- 1Department of Interventional Therapy, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Tianjin, China
- 2Tianjin's Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute & Hospital, Tianjin, China
- 3Key Laboratory of Cancer Prevention and Therapy, Tianjin Medical University Cancer Institute & Hospital, Tianjin, China
Objective: This study aimed to assess the efficacy and safety of hepatic arterial infusion chemotherapy (HAIC) combined with lenvatinib and immunotherapy and explore its potential as a conversion treatment for unresectable hepatocellular carcinoma (uHCC).
Methods: A retrospective analysis was performed on clinical data from patients with uHCC who underwent HAIC, lenvatinib, and PD-1/PD-L1 immunotherapy. Data were collected from our hospital between November 2018 and December 2022. Efficacy was assessed based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST). The primary endpoints were overall survival (OS), progression-free survival (PFS), and conversion therapy rate. Additionally, survival status curves were plotted using the Kaplan-Meier method. Lastly, prognostic risk factors affecting conversion therapy and survival outcomes were evaluated using Logistic and Cox regression models.
Results: As of December 2022, 318 patients were included, comprising 40 patients (12.6%) in BCLC stage A, 123 patients (38.7%) in BCLC stage B, and 155 patients (48.7%) in BCLC stage C. The overall objective response rate (ORR) was 47.1%, whilst the disease control rate (DCR) was 85.5%. Meanwhile, the median overall survival (mOS) for the entire cohort was 21.7 months (95% CI: 19.7-24.3), with a median progression-free survival (mPFS) of 11.4 months (95% CI: 9.4-13.4). A total of 110 patients (34.6%) underwent conversion surgery. Multivariate logistic regression analysis identified BCLC stage as the sole independent risk factor affecting eligibility for conversion therapy. Subgroup analysis revealed that BCLC-B stage patients who achieved successful conversion therapy demonstrated significantly superior outcomes compared to those who did not undergo successful conversion therapy (median OS: 29.3 months [95% CI: 24.3-NA] vs. 19.7 months [95% CI: 17.2-24.6], P = 0.0013). Multivariate regression analysis identified the BCLC stage, the presence of distant metastasis, and receipt of conversion therapy as independent prognostic factors influencing OS. Among the cohort, 169 (53.1%) experienced grade 3-4 adverse events (AEs), with the most commonly reported AEs being fatigue, fever, and pain.
Conclusion: The combination of HAIC with lenvatinib and immunotherapy yielded a high ORR, improved the conversion therapy rate, and prolonged both OS and PFS in patients with uHCC while maintaining a favorable safety profile. BCLC stage was identified as an independent prognostic factor influencing the success of conversion therapy, with patients in stage B deriving significant survival benefits post-conversion.
Introduction
As is well documented, HCC is the sixth most prevalent cancer globally and the third leading cause of cancer-related deaths. By 2025, global liver cancer incidence is projected to exceed one million cases, with China, a high-incidence region, accounting for 47% of these cases (1). The majority of HCC patients are diagnosed at intermediate to advanced stages, rendering them ineligible for curative surgery or ablation, with a median survival time of merely 1 to 1.5 years (2). In recent years, advances in non-surgical treatments have enabled more patients with advanced liver cancer to undergo downstaging through conversion therapy, leading to improved survival benefits (3, 4).
The first-line standard treatment for advanced-stage liver cancer has been upgraded to a combination of targeted and immunotherapy (5, 6). To date, multiple large-scale Phase III clinical studies have validated the efficacy and safety of immune-targeted combination therapies for the treatment of patients with advanced hepatocellular carcinoma (HCC), achieving ORR ranging between 21% to 32.8% and a median OS between 19.2 to 22.1 months, significantly surpassing the efficacy of targeted therapy or immunotherapy alone (7, 8).
Conversion therapy refers to the treatment strategy for patients with uHCC, including those with poor overall physical conditions unable to tolerate surgical trauma or with compromised liver function precluding surgical intervention. This approach also encompasses tumor-specific conversion, wherein therapy aims to reduce tumor burden or downgrade the disease stage to facilitate curative R0 resection (9, 10). While targeted and immunotherapy have been established to confer survival benefits for patients with uHCC, the local control rate of the tumor is equally important, leading to the emergence of a conversion strategy integrating local therapy and targeted immunotherapy (11–13).
HAIC is effective in decreasing the intrahepatic tumor burden, given that it allows for the targeted delivery of chemotherapy drugs to the arteries supplying the tumor. The FOLFOX-HAIC treatment regimen (oxaliplatin, leucovorin, and fluorouracil), in conjunction with targeted therapy and immunotherapy, has preliminarily shown higher tumor response rates, improved survival outcomes, and greater potential for conversion therapy in patients with intermediate to advanced HCC and has become a clinical research hotspot (14, 15).
Thus, this study aimed to evaluate the safety and efficacy of FOLFOX-HAIC combined with lenvatinib and immunotherapy in HCC patients, explore factors influencing efficacy and prognosis, and identify factors affecting conversion therapy.
Materials and methods
Patients
This study retrospectively analyzed data collected from patients with uHCC who underwent HAIC combined with targeted therapy and immunotherapy at our hospital between November 2018 and December 2022. All patients were diagnosed based on the Chinese Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2019 Edition) and confirmed as uHCC through multidisciplinary team (MDT) consultations. The inclusion criteria were as follows: 1. age between 20 and 75 years; 2. Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0-2; 3. Child-Pugh class A or B liver function; 4. absence of other malignancies. The exclusion criteria included an expected survival time of less than 3 months, severe liver dysfunction, severe splenic hyperfunction, a history of gastrointestinal bleeding, prior systemic or local treatment, and a history of liver or kidney transplantation and heart failure.
Treatment protocol
HAIC was performed by puncturing the femoral artery using the modified Seldinger technique. During the procedure, a 2.7F microcatheter was super-selectively placed into the tumor-feeding artery to administer the drugs. The FOLFOX regimen used was as follows: a 4-hour infusion of 85 mg/m2 oxaliplatin, a 2-hour infusion of 400 mg/m2 calcium folinate and a bolus injection of 400 mg/m2 fluorouracil, followed by a 23-hour infusion of 1200-mg/m2 fluorouracil on day 1 of treatment. Dosages were adjusted based on the Child-Pugh score and chemotherapy tolerance. HAIC was repeated every 3-4 weeks until disease progression, the occurrence of unacceptable toxicity, or a change in the treatment plan. Upon the occurrence of grade 3 or 4 adverse events, the dosage of oxaliplatin was adjusted to 65 mg/m², while the dose of 5-fluorouracil was modified to 300 mg for each bolus administration and 1000 mg for each cycle.
Molecular targeted and immunotherapy
Prior to or following the initial HAIC session, patients were intravenously injected with anti-PD-1 antibodies every 3 weeks, including 200 mg of sintilimab, 200 mg of tislelizumab, 200 mg of camrelizumab, 240 mg of toripalimab, or 200 mg of pembrolizumab. For anti-angiogenic therapy, patients were administered 8 mg lenvatinib orally once daily. To avoid adverse events, lenvatinib were discontinued for 2 days before and after HAIC. If the immunotherapy injection coincided with HAIC, it was postponed until 2 days after the procedure. Grade 3-4 AE measures including immunotherapy discontinuation, hormone therapy initiation, and combined immunosuppressants if necessary.
Endpoints and evaluation of treatment effectiveness
The primary endpoints of the study were OS, PFS, and conversion therapy rate. Secondary endpoints included objective response rate (ORR, comprising complete response [CR] and partial response [PR]), disease control rate (DCR, comprising ORR and stable disease [SD]), and the incidence of adverse events (AEs) defined according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Imaging assessments were conducted using the mRECIST (16). Evaluations were performed by two associate chief physicians or higher, each with more than five years of experience in imaging diagnostics for malignant tumors. Disagreements were adjudicated by a senior chief physician. Treatment continuation was considered when PR or SD was achieved, and the quality of life of patients did not decline. Conversion therapy was considered when CR or near-CR(≥90% reduction in enhancement) was achieved, and the treatment plan was accordingly adjusted in cases of PD. Treatment was suspended if severe adverse reactions occurred.
Conversion of MDT treatment recommendations
Our Multidisciplinary Team (MDT) consists of interventional radiologists, hepatobiliary surgeons, medical oncologists, and diagnostic radiologists, who collaboratively participate in the entire treatment process including conversion therapy regimens and surgical timing/indications. Conversion Criteria: Child-Pugh A/B, ECOG ≤2, and imaging meeting oncological standards, with resection requiring FLR ≥40% & ICG-R15 ≤20%, and ablation allowing ≤5 cm solitary tumor or ≤3 lesions (each ≤3 cm).
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software and R programming, using independent samples t-test, rank sum test, chi-square test, and Kaplan-Meier curve analysis. Univariate and multivariate Cox regression analyses were conducted to identify prognostic factors, with P < 0.05 considered statistically significant.
Result
Clinical characteristics of patients
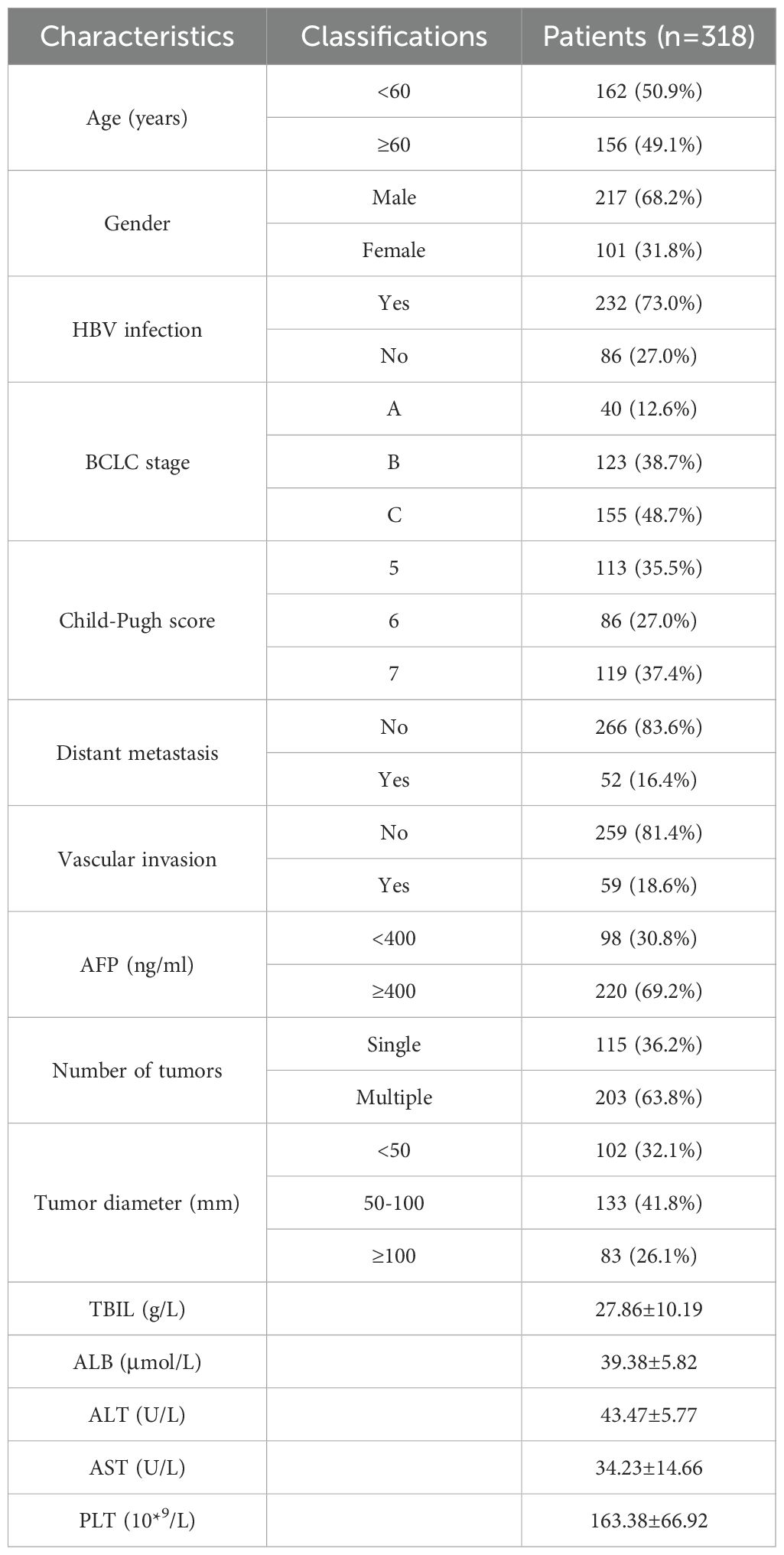
As of December 2022, 536 patients with uHCC underwent HAIC combined with molecular targeted and immunotherapy at our center, among which 318 met the inclusion criteria. Among the patients, 12.6% were in the BCLC-A stage, 38.7% in the B stage, and 48.7% in the C stage. Chronic hepatitis B virus infection was the most common cause of uHCC (73.3%). There were 115 cases (36.1%) with a single lesion and 203 cases (63.9%) with multiple lesions. Tumor size was categorized as <5 cm (31.9%), 5-10 cm (42.4%), and ≥10 cm (25.7%). Tumor location was unilateral in 144 cases (45.3%) and bilateral sides in 174 cases (54.7%). Vascular invasion was observed in 59 cases (18.6%), including 50 cases (15.7%) with portal vein tumor thrombus, and distant metastasis was noted in 52 cases (16.4%) (Table 1).
The most frequently used immunotherapy drug was sintilimab, used in 136 cases, followed by tislelizumab in 108 cases, camrelizumab in 45 cases, atezolizumab in 17 cases, and toripalimab in 12 cases.
Short-term efficacy
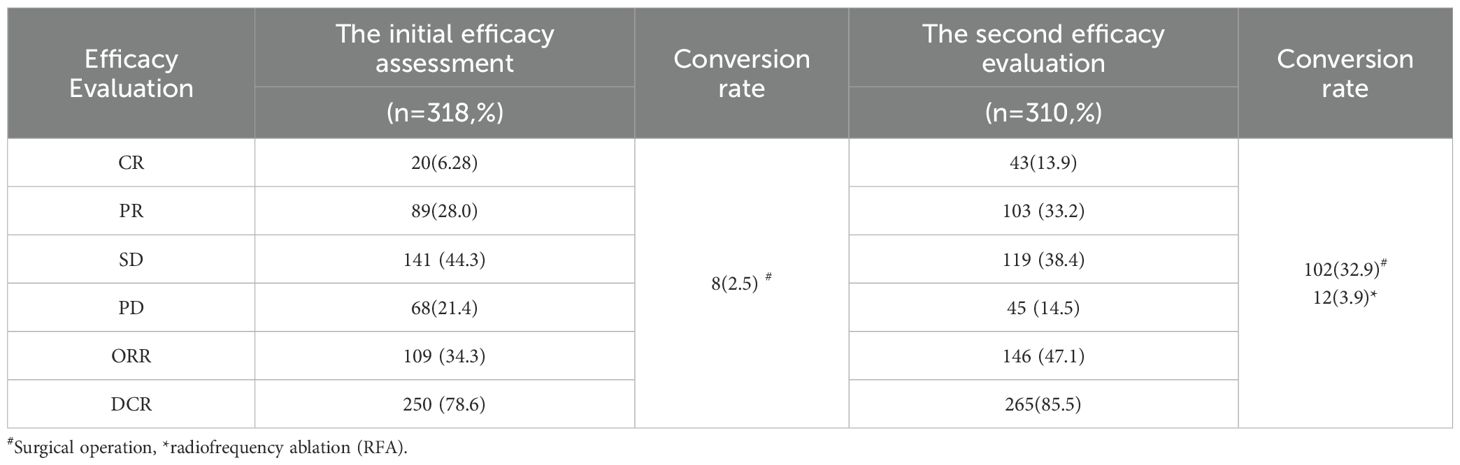
Follow-up was conducted at the end of every alternative course of HAIC, with all 318 patients completing the follow-up and receiving the next course of HAIC combined therapy. The first evaluation results revealed CR in 20 cases (6.28%), PR in 89 cases (28.0%), SD in 141 cases (44.3%), and PD in 68 cases (21.4%), with an ORR of 34.3% and a DCR of 78.6%. Among them, 8 patients underwent surgical resection after MDT discussion, and all postoperative pathological results were CR. At the second evaluation, excluding the 8 patients who underwent conversion resection, 310 patients completed the follow-up, with CR achieved in 43 cases (13.9%), PR in 103 cases (33.2%), SD in 119 cases (38.4%), and PD in 45 cases (14.5%), with an ORR and DCR of 47.1% and 85.5%, respectively. After the second evaluation, 102 additional patients underwent conversion surgery treatment following MDT discussion, among which 12 underwent radical radiofrequency ablation therapy (Table 2).
Conversion therapy analysis
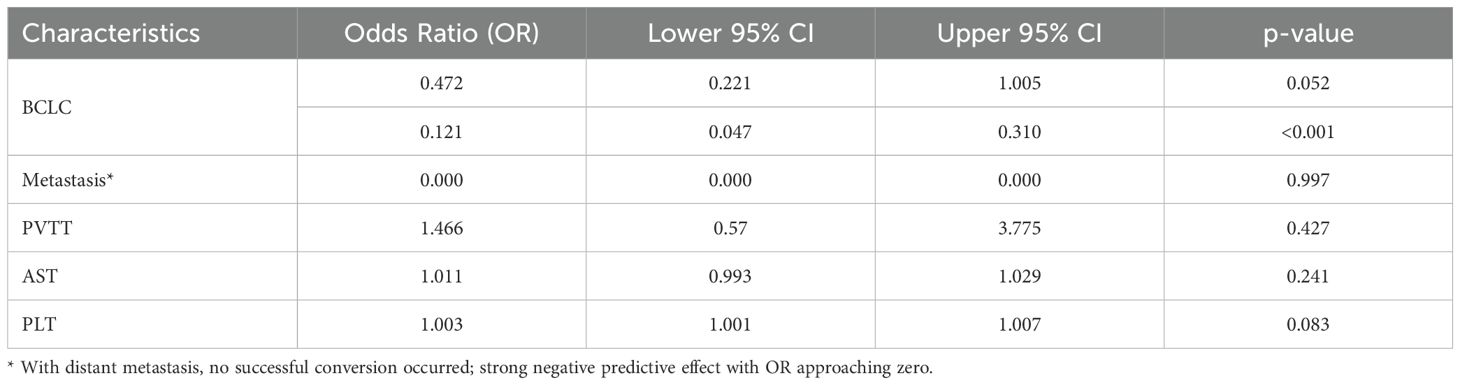
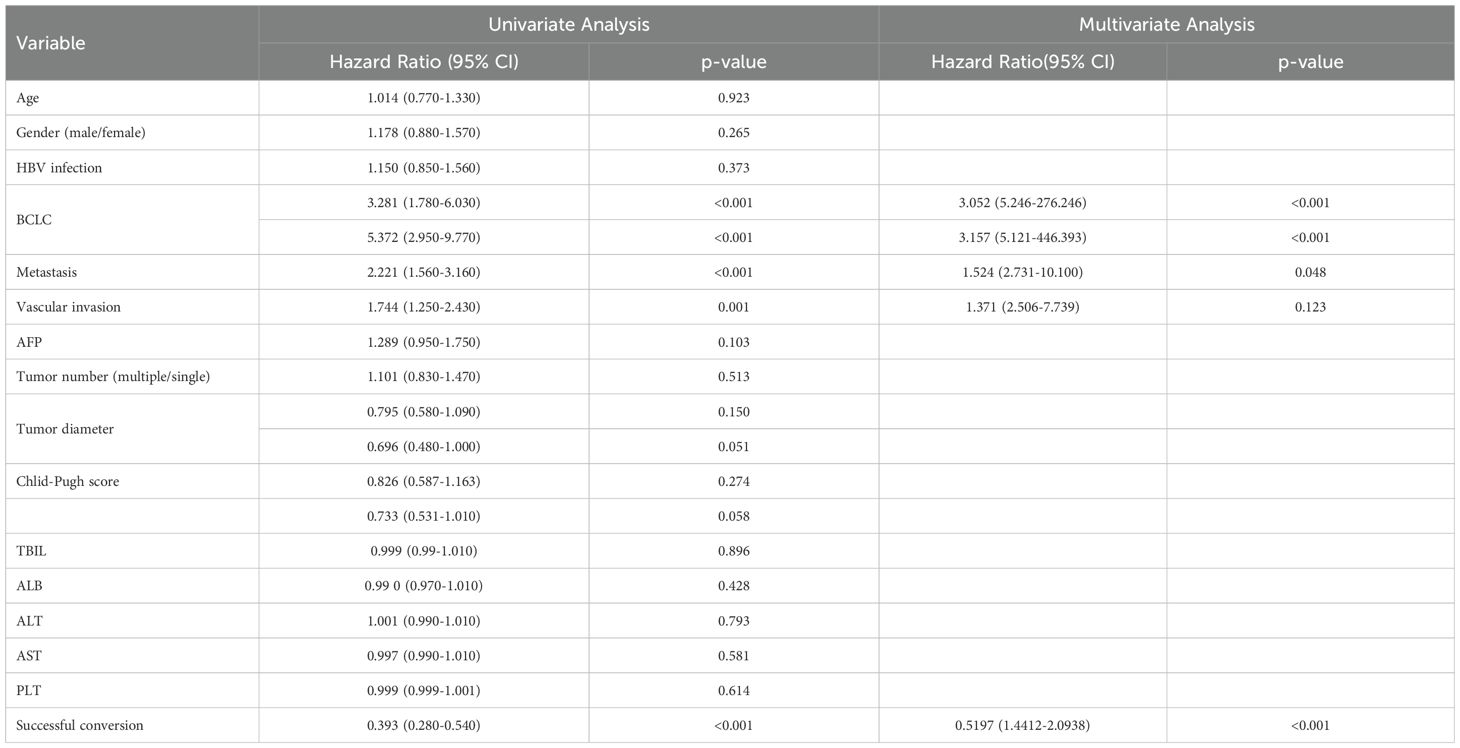
In the present study, 110 patients (34.6%) underwent conversion therapy. Among them, 8 cases (2.5%) met the criteria for conversion resection at the first evaluation, whereas 102 cases (32.9%) proceeded to conversion therapy after the second evaluation, including 12 cases of radical radiofrequency ablation. Among patients who underwent conversion therapy, the majority were classified as stage B (60 cases, 54.5%), followed by stages A and C, with 27 cases (24.5%) and 23 cases (20.9%), respectively. Among patients with vascular invasion, 12 cases (20.3%) successfully underwent conversion therapy, while those with distant metastasis did not undergo conversion therapy. Significant differences were noted between the conversion therapy group and the non-conversion therapy group in terms of BCLC staging, distant metastasis, vascular invasion, AST levels, and platelet counts. Multivariate analysis identified BCLC staging as the only independent risk factor affecting conversion therapy (Tables 3, 4).

Table 3. Comparison of clinical data between the conversion therapy group and non-conversion therapy groups.
Prognostic analysis
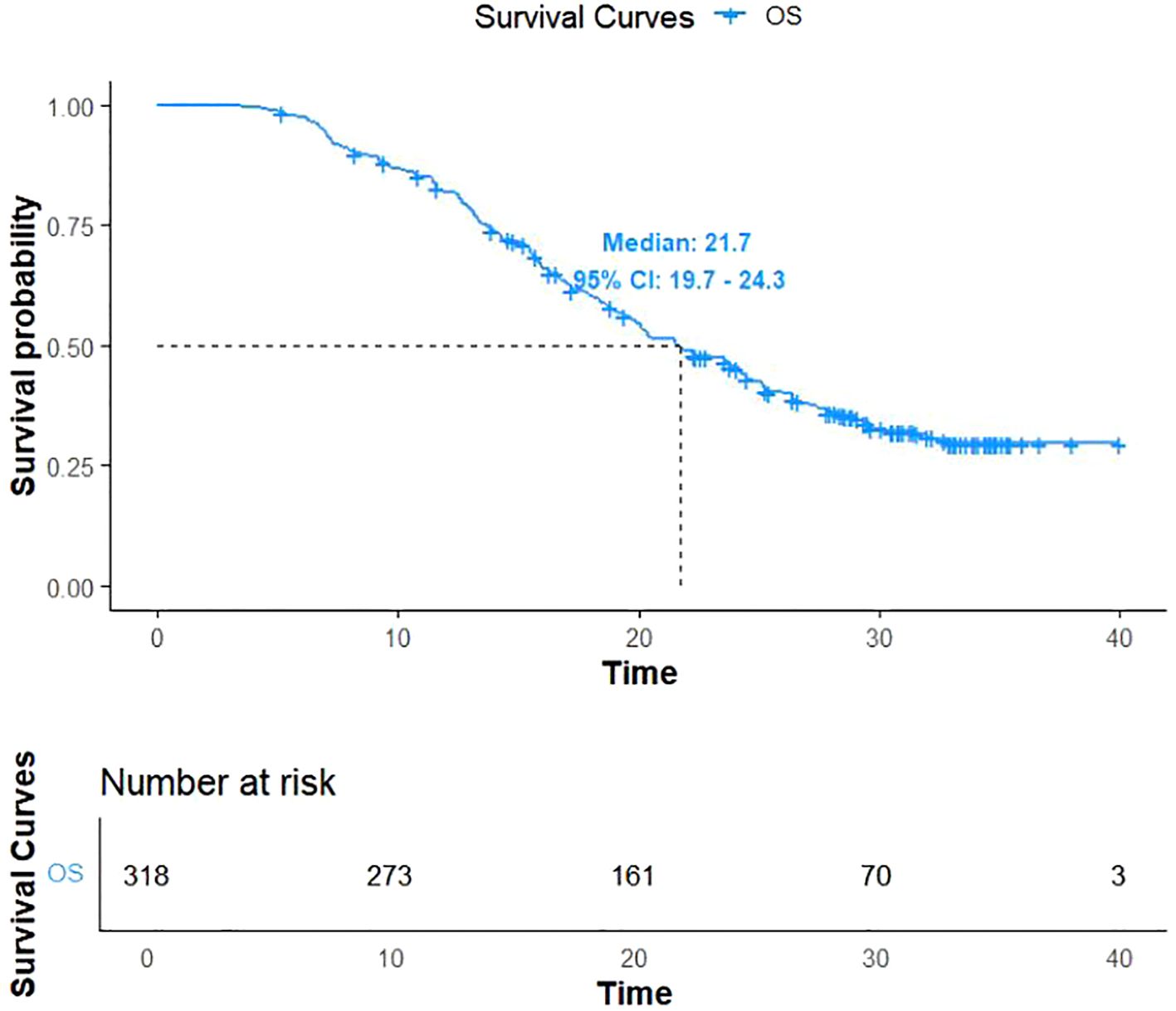
As of December 31, 2022, 206 patients (64.7%) reached the study endpoints, while the remaining 112 patients were either lost to follow-up or still alive at the time of follow-up cutoff. Kaplan-Meier curves for OS and PFS for all patients are illustrated in Figures 1, 2, respectively. The mOS and mPFS were 21.7 months (95% CI: 19.7-24.3) and 11.4 months (95% CI: 9.4-13.4), respectively.
Furthermore, Kaplan-Meier survival curves were generated to assess mOS across different BCLC stages. Interestingly, patients with BCLC stage A had not reached mOS, with over 50% remaining alive at the end of follow-up. Regarding BCLC stage B and C patients, mOS was 24.2 months (95% CI: 20.4–28.65) and 17.0 months (95% CI: 15.8-19.1), respectively. As anticipated, survival times were significantly longer in stage B patients compared to stage C patients (P < 0.001) (Figure 3).
Subgroup analysis
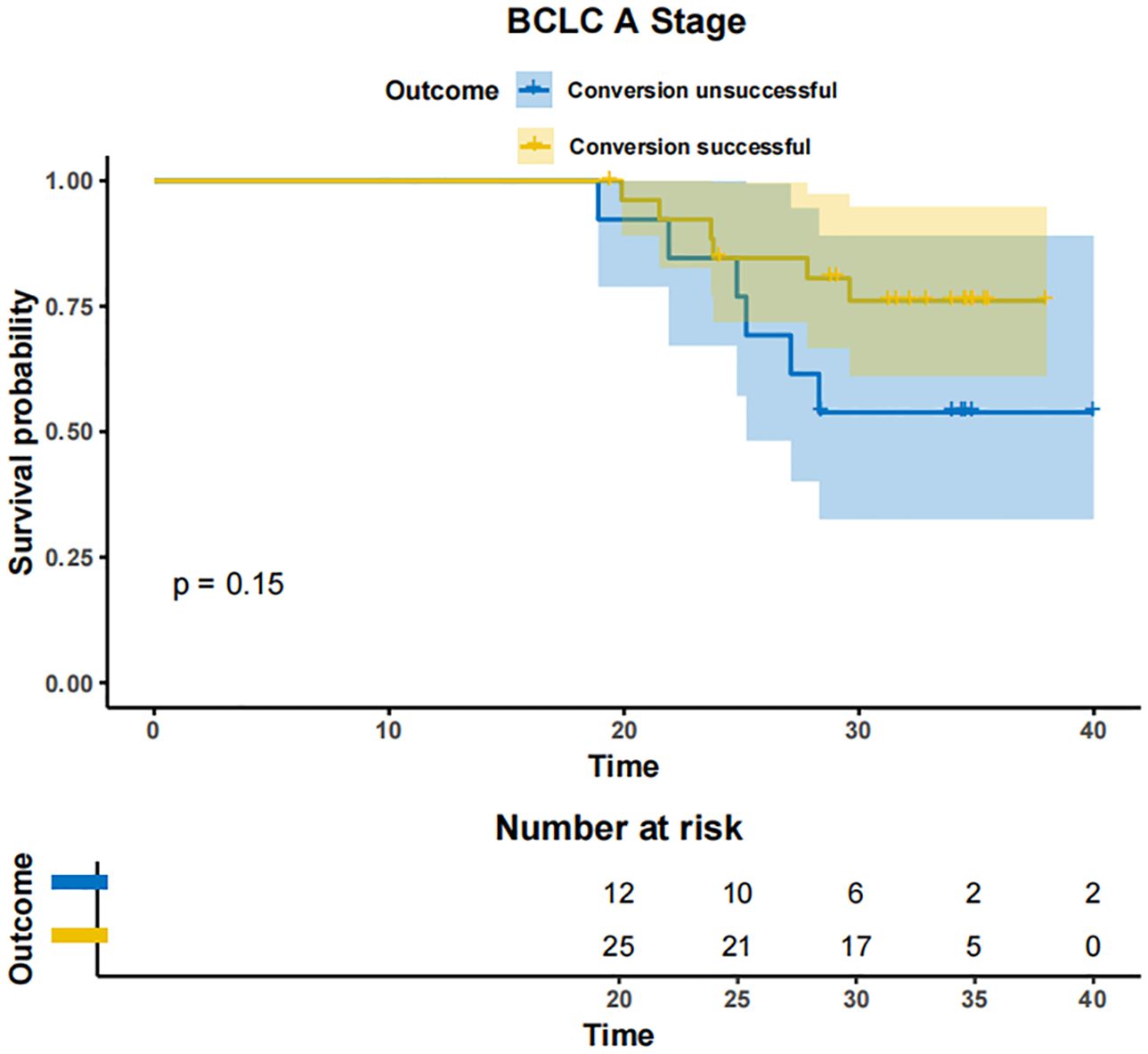
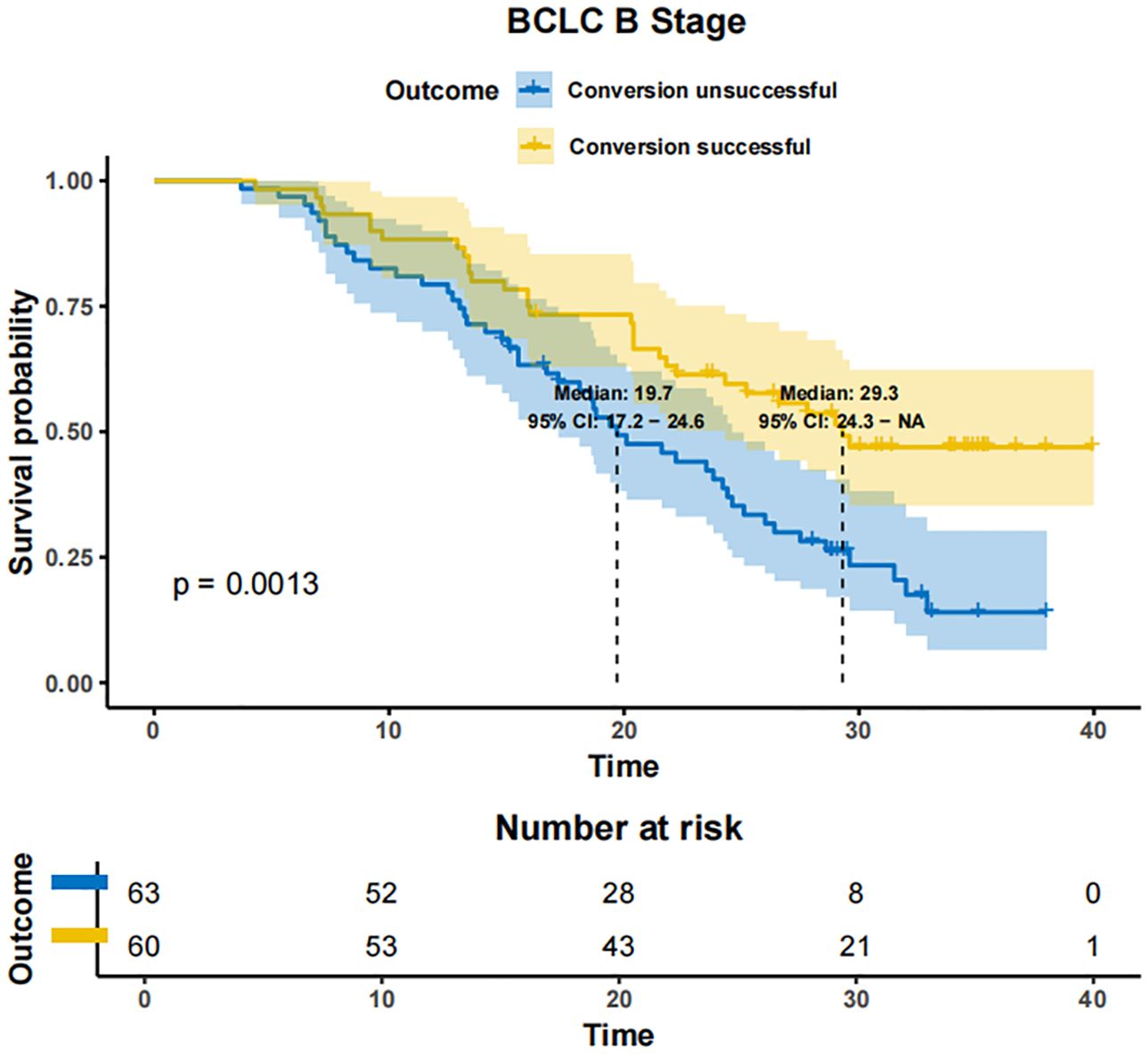
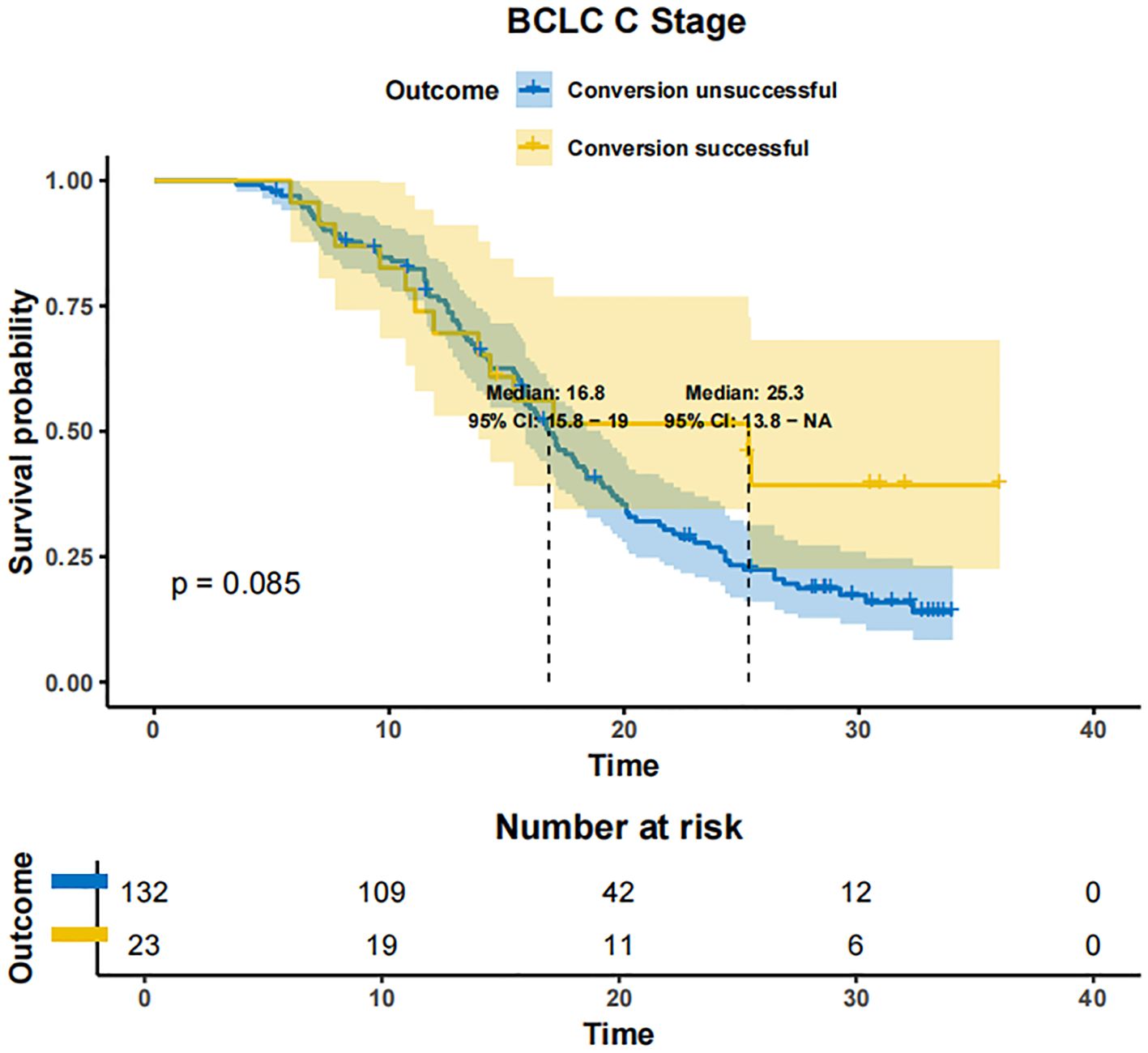
In patients with BCLC-A stage disease, the mOS was not reached in both the successfully converted treatment and non-conversion therapy groups, and no statistically significant difference was noted between the two groups according to the KM curve trend (P=0.15) (Figure 4). In contrast, in BCLC-B stage patients, the mOS was 29.3 months (95% CI: 24.3-NA) in the conversion therapy group (60 cases) and 19.7 months (95% CI: 17.2-24.6) in the non-conversion group (63 cases), with a significant difference between the two groups (P=0.0013) (Figure 5). In BCLC-C stage patients, the mOS was 25.3 months (95% CI: 13.8-NA) in the conversion therapy group (23 cases) and 16.8 months (95% CI: 15.8-19.0) in the non-conversion group (132 cases), with no statistically significant difference between the two groups (P=0.085) (Figure 6).
Univariate analysis identified four covariates associated with OS in patients, namely BCLC stage, the presence of distant metastasis, vascular invasion, and receipt of conversion therapy. These covariates were subsequently included in a multivariate Cox regression analysis using the direct entry method. The final results indicated that BCLC staging, presence of distant metastasis, and performance of conversion therapy are independent risk factors affecting OS (Table 5). A typical case is displayed in Figure 7.
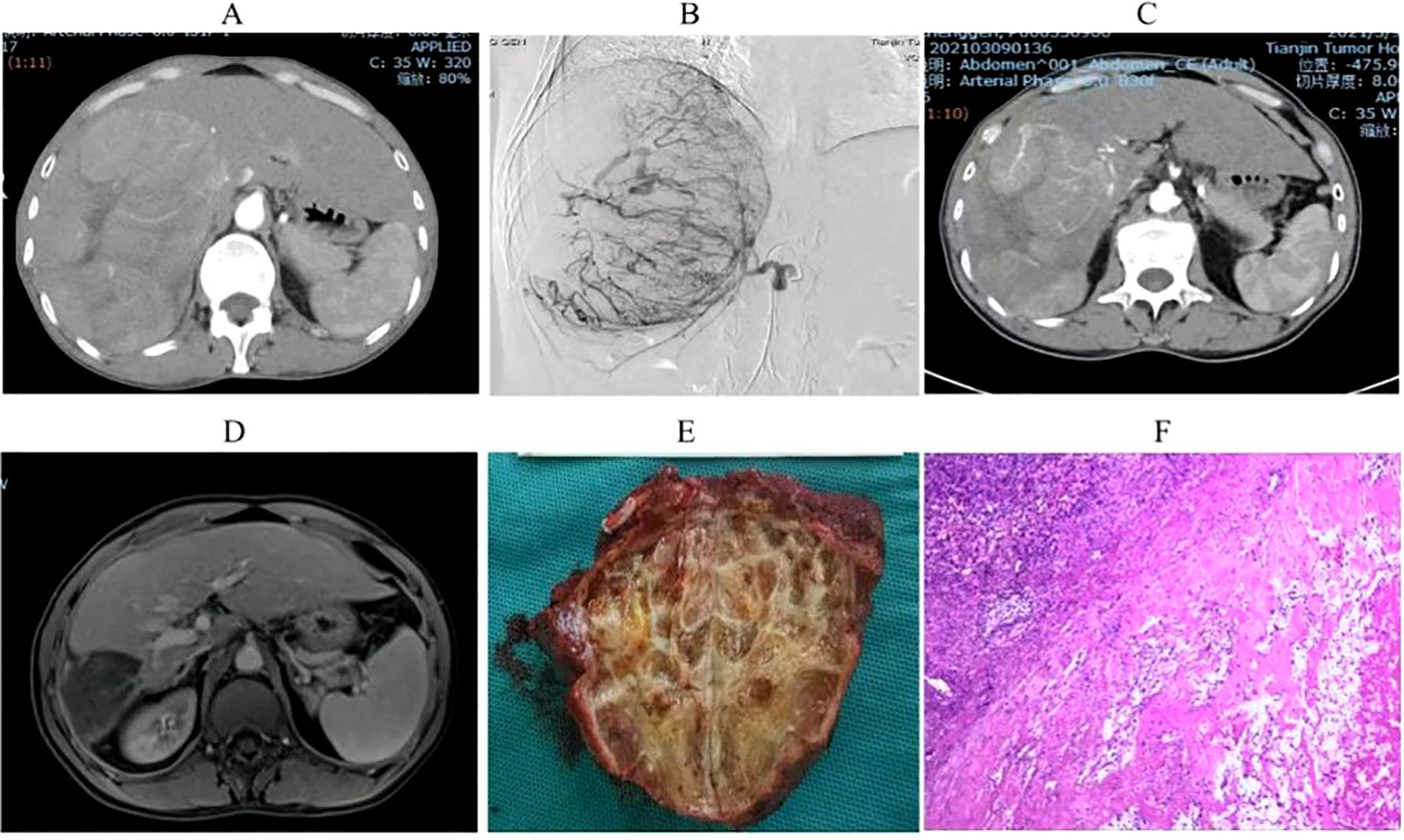
Figure 7. Imaging and pathological results of a uHCC patient who underwent conversion therapy following HAIC combined with lenvatinib and sintilimab. (A). A large HCC measuring 15 cm in size was observed in the right lobe of the liver. (B). Digital subtraction angiography (DSA) delineated a highly vascularized tumor. (C) Partial lesion shrinkage following two treatment cycles. (D) Significant reduction in tumor size after four treatment cycles. (E) Postoperative gross pathological specimens. (F). Postoperative pathological examination showed complete tumor necrosis without residual tumor.
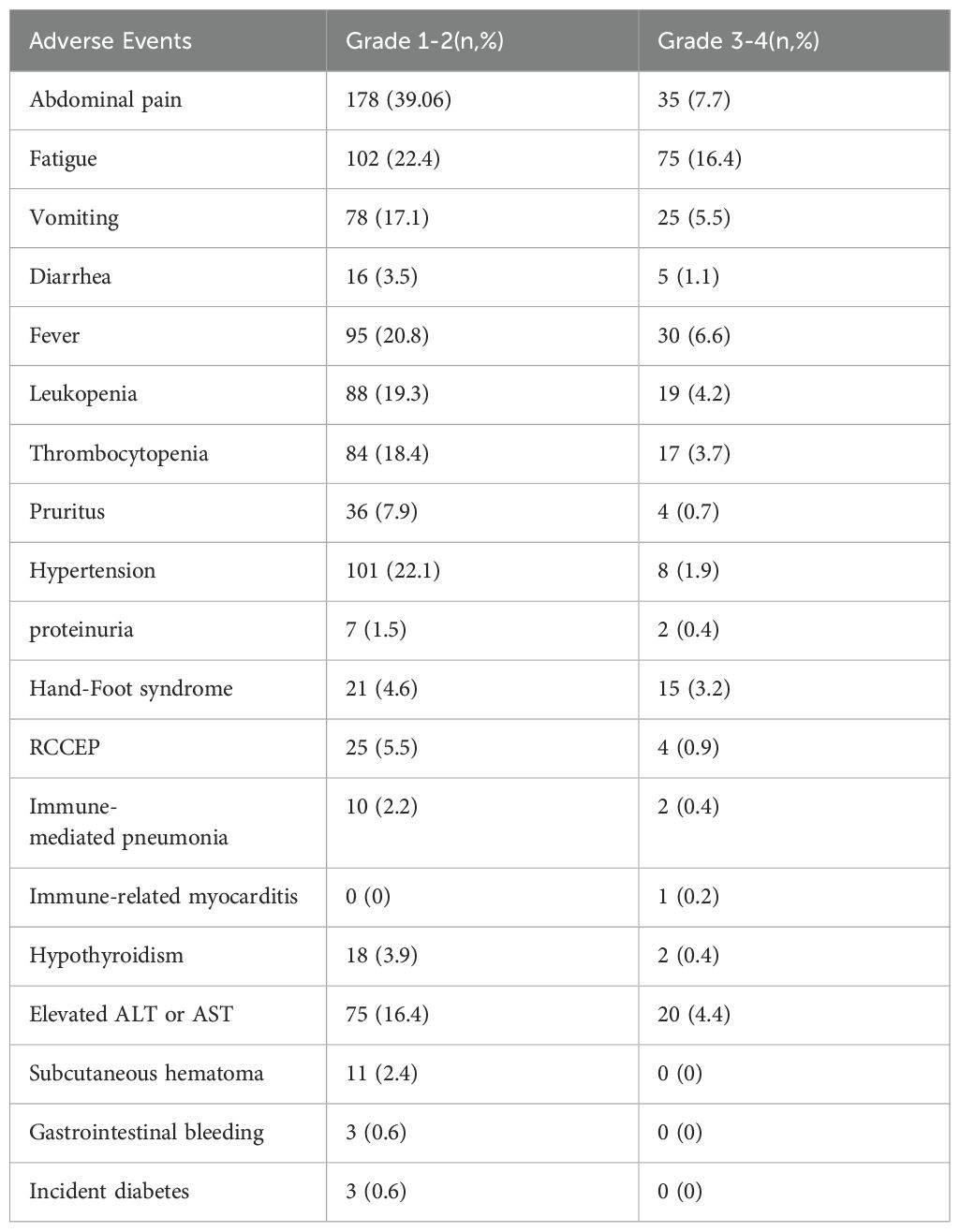
Adverse events
During the follow-up period, 456 treatment-related AEs were reported. Among these, 287 were categorized as grades 1-2 and 169 as grades 3-4, with no fatal complications. Common grade 1-2 AEs included abdominal pain, fatigue, and hypertension, while grade 3-4 AEs comprised fatigue, fever, and pain. Sixteen patients experienced hypothyroidism, among which two cases were severe but resolved after treatment with levothyroxine. Meanwhile, twenty-five patients developed reactive cutaneous capillary endothelial proliferation (RCCEP), with four requiring corticosteroid therapy. Eleven patients developed subcutaneous hematomas and three experienced gastrointestinal bleeding; all were successfully treated. Additionally, three patients developed new-onset diabetes mellitus, which was adequately managed with insulin therapy (Table 6).
Discussion
Surgical resection remains the primary treatment for early-stage HCC patients. However, over 50% of patients are diagnosed at intermediate to advanced stages, rendering them ineligible for surgical modalities. For these patients, systemic therapy remains the primary treatment approach (17). The 2024 Clinical Practice Guideline for Primary Liver Cancer recommends the following first-line systemic therapies: atezolizumab combined with bevacizumab, sintilimab combined with a bevacizumab biosimilar, and spatinib mesylate combined with camrelizumab. Additionally, lenvatinib, donafenib, sorafenib, and the FOLFOX4 chemotherapy regimen are extensively employed as first-line treatments (18). Despite significant advancements in the treatment of advanced HCC in recent years, challenges persist. The limited benefits of systemic therapies, accompanied by challenges such as drug resistance and disease progression, remain major obstacles for the majority of advanced HCC patients (19, 20).
Conversion therapy regimens are recommended to adopt multi-combination approaches and high-ORR treatment strategies while ensuring safety (3, 21). Recent studies observed that for patients with uHCC, the combination of systemic therapy with local treatments such as transarterial chemoembolization (TACE) and HAIC could further improve the ORR and conversion therapy rate (22). Notably, according to earlier studies, approximately half of patients with intermediate-to-advanced HCC may achieve conversion to surgical resection opportunities through these approaches (23, 24).
HAIC was initially recommended by Japanese guidelines as the standard treatment for HCC with portal vein tumor thrombosis (25). At present, the combination of HAIC with immunotherapy and targeted drugs has shown significant efficacy (15, 26). A retrospective study undertaken by Professor Shi Ming from the Sun Yat-sen University Cancer Center demonstrated that compared to lenvatinib alone, the triple therapy of FOLFOX-HAIC combined with toripalimab and lenvatinib significantly extended the PFS and OS of patients with advanced HCC and concurrently improved the ORR. Of note, 14.1% of patients in the triple therapy group achieved CR (27). A randomized controlled trial enrolling HCC patients with portal vein tumor thrombus compared the efficacy of combination therapy with HAIC and sorafenib versus sorafenib monotherapy. The results demonstrated that the combination therapy group achieved a significantly higher overall response rate (40.8% vs. 2.46%) and longer PFS (7.03 months vs. 2.6 months). Additionally, 12.8% of patients in the combination therapy group experienced tumor downstaging and underwent R0 resection, with 3 patients achieving a pathological complete response after treatment (28). The TRIPLET phase II study assessed the efficacy and safety of camrelizumab, apatinib, and HAIC-FOLFOX in BCLC stage C HCC patients. The treatment achieved an ORR of 77.1% (RECIST v1.1) and a median PFS of 10.38 months. More importantly, 17.1% of patients experienced downstaging, enabling curative therapies, including R0 resection in five patients and curative ablation in one (29). Similar to previous studies, this real-world study demonstrated an ORR of 47.1% and a DCR of 85.5%, with 34.6% of patients undergoing radical surgical resection and ablation. The median PFS was 11.4 months, and the median OS was 21.7 months, significantly improving patient prognosis. We posit that the synergistic effect of interventional and targeted/immunotherapy may involve mechanisms such as the activation of various antitumor immune cells or the suppression of immune cells with tumor-promoting activity following interventional procedure (30). The discrepancies between the findings of this study and previous research may attributed to the use of lenvatinib monotherapy as the targeted agent in this study, with no restriction placed on the types of immunotherapeutic agents.
HAIC combined with systemic therapy demonstrates the ability to rapidly reduce tumor size and control intrahepatic lesions while exerting a relatively minor impact on liver function (31, 32). In certain cases, it may even enhance liver function reserve following a reduction in tumor burden (33). Furthermore, HAIC is associated with a diminished tissue inflammatory response and reduced adhesion post-surgery, thereby lowering surgical risks (34). Currently, several prospective clinical studies (NCT04961918, NCT05029973, NCT04947826, and NCT05003700) are underway to assess the efficacy of HAIC in conjunction with anti-PD-1/PD-L1 immunotherapy and molecular targeted therapy for advanced HCC, as well as the effectiveness of conversion therapy. We anticipate that their results will provide higher-level evidence-based medical evidence for the effectiveness of combined therapy (35).
Herein, among the 110 patients who underwent conversion therapy, the majority were in the BCLC-B stage. Further analysis unveiled that the mOS was 29.3 months in BCLC-B patients in the conversion group compared to 19.7 months in the non-conversion group, with a statistically significant difference. On the other hand, among BCLC-C patients, 23 underwent conversion surgery, and the remaining 132 patients did not undergo conversion surgery. While the mOS was 25.3 months in the conversion group and 16.8 months in the non-conversion group, the difference was not statistically significant, likely due to the limited sample size of converted patients in stage C. These findings collectively suggest that conversion therapy should be promptly initiated in BCLC-B patients if they meet the eligibility criteria (36). In comparison, conversion therapy should be approached with caution in BCLC-C patients, considering that the primary objective should extend beyond surgical resection to the development of individualized treatment strategies based on the patient’s overall condition.
In this study, almost all patients experienced at least one side effect, with 169 patients experiencing grade 3-4 AEs. Fatigue, fever, and pain were the most common grade 3-4 AEs. Three patients developed gastrointestinal bleeding, which was successfully managed with endoscopic hemostasis. The incidence of grade 3 and 4 AEs in this study was markedly higher than in previous studies, potentially due to the inclusion of adverse reactions associated with infusion chemotherapy (37, 38). Although these adverse reactions were classified as higher grades according to the CTCAE 4.0 criteria, they were largely manageable and minimally impacted patients’ overall prognosis and quality of life (39). Despite the high incidence of common AEs, they were generally effectively managed.
Nevertheless, some limitations of this retrospective study cannot be overlooked. To begin, the application of HAIC combined with immunotherapy and lenvatinib in clinical practice is relatively recent, resulting in limited overall follow-up time and insufficient clinical evidence for recommendations. Secondly, the retrospective design of this study inevitably introduced biases-even though we mitigated these through MDT-guided inclusion/exclusion criteria, independent dual data extraction with senior adjudication, multivariate regression adjustment for known prognostic factors, and BCLC-stratified subgroup analyses. Despite these limitations, HAIC-based combination therapy has demonstrated preliminary efficacy and favorable tolerability, showing promising prospects (40). Nonetheless, further prospective, multicenter clinical studies are warranted to identify patient populations that may derive benefits from this therapy, optimize treatment strategies, and refine treatment plans.
HAIC, combined with lenvatinib and immunotherapy, has demonstrated a high ORR in patients with uHCC. Besides, this combination reduced tumor burden, offered a favorable safety profile, increased the conversion therapy rate, and prolonged both OS and PFS. Finally, BCLC staging significantly impacted the likelihood of successful conversion therapy, with stage B patients deriving substantial survival benefits post-conversion.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Tianjin Medical University Cancer Institute andHospital Review Board (No.: bc2020099). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
WZ: Conceptualization, Writing – original draft, Data curation, Writing – review & editing. XZ: Data curation, Writing – original draft. WG: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. TS: Conceptualization, Data curation, Funding acquisition, Writing – original draft. QZ: Writing – original draft, Conceptualization. XY: Methodology, Writing – original draft, Writing – review & editing. WX: Validation, Visualization, Writing – original draft. HY: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Scientific Research Project of Tianjin Education Commission (2023KJ090) and funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HAIC, hepatic arterial infusion chemotherapy; OS, Overall survival; PFS, Progression-free survival; uHCC, unresectable hepatocellular carcinoma; AE, adverse event; PVTT, portal vein tumour thrombosis; AFP, alpha-fetoprotein; mRECIST, modified Response Evaluation Criteria in Solid Tumors; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization; CR, complete response; PR, partial response; DCR, disease control rate; SD, stable disease; TKI, tyrosine kinase inhibitor.
References
1. Singal AG, Kanwal F, and Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. (2023) 20:864–84. doi: 10.1038/s41571-023-00825-3
2. Vogel A, Meyer T, Sapisochin G, Salem R, and Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
3. Vitale A, Cabibbo G, Iavarone M, Viganò L, Pinato DJ, Ponziani FR, et al. Personalised management of patients with hepatocellular carcinoma: a multiparametric therapeutic hierarchy concept. Lancet Oncol. (2023) 24:e312–22. doi: 10.1016/S1470-2045(23)00186-9
4. Sun HC and Zhu XD. Downstaging conversion therapy in patients with initially unresectable advanced hepatocellular carcinoma: an overview. Front Oncol. (2021) 11:772195. doi: 10.3389/fonc.2021.772195
5. Xu H, Zhang H, Li B, Chen K, and Wei Y. Systemic conversion therapies for initially unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Cancer. (2024) 24:1008. doi: 10.1186/s12885-024-12772-y
6. Haber PK, Puigvehí M, Castet F, Lourdusamy V, Montal R, Tabrizian P, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002-2020). Gastroenterology. (2021) 161:879–98. doi: 10.1053/j.gastro.2021.06.008
7. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
8. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
9. Arita J, Ichida A, Nagata R, Mihara Y, Kawaguchi Y, Ishizawa T, et al. Conversion surgery after preoperative therapy for advanced hepatocellular carcinoma in the era of molecular targeted therapy and immune checkpoint inhibitors. J Hepatobiliary Pancreat Sci. (2022) 29:732–40. doi: 10.1002/jhbp.1135
10. Wang MD, Xu XJ, Wang KC, Diao YK, Xu JH, Gu LH, et al. Conversion therapy for advanced hepatocellular carcinoma in the era of precision medicine: Current status, challenges and opportunities. Cancer Sci. (2024) 115:2159–69. doi: 10.1111/cas.16194
11. Iwamoto H, Shimose S, Shirono T, Niizeki T, and Kawaguchi T. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in the era of chemo-diversity. Clin Mol Hepatol. (2023) 29:593–604. doi: 10.3350/cmh.2022.0391
12. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2021) 18:293–313. doi: 10.1038/s41575-020-00395-0
13. Ai H, Gong T, Ma Y, Ma G, Ding W, Ding W, et al. Transarterial chemoembolization combined with atezolizumab plus bevacizumab conversion therapy for intermediate-stage hepatocellular carcinoma: a case report and literature review. Front Immunol. (2024) 15:1358602. doi: 10.3389/fimmu.2024.1358602
14. Zhang W, Zhang K, Liu C, Gao W, Si T, Zou Q, et al. Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: a real world study. Front Immunol. (2023) 14:1127349. doi: 10.3389/fimmu.2023.1127349
15. He M, Liu S, Lai Z, Du Z, Li Q, Xu L, et al. Hepatic arterial infusion chemotherapy for patients with hepatocellular carcinoma: Applicability in Western countries. Curr Opin Pharmacol. (2023) 70:102362. doi: 10.1016/j.coph.2023.102362
16. Llovet JM and Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. (2020) 72:288–306. doi: 10.1016/j.jhep.2019.09.026
17. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J Hepatol. (2024) 82(2):315–374. doi: 10.1016/j.jhep.2024.08.028
18. Department of Medical Administration NHCotPRoC. Clinical practice guideline for primary liver cancer(2024 edition). Med J Peking Union Med Coll Hosp. (2024) 15:532–59. doi: 10.12290/xhyxzz.2024-0304
19. Llovet JM, Pinyol R, Kelley RK, El-Khoueiry A, Reeves HL, Wang XW, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer. (2022) 3:386–401. doi: 10.1038/s43018-022-00357-2
20. Donne R and Lujambio A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology. (2023) 77:1773–96. doi: 10.1002/hep.32740
21. Long F, Chen S, Li R, Lin Y, Han J, Guo J, et al. Efficacy and safety of HAIC alone vs. HAIC combined with lenvatinib for treatment of advanced hepatocellular carcinoma. Med Oncol. (2023) 40:147. doi: 10.1007/s12032-023-02012-x
22. Singal AG, Kudo M, and Bruix J. Breakthroughs in hepatocellular carcinoma therapies. Clin Gastroenterol Hepatol. (2023) 21:2135–49. doi: 10.1016/j.cgh.2023.01.039
23. Zhang W, Tong S, Hu B, Wan T, Tang H, Zhao F, et al. Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J Immunother Cancer. (2023) 11:e007366. doi: 10.1136/jitc-2023-007366
24. Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. (2022) 11:227–52. doi: 10.21037/hbsn-21-328
25. Yamashita T. Current status of hepatocellular carcinoma treatment in Japan: hepatic arterial infusion chemotherapy. Clin Drug Investig. (2012) 32 Suppl 2:15–23. doi: 10.1007/BF03265493
26. Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. (2021) 11:618206. doi: 10.3389/fonc.2021.618206
27. Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur J Cancer. (2022) 174:68–77. doi: 10.1016/j.ejca.2022.07.005
28. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol. (2019) 5:953–60. doi: 10.1001/jamaoncol.2019.0250
29. Zhang TQ, Geng ZJ, Zuo MX, Li JB, Huang JH, Huang ZL, et al. Camrelizumab (a PD-1 inhibitor) plus apatinib (an VEGFR-2 inhibitor) and hepatic artery infusion chemotherapy for hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage C (TRIPLET): a phase II study. Signal Transduct Target Ther. (2023) 8:413. doi: 10.1038/s41392-023-01663-6
30. Kuo LF, Liu WC, Li MF, Huang FH, Chou CK, Chen TH, et al. Prognostic evaluation of conversion therapy following hepatic arterial infusion chemotherapy or immunotherapy in patients with advanced or transarterial chemoembolization unsuitable intermediate-stage hepatocellular carcinoma: A retrospective cohort study. Oncology. (2024) 1–13. doi: 10.1159/000542291
31. Yu B, Zhang N, Feng Y, Zhang Y, Zhang T, and Wang L. Hepatectomy after conversion therapy with hepatic arterial infusion chemotherapy, tyrosine kinase inhibitors and anti-PD-1 antibodies for initially unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. (2023) 10:1709–21. doi: 10.2147/JHC.S432062
32. Pang B, Zuo B, Huang L, You X, Liu T, Hao J, et al. Real-world efficacy and safety of TACE-HAIC combined with TKIs and PD-1 inhibitors in initially unresectable hepatocellular carcinoma. Int Immunopharmacol. (2024) 137:112492. doi: 10.1016/j.intimp.2024.112492
33. Ishii M, Itano O, Iwamoto H, Hibi T, and Itano S. Efficacy and safety of arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma and child-pugh class B: A retrospective cohort study. Oncology. (2022) 100:278–89. doi: 10.1159/000523703
34. Xiao Y, Deng W, Luo L, Zhu G, Xie J, Liu Y, et al. Beneficial effects of maintaining liver function during hepatic arterial infusion chemotherapy combined with tyrosine kinase and programmed cell death protein-1 inhibitors on the outcomes of patients with unresectable hepatocellular carcinoma. BMC Cancer. (2024) 24:588. doi: 10.1186/s12885-024-12355-x
35. Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: A biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol. (2022) 40:468–80. doi: 10.1200/JCO.21.01963
36. Tomonari T, Tani J, Sato Y, Tanaka H, Tanaka T, Taniguchi T, et al. Clinical features and outcomes of conversion therapy in patients with unresectable hepatocellular carcinoma. Cancers (Basel). (2023) 15:5221. doi: 10.3390/cancers15215221
37. Xin Y, Cao F, Yang H, Zhang X, Chen Y, Cao X, et al. Efficacy and safety of atezolizumab plus bevacizumab combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Front Immunol. (2022) 13:929141. doi: 10.3389/fimmu.2022.929141
38. Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang L, et al. Surgical conversion for initially unresectable locally advanced hepatocellular carcinoma using a triple combination of angiogenesis inhibitors, anti-PD-1 antibodies, and hepatic arterial infusion chemotherapy: A retrospective study. Front Oncol. (2021) 11:729764. doi: 10.3389/fonc.2021.729764
39. Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. (2012) 67:1025–39. doi: 10.1016/j.jaad.2012.02.010
40. Zang M, Hu X, Yuan G, Li R, Li W, Pang H, et al. Tyrosine kinase inhibitors, immune checkpoint inhibitors combined with hepatic arterial infusion of oxaliplatin and raltitrexed versus oxaliplatin, 5-fluorouracil and leucovorin for intermediate and advanced hepatocellular carcinoma: A retrospective study. Int Immunopharmacol. (2023) 125:111019. doi: 10.1016/j.intimp.2023.111019
Keywords: unresectable hepatocellular carcinoma, hepatic arterial infusion chemotherapy, lenvatinib, immunotherapy, conversion
Citation: Zhang W, Zhao X, Gao W, Si T, Zou Q, Yang X, Xing W and Yu H (2025) Conversion study of hepatocellular carcinoma using HAIC combined with lenvatinib and PD-1/L1 immunotherapy under the guidance of BCLC staging. Front. Immunol. 16:1596864. doi: 10.3389/fimmu.2025.1596864
Received: 20 March 2025; Accepted: 12 May 2025;
Published: 02 June 2025.
Edited by:
Tian Yang, First Affiliated Hospital of Jilin University, ChinaReviewed by:
André Mauricio De Oliveira, Federal Center for Technological Education of Minas Gerais, BrazilQiang Tu, Jiangxi Cancer Hospital, China
Koyel Kar, BCDA College of Pharmacy and Technology, India
Copyright © 2025 Zhang, Zhao, Gao, Si, Zou, Yang, Xing and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haipeng Yu, eXVoYWlwZW5nQHRqbXVjaC5jb20=
†These authors have contributed equally to this work and share first authorship
 Weihao Zhang
Weihao Zhang Xiaohui Zhao
Xiaohui Zhao Wei Gao
Wei Gao Tongguo Si1,2,3
Tongguo Si1,2,3 Xueling Yang
Xueling Yang Wenge Xing
Wenge Xing