- 1Department of Hematology, Liaoning Medical Center for Hematopoietic Stem Cell Transplantation, Second Hospital of Dalian Medical University, Dalian, China
- 2Department of Pharmacy, Second Affiliated Hospital of Dalian Medical University, Dalian, China
- 3Department of Hematology, Shanghai Ninth People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
- 4Liaoning Key Laboratory of Hematopoietic Stem Cell Transplantation and Translational Medicine, Dalian Key Laboratory of Hematology, Diamond Bay Institute of Hematology, Blood Stem Cell Transplantation Institute, Second Hospital of Dalian Medical University, Dalian, China
- 5Department of Hematology, Yingkou People’s Hospital, Yingkou, Liaoning, China
Introduction: Despite the increasing use of allogeneic hematopoietic stem cell transplantation (allo-HSCT), graft-versus-host disease (GVHD) remains the main cause of morbidity and mortality, significantly impacting HSCT outcomes. Steroids are the standard first-line treatment for acute GVHD (aGVHD); however, standardized treatment algorithms for patients who do not respond to steroid therapy are lacking. Ruxolitinib is the most promising second-line therapy for steroid-refractory (SR)-GVHD, but data on its first-line use for aGVHD are limited.
Methods: In this retrospective study, we analyzed the data of 133 patients with aGVHD who underwent transplantation at our institution. Eighty-three patients received ruxolitinib combined with methylprednisolone, while 50 received methylprednisolone alone as the initial treatment.
Results: The ruxolitinib/steroids group had a significantly higher overall response rate (ORR) on day 7 (86%) compared to the steroid-only group (68%; odds ratio [OR]=2.8, 95% confidence interval [CI]: 1.2–6.5, p=0.019). Similarly, ORR on day 14 was higher in the ruxolitinib/steroids group (92% vs. 79%; OR=2.7, 95% CI: 0.9–7.8, p=0.05). Although no statistical differences were observed in overall survival (OS), progression-free survival (PFS), and failure-free survival (FFS) between the two groups, patients who achieved early ORR on days 7 and 14 had better OS, PFS, and FFS. Additionally, in subgroup analysis of patients who underwent peripheral blood stem cell transplantations, the ruxolitinib/steroids cohort had significantly better OS (Hazard Ratio [HR]=0.34, 95% CI: 0.11–1.55, p=0.04), PFS (HR=0.37; 95% CI: 0.12–1.10, p=0.05) and FFS (HR=0.46; 95% CI: 0.19–1.11, p=0.05) compared to the steroid-only cohort. Adverse event (AEs) frequencies were comparable between groups, with the exception of neutropenia (32.5% vs. 12%, p=0.008) and CMV infection (34.9% vs. 18%, p=0.036), which were more frequent in the ruxolitinib/steroid group.
Discussion: To the best of our knowledge, this is the first real-world study to demonstrate that adding ruxolitinib to a standard methylprednisolone regimen provides an effective and safe first-line treatment for aGVHD.
1 Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a vital curative treatment modality for various malignant and benign hematologic diseases. Acute myeloid leukemia, myelodysplastic syndrome/myeloproliferative disorders, and acute lymphoblastic leukemia are the most common indications for allo-HSCT (1–3). Acute graft-versus-host disease (aGVHD) develops in 50–70% of patients following allo-HSCT with conventional prophylaxis and is a major cause of non-relapse mortality (NRM) in patients receiving transplantation, posing a significant challenge to successful transplant outcomes (4, 5).
The etiology of aGVHD is complex, and its pathophysiology can be divided into three sequential phases: (I) activation of host antigen-presenting cells (APCs); (II) activation of donor T cells, leading to their clonal expansion and differentiation; and (III) destruction of target tissue by inflammatory effector cells and cytokines, affecting multiple organs, particularly the skin, gastrointestinal tract, and liver (6–9). Human leukocyte antigen (HLA) mismatch represents the most important risk factor for aGVHD. Other risk factors include donor-recipient sex disparity, conditioning regimen intensity, underlying disease, aging, multiparous female donors, graft source, and insufficient GVHD prophylaxis (10, 11). The standard first-line treatment for aGVHD is high-dose glucocorticoids (12–14). However, approximately 50% of patients become steroid-resistant or refractory (SR), resulting in poor long-term prognosis, with an estimated NRM rate of 40% within 12 months (15, 16). To date, no consensus has been established regarding the optimal management of SR-aGVHD; however, the FDA approved ruxolitinib for SR-aGVHD in 2019, marking a significant step toward standardizing its initial treatment (17).
Ruxolitinib is an orally administered selective inhibitor of Janus kinase (JAK)1/2. JAKs are intracellular tyrosine kinases, crucial for the development and function of immune cells, and are implicated in aGVHD pathogenesis (18). Ruxolitinib-induced immunomodulation is hypothesized to involve reduced neutrophil migration during the first phase of aGVHD, decreased T-cell priming via MHC-II downregulation, reduced cytokine release during the second phase, and limited T-cell expansion in the third phase (19). Several retrospective clinical studies of ruxolitinib, as salvage therapy for SR-aGVHD, have demonstrated its clinical benefits (20, 21). The FDA approval of ruxolitinib for SR-aGVHD was based on the results of REACH1, an open-label, single-arm phase II trial that enrolled subjects from December 2016 to July 2018 (17, 22).
Studies on ruxolitinib as a first-line treatment for aGVHD are limited. Researchers from China performed a multicenter, randomized, phase III trial to evaluate the efficacy and safety of ruxolitinib plus steroids for aGVHD. The study demonstrated that adding ruxolitinib to the standard methylprednisolone regimen provided an effective and safe first-line treatment for newly diagnosed high-risk aGVHD (23). We conducted a retrospective, real-world study to investigate the therapeutic efficacy and safety of ruxolitinib combined with methylprednisolone as a first-line treatment for aGVHD.
2 Materials and methods
2.1 Patients
A retrospective analysis was conducted to evaluate the treatment outcomes of 133 patients who underwent allo-HSCT and were diagnosed with aGVHD at our center between August 2014 and June 2023. The underlying diseases included aplastic anemia (AA), acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), myelodysplastic syndrome (MDS), and congenital hemophagocytic syndrome. The marrow transplant databases of the Second Affiliated Hospital of Dalian Medical University in China were screened for aGVHD after allo-HSCT diagnosis. The patient outcomes were monitored until the end of June 2024. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Dalian Medical University.
2.2 Allo-HSCT procedure
All 133 patients underwent myeloablative (MA) conditioning regimen transplantation, except for those with AA. This included the treatment of acute leukemia and MDS based on the Beijing protocol, which utilized a modified BU/CY conditioning regimen as follows: cytarabine (2 g/m2 q12h for 2 days; qd for fully matched donors), busulfan (3.2 mg/kg/day for 4 days), cyclophosphamide (1.8 g/m2 for 3 days), and anti-thymocyte globulin (ATG) (2.5 mg/kg/day for 4 days; 2 days for fully matched donors) (24). Enhanced MA regimens, including decitabine, idarubicin, and clarithromycin, were administered according to the risk of primary disease and disease status before transplantation (25). For AA, the FLU/CY conditioning regimen, combined with ATG to suppress T cells, involved the administration of 30 mg/m2 fludarabine for 4 days, 30–50 mg/kg cyclophosphamide for 2 days, and 2.5 mg/kg/d ATG for 4 days (26). Most transplantation recipients received ATG, cyclosporine A (CsA)/Tacrolimus (FK506), mycophenolate mofetil, and short-term methotrexate for GVHD prophylaxis.
2.3 Study design
The grading and staging systems of aGVHD were based on the Glucksberg or Mount Sinai aGVHD International Consortium (MAGIC) criteria (27, 28). Individuals were divided into two treatment groups. In the ruxolitinib/steroids combination group, patients were given methylprednisolone at a dose of 0.5–2 mg/kg/day, and ruxolitinib was administered orally at a daily dose of 10–15 mg. If patients with aGVHD responded to treatment by achieving partial response (PR) or complete response (CR) within 7 days, the methylprednisolone dosage was gradually tapered. If GVHD did not recur after steroid discontinuation, the ruxolitinib dose was gradually reduced. In the steroid-only group, patients were given methylprednisolone at a dose of 0.5–2 mg/kg/day, which was gradually tapered and discontinued after CR. The initial steroid dosage for both groups was determined based on the severity of the aGVHD. For mild patients (grade I and II aGVHD, only involving the upper digestive tract), the initial dosage was 0.5 mg/kg. For grade III/IV aGVHD involving the gastrointestinal tract or hyperacuteGVHD, the initial dosage of methylprednisolone was 2 mg/kg. For most grade II-III aGVHD, the dosage was 1 mg/kg. The steroid dosage in both groups were basically balanced. According to guidelines and relevant literature (12), during the treatment of aGVHD, both groups of CNI were adjusted to effective therapeutic concentrations (cyclosporine trough concentration to 150-250ng/ml, FK506 5-15ng/ml). After aGVHD achieves CR, stop steroid first, and then tappered gradually and eventually discontinued CNI. The CNI reduction plan for both groups is the same. Second-line therapy was initiated in both groups for patients with refractory aGVHD, defined as GVHD progression after 3 days of treatment, lack of improvement within 7 days, or failure to achieve CR after 14 days.
2.4 Endpoints
The primary endpoint was the overall response rate (ORR) to aGVHD treatment at 3, 7, 14, and 28 days post-intervention. ORR was defined as the percentage of patients in each group who attained either PR or CR without requiring additional immunosuppressive agents. CR was characterized by the complete resolution of aGVHD symptoms, whereas PR was defined as an improvement in at least one stage in a single organ without worsening in others. No response (NR) was classified as no improvement, worsening symptoms in any organ, or the emergence of new GVHD-related symptoms. Additionally, GVHD progression after 3 days of therapy or lack of improvement within 7 days was considered NR. Secondary endpoints included overall survival (OS), progression-free survival (PFS), failure-free survival (FFS), cumulative incidence of relapse (CIR), NRM, and safety. OS was defined as the time from aGVHD onset to death from any cause. PFS was defined as the time from aGVHD onset to relapse of the primary disease or death from any cause, whichever occurred first. Failure-free survival (FFS) refers to the time from aGVHD onset to disease relapse or progression, NRM, or the initiation of additional therapy for aGVHD. Safety endpoints were assessed based on the frequency of adverse events (AEs), defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE 4.0).
2.5 Statistical analyses
Data were analyzed using STATA/SE 15.1 software (STATA Corp, College Station, Texas, USA). ORR, CR, and PR were analyzed using Pearson’s chi-square test. The correlation between ORR and different independent variables was calculated using regression analysis. The Kaplan-Meier method was used to estimate OS, PFS and FFS, and the Log-rank test was used to determine statistical significance. NRM and cumulative incidence of relapse were estimated by considering each other as competing risks. The reverse Kaplan-Meier method was used to calculate the median follow-up duration. Prognostic variables for OS were evaluated by univariate and multivariate analyses using Cox proportional hazard regression. Variables with statistical significance (p <0.1) in the univariate analysis were included in the multivariate analysis to adjust for potential confounding effects. A p-value <0.05 was considered statistically significant. The following variables were evaluated: sex, age at allo-HSCT (<60 years vs. ≥60 years), donor sex (female donor to male recipient vs. others), disease type (malignant vs. benign), donor type (cord blood, CBT vs. haplo-identical donor, HID vs. matched sibling donor, MSD vs. unrelated donor, URD), HLA type (fully match vs. haplo), blood type (match vs. mismatch), graft origin (CBT vs. PB+BM+CB vs. PB+BM vs. PB), hematopoietic cell transplant-comorbidity index (HCT-CI: 2 vs. 1 vs. 0), aGVHD grade (IV vs. III vs. II vs. I), aGVHD skin involvement (yes vs. no), aGVHD GI involvement (yes vs. no), aGVHD liver involvement (yes vs. no), day 3 ORR (yes vs. no), day 7 ORR (yes vs. no), day 14 ORR (yes vs. no), and day 28 ORR (yes vs. no). Pearson’s chi-square test was used to analyze AEs between different groups, including neutropenia, thrombocytopenia, cytomegalovirus (CMV) infection, Epstein-Barr virus (EBV) infection, renal toxicity, cardiac toxicity, digestive reactions, nerve toxicity, electrolyte disturbance, blood glucose disturbance, blood lipid disturbance, and hypertension.
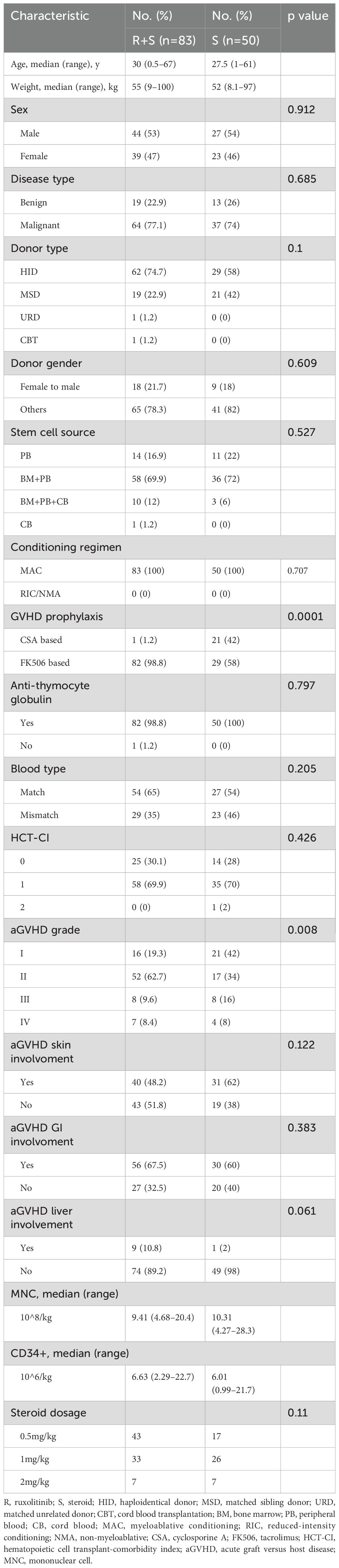
3 Results
3.1 Patient and treatment characteristics
The patient demographics and baseline disease characteristics are presented in Table 1. This study included a cohort of 133 patients with aGVHD after allo-HSCT. Of these, 83 patients were classified into the ruxolitinib/steroid group, while 50 patients were in steroid-only group. The overall median age was 30 (0.5–67) years in the ruxolitinib/steroid group and 27.5 (1–61) years in the steroid-only group. The median body weight was 55 (9–100) kg vs. 52 (8.1–97) kg, respectively. The median number of infused mononuclear cells (MNC) was 9.41 × 10^8/kg (range: 4.68–20.4) in the ruxolitinib/steroid group and 10.31×10^8/kg (range: 4.27–28.3) in the steroid-only group. The median number of infused CD34+ cells was 6.63×10^6/kg (range: 2.29–22.7) and 6.01×10^6/kg (range: 0.99–21.7), respectively. The baseline demographic, transplantation-related, and disease-related patient characteristics-including recipient sex, disease type, donor type, donor sex, stem cell source, conditioning regimen intensity, recipient/donor blood type pair, GVHD prophylaxis, ATG use, HCI-CI, aGVHD skin involvement, aGVHD GI involvement, and aGVHD liver involvement-were comparable between the two groups. All patients underwent neutrophil engraftment, with no significant difference in the time to neutrophil or platelet engraftment between the two groups. Notably, grade I aGVHD was more common in the steroid-only group, while grade II aGVHD was more frequent in the ruxolitinib/steroids group (p=0.008).
3.2 Primary endpoint
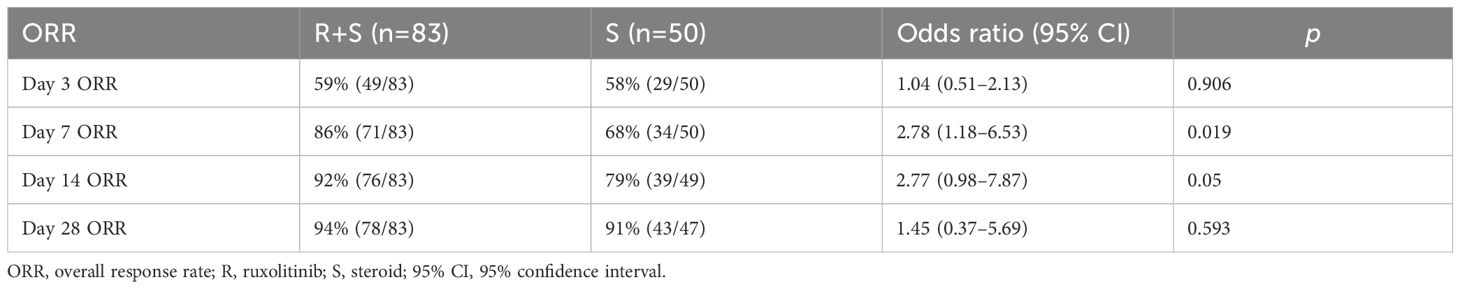
The primary endpoint was ORR at 3, 7, 14 and 28 days post-intervention. As shown in Figure 1A and Table 2, ORR on day 3 was comparable between the two groups (59% vs 58%; odds ratio [OR]=1.04, 95% confidence interval [CI]: 0.51–2.13, p=0.906). On day 7, ORR was significantly higher in the ruxolitinib/steroids combination group (86%) compared to the steroid-only group (68%; OR=2.78, 95% CI: 1.18–6.53 p=0.019; Figure 1B, Table 2). Similarly, ORR on day 14 was significantly greater in the ruxolitinib/steroids combination group (92%) compared to the steroid-only group (79%; OR=2.77, 95% CI: 0.98–7.87, p=0.05; Figure 1C, Table 2). The ruxolitinib/steroids combination cohort showed similar ORR on day 28 compared with the steroid-only cohort (94% vs 91%; OR=1.45, 95% CI: 0.37–5.69, p=0.593; Figure 1D, Table 2).
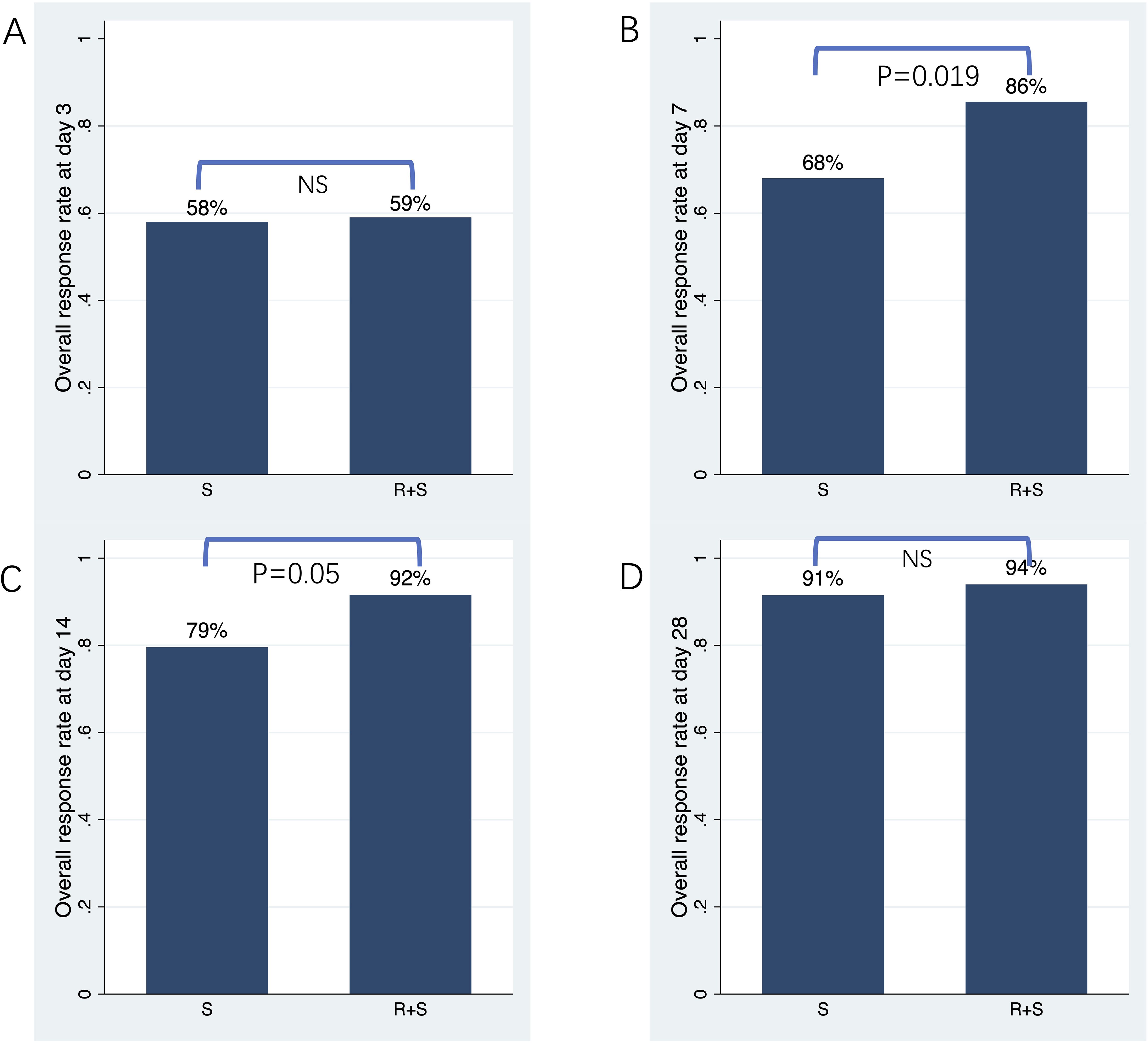
Figure 1. Overall response rate on days 3, 7, 14 and 28 post-treatment. (A) Overall response rate on day 3. (B) Overall response rate on day 7. (C) Overall response rate on day 14. (D) Overall response rate on day 28.
3.3 Secondary endpoint
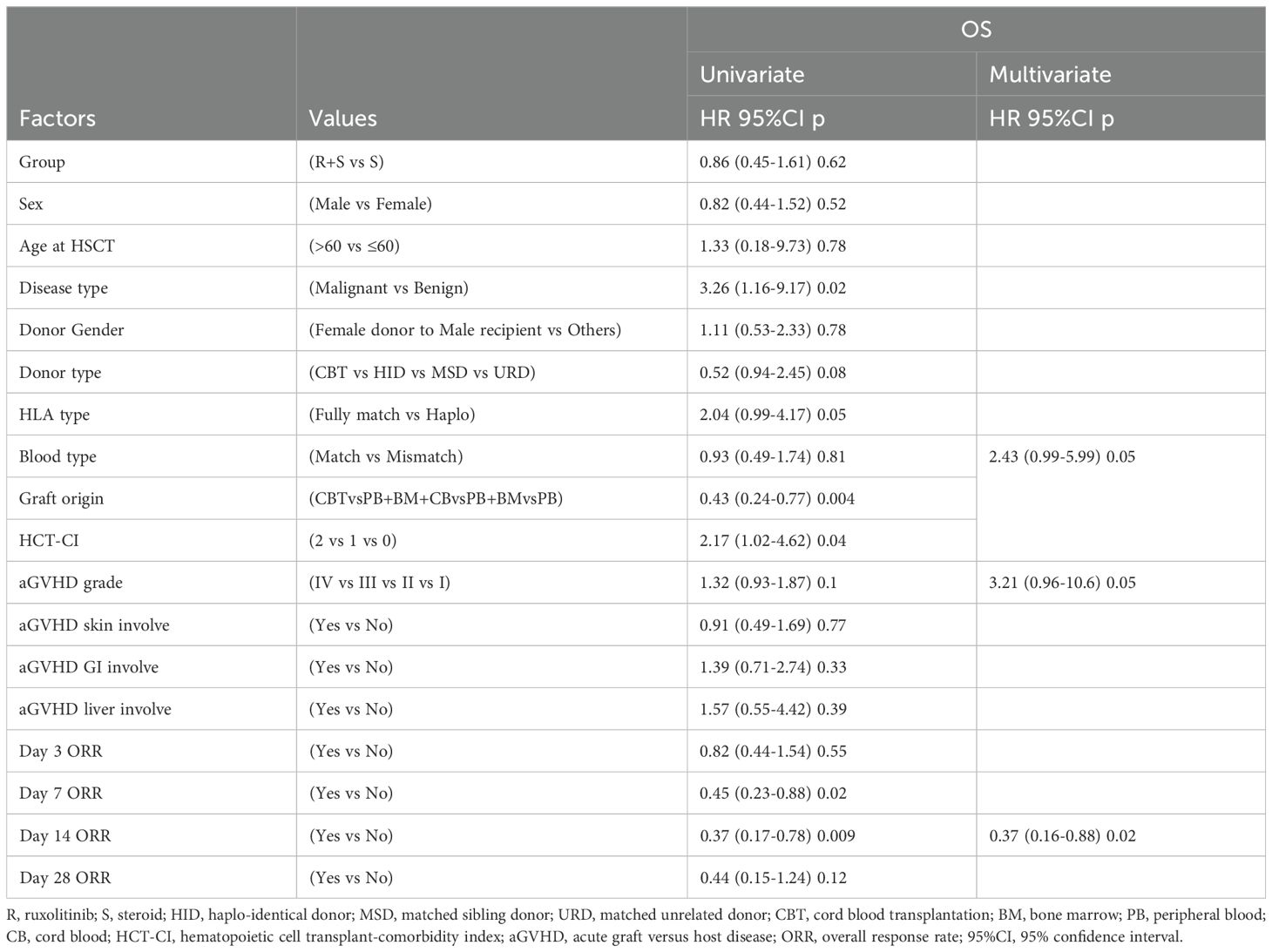
The median follow-up duration from aGVHD onset was 47 months (range: 1–80 months) in the ruxolitinib/steroids group and 90 months (range: 0.4–118 months) in steroid-only group. The 3-year OS rates were similar between two groups: 70.5% in the ruxolitinib/steroids group vs. 67.6% in the steroid-only group, both in Cox regression analysis (hazard ratio [HR]=0.86, 95% CI: 0.45–1.61, p=0.62) (Table 3) and Kaplan-Meier survival analysis (Log-rank test, p=0.65. Figure 2A). Table 3 shows the univariate and multivariate analysis results for risk factors influencing OS. Univariate analysis revealed the following: (i) ORR on day 7 and day 14 had a significantly positive effect on OS (HR=0.45, 95% CI: 0.23–0.88, p=0.02) (HR=0.37, 95% CI: 0.17–0.78, p=0.009), confirmed by Kaplan-Meier survival analysis (Figures 2B, C). (ii) Malignant hematologic disease had a significantly negative effect on OS (HR=3.26, 95% CI: 1.16–9.17, p=0.02), also confirmed by Kaplan-Meier survival analysis (Figure 2D). (iii) Other factors-including donor type (CBT vs. HID vs. MSD vs. URD), HLA type (fully matched vs. haploidentical), stem cell source (CBT vs. PB+BM+CB vs. PB+BM vs. PB), and HCI-CI-influenced OS (Table 3, Figure 3). In the multivariate analysis, only day 14 ORR (HR=0.37, 95% CI: 0.16–0.88, p=0.02), aGVHD grade (HR=3.21, 95% CI, 0.96–10.6, p=0.05), and HCI-CI (HR=2.43, 95% CI, 0.99–5.99, p=0.05) influenced OS (Table 3). Additionally, we analyzed other major risk variables for OS (Table 3). Recipient sex, age at transplantation, donor sex, recipient-donor blood pair, aGVHD skin involvement, aGVHD GI involvement, and aGVHD liver involvement had no statistically significant effect on OS. Though the 1-year PFS rates were similar between the two groups-74% in the ruxolitinib/steroids group and 72% in the steroid-only group, as shown in Kaplan-Meier survival analysis (Figure 4A, log-rank test, p=0.927)—ORR on day 7 (Figure 4B, log-rank test, p=0.03) and day 14 (Figure 4C, log-rank test, p=0.001) demonstrated a significantly more favorable impact on PFS. The 1-year FFS rates were similar between the two groups, with 56.2% in the ruxolitinib/steroids group and 55.9% in the steroid-only group (Figure 4D, log-rank test, p=0.47). Reaching ORR on day 7 (Figure 4E, log-rank test, p=0.046) and day 14 (Figure 4F, log-rank test, p=0.0004) had a significantly more favorable effect on FFS. Both groups had comparable 2-year cumulative incidence rates of relapse (ruxolitinib/steroids: 18% vs. steroid-only: 11%; Subdistribution HR, SHR=1.69, 95% CI: 0.68–4.24, p=0.257; Figure 5A) and non-relapse mortality (ruxolitinib/steroids: 15% vs. steroid-only: 23%; SHR=0.60, 95% CI: 0.24–1.45, p=0.26; Figure 5B).
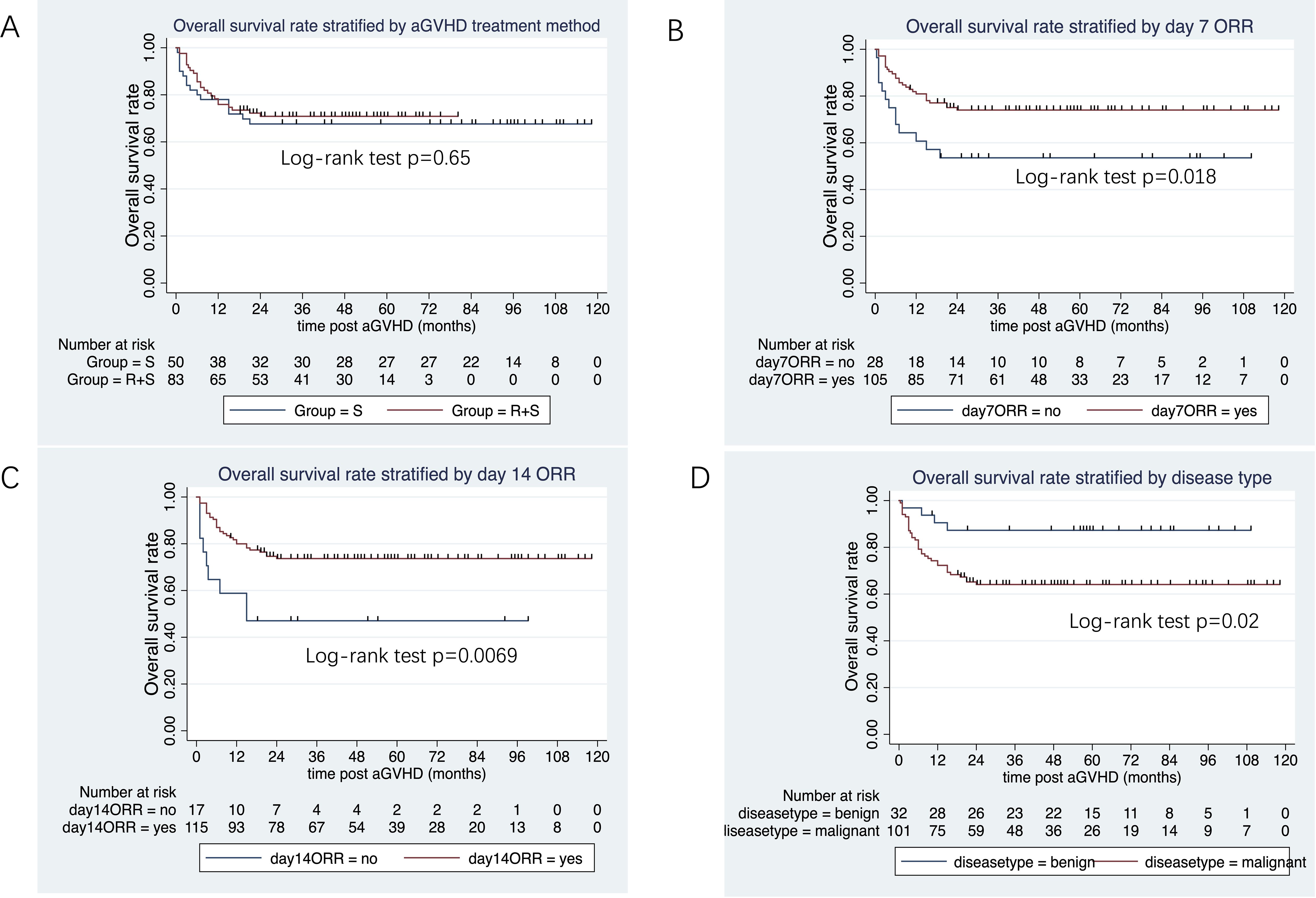
Figure 2. Overall survival rate stratified by aGVHD treatment, day 7 ORR, day 14 ORR and disease type. (A) Overall survival rate stratified by aGVHD treatment. (B) Overall survival rate stratified by day 7 ORR. (C) Overall survival rate stratified by day 14 ORR. (D) Overall survival rate stratified by disease type.
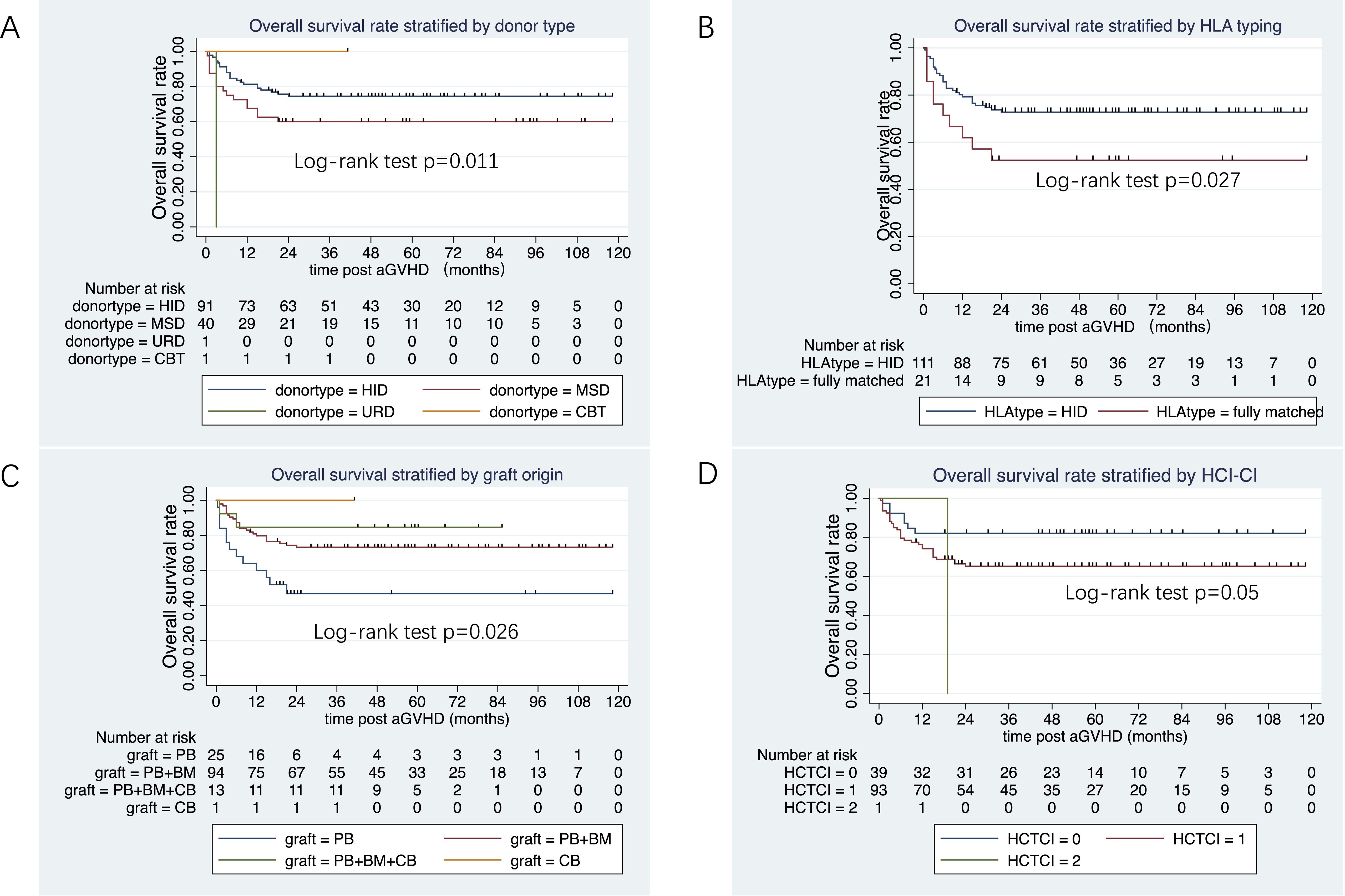
Figure 3. Overall survival rate stratified donor type, HLA typing, graft origin and HCT-CI. (A) Overall survival rate stratified by donor type. (B) Overall survival rate stratified by HLA. (C) Overall survival rate stratified by graft origin. (D) Overall survival rate stratified by HCT-CI.
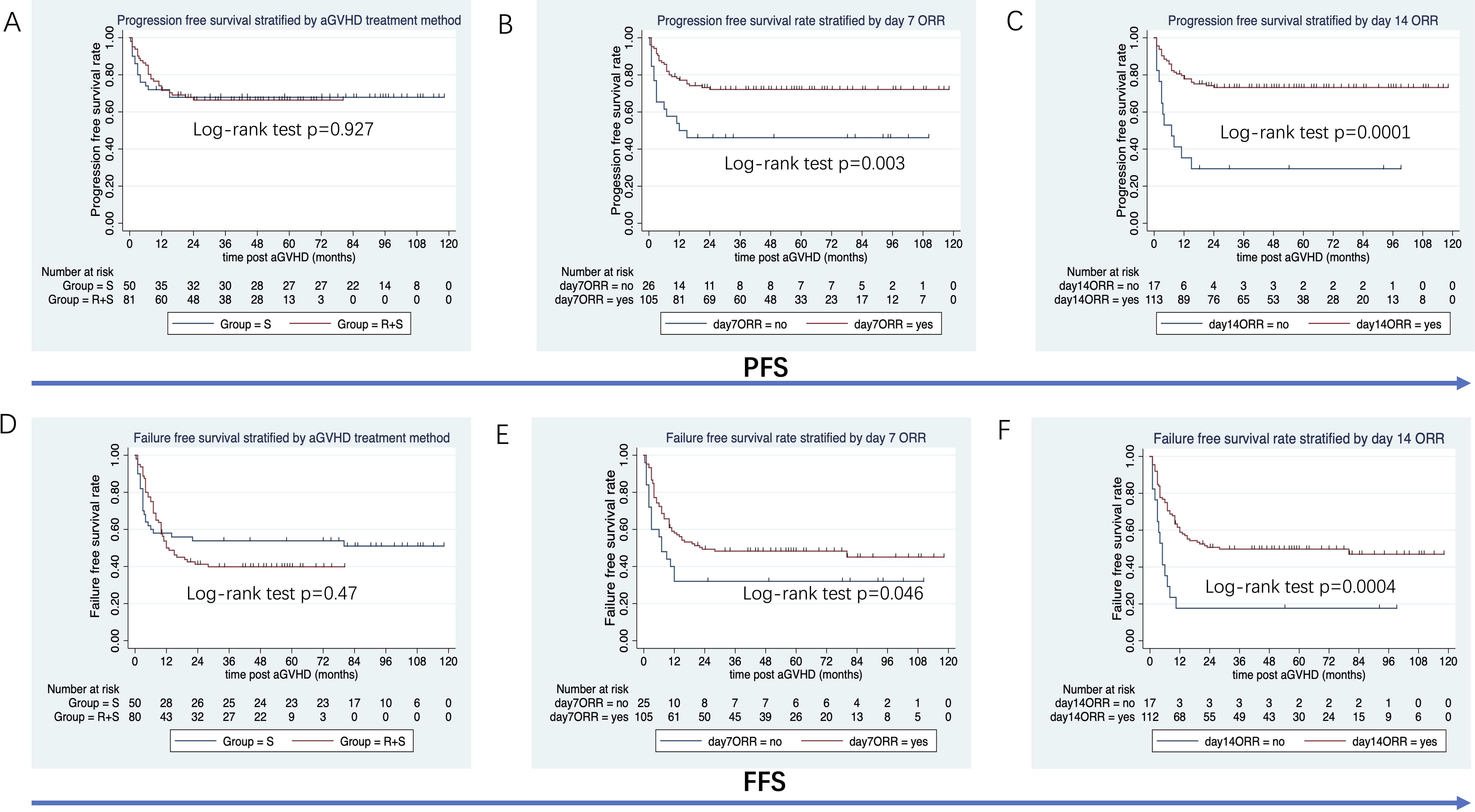
Figure 4. PFS and FFS stratified by aGVHD treatment, day 7 ORR and day 14 ORR. (A) PFS stratified by aGVHD treatment. (B) PFS stratified by day 7 ORR. (C) PFS stratified by day 14 ORR. (D) FFS stratified by aGVHD treatment. (E) FFS stratified by day 7 ORR. (F) FFS stratified by day 14 ORR.
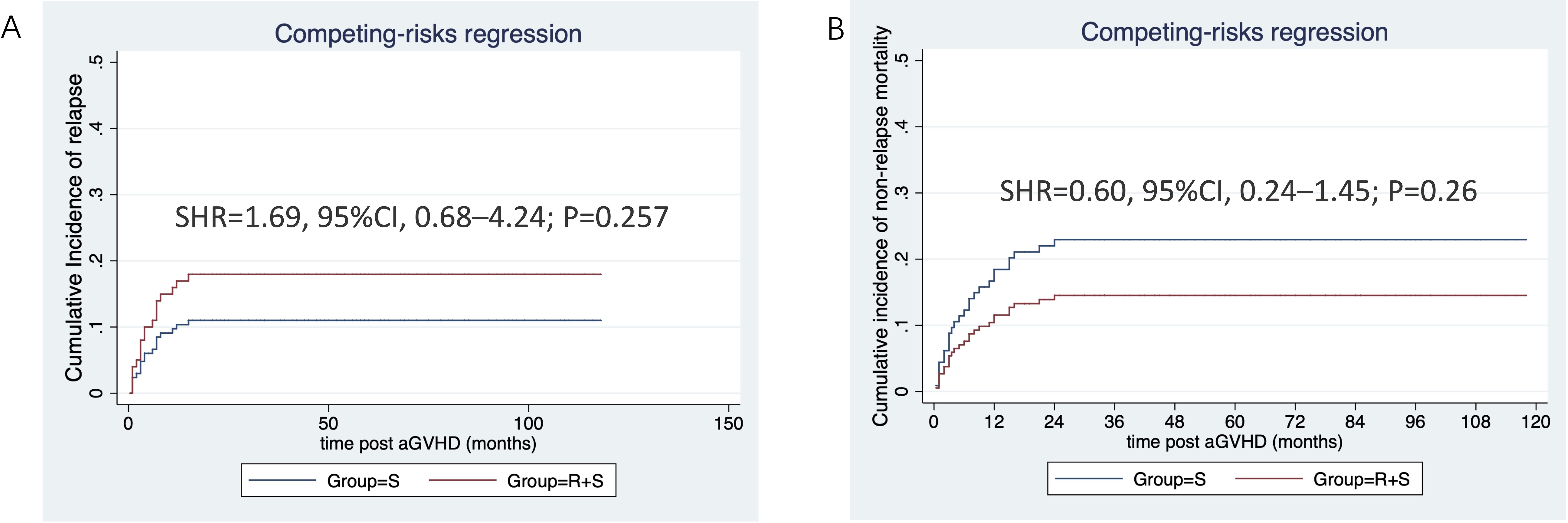
Figure 5. CIR and NRM stratified by aGVHD treatment. (A) Cumulative incidence of relapse stratified by aGVHD treatment. (B) Cumulative incidence of non-relapse mortality stratified by aGVHD treatment.
3.4 Subgroup analysis
In the subgroup of patients who received peripheral blood stem cell transplantation, the 3-year OS tended to be higher in the ruxolitinib/steroid combination group than in the steroid-only group (64.3% vs. 27.3%; log-rank test, p=0.046; Figure 6A). Similarly, the 3-year PFS was superior in the ruxolitinib/steroid group compared to the steroid-only group (57.1% vs. 27.3%; log-rank test, p=0.05; Figure 6B). Finally, the 2-year FFS was better in the ruxolitinib/steroid combination group than in the steroid-only group (21% vs. 9%; log-rank test, p=0.05; Figure 6C).
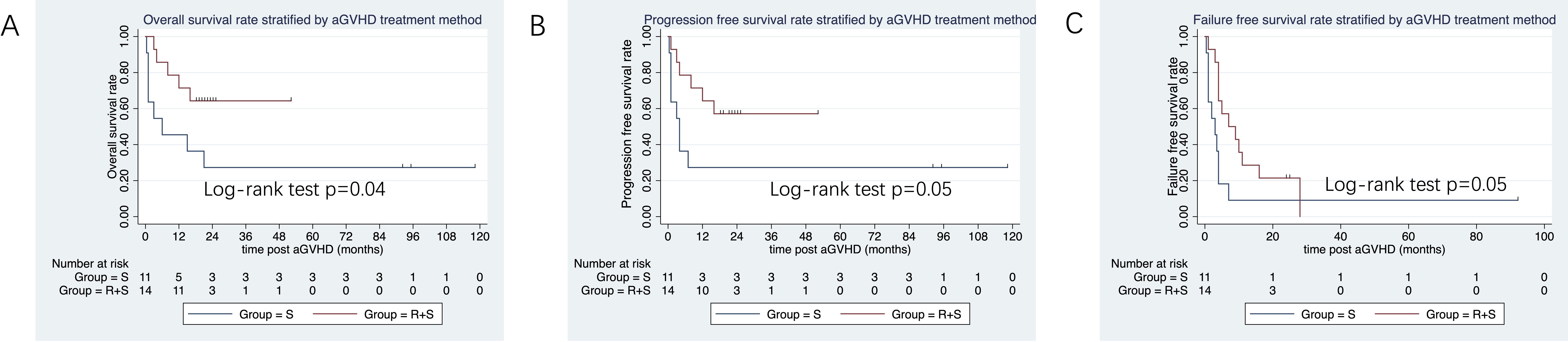
Figure 6. OS, PFS and FFS stratified by aGVHD treatment in patients who received peripheral blood (PB) grafts. (A) OS stratified by aGVHD treatment in patients with PB grafts. (B) PFS and FFS stratified by aGVHD treatment in patients with PB grafts. (C) FFS stratified by aGVHD treatment in patients with PB grafts.
3.5 Safety and tolerance analysis
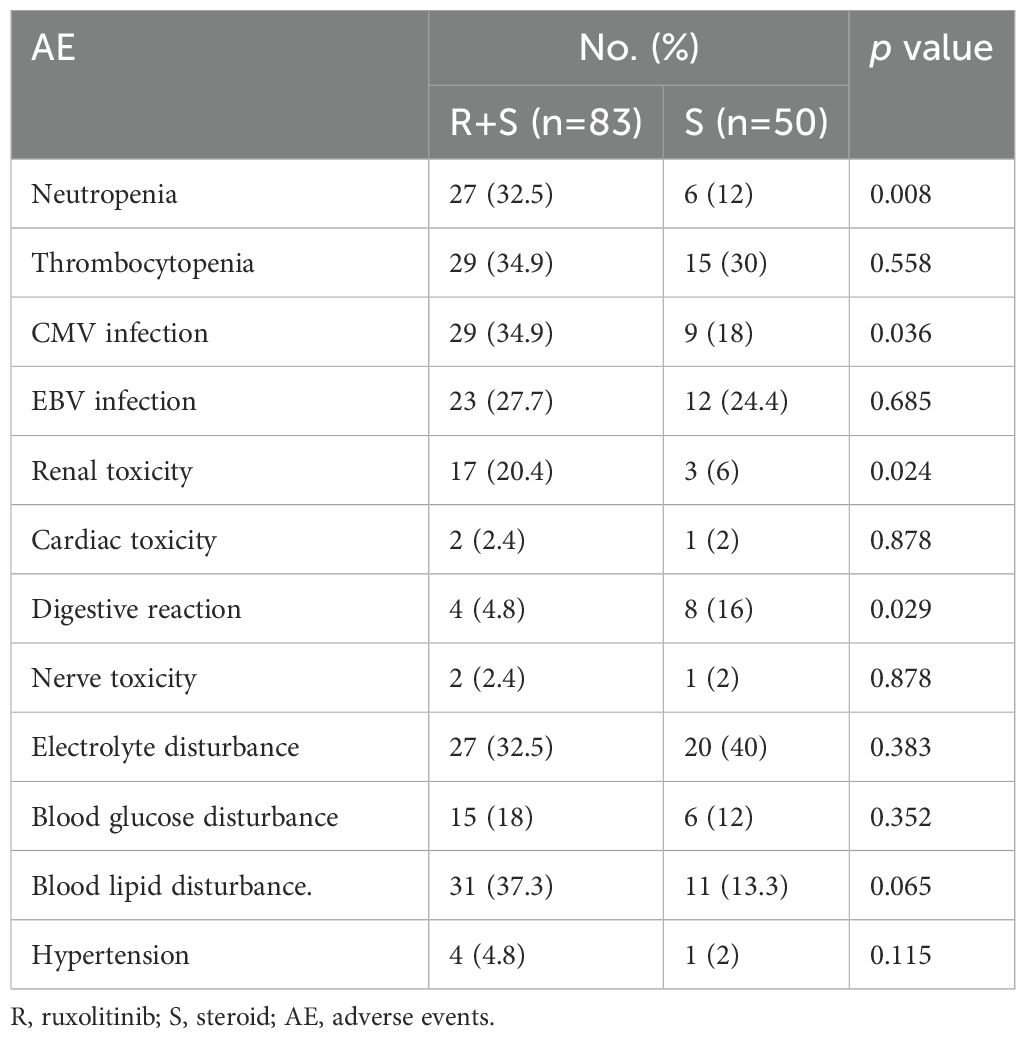
The median duration of ruxolitinib exposure was 70 days (range: 9–730 days) among the 83 patients, and none of the 83 patients discontinued ruxolitinib due to side effects, especially hepatotoxicity. No ruxolitinib toxicity related death was observed. As shown in Table 4, the most frequently observed AEs in the ruxolitinib/steroid group were neutropenia (32.5% vs. 12%, p=0.008), CMV infection (34.9% vs. 18%, p=0.036), and renal toxicity (20.4% vs. 6%, p=0.024). Other AEs, including thrombocytopenia, EBV infection, cardiac toxicity, nerve toxicity, electrolyte disturbance, blood glucose disturbance, blood lipid disturbance, and hypertension, were comparable between the two groups.
4 Discussion
With an incidence of 30–60% and a mortality rate of 15–30%, aGVHD is a serious complication of allo-HSCT (4, 29, 30). Systemic steroids are the first-line treatment for aGVHD grades II–IV, according to the European Group for Blood and Marrow Transplantation (EBMT). Unfortunately, 50% of the patients with aGVHD relapse or do not respond to steroids (31). According to the findings of the REACH1 and REACH2 trials, which assessed ruxolitinib as a second-line treatment, the overall response on day 28 was higher in the ruxolitinib group than in the control group, which included therapies such as ATG, extracorporeal photopheresis, mesenchymal stromal cells, low-dose methotrexate, mycophenolate mofetil, mammalian target of rapamycin (mTOR) inhibitor (everolimus or sirolimus), etanercept, and infliximab. Ruxolitinib is currently the only medication authorized for the treatment of SR-aGVHD (22, 32, 33). Despite the recognized benefits of first-line treatment for aGVHD, prolonged steroid therapy can lead to serious adverse effects, such as weight gain, hyperglycemia, diabetes mellitus, adrenal suppression, osteoporosis, dermatological changes, cardiovascular complications, cataracts, glaucoma, peptic ulcers, myopathy, increased propensity for infections, and neuropsychiatric disorders (34). Additionally, half of patients do not respond to steroids. Therefore, the first-line treatment of aGVHD is an unmet need in clinical practice, and novel active first-line treatments are urgent for improving efficiency and decreasing side effects in newly diagnosed patients with aGVHD. Liu et al. (23) designed the first prospective, randomized controlled trial to show that combining ruxolitinib (5mg/day) and methylprednisolone (1mg/kg/day) is a superior first-line therapy for intermediate- and high-risk aGVHD compared to the standard 2 mg/kg/day methylprednisolone regimen. This novel approach significantly improved ORRs on days 28 and 56, resulted in a more durable response at 6 months, and led to better failure-free survival than corticosteroid monotherapy. Moreover, this first-line therapy was well-tolerated and reduced exposure to steroids, opening a new chapter for the treatment of aGVHD. In real-world scenarios, the efficacy and safety of ruxolitinib and steroid combination therapy in the first-line treatment of aGVHD are still unclear, and we performed this retrospective study to focus on this issue.
We designed this study based on following reasons: 1) Considering the significant morbidity and mortality associated with aGVHD, there is an unmet need for effective and evidence based new options for the management of aGVHD. 2) Our center has noticed that the clinical research results related to the treatment of SR-GVHD with ruxolitinib internationally are encouraging. Spoerl et al. (20) reported that 6 SR-GVHD patients responded with respect to clinical GVHD symptoms and serum levels of proinflammatory cytokines after treatment with ruxolitinib in 2014; Zeiser et al. (21) reported on a multicenter clinical study in Europe and USA, which retrospectively analyzed the effectiveness of ruxolitinib in treating SR-GVHD in 2015. 3) Ruxolitinib was approved and accessible in China in March 2017. Therefore, we speculate that the first-line treatment of ruxolitinib/steroid may reduce the occurrence of SR-GVHD and increase the ORR rate. Our center first integrates ruxolitinib in the treatment strategy of aGVHD in December 2017.
In our study, ruxolitinib was administered as first-line therapy at a dosage of 10-15 mg/day combined with methylprednisolone (0.5–2 mg/kg/day). The primary endpoint, ORR on days 7 and 14, was significantly higher in the ruxolitinib/steroid combination group than that in the steroid-only group. However, ORR on day 28 was similar between the two cohorts. The ORR response in our study appeared to be achieved earlier than in Liu’s prospective trial (23). This phenomenon might be partially explained by the relatively high ruxolitinib dose used in our study. Although no statistical differences in OS, PFS, and FFS were observed between the two groups, patients with aGVHD who achieved early ORR on day 7 or 14 had superior OS, PFS, and FFS. We hypothesize that the ruxolitinib/steroid combination cohort could indirectly influence OS, PFS, and FFS by improving the early ORR on days 7 and 14. Notably, successful control of aGVHD does not always correlate with enhanced survival. For instance, Robert Zeiser (32) reported a higher ORR on day 28 in the ruxolitinib group than in the control group among patients with SR-aGVHD, but no significant difference in the 18-month OS. Similarly, in the trial by Liu et al, the 18-month OS rates were comparable between groups, at 75.4% in the RUX/steroid group and 70.5% in the steroid-only group (p=0.734). The authors mentioned that, in GVHD treatment trials, discrepancies between response and survival outcomes were likely influenced by factors such as infections, regimen-related toxicity, relapse of malignancy, and underlying conditions unrelated to GVHD (23). Interestingly, in our subgroup of patients who received peripheral blood stem cell transplantation, the 3-year OS, PFS, and 2-year FFS were superior in the ruxolitinib/steroid group than in the steroid-only group.
The most frequently reported adverse effects of ruxolitinib include infectious complications and cytopenia. In our study, neutropenia and CMV infection were more common in the ruxolitinib/steroid group, which could be managed with granulocyte colony-stimulating factor (G-CSF) and antiviral agents (ganciclovir/foscarnet). Other AEs, including thrombocytopenia, EBV infection, cardiac toxicity, nerve toxicity, electrolyte disturbance, blood glucose disturbance, blood lipid disturbance, and hypertension, were comparable between the two groups. This indicates that adding ruxolitinib to first-line steroid treatment for aGVHD does not increase severe AEs, and they remain controllable.
Real-world data play an important role in generating evidence complementary to conventional randomized controlled trials (RCT) and in improving clinical trial designs. Real-world studies have collected data from patients receiving clinical care in routine practice and provided more authentic evidence. In line with other real-world studies, one of the key limitations of our study is that the intervention of interest was not randomly assigned, which could have resulted in biased associations between the treatment and outcomes of interest. It is vital to understand the strengths and limitations of real-world and RCT evidence to implement a framework in which they can be used complementarily to create a robust evidence base for treatment decision-making. In conclusion, in our real-world clinical setting, first-line use of methylprednisolone combined with ruxolitinib proved superior to conventional methylprednisolone monotherapy for aGVHD, as demonstrated by the significantly improved overall response.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Clinical Research Ethics Committee of Second Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YY: Conceptualization, Supervision, Writing – original draft. YuH: Conceptualization, Data curation, Writing – original draft. DZ: Conceptualization, Data curation, Writing – original draft. ZK: Data curation, Writing – original draft. YaH: Data curation, Writing – original draft. YW: Data curation, Writing – original draft. GF: Supervision, Visualization, Writing – review & editing. LM: Conceptualization, Formal analysis, Visualization, Writing – review & editing. JY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded mainly by the Science and Technology Innovation Leading Talent Program of Liaoning Province (XLYC1902036 to JY), the “1+X” Program Summit Construction Project of Second Hospital of Dalian Medical University, the research projects (HXLX20240065 to JY) and (HXLX20240028 to JY).
Acknowledgments
We thank all the staff involved, including fellow doctors from other specialties, nurses, pharmacists, and laboratory and allied health personnel, for their contributions to the transplant program over the years.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Camara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transpl. (2021) 56:1651–64. doi: 10.1038/s41409-021-01227-8
2. Kroger N, Bacigalupo A, Barbui T, Ditschkowski M, Gagelmann N, Griesshammer M, et al. Indication and management of allogeneic haematopoietic stem-cell transplantation in myelofibrosis: updated recommendations by the EBMT/ELN International Working Group. Lancet Haematol. (2024) 11:e62–74. doi: 10.1016/S2352-3026(23)00305-8
3. Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American society for transplantation and cellular therapy. Biol Blood Marrow Transpl. (2020) 26:1247–56. doi: 10.1016/j.bbmt.2020.03.002
4. Zeiser R and Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. (2017) 377:2167–79. doi: 10.1056/NEJMra1609337
5. Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. (2010) 363:2091–101. doi: 10.1056/NEJMoa1004383
6. Sung AD and Chao NJ. Concise review: acute graft-versus-host disease: immunobiology, prevention, and treatment. Stem Cells Transl Med. (2013) 2:25–32. doi: 10.5966/sctm.2012-0115
8. Reddy P and Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. (2003) 17:187–94. doi: 10.1016/S0268-960X(03)00009-2
9. Choe H and Ferrara JLM. New therapeutic targets and biomarkers for acute graft-versus-host disease (GVHD). Expert Opin Ther Targets. (2021) 25:761–71. doi: 10.1080/14728222.2021.1992383
10. Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. (2012) 119:296–307. doi: 10.1182/blood-2011-06-364265
11. Aladag E, Kelkitli E, and Goker H. Acute graft-versus-host disease: A brief review. Turk J Haematol. (2020) 37:1–4. doi: 10.4274/tjh.galenos.2019.2019.0157
12. Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological Malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. (2024) 11:e147–e59. doi: 10.1016/S2352-3026(23)00342-3
13. Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transpl. (2012) 18:1150–63. doi: 10.1016/j.bbmt.2012.04.005
14. Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. (2012) 158:30–45. doi: 10.1111/j.1365-2141.2012.09129.x
15. Major-Monfried H, Renteria AS, Pawarode A, Reddy P, Ayuk F, Holler E, et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood. (2018) 131:2846–55. doi: 10.1182/blood-2018-01-822957
16. Deeg HJ. How I treat refractory acute GVHD. Blood. (2007) 109:4119–26. doi: 10.1182/blood-2006-12-041889
17. Martini DJ, Chen YB, and DeFilipp Z. Recent FDA approvals in the treatment of graft-versus-host disease. Oncologist. (2022) 27:685–93. doi: 10.1093/oncolo/oyac076
18. Schroeder MA, Choi J, Staser K, and DiPersio JF. The role of janus kinase signaling in graft-versus-host disease and graft versus leukemia. Biol Blood Marrow Transpl. (2018) 24:1125–34. doi: 10.1016/j.bbmt.2017.12.797
19. Zeiser R and Socie G. The development of ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. Blood Adv. (2020) 4:3789–94. doi: 10.1182/bloodadvances.2020002097
20. Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. (2014) 123:3832–42. doi: 10.1182/blood-2013-12-543736
21. Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. (2015) 29:2062–8. doi: 10.1038/leu.2015.212
22. Jagasia M, Perales MA, Schroeder MA, Ali H, Shah NN, Chen YB, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. (2020) 135:1739–49. doi: 10.1182/blood.2020004823
23. Dou L, Zhao Y, Yang J, Deng L, Wang N, Zhang X, et al. Ruxolitinib plus steroids for acute graft versus host disease: a multicenter, randomized, phase 3 trial. Signal Transduct Target Ther. (2024) 9:288. doi: 10.1038/s41392-024-01987-x
24. Lv M, Chang YJ, and Huang XJ. Update of the “Beijing Protocol” haplo-identical hematopoietic stem cell transplantation. Bone Marrow Transpl. (2019) 54:703–7. doi: 10.1038/s41409-019-0605-2
25. Wang J, Zhao J, Fei X, Yin Y, Cheng H, Zhang W, et al. A new intensive conditioning regimen for allogeneic hematopoietic stem cell transplantation in patients with refractory or relapsed acute myeloid leukemia. Med (Baltimore). (2018) 97:e0228. doi: 10.1097/MD.0000000000010228
26. Bacigalupo A, Socie G, Lanino E, Prete A, Locatelli F, Locasciulli A, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica. (2010) 95:976–82. doi: 10.3324/haematol.2009.018267
27. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the Mount Sinai acute GVHD international consortium. Biol Blood Marrow Transpl. (2016) 22:4–10. doi: 10.1016/j.bbmt.2015.09.001
28. Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. (1974) 18:295–304. doi: 10.1097/00007890-197410000-00001
29. Ferrara JL, Levine JE, Reddy P, and Holler E. Graft-versus-host disease. Lancet. (2009) 373:1550–61. doi: 10.1016/S0140-6736(09)60237-3
30. Cahn JY, Klein JP, Lee SJ, Milpied N, Blaise D, Antin JH, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. (2005) 106:1495–500. doi: 10.1182/blood-2004-11-4557
31. Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transpl. (2014) 49:168–73. doi: 10.1038/bmt.2013.107
32. Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. (2020) 382:1800–10. doi: 10.1056/NEJMoa1917635
33. Przepiorka D, Luo L, Subramaniam S, Qiu J, Gudi R, Cunningham LC, et al. FDA approval summary: ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease. Oncologist. (2020) 25:e328–e34. doi: 10.1634/theoncologist.2019-0627
Keywords: graft-versus-host disease, hematopoietic stem cell transplantation, ruxolitinib, steroids, leukemia
Citation: Yang Y, Huo Y, Zhou D, Kang Z, Huang Y, Wang Y, Fan G, Ma L and Yan J (2025) Efficacy and safety of ruxolitinib combined with steroids for first-line treatment of acute graft-versus-host disease after hematopoietic stem cell transplantation: a single-center, real-world experience. Front. Immunol. 16:1621708. doi: 10.3389/fimmu.2025.1621708
Received: 01 May 2025; Accepted: 27 June 2025;
Published: 14 July 2025.
Edited by:
Mahzad Akbarpour, University of Chicago Medicine, United StatesReviewed by:
Juan Carlos Olivares-Gazca, Clínica Ruiz, MexicoMolly Gallogly, University Hospitals of Cleveland, United States
Copyright © 2025 Yang, Huo, Zhou, Kang, Huang, Wang, Fan, Ma and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangjun Fan, MTc3MDk4NzU4OTlAMTYzLmNvbQ==; Liyuan Ma, ZG9jbWx5QDEyNi5jb20=; Jinsong Yan, eWFuanNkbXVAZG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Yan Yang1†
Yan Yang1† Zhijie Kang
Zhijie Kang Guangjun Fan
Guangjun Fan Liyuan Ma
Liyuan Ma Jinsong Yan
Jinsong Yan