- 1College of Ecology and Environment, Xinjiang University, Urumqi, Xinjiang, China
- 2College of Natural Resources and Environment, Northwest A&F University, Yangling, China
- 3State Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau, Institute of Soil and Water Conservation, Northwest A & F University, Yangling, China
- 4School of Geographic Sciences, Xinyang Normal University, Xinyang, China
- 5Henan Key Laboratory for Synergistic Prevention of Water and Soil Environmental Pollution, Xinyang Normal University, Xinyang, China
Robinia pseudoacacia plantations are a key component of vegetation restoration efforts on the Chinese Loess Plateau (CLP) aimed at improving the regional ecological environment. However, a major emerging threat to the sustainable growth of these plantations is drought due to the increasing pressures associated with climate change. To this end, we established standard tree ring width chronologies of R. pseudoacacia at the four sites along a north-south precipitation gradient on the CLP, aiming to determine the relationship between climate and growth as well as quantify tree resilience to drought. Results showed that water availability [precipitation and standardized precipitation evapotranspiration index (SPEI)] and maximum temperature in most seasons were the key climate factors that limited the radial growth of trees in R. pseudoacacia plantations. The relationship between temperature and tree ring width index (RWI) in both regions varied from positive (January, March, and spring) to negative (June) and then to positive (autumn) over time. Spatially, R. pseudoacacia exhibited higher resistance (Rt > 1) to drought and lower recovery (Rc < 1) in the semi-arid region compared to those in semi-humid region under the same drought event (mild, moderate, or severe). The trade-off between drought vulnerability indices indicated the recovery of R. pseudoacacia after drought had a significantly negative correlation with resistance. Resilience of R. pseudoacacia presented a distinct spatiotemporal pattern affected by variations in climate factors (temperature, precipitation and SPEI), site geographical conditions (altitude, longitude, and latitude), and tree characteristics (diameter at breast height (DBH), and RWI for one and two years before a drought event). The effect of site geographical conditions and climate factors, respectively, explained 70.6 and 41.6% of drought resistance and recovery variations. The interaction effects of climate factors and site geographical conditions accounted for 42.8 and 64.3% of the variances in resistance and recovery for R. pseudoacacia, respectively. The results demonstrated the effects of geographical and climatic conditions on the growth of plantation species such as R. pseudoacacia are important considerations that can inform future vegetation restoration efforts to improve the stability and sustainable development of plantation forests on the CLP.
1 Introduction
A central element of global climate change is climate warming, and its impact on terrestrial ecosystems has emerged as a key scientific issue (Solomon et al., 2007; Collins et al., 2013). Global average air temperatures are expected to increase by ∼1–5.7°C by 2,100, with extreme drought and rainstorm events projected to increase in both magnitude and frequency (IPCC, 2021). In the context of ongoing climate change, efforts to increase carbon stock capacity and mitigate climate change in areas considered to be ecologically fragile have included the implementation of plantation forests (Feng et al., 2022). These plantation forests significantly contribute to China’s “carbon neutrality” strategy of 2,060, provided they remain strong and healthy (Cai et al., 2022). However, climate warming can have strong negative effects on tree growth and survival, forest ecosystem function, species distribution, and overall forest productivity (Shuman et al., 2011; Garcia et al., 2014). In particular, increasing evidence indicates severe decline and mortality in many forests, especially in ecologically sensitive areas, due to warming-drying trends (Allen et al., 2010; Liu et al., 2013). Therefore, better understanding of the relationship between climate change and forest growth is necessary to maintain regional ecosystem sustainability and address the negative impacts of climate change on these forest ecosystems.
Relevant past studies have been conducted mainly based on satellite data at a large scale and lack tree growth measurement data (Qiu et al., 2021; Yang et al., 2021). Tree rings can provide a long-term record with an annual resolution of radial growth (Fritts, 1976; Salzer et al., 2009; Cao et al., 2019) and therefore, the tree ring method is widely used to study the dynamics of forest growth and its response to climate change (Liang et al., 2016; Ning et al., 2022; Che et al., 2023a; Pompa-García et al., 2023). Tree radial growth affected by climate change can demonstrate significant spatial and temporal differences (Metslaid et al., 2018; Wang A. et al., 2022; Zhang W. G. et al., 2023; Zhang W. G. et al., 2023; Ni et al., 2023). For instance, tree growth at the alpine timberline has been promoted by increased temperatures in many regions (Li et al., 2017; Yang et al., 2017). Moreover, water-sensitive trees have formed narrow rings when suffering from drought, but recovered growth with subsequent increases in rain (Fang and Zhang, 2019).
The response of trees to drought results in varied growth performance depending on the habitat and climatic conditions (Willis et al., 2018). Lloret et al. (2011) assessed this process by proposing three indices of drought vulnerability [namely “resistance (Rt), recovery (Rc), and resilience (Rs)”] based on comparing the radial growth differences of trees before and after a drought (see Methods). Compared to traditional methods of correlation analysis, this approach is better able to quantitatively assess tree response to drought and has been widely used in recent years (Rais et al., 2014; Camarero et al., 2015; Han et al., 2025; Xue et al., 2025). Multiple studies have indicated quantifying the spatiotemporal variation of tree resilience to drought across extensive regional gradients can help elucidate the primary mechanisms driving forest dynamics following climate disturbances (Fang and Zhang, 2019; Bohner and Diez, 2021). Efforts by Gazol et al. (2017) indicate these drought vulnerability indices may differ across climate regions, suggesting trees have different coping strategies related to drought, but such responses can be overwhelmed by geographical patterns of available soil moisture. Studies in the Tibetan Plateau (Fang and Zhang, 2019) show growth resilience is associated with diurnal temperature range, growth coherence among trees, and soil moisture. However, understanding of the main drivers and variation of drought resilience is very limited, especially for plantations, despite their known drought sensitivity (Che et al., 2023b; Zhang W. G. et al., 2023).
The Chinese Loess Plateau (CLP) has a particularly fragile and climate-sensitive ecosystem. Soil erosion in this region has been serious and natural forests have been destroyed due to the combined impact of anthropogenic activity and climate change (Wang et al., 2011; Feng et al., 2016; Che et al., 2022). In a concerted effort to address soil erosion and achieve ecosystem restoration, the Chinese Government implemented the “Grain for Green” project (an extensive ecological rehabilitation program) in the late 1990s (Wang A. et al., 2022). Afforestation on the CLP predominantly features Robinia pseudoacacia L., which was selected based on its fast growth and tolerance of barren and dry environments (Li et al., 2018). These plantations have effectively controlled soil erosion (Wang et al., 2019), improved the productivity of forest ecosystems, and increased the sequestration of carbon on the CLP (Feng et al., 2016; Cao and Chen, 2017; Zhu et al., 2020). However, downsides have come to light as a result of scarce precipitation and lack of understanding of R. pseudoacacia suitability in local environments and how this species responds to climate change (Jia et al., 2019; Li et al., 2021). For instance, these plantations have experienced growth degradation due to drought that is attributed to climate change (Wei et al., 2018; Yan et al., 2024). In addition, warmer and drier trends in CLP climate as a result of global warming are evident based on several decades of data (Li et al., 2010), reflecting prolonged and increasingly intense droughts and great changes in both precipitation and temperature (Zhang et al., 2012). Therefore, better understanding of the response of these plantations to drought is required to improve predictions of the impact of future climate changes on the CLP.
In this study, the overall goal was to comprehensively analyze climate-growth correlations and resilience of R. pseudoacacia plantations on the CLP to drought events. The objectives of this study were to: (1) analyze the characteristics of radial growth of R. pseudoacacia at different study sites; (2) investigate the main climate factors limiting the radial growth of R. pseudoacacia; and (3) explore variations of drought vulnerability indices and the major factors driving the resilience of these trees to drought along a precipitation gradient of the CLP. The results enrich our knowledge regarding the impacts of climate on forest growth, and are useful for developing forest management strategies for the future sustainable management of R. pseudoacacia plantations on the CLP.
2 Materials and methods
2.1 Study sites
Sampling sites were located at 107.69°E-110.22°E, 34.55°N-37.67°N along a precipitation gradient from south to north on the CLP (Figure 1). The topography of the CLP is relatively fragmented but can be categorized from southeast to northwest into three distinct regions: loess gully, loess hilly and gully, and sandy hilly regions (Yang et al., 2019). Situated deep inland and on the periphery of the monsoon-affected region, this area experiences a temperate continental monsoon climate. The mean annual precipitation (MAP) varies between 200 and 800 mm and most (more than 60%) falls between July and September. Precipitation exhibits an uneven temporal and spatial distribution that decreases from the southeast to the northwest. The mean annual temperature (MAT) ranges from 3.6 to 14.3°C (Table 1). From 1961 to 2020, the MAP in this region did not significantly change but the MAT showed a significant upward trend, indicating overall warming and drying (Wang, 2023).
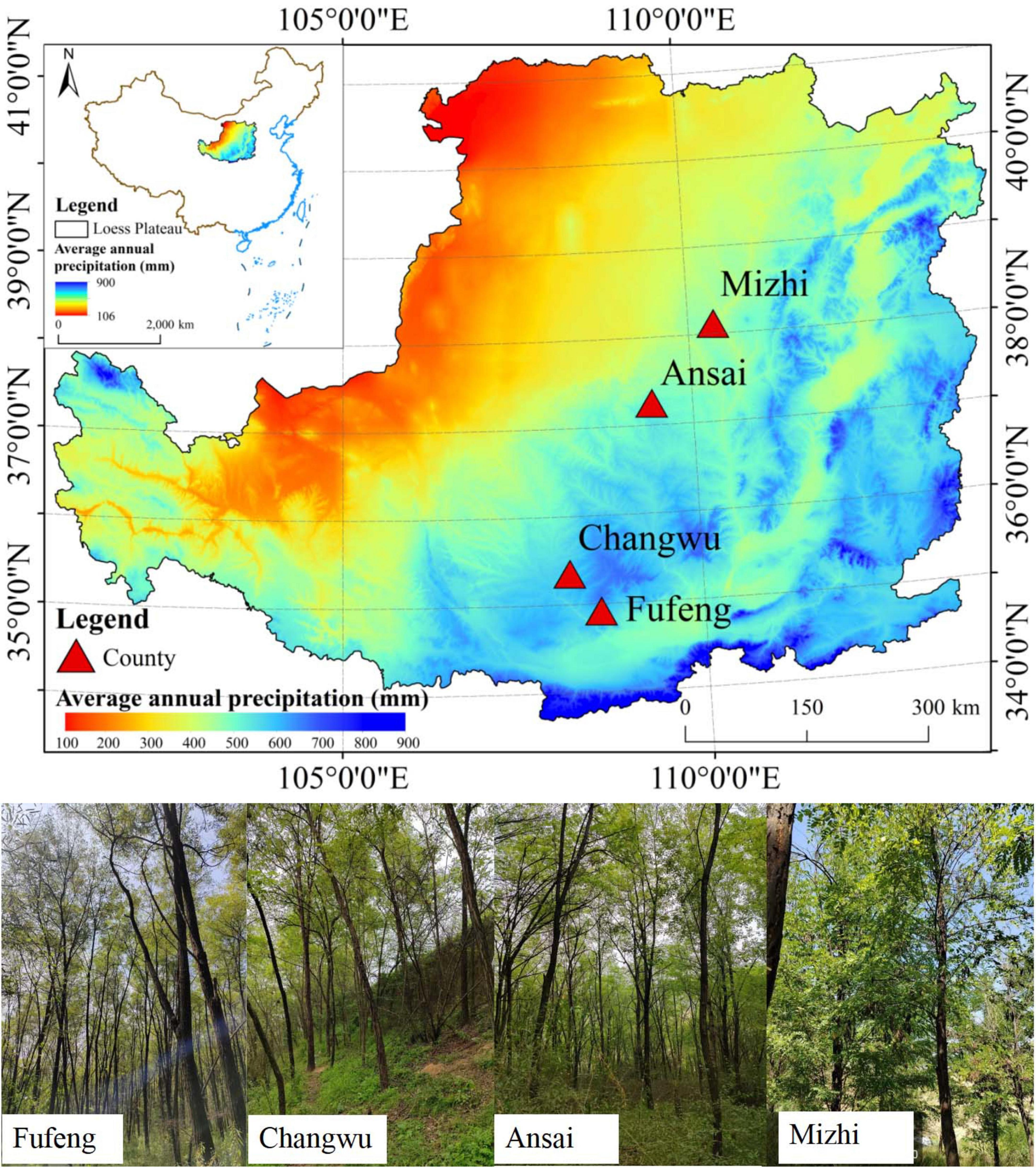
Figure 1. Location of tree ring sampling sites on the CLP. Different colored areas on the map denote the multi-year annual precipitation gradient. Sampling sites are Fufeng, Changwu, Ansai, and Mizhi along a precipitation gradient from south to north.
As a consequence of the large-scale “Grain for Green” project, most vegetation on the CLP is in the form of plantations of various species. R. pseudoacacia is the most common afforested species, with its popularity attributed to its fast growth and complex root structure. As such, it is effective at controlling soil erosion and can play a key role in improving soil quality and increasing carbon sequestration (Feng et al., 2012). Using information on the spatial distribution of R. pseudoacacia, soil texture, and MAP, we selected four study sites on the CLP located in the major distribution areas of R. pseudoacacia. These sites stretch from south to north and feature different MAP and MAT values along a precipitation gradient (Fufeng: 630.2 mm and 13.1°C; Changwu: 598.4 mm and 9.7°C; Ansai: 518.4 mm and 9.3°C; and Mizhi: 444.6 mm and 9.8°C) (Figure 1; Table 1). The climate data indicated that the four study sites had exhibited a notable warm-drying trend, characterized by a significant upward trend in temperature (P < 0.01) and no significant changes in precipitation over the past 40 years (Figure 2). The main soil textural types on the CLP as well as regional topographical features are represented by these four sites.
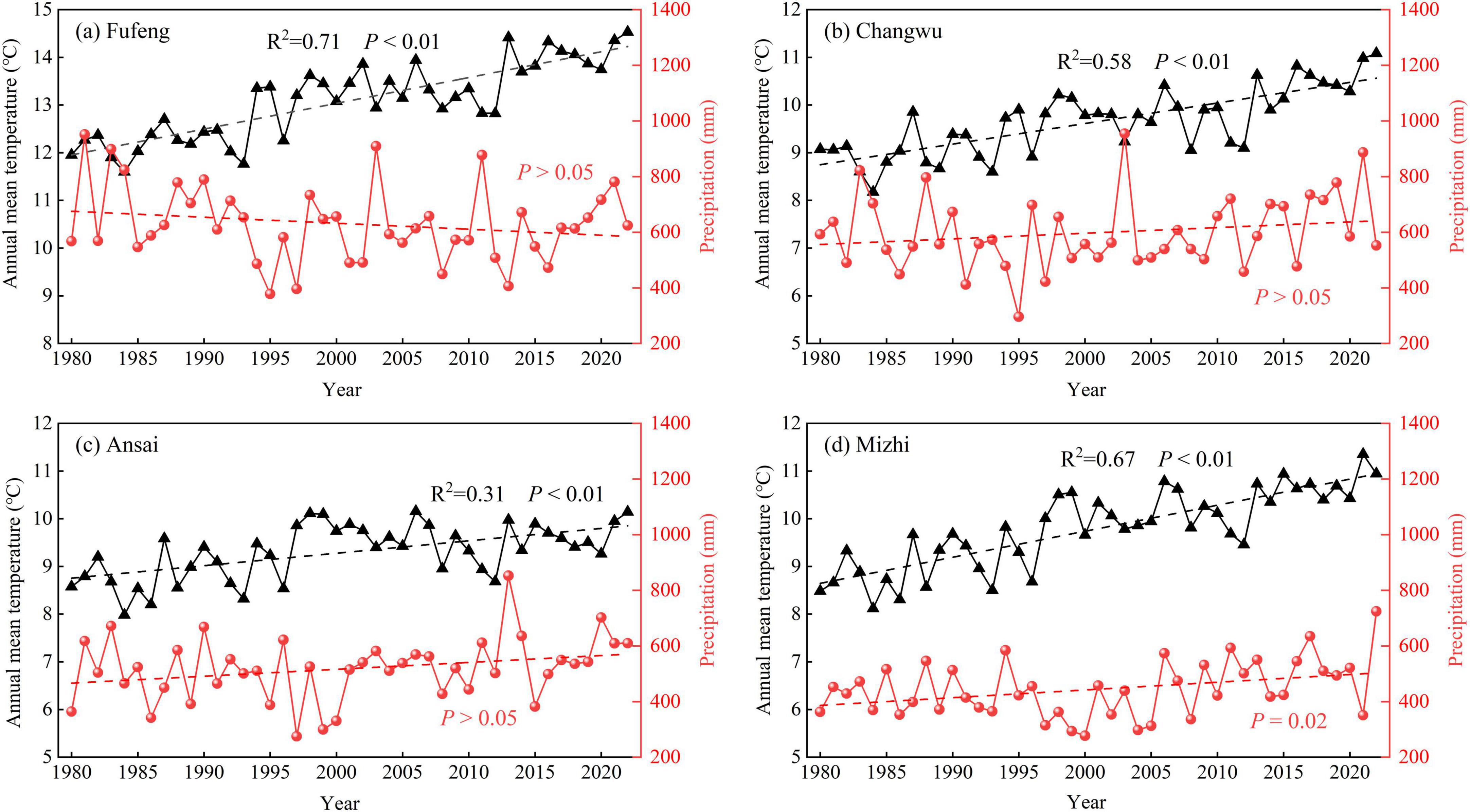
Figure 2. Variations of annual mean temperature (black line) and total precipitation (red lines) from 1980 to 2022 at the four study sites on the Loess Plateau. (a) Fufeng site, (b) Changwu site, (c) Ansai site, (d) Mizhi site. Dashed lines denoted the climate change trend.
2.2 Sample collection and processing
We conducted quadrat setting and sampling work at each sampling site in September 2023. The topographic information (latitude, longitude, and altitude) and stand characteristics of the sampling sites are recorded in Table 1. R. pseudoacacia at the sites chosen for this study exhibited minimal human interference and relatively uniform environmental conditions (slope, aspect, elevation, soil types). We avoided areas that have recently experienced significant disturbances (such as pest outbreaks and logging) as well as extreme sites with evident soil erosion or severely depleted/enriched nutrients. The Mizhi site is the only site with significant canopy dieback in the R. pseudoacacia plantations. We selected 25–30 healthy R. pseudoacacia trees (dominant trees more than one half of the total sample trees) at each site and took two cores at breast height (1.3 m) using a 5.15 mm-diameter increment borer. The cores were then stored in plastic tubes for subsequent analysis in the laboratory.
The tree ring cores were processed in the laboratory using standard dendrochronological techniques (Stokes and Smiley, 1996). The cores were first air dried, then glued into grooved wooden blocks and tied with rope to prevent warping. The samples were then polished using various grades of sandpaper (220-, 400-, and 600-grit) until the annual rings could be distinguished. Tree ring width was measured using a LINTAB 6 measurement station (Rinntech Inc., Germany; resolution 0.001 mm). Cross-dating and measurements were verified with COFECHA. Specifically, correlation coefficients of each tree ring width sequence within the main sequence were used to determine any dating or measurement errors. Any sample cores with inconsistent data were measured again or withdrawn to ensure the accurate dating of each tree ring (Holmes, 1983). The tree ring width measurements were detrended and standardized by negative exponential curve and linear regression using ARSTAN for each site (Cook and Kairiukstis, 1990). In a few cases that this method left substantial decadal variation in growth or could not be applied, a cubic smoothing spline curve of 67% of the series length was also used to fit the tree ring width (Cook and Kairiukstis, 1990). Then, ring width indices were combined using bi-weighted robust means to develop standardized ring width chronologies, reducing the effects of outliers (Cook, 1985). Dendrochronological statistics, including the first-order autocorrelation (AC1) of raw width data, mean sensitivity (MS) of indexed growth values, signal-to-noise ratio (SNR), and expressed population signal (EPS), were computed to characterize the chronologies and evaluate their quality (see Table 2). The AC1 reflects the time lag of climate effects on tree growth (Fritts, 1976), the MS reflects the magnitude of interannual variability in annual tree ring width, and the SNR reflects the amount of climate information present in the chronology (Briffa and Jones, 1990). EPS values above 0.85 indicate the constructed chronology reflects the characteristics of the theoretical chronology and can represent the overall characteristics of regional tree growth (Wigley et al., 1984).
2.3 Climate data and drought events
The monthly mean, minimum, and maximum temperatures (Tmean, Tmax, and Tmin) and total precipitation data were obtained from meteorological stations (China Meteorological Data Network)1 located nearest the sample sites. In addition, we employed two drought indices to reflect drought features on the CLP. The standardized precipitation evapotranspiration index (SPEI) was calculated using precipitation, mean temperature, and evapotranspiration data using the R package “SPEI” (Vicente-Serrano et al., 2010; Beguería et al., 2014). The self-calibrating Palmer drought severity index (scPDSI) was used to represent soil moisture conditions with additional considerations related to atmospheric input and soil evaporation (Wells et al., 2004). Gridded scPDSI data were obtained from the KNMI Explorer2 nearest to our study sites. Because drought may occur at any time of the year, we calculated SPEI and scPDSI values at various time scales (monthly, annual, seasonal, growing season) and then used different time scales for temperature, precipitation, and SPEI data to analyze the climate–growth relationships.
Based on previous studies (Che et al., 2023a) and correlation analysis, a drought year was defined based on the SPEI for March-September. We identified SPEI < −0.5 as a drought event using the drought grade classification of the SPEI and historical SPEI fluctuations for each site (Table 3; Figure 2). Drought intensity was grouped into four drought regimes (mild, moderate, severe, and extreme drought) according to SPEI scales (Chen and Sun, 2015). When drought occurs continuously for several years, we consider it as a drought event. In the case of multiple consecutive drought years interrupted by a single non-drought year, we calculated the multi-year average SPEI. Then, we determined whether 60% or more of the trees experienced growth decline in that year compared with the average RWI of the previous two non-drought years to classify the type of drought event (Schwarz et al., 2020; Che et al., 2023a). Hence, this study identified the drought years (events) as follows: 2008 and 2013–2017 (moderate drought) for the Fufeng site from 2004 to 2022; 1991 (moderate drought) and 1994–1997 (severe drought), 2000–2001, 2004–2005, 2009, 2012 (mild drought), and 2016 (moderate drought) for the Changwu site from 1989 to 2022; 1999–2000 (severe drought), 2008–2010 (mild drought), 2015–2016 (moderate drought) for the Ansai site from 1996 to 2022; 2007–2008 (moderate drought), 2015 (mild), and 2021 (severe drought) for the Mizhi site from 2005 to 2022 (Figure 3).
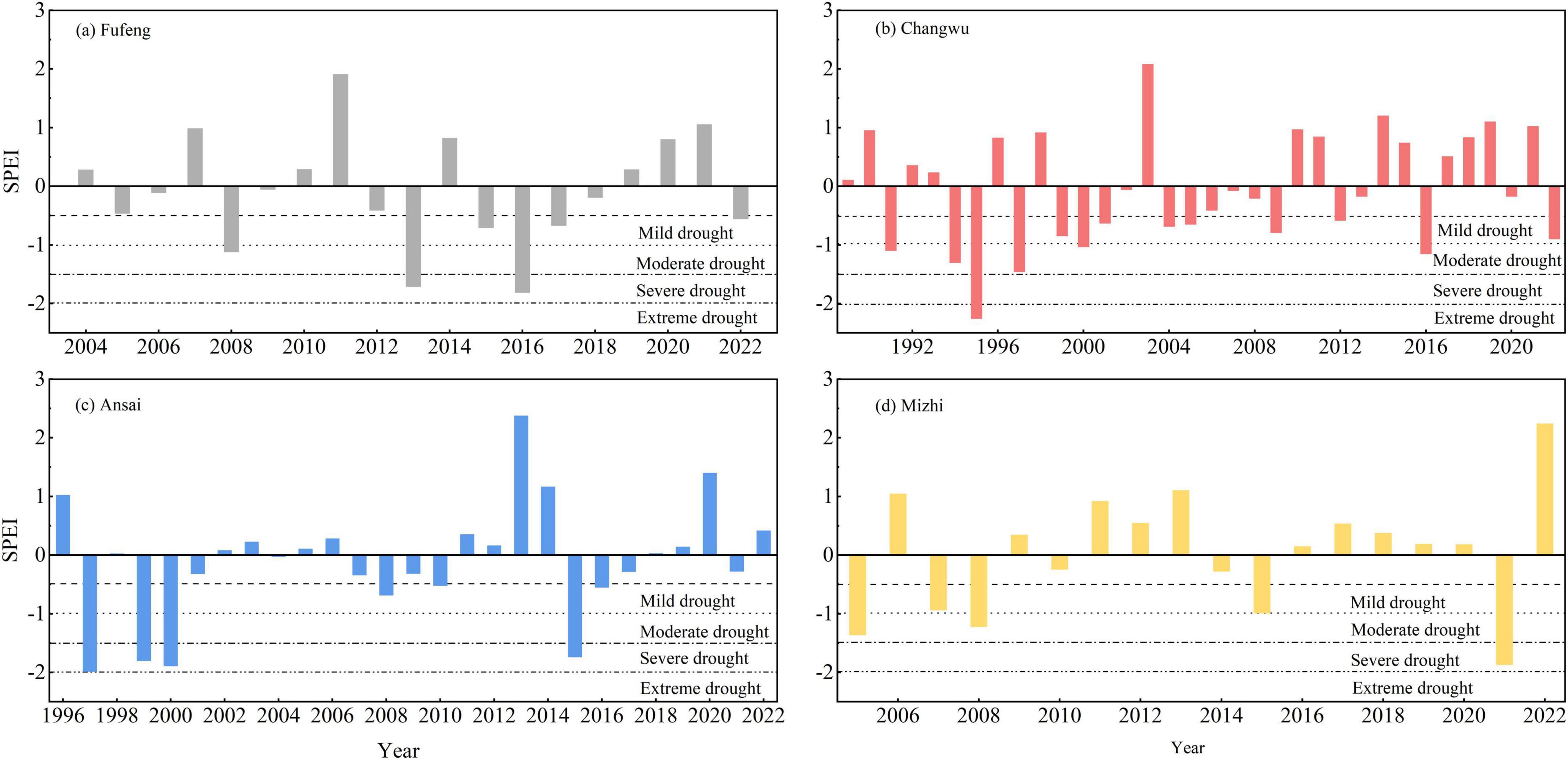
Figure 3. Interannual variationsin the standardized precipitation evapotranspiration index (SPEI) at the four sites (Fufeng, Changwu, Ansai, and Mizhi). The adjacent black dotted lines indicate a drought category, which is classified into extreme, severe, moderate, and mild drought events based on the increasing SPEI values.
2.4 Drought vulnerability indices
The vulnerability of R. pseudoacacia plantations to drought was assessed by applying three drought vulnerability indices (Lloret et al., 2011): resistance (Rt), which refers to the ability of trees to maintain growth and resist drought; recovery (Rc), which refers to the ability of trees to recover from drought events; and resilience (Rs), which refers to the ability of trees to recover to pre-drought levels after drought and quantifies trees adaptation to drought events (Fang and Zhang, 2019). The three indices were calculated using the following formulae:
where Dr indicates the RWI in the drought year; and PreDr and PostDr represent the average RWI for the 2 years before and after the drought year, respectively. The 2-year period is used to take into consideration the lag effects of drought and also minimize interference from other drought events (Schwarz et al., 2020; Chen et al., 2023). In the calculation of drought vulnerability indices, we incorporated all aforementioned types of drought events (from extreme to mild) into the equation. For the Mizhi site, a pre-drought period of 1 year for 2007–2008 and a post-drought period of 1 year for 2021 were chosen based on the data availability since we did not have periods of at least 2 years before and after the droughts. Rt > 1 was considered indicative of highly resistant to drought, with trees able to recover from drought when Rc > 1.
Schwarz et al. (2020) proposed the “Line of full resilience” framework, which assumes that trees can fully recover (i.e., Rs = 1) from a certain drought event and considers the following relationship:
Then, the “actual resilience line” was established by fitting the power functions of Rt and Rc of the trees, with the functional equation as follows:
where α and β are fitting parameters.
By comparing the deviations and intersections between the two curves, we can evaluate the capacity of the trees to recover. This approach allows for a more systematic and in-depth assessment of how trees recover in response to drought conditions.
2.5 Data analysis
Linear regression was used to fit the temporal trends of standard RWI, the SWUE and sensitivity of RWI variations to climate for R. pseudoacacia. The growth–climate relationships were quantified using correlation analysis between tree standard RWI and climate variables (Tmean, Tmax, Tmin, P, and SPEI) at different temporal scales. Our study calculated aggregated seasonal climate variables: the spring (Spr) representing March to May; the summer (Sum) representing June to August; the autumn (Aut) representing September to November; the winter (Win) representing the current year’s December to the next year’s February; and the growing season (GS) representing May to September. We also performed correlation analysis between RWI and monthly climate variables, specifically from October of the previous year to September of the current year (P10-C9).
One-way analysis of variance (ANOVA) and paired t-test were used to compare differences in tree Rt, Rc, and Rs at different spatial temporal scales. Multiple linear regression (MLR) models were used to evaluate the relationships between potential drivers and tree resilience (Rt and Rc). Specifically, we used tree resilience (Rt and Rc) as predictor variables, and potential drivers (climate factors, site geographical conditions and tree characteristic) as explanatory variables. Among them, climate variables including the mean temperature and total precipitation during the current and previous growing seasons of a drought event (GStem, PGStem, GSprep, and PGSprep), and the average SPEI of 2 years before and after a drought event, and during a drought event (SPEIpre, SPEIpos and SPEIpoi). Site geographic variables including altitude, longitude, and latitude of study sites. The tree characteristic variables including diameter at breast height (DBH), and tree ring width index for 1 and 2 years (average) before a drought event (Pre-RWI and 2Pre-RWIave). The MLR modeling was performed using the R package “lme4” (Bates et al., 2015). The step function was then used to optimize the model through a backward stepwise regression method, resulting in a simplified regression model (van der Maaten-Theunissen et al., 2015). To ensure the comparability of model parameters, the scale function was used to standardize the data before analysis. Finally, we quantified the relative importance of each explanatory variable by calculating the ratio of the absolute value of each variable’s standardized regression coefficient to the total of the absolute values of regression coefficients of all explanatory variables in the simplified regression model (Le Bagousse-Pinguet et al., 2019). Variance partitioning analysis (VPA) was conducted using a series of partial and constrained ordinations with “vegan” package in R software to identify the effects of three reconstructed variables, including climate variables, site geographic variables, and tree characteristic variables and their interactions on the changes in tree resistance and recovery (Dixon, 2003).
In addition, we utilized soil water use efficiency (SWUE) and the average sensitivity of RWI variations to evaluate the sensitivity of R. pseudoacacia growth to drought across the various study sites (Dai et al., 2004; Li et al., 2006; Zhao, 2021). The formula for calculating SWUE is as follows:
where RWIt refers to the annual ring width index of calendar year t and PDSIt refers to the value of the biological annual average PDSI corresponding to calendar year t.
The interannual sensitivity (Sχ) of RWI variations to climate is calculated as follows (Zhao, 2021):
where RWIt+1 refers to the annual ring width index of calendar year t+1.
All statistical analyses were conducted using SPSS version 25.0 (SPSS Inc., Chicago, IL, United States) and R version 4.4.1 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Radial growth and chronology characteristics
Among the statistical characteristic values of standard tree-ring width chronologies for R. pseudoacacia at the study sites, the MS and SNR values ranged from 0.19 to 0.34 and from 6.71 to 31.65, respectively (Table 2). This indicated that there were significant inter-annual variations and abundant climatic information in these chronologies. The AR1 of the sampling sites was 0.15–0.44, indicating that current year climate factors affected subsequent year radial growth in R. pseudoacacia. The EPS values were all greater than the 0.85 threshold considered to be a desirable level. These results showed the data based on the samples collected could adequately represent the overall growth characteristics of R. pseudoacacia at the study sites and therefore were suitable for dendroclimatological studies via tree ring chronologies.
The variations of radial growth for R. pseudoacacia showed obvious differences at the four study sites (Figure 4). The standard ring width chronologies varied from 0.64 to 3.35 at Fufeng, 0.62 to 1.56 at Changwu, 0.68 to 1.54 at Ansai, and 0.43 to 2.51 at Mizhi. The radial growth of R. pseudoacacia trend was relatively stable at the Changwu (R2 = 0.01, P > 0.05) and Ansai sites (R2 = 0.04, P > 0.05) but showed a slight upward trend at the Fufeng (R2 = 0.14, P > 0.05) and Mizhi sites (R2 = 0.20, P > 0.05).
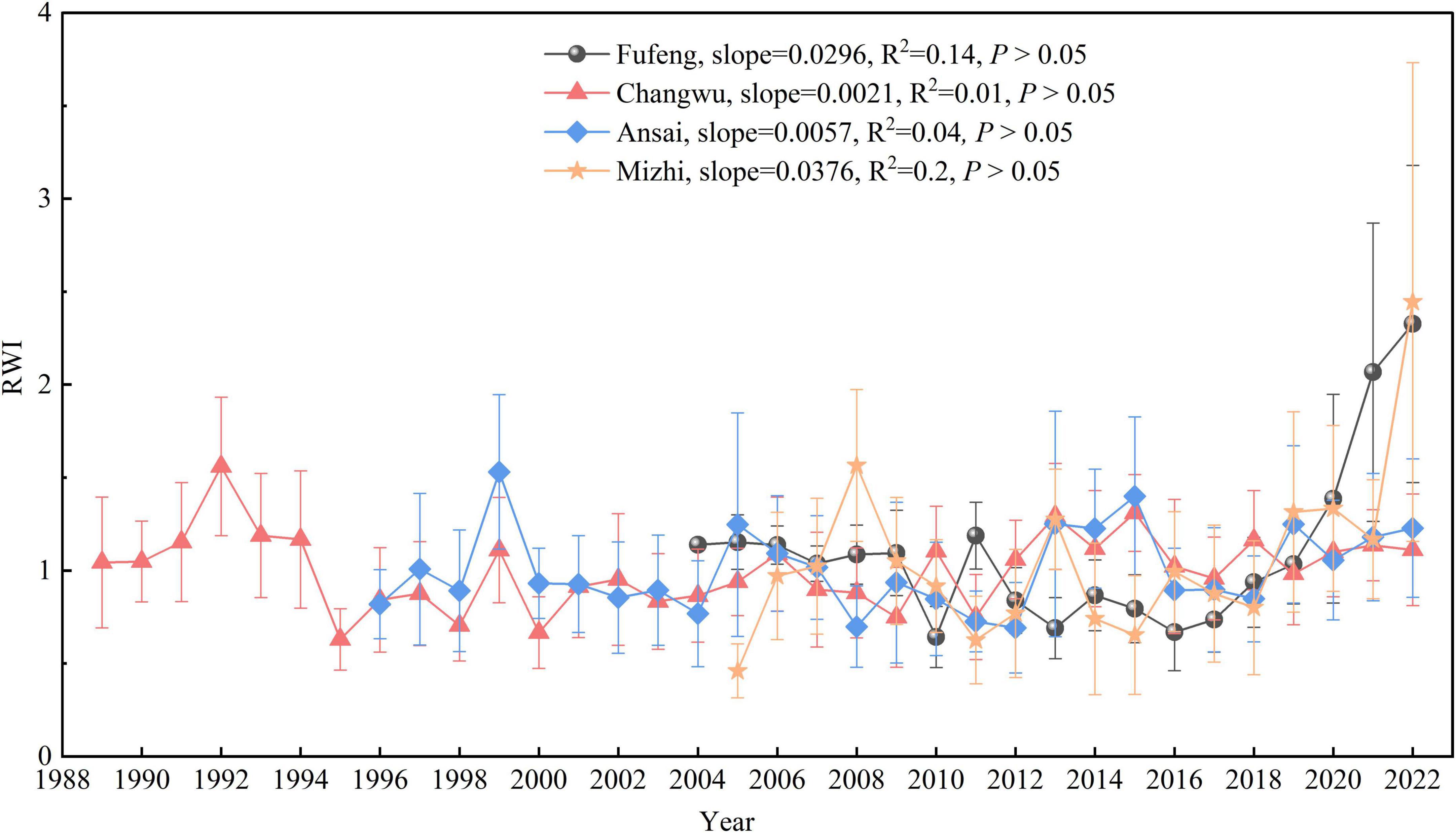
Figure 4. Interannual variations (Mean ± standard deviation) of the standard ring width chronologies for R. pseudoacacia at the four sites. RWI: tree ring width index. The slope, R2, and P-value are the primary parameters of the linear trend lines of RWI for R. pseudoacacia at each site.
3.2 Sensitivity assessment of tree growth
The SWUE and sensitivity of RWI variations to climate for R. pseudoacacia fluctuated greatly between years, while the differences of overall change trend depended on the environmental conditions of the stands (Figures 5, 6). Specifically, the average SWUE of R. pseudoacacia ranged from 0.06 to 0.26, with slight upward trends at the Fufeng and Mizhi sites and downward trends at the Changwu and Ansai sites over time. The average sensitivity of RWI variations to climate for R. pseudoacacia varied between 0.1 and 0.93, with downward trends over time except at the Fufeng site (Figure 5). Furthermore, the average sensitivity exhibited significant spatial differences in drought and non-drought years (P < 0.05), whereas the SWUE showed an opposite pattern (P > 0.05). The average sensitivity of RWI variations to climate were lower in the semi-humid region (Fufeng and Changwu sites) than in the semi-arid region (Ansai and Mizhi sites) in drought years (P < 0.05; Figure 6).
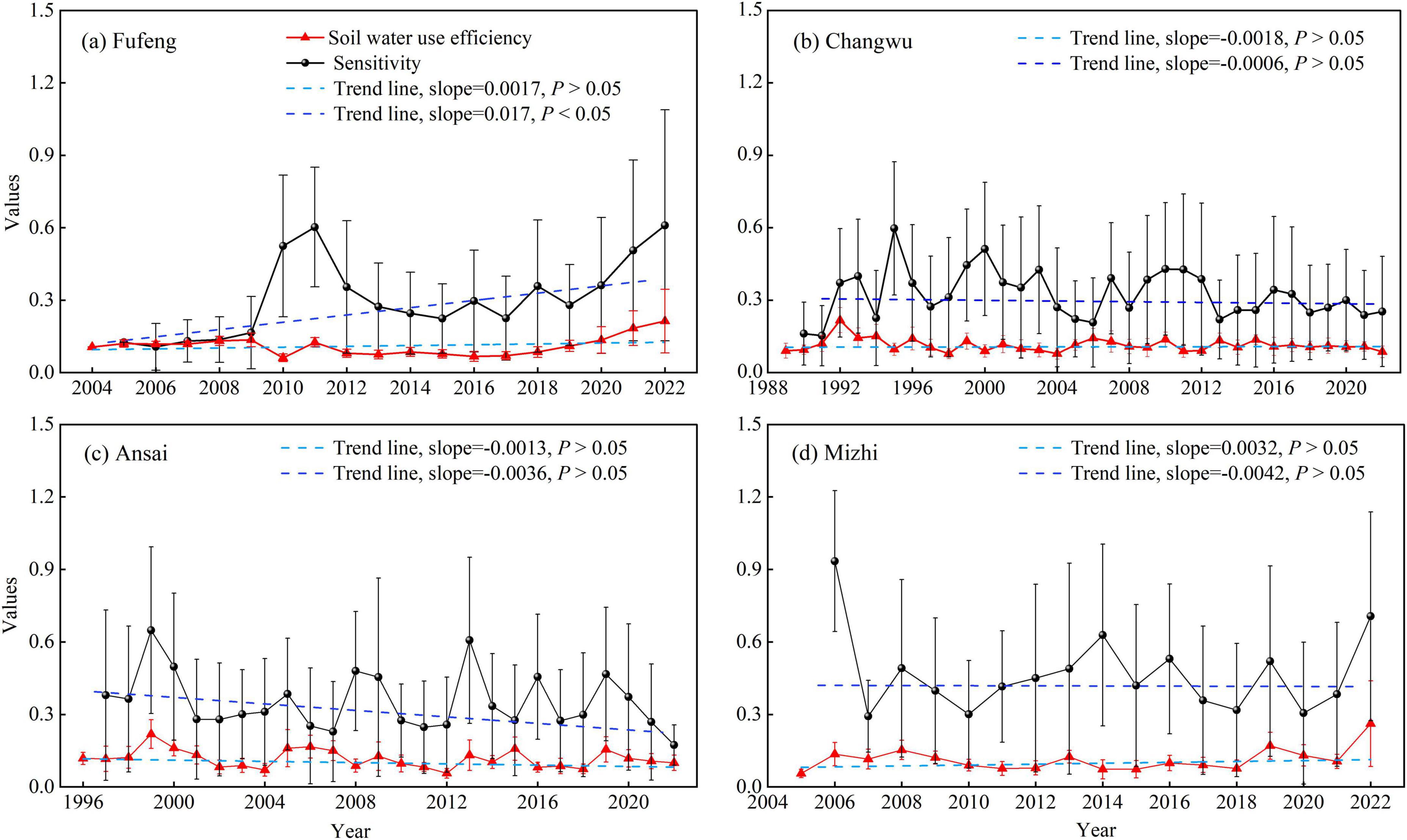
Figure 5. The interannual variations and trends of soil water use efficiency (SWUE) and sensitivity of RWI variations to climate for R. pseudoacacia at the four sites. (a) Fufeng site, (b) Changwu site, (c) Ansai site, (d) Mizhi site.
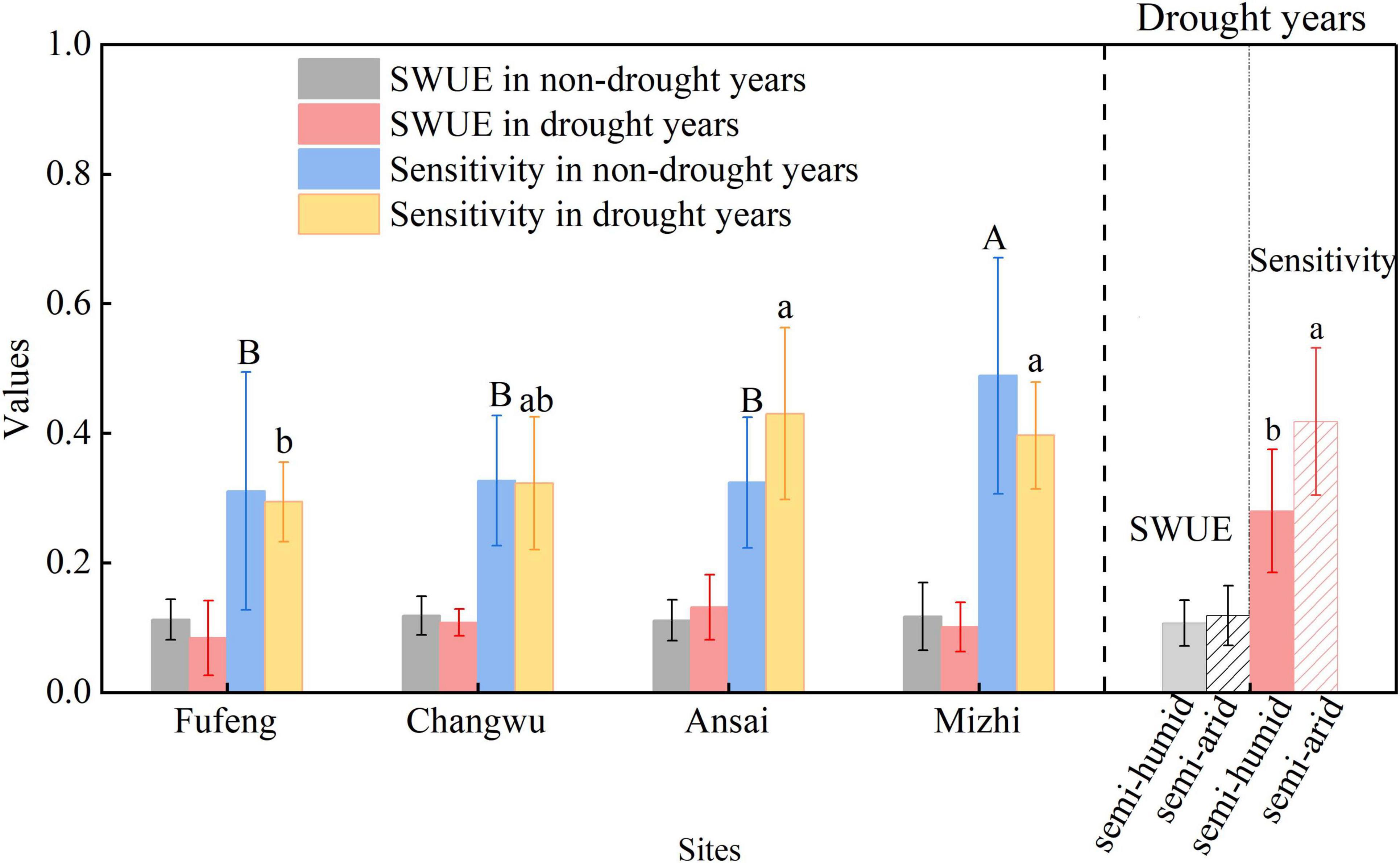
Figure 6. Comparisons of soil water use efficiency (SWUE) and sensitivity of RWI variations to climate for R. pseudoacacia across different sites and between drought and non-drought years. Different lowercase/uppercase letters denote significant differences between sites in drought/non-drought years (P < 0.05).
3.3 Radial growth response to climate factors
The growth of R. pseudoacacia in the study sites was mainly limited by climatic factors related to drought stress (Figures 7, 8). Specifically, the RWI of R. pseudoacacia at the Fufeng site was significantly positively correlated with precipitation and SPEI in August of the current year (P < 0.05) but significantly negatively correlated with Tmean and Tmax in October of the previous year (P < 0.01). Temperature and SPEI were significantly positively correlated with the RWI of R. pseudoacacia at the Changwu site, especially early in the year (January-March). Tmax in June was strongly negatively correlated with RWI (P < 0.05). At the Ansai site, RWI was significantly negatively correlated with precipitation and SPEI in October of the previous year, as well as Tmin in December. Conversely, RWI was positively correlated with Tmax in January of the current year (P < 0.05). Tmean and Tmin in March of the current year were strongly positively correlated with the growth of R. pseudoacacia at the Mizhi site (P < 0.05) (Figure 7). Moreover, radial growth of R. pseudoacacia was positively correlated with precipitation and SPEI in the spring, summer, and growing season of the previous year (Figure 8). These correlation results showed that the radial growth of R. pseudoacacia was limited by SPEI, precipitation (water availability), and maximum temperature on the CLP. The relationship between RWI of R. pseudoacacia and temperature varied from positive (January, March and spring) to negative (June) to positive (autumn) over time, indicating that the effect of temperature on radial growth transitioned from promotion to inhibition and then to promotion. Radial growth was more strongly related to climate variables at the Fufeng site than at the Mizhi site.
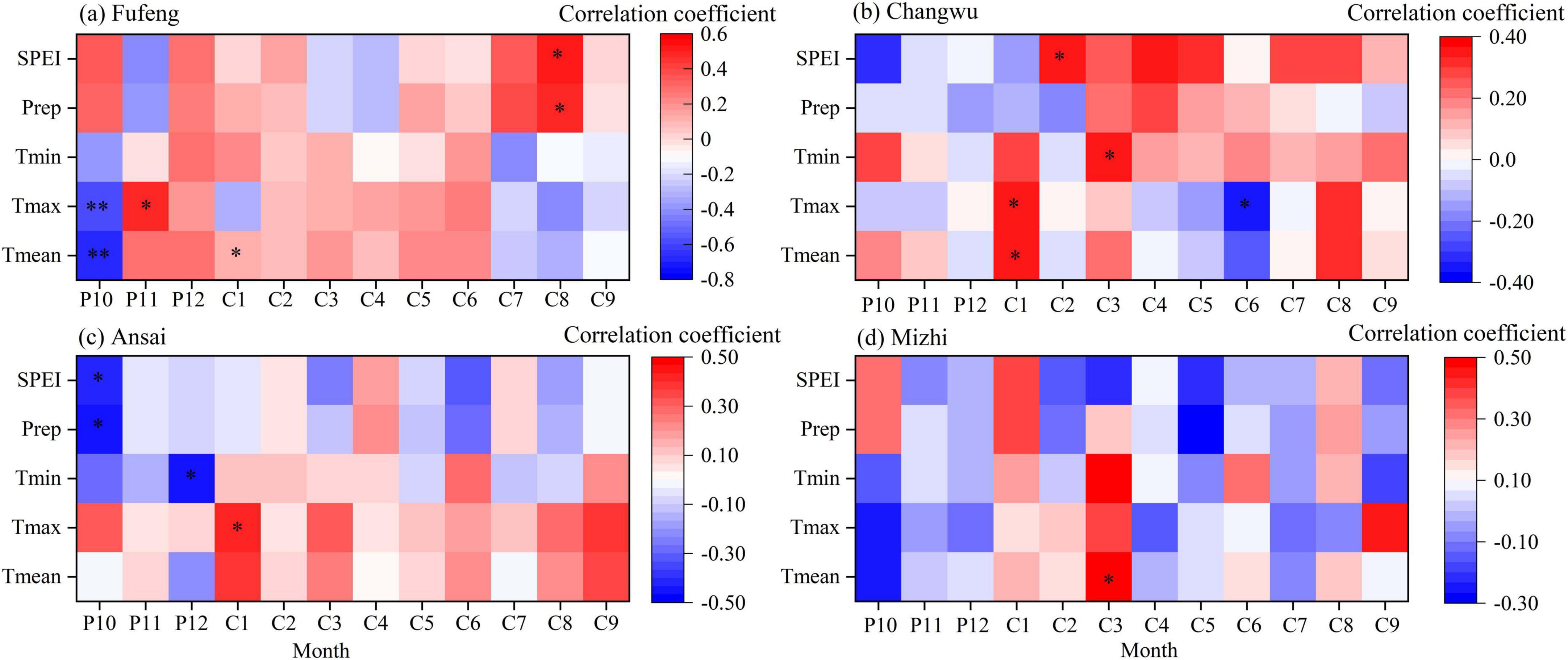
Figure 7. Correlation coefficients between the tree ring width index (RWI) of R. pseudoacacia and monthly climatic factors at the four sites. (a) Fufeng, (b) Changwu, (c) Ansai, (d) Mizhi. The x-axes (P10-C9) indicate the months from the October of the previous year to September of the current year. Tmean, monthly mean temperature; Tmax, monthly maximum temperature; Tmin, monthly minimum temperature; Prep, monthly precipitation; SPEI, standardized precipitation evapotranspiration index. “**” represents P < 0.01, and “*” represents P < 0.05.
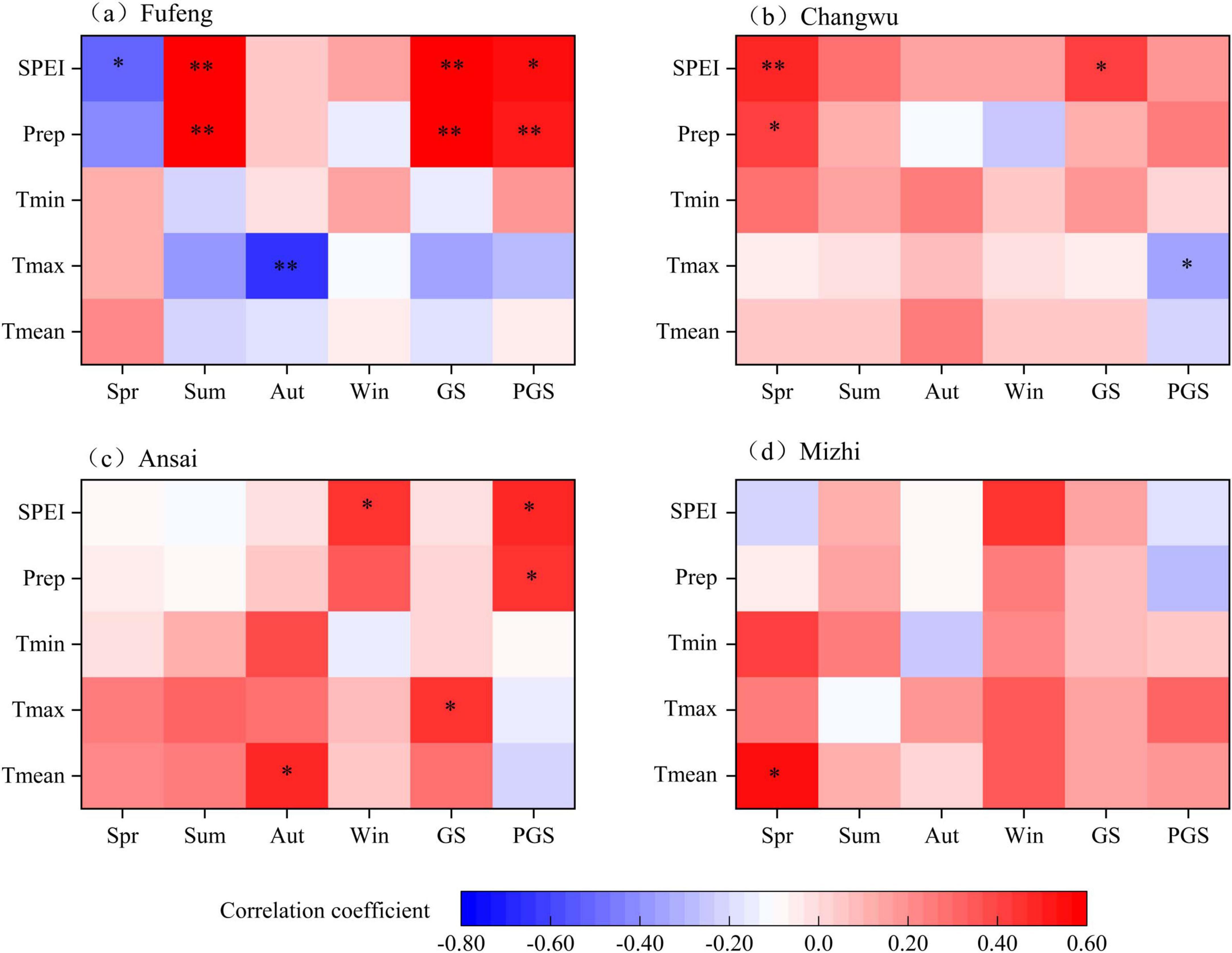
Figure 8. Correlation coefficients between the standard tree ring width index (RWI) of R. pseudoacacia and climatic factors at the four sites. (a) Fufeng site. (b) Changwu site. (c) Ansai site. (d) Mizhi site. Spring (Spr): March to May; Summer (Sum): June to August; Autumn (Aut): September to November; Winter (Win): December to February of the next year; GS and PGS: growing seasons of the current and previous year, respectively.
3.4 Variations in drought resistance, recovery, and resilience
The vulnerability of R. pseudoacacia to drought in different climate regions showed spatial heterogeneity (Figure 9). The R. pseudoacacia at the Ansai site exhibited significantly higher resistance (1.30) and lower recovery (0.72) to drought than other sites (P < 0.05). Similarly, the resilience of R. pseudoacacia at the Fufeng (0.88) and Ansai (0.89) sites to drought was also significantly lower than at other sites (P < 0.05; Figure 9a). After the mild drought event, the resistance of R. pseudoacacia at the Ansai (1.38) site to drought were significantly better than at the Changwu (0.90) site, while the opposite was true for recovery (P < 0.05; Figure 9b). The resistance (1.62) and resilience (1.23) of R. pseudoacacia at the Mizhi site after the moderate drought were significantly higher than at the Fufeng (0.99, 0.77) site. The resistance (0.99) of R. pseudoacacia at the Ansai site to drought was also significantly better than at the Changwu (0.83) site, while the recovery was significantly lower (P < 0.05; Figure 9c). Following the severe drought event, R. pseudoacacia at the Ansai site exhibited significantly higher drought resistance (1.65 vs. 0.65) and resilience (1.10 vs. 0.64) but lower recovery (1.04 vs. 0.69) than at the Changwu site (P < 0.05; Figure 9d). At the Ansai and Mizhi sites, the resistance of R. pseudoacacia to drought was greater than 1, while its post-drought recovery capacity was less than 1. Multi-year drought more negative impact on resistance and resilience for R. pseudoacacia at the Ansai site than at the Changwu site (Figures 9c,d).
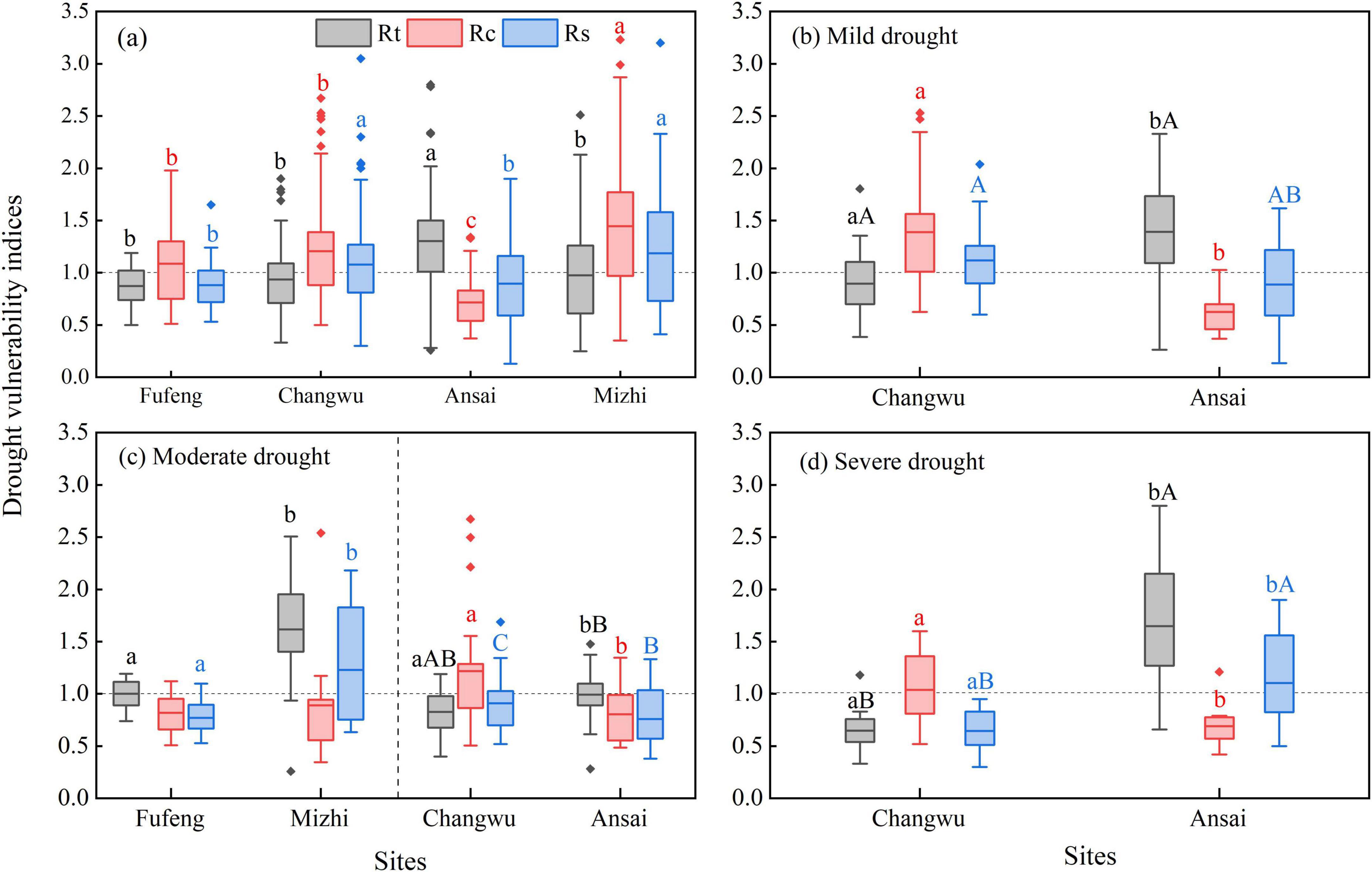
Figure 9. Variations of drought vulnerability indices (Rt: resistance; Rc: recovery; Rs: resilience) of R. pseudoacacia in response to drought events. (a) Differences of drought vulnerability indices of R. pseudoacacia at different study sites; (b) differences of Rt, Rc and Rs between Changwu (2009) and Ansai (2008–2010) sites under mild drought; (c) differences of Rt, Rc and Rs between Fufeng (2008) and Mizhi (2007–2008) sites, Changwu (2016) and Ansai (2015–2016) sites under moderate drought; (d) differences of Rt, Rc and Rs between Changwu (1994–1997) and Ansai (1999–2000) sites under severe drought. Different lowercase letters denote significant differences between sites. In (c,d), different uppercase letters denote significant differences between different drought events within the same sites (one-way ANOVA, P < 0.05).
The recovery of R. pseudoacacia after drought was significantly negatively correlated with resistance (P < 0.05; Figure 10). The deviation of the “line of actual resilience” from the “line of full resilience” decreases with decreasing resistance of R. pseudoacacia. The “line of actual resilience” intersected with the “line of full resilience” within the resistance range of 0.65–1.32, indicating that the R. pseudoacacia plantations with resistance exceeding this range could fully recover after drought. However, the resilience of R. pseudoacacia to drought was positively correlated with both resistance and recovery (P > 0.05). These results illustrated that a significantly trade-off between resistance and recovery, with more resistance to drought for R. pseudoacacia in semi-arid region than in semi-humid region.
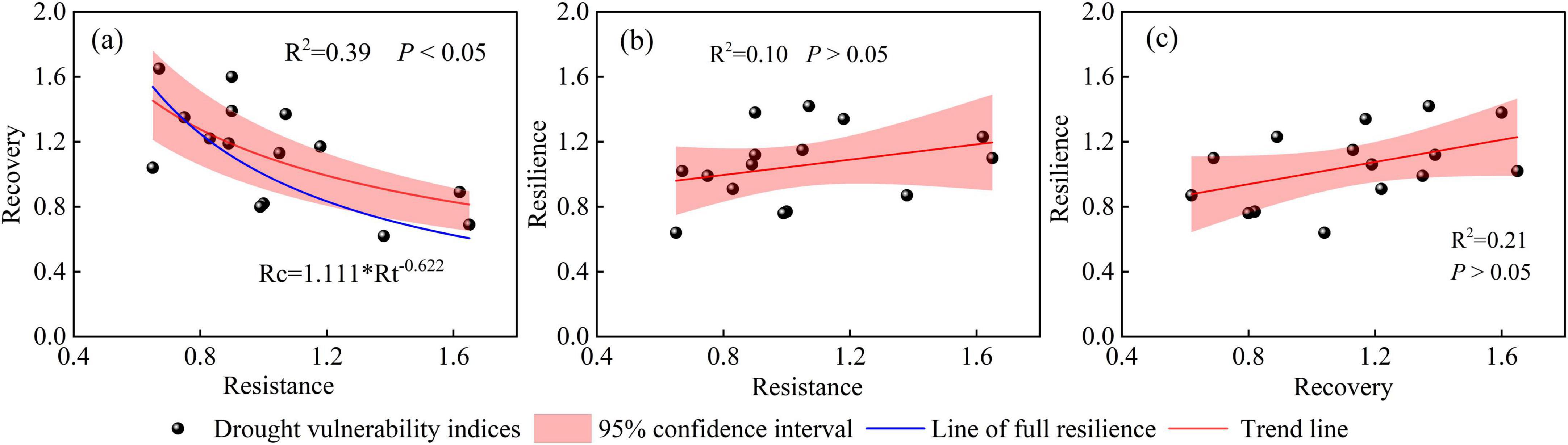
Figure 10. Relationships between resistance and recovery (a); resistance and resilience (b); and recovery and resilience (c).
3.5 Drivers of tree resilience
Climate factors (SPEIpoi, SPEIpre, PGStem, GStem, and GSprep), site geographical conditions (altitude, longitude, and latitude), and tree characteristics (DBH, Pre-RWI and 2Pre-RWI) were significantly related to the resistance and recovery of R. pseudoacacia (P < 0.01), with these factors explaining 73.5 and 84.0% of the total variance in resistance and recovery, respectively (Figure 11). The effect of altitude, longitude, and latitude on resistance and recovery accounted for 70.6 and 18.3% of the variance, respectively (P < 0.05). Tree characteristics accounted for 4.8 and 40.2% relative effects on the resistance and recovery variances of R. pseudoacacia, respectively (P < 0.05). Temperatures during the current and previous growing seasons of a drought event accounted for a combined 22.6 and 19.1% relative effects on the resistance and recovery variances. The average SPEI of 2 years prior to a drought event (SPEIpre) and during a drought event (SPEIpoi) had 14.4 % relative effect on the recovery variance of R. pseudoacacia. Total precipitation in the growing season of a drought event accounted for 1.99 and 8.1% of the variance in resistance and recovery, respectively. In addition, the interaction effects of climate factors and site geographical conditions explained 42.8 and 64.3% on resistance and recovery variances of R. pseudoacacia, respectively. The interaction effects between climatic factors and tree characteristics accounted for 16.3 and 47.2% of the variances in resistance and recovery, respectively. Similarly, the interactions between site geographical conditions and tree characteristics also explained 26.0 and 57.2% of the variances in resistance and recovery of R. pseudoacacia, respectively. However, the interaction effects of all variables weakly impact on resistance and recovery of R. pseudoacacia (Figure 12).
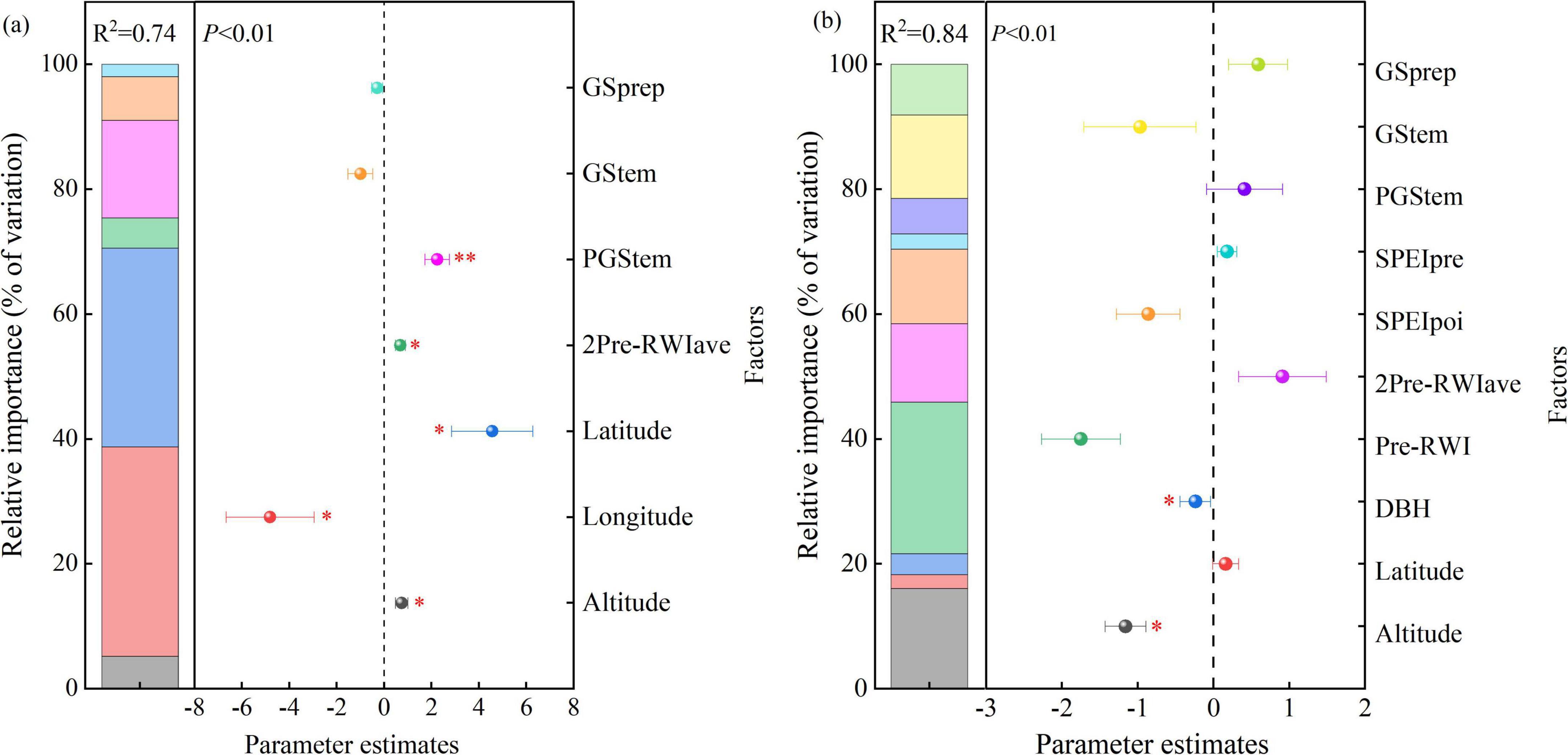
Figure 11. Relative effects of multiple predictors on the resistance (a) and recovery (b) of R. pseudoacacia to drought. Averaged parameter estimates (standardized regression coefficients) of the multiple linear regression model predictors are shown with the relative importance of each predictor. GSprep and GStem: total precipitation and mean temperature during the growing season of a drought event; PGStem: temperature of the previous growing season before a drought event; SPEIpre and SPEIpos: average standardized precipitation evapotranspiration index (SPEI) of 2 years prior to a drought event and during a drought event; Pre-RWI and 2 Pre-RWIave: tree ring width index for 1 and 2 years (average) before a drought event; DBH: diameter at breast height. * represents P < 0.05, ** represents P < 0.01.
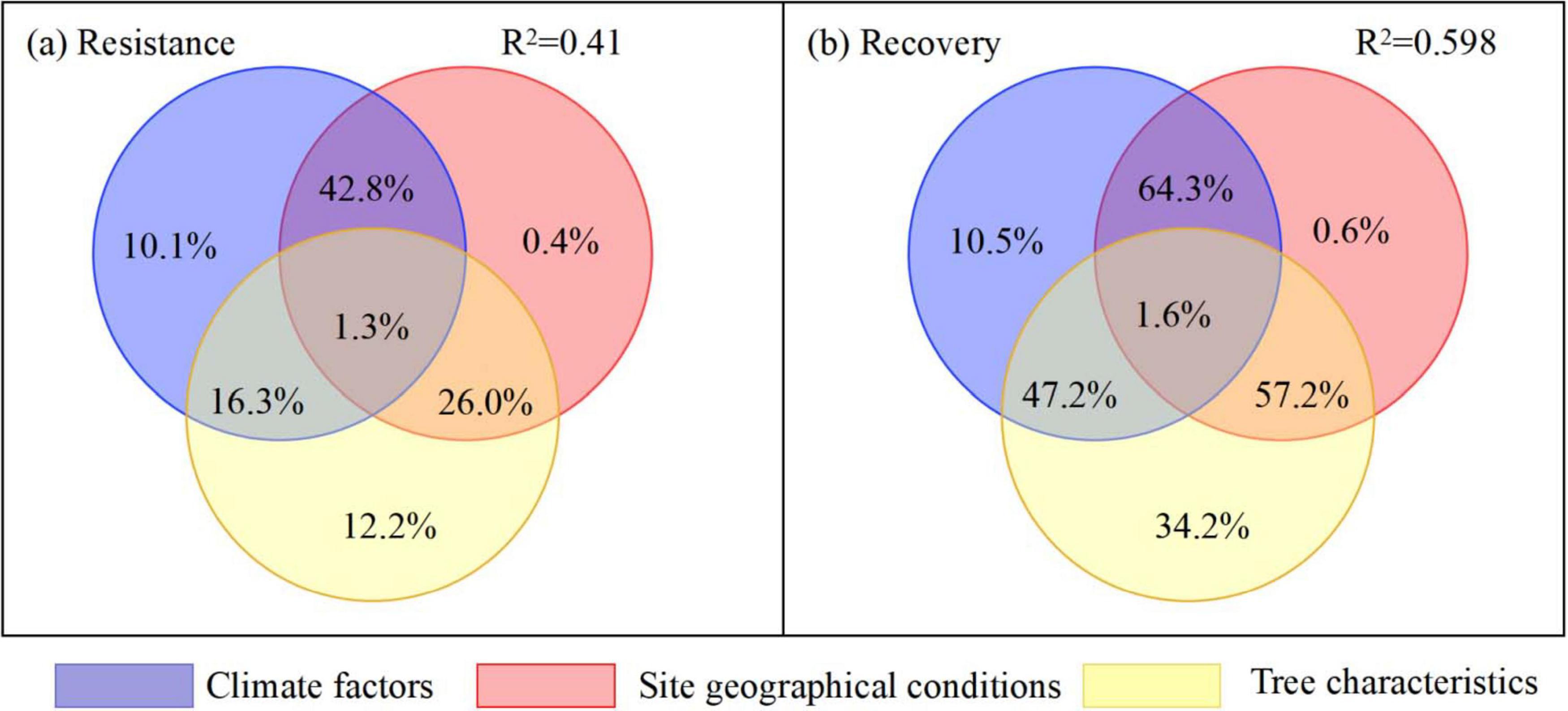
Figure 12. Venn diagram of variation partitioning analysis illustrating the contribution of climate factors (SPEIpoi, SPEIpre, PGStem, GStem, and GSprep), site geographic conditions (altitude, longitude, and latitude), tree characteristics (DBH, Pre-RWI and 2 Pre-RWIave) and their interactions on the variance of tree resistance (a) and recovery (b).
4 Discussion
4.1 Radial growth dynamics of R. pseudoacacia
The tree ring width of R. pseudoacacia showed an overall trend of first increasing and then decreasing over time. After constructing a chronology through detrending processing, we found the variations of RWI of R. pseudoacacia change with tree age differed at our four study sites (Figure 3). This is similar to the results of a previous study of R. pseudoacacia and other common species of trees on the CLP (Keyimu et al., 2021; Che et al., 2023b). Meanwhile, we also noted that the RWI of R. pseudoacacia showed a slight upward trend at the four sites, with the most significant increase observed at the Mizhi site, corresponding to the fact that only this area exhibited a significant upward trend in precipitation (Figure 2). However, previous research by Fritts (1976) indicated that trees growing in drier conditions generally exhibited higher growth variability compared to those in temperature-limited regions. Another study has also concluded that the radial growth of R. pseudoacacia showed no significant regional difference in the early stages, but the tree ring width of high-density R. pseudoacacia significantly decreased compared to low-density stands as the forest age increased (Guan et al., 2023). Therefore, the significantly spatiotemporal differences in tree growth can be attributed to the complex influences of abiotic and biotic factors. On the one hand, the terrain largely contributes to the spatial heterogeneity of regional climate change, thereby influencing the vegetation response to climate change (Zhang et al., 2020). On the other hand, the R. pseudoacacia tree is deep-rooted and requires a large amount of deep soil moisture to sustain its rapid growth during the initial growth stage. Subsequently, soil dry layers gradually develops and results in a slowdown growth rate (Jia et al., 2017). In addition, variations in soil nutrients, microbial communities and structures (Chang et al., 2017; Zhou et al., 2018; Bai et al., 2019), as well as tree anatomical structural characteristics (Levionnois et al., 2022; Song et al., 2022; Liu et al., 2023) all influenced the growth rate of R. pseudoacacia under different spatiotemporal scales.
The SWUE of R. pseudoacacia showed a slight upward trend at two sites (Fufeng and Mizhi) (Figure 5). This may be because the R. pseudoacacia was in a rapid growth stage, during which significant amounts of water and nutrients were used to support growth (Deng and Zhang, 2010; Jia et al., 2017). On a spatial scale, we found the average sensitivity of RWI variations to climate was lower under drought years in regions characterized as semi-humid (Fufeng and Changwu sites) vs. semi-arid (Ansai and Mizhi sites). This may be due to the fact that the R. pseudoacacia growing in arid environments for a long time, is more sensitive to changes in rainfall. Specifically, Wang S. F. et al. (2021) found that the root distribution of R. pseudoacacia in shallow soil layers significantly higher than in middle and deep soil layers in semi-arid region. When precipitation events occur, R. pseudoacacia can more readily shift its primary water source to shallow soil moisture due to the recharge from precipitation infiltration. This indicated that the root systems of R. pseudoacacia in arid environments may be more sensitive to changes in water availability.
4.2 Climate factor effects on radial growth
Tree ring width can provide key information about physiological processes as well as a record of environmental change, and thus reflects how climate factors affect tree growth (Fritts, 1976). The findings of this study related to the climate–growth relationship revealed large discrepancies in the response of R. pseudoacacia to climate change at the different study sites, similar to previous studies (Peng J. et al., 2019; Che et al., 2022; Chen et al., 2023; Han et al., 2023; Zhu et al., 2023). We observed positive correlations between radial growth and both the January through March temperature and the SPEI. This indicated higher temperatures before the growing season not only prompted cambium activity but also meant trees fully compensated for a previous water deficit earlier using melting soil water from the previous winter (Zweifel et al., 2010). The significant relationship between radial growth and both the Tmax in June and precipitation and SPEI in August in the semi-humid region indicated higher temperature and decreased precipitation would both reduce soil moisture and the amount of water available to trees, which would therefore form narrow rings. The results are in agreement with previous works that suggest water availability is the primary factor limiting tree growth (Yang et al., 2014; Ding et al., 2021; Jiao et al., 2021; Pompa-García et al., 2025). Our study also showed significant negative correlations between the radial growth of R. pseudoacacia and the temperature, precipitation, and SPEI in October and December of the previous year. This further illustrated how extreme climate events after the end of the growing season still greatly impact the radial growth of R. pseudoacacia in the subsequent year (i.e., legacy effects) (Wu et al., 2018).
In addition, radial growth being positively correlated with water availability (precipitation and SPEI) in the spring, summer, and growing season indicated seasonal precipitation not only reduces the evaporation rate due to increased cloud cover but also provides available water for growth and thus indirectly impacts carbohydrate storage (Liu et al., 2004; Keyimu et al., 2021; Kazimirović and Stajić, 2025). Drought during the growing season resulted in increased transpiration and decreased photosynthesis and soil moisture, which resulted in limited tree growth, also consistent with previous studies (Yang et al., 2014; Fang et al., 2017; Ding et al., 2021). To summarize the temperature–growth relationship of R. pseudoacacia in both regions, we found the correlation coefficients varied from positive (January, March, and spring) to negative (June) and then to positive (autumn) over time. This indicates that the effect of temperature on radial growth is a dynamic process, with the greatest limitation of maximum temperature on radial growth in June (Keyimu et al., 2021). Due to the uneven distribution of precipitation throughout the year in the Loess Plateau, there is a distinction between the dry season (May-June) and the rainy season (July-September; He et al., 2022). Long-term experimental studies have also demonstrated that higher temperature enhances plant physiological processes and soil evaporation during the early growth stage (May-June) of R. pseudoacacia, exacerbating water deficit and consequently imposing an indirect limitation on tree growth. With the replenishment of precipitation from July to September, the soil moisture content increases, which also promotes the growth of R. pseudoacacia (He et al., 2022; Mei and Ma, 2022; Chen, 2023). Additionally, numerous studies on the relationship between tree radial growth and climate change on the Loess Plateau have consistently demonstrated that the effect of temperature on tree growth exhibits dynamic variation (Keyimu et al., 2021; Che et al., 2022; Wang A. et al., 2022; Wang B. et al., 2022).
4.3 Tree resilience to drought
In this study, we evaluated the resilience to drought years/events by quantifying tree resilience, recovery, and resistance to gain fundamental insights into growth resilience and the interaction of various factors, both internal and external (Fang and Zhang, 2019). The drought vulnerability indices of R. pseudoacacia on the CLP exhibited spatial heterogeneity (Figure 9a), with higher resistance and recovery of R. pseudoacacia to drought at the Ansai and Mizhi sites, respectively, than at other sites. This disparity may be attributed to the Mizhi site with the lowest drought frequency and youngest tree age, while the Ansai site had a higher frequency of severe drought. This is consistent with the findings of Guan et al. (2023), who suggested that the recovery of the R. pseudoacacia at the Jiyuan site was higher than at the Minquan site on the Loess Plateau, as the tree age was younger at the Jiyuan site. Similar results were also observed in Tianshan spruces and Scots pines, where younger trees exhibited higher post-drought recovery, primarily due to their higher water use efficiency (Merlin et al., 2015). In contrast, older trees experienced a decline in water transport capacity and cumulative hydraulic damage due to drought stress, resulting in slower post-drought growth recovery (Tyree et al., 1993; Wang J. et al., 2021). The post-drought recovery of younger canopy-dominant angiosperms was also higher than old trees (Allen et al., 2016). Other studies have also suggested that older trees with narrower rings require a longer recovery time after drought to repair the lost sapwood area (Peltier and Ogle, 2020). In addition, the frequent extreme drought events have led to a sustained increase in the risk of reduced tree growth, while also limiting tree recovery (Allen et al., 2016). Therefore, these mechanisms have the potential to cause changes in the tree resistance and recovery to drought.
Under the same drought event (mild, moderate and severe), the R. pseudoacacia in the semi-arid region exhibited higher resistance (Rt > 1) compared with semi-humid region. However, the recovery of R. pseudoacacia in semi-humid region was similar to or even greater than that in semi-arid region (Figures 9b–d). These phenomena illustrate the adaptive strategies of R. pseudoacacia for local environments during their long-term evolution. Recent studies have agreed that trees acclimated to moisture-replete environments experience significantly reduced drought resistance when subjected to abrupt drought events. Consequently, trees growing under drought conditions exhibit higher drought resistance compared to those in humid environments, which may be associated with the development of stronger tree adaptive capabilities under prolonged drought conditions (McNulty et al., 2014; Gazol et al., 2017; Helman et al., 2017). The higher recovery in semi-humid region indicates that the deep soil moisture is sufficient to support the tree growth during the drought period until the climate returns to normal conditions (Longo et al., 2018). In contrast, the recovery of R. pseudoacacia in semi-arid region is less than 1, indicating that its ability to recover from drought is relatively weaker. The resistance of R. pseudoacacia at the Ansai site has shown a significantly decreasing trend after multi-years of drought over time, with the recovery values all being less than 1 (Figures 9b–d). This indicated that cumulative severe drought stress (1997, 1999–2000 and 2015) might have contributed to the delayed recovery of tree growth (Peng J.F. et al., 2019; Gao et al., 2018). Successive droughts cause prolonged hydraulic deterioration due to drought-induced cavitation (Serra-Maluquer et al., 2018) as well as carbon starvation due to carbohydrate reserve depletion (McDowell et al., 2019; He et al., 2020). However, we also found that the resistance and recovery of R. pseudoacacia at the Changwu site were lowest following severe drought events and highest following mild drought events. This finding was also reported by Lv et al. (2022), indicating that the drought resistance of Q. mongolica was lower at higher drought intensity.
The aforementioned results and relationships between drought vulnerability indices consistently indicate a strong trade-off between drought resistance and recovery (Figures 9, 10). The results align with previous studies in Northern Hemisphere forests (Gazol et al., 2017), P. orientalis pure stand, C. korshinskii pure stand, and mixed stand on the Loess Plateau (Chen et al., 2021; Che et al., 2023b), C. korshinskii in Northwest China (Che et al., 2023a), temperate broad-leaved trees (Zhu et al., 2023), indicating a significant negative correlation between tree resistance and recovery to drought. This may depend on the carbohydrate reserves of trees. Galiano et al. (2011) demonstrate that when drought resistance and recovery partially rely on carbohydrate reserves, a trade-off occurs between the two indices; however, if they are not fully reserve-dependent, trees can achieve rapid photosynthetic recovery despite resistance decline caused by reserve depletion during drought. In this study, a comparison of the deviation and intersection between the “Line of full resilience” and “Line of actual resilience” showed that R. pseudoacacia could recover fully from drought when resistance exceeded the range of 0.65–1.32. Additionally, some studies also suggested that the opposing patterns of resistance and recovery indices may be due to their mathematical relation (Gazol et al., 2017; Fang and Zhang, 2019). Assuming PreDr and PostDr are at similar levels, a significant reduction of tree growth predictably results in low resistance and high recovery. While we cannot quantify the extent to which the patterns of resistance and recovery arise from the interdependence of the two formulas, the opposing trends can be partially explained by the ecological and physiological characteristics of the trees (Zhu et al., 2023). In summary, R. pseudoacacia on the Loess Plateau still exhibits higher recovery potential following droughts, even though drought reduces the tree resistance.
Our results showed climate factors (SPEIpoi, SPEIpre, PGStem, GStem, and GSprep), site geographical conditions (altitude, longitude, and latitude), and tree characteristics (DBH, Pre-RWI and 2Pre-RWIave) had significant impacts on the response of R. pseudoacacia to drought (Figure 11). Similar to a previous study (Fang and Zhang, 2019; Zhu et al. 2023), we found that site geographical conditions and climate factors were the most important factors affecting the drought resistance (70.6%) and recovery (41.6%). Among them, resistance and recovery of trees significantly decreased or increased with increasing altitude, longitude or latitude. The R. pseudoacacia growing in higher altitude site was more resistant to drought than those growing at lower altitude, similar to the situation observed in the forests of Northeast China and southern Iberian Range (Marqu’es et al., 2016; Zhu et al., 2023). We also found an increase in average growth in the 2 years prior to a drought year enhances resistance and recovery. This suggested the effect of drought events on the resilience of R. pseudoacacia is not independent of growth in the drought year, but highly dependent on the growth in the pre-drought period (Bose et al., 2020). The resistance and recovery of trees to drought was positively affected by temperature during the growing season of the previous year; this aligns with the notion that tree resilience is influenced by “legacy” or carryover effects (Anderegg et al., 2015). The findings also showed higher temperatures during the growing season of drought years were not conducive to tree recovery after drought events. This may be because high temperatures during the main growth period can exacerbate drought in the current year, further resulting in a delayed recovery process (Li et al., 2017). In general, climate factors, site geographical conditions, and tree characteristics can influence the resilience of R. pseudoacacia. The majority of these factors typically varied across space and time, resulting in spatial and temporal variations of tree resilience. Therefore, it is noteworthy that these factors have interactive effects on resistance and recovery (Figure 12), such as the stronger interactive effects of climate factors and site geographical conditions on the tree resilience. This finding was also confirmed by Zhu et al. (2023) about the resilience of trees in four forest stands in Northeast China. However, the climate factors, site geographical conditions, and tree characteristics may strongly differ within species, and their specific mechanisms require further investigation. Additional studies also corroborate how disturbances are also related to differences in tree resilience, including considerations such as insect outbreaks, plant diseases, provenance (Seidel et al., 2016), and forest management practices (Manrique-Alba et al., 2022).
In this study, although we focused on the dynamic impacts of climatic factors, site geographical conditions, and tree characteristics on tree resilience, other biotic and abiotic factors may also have impacts on trees resistance to drought. For instance, arbuscular mycorrhizal fungi (AMF) cannot only promote the absorption of water by plant roots, but their extraradical hyphae are also capable of redistributing soil water. This process helps maintain moisture levels in the surface soil during periods of drought stress, thereby enhancing water and nutrient availability and improving plant survival (Smith et al., 2010). AMF can indirectly enhance the drought resistance of trees by promoting water and nutrient absorption and increasing root hydraulic conductivity (Shi et al., 2015). Our future studies will consider the mechanisms of the interaction between these factors, and consider stable isotopes along with quantitative aspects of wood anatomy to improve current understanding of CLP R. pseudoacacia plantation vulnerability to drought.
5 Conclusion
We used dendroecological methods to analyze climate–growth responses and quantify the effect of drought on CLP R. pseudoacacia plantation resilience. The results demonstrated the average sensitivity of RWI variations to climate for R. pseudoacacia was lower in the semi-humid region than in the semi-arid region in drought years. The climate–growth correlation analyses found water availability (precipitation and SPEI) of current year was the main climate factor limiting radial growth of R. pseudoacacia on the CLP. The influence of temperature on R. pseudoacacia radial growth transitioned from promotion (January, March and spring) to inhibition (June) and then to promotion (autumn). Significant trade-offs were evident between the recovery and resistance of R. pseudoacacia to drought. Climate factors, site geographical conditions, and tree characteristics played important roles in regulating tree growth resilience. The site geographical and climatic conditions were the notable factors affecting the drought resistance of R. pseudoacacia. The results of this study are helpful for understanding the effects of major driving forces underlying the radial growth and response of R. pseudoacacia to drought during stand development, which is important for predicting the growth trend of plantations on the CLP under the context of future climate change.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XY: Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. ZZ: Supervision, Writing – review & editing. XW: Investigation, Methodology, Writing – review & editing. MH: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Strategic Priority Research Program of Chinese Academy of Sciences and the National Natural Science Foundation of China (Nos. XDB40020202 and 42107335).
Acknowledgments
We thank the editors and reviewers for the insightful contributions by way of constructive comments and suggestions on the work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Allen, C. D., Macalady, A. K., Chenchouni, H., Bachelet, D., McDowell, N., Vennetier, M., et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage 259, 660–684. doi: 10.1016/j.foreco.2009.09.001
Allen, C. R., Angeler, D. G., Cumming, G. S., Folke, C., Twidwell, D. L., Uden, D. R., et al. (2016). Quantifying spatial resilience. J. Appl. Ecol. 53, 625–635. doi: 10.1111/1365-2664.12649
Anderegg, W. R. L., Schwalm, C., Biondi, F., Camarero, J. J., Koch, G., Litvak, M., et al. (2015). Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 349, 528–532. doi: 10.1126/science.aab1833
Bai, X., Wang, B., An, S., Zeng, Q., and Zhang, H. (2019). Response of forest species to C: N: P in the plant-litter-soil system and stoichiometric homeostasis of plant tissues during afforestation on the Loess Plateau. China. CATENA 183:104186. doi: 10.1016/j.catena.2019.104186
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. doi: 10.18637/jss.v067.i01
Beguería, S., Vicente-Serrano, S. M., Reig, F., and Latorre, B. (2014). Standardized Precipitation Evapotranspiration Index (SPEI) revisited: Parameter fitting, evapotranspiration models, tools, datasets and drought monitoring. Int. J. Climatol. 34, 3001–3023. doi: 10.1002/joc.3887
Bohner, T., and Diez, J. (2021). Tree resistance and recovery from drought mediated by multiple abiotic and biotic processes across a large geographic gradient. Sci. Total Environ. 789:147744. doi: 10.1016/j.scitotenv.2021.147744
Bose, A. K., Gessler, A., Bolte, A., Bottero, A., Buras, A., Cailleret, M., et al. (2020). Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Glob. Change Biol. 26, 4521–4537. doi: 10.1111/gcb.15153
Briffa, K. R., and Jones, P. D. (1990). “Basic chronology statistics and assessment,” in Methods of dendrochronology: Applications in the environmental sciences, eds E. R. Cook and L. A. Kairiukstis (Dordrecht: Kluwer Academic Publishers), 137–152.
Cai, W., He, N., Li, M., Xu, L., Wang, L., Zhu, J., et al. (2022). Carbon sequestration of Chinese forests from 2010 to 2060: Spatiotemporal dynamics and its regulatory strategies. Sci. Bull. 67, 836–843. doi: 10.1016/j.scib.2021.12.012
Camarero, J. J., Gazol, A., Sangüesa-Barreda, G., Oliva, J., and Vicente-Serrano, S. M. (2015). To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 103, 44–57. doi: 10.1111/1365-2745.12295
Cao, J., Liu, H., Zhao, B., Li, Z., Drew, D. M., and Zhao, X. (2019). Species-specific and elevation-differentiated responses of tree growth to rapid warming in a mixed forest lead to a continuous growth enhancement in semi-humid Northeast Asia. For. Ecol. Manage 448, 76–84. doi: 10.1016/j.foreco.2019.05.065
Cao, Y., and Chen, Y. (2017). Coupling of plant and soil C: N: P stoichiometry in black locust (Robinia pseudoacacia) plantations on the Loess Plateau. China. Trees 31, 1559–1570. doi: 10.1007/s00468-017-1569-8
Chang, E., Tian, G., and Chiu, C. (2017). Soil microbial communities in natural and managed cloud montane forests. Forests 8:33. doi: 10.3390/f8010033
Che, C., Xiao, S., Ding, A., Peng, X., and Su, J. (2022). Growth response of plantations Hippophae rhamnoides Linn. on different slope aspects and natural Caragana opulens Kom. to climate and implications for plantations management. Ecol. Ind. 138:108833. doi: 10.1016/j.ecolind.2022.108833
Che, C., Xiao, S., Peng, X., Ding, A., and Su, J. (2023a). Radial growth of Korshinsk peashrub and its response to drought in different sub-arid climate regions of northwest China. J. Environ. Manage 326:116708. doi: 10.1016/j.jenvman.2022.116708
Che, C., Xiao, S., Peng, X., Su, J., and Ding, A. (2023b). Mixed of arbor-shrub significantly enhanced the drought stress adaptive capacity of plantation forests—An interpretation based on dendroecology. J. Hydrol. 623:129785. doi: 10.1016/j.jhydrol.2023.129785
Chen, H., and Sun, J. (2015). Changes in drought characteristics over China using the standardized precipitation evapotranspiration index. J. Clim. 28, 5430–5447. doi: 10.1175/JCLI-D-14-00707.1
Chen, M., Zhang, X., Li, M., and Cao, Y. (2023). Species mixing enhances the resistance of Robinia pseudoacacia L. to drought events in semi-arid regions: Evidence from China’s Loess Plateau. Sci. Total Environ. 869:161796. doi: 10.1016/j.scitotenv.2023.161796
Chen, M., Zhang, X., Li, M., Zhang, J., and Cao, Y. (2021). Climate-growth pattern of Pinus tabulaeformis plantations and their resilience to drought events in the Loess Plateau. For. Ecol. Manage 499:119642. doi: 10.1016/j.foreco.2021.119642
Chen, Q. W. (2023). Spatial and temporal variability of soil water content and the influencing factors in two typical forest communities in Loess Hilly Region. China: Northwest A&F university.
Collins, W. J., Fry, M. M., Yu, H., Fuglestvedt, J. S., Shindell, D. T., and West, J. J. (2013). Global and regional temperature-change potentials for near-term climate forcers. Atmos. Chem. Phys. 13, 2471–2485. doi: 10.5194/acp-13-2471-2013
Cook, E. R. (1985). A time series analysis approach to tree ring standardization. Dissertation, University of Arizona: Tucson, AZ
Cook, E. R., and Kairiukstis, L. A. (1990). Methods of dendrochronology. Dordrecht: Springer Netherlands.
Dai, A., Trenberth, K. E., and Qian, T. (2004). A global dataset of Palmer drought severity index for 1870–2002: Relationship with soil moisture and effects of surface warming. J. Hydrometeor. 5, 1117–1130. doi: 10.1175/JHM-386.1
Deng, L., and Zhang, W. (2010). Natural development pattern of Robinia pseudoacacia plantations in loess hilly region. Sci. Silvae Sin. 46, 15–22.
Ding, A., Xiao, S., Peng, X., Tian, Q., and Han, C. (2021). Shrub-rings used to reconstruct drought history of the central Alxa desert, northwest China. Int. J. Climatol. 41, 4957–4965. doi: 10.1002/joc.7108
Dixon, P. (2003). VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Fang, K., Guo, Z., Chen, D., Linderholm, H. W., Li, J., Zhou, F., et al. (2017). Drought variation of western Chinese Loess Plateau since 1568 and its linkages with droughts in western North America. Clim. Dyn. 49, 3839–3850. doi: 10.1007/s00382-017-3545-9
Fang, O., and Zhang, Q. (2019). Tree resilience to drought increases in the Tibetan Plateau. Global Change Biol. 25, 245–253. doi: 10.1111/gcb.14470
Feng, X., Fu, B., Piao, S., Wang, S., Ciais, P., Zeng, Z., et al. (2016). Revegetation in China’s Loess Plateau is approaching sustainable water resource limits. Nat. Clim. Change 6, 1019–1022. doi: 10.1038/nclimate3092
Feng, X. M., Sun, G., Fu, B. J., Su, C. H., Liu, Y., and Lamparski, H. (2012). Regional effects of vegetation restoration on water yield across the Loess Plateau, China. Hydrol. Earth Syst. Sci. 16, 2617–2628. doi: 10.5194/hess-16-2617-2012
Feng, Y., Schmid, B., Loreau, M., Forrester, D. I., Fei, S., Zhu, J. X., et al. (2022). Multispecies forest plantations outyield monocultures across a broad range of conditions. Science 376, 865–868. doi: 10.1126/science.abm6363
Galiano, L., Martínez-Vilalta, J., and Lloret, F. (2011). Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol. 190, 750–759. doi: 10.1111/j.1469-8137.2010.03628.x
Gao, S., Liu, R., Zhou, T., Fang, W., Yi, C., Lu, R., et al. (2018). Dynamic responses of tree-ring growth to multiple dimensions of drought. Glob. Change Biol. 24, 5380–5390. doi: 10.1111/gcb.14367
Garcia, R. A., Cabeza, M., Rahbek, C., and Araújo, M. B. (2014). Multiple dimensions of climate change and their implications for biodiversity. Science 344:1247579. doi: 10.1126/science.1247579
Gazol, A., Camarero, J. J., Anderegg, W. R. L., and Vicente-Serrano, S. M. (2017). Impacts of droughts on the growth resilience of Northern Hemisphere forests. Global Ecol. Biogeogr. 26, 166–176. doi: 10.1111/geb.12526
Guan, C. F., Zheng, J. S., Li, Y. J., Zhang, L. X., Hu, M. J., Zhang, J. S., et al. (2023). Effects of climate and density on the radial growth and drought vulnerability of Robinia pseudoacacia. Acta Ecol. Sin. 43, 3261–3272. doi: 10.5846/stxb202112133525
Han, L., Camarero, J. J., Jia, G. D., Zhang, Z. Q., and Chen, L. X. (2025). Drought resilience and legacy effects in two forest tree species on Loess Plateau of China: Growth and water-use efficiency under different drought conditions. For. Ecosyst. 13:100297. doi: 10.1016/j.fecs.2025.100297
Han, R. Y., Gong, X. W., Li, M. Y., Leng, Q. N., Zhou, Y. J., Ning, Q. R., et al. (2023). Combined tree-ring width and wood anatomy chronologies provide insights into the radial growth and hydraulic strategies in response to an extreme drought in plantation-grown Mongolian pine trees. Environ. Exp. Bot. 208:105259. doi: 10.1016/j.envexpbot.2023.105259
He, M., Wang, Y., Wang, L., Jia, X., Zhao, C., and Zhang, P. (2022). Spatial–temporal dynamics and recovery mechanisms of dried soil layers under Robinia pseudoacacia forest based on in-situ field data from 2017 to 2020. Land Degrad. Dev. 33, 2500–2511. doi: 10.1002/ldr.4327
He, W., Liu, H., Qi, Y., Liu, F., and Zhu, X. (2020). Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Change Biol. 26, 3627–3638. doi: 10.1111/gcb.15078
Helman, D., Lensky, I. M., Yakir, D., and Osem, Y. (2017). Forests growing under dry conditions have higher hydrological resilience to drought than do more humid forests. Global Change Biol. 23, 2801–2817. doi: 10.1111/gcb.13551
Holmes, R. L. (1983). Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 43, 69–95. doi: 10.1006/biol.1999.0214
IPCC (2021). “Summary for policymakers,” in Climate change 2021: The physical science basis. contribution of working group i to the sixth assessment report of the intergovernmental panel on climate change, eds V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, et al. (Cambridge: Cambridge University Press).
Jia, X., Shao, M., Zhu, Y., and Luo, Y. (2017). Soil moisture decline due to afforestation across the Loess Plateau. China. J. Hydrol. 546, 113–122. doi: 10.1016/j.jhydrol.2017.01.011
Jia, Y. H., Li, T. C., Shao, M. A., Hao, J. H., Wang, Y. Q., Jia, X. X., et al. (2019). Disentangling the formation and evolvement mechanism of plants-induced dried soil layers on China’s Loess Plateau. Agr. Forest Meteorol. 269, 57–70. doi: 10.1016/j.agrformet.2019.02.011
Jiao, W., Wang, L., Smith, W. K., Chang, Q., Wang, H., and D’Odorico, P. (2021). Observed increasing water constraint on vegetation growth over the last three decades. Nat Commun. 12:3777. doi: 10.1038/s41467-021-24016-9
Kazimirović, M., and Stajić, B. (2025). Potential effects of climate change on growth and the implications for conservation of the endangered Serbian spruce (Picea omorika (Pančić) Purk.). For. Ecosyst. 12:100287. doi: 10.1016/j.fecs.2024.100287
Keyimu, M., Li, Z., Fu, B., Chen, W., Wei, J., Jiao, L., et al. (2021). Spatial differences in the radial growth responses of black locust (Robinia pseudoacacia Linn.) to climate on the Loess Plateau, China. Dendrochronologia 67:125832. doi: 10.1016/j.dendro.2021.125832
Le Bagousse-Pinguet, Y., Soliveres, S., Gross, N., Torices, R., Berdugo, M., and Maestre, F. T. (2019). Phylogenetic, functional, and taxonomic richness have both positive and negative effects on ecosystem multifunctionality. Proc. Natl. Acad. Sci. U. S. A. 116, 8419–8424. doi: 10.1073/pnas.1815727116
Levionnois, S., Kaack, L., Heuret, P., Abel, N., Ziegler, C., Coste, S., et al. (2022). Pit characters determine drought-induced embolism resistance of leaf xylem across 18 Neotropical tree species. Plant Physiol. 190, 371–386. doi: 10.1093/plphys/kiac223
Li, C., Fu, B., Wang, S., Stringer, L. C., Wang, Y., Li, Z., et al. (2021). Drivers and impacts of changes in China’s drylands. Nat. Rev. Earth Environ. 2, 858–873. doi: 10.1038/s43017-021-00226-z
Li, G., Zhang, X., Huang, J., Wen, Z., and Du, S. (2018). Afforestation and climatic niche dynamics of black locust (Robinia pseudoacacia). For. Ecol. Manage 407, 184–190. doi: 10.1016/j.foreco.2017.10.019
Li, J., Erickson, J. E., Peresta, G., and Drake, B. G. (2010). Evapotranspiration and water use efficiency in a Chesapeake Bay wetland under carbon dioxide enrichment. Glob. Change Biol. 16, 234–245. doi: 10.1111/j.1365-2486.2009.01941.x
Li, J., Gou, X., Cook, E. R., and Chen, F. (2006). Tree-ring based drought reconstruction for the central Tien Shan area in northwest China. Geophys. Res. Lett. 33:2006GL025803. doi: 10.1029/2006GL025803
Li, J., Shi, J., Zhang, D. D., Yang, B., Fang, K., and Yue, P. H. (2017). Moisture increase in response to high-altitude warming evidenced by tree-rings on the southeastern Tibetan Plateau. Clim. Dyn. 48, 649–660. doi: 10.1007/s00382-016-3101-z
Liang, E., Leuschner, C., Dulamsuren, C., Wagner, B., and Hauck, M. (2016). Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Clim. Change 134, 163–176. doi: 10.1007/s10584-015-1531-y
Liu, H., Park Williams, A., Allen, C. D., Guo, D., Wu, X., Anenkhonov, O. A., et al. (2013). Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Change Biol. 19, 2500–2510. doi: 10.1111/gcb.12217
Liu, Q., Wu, C., Gao, L., and Ying, L. (2023). Coordination between non-structure carbohydrates and hydraulic function under different drought duration, intensity, and post-drought recover. Environ. Exp. Bot. 215:105485. doi: 10.1016/j.envexpbot.2023.105485
Liu, Y., Shi, J., Shishov, V., Vaganov, E., Yang, Y., Cai, Q., et al. (2004). Reconstruction of May–July precipitation in the north Helan Mountain, Inner Mongolia since A.D. 1726 from tree-ring late-wood widths. Chin. Sci. Bull. 49, 405–409. doi: 10.1007/BF02900325
Lloret, F., Keeling, E. G., and Sala, A. (2011). Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120, 1909–1920. doi: 10.1111/j.1600-0706.2011.19372.x
Longo, M., Knox, R. G., Levine, N. M., Alves, L. F., Bonal, D., Camargo, P. B., et al. (2018). Ecosystem heterogeneity and diversity mitigate Amazon forest resilience to frequent extreme droughts. New Phytol. 219, 914–931. doi: 10.1111/nph.15185
Lv, P., Rademacher, T., Huang, X., Zhang, B., and Zhang, X. (2022). Prolonged drought duration, not intensity, reduces growth recovery and prevents compensatory growth of oak trees. Agr. For. Meteorol. 326:109183. doi: 10.1016/j.agrformet.2022.109183
Manrique-Alba, À, Beguería, S., and Camarero, J. J. (2022). Long-term effects of forest management on post-drought growth resilience: An analytical framework. Sci. Total Environ. 810:152374. doi: 10.1016/j.scitotenv.2021.152374
Marqu’es, L., Camarero, J. J., Gazol, A., and Zavala, M. A. (2016). Drought impacts on tree growth of two pine species along an altitudinal gradient and their use as earlywarning signals of potential shifts in tree species distributions. Forest Ecol. Manag. 381, 157–167. doi: 10.1016/j.foreco.2016.09.021
McDowell, N. G., Grossiord, C., Adams, H. D., Pinzón-Navarro, S., Mackay, D. S., Breshears, D. D., et al. (2019). Mechanisms of a coniferous woodland persistence under drought and heat. Environ. Res. Lett. 14:045014. doi: 10.1088/1748-9326/ab0921
McNulty, S. G., Boggs, J. L., and Sun, G. (2014). The rise of the mediocre forest: Why chronically stressed trees may better survive extreme episodic climate variability. New For. 45, 403–415. doi: 10.1007/s11056-014-9410-3
Mei, X. M., and Ma, L. (2022). Effect of afforestation on soil water dynamics and water uptake under different rainfall types on the Loess hillslope. CATENA 213:106216. doi: 10.1016/j.catena.2022.106216
Merlin, M., Perot, T., Perret, S., Korboulewsky, N., and Vallet, P. (2015). Effects of stand composition and tree size on resistance and resilience to drought in sessile oak and Scots pine. For. Ecol. Manag. 339, 22–33. doi: 10.1016/j.foreco.2014.11.032
Metslaid, S., Hordo, M., Korjus, H., Kiviste, A., and Kangur, A. (2018). Spatio-temporal variability in Scots pine radial growth responses to annual climate fluctuations in hemiboreal forests of Estonia. Agr. Forest Meteorol. 252, 283–295. doi: 10.1016/j.agrformet.2018.01.018
Ni, Y., Xiao, W., Liu, J., Jian, Z., Li, M., Xu, J., et al. (2023). Radial growth-climate correlations of Pinus massoniana in natural and planted forest stands along a latitudinal gradient in subtropical central China. Agr. Forest Meteorol. 334:109422. doi: 10.1016/j.agrformet.2023.109422
Ning, Q. R., Gong, X. W., Li, M. Y., and Hao, G. Y. (2022). Differences in growth pattern and response to climate warming between Larix olgensis and Pinus koraiensis in Northeast China are related to their distinctions in xylem hydraulics. Agr. Forest Meteorol. 312:108724. doi: 10.1016/j.agrformet.2021.108724
Peltier, D. M. P., and Ogle, K. (2020). Tree growth sensitivity to climate is temporally variable. Ecol. Lett. 23, 1561–1572. doi: 10.1111/ele.13575
Peng, J., Wu, C., Zhang, X., Wang, X., and Gonsamo, A. (2019). Satellite detection of cumulative and lagged effects of drought on autumn leaf senescence over the Northern Hemisphere. Glob. Change Biol. 25, 2174–2188. doi: 10.1111/gcb.14627
Peng, J. F., Li, J., Wang, T., Huo, J., and Yang, L. (2019). Effect of altitude on climate–growth relationships of Chinese white pine (Pinus armandii) in the northern Funiu Mountain, central China. Clim. Change 154, 273–288. doi: 10.1007/s10584-019-02416-7
Pompa-García, M., Camarero, J. J., Valeriano, C., and Vivar-Vivar, E. D. (2025). Variable growth responses of four tree species to climate and drought in a Madrean pine-oak forest. For. Ecosyst. 12:100292. doi: 10.1016/j.fecs.2025.100292
Pompa-García, M., Camarero, J. J., and Vivar-Vivar, E. D. (2023). Contrasting climate drivers of seasonal growth in western vs. eastern Mexican mountain conifer forests. For. Ecosyst. 10:100091. doi: 10.1016/j.fecs.2023.100091
Qiu, L., Wu, Y., Yu, M., Shi, Z., Yin, X., Song, Y., et al. (2021). Contributions of vegetation restoration and climate change to spatiotemporal variation in the energy budget in the loess plateau of China. Ecol. Indic. 127:107780. doi: 10.1016/j.ecolind.2021.107780
Rais, A., Van De Kuilen, J.-W. G., and Pretzsch, H. (2014). Growth reaction patterns of tree height, diameter, and volume of Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) under acute drought stress in Southern Germany. Eur. J. Forest Res. 133, 1043–1056. doi: 10.1007/s10342-014-0821-7
Salzer, M. W., Hughes, M. K., Bunn, A. G., and Kipfmueller, K. F. (2009). Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. U. S. A. 106, 20348–20353. doi: 10.1073/pnas.0903029106
Schwarz, J., Skiadaresis, G., Kohler, M., Kunz, J., Schnabel, F., and Vitali, V. (2020). Quantifying growth responses of trees to drought—a critique of commonly used resilience indices and recommendations for future studies. Curr. For. Rep. 6, 185–200. doi: 10.1007/s40725-020-00119-2
Seidel, H., Schunk, C., Matiu, M., and Menzel, A. (2016). Diverging drought resistance of Scots pine provenances revealed by infrared thermography. Front. Plant Sci. 7:1247. doi: 10.3389/fpls.2016.01247
Serra-Maluquer, X., Mencuccini, M., and Martínez-Vilalta, J. (2018). Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia 187, 343–354. doi: 10.1007/s00442-018-4118-2
Shi, Z. Y., Mickan, B., Feng, G., and Chen, Y. L. (2015). Arbuscular mycorrhizal fungi improved plant growth and nutrient acquisition of desert ephemeral Plantago minuta under variable soil water conditions. J. Arid Land. 7, 414–420. doi: 10.1007/s40333-014-0046-0
Shuman, J. K., Shugart, H. H., and O’Halloran, T. L. (2011). Sensitivity of Siberian larch forests to climate change. Glob. Change Biol. 17, 2370–2384. doi: 10.1111/j.1365-2486.2011.02417.x
Smith, S. E., Facelli, E., Pope, S., and Smith, F. A. (2010). Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326, 3–20. doi: 10.1007/s42832-021-0107-1
Solomon, S., Qin, D., Manning, M., Marquis, M., Averyt, K., Tignor, M. M. B., et al. (2007). Climate change 2007: The physical science basis. Cambridge: Cambridge University Press.
Song, Y., Poorter, L., Horstin, A., Delzon, S., and Sterck, F. (2022). Pit and tracheid anatomy explain hydraulic safety but not hydraulic efficiency of 28 conifer species. J. Exp. Bot. 73, 1033–1048. doi: 10.1093/jxb/erab449
Stokes, M. A., and Smiley, T. L. (1996). An introduction to tree-ring dating. Tucson, AZ: University of Arizona Press.
Tyree, M. T., Cochard, H., Cruiziat, P., Sinclair, B., and Ameglio, T. (1993). Drought-induced leaf shedding in walnut: Evidence for vulnerability segmentation. Plant Cell Environ. 16, 879–882. doi: 10.1111/j.1365-3040.1993.tb00511.x
van der Maaten-Theunissen, M., van der Maaten, E., and Bouriaud, O. (2015). pointRes: An R package to analyze pointer years and components of resilience. Dendrochronologia 35, 34–38. doi: 10.1016/j.dendro.2015.05.006
Vicente-Serrano, S. M., Beguería, S., and López-Moreno, J. I. (2010). A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 23, 1696–1718. doi: 10.1175/2009JCLI2909.1
Wang, A., Gao, X., Zhou, Z., Yang, H., Zhao, X. H., Wang, Y., et al. (2022). Dynamic responses of tree-ring growth to drought over Loess Plateau in the past three decades. Ecol. Indic. 143:109423. doi: 10.1016/j.ecolind.2022.109423
Wang, B., Chen, T., Li, C., Xu, G., Wu, G., and Liu, G. (2022). Discrepancy in growth resilience to drought among different stand-aged forests declines going from a semi-humid region to an arid region. For. Ecol. Manage 511:120135. doi: 10.1016/j.foreco.2022.120135
Wang, J., Fu, B., Jiao, L., Lu, N., Li, J., Chen, W., et al. (2021). Age-related water use characteristics of Robinia pseudoacacia on the Loess Plateau. Agr. Forest Meteorol. 301–302, 108344. doi: 10.1016/j.agrformet.2021.108344
Wang, L., Yen, H., Xinhui, E., Chen, L., and Wang, Y. (2019). Dissolved organic carbon driven by rainfall events from a semi-arid catchment during concentrated rainfall season in the Loess Plateau, China. Hydrol. Earth Syst. Sci. 23, 3141–3153. doi: 10.5194/hess-23-3141-2019
Wang, S. F. (2023). Study on the relationship between root water uptake and soil carbon sequestration in deep soils of typical plantations on the loess plateau. China: Northwest A&F university.
Wang, S. F., Yang, M., Gao, X. D., Zhang, Z. B., Wang, X. Z., Zhao, X. N., et al. (2021). Comparison of the root-soil water relationship of two typical revegetation species along a precipitation gradient on the Loess Plateau. Environ. Res. Lett. 16:064054. doi: 10.1088/1748-9326/ac00e4
Wang, Y., Shao, M., Zhu, Y., and Liu, Z. (2011). Impacts of land use and plant characteristics on dried soil layers in different climatic regions on the Loess Plateau of China. Agric. For. Meteorol. 151, 437–448. doi: 10.1016/j.agrformet.2010.11.016
Wei, J. S., Li, Z. S., Feng, X. Y., Zhang, Y., Chen, W. L., Wu, X., et al. (2018). Ecological and physiological mechanisms of growth decline of Robinia pseudoacacia plantations in the Loess Plateau of China. Chinese J. Appl. Ecol. 29, 2433–2444. doi: 10.13287/j.1001-9332.201807.037
Wells, N., Goddard, S., and Hayes, M. J. (2004). A self-calibrating palmer drought severity index. J. Clim. 17, 2335–2351. doi: 10.1175/1520-0442(2004)017<2335:ASPDSI>2.0.CO;2
Wigley, T. M. L., Briffa, K. R., and Jones, P. D. (1984). On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Clim. 23, 201–213. doi: 10.1175/1520-04501984023<0201:OTAVOC>2.0.CO;2
Willis, K. J., Jeffers, E. S., and Tovar, C. (2018). What makes a terrestrial ecosystem resilient? Science 359, 988–989. doi: 10.1126/science.aar5439
Wu, X., Liu, H., Li, X., Ciais, P., Babst, F., and Guo, W. (2018). Differentiating drought legacy effects on vegetation growth over the temperate Northern Hemisphere. Glob. Change Biol. 24, 504–516. doi: 10.1111/gcb.13920
Xue, R. H., Jiao, L., Zhang, P., Wang, X. G., Li, Q., Yuan, X., et al. (2025). Climatic habitat regulates the radial growth sensitivity of two conifers in response to climate change. For. Ecosyst. 12:100282. doi: 10.1016/j.fecs.2024.100282
Yan, X., Zhang, Z., Zhao, X., Huang, M., Wu, X., and Guo, T. (2024). Differentiated responses of plant water use regulation to drought in Robinia pseudoacacia plantations on the Chinese Loess Plateau. Agric. Water Manag. 291:108659. doi: 10.1016/j.agwat.2023.108659
Yang, B., He, M., Shishov, V., Tychkov, I., Vaganov, E., Rossi, S., et al. (2017). New perspective on spring vegetation phenology and global climate change based on Tibetan Plateau tree-ring data. Proc. Natl. Acad. Sci. U. S. A. 114, 6966–6971. doi: 10.1073/pnas.1616608114
Yang, B., Qin, C., Wang, J., He, M., Melvin, T. M., Osborn, T. J., et al. (2014). A 3,500-year tree-ring record of annual precipitation on the northeastern Tibetan Plateau. Proc. Natl. Acad. Sci. U. S. A. 111, 2903–2908. doi: 10.1073/pnas.1319238111
Yang, C., Fu, M., Feng, D., Sun, Y., and Zhai, G. (2021). Spatiotemporal changes in vegetation cover and its influencing factors in the Loess Plateau of China based on the geographically weighted regression model. Forests 12:673. doi: 10.3390/f12060673
Yang, Y., Wang, B., Wang, G., and Li, Z. (2019). Ecological regionalization and overview of the Loess Plateau. Acta Ecol. Sin. 39, 7389–7397. doi: 10.5846/stxb201909031825
Zhang, B., Wu, P., Zhao, X., Wang, Y., Wang, J., and Shi, Y. (2012). Drought variation trends in different subregions of the Chinese Loess Plateau over the past four decades. Agric. Water Manag. 115, 167–177. doi: 10.1016/j.agwat.2012.09.004
Zhang, W. G., Gou, X., Liu, W., Li, J., Su, J., Dilawar, N., et al. (2023). Divergent tree radial growth patterns of Qinghai spruce (Picea crassifolia) at the alpine timberline along a moisture gradient in the Qilian mountains, Northwest China. Agric. For. Meteorol. 328:109240. doi: 10.1016/j.agrformet.2022.109240
Zhang, X., Chen, M., Li, M., Huang, L., and Cao, Y. (2023). Drivers of Robinia pseudoacacia L. growth during stand development on the Loess Plateau, China. Dendrochronologia 81:126132. doi: 10.1016/j.dendro.2023.126132
Zhang, Z., Huang, M., Yang, Y., and Zhao, X. (2020). Evaluating drought-induced mortality risk for Robinia pseudoacacia plantations along the precipitation gradient on the Chinese Loess Plateau. Agr. Forest Meteorol. 284:107897. doi: 10.1016/j.agrformet.2019.107897
Zhao, X. (2021). Response of radial growth of Populus davidiana to drought Events in the South part of daxing’an mountains. China: Inner Mongolia Agricultural University.
Zhou, Z., Wang, C., and Luo, Y. (2018). Effects of forest degradation on microbial communities and soil carbon cycling: A global meta-analysis. Global Ecol. Biogeogr. 27, 110–124. doi: 10.1111/geb.12663
Zhu, L., Zhang, J., Camarero, J. J., Cooper, D. J., Cherubini, P., Yuan, D., et al. (2023). Drivers and spatiotemporal patterns of post-drought growth resilience of four temperate broad-leaved trees. Agric. For. Meteorol. 342:109741. doi: 10.1016/j.agrformet.2023.109741
Zhu, Y., Wang, Y., and Chen, L. (2020). Effects of non-native tree plantations on soil microarthropods and their feeding activity on the Chinese Loess Plateau. For. Ecol. Manage. 477:118501. doi: 10.1016/j.foreco.2020.118501
Keywords: tree ring width, radial growth, climate change, drought, resilience
Citation: Yan X, Zhang Z, Wu X and Huang M (2025) Radial growth-climate correlations and resilience of Robinia pseudoacacia plantations to drought on the Chinese Loess Plateau. Front. For. Glob. Change 8:1608397. doi: 10.3389/ffgc.2025.1608397
Received: 08 April 2025; Accepted: 23 June 2025;
Published: 16 July 2025.
Edited by:
Osbert Jianxin Sun, Beijing Forestry University, ChinaReviewed by:
Maria Laura Suarez, Instituto De Investigaciones En Biodiversidad Y Medioambiente-INIBIOMA-CONICET, ArgentinaXiaowei Guo, Zhejiang University, China
Copyright © 2025 Yan, Zhang, Wu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingbin Huang, aG1iZEBud3N1YWYuZWR1LmNu
 Xiaoying Yan
Xiaoying Yan Zhongdian Zhang4,5
Zhongdian Zhang4,5