- 1School of Pharmacy, Quanzhou Medical College, Quanzhou, China
- 2College of Veterinary Medicine, Northeast Agricultural University, Harbin, China
- 3Heilongjiang Key Laboratory for Animal Disease Control and Pharmaceutical Development, Northeast Agricultural University, Harbin, China
Introduction: Gerbera piloselloides (L.) Cass. and Gerbera delavayi Franch. are increasingly recognized for their medicinal properties, particularly among ethnic minority communities in southern China, where they are used for heat-clearing, detoxification, cough relief, lung expulsion, and asthma alleviation. Despite their traditional use, these species have been subjected to limited research regarding their biological activities, leaving a gap in scientific understanding.
Methods: This study was designed to investigate the essential oil (EO) compositions, as well as the antioxidant and antimicrobial properties of G. piloselloides and G. delavayi. The EOs were extracted via hydrodistillation and analyzed using gas chromatography-mass spectrometry (GC-MS). The antioxidant potential was assessed through ABTS and DPPH free radical scavenging assays, along with the ferric reducing antioxidant power (FRAP) method. The antimicrobial activity was evaluated against five bacterial strains, including two Gram-positive (Staphylococcus aureus, Listeria) and three Gram-negative (Salmonella, Escherichia coli, Pasteurella multocida) species, using the broth microdilution technique.
Results: The essential oil from G. piloselloides (EOgp) yielded 0.14% and was found to contain 24 compounds. It demonstrated high antioxidant activity in the ABTS assay and exhibited the strongest antibacterial effect against Listeria in vitro. In contrast, the essential oil from G. delavayi (EOgd) had a higher yield of 0.26% and contained a more complex composition with 100 compounds. It showed superior antioxidant activity in both the DPPH and FRAP assays and also demonstrated the highest antibacterial activity against Listeria.
Discussion: The findings of this study confirm that both G. piloselloides and G. delavayi possess significant potential as natural sources of antioxidants and antibacterial agents, warranting further exploration for their development into therapeutic products.
1 Introduction
The relentless progression of antibiotic resistance, particularly among multidrug-resistant bacterial strains, poses a significant threat to global health, underscoring the urgent need for the continuous development and discovery of new antimicrobial materials (Baran et al., 2023). While the “Antibiotic Era” may be waning, the potential of medicinal plants as a source for novel antimicrobial agents remains a beacon of hope. Through the intricate process of photosynthesis, plants synthesize a wealth of organic matter and secondary metabolites, which exhibit a broad spectrum of biological activities. These compounds lay the pharmacological groundwork for the prevention and treatment of diseases (Petric et al., 2020; Rehman et al., 2020). With many medicinal plants recognized for their safety, efficacy, and minimal side effects, the exploration of their bioactive compounds for antimicrobial properties is not only imperative but also a promising avenue in the fight against multidrug-resistant bacteria (Bouarab Chibane et al., 2019).
Essential oils (EOs), a type of secondary metabolite produced by aromatic plants, exhibit a spectrum of biological activities, including antibacterial, antioxidant, anti-inflammatory, enzyme inhibitory, sedative, anxiolytic, and antidepressant properties (Zengin et al., 2019; Liu Y. et al., 2024). EOs are utilized as natural remedies for the treatment of infectious diseases and as flavoring agents in food, offering a green and healthy alternative (Coelho et al., 2023; Wu et al., 2024). Due to their remarkable biological activities, EOs from medicinal plants are of great interest to scientists seeking to identify new phytochemical bioactive molecules that align with biodiversity and medicinal needs (Oliveira de Veras et al., 2020).
Gerbera Cass., a member of the Compositae family (Mutisieae Cass.), comprises approximately 80 species ranging from Africa to East Asia, with 20 species found in China, predominantly in the southwestern region (Zhao et al., 2024). Gerbera piloselloides (L.) Cass. and Gerbera delavayi Franch. are perennial herbs within the Gerbera genus. G. piloselloides is known for its heat-clearing, detoxifying, cough-relieving, phlegm-resolving, and circulation-regulating properties (Zhao et al., 2022; Liu C. et al., 2024). Traditionally, it is used in southwestern China to treat cough and sore throat when mixed with honey and also serves as a flavoring agent in winemaking and meat cooking due to its pleasant aroma (Zhou et al., 2022). The plant’s bioactive compounds, including caffeic acid derivatives, parasorboside derivatives, coumarins, and flavonoids, have been isolated through activity-guided isolation (Wang et al., 2014). The EO of G. piloselloides, EOgp, has been analyzed by GC-MS and found to contain fatty acids, terpenes, and aromatic compounds (Tang et al., 2003).
G. delavayi, found in open areas and forest margins at altitudes of 1800 to 3200 meters, was historically known as “ignited flowers” or “fireweed” due to its leaf’s combustion-supporting properties (Xu et al., 2017). The soft fiber on the back of its leaves is used in hand-weaving (Zheng et al., 2017). Beyond its use in spinning, G. delavayi holds significance in medicine and ornamental purposes. Gerbera species in China are noted for their antitussive, antipyretic, hemostatic, circulatory, and anti-inflammatory effects (Wu et al., 2005). The ethanol extract of G. delavayi has led to the isolation of two new coumarin compounds, gerdelavins A and B, along with 13 known compounds (Liu et al., 2010). Coumarins, characterized by their benzopyrone core, interact with various enzymes and receptors in organisms through weak bonds, conferring a broad range of medicinal potential, including antibacterial, antitumor, and anticoagulant activities (Balewski et al., 2021; Citarella et al., 2024).
In this context, our study endeavors to delve deeper into the properties of two lesser-studied Gerbera species, G. piloselloides and G. delavayi. The objective was to assess the EO compositions and to explore their antioxidant and antimicrobial potential. Notably, there is a paucity of literature documenting the biological activities of the EOs from these two plant species. Consequently, this investigation stands as the first comprehensive examination of the biological activities of the extracted EOs from G. piloselloides and G. delavayi, marking a significant contribution to the existing knowledge base.
2 Materials and methods
2.1 Plant material
To obtain a comprehensive representation of the chemical profile, the entire plants of G. piloselloides and G. delavayi, encompassing leaves, stems, roots, and rhizomes, were collected from the Stone Forest region of China. Plant materials from two Gerbera species, were meticulously collected in the Stone Forest region of China. The sampling locations were at elevations of 2316 m (24°81′10.55″ N, 103°30′12.83″ E) for G. piloselloides and 1689 m (24°46′27.55″ N, 103°17′18.83″ E) for G. delavayi, within Shilin County, Kunming, Yunnan Province, in July 2019. The taxonomic identification of these species was conducted by the Professor Huifeng Sun from Heilongjiang University of Chinese Medicine in Harbin, China.
For posterity and to facilitate future studies, voucher specimens were meticulously archived in the Herbarium of the College of Veterinary Medicine. The voucher numbers assigned to G. piloselloides and G. delavayi are 2031 and 2032, respectively. Following collection, the herbal materials were subjected to natural drying at room temperature. Subsequently, they were finely pulverized using a grinder and preserved at a refrigerated temperature of 4 °C, awaiting subsequent utilization in experimental procedures.
2.2 Extraction of essential oils
The essential oils (EOs) from G. piloselloides and G. delavayi were extracted using the hydrodistillation method, as described by Semerdjieva et al. (2019). For the extraction process, a precise amount of 100 grams of dried plant material was combined with 1000 mL of distilled water in a flask. The extraction was conducted for a duration of 8 h, commencing once the water reached boiling point. Following extraction, the EOs were separated from the aqueous phase with ethyl ether, dried over anhydrous sodium sulfate, filtered, and then subjected to evaporation of the ethyl ether in an oven at 40 °C for one hour. The resulting EO was transferred to amber vials and stored at -20 °C. The yield percentage (w/w) of the oil was determined based on the initial weight of the plant material used.
2.3 GC/MS analysis
The compositional analysis of the EOs was performed using an Agilent Technologies Gas Chromatograph model 7697A, equipped with a triple quadrupole detection system and a split-splitless injection port. The chromatographic separation was achieved on a HP-5MS fused silica capillary column (30 m × 250 μm × 0.25 μm) coupled with an Agilent MS Detector. The column temperature program began at 40 °C for 5 min, followed by an increase to 280 °C at a rate of 10 °C/min. An injection volume of 0.8 μL was used with a split ratio of 1: 20, and helium was employed as the carrier gas at a constant flow rate of 20 mL/min. Mass spectra were acquired at an electron energy of 70 eV, with the ion source temperature set at 250 °C. The mass spectra data were recorded within the mass-to-charge ratio (m/z) range of 44-550.
The identification of the EO compounds was accomplished by comparing their retention times and mass spectra with reference data in the NIST mass spectra library. The relative percentage contents of the individual compounds were quantified based on the peak areas in the GC-MS chromatograms, following the methodology described by Thabet et al. (2022).
The identification of the EO compounds was accomplished by comparing their retention times and mass spectra with reference data in the NIST mass spectra library. The retention indices were calculated using the linear retention index method, with a mixture of n-alkanes (C8-C20) at a concentration of 1 μg/mL as the reference compounds. The relative percentage contents of the individual compounds were quantified based on the peak areas in the GC-MS chromatograms, following the methodology described by Thabet et al. (2022).
2.4 Estimation of total polyphenolic content
The TPC was quantified using the Folin-Ciocalteu method adapted for a 96-well microplate format (Larrazabal-Fuentes et al., 2019). Initially, the EO sample (500 μg/mL) was combined with 10% (v/v) Folin-Ciocalteu reagent at a ratio of 1: 5 and allowed to stand for 5 min. Subsequently, a sodium carbonate solution was added to the mixture at a volume four times that of the sample and the mixture was shaken for 1 min. After incubation for 1 h at 25 °C, the absorbance was recorded at 765 nm using a microplate reader. A calibration curve was generated using gallic acid dilutions ranging from 0 to 1000 μg/mL. The results were expressed as milligrams of gallic acid equivalents per milliliter of EO.
2.5 Evaluation of antioxidant activities
The antioxidant potential of the EOs was assessed using the ferric reducing antioxidant power (FRAP) assay, 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging assay, in conjunction with the determination of the TPC. Vitamin C was employed as the standard reference. The protocols outlined below were adapted for use with 96-well microplates. All assays were conducted in triplicate.
2.5.1 DPPH radical scavenging activity assay
The DPPH radical scavenging capacity of the EOs was evaluated using the methodology of Larrazabal-Fuentes et al. (2019). The percent inhibition (I%) was calculated with the formula: I% = [(Ac - As)/(Ac)] × 100, where Ac is the absorbance of the control and As is the absorbance of the sample. The results were reported as IC50 values, representing the concentration of EO (μg/mL) required to inhibit 50% of the DPPH radicals in the solution, determined through linear regression analysis of the percentage of residual DPPH versus sample concentration.
2.5.2 FRAP assay
The FRAP assay was performed as described by Tian et al. (2019). An extract solution (500 μg/mL, 10 μL) was mixed with freshly prepared FRAP solution (70 μL), and the change in absorbance was measured at 593 nm after a 30-min incubation at 37 °C. Standard solutions of FeSO4·7H2O (0-500 μg/mL) and vitamin C (0-200 μg/mL) were used to construct the calibration curve. FRAP results were expressed as milligrams of vitamin C equivalent per milliliter of EO.
2.5.3 ABTS scavenging activity
The ABTS scavenging activity of the EO was determined following the procedures of Kıvrak (2014). ABTS radical cation (ABTS+) was generated by reacting ABTS (7 mM) with potassium persulfate (2.45 mM) at room temperature in the dark for 16 h. The ABTS+ solution was then diluted with ethanol to achieve an absorbance of 0.700 ± 0.005 at 734 nm. This solution (160 μL) was mixed with 40 μL of EO (0-20000 μg/mL), and the absorbance was measured at 734 nm after a 30-minute incubation at 30 °C. Vitamin C at various concentrations (0-200 μg/mL) served as the reference. The percentage scavenging of ABTS radicals was calculated using the equation from the DPPH assay. The results were expressed as IC50 values, calculated based on the linear regression of the percentage of residual ABTS versus sample concentration.
2.6 Evaluation of antibacterial activity
2.6.1 Microbial strains employed
The antimicrobial efficacy of the EOs was assessed against a panel of bacterial strains, including S. aureus CMCC26003, Listeria ATCC 19111, Salmonella CVCC541, E. coli CVCC10141, and P. multocida C48-1. These strains were obtained from the Harbin Institute of Veterinary Medicine (Harbin, Heilongjiang, China).
2.6.2 Determination of minimal inhibitory concentrations
The MICs of the EOs were determined using the broth microdilution method, as outlined in the CLSI protocols M60 (CLSI, 2017) and M100 (CLSI, 2018). The procedure involved preparing a stock solution of EO at 25 mg/mL in a mixture of 20% dimethyl sulfoxide (DMSO) and 80% distilled water. Initially, 100 µL of this stock solution was added to the first well of a 96-well plate, followed by serial two-fold dilutions to achieve concentrations ranging from 25 to 0.05 mg/mL (Cui et al., 2018). Subsequently, each bacterial strain was inoculated into LB broth to achieve a McFarland standard of 0.5, diluted 100-fold, and then added to the wells at a volume of 100 µL per well. The MIC was defined as the lowest concentration of EO that inhibited visible growth of the bacterial strains after an incubation period of 16–18 h at 37 °C. Chloramphenicol, at concentrations ranging from 10 to 0.04 mg/mL, was used as a positive control, while a solution of 20% DMSO-80% distilled water served as the negative control. All experiments were conducted in triplicate to ensure accuracy and reproducibility.
2.7 Statistical analysis
Significance was determined at a p-value threshold of < 0.05. Data were processed using GraphPad Prism® version 7.0 and presented as mean ± standard deviation (SD). To ascertain statistically significant differences among the groups, a one-way analysis of variance (ANOVA) was performed.
3 Results and discussion
3.1 Chemical composition of essential oils
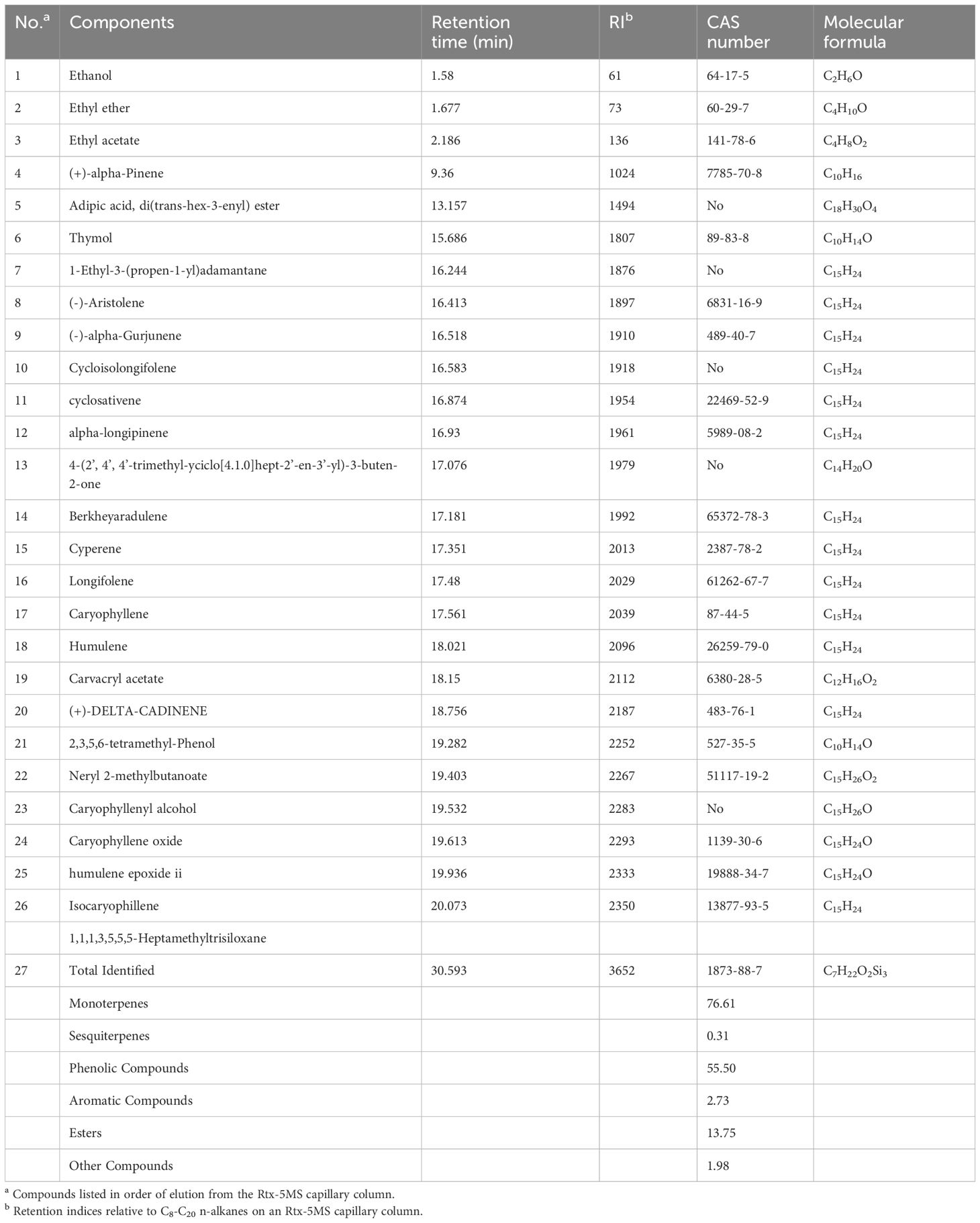
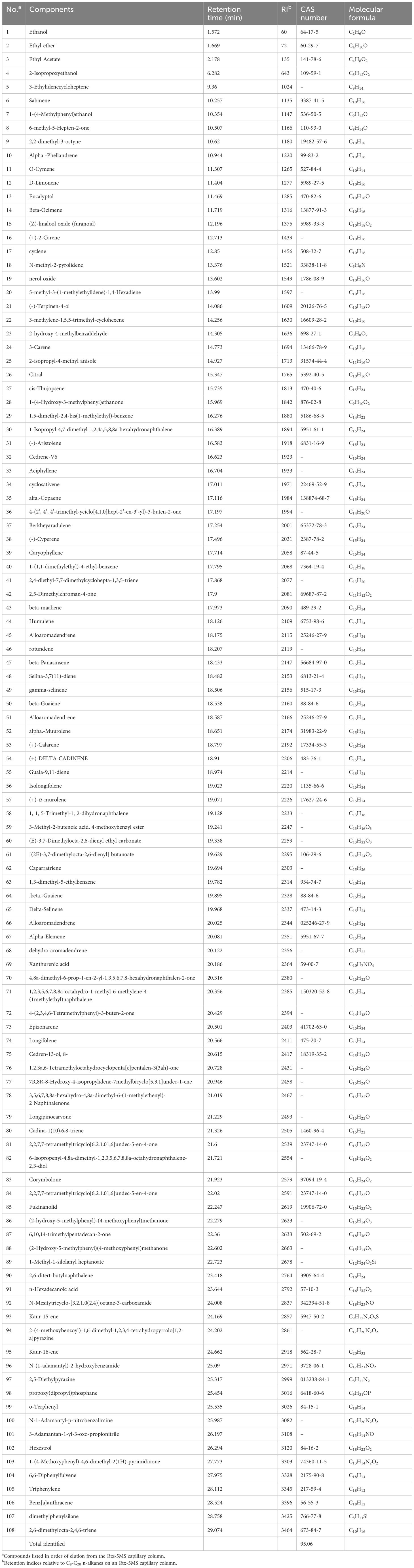
The medicinal parts of two Gerbera species, namely the whole plants of G. piloselloides and G. delavayi, were subjected to hydrodistillation, yielding yellow EOs with distinctive odors. The yields for G. piloselloides and G. delavayi were 0.14% (w/w) and 0.26% (w/w), respectively. The chemical compositions of these EOs were elucidated using GC/MS. The compositional percentages of the EOs from G. piloselloides and G. delavayi are presented in Tables 1 and 2, respectively. The total ion chromatograms for the EOs of both species (EOgp and EOgd) are depicted in Figure 1. GC/MS analysis of EOgp identified 24 components, with berkheyaradulene (32.03%), 4-(2’, 4’, 4’-trimethyl-cyclo[4.1.0]hept-2’-en-3’-yl)-3-buten-2-one (12.86%), caryophyllene (6.78%), and cycloisolongifolene (5.30%) as the principal constituents. In contrast, EOgd comprised 100 components, with butanoic acid, 3,7-dimethyl-2,6-octadienyl ester, (E)- (10.50%), cyperene (9.70%), β-panasinsene (7.13%), benzamide, N-(1-adamantyl)-2-hydroxy- (6.12%), and benzene, 1-(1,1-dimethylethyl)-4-ethyl- (5.31%) as the predominant compounds.
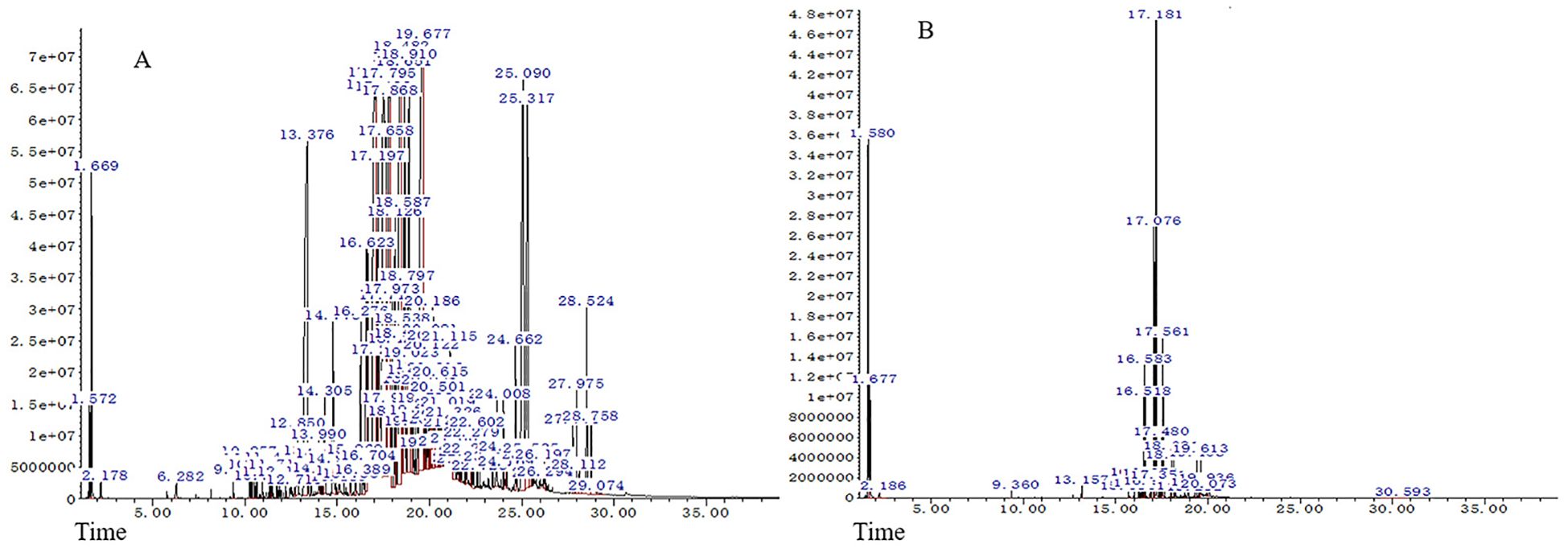
Figure 1. GC/MS profiles of the essential oils from Gerbera piloselloides (A) and Gerbera delavayi (B).
In a prior phytochemical study, the researchers documented the presence of 17 volatile organic components in G. piloselloides, encompassing fatty acids, terpenes, and aromatic compounds. Notably, neryl (S) -2-methylbutanoate (35.99%), 4-hydroxy-3-methylacetophenone (8.74%), and n-hexadecanoic acid (7.48%) emerged as the predominant constituents of the plant’s essential oil (Luo et al., 2013). Previous studies have identified various volatile organic compounds in specific parts of G. piloselloides, such as leaves, caudices, and roots (Tang et al., 2003). However, our study focused on the essential oils extracted from the whole plants of G. piloselloides and G. delavayi. The observed discrepancies in the identified compounds may be attributed to the varying environmental conditions of the plant collection sites and the inclusion of multiple plant parts in our analysis.
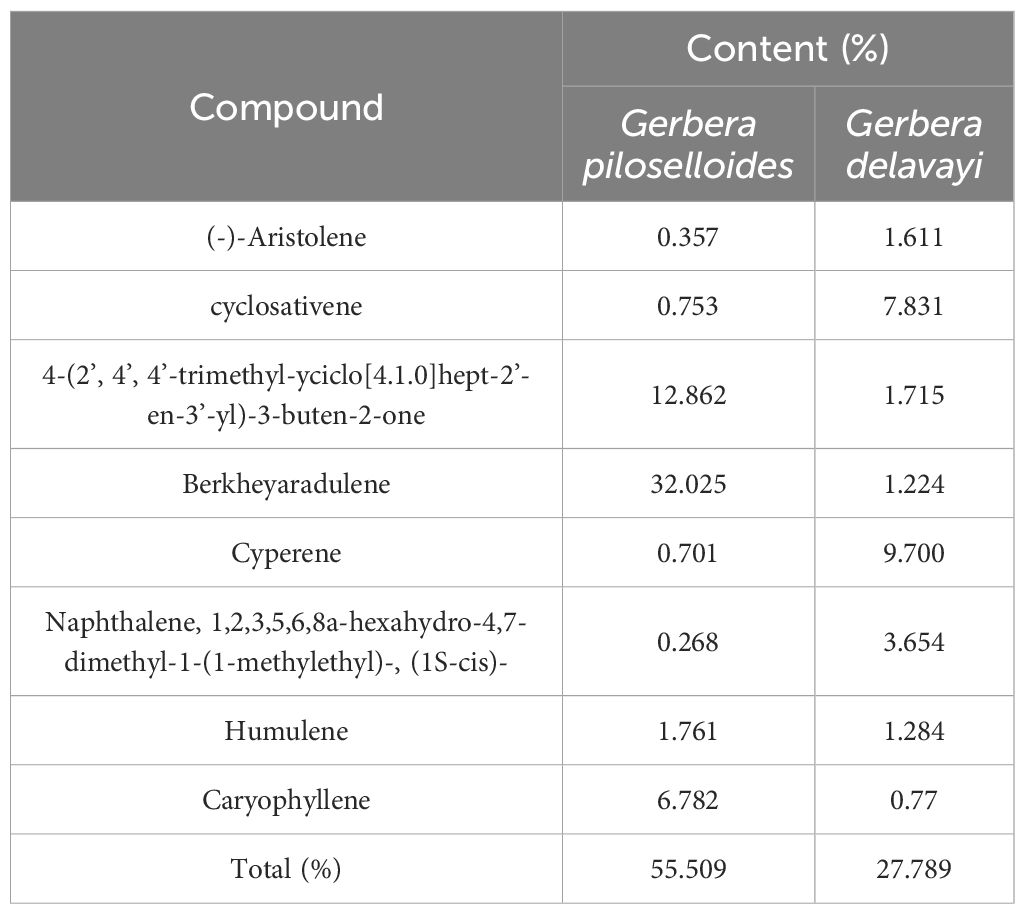
Previous research on Gerbera has revealed the presence of coumarins, sesquiterpenoids, triterpenoids, and cyanogenic glycosides (Liu et al., 2010). Despite the distinct EO profiles of the two Gerbera species, eight compounds, including (-)-aristolene, 4-(2’, 4’, 4’-trimethyl-cyclo[4.1.0]hept-2’-en-3’-yl)-3-buten-2-one, berkheyaradulene, cyclosativene, cyperene, naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)-, humulene, and caryophyllene, are common to both, as detailed in Table 3.
Berkheyaradulene is particularly abundant in G. piloselloides (10.50%), representing a sesquiterpene hydrocarbon with an unusual carbon skeleton characterized by a bridgehead carbon connected to three rings, also found in other Asteraceae plants (Szöke et al., 2004). Caryophyllene, notable for its cyclobutane ring, a rare occurrence in nature, is often accompanied by isocaryophyllene and α-humulene, its ring-opened isomer (Taherpour et al., 2010). Cyperene, a tetracyclic sesquiterpene, possesses unique properties such as sterilizing, antioxidant, anticarcinogenic, and immune-boosting functions (Skała et al., 2016; Hu et al., 2017). Thymol, with its thyme oil-like aroma, may contribute to the use of G. piloselloides in winemaking and meat cooking. Thymol’s expectorant properties have been documented, and it also exhibits bactericidal effects, suggesting its potential in treating bronchitis and whooping cough (Zhou et al., 2019). Furthermore, thymol holds promise for applications in the preservatives industry, as an insect repellent, and in the perfume industry (Roufegarinejad et al., 2018; Reyhani et al., 2022; Dadé et al., 2023).
3.2 Antioxidant capacity of essential oils
EOs are integral aromatic constituents found in herbs and spices, conferring them with a range of biological activities, including antimicrobial, antifungal, antioxidant, and anti-inflammatory effects (Valdivieso-Ugarte et al., 2019). However, the composition of EOs in these herbs is intricate, lacking a straightforward and precise method for a comprehensive and objective evaluation of the antioxidant capacity of traditional Chinese medicines. Consequently, a variety of antioxidant assays are necessary to profile the total antioxidant potential of natural extracts in this context.
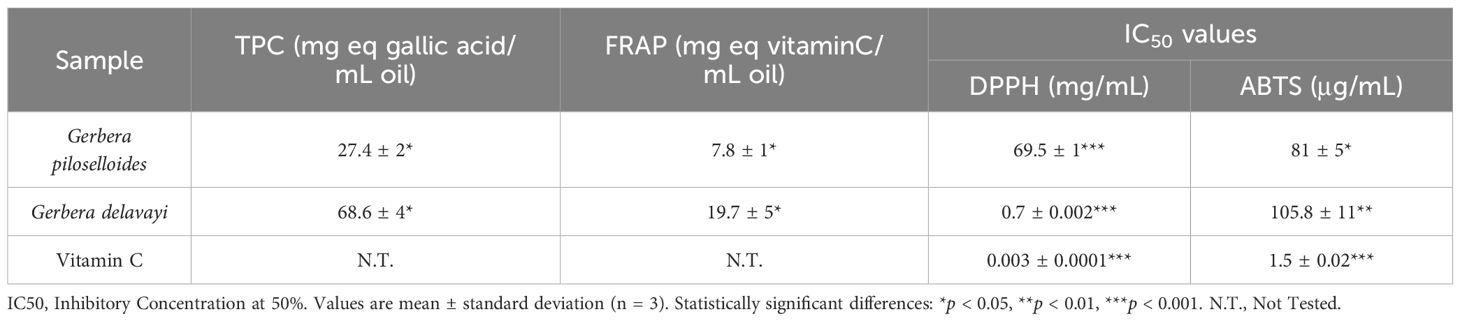
We assessed the antioxidant potential of EOgp and EOgd by evaluating their efficacy in scavenging the stable free radicals ABTS and DPPH. The radical scavenging activities of the EOs are depicted in Figures 2A and 2B, respectively. The concentrations of the EOs required to inhibit each radical by 50% (IC50) are presented in Table 4. Notably, G. delavayi exhibited a significantly higher DPPH free radical scavenging ability (IC50 0.7 mg/mL) compared to G. piloselloides (IC50 69.5 mg/mL). However, both G. piloselloides and G. delavayi demonstrated similar ABTS free radical scavenging activity, with IC50 values of 81 µg/mL and 105.8 µg/mL, respectively. It is documented that certain compounds with ABTS scavenging capability may not exhibit DPPH scavenging activity, which could account for the observed results (Borah et al., 2019).
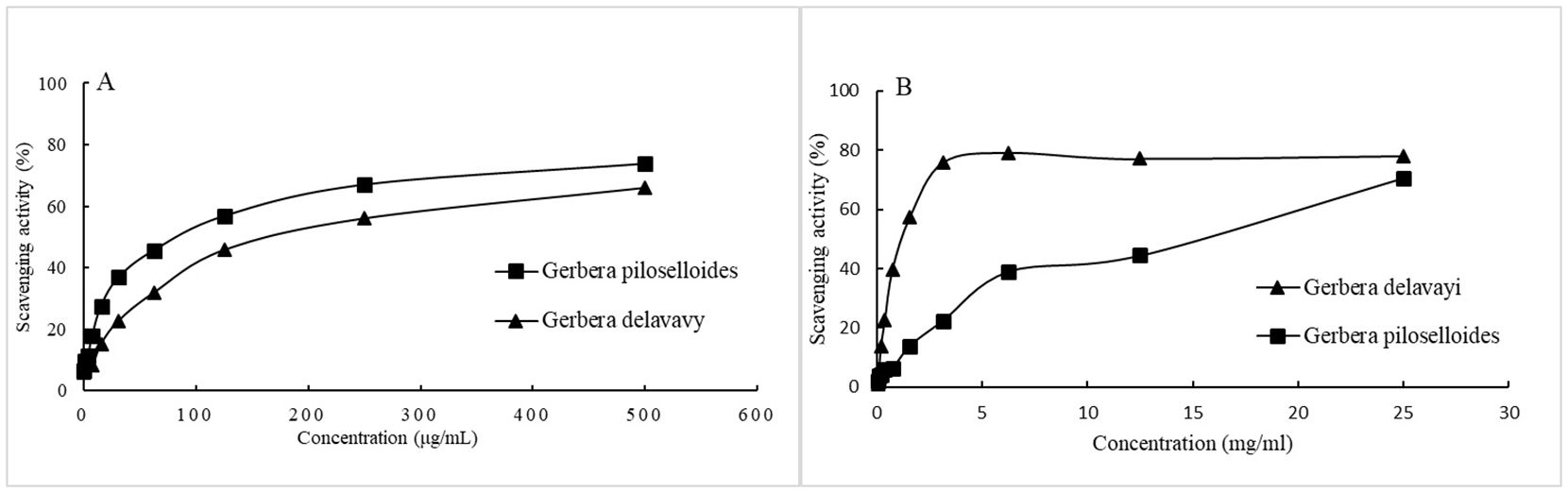
Figure 2. Radical scavenging activity of the essential oils from Gerbera piloselloides and Gerbera delavayi against the ABTS radical (A) and DPPH (B). Data are represented as mean standard deviation (SD) of triplicate experiments.
The reducing capability of the extracts was determined using a microplate reader to track the conversion of Fe3+ to Fe2+ in the presence of the extracts. An increase in absorbance is indicative of the extract’s reducing power. The influence of antioxidant concentration on FRAP inhibition is summarized in Figure 3. FRAP results were expressed as milligrams of vitamin C equivalent per milliliter of oil, and TPC data are provided in Table 4. G. delavayi displayed a superior antioxidant capacity, with 19.7 mg eq vitamin C/mL oil, compared to G. piloselloides (7.8 mg eq vitamin C/mL oil). The FRAP antioxidant activities were directly proportional to the TPC, with G. piloselloides and G. delavayi exhibiting TPC values of 27.4 mg eq gallic acid/mL oil and 68.6 mg eq gallic acid/mL oil, respectively. Studies by other researchers have also linked the antioxidant activity of Solanum elaeagnifolium to its TPC (Bouslamti et al., 2022). These findings suggest that TPC compounds contribute significantly to the antioxidant activity of G. delavayi.
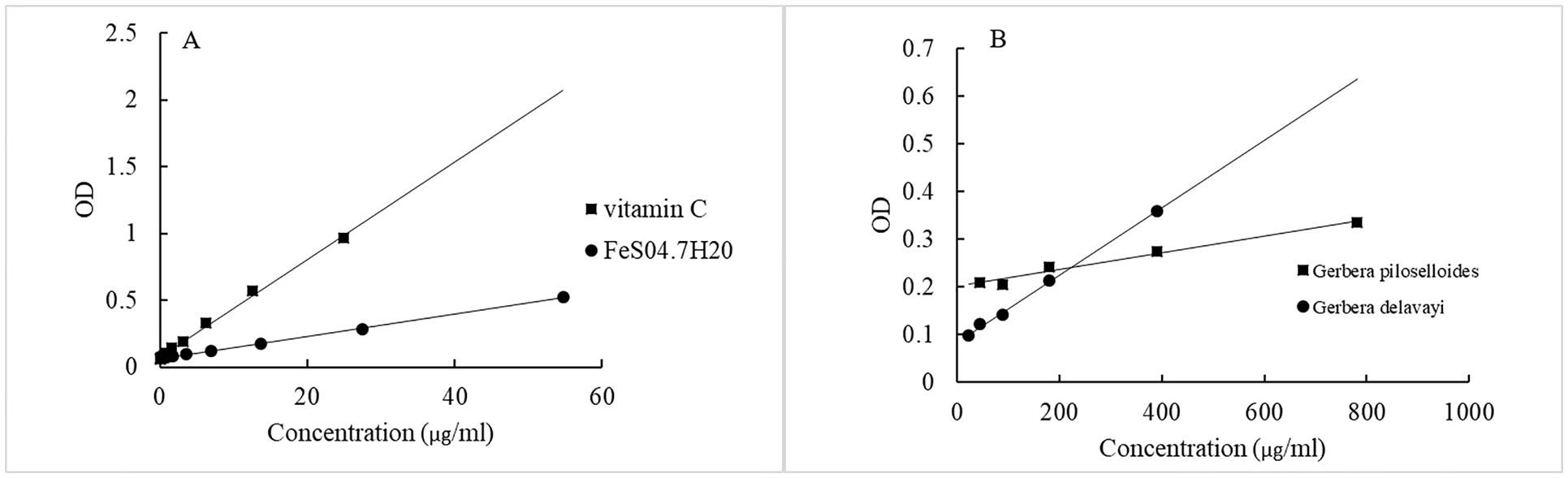
Figure 3. Concentration-dependent effects of antioxidants on the inhibition of the FRAP assay. (A) shows the correlation coefficients (r²) for vitamin C (r² = 0.996) and FeSO4·7H2O (r² = 0.999); (B) depicts the correlation coefficients for Gerbera piloselloides (r² = 0.978) and FeSO4·7H2O (r² = 0.998).
3.3 Antimicrobial activity of essential oils
Bacterial infections continue to be a leading cause of mortality worldwide, a situation exacerbated by the persistent emergence of antibiotic resistance (Huemer et al., 2020). EO components derived from medicinal plants are noted for their high biological activity, and the quest for alternative antimicrobial agents to replace antibiotics has become a focal point of contemporary research (Coimbra et al., 2022).
This study assessed the antimicrobial potential of G. piloselloides and G. delavayi by evaluating their inhibitory effects against Listeria, S. aureus, Salmonella, P. multocida, and E. coli. The minimum inhibitory concentrations (MICs) of the EOs against these microbial strains are detailed in Table 5. The data reveal that EOgp demonstrated inhibitory activity against Listeria ATCC 19111, S. aureus CMCC26003, Salmonella CVCC541, P. multocida C48-1, and E. coli CVCC10141 with MICs of 6.3 mg/mL, 12.5 mg/mL, 12.5 mg/mL, 6.3 mg/mL, and 12.5 mg/mL, respectively. Similarly, Eogd exhibited efficacy against the same pathogens with MIC values of 6.3 mg/mL, 12.5 mg/mL, 12.5 mg/mL, 6.3 mg/mL, and 6.3 mg/mL, respectively. Notably, the antimicrobial potency of both EOs against Listeria surpassed that of chloramphenicol.
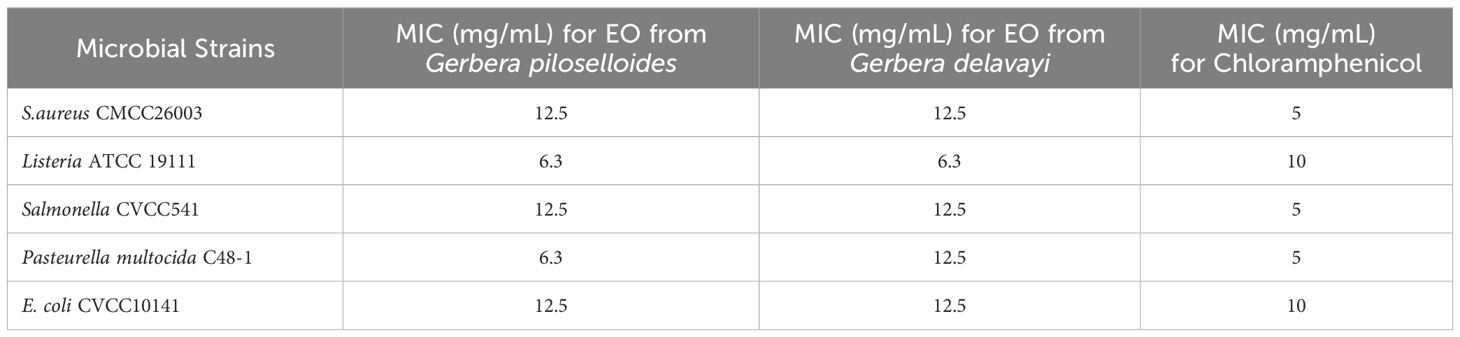
Table 5. Minimum Inhibitory Concentrations (MICs) of essential oils from Gerbera piloselloides and Gerbera delavayi Franch, and Chloramphenicol against selected strains.
The biological effects of EOs are a consequence of the synergistic interaction of all molecules within the oil, and it is erroneous to attribute these effects to a single compound (Melo et al., 2020). The predominant components identified in both plant EOs were terpenes, natural products that serve diverse roles in various organisms and exhibit a wide array of structural diversity. Listeria has long been implicated as a primary agent of foodborne diseases in humans and animals. Cho et al. (2020) reported on the combined activities of gaseous oregano and thyme thymol EOs against Listeria monocytogenes. In the study by Said et al. (2016), oxygenated terpenes such as chamazulene-a degradation product, β-thujone, and camphor were identified as the main components of bioactive oils with antibacterial activity against Listeria monocytogenes. In the present manuscript, we also observed a high terpenoid content in both EOgp and EOgd, which may be responsible for their significant inhibitory effects against Listeria. The presence of these oxygenated terpenes in our extracts aligns with the findings of Said et al. (2016), suggesting that these compounds could be key contributors to the antibacterial properties observed. Our data further support the potential of natural plant-derived EOs as agents for controlling Listeria monocytogenes in antibacterial applications. However, it is important to note that while these EOs show promise, their safety profile must be thoroughly investigated before they can be considered for practical use.
4 Conclusion
In the present investigation, we assessed the chemical constituents, as well as the antioxidant and antimicrobial properties, of EOs extracted from the whole plants of G. piloselloides (EOgp) and G. delavayi (EOgd) via hydrodistillation. Our findings reveal that both EOgd and EOgp exhibit significant antioxidant capabilities and demonstrate differential inhibitory effects against five tested microbial strains. Notably, both essential oils exerted potent antibacterial effects against Listeria monocytogenes in vitro. These findings contribute to the growing body of evidence supporting the potential of these species as natural sources for the development of therapeutic products. Further research is needed to explore the specific mechanisms of action and safety profiles of these essential oils.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Author contributions
JW: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft. WH: Data curation, Formal analysis, Resources, Software, Writing – original draft. JC: Formal analysis, Investigation, Visualization, Writing – review & editing. JH: Methodology, Supervision, Validation, Writing – review & editing. CK: Data curation, Resources, Visualization, Writing – original draft. ZS: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was financially supported by the National Natural Science Foundation of China (31572559), postdoctoral scientific research developmental fund of Heilongjiang Province in 2008 (LBH-Q18020) and the Scientific Research Funds of Quanzhou Medical College (XJY2412).
Acknowledgments
Special thanks to reviewers for their valuable comments. In addition, the authors gratefully acknowledge every teacher, classmate, and friend who helped the authors with their experiment and writing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
EO, essential oil; FRAP, ferric reducing antioxidant power; ABTS, 2,29-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid; DPPH, 1,1-diphenyl-2-picrylhydrazyl; EOgp, essential oil from Gerbera piloselloides; EOgd, oil from Gerbera delavay; TPC, total polyphenolic content.
References
Balewski, Ł., Szulta, S., Jalińska, A., Kornicka, A. (2021). Recent advances in coumarin-metal complexes with biological properties. Front. Chem. 9. doi: 10.3389/fchem.2021.781779
Baran, A., Kwiatkowska, A., Potocki, L. (2023). Antibiotics and bacterial resistance-A short story of an endless arms race. Int. J. Mol. Sci. 24, 5777. doi: 10.3390/ijms24065777
Borah, A., Paw, M., Gogoi, R., Loying, R., Sarma, N., Munda, S., et al. (2019). Chemical composition, antioxidant, anti-inflammatory, anti-microbial and in-vitro cytotoxic efficacy of essential oil of Curcuma caesia Roxb. leaves: An endangered medicinal plant of North East India. Ind. Crops Prod. 129, 448–454. doi: 10.1016/j.indcrop.2018.12.035
Bouarab Chibane, L., Degraeve, P., Ferhout, H., Bouajila, J., Oulahal, N. (2019). Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 99, 1457–1474. doi: 10.1002/jsfa.9357
Bouslamti, M., El Barnossi, A., Kara, M., Alotaibi, B. S., Al Kamaly, O., Assouguem, A., et al. (2022). Total polyphenols content, antioxidant and antimicrobial activities of leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 27, 4322. doi: 10.3390/molecules27134322
Cho, Y., Kim, H., Beuchat, L. R., Ryu, J. H. (2020). Synergistic activities of gaseous oregano and thyme thymol essential oils against Listeria monocytogenes on surfaces of a laboratory medium and radish sprouts. Food Microbiol. 86, 103357. doi: 10.1016/j.fm.2019.103357
Citarella, A., Vittorio, S., Dank, C., Ielo, L. (2024). Syntheses, reactivity, and biological applications of coumarins. Front. Chem. 12. doi: 10.3389/fchem.2024.1362992
CLSI (2017). “Performance standards for antifungal susceptibility testing of yeasts,” in CLSI Supplement M60, 1 th (Clinical Laboratory Standards Institute, Wayne, PA).
CLSI (2018). “Performance standards for antimicrobial susceptibility testing,” in CLSI Supplement M100, 28 th (Clinical Laboratory Standards Institute, Wayne, PA).
Coelho, M. G., da Silva, A. P., de Toledo, A. F., Cezar, A. M., Tomaluski, C. R., Barboza, R. D. F., et al. (2023). Essential oil blend supplementation in the milk replacer of dairy calves: Performance and health. PloS One 18, e0291038. doi: 10.1371/journal.pone.0291038
Coimbra, A., Ferreira, S., Duarte, A. P. (2022). Biological properties of Thymus zygis essential oil with emphasis on antimicrobial activity and food application. Food Chem. 393, 133370. doi: 10.1016/j.foodchem.2022.133370
Cui, H., Pan, H. W., Wang, P. H., Yang, X. D., Zhai, W. C., Dong, Y., et al. (2018). Essential oils from Carex meyeriana Kunth: Optimization of hydrodistillation extraction by response surface methodology and evaluation of its antioxidant and antimicrobial activities. Ind. Crops Prod. 124, 669–676. doi: 10.1016/j.indcrop.2018.08.041
Dadé, M. M., Daniele, M., Reyes-Novelo, E., Rodriguez-Vivas, R. I. (2023). Lethal and repellent effect of amitraz, eugenol and thymol against Triatoma infestans, the main vector of Trypanosoma cruzi in the southern of America. Med. Vet. Entomol. 37, 574–580. doi: 10.1111/mve.12655
Hu, Q. P., Cao, X. M., Hao, D. L., Zhang, L. L. (2017). Chemical composition, antioxidant, DNA damage protective, cytotoxic and antibacterial activities of Cyperus rotundus rhizomes essential oil against foodborne pathogens. Sci. Rep. 7, 45231. doi: 10.1038/srep45231
Huemer, M., Mairpady Shambat, S., Brugger, S. D., Zinkernagel, A. S. (2020). Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 21, e51034. doi: 10.15252/embr.202051034
Kıvrak, İ. (2014). Analytical methods applied to assess chemical composition, nutritional value and in vitro bioactivities of Terfezia olbiensis and Terfezia claveryi from Turkey. Food Anal. Methods 8, 1279–1293. doi: 10.1007/s12161-014-0009-2
Larrazabal-Fuentes, M., Palma, J., Paredes, A., Mercado, A., Neira, I., Lizama, C., et al. (2019). Chemical composition, antioxidant capacity, toxicity and antibacterial activity of the essential oils from Acantholippia deserticola (Phil.) Moldenke (Rica rica) and Artemisia copa Phil. (Copa copa) extracted by microwave-assisted hydrodistillation. Ind. Crops Prod. 142, 111830. doi: 10.1016/j.indcrop.2019.111830
Liu, S. Z., Feng, J. Q., Wu, J., Zhao, W. M. (2010). A new monoterpene–coumarin and a new monoterpene–chromone from Gerbera delavayi. Verlag Helv. Chim. Acta 93, 2026–2029. doi: 10.1002/hlca.201000017
Liu, C., He, Y., Zhou, K., Wang, H., Zhou, M., Sun, J., et al. (2024). Mitigation of allergic asthma in mice: A compound mixture comprising luteolin, arbutin, and marmesin from Gerbera Piloselloides Herba by suppression of PI3K/Akt pathway. Heliyon 10, e37632. doi: 10.1016/j.heliyon.2024.e37632
Liu, Y., Ren, H., Li, K. (2024). Litsea cubeba essential oil: Extraction, chemical composition, antioxidant and antimicrobial properties, and applications in the food industry. J. Food Sci. 89, 4583–4603. doi: 10.1111/1750-3841.17236
Luo, L., Deng, J. M., Liao, H. W. (2013). Analysis of volatile oil from Gerbera piloselloides by GC-MS. J. Chin. Medicinal Materials 36, 944–945. doi: 10.13863/j.issn1001-4454.2013.06.030
Melo, C. R., Oliveira, B. M. S., Santos, A. C. C., Silva, J. E., Ribeiro, G. T., Blank, A. F., et al. (2020). Synergistic effect of aromatic plant essential oils on the ant Acromyrmex balzani (Hymenoptera: Formicidae) and antifungal activity on its symbiotic fungus Leucoagaricus gongylophorus (Agaricales: Agaricaceae). Environ. Sci. Pollut. Res. Int. 27, 17303–17313. doi: 10.1007/s11356-020-08170-z
Oliveira de Veras, B., Melo de Oliveira, M. B., Granja da Silva Oliveira, F., Queiroz Dos Santos, Y., Saturnino de Oliveira, J. R., Lucia de Menezes Lima, V., et al. (2020). Chemical composition and evaluation of the antinociceptive, antioxidant and antimicrobial effects of essential oil from Hymenaea cangaceira (Pinto, Mansano & Azevedo) native to Brazil: A natural medicine. J. Ethnopharmacol. 247, 112265. doi: 10.1016/j.jep.2019.112265
Petric, D., Mravcakova, D., Kuckova, K., Cobanova, K., Kisidayova, S., Cieslak, A., et al. (2020). Effect of dry medicinal plants (wormwood, chamomile, fumitory and mallow) on in vitro ruminal antioxidant capacity and fermentation patterns of sheep. J. Anim. Physiol. Anim. Nutr. (Berl.) 104, 1219–1232. doi: 10.1111/jpn.13349
Rehman, A., Jafari, S. M., Aadil, R. M., Assadpour, E., Randhawa, M. A., Mahmood, S. (2020). Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 101, 106–121. doi: 10.1016/j.tifs.2020.05.001
Reyhani, Y., Iranshahi, M., Taghizadeh, S. F., Saberi, S., Farhadi, F. (2022). An evaluation of qH NMR: A complementary approach to GC-FID for quantification of Thymol and trans-Anethole in essential oils and supplements. J. Pharm. Biomed. Anal. 220, 114992. doi: 10.1016/j.jpba.2022.114992
Roufegarinejad, L., Jahanban-Esfahlan, A., Sajed-Amin, S., Panahi-Azar, V., Tabibiazar, M. (2018). Molecular interactions of thymol with bovine serum albumin: Spectroscopic and molecular docking studies. J. Mol. Recognit. 31, e2704. doi: 10.1002/jmr.2704
Said, M.-A., Militello, M., Saia, S., Settanni, L., Aleo, A., Mammina, C., et al. (2016). Artemisia arborescens essential oil composition, enantiomeric distribution, and antimicrobial activity from different wild populations from the mediterranean area. Chem. Biodivers. 13, 1095–1102. doi: 10.1002/cbdv.201500510
Semerdjieva, I. B., Shiwakoti, S., Cantrell, C. L., Zheljazkov, V. D., Astatkie, T., Schlegel, V., et al. (2019). Hydrodistillation extraction kinetics regression models for essential oil yield and composition in Juniperus virginiana, J. excelsa, and J. sabina. Molecules 24, 986. doi: 10.3390/molecules24050986
Skała, E., Rijo, P., Garcia, C., Sitarek, P., Kalemba, D., Toma, M., et al. (2016). The essential oils of Rhaponticum carthamoides hairy roots and roots of woil-grown plants: Chemical composition and antimicrobial, anti-inflammatory, and antioxidant activities. Oxid. Med. Cell Longev. 2016, 8505384. doi: 10.1155/2016/8505384
Szöke, E., Máday, E., Marczal, G., Lemberkovics, E. (2004). Analysis of biologically active essential oil components of chamomiles in Hungary (in vivo - in vitro). Acta Hortic. 597, 275–284. doi: 10.17660/ActaHortic.2003.597.40
Taherpour, A. A., Maroofi, H., Bajelani, O., Larijani, K. (2010). Chemical composition of the essential oil of Valeriana alliariifolia Adams of Iran. Nat. Prod. Res. 24, 973–978. doi: 10.1080/14786410902900010
Tang, X. J., Zhang, Y., Huang, H. R., Fang, T. Z., Xu, S. B. (2003). Analysis and comparison of the essential oil from the leaves, caudexes and roots of Gerbera piloselloides cass. Acta Scientiarum Naturalium Universitatis Sunyatseni 42, 125.
Thabet, A. A., Moghannem, S., Ayoub, I. M., Youssef, F. S., Al Sayed, E., Singab, A. N. B. (2022). GC/MS profiling of essential oils from Bontia daphnoides L., chemometric discrimination, isolation of dehydroepingaione and evaluation of antiviral activity. Sci. Rep. 12, 17707. doi: 10.1038/s41598-022-22174-4
Tian, H., Zhao, H., Zhou, H., Zhang, Y. (2019). Chemical composition and antimicrobial activity of the essential oil from the aerial part of Dictamnus dasycarpus Turcz. Ind. Crops Prod. 140, 111713. doi: 10.1016/j.indcrop.2019.111713
Valdivieso-Ugarte, M., Gomez-Llorente, C., Plaza-Díaz, J., Gil, Á. (2019). Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 11, 2786. doi: 10.3390/nu11112786
Wang, J., Petrova, V., Wu, S. B., Zhu, M., Kennelly, E. J., Long, C. (2014). Antioxidants from Gerbera piloselloides: an ethnomedicinal plant from southwestern China. Nat. Prod. Res. 28, 2072–2075. doi: 10.1080/14786419.2014.924000
Wu, Z. Y., Raven, P. H., Hong, D. Y. (2005). Flora of China. Vol 14: Apiaceae through Ericaceae (Beijing, China: Science Press), 451–471.
Wu, H., Zhao, F., Li, Q., Huang, J., Ju, J. (2024). Antifungal mechanism of essential oil against foodborne fungi and its application in the preservation of baked food. Crit. Rev. Food Sci. Nutr. 64, 2695–2707. doi: 10.1080/10408398.2022.2124950
Xu, X. D., Zheng, W., Chen, L. Q., Wen, J. (2017). Genetic diversity and population structure of Gerbera delavayi (Asteraceae) in Southwest China: Implications for conservation. Ann. Bot. Fenn. 54, 409–422. doi: 10.5735/085.054.0623
Zengin, G., Atasagun, B., Zakariyyah Aumeeruddy, M., Saleem, H., Mollica, A., Babak Bahadori, M., et al. (2019). Phenolic profiling and in vitro biological properties of two Lamiaceae species (Salvia modesta and Thymus argaeus): A comprehensive evaluation. Ind. Crops Prod. 128, 308–314. doi: 10.1016/j.indcrop.2018.11.027
Zhao, C., Gao, H., Li, J., Yu, M., Wu, J., Zhang, H., et al. (2022). Bioactive constituents from Gerbera piloselloides with anti-inflammatory and antiproliferative activities. Fitoterapia 161, 105258. doi: 10.1016/j.fitote.2022.105258
Zhao, C., Li, J., Hu, Y., Li, L., Yu, M., Huang, Y., et al. (2024). (+)/(-)-Gerbeloid A, a pair of unprecedented coumarin-based polycyclic meroterpenoid enantiomers from Gerbera piloselloides: Structural elucidation, semi-synthesis, and lipid-lowering activity. Acta Pharm. Sin. B 14, 2657–2668. doi: 10.1016/j.apsb.2024.03.035
Zheng, W., Xu, X., Wen, J. (2017). The ethnic textile use of natural fibers from fireweed (Gerbera delavayi) in Southwest China. Econ. Bot. 71, 380–386. doi: 10.1007/s12231-017-9394-y
Zhou, K., Lu, D., You, J., Liu, T., Sun, J., Lu, Y., et al. (2022). Integrated plasma pharmacochemistry and network pharmacology to explore the mechanism of Gerberae Piloselloidis Herba in treatment of allergic asthma. J. Ethnopharmacol. 298, 115624. doi: 10.1016/j.jep.2022.115624
Keywords: essential oil, Gerbera delavayi Franch., Gerbera piloselloides, chemical composition, antioxidant activity, antibacterial activity
Citation: Wu J, Hu W, Chen J, Hu J, Ke C and Sheng Z (2025) Characterizing the essential oil composition and assessing the antioxidant and antimicrobial properties of two compositae taxa: Gerbera delavayi Franch. and Gerbera piloselloides (L.) Cass. Front. Plant Sci. 16:1527525. doi: 10.3389/fpls.2025.1527525
Received: 13 November 2024; Accepted: 14 April 2025;
Published: 08 May 2025.
Edited by:
Wilhelm Boland, Max Planck Institute for Chemical Ecology, GermanyReviewed by:
Antonio Giovino, Council for Agricultural Research and Agricultural Economy Analysis | CREA, ItalyShihong Luo, Shenyang Agricultural University, China
Copyright © 2025 Wu, Hu, Chen, Hu, Ke and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zunlai Sheng, c2hlbmd6dW5sYWlAbmVhdS5lZHUuY24=
 Junkai Wu
Junkai Wu Wanjun Hu2,3
Wanjun Hu2,3 Zunlai Sheng
Zunlai Sheng