- Hawkesbury Institute for the Environment, Western Sydney University, Richmond, NSW, Australia
Many scarab beetles spend the majority of their lives belowground as larvae, feeding on grass roots. Many of these larvae are significant pests, causing damage to crops and grasslands. Damage by larvae of the greyback cane beetle (Dermolepida albohirtum), for example, can cause financial losses of up to AU$40 million annually to the Australian sugarcane industry. We review the ecology of some scarab larvae in Australasia, focusing on three subfamilies; Dynastinae, Rutelinae, and Melolonthinae, containing key pest species. Although considerable research on the control of some scarab pests has been carried out in Australasia, for some species, the basic biology and ecology remains largely unexplored. We synthesize what is known about these scarab larvae and outline key knowledge gaps to highlight future research directions with a view to improve pest management. We do this by presenting an overview of the scarab larval host plants and feeding behavior; the impacts of abiotic (temperature, moisture, and fertilization) and biotic (pathogens, natural enemies, and microbial symbionts) factors on scarab larvae and conclude with how abiotic and biotic factors can be applied in agriculture for improved pest management, suggesting future research directions. Several host plant microbial symbionts, such as arbuscular mycorrhizal fungi and endophytes, can improve plant tolerance to scarabs and reduce larval performance, which have shown promise for use in pest management. In addition to this, several microbial scarab pathogens have been isolated for commercial use in pest management with particularly promising results. The entomopathogenic fungus Metarhizium anisopliae caused a 50% reduction in cane beetle larvae while natural enemies such as entomopathogenic nematodes have also shown potential as a biocontrol. Key abiotic factors, such as soil water, play an important role in affecting both scarab larvae and these control agents and should therefore feature in future multi-factorial experiments. Continued research should focus on filling knowledge gaps including host plant preferences, attractive trap crops, and naturally occurring pathogens that are locally adapted, to achieve high efficacy in the field.
Introduction
Worldwide there are over 31,000 species of scarab beetles (Coleoptera: Scarabaeidae; Jameson, 2015) and within Australia alone there are well over 2,200 described species (Hangay and Zborowski, 2010). These scarabs can be found across tropical, subtropical and temperate regions of Australia and New Zealand in a broad range of ecosystem types including agroecosystems (Allsopp, 1999). Many scarabs have become destructive pests of grasslands as root-feeders (Potter and Braman, 1991). There are also instances where introduced plant species have become the preferred host to a number of native scarabs such as greyback cane beetle larvae (Dermolepida albohirtum Waterhouse, subfamily: Melolonthinae) feeding on sugarcane (Saccharum sp.). Moreover, the problem of such species becoming pests has been exacerbated by agriculture (Robertson et al., 1995), such as large-scale transition of grassland into arable crop production, or of forests and woodlands into pastures. Crop losses due to scarab larval damage for sugarcane in Australia alone can result in losses up to AU$40 million annually (Chandler, 2002). Historically, this problem has been addressed by using chemical pesticides, which can have serious collateral effects on non-target organisms and the environment (Jackson and Klein, 2006). As such, alternative management strategies are being continually investigated (Goldson et al., 2015).
Understanding the biology and behavior of scarab larvae, including their interactions with host plants and the soil environment (or rhizosphere) is an essential component to enabling effective management and control, both in Australia and at a global scale. There are numerous studies on these larvae within Australasia, some of which have elucidated core biology, behavior and even responses to future environment such as climate change (Johnson et al., 2014). However, for many scarab species this work was carried out some time ago, while for others the majority of their ecology has yet to be described. This is partly due to their soil-dwelling habit which has made culturing and experimentation particularly challenging. It is therefore timely to synthesize the fragmented information available on this group of root-feeding pests in Australasia. In this review we identify where knowledge is lacking, highlight promising research avenues into pest management, to suggest where continued research should be focused. In particular, this review focuses on belowground influences which impact larval development and survival. Edaphic variables such as soil moisture and temperature alongside biotic interactions with microbiota both in the soil and with host plants show most promise for improved pest management.
We concentrate on three subfamilies belonging to the family Scarabaeidae: Dynastinae (e.g., African black beetle Heteronychus arator Fabricius and Argentine scarab Cyclocephala signaticollis Burmeister), Rutelinae (e.g., Christmas beetles Anoplognathus sp. Leach) and Melolonthinae (e.g., dusky pasture scarab Sericesthis nigrolineata Boisduval and greyback cane beetle D. albohirtum). Within these subfamilies we focus on the key pest species/genera examples mentioned, while including any relevant information from other species within the subfamilies. The redheaded cockchafer, Adoryphorus couloni Burmeister (subfamily: Dynastinae) is also a significant pasture pest within Australia and was comprehensively reviewed recently (Berg et al., 2014). Hence, we do not include this species within the review. Within the three subfamilies we specifically focus on:
1. Host plants and feeding behavior
2. Abiotic soil factors (temperature, moisture, and fertilization)
3. Biotic soil factors (pathogens, natural enemies, and symbionts)
4. Applied perspectives
5. Directions for future research
Host Plants and Feeding Behavior
While the majority of scarabs are grass root-feeders in their larval stages (Figures 1 and 2; Goodyer and Nicholas, 2007), some larvae feed on organic matter in the soil litter (Jackson and Klein, 2006). For some pest scarab species, feeding ecology has been documented relatively well. Across the subfamilies discussed here the most damaging and voracious feeding occurs during the third instar, therefore the timing of development of pest scarab larvae is important to consider from a pest management perspective (Figure 3). Indeed, the ability of all scarab larvae to locate suitable hosts is equally as important as the nutritional value of the host plant. Carbon dioxide emissions by the host plant is an important root exudate that plays a role in host plant location by root herbivores (Johnson and Gregory, 2006); however, other volatile root exudates are clearly critical in host plant location by scarab larvae (Eilers et al., 2012). The topic of host plant location by root-feeders was reviewed by Johnson and Gregory (2006) and revised by Johnson and Nielsen (2012), and we will not discuss this in detail here. Here we will present what is known regarding the feeding behavior of some of the key species from within Dynastinae, Rutelinae, and Melolonthinae.
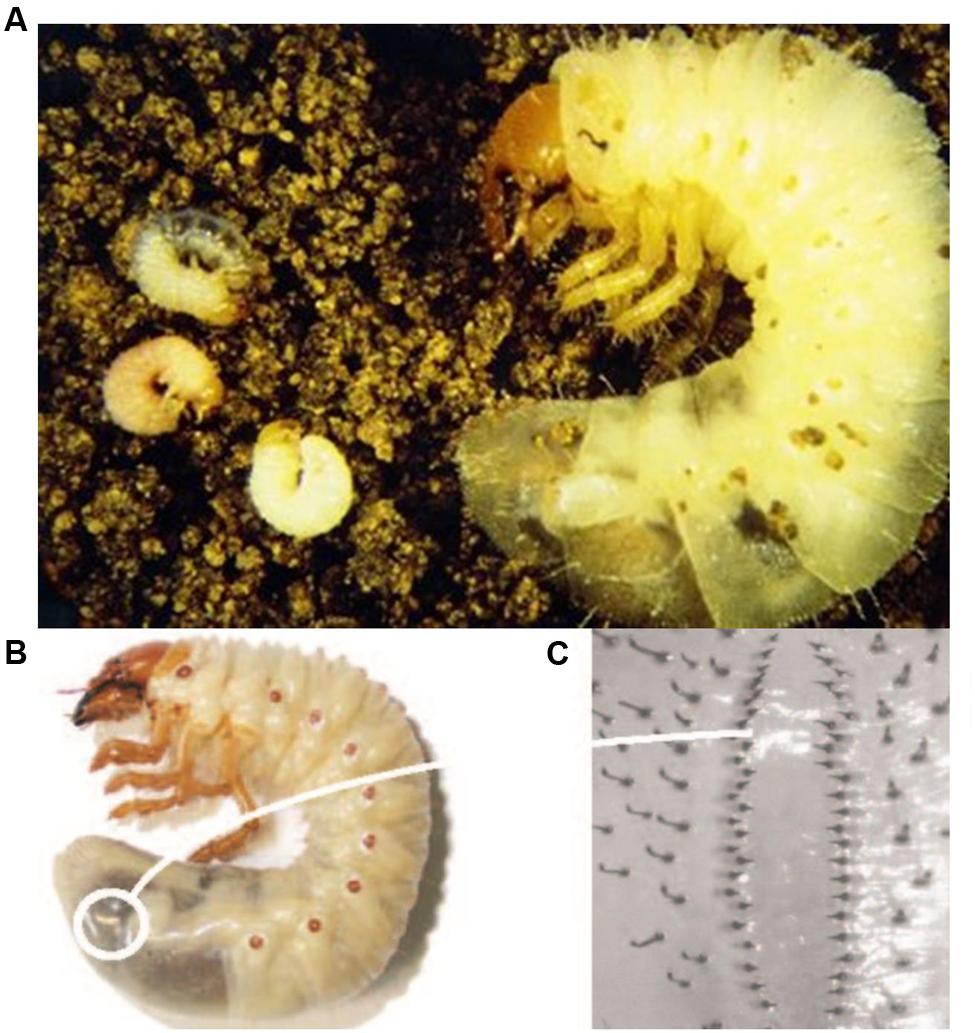
FIGURE 1. Scarab larvae: (A) African black beetle larvae Heteronychus arator, (B) greyback cane beetle larva Dermolepida albohirtum, (C) close-up of hair pattern (raster) used to identify greyback cane beetle larvae. Images supplied by Western Australian Department of Agriculture and Food (African black beetle) and Sugar Research Australia (greyback cane beetle larva).

FIGURE 2. Third instar larva of the greyback cane beetle (D. albohirtum). Image supplied by Adam Frew.
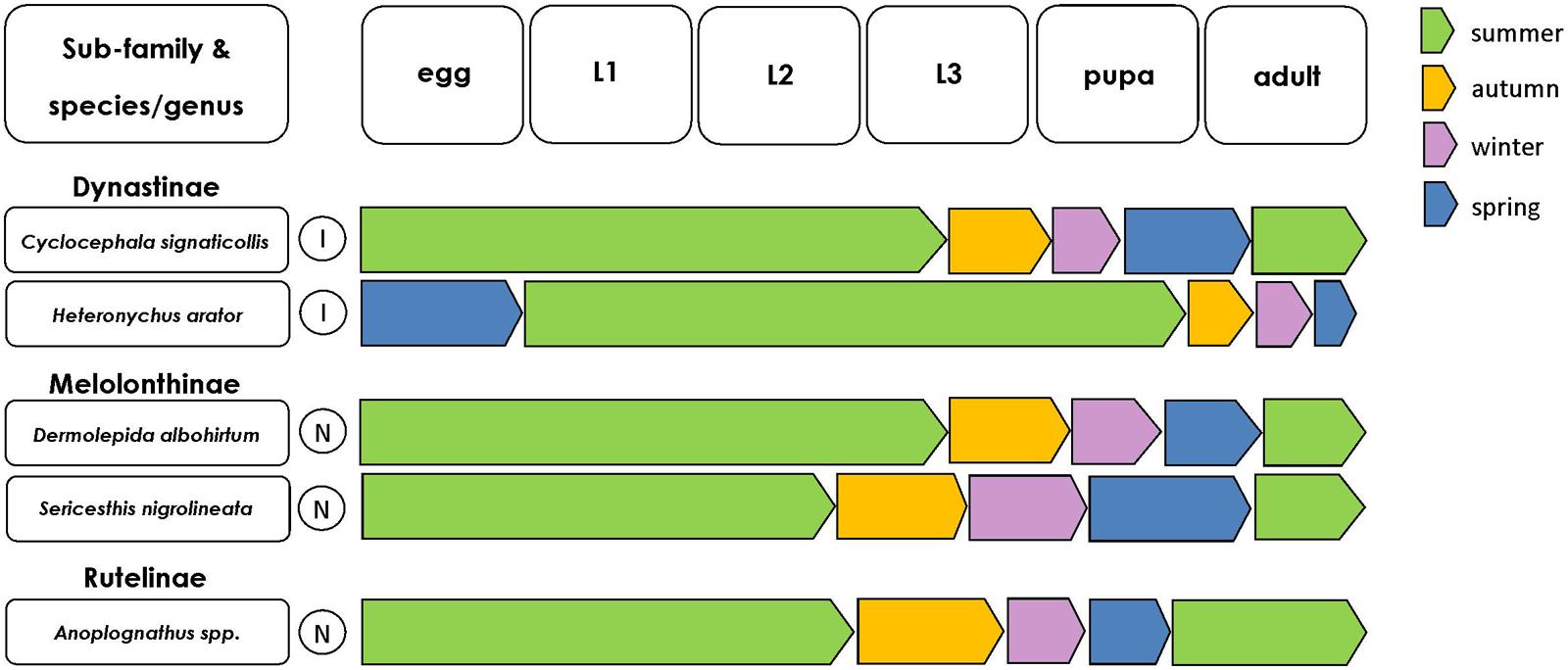
FIGURE 3. Seasonal occurrence of scarab life stages for each of the key scarab pest species. Such information can help to design life-stage specific, targeted pest control programs. Color of arrows indicates the season in which each scarab life stage typically occurs (within Australia and New Zealand). Circles with I indicate species invasive to Australasia, circles with N indicate species native to Australasia.
Dynastinae
The African black beetle has been described as a sporadic pest of pastures and crops across New Zealand and Australia (Matthiessen and Ridsdill-Smith, 1991). Plant species composition influences the distribution of the African black beetle across the landscape (King and Kain, 1974; King et al., 1982). The larvae seem to have reduced performance on species such as Medicago sativa (King et al., 1975) and tend to avoid feeding on Trifolium repens (Sutherland and Greenfield, 1978), which is due, at least in part, to the feeding deterrents medicarpin and vestitol present in the roots (Russell et al., 1982). That said, larvae will eat T. repens roots if given no other choice (King et al., 1981c). Despite this, T. repens is a common food source for other scarab larvae such as Costelytra zealandica White (subfamily: Melolonthinae; King et al., 1981a; Russell et al., 1982; Prestidge et al., 1985).
By contrast, the grasses Lolium perenne and Paspalum dilatatum have been shown to be a preferred food choice of pasture grass species (King, 1977; King et al., 1981a). King (1977) found that African black beetle larval mass gain was greater on L. perenne when compared with T. repens and Lotus pedunculatus, but also that organic matter in the soil stimulated this feeding and increased weight gain. The organic content of the soil acting as a feeding stimulant has therefore been suggested as having implications for damage in soil with high peat content (Bell et al., 2011). Indeed the African black beetle is a significant pest of L. perenne pastures, both as larvae and adults, feeding on below- and aboveground portions of the plant, respectively (Popay and Bonos, 2008). The endophytic fungus Neotyphodium lolii, forms a mutualistic relationship with L. perenne (Raman et al., 2012). Feeding by adult African black beetles is well documented to be deterred by N. lolii infected L. perenne (Popay and Baltus, 2001), which has been attributed to the presence of alkaloids (Thom et al., 2014). More recently, Qawasmeh et al. (2015) found that different strains of N. lolii had an impact on the aboveground volatile profile of L. perenne and the attractiveness of this host plant to adult African black beetles.
The majority of research into endophyte (Table 1) induced protection has focused on aboveground herbivores (Popay and Baltus, 2001). One study on a specific N. lolii strain noted that the African black beetle larvae were observed to have a reduced occurrence in N. lolii infected grasses (Hume et al., 2007). More recently, another study has found changes in the root volatile profile in response to Neotyphodium uncinatum infection and found decreased attraction to C. zealandica larvae belowground (Rostás et al., 2015).
Considering damage can be significant, more research focusing on the efficacy of N. lolii strains in deterring African black beetle larvae would be the logical next steps. In the field, replacing turfgrass or pasture with N. lolii infected L. perenne could convey protection against African black beetle adults at the very least, perhaps reducing oviposition, and indeed may deter all alkaloid sensitive insect herbivores (see ‘Applied perspectives’ section).
The feeding behavior of Argentine scarab larvae has not received significant attention in the literature despite its pest status on turf and pastures (Carne, 1957a). Within Argentina, the larvae are known as pests particularly of potato crops (Berón and Diaz, 2005), but are known to feed on roots of flax, lucerne, sunflower, and carrot crops as well (Mondito et al., 1997). In Australia, however, the larvae feed mainly on grass roots. Carne (1957a) noted that the larvae were found in the greatest numbers in grasslands with Cynodon dactylon and P. dilatatum. It was also noted that this scarab could successfully develop on a diet composed solely of decomposing organic matter; however, the abundance found in pastures indicates some of their nutrient requirements are derived from grass roots. It is evident the Argentine scarab larvae feed on both organic matter and actively on grass roots but other than a few studies no other feeding behavior investigation has been carried out on the Argentine scarab in Australian grasslands. The lack of context specific studies on the larval feeding preferences of this scarab species, alongside the efficacy of management practices, calls for initial host preference studies to be conducted before any control initiatives can effectively be researched and applied.
Rutelinae
The feeding behavior of adult Anoplognathus spp., which consume the leaves of eucalypts, is addressed well within the literature (Carne et al., 1974; Edwards et al., 1993; Steinbauer and Wanjura, 2002; Johns et al., 2004; Steinbauer and Weir, 2007), in contrast to the information on larval feeding behavior, which is relatively scarce.
Anoplognathus larvae are known to feed on organic matter in the soil, grass roots, and crop roots (Carne, 1957b; Sallam et al., 2011). Some species within the genus, such as Anoplognathus montanus, will commonly feed on rotting organic material such as timber, but will also feed on the finer roots of eucalypts (Carne, 1957b). Carne et al. (1974) stated that larvae of Anoplognathus feed primarily on organic matter in the soil and tend not to seek out plant roots. While Davidson and Roberts (1968a) confirmed this, they nonetheless stated that the organic matter they feed on is composed mainly of plant roots. Here, they also found that when Christmas beetle larvae fed on the grass Phalaris tuberosa and T. repens, larvae often failed to reach pupation, which could be due to secondary metabolites in the plant. In a further study that year, it was found that Christmas beetle larvae avoided feeding on T. repens altogether (Davidson and Roberts, 1968b), a behavior also exhibited by African black beetle larvae.
The larvae of Anoplognathus spp. have been reported as pests of sugarcane, although only when numbers are high (Samson et al., 2013). Significant damage to pastures by Christmas beetle larvae is well known, particularly by the third instar (Urquhart, 1995). Feeding populations of larvae can be influenced by aboveground herbivores. A study by Roberts and Morton (1985) investigated the effects of grazing pressure on the biomass of Anoplognathus sp. larvae, and found that larval abundance peaked under low to intermediate grazing pressure. Therefore, low pasture damage by larvae may be exacerbated by moderate grazing of livestock aboveground.
Melolonthinae
The greyback cane beetle is a long standing pest within sugarcane and the larvae can cause devastating damage to crops (Chandler, 2002). Initial uncertainties regarding the feeding of mainly organic material in the soil (Illingworth and Dodd, 1921) have been resolved as there is compelling evidence for grass roots as the main resource (Sallam, 2011). Root feeding was shown by Logan and Kettle (2002) who investigated the effect of food type on the survival and development of first instar greyback cane beetle larvae. Larval survival and development was highest in treatments with grass seedlings and lowest in soil alone. This result was confirmed by a second experiment using sugarcane, Guinea grass (Panicum maximum), cane trash (mulch), and a soil only environment, where larval survival and mass was lowest in the soil only treatment and highest when cane or grass were available (Figure 4).
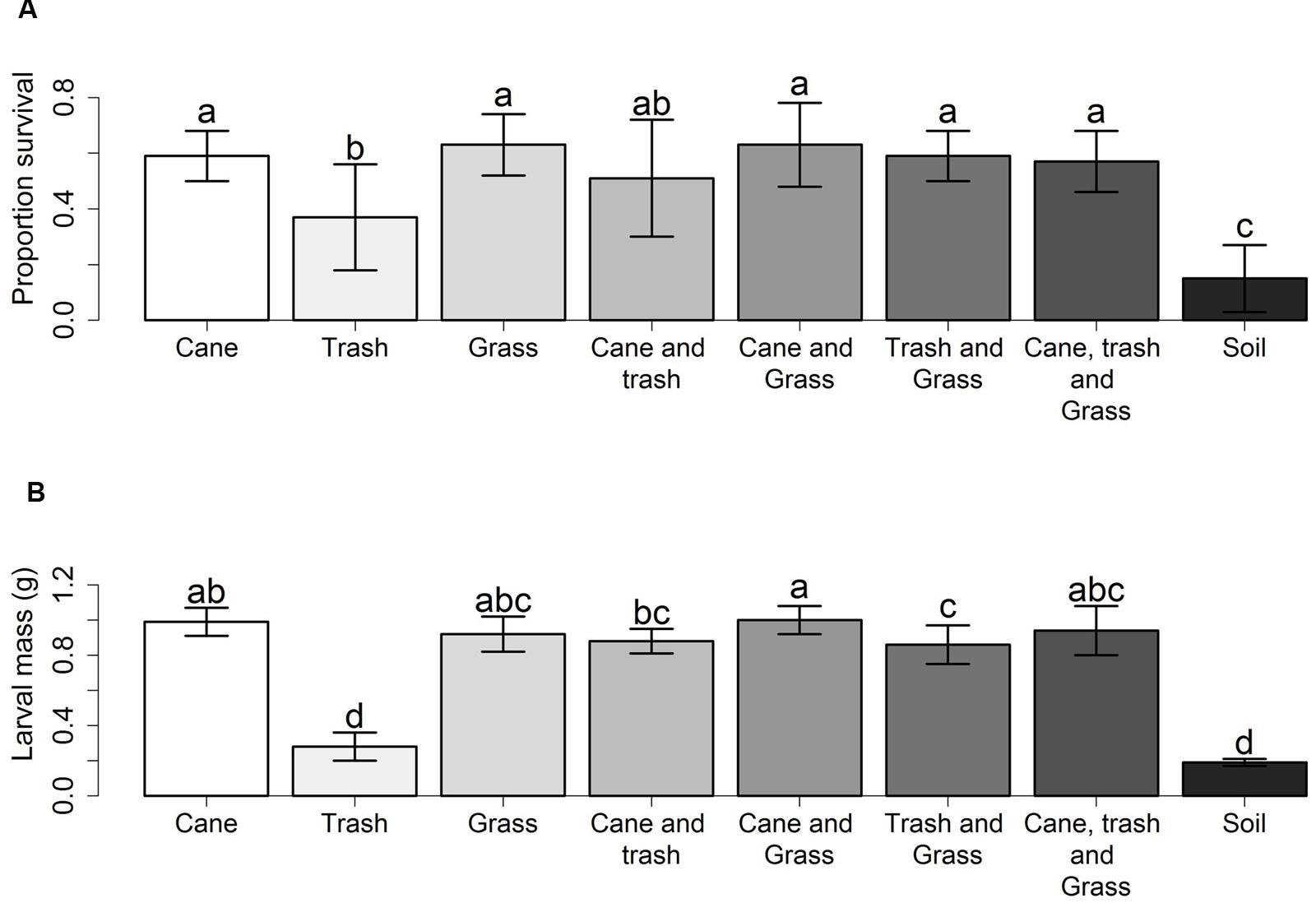
FIGURE 4. Survivorship and mass of early instar larvae of D. albohirtum. (A) Mean proportion survival (±SD), and (B) mean larval mass in grams (±SD), of larvae after 4 weeks in bins with food of either sugarcane, Guinea grass, cane trash, combinations of two or three of these, or none of these. Different letters indicate significant effects of treatments. Adapted from Logan and Kettle (2002).
In Australia, cane beetles are the major pests to the sugar industry (McLeod et al., 1999; Horsfield et al., 2008) and as a result there have been several studies into pest management and environmental conditions that may impact on larval induced damage to sugarcane (Robertson et al., 1995; Robertson and Walker, 2001; Chandler, 2002). Coupled with the development of pest management strategies, Allsopp (1991) investigated feeding stimulants of greyback cane beetle larvae, which could be used to enhance the efficacy of larval baits. Larvae showed a strong feeding response to fructose and sucrose. Both sucrose and fructose, along with glucose, are the most abundant sugars found in sugarcane, and are both at higher concentrations in the lower stem of sugarcane compared with the roots (Irvine, 1977).
Estimates of population size and density within sugarcane fields vary from three or four larvae per cane plant (Ward and Robertson, 1999) to numbers of 15 per plant, or more (Jarvis, 1933; Sallam, 2011). Some Melolonthinae larvae have shown specific soil type preferences. A study by Cherry and Allsopp (1991) found distinct soil type preferences between different species, with some larval populations of some species positively correlated with clay and silt, and negatively with sand content, while other species showed opposing correlations. For yet other species, such as the greyback cane beetle, soil type has little influence on the distribution (Robertson and Walker, 2001). Overall there is no ‘one soil type fits all’ for scarab species as studies have shown species specific preferences (Gordon and Anderson, 1981; Cherry and Hall, 1986).
Studies conducted into the feeding behavior of dusky pasture scarab larvae have focused on climatic and abiotic influences rather than host preference. The larvae can feed and survive in soil in the absence of plant roots (Ridsdill-Smith, 1975; Smith and Porter, 1980), however, it is not clear if they are able to develop into adults on soil organic matter alone. The feeding behavior, and relative consumption of food is largely influenced by temperature (Davidson et al., 1972b; Ridsdill-Smith et al., 1975; Cairns, 1978) and under field conditions there is often a seasonal pattern of larval feeding as a result of local temperatures. Ridsdill-Smith (1975) carried out an investigation into the feeding behavior of dusky pasture scarab larvae using slices of carrot under different temperatures. It was found that the larval consumption of food peaked at 30°C while, interestingly, the efficiency of conversion of ingested food (which accounts for larval growth and the mass of food consumed) peaked at a temperature of 14°C. To build upon this, a follow-up study utilizing larvae that had been fed on living roots and a variety of food sources was conducted by Ridsdill-Smith (1977). It found that the feeding of dusky pasture scarab larvae declined when the population densities were high, although this was likely a result of a lack of young living roots. This was confirmed by Ridsdill-Smith and Roberts (1976), who also showed that larval growth reduced as density increased, which was also likely to be due to a limited food supply. The study also suggested that the larvae preferred to feed on younger roots.
One recent study by Johnson et al. (2014) provided evidence of compensatory feeding by the dusky pasture scarab larvae under elevated atmospheric CO2 (eCO2) on Microlaena stipoides, a C3 grass. Despite this increased feeding, the performance of the dusky pasture scarab was much lower under these eCO2 conditions, which was likely due to a reduction in the root nitrogen concentrations. Interestingly, under ambient CO2, larvae consumed 48% more material from M. stipoides than from Cymbopogon refractus, a C4 grass. Generally, C3 grasses are thought to be more susceptible to herbivory than C4 grasses (Caswell et al., 1973). More studies of this type are necessary to elucidate the relationship between scarabs and their host plants, particularly when considering changes in feeding behaviors as a result of climate change. It can be concluded from these studies that the feeding behavior of the dusky pasture scarab larvae is strongly influenced by abiotic factors such as temperature and, indirectly, atmospheric CO2. As such, future research should investigate host plant preferences alongside abiotic and biotic interactions, including changes in atmospheric CO2 concentrations.
Abiotic Soil Factors
Abiotic factors have been seen to have a strong influence on insect pests of Australasia (Powell et al., 2003). All root-feeding insects respond directly to their immediate physical and chemical environment (Barnett and Johnson, 2013). Here, we review some significant abiotic factors impacting on scarab larvae: temperature, moisture, and fertilization. We focus on species within Dynastinae, Rutelinae, and Melolonthinae found in Australasia. We also draw on studies of other species within these subfamilies outside Australasia to indicate the general impact of abiotic rhizospheric factors on scarab larvae. These factors are considered with a view to highlight where agricultural practices could be modified to reduce damage by scarab larvae (discussed in more detail in ‘Applied perspectives’ section).
Temperature
The temperature of the soil can impact significantly on scarabs, particularly in the egg and early larval stages. For example, temperature has been seen to have an impact on population fluctuations of the African black beetle (East et al., 1981; King et al., 1981b). Despite this importance, few studies have focused on the temperature preferences for oviposition by scarab females.
Regarding larval stages, a single exposure of 35°C for 24 h has been shown to kill 100% of first instar larvae of Anoplognathus spp. and the dusky pasture scarab, while around 62% survive when exposure to such temperatures is only for 12 h (Hassan and Hilditch, 1976). Within the same study, second instar larvae showed a higher tolerance for high temperatures, for example at 37.5°C, 73% of first instar larvae died while only 40% of second instar died. Regarding the lower temperature threshold it is generally understood that at low temperatures (below 16°C) scarab eggs will take longer to hatch and larvae will take longer to develop (Davidson et al., 1972b). This relationship between temperature and development was investigated in greyback cane beetle pupae (Logan and Kettle, 2007), where the minimum and maximum time for pupal development was found to be 26 days at 30°C and 75 days at 18°C, respectively. The low temperature threshold, at and below which no development occurs was 12°C. There have several studies showing the influence of temperature on the growth and development of the dusky pasture scarab (Davidson et al., 1972b; Ridsdill-Smith, 1975; Ridsdill-Smith et al., 1975; Cairns, 1978). The relative growth rate of these larvae was found to have lower and upper temperature limits of 5°C and 32°C, respectively, with optimum growth occurring around 17.5°C (Ridsdill-Smith et al., 1975). One study on Rhizotragus majalis Razoumowsky (subfamily: Melolonthinae), indicated that later instar larvae have much greater mobility and therefore older scarab larvae are likely to be less susceptible to temperature stress through avoidance behavior (Villani and Nyrop, 1991). This was confirmed by Zhang et al. (2003) who confirmed higher mobility in second and third instars by monitoring their acoustic sounds, which also increased with soil temperature, while below 9°C sound production fell to a minimum. Overall, temperature plays an important role in the survival, and the rate of development of scarab larvae. Generally, larval growth rate increases with temperature, where upper limits tend to be between 35 and 40°C, and as temperatures drop to 16°C or below, development is significantly reduced. First instar larvae tend to be the most sensitive to temperatures stress, while scarab eggs and later instar larvae are more tolerant.
These larval responses to temperature indicate how significant climate can be to larval populations. Indeed, high temperatures at a particular time of development can have particularly large impacts on greyback cane beetle populations. Horsfield et al. (2008) analyzed larval damage records and climatic averages from 1989 to 2003 and showed that prolonged hot and dry conditions during the late spring can limit population numbers by impacting on emergence, as well as synchrony of emergence with feeding, mating and egg laying. Conversely, milder and wetter spring season can promote adult emergence and the ability of the adults to successfully feed, mate and lay eggs. This would directly impact on successive larval populations and therefore damage to cane the following year.
Moisture
Soil moisture is often referred to as the most important property that affects the development and survival of scarab larvae belowground (Brown and Gange, 1990; Barnett and Johnson, 2013). Indeed, eggs of many scarab species must absorb water before hatching (Potter, 1983), and hence the availability of water in the soil can be critical to scarab population dynamics. Soil moisture is also the factor best examined in the literature with regards to female oviposition in scarabs (Potter, 1983; Cherry et al., 1990; Allsopp et al., 1992; Logan, 2007). Several studies have shown different optimal soil moisture conditions for maximum oviposition. Some Melolonthinae scarabs are known to oviposit in soils around field capacity (-74 kPa; Logan, 2007), while others within the same subfamily prefer a range between field capacity and dry soil near wilting point (-1500 kPa; Logan, 2007). Ward and Rogers (2007) carried out a study on soil moisture ovipositional preferences in four Melolonthinae scarabs found in Australia, including the greyback cane beetle. It was concluded that those species adapted to the semi-arid tropics, where rainfall is unreliable, have little or no preferences observed beyond a reduction in oviposition in very dry soil (-1500 kPa). However, in subtropical and temperate (with less seasonal rainfall) adapted species there were clear preferences for drier soils (-1000 kPa). This suggests that the climates in which key/target pest species have originated and are adapted to, must be considered in attempts to manage populations. It also indicates that for those tropically adapted species, moisture control as a form of pest management may not be the way forward, as their ovipositional preferences are likely to be driven by factors other than soil moisture.
Moisture content of the soil can directly impact on scarab larvae populations. African black beetle populations, for example, have been shown to be suppressed in regions with early summer rainfall (Matthiessen and Ridsdill-Smith, 1991), as first instar larvae are more moisture sensitive than egg stage or later instars (King, 1979; King et al., 1981b). In periods of seasonal drought, the larval populations are no longer suppressed by the normally high moisture content, resulting in damaging outbreaks (Matthiessen and Ridsdill-Smith, 1991). Whether these population responses would be the same in different soils is uncertain. Matthiessen (1999) showed that soil type had a significant impact on African black beetle larval survival, and that this factor interacted with soil moisture, where larval survival was higher under regular watering treatments compared with no watering, but only in some soil types. With these studies in mind, investigations are necessary to elucidate the interaction between soil moisture and soil texture, where larval populations are monitored under different common soil types in the field, under a range of soil moisture treatments. Future work should also include extreme climate events, such as drought and flooding, as the frequency of such events are predicted to increase in the future (Pachauri et al., 2014). This way, we can gain a better picture of how belowground scarab pest status will change in the future.
Several studies have reported responses from other scarabs to soil moisture. For example, within the genus Cyclocephala, larvae are significantly more abundant and also have higher mass in irrigated, compared to non-irrigated plots (Potter et al., 1996). Survival of dusky pasture scarab larvae have been shown to be optimal between -100 and -150 kPa, while in saturated soils, larval survival is negatively proportional to the length of exposure (Davidson et al., 1972b). While studies involving R. majalis, have shown that larvae move quickly toward the surface when the moisture content of the soil is increased, yet little movement is exhibited in response to drought conditions (Villani and Wright, 1988).
Changes in soil moisture will also impact the host plants of scarab larvae. In addition to this, the diffusion of plant root volatiles is reduced in high soil moisture, however, some moisture is required to prevent total vertical diffusion (Hiltpold and Turlings, 2008). Indeed, natural enemies of scarab larvae, such as entomopathogenic nematodes (Table 1) (EPNs), are more effectively recruited by plant volatiles and have higher virulence in soils with high moisture content (Grant and Villani, 2003). Therefore future studies into the effects of different soil moisture contents within a variety of soil types, would also benefit to consider how the natural enemies and pathogens respond under these conditions. This way a more holistic and ecologically relevant picture can be constructed.
Fertilization
The response of soil dwelling root-feeders to fertilization has received some attention within the literature. Frew et al. (2013) found that the application of nitrogen, phosphorus, and potassium (NPK) fertilizers promoted more nutritionally superior grass species, which in turn increased abundance of dusky pasture scarab larvae. However, Potter et al. (1996) who investigated the effects of different agricultural practices on scarab populations over 3 years and found no significant effect of NPK fertilizer on Cyclocephala spp. density or growth. On the other hand, Radcliffe (1970) added organic (cow dung) fertilizer to the soil and found that this lessened the damage to grass roots by C. zealandica. This may have been where the larvae switched from feeding on the grass roots to the increased provision of organic matter in the soil, or the addition of excess organic matter may have contributed to better compensatory root growth in response to damage, or a combination of both. In the same study it was found that larvae development was more advanced when treated with nitrogen fertilizer (Radcliffe, 1970). It has also been shown that the addition of organic fertilizer increases the mass gain of C. zealandica larvae (Wightman, 1974). In contrast to these findings, other studies on C. zealandica have shown the addition of nitrogen fertilizers has had no effect on larval feeding and survival (Prestidge et al., 1985) or population density (Prestidge and East, 1984), with similar responses found with Popillia japonica Newman (subfamily: Rutelinae) to the application of NPK fertilizer (Crutchfield et al., 1995). Other root feeding insects have been shown to respond positively to the addition of nitrogen fertilizer, such as the rice weevil larvae [Lissorhoptrus oryzophilus Kuschel (Curculionidae, Erirhininae)] and the western corn rootworm larvae [Diabrotica virgifera virgifera LeConte (Chrysomelidae, Galerucinae)] (Spike and Tollefson, 1988). In the comprehensive review of belowground herbivores by Brown and Gange (1990), it was suggested that the timing of fertilization is important to the effect on the root feeding larvae. They suggested that if nitrogen fertilizer is applied before larvae are present then this promotes root growth, which in turn gives a greater food supply to larvae, while if fertilizer is added after larval establishment then the damage to grasses is less (Spike and Tollefson, 1988).
It is known in some plants that when nitrogen is limiting in the soil, plant defense investment increases in the leaves (Schmelz et al., 2003; Chen et al., 2008). Low soil nitrogen content could similarly affect root defense investment allocation, thereby impacting the root-feeding scarab beetle larvae populations. It has been suggested that fertilization may cause a reduction in the defensive root compounds (Hol, 2011; Erb and Lu, 2013). These may be direct secondary defenses affecting scarab feeding or performance, or indirect defenses involving recruitment of natural enemies such as EPNs (see section on ‘Pathogens, natural enemies and symbionts’ below). Such plant responses to fertilization addition could be linked to arbuscular mycorrhizal fungal (AMF) associations. AMF associations have been shown to increase induced plant defense responses (Pozo and Azcón-Aguilar, 2007), but root colonization by AMF is known to be reduced when soil nutrients (particularly nitrogen and phosphorus) are high (Vannette and Hunter, 2009; Smith and Read, 2010). Therefore any decrease in plant defenses in response to high nitrogen, could be mediated by limited AMF colonization.
Overall, the literature is not consistent regarding the impact of fertilization on scarab larvae and similar species, although both positive and null effects seem to be the most common responses reported. Any positive effect is likely to be due to an increase in organic matter for younger instar scarabs to ingest and an increase in the nutritional value of host plant species. An increase in nutrient availability may also result in an increase in the tolerance of the host plant to herbivory, although this is likely to be dependent on the nutrient and specific herbivore in question (Wise and Abrahamson, 2007). This may also impact on important microbial plant associations in the soil (Smith and Read, 2010), which can indirectly impact on herbivores (Bennett and Bever, 2007; Biere and Bennett, 2013). Therefore soil fertility may promote root-feeding scarabs, but also may increase plant tolerance to herbivory as well as benefit the natural enemies of scarabs belowground. Continued research should aim to include as many contributing factors to plant–insect interactions within the soil (such as AMF and EPNs) as possible, as these are likely to produce outcomes more relevant in the field.
Biotic Soil Factors
Pathogens, Natural Enemies and Symbionts
Scarabs have a number of natural enemies and insect pathogens that threaten their survival. Scarab larvae have evolved within the soil environment, which naturally brings them in close contact with numerous soil organisms and microbiota, some of which are pathogens (Jackson and Klein, 2006). Here we discuss some pathogens and natural enemies that have been identified to hold potential as biocontrol agents against scarab larval pests in the field.
Entomopathogenic fungi are ubiquitous in soils, particularly those within the genera Metarhizium and Beauveria. Greyback cane beetle larvae are easily infected by the entomopathogenic fungus (Table 1) M. anisopliae. The impact of this naturally occurring fungus on the larval populations is not density dependent and as such has been shown to account for a fixed mortality rate, regardless of the population density, while the spores are known to be resistant to many agricultural practices (Sallam et al., 2003, 2007). This fungus has been isolated and commercialized as BioCaneTM and used as a fungal biocontrol that in trials has shown more than 50% control of the canegrub after 6 months of a single application (Logan et al., 2000). Interestingly Berón and Diaz (2005) carried out susceptibility trials of the Argentine scarab larvae to different strains of M. anisopliae. All strains showed low virulence against the larvae, possibly due to the lack of host specificity to the Argentine scarab. However, a particular strain of the entomopathogenic fungus Beauveria bassiana, did show up to 70% mortality in Argentine scarab larvae. The differences in virulence of M. anisopliae toward different scarab larvae species shows how the insect response to microbial pathogens can often be species specific, and can vary significantly. Another Beauveria sp. that has shown success as a biocontrol is B. brongniartii, which has been successful acting against a broad range of hosts. Some native strains have been isolated from Melolontha melolontha Linneaus (subfamily: Melolonthinae) and used as pest controls across Europe with good success (Dolci et al., 2006). Similar work with Beauveria strains isolated from Madagascar and Turkey have also seen success (Maurer et al., 1997; Sevim et al., 2010). These are further examples of successful isolation and application of naturally occurring scarab pathogens.
A significant pathogenic microorganism, particularly noted in efficacy against the greyback cane beetle larvae, is the protozoan Adelina sp. which is a density dependent pathogen (Robertson et al., 1998). High Adelina incidence causes a drop in the larval population which in turn impacts on the Adelina incidence in the soil. Interestingly Sallam et al. (2003) found that Adelina incidence was higher in soil with grass cover compared to bare soil areas, which could be due to higher moisture retention and cooler temperatures. Responses such as these should be taken into account when managing larval populations in agriculture to optimize natural pathogen efficacy.
Within New Zealand, the bacteria Serratia entomophila and S. proteamaculans were isolated from C. zealandica as the cause of amber disease, which leads to the cessation of feeding of the scarab grub resulting in eventual death (Hurst et al., 2004). These bacteria were developed as biopesticides against scarabs and have been used for almost 20 years as biocontrol agents. These are further examples of microbial pathogens adapted to their host, and their host range which were used to great success as a control method of scarabs (Hurst et al., 2000).
There are a number of viruses that infect scarabs, such as pox viruses and iridescent viruses; however, little research has been done on their potential as biocontrols, and their presence and effect on scarab populations under natural conditions has not yet been documented (Jackson and Glare, 1992). Damage by the Dynastinae scarab larvae within the genus Oryctes has been successfully mitigated via the Oryctes virus (Huger, 2005), which is a unique virus, in that it was identified as the first rod-shaped, non-occluded insect virus, and is highly infectious. It has been isolated, purified and used in pest control for over 10 years, but it has low success on any species outside of the target scarab genus Oryctes (Huger, 2005). Current research is focused on selecting strains of the virus for greatest persistence in the environment.
One of the major natural enemies of scarabs are EPNs, which are internal parasites of scarabs. They do not act alone, but rather it is their association with entomopathogenic bacteria that kill the scarab hosts. Steinernema and Heterorhabditis are the two genera of EPNs and there are a number of species within both genera that infect scarabs (Klein, 1993). The EPNs kill the larvae via their symbiotic bacteria Xenorhabdus sp. Several species have been isolated from scarab grubs, such as Steinernema glaseri, S. anomaly, Heterorhabditis megidis, and several different strains of S. carpocapsae and H. bacteriophora (Klein, 1993), their potential to control scarab larvae populations is being investigated. Some nematodes have shown success in laboratory and field trials against scarab larvae, with particular interest in S. scarabaei as an effective control against a range of scarabs dominant in North America and Asia (Stock and Koppenhöfer, 2003). However, other efforts to use EPNs in the field have not been successful, which have been attributed to a lack of understanding of the nematode–bacterium complex and differences in target species susceptibility, biology or behavior (Klein, 1993; Georgis et al., 2006). Recently Wu et al. (2014) tested and compared the virulence of four EPN species and their interactive effects with entomopathogenic fungi against the scarab larvae of Cyclocephala lurida Bland (subfamily: Dynastinae). They concluded that the impact of H. bacteriophora alone or in combination with the fungal pathogens was comparable to that of an imidacloprid insecticide against the larvae. This indicates the potential EPNs have as biocontrols and that further work is warranted to fully elucidate the interaction between natural enemies, pathogens, and host. Plants can recruit EPNs via attractive volatile signals as a natural defense strategy (Grewal et al., 1994; Rasmann et al., 2005). It has been shown that EPNs can be selectively bred for enhanced responsiveness to these volatile cues (Hiltpold et al., 2010), meaning that improved efficacy of the commercial EPN use is still ongoing and holds great potential as a biological control method of scarabs in agriculture and industry.
Finally, diverse communities of endosymbiotic bacteria (Table 1) that assist with the digestion of plant material, particularly cellulose and hemicelluloses, live within the hindguts of scarab larvae (Cazemier et al., 2003; Huang et al., 2010). Pittman et al. (2008b) found that there were species within the bacterial community of the greyback cane beetle larvae hindgut that were consistently found within the larvae across their geographical distribution. These bacteria were successfully transformed and reintroduced into the hindgut of the larvae, which indicates they are strong candidates to control the populations of greyback cane beetle larvae through the expression of anti-feeding compounds within the larval gut (Pittman et al., 2008a). Non-resident bacteria are normally not useful in such paratransgenic control methods because they are unable to remain established within the gut (Chapco and Kellin, 1994). Therefore the discovery, successful transformation and establishment of these candidate bacteria within the greyback cane beetle larval gut provides good grounding for the future development of paratransgenic control methods of the larvae.
Applied Perspectives
We have discussed the impacts of some abiotic and biotic factors within the soil environment that impact on scarab larval populations. Many agricultural practices interact with these factors within the soil, and could potentially mitigate or exacerbate scarab damage to grasses and crops (Barnett and Johnson, 2013; see Figure 5 for a summary of key interactions within an applied context).
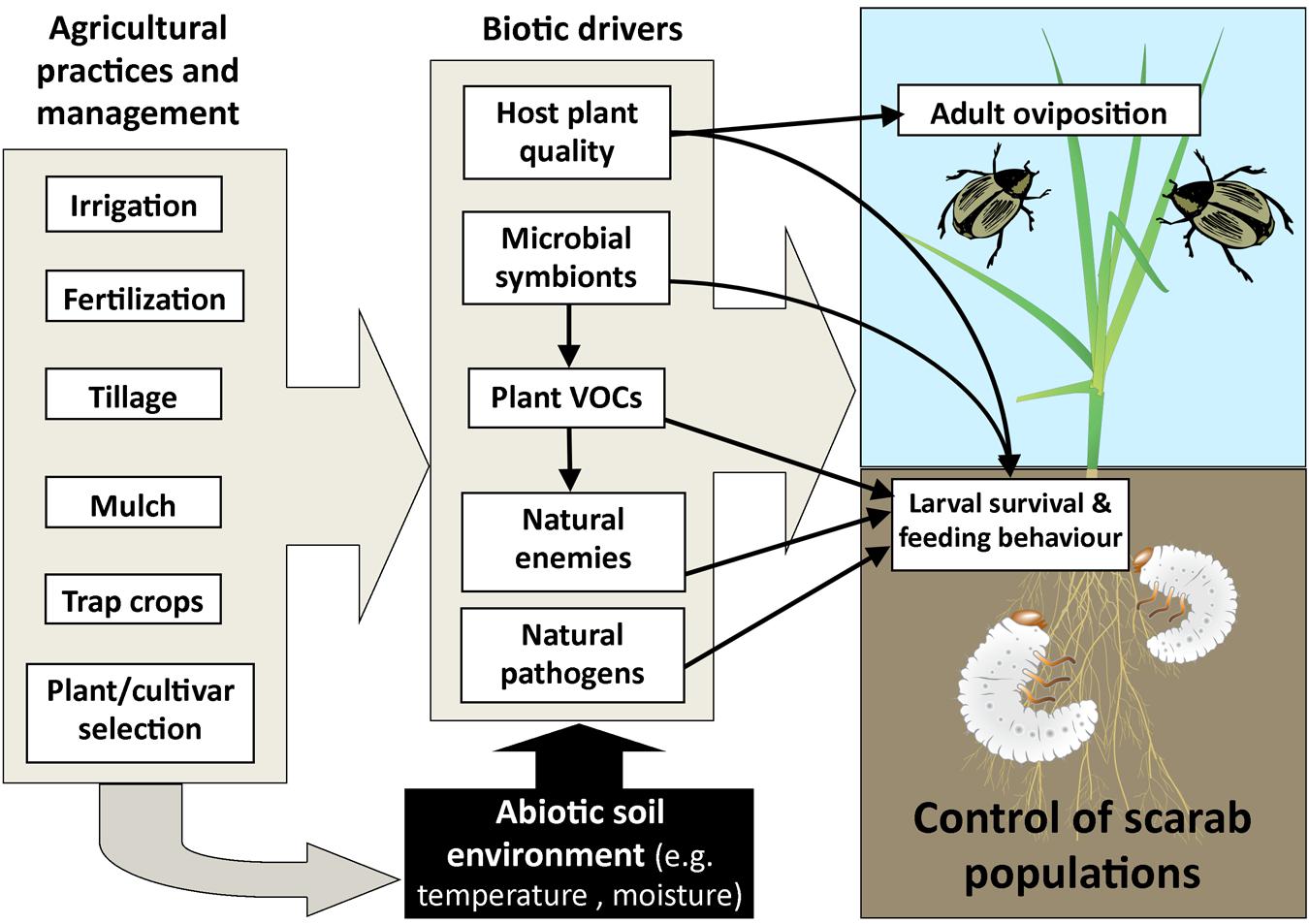
FIGURE 5. Diagram of agricultural practices and management factors that impact on plant and soil factors (abiotic and biotic), which in turn can influence oviposition by adults together with larval survival and feeding behavior. Arrows indicate key linkages between interacting factors.
Scarab larvae have been shown to respond to the application of fertilizers (Wightman, 1974; Frew et al., 2013). However, it is important to note that AMF plant associations can be negatively impacted by fertilization (Smith and Read, 2010). Therefore, the application of NPK fertilizer, particularly to newly establishing crops or pastures should be kept to a minimum, to minimize any positive impacts on scarab populations and to ensure effective AMF colonization to enhance grass productivity and defenses. The addition of mulch, is commonly used to conserve moisture and generally improve soil fertility, and therefore could reduce the priming of plant defenses to herbivores by reducing AMF colonization (Grant et al., 2005; Smith and Read, 2010).
Mulch also affects temperature, which in turn may influence scarab beetle larvae. Different types of mulch have been shown to have different effects on the temperature of the soil (Ramakrishna et al., 2006). For example, polythene mulch has been shown to increase soil temperature by 6°C, while straw mulch also increased soil temperature, but to a lesser extent (Ramakrishna et al., 2006). Contrastingly, a study by Lal (1974) found that mulch consistently decreased the maximum soil temperature across a range of depths (5, 10, and 20 cm), with the biggest difference of 8°C, seen at 5 cm below the soil surface. Tillage is another agricultural practice which has been shown to affect soil temperature (Griffith et al., 1973; Malhi and O’Sullivan, 1990; Licht and Al-Kaisi, 2005). Conventional tillage increases top soil temperatures by 2.8°C compared with no tillage (Malhi and O’Sullivan, 1990), although smaller increases in temperature of 1.9°C have also been reported (Licht and Al-Kaisi, 2005). Higher soil temperatures (depending on climatic conditions) reduce greyback cane beetle populations (Horsfield et al., 2008), and first instar larvae of the dusky pasture scarab have been found to be the most temperature sensitive (Davidson et al., 1972a). However, other common practices such as irrigation are known to lower soil temperatures by up to 3.8°C (Wang et al., 2000).
Taking these effects into account, the timely refrain from irrigation alongside the application of polythene or straw mulch coupled with tillage, for example, could raise soil temperature sufficiently to impact on larval populations. However, limiting soil moisture could decrease the efficacy of EPN populations within the soil at controlling scarab populations. The effects of raising temperatures in this manner on crop health and yield, however, should also be investigated.
The effects of other land management practices on scarab larvae populations have been reported such as the study by Potter et al. (1996) who found that intense mowing of grasses and the addition of aluminum sulfate treatments significantly decreased populations of Cyclocephala spp., as well as the average larval mass. This study, however, only was done within one soil type, which is a critical factor (Cherry and Allsopp, 1991; Matthiessen, 1999), and scarab responses may differ under different soils.
Many crops have irrigation systems in place to ensure sufficient water is supplied, which can lead to very different soil conditions compared to natural systems. Mulch, as discussed, is commonly used in agriculture to conserve moisture and increase fertility of soil, and so it naturally follows that in mulched systems, moisture retention of the soil will be higher (Moody et al., 1963; Lal, 1974; Ramakrishna et al., 2006). Host plant location by larvae beneath the soil surface could be improved under these moist soil conditions due to the fluid dynamics of root exudates (Gouinguené and Turlings, 2002; Hiltpold and Turlings, 2008). However, at the same time, natural enemies such as EPNs will also benefit from this phenomenon as it has been shown across several species that EPN virulence increases with soil moisture content (Kung et al., 1991; Grant and Villani, 2003; Frew et al., 2013). Therefore, as practices such as fertilization may decrease EPN attracting volatiles while irrigation enhances EPN mobility and survival, effective strains of host specific EPNs should be applied to pastures or crops requiring little fertilization alongside ample irrigation to effectively repress scarab larval populations.
Other soil antagonists can be impacted by land use practices. For example, larvae of the scarab Ataenius spretulus Haldeman (subfamily: Aphodiinae) were found, within a golf course environment, to be in greater abundance where the turf had been mowed to fairway height (1.6 cm), compared with turf mowed to rough height (5.1 cm). This correlated with the number of larvae found to be infected with a bacterial pathogen, Bacillus sp., where 68% of larvae were infected in the turf mowed to rough height, compared to 34% of larvae infected in turf mowed to fairway height. In addition to this, Anoplognathus spp. and Sericesthis spp. larval populations have been shown to peak under moderate grazing pressure, yet were lowest under high intensity grazing (Roberts and Morton, 1985). These findings alone are unlikely to have a direct applied significance to all scarab larval pest management. However, they may provide critical information for other managed grassland systems, where decreasing regular mowing or allowing high intensity grazing may mitigate larval infestations in future years. Common practices as mowing should be investigated for their impacts on critical soil abiotic factors such as moisture alongside scarab larval populations and their interactions with natural pathogens.
In direct attempts to mitigate damage caused by insect herbivores, the ‘push–pull’ system is a method which aims to utilize repellant or unattractive plants while simultaneously using attractive yet less valuable plants to attract pests away from valuable crops or pastures (Pickett et al., 2014). A similar system could be utilized against scarab larval pests. For example, where African black beetle populations are problematic, the use of T. repens and N. lolii infected L. perenne could be used as a repellant [the former of which may also be effective against Christmas beetle larvae (Davidson and Roberts, 1968a)], while L. perenne and P. dilatatum could be utilized within ‘trap crops,’ particularly as areas with P. dilatatum are also preferred sites for oviposition. Indeed, P. dilatatum could also be useful, alongside C. dactylon in ‘trap cultures’ for other Dynastinae species such as the Argentine scarab (Carne, 1957a). It has been suggested, however, that the efficacy of ‘push–pull’ systems would be improved if a better understanding of the mechanisms were obtained, for example the specificity and distance ranges of plant volatile cues (Eigenbrode et al., 2016).
In the end, where effective biocontrol methods are commercially available, these should be employed in conjunction with the use of agricultural and land-use practices, such as irrigation and mowing (where applicable) to create optimal conditions for efficacy and infectivity. Where scarab plant host preferences are known (for feeding or oviposition), these can be employed in ‘push–pull’ strategies, to limit larval populations in areas of interest. Where either of these are unavailable or remain unknown, such is the case for some of our focal species, timely utilization of certain land-use practices can be applied to create poor conditions for the scarab populations (e.g., during the first instar, when larvae are most vulnerable to temperature stress). Indeed, in either situation, encouragement of natural beneficial soil microbes (such as AMF) should also be applied. However, as there are gaps in the knowledge for ecology of many scarab species, the direction of future research is of primary importance in improving strategies to limit pest scarab larvae in grasses across Australasia.
Directions for Future Research
Basic Ecology
Some of the work on the basic ecology of scarab larval pests to grasses was carried out over 20 years ago (Carne, 1957a; Carne and Chinnick, 1957; Ridsdill-Smith, 1975), with little research on particular species since. It is our belief that for those species where there remains some paucity of knowledge in their basic ecology, feeding trials looking at host preference alongside population monitoring under different conditions (this includes monitoring of abiotic factors and microbial sampling) should be prioritized. With this knowledge, more effective implementation of strategies such as ‘push–pull’ systems or other agricultural practices that suppress scarab beetle populations can be applied within context. This means management systems could take into account species specific responses, accounting for local abiotic and biotic interactions.
Volatile Cues
The effectiveness of classic pest management strategies such as ‘push–pull’ systems have recently been criticized, particularly for focusing too much on long-range effects, and should consider all cues that can work synergistically (Eigenbrode et al., 2016). Indeed we would concur with this framework for application to belowground pests, but such behavioral cues would first require investigation. We recommend that future research should investigate olfactory cues of pest larvae and their natural enemies belowground to plant roots, and how these may interact with common agricultural and land-use practices. Experiments such as those carried out by Rasmann et al. (2005) using six-arm olfactometers are an ideal starting point to determine attractiveness of plant species to scarab larval pests and/or their natural enemies.
Pathogens and Microbes
Biocontrol of scarab pests has been particularly successful where a naturally occurring pathogen is identified, isolated and then applied within its naturally occurring range (Maurer et al., 1997; Hurst et al., 2000; Sallam et al., 2003, 2007; Dolci et al., 2006; Sevim et al., 2010). Hence, knowledge of belowground community composition is important if native microbes or EPNs are to be utilized in the control of insect pests in the soil. Using methods similar to that of Sevim et al. (2010), the presence of naturally occurring scarab pathogens could be identified using a baiting method (Zimmermann, 1986). The pathogen can then be isolated from infected larvae and the DNA sequenced; effective isolates can then be used in bioassays to test pathogenicity against the target pest species. We recommend the isolation, identification and ultimately the application of natural pathogens, where possible. The persistence of scarab pathogens in the soil indicates some level of evolutionary success, which should be exploited in efforts to control problematic species.
Concluding Remarks
Here, we have presented information on several key scarab larval species within three subfamilies, known to cause significant damage to grasslands and crops within Australia and New Zealand. While the ecology of some species has been well researched, information on others, including the Argentine scarab, has not been described in any detail. The feeding behavior and general ecology has been investigated for species such as African black beetle larvae and greyback cane beetle larvae. These pests have had significant attention as a result of their impact on agriculture, and control methods such as the application of natural pathogens, or the application of host plant endophytes have shown noteworthy promise. Although our knowledge is somewhat limited for some species, there is good evidence that changes in management can potentially have a large impact in limiting damage to crops and grasslands. Overall it seems clear that, in terms of improved pest management of scarab larvae, it does not make sense to run before we can walk. Immediate research concerns should lie with filling knowledge gaps in the ecology of scarab species within Australasia. This should include assessing population dynamics, interactions and influences with abiotic factors within the local environment. In addition to this, successful biocontrol strategies, both within and outside Australasia, have utilized naturally occurring pathogens and natural enemies, which are adapted to their host and local environment. Therefore, similar strategies need to be central to future biocontrol research on Australasian scarab pests. This will necessitate multi-factorial studies to investigate how best to integrate these antagonists under different abiotic conditions. Overall, pest management strategies that are applied within context would be more effective with an improved fundamental ecological understanding of key scarab pests.
Author Contributions
AF wrote the main body of the review, contributing the majority of the intellectual content and concept of the review. KB read the review in detail, giving advice and contributing important intellectual content. KB was responsible for the production of Figure 3. MR read the review in detail, giving advice and contributing intellectual content. UN read the review in detail, giving advice and contributing intellectual content. SJ aided in the concept of the review, contributing important intellectual content.
Funding
This work was produced as part of a research project funded by Sugar Research Australia and the Hawkesbury Institute for the Environment, Western Sydney University.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This paper is as part of a series of articles from the ninth Australasian Congress of Grassland Invertebrate Ecology and with sponsorship from HIE. The authors would like to thank the reviewers for their invaluable comments.
References
Allsopp, P. G. (1991). Assessment of Various Food Constituents as Feeding Attractants for Canegrubs in a Pest Control Program. Brisbane: Bureau of Sugar Experiment Stations.
Allsopp, P. G. (1999). How localized are the distributions of Australian scarabs (Coleoptera: Scarabaeoidea)? Divers. Distrib. 5, 143–149. doi: 10.1046/j.1472-4642.1999.00050.x
Allsopp, P. G., Klein, M. G., and McCoy, E. L. (1992). Effect of soil moisture and soil texture on oviposition by Japanese beetle and rose chafer (Coleoptera: Scarabaeidae). J. Econ. Entomol. 85, 2194–2200. doi: 10.1093/jee/85.6.2194
Barnett, K., and Johnson, S. N. (2013). Living in the soil matrix: abiotic factors affecting root herbivores. Advan. Insect Physiol. 45, 1–52. doi: 10.1016/B978-0-12-417165-7.00001-5
Bell, N. L., Townsend, R. J., Popay, A. J., Mercer, C. F., and Jackson, T. A. (2011). “Black beetle: lessons from the past and options for the future,” in Proceedings of the Pasture Persistence Symposium. Grassland Research and Practice Series, Vol. 15, Dunedin, 119–124.
Bennett, A. E., and Bever, J. D. (2007). Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 88, 210–218. doi: 10.1890/0012-9658(2007)88[210:MSDAPG]2.0.CO;2
Berg, G., Faithfull, I. G., Powell, K. S., Bruce, R. J., Williams, D. G., and Yen, A. L. (2014). Biology and management of the redheaded pasture cockchafer Adoryphorus couloni (Burmeister) (Scarabaeidae: Dynastinae) in Australia: a review of current knowledge. Aus. Entomol. 53, 144–158. doi: 10.1111/aen.12062
Berón, C. M., and Diaz, B. M. (2005). Pathogenicity of hyphomycetous fungi against Cyclocephala signaticollis. BioControl 50, 143–150. doi: 10.1007/s10526-004-0586-x
Biere, A., and Bennett, A. E. (2013). Three-way interactions between plants, microbes and insects. Funct. Ecol. 27, 567–573. doi: 10.1111/1365-2435.12100
Brown, V. K., and Gange, A. C. (1990). Insect herbivory below ground. Advances in Ecological Research 20, 1–58. doi: 10.1016/S0065-2504(08)60052-5
Cairns, S. C. (1978). Growth, respiration and the utilization of assimilated energy in the larvae of Sericesthis nigrolineata (Coleoptera). Oikos 31, 142–152.
Carne, P. B. (1957a). Cyclocephala signaticollis Burmeister, an introduced pasture scarab (Coleoptera). Proc. Linn. Soc. N. S. W. 81, 217–221.
Carne, P. B. (1957b). A revision of the ruteline genus Anoplognathus Leach (Coleoptera: Scarabaeidae). Aust. J. Zool. 5, 88–144. doi: 10.1071/ZO9570088
Carne, P. B., and Chinnick, L. J. (1957). The pruinose scarab (Sericesthis pruinose Dalman) and its control in turf. Aust. J. Agric. Res. 8, 604–616. doi: 10.1071/AR9570604
Carne, P. B., Greaves, R. T. G., and McInnes, R. S. (1974). Insects damage to plantation-grown eucalypts in north coastal New South Wales, with particular reference to Christmas beetles (Coleoptera: Scarabaeidae). Aust. J. Entomol. 13, 189–206. doi: 10.1111/j.1440-6055.1974.tb02173.x
Caswell, H., Reed, F., Stephens, S. N., and Werner, P. A. (1973). Photosynthetic pathways and selective herbivory: a hypothesis. Am. Nat. 107, 465–480. doi: 10.1086/282851
Cazemier, A. E., Verdoes, J. C., Reubsaet, F. A. G., Hackstein, J. H. P., Van Der Drift, C., and Op den Camp, H. J. M. (2003). Promicromonospora pachnodae sp. nov., a member of the (hemi)cellulolytic hindgut flora of larvae of the scarab beetle Pachnoda marginata. Antonie Van Leeuwenhoek. 83, 135–148. doi: 10.1023/A:1023325817663
Chandler, K. J. (2002). Strategies to Control Greyback Canegrub in Early Harvested Ratoon Crops. SRDC Final Report SD02022. Brisbane, QLD: Bureau of Sugar Experiment Station.
Chapco, W., and Kellin, R. A. (1994). Persistence of ingested bacteria in the grasshopper gut. J. Invertebr. Pathol. 64, 149–150. doi: 10.1006/jipa.1994.1086
Chen, Y., Ruberson, J. R., and Olson, D. M. (2008). Nitrogen fertilization rate affects feeding, larval performance, and oviposition preference of the beet armyworm, Spodoptera exigua, on cotton. Entomol. Exp. Appl. 126, 244–255. doi: 10.1111/j.1570-7458.2007.00662.x
Cherry, R. H., and Allsopp, P. G. (1991). Soil texture and distribution of Antitrogus parvulus Britton, Lepidiota crinita Brenske and L. negatoria Blackburn (Coleoptera, Scarabaeidae) in South Queensland sugarcane fields. J. Aust. Entomol. Soc. 30, 89–92. doi: 10.1111/j.1440-6055.1991.tb02201.x
Cherry, R. H., Coale, F. J., and Porter, P. S. (1990). Oviposition and survivorship of sugarcane grubs (Coleoptera: Scarabaeidae) at different soil moistures. J. Econ. Entomol. 83, 1355–1359. doi: 10.1093/jee/83.4.1355
Cherry, R. H., and Hall, D. G. (1986). Flight activity of Melanotus communis (Coleoptera: Elateridae) in Florida sugar cane fields. J. Econ. Entomol. 79, 626–628. doi: 10.1093/jee/79.3.626
Crutchfield, B. A., Potter, D. A., and Powell, A. J. (1995). Irrigation and nitrogen fertilization effects on white grub injury to Kentucky bluegrass and tall fescue turf. Crop Sci. 35, 1122–1126. doi: 10.2135/cropsci1995.0011183X003500040034x
Davidson, R. L., and Roberts, R. J. (1968a). Influence of plants, manure and soil moisture on survival and liveweight gain of two scarabaeid larvae. Entomol. Exp. Appl. 11, 305–314. doi: 10.1111/j.1570-7458.1968.tb02059.x
Davidson, R. L., and Roberts, R. J. (1968b). Species differences in scarab–pasture relationships. Bull. Entomol. Res. 58, 315–324. doi: 10.1017/S0007485300056868
Davidson, R. L., Wiseman, J. R., and Wolfe, V. J. (1972a). Environmental stress in the pasture scarab Sericesthis nigrolineata Boisd. I. Mortality in larvae caused by high temperature. J. Appl. Ecol. 9, 783–797. doi: 10.2307/2401904
Davidson, R. L., Wiseman, J. R., and Wolfe, V. J. (1972b). Environmental stress in the pasture scarab Sericesthis nigrolineata Boisd. II. Effects of soil moisture and temperature on survival of first-instar larvae. J. Appl. Ecol. 9, 799–806. doi: 10.2307/2401905
Dolci, P., Guglielmo, F., Secchi, F., and Ozino, O. I. (2006). Persistence and efficacy of Beauveria brongniartii strains applied as biocontrol agents against Melolontha melolontha in the Valley of Aosta (northwest Italy). J. Appl. Microbiol. 100, 1063–1072. doi: 10.1111/j.1365-2672.2006.02808.x
East, R., King, P. D., and Watson, R. N. (1981). Population studies of grass grub (Costelytra zealandica) and black beetle (Heteronychus arator) (Coleoptera, Scarabaeidae). N. Z. J. Ecol. 4, 56–64.
Edwards, P. B., Wanjura, W. J., and Brown, W. V. (1993). Selective herbivory by Christmas beetles in response to intraspecific variation in Eucalyptus terpenoids. Oecologia 95, 551–557. doi: 10.1007/BF00317440
Eigenbrode, S. D., Birch, A. N. E., Lindzey, S., Meadow, R., and Snyder, W. E. (2016). A mechanistic framework to improve understanding and applications of push-pull systems in pest management. J. Appl. Ecol. 53, 202–212. doi: 10.1111/1365-2664.12556
Eilers, E. J., Talarico, G., Hansson, B. S., Hilker, M., and Reinecke, A. (2012). Sensing the underground – ultrastructure and function of sensory organs in root-feeding Melolontha melolontha (Coleoptera: Scarabaeinae) larvae. PLoS ONE 7:e41357. doi: 10.1371/journal.pone.0041357
Erb, M., and Lu, J. (2013). Soil abiotic factors influence interactions between belowground herbivores and plant roots. J. Exp. Bot. 64, 1295–1303. doi: 10.1093/jxb/ert007
Frew, A., Nielsen, U. N., Riegler, M., and Johnson, S. N. (2013). Do eucalypt plantation management practices create understory reservoirs of scarab beetle pests in the soil? For. Ecol. Manag. 306, 275–280. doi: 10.1016/j.foreco.2013.06.051
Georgis, R., Koppenhöfer, A. M., Lacey, L. A., Bélair, G., Duncan, L. W., Grewal, P. S., et al. (2006). Successes and failures in the use of parasitic nematodes for pest control. Biol. Control 38, 103–123. doi: 10.1016/j.biocontrol.2005.11.005
Goldson, S. L., Bourdôt, G. W., Brockerhoff, E. G., Byrom, A. E., Clout, M. N., McGlone, M. S., et al. (2015). New Zealand pest management: current and future challenges. J. R. Soc. N. Z. 45, 31–58. doi: 10.1080/03036758.2014.1000343
Goodyer, G. J., and Nicholas, A. (2007). Scarab Grubs in Northern Tableland Pastures - Primefact 512. Available at: http://www.dpi.nsw.gov.au/__data/assets/pdf_file/0008/110213/scarab-grubs-in-northern-tableland-pastures.pdf [Accessed 01/11/2014].
Gordon, R. D., and Anderson, D. M. (1981). The species of Scarabaeidae (Coleoptera) associated with sugarcane in south Florida. Florida Entomol. 64, 119–138. doi: 10.2307/3494604
Gouinguené, S. P., and Turlings, T. C. J. (2002). The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 129, 1296–1307. doi: 10.1104/pp.001941
Grant, C., Bittman, S., Montreal, M., Plenchette, C., and Morel, C. (2005). Soil and fertilizer phosphorus: effects on plant P supply and mycorrhizal development. Can. J. Plant Sci. 85, 3–14. doi: 10.4141/P03-182
Grant, J. A., and Villani, M. G. (2003). Soil moisture effects on entomopathogenic nematodes. Environ. Entomol. 32, 80–87. doi: 10.1603/0046-225X-32.5.983
Grewal, P. S., Lewis, E. E., Gaugler, R., and Campbell, J. F. (1994). Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology 108, 207–215. doi: 10.1017/S003118200006830X
Griffith, D. R., Mannering, J. V., Galloway, H. M., Parsons, S. D., and Richey, C. B. (1973). Effect of eight tillage-planting systems on soil temperature, percent stand, plant growth, and yield of corn on five Indiana soils. Agron. J. 65, 321–326. doi: 10.2134/agronj1973.00021962006500020040x
Hangay, G., and Zborowski, P. (2010). A Guide to the Beetles of Australia. Collingwood, VIC: CSIRO Publishing.
Hassan, S. T., and Hilditch, J. A. (1976). Survival of larvae of Anoplognathus porosus (Dalman) and Sericesthis nigrolineata Boisd. (Coleoptera: Scarabaeidae). J. Appl. Ecol. 13, 333–339. doi: 10.2307/2401783
Hiltpold, I., Baroni, M., Toepfer, S., Kuhlmann, U., and Turlings, T. C. J. (2010). Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest. J. Exp. Biol. 213, 2417–2423. doi: 10.1242/jeb.041301
Hiltpold, I., and Turlings, T. C. J. (2008). Belowground chemical signaling in maize: when simplicity rhymes with efficiency. J. Chem. Ecol. 34, 628–635. doi: 10.1007/s10886-008-9467-6
Hol, W. H. G. (2011). The effect of nutrients on pyrrolizidine alkaloids in Senecio plants and their interactions with herbivores and pathogens. Phytochem. Rev. 10, 119–126. doi: 10.1007/s11101-010-9188-7
Horsfield, A., Sallam, M. N. S., Drummond, F. A., Williams, D. J., and Schultz, R. J. (2008). Role of climatic factors on damage incidence by Dermolepida albohirtum (Coleoptera: Scarabaeidae), in Burdekin sugarcane fields, Australia. J. Econ. Entomol. 101, 334–340. doi: 10.1093/jee/101.2.334
Huang, S.-W., Zhang, H.-Y., Marshall, S., and Jackson, T. A. (2010). The scarab gut: a potential bioreactor for bio-fuel production. Insect Sci. 17, 175–183. doi: 10.1111/j.1744-7917.2010.01320.x
Huger, A. M. (2005). The Oryctes virus: its detection, identification, and implementation in biological control of the coconut palm rhinoceros beetle, Oryctes rhinoceros (Coleoptera: Scarabaeidae). J. Invertebr. Pathol. 89, 78–84. doi: 10.1016/j.jip.2005.02.010
Hume, D. E., Ryan, D. L., Cooper, B. M., and Popay, A. J. (2007). Agronomic performance of AR37-infected ryegrass in northern New Zealand. Proc. N. Z. Grassland Assoc. 69, 201–205.
Hurst, M. R. H., Glare, T. R., and Jackson, T. A. (2004). Cloning Serratia entomophila antifeeding genes—a putative defective prophage active against the grass grub Costelytra zealandica. J. Bacteriol. 186, 5116–5128. doi: 10.1128/JB.186.15.5116-5128.2004
Hurst, M. R. H., Glare, T. R., Jackson, T. A., and Ronson, C. W. (2000). Plasmid-located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. J. Bacteriol. 182, 5127–5138. doi: 10.1128/JB.182.18.5127-5138.2000
Illingworth, J. F., and Dodd, A. P. (1921). Australian sugar-cane beetles and their allies. Bull. Qld. Bureau Sugar Exp. Stat. Division Entomol. 16, 1–104.
Irvine, J. E. (1977). “Composition of cane and juice,” in Sugarcane Hand Book, eds C. P. Meade and J.C. P. Chen (New York, NY: John Wiley & Sons, Inc.), 15.
Jackson, T. A., and Glare, T. R. (1992). Use of Pathogens in Scarab Pest Management. Andover: Intercept Ltd.
Jackson, T. A., and Klein, M. G. (2006). Scarabs as pests: a continuing problem. Coleopterists Bull. 60, 102–119. doi: 10.1649/0010-065X(2006)60[102:SAPACP]2.0.CO;2
Jameson, M. L. (2015). Symbiota Collections of Athropods Network. Available at: http://symbiota4.acis.ufl.edu/scan/portal/collections/misc/collprofiles.php?collid = 16 [Accessed May 05, 2015].
Jarvis, E. (1933). Monthly Notes on the Greyback Cane Beetle and Its Control. Issue 9 of Farm Bulletin (Brisbane: Bureau of Sugar Experiment Stations. Division of Soils and Agriculture) 9, 1–40.
Johns, C. V., Stone, C., and Hughes, L. (2004). Feeding preferences of the Christmas beetle Anoplognathus chloropyrus (Coleoptera: Scarabaeidae) and four paropsine species (Coleoptera: Chrysomelidae) on selected Eucalyptus grandis clonal foliage. Aust. For. 67, 184–190. doi: 10.1080/00049158.2004.10674932
Johnson, S. N., and Gregory, P. J. (2006). Chemically-mediated host-plant location and selection by root-feeding insects. Physiol. Entomol. 31, 1–13. doi: 10.1111/j.1365-3032.2005.00487.x
Johnson, S. N., Lopaticki, G., and Hartley, S. E. (2014). Elevated atmospheric CO2 triggers compensatory feeding by root herbivores on a C3 but not a C4 grass. PLoS ONE 9:e90251. doi: 10.1371/journal.pone.0090251
Johnson, S. N., and Nielsen, U. N. (2012). Foraging in the dark – chemically mediated host plant location by belowground insect herbivores. J. Chem. Ecol. 38, 604–614. doi: 10.1007/s10886-012-0106-x
King, P. D. (1977). Effect of plant species and organic matter on feeding behaviour and weight gain of larval black beetle, Heteronychus arator (Coleoptera: Scarabaeidae). N. Z. J. Zool. 4, 445–448. doi: 10.1080/03014223.1977.9517968
King, P. D. (1979). Aspects of the Ecology of Black Beetle Heteronychus arator (F.), (Coleoptera: Dynastinae). D.Phil. thesis, University of Waikato, Hamilton.
King, P. D., and Kain, W. M. (1974). “Aspects of the feeding mechanisms of two Scarabaeid pasture pests,” in Proceedings of the Abstracts 1st Australasian Conference on Grassland Invertebrate Ecology, Armidale, 9–10.
King, P. D., Meekings, J. S., and Mercer, C. F. (1982). Effects of whitefringed weevil (Graphognathus leucoloma) and black beetle (Heteronychus arator) populations on pasture species. N. Z. J. Agric. Res. 25, 405–414. doi: 10.1080/00288233.1982.10417904
King, P. D., Mercer, C. F., and Meekings, J. S. (1981a). Ecology of black beetle, Heteronychus arator (Coleoptera, Scarabaeidae) - influence of temperature on feeding, growth, and survival of the larvae. N. Z. J. Zool. 8, 113–117. doi: 10.1080/03014223.1981.10427949
King, P. D., Mercer, C. F., and Meekings, J. S. (1981b). Ecology of black beetle, Heteronychus arator (Coleoptera: Scarabaeidae)—population studies. N. Z. J. Agric. Res. 24, 87–97. doi: 10.1080/00288233.1981.10420876
King, P. D., Mercer, C. F., and Meekings, J. S. (1981c). Ecology of black beetle, Heteronychus arator (Coleoptera: Scarabaeidae)—relative consumption of pasture plant roots by larvae. N. Z. J. Zool. 8, 123–125. doi: 10.1080/03014223.1981.10427949
King, P. D., Mercer, C. F., Stirling, J., and Meekings, J. S. (1975). “Resistance of lucerne to black beetle,” in Proceedings of the 28th New Zealand Weed & Pest Control Conference, Hastings, 161–164.
Klein, M. G. (1993). “Biological control of scarabs with entomopathogenic nematodes,” in Nematodes and the Biological Control of Insect Pests, eds R. Bedding, R. Akhurst, and H. Kaya (Melbourne, VIC: CSIRO), 49–57.
Kung, S.-P., Gaugler, R., and Kaya, H. K. (1991). Effects of soil temperature, moisture, and relative humidity on entomopathogenic nematode persistence. J. Invertebr. Pathol. 57, 242–249. doi: 10.1016/0022-2011(91)90123-8
Lal, R. (1974). Soil temperature, soil moisture and maize yield from mulched and unmulched tropical soils. Plant Soil 40, 129–143. doi: 10.1007/BF00011415
Licht, M. A., and Al-Kaisi, M. (2005). Strip-tillage effect on seedbed soil temperature and other soil physical properties. Soil Tillage Res. 80, 233–249. doi: 10.1016/j.still.2004.03.017
Logan, D. P. (2007). Effect of soil moisture on oviposition by Childers canegrub, Antitrogus parvulus Britton (Coleoptera: Scarabaeidae). Aust. J. Entomol. 36, 175–178. doi: 10.1111/j.1440-6055.1997.tb01451.x
Logan, D. P., and Kettle, C. G. (2002). Effect of food and larval density on survival and growth of early instar greyback canegrub, Dermolepida albohirtum (Waterhouse) (Coleoptera : Scarabaeidae). Aust. J. Entomol. 41, 253–261. doi: 10.1046/j.1440-6055.2002.00294.x
Logan, D. P., and Kettle, C. G. (2007). Temperature-dependent development and distribution in the soil profile of pupae of greyback canegrub Dermolepida albohirtum (Waterhouse) (Coleoptera: Scarabaeidae) in Queensland sugarcane. Aust. J. Entomol. 46, 17–22. doi: 10.1111/j.1440-6055.2007.00578.x
Logan, D. P., Robertson, L. N., and Milner, R. J. (2000). Review of the development of Metarhizium anisopliae as a microbial insecticide, BioCaneTM, for the control of greyback canegrub Dermolepida albohirtum (Waterhouse) (Coleoptera: Scarabaeidae) in Queensland sugarcane. IOBC/WPRS Bull. 23, 131–137.
Malhi, S. S., and O’Sullivan, P. A. (1990). Soil temperature, moisture and penetrometer resistance under zero and conventional tillage in central Alberta. Soil Tillage Res. 17, 167–172. doi: 10.1016/0167-1987(90)90014-5
Matthiessen, J. N. (1999). Late immature mortality is the major influence on reproductive success of African black beetle, Heteronychus arator (Fabricius) (Coleoptera: Scarabaeidae), in a Mediterranean-climate region of Australia. Aust. J. Entomol. 38, 348–353. doi: 10.1046/j.1440-6055.1999.00123.x
Matthiessen, J. N., and Ridsdill-Smith, T. J. (1991). Populations of African black beetle, Heteronychus arator (Coleoptera, Scarabaeidae) in a Mediterranean climate region of Australia. Bull. Entomol. Res. 81, 85–91. doi: 10.1017/S000748530005327X
Maurer, P., Couteaudier, Y., Girard, P. A., Bridge, P. D., and Riba, G. (1997). Genetic diversity of Beauveria bassiana and relatedness to host insect range. Mycol. Res. 101, 159–164. doi: 10.1017/S0953756296002213
McLeod, R. S., Mcmahon, G. G., and Allsopp, P. G. (1999). Costs of major pests and diseases to the Australian sugar industry. Plant Protect. Q. 14, 42–46.
Mondito, E. A., López, A. N., Alvarez-Castillo, H. A., and Carmona, D. M. (1997). The life cycle of Cyclocephala signaticollis Burmeister, 1847 (Coleoptera: Scarabaeidae: Dynastinae) and its relationship with some environmental factors. Elytron 11, 145–156.
Moody, J. E., Jones, J. N., and Lillard, J. H. (1963). Influence of straw mulch on soil moisture, soil temperature and the growth of corn. Soil Sci. Soc. Am. J. 27, 700–703. doi: 10.2136/sssaj1963.03615995002700060038x
Pachauri, R. K., Allen, M. R., Barros, V. R., Broome, J., Cramer, W., Christ, R., et al. (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, 151.
Pickett, J. A., Woodcock, C. M., Midega, C. A. O., and Khan, Z. R. (2014). Push–pull farming systems. Curr. Opin. Biotechnol. 26, 125–132. doi: 10.1016/j.copbio.2013.12.006
Pittman, G. W., Brumbley, S. M., Allsopp, P. G., and O’Neill, S. L. (2008a). Assessment of gut bacteria for a paratransgenic approach to control Dermolepida albohirtum larvae. Appl. Environ. Microbiol. 74, 4036–4043. doi: 10.1128/AEM.02609-07
Pittman, G. W., Brumbley, S. M., Allsopp, P. G., and O’Neill, S. L. (2008b). “Endomicrobia” and other bacteria associated with the hindgut of Dermolepida albohirtum larvae. Appl. Environ. Microbiol. 74, 762–767. doi: 10.1128/AEM.01831-07
Popay, A. J., and Baltus, J. G. (2001). “Black beetle damage to perennial ryegrass infected with AR1 endophyte,” in Proceedings of the New Zealand Grassland Association, Hamilton, 267–272.
Popay, A. J., and Bonos, S. A. (2008). “Biotic responses in endophytic grasses,” in Neotyphodium in Cool-Season Grasses, eds C. A. Roberts, C. A. West, and D. E. Spiers (Ames, IA: Blackwell Publishing), 163–185.
Potter, D. A. (1983). Effect of soil moisture on oviposition, water absorption, and survival of southern masked chafer (Coleoptera: Scarabaeidae) eggs. Environ. Entomol. 12, 1223–1227. doi: 10.1093/ee/12.4.1223
Potter, D. A., and Braman, S. K. (1991). Ecology and management of turfgrass insects. Annu. Rev. Entomol. 36, 383–406. doi: 10.1146/annurev.en.36.010191.002123
Potter, D. A., Powell, A. J., Spicer, P. G., and Williams, D. W. (1996). Cultural practices affect root-feeding white grubs (Coleoptera: Scarabaeidae) in turfgrass. J. Econ. Entomol. 89, 156–164. doi: 10.1093/jee/89.1.156
Powell, K. S., Slattery, W. F., Deretic, J., Herbert, K., and Hetherington, S. (2003). Influence of soil type and climate on the population dynamics of grapevine phylloxera in Australia. Acta Hortic. 617, 33–41. doi: 10.17660/ActaHortic.2003.617.5
Pozo, M. J., and Azcón-Aguilar, C. (2007). Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 10, 393–398. doi: 10.1016/j.pbi.2007.05.004
Prestidge, R. A., and East, R. (1984). Use of fertiliser nitrogen to manipulate pasture plant quality and compensate for damage by grass grub, Costelytra zealandica (Coleoptera: Scarabaeidae). N. Z. Entomol. 8, 24–29. doi: 10.1080/00779962.1984.9722457
Prestidge, R. A., Van Der Zijpp, S., and Badan, D. (1985). Effects of plant species and fertilizers on grass grub larvae, Costelytra zealandica. N. Z. J. Agric. Res. 28, 409–417. doi: 10.1080/00288233.1985.10430446
Qawasmeh, A., Raman, A., and Wheatley, W. (2015). Volatiles in perennial ryegrass infected with strains of endophytic fungus: impact on African black beetle host selection. J. Appl. Entomol. 139, 94–104. doi: 10.1111/jen.12140
Radcliffe, J. E. (1970). Some effects of grass grub (Costelytra zealandica (White)) larvae on pasture plants. N. Z. J. Agric. Res. 13, 87–104. doi: 10.1080/00288233.1970.10421199
Ramakrishna, A., Tam, H. M., Wani, S. P., and Long, T. D. (2006). Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crops Res. 95, 115–125. doi: 10.1016/j.fcr.2005.01.030
Raman, A., Wheatley, W., and Popay, A. J. (2012). Endophytic fungus—vascular plant—insect interactions. Environ. Entomol. 41, 433–447. doi: 10.1603/EN11317
Rasmann, S., Köllner, T. G., Degenhardt, J., Hiltpold, I., Toepfer, S., Kuhlmann, U., et al. (2005). Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434, 732–737. doi: 10.1038/nature03451
Ridsdill-Smith, T. J. (1975). Selection of living grass roots in the soil by larvae of Sericesthis nigrolineata (Coleoptera: Scarabaeidae). Entomol. Exp. Appl. 18, 75–86. doi: 10.1111/j.1570-7458.1975.tb00388.x
Ridsdill-Smith, T. J. (1977). Effects of root-feeding by scarabaeid larvae on growth of perennial rye-grass plants. J. Appl. Ecol. 14, 73–80. doi: 10.2307/2401828
Ridsdill-Smith, T. J., Porter, M. R., and Furnival, A. G. (1975). Effects of temperature and developmental stage on feeding by larvae of Sericesthis nigrolineata (Coleoptera: Scarabaeiae). Entomol. Exp. Appl. 18, 244–254. doi: 10.1111/j.1570-7458.1975.tb02376.x
Ridsdill-Smith, T. J., and Roberts, R. J. (1976). Insect density effects in root feeding by larvae of Sericesthis nigrolineata (Coleoptera: Scarabaeidae). J. Appl. Ecol. 13, 423–428. doi: 10.2307/2401791
Roberts, R. J., and Morton, R. (1985). Biomass of larval Scarabaeidae (Coleoptera) in relation to grazing pressures in temperate, sown pastures. J. Appl. Ecol. 22, 863–874. doi: 10.2307/2403235
Robertson, L. N., Allsopp, P. G., Chandler, K. J., and Mullins, R. T. (1995). Integrated management of canegrubs in Australia: current situation and future research directions. Aus. J. Agric. Res. 46, 1–16. doi: 10.1071/AR9950001
Robertson, L. N., Dall, D. J., Lai-Fook, J., Kettle, C. G., and Bakker, P. (1998). Key Factors in Control of Greyback Canegrub Populations. Final Report – SRDC Project BSS120. Indooroopilly, QLD: BSES Publication SD98014.
Robertson, L. N., and Walker, P. W. (2001). Distribution of greyback canegrub, Dermolepida albohirtum (Coleoptera: Scarabaeidae), larvae in sugarcane soil. Proc. Int. Soc. Sugarcane Technol. 24, 361–365.
Rostás, M., Cripps, M. G., and Silcock, P. (2015). Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia 177, 487–497. doi: 10.1007/s00442-014-3104-6
Russell, G. B., Sutherland, O. R. W., Christmas, P. E., and Wright, H. (1982). Feeding deterrents for black beetle larvae, Heteronychus arator (Scarabaeidae), in Trifolium repens. N. Z. J. Zool. 9, 145–150. doi: 10.1080/03014223.1982.10423844
Sallam, M. N., Bakker, P., and Dall, D. J. (2003). “Prevalence of soil-borne diseases of greyback canegrubs with special reference to Adelina sp.,” in Proceedings of Australian Society of Sugarcane Technologists, Townsville, 24.
Sallam, M. N., McAvoy, C. A., Samson, P. R., and Bull, J. J. (2007). Soil sampling for Metarhizium anisopliae spores in Queensland sugarcane fields. BioControl 52, 491–505. doi: 10.1007/s10526-006-9038-0
Sallam, N. (2011). Review of current knowledge on the population dynamics of Dermolepida albohirtum (Waterhouse) (Coleoptera: Scarabaeidae). Aust. J. Entomol. 50, 300–308.
Sallam, N., Burgess, D. J. W., Lowe, G. E., Peck, D. R., and Bruce, R. C. (2011). “Survey of sugarcane pests and their natural enemies on the Atherton Tableland, far north Queensland,” in Proceedings of Australian Society of Sugar Cane Technologists, Mackay.
Samson, P., Sallam, N., and Chandler, K. (2013). Pests of Australian Sugarcane. Indooroopilly, QLD: BSES.
Schmelz, E. A., Alborn, H. T., Engelberth, J., and Tumlinson, J. H. (2003). Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol. 133, 295–306. doi: 10.1104/pp.103.024174
Sevim, A., Demir, I., Höfte, M., Humber, R. A., and Demirbag, Z. (2010). Isolation and characterization of entomopathogenic fungi from hazelnut-growing region of Turkey. Biocontrol 55, 279–297. doi: 10.1007/s10526-009-9235-8
Smith, T. J. R. and Porter, M. R. (1980). Influence of soil moisture on root feeding and growth rate of Sericesthis nigrolineata larvae (Scarabaeidae: Coleoptera). Aust. J. Entomol. 19, 73–77. doi: 10.1111/j.1440-6055.1980.tb00965.x
Spike, B. P., and Tollefson, J. J. (1988). Western corn rootworm (Coleoptera, Chrysomelidae) larval survival and damage potential to corn subjected to nitrogen and plant-density treatments. J. Econ. Entomol. 81, 1450–1455. doi: 10.1093/jee/81.5.1450
Steinbauer, M. J., and Wanjura, W. J. (2002). Christmas beetles (Anoplognathus spp., Coleoptera: Scarabaeidae) mistake peppercorn trees for eucalypts. J. Nat. History 36, 119–125. doi: 10.1080/00222930010022917
Steinbauer, M. J., and Weir, T. A. (2007). Summer activity patterns of nocturnal Scarabaeoidea (Coleoptera) of the southern tablelands of New South Wales. Aust. J. Entomol. 46, 7–16. doi: 10.1111/j.1440-6055.2007.00579.x
Stock, S. P., and Koppenhöfer, A. M. (2003). Steinernema scarabaei n. sp. (Rhabditida: Steinernematidae), a natural pathogen of scarab beetle larvae (Coleoptera: Scarabaeidae) from New Jersey, USA. Nematology 5, 191–204. doi: 10.1163/156854103767139680
Sutherland, O. R. W., and Greenfield, W. J. (1978). Effect of root extracts of resistant pasture plants on the feeding and survival of black beetle larvae, Heteronychus arator (Scarabaeidae). N. Z. J. Zool. 5, 173–175. doi: 10.1080/03014223.1978.10423748
Thom, E. R., Popay, A. J., Waugh, C. D., and Minneé, E. M. K. (2014). Impact of novel endophytes in perennial ryegrass on herbage production and insect pests from pastures under dairy cow grazing in northern New Zealand. Grass Forage Sci. 69, 191–204. doi: 10.1080/00480169.2012.715379
Urquhart, C. A. (1995). “Forest protection – Christmas beetles (Coleoptera:: Scarabaeidae),” in Research Division State Forests of New South Wales (Beecroft, NSW: State Forests of NSW).
Vannette, R. L., and Hunter, M. D. (2009). Mycorrhizal fungi as mediators of defence against insect pests in agricultural systems. Agric. For. Entomol. 11, 351–358. doi: 10.1111/j.1461-9563.2009.00445.x
Villani, M. G., and Nyrop, J. P. (1991). Age-dependent movement patterns of Japanese beetle and European chafer (Coleoptera: Scarabeidae) grubs in the soil–turfgrass microcosms. Environ. Entomol. 20, 241–251. doi: 10.1093/ee/20.1.241
Villani, M. G., and Wright, R. J. (1988). Use of radiography in behavioral studies of turfgrass-infesting scarab grub species (Coleoptera: Scarabaeidae). Bull. Entomol. Soc. Am. 34, 132–144. doi: 10.1093/besa/34.3.132
Wang, D., Shannon, M. C., Grieve, C. M., and Yates, S. R. (2000). Soil water and temperature regimes in drip and sprinkler irrigation, and implications to soybean emergence. Agric. Water Manag. 43, 15–28. doi: 10.1016/S0378-3774(99)00057-8
Ward, A. L., and Robertson, L. N. (1999). “Evidence for density-stabilising effects influencing the population dynamics of greyback canegrub in northern Queensland,” in Proceedings of Australian Society of Sugar Cane Technologists, Townsville, 164–170.
Ward, A. L., and Rogers, D. J. (2007). Oviposition response of scarabaeids: does ‘mother knows best’ about rainfall variability and soil moisture? Physiol. Entomol. 32, 357–366. doi: 10.1111/j.1365-3032.2007.00587.x
Wightman, J. A. (1974). Rearing Costelytra zealandica (Coleoptera: Scarabaeidae) 4. Some effects of different larval densities and food availability on larval survival and weight change. N. Z. J. Zool. 1, 217–223. doi: 10.1080/03014223.1974.9517830
Wise, M. J., and Abrahamson, W. G. (2007). Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. Am. Nat. 169, 443–454. doi: 10.1086/512044
Wu, S., Youngman, R. R., Kok, L. T., Laub, C. A., and Pfeiffer, D. G. (2014). Interaction between entomopathogenic nematodes and entomopathogenic fungi applied to third instar southern masked chafer white grubs, Cyclocephala lurida (Coleoptera: Scarabaeidae), under laboratory and greenhouse conditions. Biol. Control 76, 65–73. doi: 10.1016/j.biocontrol.2014.05.002
Zhang, M. L., Crocker, R. L., Mankin, R. W., Flanders, K. L., and Brandhorst-Hubbard, J. L. (2003). Acoustic identification and measurement of activity patterns of white grubs in soil. J. Econ. Entomol. 96, 1704–1710. doi: 10.1093/jee/96.6.1704
Keywords: Anoplognathus, belowground herbivory, Cyclocephala signaticollis, Dermolepida albohirtum, Heteronychus arator, pasture, pest management, Sericesthis nigrolineata
Citation: Frew A, Barnett K, Nielsen UN, Riegler M and Johnson SN (2016) Belowground Ecology of Scarabs Feeding on Grass Roots: Current Knowledge and Future Directions for Management in Australasia. Front. Plant Sci. 7:321. doi: 10.3389/fpls.2016.00321
Received: 21 October 2015; Accepted: 01 March 2016;
Published: 22 March 2016.
Edited by:
Michael Rostás, Lincoln University, New ZealandReviewed by:
Karl Kunert, University of Pretoria, South AfricaAndreas Reinecke, Max Planck Institute for Ornithology, Germany
Copyright © 2016 Frew, Barnett, Nielsen, Riegler and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam Frew, YS5mcmV3QHdlc3Rlcm5zeWRuZXkuZWR1LmF1
 Adam Frew
Adam Frew Kirk Barnett
Kirk Barnett Uffe N. Nielsen
Uffe N. Nielsen Markus Riegler
Markus Riegler Scott N. Johnson
Scott N. Johnson