- 1Department of Biology, College of Science and Technology, Wenzhou-Kean University, Wenzhou, China
- 2Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, United Arab Emirates
- 3Department of Applied Biology, College of Sciences, University of Sharjah, Sharjah, United Arab Emirates
- 4Department of Agronomy, Faculty of Agriculture, University of Khartoum, Khartoum North, Sudan
In the context of climate change, abiotic stresses are recognized as significant environmental challenges that limit agricultural productivity globally. These conditions disrupt normal plant growth and development processes. The ability of plants to tolerate these stressors is linked to their resilience mechanisms. Glycine betaine (GB), also known as betaine, is a derivative of methylated glycine identified in numerous plant species as a substance that mitigates the detrimental effects of stressful environments. GB is synthesized in the cytosol as an initial response to abiotic stress, and signaling molecules, such as jasmonic acid and methyl jasmonate, primarily initiate its production. Recent studies have highlighted their role in stimulating GB synthesis and its subsequent accumulation. The concentration of GB within a plant system can effectively indicate tolerance levels, ultimately contributing to the understanding of resilience mechanisms. GB plays a crucial role in reducing the accumulation and detoxification of reactive oxygen species (ROS), which aids in restoring photosynthesis and alleviating oxidative stress. It contributes to the stabilization of membranes and macromolecules and is essential for the protection and stabilization of photosynthetic components, such as ribulose-1,5-bisphosphate carboxylase/oxygenase, photosystem II, and quaternary enzyme and protein complex structures, under environmental stress conditions. Furthermore, GB can enhance stress tolerance even at minimal concentrations by activating the genes associated with stress defense mechanisms. Recent studies have demonstrated that the application of GB can protect against environmental challenges, thereby improving both crop yield and quality. This review concentrates on the role of GB in promoting abiotic stress tolerance and explores potential strategies for engineering GB biosynthesis in plants.
1 Introduction
Extreme weather is one of the main concerns in the twenty-first century. According to several available data, the Earth’s average surface temperature has risen by roughly 1.18°C since the late 19th century and is expected to rise by an additional 0.2°C every decade for the next 30 years (Bailey-Serres et al., 2019). The beginning and end of the crop growing season have changed significantly over the past 20 years, which has decreased crop output, reduced the amount of fresh water available for irrigation, and exacerbated biodiversity loss (Brown and Cunningham, 2019). This, in turn, threatens available natural resources, in addition to having a negative impact on crop production. Severe weather patterns and various abiotic stressors, including salt, cold, heat, freezing, and draft flooding, have decreased crop yield and food security worldwide. Urbanization, global population growth, and land use patterns have rapidly changed as a result of industrialization. Providing food to the growing population, even if the amount of land under cultivation is decreasing, is one of the most significant obstacles in the scientific community. Thus, it is essential to create climate-resilient crops, and the only way to ensure a continuous supply of food worldwide is to develop environmentally friendly techniques for managing plant stress (Basit et al., 2024b; Naeem et al., 2024).
The production and accumulation of suitable solutes are among the most well-established stress-responsive processes in plants. Small organic metabolites that are water soluble and membrane-impermeable are compatible solutes. They can accumulate significantly in the cytoplasm at high concentrations (0.2 M), corresponding to stress responses (Kurepin et al., 2015). Numerous prokaryotic and eukaryotic cells, from bacteria to higher species such as plants and animals, have been shown to contain compatible osmolytes, and these cells exhibit a great deal of chemical variety among different organisms (Rasheed et al., 2024). Numerous compatible solutes have been found in plant cellular systems, such as quaternary amines (betaine, polyamines, and dimethyl sulfoniopropionate), sugars (trehalose), polyols, including sugar alcohols (mannitol, sorbitol, pinitol, glycerol, and galactinol), amino acids (proline, glutamate, glutamine, and alanine), and their derivatives (ectoine and hydroxyectoine) (Khan et al., 2009; Narwal et al., 2024).
Glycine betaine (GB), a low-molecular-weight molecule with high solubility and low viscosity, can accumulate significantly in the photosynthetic apparatus, including chloroplasts and plastids (Ali et al., 2020). Because of these exceptional qualities, GB is among the most effective osmoprotectants (Annunziata et al., 2019). According to previous reports, the degree of stress tolerance is generally related to the buildup of the GB (Table 1). However, several plants do not accumulate GB under stressful or normal conditions (Zulfiqar et al., 2022). According to previous reports, GB is one of the most suitable solutes for effectively protecting plants from a variety of abiotic stressors (Ali et al., 2020; Zulfiqar et al., 2022). Plants’ ability to withstand abiotic stress can be enhanced using exogenous GB application and transgenic plant techniques. GB is easily absorbed by the root or leaf tissue when applied exogenously through nutrient or foliar spray (Ibrahim et al., 2023). It also effectively stabilizes the quaternary structure of proteins and enzymes, oxygen-evolving photosystem II (PSII), and components of the photosynthetic machinery such as ribulose-1,5-biphosphate carboxylase/oxygenase (RuBisCO). GB helps to stabilize the cellular structures, particularly membranes and proteins, under conditions of high salinity and temperature stress. By maintaining membrane integrity and enzyme function, GB enhances plant resilience to abiotic stresses (Tian et al., 2017; Wang et al., 2019).
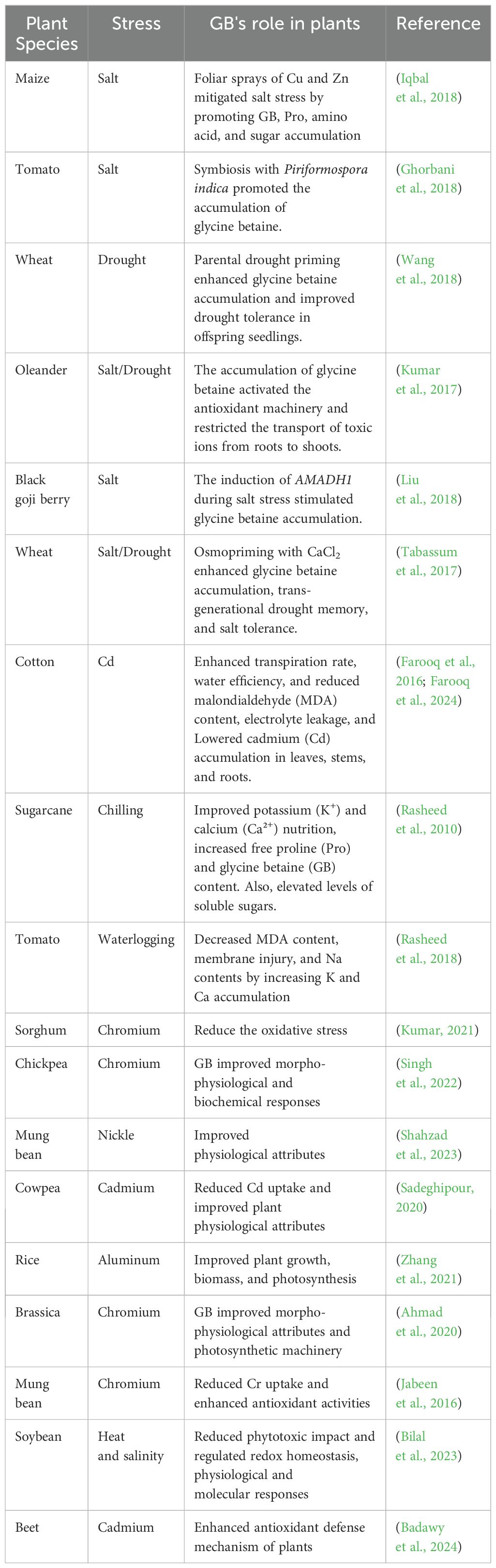
Table 1. The positive impact of glycine betaine (GB) supplementation on various plant species under different abiotic stresses.
Abiotic stress often triggers the overproduction of reactive oxygen species (ROS), which can result in oxidative stress (Basit et al., 2022a, b; Rao et al., 2025). GB has been found to have a positive effect in reducing oxidative damage caused by abiotic stressors in plants. According to Alcázar et al. (2010), exogenous hydroxyl radicals (OH•) caused a significant, dose-dependent outflow of K+ ions from epidermal cells in the elongation zone when applied to Arabidopsis roots. Nevertheless, this outflow of K+ ions was considerably decreased when GB was added to the incubation medium at a concentration of 5 mM. Exogenously administered GB also decreased cell membrane damage in cotton (Cheng et al., 2018) and the chilling-induced generation of H2O2 (Su and Wu, 2004) in tomato plants. By activating or stabilizing ROS-scavenging and/or suppressing the generation of ROS through other mechanisms, GB lessens the harmful consequences of oxidative stress even if it does not directly scavenge ROS. Fewer studies, nevertheless, have noted how ROS regulates plant growth and development and confers stress tolerance through its signaling function at lower cellular concentrations (Ali et al., 2020). The antioxidant system, which consists of both enzymatic and non-enzymatic components that scavenge or detoxify ROS (Ali et al., 2020; Zulfiqar et al., 2022), is one of the remarkable mechanisms that plants have evolved to maintain the cellular redox state and return oxidized macromolecules to their previous reduced states (Tian et al., 2017).
Thus, in this review, we have highlighted the biosynthesis, regulation, and protective mechanisms of GB in plants, emphasizing its various mechanisms for improving abiotic stress tolerance in plants. Additionally, we have provided information on how it can boost the growth and yield of different crop plants under stressful circumstances, as well as its synergistic effects with other genes that defend against stress. Furthermore, we discussed the interactive role of GB with phytohormones and other signaling networks to confer plant resistance to abiotic stress in order to boost productivity.
2 Biosynthesis and regulation mechanism of glycine betaine
2.1 Biosynthesis mechanism of glycine betaine in plants
Numerous data on GB synthesis in plants have been gathered, indicating that the cytoplasm, peroxisomes, and chloroplasts are the locations of GB production; nevertheless, the pathways by which GB is degraded remain unclear (Valenzuela-Soto and Figueroa-Soto, 2019). GB synthesis has been observed to influence the production of crucial plant growth regulators (PGRs), including auxin and ethylene (Annunziata et al., 2019). In the process of elucidating the synthesis of GB, it was discovered that phospho-ethanolamine N-methyltransferase (PEAMT), a cytosolic enzyme, catalyzes three consecutive adenosyl-methionine-dependent methylations of phospho-ethanolamine, which leads to the production of phospho-choline. Choline is subsequently produced from PEAMT-induced phosphocholine (PC), which has been proposed as the initial step in GB production (Ali et al., 2020). Plant-specific processes and distinct mechanisms are used to produce choline from PC. PC is promptly dephosphorylated to choline in spinach, but it is first incorporated into phosphatidylcholine in tobacco and then transformed into choline (McNeil et al., 2001). The bulk of the metabolic flux to choline occurs through PEAMT, which is subject to feedback regulation by PC. Therefore, PEAMT is a prime candidate for the genetic modification of plants to improve choline production (Annunziata et al., 2019).
Choline is converted into GB by a process known as the GB biosynthesis pathway, which can be one-step in certain organisms, such as Arthrobacter Spp., or two-step in most organisms including plants. Members of the two angiosperm families Chenopodiaceae and Gramineae provided the first information regarding this route in higher plants. In these plants, choline is converted to GB in two steps with an intermediate called betaine aldehyde (Valenzuela-Soto and Figueroa-Soto, 2019). The oxidation of choline to betaine aldehyde in plants is mediated by choline monooxygenase (CMO) in the first stage, whereas the subsequent oxidation of betaine aldehyde is mediated by betaine aldehyde dehydrogenase in the second step (Figure 1). According to Weigel et al. (1986), the two enzymes involved in this process are BADH (an NAD+-dependent betaine aldehyde dehydrogenase) and CMO (a ferredoxin-dependent cholinexygenase). In certain plants, the chloroplast stroma contains both enzymes (Annunziata et al., 2019). There is evidence of a peroxisomal NADPH-dependent CMO in certain other plants, such as barley, whereas betaine aldehyde dehydrogenase (BADH) is found in both the cytosol and chloroplasts (Annunziata et al., 2019; Valenzuela-Soto and Figueroa-Soto, 2019).
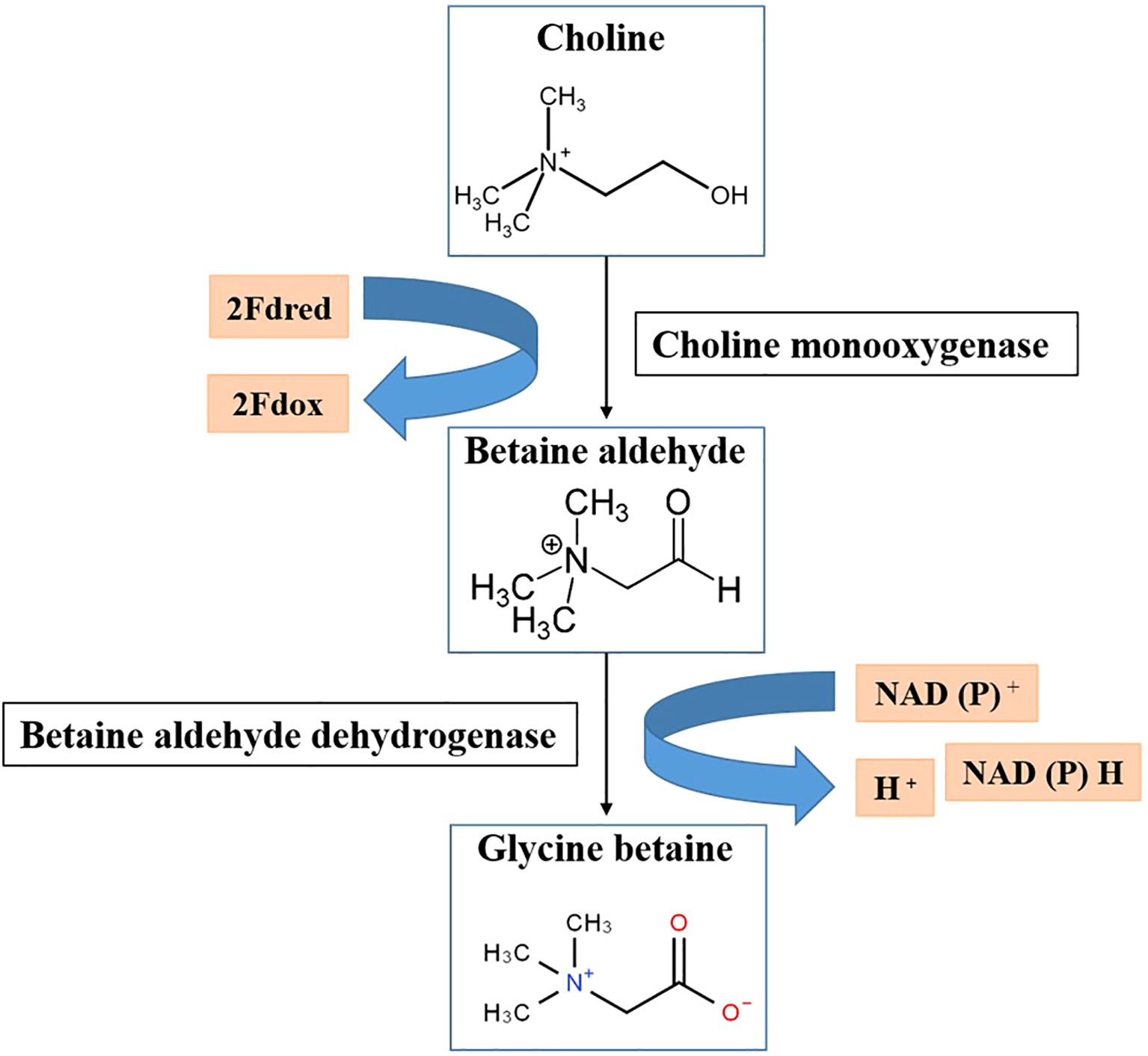
Figure 1. Biosynthesis of glycine betaine (GB) in plants. GB is synthesized from choline in two steps: choline is first converted to betaine aldehyde by choline monooxygenase (CMO), and then betaine aldehyde is further converted to GB by betaine aldehyde dehydrogenase (BADH). This pathway is crucial for stress tolerance, as GB stabilizes cellular structures under abiotic stress conditions. 2Fdred, 2Fe-ferredoxin reductase; 2Fdox, 2Fe-ferredoxin oxidized.
Despite having orthologs of both CMO and BADH, rice is regarded as a typical plant species that does not accumulate GB. Northern blotting showed increased OsCMO expression of the rice gene OsCMO. Higher GB content and improved resistance to salt stress are the outcomes of heterologous transgenic expression of OsCMO in tobacco. Although it was infrequently accumulated in wild-type plants, immunoblotting showed that a functional OsCMO protein of the proper size was present in transgenic tobacco. When rice seedlings were exposed to salt stress, a significant number of abridged OsCMO proteins were produced, indicating a deficiency in functional OsCMO (Sharma et al., 2023).
2.2 Regulation mechanism of glycine betaine biosynthesis in plants
According to previous reports, several plant-catalyzing enzymes use betaine aldehyde to synthesize GB from choline. According to previous reports, CMO, a ferredoxin-dependent stromal enzyme, is the initial stage of GB synthesis in spinach (Wu et al., 2023). This enzyme, also known as an unusual iron-sulfur (Fe-S) enzyme, has been shown to be triggered in spinach under salinity stress. The osmoregulatory GB production pathway involves the GB aldehyde dehydrogenase gene (gbsA), based on an investigation (Table 2). The GbsA enzyme is selective for GB aldehyde and prefers NAD+ as compared to NADP+ as a cofactor (Ghorbani et al., 2023). It was shown that the GbsA enzyme’s activity increased GB levels, which in turn conferred high salt tolerance. Based on emerging research, gbsA is now known as the betaine aldehyde dehydrogenase gene (BADH). Additionally, GB-induced abiotic stress tolerance in watermelon suspension cells is mediated by PGR-mediated signaling. The conserved CGTCA pattern found in the promoter regions of the CMO and BADH genes of watermelon, which are involved in GB production, responds to methyl jasmonate (MeJA) (Sharma et al., 2023). Additionally, MeJA was successful in eliciting the expression of both CMO and BADH, which resulted in the buildup of GB, comparable to the osmotic stress induced by mannitol. Furthermore, GB buildup is considerably reduced when ibuprofen, an inhibitor of JA production, is administered prior to osmotic stress (Xu et al., 2018).
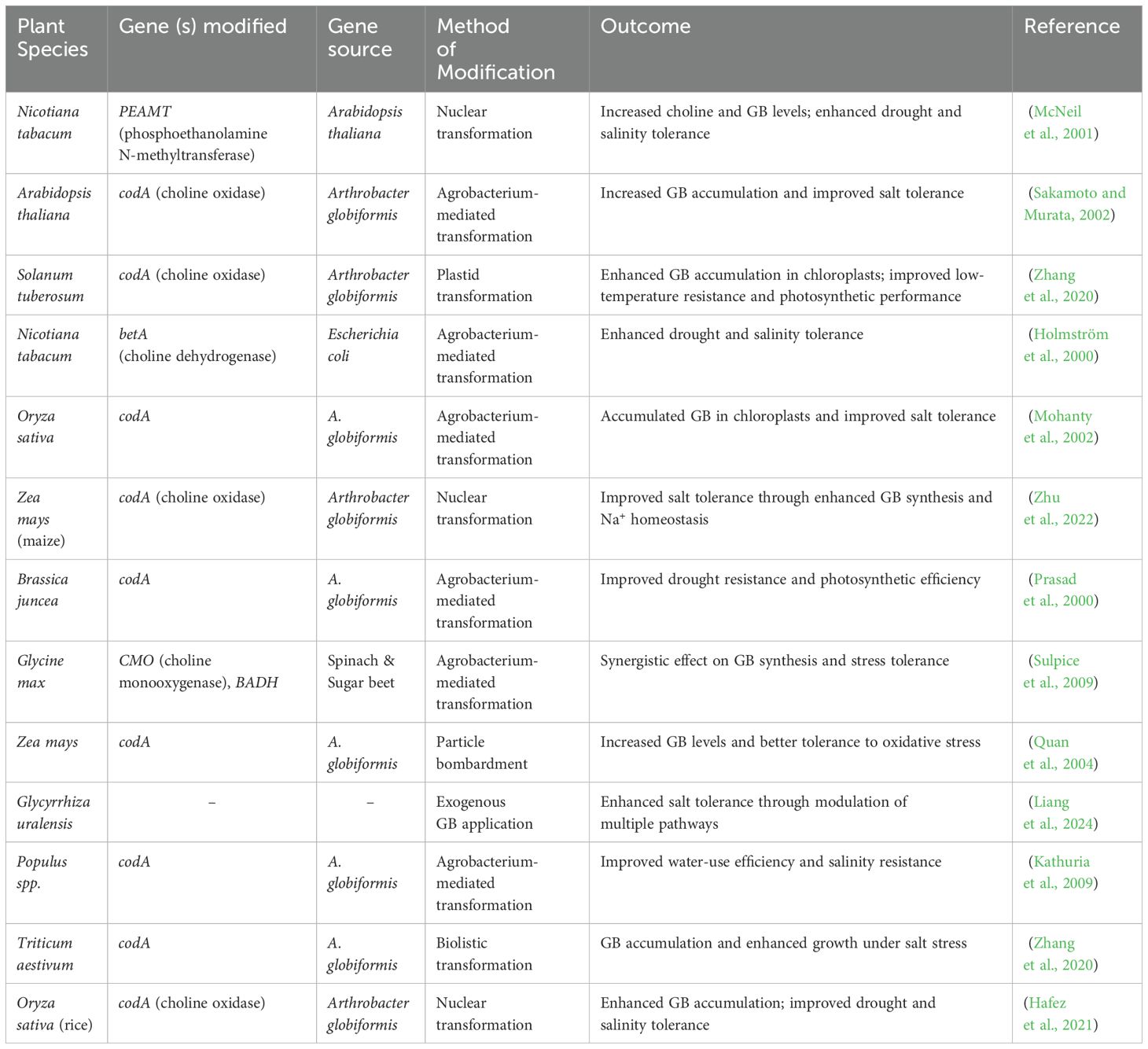
Table 2. Genetic engineering approaches to enhance glycine betaine (GB) synthesis in various plant species.
Gao et al. (2020) demonstrated that changes in lipid metabolism and GB synthesis play a key role in the development of salt tolerance in the halophytic seashore paspalum grass (Paspalum vaginatum) by regulating choline and genotypic variants. Through increases in digalactosyl diacylglycerol levels and phosphatidylinositol and phosphatidic acid synthesis, upregulation of GB synthesis led to choline-induced salt tolerance in the grass, which in turn led to changes in lipid metabolism.
3 Protective mechanism of glycine betaine in plants
3.1 Protective role of glycine betaine for translational and transcriptional machinery
Reinforcement of photosynthesis, osmoregulation, membrane integrity, detoxification of reactive oxygen radicals produced under stress conditions, and maintenance of the native structure of proteins and enzymes are suggested mechanisms for GB-mediated abiotic stress resistance (Hossain et al., 2021). However, due of the modest levels of GB accumulation in transgenic plants, these pathways might not fully account for the documented stress tolerance in these plants. Similar to plant hormones, the effective concentrations of GB upon absorption of exogenously applied GB or as a result of genetically modified GB production in vivo are within the millimolar range (Figueroa-Soto and Valenzuela-Soto, 2018). Therefore, it is equally logical to hypothesize that GB’s role in protecting the transcriptional and translational machinery may be mediated by activation of the expression of particular genes under a stress environment, whose products contribute to the establishment of stress tolerance (Hossain et al., 2021). GB might act as a modulator or signaling molecule under environmental stress, increasing the expression of genes related to osmoprotection, antioxidant defense, and protein stability. These genes produce antioxidant enzymes, heat shock proteins, molecular chaperones, and other defense mechanisms that preserve the integrity of ribosomes, RNA polymerases, and related regulatory proteins. Moreover, GB helps to preserve the structure and function of macromolecular complexes i.e., transcription factors and ribosomes. By protecting these complexes from denaturation or aggregation during abiotic stress, GB ensures the continuity of gene expression and protein synthesis, thereby enhancing cellular stress tolerance. This dual functionality positions GB as a key osmoprotectant and regulatory molecule in stress adaptation mechanisms (Sakamoto and Murata, 2002).
These gene products enhance the organism’s general ability to withstand stress by reducing oxidative damage and stabilizing macromolecular structures. Furthermore, GB might have an impact on transcription factors and epigenetic regulators, which would further optimize the cellular reaction to stress. As a result, its function goes beyond just stabilizing cellular machinery; it also actively participates in stress-adaptive gene expression networks, which eventually improves productivity and survival in challenging circumstances (Hossain et al., 2021).
GB has been shown to bind to double-stranded DNA in vitro, suggesting a potential role in modulating nucleic acid structure and function. Under high-salinity conditions, GB acts as a regulator of gene expression by influencing transcription and DNA replication processes. Transcriptomic analyses using cDNA microarrays have revealed that transgenic plants accumulating GB exhibit altered expression of endogenous genes involved in stress responses, particularly those related to osmolyte biosynthesis and antioxidant defense (Kahraman et al., 2019). In tomato and wheat plants, exogenous application of GB led to significant changes in transcript levels of stress-responsive genes. For instance, GB treatment upregulated the cat1 gene, which encodes catalase, an essential antioxidant enzyme in tomato, enhancing catalase activity especially under low-temperature stress (Park et al., 2006). Similarly, in wheat, GB influenced the expression of cold-responsive proteins such as WCOR410 and WCS120, which are associated with cellular protection during freezing conditions (Hossain et al., 2022). These findings indicate that GB not only functions as an osmoprotectant but also modulates the transcription of key genes involved in abiotic stress tolerance pathways.
Additionally, gene expression in the flower buds of wild-type and cod-A transgenic tomato was compared using cDNA microarray, wherein the expression of 29 genes was suppressed and the expression of 30 genes was enhanced (Park et al., 2007). Furthermore, Jarin et al. (2024) showed that GB functions similarly to chaperonins in vivo. This seems to imply that GB may maintain the translational and transcriptional machinery required for effective gene expression in stressed situations. The ability of GB and its products to activate specific genes in transgenic plants may aid in developing an understanding of GB-enhanced stress tolerance in plants.
3.2 GB-mediated protection of photosynthetic machinery
The repair of PSII is inhibited by different abiotic stressors that produce ROS. Heavy metals, low temperatures, mild heat, salinity, and CO2 limitation produce ROS by inhibiting CO2 fixation and lowering 3-phosphoglyceric acid levels. The amount of NADP+ decreases when photosynthetic fixation of CO2 is suppressed. Because PSI lacks the primary electron acceptor (NADP+), electrons are transported to molecular oxygen more quickly, producing H2O2 via O2.- manufacturing (Chauhan et al., 2023). Consequently, these ROS prevent PSII repair by blocking protein synthesis. Therefore, by preventing PSII repair, ROS increases the degree of photoinhibition. While the repair of the PSII complex entails several procedures that guarantee the removal and replacement of the damaged D1 protein, photodamage to PSII is defined by photochemical damage to a component of the PSII reaction center, namely, the D1 protein (Chauhan et al., 2023).
Furthermore, surplus electrons from PSI are transformed into ROS when photosynthetic fixation of CO2 is reduced under abiotic stress. This prevents the translation stage of the pre-D1 protein from being synthesized, which consequently prevents the repair of the photodamaged PSII. GB may maintain the fixation of CO2 by shielding CO2-fixing enzymes (Rubisco and Rubisco activase) from abiotic stress, which effectively inhibits the generation of ROS. Additionally, GB modulates the genes encoding ROS-scavenging enzymes, which break down ROS and lower their concentrations in cells, thereby lessening the impact of abiotic stress on the photosynthetic apparatus (Didaran et al., 2024). Ohnishi and Murata (2006) documented the interaction between GB synthesis and the effect of salt stress on PSII photoinhibition. While GB, which had been produced in vivo, shielded PSII from photoinhibition under these circumstances, salt stress from 220 mM NaCl increased PSII photoinhibition. However, the photodamage to PSII was unaffected by salt stress or GB synthesis. In contrast, GB reversed the inhibitory effect of salt stress, which prevented the effective repair of the photodamaged PSII. According to pulse-chase labeling tests, salt stress decreased both the de novo synthesis of the D1 protein and its degradation in photodamaged PSII. However, under salt stress, GB shielded PSII from suppressing D1 protein synthesis and degradation. GB and salt stress had no effect on psbA transcript levels. These findings imply that GB might accelerate the repair of photodamaged PSII by counteracting the inhibitory effects of salt stress.
Furthermore, Yang and Lu (2005) investigated the effects of mild heat stress and GB production in tobacco plants that had been modified to produce GB in vivo. Moderate heat stress was observed to block RuBisCO activase, which ultimately restricted the fixation of CO2. ROS production was accelerated by these conditions, preventing PSII from being repaired. Rubisco activase is likely protected against moderate heat-induced inhibition by GB. The defense mechanisms of the PSII repair system against mild heat stress and salt stress appear to possess certain characteristics, i.e., the upregulation of protective proteins, enhanced expression and synthesis of the D1 protein, and activation of chloroplast proteases and chaperones that facilitate the efficient degradation and replacement of damaged PSII components. Together, these responses help maintain photosynthetic efficiency by ensuring dynamic recovery of PSII under fluctuating environmental conditions (Nishiyama et al., 2006). Further research is required to thoroughly understand the mechanisms underlying how different types of environmental stressors affect photoinhibition and photosynthesis.
4 Role of exogenous GB application in the plant’s abiotic stress tolerance mechanism
Numerous environmental stressors typically affect the growth, development, yield, and productivity of different plants. Abiotic stress has become a serious global issue that significantly limits the yield of food and economic crops (Liang et al., 2024). Plants have evolved a number of natural stress-protective mechanisms to combat abiotic stress (Figure 2). However, plants cannot withstand or adapt to higher levels of abiotic stressors or many abiotic stresses (Ghorbani et al., 2023). Therefore, depending on the crops and their diverse species, farmers use a variety of exogenous treatments with different agrochemicals, pesticides, PGRs, growth stimulators, osmolytes, or osmoprotectants, among others, to ensure that plants survive harsh conditions. The crucial role of the accumulation of osmoprotectants, such as GB and proline, in osmotic adjustments and considerable improvements in plant stress physiology under stress- and tissue-specific regulation in response to multiple abiotic stresses has now been fully documented (Dutta et al., 2019).
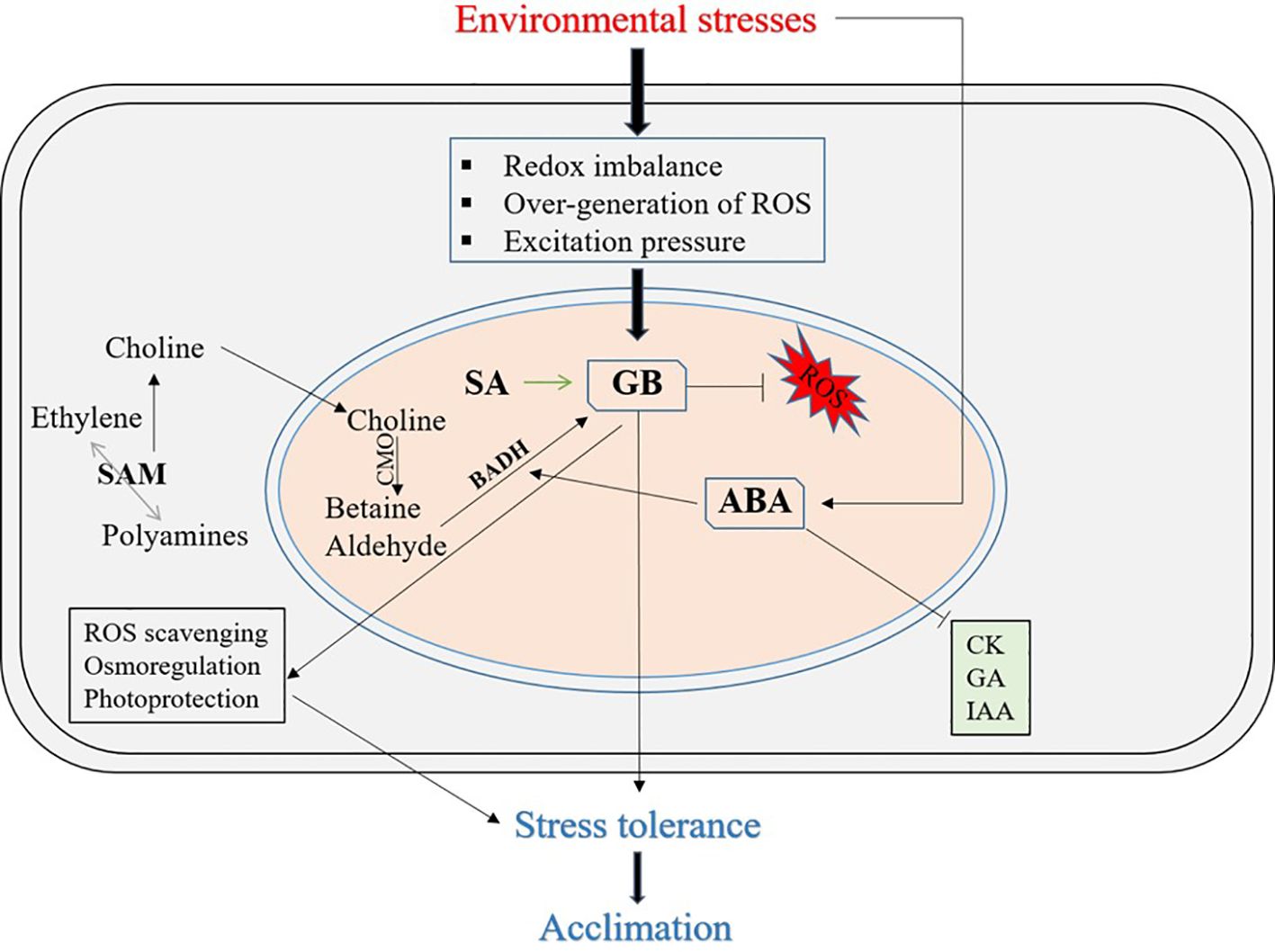
Figure 2. A schematic model illustrating the role of GB in mitigating abiotic stress effects in plants, highlighting its interactions with photosynthesis, plant hormones, and reactive oxygen species (ROS). The figure highlights GB's interactions with key physiological processes under stress conditions, including its influence on plant hormones and reactive oxygen species (ROS). GB helps stabilize cellular structures, protect chloroplasts, and regulate hormonal balance, thereby enhancing the plant’s ability to withstand environmental stressors. Additionally, GB modulates ROS scavenging mechanisms, preventing oxidative damage and maintaining osmoregulation. ROS, Reactive Oxygen Species; SAM, S-adenosylmethionine; SA, Salicylic Acid; CMO, Choline Monooxygenase; CK, Cytokinins; GA, Gibberellins; IAA, Indole-3-acetic acid; ABA, Abscisic Acid; BADH, Betaine Aldehyde Dehydrogenase.
It has been found that GB foliar application topically to onion plants increases their resistance to salt stress by boosting their antioxidant defense systems, which are essential for reducing oxidative damage and scavenging reactive oxygen species (ROS). Furthermore, GB strengthens the plant’s defensive mechanisms by encouraging the accumulation of non-enzymatic antioxidants such as glutathione, ascorbic acid, and phenolic substances. Lower levels of malondialdehyde (MDA) indicate a reduction in lipid peroxidation, which GB helps to maintain membrane integrity and protects against cellular damage under salt stress. Additionally, it prevents excessive ion toxicity by controlling the balance of potassium (K+) and sodium (Na+), which helps maintain ion homeostasis. By these means, GB helps onion plants better tolerate saline conditions by reducing oxidative stress and promoting general plant development and physiological performance (Rady et al., 2018). Exogenous GB supplementation has been shown to reduce salt stress in Phaseolus vulgaris (common bean) by preserving a high K+/Na+ ratio, limiting the uptake of Na+ ions, and promoting antioxidant defense mechanisms to reduce salinity-induced toxicity. However, by improving K+ uptake and maintaining a higher K+/Na+ ratio, GB treatments simultaneously produced salt stress tolerance, which in turn caused the common bean plants to experience osmoregulation (Sofy et al., 2020). Additionally, it was found that GB-induced salt stress protection in common beans is more closely linked to ion uptake regulation than to the capacity of the antioxidant defense system (Table 1). It is stated that GB stabilizes plasma membrane integrity and transporter proteins, thereby reducing nonspecific ion influx and leakage (Chen and Murata, 2008; Chen and Murata, 2011). Also, GB modulates the activity and expression of specific ion transporters and channels, such as H+-ATPases and metal transporter families (ZIP, NRAMP), enhancing selective uptake and sequestration of essential ions while limiting toxic metal accumulation (Hasanuzzaman et al., 2020). Additionally, GB enhances antioxidant defense systems, indirectly supporting ion homeostasis by protecting transporters from oxidative damage (Ali et al., 2020). These combined effects enable plants to maintain nutrient uptake and reduce heavy metal toxicity under stress conditions.
Furthermore, GB has been shown to control several cellular metabolism-related functions in addition to acting as an osmolyte (Figueroa-Soto and Valenzuela-Soto, 2018). GB plays a major role in protein synthesis and is actively involved in the control of homocysteine/methionine pathway enzymes, metabolism of carbohydrates (glucose, sucrose, and fructose), glycogen, lipogenesis, and lipid oxidation. Based on previous research on the underlying mechanisms of GB actions, GB may alter the phosphorylation status of certain kinases or cause changes in the methylation status of target gene promoters to alter the activities of enzymes. By enhancing growth, development, photosynthesis, antioxidant enzyme activity, nutrient or mineral uptake, regulating antioxidant levels, and lowering the uptake of excessive heavy metals, GB has been proposed to enable plants to withstand heavy metal stress, thereby limiting oxidative stress (Ali et al., 2020).
In addition, Huang et al. (2020) determined that GB increases PSII’s oxygen-evolving activity of PSII, in addition to stabilizing its structure. The oxygen-evolving complex (OEC), which is essential for effective water splitting and oxygen synthesis, is kept intact in part by GB. GB shields PSII from oxidative degradation and stops photoinhibition by stabilizing important PSII proteins like D1 and D2. Furthermore, GB ensures the best possible electron transfer efficiency by assisting in the maintenance of the correct configuration of PSII-associated cofactors, such as manganese (Mn) clusters. In the end, this structural stabilization improves energy generation and photosynthetic efficiency, enabling plants to maintain improved growth and stress tolerance. Under drought stress conditions, GB treatment remarkably decreased the levels of hydrogen peroxide, superoxide ions, and malonaldehyde in maize. Moreover, it has been shown that GB treatment increases maize resistance to salinity stress by controlling physiological characteristics, ionic homeostasis, and antioxidant defense systems (Dustgeer et al., 2021). A recent study by Zulfiqar et al. (2022) emphasized the key role of GB in providing plant thermotolerance and claimed that exogenous GB treatments, such as seed priming or plant priming, increase endogenous levels of GB, which helps plants to tolerate heat stress. Likewise, it has been stated that foliar application of GB greatly improves the morphology, growth, chlorophyll levels, relative water content, and gas-exchange characteristics of maize under conditions of water deficit, with the exception of intercellular CO2 concentration, osmolytes, ROS accumulation, antioxidant enzyme activities, and yield (Shemi et al., 2021). Wang et al. (2024) recently demonstrated that GB synthesis by genetic engineering might increase the tolerance of tobacco photosynthetic apparatus against drought stress. This study compared the ability of transgenic tobacco to withstand drought stress by accumulating GB in wild-type tobacco plants. By measuring photosynthetic gas exchange parameters, thylakoid membrane protein function, chloroplast structure, thylakoid membrane/lamellae structure or stability, and chlorophyll fluorescence, it was discovered that transgenic tobacco improved the photosynthetic apparatus’s resistance to drought. In addition, through greater oxidation of the xanthophyll cycle, genetic engineering of GB synthesis could improve the unsaturated fatty acid index, increase digalactosyl diacylglycerol and phosphatidylglycerol levels, and reduce the photoinhibition of PSII in tobacco exposed to drought stress.
5 GB cross-talk with phytohormone and signaling molecules
Phytohormones are the primary signaling molecules in plants and play a crucial role in controlling abiotic stress responses in all plant species. In essence, stress hormones, such as ABA, ethylene, salicylic acid (SA), and other conventional plant growth regulators (PGRs), play a role in controlling plant signaling (Basit et al., 2021; Aerts et al., 2021). Other molecules, such as charged PAs, the gasotransmitter nitric oxide (NO), and even plant nutrients, balance the cellular environment when the body reacts to unfavorable conditions (Wu et al., 2024). During abiotic stress, glycine betaine stabilizes the cellular osmoticum and detoxifies ROS molecules by inducing antioxidant machinery. This process requires complex interactions between signaling-related molecular components (Table 3).
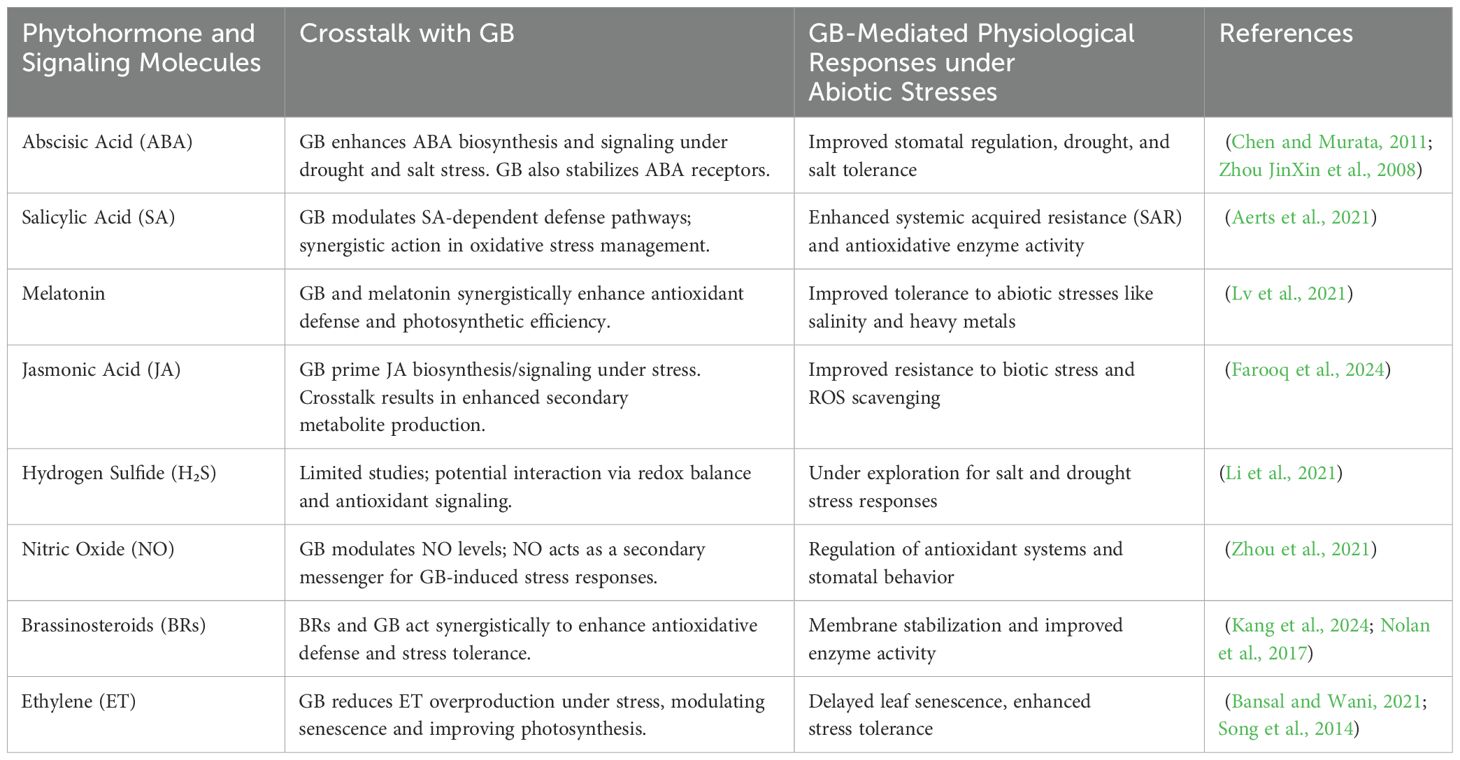
Table 3. Crosstalk between glycine betaine and phytohormones/signaling molecules in regulating plant stress responses.
5.1 Phytohormones
5.1.1 Ethylene
The gaseous hormone ethylene is necessary for plant growth and development (Khan et al., 2024). Additionally, by encouraging stem elongation, leaf area expansion, stem radial growth, and leaf thickness, ethylene increases the resistance to extreme forms of freezing that are relatively severe (Kurepin et al., 2017). As choline, a common mediator molecule, is involved, ethylene and GB production are closely related. A methyl group is transferred from S-adenosyl methionine (SAM) to choline, which facilitates ethylene production. Therefore, the production of ethylene may occur at the expense of GB synthesis or alternatively. To ensure development and survival, plants under extreme abiotic stress conditions produce endogenous GB (Wang et al., 2024). This may occur at the expense of ethylene production because choline is channeled into GB synthesis. Tobacco and tomatoes, which are high ethylene-accumulating species, did not accumulate large amounts of GB under cold or other stresses. Additionally, in response to abiotic stresses, species with minimal ethylene accumulation showed higher GB production (Kurepin et al., 2017).
5.1.2 Abscisic acid
Abscisic acid (ABA) is considered a universal stress phytohormone that regulates a wide range of physiological processes under abiotic stressors. In response to stress, plant tissues assemble relatively high levels of ABA (Roychoudhury and Banerjee, 2017). In addition to preventing unintended desiccation and water loss, this enables quick stomatal closure. Exogenous ABA treatment promotes GB accumulation in barley plants under drought, salt, and low-temperature stresses (Jagendorf and Takabe, 2001; Holmström et al., 2000). An intriguing study demonstrated that the exogenous application of either ABA or GB improved the ability of Arabidopsis to withstand freezing temperatures. Nevertheless, GB production was boosted by ABA-only treatment, suggesting that ABA may function upstream of the GB biosynthesis pathway. An investigation regarding the temporal regulation between ABA and GB determined that drought stress initially augmented the endogenous ABA content in the cells of Zea mays seedlings, followed by BADH transcription and GB content (Zhang et al., 2012). Reduced photosynthetic activity may result from the efficient induction of stomatal closure caused by ABA accumulation under abiotic stress. Nonetheless, the functions of GB in enhancing photosynthetic efficiency under abiotic stress have been addressed earlier. The roles of ABA and GB might seem incompatible because ABA positively regulates GB production. However, exogenous ABA application decreased stomatal conductance without decreasing Tradescantia virginiana’s photosynthetic ability, according to the investigation of Franks and Farquhar Franks and Farquhar (2001). This inference most probably emphasizes the correlated functions of ABA and GB in response to abiotic stresses.
5.1.3 Salicylic Acid
Abiotic stress tolerance in plants is also linked to endogenous salicylic acid (SA) accumulation. Salicylic acid promotes the synthesis of both enzymatic and non-enzymatic antioxidants, which effectively scavenge ROS and provide a defense against oxidative stress (Salinas et al., 2025). Additionally, exogenous SA supplementation has helped to develop tolerance to drought, salt, heavy metal toxicity, and temperature extremes (Paul et al., 2024). In contrast to the amount of grain production in the stressed plants treated separately with SA or GB, Aldesuquy et al. (2012) demonstrated that the co-application of SA and GB further increased the yield in drought-stressed wheat plants.
5.1.4 Other Traditional Plant Growth Regulators
Auxins, gibberellic acids (GAs), and cytokinins (CKs) are traditional plant growth regulators (PGRs). They are involved in growth, development, stem elongation, polar solute transport, phototropism and gravitropism, cell division, and initiation of reproduction (Singh et al., 2024). ABA and GA are known to have antagonistic relationships. GA production is suppressed during abiotic stress when endogenous ABA levels increase (Kurepin et al., 2017). As GB synthesis is triggered by ABA accumulation, it may be concluded that GB and GA have a negative relationship. Bao et al. (2011) noticed that transgenic Lolium perenne plants that overexpressed CMO and BADH genes from spinach chloroplasts had reduced GA1 levels, which is consistent with this hypothesis. Additionally, there was a negative correlation between cytokinin levels and GB accumulation. Kathuria et al. (2009) found that transgenic rice plants overexpressing choline oxidase (coda) showed higher production of CK dehydrogenase 1 (CKX1), an enzyme that breaks down CK. Plants had low CK concentrations and significant GB accumulation. GB synthesis is also negatively regulated by auxin. In Kochia scoparia plants subjected to salt stress, exogenous administration of the synthetic auxin dicamba decreased CMO expression and, consequently, GB accumulation (Kern and Dyer, 2004).
5.2 Polyamines
Glycine betaine and polyamine crosstalk as compatible solutes to control the cellular osmoticum during abiotic stressors (Paul et al., 2017). GB is associated with polyamines, such as putrescine, spermidine, and spermine, because they perform their corresponding functions. Prior to salt treatment, seedlings primed with spermidine and spermine exhibited higher levels of BADH1 expression in the shoots of the salt-tolerant rice cultivar Nonabokra than in the salt-sensitive rice cultivar IR-64. This was in contrast to seedlings that were not primed under stress. Conversely, priming with spermidine and spermine promoted the accumulation of BADH1 transcripts in Nonabokra roots and decreased gene expression in IR-64 roots subjected to 75 mM NaCl (Banerjee and Roychoudhury, 2018).
In the roots and shoots of Gobindobhog, another salt-sensitive, aromatic rice cultivar, polyamine priming of salt-stressed seedlings increased BADH1 expression (Paul and Roychoudhury, 2017). These findings unequivocally demonstrate how PAs and the mechanism involved in GB biosynthesis interact when rice is subjected to salt stress. Under salt stress, the amount of GB in Arabidopsis increases due to the overexpression of aldehyde dehydrogenase 10A8 (ALDH10A8) and ALDH10A9 (encoding functional BADH). Additionally, transgenic plants showed a decrease in putrescine and spermidine content during salinization, as well as an increase in free PAs such as agmatine, cadaverine, and tyramine (Rippa et al., 2012). The ability of BADHs to oxidize 3-aminopropanal, an intermediate molecule in PA catabolism, was demonstrated by Rippa et al (Bansal and Wani, 2021). 3-Aminopropanal is produced when polyamine oxidase (PAO) oxidizes spermine, and ALDH10A8 and ALDH10A9 are used as substrates (Missihoun et al., 2015). This indicated the direct involvement of BADH isoforms in spermine degradation. Choudhary et al. (2012) reported that exogenous supplementation with spermidine and epibrassinolide (an active brassinosteroid) markedly elevated the titers of cellular antioxidants, such as GB, Pro, AsA, GSH, and total phenolics, in Raphanus sativus seedlings subjected to Cr stress. This led to the effective scavenging of excess ROS and the acquisition of stress tolerance. This demonstrates that PAs, GB, and brassinosteroids are regulated in three steps to induce stress-induced responses. When Stevia rebaudiana, a perennial medicinal herb, was supplemented with synthetic PA (diamino hydroxyl ethyl phospho mineral and diaminohexanoic mineral amino ethanoic acid), the endogenous GB and Pro levels amplified in response to cold stress (Moradi Peynevandi et al., 2018).
5.3 Gasotransmitters
Nitric oxide (NO) and hydrogen sulfide (H2S) are gaseous chemicals involved in various plant-signaling systems. Additionally, these gasotransmitters help plants to become more resilient to multiple abiotic stressors (Basit et al., 2023). They are essential, concentration-dependent redox-related signaling molecules (Basit et al., 2024a). Cellular generation of H2S was elevated in Spinacia oleraceaseedlings when sodium hydrosulfide (NaHS) was applied exogenously. Following NaHS treatment, there was an increase in the transcription of BADH and CMO genes. In treated plants, the H2S-induced buildup of GB programmable the antioxidant machinery and enhances drought resistance (Chen et al., 2016). Unknown signaling connections between H2S and GB that have not been thoroughly studied have been suggested by these studies. Zanganeh et al. (2018) recently demonstrated that seed priming with SA and NaHS caused GB and NO accumulation in Z. mays seedlings grown under Pb stress. Additionally, treated plants showed decreased expression of 1-aminocyclopropane-1-carboxylate synthase 6 (ACS6), a gene involved in ethylene biosynthesis. Choline channelization toward GB synthesis rather than ethylene generation was the cause of this phenomenon. Arginine metabolism is most likely directed toward NO formation rather than PA synthesis, as evidenced by the decrease in the PA biosynthesis gene, SAM decarboxylase (SAMDC), and the generation of NO during Pb stress (Zanganeh et al., 2018). Therefore, the combined effects of H2S, NO, and GB on Pb tolerance in maize plants were investigated. GB and NO have a positive regulatory relationship in maize seedlings under oxidative stress (Phillips et al., 2018). Nitric oxide synthase (NOS) inhibitor-treated seedlings showed decreased BADH expression and increased GB. As a result of decreased GB production, the activity of antioxidant enzymes is also inhibited. In seedlings treated with NOS inhibitors, there was an increase in H2O2 accumulation due to a decrease in CAT expression (Phillips et al., 2018).
6 Plant engineering to improve GB biosynthesis
Abiotic stresses can reduce plant growth, yield loss, and even plant death, severely affecting agricultural productivity. Factors, such as prolonged drought, high salinity, and extreme temperatures, disturb plant metabolism and cause cellular damage, including oxidative stress, dehydration, and ionic imbalances. Recent biotechnological advancements, particularly in the regulation of molecular targets and the development of transgenic plants, have facilitated a deeper understanding of GB-mediated tolerance to abiotic stress. However, the molecular biology of GB production has not yet been thoroughly explored. Recent studies have confirmed the molecular mechanisms underlying GB accumulation in response to stress. Proteomic studies have identified BADH as a marker for salt stress in T. monococcum seedlings (Lv et al., 2016). The proteome of the seedlings subjected to salt stress was examined using two-dimensional gel electrophoresis (2-DE) in conjunction with MALDI-TOF-TOF mass spectrometry. This analysis led to the identification of proteins associated with regulatory functions, stress protection, protein dynamics, and metabolic pathways. Subsequent investigations have confirmed that BADH, leucine aminopeptidase 2, Cu/Zn superoxide dismutase, and 2-Cys peroxiredoxin BAS1 serve as molecular markers indicative of the response to salt stress (Hasanuzzaman et al., 2019). The significance of BADH in abiotic stress tolerance was demonstrated through a knockdown experiment employing RNA interference (RNAi). In rice, BADH1-RNAi repression lines showed diminished yield and reduced tolerance to salt, drought, and cold conditions. This phenomenon has been attributed to increased levels of MDA and H2O2 accumulation in plant tissues (Tang et al., 2014). Notably, the repression lines did not exhibit alterations in the endogenous GB content. Therefore, the observed decline in stress tolerance may be linked to decreased dehydrogenation of aldehydes that accumulate in various metabolic pathways.
Major cereal crops, including barley, wheat, and maize, exhibit limited capacity to accumulate GB under normal conditions. Consequently, transgenic strategies aimed at overexpressing genes involved in GB biosynthesis have been implemented to improve plant resilience to abiotic stress. Rice, a key staple food crop, possesses one CMO and two BADH homologs (Baicharoen et al., 2018; Ghosh and Roychoudhury, 2018; Hernandez-Leon and Valenzuela-Soto, 2023). However, the deletion of the translational initiation codon, loss of essential functional domains, and emergence of premature stop codons due to frameshift mutations collectively lead to the production of non-functional BADH2 homologs. This gene plays a crucial role in the synthesis of the aromatic compound 2-acetyl-1-pyrroline found in aromatic rice varieties (Baicharoen et al., 2018). In contrast, the functional BADH1 homolog is involved in the production of GB under osmotic stress (Ghosh and Roychoudhury, 2018). Genetic engineering of GB biosynthesis in GB non- or low-accumulators has been tested in diverse plant species, including Arabidopsis, tobacco, Brassica, tomato, maize, rice, potato, and wheat. This results in an increased tolerance to multiple stresses (Sharma et al., 2024). The association between ion channel regulation and GB has been documented by Ahire et al. (2018). This study demonstrated that overexpression of vacuolar proton pyrophosphatase (VPPase) derived from Sorghum bicolor in Bacopa monnieri resulted in increased salt tolerance, which was attributed to the enhanced production of GB. Furthermore, transgenic plant shoots showed reduced levels of malondialdehyde (MDA) and exhibited diminished membrane damage, which can be ascribed to the GB-mediated activation of the antioxidant system and the efficient scavenging of harmful reactive oxygen species (ROS) (Ahire et al., 2018).
Overexpression of genes responsible for stress-related proteins has been shown to stimulate the synthesis of GB in transgenic plant tissues (Zulfiqar et al., 2022). For instance, the introduction of the tobacco osmotin gene into chilli pepper plants led to improved salt tolerance, which was attributed to the increased production of GB and proline. These transgenic plants demonstrated elevated activities of superoxide dismutase (SOD), glutathione reductase (GR), and ascorbate peroxidase (APX), along with a higher relative water content (RWC) under stress conditions when compared to control plants (Subramanyam et al., 2011). Additionally, the G-protein RabAc4, which plays a role in membrane trafficking, has been identified to work in conjunction with GB to enhance chilling tolerance in tomato seedlings (Subramanyam et al., 2011). Summary of transgenic strategies aimed at boosting GB accumulation and enhancing tolerance to abiotic stress.
7 Conclusion and future perspectives
Glycine betaine, the principal organic osmolyte, plays multiple roles in plant growth and development. Many higher plants naturally synthesize GB as a protective measure in response to various environmental stresses. In addition to its function as an osmoprotectant, GB plays a crucial role in stabilizing cytosolic pH, proteins, enzymes, and membranes while also scavenging harmful ROS and preserving the redox balance within cells, thereby protecting subcellular structures in plants under stress. Furthermore, GB helps maintain ionic equilibrium in plants under different stress conditions. Recent research has extensively demonstrated that both endogenous and exogenous GB enhance plant tolerance to various abiotic stress events. Recent research suggests that differentially expressed endogenous genes play a crucial role in the stress tolerance of higher plants mediated by GB. This process involves scavenging of ROS, management of oxidative stress, modulation of gene expression, and enhanced accumulation of GB under abiotic stress conditions. Owing to the diverse functions of the genes involved in the GB biosynthesis pathway, efforts are underway to create transgenic plants that can efficiently accumulate GB, thus exhibiting improved resilience to various abiotic stresses, including secondary oxidative bursts. Despite ongoing research on GB, its signaling mechanisms remain poorly understood and require further exploration.
Author contributions
FB: Conceptualization, Writing – original draft. MA: Writing – review & editing. FH: Writing – review & editing. WA: Writing – review & editing. AE: Writing – review & editing. SS: Writing – review & editing. MS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aerts, N., Pereira Mendes, M., and Van Wees, S. C. (2021). Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 105, 489–504. doi: 10.1111/tpj.15124
Ahire, M., Anil Kumar, S., Punita, D., Mundada, P., Kavi Kishor, P., and Nikam, T. (2018). The vacuolar proton pyrophosphatase gene (SbVPPase) from the Sorghum bicolor confers salt tolerance in transgenic Brahmi [Bacopa monnieri (L.) Pennell. Physiol. Mol. Biol. Plants. 24, 809–819. doi: 10.1007/s12298-018-0586-4
Ahmad, R., Ali, S., Abid, M., Rizwan, M., Ali, B., Tanveer, A., et al. (2020). Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ. Sci. pollut. Res. 27, 1101–1111. doi: 10.1007/s11356-019-06761-z
Alcázar, R., Altabella, T., Marco, F., Bortolotti, C., Reymond, M., Koncz, C., et al. (2010). Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231, 1237–1249. doi: 10.1007/s00425-010-1130-0
Aldesuquy, H. S., Abbas, M. A., Abo-Hamed, S. A., Elhakem, A. H., and Alsokari, S. S. (2012). Glycine betaine and salicylic acid induced modification in productivity of two different cultivars of wheat grown under water stress. J. Stress Physiol. Biochem. 8, 72–89.
Ali, S., Abbas, Z., Seleiman, M. F., Rizwan, M., YavaŞ, İ., Alhammad, B. A., et al. (2020). Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 9, 896. doi: 10.3390/plants9070896
Annunziata, M. G., Ciarmiello, L. F., Woodrow, P., Dell’Aversana, E., and Carillo, P. (2019). Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 10, 230. doi: 10.3389/fpls.2019.00230
Badawy, A. A., Alamri, A. A., Hussein, H.-A. A., Salem, N. F., Mashlawi, A. M., Kenawy, S. K., et al. (2024). Glycine betaine mitigates heavy metal toxicity in beta vulgaris (L.): an antioxidant-driven approach. Agronomy 14, 797. doi: 10.3390/agronomy14040797
Baicharoen, A., Vijayan, R., and Pongprayoon, P. (2018). Structural insights into betaine aldehyde dehydrogenase (BADH2) from Oryza sativa explored by modeling and simulations. Sci. Rep. 8, 12892. doi: 10.1038/s41598-018-31204-z
Bailey-Serres, J., Parker, J. E., Ainsworth, E. A., Oldroyd, G. E., and Schroeder, J. I. (2019). Genetic strategies for improving crop yields. Nature 575, 109–118. doi: 10.1038/s41586-019-1679-0
Banerjee, A. and Roychoudhury, A. (2018). Seed priming technology in the amelioration of salinity stress in plants. Adv. seed priming, 81–93. doi: 10.1007/978-981-13-0032-5_5
Bansal, M. and Wani, S. H. (2021). “Cross-talk of compatible solutes with other signalling pathways in plants,” in Bansal, M. and Wani, S. H. (Eds.), Compatible Solutes Engineering for Crop Plants Facing Climate Change (Cham, Switzerland: Springer Nature), pp. 205–222. doi: 10.1007/978-3-030-49325-7_12
Bao, Y., Zhao, R., Li, F., Tang, W., and Han, L. (2011). Simultaneous expression of Spinacia oleracea chloroplast choline monooxygenase (CMO) and betaine aldehyde dehydrogenase (BADH) genes contribute to dwarfism in transgenic Lolium perenne. Plant Mol. Biol. Rep. 29, 379–388. doi: 10.1007/s11105-010-0243-8
Basit, F., Abbas, S., Sheteiwy, M. S., Bhat, J. A., Alsahli, A. A., and Ahmad, P. (2024a). Deciphering the alleviation potential of nitric oxide, for low temperature and chromium stress via maintaining photosynthetic capacity, antioxidant defence, and redox homeostasis in rice (Oryza sativa). Plant Physiol. Biochem. 214, 108957. doi: 10.1016/j.plaphy.2024.108957
Basit, F., Bhat, J. A., AlYemeni, M. N., Shah, T., and Ahmad, P. (2023). Nitric oxide mitigates vanadium toxicity in soybean (Glycine max L.) by modulating reactive oxygen species (ROS) and antioxidant system. J. Hazard. Mater. 451, 131085. doi: 10.1016/j.jhazmat.2023.131085
Basit, F., Bhat, J. A., Guan, Y., Jan, B. L., Tyagi, A., and Ahmad, P. (2022a). Nitric oxide and spermine revealed positive defense interplay for the regulation of the chromium toxicity in soybean (Glycine max L.). Environ. pollut. 308, 119602. doi: 10.1016/j.envpol.2022.119602
Basit, F., Bhat, J. A., Ulhassan, Z., Noman, M., Zhao, B., Zhou, W., et al. (2022b). Seed priming with spermine mitigates chromium stress in rice by modifying the ion homeostasis, cellular ultrastructure and phytohormones balance. Antioxidants 11, 1704. doi: 10.3390/antiox11091704
Basit, F., Khalid, M., El-Keblawy, A., Sheteiwy, M. S., Sulieman, S., Josko, I., et al. (2024b). Hypoxia stress: plant’s sensing, responses, and tolerance mechanisms. Environ. Sci. pollut. Res. 31(55), 63458–63472. doi: 10.1007/s11356-024-35439-4
Basit, F., Liu, J., An, J., Chen, M., He, C., Zhu, X., et al. (2021). Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ. Sci. pollut. Res. 28, 44768–44779. doi: 10.1007/s11356-021-15087-8
Bilal, S., Shahzad, R., Asaf, S., Imran, M., Al-Harrasi, A., and Lee, I.-J. (2023). Efficacy of endophytic SB10 and glycine betaine duo in alleviating phytotoxic impact of combined heat and salinity in Glycine max L. via regulation of redox homeostasis and physiological and molecular responses. Environ. pollut. 316, 120658. doi: 10.1016/j.envpol.2022.120658
Brown, J. and Cunningham, S. A. (2019). Global-scale drivers of crop visitor diversity and the historical development of agriculture. Proc. R. Soc B 286, 20192096. doi: 10.1098/rspb.2019.2096
Chauhan, J., Singh, P., Choyal, P., Mishra, U. N., Saha, D., Kumar, R., et al. (2023). Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 10, 100296. doi: 10.1016/j.stress.2023.100296
Chen, T. H. and Murata, N. (2008). Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 13, 499–505. doi: 10.1016/j.tplants.2008.06.007
Chen, T. H. and Murata, N. (2011). Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ. 34, 1–20. doi: 10.1111/j.1365-3040.2010.02232.x
Chen, J., Shang, Y.-T., Wang, W.-H., Chen, X.-Y., He, E.-M., Zheng, H.-L., et al. (2016). Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front. Plant Sci. 7, 1173. doi: 10.3389/fpls.2016.01173
Cheng, C., Pei, L., Yin, T., and Zhang, K. (2018). Seed treatment with glycine betaine enhances tolerance of cotton to chilling stress. J. Agric. Sci. 156, 323–332. doi: 10.1017/S0021859618000278
Choudhary, S. P., Kanwar, M., Bhardwaj, R., Yu, J.-Q., and Tran, L.-S. P. (2012). Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PloS One 7, e33210. doi: 10.1371/journal.pone.0033210
Didaran, F., Kordrostami, M., Ghasemi-Soloklui, A. A., Pashkovskiy, P., Kreslavski, V., Kuznetsov, V., et al. (2024). The mechanisms of photoinhibition and repair in plants under high light conditions and interplay with abiotic stressors. J. Photochem. Photobiol. B. 259, 113004. doi: 10.1016/j.jphotobiol.2024.113004
Dustgeer, Z., Seleiman, M. F., Imran, K., Chattha, M. U., ALHAMMAD, B. A., Jalal, R. S., et al. (2021). Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. 49, 12248–12248. doi: 10.15835/nbha49112248
Dutta, T., Neelapu, N. R. R., Wani, S. H., and Surekha, C. (2019). Role and regulation of osmolytes as signaling molecules to abiotic stress tolerance. Plant Signal. Mo l, 459–477. doi: 10.1016/B978-0-12-816451-8.00029-0
Farooq, M., Ali, S., Hameed, A., Bharwana, S., Rizwan, M., Ishaque, W., et al. (2016). Cadmium stress in cotton seedlings: physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S. Afr. J. Bot. 104, 61–68. doi: 10.1016/j.sajb.2015.11.006
Farooq, M., Rafique, S., Zahra, N., Rehman, A., and Siddique, K. H. (2024). Root system architecture and salt stress responses in cereal crops. J. Agron. Crop Sci. 210, e12776. doi: 10.1111/jac.12776
Figueroa-Soto, C. G. and Valenzuela-Soto, E. M. (2018). Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie. 147, 89–97. doi: 10.1016/j.biochi.2018.01.002
Franks, P. J. and Farquhar, G. D. (2001). The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 125, 935–942. doi: 10.1104/pp.125.2.935
Gao, Y., Li, M., Zhang, X., Yang, Q., and Huang, B. (2020). Up‐regulation of lipid metabolism and glycine betaine synthesis are associated with choline‐induced salt tolerance in halophytic seashore paspalum. Plant Cell Environ. 43, 159–173.
Ghorbani, A., Ghasemi-Omran, V. O., and Chen, M. (2023). The effect of glycine betaine on nitrogen and polyamine metabolisms, expression of glycoside-related biosynthetic enzymes, and K/Na balance of stevia under salt stress. Plants 12, 1628. doi: 10.3390/plants12081628
Ghorbani, A., Razavi, S., Ghasemi Omran, V., and Pirdashti, H. (2018). Piriformospora indica inoculation alleviates the adverse effect of NaCl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.). Plant Biol. 20, 729–736. doi: 10.1111/plb.12717
Ghosh, P. and Roychoudhury, A. (2018). Differential levels of metabolites and enzymes related to aroma formation in aromatic indica rice varieties: comparison with non-aromatic varieties. 3 Biotech. 8, 1–13. doi: 10.1007/s13205-017-1045-6
Hafez, E. M., Gowayed, S. M., Nehela, Y., Sakran, R. M., Rady, A. M., Awadalla, A., et al. (2021). Incorporated biochar-based soil amendment and exogenous glycine betaine foliar application ameliorate rice (Oryza sativa L.) tolerance and resilience to osmotic stress. Plants 10, 1930. doi: 10.3390/plants10091930
Hasanuzzaman, M., Banerjee, A., Bhuyan, M. B., Roychoudhury, A., Al Mahmud, J., and Fujita, M. (2019). Targeting glycinebetaine for abiotic stress tolerance in crop plants: physiological mechanism, molecular interaction and signaling. Phyton 88, 185. doi: 10.32604/phyton.2019.07559
Hasanuzzaman, M., Bhuyan, M. B., Zulfiqar, F., Raza, A., Mohsin, S. M., Mahmud, J. A., et al. (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 9, 681. doi: 10.3390/antiox9080681
Hernandez-Leon, S. G. and Valenzuela-Soto, E. M. (2023). Glycine betaine is a phytohormone-like plant growth and development regulator under stress conditions. J. Plant Growth Regul. 42, 5029–5040. doi: 10.1007/s00344-022-10855-3
Holmström, K. O., Somersalo, S., Mandal, A., Palva, T. E., and Welin, B. (2000). Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 51, 177–185. doi: 10.1093/jexbot/51.343.177
Hossain, A., Pramanick, B., Bhutia, K. L., Ahmad, Z., Moulick, D., Maitra, S., et al. (2021). Emerging roles of osmoprotectant glycine betaine against salt-induced oxidative stress in plants: A major outlook of maize (Zea mays L.), Frontiers in plant-soil interaction. Elsevier, 567–587. doi: 10.1016/B978-0-323-90943-3.00015-8
Hossain, A., Syed, M. A., Maitra, S., Garai, S., Mondal, M., Akter, B., et al. (2022). Genetic regulation, biosynthesis, and the roles of osmoprotective compounds in abiotic stress tolerance in plants, Plant Abiotic Stress Physiology. Apple Acad. Press, 61–114. doi: 10.1201/9781003180579-4
Huang, S., Zuo, T., and Ni, W. (2020). Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta 251, 36. doi: 10.1007/s00425-019-03330-z
Ibrahim, E. A., Ebrahim, N. E., and Mohamed, G. Z. (2023). Effect of water stress and foliar application of chitosan and glycine betaine on lettuce. Sci. Rep. 13, 17274. doi: 10.1038/s41598-023-43992-0
Iqbal, M. N., Rasheed, R., Ashraf, M. Y., Ashraf, M. A., and Hussain, I. (2018). Exogenously applied zinc and copper mitigate salinity effect in maize (Zea mays L.) by improving key physiological and biochemical attributes. Environ. Sci. pollut. Res. 25, 23883–23896. doi: 10.1007/s11356-018-2383-6
Jabeen, N., Abbas, Z., Iqbal, M., Rizwan, M., Jabbar, A., Farid, M., et al. (2016). Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch. Agron. Soil Sci. 62, 648–662. doi: 10.1080/03650340.2015.1082032
Jagendorf, A. T. and Takabe, T. (2001). Inducers of glycinebetaine synthesis in barley. Plant Physiol. 127, 1827–1835. doi: 10.1104/pp.010392
Jarin, A., Ghosh, U. K., Hossain, M. S., Mahmud, A., and Khan, M. A. R. (2024). Glycine betaine in plant responses and tolerance to abiotic stresses. Discov. Agric. 2, 127. doi: 10.1007/s44279-024-00152-w
Kahraman, M., Sevim, G., and Bor, M. (2019). “The role of proline, glycinebetaine, and trehalose in stress-responsive gene expression,” in Hasanuzzaman, H. K., Fujita, R., Nahar, M., and Bor, M. (Eds.), Osmoprotectant-mediated abiotic stress tolerance in plants: recent advances and future perspectives (Singapore: Springer Nature), pp. 241–256.
Kang, Y., Jiang, Z., Meng, C., Ning, X., Pan, G., Yang, X., et al. (2024). A multifaceted crosstalk between brassinosteroid and gibberellin regulates the resistance of cucumber to phytophthora melonis. Plant J. 119, 1353–1368. doi: 10.1111/tpj.16855
Kathuria, H., Giri, J., Nataraja, K. N., Murata, N., Udayakumar, M., and Tyagi, A. K. (2009). Glycinebetaine-induced water-stress tolerance in codA-expressing transgenic indica rice is associated with up-regulation of several stress responsive genes. Plant Biotech. J. 7, 512–526. doi: 10.1111/j.1467-7652.2009.00420.x
Kern, A. J. and Dyer, W. E. (2004). Glycine betaine biosynthesis is induced by salt stress but repressed by auxinic herbicides in Kochia scoparia. J. Plant Growth Regul. 23, 9–19. doi: 10.1007/s00344-003-0045-4
Khan, S., Alvi, A. F., and Khan, N. A. (2024). Role of ethylene in the regulation of plant developmental processes. Stresses 4, 28–53. doi: 10.3390/stresses4010003
Khan, M. S., Yu, X., Kikuchi, A., Asahina, M., and Watanabe, K. N. (2009). Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotech. 26, 125–134. doi: 10.5511/plantbiotechnology.26.125
Kumar, P. (2021). Soil applied glycine betaine with Arbuscular mycorrhizal fungi reduces chromium uptake and ameliorates chromium toxicity by suppressing the oxidative stress in three genetically different Sorghum (Sorghum bicolor L.) cultivars. BMC Plant Biol. 21, 1–16. doi: 10.1186/s12870-021-03113-3
Kumar, D., Al Hassan, M., Naranjo, M. A., Agrawal, V., Boscaiu, M., and Vicente, O. (2017). Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PloS One 12, e0185017. doi: 10.1371/journal.pone.0185017
Kurepin, L. V., Ivanov, A. G., Zaman, M., Pharis, R. P., Allakhverdiev, S. I., Hurry, V., et al. (2015). Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth. Res. 126, 221–235. doi: 10.1007/s11120-015-0125-x
Kurepin, L. V., Ivanov, A. G., Zaman, M., Pharis, R. P., Hurry, V., and Hüner, N. P. (2017). Interaction of glycine betaine and plant hormones: Protection of the photosynthetic apparatus during abiotic stress. In Hou, H. J. M., Najafpour, M. M., Moore, G. F., and Allakhverdiev, S. I. (Eds.), Photosynthesis: Structures, Mechanisms, and Applications (Springer), pp. 185–202. doi: 10.1007/978-3-319-48873-8_9
Li, Z.-G., Xiang, R.-H., and Wang, J.-Q. (2021). Hydrogen sulfide–phytohormone interaction in plants under physiological and stress conditions. J. Plant Growth Regul. 40, 2476–2484. doi: 10.1007/s00344-021-10350-1
Liang, X., Li, J., Yang, Y., Jiang, C., and Guo, Y. (2024). Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 66, 303–329. doi: 10.1111/jipb.13599
Liu, Y., Song, Y., Zeng, S., Patra, B., Yuan, L., and Wang, Y. (2018). Isolation and characterization of a salt stress-responsive betaine aldehyde dehydrogenase in Lycium ruthenicum Murr. Physiol. Plant 163, 73–87. doi: 10.1111/ppl.12669
Lv, Y., Pan, J., Wang, H., Reiter, R. J., Li, X., Mou, Z., et al. (2021). Melatonin inhibits seed germination by crosstalk with abscisic acid, gibberellin, and auxin in Arabidopsis. J. Pineal Res. 70, e12736. doi: 10.1111/jpi.12736
Lv, D.-W., Zhu, G.-R., Zhu, D., Bian, Y.-W., Liang, X.-N., Cheng, Z.-W., et al. (2016). Proteomic and phosphoproteomic analysis reveals the response and defense mechanism in leaves of diploid wheat T. monococcum under salt stress and recovery. J. Proteom. 143, 93–105. doi: 10.1016/j.jprot.2016.04.013
McNeil, S. D., Nuccio, M. L., Ziemak, M. J., and Hanson, A. D. (2001). Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase. Proc. Natl. Acad. Sci. U.S.A. 98, 10001–10005. doi: 10.1073/pnas.171228998
Missihoun, T. D., Willée, E., Guegan, J.-P., Berardocco, S., Shafiq, M. R., Bouchereau, A., et al. (2015). Overexpression of ALDH10A8 and ALDH10A9 genes provides insight into their role in glycine betaine synthesis and affects primary metabolism in Arabidopsis thaliana. Plant Cell Physiol. 56, 1798–1807. doi: 10.1093/pcp/pcv105
Mohanty, A., Kathuria, H., Ferjani, A., Sakamoto, A., Mohanty, P., Murata, N., et al. (2002). Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theor. Appl. Genet. 106, 51–57. doi: 10.1007/s00122-002-1063-5
Moradi Peynevandi, K., Razavi, S. M., and Zahri, S. (2018). The ameliorating effects of polyamine supplement on physiological and biochemical parameters of Stevia rebaudiana Bertoni under cold stress. Plant Prod. Sci. 21, 123–131. doi: 10.1080/1343943X.2018.1437756
Naeem, M., Gill, S. S., Aftab, T., and Tuteja, N. (2024). Crop improvement & Plant resilience to abiotic stresses. Elsevier p, 111958. doi: 10.1016/j.plantsci.2023.111958
Narwal, P., Negi, N. P., and Kumar, D. (2024). Boosting banana resilience: Calcium supplementation enhances osmolyte and secondary metabolites production and strengthens the antioxidant machinery in drought and cold-exposed banana plants. Environ. Exp. Bot. 226, 105946. doi: 10.1016/j.envexpbot.2024.105946
Nishiyama, Y., Allakhverdiev, S. I., and Murata, N. (2006). A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta (BBA)-Bioenergetics 1757, 742–749. doi: 10.1016/j.bbabio.2006.05.013
Nolan, T., Chen, J., and Yin, Y. (2017). Cross-talk of Brassinosteroid signaling in controlling growth and stress responses. Biochem. J. 474, 2641–2661. doi: 10.1042/BCJ20160633
Ohnishi, N. and Murata, N. (2006). Glycinebetaine counteracts the inhibitory effects of salt stress on the degradation and synthesis of D1 protein during photoinhibition in Synechococcus sp. PCC 7942. Plant Physiol. 141, 758–765. doi: 10.1104/pp.106.076976
Park, E.-J., Jeknic, Z., and Chen, T. H. (2006). Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 47, 706–714. doi: 10.1093/pcp/pcj041
Park, E. J., Jeknić, Z., PINO, M. T., Murata, N., and CHEN, T. H. H. (2007). Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 30, 994–1005. doi: 10.1111/j.1365-3040.2007.01694.x
Paul, A., Kakoti, M., Dutta, P., Hazarika, B., Robertson, A., Talukdar, N., et al. (2024). Role of salicylic acid in mitigating stress and improving productivity of crops: A review. J.Adv. Bio. Biotech. 27, 1351–1361. doi: 10.9734/jabb/2024/v27i71097
Paul, S. and Roychoudhury, A. (2017). Effect of seed priming with spermine/spermidine on transcriptional regulation of stress-responsive genes in salt-stressed seedlings of an aromatic rice cultivar. Plant Gene 11, 133–142. doi: 10.1016/j.plgene.2017.05.007
Paul, S., Roychoudhury, A., Banerjee, A., Chaudhuri, N., and Ghosh, P. (2017). Seed pre-treatment with spermidine alleviates oxidative damages to different extent in the salt (NaCl)-stressed seedlings of three indica rice cultivars with contrasting level of salt tolerance. Plant Gene 11, 112–123. doi: 10.1016/j.plgene.2017.04.002
Phillips, K., Majola, A., Gokul, A., Keyster, M., Ludidi, N., and Egbichi, I. (2018). Inhibition of NOS-like activity in maize alters the expression of genes involved in H2O2 scavenging and glycine betaine biosynthesis. Sci. Rep. 8, 12628. doi: 10.1038/s41598-018-31131-z
Prasad, K., Sharmila, P., Kumar, P., and Saradhi, P. P. (2000). Transformation of Brassica juncea (L.) Czern with bacterial codA gene enhances its tolerance to salt stress. Mol. Breed. 6, 489–499. doi: 10.1023/A:1026542109965
Quan, R., Shang, M., Zhang, H., Zhao, Y., and Zhang, J. (2004). Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant biotech. J. 2, 477–486. doi: 10.1111/j.1467-7652.2004.00093.x
Rady, M. O., Semida, W. M., Abd El-Mageed, T. A., Hemida, K. A., and Rady, M. M. (2018). Up-regulation of antioxidative defense systems by glycine betaine foliar application in onion plants confer tolerance to salinity stress. Sci. Horti. 240, 614–622. doi: 10.1016/j.scienta.2018.06.069
Rao, M. J., Duan, M., Zhou, C., Jiao, J., Cheng, P., Yang, L., et al. (2025). Antioxidant defense system in plants: reactive oxygen species production, signaling, and scavenging during abiotic stress-induced oxidative damage. Horticulturae 11, 477. doi: 10.3390/horticulturae11050477
Rasheed, R., Iqbal, M., Ashraf, M. A., Hussain, I., Shafiq, F., Yousaf, A., et al. (2018). Glycine betaine counteracts the inhibitory effects of waterlogging on growth, photosynthetic pigments, oxidative defence system, nutrient composition, and fruit quality in tomato. J. Hortic. Sci. Biotechnol. 93, 385–391. doi: 10.1080/14620316.2017.1373037
Rasheed, Y., Khalid, F., Ashraf, H., Asif, K., Maqsood, M. F., Naz, N., et al. (2024). Enhancing plant stress resilience with osmolytes and nanoparticles. J. Soil Sci. Plant Nutr., 1–36. doi: 10.1007/s42729-024-01821-x
Rasheed, R., Wahid, A., Ashraf, M., and Basra, S. M. (2010). Role of proline and glycinebetaine in improving chilling stress tolerance in sugarcane buds at sprouting. Int. J. Agric. Biol. 12, 1–8.
Rippa, S., Zhao, Y., Merlier, F., Charrier, A., and Perrin, Y. (2012). The carnitine biosynthetic pathway in Arabidopsis thaliana shares similar features with the pathway of mammals and fungi. Plant Physiol. Biochem. 60, 109–114. doi: 10.1016/j.plaphy.2012.08.001
Roychoudhury, A. and Banerjee, A. (2017). Abscisic acid signaling and involvement of mitogen activated protein kinases and calcium-dependent protein kinases during plant abiotic stress. Mech. Plant hormone Signaling under Stress 1, 197–241. doi: 10.1002/9781118889022.ch9
Sadeghipour, O. (2020). Cadmium toxicity alleviates by seed priming with proline or glycine betaine in cowpea (Vigna unguiculata (L.) Walp.). Egypt. J. Agron. 42, 163–170. doi: 10.21608/agro.2020.23667.1204
Sakamoto, A. and Murata, N. (2002). The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 25, 163–171. doi: 10.1046/j.0016-8025.2001.00790.x
Salinas, P., Velozo, S., and Herrera-Vásquez, A. (2025). Salicylic acid accumulation: emerging molecular players and novel perspectives on plant development and nutrition. J. Exp. Bot. 76, 1950–1969. doi: 10.1093/jxb/erae309
Shahzad, K., Ali, A., Ghani, A., Nadeem, M., Khalid, T., Nawaz, S., et al. (2023). Exogenous application of proline and glycine betaine mitigates nickel toxicity in mung bean plants by up-regulating growth, physiological and yield attributes. Pak J. Bot. 55, 10.30848. doi: 10.30848/PJB2023-SI(3)
Sharma, J., Kumar, S., Singh, P., Kumar, V., Verma, S., Khyalia, P., et al. (2024). Emerging role of osmoprotectant glycine betaine to mitigate heavy metals toxicity in plants: A systematic review. Biol. Futura. 75, 159–176. doi: 10.1007/s42977-023-00198-9
Sharma, A., Pathania, A., Sharma, P., Bhardwaj, R., and Sharma, I. (2023). “Role of glycine betaine in regulating physiological and molecular aspects of plants under abiotic stress.” The Role of Growth Regulators and Phytohormones Overcoming Environmental Stress, 327–353. doi: 10.1016/B978-0-323-98332-7.00017-2
Shemi, R., Wang, R., Gheith, E.-S. M., Hussain, H. A., Hussain, S., Irfan, M., et al. (2021). Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 11, 3195. doi: 10.1038/s41598-021-82264-7
Singh, D., Singh, C. K., Singh, D., Sarkar, S. K., Prasad, S. K., Sharma, N. L., et al. (2022). Glycine betaine modulates chromium (VI)-induced morpho-physiological and biochemical responses to mitigate chromium toxicity in chickpea (Cicer arietinum L.) cultivars. Sci. Rep. 12, 8005. doi: 10.1038/s41598-022-11869-3
Singh, S., Thejesh, C., and Mathpal, B. (2024). Improving wheat grain quality through zn, cytokinin and gibberellic acid applications. J. Soil Sci. Plant Nutr. 24, 7117–7128. doi: 10.1007/s42729-024-02028-w
Sofy, M. R., Elhawat, N., and Alshaal, T. (2020). Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 200, 110732. doi: 10.1016/j.ecoenv.2020.110732
Song, S., Qi, T., Wasternack, C., and Xie, D. (2014). Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 21, 112–119. doi: 10.1016/j.pbi.2014.07.005
Su, J. and Wu, R. (2004). Stress-inducible synthesis of proline in transgenic rice confers faster growth under stress conditions than that with constitutive synthesis. Plant Sci. 166, 941–948. doi: 10.1016/j.plantsci.2003.12.004
Subramanyam, K., Sailaja, K., Subramanyam, K., Muralidhara Rao, D., and Lakshmidevi, K. (2011). Ectopic expression of an osmotin gene leads to enhanced salt tolerance in transgenic chilli pepper (Capsicum annum L.). Plant Cell Tissue Organ Culture (PCTOC) 105, 181–192. doi: 10.1007/s11240-010-9850-1
Sulpice, R., Pyl, E.-T., Ishihara, H., Trenkamp, S., Steinfath, M., Witucka-Wall, H., et al. (2009). Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. U. S. A. 106, 10348–10353. doi: 10.1073/pnas.0903478106
Tabassum, T., Farooq, M., Ahmad, R., Zohaib, A., and Wahid, A. (2017). Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol. Biochem. 118, 362–369. doi: 10.1016/j.plaphy.2017.07.007
Tang, W., Sun, J., Liu, J., Liu, F., Yan, J., Gou, X., et al. (2014). RNAi-directed downregulation of betaine aldehyde dehydrogenase 1 (OsBADH1) results in decreased stress tolerance and increased oxidative markers without affecting glycine betaine biosynthesis in rice (Oryza sativa). Plant Mol. Biol. 86, 443–454. doi: 10.1007/s11103-014-0239-0
Tian, F., Wang, W., Liang, C., Wang, X., Wang, G., and Wang, W. (2017). Overaccumulation of glycine betaine makes the function of the thylakoid membrane better in wheat under salt stress. Crop J. 5, 73–82. doi: 10.1016/j.cj.2016.05.008
Valenzuela-Soto, E. M. and Figueroa-Soto, C. G. (2019). “Biosynthesis and degradation of glycine betaine and its potential to control plant growth and development,” in Hasanuzzaman, H. K., Fujita, R., Nahar, M., and Bor, M. (Eds.), Osmoprotectant-mediated abiotic stress tolerance in plants: recent advances and future perspectives (Singapore: Springer Nature), pp. 123–140. doi: 10.1007/978-981-13-8854-3_7
Wang, L., Bokhary, S. U. F., Xie, B., Hu, S., Jin, P., and Zheng, Y. (2019). Biochemical and molecular effects of glycine betaine treatment on membrane fatty acid metabolism in cold stored peaches. Postharvest Biol. Technol. 154, 58–69. doi: 10.1016/j.postharvbio.2019.04.007
Wang, L., Shi, K., Song, Q., Wang, Y., Wu, T., Wang, X., et al. (2024). Glycine betaine enhances chilling tolerance in peach fruit by modulating PpbHLH130-mediated antioxidant metabolism. Postharvest Biol. Technol. 218, 113166. doi: 10.1016/j.postharvbio.2024.113166
Wang, X., Zhang, X., Chen, J., Wang, X., Cai, J., Zhou, Q., et al. (2018). Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front. Plant Sci. 9, 261. doi: 10.3389/fpls.2018.00261
Weigel, P., Weretilnyk, E. A., and Hanson, A. D. (1986). Betaine aldehyde oxidation by spinach chloroplasts. Plant Physiol. 82, 753–759. doi: 10.1104/pp.82.3.753
Wu, X., Gong, D., Zhao, K., Chen, D., Dong, Y., Gao, Y., et al. (2024). Research and development trends in plant growth regulators. Advanced Agrochem. 3, 99–106. doi: 10.1016/j.aac.2023.11.005
Wu, H., Zhang, Z., Liu, Z., Meng, Q., Xu, Z., Zhang, H., et al. (2023). Comparative transcriptome analysis of gene expression and regulatory characteristics associated with different bolting periods in spinacia oleracea. Genes 15, 36. doi: 10.3390/genes15010036
Xu, Z., Sun, M., Jiang, X., Sun, H., Dang, X., Cong, H., et al. (2018). Glycinebetaine biosynthesis in response to osmotic stress depends on jasmonate signaling in watermelon suspension cells. Front. Plant Sci. 9, 1469. doi: 10.3389/fpls.2018.01469
Yang, X. and Lu, C. (2005). Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol. Plant 124, 343–352. doi: 10.1111/j.1399-3054.2005.00518.x
Zanganeh, R., Jamei, R., and Rahmani, F. (2018). Impacts of seed priming with salicylic acid and sodium hydrosulfide on possible metabolic pathway of two amino acids in maize plant under lead stress. Mol. Biol. Res. 7, 83. doi: 10.22099/mbrc.2018.29089.1317
Zhang, L., Gao, M., Hu, J., Zhang, X., Wang, K., and Ashraf, M. (2012). Modulation role of abscisic acid (ABA) on growth, water relations and glycinebetaine metabolism in two maize (Zea mays L.) cultivars under drought stress. Int. J. Mol. Sci. 13, 3189–3202. doi: 10.3390/ijms13033189
Zhang, T., Li, Z., Li, D., Li, C., Wei, D., Li, S., et al. (2020). Comparative effects of glycinebetaine on the thermotolerance in codA-and BADH-transgenic tomato plants under high temperature stress. Plant Cell Rep. 39, 1525–1538. doi: 10.1007/s00299-020-02581-5
Zhang, T., Zhang, W., Li, D., Zhou, F., Chen, X., Li, C., et al. (2021). Glycinebetaine: a versatile protectant to improve rice performance against aluminium stress by regulating aluminium uptake and translocation. Plant Cell Rep. 40, 2397–2407. doi: 10.1007/s00299-021-02780-8
Zhou, X., Joshi, S., Khare, T., Patil, S., Shang, J., and Kumar, V. (2021). Nitric oxide, crosstalk with stress regulators and plant abiotic stress tolerance. Plant Cell Rep. 40, 1395–1414. doi: 10.1007/s00299-021-02705-5
Zhou Jin Xin, Z. J., Hu XinWen, H. X., Zhang HaiWen, Z. H., and Huang RongFeng, H. R. (2008). Regulatory role of ABA in plant response to biotic stresses. 169–174.
Zhu, M., Li, Q., Zhang, Y., Zhang, M., and Li, Z. (2022). Glycine betaine increases salt tolerance in maize (Zea mays L.) by regulating Na+ homeostasis. Front. Plant Sci. 13, 978304. doi: 10.3389/fpls.2022.978304
Keywords: osmolytes, stress tolerance, biosynthesis, reactive oxygen species (ROS), stress signaling network
Citation: Basit F, Alyafei M, Hayat F, Al-Zayadneh W, El-Keblawy A, Sulieman S and Sheteiwy MS (2025) Deciphering the role of glycine betaine in enhancing plant performance and defense mechanisms against environmental stresses. Front. Plant Sci. 16:1582332. doi: 10.3389/fpls.2025.1582332
Received: 24 February 2025; Accepted: 16 June 2025;
Published: 23 July 2025.
Edited by:
Kai-Hua Jia, Shandong Academy of Agricultural Sciences, Jinan, ChinaReviewed by:
Mudassir Iqbal Shad, Government College University, Faisalabad, Faisalabad, PakistanHonghai Zhu, Zhejiang Chinese Medical University, Hangzhou, China
Copyright © 2025 Basit, Alyafei, Hayat, Al-Zayadneh, El-Keblawy, Sulieman and Sheteiwy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed S. Sheteiwy, bXNhbGFoQHVhZXUuYWMuYWU=
 Farwa Basit
Farwa Basit Mohammed Alyafei2
Mohammed Alyafei2 Faisal Hayat
Faisal Hayat Wasef Al-Zayadneh
Wasef Al-Zayadneh Ali El-Keblawy
Ali El-Keblawy Mohamed S. Sheteiwy
Mohamed S. Sheteiwy