- 1Department of Plant and Environmental Sciences, Faculty of Science, DNRF Center DynaMo, University of Copenhagen, Frederiksberg, Denmark
- 2Department of Plant and Environmental Sciences, Faculty of Science, Copenhagen Plant Science Centre, University of Copenhagen, Frederiksberg, Denmark
- 3Department of Plant Sciences, University of California, Davis, Davis, CA, USA
Naturally variable regulatory networks control different biological processes including reproduction and defense. This variation within regulatory networks enables plants to optimize defense and reproduction in different environments. In this study we investigate the ability of two enzyme-encoding genes in the glucosinolate pathway, AOP2 and AOP3, to affect glucosinolate accumulation and flowering time. We have introduced the two highly similar enzymes into two different AOPnull accessions, Col-0 and Cph-0, and found that the genes differ in their ability to affect glucosinolate levels and flowering time across the accessions. This indicated that the different glucosinolates produced by AOP2 and AOP3 serve specific regulatory roles in controlling these phenotypes. While the changes in glucosinolate levels were similar in both accessions, the effect on flowering time was dependent on the genetic background pointing to natural variation in cross-talk between defense chemistry and onset of flowering. This variation likely reflects an adaptation to survival in different environments.
Introduction
To maximize plant fitness in challenging, ever-changing and unpredictable environments, organisms must coordinate growth and defense to respond to diverse combinations of biotic and abiotic factors. Interactions between biotic and abiotic factors force growth and defense to co-evolve for local adaptation. Optimizing this necessary co-evolution requires the combination of various mechanistic solutions including the plasticity provided by regulatory networks to respond to environmental changes. Additional solutions are provided by the diversity inherent in genetic variation within a plant species, consequently providing different solutions depending on the environment (Pigliucci, 2003). Together, regulatory networks and genetic variation establish the potential for diverse solutions across a species to optimize growth and defense across highly varied environments (Burow et al., 2010; Paul-Victor et al., 2010; Woods et al., 2012).
To identify genes involved in cross-talk between growth and defense, we focused on Arabidopsis natural variation studies. Numerous studies have identified key genes controlling natural variation in the plastic timing of flowering time and thereby reproduction (Koornneef et al., 1991; Michaels and Amasino, 1999; Johanson et al., 2000; El-Din El-Assal et al., 2001; Salomé et al., 2011; Ward et al., 2012; Grillo et al., 2013; Méndez-Vigo et al., 2013). Similarly, natural variation studies in the primary Arabidopsis defense compounds, the glucosinolates produced from tryptophan (indole glucosinolates) and methionine (aliphatic glucosinolates), have aimed at understanding the diversity in glucosinolate profiles (Kliebenstein et al., 2001a,b; Hirai et al., 2005; Keurentjes et al., 2006; Wentzell et al., 2007; Rowe et al., 2008; Jensen et al., 2014). Cross-talk between the networks controlling flowering and glucosinolate profiles seems to occur, as glucosinolate biosynthetic genes within the GS-AOP locus have been associated with not only glucosinolate biosynthesis, but also the control of onset of flowering (Figure 1), (Atwell et al., 2010; Kerwin et al., 2011). Hence, the genes in this locus may help illuminate the cross-talk between defense and flowering time in Arabidopsis.
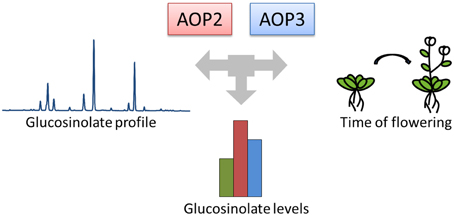
Figure 1. AOP2 and AOP3 have been associated with natural variation in different phenotypes. The GS-AOP locus encoding the glucosinolate biosynthetic enzymes AOP2 and AOP3 has been associated with variation in glucosinolate profiles due to their enzymatic activities. The same genes have been linked to changes in glucosinolate levels and onset of flowering in different natural variation studies.
The GS-AOP locus encodes the two 2-oxoglutarate-dependent dioxygenases AOP2 and AOP3, which convert specific short-chained (SC) methylsulfinylalkyl glucosinolate precursors to alkenyl glucosinolates and hydroxyalkyl glucosinolates, respectively (Figure 2), (Mithen et al., 1995; Kliebenstein et al., 2001c). While AOP2 catalyzes the conversion of the 3-methylsulfinylpropyl (3msp) to the 2-propenyl glucosinolate as well as the conversion of 4-methylsulfinylbutyl (4msb) to 3-butenyl glucosinolate, AOP3 activity has only been detected for 3msp, which is converted by AOP3 to form 3-hydroxypropyl glucosinolate (3ohp), (Kliebenstein et al., 2001c). Natural variation in sequence and expression patterns of AOP2 and AOP3 leads to different glucosinolate profiles among Arabidopsis accessions. Studies investigating natural AOP alleles have linked the expression of an enzymatically functional AOP2 or AOP3 in leaves to increased glucosinolate content, however, with different regulatory potential (Kliebenstein et al., 2001b; Wentzell et al., 2007; Rohr et al., 2009, 2012). Overall, accessions expressing a functional AOP2 enzyme show the highest glucosinolate levels, followed by AOP3 accessions, which still accumulate glucosinolates to higher levels than AOPnull accessions (Kliebenstein et al., 2001b).
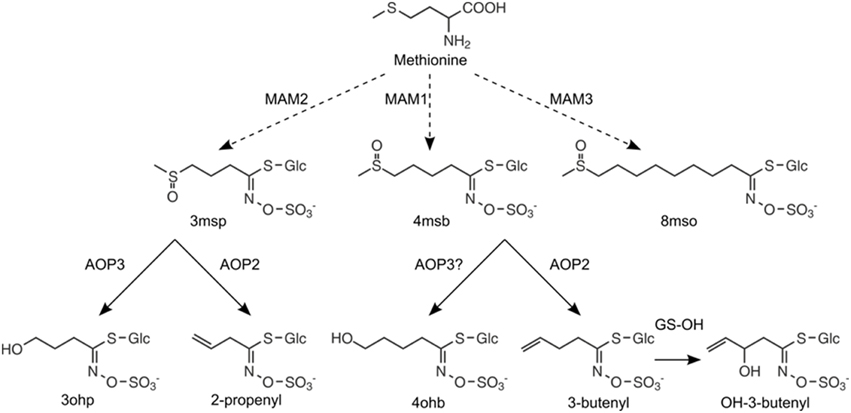
Figure 2. Enzymatic functions of MAMs and AOPs in the aliphatic glucosinolate pathway. The chain length of aliphatic glucosinolates is controlled by GS-ELONG: expression of MAM2 in the absence of MAM1 leads to C3 glucosinolates, MAM1 is required for the production of C4 glucosinolates, and MAM3 is responsible for the production of LC glucosinolates with C8 as the predominant chain length. The C3 glucosinolate, 3-methylsulfinylpropyl glucosinolate (3msp), can be converted to 3-hydroxypropyl glucosinolate (3ohp) by AOP3 or to 2-propenyl glucosinolate (2-prop) by AOP2. The C4 glucosinolate, 4-methylsulfinylbutyl glucosinolate (4msb), is converted to 3-butenyl by AOP2 glucosinolate (3-but), which is further converted by GS-OH to 2(R/S)-hydroxy-3-butenyl glucosinolate. Here, we report that 4msb can also be converted by AOP3 to give 4-hydrozybutyl glucosinolate (4ohb), (see Figure 3).
Based on the catalytic properties of AOP2 and AOP3, we hypothesized that the previously observed difference in these two genes' potential regulatory effects may depend on their enzymatic function and substrate availability, i.e., the levels of 3msp and 4msb. The production of these substrates is largely controlled by the allelic status of GS-ELONG encoding different methylthioalkylmalate synthases. In the absence of a functional AOP2 or AOP3, the presence of MAM1 (methylthioalkylmalate synthase 1) leads to accumulation of 4msb as the major SC aliphatic glucosinolate, while accumulation of 3msp is attributed to the presence of MAM2 (Figure 2), (de Quiros et al., 2000; Kroymann et al., 2001, 2003). GS-ELONG and GS-AOP show an epistatic interaction for glucosinolate accumulation (Kliebenstein et al., 2001b). This epistasis might be linked to the differences in AOP2/3 substrate availability depending on the allelic state at GS-ELONG, which would hint at specific feedback effects of the different products formed by AOP2 and AOP3.
In addition to their enzymatic and regulatory role in glucosinolate biosynthesis, both AOP2 and AOP3 have also been linked to flowering time control. Introduction of a functional AOP2 to the AOPnull accession Col-0 shortened the circadian clock period and altered flowering time (Kerwin et al., 2011). In contrast, the AOP3 gene was found to have a hit in a genome wide association study to the control of the transcript level for a key flowering regulator, the MADS-box transcription factor FLC (Flowering Locus C), (Atwell et al., 2010). Yet the associations between the AOPs and flowering time remain to be tested in different accessions. However, AOP2 and AOP3 may mediate cross-talk between chemical defense i.e., glucosinolates and onset of flowering, which is critical for adaptation to environmental settings.
Hence, we tested if these two enzyme encoding genes, AOP2 and AOP3, have different effects on glucosinolate levels and flowering time in two different accessions, Col-0 and Cph-0, which express neither AOP2 nor AOP3. We introduced AOP2 and AOP3 into the two AOPnull accessions that differ at the GS-ELONG locus and thus accumulate different AOP substrates. The use of multiple transgenic lines enable direct comparison of the effects of the three different AOP alleles (AOP2, AOP3, AOPnull) on glucosinolate accumulation and flowering time. Thus, this approach allowed us to systematically investigate the differences in the regulatory roles of all three AOP alleles, which is not possible in natural variation studies such as GWAS or in QTL mapping e.g., using RILs or F2 populations. The introduction of AOP2 and AOP3 led to different changes in the glucosinolate profiles in the two accessions. While the regulatory effects on glucosinolate levels were similar in both accessions, our study shows that AOP2 and AOP3 possess different abilities to change flowering time dependent on the genetic background.
Materials and Methods
Generation of Expression Constructs
For generation of expression constructs, we extracted genomic DNA from leaf tissue using the CTAB method (Clarke, 2009). The genomic sequence of AOP2 was amplified from Col-0 gDNA expressing the AOP2 allele of B. oleracea BoGSL-ALK under the control of the CaMV 35S promoter (Li and Quiros, 2003) with the primers 5′-ggcttaauATGGGTG CAGACACTCCTCAAC-3′ and 5′-ggtttaauTTATGCT CCAGAGACGGCAC-3′. AOP3 was amplified from Ler gDNA using primers designed based on the reference sequence from TAIR9, 5′-ggcttaauATGGGTTCATGCAGTCCTCA-3′ and 5′-ggtttaauTTATTTCCCAGCAGAGACGC-3′. AOP2 and AOP3 were inserted into pCAMBIA2300-35Su and pCAMBIA3300-35Su, respectively, downstream of the CaMV 35S promoter (Nour-Eldin et al., 2006). The constructs were verified by sequencing.
Generation of Transgenic Plants
Agrobacterium tumefaciens (strain PGV38 c58) was transformed with either the 35S:AOP2 or the 35S:AOP3 expression construct for transformation of Col-0 and Cph-0 by floral-dip (Clough and Bent, 1998). Positive transformants carrying the 35S:AOP2 construct were selected on MS plates with 50 μM kanamycin. T1 seeds carrying the 35S:AOP3 construct were selected by repeated spraying with 300 μM Basta at the 4-leaf stage. In the T1 and subsequent generations, the presence of the transgenes was confirmed by PCR on genomic DNA. For AOP2, we obtained multiple insertion lines in both Col-0 and Cph-0, whereas for AOP3 multiple lines were obtained in Cph-0, but only one line in Col-0.
Plant Growth
For all experiments, seeds were sown in a randomized design and cold stratified at 4°C for at least 2 days. The plants were grown in climate chambers at 80–120 μE/(m2* s) light intensity, 16 h light, 20°C, and 70% relative humidity. Flowering time was measured as the period between cold stratification and the day, when the inflorescence reached 1 cm in height and normalized to the corresponding WT.
Glucosinolate Analysis
Glucosinolates were extracted from fresh mature rosette leaves around 22 (Col-0) or 27 (Cph-0) days after stratification and analyzed as desulfo-glucosinolates for some experiments by HPLC/DAD as previously described (Kliebenstein et al., 2001c; Andersen et al., 2013) using p-hydroxybenzyl glucosinolate as an internal standard. Separation of desulfo-glucosinolates by HPLC was achieved on a Supelcosil LC-18-DB column, 25 cm × 4.6 mm, 5 μm particle size (Supelco, Bellefonte, PA, USA) or a ZORBAX SB-Aq column, 25 cm × 4.6 mm, 5 μm particle size (Agilent Technologies).
Alternatively, desulfo-glucosinolates were analyzed by UHPLC/TQ-MS on an Advance™-UHPLC/EVOQ™Elite-TQ-MS instrument (Bruker) equipped with a C-18 reversed phase column (Kinetex 1.7 u XB-C18, 10 cm × 2.1 mm, 1.7 μm particle size, Phenomenex) by using a 0.05% formic acid in water (v/v) (solvent A)-0.05% formic acid in acetonitrile (v/v) (solvent B) gradient at a flow rate of 0.4 ml/min at 40°C. The gradient applied was as follows: 2% B (0.5 min), 2–30% (0.7 min), 30–100% (0.8 min), 100% B (0.5 min), 100–102% B (0.1 min), and 2% B (1.4 min). Compounds were ionized by ESI with a spray voltage of +3500 V, heated probe temperature 400°C, cone temperature 250°C.
Desulfo-glucosinolates were monitored based on the following MRM transitions: 3-methylthiopropyl (3mtp), (+)328 > 166 [5V]; 3-methylsulfinyl (3msp), (+)344 > 182 [10V]; 2-propenyl (2-prop), (+)280 > 118 [5V]; 3-hydroxypropyl (3ohp), (+)298 > 118 [15V]; 4-methylthiobutyl (4mtb), (+)342 > 132 [15V]; 4-methylsulfinylbutyl (4msb), (+)358 > 196 [5V]; 3-butenyl (3-but), (+)294 > 132 [15V]; 2R/2S-2-hydroxy-3-butenyl, (+)310 > 130 [15V]; 4-hydroxybutyl (4ohb), (+)312 > 132 [15V]; 5-methylsulfinylpentyl (5msp), (+)372 > 210 [5V]; 7-methylthioheptyl (7mth), (+)384 > 222 [5V]; 7-methylsulfinylheptyl (7msh), (+)400 > 238 [7V]; 8-methylthiooctyl (8mto), (+)398 > 236 [5V]; 8-methylsulfinyloctyl (8mso), (+)414 > 252 [5V]; indol-3-ylmethyl (I3M), (+)369 > 207 [10V]; N-methoxy-indol-3-ylmethyl (NMOI3M), (+)399>237 [10V]; 4-methoxy-indol-3-ylmethyl (4MOI3M), (+)399 > 237 [10V]; and p-hydroxybenzyl (pOHB), (+)346 > 184 [10V] (internal standard). 2R- and 2S-2-hydroxy-3-butenyl glucosinolate and N- and 4-methoxy-indol-3-ylmethyl glucosinolate were distinguished based on retention times in comparison to those of known standards. Quantification of the individual glucosinolates was based on response factors relative to pOHB calculated from standard curves in control extracts.
Statistics
We used R version 3.0.1 (2013-05-16) for statistical analysis (Team, 2013). For the WT and insertion lines significance was tested using the lm and Anova function for the following linear model GLS = Experiment + Genotype + insertion line nested within Genotype + Experiment:Genotype with specific differences tested post-hoc using the pairwise t-test function with a Holm-adjustment for multiple testing. Summary statistics was calculated using the SummaryBy from the doBy package (Højsgaard and Halekoh, 2014).
Generation of the Col-0 × Cph-0 F2 Population
To create the Col-0 × Cph-0 F2 mapping population, Col-0 and Cph-0 WT plants were grown until the flowering stage and Cph-0 was used to pollinate Col-0. The F1 plants were genotyped for MAM1 and MAM2 to insure the presence of a copy of each allele, i.e., heterozygosity, before the F1 plants were selfed. The F2 population was investigated for flowering time by measuring the number of days from stratification till the primary inflorescence reached 1 cm (File S1 in Supplementary Table 1). For 171 plants, we obtained both genotype and phenotype results, which could be used in QTL mapping.
Genotyping by MassARRAY
For genotyping, genomic DNA was extracted using the Qiagen DNeasy 96 Plant Kit (Qiagen) according to manufacturer's protocol. 100 sites were chosen for Sequenom MassARRAY in an attempt to get full coverage of the genome. However, we did not have any previous knowledge on the Cph-0 accession and only 53 of the SNPs turned out being polymorphic between the parents. These SNPs were used to generate genetic maps for each mapping population using the Haldane function (File S2 in Supplementary Table 1).
QTL Mapping
Windows QTL Cartographer Version 2.5 was used for composite interval mapping determining significant thresholds for flowering time by doing 1000 permutations to estimate the 0.05 significance levels (Wang et al., 2012). The main-effect markers were validated and tested for Two-Way epistatic interactions using lm type II and ANOVA in R version 3.0.1 (2013-05-16) including the most significant marker for each QTL.
Quantitative Real-time PCR
RNA was extracted from three pools of leaves for each genotype with Sigma Spectrum Plant Total RNA kit, treated with Sigma DNAse1 and reverse transcribed with iScript (Bio-rad). Expression was assayed by quantitative real-time PCR using SYBR Green and the data was normalized to UBC expression of each pool. The following primers were used: UBC (At5g25760), 5′-CTGAGCCGGACA GTCCTCTTAACTG-3′ and 5′-CGGCGAGGC GTGTATACATTTGTG 3′; FT, 5′-TGGTGGAGA AGACCTCAGGA-3′ and 5′-GAGGTGAGGGTT GCTAGGAC-3′; FLC in Col-0, 5′-GAGCCAAGAA GACCGAACTCAT-3′ and 5′-GAGATTTGTCCA GCAGGTGAC-3′; FLC in Cph-0: 5′-TGACTAGAG CCAAGAAGACCG-3′ and 5′-AGCAGGTGA CATCTCCATCTC-3′. Gene expression levels are presented as mean fold difference for two individual experiments. Results obtained in two independent experiments were pooled for statistical analysis lm. Anova and pairwise t-test with holm adjustment was used to test for significant differences at each time point and between experiments in R version 3.0.1 (2013-05-16) (Team, 2013).
Results
AOP2 and AOP3 Alter Glucosinolate Profiles in Col-0 and Cph-0
Biosynthesis of the glucosinolate core structure gives rise to methylthioalkyl glucosinolates, which are further converted to methylsulfinylalkyl glucosinolates by GS-OX FMO1-5 (Hansen et al., 2007; Sønderby et al., 2010). Methylsulfinylalkyl glucosinolates accumulate in AOPnull accessions, i.e., accessions that express neither a functional AOP2 nor AOP3 in leaves (Figure 2). To investigate the relative effects of AOP2 and AOP3, we introduced them to two AOPnull accessions accumulating different SC methylsulfinylalkyl glucosinolates. We used the Col-0 accession accumulating mainly 4msb and a so far undescribed accession accumulating 3msp as its major SC glucosinolate, Copenhagen-0 (Cph-0).
In agreement with previous work using the Brassica oleracea or Arabidopsis accession Pi AOP2 (Li and Quiros, 2003; Wentzell et al., 2007; Neal et al., 2010), constitutive expression of AOP2 in the Col-0 background lead to the formation of 2-propenyl and 3-butenyl glucosinolate from 3msp to 4msb, respectively (Figures 2, 3B,C, for all individual glucosinolates see Figure S1). The presence of a functional GS-OH within Col-0 further modified the 3-butenyl side chain to 2R- and 2S-2-hydroxy-3-butenyl (Figure 3C), (Hansen et al., 2008). AOP3 expression in Col-0 led to the conversion of 3msp to 3ohp and interestingly, we also detected small amounts of 4-hydroxybutyl glucosinolate (4ohb), (Figures 3B,C). Thus, AOP3 appears to be able to convert 4msb to 4ohb in planta, an activity that could not be detected in vitro (Kliebenstein et al., 2001c). However, based on the ratios of substrates and products in Col-0, AOP3 seems to have a preference for 3msp.
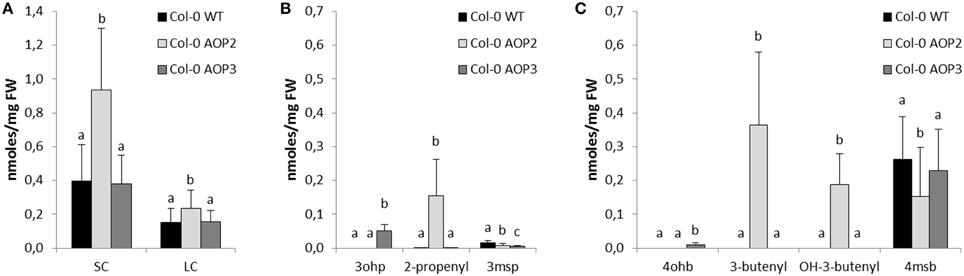
Figure 3. Effects of AOP2 and AOP3 on glucosinolate accumulation in Col-0. Glucosinolate concentrations in leaves of Col-0 WT (black), n = 28, Col-0 AOP2 (light gray), n = 18 (2 independent insertion lines), and Col-0 AOP3 (dark gray), n = 25 (1 line), as (A) Total SC and LC, (B) C3 glucosinolates, and (C) C4 substrate and products. Means (+ standard deviations) are shown for two experimental repeats of the same lines. Nested ANOVA across independent lines and post testing was used for statistical analysis. Letters indicate significant differences (P < 0.05) between genotypes.
In the Cph-0 background, 4msb is absent and instead, 3msp is the major SC glucosinolate. Consequently, introduction of the enzymes into the Cph-0 led to fewer different glucosinolate structures than in Col-0. As expected, AOP2 expression resulted in the formation of 2-propenyl glucosinolate from 3msp, whereas AOP3 formed 3ohp from the same substrate (Figure 4B, for all individual glucosinolates see Figure S1).
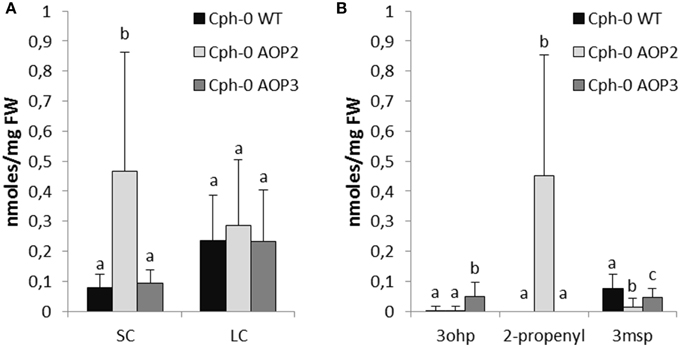
Figure 4. Effects of AOP2 and AOP3 on glucosinolate accumulation in Cph-0. Glucosinolate concentrations in leaves of Cph-0 WT including empty vector controls (black), n = 104, Cph-0 AOP2 (light gray), n = 73 (3 independent insertion lines), and Cph-0 AOP3 (dark gray), n = 60 (3 independent insertion lines), as (A) Total SC and LC, and (B) The C3 substrate and products. Means (+ standard deviations) are shown for analysis of two experimental repeats of the lines. Nested ANOVA across independent lines and post testing was used for statistical analysis. Letters indicate significant differences (P < 0.05) between genotypes.
AOP2 and AOP3 have Differential Effects on Glucosinolate Levels in Col-0 and Cph-0
Several studies have suggested that both AOP2 and AOP3 influence the total level of glucosinolate accumulation as well as the specific structures being produced (Kliebenstein et al., 2001b; Wentzell et al., 2007; Rohr et al., 2009, 2012; Brachi et al., 2015). To directly compare the regulatory capacity of both genes, we quantified the glucosinolates in our different AOP2 and AOP3 lines. The introduction of AOP2 to Col-0 led to a several fold increase in SC glucosinolates (Figure 3A), which is in agreement with previously published studies (Wentzell et al., 2007; Burow et al., 2015). The high levels of SC glucosinolates correlate with high accumulation of glucosinolates down-stream of AOP2, i.e., 2-propenyl, 3-butenyl, and 2R/2S-2-hydroxy-3-butenyl (Figures 3B,C). Introduction of AOP2 to Col-0 does, however, not only change the levels of SC but also LC glucosinolates, which are not AOP2 substrates (Figure 3A). In contrast to AOP2, the introduction of AOP3 into Col-0 did not change the total accumulation of SC or LC glucosinolates (Figure 3A). Thus, in the Col-0 background, AOP2 and AOP3 might differ in their ability to regulate glucosinolate accumulation possibly depending on their difference in enzymatic properties. However, as only one AOP3 line was obtained in Col-0, we cannot completely rule out a regulatory effect of AOP3 on glucosinolate accumulation in this background. In this line, AOP3 expression caused a larger decrease in 3msp than AOP2 and 4msb levels were only significantly decreased in the AOP2 lines (Figures 3B,C). This difference may be critical in case the removal of substrates is important for the regulatory role through a feed-back mechanism. However, if the regulatory role of AOP2 depends on its enzymatic activity, not only the removal of substrates but also the accumulation of the specific glucosinolate products may mediate this function.
Next, we investigated potential regulatory effects of AOP2 and AOP3 in Cph-0 that accumulates 3msp as the major SC glucosinolate. Cph-0 expressing AOP2 accumulated high levels of SC glucosinolates (Figure 4A). Similar to the effect of AOP2 expression in Col-0, increased SC glucosinolate levels correlated with the amounts of the AOP2 product (Figure 4B). In Cph-0, AOP2 only converted 3msp to 2-propenyl, suggesting that the effect of AOP2 on SC may not depend on the chain length of the available substrate. In contrast to Col-0, introduction of AOP2 to Cph-0 did not significantly affect LC glucosinolate accumulation. Thus, the regulatory effect of AOP2 on LC glucosinolates might differ between the two accessions. Similar to the Col-0 accession, AOP3 did not change the levels of total SC or LC glucosinolates in Cph-0 (Figure 4A). Even though the AOP2 and AOP3 can both convert 3msp, AOP2 seems to more efficiently pull from the 3msp pool than AOP3 and possibly thereby increase the flux through the pathway in the Cph-0 accession. Taken together, AOP2 has a larger effect on the regulation of glucosinolate levels than AOP3 in both accessions, which might be explained by the difference in their catalytic activities.
Effects on Flowering Time Differ between AOP2 and AOP3 and Depend on the Genetic Background
The glucosinolate biosynthetic genes AOP2 and AOP3 represent candidate genes for the integration of defense with reproduction as they are associated with the control of flowering time in both the laboratory and the field (Atwell et al., 2010; Kerwin et al., 2011, 2015). To test the ability of AOP2 and AOP3 to link glucosinolates and flowering time in different backgrounds, we measured flowering time in all of our lines (Figure 5). AOP2 has been identified as a QTL for altering circadian clock parameters and thereby flowering time (Kerwin et al., 2011). Accordingly, introduction of a functional AOP2 into Col-0 under 16 h light delayed flowering time by several days (Figure 5A). AOP3 has been associated with natural variation in flowering time and gene expression level of the MADS-box transcription factor FLC (Flowering Locus C), which is one of the major determinants of flowering (Shindo et al., 2005; Atwell et al., 2010). Analysis of the Col-0 AOP3 line showed no significant difference between Col-0 WT and Col-0 AOP3 lines (Figure 5A). Thus, AOP2 but not AOP3 seems to influence onset of flowering in Col-0.
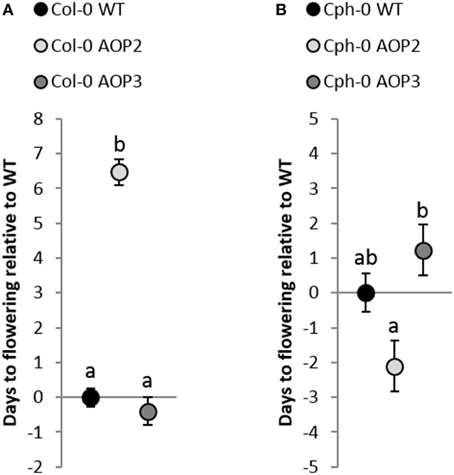
Figure 5. Effects of AOP2 and AOP3 on flowering. (A) Average (+ standard error) of flowering time in days relative to Col-0 WT (28.4 days ± 5.3). Black Col-0 WT, n = 110, light gray Col-0 AOP2, n = 50, (2 independent insertion lines), and dark gray Col-0 AOP3, n = 32, (1 line). ANOVA with nesting and experiment interaction (min 2 repeats) shows that Col-0 AOP2 is significantly different from Col-0 WT and Col-0 AOP3, P < 0.001, whereas P = 0.45 for the Col-0 WT and Col-0 AOP3 comparison. (B) Flowering time relative to Cph-0 WT (41.6 days ±5.3). Cph-0 WT (black), n = 60, Cph-0 AOP2 (light gray), n = 73 (3 independent insertion lines), and Cph-0 AOP3 (dark gray), n = 60 (3 independent insertion lines). ANOVA with nesting of the different insertion lines and experiment interaction (two repeats) showed no significant difference between Cph-0 WT and the insertion lines; Cph-0 WT and Cph-0 AOP2 (P = 0.06) and Cph-0 WT and Cph-0 AOP3 (P = 0.22). Cph-0 AOP2 and Cph-0 AOP3 showed a significant difference (P < 0.01).
In contrast to its pronounced delay on flowering in Col-0, AOP2 had a suggestive ability to speed up flowering in Cph-0 using three independent transgenic lines (nested ANOVA; P = 0.06; Figure 5B). Similarly, AOP3 expression in the Cph-0 background did not significantly change flowering time compared to the WT (Figure 5B). However, the AOP2 and AOP3 lines in Cph-0 showed a significant difference in flowering time from each other (P < 0.01), indicating that AOP2 and AOP3 have significant opposite effects on flowering time in Cph-0, which differs from the effects in Col-0.
Flowering Time Network Architecture is Critical for the Regulatory Effects of AOP2 and AOP3
While AOP2 and AOP3 had similar effects on glucosinolate accumulation across Col-0 and Cph-0, each gene differs in its ability to alter flowering time in the two accessions. One factor that may contribute to this difference is variation in the internal flowering time pathways in the two accessions. The Col-0 WT flowers earlier than Cph-0 WT indicating differences in the flowering networks. To identify the underlying loci that contribute to these differences and the variation in the AOP2/3 effect on flowering, we established a Col-0 × Cph-0 F2 population to map QTLs that control the major difference in flowering time between Col-0 and Cph-0. We genotyped 171 F2 plants for 100 SNPs using Sequenom MassARRAY® and this resulted in identification of 53 polymorphic sites. For the Cph-0 accession with no prior sequence knowledge, we compared the SNP combination to the 1001 genome accession database (Cao et al., 2011; Schneeberger et al., 2011; Long et al., 2013) and did not find any annotated accession to have the same combination. The F2 population was also phenotyped for flowering time.
QTL mapping for flowering time revealed two loci (X29 and X188) as the major QTLs (Figure 6, Table 1). We found FT and FLC as the top candidate genes in these loci (candidate gene list from Grillo et al., 2013). Therefore, we analyzed the transcript levels of FT and FLC in the two accessions and found FT expression in Cph-0 to be 1–2% of the transcript level in Col-0, while FLC transcript levels were around 500 times higher in Cph-0 than in Col-0 (Table 2). This is in agreement with the observed difference in flowering time between the two accessions, as FLC delays flowering time by repressing FT expression. This difference suggests that the ability to detect the influence of the AOP2 and AOP3 genes on flowering may depend on the allelic status at these known major effect flowering time genes in Arabidopsis. Further work is needed to assess how the AOP2 or AOP3 genes interplay with these known flowering time genes.
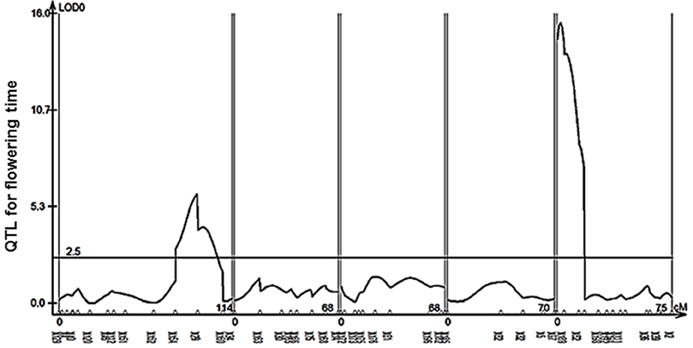
Figure 6. QTL mapping for flowering time in the Col-0 × Cph-0. QTL mapping in an F2 population derived from a Col-0 × Cph-0 cross revealed two significant loci (X129 and X188) controlling flowering time.
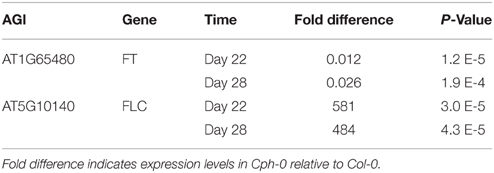
Table 2. Expression analysis for flowering time genes in QTLs identified in the Col-0 × Cph-0 F2 population.
Discussion
In the field, glucosinolate profiles and herbivory resistance are strongly dependent on GS-AOP and GS-ELONG (Bidart-Bouzat and Kliebenstein, 2008; Züst et al., 2012; Brachi et al., 2015; Kerwin et al., 2015). Hence, the structures and levels of the glucosinolates produced depend on the allelic status of these two loci and their interaction plays an important role for plant fitness. The introduction of AOP2 into the AOPnull accessions Col-0 and Cph-0 causes accumulation of alkenyl glucosinolates together with increased levels of aliphatic glucosinolates (Figures 3, 4), (Li and Quiros, 2003; Wentzell et al., 2007; Burow et al., 2015). In contrast, AOP3 expression in the same backgrounds led to formation of hydroxyalkyl glucosinolates without associated changes in glucosinolate levels. Thus, the production of alkenyl but not hydroxyalkyl glucosinolates influences the feedback regulation of glucosinolate biosynthesis in Cph-0 and possibly also in Col-0. Both AOP2 and AOP3 convert the same substrates, which suggests that it is the product being produced and not the substrate that is the determining factor. Consequently, the increased flux from primary metabolism into specialized metabolism in the presence of AOP2 may depend on the products of the enzyme mediating positive feedback regulation of the pathway. Recent work has suggested that this may occur by altering the jasmonate signal transduction pathway in lines that contain a functional AOP2 gene (Burow et al., 2015).
AOP2 significantly increased levels of aliphatic glucosinolates in both Col-0 and Cph-0 that differ in their GS-ELONG allelic status. This suggests that variation in the presence of 3C or 4C substrate availability is not the major determinant for the control of SC glucosinolates by the interaction of AOP2 and GS-ELONG. Only in the Col-0 background, LC glucosinolate levels were also significantly increased in the AOP2 lines, which could suggest that this effect requires the presence of C4 glucosinolates or variation in the MAM3, which catalyzes the production of the LC glucosinolates. The fact that LC levels differ between the two WTs suggests that there is MAM3 variation or in the regulatory network controlling LC glucosinolate accumulation.
As previously found, we could show that AOP2 delays flowering time in Col-0 (Kerwin et al., 2011), but there was only a suggestive effect of AOP2 on flowering in the Cph-0 background (Figure 5). Mapping flowering time variation between Col-0 and Cph-0 suggested that these two accessions have natural variation in FLC and FT suggesting that this difference in the AOP2 effect on flowering time may be due to interactions with the known flowering time pathways. Supporting this hypothesis, AOP2 has been shown to alter the circadian clock pathway that affects flowering time via regulation of FT (Kerwin et al., 2011). Thus, the regulatory effect of AOP2 on flowering time in Col-0 through FT might be altered by the competing regulation by the 500 times higher FLC levels in Cph-0. Recently, AOP2 was moreover found to mediate positive feedback regulation between the aliphatic glucosinolate biosynthetic pathway and jasmonate signaling (Burow et al., 2015). More specifically, expression of AOP2 in Col-0 led to increased transcript levels of MYC2. Interestingly, MYC2 is not only a key regulator of jasmonate-mediated plant responses (Lorenzo et al., 2004; Dombrecht et al., 2007) including glucosinolate biosynthesis, but also involved in circadian oscillation of jasmonate signaling through direct interaction with the clock component TIME FOR COFFEE (Shin et al., 2012). The ability of AOP2 to alter flowering time in Col-0 might thus be linked to its regulatory input into jasmonate signaling. In contrast to AOP2, no clear effect was observed for AOP3 in either accession. Yet, AOP3 has been linked to natural variation in FLC expression (Atwell et al., 2010) indicating that AOP3 provides input to the flowering time network through a different mechanism than AOP2. If AOP3 can play a regulatory role in flowering time at all, the effect is minor and possibly requires a specific allelic status at the flowering time genetic loci.
Our study illustrates natural variation in the cross-talk between glucosinolate accumulation and flowering time. The ability to fine-tune this cross-talk differs between the two enzyme-encoding genes, AOP2 and AOP3, even though they arose from a recent gene duplication event and share high sequence similarity (Kliebenstein et al., 2001c). To fully understand the role of the GS-AOP locus in fine-tuning the regulatory cross-talk between glucosinolate profiles, jasmonate signaling, and the onset of flowering time, future studies in a larger number of genetic backgrounds will be required. Most importantly, studies in accessions expressing a functional AOP2 or AOP3 will reveal potential differences in the architecture of the regulatory networks in these backgrounds. Nevertheless, the specific regulatory effects and the dependency on the genetic background possibly reflect the plant's need to coordinate defense and reproduction when faced with different combinations of biotic and abiotic challenges. Hence, Arabidopsis seems to have evolved different cross-talk mechanisms linking defense and flowering time phenotypes to adapt to different environments.
Author Contributions
LJ, DK, and MB designed the study and interpreted the data. LJ and MB conducted the plant work. LJ, MB, and DK did the statistical analyses. HJ designed and carried out expression analyses. LJ and MB wrote the paper. DK and BH commented on the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Financial support for the work was provided by the Danish National Research Foundation DNRF grant 99 (BH, MB, DK, LJ, HJ) and IEE Marie Curie stipend PIEF-GA-2008-221236 (MB).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00697
References
Andersen, T. G., Nour-Eldin, H. H., Fuller, V. L., Olsen, C. E., Burow, M., and Halkier, B. A. (2013). Integration of biosynthesis and long-distance transport establish organ-specific glucosinolate profiles in vegetative arabidopsis. Plant Cell 25, 3133–3145. doi: 10.1105/tpc.113.110890
Atwell, S., Huang, Y. S., Vilhjalmsson, B. J., Willems, G., Horton, M., Li, Y., et al. (2010). Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465, 627–631. doi: 10.1038/nature08800
Bidart-Bouzat, M. G., and Kliebenstein, D. J. (2008). Differential levels of insect herbivory in the field associated with genotypic variation in glucosinolates in Arabidopsis thaliana. J. Chem. Ecol. 34, 1026–1037. doi: 10.1007/s10886-008-9498-z
Brachi, B., Meyer, C. G., Villoutreix, R., Platt, A., Morton, T. C., Roux, F., et al. (2015). Coselected genes determine adaptive variation in herbivore resistance throughout the native range of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 112, 4032–4037. doi: 10.1073/pnas.1421416112
Burow, M., Atwell, S., Francisco, M., Kerwin, R. E., Halkier, B. A., and Kliebenstein, D. J. (2015). The glucosinolate biosynthetic gene Aop2 mediates feed-back regulation of jasmonic acid signaling in Arabidopsis. Mol. Plant 8, 1201–1212. doi: 10.1016/j.molp.2015.03.001
Burow, M., Halkier, B. A., and Kliebenstein, D. J. (2010). Regulatory networks of glucosinolates shape Arabidopsis thaliana fitness. Curr. Opin. Plant Biol. 13, 348–353. doi: 10.1016/j.pbi.2010.02.002
Cao, J., Schneeberger, K., Ossowski, S., Gunther, T., Bender, S., Fitz, J., et al. (2011). Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat. Genet. 43, 956–963. doi: 10.1038/ng.911
Clarke, J. D. (2009). Cetyltrimethyl ammonium bromide (CTAB) DNA miniprep for plant DNA isolation. Cold Spring Harb Protoc. 3:Pdb.prot5177. doi: 10.1101/pdb.prot5177
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
de Quiros, H. C., Magrath, R., McCallum, D., Kroymann, J., Scnabelrauch, D., Mitchell-Olds, T., et al. (2000). Alpha-keto acid elongation and glucosinolate biosynthesis in Arabidopsis thaliana. Theor. Appl. Genet. 101, 429–437. doi: 10.1007/s001220051500
Dombrecht, B., Xue, G. P., Sprague, S. J., Kirkegaard, J. A., Ross, J. J., Reid, J. B., et al. (2007). MYC2 differentially modulates diverse jasmonatedependent functions in Arabidopsis. Plant Cell 19, 2225–2245. doi: 10.1105/tpc.106.048017
El-Din El-Assal, S., Alonso-Blanco, C., Peeters, A. J., Raz, V., and Koornneef, M. (2001). A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat. Genet. 29, 435–440. doi: 10.1038/ng767
Grillo, M. A., Li, C., Hammond, M., Wang, L., and Schemske, D. W. (2013). Genetic architecture of flowering time differentiation between locally adapted populations of Arabidopsis thaliana. New Phytol. 197, 1321–1331. doi: 10.1111/nph.12109
Hansen, B. G., Kerwin, R. E., Ober, J. A., Lambrix, V. M., Mitchell-Olds, T., Gershenzon, J., et al. (2008). A novel 2-oxoacid-dependent dioxygenase involved in the formation of the goiterogenic 2-hydroxybut-3-enyl glucosinolate and generalist insect resistance in Arabidopsis. Plant Physiol. 148, 2096–2108. doi: 10.1104/pp.108.129981
Hansen, B. G., Kliebenstein, D. J., and Halkier, B. A. (2007). Identification of a flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J. 50, 902–910. doi: 10.1111/j.1365-313X.2007.03101.x
Hirai, M. Y., Klein, M., Fujikawa, Y., Yano, M., Goodenowe, D. B., Yamazaki, Y., et al. (2005). Elucidation of gene-to-gene and metabolite-to-gene networks in Arabidopsis by integration of metabolomics and transcriptomics. J. Biol. Chem. 280, 25590–25595. doi: 10.1074/jbc.M502332200
Højsgaard, S., and Halekoh, U. (2014). DoBy: Groupwise Statistics, LSmeans, Linear Contrasts, Utilities. Aalborg.
Jensen, L. M., Halkier, B. A., and Burow, M. (2014). How to discover a metabolic pathway? An update on gene identification in aliphatic glucosinolate biosynthesis, regulation and transport. Biol. Chem. 395, 529–543. doi: 10.1515/hsz-2013-0286
Johanson, U., West, J., Lister, C., Michaels, S., Amasino, R., and Dean, C. (2000). Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290, 344–347. doi: 10.1126/science.290.5490.344
Kerwin, R. E., Jimenez-Gomez, J. M., Fulop, D., Harmer, S. L., Maloof, J. N., and Kliebenstein, D. J. (2011). Network quantitative trait loci mapping of circadian clock outputs identifies metabolic pathway-to-clock linkages in Arabidopsis. Plant Cell 23, 471–485. doi: 10.1105/tpc.110.082065
Kerwin, R., Feusier, J., Corwin, J., Rubin, M., Lin, C., Muok, A., et al. (2015). Natural genetic variation in Arabidopsis thaliana defense metabolism genes modulates field fitness. Elife 4:e05604. doi: 10.7554/eLife.05604
Keurentjes, J. J., Fu, J., de Vos, C. H., Lommen, A., Hall, R. D., Bino, R. J., et al. (2006). The genetics of plant metabolism. Nat. Genet. 38, 842–849. doi: 10.1038/ng1815
Kliebenstein, D. J., Gershenzon, J., and Mitchell-Olds, T. (2001a). Comparative quantitative trait loci mapping of aliphatic, indolic and benzylic glucosinolate production in Arabidopsis thaliana leaves and seeds. Genetics 159, 359–370. Available online at: http://www.genetics.org/content/159/1/359.long
Kliebenstein, D. J., Kroymann, J., Brown, P., Figuth, A., Pedersen, D., Gershenzon, J., et al. (2001b). Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126, 811–825. doi: 10.1104/pp.126.2.811
Kliebenstein, D. J., Lambrix, V. M., Reichelt, M., Gershenzon, J., and Mitchell-Olds, T. (2001c). Gene duplication in the diversification of secondary metabolism: tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 13, 681–693. doi: 10.1105/tpc.13.3.681
Koornneef, M., Hanhart, C. J., and Van der veen, J. H. (1991). A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229, 57–66. doi: 10.1007/BF00264213
Kroymann, J., Donnerhacke, S., Schnabelrauch, D., and Mitchell-Olds, T. (2003). Evolutionary dynamics of an Arabidopsis insect resistance quantitative trait locus. Proc. Natl. Acad. Sci. U.S.A. 100, 14587–14592. doi: 10.1073/pnas.1734046100
Kroymann, J., Textor, S., Tokuhisa, J. G., Falk, K. L., Bartram, S., Gershenzon, J., et al. (2001). A gene controlling variation in arabidopsis glucosinolate composition is part of the methionine chain elongation pathway. Plant Physiol. 127, 1077–1088. doi: 10.1104/pp.010416
Li, G., and Quiros, C. F. (2003). In planta side-chain glucosinolate modification in Arabidopsis by introduction of dioxygenase Brassica homolog BoGSL-ALK. Theor. Appl. Genet. 106, 1116–1121. doi: 10.1007/s00122-002-1161-4
Long, Q., Rabanal, F. A., Meng, D., Huber, C. D., Farlow, A., Platzer, A., et al. (2013). Massive genomic variation and strong selection in Arabidopsis thaliana lines from Sweden. Nat. Genet. 45, 884–890. doi: 10.1038/ng.2678
Lorenzo, O., Chico, J. M., Sánchez-Serrano, J. J., and Solano, R. (2004). JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16, 1938–1950. doi: 10.1105/tpc.022319
Méndez-Vigo, B., Martínez-Zapater, J. M., and Alonso-Blanco, C. (2013). The flowering repressor SVP underlies a novel Arabidopsis thaliana QTL interacting with the genetic background. PLoS Genet. 9:e1003289. doi: 10.1371/journal.pgen.1003289
Michaels, S. D., and Amasino, R. M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11, 949–956. doi: 10.1105/tpc.11.5.949
Mithen, R., Clarke, J., Lister, C., and Dean, C. (1995). Genetics of aliphatic glucosinolates.III. Side-chain structure of aliphatic glucosinolates in Arabidopsis-thaliana. Heredity 74, 210–215. doi: 10.1038/hdy.1995.29
Neal, C. S., Fredericks, D. P., Griffiths, C. A., and Neale, A. D. (2010). The characterisation of AOP2: a gene associated with the biosynthesis of aliphatic glucosinolates in Arabidopsis thaliana. BMC Plant Biol. 10:170. doi: 10.1186/1471-2229-10-170
Nour-Eldin, H. H., Hansen, B. G., Nørholm, M. H. H., Jensen, J. K., and Halkier, B. A. (2006). Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res. 34, e122. doi: 10.1093/nar/gkl635
Paul-Victor, C., Züst, T., Rees, M., Kliebenstein, D. J., and Turnbull, L. A. (2010). A new method for measuring relative growth rate can uncover the costs of defensive compounds in Arabidopsis thaliana. New Phytol. 187, 1102–1111. doi: 10.1111/j.1469-8137.2010.03325.x
Pigliucci, M. (2003). Selection in a model system: ecological genetics of flowering time in Arabidopsis thaliana. Ecology 84, 1700–1712. doi: 10.1890/0012-9658(2003)084[1700:SIAMSE]2.0.CO;2
Rohr, F., Ulrichs, C., and Mewis, I. (2009). Variability of aliphatic glucosinolates in Arabidopsis thaliana (L.) - impact on glucosinolate profile and insect resistance. J. Appl. Bot. Food Quality 82, 131–135. Available online at: http://pub.jki.bund.de/index.php/JABFQ/article/view/2091/2476
Rohr, F., Ulrichs, C., Schreiner, M., Zrenner, R., and Mewis, I. (2012). Responses of Arabidopsis thaliana plant lines differing in hydroxylation of aliphatic glucosinolate side chains to feeding of a generalist and specialist caterpillar. Plant Physiol. Biochem. 55, 52–59. doi: 10.1016/j.plaphy.2012.03.005
Rowe, H. C., Hansen, B. G., Halkier, B. A., and Kliebenstein, D. J. (2008). Biochemical networks and epistasis shape the Arabidopsis thaliana metabolome. Plant Cell 20, 1199–1216. doi: 10.1105/tpc.108.058131
Salomé, P. A., Bomblies, K., Laitinen, R. A. E., Yant, L., Mott, R., and Weigel, D. (2011). Genetic architecture of flowering-time variation in Arabidopsis thaliana. Genetics 188, 421–433. doi: 10.1534/genetics.111.126607
Schneeberger, K., Ossowski, S., Ott, F., Klein, J. D., Wang, X., Lanz, C., et al. (2011). Reference-guided assembly of four diverse Arabidopsis thaliana genomes. Proc. Natl. Acad. Sci. U.S.A. 108, 10249–10254. doi: 10.1073/pnas.1107739108
Shin, J., Heidrich, K., Sanchez-Villarreal, A., Parker, J. E., and Davis, S. J. (2012). TIME FOR COFFEE represses accumulation of the MYC2 transcription factor to provide time-of-day regulation of jasmonate signaling in Arabidopsis. Plant Cell 24, 2470–2482, doi: 10.1105/tpc.111.095430
Shindo, C., Aranzana, M. J., Lister, C., Baxter, C., Nicholls, C., Nordborg, M., et al. (2005). Role of FRIGIDA and FLOWERING LOCUS C in determining variation in flowering time of Arabidopsis. Plant Physiol. 138, 1163–1173. doi: 10.1104/pp.105.061309
Sønderby, I. E., Geu-Flores, F., and Halkier, B. A. (2010). Biosynthesis of glucosinolates - gene discovery and beyond. Trends Plant Sci. 15, 283–290. doi: 10.1016/j.tplants.2010.02.005
Team, R. C. (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Wang, S., Basten, C., and Zeng, Z. (2012). Windows QTL Cartographer 2.5. Raleigh, NC: Department of Statistics, North Carolina State University.
Ward, J. K., Samanta Roy, D., Chatterjee, I., Bone, C. R., Springer, C. J., and Kelly, J. K. (2012). Identification of a major QTL that alters flowering time at elevated [CO(2)] in Arabidopsis thaliana. PLoS ONE 7:e49028. doi: 10.1371/journal.pone.0049028
Wentzell, A. M., Rowe, H. C., Hansen, B. G., Ticconi, C., Halkier, B. A., and Kliebenstein, D. J. (2007). Linking metabolic QTLs with network and cis-eQTLs controlling biosynthetic pathways. PLoS Genet. 3, 1687–1701. doi: 10.1371/journal.pgen.0030162
Woods, E. C., Hastings, A. P., Turley, N. E., Heard, S. B., and Agrawal, A. A. (2012). Adaptive geographical clines in the growth and defense of a native plant. Ecol. Monogr. 82, 149–168. doi: 10.1890/11-1446.1
Keywords: glucosinolates, AOP2, AOP3, flowering time, regulation, cross-talk
Citation: Jensen LM, Jepsen HSK, Halkier BA, Kliebenstein DJ and Burow M (2015) Natural variation in cross-talk between glucosinolates and onset of flowering in Arabidopsis. Front. Plant Sci. 6:697. doi: 10.3389/fpls.2015.00697
Received: 25 June 2015; Accepted: 21 August 2015;
Published: 08 September 2015.
Edited by:
Stanislav Kopriva, University of Cologne, GermanyReviewed by:
Jens Rohloff, Norwegian University of Science and Technology, NorwayHenning Frerigmann, Universität zu Köln, Germany
Copyright © 2015 Jensen, Jepsen, Halkier, Kliebenstein and Burow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meike Burow, Department of Plant and Environmental Sciences, DNRF Center DynaMo, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg, Denmark,bWJ1QHBsZW4ua3UuZGs=
 Lea M. Jensen
Lea M. Jensen Henriette S. K. Jepsen1,2
Henriette S. K. Jepsen1,2 Barbara A. Halkier
Barbara A. Halkier Daniel J. Kliebenstein
Daniel J. Kliebenstein Meike Burow
Meike Burow