- Zibo Central Hospital, Zibo, China
Background: To investigate the effect of multiple intravitreal injections of conbercept on the cornea in patients with branch retinal vein occlusion (BRVO)-induced macular edema.
Methods: The retrospective study analyzed the clinical data of 40 patients (40 eyes) with BRVO-induced macular edema between March 2020 and March 2023. All patients received intravitreal injections of conbercept according to the “3 + PRN” regimen and were followed for at least 6 months. Corneal analysis was performed using a corneal endothelial microscope and a Pentacam corneal topographic map. The best-corrected vision acuity (BCVA), central retinal thickness, central corneal thickness, corneal endothelial cell density, hexagonal cell percentage, and coefficient of variation before and at 1, 3, and 6 months after the injection were compared.
Results: The study included 40 patients (22 males and 18 females) with an average age of 50.80 ± 7.35 years (range, 38–67 years). The corneal endothelial cell densities, central corneal thicknesses, hexagonal cell percentages, and coefficients of variation before and at 1, 3, and 6 months after the injection were not significantly different (P > 0.05). The average BCVA was significantly higher at 1, 3, and 6 months after than before the injection (P < 0.05 each). The average central retinal thickness was significantly lower at 1, 3, and 6 months after than before the injection (P < 0.05 each). The number of injections was 3.08 ± 0.27 at the last follow-up. No adverse reactions, such as endophthalmitis, retinal detachment, or thrombus, were observed in any patient after treatment.
Conclusion: Multiple intravitreal injections of conbercept can improve the BCVA and reduce macular edema in patients with BRVO and have no significant effect on the cornea.
1 Background
Retinal vein occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy. It is clinically characterized by tortuous dilatation of retinal veins and edema, bleeding, and exudation in the areas distributed along the veins (1). Depending on the location of the occlusion, RVO can be divided into branch (BRVO) and central. Arteriovenous cross-compression is the predominant pathogenic mechanism underlying BRVO. The pathogenesis of RVO is still unclear, and the main risk factors include hypertension, hyperlipidemia, diabetes, and systemic inflammation. Neovascularization and macular edema (ME) are the main complications of RVO, but ME is the main cause of vision loss (2–5). Persistent ME and the resulting damage to the fundal structure may lead to irreversible impairment of visual function.
Vascular endothelial growth factor (VEGF) plays an important role in the pathogenesis of ME secondary to BRVO (6). The main treatment for BRVO-induced ME has been anti-VEGF therapy (7–9). In clinical practice, the commonly used anti-VEGF drugs include ranibizumab (Lucentis; Genentech Inc., South San Francisco, CA, United States), conbercept (KH902; Chengdu Kanghong Biotech Co., Ltd., Sichuan, China), aflibercept (EYLEA; Bayer HealthCare, Berlin, Germany), and bevacizumab (Avastin; Genentech Inc.). Conbercept is a fusion protein composed of extracellular domain 2 of VEGF receptor (VEGFR)-1 and extracellular domains 3 and 4 of VEGFR-2, which are fused with the Fc fragment of human immunoglobulin G1 (IgG1).
Clinical studies have reported that VEGF and anti-VEGF drugs may affect the cornea, in addition to neovascular diseases, which should arouse the attention of clinicians (10). It has been reported that corneal endothelial cell density (ECD) in patients with age-related macular degeneration can be significantly reduced by ranibizumab and aflibercept (11). However, other studies have found that ranibizumab and bevacizumab are not toxic to corneal endothelial cells (12). At present, there are few reports about the effects of intravitreal injections of conbercept on the cornea.
This study aimed to ascertain the effect of multiple intravitreal injections of conbercept on the cornea in patients with BRVO-induced ME.
2 Materials and methods
2.1 Study protocol
The study received ethical approval from an institutional review board (IRB). All patients provided informed consent.
2.2 Patients
The clinical data of 40 patients (40 eyes) with BRVO-induced ME admitted to the Department of Ophthalmology of Zibo Central Hospital between March 2020 and March 2023 were retrospectively analyzed. All patients received intravitreal injections of conbercept according to the 3 + pro re nata (PRN) treatment strategy and were followed for at least 6 months.
The inclusion criteria were as follows: (a) patients who were clinically diagnosed with BRVO and needed anti-VEGF therapy; (b) patients who had not received any intraocular injection before; (c) patients with no history of wearing contact lenses; (d) no history of eye diseases affecting corneal endothelial function; (e) no history of eye surgery such as those for the internal eye and ocular surface; and (f) no history of ocular laser therapy within 3 months before anti-VEGF injection. The exclusion criteria were: (a) patients with any corneal or ocular surface disease; (b) patients with concurrent retinal or optic nerve diseases; (c) endothelial cell count less than 1000/mm2; (d) patients with incomplete clinical data; (e) age of < 18 years; (f) patients who had received anti-VEGF drugs, triamcinolone, or laser photocoagulation therapy.
2.3 Examination and treatment
The examinations included tonometry, best-corrected visual acuity (BCVA) (logMAR), slit lamp biomicroscopy, corneal endothelial microscopy, ocular fundus examination, and optical coherence tomography at baseline and 1, 3, and 6 months. Intraocular pressure was measured using non-contact tonometry (TX-20, Canon, Japan). A corneal endothelial microscope (KONAN MEDICAL, INC.) was used to determine the ECD, proportion of hexagonal cells (Hex%), and coefficient of variation (CV). Central corneal thickness (CCT) was measured with a Pentacam corneal topographic map (OCULUS Optikgerate GmbH). The inspection area was the central cornea, and the clearest image quality was taken to record the data. Central retinal thickness (CRT) was measured using optical coherence tomography (Optovue, Inc., Fremont, CA, United States). The device had an accompanying program to measure the CRT (distance from the inner boundary membrane of the retina to the retinal pigment epithelium), which is the thickness of the retinal nerve epithelium.
All patients received levofloxacin eye drops four times a day for 7 days before the injection, and all intravitreal injections were administered in the operating room of the bacteria-free laminar Flow center. The patient was placed in a supine position and administered surface anesthesia. The conjunctival sac was rinsed with Povidone iodine solution for 3 min, followed by 100 mL of normal saline. A 30G sterile syringe needle was inserted into the vitreous at 3.5–4.0 mm behind the corneal limbus perpendicular to the sclera, and conbercept (0.05 mL containing conbercept 0.5 mg) was injected into the vitreous body. A sterile cotton swab was used to press the tip of the needle subsequently, and tobramycin and dexamethasone eye ointments were applied and covered with gauze. All injection procedures were performed by the same ophthalmologist to minimize procedural variability. The tobramycin and dexamethasone eye ointments were also applied to the conjunctival sac, and gauze was used to cover the operative eye for 1 day. Levofloxacin eye drops were administered for 1 week after the procedure. The first follow-up was scheduled a week after the injection, and the follow-up frequency was once a month.
The criteria for reinjection included an increase in CRT by > 100 μm, new retinal fluid, a decrease in BCVA by more than one row, or new areas of retinal neovascularization.
2.4 Observed parameters
The BCVA (logMAR), CRT, CCT, ECD, Hex%, and CV were compared before and at 1, 3, and 6 months after the injection.
2.5 Statistical analysis
SPSS17.0 statistical software was used for the analysis. Quantitative data are expressed as mean ± standard deviation (SD). The BCVA was converted to the logarithm of the minimum angle of resolution (logMAR). Repeated-measures analysis of variance (ANOVA) was used to compare the changes in BCVA, CRT, CCT, ECD, Hex%, and CV before and at 1, 3, and 6 months after the injection. If the results of ANOVA were significant, the LSD-t-test was used for pairwise comparison of the sample means. P-values of < 0.05 denoted statistical significance.
3 Results
3.1 Baseline characteristics
The study included 40 patients (22 males and 18 females) with an average age of 50.80 ± 7.35 years (range, 38–67 years).
3.2 BCVA
The baseline BCVA was 0.55 ± 0.13 logMAR, decreasing significantly to 0.34 ± 0.13, 0.31 ± 0.13, and 0.27 ± 0.11 logMAR at 1, 3, and 6 months, respectively (P < 0.05) (Table 1).
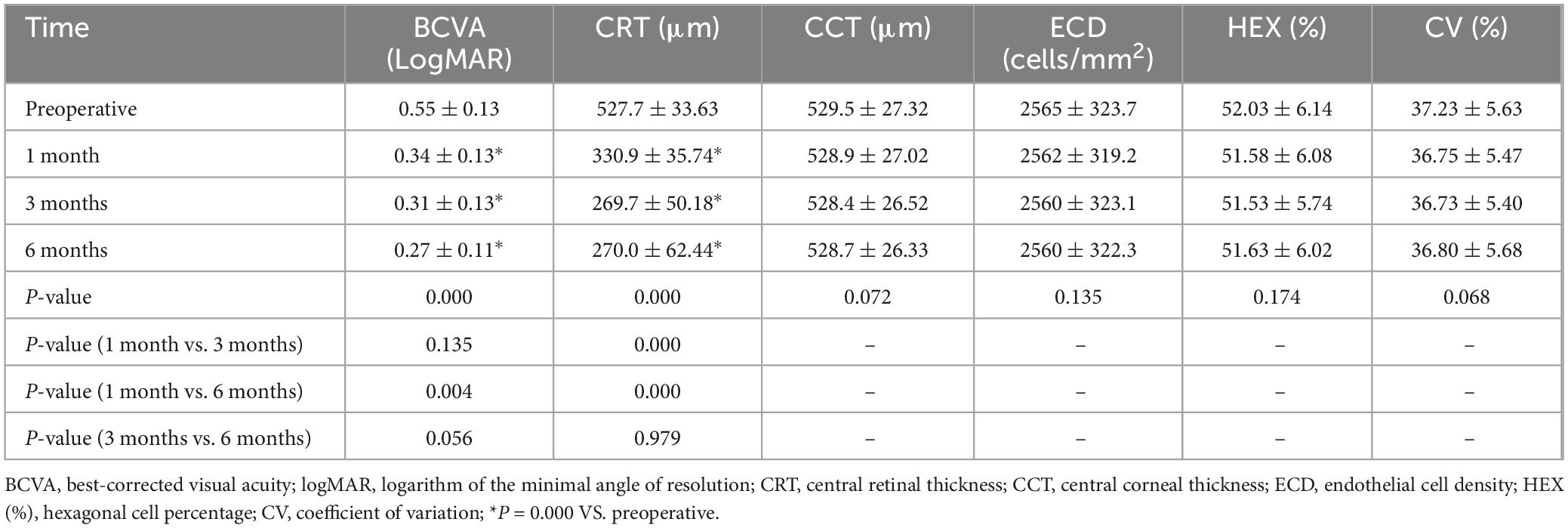
Table 1. Best-corrected visual acuity (BCVAs), CRTs, CCTs, ECDs, Hex% values, and CVs before and after the injection.
3.3 CRT
The average CRT was 527.7 ± 33.63 μm before treatment, decreasing sig nificantly to 330.9 ± 35.74 μm, 269.7 ± 50.18 μm, and 270.0 ± 62.44 μm at 1, 3, and 6 months, respectively (P < 0.05) (Table 1).
3.4 CCT, ECD, Hex%, and CV
The average CCT of the affected eye was 529.5 ± 27.32 μm before the injection and 528.9 ± 27.02 μm, 528.4 ± 26.52 μm, and 528.7 ± 26.33 μm, at 1, 3, and 6 months after the injection, respectively. The CCT did not change significantly at any of the time points (P > 0.05). The average ECD of the affected eye was 2565 ± 323.7 cells/mm2 before the injection and 2562 ± 319.2 cells/mm2, 2560 ± 323.1 cells/mm2, and 2560 ± 322.3 cells/mm2 at 1, 3, and 6 months after the injection, respectively. The ECD did not change significantly at any of the time points (P > 0.05). The average Hex% of the affected eye was 52.03 ± 6.14 before the injection and 51.58 ± 6.08, 51.53 ± 5.74, and 51.63 ± 6.02, at 1, 3, and 6 months after the injection, respectively; the Hex% did not change significantly at any of the time points (P > 0.05). The average CV of the affected eye was 37.23 ± 5.63 before the injection and 36.75 ± 5.47, 36.73 ± 5.40, and 36.80 ± 5.68 1, 3, and 6 months after the injection, respectively; it did not change significantly at any time point (P > 0.05) (Table 1).
3.5 Complications
Over the six postoperative months, subconjunctival hemorrhage was observed in four patients, transient intraocular pressure elevation occurred in one patient, and corneal epithelial injury was observed in one patient. None of the patients included in this study had severe ocular or systemic adverse reactions related to the intravitreal injection or drugs.
3.6 Number of injections
Over the six postoperative months, the average number of injections was 3.08 ± 0.27.
4 Discussion
Branch retinal vein occlusion has various ocular sequelae, with ME being the most common. In patients with BRVO, retinal photoreceptor function is impaired due to the dysfunction of macular cells, which can further cause visual loss if not controlled in time. Therefore, effectively reducing the degree of retinal ischemia and hypoxia and accelerating the absorption of macular edema have become the key principles of the clinical treatment of BRVO-induced ME. It has been suggested that exudate accumulation (extracellular edema) caused by blood-retinal barrier (BRB) dysfunction is the main pathological mechanism underlying macular edema (13). VEGF may destroy the BRB and cause macular edema (14). For a long time, macular grating photocoagulation has been mainly used to treat ME secondary to BRVO. Despite its efficacy, it may damage visual cells, and it has limited effect on improving the visual acuity of patients (15). It has been gradually replaced by intravitreal injection of anti-VEGF drugs (16–19) and anti-inflammatory therapy (20, 21).
Intravitreal injection of anti-VEGF drugs not only effectively reduces macular edema, but also improves the visual acuity of patients (22). The principle is that the drug can reduce the VEGF concentration in the eye, inhibit the formation of new blood vessels, and reduce the permeability of blood vessels, thereby reducing the leakage of blood vessels and promoting the absorption of edema.
Corneal endothelial cells play an important role in maintaining corneal transparency, and corneal transparency is one of the important conditions for the normal physiological function of visual organs. As the innermost layer of the cornea, the corneal endothelium is composed of a single layer of endothelial cells, which are in contact with the anterior aqueous humor. Some scholars have found that corneal endothelial cells can express VEGF receptors (23). Some scholars have reported that the presence of anti-VEGF drugs can be detected in the anterior chamber water after an intravitreal injection (24). Therefore, the effect of multiple intravitreal injections of anti-VEGF drugs on the cornea requires further study.
The corneal morphology and CCT were not significantly affected by the short-term injection of aflibercept in a study (25). However, other scholars have come to a different conclusion (26).
Conbercept is a recombinant fusion protein produced using the hamster ovarian cell system. It exhibits a high affinity for VEGF and tightly binds to various VEGF-A subtypes, as well as VEGF-B and PIGF simultaneously (27). Conbercept is currently widely used to treat diabetic ME (DME) and ME secondary to RVO. A large number of studies have shown that conbercept is effective in treating RVO (28–31). However, we should be wary of the effect of conbercept on the cornea in the clinic.
To our knowledge, this study is the first to ascertain the safety of conbercept in the treatment of patients with ME secondary to BRVO using corneal endothelial microscopy and a Pentacam corneal topographic map.
In this study, 40 patients with BRVO-induced ME received intravitreal injections of conbercept according to the “3 + PRN” regimen and were followed for at least 6 months. The BCVA, CRT, CCT, ECD, Hex%, and CV were compared before and 1, 3, and 6 months after the injection. The results of this study showed that the ECD, Hex%, and CV at 1, 3, and 6 months after were not statistically significantly different from those before the injection. This suggests that conbercept has no significant effect on the corneal endothelium in patients with BRVO. There were no significant differences between the CCTs before and after treatment. This is not entirely consistent with the results of previous studies on the effects of ranibizumab and aflibercept on the cornea of patients with age-related macular degeneration (11). However, the conclusions of this study are consistent with previous studies on the effect of ranibizumab on the corneal endothelium in patients with age-related macular degeneration (32). In addition, the results of this study also showed that BCVA and CRT at 1, 3, and 6 months after treatment were significantly different from those before treatment. This suggests that conbercept can reduce ME in patients with BRVO and improve the BCVA. This is consistent with the reports of previous studies (33, 34). None of the patients included in this study had severe ocular or systemic adverse reactions related to intravitreal injection or drugs, indicating the safety of this treatment.
The main limitations of this study include its retrospective nature, small sample size, and short follow-up duration. While the PRN (pro re nata) regimen in the study reflects real-world clinical practice, it may introduce variability in treatment intervals. The absence of a fixed-dosing control group limits direct comparisons of treatment efficacy and safety. This retrospective study relied on existing data, which may be subject to incompleteness or missing records. The small sample size increases susceptibility to random variations, potentially compromising the stability and reliability of the conclusions. Additionally, the short follow-up duration might be insufficient to fully evaluate the long-term effects of the treatment. The next step is to increase the sample size, extend the follow-up duration, and conduct prospective multi-center randomized controlled trials to validate the findings.
5 Conclusion
Multiple intravitreal injections of conbercept can improve the BCVA and reduce macular edema in patients with BRVO. They demonstrated no significant effect on the morphology of corneal endothelium and CCT.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Zibo Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
GZ: Formal Analysis, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review and editing. TS: Methodology, Writing – original draft, Writing – review and editing. YS: Software, Writing – original draft, Writing – review and editing. NL: Resources, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tah V, Orlans H, Hyer J, Casswell E, Din N, Sri Shanmuganathan V, et al. Anti-VEGF therapy and the retina: An update. J Ophthalmol. (2015) 2015:627674. doi: 10.1155/2015/627674
2. Konidaris V, Tsaousis K, Anzidei R, de la Mata G, Brent AJ. Real-world results of switching treatment from ranibizumab to aflibercept in macular oedema secondary to branch retinal vein occlusion. Ophthalmol Ther. (2018) 7:387–95. doi: 10.1007/s40123-018-0149-3
3. Yau J, Lee P, Wong T, Best J, Jenkins A. Retinal vein occlusion: An approach to diagnosis, systemic risk factors and management. Intern Med J. (2009) 38:904–10. doi: 10.1111/j.1445-5994.2008.01720.x
4. Rogers S, McIntosh R, Lim L, Mitchell P, Cheung N, Kowalski J, et al. Natural history of branch retinal vein occlusion: An evidence-based systematic review. Ophthalmology. (2010) 117:1094–1101.e5. doi: 10.1016/j.ophtha.2010.01.058
5. McIntosh R, Rogers S, Lim L, Cheung N, Wang J, Mitchell P, et al. Natural history of central retinal vein occlusion: An evidence-based systematic review. Ophthalmology. (2010) 117:1113–1123.e15. doi: 10.1016/j.ophtha.2010.01.060
6. Campochiaro P, Brown D, Awh C, Lee S, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology. (2011) 118:2041–9. doi: 10.1016/j.ophtha.2011.02.038
7. Chen X, Hu T, Zuo J, Wu H, Liu Z, Zhan Y, et al. Intravitreal conbercept for branch retinal vein occlusion induced macular edema: One initial injection versus three monthly injections. BMC Ophthalmol. (2020) 20:225. doi: 10.1186/s12886-020-01494-x
8. Ratra D, Murari S, Dalan D, Agarwal V. Immediate response to intravitreal treatment for macular edema due to diabetes and retinal vein occlusion. Eur J Ophthalmol. (2024) 35:298–305. doi: 10.1177/11206721241255721
9. Shor R, Segal O, Barequet D, Greenbaum E, Trivizki O, Loewenstein A, et al. Branch retinal vein occlusion treated with anti-VEGF: To switch or not to switch? Can J Ophthalmol. (2024) 59:e824–9. doi: 10.1016/j.jcjo.2024.01.016
10. Chalam K, Agarwal S, Brar V, Murthy R, Sharma R. Evaluation of cytotoxic effects of bevacizumab on human corneal cells. Cornea. (2009) 28:328–33. doi: 10.1097/ICO.0b013e31818b8be0
11. Urban B, Szwabowicz M, Bakunowicz-Łazarczyk A. Effect of repeated intravitreal ranibizumab and aflibercept injections on the cornea in patients with age-related macular degeneration. J Ophthalmol. (2020) 2020:4928905. doi: 10.1155/2020/4928905
12. Merz P, Röckel N, Ballikaya S, Auffarth G, Schmack I. Effects of ranibizumab (Lucentis®) and bevacizumab (Avastin®) on human corneal endothelial cells. BMC Ophthalmol. (2018) 18:316. doi: 10.1186/s12886-018-0978-9
13. Zhang X, Bao S, Lai D, Rapkins R, Gillies M. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. (2008) 57:1026–33. doi: 10.2337/db07-0982
14. Tababat-Khani P, Bengtsson B, Agardh E. Effects of focal/grid laser treatment on the central visual field in diabetic macular oedema: A 2-year follow-up study. Acta Ophthalmol. (2016) 94:240–5. doi: 10.1111/aos.12956
15. The Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. the central vein occlusion study group M report. Ophthalmology. (1995) 102:1425–33. doi: 10.1016/s0161-642030849-4
16. Nonaka R, Noma H, Yasuda K, Sasaki S, Goto H, Shimura M. Visual acuity and retinal thickness and sensitivity after intravitreal ranibizumab injection for macular edema in branch retinal vein occlusion. J Clin Med. (2024) 13:2490. doi: 10.3390/jcm13092490
17. Delsoz M, Mousavi S, Aslam S. Treatment outcomes for maculopathy secondary to retinal vein occlusion in Afghanistan. Oman J Ophthalmol. (2024) 17:43–6. doi: 10.4103/ojo.ojo_328_22
18. Song W, Jiao W, Li F, Ma A, Zhao B. Evaluation of microvascular structure changes after conbercept treatment on macular edema secondary to retinal vein occlusion. Biomed Res Int. (2020) 2020:9046781. doi: 10.1155/2020/9046781
19. Zhu X, Yu Y, Zhuoga S, Dawa X, Zhou Y, Wangmu O, et al. Real-world outcomes of anti-vascular endothelial growth factor therapy for retinal vascular vein occlusion in Tibet, China. Int J Ophthalmol. (2022) 15:1814–20. doi: 10.18240/ijo.2022.11.12
20. Haller J, Bandello F, Belfort R, Blumenkranz M, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. (2011) 118:2453–60. doi: 10.1016/j.ophtha.2011.05.014
21. Kuppermann B, Blumenkranz M, Haller J, Williams G, Weinberg D, Chou C, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. (2007) 125:309–17. doi: 10.1001/archopht.125.3.309
22. Qian T, Zhao M, Xu X. Comparison between anti-VEGF therapy and corticosteroid or laser therapy for macular oedema secondary to retinal vein occlusion: A meta-analysis. J Clin Pharm Ther. (2017) 42:519–29. doi: 10.1111/jcpt.12551
23. Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. (2000) 41:2514–22.
24. Wang X, Sawada T, Kakinoki M, Miyake T, Kawamura H, Saishin Y, et al. Aqueous vascular endothelial growth factor and ranibizumab concentrations after monthly and bimonthly intravitreal injections of ranibizumab for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. (2013) 252:1033–9. doi: 10.1007/s00417-013-2505-2
25. Lin DS, Zhou ZT, Huang XQ, Xiao LY, Zhang SB. Effect of repeated intravitreal aflibercept injections on the cornea in patients with macular edema. Rec Adv Ophthalmol. (2021) 41:368–70.
26. Arslan G, Guven D, Alkan A, Kacar H, Demir M. Short term effects of intravitreal anti-vascular endothelial growth factor agents on cornea, anterior chamber, and intraocular pressure. Cutan Ocul Toxicol. (2019) 38:344–8.
27. Yang X, Xu J, Wang R, Mei Y, Lei H, Liu J, et al. A randomized controlled trial of conbercept pretreatment before vitrectomy in proliferative diabetic retinopathy. J Ophthalmol. (2016) 2016:2473234. doi: 10.1155/2016/2473234
28. Zhang Q, Hou Y, Cao X, Zhang R, Liu Y, Wei C, et al. Predictors of visual recovery in patients with macular edema secondary to central retinal vein occlusion after treatment with Conbercept. BMC Ophthalmol. (2021) 21:402. doi: 10.1186/s12886-021-02174-0
29. Ding X, Wang Y, Zou B, Zang D, Hao Y. Effect of conbercept treatment on macular edema and microvascular structure in eyes with retinal vein occlusions. Int J Gen Med. (2022) 15:7311–8. doi: 10.2147/IJGM.S373015
30. Huang Y, Linghu M, Hu W, Huang X. Conbercept improves macular microcirculation and retinal blood supply in the treatment of nonischemic branch retinal vein occlusion macular edema. J Clin Lab Anal. (2022) 36:e24774. doi: 10.1002/jcla.24774
31. Xing Q, Dai Y, Huang X, Peng L. Comparison of efficacy of conbercept, aflibercept, and ranibizumab ophthalmic injection in the treatment of macular edema caused by retinal vein occlusion: A meta-analysis. Int J Ophthalmol. (2023) 16:1145–54. doi: 10.18240/ijo.2023.07.21
32. Pérez-Rico C, Benítez-Herreros J, Castro-Rebollo M, Gómez-Sangil Y, Germain F, Montes-Mollón M, et al. Effect of intravitreal ranibizumab on corneal endothelium in age-related macular degeneration. Cornea. (2010) 29:849–52. doi: 10.1097/ICO.0b013e3181ca33d2
33. Bai J, Wang W, Dou Z, Geng B, Xu X, Zhu Y, et al. Efficacy of intravitreal conbercept injection on short– and long-term macular edema in branch retinal vein occlusion. Int J Ophthalmol. (2022) 15:489–94. doi: 10.18240/ijo.2022.03.18
Keywords: conbercept, branch retinal vein occlusion, macular edema, cornea, best-corrected vision acuity
Citation: Zhai G, Shen T, Su Y and Liu N (2025) Effect of multiple intravitreal injections of conbercept on the cornea in patients with branch retinal vein occlusion-induced macular edema. Front. Med. 12:1595543. doi: 10.3389/fmed.2025.1595543
Received: 18 March 2025; Accepted: 09 June 2025;
Published: 25 June 2025.
Edited by:
Giovanni Luca Romano, Kore University of Enna, ItalyReviewed by:
Rosalia Battaglia, University of Catania, ItalyChiara Pennisi, Kore University of Enna, Italy
Copyright © 2025 Zhai, Shen, Su and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Liu, NDE3MDUxODgzQHFxLmNvbQ==
 Gaixia Zhai
Gaixia Zhai Tiehui Shen
Tiehui Shen Yuanzhen Su
Yuanzhen Su Na Liu*
Na Liu*