- 1Department of Nephrology, Rheumatology and Immunology, Childrens Hospital of Jiangnan University, Wuxi, Jiangsu, China
- 2Department of Nephrology, Rheumatology and Immunology, Wuxi Childrens Hospital, Wuxi, Jiangsu, China
Introduction: Neuromyelitis optica spectrum disorder (NMOSD) is an immune-mediated, typically relapsing central nervous system demyelinating disorder characterized by optic neuritis (ON) and transverse myelitis (TM). While systemic or organ-specific autoimmune comorbidities are well-documented in 20–30% of adult NMOSD cases, such associations remain rarely reported in pediatric populations.
Case Report: We present a 14-year-old girl with NMOSD coexisting with primary Sjögren’s syndrome (pSS). At 11 years of age, she presented with acute right-sided headache, painful eye movements, and vision loss. Diagnostic workup confirmed AQP4-IgG seropositivity, ON, and corresponding T2-hyperintense lesions on optic nerve MRI, meeting 2023 Neuromyelitis Optica Study Group (NEMOS) revised recommendations. Acute-phase treatment included intravenous methylprednisolone and intravenous immunoglobulin, followed by maintenance therapy with oral prednisone and mycophenolate mofetil (MMF), with gradual prednisolone tapering. Right-eye vision normalized after intervention. Initial workup revealed positive antinuclear antibody (ANA), anti-Ro/SSA, anti-La/SSB, and elevated alanine aminotransferase (ALT)/aspartate aminotransferase (AST). Aged 12.5 years, labial salivary gland biopsy for persistent transaminitis showed focal lymphocytic sialadenitis (focus score ≥1 focus/4 mm²), satisfying the 2016 ACR/EULAR criteria for pSS. At 13.5 years, MMF was switched to tacrolimus due to persistent ALT/AST elevation, leading to biochemical normalization. No NMOSD relapses occurred post-initial episode.
Conclusion: This case highlights the rare but clinically important co-occurrence of NMOSD and pSS in children. Routine screening for autoantibodies (e.g., ANA, organ-specific antibodies) in pediatric NMOSD is warranted to detect comorbid autoimmune disorders. Targeted therapy for concurrent connective tissue diseases can optimize clinical outcomes and quality of life.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare and severe inflammatory autoimmune disease of the central nervous system (CNS) that is associated with serum aquaporin-4 (AQP4-IgG) antibodies directed against the AQP4 channel found on the foot processes of astrocytes (1–3). AQP4-IgG is key in NMOSD pathophysiology and causes astrocytopathy, demyelination, and neuropathy through complement activation and cell-mediated immunity (4–6). NMOSD has six core clinical characteristics: optic neuritis (ON), acute myelitis, area postrema syndrome, acute brainstem syndrome, acute diencephalic clinical syndrome and symptomatic cerebral syndrome. Among these, three main clinical features are recognized: isolated longitudinally extensive TM or isolated ON; various forms of brainstem encephalitis found in adults; and a broad variety of cerebral symptoms mostly found in children (7–9). Presentations are uncommon in the pediatric age range, accounting for approximately 3%-5% of cases (10).
Connective tissue disease (CTD) is a broad category of rheumatic diseases related to autoimmune dysfunction that can cause damage to multiple organs and systems in the body, including the nervous system. Although demyelination of the central nervous system can be caused by CTD alone, an increasing number of reports have revealed that NMOSD often coexists with CTDs in adults, particularly primary Sjögren syndrome (SS) and systemic lupus erythematosus (SLE) (11–13). An analysis of studies that recruited unselected patients with SLE and SS revealed pooled overlapping percentages of NMOSD of 0.6% and 6.5%, respectively (9). Studies enrolling rheumatologic patients with nervous system symptom involvement reported a higher percentage of NMOSD, with a pooled percentage of 26.5% in SS patients (9). Similarly, when patients with NMOSD were recruited, the pooled percentages of SS or SLE patients were 7.0% and 3.5%, respectively (9). Although the coexistence of NMOSD and pSS is relatively common in adults, this condition is rarely observed in children. Here, we report a child with NMOSD who also has coexisting pSS.
Case report
The patient, a 14-year-old Chinese girl, presented with acute onset of right-sided headaches, pain with eye movements, and vision loss in February 2021 (11 years old). She was admitted to the ophthalmic department of the local hospital. Head and orbital MRI revealed high T2 signals with enhancement in the inner segment of the right optic nerve frame and multiple abnormal signals in the left basal ganglia area and subcortical regions of both the frontal and parietal lobes (Figures 1A, B). Neuro-ophthalmology examination revealed visual acuity of 20/40 in the right eye (oculus dexter, OD), with a right relative afferent pupillary defect (RAPD) and 20/30 in the left eye (oculus sinister, OS). The color vision (Hardy–Rand–Rittler) was 4/14 OD and5/14 OS. Intraocular pressures were within normal limits in both eyes. Slit-lamp examination was unremarkable. Extraocular motility was full, but there was left-eye discomfort with eye movements. A dilated fundus exam revealed circumferential optic disc edema with peripapillary hemorrhage OD and a normal disc in the OS.
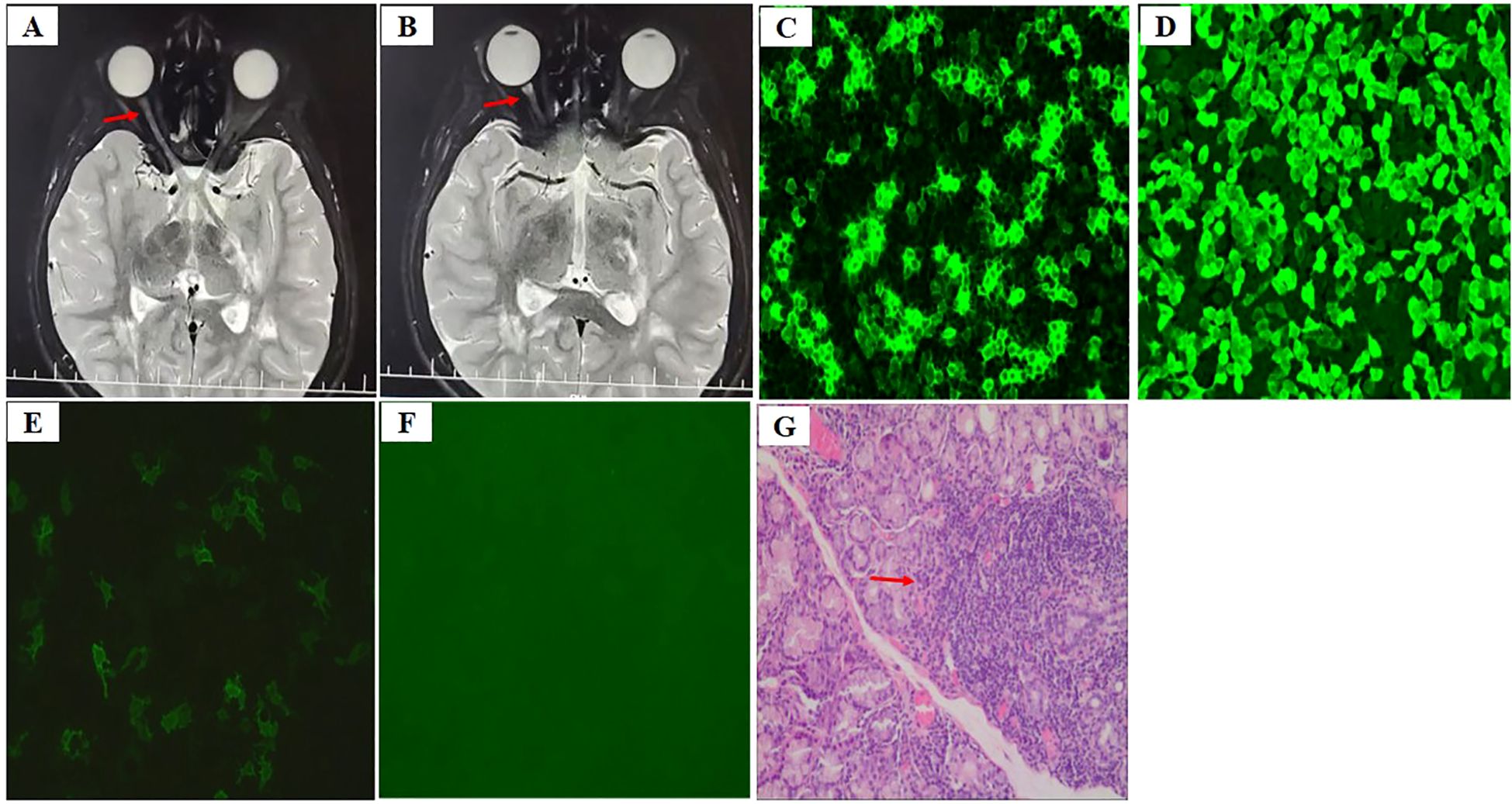
Figure 1. (A, B) Axial T2-weighted image shows thickening of the right optic nerve (A) and hyperintense lesion in the optic nerve sheath (B) [arrows in (A, B)], (C) AQP4-IgG in the serum at an AQP4 titer of 1:1000 by cell-based assay (CBA) before therapy, (D) AQP4-IgG for positive control, (E) AQP4-IgG in the serum at an AQP4 titer of 1:100 during preventive therapy, (F) AQP4-IgG for negative control, (G) Labial salivary gland biopsy displays focal lymphocytic sialadenitis with 1 focus/4 mm2 [arrow in (G)].
The serological workup for tuberculosis, syphilis, and myelin oligodendrocyte glycoprotein (MOG) was negative. The anti-aquaporin-4 immunoglobulin G (AQP4-IgG) was detected in the serum at an AQP4 titer of 1:1000 (Figures 1C, D). Cerebrospinal fluid (CSF) analysis was normal. CSF culture was negative, as were all polymerase chain reaction tests for fungal and viral infections. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level were normal before the initiation of steroids. ANA was positive at a titer of 1:320 in a speckled pattern, and anti-Ro/SSA and anti-La/SSB were positive. Others antibodies, including the remainder of the extractable nuclear antigen (ENA) panel, anti-double-stranded DNA antibodies (anti-ds-DNA), anti-neutrophil cytoplasmic antibody (ANCA), antiphospholipid (aPL) antibodies, and rheumatoid factor (RF), were negative. Complement 3 and 4 levels were normal. Urinalysis revealed no abnormalities. Quantitative immunoglobulins were within normal limits.
She was diagnosed with NMOSD on the basis of a positive test for AQP-IgG and acute optic neuritis and was treated with intravenous pulse methylprednisolone (IVMP) at 500 mg per day for 3 consecutive days. She continued to be administered IVMP at 250 mg per day for 3 consecutive days combined with intravenous immunoglobulins (IVIGs) at 400 mg/kg per day for 5 consecutive days because of a poor response to the first dose of IVMP. Her vision gradually returned to normal after acute treatment. Oral prednisone and mycophenolate mofetil (MMF; 1000 mg) were administered for preventive treatment after acute treatment, and the dose of prednisolone was gradually tapered. She was admitted to our hospital for evaluation because of persistently elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in June 2022. Physical examination revealed Cushing’s appearance. Other physical findings were unremarkable. Schirmer test results was normal (>5 mm/5 min) and ocular surface staining was negative. Her medical history and family history were also unremarkable. Laboratory testing revealed elevated ALT (272 U/l, normal range 7–40 U/l) and AST (1–42 U/l, normal range 13–35 U/l) levels. Serum bilirubin levels were normal. The AQP4- IgG was tested in the serum at an AQP4 titer of 1:100 (Figures 1E, F). Autoantibodies were positive for ANA (1:640), anti-Ro/SSA and anti-La/SSB, while tests for other autoantibodies, including the remainder of the ENA panel, anti-ds-DNA, ANCA, aPL, RF, lupus anticoagulant (LAC), anti-β2 glycoprotein-I antibody (anti-β2GPI) and autoimmune hepatitis antibodies, were negative. Labial salivary gland biopsy was performed to identify possible reasons for the elevated ALT and AST levels, and focal lymphocytic sialadenitis with 1 focus/4 mm2 (1 focus ≥50 lymphocytes/4 mm²) was detected (Figure 1G). The patient also met the 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for primary Sjögren’s syndrome on the basis of positive anti-SSA and labial salivary glands with focal lymphocytic sialadenitis and a focus score of ≥1 foci/4 mm2 (14). MMF was switched to tacrolimus because of persistently elevated ALT and AST levels at the age of 13.5 years. ALT and AST levels subsequently returned to normal. NMOSD did not occur after the first episode.
Discussion
Herein, we report a Chinese girl with NMOSD coexisting with SS. She was 11 years old, had unilateral vision loss (right) and pain with eye movements, and presented with ON. She fulfilled both the 2015 International Panel for NMO Diagnosis (IPND) criteria and the 2023 revised recommendations by the Neuromyelitis Optica Study Group (NEMOS), demonstrating AQP4-IgG seropositivity along with optic neuritis and corresponding T2-hyperintense lesions on optic nerve MRI (8, 15).
The incidence of NMOSD in China per 100,000 person-years was 0.278, with 0.075 in children and 0.347 in adults (16). Therefore, NMOSD is a rare disease in adults and is more rare in children. The onset of the disorder typically occurs at a median age of 40 years in adults, whereas the typical age at onset is between 10 and 12 years in pediatric patients with NMOSD (1, 10, 17, 18).
NMOSD can be divided into three groups according to the presence of serum antibodies: anti–AQP4-IgG antibodies, anti-myelin oligodendrocyte glycoprotein (MOG-IgG) antibodies, and seronegative NMOSD (7, 19). Similar to other autoimmune diseases, seropositive NMOSD is more common in females than in males, with a female-to-male ratio of nearly 10:1 in adults, whereas seronegative cases exhibit an equal sex distribution (17, 20). Serum anti-AQP4 antibodies are present in 70–90% of patients with adult NMOSD and are associated with high specificity (21). Approximately 31% of pediatric patients with NMOSD have detectable serum anti–AQP4-IgG, 57% have serum MOG-IgG, and approximately 12% are double seronegative (22, 23). Therefore, the frequency of AQP4-IgG antibody seropositivity in patients with NMOSD is generally lower in children than in adults (24).
The most frequent clinical features of NMOSD are visual, motor, sensory and constitutional symptoms, such as fever, vomiting, and seizures (1, 25). ON occurs as the first clinical symptom in 50–75% of pediatric patients, and TM occurs in 30–50%, either alone or in combination (3, 25, 26). Other manifestations included ataxia, encephalopathy, and cranial nerve dysfunction, such as ophthalmoparesis or area postrema syndrome (2, 3).
A recurrent course of NMOSD, with exacerbations and incomplete remissions, leads to the development of disability, which has a profound impact on patients’ quality of life. However, recently, a number of randomized controlled trials have demonstrated that biological therapies that act on key elements of NMOSD pathogenesis, such as the interleukin-6 pathway, B cells, and complement, have impressive efficacy in preventing the occurrence of clinical relapses (6, 27). Therefore, four preventive immunotherapies have now been approved for AQP4-IgG-positive NMOSD in many regions of the world: eculizumab, ravulizumab, inebilizumab, and satralizumab. These new drugs may potentially substitute rituximab and classical immunosuppressive therapies, which were as yet the mainstay of treatment for both, AQP4-IgG-positive and -negative NMOSD (23). Early diagnosis and aggressive treatment are crucial for managing symptoms and preventing long-term disability in NMOSD patients.
In addition to AQP4-IgG antibodies, antinuclear autoantibody (ANA) and other non-organ-specific autoantibodies are also often detectable in patients with NMOSD who do not have clinical evidence of a systemic autoimmune disease (28, 29). Although some vaccinations, neoplasms, infections, medications or systemic autoimmune diseases can mimic manifestations of NMOSD (16, 30–32), the coexistence of NMOSD with certain systemic diseases, such as myasthenia gravis, autoimmune hypothyroidism, SLE or pSS, is currently well established (11, 33).
At the beginning of the disease, ANA, anti-Ro/SSA and anti-La/SSB autoantibodies were detected in our patient. She had no clinical features involving the exocrine glands. However, persistently elevated ALT and AST levels were observed in the patient and unexplainable by NMOSD. In addition, specific autoimmune hepatitis markers (such as anti-LKM and anti-SMA antibodies) were negative, ruling out autoimmune hepatitis as the cause of elevated ALT and AST levels. The ALT and AST levels were already elevated prior to MMF treatment and did not further increase after its initiation, making drug-induced liver injury unlikely. Labial salivary gland biopsy was performed, revealing focal lymphocytic sialadenitis with a focus score ≥1 focus/4 mm2. The patient also met the 2016 ACR/EULAR classification criteria for primary Sjögren’s syndrome on the basis of positive anti-SSA and labial salivary glands with focal lymphocytic sialadenitis and a focus score of ≥1 foci/4 mm2 (14).
pSS is rare in children and has a different clinical presentation than in adults (34–37). Adult pSS is particularly prominent with glandular symptoms (such as dry mouth and dry eyes), whereas pediatric pSS is more commonly associated with constitutional symptoms (fever, rash or purpura) and extraglandular manifestations, such as hematological, hepatic, articular and renal involvement (34–37).
The prevalence of neurological manifestations in primary SS varies between 10% and 70%, of which 2–25% of adults exhibit central nervous system (CNS) involvement (37). A wide spectrum of neurologic manifestations in pSS can range from asymptomatic brain lesions on MRI to symptomatic brain lesions, meningitis, myelopathy, cranial neuropathy, sensorimotor polyneuropathy and mononeuritis multiplex (37, 38). Neurological involvement is not common in pediatric patients with pSS, and CNS symptoms are present in 8.7% of patients (39). Headache is the most common symptom of CNS involvement, which is among the rarest systemic involvements in pediatric pSS patients (37, 39).
Our patient met the diagnosis of the coexistence of NMOSD and pSS. Studies in adults revealed that NMOSD is most frequently associated with SS and anti-Ro/SSA antibody (SSA-Ab) positivity in Chinese populations (40, 41). The prevalence of SS is greater among AQP4-IgG-positive patients than among AQP4-IgG-negative patients, with a potential prevalence of 10–20% at the time of diagnosis of AQP4-IgG-positive NMOSD (41). Prasad CB et al. performed a systematic review and found that NMOSD preceded primary SS onset in 45.5% of patients, that NMOSD occurred after primary SS onset in 29.5% of patients, and that 25% of patients presented simultaneously (42).
The reported prevalence of SS in adult patients with NMOSD is estimated to be between 2% and 30%, representing is a highly underrecognized association (42). Although the coexistence of NMOSD and SS is not uncommon in adults, this condition is very rare in children.
Five patients (including our patient) with both NMOSD and pSS were identified via a literature review of PubMed (Table 1) (43–46). They were all female. NMOSD preceded pSS onset in one patient and occurred after pSS onset in one patient. The diagnosis of NMOSD preceded the diagnosis of pSS in our patient, but the symptoms of the two diseases occurred simultaneously (43–46). Three patients presented simultaneously. The median age of NMOSD onset was 11.4 years (range 9-17), and the median age of pSS onset was 12.8 years (range 9-17). Among these five patients, two had autoimmune diseases other than pSS and NMOSD. One patient had SLE, and the other had multiple autoimmunity disorders, including Hashimoto’s disease, celiac disease, and vitiligo (45, 46). Four of the five patients had ON, and three had TM. pSS symptoms differed across the five patients. One patient had parotid gland enlargement, multiple oral cavity caries and fever. In addition, one patient had distal renal Tubular acidosis (dRTA), and one patient had elevated ALT and AST levels. The other two patients had no symptoms and were diagnosed by salivary gland biopsy (43–46). Four of the five patients achieved complete remission after acute and preventive therapy, and one achieved no remission, with a recurrent course and the accumulation of disability (43–46).
The international panel of NMOSD diagnoses concluded that the presence of CTD in NMOSD patients is a coexistence, rather than a complication, of connective tissue disease. NMOSD patients with anti-SSA/Ro antibodies have a significantly greater risk of relapse (47), and anti-SSA/Ro antibodies may be associated with disease activity and severe disability in patients with NMOSD (48). When first-attack NMOSD patients are complicated with CTD, they have a higher recurrence rate, more recurrences, and earlier first recurrence (49). The authors agreed that autoantibodies, ANA and other organ-specific or non-organ-specific autoantibodies should be detected in patients with NMOSD to identify systemic or organ-specific autoimmune diseases and to help predict the prognosis of patients with NMOSD. The coexistence of NMOSD and pSS can present complex challenges in terms of management and treatment. Diagnosing NMOSD in pSS patients is crucial, as it has a highly relapsing course requiring indefinite immunosuppression. Moreover, if not diagnosed early, damage accrual occurs over time, leading to permanent disability and morbidity. CNS symptoms may occur during the course of the disease in children with pSS, and AQP4-IgG should be tested as early as possible to avoid missed diagnoses. Once CTDs coexisting with NMOSD are diagnosed, targeted treatment should be actively administered to improve the prognosis and quality of life of patients.
Conclusion
NMOSD is an autoimmune condition that commonly co-occurs with systemic or organ-specific autoimmune diseases in adult populations, though such associations are infrequently observed in pediatric cases. Comprehensive autoantibody screening, including ANA and organ-specific/non-organ-specific autoantibodies, is essential in NMOSD patients to identify concurrent autoimmune disorders and facilitate prognostic evaluation. Clinicians should maintain a high index of suspicion for autoimmune comorbidities during long-term follow-up. Importantly, AQP4-IgG testing should be performed in patients with underlying autoimmune diseases who develop neurological symptoms suggestive of NMOSD. Accurate diagnosis of NMOSD is particularly crucial for the effective management of patients with CTD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Children’s Hospital of Jiangnan University, Wuxi, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
G-QZ: Data curation, Writing – review & editing. R-XH: Formal analysis, Writing – review & editing. YP: Formal analysis, Writing – review & editing. YY: Investigation, Writing – review & editing. G-ML: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the patient and her parents, who gave us written consent to use and publish the patient’s clinical data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wingerchuk DM and Lucchinetti CF. Neuromyelitis optica spectrum disorder. N Engl J Med. (2022) 387:631–9. doi: 10.1056/NEJMra1904655
2. Siriratnam P, Huda S, Butzkueven H, van der Walt A, Jokubaitis V, and Monif M. A comprehensive review of the advances in neuromyelitis optica spectrum disorder. Autoimmun Rev. (2023) 22:103465. doi: 10.1016/j.autrev.2023.103465
3. Bagherieh S, Afshari-Safavi A, Vaheb S, Kiani M, Ghaffary EM, Barzegar M, et al. Worldwide prevalence of neuromyelitis optica spectrum disorder (NMOSD) and neuromyelitis optica (NMO): a systematic review and meta-analysis. Neurol Sci. (2023) 44:1905–15. doi: 10.1007/s10072-023-06617-y
4. Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. (2008) 4:202–14. doi: 10.1038/ncpneuro0764
5. Cacciaguerra L and Flanagan EP. Updates in NMOSD and MOGAD diagnosis and treatment: A tale of two central nervous system autoimmune inflammatory disorders. Neurol Clin. (2024) 42:77–114. doi: 10.1016/j.ncl.2023.06.009
6. Hu RX, Yao Y, Xu DD, Bao YQ, Liu XW, Zhu GQ, et al. A Chinese child with both systemic lupus erythematosus coexisting with neuromyelitis optica spectrum disorder: a case report. Pediatr Rheumatol Online J. (2024) 22:107. doi: 10.1186/s12969-024-01045-4
7. Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, and Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. (2020) 6:85. doi: 10.1038/s41572-020-0214-9
8. Jarius S, Aktas O, Ayzenberg I, Bellmann-Strobl J, Berthele A, Giglhuber K, et al. Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis. J Neurol. (2023) 270:3341–68. doi: 10.1007/s00415-023-11634-0
9. Esposito JE, Annoni G, D'Amato M, Graziosi A, Troilo F, Di Risio A, et al. Systemic connective tissue disease and neuromyelitis optica spectrum disorder coexistence: A systematic review and meta-analysis. J Integr Neurosci. (2024) 23:35. doi: 10.31083/j.jin2302035
10. Poisson K, Moeller K, and Fisher KS. Pediatric neuromyelitis optica spectrum disorder. Semin Pediatr Neurol. (2023) 46:101051. doi: 10.1016/j.spen.2023.101051
11. Cruz RA, Chaudhary S, Guevara M, and Meltzer E. Neuromyelitis optica spectrum disorders (NMOSD) and connective tissue disease (CTD): an update for the rheumatologist. Curr Rheumatol Rep. (2021) 23:33. doi: 10.1007/s11926-021-01000-2
12. Akaishi T, Tarasawa K, Matsumoto Y, Sandhya P, Misu T, Fushimi K, et al. Associations between neuromyelitis optica spectrum disorder, Sjogren's syndrome, and conditions with electrolyte disturbances. J Neurol Sci. (2023) 452:120742. doi: 10.1016/j.jns.2023.120742
13. Lin J, Xue B, Li J, Xie D, Weng Y, Zhang X, et al. The relationship between neuromyelitis optica spectrum disorder and autoimmune diseases. Front Immunol. (2024) 15:1406409. doi: 10.3389/fimmu.2024.1406409
14. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. (2017) 76:9–16. doi: 10.1136/annrheumdis-2016-210571
15. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
16. Tian DC, Li Z, Yuan M, Zhang C, Gu H, Wang Y, et al. Incidence of neuromyelitis optica spectrum disorder (NMOSD) in China: A national population-based study. Lancet Reg Health West Pac. (2020) 2:100021. doi: 10.1016/j.lanwpc.2020.100021
17. Waliszewska-Prosół M, Chojdak-Łukasiewicz J, Budrewicz S, and Pokryszko-Dragan A. Neuromyelitis optica spectrum disorder treatment-current and future prospects. Int J Mol Sci. (2021) 22:2801. doi: 10.3390/ijms22062801
18. Preziosa P, Amato MP, Battistini L, Capobianco M, Centonze D, Cocco E, et al. Moving towards a new era for the treatment of neuromyelitis optica spectrum disorders. J Neurol. (2024) 271:3879–96. doi: 10.1007/s00415-024-12426-w
19. Ding M, Lang Y, and Cui L. AQP4-IgG positive paraneoplastic NMOSD: A case report and review. Brain Behav. (2021) 11:e2282. doi: 10.1002/brb3.v11.10
20. Ferilli MAN, Paparella R, Morandini I, Papetti L, Figà Talamanca L, Ruscitto C, et al. Pediatric neuromyelitis optica spectrum disorder: case series and literature review. Life (Basel). (2021) 12:19. doi: 10.3390/life12010019
21. Legge AC and Hanly JG. Recent advances in the diagnosis and management of neuropsychiatric lupus. Nat Rev Rheumatol. (2024) 20:712–28. doi: 10.1038/s41584-024-01163-z
22. Chitnis T. Pediatric central nervous system demyelinating diseases. Continuum Lifelong Learn Neurol. (2019) 25:793–814. doi: 10.1212/CON.0000000000000730
23. Hacohen Y, Mankad K, Chong WK, Barkhof F, Vincent A, Lim M, et al. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology. (2017) 89:269–78. doi: 10.1212/WNL.0000000000004117
24. Dahan A, Brilot F, Leventer R, Kornberg AJ, Dale RC, and Yiu EM. Neuromyelitis optica spectrum disorder and anti-aquaporin 4 channel immunoglobulin in an Australian pediatric demyelination cohort. J Child Neurol. (2020) 35:291–6. doi: 10.1177/0883073819895191
25. Gise RA and Heidary G. Update on pediatric optic neuritis. Curr Neurol Neurosci Rep. (2020) 20:4. doi: 10.1007/s11910-020-1024-x
26. Lock JH, Newman NJ, Biousse V, and Peragallo JH. Update on pediatric optic neuritis. Curr Opin Ophthalmol. (2019) 30:418–25. doi: 10.1097/ICU.0000000000000607
27. Kümpfel T, Giglhuber K, Aktas O, Ayzenberg I, Bellmann-Strobl J, Häußler V, et al. Update on the diagnosis and treatment of neuromyelitis optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part II: Attack therapy and long-term management. J Neurol. (2024) 271:141–76. doi: 10.1007/s00415-023-11910-z
28. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, and Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. (2007) 6:805–15. doi: 10.1016/S1474-4422(07)70216-8
29. Yang CS, Zhang QX, Chang SH, Zhang LJ, Li LM, Qi Y, et al. Neuromyelitis optica spectrum disorders with and without connective tissue disorders. BMC Neurol. (2018) 18:177. doi: 10.1186/s12883-018-1182-5
30. Khimani K, Patel SP, Whyte A, and Al-Zubidi N. Case report: neuromyelitis optica after treatment of uveal melanoma with nivolumab and ipilimumab. Front Oncol. (2022) 12:806501. doi: 10.3389/fonc.2022.806501
31. Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, and Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord. (2017) 10:265–89. doi: 10.1177/1756285617709723
32. Shahmohammadi S, Doosti R, Shahmohammadi A, Mohammadianinejad SE, Sahraian MA, Azimi AR, et al. Autoimmune diseases associated with Neuromyelitis Optica Spectrum Disorders: a literature review. Mult Scler Relat Disord. (2019) 27:350–63. doi: 10.1016/j.msard.2018.11.008
33. Taheri N, Sarrand J, and Soyfoo MS. Neuromyelitis optica: pathogenesis overlap with other autoimmune diseases. Curr Allergy Asthma Rep. (2023) 23:647–54. doi: 10.1007/s11882-023-01112-y
34. Hou L, Wang N, Zhao C, Wang X, and Du Y. A single-center study of clinical features of pediatric Sjogren's syndrome. Pediatr Rheumatol Online J. (2023) 21:119. doi: 10.1186/s12969-023-00902-y
35. Gong Y, Liu H, Li G, Zhang T, Li Y, and Guan W. Childhood-onset primary Sjögren's syndrome in a tertiary center in China: clinical features and outcome. Pediatr Rheumatol Online J. (2023) 21:11. doi: 10.1186/s12969-022-00779-3
36. Liu C, Jin Y, Huang H, Ding F, Xu X, Bao S, et al. Clinical and laboratory features of childhood-onset primary Sjögren's syndrome: A retrospective study from China. Front Pediatr. (2023) 10:1044812. doi: 10.3389/fped.2022.1044812
37. Baldini C, Fulvio G, La Rocca G, and Ferro F. Update on the pathophysiology and treatment of primary Sjogren syndrome. Nat Rev Rheumatol. (2024) 20:473–91. doi: 10.1038/s41584-024-01135-3
38. Barsottini OGP, Moraes MPM, Fraiman PHA, Marussi VHR, Souza AWS, Braga Neto P, et al. Sjogren's syndrome: a neurological perspective. Arq Neuropsiquiatr. (2023) 81:1077–83. doi: 10.1055/s-0043-1777105
39. Yilmaz FC, Kisaoglu H, Baba O, and Kalyoncu M. Spectrum of central nervous system involvement in childhood-onset sjögren's syndrome: A case-based review. Mediterr J Rheumatol. (2024) 35:298–304. doi: 10.31138/mjr.241123.soc
40. Chen C, Xiaobo S, Yuge W, Yaqing S, Ling F, Lisheng P, et al. Multiple autoantibodies and neuromyelitis optica spectrum disorders. Neuroimmunomodulation. (2016) 23:151–6. doi: 10.1159/000448286
41. Akaishi T, Takahashi T, Fujihara K, Misu T, Fujimori J, Takai Y, et al. Impact of comorbid Sjögren syndrome in anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders. J Neurol. (2021) 268:1938–44. doi: 10.1007/s00415-020-10377-6
42. Prasad CB, Kopp CR, Naidu G, Sharma V, Misra DP, Agarwal V, et al. Overlap syndrome of anti-aquaporin 4 positive neuromyelitis optica spectrum disorder and primary Sjögren's syndrome: a systematic review of individual patient data. Rheumatol Int. (2024) 44:2807–15. doi: 10.1007/s00296-023-05397-0
43. Arabshahi B, Pollock AN, Sherry DD, Albert DA, Kreiger PA, and Pessler F. Devic disease in a child with primary Sjogren syndrome. J Child Neurol. (2006) 21:285–6. doi: 10.1177/08830738060210040701
44. Gökçay F, Celebisoy N, Gökçay A, Kabasakal Y, and Oder G. Primary Sjogrens syndrome presenting as neuromyelitis optica. Pediatr Neurol. (2007) 36:58–60. doi: 10.1016/j.pediatrneurol.2006.07.008
45. Bruns N, Finkelberg I, Al-Attrach I, Hoyer PF, and Büscher R. Unusual presentation of polyautoimmunity and renal tubular acidosis in an adolescent with hashimoto's thyroiditis and central pontine myelinolysis. Front Endocrinol (Lausanne). (2020) 11:548877. doi: 10.3389/fendo.2020.548877
46. Liu L, Tang L, Zhang L, Li X, Huang P, Xiong J, et al. The first case report of preschool-onset SS/SLE coexisting with NMOSD of chinese origin. Front Immunol. (2022) 13:887041. doi: 10.3389/fimmu.2022.887041
47. Park JH, Hwang J, Min JH, Kim BJ, Kang ES, and Lee KH. Presence of anti-Ro/SSA antibody may be associated with anti-aquaporin-4 antibody positivity in neuromyelitis optica spectrum disorder. J Neurol Sci. (2015) 348:132–5. doi: 10.1016/j.jns.2014.11.020
48. Lin L, Hang H, Zhang J, Lu J, Chen D, and Shi J. Clinical significance of anti-SSA/Ro antibody in Neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. (2022) 58:103494. doi: 10.1016/j.msard.2022.103494
Keywords: aquaporin 4, children, neuromyelitis optica spectrum disorders, optic neuritis, Sjögren syndrome
Citation: Zhu G-q, Hu R-x, Peng Y, Yao Y and Li G-m (2025) A Chinese girl with neuromyelitis optica spectrum disorder coexisting with primary Sjogren’s syndrome: a case report and literature review. Front. Immunol. 16:1559825. doi: 10.3389/fimmu.2025.1559825
Received: 13 January 2025; Accepted: 25 June 2025;
Published: 08 July 2025.
Edited by:
Ban Luo, Huazhong University of Science and Technology, ChinaReviewed by:
Lingjuan Xu, Huazhong University of Science and Technology, ChinaChandra Bhushan Prasad, Healthway Hospitals, India
Copyright © 2025 Zhu, Hu, Peng, Yao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-min Li, bGlndW9taW40ODZAc2luYS5jb20=
 Guo-qin Zhu
Guo-qin Zhu Rong-xuan Hu1,2
Rong-xuan Hu1,2 Guo-min Li
Guo-min Li