- 1College of Life Sciences, Xinyang Normal University, Xinyang, China
- 2Henan Dabieshan National Field Observation and Research Station of Forest Ecosystem, Zhengzhou, China
- 3Xinyang Academy of Ecological Research, Xinyang, China
- 4School of Life Sciences, Henan University, Kaifeng, China
Biological invasions can lead to changes in the interspecific association and coexistence patterns of plant species; however, the differences in the interspecific association characteristics of invaded plant communities in heterogeneous habitats and their variations along latitudinal gradients remain unclear. Here, we established 40 terrestrial plots and 40 aquatic plots invaded by the amphibious invasive species Alternanthera philoxeroides (Mart.) Griseb. within the range of 21–37°N in China to explore the interspecific associations and association intensities of A. philoxeroides-invaded plant communities and their latitudinal trends. We found that there was a significantly positive interspecific association in the overall terrestrial communities, with A. philoxeroides having a strong association with many accompanying species. However, there was a nonsignificant negative interspecific association in the overall aquatic communities. The ratio of species pairs with positive/negative associations (PNR) in terrestrial communities dramatically decreased at higher latitudes. The values of the Jaccard index (JA), Ochiai index (OI), and Dice index (DI) which represent the interspecific association intensity in terrestrial communities, were extremely significantly greater than those in aquatic communities. The association intensity between terrestrial accompanying species significantly decreased with increasing latitude, and the association intensity between aquatic accompanying species varied weakly along the latitudinal gradient. Our study revealed that A. philoxeroides invasion aggravated interspecific competition among accompanying species in terrestrial plant communities in higher latitude regions, whereas the aquatic A. philoxeroides-invaded communities presented more mutualistic relationships to resist abiotic stress in higher latitude regions.
1 Introduction
Under current rapid global changes, increasing alien plant invasions have led to increased homogenization of communities and have severely damaged the inherent structure, processes, and functions of ecosystems. Plant invasions are also considered important causes of native species extinction (Gilbert and Levine, 2013; Waheed et al., 2024). Frequent resource fluctuations and species migration caused by human activities have accelerated the spread of invasive plants (Gao et al., 2021a; Borokini et al., 2023; Qian, 2023). Compared with native species, many invasive plants usually have a greater growth rate, reproductive ability, ecological adaptability and allelopathy, which exacerbates the ability of invasive plants to occupy living space and squeeze out accompanying species, thereby altering the interspecific competition pattern of invaded communities (Carboni et al., 2021; Bando et al., 2023; Sun et al., 2024). However, some native plants with functional traits similar to those of invaders can coexist with them (Zheng et al., 2018). The relationship between invasive and native plants is affected by various environmental factors (Liu et al., 2022; Zhang et al., 2023a), e.g., as opportunists, invasive plants have a stronger response to climate change than native species do, and the ecological plasticity of invasive plants could reduce the limitations imposed by adverse climates on their spread and expand their potential distributions (Wang et al., 2019; Liu et al., 2022). During drought stress, invasive plants can still maintain relatively high root water potential to reduce transpiration and inhibit the growth of native plant roots (Li et al., 2023). In addition, invasive plants can accumulate biomass by absorbing relatively high amounts of soil nitrogen and phosphorus, as well as experiencing mutual benefits with mycorrhizal fungi, thereby suppressing the ability of native plants to acquire resources (Chen et al., 2023; Li et al., 2024a). The performance of invasive plants is closely related to the habitat types, and the impact of invaders on native plants also depends on the geographical spatial effects caused by habitat changes, established studies have shown that changes in habitat types along latitudinal gradients significantly influence the coexistence of invasive and native plants and drive variations in their interspecific relationships (Catford et al., 2022; Ahmad et al., 2025). Thus, exploring the changes in interspecific relationships between invasive and native plant species in heterogeneous habitats is beneficial for predicting plant invasion trends and more effectively protecting native biodiversity.
Interspecific association refers to the interrelation in the spatial distribution of different species caused by habitat heterogeneity; comprehensively represents the interdependence, competition or independence between species; and is the foundation of community formation and evolution (Dou et al., 2022; Zhang et al., 2023a). The competitive or facilitative interactions between invasive and native plants determine species coexistence patterns in invaded communities (Kinlock, 2021; Yang et al., 2022). Studies have revealed that there is a strong positive association between different invasive plants, which accelerates the degradation of native plant communities (Gilbert and Levine, 2013; Zhang et al., 2023a). However, invasive and native plant species usually show a strong negative association (Fridley et al., 2007; Kuebbing and Nunez, 2015; Waller et al., 2016), e.g., in China the native Imperata cylindrica and the invasive Solidago canadensis cannot stably coexist due to intense resource competition, leading to a significant negative association (Lei et al., 2018). Invasive Alliaria petiolata and Rhamnus cathartica can efficiently capture light sources and nutrients, which strongly inhibits native plant growth and results in a more negative association, whereas habitat disturbance strengthens this negative association (Waller et al., 2016). Some studies have also shown that positive associations between native plants during community succession could effectively weaken the invasional meltdown caused by mutual assistance among invasive plant species (Yin et al., 2022; Sun et al., 2024). In addition, few native plants with functional traits and resource utilization capabilities similar to those of invasive plants could form positive associations with invaders; these native species reduce the dominance of invaders in communities and increase plant diversity (Loiola et al., 2018; Jin et al., 2022). Interspecific association is also influenced by habitat types and geographical space. In low-latitude terrestrial environments, the high plant diversity intensifies resource competition between native and invasive plants (Guo et al., 2021), while in high-latitude terrestrial environments, severe abiotic stress may cause the interspecific relationships between native and invasive plants shifting toward neutrality or even positive association (Chiuffo et al., 2022). However, compared to the terrestrial environment, the lower species diversity of aquatic plants makes aquatic ecosystem more vulnerable to plant invasions, which results in the universal interspecific mutualism among native aquatic plants to resist invaders (Guo et al., 2013; Petruzzella et al., 2020). Studying the interspecific association characteristics of invaded plant communities in different geographical spaces can help to clarify species coexistence mechanisms in invaded ecosystems and predict community succession dynamics.
Hydrothermal fluctuations and changes in interference intensity caused by latitudinal variations significantly affect the interspecific relationships between invasive and native plant species (Guo et al., 2021; Chiuffo et al., 2022). The abundant resources available in low-latitude regions alleviate intense interspecific competition, so plants in these areas have strong potential competitiveness, while the abiotic stress caused by increasing latitude intensifies interspecific competition, plants in high-latitude regions thus adopt more conservative survival strategies by enhancing their defensive capabilities and reducing palatability to avoid great tissue loss (Martin-Albarracin and Amico, 2021; Guo et al., 2023; Zhou et al., 2023). Due to the long evolutionary history of native plants, their interspecific relationships usually show a negative association with increasing latitude. However, because invasive plants have a strong growth–defense tradeoff along latitudinal gradients, the latitudinal trend of interspecific associations between invasive and native plants may exhibit variability (Lu et al., 2018; Xiao et al., 2020; Gao et al., 2021b; Guo et al., 2023). For example, the invasive Erigeron canadensis preferentially allocates resources to the leaf structure in high-latitude regions and enhances its competitiveness by secreting relatively high concentrations of allelochemicals, and the negative association between E. canadensis and native species intensifies with increasing latitude (Wang et al., 2017; Chiuffo et al., 2022). While the invasive Spartina alterniflora performs similarly to its native accompanying species Phragmites australis along the latitudinal gradient, as latitude increases, both their carbon: phosphorus (C:P) ratio, nitrogen: phosphorus (N:P) ratio and flavonoid contents increase but palatability decreases, and the process of biotic homogenization caused by S. alterniflora invasion may slow with increasing latitude (Zhang et al., 2019; Guo et al., 2023). Moreover, the continuous spread of species to higher latitudes under global warming further exacerbates the complexity and uncertainty of interspecific associations in invaded ecosystems (Wang et al., 2019). However, the variations in the interspecific association characteristics between invasive and native plant species in heterogeneous habitats across a large latitudinal gradient are still unclear.
Alternanthera philoxeroides (Mart.) Griseb., also known as alligator weed, is a kind of weed in the family Amaranthaceae that is native to South America. Due to its amphibious attributes, strong clonal reproduction and ecological plasticity, A. philoxeroides has been globally invasive, and can be found in many countries including the United States, Australia, India, New Zealand, and China, causing serious threats to the environment, economic development, and ecosystem function (Gao et al., 2021b). In China, A. philoxeroides has invaded more than 20 provinces, with invasion ranges spanning nearly 20 degrees of latitude, significantly altering the inherent structure of native communities and weakening plant diversity. In 2023, A. philoxeroides was included in the list of invasive species under key management in China (Wu et al., 2016, 2023a). Many previous studies have explored how the performance of A. philoxeroides varies along the latitudinal gradient. For example, the importance value of A. philoxeroides increases with increasing latitude, and warming increases the interspecific competitiveness of A. philoxeroides in high-latitude regions (Wu et al., 2016, 2017). With increasing latitude, while the root length and germination rate of A. philoxeroides decrease and the chemical defense of invasive A. philoxeroides populations decreases, the chemical defense of its native population increases (Yang et al., 2021; Hu et al., 2023). The β species diversity and niche overlap of the terrestrial A. philoxeroides- invaded plant communities increase with increasing latitude, indicating that the native communities would further suffer more biotic homogenization caused by A. philoxeroides invasion in high-latitude regions (Wu et al., 2022, 2023b). In addition, the diversity of soil microorganisms and the richness of soil arthropods in A. philoxeroides-invaded habitats also show various latitudinal trends (Lu et al., 2018; Gao et al., 2021b).
Currently, the interspecific association of the A. philoxeroides communities is still not well understood (but see Nan et al., 2023), especially its latitudinal trend at a large spatial scale, which has not yet been clarified. In this study, we conducted a survey on the plant communities invaded by A. philoxeroides in the wild within the latitudinal range from 21–37°N in China, and we hypothesize that the interspecific association characteristics of A. philoxeroides-invaded communities in both aquatic and terrestrial habitats exhibit different trends along the latitudinal gradient. Specifically, we address the following questions: (1) Are there differences in the interspecific associations and association intensities of A. philoxeroides between terrestrial and aquatic habitats? (2) Are there differences in the latitudinal trends of the above indicators between terrestrial and aquatic habitats?
2 Materials and methods
2.1 Site selection
From July to August of 2019-2020, we completed surveys of Alternanthera philoxeroides-invaded plant communities spanning the 21–37°N latitudinal range in the Chinese mainland in batches (without repeated survey). We selected areas more than 100 m2 that were continuously invaded by A. philoxeroides to establish the sampling plots, and the entire study area covered 18 cities within 10 provinces in China. Starting from 21°N, a total of eight latitudinal clusters were set, and each cluster was 2° apart (low latitude: Cluster 1: 21–23°N and Cluster 2: 23–25°N; middle latitude: Cluster 3: 25–27°N, Cluster 4: 27–29°N, Cluster 5: 29–31°N and Cluster 6: 31–33°N; higher latitude: Cluster 7: 33–35°N and Cluster 8: 35–37°N). Five terrestrial plots and five aquatic plots were established in each latitudinal cluster, the area of each plot was 10 × 10 m, and the distance between plots with the same ecotype was more than 10 km (Wu et al., 2016, 2023b). In total, 40 terrestrial plots and 40 aquatic plots were established, and their geographical distributions are shown in Figure 1.
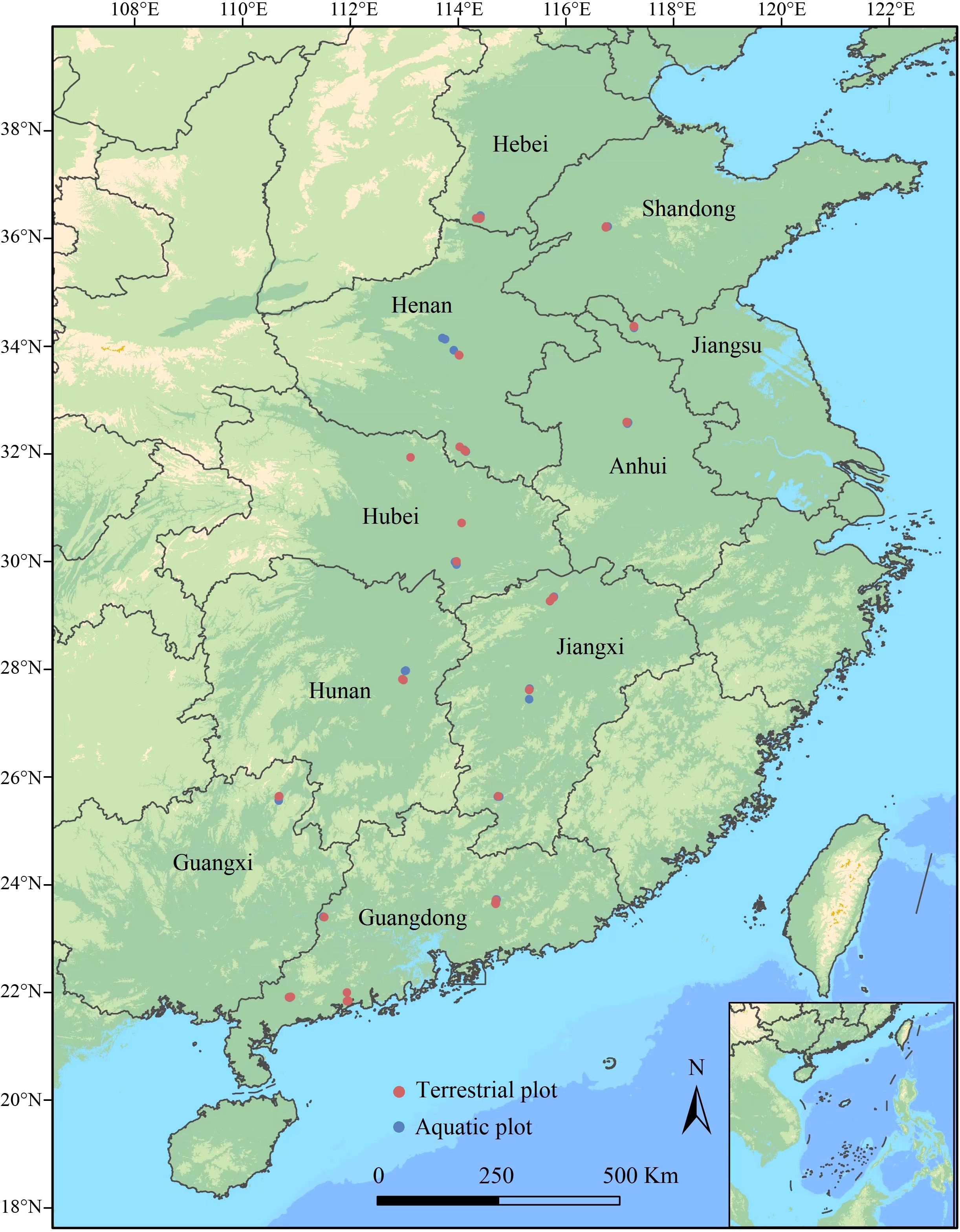
Figure 1. Distribution of sampling plots for the A. philoxeroides-invaded communities in China along latitudinal gradients (21° N to 37° N). Plot size: 10 × 10 m; transect size: 10 m; quadrat size: 0.5 × 0.5 m.
2.2 Field survey
Three 10 m long transects were evenly established in each plot, and five small quadrats with areas of 0.5 × 0.5 m were evenly distributed along each transect for plant community investigation. We recorded the names, heights, abundances and coverages of the plant species in every quadrat. For each plant species, we randomly selected 10 individuals and measured their average height; if the population size was less than 10 individuals, all of their heights were measured. The abundance was measured by the numbers of asexual branches (for clonal plants), tillers (for graminaceous plants) or individuals (for nonclonal plants). The coverage was measured via a 0.5 × 0.5 m metal frame with 100 cells (Wu et al., 2016). We took photos or collected samples of unknown plant species during field surveys and then identified them via the databases of ‘Chinese Virtual Herbarium’ (https://www.cvh.ac.cn) and the online ‘Flora Reipublicae Popularis Sinicae’ (http://www.cn-flora.ac.cn). We used a handheld GPS receiver (Garmin Inc., Olathe, KS, USA) to record the longitude, latitude and altitude of each plot.
2.3 Data calculation
2.3.1 Importance value
We used the importance value (IV) as a comprehensive index to measure the dominance of plant species in A. philoxeroides-invaded communities. The calculation formula for IV is IV = (relative abundance + relative height + relative coverage)/3 (Wu et al., 2016). The total IV was the sum of the IVs for a plant species in all terrestrial or aquatic plots.
2.3.2 Overall interspecific associations
We used the variance ratio (VR) method to measure the overall interspecific association and tested its significance by using a statistical measure (W) (Jin et al., 2022). For the overall community, we selected the main plant species with a total frequency ≥9 in terrestrial communities and ≥4 in aquatic communities for analysis (see Supplementary Table S1). For the communities at different latitudes, we selected the dominant plant species with a total IV≥0.05 in each latitudinal cluster for analysis (see Supplementary Table S2). The calculation formula was as follows (Zhang et al., 2023a):
where S is the total number of species, N is the total number of plots, Tj is the number of species appearing in plot j, t is the average number of species in all plots, t=(T1+T2+…+Tn)/N, and ni is the number of plots where species i appears. VR>1 indicates a positive association, VR<1 indicates a negative association, and VR=1 indicates no association between species. Using the statistical measure (W) to test the deviation of VR from 1, χ20.95N<W<χ20.05N indicates that the interspecific association is not significant; conversely, there is a significant association between species.
2.3.3 χ2 Test
We established a 2×2 contingency table between species and then calculated the values of a, b, c, and d. a represents the number of plots where both species 1 and 2 appeared, b represents the number of plots where only species 1 appeared, c represents the number of plots where only species 2 appeared, and d represents the number of plots where neither of these two species were present. The calculation formula for the χ2 test was as follows (Dai et al., 2020):
χ2>6.635 indicates that the interspecific association is extremely significant (P<0.01), 3.841<χ2<6.635 indicates that the association is significant (P<0.05), and χ2<3.841 indicates that the association is not significant. Based on the results of the χ2 test, we constructed a constellation diagram of interspecific associations in terrestrial and aquatic communities. Because the χ2 test cannot quantitatively distinguish between positive and negative associations, further analysis was needed in conjunction with the interspecific association coefficient.
2.3.4 Interspecific association coefficient
(1) Association coefficient (AC)
The calculation formula for AC was as follows (Jin et al., 2022; Zhang et al., 2023a):
when ad≥bc, ; when bc>ad and d≥a, ; and when bc>ad and d<a, .
(2) Spearman correlation coefficient
The calculation formula was as follows (Lei et al., 2018):
where xij and xkj are the IVs of species i and k in plot j, respectively
(3) Pearson correlation coefficient
The calculation formula was as follows (Lei et al., 2018):
where and are the average IVs of species i and k in all plots.
2.3.5 Ratio of species pairs with positive/negative associations
Based on the values of the AC, Spearman coefficient and Pearson coefficient, we calculated the PNR along latitudinal Clusters 1–8 as follows (Liu et al., 2017):
PNR= (total number of positively associated species pairs)/(total number of negatively associated species pairs)
2.3.6 Index of interspecific association intensity
1. The Jaccard index (JA) was calculated as follows: ;
2. The Ochiai index (OI) was calculated as follows: ;
3. The Dice index (DI) was calculated as follows: (Sidabukke et al., 2021).
The closer the index value is to 1, the greater the degree of interspecific association. Based on the calculation results, we constructed semimatrix diagrams of the association intensity in terrestrial and aquatic communities.
2.4 Statistical analyses
We performed an independent sample t test (subset for α= 0.05) to compare the differences in interspecific association intensity between terrestrial and aquatic communities using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA), as well as the differences in association intensity between A. philoxeroides and accompanying species and between each accompanying species. We conducted one-way ANOVA and LSD (Fisher’s protected least significant difference) multiple comparisons to examine the variations in association intensity between A. philoxeroides and accompanying species and between each accompanying species along the latitudinal gradient.
3 Results
3.1 Interspecific associations of overall communities
A total of 178 plant species were recorded in the terrestrial communities, and 104 plant species were recorded in the aquatic communities (Supplementary Table S3). The VR of the overall terrestrial communities was 2.434, and the W was 97.359, indicating a significantly positive association; however, the positive and negative interspecific associations in each cluster strongly fluctuated along the latitudinal gradient (Table 1). The VR of the overall aquatic communities was 0.887, and W was 35.479, indicating a nonsignificant negative correlation (Table 1). The aquatic communities in the low- and middle- latitude clusters mostly presented positive interspecific associations; with increasing latitude, the associations became negative, especially for the plant species in Cluster 7 at higher latitudes, which presented a significantly negative association (Table 1).
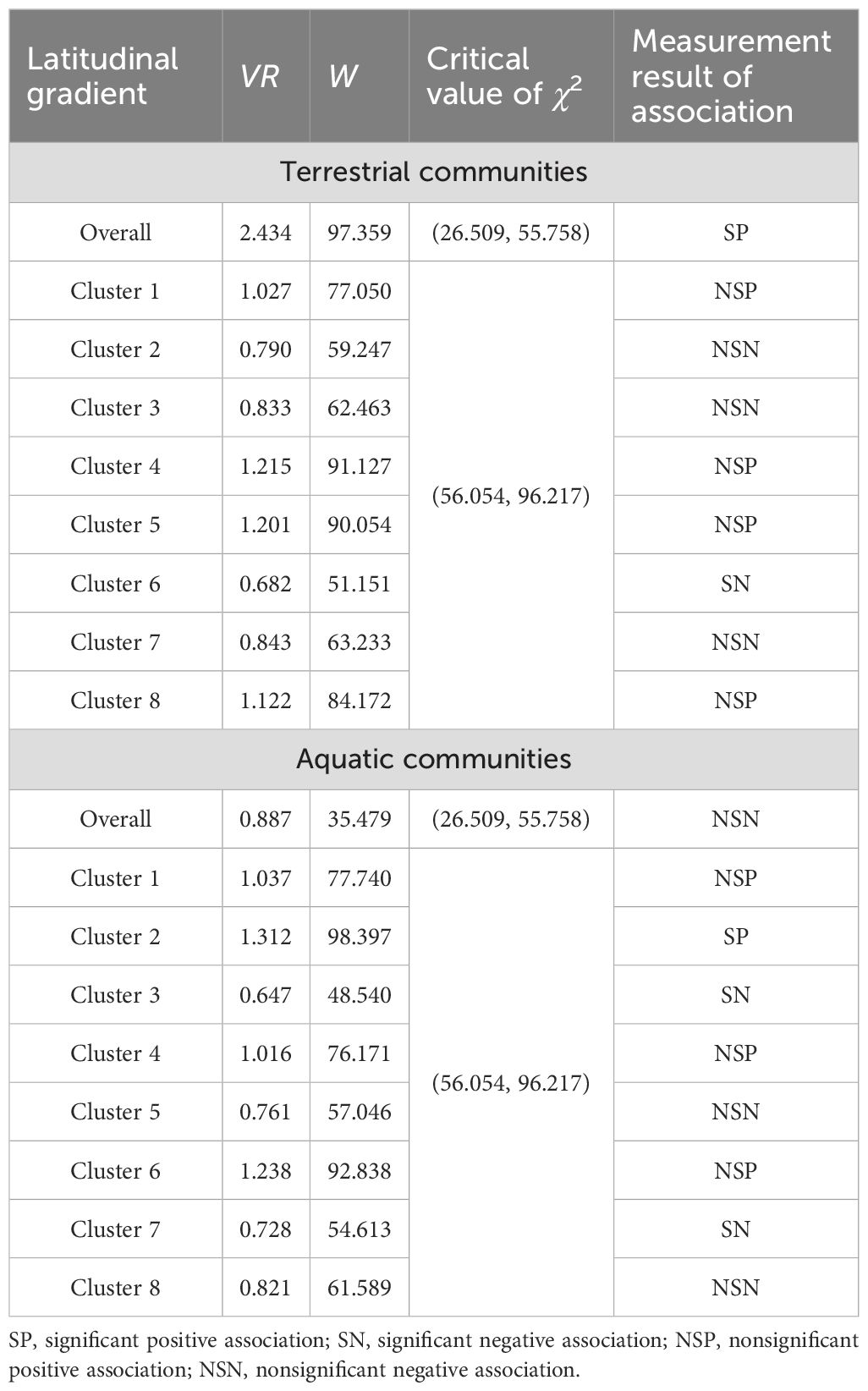
Table 1. Overall interspecific associations and significance test of the A. philoxeroides-invaded communities.
In terrestrial communities, a total of 300 species pairs were established; among them, there were 167 positive association pairs, 131 negative association pairs, and 2 independent species pairs (upper right corner of Figure 2a). The χ2 test revealed 4 significantly positive association pairs: Bidens pilosa–Cyperus rotundus, Setaria viridis–Erigeron canadensis, S. viridis–Broussonetia papyrifera, and Erigeron annuus–B. papyrifera, and 2 extremely significantly positive association pairs: S. viridis–Humulus scandens and Artemisia argyi–B. papyrifera. There were 4 significantly negatively associated pairs: Ipomoea nil–Amaranthus lividus, S. viridis–Polygonum hydropiper, C. rotundus–H. scandens, and A. argyi–Paspalum paspaloides, and 2 extremely significantly negatively associated pairs: Digitaria sanguinalis–E. canadensis and E. canadensis–Eclipta prostrata (lower left corner of Figure 2a).
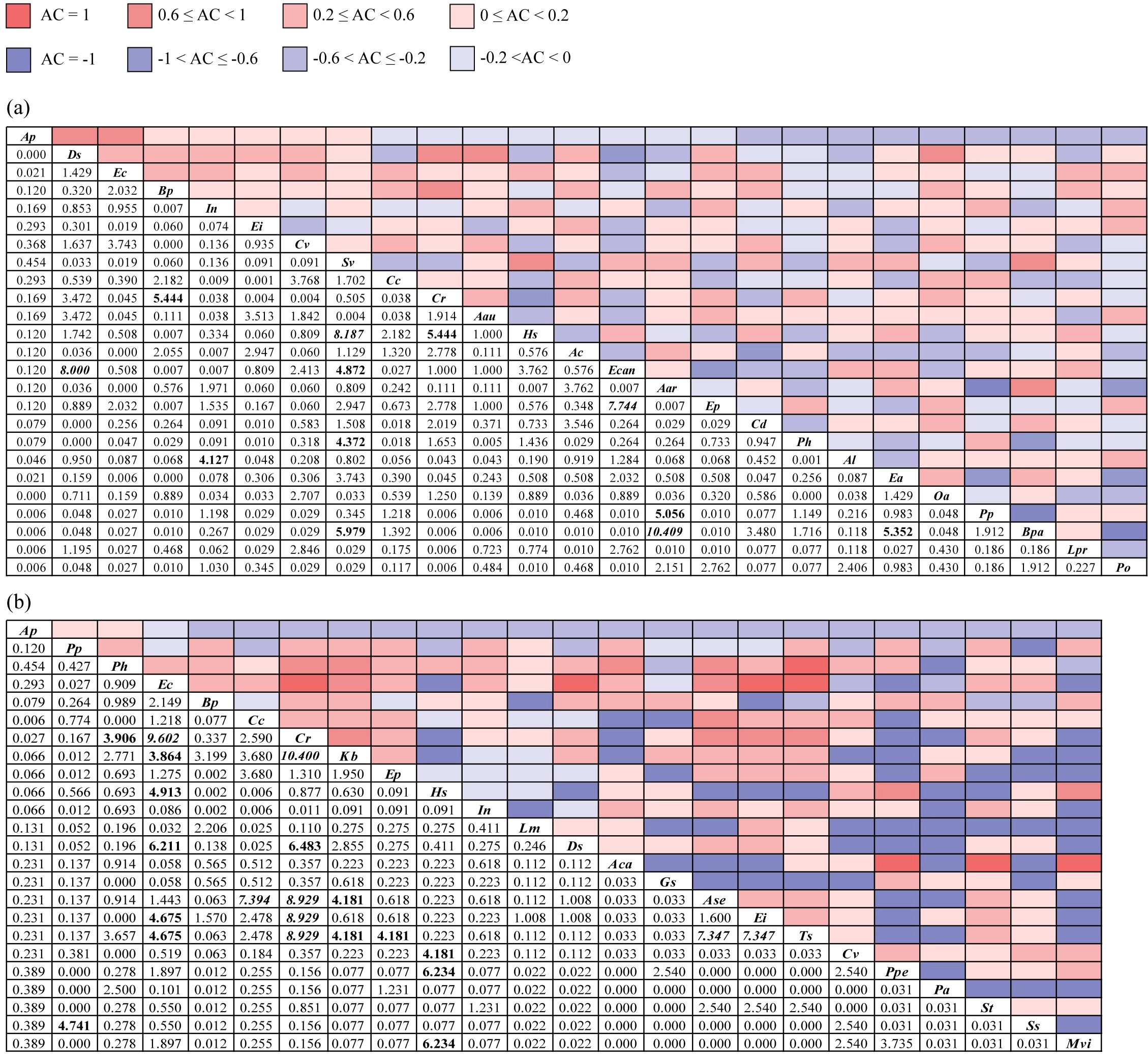
Figure 2. The χ2 test (lower left) and association coefficient (AC, upper right) of overall interspecific associations in terrestrial (a) and aquatic (b) A. philoxeroides-invaded communities. The bold font indicates that the χ2 test is significant at the P<0.05 level, and the italic font indicates that the χ2 test is extremely significant at the P<0.01 level. Aar, Artemisia argyi; Aau, Acalypha australis; Ac, Ageratum conyzoides; Aca, Acorus calamus; Al, Amaranthus lividus; Ap, Alternanthera philoxeroides; Ase, Alternanthera sessilis; Bp, Bidens pilosa; Bpa, Broussonetia papyrifera; Cc, Commelina communis; Cd, Cynodon dactylon; Cr, Cyperus rotundus; Cv, Cyperus votundus; Ds, Digitaria sanguinalis; Ea, Erigeron annuus; Ec, Echinochloa crusgalli; Ecan, Erigeron canadensis; Ei, Eleusine indica; Ep, Eclipta prostrata; Gs, Glycine soja; Hs, Humulus scandens; In, Ipomoea nil; Kb, Kyllinga brevifolia; Lm, Lemna minor; Lpr, Ludwigia prostrata; Mvi, Microstegium vimineum; Oa, Oxalis articulata; Pa, Phragmites australis; Ph, Polygonum hydropiper; Po, Portulaca oleracea; Pp, Paspalum paspaloides; Ppe, Polygonum perfoliatum; Ss, Symphyotrichum subulatum; St, Senna tora; Sv, Setaria viridis; Ts, Trigastrotheca stricta.
A total of 276 species pairs were established in aquatic communities, including 148 positive association pairs, 122 negative association pairs, and 6 independent species pairs (upper right corner of Figure 2b). The χ2 test indicated that the significantly interspecific association pairs in aquatic communities were mostly positive, while there were 2 significantly negative pairs: P. paspaloides–Symphyotrichum subulatum and Echinochloa crusgalli–H. scandens (lower left corner of Figure 2b).
3.2 Latitudinal trends in interspecific associations
A total of 973 species pairs were established in terrestrial communities across different latitudinal clusters. The χ2 test (Figure 3a) and AC values (Figure 4a) indicated that the number of species pairs with significantly positive associations was highest in Cluster 4, with 16 pairs, and the number of species pairs with significantly negative associations was highest in Cluster 6, with five pairs. Digitaria sanguinalis was significantly or extremely significantly positively associated with Ipomoea nil, Cyperus rotundus, Echinochloa crusgalli, Acalypha australis, Cynodon dactylon, Phyllanthus urinaria, and Trigastrotheca stricta at low and middle latitudes, respectively, whereas D. sanguinalis was extremely significantly negatively associated with Humulus scandens in Cluster 7 at higher latitudes (Figure 3a). Ageratum conyzoides was extremely significantly positively associated with Bidens pilosa and Cyperus votundus in Cluster 2; however, this association disappeared at middle and higher latitudes (Figure 3a). Alternanthera philoxeroides had a significantly negative association with its native congener Alternanthera sessilis in Cluster 6 but had no significant association with other accompanying species (Figure 3a). Compared with those at low and middle latitudes, the number of species pairs with significant associations at higher latitudes decreased (Figure 3a).
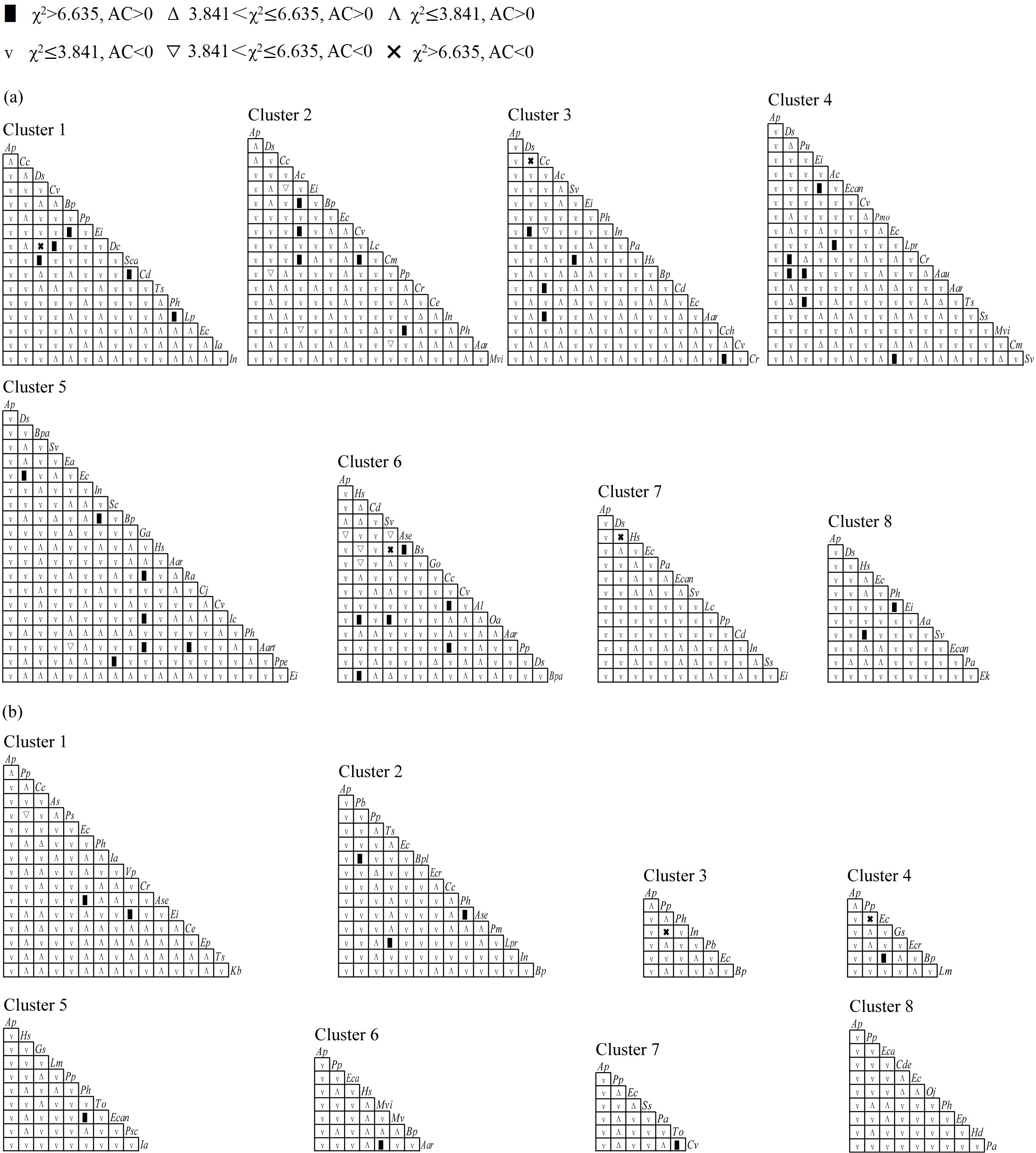
Figure 3. χ2 test of interspecific associations in terrestrial (a) and aquatic (b) A. philoxeroides-invaded communities along the latitudinal gradient. Aa, Alopecurus aequalis; Aar, Artemisia argyi; Aart, Ambrosia artemisiifolia; Aau, Acalypha australis; Ac, Ageratum conyzoides; Al, Amaranthus lividus; Ap, Alternanthera philoxeroides; As, Asarum sagittarioides; Ase, Alternanthera sessilis; Bp, Bidens pilosa; Bpa, Broussonetia papyrifera; Bpl, Bolboschoenus planiculmis; Bs, Beckmannia syzigachne; Cc, Commelina communis; Cch, Clinopodium chinense; Cd, Cynodon dactylon; Cde, Ceratophyllum demersum; Ce, Colocasia esculenta; Cj, Cayratia japonica; Cm, Cucurbita moschata; Cr, Cyperus rotundus; Cv, Cyperus votundus; Dc, Drymaria cordata; Ds, Digitaria sanguinalis; Ea, Erigeron annuus; Ec, Echinochloa crusgalli; Eca, Echinochloa caudata; Ecan, Erigeron canadensis; Ecr, Eichhornia crassipes; Ei, Eleusine indica; Ek, Elymus kamoji; Ep, Eclipta prostrata; Ga, Geum aleppicum; Go, Galium odoratum; Gs, Glycine soja; Hd, Hydrocharis dubia; Hs, Humulus scandens; Ia, Ipomoea aquatica; Ic, Imperata cylindrica; In, Ipomoea nil; Kb, Kyllinga brevifolia; Lc, Leptochloa chinensis; Lm, Lemna minor; Lp, Leptochloa paniceae; Lpr, Ludwigia prostrata; Mv, Myriophyllum verticillatum; Mvi, Microstegium vimineum; Oa, Oxalis articulata; Oj, Oenanthe javanica; Pa, Phragmites australis; Pb, Panicum bisulcatum; Ph, Polygonum hydropiper; Pm, Pilea microphylla; Pmo, Pueraria montana; Pp, Paspalum paspaloides; Ppe, Polygonum perfoliatum; Ps, Pistia stratiotes; Psc, Paederia scandens; Pu, Phyllanthus urinaria; Ra, Rumex acetosa; Sc, Echinochloa crusgalli; Sca, Sphagneticola calendulacea; Ss, Symphyotrichum subulatum; Sv, Setaria viridis; To, Typha orientalis; Ts, Trigastrotheca stricta; Vp, Veronica polita.
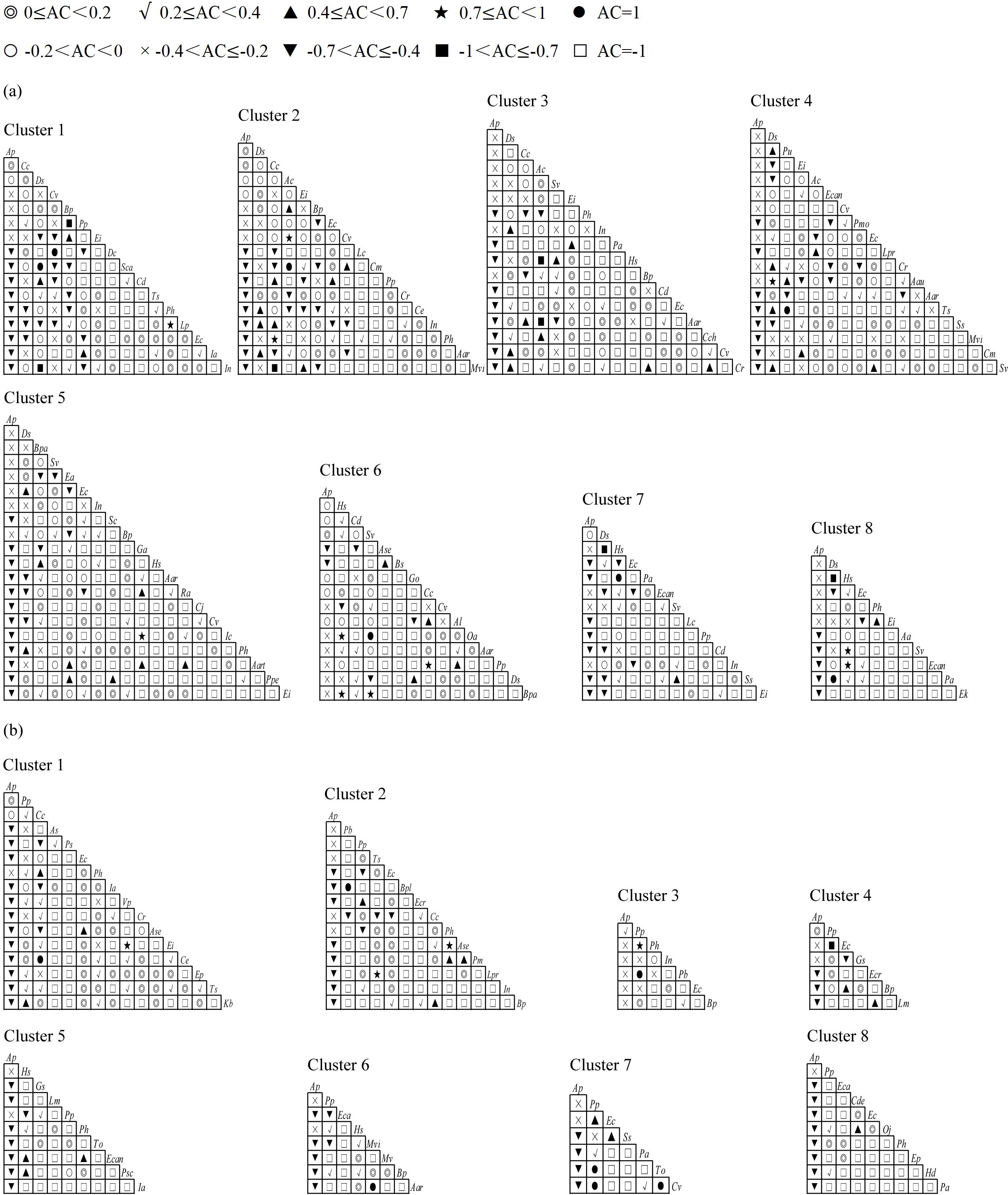
Figure 4. The AC values of terrestrial (a) and aquatic (b) A. philoxeroides-invaded communities along the latitudinal gradient. The definitions of abbreviations are the same as those in Figure 3.
A total of 392 species pairs were established in the aquatic communities at 8 latitudinal clusters. The χ2 test (Figure 3b) and AC values (Figure 4b) revealed that the number of species pairs with significantly positive associations was mainly concentrated at low and higher latitudes, whereas few species pairs with significantly negative associations: Paspalum paspaloides–Pistia stratiotes in Cluster 1, P. paspaloides–I. nil in Cluster 3 and P. paspaloides–E. crusgalli in Cluster 4 (Figure 3b); however, the species pair P. paspaloides–E. crusgalli was significantly positively associated in Cluster 7 (Figure 3b). A. philoxeroides was not significantly associated with any accompanying species in any of the latitudinal clusters.
The terrestrial ratio of species pairs with positive/negative associations (PNR) fluctuated slightly at low and middle latitudes and dramatically decreased at higher latitudes (Figure 5a), and the average PNR values of the Spearman coefficient, Pearson coefficient, and AC at higher latitudes decreased by 38.57%, 33.48%, and 47.02%, respectively, compared with those at low and middle latitudes (Supplementary Table S4). The aquatic PNR strongly fluctuated along the latitudinal gradient, with higher values in Cluster 1 and Cluster 7 and lower values in Cluster 8 (Figure 5b).
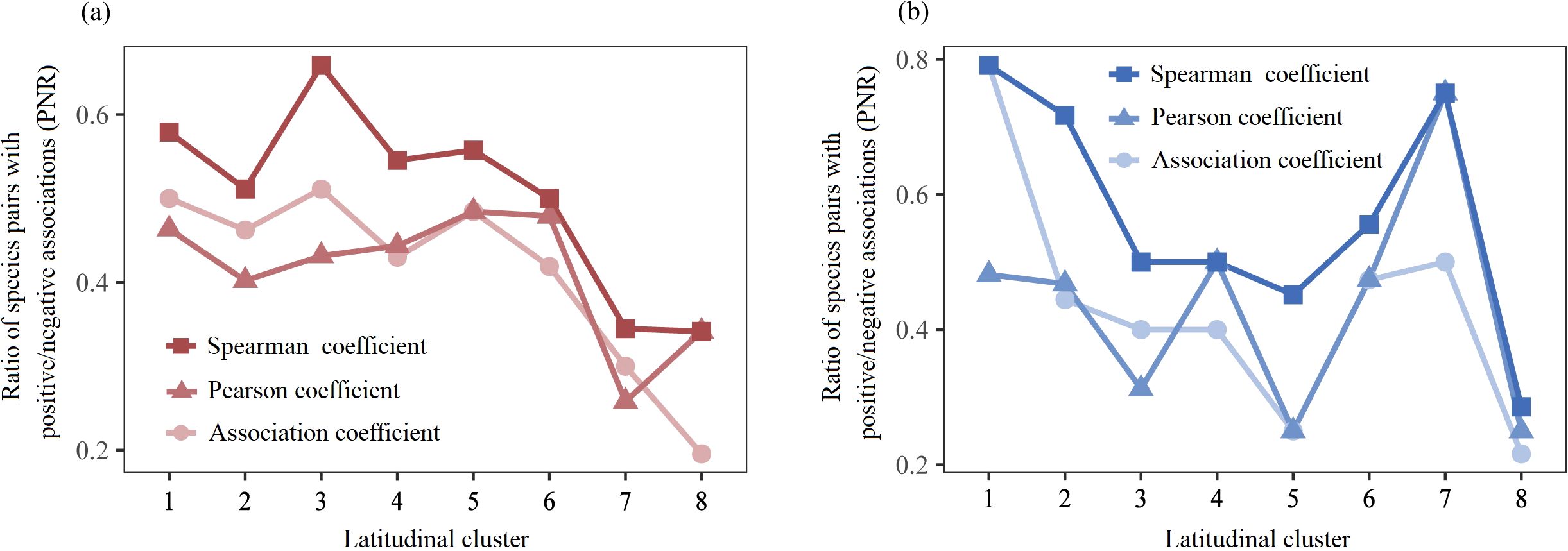
Figure 5. The latitudinal trends of PNR in terrestrial (a) and aquatic (b) A. philoxeroides-invaded communities.
3.3 Latitudinal trend of the association intensity
The semimatrix diagram of association intensity (Figures 6a–c) and the constellation diagram of interspecific associations (Figure 7a) revealed that the plant species in the terrestrial Alternanthera philoxeroides-invaded communities were closely associated and formed a continuum. There were 18 species pairs with high co-occurrence frequency and strong association intensity, including 7 species pairs established by A. philoxeroides and Digitaria sanguinalis, Echinochloa crusgalli, Bidens pilosa, Ipomoea nil, Eleusine indica, Cyperus votundus, and Setaria viridis; 5 species pairs established by D. sanguinalis and E. crusgalli, B. pilosa, I. nil, C. votundus, and Eleusine indica; 3 species pairs established by E. crusgalli and I. nil, B. pilosa, and C. votundus; and 3 other species pairs: B. pilosa–Cyperus rotundus, S. viridis- Humulus scandens, and C. votundus–Commelina communis (Figure 7a). The association intensity of the A. philoxeroides–D. sanguinalis pair was extremely strong at low latitudes; however, it decreased at middle and higher latitudes (Supplementary Figure S1a, S2a, S3a).
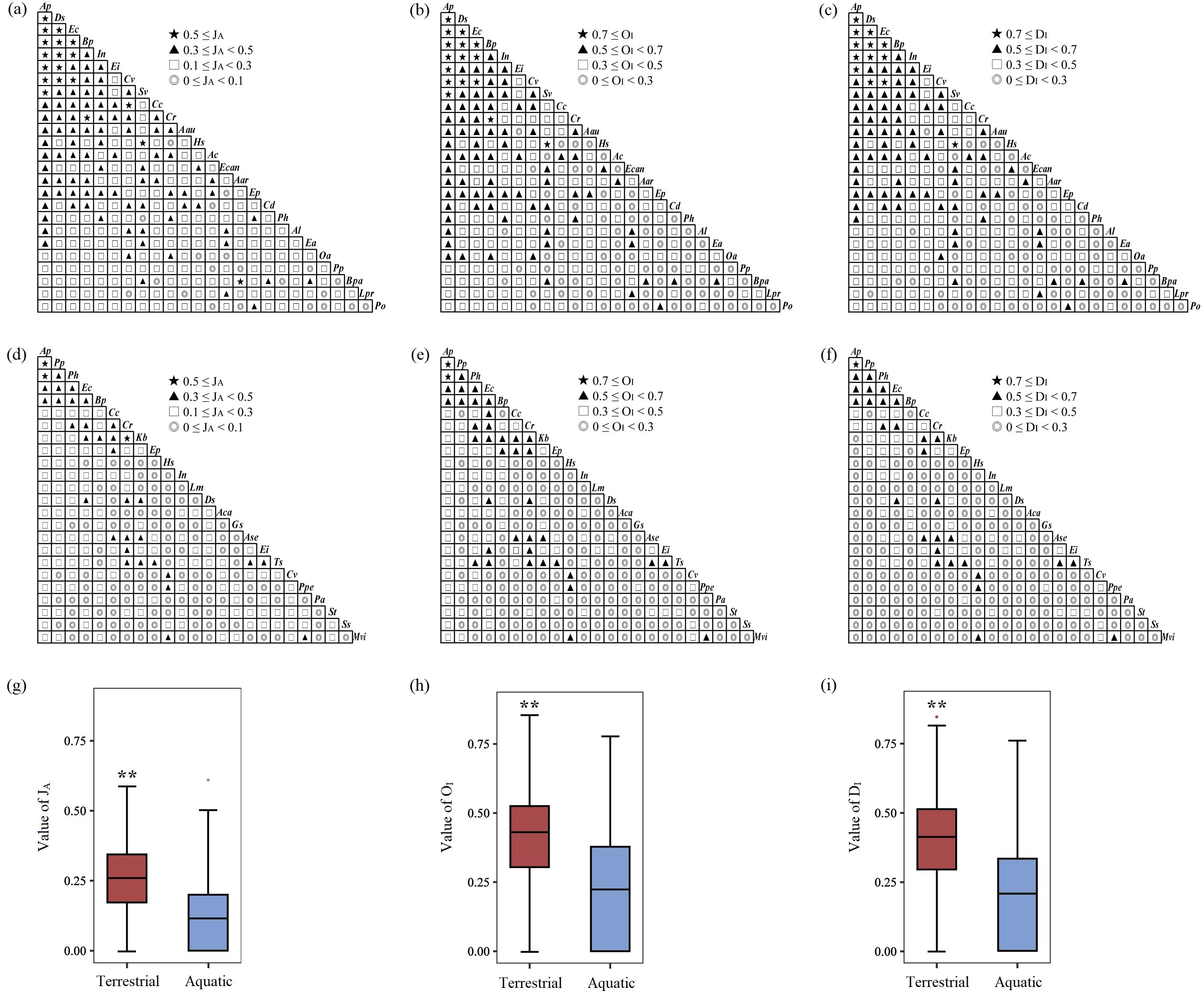
Figure 6. The values of the Jaccard index (JA), Ochiai index (OI), and Dice index (DI) in terrestrial (a–c) and aquatic (d–f) A. philoxeroides-invaded communities and comparisons of their respective differences (g–i). The height of the box plot represents the 25% and 75% percentiles, with whiskers extending to the 5% and 95% percentiles, respectively. The line in the middle represents the median, and the solid dots represent outliers. ** indicates that the difference is extremely significant at the P<0.01 level. The same applies below. The definitions of abbreviations are the same as those in Figure 2.
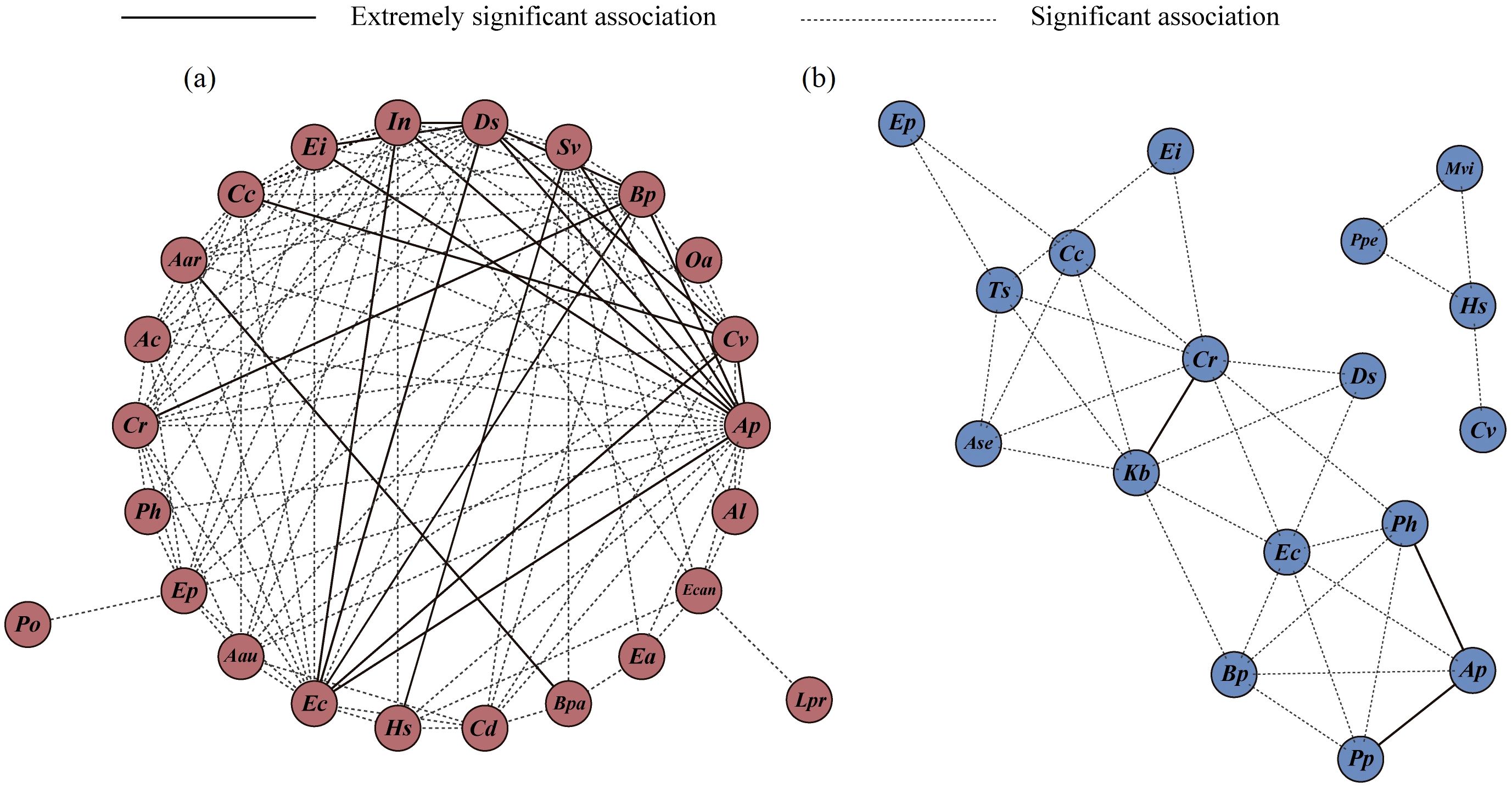
Figure 7. Constellation diagram of interspecific associations in terrestrial (a) and aquatic (b) A. philoxeroides-invaded communities. The definitions of abbreviations are the same as those in Figure 2.
The interspecific association intensity of the aquatic communities was weak, and its species co-occurrence frequency was low (Figures 6d–f, 7b). Among them, three species pairs had strong associations, A. philoxeroides–Paspalum paspaloides, A. philoxeroides–Polygonum hydropiper, and C. rotundus–Kyllinga brevifolia, and their distributions had clear boundaries in the constellation diagram (Figure 7b). Polygonum perfoliatum, Microstegium vimineum, H. scandens, and C. votundus formed a separate association group (Figure 7b). The A. philoxeroides–P. paspaloides pair had a strong association intensity in all latitudinal clusters, whereas the A. philoxeroides–P. hydropiper pair had a strong association intensity at low and middle latitudes (Supplementary Figure S1b, S2b, S3b).
Independent sample t tests revealed that the values of the JA (t=12.272, P<0.001), OI (t=11.944, P<0.001), and DI (t=13.015, P<0.001) in the overall terrestrial communities were extremely significantly greater than those in the overall aquatic communities (Figures 6g–i). In the terrestrial communities, the values of the JA (t=9.056, P<0.001), OI (t=17.675, P<0.001), and DI (t=9.523, P<0.001) between A. philoxeroides and its accompanying species were extremely significantly greater than those between each accompanying species (Figure 8a). In the aquatic communities, the values of the JA (t=5.414, P<0.001), OI (t=11.274, P<0.001), and DI (t=6.174, P<0.001) between A. philoxeroides and its accompanying species were also extremely significantly greater than those between each accompanying species (Figure 8b).
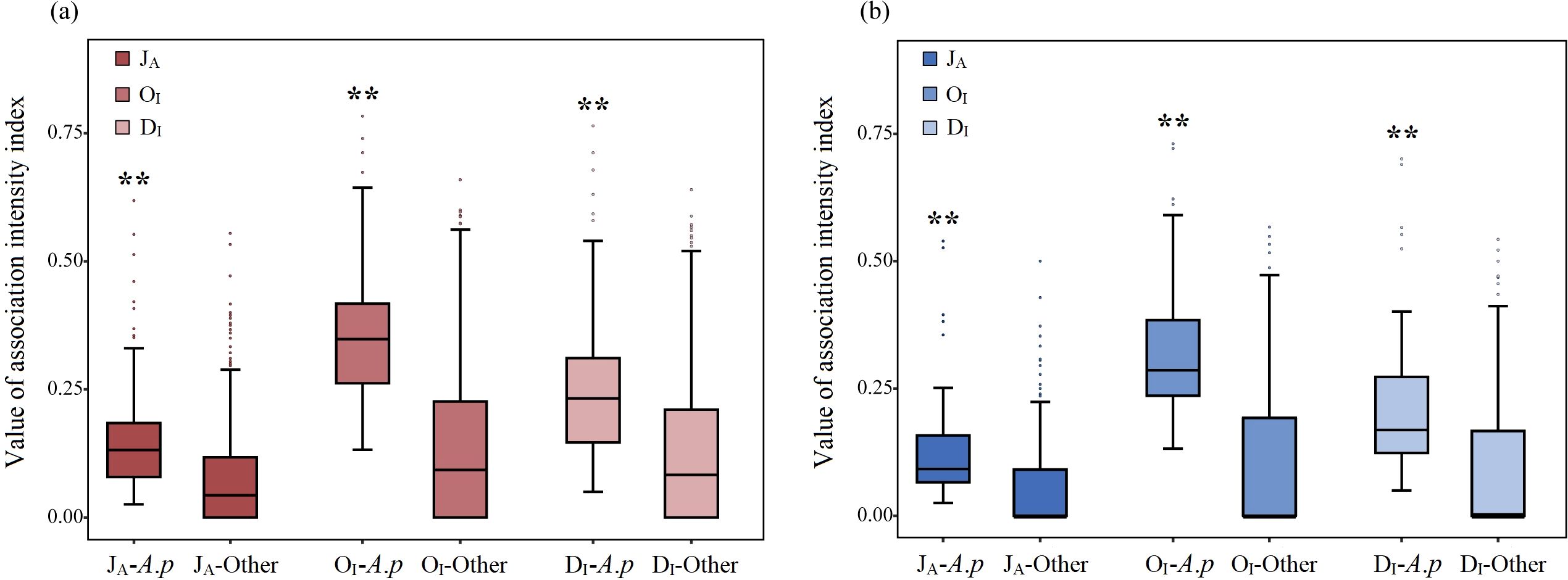
Figure 8. Differences in the association intensity of A. philoxeroides-accompanying species pair and that of each accompanying species pairs in terrestrial (a) and aquatic (b) communities. ** indicates that the difference is extremely significant at the P<0.01 level.
One-way ANOVA revealed that the association intensity between A. philoxeroides and its accompanying species was not significantly different in latitudinal Cluster 1 to 8 (Figures 9a, b). In the terrestrial communities, the association intensity between each accompanying species significantly differed along the latitudinal gradient (Figure 9c); specifically, the values of the JA (F=2.394, P=0.020), OI (F=3.171, P=0.003), and DI (F=2.836, P=0.006) significantly decreased with increasing latitude, especially the association intensity at low latitudes, which was much greater than that at higher latitudes (Figure 9c). In the aquatic communities, the association intensity between each accompanying species varied weakly along the latitudinal gradient; however, the OI (F=2.414, P=0.020) significantly decreased in Cluster 8 compared with Clusters 1, 3 and 7 (Figure 9d).
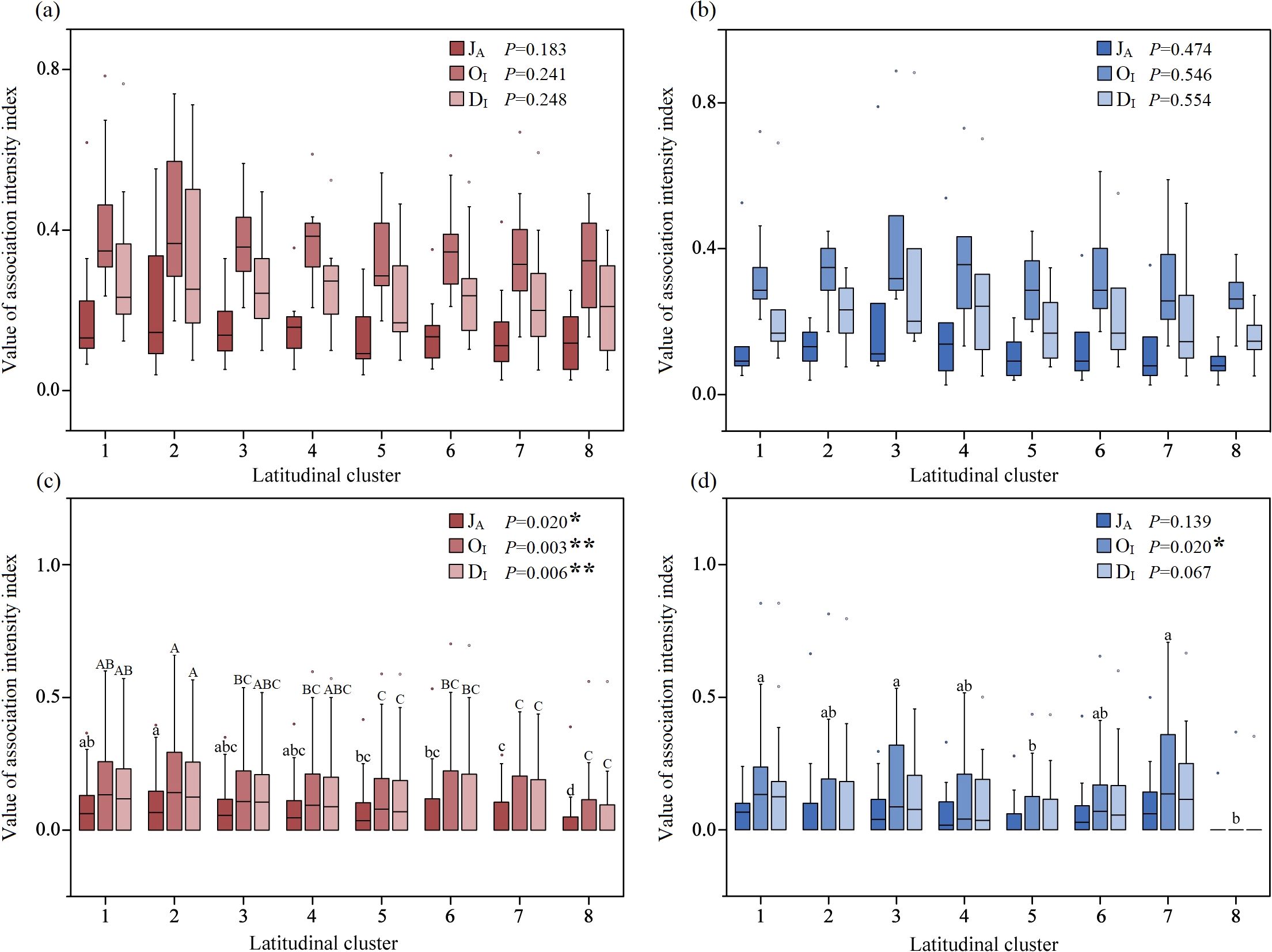
Figure 9. The latitudinal trend of association intensity between A. philoxeroides and accompanying species in terrestrial (a) and aquatic (b) communities and that between each accompanying species in terrestrial (c) and aquatic (d) communities. Different lowercase letters represent significant differences at the P<0.05 level, whereas different capital letters represent extremely significant differences at the P<0.01 level.
4 Discussion
4.1 Causes of different interspecific associations in heterogeneous habitats
Interspecific associations represent the ecological adaptability of the organic whole formed by interactions between species and habitats (Dou et al., 2022). In our study, the overall terrestrial communities had a significantly positive interspecific association, indicating that terrestrial plant species are in a stable state of mutual promotion, which might be due to environmental filtering at large spatial scales promoting native plants and invasive Alternanthera philoxeroides to resist external interference with similar survival strategies (Jin et al., 2022), and weak interspecific competition facilitates convergent evolution to form a positive association for coexistence (Wang et al., 2018; Park et al., 2020; Fridley et al., 2021). Moreover, high plant species diversity in terrestrial habitats decreases contact opportunities between plant hosts and natural enemies and pathogens, which would help maintain positive interspecific associations among terrestrial communities species at large spatial scales (Zhou et al., 2019; Wang et al., 2020). However, we found that the terrestrial communities also presented varying degrees of negative interspecific associations within small spatial scales along the latitudinal gradient, which is consistent with the invasion paradox proposed by Fridley et al. (2007); that is, the interspecific relationship between native and invasive species depends on the spatial scale. The plant species in terrestrial invaded communities have strong negative interactions at local scales, especially at higher latitudes, with stressful environments, while they usually have positive associations at large spatial scales (Fridley et al., 2007; Wu et al., 2023b). Our findings also support these results.
The overall aquatic communities had a nonsignificant negative interspecific association in this study, which indicates that the aquatic plant species are in an unstable competitive exclusion state; this occurred because the aquatic ecotype A. philoxeroides efficiently utilizes resources and can form dense blanket patches that massively squeeze out native plants, resulting in an overall interspecific negative association of the aquatic A. philoxeroides-invaded communities (Duffin et al., 2019; Wu and Ding, 2019). Similar to terrestrial communities, the interspecific associations of aquatic communities also fluctuate along latitudinal gradients because sufficient resources at low and middle latitudes enable the coexistence of many plants and maintain their stable positive associations (Chiuffo et al., 2022; Wu et al., 2022, 2023b). However, with increasing latitude, increased abiotic stress leads to a negative interspecific association, and the structure of the aquatic communities is thus loose and unstable at higher latitudes. The invader is the driver of community dynamics at this time and determines the community succession process (Yin et al., 2022). This finding also implies that aquatic ecosystems may be more vulnerable to A. philoxeroides invasion and that the negative interspecific associations lead to a long-term suppression of aquatic plants by invaders and may result in native species extinction, thus causing a serious threat to native biodiversity (Muthukrishnan and Larkin, 2020). In our study, the PNR of both the terrestrial and aquatic communities was less than 1, indicating that the overall interspecific relationship of the A. philoxeroides-invaded communities was dominated by competition because the plundering of limited resources by invaders intensifies interspecific competition (Lei et al., 2018; Carboni et al., 2021; Wu et al., 2023b). In addition, interspecific competition is more frequent than facilitation between plant species worldwide, and this competitive coexistence is also an important mechanism for community construction (Kinlock, 2021; Losapio et al., 2021; Yang et al., 2022).
4.2 Causes of latitudinal trends in interspecific associations
The PNR of the terrestrial A. philoxeroides communities was stable at low and middle latitudes, possibly because the high resource availability in these regions could relieve intense interspecific competition (Martin-Albarracin and Amico, 2021). Digitaria sanguinalis had the highest IV among the accompanying plant species of the terrestrial A. philoxeroides communities and had extensive positive associations with many native plants, indicating that these species have the ability to adapt to and coexist with each other (Jin et al., 2022; Wu et al., 2022) and suggesting that D. sanguinalis could be used as a native biotic alternative to A. philoxeroides invasion (Nan et al., 2023; Wu et al., 2023b). Studies on interspecific competition have shown that the similar or dissimilar requirements of plant species for limited resources lead to varied interspecific relationships across different latitudes (Jin et al., 2022; Puglielli et al., 2023). In our study, the terrestrial communities had the greatest number of positive association pairs in Cluster 4 at middle latitudes, indicating that the plant species in the terrestrial A. philoxeroides communities exhibited strong mutual assistance in this region. The terrestrial ecotype A. philoxeroides and Alternanthera sessilis formed significantly negative interspecific associations in Cluster 6 at middle latitudes, possibly because A. sessilis, as the native congener has similar functional traits as A. philoxeroides does, which makes A. sessilis more resistant to A. philoxeroides invasion; however, the clonal integration of A. philoxeroides enhances its interspecific competitiveness (Loiola et al., 2018; You et al., 2023). D. sanguinalis had an extremely significant negative association with Humulus scandens in Cluster 7 because resource allocation and competition are the dominant factors determining community structure in high-latitude stress environments (Arnst et al., 2021), and the demand for the same resources between D. sanguinalis and H. scandens may lead to their strong competitive exclusion. Our previous study also revealed that the niche overlap between the terrestrial ecotype A. philoxeroides and its accompanying species was greatest at high latitudes (Wu et al., 2023b), suggesting that A. philoxeroides invasion may exacerbate interspecific competition among native plant species in the terrestrial communities (Wu et al., 2024).
As latitude increases, the aquatic A. philoxeroides communities have a high rate of species replacement, and the interspecific association of the aquatic communities may be more sensitive to environmental changes than that of the terrestrial communities (Wu et al., 2022). The PNR of the aquatic A. philoxeroides communities in Clusters 5–7 trended to increase, indicating that more species shifted from competitive relationships to mutualistic effects in this region. In addition, the interspecific relationship between Paspalum paspaloides and Echinochloa crusgalli shifted from a negative association in Cluster 4 to a positive association in Cluster 7. These findings support the stress gradient hypothesis (SGH), which states that competition between species dominates under low-stress conditions, whereas as stress increases, species usually exhibit more mutualistic effects to resist stressful environments jointly (Spake et al., 2021; Chiuffo et al., 2022). However, the resource constraints and high invasion dominance of the aquatic ecotype A. philoxeroides in Cluster 8 may strongly suppress other accompanying species, causing a sharp decrease in PNR (Arnst et al., 2021; Wu et al., 2022; Zhang et al., 2023b). We found a significant negative association between P. paspaloides and Pistia stratiotes, Ipomoea nil, and E. crusgalli in the aquatic communities at low and middle latitudes, which was due to the malignant invasive P. stratiotes strongly squeezing the living space of native plants, as well as fierce competition for available resources between the dominant accompanying species P. paspaloides and I. nil and E. crusgalli (Wu et al., 2023b).
4.3 Causes of latitudinal trend in association intensity
Dispersal restriction is a crucial factor affecting the degree of species aggregation in communities; the greater the opportunity to approach two species is, the stronger their interactions are (He et al., 2022). In this study, the values of the JA, OI, and DI in the terrestrial communities were significantly greater than those in the aquatic communities, indicating that the terrestrial plant species presented greater association intensity. In terrestrial habitats, high levels of plant diversity lead to relatively high co-occurrence frequencies of plant species, such as the strong associations between A. philoxeroides and D. sanguinalis, E. crusgalli, and Bidens pilosa, and their convergent adaptation to terrestrial habitats enables these species to coexist widely (Wu et al., 2022). In aquatic habitats, severe A. philoxeroides invasion dramatically weakens native plant species diversity, and strong associations, such as A. philoxeroides–P. paspaloides and A. philoxeroides–Polygonum hydropiper, are only observed in a few species pairs; however, most species pairs with weak associations, such as the JA values within Erigeron canadensis–Eclipta prostrata and Erigeron annuus–P. paspaloides, are less than 0.05, which may be due to the random colonization of species within the boundaries of dispersal restrictions (Martin-Albarracin and Amico, 2021; Sidabukke et al., 2021; Liu et al., 2023). In our study, the association intensity between A. philoxeroides and the accompanying species had no obvious latitudinal trend and was significantly greater than that between each accompanying species, indicating that the interspecific interaction between A. philoxeroides and other plants in the invaded communities might not be sensitive to the latitudinal gradient, and that the strong tolerance and wide ecological niche of A. philoxeroides increase its probability of occurrence in all latitudinal clusters (Muthukrishnan et al., 2018; Mod et al., 2023; Wu et al., 2023b). Moreover, the high morphological plasticity of A. philoxeroides makes its co-occurrence with native plants less dependent on trait matching, which also promotes its widespread coexistence with many native plants on a large spatial scale (Peralta et al., 2020; Coux et al., 2021).
Interspecific associations are also influenced by a variety of other factors, such as climate, spatial heterogeneity, and plant-herbivore interactions (Cordero and Jackson, 2019). Compared with the enemy release of invasive A. philoxeroides at higher latitudes, the native accompanying plants in the invaded communities may suffer from stronger herbivory pressure and thus lead to slower growth and reproduction, resulting in a lower interspecific association between native species and a weaker competitive relationship for their coexistence (Neill et al., 2020; Sun et al., 2023). We found that the association intensity between accompanying species in the terrestrial A. philoxeroides communities significantly decreased with increasing latitude, because low-latitude regions with excellent hydrothermal resources could support greater plant diversity and stronger interspecific interactions, which would allow multiple plant species to share similar resources and promote spatial aggregation to increase co-occurrence rates, thus increasing the interspecific association intensity (Chiuffo et al., 2022; Cordero and Jackson, 2019). However, as latitude increases, various plant species with different life forms may exhibit differences in their tolerance to abiotic stress, and the distribution of terrestrial rare species becomes more scattered, leading to a decrease in interspecific association intensity (Lu et al., 2018; Zhang et al., 2019). In our studied aquatic communities, the association intensity of the accompanying plant species was nearly 0 in Cluster 8, possibly because the aquatic communities at higher latitudes suffer from stronger abiotic stress, and serious resource limitations and A. philoxeroides invasion lead to competition-dominated interactions in the aquatic invasion communities, which further exacerbates the loss of accompanying species and decreases the association intensity (Muthukrishnan and Larkin, 2020).
4.4 Limitations and future research needs
In natural ecosystems, native plant communities may be co-invaded by multiple alien plant species, the invasional meltdown hypothesis (IMH) states that multiple invaders can mutually promote their invasion processes when invade into the same ecosystem, and thus exacerbat the negative impact on native communities (Zhang et al., 2020). The coexisting invasive plants could also reshape interspecific relationships by altering the structure and stability of native communities (Ahmad et al., 2025). In the field investigation, although we have artificially ensured the status of studied species, A. philoxeroides, as the constructive species in the invaded communities, the presence of other accompanying invasive plants in the plots, such as E. canadensis and E. annuus, may also affect the interspecific relationships in co-invaded habitats (Wang et al., 2022; Li et al., 2024b). This new type of interspecific interaction network caused by invasional meltdown may weaken the accurate assessment of interspecific association (Vizentin-Bugoni et al., 2021), and the single effect of A. philoxeroides invasion on interspecific association would be further examined by artificially constructing plant communities.
5 Conclusions
We found that in terrestrial communities, Alternanthera philoxeroides had a strong interspecific association with many native plants, and the number of species pairs with positive associations and association intensity significantly decreased at higher latitudes. In aquatic communities, the interspecific associations between A. philoxeroides and native plants, as well as the latitudinal trend of the association intensity, were relatively weak. In addition, the association intensity between A. philoxeroides and its accompanying plants was greater than that between the accompanying plants themselves. These results indicate that high resource availability at low and middle latitudes may alleviate the competition between A. philoxeroides and accompanying plants in terrestrial communities, whereas the terrestrial A. philoxeroides invasion exacerbates the competition between accompanying plant species. Compared with low-latitude regions, abiotic stress at higher latitudes decreases the association intensity between native accompanying plants; however, the plant species in the aquatic A. philoxeroides communities exhibit more mutualistic relationships for jointly resisting stress. Thus, under global environmental changes, we should intensify plant diversity protection at higher latitudes and combine artificial community with the implementation of biotic substitutions to promote vegetation restoration in invaded habitats, for preventing the spread of A. philoxeroides effectively by enhancing biotic resistance and regulating interspecific associations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. SD: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. MY: Investigation, Writing – original draft. YL: Investigation, Writing – original draft. BR: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research received financial support from the National Natural Science Foundation of China (31800460), the Xinyang Academy of Ecological Research Open Foundation (2023XYMS16), the Science and Technology Research Project of Henan Province (232102110062), the Key Scientific Research Projects of Higher Education Institutions of Henan Province (24A180028), and the Nanhu Scholars Program for Young Scholars of Xinyang Normal University (XYNU) (2023A017, 2018B051).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1602762/full#supplementary-material
References
Ahmad R., Lone S. A., Rashid I., and Khuroo A. A. (2025). A global synthesis of the ecological effects of co-invasions. J. Ecol. 113, 570–581. doi: 10.1111/1365-2745.14475
Arnst E. A., Wiser S. K., Sullivan J. J., Buckley H. L., and Roxburgh S. (2021). Resource competition, not facilitation, structures gravel beach plant communities. J. Veg. Sci. 32, e13099. doi: 10.1111/jvs.13099
Bando F. M., Figueiredo B. R. S., Moi D. A., Thomaz S. M., Michelan T. S., García-Girón J., et al. (2023). Invasion by an exotic grass species homogenizes native freshwater plant communities. J. Ecol. 111, 799–813. doi: 10.1111/1365-2745.14061
Borokini I. T., Kortz A., Anibaba Q. A., Witt A., Aigbokhan E. I., Hejda M., et al. (2023). Alien flora of Nigeria: taxonomy, biogeography, habitats, and ecological impacts. Biol. Invasions 25, 3677–3696. doi: 10.1007/s10530-023-03140-1
Carboni M., Livingstone S. W., Isaac M. E., and Cadotte M. W. (2021). Invasion drives plant diversity loss through competition and ecosystem modification. J. Ecol. 109, 3587–3601. doi: 10.1111/1365-2745.13739
Catford J. A., Wilson J. R., Pyšek P., Hulme P. E., and Duncan R. P. (2022). Addressing context dependence in ecology. Trends Ecol. Evol. 37, 158–170. doi: 10.1016/j.tree.2021.09.007, PMID: 34756764
Chen L., Wang M. Q., Shi Y., Ma P. P., Xiao Y. L., Yu H. W., et al. (2023). Soil phosphorus form affects the advantages that arbuscular mycorrhizal fungi confer on the invasive plant species, Solidago canadensis, over its congener. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1160631, PMID: 37125154
Chiuffo M. C., Moyano J., Policelli N., Torres A., Vitali A., Nuñez M. A., et al. (2022). Importance of invasion mechanisms varies with abiotic context and plant invader growth form. J. Ecol. 110, 1957–1969. doi: 10.1111/1365-2745.13929
Cordero R. D. and Jackson D. A. (2019). Species-pair associations, null models, and tests of mechanisms structuring ecological communities. Ecosphere 10, e02797. doi: 10.1002/ecs2.2797
Coux C., Donoso I., Tylianakis J. M., García D., Martínez D., Dehling D. M., et al. (2021). Tricky partners: native plants show stronger interaction preferences than their exotic counterparts. Ecology 102, e03239. doi: 10.1002/ecy.3239, PMID: 33125718
Dai J., Liu H. Y., Xu C. Y., Qi Y., Zhu X. R., Zhou M., et al. (2020). Divergent hydraulic strategies explain the interspecific associations of co-occurring trees in forest–Steppe Ecotone. Forests 11, 942. doi: 10.3390/f11090942
Dou Z. G., Cui L. J., Li W., Lei Y., Zuo X. Y., Cai Y., et al. (2022). Effect of freshwater on plant species diversity and interspecific associations in coastal wetlands invaded by Spartina alterniflora. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.965426, PMID: 36212281
Duffin K. I., Li S. P., and Meiners S. J. (2019). Species pools and differential performance generate variation in leaf nutrients between native and exotic species in succession. J. Ecol. 107, 595–605. doi: 10.1111/1365-2745.13043
Fridley J. D., Jo I., Hulme P. E., and Duncan R. P. (2021). A habitat-based assessment of the role of competition in plant invasions. J. Ecol. 109, 1263–1274. doi: 10.1111/1365-2745.13553
Fridley J. D., Stachowicz J. J., Naeem S., Sax D. F., Seabloom E. W., Smith M. D., et al. (2007). The invasion paradox: reconciling pattern and process in species invasions. Ecology 88, 3–17. doi: 10.1890/0012-9658(2007)88, PMID: 17489447
Gao F. L., He Q. S., Zhang Y. D., Hou J. H., and Yu F. H. (2021a). Effects of soil nutrient heterogeneity on the growth and invasion success of alien plants: A multi-species study. Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.619861
Gao L. L., Wei C. Q., Xu H., Liu X. Y., Siemann E., and Lu X. M. (2021b). Latitudinal variation in the diversity and composition of various organisms associated with an exotic plant: the role of climate and plant invasion. New Phytol. 231, 1559–1569. doi: 10.1111/nph.17479, PMID: 34018617
Gilbert B. and Levine J. M. (2013). Plant invasions and extinction debts. P. Natl. Acad. Sci. U.S.A. 110, 1744–1749. doi: 10.1073/pnas.1212375110, PMID: 23297239
Guo H., Zhang Y., Lan Z., and Pennings S. C. (2013). Biotic interactions mediate the expansion of black mangrove (Avicennia germinans) into salt marshes under climate change. Global Change Biol. 19, 2765–2774. doi: 10.1111/gcb.12221, PMID: 23580161
Guo Q. F., Cade B. S., Dawson W., Essl F., Kreft H., Pergl J., et al. (2021). Latitudinal patterns of alien plant invasions. J. Biogeogr. 48, 253–262. doi: 10.1111/jbi.13943
Guo Y. L., Zhang Y. Z., Wu J. H., Richards C. L., Bossdorf O., Li B., et al. (2023). Geographic variation of litter chemistry and palatability in an invasive plant versus its native competitor. J. Biogeogr. 50, 1139–1150. doi: 10.1111/jbi.14604
He C. M., Jia S. H., Luo Y., Hao Z. Q., and Yin Q. L. (2022). Spatial Distribution and species association of dominant tree species in huangguan plot of Qinling Mountains, China. Forests 13, 866. doi: 10.3390/f13060866
Hu S. Y., Gao H., Li J., Wang Y. H., Gao A. G., Wen J. H., et al. (2023). The latitudinal and longitudinal allelopathic patterns of an invasive alligator weed (Alternanthera philoxeroides) in China. PloS One 18, e0280866. doi: 10.1371/journal.pone.0280866, PMID: 36689420
Jin S. S., Zhang Y. Y., Zhou M. L., Dong X. M., Chang C. H., Wang T., et al. (2022). Interspecific association and community stability of tree species in natural secondary forests at different altitude gradients in the southern Taihang Mountains. Forests 13, 373. doi: 10.3390/f13030373
Kinlock N. L. (2021). Uncovering structural features that underlie coexistence in an invaded woody plant community with interaction networks at multiple life stages. J. Ecol. 109, 384–398. doi: 10.1111/1365-2745.13489
Kuebbing S. E. and Nunez M. A. (2015). Negative, neutral, and positive interactions among nonnative plants: patterns, processes, and management implications. Global Change Biol. 21, 926–934. doi: 10.1111/gcb.12711, PMID: 25142018
Lei Y., Wu Z. L., Wu L. Z., Shi H. L., Bai H. T., Fu W., et al. (2018). Interspecific correlation between exotic and native plants under artificial wetland forests on the Dianchi lakeside, south-west China. Mar. Freshwater Res. 69, 669–676. doi: 10.1071/MF17177
Li J., He J. Z., Liu M., Yan Z. Q., Xu X. L., and Kuzyakov Y. (2024a). Invasive plant competitivity is mediated by nitrogen use strategies and rhizosphere microbiome. Soil Biol. Biochem. 192, 109361. doi: 10.1016/j.soilbio.2024.109361
Li C., Li Y., Xu Z. L., Zhong S. S., Cheng H. Y., Liu J., et al. (2024b). The effects of co-invasion by three Asteraceae invasive alien species on plant taxonomic and functional diversity in herbaceous ruderal communities in southern Jiangsu, China. Biol. Futura 75, 205–217. doi: 10.1007/s42977-024-00202-w, PMID: 38300414
Li W. R., Wang L. W., Qian S. F., He M. Y., Cai X. J., and Ding J. Q. (2023). Root characteristics explain greater water use efficiency and drought tolerance in invasive Compositae plants. Plant Soil 483, 209–223. doi: 10.1007/s11104-022-05734-5
Liu X. Y., He D., Tian W. B., Song Y. J., Yin F., Xu M. S., et al. (2017). Patterns of species associations in woody plants in forest communities of Putuoshan Island, Zhejiang, China. Chin. J. Plant Ecol. 41, 1219–1227. doi: 10.17521/cjpe.2017.0170
Liu C. L., Wolter C., Courchamp F., Roura-Pascual N., and Jeschke J. M. (2022). Biological invasions reveal how niche change affects the transferability of species distribution models. Ecology 103, e3719. doi: 10.1002/ecy.3719, PMID: 35388469
Liu Z. D., Zhou X. L., Tian J. J., Yang L., Wang Y. H., and Shen S. K. (2023). Mechanism of terrestrial plant community assembly under different intensities of anthropogenic disturbance in Dianchi Lakeside. Forests 14, 670. doi: 10.3390/f14040670
Loiola P. P., de Bello F., Chytry M., Gotzenberger L., Carmona C. P., Pysek P., et al. (2018). Invaders among locals: alien species decrease phylogenetic and functional diversity while increasing dissimilarity among native community members. J. Ecol. 106, 2230–2241. doi: 10.1111/1365-2745.12986
Losapio G., Schoeb C., Staniczenko P. P. A., Carrara F., Palamara G. M., De Moraes C. M., et al. (2021). Network motifs involving both competition and facilitation predict biodiversity in alpine plant communities. P. Natl. Acad. Sci. U.S.A. 118, e2005759118. doi: 10.1073/pnas.2005759118, PMID: 33526655
Lu X. M., He M. Y., Ding J. Q., and Siemann E. (2018). Latitudinal variation in soil biota: testing the biotic interaction hypothesis with an invasive plant and a native congener. ISME J. 12, 2811–2822. doi: 10.1038/s41396-018-0219-5, PMID: 30013163
Martin-Albarracin V. L. and Amico G. C. (2021). Plant origin and fruit traits shape fruit removal patterns by native birds in invaded plant communities. Biol. Invasions 23, 857–870. doi: 10.1007/s10530-020-02407-1
Mod H. K., Rissanen T., Niittynen P., Soininen J., and Luoto M. (2023). The relationships of plant species occupancy to niches and traits vary with spatial scale. J. Biogeogr. 50, 1013–1025. doi: 10.1111/jbi.14608
Muthukrishnan R., Hansel-Welch N., and Larkin D. J. (2018). Environmental filtering and competitive exclusion drive biodiversity-invasibility relationships in shallow lake plant communities. J. Ecol. 106, 2058–2070. doi: 10.1111/1365-2745.12963
Muthukrishnan R. and Larkin D. J. (2020). Invasive species and biotic homogenization in temperate aquatic plant communities. Global Ecol. Biogeogr. 29, 656–667. doi: 10.1111/geb.13053
Nan Q. R., Zhang Q., Li X. H., Zheng D. N., Li Z. H., and Zhao L. Y. (2023). Niche and interspecific association of the dominant species during the invasion of Alternanthera philoxeroides in the Yangtze River Basin, China. Agriculture-Basel 13, 621. doi: 10.3390/agriculture13030621
Neill P. E., Rozbaczylo N., Villasenor-Parada C., Guzmán-Rendón G., Sampértegui S., and Hernández C. E. (2020). Patterns of association of native and exotic boring polychaetes on the southeastern Pacific coast of Chile: the combined importance of negative, positive and random interactions. Peer J. 8, e8560. doi: 10.7717/peerj.8560, PMID: 32411504
Park D. S., Feng X., Maitner B. S., Ernst K. C., and Enquist B. J. (2020). Darwin’s naturalization conundrum can be explained by spatial scale. P. Natl. Acad. Sci. U.S.A. 117, 10904–10910. doi: 10.1073/pnas.1918100117, PMID: 32366659
Peralta G., Perry G. L. W., Vazquez D. P., Dehling D. M., and Tylianakis J. M. (2020). Strength of niche processes for species interactions is lower for generalists and exotic species. J. Anim. Ecol. 89, 2145–2155. doi: 10.1111/1365-2656.13274, PMID: 32495955
Petruzzella A., Rodrigues T.A.D.S.R., van Leeuwen C. H. A., Esteves F. D., Figueiredo-Barros M. P., and Bakker E. S. (2020). Species identity and diversity effects on invasion resistance of tropical freshwater plant communities. Sci. Rep. 10, 5626. doi: 10.1038/s41598-020-62660-1, PMID: 32221401
Puglielli G., Tordoni E., Laanisto L., Kalwij J. M., Hutchings M. J., and Humphreys A. M. (2023). Abiotic stress tolerance can explain range size and filling in temperate woody plants. Perspect. Plant Ecol. 59, 125734. doi: 10.1016/j.ppees.2023.125734
Qian H. (2023). Patterns of phylogenetic relatedness of non-native plants across the introduction-naturalization-invasion continuum in China. Plant Diversity 45, 169–176. doi: 10.1016/j.pld.2022.12.005, PMID: 37069929
Sidabukke S. H., Barus T. A., Utomo B., and Aulin F. R. (2021). The effect of forest land allocation on understory plant species associations. IOP Conf. Ser: Earth Environ. Sci. 912, 12082. doi: 10.1088/1755-1315/912/1/012082
Spake R., Soga M., Catford J. A., and Eigenbrod F. (2021). Applying the stress-gradient hypothesis to curb the spread of invasive bamboo. J. Appl. Ecol. 58, 1993–2003. doi: 10.1111/1365-2664.13945
Sun Y., Ren Z. K., Muller-Scharer H., Callaway R. M., van Kleunen M., and Huang W. (2024). Increasing and fluctuating resource availability enhances invasional meltdown. Ecology 105, 4387. doi: 10.1002/ecy.4387, PMID: 39016245
Sun X., Sun Y. M., Cao X. Y., Zhai X. C., Callaway R. M., Wan J. L., et al. (2023). Trade-offs in non-native plant herbivore defences enhance performance. Ecol. Lett. 26, 1584–1596. doi: 10.1111/ele.14283, PMID: 37387416
Vizentin-Bugoni J., Sperry J. H., Kelley J. P., Gleditsch J. M., Foster J. T., Drake D. R., et al. (2021). Ecological correlates of species’ roles in highly invaded seed dispersal networks. P. Natl. Acad. Sci. U.S.A. 118, e2009532118. doi: 10.1073/pnas.2009532118, PMID: 33431649
Waheed M., Walas L., Alipour S., Arshad F., Jameel M. A., Siddiqui M. H., et al. (2024). Global climate change increases the risk of invasion and the expansion of paper mulberry in the subtropical region. Glob. Ecol. Conserv. 54, e03088. doi: 10.1016/j.gecco.2024.e03088
Waller D. M., Mudrak E. L., Amatangelo K. L., Klionsky S. M., and Rogers D. A. (2016). Do associations between native and invasive plants provide signals of invasive impacts? Biol. Invasions 18, 3465–3480. doi: 10.1007/s10530-016-1238-7
Wang C. Y., Jiang K., Liu J., Zhou J. W., and Wu B. D. (2018). Moderate and heavy Solidago canadensis L. invasion are associated with decreased taxonomic diversity but increased functional diversity of plant communities in East China. Ecol. Eng. 112, 55–64. doi: 10.1016/j.ecoleng.2017.12.025
Wang C. Y., Jiang K., Zhou J. W., and Liu J. (2017). Allelopathic suppression by Conyza canadensis depends on the interaction between latitude and the degree of the plant’s invasion. Acta Bot. Bras. 31, 212–219. doi: 10.1590/0102-33062017abb0045
Wang C. J., Li Q. F., and Wan J. Z. (2019). Potential invasive plant expansion in global ecoregions under climate change. Peer J. 7, e6479. doi: 10.7717/peerj.6479, PMID: 30863672
Wang C. Y., Wei M., Wang S., Wu B. D., and Cheng H. Y. (2020). Erigeron annuus (L.) Pers. and Solidago canadensis L. antagonistically affect community stability and community invasibility under the co-invasion condition. Sci. Total Environ. 716, 137128. doi: 10.1016/j.scitotenv.2020.137128, PMID: 32045766
Wang C. Y., Yu Y. L., Cheng H. Y., and Du D. L. (2022). Which factor contributes most to the invasion resistance of native plant communities under the co-invasion of two invasive plant species? Sci. Total Environ. 813, 152628. doi: 10.1016/j.scitotenv.2021.152628, PMID: 34963604
Wu H., Carrillo J., and Ding J. Q. (2016). Invasion by alligator weed, Alternanthera philoxeroides, is associated with decreased species diversity across the latitudinal gradient in China. J. Plant Ecol. 93, 311–319. doi: 10.1093/jpe/rtv060
Wu H. and Ding J. Q. (2019). Global change sharpens the double-edged sword effect of aquatic alien plants in China and beyond. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00787, PMID: 31249587
Wu H., Dong S. J., and Rao B. Q. (2022). Latitudinal trends in the structure, similarity and beta diversity of plant communities invaded by Alternanthera philoxeroides in heterogeneous habitats. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1021337, PMID: 36275507
Wu H., Dong S. J., Wang Y. Y., Wang L., and Rao B. Q. (2023b). Niche Characteristics of Alternanthera philoxeroide-invaded plant communities in heterogeneous habitats and their latitudinal trends. Diversity-Basel 15, 651. doi: 10.3390/d15050651
Wu H., Dong S. J., Wang L., Zhu Y., Jia S., and Rao B. Q. (2023a). Nitrogen enrichment alters the resistance of a noninvasive alien plant species to Alternanthera philoxeroides invasion. Front. Ecol. Evol. 11. doi: 10.3389/fevo.2023.1215191
Wu H., Ismail M., and Ding J. Q. (2017). Global warming increases the interspecific competitiveness of the invasive plant alligator weed, Alternanthera philoxeroides. Sci. Total Environ. 575, 1415–1422. doi: 10.1016/j.scitotenv.2016.09.226, PMID: 27720597
Wu T. T., Li Y. Z., Cadotte M. W., Godoy O., and Chu C. J. (2024). Can competitive effects and responses of alien and native species predict invasion outcomes? Fund Res. 88, 1066–1078. doi: 10.1016/j.fmre.2024.05.001
Xiao L., Ding J. Q., Zhang J. L., Huang W., and Siemann E. (2020). Chemical responses of an invasive plant to herbivory and abiotic environments reveal a novel invasion mechanism. Sci. Total Environ. 741, 140452. doi: 10.1016/j.scitotenv.2020.140452, PMID: 32886966
Yang X. J., Gomez-Aparicio L., Lortie C. J., Verdú M., Cavieres L. A., Huang Z. Y., et al. (2022). Net plant interactions are highly variable and weakly dependent on climate at the global scale. Ecol. Lett. 25, 1580–1593. doi: 10.1111/ele.14010, PMID: 35460586
Yang Y., Liu M., Pan Y. F., Huang H. Y., Pan X. Y., Sosa A., et al. (2021). Rapid evolution of latitudinal clines in growth and defence of an invasive weed. New Phytol. 230, 845–856. doi: 10.1111/nph.17193, PMID: 33454953
Yin D. Y., Meiners S. J., Ni M., Ye Q., He F. L., and Cadotte M. W. (2022). Positive interactions of native species melt invasional meltdown over long-term plant succession. Ecol. Lett. 25, 2584–2596. doi: 10.1111/ele.14127, PMID: 36310402
You W. H., Li N. N., Zhang J., Song A., and Du D. L. (2023). The plant invader Alternanthera philoxeroides benefits from clonal integration more than its native co-genus in response to patch contrast. Plants-Basel 12, 2371. doi: 10.3390/plants12122371, PMID: 37375996
Zhang T. J., Chen Y. N., and Ali S. (2023a). Abiotic stress and human activities reduce plant diversity in desert riparian forests. Ecol. Indic. 152, 110340. doi: 10.1016/j.ecolind.2023.110340
Zhang Z. J., Liu Y. J., Brunel C., and van Kleunen M. (2020). Soil-microorganism-mediated invasional meltdown in plants. Nat. Ecol. Evol. 4, 1612–1621. doi: 10.1038/s41559-020-01311-0, PMID: 33020599
Zhang Y. Z., Pennings S. C., Li B., and Wu J. H. (2019). Biotic homogenization of wetland nematode communities by exotic Spartina alterniflora in China. Ecology 100, e02596. doi: 10.1002/ecy.2596, PMID: 30861108
Zhang R. Y., Tian D. S., Wang J. S., and Niu S. L. (2023b). Critical role of multidimensional biodiversity in contributing to ecosystem sustainability under global change. Geogr. Sustain. 4, 232–243. doi: 10.1016/j.geosus.2023.05.002
Zheng Y. L., Burns J. H., Liao Z. Y., Li Y. P., Yang J., Chen Y. J., et al. (2018). Species composition, functional and phylogenetic distances correlate with success of invasive Chromolaena odorata in an experimental test. Ecol. Lett. 21, 1211–1220. doi: 10.1111/ele.13090, PMID: 29808558
Zhou Q., Shi H., Shu X., Xie F. L., Zhang K. R., Zhang Q. F., et al. (2019). Spatial distribution and interspecific associations in a deciduous broad-leaved forest in north-central China. J. Veg. Sci. 30, 1153–1163. doi: 10.1111/jvs.12805
Keywords: alligator weed, habitat heterogeneity, interspecific relationship, latitude, plant invasions, species coexistence
Citation: Wu H, Dong S, Yu M, Liu Y and Rao B (2025) Interspecific association characteristics of Alternanthera philoxeroides-invaded communities and their latitudinal variations. Front. Ecol. Evol. 13:1602762. doi: 10.3389/fevo.2025.1602762
Received: 30 March 2025; Accepted: 23 June 2025;
Published: 09 July 2025.
Edited by:
Wen-Hua You, Jiangsu University, ChinaReviewed by:
Hongwei Yu, Hebei Agricultural University, ChinaZhiguo Dou, National Marine Environmental Monitoring Center, China
Copyright © 2025 Wu, Dong, Yu, Liu and Rao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wu, d3VoYW84Njg2ODY4NkAxNjMuY29t; Benqiang Rao, cmJxeHlAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Hao Wu
Hao Wu Sijin Dong1,4†
Sijin Dong1,4†