doi: 10.1073/pnas.1513602112
Sineshchekov, O. A., Govorunova, E. G., Wang, J., Li, H., and Spudich, J. L. (2013). Intramolecular proton transfer in channelrhodopsins. Biophys. J. 104, 807–817. doi: 10.1016/j.bpj.2013.01.002
Sineshchekov, O. A., Jung, K. H., and Spudich, J. L. (2002). Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A. 99, 8689–8694. doi: 10.1073/pnas.122243399
Singh, S., Agrawal, S. B., and Agrawal, M. (2014). UVR8 mediated plant protective responses under low UV-B radiation leading to photosynthetic acclimation. J. Photochem. Photobiol. B: Biol. 137, 67–76. doi: 10.1016/j.jphotobiol.2014.03.026
Slamovits, C. H., Okamoto, N., Burri, L., James, E. R., and Keeling, P. J. (2011). A bacterial proteorhodopsin proton pump in marine eukaryotes. Nat. Commun. 2, 183. doi: 10.1038/ncomms1188
Smith, R. C. and Baker, K. S. (1981). Optical properties of the clearest natural waters (200–800 nm). Appl. Opt. 20, 177–184. doi: 10.1364/AO.20.000177
Soppa, J. (1994). Two hypotheses - one answer. FEBS Lett. 342, 7–11. doi: 10.1016/0014-5793(94)80573-3
Spalding, E. P. (2000). Ion channels and the transduction of light signals. Plant Cell Environ. 23, 665–674. doi: 10.1046/j.1365-3040.2000.00594.x
Spudich, J. L. (2013). “Sensory rhodopsin I,” in Encyclopedia of biophysics. Ed. Roberts, G. C. K. (Springer Berlin Heidelberg, Berlin, Heidelberg), 2309–2312.
Spudich, J. L., Yang, C. S., Jung, K. H., and Spudich, E. N. (2000). Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 16, 365–392. doi: 10.1146/annurev.cellbio.16.1.365
Sridharan, S., Gajowa, M. A., Ogando, M. B., Jagadisan, U. K., Abdeladim, L., Sadahiro, M., et al. (2022). High-performance microbial opsins for spatially and temporally precise perturbations of large neuronal networks. Neuron 110, 1139–1155, e1136. doi: 10.1016/j.neuron.2022.01.008
Suetsugu, N., Mittmann, F., Wagner, G., Hughes, J., and Wada, M. (2005). A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc. Natl. Acad. Sci. U.S.A. 102, 13705–13709. doi: 10.1073/pnas.0504734102
Suetsugu, N. and Wada, M. (2013). Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: phototropin, ZTL/FKF1/LKP2 and aureochrome. Plant Cell Physiol. 54, 8–23. doi: 10.1093/pcp/pcs165
Sugiura, M., Tsunoda, S. P., Hibi, M., and Kandori, H. (2020). Molecular properties of new enzyme rhodopsins with phosphodiesterase activity. ACS Omega 5, 10602–10609. doi: 10.1021/acsomega.0c01113
Sullivan, J. A. and Deng, X. W. (2003). From seed to seed: the role of photoreceptors in development. Dev. Biol. 260, 289–297. doi: 10.1016/S0012-1606(03)00212-4
Suzuki, T., Yamasaki, K., Fujita, S., Oda, K., Iseki, M., Yoshida, K., et al. (2003). Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization. Biochem. Biophys. Res. Commun. 301, 711–717. doi: 10.1016/S0006-291X(02)03079-6
Taguchi, M., Sakuraba, S., Chan, J., and Kono, H. (2024). Unveiling the photoactivation mechanism of BLUF photoreceptor protein through hybrid quantum mechanics/molecular mechanics free-energy calculation. ACS Phys. Chem. Au 4, 647–659. doi: 10.1021/acsphyschemau.4c00040
Takahashi, F. (2016). Blue-light-regulated transcription factor, Aureochrome, in photosynthetic stramenopiles. J. Plant Res. 129, 189–197. doi: 10.1007/s10265-016-0784-5
Takahashi, M. and Mikami, K. (2016). Phototropism in the marine red macroalga Pyropia yezoensis. Am. J. Plant Sci. 7, 2412–2428. doi: 10.4236/ajps.2016.717211
Takahashi, M. and Mikami, K. (2019). A simple procedure to observe phototropic responses in the red seaweed. Phototropism 1926, 121–130. doi: 10.1007/978-1-4939-9015-3_9
Takahashi, F., Yamagata, D., Ishikawa, M., Fukamatsu, Y., Ogura, Y., Kasahara, M., et al. (2007). AUREOCHROME, a photoreceptor required for photomorphogenesis in stramenopiles. Proc. Natl. Acad. Sci. U.S.A. 104, 19625–19630. doi: 10.1073/pnas.0707692104
Tan, P., He, L., Huang, Y., and Zhou, Y. (2022). Optophysiology: Illuminating cell physiology with optogenetics. Physiol. Rev. 102, 1263–1325. doi: 10.1152/physrev.00021.2021
Tang, F., Yan, F., Zhong, Y., Li, J., Gong, H., and Li, X. (2024). Optogenetic brain-computer interfaces. Bioengineering (Basel) 11, 821. doi: 10.3390/bioengineering11080821
Tedetti, M. and Sempere, R. (2006). Penetration of ultraviolet radiation in the marine environment. A review. Photochem. Photobiol. 82, 389–397. doi: 10.1562/2005-11-09-IR-733
Tian, Y. (2018). Charakterisierung neuartiger Rhodopsine mit Licht-regulierter cGMP-Produktion oder cGMP-Degradation. Würzburg, Germany: Julius-Maximilians-Universität Würzburg.
Tian, Y., Gao, S., Von Der Heyde, E. L., Hallmann, A., and Nagel, G. (2018a). Two-component cyclase opsins of green algae are ATP-dependent and light-inhibited guanylyl cyclases. BMC Biol. 16, 144. doi: 10.1186/s12915-018-0613-5
Tian, Y., Gao, S., Yang, S., and Nagel, G. (2018b). A novel rhodopsin phosphodiesterase from Salpingoeca rosetta shows light-enhanced substrate affinity. Biochem. J. 475, 1121–1128. doi: 10.1042/BCJ20180010
Tian, Y., Yang, S., Nagel, G., and Gao, S. (2022). Characterization and modification of light-sensitive phosphodiesterases from choanoflagellates. Biomolecules 12, 88. doi: 10.3390/biom12010088
Tilbrook, K., Arongaus, A. B., Binkert, M., Heijde, M., Yin, R., and Ulm, R. (2013). The UVR8 UV-B photoreceptor: perception, signaling and response. Arabidopsis Book 11, e0164. doi: 10.1199/tab.0164
Timmis, J. N., Ayliffe, M. A., Huang, C. Y., and Martin, W. (2004). Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 5, 123–135. doi: 10.1038/nrg1271
Tischer, D. and Weiner, O. D. (2014). Illuminating cell signalling with optogenetic tools. Nat. Rev. Mol. Cell Biol. 15, 551–558. doi: 10.1038/nrm3837
Tomita, H., Sugano, E., Murayama, N., Ozaki, T., Nishiyama, F., Tabata, K., et al. (2014). Restoration of the majority of the visual spectrum by using modified Volvox channelrhodopsin-1. Mol. Ther. 22, 1434–1440. doi: 10.1038/mt.2014.81
Trippens, J., Greiner, A., Schellwat, J., Neukam, M., Rottmann, T., Lu, Y., et al. (2012). Phototropin influence on eyespot development and regulation of phototactic behavior in Chlamydomonas reinhardtii. Plant Cell 24, 4687–4702. doi: 10.1105/tpc.112.103523
Tsunoda, S. P., Sugiura, M., and Kandori, H. (2021). Molecular properties and optogenetic applications of enzymerhodopsins. Adv. Exp. Med. Biol. 1293, 153–165.
Ulijasz, A. T. and Vierstra, R. D. (2011). Phytochrome structure and photochemistry: recent advances toward a complete molecular picture. Curr. Opin. Plant Biol. 14, 498–506. doi: 10.1016/j.pbi.2011.06.002
Ulm, R. and Jenkins, G. I. (2015). Q&A: How do plants sense and respond to UV-B radiation? BMC Biol. 13, 45. doi: 10.1186/s12915-015-0156-y
Vaidya, A. T., Chen, C. H., Dunlap, J. C., Loros, J. J., and Crane, B. R. (2011). Structure of a light-activated LOV protein dimer that regulates transcription. Sci. Signal. 4, ra50. doi: 10.1126/scisignal.2001945
Vecchi, V., Barera, S., Bassi, R., and Dall'osto, L. (2020). Potential and challenges of improving photosynthesis in algae. Plants (Basel) 9, 67. doi: 10.3390/plants9010067
Venkatachalam, V. and Cohen, A. E. (2014). Imaging GFP-based reporters in neurons with multiwavelength optogenetic control. Biophys. J. 107, 1554–1563. doi: 10.1016/j.bpj.2014.08.020
Vierock, J. and Hegemann, P. (2023). “Sensory photoreceptors in Chlamydomonas (Chapter 6),” in The Chlamydomonas Sourcebook, 3rd ed. Eds. Grossman, A. R. and Wollman, F.-A. (Academic Press, London), 205–222.
Vlasova, A. D., Bukhalovich, S. M., Bagaeva, D. F., Polyakova, A. P., Ilyinsky, N. S., Nesterov, S. V., et al. (2024). Intracellular microbial rhodopsin-based optogenetics to control metabolism and cell signaling. Chem. Soc. Rev. 53, 3327–3349. doi: 10.1039/D3CS00699A
Wakabayashi, K. I., Isu, A., and Ueki, N. (2021). Channelrhodopsin-dependent photo-behavioral responses in the unicellular green alga Chlamydomonas reinhardtii. Adv. Exp. Med. Biol. 1293, 21–33. doi: 10.1007/978-981-15-8763-4_2
Wang, D., Cai, H., and Yang, O. (2021). Full solar-spectrum power-generation system based on high efficiency and wide spectral splitter film and fresnel lens. IEEE Access 9, 142147–142155. doi: 10.1109/ACCESS.2021.3119287
Wang, X. and Lin, C. (2025). The two action mechanisms of plant cryptochromes. Trends Plant Sci. 30, 775–791. doi: 10.1016/j.tplants.2024.12.001
Wang, T., Nonomura, T., Lan, T. H., and Zhou, Y. (2025). Optogenetic engineering for ion channel modulation. Curr. Opin. Chem. Biol. 85, 102569. doi: 10.1016/j.cbpa.2025.102569
Wang, X., Wang, Q., Nguyen, P., and Lin, C. (2014). Cryptochrome-mediated light responses in plants. Enzymes 35, 167–189. doi: 10.1016/B978-0-12-801922-1.00007-5
Watanabe, Y., Sugano, E., Tabata, K., Hatakeyama, A., Sakajiri, T., Fukuda, T., et al. (2021). Development of an optogenetic gene sensitive to daylight and its implications in vision restoration. NPJ Regener. Med. 6, 64. doi: 10.1038/s41536-021-00177-5
Watari, M., Ikuta, T., Yamada, D., Shihoya, W., Yoshida, K., Tsunoda, S. P., et al. (2019). Spectroscopic study of the transmembrane domain of a rhodopsin-phosphodiesterase fusion protein from a unicellular eukaryote. J. Biol. Chem. 294, 3432–3443. doi: 10.1074/jbc.RA118.006277
Weissbecker, J., Boumrifak, C., Breyer, M., Wiessalla, T., Shevchenko, V., Mager, T., et al. (2021). The voltage dependent sidedness of the reprotonation of the retinal schiff base determines the unique inward pumping of xenorhodopsin. Angew. Chem. Int. Ed. Engl. 60, 23010–23017. doi: 10.1002/anie.202103882
Weissig, H. and Beck, C. F. (1991). Action spectrum for the light-dependent step in gametic differentiation of Chlamydomonas reinhardtii. Plant Physiol. 97, 118–121. doi: 10.1104/pp.97.1.118
Werner, R. (2002). Chlamydomonas reinhardtii as a unicellular model for circadian rhythm analysis. Chronobiol. Int. 19, 325–343. doi: 10.1081/CBI-120002981
Wietek, J. and Prigge, M. (2016). Enhancing channelrhodopsins: An overview. Methods Mol. Biol. 1408, 141–165. doi: 10.1007/978-1-4939-3512-3_10
Wietek, J., Wiegert, J. S., Adeishvili, N., Schneider, F., Watanabe, H., Tsunoda, S. P., et al. (2014). Conversion of channelrhodopsin into a light-gated chloride channel. Science 344, 409–412. doi: 10.1126/science.1249375
Wright, W., Gajjeraman, S., Batabyal, S., Pradhan, S., Bhattacharya, S., Mahapatra, V., et al. (2017a). Restoring vision in mice with retinal degeneration using multicharacteristic opsin. Neurophotonics 4, 041505. doi: 10.1117/1.NPh.4.4.049801
Wright, W., Pradhan, S., Bhattacharya, S., Mahapatra, V., Tripathy, A., Gajjeraman, S., et al. (2017b). Multi-characteristic opsin enabled vision restoration. Proc. SPIE 10052, Optogenetics and Optical Manipulation, 100520Q. doi: 10.1117/12.2257527
Wrobel, C., Dieter, A., Huet, A., Keppeler, D., Duque-Afonso, C. J., Vogl, C., et al. (2018). Optogenetic stimulation of cochlear neurons activates the auditory pathway and restores auditory-driven behavior in deaf adult gerbils. Sci. Transl. Med. 10, eaao0540.
Wu, Q. and Gardner, K. H. (2009). Structure and insight into blue light-induced changes in the BlrP1 BLUF domain. Biochemistry 48, 2620–2629. doi: 10.1021/bi802237r
Wu, S. H., Mcdowell, M. T., and Lagarias, J. C. (1997). Phycocyanobilin is the natural precursor of the phytochrome chromophore in the green alga Mesotaenium caldariorum. J. Biol. Chem. 272, 25700–25705. doi: 10.1074/jbc.272.41.25700
Yan, L., Cao, X., Wang, L., Chen, J., Sancar, A., and Zhong, D. (2024). Dynamics and mechanism of DNA repair by a bifunctional cryptochrome. Proc. Natl. Acad. Sci. U.S.A. 121, e2417633121. doi: 10.1073/pnas.2417633121
Yang, X., Kuk, J., and Moffat, K. (2008). Crystal structure of Pseudomonas aeruginosa bacteriophytochrome: photoconversion and signal transduction. Proc. Natl. Acad. Sci. U.S.A. 105, 14715–14720. doi: 10.1073/pnas.0806718105
Yin, R. and Ulm, R. (2017). How plants cope with UV-B: from perception to response. Curr. Opin. Plant Biol. 37, 42–48. doi: 10.1016/j.pbi.2017.03.013
Yizhar, O., Fenno, L. E., Prigge, M., Schneider, F., Davidson, T. J., O'shea, D. J., et al. (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178. doi: 10.1038/nature10360
Yoshida, K., Tsunoda, S. P., Brown, L. S., and Kandori, H. (2017). A unique choanoflagellate enzyme rhodopsin exhibits light-dependent cyclic nucleotide phosphodiesterase activity. J. Biol. Chem. 292, 7531–7541. doi: 10.1074/jbc.M117.775569
Yu, X., Liu, H., Klejnot, J., and Lin, C. (2010). The cryptochrome blue light receptors. Arabidopsis Book 8, e0135. doi: 10.1199/tab.0135
Yuan, Y., Iannetta, A. A., Kim, M., Sadecki, P. W., Arend, M., Tsichla, A., et al. (2025). Phototropin connects blue light perception to starch metabolism in green algae. Nat. Commun. 16, 2545. doi: 10.1038/s41467-025-57809-3
Zabelskii, D., Alekseev, A., Kovalev, K., Rankovic, V., Balandin, T., Soloviov, D., et al. (2020). Viral rhodopsins 1 are an unique family of light-gated cation channels. Nat. Commun. 11, 5707. doi: 10.1038/s41467-020-19457-7
Zachleder, V., Bišová, K., Vítová, M., Kubín, Š., and Hendrychová, J. (2002). Variety of cell cycle patterns in the alga Scenedesmus quadricauda (Chlorophyta) as revealed by application of illumination regimes and inhibitors. Eur. J. Phycol. 37, 361–371. doi: 10.1017/S0967026202003815
Zarbin, M. A., Montemagno, C., Leary, J. F., and Ritch, R. (2013). Nanomedicine for the treatment of retinal and optic nerve diseases. Curr. Opin. Pharmacol. 13, 134–148. doi: 10.1016/j.coph.2012.10.003
Zarekarizi, A., Hoffmann, L., and Burritt, D. J. (2023). The potential of manipulating light for the commercial production of carotenoids from algae. Algal Res. 71, 103047. doi: 10.1016/j.algal.2023.103047
Zhang, H., Fang, H., Liu, D., Zhang, Y., Adu-Amankwaah, J., Yuan, J., et al. (2022). Applications and challenges of rhodopsin-based optogenetics in biomedicine. Front. Neurosci. 16, 966772. doi: 10.3389/fnins.2022.966772
Zhang, Q., Li, T., Xu, M., Islam, B., and Wang, J. (2024b). Application of optogenetics in neurodegenerative diseases. Cell Mol. Neurobiol. 44, 57. doi: 10.1007/s10571-024-01486-1
Zhang, Q., Lin, L., Fang, F., Cui, B., Zhu, C., Luo, S., et al. (2023). Dissecting the functions of COP1 in the UVR8 pathway with a COP1 variant in Arabidopsis. Plant J. 113, 478–492. doi: 10.1111/tpj.v113.3
Zhang, Q., Song, L., Fu, M., He, J., Yang, G., and Jiang, Z. (2024c). Optogenetics in oral and craniofacial research. J. Zhejiang Univ. Sci. B 25, 656–671. doi: 10.1631/jzus.B2300322
Zhang, F., Vierock, J., Yizhar, O., Fenno, L. E., Tsunoda, S., Kianianmomeni, A., et al. (2011). The microbial opsin family of optogenetic tools. Cell 147, 1446–1457. doi: 10.1016/j.cell.2011.12.004
Zhang, Q. R., Wang, S. X., and Chen, R. (2024d). Integrated bioelectronic and optogenetic methods to study brain-body circuits. ACS Nano 18, 30117–30122. doi: 10.1021/acsnano.4c07256
Zhang, H., Xiong, X., Guo, K., Zheng, M., Cao, T., Yang, Y., et al. (2024a). A rapid aureochrome opto-switch enables diatom acclimation to dynamic light. Nat. Commun. 15, 5578. doi: 10.1038/s41467-024-49991-7
Zhou, Y., Wei, Y., Li, L., Yan, T., and Ye, H. (2025). Optogenetics in medicine: innovations and therapeutic applications. Curr. Opin. Biotechnol. 92, 103262. doi: 10.1016/j.copbio.2025.103262
Ziegler, T., Schumacher, C. H., and Möglich, A. (2016). Guidelines for photoreceptor engineering. Methods Mol. Biol. 1408, 389–403. doi: 10.1007/978-1-4939-3512-3_27
Zoltowski, B. D., Motta-Mena, L. B., and Gardner, K. H. (2013). Blue light-induced dimerization of a bacterial LOV-HTH DNA-binding protein. Biochemistry 52, 6653–6661. doi: 10.1021/bi401040m
Zou, Y., Müller, N., and Mittag, M. (2015). The role of an animal-like cryptochrome in the life cycle of the unicellular green alga Chlamydomonas reinhardtii. Eur. J. Phycology 50, 69–69.
Keywords: algae, channelrhodopsins, photobiology, light perception, light sensing, photoreceptors, photosensors, optogenetics
Citation: Hallmann A (2025) Sensing a rainbow of colors: algal photoreceptors. Front. Plant Sci. 16:1684559. doi: 10.3389/fpls.2025.1684559
Received: 12 August 2025; Accepted: 23 September 2025;
Published: 14 October 2025.
Edited by:
Rei Narikawa, Shizuoka University, JapanReviewed by:
Nathan C. Rockwell, University of California, Davis, United StatesAndrea Flores-Ibarra, University of Cologne, Germany
Copyright © 2025 Hallmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Armin Hallmann, YXJtaW4uaGFsbG1hbm5AdW5pLWJpZWxlZmVsZC5kZQ==
†ORCID: Armin Hallmann, orcid.org/0000-0001-5977-6143
 Armin Hallmann
Armin Hallmann