- 1Institute for Agro-food Standards and Testing Technology, Shanghai Academy of Agricultural Sciences, Shanghai, China
- 2School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai, China
- 3Technical Center for Animal Plant and Food Inspection and Quarantine, Shanghai Customs, Shanghai, China
A systematic study was carried out on 638 wheat and paddy grains (including fresh and stored samples) collected in 2021 from Shanghai, China, to identify the major mycobiota and their toxigenic abilities. A total of 349 fungi, namely, 252 Fusarium, 53 Aspergillus, and 44 Alternaria, were characterized by morphological and molecular identification. Fusarium and Aspergillus were more frequently isolated in paddy with Fusarium sambucinum species complex and Aspergillus section flavi as the predominant species, respectively. The genus Alternaria was the most frequently isolated fungal species in wheat. The toxin-producing potentials of the identified fungi were further evaluated in vitro. Deoxynevalenol (DON) was produced by 34.5% of Fusarium isolates and zearalenone (ZEN) was produced by 47.6% of them, and one isolate also processed the abilities for fumonisin B1 (FB1), B2 (FB2), and B3 (FB3) productions. Aflatoxin B1 (AFB1), B2 (AFB2), and G1 (AFG1) were only generated by Aspergillus section flavi, with the production rate of 65.5%, 27.6%, and 13.8%, respectively. Alternariol (AOH) was the most prevalent Alternaria toxin, which could be produced by 95.5% of the isolates, followed by alternariol monomethyl ether (AME) (72.7%), altenuene (ALT) (52.3%), tenuazonic acid (TeA) (45.5%), tentoxin (TEN) (29.5%), and altenusin (ALS) (4.5%). A combinational analysis of mycobiota and toxigenic ability allowed us to provide comprehensive information about the production mechanisms of mycotoxins in wheat and paddy in a specific geographic area, and will be helpful for developing efficient prevention and control programs.
1 Introduction
Paddy (Oryza sativa L.) and wheat (Triticum aestivum L.), widely cultured crops in the world, are considered as the most important staple foods in China (Hou et al., 2015). Shanghai, the center of China’s economy and trade (Wang and Zhang, 2005), imports most of its consumed wheat and paddy grains (approximately 80.0%) from other cities, such as Anhui, Shandong, and Heilongjiang. The quality and safety of the stored grains are essential, and any deterioration may lead to a significant impact on consumer and animal health.
In Shanghai, the typical subtropical monsoon climate provides favorable conditions for fungal infections of grains (Chen et al., 2008; Qiu et al., 2014). With suitable temperature and surface moisture, fungal spores present on the kernels can germinate and grow, and then destroy the kernels, leading to yield loss and quality reduction (Lacey and Magan, 1991; Kosiak et al., 2004). Some fungal species can also produce a range of mycotoxins (Luo et al., 2021), which are chemically or heat stable, and difficult to be degraded, leading to various adverse health effects including teratogenicity, carcinogenicity, mutagenicity, immunotoxicity, or neurotoxicity (Haque et al., 2020; Suman, 2021). Mycotoxin contamination is considered to be one of the most serious food safety problems in the world (Ali et al., 2022).
In China, many studies have reported the serious contaminations of mycotoxins in wheat and paddy grains, mainly focusing on Fusarium toxins [e.g., deoxynevalenol (DON), zearalenone (ZEN), fumonisin B1 (FB1), B2 (FB2), and B3 (FB3)] (Han et al., 2014; Qiu et al., 2019; Yan et al., 2020), Aspergillus toxins [e.g., aflatoxin B1 (AFB1), B2 (AFB2), G1 (AFG1), and G2 (AFG2)] (Sun et al., 2011; Li et al., 2014), and Alternaria toxins [e.g., alternariol (AOH), alternariol monomethyl ether (AME), tenuazonic acid (TeA), tentoxin (TEN), altenuene (ALT), and altenusin (ALS)] (Li et al., 2001; Xu et al., 2016; Jiang et al., 2021). A 3-year (2010–2012) survey, conducted in Jiangsu province, China, showed that DON was the most important mycotoxin, which was found in 74.4% of wheat samples at levels ranging from 14.5 to 41,157.1 μg/kg (mean, 488.0 μg/kg), while ZEN was detected in 12.8% of samples at levels ranging from 10.1 to 3,048.9 μg/kg (mean, 73.0 μg/kg) (Ji et al., 2014). Owing to the widespread occurrence and high toxicities, comprehensive information on the production mechanisms of typical mycotoxins have become a critical issue.
In general, production of particular mycotoxins by fungi primarily depends on the fungal species. As reported, DON and ZEN are mainly produced by F. graminearum and F. culmorum (Yang et al., 2018; Ekwomadu et al., 2021); AFB1, AFB2, AFG1, and AFG2 are mainly produced by A. flavus and A. parasiticus (Tsai and Yu, 1999; Diaz et al., 2009); Alternaria toxins are mainly produced by A. alternata, A. padwickii, etc. (Ntasiou et al., 2015; Turzhanova et al., 2020). Certain mycotoxins in grains could also be produced by others fungal species. Fumonisins are mainly produced by species of Fusarium fujikuroi complex such as F. verticillioides, F. proliferatum, and F. fujikuroi, but they could also be produced by Aspergillus spp. (Frisvad et al., 2007). The toxigenic abilities of the strains belonging to the same species vary in types and concentrations of the produced mycotoxins. The same fungi might even produce different mycotoxins under different environmental conditions. A. alternata was known to produce Alternaria toxins, but it could also produce fumonisins (Chen et al., 1993; Abbas and Riley, 1996; Mirocha et al., 1996). To date, although mycotoxin contamination in wheat and paddy grains in Shanghai, China has been reported (Xing et al., 1997; Fan et al., 2021; Huang et al., 2022), little was known about the occurrence of the main toxigenic fungi and their abilities for mycotoxin production.
Based on these considerations, the aims of this work were to (1) investigate the presence of fungal microorganisms, with particular attention to toxigenic species, in 638 wheat and paddy samples collected from Shanghai, China in 2021; and (2) evaluate the mycotoxin-producing potentials of the main isolates including Aspergillus spp., Fusarium spp., and Alternaria spp.
2 Materials and methods
2.1 Chemicals and reagents
The mycotoxin standards (purity>98%) of AFB1, AFB2, AFG1, AFG2, DON, ZEN, AOH, AME, TeA, TEN, ALT, ALS, FB1, FB2, and FB3 (Supplementary Figure 1) were purchased from Qingdao pribolab (Qingdao, China). All standards were dissolved in acetonitrile to prepare 1.0 mg/ml of stock solutions and stored at −20 ± 2°C. Water was purified by a Milli-Q system (Millipore, Brussels, Belgium).
Methanol, acetonitrile, formic acid, and ammonium acetate (HPLC grade) were purchased from Merck (Darmstadt, Germany). Sodium chloride (NaCl, analytical grade) and anhydrous magnesium sulfate (MgSO4, analytical grade) were supplied by ANPEL (Shanghai, China).
2.2 Grain samples
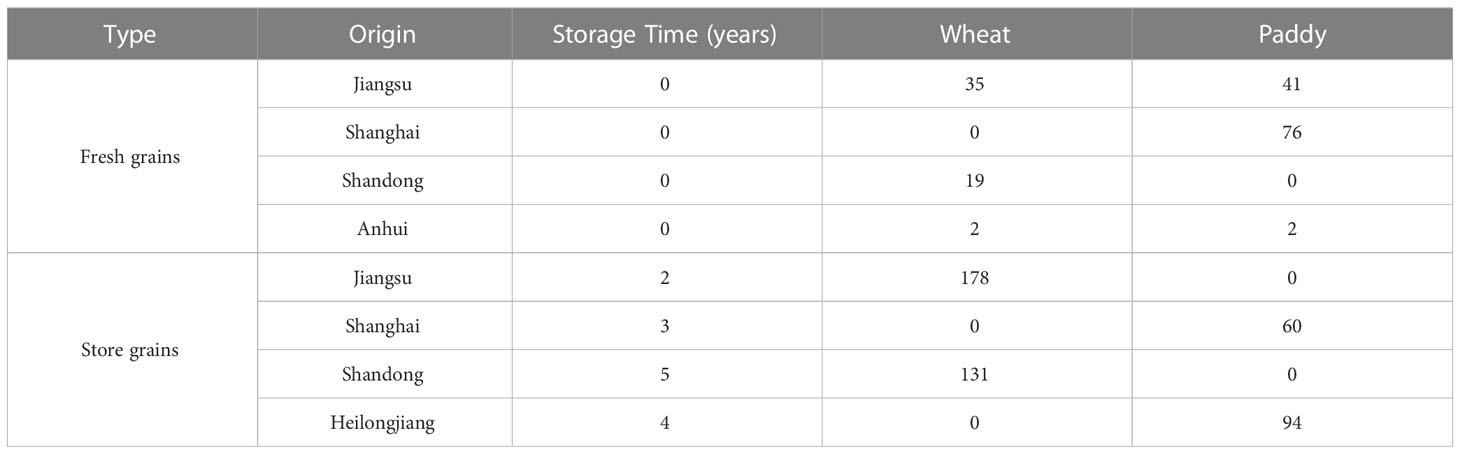
A total of 638 grain samples, namely, 365 wheat (56 fresh wheat and 309 stored wheat) and 273 paddy (119 fresh paddy and 154 stored paddy), were collected from Shanghai Pujiang Warehousing Co., Ltd. (Shanghai, China) in 2021. The fresh grains were the samples freshly collected from different parts of China (Supplementary Figure 2) and shipped to Shanghai for storage (Table 1). The stored grains were the samples stored in the barns of Shanghai for 2–5 years with good ventilation and controllable temperature (10–20°C) and humidity (50%–60%) conditions. All collected samples (each approximately 500 g) were stored in pre-sterilized polyethylene bags at 4.0 ± 0.5°C until analysis.
2.3 Isolation and identification of fungal strains
Seeds were soaked in 75% ethanol for 2 min, and then were rinsed three times by sterile water. The surface moisture of the seeds was wiped with sterilized absorbent paper. The seeds were then placed on the surface of potato dextrose agar (PDA) in petri dishes (90 mm diameter, 8 kernels/plate) and incubated at 28 ± 2°C for 4 days. The fungal strains were purified by subculture of single conidia (Dong et al., 2021) and stored as spores in 30% glycerol at −80 ± 2°C.
The isolated fungi were firstly identified by the morphological observations according to the previous studies (Reddy et al., 2010; Nagaraja et al., 2016; Tralamazza et al., 2018) and then were validated by PCR analysis (Munitz et al., 2014). All fungal strains were inoculated on PDA and cultured for 7 days at 28 ± 2°C in the dark. DNA was extracted from fungal strains according to the CTAB protocol and dissolved in 50 µl of TE (pH 8.0, 10 mM Tris and 1 mM EDTA) (Brandfass and Karlovsky, 2008). The universal primers ITS1 (5-TCCGTAGGTGAACCTGCGG-3) and ITS4 (5-TCCTCCGCTTATTGATATGC-3) were selected and the total volume of the PCR amplification was 50 μl. The PCR reaction conditions were as follows: 95°C for 5 min, followed by 35 cycles at 95°C for 30 s, 58°C for 30 s, 72°C for 1 min, and finally 72°C extension for 7 min. PCR products were purified by the AxyPrep DNA gel recovery kit, and sequenced with ABI 3730XL Analyzer (Applied Biosystems) (Sunagawa et al., 2021). The ITS sequences were compared with the sequences in the National Center for Biotechnology Information (NCBI) GenBank database by the Basic Local Alignment Search Tool (BLAST) (Supplementary Table 1). The isolates were identified with the sequences similarity in the range of 99%–100%.
2.4 Mycotoxin production by the isolated fungal strains
The toxigenic abilities of the fungal strains, which were confirmed as Fusarium, Aspergillus, or Alternaria, were evaluated in PDA. Samples were prepared following the method described previously (Fan et al., 2021). Briefly, the isolated fungal strains were cultured on PDA (9 mm diameter agar disc) at 28 ± 2°C for 7 days in quintuplicate (n = 5). The medium was dried at 50 ± 2°C (Shi et al., 2016), and the weight of medium was recorded for calculation of the mycotoxin production. Then, it was transferred into a 50-ml centrifuge tube and extracted with 10 ml of acetonitrile/water/formic acid (84/15/1, v/v/v) by shaking for 30 min and ultrasonicating for 40 min. After centrifugation at 4,000 g for 10 min, the supernatant was collected and evaporated under a soft stream of nitrogen gas at 45°C. The residues were redissolved in 1 ml of acetonitrile/water containing 5 mmol/L ammonium acetate (20/80, v/v). Finally, the solution was passed through a 0.22-μm filter membrane prior to ultrahigh-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis.
2.5 UPLC-MS/MS analysis
UPLC analysis was performed on a Waters Acquity UPLC system (Waters, Milford, MA, USA). Separation was achieved on a Waters XBridge® BEH-C18 XP column (130 Å, 2.5 µm, 3.0 × 100 mm, PN: 186006035) at 40°C. The mobile phase consisted of (A) acetonitrile and (B) water containing 5 mmol/L ammonium acetate, and a linear gradient elution program was applied as follows: initial, 10% A; 1 min, 10% A; 3 min, 70% A; 5 min, 90% A; 6 min, 90% A; 6.1 min, 10%; 8 min, 10% A. The mobile phase flow rate was 0.4 ml/min.
The separated compounds were analyzed by a Waters XEVO TQMS mass spectrometer (Waters, Milford, MA, USA) with an electrospray ionization source operated in negative mode (ESI−) for ZEN and ALS, and in positive mode (ESI+) for the other mycotoxins. Multiple reaction monitoring (MRM) mode was established as shown in Supplementary Table 2. The source parameters are set as follows: capillary voltage of 2.5 kV for ESI+ and 1.5 kV for ESI−, ion source temperature of 150°C, and desolvation temperature of 500°C. The gas flow rates were 7.0 bar for nebulizing gas and 1,000 L/h for desolvation gas, respectively. TargetLynx XS software was used to process the data (Waters Corporation, Milford, MA, USA).
2.6 Statistics
Tables were plotted using Microsoft Office Excel 2019 (Microsoft Corp., Redmond, WA, USA). Mycotoxin analysis was performed using TargetLynx XS software (Waters Corporation, Milford, MA, USA). The statistical analysis was performed using IBM SPSS Statistics soft version 26.0 (SPSS Inc., Chicago, IL, USA). The effect of fungal species on the production of mycotoxins was analyzed by a Chi-square test. Meanwhile, the effect of wheat/paddy and fresh/stored on the production of mycotoxins by fungi was analyzed by one-way ANOVA based on t-test, differences with p value ≤ 0.05 were considered significant. The DNA sequences were edited and aligned by BLAST at NCBI (http://www.ncbi.nlm.nih.gov/).
3 Results
3.1 Occurrence of fungal species from 638 wheat and paddy samples in Shanghai, China
A total of 349 fungal isolates (see Supplementary Table 1 for details) were obtained from 638 wheat and paddy samples in Shanghai, China (Table 2). The number of isolates (298) from paddy were much more than those (51) from wheat. The most prevalent genus was Fusarium with 252 isolates recovered. Among these, 242 isolates were isolated from fresh paddy grains. According to the morphological study and ITS sequences, Fusarium species were further characterized as members of the certain species complex (SC). Fusarium sambucinum SC were identified to be the predominant fungi. It is worth noting that 45.4% of the samples were infected with more than one Fusarium strain.
Aspergillus spp. (53) were also isolated but at a relatively lower frequency (15.2%) compared to Fusarium spp. (72.2%). All Aspergillus isolates were identified from stored grains with more strains (40) from paddy than that (13) from wheat. Aspergillus section flavi and A. fumigatus were the dominant species. Conversely, a total of 44 Alternaria spp. were isolated, most of which were from fresh grains (32), and no strains were found in stored paddy grains.
3.2 Toxigenic abilities of the main isolates
The toxin-producing potentials of the 349 isolates belonging to Fusarium spp., Aspergillus spp., and Alternaria spp. were evaluated (Supplementary Table 3). All the isolates were cultured in PDA for 7 days at 28 ± 2°C. As shown in Table 3, among 252 Fusarium strains, 87 (34.5%) isolates could produce DON, and 120 (47.6%) could produce ZEN. One out of three Fusarium isolates from stored wheat could produce DON, with a level of 44.2 mg/kg, much higher than those from fresh wheat grains. As potential producers of fumonisins, all the Fusarium isolates were further analyzed. Only one Fusarium fujikuroi SC stain isolated from fresh paddy was found to produce fumonisins with the concentrations of 128.1 mg/kg for FB1, 39.2 mg/kg for FB2, and 38.9 mg/kg for FB3, respectively.
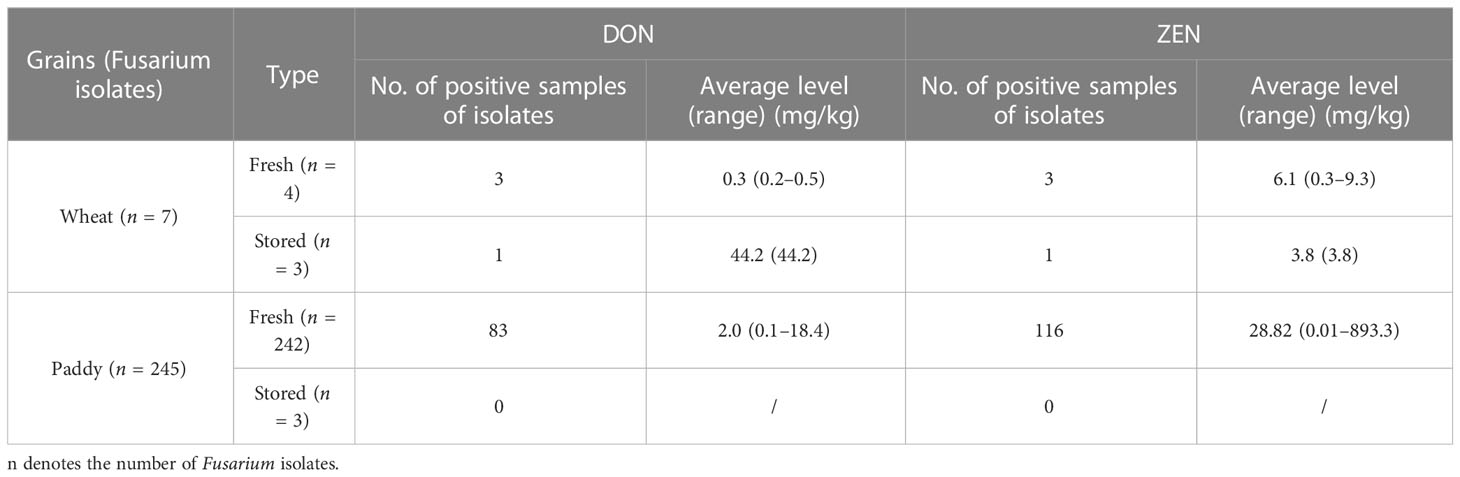
Table 3 Fusarium toxin-producing potentials of Fusarium species isolated from different grains in Shanghai, China.
The abilities of Aspergillus isolates to produce AFB1, AFB2, AFG1, and AFG2 were evaluated (Table 4). Among 53 Aspergillus strains, only 29 Aspergillus section flavi could produce AFBS, from which, 19 (35.8%) produced AFB1, 8 (15.1%) produced AFB2, 4 (7.5%) produced AFG1, and no one could produce AFG2. The highest levels of AFB1, AFB2, and AFG1 were 155.5 mg/kg, 19.0 mg/kg, and 0.6 mg/kg, respectively, which were produced by the same Aspergillus section flavi isolate.
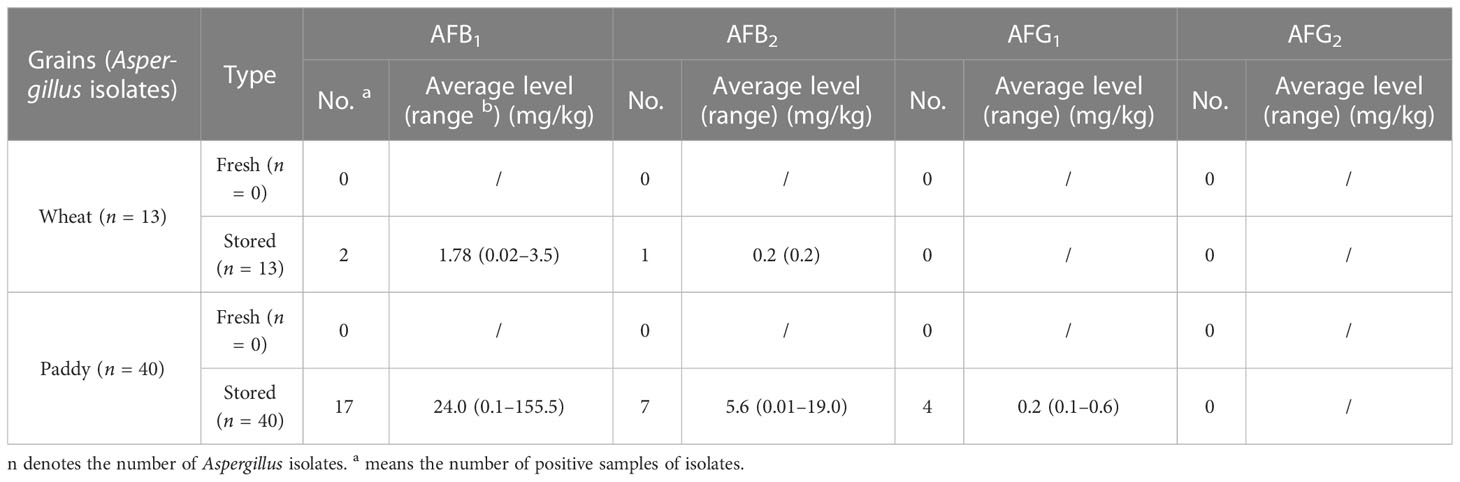
Table 4 Aspergillus toxin-producing potentials of Aspergillus isolates from different grains in Shanghai, China.
The abilities of Alternaria isolates (44) were also assayed for their productions of six Alternaria toxins, including AOH, AME, TeA, TEN, ALT, and ALS (Table 5). A total of 95.5% Alternaria isolates could produce at least one Alternaria toxin. AOH was the most prevalent Alternaria toxin, which could be produced by 95.5% of the isolates, followed by AME (72.7%), ALT (52.3%), TeA (45.5%), and TEN (29.5%). Only two Alternaria isolates produced ALS, both of which were isolated from wheat grains.
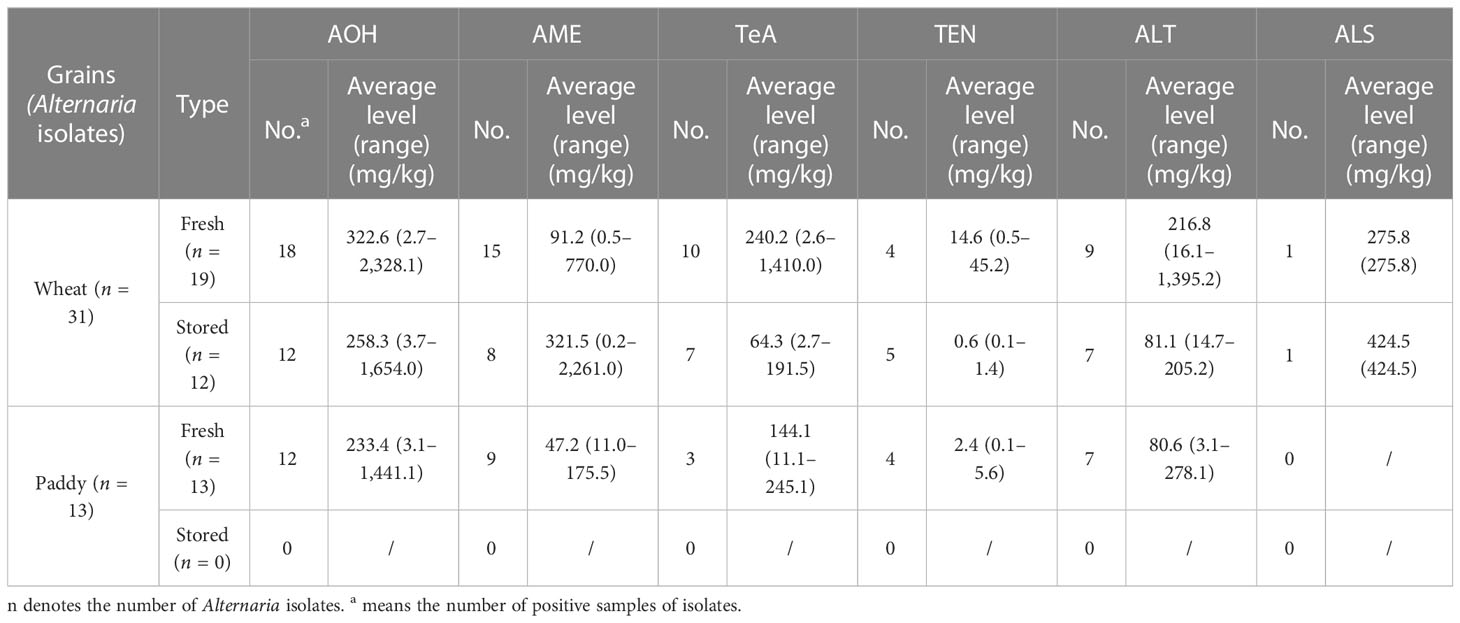
Table 5 Alternaria toxin-producing potentials of Alternaria isolates from different grains in Shanghai, China.
4 Discussion
Shanghai is the economic and financial center of China rather than a major agricultural city and most of wheat and paddy consumed in this city were supplied by other areas of China. Mycotoxins, a series of secondary metabolites produced by various mold species in grains, especially wheat and paddy during storage, have become important impactors on human and animal health. Consequently, the presence and toxigenic abilities of the harmful fungi in the stored grains are of great concern. In this study, a total of 638 wheat and paddy grains were collected from Shanghai, China in 2021, and the presence of probable toxigenic fungi including Fusarium spp., Aspergillus spp., and Alternaria spp. And their toxin-producing potentials were thoroughly investigated (Figure 1). To the best of our knowledge, this is the first survey conducted on wheat and rice grains (including fresh and stored samples) consumed in Shanghai, China that took into consideration both contaminating fungi and their toxigenic abilities. However, the morphological study in combination with ITS region analysis was insufficient to distinguish the specific sections but only provided genera information. Further molecular identification is required to accurately identify the species of Fusarium, Aspergillus, and Alternaria.
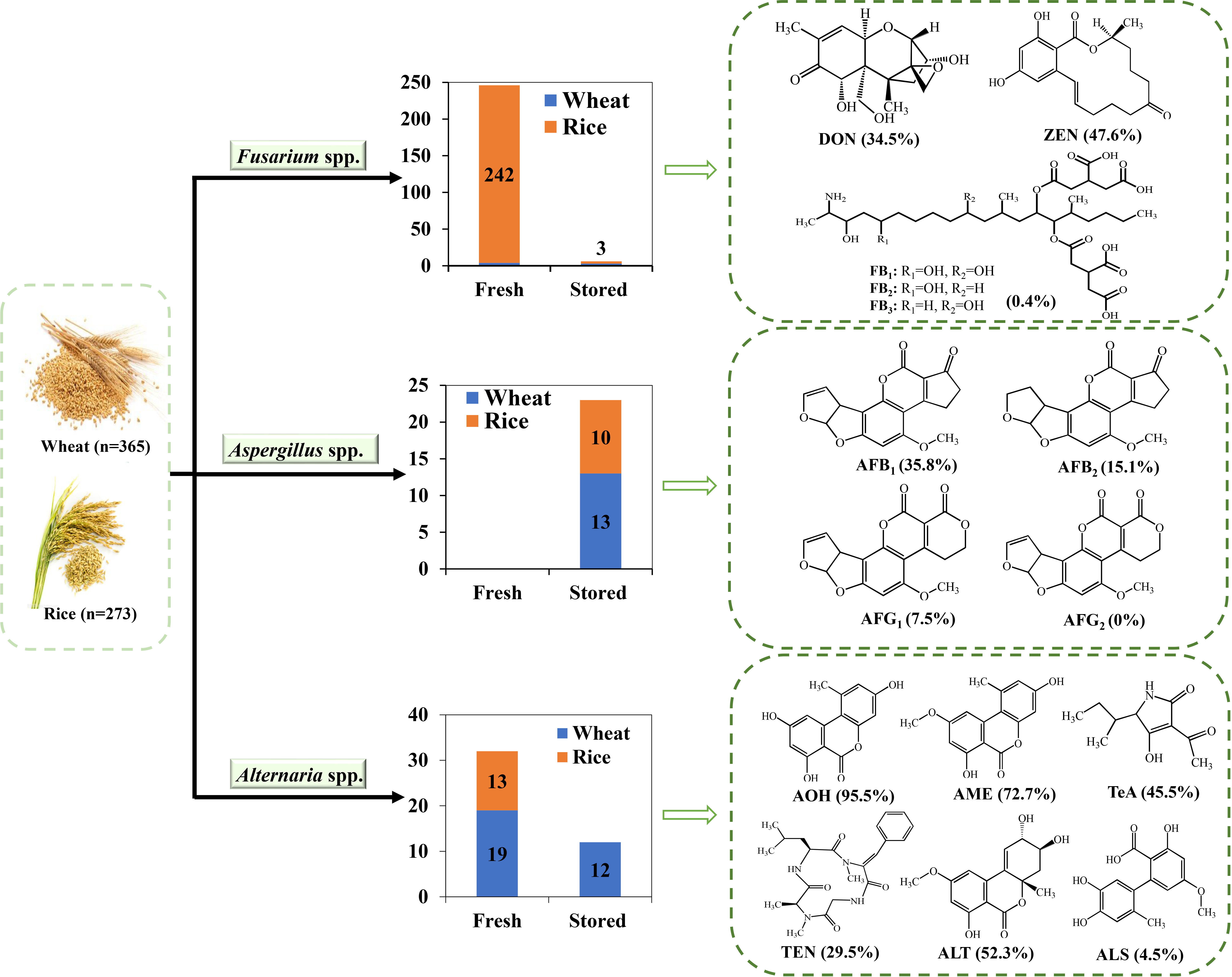
Figure 1 The distributions of Fusarium spp., Aspergillus spp., and Alternaria spp. in different grains in Shanghai, China, and their toxigenic abilities.
The differences of occurrence and toxigenic abilities of Fusarium, Aspergillus, and Alternaria species from wheat and paddy grains in Shanghai, China, were compared. The results showed that Fusarium spp. were the predominant species in fresh grains, and Aspergillus spp. were predominant in stored grains. The serious contaminations of Fusarium spp. in fresh samples might be due to the colonization of the fungi in the field, poor management, or damp conditions during the harvesting phase and transportation (Magan and Aldred, 2007; Magan et al., 2010). Aspergillus spp. is normally considered as the fungi developed in stored commodities and widely discovered in stored grains (Medina et al., 2006; Riba et al., 2010; Alkuwari et al., 2022; Tournas and Niazi, 2018; Zhao et al., 2020). All Aspergillus strains were isolated from stored grains, and 47.5% could produce AFBs. Interestingly, all the isolated Alternaria spp. were capable of producing at least one Alternaria toxin, whether in fresh or in stored grains.
Among the fungal communities recovered, Fusarium spp. were the dominant fungi in paddy grains. Most of the isolated Fusarium spp. produced DON and ZEN, and a large number of the Fusarium isolates could even co-produce DON and ZEN. The co-contaminations of DON and ZEN in wheat and paddy grains have been widely reported in literatures (Qiu and Shi, 2014; Dong et al., 2020; Fan et al., 2021), proving these organisms to be the common spoilers of grains. On the other hand, the co-occurrence of different Fusarium toxins might cause joint toxicities to humans and animals, which should be paid more attention in the future.
With regard to Aspergillus spp., the incidence was lower than that indicated by other authors, who collected the samples mainly from diseased grains (Chehri et al., 2015). As the predominant Aspergillus spp., Aspergillus section flavi might be associated with warmer geographical regions, similar to the previous studies conducted in Turkey, Iran, Australia, and Argentina (Berghofer et al., 2003; Vaamonde et al., 2003; Baydar et al., 2005; Chehri et al., 2015). Among 53 Aspergillus isolates, only Aspergillus section flavi could produce AFBs. The same results were discovered by Riba et al. in Algerian wheat, in which A. flavus was the only aflatoxigenic fungus among all the Aspergillus isolates (Riba et al., 2010). Different toxigenic abilities have also been described, in that some fungi could produce four AFBs (AFB1, AFB2, AFG1, and AFG2), while others only produced either three or two AFBs (Saleemi et al., 2010).
In recent years, Alternaria spp. have been pointed out as important contaminants in grains, especially in some regions with warm and humid climates (Li and Yoshizawa, 2000; Li et al., 2001). The incidence of Alternaria spp. in the current study was lower than that in Anhui province (100.0%), where the temperature and humidity were higher (Xu et al., 2016). In comparison to paddy, Alternaria spp. were more frequently found in wheat samples, which was in good agreement with the previous studies in China (Li et al., 2001; Xu et al., 2016). Almost all the isolates (95.5%) could produce at least one Alternaria toxin, among which AOH, AME, TeA, and TEN were the most frequently found, similar to the surveys from Germany (Muller and Korn, 2013), Canada (Scott et al., 2012), and Russia (Orina et al., 2022). Potential health risks related to the contaminations of Alternaria toxins in grains were thus proposed.
5 Conclusions
In the present study, the occurrence and toxigenic abilities of Fusarium, Aspergillus, and Alternaria species from wheat and paddy grains in Shanghai, China, were evaluated. Fusarium spp. were the main species in fresh grains, and Aspergillus spp. were predominant in stored grains. Toxin-producing potentials were different depending on the types and sources of the isolated fungi, from which a series of typical mycotoxins including DON, ZEN, AFBs, FBs, and Alternaria toxins could be generated. Co-productions of different secondary metabolites by toxigenic fungi could lead to co-contaminations of multiple mycotoxins, posing potentially additional health risks to humans and animals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author contributions
JM and ZH performed the entire project together, collected the data, performed the data analysis, and wrote the manuscript. RL, QH, JZ, XZ, XC, and CC processed the samples. KF, MW, and DG contributed to the data analysis. DN, ZZ, and ZH supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Shanghai Agriculture Applied Technology Development Program, China (Grant No. X20210302) and the National Natural Science Foundation of China (Grant number 32202201).
Acknowledgments
Shanghai Pujiang Warehousing Co., Ltd. is highly acknowledged for supporting wheat and paddy samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1202738/full#supplementary-material
References
Abbas, H. K., Riley, R. T. (1996). The presence and phytotoxicity of fumonisins and AAL-toxin in Alternaria alternata. Toxicon 34, 133–136. doi: 10.1016/0041-0101(95)00124-7
Ali, A., Kumar, R. R., Vinutha, T., Singh, T., Singh, S. P., Satyavathi, C. T., et al. (2022). Grain phenolics: critical role in quality, storage stability and effects of processing in major grain crops-a concise review. Eur. Food Res. Technol. 248, 2197–2213. doi: 10.1007/s00217-022-04026-7
Alkuwari, A., Hassan, Z. U., Zeidan, R., Al-Thani, R., Jaoua, S. (2022). Occurrence of mycotoxins and toxigenic fungi in cereals and application of yeast volatiles for their biological control. Toxins 14, 404. doi: 10.3390/toxins14060404
Baydar, T., Engin, A. B., Girgin, G., Aydin, S., Sahim, G. (2005). Aflatoxin and ochratoxin in various types of commonly consumed retail ground samples in Ankara, Turkey. Ann. Agric. Environ. Med. 12, 193–197. doi: 10.0000/PMID16457473
Berghofer, L. K., Hocking, A. D., Miskelly, D., Jansson, E. (2003). Microbiology of wheat and flour milling in Australia. Int. J. Food Microbiol. 85, 137–149. doi: 10.1016/s0168-1605(02)00507-x
Brandfass, C., Karlovsky, P. (2008). Upscaled CTAB-based DNA extraction and real-time PCR assays for Fusarium culmorum and F. graminearum DNA in plant material with reduced sampling error. Int. J. Mol. Sci. 9, 2306–2321. doi: 10.3390/ijms9112306
Chehri, K., Azami, E., Mosaber, A. (2015). Aspergillus and aflatoxin B1 contamination of stored corn grains in western Iran. Global Veterinaria 14, 39–42. doi: 10.5829/idosi.gv.2015.14.01.91150
Chen, J., Mirocha, C. J., Xie, W., Hogge, L., Olson, D. (1993). Production of the mycotoxin fumonisin B1 by Alternaria alternata f. sp. lycopersici. Appl. Environ. Microbiol. 58, 3928–3931. doi: 10.1128/AEM.58.12.3928-3931.1992
Chen, Y., Wan, J., Yin, H., Xu, X., Wang, Z. (2008). Analysis of climate characteristics and synoptic systems of high temperatures of Shanghai in summer. Atmospheric Sci. Res. Appl. 2, 35–42.
Diaz, G. J., Lozano, M. C., Acuna, A. (2009). Prevalence of Aspergillus species on selected Colombian animal feedstuffs and ability of Aspergillus section Flavi to produce aflatoxins. World Mycotoxin J. 2, 31–34. doi: 10.1128/aem.58.12.3928-3931.1992
Dong, F., Li, Y., Chen, X., Wu, J., Wang, S., Zhang, X., et al. (2021). Analysis of the Fusarium graminearum species complex from gramineous weeds near wheat fields in Jiangsu Province, China. Plant Dis. 105, 3269–3275. doi: 10.1094/PDIS-11-20-2376-RE
Dong, F., Xing, Y. J., Lee, Y. W., Mokoena, M. P., Olaniran, A. O., Xu, J. H., et al. (2020). Occurrence of Fusarium mycotoxins and toxigenic Fusarium species in freshly harvested rice in Jiangsu, China. World Mycotoxin J. 13, 201–211. doi: 10.3920/WMJ2019.2477
Ekwomadu, T. I., Akinola, S. A., Mwanza, M. (2021). Fusarium mycotoxins, their metabolites (free, emerging, and masked), food safety concerns, and health impacts. Int. J. Environ. Res. Public Health 18, 11741. doi: 10.3390/ijerph182211741
Fan, K., Ji, F., Xu, J. H., Qian, M. R., Duan, J. S., Nie, D. X., et al. (2021). Natural occurrence and characteristic analysis of 40 mycotoxins in agroproducts from Yangtze delta region. Scientia Agricultura Sin. 54, 2870–2884. doi: 10.3864/j.issn.0578-1752.2021.13.015
Frisvad, J. C., Smedsgaard, J., Samson, R. A., Larsen, T. O., Thrane, U. (2007). Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 55, 9727–9732. doi: 10.1021/jf0718906
Han, Z., Nie, D., Ediage, E. N., Yang, X., Wang, J., Chen, B., et al. (2014). Cumulative health risk assessment of co-occurring mycotoxins of deoxynivalenol and its acetyl derivatives in wheat and maize: case study, Shanghai, China. Food Chem. Toxicol. 74, 334–342. doi: 10.1016/j.fct.2014.10.018
Haque, A., Wang, Y. H., Shen, Z. Q., Li, X. H., Saleemi, M. K., He, C. (2020). Mycotoxin contamination and control strategy in human, domestic animal and poultry: a review. Microb. Pthogen. 142, 104095. doi: 10.1016/j.micpath.2020.104095
Hou, P. F., Ding, Y. F., Zhang, G. F., Li, Q., Wang, S. H., Tang, S., et al. (2015). Effect of rice or wheat residue retention on the quality of milled japonica rice in a rice-wheat rotation system in China. Crop J. 3, 67–73. doi: 10.1016/j.cj.2014.08.003
Huang, Q., Guo, W., Zhao, X., Cao, H., Fan, K., Meng, J., et al. (2022). Universal screening of 200 mycotoxins and their variations in stored cereals in Shanghai, China by UHPLC-Q-TOF MS. Food Chem. 387, 132869. doi: 10.1016/j.foodchem.2022
Ji, F., Xu, J., Liu, X., Yin, X., Shi, J. (2014). Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province, China. Food Chem. 157, 393–397. doi: 10.1016/j.foodchem.2014.02.058
Jiang, D., Wei, D., Li, H., Wang, L., Jiang, N., Li, Y., et al. (2021). Natural occurrence of Alternaria mycotoxins in wheat and potential of reducing associated risks using magnolol. J. Sci. Food Agric. 101, 3071–3077. doi: 10.1002/jsfa.10901
Kosiak, B., Torp, M., Skjerve, E., Andersen, B. (2004). Alternaria and Fusarium in Norwegian grains of reduced quality-a matched pair sample study. Int. J. Food Microbiol. 93, 51–62. doi: 10.1016/j.ijfoodmicro.2003.10.006
Lacey, J., Magan, N. (1991). “Fungi in cereal grains: their occurrence and water and temperature relationships,” in Cereal Grain. Mycotoxins, Fungi and Quality in Drying and Storage. Ed. Chelkowski, J. (Amsterdam: Elsevier), 77–118.
Li, F. Q., Toyazaki, N., Yoshizawa, T. (2001). Production of Alternaria mycotoxins by Alternaria alternata isolated from weather-damaged wheat. J. Food Prot. 64, 567–571. doi: 10.4315/0362-028x-64.4.567
Li, R., Wang, X., Zhou, T., Yang, D. X., Wang, Q., Zhou, Y. (2014). Occurrence of four mycotoxins in cereal and oil products in Yangtze delta region of China and their food safety risks. Food Control 35, 117–122. doi: 10.1016/j.foodcont.2013.06.042
Li, F. Q., Yoshizawa, T. (2000). Alternaria mycotoxins in weathered wheat from China. J. Agric. Food Chem. 48, 2920–2924. doi: 10.1021/jf0000171
Luo, S., Du, H., Kebede, H., Liu, Y., Xing, F. (2021). Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control 127, 108120. doi: 10.1016/j.foodcont.2021.108120
Magan, N., Aldred, D. (2007). Post-harvest control strategies: minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 119, 131–139. doi: 10.1016/j.ijfoodmicro.2007.07.034
Magan, N., Aldred, D., Mylona, K., Lambert, R. J. W. (2010). Review: limiting mycotoxins in stored wheat. Food Addit. Contam. 27, 644–650. doi: 10.1080/19440040903514523
Medina, A., Valle-Algarra, F. M., Mateo, R., Gimeno-Adelantado, J. V., Mateo, F., Jiménez, M. (2006). Survey of the mycobiota of Spanish malting barley and evaluation of the mycotoxin producing potential of species of Alternaria, Aspergillus and Fusarium. Int. J. Food Microbiol. 108, 196–203. doi: 10.1016/j.ijfoodmicro.2005.12.003
Mirocha, C. J., Chen, J., Xie, W., Xu, Y., Abbas, H. K., Hogge, L. R. (1996). Biosynthesis of fumonisin and AAL derivatives by Alternaria and Fusarium in laboratory culture. Adv. Exp. Med. Biol. 392, 213–224. doi: 10.1007/978-1-4899-1379-1_19
Muller, M. E. H., Korn, U. (2013). Alternaria mycotoxins in wheat- a 10 years survey in the northeast of Germany. Food Control 34, 191–197. doi: 10.1016/j.foodcont.2013.04.018
Munitz, M. S., Resnik, S. L., Pacin, A., Salas, P. M., Gonzalez, H. H., Montti, M. I., et al. (2014). Mycotoxigenic potential of fungi isolated from freshly harvested Argentinean blueberries. Mycotoxin Res. 30, 221–229. doi: 10.1007/s12550-014-0206-2
Nagaraja, H., Chennappa, G., Rakesh, S., Naik, M. K., Amaresh, Y. S., Sreenivasa, M. Y. (2016). Antifungal activity of Azotobacter nigricans against trichothecene-producing Fusarium species associated with cereals. Food Sci. Biotechnol. 25, 1197–1204. doi: 10.1007/s10068-016-0190-8
Ntasiou, P., Myresiotis, C., Konstantinou, S., Papadopoulou-Mourkidou, E., Karaoglanidis, G. S. (2015). Identification, characterization and mycotoxigenic ability of Alternaria spp. causing core rot of apple fruit in Greece. Int. J. Food Microbiol. 197, 22–29. doi: 10.1016/j.ijfoodmicro.2014.12.008
Orina, A. S., Gavrilova, O. P., Gogina, N. N., Gannibal, P. B., Gagkaeva, T. Y. (2022). Natural occurrence of Alternaria fungi and associated mycotoxins in small-grain cereals from the Urals and West Siberia regions of Russia. Toxins 13, 681. doi: 10.3390/toxins13100681
Qiu, J. B., Shi, J. R. (2014). Genetic relationships, carbendazim sensitivity and mycotoxin production of the Fusarium graminearum populations from maize, wheat and rice in eastern China. Toxins 6, 2291–2309. doi: 10.3390/toxins6082291
Qiu, J. B., Xu, J. H., Shi, J. R. (2014). Molecular characterization of the Fusarium graminearum species complex in Eastern China. Eur. J. Plant Pathol. 139, 811–823. doi: 10.1007/s10658-014-0435-4
Qiu, J. B., Xu, J. H., Shi, J. R. (2019). Fusarium toxins in Chinese wheat since the 1980s. Toxins 11, 248. doi: 10.3390/toxins11050248
Reddy, K. R., Farhana, N. I., Wardah, A. R., Salleh, B. (2010). Morphological identification of foodborne pathogens colonizing rice grains in south Asia. Pak. J. Biol. Sci. 13, 794–801. doi: 10.3923/pjbs.2010.794.801
Riba, A., Bouras, N., Mokrane, S., Mathieu, F., Lebrihi, A., Sabaou, N. (2010). Aspergillus section Flavi and aflatoxins in Algerian wheat and derived products. Food Chem. Toxicol. 48, 2772–2777. doi: 10.1016/j.fct.2010.07.005
Saleemi, M. K., Khan, M. Z., Khan, A., Javed, I. (2010). Mycoflora of poultry feeds and mycotoxins producing potential of Aspergillus species. Pak. J. Bot. 42, 427–434. doi: 10.1016/j.vetmic.2005.10.031
Scott, P. M., Zhao, W., Feng, S., Lau, P. Y. (2012). Alternaria toxins alternariol and alternariol monomethyl ether in grain foods in Canada. Mycotoxin Res. 28, 261–266. doi: 10.1007/s12550-012-0141-z
Shi, W., Tan, Y. L., Wang, S. X., Gardiner, D. M., De Saeger, S., Liao, Y. C., et al. (2016). Mycotoxigenic potentials of Fusarium species in various culture matrices revealed by mycotoxin profiling. Toxins 9, 6. doi: 10.3390/toxins9010006
Suman, M. (2021). Last decade studies on mycotoxins' fate during food processing: an overview. Curr. Opin. Food Sci. 41, 70–80. doi: 10.1016/j.cofs.2021.02.015
Sun, G., Wang, S., Hu, X., Su, J., Zhang, Y., Xie, Y., et al. (2011). Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 28, 461–470. doi: 10.1080/19440049.2010.544678
Sunagawa, K., Nakamura, S., Sato, Y., Iida, S., Miyazaki, Y., Suzuki, T., et al. (2021). Morphological and genetic identification of fungal genus/species in formalin-fixed, paraffin-embedded specimens obtained from patients with histologically proven fungal infection. Mycoses 64, 851–859. doi: 10.1111/myc.13325
Tournas, V. H., Niazi, N. S. (2018). Potentially toxigenic fungi from selected grains and grain products. J. Food Saf. 38, e12422. doi: 10.1111/jfs.12422
Tralamazza, S. M., Piacentini, K. C., Iwase, C. H. T., Rocha, L. D. (2018). Toxigenic Alternaria species: impact in cereals worldwide. Curr. Opin. Food Sci. 23, 57–63. doi: 10.1016/j.cofs.2018.05.002
Tsai, G. J., Yu, S. C. (1999). Detecting Aspergillus parasiticus in cereals by an enzyme-linked immunosorbent assay. Int. J. Food Microbiol. 50, 181–189. doi: 10.1016/S0168-1605(99)00084-7
Turzhanova, A., Khapilina, O. N., Tumenbayeva, A., Shevtsov, V., Raiser, O., Kalendar, R. (2020). Genetic diversity of Alternaria species associated with black point in wheat grains. PeerJ 8, e9097. doi: 10.7717/peerj.9097
Vaamonde, G., Patriarca, A., Fernandez Pinto, V., Comerio, R., Degrossi, C. (2003). Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section Flavi from different substrates in Argentina. Int. J. Food Microbiol. 88, 79–84. doi: 10.1016/s0168-1605(03)00101-6
Wang, S., Zhang, Y. (2005). The new retail economy of shanghai. Growth Change 36 (1), 41–73. doi: 10.1111/j.1468-2257.2005.00266.x
Xing, L., Wei, G. H., Jie, X. (1997). Studies on mycotoxins contamination or wheat in Shanghai. Shanghai J. Prev. Med. 9, 413–415. doi: 10.19428/j.cnki.sjpm.1997.09.012
Xu, W., Han, X., Li, F., Zhang, L. (2016b). Natural occurrence of Alternaria toxins in the 2015 wheat from Anhui province, China. Toxins 8, 308. doi: 10.3390/toxins8110308
Xu, W. J., Han, X. M., Zhang, J., Pan, Z., Li, F. Q., Zhang, L. S. (2016a). Survey on fungi contamination of wheat harvested in 2015 from Anhui province of China. Health Sci. 54, 92–96. doi: 10.6040/j.issn.1671-7554.0.2016.063
Yan, P., Liu, Z., Liu, S., Yao, L., Liu, Y., Wu, Y., et al. (2020). Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017. Toxins 12, 200. doi: 10.3390/toxins12030200
Yang, M., Zhang, H., Kong, X., vander Lee, T., Waalwijk, C., van Diepeningen, A., et al. (2018). Host and cropping system shape the Fusarium population: 3ADON-producers are ubiquitous in wheat whereas NIV-producers are more prevalent in rice. Toxins 10, 115. doi: 10.3390/toxins10030115
Keywords: mycotoxins, toxigenic ability, Fusarium spp., Aspergillus spp., Alternaria spp
Citation: Meng J, Li R, Huang Q, Guo D, Fan K, Zhang J, Zhu X, Wang M, Chen X, Nie D, Cao C, Zhao Z and Han Z (2023) Survey and toxigenic abilities of Aspergillus, Fusarium, and Alternaria fungi from wheat and paddy grains in Shanghai, China. Front. Plant Sci. 14:1202738. doi: 10.3389/fpls.2023.1202738
Received: 09 April 2023; Accepted: 05 July 2023;
Published: 25 July 2023.
Edited by:
Francesco Tini, University of Perugia, ItalyReviewed by:
Qiya Yang, Jiangsu University, ChinaSomenath Das, Burdwan Raj College, India
Dinorah Pan, University of the Republic, Uruguay
Copyright © 2023 Meng, Li, Huang, Guo, Fan, Zhang, Zhu, Wang, Chen, Nie, Cao, Zhao and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Han, aGFuemhlbmdAc2Fhcy5zaC5jbg==
 Jiajia Meng1
Jiajia Meng1 Kai Fan
Kai Fan Zheng Han
Zheng Han