- 1Department of Genetics and Plant Breeding, College of Agriculture, Thiruvananthapuram, Kerala, India
- 2Department of Agronomy, College of Agriculture, Thiruvananthapuram, Kerala, India
- 3Department of Agricultural Statistics, College of Agriculture, Thiruvananthapuram, Kerala, India
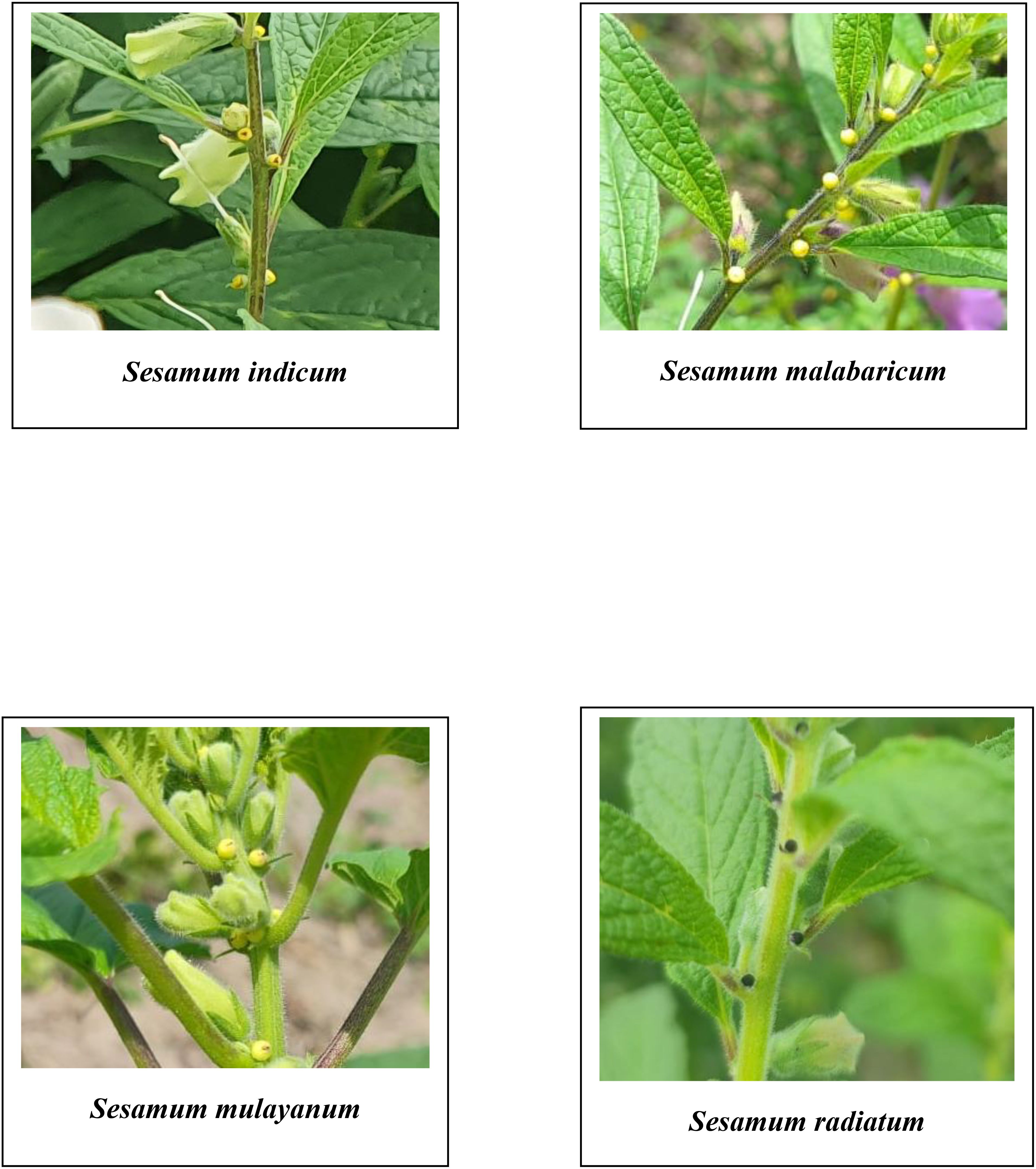
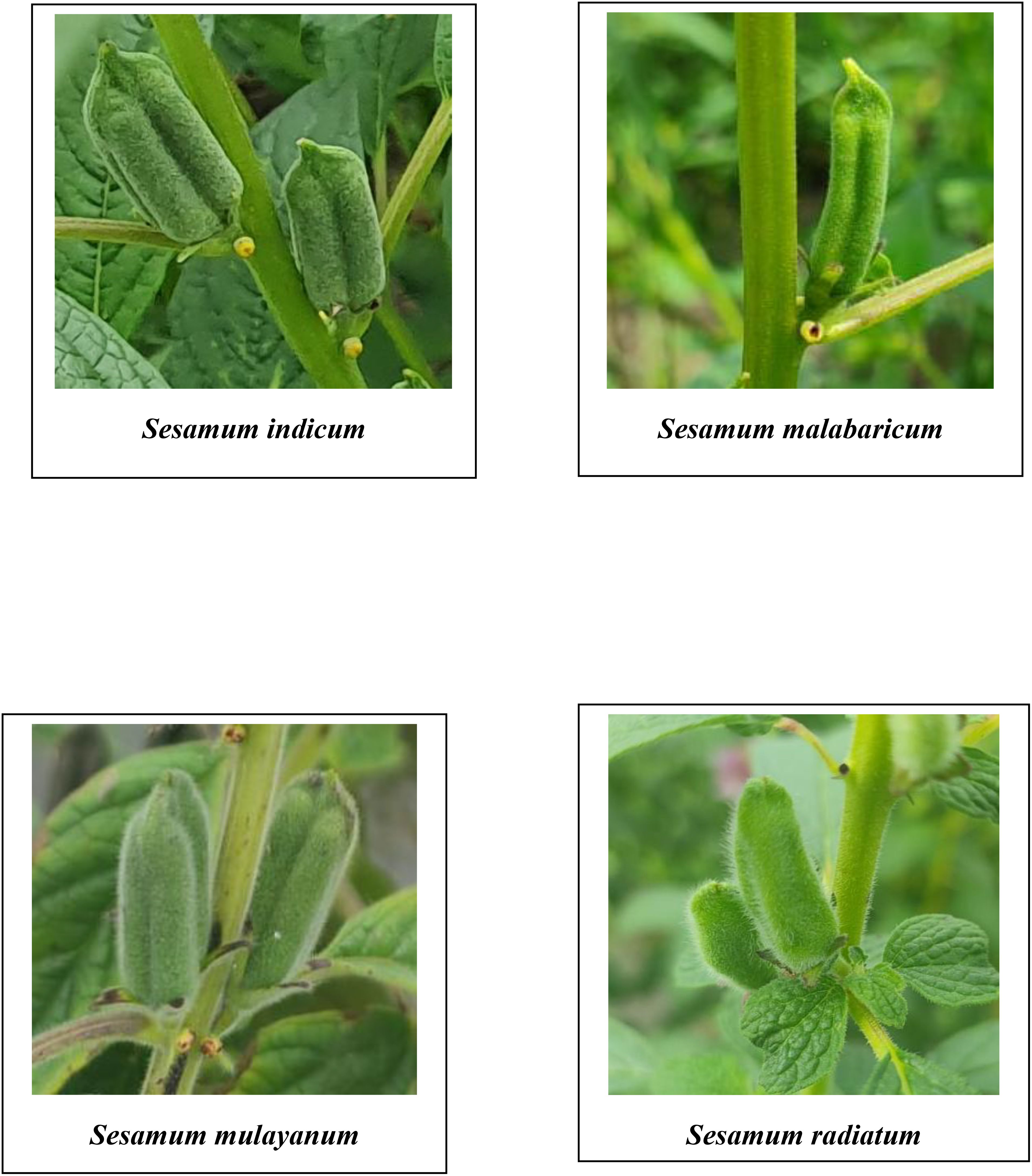
The study aimed to characterize wild relatives and traditional cultivars of sesame based on their morphology and biochemical properties, including crude protein, oil content, fatty acid profiles, and total phenol content. The study included accessions of Sesamum indicum, S. radiatum, S. mulayanum, and S. malabaricum. A comparative analysis revealed significant variation in flower color among the species. S. indicum had pale purplish-pink flowers, while S. mulayanum and S. malabaricum had dark violet flowers, and S. radiatum had white flowers with violet borders. Differences in capsule hairiness were also noted, with cultivated species having glabrous capsules, while wild species exhibited hairy capsules. The hairiness ranged from weakly hairy capsules in S. mulayanum and S. malabaricum to strongly hairy capsules in S. radiatum. All 27 genotypes produced a single flower per axil, a trait common across all sesame species. The S. indicum accession, Ayali 1, produced the most capsules per plant, while S. radiatum (IC 256273) produced the fewest. S. radiatum accessions had the longest and broadest capsules, while the shortest and narrowest capsules were found in S. malabaricum. Cultivated sesame varieties produced larger and heavier seeds compared to wild species. The highest phenol content was recorded in S. radiatum (IC 210433), while the lowest was observed in Kayamkulam 1. Seed yield per plant showed a strong positive correlation with the number of capsules per plant, 1000 seed weight, and oil content, while a significant negative correlation was found between phenol content, plant height, and seed yield. Oil content analysis revealed that the highest oil yield came from Thilak seeds, while the lowest yield was observed in S. malabaricum (IC 557243). Fatty acid profiling showed the presence of both saturated fatty acids (palmitic acid, stearic acid, behenic acid, margaric acid, and arachidic acid) and unsaturated fatty acids (oleic acid, linoleic acid, linolenic acid, eicosanoic acid, 11-eicosenoic acid, and linolelaidic acid) in varying proportions across sesame samples.
Introduction
Sesame (Sesamum) belongs to the Pedaliaceae family, which comprises 16 genera and 60 species. Out of these, 37 species are classified under Sesamum. In India there are about nine taxa of sesame of which one is widely under cultivation and the rest are wild or naturalized. In Kerala six species of sesame are found viz. S. indicum (2n=26), S. malabaricum (2n=26), S. mulayanum (2n=26), S. radiatum (2n=64), S. laciniatum (2n=26) and S. prostratum (2n=32). S. malabaricum or S. indicum subsp. malabaricum, is considered the wild progenitor of cultivated sesame. Both cultivated sesame and S. malabaricum are diploid with a chromosome number of 2n=2x=26 and produce fertile offspring when crossed. This genetic evidence supports the idea that S. malabaricum is the progenitor of cultivated sesame, unlike other sesame species (Bedigian, 2014). The progeny derived from the cross between S. malabaricum and the cultivated sesame exhibited high fertility rate under natural growth conditions (Nimmakayala et al., 2011). It is also reported to have resistance to phyllody disease and hence can be used as a donor parent in resistant breeding programmes (Saravanan and Nadarajan, 2005).
S. mulayanum is sometimes seen in sesame crop field as an associated weed (Tanesaka et al., 2012). This species shows deep seed dormancy and is characterized by conspicuous purple pigmentation on the lower lip of the corolla. The seed dormancy of S. mulayanum may be due to its seed coat structure (coat-enhanced dormancy). The character purple pigmentation of the lower lip of corolla was found to be linked with the major gene controlling seed dormancy. Tarihal et al. (2003) reported absence of any fertilization barriers in crosses between S. indicum and S. mulayanum with the latter being the female parent. The flowers of the F1 plants were characterized by pansy violet corolla color (intermediate between the parents). Based on their studies Biswas and Mitra (1990) opined that S. indicum and S. mulayanum are closely related. The seeds of S. indicum and F1 of interspecific cross S. indicum X S. mulayanum showed good germination also.
The center of origin of S. radiatum is west tropical Africa. This species was introduced into India as a part of breeding programmes in 1933 at Tamil Nadu Agricultural University, Coimbatore (John and Rao, 1941). It is cultivated and consumed as a leafy vegetable in many parts of the world (Adéoti et al., 2012). The leaves of S. radiatum are known to have phenols, antioxidant properties and minerals (Oduntan et al., 2011). It has stronger tap root with more stout fibrous roots. Correspondingly, S. radiatum has higher tolerance to biotic and abiotic stresses, compared with the cultivated sesame and some other wild species. S. laciniatum is perennial, prostrate, profusely branched, having deeply dissected coarse leaves with deep purple flowers with purple anthers where yellow glands absent, small laterally compressed tough capsules and deeply reticulated seeds with thick testa (Salunke et al., 2023).
S. indicum is the only cultivated species representing the sesame germplasm (Venkataramana Bhat et al., 1999). Sesame cultivation dates back to the ancient Indus Valley Civilization, making it one of the earliest cultivated crops. These small seeds, often referred to as the “seeds of immortality,” contain 44-58% oil, 18-25% protein, 13.5% carbohydrates, and 5% ash (Borchani et al., 2010). Sesame seeds are a rich source of energy, and they are packed with vitamins E, A, B complex, and minerals such as calcium, phosphorus, iron, copper, magnesium, zinc, and potassium. They are also an excellent substitute for mother’s milk, especially in cases of milk allergies (Ranganatha et al., 2010). Sesame oil, due to its nourishing, calming, and warming properties, is widely used as the base in over 90% of Ayurvedic treatments, making it an ideal massage oil (Shamloo et al., 2019; Biswas et al., 2018).
Sesame is a highly valued crop worldwide because of its various uses for its seeds and oil in the food, nutraceutical, and pharmaceutical industries. Global sesame seed production exceeded 7 million tons in 2022, with Africa and Asia producing over 95% of the world’s supply of sesame. In 2022, the highest producing countries were Sudan, India, Myanmar, the United Republic of Tanzania, and China, accounting for over 60% of global production (Anyogu et al., 2024).
Known as “gingelly” in some regions, S. indicum is referred to as the “queen of oilseed crops” (Biswas et al., 2018) and is traditionally cultivated in the Onattukara region of Kerala, India. This region is particularly famous for the quality of its sesame seeds and oil, grown in uplands and summer rice fallows. Three species of wild sesame—S. malabaricum, S. mulayanum and S. radiatum—are distributed throughout the Onattukara region, suggesting it may be a secondary center of origin for sesame. A preliminary study on the morphological and biochemical traits of sesame collections from this region was attempted which indicates significant variation and considerable genetic diversity. Documenting these characteristics is crucial for the conservation of sesame. The study aims to validate the genetic diversity and assess the therapeutic and agronomic potential of sesame in the Onattukara region.
Materials and methods
The study involved accessions of the widely distributed Sesamum species in the sesame growing tract of Onattukara region. Six accessions of S. radiatum, seven accessions of S. mulayanum, five accessions of S. malabaricum, five accessions of the traditional sesame cultivar Ayali, and four cultivated sesame varieties from the Onattukara region were evaluated in the study. The seeds of these 27 genotypes were sown in a randomized block design with three replications in January 2022 with rows spaced at 30 cm x 30 cm. The experiment was conducted at the Onattukara Regional Agricultural Research Station, Kayamkulam, under the Kerala Agricultural University. The station is located in the Onattukara tract, which lies between 8°55′44″ to 9°21′09″ N latitude and 76°23′13″ to 76°41′16″ E longitude.
Onattukara is a region characterized by a humid tropical climate. The predominant soil type is loamy sand, comprising 83–89% sand, 5% silt, and 5.8% clay, with a field capacity of 16.05%. The soil is porous, acidic in nature, and has low organic matter and nutrient content. Nutrient levels include 0.145% nitrogen, 0.121% phosphorus, 0.0185% potassium, 0.098% calcium, and 0.035% magnesium. Due to its low water-holding capacity, the region faces waterlogging issues during the rainy season and drought conditions during the summer. Temperatures are higher from January to April and lower between June and September. The region experiences a moderate climate, with mean minimum temperatures ranging from 22.0°C to 25.2°C and mean maximum temperatures ranging from 29.0°C to 32.9°C. Relative humidity is higher in the morning and lower in the evening, ranging between 70.0% and 80.1%. Monthly rainfall in the Onattukara region varies widely, from 21.5 cm to 473.2 cm.
Pre-treatment of seeds
Both S. radiatum and S. mulayanum are wild sesame species that exhibit innate dormancy, resulting in poor germination. To overcome this, seeds were pretreated with gibberellic acid (GA3). Specifically, the seeds were soaked in a 300 ppm solution of GA3 for 18 hours, and then they were sown in the field.
Field management
All intercultural operations were conducted according to the Package of Practices recommendations of Kerala Agricultural University (KAU, 2016). Wild cultivars of the same species were planted together, maintaining a spacing of 30 cm x 30 cm, with an alleyway of 60 cm left bare between cultivars from different species. Fertilization was done at the time of land preparation using a 30:15:30 kg/ha NPK ratio. Thinning and earthing-up were performed 15 days after sowing, while weeding was done at 15 and 30 days after sowing (DAS).
Morphological and quality parameter measurements
Biometric data on various morphological parameters were recorded from five randomly selected plants of each genotype, following standard descriptors (IPGRI and NBPGR, 2004. and protocols. Quality parameters relevant to sesame, including oil content, crude protein content, fatty acid profiling, and total phenol content, were analyzed from the seeds as described below:
Oil content (%)
Oil was extracted from sesame seeds using a cold hexane extraction method. A 1-gram sample of dried and ground sesame seeds was placed in a thimble and subjected to hexane extraction in a Soxhlet apparatus for 6 hours. The hexane was then removed under reduced pressure using a rotary evaporator, and the oil content was calculated following the method described by Latif and Anwar (2011).
Crude protein content (%)
Crude protein content was estimated using the Bradford method. Sesame seeds were ground and extracted (1:5, w/v) overnight in 4% sodium bicarbonate at 4°C. The extracts were centrifuged, filtered, and the protein content was measured using serum albumin as a standard (Leduc et al., 2006).
Fatty acid profiling
For fatty acid profiling, 50 mg of sesame oil was placed in 50 ml screw-capped Pyrex tubes (50 cm length, 1 cm internal diameter). To each tube, 2 ml of methanolic sulfuric acid was added, and the tubes were heated in an oil bath at 80°C for 2 hours, with stirring. After cooling, 2 ml of distilled water were added to stop the reaction. The esterified fatty acids were extracted twice with HPLC-grade hexane. The hexane layer was washed with 2% sodium bicarbonate, dried over sodium sulfate, and stored at -20°C before analysis by GC-MS to obtain the fatty acid profile (IUPAC, 1987).
GC-MS analysis of the oil extracted from the seeds was performed using the equipment Shimadzu GC-20101, Kyoto, Japan. The column used was Stabilwax GC Capillary Column with dimensions of 60 mm, ID × 0.25 μm film. The carrier gas used is Helium with at low of 1.0 ml/min. The oven temperature was programmed as follows: 70°C initial temperature, then gradually increased to 140°C at 10°C/min and to 240°C @3°C per min. The identification of components was based on NIST17 libraries.
Total phenol content
One gram of sesame seeds was ground and soaked in methanol, stirred at 200 rpm for 18 hours. The methanolic extract was then analyzed for total phenol content using the Folin-Ciocalteau reagent. Phenols react with phosphomolybdic acid in an alkaline medium to form a blue-colored complex (Meda et al., 2005).
Fatty acid composition and oil stability
The total saturated and unsaturated fatty acid contents were determined directly from the GC chromatogram as percentages of the respective fatty acids. Data from the GC analysis were used to compute the Polyunsaturated Fatty Acid (PUFA) content, Oleic Desaturation Ratio (ODR), and Linoleic Desaturation Ratio (LDR) using the equations from Pleines and Friedt (1988) and Fatemi and Hammond (1980).
Oil composition
The oil composition refers to the ratio of oleic to linoleic acid. A higher oil composition ratio indicates better oxidative stability of the oil, which is a key factor for evaluating the quality and longevity of sesame oil.
Oil stability
The oil stability is indicated by two parameters viz., oleic desaturation ratio (ODR) and linoleic desaturation ratio (LDR). It is calculated as follows:
PUFA
Poly unsaturated fatty acid composition can be calculated as follows:
Statistical analysis
The data were subjected to analysis of variance (ANOVA) and various genetic parameters were worked out using GRAPES software, version 1.1.0 (Gopinath et al., 2020).
Results and discussion
Morphological characters
A comprehensive analysis of qualitative morphological traits, such as plant growth type, exterior corolla color, interior corolla color, lower lip color, capsule hairiness, and seed coat color, revealed considerable variation across 27 genotypes from four different species (Figures 1–5). These characters displayed significant variation between cultivated varieties, the traditional cultivar Ayali, and wild relatives (Table 1).
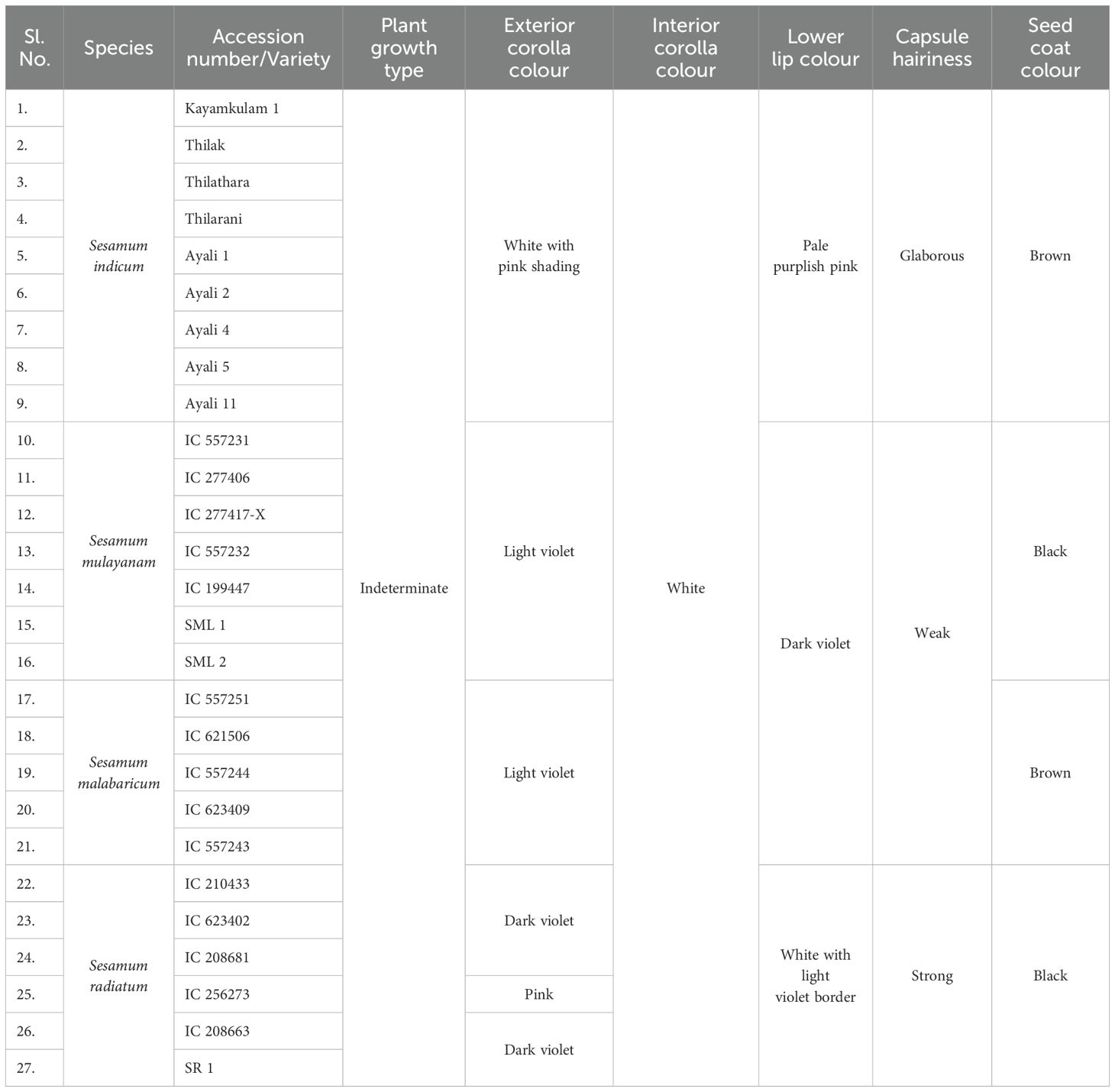
Table 1. Differences between accessions of Sesamum species with respect to morphological characters.
The plant growth type of both cultivated and wild genotypes, including the traditional cultivar Ayali, exhibited a common indeterminate growth habit. All genotypes showed a prolonged flowering stage, non-synchronous capsule ripening, and non-synchronous capsule shattering. This indeterminate growth habit aligns with findings by Kumari and Ganesamurthy (2015) and Tesfaye et al. (2021), who also reported similar growth characteristics in sesame.
The exterior corolla color displayed a wide spectrum, ranging from white to dark violet. Cultivated varieties and the traditional Ayali cultivar had white corollas with pink shading, while the wild varieties exhibited hues from light violet to dark violet, with varying shades within each species. Specifically, S. malabaricum and S. mulayanum showed light violet exterior corolla colors, with some variation in shade. In contrast, S. radiatum stood out with a distinct dark violet exterior corolla color, also showing variation in its shading. This observation is consistent with Kumari and Ganesamurthy (2015), who noted violet shades in wild sesame species and white shades in cultivated varieties. Tesfaye et al. (2021) documented that 6.78% of cultivated sesame in Ethiopia had a white corolla, 70.85% had a white corolla with pink shading, and 22.37% had a white corolla with deep pink shading.
The interior corolla color was uniformly white across all genotypes, with variations only in the degree of pigmentation. Cultivated and traditional varieties exhibited light pigmentation, while wild relatives displayed deeper pigmentation in the interior corolla. These findings align with those of Tesfaye et al. (2021), who reported similar pigmentation patterns in S. indicum.
The lower lip color of the flower varied across species. In S. radiatum, the lower lip was white with a violet border, while in S. malabaricum and S. mulayanum, it appeared as dark purplish-pink. For cultivated and traditional varieties, the lower lip was pale purplish-pink. Regarding capsule hairiness, S. radiatum exhibited profuse hairiness, whereas both S. malabaricum and S. mulayanum had weak capsule hairiness. In contrast, the capsules of both traditional and cultivated varieties were glabrous. These results are consistent with previous studies by Kumari and Ganesamurthy (2015) and Tesfaye et al. (2021), who observed similar trends in capsule hairiness and lower lip color.
Seed coat color varied from brown to black. S. malabaricum, traditional, and cultivated varieties produced brown seeds, while S. radiatum consistently yielded black seeds. Kumari and Ganesamurthy (2015) reported dull black seeds for S. malabaricum, bright black seeds for S. radiatum, and seeds with varying colors for S. indicum.
Biometric characters
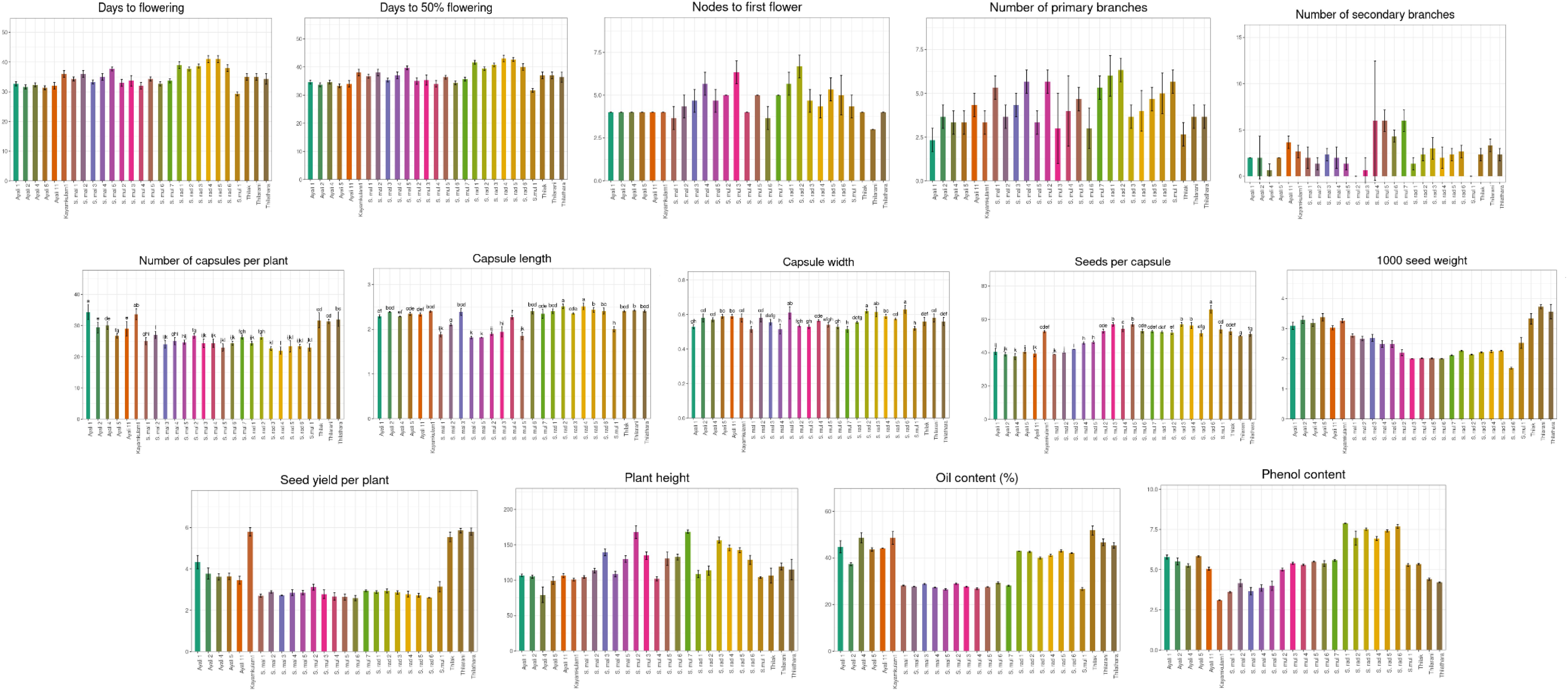
The biometric parameters of various Sesamum accessions were recorded, and their mean values are presented in Table 2. All traits exhibited substantial variation among the genotypes, indicating a broad genetic base (Figure 6).
Among the four sesame species studied, late flower initiation was observed in S. radiatum accessions IC 256273 and IC 208663, with flowering occurring at 41 days after sowing. Dansi et al. (2012) recorded flowering times of 56.75 and 46.33 days for S. radiatum accessions in their study. In contrast, early flowering was observed in S. mulayanum accession IC 557231, which flowered at 29 days. The average number of days to 50% flowering for the 27 genotypes was 36.85, closely aligning with Kalaiyarasi et al. (2019), who recorded an average of 35.40 days to 50% flowering. The latest 50% flowering occurred in S. radiatum accession IC 256273 at 43 days, while S. mulayanum accession IC 557231 reached 50% flowering at 31.67 days. The observed variation in flowering times is likely due to genetic diversity among the accessions, which belong to different species. Similar variations in flowering times have been reported by Hukumchand and Parameshwarappa (2020); Teklu et al. (2021); Kumar et al. (2022), and Gedifew et al. (2023). Within-species variation may be attributed to both environmental factors and genetic differences among accessions.
All 27 sesame genotypes produced a single flower per axil, indicating that this trait is universal across all sesame species. The average number of nodes to first flower across the 27 genotypes was 4.56. The first flower was located farthest from the base in S. radiatum accession IC 623402, while it was closest to the base in the traditional cultivar Thilarani. The variation in the number of nodes to first flower is likely a result of genetic differences among the species.
The number of primary branches varied widely among the 27 genotypes, with an average of 4.21 branches. The maximum number of primary branches was observed in S. radiatum accession IC 623402, while the fewest primary branches were produced by the traditional cultivar Ayali 1. Other accessions, including S. radiatum (IC 210433), S. mulayanum (IC 557231, IC 277406, SML 2), and S. malabaricum (IC 557251, IC 623409), had a comparable number of primary branches to IC 623402. In contrast, Thilak, Ayali 5, Ayali 4, Kayamkulam 1, S. malabaricum (IC 557243), and S. mulayanum (IC 277417-X, SML 1) were on par with Ayali 1. Secondary branch numbers were higher in S. mulayanum (IC 557232, IC 199447, SML 2), while IC 557231 and IC 277406 (S. mulayanum) did not produce secondary branches. The variation in the number of branches can be attributed to genetic differences between genotypes and environmental influences. Teklu et al. (2021) reported a mean of 4 primary branches and 2 secondary branches, and Rahna et al. (2023) found minimal environmental impact on branch numbers in sesame.
S. mulayanum accessions were the tallest among the four sesame species, with Ayali 4 being the shortest. The variation in plant height can be attributed to both genetic and environmental influences, in line with findings by Dansi et al. (2012); Kalaiyarasi et al. (2019), and Sultana et al. (2019).
The highest number of capsules per plant was observed in Ayali 1, while the lowest number was observed in S. radiatum accession IC 256273. S. mulayanum (IC 557231, IC 199447), S. radiatum (IC 208681, IC 208663, and SR 1), all produced similar numbers of capsules to IC 256273 of S. radiatum. The significant variation in capsule production is likely due to genotypic differences, as similarly noted by Sultana et al. (2019); Kumar et al. (2022), and Akkiligunta et al. (2024). All 27 genotypes exhibited four locules per capsule, a trait consistent across all sesame species.
In terms of capsule morphology, S. radiatum accessions produced the longest and broadest capsules, while S. malabaricum accessions had the shortest and narrowest capsules. Some S. mulayanum accessions were comparable to S. malabaricum in capsule dimensions. The variation in capsule length and width is mainly attributed to genetic differences. Dansi et al. (2012) observed capsule length ranging from 2.74 to 3.30 cm and width from 0.7 to 0.8 cm among S. radiatum genotypes. Hukumchand and Parameshwarappa (2020) reported capsule length variation between 1.74 and 2.93 cm, and Sultana et al. (2019) found variation between 2.3 and 2.81 cm among different sesame genotypes.
Seeds per capsule averaged 49.36, with the most seeds found in S. radiatum accession SR 1, and the least number in Ayali 4. This variation is largely due to genetic differences among genotypes, as confirmed by Saravanan et al. (2020); Manjeet et al. (2020), and Teklu et al. (2021). The heaviest seeds were observed in the cultivated variety Thilarani, while the lightest seeds were found in S. radiatum accessions. This variation in 1000-seed weight can be attributed to genetic differences. Overall, cultivated varieties generally had heavier and larger seeds compared to wild species. Similar variations in 1000-seed weight have been reported by Umamaheswari et al. (2019); Hukumchand and Parameshwarappa (2020); Kumar et al. (2022), and Mahla et al. (2024). Thilarani exhibited the highest yield per plant, while S. mulayanum accession SML 1 produced the lowest yield. The variation in seed yield per plant is due to both genetic and environmental factors, as reported by Saravanan et al. (2020); Manjeet et al. (2020) and Kumar et al. (2022).
Biochemical characters
Biochemical parameters, namely, oil content, phenolic compounds, protein levels, and fatty acid profiles were analyzed across the different species and are presented in Tables 2–4.
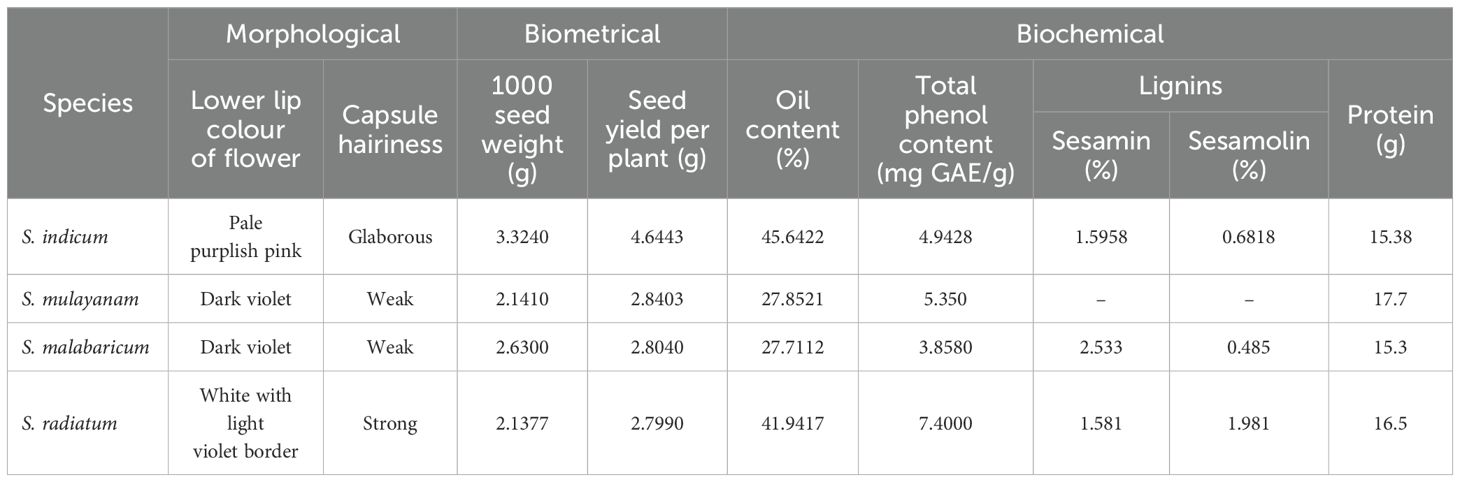
The oil content across the 27 sesame genotypes averaged 36.89%. The highest oil yield was recorded for Thilak, while the lowest was found in S. malabaricum (IC 557243). These findings are consistent with the results of Valarmathi et al. (2003); Hiremath et al. (2007) and Akhila and Beevy (2015). Oil yield in S. malabaricum (IC 621506, IC 623409) and S. mulayanum (IC 557231, IC 277417-X, IC 557232, IC 199447) was similar to IC 557243 of S. malabaricum.
Phenolic compounds, such as flavonoids, phenolic acids, and tannins, play a crucial role in stabilizing lipid oxidation and contribute to antioxidant activity. The mean phenol content across the 27 genotypes was 5.39 mg GAE/g. S. radiatum (IC 210433) had the highest phenol content, while the lowest was found in Kayamkulam 1. The high phenol content in S. radiatum aligns with findings by Pathak et al. (2020) and Oduntan et al. (2011). In contrast, Akhila and Beevy (2015) reported higher phenol content in S. indicum oils. The phenol content exhibited minimal environmental variation, as evidenced by the small difference between PCV (24.527) and GCV (24.408), along with high heritability (99%) and genetic advance (50.04%).
Fatty acid profiling
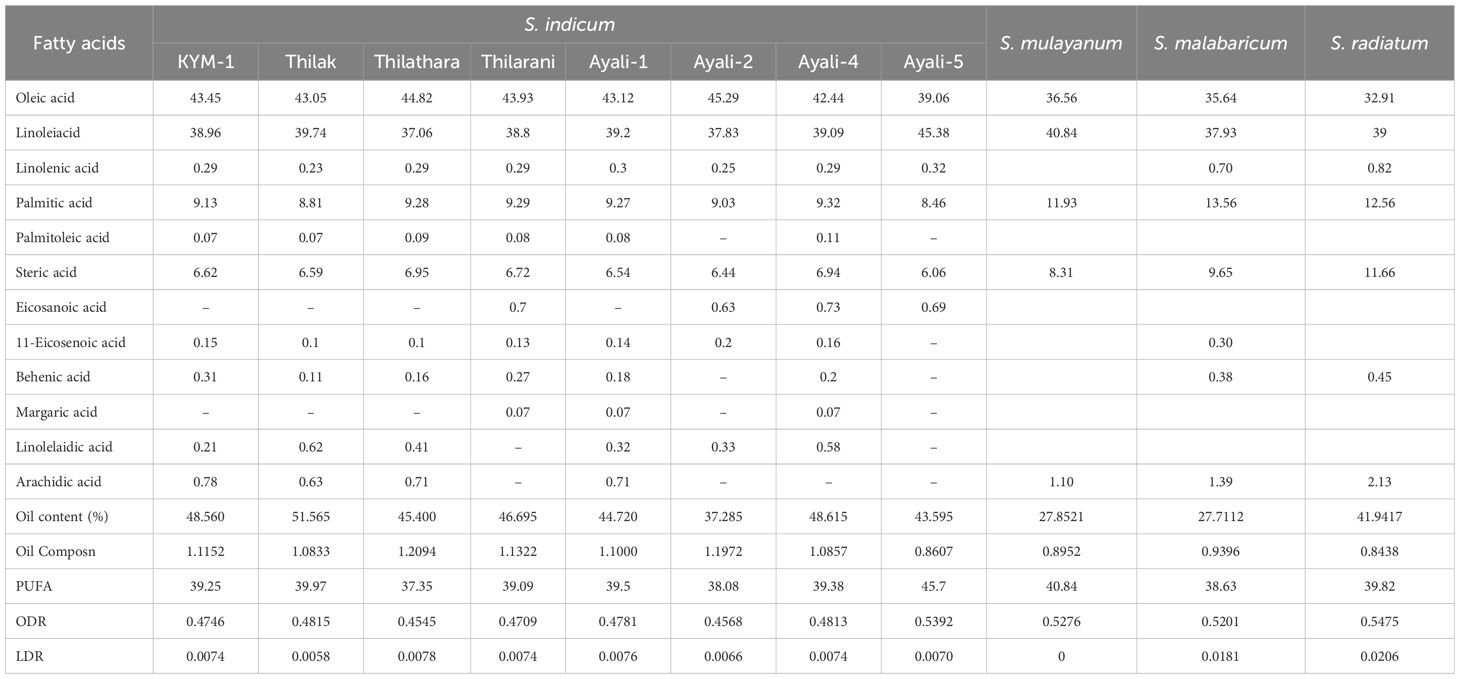
Fatty acids, major components of plant, animal, and microbial lipids, play a crucial role in oil quality. Oleic and linoleic acids are the predominant fatty acids in sesame oil, comprising approximately 80% of the total oil content. Studies on fatty acid composition in various germplasm collections have revealed significant variation in the proportions of saturated and unsaturated fatty acids, which could potentially lead to the development of higher-quality edible oils.
The fatty acid profiles of four cultivated varieties (KYM 1, Thilak, Thilathara, Thilarani), four traditional varieties (Ayali 1, Ayali 2, Ayali 4, Ayali 5), and wild relatives (S. malabaricum, S. mulayanum, and S. radiatum) were examined. The analysis revealed the presence of both saturated fatty acids (e.g., palmitic acid, stearic acid, behenic acid, margaric acid, and arachidic acid) and unsaturated fatty acids (e.g., oleic acid, linoleic acid, linolenic acid, eicosanoic acid, 11-eicosenoic acid, and linolelaidic acid) in varying proportions across the seed oils of the sesame samples.
Oleic acid, the primary unsaturated fatty acid in sesame oil, was found in the highest amounts in Ayali 2, a traditional cultivar from the Onattukara region. Wild sesame relatives exhibited lower oleic acid content, with S. radiatum showing the least amount. These results are consistent with Mondal et al. (2010) and Hiremath et al. (2007). Linoleic acid content was highest in Ayali 5 (45.38%), while Thilathara exhibited the least. Sesame cultivars with higher linoleic acid content, such as Ayali 5, could be valuable for breeding programs aimed at enhancing linoleic acid content in sesame. S. radiatum had the highest linolenic acid content, while S. indicum showed the lowest.
S. malabaricum (13.56%) exhibited the highest palmitic acid content, while Thilak had the lowest. Mondal et al. (2010) reported different results in terms of palmitic acid content. Palmitoleic acid was absent in the oil of wild sesame species, while Ayali 4 contained relatively higher amounts (0.11%). Kayamkulam 1 and Thilak showed the lowest levels (0.07%) of palmitoleic acid. A high 11.66% of stearic acid was found in S. radiatum oil, while Ayali 5 exhibited the lowest (6.06%). These findings align with Hiremath et al. (2007).
Eicosanoic acid was found only in Thilak among the cultivated varieties, while it was more prevalent in the traditional cultivar Ayali, particularly Ayali 4 (0.73%). 11-Eicosenoic acid was predominantly found in S. malabaricum (0.30%), while minimal amounts were found in both Thilak and Thilathara. S. radiatum contained the highest levels of linolelaidic acid, particularly in IC 256273. These findings contribute to the understanding of sesame fatty acid composition and offer valuable insights for sesame breeding programs focused on improving oil quality.
Table 4 presents the mean values of morphological and biochemical traits in Sesamum species, highlighting key differences that can be used to effectively distinguish among them. These variations serve as important diagnostic features for species identification and have potential implications for breeding and conservation strategies.
Conclusion
The analysis of variance performed on various morphological and biochemical parameters of S. indicum, S. malabaricum, S. mulayanum, and S. radiatum revealed significant differences, indicating substantial variability among the genotypes for the traits studied. All 27 sesame genotypes produced a single flower per axil, demonstrating that this characteristic is consistent across all sesame species. A comparative analysis of the different sesame species highlighted notable variations in the color of the lower lip of the flowers. The cultivated species S. indicum displayed pale purplish-pink flowers, while S. mulayanum and S. malabaricum had dark violet flowers, and S. radiatum exhibited white flowers with a violet border. Differences in capsule hairiness were also observed: cultivated species had glabrous capsules, while wild species produced hairy capsules, ranging from weakly hairy in S. mulayanum and S. malabaricum to strongly hairy in S. radiatum.
In terms of capsule production, the highest number of capsules per plant was found in Ayali 1, while S. radiatum (IC 256273) produced the fewest. S. radiatum accessions had the longest and broadest capsules, while S. malabaricum accessions produced the shortest and narrowest capsules. The cultivated varieties had heavier and larger seeds compared to the wild species.
Oil content analysis revealed the highest oil yield in Thilak seeds, while S. malabaricum (IC 557243) had the lowest oil yield. Fatty acid profiling of the four cultivated varieties and the wild relatives revealed the presence of both saturated fatty acids (palmitic, stearic, behenic, margaric, and arachidic acids) and unsaturated fatty acids (oleic, linoleic, linolenic, eicosanoic, 11-eicosenoic, and linolelaidic acids) in varying proportions. The highest phenol content was observed in S. radiatum (IC 210433), with the lowest in Kayamkulam 1. Lignin analysis revealed that sesamin content was highest in S. malabaricum (2.533), while sesamolin content was highest in S. radiatum (1.981). In S. indicum and S. malabaricum, sesamin constituted the major portion of lignin, whereas in S. radiatum, sesamolin content slightly exceeded that of sesamin.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
VV: Formal analysis, Methodology, Writing – original draft. LB: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AK: Conceptualization, Investigation, Supervision, Writing – review & editing. AJ: Formal analysis, Supervision, Writing – review & editing. PG: Data curation, Software, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The first author expresses sincere gratitude to Kerala Agricultural University for providing the Junior Research Fellowship for the research program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors would like to extend their thanks to ChatGPT, an AI language model developed by OpenAI, for its assistance in editing and refining the manuscript. The model’s support in improving the clarity, grammar, and overall structure of the paper was highly appreciated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1571363/full#supplementary-material
References
Adéoti, K., Dansi, A., Ahoton, L., Vodouhe, R. S., Ahohuendo, B. C., Rival, A., et al. (2012). Agromorphological characterization of Sesamum radiatum (Schum. and Thonn.), a neglected and underutilized species of traditional leafy vegetable of great importance in Benin. Afr. J. Agric. Res. 7, 3569–3578. doi: 10.5897/AJAR11.1845
Akhila, H. and Beevy, S. S. (2015). Quantification of seed oil and evaluation of antioxidant properties in the wild and cultivated species of Sesamum L.(Pedaliaceae). Int. J. Pharm. Pharm. Sci. 7, 136–142. Available online at: https://www.europub.co.uk/articles/-A-578419.
Akkiligunta, P., Jawaharlal Jatothu, Usha Kiran., B., and Sameer Kumar, C. V. (2024). Unveiling the sesame germplasm- a study of genetic variability for yield (Sesamum indicum. L). J. Exp. Agric. Int. 46, 693–699. doi: 10.9734/jeai/2024/v46i82752
Anyogu, A., Somorin, Y., Oladipo, A., and Raheem, S. (2024). Food safety issues associated with sesame seed value chains: Current status and future perspectives. Heliyon 10, (2024) e36347. doi: 10.1016/j.heliyon.2024.e36347, PMID: 39253262
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Bedigian, D. (2014). A new combination for the Indian progenitor of sesame, Sesamum indicum (Pedaliaceae). Novon 23, 5–13. doi: 10.3417/2012062
Biswas, A. K. and Mitra, A. K. (1990). Interspecific hybridization in three species of Sesamum. Indian J. Genet. Plant Breed. 50, 307–309. doi: 10.1007/s12041-021-01335-w
Biswas, S., Natta, S., Ray, D. P., Mondal, P., and Saha, U. (2018). Til (Sesamum indicum L.) - An underexploited but promising oilseed with multifarious applications: A review. Int. J. Biores. Sci. 5, 127–139. doi: 10.30954/2347-9655.02.2018.8
Borchani, C., Besbes, S., Blecker, C. H., and Attia, H. (2010). Chemical characteristics and oxidative stability of sesame seed, sesame paste, and olive oils. J. Agric. Sci. Technol. 12, 585–596. Available online at: https://sid.ir/paper/590205/en.
Dansi, A., Etèka, C. A., Adéoti, K., Orkwor, G. C., Ahohuendo, B. C., Loko, Y. L., et al. (2012). Black benniseed (Sesamum radiatum Schum. et Thonn.) cultivated as leafy vegetable in Benin. Genet. Resour. Crop Evol. 59, 955–964. doi: 10.1007/s10722-012-9816-8
Fatemi, S. H. and Hammond, E. G. (1980). Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 15, 379–385. doi: 10.1007/BF02533555
Gedifew, S., Abate, A., and Abebe, T. (2023). Genetic variability in sesame (Sesamum indicum L.) for yield and yield related traits. Harran Tarım ve Gıda Bilimleri Dergisi 27, 153165. doi: 10.29050/harranziraat.1251060
Gopinath, P. P., Parsad, R., Joseph, B., and Adarsh, V. S. (2020). GRAPES: General Rshiny based analysis platform empowered by statistics. Available online at: https://www.kaugrapes.com/home (Accessed August 28, 2021).
Hiremath, S. C., Patil, C. G., Patil, K. B., and Nagasampige, M. H. (2007). Genetic diversity of seed lipid content and fatty acid composition in some species of Sesamum L.(Pedaliaceae). Afr. J. Biotechnol. 6, 539–543. doi: 10.5897/AJB2007.000-2044
Hukumchand and Parameshwarappa, S. G. (2020). Genetically potential sesame (Sesamum indicum L.) breeding lines. J. Farm Sci. 33, 302–305. doi: 10.61475/jfm.v33i03.224
IPGRI and NBPGR (2004). Descriptors for Sesame (Sesamum spp.) (Rome, Italy: International Plant Genetic Resources Institute). National Bureau of Plant Genetic Resources, New Delhi, India.
IUPAC (International Union of Pure and Applied Chemistry) (1987). “Standard methods for the analysis of oils, fats and derivatives,” in International Union of Pure and Applied Chemistry Division Commission on Oils, Fats and Derivatives (7th Ed.) (Blackwell Jevent Publishers, Oxford).
John, C. M. and Rao, U. N. (1941). Chromosome number of Sesamum radiatum, Schum and Thonn. Beskr. Curr. Sci. 10, 364–365.
Kalaiyarasi, R., Venmuhil, R., Malaiarasi, M., Dinesh, S., and Abinaya, N. (2019). Genetic variability, heritability and genetic advance in sesame (Sesamum indicum L.) Genotypes. Int. J. Curr. Microbiol. App. Sci. 8, 1976–1983. doi: 10.20546/ijcmas.2019.807.235
KAU (Kerala Agricultural University) (2016). Package of Practices Recommendations: Crops. 14th Ed (Thrissur: Kerala Agricultural University), 360p.
Kumar, V., Sinha, S., Sinha, S., Singh, R. S., and Singh, S. N. (2022). Assessment of genetic variability, correlation and path analysis in sesame (Sesamum indicum L.). Electron. J. Plant Breed. 13, 208–215. doi: 10.37992/2022.1301.029
Kumari, B. M. and Ganesamurthy, K. (2015). Study of reproductive compatibility and morphological characterization of interspecific hybrids in Sesamum sp. Afr. J. Agric. Res. 10, 911–918. doi: 10.5897/AJAR2014.8592
Latif, S. and Anwar, F. (2011). Aqueous enzymatic sesame oil and protein extraction. Food Chem. 125, 679–684. doi: 10.1016/j.foodchem.2010.09.064
Leduc, V., Moneret-Vautrin, D. A., Tzen, J. T. C., Morisset, M., Guerin, L., and Kanny, G. (2006). Identification of oleosins as major allergens in sesame seed allergic patients. Allergy 61, 349–356. doi: 10.1111/j.1398-9995.2006.01013.x, PMID: 16436145
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Mahla, N. U., Jagtap, P. K., and Patel., H. R. (2024). Genetic variability and association among yield and yield related traits of sesame (Sesamum indicum L.) genotype. Int. J. Plant Sci. 36, 197–206. doi: 10.9734/ijpss/2024/v36i24381
Manjeet, P. K., Sheoran, R. K., Nain, M., and Avtar, R. (2020). Evaluation of sesame (Sesamum indicum L.) genotypes for genetic variability based on different traits under rainfed conditions. Electron. J. Plant Breed. 11, 54–59. doi: 10.37992/2020.1101.009
Meda, A., Lamien, C. E., Romito, M., Millogo, J., and Nacoulma, O. G. (2005). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 91, 571–577. doi: 10.1016/j.foodchem.2004.10.006
Mondal, N., Bhat, K. V., and Srivastava, P. S. (2010). Variation in fatty acid composition in Indian germplasm of sesame. J. Am. Oil Chem. Soc 87, 1263–1269. doi: 10.1007/s11746-010-1615-9
Nimmakayala, P., Perumal, R., Mulpuri, S., and Reddy, U. K. (2011). “Sesamum,” in Wild crop relatives: genomic and breeding resources, vol Oilseeds. Ed. Kole, C. (Springer, Berlin, Heidelberg), 261–273.
Oduntan, A. O., Akinwande, B. A., and Olaleye, O. (2011). Effect of plant maturity on the antioxidant properties, total phenolic and mineral contents of Sesamum radiatum leaves. Afr. J. Food Sci. 5, 914–920. doi: 10.5897/AJFS11.182
Pathak, N., Verma, N., Singh, A., Bhat, K. V., and Lakhanpaul, S. (2020). Investigations on diverse sesame (S. indicum L.) germplasm and its wild allies reveal wide variation in antioxidant potential. Physiol. Mol. Biol. Plants. 26, 697–704. doi: 10.1007/s12298-020-00784-4, PMID: 32255933
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Pleines, S. and Friedt, W. (1988). Breeding for improved C 18-fatty acid composition in rapeseed (Brassica napus L.). Lipid/Fett 90, 167–171. doi: 10.1002/lipi.19880900502
Rahna, K., Kalaiyarasi, R., Umadevi, M., Senthil, A., and Sudha, M. (2023). Genetic variability and diversity analysis in sesame Sesamum indicum L. Germplasm. Electron. J. Plant Breed. 14, 1055–1062. doi: 10.37992/2023.1403.118
Ranganatha, A. R. G., Jyotishi, A., Deshmukh, M. R., Bisen, R., Panday, A. K., and Gupta, K. N. (2010). Improved Technology for Maximizing Production of Sesame (Jabalpur: Project Coordinator, AICRP on Sesame & Niger, ICAR, JNKVV Campus), 1–17.
Salunke, J. D., Rathod, G. M., and Kale, S. S. (2023). Determination and comparison of phytochemical in Sesamum laciniatum Klein ex Wild by using FTIR spectroscopy. J. Pharmacogn. Phytochem. 12, 301–305. doi: 10.22271/phyto.2023.v12.i6d.14794
Saravanan, M., Kalaiyarasi, R., and Viswanathan, P. L. (2020). Assessment of genetic variability, character association and path analysis in F2 population of sesame (Sesamum indicum L.). Electron. J. Plant Breed. 11, 447–450. doi: 10.37992/2020.1102.077
Saravanan, S. and Nadarajan, N. (2005). Pathogenicity of mycoplasma like organism of sesame (Sesamum indicum L.) and its wild relatives. Agric. Sci. Digest. 25, 77–78. doi: 10.13140/RG.2.2.32350.04162
Shamloo, M. B. B., Nasiri, M., Maneiy, M., Dorchin, M., Mojab, F., Bahrami, H., et al. (2019). Effects of topical sesame (Sesamum indicum) oil on the pain severity of chemotherapy-induced phlebitis in patients with colorectal cancer: A randomized controlled trial. Complement. Ther. Clin. Pract. 35, 78–85. doi: 10.1016/j.ctcp.2019.01.016, PMID: 31003690
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Sultana, S., Mahmud, F., and Rahim, M. A. (2019). Genetic variability studies for selection of elite germplasm in sesame (Sesamum indicum L.). Agronomski glasnik: Glasilo Hrvatskog agronomskog društva. 81, 87–104. doi: 10.33128/ag.81.2.3
Tanesaka, E., Umeda, E., Yamamoto, M., Masuda, K., and Yamada, K. (2012). Inheritance mode of seed dormancy in the hybrid progeny of sesame, Sesamum indicum, and its wild relative. Sesamum mulayanum Nair. Weed Biol. Manage. 12, 91–97. doi: 10.1111/j.1445-6664.2012.00438.x
Tarihal, R., Sridevi, O., Shenoyand, V. V., and Salimath, P. M. (2003). Study of fertilization barriers in crosses between Sesamum indicum and its wild relatives. Indian J. Genet. Plant Breed. 63, 132–136.
Teklu, D. H., Shimelis, H., Tesfaye, A., and Mashilo, J. (2021). Genetic diversity and association of yield-related traits in sesame. Plant Breed. 140, 331–341. doi: 10.1111/pbr.v140.2
Tesfaye, T., Tesfaye, K., Keneni, G., and Alemu, T. (2021). Morphological characteristics and genetic diversity of Ethiopian sesame genotypes. Afr. Crop Sci. J. 29, 59–76. doi: 10.4314/acsj.v29i1.5
Umamaheswari, S., Suganthi, S., Sathiskumar, P., and Kamaraj, A. (2019). Genetic variability, correlation and path analysis in sesame (Sesamum indicum L.). Plant Arch. 19, 4543–4548. doi: 10.51470/PLANTARCHIVES.2025.v25.supplement-1.118
Valarmathi, G., Surendran, C., Vanniarajan, C., Kumar, M., and Saravanan, N. A. (2003). Morphological and biochemical characterization of sesame (Sesamum indicum L. and S. mulayanum L). Sesame Safflower Newsl. 18, 42–46. doi: 10.22103/gpb.2024.23161.1007
Keywords: Sesamum indicum, Sesamum radiatum, Sesamum mulayanum, Sesamum malabaricum, morphology, biochemical, phenol content
Citation: V. P. VG, B. L, K. A, Jayapal A and Gopinath PP (2025) Integrated morphological and biochemical analysis of selected sesame (Sesamum spp.) species. Front. Plant Sci. 16:1571363. doi: 10.3389/fpls.2025.1571363
Received: 06 February 2025; Accepted: 29 May 2025;
Published: 10 July 2025.
Edited by:
Eman. A. Mahmoud, Damietta University, EgyptReviewed by:
Senouwa Segla Koffi Dossou, Chinese Academy of Agricultural Sciences, ChinaAntim Maurya, University of Mississippi, United States
Copyright © 2025 V. P., B., K., Jayapal and Gopinath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lovely B., bG92ZWx5LmJAa2F1Lmlu
 Varadha Gayathri V. P.1
Varadha Gayathri V. P.1 Lovely B.
Lovely B.