- 1Department of Plant Science and Landscape Architecture, University of Maryland, College Park, MD, United States
- 2Department of Horticulture and Landscape Architecture, Purdue University, West Lafayette, IN, United States
A Corrigendum on
The Arabidopsis ATP-BINDING CASSETTE Transporter ABCB21 Regulates Auxin Levels in Cotyledons, the Root Pericycle, and Leaves
by Jenness, M. K., Carraro, N., Pritchard, C. A., and Murphy, A. S. (2019). Front. Plant Sci. 10:806. doi: 10.3389/fpls.2019.00806
In the original article, there was a mistake in Figure 7F as published. The red light source used was subsequently found to emit a small amount of far-red. The experiments were repeated with a light setup that eliminated all far-red spectra. Under these conditions, abcb21 mutants were still not different from Col-0. A correction has been made to Figure 7, its legend and the Results section.
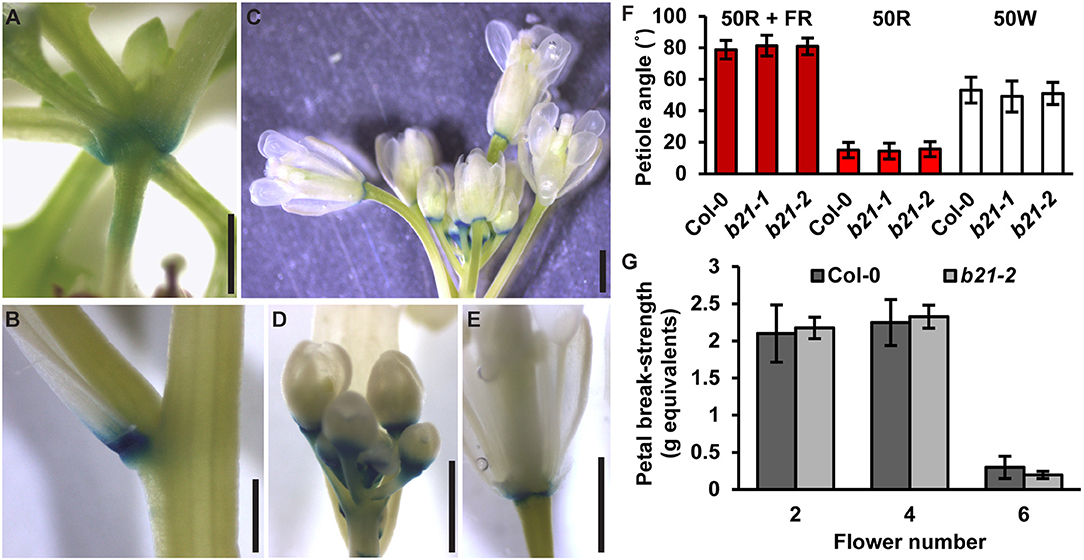
Figure 7. Expression of ABCB21 in abscission zones. proABCB21:GUS is expressed in the abscission zones of (A) rosette leaves, (B) cauline leaves, and (C) floral organs. (D,E) proABCB21:GUS expression domain is expanded in (D) young flowers and (E) restricted to the abscission zone in mature flowers. Plants were GUS stained for 16 h. (F) True leaf petiole angles in abcb21. Plants were grown on soil under 80 μmol m−2 s−1 white light, 16 h photoperiod. When plants reached stage 1.01 they were transferred to continuous 50 μmol m−2 s−1 red plus far-red light (50R + FR; burgundy bars), 50 μmol m−2 s−1 red light (50R; red bars), or 50 m−2 s−1 white light (50W; white bars) and allowed to grow an additional 3 d. Angle was determined by measuring the angle formed between the hypocotyl and the two first true leaf petioles minus 90°. Data shown are means ± SD (n = 60). (G) Flower petal break-strength in abcb21. Flower 1 was designated as the first flower with visible flower petals. Methods are detailed the methods section and Supplementary Figure 7. Data shown are means ± SD (n = 15). Scale bars: 1 mm.
The Results section, subsection ABCB21 Expression Is Rapidly Induced During Wounding:
“As reported previously (Kamimoto et al., 2012), proABCB21:GUS expression in late stage mature tissues is restricted to the abscission zones of flowers, as well as rosette and cauline leaves (Figures 7A–E). Auxin regulation of leaf positioning (Peeters et al., 2009; de Carbonnel et al., 2010) and floral organ shedding/abscission (Tang et al., 2013) suggests a possible role for ABCB21 in regulation of localized auxin accumulations in these tissues. However, no differences in light-mediated leaf positioning were observed in abcb21 mutants when responses under continuous 50 μmol m−2 s−1 red plus far red light, 50 μmol m−2 s−1 red light, or 50 μmol m−2 s−1 white light were examined (Figure 7F), and measurements of petal break-strength was not different between Col-0 and abcb21-2 (Figure 7G). It is unclear whether ABCB21 expression at these junction sites is responsive or causal. However, wounding increases ABCB21 expression ~1.7X between 30 and 60 min before returning to pre-wound levels or below (Kilian et al., 2007). Rapid induction of proABCB21:GUS expression is observed in stem tissues after wounding (Figure 8A). No GUS staining was observed in Col-0 indicating staining was not due to non-specific enzymatic activity. However, similar discrete DR5:GUS signals are initially observed in both Col-0 and abcb21-2 suggesting initial auxin accumulations are not affected (Figure 8B). A downstream role in wound-induced vascularization is possible, but does not appear to involve monolignol transport, as is observed with ABCG29 (Alejandro et al., 2012). No differences in seedling root growth on p-coumaryl alcohol were observed in abcb21 under conditions where abcg29 root growth is more inhibited than Col-0 (Supplementary Table 1), and no differences in lignin content or speciation were detected in seedling roots (Supplementary Table 2). A more localized impact on auxin-dependent vascularization is possible, but could not be reproducibly verified.”
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
References
Alejandro, S., Lee, Y., Tohge, T., Sudre, D., Osorio, S., Park, J., et al. (2012). AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr. Biol. 22, 1207–1212. doi: 10.1016/j.cub.2012.04.064
de Carbonnel, M., Davis, P., Roelfsema, M. R. G., Inoue, S.-I., Schepens, I., Lariguet, P., et al. (2010). The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 152, 1391–1405. doi: 10.1104/pp.109.150441
Kamimoto, Y., Terasaka, K., Hamamoto, M., Takanashi, K., Fukuda, S., Shitan, N., et al. (2012). Arabidopsis ABCB21 is a facultative auxin importer/exporter regulated by cytoplasmic auxin concentration. Plant Cell Physiol. 53, 2090–2100. doi: 10.1093/pcp/pcs149
Kilian, J., Whitehead, D., Horak, J., Wanke, D., Weinl, S., Batistic, O., et al. (2007). The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 50, 347–363. doi: 10.1111/j.1365-313X.2007.03052.x
Peeters, A. J., van Zanten, M., Millenaar, F. F., Voesenek, L. A., Pierik, R., and Cox, M. C. (2009). Differential petiole growth in Arabidopsis thaliana: photocontrol and hormonal regulation. New Phytol. 184, 141–152. doi: 10.1111/j.1469-8137.2009.02921.x
Tang, S., Shahid, A. A., González-Carranza, Z. H., Roberts, J. A., Basu, M. M., and Azam-Ali, S. (2013). The manipulation of auxin in the abscission zone cells of Arabidopsis flowers reveals that indoleacetic acid signaling is a prerequisite for organ shedding. Plant Physiol. 162, 96–106. doi: 10.1104/pp.113.216234
Keywords: ABCB transporter, Arabidopsis thaliana, auxin, development, seedling
Citation: Jenness MK, Carraro N, Pritchard CA and Murphy AS (2020) Corrigendum: The Arabidopsis ATP-BINDING CASSETTE Transporter ABCB21 Regulates Auxin Levels in Cotyledons, the Root Pericycle, and Leaves. Front. Plant Sci. 11:351. doi: 10.3389/fpls.2020.00351
Received: 19 February 2020; Accepted: 10 March 2020;
Published: 09 April 2020.
Edited and reviewed by: Markus Geisler, Université de Fribourg, Switzerland
Copyright © 2020 Jenness, Carraro, Pritchard and Murphy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angus S. Murphy, YXNtdXJwaHlAdW1kLmVkdQ==
 Mark K. Jenness
Mark K. Jenness Nicola Carraro
Nicola Carraro Candace A. Pritchard
Candace A. Pritchard Angus S. Murphy
Angus S. Murphy