- 1Yunnan Key Laboratory for Plateau Mountain Ecology and Restoration of Degraded Environments, School of Ecology and Environmental Science, Yunnan University, Kunming, China
- 2College of Biological Science & Engineering, Qinling-Bashan Mountains Bioresources Comprehensive Development C.I.C, State Key Laboratory of Biological Resources and Ecological Environment Jointly Built By Qinba Province and Ministry, Shaanxi Province Key Laboratory of Bio-resources, Shaanxi University of Technology, Hanzhong, China
- 3Department of Health Management, Liaoning Vocational College Of Medicine, Shenyang, China
- 4Key Laboratory of Pollution Ecology and Environment Engineering, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, China
- 5Institute of Biology, Centre for Molecular Biology and Biotechnology, University of Szczecin, Szczecin, Poland
Introduction: Solanum nigrum L. has been a widely studied Cd hyperaccumulator. Solanum lycopersicum L. also belongs to the same family (Solanaceae) with S. nigrum. Compared to S. nigrum, the Cd accumulation characteristics and remediation potentials of seven S. lycopersicum cultivars at their maximum vegetative growth period stages (MVGPS) were determined. The advantage of the remediating method at MVGPS is to avoid Cd entering food chain via fruits at mature period.
Methods: Soil pot experiments were conducted and used to determine Cd concentration in all test plants. Biomasses and some physiology index were measured either.
Results: The results showed that the enrichment factors (EFs, the Cd concentration ratio of shoots to soils) of the tested S. nigrum and all cultivars were greater than 1. However, only the translocation factors (TFs, the Cd concentration ratio of shoots to roots) of S. nigrum and Baishite (S. lycopersicum cultivar) were higher than 1, indicating that only Baishite exhibited the main properties of a Cd hyperaccumulator. In particular, the Cd concentration (20.27 mg kg-1) in shoot of Baishite was same as that of S. nigrum, and they were the highest. Cd shoot accumulation capacity (96.1 µg plant-1) of Baishite was 17.6 % higher than S. nigrum due to its higher shoot biomass (an increase of 10.4 %). The shoot of biomass (g plant-1) in Baishite was higher than in S. nigrum. Furthermore, some index like chlorophyll content and SOD activity of Baishite were higher either, which might be part reasons for Baishite highly accumulated Cd, but other mechanisms could play more important roles.
Discussion: These findings suggested that some S. lycopersicum cultivars could have a higher Cd remediation potential, and further screening for more ideal remediation plants from crop was possible.
Introduction
With the continuous human demand for resources and the increasing production intensity, wastes containing heavy metals are constantly found in the environment, and the rate of pollution to the environment is still increasing (Ogugua et al., 2022). Among them, Cd pollution in the soil environment is becoming more and more serious, and it is harmful to people and animals (Janifer et al., 2021; Dai et al., 2022). Under this background, phytoremediation of soil heavy metal pollution has gradually become a hot research topic at home and abroad (Liu et al., 2023). Phytoremediation technology is considered to be a green, cheap, and non-interference in situ remediation technology. In order to promote phytoremediation efficiency, enhanced hyperaccumulator biomass and shortened remediation cycle are needed (Dai et al., 2022; Dou et al., 2023). Solanum nigrum L. is a widely studied Cd hyperaccumulator. Wu et al. (2020) summarized its remediation potential, higher heavy metal accumulation capacities, and some strengthened measurements like suitable microbes and integrated agricultural practices. Solanum lycopersicum L. also belongs to Solanaceae just as S. nigrum. Some cultivars of S. lycopersicum were with higher biomasses. Thus, their remediation potentials may be higher if they owned Cd hyperaccumulator characteristics like S. nigrum.
In fact, there were too many studies concerning S. lycopersicum heavy metal accumulation. In general, the research scope can be roughly divided into the following four categories. The first category concerned the metals’ effects on physiological indices. Akinc et al. explored the effects of Pb on S. lycopersicum growth as well as chlorophyll and water content (Akinci et al., 2010). Wang et al. determined the toxic effect of excess Cu and Zn on some S. lycopersicum antioxidant enzymes (Wang et al., 2022). Sarwar et al. determined the influence of Cd on the anatomy of internodes and leaves of S. lycopersicum (Sarwar et al., 2023). Other studies concerned the effect of Pb and Cr on S. lycopersicum (Almaroai and Eissa, 2020), estimation of Cd tolerance (Rodríguez-Rodríguez et al., 2023), mechanisms of Cd stress avoidance by Se (Zhang et al., 2022), and association of phytohormones with growth (Sun et al., 2023). Only two S. lycopersicum cultivars were compared physiologically and biochemically (Al-Lahham et al., 2007). The second research category on S. lycopersicum concerned mainly the reduction of heavy metal toxicity to this plant. Some arbuscular mycorrhizal fungi could alleviate Cd stress in S. lycopersicum (Pan et al., 2023). Graft reduced Cd accumulation of S. lycopersicum (El-Sappah et al., 2021). Su et al. (2021) showed that the cherry tomato cultivar Hanluzhe has lower cadmium accumulation contents in the leaves as compared to Lvfeicui, and different expression genes were mainly involved in plant hormone signal transduction (Ayhan, 2010), antioxidant enzymes, cell wall biosynthesis, and metal transportation. The studies of El-Sappah et al. (2021) focused on the SlMTP genes that displayed different responses in either S. lycopersicum leaf or root treatments by five divalent heavy metals. The reciprocal interaction between endophytic Curvularia lunata CSL1 and tomato was revealed through transcriptomics research to reduce Cd toxicity (Asaf et al., 2023), genotoxicity induced by heavy metal in two cultivars (Su et al., 2021) or trico-synthesized silicon nanoparticles, and Trichoderma metabolites induced by heavy metal (Hasan et al., 2022; Raja et al., 2023). The fourth category research on S. lycopersicum involved heavy metal accumulation differences and the remediation potential of different S. lycopersicum genotypes differing in Cd accumulation (Wei et al., 2018). The role of plant growth-promoting rhizobacteria (PGPR) and its potential mechanisms to promote plant growth and enhance plant repair ability have received attention (Bhanse et al., 2022). The same goes for the suitability assessment of S. lycopersicum in remediating Zn-polluted soil (Akanbi-Gada et al., 2019) and enhancement of Cr phytoremediation by arbuscular mycorrhiza fungi and Aspergillus terreus (Pan et al., 2023). However, there were rarely studies concerning Cd hyperaccumulation characteristics and the remediation potential of S. lycopersicum, especially for several cultivar species compared to S. nigrum. In the vegetative growth period, S. lycopersicum gradually adapts to Cd in the seedling stage, and all physiological indexes basically reach the most vigorous state. Selecting this period can more appropriately reflect the maximum accumulation of Cd in tomato plants. Especially in the maximum vegetative growth period, tomato leaves are basically of the same size during the maturity period. Cd mainly accumulates in the stems and leaves. If the plants are harvested during this period and the next remediation process is repeated, it is possible to shorten the remediation cycle and improve the phytoremediation efficiency within a year. At the same time, because no fruit is produced and due to be harvested at the maximum vegetative growth period, without any fruits the food chain risk on human health of heavy metals is also avoided. Therefore, this study has a very important practical significance.
Materials and methods
Conduct of the experiment
Mature seeds of S. nigrum were collected from an agricultural field in Hanzhong City of China. The seven cultivar seeds of S. lycopersicum were procured from the seed markets in Hanzhong and Shenyang cities of China, including Meihao, L-404, Baisite, Zeibutou, Ziyu, Liaoyuanduoli, and L-402.
Top (0–20 cm) soil samples (cinnamon soil) also from the field were used in the pot experiment, with pH 6.82, 0.21 mg kg-1 Cd, available potassium content 16.9 mg kg-1, organic matter 27.2 g kg-1, and available phosphorus 16.7 mg kg-1. The soil quality is as follows: 19.4% clay, 40.5% silt, and 32.6% sand. According to NSEQS, the collected soil is pure and therefore considered as control soil (CK). According to the NSEQS standard, polluted soil (PS) was prepared by adding 3.12 mg kg-1 of CdCl2. Soil was mixed with Cd to a total of 3.09 mg kg-1, and each pot was filled with 2.5 kg of treated soil. (NH4)2SO4 was used as fertilizer for each pot (3 g). All test pots were kept for 2 months. After treatment with 0.1% sodium hypochlorite, all plant seeds were put into CK and PS soils. There were three replicates.
This experimental material was cultivated in a greenhouse of the State Key Laboratory of Biological Resources and Ecological Environment of Qinba. The culture parameters include radiation intensity of 1,001 μm m-2 s-1, temperature of 21°C–28°C, day/night period of 12/12 h, and relative air humidity of 66%. When the seedlings were growing to about 7 cm in height, seven seedlings were preserved. Tap water was supplemented daily. After 70 days at their maximum vegetative growth stages (without flowers), in order to avoid Cd from entering the food chain through fruits during the maturity period, all plants were collected.
Another reason for selecting the maximum vegetative growth stages as the time of harvest is that the main organs of plants accumulating Cd are the leaves and stems of the hyperaccumulator. At the maximum vegetative growth stages of the plants, the biomass values of leaves and stems are nearly the biggest in its whole growth period. Thus, the remediation potential at the maximum vegetative growth stages of the hyperaccumulator is also near the highest (Wei et al., 2009).
Determination of physical, chemical, and physiological indices
Rhizospheric soils were collected through gentle and manual shaking. According to this method by Dai et al. (2022), the extractable Cd in rhizosphere soil and the total Cd concentration in all samples were determined. GBW07405 (GSS-5) and GBW10015 (GSB-6) standard reference materials were used as QA/QC.
The biomass of harvested plant will be measured by collecting them according to their roots, stems, leaves, and flowers. After drying at 105°C for 5 min, the samples were dried for about 2 days until counterweight under 70°C and weighed (Dai et al., 2021).
The photosynthetic pigment content in plant leaves was determined by Dai et al. (2022). The chlorophyll content was determined as described by Dai et al. (2022). The measurement of SOD activities and MDA contents was based on the methods by Jia et al. (2023). SOD and MDA were determined because they are closely related to a plant’s resistance to heavy metals (Wang et al., 2022; Sarwar et al., 2023).
Data analysis
SPSS 12 software was used for data processing and analysis. LSD method was used to analyze at the 0.05 level of significance.
Results
Cd concentration differences in different parts of plants
Table 1 indicates that the Cd levels (mg kg-1) in Solanum roots in the CK treatment were similar (p < 0.05). The Cd concentration differences in the stems, leaves, and shoots were not big.
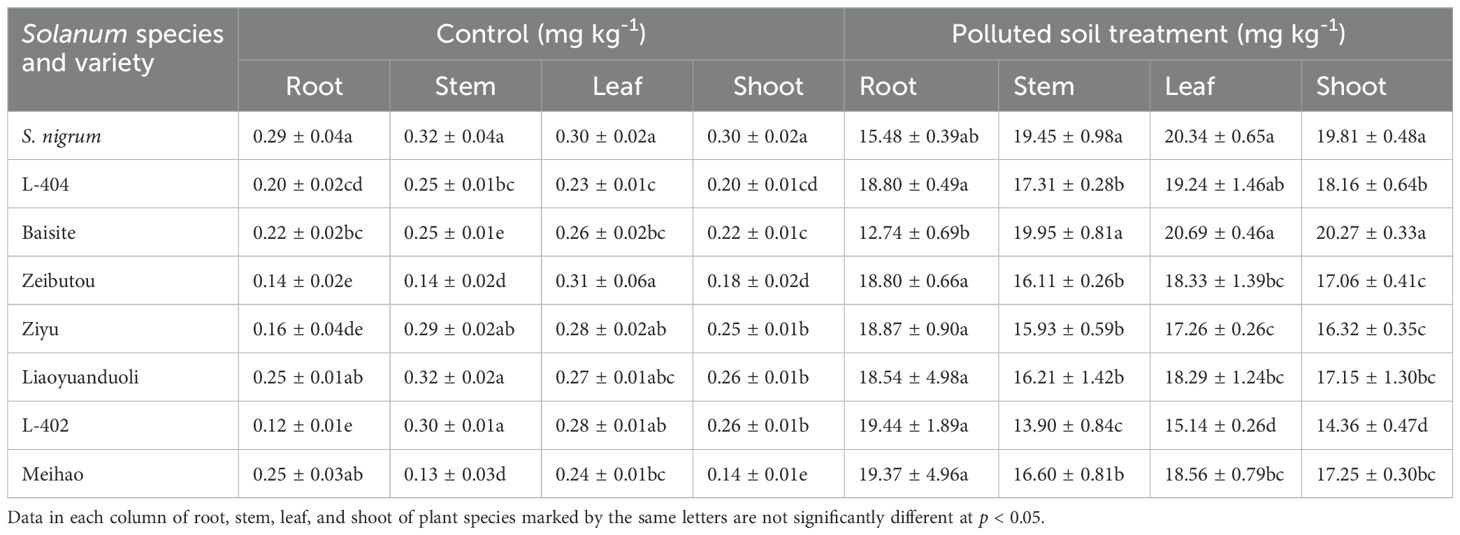
Table 1. Cd levels in Solanum nigrum and Solanum lycopersicum cultivars in control (CK) and polluted soil (PS) treatments.
The Cd concentration (mg kg-1) differences in various organs of different plants in PS treatment were greater. The Cd concentrations in the stem of S. nigrum and Baisite were the highest (p < 0.05); the Cd concentrations in the leaves of S. nigrum, L-404, and Baisite were the highest (p < 0.05). The Cd concentration in the shoots of Baishite and S. nigrum were the highest.
EF and TF of all test plants
Under the PS treatment, the EFs (the Cd concentration ratio of shoots to soils) of all plant species were higher than 1, and the EFs of S. nigrum and Baisite were the highest (7.49–7.57), followed by the values obtained in Ziyu (7.37) (Figure 1). Other EFs of S. lycopersicum cultivars ranged from 5.04 to 6.99.
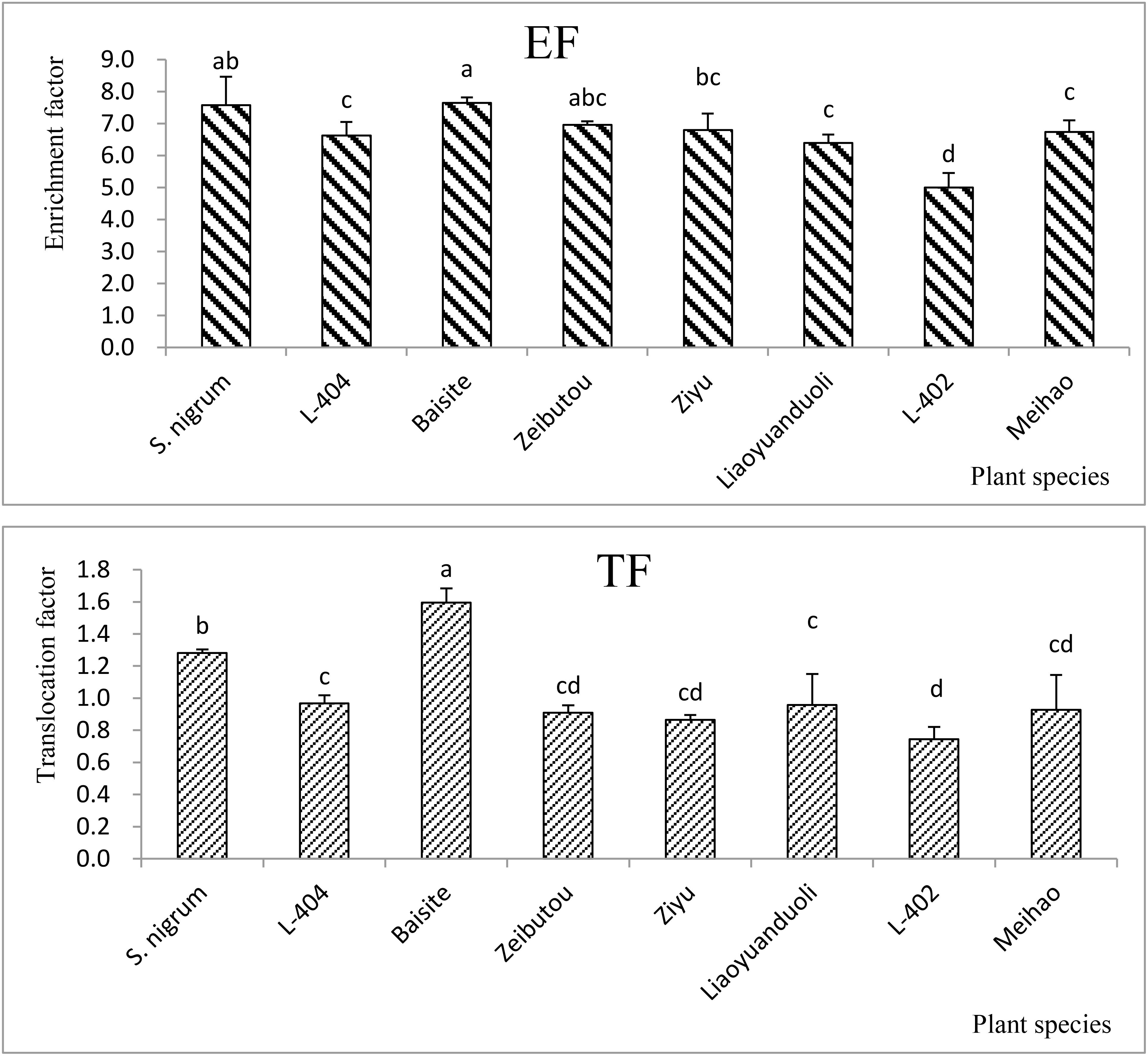
Figure 1. EFs and TFs of all tested Solanum plants. Data in each column of plant species marked by the same letters are not significantly different at p < 0.05.
There were only two TFs (the Cd concentration ratio of shoots to roots) higher than 1, i.e., S. nigrum (1.28) and Baisite (1.56). The values of other TFs of S. lycopersicum cultivars were from 0.75 to 0.98 (Figure 1).
Biomass differences among different plants
The root and shoot biomasses differences in the CK treatments of different plants were not big (p < 0.05) compared to PS treatments (Figure 2). The change trend of root biomass was the same as that aboveground (shoots) (Figure 2). However, there are significant differences between different plant species treated with CK and PS. The aboveground biomass of Baisite was the highest. S. nigrum was the second and L-404 was the lowest (p < 0.05). The aboveground biomass of Baisite was 10.4% higher than that of S. nigrum and 54.3% higher than L-404. Among them, there were other biomass data of S. lycopersicum cultivars (Figure 2).
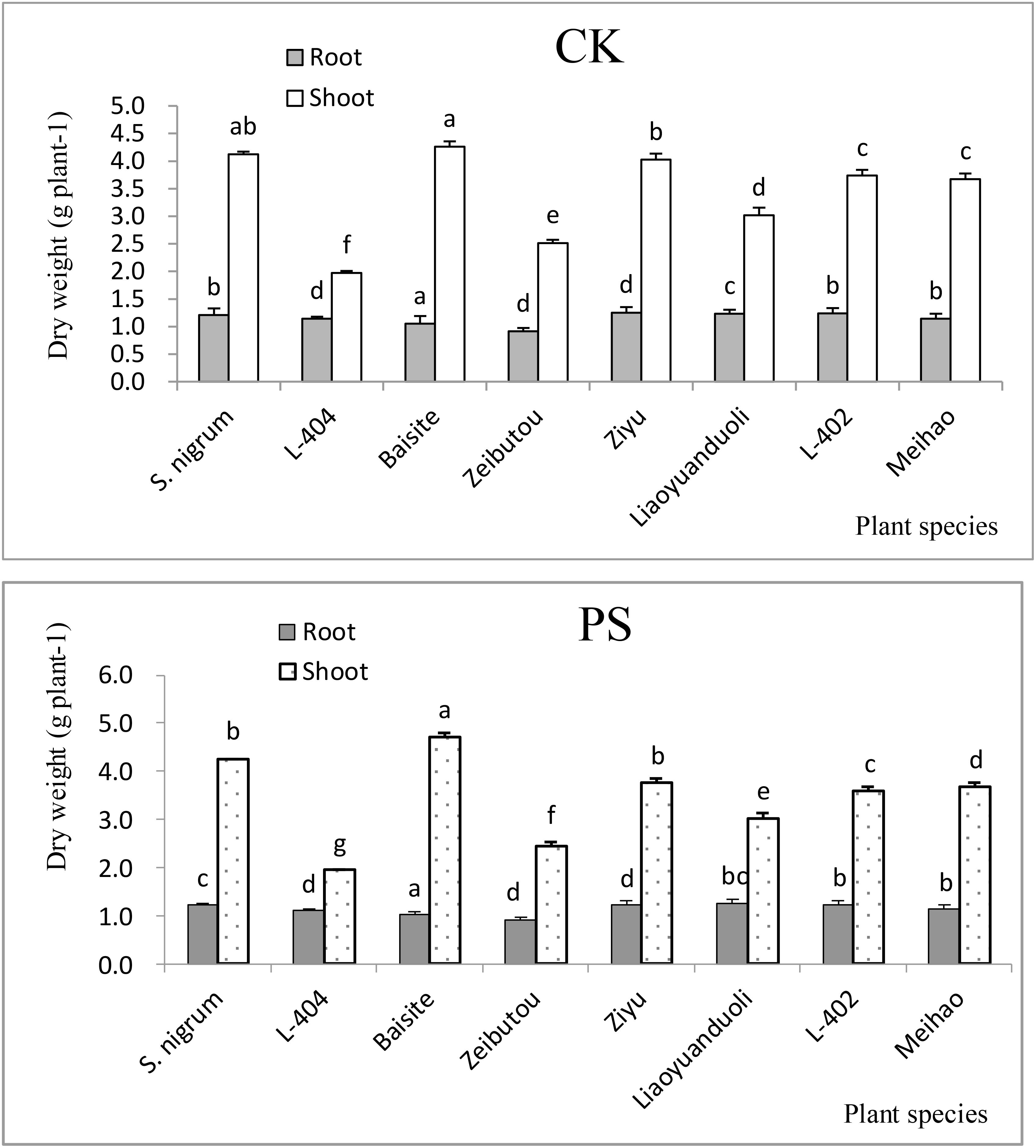
Figure 2. Biomasses of plants. Data in each column of plant species marked by the same letters are not significantly different at p < 0.05.
Cd accumulation capacity in different plants
As shown in Figure 3, the highest aboveground Cd accumulation was that of Baisite (96.1 µg plant-1), and it was 17.6% higher compared to the second Cd capacity of S. nigrum shoots (81.7 µg plant-1) (p < 0.05). The others were all smaller than that of S. nigrum.
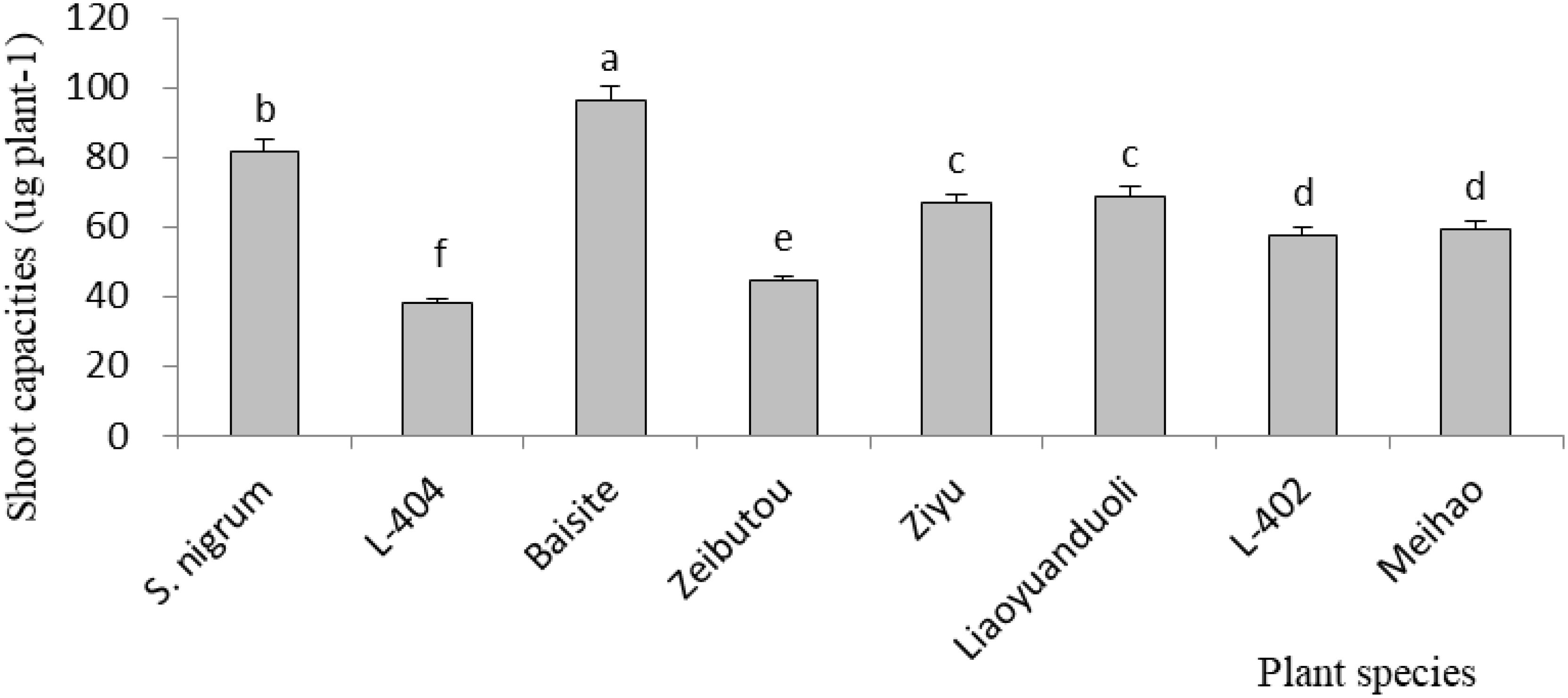
Figure 3. Cd accumulation capacities of aboveground parts. Data in each column of plant species marked by the same letters are not significantly different at p < 0.05.
Changes in extractable Cd concentrations and physiological parameters
The ECCs in soils in PS pots are demonstrated in Figure 4. The greatest ECCs were those of S. nigrum, Baisite, and Ziyu. The ECCs of other S. lycopersicum cultivars were essentially the same.
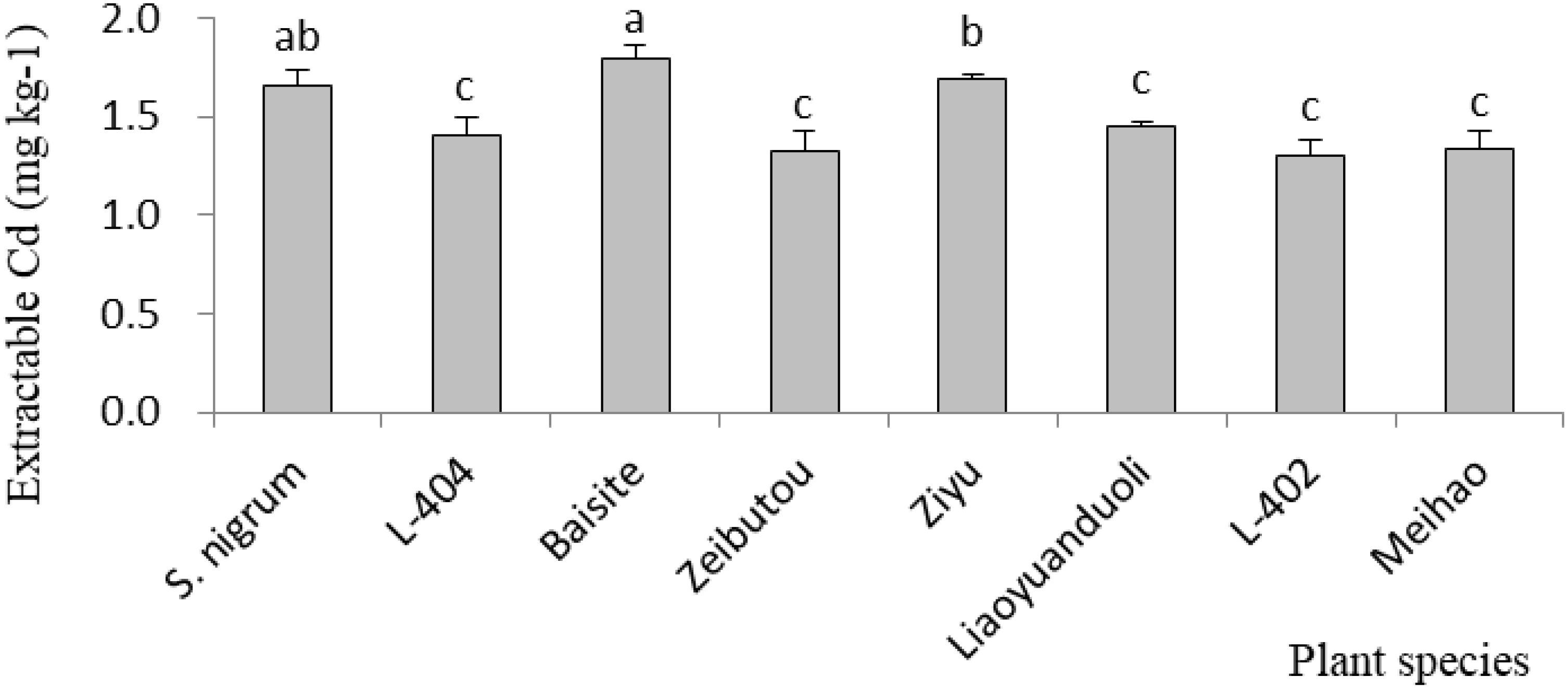
Figure 4. Cd content of extractable species in soil. Data in each column of plant species marked by the same letters are not significantly different at p < 0.05.
The chlorophyll contents in CK and PS treatments are shown in Figure 5. The chlorophyll contents of all plants in CKs were basically the same as those in PS treatments (p < 0.05). The chlorophyll concentrations of Baisite was the greatest (p < 0.05), while the S. nigrum chlorophyll contents were the second (p < 0.05). The chlorophyll concentrations of other S. lycopersicum cultivars were smaller than the previous ones, with Ziyu, Liaoyuanduoli, L-402, and Meihao cultivars being the third and similar to each other (p < 0.05); the chlorophyll concentration of L-404 was the lowest (p < 0.05) (Figure 5).
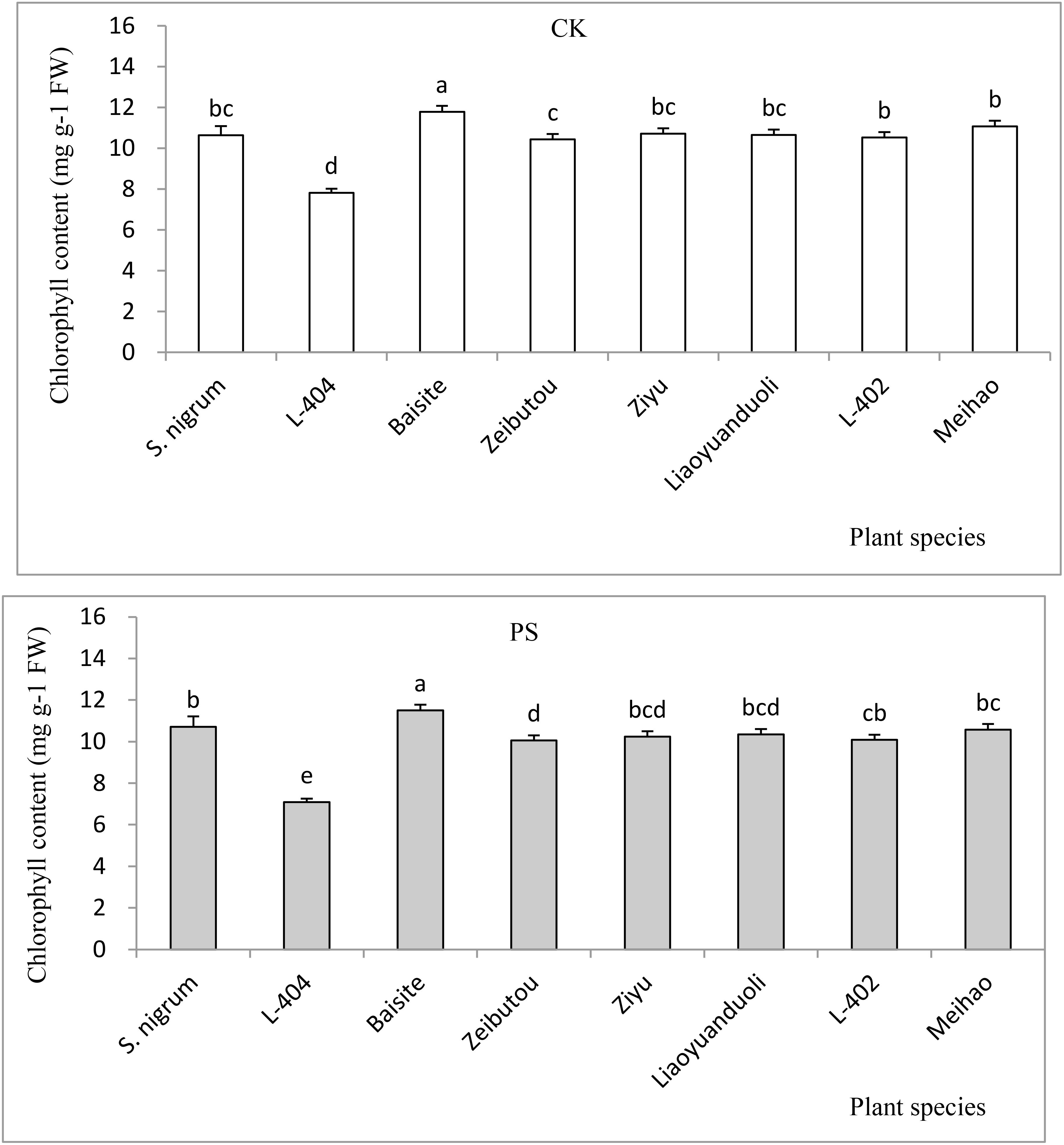
Figure 5. Chlorophyll contents of test plants. Data in each column of plant species marked by the same letters are not significantly different at p < 0.05.
The SOD values of plants in the CK and PS treatments are presented in Figure 6. The SOD data in CK were basically the same as those in the PS treatments (p < 0.05). The SOD of Baisite was the highest, with S. nigrum being the second. The SOD activities of Zeibutou and Meihao were the lowest (p < 0.05) (Figure 6).
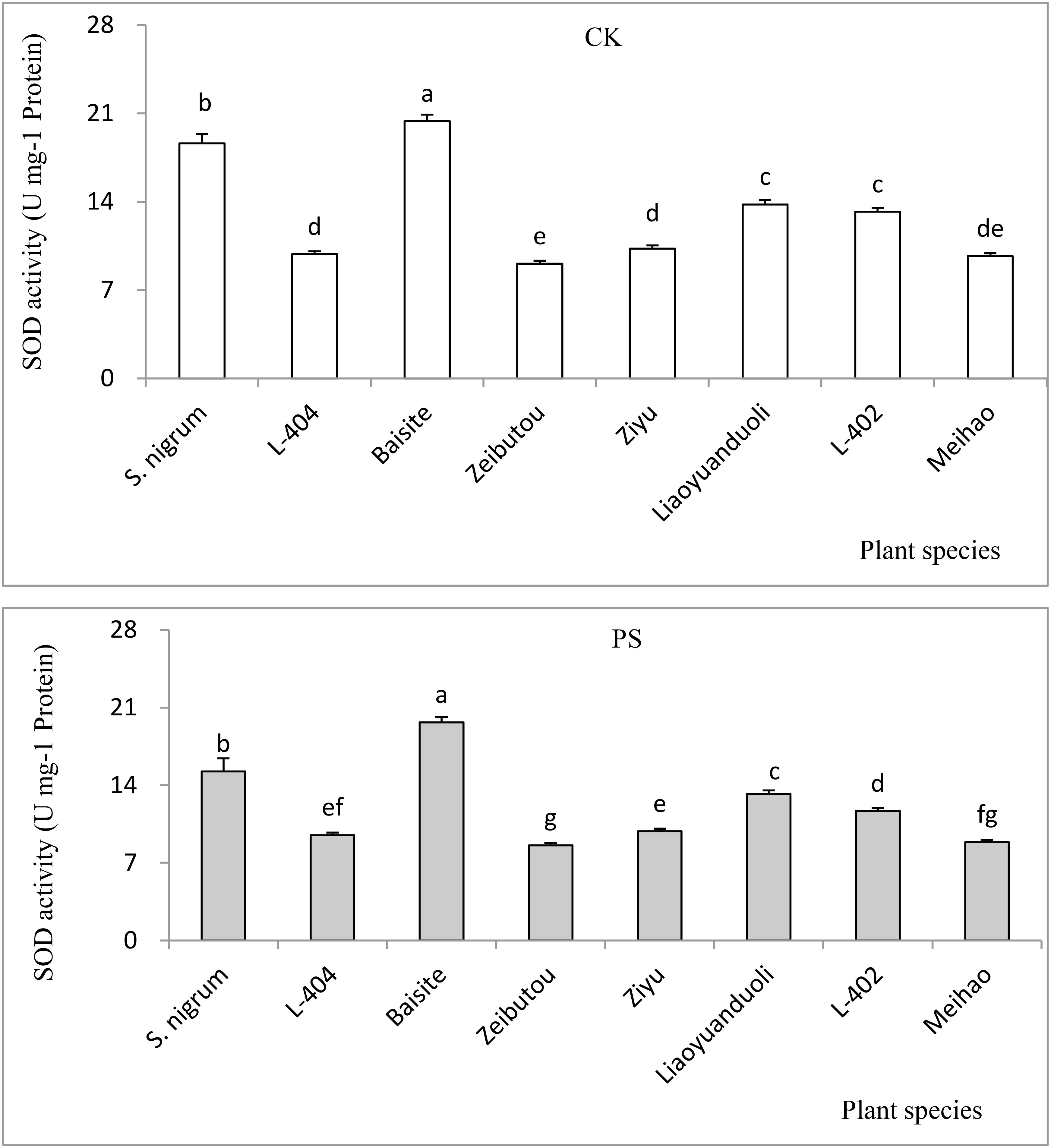
Figure 6. SOD activities of different plants. Data in each column of plant species marked by the same letters are not significantly different at p < 0.05.
Discussion
Heavy metal pollution, especially Cd pollution, has become a major obstacle to the sustainable development of China’s agriculture (Wei et al., 2009). Fortunately, the safe production of agricultural products with moderate and slight soil pollution has attracted more and more attention (Wei and Twardowska, 2013). Another key to screening and identifying Cd-accumulating plants is to develop a practical scientific method system. Environmental factors can change the activity and availability of heavy metal Cd in soil, thereby affecting the accumulation of Cd by plants. It is considered to be another index about the absorption and enrichment of Cd by crops besides genotype (Wei et al., 2009). The EFs of all S. lycopersicum cultivars in the PS treatments were all higher than 1 (Figure 1). However, the TFs of only S. nigrum and the cultivar Baishite were higher than 1 (Figure 1). Furthermore, the biomasses of all tested plant cultivars in the PS treatments were not significantly decreased compared to CKs (Figure 2). These data indicated that S. nigrum as well as the cultivar Baishite showed basic Cd hyperaccumulator characteristics (Wei et al., 2009; Wei and Twardowska, 2013). Obviously, S. lycopersicum cultivar Baishite showed the most optimal Cd hyperaccumulation properties (Figures 1, 2). Al-Lahham et al. (2007) reached a similar conclusion as well and demonstrated the suitability of one cultivar of S. lycopersicum for the stabilization of sewage sludge.
Usually, heavy metal concentration extracted by plants was increased with their extractable content in rhizospheric soil (Dou et al., 2022). The extractable Cd concentrations in soils of S. nigrum and Baishite treatment were the highest (Figure 4), but the Cd concentration in Baishite was statistically similar to those in S. nigrum (Table 1). The chlorophyll contents and biomasses of Baishite were the highest (Figures 2, 5). Their Cd concentration was the highest (Table 1). SOD scavenges these ROS in order to protect plant cells and avoid or reduce the damage to plants by stress (Dai et al., 2022).
In general, Cd accumulation capacity (µg plant-1) in the shoot of a plant represents its phytoremediation potential due to the main removal mass being equal to the products of its concentration and biomass (Dai et al., 2022). Figure 3 shows that Cd (µg plant-1) was the highest in the cultivar Baishite shoot. This was due to the fact that Baishite had the highest biomass (Figure 2). A higher biomass can also cause high Cd accumulation due to the growth force and greater efficiency of antioxidant enzymes (Wei et al., 2018). There were no positive relationships between extractable Cd content in soil, chlorophyll content and SOD activities in leaves, and Cd concentration in shoots and roots (Table 1; Figures 4-6), which indicated that some other mechanisms could play more important roles in Cd accumulation differences between various cultivars. Obviously, some unknown genes concerning Cd accumulation differences among different cultivars may be more important, which might be a future research direction.
Conclusions
Among the seven tested tomato cultivars, the cultivar Baishite showed basic Cd hyperaccumulation properties. Moreover, cultivar Baishite showed the highest biomass and the highest Cd capacity in shoots. These results indicated that the Cd level did not limit the growth of S. nigrum and S. lycopersicum. No significant differences between CK and PS were found in the other index. It is necessary to search for more S. lycopersicum species with greater Cd accumulation capacity and explanation of the mechanisms of Cd accumulation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JJ: Formal analysis, Writing – original draft. HD: Data curation, Resources, Writing – review & editing. JZ: Data curation, Validation, Writing – review & editing. SW: Conceptualization, Data curation, Supervision, Writing – review & editing. LS: Validation, Writing – review & editing. JC: Data curation, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Sanqin Talents, Shaanxi Provincial First-class Team—“Contaminated Soil Remediation and Resource Utilization Innovation Team at Shaanxi University of Technology”, the project of Foreign Experts Bureau of China (S20240182, 2025WZ-YBXM-19, and 2025WZ-YBXM-29), Shaanxi University of Technology Research Ability Enhancement Project (SLGNL202405), Qin Chuangyuan “Scientists + Engineers” Team Construction in Shaanxi Province (2024QCY-KXJ-104), Shaanxi Province City-University Co-construction Project (SXZJ-2301), Qinba Bioremediation and Resource Development Research Innovation and Introduction Base (2025YZ-YIPT-41 and S2022-ZC-GXYZ-0029), and the program financed by Poland Minister of Science under the “Regional Excellence Initiative” for 2024–2027.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akanbi-Gada, M. A., Ogunkunle, C. O., Vishwakarma, V., Viswanathan, K., and Fatoba, P. O. (2019). Phytotoxicity of nano-zinc oxide to tomato plant (Solanum lycopersicum L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environ. Technol. Inno. 14, 100325. doi: 10.1016/j.eti.2019.100325
Akinci, I.E., Akinci, S., and Yilmaz, K. (2010). Response of tomato ((Solanum lycopersicum L.) to lead toxicity: Growth, element uptake, chlorophyll and water content. Afr. J. Agr. Res. 5, 416–423. doi: 10.5897/AJAR10.016
Al-Lahham, O., El Assi, N. M., and Fayyad, M. (2007). Translocation of heavy metals to tomato (Solanum lycopersicom L.) fruit irrigated with treated wastewater. Sci. Hortic. 113, 250–254. doi: 10.1016/j.scienta.2007.03.017
Almaroai, Y. A. and Eissa, M. A. (2020). Effect of biochar on yield and quality of tomato grown on a metal-contaminated soil. Sci. Hortic. 265, 109210. doi: 10.1016/j.scienta.2020.109210
Asaf, S., Jan, R., Khan, M. A., Lubna, Khan, A. L., Asif, S., et al. (2023). Unraveling the mutualistic interaction between endophytic Curvularia lunata CSL1 and tomato to mitigate cadmium (Cd) toxicity via transcriptomic insights. Sci. Total Environ. 861, 160542. doi: 10.1016/j.scitotenv.2022.160542
Ayhan, D. (2010). Oil, micronutrient and heavy metal contents of tomatoes. Food Chem. 118, 504–507. doi: 10.1016/j.foodchem.2009.05.007
Bhanse, P., Kumar, M., Singh, L., Awasthi, M. K., and Qureshi, A. (2022). Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: Opportunities, challenges, and prospects. Chemosphere 303, 134954. doi: 10.1016/j.chemosphere.2022.134954
Dai, H. P., Wei, S. H., Twardowska, I., Hou, N., and Zhang, Q. (2022). Cosmopolitan cadmium hyperaccumulator Solanum nigrum exploring cadmium uptake transport and physiological mechanisms of accumulation in different ecotypes as a way of enhancing its hyperaccumulative capacity. J. Environ. Manage 320, 115878. doi: 10.1016/j.jenvman.2022.115878
Dai, H. P., Wei, S. H., Twardowska, I., and Zhang, Q. (2021). In search of the exclusion/low-accumulation mechanisms: Cadmium uptake and accumulation from soil by cultivated (Solanum Melongena L) and wild eggplants(Solanum torvum L). J. Clean Prod. 323, 129141. doi: 10.1016/j.jclepro.2021.129141
Dou, X. K., Dai, H. P., Grzebelus, D., Lidia, S., and Wei, S. H. (2023). Cadmium phytoextraction efficiency of hyperaccumulator as affected by harvest stage in three continuous years. Chemosphere 313, 137639. doi: 10.1016/j.chemosphere.2022.137639
Dou, X. K., Dai, H. P., Lidia, S., and Wei, S. H. (2022). Cadmium removal potential of hyperaccumulator Solanum nigrum L. under two planting modes in three years continuous phytoremediation. Environ. pollut. 307, 119493. doi: 10.1016/j.envpol.2022.119493
El-Sappah, A. H., Elrys, A. S., Desoky El-Sayed, M., Zhao, X., Wang, B. W., El-Sappah, H. H., et al. (2021). Comprehensive genome wide identification and expression analysis of MTP gene family in tomato (Solanum lycopersicum) under multiple heavy metal stress. Saudi J. Biol. Sci. 28, 6946–6956. doi: 10.1016/j.sjbs.2021.07.073
Hasan, M., Islam, S., Bhuiyan, F. H., Arefin, S., Hoque, H., Jewel, N. A., et al. (2022). Genome wide analysis of the heavy-metal-associated (HMA) gene family in tomato and expression profiles under different stresses. Gene 835, 146664. doi: 10.1016/j.gene.2022.146664
Janifer, R. X., Roopa, N., Pal Murugan, M., Om Prakash, C., and Prakash, E. P. (2021). Data on the detection of toxic metals, essential nutrients, and microbial load of Solanum lycopersicum L. (tomato) and Abelmoschus esculentus (L.) Moench (okra) in Southern India and their impact on human health—A comparison between organic and conventional vegetables. Chem. Data Collections 35, 100765. doi: 10.1016/j.cdc.2021.100765
Jia, J. B., Dai, H. P., Wei, S. H., Xue, J. M., Skuza, L., Sun, Q., et al. (2023). Toxicity of emerging contaminant antibiotics in soil to Capsicum annuum L. growth and their effects on it accumulating copper. Plant Physiol. Biochem. 196, 661–667. doi: 10.1016/j.plaphy.2023.02.019
Liu, Y. R., Huang, L. K., Wen, Z. Y., Fu, Y. Y., Liu, Q. Z., Xu, S. A., et al. (2023). Effects of intercropping on safe agricultural production and phytoremediation of heavy metal-contaminated soils. Sci. Total Environ. 875, 162700. doi: 10.1016/j.scitotenv.2023.162700
Ogugua, U. V., Kanu, S. A., and Ntushelo, K. (2022). Gibberellic acid improves growth and reduces heavy metal accumulation: A case study in tomato (Solanum lycopersicum L.) seedlings exposed to acid mine water. Heliyon 8, e12399. doi: 10.1016/j.heliyon.2022.e12399
Pan, J., Cao, S., Xu, G. F., Rehman, M., Li, X., Luo, D. J., et al. (2023). Comprehensive analysis reveals the underlying mechanism of arbuscular mycorrhizal fungi in kenaf cadmium stress alleviation. Chemosphere 314, 137566. doi: 10.1016/j.chemosphere.2022.137566
Raja, A. A. K., Syed, S. A., Saba, N., Asad, A., Ajaz, A., Awais, S., et al. (2023). Mitigating Cd and bacterial wilt stress in tomato plants through trico-synthesized silicon nanoparticles and Trichoderma metabolites. Environ. pollut. 333, 122041. doi: 10.1016/j.envpol.2023.122041
Rodríguez-Rodríguez, I., Pérez-Vázquez, L., Pablos-Pons, F., and Fernández-Espinosa, A. J. (2023). Toxic metals from atmospheric particulate matter in food species of tomato (Solanum lycopersicum) and strawberry (Fragaria x ananassa) used in urban gardening. A closed chamber study. Chemosphere 340, 139921. doi: 10.1016/j.chemosphere.2023.139921
Sarwar, M. J., Zahir, Z. A., Asghar, H. N., Shabaan, M., and Ayyub, M. (2023). Co-application of organic amendments and Cd-tolerant rhizobacteria for suppression of cadmium uptake and regulation of antioxidants in tomato. Chemosphere 327, 138478. doi: 10.1016/j.chemosphere.2023.138478
Su, L. H., Xie, Y. D., He, Z. Q., Zhang, J. W., Tang, Y., and Zhou, X. T. (2021). Network response of two cherry tomato (Lycopersicon esculentum) cultivars to cadmium stress as revealed by transcriptome analysis. Ecotox Environ. Saf. 222, 112473. doi: 10.1016/j.ecoenv.2021.112473
Sun, X. H., Wang, S. T., Tian, J. Y., Xiang, X. B., Zheng, H. Y., Liu, H. J., et al. (2023). Synergistic interplay between ABA-generating bacteria and biochar in the reduction of heavy metal accumulation in radish, pakchoi, and tomato. Environ. pollut. 333, 122084. doi: 10.1016/j.envpol.2023.122084
Wang, P. J., Xu, Z. J., Zhang, Y., Ma, Y. B., Yang, J. Y., Zhou, F., et al. (2022). Over-expression of spermidine synthase 2 (SlSPDS2) in tomato plants improves saline-alkali stress tolerance by increasing endogenous polyamines content to regulate antioxidant enzyme system and ionic homeostasis. Plant Physiol. Biochem. 192, 172–185. doi: 10.1016/j.plaphy.2022.09.025
Wei, S. H., Hu, Y. H., Srivastava, M., Zhou, Q. X., Niu, R. C., Li, Y. M., et al. (2009). Seed germination of a newly discovered hyperaccumulator Solanum nigrum L. affected by illumination and seed-soaking reagent. J. Hazard Mater. 170, 1256–1259. doi: 10.1016/j.jhazmat.2009.05.014
Wei, T., Lv, X., Jia, H. L., Hua, L., Xu, H. H., Zhou, R., et al. (2018). Effects of salicylic acid, Fe(II) and plant growth-promoting bacteria on Cd accumulation and toxicity alleviation of Cd tolerant and sensitive tomato genotypes. J. Environ. Manage. 214, 164–171. doi: 10.1016/j.jenvman.2018.02.100
Wei, S. H. and Twardowska, I. (2013). Main rhizosphere characteristics of the Cd hyperaccumulator Rorippa globosa (Turcz.) Thell. Plant Soil. 372, 669–681. doi: 10.1007/s11104-013-1783-0
Wu, Y. J., Ma, L. Y., Liu, Q. Z., Vestergård, M., Topalovic, O., Wang, Q., et al. (2020). The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J. Hazard Mater 395, 122661. doi: 10.1016/j.jhazmat.2020.122661
Zhang, Y. M., Lin, C. Y., Li, B. Z., Cheng, Y. X., Xu, W. B., Xiao, Y., et al. (2022). The health risk for consumers under heavy metal scenarios: Reduce bioaccumulation of Cd in estuary mud crab (Scylla paramamosain) through the antagonism of Se. Sci. Total Environ. 844, 157149. doi: 10.1016/j.scitotenv.2022.157149
Keywords: Solanum lycopersicum L., Solanum nigrum L., phytoremediation, cadmium, cultivar
Citation: Jia J, Dai H, Zhan J, Wei S, Skuza L and Chang J (2025) Assessing the cadmium phytoremediation potential of different Solanum lycopersicum L. cultivars during their vegetative growth phase. Front. Plant Sci. 16:1587253. doi: 10.3389/fpls.2025.1587253
Received: 04 March 2025; Accepted: 28 April 2025;
Published: 21 May 2025.
Edited by:
Khurram Bashir, Lahore University of Management Sciences, PakistanReviewed by:
Taras P. Pasternak, Miguel Hernández University of Elche, SpainVijay Sheri, Texas Tech University, United States
Copyright © 2025 Jia, Dai, Zhan, Wei, Skuza and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhe Wei, c2h1aGV3ZWlAaWFlLmFjLmNu; Junjun Chang, Y2hhbmdqdW5qdW5AeW51LmVkdS5jbg==
 Jibao Jia1
Jibao Jia1 Huiping Dai
Huiping Dai Shuhe Wei
Shuhe Wei Lidia Skuza
Lidia Skuza