- 1Basic Medical Research Centre, School of Medicine, Nantong University, Nantong, Jiangsu, China
- 2Key Laboratory of Microbial Resources Exploitation and Application, Institute of Biology, Gansu Academy of Sciences, Lanzhou, Gansu, China
Introduction: The exogenous application of phytohormones is a widely adopted approach to enhance crop productivity. However, the precise regulatory effects of different phytohormones on plant antioxidant systems, rhizosphere microbial subcommunities (abundant, moderate, and rare), and their interactions with plant growth remain poorly understood.
Methods: This study explored the effects of melatonin (MT), strigolactone (SL), and 24-epibrassinolide (BR) on the phenotypic traits, physiological properties, and rhizosphere microbial subcommunities of Paris polyphylla var. chinensis (Franch.) Hara (PPC) through controlled pot experiments.
Results: Our study demonstrated that MT and SL significantly increased PPC biomass by 69.32% and 15.23%, respectively, whereas BR at 2 mg/L inhibited root development. MT and SL may influence the growth of PPC by modulating the antioxidant system. In addition, phytohormone treatments distinctly altered the structure of soil microbial subcommunities, with partial least squares path modeling (PLS-PM) revealing that MT exerted a dominant influence on PPC biomass by regulating the structure of abundant bacterial subcommunities. Furthermore, linear discriminant analysis effect size identified key microbial taxa associated with the application of phytohormones, further substantiating their roles in biomass enhancement.
Conclusion: These findings provide significant insights into the ecological management of phytohormones for sustainable agricultural practices.
1 Introduction
Paris polyphylla var. chinensis (Franch.) Hara (PPC), a perennial flowering herb in the Melanthiaceae family (formerly classified under Liliaceae), is endemic to China and listed on the International Union for Conservation of Nature’s (IUCN) Red List (Wang et al., 2019). The rhizome of PPC is widely used in traditional Chinese medicine for treating fractures, sore throats, and snake bites (Commission, 2020). Modern pharmacological studies have identified steroid saponins as the primary active component, exhibiting anti-tumor, anti-inflammatory, and hemostatic properties (Guan et al., 2024). The market demand for this species is substantial. PPC typically grows in forest understories, or rocky slopes, where its growth is frequently challenged by climatic variability. The early seedling stage of PPC is particularly critical for establishing competitive growth, as robust development during this phase enhances subsequent yield (Carrera-Castaño et al., 2020). However, this stage is highly sensitive to environmental stresses, often resulting in poor seedling development under natural conditions, which significantly limits its yield potential.
Phytohormones are a class of small-molecule compounds synthesized and perceived by plants, with exogenous application proven to enhance crop adaptability and consequently improve yield under abiotic stress conditions (Nakano et al., 2022). In recent years, brassinosteroids (BR), strigolactones (SL), and melatonin (MT) have been extensively studied and applied due to their significant roles in plant growth and development. These hormones are widely involved in various aspects of plant growth, including seedling development, bud branching, stem elongation, and lateral root formation (Gao et al., 2022; Zhang et al., 2025; Yin et al., 2025). Typically, these hormones act as growth signals or nutrient sources, directly influencing plant growth and development (Ali et al., 2024). On the other hand, they can also indirectly promote plant growth by enhancing antioxidant enzyme activities (Singh et al., 2024) and regulating rhizosphere microbial communities (Xiao et al., 2022a). For instance, SLs have been shown to enhance the activities of superoxide dismutase (SOD) and catalase (CAT) in Capsicum chinense, and to alleviate drought-induced oxidative damage by reducing the accumulation of hydrogen peroxide (H2O2) by 24-57% and malondialdehyde (MDA) by 79-89% (Shu et al., 2024). Similarly, MT and BRs have been reported to enhance the activities of antioxidant enzymes such as SOD and CAT, thereby mitigating reactive oxygen species (ROS) bursts and maintaining redox homeostasis during the active growth phase of plants (Jiang et al., 2025; Liang et al., 2025). The effects of BRs, SLs, and MT on rhizosphere microbial communities often exhibit distinct specificities. For example, SLs primarily regulate rhizosphere microbial communities by promoting symbiosis with arbuscular mycorrhizal fungi (AMF) (Wang et al., 2024), enhancing phosphorus uptake (Bradley et al., 2024), and influencing root exudation profiles. A study by Xiaoli Zhang demonstrated that in the rhizosphere of kiwifruit (Actinidia chinensis), 14-hydroxybrassinosteroid (14-HBR) significantly enriched Dadabacteria and Acidobacteria, whereas MT notably enriched Rokubacteriales (Zhang et al., 2024). Therefore, a comprehensive understanding of the individual effects of different phytohormones on the antioxidant system and rhizosphere soil microbial communities is crucial for elucidating their potential mechanisms regulating crop yield.
Microbial communities are unevenly distributed, comprising high-abundance taxa and numerous low-abundance taxa, each with distinct characteristics and functions (Jiao and Lu, 2020). High-abundance taxa occupy broader ecological niches, exhibit functional redundancy, and provide stability to microbial communities under environmental changes. In contrast, rare taxa play critical roles in soil fertility (Yang et al., 2022b), organic matter decomposition (Sauret et al., 2014), and maintaining community diversity (Mikkelson et al., 2016). These diverse functional roles suggest that abundant and rare taxa may exhibit distinct responses to phytohormone treatments, forming intricate microbial networks that influence plant growth. However, the effects of MT, SL, and BR on the composition of different rhizosphere microbial subcommunities and their potential contributions to plant development remain largely unexplored. Therefore, investigating PPC rhizosphere microbial communities under varying phytohormone treatments should account for the distinct dynamics among abundant, moderate, and rare microbial subcommunities.
In this study, a pot experiment was conducted with the following objectives: (1) to investigate the effects of exogenous application of MT, SL, and BR on the morphological parameters of PPC. (2) to examine the responses of the plant antioxidant system and the rhizosphere soil fungal and bacterial subcommunities to the three phytohormones. (3) to explore the potential contributions of the antioxidant system and microbial subcommunities to PPC biomass accumulation.
2 Materials and methods
2.1 Plant materials, soil preparation, and growing conditions
The study was carried out at Biological Research Institute of Gansu Academy of Sciences and Heping testing base, Longnan (36.008 N, 103.970 E), Gansu, China. The experimental site is in the eastern monsoon region, and has a cold, semi-humid, and rainy climate, with an altitude of 1700 m. The growing season of PPC seedlings is from December 2018 to October 2019. The soil is classified as Calcaric Cambisol according to the Food and Agricultural Organization (FAO) classification system. The initial physicochemical characteristics of the soil were: pH = 7.84, organic matter = 1.19 g/kg, TN = 0.63 g/kg and available K = 3.42 mg/kg. During the process of collecting the soil, large particles of impurities, including plant litter, plant roots, and gravel, were removed. The soil was thoroughly mixed before being filled into the flower pots.
The PPC seeds were collected from Guanshan, Huating City, Gansu Province, China, at an elevation of 2000 meters (35°200 N, 106°396 E). Fresh seeds were subjected to cold sand storage at 4°C for three months, followed by dehusking and disinfection through immersion in 5% NaClO for 10 minutes. Subsequently, the seeds were rinsed five times with sterile distilled water and dried using sterile absorbent paper before being transplanted into plastic pots (17.6 cm × 26.5 cm × 23 cm). The pre-germination treatment of seeds is conducted under moist and cool conditions (with a 70% shade net) to ensure rapid and uniform germination after sowing. Following germination, the seedlings are grown under natural temperature conditions and managed according to standard practices.
Melatonin (purity ≥ 98%) and brassinosteroid (24-epibrassinolide, purity ≥ 90%) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and strigolactone (GR24, purity ≥ 97%) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China).
2.2 Experimental design and sample collection
This study included four treatment groups: individual inoculation with MT, SL, and BR, along with a control (CK) treated with an equal volume of sterile distilled water. The experiment was conducted using a randomized block design with three replicates per treatment, maintaining three seedlings per pot. The concentrations of MT, SL, and BR were standardized based on previous studies (Park et al., 2021; He et al., 2023b; Dong et al., 2024; Ahsan et al., 2023; Atteya et al., 2022; Sun et al., 2020), with each phytohormone applied at 2 mg/L. For each application, 15 mL per pot of the respective solution was evenly dispensed onto the potting soil surface around the stem base to target the rhizosphere. Treatments were administered once a week for five consecutive weeks, starting from the 8th week after seedling establishment.
Soil and seeding were sampled in October 10th, 2019. The seedlings were taken out of the soil and shaken gently, and the fallen soil was collected as rhizosphere soil. The collected soils and seedings were kept in -80 °C and 4 °C refrigerators for soil microbial genomic DNA extraction, chemical property analysis and plant physiological indices, respectively.
2.3 Measurement of morphological, physiological indices and hormone content of PPC
After hormone treatment, 3 pots were randomly selected from each treatment group and the root length, shoot length, and root fresh weight were measured. Plant samples were placed in an oven at 105°C for 30 min to inactivate the enzymes, and then dried at 70°C to a constant weight and the biomass was recorded. All measurements were calculated as the average per pot, based on three plants per pot.
The samples of mixed plant (1.0 g) were transferred to a plastic centrifuge tube containing 18 mL of precooled 10 mM phosphate buffered saline (PBS) (130 mM NaCl, 7 mM Na2HPO4, and 3 mM NaH2PO4; pH 7.4). The plants were subsequently homogenized using a homogenizer. The homogenized sample was centrifuged (3000 rpm/min) for 20 min at 4°C, following which the supernatant was collected and stored at 4°C for measuring the physiological indices. The activities of SOD, CAT, malondialdehyde (MDA), and H2O2 were measured following the method of Wang and Huang (Wang and Huang, 2015). MDA content was measured at 532 nm after reacting with thiobarbituric acid (TBA). H2O2 concentration was determined at 390 nm using potassium iodide. SOD activity was assessed at 560 nm via nitroblue tetrazolium (NBT) reduction, and CAT activity was quantified at 240 nm by monitoring H2O2 decomposition. Measurements were performed in triplicate.
The contents of SA, MT, SL, and BR in plants were determined using plant ELISA kits (JM-09813P1, JM-01089P1, JM-09830P1, and JM-01090P1; Shenzhen Zikerbio Biotechnology Co., Ltd., Shenzhen, China) following the manufacturer’s instructions. Briefly, the assay was based on a double-antibody sandwich ELISA. Purified plant hormone-specific antibodies were pre-coated onto microplates as solid-phase antibodies. Plant samples containing SA, MT, SL, or BR were sequentially added to the wells and bound to the immobilized antibodies, followed by binding with horseradish peroxidase (HRP)-labeled antibodies to form an antibody-antigen-enzyme complex. After thorough washing, substrate solution (TMB) was added for color development, and absorbance was measured at 450 nm using a microplate reader.
2.4 Soil microbial DNA extraction and sequencing
Soil DNA was extracted using a DNeasy PowerSoil DNA Extraction Kit according to the manufacturer’s instructions (QIAGEN). The identification region for bacterial diversity was the 16S V3-V4 region (primers: 343F5-TACGGRAGGCAGCAG-3’; 798R 5’-AGGGTATCTAATCCT-3’) (Langille et al., 2013). For fungal diversity analysis, ITS I variable regions were amplified with universal primers (primers: ITS1F 5’- CTTGGTCATTTAGAGGAAGTAA-3’; ITS2 5’- GCTGCGTTCTTCATCGATGC-3’) (Zhao et al., 2019). Sequencing was performed on an Illumina NovaSeq 6000 instrument following PCR amplification and purification. The sequencing reads first need to be processed to obtain effective sequences for all samples using barcodes. Then, software such as Pandaseq, Prinseq, and Usearch are used to align, filter, and remove chimeras from the reads. Cluster the sequences using usearch with a similarity threshold of 0.97, filter chimeras from the clustered sequences, and obtain OTU (Operational Taxonomic Units) for species classification. Then, use singletons sequences with a similarity threshold of 0.97 to align with the representative sequences of the OTU for further analysis. Annotation of taxonomic data for representative sequences of bacteria and fungi was processed using the SILVA (https://www.arb-silva.de/) and UNITE (version 8.0, https://unite.ut.ee) databases, respectively. The microbial community structure in the rhizosphere soil was determined using the Illumina NovaSeq 6000 platform (Nanjing GENEPIONEER Biotechnology Co., Ltd., China) for high-throughput sequencing of bacterial 16S rRNA and fungal ITS rRNA.
2.5 Statistical analysis
To gain deeper insights into the responses of PPC rhizosphere soil microbial communities to plant hormones, we categorized the bacterial and fungal taxonomic groups into subcommunities based on the previously described criteria (Jiao and Lu, 2020; Xiao et al., 2022b). We designated a taxonomic unit as “abundant” when its relative abundance exceeded 0.1% of the total sequences within a single sample, and as “rare” when its abundance was below 0.01% of the total sequences; all others were classified as “moderate”.
α-diversity indices refer to community richness (Chao1) and community diversity (Shannon), were conducted to indicate the dissimilarity among samples. To evaluate the differences in species composition between different soil samples, cumulative percentage bar charts were used to display the community structure at the genus level were constructed using the heatmap package in R.3.1.3 software. Non-metric multidimensional scaling (NMDS) and multivariate permutational analysis of variance (PERMANOVA) were employed to reveal the succession of soil bacterial and fungi communities (abundant, moderate, and rare taxa), as well as to measure differences in community structure among different groups. Partial least squares path modelling (PLS-PM) was performed using the ‘plspm’ package in R to investigate the relationships among plant hormone application, soil enzyme activity, various subcommunities of bacterial and fungal diversity, and biomass. Significant pearson correlation (p < 0.05) between plant hormone and microbial taxa were analyzed and visualized using the interactive networks in R.3.1.3, Cytoscape 3.3.0.
Data were subjected to analysis of variance (ANOVA) using SPSS 17.0 for Windows (IBM SPSS Inc., Chicago, USA) and are expressed as the mean ± standard deviation. A two-tailed Student’s t-test in SPSS was used to compare metabolite levels between groups. p value < 0.05 was suggestive of significant differences and p value < 0.01 indicated extremely significant differences.
3 Results
3.1 Effect of phytohormones on morphological parameters
We observed significant morphological changes in PPC plants treated with MT, SL, and BR compared with the CK at week 5 (Figure 1). Overall, compared to the control group, plants treated with MT exhibited significantly enhanced robustness, with respective increases of 39.45%, 10.34%, 67.11%, and 42.48% in root length, shoot length, fresh weight, and biomass (p < 0.05). SL has no significant effect on the primary root growth, but it leads to a significant increase in shoot length, fresh weight, and biomass. BR significantly enhanced shoot growth, exerted a significant inhibitory effect on primary root growth, and did not significantly affect biomass accumulation.
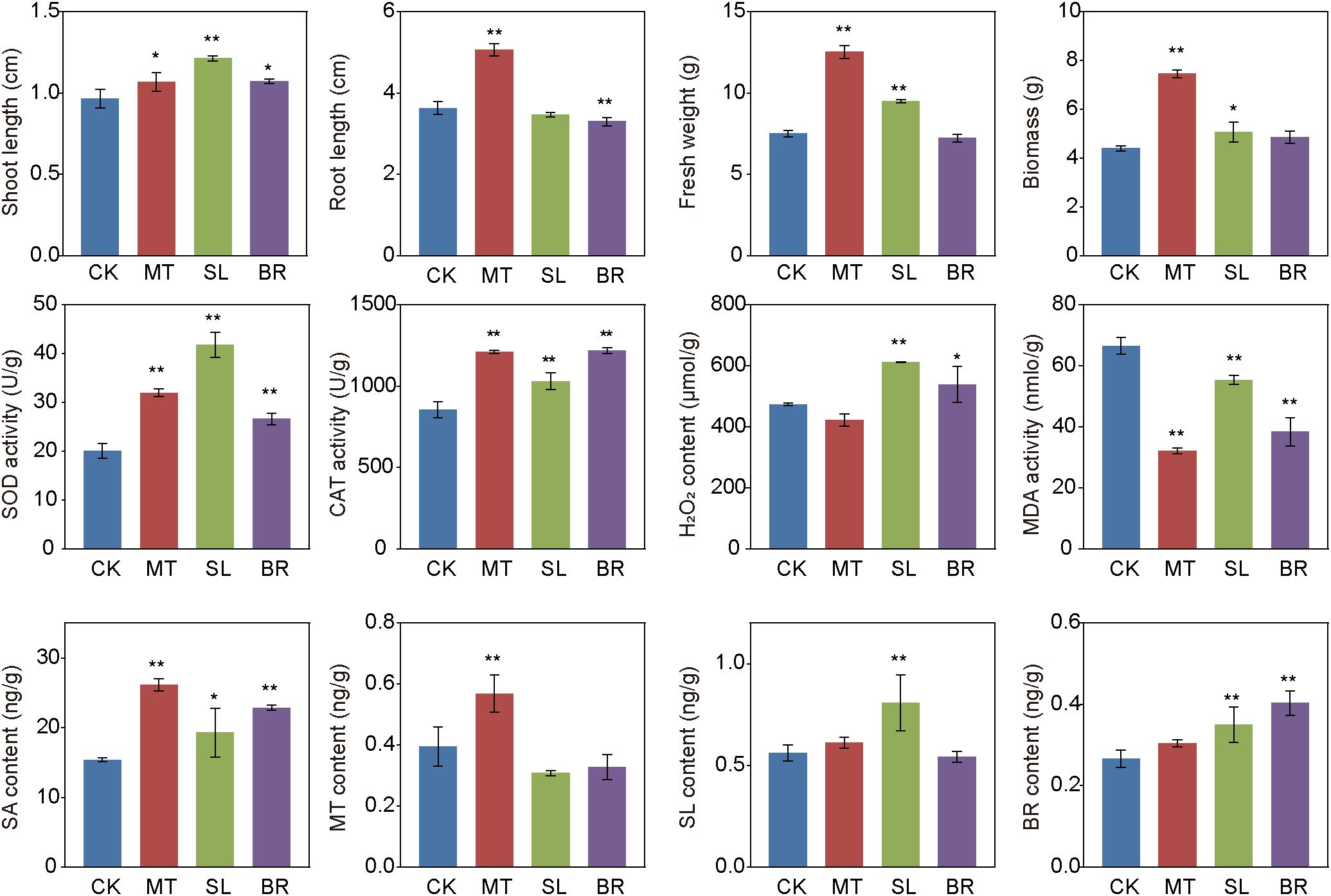
Figure 1. Morphological, physiological properties and hormone content of PPC under different phytohormones treatments. Data represent the means of three replicates with standard deviations (± SD). ** and * indicate significant differences (Dunn’s test, p < 0.05 and p < 0.01, respectively) the treatments compared to CK. CK, sterile water; MT, Melatonin; SL, strigolactone; BR, 24-epibrassinolide.
3.2 Effect of phytohormones on physiological characteristics
In this study, all phytohormone treatments enhanced SOD and CAT activities by 32.81–108.48% and 20.63–42.86%, respectively, compared to the CK (Figure 1), indicating an improved antioxidant defense capacity. Notably, MT treatment significantly reduced MDA content, suggesting a mitigation of lipid peroxidation and oxidative damage. In contrast, SL and BR treatments significantly increased H2O2 levels compared to CK, implying a possible role of H2O2 as a signaling molecule in stress response and growth regulation. All hormone treatments significantly elevated SA content, which may contribute to defense signaling. The concentrations of MT, SL, and BR in treated plants reached 0.57, 0.81, and 0.43 ng/g, respectively, representing increases of 44.12%, 44.22%, and 51.53% relative to CK. (Figure 1).
3.3 Effects of phytohormones on the diversity of rhizosphere microbial subcommunities
Using 16S rRNA gene and ITS amplicon sequencing, a total of 2,366 bacterial operational taxonomic units (OTUs) and 2,151 fungal OTUs were identified in rhizosphere soil samples. These OTUs were classified into abundant, moderate, and rare subcommunities, accounting for 9.04%, 53.25%, and 37.74% of bacterial OTUs and 5.21%, 15.02%, and 79.82% of fungal OTUs, respectively (Supplementary Table S1). Alpha diversity indices, including the Shannon index and Chao1 index, were significantly higher in the rare subcommunity compared to the abundant subcommunity (Figure 2). The Shannon index, which reflects species diversity and evenness, showed no significant changes in the abundant bacterial subcommunity under SL and BR treatments. In contrast, all three phytohormones (MT, SL, and BR) significantly influenced the Shannon index of the rare subcommunity.
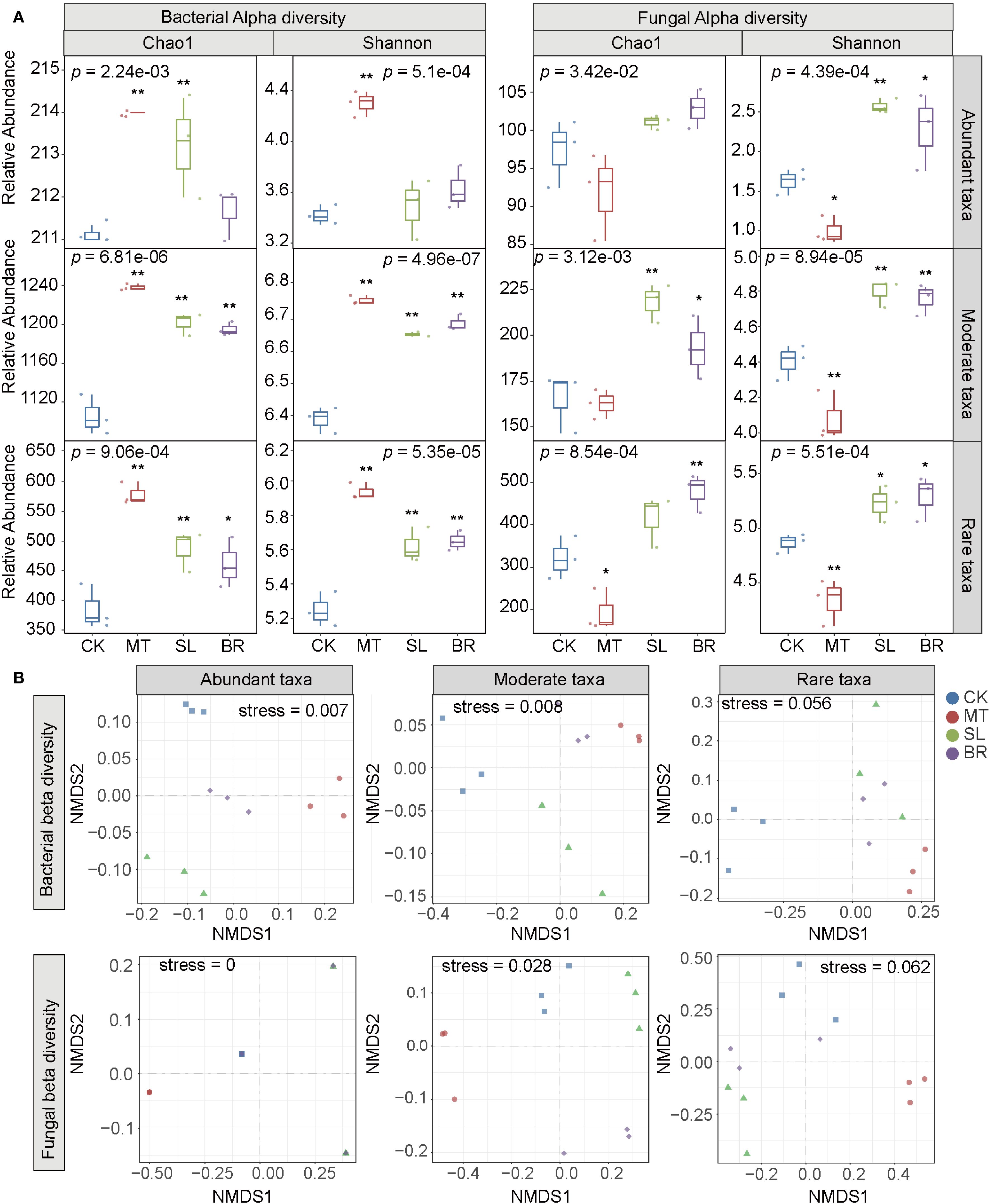
Figure 2. Diversity of rhizosphere microbial subcommunities in PPC under different phytohormone treatments. (A) Alpha diversity metrics (Chao1 and Shannon index) analyzed using the Kruskal-Wallis test, with significant differences indicated by p < 0.05 (*) and p < 0.01 (**) based on Dunn’s test. (B) Non-metric multidimensional scaling (NMDS). CK, sterile water; MT, Melatonin; SL, strigolactone; BR, 24-epibrassinolide.
Further assessment using non-metric multidimensional scaling (NMDS) and permutational multivariate analysis of variance (PERMANOVA) revealed the impacts of phytohormones on microbial community structures (Figure 2B). The results indicated that the abundant and moderate bacterial and fungal subcommunity samples exhibited significant differences compared to CK, whereas the rare subcommunities (both bacterial and fungal) showed no significant differences from CK.
3.4 Effects of phytohormones on the composition of rhizosphere microbial subcommunities
Figure 3 illustrates the compositional dynamics of abundant, moderate, and rare microbial taxa within the rhizosphere community (Supplementary Table S2). Significant shifts were observed in the dominant genera across different bacterial subcommunities. In the abundant taxa, Pseudomonas (34.44%) and Rhizobium (6.55%) were the predominant genera. The moderate taxa were primarily represented by Pirellula (36.91%) and Gemmata (24.92%), whereas Aquicella (2.41%) and SM1A02 (1.92%) exhibited relatively higher abundances among the rare taxa. Within the fungal community, certain genera, including Fusarium, Trichoderma, and Claussenomyces, exhibited a stable presence across all subcommunities. In contrast, Gibellulopsis, Rhinocladiella, Purpureocillium, Plectosphaerella, Mycothermus, and Cladosporium were exclusively detected within the moderate subcommunity, indicating their niche specificity.

Figure 3. Relative abundance of dominant genus of bacterial and fungal subcommunities under different phytohormone treatments. Only the top 15 taxa were shown. CK, sterile water; MT, Melatonin; SL, strigolactone; BR, 24-epibrassinolide.
LEfSe analysis identified key taxa driving differences in rhizosphere microbial subcommunities across phytohormone treatments (Figure 4). Among the three phytohormones, MT had the most substantial impact on bacterial composition. In fungal subcommunities, SL exhibited the most pronounced effects, particularly on abundant and rare taxa, including members of the Helotiales order, Stachybotryaceae family, Eurotiales order, and Nectriaceae family. For moderate fungal subcommunities, BR primarily influenced taxa in the Saccharomycetes class, Saccharomycetales order, and Saccharomycetales_fam_Incertae_sedis family. These findings indicate that SL and BR selectively modulate fungal subcommunities, while MT primarily affects bacterial subcommunities.
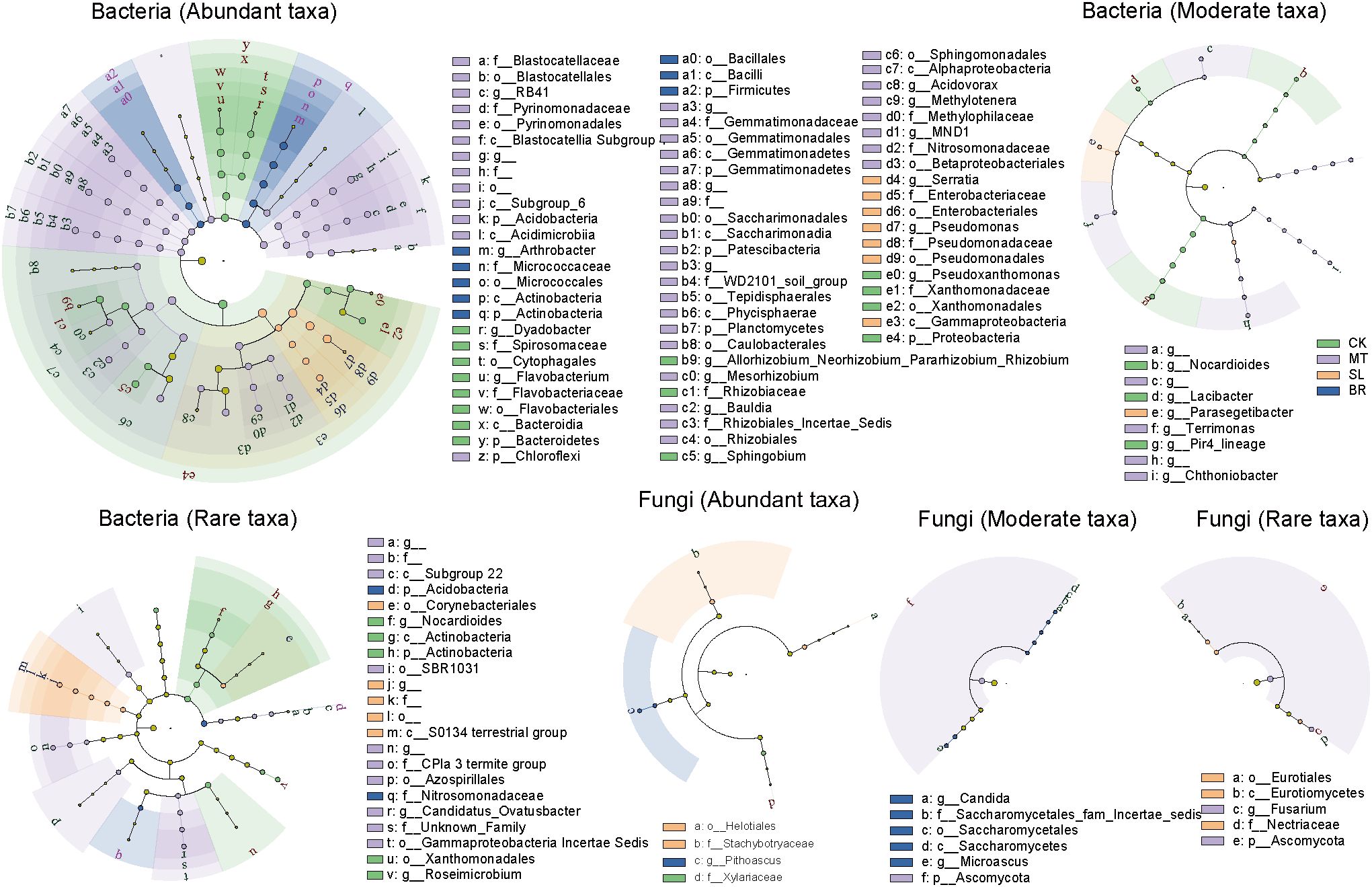
Figure 4. Cladogram of linear discriminant analysis of effects (LEfSe) for rhizosphere microbial subcommunities of different phytohormone treatments of PPC. Circles represent, from the center outwards, the phylum, class, order, family, and genus, and the size of the circle represents the relative abundance. Only taxa with LDA >4 and Wilcoxon, p < 0.05 are shown.
3.5 Predicting the PPC morphological parameters with the antioxidant system
With pronounced changes in the antioxidant system of plants treated with different hormones, we further investigated their potential association with the PPC phenotype (Figure 5). Both H2O2 and MDA showed a negative correlation with PPC biomass (H2O2: R = 0.58, p = 0.048; MDA: R = 0.74, p = 0.006), suggesting that excessive ROS accumulation and lipid peroxidation may hinder biomass accumulation. Conversely, SOD activity correlated positively with shoot length (R = 0.91, p < 0.001), highlighting the importance of enzymatic ROS scavenging for shoot growth. Interestingly, H2O2 also showed a weak positive correlation with shoot length (R = 0.63, p = 0.029), which may reflect its dual role as both a growth signal and a stress factor. No significant correlation was observed between antioxidant indices and root length, indicating that root growth at this stage may be regulated by other factors.
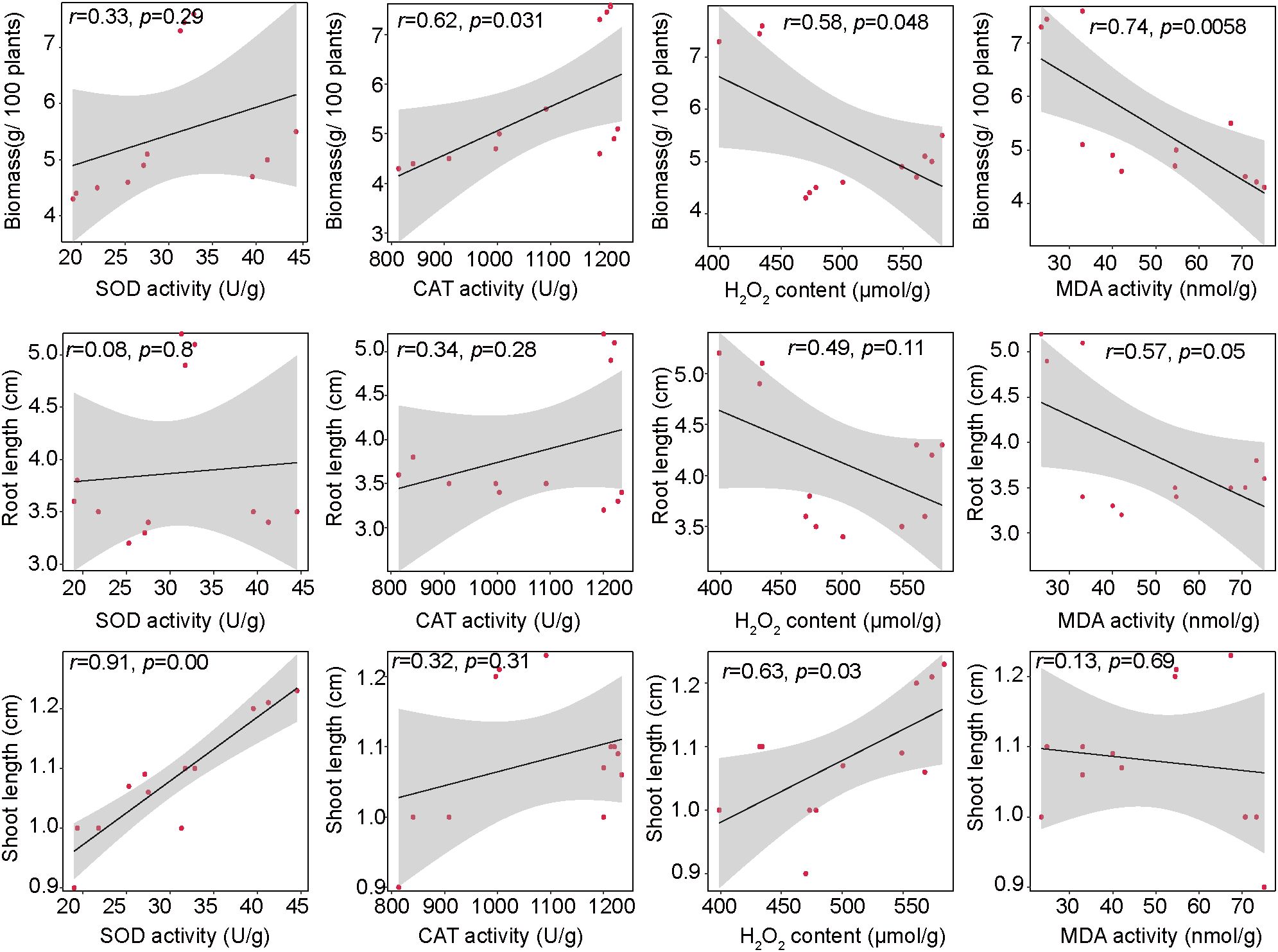
Figure 5. Linear regression analysis between the PPC morphological and the antioxidant system. The lines represent the least squares regression fit, and the shaded area are used to indicate the 95% confidence intervals.
3.6 Potential direct and indirect effects of phytohormone content and microbial subcommunities on PPC biomass
To explore the potential direct and indirect effects of soil hormone contents (MT, SL, and BR) and bacterial and fungal taxa on PPC biomass, we conducted the PLS-PM analysis based on the known effects and relationships among the predictors. The final model fit our dataset (Figure 6A) and indicated that bacterial rich taxa and fungal moderate taxa may be the most significant parameters directly promoting PPC biomass (p < 0.05) in addition to the direct effects of MT and BR hormones. SL may also indirectly promote PPC biomass by altering the rich taxa diversity of bacteria and fungi. Fungal rich taxa had a significant negative impact on the accumulation of PPC biomass (Figure 6B). Bacteria and fungi rare subcommunities had poor direct impact on the PPC biomass. Collectively, these results suggest that phytohormones not only act directly on plant physiology but also indirectly influence biomass through shaping key microbial subcommunities.
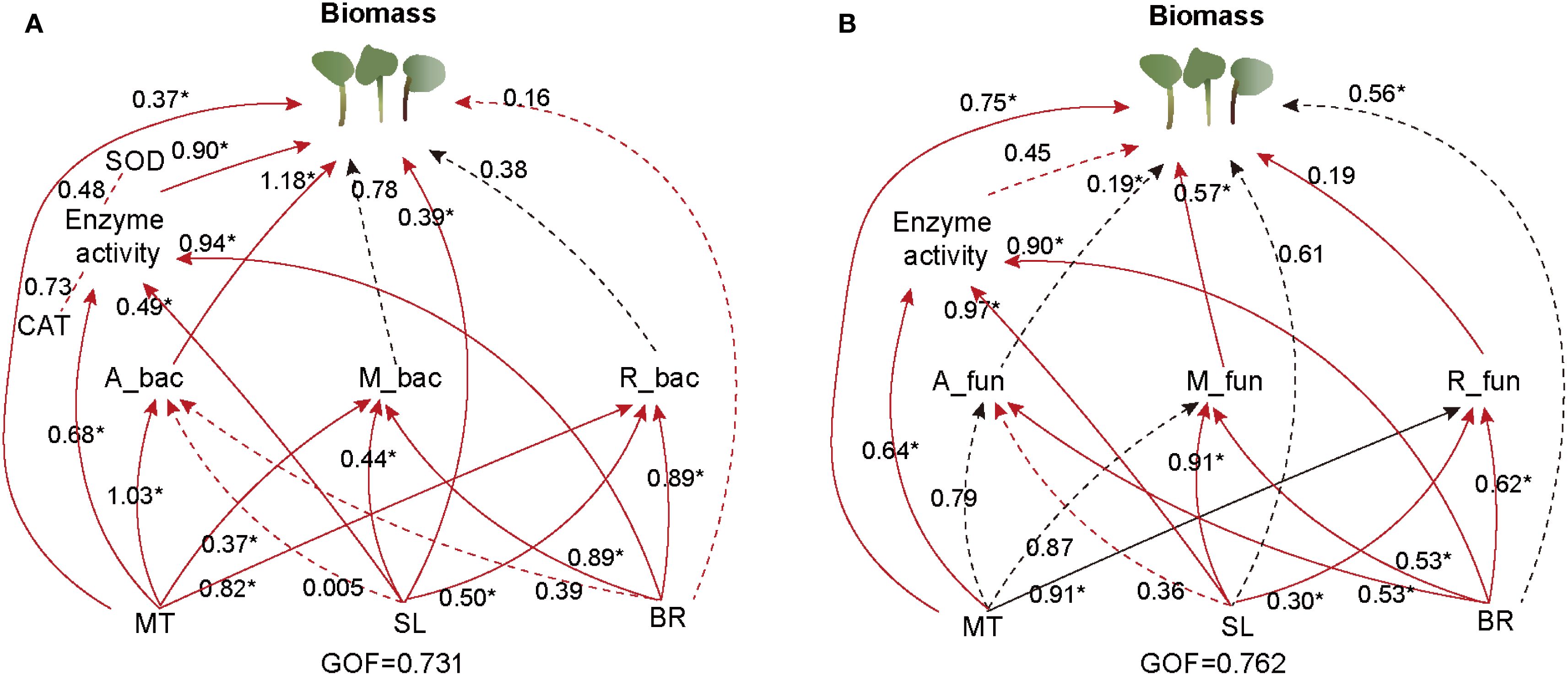
Figure 6. Partial least squares-path modelling (PLS-PM) analysis of direct and indirect influences of soil hormone contents, and bacterial (A) and fungal (B) subcommunities on PPC biomass. Positive and negative effects are presented by red and black arrows, respectively. Path coefficients that were insignificantly different from zero are shown as dashed lines; *p <0.05, and **p <0.01. The letters: A, M and R stand for abundance, moderate and rare, respectively. The goodness-of-fit was used to assess the model.
3.7 Predicting the PPC biomass with the abundances of abundant, moderate and rare taxa
With the noticeable changes in the soil’s rare and abundant communities due to inoculation, we further examined their potential connections to the increased PPC biomass. We discovered that among the key groups identified that had significant impacts on the variations in rhizosphere microbial subcommunities across different treatment groups, the most significant effects on biomass came from the abundant bacterial subcommunity. The prominent taxa within this abundant community, including Acidovorax (R = 0.82, p = 0.001), Bauldia (R = 0.91, p = 0.00), Gemmatimonadaceae (R = 0.83, p = 0.00), Methylotenera (R = 0.82, p = 0.001), MND1(R = 0.79, p = 0.02), Saccharimonadales (R = 0.97, p = 0.00), Blastocatellaceae (R = 0.62, p = 0.031), RB41(R = 0.89, p = 0.00) and Subgroup 6 (R = 0.85, p = 0.00) all showed a positive relationship with the PPC biomass (Figure 7). On the other hand, within the rare fungal subcommunity, prominent groups such as Fusarium (R = -0.59, p = 0.043) all demonstrated a negative correlation with the PPC biomass.
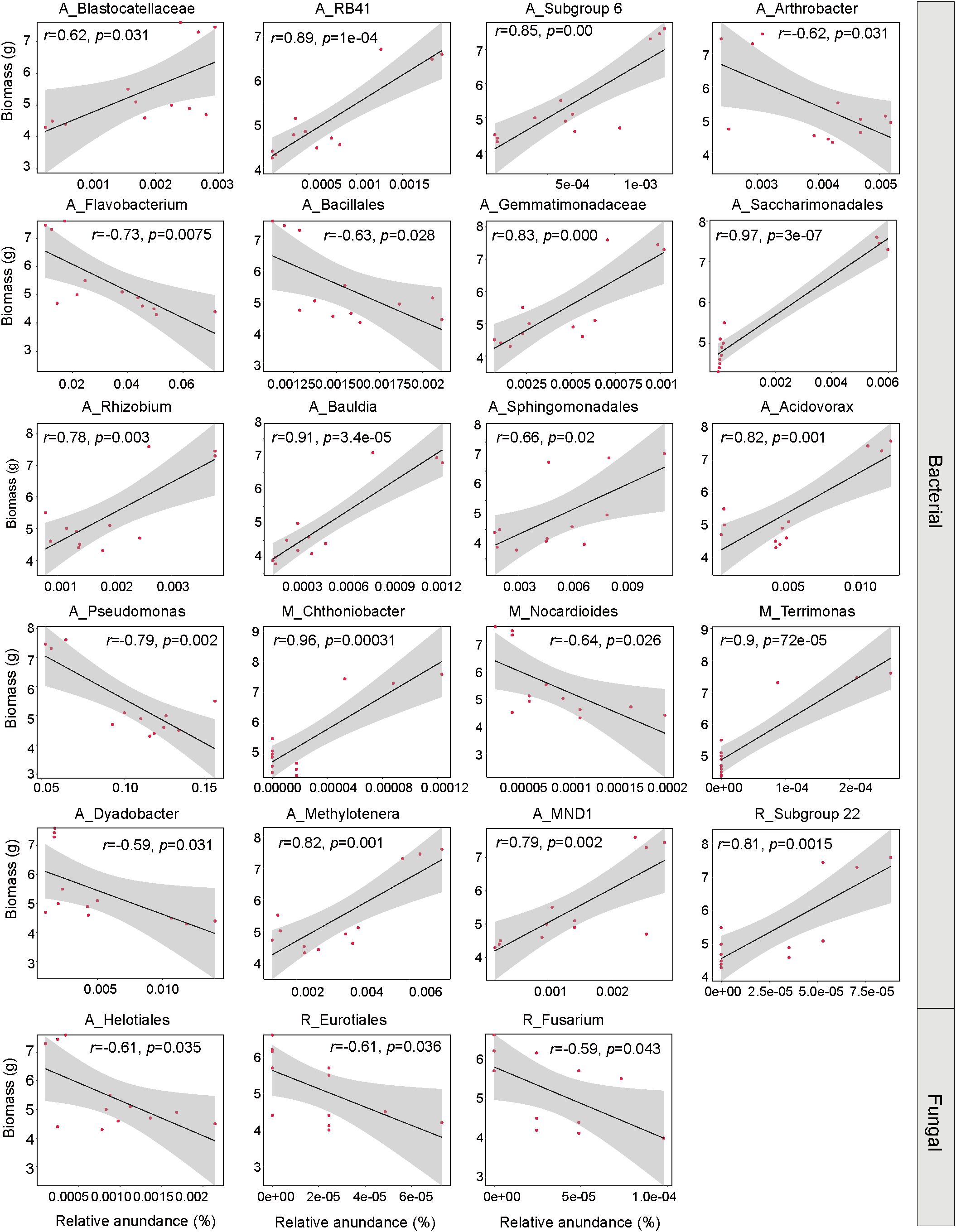
Figure 7. Linear regression analysis between the relative abundances of abundant, moderate, and rare subcommunities and PPC biomass. The lines represent least squares regression fits, and shaded areas indicate 95% confidence intervals. The letters A, M, and R denote abundant, moderate, and rare taxa, respectively.
Pearson correlation analysis revealed a significant correlation between plant hormone content (MT, SL, and BR) and the abundance of key taxa in different subcommunities of bacteria and fungi (Figure 8). MT significantly affected the relative abundance of bacteria, showing a significant positive correlation with the presence of numerous bacterial taxa, including 10 abundant taxa, 2 moderate taxa, and 1 rare species. In contrast, MT content was significantly negatively correlated with the abundance of Fusarium, Nectriaceae, and Helotiales. The abundance of the bacterial abundant species Arthrobacter was significantly positively correlated with BR content. SL content was significantly negatively correlated with the bacterial abundant species Serratia and the fungal rare species Eurotiales.
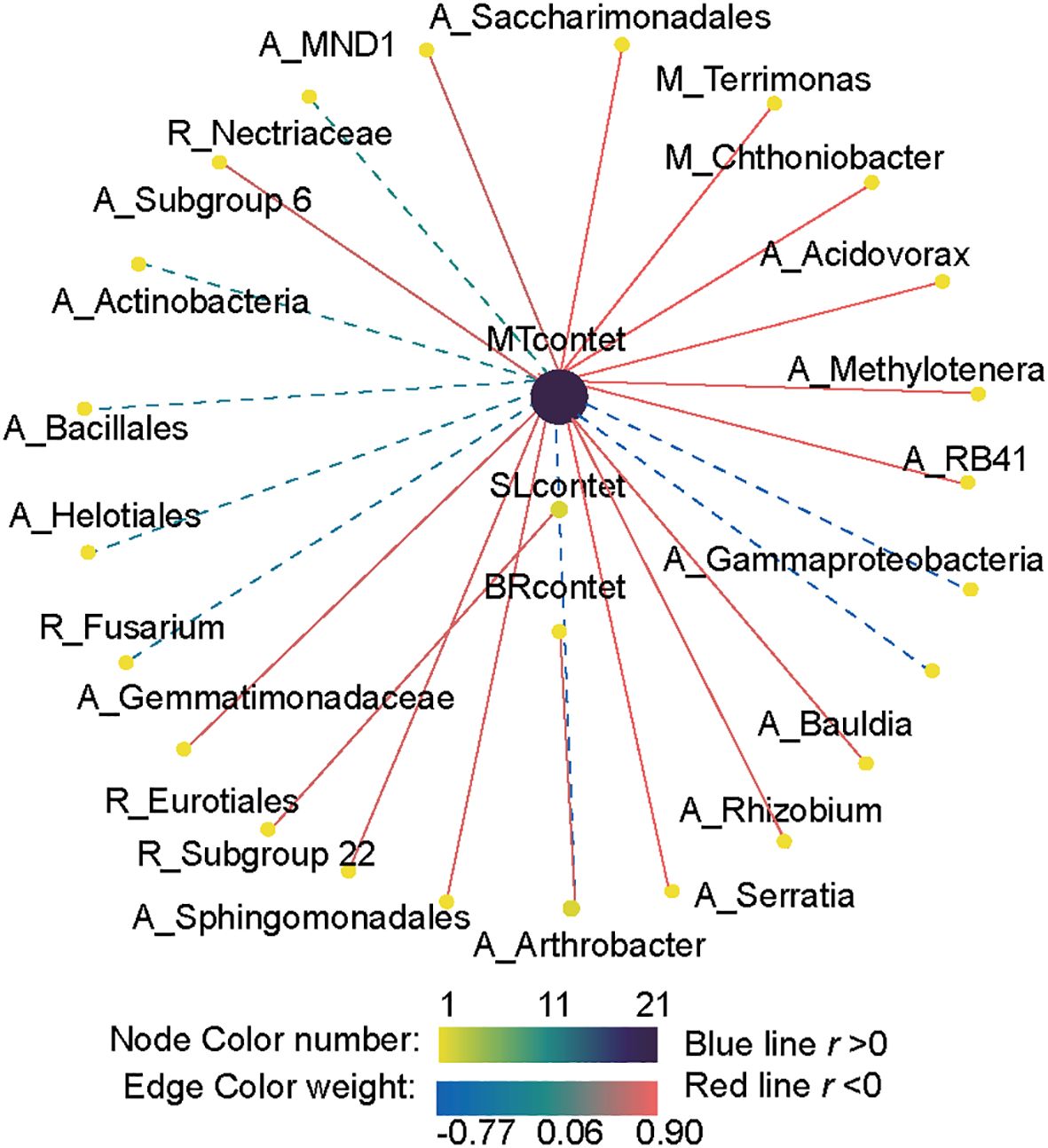
Figure 8. Correlation analysis between key bacterial and fungal taxa and phytohormone contents. A link indicates a significant correlation (Pearson’s |R| > 0.5 and FDR-corrected p < 0.05). Red and blue links represent positive and negative correlations, respectively. The differently colored dots represent the taxa belonging to distinct subcommunities. The letters A, M, and R denote abundant, moderate, and rare taxa, respectively.
4 Discussion
4.1 Phytohormone indirectly promote the growth and development of PPC by regulating the antioxidant system
Phytohormones play a crucial role in plant growth and development by regulating the antioxidant system, which in turn influences key agronomic traits (Devireddy et al., 2021). In this study, SL treatment significantly promoted stem elongation, consistent with previous findings (Chen et al., 2009). However, SL had no significant effect on primary root growth, which may be attributed to SL-induced H2O2 accumulation, potentially leading to oxidative stress that limits primary root elongation (Tian et al., 2018). The inhibitory effect of BR on root growth may be due to the relatively high concentration used in this study, which may not be optimal for PPC plants (Lv et al., 2018). Among the three phytohormones tested, MT exhibited the most pronounced effect on biomass accumulation, increasing PPC biomass by 69.32%. This effect can be attributed to its strong role in ROS scavenging and oxidative stress mitigation. MT treatment significantly enhanced superoxide dismutase (SOD) and catalase (CAT) activity, resulting in reduced MDA content, which indicates improved cellular stability and stress resistance. These findings are consistent with previous studies demonstrating that MT promotes plant growth primarily by maintaining redox homeostasis and protecting cells from oxidative damage. Extensive research has shown that MT protects plants from abiotic and biotic stress by scavenging ROS and regulating antioxidant levels (Tan et al., 2012). While MT has little effect on membrane lipid peroxidation (MDA and H2O2) under normal growth conditions, it effectively reduces their levels under oxidative stress (He et al., 2023b; Darré et al., 2024). These results suggest that the slow growth of wild PPC seedlings may be attributed to oxidative stress in their natural environment, underscoring the importance of exogenous growth regulators in enhancing ROS scavenging capacity. In conclusion, the regulatory effects of MT and SL on PPC growth can be attributed to their indirect influence through antioxidant system modulation.
4.2 Phytohormones promotes PPC biomass by regulating the soil microbial subcommunities
We classified OTUs into taxa of abundant, moderate, and rare subcommunities. Our observations revealed a dominance of rare taxa in both bacteria and fungi. This finding aligns with prior results reported in tropical forests and farmland systems (Jiao et al., 2019; He et al., 2023a). Beyond differences in species numbers, distinct rhizosphere microbial subcommunities structure also had varied responses to hormone administration. The Shannon index of abundant bacterial subsets exhibited no sensitivity to SL and BR application. This index is a measure of both evenness and abundance of species. It is likely that the abundant taxa in soil microorganisms, which typically occupy a wide range of ecological niches and use various resources, adapted effectively to their changing environment (Wu et al., 2017). Conversely, rare microorganisms, having narrower ecological niches, were more susceptible to environmental changes (Liang et al., 2020).
We applied PLS-PM to observe the relationship among hormone content, different subcommunity structures, and biomass. Results indicated that MT significantly influenced biomass by affecting the structure of bacterial abundant taxa, while other subcommunity structures lacked significant effects on biomass. Bacterial abundant subcommunities are generally considered to be the most active group in biogeochemical cycles, particularly in carbohydrate metabolism (Cottrell and David, 2003). Consequently, the abundant bacterial community makes a significant contribution to plant biomass, whereas the rare bacteria do not, which is a reasonable finding. We utilized LEFSe analysis to identify potential biomarkers for each rhizosphere soil subcommunity treated with various plant hormones. These hormones are generally considered vital sources of microbial community structure differences and the cause of distinct effects on plant growth. MT produced several biomarkers significantly related to biomass in each subcommunity, for instance, in the bacteria abundant subcommunity, Subgroup 6, MND1, RB41, Gemmatimonadaceae, and Saccharimonadales notably promoted PPC biomass. These bacteria are recognized as plant growth promoting rhizobacteria (PGPR) and can stimulate growth via carbon, nitrogen, phosphorus, or through the inhibition of pathogenic bacteria cycling within each ecosystem (Lu et al., 2020; Yang et al., 2022a; Hao et al., 2023; Li et al., 2023; Yang et al., 2023). Our pearson results showed a significant positive correlation with MT content. Prior research indicates that Fusarium, a widely distributed genus, can induce root rot in multiple plant species, thereby influencing crop growth (Franke-Whittle et al., 2015). In our study, MT significantly reduced Fusarium abundance in rare fungal communities, consequently lessening its negative impact on biomass. SL reportedly play a broad role as soil chemical signals in Ascomycetes, and in our study, SL also led to the enrichment of two Ascomycetes biomarkers, namely Nectriaceae and Eurotiales. Earlier studies highlighted these biomarkers as potential plant pathogens that could negatively affect growth (Mukasa et al., 2019; Chen et al., 2024). Significantly, MT significantly increased the relative abundance of Proteobacteria, specifically Serratia and Pseudomonas, in the bacterial abundant subcommunity and had a significant negative effect on the PPC biomass. Similarly, BR enriched Actinobacteria, particularly Arthrobacter, which also inhibited the PPC biomass. It has been reported that this might be due to the rapid growth of Proteobacteria and Actinobacteria, which can effectively compete for nutrients in rhizosphere soil (Bardgett et al., 2003; Boubekri et al., 2022). In summary, MT can promote the accumulation of PPC biomass by regulating the structure of bacterial subcommunities and the abundance of key microbial taxa, whereas the potential influence of SL and BR on PPC biomass is not correlated with alterations in microbial community structure but is rather linked to the succession of specific key taxa.
5 Conclusion
This study highlights the significant roles of MT, SL, and BR in regulating plant growth, antioxidant responses, and rhizosphere microbial subcommunities in PPC. Phytohormones MT and SL significantly increased PPC biomass by 69.32% and 15.23%, respectively. A concentration of 2 mg/L BR inhibited the development of PPC roots, which may not be suitable for the post-germination growth application of PPC seeds. MT and SL enhanced the growth and development of PPC by modulating the antioxidant system, with MT further contributing to biomass accumulation by influencing the structure of abundant bacterial subcommunities and key microbial groups. Although SL and BR did not significantly affect biomass through changes in microbial community structure, key biomarker taxa showed potential contributions to biomass accumulation. These findings provide valuable insights into the application of phytohormones and the underlying mechanisms by which they promote crop yield, offering important implications for agricultural practices.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1148787 https://www.ncbi.nlm.nih.gov/, PRJNA1149856.
Author contributions
TP: Investigation, Methodology, Writing – original draft. CW: Formal Analysis, Visualization, Writing – original draft. TY: Funding acquisition, Resources, Supervision, Writing – review & editing. JS: Data curation, Project administration, Writing – review & editing. JZ: Methodology, Writing – review & editing. LZ: Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Key research and development project of Gansu Academy of Sciences (2023ZDYF-01), Industrialization application Project of Gansu Academy of Sciences (2024YLCY-01), National Natural Science Foundation of China (Nos. 82225047, 82170274) and Pingliang city Science and Technology Program project (PL-STK-2024A-025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1576843/full#supplementary-material
Supplementary Table S1 | The OUTs number of abundant, moderate and rare taxa of bacteria and fungi.
Supplementary Table S2 | The relative abundance of abundant, moderate and rare taxa of bacteria and fungi.
References
Ahsan, M., Zulfiqar, H., Farooq, M. A., Ali, S., Tufail, A., Kanwal, S., et al. (2023). Strigolactone (GR24) Application Positively Regulates Photosynthetic Attributes, Stress-Related Metabolites and Antioxidant Enzymatic Activities of Ornamental Sunflower (Helianthus annuus cv. Vincent’s Choice) under Salinity Stress. Agriculture 13, 50. doi: 10.3390/agriculture13010050
Ali, J., Mukarram, M., Ojo, J., Dawam, N., Riyazuddin, R., Ghramh, H. A., et al. (2024). Harnessing Phytohormones: Advancing Plant Growth and Defence Strategies for Sustainable Agriculture. Physiol. Plant 176, e14307. doi: 10.1111/ppl.14307
Atteya, A. K. G., El-Serafy, R. S., El-Zabalawy, K. M., Elhakem, A., and Genaidy, E. A. E. (2022). Brassinolide Maximized the Fruit and Oil Yield, Induced the Secondary Metabolites, and Stimulated Linoleic Acid Synthesis of Opuntia ficus-indica Oil. Horticulturae 8, 452. doi: 10.3390/horticulturae8050452
Bardgett, R. D., Streeter, T. C., and Bol, R. (2003). Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 84, 1277–1287. doi: 10.1890/0012-9658(2003)084[1277:SMCEWP]2.0.CO;2
Boubekri, K., Soumare, A., Mardad, I., Lyamlouli, K., Ouhdouch, Y., Hafidi, M., et al. (2022). Multifunctional role of Actinobacteria in agricultural production sustainability: A review. Microbiol Res. 261, 127059. doi: 10.1016/j.micres.2022.127059
Bradley, J. M., Bunsick, M., Ly, G., Aquino, B., Wang, F. Z., Holbrook-Smith, D., et al. (2024). Modulation of fungal phosphate homeostasis by the plant hormone strigolactone. Mol. Cell. 84, 4031–4047.e11. doi: 10.1016/j.molcel.2024.09.004
Carrera-Castaño, G., Calleja-Cabrera, J., Pernas, M., Gómez, L., and Oñate-Sánchez, L. (2020). An Updated Overview on the Regulation of Seed Germination. Plants 9, 703. doi: 10.3390/plants9060703
Chen, C., Zou, J., Zhang, S., Zaitlin, D., and Zhu, L. (2009). Strigolactones are a new-defined class of plant hormones which inhibit shoot branching and mediate the interaction of plant-AM fungi and plant-parasitic weeds. Sci. China C Life Sci. 52, 693–700. doi: 10.1007/s11427-009-0104-6
Chen, H., White, J. F., Malik, K., and Li, C. (2024). Molecular assessment of oat head blight fungus, including a new genus and species in a family of Nectriaceae. Int. J. Food Microbiol. 417, 110715. doi: 10.1016/j.ijfoodmicro.2024.110715
Commission, C. P. (2020). Pharmacopoeia of People’s Republic of China. Volume 1. China Med. Sci. Technol. Press 1, 274–275.
Cottrell, M. T. and David, K. L. (2003). Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware estuary. Limnol Oceanogr 48, 168–178. doi: 10.4319/lo.2003.48.1.0168
Darré, M., Zaro, M. J., Guijarro-Fuertes, M., Careri, L., and Concellón, A. (2024). Melatonin Combined with Wax Treatment Enhances Tolerance to Chilling Injury in Red Bell Pepper. Metabolites 14, 330. doi: 10.3390/metabo14060330
Devireddy, A. R., Zandalinas, S. I., Fichman, Y., and Mittler, R. (2021). Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 105, 459–476. doi: 10.1111/tpj.15010
Dong, J., Ding, C., Chen, H., Fu, H., Pei, R., Shen, F., et al. (2024). Functions of exogenous strigolactone application and strigolactone biosynthesis genes GhMAX3/GhMAX4b in response to drought tolerance in cotton (Gossypium hirsutum L.). BMC Plant Biol. 24, 1008. doi: 10.1186/s12870-024-05726-w
Franke-Whittle, I. H., Manici, L. M., Insam, H., and Stres, B. (2015). Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 395, 317–333. doi: 10.1007/s11104-015-2562-x
Gao, T., Liu, X., Tan, K., Zhang, D., Zhu, B., Ma, F., et al. (2022). Introducing melatonin to the horticultural industry: physiological roles, potential applications, and challenges. Hortic. Res. 9, uhac094. doi: 10.1093/hr/uhac094
Guan, L., Zheng, Z., Guo, Z., Xiao, S., Liu, T., Chen, L., et al. (2024). Steroidal saponins from rhizome of Paris polyphylla var. chinensis and their anti-inflammatory, cytotoxic effects. Phytochemistry 219, 113994. doi: 10.1016/j.phytochem.2024.113994
Hao, Y., Cai, Z., Ma, C., White, J. C., Cao, Y., Chang, Z., et al. (2023). Root Exposure of Graphitic Carbon Nitride (g-C3N4) Modulates Metabolite Profile and Endophytic Bacterial Community to Alleviate Cadmium- and Arsenate-Induced Phytotoxicity to Rice (Oryza sativa L.). ACS Nano 17, 19724–19739. doi: 10.1021/acsnano.3c03066
He, J., Tan, X., Nie, Y., Ma, L., Liu, J., Lu, X., et al. (2023a). Distinct Responses of Abundant and Rare Soil Bacteria to Nitrogen Addition in Tropical Forest Soils. Microbiol. Spectr 11, e0300322. doi: 10.1128/spectrum.03003-22
He, X., Yin, B., Zhang, J., Zhou, S., Li, Z., Zhang, X., et al. (2023b). Exogenous melatonin alleviates apple replant disease by regulating rhizosphere soil microbial community structure and nitrogen metabolism. Sci. Total Environ. 884, 163830. doi: 10.1016/j.scitotenv.2023.163830
Jiang, D., Ou, Y., Jiang, G., Dai, G., Liu, S., and Chen, G. (2025). Melatonin-priming ameliorates aluminum accumulation and toxicity in rice through enhancing aluminum exclusion and maintaining redox homeostasis. Plant Physiol. Biochem. 219, 109433. doi: 10.1016/j.plaphy.2024.109433
Jiao, S. and Lu, Y. (2020). Soil pH and temperature regulate assembly processes of abundant and rare bacterial communities in agricultural ecosystems. Environ. Microbiol. 22, 1052–1065. doi: 10.1111/1462-2920.14815
Jiao, S., Wang, J., Wei, G., Chen, W., and Lu, Y. (2019). Dominant role of abundant rather than rare bacterial taxa in maintaining agro-soil microbiomes under environmental disturbances. Chemosphere 235, 248–259. doi: 10.1016/j.chemosphere.2019.06.174
Langille, M. G., Zaneveld, J., Caporaso, J. G., McDonald, D., Knights, D., Reyes, J. A., et al. (2013). Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814–821. doi: 10.1038/nbt.2676
Li, Z., Chi, Y., Su, X., Ye, Z., and Ren, X. (2023). Rhizobium Soaking Promoted Maize Growth by Altering Rhizosphere Microbiomes and Associated Functional Genes. Microorganisms 11, 1654. doi: 10.3390/microorganisms11071654
Liang, Z., Li, J., Feng, J., Li, Z., Nangia, V., Mo, F., et al. (2025). Brassinosteroids improve the redox state of wheat florets under low-nitrogen stress and alleviate degeneration. J. Integr. Agriculture 24, 2920–2939. doi: 10.1016/j.jia.2024.03.035
Liang, Y., Xiao, X., Nuccio, E. E., Yuan, M., Zhang, N., Xue, K., et al. (2020). Differentiation strategies of soil rare and abundant microbial taxa in response to changing climatic regimes. Environ. Microbiol. 22, 1327–1340. doi: 10.1111/1462-2920.14945
Lu, H., Wu, Y., Liang, P., Song, Q., Zhang, H., Wu, J., et al. (2020). Alkaline amendments improve the health of soils degraded by metal contamination and acidification: Crop performance and soil bacterial community responses. Chemosphere 257, 127309. doi: 10.1016/j.chemosphere.2020.127309
Lv, B., Tian, H., Zhang, F., Liu, J., Lu, S., Bai, M., et al. (2018). Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PloS Genet. 14, e1007144. doi: 10.1371/journal.pgen.1007144
Mikkelson, K. M., Bokman, C., and Sharp, J. O. (2016). Rare taxa maintain microbial diversity and contribute to terrestrial community dynamics throughout bark beetle infestation. Appl. Environ. Microbiol. 82, 6912–6919. doi: 10.1128/aem.02245-16
Mukasa, Y., Kyamanywa, S., Sserumaga, J. P., Otim, M., Tumuhaise, V., Erbaugh, M., et al. (2019). An atoxigenic L-strain of Aspergillus flavus (Eurotiales: Trichocomaceae) is pathogenic to the coffee twig borer, (Coleoptera: Curculionidea: Scolytinae). Environ. Microbiol. Rep. 11, 508–517. doi: 10.1111/1758-2229.12705
Nakano, M., Omae, N., and Tsuda, K. (2022). Inter-organismal phytohormone networks in plant-microbe interactions. Curr. Opin. Plant Biol. 68, 102258. doi: 10.1016/j.pbi.2022.102258
Park, H. S., Kazerooni, E. A., Kang, S. M., Al-Sadi, A. M., and Lee, I. J. (2021). Melatonin Enhances the Tolerance and Recovery Mechanisms in Brassica juncea (L.) Czern. Under Saline Conditions. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.593717
Sauret, C., Séverin, T., Vétion, G., Guigue, C., Goutx, M., Pujo-Pay, M., et al. (2014). 'Rare biosphere' bacteria as key phenanthrene degraders in coastal seawaters. Environ. pollut. 194, 246–253. doi: 10.1016/j.envpol.2014.07.024
Shu, H., Xu, K., Li, X., Liu, J., Altaf, M. A., Fu, H., et al. (2024). Exogenous strigolactone enhanced the drought tolerance of pepper (Capsicum chinense) by mitigating oxidative damage and altering the antioxidant mechanism. Plant Cell Rep. 43, 106. doi: 10.1007/s00299-024-03196-w
Singh, T., Bisht, N., Ansari, M. M., and Chauhan, P. S. (2024). The hidden harmony: Exploring ROS-phytohormone nexus for shaping plant root architecture in response to environmental cues. Plant Physiol. Biochem. 206, 108273. doi: 10.1016/j.plaphy.2023.108273
Sun, Y., He, Y., Irfan, A. R., Liu, X., Yu, Q., Zhang, Q., et al. (2020). Exogenous Brassinolide Enhances the Growth and Cold Resistance of Maize (Zea mays L.) Seedlings under Chilling Stress. Agronomy 10, 488. doi: 10.3390/agronomy10040488
Tan, D. X., Hardeland, R., Manchester, L. C., Korkmaz, A., Ma, S., Rosales-Corral, S., et al. (2012). Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 63, 577–597. doi: 10.1093/jxb/err256
Tian, Y., Fan, M., Qin, Z., Lv, H., Wang, M., Zhang, Z., et al. (2018). Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 9, 1063. doi: 10.1038/s41467-018-03463-x
Wang, X. K. and Huang, J. L. (2015). Principles and Techniques of Plant Physiology and Biochemistry Experiments. (Beijing: Higher Education Press).
Wang, X., Peng, C., Liang, J., Liang, Q., Xu, C., and Guo, W. (2019). The complete chloroplast genome of Paris polyphylla var. chinensis, an endemic medicinal herb in China. Mitochon DNA Part B. 4, 3888–3889. doi: 10.1080/23802359.2019.1687351
Wang, L., Xia, Y., and Hou, Y. (2024). Candy or poison: Plant metabolites as swing factors against microbes. Mol. Plant 17, 1341–1343. doi: 10.1016/j.molp.2024.08.005
Wu, W., Logares, R., Huang, B., and Hsieh, C. H. (2017). Abundant and rare picoeukaryotic sub-communities present contrasting patterns in the epipelagic waters of marginal seas in the northwestern Pacific Ocean. Environ. Microbiol. 19, 287–300. doi: 10.1111/1462-2920.13606
Xiao, R., Han, Q., Liu, Y., Zhang, X., Hao, Q., Chai, Q., et al. (2022a). Melatonin Attenuates the Urea-Induced Yields Improvement Through Remodeling Transcriptome and Rhizosphere Microbial Community Structure in Soybean. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.903467
Xiao, X., Zhu, Y., Gao, C., Zhang, Y., Gao, Y., and Zhao, Y. (2022b). Microbial inoculations improved rice yields by altering the presence of soil rare bacteria. Microbiol. Res. 254, 126910. doi: 10.1016/j.micres.2021.126910
Yang, Y., Cheng, K., Li, K., Jin, Y., and He, X. (2022b). Deciphering the diversity patterns and community assembly of rare and abundant bacterial communities in a wetland system. Sci. Total Environ. 838, 156334. doi: 10.1016/j.scitotenv.2022.156334
Yang, F., Jiang, H., Chang, G., Liang, S., Ma, K., Cai, Y., et al. (2023). Effects of Rhizosphere Microbial Communities on Cucumber Fusarium wilt Disease Suppression. Microorganisms 11. doi: 10.3390/microorganisms11061576
Yang, X., Leng, Y., Zhou, Z., Shang, H., Ni, K., Ma, L., et al. (2022a). Ecological management model for the improvement of soil fertility through the regulation of rare microbial taxa in tea (Camellia sinensis L.) plantation soils. J. Environ. Manage. 308, 114595. doi: 10.1016/j.jenvman.2022.114595
Yin, W., Dong, N., Li, X., Yang, Y., Lu, Z., Zhou, W., et al. (2025). Understanding brassinosteroid-centric phytohormone interactions for crop improvement. J. Integr. Plant Biol. 67, 563–581. doi: 10.1111/jipb.13849
Zhang, X., Huang, T., Liang, Y., Hussain, S., Peng, R., Wang, T., et al. (2024). Melatonin and 14-hydroxyed brassinosteroid combined promote kiwifruit seedling growth by improving soil microbial distribution, enzyme activity and nutrients uptake. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1336116
Zhang, J., Wang, B., Xu, H., Liu, W., Yu, J., Wang, Q., et al. (2025). Root microbiota regulates tiller number in rice. Cell 188, 3152–3166.e16. doi: 10.1016/j.cell.2025.03.033
Keywords: phytohormone, rhizosphere, morphology, microbes subcommunity, antioxidant system
Citation: Peng T, Yang T, Wang C, Sha J, Zhao J and Zhang L (2025) Exogenous phytohormones enhance Paris polyphylla var. chinensis (Franch.) Hara biomass by regulating antioxidant systems and rhizosphere microbial subcommunities. Front. Plant Sci. 16:1576843. doi: 10.3389/fpls.2025.1576843
Received: 21 February 2025; Accepted: 25 August 2025;
Published: 05 September 2025.
Edited by:
Muhammad Qadir, Hunan University, ChinaReviewed by:
Magdalena Wróbel-Kwiatkowska, Wroclaw University of Environmental and Life Sciences, PolandAsadullah Khan, Shenzhen University, China
Copyright © 2025 Peng, Yang, Wang, Sha, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengniu Wang, d2FuZ2NoZW5nbml1QDE2My5jb20=; Lei Zhang, bGVpemhhbmcxMDBAbnR1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Tong Peng
Tong Peng Tao Yang2†
Tao Yang2† Chengniu Wang
Chengniu Wang