- 1Laboratoire de Biotechnologie Valorisation et Protection des Agroressources, Faculté des Sciences et Techniques Guéliz, Université Cadi Ayyad, Marrakech, Morocco
- 2Tree and Timber Institute, National Research Council – Istituto per la Valorizzazione del Legno e delle Specie Arboree, Florence, Italy
- 3Institute of Agro-Environmental and Forest Biology, National Research Council – Istituto di Biologia Agroambientale e Forestale, Porano, Italy
- 4Laboratoire de Biotechnologie et Physiologie Végétales, Faculté des Sciences Semlalia, Université Cadi Ayyad, Marrakech, Morocco
The argan tree (Argania spinosa) occurs in a restricted area of Southwestern Morocco characterized by low water availability and high evapotranspirative demand. Despite the adaptation of the argan tree to drought stress, the extent of the argan forest has declined markedly due to increased aridity, land use changes and the expansion of olive cultivation. The oil of the argan seed is used for cooking and as the basis for numerous cosmetics. The identification of argan tree varieties with enhanced drought tolerance may minimize the economic losses associated with the decline of the argan forest and constrain the spread of desertification. In this study we collected argan ecotypes from four contrasting habitats and grew them under identical controlled environment conditions to investigate their response to drought. Leaf gas exchange analysis indicated that the argan ecotypes showed a high degree of adaptation to drought stress, maintaining photosynthetic activity at low levels of foliar water content and co-ordinating photosynthesis, stomatal behavior and metabolism. The stomata of the argan trees were highly sensitive to increased leaf to air vapor pressure deficit, representing an adaptation to growth in an arid environment where potential evapotranspiration is high. However, despite originating in contrasting environments, the four argan ecotypes exhibited similar gas exchange characteristics under both fully irrigated and water deficit conditions. Population genetic analyses using microsatellite markers indicated a high degree of relatedness between the four ecotypes; indicative of both artificial selection and the transport of ecotypes between different provinces throughout centuries of management of the argan forest. The majority of genetic variation across the four populations (71%) was observed between individuals, suggesting that improvement of argan is possible. Phenotypic screening of physiological responses to drought may prove effective in identifying individuals and then developing varieties with enhanced drought tolerance to enable the maintenance of argan production as climate change results in more frequent and severe drought events in Northern Africa.
Introduction
The argan tree (Argania spinosa) is endemic to Southwestern Morocco (Figure 1) occupying a semi-arid to arid habitat (Lefhaili, 2010). The fruit of the argan tree is an important livestock feed and the oil produced by the seed has become increasingly valued for cosmetic purposes (Charrouf and Guillaume, 2008; Lybbert et al., 2010). Despite the social, agricultural and economic importance of the argan tree (Lybbert et al., 2002), the area of argan forest decreased 44.5% between 1970 and 2007 (de Waroux and Lambin, 2012) as part of a longer decline since the 18th century (McGregor et al., 2009). A major cause of this loss of argan forest has been attributed to increased aridity leading to desertification (de Waroux and Lambin, 2012; Alba-Sanchez et al., 2015) and the expansion of olive cultivation in the native argan forest (Charrouf and Guillaume, 2009). Nonetheless, the argan tree is highly adapted to growth in conditions characterized by drought and high temperatures (Diaz-Barradas et al., 2010) where mean annual precipitation ranges from 150 to 400 mm and temperatures can rise above 40°C (Bani-Aameur and Zahidi, 2005; Msanda et al., 2005). The argan tree has a highly effective water transport system to exploit the available soil moisture (Ain-Lhout et al., 2016) and during severe drought sheds leaves to reduce transpirative water-loss (Diaz-Barradas et al., 2010; Zahidi and Bani-Aameur, 2013). There are comparatively few studies that investigate the photosynthetic and stomatal responses of the argan tree to drought. Previous studies have indicated differences in the leaf water potential, antioxidant activity (Diaz-Barradas et al., 2010; Chakhchar et al., 2015, 2016), leaf morphology (Diaz Barradas et al., 2013; Chakhchar et al., 2015) and chlorophyll fluorescence parameters (Diaz-Barradas et al., 2010) response to drought of argan trees collected from different habitats. Despite the restricted range of the argan tree (950,000 ha: Lefhaili, 2010), genetic analyses have indicated variation between accessions collected from different habitats (hereafter referred to as ecotypes; El Bahloul et al., 2014) that may underpin this variety of response to water deficit. In this study we analyzed the gas exchange responses to water deficit of four argan ecotypes collected from contrasting habitats (Figure 1 and Table 1). We hypothesize that the ecotypes from the most arid environments characterized by high evapotranspirative demand will exhibit the greatest tolerance to drought and enhanced water use efficiency (WUE). Alongside efforts to stabilize the argan forest through the creation of new plantations (Nouaim et al., 2002), analysis of physiological and genetic variability associated with increased drought tolerance may enable the identification of varieties that are more tolerant of increased aridity to prevent the further loss of argan forest area and to maintain the production of argan fruit, seeds and oil in its native habitat.
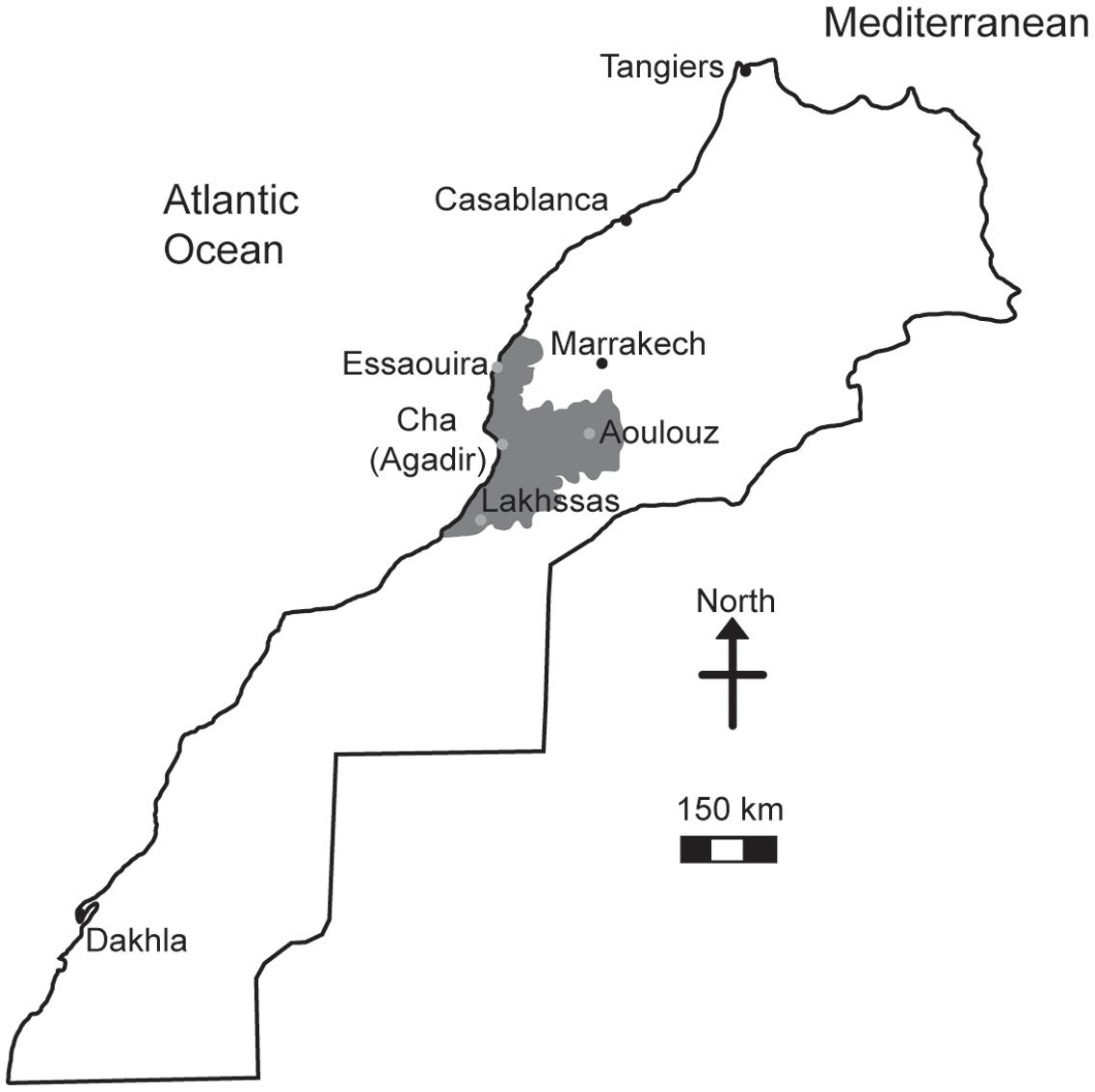
FIGURE 1. Map of Morocco showing the distribution of the argan forest (dark gray shading) and the four locations where the argan ecotypes were collected (light gray circles).
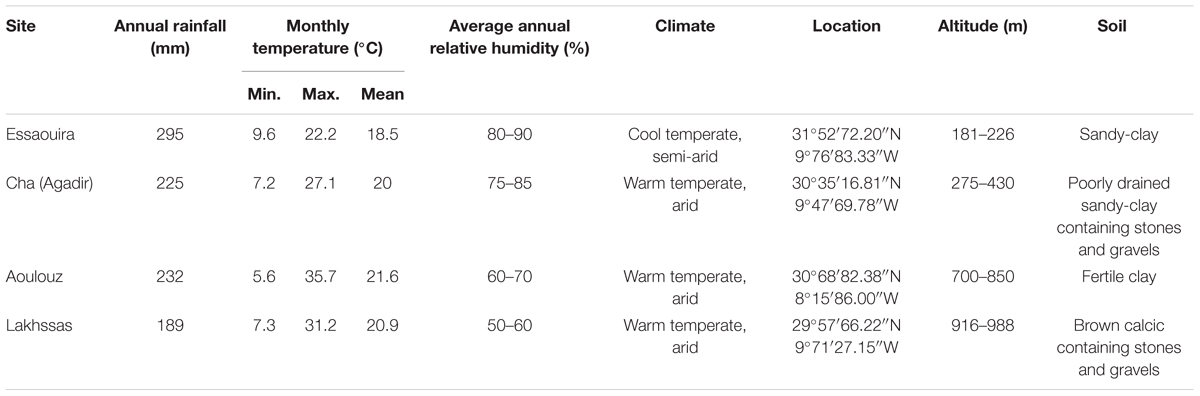
TABLE 1. A description of the sites where the argan ecotypes were collected (Figure 1).
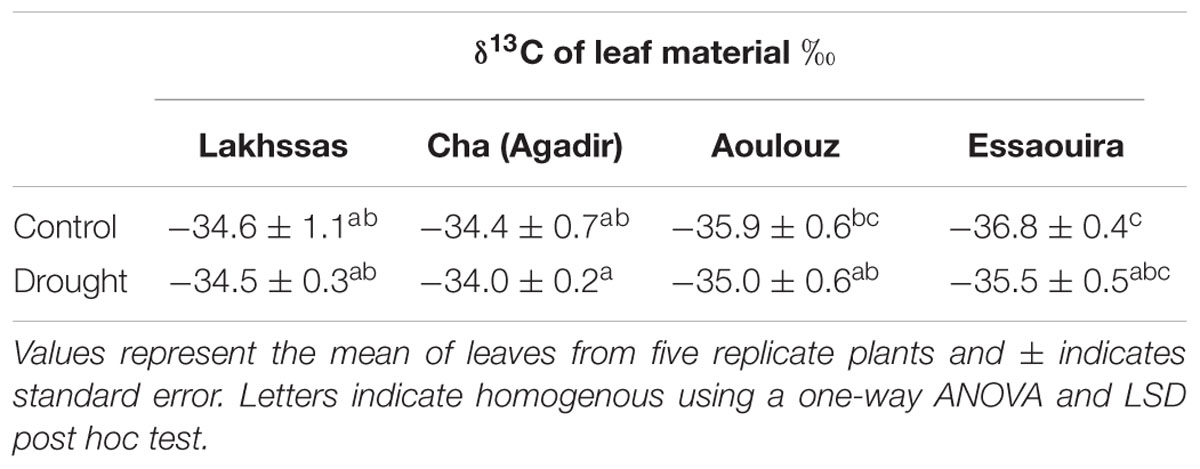
During episodes of drought, the level of water available in the soil for uptake by plants declines (Jones, 2007; Killi et al., 2016). As the root-zone soil dries, levels of free abscisic acid (ABA) in the leaves increase due to generation of ABA in the roots and transport in the xylem (Davies and Zhang, 1991), pH changes in the xylem sap (Wilkinson et al., 1998) and the conversion of glucose-conjugated ABA in the vacuole to free-ABA in the cytosol of leaf cells (Seiler et al., 2011). Higher [ABA] induces stomatal closure, reducing transpirative water-loss but also CO2-uptake for photosynthesis (PN; Tardieu and Davies, 1992; Sorrentino et al., 2016). As the concentration of CO2 in the sub-stomatal air-space (Ci) falls, an increasing proportion of CO2 composed of the heavier carbon-13 isotope (13C) is taken up for PN. Exposure to water deficit over a sufficient period of time can result in a shift in the carbon isotopic composition of the leaf as tissues become enriched in 13C (Farquhar and Richards, 1984). Analysis of foliar carbon isotopic composition can therefore provide an indication of long-term WUE (Farquhar et al., 1989b).
Argan trees collected from coastal, inland and mountainous regions of Morocco exhibited differential responses to seasonal changes in temperature, relative humidity (RH) and water availability. Despite occupying the habitat with the greatest mean annual precipitation, the argan trees from the mountainous habitat exhibited the largest reduction in leaf water potential during the summer, corresponding to the lowest PN values and quantum efficiency of CO2 assimilation of the three ecotypes (Diaz-Barradas et al., 2010). The argan trees growing under natural conditions showed close co-ordination of stomatal conductance (Gs) with leaf to air vapor pressure deficit (VPD; Diaz Barradas et al., 2013) and leaf water potential (Diaz-Barradas et al., 2010). This suggests that the physiological and gas exchange responses of argan trees are highly adapted to growth in a habitat characterized by high evaporative demand and low water availability. The stomata of many plants close in response to a reduction in atmospheric humidity which induces an increase in leaf to air VPD (Mott and Peak, 2013). However, the stomatal response to increased leaf to air VPD depends upon the adaptation of a plant to the prevailing growth conditions, with plants grown under conditions of high evapotranspirative demand exhibiting greater stomatal sensitivity to VPD (Bauerle et al., 2004). Comparison of argan ecotypes under common garden conditions suggested that ecotypes from more arid habitats exhibited greater foliar levels of anti-oxidant activity and lower water potential values than their counterparts from regions with higher levels of rainfall (Chakhchar et al., 2015, 2016). It may therefore be expected that ecotypes from diverse habitats possess contrasting photosynthetic and stomatal responses to drought associated with underlying genetic variation.
Phenotypic screening of the physiological characteristics of plants combined with genetic sequencing can enable the identification and development of traits and/or varieties with desirable attributes such as high productivity or tolerance to abiotic stress (Flexas, 2016). We selected argan ecotypes from habitats with contrasting growth conditions (Table 1). These ecotypes were grown under controlled environmental conditions in a common garden experiment and exposed to water deficit. This study aimed to: (i) investigate the PN and Gs responses of the argan tree to water deficit; (ii) gauge the stomatal adaptation of argan to growth in conditions of high evapotranspirative demand; (iii) characterize the WUE of the argan ecotypes to identify any traits/varieties that may confer improved tolerance to drought stress, and; (iv) assess genetic differences between the ecotypes that alongside their phenotypic responses to water deficit may be used to develop varieties of argan tree with enhanced tolerance to growth under water deficit conditions, and thus promote the stability of the native argan forest in Western Morocco (Figure 1).
Materials and Methods
Plant Material and Growth Conditions
Argan tree seedlings were collected from four localities: Lakhssas, a mountainous area of the Anti-Atlas mountains with the lowest mean annual precipitation of the sites; Cha is located at Agadir where the argan trees grow in a strongly maritime climate close to the Souss river, where the shallow water table provides high water availability for plant growth; Aoulouz is inland, upstream of the Souss valley, where RH is lower and water available for plant growth is lower than Cha (Agadir), and; Essaouira is the most northerly locality with an oceanic climate and highest annual rainfall. The location and description of the climate and soil types found at the sites are summarized in Figure 1 and Table 1. One hundred seedlings were collected from trees used for agricultural production of argan fruit in each province in conjunction with The Regional Centre for Forest Research, Marrakech. Forty five seedlings of equal height were then selected for comparison of physiological responses to drought. The argan seedlings were approximately 15 cm high and grown in pots (4 L, 15 cm diameter) filled with a 45:45:10 mixture of soil, peat, and perlite in a common garden in Marrakech for 2 months prior to the experimental study. The argan tree ecotypes were transferred to a large walk-in growth chamber to compare their gas exchange responses under identical growth conditions. The growth chamber conditions were 400 μmol m-2 s-1 photosynthetically active radiation (PAR) for 16 h per day, a day/night temperature regime of 28/25°C and RH of 80%. After a period of 3 months to fully acclimate to the conditions in the growth chambers, water was withheld to half of the plants until the soil water content reached 25% of the full soil water holding capacity. Soil water holding capacity was determined by filling the pots with water and allowing them to free drain for 24 h before being weighed, this weight was assumed to represent the soil water holding capacity of the pots (Killi et al., 2014). Water in the pots was replenished every 2 days. Soil water was maintained at 100% of soil holding capacity in the control plants. This drought treatment was maintained for 2 months prior to the collection of gas exchange measurements.
Leaf Gas Exchange and Analysis of Carbon Isotopic Composition
Measurement of leaf gas exchange was performed using a Li-Cor Li-6400 equipped with a 6400-05 conifer chamber (Li-Cor, Lincoln, NE, USA) between 08:00 and 12:00 each day. A metal halide light source was placed above the conifer cuvette at a height where PAR levels within the cuvette were 1000 μmol m-2 s-1. After each measurement, the area of the leaves within the cuvette was measured using a Li-Cor Li-3000 leaf area meter. Gas exchange parameters were then re-calculated using the corrected leaf area. Point measurements of PN, Gs and the internal sub-stomatal concentration of CO2 (Ci) were taken on five replicate plants for each ecotype/treatment. Conditions within the cuvette were 1000 μmol m-2 s-1 PAR, 28°C and a RH of 60%. Instantaneous water use efficiency (WUEi) was calculated as the ratio of PN to transpiration. The rate of respiration in the dark (RN) was determined by shutting off the light source and shading the leaves within the conifer cuvette for approximately 10 to 15 min until CO2 emission stabilized. This rate of CO2 emission was considered to represent RN (Lauteri et al., 2014). To investigate the effect of leaf to air VPD on stomatal behavior further instantaneous measurements of PN and Gs were conducted by reducing the RH of the air entering the cuvette (allowing a higher proportion of air to pass through the desiccant scrub tube) to increase leaf to air VPD. As stomata closed this resulted in an average 1.455 ± 0.0595°C increase in leaf temperature. After point measurements of gas exchange and measurement of leaf area, the leaves were destructively sampled and the relative water content of the leaves determined following Diaz-Pérez et al. (1995). To assess whether the argan ecotypes exhibit differences in photosynthetic capacity the response of PN to increasing Ci was determined in the two ecotypes from latitudinal extremes (Essaouira and Lakhssas). To prevent any stomatal limitation to PN the plants were exposed to a [CO2] level of 50 ppm for approximately 60 min to allow full stomatal opening. The level of [CO2] in the leaf cuvette was then rapidly increased in the following stages: 50, 100, 150, 200, 300, 500, 800, 1200, 1800, and 2200 ppm. Gas exchange parameters were recorded at each [CO2] level when PN had remained stable for approximately 1 min (Centritto et al., 2003). The response of PN to Ci was determined on five well-watered plants for each of the two argan ecotypes. The carboxylation capacity of ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO; Vcmax), the maximum rate of electron transport required for ribulose-1,5-bisphosphate (RuBP) regeneration (Jmax) and the conductance of CO2 across the mesophyll (Gm) were calculated following Ethier and Livingston (2004).
At the end of the experiment, leaf samples were collected for analysis of their stable carbon isotope composition. After collection, the leaves were dried at 60°C for 48 h until their weight remained stable. The leaves were then ground to a fine powder using a glass pestle and mortar. The ground leaf samples were then combusted in an elemental analyser (Model NA 1500, Carlo Erba, Milan, Italy) and the CO2 transferred in a helium flow to a continuous flow triple collector isotope ratio mass spectrometer (ISOPRIME, Manchester, UK). The isotope ratio 13C/12C was measured to calculate the samples’ carbon isotope composition (δ13C) relative to the VPDB (Vienna Pee Dee Belemnite) scale (Farquhar et al., 1989a).
Analysis of Genomic DNA
Microsatellite markers are highly effective in population genetics studies due to their high polymorphism, co-dominance, multiallelism, abundance, and uniform dispersion in plant genomics (Gupta et al., 1996). Genomic DNA of the argan ecotypes was isolated by grinding 50–60 mg of fresh leaf tissue in a 2 mL microcentrifuge tube containing a 5 mm diameter steel ball. The ground tissue was cooled in liquid nitrogen and then homogenized using a Mixer Mill 300 (Qiagen, Hilden, Germany). Genomic DNA was then extracted and purified using the DNeasy96 Plant Kit (Qiagen). Four microsatellite primers (Mh04; Mh07; mVpCIRB03; mVpCIRB05) developed on Manilkara huberi and Vitellaria paradoxa (Azevedo et al., 2005; Cardi et al., 2005) of the Sapotaceae family alongside A. spinosa were used to determine the genetic diversity. The unbiased probability of identity (PIunb; Peakall and Smouse, 2006) was computed for the combination of the six markers was between 0.01 and 0.08. This value indicates the probability that two unrelated trees selected at random from a population would have identical genotypes at multiple loci: the lower this value, the higher the capacity of the markers used to capture the variability in the data set. Polymerase chain reactions were conducted using a GeneAmp 2700 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). Twenty nanograms of genomic DNA was placed in 20 mL of reaction mix (Qiagen multiplex type-it kit) and exposed to the following cycles: 15 min at 95°C, 30 cycles for 30 s at 94°C, 90 s at 57°C, 1 min at 72°C and 30 min at 72°C. Amplification products (0.1 to 1 μL) were added to 20 μL formamide and 0.3 μL Genescan-500 ROX (Applied Biosystems, Foster City, CA, USA) and denatured at 95°C for 5 min. The samples were then run on an ABI PRISM 3100 DNA sequencer (Applied Biosystems).
Genotyper 3.7 software was used to score the alleles (Applied Biosciences). The programs Popgene 3.2 (Yeh et al., 1997) and GeneAlEx6 (Peakall and Smouse, 2006) were used to statistically assess intra and inter population genetic diversity. The total (N), observed (Na) and effective (Ne) number of alleles and then observed (Ho) and expected (He) heterozygosity were calculated. The Shannon Index (I) was calculated to characterize species diversity (Keylock, 2005) and unbiased heterozygosity (UHe) and the inbreeding co-efficient (Fis) were determined (Nei, 1978). The Nei genetic distance (Nei, 1978) and Unbiased Nei genetic distance (Nei and Roychoudhury, 1974) values were then used to generate unweighted pair group clustering (UPGMA: Unweighted Pair Group Method with Arithmetic Mean) using the software program POPTREE 2 (Takezaki et al., 2010). Analysis of molecular variance (AMOVA) was also performed to assess differences in population genetics between the argan ecotypes (Excoffier et al., 1992).
Results
Leaf Gas Exchange and Water Use Efficiency
Growth in soils with water levels of 25% soil holding capacity induced reductions of 25 to 31% in the RWC of argan leaves; despite the moderate nature of the differences between ecotypes these were significant (Figure 2). The Aoulouz ecotype from the mountainous inland habitat showed the most pronounced reduction in RWC. The maritime influenced Essaouira and Lakhssas ecotypes from the latitudinal extremes of the argan forest (Figure 1) with the highest and lowest levels of precipitation both showed the lowest proportional reductions in RWC of ∼25% following water deficit (Figure 2). Under control and drought stress conditions the four argan ecotypes exhibited largely identical gas exchange characteristics (Figure 3). No statistical difference was observed in PN (control, P = 0.556, F3,15 = 0.733; drought P = 0.773, F3,15 = 0.375), Gs (control, P = 0.711, F3,15 = 0.468; drought P = 0.770, F3,15 = 0.379), WUEi (control, P = 0.293, F3,15 = 1.422; drought P = 0.704, F3,15 = 0.379) or Ci (control, P = 0.353, F3,15 = 1.220; drought P = 0.738, F3,15 = 0.428) between the four ecotypes under control or drought growth conditions. Water deficit reduced levels of PN by 42–53% from 6–7.4 to 3.0–3.5 μmol m-2 s-1 (Figure 3A). This corresponded to a reduction in Gs values by 64–69% (Figure 3B). The reduction in levels of both PN and Gs in plants grown in soils with lower water availability did not result in any change in WUEi values (Figure 3C). This was consistent with no significant alteration in the δ13C values of the leaves of the argan ecotypes that experienced drought stress (Table 2). Analysis of the response of PN to Ci in the Essaouira and Lakhssas ecotypes from the latitudinal extremes of the range of the argan tree indicated that there was no significant difference in underlying photosynthetic capacity between the two ecotypes (Figure 4), consistent with the similarity in PN values recorded during point measurements of leaf gas exchange (Figure 3A). However, significant differences were found in the foliar δ13C values of the argan ecotypes under control conditions. The Essaouira ecotype from the habitat with the highest mean annual precipitation exhibited the most negative δ13C values, whilst the leaves of the Lakhssas ecotype exhibited the most positive δ13C values. However, a negative relationship between foliar δ13C and the mean annual precipitation of the source region of the ecotypes was not observed (linear regression, F1,2 = 5.910; P = 0.136). PN rates of the argan trees were positively related to Gs (Figure 5A). Levels of Rn were also positively related to PN (Figure 5B), indicative of co-ordination of metabolic and photosynthetic activity. Levels of both PN (Figure 5C) and Gs (Figure 5D) declined with increasing leaf to air VPD, indicative of the modification of stomatal behavior to increasing potential evapotranspiration.
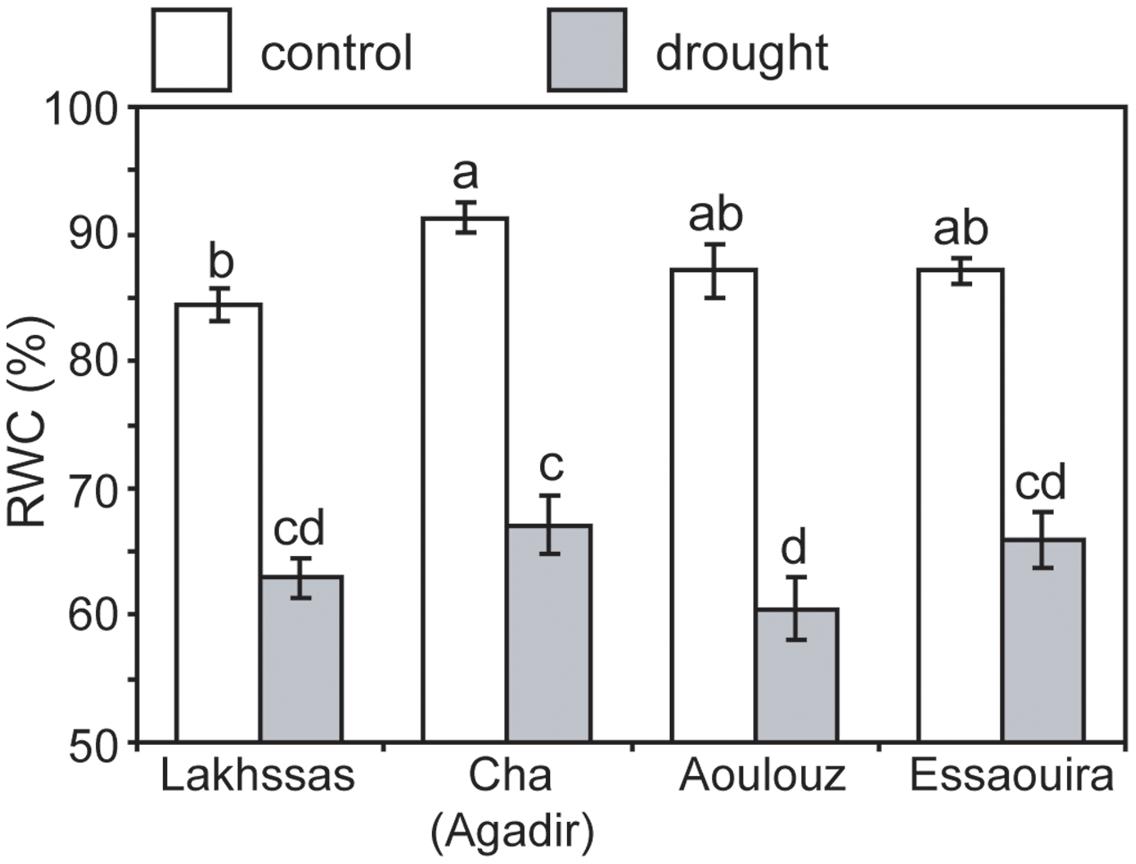
FIGURE 2. Relative water content of argan leaves of the four ecotypes under control (white) and drought (gray) conditions. Error bars indicate the standard error of five replicates. Letters denote homogenous groups using a one-way ANOVA and LSD post hoc test.
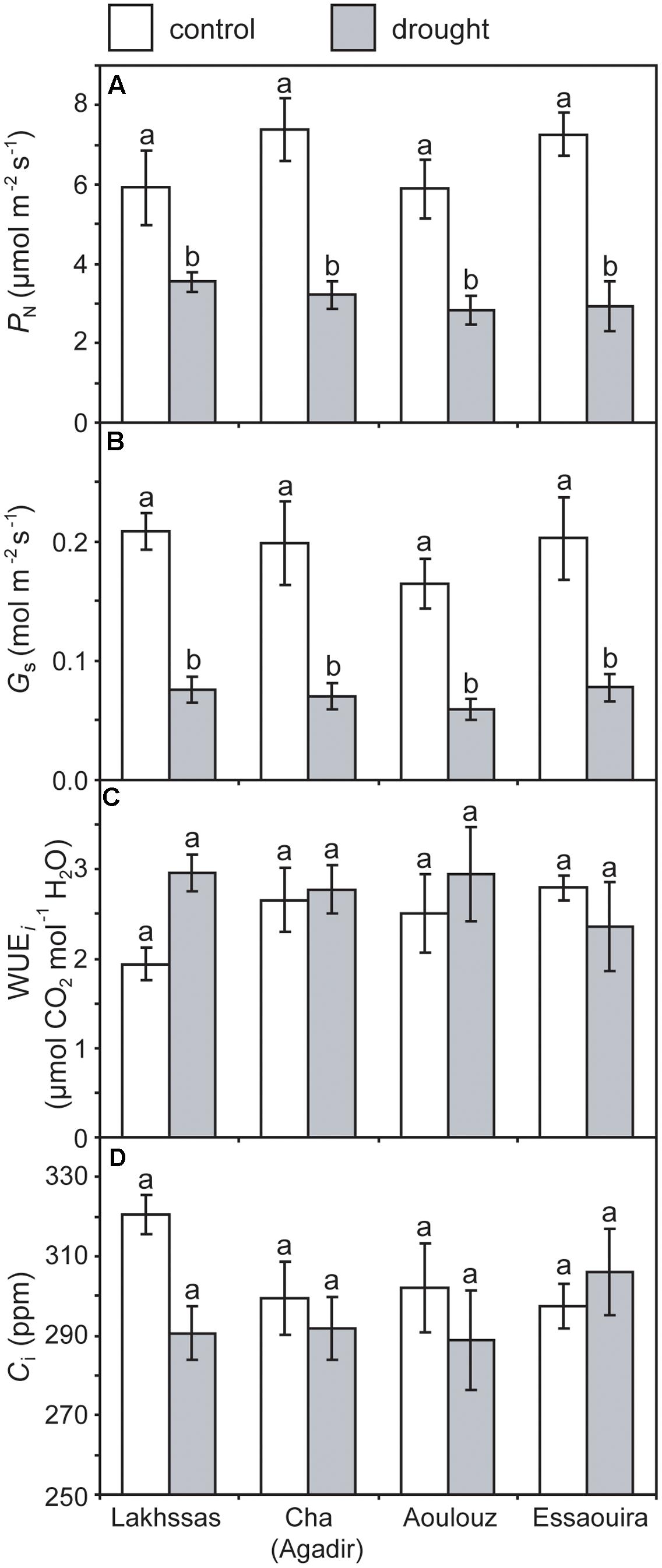
FIGURE 3. Photosynthesis (PN) (A), stomatal conductance (Gs) (B), instantaneous water use efficiency (WUEi) (C) and internal sub-stomatal concentration of CO2 (D) of the four argan ecotypes under control (white) and drought (gray) conditions. Error bars indicate the standard error of five replicates. Letters denote homogenous groups using a one-way ANOVA and LSD post hoc test.
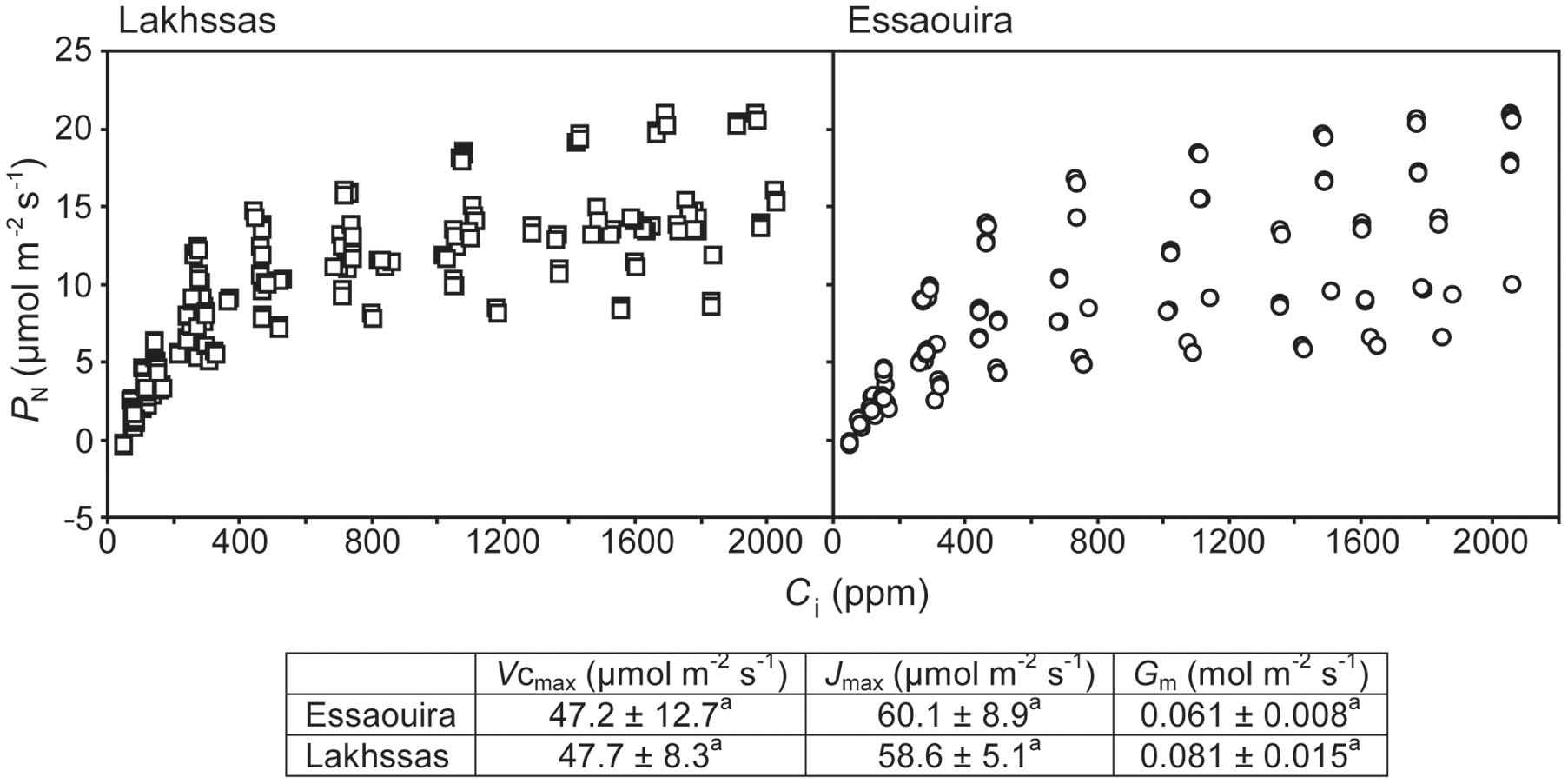
FIGURE 4. The relationship between PN and sub-stomatal [CO2] (Ci) of the Lakhssas and Essaouira argan ecotypes from the latitudinal extremes of the range of the argan forest (Figure 1). The carboxylation capacity of ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO; Vcmax), the maximum rate of electron transport required for ribulose-1,5-bisphosphate (RuBP) regeneration (Jmax) and the conductance of CO2 across the mesophyll (Gm) were calculated following Ethier and Livingston (2004). ± indicates one standard error. Letters denote homogenous groups using a one-way ANOVA and LSD post hoc test.
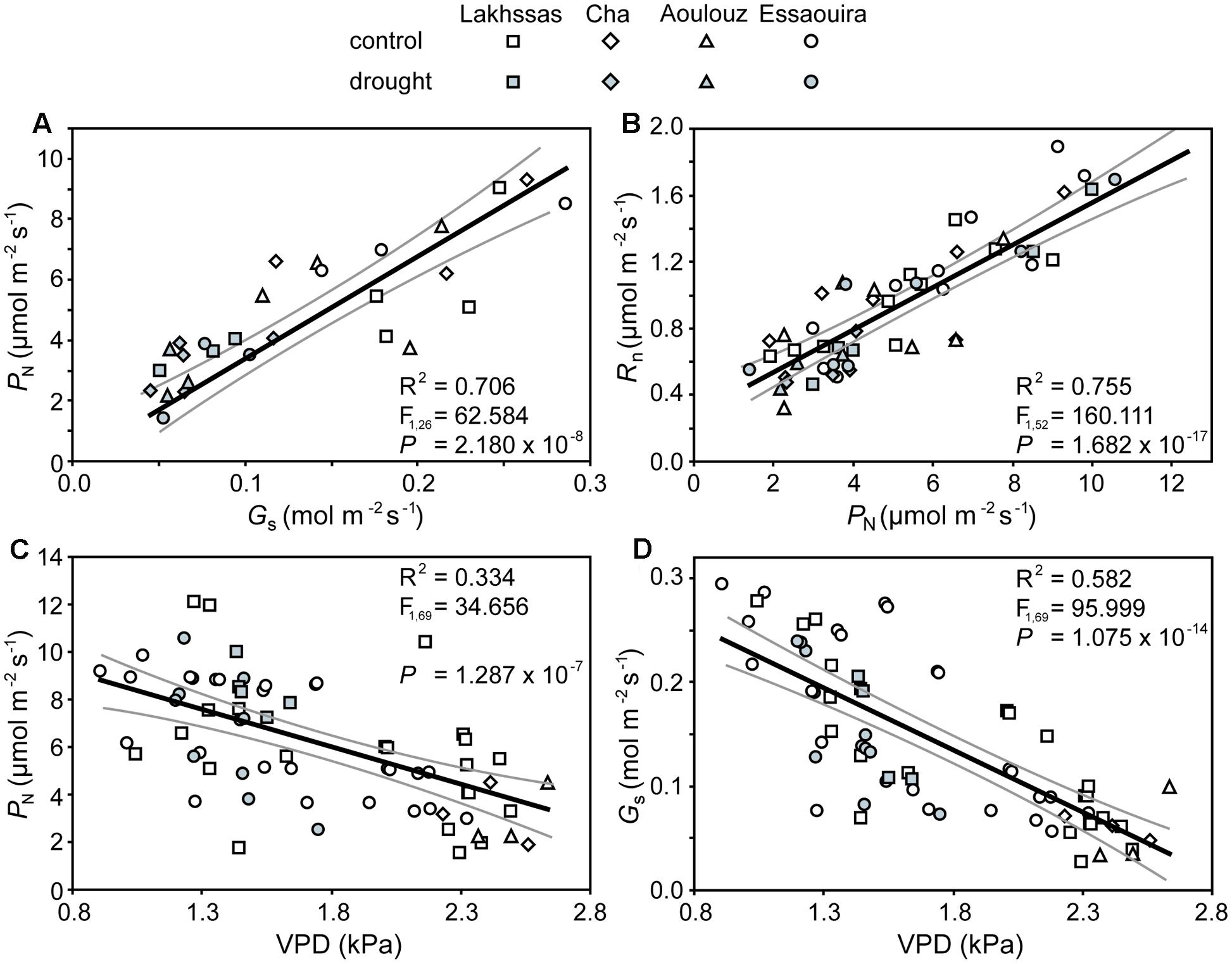
FIGURE 5. Co-ordination of PN, Gs and respiration in the dark (RN) of the argan leaves in response to drought and increased leaf to air vapour pressure deficit (VPD): (A) rates of PN and Gs; (B) the relationship between RN and PN; (C) the effect of increased leaf to air VPD on PN, and; (D) the effect of increased leaf to air VPD on Gs. Linear regression was performed to determine the significance of these relationships. The central black line indicates the line of best fit. The gray lines either side of the best fit line indicate 95% confidence intervals of the mean.
Genetic Analyses
The four SSR markers produced reproducible amplifications that allowed the argan ecotypes to be distinguished. The mean number of alleles per locus was 2.85. Heterozygosis ranged from 0.00 (locus mVpCIRB03) to 0.65 (locus Mh07) with a mean of 0.305 per population (Table 3). For all loci, the He was larger than the observed (Ho) with the exception of Aoulouz, possibly indicative of non-random mating due to the presence of null alleles. At the population level, higher values of heterozygosity were observed for the Essaouira ecotype; however, this and the Agadir ecotypes showed significant and positive Fis consistent with a population inbreeding. All loci were polymorphic in the Essaouira ecotype, while the lowest level of polymorphism (50%) was observed in the Aoulouz ecotype (Table 3).
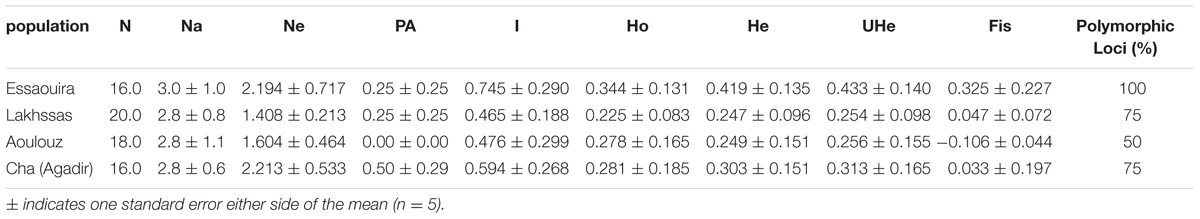
TABLE 3. Genetic diversity of four Argan populations: N, number of individuals, Ne, mean effective number of alleles per locus; Na, mean number of alleles per locus; PA, number of private alleles; Ho, observed heterozygosity; He, expected heterozygosity; I, Shannon index; UHe, unbiased heterozygosity, and; Fis, inbreeding coefficient.
A higher number of effective alleles were observed in the Essaouira and Cha (Agadir) ecotypes in comparison to those derived from Lakhssas and Aoulouz (Table 3). The Cha (Agadir) ecotype showed the highest number of private alleles (PRA = 0.5), and alongside the Essaouira ecotype showed the highest level of genetic diversity (Cha, I = 0.594; Essaouira, I = 0.745). The lowest genetic variability was observed in the plants of the Lakhssas ecotype (I = 0.465). The Essaouira ecotype exhibited a significantly positive Fis-value of 0.325, indicative of inbreeding within the population. The UPGMA analysis indicated a low level of genetic divergence between the populations of ecotypes and no correspondences between the genetic and geographic distance (Table 4); the Nei biased (Figure 6A) and unbiased (Figure 6B) analyses produced contrasting results. The Nei biased analysis indicated that Essaouira and Cha (Agadir) were the two most divergent populations of ecotypes (Figure 6A) despite both occupying coastal habitats (Figure 1). The unbiased Nei analysis instead suggested that the coastal Essaouira and mountainous Aoulouz ecotypes were the most divergent (Figure 6B). The AMOVA analysis (Figure 6C) indicated 71% of variation occurred within the individuals analyzed, while the variation between different ecotype populations amounted to 23%.
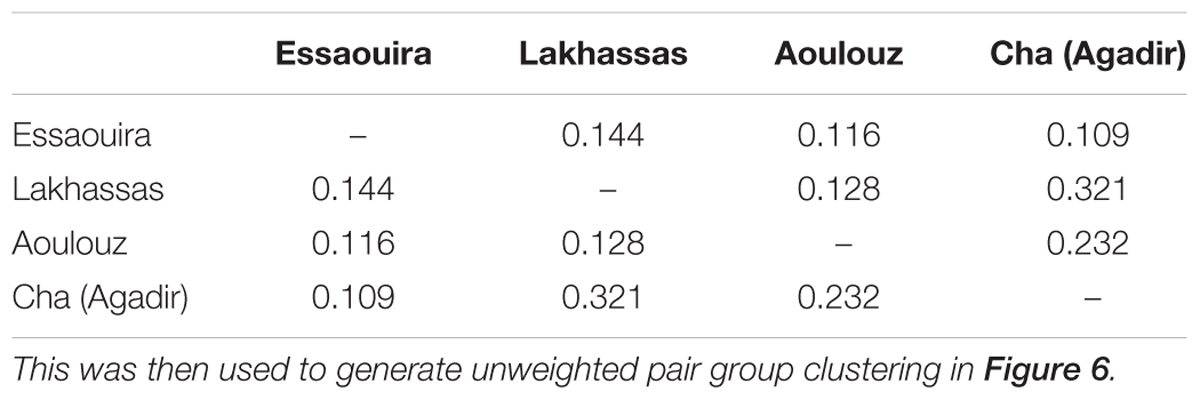
TABLE 4. Pairwise population dissimilarity matrix of inbreeding co-efficient (Fis) values of the argan ecotypes.
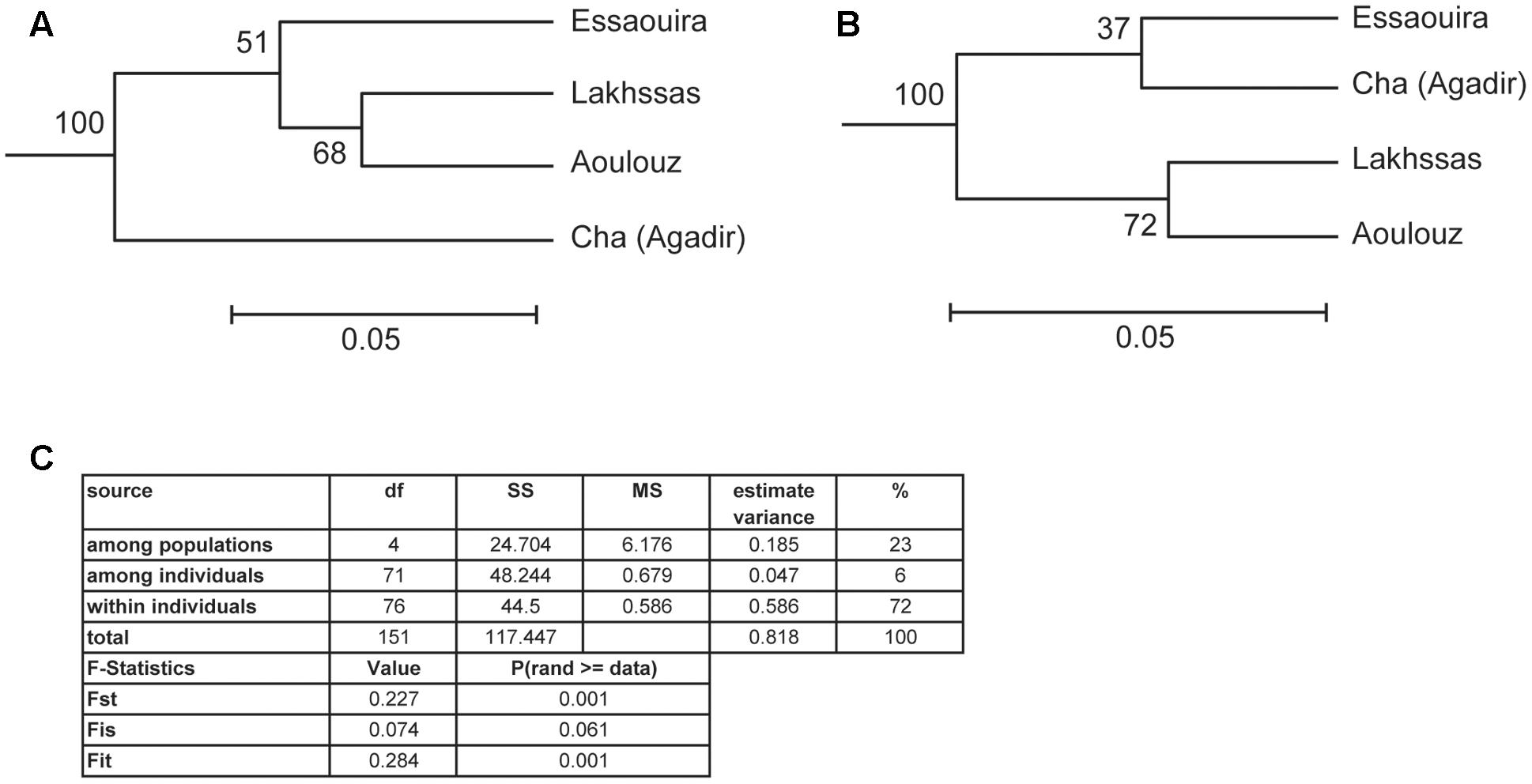
FIGURE 6. Unweighted Pair Group Method with Arithmetic Mean (UPGMA) clustering analysis of the argan ecotypes based on: (A) Nei biased, and (B) Nei unbiased. Scale indicates the genetic distance of Nei (1978) and suggests low genetic distance between the argan ecotypes collected from different provinces. (C) Analysis of molecular variance (AMOVA) analysis and F statistic of the argan ecotypes (Excoffier et al., 1992).
Discussion
The native argan forest of Western Morocco covers an area of 950,000 ha (Lefhaili, 2010). Despite this relatively restricted distribution, the argan forest occupies a range of diverse habitats ranging from cooler mountainous regions to plains where the climate is considerably warmer, and coastal to inland environments where RH and leaf to air VPD vary widely. It may therefore be expected to observe physiological and genetic variability between argan ecotypes adapted to these contrasting habitats; particularly, as under natural growth conditions argan trees exhibit differential photosynthetic and stomatal characteristics in this range of habitats (Diaz-Barradas et al., 2010). However, the results of this study showed little evidence to suggest physiological or genetic divergence between the populations of argan ecotypes analyzed; although a higher resolution of measurements may show differences in the progressive response of the argan ecotypes as soil dries.
Leaf Gas Exchange Responses of Argan Ecotypes to Drought
Under drought stress argan dramatically modifies the level of osmolytes within leaves (Chakhchar et al., 2015). This may account for the maintenance of PN and Gs to 3.5 μmol m-2 s-1 and 0.075 mol m-2 s-1 (Figure 3), respectively, when foliar RWC had fallen to ∼60% (Figure 2). Foliar water content is a strong controller of stomatal opening in many plants (Brown et al., 1976; Saliendra et al., 1995; Franks, 2013). In other drought adapted species such as olive (Olea europaea), a decline in RWC of 25 to 30%, equivalent to that observed in the argan ecotypes (Figure 2), resulted in a 94% reduction in Gs values and rates of respiration to exceed photosynthetic CO2 assimilation (i.e., negative PN values; Sun et al., 2014). This indicates that argan possesses a high degree of osmoregulation, effective root systems for water uptake and also the capacity to maintain carboxylation and protective secondary metabolism during episodes of water deficit. The decline in Gs values following growth at 25% soil holding capacity for 2 months did not induce a significant reduction in Ci values (Figure 3D). Lower Ci under drought stress results in lower availability of CO2 for PN (Flexas et al., 2002; Lauteri et al., 2014). The maintenance of Ci values under drought stress may be a function of lower demand associated with reduced carboxylation negating the impact of lower diffusion of on Ci values (accounting for the similarity in WUEi values under control and drought stress conditions), the plants not closing stomata fully to maintain a degree of Gs (e.g., Haworth et al., 2015) or may reflect an adaptation of argan to drought stress that allows the retention of photosynthetic activity during episodes of reduced water availability (Figure 3). The lack of any significant alteration in the δ13C of the leaves of the argan ecotypes (Table 2) is likely associated with the constancy of Ci levels between control and drought treatments resulting in no change in discrimination of CO2 composed of the heavier 13C isotope (Farquhar et al., 1989b). The carbon isotopic measurements performed in this study involved analysis of the bulk leaf material. As such, the structural material within the leaf may reflect pre-stress growth conditions. Compound specific analysis of recently synthesized sugars may enable identification of the impact of drought stress on discrimination of carbon isotopes during CO2 assimilation in the argan ecotypes (e.g., Lauteri et al., 2014). The current dataset is comparatively variable, with a 2.4‰ range of δ13C values in control plants; this may constrain the effectiveness of bulk leaf carbon isotopes as a phenotyping tool in studies of argan and other sclerophylls such as olive (e.g., Sun et al., 2014). The soil water content of the pots was controlled every 2 days, this may have allowed the plants to utilize the proportion of water available for growth before water levels were replenished. The high frequency replacement of lost water to maintain a constant field capacity may not represent drought stress likely to occur under field conditions (Earl, 2003; Nemali and van Iersel, 2006). A lower field capacity or less frequent replenishment of soil water levels may result in the further stomatal closure and corresponding decline in Ci and PN recorded in olive trees (Marino et al., 2014; Sun et al., 2014; Dbara et al., 2016).
The argan ecotypes showed identical reductions in Gs to reduce transpirative water-loss following growth under water deficit conditions (Figure 3B). Gs can be regulated via physiological regulation of stomatal aperture or modification of stomatal numbers in developing leaves (Haworth et al., 2015). Analysis of stomatal numbers in argan ecotypes collected from three contrasting regions indicated no population effect on stomatal density values (Bani-Aameur and Zahidi, 2005), suggesting that any ecotypic difference in stomatal control would be through active physiological behavior (e.g., Tomimatsu and Tang, 2012). The argan ecotypes analyzed in this study exhibited a high degree of active physiological stomatal behavior that allowed modification of Gs in response to changes in water availability (Figure 3B) and transportive demand (Figure 5D). The close co-ordination of PN and Gs (Figure 5A) under control and water deficit conditions is consistent with other species adapted to growth in arid environments (e.g., Flexas et al., 2002; Marino et al., 2014; Sun et al., 2014). The link between PN and the diffusive resistance to CO2 is also present during short-term variation in leaf to air VPD (Figure 5C) as the stomata close when evapotranspirative demand increases (Figure 5D). Over a range of leaf to air VPD of 0.8 to 2.8 the argan ecotypes exhibited a reduction of Gs values of 90%. In comparison, species from more mesic environments with higher water availability and lower potential evapotranspiration such as beech (Fagus sylvatica), chestnut (Castanea sativa) and oak (Quercus robur) showed respective Gs reductions of 33, 52, and 43% to an equivalent increase in leaf to air VPD (Heath, 1998). This stomatal sensitivity to VPD indicates that the argan tree possesses highly functional stomata. Moreover, the relationship between PN, Gs and RN is indicative of a high level of co-ordination between mesophyll PN and the regulation of stomatal aperture (Messinger et al., 2006; Hu et al., 2010; Engineer et al., 2016), as would be expected for a tree growing in an arid environment with high levels of evapotranspiration (Lefhaili, 2010) and risk of xylem embolism (Meinzer et al., 2009).
Population Genetics of the Argan Ecotypes
Despite being collected from diverse habitats, the four argan ecotypes exhibited similar phenotypic responses to water deficit (Figure 3 and Table 2). The four argan ecotypes exhibited similar photosynthetic and stomatal responses to growth in drought stressed conditions. This may suggest that the selective pressures experienced by the populations of argan ecotypes resulted in similar gas exchange responses as all of the habitats were characterized by comparatively low water availability (<300 mm per annum) and high evaporative demand (Table 1). Our results confirm the efficiency of using non-species-specific SSR markers in studies of genetic diversity. The possibility of using SSRs markers to perform cross-species amplification is an important tool to study the genetic characteristics of two or more species (Curtu et al., 2004; Sharma et al., 2009) Molecular ecologists increasingly require ‘universal’ genetic markers that can easily be transferred between species (Barbara et al., 2007). Moreover, despite the limited number of primers used the low value of PI confirms the reliability of the markers used in this study. Analysis of SSR markers in nine populations of argan trees suggested no difference in the number of observed (Ho) and expected (He) alleles (El Bahloul et al., 2014); indicative of the absence of evolutionary selective pressures influencing allele and genotype frequencies (Emigh, 1980). Previous analyses of isozymes (El Mousadik and Petit, 1996b), chloroplast DNA (El Mousadik and Petit, 1996a) and SSR markers (Majourhat et al., 2008) have suggested low diversity in the argan ecotypes studied. The analysis of SSR markers in this study would also be consistent with low genetic diversity among the argan ecotypes (Figure 6 and Table 4). The population genetics of the argan ecotypes suggests a high degree of artificial selection (cf. El Bahloul et al., 2014), possibly associated with human management of the argan forest over 100s of years (Ruas et al., 2016). As the native argan forest occurs over a comparatively small area (Lefhaili, 2010) the population genetic analyses undertaken in this study would suggest that seedlings have been traded and moved between regions. Moreover, the increase in the intensity of grazing in recent years (Mellado, 1989; Alados and El Aich, 2008) may have prevented the establishment of smaller trees that have resulted from sexual reproduction; thus preventing the operation of drought induced selective pressures on any genetic variation resulting from sexual reproduction.
Development of Increased Drought Tolerance in Argan
The native argan forest of Western morocco has been managed for hundreds of years (Ruas et al., 2011, 2016). This has resulted in argan populations from different areas being strongly related (Tables 3, 4) (El Mousadik and Petit, 1996a,b; Majourhat et al., 2008). The argan ecotypes analyzed in this study showed a similar high degree of adaptation to drought stress in terms of gas exchange, metabolism, and photosynthetic activity (Figures 3–5). Nevertheless, this study has indicated that the vast majority of the variation within the argan ecotypes (71%) occurred within individuals (Figure 6). This raises the possibility that phenotypic screening (Kamoshita et al., 2008) and analysis of DNA/RNA (Deyholos, 2010) could be used to identify and develop traits/varieties that confer further drought tolerance. The use of rapid phenotyping techniques such as chlorophyll fluorescence and reflectance (Furbank and Tester, 2011; Fiorani and Schurr, 2013) would be highly effective in quickly assessing large numbers of individuals to identify those with favorable performance during water deficit for more in-depth gas exchange and molecular analysis. A series of common garden experiments at different locations within the range of the argan forest would permit selection of individuals suited to growth during water deficit in the specific conditions of mountains, coastal and inland habitats that make-up the argan forest. The identification of individual argan trees with enhanced drought tolerance could be an effective tool in minimizing the further loss of argan forest and preventing desertification.
Author Contributions
AC, CEM, ML, CM, SW, and MC conducted the experiment. MH and MC wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We gratefully acknowledge funding from the European Commission FP7 project WATBIO (311929).
References
Ain-Lhout, F., Boutaleb, S., Diaz-Barradas, M. C., Jauregui, J., and Zunzunegui, M. (2016). Monitoring the evolution of soil moisture in root zone system of Argania spinosa using electrical resistivity imaging. Agric. Water Manag. 164, 158–166. doi: 10.1016/j.agwat.2015.08.007
Alados, C. L., and El Aich, A. (2008). Stress assessment of argan (Argania spinosa (L.) Skeels) in response to land uses across an aridity gradient: translational asymmetry and branch fractal dimension. J. Arid Environ. 72, 338–349. doi: 10.1016/j.jaridenv.2007.06.015
Alba-Sanchez, F., Antonio Lopez-Saez, J., Nieto-Lugilde, D., and Svenning, J.-C. (2015). Long-term climate forcings to assess vulnerability in North Africa dry argan woodlands. Appl. Veg. Sci. 18, 283–296. doi: 10.1111/avsc.12133
Azevedo, V., Vinson, C., and Ciampi, A. (2005). Twelve microsatellite loci in Manilkara huberi (Ducke) standl (Sapotaceae), an Amazonian timber species. Mol. Ecol. Notes 5, 13–15. doi: 10.1111/j.1471-8286.2004.00815.x
Bani-Aameur, F., and Zahidi, A. (2005). Variability of leaf stomatal density of adult trees of Argania spinosa (L.) Skeels in the field. Acta Bot. Gallica 152, 281–288. doi: 10.1080/12538078.2005.10515490
Barbara, T., Palma-Silva, C., Paggi, G. M., Bered, F., Fay, M. F., and Lexer, C. (2007). Cross-species transfer of nuclear microsatellite markers: potential and limitations. Mol. Ecol. 16, 3759–3767. doi: 10.1111/j.1365-294X.2007.03439.x
Bauerle, W., Whitlow, T., Setter, T., and Vermeylen, F. (2004). Abscisic acid synthesis in Acer rubrum L. leaves—a vapour-pressure-deficit-mediated response. J. Am. Soc. Hortic. Sci. 129, 182–187.
Brown, K., Jordan, W., and Thomas, J. (1976). Water stress induced alterations of the stomatal response to decreases in leaf water potential. Physiol. Plant. 37, 1–5. doi: 10.1111/j.1399-3054.1976.tb01863.x
Cardi, C., Vaillant, A., Sanou, H., Kelly, B. A., and Bouvet, J. (2005). Characterisation of microsatellite markers in the shea tree (Vitellaria paradoxa C. F Gaertn) in Mali. Mol. Ecol. Notes 5, 524–526. doi: 10.1111/j.1471-8286.2005.00980.x
Centritto, M., Loreto, F., and Chartzoulakis, K. (2003). The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ. 26, 585–594. doi: 10.1046/j.1365-3040.2003.00993.x
Chakhchar, A., Lamaoui, M., Aissam, S., Ferradous, A., Wahbi, S., El Mousadik, A., et al. (2016). Differential physiological and antioxidative responses to drought stress and recovery among four contrasting Argania spinosa ecotypes. J. Plant Interact. 11, 30–40. doi: 10.1080/17429145.2016.1148204
Chakhchar, A., Lamaoui, M., Wahbi, S., Ferradous, A., El Mousadik, A., Ibnsouda-Koraichi, S., et al. (2015). Leaf water status, osmoregulation and secondary metabolism as a model for depicting drought tolerance in Argania spinosa. Acta Physiol. Plant. 37, 80. doi: 10.1007/s11738-015-1833-8
Charrouf, Z., and Guillaume, D. (2008). Argan oil: occurrence, composition and impact on human health. Eur. J. Lipid Sci. Technol. 110, 632–636. doi: 10.1002/ejlt.200700220
Charrouf, Z., and Guillaume, D. (2009). Sustainable development in Northern Africa: the argan forest case. Sustainability 1, 1012–1022. doi: 10.3390/su1041012
Curtu, A.-L., Finkeldey, R., and Gailing, O. (2004). Comparative sequencing of a microsatellite locus reveals size homoplasy within and between European oak species (Quercus spp.). Plant Mol. Biol. Rep. 22, 339–346. doi: 10.1007/BF02772677
Davies, W. J., and Zhang, J. H. (1991). Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 55–76. doi: 10.1146/annurev.pp.42.060191.000415
Dbara, S., Haworth, M., Emiliani, G., Mimoun, M. B., Gómez-Cadenas, A., and Centritto, M. (2016). Partial root-zone drying of olive (Olea europaea var.’Chetoui’) induces reduced yield under field conditions. PLoS ONE 11:e0157089. doi: 10.1371/journal.pone.0157089
de Waroux, Y. P., and Lambin, E. F. (2012). Monitoring degradation in arid and semi-arid forests and woodlands: the case of the argan woodlands (Morocco). Appl. Geogr. 32, 777–786. doi: 10.1016/j.apgeog.2011.08.005
Deyholos, M. K. (2010). Making the most of drought and salinity transcriptomics. Plant Cell Environ. 33, 648–654. doi: 10.1111/j.1365-3040.2009.02092.x
Diaz-Barradas, M. C., Zunzunegui, M., Ain-Lhout, F., Jauregui, J., Boutaleb, S., Alvarez-Cansino, L., et al. (2010). Seasonal physiological responses of Argania spinosa tree from Mediterranean to semi-arid climate. Plant Soil 337, 217–231. doi: 10.1007/s11104-010-0518-8
Diaz Barradas, M. C., Zunzunegui, M., Paz Esquivias, M., Boutaleb, S., Valera-Burgos, J., Tagma, T., et al. (2013). Some secrets of Argania spinosa water economy in a semiarid climate. Nat. Prod. Commun. 8, 11–14.
Diaz-Pérez, J. C., Shackel, K. A., and Sutter, E. G. (1995). Relative water content and water potential of tissue 1. J. Exp. Bot. 46, 111–118. doi: 10.1093/jxb/46.1.111
Earl, H. J. (2003). A precise gravimetric method for simulating drought stress in pot experiments. Crop Sci. 43, 1868–1873. doi: 10.2135/cropsci2003.1868
El Bahloul, Y., Dauchot, N., Machtoun, I., Gaboun, F., and Van Cutsem, P. (2014). Development and characterization of microsatellite loci for the Moroccan endemic endangered species Argania spinosa (sapotaceae). Appl. Plant Sci. 2:1300071. doi: 10.3732/apps.1300071
El Mousadik, A., and Petit, R. J. (1996a). Chloroplast DNA phylogeography on the argan tree of Morocco. Mol. Ecol. 5, 547–555.
El Mousadik, A., and Petit, R. J. (1996b). High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor. Appl. Genet. 92, 832–839. doi: 10.1007/BF00221895
Emigh, T. H. (1980). A comparison of tests for Hardy-Weinberg equilibrium. Biometrics 36, 627–642. doi: 10.2307/2556115
Engineer, C. B., Hashimoto-Sugimoto, M., Negi, J., Israelsson-Nordström, M., Azoulay-Shemer, T., Rappel, W.-J., et al. (2016). CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci. 21, 16–30. doi: 10.1016/j.tplants.2015.08.014
Ethier, G. J., and Livingston, N. J. (2004). On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant Cell Environ. 27, 137–153. doi: 10.1111/j.1365-3040.2004.01140.x
Excoffier, L., Smouse, P. E., and Quattro, J. M. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131, 479–491.
Farquhar, G. D., Ehleringer, J. R., and Hubick, K. T. (1989a). Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537. doi: 10.1146/annurev.pp.40.060189.002443
Farquhar, G. D., Hubick, K. T., Condon, A. G., and Richards, R. A. (1989b). “Carbon isotope fractionation and plant water-use efficiency,” in Stable Isotopes in Ecological Research, eds P. W. Rundel and J. R. Ehleringer (Berlin: Springer), 21–40.
Farquhar, G. D., and Richards, R. A. (1984). Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct. Plant Biol. 11, 539–552. doi: 10.1071/pp9840539
Fiorani, F., and Schurr, U. (2013). Future scenarios for plant phenotyping. Annu. Rev. Plant Biol. 64, 267–291. doi: 10.1146/annurev-arplant-050312-120137
Flexas, J. (2016). Genetic improvement of leaf photosynthesis and intrinsic water use efficiency in C 3 plants: why so much little success? Plant Sci. 251, 155–161. doi: 10.1016/j.plantsci.2016.05.002
Flexas, J., Bota, J., Escalona, J. M., Sampol, B., and Medrano, H. (2002). Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 29, 461–471. doi: 10.1071/PP01119
Franks, P. J. (2013). Passive and active stomatal control: either or both? New Phytol. 198, 325–327. doi: 10.1111/nph.12228
Furbank, R. T., and Tester, M. (2011). Phenomics–technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 16, 635–644. doi: 10.1016/j.tplants.2011.09.005
Gupta, P., Balyan, H., Sharma, P., and Ramesh, B. (1996). Microsatellites in plants: a new class of molecular markers. Curr. Sci. 70, 45–54.
Haworth, M., Killi, D., Materassi, A., and Raschi, A. (2015). Co-ordination of stomatal physiological behavior and morphology with carbon dioxide determines stomatal control. Am. J. Bot. 102, 677–688. doi: 10.3732/ajb.1400508
Heath, J. (1998). Stomata of trees growing in CO2-enriched air show reduced sensitivity to vapour pressure deficit and drought. Plant Cell Environ. 21, 1077–1088. doi: 10.1046/j.1365-3040.1998.00366.x
Hu, H., Boisson-Dernier, A., Israelsson-Nordstrom, M., Bohmer, M., Xue, S., Ries, A., et al. (2010). Carbonic anhydrases are upstream regulators of CO2 controlled stomatal movements in guard cells. Nat. Cell Biol. 12, 87–93. doi: 10.1038/ncb2009
Jones, H. G. (2007). Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 58, 119–130. doi: 10.1093/jxb/erl118
Kamoshita, A., Babu, R. C., Boopathi, N. M., and Fukai, S. (2008). Phenotypic and genotypic analysis of drought-resistance traits for development of rice cultivars adapted to rainfed environments. Field Crops Res. 109, 1–23. doi: 10.1016/j.fcr.2008.06.010
Keylock, C. (2005). Simpson diversity and the Shannon–Wiener index as special cases of a generalized entropy. Oikos 109, 203–207. doi: 10.1111/j.0030-1299.2005.13735.x
Killi, D., Anlauf, R., Kavdir, Y., and Haworth, M. (2014). Assessing the impact of agro-industrial olive wastes in soil water retention: implications for remediation of degraded soils and water availability for plant growth. Int. Biodeterior. Biodegrad. 94, 48–56. doi: 10.1016/j.ibiod.2014.06.019
Killi, D., Bussotti, F., Raschi, A., and Haworth, M. (2016). Adaptation to high temperature mitigates the impact of water deficit during combined heat and drought stress in C3 sunflower and C4 maize varieties with contrasting drought tolerance. Physiol. Plant. 159, 130–147. doi: 10.1111/ppl.12490
Lauteri, M., Haworth, M., Serraj, R., Monteverdi, M. C., and Centritto, M. (2014). Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. PLoS ONE 9:e109054. doi: 10.1371/journal.pone.0109054
Lybbert, T. J., Barrett, C. B., and Narjisse, H. (2002). Market-based conservation and local benefits: the case of argan oil in Morocco. Ecol. Econ. 41, 125–144. doi: 10.1016/S0921-8009(02)00020-4
Lybbert, T. J., Magnan, N., and Aboudrare, A. (2010). Household and local forest impacts of Morocco’s argan oil bonanza. Environ. Dev. Econ. 15, 439–464. doi: 10.1073/pnas.1106382108
Majourhat, K., Jabbar, Y., Hafidi, A., and Martínez-Gómez, P. (2008). Molecular characterisation and genetic relationships among most common identified morphotypes of critically endangered rare Moroccan species Argania spinosa (Sapotaceae) using RAPD and SSR markers. Ann. For. Sci. 65, 805. doi: 10.1051/forest:2008069
Marino, G., Pallozzi, E., Cocozza, C., Tognetti, R., Giovannelli, A., Cantini, C., et al. (2014). Assessing gas exchange, sap flow and water relations using tree canopy spectral reflectance indices in irrigated and rainfed Olea europaea L. Environ. Exp. Bot. 99, 43–52. doi: 10.1016/j.envexpbot.2013.10.008
McGregor, H. V., Dupont, L., Stuut, J.-B. W., and Kuhlmann, H. (2009). Vegetation change, goats, and religion: a 2000-year history of land use in southern Morocco. Quat. Sci. Rev. 28, 1434–1448. doi: 10.1016/j.quascirev.2009.02.012
Meinzer, F. C., Johnson, D. M., Lachenbruch, B., McCulloh, K. A., and Woodruff, D. R. (2009). Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol. 23, 922–930. doi: 10.1111/j.1365-2435.2009.01577.x
Mellado, J. (1989). SOS Souss: argan forest destruction in Morocco. Oryx 23, 87–93. doi: 10.1017/S0030605300022754
Messinger, S. M., Buckley, T. N., and Mott, K. A. (2006). Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol. 140, 771–778. doi: 10.1104/pp.105.073676
Mott, K. A., and Peak, D. (2013). Testing a vapour-phase model of stomatal responses to humidity. Plant Cell Environ. 36, 936–944. doi: 10.1111/pce.12026
Msanda, F., El Aboudi, A., and Peltier, J.-P. (2005). Biodiversité et biogéographie de l’arganeraie Marocaine. Cah. Agric. 14, 357–364.
Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89, 583–590.
Nei, M., and Roychoudhury, A. (1974). Sampling variances of heterozygosity and genetic distance. Genetics 76, 379–390.
Nemali, K. S., and van Iersel, M. W. (2006). An automated system for controlling drought stress and irrigation in potted plants. Sci. Hortic. 110, 292–297. doi: 10.1016/j.scienta.2006.07.009
Nouaim, R., Mangin, G., Breuil, M., and Chaussod, R. (2002). The argan tree (Argania spinosa) in Morocco: propagation by seeds, cuttings and in-vitro techniques. Agrofor. Syst. 54, 71–81. doi: 10.1023/A:1014236025396
Peakall, R., and Smouse, P. E. (2006). GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295. doi: 10.1111/j.1471-8286.2005.01155.x
Ruas, M.-P., Ros, J., Terral, J.-F., Ivorra, S., Andrianarinosy, H., Ettahiri, A. S., et al. (2016). History and archaeology of the emblematic argan tree in the medieval Anti-Atlas Mountains (Morocco). Quat. Int. 404, 114–136. doi: 10.1016/j.quaint.2015.09.030
Ruas, M.-P., Tengberg, M., Ettahiri, A. S., Fili, A., and Van Staëvel, J.-P. (2011). Archaeobotanical research at the medieval fortified site of Îgîlîz (Anti-Atlas, Morocco) with particular reference to the exploitation of the argan tree. Veg. Hist. Archaeobot. 20, 419–433. doi: 10.1007/s00334-011-0306-2
Saliendra, N. Z., Sperry, J. S., and Comstock, J. P. (1995). Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196, 357–366. doi: 10.1007/BF00201396
Seiler, C., Harshavardhan, V. T., Rajesh, K., Reddy, P. S., Strickert, M., Rolletschek, H., et al. (2011). ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J. Exp. Bot. 62, 2615–2632. doi: 10.1093/jxb/erq446
Sharma, V., Bhardwaj, P., Kumar, R., Sharma, R. K., Sood, A., and Ahuja, P. S. (2009). Identification and cross-species amplification of EST derived SSR markers in different bamboo species. Conserv. Genet. 10, 721–724. doi: 10.1007/s10592-008-9630-1
Sorrentino, G., Haworth, M., Wahbi, S., Mahmood, T., Zuomin, S., and Centritto, M. (2016). Abscisic acid induces rapid reductions in mesophyll conductance to carbon dioxide. PLoS ONE 11:e0148554. doi: 10.1371/journal.pone.0148554
Sun, P., Wahbi, S., Tsonev, T., Haworth, M., Liu, S., and Centritto, M. (2014). On the use of leaf spectral indices to assess water status and photosynthetic limitations in Olea europaea L. during water-stress and recovery. PLoS ONE 9:e105165. doi: 10.1371/journal.pone.0105165
Takezaki, N., Nei, M., and Tamura, K. (2010). POPTREE2: software for constructing population trees from allele frequency data and computing other population statistics with windows interface. Mol. Biol. Evol. 27, 747–752. doi: 10.1093/molbev/msp312
Tardieu, F., and Davies, W. J. (1992). Stomatal response to abscisic acid is a function of current plant water status. Plant Physiol. 98, 540–545. doi: 10.1104/pp.98.2.540
Tomimatsu, H., and Tang, Y. (2012). Elevated CO2 differentially affects photosynthetic induction response in two Populus species with different stomatal behavior. Oecologia 169, 869–878. doi: 10.1007/s00442-012-2256-5
Wilkinson, S., Corlett, J. E., Oger, L., and Davies, W. J. (1998). Effects of xylem pH on transpiration from wild-type and flacca tomato leaves - A vital role for abscisic acid in preventing excessive water loss even from well-watered plants. Plant Physiol. 117, 703–709. doi: 10.1104/pp.117.2.703
Yeh, F. C., Yang, R. C., Boyle, T. B., Ye, Z., and Mao, J. X. (1997). POPGENE, the User-Friendly Shareware for Population Genetic Analysis. Edmonton, AB: University of Alberta.
Keywords: water deficit, stomatal conductance, vapor pressure deficit, carbon isotope discrimination, argan oil, simple sequence repeat markers, population genetics
Citation: Chakhchar A, Haworth M, El Modafar C, Lauteri M, Mattioni C, Wahbi S and Centritto M (2017) An Assessment of Genetic Diversity and Drought Tolerance in Argan Tree (Argania spinosa) Populations: Potential for the Development of Improved Drought Tolerance. Front. Plant Sci. 8:276. doi: 10.3389/fpls.2017.00276
Received: 30 September 2016; Accepted: 14 February 2017;
Published: 02 March 2017.
Edited by:
Iker Aranjuelo, Agribiotechnology Institute (IdAB)-CSIC-UPNA, SpainReviewed by:
Juan De Dios Alché, Spanish National Research Council, SpainRafael Ribeiro, University of Campinas, Brazil
Copyright © 2017 Chakhchar, Haworth, El Modafar, Lauteri, Mattioni, Wahbi and Centritto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew Haworth, aGF3b3J0aEBpdmFsc2EuY25yLml0
 Abdelghani Chakhchar
Abdelghani Chakhchar Matthew Haworth
Matthew Haworth Cherkaoui El Modafar1
Cherkaoui El Modafar1 Said Wahbi
Said Wahbi Mauro Centritto
Mauro Centritto