- Institute of Millet Crops, Hebei Academy of Agriculture and Forestry Sciences, Hebei Branch of China National Sorghum Improvement Center, Shijiazhuang, China
Sorghum, one of the world’s five major cereal crops, faces significant yield losses due to aphid infestations, particularly from the sorghum aphid (Melanaphis sacchari) and the greenbug (Schizaphis graminum). These pests not only cause a reduction in grain yield, but also transmit plant viruses, posing a serious threat to global food security. Current strategies to mitigate aphid damage include large-scale insecticide applications, biological control through natural enemies, and the development of aphid-resistant sorghum varieties. However, the resistance genes of aphids and their mechanisms are still unclear, which poses a major challenge to breeding programs. This review synthesizes recent advances in understanding the interactions between sorghum and these two major aphid species, exploring topics such as aphid classification, quantitative trait locus (QTL) mapping of resistance genes, and the molecular mechanisms of sorghum-aphid interactions. We also discuss conventional and emerging insecticide methods, biological control strategies, and their associated challenges. Looking ahead, the integration of molecular breeding techniques, including genetic engineering and genome editing, holds promise for accelerating the development of aphid-resistant sorghum varieties. These innovative approaches aim to minimize aphid damage, enhance sorghum productivity, and contribute to global food security in the face of climate change and evolving pest pressures.
1 Introduction
Sorghum [Sorghum bicolor (L.) Moench], originating from sub-Saharan Africa, is the world’s fifth largest cereal crop, following wheat, rice, maize, and barley, which is widely cultivated globally (Brown et al., 2006; Takanashi, 2023). Global sorghum production is approximately 61 million tons every year, serving as a staple food for more than 500 million people in 30 countries across Africa and Asia. In China, the annual production is around 3 million tons (Khoddami et al., 2023). As a C4 model crop, sorghum is different from other cereals and can be grown in extreme environments under abiotic stresses such as drought and heat (Jeff and Dahlberg, 2019; Rudo et al., 2021). Facing the challenges of a growing global population and intensifying global climate change, sorghum as a resilient crop, plays a key role in feeding more impoverished people and safeguarding global food security (Al-Salman et al., 2024). Despite its advantages in resisting abiotic stresses, sorghum faces severe challenges from biotic stresses (Erpen et al., 2018; Baillo et al., 2019; Min et al., 2023; Zhang et al., 2023). During its growth period, sorghum is susceptible to over 150 pests (Kruger et al., 2008; Guo et al., 2011). With the global expansion of sorghum cultivation, aphid (Acyrthosiphon pisum) infestations have become a significant issue in regions such as China, North America, and South Africa, posing a major threat to sorghum production (Singh et al., 2004; Bowling et al., 2017; Craigie et al., 2024). There are about 5500 species of aphids, with approximately 250 species considered economically significant pests that can cause serious harm to plants (Powell, 1992; Blackman and Eastop, 2006; Isaacs and Woodford, 2008). Among them, the sorghum aphid [Melanaphis sacchari (Zehntner)], the greenbug [Schizaphis graminum (Rondani)], the corn leaf aphids [Rhopalosiphum maidis (Fitch)], and the bird cherry-oat aphid [Rhopalosiphum padi (Linnaeus)] are the four main aphid species that cause significant damage to sorghum production (Kariyat et al., 2019; Lopes et al., 2022; Carl et al., 2024; Zhao et al., 2024a). Two of those four species, the sorghum aphid and the greenbug have a profound impact on sorghum yield and cause serious damage (Zhao et al., 2024b). Among them, sorghum aphid can reduce sorghum yield by 50% to 100% (Thudi et al., 2024).
The sorghum aphid, a member of the Homoptera: Aphididae family, has a complex taxonomic history. The sugarcane aphid (Melanaphis sacchari) was first identified on sugarcane in Java, Indonesia, in 1897, while the sorghum aphid (Melanaphis sorghi) was initially discovered on sorghum in Sudan in 1904. Although these two species have often been considered synonymous, recent studies suggest distinct host preferences: the sugarcane aphid primarily infests sugarcane, whereas the sorghum aphid is more commonly associated with sorghum (Paudyal et al., 2019). Nibouche et al. (2021) analyzed several aphid samples from the United States and identified morphological differences between sorghum aphids and sugarcane aphids (Nibouche et al., 2021). This research provides preliminary evidence supporting the existence of distinct differences between the two species. However, recent studies have revealed that sugarcane aphids can also experience large outbreaks in sorghum fields, this finding suggests that the distinction between the two feeding species is not well-defined (Paudyal et al., 2019). Therefore, researchers still tend to categorize the two species as a single species, named the sorghum aphid (M. sorghi).
The sorghum aphid severely impacts global sorghum yields by feeding on the sap within the phloem of stems and leaves throughout all growth stages of the plant (Xoconostle-Cázares et al., 2023). While feeding on sap in the phloem, the sorghum aphid can secrete honeydew, which can reduce plant photosynthesis and affects metabolic reactions (Vasquez et al., 2024). Furthermore, sorghum aphids can transmit various plant viruses, such as the cereal red leaf virus, sugarcane yellow leaf virus, and sugarcane mosaic virus (Kondaiah and Nayudu, 1984; Gupta and Virendra, 1994; Schenck and Lehrer, 2000). These viruses can lead to significant losses in sorghum production and quality, compromising the safety of food and feed, and posing risks to the health of both humans and animals (Bowling et al., 2017; Shabbir et al., 2022). Sorghum aphids, with their high reproductive capacity, can quickly disperse by flight when encountering resistance. Traditional chemical control methods, while effective, are labor-intensive, time-consuming, and pose risks of environmental pollution, making complete eradication of aphids a persistent challenge (Pekarcik and Jacobson, 2021).
The greenbug (Hemiptera: Aphididae), was first reported in 1907 (Webster, 1907). As well as the sorghum aphid, it is one of the harmful aphid species that reduce global sorghum production (Bonnie et al., 2009; Zhang and Huang, 2024). Based on the different host and plant responses to greenbug, they can be categorized as A, B, C, E, F, G, H, I, J, K, Chn1, NY, WY10MC, WY81, WY10 B, WY12 MC, and WY86 (Royer et al., 2015). The greenbug uses its needle-like mouthparts to pierce the plant’s phloem, extracting sap while injecting toxic saliva into the plant. This dual action causes significant damage to sorghum, impacting its growth and productivity (Burton and Burd, 1993; Bonnie et al., 2009; Xu et al., 2024). After feeding on sorghum, greenbugs cause the leaves to develop red spots, which gradually turn yellow and eventually lead to the death of the affected tissue (Teetes and Johnson, 1974). In one study, the result indicates that after feeding by Biotype C greenbugs, the organelle recognition function of nearby phloem cells is disrupted. Chloroplast membranes are damaged, and mitochondria undergo gradual degeneration, resulting in severe structural and functional impairment of the organelles (Al-Mousawi et al., 1983). With a strong reproductive capacity, a single female greenbug can produce 60–80 offspring and the population of greenbug can double every two days under ideal conditions (Royer et al., 2015). Therefore, finding efficient ways to control greenbug is crucial for mitigating their impact on global sorghum production in the future.
In agricultural management, it is common to use insecticides to address and prevent aphid infestations. While the large-scale application of insecticides can yield the desired results, it also poses several risks. Firstly, the excessive use of insecticides can lead to genetic mutations in aphids, allowing them to develop resistance to these chemicals. Moreover, due to their rapid reproduction rate, the misuse of insecticides may further accelerate the mutation rate of aphids, making it increasingly challenging to control new aphid populations. Additionally, the widespread use of potentially hazardous insecticides contradicts our current principles of environmental protection. Therefore, it is essential to explore alternative methods for aphid control to achieve a more sustainable approach to agricultural production. Sorghum has a relatively small genome (730M) that has been sequenced and can be accessed through online databases like the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov) and Phytozome (https://phytozome-next.jgi.doe.gov). These databases are of significant importance for identifying molecular markers associated with aphid-related genes and their practical applications in agriculture (Goodstein et al., 2012; Agarwala et al., 2018; Rudo et al., 2021). Leveraging diverse sorghum germplasm resources, identifying key aphid resistance genes, and unraveling the molecular mechanisms of sorghum resistance are essential steps to accelerate the development of resistant sorghum varieties and revitalize the sorghum seed industry. This review synthesizes prior research on the classification of sorghum aphids and greenbugs, plant responses to aphid feeding, and the identification of plant resistance genes against aphids. It also highlights future directions in sorghum breeding for aphid resistance, emphasizing the strategic use of molecular markers and advanced tools to accelerate the discovery of resistance genes and develop high-yielding, aphid-resistant sorghum varieties-a critical challenge for the future.
2 Aphid resistance materials
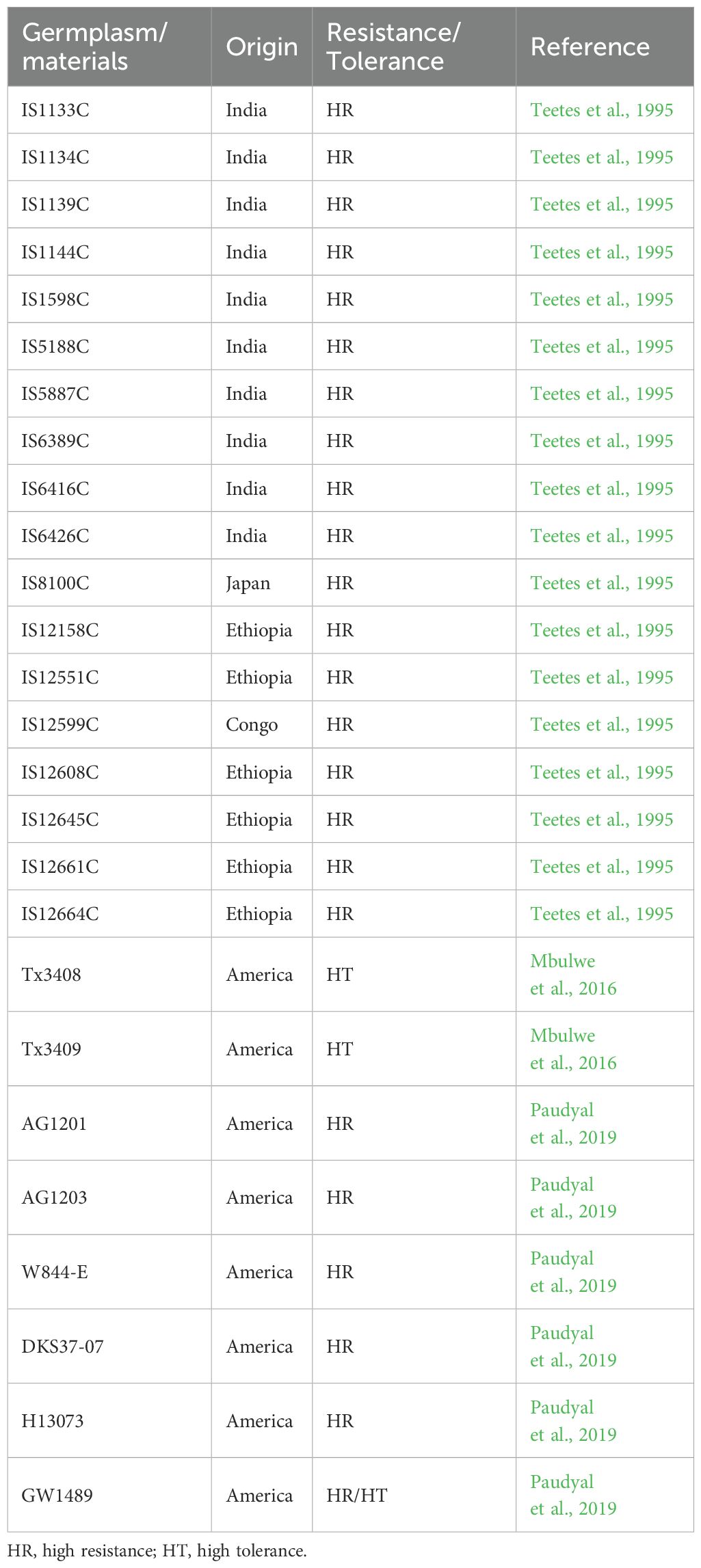
Previous studies have indicated a scarcity of aphid-resistant sorghum germplasm resources. Based on the survival rate of seedlings after aphid treatment, Singh et al. (2004) reported 18 sorghum germplasm resources with high resistance from Ethiopia, Congo, Malawi, the United States, Mexico, India, and Japan (Singh et al., 2004). Lu and Dahlberg (2001) identified approximately 5,000 germplasm resources from China, with only 1 material showing high resistance to aphid, which was homologous to the US sorghum TAM428 (Lu and Dahlberg, 2001). In recent years, several studies have conducted aphid resistance identification on more sorghum materials, the results showed that through the assays by using the nylon net, clip cage and leaf disc, 10 with moderate resistance and 6 with high resistance were identified. Another approach was to evaluate the aphid damage level by using plant height, the number of leaves and chlorophyll loss, and 2 materials with high tolerance were identified (Sharma et al., 2014; Mbulwe et al., 2016; Paudyal et al., 2019). Information on germplasm resources and sorghum materials exhibiting high resistance is summarized in Table 1. Knoll et al. (2023) identified three aphid-resistant sweet sorghum varieties, named GTS1903, GTS1904, and GTS1905, through field selection and trait observation (Knoll et al., 2023). These varieties share a common origin, all being derived from the resistant parent PI 257599, which carries known resistance loci on SBI-06, as confirmed through genetic marker identification. Huang and Huang (2023) also discovered the PI550607 line, which possessed resistance to greenbug (Huang and Huang, 2023). Guden et al. (2019) screened 561 sorghum materials and identified 26 sweet sorghum varieties with excellent agronomic traits. Through field trials and aphid population statistics, they ultimately obtained the aphid-resistant sweet sorghum variety BSS507, which showed high resistance at two experimental sites (Guden et al., 2019). In addition, previous studies identified aphid resistant/tolerant sorghum lines, including IS1144C, IS12664C, IS12609C, and TAM428 as resistant lines (Singh et al., 2004), while TX3408, TX3409 (Mbulwe et al., 2016), AG1201, AG1301, W844-E, and DKS37-07 (Paudyal et al., 2019) are tolerant lines. It is important to note that the origin and mechanisms of resistance or tolerance among these materials remain unclear and require further investigation. These sorghum lines exhibiting resistance to aphids hold potential for future breeding development as restorer lines (Restorer line is used to restore the fertility in hybrid after crossing with ms A line in hybrid production plot).
3 Aphids genotypes
3.1 Sorghum aphid genotypes
Sorghum aphid (M. sacchari) was first studied in terms of its classification in 2014 by Nibouche. They conducted genetic typing of aphids by analyzing 1333 individuals collected from sugarcane and sorghum between 2002 and 2009. They defined five multi-locus lineages (MLL) for M. sacchari based on their origin: MLL-A, Africa; MLL-B, Australia; MLL-C, South America, the Caribbean, and the Indian Ocean (including East Africa); MLL-D, USA; MLL-E, China (Nibouche et al., 2014); Following outbreaks of aphids in the United States and Caribbean coastal countries, Nibouche et al. (2018) identified a new clone lineage through satellite markers and sequencing, named MLL-F. MLL-F has been found to spread extensively on both sugarcane and sorghum. As an invasive species in the USA, its origin remains unclear. While M. sorghi and M. sacchari have traditionally been considered synonymous, experimental evidence has not definitively established whether they are the same species. Different lineages infect different host plants, hinting at potential distinctions between the two types (Nibouche et al., 2018). Further, they analyzed 199 aphid samples collected over 14 years in the USA using morphometrics and molecular data. They concluded that MLL-B, MLL-C, and MLL-D belong to M. sacchari, while MLL-A, MLL-E, and MLL-F belong to M. sorghi. Morphological differences were observed in features such as the length of the cauda, hindtibia, siphunculi, and processus terminalis length between M. sacchari and M. sorghi (Nibouche et al., 2021). In 2022, the sorghum aphid crisis on sorghum in Brazil was confirmed to be MLL-F, the same lineage as the 2013 outbreak in the USA (Harris-Shultz et al., 2022b). In this article, the mentioned M. sacchari already includes M. sorghi, which are considered synonymous. Although these has been divided into six lineages, it remains one of the aphid species with the least known genetic diversity within such a widespread global distribution of aphids (Figure 1).
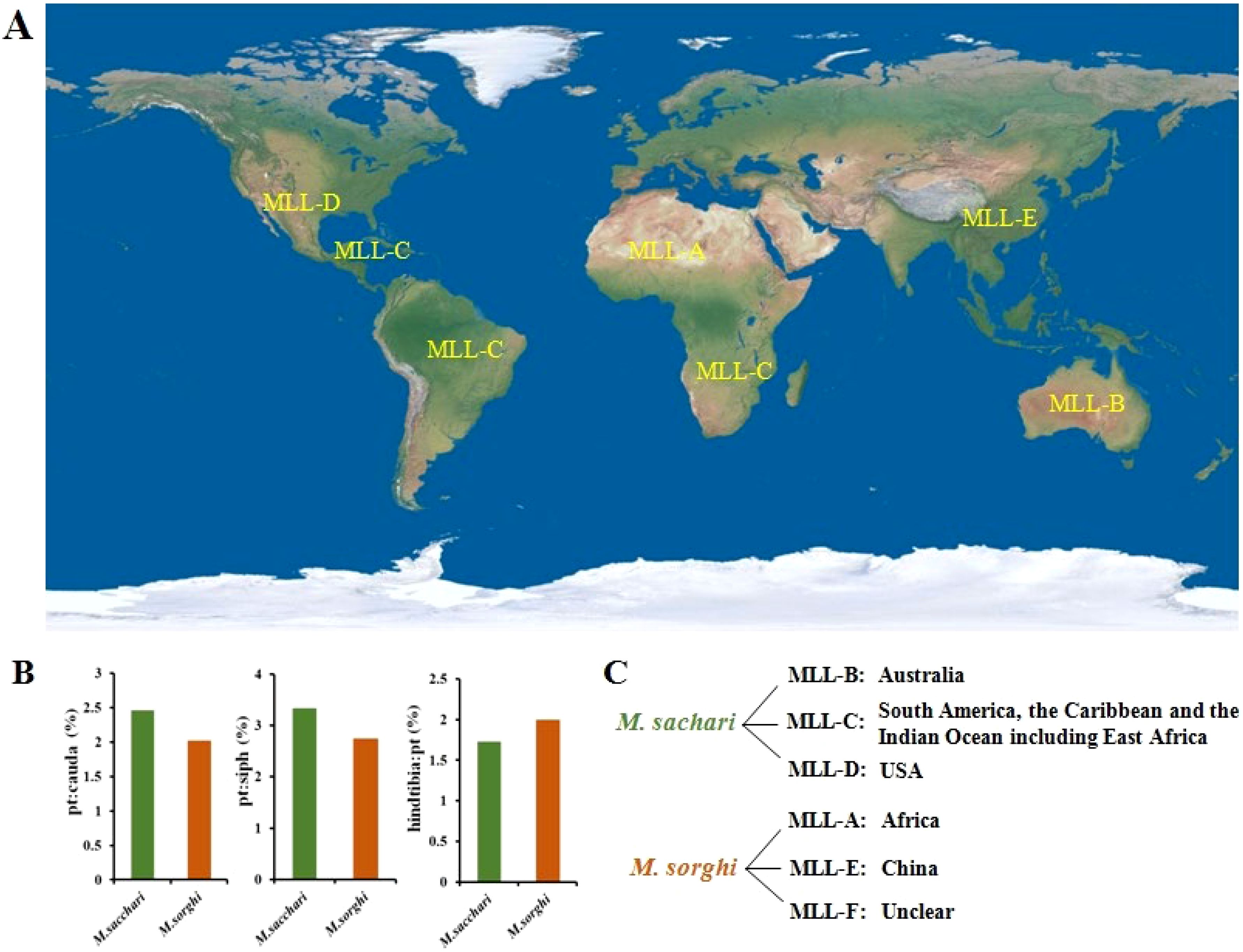
Figure 1. Lineage classification and origin of sorghum aphids. (A) Global origin distribution of six sorghum aphid lineages. (B) Differences in pt:cauda, pt:siph, and hindtibia:pt between sorghum aphids and sugarcane aphids. (C) Different lineages and origins of sorghum aphids and sugarcane aphids. pt, processus terminalis length; siph, siphunculi (Nibouche et al., 2021).
3.2 Greenbug genotypes
The greenbug (Hemiptera: Aphididae) is a major pest that threatens sorghum crops worldwide. Research on this pest began in 1961, initially focusing on classifying different types based on the plants’ reactions to greenbug infestations (Wood, 1961; Shufran et al., 2010). Wood isolated a biotype of greenbug from wheat that could harm susceptible varieties but could not grow on resistant ones, referred to as the A biotype. Further research identified a new greenbug that could damage A biotype resistant wheat, which was named the B biotype. Harvey and Hackerott (1969) isolated a new strain, the C biotype, from severely affected sorghum populations, exhibiting different sensitivities to various plants compared to the previously identified B biotype from wheat (Harvey and Hackerott, 1969). Cress and Chada (1971) found metabolic differences between the A and B biotypes, with the A biotype having a slower metabolism (Cress and Chada, 1971). Porter et al. (1982) identified a different resistance biotype, the E biotype, in wheat populations (Porter et al., 1982). Beregovoy et al. (1988) analyzed sorghum populations affected by greenbug biotype C and E in eight US regions, highlighting the E biotype higher reproduction on oats and Sudan grass but shorter survival on maize (Beregovoy et al., 1988). Kindler and Spomer (1986) discovered a new biotype, the F biotype, closely related to the A biotype in plant response, capable of killing ‘Reubens’ Canada bluegrass, L., resistant to biotypes A-E (Kindler and Spomer, 1986). Puterka et al. (1988) obtained two new strains, the G and H biotypes, from host plants in Oklahoma (SCO) and Texas (WCT) (Puterka et al., 1988). Harvey et al. (1991) isolated a severe biotype, the I biotype, from hybrid sorghum resistant to the E biotype (Harvey et al., 1991). Beregovoy and Peters (1994) identified the J biotype in a barley variety, POST, sensitive to barley but harmless to resistant wheat (Beregovoy and Peters, 1994). Harvey et al. (1997) separated the K biotype from the sorghum line PI550610 (resistant to the I biotype), with the potential for breeding due to high resistance. In addition to the A-K biotypes, several other biotypes have been reported (Harvey et al., 1997). Liu and Jin (1998) identified and named a new biotype, Chn1, on wheat varieties in Beijing, which poses significant harm to wheat but not to oats or rye (Liu and Jin, 1998). Shufran et al. (2010) isolated a new biotype, NY, from Elymus canadensis (L.) (Shufran et al., 1997). Armstrong et al. (2016) identified five new biotypes from wild barley varieties, namely WY10 MC, WY81, WY10 B, WY12 MC, and WY86. In total, there are currently 17 biotypes (Table 2), with C, E, I, and K types significantly impacting sorghum yield (Armstrong et al., 2016). Understanding the differences in resistance of various sorghum varieties to different biotypes of the greenbug is crucial for the application of resistance genes in breeding efforts.
4 Progress in molecular mechanisms of aphid resistance in sorghum
In the following section, we provide a detailed overview of recent research advancements related to sorghum aphids and greenbugs, focusing on aphid classification, plant resistance genes against aphids, and plant responses to aphid infestation.
4.1 Molecular mechanisms of sorghum resistance to sorghum aphid
4.1.1 Genetic studies of sorghum aphid resistant genes and QTLs
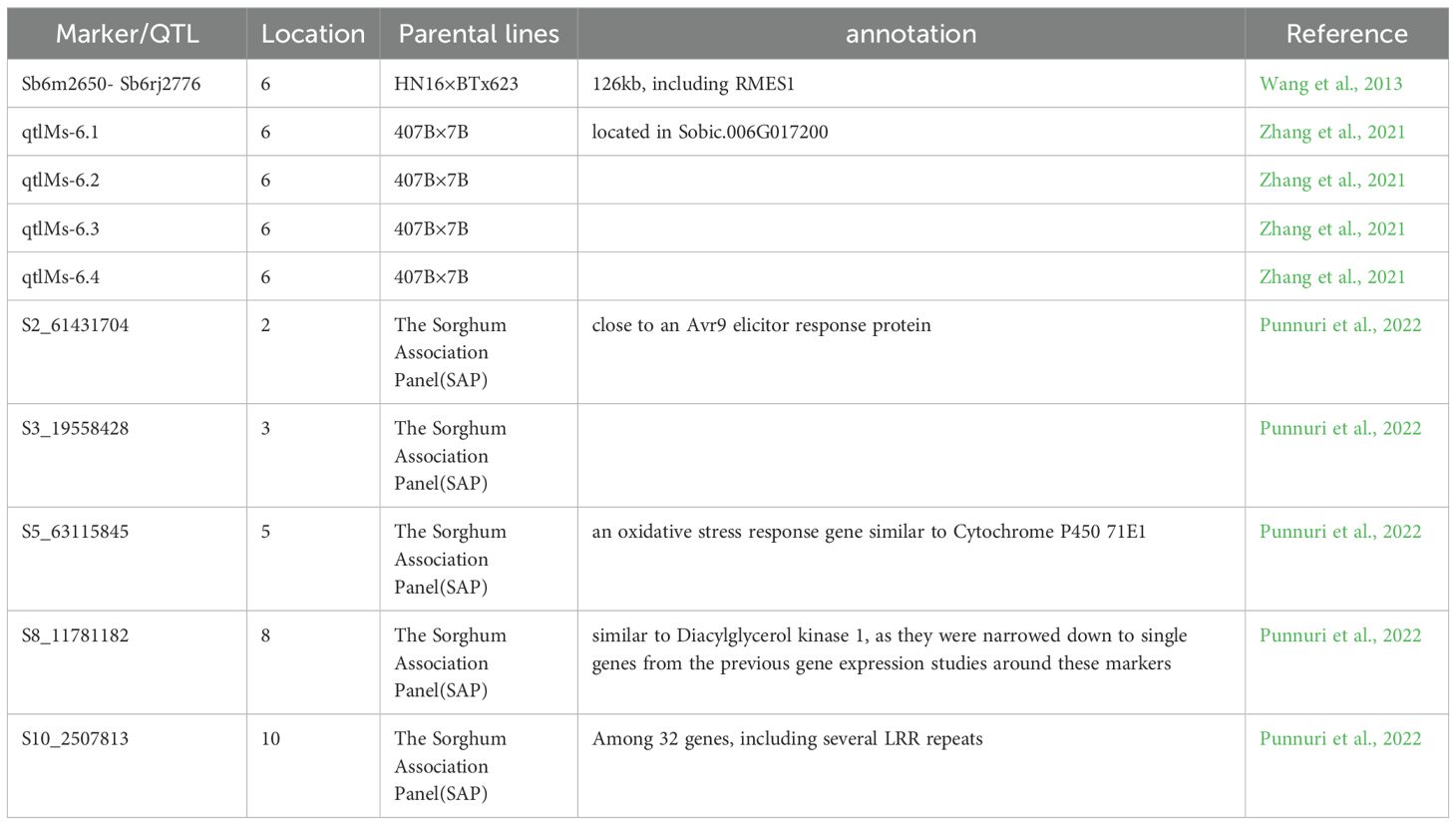
Currently, the candidate genes related to aphid resistance in sorghum and the molecular responses of sorghum to aphid infestation are not fully understood. Chang (1981) demonstrated that sorghum resistance to sorghum aphids is controlled by a single dominant gene (Chang, 1981). This conclusion was also supported by crossing the high aphid resistance sorghum variety HN-16 with QS, the aphid resistance gene mapping study was conducted for the F1 and F2 generations (Chang et al., 2006). through experiments. In recent years, an increasing number of molecular markers closely linked to aphid resistance genes have been developed. Wang et al. (2013) utilized two molecular markers, Sb6m2650 and Sb6rj2776, to map a candidate region for the sorghum resistance gene RMES1 (Resistance to M. sacchari 1) on the short arm of chromosome 6, spanning 126kb and containing 5 candidate genes, namely Sb06g001620 (Sobic.006G017000), Sb06g001630 (Sobic.006G017100), Sb06g001640 (Sobic.006G017200), Sb06g001645 (Sobic.006G017400) and Sb06g001650 (Sobic.006G017500) (Wang et al., 2013). Wang et al. (2021) using Tx2783 as a reference, combined with materials such as BTx623, RTx430, and Rio, a feature analysis was conducted on structural variations (SVs) including insertion (INS), delivery (DEL), inversion (INV), and copy number variation (CNV) among different varieties, and the same region was determined (Wang et al., 2021). Zhang et al. (2021) identified four QTLs from the sorghum aphid-resistant resource 407B, one of which, qtlMs-6.1, falls within the candidate region of the RMES1 gene (Zhang et al., 2021). Muleta et al. (2022) analyzed the gene sequences of the five candidate genes in the 126kb region using the aphid-resistant line PI276837 and three aphid-susceptible lines, BTx623, Tx430, and BTx642. They found 35, 32, and 29 SNP variations in the exons of Sobic.006G017200, Sobic.006G017400, and Sobic.006G017500, respectively, and 3 insertions/deletions in Sobic.006G017500 (Muleta et al., 2022). Punnuri et al. (2022) reported five markers associated with plant response to sorghum aphids, two of which were related to aphid numbers and sorghum damage, located on chromosome 8 (S8_11781182) and chromosome 10 (S10_2507813), and three were related to sorghum damage on chromosomes 2 (S2_61431704), 3 (S3_19558428), and 5 (S5_63115845) (Punnuri et al., 2022). The known markers and QTLs are summarized in Table 3.
In recent years, with the completion of sorghum genome sequencing and the continuous development of technologies such as transcriptomics and proteomics, research on the aphid resistance mechanism of sorghum has been progressing gradually. Through transcriptomic and metabolomic analyses of the aphid resistant sorghum variety HN-16 and the aphid sensitive variety BTx623, it was shown that the differentially expressed genes were mainly enriched in the flavonoid biosynthesis pathway, and the differentially expressed metabolites were mainly related to isoflavone biosynthesis and flavonoid biosynthesis. Furthermore, the observation of the epidermal cell structures of two different varieties revealed that the resistance of sorghum to aphids is positively correlated with the regularity of epidermal cells and negatively correlated with cell spacing and leaf thickness (Zhao et al., 2024b). Tetreault et al. (2019) demonstrated that jasmonic acid (JA), ethylene (ETH), and other plant hormones can regulate a plant’s resistance to sorghum aphids by comparing transcriptome results of aphid-resistant and susceptible materials under sorghum aphid stress (Tetreault et al., 2019). Grover et al. (2024) discovered that Auxin-Aspartic Acid (IAA-Asp) can enhance plant resistance to aphids in sorghum Brown midrib (Bmr) mutants, and Bmr negatively regulates IAA-Asp content (Grover et al., 2024). Subsequently, Grover et al. (2022b) conducted a proteomic analysis on the aphid-resistant genotype SC265, which was determined as a resistant line through choice and no-choice assays, revealing an upregulation of defense and signal-related proteins after 1 and 7 days of aphid feeding, including salicylic acid (SA), phospholipase, calcium signaling, and Zinc-related proteins (Grover et al., 2022b). Furthermore, studies suggested that ARFs (Auxin Response Factors), GRAS, MADS, NAC (NAM, ATAF1/2, CUC1/2), and WRKY transcription factor families are involved in sorghum’s response to aphids (Serba et al., 2021). Poosapati et al. (2022) demonstrated that overexpression of SbWRKY86 in tobacco and Arabidopsis can increase resistance to peach aphids (Myzus persicae), but did not directly prove resistance to sorghum aphids (Poosapati et al., 2022). Therefore, it can be a potential candidate gene for the resistance of sorghum aphids, and more in-depth studies are needed to understand its response pattern to sorghum aphids. The nucleotide-binding site (NBS)-leucine-rich repeat (LRR) gene family is an important plant disease resistance gene, widely present in plants, animals, and fungi, commonly involved in defense response signal transduction (Balamurugan et al., 2024). Tetreault et al. (2019) found that over 70 LRR genes were upregulated in aphid-resistant sorghum lines through transcriptome analysis, indicating that LRR proteins confer resistance to aphids in sorghum (Tetreault et al., 2019). Among the five candidate genes identified by Wang et al. (2013), three genes are LRR genes (Wang et al., 2013). Subsequent research can focus on the NBS-LRR gene family to further elucidate the function of each candidate gene.
4.1.2 Sorghum defensive response to sorghum aphid infestation
Aphids feeding can trigger the host plants to respond to the defense signaling pathways (Thudi et al., 2024). In this section, we briefly summarized the morphological and structural changes and hormone signals of sorghum after sorghum aphid infection. After being attacked by sorghum aphids, sorghum triggers a series of signal transductions internally to initiate plant defense responses. This intricate biological process involves multiple signaling pathways, including the coordinated action of hundreds of genes, various plant hormones, secondary metabolites, and other compounds that collectively respond to aphids (Smith and Boyko, 2010). Initially, plants undergo physiological changes in response to aphid feeding, such as forming cuticle layers and epicuticular waxes on leaves (Harris-Shultz et al., 2022b; Cardona et al., 2023b). To study the impact of epicuticular waxes on aphid feeding, Cardona et al. (2023a, b) used electrical penetration graph technology to detect aphid feeding process. They found that aphids tend to feed longer from phloem and subsequent experiments have shown that aphids prefer to feed on non-flowering plants with high wax content. In non-flowering plants, higher levels of 16-monoacylglycerol and c32-alcohol suggest these substances may influence aphid feeding on sorghum. This highlights the crucial role of epicuticular waxes in plant resistance against aphid feeding (Cardona et al., 2023a, b). Triplett et al. (2023) discovered that stomatal density, trichome density, and chloroplast density show a positive correlation with aphid resistance, whereas trichome length is negatively correlated with aphid resistance (Triplett et al., 2023). By comprehensively understanding these relationships, we can facilitate the breeding of plant varieties that possess greater resistance to aphids, ultimately leading to improved crop productivity and sustainability. Grover’s research found that plant hormones like JA and cytokinins (CTK) play essential roles in sorghum’s defense against aphids (Grover et al., 2022a). Additionally, plants produce volatile chemicals like alkaloids and sorghum ketone to defend against aphids (Mizuno et al., 2010). Some studies suggested that sorghum can reduce aphid populations by prolonging the presence of aphid predators. Sorghum serves as a food source for aphid predators like hoverflies and bees, which also collect honeydew produced by aphids. Planting susceptible sorghum varieties around the edges of fields may help defend against aphids effectively (Harris-Shultz et al., 2022a).
4.2 Molecular mechanisms of sorghum resistance to greenbug
4.2.1 Genetic studies of greenbug resistant genes and QTLs
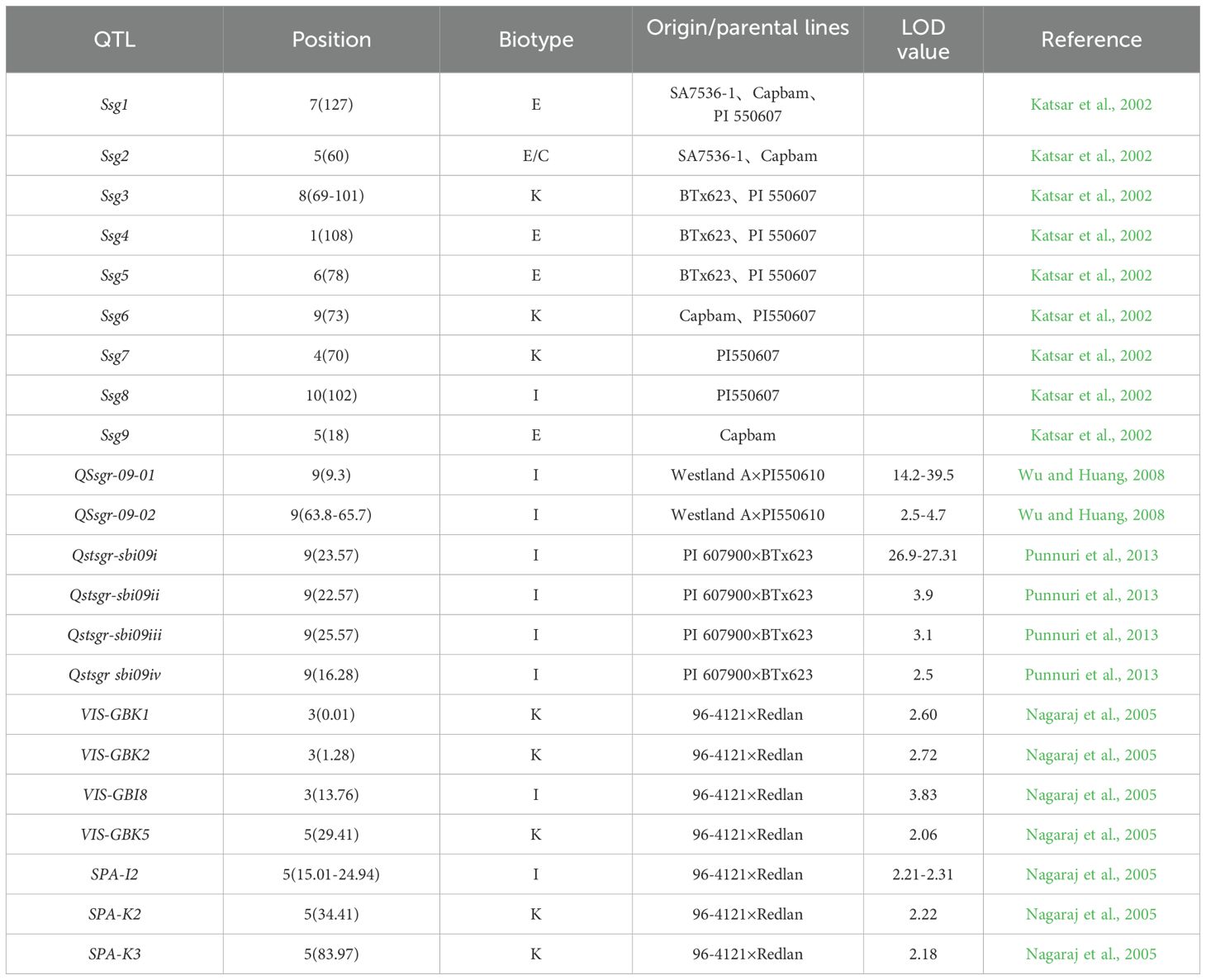
Research on the genetic mapping of the greenbug resistance can be traced back to 2002, when Katsar et al. (2002) reported 9 QTL loci associated with sorghum resistance to the greenbug. These QTLs are located on the following chromosomes: chr1 (Ssg4, bio: E), chr4 (Ssg7, bio: K), chr5 (Ssg2, bio: E/C; Ssg9, bio: E), chr6 (Ssg5, bio: E), chr7 (Ssg1, bio: E), chr8 (Ssg3, bio: K), chr9 (Ssg6, bio: K), and chr10 (Ssg8, bio: I). Notably, Ssg2 and Ssg9 can explain approximately ≈30% of the variation in the resistance phenotype, respectively (Katsar et al., 2002). Wu and Huang (2008) reported two QTL loci associated with greenbug resistance, both located on chr9 (QSsgr-09-01, PVE: 54.5-80.3%, bio: I; QSsgr-09-02, PVE: 1.3-5.9%, bio: I), from the cross Westland A×PI550610 through the construction of an F2:3 population (Wu and Huang, 2008). Next, Punnuri et al. (2013) identified four QTL loci associated with resistance to greenbug with a biotype I through the construction of an F2 population from the cross PI 607900×BTx623. These QTL, named Qstsgr-sbi09i, Qstsgr-sbi09ii, Qstsgr-sbi09iii, and Qstsgr-sbi09iv, are all located on chr9 and collectively explain 17.3-82.4% of the phenotypic variation (Punnuri et al., 2013). Subsequently, Punnuri and Huang (2017) conducted QTL mapping again using two parental lines, BTx623 (susceptible) and PI 607900 (resistant), and obtained consistent QTL analysis results with those identified using the F2 population in the previous study (Punnuri and Huang, 2017). Nagaraj et al. (2005) identified 6 QTLs using recombinant inbred lines (RILs) derived from the cross between ‘96-4121’ (resistant) and Redlan (susceptible). These QTLs were located on chr4 (VIS-GBK1, bio: K; VIS-GBK2, bio: K; VIS-GBI8, bio: I) and chr5 (VIS-GBK5, bio: K; SPA-I2, bio: I; SPA-K2, bio: K), these QTLs can explain phenotypic variation ranging from 9% to 19.6% (Nagaraj et al., 2005). The known QTLs are summarized in Table 4.
Chou and Huang (2010) first found that the expression of thaumatin-like protein (TLP) increased thousands of times after being bitten by wheat aphids, indicating that TLP may be involved in sorghum’s defense response to greenbug (Chou and Huang, 2010). TLP proteins belong to the PR gene family, widely involved in defense and development processes in plants, animals, and fungi. In plants, the TLP protein belongs to the PR-5 gene family (Irfan et al., 2020). TLPs have been found in various plants such as maize, Arabidopsis, barley, moss, and rice (Ernst et al., 2006; Zefeng et al., 2008; Liu et al., 2024). Additionally, Zhang and Huang (2024) analyzed the NAC gene family in sorghum and identified 9 SbNAC genes induced by greenbug by comparing susceptible line BTx623 and resistant line PI607900 (Zhang and Huang, 2024). This proves that the NAC transcription factor family also plays a very important role in sorghum defense processes.
4.2.2 Sorghum defensive response to greenbug infestation
Similar to the response of plants to sorghum aphid feeding, plants also take a series of defense measures to cope with the invasion of greenbug. Firstly, the phloem of sorghum will initiate defense responses. Grover et al. (2019) discovered resistance factors in the vascular tissue of resistant materials through the development of a nested association mapping (NAM) population of sorghum, which can shorten the feeding time of greenbug and thus have resistance response to greenbug (Grover et al., 2019). Harris-Shultz et al. (2019) identified a wax mutant, bloomless2 (bm2), in sorghum that is resistant to greenbug. They studied the differences in resistance of bm2 mutants to greenbug in five different backgrounds (Tx7078, P898012, P954035, BN109, PI257599) and found that sorghum had the best resistance to greenbug in the background of P898012, and moderate resistance performance in the genetic backgrounds of Tx7078 and P954035 (Harris-Shultz et al., 2019). In addition, hormones, such as SA, JA, and abscisic acid (ABA), also play a crucial role/have function in the response of sorghum to aphid stress (Zhu-Salzman et al., 2004). Zhu-Salzman et al. (2004) proved that treating at the sorghum seedlings stage (growing for 7 days) with methyl jasmonate (Me-JA) can effectively resist greenbug infestation, indicating that JA and its derivatives may play an important role in defending against greenbug (Zhu-Salzman et al., 2004). In addition to the above hormones, Park et al. (2006) found that IAA and gibberellic acid (GA) are also involved in sorghum’s resistance response to greenbug. After 72 hours of aphid feeding, the auxin-induced protein (AIP) began to be upregulated, and the gibberellin-induced protein (GIP) began to be induced (Park et al., 2006).
5 Aphids control
5.1 Chemical and biological control of sorghum aphid
Aphids, as one of the most serious threats to global sorghum production, pose a risk throughout the entire growth period of sorghum, from seedling to maturity (Xoconostle-Cázares et al., 2023). Chemical control involves the use of insecticides to reduce the populations of aphids affecting sorghum. Currently, commonly utilized insecticides include thiamethoxam, flupyradifurone, and sulfoxaflor (Barbosa et al., 2017; Colares et al., 2017). Research has demonstrated that these insecticides significantly improved efficacy and are approved for large-scale field application (Szczepaniec and Protection, A.J.C, 2018). However, it is essential to exercise caution when using broad-spectrum insecticides, particularly pyrethroids, carbamates, and organophosphates, as their use may unintentionally harm the natural enemies of sorghum aphids. This could potentially lead to outbreaks of secondary pest populations (Catchot, 2015).
In recent years, considering the impact on plants and the environment, researchers have been gradually developing new insecticides that are gentler on plants and more environmentally friendly. Sotelo-Leyva et al. (2023) discovered that extracts of Xanthotoxin from [Conyza canadensis (L.) Cronq] have a significant inhibitory effect on sorghum aphids, showing stronger aphid-killing activity at low concentrations (Sotelo-Leyva et al., 2023). Previous studies found that exogenous spraying of a 1‰ concentration of naringenin and rosmarinic acid can effectively enhance sorghum’s resistance to aphids (Zhao et al., 2024b). However, the above-mentioned methods still present risks of inducing plant mutations and environmental pollution, not aligning with current and future development directions.
As the long-term use of chemical pesticides has increasingly impacted humanity, biological control has gradually become a sought-after direction. One of the most common forms of biological control is the utilization of natural enemies of aphids, including parasitoids, parasitic wasps, pathogens, and predators (Hewlett et al., 2019). For instance, in the ecosystem of aphids, ladybugs, lacewings, and dragonfly larvae (Order Odonata: Family Aeshnidae) effectively reduce aphid populations through predation. Both Coccinella septempunctata L, (Coleoptera: Coccinellidae) and Harmonia axyridis Pallas, (Coleoptera: Coccinellidae) ladybug species significantly reduced aphid populations at low density levels (Hewlett et al., 2019). In addition to natural predation, methods for biologically controlling sorghum aphids also include the application of pathogens and parasitoids. For example, research by White (2001) demonstrated that the pathogen Verticillium lecanii and the parasitoid wasp Lysiphlebus testaceipes Cresson jointly mediate and control the sorghum aphid population in Florida (White, 2001). Although this research has only been conducted at the laboratory stage, it holds potential for large-scale field application in the future.
5.2 Chemical and biological control of greenbug
Greenbug use needle-like, piercing mouthparts to extract plant sap from the phloem of their host. While feeding, greenbug inject saliva into the plant to enhance nutrient absorption. The feeding of these greenbugs causes significant damage to wheat and sorghum. In the Great Plains region of the United States, the economic losses caused by greenbug feeding on cereal crops can exceed 100 million dollars annually (Webster, 2000; Giles et al., 2008).
Over the past 50 years, many insecticides have been used to combat greenbug in sorghum fields, mainly categorized into four groups: broad-spectrum carbamates, organophosphates, pyrethroids, and neonicotinoids (Bauernfeind, 1983; Royer et al., 2011). However, with the widespread use of insecticides, people have gradually found that aphids have developed resistance to insecticides. Shufran et al. (1997) found that specific biotypes of greenbug have developed cross-resistance to carbamates and organophosphates (Shufran et al., 1997). Furthermore, with the extensive use of organophosphates, plants have gradually developed necrotic lesions, known as sorghum organophosphate sensitivity (OPS) (Jing et al., 2021).
Here is a diverse range of natural enemies of greenbug, including ladybugs (Coleoptera: Coccinellidae), lacewings (Neuroptera: Chrysopidae), parasitic wasps (Hymenoptera: Aphidiidae, Braconidae), hoverflies (Diptera: Syrphidae), and spiders (Araneae), all of which can prey on aphids on sorghum in large numbers (Walker et al., 1973; Gilstrap et al., 1984). Previous studies showed that the release of L. testaceipes can served as an effective strategy to prevent greenbug outbreaks in sorghum (Jackson et al., 1970; Gilstrap et al., 1984). With further research and development of biological control methods, we can expect to achieve more sustainable and eco-friendly pest management strategies in agricultural production. This not only helps reduce reliance on chemical pesticides but also protects the health and balance of ecosystems.
6 Conclusion and perspectives
Aphids, as a significant threat to global crop production, have long been a subject of interest for scientists. Given the serious impact of aphids on sorghum production, breeding aphid-resistant varieties has become a primary goal for crop breeders, despite the challenges involved. Currently, researchers have identified aphid-resistant genes in sorghum within a 126KB region on chromosome 6, with the RMES1 gene’s specific location being crucial for understanding sorghum’s response to aphid feeding. However, compared with the previous research by Xie et al. (2019), which determined that the tannin 1 gene is the major locus controlling the feeding behavior of sorghum birds and revealed its bird–plant mechanism (Xie et al., 2019). More research is still needed on the aphid resistance of sorghum. Recent studies on aphid-resistant varieties have deepened efforts to mitigate the global agricultural impact of aphids. When introducing foreign varieties for aphid resistance breeding, caution is necessary to address potential risks associated with new hybrids. Notably, sorghum hormone levels change after aphid feeding, offering valuable insights into hormone roles in sorghum’s response to biotic stress. Additionally, research on physiological characteristics like stomatal density, trichome density, trichome length, chloroplast density, leaf thickness, and epidermal cell regularity provides new perspectives on sorghum’s aphid resistance mechanism. On the other hand, the extensive use of pesticides and insecticides can cause soil pollution and enhance the drug resistance of sorghum and aphids. Therefore, the dosage of drugs needs to be increased, creating a vicious cycle. This poses challenges for future research on aphid resistance genes and field management. Therefore, to solve this problem, it is necessary to explore green biological control methods, such as effectively using natural enemies to control aphids (Figure 2).
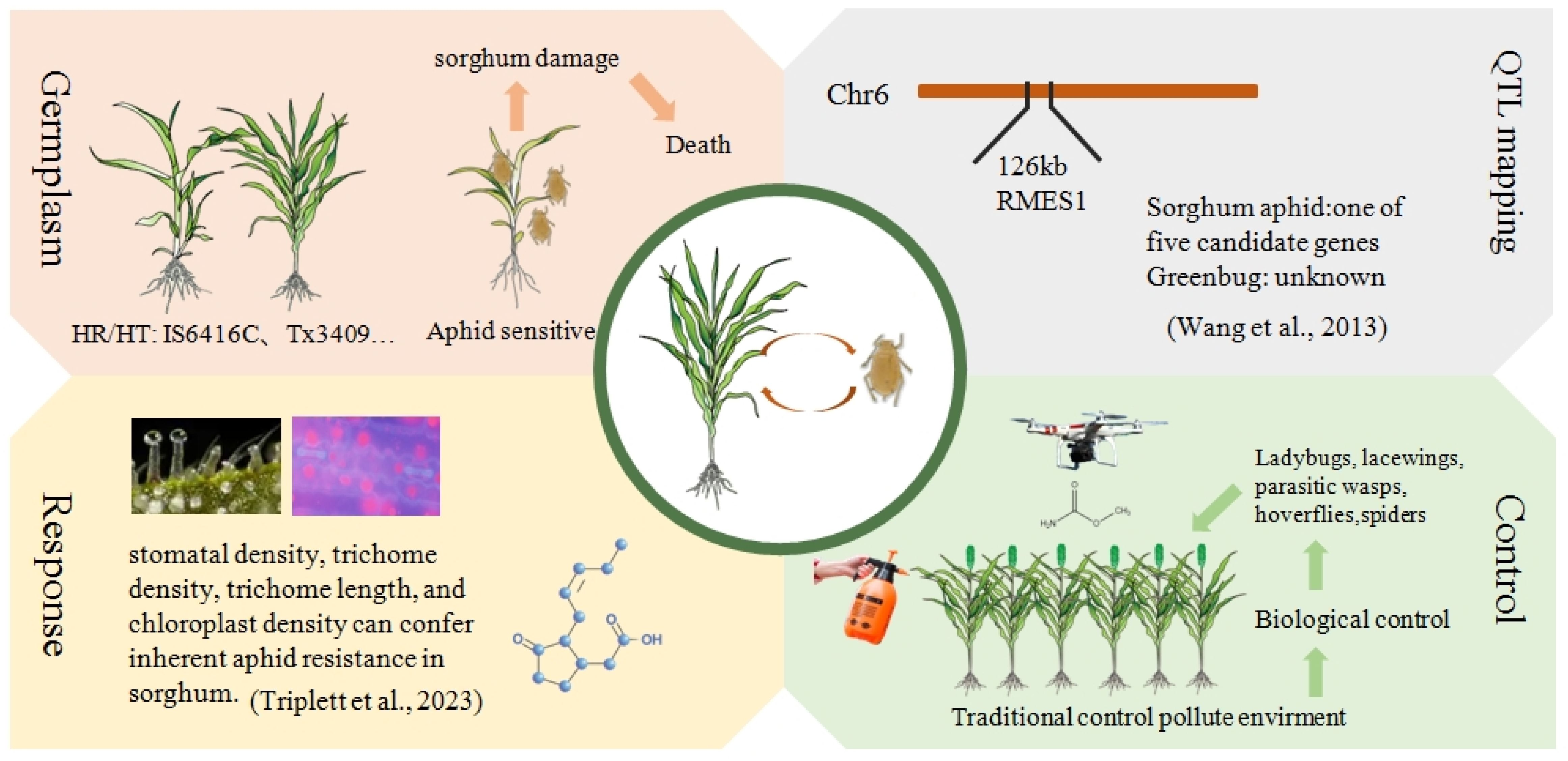
Figure 2. Strategies for enhancing aphid resistance in sorghum. Based on the selection of aphid-resistant materials, effective aphid-resistant lines can be bred. High-throughput sequencing technology can be employed to identify key genes associated with aphids resistance. Additionally, integrating chemical and biological control measures can help manage aphids populations. By utilizing these technologies and methods, the resistance and yield of sorghum can be enhanced.
In conclusion, sorghum, as one of the world’s top five crops, holds undeniable importance. However, so far, the aphid resistance genes of sorghum have not been located. Therefore, the aphid resistance genes cannot be applied to breeding research. This is also the direction that future research needs to strive for. Moving forward, greater emphasis should be placed on utilizing biological tools, conducting in-depth research on aphid-sorghum interaction mechanisms, developing more markers to facilitate breeding of aphid-resistant sorghum varieties, and striving to boost global sorghum production.
Author contributions
ZJ: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. JW: Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. XM: Formal analysis, Visualization, Writing – review & editing. YS: Data curation, Investigation, Writing – review & editing. ZW: Data curation, Validation, Writing – review & editing. YG: Formal analysis, Investigation, Writing – review & editing. PL: Funding acquisition, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The HAAFS Basic Science and Technology Contract Project (HBNKY-BGZ-02), Modern Agriculture Research System of Hebei Province (HBCT2024070201), the China Agriculture Research System (CARS-06-14.5-B5), Central Government Guides Local Funds for Science and Technology Development Project (246Z2909G).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwala, R. B. T., Beck, J., Benson, D. A., Bollin, C., Bolton, E., Bourexis, D., et al. (2018). Database resources of the national center for biotechnology information. Nucleic Acids Res. 46, D8–D13. doi: 10.1093/nar/28.1.10
Al-Mousawi, A. H., Richardson, P. E., and Burton, R. L. (1983). Ultrastructural studies of greenbug (Hemiptera: aphididae) feeding damage to susceptible and resistant wheat cultivars. Ann. Entomological Soc. America 76, 964–971. doi: 10.1093/aesa/76.6.964
Al-Salman, Y., Cano, F., Mace, E., Jordan, D., Groszmann, M., and Ghannoum, O. (2024). High water use efficiency due to maintenance of photosynthetic capacity in sorghum under water stress. J. Exp. Bot. 75, 6778–6795. doi: 10.1093/jxb/erae418
Armstrong, J., Mornhinweg, D., Payton, M., and Puterka, G. (2016). The Discovery of Resistant Sources of Spring Barley, Hordeum vulgare ssp. spontaneum, and Unique Greenbug Biotypes. J. Economic Entomology 109, 434–438. doi: 10.1093/jee/tov320
Baillo, E. H., Kimotho, R. N., Zhengbin, Z., and Ping, X. (2019). Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 10, 771. doi: 10.3390/genes10100771
Balamurugan, A., Mallikarjuna, M., Bansal, S., Nayaka, S., Rajashekara, H., Chellapilla, T., et al. (2024). Genome-wide identification and characterization of NBLRR genes in finger millet (Eleusine coracana L.) and their expression in response to Magnaporthe grisea infection. BMC Plant Biol. 24, 75. doi: 10.1186/s12870-024-04743-z
Barbosa, P., Michaud, J., Bain, C., and Torres, J. (2017). Toxicity of three aphicides to the generalist predators Chrysoperla carnea (Neuroptera: Chrysopidae) and Orius insidiosus (Hemiptera: Anthocoridae). Ecotoxicology 26, 589–599. doi: 10.1007/s10646-017-1792-5
Bauernfeind, R. ,. J. (1983). Biotype E greenbug (Homoptera: aphididae) control on wheat with insecticide sprays at low temperatures. J. Economic Entomology 76, 570–572. doi: 10.1093/jee/76.3.570
Beregovoy, V. H. and Peters, D. C. (1994). Biotype J, a unique greenbug (Homoptera: Aphididae) distinguished by plant damage characteristics. J. Kansas Entomological Soc. 67, 248–252. https://www.jstor.org/stable/25085521.
Beregovoy, V. H., Starks, K. J., and Janardan, K. G. (1988). Fecundity characteristics of the greenbug biotypes C and E cultured on different host plants. Environ. Entomology 59-62, 59–62. doi: 10.1093/ee/17.1.59
Blackman, R. L. and Eastop, V. F. (2006). Aphids on the world’s herbaceous plants and shrubs. Crop Prot. 15, 400–400.
Bonnie, B., Pendleton, Anastasia, L., Palousek, C., and G J, M., Jr (2009). Effect of biotype and temperature on fitness of greenbug (Hemiptera: Aphididae) on sorghum. J. Economic Entomology 102, 1624–1627. doi: 10.1603/029.102.0429
Bowling, R. D., B, M. J., K, D. L., G, J., S, N., E, N. E., et al. (2017). Sugarcane aphid (Hemiptera: aphididae): A new pest on sorghum in North America. J. Integr. Pest Manag. 7, 12. doi: 10.1093/jipm/pmw011
Brown, P., Klein, P., Bortiri, E., Acharya, C., Rooney, W., and Kresovich, S. (2006). Inheritance of inflorescence architecture in sorghum. Theor. Appl. Genet. 113, 931–942. doi: 10.1007/s00122-006-0352-9
Burton, R. L. and Burd, J. D. (1993). Relationship between number of greenbugs and damage to wheat seedlings. Southwestern entomologist 90588164.
Cardona, J., Grover, S., Bowman, M., Busta, L., Kundu, P., Koch, K., et al. (2023a). Sugars and cuticular waxes impact sugarcane aphid (Melanaphis sacchari) colonization on different developmental stages of sorghum. Plant Sci. 330, 111646. doi: 10.1016/j.plantsci.2023.111646
Cardona, J. B., Grover, S., Busta, L., Sattler, S. E. E., and Louis, J. (2023b). Sorghum cuticular waxes influence host plant selection by aphids. Planta 257, 22. doi: 10.1007/s00425-022-04046-3
Carl, V., Brian, R., Terry J, F., Jean Rigaud, C., Gael, P., Vamsi, N., et al. (2024). Globally deployed sorghum aphid resistance gene RMES1 is vulnerable to biotype shifts but is bolstered by RMES2. Plant Genome 17, e20452. doi: 10.1002/tpg2.20452
Catchot, A. G. (2015). Management Guidelines for SCAs in MS Grain Sorghum Vol. 4 (Mississippi: Mississippi State University Extension Service).
Chang, J. H., Xia, X. Y., Zhang, L., Li, R. G., and Luo, Y. W. (2006). Analysis of the resistance gene to the sorghum aphid, Melanaphis sacchari, with SSR marker in Sorghum bicolor. Acta Prataculturae Sinica 02. doi: 10.1360/aps040178
Chou, J. and Huang, Y. (2010). Differential expression of thaumatin-like proteins in sorghum infested with greenbugs. J. Biosci. 65, 271–276. doi: 10.1515/znc-2010-3-416
Colares, F., Michaud, J., Bain, C., and Torres, J. (2017). Relative Toxicity of Two Aphicides to Hippodamia convergens (Coleoptera: Coccinellidae): Implications for Integrated Management of Sugarcane Aphid, Melanaphis sacchari (Hemiptera: Aphididae). J. Economic Entomology 110, 52–58. doi: 10.1093/jee/tow265
Craigie, G., Hefley, T., Grijalva, I., Ciampitti, I. A., Goodin, D. G., and Mccornack, B. (2024). Detecting sorghum aphid infestation in grain sorghum using leaf spectral response. Sci. Rep. 14, 14053. doi: 10.1038/s41598-024-64841-8
Cress, D. C. and Chada, H. L. (1971). Development of a synthetic diet for the greenbug, schizaphis graminum. 3. Response of greenbug biotypes A and B to the same diet medium. Ann. Entomological Soc. America 64, 1245–1247. doi: 10.1093/aesa/64.6.1245
Ernst, R., Bernhard, S., and Wolfgang, B. (2006). cDNA sequences, MALDI-TOF analyses, and molecular modelling of barley PR-5 proteins. Phytochemistry 67, 1856–1864. doi: 10.1016/j.phytochem.2006.06.014
Erpen, D., Grosser, Hs, and Dutt, Jw (2018). Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tiss Org 132, 1–25. doi: 10.1007/s11240-017-1320-6
Giles, K., Hein, G. L., and Peairs, F. (2008). Areawide Pest Management of Cereal Aphids in Dryland Wheat Systems of the Great Plains. (USA: CABI), 441–466. doi: 10.1079/9781845933722.044
Gilstrap, F. E., Kring, T. J., and Brooks, G. W. (1984). Parasitism of aphids (Homoptera: aphididae) associated with texas sorghum. Environ. Entomology 13, 1613–1617. doi: 10.1093/ee/13.6.1613
Goodstein, D., Shu, S., Howson, R., Neupane, R., Hayes, R., Fazo, J., et al. (2012). Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186. doi: 10.1093/nar/gkr944
Grover, S., Agpawa, E., Sarath, G., Sattler, S. E., and Louis, J. (2022a). Interplay of phytohormones facilitate sorghum tolerance to aphids. Plant Mol. Biol. 109, 639–650. doi: 10.1007/s11103-020-01083-y
Grover, S., Bradenvarsani, Sureshsattler, Scott, E., and Louis, J. (2019). Resistance to greenbugs in the sorghum nested association mapping population. Anthropod-plant Interact. 13, 261–269. doi: 10.1007/s11829-019-09679-y
Grover, S., Cardona, J., Zogli, P., Alvarez, S., Naldrett, M., Sattler, S., et al. (2022b). Reprogramming of sorghum proteome in response to sugarcane aphid infestation. Plant Sci. 320, 111289. doi: 10.1016/j.plantsci.2022.111289
Grover, S., Mou, D., Shrestha, K., Puri, H., Pingault, L., Sattler, S., et al. (2024). Impaired Brown midrib12 function orchestrates sorghum resistance to aphids via an auxin conjugate indole-3-acetic acid-aspartic acid. New Phytol. 244, 1597–1615. doi: 10.1111/nph.v244.4
Guden, B., Yol, E., Ikten, C., Erdurmus, C., and Uzun, B. (2019). Molecular and morphological evidence for resistance to sugarcane aphid (Melanaphis sacchari) in sweet sorghum [Sorghum bicolor (L.) Moench. Biotech 9, 245. doi: 10.1007/s13205-019-1783-8
Guo, C., Cui, W., Feng, X., Zhao, J., and Lu, G. (2011). Sorghum insect problems and management. J. Integr. Plant Biol. 53, 178–192. doi: 10.1111/j.1744-7909.2010.01019.x
Gupta and Virendra (1994). Aphids on the world’s crops. An identification and information guide. Oriental Insects 35, 104–104. doi: 10.1080/00305316.2001.10417292
Harris-Shultz, K., Armstrong, J., Caballero, M., Hoback, W., and Knoll, J. (2022a). Sorghum bicolorInsect feeding on pollen and hymenoptera attraction to aphid-produced honeydew. Insects 13, 1152. doi: 10.3390/insects13121152
Harris-Shultz, K., Armstrong, J., Carvalho, G., Segundo, J., and Ni, X. (2022b). Melanaphis sorghi (Hemiptera: aphididae) clonal diversity in the United States and Brazil. Insects 13, 416. doi: 10.3390/insects13050416
Harris-Shultz, K., Punnuri, S., Knoll, J. E., Ni, X., and Wang, H. (2019). The sorghum epicuticular wax locus Bloomless2 reduces plant damage in P898012 caused by the sugarcane aphid. Agrosystems Geosciences Environ. 3, e20008. doi: 10.1002/agg2.20008
Harvey, T. L. and Hackerott, H. L. (1969). Recognition of a greenbug biotype injurious to Sorghum1[J]. J. Economic Entomology 1969 (4), 776–779. doi: 10.1093/jee/62.4.776
Harvey, T. L., Kofoid, K. D., Martin, T. J., and Sloderbeck, P. E. (1991). A new greenbug virulent to E-biotype resistant sorghum. Crop Sci. 31, 461–465. doi: 10.2135/cropsci1991.0011183X003100060062x
Harvey, T. L., Wilde, G. E., and Kofoid, K. D. (1997). Designation of a new greenbug, biotype K, injurious to resistant sorghum. Cropence 37, 989–991. doi: 10.2135/cropsci1997.0011183X003700030047x
Hewlett, J. A., Szczepaniec, A., and Eubanks, M. D. (2019). The effects of sugarcane aphid density in sorghum on predation by lady beetles and lacewings. Biol. Control 129, 171–177. doi: 10.1016/j.biocontrol.2018.10.015
Huang, Y. and Huang, J. (2023). Analysis of plant expression profiles revealed that aphid attack triggered dynamic defense responses in sorghum plant. Front. Genet. 14. doi: 10.3389/fgene.2023.1194273
Irfan, I., Rajiv Kumar, T., Olivia, W., and Jaswinder, S. (2020). Thaumatin-like protein (TLP) gene family in barley: genome-wide exploration and expression analysis during germination. Genes 11, 1080. doi: 10.3390/genes11091080
Jackson, H. B., Colfs, L. W., Wood, E. A., and Eikenbary, R. D. (1970). Parasites reared from the greenbug and corn leaf aphid in oklahoma in 1968 and 1969. J. Economic Entomology volume 63, 733–736(734). doi: 10.1093/jee/63.3.733
Jeff and Dahlberg (2019). The role of sorghum in renewables and biofuels. Methods Mol. Biol. 1931, 269–277. doi: 10.1007/978-1-4939-9039-9_19
Jing, Z., Wacera W, F., Takami, T., Takanashi, H., Fukada, F., Kawano, Y., et al. (2021). NB-LRR-encoding genes conferring susceptibility to organophosphate pesticides in sorghum. Sci. Rep. 11, 19828. doi: 10.1038/s41598-021-98908-7
Kariyat, R., Gaffoor, I., Sattar, S., Dixon, C., Frock, N., Moen, J., et al. (2019). Sorghum 3-deoxyanthocyanidin flavonoids confer resistance against corn leaf aphid. J. Chem. Ecol. 45, 502–514. doi: 10.1007/s10886-019-01062-8
Katsar, C. S., Paterson, R. H., Teetes, G. L., and Peterson, G. C. (2002). Molecular analysis of sorghum resistance to the greenbug (Homoptera: Aphididae). J. Economic Entomology 95, 448–457. doi: 10.1603/0022-0493-95.2.448
Khoddami, A., Messina, V., Vadabalija Venkata, K., Farahnaky, A., Blanchard, C., and Roberts, T. (2023). Sorghum in foods: Functionality and potential in innovative products. Crit. Rev. Food Sci. Nutr. 63, 1170–1186. doi: 10.1080/10408398.2021.1960793
Kindler, S. D. and Spomer, S. M. (1986). Biotypic status of six greenbug (Homoptera: aphididae) isolates. Environ. Entomology 15, 567–572. doi: 10.1093/ee/15.3.567
Knoll, J. E., Uchimiya, M., and Smith, C.M.H.M.P.R.H.S.S. (2023). Registration of three sweet sorghum lines with high tolerance to sorghum aphid (Melanaphis sorghi). J. Plant Registrations 17, 551–560. doi: 10.1002/plr2.20310
Kondaiah, E. and Nayudu, M. V. (1984). Sugarcane mosaic virus strain H - a new record from India. Short communication. Curr. Sci. 53, 273–275. doi: 10.2307/3967665
Kruger, M., Berg, J. V. D., and Plessis, H. D. (2008). Diversity and seasonal abundance of sorghum panicle-feeding Hemiptera in South Africa. Crop Prot. 27, 444–451. doi: 10.1016/j.cropro.2007.07.014
Liu, J., Feng, S., Liu, T., Mao, Y., Shen, S., Liu, Y., et al. (2024). Molecular characterization revealed the role of thaumatin-like proteins of Rhizoctonia solani AG4-JY in inducing maize disease resistance. Front. Microbiol 15. doi: 10.3389/fmicb.2024.1377726
Liu, X. and Dasheng, J. (1998). Please cite this article “A new biotype (Chn-1) of greenbug, schizaphis graminum found in Beijing[J]. Acta Entomologica Sinica. Acta Entomologica Sin. 41 (2), 141–144.
Liu, X. and Jin, D. (1998). A new biotype (Chn-1) of greenbug, schizaphis graminum found in Beijing. Acta Entomologica Sin. 41, 141–144.
Lopes, P., Souza, P., Santos, J., Borges, C., Araújo, F., Martins, J., et al. (2022). Spatiotemporal distribution of Schizaphis graminum (Rondani) and its natural enemy Coccinella septempunctata (Linnaeus) in graniferous sorghum crops. Braz. J. Biol. 84, e261972. doi: 10.1590/1519-6984.261972
Lu, Q. and Dahlberg, J. A. (2001). Chinese sorghum genetic resources. Economic Bot. 55, 401–425. doi: 10.1007/BF02866563
Mbulwe, L., Peterson, G. C., Scott-Armstrong, J., and Rooney, W. L. (2016). Registration of sorghum germplasm tx3408 and tx3409 with tolerance to sugarcane aphid [(Zehntner). J. Plant Registrations 10, 51. doi: 10.3198/jpr2015.04.0025crg
Min, T., Canghao, D., Boju, Y., Guoli, W., Yanbin, D., Yuesheng, W., et al. (2023). Current advances in the molecular regulation of abiotic stress tolerance in sorghum via transcriptomic, proteomic, and metabolomic approaches. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1147328
Mizuno, C., Rimando, A., and Duke, S. (2010). Phytotoxic activity of quinones and resorcinolic lipid derivatives. J. Agric. Food Chem. 58, 4353–4355. doi: 10.1021/jf100108c
Muleta, K., Felderhoff, T., Winans, N., Walstead, R., Charles, J., Armstrong, J., et al. (2022). The recent evolutionary rescue of a staple crop depended on over half a century of global germplasm exchange. Sci. Adv. 8, eabj4633. doi: 10.1126/sciadv.abj4633
Nagaraj, N., Reese, J., Tuinstra, M., Smith, C., Amand, P., Kirkham, M., et al. (2005). Molecular mapping of sorghum genes expressing tolerance to damage by greenbug (Homoptera: Aphididae). J. Economic Entomology 98, 595–602. doi: 10.1093/jee/98.2.595
Nibouche, S., Costet, L., Holt, J., Jacobson, A., Pekarcik, A., Sadeyen, J., et al. (2018). Invasion of sorghum in the Americas by a new sugarcane aphid (Melanaphis sacchari) superclone. PloS One 13, e0196124. doi: 10.1371/journal.pone.0196124
Nibouche, S., Costet, L., Medina, R., Holt, J., Sadeyen, J., Zoogones, A., et al. (2021). Morphometric and molecular discrimination of the sugarcane aphid, Melanaphis sacchari, (Zehntner 1897) and the sorghum aphid Melanaphis sorghi (Theobald 1904). PloS One 16, e0241881. doi: 10.1371/journal.pone.0241881
Nibouche, S., Fartek, B., Mississipi, S., Delatte, H., Reynaud, B., and Costet, L. (2014). Low genetic diversity in Melanaphis sacchari aphid populations at the worldwide scale. PloS One 9, e106067. doi: 10.1371/journal.pone.0106067
Park, S., Huang, Y., and Ayoubi, P. (2006). Identification of expression profiles of sorghum genes in response to greenbug phloem-feeding using cDNA subtraction and microarray analysis. Planta 223, 932–947. doi: 10.1007/s00425-005-0148-1
Paudyal, S., Armstrong, J. S., Giles, K., and Payton, M. E. (2019). Categories of resistance to sugarcane aphid (Hemiptera: aphididae) among sorghum genotypes. J. Economic Entomology 112, 1932–1940. doi: 10.1093/jee/toz077
Pekarcik, A. and Jacobson, A. (2021). Evaluating sugarcane aphid, melanaphis sacchari (Hemiptera: aphididae), population dynamics, feeding injury, and grain yield among commercial sorghum varieties in alabama. J. Economic Entomology 114, 757–768. doi: 10.1093/jee/toab013
Poosapati, S., Poretsky, E., Dressano, K., Ruiz, M., Vazquez, A., Sandoval, E., et al. (2022). A sorghum genome-wide association study (GWAS) identifies a WRKY transcription factor as a candidate gene underlying sugarcane aphid (Melanaphis sacchari) resistance. Planta 255, 37. doi: 10.1007/s00425-021-03814-x
Porter, K. B., Peterson, G. L., and Vise, O. (1982). A new greenbug biotype1. Crop Sci. 22, 847–850. doi: 10.2135/cropsci1982.0011183X002200040035x
Powell, J. A. (1992). Keys to the Insects of the European Part of the Ussr. Volume IV, Lepidoptera, Part 2. Ann Entomol Soc Am. 85 (3), 356–358. doi: 10.1093/aesa/85.3.356
Punnuri, S., Ayele, A., Harris-Shultz, K., Knoll, J., Coffin, A., Tadesse, H., et al. (2022). Genome-wide association mapping of resistance to the sorghum aphid in Sorghum bicolor. Genomics 114, 110408. doi: 10.1016/j.ygeno.2022.110408
Punnuri, S. and Huang, Y. (2017). Identification and confirmation of greenbug resistance loci in an advanced mapping population of sorghum. J. Agric. Sci. 155, 1610–1622. doi: 10.1017/S0021859617000685
Punnuri, S., Huang, Y., Steets, J., and Wu, Y. (2013). Developing new markers and QTL mapping for greenbug resistance in sorghum [Sorghum bicolor (L.) Moench. Euphytica 191, 191–203. doi: 10.1007/s10681-012-0755-4
Puterka, G. J., Peters, D. C., Kerns, D. L., Slosser, J. E., Bush, L., Worrall, D. W., et al. (1988). Designation of two new greenbug (Homoptera: aphididae) biotypes G and H. J. Economic Entomology 81, 1754–1759. doi: 10.1093/jee/81.6.1754
Royer, T. A., Elliott, N. C., Giles, K. L., and Kindler, S. D. (2011). Field efficacy of wintertime insecticide applications against greenbugs, Schizaphis graminum (Rondani) (Hemiptera: Aphididae) on winter wheat (Triticumaestivum L.). Crop Prot. 30, 826–832. doi: 10.1016/j.cropro.2011.03.002
Royer, T., Pendleton, B., Elliott, N. C., and Giles, K. (2015). Greenbug (Hemiptera: aphididae) biology, ecology, and management in wheat and sorghum. J. Integrated Pest Manage. 6, 19–19. doi: 10.1093/jipm/pmv018
Rudo, N., Tatenda, G., Dirk, Z. H. ,. S., and Stephen, C. (2021). Sorghum’s whole-plant transcriptome and proteome responses to drought stress: A review. Life 11, 704. doi: 10.3390/LIFE11070704
Schenck, S. and Lehrer, A. (2000). Factors Affecting the Transmission and Spread of Sugarcane yellow leaf virus. Plant Dis. 84, 1085–1088. doi: 10.1094/PDIS.2000.84.10.1085
Serba, D., Meng, X., Schnable, J., Bashir, E., Michaud, J., Vara Prasad, P., et al. (2021). Comparative transcriptome analysis reveals genetic mechanisms of sugarcane aphid resistance in grain sorghum. Int. J. Mol. Sci. 22, 7129. doi: 10.3390/ijms22137129
Shabbir, R., Zhaoli, L., Yueyu, X., Zihao, S., and Pinghua, C. (2022). Transcriptome analysis of sugarcane response to sugarcane yellow leaf virus infection transmitted by the vector. Front. Plant Sci. 13, 921674. doi: 10.3389/fpls.2022.921674
Sharma, H. C., Bhagwat, V. R., Daware, D. G., Pawar, D. B., and Gadakh, S. R. (2014). Identification of sorghum genotypes with resistance to the sugarcane aphid Melanaphis sacchari under natural and artificial infestation. Plant Breed. 133, 36–44. doi: 10.1111/pbr.2014.133.issue-1
Shufran, K. A., Burd, J. D., Anstead, J. A., and Lushai, G. (2010). Mitochondrial DNA sequence divergence among greenbug (Homoptera: aphididae) biotypes: evidence for host-adapted races. Insect Mol. Biol. 9, 179–184. doi: 10.1046/j.1365-2583.2000.00177.x
Shufran, R. A., Wilde, G. E., and Sloderbeck, P. E. (1997). Response of three greenbug (Homoptera: aphididae) strains to five organophosphorous and two carbamate insecticides. J. Economic Entomology 90, 283–286. doi: 10.1093/jee/90.2.283
Singh, B. U., Padmaja, P. G., and Seetharama, N. (2004). Biology and management of the sugarcane aphid, Melanaphis sacchari (Zehntner) (Homoptera: Aphididae), in sorghum: a review - ScienceDirect. Crop Prot. 23, 739–755. doi: 10.1016/j.cropro.2004.01.004
Smith, C. M. and Boyko, E. V. (2010). The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomologia Experimentalis Et Applicata 122, 1–16. doi: 10.1111/j.1570-7458.2006.00503.x
Sotelo-Leyva, C., Avilés-Montes, D., Manuel Rivas-González, J., Figueroa-Brito, R., Abarca-Vargas, R., Toledo-Hernández, E., et al. (2023). Xanthotoxin: An Aphicidal Coumarin From Ficus petiolaris against Melanaphis sacchari Zehntner (Hemiptera: Aphididae). J. Food Prot. 86, 100084. doi: 10.1016/j.jfp.2023.100084
Szczepaniec, and Protection, A.J.C (2018). Interactive effects of crop variety, insecticide seed treatment, and planting date on population dynamics of sugarcane aphid (Melanaphis sacchari) and their predators in late-colonized sorghum. Crop Protection 109, 72–79. doi: 10.1016/j.cropro.2018.03.002
Takanashi, H. (2023). Genetic control of morphological traits useful for improving sorghum. Breed. Sci. 73, 57–69. doi: 10.1270/jsbbs.22069
Teetes, G. L. and Johnson, J. W. (1974). Assessment of damage by the greenbug in grain sorghum hybrids of different maturities. J. Economic Entomology 67, 514–516. doi: 10.1093/jee/67.4.514
Teetes, G. L., Manthe, C. S., Peterson, G. C., Leuschner, K., and Pendleton, B. B. (1995). Sorghum resistant to the sugarcane aphid, Melanaphis sacchari (Homoptera: Aphididae), in Botswana and Zimbabwe. Insect Sci. Appl. 16, 63–71.
Tetreault, H., Grover, S., Scully, E., Gries, T., Palmer, N., Sarath, G., et al. (2019). Sorghum bicolor Global Responses of Resistant and Susceptible Sorghum (Sorghum bicolor) to Sugarcane Aphid (Melanaphis sacchari). Front. Plant Sci. 10, 145. doi: 10.3389/fpls.2019.00145
Thudi, M., Reddy, M. S. S., Naik, Y. D., Cheruku, V. K. R., Sangireddy, M. K. R., Cuevas, H. E., et al. (2024). Invasive sorghum aphid: A decade of research on deciphering plant resistance mechanisms and novel approaches in breeding for sorghum resistance to aphids. Crop Sci. 64, 2436–2458. doi: 10.1002/csc2.21301
Triplett, E., Hayes, C., Emendack, Y., Longing, S., Monclova, C., Simpson, C., et al. (2023). Leaf structural traits mediating pre-existing physical innate resistance to sorghum aphid in sorghum under uninfested conditions. Planta 258, 46. doi: 10.1007/s00425-023-04194-0
Vasquez, A., Belsky, J., Khanal, N., Puri, H., Balakrishnan, D., Joshi, N., et al. (2024). Melanaphis sacchari/sorghi complex: current status, challenges and integrated strategies for managing the invasive sap-feeding insect pest of sorghum. Pest Manage. ScienceEarly View 81, 2427–244. doi: 10.1002/ps.8291
Walker, A. L., Bottrell, D. G., and Cate, J. R. (1973). Hymenopterous parasites of biotype C greenbug in the high plains of texas. Ann. Entomological Soc. America 66, 173–176. doi: 10.1093/aesa/66.1.173
Wang, B., Jiao, Y., Chougule, K., Olson, A., and Ware, D. (2021). Pan-genome analysis in sorghum highlights the extent of genomic variation and sugarcane aphid resistance genes. Cold Spring Harbor Lab. 50, 278–284. doi: 10.1101/2021.01.03.424980
Wang, F., Zhao, S., Han, Y., Shao, Y., and Dong, Z. (2013). Efficient and fine mapping of RMES1 conferring resistance to sorghum aphid Melanaphis sacchari. Mol. Breed. 31, 777–784. doi: 10.1007/s11032-012-9832-6
Webster, J. (2000). Economic impact of the Russian wheat aphid and greenbug in the western United States 1993-1994, 1994-95, and 1997-98 (U.S. Department of Agriculture, ARS Service report PSWCRL Rep).
White, W. (2001). Melanaphis sacchari (Homoptera: aphididae), a sugarcane pest new to louisiana. Florida Entomologist 84, 435–436. doi: 10.2307/3496505
Wood, E. A. (1961). Biological studies of a new greenbug biotype1. J. Economic Entomology 54, 1171–1173. doi: 10.1093/jee/54.6.1171
Wu, Y. and Huang, Y. (2008). Molecular mapping of QTLs for resistance to the greenbug Schizaphis graminum (Rondani) in Sorghum bicolor (Moench). Theor. Appl. Genet. 117, 117–124. doi: 10.1007/s00122-008-0757-8
Xie, P., Shi, J., Tang, S., Chen, C., Khan, A., Zhang, F., et al. (2019). Control of bird feeding behavior by tannin1 through modulating the biosynthesis of polyphenols and fatty acid-derived volatiles in sorghum. Molecular Plant 12, 10. doi: 10.1016/j.molp.2019.08.004
Xoconostle-Cázares, B., Ramírez-Pool, J., Núñez-Muñoz, L., Calderón-Pérez, B., Vargas-Hernández, B., Bujanos-Muñiz, R., et al. (2023). The characterization of microbiota and antibiotic treatment effect on insects. Insects 14, 807. doi: 10.3390/insects14100807
Xu, X., Li, G., Bai, G., Bian, R., Bernardo, A., Wolabu, T. W., et al. (2024). Characterization of a new greenbug resistance gene Gb9 in a synthetic hexaploid wheat. Theor. Appl. Genet. 140. doi: 10.1007/s00122-024-04650-9
Ze, Y., Yong, Z., Xuefeng, W., Shiliang, G., Jianmin, Y., Guohua, L., et al. (2008). Genomewide comparative phylogenetic and molecular evolutionary analysis of tubby-like protein family in Arabidopsis, rice, and poplar. Genomics 92, 246–253. doi: 10.1016/j.ygeno.2008.06.001
Zhang, H. and Huang, Y. (2024). Genome-wide identification and characterization of greenbug-inducible NAC transcription factors in sorghum. Mol. Biol. Rep. 51, 207. doi: 10.1007/s11033-023-09158-x
Zhang, J., Li, W., Lv, P., Yang, X., Xu, W., Ni, X., et al. (2021). Whole-genome resequencing and transcriptome analysis provide insights on aphid-resistant quantitative trait loci/genes in Sorghum bicolor. Plant Breed. 140, 618–629. doi: 10.1111/pbr.v140.4
Zhang, H., Yu, F., Xie, P., Sun, S., Qiao, X., Tang, S., et al. (2023). A Gγ protein regulates alkaline sensitivity in crops. Science 379 (6638), eade8416. doi: 10.1126/science.ade8416
Zhao, J., Xie, L., Zhao, X., Li, L., Cui, J., and Chen, J. (2024a). Genome sequence of the sugarcane aphid, Melanaphis sacchari (Hemiptera: Aphididae). G3 14, jkae223. doi: 10.1093/g3journal/jkae223
Zhao, X., Zhao, D., Zhang, L., Chang, J., and Cui, J. (2024b). Combining transcriptome and metabolome analysis to understand the response of sorghum to Melanaphis sacchari. BMC Plant Biol. 24, 529. doi: 10.1186/s12870-024-05229-8
Keywords: sorghum, sorghum aphid, greenbug, aphid resistance mechanisms, QTL mapping, molecular breeding, chemical and biological control, food security
Citation: Jiao Z, Wang J, Ma X, Shi Y, Wang Z, Guo Y and Lv P (2025) Sorghum aphid/greenbug: current research and control strategies to accelerate the breeding of aphid-resistant sorghum. Front. Plant Sci. 16:1588702. doi: 10.3389/fpls.2025.1588702
Received: 06 March 2025; Accepted: 07 May 2025;
Published: 03 June 2025.
Edited by:
Yuxiang Zeng, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Pritha Kundu, University of Nebraska-Lincoln, United StatesPeng Xie, Sun Yat-sen University, China
Copyright © 2025 Jiao, Wang, Ma, Shi, Wang, Guo and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Lv, UEVOR0wwMDFAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Zhiyin Jiao
Zhiyin Jiao Jinping Wang†
Jinping Wang† Yannan Shi
Yannan Shi Peng Lv
Peng Lv