- 1Department of Orthopedics, Changde First Hospital of Traditional Chinese Medicine, Changde, Hunan, China
- 2Department of Graduate School, Hunan University of Traditional Chinese Medicine, Changsha, Hunan, China
- 3Department of Orthopedics, Dongguan Hospital of Guangzhou University of Chinese Medicine, Dongguan, Guangzhou, China
Background: The relationship between serum vitamins, carotenoids, and retinyl esters and mortality risk among individuals with osteoarthritis (OA) remains unclear. This study aimed to investigate these associations.
Methods: Based on data from NHANES 2001 to 2018 and the National Death Index (NDI), a total of 3,626 patients with OA were included. Cox proportional hazards models were used to evaluate the associations between serum nutrient levels and all-cause, cardiovascular, and cancer mortality. Nonlinear effects were assessed using smooth curve fitting and piecewise regression models. Subgroup analyses, sensitivity analyses, and validation in non-OA populations were conducted. Additionally, interactions between vitamin levels and OA status were examined.
Results: Higher levels of vitamin D, retinyl palmitate, and stearic acid were associated with a reduced risk of all-cause mortality in patients with OA, with consistent results in sensitivity analyses. A nonlinear inverse association was observed between vitamin D and all-cause mortality in women, with a threshold at 30.5 nmol/L. Vitamin C was associated with cardiovascular mortality, while retinyl palmitate and stearic acid were linked to a reduced risk of cancer-related death. The protective effect of vitamin D was stronger among individuals with lower educational levels. In the non-OA population, only vitamin D was associated with mortality. Interaction analysis indicated that high vitamin levels may attenuate the adverse impact of OA on mortality risk.
Conclusions: Elevated serum levels of vitamin D, retinyl palmitate, and stearic acid may be associated with reduced all-cause and cause-specific mortality among individuals with OA, highlighting their potential role in the management of OA.
1 Introduction
Osteoarthritis (OA), a chronic joint disorder characterized by progressive cartilage degeneration, significantly impairs the quality of life and functional status among middle-aged and older adults worldwide (1, 2). Since the beginning of the 21st century, the global burden of OA has continued to rise in parallel with population growth and accelerated aging. This trend has also been accompanied by disparities in regional case distributions and disease management capacities (3, 4). Previous studies have predominantly focused on the disabling effects and quality-of-life consequences of OA (5, 6), while limited attention has been paid to the role of nutritional strategies in the long-term prognostic management of OA, representing a crucial gap in the literature.
Serum vitamins, carotenoids, and retinyl esters, as essential antioxidant micronutrients, are actively involved in immune regulation, inflammation control, and cellular homeostasis (7–9). Emerging evidence suggests that folate, vitamin B12, and vitamin D may play critical roles in the progression and clinical outcomes of OA (10–13). Importantly, the intake and availability of these nutrients are relatively less influenced by socioeconomic disparities, implying that adequate nutritional status could be achievable even in resource-limited settings. Therefore, identifying modifiable, nutrition-related factors may offer new perspectives for the long-term management of OA, especially in underserved populations.
In recent years, a growing number of studies have explored the associations between serum vitamins, carotenoids, and retinyl esters and mortality risks in various health conditions, including SARS-CoV-2 infection, prediabetes, and depression (14–16). However, the potential impact of these micronutrients on mortality risk among individuals with OA remains largely unexplored. Based on these findings, this study systematically explored the associations between various serum vitamins, carotenoids, and vitamin A esters and mortality risk in patients with OA. Through multi-level analyses, the robustness and specificity of the results were validated, aiming to provide epidemiological evidence for precision nutritional interventions and long-term health management in the OA population.
2 Methods
2.1 Study population
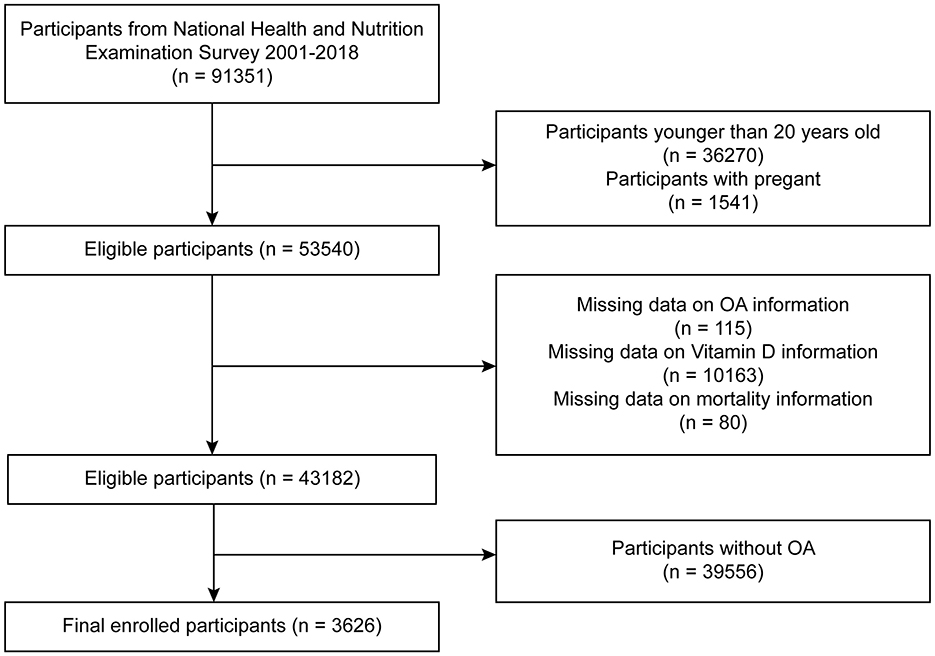
This study was based on publicly available data from the NHANES, which employs a stratified multistage sampling design to represent the non-institutionalized U.S. population. We utilized data from nine consecutive NHANES cycles spanning from 2001 to 2018 (each cycle representing a 2-year interval). A total of 91,351 participants were initially identified. We sequentially excluded individuals who met the following criteria: age <20 years (n = 36,270), pregnant women (n = 1,541), missing OA status (n = 115), missing serum vitamin D data (n = 10,163), missing mortality follow-up information (n = 80), and individuals without OA (n = 39,556). The final analytic sample included 3,626 participants with diagnosed OA (Figure 1). Participants with missing serum vitamin D data were excluded because vitamin D was the main exposure variable. To avoid potential bias, we did not use multiple imputation for this variable, as imputing primary exposures with non-random missingness may introduce error. This is a common approach in epidemiologic studies focused on exposure-outcome associations. To maximize the use of available data, individual datasets were separately cleaned for each specific vitamin or carotenoid variable. Detailed procedures are illustrated in Supplementary Figures 1A, B.
2.2 Definition of study variables
Data on serum vitamins (C, D, and E), vitamin A, and its related compounds—including carotenoids (α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and trans-lycopene) and vitamin A esters (retinyl palmitate and retinyl stearate)—were obtained from laboratory measurements in NHANES. These biomarkers were quantified using high-performance liquid chromatography (HPLC), and they provide an objective assessment of individuals' nutritional status. OA status was determined based on the Medical Conditions Questionnaire (MCQ) module in NHANES. Participants were classified as having OA if they answered “Yes” to the question, “Doctor ever said you had Osteoarthritis.” Those who answered “No” were defined as non-OA participants, indicating that they had not been diagnosed with OA by a healthcare professional. The questionnaire was administered by trained interviewers in participants' homes using a computer-assisted personal interviewing (CAPI) system, which included built-in consistency checks and terminology prompts to improve data quality and minimize interview bias.
Covariates included in the analysis were as follows: (1) Demographic variables: age, gender, race/ethnicity, education level, marital status, and the poverty-income ratio (PIR), categorized as ≤ 1.3 (low), ≤ 3.5 (middle), and >3.5 (high), all derived from NHANES demographic datasets. (2) Anthropometric and laboratory variables: alanine aminotransferase (ALT), aspartate aminotransferase (AST), body mass index (BMI), and waist circumference. (3) Comorbidities and lifestyle factors: hypertension, diabetes, cardiovascular disease (CVD), smoking status, and drinking status. The detailed definitions and diagnostic criteria for these variables are provided in Supplementary material 1.
2.3 Mortality data
Mortality data were obtained through the linkage between NHANES and the NDI, with follow-up through December 31, 2019. Deaths were identified by matching population variables, and causes of death were classified according to ICD-10 codes. Participants without a matching record were considered alive. Among the included OA participants, there were a total of 734 all-cause deaths, including 202 deaths due to cardiovascular disease and 165 cancer-related deaths. As the database did not provide information on specific cancer types, cancer mortality was broadly defined and encompassed all deaths attributable to malignant neoplasms.
2.4 Statistical analysis
The analysis accounted for the complex sampling design of NHANES by using weighted methods to enhance representativeness. Continuous variables were presented as weighted means with 95% confidence intervals (CIs), and comparisons between groups were conducted using weighted linear regression. Categorical variables were expressed as weighted percentages and compared using the chi-square test. Kaplan–Meier curves and three Cox proportional hazards models were used to assess the associations between serum nutrients and mortality risk. Nonlinear relationships were examined using smooth curve fitting and segmented Cox models, with threshold effects determined by log-likelihood ratio tests. Stratified and interaction analyses were performed, and sensitivity analyses included excluding participants who died within the first 2 years of follow-up and those with baseline preCVD. The main analyses were replicated in the non-OA population, and interaction effects were further explored by grouping participants according to nutrient levels and OA status. All analyses were conducted using R version 4.2.0 and EmpowerStats version 2.0, with a two-sided P-value < 0.05 considered statistically significant.
3 Results
3.1 Baseline characteristics of participants with OA
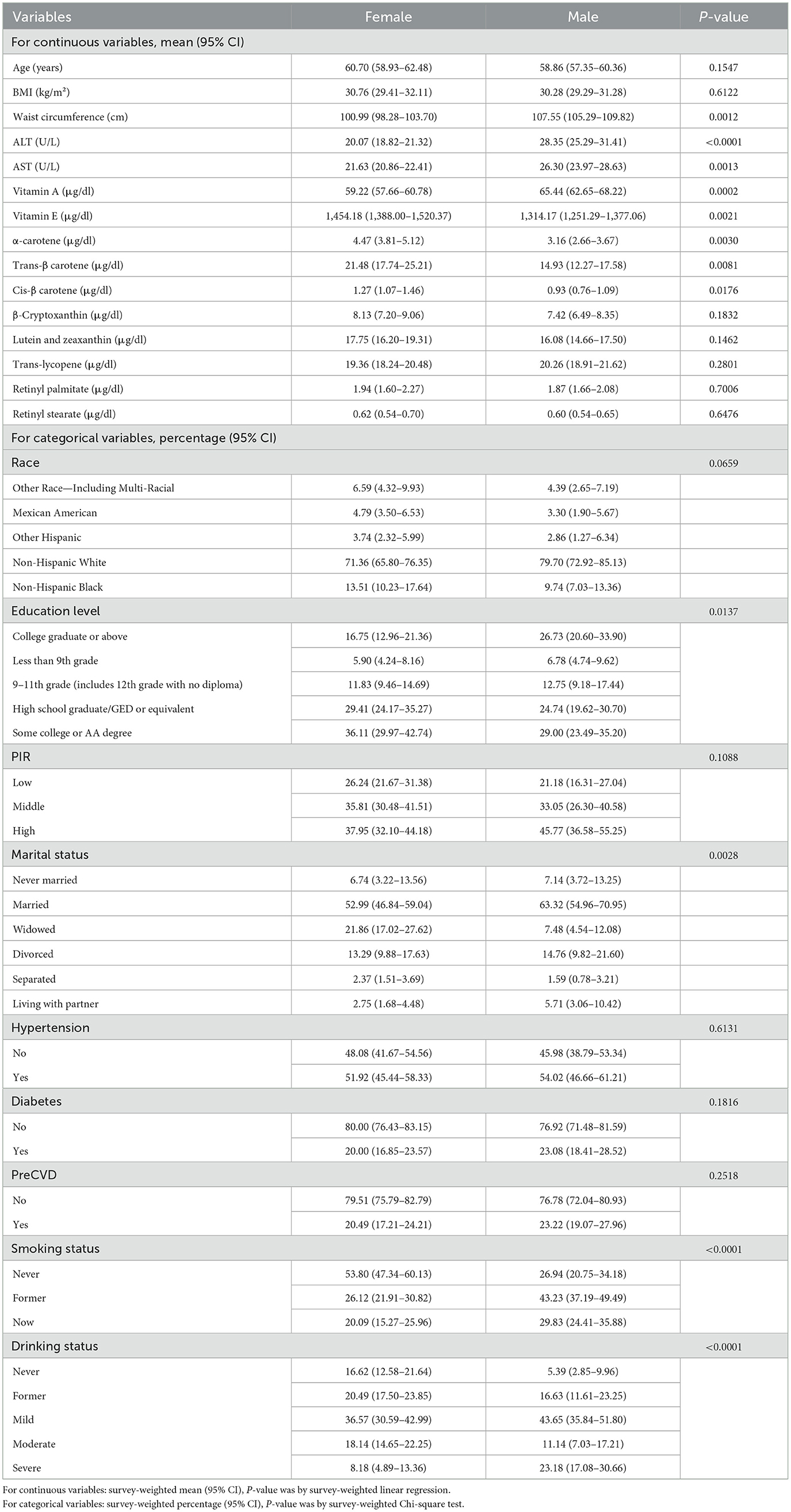
Gender-stratified analysis of baseline characteristics among participants with OA revealed significant differences across multiple anthropometric and biochemical indicators. Male participants exhibited significantly higher waist circumference, ALT and AST levels compared to females. In contrast, female participants had notably higher serum levels of vitamins A, C, D, and E, as well as several carotenoids, including α-carotene, trans-β-carotene, cis-β-carotene, and lutein + zeaxanthin (Table 1).
Regarding sociodemographic factors, education level differed significantly between gender, with a higher proportion of males having attained post-secondary education. In terms of lifestyle and clinical characteristics, smoking status varied markedly: a much greater proportion of females reported never smoking, whereas the prevalence of “now” and “former” smokers was substantially higher among males. Similarly, significant gender differences were observed in alcohol consumption, with a higher proportion of heavy drinkers among males. No significant gender differences were observed for age, β-cryptoxanthin, trans-lycopene, retinyl palmitate, retinyl stearate, race/ethnicity, hypertension, diabetes, or cardiovascular disease history. Detailed baseline characteristics stratified by gender and serum vitamin C or vitamin D levels are presented in Supplementary Table S1.
3.2 Associations between serum vitamins, carotenoids, retinyl esters, and mortality risk in OA patients
Kaplan–Meier survival curves were generated to visually compare survival probabilities across exposure groups (Figure 2). Multivariable Cox proportional hazards regression models were then applied to examine the associations between serum nutrient levels and mortality risk, adjusting for a range of covariates (Table 2). In the analysis of all-cause mortality, Model 3 (fully adjusted) revealed that higher serum levels of vitamin D, retinyl palmitate, and retinyl stearate were significantly associated with reduced mortality risk.
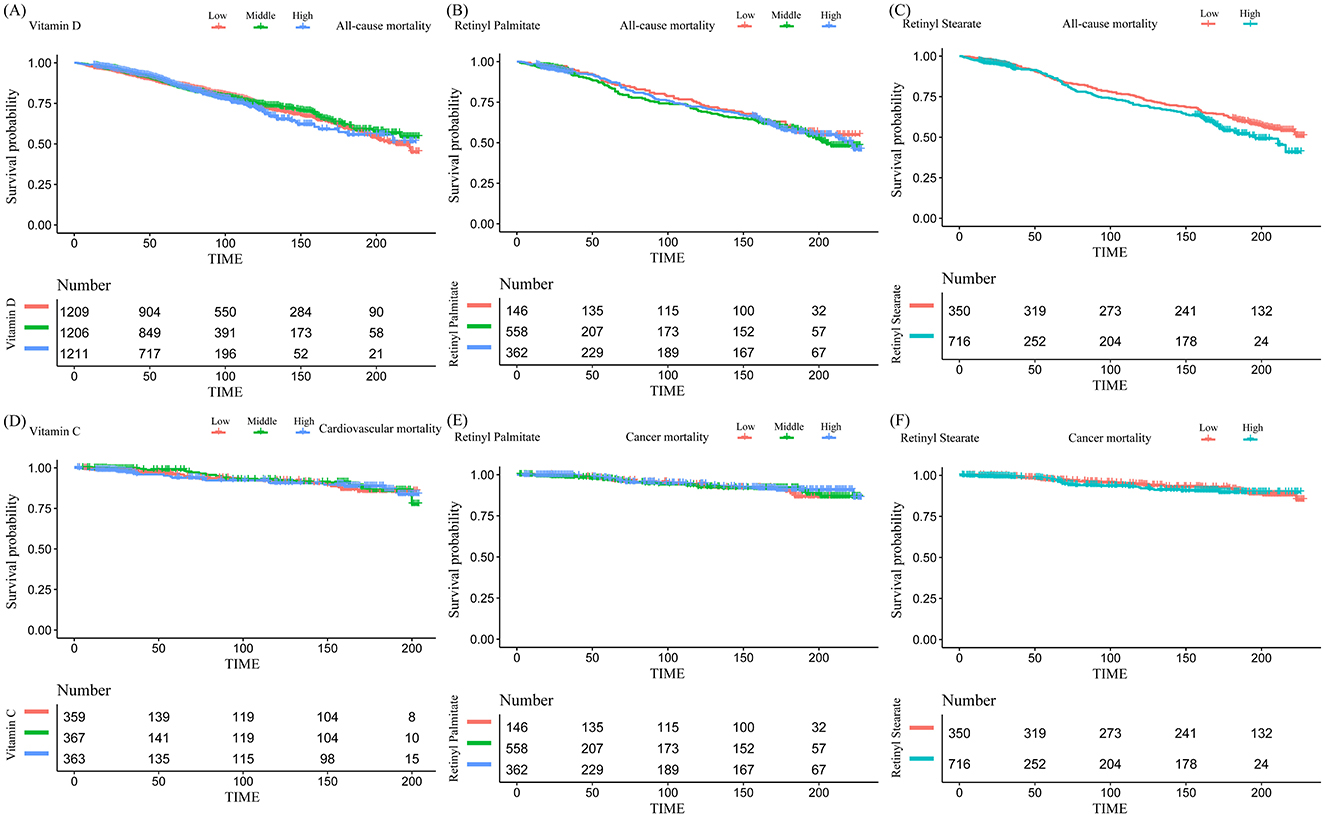
Figure 2. Kaplan–Meier curves for the associations between serum vitamin and retinyl ester levels and mortality risk in patients with osteoarthritis. (A) Vitamin D and all-cause mortality; (B) retinyl palmitate and all-cause mortality; (C) retinyl stearate and all-cause mortality; (D) vitamin C and cardiovascular mortality; (E) retinyl palmitate and cancer mortality; (F) retinyl stearate and cancer mortality.
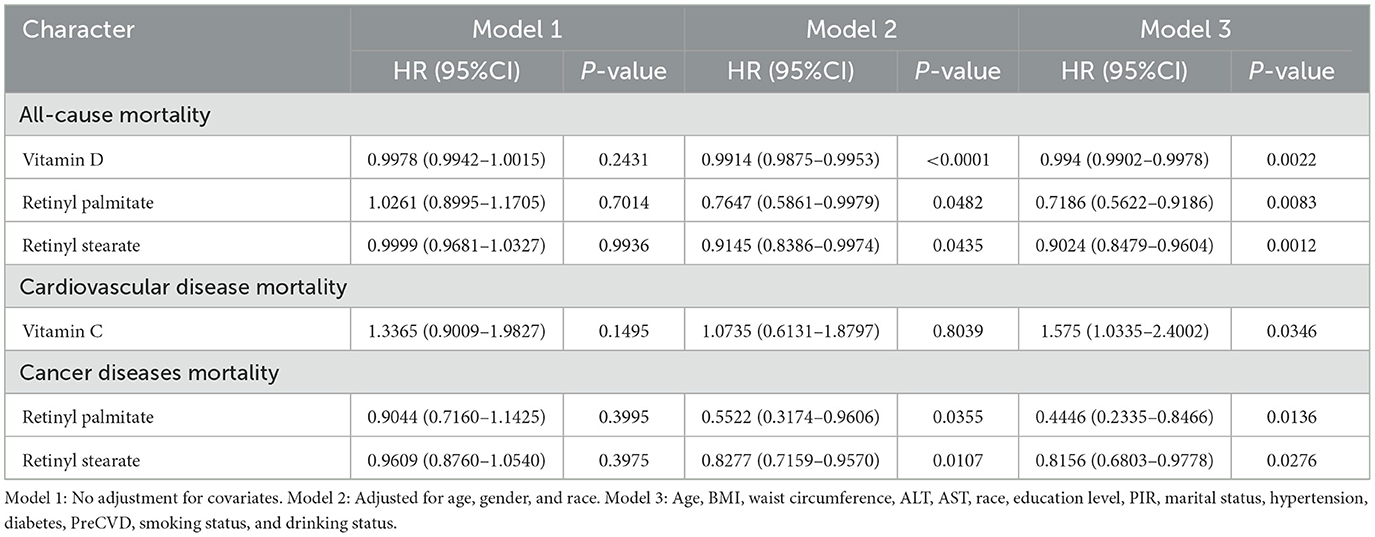
Table 2. Cox regression analysis of serum vitamins, carotenoids, and retinyl esters in relation to mortality among osteoarthritis patients.
Among the examined biomarkers, vitamin D was significantly associated with reduced all-cause mortality [HR = 0.994, 95% CI: (0.9902–0.9978), P = 0.0022]. Similarly, higher levels of retinyl palmitate [0.7186 (0.5622–0.9186), P = 0.0083] and retinyl stearate [0.9024 (0.8479–0.9604), P = 0.0012] were also associated with decreased mortality risk. In the analysis of cardiovascular mortality, elevated vitamin C concentrations were significantly associated with increased risk of cardiovascular death [1.575 (1.0335–2.4002), P = 0.0346]. Regarding cancer-specific mortality, Model 3 demonstrated that higher levels of retinyl palmitate [0.4446 (0.2335–0.8466), P = 0.0136] and retinyl stearate [0.8156 (0.6803–0.9778), P = 0.0276] were significantly associated with a lower risk of death. No significant associations were observed between other serum vitamins or carotenoids and mortality risk in OA patients. Detailed results are provided in Supplementary Tables S2A–C.
3.3 Nonlinear association analysis
Smoothing spline curves suggested potential nonlinear associations between serum vitamins, carotenoids, and retinyl esters levels and mortality risk among OA patients (Figure 3). To further examine threshold effects, two-piecewise Cox proportional hazards models were employed (Table 3). A significant inverse association was observed between serum vitamin D levels and all-cause mortality [0.99 (0.99–1.00), P < 0.0001]. The identified inflection point was 48.3 nmol/L. Below this threshold, each 1 nmol/L increase in serum vitamin D was associated with a 2% reduction in all-cause mortality risk [0.98 (0.97–0.99), P < 0.0001] above this level, the association was no longer statistically significant. A U-shaped association was detected between serum vitamin C levels and cardiovascular mortality. The inflection point was 1.15 mg/dl. Below this level, higher vitamin C concentrations were associated with decreased cardiovascular mortality (0.53, 0.23–1.25). However, above the threshold, higher levels were significantly associated with increased mortality risk [2.42 (1.45–4.04), P = 0.0007]. In subgroup analysis among female OA patients, serum vitamin D levels were inversely associated with all-cause mortality in a nonlinear pattern. The turning point was identified at 30.5 nmol/L. Below this level, each 1 nmol/L increase in vitamin D was associated with a 7% reduction in mortality risk [0.93 (0.90–0.96), P < 0.0001], whereas the association was not significant above this point (Supplementary Table S3). All log-likelihood ratio tests for nonlinear effects yielded P < 0.05, indicating statistically significant threshold effects.
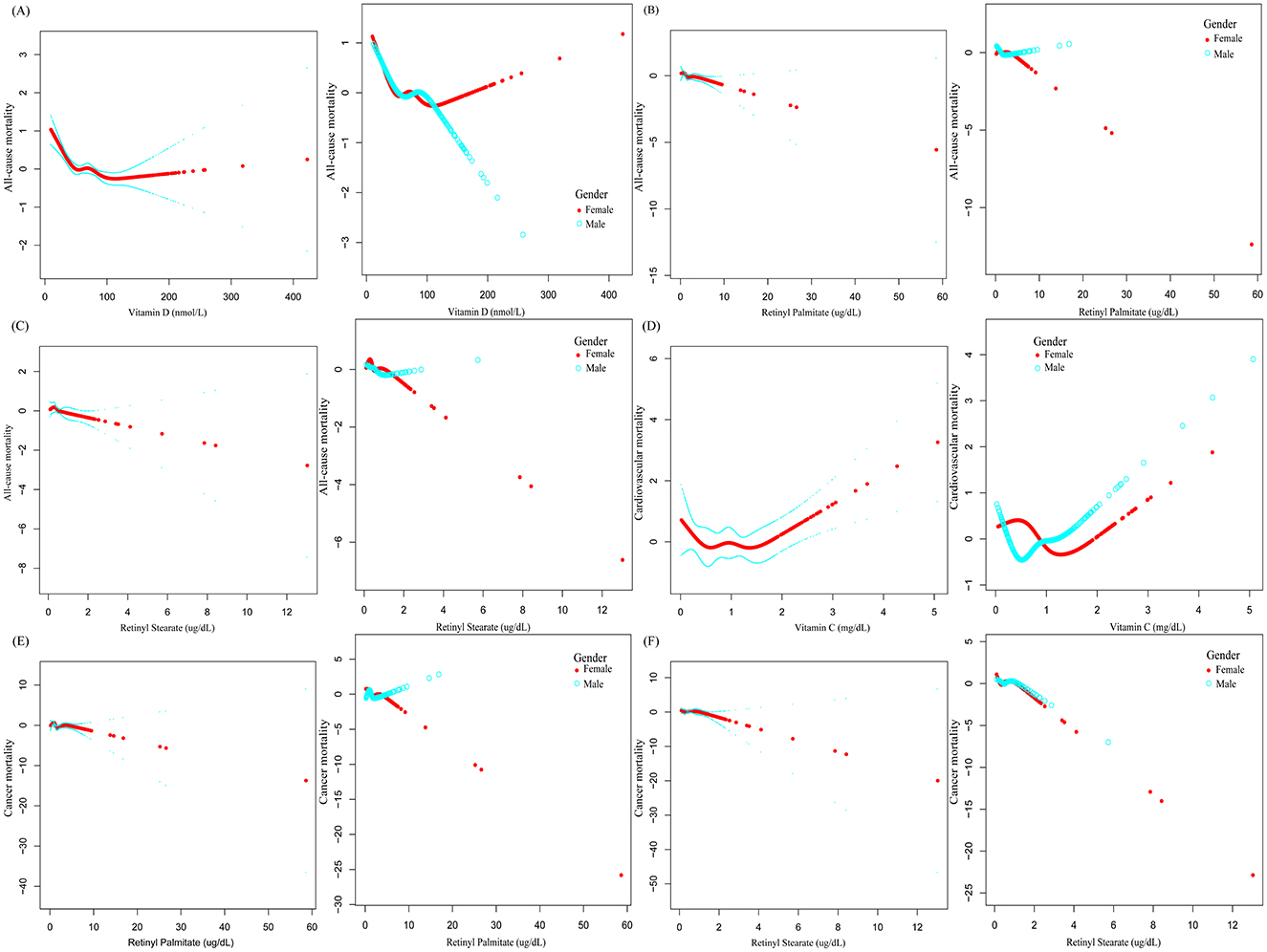
Figure 3. The association between serum vitamin, carotenoid, and retinyl ester levels and mortality risk in patients with OA.
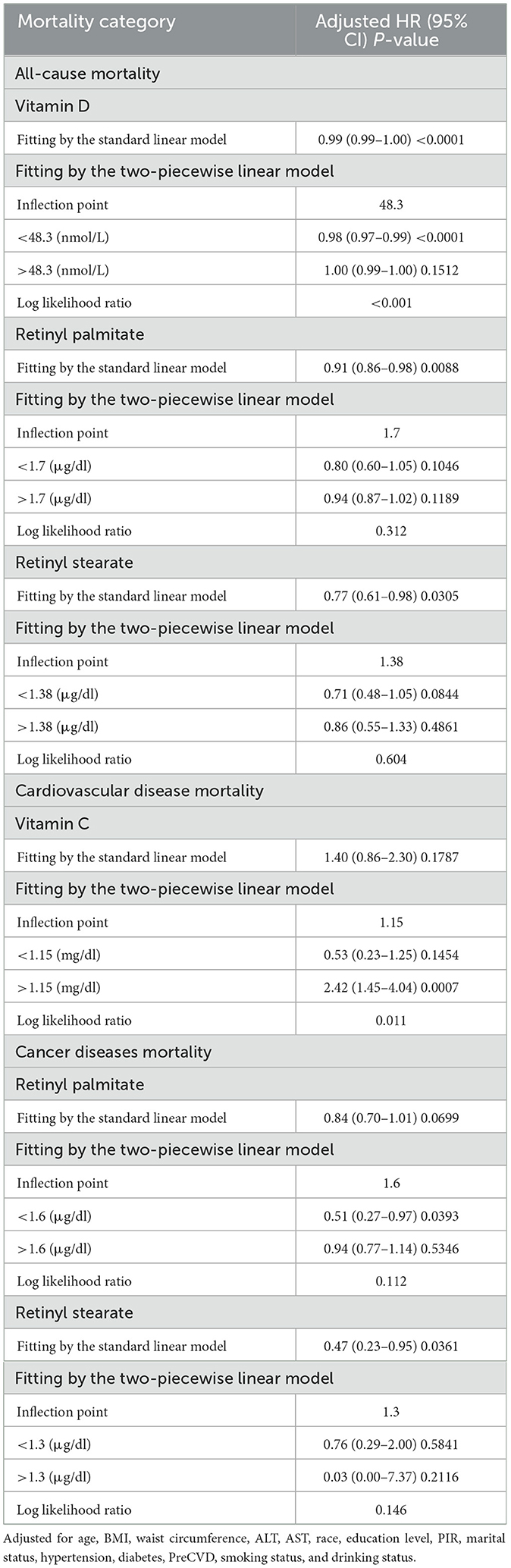
Table 3. Threshold effect analysis of serum vitamins, carotenoids, and retinyl esters in relation to mortality risk among patients with OA.
3.4 Subgroup analysis
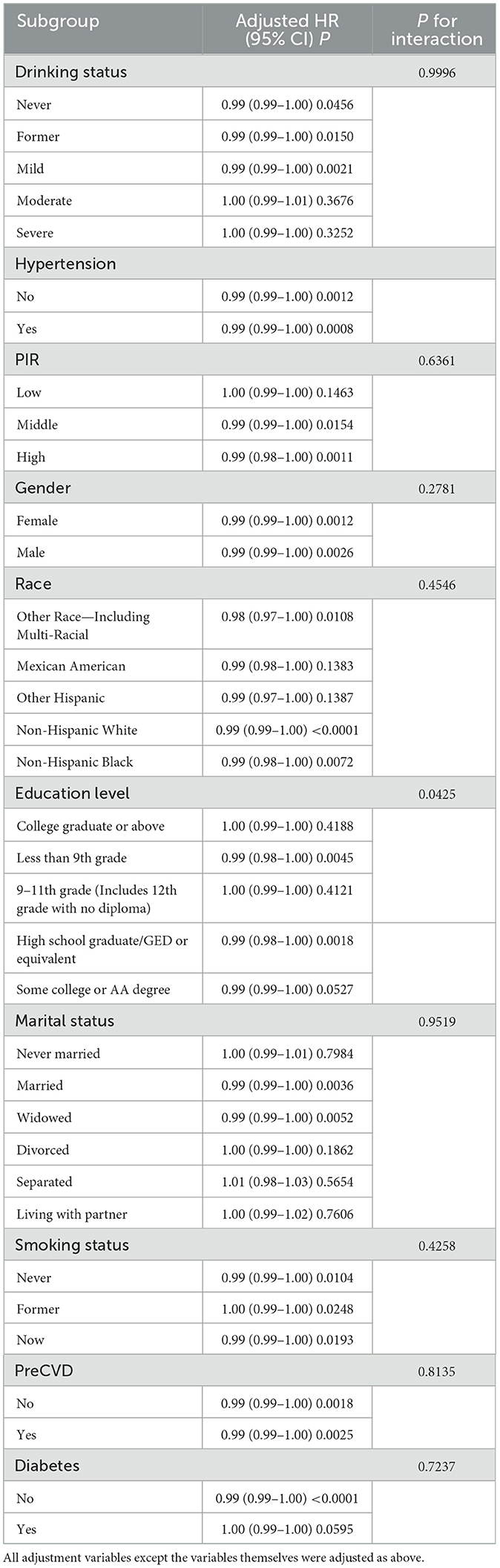
Subgroup analyses revealed that the inverse association between serum vitamin D levels and all-cause mortality in OA patients was more pronounced among individuals with lower educational attainment. Specifically, this association remained statistically significant in participants with <9th grade education (HR = 0.99, P = 0.0045) and those with a high school diploma or equivalent (HR = 0.99, P = 0.0018), but was not significant in individuals with higher education levels (Table 4). Additionally, in participants with <9th grade education, higher levels of serum retinyl stearate were significantly associated with an increased risk of all-cause mortality [1.45 (1.09–1.92), P = 0.0107], whereas no such association was observed among those with higher education (Supplementary Table S4). The P-values for interaction were <0.05 for both analyses, indicating significant effect modification by education level. No significant effect modification was observed in other predefined subgroups (detailed in Supplementary Table S4).
3.5 Sensitivity analysis
In the sensitivity analysis excluding participants who died within the first two years of follow-up, the fully adjusted Model 3 for all-cause mortality showed consistent inverse associations: vitamin D [0.9938 (0.9888–0.9989), P = 0.0162], retinyl palmitate [0.8866 (0.8281–0.9493), P = 0.0005], and retinyl stearate [0.6744 (0.4995–0.9105), P = 0.0101]. For cardiovascular mortality, the association with vitamin C was not statistically significant [1.8393 (0.7651–4.4219), P = 0.1733]. For cancer-related mortality, retinyl palmitate [0.852 (0.6871–1.0566), P = 0.1446] and retinyl stearate [0.5152 (0.2495–1.0638), P = 0.0730] showed similar trends as in the main analysis, though statistical significance was not reached. In the second sensitivity analysis excluding participants with pre-existing cardiovascular disease (Pre-CVD), the inverse association between serum vitamin D and all-cause mortality remained significant [0.9949 (0.9901–0.9997), P = 0.0374], as did the association with retinyl palmitate [0.911 (0.8326–0.9968), P = 0.0424]. The association between retinyl stearate and all-cause mortality was attenuated and no longer statistically significant [0.7607 (0.5084–1.1382), P = 0.1834]. For cardiovascular mortality, vitamin C again showed a non-significant positive association [1.7054 (0.8961–3.2455), P = 0.1040]. For cancer mortality, both retinyl palmitate [0.7851 (0.5817–1.0596), P = 0.1138] and retinyl stearate [0.5603 (0.2612–1.2015), P = 0.1367] remained non-significant but directionally consistent with the main findings. Baseline and sensitivity analysis results for all-cause, cardiovascular, and cancer-specific mortality are presented in Supplementary Tables S5, S6, 7A, B.
3.6 Associations between serum vitamins, carotenoids, retinyl esters, and mortality risk in non-OA participants
To assess whether the associations identified in Section 3.2 were specific to individuals with OA, we conducted parallel analyses in participants without OA. In the analysis of all-cause mortality using Model 3, serum vitamin D remained significantly associated with lower mortality risk [0.994 (0.9923–0.9957), P < 0.0001], while no significant associations were observed for retinyl palmitate [1.0084 (0.9903–1.0267), P = 0.3662] or retinyl stearate [1.0414 (0.9939–1.0912), P = 0.0888].
For cardiovascular mortality, vitamin C levels were not significantly associated with mortality risk [0.8834 (0.7429–1.0504), P = 0.1605]. Regarding cancer mortality, neither retinyl palmitate [1.018 (0.9846–1.0525), P = 0.2948) nor retinyl stearate [1.0376 (0.9232–1.1663), P = 0.5355) was associated with mortality risk. These findings suggest that the observed associations in OA patients may not extend to the general population. Full results are presented in Supplementary Table S8.
3.7 Combined effects of serum vitamins, carotenoids, retinyl esters levels and OA status on mortality risk
To further examine the interaction between serum nutrient levels and OA status, participants were stratified into four groups based on OA status (yes/no) and serum vitamin or carotenoid levels (low/high, dichotomized at the median). In Model 3 for all-cause mortality: participants with low vitamin D and OA [Low/OA (+)] had significantly increased mortality risk compared to the reference group [Low/OA (–)] [1.1959 (1.0297–1.3890), P = 0.0191]. In contrast, those without OA and with high vitamin D levels [High/OA (–)] had significantly reduced risk [0.7808 (0.7255–0.8402), P < 0.0001]. For retinyl palmitate, the [Low/OA (+)] group also had higher mortality risk [1.3914 (1.1195–1.7294), P = 0.0029], while the [High/OA (+)] group showed no significant difference. Similarly, for retinyl stearate, the [Low/OA (+)] group showed elevated mortality risk [1.4084 (1.0946–1.8122), P = 0.0077], with no significant association in the [High/OA (+)] group. These results suggest that higher levels of vitamin D, retinyl palmitate, and retinyl stearate may attenuate the detrimental effect of OA on all-cause mortality risk. In the analysis of cancer-specific mortality, participants with low retinyl palmitate levels and OA [Low/OA (+)] had a significantly increased risk compared to [Low/OA (–)] [1.6143 (1.0608–2.4564), P = 0.0254], while no significant association was observed in the high-level group. These findings further support a potential protective effect of higher retinyl palmitate levels in individuals with OA (Table 5).
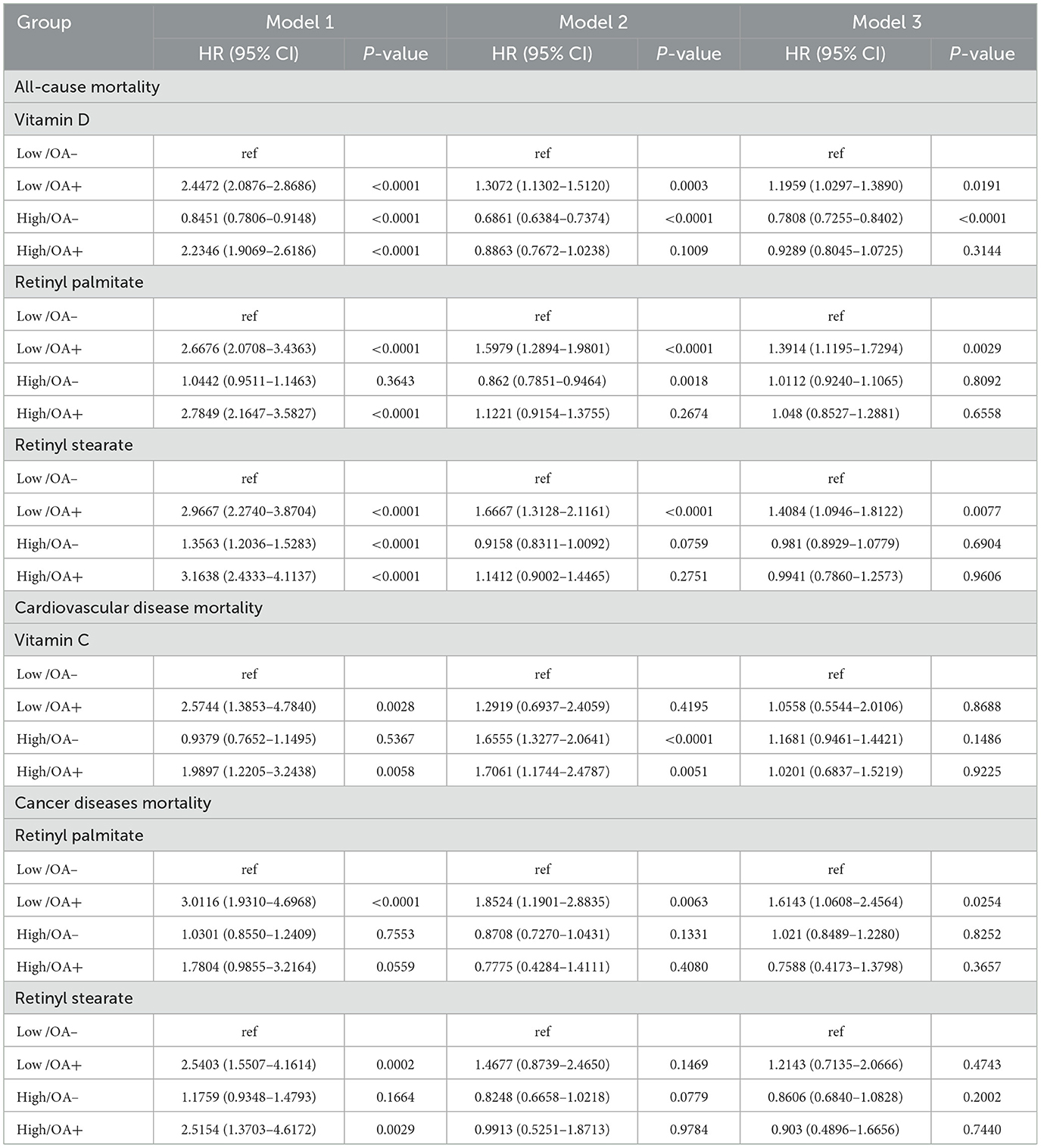
Table 5. Association between serum vitamins, carotenoids, retinyl esters, and OA status in relation to mortality.
4 Discussion
To our knowledge, this is the first nationwide, population-based study to systematically assess the associations between multiple serum vitamins, carotenoids, retinyl esters, and mortality risk among individuals with OA. This study yields three principal findings. First, elevated serum levels of vitamin D, retinyl palmitate, and retinyl stearate were significantly associated with reduced risk of all-cause mortality in OA patients. These associations remained robust across sensitivity analyses. Second, a U-shaped association was observed between serum vitamin C and cardiovascular mortality, suggesting the existence of a critical threshold in its usage and effect. Third, higher levels of retinyl palmitate and retinyl stearate were also associated with reduced cancer-specific mortality, highlighting their potential role in cancer prognosis among OA patients.
Moreover, nonlinear modeling revealed a significant threshold effect between vitamin D levels and all-cause mortality risk, with a turning point at 48.3 nmol/L. Below this threshold, vitamin D appeared protective, but no further benefit was observed beyond this level. This aligns with findings by Xu et al. (17), who reported that serum vitamin D levels exceeding 84.8 nmol/L were no longer associated with bone mineral density (BMD), with a notably lower threshold in females. Interestingly, our study similarly identified a much lower turning point for vitamin D in female OA patients—only 30.5 nmol/L. This suggests that women with OA may be more vulnerable to even mild vitamin D deficiency, and that subclinical insufficiency could elevate their mortality risk. As such, clinical strategies for vitamin D supplementation in this population should focus not only on overt deficiency but also on avoiding “borderline insufficiency.” Consistent patterns have been reported in studies of older adults with heart failure, where women were more likely to fall into low vitamin D categories and had markedly higher all-cause mortality (up to 26% in the lowest tertile group) (18). Vitamin D deficiency is also widely associated with adverse outcomes in chronic conditions such as cardiovascular disease, chronic kidney disease, and osteoporosis (19–23), underscoring its clinical importance. Notably, although higher levels of vitamin D did not appear to confer additional survival benefit in our study, “L-shaped” or even “U-shaped” relationships have been previously reported in other populations (24, 25). Our findings regarding the U-shaped association of vitamin C with cardiovascular mortality also suggest a potential threshold-dependent effect. However, a recent umbrella review analyzing multiple RCTs found that high-dose intravenous vitamin C (≥6 g/day), while occasionally associated with side effects such as hypernatremia and oxalate nephropathy, did not significantly alter the risk of major cardiovascular events compared to placebo (26). Most evidence to date suggests that normal or moderately high levels of vitamin C exert antioxidant and vasoprotective effects, particularly in populations with deficiency or high cardiovascular risk, rather than among the general healthy population (27). Nonetheless, the role of vitamin C in cardiovascular mortality remains controversial, and the U-shaped relationship observed here should be interpreted cautiously (28, 29).
To explore potential effect modifications across different strata, we conducted further subgroup analyses. The results showed that the protective effects of serum vitamin D and retinyl stearate on all-cause mortality in OA patients were more pronounced among individuals with lower educational attainment, particularly those with less than a 9th-grade education. These findings are consistent with previous studies. For example, Wang et al. (30) reported that individuals with higher vitamin D levels tended to have favorable characteristics such as higher educational attainment, nonsmoking status, and lower BMI, all of which are independently associated with reduced mortality risk. Interestingly, although educational level showed significant heterogeneity in our subgroup analysis, we did not observe similar differences across strata defined by the PIR, a common measure of economic status. This suggests that for nutrients such as vitamins—relatively easy to access—the strength of their association with mortality risk may not be primarily determined by economic conditions per se, but rather by health literacy shaped by educational background. Stormacq et al. (31) identified education as the most critical determinant of health literacy, which in turn mediates the relationship between socioeconomic status and health outcomes. In other words, it may not be that people “can't afford to eat well,” but rather that they “don't know how to eat well,” underscoring the potential public health value of promoting health education in low-education populations (32).
Vitamin D, vitamin C, as well as retinyl palmitate and retinyl stearate, are all essential micronutrients for the human body. Vitamin D is a fat-soluble vitamin that primarily exists in two forms: D2 (ergocalciferol) and D3 (cholecalciferol) (33). Its main functions include promoting the absorption of calcium and phosphorus, maintaining stable blood calcium levels, and facilitating bone mineralization. Additionally, it plays a crucial role in immune system regulation (34, 35). Vitamin D can be synthesized in the skin through exposure to ultraviolet (UV) rays, or obtained through the consumption of vitamin D-rich foods such as fatty fish, egg yolks, and liver, as well as fortified foods like vitamin D-fortified milk (36). Vitamin C (ascorbic acid) is a water-soluble vitamin with strong antioxidant properties. It scavenges free radicals in the body, reduces oxidative stress, and promotes collagen synthesis, thereby benefiting the health of bones, skin, and blood vessels (29, 37). In addition, vitamin C is involved in iron absorption and the maintenance of immune function (38). As the human body cannot synthesize vitamin C on its own, it mainly relies on dietary intake from fresh fruits (such as citrus fruits and strawberries) and vegetables (such as broccoli and spinach) (39). Retinyl Palmitate and Retinyl Stearate are esterified storage forms of vitamin A formed by the combination of retinol and fatty acids. They are mainly found in animal liver, whole-fat dairy products, and eggs (40). After ingestion, they can be hydrolyzed in the body into the active form of retinol, which participates in physiological processes such as retinal function, maintenance of skin and mucosal barriers, and immune responses (41–43).
It is worth noting that although vitamin A has been reported to benefit bone development in adolescents (44, 45), excessive intake of vitamin A—particularly through high-dose supplements—has been confirmed to be associated with neurotoxicity, hepatotoxicity, decreased bone mineral density, and an increased risk of fractures in the elderly population (46–49). Moreover, current toxicity studies on retinyl palmitate have primarily focused on its phototoxicity and the potential for inducing mood-related changes with long-term use (50–52). Research on whether retinyl palmitate or stearate themselves have potential toxic effects at appropriate doses remains limited, and no definitive conclusions have been reached. Overall, these micronutrients enjoy wide public acceptance and accessibility. Compared with other interventions, nutritional approaches are more suitable for promotion within primary healthcare systems, particularly in resource-limited settings. If well-implemented, targeted nutritional supplementation programs at the primary care level hold promise as effective strategies to improve the health of patients with OA and help reduce healthcare disparities. However, large-scale prospective studies across diverse populations and regions are still needed to validate their effectiveness and optimize application strategies.
In addition, because our primary analyses focused on individuals with OA, we conducted verification analyses among non-OA participants to assess whether the observed associations were specific to the OA population. In the non-OA group, no significant associations were observed between retinyl palmitate, retinyl stearate, and mortality outcomes. This suggests that the protective effects of these compounds on mortality may be specific to individuals with OA, possibly due to disease-specific pathological mechanisms. Currently, research on the roles of retinyl palmitate and retinyl stearate in OA remains scarce. Previous studies have only reported that retinyl palmitate may modulate cytogenotoxic events induced by cyclophosphamide (CPA) and doxorubicin (DOX) (53). Barker et al. (54) found no evidence that retinyl palmitate adversely affects fracture risk. Additionally, low levels of retinyl palmitate and retinyl stearate have been linked to peripheral artery disease and cognitive impairment in older adults (55, 56). Although the precise mechanisms remain unclear, it is plausible that these compounds may exert their protective effects in OA by mitigating inflammation, suppressing local oxidative stress in the joints, or modulating related signaling pathways. Given the paucity of research in this area, further investigations are warranted to elucidate their potential mechanistic roles and therapeutic value in OA-related outcomes.
In the final validation analysis combining serum vitamins, carotenoids, retinyl esters levels and OA status, we found that among participants with low levels of serum vitamin D, retinyl palmitate, or retinyl stearate, those with OA had significantly higher mortality risk compared to the non-OA reference group. This highlights the adverse impact of OA on survival. However, in the High/OA (+) groups, no significant difference in mortality risk was observed compared to the reference group. Notably, the High/OA (+) group for vitamin D even showed a significantly lower risk of death. These findings suggest that higher serum levels of vitamin D, retinyl palmitate, and retinyl stearate may effectively offset the detrimental effect of OA on mortality, further supporting their potential role in improving survival among OA patients. Future large-scale clinical studies are warranted to validate these findings and to explore the feasibility of these nutrients as targeted interventions in OA management.
In this study, serum data for B vitamins, folate, and vitamin K were largely missing across different NHANES cycles. Therefore, these variables were not included in the analysis. Given the potential roles of these nutrients in the pathogenesis of OA and in chronic disease management, future research should investigate these associations when complete data become available. Additionally, this study did not adjust for dietary intake or the use of vitamin supplements, which may have influenced the accuracy of serum nutrient levels and introduced potential confounding bias. Although serum concentrations can serve as an integrated marker of nutritional status, they may not fully reflect long-term exposure and are susceptible to recent dietary or supplement-related fluctuations. Thus, future studies should incorporate comprehensive dietary assessments and detailed information on supplement use to validate and expand upon our findings. Moreover, OA status in the NHANES database is based on self-reported physician diagnoses, without information on disease duration, severity, or progression. This limitation restricts our ability to assess the long-term role of serum nutrients in OA management. Therefore, the findings of this study should be interpreted with caution, and prospective cohort studies with complete clinical documentation are warranted to further explore these associations. Although the number of cancer-related deaths in this study was sufficient to support the primary analysis, statistical power may still be limited for more granular subgroup analyses, such as those focusing on specific cancer types or populations. Lastly, the identification of OA in NHANES relied on participants' responses to the question, “Has a doctor ever told you that you had osteoarthritis?” While this approach is widely used in large-scale epidemiological studies and administered by trained personnel via the CAPI system to minimize systematic bias, the risk of recall bias and misclassification remains, especially in the absence of radiographic or clinical verification. Future research should incorporate objective diagnostic criteria to enhance the accuracy of OA classification and the reliability of study outcomes.
This study has several notable strengths: (1) The use of nationally representative data from a large population-based sample enhances the generalizability of our findings. (2) To our knowledge, this is the first study to comprehensively evaluate the associations between multiple serum vitamins, carotenoids, retinyl esters, and mortality in OA patients. (3) A robust, multi-level analytic approach—including nonlinear modeling, sensitivity analyses, subgroup analyses, and replication in non-OA controls—was employed to enhance the credibility and applicability of our conclusions. Nonetheless, certain limitations should be acknowledged. First, we cannot completely rule out the possibility of residual confounding. One notable limitation of this study is the absence of urban-rural residency status as a potential confounder. Although we partially accounted for socioeconomic status using the PIR, the NHANES dataset does not explicitly classify participants based on urban or rural residence. However, substantial differences may exist between urban and rural populations in terms of dietary patterns, food sources, and lifestyle behaviors. For instance, individuals living in rural areas may consume more natural, unprocessed, or self-produced foods, whereas urban residents may have greater access to processed foods and dietary supplements. Differences in dietary and lifestyle factors may lead to variations in serum nutrient levels, potentially influencing the associations observed with bone health outcomes. The lack of this variable may introduce a degree of confounding bias, warranting further consideration and validation in future studies. Moreover, this study did not incorporate genetic factors as potential confounders. Individual genetic backgrounds may influence not only susceptibility to OA but also the absorption, metabolism, and distribution of vitamins in the body, which could in turn affect the associations between serum vitamin levels and OA or mortality observed in this study. Due to the lack of comprehensive genotyping data in the NHANES survey cycles utilized, we were unable to account for residual confounding attributable to genetic variation. Future research should aim to integrate genetic information to better elucidate the potential causal relationships between vitamin levels and the development and prognosis of OA.
5 Conclusion
Among individuals with OA, higher serum levels of vitamin D, retinyl palmitate, and retinyl stearate were associated with a lower risk of mortality, with the associations being particularly evident in women and individuals with lower educational attainment. These findings provide important epidemiological evidence to support individualized nutritional intervention strategies in OA management. Further large-scale clinical studies are warranted to validate these associations and elucidate the underlying biological mechanisms.
Data availability statement
Publicly available datasets were analyzed in this study. The raw data and analysis code supporting the findings of this study are not publicly available but can be obtained from the corresponding author upon reasonable request. Currently, the data are not deposited in any public repository and therefore do not have an accession number.
Ethics statement
The NHANES study protocols were approved by the NCHS Research Ethics Review Board. All participants provided written informed consent before participation. As this study involved the secondary analysis of publicly available de-identified data, additional institutional ethical approval was not required. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YL: Writing – original draft, Writing – review & editing. JuD: Writing – original draft. RY: Writing – original draft. ST: Writing – original draft. XQ: Writing – original draft. BW: Writing – original draft. GJ: Writing – original draft. TW: Writing – original draft. JiD: Writing – review & editing. ZY: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Hunan Province (Grant Nos. 2022JJ30087 and 2025JJ70653), the Scientific Research Project of Traditional Chinese Medicine in Hunan Province (Grant Nos. D2022033 and C2023031), the Natural Science Foundation of Changsha (Grant No. kq2402177), the Guiding Project for Scientific and Technological Innovation in Changde (Grant No. 2024ZD75), the Scientific Research Project of Hunan University of Chinese Medicine (Grant Nos. 2022XYLH045 and 2022XYLH055), and the Graduate Innovation Project of Hunan University of Chinese Medicine (Grant No. 2024CX129).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1609759/full#supplementary-material
Supplementary Figure 1A | Flow chart (vitamin C).
Supplementary Figure 1B | Flow chart (vitamin E, A, carotenoids, vitamin A esters).
Supplementary Table S1 | Baseline characteristics of the OA patient population based on sex and vitamin C and D.
Supplementary Table S2A | Cox regression analysis of serum vitamins, carotenoids, and retinyl esters and their non-significant associations with all-cause mortality in OA patients.
Supplementary Table S2B | Cox regression analysis of serum vitamins, carotenoids, and retinyl esters and their non-significant associations with cardiovascular mortality in OA patients.
Supplementary Table S2C | Cox regression analysis of serum vitamins, carotenoids, and retinyl esters and their non-significant associations with cancer mortality in OA patients.
Supplementary Table S3 | Gender-stratified threshold effect analysis of serum vitamins, carotenoids, and retinyl esters and mortality risk in OA patients.
Supplementary Table S4 | Subgroup analyses in other sections.
Supplementary Table S5 | Baseline characteristics of participants who died within the first 2 years of follow-up.
Supplementary Table S6 | Baseline characteristics excluding participants with preCVD.
Supplementary Table S7A | Cox regression analysis of serum vitamins, carotenoids, and retinyl esters and mortality in OA patients, excluding early deaths.
Supplementary Table S7B | Cox regression analysis of serum vitamins, carotenoids, and retinyl esters and mortality in OA patients, excluding participants with pre-existing CVD.
Supplementary Table S8 | Cox regression analysis of serum vitamins, carotenoids, and retinyl esters and mortality risk in participants without OA.
References
1. Tang S, Zhang C, Oo WM, Fu K, Risberg MA, Bierma-Zeinstra SM, et al. Osteoarthritis. Nat Rev Dis Primers. (2025) 11:10. doi: 10.1038/s41572-025-00594-6
2. Courties A, Kouki I, Soliman N, Mathieu S, Sellam J. Osteoarthritis year in review 2024: epidemiology and therapy. Osteoarthritis Cartilage. (2024) 32:1397–404. doi: 10.1016/j.joca.2024.07.014
3. Cao F, Xu Z, Li XX, Fu ZY, Han RY, Zhang JL, et al. Trends and cross-country inequalities in the global burden of osteoarthritis, 1990-2019: a population-based study. Ageing Res Rev. (2024) 99:102382. doi: 10.1016/j.arr.2024.102382
4. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. (2020) 72:149–62. doi: 10.1002/acr.24131
5. Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. (2014) 66:622–36. doi: 10.1002/art.38290
6. Yan H, Guo J, Zhou W, Dong C, Liu J. Health-related quality of life in osteoarthritis patients: a systematic review and meta-analysis. Psychol Health Med. (2022) 27:1859–74. doi: 10.1080/13548506.2021.1971725
7. Wei N, Dai Z. The role of nutrition in osteoarthritis: a literature review. Clin Geriatr Med. (2022) 38:303–22. doi: 10.1016/j.cger.2021.11.006
8. Zheng XY, Liang J, Li YS, Tu M. Role of fat-soluble vitamins in osteoarthritis management. J Clin Rheumatol. (2018) 24:132–7. doi: 10.1097/RHU.0000000000000587
9. Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis. Rheumatology. (2018) 57:iv61–74. doi: 10.1093/rheumatology/key011
10. Hong H, Chen L, Zhong Y, Yang Z, Li W, Song C, et al. Associations of homocysteine, folate, and vitamin B12 with osteoarthritis: a Mendelian randomization study. Nutrients. (2023) 15:1636. doi: 10.3390/nu15071636
11. Rai V, Radwan MM, Agrawal DK. IL-33, IL-37, and vitamin D interaction mediate immunomodulation of inflammation in degenerating cartilage. Antibodies. (2021) 10:41. doi: 10.3390/antib10040041
12. Sakalyte R, Denkovskij J, Bernotiene E, Stropuviene S, Mikulenaite SO, Kvederas G, et al. The expression of inflammasomes NLRP1 and NLRP3, toll-like receptors, and vitamin D receptor in synovial fibroblasts from patients with different types of knee arthritis. Front Immunol. (2021) 12:767512. doi: 10.3389/fimmu.2021.767512
13. Mende LK, Kuthati Y, Wong CS. Curcumin and vitamin D supplement attenuates knee osteoarthritis progression in ACLT + MMx rat model: effect on cartilage protection and pain reduction. Nutrients. (2025) 17:349. doi: 10.3390/nu17020349
14. D'Ecclesiis O, Gavioli C, Martinoli C, Raimondi S, Chiocca S, Miccolo C, et al. Vitamin D and SARS-CoV2 infection, severity and mortality: a systematic review and meta-analysis. PLoS ONE. (2022) 17:e0268396. doi: 10.1371/journal.pone.0268396
15. Ren W, Li Y, Lu C, Liu S, Shao Y, Shi X. Comprehensive assessment on the association of dietary vitamins with all-cause and cardiovascular mortality among individuals with prediabetes: evidence from NHANES 1999-2018. Food Funct. (2024) 15:10037–50. doi: 10.1039/D4FO02893G
16. Mao Y, Li X, Li Y, Zhu S, Han X, Zhao R, et al. Association of serum 25-hydroxyvitamin d concentrations with all-cause and cause-specific mortality among individuals with depression: a cohort study. J Affect Disord. (2024) 352:10–8. doi: 10.1016/j.jad.2024.02.018
17. Xu B, Li Q, Luo B, Liu H. Does higher serum 25-hydroxyvitamin D levels will harm bone mineral density?: a cross-sectional study. BMC Endocr Disord. (2024) 24:250. doi: 10.1186/s12902-024-01760-9
18. Yilmaz Öztekin GM, Genç A, Arslan S. Vitamin D deficiency is a predictor of mortality in elderly with chronic heart failure. Acta Endocrinol. (2021) 17:358–64. doi: 10.4183/aeb.2021.358
19. Reese JA, Davis E, Fretts AM, Ali T, Lee ET, Umans JG, et al. Vitamin D deficiency and cardiovascular disease risk factors among American Indian adolescents: the Strong Heart Family Study. Prev Chronic Dis. (2025) 22:E13. doi: 10.5888/pcd22.240354
20. Hung KC Yu TS, Hung IY, Wu JY, Yew M, Chen IW. Impact of vitamin D deficiency on postoperative outcomes in patients with chronic kidney disease undergoing surgery: a retrospective study. Sci Rep. (2025) 15:9757. doi: 10.1038/s41598-025-93807-7
21. Zhao S, Qian F, Wan Z, Chen X, Pan A, Liu G. Vitamin D and major chronic diseases. Trends Endocrinol Metab. (2024) 35:1050–61. doi: 10.1016/j.tem.2024.04.018
22. Wimalawansa SJ. Controlling chronic diseases and acute infections with vitamin D sufficiency. Nutrients. (2023) 15:3623. doi: 10.3390/nu15163623
23. Montemor CN, Fernandes M, Marquez AS, Bignardi PR, Poli RC, Gâmbaro GA, et al. Impact of reduced vitamin D levels on pain, function, and severity in knee or hip osteoarthritis. Nutrients. (2025) 17:447. doi: 10.3390/nu17030447
24. Wang J, Fan J, Yang Y, Moazzen S, Chen D, Sun L, et al. Vitamin D status and risk of all-cause and cause-specific mortality in osteoarthritis patients: results from NHANES III and NHANES 2001-2018. Nutrients. (2022) 14:4629. doi: 10.3390/nu14214629
25. Shi JW, Wu JN, Zhu XY, Zhou WH, Yang JY, Li MQ. Association of serum 25-hydroxyvitamin D levels with all-cause and cause-specific mortality among postmenopausal females: results from NHANES. J Transl Med. (2023) 21:629. doi: 10.1186/s12967-023-04413-y
26. Yanase F, Fujii T, Naorungroj T, Belletti A, Luethi N, Carr AC, et al. Harm of IV high-dose vitamin C therapy in adult patients: a scoping review. Crit Care Med. (2020) 48:e620–8. doi: 10.1097/CCM.0000000000004396
27. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. (2016) 17:1328. doi: 10.3390/ijms17081328
28. Al-Khudairy L, Flowers N, Wheelhouse R, Ghannam O, Hartley L, Stranges S, et al. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. (2017) 3:CD011114. doi: 10.1002/14651858.CD011114.pub2
29. Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, et al. Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients. (2021) 13:615. doi: 10.3390/nu13020615
30. Wang TY, Wang HW, Jiang MY. Prevalence of vitamin D deficiency and associated risk of all-cause and cause-specific mortality among middle-aged and older adults in the United States. Front Nutr. (2023) 10:1163737. doi: 10.3389/fnut.2023.1163737
31. Stormacq C, Van den Broucke S, Wosinski J. Does health literacy mediate the relationship between socioeconomic status and health disparities? Integrative review. Health Promot Int. (2019) 34:e1–17. doi: 10.1093/heapro/day062
32. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. (2011) 155:97–107. doi: 10.7326/0003-4819-155-2-201107190-00005
33. Delrue C, Speeckaert MM. Vitamin D and vitamin D-binding protein in health and disease. Int J Mol Sci. (2023) 24:4642. doi: 10.3390/ijms24054642
34. Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. (2008) 10:110–7. doi: 10.1007/s11926-008-0020-y
35. Bizzaro G, Antico A, Fortunato A, Bizzaro N. Vitamin D and autoimmune diseases: is vitamin D receptor (VDR) polymorphism the culprit. Isr Med Assoc J. (2017) 19:438–43. Available online at: https://www.ima.org.il/MedicineIMAJ/viewarticle.aspx?year=2017&month=07&page=438
36. Benedik E. Sources of vitamin D for humans. Int J Vitam Nutr Res. (2022) 92:118–25. doi: 10.1024/0300-9831/a000733
37. Pullar JM, Carr AC, Vissers M. The roles of vitamin C in skin health. Nutrients. (2017) 9:866. doi: 10.3390/nu9080866
38. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. (2017) 9:1211. doi: 10.3390/nu9111211
39. Fenech M, Amaya I, Valpuesta V, Botella MA. Vitamin C content in fruits: biosynthesis and regulation. Front Plant Sci. (2018) 9:2006. doi: 10.3389/fpls.2018.02006
40. Souganidis E, Laillou A, Leyvraz M, Moench-Pfanner R. A comparison of retinyl palmitate and red palm oil β-carotene as strategies to address vitamin A deficiency. Nutrients. (2013) 5:3257–71. doi: 10.3390/nu5083257
41. Crabtree DV, Adler AJ, Snodderly DM. Vitamin E, retinyl palmitate, and protein in rhesus monkey retina and retinal pigment epithelium-choroid. Invest Ophthalmol Vis Sci. (1996) 37:47–60.
42. Fu PP, Xia Q, Boudreau MD, Howard PC, Tolleson WH, Wamer WG. Physiological role of retinyl palmitate in the skin. Vitam Horm. (2007) 75:223–56. doi: 10.1016/S0083-6729(06)75009-9
43. Gensler HL, Aickin M, Peng YM. Cumulative reduction of primary skin tumor growth in UV-irradiated mice by the combination of retinyl palmitate and canthaxanthin. Cancer Lett. (1990) 53:27–31. doi: 10.1016/0304-3835(90)90006-J
44. Ling L. Association between serum vitamin A and bone mineral density in adolescents. Sci Rep. (2025) 15:6892. doi: 10.1038/s41598-025-91367-4
45. Zhang X, Huang J, Zhou Y, Hong Z, Lin X, Chen S, et al. Vitamin A nutritional status is a key determinant of bone mass in children. Nutrients. (2022) 14:4694. doi: 10.3390/nu14214694
46. Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr. (1999) 69:1071–85. doi: 10.1093/ajcn/69.6.1071
47. Hathcock JN, Hattan DG, Jenkins MY, McDonald JT, Sundaresan PR, Wilkening VL. Evaluation of vitamin A toxicity. Am J Clin Nutr. (1990) 52:183–202. doi: 10.1093/ajcn/52.2.183
49. de Jonge EA, Kiefte-de Jong JC, Campos-Obando N, Booij L, Franco OH, Hofman A, et al. Dietary vitamin A intake and bone health in the elderly: the Rotterdam Study. Eur J Clin Nutr. (2015) 69:1360–8. doi: 10.1038/ejcn.2015.154
50. Yan J, Xia Q, Cherng SH, Wamer WG, Howard PC, Yu H, et al. Photo-induced DNA damage and photocytotoxicity of retinyl palmitate and its photodecomposition products. Toxicol Ind Health. (2005) 21:167–75. doi: 10.1191/0748233705th225oa
51. Boudreau MD, Beland FA, Felton RP, Fu PP, Howard PC, Mellick PW, et al. Photo-co-carcinogenesis of topically applied retinyl palmitate in SKH-1 hairless mice. Photochem Photobiol. (2017) 93:1096–114. doi: 10.1111/php.12730
52. Schnorr CE, Bittencourt Lda S, Petiz LL, Gelain DP, Zeidán-Chuliá F, Moreira JC. Chronic retinyl palmitate supplementation to middle-aged Wistar rats disrupts the brain redox homeostasis and induces changes in emotional behavior. Mol Nutr Food Res. (2015) 59:979–90. doi: 10.1002/mnfr.201400637
53. de Carvalho RM, Aguiar R, Islam MT, de Alencar M, da Mata A, Braga AL, et al. Cytogenotoxicological defense of retinyl palmitate in the front damage of antineoplastics. Exp Toxicol Pathol. (2017) 69:293–7. doi: 10.1016/j.etp.2017.01.013
54. Barker ME, McCloskey E, Saha S, Gossiel F, Charlesworth D, Powers HJ, et al. Serum retinoids and beta-carotene as predictors of hip and other fractures in elderly women. J Bone Miner Res. (2005) 20:913–20. doi: 10.1359/JBMR.050112
55. Mazidi M, Wong ND, Katsiki N, Mikhailidis DP, Banach M. Dietary patterns, plasma vitamins and trans fatty acids are associated with peripheral artery disease. Lipids Health Dis. (2017) 16:254. doi: 10.1186/s12944-017-0635-y
Keywords: osteoarthritis, serum vitamins, carotenoids, mortality, NHANES
Citation: Lu Y, Duan J, You R, Tang S, Qi X, Wu B, Jian G, Wang T, Duan J and Yang Z (2025) Do serum vitamins, carotenoids, and retinyl esters influence mortality in osteoarthritis? Insights from a nationally representative study. Front. Nutr. 12:1609759. doi: 10.3389/fnut.2025.1609759
Received: 10 April 2025; Accepted: 28 May 2025;
Published: 19 June 2025.
Edited by:
Jianlin Shen, Affiliated Hospital of Putian University, ChinaReviewed by:
Wei Wei, Harbin Medical University, ChinaMayana Bsoul, Tulane University, United States
Copyright © 2025 Lu, Duan, You, Tang, Qi, Wu, Jian, Wang, Duan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuo Yang, eXo3ODAxOTlAMTYzLmNvbQ==; Jianhui Duan, ZGpoMTkxMDI1QDE2My5jb20=
 Yifan Lu
Yifan Lu Junjie Duan1
Junjie Duan1 Zhuo Yang
Zhuo Yang