- 1College of Enology, Northwest A & F University, Yangling, China
- 2College of Water Resources and Architectural Engineering, Northwest A & F University, Yangling, China
Aroma is a critical factor in determining grape quality, develops through complex interactions among various volatile compounds. This study revealed the differences of the six grape varieties with three different aroma types though the HS-SPME/GC-MS and RNA-sequencing technologies. Muscat-type grapes ('Shine 13' and 'Shine Muscat') exhibited the highest monoterpene and C13-norisoprenoid level, correlating with elevated expression of DXS, TPS, and CCD4b genes in the MEP/MVA pathways. Strawberry-type cultivars (particularly 'Hutai 8') accumulated abundant esters linked to high AAT expression, while neutral aromatic varieties showed enriched C6/C9 compounds associated with upregulated LOXA and ADH2. Muscat-type grapes dominated monoterpenes with OAVs >1, which explained the abundant Muscat flavors, while neutral aromatic aroma cultivars had the most abundant C6/C9 compounds OAVs associated with leaf-like scents. Strawberry-type cultivars exhibited the highest esters OAVs with strawberry aroma profiles. WGCNA analysis revealed four specific modules correlated with aroma compound biosynthesis correlated with alcohols (88genes), carbonyl compounds (451genes), fatty acids (110 genes), and monoterpenes (790genes) accumulation in these grapes, respectively. These findings were expected to advance our understanding of the metabolic pathways responsible for grape aroma and could provide valuable recommendations for the enhancement of grape aromatic quality.
1 Introduction
Grapes are one of the most famous fruits, typically consumed either as table grapes or processed into juice and wine (Dong et al., 2023). Grape aroma, a critical aspect of grape quality, arises from intricate chemical processes involving multiple compound classes, which shape the distinct sensory traits of various grape cultivars (Ghaste et al., 2015). Previous studies have confirmed that monoterpenes, C6/C9 compounds, C13-norisoprenoids, and esters are the dominant substances contributing to grape aromas (Wu et al., 2020; Feng et al., 2022; Wang W.-N. et al., 2023). Grapes are categorized into Muscat, strawberry, and neutral aromatic cultivars based on the level of monoterpenes. Among these compounds, monoterpenes are the primary aroma compounds to Muscat cultivars (Mateo and Jiménez, 2000), C6/C9 compounds are regarded as the fundamental, underlying scents in grapes (Wu et al., 2016); Yao et al. (2021) found that esters were the key volatile compounds in ‘Hutai 8’ (Wu et al., 2016). In grape berries, aroma compounds occur in free and glycosidically bound forms. Glycosidically bound aromas predominantly exist in glycosylated molecules that are hydrophilic, non-volatile, and flavorless. Free volatile aroma can be converted into glycosylated precursor by UDP-glycosyltransferases (UGTs) (Ghaste et al., 2015), which can directly provide grapes with floral, fruity, rose-like, and green flavors (Wang et al., 2021; Liu et al., 2022). However, until now little information about comprehensive and detailed free and bound aroma profiles of different aroma types of grape varieties has been reported.
In grape berry, the synthesis of aromatic substances included terpene metabolism (Supplementary Figure S1A), amino acid metabolism (Supplementary Figure S1B), and fatty acid metabolism (Supplementary Figure S1C). Monoterpenes, including α-terpineol, linalool, and geraniol, are considered the primary fragrant substances in Muscat grape cultivars (Luan et al., 2006). Variation in Muscat flavor among grape cultivars is attributed to the diverse monoterpene profiles (Liu et al., 2022). Monoterpenes and norisoprenoids are primarily synthesized through the methyl-erythritol phosphate (MEP) and mevalonate (MVA) pathways (Li et al., 2025). The MEP pathway takes place in plastids, whereas the MVA pathway occurs in the cytosol (Vranová et al., 2013). Terpene biosynthesis begins with the generation of isopentenyl pyrophosphate (IPP), followed by the isomerization to form dimethylallyl pyrophosphate (DMAPP) (Leng et al., 2023). The genesis of grape aroma compounds also stems from the metabolism of fatty acids, whether saturated or unsaturated, which are channeled through β-oxidation and the LOX-HPL metabolic pathway, yielding alcohols, aldehydes, ketones, acids, and esters (Kiralan et al., 2019). Moreover, these volatiles act as signaling molecules for pollination and defense against pathogen invasion (D’Auria et al., 2007). It has been indicated that alcohol acyltransferase (AAT) plays a key role in catalyzing the formation of volatile esters in fruits by linking alcohols with acyl-CoA (Beekwilder et al., 2004). Two principal types of amino acids exist, classified by their structure: aliphatic and aromatic amino acids. Leucine, isoleucine, valine, alanine, and cysteine are among the amino acids that can serve as precursors for volatile compounds in fruits, which can metabolize into alcohols, aldehydes, ketones, and esters in plants (Pan et al., 2012). However, at present, the pathway and molecular regulation mechanism of grape aroma synthesis have not been fully clear.
In this research, the neutral grape cultivars ‘Red Globe’ (V. vinifera) and ‘Moldova’ (a hybrid of V. labrusca and V. vinifera), alongside the Muscat-scented ‘Shine Muscat’ and ‘Shine 13’ (both hybrid of V. labrusca and V. vinifera), as well as the strawberry-aroma varieties ‘Summer Black’ and ‘Hutai 8’ (both hybrids of V. labrusca and V. vinifera) were selected to analyze distinct aroma compounds and identify candidate genes impart to these aroma differences in the different aroma-type grapes. The volatile compounds of grape berries were identified by GC−MS. Weighted gene co-expression network analysis (WGCNA) was conducted to explore the candidate genes related to the target traits. RNA sequencing and qRT-PCR methods were employed to examine transcriptomic signatures. By integrating transcriptome and metabolome, we identified the differences in the aroma profiles of different aroma types and elucidate the genetic basis of cultivar-specific aroma formation. The findings from this research offer valuable understanding regarding the differences among aroma-type grape cultivars, the fundamental biological processes involved, and the core molecular dynamics within grape berries. This not only enhances our understanding of the chemical basis of grape aroma diversity but also offers practical guidance for vineyard management and breeding strategy. Moreover, our findings open up several avenues for future research. The correlations we found between certain molecular markers and aroma traits can be further explored through genetic studies, potentially leading to the development of new grape cultivars with desired flavor characteristics.
2 Materials and methods
2.1 Grape cultivars
This research used six grape cultivars with different aroma types as experimental materials. These grape cultivars were collected at maturity from Weinan-based vineyards (34°50′N,109°48′E), Shanxi, China. The grape cultivars in this study were all grown on their own roots and protected by rain shelters, ensuring uniformity in vineyard management across all varieties. Three biological replicates were gathered for each type of grape, with each replicate consisting of 200 berries. These berries were chosen at random from a pool of at least 50 vines, sampled from both the North-South sides of the vineyard canopy to ensure representation. The collected samples were then flash-frozen using liquid nitrogen and preserved at a temperature of -80 degrees Celsius for subsequent analysis of the metabolome and transcriptome.
2.2 Extraction of free and glycosidically bound aroma compounds
Forty frozen grape berries were de-seeded, and the pulp and skin were mashed into a powder with 2.0g of polyvinylpyrrolidone (PVPP) and 0.5g of D-gluconolactone, using liquid nitrogen. The pulp was processed by crushing, blending, and a 4-hour soak at 4°C. Following this, it was processed by centrifugation at 8000g for 15 minutes to yield purified grape juice. At last, clear grape juice was used to detect the free volatile compounds. For each respective sample, three independent extractions were conducted.
The methods of extracting the glycosidically bound aroma compounds were consistent with (Wen et al., 2015). This process was repeated three times for each sample to create independent extracts.
2.3 HS-SPME/GC-MS analysis of aroma compounds
An automated HS-SPME system using a 2-cm DVB/CAR/PDMS fiber measuring 50/30 μm (Supelco, Bellefonte, PA, USA) and a CTC Combi PAL autosampler (CTC Analytics, Zwingen, Switzerland) was employed for extraction aromatic compounds from clear juice or mixtures. The SPME fiber underwent an activation step was consistent with (Lan et al., 2016).
Volatile compound analysis of six different grape cultivars was performed operating with an Agilent 6890 gas chromatograph integrated with an Agilent 5975 detector (GC-MS, Agilent Technologies, Santa Clara, CA). The conditions of GC-MS were consistent with (Sun et al., 2020).
The aroma compounds were pinpointed by correlating their mass spectral fingerprints with entries in the NIST14 mass spectrometry database and by aligning retention indices of the compounds against known reference values. This process was streamlined through the Automated Mass Spectral Deconvolution and Identification System (AMDIS), which seamlessly computed the retention indices and mass spectral data.
2.4 Odor activity values
OAVs were determined applying the formula OAV = c/t, with c representing the concentration of free volatiles and t referring to the odor threshold, derived from literature data.
2.5 RNA- sequencing and quantitative real-time PCR analyses
The Vazyme FastPure Universal Plant Total RNA Isolation Kit (RC411) was utilized to isolate total RNA from the pulp and skin of six grape varieties. RNA samples were used to build sequencing libraries, and subsequently examined using the BGISEQ-500 platform (Beijing Genomic Institution, www.genomics.org.cn) to produce 150-base end-sequences. Subsequently, a thorough transcriptomic analysis was conducted using the filtered initial reads sequencing data to the Vitis vinifera template genome accessible at Ensembl Plants. Gene expression was quantified using the FPKM approach. The ‘limma’ package in R (v. 4. 2. 3) was utilized to analyze expression Expression differences. A threshold of absolute log (2Fold change) ≥1 and P < 0.05 was applied for identifying differentially expressed genes (DEGs). The DEGs were employed in functional category enrichment within the KEGG pathway framework.
The primers for qRT-PCR were detailed in Supplementary Table S2.
2.6 Statistical analysis
Monoterpene concentrations of compound were compared using one-way ANOVA to evaluate differences. Subsequently, a Tukey’s HSD test was conducted with a significance level of P < 0.05, utilizing the SPSS 20.0 software (IBM, Armonk, NY). Histograms were generated using OriginPro 2021 (OriginLab Corporation, Northampton, MA). ‘pheatmap’ package in R (v. 4. 2. 3) was used to plot the heatmaps. The KEGG analysis was performed using KOBAS, a freely accessible online data analysis service (Bu et al., 2021). The ‘ggplot2’ package in R (v. 4.2.3) was used to plot the KEGG analysis enrichment pathway and KOG function classification histograms. Co-expression analysis was conducted utilizing gene expression data across the six grape cultivars.
3 Results and discussion
3.1 Free aroma compounds in six grape varieties
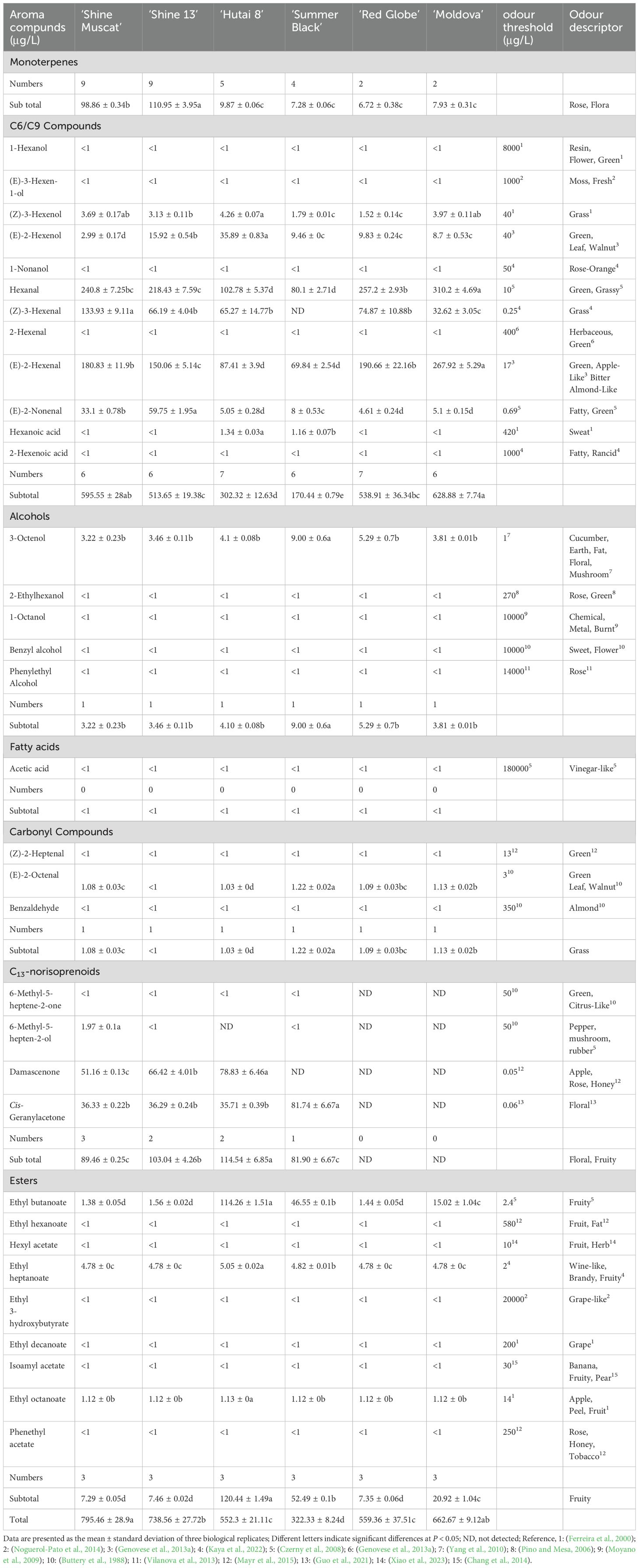
Aromatic substances reside in the grape exocarp and pulp in free and bound glycosides. The free volatiles directly impart the characteristic flavors to the grapes (Wu et al., 2019). In this research, a total of 61, 61, 57, 56, 41, and 48 free aroma compounds were found in ‘Shine Muscat’, ‘Shine 13’, ‘Hutai 8’, ‘Summer Black’, ‘Red Globe’ and ‘Moldova’, individually (Supplementary Table S2; Figure 1A). The free aroma volatiles concentration was highest for ‘Moldova’ (11814.15μg/L) and ‘Red Globe’ (8935.6μg/L), followed by ‘Shine Muscat’ (8912.43μg/L) and ‘Shine 13’ (8829.95μg/L), while it was lowest concentration for ‘Hutai 8’ (8626μg/L) and ‘Summer Black’ (6056.3μg/L) (Supplementary Table S2; Figure 1A). Of the grape cultivars included in this study, ‘Shine Muscat’ and ‘Shine 13’ among the six table grapes as Muscat varieties (Wang et al., 2021). ‘Hutai 8’ and ‘Summer Black’ were classified as strawberry-type cultivars (Wu et al., 2019).”Red Globe” and “Moldova” were categorized as neutral aromatic cultivars (Xiang et al., 2022). ‘Shine Muscat’ and ‘Shine 13’ exhibited the highest levels of free monoterpenes, with 996.23μg/L and 994.03μg/L, respectively (Supplementary Table S2; Figure 1A). Monoterpenes are considered to be the dominant compound of the Muscat cultivars (Mateo and Jiménez, 2000). In this study, linalool, trans-β-ocimene, cis-β-ocimene, β-myrcene, and geraniol were the main monoterpenes in Muscat-type cultivars. Among them, linalool was the richest monoterpene in Muscat-type cultivars. Fenoll et al. (2009) investigated composition, concentration, and aromatic impact of monoterpenes during the development of muscat humburg grape, identifying linalool as the most significant contributor to the aroma profiles. In line with our results, earlier research has indicated that linalool, geraniol, and β-myrcene were the dominant monoterpene in Muscat-type cultivars (Wang H. et al., 2023; Wang W.-N. et al., 2023; Yue et al., 2022). The concentration of free C13-norisoprenoids was highest in ‘Shine Muscat’(109.38μg/L) and ‘Shine 13’(28.09μg/L), followed by ‘Summer Black’(20.85μg/L) and ‘Hutai 8’(13.78μg/L), while in neutral aromatic cultivars were not detected. 6-Methyl-5-hepten-2-ol was the most abundant C13-norisoprenoids in Muscat-type cultivars (Supplementary Table S2; Figure 1A). Volatiles esters have been identified as the predominant volatile compounds in some hybrid cultivars derived from V. vinifera and V. labrusca, significantly contributing to the overall aromatic profile of these hybrids (Yang et al., 2011; Wu et al., 2016). In this study, the concentration of free esters was highest in ‘Hutai 8’(838.16μg/L) and ‘Summer Black’(275.55μg/L). Furthermore, ethyl 3-hydroxybutyrate was the most abundant ester in ‘Hutai 8’ and ‘Summer Black’, followed by ethyl butanoate and ethyl hexanoate (Supplementary Table S2; Figure 1A). Similarly, Yao et al. (2021) reported that the aldehydes and esters were the predominant aroma compounds in ‘Hutai 8’ grape. In accordance with our findings, previous studies reported ethyl butanoate and ethyl hexanoate were rich in strawberry-type grape cultivars (Qian et al., 2019; Yang et al., 2009). The highest levels of free C6/C9 compounds were found in ‘Moldova’ (8821.79μg/L) and ‘Red Globe’ (6828.49μg/L). Moreover, (E)-2-hexenal were the most abundant C6/C9 compounds in ‘Moldova’ and ‘Red Globe’, followed by hexanal, (E)-2-hexenol, and hexanoic acid (Supplementary Table S2; Figure 1A). Consistent with our results, previous investigations reported the neutral aromatic cultivars had the highest (E)-2-hexenal, followed by hexanal (Aubert and Chalot, 2018; Wang W.-N. et al., 2023). Terpenes and esters are present in low concentration in neutral cultivars, Koyama et al. (2022) observed that C6 compounds account for the main aroma compounds, though the concentration of terpenes and esters tends to be low in neutral grapes. It should be recognized that the concentration of C6/C9 compounds was the highest aroma compound in six grape cultivars. Wu et al. (2016) have indicated that the C6 compounds are the basic background volatile aromas, which is consistent with our findings. However, the aroma characteristics of grape berries mainly depend on the contents of monoterpenes and esters. Additionally, the neutral aromatic cultivars also had the most abundant carbonyl compounds. Among them, the concentration of benzaldehyde was the highest compound in neutral aromatic grapes.
3.2 Glycosidically-bound aroma compounds in six grape varieties
The bound aroma is mainly present in hydrophilic, non-volatile, and flavorless glycosylated molecules and the bound aroma compounds can be converted into their free form through hydrolysis (Wu et al., 2020). In grapes, the concentration of glycosylated aroma acting as a key indicator of the aromatic potential (Hernandez-Orte et al., 2015). In this research, a total of 59, 59, 55, 53, 48, and 46 bound aroma compounds were identified in ‘Shine Muscat’, ‘Shine 13’, ‘Hutai 8’, ‘Summer Black’, ‘Red Globe’, ‘Moldova’, respectively (Supplementary Table S2; Figure 1B). The bound monoterpene concentration was highest in ‘Shine 13’ (3613.85μg/L) and ‘Shine Muscat’(2512.65μg/L), succeeded by ‘Summer Black’ (1462.62μg/L) and ‘Hutai 8’ (996.78μg/L), while the neutral aromatic cultivars ‘Red Globe’ (359.66μg/L) and ‘Moldova’ (290.28μg/L) had the lowest bound monoterpene concentration. This result was consistent with the concentration of free monoterpene. This study revealed that nerol was the highest concentration bound monoterpene identified in ‘Shine 13’ and ‘Shine Muscat’, followed by linalool and geraniol. Corresponding to our results, previous studies reported that bound nerol, linalool, and geraniol were the major glycosidically monoterpenes in Muscat-type cultivars (Ruiz-García et al., 2014). Linalool and nerol can be synthesized by geraniol, are characterized by their distinctive floral, sweet, and fruit aroma, and serve as the primary compounds responsible for the aromatic profile of Muscat grapes (Fenoll et al., 2009). The abundant linalool, nerol, and geraniol may suggest the related biosynthesis pathways in Muscat grapes. Matsumoto and Ikoma (2016) found that linalool, nerol, and geraniol are the most primary aromatic compounds and responsible for the aroma in Muscat cultivars. The bound C13-norisoprenoids concentration was highest in ‘Shine 13’ (267.64μg/L) and ‘Shine Muscat’ (158.99μg/L), followed by ‘Hutai 8’ (52.63μg/L) and ‘Summer Black’ (42.97μg/L), however, ‘Red Globe’ (40.9μg/L) and ‘Moldova’ (41.07μg/L) had the lowest C13-norisoprenoids. This result was in line with the free C13-norisoprenoids, and the bound C13-norisoprenoids were higher than the free form. 6-Methyl-5-hepten-2-ol was the most abundant bound C13-norisoprenoid in Muscat-type cultivars, while strawberry and neutral cultivars were not identified. The concentration of bound esters was highest for strawberry-type cultivars, specifically rich in ‘Hutai 8’ (5190.29μg/L). Ethyl 3-hydroxybutyrate was the predominant ester in ‘Hutai 8’, which is consistent with its high concentration among the free esters. Fatty acids undergo metabolic processes within fruits to yield volatile esters (Qin et al., 2014). Therefore, alcohols, as metabolic precursors, may play important roles in influencing the compounds and distribution of volatile esters within grape berries (Qian et al., 2019). The concentration of bound alcohols was found to be the highest in ‘Hutai 8’ (6674.97μg/L) and ‘Summer Black’ (5877.63μg/L). Phenylethyl alcohol emerged as the most abundant alcohol, followed by benzyl alcohol, which in agreement with an earlier study on strawberry-type cultivars (Qian et al., 2019).
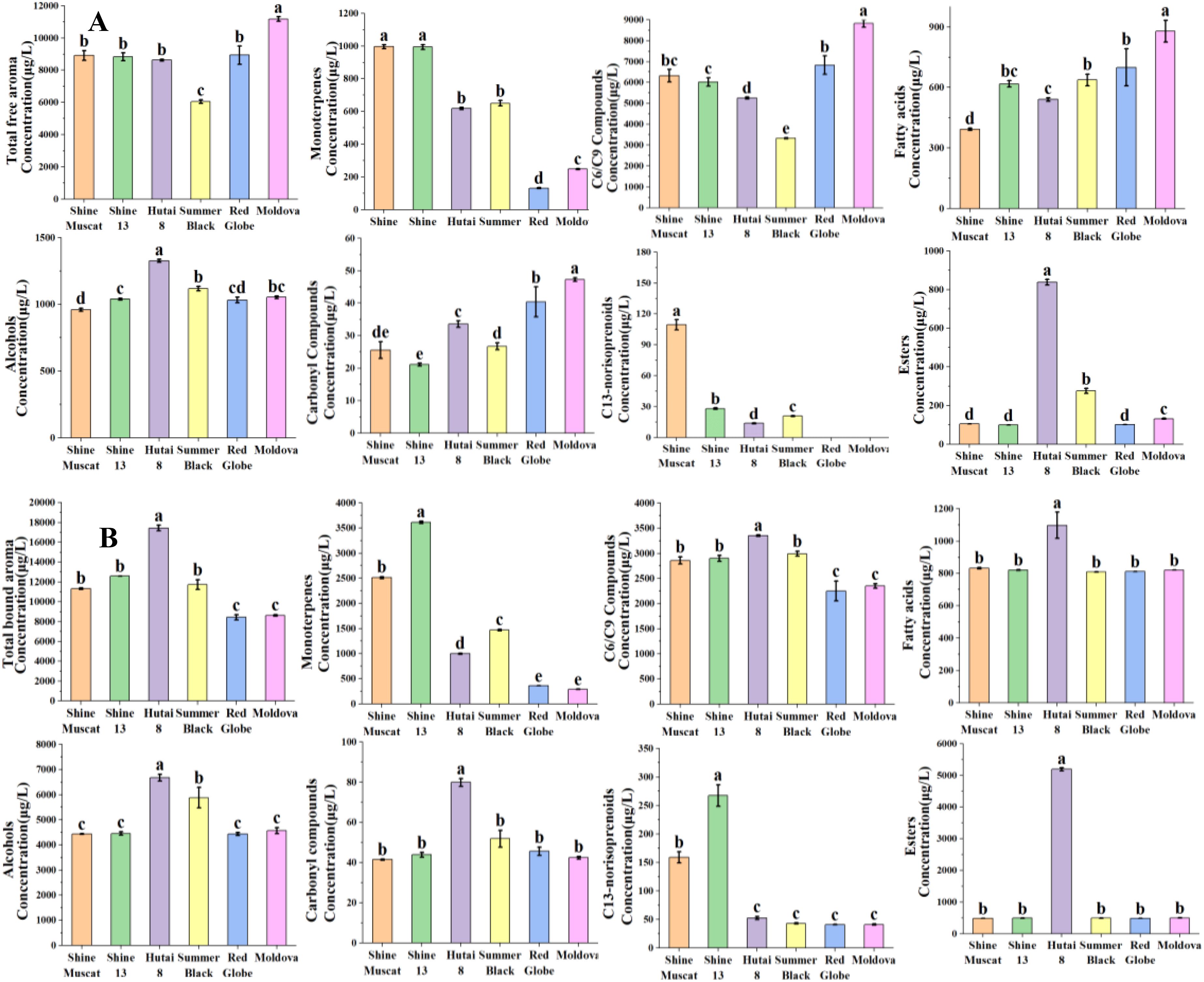
Figure 1. Concentrations of free (A) and (B) aroma compounds in six grape cultivars. Different letters in each graph indicated significant differences at P < 0.05.
3.3 Principal component analysis of free and glycosidically-bound aroma compounds in six grape cultivars
PCA was conducted on the six grape cultivars using the free and bound aroma compounds as the variables to evaluate their cultivar characteristics. The first and second principal components (PC1 and PC2) accounted for 33.42% and 27.06% of the total variance in the free aroma compounds of the six grape cultivars (Figure 2A), as well as 42.25% and 22.69% of the total variance in the bound aroma compounds of the six grape cultivars (Figure 2B), respectively. It was observed that both the free and glycosidically-bound aromas in the six grape cultivars were divided into three parts: ‘Shine Muscat’ and ‘Shine 13’, ‘Hutai 8’ and ‘Summer Black’, as well as ‘Moldova’ and ‘Red Globe’ were in the same group, respectively. Accordingly, all grapes in the same cultivar type had similar aroma profiles.
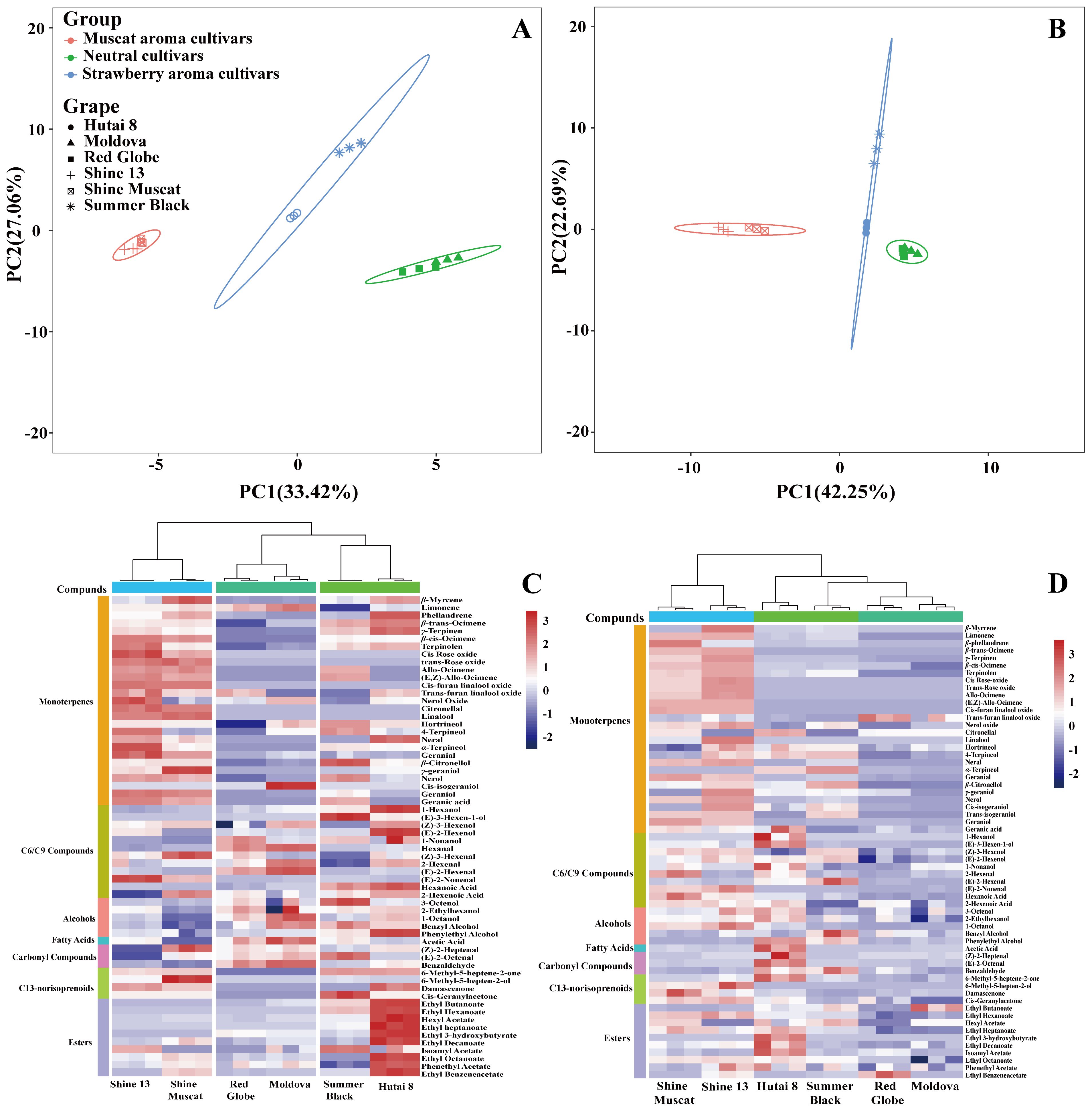
Figure 2. Principal component analysis (PCA) of free (A) and bound (B) volatile compounds of six grape cultivars. Heatmap analysis of free (C) and bound (D) aroma compounds.
3.4 Heatmap analysis of free aroma compounds
To further explore the variations of the free and bound aroma profiles of six grape cultivars, a heatmap analysis was performed. The results revealed significant inter-cultivar differences in both the abundance and diversity of aroma compounds (Figure 2C), corroborating previous findings that even cultivars sharing the same aroma classification exhibit distinct compound profiles (Wu et al., 2019). Hierarchical clustering grouped the cultivars into three distinct clusters: Muscat-flavored cultivars (‘Shine Muscat’ and ‘Shine 13’); strawberry-aroma cultivars (‘Hutai 8’ and ‘Summer Black’); neutral-aroma cultivars (‘Red Globe’ and ‘Moldova’). This observation was in alignment with the findings presented in Section 3.3, suggesting that grape cultivars of the same aroma type share similar aroma profiles and genetic backgrounds. In this study, a total of 27 monoterpenes, 12 C6/C9 compounds, 5 alcohols, 1 fatty acid, 3 carbonyl compounds, C13-norisoprenoids, and 10 esters were detected. Muscat-flavored clusters exhibited strong positive correlations with monoterpenes and C13-norisoprenoids. Strawberry-aroma clusters were predominantly associated with alcohol and esters. Neutral-aroma clusters showed higher affinity for C6/C9 compound. The distinct clustering further may support the utility of volatile compound profiling as a tool for cultivar classification and quality prediction.
3.5 Heatmap analysis of glycosidically-bound aroma compounds
Figure 2D illustrated the variability of bound aroma compounds across six grape cultivars. A total of 58 bound volatiles were identified, comprising 28 monoterpenes, 10 C6/C9 compounds, 5 alcohols, 1 fatty acid, 3 carbonyl compounds, 2 C13-norisoprenoids, and 10 esters. Hierarchical clustering revealed two distinct groups: Muscat-flavored cultivars (‘Shine Muscat’ and ‘Shine 13’); neutral-aroma cultivars (‘Red Globe’ and ‘Moldova’). This clustering aligns with their free aroma profiles, suggesting conserved biosynthetic regulation of both free and bound forms within cultivars. However, ‘Hutai 8’ and ‘Summer Black’ (strawberry-aroma cultivars) were not clustered, suggesting the lower concentration of bound aroma compounds in ‘Summer Black’. Notably, Muscat-type cultivars (‘Shine 13’ and ‘Shine Muscat’) exhibited strong positive correlations with bound monoterpene and C13-norisoprenoids, mirroring their free volatile associations. In contrast, strawberry-aroma cultivars (‘Hutai 8’ and ‘Summer Black’) showed higher affinity for fatty acid, carbonyl compounds, and ester, potentially contributing to their characteristic ripe-fruit aroma. These findings highlight the parallel between bound and free volatile profiles.
3.6 OAVs of volatile aroma compounds in six grape varieties
This study described the results of the OAVs in six grape cultivars, which were employed to evaluate the impact of each component on grape aromas (Table 1). The OAV, derived from the concentration of the aroma compound over its odor detection threshold, serves as a crucial metric for assessing the quality of grape aromas (Yang et al., 2019). However, only the OAV > 1 of volatile compounds was regarded as contributing to the aroma (Genovese et al., 2013b).
A total of 9, 9, 5, 4, 2, and 2 monoterpenes in ‘Shine Muscat’, ‘Shine 13’, ‘Hutai 8’, ‘Summer Black’, ‘Red Globe’, and ‘Moldova’ had OAVs >1, respectively. The Muscat-type cultivars exhibited the highest OAVs among the six grapes, with especially high proportions for linalool and rose oxide, which impart the floral, rose, and sweet taste characteristics (Wu et al., 2019). Corresponding to our results, previous results discovered linalool and α-terpineol with OAVs>1 as being linked to the Muscat aroma (Yang et al., 2024). It was observed that 6, 6, 7, 6, 7, and 6 free C6/C9 compounds in ‘Shine Muscat’, ‘Shine 13’, ‘Hutai 8’, ‘Summer Black’, ‘Red Globe’ and ‘Moldova’ had OAVs >1, respectively. The OAVs of C6/C9 compounds, which are known to primarily possess green leaf or floral and fruity odor (Kalua and Boss, 2009; Yue et al., 2023), were relatively high, thus may significantly influence the formation of the volatile profiles of six grape cultivars. Wu et al. (2016) reported that the C6 compounds are the basic background volatile aromas, while the aroma characteristics of grape pulp and peel mainly depend on the contents of monoterpenes and esters. Among the C6/C9 compounds, the OAV of hexanal, (Z)-3-hexenal, and (E)-2-hexenal were present in relatively high in six grape varieties. Yang et al. (2009) documented that hexanal and (E)-2-hexenal were the dominant C6 volatile compounds and they were detected in nearly all the grape samples, which were consistent with our results. A total of 3, 2, 2, 1, 0, and 0 free C13-norisoprenoids in ‘Shine Muscat’, ‘Shine 13’, ‘Hutai 8’, ‘Summer Black’, ‘Red Globe’ and ‘Moldova’ had OAVs >1, respectively. Damascenone and cis-geranylacetone were the main factors influencing the aroma profiles of both Muscat-type and strawberry-type cultivars, due to their relatively high OAVs and low thresholds. These compounds can contribute to the development of apple, rose, honey, and floral notes in these grape berries (Mayr et al., 2015; Guo et al., 2021). A total of 3, 3, 3, 3, 3, and 3 free esters in ‘Shine Muscat’, ‘Shine 13’, ‘Hutai 8’, ‘Summer Black’, ‘Red Globe’, and ‘Moldova’ had OAVs >1, respectively. However, ‘Hutai 8’ and ‘Summer Black’ exhibited the highest OAVs in esters, with ethyl butanoate as the primary compound with an OAV >1, thereby contributing to the fruity aroma profile of these grape varieties.
3.7 Characterization and functional profiling of genes showing differential expression in three grape varieties
In this research, DEGs were calculated based on the FPKM value using R (v 4.2.3) with limma package, DEG analysis revealed upregulated and downregulated genes in three grape varieties of different aroma types (adj. P. Val < 0.05, |log2FC| ≥ 1) (Figure 3). A total of 2328 DEGs were identified in ‘RG’ (‘Red Globe’) and ‘S13’ (‘Shine 13’), of which 1157 and 1171 DEGs showed upregulated and downregulated expression levels, respectively (Figure 3A); A total of 2796 DEGs were found in ‘RG’ and ‘SB’ (‘Summer Black’), of which 1358 and 1438 DEGs exhibited upregulated and downregulated expression levels, respectively (Figure 3B); A total of 2523 DEGs were selected in ‘S13’ and ‘SB’, of which 1232 and 1291 DEGs showed upregulated and downregulated expression levels, respectively (Figure 3C).
Figure 3. DEGs in three aroma types of grape varieties (A–C). RG (‘Red Globe’), S13 (‘Shine 13’), SB (‘Summer Black’), and KEGG pathway analysis of DEGs. (D) RG (‘Red Globe’) vs S13 (‘Shine 13’); (E) RG vs SB (‘Summer Black’); (F) S13 vs SB; Count represents the DEG number. Gene ratios are calculated as the ratio of DEGs in each KEGG pathway compared with the total number of DEGs.
To further investigate the functions of these DEGs concerning the biosynthesis of aromatic compounds, we performed KEGG pathway enrichment analysis. It was noticed that 7, 7, and 2 pathways that were significantly enriched (P <0.01) in ‘RG-S13’, ‘RG-SB’, and ‘SB-S13’, respectively; KEGG enrichment showed significant enrichment in limonene and pinene degradation, glycine, serine and threonine metabolism, galactose metabolism, glyoxylate and dicarboxylate metabolism, protein processing in endoplasmic reticulum and biosynthesis of secondary metabolites and metabolic pathways at ‘RG-S13’ (Figure 3D); The significantly enriched KEGG pathways included diterpenoid biosynthesis, alanine, aspartate and glutamate metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, glycine, serine and threonine metabolism, cysteine and methionine metabolism, arginine and proline metabolism and biosynthesis of secondary metabolites at ‘RG-SB’ (Figure 3E). KEGG pathway enrichment analysis of the ‘SB-S13’ revealed enrichment in protein processing in endoplasmic reticulum and plant-pathogen interaction (Figure 3F). These significantly KEGG enrichment pathways mostly relate to the anabolism of amino acids, which were implicated in the anabolic and catabolic processes related to volatile compounds, including aldehydes, alcohols, esters, and methoxypyrazines.
3.8 Gene coexpression network analyses
With the acquisition of RNA expression data, and the aroma data of ‘RG’, ‘S13’, and ‘SB’ in the three different aroma types of grape berries, the gene co-expression network with weights was established using the optimal soft threshold, and 23,445 genes were selected for WGCNA analysis (Figure 4B). The genes were categorized into 8 distinct expression clusters, and a dendrogram of the gene clusters was constructed (Figure 4A). Based on the correlation results between modules and the concentration of aromas (Figure 4C), the MEpink (790 genes) and MEgreenyellow (110 genes) showed a significant correlation with monoterpenes, the MEblack (88 genes), MEsalmon (451 genes) and MEpink (790 genes) modules showed a significant correlation with C6/C9 compounds, MEblack (88 genes), MEsalmon (451 genes) modules were positively correlated with alcohols, MEgreenyellow (110 genes), MEpink (790 genes) modules were positively correlated with fatty acids, MEsalmon(451 genes), MEgreenyellow (110 genes) and MEpink (790 genes) modules were positively correlated with C13-norisoprenoids. MEblack (88 genes), MEsalmon (451 genes), and MEpink (790 genes) were positively correlated with esters.

Figure 4. WGCNA and KOG function classification analysis in three aroma types of grape varieties. (A) Phylogenetic tree heatmap associated with volatile aroma compounds. (B) Gene co-expression network heatmap plot. (C) Heatmap of correlation between co-expressed gene modules and volatile aroma compounds traits. (D) KOG function classification in three aroma types of grape varieties.
The KOG function classification was performed on the RNA expression data (Figure 4D). There were 1373 and 1270 genes associated with lipid transport and metabolism and amino acid transport and metabolism, respectively. In addition, 1643 genes were related to secondary metabolites biosynthesis, transport and catabolism. These genes may regulate compounds related to aroma metabolism and catabolism.
3.9 Expression analysis of genes related to aroma biosynthesis in six types of grape cultivars
Monoterpenes and C13-norisoprenoids are predominantly produced through MEP and MVA pathways which occur in grapes plastids and cytosol, respectively (Wen et al., 2015). C6/C9 compounds, alcohols, carbonyl compounds, and esters are primarily biosynthesized in β-oxidation and lipoxygenase-hydroperoxide lyase (LOX-HPL) pathways (Lin et al., 2019; Schwab et al., 2008; Wang et al., 2001).
The elevated expression of key terpenoid biosynthetic genes (DXS, CMK, HDS, HDR, TPS, HMGR, CCD4a CCD4b, PNlinNer2, CSLinNer2 and LIS) in muscat-flavored cultivars (‘Shine Muscat’ and ‘Shine 13’) (Figure 5) strongly supports their role in monoterpene accumulation. Estévez et al. (2001) reported that DXS acts as the pivotal enzyme that initiates the first step in the MEP pathway. Furthermore, previous quantitative trait loci (QTL) analyses have indicated that DXS exhibited a strong correlation with the flavor profile of Muscat grape berries (Emanuelli et al., 2010), suggesting that genetic selection for DXS activity may drive cultivar-specific flavor differentiation. Overexpression of the DXS1 gene in Nicotiana benthamiana leaves was demonstrated to enhance monoterpene biosynthesis, as reported by Wang et al. (2021). Notably, Yang et al. (2024) reported that relative expression levels of CMK and HDR were higher in muscat-type cultivars compared to neutral cultivars, which is consistent with our results. As a key rate-limiting enzyme in the MEP pathway, HDR regulates the synthesis of isoprenoids in plants (Banerjee and Sharkey, 2014). A previous study reported that HDR is the most expressed gene of the MEP pathway in ‘Sangiovese’ grape (D’Onofrio et al., 2018). Similarly, in this study, HDR also exhibited high expression levels in six grape cultivars. TPSs accelerate the concluding stage of free monoterpenes in the MEP pathway (Tholl, 2006). Until now, a total of 43 TPSs (terpene synthases) have been biochemically identified and characterized in grape berries (Lücker et al., 2004; Martin et al., 2010; Martin and Bohlmann, 2004). TPS, PNlinNer2, CSLinNer, and LIS belonged to TPSs gene family. HMGR is the key enzymes that catalyze the third step of the MVA pathway. A previous study indicated that the expression of HMGR genes was considerably elevated in Muscat grape varieties compared to strawberry-type cultivars (Zheng et al., 2021). Consistent with our results, the expression levels of HMGR in Muscat-type cultivars were significantly higher than in strawberry-type cultivars and neutral aromatic cultivars. The CCD4 contributes to the creation of norisoprenoid aroma compounds and produces a range of water-soluble, low-threshold volatile compounds through the targeted cleavage of oxidized carotenoids (Zhong et al., 2020). The levels of CCD4a/4b expression were considerably high in Muscat-type cultivars. Additionally, the high abundance of CCD4a/4b suggests potential crosstalk between carotenoid cleavage and monoterpene modification pathways, which may diversify volatile profiles through secondary transformations. In line with our results, an earlier investigation found that the abundance of CCD4 was more abundant in Muscat-type grape cultivars in comparison with those with a neutral aroma (Yang et al., 2024). These gene expression patterns correlated with the levels of terpene compounds, implying that these genes may play a predominant role in the variation of Muscat aroma among the six grape cultivars.
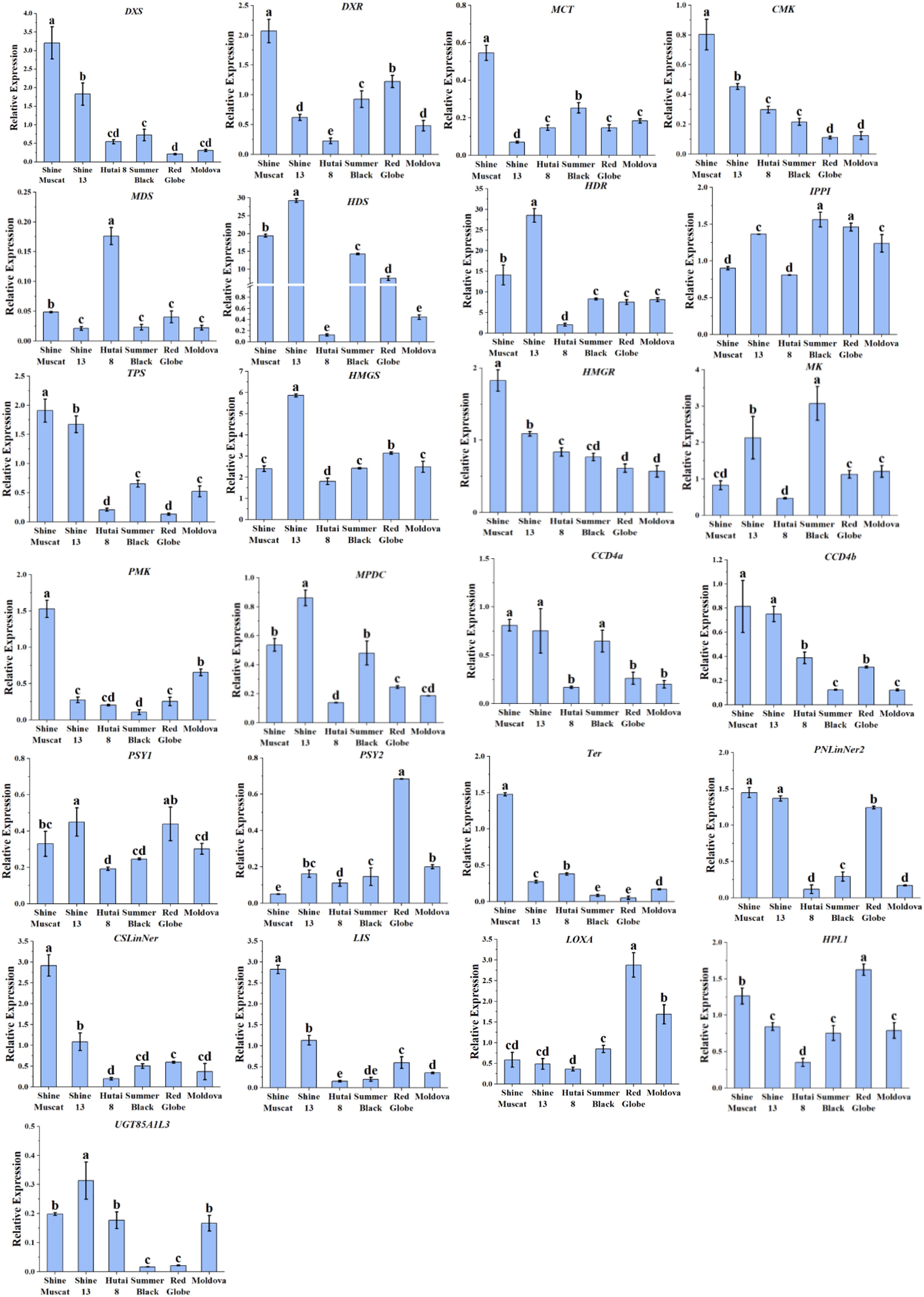
Figure 5. qRT-PCR analysis of the aroma related biosynthesis genes in six grape cultivars. Different letters in each graph indicated significant differences at P < 0.05.
Among the five genes in β-oxidation and LOX-HPL pathways, LOXA and HPL1 had the highest expression levels in ‘Red Globe’ (Figure 5). Our findings were in agreement with previous studies, which also reported that LOXA and HPL1 exhibited high expression levels in neutral aromatic cultivars (Qian et al., 2016). Significantly, ‘Hutai 8’ had the highest expression levels of AAT, followed by the ‘Summer Black’ and ‘Shine 13’. Additionally, ‘Hutai 8’ exhibited the highest concentration of free and bound esters, which was consistent with the expression levels of AAT. It was proposed that AAT genes could substantially impact the enzyme activity of AATs in fruits, thereby influencing ester production (Balbontín et al., 2010; El-Sharkawy et al., 2005; Souleyre et al., 2005).
3.10 Correlations between the expression levels of genes involved in aroma biosynthesis pathways and the composition of aroma compounds across six grape cultivar types
To explore the link between aroma concentrations and gene expression across six grape cultivars, Pearson correlation analysis was performed, and visualized with a correlation heatmap (Figure 6A). It was observed that the concentrations of linalool, γ- geraniol, and trans-rose oxide were significantly positively correlated with the expression levels of DXS. The concentrations of linalool, geranial, and cis-rose oxide were markedly related to the expression levels of HDR. The concentration of geraniol and geranic acid was substantially correlated to the expression levels of DXS. Previous studies found the expression level of VvDXS shows a positive correlation with the accumulation of monoterpenes in grape berries (Costantini et al., 2017; Wang et al., 2021). The concentration of γ-geraniol was considerably correlated with the expression levels of HMGR, LIS, and Ter. Furthermore, HMGR is a rate-limiting enzyme in the MVA pathway, controlling the monoterpene synthesis, and facilitates the enzymatic conversion of HMG-CoA to MVA (Leng et al., 2023). Additionally, previous studies have demonstrated that overexpression of HMGR (PtHMGR) in Populus trichocarpa modulates the expression of genes associated with the MVA and MEP pathways and markedly enhances the synthesis of terpenoid compounds (Wei et al., 2019). The concentration of trans-furan linalool oxide, citronellal, linalool, geranial, geraniol, and trans-rose oxide showed a marked positive association with the expression levels of TPS. Similarly, several studies reported that HDS, MVK, PNLinNer1, and DXS showed strong correlations with monoterpenes accumulation (Yang et al., 2024; Ji et al., 2021; Zhou et al., 2022).
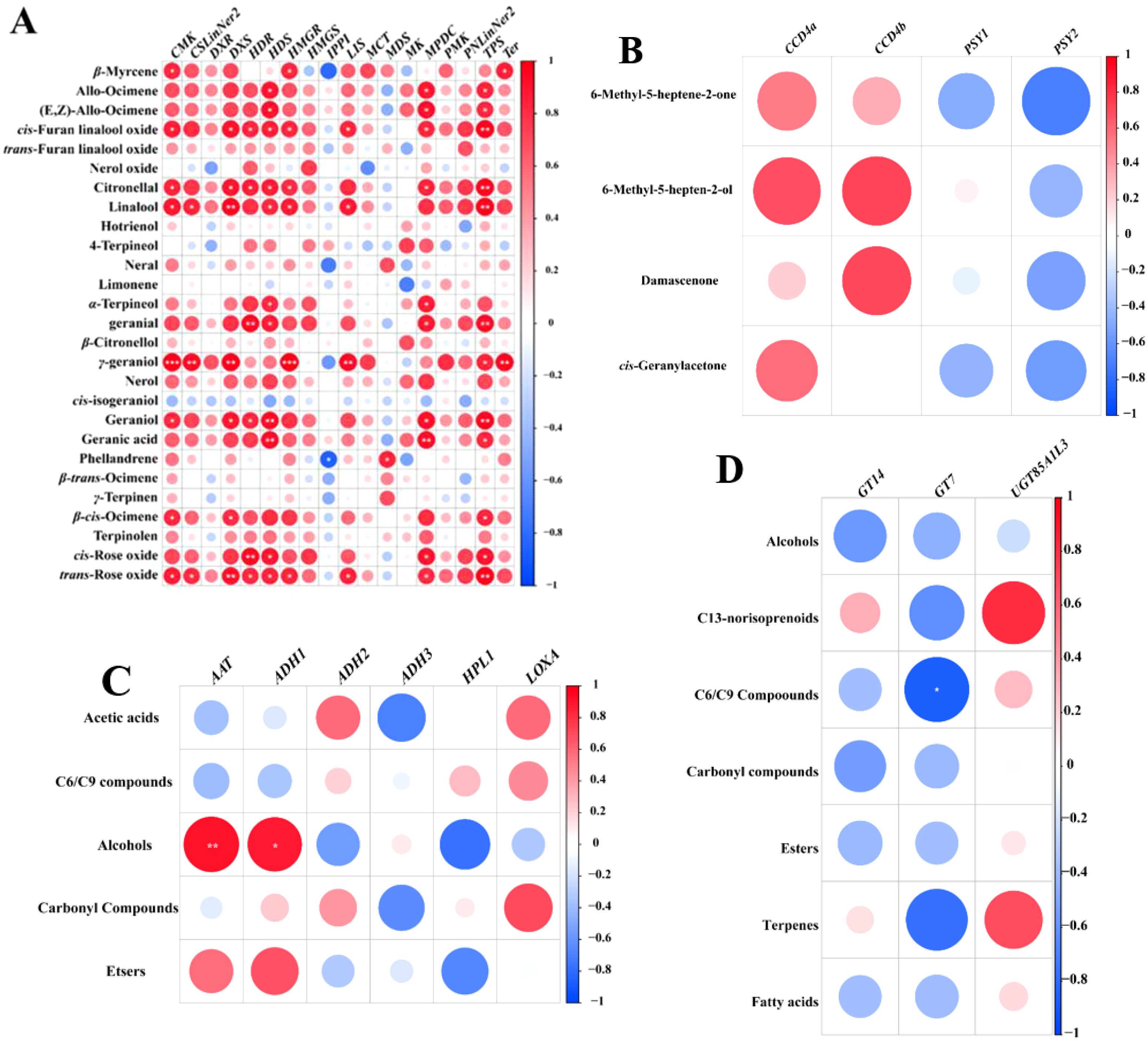
Figure 6. Correlations between the expression of aroma biosynthesis related genes and aroma compounds in six grape cultivars. (A) Correlation analysis of gene expression and monoterpene contents in MEP and MVA pathway; (B) Correlation analysis of gene expression and C13-norisoprenoids in MEP pathway; (C) Correlation analysis of gene expression and metabolite contents in LOX-HPL and β-oxidation pathway; (D) Correlation analysis of gene expression and bound aroma compounds. * indicates 0.01 < P < 0.05, ** indicates 0.001 < P < 0.01, *** indicates P ≤ 0.001.
The expression levels of ADH1 and AAT showed a marked positive association with the concentrations of alcohols and esters. (Figure 6C). Qian et al. (2019) observed that the genes (LOXA and LOXO) in the LOX pathway were positively related with their corresponding volatile esters. However, in this research, the expression levels of HPL1 were negatively correlated with the concentrations of esters and alcohols, it may be due to the differences of varieties, environment and cultivation conditions. The expression levels of CCD4a and CCD4b were positively correlated with the concentration of C13-norisoprenoids, whereas the expression levels of PSY1 and PSY2 were negatively correlated with the concentration of C13-norisoprenoids (Figure 6B). UGT85AIL3 expression correlated positively with bound C13-norisoprenoid and monoterpene concentrations, while GT7 expression showed a negative correlation with bound C6/C9 compound and monoterpene concentrations (Figure 6D). These discoveries align with the findings referred to in section 3.9 above.
4 Conclusions
This study provided comprehensive insights into the molecular and biochemical mechanisms underlying aroma diversity across six grape cultivars. There were 61, 61, 57, 56, 41, and 48 free aroma compounds as well as 59, 59, 55, 53, 48, and 46 bound aroma compounds were identified in ‘Shine Muscat’, ‘Shine 13’, ‘Hutai 8’, ‘Summer Black’, ‘Red Globe’ and ‘Moldova’, respectively. This reserach revealed distinct volatile profiles among the three aroma types, Muscat-type cultivars exhibited the highest levels of monoterpenes and C13-norisoprenoids, strawberry-type ‘Hutai 8’ was characterized by elevated ester content, while neutral aromatic cultivars showed dominance in C6/C9 and carbonyl compounds. PCA analysis further confirmed significant differences in aroma profiles, clustering the six cultivars into three distinct groups, aligning with their sensory classifications. Notably, there were 14, 13, 14, 12 12, and 11 volatiles with OAVs > 1 in six grape cultivars, respectively. Linalool, rose oxide, damascenone, and cis-geranylacetone were the main aroma compounds with OAVs >1 in Muscat-type grapes; ethyl butanoate was the main aroma compound with OAVs > 1 in strawberry-type grapes. RNA-sequencing and WGCNA analyses showed that 88 genes, 451 genes, 110 genes, and 790 genes had significantly positively correlated with alcohols, carbonyl compounds, fatty acids, and monoterpenes in three aroma types of grapes, respectively. Specifically, genes in the MEP pathway (DXS, CMK, HDS, HDR, TPS) and MVA pathway (HMGR) were highly expressed in Muscat-type cultivars, correlating strongly with monoterpenes accumulation. LOXA and ADH2 expression peaked in ‘Red Globe’, while AAT dominated in ‘Hutai 8’, consistent with their respective volatile signatures. Crucially, correlation analyses highlighted functional linkages between gene expression and aroma compound synthesis. For instance, CCD4a/b expression positively correlated with C13-norisoprenoid levels, while UGT85AIL3 was associated with bound monoterpenes and C13-norisoprenoids. Additionally, AAT and ADH1 expression aligned with ester and alcohol biosynthesis, respectively.
An understanding of the aroma profiles metabolism and genetic mechanism of volatile compounds is particularly important for breeding new grape cultivars. These findings not only elucidate the genetic basis of cultivar-specific aroma formation but also offer a framework for targeted breeding strategies to enhance flavor complexity in grape berries.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
GW: Formal Analysis, Investigation, Methodology, Software, Writing – original draft. YX: Investigation, Writing – review & editing. RR: Writing – review & editing. HC: Investigation, Software, Writing – review & editing. BY: Conceptualization, Investigation, Writing – review & editing. MG: Methodology, Project administration, Supervision, Writing – review & editing. SX: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (Grant No. 32302477), Postdoctoral Research Foundation of China (2023M732897) and Postdoctoral Research Foundation of Shaanxi Province (2023BSHEDZZ122).
Acknowledgments
We express our appreciation to Institute of Forestry and Pomology at the Beijing Academy of Agricultural and Forestry Sciences for providing access to their HS-SPME/GC-MS facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1544593/full#supplementary-material
References
Aubert, C., Chalot, G. (2018). Chemical composition, bioactive compounds, and volatiles of six table grape varieties (Vitis vinifera L.). Food Chem. 240, 524–533. doi: 10.1016/j.foodchem.2017.07.152
Balbontín, C., Gaete-Eastman, C., Fuentes, L., Figueroa, C. R., Herrera, R., Manriquez, D., et al. (2010). VpAAT1, a gene encoding an alcohol acyltransferase, is involved in ester biosynthesis during ripening of mountain papaya fruit. J. Agric. Food Chem. 58, 5114–5121. doi: 10.1021/jf904296c
Banerjee, A., Sharkey, T. D. (2014). Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 31, 1043–1055. doi: 10.1039/C3NP70124G
Beekwilder, J., Alvarez-Huerta, M., Neef, E., Verstappen, F. W. A., Bouwmeester, H. J., Aharoni, A. (2004). Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 135, 1865–1878. doi: 10.1104/pp.104.042580
Bu, D., Luo, H., Huo, P., Wang, Z., Zhang, S., He, Z., et al. (2021). KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 49, W317–W325. doi: 10.1093/nar/gkab447
Buttery, R. G., Turnbaugh, J. G., Ling, L. C. (1988). Contribution of volatiles to rice aroma. J. Agric. Food Chem. 36, 1006–1009. doi: 10.1021/jf00083a025
Chang, E.-H., Jung, S.-M., Hur, Y.-Y. (2014). Changes in the aromatic composition of grape cv. Cheongsoo wine depending on the degree of grape ripening. Food Sci. Biotechnol. 23, 1761–1771. doi: 10.1007/s10068-014-0241-y
Costantini, L., Kappel, C. D., Trenti, M., Battilana, J., Emanuelli, F., Sordo, M., et al. (2017). Drawing links from transcriptome to metabolites: The evolution of aroma in the ripening berry of Moscato Bianco (Vitis vinifera L.). Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00780
Czerny, M., Christlbauer, M., Christlbauer, M., Fischer, A., Granvogl, M., Hammer, M., et al. (2008). Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 228, 265–273. doi: 10.1007/s00217-008-0931-x
D’Auria, J. C., Pichersky, E., Schaub, A., Hansel, A., Gershenzon, J. (2007). Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 49, 194–207. doi: 10.1111/j.1365-313X.2006.02946.x
D’Onofrio, C., Matarese, F., Cuzzola, A. (2018). Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 242, 352–361. doi: 10.1016/j.foodchem.2017.09.084
Dong, Y., Duan, S., Xia, Q., Liang, Z., Dong, X., Margaryan, K., et al. (2023). Dual domestications and origin of traits in grapevine evolution. Science 379, 892–901. doi: 10.1126/science.add8655
El-Sharkawy, I., Manríquez, D., Flores, F. B., Regad, F., Bouzayen, M., Latché, A., et al. (2005). Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Mol. Biol. 59, 345–362. doi: 10.1007/s11103-005-8884-y
Emanuelli, F., Battilana, J., Costantini, L., Cunff, L. L., Boursiquot, J., This, P., et al. (2010). A candidate gene association study on muscat flavor in grapevine (Vitis vinifera L.). BMC Plant Biol. 10, 241. doi: 10.1186/1471-2229-10-241
Estévez, J. M., Cantero, A., Reindl, A., Reichler, S., León, P. (2001). 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J. Biol. Chem. 276, 22901–22909. doi: 10.1074/jbc.m100854200
Feng, M., Jin, X., Yao, H., Zhu, T., Guo, S., Li, S., et al. (2022). Evolution of volatile profile and aroma potential of ‘Gold Finger’ table grapes during berry ripening. J. Sci. Food Agric. 102, 291–298. doi: 10.1002/jsfa.11357
Fenoll, J., Manso, A., Hellin, P., Ruiz, L., Flores, P. (2009). Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 114, 420–428. doi: 10.1016/j.foodchem.2008.09.060
Ferreira, V., LóPez, R., Cacho, J. F. (2000). Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agriculture/Journal Sci. Food Agric. 80, 1659–1667. doi: 10.1002/1097-0010(20000901)80:11
Genovese, A., Gambuti, A., Lamorte, S. A., Moio, L. (2013a). An extract procedure for studying the free and glycosilated aroma compounds in grapes. Food Chem. 136 (2), 822–834. doi: 10.1016/j.foodchem.2012.08.061
Genovese, A., Lamorte, S. A., Gambuti, A., Moio, L. (2013b). Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 53 (1), 15–23. doi: 10.1016/j.foodres.2013.03.051
Ghaste, M., Narduzzi, L., Carlin, S., Vrhovsek, U., Shulaev, V., Mattivi, F. (2015). Chemical composition of volatile aroma metabolites and their glycosylated precursors that can uniquely differentiate individual grape cultivars. Food Chem. 188, 309–319. doi: 10.1016/j.foodchem.2015.04.056
Guo, X., Ho, C., Schwab, W., Wan, X. (2021). Aroma profiles of green tea made with fresh tea leaves plucked in summer. Food Chem. 363, 130328. doi: 10.1016/j.foodchem.2021.130328
Hernandez-Orte, P., Concejero, B., Astrain, J., Lacau, B., Cacho, J., Ferreira, V. (2015). Influence of viticulture practices on grape aroma precursors and their relation with wine aroma. J. Sci. Food Agric. 95, 688–701. doi: 10.1002/jsfa.6748
Ji, X., Wang, B., Wang, X., Wang, X., Liu, F., Wang, H. (2021). Differences of aroma development and metabolic pathway gene expression between Kyoho and 87-1 grapes. J. Integr. Agric. 20, 1525–1539. doi: 10.1016/s2095-3119(20)63481-5
Kalua, C. M., Boss, P. K. (2009). Evolution of Volatile Compounds during the Development of Cabernet Sauvignon Grapes (Vitis vinifera L.). J. Agric. Food Chem. 57, 3818–3830. doi: 10.1021/jf803471n
Kaya, O., Incesu, M., Ates, F., Keskin, N., Verdugo-Vásquez, N., Gutiérrez-Gamboa, G. (2022). Study of volatile organic compounds of two table grapes (cv. Italia and bronx seedless) along ripening in vines established in the aegean region (Turkey). Plants 11, 1935. doi: 10.3390/plants11151935
Kiralan, M., Çalik, G., Kiralan, S., Özaydin, A., Özkan, G., Ramadan, M. F. (2019). Stability and volatile oxidation compounds of grape seed, flax seed and black cumin seed cold-pressed oils as affected by thermal oxidation. Grasas y Aceites 70, 295. doi: 10.3989/gya.0570181
Koyama, K., Kono, A., Ban, Y., Bahena-Garrido, S. M., Ohama, T., Iwashita, K., et al. (2022). Genetic architecture of berry aroma compounds in a QTL (quantitative trait loci) mapping population of interspecific hybrid grapes (Vitis labruscana× Vitis vinifera). BMC Plant Biology 22 (1), 458.
Lan, Y., Qian, X., Yang, Z., Xiang, X., Yang, W., Liu, T., et al. (2016). Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ‘Beibinghong’ grapes in Northeastern China. Food Chem. 212, 172–182. doi: 10.1016/j.foodchem.2016.05.143
Leng, X., Cong, J., Cheng, L., Wan, H., Liu, Y., Yuan, Y., et al. (2023). Identification of key gene networks controlling monoterpene biosynthesis during grape ripening by integrating transcriptome and metabolite profiling. Hortic. Plant J. 9, 931–946. doi: 10.1016/j.hpj.2023.03.005
Li, W., Zheng, T., Zhang, J., Li, W., Chen, K., Zhang, K., et al. (2025). Supplementary light with different wavelengths improved the monoterpenes aroma and quality traits of ‘Shine Muscat’grape berries under facility cultivation. Food Chem. 474. doi: 10.1016/j.foodchem.2025.143255
Lin, J., Massonnet, M., Cantu, D. (2019). The genetic basis of grape and wine aroma. Hortic. Res. 6, 81. doi: 10.1038/s41438-019-0163-1
Liu, S., Shan, B., Zhou, X., Gao, W., Liu, Y., Zhu, B., et al. (2022). Transcriptome and metabolomics integrated analysis reveals terpene synthesis genes controlling linalool synthesis in grape berries. J. Agric. Food Chem. 70, 9084–9094. doi: 10.1021/acs.jafc.2c00368
Luan, F., Mosandl, A., Gubesch, M., Wüst, M. (2006). Enantioselective analysis of monoterpenes in different grape varieties during berry ripening using stir bar sorptive extraction- and solid phase extraction-enantioselective-multidimensional gas chromatography-mass spectrometry. J. Chromatogr. A 1112, 369–374. doi: 10.1016/j.chroma.2005.12.056
Lücker, J., Bowen, P., Bohlmann, J. (2004). Vitis vinifera terpenoid cyclases: Functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (−)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 65, 2649–2659. doi: 10.1016/j.phytochem.2004.08.017
Martin, D. M., Aubourg, S., Schouwey, M. B., Daviet, L., Schalk, M., Toub, O., et al. (2010). Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 10, 226. doi: 10.1186/1471-2229-10-226
Martin, D. M., Bohlmann, J. (2004). Identification of Vitis vinifera (−)-α-terpineol synthase by in silico screening of full-length cDNA ESTs and functional characterization of recombinant terpene synthase. Phytochemistry 65, 1223–1229. doi: 10.1016/j.phytochem.2004.03.018
Mateo, J. J., Jiménez, M. (2000). Monoterpenes in grape juice and wines. J. Chromatogr. A 881, 557–567. doi: 10.1016/S0021-9673(99)01342-4
Matsumoto, H., Ikoma, Y. (2016). Effect of postharvest temperature on the muscat flavor and aroma volatile content in the berries of ‘Shine Muscat’ (Vitis labruscana Baily×V. vinifera L.). Posth. Biol. Technol. 112, 256–265. doi: 10.1016/j.postharvbio.2015.09.004
Mayr, C. M., Capone, D. L., Pardon, K. H., Black, C. A., Pomeroy, D., Francis, I. L. (2015). Quantitative analysis by GC-MS/MS of 18 aroma compounds related to oxidative off-flavor in wines. J. Agric. Food Chem. 63, 3394–3401. doi: 10.1021/jf505803u
Moyano, L., Zea, L., Villafuerte, L., Medina, M. (2009). Comparison of odor-active compounds in sherry wines processed from ecologically and conventionally grown pedro ximenez grapes. J. Agric. Food Chem. 57, 968–973. doi: 10.1021/jf802252u
Noguerol-Pato, R., Sieiro-Sampredro, T., González-Barreiro, C., Cancho-Grande, B., Simal-Gándara, J. (2014). Effect on the aroma profile of graciano and tempranillo red wines of the application of two antifungal treatments onto vines. Molecules 19, 12173–12193. doi: 10.3390/molecules190812173
Pan, Q.-H., Chen, F., Zhu, B.-Q., Ma, L.-Y., Li, L., Li, J.-M. (2012). Molecular cloning and expression of gene encoding aromatic amino acid decarboxylase in ‘Vidal blanc’ grape berries. Mol. Biol. Rep. 39, 4319–4325. doi: 10.1007/s11033-011-1219-y
Pino, J. A., Mesa, J. (2006). Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragrance J. 21, 207–213. doi: 10.1002/ffj.1703
Qian, X., Liu, Y., Zhang, G., Yan, A., Wang, H., Wang, X., et al. (2019). Alcohol acyltransferase gene and ester precursors differentiate composition of volatile esters in three interspecific hybrids of Vitis labrusca x V. Vinifera during berry development period. Food Chem. 295, 234–246. doi: 10.1016/j.foodchem.2019.05.104
Qian, X., Xu, X., Yu, K., Zhu, B., Lan, Y., Duan, C., et al. (2016). Varietal dependence of GLVs accumulation and LOX-HPL pathway gene expression in four vitis vinifera wine grapes. Int. J. Mol. Sci. 17, 1924. doi: 10.3390/ijms17111924
Qin, G., Tao, S., Zhang, H., Huang, W., Wu, J., Xu, Y., et al. (2014). Evolution of the aroma volatiles of pear fruits supplemented with fatty acid metabolic precursors. Molecules 19, 20183–20196. doi: 10.3390/molecules191220183
Ruiz-García, L., Hellín, P., Flores, P., Fenoll, J. (2014). Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chem. 154, 151–157. doi: 10.1016/j.foodchem.2014.01.005
Schwab, W., Davidovich‐Rikanati, R., Lewinsohn, E. (2008). Biosynthesis of plant‐derived flavor compounds. Plant J. 54, 712–732. doi: 10.1111/j.1365-313X.2008.03446.x
Souleyre, E. J. F., Greenwood, D. R., Friel, E. N., Karunairetnam, S., Newcomb, R. D. (2005). An alcohol acyl transferase from apple (cv. Royal Gala), MpAAT1, produces esters involved in apple fruit flavor. FEBS J. 272, 3132–3144. doi: 10.1111/j.1742-4658.2005.04732.x
Sun, L., Zhu, B., Zhang, X., Wang, H., Yan, A., Zhang, G., et al. (2020). The accumulation profiles of terpene metabolites in three Muscat table grape cultivars through HS-SPME-GCMS. Sci. Data 7, 5. doi: 10.1038/s41597-019-0321-1
Tholl, D. (2006). Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 9, 297–304. doi: 10.1016/j.pbi.2006.03.014
Vilanova, M., Escudero, A., Graña, M., Cacho, J. (2013). Volatile composition and sensory properties of North West Spain white wines. Food Res. Int. 54, 562–568. doi: 10.1016/j.foodres.2013.07.036
Vranová, E., Coman, D., Gruissem, W. (2013). Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 64, 665–700. doi: 10.1146/annurev-arplant-050312-120116
Wang, W., Feng, J., Wei, L., Khalil-Ur-Rehman, M., Nieuwenhuizen, N. J., Yang, L., et al. (2021). Transcriptomics Integrated with Free and Bound Terpenoid Aroma Profiling during “Shine Muscat” (Vitis labrusca × V. vinifera) Grape Berry Development Reveals Coordinate Regulation of MEP Pathway and Terpene Synthase Gene Expression. J. Agric. Food Chem. 69, 1413–1429. doi: 10.1021/acs.jafc.0c06591
Wang, W.-N., Qian, Y.-H., Liu, R.-H., Liang, T., Ding, Y.-T., Xu, X.-L., et al. (2023). Effects of table grape cultivars on fruit quality and aroma components. Foods 12, 3371. doi: 10.3390/foods12183371
Wang, H., Wang, X., Yan, A., Liu, Z., Ren, J., Xu, H., et al. (2023). Metabolomic and transcriptomic integrated analysis revealed the decrease of monoterpenes accumulation in table grapes during long time low temperature storage. Food Res. Int. 174, 113601. doi: 10.1016/j.foodres.2023.113601
Wang, C., Xing, J., Chin, C.-K., Ho, C.-T., Martin, C. E. (2001). Modification of fatty acids changes the flavor volatiles in tomato leaves. Phytochemistry 58, 227–232. doi: 10.1016/S0031-9422(01)00233-3
Wei, H., Xu, C., Movahedi, A., Sun, W., Li, D., Zhuge, Q. (2019). Characterization and function of 3-hydroxy-3-methylglutaryl-CoA reductase in Populus trichocarpa: Overexpression of PtHMGR enhances terpenoids in transgenic poplar. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01476
Wen, Y.-Q., Zhong, G.-Y., Gao, Y., Lan, Y.-B., Duan, C.-Q., Pan, Q.-H. (2015). Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions. BMC Plant Biol. 15, 240. doi: 10.1186/s12870-015-0631-1
Wu, Y., Duan, S., Zhao, L., Gao, Z., Luo, M., Song, S., et al. (2016). Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 6, 31116. doi: 10.1038/srep31116
Wu, Y., Zhang, W., Song, S., Xu, W., Zhang, C., Ma, C., et al. (2020). Evolution of volatile compounds during the development of Muscat grape ‘Shine Muscat’ (Vitis labrusca × V. vinifera). Food Chem. 309, 125778. doi: 10.1016/j.foodchem.2019.125778
Wu, Y., Zhang, W., Yu, W., Zhao, L., Song, S., Xu, W., et al. (2019). Study on the volatile composition of table grapes of three aroma types. LWT 115, 108450. doi: 10.1016/j.lwt.2019.108450
Xiang, N., Xie, H., Qin, L., Wang, M., Guo, X., Zhang, W. (2022). Effect of Climate on Volatile Metabolism in ‘Red Globe’ Grapes (Vitis vinifera L.) during Fruit Development. Foods 11, 1435. doi: 10.3390/foods11101435
Xiao, Z., Li, B., Niu, Y., Xiong, J., Zhang, J. (2023). Characterization of key aroma-active compounds in yellow peach (Prunus persica L. ‘Jinxiu’) based on GC–MS–O, OAV, aroma recombination and omission experiment. J. Food Measure. Charact. 17, 4448–4461. doi: 10.1007/s11694-023-01970-0
Yang, C., Wang, Y., Wu, B., Fang, J., Li, S. (2011). Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chemistry 128 (4), 823–830.
Yang, C., Li, Y., He, L., Song, Y., Zhang, P., Liu, S. (2024). Metabolomic and transcriptomic analyses of monoterpene biosynthesis in Muscat and Neutral grape hybrids. Scientia. Hortic. 336, 113434. doi: 10.1016/j.scienta.2024.113434
Yang, C., Luo, L., Zhang, H., Yang, X., Lv, Y., Song, H. (2010). Common aroma-active components of propolis from 23 regions of China. J. Sci. Food Agric. 90, 1268–1282. doi: 10.1002/jsfa.3969
Yang, C., Wang, Y., Liang, Z., Fan, P., Wu, B., Yang, L., et al. (2009). Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC–MS. Food Chem. 114, 1106–1114. doi: 10.1016/j.foodchem.2008.10.061
Yang, Y., Zheng, F., Yu, A., Sun, B. (2019). Changes of the free and bound volatile compounds in Rubus corchorifolius L. f. fruit during ripening. Food Chem. 287, 232–240. doi: 10.1016/j.foodchem.2019.02.080
Yao, H., Jin, X., Feng, M., Xu, G., Zhang, P., Fang, Y., et al. (2021). Evolution of volatile profile and aroma potential of table grape Hutai-8 during berry ripening. Food Res. Int. 143, 110330. doi: 10.1016/j.foodres.2021.110330
Yue, X., Ju, Y., Cui, Y., Wei, S., Xu, H., Zhang, Z. (2023). Evolution of green leaf volatile profile and aroma potential during the berry development in five Vitis vinifera L. Cultivars. Food Chem.: X 18, 100676. doi: 10.1016/j.fochx.2023.100676
Yue, X., Ju, Y., Zhang, H., Wang, Z., Xu, H., Zhang, Z. (2022). Integrated transcriptomic and metabolomic analysis reveals the changes in monoterpene compounds during the development of Muscat Hamburg (Vitis vinifera L.) grape berries. Food Res. Int. 162, 112065. doi: 10.1016/j.foodres.2022.112065
Zheng, T., Guan, L., Yu, K., Haider, M. S., Nasim, M., Liu, Z., et al. (2021). Expressional diversity of grapevine 3-Hydroxy-3-methylglutaryl-CoA reductase (VvHMGR) in different grapes genotypes. BMC Plant Biol. 21, 279. doi: 10.1186/s12870-021-03073-8
Zhong, Y., Pan, X., Wang, R., Xu, J., Guo, J., Yang, T., et al. (2020). ZMCCD10A encodes a distinct type of carotenoid cleavage dioxygenase and enhances plant tolerance to low phosphate. Plant Physiol. 184, 374–392. doi: 10.1104/pp.20.00378
Keywords: grape, aroma, volatiles, HS-SPME/GC-MS, RNA-seq
Citation: Wu G, Xin Y, Ren R, Chen H, Yang B, Ge M and Xie S (2025) Comprehensive aroma profiles and the underlying molecular mechanisms in six grape varieties with different flavors. Front. Plant Sci. 16:1544593. doi: 10.3389/fpls.2025.1544593
Received: 16 December 2024; Accepted: 01 April 2025;
Published: 28 April 2025.
Edited by:
Robin Joshi, University of Pennsylvania, United StatesReviewed by:
Nan Lu, University of North Texas, United StatesPrithvi Pal, University of Georgia, United States
Copyright © 2025 Wu, Xin, Ren, Chen, Yang, Ge and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sha Xie, eGllc2hhQG53YWZ1LmVkdS5jbg==; Maosheng Ge, Z21zbm9uZ3NodWlAbndhZnUuZWR1LmNu
 Guang Wu
Guang Wu Yuchen Xin1
Yuchen Xin1 Sha Xie
Sha Xie