- Department of Agricultural Microbiology, Tamil Nadu Agricultural University, Coimbatore, India
Zinc (Zn) deficiency in major food crops has been considered as an important factor affecting the crop production and subsequently the human health. Rice (Oryza sativa) is sensitive to Zn deficiency and thereby causes malnutrition to most of the rice-eating Asian populations. Application of zinc solubilizing bacteria (ZSB) could be a sustainable agronomic approach to increase the soil available Zn which can mitigate the yield loss and consequently the nutritional quality of rice. Understanding the molecular interactions between rice and unexplored ZSB is useful for overcoming Zn deficiency problems. In the present study, the role of zinc solubilizing bacterial strain Enterobacter cloacae strain ZSB14 on regulation of Zn-regulated transporters and iron (Fe)-regulated transporter-like protein (ZIP) genes in rice under iron sufficient and deficient conditions was assessed by quantitative real-time reverse transcription PCR. The expression patterns of OsZIP1, OsZIP4, and OsZIP5 in root and shoot of rice were altered due to the Zn availability as dictated by Zn sources and ZSB inoculation. Fe sufficiency significantly reduced the root and shoot OsZIP1 expression, but not the OsZIP4 and OsZIP5 levels. Zinc oxide in the growth medium up-regulated all the assessed ZIP genes in root and shoot of rice seedlings. When ZSB was inoculated to rice seedlings grown with insoluble zinc oxide in the growth medium, the expression of root and shoot OsZIP1, OsZIP4, and OsZIP5 was reduced. In the absence of zinc oxide, ZSB inoculation up-regulated OsZIP1 and OsZIP5 expressions. Zinc nutrition provided to the rice seedling through ZSB-bound zinc oxide solubilization was comparable to the soluble zinc sulfate application which was evident through the ZIP genes’ expression and the Zn accumulation in root and shoot of rice seedlings. These results demonstrate that ZSB could play a crucial role in zinc fertilization and fortification of rice.
Introduction
Zinc (Zn) is a critical micronutrient responsible for several cellular functions in plant and its deficiency causes decrease in plant growth and yield. Zn deficiency in major food crops, apart from yield loss, reduced the Zn content of grains and subsequently causes serious problems in human nutrition. Rice, the staple diet for more than 560 million people of world, is one of the “most sensitive" crops for Zn nutrition. Nearly 50% of the rice-growing soils are under Zn-deficient and rice grown on these soils generally produce low yield with poor nutritional quality. For incidence, Zn-sufficient rice had about 40 mg/kg of Zn in its grains, whereas Zn-deficient rice accumulated less than 10 mg/kg of grain-Zn (Wissuwa et al., 2008). Soil pH especially alkalinity, low organic carbon, high carbonates (calcareous) and low redox potential of wetland primarily limit the Zn availability for rice. However, agricultural intensification, imbalanced nutrients and neglected micronutrient application further worsen the Zn-deficiency problem in rice. Indeed, zinc deficiency in rice is becoming one of the public health problems through malnutrition in many rice-based food adopting countries of Asia (Cakmak, 2008).
For rice, soil and foliar application of highly soluble zinc sulfate (ZnSO4) is a common Zn-fertilization to correct the Zn deficiency. However, the applied Zn got precipitated as hydroxides, carbonates, phosphates and sulfides as dictated by physico-chemical properties of the soil and resulted with very low fertilizer use efficiency (1–5%). Alternatively, exploring the soil bacteria, capable of solubilizing inorganic Zn and thereby increasing the availability for crop assimilation, is a viable option to achieve the objective of correcting the Zn deficiency and thereby overcoming the zinc malnutrition in human (He et al., 2010; Mäder et al., 2010). Several zinc solubilizing bacteria (ZSB) were characterized from tropical and temperate soils to provide plant available Zn (Hafeez et al., 2013). For example, Gluconacetobacter from sugarcane, Bacillus and Pseudomonas from soybean, rice and wheat capable of solubilizing zinc compounds such as oxide, carbonate, and phosphate were reported earlier (Saravanan et al., 2011). These ZSB strains produce variety of low molecular weight organic acids, particularly gluconic acid, dissolute the insoluble Zn; reduce the pH of the soil solution and thereby increase the plant available zinc (Hafeez et al., 2013). Inoculation of these bacteria enhanced the Zn uptake of rice (Vaid et al., 2014), maize (Goteti et al., 2013), wheat (Rana et al., 2012), green gram (Sharma et al., 2012), and soybean (Ramesh et al., 2014). Few studies also confirmed the ability of ZSB for biofortification in rice (Vaid et al., 2014) and wheat (Ramesh et al., 2014). However, their full potential to mitigate the zinc deficiency and to increase the grain-Zn is not yet explored due to poor understanding of microbe-soil-plant interactions.
The soil available zinc (Zn2+) is taken up by root membrane transport mechanisms in rice which include phytosiderophores (Bashir et al., 2010) and Zn-regulated transporters and iron (Fe)-regulated transporter-like protein (ZIP) family (Guerinot, 2000). In rice, several ZIPs including OsIRT1, OsIRT2, OsZIP1, OsZIP3, OsZIP4, OsZIP5, OsZIP7, and OsZIP8 were reported to be responsible for Zn uptake from soil, translocation within root and from root to shoot as well as for storage in grains (Ramesh et al., 2003; Ishimaru et al., 2005, 2006; Yang et al., 2009; Lee et al., 2010a,b). OsITR1 and OsITR2 are responsible for transport of Fe2+ from rhizosphere to root with less affinity to Zn (Ishimaru et al., 2006). OsZIP1, OsZIP3, OsZIP4, OsZIP5, and OsZIP8 are rice plasma membrane Zn transporters and are induced by Zn deficiency (Ramesh et al., 2003; Ishimaru et al., 2005; Yang et al., 2009; Lee et al., 2010a; Suzuki et al., 2012). The expression of most of the well-studied rice ZIP genes (OsZIP1, OsZIP4, OsZIP5) was controlled by the availability of divalent cations such as Zn2+, Fe2+, Cu2+, Mn2+ (Bughio et al., 2002; Ishimaru et al., 2005; Lee et al., 2010a). Few studies also confirmed that these transporter genes’ expression varied between root and shoot tissues of rice (Ishimaru et al., 2011). Similarly, Chen et al. (2008) reported the differential expression pattern of ZIP genes (OsZIP1, OsZIP3, and OsZIP4) between Zn-efficient and Zn-inefficient cultivars of rice. These ZIP genes varied their expression levels at different growth stages of rice from germination to grain filling (Ishimaru et al., 2011). The plant growth promoting rhizobacteria (PGPR) upon colonizing the roots, acidify the rhizosphere through organic acids and produce siderophores which facilitate the trace elements’ uptake by the crop plants. However, no attempts were made so far to elucidate the role of these zinc solubilizing PGPR strains to regulate the expression of metal transporter genes in the root. Understanding the interaction between rice plant and Zn solubilizing PGPR in terms of Zn transporter genes’ expression would help to alleviate the Zn deficiency as well as to improve the Zn fortification. In the present work, we have reported the root and shoot ZIP genes’ expression pattern of rice seedlings upon inoculating with a potential ZSB (Enterobacter cloacae strain ZSB14) under controlled condition. Our results suggest that the ZSB in rhizosphere of rice roots may regulate ZIP genes’ expression either directly or indirectly through Zn availability.
Materials and Methods
Bacterial Strain and Culture Condition
Enterobacter cloacae strain ZSB14, isolated and characterized from rhizosphere of rice, capable of solubilizing insoluble Zn compounds viz., ZnO (24.05 μg/ml of soluble Zn), ZnCO3 (19.37 μg/ml) and Zn3(PO4)2 (6.06 μg/ml) was used for this study. In order to maintain the Zn solubilizing potential of the strain, The culturing was routinely done in Bunt and Rovira medium containing 0.1% ZnO with and without agar (1.5%; Bunt and Rovira, 1955) at 30°C in an incubator (Lab Companion, USA).
Rice Culture and ZSB Inoculation
Rice (Orzya sativa) cultivar Co51 of Tamil Nadu Agricultural University, Coimbatore was used for this experiment. De-husked healthy seeds were surface sterilized with sodium hypochlorite with 5% available chlorine for 10 min followed by five washes with sterile distilled water. The seeds were soaked in sterile distilled water for over-night for sprouting. Uniformly sprouted seeds were placed (10 seeds per plate) on Fe sufficient (Fe+) modified Hoagland medium (5 mM KNO3, 2 mM MgSO4, 2 mM Ca(NO3)2, 2.5 mM KH2PO4, 70 μM H3BO3, 1 μM MnCl2, 0.5 μM CuSO4, 10 μM NaCl, 0.2 μM Na2MoO4, 50 μM FeEDTA, 1.0 g/l MES buffer pH 5.8; 40 mM Sucrose; 8.0 g/l plant agar). For Fe-deficient (Fe-) condition, modified Hoagland medium lacking FeEDTA was used. Both under Fe+ and Fe-, five treatments for Zn nutrition were adopted viz., (i) no-zinc control; (ii) soluble Zn as ZnSO4 (5 μM); (iii) sparingly soluble ZnO (10 μM); (iv) ZnO with ZSB inoculation; (v) ZSB inoculation alone. ZnSO4 (5 μM) or ZnO (10 μM) was supplemented directly in modified Hoagland medium depending upon the treatment and seeds were placed. The plants were grown in a growth chamber with 12 h light (200 mole/m2/s) at 28°C. After 7 days of growth, the rice seedlings were inoculated with ZSB strain depending upon the treatment. For this, the strain ZSB14 was cultivated in Bunt and Rovira medium added with ZnO to achieve a final Zn concentration of 0.1% at 30°C till reached a final concentration of approximately 1011 colony forming units (cfu) per ml. The bacteria were pelletized by centrifugation at 5000 g for 20 min at room temperature and cell pellets were re-suspended in 10 mM MgSO4 and centrifuged. This operation was repeated and afterward the cell pellets were re-suspended in 10 mM MgSO4. The bacterial titer was adjusted to the OD600 of 0.05 (108 cfu per ml) and 20 μL of bacterial suspension was then applied on each root of 7-days-old seedlings, right below the hypocotyl. After additional 7 days of incubation, the seedlings were removed carefully from the plates and assessed for ZIP gene expression.
RNA Preparation and Real-Time RT-PCR Analysis
Total RNA from shoot and root of rice was extracted separately by following the procedure of Oñate-Sánchez and Vicente-Carbajosa (2008). The residual genomic DNA in the RNA preparation was digested with RNAse-free DNase I (New England Biolabs) until no amplicons were obtained when using RNA preparations directly in the PCR reaction with the primers for the actin gene (OsACT1). The primer details are provided in Table 1. Subsequently, complementary DNA (cDNA) was synthesized from 3 μg of DNA-free total RNA using Revert Aid H minus reverse transcriptase (Thermo Scientific) by primering with oligo d(T)18 (Invitrogen) in a 40-μL reaction mixture according to the manufacturer’s instruction. Real-time PCR was performed in Roche Lightcycler 480II (Roche, Switzerland) to quantify the transcripts of OsZIP1, OsZIP4, and OsZIP5 (Primer details in Table 1) using SYBR Green (SYBR Premix ExTaq, Tli RNase H Plus, Takara) as the detection system. The constitutively expressed OsACTIN1 gene was amplified as the reference gene. Changes in expression were calculated by relative quantification (ΔΔCt) method (Livak and Schmittgen, 2001) using threshold cycle (Ct) values of target and reference genes. For all real-time RT-PCR analyses, three biological replicates and two technical replicates were used. The size and intensity of amplified fragments were confirmed by gel electrophoresis.
Determination of Zn Content in Rice Plant
Rice seedlings washed until free from agar medium were oven-dried at 70°C for 5 h and digested with 15 ml triple acid mixture (nitric, sulfuric, and perchloric acid in the ratio of 9:2:1) for overnight. The volume of cooled digest was made up to 25 ml using deionized double distilled water and the dilutions were used for Zn estimation using atomic absorption spectrophotometer (GBS Scientific, Australia) at the wavelength of 213.86 nm.
Statistical Analyses
All the data were subjected to statistical analysis with software, Microsoft Excel for Windows 2007 add-in with XLSTAT Version 2010.5.05 (XLSTAT, 2010). Statistically significant differences between the treatments were analyzed using analysis of variance (ANOVA) and Duncan’s Multiple Range Test (DMRT) at 5% significance level.
Results
OsZIP1 Expression
Both the Fe-levels and Zn-treatments significantly influenced the expression of OsZIP1 (Figure 1). The 7-days-old Fe- rice roots had higher copies of OsZIP1 transcripts than those of Fe+ seedlings. Under Fe- condition, ZnO addition recorded highest OsZIP1 transcripts followed by no-Zn control. Under Fe+ condition, no-Zn control recorded lowest OsZIP1 transcripts followed by ZnSO4 while, ZnO recorded the maximum relative expression among the treatments. Addition of ZnO in the medium up-regulated OsZIP1 expression both in Fe- and Fe+ conditions (Figure 1A). When ZSB-inoculated seedlings were assayed for OsZIP1 expression after 7 days (14 days-old seedlings), the expression pattern of root OsZIP1 was different than the earlier (Figure 1B). The ZSB inoculation substantially reduced the OsZIP1 expression both in Fe-deficient and Fe-sufficient rice roots in the presence of ZnO. When ZnO was not in the medium, ZSB inoculated rice seedlings had maximum OsZIP1 transcripts for both Fe-deficient and Fe-sufficient conditions. Under Fe+ condition, ZnSO4 amendment recorded higher OsZIP1 transcripts than No-Zn and ZnO + ZSB, but lower than ZnO and ZSB. Under Fe- condition, the same trend was noticed with the exception of ZnSO4 and ZnO with at par levels.
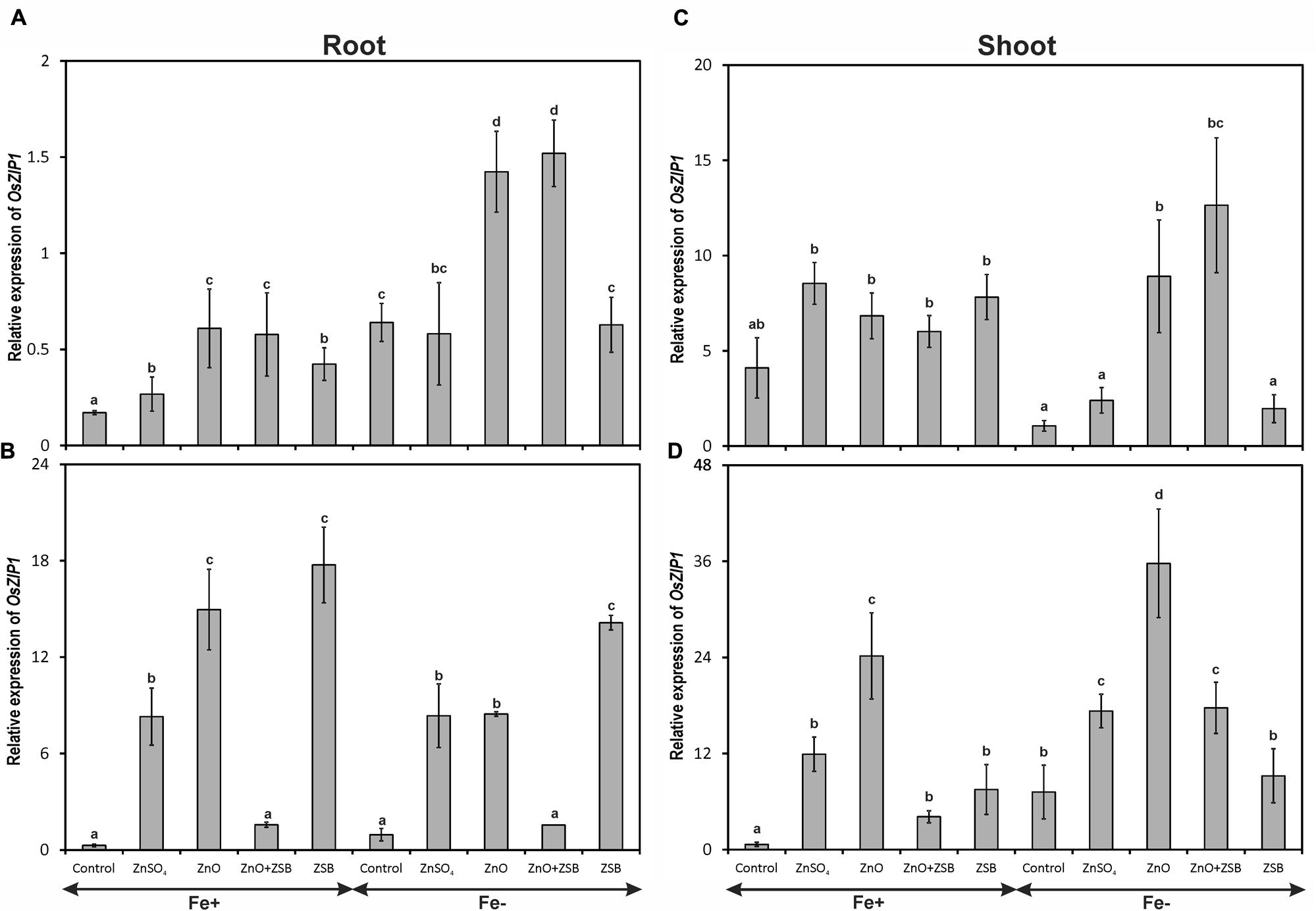
FIGURE 1. Expression pattern of OsZIP1 in root (A,B) and shoot (C,D) of rice seedlings as influenced by Fe and Zn. (A,C) 7th day expression levels; (B,D) 14th day expression levels. Fe+, Fe sufficient condition; Fe-, Fe deficient condition. Control, No-zinc control; ZnSO4 at 5 mM; ZnO at 10 mM; ZSB, Zinc solubilizing bacteria (Enterobacter cloacae strain ZSB14) inoculation on 7th day. Relative mRNA abundance of OsZIP1 was quantified and normalized with OsACTIN1 gene on 7th day and 14th day. Data from real-time RT-PCR experiments were analyzed according to the 2-ΔΔCt method. Means of six replicate values plotted, errors bars indicate the standard error. Values followed by the same letter in each panel are not significantly different from each other as determined by DMRT (p ≤ 0.05).
The OsZIP1 levels of shoot were nearly 10-fold higher than root in the 7-days-old seedlings before exposure to ZSB inoculation (Figure 1C). However, the Fe-sufficient shoots did not show any significant difference within the Zn-treatments for the level of OsZIP1 transcripts, while the Fe-deficient shoots showed significant difference between them. The no-Zn controls and ZnSO4 had lowest shoot OsZIP1 while ZnO amended Fe- rice recorded maximum expression. After 7-days of ZSB inoculation, OsZIP1 levels had significant different in the Zn-treatments. The ZSB inoculation considerably reduced the OsZIP1 expression of the rice shoot (Figure 1D). When comparing the ZnO and ZnO + ZSB, nearly 50% reduction in OsZIP1 expression was recorded due to ZSB inoculation in both Fe- and Fe+ conditions. In shoot also, the OsZIP1 expression was reduced in the presence of Fe in the medium.
OsZIP4 Expression
The expression of OsZIP4 gene was significantly influenced by different Zn-treatments but not by Fe- levels (Figure 2). Both Fe+ and Fe- rice responded similar pattern of OsZIP4 expression in the root and shoot of rice seedlings. The root of 7-days-old rice seedlings recorded significantly highest OsZIP4 transcripts due to ZnO followed by no-Zn control under Fe-deficient and sufficient conditions (Figure 2A). The ZnSO4 amendment down-regulated the OsZIP4 significantly than other treatments. When the ZSB strain was inoculated, the root OsZIP4 showed remarkable difference of expression after 7 days. Irrespective of treatments, the level of relative expression of OsZIP4 at 14 days-old seedling had been increased nearly 10-fold than 7 days-old plants (Figure 2B). Among the rice seedlings exposed to different amendments, ZnO addition significantly increased the root-OsZIP4 transcripts followed by ZnSO4, while the ZnO + ZSB and ZSB alone significantly reduced the expression. The no-Zn controls did not show any variations in their root OsZIP4 transcript levels between two observations.
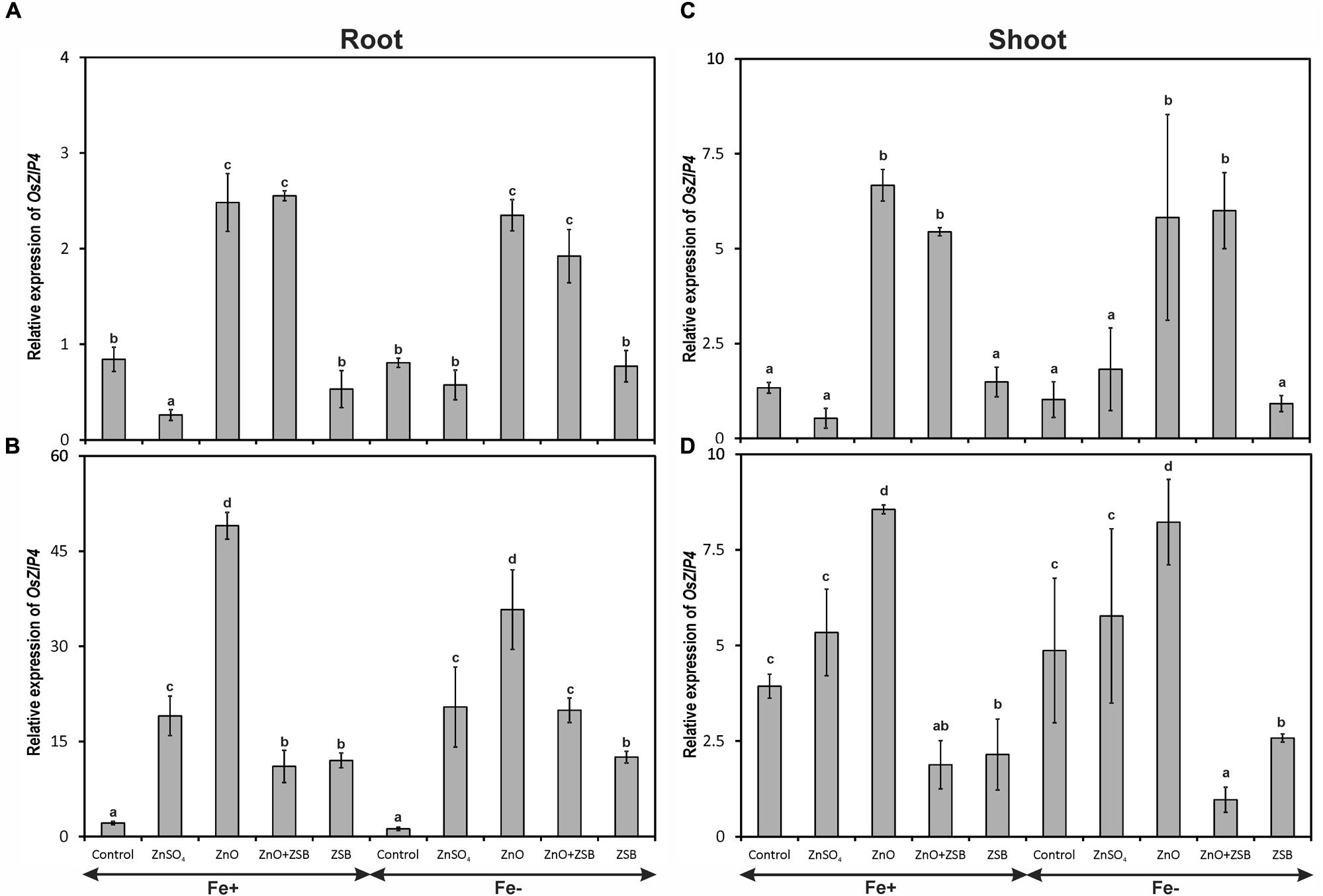
FIGURE 2. Expression pattern of OsZIP4 in root (A,B) and shoot (C,D) of rice seedlings as influenced by Fe and Zn. (A,C) 7th day expression levels; (B,D) 14th day expression levels. Fe+, Fe sufficient condition; Fe-, Fe deficient condition. Control, No-zinc control; ZnSO4 at 5 mM; ZnO at 10 mM; ZSB, Zinc solubilizing bacteria (Enterobacter cloacae strain ZSB14) inoculation on 7th day. Relative mRNA abundance of OsZIP4 was quantified and normalized with OsACTIN1 gene on 7th day and 14th day. Data from real-time RT-PCR experiments were analyzed according to the 2-ΔΔCt method. Means of six replicate values plotted, errors bars indicate the standard error. Values followed by the same letter in each panel are not significantly different from each other as determined by DMRT (p ≤ 0.05).
With reference to shoot OsZIP4, the level of expression remained same between Fe+ and Fe- seedlings after 14 days of incubation (Figures 2C,D). The shoot of 7-days-old rice seedlings before ZSB inoculation exposed to ZnO had significantly higher OsZIP4 transcripts that ZnSO4 and no-Zn controls of both Fe-deficient and sufficient rice plants (Figure 2C). When ZSB was inoculated on 7th day, the OsZIP4 significantly reduced in ZnO + ZSB treatment to a tune of 77 and 88% for Fe-sufficient and deficient rice plants, respectively as compared to ZnO treatment (Figure 2D). Irrespective to Fe-levels, ZnO amended uninoculated plants remained constant level of expression for both the assessments; whereas ZnSO4 amended plants increased their OsZIP4 expression levels to ninefold after 7 days of additional incubation.
OsZIP5 Expression
The transcripts of OsZIP5 were strongly found in shoots and weakly in roots of 7-days-old rice seedlings (Figure 3). Like OsZIP4, OsZIP5 also did not show significant response to Fe levels. In the roots of 7-days-old rice seedlings, the no-Zn and ZnO amended rice seedlings showed significantly higher levels of OsZIP5 in both Fe-sufficient and deficient conditions (Figure 3A). The ZnSO4 amended rice plants had the least expression of OsZIP5 in their roots. After ZSB inoculation and 7 days incubation, the pattern of OsZIP5 expression was different than those of before inoculation. After additional 7 days of incubation, the rice seedlings exposed to ZnO alone had nearly 60-fold increased OsZIP5 transcripts in both Fe+ and Fe- conditions (Figure 3B). However, ZSB inoculation alone also induced OsZIP5 in Fe+ and Fe- rice roots but significantly lower than ZnO amendment. The ZnO + ZSB inoculation, ZnSO4 and no-Zn controls had least OsZIP5 transcripts in their Fe+ and Fe- roots.
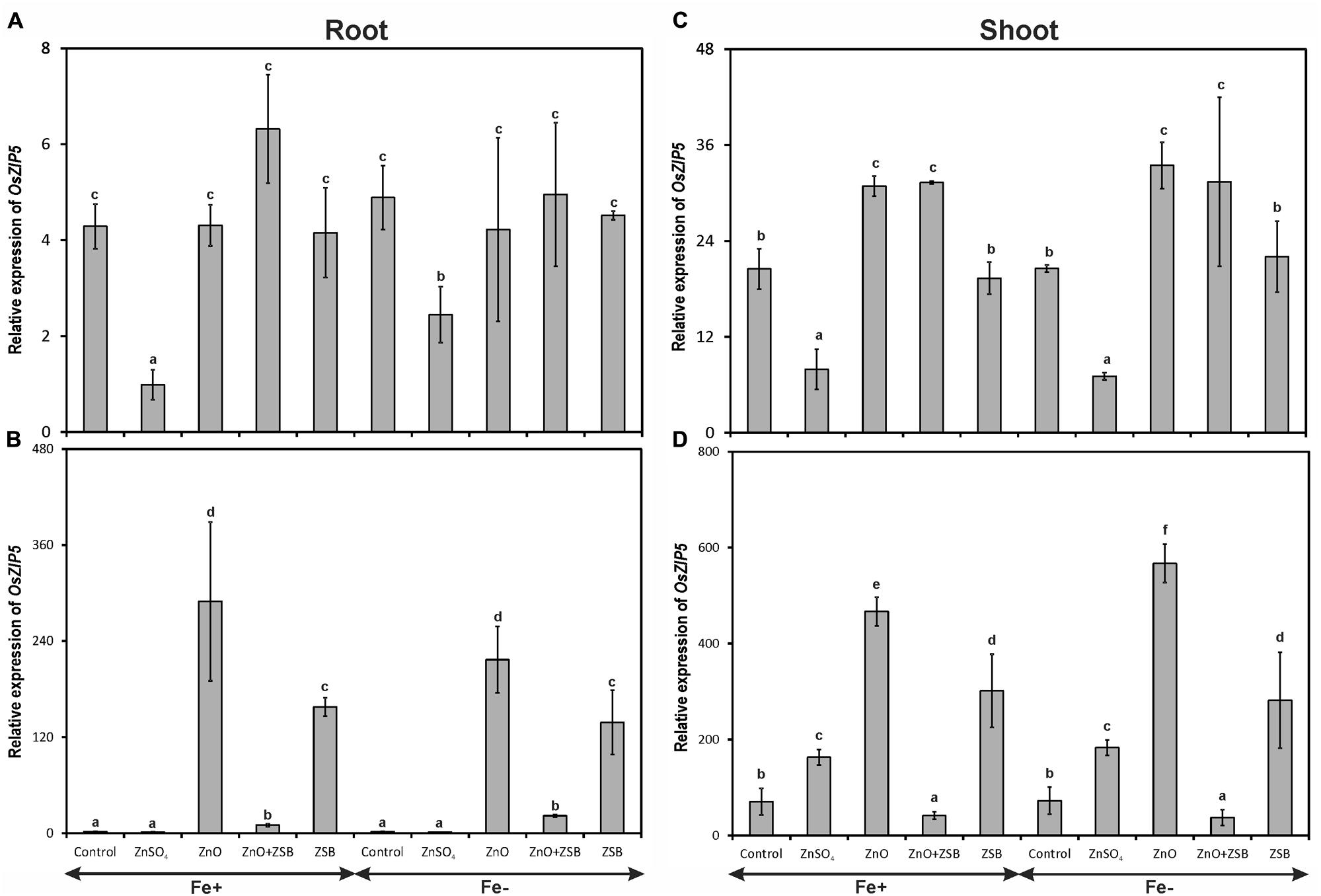
FIGURE 3. Expression pattern of OsZIP5 in root (A,B) and shoot (C,D) of rice seedlings as influenced by Fe and Zn. (A,C) 7th day expression levels; (B,D) 14th day expression levels. Fe+, Fe sufficient condition; Fe-, Fe deficient condition. Control, No-zinc control; ZnSO4 at 5 mM; ZnO at 10 mM; ZSB, Zinc solubilizing bacteria (Enterobacter cloacae strain ZSB14) inoculation on 7th day. Relative mRNA abundance of OsZIP5 was quantified and normalized with OsACTIN1 gene on 7th day and 14th day. Data from real-time RT-PCR experiments were analyzed according to the 2-ΔΔCt method. Means of six replicate values plotted, errors bars indicate the standard error. Values followed by the same letter in each panel are not significantly different from each other as determined by DMRT (p ≤ 0.05).
The response of rice shoot OsZIP5 was similar to that of root but with twofold increased levels than roots (Figures 3C,D). There was no significant difference between Fe+ and Fe- plants in terms of shoot OsZIP5 expression. The ZnSO4 amendment in the medium down-regulated the OsZIP4 in Fe+ and Fe- shoots, whereas no-Zn controls and ZnO amendments had significantly higher copies of OsZIP5 transcripts (Figure 3C). When ZSB was inoculated to their respective treatment plants, there was significant effect found due to ZSB inoculation. ZnO + ZSB inoculation significantly down-regulated the OsZIP5 to a tune of 91 and 95% for Fe+ and Fe- plants, respectively as compared to ZnO amended rice shoots (Figure 3D). The ZSB inoculation without ZnO up-regulated the OsZIP5 expression in shoots after 7-days of incubation. The no-Zn and ZnSO4 also had significantly higher levels of OsZIP5 transcripts than ZnO + ZSB treatment.
Leave Chlorosis of Rice Seedlings
We examined the role of Fe and ZSB-bound Zn availability on metal uptake of rice (Fe and Zn) in terms of chlorosis of leaves. The color intensity of rice leaves after 14-days of exposure to various Zn treatments under Fe+ and Fe- conditions showed significant difference (Figure 4). The Fe+ condition made rice leaves with dark intensity while the Fe- showed chlorosis. Under Fe+ condition, ZnO induced the chlorosis of leaves, while with ZSB inoculation, the chlorosis was reduced. The ZnSO4 and ZSB alone did not show any chlorosis at all. Under Fe- condition, no-Zn and ZnO showed severe yellowing, while the ZnSO4 and ZSB had less chlorosis.
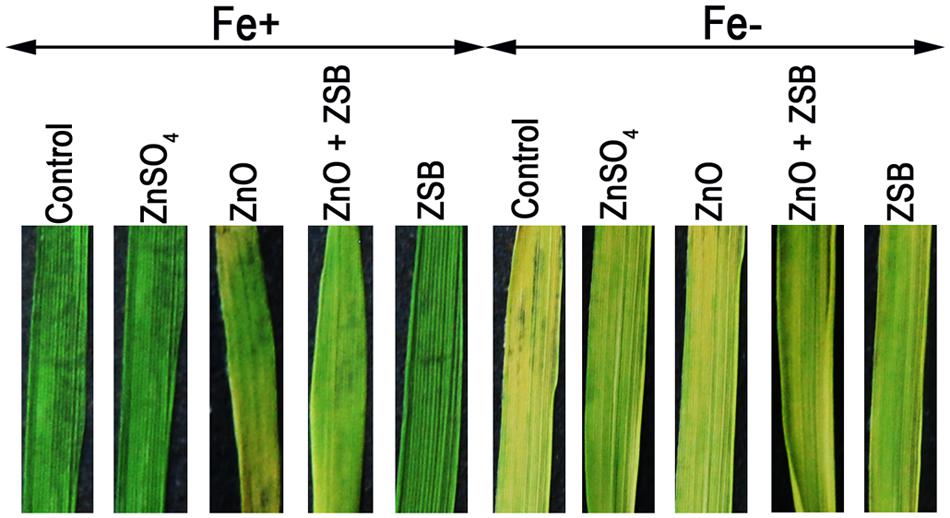
FIGURE 4. Leaf color of 14-days-old rice seedlings after exposure to different Zn treatments under Fe+ and Fe- conditions. Control, No-zinc control; ZnSO4 at 5 mM; ZnO at 10 mM; ZSB, Zinc solubilizing bacteria (Enterobacter cloacae strain ZSB14) inoculation on 7th day.
Zn Content of Rice Seedlings
The root and shoot Zn content of rice seedlings before ZSB inoculation (7th day) was significantly influenced by Zn amendments and also due to Fe conditions. In 7-days-old seedlings, ZnSO4 recorded 198 and 280% higher root Zn and 108 and 121% higher shoot Zn than Fe+ and Fe- no-Zn controls, respectively (Table 2). ZnO also increased the root and shoot Zn of rice seedlings than no-Zn controls, which were trivial (14–18%) as compared to ZnSO4. The Zn uptake measured as zinc content of rice seedlings after ZSB inoculation (14th day) was also significantly influenced by Zn sources. Fe- condition increased the Zn contents of root and shoot significantly than Fe+ for Zn treatments (ZnSO4 and ZnO + ZSB) but not for no-Zn and ZSB alone controls at 14th day (Table 2). Among the various treatments enforced, the ZnSO4 recorded maximum root and shoot Zn contents (198.25 and 154.26 mg/g for Fe- and 145.15 and 125.37 mg/g for Fe+ respectively). The ZnO + ZSB treated plants recorded 157.46 and 124.13 mg/g of Zn in root and shoot, respectively under Fe-deficient condition, and 87.24 and 90.16 mg/g for Fe-sufficient conditions. The ZnO alone treated plants had very little increase of Zn content as compared to no-Zn control. ZSB inoculation alone had at par root and shoot Zn levels as that of no-Zn controls for Fe+ and Fe- rice seedlings.
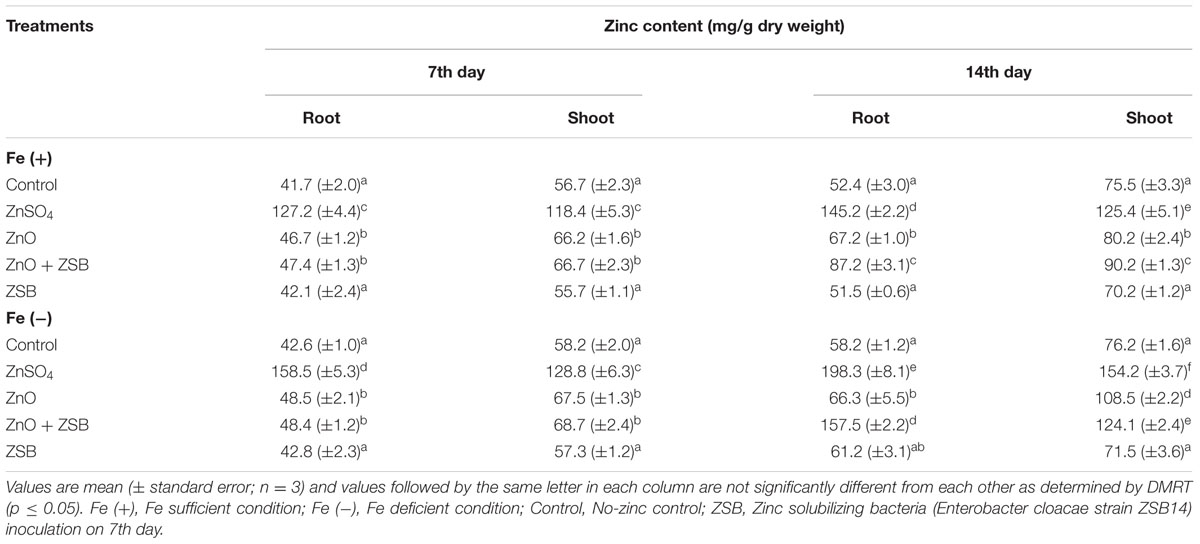
TABLE 2. Effect of different Zn sources and ZSB inoculation on the Zn content of rice seedlings (Cultivar Co51).
Discussion
Exploiting the ZSB for alleviating Zn-deficiency as well as for Zn-fortification in food grains like rice could be a promising agronomical approach to minimize the Zn-deficiency in human being. Keeping in view the unambiguous benefits of ZSB (Hafeez et al., 2013), through the present investigation, we reported that ZSB inoculation to rice could alter the expression of zinc transporting genes of rice based on the Zn solubilization and thereby regulate the uptake of zinc.
The ZIP family transporters are well-characterized and are suggested to be the primary uptake system for Zn in plants (Guerinot, 2000; Mäser et al., 2001). Most of these ZIP genes are induced by Zn deficiency (Ramesh et al., 2003; Ishimaru et al., 2005; Chen et al., 2008) and their expression pattern varied between root and shoot system. OsZIP1 was shown to be expressed higher levels in roots than shoots under Zn-deficient condition (Ramesh et al., 2003; Ishimaru et al., 2005). Chen et al. (2008) observed that OsZIP1 was up-regulated in Zn-deficient roots, but no visible transcripts detected in shoots of both Zn-efficient and Zn-inefficient rice genotypes. In contrast to these, Ramegowda et al. (2013) found that OsZIP1 over-expressing transgenic finger millet showed higher expression of OsZIP1 in leaves under Zn-sufficient condition. In the present work also, we found higher expression of OsZIP1 in shoot than root in 7-days-old rice and the OsZIP1 expression was influenced by Fe availability apart from zinc. The rice grown for 7-days under Fe-sufficient condition had relatively lower OsZIP1 transcripts than those plants grown in Fe-deficient condition. Among the two Zn-treatments, sparingly soluble ZnO up-regulated the OsZIP1 as compared to highly soluble ZnSO4 before ZSB inoculation. This is in accordance with the earlier findings that the zinc abundance reduced the root OsZIP1 expression (Ramesh et al., 2003; Ishimaru et al., 2005). However, in the present work, when the rice plants grown with ZnO had highest OsZIP1 expression in their roots after 7 days which was higher than no-zinc control. This implies that the sparingly soluble ZnO could not supply the available Zn in the growth medium of rice and subsequently cause more stress than no-Zn condition. Further investigations are needed to understand how the ZnO induced the ZIP transporters higher than no-Zn condition. However, when ZSB was inoculated on 7-days-old rice seedling, considerable reduction in OsZIP1 expression was noticed in both Fe+ and Fe- root and shoot of rice. This might be due to the ZSB-mediated Zn solubility and availability in the medium as well as the ZSB-mediated rhizospheric effects. Interestingly, ZSB inoculation increased the root and shoot OsZIP1 expression even in the absence of Zn.
In the present work, Fe+ and Fe- conditions did not alter the expressions of OsZIP4 and OsZIP5 as that of OsZIP1 which is in accordance with the earlier works (Ishimaru et al., 2005, 2007; Lee et al., 2010a). OsZIP1 is primarily associated with metal uptake from rhizosphere (Ramesh et al., 2003), while OsZIP4 and OsZIP5 are involved in the translocation of Zn with in the plant (Ishimaru et al., 2005) might be the reason, why these genes are not regulated due to Fe levels. The previous works confirmed that OsZIP4 in Zn-deficient rice was expressed in meristem and vascular bundles of roots and shoots and is responsible for Zn translocation to various plant parts that require Zn (Ishimaru et al., 2011). As the transporters involving in metal uptake from soil may have non-specific uptake of the ions such as Zn, Fe, Cu, Cd, Mn from soil to the root, these genes’ expression was regulated based upon the affinity of the metals. However, the transporters responsible for translocation of metals within the plant had less impact of other metal species. For example, the transporters OsZIP4, OsZIP5, and OzZIP8 responsible for Zn translocation in rice are not influenced by Fe+, while the OsZIP1 and OsITR1 responsible for Zn and Fe uptake from soil respectively, were also influenced by other metals (Lee and An, 2009). The present results are in accordance with these findings. In the present work, ZnSO4 in the growth medium made Zn sufficient condition and thereby reduced the OsZIP4 expressions in both root and shoot. When ZnO was amended, the relative OsZIP4 expression was significantly higher than no-Zn control which means that the addition of ZnO cause more stress to the rice than no-Zn. When the ZSB was inoculated on 7th day and incubated for additional 7-days, the relative expression of OsZIP4 got varied in those treatments which imply that rhizosphere colonization of ZSB either directly or indirectly regulates ZIP genes of rice. Compare to ZnO treatment, ZnO + ZSB reduced the OsZIP4 expression revealed that the ZSB-mediated solubilization of ZnO enhanced the uptake of Zn and thereby reduced the Zn deficiency. The down-regulation of OsZIP4 found in rice shoot due to ZSB inoculation implies that the ZSB-bound Zn release has been effectively translocated to the shoot system also. Compare to no-Zn control, ZSB inoculation in the absence of ZnO up-regulated root OsZIP4 but down-regulated the shoot OsZIP4. However, compare to OsZIP1, the ZSB-mediated regulation of OsZIP4 was relatively low. Hence, further investigation is needed to understand this variation between ZIP transporters’ response for ZSB inoculation.
OsZIP5 is a plasma membrane-bound transporter responsible for Zn translocation within the rice plant. Expression of OsZIP5 is mainly regulated by Zn levels and Zn deficient condition up-regulated the expressions in both shoot and root (Lee et al., 2010a). Over-expression of OsZIP5 over-expressed plants showed sensitive to excess Zn, while the OsZIP5 knock-out plants had high Zn tolerance (Ishimaru et al., 2011). In the present experiment, the expression pattern of OsZIP5 was differed from OsZIP1 and OsZIP4 in several treatments. Before ZSB inoculation, no-Zn and ZnO applied rice plants, which are suffered with Zn deficiency had maximum OsZIP5 expression both in root and shoot. ZnSO4 in the medium down regulated the expression of root and shoot OsZIP5. When ZSB was inoculated to ZnO and no-Zn plants, OsZIP5 was in low copies in ZnO amended plants, while No-Zn but ZSB inoculated plants had significantly higher transcripts. This result implies that ZSB had significant influence on OsZIP5 by providing soluble Zn from ZnO while in the absence of Zn, ZSB up-regulated OsZIP5 as that of OsZIP1. Hence, it is clear from these experiments that ZSB had direct impact on OsZIP1 and OsZIP5 and for OsZIP4, the regulation is dependent of Zn-availability due to the functioning of ZSB.
Several previous studies also confirmed that ZSB inoculation enhanced the exchangeable Zn in the soil or rhizosphere of crops through organic acid production and enhanced microbial processes and subsequently improved the Zn uptake (Oburger et al., 2009; Ramesh et al., 2014; Shakeel et al., 2015). As supportive to these findings, in the present work, the Zn content of shoot and root of ZnO + ZSB inoculated rice plants was higher than no-Zn, ZnO alone and ZSB alone plants, but lower than ZnSO4 amended plants. This was further evident from the observation on the chlorosis of rice leaves in the present experiment (Figure 4). Fe and Zn sufficient conditions did not show any chlorosis, while ZnO induced the chlorosis implies that ZnO may affect the Fe uptake along with Zn. Hence, inoculation of ZSB in the root zone improved the Zn uptake and translocation within the plant and thereby increased the Zn contents of root and shoot compared to no-Zn and ZnO alone controls.
Conclusion
In the present investigation, we proved that the inoculation of ZSB under controlled condition can able to regulate some of the Zn-regulated transporters family genes and thereby controlled the Zn uptake in rice seedlings. Zn sufficient condition created by ZnSO4 down regulated OsZIP4 and OsZIP5 both in root and shoot of rice. The application of sparingly soluble ZnO as Zn source created severe Zn related stress to the rice, which up-regulated all the ZIP genes. Upon inoculation of ZSB, the expression levels of OsZIP1, OsZIP4, and OsZIP5 were reduced. In the absence of Zn source, ZSB inoculation could regulate OsZIP1 and OsZIP5 but not the OsZIP4. These results are evident that the ZSB inoculation as PGPR could regulate the Zn uptake and translocation in rice plant and thereby zinc fortification in rice grains.
Author Contributions
The experiments were planned and executed together by SK and DB. SK undertook the data analysis. The interpretation of results and manuscript preparation were done by DB.
Funding
The corresponding author (DB) thanks Ministry of Human Resource Development, New Delhi, India for providing financial support through Centre of Excellence in Frontier Areas of Science and Technology on Microbes to Feed the World: Plant-microbe interaction to boost the agricultural production (F. No. 5-5/2014 – TS VII).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Dr. Corne M. J. Pieterse, Dr. Peter A. H. M. Bakker, and Dr. Roeland L. Berendsen of Plant Microbe Interactions Laboratory, Department of Biology, Utrecht University, Utrecht, The Netherlands for sufficient knowledge given through HRD training to carry out the present experiments.
References
Bashir, K., Ishimaru, Y., and Nishizawa, N. (2010). Iron uptake and loading into rice grains. Rice 3, 122–130. doi: 10.1007/s12284-010-9042-y
Bughio, N., Yamaguchi, H., Nishizawa, N. K., Nakanishi, H., and Mori, S. (2002). Cloning an iron-regulated metal transporter from rice. J. Exp. Bot. 53, 1677–1682. doi: 10.1093/jxb/erf004
Bunt, J., and Rovira, A. (1955). Microbiological studies of some subantarctic soils. J. Soil Sci. 6, 119–128. doi: 10.1111/j.1365-2389.1955.tb00836.x
Cakmak, I. (2008). Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302, 1–17. doi: 10.1007/s11104-007-9466-3
Chen, W. R., Feng, Y., and Chao, Y. E. (2008). Genomic analysis and expression pattern of OsZIP1, OsZIP3, and OsZIP4 in two rice (Oryza sativa L.) genotypes with different zinc efficiency. Russ. J. Plant Physiol. 55, 400–409. doi: 10.1134/S1021443708030175
Goteti, P. K., Emmanuel, L. D. A., Desai, S., and Shaik, M. H. A. (2013). Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013:869697. doi: 10.1155/2013/869697
Guerinot, M. L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta 1465, 190–198. doi: 10.1016/S0005-2736(00)00138-3
Hafeez, F., Abaid-Ullah, M., and Hassan, M. (2013). “Plant growth-promoting rhizobacteria as zinc mobilizers: a promising approach for cereals biofortification,” in Bacteria in Agrobiology: Crop Productivity, eds D. K. Maheshwari, M. Saraf, and A. Aeron (Berlin: Springer), 217–235.
He, C. Q., Tan, G., Liang, X., Du, W., Chen, Y., Zhi, G., et al. (2010). Effect of Zn-tolerant bacterial strains on growth and Zn accumulation in Orychophragmus violaceus. Appl. Soil. Ecol. 44, 1–5. doi: 10.1016/j.apsoil.2009.07.003
Ishimaru, Y., Bashir, K., and Nishizawa, N. (2011). Zn uptake and translocation in rice plants. Rice 4, 21–27. doi: 10.1007/s12284-011-9061-3
Ishimaru, Y., Masuda, H., Suzuki, M., Bashir, K., Takahashi, M., Nakanishi, H., et al. (2007). Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants. J. Exp. Bot. 58, 2909–2915. doi: 10.1093/jxb/erm147
Ishimaru, Y., Suzuki, M., Kobayashi, T., Takahashi, M., Nakanishi, H., Mori, S., et al. (2005). OsZIP4, a novel zinc-regulated zinc transporter in rice. J. Exp. Bot. 56, 3207–3214. doi: 10.1093/jxb/eri317
Ishimaru, Y., Suzuki, M., Tsukamoto, T., Suzuki, K., Nakazono, M., Kobayashi, T., et al. (2006). Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 45, 335–346. doi: 10.1111/j.1365-313X.2005.02624.x
Lee, S., and An, G. (2009). Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ. 32, 408–416. doi: 10.1111/j.1365-3040.2009.01935.x
Lee, S., Jeong, H., Kim, S., Lee, J., Guerinot, M., and An, G. (2010a). OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol. Biol. 73, 507–517. doi: 10.1007/s11103-010-9637-0
Lee, S., Kim, S., Lee, J., Guerinot, M., and An, G. (2010b). Zinc deficiency-inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol. Cells 29, 551–558. doi: 10.1007/s10059-010-0069-0
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Mäder, P., Kaiser, F., Adholeya, A., Singh, R., Uppal, H. S., Sharma, A. K., et al. (2010). Inoculation of root microorganisms for sustainable wheat–rice and wheat–black gram rotations in India. Soil Biol. Biochem. 43, 609–619. doi: 10.1016/j.soilbio.2010.11.031
Mäser, P., Thomine, S., Schroeder, J. I., Ward, J. M., Hirschi, K., Sze, H., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667. doi: 10.1104/pp.126.4.1646
Oburger, E., Kirk, G. J. D., Wenzel, W. W., Puschenreiter, M., and Jones, D. L. (2009). Interactive effects of organic acids in the rhizosphere. Soil Biol. Biochem. 41, 449–457. doi: 10.1016/j.soilbio.2008.10.034
Oñate-Sánchez, L., and Vicente-Carbajosa, J. (2008). DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes 1:93. doi: 10.1186/1756-0500-1-93
Ramegowda, Y., Venkategowda, R., Jagadish, P., Govind, G., Hanumanthareddy, R.-R., Makarla, U., et al. (2013). Expression of a rice Zn transporter, OsZIP1, increases Zn concentration in tobacco and finger millet transgenic plants. Plant Biotechnol. Rep. 7, 309–319. doi: 10.1007/s11816-012-0264-x
Ramesh, A., Sharma, S. K., Sharma, M. P., Yadav, N., and Joshi, O. P. (2014). Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil. Ecol. 73, 87–96. doi: 10.1016/j.apsoil.2013.08.009
Ramesh, S. A., Shin, R., Eide, D. J., and Schachtman, D. P. (2003). Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol. 133, 126–134. doi: 10.1104/pp.103.026815
Rana, A., Saharan, B., Nain, L., Prasanna, R., and Shivay, Y. S. (2012). Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Sci. Plant Nutr. 58, 573–582. doi: 10.1080/00380768.2012.716750
Saravanan, V. S., Kumar, M. R., and Sa, T. M. (2011). “Microbial zinc solubilization and their role on plants,” in Bacteria in Agrobiology: Plant Nutrient Management, ed. D. K. Maheshwari (Berlin: Springer), 47–63.
Shakeel, M., Rais, A., Hassan, M. N., and Hafeez, F. Y. (2015). Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) varieties. Front. Microbiol. 6:1286. doi: 10.3389/fmicb.2015.01286
Sharma, S. K., Sharma, M. P., Ramesh, A., and Joshi, O. P. (2012). Characterization of zinc-solubilizing Bacillus isolates and their potential to influence zinc assimilation in soybean seeds. J. Microbiol. Biotechnol. 22, 352–359. doi: 10.4014/jmb.1106.05063
Suzuki, M., Bashir, K., Inoue, H., Takahashi, M., Nakanishi, H., and Nishizawa, N. (2012). Accumulation of starch in Zn-deficient rice. Rice 5, 1–8. doi: 10.1186/1939-8433-5-9
Vaid, S., Kumar, B., Sharma, A., Shukla, A., and Srivastava, P. (2014). Effect of Zn solubilizing bacteria on growth promotion and Zn nutrition of rice. J. Soil Sci. Plant Nutr. 14, 889–910. doi: 10.4067/S0718-95162014005000071
Wissuwa, M., Ismail, A. M., and Graham, R. D. (2008). Rice grain zinc concentrations as affected by genotype, native soil-zinc availability, and zinc fertilization. Plant Soil 306, 37–48. doi: 10.1007/s11104-007-9368-4
XLSTAT (2010). XLSTAT. Paris: Addinsoft SARL. Available at: http://www.xlstat.com.
Keywords: metal transporter, rice, zinc solubilizing bacteria, zinc uptake, ZIP genes
Citation: Krithika S and Balachandar D (2016) Expression of Zinc Transporter Genes in Rice as Influenced by Zinc-Solubilizing Enterobacter cloacae Strain ZSB14. Front. Plant Sci. 7:446. doi: 10.3389/fpls.2016.00446
Received: 28 December 2015; Accepted: 21 March 2016;
Published: 06 April 2016.
Edited by:
Gero Benckiser, University of Giessen (Retired), GermanyReviewed by:
Zakira Naureen, University of Nizwa, OmanChong Zhang, University of Maryland Baltimore County, USA
Copyright © 2016 Krithika and Balachandar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dananjeyan Balachandar, ZGJhbHVAdG5hdS5hYy5pbg==
 Selvaraj Krithika
Selvaraj Krithika Dananjeyan Balachandar
Dananjeyan Balachandar