- 1Key Laboratory of Eco-environments in Three Gorges Reservoir Region (Ministry of Education), Chongqing Key Laboratory of Plant Ecology and Resources in Three Gorges Reservoir Region, School of Life Sciences, Southwest University, Chongqing, China
- 2School of Biological Science and Food Engineering, Huanghuai University, Zhumadian, China
Clonal plants are widely distributed in the riparian zone and play a very important role in the maintenance of wetland ecosystem function. Flooding is an environmental stress for plants in the riparian zone, and the response of plants varies according to the depth and duration of flooding. However, there is a lack of research on the growth response of clonal plants during flooding, and the endogenous hormone response mechanism of clonal plants is still unclear. In the present study, Alternanthera philoxeroides, a clonal plant in the riparian zone, was used to investigate the time-dependent stem elongation, the elongation of different part of the immature internodes, and the relationship between growth elongation and the phytohormone gibberellin (GA) under a series of submergence depths (0 m, 2 m, 5 m, and 9 m). The results showed that stem elongation occurred under all treatments, however, compared to 0 m (control), plants grew more under 2 m and 5 m submergence depth, while grew less under 9 m water depth. Additionally, basal part elongation of the immature internode was the predominant factor contributing to the stem growth of A. philoxeroides under different submergence depths. The phytohormone contents in basal part of the mature and immature internodes showed that GA induced the differential elongation of internode. Plant submerged at depth of 2 m had the highest GA accumulation, but plant submerged at depth of 9 m had the lowest GA concentration. These data suggested that GA biosynthesis are essential for stem elongation in A. philoxeroides, and the basal part of the immature internode was the main position of the GA biosynthesis. This study provided new information about the rapid growth and invasion of the clonal plant A. philoxeroides around the world, further clarified the effects of submergence depth and duration on the elongation of the stem, and deepened our understanding of the growth response of terrestrial plants in deeply flooded environments.
1 Introduction
Flooding is an environmental stress for plants in the riparian zone, and the response of plants varies according to the depth and duration of flooding (Müller et al., 2019). Flooding depth and duration concomitantly influence the growth traits and yield of plant (Meng et al., 2022). A longer waterlogging duration caused a greater reduction in the above parameters (Wang et al., 2017). Clonal plants are widely distributed in the riparian zone and play a very important role in the maintenance of wetland ecosystem function, and often adapt to environmental changes through phenotypic plasticity, especially invasive clonal plants (Dong et al., 2017; Guo et al., 2023). Previous studies have demonstrated that the clonal plant Alternanthera philoxeroides is an invasive amphibious weed that is native to South America but has now invaded into the temperate and tropical regions across the world (Wu et al., 2017). A. philoxeroides has rapid clonal reproduction and is phenotypically plastic (leaf area, internode length, shoot diameter, etc.) (Gao et al., 2015).
Compared to the terrestrial environmental factors, the environmental factors in the water body can change considerably, such as light, water temperature, dissolved O2 and CO2 concentrations, etc (Vervuren et al., 2003). Light quantity and quality in rivers vary with depth and turbidity, for example, in the River Rhine, light quantum flux decreases with increasing water depth both in freshwater lakes and in flooded environments (Vervuren et al., 1999; Li et al., 2024). Light is reduced to 90% of the total solar radiation entering the water column at a depth of 50 cm, and less than 1% of the light intensity is available underwater when the water depth reaches more than 1.5 m (Vervuren et al., 2003). In completely submerged environments, O2 concentration in plants is reduced and low levels of O2 stimulate ethylene biosynthesis, whose diffusion rate in water is very slow, leading to a rapid rise in ethylene levels in plants in a short period of time (Voesenek et al., 2016; Wang and Komatsu, 2022).
The clonal plant A. philoxeroides is a common plant in floodplains, riparian zones, and water-level drawdown zones of large reservoirs with inundation-disturbed habitats (Yang et al., 2019), and it is also distributed in areas with deeper inundation (Zheng et al., 2021). For example, the Three Gorges Reservoir (TGR) which is the largest hydroelectric power project in the world, the water level of the reservoir fluctuates regularly from 145 m to 175 m in elevation (Chen et al., 2021). Thus, the water-level drawdown zones with a maximum drop of 30 m are formed along the banks of the Yangtze River (Lei et al., 2017). We have been conducting long-term research on plant growth in the drawdown zone, and we have found that the clonal plant A. philoxeroides exhibits a fast-growing in shallow submerged environments and a slow-growing or even stop-growing in deep submerged conditions (Ayi et al., 2016; Jing et al., 2022), but there is little discussion on the growth response of A. philoxeroides at different water depths and its response mechanism. Our previous study has shown that the formation of pith cavity and adventitious roots, and non-structural carbohydrate metabolism play important roles in the changes of different growth strategies in A. philoxeroides (Jing et al., 2022). The stem growth of A. philoxeroides at any submergence depth was chiefly caused by the elongation of the basal parts of immature internodes, which was highly correlated to both cell proliferation and cell enlargement (Jing et al., 2024). However, the time depended stem elongation and the hormone regulatory mechanisms remain unclear, this characteristic of the plant species is crucial for explaining the successful invasion of clonal plants (You et al., 2016).
Phytohormone plays a very important role as a signalling substance in the response of plants to biotic and abiotic stresses (Taiz and Zeiger, 2010). The response and adaptation strategies of plants in flooded environments are very closely related to hormone concentrations, and the regulation of plant morphology, anatomy, physiology, ecology, molecules and signalling under low oxygen or low light stress conditions is largely influenced by hormones (Bailey-Serres and Voesenek, 2008; Lin et al., 2021). When plants were submerged, the content of ethylene increases due to the diffusion of ethylene is weakened, which inhibits abscisic acid (ABA) synthesis and promotes ABA decomposition, whereas ABA inhibits gibberellin (GA) synthesis (Fukao and Bailey-Serres, 2008), so an increase in ethylene promotes an increase in GA content, which in turn promotes stem growth (Sasidharan and Voesenek, 2015). Physiological and genetic analyses indicated that GA biosynthesis and signal transduction are essential for internode elongation in deep-water rice (Ayano et al., 2014). Its role in plant response to submergence has been widely reported (Sasidharan and Voesenek, 2015; Wang et al., 2021). However, changes in the concentration of the endogenous hormone GA in different water depth environments have not been reported for the clonal plant A. philoxeroides.
To explore the time-dependent stem elongation and the hormone regulatory mechanisms under different submergence depths, taking the clonal plant Alternanthera philoxeroides (Mart.) Griseb., a submergence-tolerant plant as a model, we hope to solve the following scientific questions in this study: (1) What are the trends in the stems of different maturity levels of A. philoxeroides as the duration of submergence changes? (2) Is there a difference in the content of endogenous hormone GA in the immature internodes of A. philoxeroides under different water-depths? In order to clarify the above questions, we measured the length of stem, immature stem of A. philoxeroides at different water-depths, and determined the endogenous hormone GA content of mature and immature stem by using high-performance liquid chromatography and quantified by tandem mass spectrometry (HPLC-MS/MS). The answers to these questions will help understanding the phytohormone regulatory mechanisms of clonal plant tolerance to extreme flooding and explain why A. philoxeroides remains highly invasive worldwide.
2 Materials and methods
2.1 Plant material and cultivation
Alternanthera philoxeroides (Mart.) Griseb. can spread quickly via clonal growth, in this study, plants were grown as described in Jing et al. (2022). A. philoxeroides plants were cultivated from cuttings obtained from plants naturally growing on the banks of the Jialing River in Chongqing, Southwest China (29○49’42’’N, 106○26’46’’E). Each selected cutting was planted in a plastic pot (diameter and depth were both 13 cm) containing riparian soil from the Jialing River banks. All plants were cultivated under the same conditions. The temperature, relative humidity, daily maximum light (PAR) intensity, and water provision were maintained at 10~15 °C, 75~85%, 600~800 µmol m–2 s–1, and approximately 80~90% of the soil water-holding capacity, respectively. After approximately one month of cultivation, plants with approx. 288 mm height and 12 internodes were selected for submergence treatments.
2.2 Experimental design
The plants subjected to complete submergence treatments were suspended at planned water depths, as described in Jing et al. (2022). The design of 2 m, 5 m, and 9 m deep submergence was based on our long-term field observation on the elevational distribution of A. philoxeroides in the water level fluctuation zone of the Three Gorges reservoir. Unsubmerged control plants were placed under dark conditions and watered regularly to ensure adequate water supply. Four submergence treatments were applied in a fully randomized design using selected plants. Control plants were placed under dark conditions and were not submerged, in this article it is called 0 m. Additionally, three groups of plants were submerged in a water-filled concrete reservoir, with the top of plants 2 m, 5 m, and 9 m beneath the water surface (Supplementary Figure S1A). The plants in pots were suspended at planned water depths as described in Jing et al. (2022). According to the previous observation and pre-experimental results, the submergence treatments lasted to 11 d in the growth measured experiments, but 4 d in the endogenous GA measurements.
To investigate the effects of submergence depth on plants, the physicochemical status of water body (light, dissolved oxygen (DO), pH, and temperature) in the concrete reservoir were kept constant at any depths as described in Jing et al. (2022). DO concentration, photosynthetically active radiation (PAR) intensity, temperature and pH of the water column at different depths in the reservoir were checked twice per day (morning and evening) using a multi-parameter water quality analyzer (Hydrolab DS5, Hach, United States). During the experiment, no significant differences in these factors were found between different water depths (Table 1).
2.3 Growth measurements
Each plant had approximately 12 internodes at the start of treatments. From the stem base upwards, the 1st to 6th internodes were relatively more mature and the 7th to 12th internodes were immature (Supplementary Figure S1B). We marked nondestructively immature stems so as to distinguish the mature, immature stems formed before treatment. The length of mature stems, immature stems were measured every day. As plants may produce gaseous substances such as ethylene during submergence, it was ensured that the measurements were taken underwater, at the same time, the plants are not exposed to the atmosphere. Once the daily measurement was completed, the plants were quickly submerged to the appropriate water-depth for continuation of the treatment. In order to investigate the submergence time-dependent growth pattern of different parts of immature internodes, we selected an immature internode (the length of the internode was usually between 2.5 and 3.2 cm, as shown in Supplementary Figure S1D) from each plant before treatments and divided the internode into three equilong parts (basal, middle, and upper part, as shown in Supplementary Figure S1E) by marking with red polyester threads (Jing et al., 2024), measured the lengths of all parts every day.
2.4 Phytohormone concentration analysis
The endogenous GA concentration in the basal part of the internode of A. philoxeroides plants were mainly composed of GA1, GA3, GA4, and GA7. According to the results of the pre-experiment and the research objectives of the present study, the growth of plants under different submergence depths had already shown significant differences at the 4th day, and the endogenous hormone content of the plants was relatively high, the sampling time was set as the 4th day. The basal parts of each internode were harvested after submergence or control treatments, frozen in liquid nitrogen immediately, and kept at -80 °C before freeze drying. Ten basal parts of the mature and immature internodes were pooled to obtain enough material per sample. There were three replicates for GAs (GA1, GA3, GA4 and GA7) analyses. Measurement of endogenous GAs concentration was performed by HPLC-MS/MS. The HPLC-MS/MS system was composed of a high-performance liquid chromatography (HPLC, Agilent Technologies 1200 series, USA) connected to AB Sciex API 6500 Qtrap mass spectrometer (Concord, ON, Canada). The Analyst 1.6.3 software (Concord, ON, Canada) controlled the HPLC-MS/MS system and Multiquant 3.2 to process the data (Pan et al., 2010).
2.5 Statistical analysis
Elongation difference of stem (or immature stem) between submergence depths were checked by one-way ANOVA. Separate ANOVA with Repeated Measures was used to detect the difference in stem length, basal, middle, and upper parts within internodes, One-way ANOVA was used to examine the difference in contents of gibberellin in mature and young stems respectively between different treatments. Logarithm data transformation was performed to equalize variance if necessary. Differences between treatments were detected using the Tukey HSD test, and the significance level was set at p = 0.05. All analyses were conducted using SPSS 22 (SPSS Inc., Chicago).
3 Results
3.1 Elongation of stem and immature stem parts
A. philoxeroides plants subjected to four treatments all elongated their stems during the experiment day by day (Figure 1A). At the end of treatments, the stem elongation were 198.00 mm, 245.67 mm, 223.17 mm, and 87.50 mm averagely in plants submerged at water depths of 0 m (control), 2 m, 5 m, and 9 m, respectively (Figure 1A). Compared to the 0 m, the stem presented apparent elongation when submerged at water depth of 2 m and 5 m, but the stem only had very slight elongation when submerged at water depth of 9 m. Stem elongation decreased with increasing submergence depth after 6 days treatment (Figure 1A). From the 7th day, the stem elongation of A. philoxeroides was significantly inhibited in the 9 m submergence depth, no elongation was observed. At this time, the stem elongation of plants submerged at water depth of 2 m and 5 m were still faster, indicating that the growth response of the stem of A. philoxeroides varied greatly at different water depths (Figure 1A). It was shown that water depth of 2 m and 5 m promoted elongation of the stems, while water depth of 9 m inhibited elongation of the stems, and this inhibition was more severe from the 7th day after the onset of submergence.
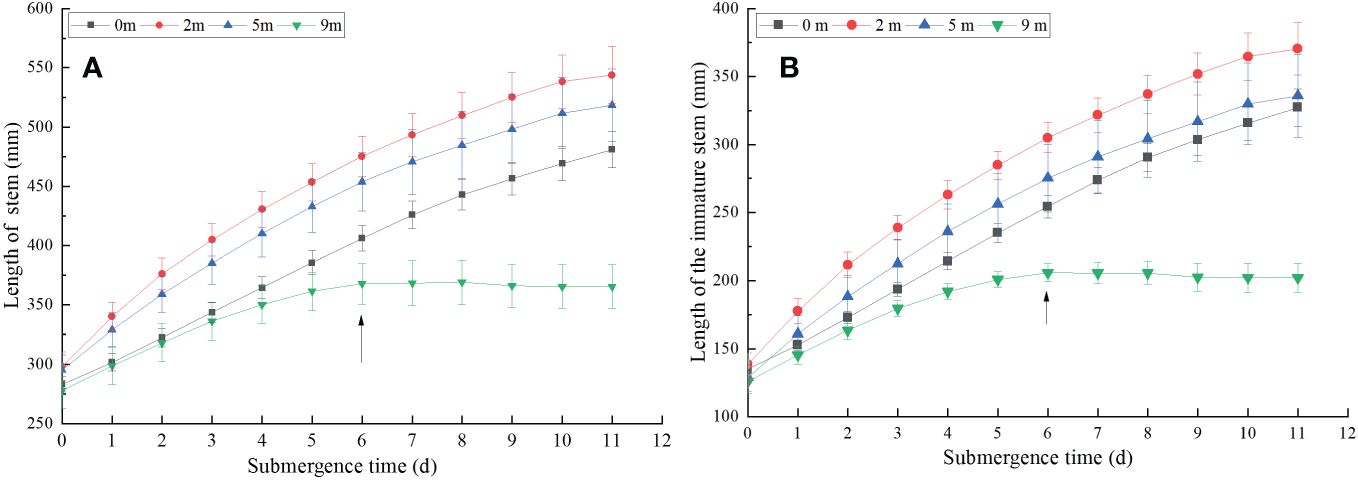
Figure 1 Length of stem (A) and immature stem (B) of Alternanthera philoxeroides after the start of different submergence treatments (Mean ± s. e.; n = 6). The arrows indicate A philoxeroides stops growing at 9 m water depth.
The immature stem elongation trend (Figure 1B, the 7th to 12th internodes) of A. philoxeroides under different water depths was consistent with that of the stems (Figure 1A). At the end of treatments, the elongation of immature stems in plants submerged at water depth of 0 m (control), 2 m and 5 m were 192.17 mm, 231.67 mm, and 206.83 mm, respectively (Figure 1B), and the immature stems were rapidly elongated throughout the treatment period. However, the water depth of 9 m promoted plant growth during the first 6 days of the experiment and then turned to inhibit plant growth as the duration of submergence increased (Figure 1B, especially on 7th day after the start of submergence), during the whole treatment period, the immature stem only grew 76.33 mm averagely.
3.2 Elongation of the different parts of immature internodes
The elongation of the basal (Figure 2A), middle (Figure 2B) and upper (Figure 2C) of immature internodes of A. philoxeroides showed different growth trends, which grew from about 8 mm at the start of the treatments to about 19 mm, 11 mm and 6 mm at 7 day under 2 m water depth, respectively. The growth rate of the basal parts was significantly faster than that of the middle parts, which in turn was faster than that of the upper parts (Figures 2A–C). However, when submergence treatment was carried out for 8 days, there was no significant growth in all parts of the marked immature internode, except for the internodes of the 0 m (unsubmerged group). In the same period of time, the elongation of basal, middle, and upper parts gradually decreased with increasing water depth (Figures 2A–C). Overall, 2 m water depth promoted the basal parts elongation of immature internodes at the early stage of treatments, while 9 m water depth had a certain inhibitory effect on the elongation of the immature internodes, and 5 m water depth had faster growth than 0 m at the beginning of submergence for 1~4 days, but the final length of all parts of the immature internodes was lower than that of the 0 m (Figure 2C).
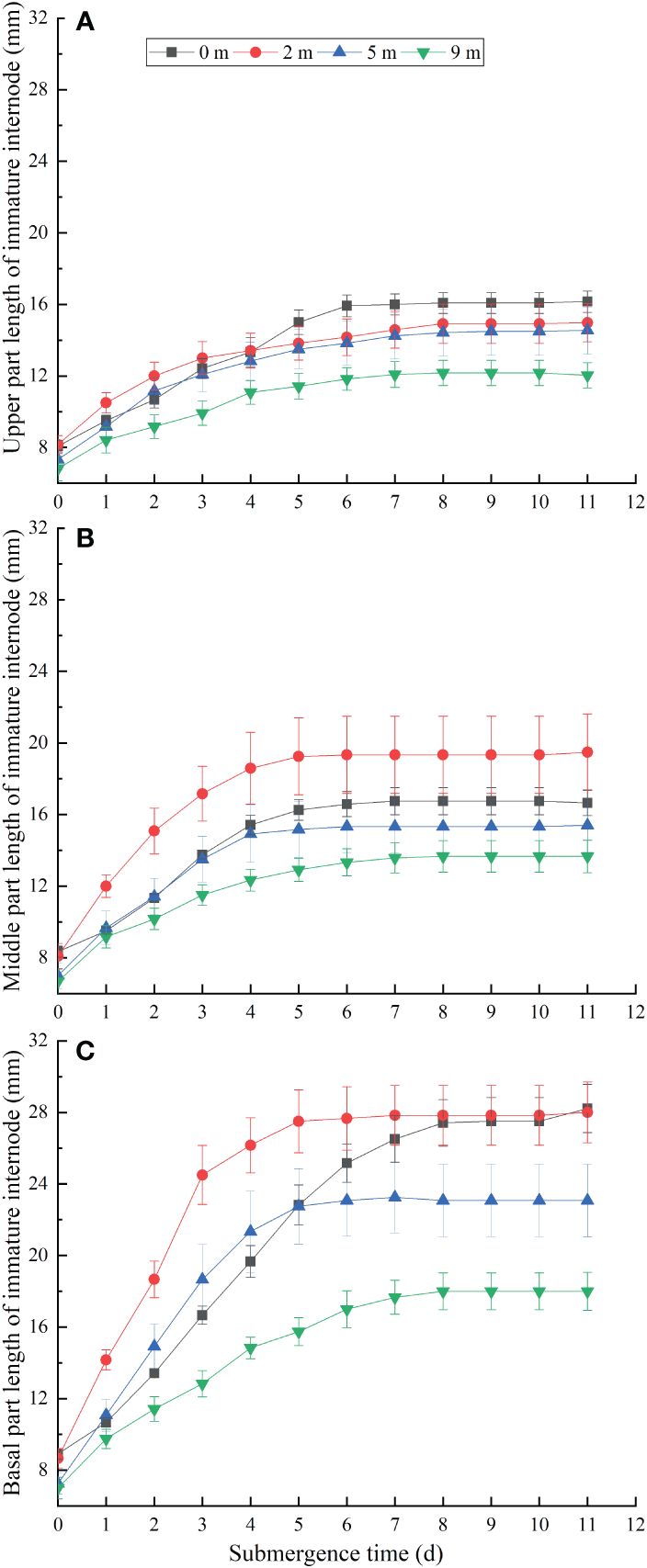
Figure 2 Upper (A), middle (B) and basal (C) length of the immature internode for Alternanthera philoxeroides after the start of different submergence treatments (Mean ± s. e.; n = 6).
3.3 Endogenous GA concentration in the basal part of the internode
The concentration of GAs in immature internodes was higher than that in the mature internodes of A. philoxeroides (Figure 3). The concentration of GAs in the basal parts of immature internodes decreased with increasing submergence depth at the fourth day of experiment (p < 0.05, Figure 3). Plants submerged at 2 m water depth had the highest GAs concentration but plants submerged at 9 m depth had the lowest GAs concentration (p < 0.05, Figure 3). However, no significant difference was found between 0 m and 5 m submergence depths in GAs concentration in immature stems of A. philoxeroides (p > 0.05, Figure 3). The concentration of GAs in mature internodes of unsubmerged plants was significantly higher than that of submerged plants, but no difference was found among the three submerged treatments (i.e., 2 m, 5 m and 9 m). This indicates that 2 m water depth promoted GAs biosynthesis in immature internodes, whereas 9 m water depth inhibited GAs biosynthesis.
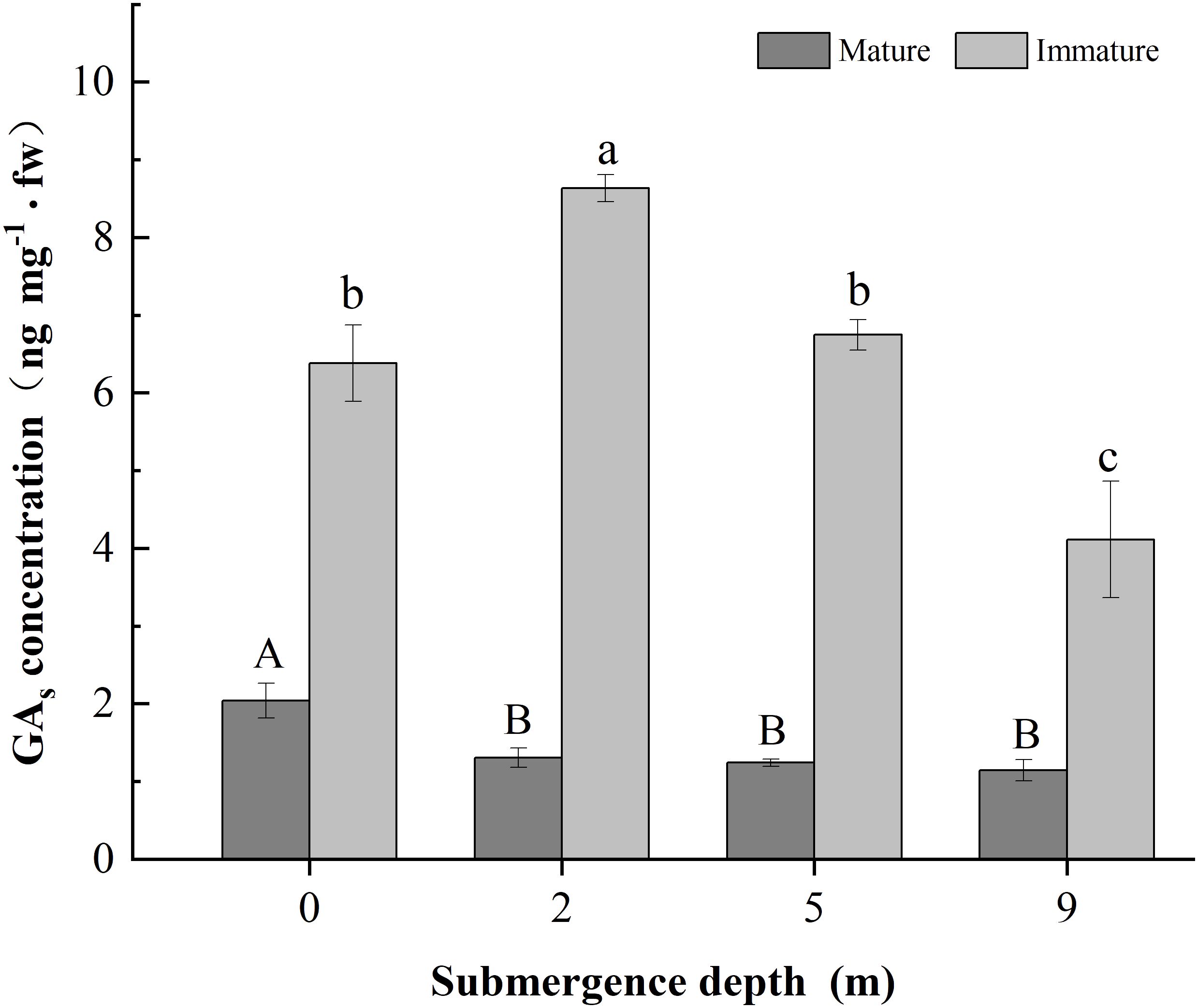
Figure 3 Concentration of GAS in the basal of mature and immature internodes of Alternanthera philoxeroides at the fourth day of the submergence treatments (Mean ± s. e.; n =3). Different upper-case letters indicate statistically significant differences (p < 0.05) between mature internode for treatments. Different lower-case letters indicate statistically significant differences (p < 0.05) between mature stems for treatments. fw indicates fresh weight.
4 Discussion
The influences of submergence on plant growth are different according to different flooding depths and durations. It has been reported that the yield of rice is almost non-existent when the flooding time is 5~6 days (Meng et al., 2022), longer flooding durations limited the basal area growth of larger trees and reduced sexual reproduction (Greet et al., 2020). In the present study, the stems of A. philoxeroides were elongating with the durations of submergence in all treatments (Figures 1A, B), this is mainly due to the growth of immature internodes. But the elongation of immature stems decreased when water depth increased (Figures 1A, B). This is consistent with the results of our previous studies, which found that immature internodes comparatively made the largest contribution to plant stem elongation (Jing et al., 2022). Moreover, as previous studies have found that the immature internodes showed intra-internodal variation in elongation among their basal, middle, and upper parts, and the variation was affected by submergence depth (Jing et al., 2024). The basal parts achieved much longer elongation than the middle and upper parts at 2 m water depth, but this elongation difference faded away when the water depth increased gradually to 9 m (Figures 2A–C).
In fact, after 7 days of submergence, the stems of A. philoxeroides were no longer growing under 9 m water depth (Figure 1A), the same to the immature stems (Figure 1B). Plants submerged at depth of 0 m, 2 m, 5 m continued to grow until the experiment was terminated after 11 days of submergence (Figures 1A, B). However, elongation of immature internodes was over by day 5 (Figure 2A–C), suggesting that A. philoxeroides produces new internodes to increase its total stem length during submergence. This is consistent with our previous findings that A. philoxeroides can produce new internodes during submergence, but very few new internodes were produced during submergence at water depth of 9 m, and 1.85, 2.40, 1.85 new internodes on average were produced at water depth of 0 m, 2 m, and 5 m, respectively (Jing et al., 2022). This is an important feature for clonal plants to be able to reproduce in stressful environments (Jing et al., 2022).
It has been shown that ethylene content in plants rises rapidly within a short period of time in a completely submerged environment (Raskin and Kende, 1984; Voesenek et al., 2013, Voesenek et al., 2016), and that ethylene markedly increases the activity of the endogenous hormone GA, which in turn promotes cell division and cell elongation, and ultimately plant growth (Sasidharan and Voesenek, 2015). Therefore, in this study, when A. philoxeroides was completely submerged in a 2 m water depth environment, as in the completely submerged environment, the stem length was increased due to the rapid increase of ethylene concentration thereby inducing an increase in the biosynthesis of GAs, which promotes elongation and growth of the plant’s immature parts (Figure 3). GA promotes cell division and cell elongation (Sauter and Kende, 1992), this is consistent with our previous study, the difference in the internode elongation is mainly due to the difference in cell growth and development. Cells in the basal parts of immature internodes were shorter and numerous, whereas those in the middle and upper parts were relatively longer and smaller in number (Jing et al., 2024). Plants possess higher concentrations of GA under 2 m water depth, but have lower concentrations of growth-promoting GA and less plant growth under 9 m water depth (Figure 3). In our experimental system, all factors except water depth were kept constant (Table 1). The main difference between 2 m and 9 m of complete submergence is the difference in hydrostatic pressure. For every 1 m increase in water depth, the pressure of water acting on an object increases by about 9.8 × 103 Pa, and the pressure under 9 m water depth is about 0.088 MPa. How do deep submergence environments affect the biosynthesis of the endogenous hormone GAs and thus inhibit plant growth?
For living cells, GAs promotes hydrolysis of hemicellulose by Xyloglucan Endotransfer glycosidase thereby softening and relaxing the cell wall and promoting cell elongation and cell division (Ayano et al., 2014; Wang and Komatsu, 2022). The percentage of S-phase cells significantly increased within 4~7 h of treating rice with GA3 through [3H] thymidine and DNA admixture experiments, suggesting that GA promotes cell division in meristematic tissues of internode and shortens the cell cycle, and that internode elongation in deep-water rice is ultimately regulated by GA (Fukao et al., 2019). Our findings are also consistent with previous studies that the response of plants in submerged environments is very closely linked to the GAs. Consequently, GAs content at the base of immature internodes of A. philoxeroides decreased with increasing water depth, and as we expected.
It was demonstrated in previous studies that mechanical stress has important effects on the biosynthesis of endogenous plant hormones (Chehab et al., 2012; Lange and Lange, 2015), such as the effect of soil stress on ethylene synthesis (Potocka and Szymanowska-Pułka, 2018). From a mechanical perspective, when the force environment is altered, the distribution of mechanical stresses within plant tissues also undergoes localized changes, followed by cascading effects at the cellular (Richter et al., 2009) and molecular (Laskowski et al., 2008) levels. Therefore, it is possible that the higher hydrostatic pressure under 9 m water depth resulted in changes in the distribution of mechanical stress in the immature internodes of A. philoxeroides, which in turn affected the biosynthesis of GAs. Hydrostatic pressure is a specific type of mechanical stress, and its effects on plant growth include the effects of submergence and the effects of force. The stress response produced by plants varies depending on the severity and duration of the stress occurrence (Shi et al., 2016). The growth response of plants should be different for different intensities of hydrostatic pressure and different times of action on the plants.
5 Conclusion
Entirely consistent with the conjecture, our results suggested that the stem elongation of A. philoxeroides responded significantly to submergence depth and duration, especially the response of immature stems. Elongation of immature stems decreased when water depth increased, which was associated with elongation at the basal of immature internodes, and the basal parts made the biggest contribution to the elongation of internodes. Moreover, the elongation of the basal part of the immature stems was related to the concentration of endogenous hormone GAs. Therefore, in flood-prone environments, A. philoxeroides was able to grow rapidly through clonal integration, and its growth response differences to water depth were regulated by endogenous hormones. The results of this study provide strong evidence to demonstrate the important role of the hydrostatic pressure induced by flooding. Investigating the growth response of plants in different water depth can deepen our understanding of clonal plant submergence tolerance mechanisms, and help to explore the future management of water level regulation in the drawdown zone of large reservoirs (e.g., Three Gorges Reservoir).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SJ: Data curation, Investigation, Writing – original draft. XR: Data curation, Investigation, Writing – original draft. FL: Writing – review & editing. HN: Data curation, Writing – original draft. QA: Formal analysis, Methodology, Writing – review & editing. BW: Data curation, Writing – original draft. BZ: Conceptualization, Funding acquisition, Methodology, Writing – original draft. XZ: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (grant numbers U22A20448, 31400480, 31800331), National Key R&D Program of China (2023YFF1305204), Chongqing Talents Program (grant number (cstc2021ycjh-bgzxm0316), the Science and Technology Major Projects of Henan Province of China (Grant number 232102321047) and Key Scientific Research Project of Colleges and Universities in Henan Province (Grant number 23B180010).
Acknowledgments
We thank Xingrong Wu, Senrui Zhang, Dongdong Ji, Meng Wang, Jine Huang, Zhao Zhang and all the people who helped with the fieldwork over the course of the experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1348080/full#supplementary-material
References
Ayano, M., Takahiro, K., Mikiko, K., Hitoshi, S., Takuya, K., Takeshi, K., et al. (2014). Gibberellin biosynthesis and signal transduction is essential for internode elongation in deepwater rice. Plant Cell Environ. 37, 2313–2324. doi: 10.1111/pce.12377
Ayi, Q., Zeng, B., Liu, J., Li, S., van Bodegom, P. M., Cornelissen, J. H. C. (2016). Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 118, 675–683. doi: 10.1093/aob/mcw051
Bailey-Serres, J., Voesenek, L. A. C. J. (2008). Flooding stress: acclimations and genetic diversity. Annu. Rev. Plant Biol. 59, 313–339. doi: 10.1146/annurev.arplant.59.032607.092752
Chehab, E. W., Yao, C., Henderson, Z., Kim, S., Braam, J. (2012). Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr. Biol. 22, 701–706. doi: 10.1016/j.cub.2012.02.061
Chen, Z., Arif, M., Wang, C., Chen, X., Li, C. (2021). Effects of hydrological regime on foliar decomposition and nutrient release in the riparian zone of the Three Gorges Reservoir, China. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.661865
Dong, B., Fu, T., Luo, F., Yu, F. (2017). Herbivory-induced maternal effects on growth and defense traits in the clonal species Alternanthera Philoxeroides. Sci. Total Environ. 605–606, 114–123. doi: 10.1016/j.scitotenv.2017.06.141
Fukao, T., Bailey-Serres, J. (2008). Submergence tolerance conferred by sub1a is mediated by SLR1 and SLRl1 restriction of gibberellin responses in rice. Proc. Natl. Acad. Sci. U. S. A. 105, 16814–16819. doi: 10.1073/pnas.0807821105
Fukao, T., Barrera-Figueroa, B. E., Juntawong, P., Peña-Castro, J. M. (2019). Submergence and waterlogging stress in plants: a review highlighting research opportunities and understudied aspects. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00340
Gao, L., Geng, Y., Yang, H., Hu, Y., Yang, J. (2015). Gene expression reaction norms unravel the molecular and cellular processes underpinning the plastic phenotypes of Alternanthera Philoxeroides in contrasting hydrological conditions. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00991
Greet, J., Fischer, S., Russell, K. (2020). Longer duration flooding reduces the growth and sexual reproductive efforts of a keystone wetland tree species. Wetl. Ecol. Manage. 28, 655–666. doi: 10.1007/s11273-020-09738-9
Guo, X., Hu, Y., Ma, J., Wang, H., Wang, K., Wang, T., et al. (2023). Nitrogen deposition effects on invasive and native plant competition: implications for future invasions. Ecotox. Environ. Safe. 259, 115029. doi: 10.1016/j.ecoenv.2023.115029
Jing, S., Ren, X., Lin, F., Niu, H., Ayi, Q., Wan, B., et al. (2024). Water depth-dependent stem elongation of completely submerged Alternanthera Philoxeroides is mediated by intra-internodal growth variations. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1323547
Jing, S., Zhang, X., Niu, H., Lin, F., Ayi, Q., Wan, B., et al. (2022). Differential growth responses of Alternanthera Philoxeroides as affected by submergence depths. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.883800
Lange, M. J. P., Lange, T. (2015). Touch-induced changes in Arabidopsis morphology dependent on gibberellin breakdown. Nat. Plants 1, 14025. doi: 10.1038/nplants.2014.25
Laskowski, M., Grieneisen, V. A., Hofhuis, H., Hove, C. A., Hogeweg, P., Maree, A. F., et al. (2008). Root system architecture from coupling cell shape to auxin transport. PLoS. Biol. 6, e307. doi: 10.1371/journal.pbio.0060307
Lei, S., Zeng, B., Xu, S., Zhang, X. (2017). Response of basal metabolic rate to complete submergence of riparian species Salix Variegata in the three gorges reservoir region. Sci. Rep. 7, 1–11. doi: 10.1038/s41598–017-13467–0
Li, H., Lv, J., He, X., Bao, Y., Nsabimana, G. (2024). Precipitation-dependent sensitivity of suspended sediment concentration to turbidity in a mountainous river in Southwestern China. Ecol. Indic. 159, 111644. doi: 10.1016/j.ecolind.2024.111644
Lin, C., Ogorek, L. L. P., Pedersen, O., Sauter, M. (2021). Oxygen in the air and oxygen dissolved in the floodwater both sustain growth of aquatic adventitious roots in rice. J. Exp. Bot. 72, 1879–1890. doi: 10.1093/jxb/eraa542
Meng, Y., Yu, S., Yu, Y., Jiang, L. (2022). Flooding depth and duration concomitantly influence the growth traits and yield of rice. Irrig. Drain. 71, 94–107. doi: 10.1002/ird.2632
Müller, J. T., van Veen, H., Bartylla, M. M., Akman, M., Pedersen, O., Sun, P., et al. (2019). Keeping the shoot above water - submergence triggers antithetical growth responses in stems and petioles of watercress (nasturtium officinale). New Phytol. 229, 140–155. doi: 10.1111/nph.16350
Pan, X., Welti, R., Wang, X. (2010). Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 5, 986–992. doi: 10.1038/nprot.2010.37
Potocka, I., Szymanowska-Pułka, J. (2018). Morphological responses of plant roots to mechanical stress. Ann. Bot. 122, 711–723. doi: 10.1093/aob/mcy010
Raskin, I., Kende, H. (1984). Role of gibberellin in the growth response of submerged deep water rice. Plant Physiol. 76, 947–950. doi: 10.1104/pp.76.4.947
Richter, G. L., Monshausen, G. B., Krol, A., Gilroy, S. (2009). Mechanical stimuli modulate lateral root organogenesis. Plant Physiol. 151, 1855–1866. doi: 10.1104/pp.109.142448
Sasidharan, R., Voesenek, L. A. C. J. (2015). Ethylene-mediated acclimations to flooding stress. Plant Physiol. 169, 3–12. doi: 10.1104/pp.15.00387
Sauter, M., Kende, H. (1992). Gibberellin-induced growth and regulation of the cell division cycle in deepwater rice. Planta 188, 362–368. doi: 10.1007/BF00192803
Shi, H., Liu, R., Xue, C., Shen, X., Wei, N., Deng, X. W., et al. (2016). Seedlings transduce the depth and mechanical pressure of covering soil using cop1 and ethylene to regulate ebf1/ebf2 for soil emergence. Curr. Biol. 26, 139–149. doi: 10.1016/j.cub.2015.11.053
Vervuren, P. J., Beurskens, S. M., Blom, C. W. (1999). Light acclimation, CO2 response and long-term capacity of underwater photosynthesis in three terrestrial plant species. Plant Cell Environ. 22, 959–968. doi: 10.1046/j.1365-3040.1999.00461.x
Vervuren, P. J., Blom, C. W., De Kroon, H. (2003). Extreme flooding events on the Rhine and the survival and distribution of riparian plant species. J. Ecol. 91, 135–146. doi: 10.1046/j.1365-2745.2003.00749.x
Voesenek, L. A. C. J., Sasidharan, R., Visser, E. J. W., Bailey-Serres, J. (2016). Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol. 209, 39–43. doi: 10.1111/nph.13775
Voesenek, L. A. C. J., Sasidharan, R., Weber, A. (2013). Ethylene-and oxygen signalling-drive plant survival during flooding. Plant Biol. 15, 426–435. doi: 10.1111/plb.12014
Wang, X., Komatsu, S. (2022). The role of phytohormones in plant response to flooding. Int. J. Mol. Sci. 23, 6383. doi: 10.3390/ijms23126383
Wang, X., Deng, Z., Zhang, W., Meng, Z., Chang, X., Lv, M. (2017). Effect of waterlogging duration at different growth stages on the growth, yield and quality of Cotton. PLoS One 12, e169029. doi: 10.1371/journal.pone.0169029
Wang, Q., Wang, L., Chandrasekaran, U., Luo, X., Zheng, C., Shu, K. (2021). ABA biosynthesis and signaling cascades under hypoxia stress. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.661228
Wu, H., Carrillo, J., Ding, J. (2017). Species diversity and environmental determinants of aquatic and terrestrial communities invaded by Alternanthera Philoxeroides. Sci. Total Environ. 581–582, 666–675. doi: 10.1016/j.scitotenv.2016.12.177
Yang, C., Yang, X., Zhang, X., Zhang, F., Zhou, C., Wang, Q., et al. (2019). Anatomical structures of alligator weed (Alternanthera Philoxeroides) suggest it is well adapted to the aquatic-terrestrial transition zone. Flora 253, 27–34. doi: 10.1016/j.flora.2019.02.013
You, W. H., Han, C. M., Fang, L. X., Du, D. L. (2016). Propagule pressure, habitat conditions and clonal integration influence the establishment and growth of an invasive clonal plant, Alternanthera Philoxeroides. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00568
Keywords: clonal plant, alligator weed, gibberellin, hydrostatic pressure, submergence times, submergence depth
Citation: Jing S, Ren X, Lin F, Niu H, Ayi Q, Wan B, Zeng B and Zhang X (2024) Stem elongation and gibberellin response to submergence depth in clonal plant Alternanthera philoxeroides. Front. Plant Sci. 15:1348080. doi: 10.3389/fpls.2024.1348080
Received: 01 December 2023; Accepted: 08 May 2024;
Published: 24 May 2024.
Edited by:
Wei Xue, Taizhou University, ChinaReviewed by:
Xiao Guo, Qingdao Agricultural University, ChinaPeiyu Zhang, Netherlands Institute of Ecology (NIOO-KNAW), Netherlands
Copyright © 2024 Jing, Ren, Lin, Niu, Ayi, Wan, Zeng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Zhang, enhwaW1tdW5Ac3d1LmVkdS5jbg==
†These authors have contributed equally to this work
 Shufang Jing
Shufang Jing Xinyi Ren
Xinyi Ren Feng Lin
Feng Lin Hangang Niu
Hangang Niu Qiaoli Ayi
Qiaoli Ayi Binna Wan
Binna Wan Bo Zeng
Bo Zeng Xiaoping Zhang1*
Xiaoping Zhang1*