- Department of Breast Diseases, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Breast cancer has become the second most common cancer after lung cancer. Patients may present with skin manifestations at the time of initial diagnosis, while erysipel-like carcinoma typically appears later, following initial treatment. This delay increases the risk of misdiagnosis.
Case presentation: The patient was a 51-year-old female. A modified radical mastectomy for left breast carcinoma (pT2N3M0, stage IIIC; tumor size 4.6 cm × 4.5 cm × 1.6 cm, 14/21 axillary lymph nodes involved), HER2-positive type, was performed on April 21, 2021. In April 2024 (three years post-surgery), the patient developed unexplained redness and swelling in the skin of the left upper limb, accompanied by increased skin temperature. This was misdiagnosed as erysipelas of the upper limb. After one week of antibiotic treatment, the redness and swelling slightly subsided. In May 2024, the patient experienced dizziness and headaches without any obvious cause. Enhanced cranial MRI revealed multiple brain metastases, with possible lymph node metastasis in the left cervical region. The patient underwent whole-brain radiotherapy. During radiotherapy, erysipelas-like rashes developed on the left chest wall, upper limb, and right breast skin. In June 2024, a skin biopsy of the chest wall confirmed cutaneous metastasis. Following systemic anti-tumor treatment, both the skin and brain metastasis improved.
Conclusion: Pathological biopsy should be emphasized when breast cancer patients develop localized rashes. Understanding the unique inflammatory manifestations of cutaneous metastasis is crucial for breast oncologists to enable early diagnosis, timely treatment, and improved overall survival.
Introduction
Global cancer statistics for 2022 indicate that breast cancer has become the second most common cancer among women after lung cancer, with an annual incidence rate increase of 0.6%-1% (1, 2). It can metastasize to atypical sites such as the gastrointestinal tract, skin, and other organs (3, 4). The clinical manifestations of skin metastasis in breast cancer include red patches, purplish-red papules, small blisters, and asymptomatic hard nodules on the breast and adjacent skin. Among these, carcinoma erysipeloides (CE) accounts for only 3% of breast cancer skin metastases (5), and its appearance closely resembles erysipelas, making it easily misdiagnosed. Skin metastases may present with clinical features resembling erysipeloid rash, complicating diagnosis and increasing the risk of misdiagnosis. This paper reports a case of advanced invasive ductal carcinoma of the breast with dermatitis-like skin metastasis, aiming to provide insights and guidance for diagnosis and treatment. Informed consent was obtained from the patient.
Case report
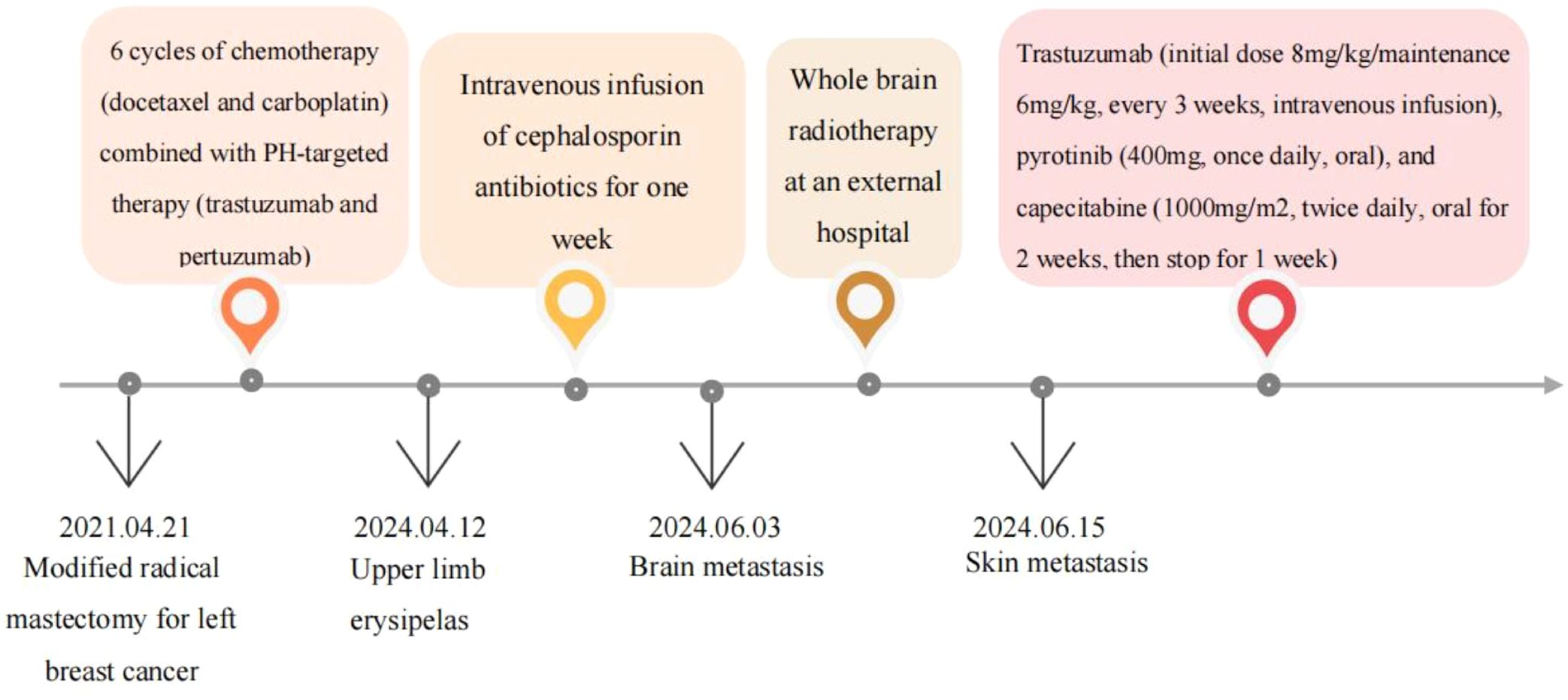
We received a referral for a 51-year-old female patient who underwent a modified radical mastectomy for left breast cancer (pT2N3M0, stage IIIC, HER-2 positive) on April 21, 2021, after being treated at another hospital three years earlier. The overall treatment process is shown in the following diagram (Figure 1). Postoperative pathology revealed invasive ductal carcinoma of the left breast, grade II, with a tumor size of 4.6 cm × 4.5 cm × 1.6 cm, vascular invasion (+), no definite nerve invasion, and lymphoplasmacytic cellular infiltration between cancerous tissues (+), and left breast carcinoma (pT2N3MO,stage IIIC; tumor size 4.6cm × 4.5cm × 1.6cm, 14/21 axillary lymph nodes involved). Immunohistochemistry showed: Estrogen Receptor (ER) (-), Progesterone Receptor (PR) (-), Human Epidermal Growth Factor Receptor2 (HER2) (3+), MKI67 Protein (Ki67) (30%+), E-cadherin (E-cad) (+), Cell Counting Kit-8 (CK8) (+). She subsequently underwent six rounds of chemotherapy (docetaxel and carboplatin) combined with PH-targeted therapy (trastuzumab and pertuzumab). After adjuvant therapy, she received regular follow-ups every 3–6 months, during which the disease remained stable.
On April 12, 2024, the patient’s arm on the side where the axillary clearance was performed—especially the back of the left hand—developed erythema and edema (Figure 2). The patient’s general condition was stable, with no obvious fever or chills. A blood test showed a white blood cell count of 9.6 × 10^9/L. She visited the dermatology department and was diagnosed with erysipelas of the upper limb. After receiving a week-long course of intravenous cephalosporin antibiotics, the localized redness and swelling in the left upper limb gradually subsided (Figure 2).
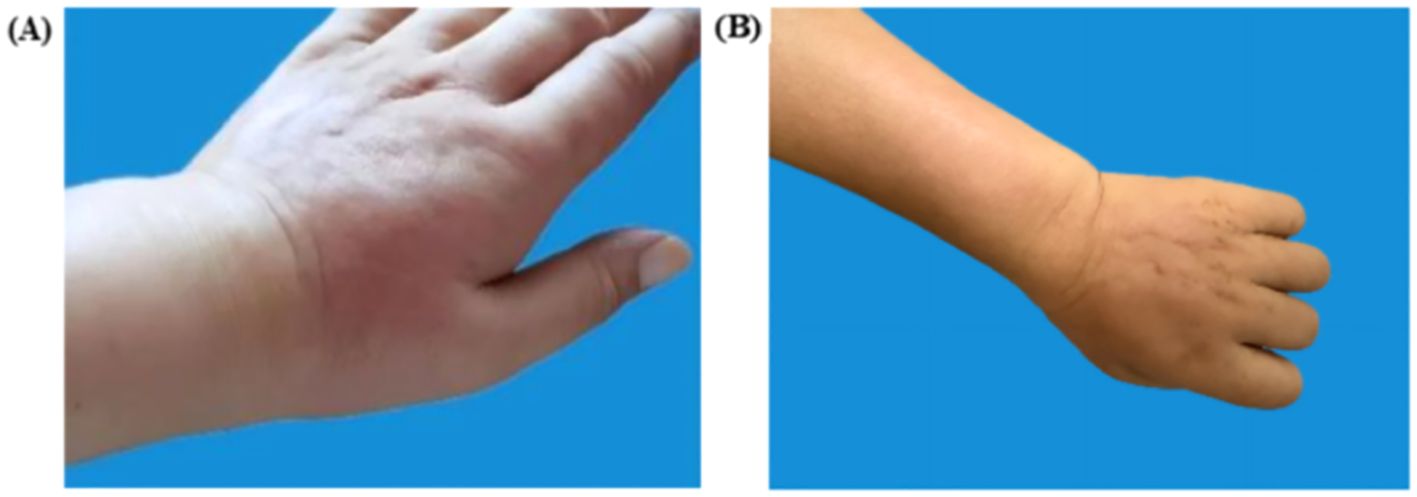
Figure 2. Left upper extremity lesions. (A) Pre-treatment erythema (April 12, 2024, the left dorsum of the hand shows redness with localized swelling and clear borders). (B) Post-treatment resolution (April 18, 2024, localized skin redness and swelling subsided after one week of antibiotic treatment).
A month later, the patient experienced dizziness, headache, nausea, and vomiting without apparent cause. Tumor markers indicated a significant increase in carcinoembryonic antigen (CEA) and carbohydrate antigen 125 (CA125) compared to previous levels. Enhanced cranial MRI suggested metastatic tumors in the right frontal lobe cortex, left parietal lobe cortex, and left cerebellar hemisphere, with the largest measuring approximately 11×13 mm (Figure 3). Whole-brain radiotherapy was performed at another hospital on June 5, 2024.
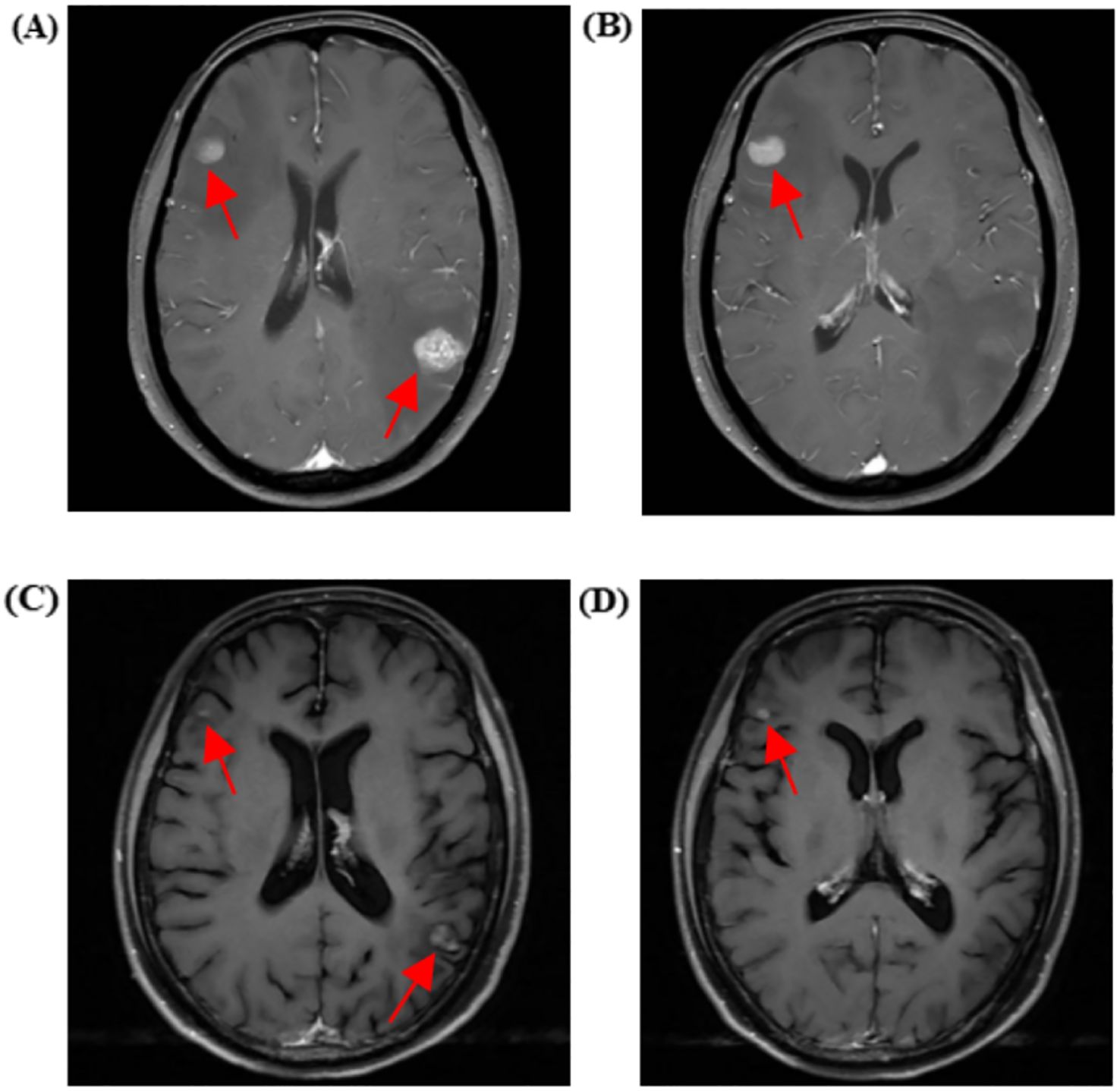
Figure 3. Cranial MRI showing metastatic lesions before and after treatment. (A, B) Cranial enhanced MRI on June 3, 2024, reveals metastases in the right frontal cortex, left parietal cortex, and left cerebellar hemisphere (the largest measuring approximately 11×13 mm). (C, D) Cranial-enhanced Magnetic Resonance Imaging (MRI) performed on July 30, 2024, shows that after whole-brain radiotherapy and antitumor treatment, the metastatic lesions in the right frontal lobe cortex, left parietal lobe cortex, and left cerebellar hemisphere (largest approximately 6*8mm) had reduced in size by 46% compared to before.
During radiotherapy, the patient’s left chest wall skin developed redness and itching, and the area of skin lesions gradually spread to the right chest wall over time. The skin lesion on the left upper limb also reappeared (Figure 4). Breast MRI enhancement suggested multiple non-mass enhancements in the right breast, involving the nipple and skin. Positron Emission Computed Tomography/Computed Tomography (PET/CT) showed multiple low-density lesions in the brain with increased Fluoro Deoxy Glucose (FDG) metabolism, as well as left cervical lymph nodes with increased FDG metabolism, suggesting multiple brain metastases and left cervical lymph node metastases. No abnormal FDG metabolism was observed in other organs (lungs, liver, bones, uterus, gallbladder, etc.).
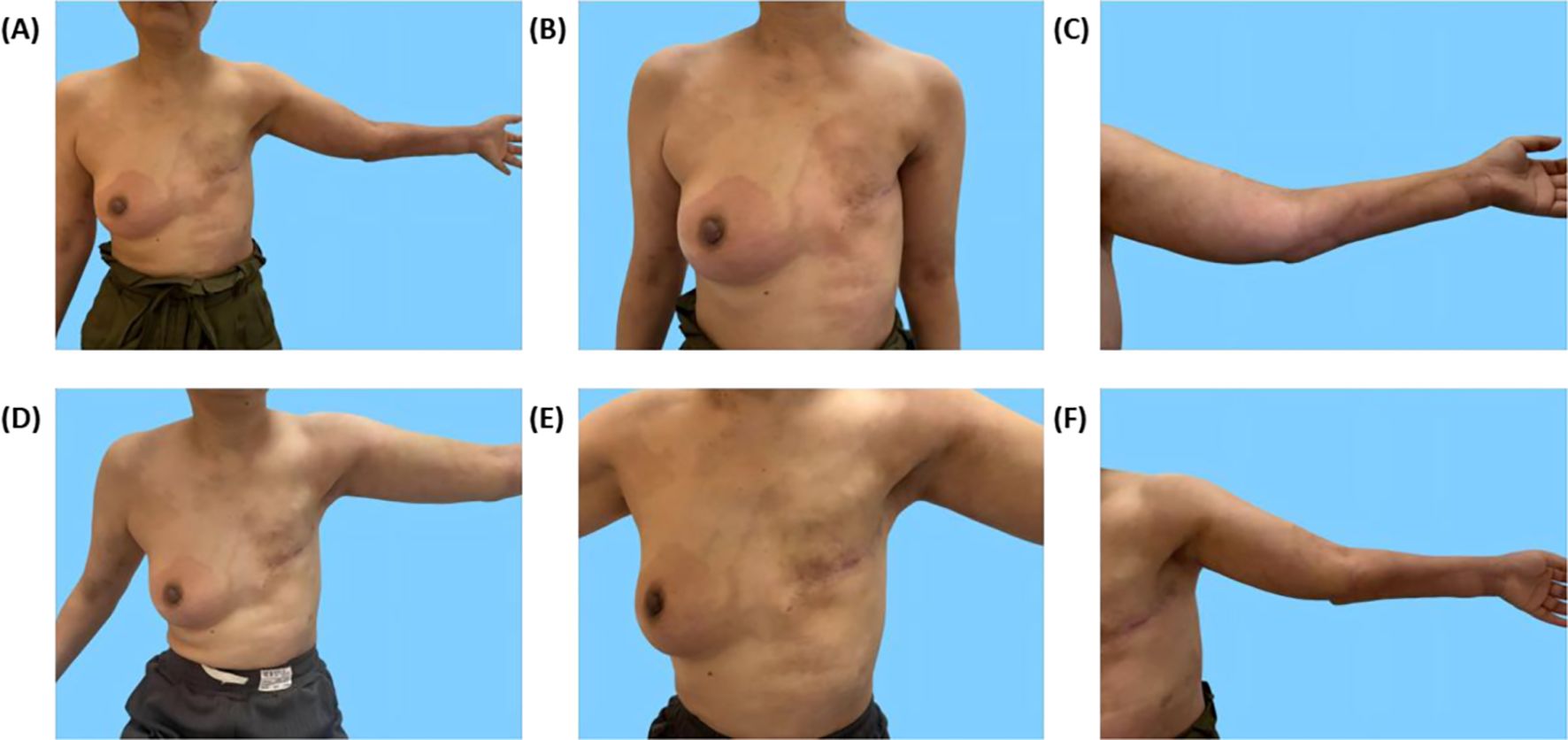
Figure 4. Skin lesions on the chest wall and left upper extremity before and after treatment. (A–C) Redness and swelling on the chest wall and left upper extremity with clear borders. (D–F) Reduction in erythema and regression of skin lesions after more than one month of systemic therapy.
On June 15, 2024, a chest wall skin biopsy was performed. Histopathology showed infiltrating carcinoma consistent with breast cancer metastasis. Immunohistochemistry were as follows: ER (-), PR (-), HER2 (3+), Ki67 (70%+), GATA binding protein 3(GATA-3)(partially ±), Broad-spectrum Cytokeratin (CK wide) (+), CK5/6 (-), E-cad (-), and TP53 tumor protein (p53) (-) (Figures 4, 5). At this point, the patient was diagnosed with multiple metastases of malignant tumors in the left breast, brain, lymph nodes, and skin—HER2-positive type. On June 30, 2024, she began treatment with inetetamab trastuzumab (first dose 8 mg/kg/, maintenance dose 6mg/kg, once every 3 weeks, intravenous infusion), pyrotinib (400mg, once daily, oral), and capecitabine (1000mg/m², twice daily, oral for 2 weeks followed by 1 week off). After one cycle of treatment with this regimen, the patient’s skin metastatic lesions showed regression, with large areas of infiltrative erythema darkening and shrinking in size. Tumor markers CEA and CA125 also decreased. A follow-up enhanced MRI of the head indicated that multiple metastatic lesions had shrunk, with the largest lesion reduced to approximately 6*8mm—representing a 46% regression, assessed as partial response (PR). As of the last follow-up in September 2024, the patient’s condition remains stable.
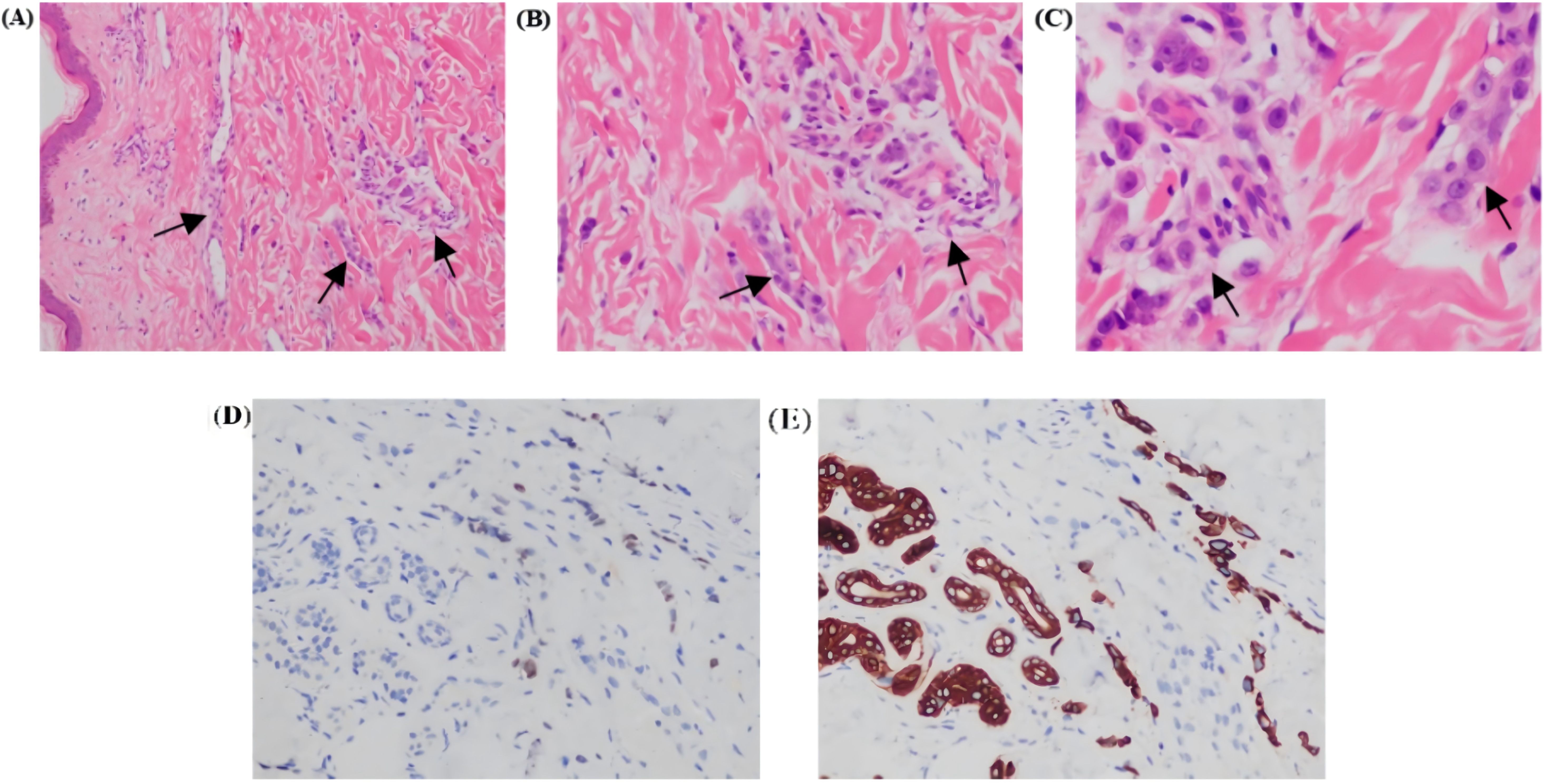
Figure 5. Photomicrographs of Hematoxylin and Eosin (H&E) stained sections revealed tumor cells arranged in cords and nests within the dermis (A, B); H&E, (10x and 20×). Tumor cells separated by dermal collagen fiber bundles show marked atypia [(C); H&E, 40×]. Tumor cells showed partial GATA-3 positivity [(D); 20×]. Broad spectrum CK-20 immunopositivity seen in tumor cells [(E); 20x]. All figures are provided in high-resolution TIFF format (300 dpi).
Discussion
Skin metastasis is a type of secondary cancer in which malignant cells spread from the primary tumor to the skin through blood circulation or lymphatic vessels. Its incidence is approximately 0.6%-9% (6), typically occurring in the later stages of cancer. About 61.9% of patients with skin metastasis also present with one or more visceral organ metastases (7), and the median survival rate is only 6–8 months (8). In this case, the patient was diagnosed alongside extensive brain metastasis including rapid disease progression. Therefore, early identification of skin metastasis is critical to securing valuable time for systemic anti-tumor treatment.
Skin metastases can originate from a wide range of primary tumors, including those of the lungs, breasts, gastrointestinal tract, kidneys, liver, prostate, bladder, uterus, and ovaries (9). Regardless of the origin, the most common histological subtype of the primary tumor associated with skin metastasis is adenocarcinoma (10). Adenocarcinoma appears more likely to metastasize to the skin compared to other histological types, though the mechanism remains unclear. Some scholars speculate (11) that this may be related to the affinity of adenocarcinoma for dermal lymphatic vessels, which allows them to adhere to the lymphatic endothelial cells, form clusters, block lymphatic flow, and evade immune surveillance by lymphocytes.
Compared with lung cancer—which more commonly spreads to the trunk, scalp, and limbs—breast cancer skin metastasis is most commonly found on the trunk (10). Approximately 84.8% of patients present with metastasis on the same side as primary breast cancer, and about 9.1% of patients have metastasis on both sides of the chest wall (8). In this case, skin metastasis initially appeared on the left limb and later involved both sides of the chest wall. A single patient may exhibit one or more clinical types of skin metastasis. Nodular carcinoma is the most common, accounting for approximately 80% of cases. Clinically, it often presents as painless, firm, solitary or multiple pale red or red nodules of the body surface. Pathologically, it shows cord-like or lump-like infiltration of tumor cells in the dermis layer (12, 13). Erysipelas-like carcinoma accounts for only about 3% of skin metastases. Clinically, it presents as red or erythematous, infiltrative, hard, and tough plaques, with low skin temperature. Pathologically, it features tumor cells in clusters or cords within the full thickness of the dermis and lymphatic vessels, with minimal or no inflammatory cell infiltration (14). Tumor cells can adhere to lymphatic endothelial cells through specific adhesion molecules, forming cell clusters that retrograde spread and invade lymphatic vessels extensively. The dermal and subcutaneous lymphatic pathways may then become filled with nest-like and cord-like polymorphic tumor cells leading to lymphatic obstruction, capillary congestion, and lymphedema. In some patients, localized skin temperature may be elevated, further mimicking erysipelas and making it easy to misdiagnose. In this case, the patient was initially misdiagnosed with simple erysipelas of the left upper limb. Several factors contributed to this error. First, the clinical manifestations of erysipelas-like cancer are similar to those of simple erysipelas—both presenting with redness and swelling. Second, the condition occurred during summer, a season when lymphatic edema is more common in post-surgical breast cancer patients. The fact that the lesion appeared on the previously affected side added to the confusion. Third, the patient had an elevated white blood cell count. Finally, there was clear positive feedback on the antibiotics, as the redness and swelling subsided temporarily.
Clinical observations indicate that for patients with inflammatory skin lesions who initially respond to treatment (15), particularly those with a history of malignant tumors, it is crucial to maintain a high degree of vigilance regarding the potential metastasis of the primary tumor (10, 16–18). In cases of suspected erysipelas, performing local skin pathology and immunohistochemistry may risk aggravating the lesions or triggering systemic infection. Therefore, some scholars have suggested the use of dermoscopy before invasive procedures such as biopsy and immunohistochemistry (19, 20). If polymorphic vascular structures appear in the skin tissue under a microscope, this should prompt further histopathological and immunohistochemical investigation. Additionally, since skin metastasis is a manifestation of late-stage tumors and is often accompanied by multiple visceral metastases, a comprehensive systemic tumor evaluation should be conducted to avoid missing metastatic foci.
A study involving 27 patients with skin metastasis from breast cancer found that luminal-type breast cancer is the most common molecular subtype for skin metastasis, followed by HER2-positive and triple-negative breast cancer (21). The vast majority of skin metastases from breast cancer can achieve significant relief after systemic anti-tumor therapy combined with local treatment. For patients presenting with isolated skin metastases, local treatments include traditional surgery and radiotherapy, as well as newer approaches such as photodynamic therapy, direct injection of antitumor agents into the tumor, and application of antitumor agents on the surface of skin lesions, which have been continuously developed and refined in recent years. However, in cases where skin metastasis is found along with other visceral metastases, systemic treatment should still be the primary therapeutic approach, with local treatment as an adjunct. Treatment planning should follow the principles for advanced breast cancer. In this case, the patient was diagnosed with HER2-positive advanced breast cancer with skin, brain, and lymphatic metastasis. After the whole-brain radiotherapy, a systemic treatment regimen combining small molecule Tyrosine Kinase Inhibitor (TKI), chemotherapy, and large-molecule monoclonal antibodies. Following this treatment, both skin and brain metastases showed measurable improvement, indicating that the anti-tumor therapy was effective.
Conclusion
Skin is a relatively rare site for metastasis in breast cancer and other primary tumors. During diagnosis and treatment, cutaneous metastases are often misdiagnosed due to its clinical similarity to skin conditions such as erysipelas-like rash—especially when antibiotic treatment appears effective. To improve diagnostic accuracy breast surgeons should collaborate with dermatopathologists, radiologists, and oncologists. This is just a single case report, and a relatively short follow-up period. Therefore, we need more cases and cohort studies with longer follow-up periods to support these observations. Future multi-center studies with extended follow-up periods are warranted to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This article is a case report and has been approved by the patient. The studies were conducted in accordance with the local legislation and institutional requirements. The patients are those diagnosed and treated in our department of the hospital. The tests and examination results obtained were conducted during the patients’ hospital stay for the purpose of making a clear diagnosis and subsequent treatment, and informed consent from the patients has been obtained. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WG: Writing – original draft, Data curation. JY: Writing – original draft. MD: Writing – review & editing, Supervision. JS: Writing – review & editing, Methodology, Supervision. KJ: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China Youth Program (No. 82104952); the Special Project of Medical Innovation Research of Shanghai Science and Technology Commission (No. 21Y11923600, No. 23Y11921600); the Yueyang Hospital Translational Medicine Research Institute Grade Fund (No.2024yyzh05, No.2024YJJB12); and the Yueyang Hospital Youth Talent Training Program (No.RY411.07.02.04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, and Jemal A. Cancer statistics. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Giaquinto AN, Sung H, Newman LA, Freedman RA, Smith RA, Star J, et al. Breast cancer statistics. CA Cancer J Clin. (2024) 74:477–95. doi: 10.3322/caac.21863
3. Dayan D, Lukac S, Rack B, Ebner F, Fink V, Leinert E, et al. Effect of histological breast cancer subtypes invasivelobular versus non-special type on survival in early intermediate-to-high-risk breast carcinoma: results from the SUCCESS trials. Breast Cancer Res. (2023) 25:153. doi: 10.1186/s13058-023-01750-0
4. Dilawar H, Ahmed A, Habib S, Iqbal J, Abdul Rehman T, Hadi I, et al. Gastric metastasis from invasive lobular breast cancer, resembling primary gastric cancer. J Nucl Med Technol. (2024) 52:68–70. doi: 10.2967/jnmt.123.266035
5. Rehman S and Naveed MA. Skin metastasis in breast cancer patients; a case series. J Cancer Allied Spec. (2020) 6:307. doi: 10.37029/jcas.v6i1.307
6. Lian Z, Zhifang Z, Hongzhi G, Xin W, and Juan W. Clinical and histopathological analysis of 62 cases of cutaneous metastatic carcinoma. Chin J Lepr Skin Dis. (2022) 38:803–6. doi: 10.12144/zgmfskin202211803
7. Jaros J, Hunt S, Mose E, Lai O, and Tsoukas M. Cutaneous metastases: A great imitator. Clin Dermatol. (2020) 38:216–22. doi: 10.1016/j.clindermatol.2019.10.004
8. Shin DM, Jung YJ, Kim H, Oh SJ, Shim J, Lee JH, et al. Clinical characteristics and survival analysis of cutaneous metastases in a single tertiary centre in Korea. J Eur Acad Dermatol Venereol. (2023) 37:2311–8. doi: 10.1111/jdv.19361
9. Sivakanthan T, Tanner J, Mahata B, and Agrawal A. Investigating the role of tumour-to-skin proximity in predicting nodal metastasis in breast cancer. Breast Cancer Res Treat. (2024) 205:109–16. doi: 10.1007/s10549-023-07230-5
10. Eroğlu İ, Üner A, Gürler F, Yazıcı O, Özet A, and Özdemir N. Carcinoma erysipeloides, a case-report and review of the sixty-nine cases in the literature. Am J Med Sci. (2025) 369:485–90. doi: 10.1016/j.amjms.2024.10.008
11. Wenlin W, He Z, Zhenfeng H, Zhikuan X, and Rongyan Y. Clinical and literature analysis of erysipelas skin metastases. J Pract Dermatol. (2020) 13:201–4. doi: 10.11786/sypfbxzz.1674-1293.20200403
12. Gonzálezmartínez S, Pizarro D, Pérezmies B, Caniegocasas T, Curigliano G, Cortés J, et al. Clinical, pathological, and molecular features of breast carcinoma cutaneous metastasis. Cancers Basel. (2021) 13:5416. doi: 10.3390/cancers13215416
13. Mulvaney PM and Schmults CD. Molecular prediction of metastasis in cutaneous squamous cell carcinoma. Curr Opin Oncol. (2020) 32:129–36. doi: 10.1097/CCO.0000000000000609
14. Xiaobin L and Bin W. Analysis of the diagnostic process of a case of breast cancer with skin metastasis misdiagnosed as sebaceous gland cyst with infection. Chin Gen Pract. (2020) 23:2604–6. doi: 10.12114/j.issn.1007-9572.2020.00.164
15. Junak M, Jecius H, and Erdrich J. Cutaneous metastasis in the setting of both colon and breast primary Malignancies. Case Rep Gastrointest Med. (2020) 2020:8852459. doi: 10.1155/2020/8852459
16. Tan WF and Voo SYM. A sinister rash in a lady with breast Malignancy. Med J Malaysia. (2021) 76:275–7. Available at: https://openurl.ebsco.com/contentitem/gcd:149427828?sid=ebsco:plink:crawler&id=ebsco:gcd:149427828
17. Pliakou E, Lampropoulou DI, Nasi D, and Aravantinos G. Skin metastases from gastric cancer, a rare entity masquerading as erysipelas: A case report. Mol Clin Oncol. (2022) 16:110. doi: 10.3892/mco.2022.2543
18. Kurmus GI, Canpolat F, Gönül M, Gökçe A, and Kartal SP. Cutaneous metastases of signet-ring cell gastric carcinoma: A case report. Curr Health Sci J. (2024) 50:444–7. doi: 10.12865/CHSJ.50.03.12
19. Alexis F, Leggett LR, Agarwal N, Bakhtin Z, and Farabi B. Carcinoma erysipeloides with clinical and dermatoscopic features: an overlooked clinical manifestation of breast cancer. Cureus. (2022) 14:e23445. doi: 10.7759/cureus.23445
20. Tiodorovic D, Stojkovic-Filipovic J, Marghoob A, Argenziano G, Puig S, Malvehy J, et al. Dermatoscopic patterns of cutaneous metastases: A multicentre cross-sectional study of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. (2024) 38:1432–8. doi: 10.1111/jdv.19962
Keywords: breast carcinoma, cutaneous metastasis, erysipeloid, pathology, immunohistochemistry
Citation: Gu W, Yuan J, Dong M, Sheng J and Jiang K (2025) Case Report: Advanced breast invasive ductal carcinoma with erysipeloid cutaneous metastasis misdiagnosed as erysipelas. Front. Oncol. 15:1535421. doi: 10.3389/fonc.2025.1535421
Received: 27 November 2024; Accepted: 20 June 2025;
Published: 14 July 2025.
Edited by:
Cherry Bansal, Tantia University, IndiaReviewed by:
Tanvi Jha, Atal Bihari Vajpayee Institute of Medical Sciences and Dr. Ram Manohar Lohia Hospital, Indiaİmdat Eroğlu, Gazi University, Türkiye
Copyright © 2025 Gu, Yuan, Dong, Sheng and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Jiang, c3VyZ2VvbmprQDE2My5jb20=; Jiayu Sheng, c2p5MTk4M3NoQDEyNi5jb20=
†These authors share first authorship
 Weiju Gu
Weiju Gu Jing Yuan
Jing Yuan Mengting Dong
Mengting Dong Jiayu Sheng
Jiayu Sheng