- Marine Bio-Products Research Laboratory, Department of Plant, Food and Environmental Sciences, Faculty of Agriculture, Dalhousie University, Truro, NS, Canada
Downy mildew, caused by Plasmopara viticola, poses a major threat to grapevine production, necessitating effective and sustainable disease management strategies. Ascophyllum nodosum extract (ANE) and Pseudomonas fluorescens CHA0 possess biostimulant and biocontrol properties, respectively, with reported potential to induce plant defense against various pathogens. However, their combined application and potential synergistic effects in grapevine disease management remain largely unexplored. In this study, their efficacy in controlling downy mildew in grapevines was investigated. Our results demonstrate that both ANE and CHA0, individually and in combination, significantly reduced downy mildew severity. In vitro assays revealed that grape leaves treated with ANE, CHA0, or their combination suppressed disease establishment and progression, with the combined treatment showing the greatest reduction in spore count (~66%), zoospore numbers (~89–93%), and empty sporangia (~53–69%). In vivo greenhouse experiments confirmed these findings, showing that combined foliar applications of ANE and CHA0 reduced downy mildew incidence to approximately 22%, compared to 40–50% with individual treatments and 70% in untreated controls, implying a synergistic interaction between these treatments. In addition to disease suppression, combined ANE and CHA0 applications triggered robust biochemical and molecular defense responses in grapevines. Key defense enzymes, including phenylalanine ammonia lyase, peroxidase, and polyphenol oxidase, showed significantly enhanced activities, accompanied by elevated levels of phenolics and hydrogen peroxide. At the molecular level, the combination significantly upregulated stress-responsive genes such as CHI, GLP2 and GLP7, along with jasmonic acid-responsive genes including LOX9, OSM1, and PR4, suggesting a priming effect that reinforces the plant’s innate immune responses. Overall, these findings highlight the potential of combined ANE and CHA0 treatments in enhancing grapevine resistance to downy mildew through the activation of defense mechanisms and modulation of key biochemical markers. The synergistic interplay between the biostimulant and biocontrol agent offers a promising eco-friendly alternative to conventional chemical-based disease management in viticulture.
1 Introduction
Grapes (Vitis vinifera L.) are one of the most widely cultivated fruit crops worldwide. They can be consumed fresh, dried to make raisins, crushed for juice, or fermented into alcoholic beverages, highlighting the grape industry’s extensive reach and economic significance. With increasing popularity, grapes have become the world’s third most valuable horticultural crop (Alston and Sambucci, 2019). Over the past decade, the global grape industry has experienced rapid growth, with grape production increasing from 69.1 million to 73.5 million tons (FAO, 2022). However, grape productivity is affected by various biotic and abiotic stresses, with downy mildew caused by the oomycete Plasmopara viticola being a significant concern. This pathogen poses a major threat to viticulture, impacting nearly all grapevine varieties. Symptoms of the disease include yellow circular spots on the upper surface of leaves and white, downy fungal growth on the lower surface (Peng et al., 2023). Although fungicides are crucial for managing downy mildew, their overuse has contributed to the resurgence of the pathogen and raised environmental concerns. Additionally, the growing demand for organic grapes and grape-based products is driving growth in the global organic grape market (Jindo et al., 2022). Therefore, exploring environmentally friendly alternatives for disease management is essential to minimize the chemical impact on grape production and promote sustainable practices.
An effective alternative to the use of pesticides and synthetic fertilizers is the application of biostimulants and biologicals. Biostimulants, widely used in organic vineyards, can reduce dependence on synthetic fertilizers, pesticides, and fungicides. Among these, seaweed extracts are particularly notable for their environmental sustainability, efficacy, and long shelf life. Ascophyllum nodosum extracts (ANE) have been extensively researched for their beneficial effects on plant health. At low concentrations, these extracts enhance plant resistance to a wide range of fungal and bacterial pathogens (Jardin, 2015; Bajpai et al., 2019; Bahmani et al., 2023b). A. nodosum contains several biologically active components, including phlorotannins, alginic acid, fucoidans, and laminarin (Shukla et al., 2019). Previous studies have demonstrated that seaweed extracts from various species can effectively control downy mildew in grapes. Raj et al. (2019) investigated the effects of extracts from brown, green, and red seaweeds on downy mildew and found that the extract from Hydroclathrus clathratus, a brown seaweed, was the most effective in preventing the disease. Extracts from A. nodosum have been shown to enhance grape growth by improving copper uptake (Turan and Köse, 2004) and increasing freezing tolerance (Wilson, 2001). However, to date, there have been no documented reports of using Ascophyllum extracts for managing downy mildew in grapevines.
In addition to biostimulants, biocontrol agents have also proven effective in managing different plant diseases. Different species of Pseudomonas have biocontrol properties and have been found to control various plant pathogens. Pseudomonas fluorescens is a beneficial plant growth-promoting rhizobacterium (PGPR) known for enhancing plant health through induced systemic resistance and biological control of pathogens (Ganeshan and Kumar, 2005). Different strains of P. fluorescens have been reported to be effective against downy mildew disease in various crops such as maize (Kamalakannan and Shanmugam, 2009), pearl millet (Lavanya et al., 2022), and grapes (Lakkis et al., 2019). The downy mildew of grapevine was controlled by applying P. fluorescens PTA-CT2, which induced systemic resistance in the grapevine against P. viticola (Lakkis et al., 2019). Currently, there are no reports of P. fluorescens CHA0 (hereafter referred to as CHA0) being used to manage P. viticola for the control of downy mildew disease. However, CHA0 has demonstrated potentially control several plant diseases caused by soil-borne pathogens (Keel et al., 1992; Jousset et al., 2006).
Plants possess inducible defense mechanisms to cope with pathogen infection challenges (Conrath et al., 2002; Wiesel et al., 2014) including systemic acquired resistance (SAR) and induced systemic resistance (ISR). SAR is mediated by salicylic acid (SA) and the expression of pathogenesis-related (PR) genes, while jasmonic acid (JA) and ethylene (ET) signaling pathways are involved in ISR (Lemarié et al., 2015). Biostimulants and biocontrol agents have been shown to improve the plant’s resistance to pathogens by triggering these mechanisms (Compant et al., 2005; Shukla et al., 2019).
Various defense enzymes play crucial roles in these mechanisms. Among them, polyphenol oxidase (PPO) is involved in lignification and the oxidative stress response, contributing to pathogen resistance by catalyzing the oxidation of phenolic compounds (Hiraga et al., 2001; Mayer, 2006). Peroxidase (PO) reinforces cell walls through the synthesis of lignin and suberin while also generating reactive oxygen species (ROS) to combat pathogen invasion (Almagro et al., 2009). Phenylalanine ammonia-lyase (PAL) is a key enzyme in the phenylpropanoid pathway, facilitating the biosynthesis of secondary metabolites such as phytoalexins and lignin, which enhance structural defense (Potin et al., 1999; Hemm et al., 2004; Kim and Hwang, 2014). In addition to enzymatic defense, pathogenesis-related (PR) proteins contribute to plant immunity through various mechanisms. Lipoxygenase (LOX) is involved in the oxidation of polyunsaturated fatty acids and plays a central role in the jasmonic acid (JA) signaling pathway, mediating defense responses against pathogen invasion (Park et al., 2010; Podolyan et al., 2010; Wasternack and Song, 2017). Plant defensins (PDFs) exhibit antimicrobial activity by disrupting pathogen membranes, thereby limiting fungal and bacterial infections (Sher Khan et al., 2019). Germin-like proteins (GLPs) are a diverse group of water-soluble glycoproteins that provide antifungal activity, participate in oxidative stress responses, and reinforce cell walls, thereby impeding pathogen penetration (Dunwell et al., 2008). Furthermore, thaumatin-like proteins (TLPs) act as antifungal agents by disrupting fungal cell membranes and inhibiting pathogen growth (Liu et al., 2010). These proteins function within the salicylic acid (SA)-mediated defense pathway, the jasmonic acid (JA)/ethylene (ET)-mediated defense pathway, or both, depending on the nature of the pathogen and the plant species (Thomma et al., 1998; Zhu et al., 2002; Lemarié et al., 2015; Yan et al., 2017). Previous studies have shown that enhanced plant resistance to pathogens is often correlated with elevated activities of these defense enzymes and increased expression of pathogenesis-related (PR) genes (Jayaraj et al., 2008; Subramanian et al., 2011; Esserti et al., 2016; Bahmani et al., 2023b).Despite reports highlighting the effectiveness of ANE and CHA0 in controlling various plant diseases, their potential for managing downy mildew in grapevine remains unexplored. Additionally, potential synergistic interactions between ANE and CHA0 have not been investigated.
The goals of the current study were to investigate the antifungal potential of ANE and CHA0 through both direct and indirect modes of action. This included conducting in vitro assays using the leaf disc treatment method and spore assays, studying the defense mechanisms in grapevines treated with ANE and CHA0, either alone or in combination, and evaluating the effectiveness of their combined application in reducing downy mildew symptoms. The results demonstrated that the application of ANE and CHA0 inhibited the occurrence and spread of Plasmopara viticola and enhanced grape resistance to downy mildew by activating various plant defense mechanisms.
2 Materials and methods
2.1 Plant material and growth condition
Grapevines of the Chardonnay variety were obtained from VineTech, Canada. The grapevines were acclimated and potted in Pro-Mix. The vines were grown in a greenhouse (Conviron, Canada) maintained at 70–80% relative humidity (RH) and a day/night temperature of 24/21 ± 2°C with a 16-h light/8-h dark photoperiod. Plants were irrigated with a fertilizer solution (1 g/L of 20-20–20 nitrogen, phosphorus, and potassium (NPK)) at 50 mL per plant once every two weeks. Pots were irrigated with water on alternate days or as needed.
2.2 Isolation and maintenance of disease inoculum
Inoculum of downy mildew (Plasmopara viticola) was collected from naturally infected grapevines in vineyards located in Wolfville, Nova Scotia, Canada. The inoculum was maintained by repeated inoculations on Chardonnay grape plants grown under greenhouse conditions, as described above. The P. viticola spore suspension was prepared in sterile distilled water and sprayed on the abaxial surface of the grape leaves. After spraying, the plants were placed in a mist chamber with continuous misting at 21°C overnight with >90% RH. After 12 hours, the misting was stopped, and the plants were subjected to dry conditions. This cycle was repeated every 12 hours for seven days. After seven days, infection symptoms appeared on the grape leaves as sporulating lesions on the abaxial surface. Sporulation increased until day 10. Leaves with freshly sporulating lesions were collected and washed in cold (4°C) sterile distilled water to collect the sporangia. The sporangia suspension was filtered through sterile cotton wool and adjusted to a concentration of 2 × 15 spores/mL by counting with a hemocytometer.
2.3 Pseudomonas protegens CHA0 and ANE treatments
The Pseudomonas protegens CHA0 (hereafter referred to as CHA0) strain was obtained from Dr. Zhenyu Cheng at Dalhousie University. CHA0 was cultured and maintained on King’s B agar (10 g agar, 20 g peptone mix, 1.5 g anhydrous K2HPO4, 1.5 g MgSO4, and 10 mL glycerol in 1 L distilled water, pH 7.2) at 28 ± 2°C. A glycerol stock solution was prepared from this culture and used to prepare a bacterial suspension of CHA0 at a concentration of 107 CFU/mL. ANE (Acadian Seaplants Limited) stock solution (10%) was prepared in distilled water and stored at 4°C until further use. Foliar treatments were applied until runoff occurred, and soil drench applications were performed by adding 400 mL of either ANE or CHA0 to achieve the desired final concentration.
2.4 Leaf disc assay
The disc assay was performed using the moist-chamber Petri dish method with minor modifications (Cohen, 1993). Leaf discs (1 cm in diameter) were placed in Petri dishes lined with two layers of Whatman filter paper wetted with 8 mL of the treatment solution, which included ANE (0.01% v/v), CHA0 (107 CFU/mL), and their combination. For the combination treatment, 800 µL of CHA0 from a 108 CFU/mL stock and 800 µL of ANE from a 0.1% (v/v) stock were combined in 8 mL of King’s B liquid medium to achieve final concentrations of 107 CFU/mL for CHA0 and 0.01% (v/v) for ANE, respectively. Distilled water was used as the control treatment. Each leaf disc was floated on the treatment solution. A 50 µL aliquot of the downy mildew spore suspension was placed at the center of each leaf disc. To maintain humidity, the plates were sealed with Parafilm and incubated in the dark at 21°C for 24 h. After 24 h, the suspension droplet was removed using sterile filter paper to prevent secondary contamination, and the plates were returned to the incubator for 7 days. At the end of the incubation period, leaf discs were examined for sporulation and disease symptoms. Each treatment was performed with three independent biological replicates, with each replicate consisting of five leaf discs.
2.5 Spore assay
The downy mildew spore suspension was amended with ANE (0.01% v/v), CHA0 (107 CFU/mL), and their combination, then incubated at 18°C. The suspension was observed under a microscope at 2 h and 6 h intervals. The number of released and actively swimming zoospores was counted at 10× magnification using a hemocytometer. Additionally, the number of empty sporangia was recorded and compared across treatments. Each treatment was performed with three independent biological replicates.
2.6 Downy mildew disease evaluation under greenhouse condition
The downy mildew disease evaluation was performed as previously described by Lazazzara et al. (2021) with some modifications. The grape plants were grown and maintained in the greenhouse as described above. Uniform, healthy plants were selected for the experiments. The plants were treated as follows: 0.1% ANE as a foliar spray, 0.3% ANE as a soil drench, CHA0 as a soil drench (107 CFU/g of soil), CHA0 as a foliar spray (107 CFU/mL), 0.1% ANE + CHA0 (107 CFU/mL) as a foliar spray, 0.3% ANE + CHA0 (107 CFU/g of soil) as a soil drench, non-treated plants were used as controls. Six hours after treatment application, plants were inoculated with a P. viticola spore suspension (2 × 15 spores/mL) on the abaxial surfaces of the leaves. Following pathogen inoculation, the plants were incubated in a mist chamber under continuous mist in dark conditions at 21°C overnight with >90% RH. After 12 h, the misting was stopped, and plants were transferred to controlled greenhouse conditions as previously described. The severity of the disease was visually assessed based on the percentage of abaxial leaf area covered by sporulation and scored according to the protocol described by European and Mediterranean Plant Protection Organization (2001) as follows: 1 = No infection, 2 = < 5% leaf area infected, 3 = 5–10% leaf area infected, 4 = 10–25% leaf area infected, 5 = 25–50% leaf area infected, 6 = 50–75% leaf area infected, 7 = >75% leaf area infected. Five replicates were analyzed for each treatment, and the experiment was conducted three times independently.
2.7 Plant growth and treatments for gene expression analysis and biochemical assays
Three-month-old, uniform, and healthy grape plants were treated with 0.1% ANE, CHA0 (107 CFU/mL), and 0.1% ANE + CHA0 (107 CFU/mL) as a foliar spray and maintained in the greenhouse, as described above. Leaf samples were harvested at 24, 48, and 72 h post-treatment, ground in liquid nitrogen, and stored at −80°C for gene expression analyses and biochemical assays.
2.8 Gene expression analysis
The expression of defense-related genes was examined using qRT-PCR. Total RNA was isolated from the leaf samples (100 mg) using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. Two micrograms of RNA were treated with DNase (Promega) and converted to cDNA using a RevertAid cDNA Synthesis Kit, following the manufacturer’s instructions. The qRT-PCR analysis was performed as previously described (Bahmani and Prithiviraj, 2024) using a StepOne Plus Real-Time PCR System (Applied Biosystems). Gene-specific primer sequences were designed using Primer3 Plus Software. ACTIN was used as the endogenous control for normalization. The experiment was performed with three independent biological replicates, each consisting of three technical replicates. The sequences of gene-specific primers are provided in Supplementary Table S1.
2.9 Assessment of peroxidase and polyphenol oxidase activity
Briefly, ground leaf samples (500 mg) were extracted in 50 mM sodium phosphate buffer supplemented with 0.05% polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 12,000 rpm for 15 minutes at 4°C, and the resulting supernatant was collected as the crude enzyme extract. Total protein content was determined using the Bradford assay (Bradford, 1976). Peroxidase (PO) and Polyphenol Oxidase (PPO) activities were measured using guaiacol and catechol as substrates, respectively, following the previously described method (Ngadze et al., 2012). For PO activity, the reaction mixture contained 0.5 ml of crude enzyme extract, 1.5 ml of 50 mM sodium phosphate buffer, 1 ml of guaiacol, and 1 ml of 2% hydrogen peroxide (H2O2). The absorbance was measured at 470 nm for 5 minutes, with readings taken at 30-second intervals. For PPO activity, the reaction mixture contained 1 ml of crude enzyme extract, 2.9 ml of 50 mM sodium phosphate buffer, and 1 ml of 100 mM catechol (Sigma). The reaction was monitored by measuring absorbance at 546 nm at 30-second intervals for 5 minutes using a Cytation™ 5 multimode plate reader (BioTek, USA). Enzyme activities were expressed as the change in absorbance per minute per milligram of protein. The experiment was performed with three independent biological replicates.
2.10 Phenylalanine ammonia lyases activity assay
PAL activity was assessed using 500 mg of leaf tissue and phenylalanine as the substrate following the method described in detail earlier (Subramanian et al., 2011). Enzyme activity was measured as the change in absorbance at 290 nm, corresponding to the conversion of L-phenylalanine to trans-cinnamic acid, using a Cytation™ 5 multimode plate reader (BioTek, USA). The amount of trans-cinnamic acid produced was calculated using a standard curve of trans-cinnamic acid and expressed as nmol of trans-cinnamic acid per mg of tissue. The experiment was performed with three independent biological replicates.
2.11 Total phenolic content
Total phenolic content in leaf samples (1 g) was determined using the Folin-Ciocalteu reagent (Zieslin and Ben-Zaken, 1993). Briefly, plant samples were extracted in 85% ice-cold methanol (v/v) and incubated at room temperature in the dark for 48 hours. The homogenate was then centrifuged, and the resulting supernatant was collected and mixed with 10% Folin-Ciocalteu reagent by vortexing. Subsequently, 0.7 M sodium carbonate (Na2CO3) was added, and the reaction mixture was further incubated for 2 hours. After incubation, the absorbance of the samples was measured at 725 nm using a Cytation™ 5 plate reader (BioTek, USA), and total phenolic content was calculated using a standard curve of gallic acid (0–10 µg/mL). Results were expressed as µg of gallic acid per g fresh weight (µg GAE/g FW). The experiment was performed with three independent biological replicates.
2.12 Measurement of hydrogen peroxide
The hydrogen peroxide (H2O2) content in leaf tissues (100 mg) was assessed using a spectrophotometric method described earlier (Bahmani et al., 2023a) using a Cytation™ 5 plate reader (BioTek, USA). The assay included 500 µL of plant extract supernatant (prepared in 0.1% trichloroacetic acid), 500 µL of 10 mM potassium phosphate buffer (pH 7.0), and 1 mL of 1 M potassium iodide. A blank sample containing all components except the plant extract was used for calibration. H2O2 levels were quantified using a standard curve of known H2O2 concentrations. The experiment was performed with three independent biological replicates.
2.13 Statistical analysis
Data were analyzed using one-way or two-way analysis of variance (ANOVA), depending on the number of factors involved in each experiment. For experiments involving a single factor, one-way ANOVA was applied (Supplementary Table S2). When both treatment and time factors were considered, two-way ANOVA was employed to evaluate their individual effects and potential interactions (Supplementary Tables S3 and S4). Post-hoc comparisons were conducted using Tukey’s test, with statistical significance set at P ≤ 0.05. All analyses were performed using Minitab software (version 22).
3 Results
3.1 ANE and CHA0 applications reduced downy mildew disease severity in grapevines
To examine the effectiveness of ANE and CHA0 in controlling downy mildew in grapevines, pathogen inhibition under in vitro conditions and disease incidence under in vivo conditions were assessed following their individual and combined treatments. In the leaf disc assay, grape leaves treated with ANE, CHA0, or their combination showed enhanced resistance to downy mildew at 7 days post-inoculation (Figure 1A). A significant reduction in spore count was observed in ANE (~35%) and CHA0 (~50%) single treatments compared to untreated control. However, the combined application of ANE and CHA0 resulted in a significantly greater reduction in spore count (~66%) compared to either treatment alone (Figure 1B). Further analysis revealed a significant reduction in zoospore numbers and empty sporangia across all treatments at both 2- and 6-hour post-inoculation, except for the 0.01% ANE treatment, where no significant difference was observed compared to the control (Figures 2A, B). The combination of 0.01% ANE and CHA0 significantly reduced zoospore numbers and empty sporangia compared to individual treatments at 2 hours post-inoculation. However, at 6 hours post-inoculation, no significant improvement was observed over CHA0 alone (Figure 2A). To assess the effectiveness of ANE and CHA0 in controlling downy mildew under greenhouse conditions, disease incidence was evaluated following foliar spray and soil drench treatments, where grape plants were treated with 0.1% and 0.3% concentrations of ANE, CHA0, and their combinations. These treatments exhibited enhanced resistance to downy mildew (Figure 3A). Disease incidence was significantly lower across all treatments compared to control plants. No significant differences were observed between the individual treatments of ANE and CHA0. Additionally, soil drench applications of ANE and CHA0 did not differ significantly from their respective foliar applications. However, when ANE and CHA0 were applied in combination, the foliar spray treatment exhibited superior efficacy compared to the soil drench application. Notably, the combined soil drench treatment showed a performance similar to that of the individual ANE or CHA0 treatments, regardless of whether applied as soil drench or foliar spray. Among all treatments, the combined foliar application of 0.1% ANE and CHA0 resulted in the greatest reduction in disease incidence (~22%), which was significantly lower than the control (~70%) and other individual treatments (~40-50%) (Figure 3B). Collectively, these results demonstrate the potential of combined ANE and CHA0 treatments in enhancing grapevine resistance to downy mildew.
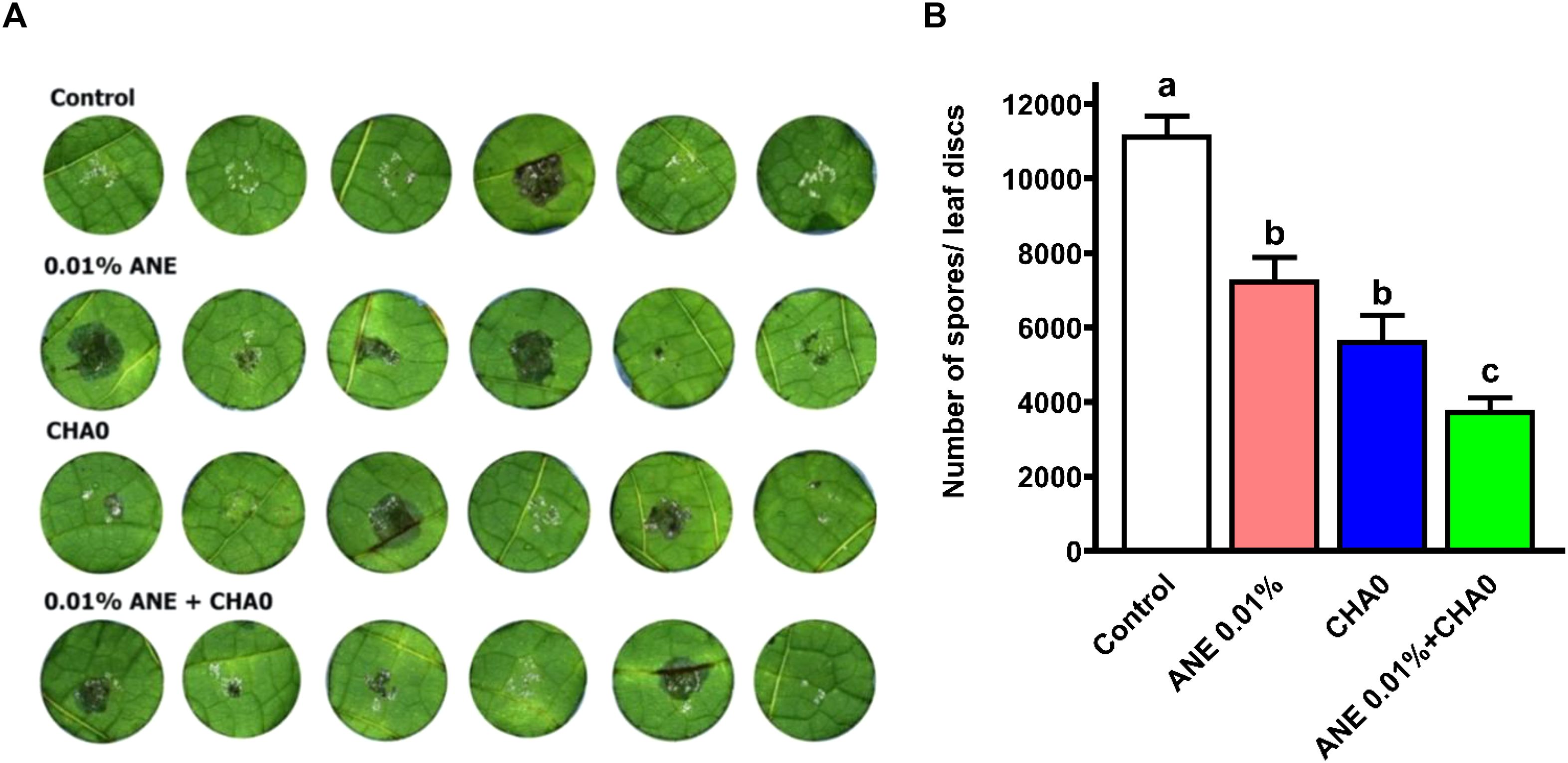
Figure 1. The combined application of Ascophyllum nodosum extract (ANE) and Pseudomonas fluorescens CHA0 reduces downy mildew disease development on grapevine leaf discs. (A) Representative photographs of grapevine leaf discs treated with 0.01% (v/v) ANE, Pseudomonas fluorescens CHA0 (107 CFU/mL), and their combination, showing necrotic lesions and downy mildew growth. Leaf discs were inoculated with Plasmopara viticola sporangia and incubated for 7 days, after which they were photographed and examined for disease symptoms. (B) Bar graph representing the number of P. viticola spores per leaf disc following treatment with ANE, CHA0, or their combination. Data were analyzed using one-way ANOVA, and values are presented as the mean ± standard error (SE) from three biological replicates (n = 3). Different letters above the bars indicate significant differences according to Tukey’s test (p ≤ 0.05).
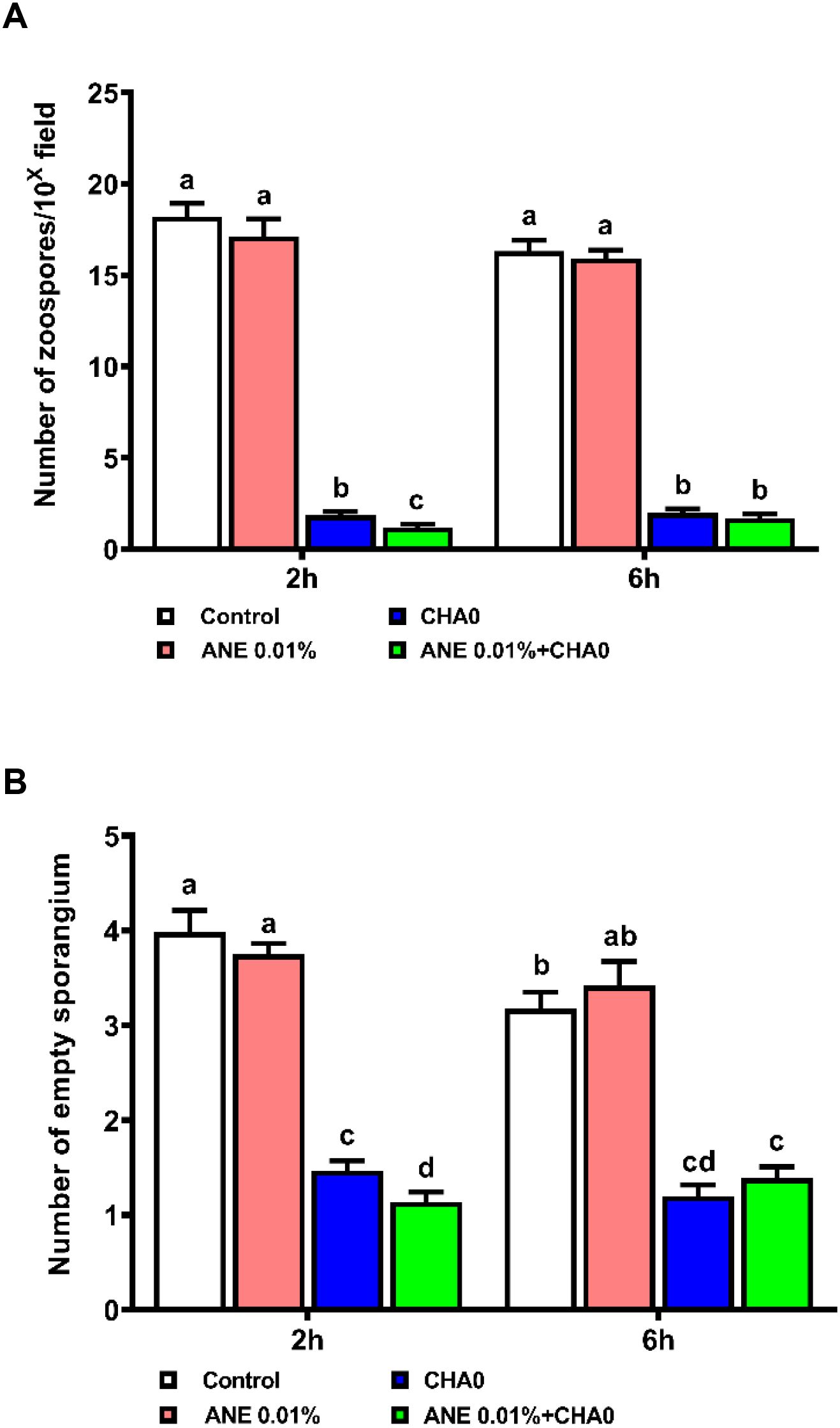
Figure 2. Ascophyllum nodosum extract (ANE) and Pseudomonas fluorescens CHA0 influence the sporulation dynamics of Plasmopara viticola over time post-inoculation. (A) Bar graph representing the number of zoospores, and (B) the number of empty sporangia, following treatment with 0.01% (v/v) ANE, Pseudomonas fluorescens CHA0 (107 CFU/mL), and their combination at 2 h and 6 h post-inoculation. Data were analyzed using two-way ANOVA, and values are presented as the mean ± standard error (SE) from three biological replicates (n = 3). Different letters above the bars indicate significant differences according to Tukey’s test (p ≤ 0.05).
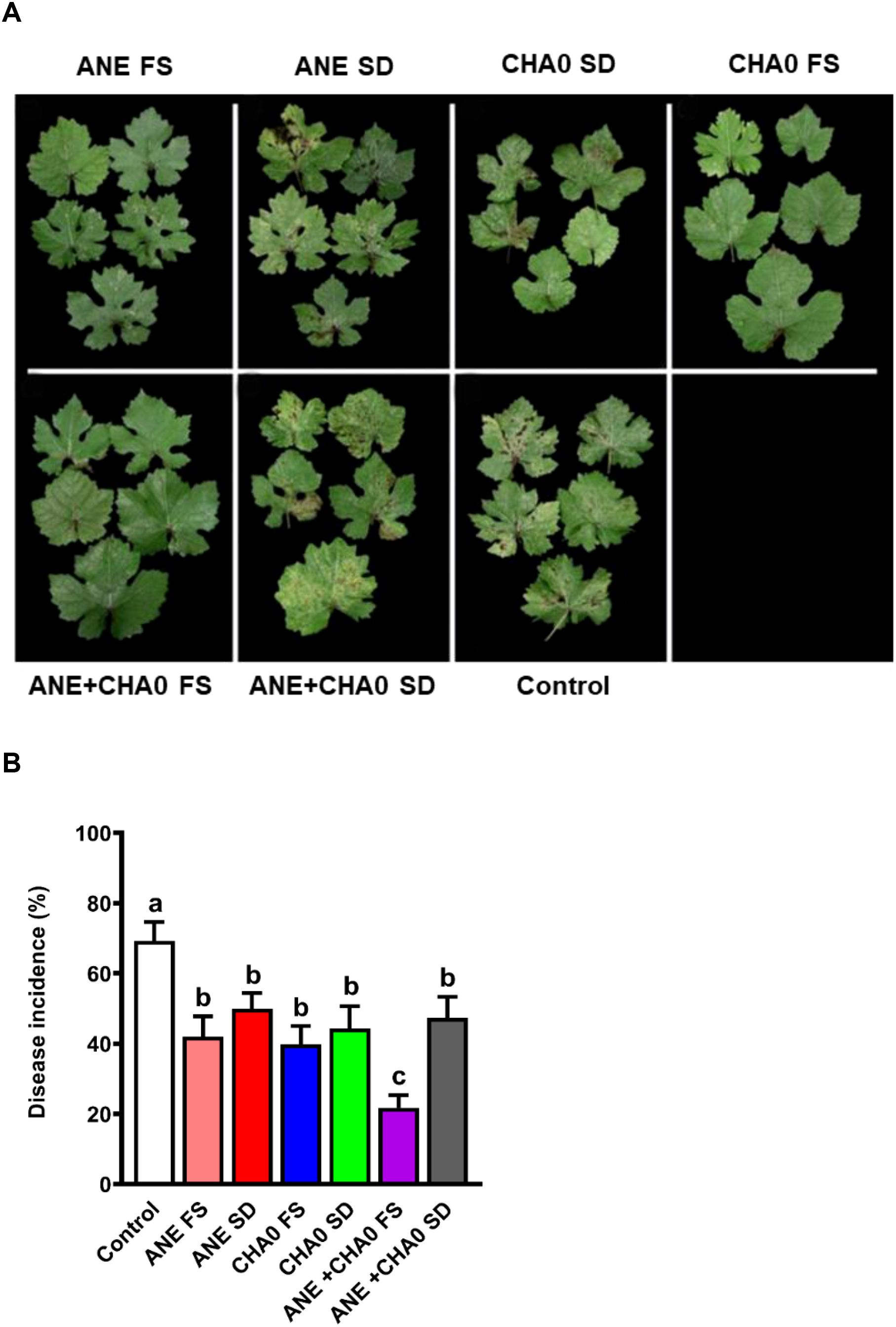
Figure 3. Ascophyllum nodosum extract (ANE) and Pseudomonas fluorescens CHA0 reduce downy mildew disease incidence in grapevine. (A) Representative images of grapevine leaves exhibiting disease symptoms following treatment with ANE, Pseudomonas fluorescens CHA0, and their combination, applied via soil drench or foliar spray. The plants were inoculated with a P. viticola spore suspension (2 × 15 spores/mL) and maintained under greenhouse conditions as described in Materials and Methods. (B) Bar graph indicating disease incidence (%), calculated as the percentage of infected leaves per plant in each treatment. ANE FS: 0.1% ANE foliar spray, ANE SD: 0.3% ANE soil drench, CHA0 SD: CHA0 (107 CFU/mL) soil drench, CHA0 FS: CHA0 foliar spray (107 CFU/mL), ANE+CHA0 FS: 0.1% ANE + CHA0 (107 CFU/mL) foliar spray, ANE+CHA0 SD: 0.3% ANE + CHA0 (107 CFU/mL) soil drench, control: non-treated plants. Data were analyzed using one-way ANOVA, and values are presented as the mean ± standard error (SE) from three biological replicates (n = 5). Different letters above the bars indicate significant differences according to Tukey’s test (p ≤ 0.05).
3.2 Enhanced plant defense responses triggered by combined ANE and CHA0 applications
To examine the effect of ANE and CHA0 on plant defense responses, the activity of key defense enzymes and markers in grapevines was assessed over a 72-hour period. Both ANE and CHA0, when applied individually or in combination, synergistically stimulated the activities of phenylalanine ammonia lyase (PAL), peroxidase (PO), polyphenol oxidase (PPO), as well as the total phenolic content (TPC) and hydrogen peroxide (H2O2) levels. The enzyme activities of PAL, PO, and PPO gradually increased from 24 to 72 hours following treatment, whereas no significant changes were observed in untreated control plants (Figures 4A, C). PAL activity was significantly higher in all treatments compared to control plants, with the most pronounced increase observed at 72 hours when ANE and CHA0 were applied in combination. Across all time points, individual treatments of ANE showed higher PAL activity compared to CHA0. The combined treatment resulted in a notable enhancement at 48 and 72 hours, showing a significant increase compared to individual treatments (Figure 4A). At 24 hours, no significant differences were observed across the individual or combined treatments (Figure 4A). PO activity was significantly increased across all treatments and time points compared to control plants, with the combined application of ANE and CHA0 showing a marked increase at 24, 48, and 72 hours compared to individual treatments (Figure 4B). PO activity sharply increased from 24 to 48 hours and then plateaued, with no significant changes thereafter. Similar to PAL activity, individual treatments of ANE showed higher PO activity compared to CHA0 at all time points. PPO activity was significantly elevated in all treatments compared to control plants, with the combined application resulting in the highest levels of PPO activity at 48 and 72 hours compared to individual treatments (Figure 4C). At 24 hours, no significant differences were noted between individual and combined applications of ANE and CHA0. Additionally, no significant differences were observed between the individual treatments of ANE and CHA0 at any time point (Figure 4C). Like the defense enzyme activities, TPC and H2O2 content significantly increased from 24 to 48 hours in all treatments, but no significant difference was observed between 48 and 72 hours. These levels remained unchanged in control plants (Figures 4D, E). All treatments resulted in a significant increase in TPC and H2O2 accumulation compared to control plants at all time points (Figures 4D, E). While no significant differences were observed between the individual applications of ANE and CHA0, the combined application significantly enhanced TPC and H2O2 content at all time points compared to their individual treatments (Figures 4D, E). These findings demonstrate that treatment with ANE and CHA0 primes the physiological state of grapevines, leading to the effective activation of defense mechanisms and modulation of key biochemical markers associated with plant immunity.
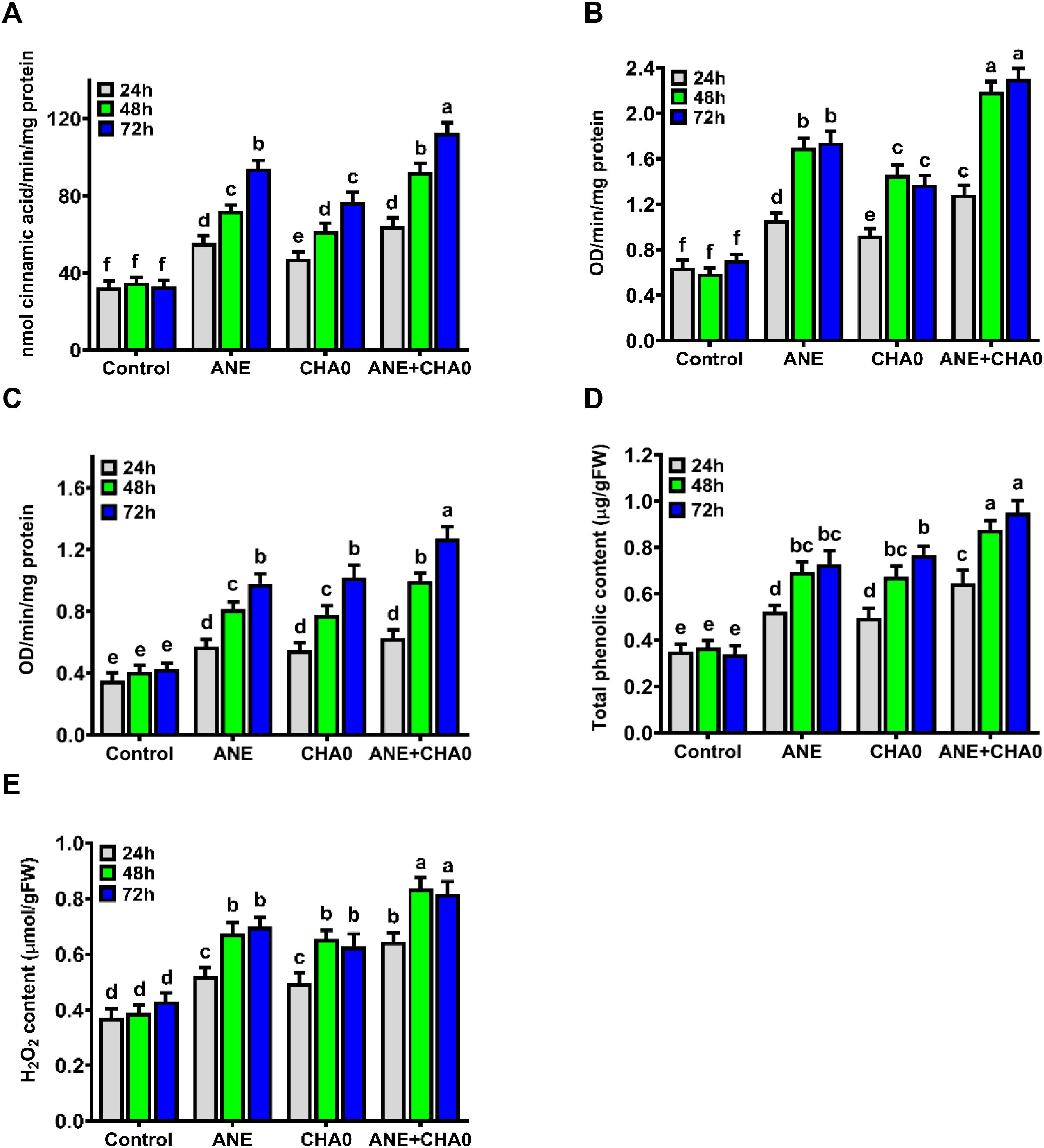
Figure 4. Ascophyllum nodosum extract (ANE) and Pseudomonas fluorescens CHA0 modulate enzymatic and biochemical defense responses in grapevine. Bar graphs showing the activity of defense-related enzymes and biochemical responses in grapevine leaves following foliar application of 0.1% (v/v) ANE, Pseudomonas fluorescens CHA0 (107 CFU/mL), and their combination at 24 h, 48 h, and 72 h post-treatment. (A) Phenylalanine ammonia lyase (PAL) activity, (B) Peroxidase (PO) activity, (C) Polyphenol oxidase (PPO) activity, (D) Total phenolic content, and (E) Hydrogen peroxide (H2O2) accumulation. Data were analyzed using two-way ANOVA, and values are presented as the mean ± standard error (SE) from three biological replicates (n = 3). Different letters above the bars indicate significant differences according to Tukey’s test (p ≤ 0.05).
3.3 ANE and CHA0 applications altered the expression of genes involved in defense response mechanisms
Pathogen-responsive genes are essential in plant defense against biotic stresses. To evaluate whether ANE or CHA0 primes grapevine at the molecular level, we quantified the transcript levels of several key pathogen-responsive genes using qRT-PCR. Both ANE and CHA0 treatments, whether individually or in combination, triggered significant molecular responses in grapevines, likely contributing to enhanced defense against downy mildew. The expression of LOX9, a JA-responsive gene, was significantly upregulated across all treatments and time points compared to control plants. While no significant differences were observed between the individual treatments of ANE and CHA0 at any time point, LOX9 expression exhibited a marked increase from 48 to 72 hours in all treatments. Notably, the combined application of ANE and CHA0 resulted in significantly higher LOX9 expression at all time points compared to their individual treatments, with an early induction observed between 24 and 48 hours (Figure 5A). The expression levels of CHI and GLP7 also showed significant upregulation from 24 to 72 hours across all treatments, with no significant changes in untreated control plants (Figures 5C, D). For CHI, expression was significantly lower in the CHA0 treatment at 48 and 72 hours compared to ANE alone, while no differences were observed at 24 hours (Figure 5C). For GLP7, no significant differences were observed between the individual treatments of ANE and CHA0. However, the combined treatment consistently showed significantly higher expression levels for both CHI and GLP7 at all time points compared to their individual applications (Figures 5C, D).
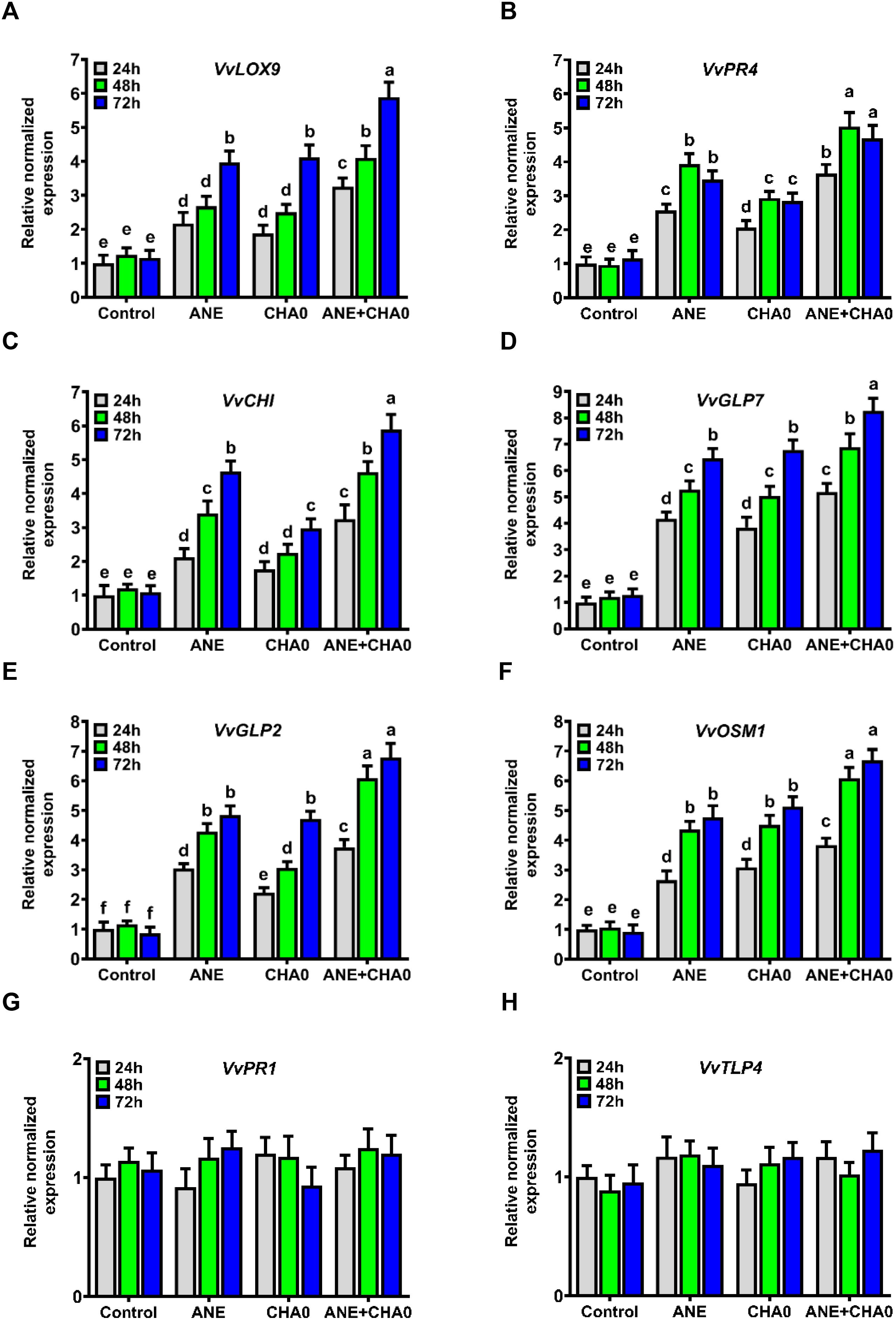
Figure 5. Ascophyllum nodosum extract (ANE) and Pseudomonas fluorescens CHA0 modulate the expression of pathogen-responsive genes in grapevine. Bar graphs showing the transcript levels of selected pathogen-responsive genes in grapevine leaves following foliar application of 0.1% (v/v) ANE, Pseudomonas fluorescens CHA0 (107 CFU/mL), and their combination at 24 h, 48 h, and 72 h post-treatment. Gene expression was analyzed using quantitative real-time PCR (qRT-PCR), with Actin used as an endogenous reference gene for normalization. Transcript levels were normalized to the control (water-treated plants). Data were analyzed using two-way ANOVA, and values are presented as the mean ± standard error (SE) of three biological replicates (n = 3). (A) VvLOX9 (lipoxygenase 9), (B) VvPR4 (pathogenesis-related 4), (C) VvCHI (chalcone isomerase), (D) VvGLP7 (germin-like protein 7), (E) VvGLP2 (germin-like protein 2), (F) VvOSM1 (osmotin 1), (G) VvPR1 (pathogenesis-related 1), (H) VvTLP4 (thaumatin-like protein 4), all genes from Vitis vinifera (Vv). Different letters above the bars indicate significant differences according to Tukey’s test (p ≤ 0.05).
The expression of GLP2 and OSM1 followed a similar pattern, with significantly higher transcript levels in all treatments compared to control plants (Figures 5E, F). Both genes showed upregulation from 24 to 48 hours under all treatments, with no further increase at 72 hours, except for GLP2, which exhibited significantly higher expression in the CHA0 treatment at 72 hours (Figure 5E). Interestingly, GLP2 expression was significantly lower in the CHA0 treatment at 24 and 48 hours compared to ANE alone, but no differences were observed at 72 hours (Figure 5E). For OSM1, no significant differences were noted between ANE and CHA0 individual treatments at any time point. However, both GLP2 and OSM1 showed significantly higher expression levels under the combined application of ANE and CHA0 compared to the individual treatments at all time points (Figures 5E, F). The expression of PR4 was significantly upregulated across all treatments and time points compared to control plants (Figure 5B). This upregulation was most pronounced between 24 and 48 hours, with no significant differences at 72 hours. Additionally, PR4 expression was consistently lower in the CHA0 treatment compared to ANE alone at all time points. The combined treatment of ANE and CHA0 resulted in significantly higher PR4 expression compared to the individual treatments (Figure 5B). In contrast, the expression of the SA-responsive genes PR1 and TLP4 remained unchanged across all treatments, including both individual and combined applications of ANE and CHA0 (Figures 5G, H).
Taken together, these findings demonstrate that ANE and CHA0 treatments primarily activate JA-responsive genes such as LOX9, PR4, GLP2, and OSM1, while SA-dependent pathways remain unaffected. The significant upregulation of JA-responsive genes, particularly under the combined application of ANE and CHA0, suggests that these treatments may prime grapevine defense through the JA signaling pathway, thereby enhancing the plant’s ability to resist pathogen attacks.
4 Discussion
Downy mildew, caused by Plasmopara viticola, poses a significant threat to grapevine cultivation worldwide, leading to considerable economic losses. The most common method for controlling this pathogen primarily involves the use of fungicides, which, despite their efficacy, have been associated with adverse effects on soil health, environmental contamination, and the emergence of resistant pathogen strains (Pimentel et al., 1992; Chen et al., 2007). As a result, there is an urgent need for sustainable and less invasive strategies to manage downy mildew in grapevines. This study highlights the effectiveness of ANE and CHA0 in controlling downy mildew in grapevines. Both in vitro and in vivo trials demonstrated a significant reduction in pathogen establishment and disease incidence following the application of these treatments (Figures 1-3). Notably, the combined application of ANE and CHA0 consistently outperformed individual treatments, suggesting a synergistic interaction that enhances grapevine resistance to the pathogen. In the greenhouse experiment, applying ANE or CHA0 individually as a foliar treatment was not significantly different from their use as a soil drench. However, when ANE and CHA0 were applied together via foliar application, a substantial improvement was observed compared to the soil drench approach (Figure 3). These findings suggest that foliar application is more effective than root drenching, possibly because the elicitor molecules are more mobile or readily available when applied via spraying (Abkhoo and Sabbagh, 2015). The ANE-induced resistance to downy mildew observed in this study can be attributed to the presence of bioactive compounds such as laminarin, fucoidan, alginates, and phytohormones in ANE, which are known to act as elicitors of plant defense pathways (Jayaraj et al., 2008; Sharma et al., 2013; Shukla et al., 2019). By activating signaling pathways involving salicylic acid, jasmonic acid, and ethylene, these compounds trigger ISR or SAR (Subramanian et al., 2011; Ali et al., 2016). The resulting resistance mechanisms enhance the activity of defense enzymes and stimulate the accumulation of phenolic compounds, further strengthening the plant’s ability to resist pathogen invasion (Modafar et al., 2012). Supporting our findings, ANE has been shown to enhance fungal disease resistance in various plants. The application of ANE significantly reduced the incidence of damping-off in cucumber (Abkhoo and Sabbagh, 2015), early blight and bacterial spot in sweet pepper and tomato (Ali et al., 2019), leaf spot, gummy stem blight, and grey mold in cucumber (Jayaraman et al., 2010), powdery mildew in strawberry (Bajpai et al., 2019), and black rot in carrot (Jayaraj et al., 2008).
Pseudomonas spp., such as the Pseudomonas fluorescens CHA0 strain, are widely recognized as biocontrol agents and have been shown to enhance plant resistance to pathogens by inducing ISR and producing antimicrobial compounds and secondary metabolites (Haas et al., 1991; Haas and Keel, 2003; de Werra et al., 2009; Alattas et al., 2024). Our study demonstrated that the application of Pseudomonas fluorescens CHA0 significantly reduced the severity of downy mildew disease in grapevines. This finding is consistent with previous research highlighting the efficacy of CHA0 in controlling various fungal diseases across different plant species, including take-all disease in wheat caused by Gaeumannomyces graminis var. tritici (Keel et al., 1992; Sari et al., 2008), black root rot in tobacco caused by Thielaviopsis basicola (Keel et al., 1992), and damping-off and root rot in cucumber caused by Pythium ultimum (Péchy-Tarr et al., 2005).
In the present study, the application of ANE, CHA0, or their combination significantly boosted the activity of key defense enzymes (PAL, PPO, and PO) and the accumulation of phenolic compounds in grapevines. The combined treatment demonstrated a synergistic effect, leading to the most substantial enhancement of these defense traits. These improvements were observed as early as 24 hours post-treatment and remained consistent up to 72 hours (Figure 4). The observed activation of defense enzymes can be attributed to the elicitors present in the seaweed extracts, which trigger plant defense mechanisms (Shukla et al., 2019; Bahmani et al., 2023b). The roles of these enzymes in plant defense further support these findings. PAL, a critical enzyme in the phenylpropanoid pathway, facilitates the synthesis of phenolic compounds, phytoalexins, and lignin, which are essential for reinforcing cell walls and hindering pathogen progression (Potin et al., 1999; Hemm et al., 2004; Kim and Hwang, 2014). Similarly, the observed increase in PO activity contributes to lignification and cell wall cross-linking, strengthening plant tissues against enzymatic degradation by pathogens (Bradley et al., 1992). Enhanced PPO activity, which catalyzes the oxidation of phenolic compounds into antimicrobial quinones, underscores the activation of plant defenses induced by these treatments (Hiraga et al., 2001; Mayer, 2006). These enzymatic responses are consistent with prior studies on ANE-treated plants, where increased activities of PAL, PPO, and PO were linked to enhanced resistance against fungal diseases, including early blight (Alternaria solani) in sweet pepper and tomato (Ali et al., 2016), powdery mildew (Podosphaera aphanis) in strawberry (Bajpai et al., 2019), Alternaria black rot (Alternaria radicina) in carrot (Jayaraj et al., 2008), and fusarium root and stem rot (Fusarium oxysporum) and botrytis blight (Botrytis cinerea) in cucumber (Jayaraman et al., 2010). Furthermore, CHA0-induced enhancement of these enzyme activities highlights its role in ISR, a mechanism by which beneficial microbes prime the plant’s immune system (Bakker et al., 2007). Similar findings have been reported in other studies, where P. fluorescens-mediated resistance to take-all disease in wheat (Sari et al., 2008) and foot rot disease in black pepper (Paul and Sarma, 2005) was correlated with increased activities of defense enzymes such as PAL, PPO, and PO.
In addition to enzymatic defense, the increased phenolic content observed in this study (Figure 4) highlights the activation of secondary metabolism by ANE and CHA0, which likely contributed to mitigating downy mildew disease. This increase in phenolic content can be attributed to the activation of phenylpropanoid pathway enzymes such as PAL, PPO, and PO, whose induction was also evident in this study. These enzymes play pivotal roles in phenolic metabolism, with their elevated activity likely contributing to an expanded free phenolic pool. Beyond their antimicrobial activity, phenolic compounds also promote lignin biosynthesis, reinforcing the cell wall and establishing stronger physical barriers against pathogen penetration. The dual functionality of phenolic compounds, induced by ANE and CHA0, enhances the plant’s overall defense response and resistance to downy mildew (Vidhyasekaran, 2007; Jayaraman et al., 2010; Miedes et al., 2014; Carvalho et al., 2018). Consistent with our findings, previous studies have demonstrated that enhanced phenolic content in plants treated with ANE (Jayaraman et al., 2010; Ali et al., 2019; Bajpai et al., 2019) and Pseudomonas fluorescens (Paul and Sarma, 2005; Sari et al., 2008) is associated with improved resistance to various fungal pathogens.
The increase in H2O2 content observed in grapevines treated with ANE and CHA0 (Figure 4) likely results from the activation of plant innate immunity mechanisms. Both ANE and CHA0 appear to trigger the production of reactive oxygen species (ROS) as part of the plant’s early defense response to pathogen attack. Among ROS, H2O2 plays a central role in signaling the plant’s immune system and enhancing resistance to pathogens. It contributes to defense by participating in the oxidation of membrane lipids, which initiates the synthesis of various antifungal substances, such as phytoalexins, reinforcing cell walls, and directly inhibiting pathogen growth by damaging cellular structures (Foyer and Noctor, 2005; Dumanovic et al., 2020). Our findings align with earlier studies demonstrating that treatment with ANE and other brown seaweed extracts induces H2O2 production, thereby enhancing resistance to fungal and bacterial pathogens (Jayaraj et al., 2008).
Furthermore, single or combined treatments with ANE and CHA0 notably upregulated several pathogen-responsive genes, including LOX9, CHI, GLP2, GLP7, PR4, and OSM1, whereas the expression of PR1 and TLP4 did not change significantly (Figure 5). This induction was particularly pronounced when ANE and CHA0 were applied together (Figure 5). The enhanced expression of these genes in response to ANE and CHA0 treatment correlates with the observed resistance of grapevine in this study. Notably, previous studies have shown that downy mildew-resistant grapevines exhibit higher expression levels of these pathogen-responsive genes (Gessler et al., 2011; Malacarne et al., 2011), further supporting the role of ANE and CHA0 in activating defense pathways. PR1 and TLP4 (thaumatin-like proteins) are pathogenesis-related (PR) proteins involved in systemic acquired resistance (SAR), mediating pathogen resistance through their antifungal activities. These proteins hydrolyze the cell walls of invading fungi and disrupt fungal cell membrane integrity, thereby inhibiting fungal growth (Leubner-Metzger and Meins, 1999; Liu et al., 2010; Ali et al., 2018; Goyal et al., 2021). These genes are widely used as markers of salicylic acid (SA) signal transduction pathways, with their upregulation attributed to the accumulation of SA and the activation of SA-mediated defense responses (van Loon et al., 2006; Dufour et al., 2012). Interestingly, neither ANE nor CHA0 induced the expression of these genes, suggesting that ANE/CHA0-mediated enhanced resistance of grapevine to downy mildew is independent of the SA pathway and may not act through SAR (Figure 5). This could be explained by the presence of polysaccharides such as laminarin in ANE, which are known to impair SA accumulation and, consequently, affect the expression of these genes (Mercier et al., 2001). These findings are consistent with previous studies showing that disease suppression by ANE treatment does not rely on the SA-dependent pathway (Subramanian et al., 2011; Ali et al., 2016).
The upregulation of jasmonic acid (JA)-responsive genes, including LOX9, PR4, and OSM1, by ANE and CHA0 demonstrates the activation of the JA signaling pathway, which plays a crucial role in plant defense mechanisms (Xu et al., 1994; Thomma et al., 1998; Dufour et al., 2012; Ali et al., 2018). LOX9 is a lipoxygenase enzyme involved in the oxidation of polyunsaturated fatty acids, contributing to the synthesis of bioactive molecules such as jasmonic acid and other oxylipins, which are essential for the plant’s defense against pathogen invasion (Park et al., 2010; Podolyan et al., 2010; Wasternack and Song, 2017). Similarly, PR4, a pathogenesis-related protein with chitinase activity, directly supports plant defense by breaking down the cell walls of invading fungal pathogens, thereby inhibiting their growth (Enoki and Suzuki, 2016). Furthermore, OSM1, which encodes osmotin, a PR-5 family protein, plays a crucial role in plant defense under stress by activating the MAPK pathway, disrupting cellular structures, inducing ROS-mediated cell death, and exhibiting antifungal activity (Freitas et al., 2015; Hakim et al., 2018). In agreement with our findings, the expression of LOX isoforms in Arabidopsis, VpPR4 in grapevines, and OsOSM1 in rice has been shown to enhance resistance to Alternaria leaf spot, powdery mildew, and sheath blight, respectively (Hwang and Hwang, 2010; Dai et al., 2016; Xue et al., 2016). Chalcone isomerase (CHI) is an essential enzyme in the flavonoid biosynthesis pathway, significantly contributing to plant defenses against a range of pathogens (Liu et al., 2015). CHI-overexpressing soybean plants demonstrated higher resistance to the Phytophthora sojae pathogen (Zhou et al., 2018). CHI expression can be enhanced by both the SA and JA pathways (Yin et al., 2019). However, since SA and JA often exhibit antagonistic interactions (Yang et al., 2019) and given that the SA-responsive gene examined in this study showed no significant change, the increased expression of CHI observed is more likely associated with the activation of the JA signaling pathway. Germin-like proteins (GLPs) represent a broad group of water-soluble glycoproteins found across a wide range of plant species (Barman and Banerjee, 2015). They play a crucial role in plant defense by reinforcing cell walls, exhibiting antifungal properties, and mediating oxidative stress responses. Through the production of reactive oxygen species (ROS), regulation of physiological processes, and their enzymatic activity, GLP2 and GLP7 promote plant defense responses against pathogens (Bernier and Berna, 2001; Dunwell et al., 2008). Therefore, the priming effect of ANE and CHA0 in increasing the expression of GLP2 and GLP7 can be linked to the increased resistance of grapevine to downy mildew observed in the present study. Supporting this, the overexpression of OsGLP2–1 in rice enhanced resistance to leaf blast, panicle blast, and bacterial blight by stimulating SOD enzyme activity and increasing H2O2 production (Liu et al., 2016). Moreover, the overexpression of OsRGLP1 in Medicago truncatula, and GhGLP2 in cotton has been shown to enhance resistance to Fusarium oxysporum and Verticillium wilt, respectively (Sultana et al., 2016; Pei et al., 2020). In addition, the enhanced H2O2 content observed in this study can be correlated with higher expression of GLP genes, as the involvement of many GLPs in H2O2 production has been reported (Barman and Banerjee, 2015).
In support of our data, increased transcript levels of pathogen-responsive genes including CHI, PR1, and PR5, have been associated with ANE-induced resistance in carrot plants against the fungal pathogens Alternaria radicina and Botrytis cinerea (Jayaraj et al., 2008). Similarly, treatment with Stimplex™, a seaweed extract derived from Ascophyllum nodosum, reduced the incidence of fungal diseases such as Alternaria cucumerinum, Didymella applanata, Fusarium oxysporum, and Botrytis cinerea in cucumber, and this resistance was linked to the upregulation of CHI, and LOX defense genes (Jayaraman et al., 2010). The activation of these genes by CHA0 treatment is consistent with findings from other studies, where Pseudomonas fluorescens PTA-CT2 treatment induced stress-responsive genes, including LOX9, CHI, and PR1/6, in the presence or absence of Botrytis cinerea and Plasmopara viticola pathogens in grapevines (Gruau et al., 2015; Lakkis et al., 2019). Similarly, tomato plants inoculated with Pseudomonas putida exhibited increased levels of LOXD and LOXF genes (Mariutto et al., 2011). Overall, the priming effect of ANE and CHA0 in grapevine against downy mildew observed in this study appears to be primarily dependent on the jasmonic acid (JA) pathway rather than the salicylic acid (SA) pathway. This observation is intriguing given that Plasmopara viticola, the causal agent of grapevine downy mildew, is a biotrophic pathogen typically associated with SA-mediated resistance (Glazebrook, 2005; Pieterse et al., 2009; Peng et al., 2023). However, the role of JA signaling in grapevine resistance against P. viticola has been documented (Figueiredo et al., 2015), suggesting that JA-mediated pathways can also contribute to defense against this biotrophic pathogen. This aligns with the complex interplay between SA and JA signaling pathways, which are known to exhibit both antagonistic and synergistic interactions (Pieterse et al., 2014).
5 Conclusion
In conclusion, the integrated application of ANE and CHA0 significantly enhances grapevine resilience against downy mildew by inducing the defensive enzyme activities and secondary metabolites, and upregulating pathogen-responsive genes. The synergy between ANE and CHA0 suggests a promising pathway for developing highly effective biocontrol strategies that leverage the strengths of natural products and microbial inoculants. The adoption of such strategies can contribute to the development of sustainable and environmentally friendly disease management practices in viticulture and beyond. Future research should extend to field trials to confirm these promising results and further elucidate the molecular mechanisms involved in these enhanced defensive responses in grapevines.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RB: Data curation, Methodology, Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Validation, Visualization. PR: Formal Analysis, Investigation, Methodology, Writing – original draft. PM: Writing – original draft. BP: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. B.P.’s lab was supported by the financial assistance of the Nova Scotia Department of Agriculture through the Sustainable Canadian Agricultural Partnership (Sustainable CAP) program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1568426/full#supplementary-material
References
Abkhoo, J. and Sabbagh, S. K. (2015). Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J. Appl. Phycology 28, 1333–1342. doi: 10.1007/s10811-015-0693-3
Alattas, H., Glick, B. R., Murphy, D. V., and Scott, C. (2024). Harnessing Pseudomonas spp. for sustainable plant crop protection. Front. Microbiol 15. doi: 10.3389/fmicb.2024.1485197
Ali, N., Ramkissoon, A., Ramsubhag, A., and Jayaraj, J. (2016). Ascophyllum extract application causes reduction of disease levels in field tomatoes grown in a tropical environment. Crop Prot. 83, 67–75. doi: 10.1016/j.cropro.2016.01.016
Ali, O., Ramsubhag, A., and Jayaraman, J. (2019). Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS One 14, e0216710. doi: 10.1371/journal.pone.0216710
Ali, S., Ganai, B. A., Kamili, A. N., Bhat, A. A., Mir, Z. A., Bhat, J. A., et al. (2018). Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol Res. 212-213, 29–37. doi: 10.1016/j.micres.2018.04.008
Almagro, L., Gómez Ros, L. V., Belchi-Navarro, S., Bru, R., Ros Barceló, A., and Pedreño, M. A. (2009). Class III peroxidases in plant defence reactions. J. Exp. Bot. 60, 377–390. doi: 10.1093/jxb/ern277
Alston, J. M. and Sambucci, O. (2019). Grapes in the world economy. In The Grape Genome. Compendium of Plant Genomes. Cantu, D. and Walker, M. (eds) (Cham: Springer), 1–24. doi: 10.1007/978-3-030-18601-2_1
Bahmani, R., Kim, D., Modareszadeh, M., and Hwang, S. (2023a). Ethylene and ROS mediate root growth inhibition induced by the endocrine disruptor bisphenol A (BPA). Plant Physiol. Biochem. 205, 108212. doi: 10.1016/j.plaphy.2023.108212
Bahmani, R., More, P., Babarinde, S., Zhou, M., and Prithiviraj, B. (2023b). Seaweeds for plant disease management: current research advances and future perspectives. Phytoparasitica 51, 783–802. doi: 10.1007/s12600-023-01074-x
Bahmani, R. and Prithiviraj, B. (2024). A Plant biostimulant prepared from Ascophyllum nodosum Induces Flowering by Regulating the MIR156-mediated Age Pathway in Arabidopsis. Physiologia Plantarum 176. doi: 10.1111/ppl.14531
Bajpai, S., Shukla, P. S., Asiedu, S., Pruski, K., and Prithiviraj, B. (2019). A biostimulant preparation of brown seaweed ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol. J. 35, 406–416. doi: 10.5423/PPJ.OA.03.2019.0066
Bakker, P. A. H. M., Pieterse, C. M. J., and Loon, L. C. V. (2007). Induced systemic resistance by fluorescent pseudomonas spp. Phytopathology 97, 239–243. doi: 10.1094/PHYTO-97-2-0239
Barman, A. R. and Banerjee, J. (2015). Versatility of germin-like proteins in their sequences, expressions, and functions. Funct. Integr. Genomics 15, 533–548. doi: 10.1007/s10142-015-0454-z
Bernier, F. and Berna, A. (2001). Germins and germin-like proteins: Plant do-all proteins. But what do they do exactly? Plant Physiol. Biochem. 39, 545–554. doi: 10.1016/S0981-9428(01)01285-2
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Bradley, D. J., Kjellbom, P., and Lamb, C. J. (1992). Elicitor-and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70, 21–30. doi: 10.1016/0092-8674(92)90530-P
Carvalho, R. S., Carollo, C. A., Magalhães, J. C. D., Palumbo, J. M. C., Boaretto, A. G., Sá, I. C. N. E., et al. (2018). Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tannin and gallic acid. South Afr. J. Bot. 114, 181–187. doi: 10.1016/j.sajb.2017.11.010
Chen, W.-J., Delmotte, F. O., Cervera, S. R., Douence, L., Greif, C., and Corio-Costet, M.-F. (2007). At least two origins of fungicide resistance in grapevine downy mildew populations. Appl. Environ. Microbiol. 73, 5162–5172. doi: 10.1128/AEM.00507-07
Cohen, R. (1993). A leaf disk assay for detection of resistance of melons to Sphaerotheca fuliginea race 1. Plant Dis. 77, 513–517. doi: 10.1094/PD-77-0513
Compant, S., Duffy, B., Nowak, J., Clement, C., and Barka, E. A. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol 71, 4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005
Conrath, U., Pieterse, C. M. J., and Mauch-Mani, B. (2002). Priming in plant–pathogen interactions. Trends Plant Sci. 7, 210–216. doi: 10.1016/S1360-1385(02)02244-6
Dai, L., Wang, D., Xie, X., Zhang, C., Wang, X., Xu, Y., et al. (2016). The Novel Gene VpPR4–1 from Vitis pseudoreticulata Increases Powdery Mildew Resistance in Transgenic Vitis vinifera L. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00695
de Werra, P., Pechy-Tarr, M., Keel, C., and Maurhofer, M. (2009). Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl. Environ. Microbiol 75, 4162–4174. doi: 10.1128/AEM.00295-09
Dufour, M. C., Lambert, C., Bouscaut, J., Mérillon, J. M., and Corio-Costet, M. F. (2012). Benzothiadiazole-primed defence responses and enhanced differential expression of defence genes in Vitis vinifera infected with biotrophic pathogens Erysiphe necator and Plasmopara viticola. Plant Pathol. 62, 370–382. doi: 10.1111/j.1365-3059.2012.02628.x
Dumanovic, J., Nepovimova, E., Natic, M., Kuca, K., and Jacevic, V. (2020). The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.552969
Dunwell, J. M., Gibbings, J. G., Mahmood, T., and Saqlan Naqvi, S. M. (2008). Germin and germin-like proteins: evolution, structure, and function. Crit. Rev. Plant Sci. 27, 342–375. doi: 10.1080/07352680802333938
Enoki, S. and Suzuki, S. (2016). “Pathogenesis-related proteins in grape,” in Grape and Wine Biotechnology. (London: IntechOpen), 43–57.
Esserti, S., Smaili, A., Rifai, L. A., Koussa, T., Makroum, K., Belfaiza, M., et al. (2016). Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. J. Appl. Phycology 29, 1081–1093. doi: 10.1007/s10811-016-0996-z
European and Mediterranean Plant Protection Organization. (2001). Guidelines for the efficacy evaluation of fungicides: Plasmopara viticola. EPPO Bulletin 31, 313–317.
FAO (2022). Agricultural production statistics 2000–2020 (Rome, Italy: FAOSTAT analytical brief series no. 60).
Figueiredo, A., Monteiro, F., and Sebastiana, M. (2015). First clues on a jasmonic acid role in grapevine resistance against the biotrophic fungus Plasmopara viticola. Eur. J. Plant Pathol. 142, 645–652. doi: 10.1007/s10658-015-0634-7
Foyer, C. H. and Noctor, G. (2005). Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17, 1866–1875. doi: 10.1105/tpc.105.033589
Freitas, C. D. T., Silva, M. Z. R., Bruno-Moreno, F., Monteiro-Moreira, A. C. O., Moreira, R. A., and Ramos, M. V. (2015). New constitutive latex osmotin-like proteins lacking antifungal activity. Plant Physiol. Biochem. 96, 45–52. doi: 10.1016/j.plaphy.2015.07.012
Ganeshan, G. and Kumar, A. M. (2005). Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J. Plant Interact. 1, 123–134. doi: 10.1080/17429140600907043
Gessler, C., Pertot, I., and Perazzolli, M. (2011). Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 50, 3–44. doi: 10.14601/Phytopathol_Mediterr-9360
Glazebrook, J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. doi: 10.1146/annurev.phyto.43.040204.135923
Goyal, N., Bhatia, G., Garewal, N., Upadhyay, A., Singh, K., Goyal, N., et al. (2021). Identification of defense related gene families and their response against powdery and downy mildew infections in Vitis vinifera. BMC Genomics 22, 776. doi: 10.1186/s12864-021-08081-4
Gruau, C., Trotel-Aziz, P., Villaume, S., Rabenoelina, F., Clément, C., Baillieul, F., et al. (2015). Pseudomonas fluorescensPTA-CT2 Triggers Local and Systemic Immune Response AgainstBotrytis cinereain Grapevine. Mol. Plant-Microbe Interactions® 28, 1117–1129. doi: 10.1094/mpmi-04-15-0092-r
Haas, D. and Keel, C. (2003). Regulation of antibiotic production in root-colonizing Peudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 41, 117–153. doi: 10.1146/annurev.phyto.41.052002.095656
Haas, D., Keel, C., Laville, J., Maurhofer, M., OberhÄnsli, T., Schnider, U., et al. (1991). Secondary Metabolites of Pseudomonas fluorescens Strain CHA0 Involved in the Suppression of Root Diseases. In: Hennecke, H. and Verma, D. P. S.. (eds) Advances in Molecular Genetics of Plant-Microbe Interactions Vol. 1. Current Plant Science and Biotechnology in Agriculture, 10. (Dordrecht: Springer). doi: 10.1007/978-94-015-7934-6_68
Hakim, Ullah, A., Hussain, A., Shaban, M., Khan, A. H., Alariqi, M., et al. (2018). Osmotin: A plant defense tool against biotic and abiotic stresses. Plant Physiol. Biochem. 123, 149–159. doi: 10.1016/j.plaphy.2017.12.012
Hemm, M. R., Rider, S. D., Ogas, J., Murry, D. J., and Chapple, C. (2004). Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J. 38, 765–778. doi: 10.1111/j.1365-313X.2004.02089.x
Hiraga, S., Sasaki, K., Ito, H., Ohashi, Y., and Matsui, H. (2001). A large family of class III plant peroxidases. Plant Cell Physiol. 42, 462–468. doi: 10.1093/pcp/pce061
Hwang, I. S. and Hwang, B. K. (2010). The pepper 9-lipoxygenase gene CaLOX1 functions in defense and cell death responses to microbial pathogens. Plant Physiol. 152, 948–967. doi: 10.1104/pp.109.147827
Jardin, P. D. (2015). lant biostimulants: Definition, concept, main categories and regulation. Scientia Hortic. 196, 3–14. doi: 10.1016/j.scienta.2015.09.021
Jayaraj, J., Wan, A., Rahman, M., and Punja, Z. K. (2008). Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot. 27, 1360–1366. doi: 10.1016/j.cropro.2008.05.005
Jayaraman, J., Norrie, J., and Punja, Z. K. (2010). Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycology 23, 353–361. doi: 10.1007/s10811-010-9547-1
Jindo, K., Goron, T. L., Pizarro-Tobías, P., Sánchez-Monedero, M.Á., Audette, Y., Deolu-Ajayi, A. O., et al. (2022). Frontiers | Application of biostimulant products and biological control agents in sustainable viticulture: A review. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.932311
Jousset, A., Lara, E., Wall, L. G., and Valverde, C. (2006). Secondary metabolites help biocontrol strain pseudomonas fluorescens CHA0 to escape protozoan grazing. Appl. Environ. Microbiol. 72, 7083–7090. doi: 10.1128/AEM.00557-06
Kamalakannan, A. and Shanmugam, V. (2009). Management approaches of maize downy mildew using biocontrol agents and plant extracts. Acta Phytopathologica Entomologica Hungarica 44, 255–266. doi: 10.1556/aphyt.44.2009.2.4
Keel, C., Schnider, U., Maurhofer, M., Voisard, C., Laville, J., Burger, U., et al. (1992). Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2, 4-diacetylphloroglucinol. Mol. Plant-Microbe Interact. 5, 4–13. doi: 10.1094/MPMI-5-004
Kim, D. S. and Hwang, B. K. (2014). An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 65, 2295–2306. doi: 10.1093/jxb/eru109
Lakkis, S., Trotel-Aziz, P., Rabenoelina, F., Schwarzenberg, A., Nguema-Ona, E., Clément, C., et al. (2019). Frontiers | Strengthening grapevine resistance by pseudomonas fluorescens PTA-CT2 relies on distinct defense pathways in susceptible and partially resistant genotypes to downy mildew and gray mold diseases. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01112
Lavanya, S. N., Niranjan-Raj, S., Jadimurthy, R., Sudarsan, S., Srivastava, R., Tarasatyavati, C., et al. (2022). Immunity elicitors for induced resistance against the downy mildew pathogen in pearl millet. Sci. Rep. 12, 4078. doi: 10.1038/s41598-022-07839-4
Lazazzara, V., Vicelli, B., Bueschl, C., Parich, A., Pertot, I., Schuhmacher, R., et al. (2021). Trichoderma spp. volatile organic compounds protect grapevine plants by activating defense-related processes against downy mildew. Physiologia Plantarum 172, 1950–1965. doi: 10.1111/ppl.13406
Lemarié, S., Robert-Seilaniantz, A., Lariagon, C., Lemoine, J., Marnet, N., Jubault, M., et al. (2015). Both the jasmonic acid and the salicylic acid pathways contribute to resistance to the biotrophic clubroot agent plasmodiophora brassicae in arabidopsis. Plant Cell Physiol. 56, 2158–2168. doi: 10.1093/pcp/pcv127
Leubner-Metzger, G. and Meins, F. (1999). “Functions and regulation of plant ß-1, 3-glucanases (PR-2),” in Pathogenesis-related proteins in plants. (CRC Press, Boca Raton, FL), 49–76.
Liu, J.-J., Sturrock, R., Ekramoddoullah, A. K. M., Liu, J.-J., Sturrock, R., and Ekramoddoullah, A. K. M. (2010). The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Rep. 29, 419–436. doi: 10.1007/s00299-010-0826-8
Liu, Q., Yang, J., Yan, S., Zhang, S., Zhao, J., Wang, W., et al. (2016). The germin-like protein OsGLP2–1 enhances resistance to fungal blast and bacterial blight in rice. Plant Mol. Biol. 92, 411–423. doi: 10.1007/s11103-016-0521-4
Liu, Y., Zhao, S., Wang, J., Zhao, C., Guan, H., Hou, L., et al. (2015). Molecular cloning, expression, and evolution analysis of type II CHI gene from peanut (Arachis hypogaea L.). Dev. Genes Evol. 225, 1–10. doi: 10.1007/s00427-015-0489-0
Malacarne, G., Vrhovsek, U., Zulini, L., Cestaro, A., Stefanini, M., Mattivi, F., et al. (2011). Resistance to Plasmopara viticolain a grapevine segregating population is associated with stilbenoid accumulation and with specific host transcriptional responses. BMC Plant Biol. 11, 114. doi: 10.1186/1471-2229-11-114
Mariutto, M., Duby, F., Adam, A., Bureau, C., Fauconnier, M.-L., Ongena, M., et al. (2011). The elicitation of a systemic resistance by Pseudomonas putidaBTP1 in tomato involves the stimulation of two lipoxygenase isoforms. BMC Plant Biol. 11, 29. doi: 10.1186/1471-2229-11-29
Mayer, A. M. (2006). Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 67, 2318–2331. doi: 10.1016/j.phytochem.2006.08.006
Mercier, L., Lafitte, C., Borderies, G., Briand, X., Esquerré-Tugayé, M.-T., and Fournier, J. (2001). The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 149. doi: 10.1046/j.1469-8137.2001.00011.x
Miedes, E., Vanholme, R., Boerjan, W., and Molina, A. (2014). The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00358
Modafar, C. E., Elgadda, M., Boutachfaiti, R. E., Abouraicha, E., Zehhar, N., Petit, E., et al. (2012). Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Scientia Hortic. 138, 55–63. doi: 10.1016/j.scienta.2012.02.011
Ngadze, E., Icishahayo, D., Coutinho, T. A., and Waals, J. E. V. D. (2012). Role of polyphenol oxidase, peroxidase, phenylalanine ammonia lyase, chlorogenic acid, and total soluble phenols in resistance of potatoes to soft rot. Plant Dis. 96, 186–192. doi: 10.1094/PDIS-02-11-0149
Park, Y.-S., Kunze, S., Ni, X., Feussner, I., and Kolomiets, M. V. (2010). Comparative molecular and biochemical characterization of segmentally duplicated 9-lipoxygenase genes ZmLOX4 and ZmLOX5 of maize. Planta 231, 1425–1437. doi: 10.1007/s00425-010-1143-8
Paul, D. and Sarma, Y. (2005). Pseudomonas fluorescens mediated systemic resistance in black pepper (Piper nigrum L.) is driven through an elevated synthesis of defence enzymes. Arch. Of Phytopathol. And Plant Prot. 38, 139–149. doi: 10.1080/03235400500094324
Péchy-Tarr, M., Bottiglieri, M., Mathys, S., Lejbølle, K. B., Schnider-Keel, U., Maurhofer, M., et al. (2005). RpoN (σ54) controls production of antifungal compounds and biocontrol activity in Pseudomonas fluorescens CHA0. Mol. Plant-Microbe Interact. 18, 260–272. doi: 10.1094/MPMI-18-0260
Pei, Y., Zhu, Y., Jia, Y., Ge, X., Li, X., Li, F., et al. (2020). Molecular evidence for the involvement of cotton GhGLP2, in enhanced resistance to Verticillium and Fusarium Wilts and oxidative stress. Sci. Rep. 10, 12510. doi: 10.1038/s41598-020-68943-x
Peng, J., Wang, X., Wang, H., Li, X., Zhang, Q., Wang, M., et al. (2023). Advances in understanding grapevine downy mildew: From pathogen infection to disease management. Mol. Plant Pathol. 25, e13401. doi: 10.1111/mpp.13401
Pieterse, C. M. J., Leon-Reyes, A., van der Ent, S., and Van Wees, S. C. M. (2009). Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316. doi: 10.1038/nchembio.164
Pieterse, C. M., Zamioudis, C., Berendsen, R. L., Weller, D. M., Van Wees, S. C., and Bakker, P. A. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375. doi: 10.1146/annurev-phyto-082712-102340
Pimentel, D., Acquay, H., Biltonen, M., Rice, P., Silva, M., Nelson, J., et al. (1992). Environmental and economic costs of pesticide use. BioScience 42, 750–760. doi: 10.2307/1311994
Podolyan, A., White, J., Jordan, B., and Winefield, C. (2010). Identification of the lipoxygenase gene family from Vitis vinifera and biochemical characterisation of two 13-lipoxygenases expressed in grape berries of Sauvignon Blanc. Funct. Plant Biol. 37, 767–784. doi: 10.1071/fp09271
Potin, P., Bouarab, K., Küpper, F., and Kloareg, B. (1999). Oligosaccharide recognition signals and defence reactions in marine plant-microbe interactions. Curr. Opin. Microbiol. 2, 276–283. doi: 10.1016/S1369-5274(99)80048-4
Raj, T. S., Muthukumar, A., Vignesh, S., Charumathi, M., and Suji, H. (2019). Efficacy of seaweed extract against downy mildew of grapes caused by plasmopara viticola. Plant Arch. (09725210) 19, 2877–2882.
Sari, E., Etebarian, H. R., and Aminian, H. (2008). Effects of Pseudomonas fluorescens CHA0 on the Resistance of Wheat Seedling Roots to the Take-all Fungus Gaeumannomyces graminis var. tritici. Plant Production Sci. 11, 298–306. doi: 10.1626/pps.11.298
Sharma, H. S. S., Fleming, C., Selby, C., Rao, J. R., Martin, T., Sharma, H. S. S., et al. (2013). Plant biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycology 26, 465–490. doi: 10.1007/s10811-013-0101-9
Sher Khan, R., Iqbal, A., Malak, R., Shehryar, K., Attia, S., Ahmed, T., et al. (2019). Plant defensins: types, mechanism of action and prospects of genetic engineering for enhanced disease resistance in plants. 3 Biotech. 9, 192. doi: 10.1007/s13205-019-1725-5
Shukla, P. S., Mantin, E. G., Adil, M., Bajpai, S., Critchley, A. T., and Prithiviraj, B. (2019). Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00655
Subramanian, S., Sangha, J. S., Gray, B. A., Singh, R. P., Hiltz, D., Critchley, A. T., et al. (2011). Extracts of the marine brown macroalga, Ascophyllum nodosum, induce jasmonic acid dependent systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato DC3000 and Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 131, 237–248. doi: 10.1007/s10658-011-9802-6
Sultana, T., Deeba, F., Naz, F., Rose, R. J., and Saqlan Naqvi, S. M. (2016). Expression of a rice GLP in Medicago truncatula exerting pleiotropic effects on resistance against Fusarium oxysporum through enhancing FeSOD-like activity. Acta Physiologiae Plantarum 38, 255. doi: 10.1007/s11738-016-2273-9
Thomma, B. P., Eggermont, K., Penninckx, I. A., Mauch-Mani, B., Vogelsang, R., Cammue, B. P., et al. (1998). Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. 95, 15107–15111. doi: 10.1073/pnas.95.25.15107
Turan, M. and Köse, C. (2004). Seaweed extracts improve copper uptake of grapevine. Acta Agriculturae Scandinavica Section B - Soil Plant Sci. 54, 213–220. doi: 10.1080/09064710410030311
van Loon, L. C., Rep, M., and Pieterse, C. M. (2006). Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. doi: 10.1146/annurev.phyto.44.070505.143425
Vidhyasekaran, P. (2007). Fungal Pathogenesis in Plants and Crops: Molecular Biology and Host Defense Mechanisms, 2nd ed. (Boca Raton: CRC Press). doi: 10.1201/9781420021035
Wasternack, C. and Song, S. (2017). Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 68, 1303–1321. doi: 10.1093/jxb/erw443
Wiesel, L., Newton, A. C., Elliott, I., Booty, D., Gilroy, E. M., Birch, P. R. J., et al. (2014). Frontiers | Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00655
Wilson, S. (2001). Frost management in cool climate vineyards. Univ. Tasmania Res. Rep. UT 99, 1–34.
Xu, Y., Chang, P., Liu, D., Narasimhan, M. L., Raghothama, K. G., Hasegawa, P. M., et al. (1994). Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6, 1077–1085. doi: 10.1105/tpc.6.8.1077
Xue, X., Cao, Z. X., Zhang, X. T., Wang, Y., Zhang, Y. F., Chen, Z. X., et al. (2016). Overexpression of osOSM1 enhances resistance to rice sheath blight. Plant Dis. 100, 1634–1642. doi: 10.1094/PDIS-11-15-1372-RE
Yan, X., Qiao, H., Zhang, X., Guo, C., Wang, M., Wang, Y., et al. (2017). Analysis of the grape (Vitis vinifera L.) thaumatin-like protein (TLP) gene family and demonstration that TLP29 contributes to disease resistance. Sci. Rep. 7, 4269. doi: 10.1038/s41598-017-04105-w
Yang, J., Duan, G., Li, C., Liu, L., Han, G., Zhang, Y., et al. (2019). The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01349
Yin, Y.-c., Zhang, X.-D., Gao, Z.-Q., Hu, T., and Liu, Y. (2019). The research progress of chalcone isomerase (CHI) in plants. Mol. Biotechnol. 61, 32–52. doi: 10.1007/s12374-018-0017-7
Zhou, Y., Huang, J. L., Zhang, X. L., Zhu, L. M., Wang, X. F., Guo, N., et al. (2018). Overexpression of Chalcone Isomerase (CHI) Increases Resistance Against Phytophthora sojae in Soybean. J. Plant Biol. 61 (5), 309–319. doi: 10.1007/s12374-018-0017-7
Zhu, J., Gong, Z., Zhang, C., Song, C.-P., Damsz, B., Inan, G., et al. (2002). OSM1/SYP61: A syntaxin protein in arabidopsis controls abscisic acid–mediated and non-abscisic acid–mediated responses to abiotic stress. Plant Cell 14, 3009–3028. doi: 10.1105/tpc.006981
Keywords: biostimulant, defense enzymes, induced systemic resistance, jasmonic acid signaling, pathogen-responsive genes
Citation: Bahmani R, Rathor P, More P and Prithiviraj B (2025) Synergistic activation of grapevine defense mechanisms against downy mildew by Ascophyllum nodosum extract and Pseudomonas fluorescens CHA0. Front. Plant Sci. 16:1568426. doi: 10.3389/fpls.2025.1568426
Received: 29 January 2025; Accepted: 13 May 2025;
Published: 05 June 2025.
Edited by:
Giuseppe Mannino, University of Turin, ItalyReviewed by:
Sonia Cacini, Council for Agricultural and Economics Research (CREA), ItalyNoemi Gatti, University of Turin, Italy
Copyright © 2025 Bahmani, Rathor, More and Prithiviraj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Balakrishnan Prithiviraj, YnByaXRoaXZpcmFqQGRhbC5jYQ==
 Ramin Bahmani
Ramin Bahmani Pramod Rathor
Pramod Rathor Prashant More
Prashant More Balakrishnan Prithiviraj
Balakrishnan Prithiviraj