- 1Department of Tobacco Agriculture, Zhengzhou Tobacco Research Institute of China National Tobacco Corporation (CNTC), Zhengzhou, China
- 2Department of Research Center on Tobacco Cultivating and Curing, Tobacco Research Institute of Hubei, Wuhan, China
- 3Department of Agronomy, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, China
- 4China Tobacco Gene Research Center, Zhengzhou Tobacco Research Institute of China National Tobacco Corporation (CNTC), Zhengzhou, China
- 5Tasmanian Institute of Agriculture, University of Tasmania, Hobart, TAS, Australia
The lack of irrigation water in agricultural soils poses a significant constraint on global crop production. In-depth investigation into microRNAs (miRNAs) has been widely used to achieve a comprehensive understanding of plant defense mechanisms. However, there is limited knowledge on the association of miRNAs with drought tolerance in cigar tobacco. In this study, a hydroponic experiment was carried out to identify changes in plant physiological characteristics, miRNA expression and metabolite profile under drought stress, and examine the mitigating effects of selenium (Se) application. The shoot dry weight of drought-stressed plants was approximately half (50.3%) of that in non-stressed (control) conditions. However, plants supplied with Se attained 38.8% greater shoot dry weight as compared to plants with no Se supply under drought stress. Thirteen miRNAs were identified to be associated with drought tolerance. These included 7 known (such as nta-miR156b and nta-miR166a) and 6 novel miRNAs (such as novel-nta-miR156-5p and novel-nta-miR209-5p) with the target genes of squamosa promoter-binding-like protein 4 (SPL4), serine/threonine protein phosphatase 2A (PPP2A), cation/calcium exchanger 4-like (CCX4), extensin-1-like (EXT1) and reduced wall acetylation 2 (RWA2). Further investigation revealed that the expression levels of Ext1 and RWA2 were significantly decreased under drought stress but increased with Se addition. Moreover, key metabolites such as catechin and N-acetylneuraminic acid were identified, which may play a role in the regulation of drought tolerance. The integrated analysis of miRNA sequencing and metabolome highlighted the significance of the novel-nta-miR97-5p- LRR-RLK- catechin pathway in regulating drought tolerance. Our findings provide valuable insights into the molecular mechanisms underlying drought tolerance and Se-induced stress alleviation in cigar tobacco.
1 Introduction
In the past few decades, the Earth’s climate has been seriously impacted by global warming due to increasing levels of greenhouse gases in the Earth’s atmosphere. This has affected normal rainfall patterns, which aggravated drought to extreme levels in many parts of the world, including China. Drought, as a major abiotic constraint on plant growth, has the potential to reduce crop yield by up to 15% (Leng and Hall, 2019). In China, annual losses in agricultural production caused by drought could reach up to 4.2 billion US dollars (USD), equating 0.23% of the national GDP (Su et al., 2018).
Drought stress induces oxidative stress in plants, leading to leaf structure damage and decreased photosynthesis and stomatal activity (Khan et al., 2023). Plants have evolved diverse strategies to cope with the harmful effects of drought, which involve the reprogramming of their transcriptome, proteome and metabolome profiles to enhance stress tolerance. Post-transcriptional regulation by microRNAs (miRNAs) is a crucial regulatory process underlying this reprogramming. The length of miRNAs ranges from 20 to 24 nucleotides in length and these miRNAs are involved in gene silencing in plants (Cuperus et al., 2011), many biological processes, such as plant growth, development, biotic and abiotic stress resistance (Chen, 2005; Cuperus et al., 2011; Teng et al., 2023). Teng et al. (2023) identified 23 differentially expressed miRNAs which were highly associated with Cd tolerance in maize. The target genes of these miRNAs were mainly involved in GSH metabolism, antioxidant enzyme activity and metal transport. Hormones such as abscisic acid and auxin play key roles in regulating drought tolerance in plants. For instance, miR167, which targets auxin response factor, was downregulated by abscisic acid application under drought stress (Liu et al., 2009). Several miRNAs (e.g., miRNA156, miR166, miR167 and miR168) have been demonstrated in certain plant species to regulate the expression of drought-responsive genes, including SQUAMOSA promoter-binding protein-like (SPL), cation/Ca2+ exchanger (CCX), REDUCED WALL ACETYLATION (RWA), protein phosphatase 2 (PP2) and exostosin glycosyltransferase (EXT) (Gao et al., 2016; Zhang et al., 2018; Feyissa et al., 2019; Liu et al., 2020). Further investigation of drought regulated miRNAs and their target genes would greatly contribute to the understanding of the molecular basis underlying drought-stress tolerance in tobacco.
Tobacco (Nicotiana tabacum L.) is an annual or limited perennial herbaceous plant species that belongs to the Solanaceae family. It is a commercial crop extensively cultivated worldwide, including China. In recent years, the global consumption and demands for cigar tobacco have shown steady increase particularly in China and United States (Yan et al., 2021; Wang et al., 2022). Tobacco is primarily cultivated in semi-arid and rain-fed areas, heavily reliant on rainwater to meet the crop water demands. As tobacco is highly susceptible to drought stress, improving tolerance to drought stress in tobacco is of significant economic importance. A comprehensive understanding of drought-responsive transcriptional networks would facilitate the identification of integrated biological pathways involved in mitigating drought stress. Although some key drought responsive miRNAs such as miR160, miR162, miR166, miR168, 390, miR395 and miR398 (Rabara et al., 2015; Yin et al., 2015; Chen et al., 2017) have been identified in tobacco, none of the predicted targets have been further validated through cloning or sequence alignment.
Selenium (Se) is an essential micronutrient in plants and plays a significant role in mitigating the effects of abiotic stress (Cao et al., 2013; Lanza and Dos Reis, 2021). Previous reports have demonstrated the positive effects of Se application on plant drought stress tolerance, including increases in proline and chlorophyll content, accumulation of osmoprotectant and enhancement in antioxidant enzyme activity in plants (Ahmad et al., 2021; Han et al., 2022). Moreover, Se application in plants has been shown to reduce reactive oxygen species (ROS) toxicity under drought stress conditions (Han et al., 2022). Although several studies have reported improved plant performance in tobacco with Se application under drought stress through physiological assessments (Han et al., 2022), the molecular basis underlying this phenomenon remains largely unknown. Therefore, the objectives of this study were: (i) to investigate the negative effect of drought stress on plant growth, (ii) to identify metabolites, miRNAs and prediction of their targeted genes associated with increased drought tolerance, (iii) to demonstrate the effect of Se addition in mitigating plant drought tolerance by inducing the selected miRNAs, target genes and metabolite accumulation in tobacco.
2 Materials and methods
2.1 Plant materials and growth condition
The commercially grown tobacco cultivar ‘CX26’ is widely used for cigar wrapper production in Hubei Province, China. This cultivar was used in this experiment. Firstly, the seeds were germinated in pots filled with vermiculite and routinely irrigated to maintain adequate moisture levels (Bukhari et al., 2016). Forty-day seedlings were then transplanted to containers containing 12L nutrient solution. The composition of the nutrient solution was set according to Bukhari et al. (2016), which is represented in mg L-1: (NH4)2SO4, 48.2; K2SO4, 15.9; KNO3, 18.5; KH2PO4, 24.8; MgSO4.7H2O, 154.88; Ca(NO3)2.4H2O, 86.17; ZnSO4.7H2O, 0.11; Fe-Citrate, 7.0; CuSO4.5H2O, 0.04; MnCl2.4H2O, 0.9; H2MoO4, 0.01 and H3BO3, 2.9 (pH, 5.8). Plants were allowed to grow for 7 days in a glasshouse at the Zhengzhou Tobacco Research Institute before the treatments were added. Three treatments (including control) were used in this study: 20% PEG-6000 (Zhang et al., 2017) was added in the nutrient solution for drought stress (DS), with an additional dose of 3 μM Se (as Na2SeO3, Huang et al., 2021) for Se treatment (DSSe). There was no addition of PEG and Se in control plants (CK). The nutrient solutions were renewed weekly and aerated by using air pumps. Plants were harvested after 6 days of respective treatments and further separated into shoots and roots, by keeping three biological replicates for each treatment. Harvested shoots were immediately stored at -80°C until further use.
2.2 Total RNA extraction, library construction and sequencing of small RNA
RNA was extracted from shoot samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Nine small RNA libraries were constructed from the collected samples (three samples each per treatment). Total RNA was isolated using small RNA sample pre kit (Illumina, San Diego, California, America) according to the manufacturer’s instructions. The adaptors were added to the small RNAs to reverse transcribe them into cDNA followed by amplification. Further, the PCR products were purified, and the libraries were obtained and quality was assessed using Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA, USA). Lastly, Illumina sequencing of small RNAs was carried out using HiSeq 4000 (Illumina, San Diego, CA, USA).
2.3 Identification of miRNAs
The identification of known and novel miRNAs were performed according to Xu et al. (2013). Briefly, small RNA reads were generated after sequencing and raw data was obtained. The adaptors and low-quality reads were discarded using the ACGT-miR program, and comparisons of clean reads were made using Rfam and GenBank databases. The matched non-coding snRNA, rRNA, tRNA and snoRNA sequences were removed and the remaining clean reads were mapped to the tobacco genome by using Bowtie software v1.1.2 with specific parameter settings according to Langmead et al. (2009). Those sequence reads identical to the known mature miRNAs were selected for subsequent analysis. The unannotated unique miRNAs with fold to stem-loop structure were regarded as novel miRNAs.
2.4 Identification of miRNAs in response to drought and selenium treatments with the prediction of target genes
Transcripts per million (TPM) was used to normalize the frequency of miRNAs according to He et al. (2016). Differentially expressed miRNAs (DEMs) between all treatments were detected using DEGseq. The screening criteria were set as: log2(DS vs CK) or log2(DSSe vs DS) > 1 (upregulated) or <−1 (downregulated), and p value ≤0.05. psRNATarget was used to predict target genes of miRNAs (Dai and Zhao, 2011). The prediction of potential target genes for the miRNAs was based on the following criteria: (1) less than four nucleotide mismatches between miRNAs and their target genes; (2) less than three consecutive nucleotide mismatches; and (3) no mismatches at the complementary site between the miRNA sequence and the target mRNA, specifically at positions 10 or 11 of the miRNA sequence, which are considered putative cleavage sites (Kantar et al., 2010).
Total RNA was extracted from stored leaf samples. qRT-PCR was carried out according to Lin et al. (2022). The PCR conditions were comprised of an initial denaturation step at 95°C for 3 min, second denaturation at 94°C for 60 s (40 cycles), annealing at 58°C for 30 s, extension at 72°C for 30 s, and finally extension at 72°C for 5 min. The relative expression level was determined using the ΔΔCT method. The tobacco NttubA1 was used as a reference gene. The primers of NtRWA2 and NtEXT1 were listed in Table S1.
2.5 Metabolomic analysis
The extraction, identification and quantification of leaf metabolites were carried out according to Lin et al. (2022). Briefly, the samples were placed in tubes containing 1 ml extraction solution (methanol: acetonitrile: water, 2: 2: 1). After 40 Hz trituration for 4 min and 5 min sonification, the sample extracts were centrifuged at 12,000 rpm at 4°C for 20 min and supernatants were collected and further analyzed using a liquid chromatography–mass spectrometry (LC-MS) system. The metabolites were separated by a UHPLC system with a UPLC HSS T3 column. Briefly, the chromatographic separation was carried out using Hypersil GOLD aQ Dim column (Thermo Fisher Scientific, USA). The mobile phase consisted of a 0.1% formic acid aqueous solution (A solvent) and acetonitrile containing 0.1% formic acid (B solvent). The elution was conducted using the following gradient: 0-2 minutes, 5% B solvent; 2-22 minutes, 5-95% B solvent; 22-27 minutes, 95% B solvent; 27-27.1 minutes, 95-5% B solvent; 27.1-30 minutes, 5% B solvent. The flow rate was set at 0.3 ml min-1. The temperature of column was set at 40°C, and the volume of injection was 5 μl. The metabolites were detected using a high-resolution tandem mass spectrometer. The data of mass spectrometry was then imported into Compound Discoverer 3.2 (Thermo Fisher Scientific, USA) software. By mapping the BMDB (BGI Metabolome Database, China), mzCloud database, and ChemSpider database, a data matrix that included information such as metabolite peak areas and identification results was obtained. Metabolites with fold change > 1.20 or < 0.83, P value < 0.05, were regarded as differentially accumulated metabolites, which were monitored in DS vs CK and DSSe vs DS treatments.
2.6 Statistical analysis
The illustration of graphs was performed using Origin software (Origin Lab Corporation, Wellesley Hills, Wellesley, MA, USA) and statistical analyses were conducted using Data Processing System (DPS) statistical software. One-way ANOVA multiple comparisons and Duncan’s multiple range test were used to compare the means of all the treatments used in this study. The significance of all tests was determined at a level of P < 0.05.
3 Results
3.1 Effects of drought stress and selenium treatment on plant growth
Drought stress markedly inhibited plant growth after 6 days of stress application (Figure 1) with the seedlings exhibiting clear symptoms of leaf chlorosis. The shoot dry weight of DS seedlings was only 50.3% of that of non-stressed (CK) seedlings (Figure 1), albeit no significant difference was found in their root dry weight. The shoot dry weight of DSSe seedlings was 38.8% higher than that of DS seedlings (Figure 1), with no significant differences were recorded in root dry weight.
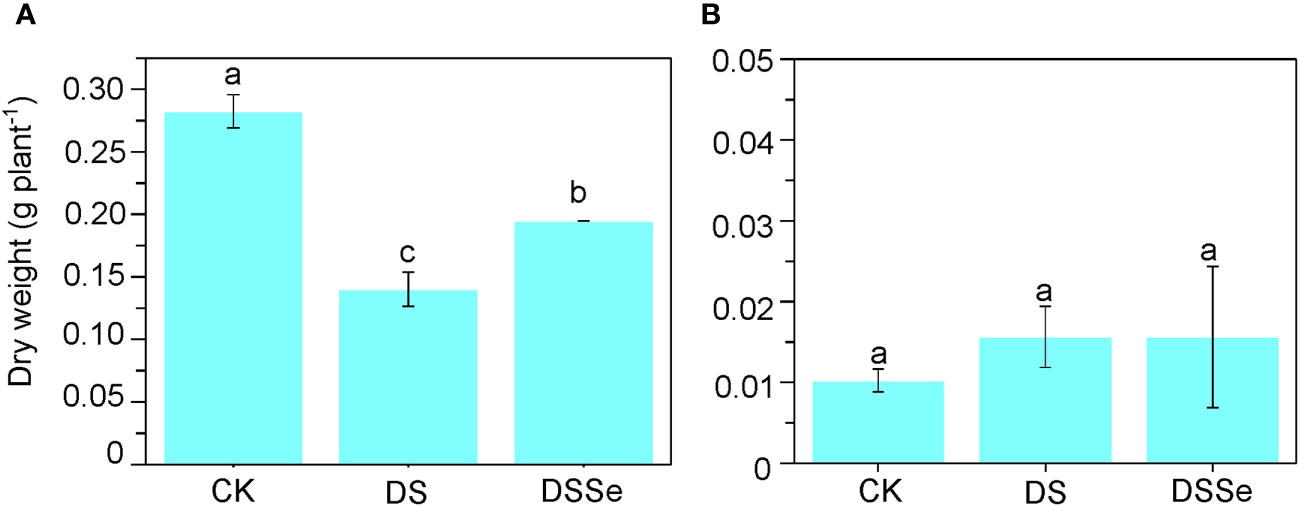
Figure 1 Exogenous application of Se drought stress tolerance in tobacco. The dry weight of shoot (A) and root (B) of CX26 grown under control (CK), drought stress (DS) and drought stress+Se (DSSe) conditions after 40 DAS. Drought stress was applied by adding 20% PEG-6000 into plant nutrient solution, while additional dose of 3 μM Na2SeO3 was supplied to drought + Se treatment. Data are presented as mean ± SD (n = 3). Different letters indicate statistically significant differences. DAS, days after sowing; Se, selenium; PEG, polyethylene glycol.
3.2 Analysis of small RNAs
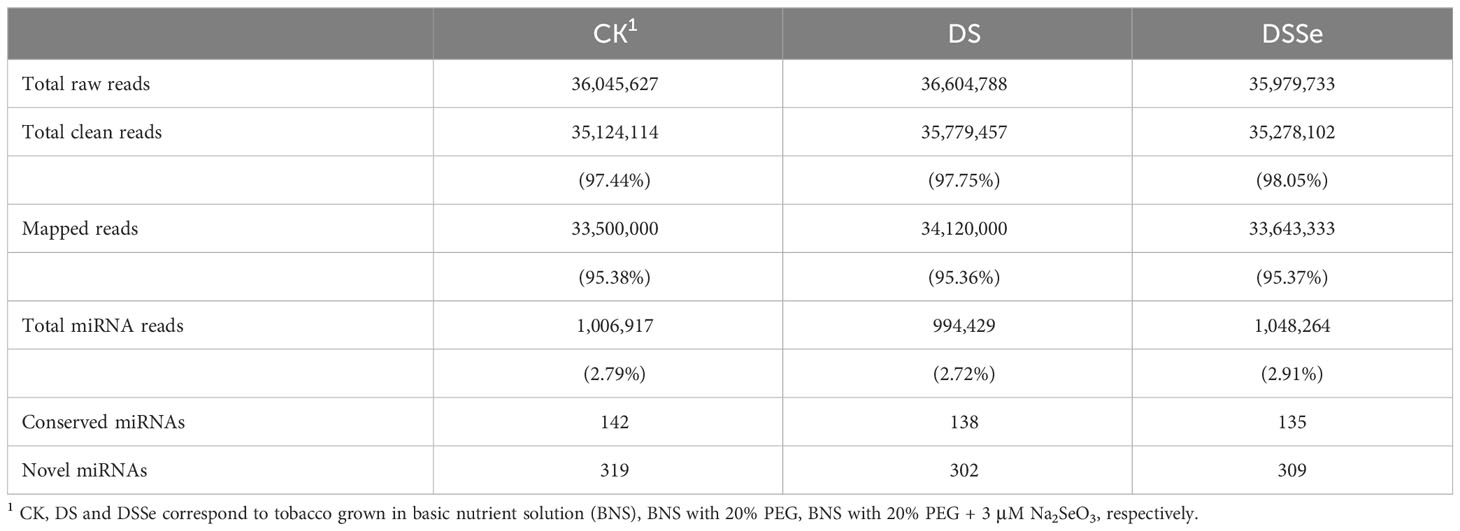
The differences in plant growth under different treatments led to the identification of drought tolerance-related miRNAs and their target genes. A total of 36,045,627, 36,604,788 and 35,979,733 raw reads were generated from the CK, DS and DSSe treatments, respectively (Table 1) and 35,124,114 (97.44%), 35,779,457 (97.75%) and 35,278,102 (98.05%) reads were remained after filtering and trimming of the CK, DS and DSSe groups, respectively. Of the high-quality sequence reads obtained, 95.38%, 95.36% and 95.37% of the CK, DS and DSSe groups were mapped to the tobacco genome (Table 1). In total, 1,006,917 (2.79%), 994,429 (2.72%) and 1,048,264 (2.91%) miRNA reads were mapped to the reference genome in the CK, DS and DSSe treatments, respectively. Of these, 142, 138 and 135 were conserved miRNAs, whereas 319, 302 and 309 were novel miRNAs identified in control, DS and DSSe treatments, respectively (Table 1).
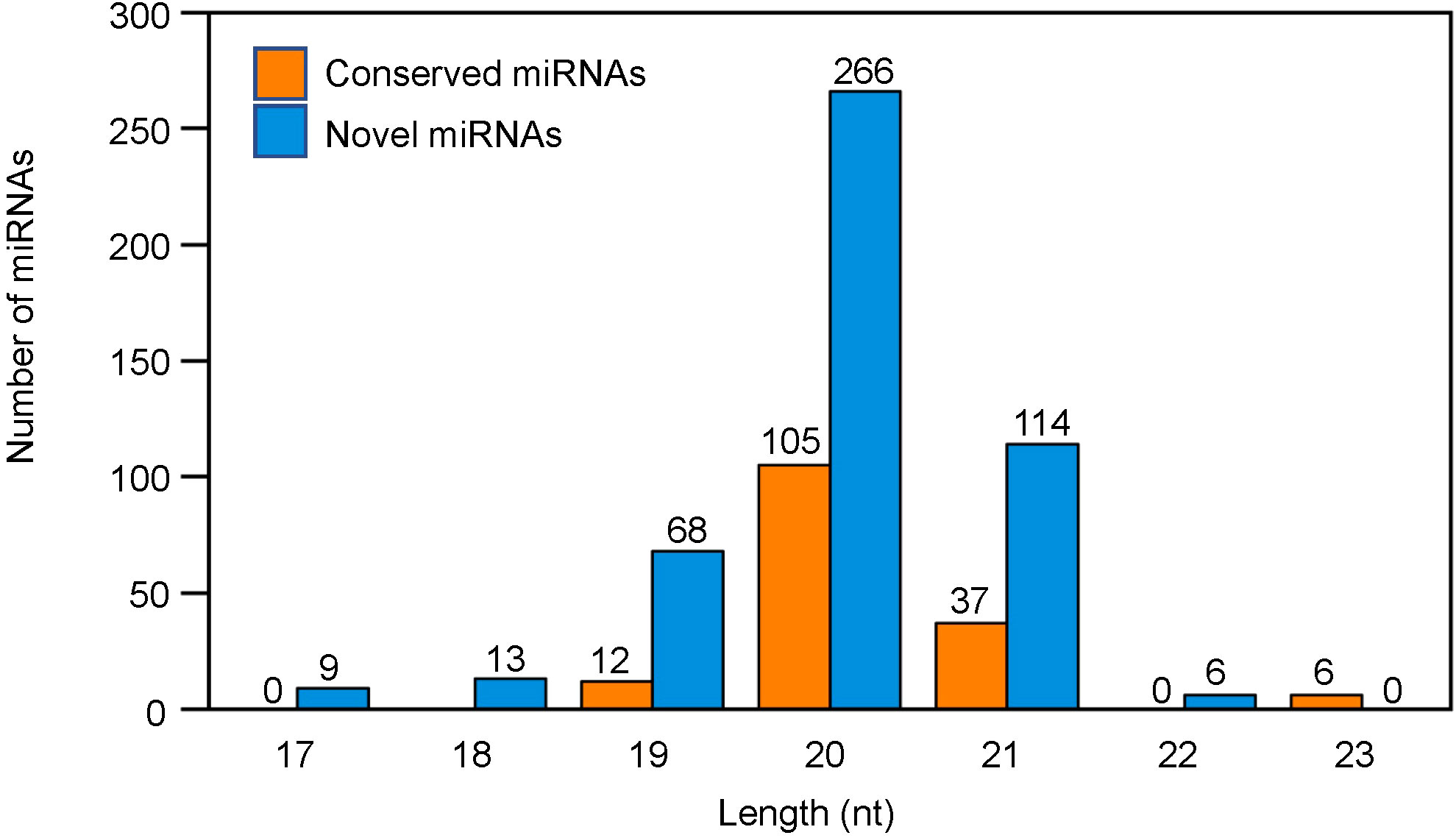
Regarding the length distribution of miRNAs, the 21-24 nt sequences were found to be dominant with 24 nt sequences being the most abundant length amongst all the treatments (Figure 2). The conserved miRNAs were mainly distributed at 20 nt and 21 nt, whereas the novel miRNAs were mainly distributed at 19 nt, 20 nt and 21 nt (Figure 3).
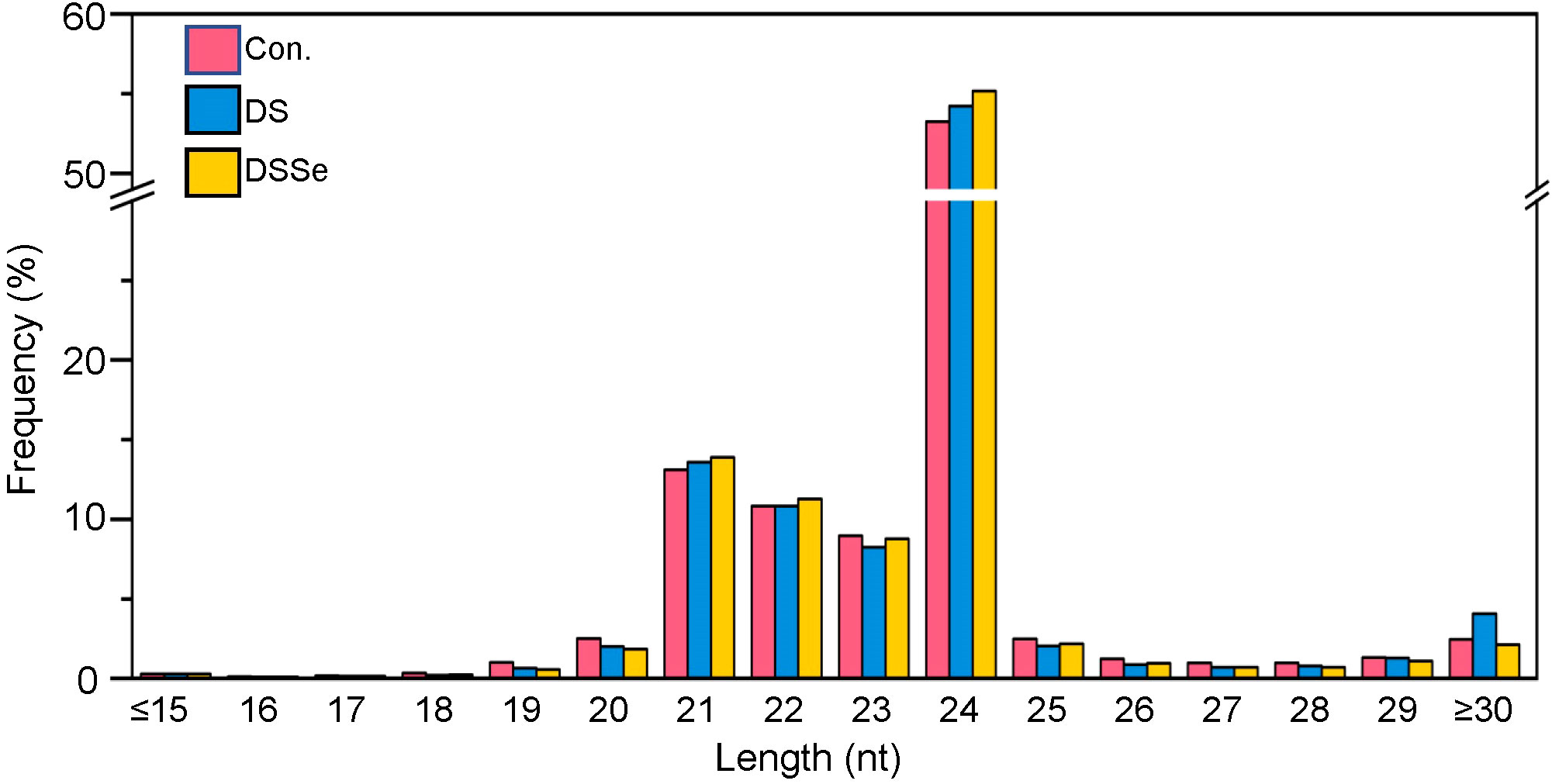
Figure 2 Length distribution of small RNAs in shoots of CX26. The X-axis represents the small RNA length (nucleotide) and the Y-axis represents the percentage of small RNA reads. Control (pink), DS (blue) and DSSe (yellow) represent control, drought (20% PEG-6000 treatment) and drought + 3 μM Na2SeO3.
3.3 Analysis of differentially expressed miRNAs under different treatments
The miRNA expression profiles were greatly influenced by the growth condition with 16 and 27 miRNAs being upregulated and downregulated, respectively, under drought treatment as compared to the control (Figure 4, Tables S1, S2). Among the downregulated miRNAs, 12 were known and 15 were novel. These novel miRNAs included miRNA156, miR166, miR167 and miR168 family miRNAs (Table S1). More than 75% (10) of the upregulated miRNAs were known, with only 3 novel miRNAs (Table S2). Those known miRNAs belonged to 12 miRNA families. Moreover, 28 upregulated and 20 downregulated miRNAs were detected in the DSSe as compared to DS (Tables S3, S4). Amongst 20 downregulated miRNAs identified, 11 were known and 9 were novel (Table S3). The downregulated miRNAs between DSSe and DS consisted of nta-miR156a (25.0-fold), nta-miR399f (24.7-fold) and novel-nta-miR27-5p (22.8-fold). Further, we recorded 12 known and 16 novel upregulated miRNAs in the DSSe as compared to DS and these included miR156b, miR156d, miR166a and miR390a (Table S4).

Figure 4 Differential expression miRNAs under DS and DSSe treatments. The CK, DS and DSSe represent control, drought and drought+Se.
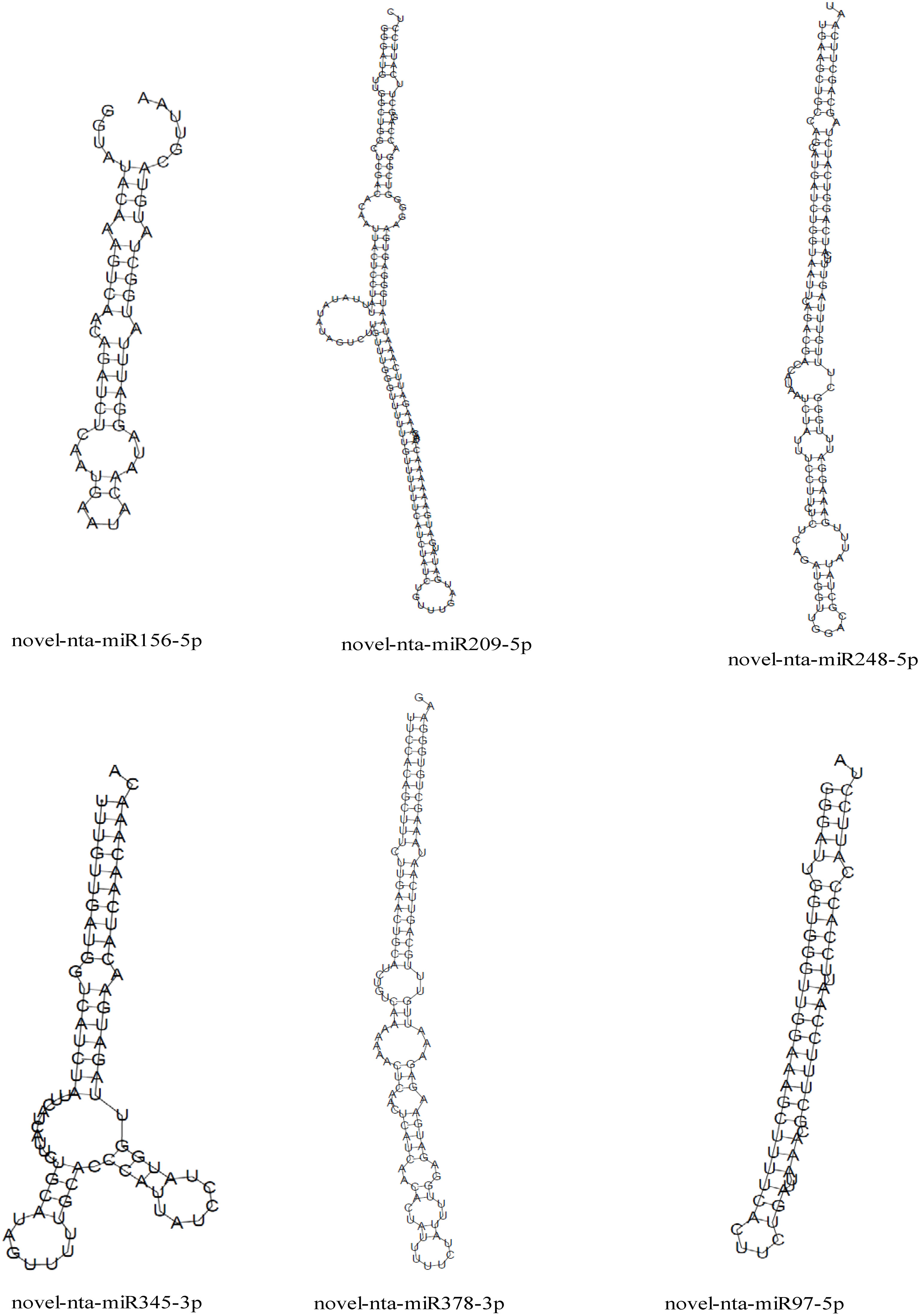
Interestingly, nine miRNAs were downregulated in DS vs CK but upregulated in DSSe vs DS (Group I, Figures 4, 5). These miRNAs were comprised of six novel (novel-nta-miR156-5p, novel-nta-miR209-5p, novel-nta-miR248-5p, novel-nta-miR345-3p, novel-nta-miR378-3p and novel-nta-miR97-5p) and three known miRNAs (nta-miR156b, nta-miR166a and nta-miR482b-3p). Furthermore, nta-miR156b was found to be downregulated (-24.5-fold) in DS but upregulated (22.8-fold) in DSSe (Table 2). Meanwhile, four miRNAs were upregulated in DS vs CK, while the four miRNAs were downregulated in DSSe vs DS (Group II, Table 3). All those miRNAs were known, including nta-miR156c, nta-miR482c, nta-miR6019b and nta-miR6154a. The miRNAs of Group I and II may play key roles in Se-induced drought alleviation.

Figure 5 Gene Ontology (GO) (A) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (B) analysis for target genes of differently expressed miRNA under different conditions in tobacco.
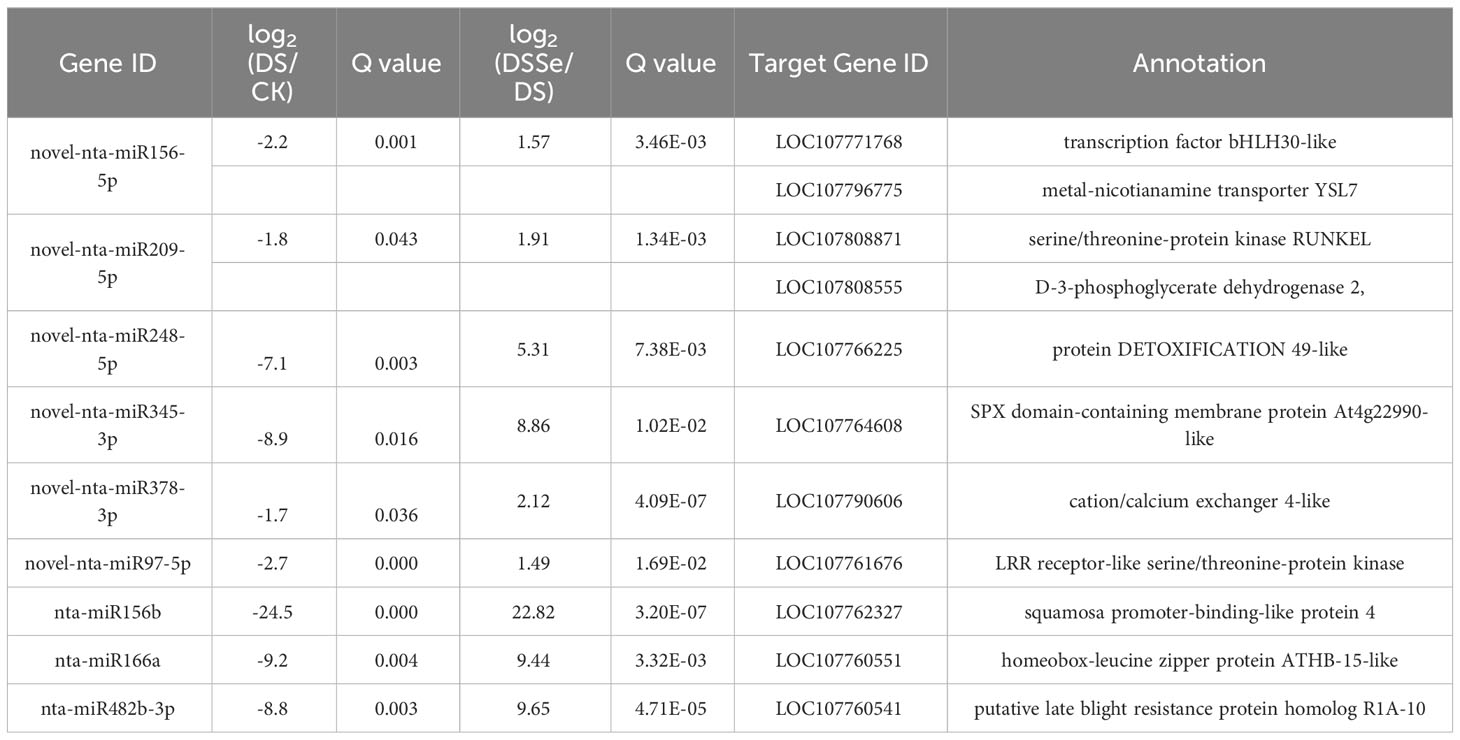
Table 2 List of miRNAs downregulated under DS treatment (DS vs control) but upregulated under DSSe treatment (DSSe vs D).
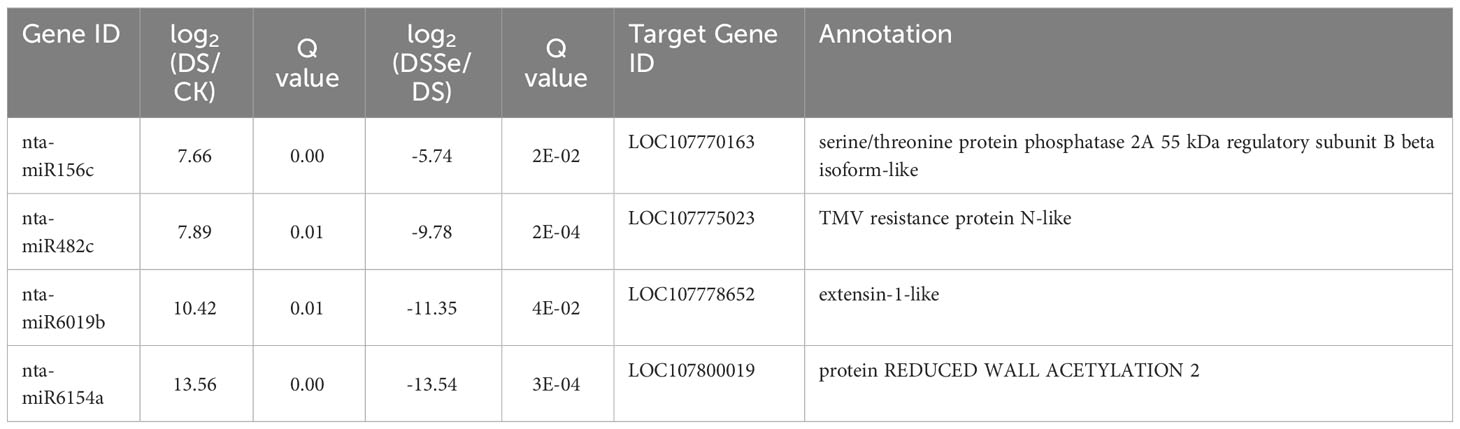
Table 3 List of miRNAs upregulated under drought treatment (DS vs CK) but downregulated under DSSe treatment (DSSe vs D).
3.4 Identification of target genes and function categories of DEMs
We predicted the target genes of the identified miRNAs using psRNATarget. Gene Ontology (GO) analysis showed that these target genes are mainly involved in cellular process, metabolic process, cell, cell part, organelle, binding and catalytic activity (Figure 5A). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that these target genes were involved in translation, transcription and carbohydrate metabolism (Figure 5B). Moreover, these target genes were involved in cellular processes, environmental information processes and organismal systems (Figure 5B). We classified the resultant target genes into Groups I and II based on their predicted functions. Group I contained eleven target genes, including transcription factors, metal transporters, protein kinases and detoxification-related proteins (Table 2), with the identification of three transcription-related genes e.g., transcription factor bHLH30-like, squamosa promoter-binding-like protein 4 (SPL4) and homeobox-leucine zipper protein ATHB-15-like. We also detected two metal transporters (metal-nicotianamine transporter YSL7 and cation/calcium exchanger 4-like SlCCX4) and two protein kinase genes (serine/threonine-protein kinase RUNKEL and LRR receptor-like serine/threonine-protein kinase). The other four genes included D-3-phosphoglycerate dehydrogenase 2, protein DETOXIFICATION 49-like, SPX domain-containing membrane protein At4g22990-like (SPX) and late blight resistance protein homolog R1A-10. In Group II, the targets of the four miRNAs were serine/threonine protein phosphatase 2A 55 kDa regulatory subunit B beta isoform-like (PP2A), TMV resistance protein N-like, extensin-1-like (EXT1) and Reduced Wall Acetylation 2 (RWA2). The putative secondary structures of the six novel miRNAs were also predicted (Figure 6).
To further investigate whether the differentially expressed miRNA target genes were involved in drought tolerance, we selected two target genes (NtRWA2 and NtEXT1) for qRT-PCR analysis. The relative gene expression of NtRWA2 and NtEXT1 was significantly decreased in DS vs CK and inceased in DSSe vs DS (Figure 7). These results suggested that NtRWA2 and NtEXT1 might play an important function in regulating drought tolerance.
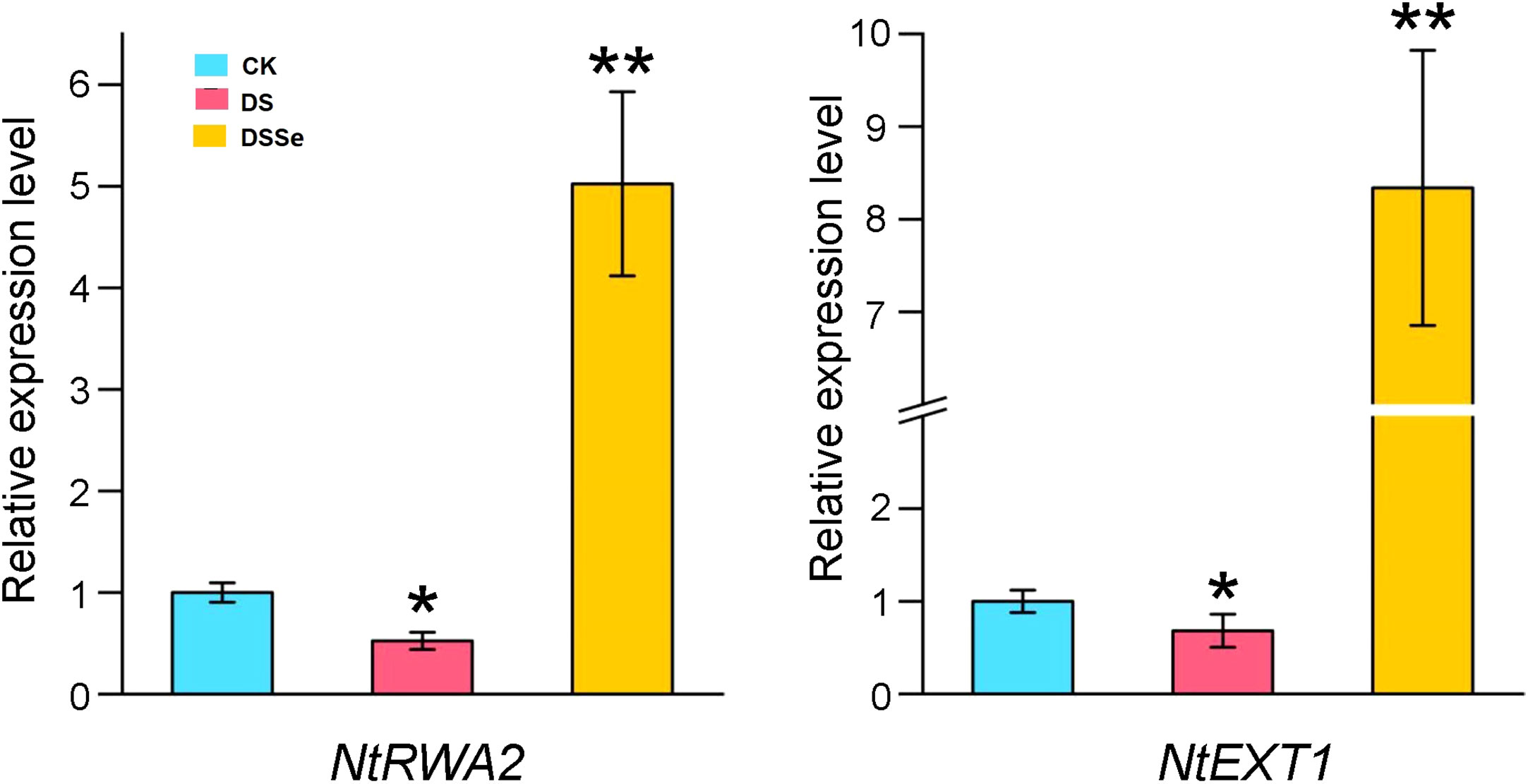
Figure 7 Relative expression of NtRWA2 and NtEXT1 under DS and DSSe treatments. * and ** represent significant difference at P <0.05 and 0.01, respectively.
3.5 Analysis of differentially accumulated metabolites under different treatments
This study recorded 130 upregulated and 77 downregulated DAMs after drought treatment (DS vs CK), while there were 64 upregulated and 63 downregulated DAMs after the addition of Se (DSSe vs DS) (Figure S1). Interestingly, eight metabolites including ur-144 n-(2-hydroxypentyl) metabolite and o-[(2e)-hexenedioyl] carnitine were downregulated in DS treatment but were upregulated in DSSe treatment (Table 4). We also identified 14 metabolites were upregulated in DS treatment but downregulated in DSSe treatment, which included 9,10-dibromostearic acid, quinoline-3-carboxamides, lactose, N-acetylneuraminic acid and catechin (Table 5). These DAMs might play key roles in drought tolerance in tobacco.
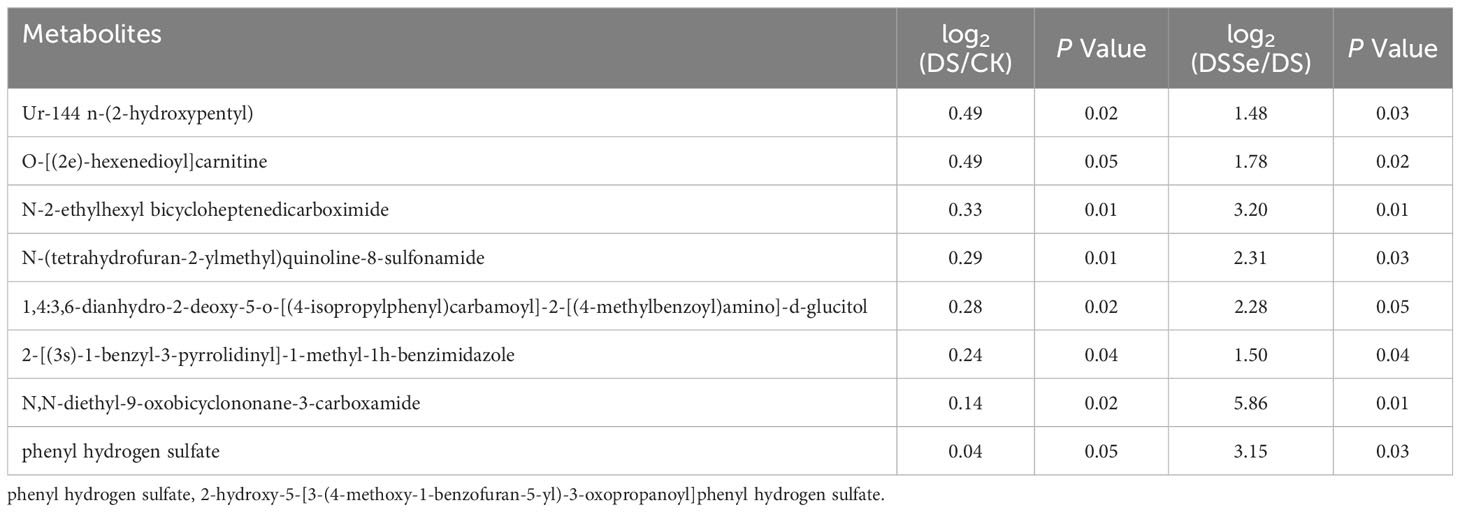
Table 4 List of metabolites downregulated under drought treatment (DS vs CK) but upregulated under DSSe treatment (DSSe vs D).
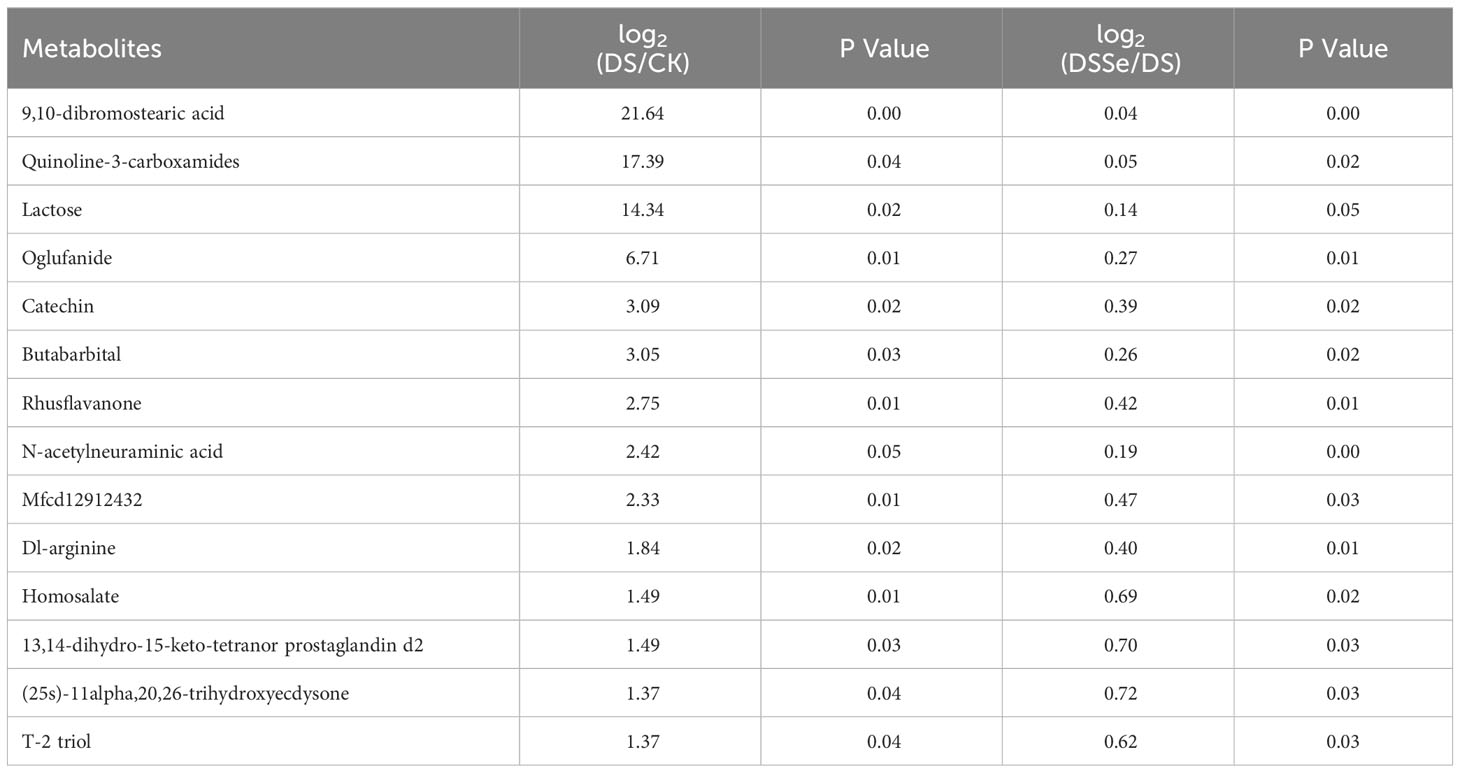
Table 5 List of metabolites upregulated under drought treatment (DS vs CK) but downregulated under DSSe treatment (DSSe vs D).
4 Discussion
This study revealed differences between non-stressed and drought-stressed plants in their biomass production, miRNA expression and metabolite profiling. In addition, these differences were better visualized and understood by adding another treatment of Se, which helped to reduce the yield losses under drought conditions. Our results demonstrated that Se application can be used as a useful strategy to improve drought tolerance in tobacco. Recently, Han et al. (2022) compared the alleviation effects of selenite on drought stress in tobacco and found that different Se species could alleviate drought-induced growth inhibition via elevating photosynthesis, osmotic substance content, antioxidant enzyme activity and stress-responsive genes. However, the regulatory mechanisms of miRNAs conferring drought tolerance in tobacco are largely unknown. Our study has highlighted several important miRNAs that may be involved in regulating drought stress tolerance in tobacco. Based on the identified miRNAs and DAMs, we proposed a tentative model involving drought tolerance (Figure 8).
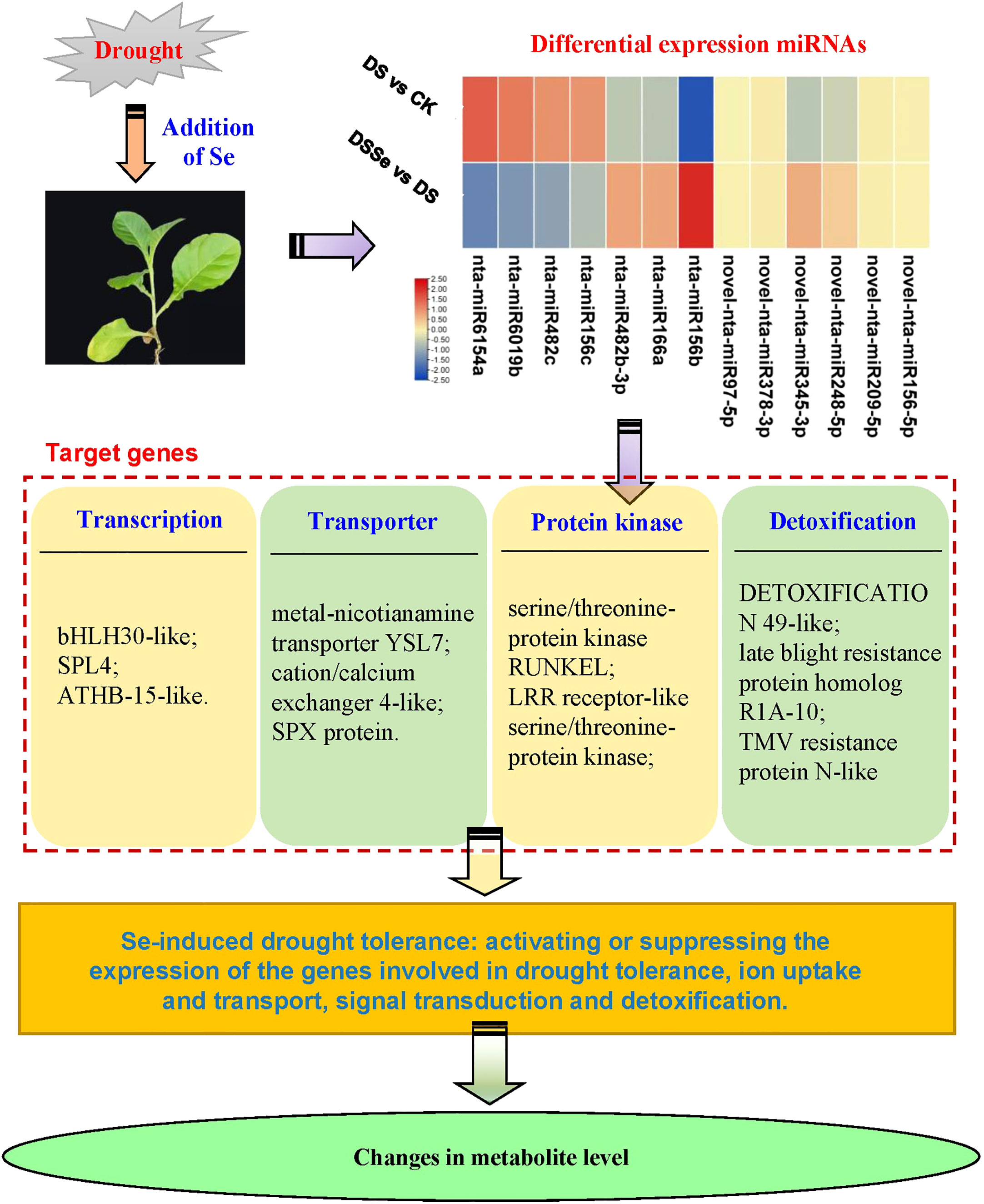
Figure 8 A working model of miRNAs and their target genes in tobacco involving in Se-induced increased drought tolerance. ATHB-15-like, homeobox-leucine zipper protein ATHB-15-like; bHLH30-like, transcription factor bHLH30-like; SPX protein, SPX domain-containing membrane protein; SPL4, squamosa promoter-binding-like protein 4.
Establishing a correlation between miRNAs and metabolites could provide a better understanding of Se-induced drought tolerance in tobacco and identify key genes for drought tolerance. In our study, we identified 2 miRNAs that showed significant positive or negative correlations with 6 metabolites (Table S6, Tables 2-5). The target genes of these miRNAs were metal-nicotianamine transporter YSL7 (YSL7) and LRR receptor-like serine/threonine-protein kinase (LRR-RLK). Previous studies have reported the involvement of OsYSL2 in the phloem transport of Fe and Mn in rice (Koike et al., 2004) and ZmYSL2 for iron distribution and development in maize (Zang et al., 2020). Additionally, OsYSL9 has been found to play a role in the distribution of Fe in developing rice grains (Senoura et al., 2017). In the present study, the novel-nta-miR156-5p exhibited significantly positive and negative correlation with phenyl hydrogen sulfate and lactose, respectively (Table S6). Similiarly, the novel-nta-miR97-5p exhibited negative correlations with butabarbital, catechin and oglufanide, and its target gene, LRR-RLK, has been implicated in regulating abiotic stress tolerance through ABA signaling activation and ROS detoxification (Ye et al., 2017). Catechin, a flavonoid, has been previously reported to enhance flooding tolerance by increasing the capacity of ROS scavenging (Yiu et al., 2011). Henschel et al. (2023) found that foliar application of L-carnitine significantly alleviated drought-induced growth inhibition by elevating membrane integrity and water balance in radish plants. In the present study, the abundance of carnitine was notably decreased under drought stress, but increased after the addition of Se, suggesting a potential positive correlation between carnitine and drought tolerance in tobacco. The accumulation of tea catechins have been found to be improved under drought stress (Lv et al., 2021). The possible mechanism by which catechin responds to drought stress may involve clearing over-accumulated reactive oxidative species (Lv et al., 2021), which is in agreement to the findings of our study. However, the abundance of catechins was found to be decreased after Se addition. This might be due to lower accumulation of ROS in plants under DSSE as compared to DS conditions. These results suggested that the novel-nta-miR97-5p- LRR-RLK- catechin pathway might play a central role in drought tolerance, which needs to be further validated in future studies.
Extensin and reduced wall acetylation proteins play numerous functions in plant cell wall (CW) (Manabe et al., 2013). Extensin is the most abundant protein in plant CW and has an important function in plant defense via strengthening the CW (Castilleux et al., 2021). The expression of extensin-like gene is significantly induced by drought stress in tolerant wheat genotypes but downregulated in sensitive genotypes under similar circumstances (Keskin, 2019). Manabe et al. (2013) found that the growth of the triple and quadruple rwa mutants was severely inhibited through affecting the cell differentiation of secondary CW, thus showing the significance of reduced wall acetylation (RWA) proteins in CW acetylation of plants. In the present study, the expression of NtRWA2 and NtEXT1 was significantly decreased in DS treatment but significantly increased in DSSe treatment (Figure 7), which reflects their importance in regulating drought tolerance in tobacco. The results presented in this study needs to be tested by conducting further studies for the better understanding of the drought tolerance mechanism in tobacco.
Several transcription factors regulate gene expression during plant growth and development when grown under stress conditions (Lin et al., 2022). Overexpression of bHLH55 increases salt tolerance in maize (Yu et al., 2021). On the contrary, the knockout of OsbHLH24 enhances salt tolerance in rice. Compared with wild type plants, the mutant plants have higher scavenging capacity of reactive oxygen species. Moreover, knock-out of OsbHLH24 also markedly upregulated salt tolerance-related genes, such as OsSOS1, OsHAK7 and OsHKT1;3 (Alam et al., 2022). In addition, the SPL gene family is involved in the activation of the conserved miR156-SPL module, which is a key regulator of plant abiotic stress resistance (Wang et al., 2019). Overexpression of MtHB2 (a homeobox-leucine zipper protein) in transgenic Arabidopsis leads to a greater more sensitivity to salt and drought compared with the wild type (Song et al., 2012). In this study, the expressions of novel-nta-miR156-5p, nta-miR156b and nta-miR166a was significantly downregulated in DS treatment but upregulated in DSSe treatment (Table 2). Their targets were transcription factor bHLH30-like, squamosa promoter-binding-like protein 4 and homeobox-leucine zipper protein ATHB-15-like. These results suggested that the transcription factors may negatively regulate drought tolerance and Se addition can inhibit the expression of these genes via regulating miRNA expression.
Drought stress affects the uptake and transport of nutrient element (Lanza and Dos Reis, 2021). The cation/calcium exchanger (CCX) family is involved in the transport of Ca and other cations (Su et al., 2021). Yang et al. (2021) found that apple MdCCX2 positively regulated salt tolerance by decreasing Na+ content and elevating the ability of ROS scavenge. SPX proteins play a negative role in regulating phosphorus signaling and are involved in inhibiting the phosphorus starvation response by PHR genes (Zhang et al., 2021). In this study, several members of metal-nicotianamine transporter (YSL), CCX and SPX family target genes were identified in tobacco (Table 2). Our results suggested that the target genes may elevate drought tolerance via regulating the uptake, transport and distribution of nutrient elements, such as phosphorus. Further research needs to be carried out in future to investigate how miRNA and target genes regulate these processes.
This study identified two protein kinase genes as miRNA targets, serine/threonine-protein kinase RUNKEL and LRR-RLK (Table 2). Their corresponding miRNAs were novel-nta-miR209-5p and novel-nta-miR97-5p. Previous studies found that serine/threonine-protein kinases were involved in abiotic stress tolerance (Luo et al., 2006). The above differentially expressed miRNAs may negatively regulate drought tolerance by inhibiting the two protein kinases. Meanwhile, we also found four miRNAs that were upregulated in DS and downregulated in DSSe treatment (Table 3). One of their targets was serine/threonine protein phosphatase 2A (PP2A) 55 kDa regulatory subunit B beta isoform-like. PP2A also positively regulates abiotic stress tolerance in wheat (Liu et al., 2019). These miRNAs and their target modules may positively regulate drought tolerance in tobacco.
This study revealed 22 differentially accumulated metabolites, which might be associated to regulate drought tolerance. We suggest that further research needs to be carried out to investigate the relationship between the identified DAMs and drought tolerance, and how miRNA operates for the activation of metabolite pathways that led to their increased accumulation.
5 Conclusions
Selenium application significantly alleviated drought-induced growth inhibition. The present study identified seven known and six novel miRNAs in tobacco seedlings associated with Se-induced drought stress alleviation, including nta-miR156c, nta-miR482c, nta-miR6019b and nta-miR6154a. Functional analysis of the identified target genes revealed that these miRNAs belonged to transcription factors, metal transporters, protein kinase, etc. Twenty-two key metabolites were identified to be involved in drought tolerance. The combined analysis of miRNA sequencing and metabolome identified a metabolic pathway associated with regulating drought tolerance. Thus far, this is the first study to investigate the effect of exogenous Se application in regulating plant drought-tolerance in tobacco using a multi-omics strategy. The implications of this research helped to better understand the physiological and molecular basis underlying Se mediated defense mechanisms in tobacco. The identified miRNAs and their targets can be used in future to achieve greater drought tolerance in tobacco.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HD: Conceptualization, Writing – original draft, Writing – review & editing, Data curation. JY: Conceptualization, Methodology, Writing – original draft. LT: Visualization, Writing – original draft. ZW: Data curation, Writing – original draft. TL: Data curation, Formal Analysis, Writing – original draft. WK: Data curation, Writing – review & editing. RY: Formal Analysis, Investigation, Writing – original draft. BQ: Investigation, Writing – original draft. YZ: Resources, Supervision, Writing – review & editing. CY: Project administration, Resources, Writing – review & editing.
Funding
This work was supported by CNTC Research Program [110202101013(XJ-05)] and the Natural Science Foundation of Henan Province (182300410055).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1255682/full#supplementary-material
References
Ahmad, Z., Anjum, S., Skalicky, M., Waraich, E. A., Muhammad, S. T. R., Ayub, M. A., et al. (2021). Selenium alleviates the adverse effect of drought in oilseed crops camelina (Camelina sativa L.) and canola (Brassica napus L.). Molecules 26 (6), 1699. doi: 10.3390/molecules26061699
Alam, M. S., Kong, J., Tao, R., Ahmed, T., Alamin, M., Alotaibi, S. S., et al. (2022). CRISPR/Cas9 mediated knockout of the OsbHLH024 transcription factor improves salt stress resistance in rice (Oryza sativa L.). Plants 11 (9), 1184. doi: 10.3390/plants11091184
Bukhari, S. A. H., Wang, R., Wang, W., Ahmed, I. M., Zheng, W., Cao, F. (2016). Genotype-dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ. Sci. pollut. Res. 23, 18229–18238. doi: 10.1007/s11356-016-7017-2
Cao, F., Wang, N., Zhang, M., Dai, H., Dawood, M., Zhang, G., et al. (2013). Comparative study of alleviating effects of GSH, Se and Zn under combined contamination of cadmium and chromium in rice (Oryza sativa). Biometals 26, 297–308. doi: 10.1007/s10534-013-9611-9
Castilleux, R., Plancot, B., Vicré, M., Nguema-Ona, E., Driouich, A. (2021). Extensin, an underestimated key component of cell wall defence? Ann. Bot. 127 (6), 709–713. doi: 10.1093/aob/mcab001
Chen, X. (2005). microRNA biogenesis and function in plants. FEBS Lett. 579 (26), 5923–5931. doi: 10.1016/j.febslet.2005.07.071
Chen, Q., Li, M., Zhang, Z., Tie, W., Chen, X., Jin, L., et al. (2017). Integrated mRNA and microRNA analysis identifies genes and small miRNA molecules associated with transcriptional and post-transcriptional-level responses to both drought stress and re-watering treatment in tobacco. BMC Genomics 18, 1–16. doi: 10.1186/s12864-016-3372-0
Cuperus, J. T., Fahlgren, N., Carrington, J. C. (2011). Evolution and functional diversification of MIRNA genes. Plant Cell 23 (2), 431–442. doi: 10.1105/tpc.110.082784
Dai, X., Zhao, P. (2011). psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 39 (suppl_2), W155–W159. doi: 10.1093/nar/gkr319
Feyissa, B. A., Arshad, M., Gruber, M. Y., Kohalmi, S. E., Hannoufa, A. (2019). The interplay between miR156/SPL13 and DFR/WD40–1 regulate drought tolerance in alfalfa. BMC Plant Biol. 19 (1), 1–19. doi: 10.1186/s12870-019-2059-5
Gao, F., Wang, N., Li, H., Liu, J., Fu, C., Xiao, Z., et al. (2016). Identification of drought-responsive microRNAs and their targets in Ammopiptanthus mongolicus by using high-throughput sequencing. Sci. Rep. 6 (1), 34601. doi: 10.1038/srep34601
Han, D., Tu, S., Dai, Z., Huang, W., Jia, W., Xu, Z., et al. (2022). Comparison of selenite and selenate in alleviation of drought stress in Nicotiana tabacum L. Chemosphere 287, 132136. doi: 10.1016/j.chemosphere.2021.132136
He, X., Zheng, W., Cao, F., Wu, F. (2016). Identification and comparative analysis of the microRNA transcriptome in roots of two contrasting tobacco genotypes in response to cadmium stress. Sci. Rep. 6 (1), 32805. doi: 10.1038/srep32805
Henschel, J. M., Dantas, E. F. O., de Azevedo Soares, V., Dos Santos, S. K., da Silva Gomes, D., Ferreira, L. M., et al. (2023). Drought stress mitigation by foliar application of L-carnitine and its effect on radish morphophysiology. Physiol. Mol. Biol. Plants 29, 579–590. doi: 10.1007/s12298-023-01308-6
Huang, H., Li, M., Rizwan, M., Dai, Z., Yuan, Y., Hossain, M. M., et al. (2021). Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 401, 123393. doi: 10.1016/j.jhazmat.2020.123393
Kantar, M., Unver, T., Budak, H. (2010). Regulation of barley miRNAs upon dehydration stress correlated with target gene expression. Funct. Integr. Genomics 10, 493–507. doi: 10.1007/s10142-010-0181-4
Keskin, B. C. (2019). Quantitative mRNA expression profiles of germin-like and extensin-like proteins under drought stress in Triticum aestivum. Int. J. Life Sci. Biotechnol. 2 (2), 95–107. doi: 10.38001/ijlsb.566942
Khan, R., Ma, X., Hussain, Q., Chen, K., Farooq, S., Asim, M., et al. (2023). Transcriptome and anatomical studies reveal alterations in leaf thickness under long-term drought stress in tobacco. J. Plant Physiol. 281, 153920. doi: 10.1016/j.jplph.2023.153920
Koike, S., Inoue, H., Mizuno, D., Takahashi, M., Nakanishi, H., Mori, S., et al. (2004). OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 39 (3), 415–424. doi: 10.1111/j.1365-313X.2004.02146.x
Langmead, B., Trapnell, C., Pop, M., Salzberg, S. L. (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. doi: 10.1186/gb-2009-10-3-r25
Lanza, M. G. D. B., Dos Reis, A. R. (2021). Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 164, 27–43. doi: 10.1016/j.plaphy.2021.04.026
Leng, G., Hall, J. (2019). Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 654, 811–821. doi: 10.1016/j.scitotenv
Lin, K., Zeng, M., Williams, D. V., Hu, W., Shabala, S., Zhou, M., et al. (2022). Integration of transcriptome and metabolome analyses reveals the mechanistic basis for cadmium accumulation in maize. Iscience 25 (12), 105484. doi: 10.1016/j.isci.2022.105484
Liu, D., Li, B., Feng, G., Mao, X., Li, A., Chang, X., et al. (2019). TaPP2AbB″;-γ, a wheat regulatory subunit of PP2A enhanced abiotic stress tolerance. Plant Growth Regul. 89, 345–355. doi: 10.1007/s10725-019-00540-z
Liu, X., Tan, C., Cheng, X., Zhao, X., Li, T., Jiang, J. (2020). miR168 targets Argonaute1A mediated miRNAs regulation pathways in response to potassium deficiency stress in tomato. BMC Plant Biol. 20 (1), 1–17. doi: 10.1186/s12870-020-02660-5
Liu, Q., Zhang, Y., Wang, C., Luo, Y., Huang, Q., Chen, S., et al. (2009). Expression analysis of phytohormone-regulated microRNAs in rice, implying their regulation roles in plant hormone signaling. FEBS Lett. 583, 723–728. doi: 10.1016/j.febslet.2009.01.020
Luo, G. Z., Wang, Y. J., Xie, Z. M., Gai, J. Y., Zhang, J. S., Chen, S. Y. (2006). The putative Ser/Thr protein kinase gene GmAAPK from soybean is regulated by abiotic stress. J. Integr. Plant Biol. 48 (3), 327–333. doi: 10.1111/j.1744-7909.2006.00169.x
Lv, Z., Zhang, C., Shao, C., Liu, B., Liu, E., Yuan, D., et al. (2021). Research progress on the response of tea catechins to drought stress. J. Sci. Food Agric. 101 (13), 5305–5313. doi: 10.1002/jsfa.11330
Manabe, Y., Verhertbruggen, Y., Gille, S., Harholt, J., Chong, S.-L., Pawar, P. M.-A., et al. (2013). Reduced wall acetylation proteins play vital and distinct roles in cell wall O-acetylation in Arabidopsis. Plant Physiol. 163 (3), 1107–1117. doi: 10.1104/pp.113.225193
Rabara, R. C., Tripathi, P., Reese, R. N., Rushton, D. L., Alexander, D., Timko, M. P., et al. (2015). Tobacco drought stress responses reveal new targets for Solanaceae crop improvement. BMC Genomics 16, 1–23. doi: 10.1186/s12864-015-1575-4
Senoura, T., Sakashita, E., Kobayashi, T., Takahashi, M., Aung, M. S., Masuda, H., et al. (2017). The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol. Biol. 95, 375–387. doi: 10.1007/s11103-017-0656-y
Song, S., Chen, Y., Zhao, M., Zhang, W. (2012). A novel Medicago truncatula HD-Zip gene, MtHB2, is involved in abiotic stress responses. Environ. Exp. Bot. 80, 1–9. doi: 10.1016/j.envexpbot.2012.02.001
Su, B., Huang, J., Fischer, T., Wang, Y., Kundzewicz, Z. W., Zhai, J., et al. (2018). Drought losses in China might double between the 1.5 C and 2.0 C warming. Proc. Natl. Acad. Sci. U.S.A. 115 (42), 10600–10605. doi: 10.1073/pnas.1802129115
Su, W., Zhang, C., Wang, D., Ren, Y., Sun, T., Feng, J., et al. (2021). The CaCA superfamily genes in Saccharum: Comparative analysis and their functional implications in response to biotic and abiotic stress. BMC Genomics 22 (1), 1–19. doi: 10.1186/s12864-021-07828-3
Teng, L., Zhang, X., Wang, R., Lin, K., Zeng, M., Chen, H., et al. (2023). miRNA transcriptome reveals key miRNAs and their targets contributing to the difference in Cd tolerance of two contrasting maize genotypes. Ecotoxicol. Environ. Saf. 256, 114881. doi: 10.1016/j.ecoenv.2023.114881
Wang, X., Kim, Y., Borowiecki, M., Tynan, M. A., Emery, S., King, B. A. (2022). Trends in Cigar sales and prices, by product and flavor type—the United States 2016–2020. Nicotine Tob. Res. 24 (4), 606–611. doi: 10.1093/ntr/ntab238
Wang, J., Ye, Y., Xu, M., Feng, L., Xu, L. (2019). Roles of the SPL gene family and miR156 in the salt stress responses of tamarisk (Tamarix chinensis). BMC Plant Biol. 19, 1–11. doi: 10.1186/s12870-019-1977-6
Xu, F., Liu, Q., Chen, L., Kuang, J., Walk, T., Wang, J., et al. (2013). Genome-wide identification of soybean microRNAs and their targets reveals their organ-specificity and responses to phosphate starvation. BMC Genomics 14, 1–30. doi: 10.1186/1471-2164-14-66
Yan, X., Wang, Y., Lei, J., Zhou, J., Pan, Y., Hu, X., et al. (2021). Classification of domestic cigars and its practical application. Acta Tabacaria Sin. 27 (5), 100–109. doi: 10.16472/j.Chinatobacco.2021.T0029
Yang, J., Guo, X., Li, W., Chen, P., Cheng, Y., Ma, F., et al. (2021). MdCCX2 of apple functions positively in modulation of salt tolerance. Environ. Exp. Bot. 192, 104663. doi: 10.1016/j.envexpbot.2021.104663
Ye, Y., Ding, Y., Jiang, Q., Wang, F., Sun, J., Zhu, C. (2017). The role of receptor-like protein kinases (RLKs) in abiotic stress response in plants. Plant Cell Rep. 36, 235–242. doi: 10.1007/s00299-016-2084-x
Yin, F., Qin, C., Gao, J., Liu, M., Luo, X., Zhang, W., et al. (2015). Genome-wide identification and analysis of drought-responsive genes and microRNAs in tobacco. Int. J. Mol. Sci. 16 (3), 5714–5740. doi: 10.3390/ijms16035714
Yiu, J., Tseng, M., Liu, C. (2011). Exogenous catechin increases antioxidant enzyme activity and promotes flooding tolerance in tomato (Solanum lycopersicum L.). Plant Soil 344, 213–225. doi: 10.1007/s11104-011-0741-y
Yu, C., Yan, M., Dong, H., Luo, J., Ke, Y., Guo, A., et al. (2021). Maize bHLH55 functions positively in salt tolerance through modulation of AsA biosynthesis by directly regulating GDP-mannose pathway genes. Plant Sci. 302, 110676. doi: 10.1016/j.plantsci.2020.110676
Zang, J., Huo, Y., Liu, J., Zhang, H., Liu, J., Chen, H. (2020). Maize YSL2 is required for iron distribution and development in kernels. J. Exp. Bot. 71 (19), 5896–5910. doi: 10.1111/j.1365-313X.2004.02146.x
Zhang, J., Zhang, H., Srivastava, A. K., Pan, Y., Bai, J., Fang, J., et al. (2018). Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 176 (3), 2082–2094. doi: 10.1104/pp.17.01432
Zhang, K., Zhou, Z., Li, J., Wang, J., Yu, L., Lin, S. (2021). SPX-related genes regulate phosphorus homeostasis in the marine phytoplankton, Phaeodactylum tricornutum. Commun. Biol. 4 (1), 797. doi: 10.1038/s42003-021-02284-x
Keywords: cigar tobacco, drought, selenium, multi-omics, target genes
Citation: Dai H, Yang J, Teng L, Wang Z, Liang T, Khan WA, Yang R, Qiao B, Zhang Y and Yang C (2023) Mechanistic basis for mitigating drought tolerance by selenium application in tobacco (Nicotiana tabacum L.): a multi-omics approach. Front. Plant Sci. 14:1255682. doi: 10.3389/fpls.2023.1255682
Received: 09 July 2023; Accepted: 04 September 2023;
Published: 19 September 2023.
Edited by:
Guowei Li, Shandong Academy of Agricultural Sciences, ChinaReviewed by:
Parviz Heidari, Shahrood University of Technology, IranFanrong Zeng, Yangtze University, China
Copyright © 2023 Dai, Yang, Teng, Wang, Liang, Khan, Yang, Qiao, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanling Zhang, emhhbmd5YW5saW5nQHp0cmkuY29tLmNu; Chunlei Yang, eWNsMTkzNzM3QDE2My5jb20=
†These authors have contributed equally to this work
 Huaxin Dai
Huaxin Dai Jinpeng Yang2†
Jinpeng Yang2† Lidong Teng
Lidong Teng Waleed Amjad Khan
Waleed Amjad Khan