- 1Plant Physiology Section, Department of Botany, Aligarh Muslim University, Aligarh, India
- 2Department of Plant and Soil Sciences, Mississippi State University, Starkville, MS, United States
- 3Feed the Future Innovation Lab for Collaborative Research on Sustainable Intensification, Kansas State University, Manhattan, KS, United States
- 4Department of Agronomy, Kansas State University, Manhattan, KS, United States
Plants are sessile organisms confronted by various abiotic stresses, including drought, salinity, heavy metals (HMs), and high/low temperatures throughout their growth cycles. In response to stress conditions, plants activate a cascade of metabolites and signalling molecules and networks. These intricate networks of signalling molecules like nitric oxide (NO), hydrogen sulfide (H2S), methyl jasmonate (MeJA), hydrogen peroxide (H2O2), ethylene (ETH), melatonin (MT), and calcium (Ca2+), play a crucial role in enhancing the production of secondary metabolites (SMs) in plants. In plants, SMs are characterized by four diverse groups’ terpenes, phenolics, alkaloids, and glucosinolates. Various environmental factors and plant developmental stages influence the production of SMs. The production and regulation of terpenes, phenolics, alkaloids, and glucosinolates in response to signalling molecules under stressed conditions provide valuable insights into stress tolerance. These insights are crucial for developing agricultural practices that improve crop resilience. They are essential for plants to cope with oxidative stress by providing defence mechanisms for improved adaptation, tolerance, and resilience strategies. Conversely, the crosstalk among the signalling molecules paves the way for new research avenues of plant stress management. This review emphasizes the essential role of SMs in plants and how the signalling molecules regulate their production under stress conditions. It also provides valuable insights into the mechanisms that facilitate plant adaptation and stress resilience.
1 Introduction
Plants constantly face a variety of environmental stress factors, including biotic (e.g., pathogens, insects) and abiotic (e.g., drought, salinity, and temperature). To cope with these stresses, plants initiate various defence mechanisms by synthesizing secondary metabolites (SMs) or compounds. Secondary metabolites are bioactive compounds in plants that are essential for biological activities (Erb and Kliebenstein, 2020; Jan et al., 2021) and play important roles in plant survival, adaptation, and resilience. Secondary molecules can alleviate the detrimental symptoms in plants caused by a stressful environment and improve their efficiency and metabolic activity (Kessler and Kalske, 2018; Salam et al., 2023). Under adverse circumstances, plants produce more than 100,000 SMs using biosynthetic pathways (Meena et al., 2017). Secondary metabolites can be categorized into 4 major groups: terpenes, phenolics, alkaloids (nitrogen-containing), and glucosinolates (sulfur-containing). Monoterpenes, inclusive of menthol, linalool, camphor, α-pinene, and β-pinene, have antimicrobial and antioxidant activities (Reshi et al., 2023). Volatile compounds like terpenoids provide stress tolerance to plants against oxidative and thermal stressors (Bai et al., 2019). Compared to normal conditions, more phenolic and flavonoid compounds are biosynthesized by plants growing in stressful environments. Noticeably, flavonoids and anthocyanin compounds (which exhibit antioxidant capacity) reduce ROS (reactive oxygen species) and associated oxidative damage (Sharma et al., 2019). Various internal and external factors, including stress and signalling molecules, significantly influence these metabolite productions. Unlike traditional signalling molecules, such as hormones, gasotransmitters (GTs) diffuse rapidly through plant tissues and interact with various cellular targets to regulate biochemical and physiological pathways. They play a significant role in modulating complex stress signalling pathways and metabolic processes (Sana et al., 2025).
Among the myriads of signalling molecules involved in regulating plant secondary products, nitric oxide (NO), hydrogen sulfide (H2S), methyl jasmonate (MeJA), hydrogen peroxide (H2O2), ethylene (ETH), melatonin (MT), and calcium (Ca2+) have emerged as key players. Nitric oxide is a signalling molecule involved in various physiological processes in plants, including growth and metabolic activities. Nitric oxide stimulates or inhibits specific enzymes and transcription factors, thereby influencing the biosynthetic pathways of SMs. Being a dynamic molecule, NO provides adaptation to plants under adverse environmental conditions (Zhou W. et al., 2021; Kumari R. et al., 2024). Hydrogen sulfide, a gaseous molecule similar to NO, has recently gained attention due to its pivotal role in plant physiology and reactivity to stress (Zhang et al., 2021; Saini et al., 2024). Hydrogen sulfide mitigates the adverse effects of abiotic stress by counteracting the accumulation of ROS, such as peroxides, hydroxyl radicals (OH−), superoxide radicals (O2•–), and singlet oxygen (O2). Reactive oxygen species disrupt nucleic acid structures and interfere with plant metabolic pathways (Amir et al., 2021). Many studies have been conducted on different plants under numerous stresses, showing that H2S application helps to cope with stress by enhancing bioactive compounds in plants (Lu et al., 2024; Shen et al., 2024). Similarly, jasmonic acid (JA) and its methyl ester derivative (MeJA) are known to produce broad categories of SMs, such as rosmarinic acid, terpenoids, plumbagin, and indole alkaloids (Almagro et al., 2014; Khan et al., 2024a). Notable increases in the expression of transcription factors and genes involved in forming SMs have been reported. For instance, WRKY (transcription factor engaged in biotic and abiotic stress responses) is the main factor that influences alkaloid production, such as taxol and artemisinin in Taxus chinensis and Artemisia annua, respectively (Subramaniyam et al., 2014). Besides, H2O2, ETH, MT, and Ca2+ also play integral roles in plant stress responses and SM production. They work individually or in a network of other molecules to enhance plant resilience. This process promotes metabolic adjustments and improves the accumulation of beneficial compounds. For instance, proline, carotenoids, glutathione, ascorbic acid, and phenolics are among the substances that are generated to control the harm that oxidative damage imparts. Ultimately, their action contributes to improved plant health and productivity in challenging environments (Chen H. et al., 2022; Zhang et al., 2021; Gupta et al., 2023). All these signalling molecules act as messengers, facilitating communication within plant tissues and activating specific pathways in response to environmental stimuli. This review discusses the multifaceted role and essentiality of signalling molecules in various abiotic stress conditions. Furthermore, it improves our understanding of the complex dynamic between signalling molecules and SMs to also improve the potency of these compounds in the agricultural sectors.
2 Secondary metabolites in plant’s defence and adaptation: an overview
Primary and secondary metabolites are the two groups in which plant metabolites are distributed. Primary metabolites, such as proteins, lipids, and carbohydrates, directly influence plant growth and development. In contrast, SMs are small organic molecules originating from primary metabolites. Under specific circumstances, SMs serve particular functions, including resistance against pathogen and insect attacks and tolerance to abiotic stresses (Isah, 2019; Upadhyay et al., 2025). They contain a molecular mass of less than 3000 daltons and have widespread application in the agriculture sectors (Elshafie et al., 2023). In fact, plants have an assortment of molecular, cellular, and signalling crosstalk in stress response, which is triggered by the detection of certain abiotic stress factors that induce the generation of SMs. These metabolites are synthesized by multiple metabolic pathways and are implicated in the activation and reinforcement of defence mechanisms in plants. According to Patil (2020), these metabolites are classified into four broad categories: terpenoids (carbon and hydrogen compounds), phenolics (containing a benzene ring), alkaloids (nitrogen-containing compounds), and glucosinolates (sulfur-containing compounds), as shown in Figure 1.
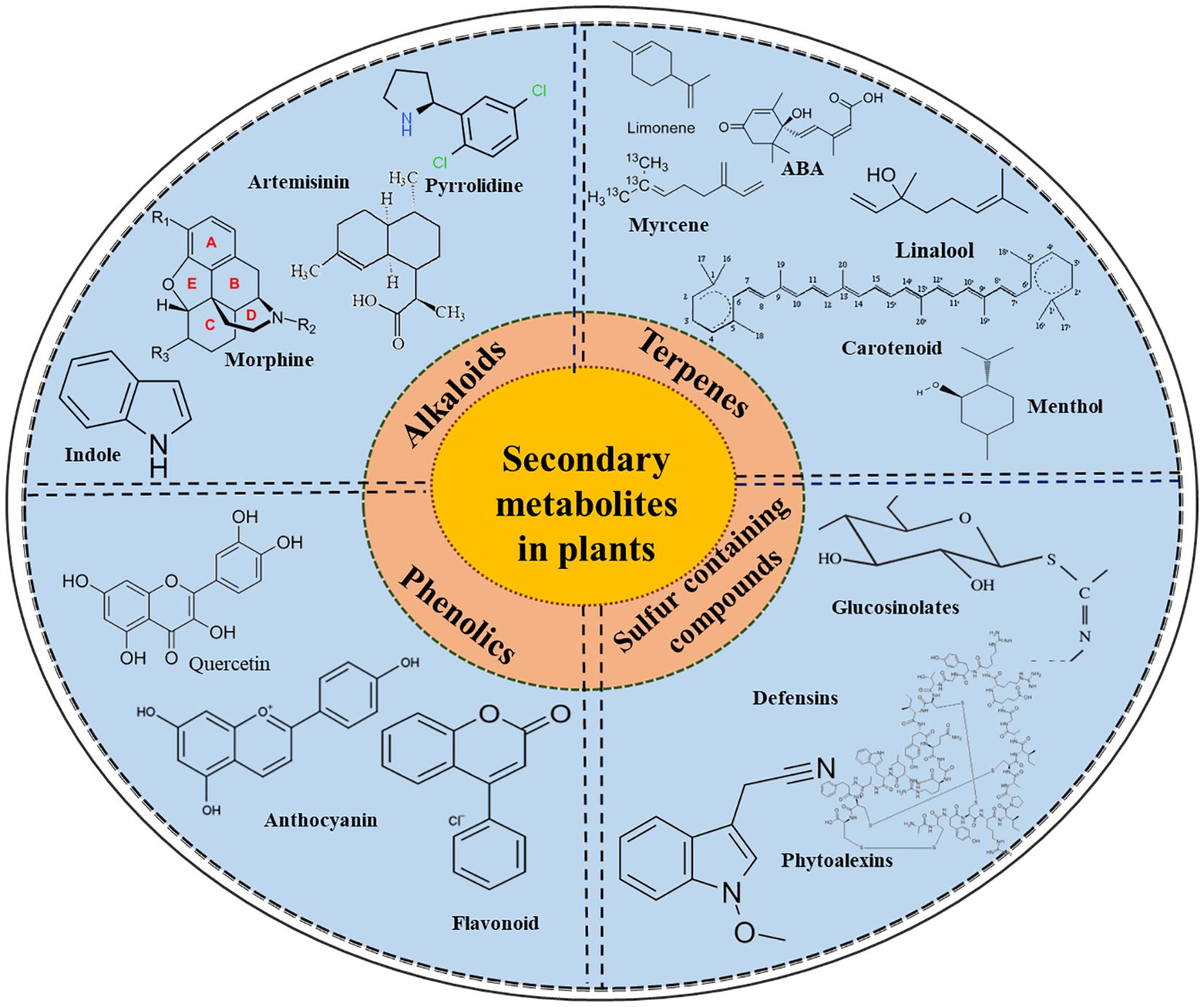
Figure 1. The schematic diagram shows the different classes of secondary metabolites, including terpenes, phenolics, alkaloids, and glucosinolates, along with some of their important members, which encompass diverse biological and physiological functions.
2.1 Terpenoids
Terpenes or terpenoids are an enormous chemical group containing nearly 22,000 compounds. Isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP) bear five-carbon precursor units, which produce a basic ring of terpenoids. Plants have an active cytosolic mevalonic acid pathway (MVA) and plastidial 2-C-Methyl-D-erythritol-4-phosphate (MEP) pathways, which are both involved in the synthesis of IPP and DMAPP precursors (Bergman et al., 2024). DMAPP and IPP condense to form sesquiterpenes, sterols, and triterpenes. Meanwhile, the MEP pathway uses glyceraldehyde-3-phosphate, pyruvate, and 1-deoxy-D-xylulose 5-phosphate synthase (DXS) to produce DXP (Kumari M. et al., 2024). Terpenoids are distributed throughout the plants and play essential roles in plant physiology and ecology. They are also involved in environmental adaptation and stress tolerance (Boncan et al., 2020; Ninkuu et al., 2021). Furthermore, the terpenes are divided into monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes (C40), and polyterpenes with more than ten carbon units (C>10) (Naji et al., 2024). Also, terpenoids encompass carotenes, carotenoids, and xanthophylls, which function primarily as light-harvesting pigments and antioxidants. These pigments protect the photosystem by eliminating singlet oxygen production, scavenging ROS, and releasing surplus energy as heat through the xanthophyll cycle (Tiku, 2018; Khan et al., 2024a). Terpenoids act as membrane stabilizing agents, preventing ion leakage, and are used in natural pesticides and herbicides due to their repellent or toxic effects on pests (Khan et al., 2024a). Moreover, isoprene biosynthesis in plants is activated by high temperature, solar radiance, and water scarcity. Isoprene is proficient in quenching several ROS and NOS species and enhancing the thylakoid membrane constancy. Diterpenes and monoterpenes act as toxins or signalling molecules to deter pathogens. For instance, diterpenes help plants defend against microbes like Magnaporthe oryzae. In the case of rice, four main labdane-related diterpenoids momilactones A and B, phytocassanes A–F, oryzalexins A–F, and oryzalexin S. that act as phytoalexins (Zhan et al., 2020). The plant-parasitic nematode Heterodera schachtii was defended against by sesquiterpenoid nootkatone through the upregulation of defence genes controlling the SA (PR1, PR5, and NPR1) and JA pathways (JAZ10, CYP82C2, and LOX) (Habash et al., 2020).
2.2 Phenolics
Phenolic compounds, also known as phenols, are a diverse class of bioactive secondary compounds. The molecules have at least one aromatic ring containing one or more hydroxyl groups, ubiquitous in plants and essential for defending against pests, diseases, and abiotic stresses (Bano et al., 2023). The hydroxyl group helps counterbalance ROS like H2O2 and singlet oxygen, catalyses oxygenation reactions by forming metal complexes, and hinders the activity of certain oxidizing enzymes. An increased number of phenolic hydroxyl groups provides more reactive sites for oxidation, thereby strengthening the compound’s free radical neutralization capacity (Naikoo et al., 2019; Wang et al., 2021). Additionally, phenolic compounds differ in their ability to chelate metals, largely determined by their reduction potentials, with gallic acid at the top of the hierarchy and coumaric acid at the bottom. They also interact with membrane phospholipids and proteins. This interaction stabilizes cell membranes by reducing fluidity and preventing lipid peroxidation. They also facilitate numerous physiological and biochemical activities, including plant protection against biotic and abiotic stress (Al Mamari, 2021). Phenolic biosynthesis in plants is initiated by two major compounds: phosphoenolpyruvate (a component of glycolysis) and erythrose-4-phosphate (a component of the pentose phosphate pathway) via the shikimate pathway, which ultimately produces more complex and diverse phenolics compounds (Santos-Sánchez et al., 2019). Phenolic compounds act as antioxidants, scavenging ROS such as O2•–, H2O2, and OH. Moreover, phenolics can chelate (bind) with metal ions, preventing them from participating in ROS formation and increasing the activity of antioxidant-forming enzymes (Dias et al., 2021). Phenolics are divided into two groups: flavonoids and non-flavonoids. Two aromatic rings and a 15-C molecule comprise the molecular structure of flavonoids. Non-flavonoids contain complex structures and high molecular mass as compared to flavonoids. The primary function of phenolics is to provide colouration to various plant parts (Caleja et al., 2017). Flavonoids play diverse roles in plants, including antimicrobial agents, repellents, and UV protectants, as well as in the growth and development of various species (Mathesius, 2018). These are primarily sourced from vacuoles, which engage in ROS scavenging in peroxisomes, chloroplasts, and mitochondria. The interplay between phenolics and flavonoids majorly contributes to effective stress management in plants. Analogously, intense light, heat, nutrient, and sugar deficiencies promote anthocyanin accumulation in plants to prevail against adverse conditions. Some phenolic compounds help in synthesizing phytoalexins, which are antimicrobial substances produced in response to infection. The buildup of coumarins has been demonstrated to strengthen resistance to bacterial, viral, and fungal infections, which is especially noticeable in the defence against pathogens, such as oomycetes. Gallic acid pre-treatment in Camellia sinensis cv. Longjing 43 against Ectropis obliqua larvae stimulates the production of antifeedant compounds, like epigallocatechin-3-gallate, naringenin, and astragalin (Zhang X. et al., 2022). Likewise, purple corn pericarp extract enriches with polyphenols, which dramatically suppresses Manduca sexta growth (Tayal et al., 2020).
Together, phenolics and terpenoids provide a complementary, multi-layered defence strategy, integrating rapid biochemical responses with oxidative defences and enabling plants to resist stresses effectively. These two classes of secondary metabolites often function synergistically. Phenolics stabilize the cellular redox environment and limit pathogen spread (Shiade et al., 2024), and terpenoids act as early, front-line deterrents, either killing or repelling invaders or signalling for further defence responses.
2.3 Alkaloids
Nitrogen-containing compounds are produced from diverse aromatic amino acids in plants and can be categorized into alkaloids, cyanogenic glycosides, and non-protein amino acids. Based on their heterocyclic ring system and biosynthetic precursors, indole, purine, quinoline, isoquinoline, tropane, and imidazole alkaloids are classified into various classes (Roy, 2017). Non-protein amino acids act as precursors by providing the nitrogen atoms for alkaloid biosynthesis, which produces quinoline and quinazoline alkaloids. Synthesized alkaloids are stored in designated cellular compartments. In response to various environmental stress signals, these alkaloids are secreted from their storage organelles or specialized glands and transported to target tissues (Bhambhani et al., 2021). Alkaloids contain various antibacterial and antiviral activities and provide defence against a variety of microbes. Specifically, aromatic amino acids (phenylalanine, tyrosine, and tryptophan) serve as precursors for certain alkaloids (isoquinoline and indole alkaloids) and are recognized as antiherbivore compounds (Debnath et al., 2018). Besides, Brassica juncea, a cadmium (Cd) accumulator, shows high biomass production under Cd-contaminated soil and alkaloid accumulation, which counteract the stress effect (Tan et al., 2021). Alkaloids are the main SMs that accumulate in Sophora alopecuroides, associated with nitrogen metabolism. Experimental studies suggest that the upregulated unigenes in the nitrogen metabolism pathway may enhance alkaloid biosynthesis in S. alopecuroides under severe drought stress conditions (Huang et al., 2024). These substances also responded to mechanical stress by diminishing pathogen proliferation and inducing a hypersensitive response by programmed cell death (PCD) (Gogoi et al., 2024).
2.4 Sulfur-containing compounds
Glucosinolates, thiosulfate, allicin, derived from cysteine sulfoxides, reactive sulfur species (RSS) (H2S and sodium sulphate), and antimicrobial peptides (defensins and thionins) are examples of sulfur-containing metabolites that shield plants from pathogenic microbes (Künstler et al., 2020). These compounds have ecological and physiological significance for plants and are valued by humans for their flavours and potential health benefits, including cancer protection. Earlier workers reported that the abundance of glucosinolates occurs in the Brassicaceae family (Blažević et al., 2015). The biosynthesis of glucosinolates begins in the early stage with the help of aliphatic, aromatic, or indole amino acids through chain elongation and, lastly, is stored in vacuoles (Sharma et al., 2024). Noticeably, various sulfur-containing metabolites, including glutathione, phytoalexins, alliins, and defensins, participate in plant defence against various abiotic stress stimuli (Twaij and Hasan, 2022). Moreover, RSS, such as H2S, plays an influential role in alleviating the negative effects of abiotic stress in plants. For instance, in maize, H2S elevated the antioxidant enzyme and proline concentration, which suppressed abiotic stress consequences (Liu Z. et al., 2019). Similarly, phytoalexins (camalexin and resveratrol), low molecular weight compounds, are produced and accumulated through pathogenic infection to protect plants. One of the glucosinolates hydrolysis, 3-butene nitrile (3BN) elicits the defence in A. thaliana, by coordinating crosstalk with JA, SA, and NO signalling (Ting et al., 2020).
3 Signalling molecules in plants: an overview
Signalling molecules, such as NO, H2S, MeJA, H2O2, ETH, MT, and Ca2+ in plants, facilitate communication within and between the cells under normal or stressful conditions and play decisive roles in physiological processes. They significantly impact the coordinated regulation of signalling networks and developmental processes under a wide range of challenging circumstances (Dikilitas et al., 2019). Optimal levels of signalling compounds trigger the activation of defence pathways, via the activation of enzymatic and non-enzymatic antioxidant systems, together with phenolics and other SM biosynthesis (Liang et al., 2018; Rao and Zheng, 2025). Signal transduction is a cascade of molecular events that convert extracellular physiological signals into intracellular responses. This response often leads to affecting the expression patterns of important genes and proteins. Figure 2 highlights the role of various signalling compounds and their donors under numerous abiotic stresses, activating key genes that influence SM production and stimulating plant defence mechanisms.
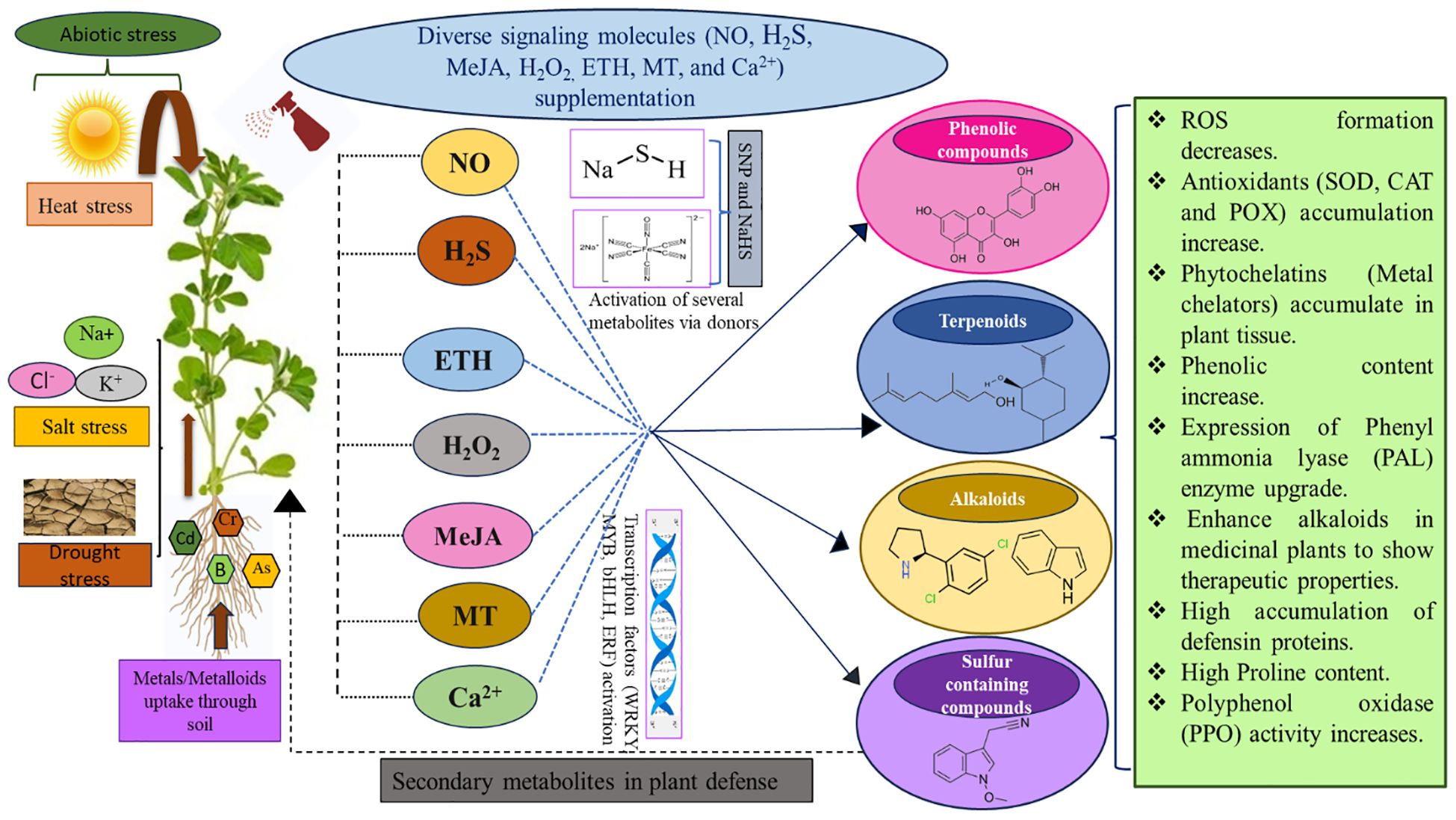
Figure 2. An overview of the role of signalling molecules (NO, H2S, ETH, H2O2, MeJA, MT, and Ca+2) under abiotic stress emphasizes their importance in strengthening plant resistance by regulating various pathways and enzymes regarding secondary metabolites. These metabolites trigger specific signalling cascades that help plants adjust to challenging environments. Fine-tuning protective mechanisms like antioxidant systems, osmotic balance, and stress-related gene expression contributes to improved plant tolerance and survival.
3.1 Role of nitric oxide in SM production under stressful environment
Abiotic stress is a strong elicitor that recuperates SM production to impart signals in the plant, stimulating defence machinery and ensuring survival in harsh environments (Yeshi et al., 2022). Under stress conditions, plants usually do catabolic degradation of ROS into non-toxic compounds with the support of some enzymes, including superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), and ascorbate peroxidase (APX). Plants have precise defence mechanisms to counteract the adverse situation and avoid the inimical consequences of stressful environments (Jan et al., 2021). As a compact free radical, NO positively contributes to plant development and growth, impacting every phase from germination to senescence (Rather et al., 2020). Nitric oxide production occurs in organelles including the chloroplast, mitochondria, peroxisome, plasma membrane, and apoplast, with the help of oxidative and reductive pathways (Nabi et al., 2019). NO effectively protects them from oxidative damage by triggering the activities of enzymes that control intracellular ROS (Hancock and Veal, 2021). Within cellular signalling, NO acts downstream of primary signals, such as Ca²+, JA, cADPR, abscisic acid, H2O2, MAPK cascades, and cGMP, serving as a secondary messenger. It is necessary to balance the concentration of NO when reacting to different physiological and environmental stresses. Earlier reports highlighted the importance of NO donors, such as sodium nitroprusside (SNP), S-nitro cysteine (CySNO), and S-nitrsoglutathione (GSNO) that are employed to improve the defence ability of plants (Rahim et al., 2022). Sodium nitroprusside (SNP), a NO donor, plays a fundamental role in plant processes and growth regulation and boosts alkaloid accumulation and antioxidant-related genes under stress conditions (Farzaei and Sayyari, 2024). Nitric oxide has been successfully employed as an elicitor to stimulate SM accumulation in plants. Nitric oxide under stressful conditions modulates non-enzymatic antioxidants, including ascorbic acid (AsA), glutathione (GSH), alkaloids, α-tocopherol, carotenoids, phenolics, flavonoids, and proline (Hasanuzzaman et al., 2020). For example, SNP applied on Calendula officinalis L. significantly enhanced the total flavonoids, phenolics, antioxidant activity, and essential oil yield (Zhang et al., 2012). Endogenously and exogenously produced NO and the application of NO donors regulate phenolics, flavonoids, and caffeic acid derivatives in several plant species (Table 1).
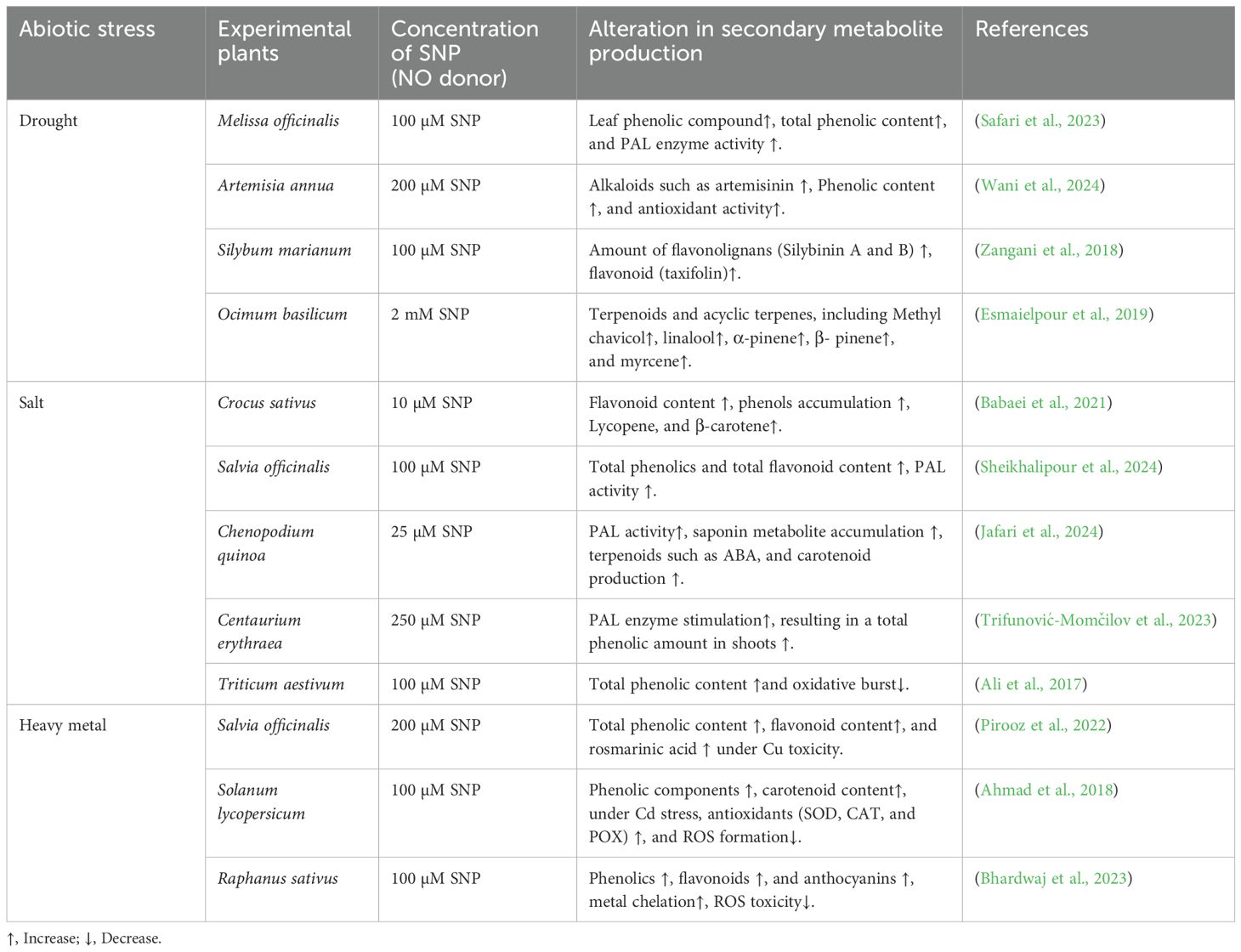
Table 1. Exploring the role of sodium nitroprusside (SNP) in managing secondary metabolite production in diverse plant species under abiotic stress.
3.1.1 NO under drought stress
Drought stress is one of the severe threats to plants. Recent climate deviations have increased the frequency, intensity, and duration of stress, making it a global concern. Supplementation of NO produces diverse SMs and essential oil components. For example, 60 μM of SNP to Origanum majorana reduced lipid peroxidation and activated the PAL (phenyl ammonia lyase) enzyme, enhancing phenolics and a broad class of antioxidants (Liang et al., 2018; Farouk and Al-Huqail, 2020). In Scrophularia striata, the crosstalk of NO with H2O2 operates signal transduction pathways to induce PAL activity to enhance the accumulation of phenolic compounds under drought stress (Falahi et al., 2018). According to Chavoushi et al. (2020), the combined application of 25 μM SNP and 250 μM SA in safflower (Carthamus tinctorius L.) regulates the expression of some flavonoids (rutin, quercetin, luteolin, and apigenin) under drought conditions. They also stimulate the anthocyanins, phenols, and PAL activity. Like in Radix saposhnikoviae, SNP applied to fresh roots amplify the efficiency of acetyl-CoA carboxylase (ACC), PAL, and chalcone synthase (CHS) enzymes along with some therapeutic constituents, such as prim-O-glucosylcimifugin, cimifugin, and sec-O-glucosylhamaudol (Song X. W. et al., 2023). Under 60% field capacity, drought-affected Brassica oleracea displayed a significant increase in total phenolics content, chlorophyll, SOD, and POX activities. Foliar application of NO in the pre-sowing period raises the biomass output of the plants (Munawar et al., 2019). Moreover, supplementation of both NO and NOSH (donor of H2S) significantly alleviates the drought stress in Medicago sativa via stimulation of major antioxidant genes (GST17, Cu/ZnSOD, FeSOD, cAPX), linked with ROS scavenging (Antoniou et al., 2020).
3.1.2 NO under salinity stress
Salinity in soil hinders major physiological processes in plants. Salinity stress causes lipid peroxidation, changes the Na+/K+ ratio, and reduces the transport of essential elements, distorting plant structure and growth. Nitric oxide synthase (NOS) is the key enzyme of the NO biosynthetic pathway, particularly expressed in abiotic stress. It is noted that unfavourable conditions induce endogenous NO production mainly through the highly expressed NOS1 enzyme and nitrate reductase (NR) pathways. Accumulation of NO in plants alleviates salt stress as it increases isoflavonoid synthesis and related gene (CHR, CHIA, C4H, and IFS) expressions (Yin et al., 2022). The application of SNP in the case of Panax ginseng and rice (Oryza sativa L.) plants significantly boosted the activity of antioxidant enzymes, such as SOD, CAT, APX, POX, and polyphenol peroxidase (PPO), to protect the plant from salt stress (Rahim et al., 2022). Similarly, in the case of Brassica napus L., NaCl exposure in plants causes phytotoxicity; however, NO treatment controls the toxic effects and improves phenolic content and PAL enzyme activity (Karimi et al., 2020). Moreover, exogenous supplementation of SNP to Silybum marianum L. seedlings enhances the therapeutic properties of phenolics and flavonoids (Zangani et al., 2018).
3.1.3 NO under heavy metal stress
Many studies stated that plants upon exposure to Heavy metals (HMs) stress exhibit ultrastructural changes, including chlorosis, disorganized photosynthesis system, root and shoot damage, and senescence. Heavy metals stimulate the plants’ endogenous SNP production. Application of SNP stimulates SMs production to resist metals (Pb and Cd)/metalloid effects, resulting in improved shoot and root dry weights, and growth indices in various plant species (Emamverdiansss et al., 2021). Under Cd stress, exposure of SNP (200µM) in Artemisia annua enhances the artemisinin (present in glandular secretory trichomes) content (Wani et al., 2023). Under stress conditions, the production of phenolic compounds decreases; however, the application of exogenous NO increased anthocyanins, flavonols, and flavonoids in seedlings of Solanum tuberosum (Nabati et al., 2024). Moreover, above the permissible limit, copper (Cu) shows toxic symptoms in plants, such as necrosis and growth retardation. In Raphanus sativus, Cu toxicity is overcome by supplementing NO (200 µM), resulting in enhanced phenolic, flavonoid, and anthocyanin concentrations to reduce free radicals (Bhardwaj et al., 2023). Overall, research shows that NO’s role becomes more pronounced in regulating the production of metabolites to alleviate stress in plants.
4 Role of hydrogen sulfide in SM production under stress conditions
Hydrogen sulfide is primarily known as a gaseous transmitter and has been recognized to play a vital role in cellular and physiological processes in plants. It involves cell proliferation, seed germination, stomatal conductance, root formation, overall plant development, and enhancing plant responses to environmental stresses (Zhang et al., 2020). Hydrogen sulfide is a highly mobile and transported molecule throughout the plants. Sub-cellular compartments, including the chloroplast, cytosol, and mitochondria, serve as the sites for H2S formation, where sulfur and cysteine metabolism-related enzymes drive its biogenesis (Zhou et al., 2020). Hydrogen sulfide and its donors (NaHS) impact SMs by influencing sulfur metabolism and modulating stress responses to counteract by eliminating ROS, thereby maintaining intracellular redox balance (Rizwan et al., 2019). It is reported that numerous H2S donors, such as sodium hydrogen sulfide (NaHS), calcium sulfide, and diallyl trisulfide (DATS), also participate under abiotic stress (Hilal et al., 2023). Hydrogen sulfide also positively affects the postharvest storage of vegetables and fruits. In tomatoes (Solanum lyocpersicum L.), it raised the level of SM and their regulation via high gene expression. Moreover, H2S increases the expression of PAL5, monodehydroascorbate reductase (MDHAR), and dehydroascorbate reductase 1 (DHAR1) genes along with flavonoid-related genes like chalcone synthase 1 or 2(CHS1 or 2), flavanone 3-dioxygenase (F3H), and flavanol synthase (FLS) (Zhang Y. et al., 2023). Besides, under Cd2+ stress, the photosynthetic system in Vigna radiata tends to be repaired by exogenous NO and H2S application, with Ca2+, which acts as an intermediate (Khan et al., 2020). These studies highlight the potential role of H2S in enhancing SMs synthesis under abiotic stress (Table 2).
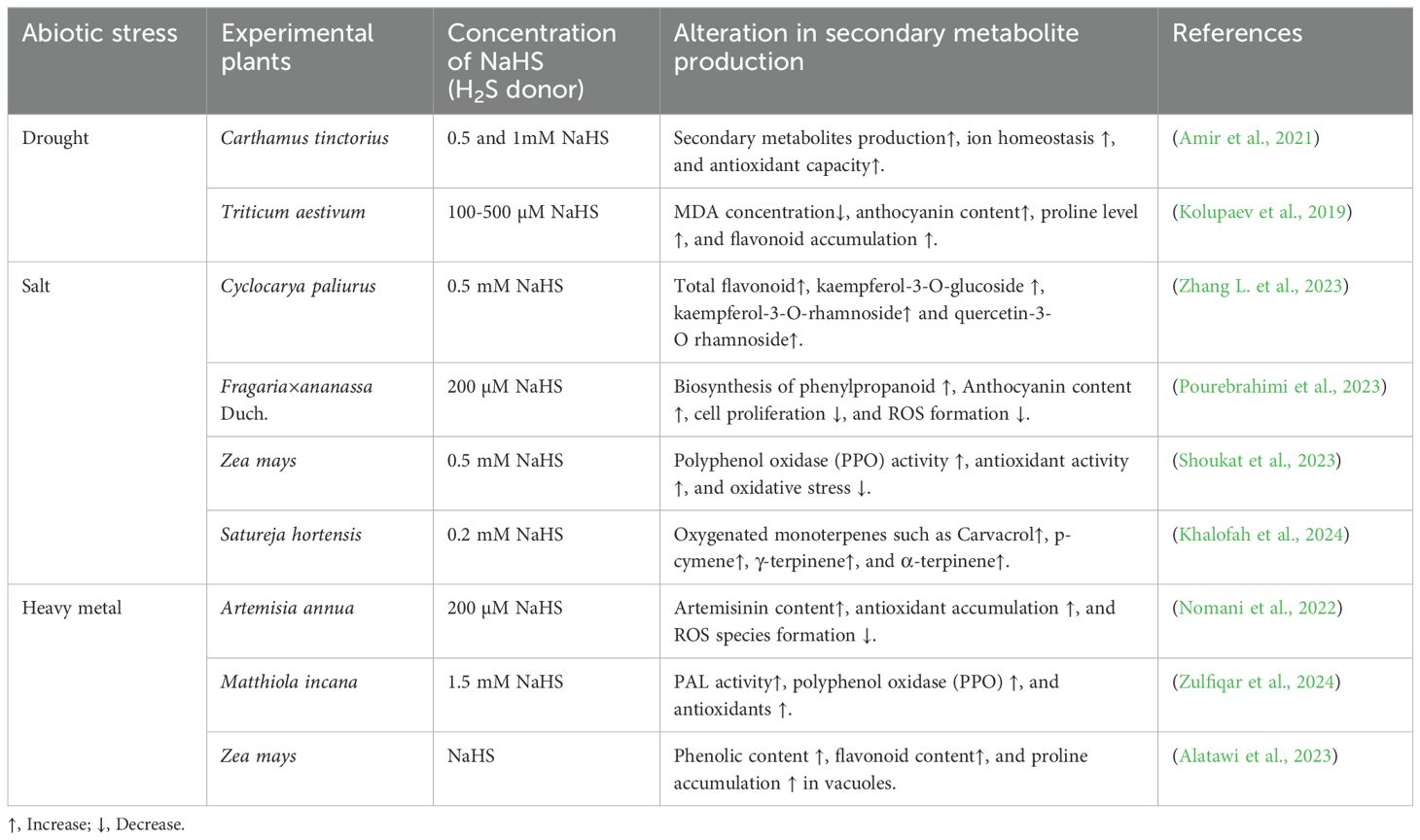
Table 2. Exploring the role of sodium hydrogen sulfide (NaHS) in managing secondary metabolite production in diverse plant species under abiotic stress.
4.1 H2S under salinity stress
High accumulation of various salts in the soil produces osmotic stress and ion toxicity, which negatively regulate nutrient uptake, physiological activity, and metabolic pathways. As a result, the accumulation of salinity in plants increases ROS at the subcellular level. Foliar spray of NaHS in batal (Cyclocarya paliurus) leaves escalates phenolic content and many other chemical constituents such as quercetin-3-0-rhamnoside and kaemferol-3-0-rhamnoside, and 3-O-caffeoylquinic acid (Chen et al., 2021). As stated, phenolic compounds, equipped with a hydroxyl group, can lessen the toxicity of ROS. Alamer (2023) demonstrated that H2S has a beneficial effect in wheat (Triticum aestivum L.) crops upon applying it and upgrades PAL enzyme activity, resulting in an improved phenolic level compared to control and NaCl-treated plants. Other research proved that Satureja hortensis treated with 0.2 mM NaHS increases oxygenated monoterpenes such as carvacrol, p-cymene, γ-terpinene, and α-terpinene under high saline conditions (Khalofah et al., 2024).
4.2 H2S under heavy metal stress
Heavy metal stress correlates with ROS accumulation and antioxidant formation in response to oxidative injury. When exposed to HM stress, SMs like flavonoids and anthocyanins significantly diminish redox imbalance within plant cells (Maleki et al., 2017; Goncharuk and Zagoskina, 2023). Under arsenic (As) stress, H2S and melatonin (MT) supplementation in tomato seedlings increases antioxidant phenolic compounds (anthocyanin, polyphenols, and flavonoids), helping reduce oxidative stress (Ghorbani et al., 2024). Besides phenolics, terpenoids also help in mitigating abiotic stress in cucumbers (Cucumus sativa L.) under chilling conditions. The tetracyclic triterpenoid known as cucurbitacin protects plants from both abiotic and biotic stress. This protective effect is notably enhanced when plants are treated with NaHS (Liu Z. et al., 2019). A similar study was conducted on Cucurbita pepo under nickel (Ni) stress with supplementation of various concentrations (50, 100, 200, and 400 μM) of NaHS, under normal conditions. Whereas, among all the applied doses, 100 μM of NaHS improved phenolics, anthocyanins, and sinigrin (glucosinolate) content in roots and aerial parts (Valivand and Amooaghaie, 2021a). Besides, combined supplementation of NO and H2S to form nitrsothiols, which significantly increases resistance against pathogenic attack (Corpas et al., 2024). Although the application of NaHS and SNP regulates the endogenous production of NO and protects Bermuda grass from lead toxicity (Shi et al., 2014).
5 Role of methyl jasmonate in SM production under stress conditions
Jasmonic acid and its derivatives (MeJA and Ja-Ile) are collectively known as jasmonate. Methyl jasmonate is highly volatile and aids in controlling a variety of abiotic and biotic stress. To overcome the penalties of environmental stress, it stimulates molecular signal transduction and gene expression regulation, which leads to the accumulation of SMs (Jeyasri et al., 2023). It induces the gene expression involved in alkaloids, phenolics, and terpenes biosynthesis to improve the plant’s survival abilities. Several transcription factors, including WRKY, bZIP, basic-helix-loop-helix (bHLH), NAC, ERF, MYB, and MYC2 families, regulate the MeJA signalling process, as well as upregulate SM production (Kumar et al., 2021). These transcription factors are essential for regulating the synthesis of compounds like flavonoids and phenols. For instance, in Salvia miltiorrhiza, the MeJA-responsive MYB factor stimulates phenolic acid accumulation (Zhou W. et al., 2021). A similar role of WRKY1 in Artemisia annua is that it regulates artemisinin biosynthesis by binding to the promoter of the sesquiterpene synthesis gene. According to Davari et al. (2018), Satureja hortensis treated with JA shows a considerable improvement in the quantities of α-pinene and monoterpene hydrocarbons in essential oil. Further, exogenous MeJA enhanced the expression of disease-resistance genes like RrRGA3, RrPPO, RrCHIT, RrPRB1, and RrRPM1. It also activated genes related to AsA biosynthesis and the AsA–GSH cycle, including RrMIXO, RrAKRC9, RrDHAR, and RrGPX, leading to an increase in AsA levels. Methyl jasmonate amplifies activities of PAL, C4H, 4CL enzymes, and gene expression (RrPAL, Rr4CL, RrCSE, RrCCR, RrPGT, RrHCT, RrDFR, RrERF114) in the phenylpropanoid pathway. This upregulation resulted in a higher accumulation of L-phenylalanine, caffeic acid, phlorizin, other phenolic compounds, and lignin in Rosa roxburghii (Ma et al., 2025).
5.1 MeJA under drought stress
Drought stress affects regular activities and manifests detrimental symptoms in stressed plants. Plants stimulate flavonoid biosynthesis, which sustains antioxidant characteristics to protect plants. Several in vitro studies have shown that drought stress is interrelated with SM accumulation in plants and associated with metabolic activities (Ghosh et al., 2018). Genome editing in SM regulatory genes can improve drought tolerance according to their functions. For example, bHLH transcription factors exist in various plant species and synchronize with flavonoid biosynthesis to improve drought effects. VvbHLH1 (members of the bHLH family) overexpressed in Arabidopsis and modulates phenolic/flavonoid biosynthesis, ABA signalling, osmolytes production, and ROS scavenging systems (Wang F. et al., 2016). For example, the application of MeJA increases SMs (anthocyanin, phenolics, and carotenoids), which act as superoxide and hydroxide radical scavengers and deplete drought stress in Ocimum basilicum (Lopes et al., 2024). MeJA administration enhances the total phenolic content in peppermint leaves (Shenavaie Zare et al., 2022). Furthermore, soybeans (Glycine max L.) under drought stress have shown a reduction in total flavonoid content, but MeJA supplementation increases the accumulation of isoflavonoids and phenolic compounds (Mohamed and Latif, 2017). Under water deficit conditions in Astragalus membranaceus, treatment of 200µM MeJA improved glycoside (calycosin-7-O-β-d-glucoside) accumulation with diverse therapeutic properties (Feng et al., 2021). Aromatic amino acids act as precursors for SM synthesis, such as phenylalanine and tyrosine, and participate in flavonoid accumulation during adverse conditions. Glucosinolates derived from sulfur-containing amino acids (methionine, tryptophan) are well-known for providing tolerance against biotic and abiotic stress. Tang et al. (2023) confirmed that applying MeJA in Prunella vulgaris L. enhanced the activity of PAL, tyrosine aminotransferase (TAT), and 4-coumaroyl CoA ligase (4-CL) enzymes that are involved in the phenylpropanoid pathway. The activation of these enzymes helps in improving membrane stability and photoprotection machinery in plants.
5.2 MeJA under salinity stress
Soil salinization is the most significant agricultural sector issue, particularly in dry and semi-arid areas. The presence of salt stress generates ROS, which can degrade both carotenoids and secondary compounds. Interestingly, MeJA treatment improves the photoprotection mechanism via the accumulation of carotenoids and regulates antioxidants (SOD, POX, DHAR, and CAT) to overcome cellular oxidative stress. Kiani et al. (2021) verified that 60 µM of MeJA enhanced the photosynthetic pigment (chlorophyll and carotenoids) and also modulated phenolic compounds. He et al. (2021) also demonstrated that higher concentrations of NaCl reduce carotenoid content in Zea mays, following MeJA supplementation, the amount of carotenoid, lutein, and zeaxanthin rises in NaCl-stressed plants. Several studies focused on the influential characteristics of MeJA in SM production, particularly members of the Lamiaceae family under saline toxicity. For instance, Mentha piperita undergoes treatment of MeJA and shows an elevation in methyl acetate, β-pinene, and 1,8-cineole (Afkar and Karimzadeh, 2014). Saeed et al. (2017) experimented with the medicinal plant Ajuga bracteosa to explore the impact of MeJA and phenylacetic acid (PAA) on its growth parameters and bioactive constituents. They observed that MeJA and PAA application increased the total phenolic (lignin and tannin) and flavonoid content in the root suspension of A. bracteosa. Previous studies documented that MeJA treatment facilitates the biosynthesis of SMs in plant cells by influencing ROS scavenging activities and activating pivotal antioxidant genes (Ho et al., 2020; Khan et al., 2024a). Furthermore, MeJA has been extensively deployed as an elicitor in various species of medicinal plants to increase the production of SMs in the cell culture system. The combined application of salicylic acid (SA) and MeJA upgrades flavonoids and phenolic yield in Phyllanthus pulcher (Danaee et al., 2015). A similar study highlights the elicitation potentiality of MeJA and SA in the case of Cuminum cyminum, where MeJA dominates the involvement of key genes and enzymes involved in phenolic compound synthesis (Rahimi et al., 2013). They suggested that this stimulation boosts cellular activities at both biochemical and molecular levels through various signalling compounds. Additionally, MeJA plays a decisive role in signal transduction, accelerating enzyme catalysis, and promoting the production of specific compounds (polyphenols, terpenoids, flavonoids, and alkaloids) (Rahimi et al., 2013; Ho et al., 2020). Table 3 summarizes the effects of MeJA treatment on the concentrations of flavonoids, polyphenols, and isoprene compounds (volatile and non-volatile) in a toxic environment.
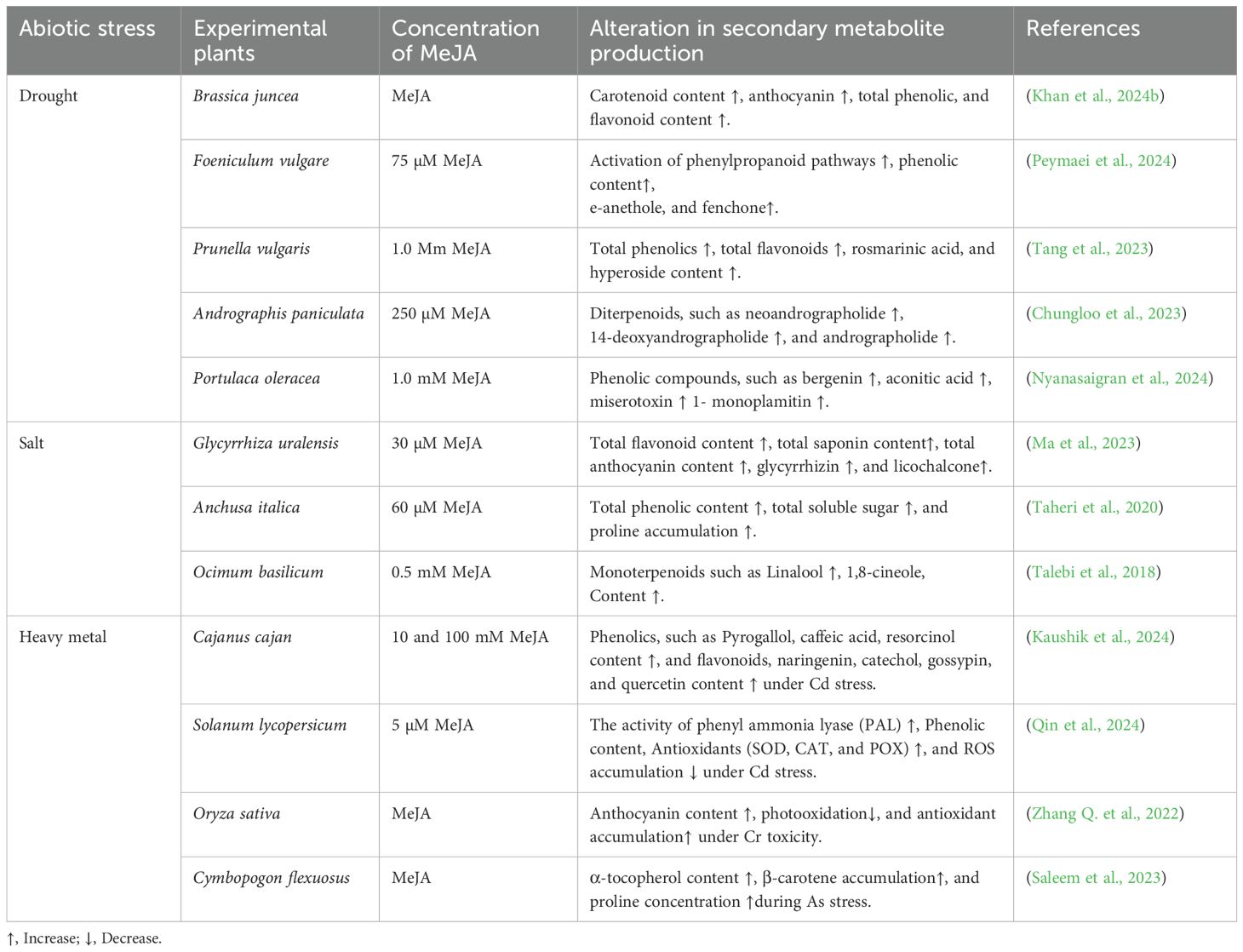
Table 3. Exploring the role of methyl jasmonate (MeJA) in managing secondary metabolite production in diverse plant species under abiotic stress.
5.3 MeJA under heavy metal stress
Plants face HMs toxicity, such as chromium (Cr), Cd, arsenic (As), and Cu in the soil that are taken up by plants through the transporters, altering physiological, biochemical, and genetic compositions (Ahmad et al., 2018). In exposure to HMs, phenols act as efficient chelators. Plant root secretes phenolic compounds (p-hydroxybenzoic and vanillic) and flavonoids (naringenin and quercetin) that help in the solubilization of metals in the soil (Quartacci et al., 2009). Additionally, antioxidants regulate ROS levels and are crucial for developing heavy metal tolerance strategies in plants. Polyphenols, terpenes, and various vitamins play a key role in downregulating O2•– and minimizing the effect of oxidative imbalance. An exogenous supply of MeJA under As stress significantly alleviates the toxicity and further amplifies the PAL and polyphenol oxidase (PPO) enzyme activities (Farooq et al., 2016). One of the antioxidants, e.g., astaxanthin (tetraterpenoids) obtained from Haematococcus pluvialis highly accumulated when exposed to MeJA, causes the astaxanthin-related genes (PSY, BKT, and CRTR-b) to express, for its high accumulation (Liu Y. et al., 2019). In a study, the supply of MeJA to Centella asiatica increased the concentration of triterpenoids via increment in PAL enzyme and antioxidant enzyme activity (Buraphaka and Putalun, 2020). Under selenium (Se) stress, the species Plantago ovata was exposed to JA (10 µM), which synergistically improved the provision of numerous SMs. For instance, hydroxycinnamic acids, flavonoids, lignins, tannins, and other similar compounds serve as facilitators in stress responses (Dey and Raychaudhuri, 2024).
6 Role of hydrogen peroxide in SM production under stress conditions
In recent years, considerable attention has been paid to H2O2, which acts as a signalling molecule, triggering various physiological and biochemical responses in plants. Hydrogen peroxide signals trigger adaptive responses affecting cell proliferation, differentiation, transportation, plant survival, and numerous metabolic processes. These physiological processes include seed germination, seedling maturation, stomatal movement, photosynthesis, cell growth and development, antioxidant systems, and senescence, which is significantly regulated by H2O2 (Nazir et al., 2020; Fatima et al., 2023). Hydrogen peroxide is a free radical belonging to the ROS family, synthesized from three main routes: photorespiration, electron transport series, and redox reaction in apoplast (Černý et al., 2018). It can initiate pathways that lead to the synthesis of SMs, which often help plant overall resilience, as mentioned in Table 4.
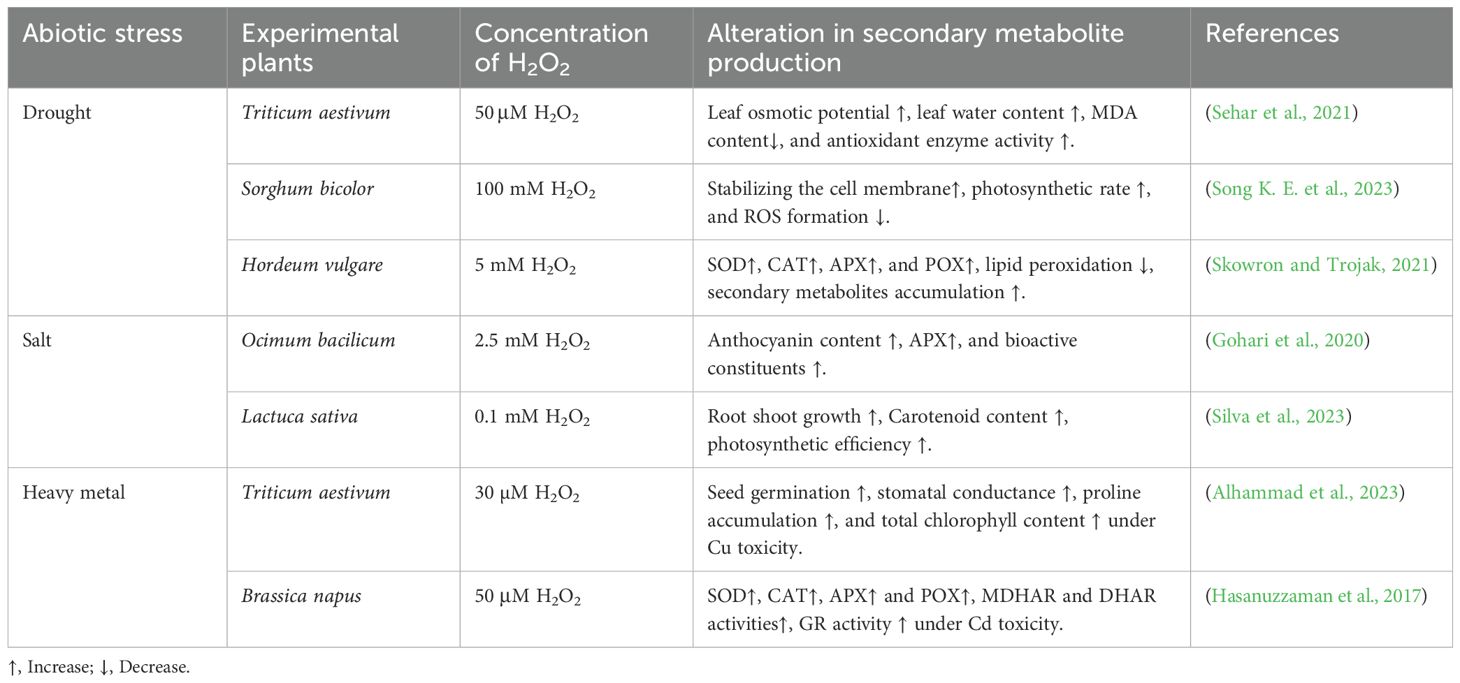
Table 4. Exploring the role of hydrogen peroxide (H2O2) in managing secondary metabolite production in diverse plant species under abiotic stress.
6.1 H2O2 under drought stress
The production of ROS under drought stress modulates plant metabolic machinery in mitochondria, cytoplasm, and peroxisome, and damages cell basic elements (lipids, proteins, and carbohydrates) (Nadarajah, 2020). Applying H2O2 in plant species positively influences their tolerance mechanisms. Hydrogen peroxide modulates the PPO activities by oxidising phenolic compounds associated with antioxidant activity. Under drought stress conditions, pre-treatment of H2O2 influences POX and PPO working mechanisms, ultimately improving phenolic content in root and shoot cells (Bhardwaj et al., 2021). Hydrogen peroxide treatment in soybeans increases the PAL enzyme activity, which boosts total phenolic accumulation compared to stress-affected plants (Darmanti et al., 2021). H2O2 (10 μM) applied on wheat plants reveals a significant elevation in osmolytes, physiological attributes, K+ accumulation, membrane stability, and reduced ROS formation (Singh et al., 2021). Using a metabolomic approach, leaf metabolites in maize were modulated by 10 mM of H2O2 and salinity. Specifically, 42 of the 51 metabolites remain unchanged in plants treated with H2O2 under non-saline conditions (Dos Santos Araújo et al., 2021).
6.2 H2O2 under salinity stress
Soil salinity is a prominent cause of land deterioration after soil erosion has posed persistent challenges to agriculture. Hydrogen peroxide influences various physiological processes, including stomatal closure, root growth, and nutrient uptake, that support plant adaptation to saline environments. The seed priming with 5 mM H2O2 under saline toxicity enhances ferulic, hesperidin, quercetin, luteolin, and rosmarinic acid in Salvia officinalis (Amooaghaie et al., 2024). Further, lettuce (Lactuca sativa L.) plants exposed to H2O2 exhibit increased levels of phenolic compounds that provide antioxidant effects attributed to the hydroxyl group in the benzene ring. It revives many structural genes (PAL, DFR, UFGT, and CHS) of phenylpropanoid metabolism (Wang et al., 2023). Sodium nitroprusside and H2O2 are the priming agents that increase plant tolerance against a range of abiotic stress. The combined treatment of 200 μM SNP + 2.5 mM H2O2 effectively alleviated salinity stress in Ocimum bacilicum via an increment in anthocyanin, APX activity, Chl a and b, and key metabolites like methyl chavicol, linalool, cadinol, and epi-α-cadinol (Gohari et al., 2020). Similarly, NO and H2O2 in Brassica napus L. during saline stress increased antioxidant activity and stimulated PAL activity, which influences flavonoid content (Karimi et al., 2020). Seeds of Brassica oleracea var. botrytis primed with H2O2 improved the defence mechanism and reduced lipid peroxidation and electrolyte leakage (Ellouzi et al., 2021). Further, foliar application of H2O2 helps to adapt strategies that minimize saline toxicity in cotton plants by providing strength to the antioxidant system (Nóbrega et al., 2024).
6.3 H2O2 under heavy metal stress
To overcome the consequences of HMs, H2O2 strengthens the defence machinery in plants to balance the regulatory function. For instance, under As toxicity, 50 μM H2O2 is applied in Oryza sativa, augmenting carotenoid content, osmolytes (proline) in vacuoles and SOD, CAT, and POX activities (Asgher et al., 2021). Controlled application of H2O2 can potentially boost metabolite levels during cultivation. In Aquilaria sinensis, the early response to wound stress involves the activation of AsTPS10, AsTPS16, and AsTPS19, which initiate a H2O2 signalling pathway that leads to the accumulation of sesquiterpenes (Lv et al., 2017). Similarly, in Ficus deltoidei, the application of H2O2, from the vegetative stage to the flowering stage, also influences the plant to generate antioxidant enzymes (POX and PPO) for rapid ROS scavenging (Nurnaeimah et al., 2020). Nazir et al. (2019) reported that H2O2 root dipping treatment had a significant and positive effect on growth and yield characteristics. Besides, following treatment, the leaf’s potential to retain water, photosynthetic pigments, stomatal movement, antioxidant system, and osmoprotectant improves against Cu stress.
7 Role of ethylene in SM production under stress conditions
Ethylene (ETH) is one of the key signalling molecules in plant development (seed germination to leaf abscission) and SM production during stress conditions. Ethylene receptors are predominantly present on the endoplasmic reticulum (ER) and are negatively regulated by various factors such as ETR1, ETR2, ERS1, ERS2, and EIN4. Besides, the AP2/ERF (ethylene-responsive elements) factor notably regulates stress-responsive genes, including Dehydrin, LEA, HMA, ALMT, SERP2, RLK, and MAPKKK3, which are recognized for controlling abiotic and biotic stress (Feng et al., 2020). Ethylene positively influences the number of SMs (phenolics, terpenoids, and alkaloids) in plants which provide resistance against biotic and abiotic stress. Ke et al. (2018) postulated that ETH modulates flavonol biosynthesis by regulating the expression of chalcone isomerase, CHS, and flavonol synthase via the MYB12 transcription factor. Further, ETH precursor (1-aminocyclopropane-1-carboxylic acid, ACC) also participated in phenolic synthesis via high expression of anthranilate synthase (AS), PAL, and isochorismate synthase (ICS) in plants (Liu et al., 2016).
7.1 ETH under drought stress
Different plant species experience drought to varying degrees, and they frequently coincide with a temperature rise. To combat drought stress, plants produce a class of SMs that reduce ionic imbalance and ROS toxicity and maintain the interrelation between phenotype and genotype of the plant (Salvi et al., 2021). Knowing the function of ETH can aid in creating plants to increase the synthesis of SM in crops during droughts. ETH plays a dual role under drought stress as a prooxidant (ROS accumulator) and antioxidant (ROS scavenger). It was noticed that applying ethephon (a diverse form of ETH) to the seeds elevates antioxidants and osmolyte production and lessens lipid peroxidation (Zhang et al., 2016; Kowsalya et al., 2025). Furthermore, ERF also controls root development and maturation under drought stress. Kumar et al. (2022) reported that under drought stress, overexpressing the OsAP2/ERF-N22 line leads to elevated stomatal conductance, transpiration movement, improved membrane stability index, higher relative water content, and osmotic potential.
7.2 ETH under salinity stress
Signalling molecules are essential for managing stress, providing tolerance, and positive regulation of plant metabolic activities throughout the life cycle. ROS generally determines how ETH reacts downstream under stress conditions because ETH signalling depends on ROS levels. Endogenous and exogenous supply of ETH-releasing compounds (ethephon and ACC) reduces salinity in diverse plant species (Iqbal et al., 2017). Applying ethephon in the Daucus carota root to accumulate more anthocyanin via raising their structural genes is likely driven by the effect of DcMYB1’s influence on the DcPAL gene (Barba-Espín et al., 2017). Furthermore, elevated ETH production or persistent activation of ETH signalling resulted in reducing ROS accumulation in root vascular tissue, which contributes to improved salt tolerance. Ethephon increases phenolic and phenylpropanoid content in Fagopyrum esculentum during high saline conditions. Phenylpropanoid provides better resistance against various types of stress, including water, UV, and wound stress (Li et al., 2017).
7.3 ETH under heavy metal stress
Ethylene helps plants cope with the adverse effects of HMs by modulating stress signalling, enhancing antioxidant production, and facilitating metal sequestration. Arabidopsis treated with zinc oxide (ZnO) nanoparticles, ETH signalling deficient mutants ein2–1 and etr1–3 exhibited higher SOD, CAT, APX, and POX activity compared to wild-type plants (Khan et al., 2019). Under Cd toxicity, Lycium chinense up-regulates the LchERF gene and GSG accumulation, which increases the production of ETH and helps overcome Cd toxicity (Guan et al., 2016). Similarly, the supplementation of ETH in Cd-stressed Catharanthus roseus impedes ROS and MDA production and promotes the accumulation of metallothionein (a cysteine-rich biomolecule) (Chen et al., 2017). An experimental study revealed that As-tolerant mutant eto1–1 has a high production of phytochelatins (PCs) as compared to the wild type, proving that ETH better responds in Arabidopsis thaliana under metal stress (Zou et al., 2024). Subsequent research also reported that, in comparison to the wild-type, the ETH-producing mutant eto1–1 produced less MDA and O2•– during As toxicity. Conversely, the application of 100 µM of ACC to Nelumbo nucifera G. under Cd exposure increases antioxidant activities, traps oxidative radicles, and reduces MDA and electrolyte leakage (Wang et al., 2020). Table 5 illustrates various examples of plant species under abiotic stress, where supplementation of ETH increases SMs.
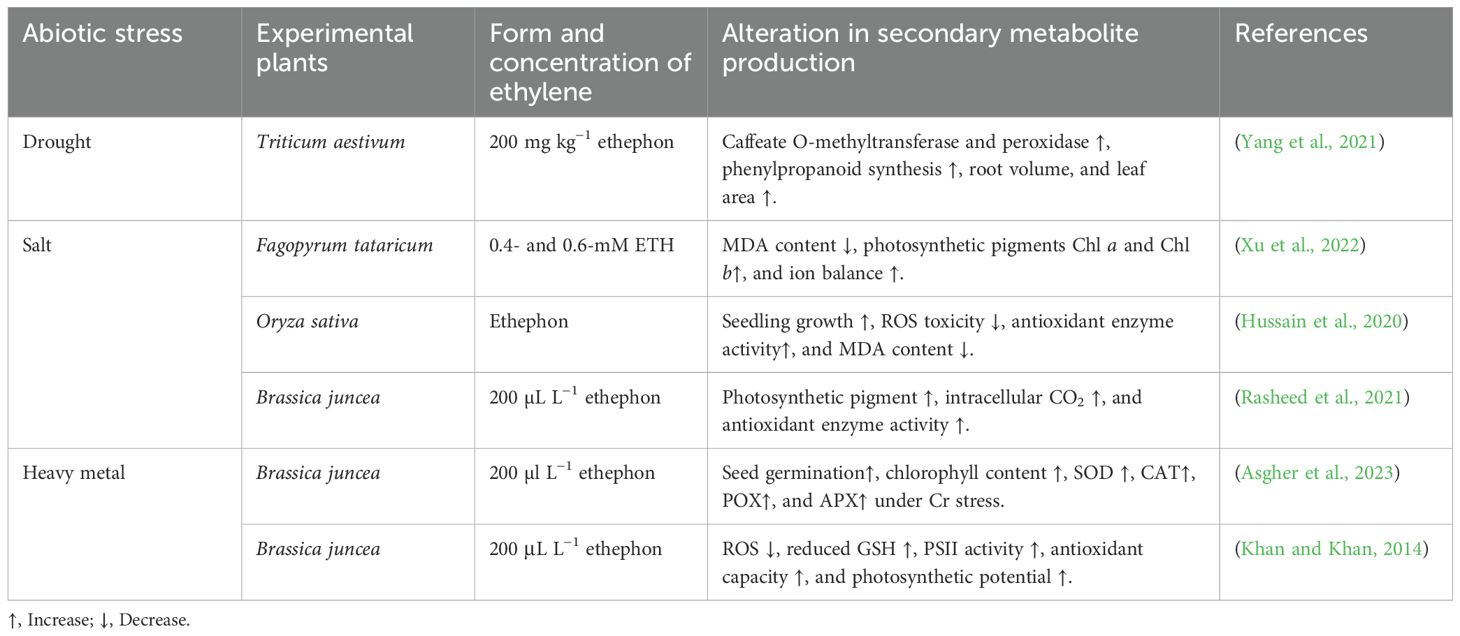
Table 5. Exploring the role of ethylene (ETH) in managing secondary metabolite production in diverse plant species under abiotic stress.
8 Role of melatonin in SM production under stress conditions
Phytomelatonin (N-acetyl-5-methoxy tryptamine, MT) is an indole compound derived from serotonin and is an emerging molecule that regulates diverse functions in plants, including osmo protectant, seed germination, photosynthesis, and delayed senescence (Zhang T. et al., 2022). Plants have two main pathways for synthesising phytomelatonin: primary and secondary pathways. The main enzymes involved in the synthesis of MT in plants are tryptophan hydroxylase (TPH), caffeic acid o-methyltransferase (COMT), tryptophan decarboxylase (TDC), and serotonin N-acyltransferase (SNAT) (He et al., 2023). In the case of plants, SNAT is found within the chloroplast, facilitating the conversion of serotonin to melatonin, whereas in vertebrates, MT production takes place in mitochondria. Melatonin supplementation in plants significantly reduced ROS-generated stress via antioxidant formation and synthesis of SMs (Arnao et al., 2022). Melatonin behaves like an efficient free radical scavenger for OH, H2O2, peroxynitrite anion (ONOO-), alkoxy radical (RO), and peroxyl radical (ROO). Moreover, MT induces a diversity of compounds including terpenoids (mono, di, tri, and polyterpenes), phenolics, anthocyanins, flavonoids, and glucosinolates. It improves the post-harvest quality of fruits and vegetables by the activation of crucial enzymes such as PAL, CHS, ANR, DFR, and OMT. This enzyme upregulates under stress conditions and accumulates phenolics and flavonoid amounts in plants (Li et al., 2020).
8.1 MT under drought stress
One of the main consequences of drought stress in plants is a reduced CO2 assimilation rate that energizes the overproduction of ROS, which causes impairment at the cellular level. Melatonin application stabilizes the defence system of plant species by promoting the production of SMs and antioxidants (Nguyen et al., 2022). For example, 50 μM of MT treatment under drought conditions increases the anthocyanin content in Helianthus annuus L. It is also linked with regulating phenolics and flavonoids in plants, where these metabolites act as ROS scavengers (Mahmood et al., 2024). In a related finding, Taxus baccata L. shows that a 100 μM concentration of MT improves phenolic content, SOD, CAT, and POX activities, thus enhancing plant resilience. Melatonin also stimulates the expression of taxol biosynthetic genes (TXS and DBAT), which proves that MT is a modulator of gene expression in both contexts (Shahmohammadi et al., 2024). A positive role of MT (200 μM) under drought conditions maximizes the accumulation of carotenoids and phenolic compounds in Brassica rapa (Hasnain et al., 2023).
8.2 MT under salinity stress
Melatonin works as a stress- reliever alleviating H2O2, inducing the Na+/K+ pump, and stimulating ROS catabolizing enzymes. According to Sheikhalipour et al. (2022), MT influences WRKY, SOAR1, and Mc5PTase7 genes, which provide stress tolerance and modulate secondary metabolism. These alterations were proceeded by MAP30, α-MMC, and PAL genes, which were expressed in the stressed and MT-treated plants of Momordica charantia L. under saline conditions. Under salt stress, Trigonella foenum- graecum L. treated with different doses of MT, i.e., 60 and 90 mg L-1, increased alkaloid content by activating Squalane synthase (SQS) and CAS gene, which is pivotal in diosgenin biosynthesis. Additionally, MT improved the levels of phenolics and flavonoids to maintain ion homeostasis (Mohamadi Esboei et al., 2022). MT positively affected the activity of flavonoids in plants facing various abiotic stress conditions. For example, MT treatment in grape berries (Vitis vinifera L.) upscale total phenol compounds, total flavonoids, and DPPH, and upregulates the expression of the PAL, STS, 4CL, and CHS genes, enhancing the accumulation of flavanones, flavanols, and flavonoids (Xu et al., 2017). Similarly, Jahan et al. (2020) explained that MT supplementation increases CHS enzyme activity under stress conditions, whereas CHS plays a role in anthocyanin production. Besides, co-exposure of MT and Pseudomonas fluorescens in Brassica juncea enhances the production of notable flavonoids, specifically kaempferol, cyanidin, naringenin, quercetin, and myricetin. These flavonoids are vital for safeguarding cells that engage in photosynthesis (Khan et al., 2023).
8.3 MT under heavy metal stress
Various signalling molecules efficiently improve photosynthetic apparatus, growth metrics, and SM biosynthesis to combat HM toxicity (Aftab and Roychoudhury, 2021). Foliar treatment of MT in strawberries (Fragaria x ananassa) against Cd (1mM) stress increases anthocyanin and phenolic enzymatic activities, and decreases lipid peroxidation and electrolyte leakage (Saqib et al., 2023). Conversely, the application of MT (100 μM) in the case of the Brassica napus plant under cobalt stress improved the expression of genes involved in SM metabolism, such as PAL, PPO, and CAD in roots and leaves, which markedly reduced oxidative stress (Ali et al., 2023). In Camellia sinensis, catechin is a major SM, which is enhanced in the presence of MT under As stress. MT elevates the transcript level of anthocyanin and catechin genes (CsCHS, CsCHI, CsF3H, CsDFR, and CsANS), which significantly increases metal tolerance (Li et al., 2021). Dou et al. (2022) also reported that an exogenous supply of MT in Solanum lycopersicum escalated 3 flavonoids (quercetin, rutin, and naringenin) and six phenolic compounds (caffeic acid, p-hydroxybenzoic acid, protocatechuic acid, chlorogenic acid, gentisic acid, and sinapic acid) to combat metal toxicity. Table 6 reveals that the MT application under various abiotic stresses alters secondary metabolite production and mitigates stress toxicity.
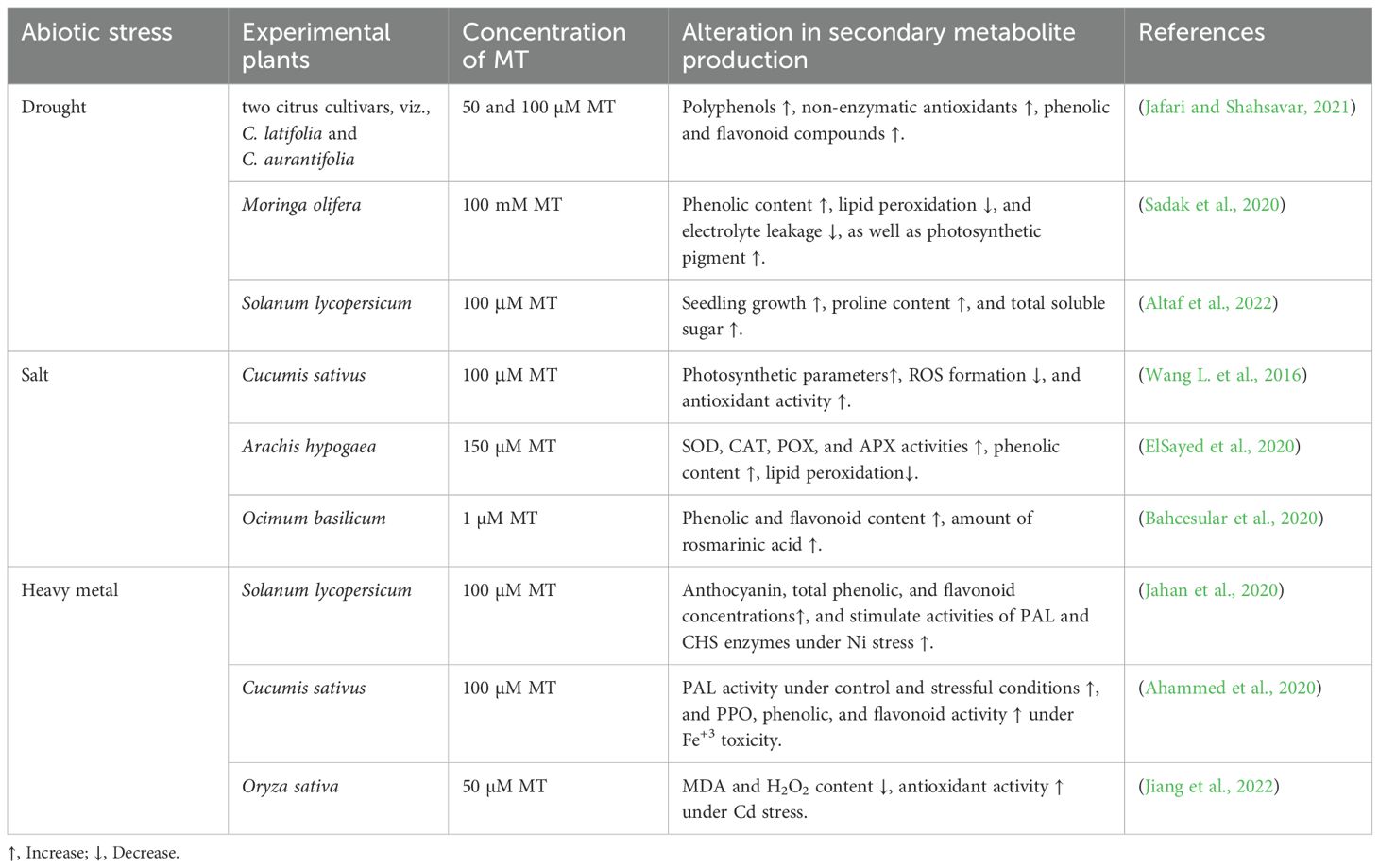
Table 6. Exploring the role of melatonin (MT) in managing secondary metabolite production in diverse plant species under abiotic stress.
9 Role of calcium in SM production under stress conditions
Calcium (Ca2+) is an essential element, present in plant shoots ranging from 0.1 to 5% on a plant dry weight basis (Thor, 2019). It is a vital component of plant cell walls, involved in cell signalling (nutrient and plant immunity signalling), and acts as a secondary messenger. The role of Ca²+ in maintaining membrane fluidity and integrity is crucial for preventing ion and metabolite leakage. It also fosters the accumulation of osmolytes (proline and glycine betaine), which assist in osmotic adjustment and protect cellular structures under stress conditions (Marques et al., 2016). Localization of Ca2+, primarily in the plasma membrane and other cellular compartments such as endoplasmic reticulum, golgi bodies, and plant vacuoles. In response to abiotic stress, protein kinase and phosphatase, both enzymes, mediate the coupling of Ca2+ and ROS signalling pathways using calmodulin-like protein (CAL) and CaM (calmodulin, a calcium-binding protein) as Ca2+ sensors (Zeng et al., 2015). Plant signalling processes are driven by the interaction of Ca²+ with its sensors. Primary Ca²+ sensors, such as calmodulin-like proteins (CMLs), calmodulin (CaM), calcineurin B-like proteins (CBLs), and calcium-dependent protein kinases (CDPKs/CPKs), are instrumental in mediating hormonal responses and stress signals (Zhu et al., 2022). Calcium chloride (CaCl2) applied topically proved more effective than calcium oxide and calcium chelate. For example, CaCl2 enhances PAL activity and up-regulates expressions of related genes such as LcPAL1 and LcPAL2, thereby increasing the total phenolic content in Cucumis melo. It is reported that CaCl2 is advantageous for activating the phenylpropane pathway (You et al., 2024). Similarly, Fagopyrum esculentum was treated with 3% sucrose and 7.5 mM CaCl2 at the sprouting stage, and there was a high elevation in total phenolics and total flavonoid content. Dominant flavonoids occur in Fagopyrum sprouts, including C- glycosylflavonones (vitexin, orietin, isovitexin) and rutin (Sim et al., 2020). Table 7 summarizes the role of Ca2+ and its donors in overcoming abiotic stress via the upregulation of SMs in different plant species.
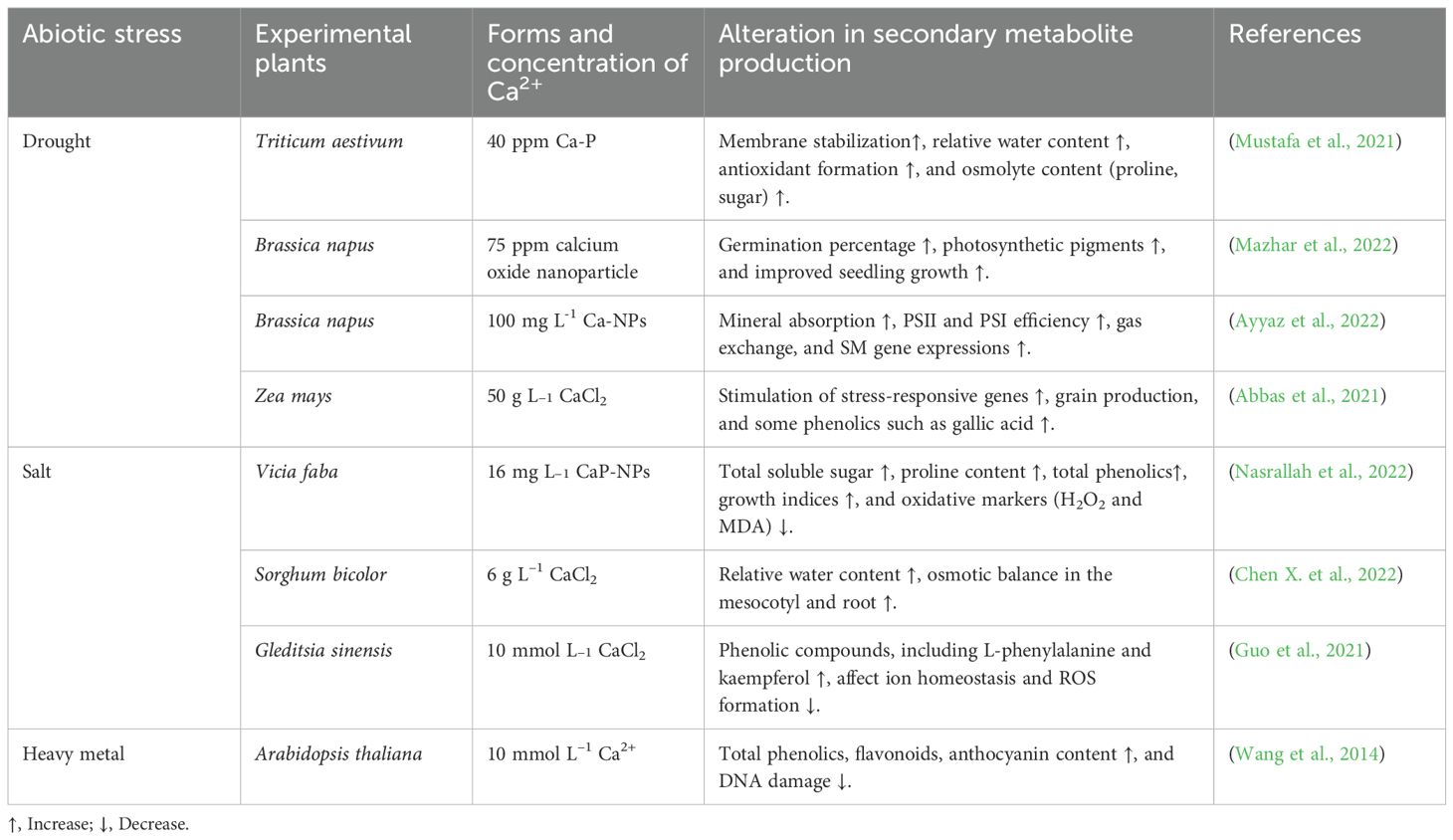
Table 7. Exploring the role of calcium (Ca2+) in managing secondary metabolite production in diverse plant species under abiotic stress.
9.1 Ca2+ under drought stress
Drought conditions are first perceived response through the root, resulting in the transmission of stress signals from the root to the aerial parts. Ca2+ promotes the synthesis of osmolytes, such as proline and glycine betaine, which help maintain osmotic balance and protect cells during drought. MYB, bZIP, WRKY, bHLH, CAMTAs, NAC, DREB, and MADS-box transcription factors, which are regulated by CaM, perform essential roles in maintaining normal physicochemical functions and enhancing stress resistance (Baek et al., 2023). In stress conditions, Ca2+ accumulation is high in the cytosol where it binds with CaM, thus regulating antioxidant activities and improving seed germination (Qin et al., 2019). Foliar-applied Ca2+ (50 mg/L) increased seed yield, sugar and starch content, and ionic balance in Zea mays under drought conditions (Abbas et al., 2021). Naeem et al. (2018) also reported in Zea mays that foliar spray of Ca2+ promotes the synthesis of osmolytes (proline and glycine betaine), which helps in maintaining osmotic balance and protects cells from drought. Furthermore, foliar treatment of CaCl2 in Musa sp. significantly raises the phenolics and flavonoids to overcome the toxicity of abiotic stress (Narwal et al., 2024). Applying CaCl2 in Zoysia japonica positively influences growth variables (seedling growth, chlorophyll, carotenoid content, and antioxidants) (Xu et al., 2013). In transgenic tobacco (Nicotiana tabacum L.) plants, the overexpression of StCaM2 (an isoform of CaM) increases their resistance to drought and salt conditions through the improvement in the functioning of PSII, which ultimately diminishes ROS formation and increases anti-oxidative enzyme activity in tobacco plants (Raina et al., 2021).
9.2 Ca2+ under salinity stress
Previous research demonstrated that salt stress stimulates Ca2+ accumulation, which acts as a bivalent cation for Na+ influx through a monovalent cation channel to maintain ion homeostasis. High accumulation of Na+ ions is the main toxicity in the soil. To achieve ion balance and control the outflow of excess Na+ ions, many transporters, ions, Ca2+ sensors, and their downstream interacting counterparts work in concert. Ca2+ is essential for enhancing salinity tolerance in plants through its role in signalling, root development, nutrient uptake, membrane stability, osmotic adjustment, and formation of antioxidants. Applying calcium phosphate nanoparticles (Cap-NPS) under saline conditions enhances the content of phenolic and flavonoid and antioxidant enzyme activities, and diminishes H2O2 accumulation (Nasrallah et al., 2022). Ca2+ concentrations (5, 15, 35 mM) significantly mitigate the saline toxicity in Sorghum bicolor via improved seedling growth, ion balancing, and antioxidant enzyme activity (Mulaudzi et al., 2020). Moreover, applying CaCl2 under salt stress increased the number of flower clusters per plant, several fruits per cluster, and the number of fruits per plant in Olea europaea L (El-Hady et al., 2020). CaCl2 elevated the level of various phenolic production, including L-phenylalanine, kaempferol, ferulic acid, and catechin, which responded negatively to the salinity stress in the case of Gleditsia sinensis Lam (Guo et al., 2021). These findings suggested that Ca2+ and its isoforms counteract the adverse effects of salinity via the synthesis and accumulation of some specific phenolic compounds in stressed plants. Moreover, overexpression of the CaM gene (OsCam1-1) under high salinity in rice significantly alters the expression of genes involved in cellular metabolism, hormone regulation, lipid and carbohydrate metabolism, secondary metabolism, and key cycles like glycolysis, tricarboxylic acid, glyoxylate, and signalling cascade (Yuenyong et al., 2018).
9.3 Ca2+ under heavy metal stress
For plants to respond to HM exposure, Ca2+ signalling is necessary for sensing, defence mechanism activation, oxidative stress control, and cellular homeostasis maintenance. Ca2+ alone or combined with other signalling molecules reduces metal uptake through the soil and minimizes ROS generation. For instance, foliage spray of CaCl2 enhances SOD, CAT, POX, and APX formation, stimulates NR activity, and enhances protein content in Cucurbita pepo under Ni stress (Valivand and Amooaghaie, 2021b). Similarly, CaCl2 was applied in the Cicer arietinum L. under Cd stress, and it invigorated the phenolic and flavonoid content to tolerate Cd toxicity by the formation of antioxidant molecules, encompassing both enzymatic and non-enzymatic categories (Ahmad et al., 2016). In another study, combined crosstalk of Ca2+ and MT synergistically increases the resistance in Vicia faba against As toxicity through regulation of the ascorbate glutathione cycle (Siddiqui et al., 2020). Role of calcium oxide nanoparticles (CaO NPs) attributed to its enhancement of Ca2+ absorption, photosynthetic pigments, ROS scavenging ability, reduction of As uptake, and translocation from roots to aerial organs in Hordeum vulgare L (Nazir et al., 2022). Similarly, Ca2+ not only decreases the uptake of Cd but also reduces Cd accumulation in plant cells by induction of the Ca2+ channel, enhances micronutrients like Na, P, K, and Mg through the soil via root absorption in Fagopyrum esculentum (Hakeem et al., 2022). These relevant findings underline the importance of exogenous Ca²+ in lowering metal accumulation, improving photosynthetic parameters, and higher the accumulation of SMs to achieve better yields.
10 Signalling molecules crosstalk for the regulation of the plant defence system
Signalling molecules (NO, H2S, MeJA, H2O2, ETH, MT, and Ca2+) respond in multiple interaction nodes, which conjointly upregulate prodigious metabolic activities in plants. In the context of prolonged NaCl stress, new insights emerge regarding the role of Ca2+ and H2S interactions in maintaining ion balance, regulating redox states, and influencing both primary and secondary metabolism (Khan et al., 2021). Plants use different sensors and signalling components against stress. One secondary messenger, cyclic ADP ribose (cADPR), triggers Ca2+ secretion from internal stores (endoplasmic reticulum) and operates effortless signalling in many plant and animal cells (Grams et al., 2024). Initial research in plants underscored the importance of cADPR in facilitating the action of NO on the activation of defence gene expression (Zhou X. et al., 2021; Naz et al., 2024). Cyclic ADP ribose activates NO synthase and catalyses NO production, upregulating CaM and Ca2+ signalling, influencing many other essential molecules in stress management. Calcium signalling activates many genes, including CAMTA (CaM binding transcription activator) and MYB, which implies a potential role during abiotic stress in Aegilops tauschii (Seifikalhor et al., 2019). Moreover, MT and other signalling molecules displayed positive interaction, which influences NO production through the activation of NOS-like enzymes in the arginine metabolic pathway (Aghdam et al., 2019; Hussain et al., 2024). Combinations of MT and NO produced NOMT (N-nitroso MT), which was recently discovered to play functions in the morpho-physiological activity of plants (Hussain et al., 2024). Melatonin and SNP-triggered NO significantly elevate isoflavone content by overexpression of cinnamic acid 4-hydroxylase (C4H) and PAL. It also amplified the gene expression of PAL, C4H, IFS, and CHI1A, which regulate isoflavone biosynthesis under abiotic stress (Yin et al., 2022). An experimental study (Sehar et al., 2023a) demonstrates that MT and MeJA synergistically influence S- assimilation, which upregulates ETH synthesis to dimmish the effects of heat stress. Ethylene regulates SOD, ascorbic acid activity and improves photosynthetic mechanisms in plants. Melatonin also contributes to preserving the amount of psbA and D1 protein in photosynthetic plants (Sehar et al., 2023b). Further, JAZs and MYC2, key regulators have been shown to play a crucial role in stress response by mediating JA signalling. Additionally, EIN3, its homolog EIL1, and the ERF-domain transcription factor ORA59 in A. thaliana demonstrate a positive interaction between JA and ETH signalling pathways under stress conditions (Zhu and Lee, 2015). Some studies revealed that NO and ETH are linked through mitogen-activated protein kinase (MAPK) signalling during stress (Wu et al., 2021). Mitogen-activated protein kinase positively regulates NO biosynthesis and NR activity in stress tolerance. It has also been reported that NO and MAPK markedly upscale the biosynthesis of H2S, which proves the positive correlation between NO and H2S (Bhuyan et al., 2020). However, the interplay between NO and H2S in PCD requires further exploration. While NO typically reduces oxidative stress, high levels of NO3- may trigger ROS and MDA accumulation. H2S counters this via CsNMAPK signalling, but the MAPK inhibitor PD98059 weakens NO function and disrupts NO-H2S signalling, with underlying mechanisms still uncertain (Qi et al., 2019). Besides, the combined application of H2O2, CaCl2, and SNP at the germination stage reversed the effects of saline toxicity in Chenopodium quinoa. Furthermore, their crosstalk improved α-amylase activity to promote seed germination, altering physiological mechanisms that help plants tolerate adverse conditions (Hajihashemi et al., 2020). It is well established that these vital signalling molecules regulate diverse aspects of plant development and positively react against stress conditions.
The above studies revealed that signalling molecules play significant roles in regulating SM production through their effects on enzyme activity, stress responses, signalling pathways, and oxidative cellular redox states. The crosstalk among diverse molecules, such as NO, H2S, MeJA, H2O2, ETH, MT, and Ca2+, as stated in Figure 3, maintains a sophisticated signalling network that enables plants to respond to abiotic stresses effectively. Understanding their interactions could provide better ideas for enhancing stress tolerance in crops, ultimately improving agricultural resilience in challenging environmental conditions. The increasing intensity of numerous environmental stress factors makes plant survival much more challenging and crucial. We hope the signalling molecules will bring an entirely novel viewpoint to the field of study and encourage the scientific community to further research.
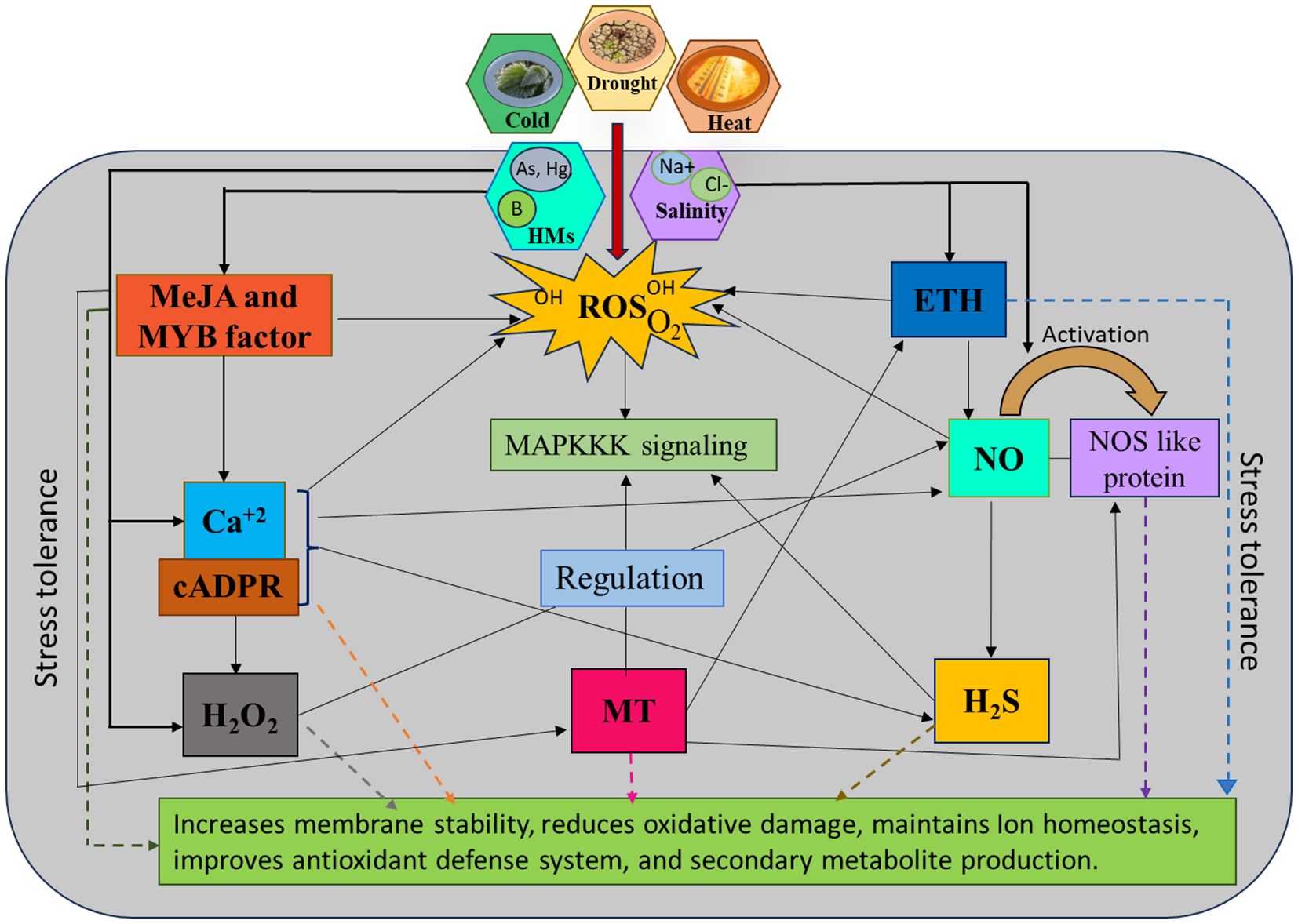
Figure 3. This intricate interplay between signalling molecules and their downstream effectors exemplifies the sophisticated regulatory systems plants have evolved to survive and thrive under abiotic stress. When plants face stress, they experience heightened levels of reactive oxygen species (ROS) accumulation, which can be alleviated by secondary metabolites activated by these signalling compounds. Arrows in the diagram indicate interactions between pathways, key donors and mediators involved in these processes including MYB transcription factors, sodium hydrosulfide (NaHS) or nitric oxide synthase (NOS) enzyme for H2S and NO production, sodium nitroprusside (SNP) as an exogenous NO donor, and cyclic ADP ribose (cADPR) for Ca²+ signalling which implement stress tolerance strategies through augmenting membrane stability, ion balance, and the generation of antioxidants (SOD, CAT, POX, and APX), and enhance metabolic reprogramming, ultimately contributing to plant resilience under harsh environmental conditions.
11 An innovative field in plant biology delving into the dynamic interplay between signalling pathways and secondary metabolite networks
11.1 Multi-omics approaches
Multi-omics approaches (genomics, transcriptomics, metabolomics, and proteomics) to map real-time interactions between signalling pathways.
Metabolomics provides direct insights into the status of metabolites and serves as a core point for connecting with other omics technologies to discover plant signalling molecules.
The interplay between plant metabolism and SMs is complex and multifaceted, and understanding these interactions is foremost for developing strategies to refine plant health and productivity in an altered environment.
11.2 Role of non-classical signalling molecules
Microbial VOCs (volatile organic compounds) are being found to modulate the production of secondary metabolites.
Bacterial volatile organic compounds (alcohols, aldehydes, alkenes, alkynes, benzenes, esters, heterocycles, ketones, sulphides, and terpenoids) induce defence and protect the plants from phytopathogens. The signalling pathways involving these are not well mapped.
11.3 Epigenetic regulation in response to stress
Epigenetic modification is a key tool for gene expression and SM production. Despite the advanced research, tools, and techniques in molecular biology and biotechnology, some questions regarding epigenetic modification remain unresolved. There are stress-induced epigenetic modifications (e.g., histone modification, DNA methylation) regulating signalling pathways and metabolite production, which remain unclear.
12 Conclusions and future perspectives
This review highlights the potentiality of signalling molecules (NO, H2S, MeJA, H2O2, ETH, MT, and Ca2+) in plant growth and development. These molecules regulate primary and secondary metabolic pathways to improve plant biochemical and physiological function and improve tolerance and resilience against abiotic stresses. It elucidates a better understanding of various signalling molecules that play putative roles in SM production by influencing plant responses specifically under stressful conditions. Under adverse conditions, signalling compounds typically rise, leading to the activation of SM-responsive genes and pathways that help to cope with stress. Subsequent research ought to focus on the intricate interplay among various compounds, such as NO, H2S, MeJA, H2O2, ETH, MT, and Ca2+ in regulating the synthesis of SM. Meanwhile, advancements in the medicinal plant field will come from using cutting-edge analytical techniques to identify novel SMs and their large-scale production. Biotechnological approaches, such as plant tissue culture techniques and CRISPR/Cas9 techniques, are useful for modulating and further identifying the genes involved in SMs synthesis. This could provide promising results and thereby overcome the issue of commercial exploitation of medicinal plants. Moreover, in changing climatic conditions where plants are being exposed to diverse stress situations, the inherent ability to produce SM can be increased by supplementing numerous signalling molecules. Such application would reduce the stress via SMs synthesis and help to achieve fundamental bioactive compounds that can be used in biopharmaceuticals. Further research is needed to deepen our understanding of the specific roles and mechanisms of various signalling molecules that offer the potential for developing stress-tolerant crop varieties with enhanced SM profiles.
Author contributions
S: Formal analysis, Resources, Writing – review & editing, Data curation, Funding acquisition, Software, Writing – original draft. TA: Conceptualization, Investigation, Validation, Visualization, Writing – review & editing. MN: Conceptualization, Investigation, Validation, Visualization, Writing – review & editing, Formal analysis, Project administration, Resources, Supervision. PJ: Validation, Visualization, Writing – review & editing, Conceptualization. PP: Conceptualization, Funding acquisition, Software, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. First author acknowledges the financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, India, in the form of Junior Research Fellowship (JRF) under the JRF scheme (Grant No. 231610038665). Contribution number 26-014-J from the Kansas Agricultural Experiment Station, Kansas State University, Manhattan, KS, United States, is gratefully acknowledged for providing financial support to publish this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, M., Abdel-Lattif, H., and Shahba, M. (2021). Ameliorative effects of calcium sprays on yield and grain nutritional composition of maize (Zea mays L.) cultivars under drought stress. Agriculture 11, 285. doi: 10.3390/agriculture11040285
Afkar, S. and Karimzadeh, G. (2014). Changing in the chemical composition of the Essential oils of Mentha piperita after MeJA treatment. Int. J. Agric. Crop Sci. 7, 1493.
Aftab, T. and Roychoudhury, A. (2021). Crosstalk among plant growth regulators and signalling molecules during biotic and abiotic stresses: molecular responses and signalling pathways. Plant Cell Rep. 40, 2017–2019. doi: 10.1007/s00299-021-02791-5
Aghdam, M. S., Luo, Z., Jannatizadeh, A., Sheikh-Assadi, M., Sharafi, Y., Farmani, B., et al. (2019). Employing exogenous melatonin application confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chem. 275, 549–556. doi: 10.1016/j.foodchem.2018.09.157
Ahammed, G. J., Wu, M., Wang, Y., Yan, Y., Mao, Q., Ren, J., et al. (2020). Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense, and secondary metabolism in cucumber. Sci. Hortic. 265, 109205. doi: 10.1016/j.scienta.2020.109205
Ahmad, P., Abdel Latef, A. A., Abd_Allah, E. F., Hashem, A., Sarwat, M., Anjum, N. A., et al. (2016). Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00513
Ahmad, P., Ahanger, M. A., AlYemeni, M. N., Wijaya, L., and Alam, P. (2018). The exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255, 79–93. doi: 10.1007/s00709-017-1132-x
Alamer, K. H. (2023). Exogenous hydrogen sulfide supplementation alleviates the salinity-stress-mediated growth decline in wheat (Triticum aestivum L.) by modulating tolerance mechanisms. Plants 12, 3464. doi: 10.3390/plants12193464
Alatawi, A., Mfarrej, M. F. B., Alshegaihi, R. M., Asghar, M. A., Mumtaz, S., Yasin, G., et al. (2023). Application of silicon and sodium hydrosulfide alleviates arsenic toxicity by regulating the physio-biochemical and molecular mechanisms of Zea mays. Environ. Sci. pollut. Res. 30, 76555–76574. doi: 10.1007/s11356-023-27739-y
Alhammad, B. A., Seleiman, M. F., and Harrison, M. T. (2023). Hydrogen peroxide mitigates cu stress in wheat. Agriculture 13, 862. doi: 10.3390/agriculture13040862
Ali, Q., Daud, M. K., Haider, M. Z., Ali, S., Rizwan, M., Aslam, N., et al. (2017). Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 119, 50–58. doi: 10.1016/j.plaphy.2017.08.010
Ali, S., Gill, R. A., Ulhassan, Z., Zhang, N., Hussain, S., Zhang, K., et al. (2023). Exogenously applied melatonin enhanced the tolerance of Brassica napus against cobalt toxicity by modulating antioxidant defense, osmotic adjustment, and expression of stress response genes. Ecotoxicol. Environ. Saf. 252, 114624. doi: 10.1016/j.ecoenv.2023.114624
Almagro, L., Gutierrez, J., Pedreño, M. A., and Sottomayor, M. (2014). Synergistic and additive influence of cyclodextrins and methyl jasmonate on the expression of the terpenoid indole alkaloid pathway genes and metabolites in Catharanthus roseus cell cultures. Plant Cell. Tissue Organ Cult. (PCTOC). 119, 543–551. doi: 10.1007/s11240-014-0554-9
Al Mamari, H. H. (2021). “Phenolic compounds: classification, chemistry, and updated techniques of analysis and synthesis,” In Phenolic Compounds-Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications. IntechOpen, 10. doi: 10.5772/intechopen.98958
Altaf, M. A., Shahid, R., Ren, M. X., Naz, S., Altaf, M. M., Khan, L. U., et al. (2022). Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 11, 309. doi: 10.3390/antiox11020309
Amir, S. B., Rasheed, R., Ashraf, M. A., Hussain, I., and Iqbal, M. (2021). Hydrogen sulfide mediates defense response in safflower by regulating secondary metabolism, oxidative defense, and elemental uptake under drought. Physiol. Plant. 172, 795–808. doi: 10.1111/ppl.13267
Amooaghaie, R., Mardani Korrani, F., Ghanadian, M., Ahadi, A., Pak, A., and Mardani, G. (2024). Hybrid priming with He–Ne laser and hydrogen peroxide advances the phenolic composition and antioxidant quality of Salvia officinalis under saline and non-saline conditions. J. Plant Growth Regul. 43, 1012–1025. doi: 10.1007/s00344-023-11156-z
Antoniou, C., Xenofontos, R., Chatzimichail, G., Christou, A., Kashfi, K., and Fotopoulos, V. (2020). Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomolecules 10, 120. doi: 10.3390/biom10010120
Arnao, M. B., Cano, A., and Hernández-Ruiz, J. (2022). Phytomelatonin: an unexpected molecule with amazing performances in plants. J. Exp. Bot. 73, 5779–5800. doi: 10.1093/jxb/erac009
Asgher, M., Ahmed, S., Sehar, Z., Gautam, H., Gandhi, S. G., and Khan, N. A. (2021). Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mater. 401, 123365. doi: 10.1016/j.jhazmat.2020.123365
Asgher, M., Sehar, Z., Fatma, M., Hanief, M., Shah, A. A., and Khan, N. A. (2023). Ethylene and spermine attenuate chromium-inhibited photosynthetic functions by improving nitrogen and sulfur assimilation and antioxidant system in mustard. Plant Stress 9, 100196. doi: 10.1016/j.stress.2023.100196
Ayyaz, A., Fang, R., Ma, J., Hannan, F., Huang, Q., Sun, Y., et al. (2022). Calcium nanoparticles (Ca-NPs) improve drought stress tolerance in Brassica napus by modulating the photosystem II, nutrient acquisition, and antioxidant performance. NanoImpact 28, 100423. doi: 10.1016/j.impact.2022.100423
Babaei, S., Niknam, V., and Behmanesh, M. (2021). Comparative effects of nitric oxide and salicylic acid on salinity tolerance in saffron (Crocus sativus). Plant Biosystems-An. Int. J. Dealing. all Aspects. Plant Biol. 155, 73–82. doi: 10.1080/11263504.2020.1727975
Baek, D., Cho, H. M., Cha, Y. J., Jin, B. J., Lee, S. H., Park, M. S., et al. (2023). Soybean calmodulin-binding transcription activators, GmCAMTA2 and GmCAMTA8, coordinate the circadian regulation of developmental processes and drought stress responses. Int. J. Mol. Sci. 24, 11477. doi: 10.3390/ijms241411477
Bahcesular, B., Yildirim, E. D., Karaçocuk, M., Kulak, M., and Karaman, S. (2020). Seed priming with melatonin effects on growth, essential oil compounds, and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crops Products. 146, 112165. doi: 10.1016/j.indcrop.2020.112165
Bai, L., Li, J., Li, H., Song, J., Zhou, Y., Lu, R., et al. (2019). Renoprotective effects of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via suppressing NF-κB signaling and NLRP3 inflammasome activation by exosomes in rats. Biochem. Pharmacol. 169, 113619. doi: 10.1016/j.bcp.2019.08.021
Bano, A., Qadri, T. A., and Khan, N. (2023). Bioactive metabolites of plants and microbes and their role in agricultural sustainability and mitigation of plant stress. South Afr. J. Bot. 159, 98–109. doi: 10.1016/j.sajb.2023.05.049
Barba-Espín, G., Glied, S., Crocoll, C., Dzhanfezova, T., Joernsgaard, B., Okkels, F., et al. (2017). Foliar-applied ethephon enhances the content of anthocyanin of black carrot roots (Daucus carota ssp. sativus var. atrorubens Alef.). BMC Plant Biol. 17, 1–11. doi: 10.1186/s12870-017-1021-7
Bergman, M. E., Kortbeek, R. W., Gutensohn, M., and Dudareva, N. (2024). Plant terpenoid biosynthetic network and its multiple layers of regulation. Prog. Lipid Res. 95, 101287. doi: 10.1016/j.plipres.2024.101287
Bhambhani, S., Kondhare, K. R., and Giri, A. P. (2021). Diversity in chemical structures and biological properties of plant alkaloids. Molecules 26, 3374. doi: 10.3390/molecules26113374
Bhardwaj, R. D., Singh, N., Sharma, A., Joshi, R., and Srivastava, P. (2021). Hydrogen peroxide regulates antioxidant responses and redox-related proteins in drought stressed wheat seedlings. Physiol. Mol. Biol. Plants 27, 151–163. doi: 10.1007/s12298-021-00937-z
Bhardwaj, S., Verma, T., Raza, A., and Kapoor, D. (2023). Silicon and nitric oxide-mediated regulation of growth attributes, metabolites and antioxidant defense system of radish (Raphanus sativus L.) under Arsenic Stress. Phyton. (0031-9457) 92, 92. doi: 10.32604/phyton.2023.025672
Bhuyan, M. B., Hasanuzzaman, M., Parvin, K., Mohsin, S. M., Al Mahmud, J., Nahar, K., et al. (2020). Nitric oxide and hydrogen sulfide: two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 90, 409–424. doi: 10.1007/s10725-020-00594-4
Blažević, I., Montaut, S., Nicola, G. R. D., and Rollin, P. (2015). Long-chain glucosinolates from Arabis turrita: Enzymatic and non-enzymatic degradations. Natural Product. Commun. 10. doi: 10.1177/1934578X1501000662
Boncan, D. A. T., Tsang, S. S., Li, C., Lee, I. H., Lam, H. M., Chan, T. F., et al. (2020). Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 21, 7382. doi: 10.3390/ijms21197382
Buraphaka, H. and Putalun, W. (2020). Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) Urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind. Crops Products. 146, 112171. doi: 10.1016/j.indcrop.2020.112171
Caleja, C., Ribeiro, A., Filomena Barreiro, M., and CFR Ferreira, I. (2017). Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Design. 23, 2787–2806. doi: 10.2174/1381612822666161227153906
Černý, M., Habánová, H., Berka, M., Luklová, M., and Brzobohatý, B. (2018). Hydrogen peroxide: its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 19, 2812. doi: 10.3390/ijms19092812
Chavoushi, M., Najafi, F., Salimi, A., and Angaji, S. A. (2020). Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Sci. Hortic. 259, 108823. doi: 10.1016/j.scienta.2019.108823
Chen, H., Bullock, D. A., Jr., Alonso, J. M., and Stepanova, A. N. (2022). To fight or to grow: the balancing role of ethylene in plant abiotic stress responses. Plants 11, 33. doi: 10.3390/plants11010033
Chen, Q., Wu, K., Tang, Z., Guo, Q., Guo, X., and Wang, H. (2017). Exogenous ethylene enhanced the cadmium resistance and changed the alkaloid biosynthesis in Catharanthus roseus seedlings. Acta Physiol. Plant. 39, 1–12. doi: 10.1007/s11738-017-2567-6
Chen, P., Yang, W., Jin, S., and Liu, Y. (2021). Hydrogen sulfide alleviates salinity stress in Cyclocarya paliurus by maintaining chlorophyll fluorescence and regulating nitric oxide level and antioxidant capacity. Plant Physiol. Biochem. 167, 738–747. doi: 10.1016/j.plaphy.2021.09.004
Chen, X., Zhang, R., Li, B., Cui, T., Liu, C., Liu, C., et al. (2022). Alleviation of oxidative damage induced by CaCl2 priming is related to osmotic and ion stress reduction rather than enhanced antioxidant capacity during germination under salt stress in sorghum. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.881039
Chungloo, D., Tisarum, R., Sotesaritkul, T., Praseartkul, P., Himanshu, S. K., Datta, A., et al. (2023). Exogenous foliar application of methyl jasmonate alleviates water-deficit stress in Andrographis paniculata. J. Soil Sci. Plant Nutr. 23, 5468–5481. doi: 10.1007/s42729-023-01414-0
Corpas, F. J., Rivero, R. M., Freschi, L., and Palma, J. M. (2024). Functional interactions among H2O2, NO, H2S, and melatonin in the physiology, metabolism, and quality of horticultural Solanaceae. J. Exp. Bot., erae513. doi: 10.1093/jxb/erae513
Danaee, M., Farzinebrahimi, R., Kadir, M. A., Sinniah, U. R., Mohamad, R., and Mat Taha, R. (2015). Effects of MeJA and SA elicitation on secondary metabolic activity, antioxidant content and callogenesis in Phyllanthus pulcher. Braz. J. Bot. 38, 265–272. doi: 10.1007/s40415-015-0140-3
Darmanti, S., Hastuti, E. D., and Suedy, S. W. A. (2021). Exogenous hydrogen peroxide induces an antioxidative defense system against drought stress in soybean [Glycine max (L.) merr.] crops. J. Anim. Plant Sci. 31, 213–220. doi: 10.36899/JAPS.2021.1.0208
Davari, A., Solouki, M., and Fazeli-Nasab, B. (2018). Effects of jasmonic acid and titanium dioxide nanoparticles on process of changes of phytochemical and antioxidant in genotypes of Satureja hortensis L. Eco-Phytochem. J. Med. Plants 5, 1–20.
Debnath, B., Singh, W. S., Das, M., Goswami, S., Singh, M. K., Maiti, D., et al. (2018). Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 9, 56–72. doi: 10.1016/j.mtchem.2018.05.001
Dey, S. and Raychaudhuri, S. S. (2024). Methyl jasmonate improves selenium tolerance via regulating ROS signalling, hormonal crosstalk, and phenylpropanoid pathway in Plantago ovata. Plant Physiol. Biochem. 209, 108533. doi: 10.1016/j.plaphy.2024.108533
Dias, M. C., Pinto, D. C., and Silva, A. M. (2021). Plant flavonoids: Chemical characteristics and biological activity. Molecules 26, 5377. doi: 10.3390/molecules26175377
Dikilitas, M., Simsek, E., and Karakas, S. (2019). “Stress responsive signaling molecules and genes under stressful environments in plants,” in Plant signaling molecules (New Delhi India: Woodhead Publishing), 19–42. doi: 10.1016/B978-0-12-816451-8.00002-2
Dos Santos Araújo, G., de Oliveira Paula-Marinho, S., de Paiva Pinheiro, S. K., de Castro Miguel, E., de Sousa Lopes, L., Marques, E. C., et al. (2021). H2O2 priming promotes salt tolerance in maize by protecting chloroplasts ultrastructure and primary metabolites modulation. Plant Sci. 303, 110774. doi: 10.1016/j.plantsci.2020.110774
Dou, J., Wang, J., Tang, Z., Yu, J., Wu, Y., Liu, Z., et al. (2022). Application of exogenous melatonin improves tomato fruit quality by promoting the accumulation of primary and secondary metabolites. Foods 11, 4097. doi: 10.3390/foods11244097
El-Hady, E. S., Merwad, M. A., Shahin, M. F., and Hagagg, L. F. (2020). Influence of foliar spray with some calcium sources on flowering, fruit set, yield and fruit quality of olive Kalmata and Manzanillo cultivars under salt stress. Bull. Natl. Res. Centre. 44, 1–6. doi: 10.1186/s42269-020-00452-3
Ellouzi, H., Oueslati, S., Hessini, K., Rabhi, M., and Abdelly, C. (2021). Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hortic. 288, 110360. doi: 10.1016/j.scienta.2021.110360
ElSayed, A. I., Boulila, M., Rafudeen, M. S., Mohamed, A. H., Sengupta, S., Rady, M., et al. (2020). Melatonin regulatory mechanisms and phylogenetic analyses of melatonin biosynthesis related genes extracted from peanut under salinity stress. Plants 9, 854. doi: 10.3390/plants9070854
Elshafie, H. S., Camele, I., and Mohamed, A. A. (2023). A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 24, 3266. doi: 10.3390/ijms24043266
Emamverdiansss, A., Ding, Y., Barker, J., Mokhberdoran, F., Ramakrishnan, M., Liu, G., et al. (2021). Nitric oxide ameliorates plant metal toxicity by increasing antioxidant capacity and reducing Pb and Cd translocation. Antioxidants 10, 1981. doi: 10.3390/antiox10121981
Erb, M. and Kliebenstein, D. J. (2020). Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol. 184, 39–52. doi: 10.1104/pp.20.00433
Esmaielpour, B., Fatemi, H., and Moradi, M. (2019). Effects of nitric oxide on some morphophysiological and biochemical properties of basil (Ocimum basilicum L.) under drought stress conditions. Iranian. J. Med. Aromatic. Plants 35, 4. doi: 10.22092/ijmapr.2019.124983.2482
Falahi, H., Sharifi, M., Chashmi, N. A., and Maivan, H. Z. (2018). Water stress alleviation by polyamines and phenolic compounds in Scrophularia striata is mediated by NO and H2O2. Plant Physiol. Biochem. 130, 139–147. doi: 10.1016/j.plaphy.2018.07.004
Farooq, M. A., Gill, R. A., Islam, F., Ali, B., Liu, H., Xu, J., et al. (2016). Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00468
Farouk, S. and Al-Huqail, A. A. (2020). Sodium nitroprusside application regulates antioxidant capacity, improves phytopharmaceutical production and essential oil yield of marjoram herb under drought. Ind. Crops Products. 158, 113034. doi: 10.1016/j.indcrop.2020.113034
Farzaei, L. and Sayyari, M. (2024). Elicitation with sodium nitroprusside and Trichoderma improves vincristine and vinblastine yield in Catharanthus roseus cell suspension culture by modulating terpenoid indole alkaloid pathway genes. Plant Cell. Tissue Organ Cult. (PCTOC). 157, 1–16. doi: 10.1007/s11240-024-02727-3
Fatima, H., Ishaque, S., Hashim, M., Hano, C., Abbasi, B. H., and Anjum, S. (2023). “Role of hydrogen peroxide in plant and crosstalk with signaling networks, growth, and development,” in Hormonal Cross-Talk, Plant Defense and Development (Cambridge, Massachusetts, United States: Academic Press), 195–224. doi: 10.1016/B978-0-323-95375-7.00002-1
Feng, K., Hou, X. L., Xing, G. M., Liu, J. X., Duan, A. Q., Xu, Z. S., et al. (2020). Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 40, 750–776. doi: 10.1080/07388551.2020.1768509
Feng, Y., Zhao, Y., Ha, Y., Li, J., Su, Z., Quan, X., et al. (2021). Drought stress-induced methyl jasmonate accumulation promotes calycosin-7-O-β-d-glucoside production in Astragalus membranaceus adventitious roots. Plant Cell. Tissue Organ Cult. (PCTOC). 147, 561–568. doi: 10.1007/s11240-021-02147-7
Ghorbani, A., Pehlivan, N., Zargar, M., and Chen, M. (2024). Synergistic role of melatonin and hydrogen sulfide in modulating secondary metabolites and metal uptake/sequestration in arsenic-stressed tomato plants. Sci. Hortic. 331, 113159. doi: 10.1016/j.scienta.2024.113159
Ghosh, S., Watson, A., Gonzalez-Navarro, O. E., Ramirez-Gonzalez, R. H., Yanes, L., Mendoza-Suárez, M., et al. (2018). Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 13, 2944–2963. doi: 10.1038/s41596-018-0072-z
Gogoi, K., Gogoi, H., Borgohain, M., Saikia, R., Chikkaputtaiah, C., Hiremath, S., et al. (2024). The molecular dynamics between reactive oxygen species (ROS), reactive nitrogen species (RNS), and phytohormones in plant’s response to biotic stress. Plant Cell Rep. 43, 1–25. doi: 10.1007/s00299-024-03343-3
Gohari, G., Alavi, Z., Esfandiari, E., Panahirad, S., Hajihoseinlou, S., and Fotopoulos, V. (2020). Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol. Plant. 168, 361–373. doi: 10.1111/ppl.13020
Goncharuk, E. A. and Zagoskina, N. V. (2023). Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium. Molecules 28, 3921. doi: 10.3390/molecules28093921
Grams, R. J., Santos, W. L., Scorei, I. R., Abad-García, A., Rosenblum, C. A., Bita, A., et al. (2024). The rise of boron-containing compounds: advancements in synthesis, medicinal chemistry, and emerging pharmacology. Chem. Rev. 124, 2441–2511. doi: 10.1021/acs.chemrev.3c00663
Guan, C., Ji, J., Li, X., Jin, C., and Wang, G. (2016). LcMKK, a MAPK kinase from Lycium chinense, confers cadmium tolerance in transgenic tobacco by transcriptional upregulation of ethylene responsive transcription factor gene. J. Genet. 95, 875–885. doi: 10.1007/s12041-016-0710-6
Guo, Y., Liu, Y., Zhang, Y., Liu, J., Gul, Z., Guo, X. R., et al. (2021). Effects of exogenous calcium on adaptive growth, photosynthesis, ion homeostasis and phenolics of Gleditsia sinensis Lam. plants under salt stress. Agriculture 11, 978. doi: 10.3390/agriculture11100978
Gupta, S., Kaur, N., Kant, K., Jindal, P., Ali, A., and Naeem, M. (2023). Calcium: A master regulator of stress tolerance in plants. South Afr. J. Bot. 163, 580–594. doi: 10.1016/j.sajb.2023.10.047
Habash, S. S., Könen, P. P., Loeschcke, A., Wüst, M., Jaeger, K. E., Drepper, T., et al. (2020). The plant sesquiterpene nootkatone efficiently reduces Heterodera schachtii parasitism by activating plant defense. Int. J. Mol. Sci. 21, 9627. doi: 10.3390/ijms21249627
Hajihashemi, S., Skalicky, M., Brestic, M., and Pavla, V. (2020). Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem. 154, 657–664. doi: 10.1016/j.plaphy.2020.07.022
Hakeem, K. R., Alharby, H. F., and Pirzadah, T. B. (2022). Exogenously applied calcium regulates antioxidative system and reduces cadmium-uptake in Fagopyrum esculentum. Plant Physiol. Biochem. 180, 17–26. doi: 10.1016/j.plaphy.2022.03.011
Hancock, J. T. and Veal, D. (2021). Nitric oxide, other reactive signalling compounds, redox, and reductive stress. J. Exp. Bot. 72, 819–829. doi: 10.1093/jxb/eraa331
Hasanuzzaman, M., Bhuyan, M. B., Parvin, K., Bhuiyan, T. F., Anee, T. I., Nahar, K., et al. (2020). Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 21, 8695. doi: 10.3390/ijms21228695
Hasanuzzaman, M., Nahar, K., Gill, S. S., Alharby, H. F., Razafindrabe, B. H., and Fujita, M. (2017). Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00115
Hasnain, Z., Zafar, S., Usman, S., Zhang, L., and Elansary, H. O. (2023). Elucidating role of melatonin foliar spray in ameliorating adverse effects of drought stress on growth and physio-biochemical attributes of Brassica rapa plants. Sci. Hortic. 321, 112336. doi: 10.1016/j.scienta.2023.112336
He, W., Luo, H., Xu, H., Zhou, Z., Li, D., Bao, Y., et al. (2021). Effect of exogenous methyl jasmonate on physiological and carotenoid composition of yellow maize sprouts under NaCl stress. Food Chem. 361, 130177. doi: 10.1016/j.foodchem.2021.130177
He, Z., Wen, C., and Xu, W. (2023). Effects of endogenous melatonin deficiency on the growth, productivity, and fruit quality properties of tomato plants. Horticulturae 9, 851. doi: 10.3390/horticulturae9080851
Hilal, B., Khan, T. A., and Fariduddin, Q. (2023). Recent advances and mechanistic interactions of hydrogen sulfide with plant growth regulators in relation to abiotic stress tolerance in plants. Plant Physiol. Biochem. 196, 1065–1083. doi: 10.1016/j.plaphy.2023.03.006
Ho, T. T., Murthy, H. N., and Park, S. Y. (2020). Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int. J. Mol. Sci. 21, 716. doi: 10.3390/ijms21030716
Huang, X., Rong, W., Zhang, X., Gao, Y., Zhou, Y., Su, J., et al. (2024). Transcriptome and metabolome analysis reveal the dynamic changes and biosynthesis pathways of alkaloids in Sophora alopecuroides L. under drought stress. Ind. Crops Products. 212, 118365. doi: 10.1016/j.indcrop.2024.118365
Hussain, A., Faheem, B., Jang, H. S., Lee, D. S., Mun, B. G., Rolly, N. K., et al. (2024). Melatonin–nitric oxide crosstalk in plants and the prospects of NOMela as a nitric oxide donor. Int. J. Mol. Sci. 25, 8535. doi: 10.3390/ijms25158535
Hussain, S., Zhu, C., Huang, J., Huang, J., Zhu, L., Cao, X., et al. (2020). Ethylene response of salt stressed rice seedlings following Ethephon and 1-methylcyclopropene seed priming. Plant Growth Regul. 92, 219–231. doi: 10.1007/s10725-020-00632-1
Iqbal, N., Umar, S., Per, T. S., and Khan, N. A. (2017). Ethephon increases photosynthetic-nitrogen use efficiency, proline, and antioxidant metabolism to alleviate decrease in photosynthesis under salinity stress in mustard. Plant Signaling Behav. 12, e1297000. doi: 10.1080/15592324.2017.1297000
Isah, T. (2019). Stress and defense responses in plant secondary metabolites production. Biol. Res. 52. doi: 10.1186/s40659-019-0246-3
Jafari, T., Iranbakhsh, A., Aliabad, K. K., Daneshmand, F., and Seifati, S. E. (2024). Nitric Oxide Reduced Saponin Metabolite in Chenopodium quinoa Seedlings Cultivated under Salinity. Russian J. Plant Physiol. 71, 66. doi: 10.1134/S1021443723603518
Jafari, M. and Shahsavar, A. (2021). The effect of foliar application of melatonin on changes in secondary metabolite contents in two citrus species under drought stress conditions. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.692735
Jahan, M. S., Guo, S., Baloch, A. R., Sun, J., Shu, S., Wang, Y., et al. (2020). Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 197, 110593. doi: 10.1016/j.ecoenv.2020.110593
Jan, R., Asaf, S., Numan, M., and Lubna and Kim, K. M. (2021). Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 11, 968. doi: 10.3390/agronomy11050968
Jeyasri, R., Muthuramalingam, P., Karthick, K., Shin, H., Choi, S. H., and Ramesh, M. (2023). Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: an updated review. Plant Cell. Tissue Organ Cult. (PCTOC). 153, 447–458. doi: 10.1007/s11240-023-02485-8
Jiang, Y., Huang, S., Ma, L., Kong, L., Pan, S., Tang, X., et al. (2022). Effect of exogenous melatonin application on the grain yield and antioxidant capacity in aromatic rice under combined lead–cadmium stress. Antioxidants 11, 776. doi: 10.3390/antiox11040776
Karimi, Z., Khara, J., and Habibi, G. (2020). Combined hydrogen peroxide and nitric oxide priming modulate salt stress tolerance in acclimated and non-acclimated oilseed rape (Brassica napus L.) plants. J. Plant Physiol. Breed. 10, 27–43. doi: 10.22034/jppb.2020.13099
Kaushik, S., Ranjan, A., Singh, A. K., and Sirhindi, G. (2024). Methyl jasmonate reduces cadmium toxicity by enhancing phenol and flavonoid metabolism and activating the antioxidant defense system in pigeon pea (Cajanus cajan). Chemosphere 346, 140681. doi: 10.1016/j.chemosphere.2023.140681
Ke, S. W., Chen, G. H., Chen, C. T., Tzen, J. T., and Yang, C. Y. (2018). Ethylene signaling modulates contents of catechin and the ability of antioxidants in Camellia sinensis. Bot. Stud. 59, 1–8. doi: 10.1186/s40529-018-0226-x
Kessler, A. and Kalske, A. (2018). Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 49, 115–138. doi: 10.1146/annurev-ecolsys-110617-062406
Khalofah, A., Bamatov, I., and Zargar, M. (2024). Interaction of melatonin and H2S mitigates NaCl toxicity summer savory (Satureja hortensis L.) through Modulation of biosynthesis of secondary metabolites and physio-biochemical attributes. Environ. Sci. pollut. Res. 31, 1–14. doi: 10.1007/s11356-024-34356-w
Khan, V., Jha, A., Seth, T., Iqbal, N., and Umar, S. (2024a). Exploring the role of jasmonic acid in boosting the production of secondary metabolites in medicinal plants: Pathway for future research. Ind. Crops Products. 220, 119227. doi: 10.1016/j.indcrop.2024.119227
Khan, M. I. R. and Khan, N. A. (2014). Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 251, 1007–1019. doi: 10.1007/s00709-014-0610-7
Khan, V., Mubashshir, M., Umar, S., and Iqbal, N. (2024b). Methyl jasmonate and pseudomonas fluorescens synergistically boost antioxidative defense, secondary metabolites, and osmolyte production to enhance drought resilience in mustard. J. Plant. Growth. Regul., 1–19. doi: 10.1007/s00344-024-11310-1
Khan, M. N., Siddiqui, M. H., AlSolami, M. A., Alamri, S., Hu, Y., Ali, H. M., et al. (2020). Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiol. Biochem. 156, 278–290. doi: 10.1016/j.plaphy.2020.09.017
Khan, M. N., Siddiqui, M. H., Mukherjee, S., Alamri, S., Al-Amri, A. A., Alsubaie, Q. D., et al. (2021). Calcium-hydrogen sulfide crosstalk during K+-deficient NaCl stress operates through regulation of Na+/H+ antiport and antioxidative defense system in mung bean roots. Plant Physiol. Biochem. 159, 211–225. doi: 10.1016/j.plaphy.2020.11.055
Khan, V., Umar, S., and Iqbal, N. (2023). Synergistic action of Pseudomonas fluorescens with melatonin attenuates salt toxicity in mustard by regulating antioxidant system and flavonoid profile. Physiol. Plant. 175, e14092. doi: 10.1111/ppl.14092
Khan, A. R., Wakeel, A., Muhammad, N., Liu, B., Wu, M., Liu, Y., et al. (2019). Involvement of ethylene signaling in zinc oxide nanoparticle-mediated biochemical changes in Arabidopsis thaliana leaves. Environ. Sci.: Nano. 6, 341–355. doi: 10.1039/C8EN00971F
Kiani, R., Arzani, A., and Mirmohammady Maibody, S. A. M. (2021). Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.646221
Kolupaev, Y. E., Firsova, E. N., Yastreb, T. O., Ryabchun, N. I., and Kirichenko, V. V. (2019). Effect of hydrogen sulfide donor on antioxidant state of wheat plants and their resistance to soil drought. Russian J. Plant Physiol. 66, 59–66. doi: 10.1134/S1021443719010084
Kowsalya, K., Halka, J., Anand, M., Sahayarayan, J. J., Rajkumar, R., and Arun, M. (2025). Unraveling the multifaceted role of ethephon in plant physiology: from seed germination to crop maturation and harvesting. J. Plant Biochem. Biotechnol., 1–26. doi: 10.1007/s13562-024-00953-6
Kumar, R., Das, S., Mishra, M., Choudhury, D. R., Sharma, K., Kumari, A., et al. (2021). Emerging roles of NAC transcription factor in medicinal plants: progress and prospects. 3. Biotech. 11, 1–14. doi: 10.1007/s13205-021-02970-x
Kumar, V., Kumar, A., Tewari, K., Garg, N. K., Changan, S. S., and Tyagi, A. (2022). Isolation and characterization of drought and ABA responsive promoter of a transcription factor encoding gene from rice. Physiol. Mol. Biol. Plants 28, 1813–1831. doi: 10.1007/s12298-022-01246-9
Kumari, M., Checker, V. G., Kathpalia, R., Srivastava, V., Singh, I. K., and Singh, A. (2024). Metabolic engineering for enhanced terpenoid production: Leveraging new horizons with an old technique. Plant Physiol. Biochem., 108511. doi: 10.1016/j.plaphy.2024.108511
Kumari, R., Kapoor, P., Mir, B. A., Singh, M., Parrey, Z. A., Rakhra, G., et al. (2024). Unlocking the versatility of Nitric Oxide in plants and insights into its molecular interplays under biotic and abiotic stress. Nitric. Oxide 150, 1–17. doi: 10.1016/j.niox.2024.07.002
Künstler, A., Gullner, G., Ádám, A. L., Kolozsváriné Nagy, J., and Király, L. (2020). The versatile roles of sulfur-containing biomolecules in plant defense-A road to disease resistance. Plants (Basel). 9, 1705. doi: 10.3390/plants9121705
Li, X., Ahammed, G. J., Zhang, X. N., Zhang, L., Yan, P., Zhang, L. P., et al. (2021). Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 403, 123922. doi: 10.1016/j.jhazmat.2020.123922
Li, D., Guo, Y., Zhang, D., He, S., Gong, J., Ma, H., et al. (2020). Melatonin represses oil and anthocyanin accumulation in seeds. Plant Physiol. 183, 898–914. doi: 10.1104/pp.20.00117
Li, X., Thwe, A. A., Park, C. H., Kim, S. J., Arasu, M. V., Abdullah Al-Dhabi, N., et al. (2017). Ethephon-induced phenylpropanoid accumulation and related gene expression in tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) hairy root. Biotechnol. Biotechnol. Equip. 31, 304–311. doi: 10.1080/13102818.2017.1282835
Liang, D., Shen, Y., Ni, Z., Wang, Q., Lei, Z., Xu, N., et al. (2018). Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00426
Liu, Y. H., Alimujiang, A., Wang, X., Luo, S. W., Balamurugan, S., Yang, W. D., et al. (2019). Ethanol induced jasmonate pathway promotes astaxanthin hyperaccumulation in Haematococcus pluvialis. Bioresource. Technol. 289, 121720. doi: 10.1016/j.biortech.2019.121720
Liu, Z., Li, Y., Cao, C., Liang, S., Ma, Y., Liu, X., et al. (2019). The role of H2S in low temperature-induced cucurbitacin C increases in cucumber. Plant Mol. Biol. 99, 535–544. doi: 10.1007/s11103-019-00834-w
Liu, J., Liu, Y., Wang, Y., Zhang, Z. H., Zu, Y. G., Efferth, T., et al. (2016). The combined effects of ethylene and MeJA on metabolic profiling of phenolic compounds in Catharanthus roseus revealed by metabolomics analysis. Front. Physiol. 7. doi: 10.3389/fphys.2016.00217
Lopes, A. S., Dias, T. J., Henschel, J. M., da Silva, J. H. B., de Oliveira Sousa, V. F., Targino, V. A., et al. (2024). Methyl jasmonate mitigates drought stress in purple basil by enhancing photosynthesis and secondary metabolism. J. Plant Growth Regul. 44, 1–14. doi: 10.1007/s00344-024-11392-x
Lu, J. L., Hu, Y. C., Chen, Y., Liu, R., Di, J. J., Feng, T. T., et al. (2024). Hydrogen sulfide promoted cell differentiation, antioxidant ability, and flavonoid accumulation in Ginkgo biloba L. suspension cells. Plant Cell. Tissue Organ Cult. (PCTOC). 156, 36. doi: 10.1007/s11240-023-02631-2
Lv, F. F., Sun, P. W., Liu, P. W., Li, J., Xu, Y. H., and Wei, J. H. (2017). Study on healthy and wound induced expression of 22 sesquiterpene synthase genes of Aquilaria sinensis. Modern. Chin. Med. 8, 1076–1082.
Ma, J., Liu, S., Zeng, J., Zhang, Y., Chang, W., Meng, Z., et al. (2025). Comparative metabolome and transcriptome analyses reveal the role of MeJA in improving postharvest disease resistance and maintaining the quality of Rosa roxburghii fruit. Postharvest. Biol. Technol. 220, 113314. doi: 10.1016/j.postharvbio.2024.113314
Ma, X., Yu, X., Cui, G., Guo, Z., Lang, D., and Zhang, X. (2023). Methyl jasmonate mitigates osmotic stress by regulating carbon and nitrogen metabolism of Glycyrrhiza uralensis seedlings subjected to salt stress. Acta Physiol. Plant. 45. doi: 10.1007/s11738-023-03574-z
Mahmood, S., Afzal, B., Bashir, R., Shakoor, M. B., Nisa, Z. U., Rizwan, M., et al. (2024). Melatonin priming could modulate primary and secondary metabolism of sunflower with better nutraceutical value and tolerance against water deficit environment. Plant Stress 13, 100533. doi: 10.1016/j.stress.2024.100533
Maleki, M., Ghorbanpour, M., and Kariman, K. (2017). Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene 11, 247–254. doi: 10.1016/j.plgene.2017.04.006
Marques, D. J., Ferreira, M. M., Lobato, A. K. D. S., Freitas, W. A. D., Carvalho, J. D. A., Ferreira, E. D., et al. (2016). Potential of calcium silicate to mitigate water deficiency in maize. Bragantia 75, 275–285. doi: 10.1590/1678-4499.446
Mathesius, U. (2018). Flavonoid functions in plants and their interactions with other organisms. Plants 7, 30. doi: 10.3390/plants7020030
Mazhar, M. W., Ishtiaq, M., Maqbool, M., and Akram, R. (2022). Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence, and yield traits of canola plants under drought stress. South Afr. J. Bot. 151, 889–899. doi: 10.1016/j.sajb.2022.11.017
Meena, K. K., Sorty, A. M., Bitla, U. M., Choudhary, K., Gupta, P., Pareek, A., et al. (2017). Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00172
Mohamadi Esboei, M., Ebrahimi, A., Amerian, M. R., and Alipour, H. (2022). Melatonin confers fenugreek tolerance to salinity stress by stimulating the biosynthesis processes of enzymatic, and non-enzymatic antioxidants, and diosgenin content. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.890613
Mohamed, H. I. and Latif, H. H. (2017). Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol. Mol. Biol. Plants 23, 545–556. doi: 10.1007/s12298-017-0451-x
Mulaudzi, T., Hendricks, K., Mabiya, T., Muthevhuli, M., Ajayi, R. F., Mayedwa, N., et al. (2020). Calcium improves germination and growth of Sorghum bicolor seedlings under salt stress. Plants 9, 730. doi: 10.3390/plants9060730
Munawar, A., Akram, N. A., Ahmad, A., and Ashraf, M. (2019). Nitric oxide regulates the oxidative defense system, key metabolites, and growth of broccoli (Brassica oleracea L.) plants under water-limited conditions. Sci. Hortic. 254, 7–13. doi: 10.1016/j.scienta.2019.04.072
Mustafa, H., Ilyas, N., Akhtar, N., Raja, N. I., Zainab, T., Shah, T., et al. (2021). Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol. Environ. Saf. 223, 112519. doi: 10.1016/j.ecoenv.2021.112519
Nabati, J., Nemati, Z., and Rezazadeh, E. B. (2024). Involvement of nitric oxide in biochemical and physiological response of potato seedling under cold stress. J. Plant Growth Regul. 43, 1–12. doi: 10.1007/s00344-024-11401-z
Nabi, R. B. S., Tayade, R., Hussain, A., Kulkarni, K. P., Imran, Q. M., Mun, B. G., et al. (2019). Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 161, 120–133. doi: 10.1016/j.envexpbot.2019.02.003
Nadarajah, K. K. (2020). ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 21, 5208. doi: 10.3390/ijms21155208
Naeem, M., Naeem, M. S., Ahmad, R., Ihsan, M. Z., Ashraf, M. Y., Hussain, Y., et al. (2018). Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch. Agron. Soil Sci. 64, 116–131. doi: 10.1080/03650340.2017.1327713
Naikoo, M. I., Dar, M. I., Raghib, F., Jaleel, H., Ahmad, B., Raina, A., et al. (2019). Role and regulation of plants phenolics in abiotic stress tolerance: An overview. Plant Signaling Mol., 157–168. doi: 10.1016/B978-0-12-816451-8.00009-5
Naji, E. F., Abdulfatah, H. F., and Hashim, K. S. (2024). Plant secondary metabolites, their classification and biological roles: a review. J. Univ. Anbar. Pure. Sci. 18, 106–115. doi: 10.37652/juaps.2023.144549.1164
Narwal, P., Negi, N. P., and Kumar, D. (2024). Boosting banana resilience: Calcium supplementation enhances osmolyte and secondary metabolites production and strengthens the antioxidant machinery in drought and cold-exposed banana plants. Environ. Exp. Bot. 226, 105946. doi: 10.1016/j.envexpbot.2024.105946
Nasrallah, A. K., Kheder, A. A., Kord, M. A., Fouad, A. S., El-Mogy, M. M., and Atia, M. A. (2022). Mitigation of salinity stress effects on broad bean productivity using calcium phosphate nanoparticles application. Horticulturae 8, 75. doi: 10.3390/horticulturae8010075
Naz, M., Afzal, M. R., Raza, M. A., Pandey, S., Qi, S., Dai, Z., et al. (2024). Calcium (Ca2+) signaling in plants: A plant stress perspective. South Afr. J. Bot. 169, 464–485. doi: 10.1016/j.sajb.2024.04.047
Nazir, F., Fariduddin, Q., and Khan, T. A. (2020). Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 252, 126486. doi: 10.1016/j.chemosphere.2020.126486
Nazir, F., Hussain, A., and Fariduddin, Q. (2019). Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 230, 544–558. doi: 10.1016/j.chemosphere.2019.05.001
Nazir, M. M., Li, Q., Noman, M., Ulhassan, Z., Ali, S., Ahmed, T., et al. (2022). Calcium oxide nanoparticles have the role of alleviating arsenic toxicity of barley. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.843795
Nguyen, L. V., Bertero, D., Hoang, D. T., and Long, N. V. (2022). Variation in quinoa roots growth responses to drought stresses. J. Agron. Crop Sci. 208, 830–840. doi: 10.1111/jac.12528
Ninkuu, V., Zhang, L., Yan, J., Fu, Z., Yang, T., and Zeng, H. (2021). Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 22, 5710. doi: 10.3390/ijms22115710
Nóbrega, J. S., Gomes, V. R., Soares, L. A. D. A., Lima, G. S. D., Silva, A. A. R. D., Gheyi, H. R., et al. (2024). Hydrogen peroxide alleviates salt stress effects on gas exchange, growth, and production of naturally colored cotton. Plants 13, 390. doi: 10.3390/plants13030390
Nomani, L., Zehra, A., Choudhary, S., Wani, K. I., Naeem, M., Siddiqui, M. H., et al. (2022). Exogenous hydrogen sulphide alleviates copper stress impacts in Artemisia annua L.: Growth, antioxidant metabolism, glandular trichome development and artemisinin biosynthesis. Plant Biol. 24, 642–651. doi: 10.1111/plb.13242
Nurnaeimah, N., Mat, N., Suryati Mohd, K., Badaluddin, N. A., Yusoff, N., Sajili, M. H., et al. (2020). The effects of hydrogen peroxide on plant growth, mineral accumulation, as well as biological and chemical properties of Ficus deltoidea. Agronomy 10, 599. doi: 10.3390/agronomy10040599
Nyanasaigran, L., Ramasamy, S., Gautam, A., Guleria, P., Kumar, V., and Yaacob, J. S. (2024). Methyl jasmonate elicitation improves the growth performance and biosynthesis of antioxidant metabolites in Portulaca oleracea through ROS modulation. Ind. Crops Products. 216, 118709. doi: 10.1016/j.indcrop.2024.118709
Patil, A. S. (2020). Plant Secondary Metabolites: Isolation, Characterization & Biological Properties (Delhi, India: Studera Press).
Peymaei, M., Sarabi, V., and Hashempour, H. (2024). Improvement of the yield and essential oil of fennel (Foeniculum vulgare Mill.) using external proline, uniconazole and methyl jasmonate under drought stress conditions. Sci. Hortic. 323, 112488. doi: 10.1016/j.scienta.2023.112488
Pirooz, P., Amooaghaie, R., Ahadi, A., Sharififar, F., and Torkzadeh-Mahani, M. (2022). Silicon and nitric oxide synergistically modulate the production of essential oil and rosmarinic acid in Salvia officinalis under Cu stress. Protoplasma 255, 1–12. doi: 10.1007/s00709-021-01708-z
Pourebrahimi, M., Eshghi, S., Ramezanian, A., and Faghih, S. (2023). Effect of combined application of selenium and hydrogen sulfide under salinity stress on yield, physiological traits and biofortification of strawberries in hydroponic cultivation. Sci. Hortic. 315, 111982. doi: 10.1016/j.scienta.2023.111982
Qi, Q., Guo, Z., Liang, Y., Li, K., and Xu, H. (2019). Hydrogen sulfide alleviates oxidative damage under excess nitrate stress through MAPK/NO signaling in cucumber. Plant Physiol. Biochem. 135, 1–8. doi: 10.1016/j.plaphy.2018.11.017
Qin, K., Fan, S., Zhang, F., and Wang, Y. (2019). Effects of exogenous calcium on datura seed germination under drought stress. J. Bot. Res. 1, 8–14. doi: 10.30564/jrb.v1i2.862
Qin, C., Lian, H., Alqahtani, F. M., and Ahanger, M. A. (2024). Chromium-mediated damaging effects on growth, nitrogen metabolism and chlorophyll synthesis in tomato can be alleviated by foliar application of melatonin and jasmonic acid priming. Sci. Hortic. 323, 112494. doi: 10.1016/j.scienta.2023.112494
Quartacci, M. F., Irtelli, B., Gonnelli, C., Gabbrielli, R., and Navari-Izzo, F. (2009). Naturally-assisted metal phytoextraction by Brassica carinata: Role of root exudates. Environ. pollut. 157, 2697–2703. doi: 10.1016/j.envpol.2009.04.035
Rahim, W., Khan, M., Al Azzawi, T. N. I., Pande, A., Methela, N. J., Ali, S., et al. (2022). Exogenously applied sodium nitroprusside mitigates lead toxicity in rice by regulating antioxidants and metal stress-related transcripts. Int. J. Mol. Sci. 23, 9729. doi: 10.3390/ijms23179729
Rahimi, A. R., Rokhzadi, A., Amini, S., and Karami, E. (2013). Effect of salicylic acid and methyl jasmonate on growth and secondary metabolites in Cuminum cyminum L. J. Biodivers. Environ. Sci. 3, 140–149.
Raina, M., Kumar, A., Yadav, N., Kumari, S., Yusuf, M. A., Mustafiz, A., et al. (2021). StCaM2, a calcium binding protein, alleviates negative effects of salinity and drought stress in tobacco. Plant Mol. Biol. 106, 85–108. doi: 10.1007/s11103-021-01131-1
Rao, M. J. and Zheng, B. (2025). The role of polyphenols in abiotic stress tolerance and their antioxidant properties to scavenge reactive oxygen species and free radicals. Antioxidants 14, 74. doi: 10.3390/antiox14010074
Rasheed, F., Sehar, Z., Fatma, M., Iqbal, N., Masood, A., Anjum, N. A., et al. (2021). Involvement of ethylene in reversal of salt stress by salicylic acid in the presence of sulfur in mustard (Brassica juncea L.). J. Plant Growth Regul. 41, 1–18. doi: 10.1007/s00344-021-10526-9
Rather, B. A., Mir, I. R., Masood, A., Anjum, N. A., and Khan, N. A. (2020). Nitric oxide pre-treatment advances seed germination and alleviates copper-induced photosynthetic inhibition in Indian mustard. Plants 9, 776. doi: 10.3390/plants9060776
Reshi, Z. A., Ahmad, W., Lukatkin, A. S., and Javed, S. B. (2023). From Nature to Lab: A review of secondary metabolite biosynthetic pathways, environmental influences, and in vitro approaches. Metabolites 13, 895. doi: 10.3390/metabo13080895
Rizwan, M., Mostofa, M. G., Ahmad, M. Z., Zhou, Y., Adeel, M., Mehmood, S., et al. (2019). Hydrogen sulfide enhances rice tolerance to nickel through the prevention of chloroplast damage and the improvement of nitrogen metabolism under excessive nickel. Plant Physiol. Biochem. 138, 100–111. doi: 10.1016/j.plaphy.2019.02.023
Roy, A. (2017). A review on the alkaloids an important therapeutic compound from plants. Int. J. Plant Biotechnol. 3, 1–9.
Sadak, M. S., Abdalla, A. M., Abd Elhamid, E. M., and Ezzo, M. I. (2020). Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Centre. 44, 1–13. doi: 10.1186/s42269-020-0275-7
Saeed, S., Ali, H., Khan, T., Kayani, W., and Khan, M. A. (2017). Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiol. Mol. Biol. Plants 23, 229–237. doi: 10.1007/s12298-016-0406-7
Safari, F., Akramian, M., Salehi-Arjmand, H., and Ghorbanpour, M. (2023). Nitric oxide-induced physiochemical alterations and gene expression in lemon balm (Melissa officinalis L.) under water deficit stress. J. Plant Growth Regul. 42, 5438–5451. doi: 10.1007/s00344-022-10673-7
Saini, N., Modolo, L. V., Deswal, R., Sehrawat, A., Yadav, N., and Sangwan, N. S. (2024). Expanding roles of cross-talk between hydrogen sulfide and nitric oxide under abiotic stress in plants. Plant Physiol. Biochem. 214, 108852. doi: 10.1016/j.plaphy.2024.108852
Salam, U., Ullah, S., Tang, Z. H., Elateeq, A. A., Khan, Y., Khan, J., et al. (2023). Plant metabolomics: an overview of the role of primary and secondary metabolites against different environmental stress factors. Life 13, 706. doi: 10.3390/life13030706
Saleem, K., Asghar, M. A., Javed, H. H., Raza, A., Seleiman, M. F., Ullah, A., et al. (2023). Alleviation of arsenic toxicity-induced oxidative stress in lemon grass by methyl jasmonate. South Afr. J. Bot. 160, 547–559. doi: 10.1016/j.sajb.2023.07.034
Salvi, P., Manna, M., Kaur, H., Thakur, T., Gandass, N., Bhatt, D., et al. (2021). Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 40, 1305–1329. doi: 10.1007/s00299-021-02683-8
Sana, A. U., Aftab, T., Gill, R., Gill, S. S., and Naeem, M. (2025). Harnessing gasotransmitters for enhanced plant resilience: strategies for managing metalloid (s) stress. J. Plant Growth Regul., 1–24. doi: 10.1007/s00344-025-11676-w
Santos-Sánchez, N. F., Salas-Coronado, R., Hernández-Carlos, B., and Villanueva-Cañongo, C. (2019). Shikimic Acid Pathway in Bbiosynthesis of Phenolic Compounds (London U.K: Intech Open), 1–15.
Saqib, M., Shahzad, U., Zulfiqar, F., Tiwari, R. K., Lal, M. K., Naz, S., et al. (2023). Exogenous melatonin alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in strawberry. South Afr. J. Bot. 157, 10–18. doi: 10.1016/j.sajb.2023.03.039
Sehar, Z., Fatma, M., Khan, S., Mir, I. R., Abdi, G., and Khan, N. A. (2023a). Melatonin influences methyl jasmonate-induced protection of photosynthetic activity in wheat plants against heat stress by regulating ethylene-synthesis genes and antioxidant metabolism. Sci. Rep. 13, 7468. doi: 10.1038/s41598-023-34682-y
Sehar, Z., Gautam, H., Masood, A., and Khan, N. A. (2023b). Ethylene-and proline-dependent regulation of antioxidant enzymes to mitigate heat stress and boost photosynthetic efficacy in wheat plants. J. Plant Growth Regul. 42, 2683–2697. doi: 10.1007/s00344-022-10737-8
Sehar, Z., Jahan, B., Masood, A., Anjum, N. A., and Khan, N. A. (2021). Hydrogen peroxide potentiates defense system in presence of sulfur to protect chloroplast damage and photosynthesis of wheat under drought stress. Physiol. Plant. 172, 922–934. doi: 10.1111/ppl.13225
Seifikalhor, M., Aliniaeifard, S., Shomali, A., Azad, N., Hassani, B., Lastochkina, O., et al. (2019). Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signaling Behav. 14, 1665455. doi: 10.1080/15592324.2019.1665455
Shahmohammadi, F., Ghanbari Jahromi, M., Farhadpour, M., Kalateh Jari, S., and Torkashvand, A. M. (2024). Biochemical responses and dynamics of the taxol biosynthesis pathway genes in Taxus baccata L. plants sprayed with melatonin under drought stress. Plant Soil 509, 1–19. doi: 10.1007/s11104-024-06890-6
Sharma, S., Rani, H., Kaur, G., Kumar, S., Sheikh, S., and Samota, M. K. (2024). Comprehensive overview of glucosinolates in crucifers: Occurrence, roles, metabolism, and transport mechanisms—A review. Phytochem. Rev. 24, 1–28. doi: 10.1007/s11101-024-10021-5
Sharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M., and Zheng, B. (2019). Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24, 2452. doi: 10.3390/molecules24132452
Sheikhalipour, M., Kulak, M., Mohammadi, S. A., Esmaielpour, B., Nouraein, M., Kocak, M. Z., et al. (2024). Foliar application of either melatonin or sodium nitroprusside regulates the antioxidant status, and the morpho-physiological attributes and essential oil production in sage (Salvia officinalis L.) under salinity stress. Sci. Hortic. 323, 112526. doi: 10.1016/j.scienta.2023.112526
Sheikhalipour, M., Mohammadi, S. A., Esmaielpour, B., Zareei, E., Kulak, M., Ali, S., et al. (2022). Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system, and secondary metabolism-related genes. BMC Plant Biol. 22, 380. doi: 10.1186/s12870-022-03728-0
Shen, X., Liu, Y., Zeng, Y., Zhao, Y., Bao, Y., Wu, Z., et al. (2024). Hydrogen sulfide alleviates the chilling-induced lignification in loquat fruit by regulating shikimate, phenylpropanoid, and cell wall metabolisms. Postharvest. Biol. Technol. 214, 113012. doi: 10.1016/j.postharvbio.2024.113012
Shenavaie Zare, A., Ganjeali, A., Vaezi Kakhki, M. R., Cheniany, M., and Mashreghi, M. (2022). Plant elicitation and TiO2 nanoparticles application as an effective strategy for improving the growth, biochemical properties, and essential oil of peppermint. Physiol. Mol. Biol. Plants 28, 1391–1406. doi: 10.1007/s12298-022-01215-2
Shi, H., Ye, T., and Chan, Z. (2014). Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in Bermudagrass (Cynodon dactylon (L.) Pers.). Plant Physiol. Biochem. 74, 99–107. doi: 10.1016/j.plaphy.2013.11.001
Shiade, S. R. G., Zand-Silakhoor, A., Fathi, A., Rahimi, R., Minkina, T., Rajput, V. D., et al. (2024). Plant metabolites and signaling pathways in response to biotic and abiotic stresses: Exploring bio stimulant applications. Plant Stress 12, 100454. doi: 10.1016/j.stress.2024.100454
Shoukat, S., Tassawar, A., Keyani, R., Zafar, M., Naz, R., Nosheen, A., et al. (2023). Exogenous application of sodium hydrosulfide and salicylic acid mitigate salinity stress in maize by regulating ionic balance, biochemical attributes, photosynthetic pigments, and some key antioxidants. South Afr. J. Bot. 158, 393–404. doi: 10.1016/j.sajb.2023.05.016
Siddiqui, M. H., Alamri, S., Khan, M. N., Corpas, F. J., Al-Amri, A. A., Alsubaie, Q. D., et al. (2020). Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 398, 122882. doi: 10.1016/j.jhazmat.2020.122882
Silva, P. C., Gheyi, H. R., Jesus, M.J.D.S.D., Correia, M. R., and Azevedo, A. D. D. (2023). Seed priming with hydrogen peroxide enhances tolerance to salt stress of hydroponic lettuce. Rev. Bras. Engenharia. Agrícola. e Ambiental. 27, 704–711. doi: 10.1590/1807-1929/agriambi.v27n9p704-711
Sim, U., Sung, J., Lee, H., Heo, H., Jeong, H. S., and Lee, J. (2020). Effect of calcium chloride and sucrose on the composition of bioactive compounds and antioxidant activities in buckwheat sprouts. Food Chem. 312, 126075. doi: 10.1016/j.foodchem.2019.126075
Singh, S., Prakash, P., and Singh, A. K. (2021). Salicylic acid and hydrogen peroxide improve antioxidant response and compatible osmolytes in wheat (Triticum aestivum L.) under water deficit. Agric. Res. 10, 175–186. doi: 10.1007/s40003-020-00490-3
Skowron, E. and Trojak, M. (2021). Effect of exogenously-applied abscisic acid, putrescine and hydrogen peroxide on drought tolerance of barley. Biologia 76, 453–468. doi: 10.2478/s11756-020-00644-2
Song, K. E., Hwang, H. R., Hong, E. S. H., Konvalina, P., Jun, W. J., Jung, J. W., et al. (2023). Hydrogen peroxide ameliorates the adversities of drought stress during germination and seedling growth in sorghum (Sorghum bicolor L.). Agronomy 13, 330. doi: 10.3390/agronomy13020330
Song, X. W., Yao, Y., Yu, P. C., Zhang, W., Liu, W. F., Wang, L. Y., et al. (2023). Sodium nitroprusside improved the quality of Radix Saposhnikoviae through constructed physiological response under ecological stress. Sci. Rep. 13, 15823. doi: 10.1038/s41598-023-43153-3
Subramaniyam, S., Mathiyalagan, R., Natarajan, S., Kim, Y. J., Jang, M. G., Park, J. H., et al. (2014). Transcript expression profiling for adventitious roots of Panax ginseng Meyer. Gene 546, 89–96. doi: 10.1016/j.gene.2014.05.024
Taheri, Z., Vatankhah, E., and Jafarian, V. (2020). Methyl jasmonate improves physiological and biochemical responses of Anchusa italica under salinity stress. South Afr. J. Bot. 130, 375–382. doi: 10.1016/j.sajb.2020.01.026
Talebi, M., Moghaddam, M., and Pirbalouti, A. G. (2018). Methyl jasmonate effects on volatile oil compounds and antioxidant activity of leaf extract of two basil cultivars under salinity stress. Acta Physiol. Plant. 40, 1–11. doi: 10.1007/s11738-018-2611-1
Tan, P., Zeng, C., Wan, C., Liu, Z., Dong, X., Peng, J., et al. (2021). Metabolic profiles of Brassica juncea roots in response to cadmium stress. Metabolites 11, 383. doi: 10.3390/metabo11060383
Tang, H., Hu, J., Zhao, M., Cao, L., and Chen, Y. (2023). Comparative study of the physiological responses, secondary metabolites, and gene expression of medicinal plant Prunella vulgaris L. treated with exogenous methyl jasmonate and salicylic acid. Acta Physiol. Plant. 45, 20. doi: 10.1007/s11738-022-03498-0
Tayal, M., Somavat, P., Rodriguez, I., Thomas, T., Christoffersen, B., and Kariyat, R. (2020). Polyphenol-rich purple corn pericarp extract adversely impacts herbivore growth and development. Insects 11, 98. doi: 10.3390/insects11020098
Tiku, A. R. (2018). “Antimicrobial compounds and their role in plant defense,” in Molecular Aspects of Plant-Pathogen Interaction, 283–307. doi: 10.1007/978-981-10-7371-7_13
Ting, H. M., Cheah, B. H., Chen, Y. C., Yeh, P. M., Cheng, C. P., Yeo, F. K. S., et al. (2020). The role of a glucosinolate-derived nitrile in plant immune responses. Front. Plant Sci. 11, 257. doi: 10.3389/fpls.2020.00257
Trifunović-Momčilov, M., Stamenković, N., Đurić, M., Milošević, S., Marković, M., Giba, Z., et al. (2023). Role of sodium nitroprusside on potential mitigation of salt stress in centaury (Centaurium erythraea Rafn) shoots grown in vitro. Life 13, 154. doi: 10.3390/life13010154
Twaij, B. M. and Hasan, M. N. (2022). Bioactive secondary metabolites from plant sources: types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 13, 4–14. doi: 10.3390/ijpb13010003
Upadhyay, R., Saini, R., Shukla, P. K., and Tiwari, K. N. (2025). Role of secondary metabolites in plant defense mechanisms: A molecular and biotechnological insights. Phytochem. Rev. 24, 953–983. doi: 10.1007/s11101-024-09976-2
Valivand, M. and Amooaghaie, R. (2021a). Foliar spray with sodium hydrosulfide and calcium chloride advances dynamic of critical elements and efficiency of nitrogen metabolism in Cucurbita pepo L. under nickel stress. Sci. Hortic. 283, 110052. doi: 10.1016/j.scienta.2021.110052
Valivand, M. and Amooaghaie, R. (2021b). Sodium hydrosulfide modulates membrane integrity, cation homeostasis, and accumulation of phenolics and osmolytes in Zucchini under nickel stress. J. Plant Growth Regul. 40, 313–328. doi: 10.1007/s00344-020-10101-8
Wang, X., Li, Y., Han, L., Li, J., Liu, C., and Sun, C. (2021). Role of flavonoids in the treatment of iron overload. Front. Cell Dev. Biol. 9, 685364. doi: 10.3389/fcell.2021.685364
Wang, W., Lin, Z., Wang, W., Shang, M., Lv, H., Zong, Q., et al. (2023). Elicitation with hydrogen peroxide promotes growth, phenolic-enrichment, antioxidant activity and nutritional values of two hydroponic lettuce genotypes. Food Chem.: X. 19, 100847. doi: 10.1016/j.fochx.2023.100847
Wang, L. Y., Liu, J. L., Wang, W. X., and Sun, Y. (2016). Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54, 19–27. doi: 10.1007/s11099-015-0140-3
Wang, C., Wang, Y., Zeng, W., and Li, S. (2014). Alleviation of Cd toxicity in Arabidopsis thaliana seedlings by exogenous Ca2+ or K+. Chin. Bull. Bot. 49, 262. doi: 10.3724/SP.J.1259.2014.00262
Wang, Y., Yuan, M., Li, Z., Niu, Y., Jin, Q., Zhu, B., et al. (2020). Effects of ethylene biosynthesis and signaling on oxidative stress and antioxidant defense system in Nelumbo nucifera G. under cadmium exposure. Environ. Sci. pollut. Res. 27, 40156–40170. doi: 10.1007/s11356-020-09918-3
Wang, F., Zhu, H., Chen, D., Li, Z., Peng, R., and Yao, Q. (2016). A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell. Tissue Organ Cult. (PCTOC). 125, 387–398. doi: 10.1007/s11240-016-0953-1
Wani, K. I., Naeem, M., and Aftab, T. (2024). Understanding the interplay between strigolactone and nitric oxide in alleviating cadmium-induced toxicity in Artemisia annua. Environ. Exp. Bot. 225, 105856. doi: 10.1016/j.envexpbot.2024.105856
Wani, K. I., Naeem, M., Khan, M. M. A., and Aftab, T. (2023). Nitric oxide induces antioxidant machinery, PSII functioning, and artemisinin biosynthesis in Artemisia annua under cadmium stress. Plant Sci. 334, 111754. doi: 10.1016/j.plantsci.2023.111754
Wu, X., Liu, Z., and Liao, W. (2021). The involvement of gaseous signaling molecules in plant MAPK cascades: function and signal transduction. Planta 254, 127. doi: 10.1007/s00425-021-03792-0
Xu, C., Li, X., and Zhang, L. (2013). The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PloS One 8, e68214. doi: 10.1371/journal.pone.0068214
Xu, L., Yue, Q., Bian, F. E., Sun, H., Zhai, H., and Yao, Y. (2017). Melatonin enhances phenolics accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01426
Xu, C., Zhang, Y. P., and Yang, H. B. (2022). Effects of ethephon on physiological characteristics and gene expression of Tartary buckwheat under salt stress. Chilean. J. Agric. Res. 82, 234–243. doi: 10.4067/S0718-58392022000200234
Yang, H., Hu, W., Zhao, J., Huang, X., Zheng, T., and Fan, G. (2021). Genetic improvement combined with seed ethephon priming improved grain yield and drought resistance of wheat exposed to soil water deficit at tillering stage. Plant Growth Regul. 95, 399–419. doi: 10.1007/s10725-021-00749-x
Yeshi, K., Crayn, D., Ritmejerytė, E., and Wangchuk, P. (2022). Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 27, 313. doi: 10.3390/molecules27010313
Yin, Y., Hu, J., Tian, X., Yang, Z., and Fang, W. (2022). Nitric oxide mediates melatonin-induced isoflavone accumulation and growth improvement in germinating soybeans under NaCl stress. J. Plant Physiol. 279, 153855. doi: 10.1016/j.jplph.2022.153855
You, W., Zhang, J., Ru, X., Xu, F., Wu, Z., Jin, P., et al. (2024). CaCl2 promoted phenolics accumulation via the CmCAMTA4-mediated transcriptional activation of phenylpropane pathway and energy metabolism in fresh-cut cantaloupe. Postharvest. Biol. Technol. 207, 112599. doi: 10.1016/j.postharvbio.2023.112599
Yuenyong, W., Chinpongpanich, A., Comai, L., Chadchawan, S., and Buaboocha, T. (2018). Downstream components of the calmodulin signaling pathway in the rice salt stress response revealed by transcriptome profiling and target identification. BMC Plant Biol. 18, 1–23. doi: 10.1186/s12870-018-1538-4
Zangani, E., Zehtab-Salmasi, S., Andalibi, B., and Zamani, A. A. (2018). Protective effects of nitric oxide on photosynthetic stability and performance of Silybum marianum under water deficit conditions. Agron. J. 110, 555–564. doi: 10.2134/agronj2017.07.0396
Zeng, H., Xu, L., Singh, A., Wang, H., Du, L., and Poovaiah, B. W. (2015). Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00600
Zhan, C., Lei, L., Liu, Z., Zhou, S., Yang, C., Zhu, X., et al. (2020). Selection of a subspecies-specific diterpene gene cluster implicated in rice disease resistance. Nat. Plants 6, 1447–1454. doi: 10.1038/s41477-020-00816-7
Zhang, Q., Feng, Y. X., Tian, P., Lin, Y. J., and Yu, X. Z. (2022). Proline-mediated regulation on jasmonate signals repressed anthocyanin accumulation through the MYB-bHLH-WDR complex in rice under chromium exposure. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.953398
Zhang, L., Liu, Y., Zhang, Z., and Fang, S. (2023). Physiological response and molecular regulatory mechanism reveal a positive role of nitric oxide and hydrogen sulfide applications in salt tolerance of Cyclocarya paliurus. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1211162
Zhang, X., Ran, W., Li, X., Zhang, J., Ye, M., Lin, S., et al. (2022). Exogenous application of gallic acid induces the direct defense of tea plant against Ectropis obliqua caterpillars. Front. Plant Sci. 13, 833489. doi: 10.3389/fpls.2022.833489
Zhang, M., Smith, J. A. C., Harberd, N. P., and Jiang, C. (2016). The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 91, 651–659. doi: 10.1007/s11103-016-0488-1
Zhang, T., Wang, J., Sun, Y., Zhang, L., and Zheng, S. (2022). Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 41, 1–17. doi: 10.1007/s00344-021-10317-2
Zhang, Y., Yun, F., Man, X., Huang, D., and Liao, W. (2023). Effects of hydrogen sulfide on sugar, organic acid, carotenoid, and polyphenol level in tomato fruit. Plants 12, 719. doi: 10.3390/plants12040719
Zhang, B., Zheng, L. P., and Wang, J. W. (2012). Nitric oxide elicitation for secondary metabolite production in cultured plant cells. Appl. Microbiol. Biotechnol. 93, 455–466. doi: 10.1007/s00253-011-3658-8
Zhang, J., Zhou, M., Zhou, H., Zhao, D., Gotor, C., Romero, L. C., et al. (2021). Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 63, 146–160. doi: 10.1111/jipb.13022
Zhang, N. N., Zou, H., Lin, X. Y., Pan, Q., Zhang, W. Q., Zhang, J. H., et al. (2020). Hydrogen sulfide and rhizobia synergistically regulate nitrogen (N) assimilation and remobilization during N deficiency-induced senescence in soybean. Plant. Cell Environ. 43, 1130–1147. doi: 10.1111/pce.13736
Zhou, H., Chen, Y., Zhai, F., Zhang, J., Zhang, F., Yuan, X., et al. (2020). Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol. Biochem. 155, 213–220. doi: 10.1016/j.plaphy.2020.07.038
Zhou, X., Joshi, S., Khare, T., Patil, S., Shang, J., and Kumar, V. (2021). Nitric oxide, crosstalk with stress regulators, and plant abiotic stress tolerance. Plant Cell Rep. 40, 1395–1414. doi: 10.1007/s00299-021-02705-5
Zhou, W., Shi, M., Deng, C., Lu, S., Huang, F., Wang, Y., et al. (2021). The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in Salvia miltiorrhiza. Horticult. Res. 8. doi: 10.1038/s41438-020-00443-5
Zhu, Z. and Lee, B. (2015). Friends or foes: new insights in jasmonate and ethylene co-actions. Plant Cell Physiol. 56, 414–420. doi: 10.1093/pcp/pcu171
Zhu, X., Wang, P., Bai, Z., Herde, M., Ma, Y., Li, N., et al. (2022). Calmodulin-like protein CML24 interacts with CAMTA2 and WRKY46 to regulate ALMT1-dependent Al resistance in Arabidopsis thaliana. New Phytol. 233, 2471–2487. doi: 10.1111/nph.17812
Zou, Y., Liu, Y., Li, W., Cao, Q., Wang, X., Hu, Z., et al. (2024). Ethylene is the key phytohormone to enhance arsenic resistance in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 281, 116644. doi: 10.1016/j.ecoenv.2024.116644
Keywords: abiotic stress, antioxidants, cross-talk, secondary metabolites, signalling molecules, metabolic regulation
Citation: Sana, Aftab T, Naeem M, Jha PK and Prasad PVV (2025) Production of secondary metabolites under challenging environments: understanding functions and mechanisms of signalling molecules. Front. Plant Sci. 16:1569014. doi: 10.3389/fpls.2025.1569014
Received: 03 February 2025; Accepted: 30 June 2025;
Published: 11 August 2025.
Edited by:
Jinda Wang, Fujian Agriculture and Forestry University, ChinaReviewed by:
Mohd Irfan Naikoo, King Fahd University of Petroleum and Minerals, Saudi ArabiaSheylla Susan Moreira da Silva de Almeida, Universidade Federal do Amapá, Brazil
Copyright © 2025 Sana, Aftab, Naeem, Jha and Prasad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Naeem, bmFlZW1nYXVyQGdtYWlsLmNvbQ==
 Sana1
Sana1 Tariq Aftab
Tariq Aftab M. Naeem
M. Naeem Prakash Kumar Jha
Prakash Kumar Jha P. V. Vara Prasad
P. V. Vara Prasad