- 1College of Agriculture, Guizhou University, Guiyang, China
- 2Institute of Vegetable Industry Technology Research, Guizhou University, Guiyang, China
- 3Guizhou Institute of Biotechnology, Guizhou Province Academy of Agricultural Sciences, Guiyang, China
Radish (Raphanus sativus L.) is an important root vegetable crop belonging to the Brassicaceae family. Anthocyanin rich radish varieties are popular among consumers because of their bright color and high nutritional value. However, the underlying molecular mechanism responsible for skin and flesh induce anthocyanin biosynthesis in transient overexpression, gene silencing and transcriptome sequencing were used to verify its function in radish anthocyanin accumulation, radish remains unclear. Here, we identified a long noncoding RNA LINC15957, overexpression of LINC15957 was significantly increased anthocyanin accumulation in radish leaves, and the expression levels of structural genes related to anthocyanin biosynthesis were also significantly increased. Anthocyanin accumulation and expression levels of anthocyanin biosynthesis genes were significantly reduced in silenced LINC15957 flesh when compared with control. By the transcriptome sequencing of the overexpressed LINC15957 plants and the control, 5,772 differentially expressed genes were identified. A total of 3,849 differentially expressed transcription factors were identified, of which MYB, bHLH, WD40, bZIP, ERF, WRKY and MATE were detected and differentially expressed in the overexpressed LINC15957 plants. KEGG enrichment analysis revealed the genes were significant enriched in tyrosine, L-Phenylalanine, tryptophan, phenylpropanol, and flavonoid biosynthesis. RT-qPCR analysis showed that 8 differentially expressed genes (DEGs) were differentially expressed in LINC15957-overexpressed plants. These results suggested that LINC15957 involved in regulate anthocyanin accumulation and provide abundant data to investigate the genes regulate anthocyanin biosynthesis in radish.
1 Introduction
Color is one of the most significant quality traits of plants, and is the most adaptive phenotypic trait in the evolution of plants. Anthocyanin are an important pigment that gives plants their color, and color trait is one of the important research directions of plant genetic improvement (Dai and Hong, 2016). Anthocyanin as common antioxidant substances plays important role in scavenging free radicals, accelerating human metabolism, protect vision, smooth blood sugar, fight obesity and inflammation, and delaying aging and preventing cardiovascular disease (Nomi et al., 2019). In addition, anthocyanin is involved in many biological processes, such as plant environmental stress response, pathogenic bacteria and insect stress response. Anthocyanin synthesis is regulated by several transcription factors, of which a protein complex (MBW) consisting of R2R3-MYB, bHLH and WD40 transcription factors binds to the promoters of structural genes in plants (Ramsay and Glover, 2005).
Radish (Raphanus sativus L., 2n = 2x = 18) is an important vegetable crop. Radish has plentiful germplasm resources and a long history of cultivation. After long-term exposure to natural and artificial selection, different varieties with different skin and flesh colors have been formulated. Among them, anthocyanin-rich radish varieties are popular among consumers because of their bright color and high nutritional value. Anthocyanin have been widely used as natural pigments because of their good thermal stability and beneficial antioxidant activity in radish (Rahman et al., 2006; Matsufuji et al., 2007). AtPAP1/2 is a key transcription factor regulating anthocyanin biosynthesis in Arabidopsis. The homologous gene of AtPAP1/2 in radish is generally involved in the regulation of anthocyanin biosynthesis in radish, and RsMYB1 was demonstrated to be a key transcription factor regulating anthocyanin synthesis by heterologous overexpression in Arabidopsis, tobacco, and Petunia (Lim et al., 2016; Ai et al., 2017). A key gene controlling anthocyanin biosynthesis in the fleshy root of radish was mapped by QTL-seq and RNA-seq techniques and validated the function of RsMYB1 (Wang et al., 2020a). Previous studies found that the homologs of AtPAP1/2, RsMYB41, RsMYB117, and RsMYB132, were found in the red radish genome (Muleke et al., 2021). RsMYB132 were highly expressed in the root bark of red-skinned radish fleshy roots, whereas RsMYB65 and RsMYB159 were highly expressed in the root bark of purple-skinned radish fleshy roots, indicating these genes are involved in regulating anthocyanin synthesis in radish fleshy roots. Several key members of the v-myb avian myeloblastosis viral oncogene homolog (MYB), basic helix-loophelix (bHLH) and WRKY families are major drivers of transcriptional changes between purple and green radish (Zhuang et al., 2019). Previous studies showed that RsGSTF12-1 and RsGSTF12-2 may participated in anthocyanin transport in carmine radish (Gao et al., 2020a). The expression pattern of RsTT19 was consistent with key genes of the anthocyanin synthesis pathway in radish (Liu et al., 2019b). Radish multidrug and toxic compound extrusion (MATE) gene family members RsMATE2, RsMATE3, RsMATE7, RsMATE8, and RsMATE9 was participated in radish anthocyanin translocation through phylogenetic tree and expression analysis (M’mbone et al., 2018). However, the molecular mechanism underlying radish anthocyanin biosynthesis in radish remains unclear.
Long noncoding RNAs (lncRNAs) are a class of RNA molecules that do not encode proteins and can interact with proteins, DNA and RNA (Ponting et al., 2009). Eukaryotic genomes encode thousands of lncRNAs, which play important roles in vital biological processes (Wu et al., 2020a). To date, at least four different lncRNA-mediated regulatory mechanisms have been revealed, including target mimicry, transcriptional interference, PRC2-associated histone methylation, and DNA methylation. Although lncRNAs have roles in both the nucleus and cytoplasm, they are mostly found in the nucleus (Chekanova, 2015). LncRNAs have been shown to act as transcriptional regulators and competitive endogenous RNAs (ceRNAs) as molecular cargoes for protein relocalization and as modular scaffolds to recruit the assembly of multiple protein complexes for chromatin modification. Many studies have indicated that lncRNAs have involved in regulating flowering, male sterility, nutrient metabolism, and biotic and abiotic stress responses in plants (Zhang et al., 2013; Liu et al., 2015; Wang et al., 2018). Previous studies found that an endogenous rice lncRNA, TWISTED LEAF (TL) play a cis-regulatory role on OsMYB60 in leaf morphological development (Liu et al., 2018a). Silencing of two novel intergenic lncRNAs in tomato, lncRNA1459 and lncRNA1840, resulted in a significant delay in wild-type fruit ripening, suggesting lncRNAs may be important regulators of tomato fruit ripening (Zhu et al., 2015). The apple lncRNA MSTRG.85814 positively promoted SAUR32 expression, which then activated proton extrusion involved in the Fe-deficiency response (Sun et al., 2020). In strawberry, 50,601 putative lncRNAs associated with anthocyanin were identified, 68 lncRNAs were differentially expressed and co-expressed with anthocyanin-related mRNAs (Lin et al., 2018). LNC1 and LNC2 were identified as targets of miR156a and miR828a to reduce SPL9 expression and induce MYB114 expression, respectively, which lead to increased and decreased anthocyanin content (Zhang et al., 2018). Through Weighted correlation network analysis (WGCNA analysis) miRNA-lncRNA-mRNA expression regulation network construction, and gene function verification, confirmed that lncRNA MLNC3.2 and MLNC4.6 are potential targets of miRNA156a, and prevented the degradation of SPL2-like and SPL33 by miR156a under light induction, promoting the expression of SPL2-like and SPL33 and the accumulation of anthocyanin (Yang et al., 2019). A total of 2,070 co-expressed lncRNA-mRNA pairs were generated, MdLNC610, a positive regulator promoting MdACO1 expression and ethylene biosynthesis, was involved in the regulation of strong light-induced anthocyanin production in apple (Yu et al., 2022). Most of the flavonoid synthesis pathway genes may also be regulated by lncRNAs, and constructed sly-miR5303, stu-miR5303g, stu-miR7997a, and stu-miR7997c three “lncRNA-miRNA-mRNA” regulatory networks, including 28 differentially expressed mRNAs and 6 differentially expressed lncRNAs (Zhou et al., 2022). MdLNC499 in apple pericarp positively regulates the expression of MdERF109 to promote light-induced anthocyanin biosynthesis (Ma et al., 2021). A specific lncRNA-miRNA-mRNA network was formed in different tissues of apple, and MSTRG.60895.2-mdm-miR393-MD17G1009000 may be involved in anthocyanin metabolism in fruit (Wang et al., 2022).
Based on our previous lncRNA sequencing results with a high and low anthocyanin content varieties, a long noncoding RNA LINC15957 was found to be differentially expressed, and selected for further explored. In this study, LINC15957 was conducted to functional assays by transient overexpression and virus-induced gene silencing technology. The expression of anthocyanin structural genes was validated in LINC15957 overexpressed plants and control by qRT-PCR. Transcriptome sequencing of LINC15957 overexpressed plants and control were performed to investigate the differentially expressed genes in the accumulation of anthocyanin. These results provide a theoretical basis for further investigation of the molecular mechanism of lncRNAs involvement in anthocyanin accumulation in radish.
2 Materials and methods
2.1 Materials and treatments
The full seeds of the high generation inbred plant with red-skin and flesh radish (YZH) were selected and sown in cavity trays (5 × 10). The seedlings were raised in an artificial climate chamber (day temperature 25°C, 16 h; night temperature 16°C, 8 h; humidity 75%), and three true leaves were transplanted into plastic pots (outer diameter 29.2 cm, height 23.5 cm), with 15 pots of each material. The fleshy roots were allowed to expand to about 5 cm. Three plants of test materials were taken (three biological replicates), and two portions of root bark were taken from each plant, 1 g each, wrapped in tin foil and immediately snap-frozen in liquid nitrogen and stored at -80°C. Healthy two-leafed plants were selected as Agrobacterium-mediated transient transformation material for radish.
2.2 Total RNA extraction and cDNA synthesis
RNA was extracted from 0.1 g radish root skin using RNAiso Plus (TaKaRa, Beijing, China) liquid nitrogen extraction method. The quality was identified by 1.0% agarose gel electrophoresis, and the RNA concentration and purity were detected by ultra-micro spectrophotometer. cDNA was synthesized using StarScript II RT Mix with gDNA Remover (GeneStar, Beijing, China) according to the reagent instructions. The cDNA was stored for further gene cloning and quantitative real-time PCR (RT-qPCR) analysis.
2.3 LINC15957 overexpression vector construction and plant transformation
The full sequence of LINC15957 was cloned into the pGreenII 62-SK vectors with restriction endonuclease BamH I and Kpn I. The recombinant plasmids were transformed into Agrobacterium tumefaciens GV3101 by freeze-thaw method. “Degaohongmeizan” radish cotyledons and true leaves were treated by injection according to the method of Fan et al. (2020). The cotyledons injected with pGreenII 62-SK were used as controls. The infiltrated cotyledons were cultured in the dark for 2 days and then transferred to normal light conditions. The coloration of cotyledons near the injection site was closely monitored and photographed. The infiltrated cotyledons with red color were sampled and preserved for anthocyanin content assay and RT-qPCR analysis.
2.4 Virus-induced gene silencing
To functionally characterize LINC15957, a TYMV-based virus-induced gene silencing (VIGS) system was used (Pflieger et al., 2008; Yu et al., 2018). A palindromic DNA fragment of 80 nt corresponding to LINC15957 genes was designed and synthesized, and 15-nt homologous sequences corresponding to the vector were introduced to both 3′ and 5′ ends. The pTY-S VIGS vector plasmid was digested with SnaB I restriction endonuclease. Positive clones were identified using amplification of the TYMV-CP gene of the expected size (522 bp). Ten μL of purified pTY-S carrying the target gene plasmid DNA was injected into the root flesh of red fleshed radish. Plants injected with empty pTY-S vector were used as controls. Injected plants were maintained in an artificial climate chamber at 25°C/22°C with a 16 h/8 h light/dark cycle. The phenotype was assessed after three weeks. Primers used to detect clones that silence the LINC15957 gene are listed in Supplementary Table 1.
2.5 Determination of anthocyanin content
The pH difference method was performed as described by Mao et al. (2021). 1 g of the samples were well ground in liquid nitrogen, 0.05% hydrochloric acid monomethanol solution (pre-chilled at 4°C) was added with full tube, mixed well and placed in the dark at 4°C. Two test tubes were set up (1 ml of extract in each tube), and 4 ml of 0.4 mol/L KCl-HCl buffer (pH 1.0) and 0.4 mol/L citric acid/disodium hydrogen phosphate buffer (pH 5.0) were added, mixed well and allowed to stand for 20 min at room temperature. The 510 nm and 700 nm absorbance values were measured by a UV spectrophotometer. Each sample was repeated with three times.
2.6 Gene expression analysis
The PCR and amplification was conducted as described previously (Luo et al., 2020). Real-time quantitative PCR analysis (RT-qPCR) was performed on SYBR Green Master Mix (GeneStar, Hangzhou, China) using a BoriPlantGene 9600 PlUS (FOD-96A) Fluorescence Quantitative (Hangzhou, China). The radish Actin gene was used as an internal control. The relative expression levels of candidate genes were determined using Equation 2-ΔΔCt. Three biological replicates were performed for all reactions. All primers are listed in Supplementary Table 1.
2.7 Transcriptome sequencing and data analysis
The total RNA was extracted from the LINC15957 overexpressed plants and control radish leaves using RNAprep Pure Plant Kit (TIANGEN, Beijing, China). A cDNA library was constructed for each sample using enriched mRNA. The libraries were sequenced using the Illumina HiSeq-PE150 sequencing platform from BeijingNovogene Co. Ltd. Reference genome and gene model annotation files were downloaded from genome website directly (http://39.100.233.196:82/download_genome/Brassica_Genome_data/Rapsa_Xiang_V1.0/). Index of the reference genome was built using Hisat2 v2.0.5 and paired-end clean reads were aligned to the reference genome using Hisat2 v2.0.5.
Differential expression analysis of two processing samples (three biological replicates per process) was performed using the DESeq2 R package (1.20.0). Gene Ontology (GO) enrichment analysis of differentially expressed genes was implemented by the clusterProfiler R package, in which gene length bias was corrected. GO terms with corrected Pvalue less than 0.05 were considered significantly enriched by differentially expressed genes. ClusterProfiler R package was used to test the statistical enrichment of differential expression genes in Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
2.8 Statistical analysis
All anthocyanin determinations and real-time quantitative polymerase chain reaction (RT-qPCR) were performed in triplicate, and the data are expressed as the mean ± standard deviation (SD). Student’s t test was used to compare different samples. A difference was considered statistically significant when P < 0.05 or very significant when P < 0.01.
3 Results
3.1 Acquiring and cloning of LINC15957
The anthocyanin was accumulated in the root skin and flesh stem of “YZH” radish (2.12 mg/g), while almost no anthocyanin was accumulated in white skinned and flesh radish “WN07” (0.04 mg/g) (Figures 1A, B). To explore lncRNAs involve in regulation of anthocyanin biosynthesis in radish, “YZH” and “WN07” was used to lncRNA sequencing. The result showed that LINC15957 were differentially expressed lncRNA in “YZH” and “WN07” (data not shown). RT-qPCR results showed that LINC15957 was highly expressed in the root bark of red skinned and flesh radish, while the expression level was lower in white skinned and flesh radish (Figure 1C). According to genome and transcriptome sequences, primers were designed to amplify the full length of LINC15957, a 314-bp fragment were obtained (Supplementary Table 2).
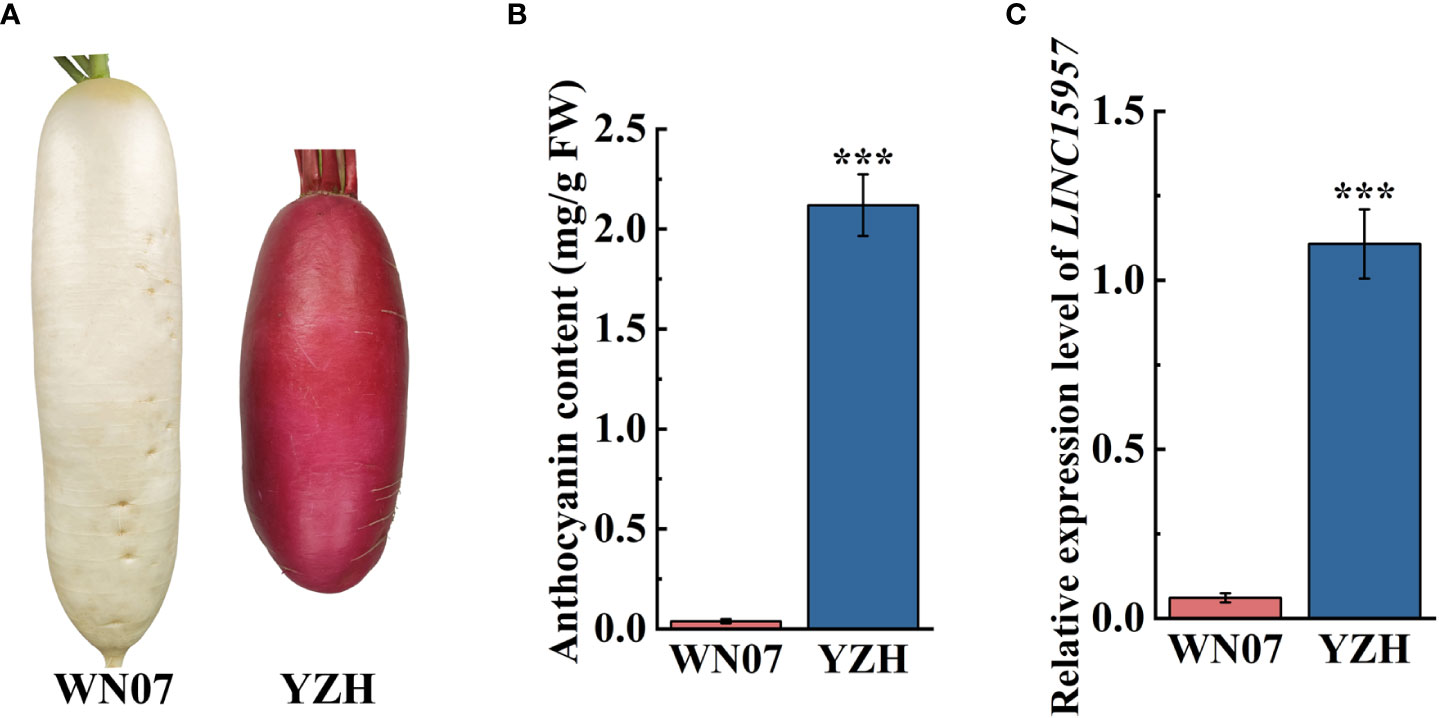
Figure 1 Anthocyanin content and related gene expression in radish with different colors. (A) Two radish cultivars “WN07” and “YZH” with different colored radish. (B) Content of anthocyanin in root skin of “WN07” and “YZH” radish. (C) The relative expression of LINC15957 in “WN07” and “YZH” radish root skin. Error bars represent the mean ± SE of three biological replicates. ***p < 0.001, Student’s t-test.
3.2 LINC15957 positively regulates the biosynthesis of anthocyanin in radish
To further investigate the role of LINC15957 involve in the anthocyanin biosynthetic pathway, the overexpressed vectors of LINC15957-pGreenII62-SK with the constitutive 35S promoter was constructed. Two cotyledon of radish were selected for transient overexpression of LINC15957 and pGreenII62-SK (control). PCR amplification indicated that LINC15957 was successfully transferred into radish leaves (Figure 2A). The pigmentation was observed in injection sites of radish leaves after transformation with LINC15957 (Figure 2B). The anthocyanin content of OE- LINC15957 plants was significantly higher than that of the empty vector 6 - 7 times (Figure 2C). The gene expression level of LINC15957 in OE-LINC15957 plants was almost 32.5-fold greater than in control (Figure 2D). The relative expression level of the anthocyanin biosynthetic genes was conducted by qRT-PCR. The results showed that a significant increase in expression of RsANS (anthocyanidin synthase), RsUFGT (UDP-glucose:flavonoid 3-O-glucosyltransferase), RsPAL (phenylalanine aminolyase), RsF3H (flavanone 3β-hydroxylase), RsDFR (dihydroflavonol 4-reductase), RsC4H (Cinnamate 4-Hydroxylase) and RsCHS (chalcone synthase), when LINC15957 were overexpressed. In addition, the expression level of RsMYB1 in OE-LINC15957 plants was 2.0-fold greater than in control (Figure 2E).
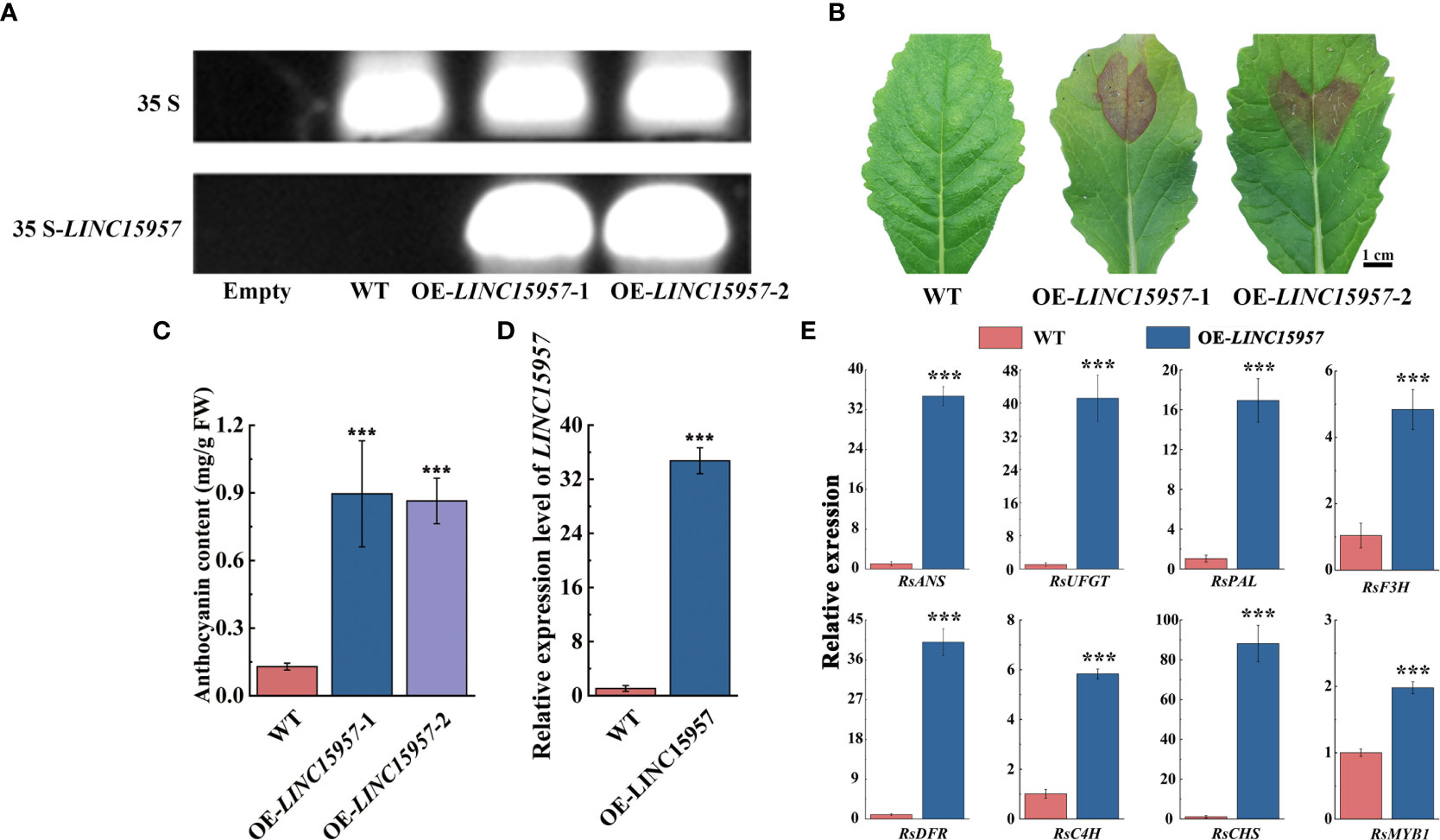
Figure 2 The transiently expressed LINC15957 in radish leaves. (A) The PCR validation of LINC15957 in OE-LINC15957 and control. The upper band indicates 35S and the lower band indicates LINC15957 is successfully transiently expressed in radish leaves. “Empty” is leaves without injection infection, “WT” is empty vector. (B) LINC15957 is transiently expressed in radish leaves. (C) Anthocyanin content in the tissue of injection empty, OE-LINC15957-1 and OE-LINC15957-2. (D) The gene expression level in OE-LINC15957. (E) Expression pattern of key anthocyanin biosynthetic genes in OE-LINC15957. RsACTIN was used as reference gene for normalization. Error bars represent the mean ± SE of three biological replicates. ***p < 0.001, Student’s t-test.
3.3 LINC15957 silencing reduced anthocyanin accumulation in radish root flesh
LINC15957-pTY recombinant plasmid is used to silence the expression of LINC1595 in red skin and flesh radish. Decreased coloration was displayed at the infiltration sites 4 weeks after transformation with LINC15957-pTY, while no color change was observed with transformation of pTY (Figure 3A). The anthocyanin content in LINC15957-pTY was significantly lower than that of the control (Figure 3B), indicating the accumulation of anthocyanin in the root flesh of radish infected with LINC15957-pTY was inhibited. The expression level of LINC15957 in fruit infected with LINC15957-pTY was significantly lower than that of the control (Figure 3C). In comparison with the control, the anthocyanin related structural genes (RsANS, RsUFGT, RsPAL, RsF3H, RsDFR, RsC4H and RsCHS) in fruit infected with LINC15957-pTY was significantly decreased (Figure 3D). The expression level of RsMYB1 in LINC15957-pTY were reduced in comparison with the control. Taken together, these results demonstrate that LINC15957 is positively regulate anthocyanin synthesis in radish.
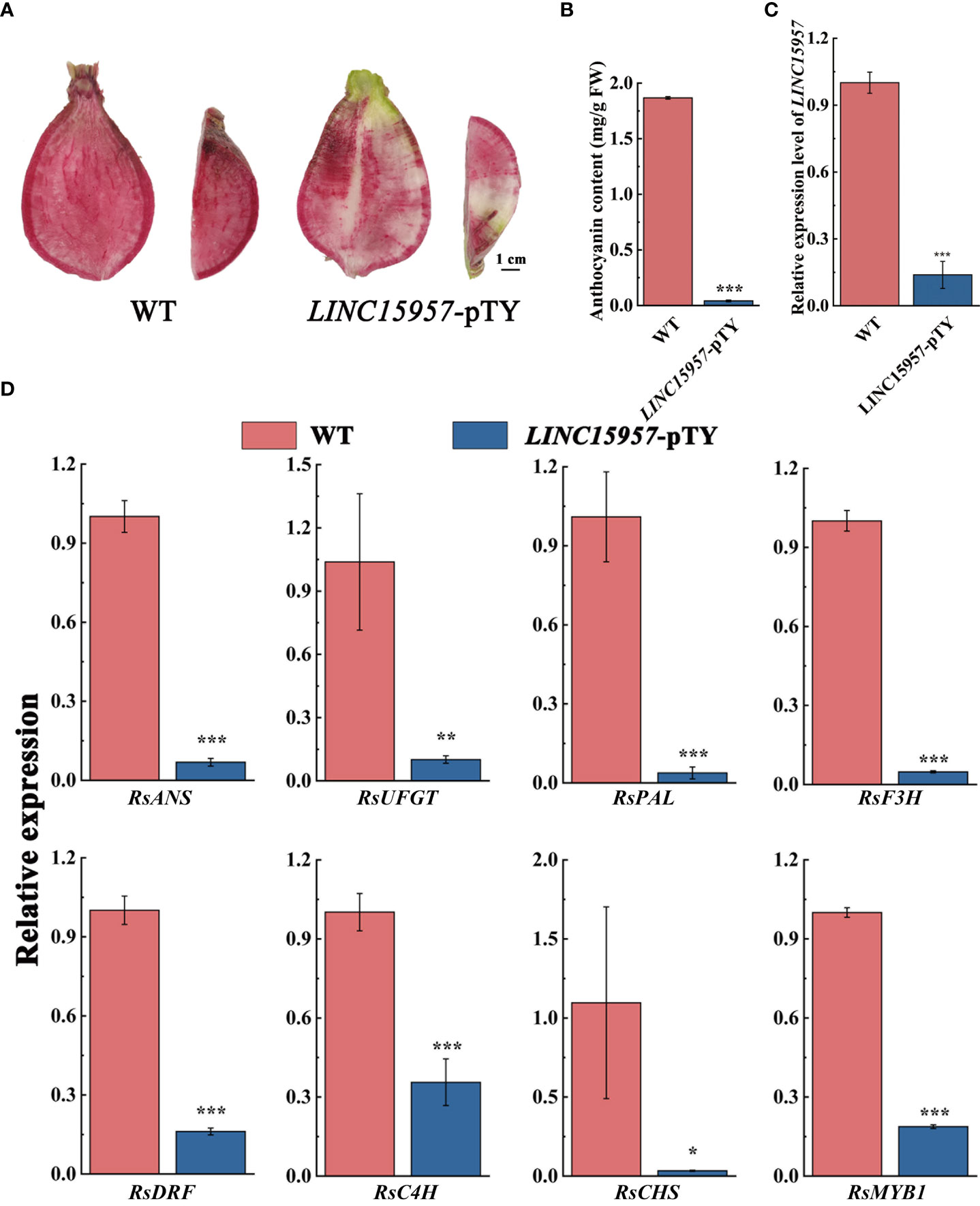
Figure 3 Viral induced silencing of the LINC15957 genes in radish flesh. (A) Radish were inoculated with pTY (WT) or LINC15957-pTY (from left to right). Photographs of root were taken at 4 week post-inoculation. (B) Anthocyanin content in the tissue of injection empty and LINC15957-pTY. (C) The gene expression level in LINC15957-pTY. (D) Expression pattern of key anthocyanin biosynthetic genes in LINC15957-pTY. RsACTIN was used as reference gene for normalization. Error bars represent the mean ± SE of three biological replicates. *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test.
3.4 Transcriptome sequencing and DGE analysis
To further identify genes regulated by LINC15957 in radish anthocyanin accumulation, six libraries (LINC15957-overexpressed plants and control) were constructed for transcriptome sequencing. The raw data (Q20 ≥ 97.09% and Q30 ≥ 92.17%) for overexpression and control were 6.68 Gb and 7.06 Gb, respectively. After trimming the barcode, 6.53 and 6.89 Gb of clean data were obtained (Supplementary Table 3), respectively. The average for all samples with GC percentage was 46.78% (Supplementary Table 3). Approximately 85% of the clean reads were mapped to 38,022,061 genes in radish reference genome (Supplementary Table 4), of which 3,547 genes were found to be novel loci that were not annotated in the radish genome (Supplementary Table 5). To investigate DEGs and anthocyanin biosynthesis or regulatory genes in the candidate region, we compared the transcriptomes of LINC15957-overexpressed plants and WT. A total of 5,772 differential genes were identified, including 2,570 up-regulated and 3,202 down-regulated genes (Figure 4A). Early genes include PAL, CHS, F3H, chalcone flavonol ketone isomerase (CHI), flavonoid 3′-hydroxylase (F3′H) and Flavonoid 3’5’-hydroxylase (F3′5′H), which contribute to the formation of dihydroflavonol. The late genes DFR, ANS, UFGT, methyltransferase (MT), and rhamnosyltransferase (RT) plays a role in anthocyanin production. In the present study, we identified 12 core genes that are up-regulated and associated with anthocyanin biosynthesis. Notably, both early (PAL, F3H and CHS) and late (DFR, ANS and UFGT) genes were significantly up-regulated in LINC15957-overexpressed plants compared to controls (Figure 4B).
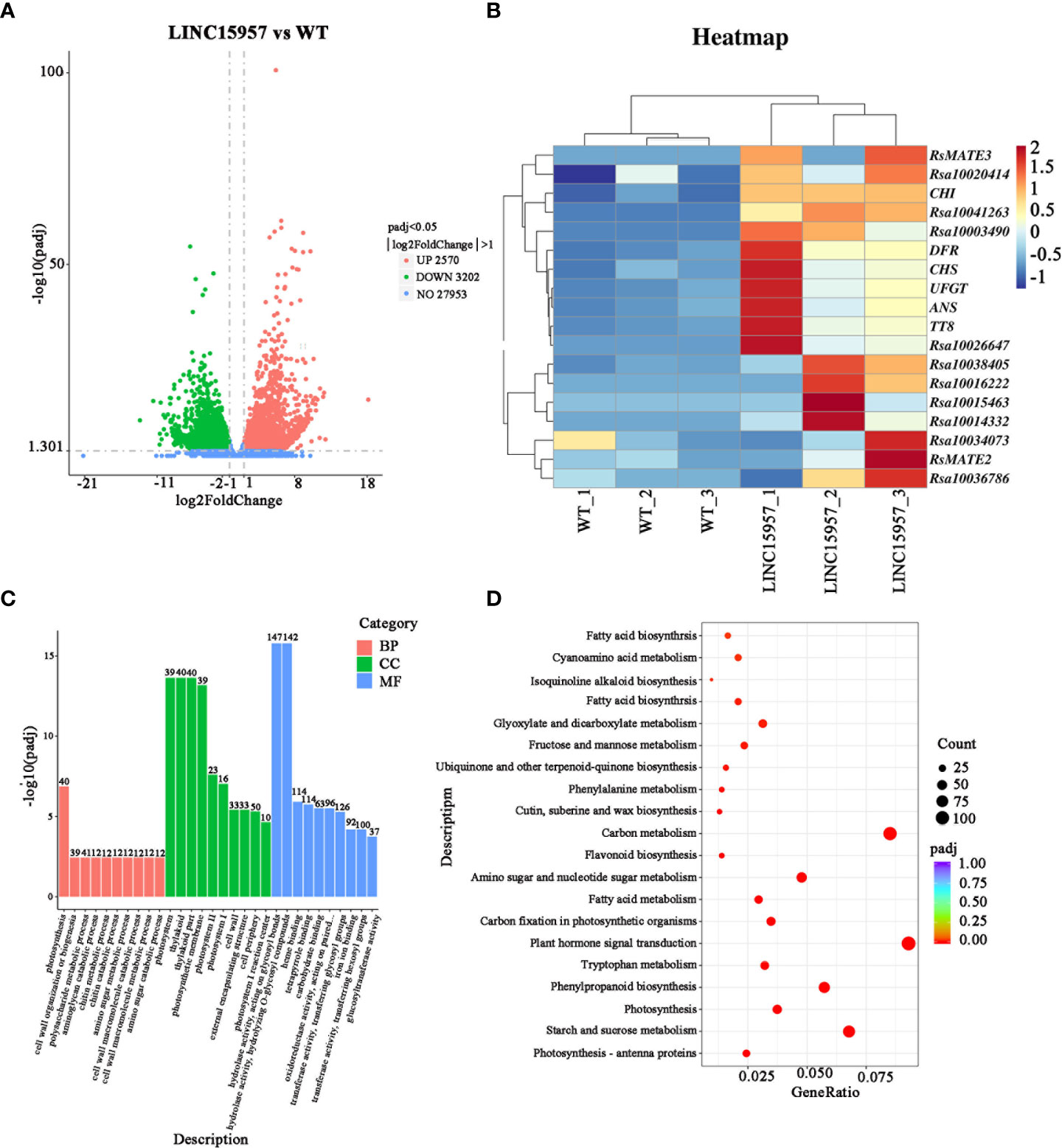
Figure 4 Transcriptome analysis in WT vs. OE-LINC15957. (A) Volcano plot showing the differentially expressed genes (DEGs) in red and green. The X-axis represents the fold change in WT vs. OE-LINC15957 (on a log2 scale). The Y-axis represents the negative log10 transformed average FPKM values. (B) Expression levels of the 6 core genes and 12 transcription factors involved in anthocyanin biosynthesis in radish leaves. Each square represents the transcription level in the two processes (WT and overexpressing LINC15957) at radish leaves. Different color indicates differences in the level of gene expression, from high (red) to low (blue). (C) Gene Ontology (GO) enrichment analysist for differentially expressed genes (DEGs) between WT and OE-LINC15957. (D) Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the DEGs.
To further identify the function of DEGs, Gene Ontology (GO) term and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of the identified DEGs were carried out. GO annotations revealed that all DEGs were assigned to 76 GO terms. In the molecular function (39 terms), the major subcategories were hydrolase activity, heme binding, tetrapyrrole binding. For biological process (21 terms), photosynthesis, cell wall organization or biogenesis and polysaccharide metabolic process were the dominant terms. The ‘photosystem’ and ‘thylakoid’ and ‘thylakoid part’ terms were extraordinarily remarkable in the cellular component (16 terms) (Figure 4C). KEGG pathway enrichment analysis showed that tyrosine, phenylalanine, tryptophan, phenylpropanoid, flavonoid biosynthesis was significantly enriched, which indicated that anthocyanin were synthesized from the precursor phenylalanine by the biosynthesis of phenylpropanoid and flavonoid (Figure 4D).
3.5 Transcription factors involve in anthocyanin biosynthesis
Many studies have indicated that many transcription factors were involved in anthocyanin biosynthesis, such as MYB, bHLH, WD40 (Liu et al., 2018b), basic domain leucine zipper (bZIP) (Fan et al., 2019), MATE (Gomez et al., 2011), WRKY (Cong et al., 2021) and ethylene responsive factor (ERF) (Wu et al., 2020b). Transcriptome analysis in LINC15957-overexpressed revealed 12,167 transcription factors were identified, of which 3,849 were found to be differentially expressed (Supplementary Tables 6, 7), of which 1,934 were upregulated and 1,915 were downregulated. A total of 87 MYB transcription factors were identified, of which 55 were upregulated (Figure 5A). Totally, 56 bHLH transcription factors were detected, of which 13 were upregulated. A total of 16 WD40 transcription factors were identified, of which 6 were upregulated (Figure 5B). A total of 27 bZIP and 36 MATE transcription factors, of which 6 and 18 were upregulated, respectively (Figure 5C). In all, 67 WRKY transcription factors, of which 61 were upregulated (Figure 5D). A total of 61 ERF transcription factors, of which 19 were upregulated (Figure 5E). Most of the transcription factors involved in the regulation of genes related to the anthocyanin biosynthetic pathway belong to the MYB, bHLH, and WD40 families. These transcription factors identified in this study may be involved in anthocyanin accumulation in radish.
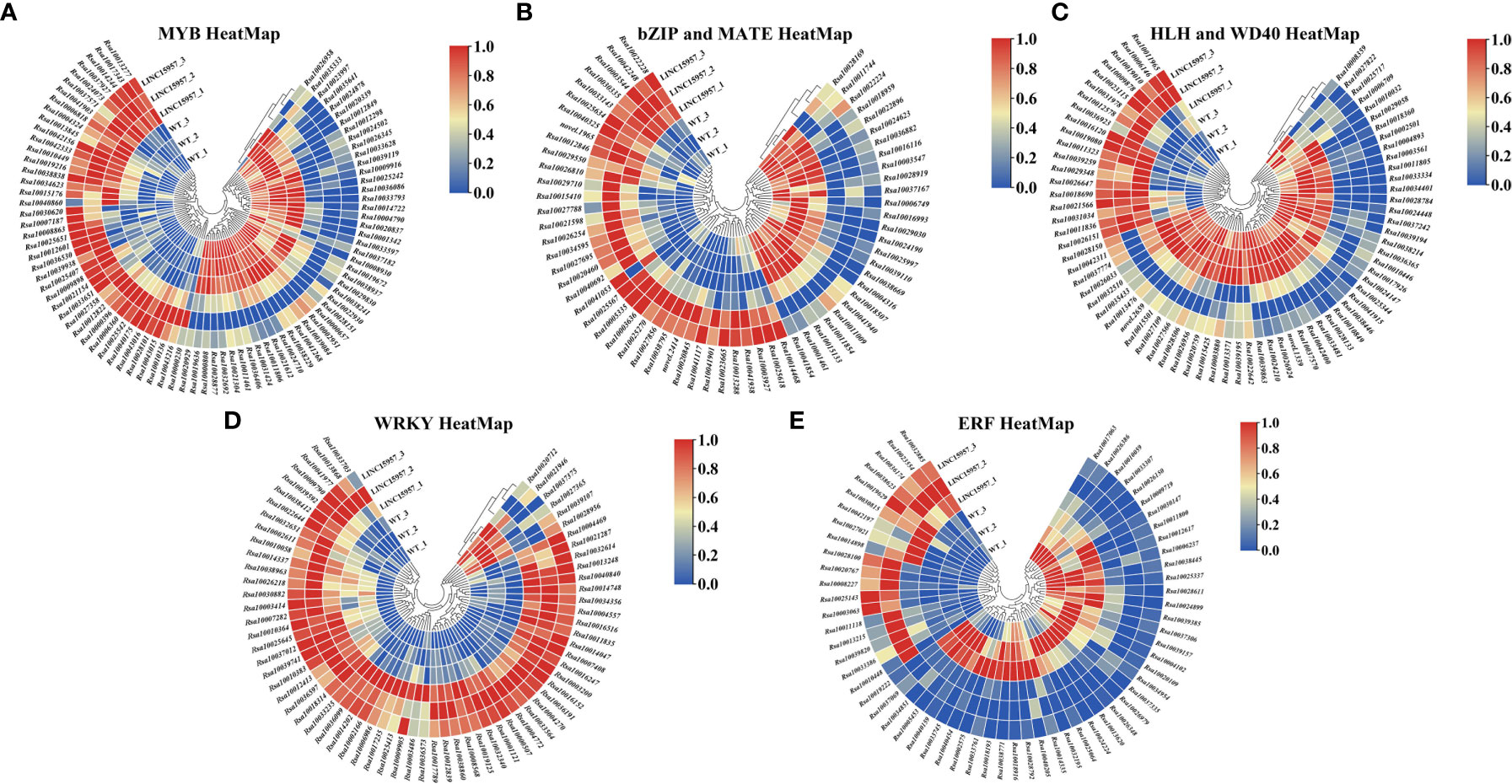
Figure 5 Heat-map depicting normalized log2-fold changes in mRNA expression inferred from RNAseq data for transcripts involved in transcription factor family. (A) Differential MYB transcription factor heat-map. (B) Differential bZIP and MATE transcription factor heat-map. (C) Differential HLH and WD40 transcription factor heat-map. (D) Differential WRKY transcription factor heat-map. (E) Differential ERF transcription factor heat-map.
3.6 RT-qPCR assay
To determine LINC15957 regulates anthocyanin accumulation in radish, the expression of anthocyanin biosynthetic structure gene and transcription factors in LINC15957-overexpressed plants was analyzed by RT-qPCR. A total of 8 genes were selected to validated (Figure 6). The expression levels of these 8 genes in different treatments were consistent with those determined by RNA-Seq, indicating the reliability of the transcriptome sequencing results. It was found that the expression level of all 8 genes in LINC15957-overexpressed plants was higher than that in control. Therefore, these results indicate that the 8 genes can be regulated by LINC15957 through the radish anthocyanin biosynthesis pathway.
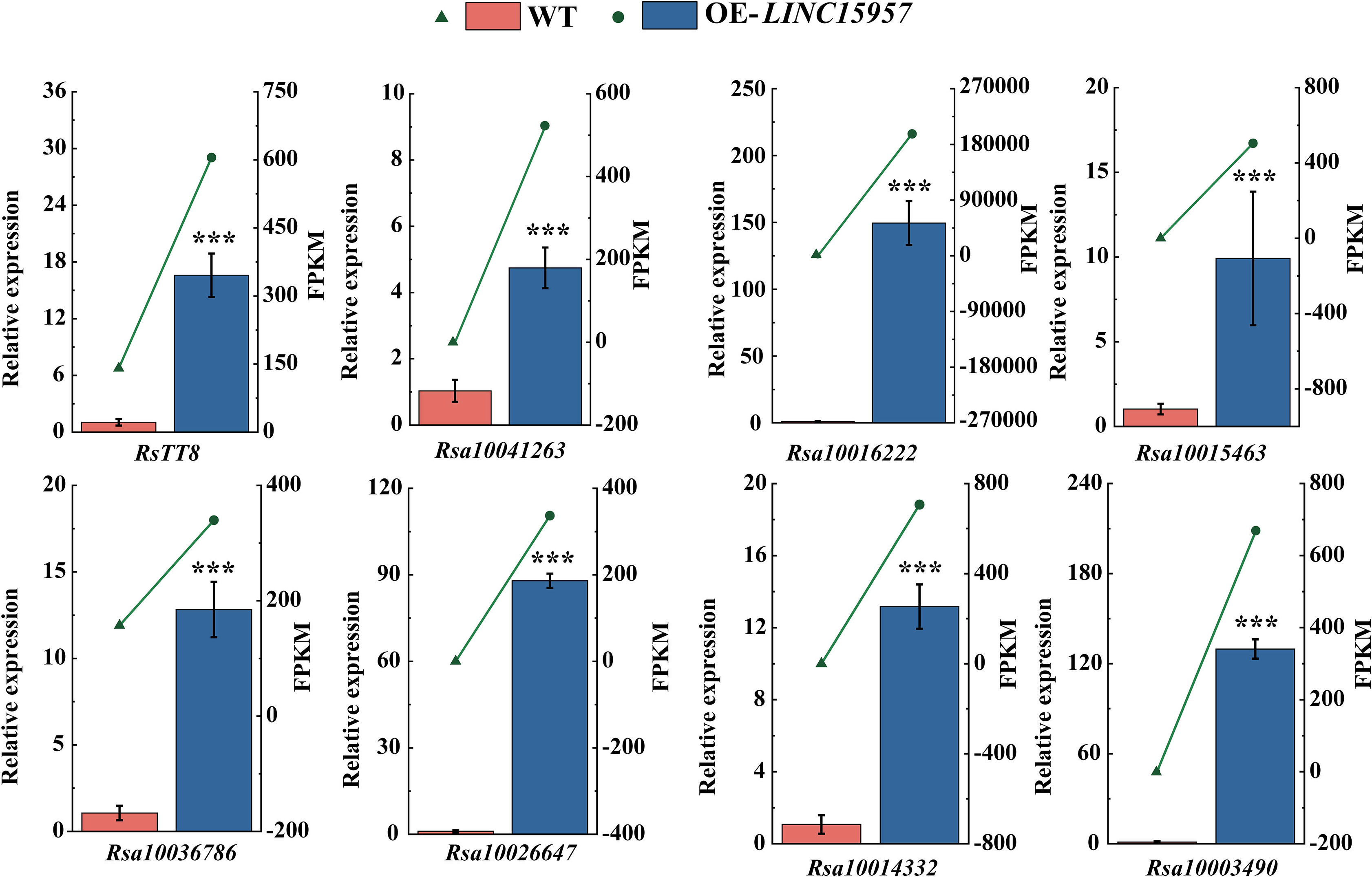
Figure 6 The genes related to anthocyanin biosynthesis in OE-LINC15957 were analyzed by RT-qPCR. Relative gene expression levels were standardized relative to actin transcription levels. Error bars represent the mean ± SE of three biological replicates. ***p < 0.001, Student’s t-test.
4 Discussion
The anthocyanin-rich radish varieties have good appearance quality, and also have high nutrition and value. The genes related to anthocyanin synthesis in radish have been widely characterized (Lim et al., 2016; Lai et al., 2021). It was found that genes homologous to Arabidopsis AtPAP1/2 in radish were involved in the regulation of radish anthocyanin synthesis (Lim et al., 2016). RsMYB1 was shown to be a key transcription factor regulating anthocyanin synthesis by heterologous overexpression in Arabidopsis, tobacco, and Petunia (Lim et al., 2016; Ai et al., 2017). The key gene RsMYB1.1 controlling the purple skin trait of radish fleshy roots was identified using QTL-seq and QTL mapping, and identified four homologs of Arabidopsis PAP1 (RsMYB1.1 to RsMYB1.4) in the radish genome (Liu et al., 2019a). RsMYB1, a key gene controlling anthocyanin biosynthesis in the fleshy root of radish was mapped by combined QTL-seq and RNA-seq technique, and verified their function by VIGS (Wang et al., 2020b). The key gene RsMYB90 was identified, which controls the red-skinned trait of fleshy roots of radish (Luo et al., 2020). Transient overexpression of RsGST1 and the key anthocyanin biosynthesis regulator RsMYB1a in radish leaves were significantly enhanced anthocyanin biosynthesis. Dual luciferase and yeast single hybridization assays showed that RsMYB1a binds to the promoter and activates RsGST1 expression. However, the molecular mechanisms regulating radish anthocyanin biosynthesis are not yet resolved.
Long noncoding RNAs are a potential regulator in pigment formation (Jin et al., 2013) and can activate multiple mechanisms or affect distal genes in a trans manner (Ørom et al., 2010; Ariel et al., 2014). Many studies found that lncRNAs recruited epigenetic complexes or act as target mimics (Liu et al., 2015; Yang et al., 2019). It was shown that the lncRNA mutant lncRNA1459 associated with tomato ripening significantly reduced lycopene accumulation and decreased the expression of related genes. It is predicted that lncRNA1459 indirectly regulates gene transcription by interacting with target proteins (Li et al., 2018). LncRNAs MLNC3.2 and MLNC4.6 act as endogenous targeting mimics (eTM) of miR156a and promote anthocyanin accumulation in apple fruit (Yang et al., 2019). The expression of lncRNA-MdLNC610 was activated under light treatment, which in turn activated the expression of MdACO1 and increased ethylene production and anthocyanin accumulation in apple fruits. Overexpression of MdLNC499 promoted anthocyanin accumulation and induced the expression of MdERF109 and MdWRKY1 (Ma et al., 2021). MdLNC610 was physically located downstream with the 1-aminocyclopropane-1-carboxylate oxygenase (ACO) ethylene biosynthesis gene MdACO1 and affected anthocyanin biosynthesis under high-light treatment (Yu et al., 2022). In this study, the anthocyanin accumulation was increased in transient overexpression of LINC15957 in radish leaves, while anthocyanin accumulation was significantly reduced in red flesh radish root by silencing LINC15957. A total of 5,772 differentially expressed genes were identified in transcriptome data. The anthocyanin related structural genes (RsANS, RsUFGT, RsPAL, RsF3H, RsDFR, RsC4H and RsCHS) and RsMYB1 were changed significantly with anthocyanin (Figure 7). KEGG enrichment analysis revealed the genes were significant enriched in tyrosine, L-Phenylalanine, tryptophan, phenylpropanol, and flavonoid biosynthesis. These results suggested that LINC15957 can play an important role in anthocyanin accumulation.
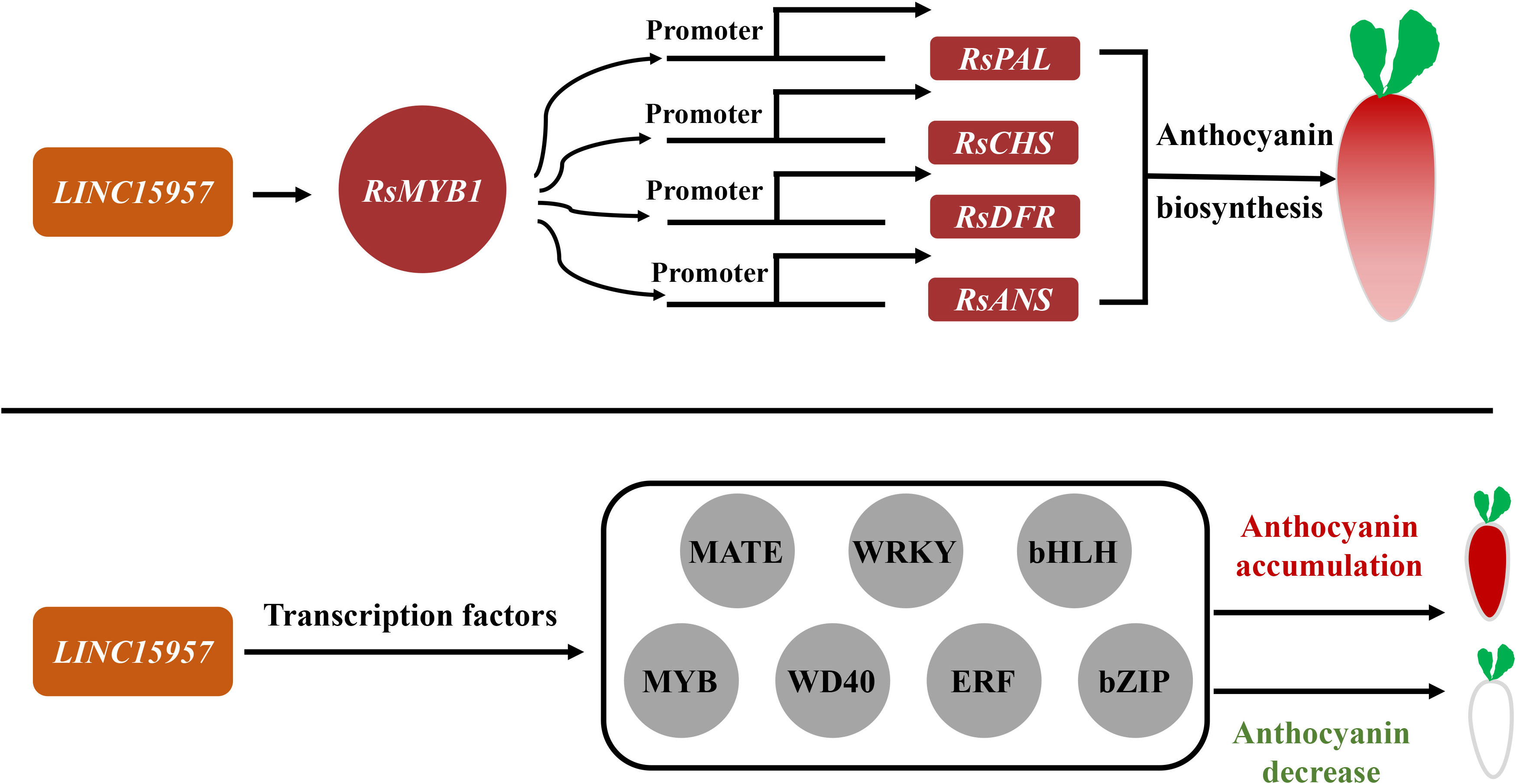
Figure 7 The regulatory pattern of LINC15957 with transcription factor and anthocyanin synthesis-related genes. Red represents up-regulation, gray represents up-regulation or down-regulation, rectangle represents genes related to anthocyanin synthesis, and circle represents transcription factors.
Anthocyanin biosynthesis in plants was regulated by various transcription factors, such as MYB, bHLH, WRKY, ERF, bZIP and BBX family members (Gonzalez et al., 2008; Jaakola et al., 2010). The transcriptional activator MBW complex (MYB-bHLH-WD40) regulates the activation of genes associated with anthocyanin biosynthesis in plants (Zhao et al., 2013). The R2R3 MYB transcription factor was involved in a variety of plant functions, including activation or repression of anthocyanin biosynthesis (Niu et al., 2010; Zhao et al., 2013; Chen et al., 2022). In general, bHLH transcription factors regulated structural genes responsible for anthocyanin biosynthesis (Zhang et al., 2019; Deng et al., 2020). CmbHLH2 and CmMYB6 interaction played a key role in anthocyanin pigmentation changes in chrysanthemum leaves and seeds (Lim et al, 2021). Overexpression of PbbHLH2 in “Red Zaosu” increased anthocyanin content and the gene expression levels of late biosynthetic genes. The WD40 repeat protein in Medicago truncatula is required for tissue-specific anthocyanin and proanthocyanidin biosynthesis but not for trichome development (Pang et al., 2009). Light promoted anthocyanin accumulation in dendrobium seedlings through the upregulation of the WD40 repeat transcription factor DcTTG1, which induced the expression of anthocyanin synthesis-related genes (Jia et al., 2021). In Arabidopsis, the WD40 repeat protein TTG1 forms a complex with bHLH (GL3, EGL3, or TT8) and R2R3-MYB (PAP1, PAP2, MYB113, and MYB114) TFs regulated anthocyanin synthesis (Zhang et al., 2003; Gonzalez et al., 2008). It was found that RsMYB41, RsMYB117, and RsMYB132, homologs of AtPAP1/2, were highly expressed in the root bark of red-skinned radish fleshy roots, while RsMYB65 and RsMYB159 were highly expressed in the root bark of purple-skinned radish fleshy roots, and presumably involved in the regulation of the anthocyanin synthesis of radish fleshy roots. The expression analysis revealed that two of the 135 bZIP gene family members (RsbZIP011 and RsbZIP102) might be involved in the regulation of anthocyanin biosynthesis in radish (Fan et al., 2019). Transcriptome analysis revealed that some members of WRKY, ERF, GRAS, NF-YA, C2H2-Dof, HD-ZIP, AP2, zinc finger protein, Tify, HB and LBD gene families may be involved in the regulation of radish anthocyanin biosynthesis (Sun et al., 2018; Gao et al., 2019; Yu et al., 2020; Gao et al., 2020b). In this study, A total of 87 MYB, 56 bHLH,16 WD40, 27 bZIP, 36 MATE, 67 WRKY and 61 ERF transcription factors were differential expression in OE-LINC15957 plants (Figure 7). These results suggested that transcription factors could involve in the regulation of anthocyanin accumulation in radish.
5 Conclusion
In this study, the overexpression of LINC15957 were improved anthocyanin accumulate in radish leaves, and silence of LINC15957 were decreased anthocyanin accumulate in red-fleshed radish by VIGS, indicating LINC15957 were positively regulates anthocyanin accumulate in radish. Transcriptome sequencing analysis revealed that the genes ANS, PAL, C4H, CHS, and DFR, which are related to anthocyanin biosynthesis, were differentially expressed in LINC15957 overexpressed leaves. RT-qPCR results also verified that these structural genes were highly expressed in LINC15957 overexpressed leaves, demonstrating that LINC15957 plays important roles in radish anthocyanin biosynthesis. These results in the present study provide a theoretical basis for understanding the regulatory mechanisms of lncRNAs involve in anthocyanin biosynthesis and breeding varieties with high anthocyanin content in radish.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA916343.
Author contributions
HT: writing original draft preparation. HT and XL: writing – review and editing. HT, XL, JLu, LW, YL, YJ and XP: methodology. XX, JLi and WZ: resources. HT, XL and JLu: data analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (31960598), Guizhou Highland Specialty Vegetable Green Production Science and Technology Innovation Talent Team (Qiankehe Platform Talent-CXTD [2022]003) and Guizhou Modern Agriculuture Research System(GZMARS)-Plateau characteristic vegetable industry.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1139143/full#supplementary-material
References
Ørom, U. A., Derrien, T., Beringer, M., Gumireddy, K., Gardini, A., Bussotti, G., et al. (2010). Long noncoding RNAs with enhancer-like function in human cells. Cell 143 (1), 46–58. doi: 10.1016/j.cell.2010.09.001
Ai, T. N., Naing, A. H., Arun, M., Jeon, S. M., Kim, C. K. (2017). Expression of RsMYB1 in Petunia enhances anthocyanin production in vegetative and floral tissues. Sci. Hortic. 214, 58–65. doi: 10.1016/j.scienta.2016.11.016
Ariel, F., Jegu, T., Latrasse, D., Romero-Barrios, N., Christ, A., Benhamed, M., et al. (2014). Noncoding transcription by alternative RNA polymerases dynamically regulates an auxin-driven chromatin loop. Mol. Cell. 55 (3), 383–396. doi: 10.1016/j.molcel.2014.06.011
Chekanova, J. A. (2015). Long non-coding RNAs and their functions in plants. Curr. Opin. Plant Biol. 27, 207–216. doi: 10.1016/j.pbi.2015.08.003
Chen, D. Z., Chen, H. D., Dai, G. Q., Zhang, H. M., Liu, Y., Shen, W. J., et al. (2022). Genome-wide identification of R2R3-MYB gene family and association with anthocyanin biosynthesis in Brassica species. BMC Genomics 23 (1), 1–13. doi: 10.1186/s12864-022-08666-7
Cong, L., Qu, Y. Y., Sha, G. Y., Zhang, S. C., Ma, Y. F., Chen, M., et al. (2021). PbWRKY75 promotes anthocyanin synthesis by activating PbDFR, PbUFGT, and PbMYB10b in pear. Physiol. Plant 173 (4), 1841–1849. doi: 10.1111/ppl.13525
Dai, S. L., Hong, Y. (2016). Molecular breeding for flower colors modification on ornamental plants based on the mechanism of anthocyanin biosynthesis and coloration. Sci. Agric. Sin. 49 (3), 529–542. doi: 10.3864/j.issn.0578-1752.2016.03.011
Deng, J., Li, J. J., Su, M. Y., Lin, Z. Y., Chen, L., Yang, P. F.. (2020). A bHLH gene NnTT8 of Nelumbo nucifera regulates anthocyanin biosynthesis. Plant Physiology and Biochemistry. 158: 518–523. doi: 10.1016/j.plaphy.2020.11.038
Fan, L. X., Wang, Y., Xu, L., Tang, M. J., Zhang, X. L., Ying, J. L., et al. (2020). A genome-wide association study uncovers a critical role of the RsPAP2 gene in red-skinned Raphanus sativus l. Hortic. Res. 7 (1), 13. doi: 10.1038/s41438-020-00385-y
Fan, L., Xu, L., Wang, Y., Tang, M., Liu, L. (2019). Genome- and transcriptome-wide characterization of bZIP gene family identifies potential members involved in abiotic stress response and anthocyanin biosynthesis in radish (Raphanus sativus l.). Int. J. Mol. Sci. 20 (24), 6334. doi: 10.3390/ijms20246334
Gao, J., Chen, B. W., Lin, H. J., Liu, Y., Wei, Y., Chen, F. B., et al. (2020a). Identification and characterization of the glutathione s-transferase (GST) family in radish reveals a likely role in anthocyanin biosynthesis and heavy metal stress tolerance. Gene 743, 144484. doi: 10.1016/j.gene.2020.144484
Gao, J., Li, W. B., Liu, H. F., Chen, F. B. (2020b). Identification of differential expression genes related to anthocyanin biosynthesis in carmine radish (Raphanus sativus l.) fleshy roots using comparative RNA-seq method. PloS One 15 (4), e0231729. doi: 10.1371/journal.pone.0231729
Gao, J., Peng, H., Chen, F. B., Luo, M., Li, W. B. (2019). Genome-wide analysis of transcription factors related to anthocyanin biosynthesis in carmine radish (Raphanus sativus l.) fleshy roots. PeerJ 7 (1), e8041. doi: 10.7717/peerj.8041
Gomez, C., Conejero, G., Torregrosa, L., Cheynier, V., Terrier, N., Ageorges, A. (2011). In vivo grapevine anthocyanin transport involves vesicle-mediated trafficking and the contribution of anthoMATE transporters and GST. Plant J. 67 (6), 960–970. doi: 10.1111/j.1365-313X.2011.04648.x
Gonzalez, A., Zhao, M., Leavitt, J. M., Lloyd, A. M. (2008). Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in arabidopsis seedlings. Plant J. 53 (5), 814–827. doi: 10.1111/j.1365-313X.2007.03373.x
Jaakola, L., Poole, M., Jones, M., Kamarainen-Karppinen, T., Koskimaki, J. J., Hohtola, A., et al. (2010). A SQUAMOSA MADS box gene involved in the regulation of anthocyanin accumulation in bilberry fruits. Plant Physiol. 153 (4), 1619–1629. doi: 10.1104/pp.110.158279
Jia, N., Wang, J., Wang, Y., Ye, W., Liu, J., Jiang, J., et al. (2021). The light-induced WD40-repeat transcription factor DcTTG1 regulates anthocyanin biosynthesis in Dendrobium candidum. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.633333
Jin, J. J., Liu, J., Wang, H., Wong, L., Chua, N. H. (2013). PLncDB: plant long non-coding RNA database. Bioinformatics 29 (8), 1068–1071. doi: 10.1093/bioinformatics/btt107
Lai, B., You, Y., Zhang, L., Wang, Q. X., Chen, F. B., Luo, G. J., et al. (2021). Identification and functional characterization of RsGST1, an anthocyanin-related glutathione s-transferase gene in radish. J. Plant Physiol. 263 (3), 153468. doi: 10.1016/j.jplph.2021.153468
Li, R., Fu, D. Q., Zhu, B. H., Luo, Y. B., Zhu, H. L. (2018). CRISPR/Cas9-mediated mutagenesis of lncRNA1459 alters tomato fruit ripening. Plant J. 94, 513–524. doi: 10.1111/tpj.13872
Lim, S. H., Kim, D. H., Jung, J. A., Lee, J. Y. (2021). Alternative splicing of the basic helix–Loop–Helix transcription factor gene CmbHLH2 affects anthocyanin biosynthesis in ray florets of chrysanthemum (Chrysanthemum morifolium). Front. Plant Sci. 12. doi: 10.3389/fpls.2021.669315
Lim, S. H., Song, J. H., Kim, D. H., Kim, J. K., Lee, J. Y., Kim, Y. M., et al. (2016). Activation of anthocyanin biosynthesis by expression of the radish R2R3-MYB transcription factor gene RsMYB1. Plant Cell Rep. 35 (3), 641–653. doi: 10.1007/s00299-015-1909-3
Lin, Y. X., Jiang, L. Y., Chen, Q., Li, Y. L., Zhang, Y. T., Luo, Y., et al. (2018). Comparative transcriptome profiling analysis of red- and white-fleshed strawberry (Fragaria × ananassa) provides new insight into the regulation of the anthocyanin pathway. Plant Cell Physiol. 59 (9), 1844–1859. doi: 10.1093/pcp/pcy098
Liu, X., Hao, L. L., Li, D. Y., Zhu, L. H., Hu, S. N. (2015). Long non-coding RNAs and their biological roles in plants. Genom Proteom Bio. 13, 137–147. doi: 10.1016/j.gpb.2015.02.003
Liu, Y. J., Hou, H., Jiang, X. L., Wang, P. Q., Dai, X. L., Chen, W., et al. (2018b). A WD40 repeat protein from Camellia sinensis regulates anthocyanin and proanthocyanidin accumulation through the formation of MYB-bHLH-WD40 ternary complexes. Int. J. Mol. Sci. 19 (6), 1686. doi: 10.3390/ijms19061686
Liu, X., Li, D. Y., Zhang, D. L., Yi, D. D., Zhao, Y., Ji, C. J., et al. (2018a). A novel antisense long noncoding RNA, TWISTED LEAF, maintains leaf blade flattening by regulating its associated sense R2R3-MYB gene in rice. New Phytol. 218 (2), 774–788. doi: 10.1111/nph.15023
Liu, T. J., Wang, J. L., Wu, C. H., Zhang, Y. J., Zhang, X. H., Li, X. M., et al. (2019a). Combined QTL-seq and traditional linkage analysis to identify candidate genes for purple skin of radish fleshy taproots. Front. Genet. 10. doi: 10.3389/fgene.2019.00808
Liu, T. J., Zhang, Y. J., Zhang, X. H., Sun, Y. Y., Wang, H. P., Song, J. P., et al. (2019b). Transcriptome analyses reveal key genes involved in skin color changes of ‘Xinlimei’ radish taproot. Plant Physiol. Bioch. 139, 528–539. doi: 10.1016/j.plaphy.2019.04.006
Luo, X. B., Xu, L., Wang, Y., Dong, J. H., Chen, Y. L., Tang, M. J., et al. (2020). An ultra-high-density genetic map provides insights into genome synteny, recombination landscape and taproot skin colour in radish (Raphanus sativus l.). Plant Biotechnol. J. 18, 274–286. doi: 10.1111/pbi.13195
Ma, H. Y., Yang, T., Li, Y., Zhang, J., Wu, T., Song, T. T., et al. (2021). The long noncoding RNA MdLNC499 bridges MdWRKY1 and MdERF109 function to regulate early-stage light-induced anthocyanin accumulation in apple fruit. Plant Cell. 33, 3309–3330. doi: 10.1093/plcell/koab188
Mao, Z. L., Jiang, H. Y., Wang, S., Wang, Y. C., Yu, L., Zou, Q., et al. (2021). The MdHY5-MdWRKY41-MdMYB transcription factor cascade regulates the anthocyanin and proanthocyanidin biosynthesis in red-fleshed apple. Plant Sci. 306, 110848. doi: 10.1016/j.plantsci.2021.110848
Matsufuji, H., Kido, H., Misawa, H., Yaguchi, J., Otsuki, T., Chino, M., et al. (2007). Stability to light, heat, and hydrogen peroxide at different pH values and DPPH radical scavenging activity of acylated anthocyanin from red radish extract. J. Agric. Food Chem. 55 (9), 3692–3701. doi: 10.1021/jf063598o
M'mbone, M. E., Cheng, W., Xu, L., Wang, Y., Karanja, B. K., Zhu, X., et al. (2018). Identification and transcript analysis of MATE genes involved in anthocyanin transport in radish (Raphanus sativus L.). Sci. Hortic. 238: 195–203. doi: 10.1016/j.scienta.2018.04.029
Muleke, E. M. M., Wang, Y., Zhang, W. T., Liang, X. U., Liu, L. W. (2021). Genome-wide identification and expression profiling of MYB transcription factor genes in radish (Raphanus sativus l.). J. Integr. Agr. 20 (1), 120–131. doi: 10.1016/S2095-3119(20)63308-1
Niu, S. S., Xu, C. J., Zhang, W. S., Zhang, B., Li, X., Lin-Wang, K., et al. (2010). Coordinated regulation of anthocyanin biosynthesis in Chinese bayberry (Myrica rubra) fruit by a R2R3 MYB transcription factor. Planta 231 (4), 887–899. doi: 10.1007/s00425-009-1095-z
Nomi, Y., Iwasaki-Kurashige, K., Matsumoto, H. (2019). Therapeutic effects of anthocyanin for vision and eye health. Molecules 24 (18), 3311. doi: 10.3390/molecules24183311
Pang, Y., Wenger, J. P., Saathoff, K., Peel, G. J., Wen, J. Q., Huhman, D., et al. (2009). A WD40 repeat protein from Medicago truncatula is necessary for tissue-specific anthocyanin and proanthocyanidin biosynthesis but not for trichome development. Plant Physiol. 151 (3), 1114–1129. doi: 10.1104/pp.109.144022
Pflieger, S., Blanchet, S., Camborde, L., Drugeon, G., Rousseau, A., Noizet, M., et al. (2008). Efficient virus-induced gene silencing in arabidopsis using a 'one-step' TYMV-derived vector. Plant J. 56 (4), 678–690. doi: 10.1111/j.1365-313X.2008.03620.x
Ponting, C. P., Oliver, P. L., Reik, W. (2009). Evolution and functions of long noncoding RNAs. Cell 136, 629–641. doi: 10.1016/j.cell.2009.02.006
Rahman, M. M., Ichiyanagi, T., Komiyama, T., Hatano, Y., Konishi, T. (2006). Superoxide radical- and peroxynitrite-scavenging activity of anthocyanin; structure-activity relationship and their synergism. Free Radic. Res. 40 (9), 993–1002. doi: 10.1080/10715760600815322
Ramsay, N. A., Glover, B. J. (2005). MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 10 (2), 63–70. doi: 10.1016/j.tplants.2004.12.011
Sun, Y., Hao, P., Lv, X., Tian, J., Wang, Y., Zhang, X., et al. (2020). A long non-coding apple RNA, MSTRG.85814.11, acts as a transcriptional enhancer of SAUR32 and contributes to the fe-deficiency response. Plant J. 103 (1), 53–67. doi: 10.1111/tpj.14706
Sun, Y. Y., Wang, J. L., Qiu, Y., Liu, T. J., Song, J. P., Li, X. X. (2018). Identification of ‘Xinlimei’ radish candidate genes associated with anthocyanin biosynthesis based on a transcriptome analysis. Gene 657, 81–91. doi: 10.1016/j.gene.2018.03.001
Wang, D., Gao, Y., Sun, S., Li, L., Wang, K. (2022). Expression profiles and characteristics of apple lncRNAs in roots, phloem, leaves, flowers, and fruit. Int. J. Mol. Sci. 23 (11), 5931. doi: 10.3390/ijms23115931
Wang, Y., Liu, W., Wang, X., Yang, R., Wu, Z., Wang, H., et al. (2020b). MiR156 regulates anthocyanin biosynthesis through SPL targets and other microRNAs in poplar. Hortic. Res. 7, 118. doi: 10.1038/s41438-020-00341-w
Wang, Y., Luo, X. J., Sun, F., Hu, J. H., Zha, X. J., Su, W., et al. (2018). Overexpressed lncRNA LAIR increases grain yield and regulates neighbouring gene cluster expression in rice. Nat. Commun. 9 (1), 3516. doi: 10.1038/s41467-018-05829-7
Wang, Q. B., Wang, Y. P., Sun, H. H., Sun, L., Zhang, L. (2020a). Transposon-induced methylation of the RsMYB1 promoter disturbs anthocyanin accumulation in red-fleshed radish. J. Exp. Bot. 71 (9), 2537–2550. doi: 10.1093/jxb/eraa010
Wu, L., Liu, S., Qi, H., Cai, H., Xu, M. (2020a). Research progress on plant long non-coding RNA. Plants 9, 408. doi: 10.3390/plants9040408
Wu, T., Liu, H. T., Zhao, G. P., Song, J. X., Wang, X. L., Yang, C. Q., et al. (2020b). Jasmonate and ethylene-regulated ethylene response factor 22 promotes lanolin-induced anthocyanin biosynthesis in 'Zaosu' pear (Pyrus bretschneideri rehd.) fruit. Biomolecules 10 (2), 278. doi: 10.3390/biom10020278
Yang, T., Ma, H., Zhang, J., Wu, T., Song, T. T., Tian, J., et al. (2019). Systematic identification of long noncoding RNAs expressed during light-induced anthocyanin accumulation in apple fruit. Plant J. 100 (3), 572–590. doi: 10.1111/tpj.14470
Yu, R. G., Du, X. L., Li, J., Liu, L., Hu, C. M., Yan, X. L., et al. (2020). Identification and differential expression analysis of anthocyanin biosynthetic genes in root-skin color variants of radish (Raphanus sativus l.). Genes Genom. 42 (4), 413–424. doi: 10.1007/s13258-020-00915-x
Yu, J., Qiu, K., Sun, W., Yang, T., Wu, T., Song, T., et al. (2022). A long noncoding RNA functions in high-light-induced anthocyanin accumulation in apple by activating ethylene synthesis. Plant Physiol. 189 (1), 66–83. doi: 10.1093/plphys/kiac049
Yu, J., Yang, X. D., Wang, Q., Gao, L. W., Yang, Y., Xiao, D., et al. (2018). Efficient virus-induced gene silencing in Brassica rapa using a turnip yellow mosaic virus vector. Biol. Plant 62, 826–834. doi: 10.1007/s10535-018-0803-6
Zhang, G. Y., Chen, D. G., Zhang, T., Duan, A. G., Zhang, J. G., He, C. Y. (2018). Transcriptomic and functional analyses unveil the role of long non-coding RNAs in anthocyanin biosynthesis during sea buckthorn fruit ripening. DNA Res. 25 (5), 465–476. doi: 10.1093/dnares/dsy017
Zhang, F., Gonzalez, A., Zhao, M., Payne, C. T., Lloyd, A. (2003). A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130, 4859–4869. doi: 10.1242/dev.00681
Zhang, Y. J., Li, Y., Li, W. P., Hu, Z. L., Yu, X. H., Tu, Y., et al. (2019). Metabolic and molecular analysis of nonuniform anthocyanin pigmentation in tomato fruit under high light. Hortic. Res. 6, 56. doi: 10.1038/s41438-019-0138-2
Zhang, J., Mujahid, H., Hou, Y., Nallamilli, B. R., Peng, Z. (2013). Plant long ncRNAs: A new frontier for gene regulatory control. Am. J. Plant Sci. 4, 1038–1045. doi: 10.4236/ajps.2013.45128
Zhao, L., Gao, L. P., Wang, H. X., Chen, X. T., Wang, Y. S., Yang, H., et al. (2013). The R2R3-MYB, bHLH, WD40, and related transcription factors in flavonoid biosynthesis. Funct. Integr. Genomic. 13 (1), 75–98. doi: 10.1007/s10142-012-0301-4
Zhou, Y., Mumtaz, M. A., Zhang, Y., Yang, Z., Hao, Y., Shu, H., et al. (2022). Response of anthocyanin biosynthesis to light by strand-specific transcriptome and miRNA analysis in Capsicum annuum. BMC Plant Biol. 22 (1), 79. doi: 10.1186/s12870-021-03423-6
Zhu, B., Yang, Y., Li, R., Fu, D., Wen, L., Luo, Y., et al. (2015). RNA Sequencing and functional analysis implicate the regulatory role of long non-coding RNAs in tomato fruit ripening. J. Exp. Bot. 66, 4483–4495. doi: 10.1093/jxb/erv203
Keywords: radish, anthocyanin, lncRNA, transcriptome, differentially expressed genes (DEGs)
Citation: Tan H, Luo X, Lu J, Wu L, Li Y, Jin Y, Peng X, Xu X, Li J and Zhang W (2023) The long noncoding RNA LINC15957 regulates anthocyanin accumulation in radish. Front. Plant Sci. 14:1139143. doi: 10.3389/fpls.2023.1139143
Received: 06 January 2023; Accepted: 13 February 2023;
Published: 27 February 2023.
Edited by:
Mengyao Li, Sichuan Agricultural University, ChinaReviewed by:
Kaijing Zhang, Anhui Science and Technology University, ChinaXiaochuan Sun, Huaiyin Institute of Technology, China
Bo Sun, Sichuan Agricultural University, China
Copyright © 2023 Tan, Luo, Lu, Wu, Li, Jin, Peng, Xu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wanping Zhang, MTIyNjE5MDM2OEBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Huping Tan
Huping Tan Xiaobo Luo
Xiaobo Luo Jinbiao Lu1,2
Jinbiao Lu1,2 Jingwei Li
Jingwei Li