- 1Department of Agronomy, Food, Natural Resources, Animals and the Environment, University of Padua, Padova, Italy
- 2Department of Land, Environment, Agriculture and Forestry, University of Padua, Padova, Italy
The SDHI fungicide Sedaxane has shown to efficiently control Rhizoctonia spp. growth and to possess biostimulant properties in cereal crops. As a first, the present study investigated its effectiveness as a seed treatment of the dicot species oilseed rape (Brassica napus var. oleifera). For this, seeds were treated with different fungicides: (i) the conventionally used active ingredient Thiram, (ii) Sedaxane, or (iii) Sedaxane in combination with Fludioxonil and Metalaxyl-M, and later sown in soil inoculated with Rhizoctonia solani. The resulting shoot and root growth from the treated seeds were recorded in early growth stages and the presence of Rhizoctonia DNA in the basal stem tissue was quantified. Here we demonstrate that all the fungicide treatments were effective in greatly reducing the presence of Rhizoctonia DNA, with Thiram confirming to have high fungicidal effects. Following seed treatment, shoot and root growth at the 2-leaf stage was reduced regardless of inoculation, indicating that the fungicides became phytotoxic, with particular respect to Thiram. In seedlings grown in inoculated soil, significant biostimulation of the roots was observed at the 4-leaf stage of treatments containing both Sedaxane alone and in a mixture. Leaf area was stimulated in control soil not inoculated with Rhizoctonia, likely due to improved PSII efficiency, stomatal conductance, and CO2 assimilation rate. Young oilseed rape seedlings are thus highly sensitive to seed treatments with these fungicides, and in particular to Thiram. The retardation in growth is quickly overcome by the 4-leaf stage however. We confirm that Sedaxane indeed possesses root biostimulant properties in oilseed rape, which are enhanced in combination with other fungicides. Such biostimulating properties impose its greatest effects under conditions of biotic stress.
1. Introduction
Seed coating with fungicides is known to provide effective protection from soil and seed-borne pathogens during germination and early growth stages of field crops (Mathre et al., 2001). Modern fungicide formulations for seed dressing often constitute a mixture of active ingredients (a.i.) each with different modes of action, thereby contrasting a wide range of pathogens (Kitchen et al., 2016). The soil-borne Rhizoctonia solani Kühn fungus (teleomorph: Thanatephorus cucumeris) is one of the major pathogens of oilseed rape (Huber et al., 1992). It is responsible for significant yield losses caused by reduced seed germination and plant growth (Acharya et al., 1984; Gugel et al., 1987). Oilseed rape can be infected by two different anastomosis groups (AGs) of R. solani, namely AG2-1 and AG4, which are both associated to damping-off, root rot and yield reductions. AG2-1 is the most virulent, aggravating oil production losses of up to 30% (Tahvonen et al., 1984; Kataria and Verma, 1992; Khangura et al., 1999). R. solani mycelium, which originates from wintering sclerotia, grows towards the plant and penetrates the epidermal cells of the roots and stem base (Sherwood, 1970; Armentrout and Downer, 1987). The fungi can enter the plant either passively through wounds, lenticels and stomata (Nakayama, 1940; Murray, 1982), or actively, via enzymatic and mechanical pathways (Trail and Kolller, 1990).
In recent years, various active ingredients (a.i.) have been made commercially available to implement chemical control of Rhizoctonia. Metalaxyl-M is effective against both Fusarium spp. and R. solani in oilseed rape. It reduces damping-off during germination by inhibiting RNA polymerase activity via a systemic translocation pathway (Hwang et al., 2007). Seed application of the a.i. Fludioxonil is associated with a reduction of conidial germination in several fungi, such as snow mould (Microdochium nivale), Botrytis cinerea and Penicillium expansum. Fludioxonil inhibits the transport-associated phosphorylation of glucose and prevents glycerol synthesis (Leroux, 1996; Errampalli, 2004; Knauf-Beiter and Zeun, 2012). The combination of various fungicides has successfully been applied in various cereal crops, as for example the mixture Metalaxyl-M and Fludioxonil against root rot in buckwheat (Mondal, 2004). Nonetheless, some of these effective a.i. have already been withdrawn from the market in various countries (e.g., Thiram) or are in immediate risk of being revoked (e.g., Fludioxonil) due to their environmental impact and toxicity, therefore it is needed to search for new a.i.
Besides identifying the highly effective a.i. of fungicides, there has been a growing interest in exploring their plant biostimulant properties as supplementary effects when used in seed or leaf treatments. Fungal compounds conferring biostimulant effects relating to improved tolerance to abiotic stresses have already been discovered and characterised in various fungicides, including the ubiquinol oxidase inhibitors (Qol) strobilurins and azoles (Berdugo et al., 2012). The biostimulant effects of Sedaxane, a fungicide recently released on the market and classified as a pyrazole-carboxamide succinate dehydrogenase inhibitor (SDHI) have also been demonstrated (Zeun et al., 2013; Ajigboye et al., 2016). Sedaxane has shown to be able to efficiently control both seed- and soil-borne pathogenic fungi infection and growth, while also improving root growth, drought tolerance and shoot biomass in various cereal crops (Swart, 2011; Dal Cortivo et al., 2017). However, little is known on its concurrent effectiveness as a fungicide and biostimulant agent when used as a seed treatment in dicotyledonous species.
Given this background, the goals of this study were to (i) discriminate the antifungal efficacy against R. solani versus the biostimulant effect of Sedaxane in the dicotyledonous oilseed rape (Brassica napus var. oleifera), in comparison with the conventional fungicide Thiram (withdrawn since 2019 in Italy); (ii) assess whether the application of Sedaxane in combination with other active ingredients has greater efficacy as compared to Sedaxane alone in both controlling the pathogen and promoting plant growth; and (iii) highlight any physiological response, particularly relating to photosynthesis, to the tested fungicides in young oilseed rape plants. To achieve these goals, Sedaxane was applied as seed treatment on oilseed rape alone or in combination with Fludioxonil and Metalaxyl-M and compared to seeds treated with Thiram. The effects were measured in early growth stages of plants grown in rhizoboxes (2-leaf) and pots (4-leaf) containing soil inoculated with R. solani and compared to controls not inoculated. Root (depth, surface area and diameter, biomass) and shoot (leaf area and biomass, photosynthetic activity) parameters were assessed at plant harvest together with the amount of DNA of R. solani in the stem base tissues of oilseed rape through quantitative PCR (qPCR) analysis.
2. Materials and methods
2.1. Rhizobox and pot trials set-up
Plants of the oilseed rape variety SY-Harnas (Syngenta, Basel, Switzerland), provided by CETAPP (France), were grown both in transparent-wall rhizoboxes (45 cm high, 30 cm wide, 2.5 cm thick, 3.3 L volume) until 2-leaf stage, and in cylindrical PVC pots (50 cm high, 9 cm diameter, 3.1 L volume) until 4-leaf stage. Plants grown in both types of containers were cultured in a greenhouse at the “Lucio Toniolo” experimental farm of the University of Padua (Legnaro, Padua, NE Italy). Rhizoboxes and pots were filled with a sterilized mixture (48 h in an oven at 105°C) of silty-loam soil (pH 8.4) and fine sand (1:1 w/w). A recommended dose of fertilizers, corresponding to 100 kg N, 150 kg P2O5 and 300 kg K2O per hectare was added and carefully mixed with the substrate before rhizobox/pot filling.
Three fungicidal seed treatments, i.e the conventional fungicide Thiram, Sedaxane alone (Sdx) and Sdx in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx) were investigated under conditions in which the soil had been inoculated with R. solani (+ Rhizoctonia) or not (− Rhizoctonia), and results were compared with controls left untreated with fungicides (Table 1). Seed dressing was performed at Syngenta (CETAPP, France) by injecting the slurry formulation of fungicides into a chamber equipped with an air influx to allow for thorough seed mixing. The action of adhesive co-formulants ensured a complete and homogeneous distribution of the a.i. dose. This resulted in 8 treatments in both rhizobox and pot trials. The trials were arranged following a completely randomized experimental design with 3 replicates. Each replicate (i.e., rhizobox/pot) consisted of 3 subsamples (individual plants) (Figure S1).
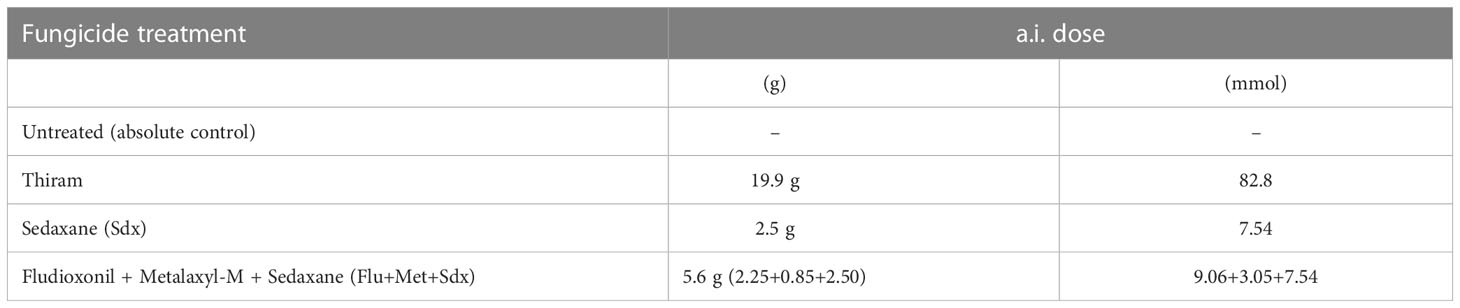
Table 1 List of seed treatments applied with or without R. solani in both rhizobox and pot trials and the amount of added active ingredients (a.i.) per 1 million (1 M) seeds.
Soil inoculation was performed before sowing by using barley seeds infected with R. solani strain RS 22 (AG2-1). Four infected barley seeds were applied to each rhizobox and pot as follows: seeds were pestled in a mortar and the resulting flour was mixed with 1 mL of milliQ water. The inoculum solution was equally divided and added to soil at three equally spaced positions, wherein oilseed rape seeds were sown a few days later. In order to avoid artefacts due to organic matter supply, four infected and sterilized (in autoclave at 120°C for 20 min) barley seeds were similarly applied in the treatments not inoculated (− Rhizoctonia) of both rhizoboxes and pots. As such, the treatments constituting non-treated soils contained inactivated pathogens. After soil inoculation, rhizoboxes and pots were kept at a constant temperature of 15°C for 10 days prior sowing to allow the development of the Rhizoctonia mycelium.
Three seeds of oilseed rape per rhizobox and pot were sown at 1 cm depth and equally spaced apart, in correspondence with the positions of pathogen inoculation. Plants were kept in the greenhouse at 20°C/15°C (day/night), 12h/12h (light/dark) and 70% air humidity. The whole rhizoboxes were covered with a black film to avoid any interaction of light with root growth through the transparent walls, and the rhizoboxes were placed at a 45° angle to facilitate un-destructive root observations. Oilseed rape plants were grown until 24 Days After Sowing (DAS; 2-leaf stage) in rhizoboxes and 28 DAS in the pot trial (4-leaf stage). Plants were regularly watered in both rhizoboxes and pots throughout the experiment.
2.2. Root growth analysis
Root growth parameters were revealed in the rhizobox trial only. Root depth was measured at 2-day intervals, from 6 to 17 DAS, by means of a ruler leaned on the lower transparent wall of the rhizoboxes. The dynamics of root deepening (Y) was fit with the Gompertz equation, as follows:
where a (asymptote), b (value of x at ½ a), and c (deepening rate) are coefficients and x is time (DAS).
At the end of the rhizobox trial, plants were harvested and their shoots and roots were separated. The roots were gently washed, separated from soil particles and stored in a 15% v/v ethanol solution until further processing. Root length, surface area and diameter were measured by analysis of 1-bit 300-DPI TIFF images of the root systems acquired through a flatbed scanner (Epson Expression 11000XL, Epson, Suwa, Japan) using the WinRhizo software (Regent Instruments, Ville de Québec, QC, Canada).
The dry weight of the roots was later measured in plants grown both in rhizoboxes and pots, after oven-drying for 48 h at 105°C.
2.3. Shoot parameters
Due to their greater development in comparison to plants grown in rhizoboxes, the photosynthetic activity was measured at 27 DAS only in plants grown in pots by means of an infrared gas analyzer LI-6800 (Li-COR Inc., Lincoln, Nebraska, USA). PSII Photosynthetic Efficiency (Fv′/Fm′), stomatal conductance, electron transport rate (ETR) and CO2 Net Assimilation (A) were determined following Murchie and Lawson (2013).
The Fv′/Fm′ ratio was measured as an index of efficiency in energy harvesting by the oxidized (open) reaction centres of photosystem II (PSII) in the last developed leaf, where Fv′ and Fm′ represent variable and maximal fluorescence, respectively. As Fm′ includes minimal fluorescence (F0′) of a dark-adapted leaf, Fv′ is calculated as Fm′ – F0′. Dark adaptation was set with the use of a far-red light to excite photosystem I (PSI), thus forcing electrons to drain from PSII. Only a few seconds of far-red light are needed to obtain this effect. The fluorimeter provides a “dark pulse” routine used to determine F0′. Five Fv′/Fm′ records were registered for each leaf of each replicate.
Leaf area was assessed after plant harvest at the end of both rhizobox and pot trials, by means of the LI-3100C Area Meter (Li-COR Inc., Lincoln, Nebraska, USA). Shoot dry biomass was determined after oven-drying for 48 h at 105°C.
2.4. Quantitative PCR of R. solani in stem base tissues
In order to quantify the presence of R. solani in oilseed rape plants, 1cm long portions of the stem base were collected from each plant grown in the pot trial. Three biological replicates (n = 3) per treatment were analyzed, each obtained by mixing 3 subsamples (plants) from every pot. Two technical replicates were performed on each biological replicate. Genomic DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Milan, Italy), quantified by agarose gel electrophoresis, and then used as template in a qPCR reaction. The qPCR was performed on a Rotor-Gene Q 2plex (Qiagen) using primers specific for Brassica napus ENTH gene (Yang et al., 2014), as the internal housekeeping control gene, and for the R. solani ARSF4 gene (Dubey et al., 2016). Primers used are reported in Table S1. The reaction mixture of 20 µL contained 10 μL of 2X Rotor-Gene SYBR Green PCR MasterMix (Qiagen), 0.5 μM of each specific primer and 1 μL of DNA template. The qPCR was performed by repeating the following cycle 40 times: 95°C for 30 s; 60°C for 30 s; 72°C for 45 s. Reactions were performed in triplicates.
Relative DNA quantification was performed using the Rotor-Gene v. 2.0.3.2 software and the tool REST (Qiagen).
2.5. Statistical analysis
Analysis of variance (ANOVA) was performed using CoStat software (CoHort software, Birmingham, UK) with Student Newmal-Keuls test in order to highlight significant differences among means at p ≤ 0.05, and significance of the main effects “inoculum”, “treatment” and “inoculum × treatment” interaction.
Principal component analysis (PCA) and factorial discriminant analysis (Multigroup Discriminant Analysis (MDA) with Wilks’ lambda and Pillai’s trace tests (Podani, 2007) were carried out using MS Excel XLSTAT (Addinsoft, Paris, France) to describe the response of oilseed rape in pots (4-leaf stage) to fungicide seed treatment and Rhizoctonia soil inoculum, in terms of shoot and root growth and photosynthetic parameters. Before analysis, multivariate data normality was verified by the Shapiro test using R 3.0.1 software (Ihaka and Gentleman, 1996), and data were standardized by subtracting the mean and dividing by the standard deviation within each variable.
3. Results
3.1. Rhizobox trial
3.1.1. Dynamics of root deepening
The inoculum with R. solani did not significantly affect the initial rooting of oilseed rape plants, which reached a maximum root depth of ~40 cm in absence of the pathogen (− Rhizoctonia) and ~35 cm in presence of the pathogen (+ Rhizoctonia) at 17 DAS (Figure 1). In non-inoculated soil, after an initial gap, the application of Flu+Met+Sdx and Sdx led to greater root depth as compared to the absolute control starting from 8 DAS. Instead, the use of Thiram caused retarded root deepening within the first 2 weeks (p ≤ 0.05), but similar values to controls at the end of the trial.
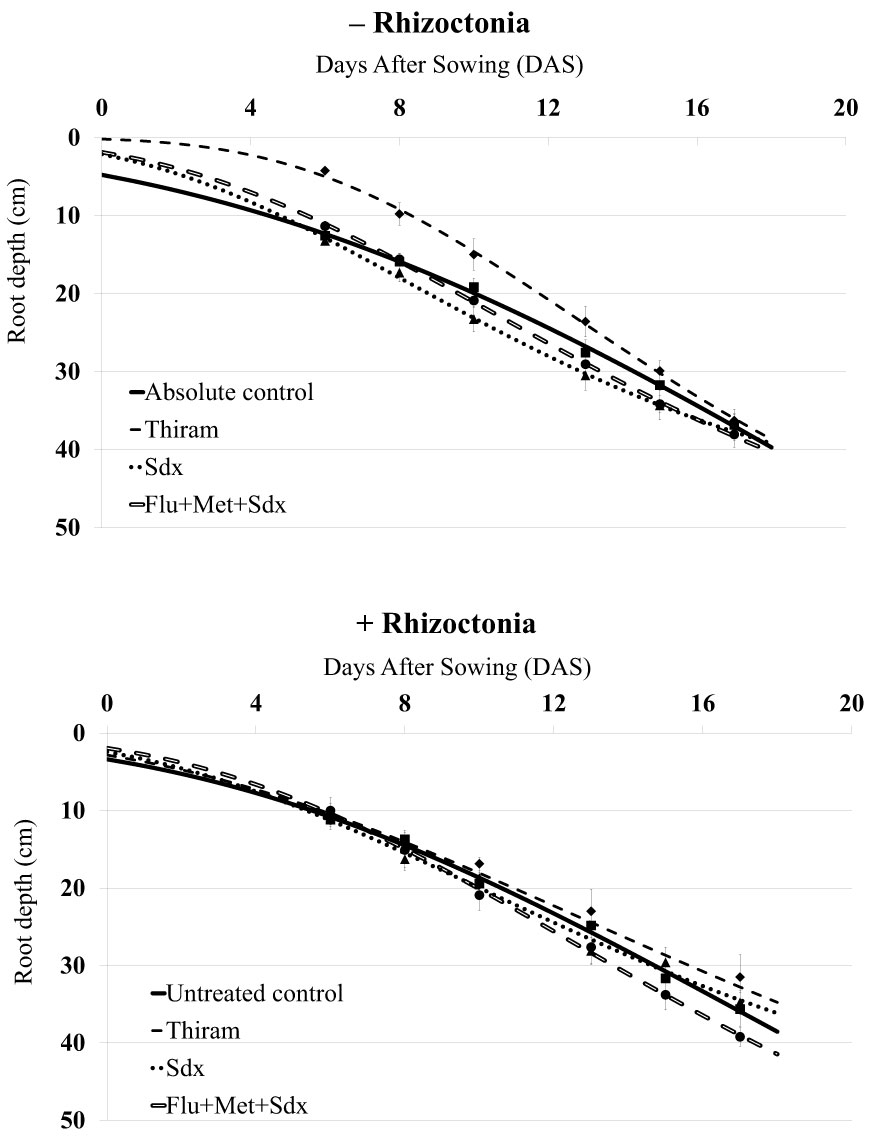
Figure 1 Dynamics of root deepening (cm; mean ± S.E.; n = 3) over 20 days after sowing (DAS) in oilseed rape plants grown in rhizoboxes, in soil inoculated (+ Rhizoctonia) and not inoculated (– Rhizoctonia) with R. solani, under three different seed treatments, i.e. Thiram and Sedaxane alone (Sdx), and Sdx in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx). Untreated control comprises plants grown in inoculated soil but without any seed treatment; absolute control comprises plants grown in non-inoculated soil and which received no seed treatment.
In R. solani inoculated soil (+ Rhizoctonia), only the seed treatment with Flu+Met+Sdx allowed for increased root depth starting from 10 DAS, as compared to all the other treatments. The plants treated with Thiram and Sdx showed substantially similar root deepening pattern to untreated controls (Figure 1). According to ANOVA (Table S2), the type of treatment had a significant effect (p ≤ 0.05), with Sedaxane alone or in combination with the other two a.i. (Flu+Met+Sdx) significantly improving the average root depth in comparison with Thiram over the investigated period.
3.1.2. Root surface area and diameter
In absence of any fungicide treatment, R. solani inoculum significantly reduced the root surface area (−44% vs. absolute control) and increased root diameter (+27%) of oilseed rape plants grown in rhizoboxes (Figure 2; Table S2).
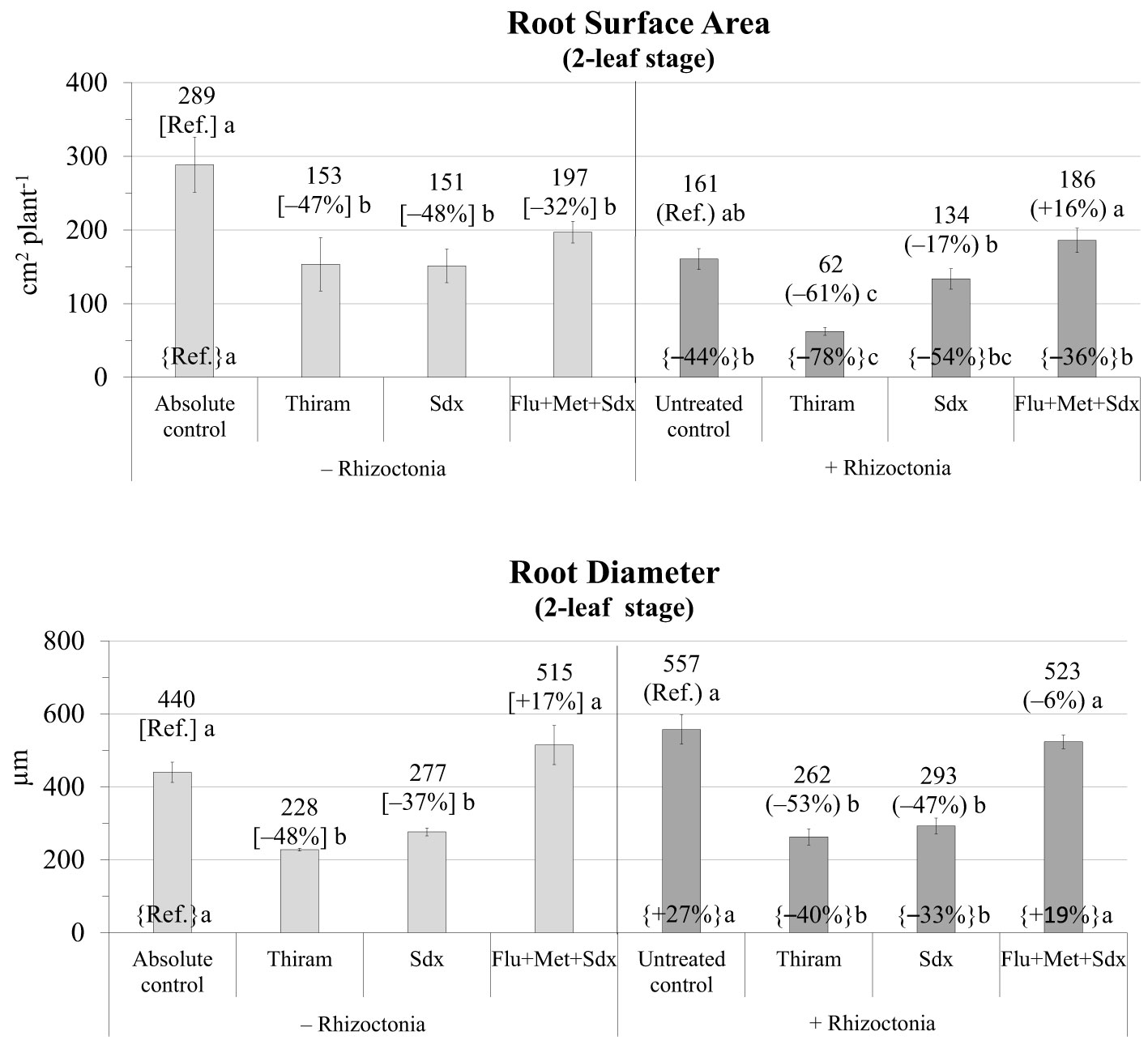
Figure 2 Root surface area (cm2 plant-1; mean ± S.E.; n=3) and diameter (µm; mean ± S.E; n = 3) of oilseed rape plants at 2-leaf stage (24 days after sowing) grown in rhizoboxes containing soil inoculated (+ Rhizoctonia) and not inoculated (– Rhizoctonia) with R. solani, under three different seed treatments, i.e. Thiram, Sedaxane alone (Sdx) and Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx). Different letters indicate significant differences among treatments (Newman–Keuls test, p ≤ 0.05) within the same soil conditions, i.e. + Rhizoctonia (round brackets) or – Rhizoctonia (squared brackets), and among + Rhizoctonia treatments and absolute control (brace brackets).
The two main fixed effects treatment and inoculum were statistically significant (p ≤ 0.001) in explaining root surface area, as well as their interaction (p ≤ 0.05) (Table S2). In particular, in not inoculated soil, root surface area was significantly reduced by all the fungicide treatments, with Thiram and Sedaxane having the most detrimental effect (–47% and –48% respectively, vs. absolute control, p ≤ 0.05). Thiram also induced a significant reduction in root surface area in inoculated soil (–61% vs. untreated control, p ≤ 0.05), Sedaxane a slight reduction (-17%), and Flu+Met+Sdx a slight increase (+16%) but which were both not statistically different from the untreated control.
Similarly, the ANOVA showed that both Inoculum (p ≤ 0.05) and Treatment (p ≤ 0.001) also strongly affected root diameter (Table S2). Root diameter was markedly (p ≤ 0.05) reduced by Thiram and Sdx treatments in both inoculated (–48% and –37%, respectively vs. absolute control) and not inoculated soil (–53% and –47%, respectively vs. untreated control). A full recovery of root diameter was recorded with the use of Sdx in combination with Fludioxonil+Metalaxyl-M (Flu+Met+Sdx), even leading to slight improvements vs. not inoculated soil (+19% vs. absolute control) (Figure 2).
3.1.3. Shoot growth parameters
The ANOVA revealed that leaf area and shoot biomass were significantly affected by both Treatment (p ≤ 0.001) and Inoculum and their interaction (p ≤ 0.05) (Table S2). Without any seed treatment, inoculum with R. solani significantly reduced the leaf area (–14% vs. absolute control) and particularly the shoot dry biomass (–34%) of oilseed rape plants cultivated in rhizoboxes (Figure 3; Table S2). Leaf area was only slightly reduced (p > 0.05) with all the fungicides in both not inoculated and R. solani inoculated soil however, while Thiram showed marked phytotoxicity in infected soil (–68% vs. untreated control, p ≤ 0.05).
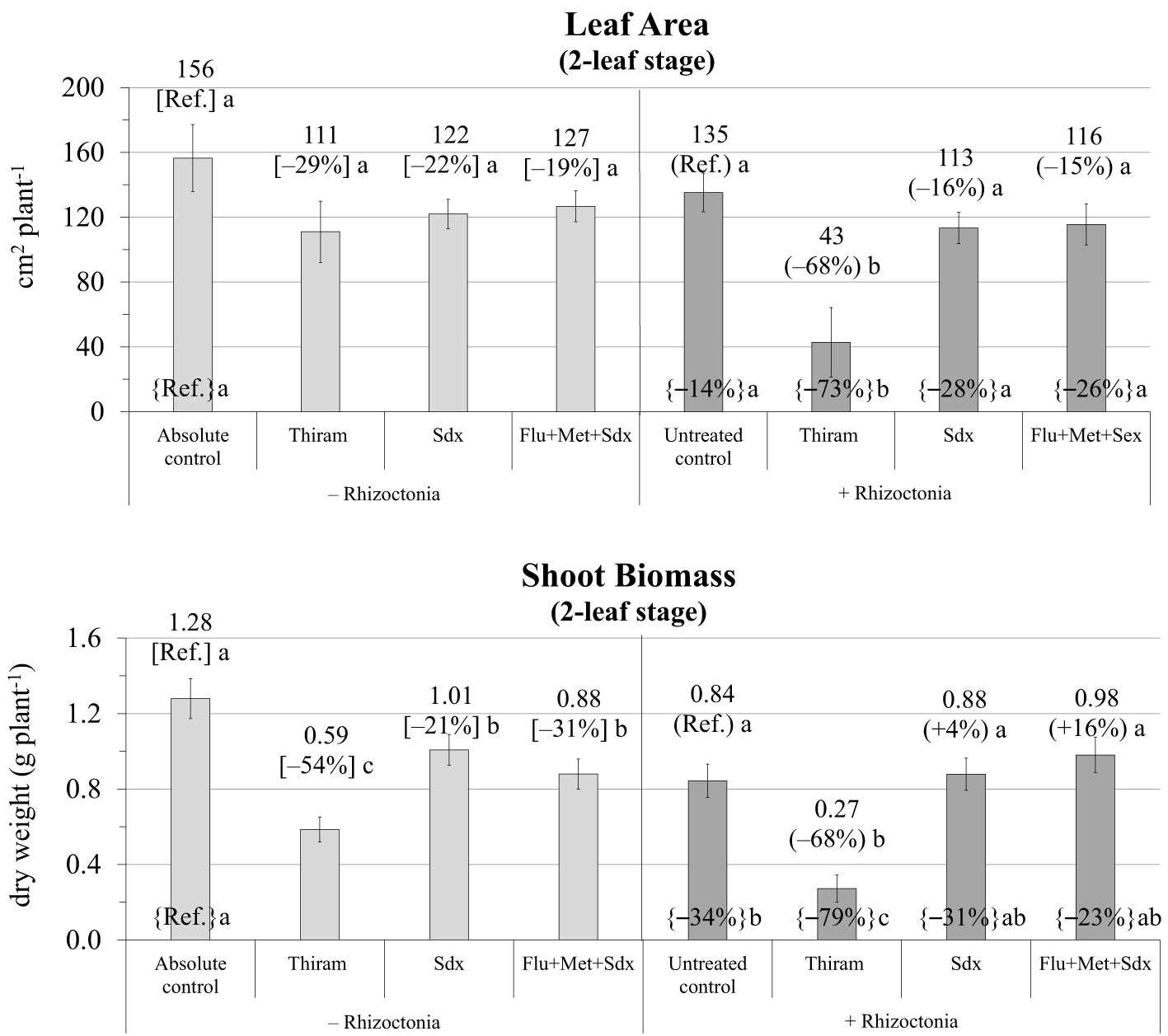
Figure 3 Leaf area (cm2 plant-1; mean ± S.E.; n=3) and shoot biomass (g dry weight plant-1; mean ± S.E.; n = 3) of oilseed rape plants at 2-leaf stage (24 days after sowing) grown in rhizoboxes, containing soil inoculated (+ Rhizoctonia) and not inoculated (– Rhizoctonia) with R. solani under three different seed treatments, i.e. with Thiram, Sedaxane alone (Sdx) and Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx). Different letters indicate significant differences among treatments (Newman–Keuls test, p ≤ 0.05) within the same soil conditions, i.e. + Rhizoctonia (round brackets) or – Rhizoctonia (squared brackets), and among + Rhizoctonia treatments and absolute control (brace brackets).
As regards shoot biomass, a similar trend as leaf area was observed; a significant decrease by Thiram in both inoculated (–68% vs. untreated control) and non-inoculated soil (–54% vs. absolute control). Under soil infection, Sedaxane alone and in combination with other fungicides (Flu+Met+Sdx) allowed an appreciable recovery of shoot dry biomass, which was statistically similar to the absolute control (Figure 3).
3.2. Pot trial
3.2.1. Root biomass
According to the ANOVA in the pot trial, the effect of Treatment and the Treatment × Inoculum interaction both had a significant effect on root biomass (p ≤ 0.05) (Table S3). Indeed, at the later growth stage of 4 leaves in the pot trail, root biomass of plants cultivated in soil infected with R. solani and without any fungicide treatment (untreated control) was still reduced (–20% vs. absolute control) (Figure 3; Table S3). The application of Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx) allowed maximal root dry weight both in R. solani inoculated (+48% vs. untreated control, p ≤ 0.05) and non-inoculated soil (+19% vs. absolute control). In inoculated soil (+ Rhizoctonia), also the application of Sedaxane alone (Sdx) significantly increased root dry biomass by +37% (p ≤ 0.05) (Figure 4).
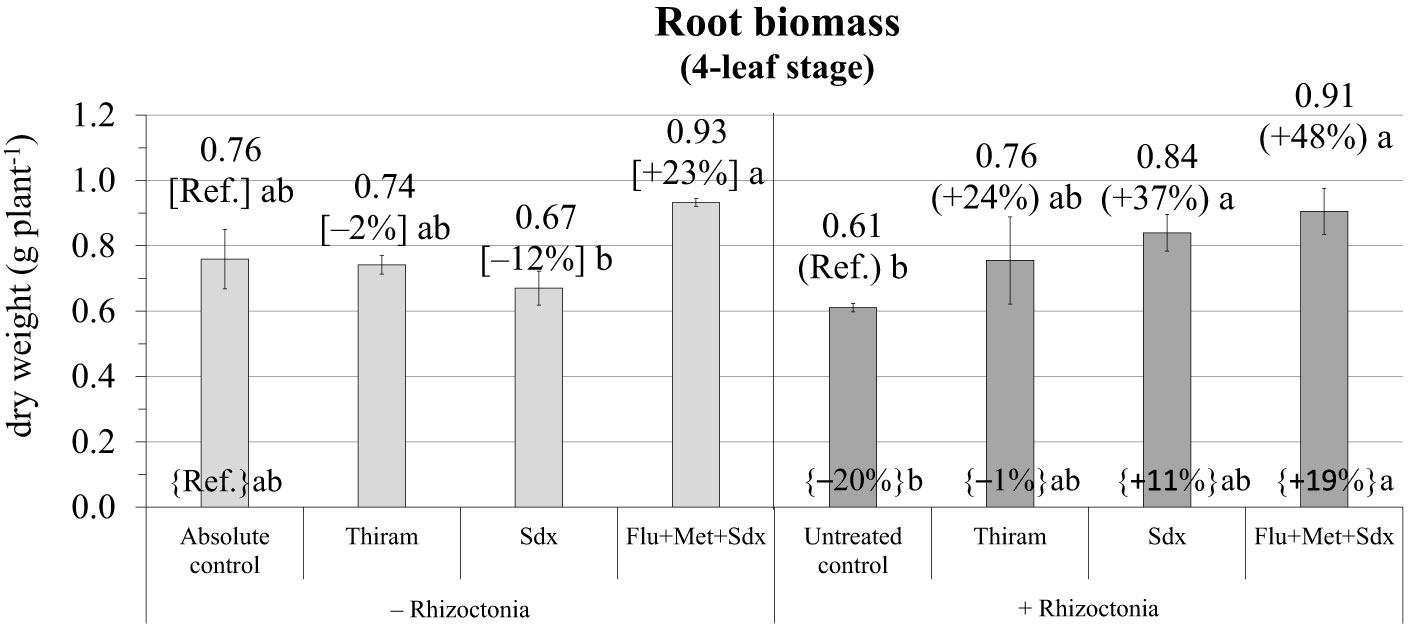
Figure 4 Root biomass (g dry weight plant-1; mean ± S.E.; n = 3) of oilseed rape plants at 4-leaf stage (28 days after sowing) grown in pots, containing soil inoculated (+ Rhizoctonia) and not inoculated (– Rhizoctonia) with R. solani, under three different seed treatments, i.e. Thiram, Sedaxane alone (Sdx) and Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx). Different letters indicate significant differences among treatments (Newman–Keuls test, p ≤ 0.05) within the same soil conditions, i.e. + Rhizoctonia (round brackets) and – Rhizoctonia (squared brackets), and among + Rhizoctonia treatments and absolute control (brace brackets).
3.2.2. qPCR Quantification of R. solani in the Stem Base Tissues
The presence of R. solani DNA was quantified in the stem base tissue of oilseed rape plants of the pot trial. The quantification was carried out in plants grown in inoculated soil (+ Rhizoctonia) and which had received a seed treatment, and compared to untreated plants as well as the absolute control (– Rhizoctonia). All the fungicides drastically reduce the presence of the fungal DNA in the plant tissues (Figure 5). There was an absence of visible (shoot) symptoms/injuries by Rhizoctonia on all plants regardless of treatment and soil condition. Compared to untreated controls, Thiram and Flu+Met+Sdx reduced the amount of fungal DNA by ~99% leading to a complete absence of R. solani DNA, similarly to the absolute control, where the pathogen was never applied to the soil. Sedaxane was also very effective at reducing the presence of R. solani DNA by ~97% (Figure 5).
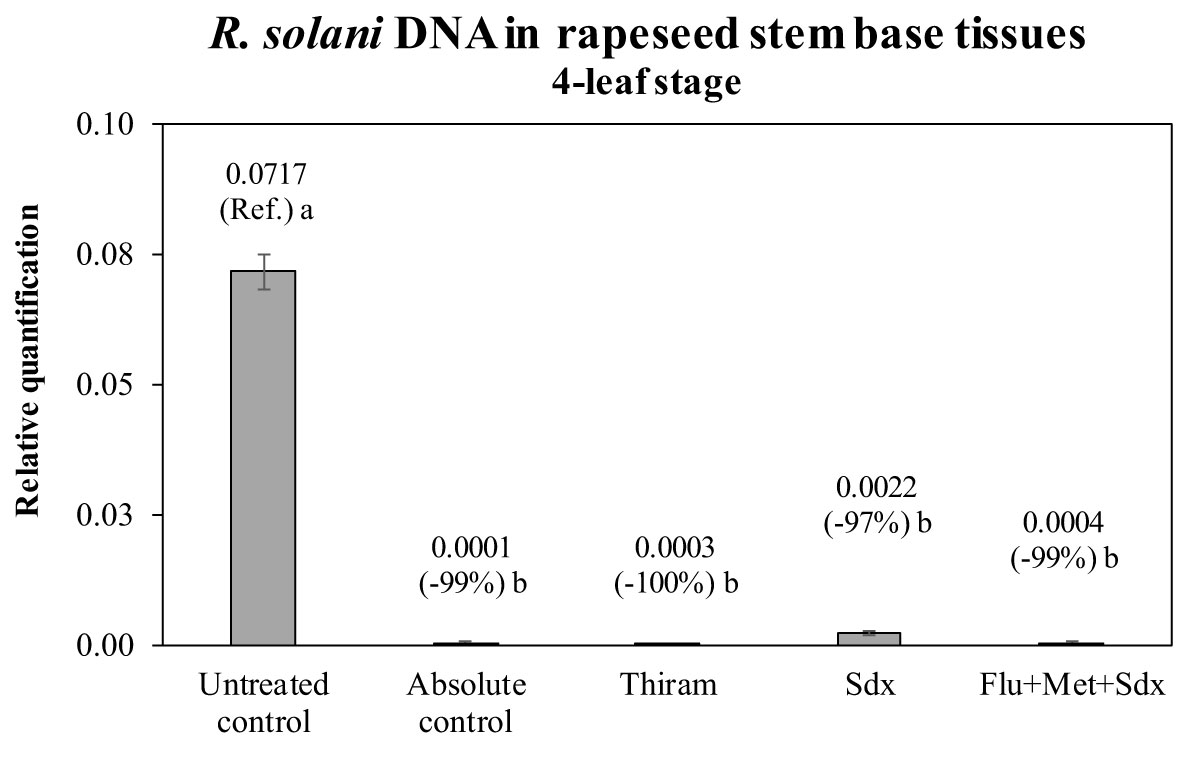
Figure 5 Relative quantification of R. solani DNA (mean ± S.E.; n = 3) in stem base tissues of oilseed rape plants at the 4-leaf stage (28 days after sowing) grown in pots containing soil inoculated with R. solani (+ Rhizoctonia), under three different seed treatments, i.e. Thiram, Sedaxane alone (Sdx) and Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx). Untreated control comprises plants grown in inoculated soil but without any seed treatment; absolute control comprises plants grown in non-inoculated soil and which received no seed treatment. Different letters indicate significant differences among treatments (Newman–Keuls test, p ≤ 0.05).
3.2.3. Shoot parameters
The ANOVA disclosed Treatment as being the only fixed effect with a significant (p ≤ 0.05) effect on leaf area (Table S3). Indeed, oilseed rape plants grown in non-inoculated soil (– Rhizoctonia) showed increased leaf area at the 4-leaf stage following seed treatment with all the tested fungicides. In particular, significant biostimulation by Sedaxane in combination with Fludioxonil and Metalaxyl-M was observed (+53%; p ≤ 0.05) (Figure 6). However, no effect of fungicide treatment was disclosed under conditions of soil infection, although treatment with Flu+Met+Sdx slightly, but insignificantly, increased leaf area compared to untreated controls (+7%, p > 0.05).
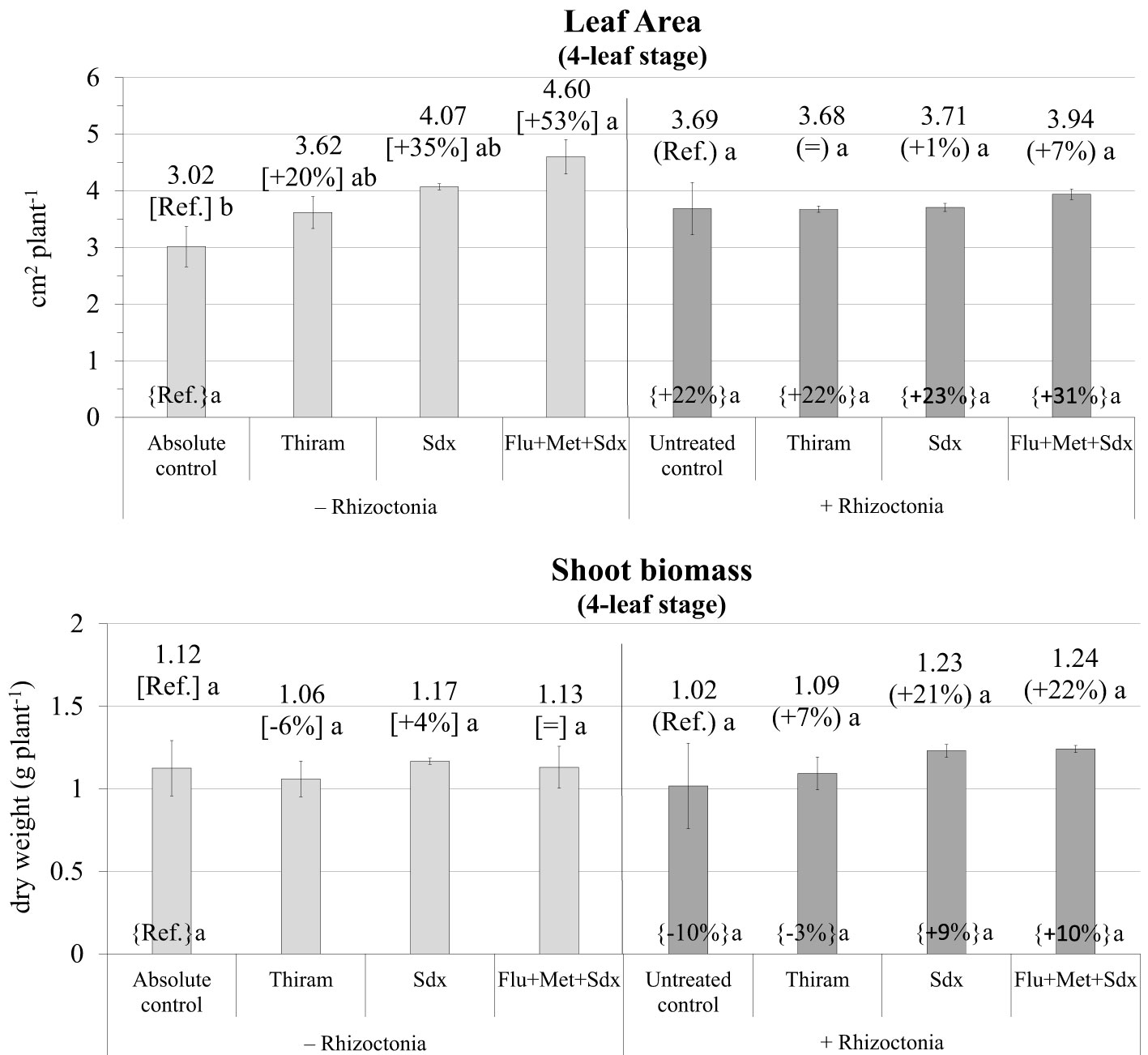
Figure 6 Leaf Area (cm2 plant-1; mean ± S.E.; n=3) and shoot biomass (g dry weight plant-1; mean ± S.E.; n = 3) of oilseed rape plants at 4-leaf stage (28 days after sowing) grown in pots containing soil inoculated (+ Rhizoctonia) and not inoculated (– Rhizoctonia) with R. solani under three different seed treatments, i.e. with Thiram, Sedaxane alone (Sdx) and Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx). Different letters indicate significant differences among treatments (Newman–Keuls test, p ≤ 0.05) within the same soil conditions, i.e. + Rhizoctonia (round brackets) or – Rhizoctonia (squared brackets), and among + Rhizoctonia treatments and absolute control (brace brackets).
As regards shoot biomass, the ANOVA did not reveal any significant effect from neither Inoculum nor Treatment (Table S3). In fact, no significant variations were observed in shoot dry biomass regardless of soil inoculation and fungicide treatment. However, with soil inoculation, both Sdx and Flu+Met+Sdx treatments increased shoot biomass by -20% compared to untreated controls although this was statistically not significant (p > 0.05).
3.2.4. Leaf photosynthetic efficiency
According to the ANOVA, PSII efficiency, expressed as ratio between variable and maximum fluorescence (Fv′/Fm′), was significantly affected by Treatment (p ≤ 0.001) and to a lesser extent by Inoculum (p ≤ 0.05) at the 4-leaf stage. Neither Inoculum nor Treatment main effects significantly affected ETR or any other physiological parameter (Table S3). The efficiency of photosystem II, and the electron transport rate were fairly stable across seed treatments, with no significant effect from Rhizoctonia inoculation. Only Sedaxane applied alone reduced the two parameters within a range from –4% to –5% in non-inoculated (p > 0.05; n.s.) and from –10% to –16% in inoculated soil (p ≤0.05) for the PSII efficiency and ETR respectively. An increase trend was observed in the PSII efficiency and ETR (p > 0.05) when the Flu+Met+Sdx seed treatment was given to plants grown in non-inoculated soil (Figure 7).
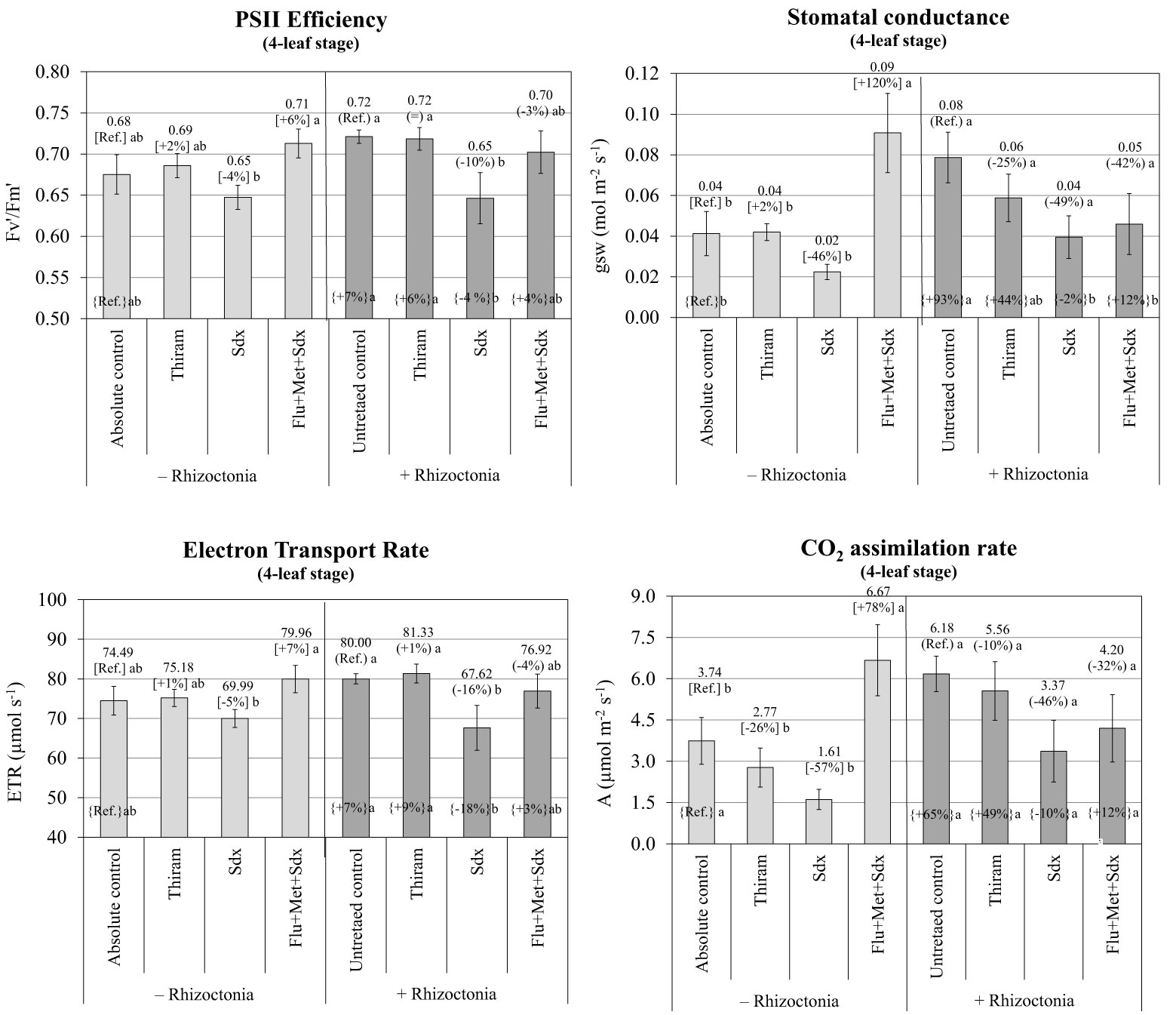
Figure 7 Photosynthetic efficiency of photosystem II (PSII) (Fv′/Fm′), stomatal conductance (gsw), electron transport rate (ETR) and CO2 assimilation rate (A) (mean ± S.E.; n=3) of oilseed rape plants at 4-leaf stage (28 days after sowing) grown in pots containing soil inoculated (+ Rhizoctonia) and not inoculated (– Rhizoctonia) with R. solani under three different seed treatments, i.e. with Thiram, Sedaxane alone (Sdx) and Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx). Different letters indicate significant differences among treatments (Newman–Keuls test, p ≤ 0.05) within the same soil conditions, i.e. + Rhizoctonia (round brackets) or – Rhizoctonia (squared brackets), and among + Rhizoctonia treatments and absolute control (brace brackets).
A similar trend was observed in regards to the variation in stomatal conductance (gsw) and CO2 assimilation rate (A) in non-inoculated soil (– Rhizoctonia). Here, a +120% (p ≤0.05) and +78% (p ≤ 0.05) increase followed seed treatment with Sedaxane in combination with Fludioxonil and Metalaxyl-M for gsw and CO2 assimilation rate respectively (Figure 7). In this soil condition, the use of Sedaxane alone was instead associated with a relevant decrease, but insignificant, of both stomatal conductance (–46%) and CO2 assimilation rate (-57%) in comparison to the absolute control, and lower and not significant variations were observed with Thiram.
Noteworthy, soil inoculation by R. solani allowed for a relevant increase in both stomatal conductance (+93%; p ≤0.05) and CO2 assimilation rate (+65%; p > 0.05) as compared to the absolute control. Under soil infection, a decrease of both stomatal conductance and CO2 assimilation was revealed following all the fungicide treatments, in particular with Sedaxane alone and in a mixture (Flu+Met+Sdx). However, these treatments merely permitted stomatal conductance and CO2 assimilation rates to reach comparable levels to those of the absolute control.
3.2.5. MDA and PCA
Principal Component Analysis (PCA) applied to the pot trial allowed for the identification of two synthetic variables, F1 (64.42%) and F2 (18.59%), which explained an overall variability of 81.02% (Figure 8). The most significant variables (loadings > |0.5|) were shoot and root biomass, stomatal conductance (gsw) and CO2 assimilation rate (A) associated to F2. According to the vector direction of each variable in PCA, there is strong positive correlation among all the parameters related to the photosynthetic activity, and a clear negative correlation between shoot dry biomass and leaf area.
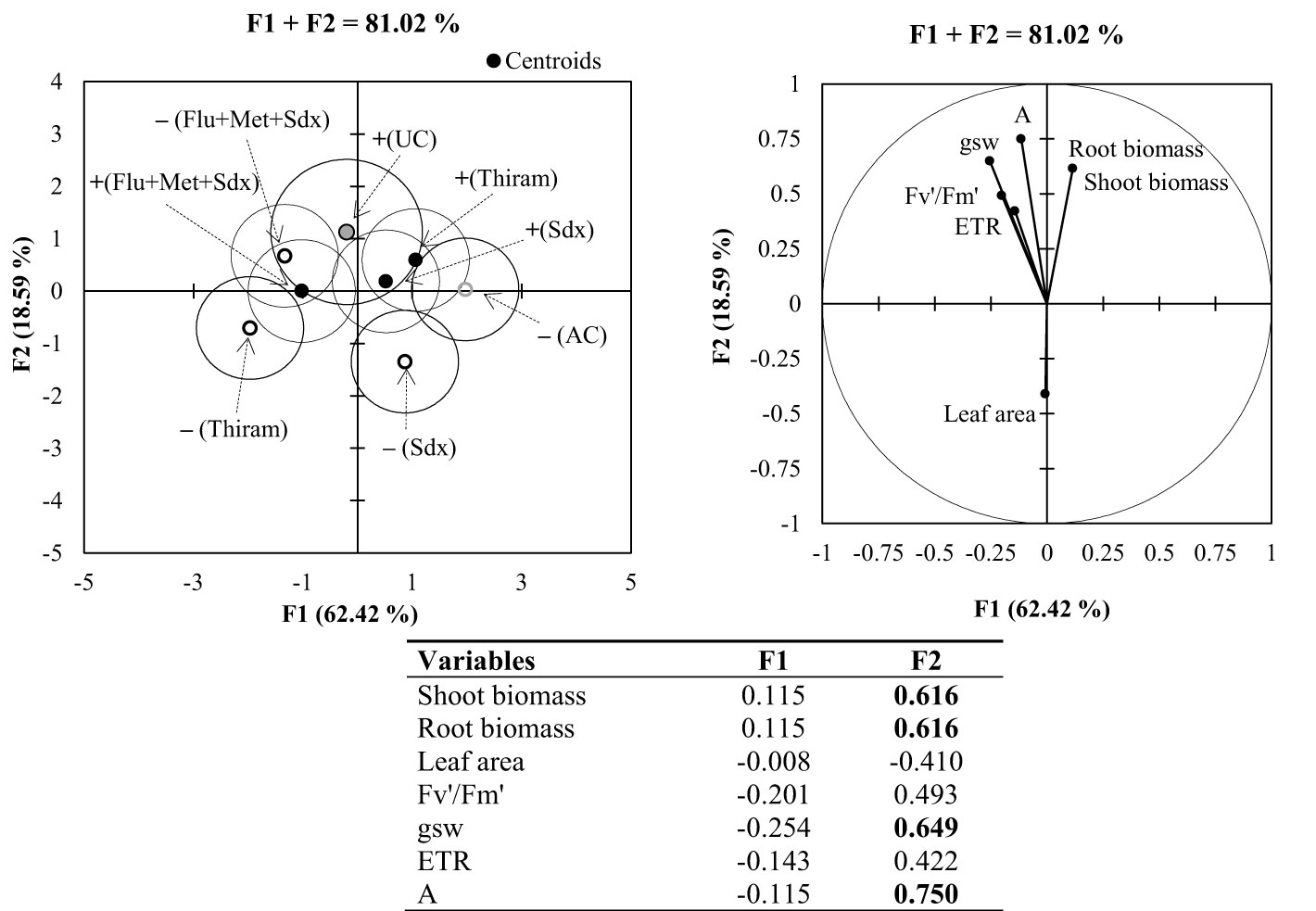
Figure 8 Multigroup discriminant analysis (MDA; left) and principal component analysis (PCA; right) for shoot and root parameters of oilseed rape plants at 4-leaf stage (28 days after sowing) grown in pots, containing soil inoculated (+ in the MDA) and not inoculated (– in the MDA) with R. solani under three different seed treatments, i.e. Thiram, Sedaxane alone (Sdx) and Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx). In MDA: UC: untreated control (+Rhizoctonia), AC: absolute control (–Rhizoctonia). In the PCA: Fv′/Fm′: photosynthetic efficiency of photosystem II (PSII); gsw: stomatal conductance; ETR: electron transport rate; A: CO2 assimilation rate. The isodensity confidence circles contain 75% of the variability. In the bottom table, highly informative variables (loadings > |0.5|) highlighted in bold, within synthetic variables F1 and F2.
According to the centroids position and cluster separation in MDA, a different plant response was observed under the investigated treatments. Within non-inoculated soil, the effects of seed treatment with Sedaxane in combination with Fludioxonil and Metalaxyl-M (Flu+Met+Sdx) was mostly related to variations in physiological parameters of leaf photosynthesis. Instead, the effects of Thiram and Sedaxane alone were mostly associated to variations in plant biomass (both root and shoot) under soil inoculation, and leaf area in non-inoculated soil.
4. Discussion
The use of antifungal ingredients for seed treatment has been receiving increasing interest in recent years not only due to their effective control of seed- and soil-borne pathogens during early growth stages, but also due to their possible biostimulant properties, particularly on plant roots. Among recently released fungicides, Sedaxane has proven to have a broad-spectrum activity, particularly in regards to R. solani and Mycosphaerella reliana (Swart, 2011; Dal Cortivo et al., 2017) growth control, and its registration approval in cereal crops is granted more frequently worldwide.
In the present study, we innovatively investigated the effects of Sedaxane on oilseed rape, as a first example of application in seed treatment in a dicotyledonous species. This was done to compare its effects with already existing and commercially available fungicides. The objective to distinguish the protective effect from possible biostimulant properties is also innovative, with this approach being scarcely applied in literature. Accordingly, the trials were conducted in R. solani inoculated and non-inoculated soil of previously sterilized substrate. It cannot be ruled out however, that sterilization may have altered plant growth to a certain extent due to inactivation of soil microbes.
We here demonstrate that all the investigated fungicides efficiently protected against R. solani; the level of Rhizoctonia DNA at plant collar was reduced by at least 97%. This justifies the absence of visible symptoms of pathological infection on the plants. Since roots are the main plant organ targeted by Rhizoctonia, without seed protection the infection caused marked root growth impairments (–45% in surface area at 2 leaves and –20% of biomass at 4 leaves). The full recovery of root biomass at the 4-leaf stage in plants that received seed treatment therefore illustrates the effectiveness of the formulations and doses of all tested fungicides.
The possession of secondary biostimulation effects in the fungicides were assessed as additional growth compared with the absolute control (no Rhizoctonia, no seed treatment). As such, Sedaxane mixed with Fludioxonil and Metalaxyl-M had ultimately (4-leaf stage, end of the trial) a clear root biostimulating power, as conveyed by the increased root biomass in both the R. solani inoculated soil and non-inoculated soil. This might be due to a synergistic effect of the three fungicides due to their differing modes of action (MOA) and target sites in pathogens. Indeed, Fludioxonil is a preventive fungicide, while Metalaxyl-M and Sedaxane have systemic activity. Fludioxonil targets the histidine kinase enzyme involved in osmotic signal transduction (MOA E2), Metalaxyl-M the RNA polymerase I enzyme (MOA A1), while Sedaxane is a succinate dehydrogenase inhibitor (SDHI) blocking fungus respiration (MOA C2). When applied alone, Sedaxane showed significant biostimulating effects on root biomass under Rhizoctonia-inoculated soil, but not in non-inoculated soil. Indeed, sedaxane has already been reported to have auxin- and gibberellin-like activities in various cereals crops (Zeun et al., 2013; Dal Cortivo et al., 2017). Auxins are known to exert a central role in primary root elongation, lateral root initiation, and root hair development, and this is expected to be the main mechanism of root stimulation by Sedaxane. The gibberellic activity, on the other hand, could be responsible for better shoot growth and leaf expansion (Fleet and Sun, 2005), which are both strategic in the open field to ensure fast plant establishment in the sensitive early phases of growth. Although in early oilseed rape stages (2 leaves) all the tested fungicide formulations exerted a significant shoot and root phytotoxicity, within the 4-leaf stage a full growth recovery was ascertained. At this time, Sedaxane either alone and in a mixture with other fungicides allowed improved shoot growth of oilseed rape particularly under Rhizoctonia pressure, possibly due to its gibberellinic-like activity. In the same way, in non-inoculated soil, improved leaf expansion, PSII efficiency and stomatal conductance could be observed as the main beneficial effects of the Sedaxane fungicide mixture.
The initial shoot and root phytotoxicity was associated particularly to the use of Thiram, possibly due to its higher dosage as compared to the other a.i. used in these trials. An over-dosage of fungicides has been reported to negatively affect plant growth and development, with evident alteration of plant physiological processes, such as nitrogen metabolism and photosynthetic activity (Saladin et al., 2003; Petit et al., 2008). For instance, the application of strobilurin analogs was found to reduce the Fv′/Fm′ fluorescence ratio in soybean, linked to an electron transport block from PSII to PSI (Nason et al., 2007). A decrease in net photosynthesis was also correlated to an increase in fungicide doses in grapevine and pea (Saladin et al., 2003; Nason et al., 2007). Indeed, fungicides could reduce the activity of enzymes involved in the synthesis of chlorophyll and other foliar pigments (i.e., carotenoids), thus causing reduced CO2 assimilation, and thereby biomass accumulation and yield (Shahid et al., 2018). These effects were clearly found at 2-leaf stage in our rhizobox trial, with reduced shoot biomass as well as leaf area with the use of Thiram, regardless of soil inoculation.
Although the initial phytotoxicity was found to be transiently affecting oilseed rape with the use of all the tested fungicide formulations, the reasons as to why root growth was retarded (but to a lesser extent shoot) with Sedaxane use in either lone use or in mixture, in absence of soil inoculation remain unclear. In previous studies (Rose et al., 2018; Shahid et al., 2018), decreases in shoot biomass in pea and lupin were associated with a modification or inhibition of the activity of various enzymes involved in growth, development and metabolism when treated with systemic fungicides, such as Kitazin, Hexaconazole and Carbendazim, following their translocation from root to shoot through the xylematic sap flow. This cannot be the case for Thiram as it is not a systemic a.i. Indeed it is thought that fungicide phytoxicity is related to cellular damage and production of Reactive Oxygen Species (ROS), which can reduce synthesis of lipids, proteins, and nucleic acids, and provoke inefficient water and nutrient uptake (Yilmaz et al., 2017; Shahid et al., 2018). This would explain transient below-ground phytotoxicity of Sedaxane in correspondence with the penetration/uptake sites of the fungicide, even in absence of pathogen pressure. We therefore suspect that oilseed rape is highly sensitive to seed treatments with fungicides, regardless of a.i. choice, and the observed growth retard was likely exacerbated by the small soil volume in rhyzoboxes used for assessing plant growth at the early stage.
Despite efficient exclusion of Rhizoctonia DNA from the stem base tissues of oilseed rape, the presence of mycelium in the rhizosphere is also hypothesized to secrete toxic compounds which affect root growth and development in the host plant. However, this requires further specific investigations to be confirmed. Through secondary metabolism, R. solani can produce several compounds that aid the infection process, such as fatty acids, steroids, phenolic compounds and glycoproteins, all of which are also associated to root phytotoxicity (Mandava et al., 1980; Adachi and Inagaki, 1988; Velazhahan and Vidhyasekaran, 2000; Ma et al., 2004; Aliferis and Jabaji, 2010). However, Sedaxane in combination with Fludioxonil and Metalaxyl-M, was capable of partially recovering the root surface area at the 2-leaf stage under R. solani pressure.
The question which arises is why root biostimulation with Sedaxane is better expressed under Rhizoctonia soil infection rather than without the pathogen. Actually, we argue that in presence of this pathogen a priming effect probably exists in oilseed rape plants, which involves a physiochemical response capable of corroborating the biostimulant effect of the fungicide. Priming is known to activate systemic defense responses only when the plant is challenged by a pathogen or is exposed to abiotic stress (Aranega-Bou et al., 2014). Since natural or synthetic chemicals and infection by pathogens can induce priming effects in plants (Aguado et al., 2019; Westman et al., 2019; De Vega et al., 2021), we hypothesize that the greater positive effect observed with the use of Sedaxane alone, and to a greater extent when in combination with Fludioxonil and Metalaxil-M and in the presence of R. solani could be ascribed to a plant response related to priming. While this hypothesis requires further investigation to clarify the possible involved mechanisms, Sedaxane in combination with Fludioxonil and Metalaxyl-M can be agronomically exploited in a dicot species such as oilseed rape to control Rhizoctonia. A faster growth within the 4-leaf stage would also allow oilseed rape to quickly overcome the initial delicate phases of growth, with possible improved tolerance against abiotic stresses as well. Soil sterilization of rhizoboxes and pot trials was necessary to disentangle the fungal protection properties from the biostimulating effects of Sedaxane, while further investigations in real field conditions will be necessary to ascertain possible interactions with the soil microbiota and the molecular cross-talk of biopriming.
5. Conclusions
Seed treatment with Sedaxane is consolidating in cereal crops, showing successful control of a wide spectrum of fungal pathogens, particularly R. solani, which can be exploited agronomically. Here we confirm an efficient control of this pathogen also in the dicot oilseed rape by Sedaxane either alone or in combination with Fludioxonil and Metalyl-M. Commercial formulations with low contents of these a.i are good candidates for replacing the over-used conventional fungicide Thiram, which is being withdrawn in various countries worldwide. Although all the fungicides tested here had initial phytotoxicity, Sedaxane exhibited clear root biostimulant properties as a supplementary effect to its antifungal features, like similarly observed in cereal crops. This suggests new opportunities for exploiting the biostimulant value of this fungicide in other Brassicaceae species, such as turnip, cabbage, kale, and leaf rape. The optimal biostimulant effects of Sedaxane are observed in combination with other a.i. and under high Rhizoctonia pressure however. As seed treatments require small amounts of fungicides in the field, the discovery of new a.i. or natural compounds with double effects at low doses is expected to reduce the impact of agrochemicals on the environment. This is advantageous both for the agricultural industry and for improving sustainable practices.
Our preliminary results suggest that young oilseed rape seedlings are highly sensitive to seed treatments with fungicides, particularly Thiram, but the growth retardation is quickly overcome within the 4-leaf stage. At this developmental stage, the biostimulant properties could be observed aboveground as well. While fungicide biostimulation is associated with improved photosynthetic efficiency and stomatal conductance in absence of Rhizoctonia, further investigations will be necessary to clarify the physiological mechanisms sustaining the maximal stimulating effect under high pathogen pressure and whether these results are confirmed in the open field.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Conceptualization, TV: Methodology, TV and LS: Investigation, FC, ADS: Resources, TV: Data curation, FC, ADS. and GB: Writing—original draft preparation, FC, ADS and AP: Writing-review and editing, AP, SSM, LS and TV: Supervision, TV: Project administration, TV: Funding acquisition, TV. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Syngenta Crop Protection (Milan, Italy), research title “Root protection and biostimulant effects of seed-applied fungicides in oilseed rape under high Rhizoctonia pressure”.
Acknowledgments
The project BIRD228113/22 from the University of Padova, entitled "Fermentation of plant products and by-products of their processing to obtain functional foods and bioactive compounds", is gratefully acknowledged. The authors wish to thank Adriano Massignan for his help in the experimental management. Also, a special thanks to Silvana Odorizzi and Carla Castiglioni for carrying out q-PCR analysis.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1130825/full#supplementary-material
References
Acharya, S. N., Verma, P. R., Dueck, J., Downey, R. K. (1984). Screening rapeseed/canola for resistance to damping-off and seedling root rot caused by Rhizoctonia solani. Can. J. Plant Pathol. 6, 325–328. doi: 10.1080/07060668409501538
Adachi, T., Inagaki, K. (1988). Phytotoxin produced by Rhizoctonia oryzae ryker et gooch. Agr. Biol. Chem. 52, 2625. doi: 10.1271/bbb1961.52.2625
Aguado, A., Savoie, J. M., Chéreau, S., Ducos, C., Aguilar, M., Ferrer, N., et al. (2019). Priming to protect maize from Fusarium verticillioides and its fumonisin accumulation. J. Sci. Food Agric. 99, 64–72. doi: 10.1002/jsfa.9142
Ajigboye, O. O., Lu, C., Murchie, E. H., Schlatter, C., Swart, G., Ray, R. V. (2016). Altered gene expression by sedaxane increases PSII efficiency, photosynthesis and growth and improves tolerance to drought in wheat seedlings. Pestic. Biochem. Physiol. 137, 49–61. doi: 10.1016/j.pestbp.2016.09.008
Aliferis, K. A., Jabaji, S. (2010). 1H NMH and GC-MS metabolic fingerprinting of developmental stages of Rhizoctonia solani sclerotia. Metabolomics 6, 96–108. doi: 10.1007/s11306-009-0180-4
Aranega-Bou, P., de la O Leyva, M., Finiti, I., García-Agustín, P., González-Bosch, C. (2014). Priming of plant resistance by natural compounds. hexanoic acid as a model. Front. Plant Sci. 488, 1–12. doi: 10.3389/fpls.2014.00488
Armentrout, V. N., Downer, A. J. (1987). Infection cushion development by Rhizoctonia solani on cotton. Phytopathology 77, 619–623. doi: 10.1094/Phyto-77-619
Berdugo, C. A., Steiner, U., Dehne, H. W., Oerke, E. C. (2012). Effect of bixafen on senescence and yield formation of wheat. Pestic. Biochem. Physiol. 104, 1717–1177. doi: 10.1016/j.pestbp.2012.07.010
Dal Cortivo, C., Conselvan, G. B., Carletti, P., Barion, G., Sella, L., Vamerali, T. (2017). Biostimulant effects of seed-applied sedaxane fungicide: morphological and physiological changes in maize seedlings. Front. Plant Sci. 8, 1–11. doi: 10.3389/fpls.2017.02072
De Vega, D., Holden, N., Hedley, P. E., Morris, J., Luna, E., Newton, A. (2021). Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant Cell Environ. 44, 290–303. doi: 10.1111/pce.13921
Dubey, S. C., Tripathi, A., Upadhyay, B. K., Kumar, A. (2016). Development of conventional and real time PCR assay for detection and quantification of Rhizoctonia solani infecting pulse crops. Biologia 71 (2), 133–138. doi: 10.1515/biolog-2016-0027
Errampalli, D. (2004). Effect of fludioxonil on germination and growth of penicillium expansum and decay in apple cvs. empire gala. Crop Prot. 23, 811–817. doi: 10.1016/j.cropro.2003.12.010
Fleet, C. M., Sun, T. P. (2005). A DELLAcate balance: The role of gibberellin in plant morphogenesis. Curr. Opin. Plant Biol. 8 (1), 77–85. doi: 10.1016/j.pbi.2004.11.015
Gugel, R. K., Yitbarek, S. M., Verma, P. R., Morrall, R. A. A., Sadasivaiah, R. S. (1987). Etiology of the Rhizoctonia root rot complex of canola in the peace river region of Alberta. Can. J. Plant Pathol. 9, 119–128. doi: 10.1080/07060668709501891
Huber, D. M., Christmas, E. P., Herr, L. J., McCay-Buis, T. S., Baird, R. (1992). Rhizoctonia crown rot of canola in Indiana. Plant Dis. 76, 1251–1253. doi: 10.1094/PD-76-1251
Hwang, S. F., Gossen, B. D., Conner, R. L., Chang, K. F., Turnbull, G. D., Lopetinsky, K., et al. (2007). Management strategies to reduce losses caused by Rhizoctonia seedling blight of field pea. Can. J. Plant Sci. 87, 145–155. doi: 10.4141/P04-172
Ihaka, R., Gentleman, R. (1996). R: A language for data analysis and graphics. J.Comput. Graph. Stat. 5, 299–314.
Kataria, H., Verma, P. (1992). Rhizoctonia solani damping off and root rot in oilseed rape and canola. Crop Prot. 11, 8–13. doi: 10.1016/0261-2194(92)90072-D
Khangura, R. K., Barbetti, M. J., Sweetingham, M. W. (1999). Characterization and pathogenicity of rhizoctonia species on canola. Plant Dis. 83, 714–721. doi: 10.1094/PDIS.1999.83.8.714
Kitchen, J. L., Van Den Bosch, F., Paveley, N. D., Helps, J., Van Den Berg, F. (2016). The evolution of fungicide resistance resulting from combinations of foliar-acting systemic seed treatments and foliar-applied fungicides: a modeling analysis. PloS One 11, e0161887. doi: 10.1371/journal.pone.0161887
Knauf-Beiter, G., Zeun, R. (2012). “Chemistry, biology of fludioxonil, fenpiclonil, and quinoxyfen,” in Modern crop protection compounds, vol. 2 . Eds. Kraemer, W., Schirmer, U. (Weinheim, Germany: Wiley-VCH), 721–737.
Leroux, P. (1996). Recent developments in the mode of action of fungicides. Pestic Sci. 47, 191–197. doi: 10.1002/(SICI)1096-9063(199606)47:2<191::AID-PS415>3.0.CO;2-I
Ma, Y. M., Li, Y., Liu, J. Y., Song, Y. C., Tan, R. X. (2004). Anti-Helicobacter pylori metabolites from rhizoctonia sp. Cy064, an endophytic fungus in Cynodon dactylon. Fitoterapia 75, 451–456. doi: 10.1016/j.fitote.2004.03.007
Mandava, N. B., Orellana, R. G., Warthen, J. D., Worley, J. F., Dutky, S. R., Finegold, H., et al. (1980). Phytotoxins in Rhizoctonia solani: isolation and biological activity of m-hydroxy- and m-methoxyphenylacetic acids. J. Agr. Food Chem. 28, 71–75. doi: 10.1021/jf60227a009
Mathre, D. E., Johnston, R. H., Grey, W. E. (2001). Small grain cereal seed treatment (Saint Paul, MN: American Phytopathological Society).
Mondal, K. K. (2004). Evaluation of seed-dressing fungicides against sclerotinia root rot of buckwheat. Fagopyrum 21, 105–107.
Murchie, E. H., Lawson, T. (2013). Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J. Exp. Bot. 64, 3983–3998. doi: 10.1093/jxb/ert208
Murray, D. I. L. (1982). Penetration of barley root and coleoptile surfaces by Rhizoctonia solani. Trans. Brit. Mycol. Soc 79, 354–360. doi: 10.1016/S0007-1536(82)80130-7
Nakayama, T. A. (1940). Study on the infection of cotton seedlings by Rhizoctonia solani. Ann. Phytopathol. Soc Japan. 10, 93–103. doi: 10.3186/jjphytopath.10.93
Nason, M. A., Farrar, J., Bartlett, D. (2007). Strobilurin fungicides induce changes in photosynthetic gas exchange that do not improve water use efficiency of plants grown under conditions of water stress. Pest Manage. Sci. 3, 1191–1200. doi: 10.1002/ps.1443
Petit, A. N., Fontaine, F., Clément, C., Vaillant-Gaveau, N. (2008). Photosynthesis limitations of grapevine after treatment with the fungicide fludioxonil. J. Agric. Food Chem. 56, 67661–66767. doi: 10.1021/jf800919u
Podani, J. (2007). “PCA. ordinamento,” in Analisi ed esplorazione multivariate dei dati in ecologia e biologia (Napoli, Italy: Liguori Editore), 333–334.
Rose, M. T., Van Zwieten, L., Claassens, A., Scanlan, C., Rose, M. T. (2018). Phytotoxicity of soil borne glyphosate residues is influenced by the method of phosphorus fertilizer application. Plant Soil 422, 455–465. doi: 10.1007/s11104-017-3482-8
Saladin, G., Magnè, C., Clémenent, C. (2003). Effects of fludioxonil and pyrimethanil, two fungicides used against Botrytis cinerea, on carbohydrate physiology in Vitis vinifera l. Pest Manage. Sci. 59, 1083–1092. doi: 10.1002/ps.733
Shahid, M., Ahmed, B., Zaidi, A., Saghir, Khan, M. (2018). Toxicity of fungicides to Pisum sativum: a study of oxidative damage, growth suppression, cellular death and morpho-anatomical changes. R. Soc Chem. 8, 38483–38498. doi: 10.1039/C8RA03923B
Sherwood, R. T. (1970). “Physiology of rhizoctonia solani,” in Rhizoctonia solani: Biology and pathology. Ed. Parmeter, J. R. (Berkeley, CA, USA: University of California Press), 69–92.
Tahvonen, R., Hollo, J., Hannukkala, A., Kurppa, A. (1984). Rhizoctonia solani damping-off on spring turnip rape and spring rape (Brassica spp.) in Finland. J. Agr. Sci. Finland. 56, 143–154. doi: 10.23986/afsci.72165
Trail, F., Kolller, W. (1990). Diversity of cutinases from plant pathogenic fungi: Evidence for relationship between enzyme properties and tissue specificity. Physiol. Mol. Plant Pathol. 36, 495–508. doi: 10.1016/0885-5765(90)90022-P
Velazhahan, R., Vidhyasekaran, P. (2000). Isolation of an elicitor from Rhizoctonia solani, the rice sheath blight pathogen which activates phenylpropanoid metabolism in suspension-cultured rice cells. J. Plant Dis. Protect. 107, 135–144.
Westman, S. M., Kloth, K. J., Hanson, J., Ohlsson, A. B., Albrectsen, B. R. (2019). Defence priming in Arabidopsis – a meta-analysis. Sci. Rep. 9, 13309. doi: 10.1038/s41598-019-49811-9
Yang, H., Liu, J., Huang, S., Guo, T., Deng, L., Hua, W. (2014). Selection and evaluation of novel reference genes for quantitative reverse transcription PCR (qRT-PCR) based on genome and transcriptome data in Brassica napus l. Gene 538, 113–122. doi: 10.1016/j.gene.2013.12.057
Yilmaz, S., Kaya, E., Kisacam, M. A. (2017). “The effect of oxidative stress of aflatoxin and protective effect of lycopene on aflatoxin damage,” in Aflatoxin-control, analysis, detection and health risks (London, UK: In Tech) 2017, 67–90.
Keywords: biostimulant, hormone-like activity, photosynthesis, root growth, shoot phytotoxicity
Citation: Panozzo A, Barion G, Moore SS, Cobalchin F, Di Stefano A, Sella L and Vamerali T (2023) Early morpho-physiological response of oilseed rape under seed applied Sedaxane fungicide and Rhizoctonia solani pressure. Front. Plant Sci. 14:1130825. doi: 10.3389/fpls.2023.1130825
Received: 23 December 2022; Accepted: 13 February 2023;
Published: 22 February 2023.
Edited by:
Alessandro Vitale, University of Catania, ItalyReviewed by:
Frank Walker, University of Hohenheim, GermanyShaikhul Islam, Bangladesh Agricultural Research Council, Bangladesh
Copyright © 2023 Panozzo, Barion, Moore, Cobalchin, Di Stefano, Sella and Vamerali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Panozzo, YW5uYS5wYW5venpvQHVuaXBkLml0
 Anna Panozzo
Anna Panozzo Giuseppe Barion
Giuseppe Barion Selina Sterup Moore
Selina Sterup Moore Francesca Cobalchin1
Francesca Cobalchin1 Luca Sella
Luca Sella Teofilo Vamerali
Teofilo Vamerali