- 1Texas A&M AgriLife Research and Extension Center, Weslaco, TX, United States
- 2Department of Veterinary Pathology, Dr. GCN College of Veterinary & Animal Sciences, CSK Himachal Pradesh Agricultural University, Palampur, India
- 3Department of Plant Pathology and Microbiology, Texas A&M University, College Station, TX, United States
- 4Institute for Advancing Health Through Agriculture, Texas A&M AgriLife, College Station, TX, United States
Plant-based heterologous expression systems can be leveraged to produce high-value therapeutics, industrially important proteins, metabolites, and bioproducts. The production can be scaled up, free from pathogen contamination, and offer post-translational modifications to synthesize complex proteins. With advancements in molecular techniques, transgenics, CRISPR/Cas9 system, plant cell, tissue, and organ culture, significant progress has been made to increase the expression of recombinant proteins and important metabolites in plants. Methods are also available to stabilize RNA transcripts, optimize protein translation, engineer proteins for their stability, and target proteins to subcellular locations best suited for their accumulation. This mini-review focuses on recent advancements to enhance the production of high-value metabolites and proteins necessary for therapeutic applications using plants as bio-factories.
1 Introduction
Many plant-based expression systems were developed for the large-scale production of valuable proteins and metabolites used in the pharmaceuticals, nutraceuticals, and cosmetics industries. These platforms are relatively cost-efficient, free from pathogens affecting humans, can synthesize complex proteins with post-translational modifications, and are scalable (Buyel, 2019; Burnett and Burnett, 2020). They can be used to produce vaccines, antibodies, antimicrobial peptides, hormones, growth factors, and industrially essential enzymes. Plants can be also used to produce high-value secondary metabolites (SMs). The SMs are produced from primary metabolic pathways and are induced in limited quantities during plant growth, development, and abiotic/biotic interactions (Chen et al., 2022). SMs have broad activities against viral, bacterial, and fungal infections and are used to treat various diseases like cancer, arthritis, diabetes, and neurological and respiratory disorders (De Filippis, 2016). Since chemical synthesis is expensive and challenging for many SMs, pharmaceutical industries depend on medicinal plants for sourcing SMs (Isah, 2019). With advancements in molecular techniques and synthetic biology tools, researchers have increased the quantity and quality of plant-made recombinant proteins and SMs. This mini-review summarizes different strategies for the enhanced production of valuable proteins and metabolites using plants as bio-factories for heterologous expression.
2 Heterologous expression of recombinant proteins in plants
Plant molecular farming is the practice of using plant-based platforms to produce high-value recombinant peptides and proteins. These proteins can either be stably or transiently produced, and as per need, they can be directed to accumulate in whole plants, seeds, chloroplasts, fruits, or roots (Xu et al., 2018). Moreover, many plant transformation methods based on Agrobacterium or polyethylene glycol (PEG)-mediated transformation, particle-bombardment, vacuum, and virus-based infiltrations are well established (Joung et al., 2015).
2.1 Approaches to enhance expression of recombinant proteins
2.1.1 Promotor engineering and combinatorial stacking
The promoter is an essential element in regulating transgene expression. As per the need, many different types of promoters, like constitutive, inducible, tissue-specific, and synthetic promoters, are employed. The cauliflower mosaic virus 35S promoter (CaMV 35S), a strong constitutive promoter in either single or multiple copies, is widely used in dicot plants, whereas the maize ubiquitin-1 (Ubi-1) promoter is used to express therapeutic proteins in monocots (Phakham et al., 2021; Mirzaee et al., 2022). Recently, Damaj et al. (2020) reported a combinatorial stacked promoter system to enhance the expression of recombinant bovine lysozyme (BvLz). BvLz is a potent broad-spectrum antimicrobial enzyme used in the food, cosmetic, and agricultural industries. Combinatorial plant transformation and co-expression of BvLz under the control of various constitutive and culm-regulated promoters yielded high levels of expression (up to 11.5% of total soluble protein) in sugarcane culms (Damaj et al., 2020). Similarly, Padilla et al. (2020) successfully enhanced the expression of recombinant Galanthus nivalis L. (snowdrop) agglutinin (GNA) in sugarcane and energy cane. GNA possesses antiviral, antifungal, and antitumor activities. Under a single constitutive Ubi-1 promoter, GNA accumulated 0.04% and 0.3% of total soluble protein (TSP) in sugarcane culms and leaves, respectively. Its expression was further increased to 1.8% TSP and 2.3% TSP in sugarcane and energy cane lines, respectively, by co-expressing recombinant GNA under multiple promoters (pUbi-1 and culm-regulated promoters from sugarcane dirigent5-1 and sugarcane bacilliform virus; pUBD5:GNA) from different expression vectors. Moreover, the expression of recombinant GNA in the triple promoter transgenic lines (pUBD5:GNA) was further boosted to 2.7% TSP by inducing promoter activity with salicylic acid (Padilla et al., 2020). These studies demonstrate the great potential of the inducible promoters and combinatorial promoter stacking system to increase the accumulation of high-value therapeutic proteins in plants.
2.1.2 Codon optimization
The native amino-acid codon degeneracy enables the optimization of non-favorable codons within an open reading frame of a protein. Some mRNAs possess cryptic splicing sites, secondary structures, mRNA stability elements, and alternative translation start sites that may negatively affect protein translation and accumulation. The codon optimization process uses synonymous codons without altering the protein amino acid sequence to increase translational efficiency (Webster et al., 2017). For instance, through codon optimization, the expression of stem cell factor (SCF) for ex vivo RBC production increased 25- to 30-fold in tobacco Bright Yellow-2 (BY-2) cells (Wang et al., 2021). In another study, a codon-optimized BvLz was stably expressed in sugarcane culms (Damaj et al., 2020). Further, codon optimization and other approaches enhanced the expression of human interferon-gamma (IFNγ) via bamboo mosaic virus (BaMV) mediated transient expression in Nicotiana benthamiana (Jiang et al., 2019).
2.1.3 Expression using plant virus vectors
Plant viruses infect many crops, ornamentals, and medicinal plant species (Kulshreshtha et al., 2017; Sharma et al., 2019). Many asymptomatic or inactivated viruses have been engineered as chimeric expression vectors to transiently express therapeutic proteins in different plant species. For instance, the tobacco mosaic virus (TMV) was employed to express the receptor binding domain (RBD) of the SARS-CoV-2 spike protein in glycoengineered N. benthamiana. After purification of the protein and vaccination, mice produced RBD-specific antibodies that neutralized the SARS-CoV-2 virus infection in Vero E6 cells (Maharjan et al., 2021). Likewise, the bean yellow dwarf virus (BeYDV) expressed SARS-CoV-2 RBD and basic fibroblast growth factor (bFGF) in two microalgae species; Chlamydomonas reinhardtii and Chlorella vulgaris (Malla et al., 2021). Similarly, the cowpea mosaic virus (CPMV) and the potato virus X (PVX) expressed vaccine candidates in the N. benthamiana against the hepatitis E virus and the influenza virus, respectively (Mardanova et al., 2017; Zahmanova et al., 2021). These findings underscore the utility of plant viruses to express a wide range of biologics to produce therapeutic molecules rapidly during pandemics.
2.1.4 Suppression of RNA silencing
A potential limitation in any eukaryotic expression system is the inherent host RNA silencing mechanism that may suppress the expression of foreign genes. Fortunately, many plant virus-encoded suppressor proteins can be co-expressed with the gene of interest to suppress host silencing, increasing recombinant protein expression by several folds (Gao et al., 2013). For instance, the co-expression of tombusvirus P19 suppressor led to a 40% increase in the expression of truncated human IFNγ accumulation in N. benthamiana using a BaMV-based vector (Jiang et al., 2019). In another example, PVX and a P19 suppressor were used to express a fusion protein (lhmlt) of melittin peptide and gonadotropin-releasing hormone receptor (GnRHR) in N. benthamiana. The purified protein was functional and inhibited the cancerous cells in the MTT assay (Naseri et al., 2020). In a recent study, CRISPR/Cas9 was also employed to knockout N. benthamiana dicer-like proteins 2 and 4 (NbDCL2 and NbDCL4). The knockout plants produced 6.96 folds higher expression of human fibroblast growth factor 1 (FGF1) than wild-type plants (Matsuo, 2022).
2.1.5 Optimization of downstream processing
To enable efficient purification of the recombinant proteins from plant cells, often downstream extraction and processing steps need to be optimized. In the extraction phase, tissue homogenization releases impurities like host cell proteins, enzymes, and phenolic compounds. The phenolic compounds form covalent complexes with recombinant protein in the presence of plant polyphenol oxidases (PPO) and could result in aggregation and precipitation of recombinant protein which ultimately reduces protein yield and quality. Furthermore, many plant proteases can degrade target proteins. These factors can be partially addressed by including broad-spectrum protease inhibitors and antioxidants in the extraction steps (Buyel et al., 2015). In addition, CRISPR/Cas9-based target editing of PPO genes in the host cells can be employed to enhance in planta expression of recombinant therapeutic proteins (González et al., 2020). Another alternative is to target proteins via. signal sequences to accumulate into specific subcellular compartments such as apoplast, chloroplast, and endoplasmic reticulum (Habibi et al., 2017). Recombinant proteins can subsequently be purified with affinity tags such as maltose-binding protein, glutathione S-transferase (GST), thioredoxin, staphylococcal protein A, and poly-histidine tag (Pina et al., 2021).
2.2 Approaches to optimizing post-translational modifications
Glycosylation is a key post-translational modification needed in many human therapeutic proteins. Glycosylation in plants differs from humans and possesses additional α 1,3 fucose and β 1,2 xylose modifications. This may affect the activity, stability, and immunogenic responses of the therapeutic protein (Grabowski et al., 2014). Therefore, transgenic plants and plant cell cultures were developed to remove plant-specific glycan and to introduce human glycosylation pathways to produce more complex proteins like monoclonal antibodies (Castilho and Steinkellner, 2012). For instance, antibodies (CAP256-VRC26) against human immunodeficiency virus type 1 were expressed in glycoengineered N. benthamiana. These antibodies showed equivalent neutralizing activity to mammalian-produced antibodies (Singh et al., 2020). Similarly, human-like glycosylated granulocyte colony-stimulating factor (G-CSF) was produced in N. benthamiana by co-expressing genes needed for human-specific O-glycosylated G-CSF (Ramírez-Alanis et al., 2018).
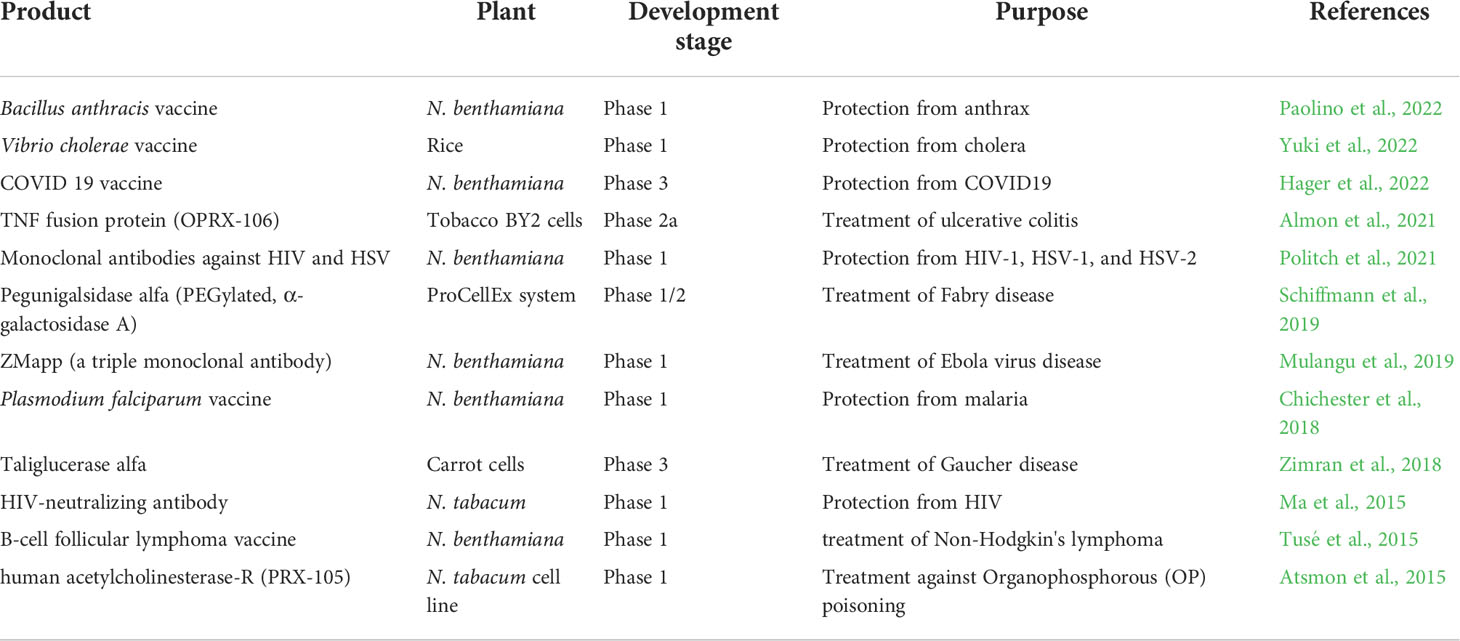
2.3 Examples of key plant-made therapeutic proteins
Many essential therapeutic proteins, vaccines, and monoclonal antibodies are produced in plant systems. A handful of these biologics are commercialized or in clinical trials (Table 1). The first plant-made drug approved for human use by U.S. Food and Drug Administration (FDA) is Elelyso (taliglucerase alfa). It was produced in genetically modified carrot cells by Protalix Biotherapeutics to treat heritable type I Gaucher’s disease (Fox, 2012). Another drug is ZMapp, a cocktail of three monoclonal antibodies produced in transgenic tobacco. Its administration completely cured Ebola infection in Rhesush macaques (Qiu et al., 2014). The other plant-made commercialized proteins include bovine trypsin (expressed in corn and marketed by Sigma Aldrich), human and animal growth factors (expressed in barley seeds and marketed by ORF Genetics), and recombinant human serum albumin (expressed in rice and marketed by ScienCell Research Laboratories). Recently, a large-scale phase 3 clinal trial of N. benthamiana produced a quadrivalent influenza vaccine that demonstrated substantial protection against influenza viruses in adults (Ward et al., 2020). These findings conclude that plant-made platforms to produce biopharmaceuticals have broad potential but need more research and optimizations to meet market demand.
3 Heterologous expression of secondary metabolites in plants
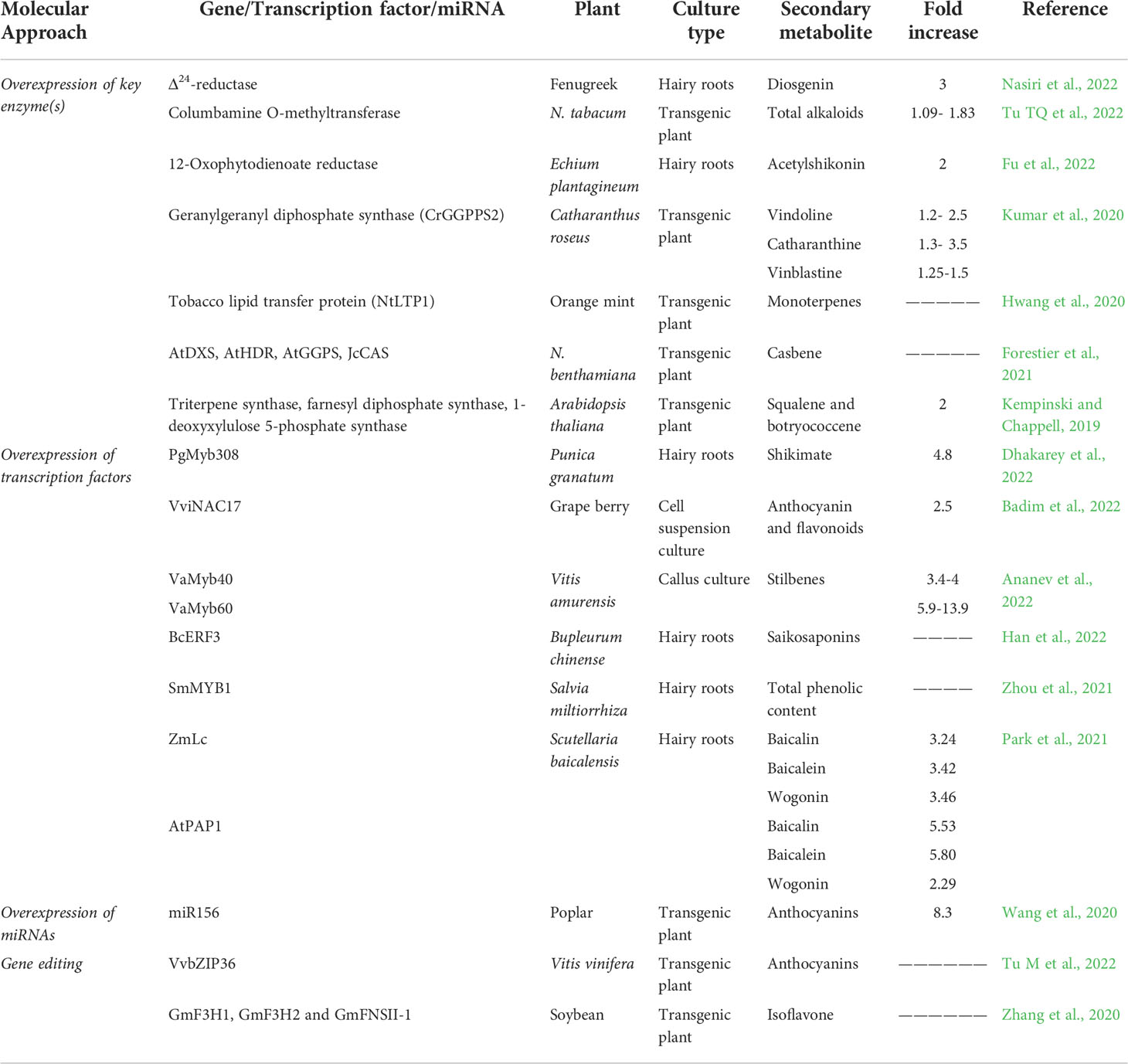
SMs are produced from primary metabolic pathways in response to growth, development, and biotic and abiotic stresses. Several SMs have utility as therapeutics for human diseases. Many plant expression platforms like cell/suspension culture, callus culture, organ culture, hairy root culture, shoot culture, and transgenics are established for the heterologous production of SMs (Fazili et al., 2022). However, the accumulation of SMs can be enhanced further by a better understanding and optimization of metabolic pathways, the rate-limiting step(s), and genetic regulations.
3.1 Approaches to enhance the production of secondary metabolites
3.1.1 Overexpression of rate-limiting enzyme(s)
The metabolic pathways may have single or multiple rate-limiting steps, and the overexpression of crucial rate-limiting enzyme(s) may improve the production of the desired metabolite. For instance, Atropa belladonna ornithine decarboxylase (AbODC) is a rate-limiting enzyme in the biosynthesis of tropane alkaloids. Tropane alkaloids have several clinical uses such as treating Alzheimer’s disease, postoperative nausea, and motion sickness. Overexpression of AbODC significantly increased the accumulation of putrescine, N-methylputrescine, hyoscyamine, and anisodamine in A. belladonna hairy roots and transgenic plants (Zhao et al., 2020). Similarly, overexpression of hyoscyamine six hydroxylase, a key enzyme in the scopolamine biosynthetic pathway, increased the production of scopolamine alkaloid in the hairy roots of Datura innoxia (Li et al., 2020). Camptothecin is an FDA-approved pharmaceutically important monoterpenoid indole alkaloid having potent anticancer properties. The expression of two key camptothecin biosynthetic genes, OpG10H and OpSLS, greatly enhanced its production (up to 3.5 mg/g) in transgenic Ophiorrhiza pumila hairy roots (Shi et al., 2020).
3.1.2 Overexpression of transcription factors
Transcription factors are critical regulators of many metabolic pathways, and their expression can enhance SMs production. The overexpression of OpWRKY2 activated a camptothecin pathway gene, OpTDC. It resulted in a three-fold increase in camptothecin accumulation in Ophiorrhiza pumila hairy roots (Hao et al., 2021). In another study, astragalosides production was increased in Astragalus membranaceus hairy roots by overexpressing Arabidopsis transcription factor MYB12, Production of anthocyanin pigment 1 (PAP1), and maize leaf color (Lc) transcription factors (Li et al., 2022).
3.1.3 Overexpression of MicroRNAs
MicroRNAs (miRNAs) are small noncoding RNAs important for gene regulation. They play crucial roles in growth, development, and stress responses, as well as the regulation of secondary metabolite pathways (Hossain et al., 2022). Small RNA sequencing of three grape varieties with different anthocyanin and flavonoid content identified that the grape lines with high anthocyanin content abundantly express two miRNAs, miR828 and miR858. These miRNAs target the MYB114 transcription factor, which is a negative regulator of anthocyanin biosynthesis. Hence, overexpression of these miRNAs increased anthocyanin accumulation (Tirumalai et al., 2019). In another study, overexpression of miR156 isolated from Medicago truncatula increased 8.3 folds in total anthocyanins production in transgenic poplar plants compared to wild-type plants (Wang et al., 2020).
3.1.4 Gene editing
CRISPR/Cas9 mediated targeted mutagenesis has been developed to enhance the production of valuable metabolites in several plant species. Atropa belladonna produces a small amount of hyoscyamine and its structural analogs, anisodamine, and scopolamine. Zeng et al. (2021) used CRISPR/Cas9 to disrupt hyoscyamine 6β-hydroxylase (AbH6H). The transgenic plants produced a significantly higher hyoscyamine content but without anisodamine and scopolamine alkaloids (Zeng et al., 2021). In another study, Karlson et al. (2022) used CRISPR/Cas9 to silence cinnamate-4-hydroxylase (C4H) for enhanced flavonoid production into N. tabacum cell suspension. C4H-silenced cells produced a significantly higher concentration of cinnamic acid, chlorogenic acid, pinostrobin, and naringenin than wild-type cells (Karlson et al., 2022).
3.1.5 Expression in genetically engineered microalgae
Although not plants, microalgae which are also photosynthetic organisms can be leveraged to produce a broad range of secondary metabolites of pharmaceutical importance (Sreenikethanam et al., 2022). The Cannabis sativa plant naturally produces cannabinoids that treat nausea and vomiting caused by cancer chemotherapy, neuropathic pain, and spasticity. Furthermore, cannabinoids also possess anticancer properties (Mangal et al., 2021). Genetically engineered Microalgae were recently used for the heterologous production of cannabinoids (Bolaños-Martínez et al., 2022). Microalgae system is advantageous as they have limited growth needs such as CO2 and few organic compounds.
3.2 Examples of key plant-derived secondary metabolites
Plant SMs are useful as treatments for several diseases, including cancer, diabetes, COVID-19, arthritis, and neurological and cardiovascular disorders (De Filippis, 2016). To date, only paclitaxel has been produced on a commercial scale. It is included in the WHO list of essential medicines and is used to treat different cancers. Phyton Biotech is commercially producing paclitaxel (trade name Taxol® by Bristol-Myers Squibb) using plant cell fermentation technology based on cell lines of Taxus chinensis v. marei (https://phytonbiotech.com/). The other SMs produced in different expression systems showed higher expression than native plants (Table 2), but more efforts are still needed to produce SMs at an industrial scale.
4 Conclusion
Despite the advantages of plant-based expression platforms, only a few commercial products passed the regulatory approvals and reached the market (Schillberg et al., 2019). In our perspective, a critical barrier to commercialization using plant-based expression platforms comes down to investment returns. Any profitable company wants products of high quality, reliability, and quantity at a low cost. Currently, the market favors mammalian and bacterial platforms as they have a long and successful history of making pharmaceuticals. Furthermore, these platforms have demonstrated batch-to-batch consistency and safety, which is especially important for drug formulations for human use. Plant-based expression platforms face higher capital costs for raw materials and infrastructure, downstream process optimization costs, lower market demand, public acceptance, more biosafety needs, and regulatory approvals. Industries often are wary of switching from well-established platforms to plant-based platforms. Plant-based expression platforms need to demonstrate greater net economic return compared to prokaryotic and mammalian systems to be competitive. With recent advancements in genetic and genomic tools, the heterologous production of high-value proteins and metabolites in plant expression systems has gained traction. The platforms can be deployed to mass-produce (scalable) biopharmaceuticals in a shorter timeframe and can be relatively cost-effective compared to other conventional cell culture-based systems. This is particularly helpful during rapid response situations such as during pandemics. New approaches have also allowed for improvements in target protein stability and accumulation, post-translational modifications, and downstream recovery/purification of the proteins. We anticipate increasing market demand for high-value therapeutics and bioproducts that can boost commercial interest in plant-based expression platforms.
Author contributions
KM conceptualized and supervised the study. AK, SS, and CP conducted the study and prepared the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported in part by funds from USDA NIFA (HATCH 1023984), Texas A&M AgriLife Research Insect-vectored Disease Seed Grants (114185-96210), and the Texas A&M AgriLife Institute for Advancing Health Through Agriculture to KM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almon, E., Shaaltiel, Y., Sbeit, W., Fich, A., Schwartz, D., Waterman, M., et al. (2021). Novel orally administered recombinant anti-TNF alpha fusion protein for the treatment of ulcerative colitis: results from a phase 2a clinical trial. J. Clin. Gastroenterol. 55, 134–140. doi: 10.1097/MCG.0000000000001314
Ananev, A. A., Suprun, A. R., Aleynova, O. A., Nityagovsky, N. N., Ogneva, Z. V., Dubrovina, A. S., et al. (2022). Effect of VaMyb40 and VaMyb60 overexpression on stilbene biosynthesis in cell cultures of grapevine vitis amurensis rupr. Plants 11, 1916. doi: 10.3390/plants11151916
Atsmon, J., Brill-Almon, E., Nadri-Shay, C., Chertkoff, R., Alon, S., Shaikevich, D., et al. (2015). Preclinical and first-in-human evaluation of PRX-105, a PEGylated, plant-derived, recombinant human acetylcholinesterase-r. Toxicol. Appl. Pharmacol. 287, 202–209. doi: 10.1016/j.taap.2015.06.004
Badim, H., Vale, M., Coelho, M., Granell, A., Gerós, H., Conde, A. (2022). Constitutive expression of VviNAC17 transcription factor significantly induces the synthesis of flavonoids and other phenolics in transgenic grape berry cells. Front. Plant Sci. 13, 1–12. doi: 10.3389/fpls.2022.964621
Bolaños-Martínez, O. C., Malla, A., Rosales-Mendoza, S., Vimolmangkang, S. (2022). Harnessing the advances of genetic engineering in microalgae for the production of cannabinoids. Crit. Rev. Biotechnol. 1–12. doi: 10.1080/07388551.2022.2071672
Burnett, M. J., Burnett, A. C. (2020). Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet 2, 121–132. doi: 10.1002/ppp3.10073
Buyel, J. F. (2019). Plant molecular farming–integration and exploitation of side streams to achieve sustainable biomanufacturing. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01893
Buyel, J. F., Twyman, R. M., Fischer, R. (2015). Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 33, 902–913. doi: 10.1016/j.biotechadv.2015.04.010
Castilho, A., Steinkellner, H. (2012). Glyco-engineering in plants to produce human-like n-glycan structures. Biotechnol. J. 7, 1088–1098. doi: 10.1002/biot.201200032
Chen, D., Mubeen, B., Hasnain, A., Rizwan, M., Adrees, M., Naqvi, S. A. H., et al. (2022). Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: Current scenario and future perspectives. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.881032
Chichester, J. A., Green, B. J., Jones, R. M., Shoji, Y., Miura, K., Long, C. A., et al. (2018). Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A phase 1 dose-escalation study in healthy adults. Vaccine 36, 5865–5871. doi: 10.1016/j.vaccine.2018.08.033
Damaj, M. B., Jifon, J. L., Woodard, S. L., Vargas-Bautista, C., Barros, G. O., Molina, J., et al. (2020). Unprecedented enhancement of recombinant protein production in sugarcane culms using a combinatorial promoter stacking system. Sci. Rep. 10, 13713. doi: 10.1038/s41598-020-70530-z
De Filippis, L. F. (2016). “Plant secondary metabolites: From molecular biology to health products,” in Plant-Environment Interaction: Responses and Approaches to Mitigate Stress, ed. Azooz, M. M., Ahmad, P.. 263–299. doi: 10.1002/9781119081005.ch15
Dhakarey, R., Yaritz, U., Tian, L., Amir, R. (2022). A myb transcription factor, PgMyb308-like, enhances the level of shikimate, aromatic amino acids, and lignins, but represses the synthesis of flavonoids and hydrolyzable tannins, in pomegranate (Punica granatum l.). Hortic. Res. 9, uhac008. doi: 10.1093/hr/uhac008
Fazili, M. A., Bashir, I., Ahmad, M., Yaqoob, U., Geelani, S. N. (2022). In vitro strategies for the enhancement of secondary metabolite production in plants: a review. Bull. Natl. Res. Cent. 46, 35. doi: 10.1186/s42269-022-00717-z
Forestier, E. C., Czechowski, T., Cording, A. C., Gilday, A. D., King, A. J., Brown, G. D., et al. (2021). Developing a nicotiana benthamiana transgenic platform for high-value diterpene production and candidate gene evaluation. Plant Biotechnol. J. 19, 1614–1623. doi: 10.1111/pbi.13574
Fox, J. (2012). First plant-made biologic approved. Nat. Biotechnol. 30, 472. doi: 10.1038/nbt0612-472
Fu, J., Ren, R., Jin, S., Fang, R., Wen, Z., Yang, M., et al. (2022). Overexpression of a putative 12-oxophytodienoate reductase gene, EpOPR1, enhances acetylshikonin production in echium plantagineum. In Vitro Cell. Dev. Biol. Plant 58, 311–320. doi: 10.1007/s11627-022-10259-8
Gao, S. J., Damaj, M. B., Park, J. W., Beyene, G., Buenrostro-Nava, M. T., Molina, J., et al. (2013). Enhanced transgene expression in sugarcane by co-expression of virus-encoded RNA silencing suppressors. PLos One 8, e66046. doi: 10.1371/journal.pone.0066046
González, M. N., Massa, G. A., Andersson, M., Turesson, H., Olsson, N., Fält, A. S., et al. (2020). Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complexes delivery of the CRISPR/Cas9 system. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01649
Grabowski, G. A., Golembo, M., Shaaltiel, Y. (2014). Taliglucerase alfa: an enzyme replacement therapy using plant cell expression technology. Mol. Genet. Metab. 112, 1–8. doi: 10.1016/j.ymgme.2014.02.011
Habibi, P., Prado, G. S., Pelegrini, P. B., Hefferon, K. L., Soccol, C. R., Grossi-de-Sa, M. F. (2017). Optimization of inside and outside factors to improve recombinant protein yield in plant. Plant Cell Tissue. Organ Cult. 130, 449–467. doi: 10.1007/s11240-017-1240-5
Hager, K. J., Pérez Marc, G., Gobeil, P., Diaz, R. S., Heizer, G., Llapur, C., et al. (2022). Efficacy and safety of a recombinant plant-based adjuvanted covid-19 vaccine. N. Engl. J. Med. 386, 2084–2096. doi: 10.1056/NEJMoa2201300
Han, W., Xu, J., Wan, H., Zhou, L., Wu, B., Gao, J., et al. (2022). Overexpression of BcERF3 increases the biosynthesis of saikosaponins in bupleurum chinense. FEBS Open Bio. 12, 1344–1352. doi: 10.1002/2211-5463.13412
Hao, X., Xie, C., Ruan, Q., Zhang, X., Wu, C., Han, B., et al. (2021). The transcription factor OpWRKY2 positively regulates the biosynthesis of the anticancer drug camptothecin in ophiorrhiza pumila. Hortic. Res. 8, 7. doi: 10.1038/s41438-020-00437-3
Hossain, R., Quispe, C., Saikat, A. S. M., Jain, D., Habib, A., Janmeda, P., et al. (2022). Biosynthesis of secondary metabolites based on the regulation of microRNAs. BioMed. Res. Int. 2022, 9349897. doi: 10.1155/2022/9349897
Hwang, H. S., Adhikari, P. B., Jo, H. J., Han, J. Y., Choi, Y. E. (2020). Enhanced monoterpene emission in transgenic orange mint (Mentha× piperita f. citrata) overexpressing a tobacco lipid transfer protein (NtLTP1). Planta 252, 44. doi: 10.1007/s00425-020-03447-6
Isah, T. (2019). Stress and defense responses in plant secondary metabolites production. Biol. Res. 52, 39. doi: 10.1186/s40659-019-0246-3
Jiang, M. C., Hu, C. C., Lin, N. S., Hsu, Y. H. (2019). Production of human IFNγ protein in nicotiana benthamiana plant through an enhanced expression system based on bamboo mosaic virus. Viruses. 11, 509. doi: 10.3390/v11060509
Joung, Y. H., Choi, P. S., Kwon, S. Y., Harn, C. H. (2015). “Plant transformation methods and applications,” in Current technologies in plant molecular breeding (Dordrecht: Springer), 297–343. doi: 10.1007/978-94-017-9996-6_9
Karlson, C. K. S., Mohd Noor, S. N., Khalid, N., Tan, B. C. (2022). CRISPRi-mediated down-regulation of the cinnamate-4-hydroxylase (C4H) gene enhances the flavonoid biosynthesis in nicotiana tabacum. Biology 11, 1127. doi: 10.3390/biology11081127
Kempinski, C., Chappell, J. (2019). Engineering triterpene metabolism in the oilseed of Arabidopsis thaliana. Plant Biotechnol. J. 17, 386–396. doi: 10.1111/pbi.12984
Kulshreshtha, A., Roshan, P., Sharma, D., Hallan, V. (2017). Molecular characterization of a new begomovirus infecting mirabilis jalapa in northern India. Arch. Virol. 162, 2163–2167. doi: 10.1007/s00705-017-3330-4
Kumar, S. R., Rai, A., Bomzan, D. P., Kumar, K., Hemmerlin, A., Dwivedi, V., et al. (2020). A plastid-localized bona fide geranylgeranyl diphosphate synthase plays a necessary role in monoterpene indole alkaloid biosynthesis in catharanthus roseus. Plant J. 103, 248–265. doi: 10.1111/tpj.14725
Li, X. H., Kim, J. K., Park, S. U. (2022). Heterologous expression of three transcription factors differently regulated astragalosides metabolic biosynthesis in astragalus membranaceus hairy roots. Plants 11, 1897. doi: 10.3390/plants11141897
Li, Q., Zhu, T., Zhang, R., Bu, Q., Yin, J., Zhang, L., et al. (2020). Molecular cloning and functional analysis of hyoscyamine 6β-hydroxylase (H6H) in the poisonous and medicinal plant datura innoxia mill. Plant Physiol. Biochem. 153, 11–19. doi: 10.1016/j.plaphy.2020.04.021
Ma, J. K. C., Drossard, J., Lewis, D., Altmann, F., Boyle, J., Christou, P., et al. (2015). Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 13, 1106–1120. doi: 10.1111/pbi.12416
Maharjan, P. M., Cheon, J., Jung, J., Kim, H., Lee, J., Song, M., et al. (2021). Plant-expressed receptor binding domain of the SARS-CoV-2 spike protein elicits humoral immunity in mice. Vaccines 9, 978. doi: 10.3390/vaccines9090978
Malla, A., Rosales-Mendoza, S., Phoolcharoen, W., Vimolmangkang, S. (2021). Efficient transient expression of recombinant proteins using DNA viral vectors in freshwater microalgal species. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.650820
Mangal, N., Erridge, S., Habib, N., Sadanandam, A., Reebye, V., Sodergren, M. H. (2021). Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. Oncol. 147, 2507–2534. doi: 10.1007/s00432-021-03710-7
Mardanova, E. S., Blokhina, E. A., Tsybalova, L. M., Peyret, H., Lomonossoff, G. P., Ravin, N. V. (2017). Efficient transient expression of recombinant proteins in plants by the novel pEff vector based on the genome of potato virus X. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00247
Matsuo, K. (2022). CRISPR/Cas9-mediated knockout of the DCL2 and DCL4 genes in nicotiana benthamiana and its productivity of recombinant proteins. Plant Cell Rep. 41, 307–317. doi: 10.1007/s00299-021-02809-y
Mirzaee, M., Osmani, Z., Frébortová, J., Frébort, I. (2022). Recent advances in molecular farming using monocot plants. Biotechnol. Adv. 58, 107913. doi: 10.1016/j.biotechadv.2022.107913
Mulangu, S., Dodd, L. E., Davey, R. T., Jr., Tshiani Mbaya, O., Proschan, M., Mukadi, D., et al. (2019). A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 381, 2293–2303. doi: 10.1056/NEJMoa1910993
Naseri, Z., Dorani Uliaei, E., Ofoghi, H., Davarpanah, S. J. (2020). Expression of novel lhmlt fusion protein using plant viral vector and study of its anticancer effect. Plant Cell Tiss. Organ Cult. 143, 97–108. doi: 10.1007/s11240-020-01900-8
Nasiri, A., Rashidi-Monfared, S., Ebrahimi, A., Charkhabi, N. F., Moieni, A. (2022). Metabolic engineering of the diosgenin biosynthesis pathway in trigonella foenum-graceum hairy root cultures. Plant Sci. 323, 111410. doi: 10.1016/j.plantsci.2022.111410
Padilla, C. S., Damaj, M. B., Yang, Z. N., Molina, J., Berquist, B. R., White, E. L., et al. (2020). High-level production of recombinant snowdrop lectin in sugarcane and energy cane. Front. bioeng. Biotechnol. 8. doi: 10.3389/fbioe.2020.00977
Paolino, K. M., Regules, J. A., Moon, J. E., Ruck, R. C., Bennett, J. W., Remich, S. A., et al. (2022). Safety and immunogenicity of a plant-derived recombinant protective antigen (rPA)-based vaccine against bacillus anthracis: a phase 1 dose-escalation study in healthy adults. Vaccine 40, 1864–1871. doi: 10.1016/j.vaccine.2022.01.047
Park, C. H., Xu, H., Yeo, H. J., Park, Y. E., Hwang, G. S., Park, N. I., et al. (2021). Enhancement of the flavone contents of scutellaria baicalensis hairy roots via metabolic engineering using maize lc and arabidopsis PAP1 transcription factors. Metab. Eng. 64, 64–73. doi: 10.1016/j.ymben.2021.01.003
Phakham, T., Bulaon, C. J. I., Khorattanakulchai, N., Shanmugaraj, B., Buranapraditkun, S., Boonkrai, C., et al. (2021). Functional characterization of pembrolizumab produced in nicotiana benthamiana using a rapid transient expression system. Front. Plant Sci. 12, 736299. doi: 10.3389/fpls.2021.736299
Pina, A. S., Batalha, Í.L., Dias, A. M. G. C., Roque, A. C. A. (2021). “Affinity tags in protein purification and peptide enrichment: An overview,” in Protein downstream processing, vol. 2178 . Ed. Labrou, N. E. (New York, NY: Humana). doi: 10.1007/978-1-0716-0775-6_10
Politch, J. A., Cu-Uvin, S., Moench, T. R., Tashima, K. T., Marathe, J. G., Guthrie, K. M., et al. (2021). Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): A phase I randomized trial. PLos Med. 18, e1003495. doi: 10.1371/journal.pmed.1003495
Qiu, X., Wong, G., Audet, J., Bello, A., Fernando, L., Alimonti, J. B., et al. (2014). Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514, 47–53. doi: 10.1038/nature13777
Ramírez-Alanis, I. A., Renaud, J. B., García-Lara, S., Menassa, R., Cardineau, G. A. (2018). Transient co-expression with three O-glycosylation enzymes allows production of GalNAc-o-glycosylated granulocyte-colony stimulating factor in n. benthamiana. Plant Methods 14, 1–14. doi: 10.1186/s13007-018-0363-y
Schiffmann, R., Goker-Alpan, O., Holida, M., Giraldo, P., Barisoni, L., Colvin, R. B., et al. (2019). Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year phase 1/2 clinical trial. J. Inherit. Metab. Dis. 42, 534–544. doi: 10.1002/jimd.12080
Schillberg, S., Raven, N., Spiegel, H., Rasche, S., Buntru, M. (2019). Critical analysis of the commercial potential of plants for the production of recombinant proteins. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00720
Sharma, D., Kulshreshtha, A., Kumar, R., Hallan, V. (2019). First report of natural infection of alternanthera yellow vein virus and cotton leaf curl multan betasatellite on a new host picrorhiza kurroa, an important endangered medicinal herb. J. Plant Pathol. 101, 149–153. doi: 10.1007/s42161-018-0123-x
Shi, M., Gong, H., Cui, L., Wang, Q., Wang, C., Wang, Y., et al. (2020). Targeted metabolic engineering of committed steps improves anti-cancer drug camptothecin production in ophiorrhiza pumila hairy roots. Ind. Crops Prod. 148, 112277. doi: 10.1016/j.indcrop.2020.112277
Singh, A. A., Pooe, O., Kwezi, L., Lotter-Stark, T., Stoychev, S. H., Alexandra, K., et al. (2020). Plant-based production of highly potent anti-HIV antibodies with engineered posttranslational modifications. Sci. Rep. 10, 6201. doi: 10.1038/s41598-020-63052-1
Sreenikethanam, A., Raj, S., Gugulothu, P., Bajhaiya, A. K. (2022). Genetic engineering of microalgae for secondary metabolite production: Recent developments, challenges, and future prospects. Front. bioeng. Biotechnol. 10. doi: 10.3389/fbioe.2022.836056
Tirumalai, V., Swetha, C., Nair, A., Pandit, A., Shivaprasad, P. V. (2019). miR828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. J. Exp. Bot. 70, 4775–4792. doi: 10.1093/jxb/erz264
Tu, T. Q., Do, P. T., Van Nguyen, D., Pham, N. T. T., Nguyen, T. T., Chu, M. H. (2022). The columbamine O-methyltransferase gene (CoOMT) is capable of increasing alkaloid content in transgenic tobacco plants. Mol. Biol. Rep. 49, 2667–2675. doi: 10.1007/s11033-021-07074-6
Tu, M., Fang, J., Zhao, R., Liu, X., Yin, W., Wang, Y., et al. (2022). CRISPR/Cas9-mediated mutagenesis of VvbZIP36 promotes anthocyanin accumulation in grapevine (Vitis vinifera). Hortic. Res. 9, uhac022. doi: 10.1093/hr/uhac022
Tusé, D., Ku, N., Bendandi, M., Becerra, C., Collins, R., Langford, N., et al. (2015). Clinical safety and immunogenicity of tumor-targeted, plant-made id-KLH conjugate vaccines for follicular lymphoma. Biomed. Res. Int. 2015, 648143. doi: 10.1155/2015/648143
Wang, X., Karki, U., Abeygunaratne, H., UnnoldCofre, C., Xu, J. (2021). Plant cell-secreted stem cell factor stimulates expansion and differentiation of hematopoietic stem cells. Process Biochem. 100, 39–48. doi: 10.1016/j.procbio.2020.09.029
Wang, Y., Liu, W., Wang, X., Yang, R., Wu, Z., Wang, H., et al. (2020). MiR156 regulates anthocyanin biosynthesis through SPL targets and other microRNAs in poplar. Hortic. Res. 7, 118. doi: 10.1038/s41438-020-00341-w
Ward, B. J., Makarkov, A., Séguin, A., Pillet, S., Trépanier, S., Dhaliwall, J., et al. (2020). Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (≥ 65 years): Two multicentre, randomised phase 3 trials. Lancet 396, 1491–1503. doi: 10.1016/S0140-6736(20)32014-6
Webster, G. R., Teh, A. Y. H., Ma, J. K. C. (2017). Synthetic gene design-the rationale for codon optimization and implications for molecular pharming in plants. Biotechnol. Bioeng. 114, 492–502. doi: 10.1002/bit.26183
Xu, J., Towler, M., Weathers, P. J. (2018). “Platforms for plant-based protein production,” in Bioprocessing of plant in vitro systems (Cham: Springer), 509–548. doi: 10.1007/978-3-319-54600-1_14
Yuki, Y., Nojima, M., Kashima, K., Sugiura, K., Maruyama, S., Kurokawa, S., et al. (2022). Oral MucoRice-CTB vaccine is safe and immunogenic in healthy US adults. Vaccine 40, 3372–3379. doi: 10.1016/j.vaccine.2022.04.051
Zahmanova, G., Mazalovska, M., Takova, K., Toneva, V., Minkov, I., Peyret, H., et al. (2021). Efficient production of chimeric hepatitis b virus-like particles bearing an epitope of hepatitis e virus capsid by transient expression in nicotiana benthamiana. Life 11, 64. doi: 10.3390/life11010064
Zeng, L., Zhang, Q., Jiang, C., Zheng, Y., Zuo, Y., Qin, J., et al. (2021). Development of atropa belladonna l. plants with high-yield hyoscyamine and without its derivatives using the CRISPR/Cas9 system. Int. J. Mol. Sci. 22, 1731. doi: 10.3390/ijms22041731
Zhang, P., Du, H., Wang, J., Pu, Y., Yang, C., Yan, R., et al. (2020). Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 18, 1384–1395. doi: 10.1111/pbi.13302
Zhao, T., Li, S., Wang, J., Zhou, Q., Yang, C., Bai, F., et al. (2020). Engineering tropane alkaloid production based on metabolic characterization of ornithine decarboxylase in atropa belladonna. ACS Synth. Biol. 9, 437–448. doi: 10.1021/acssynbio.9b00461
Zhou, W., Shi, M., Deng, C., Lu, S., Huang, F., Wang, Y., et al. (2021). The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in salvia miltiorrhiza. Hortic. Res. 8, 10. doi: 10.1038/s41438-020-00443-5
Zimran, A., Gonzalez-Rodriguez, D. E., Abrahamov, A., Cooper, P. A., Varughese, S., Giraldo, P., et al. (2018). Long-term safety and efficacy of taliglucerase alfa in pediatric gaucher disease patients who were treatment-naive or previously treated with imiglucerase. Blood Cells Mol. Dis. 68, 163–172. doi: 10.1016/j.bcmd.2016.10.005
Keywords: plant secondary metabolites, heterologous production, molecular farming, plant-made secondary metabolites, plant-made therapeutic proteins
Citation: Kulshreshtha A, Sharma S, Padilla CS and Mandadi KK (2022) Plant-based expression platforms to produce high-value metabolites and proteins. Front. Plant Sci. 13:1043478. doi: 10.3389/fpls.2022.1043478
Received: 13 September 2022; Accepted: 10 October 2022;
Published: 08 November 2022.
Edited by:
Polavarapu Bilhan Kavi Kishor, Osmania University, IndiaReviewed by:
T D Nikam, Savitribai Phule Pune University, IndiaCopyright © 2022 Kulshreshtha, Sharma, Padilla and Mandadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kranthi K. Mandadi, a2ttYW5kYWRpQHRhbXUuZWR1
†These authors have contributed equally to this work and share first authorship
 Aditya Kulshreshtha
Aditya Kulshreshtha Shweta Sharma
Shweta Sharma Carmen S. Padilla
Carmen S. Padilla Kranthi K. Mandadi
Kranthi K. Mandadi