- Jiangxi Provincial Key Laboratory of Plant Germplasm Resources Innovation and Genetic Improvement, Lushan Botanical Garden, Chinese Academy of Sciences, Jiujiang, China
Ralstonia solanacearum, the causal agent of bacterial wilt, is recognized as one of the most destructive vascular pathogens. Plant defense responses are gradually developed through long-term interactions with R. solanacearum. The plant cell wall integrity (CWI) system has evolved to initiate defense responses via a diverse array of plasma membrane-resident sensors. These defense responses result primarily from physical and chemical actions that counteract infection with R. solanacearum. The plant cell wall serves as a defensive barrier against the pathogen, including cellulose, hemicellulose, pectin, lignin, and suberin. Various modifications to the cell wall and multiple changes in its composition are employed by plants resistant to R. solanacearum. Physical confinement vertically or horizontally induced in xylem tissues is the most effective method of defense against R. solanacearum. The timely formation of tyloses and gels within the vessel lumen contributes to the suppression of R. solanacearum. In addition, the deposition of callose at the infected sites reinforces the cell wall, thereby preventing the further spread of R. solanacearum. Morphological modifications, such as the thickening of the pit membranes and the increased number of larger xylem vessels, play crucial roles in conferring resistance to R. solanacearum. Secondary metabolites act as phytoalexins used by plants against R. solanacearum. In this review, we discuss the strategies deployed by plants resistant to R. solanacearum. In particular, we outline the physical and chemical restrictions, as well as the tissue constraints, against the vascular pathogen.
1 Introduction
Soil-borne pathogens are a significant cause of crop losses in agricultural species, posing a threat to global agriculture and food security (McCann, 2020). As the causal agent of bacterial wilt, Ralstonia solanacearum is in the list of the most scientifically significant plant pathogens (Mansfield et al., 2012). This pathogen has an extremely broad host range and infects more than 250 plant species, including tomato, tobacco, potato, eggplant, and peanut (Genin, 2010; Genin and Denny, 2012; Peeters et al., 2013; Xiao et al., 2023). The R. solanacearum species complex (RSSC) comprises three distinct species—R. solanacearum, Ralstonia pseudosolanacearum, and Ralstonia syzygii—all of which share a core genome (Paudel et al., 2020). Among the virulent determinants of R. solanacearum, swimming/motility, the cell wall-degrading enzymes (CWDEs), the type III secretion system (T3SS), and exopolysaccharide (EPS) are critical for its pathogenicity (Valls et al., 2006; Milling et al., 2011; Coll and Valls, 2013; Vailleau and Genin, 2023). The bacteria gain entry into the root cortex through the root tips, wounds, or cracks at sites of lateral root emergence and subsequently invade the xylem vessels (Vailleau et al., 2007; Digonnet et al., 2012). R. solanacearum in the xylem vessels proliferate up to high cell densities, ultimately disrupting the water conductance and inducing wilting symptoms (Xue et al., 2020). Plants are persistently challenged by R. solanacearum during their entire growth period, but develop an innate immune system to counteract the threat (Martin et al., 2003; Jones and Dangl, 2006). The innate immune system of plants consists of two layers (Chisholm et al., 2006; Boller and Felix, 2009). The first layer is known as pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI), which recognize PAMPs by corresponding pattern recognition receptors (PRRs) in the plasma membrane. Recognition by plant PRRs initiates the downstream defense responses such as the production of reactive oxygen species (ROS), cytosolic Ca2+ burst, the activation of mitogen-activated protein kinases (MAPKs), and the expression of defense-related genes (Dodds and Rathjen, 2010; Zipfel, 2014). The other layer is effector-triggered immunity (ETI), which is based on the direct or indirect recognition of specific effectors by resistant proteins containing nucleotide-binding leucine-rich repeats (NB-LRRs). ETI produces faster, longer, and stronger responses than PTI, thereby quickly triggering a hypersensitive response (HR), i.e., an induced cell death (Stuart et al., 2013; Huet, 2014). ETI cooperates with PTI to protect plants from pathogenic attacks (Ngou et al., 2021). The two-layer immune system of plants initiates a series of resistant responses at the cellular and tissue levels (Planas-Marquès et al., 2020; Shi et al., 2023). The regulatory responses are involved in tissue constraints, modifications of the cell wall, inducible defenses, and resistant metabolites to counteract the invasion of R. solanacearum (Kashyap et al., 2021; Shi et al., 2023). This review focuses on a summary of the strategies deployed by plants resistant to R. solanacearum, but also provides an overview of the regulatory mechanisms against bacterial wilt disease.
2 Different tissue constraints deployed by plants
R. solanacearum infects the roots through wounds or natural openings and rapidly multiplies in the xylem vessels (Schell, 2000; Mansfield et al., 2012). The bacteria accumulate in xylem ducts, potentially obstructing the water flow and eventually causing the plant to wilt (Hikichi et al., 2017; Mori et al., 2018). Grafting tests have confirmed that Hawaii 7996 rootstock is able to restrict R. solanacearum up to the stem in tomato (Nakaho et al., 2004; Truong et al., 2015). In susceptible tomato roots, R. solanacearum diffuses more rapidly from the cortex to the vascular system when compared with resistant Hawaii 7996 plants (Caldwell et al., 2017). Upon infection with R. solanacearum, petunia forms more lateral root structures. However, these elongated lateral roots do not contribute to resistance against the pathogen (Zolobowska and Van Gijsegem, 2006). Resistance to R. solanacearum relies on four different steps (Figure 1), which limit the bacterial spread: i) invasion of the plant root; ii) vertical movement upward to the stem; iii) circular passage from vessel to vessel; and iv) radial movement from xylem vessels into the pith/cortex (Planas-Marquès et al., 2020). Morphological changes between susceptible and resistant roots are observed upon infection with R. solanacearum (Xue et al., 2020). Primary root growth is significantly inhibited after infection with R. solanacearum (Digonnet et al., 2012; Xue et al., 2020). An increased number of larger xylem vessels within resistant roots are observed when compared with susceptible plants via histological staining (Caldwell et al., 2017). It is credible that the larger diameter of xylem vessels impedes bacterial colonization. The ability to limit R. solanacearum spreading up to tobacco stem is one of the effective strategies used by plants resistant to the pathogen (Bittner et al., 2016). The resistant rootstock cultivar LS-89, which limits the movement of R. solanacearum between xylem vessels, exhibits thickened pit membranes (Nakaho et al., 2000). R. solanacearum-resistant rootstocks promote a significant effect on yield in field trials compared with the non-grafted “BHN 602” (McAvoy et al., 2012). Bioluminescence imaging arrays have demonstrated that the colonization and multiplication of R. solanacearum are confined within plant roots; however, a limited number of bacteria are detected in stem ducts (Ferreira et al., 2017). After inoculation with R. solanacearum, Solanum dulcamara exhibits delayed symptomatology; moreover, the bacterial progression is notably restricted within the roots (Sebastià et al., 2021). The distinct morphology of the stem plays vital roles in limiting bacterial colonization and movement (Planas-Marquès et al., 2020). Once R. solanacearum penetrates the vascular cylinder of susceptible plants, these bacteria are able to proliferate rapidly (Shi et al., 2023). When the R. solanacearum populations reach 5 × 108 CFU/g, approximately half of the xylem vessels from the stem are clogged, thus correlating with the onset of wilt symptoms (Ingel et al., 2022). When compared with the susceptible cultivar Ponderosa, R. solanacearum is merely observed in the primary xylem tissues and less in the secondary xylem of resistant LS-89 plants (Nakaho et al., 2000; Ishihara et al., 2012). In addition, thickening of the pit membranes is observed in the LS-89 stems using scanning electron microscopy (Nakaho et al., 2000; Ishihara et al., 2012). These studies demonstrate that morphological constraints contribute to resistance to R. solanacearum.
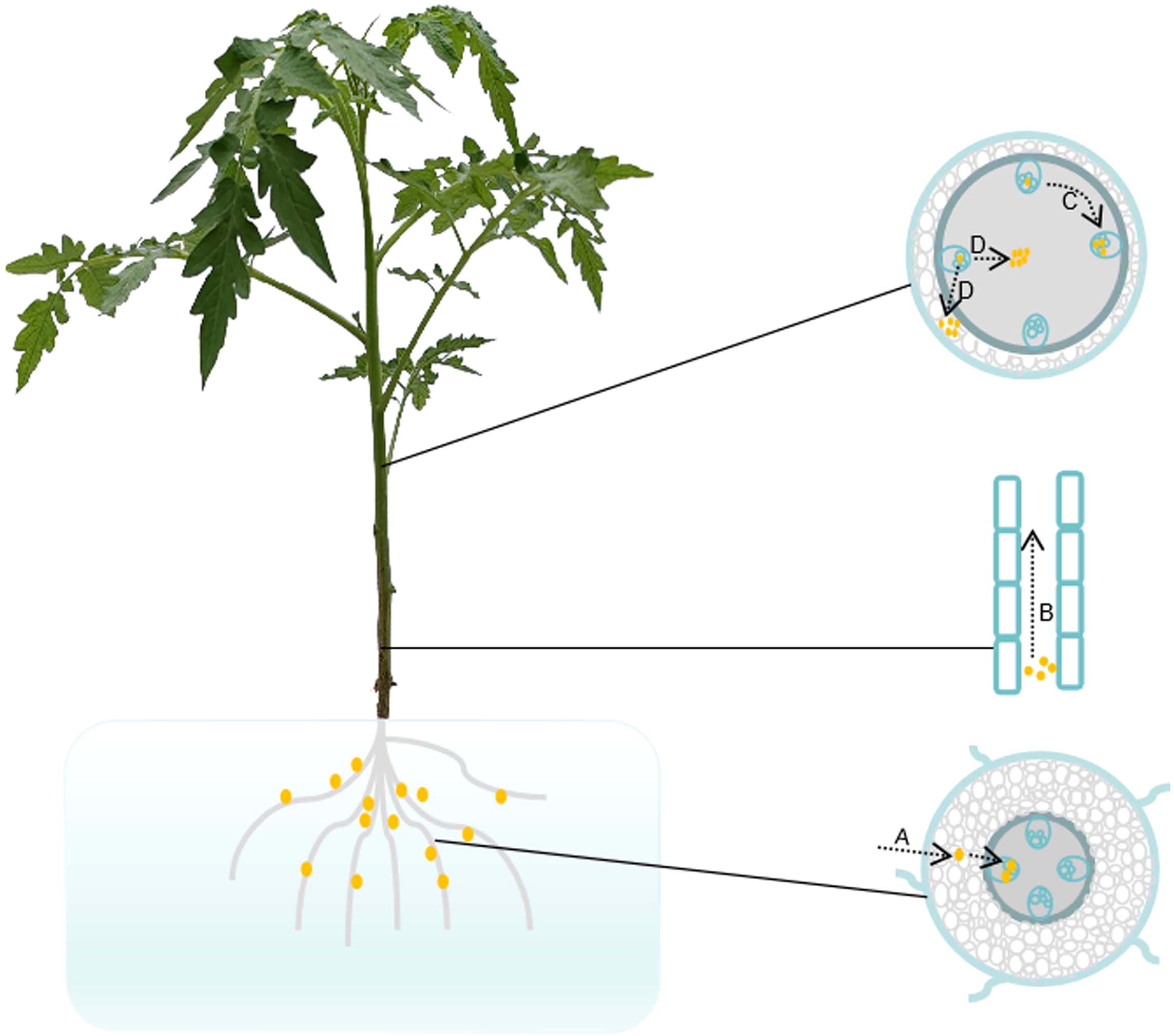
Figure 1. Pathogenic progress of Ralstonia solanacearum in tomato plant. (A) Tomato roots infected with R. solanacearum. R. solanacearum enters the root cortex through the tips, wounds, or cracks at infected sites, subsequently invading and proliferating within the root vasculature. (B) Vertical movement of R. solanacearum upward to the stem. R. solanacearum in the roots spreads vertically up to the stem vascular bundles. (C) Circular passage of R. solanacearum between xylem vessels. R. solanacearum in the xylem ducts moves circularly and proliferates among the xylem vessels. (D) Radial/apoplastic movements of R. solanacearum into the pith/cortex tissue. R. solanacearum spreads in the intercellular spaces, multiplies out of the xylem vessels into the pith and cortex, and finally causes the plant to wilt. Brown dots represent R. solanacearum.
3 Structural barriers induced by R. solanacearum
The number of R. solanacearum in the xylem vessels of plants increase up to a high density, subsequently causing plant wilting. The vascular barriers induced by R. solanacearum comprise one of the important defenses against bacterial wilt disease. Among the inducible defenses, we focused on the formation of tyloses and the deposition of gels and callose against the invasion of R. solanacearum (Figure 2A). Parenchyma cells protrude into the xylem vessels through the pit membranes and form tyloses to limit the spread of the pathogen. In contrast, gels and callose are deposited in the xylem vessels in response to pathogenic invasion. The tyloses and gels occlude vascular vessels to restrict the vertical progression of R. solanacearum. The timely formation of physical barriers upon pathogenic perception results in the confinement of R. solanacearum at the infected vessel and effectively prevents bacterial movement.
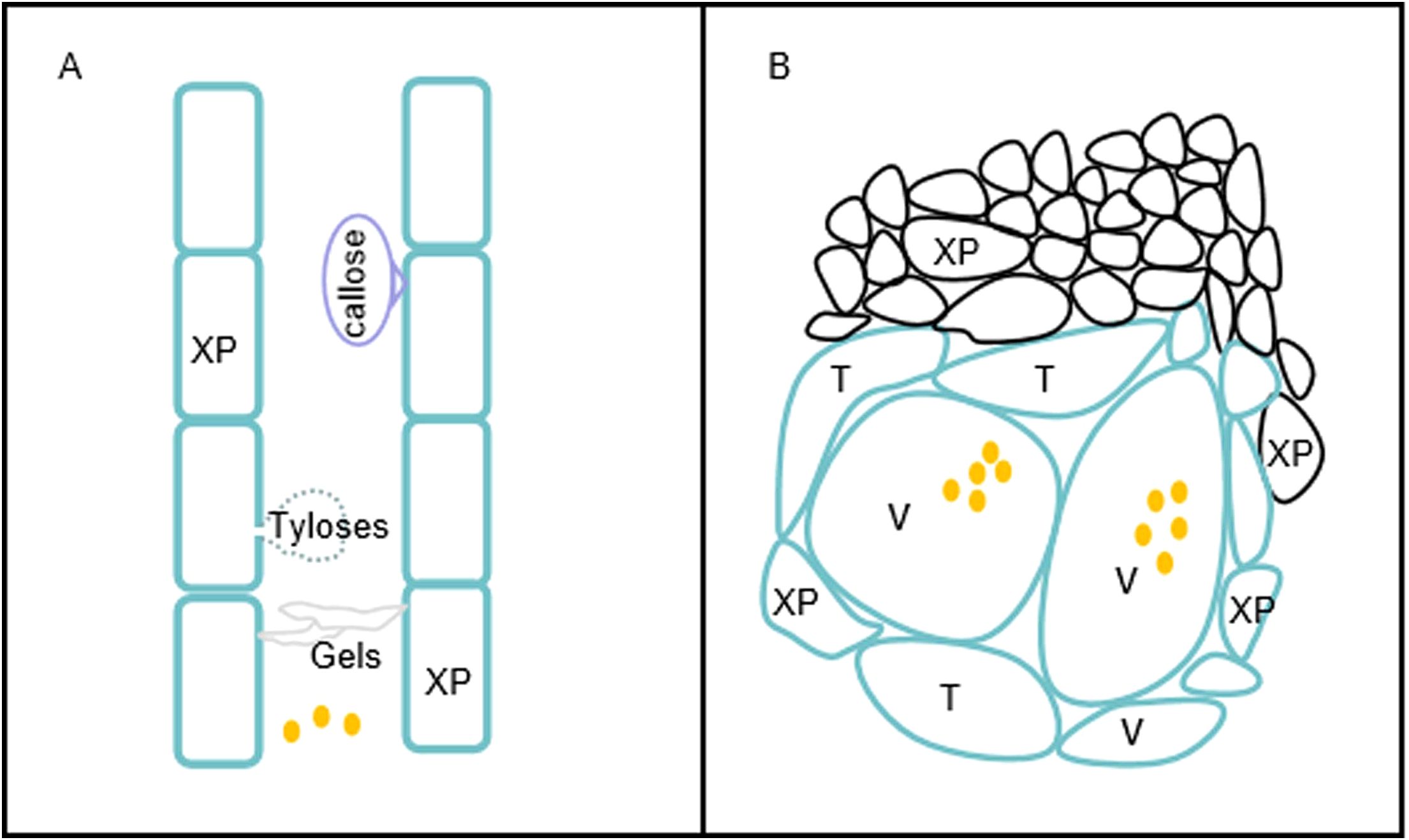
Figure 2. Physical barriers induced by Ralstonia solanacearum. (A) Vertical restriction of the movement of R. solanacearum in the xylem duct. Resistant plants produce tyloses, gels, and callose that restrict the vertical movement of R. solanacearum. (B) Horizontal restriction of R. solanacearum spread in xylem vessels. Vascular coating with lignin and suberin and reinforcement of the xylem vessels (V), the surrounding xylem parenchyma (XP), and tracheids (T) impede the horizontal spread of R. solanacearum to the surrounding tissues. Brown dots represent R. solanacearum.
3.1 Formation of tyloses
Tyloses are balloon-like structures of parenchyma cells that grow out into the lumen of the xylem vessels (Lowe-Power et al., 2018). These outgrowths form a physical barrier and prevent R. solanacearum from spreading. In order to prevent the pathogenic spread, the tyloses in tomato varieties are induced at the infected sites (Grimault et al., 1994; Leśniewska et al., 2017). A lower density of tyloses is found in R. solanacearum-susceptible tomato plants using light and electron microscopy (Grimault et al., 1994). In addition, the formation of tyloses in susceptible stems is significantly delayed (Grimault et al., 1994). However, extensive formation of tyloses in Pierce’s disease (PD)-susceptible grapevines occludes the xylem vessels and impairs water conductance (Sun et al., 2013). Furthermore, the tyloses in PD-resistant grapevines develop specifically and emerge at the sites of inoculation (Sun et al., 2013). The process of tylosis formation is precisely and tightly regulated by hormones such as auxin, ethylene (ET), and jasmonate (JA) (Kashyap et al., 2021; Shi et al., 2023). Grapevine plants treated with ET exhibit an increased density of tyloses, suggesting that ET is required for tylosis formation (Pérez-Donoso et al., 2007). Similarly, ET inhibitors abolish tylosis formation in grape stems, suggesting that ET is essential for the formation of tyloses (Sun et al., 2008). JA acts synergistically with ET to promote tylosis formation (Leśniewska et al., 2017). These findings suggest that the timely formation of tyloses is essential for resistance against pathogenic attacks. Wide xylem vessels that allow for concentrated tyloses are less efficient in forming compartmentalization, thereby conferring susceptibility of grapevine to the vascular pathogen Phaeomoniella chlamydospora when compared with xylem vessels with a narrow diameter (Pouzoulet et al., 2017). In addition to pathogenic invasion, tyloses are formed in response to abiotic stresses, such as freezing and wounding (Kashyap et al., 2021; Shi et al., 2023). Tyloses produce organs of antimicrobial compounds in addition to serving as a structural barrier. Compounds in the tyloses of tomato plants such as elemental S, which is detected using gas chromatography–mass spectroscopy (GC-MS), act as fungicides to inhibit spore germination of the fungal pathogen Verticillium dahliae (Williams et al., 2002).
3.2 Deposition of gels
The deposition of gels within the lumen of xylem vessels is observed during the invasion of vascular pathogens and functions as an inducible defense (Shi et al., 2023). The secretion of gels in xylem vessels often coincides with the formation of tyloses. Pectin constitutes the primary component of the gels, which contain antimicrobial compounds such as elemental sulfur and phytoalexins (Clérivet et al., 2000; Williams et al., 2002). Gels appear as translucent fibers and contain continuous pectin-rich substances that obstruct the xylem vessels (Sun et al., 2008). Both xylem parenchyma cells and tyloses secrete the gels, which are then transported through pit membranes into vessel elements (Bishop and Cooper, 1984; Sun, 2022). Furthermore, the gels are strengthened by the cross-linking of lignin and phenolic compounds, thereby forming strong physical barriers that impede the movement of R. solanacearum (Kashyap et al., 2021). Vascular gels induced by R. solanacearum are associated with the defense response (Kashyap et al., 2021). The formation of vascular gels is induced in R. solanacearum-resistant tomato cultivars, suggesting a correlation with resistance to R. solanacearum (Bishop and Cooper, 1984; Kim et al., 2016).
3.3 Deposition of callose
Callose is composed of hundreds of polysaccharides linked by β-1,3 glycosidic bond (Kashyap et al., 2021). The homopolysaccharide that is deposited between the plasma membrane and the cell wall plays a crucial role in biological processes such as plant development and stress response. Callose strengthens the cell wall structure by increasing its rigidity at the infected site and by diminishing the activity of pathogen-secreted CWDEs (Wang et al., 2021). Wang et al. (2021) speculated that the deposition of callose acts as one of the early defense responses of plants against pathogenic invasion (Wang et al., 2021). Numerous studies have shown that callose deposition is correlated with the defense of plants against bacterial, fungal, and viral infection. Upon treatment with bacterial pathogens such as Xanthomonas campestris pv. vesicatoria and Pseudomonas syringae pv. phaseolicola, callose is deposited to reinforce the plant cell wall at the infected sites (Bestwick et al., 1995; Brown et al., 1998; Wang et al., 2021). Callose is also induced by PAMPs including flagellin (flg22), elongation factor Tu (EF-Tu; elf18), chitin, and chitosan (Couto and Zipfel, 2016; Zipfel and Oldroyd, 2017). Barley papillae, which contain callose and high concentrations of cellulose, serve as physical barriers preventing the penetration of the fungus Blumeria graminis f. sp. hordei (Chowdhury et al., 2014). During the incompatible interaction between soybean and soybean mosaic virus (SMV), callose is deposited in the plasmodesmata to restrict viral movement between cells (Li et al., 2012). The exogenous application of salicylic acid (SA) induces callose deposition in the plasmodesmata, indicating that SA plays a crucial role in the deposition of callose (Wang et al., 2013). Potato plants overexpressing NACb4 are capable of inducing callose deposition and enhancing tolerance against R. solanacearum (Chang et al., 2020). The endophytic bacterium has the potential to prime callose deposition upon R. pseudosolanacearum GMI1000 infection (Rodriguez et al., 2019). It was found that R. solanacearum-resistant potatoes display similar callose deposition density to plants not inoculated with the pathogen, suggesting that the callose preexists in resistant plants (Ferreira et al., 2017). Ferreira et al. (2017) proposed that the preexisting callose is deposited into the cell wall of plants resistant to R. solanacearum.
4 Alterations of plant cell wall resistant to R. solanacearum
The plant cell wall serves as a crucial barrier against pathogenic invasion (Malinovsky et al., 2014). Pathogens secrete CWDEs that hydrolyze the linkages between glycan moieties, thereby breaking down the barrier. The development of the plant cell wall is a dynamic process by which the synthesis and modifications are integrated to regulate the resistance to pathogens (Bacete et al., 2018). The composition of the plant cell wall that is integral to the defense against pathogens mainly comprises cellulose, hemicellulose, pectin, lignin, and suberin (Shi et al., 2023). The plant cell wall integrity (CWI) has a significant effect on abiotic and biotic stresses (Miedes et al., 2014; Kesten et al., 2017; Bacete et al., 2018). Modifications to the cell wall have been shown to affect pathogen resistance (Malinovsky et al., 2014). Plants have evolved a specialized mechanism to maintain CWI, thereby providing effective resistance to diseases (Bellincampi et al., 2014; Malinovsky et al., 2014). When the CWI of plants is compromised, the CWI system monitors the state of the cell wall and subsequently activates innate immune responses (Shi et al., 2023). The oligogalacturonides (OGAs) derived from pectic homogalacturonan (HGA) are recognized by wall-associated kinases (WAKs) that sense the integrity of pectin (Ferrari et al., 2013). The xylem vessels of resistant plants exhibit reinforced pit membranes, thereby impeding the movement of pathogens between vessels and the vessel/parenchyma (Choat et al., 2008; Sun et al., 2011; Kashyap et al., 2021). Reinforcement of the cell wall is able to limit the horizontal movement of R. solanacearum between xylem vessels (Kashyap et al., 2021). Moreover, the composition and the structure of xylem pit membranes are altered in resistant plants (Kashyap et al., 2021). When compared with PD-susceptible plants, the pit membranes of resistant grapevine are found to lack fucosylated xyloglucans and weakly methyl-esterified homogalacturonans (ME-HGs) and to encompass a small amount of heavily ME-HGs (Sun et al., 2011).
Cellulose, which is synthesized by plasma membrane-localized cellulose synthase complexes (CSCs), plays an important role in the defense against pathogenic attacks (Polko and Kieber, 2019). Each unit of the CSC is composed of at least three different cellulose synthases (CESAs) (Somerville, 2006). CESA1, CESA3, and CESA6 are required in the formation of cellulose in primary walls, while CESA4, CESA7, and CESA8 are responsible for the production of secondary wall cellulose (Hernandez-Blanco et al., 2007; Endler and Persson, 2011). Arabidopsis mutants deficient in CESA4/7/8 exhibit increased resistance to R. solanacearum (Hernandez-Blanco et al., 2007; Wan et al., 2021). The loss of function of MYB46, which positively regulates the expression of CESA4/7/8, enhances the resistance of Arabidopsis plants to Botrytis cinerea (Wan et al., 2021). There are a large number of cases showing that the inhibition of cellulose synthesis results in increased susceptibility to plant diseases (Shi et al., 2023). When cellulose synthase-like D2 is silenced, transgenic plants show enhanced susceptibility to powdery mildew (Douchkov et al., 2016). The transcription factor WRKY53 promotes the expression of three secondary cell wall-related cellulose synthase genes, thereby conferring rice resistance to Xanthomonas oryzae pv. oryzae (Xoo) by strengthening the sclerenchyma cell walls surrounding the xylem vessel (Xie et al., 2021).
Hemicellulose is composed of polysaccharides with β-1,4-linked backbones of xylose, mannose, and glucose (Malinovsky et al., 2014). Changes in the content and the acetylation of hemicellulose in the cell wall confer plants resistance to phytopathogenic microbes (Bacete et al., 2018). In contrast to wild-type plants, Arabidopsis det3 and irx6 mutants with increased levels of xylose confer enhanced resistance to the fungus Plectosphaerella cucumerina (Brown et al., 2005; Wan et al., 2021). Sufficient evidence suggests that the degree of xylan acetylation affects the resistance of plants to fungal and bacterial pathogens (Shi et al., 2023). The Arabidopsis mutant rwa2 with decreased levels of xylan acetylation exhibits enhanced tolerance to B. cinerea (Manabe et al., 2011). Once pathogens breach the cutin layer in plants, pectin functions as a barrier to impede invasion (Wan et al., 2021). The altered pectin biosynthetic pathway in Arabidopsis thaliana results in the susceptibility of plants to P. syringae and B. cinerea (Zhang et al., 2016). It is suggested that changes in the pectin content or its modification plays a crucial role in plant resistance to pathogenic attacks (Bacete et al., 2018). Pectin methylesterases (PMEs), whose activity is controlled by protein inhibitors (pectin methylesterase inhibitors, PMEIs), regulate the degree of pectin methyl esterification (Lionetti et al., 2017). A highly methylated pectin is associated with strong tolerance to CWDEs (Wan et al., 2021). Immunological staining revealed that the R. solanacearum-resistant Hawaii 7996 cultivar exhibits a higher degree of HGA methyl esterification compared with the susceptible cultivar Wva700 (Wydra and Beri, 2006). The overexpression of PMEIs confers plants enhanced resistance to pathogens (Shi et al., 2023). The silencing of CaPMEI1, which encodes a PMEI protein, increases the susceptibility of pepper to X. campestris pv. vesicatoria (An et al., 2008). Polygalacturonases (PGs) depolymerize the HGA, thereby compromising the CWI. OGAs are released from the HGA backbone and function as elicitors to trigger the plant defense responses (Malinovsky et al., 2014). The wall-associated kinase 1 (WAK1) in A. thaliana has been identified as the OGA receptor, suggesting an OGA-induced immunity (Brutus et al., 2010). There are a few demonstrations of the involvement of pectin acetylation in plant biotic stresses. When pectin acetylesterases (CsPAEs) are silenced, transgenic citrus plants show increased resistance to bacterial canker disease (Li et al., 2020).
Lignification is capable of increasing the mechanical strength of the plant cell wall and improving the resistance of plants to CWDE-secreting pathogens (Kashyap et al., 2022). The overexpression of genes involved in lignin biosynthesis confers tomato resistance to bacterial wilt disease (Kashyap et al., 2022). Transcriptomic analysis indicated that the lignin biosynthesis genes of tomato are upregulated upon infection with R. solanacearum, suggesting a relationship between R. solanacearum resistance and lignin biosynthesis (Ishihara et al., 2012). When the phenylalanine ammonia lyase gene (PAL1) is knocked out, the mutant exhibits a significant reduction in lignin accumulation and a weakened resistance to P. syringae (Rohde et al., 2004; Huang et al., 2010). The knockout of the transcription factor MYB15 results in decreased levels of lignin, thereby enhancing the susceptibility of Arabidopsis to P. syringae (Chezem et al., 2017). There are a few examples showing that an increased lignin content in the cell wall renders plants susceptible to pathogens. The NAC transcription factor RD26 positively regulates the resistance of Arabidopsis against R. solanacearum by inhibiting lignin biosynthesis (Wang et al., 2025). A reduced lignin content is found in GhMYB4-overexpressing cotton, enhancing the plant resistance to V. dahliae (Xiao et al., 2021). This is explained by the decreased lignification changing the CWI and amplifying the release of OGAs, thereby strengthening plant immunity. Suberin is a lipid–phenolic heteropolyester that is deposited between the plasma membrane and the cell wall (Shi et al., 2023). In addition to mitigating water loss, suberin acts as a barrier restricting the horizontal colonization of pathogens (Kashyap et al., 2022). The involvement of abscisic acid (ABA) and ET in suberin formation highlights a significant correlation between plant phytohormones and immune responses (Cottle and Kolattukudy, 1982; Leśniewska et al., 2017). Suberin, as a vascular coating, is induced upon infection with R. solanacearum, thereby impeding the spread of pathogens in tomato plants (Shi et al., 2023). Suberin deposition in the xylem vessels functions as a barrier that contributes to pathogenic resistance (Kashyap et al., 2022). For example, suberin reinforcement in paravascular parenchyma cells prevents the movement of the fungus P. chlamydospora from one vessel to the adjacent vessel (Pouzoulet et al., 2013, 2017).
5 Plant metabolites involved in the resistance against R. solanacearum
Plants produce a variety of secondary metabolites that act as protectors inhibiting pathogenic growth and reproduction (Yang et al., 2021b). The metabolites known as phytoalexins exhibit diverse structures and antimicrobial activities (Kumar et al., 2023). The phytoalexins include alkaloids, isoprenoids, and phenylpropanoids (Kumar et al., 2023). In tobacco, coumarin diminishes the activity of acyl homoserine lactone, antagonizes the regulatory proteins of quorum sensing (QS), and eventually restricts the adhesion and colonization of R. solanacearum (Qais et al., 2021). The plant secondary metabolite daphnetin weakens the virulence of R. solanacearum in tobacco through inhibiting the EPS production and biofilm formation (Yang et al., 2021b). 6-Methylcoumarin functions as an antibacterial metabolite by disrupting the cell division of R. solanacearum (Yang et al., 2021a). Three root exudates from mulberry plants, including erucamide, oleamide, and camphor bromide, inhibit the growth of R. Solanacearum by inducing oxidative stress (Li et al., 2024). Plant-derived hydroxycoumarins are recognized as phytoalexins that defend against attacks from R. solanacearum (Yang et al., 2016). Caffeic acid derived from root exudates inhibits the biofilm formation of R. solanacearum through repressing the expression of the lecM and epsE genes (Li et al., 2021). In addition to its antimicrobial activity, caffeic acid effectively activates phenylalanine ammonia lyase (PAL) and peroxidase (POD), which subsequently regulate the accumulation of lignin and hydroxyproline (Li et al., 2021). Caffeic acid is therefore engineered as a potential and effective antibacterial agent for the control of bacterial wilt disease. Biochemical analysis showed that phytoalexins exhibit various antibacterial activities such as inhibition of biofilm and formation of EPS, damage to the cell wall, and disruption of bacterial cell division.
Aside from phytoalexins, plants combat R. solanacearum by reducing the production of metabolites used for pathogen virulence (Shen et al., 2020). R. solanacearum utilizes plant-derived metabolites to promote the production of virulent factors (Shen et al., 2020). R. solanacearum-resistant tomato varieties impede bacterial reproduction by diminishing the production of l-glutamic acid (Shen et al., 2020). Metabolites in the xylem sap that are required as carbon or nitrogen sources for R. solanacearum growth, such as putrescine, alpha-d-glucopyranoside, and arabinitol, are significantly decreased in the resistant cultivar K326, suggesting a defense strategy of the plant against R. solanacearum (Yang et al., 2022).
6 Discussion
R. solanacearum-resistant plants predominantly depend on inducible structural barriers. These barriers, which are activated in response to R. solanacearum infection, include the formation of tyloses, gels, and callose and the reinforcement of the cell wall. These defense responses in R. solanacearum-resistant plants are primarily regulated by phytohormones, particularly SA, JA, and ET. ET acts synergistically with JA to promote the formation of tyloses in xylem vessels, whereas SA abolishes the JA-induced formation of tyloses. Furthermore, ET and JA play a significant role in reinforcing the cell walls deposited with lignin and suberin (Kashyap et al., 2021; Wan et al., 2021).
In contrast to that in resistant plants, the bacteria proliferate more rapidly within the xylem vessels of susceptible plants. The timely establishment of these barriers is critically important for confining the bacteria at the infected sites. In addition, plants use secondary metabolites as part of their resistance strategy against R. solanacearum. Physicochemical defense responses, both vertical and horizontal, are key strategies employed by plants to prevent bacterial wilt disease (Figure 2). Horizontal reinforcement of the cell wall and vascular coating with lignin and suberin restrict the bacterial movement between xylem vessels, thereby preventing plant wilting. Furthermore, plants resistant to R. solanacearum exhibit morphological changes, suggesting that constitutive barriers contribute to resistance to the pathogen. As crop yield often decreases in highly resistant cultivars due to resource allocation trade-offs, plant molecular biologists frequently release resistant germplasms. The efficient utilization of resistant traits is crucial for the development of R. solanacearum-resistant cultivars. The CRISPR-Cas9 technology has been efficiently employed to produce transgenic crops resistant to R. solanacearum, including tomato, peanut, and potato (Kashyap et al., 2022). The cultivation of resistant cultivars is an economical and effective strategy to mitigate bacterial wilt disease. For instance, tomato plants expressing the NPR1 gene from A. thaliana exhibit enhanced resistance to bacterial wilt disease (Lin et al., 2004). Moreover, grafting the scion of crop onto an R. solanacearum-resistant rootstock represents an effective management strategy for the control of bacterial wilt disease. Understanding the resistance mechanisms employed by plants against R. solanacearum will not only provide valuable insights for related research but also facilitate the breeding of R. solanacearum-resistant cultivars.
Author contributions
DX: Writing – original draft, Writing – review & editing. WW: Writing – review & editing. DK: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Jiujiang Basic Research Program Project (S2024KXJJ0001) and Lushan Botanical Garden Basal Research Fund (2023ZWZX11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, S. H., Sohn, K. H., Choi, H. W., Hwang, I. S., Lee, S. C., and Hwang, B. K. (2008). Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228, 61–78. doi: 10.1007/s00425-008-0719-z
Bacete, L., Melida, H., Miedes, E., and Molina, A. (2018). Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J. 93, 614–636. doi: 10.1111/tpj.13807
Bellincampi, D., Cervone, F., and Lionetti, V. (2014). Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00228
Bestwick, C. S., Bennett, M. H., and Mansfield, J. W. (1995). Hrp mutant of Pseudomonas syringae pv phaseolicola induces cell wall alterations but not membrane damage leading to the hypersensitive reaction in lettuce. Plant Physiol. 108, 503–516. doi: 10.1104/pp.108.2.475
Bishop, C. D. and Cooper, R. M. (1984). Ultrastructure of vascular colonization by fungal wilt pathogens. II. Invasion Resist. Cultiv. Physiol. Plant Pathol. 24, 277–289. doi: 10.1016/0048-4059(84)90002-X
Bittner, R. J., Arellano, C., and Mila, A. L. (2016). Effect of temperature and resistance of tobacco cultivars to the progression of bacterial wilt, caused by Ralstonia solanacearum. Plant Soil 408, 299–310. doi: 10.1007/s11104-016-2938-6
Boller, T. and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Brown, I., Trethowan, J., Kerry, M., Mansfield, J., and Bolwell, G. P. (1998). Localization of components of the oxidative cross-linking of glycoproteins and of callose synthesis in papillae formed during the interaction between non-pathogenic strains of Xanthomonas campestris and French bean mesophyll cells. Plant J. 15, 333–343. doi: 10.1046/j.1365-313X.1998.00215.x
Brown, D. M., Zeef, L. A., Ellis, J., Goodacre, R., and Turner, S. R. (2005). Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17, 2281–2295. doi: 10.1105/tpc.105.031542
Brutus, A., Sicilia, F., Macone, A., Cervone, F., and De Lorenzo, G. (2010). A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. 107, 9452–9457. doi: 10.1073/pnas.1000675107
Caldwell, D., Kim, B. S., and Iyer-Pascuzzi, A. S. (2017). Ralstonia solanacearum differentially colonizes roots of resistant and susceptible tomato plants. Phytopathology 107, 528–536. doi: 10.1094/PHYTO-09-16-0353-R
Chang, Y., Yu, R., Feng, J., Chen, H., Eri, H., and Gao, G. (2020). NAC transcription factor involves in regulating bacterial wilt resistance in potato. Funct. Plant Biol. 47, 925–936. doi: 10.1071/FP19331
Chezem, W. R., Memon, A., Li, F. S., Weng, J. K., and Clay, N. K. (2017). SG2-type R2R3-MYB transcription factor MYB15 controls defense-induced lignification and basal immunity in Arabidopsis. Plant Cell 29, 1907–1926. doi: 10.1105/tpc.16.00954
Chisholm, S. T., Coaker, G., Day, B., and Staskawicz, B. J. (2006). Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 124, 803–814. doi: 10.1016/j.cell.2006.02.008
Choat, B., Cobb, A. R., and Jansen, S. (2008). Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol. 177, 608–626. doi: 10.1111/j.1469-8137.2007.02317.x
Chowdhury, J., Henderson, M., Schweizer, P., Burton, R. A., Fincher, G. B., and Little, A. (2014). Differential accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp. Hordei. New Phytol. 204, 650–660. doi: 10.1111/nph.12974
Clérivet, A., Déon, V., Alami, I., Lopez, F., Geiger, J. P., and Nicole, M. (2000). Tyloses and gels associated with cellulose accumulation in vessels are responses of plane tree seedlings (Platanus×acerifolia) to the vascular fungus Ceratocystis fimbriata f. sp platani. Trees 15, 25–31. doi: 10.1007/s004680000063
Coll, N. S. and Valls, M. (2013). Current knowledge on the Ralstonia solanacearum type III secretion system. Microb. Biotechnol. 6, 614–620. doi: 10.1111/1751-7915.12056
Cottle, W. and Kolattukudy, P. E. (1982). Abscisic acid stimulation of suberization: induction of enzymes and deposition of polymeric components and associated waxes in tissue cultures of potato tuber. Plant Physiol. 70, 775–780. doi: 10.1104/pp.70.3.775
Couto, D. and Zipfel, C. (2016). Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. doi: 10.1038/nri.2016.77
Digonnet, C., Martinez, Y., Denancé, N., Chasseray, M., Dabos, P., Ranocha, P., et al. (2012). Deciphering the route of Ralstonia solanacearum colonization in Arabidopsis thaliana roots during a compatible interaction: focus at the plant cell wall. Planta 236, 1419–1431. doi: 10.1007/s00425-012-1694-y
Dodds, P. N. and Rathjen, J. P. (2010). Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11, 539–548. doi: 10.1038/nrg2812
Douchkov, D., Lueck, S., Hensel, G., Kumlehn, J., Rajaraman, J., Johrde, A., et al. (2016). The barley (Hordeum vulgare) cellulose synthase-like D2 gene (HvCslD2) mediates penetration resistance to host-adapted and nonhost isolates of the powdery mildew fungus. New Phytol. 212, 421–433. doi: 10.1111/nph.14065
Endler, A. and Persson, S. (2011). Cellulose synthases and synthesis in Arabidopsis. Mol. Plant 4, 199–211. doi: 10.1093/mp/ssq079
Ferrari, S., Savatin, D. V., Sicilia, F., Gramegna, G., Cervone, F., and Lorenzo, G. D. (2013). Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00049
Ferreira, V., Pianzzola, M. J., Vilaró, F. L., Galván, G. A., Tondo, M. L., Rodriguez, M. V., et al. (2017). Interspecific potato breeding lines display differential colonization patterns and induced defense responses after Ralstonia solanacearum infection. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01424
Genin, S. (2010). Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 187, 920–928. doi: 10.1111/j.1469-8137.2010.03397.x
Genin, S. and Denny, T. P. (2012). Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 50, 67–89. doi: 10.1146/annurev-phyto-081211-173000
Grimault, V., Gélie, B., Lemattre, M., Prior, P., and Schmit, J. (1994). Comparative histology of resistant and susceptible tomato cultivars infected by Pseudomonas solanacearum. Physiol. Mol. Plant Pathol. 44, 105–123. doi: 10.1016/S0885-5765(05)80105-5
Hernandez-Blanco, C., Feng, D. X., Hu, J., Sanchez-Vallet, A., Deslandes, L., Llorente, F., et al. (2007). Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 19, 890–903. doi: 10.1105/tpc.106.048058
Hikichi, Y., Mori, Y., Ishikawa, S., Hayashi, K., Ohnishi, K., Kiba, A., et al. (2017). Regulation involved in colonization of intercellular spaces of host plants in Ralstonia solanacearum. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00967
Huang, J., Gu, M., Lai, Z., Fan, B., Shi, K., Zhou, Y. H., et al. (2010). Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 153, 1526–1538. doi: 10.1104/pp.110.157370
Huet, G. (2014). Breeding for resistances to Ralstonia solanacearum. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00715
Ingel, B., Caldwell, D., Duong, F., Parkinson, D. Y., McCulloh, K. A., Iyer-Pascuzzi, A. S., et al. (2022). Revisiting the source of wilt symptoms: X-ray microcomputed tomography provides direct evidence that Ralstonia biomass clogs xylem vessels. PhytoFrontiers™ 2, 41–51. doi: 10.1101/2021.03.19.436187
Ishihara, T., Mitsuhara, I., Takahashi, H., and Nakaho, K. (2012). Transcriptome analysis of quantitative resistance-specific response upon Ralstonia solanacearum infection in tomato. PloS One 7, e46763. doi: 10.1371/journal.pone.0046763
Jones, J. D. and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Kashyap, A., Jiménez-Jiménez, A. L., Zhang, W., Capellades, M., Srinivasan, S., Laromaine, A., et al. (2022). Induced ligno-suberin vascular coating and tyramine-derived hydroxycinnamic acid amides restrict Ralstonia solanacearum colonization in resistant tomato. New Phytol. 234, 1411–1429. doi: 10.1111/nph.17982
Kashyap, A., Planas-Marquès, M., Capellades, M., Valls, M., and Coll, N. S. (2021). Blocking intruders: inducible physico-chemical barriers against plant vascular wilt pathogens. J. Exp. Bot. 72, 184–198. doi: 10.1093/jxb/eraa444
Kesten, C., Menna, A., and Sánchez-Rodríguez, C. (2017). Regulation of cellulose synthesis in response to stress. Curr. Opin. Plant Biol. 40, 106–113. doi: 10.1016/j.pbi.2017.08.010
Kim, S. G., Hur, O. S., Ro, N. Y., Ko, H. C., Rhee, J. H., Sung, J. S., et al. (2016). Evaluation of resistance to Ralstonia solanacearum in tomato genetic resources at seedling stage. Plant Pathol. J. 32, 58–64. doi: 10.5423/PPJ.NT.06.2015.0121
Kumar, S., Korra, T., Thakur, R., Arutselvan, R., Kashyap, A. S., Nehela, Y., et al. (2023). Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 8, 100154. doi: 10.1016/j.stress.2023.100154
Leśniewska, J., Öhman, D., Krzesłowska, M., Kushwah, S., Barciszewska-Pacak, M., Kleczkowski, L. A., et al. (2017). Defense responses in aspen with altered pectin methylesterase activity reveal the hormonal inducers of tyloses. Plant Physiol. 173, 1409–1419. doi: 10.1104/pp.16.01443
Li, Q., Fu, J., Qin, X., Yang, W., Qi, J., Li, Z., et al. (2020). Systematic analysis and functional validation of citrus pectin acetylesterases (CsPAEs) reveals that CsPAE2 negatively regulates citrus bacterial canker development. Int. J. Mol. Sci. 21, 9429. doi: 10.3390/ijms21249429
Li, S., Pi, J., Zhu, H., Yang, L., Zhang, X., and Ding, W. (2021). Caffeic acid in tobacco root exudate defends tobacco plants from infection by Ralstonia solanacearum. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.690586
Li, P., Wang, S., Liu, M., Dai, X., Shi, H., Zhou, W., et al. (2024). Antibacterial Activity and Mechanism of Three Root Exudates from Mulberry Seedlings against Ralstonia pseudosolanacearum. Plants 13, 482. doi: 10.3390/plants13040482
Li, W., Zhao, Y., Liu, C., Yao, G., Wu, S., Hou, C., et al. (2012). Callose deposition at plasmodesmata is a critical factor in restricting the cell-to-cell movement of Soybean mosaic virus. Plant Cell Rep. 31, 905–916. doi: 10.1007/s00299-011-1211-y
Lin, W. C., Lu, C. F., Wu, J. W., Cheng, M. L., Lin, Y. M., Cheng, C. P., et al. (2004). Transgenic tomato plants expressing the Arabidopsis NPR1 gene display enhanced resistance to a spectrum of fungal and bacterial diseases. Transgenic Res. 13, 567–581. doi: 10.1007/s11248-004-2375-9
Lionetti, V., Fabri, E., De Caroli, M., Hansen, A. R., Willats, W. G., Piro, G., et al. (2017). Three pectin methylesterase inhibitors protect cell wall integrity for Arabidopsis immunity to Botrytis. Plant Physiol. 173, 1844–1863. doi: 10.1104/pp.16.01185
Lowe-Power, T. M., Khokhani, D., and Allen, C. (2018). How Ralstonia solanacearum exploits and thrives in the flowing plant xylem environment. Trends Microbiol. 26, 929–942. doi: 10.1016/j.tim.2018.06.002
Malinovsky, F. G., Fangel, J. U., and Willats, W. G. (2014). The role of the cell wall in plant immunity. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00178
Manabe, Y., Nafisi, M., Verhertbruggen, Y., Orfila, C., Gille, S., Rautengarten, C., et al. (2011). Loss-of-function mutation of REDUCED WALL ACETYLATION2 in Arabidopsis leads to reduced cell wall acetylation and increased resistance to Botrytis cinerea. Plant Physiol. 155, 1068–1078. doi: 10.1104/pp.110.168989
Mansfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., Ronald, P., et al. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13, 614–629. doi: 10.1111/j.1364-3703.2012.00804.x
Martin, G. B., Bogdanove, A. J., and Sessa, G. (2003). Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 54, 23–61. doi: 10.1146/annurev.arplant.54.031902.135035
McAvoy, T., Freeman, J. H., Rideout, S. L., Olson, S. M., and Paret, M. L. (2012). Evaluation of grafting using hybrid rootstocks for management of bacterial wilt in field tomato production. HortScience 47, 621–625. doi: 10.21273/HORTSCI.47.5.621
McCann, H. C. (2020). Skirmish or war: the emergence of agricultural plant pathogens. Curr. Opin. Plant Biol. 56, 147–152. doi: 10.1016/j.pbi.2020.06.003
Miedes, E., Vanholme, R., Boerjan, W., and Molina, A. (2014). The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00358
Milling, A., Babujee, L., and Allen, C. (2011). Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS One 6, e15853. doi: 10.1371/journal.pone.0015853
Mori, Y., Ishikawa, S., Ohnishi, H., Shimatani, M., Morikawa, Y., Hayashi, K., et al. (2018). Involvement of ralfuranones in the quorum sensing signalling pathway and virulence of Ralstonia solanacearum strain OE1-1. Mol. Plant Pathol. 19, 454–463. doi: 10.1111/mpp.12537
Nakaho, K., Hibino, H., and Miyagawa, H. (2000). Possible mechanisms limiting movement of Ralstonia solanacearum in resistant tomato tissues. J. Phytopathol. 148, 181–190. doi: 10.1046/j.1439-0434.2000.00476.x
Nakaho, K., Inoue, H., Takayama, T., and Miyagawa, H. (2004). Distribution and multiplication of Ralstonia solanacearum in tomato plants with resistance derived from different origins. J. Gen. Plant Pathol. 70, 115–119. doi: 10.1007/s10327-003-0097-0
Ngou, B. P. M., Ahn, H. K., Ding, P., and Jones, J. D. (2021). Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115. doi: 10.1038/s41586-021-03315-7
Paudel, S., Dobhal, S., Alvarez, A. M., and Arif, M. (2020). Taxonomy and phylogenetic research on Ralstonia solanacearum species complex: a complex pathogen with extraordinary economic consequences. Pathogens 9, 886. doi: 10.3390/pathogens9110886
Peeters, N., Guidot, A., Vailleau, F., and Valls, M. (2013). Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant Pathol. 14, 651–662. doi: 10.1111/mpp.12038
Pérez-Donoso, A. G., Greve, L. C., Walton, J. H., Shackel, K. A., and Labavitch, J. M. (2007). Xylella fastidiosa infection and ethylene exposure result in xylem and water movement disruption in grapevine shoots. Plant Physiol. 143, 1024–1036. doi: 10.1104/pp.106.087023
Planas-Marquès, M., Kressin, J. P., Kashyap, A., Panthee, D. R., Louws, F. J., Coll, N. S., et al. (2020). Four bottlenecks restrict colonization and invasion by the pathogen Ralstonia solanacearum in resistant tomato. J. Exp. Bot. 71, 2157–2171. doi: 10.1093/jxb/erz562
Polko, J. K. and Kieber, J. J. (2019). The regulation of cellulose biosynthesis in plants. Plant Cell 31, 282–296. doi: 10.1105/tpc.18.00760
Pouzoulet, J., Jacques, A., Besson, X., Dayde, J., and Mailhac, N. (2013). Histopathological study of response of Vitis vinifera cv. Cabernet Sauvignon to bark and wood injury with and without inoculation by Phaeomoniella chlamydospora. Phytopathol. Mediterr. 5, 313–323. doi: 10.14601/Phytopathol_Mediterr-12581
Pouzoulet, J., Scudiero, E., Schiavon, M., and Rolshausen, P. E. (2017). Xylem vessel diameter affects the compartmentalization of the vascular pathogen Phaeomoniella chlamydospora in grapevine. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01442
Qais, F. A., Khan, M. S., Ahmad, I., Husain, F. M., Khan, R. A., Hassan, I., et al. (2021). Coumarin exhibits broad-spectrum antibiofilm and antiquorum sensing activity against gram-negative bacteria: in vitro and in silico investigation. ACS Omega 6, 18823–18835. doi: 10.1021/acsomega.1c02046
Rodriguez, M. V., Tano, J., Ansaldi, N., Carrau, A., Srebot, M. S., Ferreira, V., et al. (2019). Anatomical and biochemical changes induced by Gluconacetobacter diazotrophicus stand up for Arabidopsis thaliana seedlings from Ralstonia solanacearum infection. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01618
Rohde, A., Morreel, K., Ralph, J., Goeminne, G., Hostyn, V., De Rycke, R., et al. (2004). Molecular phenoty of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 16, 2749–2771. doi: 10.1105/tpc.104.023705
Schell, M. A. (2000). Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu. Rev. Phytopathol. 38, 263–292. doi: 10.1146/annurev.phyto.38.1.263
Sebastià, P., de Pedro-Jové, R., Daubech, B., Kashyap, A., Coll, N. S., and Valls, M. (2021). The bacterial wilt reservoir host Solanum dulcamara shows resistance to Ralstonia solanacearum infection. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.755708
Shen, F., Yin, W., Song, S., Zhang, Z., Ye, P., Zhang, Y., et al. (2020). Ralstonia solanacearum promotes pathogenicity by utilizing l-glutamic acid from host plants. Mol. Plant Pathol. 21, 1099–1110. doi: 10.1111/mpp.12963
Shi, H., Liu, Y., Ding, A., Wang, W., and Sun, Y. (2023). Induced defense strategies of plants against Ralstonia solanacearum. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1059799
Somerville, C. (2006). Cellulose synthesis in higher plants. Annu. Rev. Cell Dev. Biolol 22, 53–78. doi: 10.1146/annurev.cellbio.22.022206.160206
Stuart, L. M., Paquette, N., and Boyer, L. (2013). Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nat. Rev. Immunol. 13, 199–206. doi: 10.1038/nri3398
Sun, Q. (2022). Structural variation and spatial polysaccharide profiling of intervessel pit membranes in grapevine. Ann. Bot. 130, 595–609. doi: 10.1093/aob/mcac096
Sun, Q., Greve, L. C., and Labavitch, J. M. (2011). Polysaccharide compositions of intervessel pit membranes contribute to Pierce’s disease resistance of grapevines. Plant Physiol. 155, 1976–1987. doi: 10.1104/pp.110.168807
Sun, Q., Rost, T. L., and Matthews, M. A. (2008). Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): Tyloses in summer and gels in winter. Am. J. Bot. 95, 1498–1505. doi: 10.3732/ajb.0800061
Sun, Q., Sun, Y., Walker, M. A., and Labavitch, J. M. (2013). Vascular occlusions in grapevines with Pierce’s disease make disease symptom development worse. Plant Physiol. 161, 1529–1541. doi: 10.1104/pp.112.208157
Truong, H. T. H., Kim, S., Tran, H. N., Nguyen, T. T. T., Nguyen, L. T., and Hoang, T. K. (2015). Development of a SCAR marker linked to bacterial wilt (Ralstonia solanacearum) resistance in tomato line Hawaii 7996 using bulked-segregant analysis. Horticult. Environ. Biotechnol. 56, 506–515. doi: 10.1007/s13580-015-1050-9
Vailleau, F. and Genin, S. (2023). Ralstonia solanacearum: An arsenal of virulence strategies and prospects for resistance. Annu. Rev. Phytopathol. 61, 25–47. doi: 10.1146/annurev-phyto-021622-104551
Vailleau, F., Sartorel, E., Jardinaud, M. F., Chardon, F., Genin, S., Petitprez, M., et al. (2007). Characterization of the interaction between the bacterial wilt pathogen Ralstonia solanacearum and the model legume plant Medicago truncatula. Mol. Plant-Microbe Interact. 20, 159–167. doi: 10.1094/MPMI-20-2-0159
Valls, M., Genin, S., and Boucher, C. (2006). Integrated regulation of the type III secretion system and other virulence determinants in Ralstonia solanacearum. PLoS Pathog. 2, e82. doi: 10.1371/journal.ppat.0020082
Wan, J., He, M., Hou, Q., Zou, L., Yang, Y., Wei, Y., et al. (2021). Cell wall associated immunity in plants. Stress Biol. 1, 3. doi: 10.1007/s44154-021-00003-4
Wang, Y., Li, X., Fan, B., Zhu, C., and Chen, Z. (2021). Regulation and function of defense-related callose deposition in plants. Int. J. Mol. Sci. 22, 2393. doi: 10.3390/ijms22052393
Wang, B., Luo, C., Li, X., Jimenez, A., Cai, J., Chen, J., et al. (2025). The FERONIA-RESPONSIVE TO DESICCATION 26 module regulates vascular immunity to Ralstonia solanacearum. Plant Cell 37(1), koae302. doi: 10.1093/plcell/koae302
Wang, X., Sager, R., Cui, W., Zhang, C., Lu, H., and Lee, J. Y. (2013). Salicylic acid regulates plasmodesmata closure during innate immune responses in Arabidopsis. Plant Cell 25, 2315–2329. doi: 10.1105/tpc.113.110676
Williams, J. S., Hall, S. A., Hawkesford, M. J., Beale, M. H., and Cooper, R. M. (2002). Elemental sulfur and thiol accumulation in tomato and defense against a fungal vascular pathogen. Plant Physiol. 128, 150–159. doi: 10.1104/pp.128.1.150
Wydra, K. and Beri, H. (2006). Structural changes of homogalacturonan, rhamnogalacturonan I and arabinogalactan protein in xylem cell walls of tomato genotypes in reaction to Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 68, 41–50. doi: 10.1016/j.pmpp.2006.06.001
Xiao, S., Hu, Q., Shen, J., Liu, S., Yang, Z., Chen, K., et al. (2021). GhMYB4 downregulates lignin biosynthesis and enhances cotton resistance to Verticillium dahliae. Plant Cell Rep. 40, 735–751. doi: 10.1007/s00299-021-02672-x
Xiao, X. O., Lin, W., Feng, E., and Ou, X. (2023). Transcriptome and metabolome response of eggplant against Ralstonia solanacearum infection. PeerJ 11, e14658. doi: 10.7717/peerj.14658
Xie, W., Ke, Y., Cao, J., Wang, S., and Yuan, M. (2021). Knock out of transcription factor WRKY53 thickens sclerenchyma cell walls, confers bacterial blight resistance. Plant Physiol. 187, 1746–1761. doi: 10.1093/plphys/kiab400
Xue, H., Lozano-Durán, R., and Macho, A. P. (2020). Insights into the root invasion by the plant pathogenic bacterium Ralstonia solanacearum. Plants 9, 516–525. doi: 10.3390/plants9040516
Yang, L., Ding, W., Xu, Y., Wu, D., Li, S., Chen, J., et al. (2016). New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules 21, 468. doi: 10.3390/molecules21040468
Yang, L., Wang, Y., He, X., Xiao, Q., Han, S., Jia, Z., et al. (2021a). Discovery of a novel plant-derived agent against Ralstonia solanacearum by targeting the bacterial division protein FtsZ. Pesticide Biochem. Physiol. 177, 104892. doi: 10.1016/j.pestbp.2021.104892
Yang, L., Wei, Z., Li, S., Xiao, R., Xu, Q., Ran, Y., et al. (2021b). Plant secondary metabolite, daphnetin reduces extracellular polysaccharides production and virulence factors of Ralstonia solanacearum. Pesticide Biochem. Physiol. 179, 104948. doi: 10.1016/j.pestbp.2021.104948
Yang, L., Wei, Z., Valls, M., and Ding, W. (2022). Metabolic profiling of resistant and susceptible tobaccos response incited by Ralstonia pseudosolanacearum causing bacterial wilt. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.780429
Zhang, H., Hong, Y., Huang, L., Li, D., and Song, F. (2016). Arabidopsis AtERF014 acts as a dual regulator that differentially modulates immunity against Pseudomonas syringae pv. Tomato Botrytis Cinerea Sci. Rep. 6, 30251. doi: 10.1038/srep30251
Zipfel, C. (2014). Plant pattern-recognition receptors. Trends Immunol. 35, 345–351. doi: 10.1016/j.it.2014.05.004
Zipfel, C. and Oldroyd, G. E. (2017). Plant signalling in symbiosis and immunity. Nature 543, 328–336. doi: 10.1038/nature22009
Keywords: Ralstonia solanacearum, inducible defense, cell wall integrity, plant structural barrier, vascular pathogen, bacterial wilt
Citation: Xue D, Wu W and Kong D (2025) Strategies utilized by plants to defend against Ralstonia solanacearum. Front. Plant Sci. 16:1510177. doi: 10.3389/fpls.2025.1510177
Received: 12 October 2024; Accepted: 01 May 2025;
Published: 26 May 2025.
Edited by:
Youxiong Que, Chinese Academy of Tropical Agricultural Sciences, ChinaReviewed by:
Dang Fengfeng, South China Agricultural University, ChinaGamze Boluk-Sari, Republic of Türkiye Ministry of Agriculture and Forestry, Türkiye
Copyright © 2025 Xue, Wu and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danyu Kong, a29uZ2R5QGxzYmcuY24=
 Dexing Xue
Dexing Xue Weifeng Wu
Weifeng Wu