- Resilient Agriculture, AgResearch Ltd., Palmerston North, New Zealand
The global challenges of climate change and rising energy demands necessitate innovative agricultural solutions. One promising strategy is the transformation of photosynthetic tissues into lipid-rich organs, providing energy-dense biomass for biofuel production while enhancing carbon sequestration. However, these metabolic shifts require substantial NADPH and ATP, reshaping cellular processes such as the Calvin-Benson cycle, glycolysis, and oxidative pentose phosphate pathways. This review explores the intricate metabolic and regulatory networks underpinning lipid accumulation, with a focus on carbon/nitrogen partitioning, redox regulation, and their implications for plant stress tolerance and productivity. Furthermore, we highlight recent progress in field applications, multi-omics integration, and emerging strategies to optimize lipid accumulation in crops while mitigating trade-offs in biomass yield and agronomic performance. Understanding these complex interactions will be essential for developing sustainable, high-lipid crops that support bioenergy production and climate-resilient agriculture.
Highlights
● The review explores the intricate metabolic and regulatory networks underlying lipid accumulation in non-seed tissues, emphasizing carbon/nitrogen partitioning, redox regulate on, and their impact on plant stress tolerance and productivity.
● Key future research directions are outlined, including integrative multi-omics approaches, environmental stress adaptation, carbon-nitrogen interactions, and extensive field trials.
● The potential of alternative crop species and systems is discussed, highlighting opportunities to broaden the applications of these technologies for sustainable agriculture and bioenergy production.
1 Introduction
The growing global demand for food, forage, and biofuels calls for innovative strategies to enhance crop productivity. One promising approach is increasing lipid biosynthesis in non-seed tissues, offering a dual benefit: boosting the energy density of plant biomass and reducing dependency on carbohydrate-dominated metabolism (Heaton et al., 2008; Vanhercke et al., 2019). Triacylglycerol (TAG) is an energy-dense carbon (C) sink, storing over twice as much energy per gram as carbohydrates, making it ideal for biofuel and feedstock production (Durrett et al., 2008; Xu and Shanklin, 2016).
Bioenergy feedstocks offer a solution to climate adaptation and sustainable energy challenges. Oils, being hydrophobic, help maintain cellular integrity under drought or heat stress, thereby enhancing the plant’s overall resilience (Lu et al., 2020; Perlikowski et al., 2022). Additionally, increasing vegetative oil storage can boost the plant’s C sink capacity, contributing to reduced net greenhouse gas emissions when these crops are cultivated at scale (Paul and Eastmond, 2020; Beechey-Gradwell et al., 2020). Moreover, leaf oil-rich biomass can serve as a dual-purpose feedstock, supporting biofuel production while simultaneously providing valuable co-products, such as high-energy livestock feed (Winichayakul et al., 2020; Beechey-Gradwell et al., 2022).
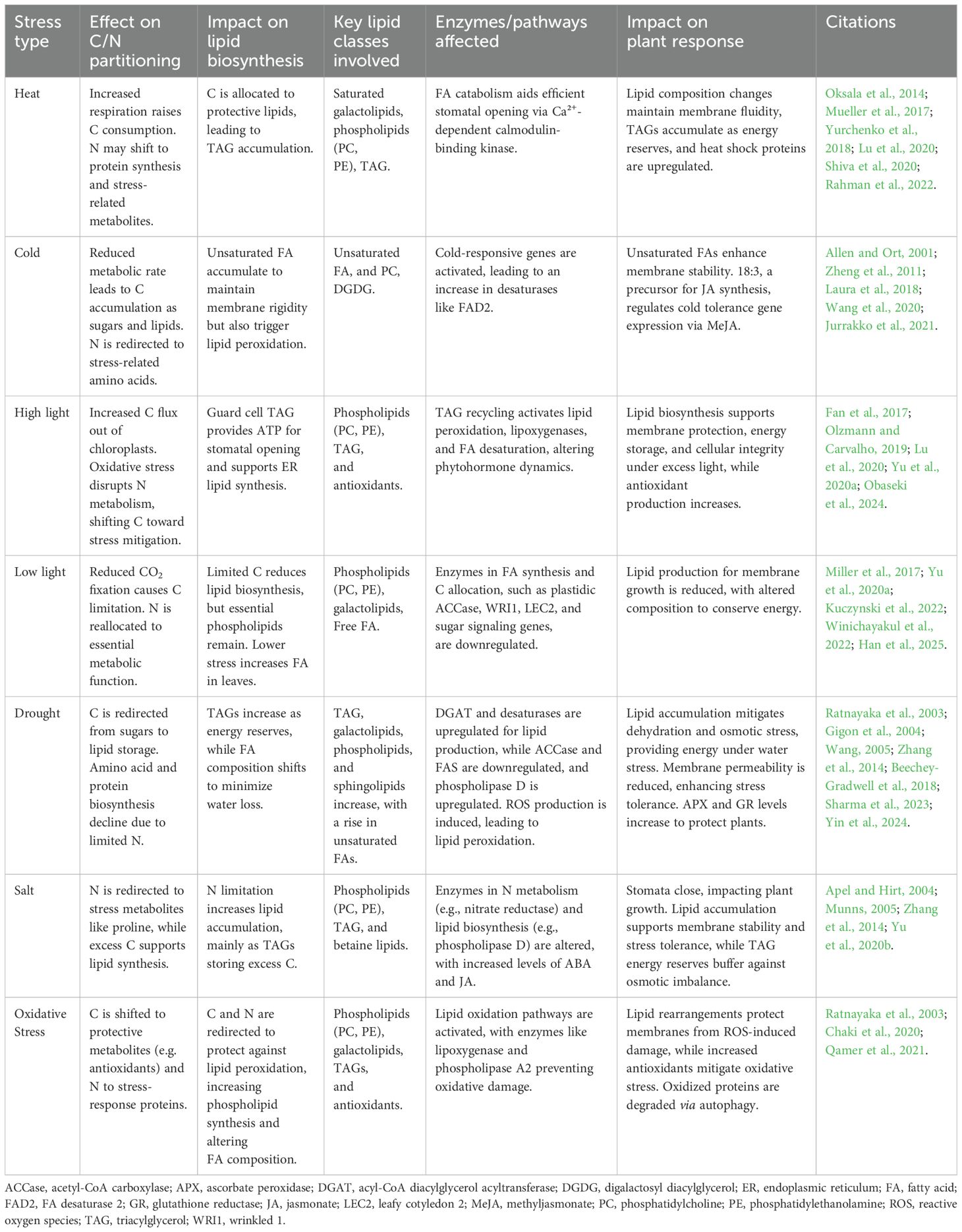
In this review, when referring to “high-lipid plants”, we specifically mean vegetative oil storage plants—those that accumulate significant amounts of lipids in their non-seed tissues, such as leaves, stems, or roots. Unlike traditional oilseed crops, which store lipids primarily in seeds, these high-lipid plants are engineered to store oils in their vegetative tissues, making them a novel and promising resource for various applications in bioenergy, biofuels, and livestock feed. For examples, high-lipid perennial ryegrass (Lolium perenne) has been engineered to enhance forage energy efficiency, reducing reliance on expensive lipid supplements (e.g., oilseeds, vegetable oils) while improving the nutritional quality of animal-derived products (Bayat et al., 2018; Beechey-Gradwell et al., 2022). Beyond forage applications, other high-biomass species—such as tobacco (Nicotiana tabacum) and C4 sorghum (Sorghum bicolor)—have been targeted for sustainable bioenergy production due to their resilience under abiotic stresses (e.g., drought, heat) and capacity for lipid engineering in grains, stems, and leaves (Mullet et al., 2014; Vanhercke et al., 2018 & 2019). These modifications aim to optimize lipid yields for industrial applications, including biodiesel, biolubricants, and aviation fuels. Table 1 summarizes the lipid content improvements, key genetic transformation events, and target applications for these engineered crops, highlighting their diverse roles in agriculture and bioeconomy.
Recent biotechnological advances have enabled the introduction and optimization of TAG biosynthesis pathways in these vegetative tissues, transforming them into efficient lipid-accumulating organs. This process requires the coordinated regulation of multiple biological processes, including chloroplastic de novo fatty acid (FA) biosynthesis, TAG assembly in the endoplasmic reticulum (ER), lipid droplet (LD) formation, and cytoplasmic LD storage (reviewed in Vanhercke et al., 2019). Additionally, significant progress has been made in redirecting C flux away from starch and sucrose biosynthesis and toward oil biosynthesis pathways (Table 1), further enhancing lipid yields (Sanjaya et al., 2011; Zhai et al., 2017).
However, redirecting C flux to lipid biosynthesis demands significant NADPH and ATP, affecting pathways like the Calvin-Benson cycle, glycolysis, and the oxidative pentose phosphate pathway (OxPPP) (Li-Beisson et al., 2013). Additionally, C and nitrogen (N) partitioning are tightly interconnected, with N assimilation playing a pivotal role in photosynthetic efficiency and plant growth (Bloom et al., 1985; Nunes-Nesi et al., 2010). Redox balance and C/N allocation regulate carbohydrate and lipid production, influencing how plants respond to their environment (Chaput et al., 2020).
This review examines the intricate metabolic and regulatory networks governing vegetative lipid biosynthesis, focusing on the interplay of C/N partitioning, energy metabolism, and redox regulation. We discuss how these dynamics shape plant responses to environmental conditions and highlight the potential of field applications to address global food and energy demands. Additionally, advancing integrative multi-omics approaches, understanding the role of lipid accumulation in stress adaptation, and conducting extensive field trials will be pivotal for scaling up these technologies and validating their benefits in real-world agricultural systems.
2 Coordination of carbon partitioning in high-lipid plants
Efficient C partitioning is a cornerstone of plant productivity, with far-reaching implications for agriculture, particularly in enhancing crop yield, stress tolerance, and resource use efficiency. In high-lipid plants, C partitioning is tightly regulated to balance the demands of growth, storage, and stress responses. This section explores the regulatory networks governing C flow in plants, emphasizing triose phosphate/phosphate translocator (TPT), glycolysis, and lipid biosynthesis, and their roles in optimizing vegetative lipid accumulation.
2.1 Triose phosphate transport and carbon partitioning
Dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, key triose phosphate (TP) metabolites, play a crucial role in C partitioning between starch, sucrose, and lipids (Figure 1). Under high photosynthetic activity, increased TP levels coincide with decreased inorganic phosphate (Pi) availability, as Pi is consumed in ATP synthesis. TPT, an antiporter located at the inner chloroplast membrane envelope, exchanges stromal TPs with cytosolic Pi, facilitating the distribution of photosynthetic C from chloroplasts to the cytosol (Fliege et al., 1978). When sink tissues require fewer exported carbohydrates than the photosynthesis rate generates, or when Pi and sucrose synthesis rates are limited, TPs are temporarily stored as starch (Zeeman et al., 2002). Optimizing TPT to enhance sucrose export while maintaining adequate starch reserves could potentially boost biomass and improve stress resilience (Schneider et al., 2002).
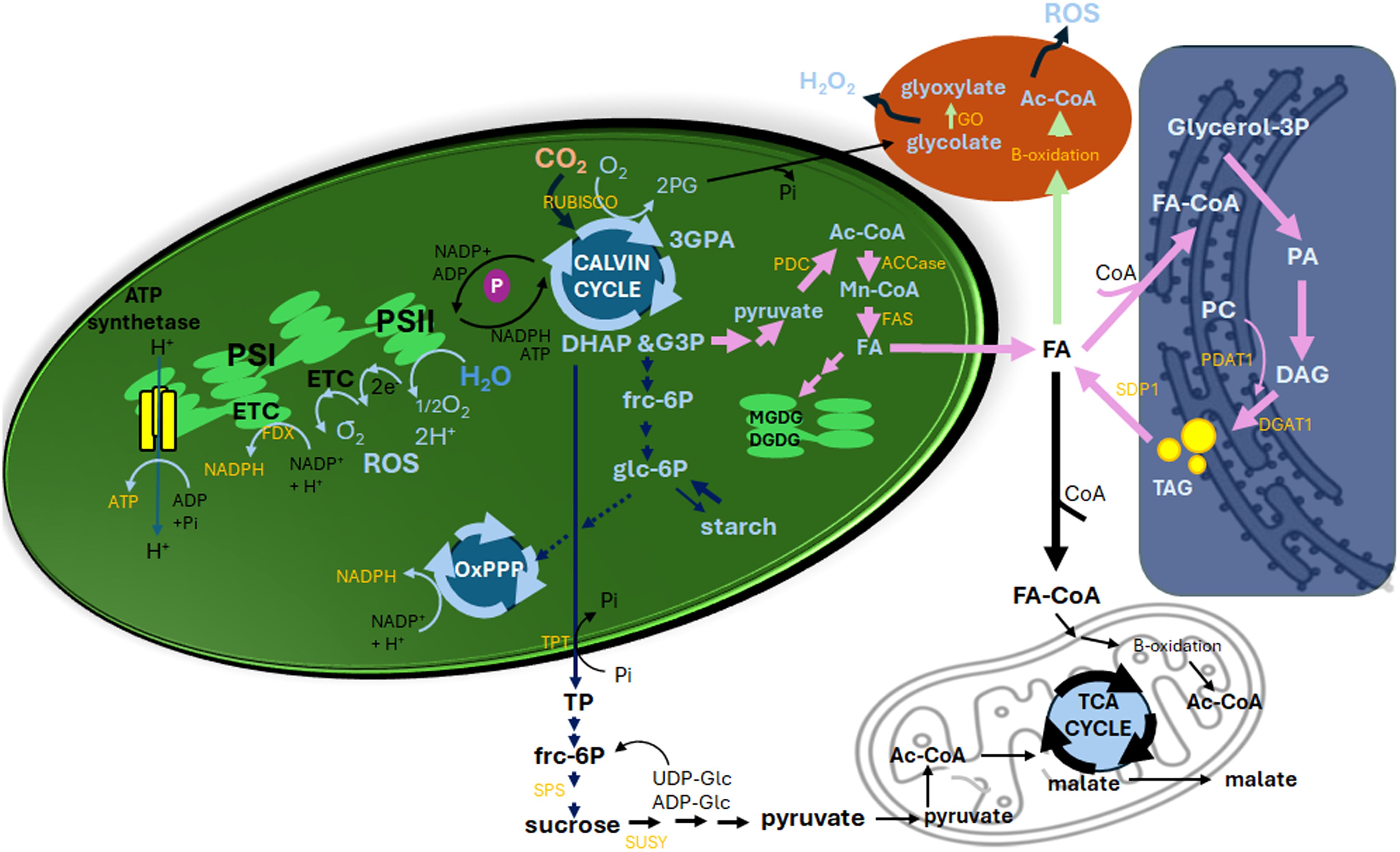
Figure 1. Interplay between photosynthesis, carbon metabolism, lipid biosynthesis, and redox homeostasis in high-lipid plant cells. Upon illumination, photosystem II (PSII) captures light energy, initiating the photolysis of water (H2O) and releasing oxygen (O2). Reactive oxygen species (ROS) are generated by the chloroplast electron transport chain (ETC), producing 1/2O2 at PSII and O2-· at PSI. PSI also facilitates ATP synthesis and generates NADPH via ferredoxin (FDX)-NADP reductase. These products fuel the Calvin cycle, where CO2 is fixed by ribulose-1,6-bisphosphate carboxylase/oxygenase (RUBISCO) to form 3-phosphoglyceric acid (3-PGA). Using ATP and NADPH, 3-PGA is reduced to glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP), collectively termed triose phosphates (TPs). During photorespiration, when O2 levels are high and CO2 levels are low, RUBISCO produces 2-phosphoglycolate (2PG) in coupling with the release of inorganic phosphate (Pi). This is transported to peroxisomes, where its metabolism by glycolate oxidase (GO) generates H2O2. When carbon metabolism favours sugar and starch synthesis, two G3P molecules form six-carbon fructose-6-phosphate (frc-6P) and glucose-6-phosphate (glc-6P) with total 18 ATP and 12 NADPH consumption for starch synthesis in the chloroplasts or TPs may be transported to the cytosol via the TP/phosphate translocator (TPT) and converted to sucrose by sucrose phosphate synthase (SPS) with an exchange to the cytosol Pi. Starch synthesized during the day is degraded at night into sucrose for energy needs. In high-lipid plants, carbon flux may shift toward lipid biosynthesis. G3P undergoes glycolysis, producing pyruvate, which is converted to acetyl-CoA (Ac-CoA) by the pyruvate dehydrogenase complex (PDC). Ac-CoA is carboxylated to malonyl-CoA by acetyl-CoA carboxylase (ACCase), the first step in fatty acid (FA) synthesis. This process requires NADPH, and the resulting FAs follow multiple pathways: FAs undergo multiple pathways: (1) Membrane Lipid Synthesis: FAs are incorporated into monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG). (2) β-Oxidation in Mitochondria: FAs are activated to FA-CoA and transported via the carnitine shuttle for oxidation, yielding Ac-CoA, NADH, and FADH2. Ac-CoA enters the TCA cycle, while NADH and FADH2 fuel the mitochondrial ETC. (3) Glycerolipid Assembly: FA-CoA derivatives participate in forming phosphatidic acid (PA), phosphatidylcholine (PC), diacylglycerol (DAG), and triacylglycerol (TAG), involving Ac-CoA: diacylglycerol acyltransferase 1 (DGAT1) and phosphatidylcholine: diacylglycerol acyltransferase 1 (PDAT1) activities. TAG turnover is dynamic, with sugar-dependent lipase (SDP1) mediating its degradation. (4) β-Oxidation in Peroxisomes: This process regenerates Ac-CoA and releases ROS, which, if exceeding catalase capacity, may contribute to oxidative stress. Sucrose breakdown by sucrose synthase (SUSY) produces UDP-glucose and fructose, fuelling glycolysis to generate pyruvate, ATP, and NADH. A portion of glucose and fructose enters the oxidative pentose phosphate pathway (OxPPP) to supply NADPH for FA biosynthesis and redox balance. Pyruvate also enters the TCA cycle, generating intermediates like malate and citrate. Malate may be exported between mitochondria and cytosol through the malate-aspartate shuttle, balancing redox states via NADH transfer. Arrows may indicate reactions involving more than one step. Thick arrows indicate pathways favoured in high-lipid plants, highlighting metabolic flexibility and integration.
Interestingly, impaired TPT function does not impair growth under normal conditions. However, overexpression of TPT and cytosolic fructose-1,6-bisphosphatase activates sucrose-phosphate synthase (SPS), accelerating sucrose synthesis and promoting growth (Huber and Huber, 1992; Cho et al., 2012). In rice mutants with reduced SPS activity (84% decrease), starch content increased, but growth remained unaffected, suggesting that higher SPS activity promotes more efficient TP export, limiting C availability for starch synthesis (Hashida et al., 2016). RNA interference-mediated downregulation of ADP-glucose pyrophosphorylase, a key enzyme in starch biosynthesis, resulted in a dramatic 16-fold increase in TAG and other lipid accumulation in both wild-type and high-oil potato lines (Xu et al., 2019). This metabolic shift was accompanied by substantial alterations in sugar profiles and starch content across both tuber and leaf tissues, along with significant changes in tuber starch properties. The study demonstrates how redirecting C flux from starch synthesis toward lipid biosynthesis can dramatically enhance oil accumulation in vegetative tissues. These findings underscore the critical importance of balancing starch and sucrose synthesis to maintain C availability for lipid production, as these competing pathways both depend on photoassimilates generated by the Calvin cycle (Sanjaya et al., 2011).
2.2 Glycolysis and lipid biosynthesis
Glycolysis is a fundamental metabolic pathway linking C partitioning, providing substrates for FA biosynthesis via acetyl-CoA carboxylase (ACCase) and the FA synthase (FAS) complex in the chloroplasts (Figure 1, pink arrows). Cytosolic pyruvate, derived from glycolysis, feeds into FA synthesis within plastids, where plastidic pyruvate is converted to acetyl-CoA (Ac-CoA) by the pyruvate dehydrogenase complex (PDC). This tightly regulated process ensures sufficient Ac-CoA for FA biosynthesis. Studies in tobacco using ¹³CO2 labelling and metabolic flux analysis reveal that starch and sucrose cycling support lipid biosynthesis demands (Chu et al., 2022). In high-lipid ryegrass lines, lower shoot sugar levels were associated with reduced fructan biosynthesis and upregulated PDC transcripts (Winichayakul et al., 2022).
Feedback mechanisms further highlight the interconnectedness of glycolysis and lipid biosynthesis. In high-lipid ryegrass, upregulated hexokinase expression suggests enhanced glycolysis, providing substrates for pyruvate and Ac-CoA production (Winichayakul et al., 2022). Elevated hexose phosphate levels may also fuel the OxPPP, the primary source of lipogenic NADPH, as shown by ¹³C metabolic flux analysis in oleaginous yeast cells (Zhang et al., 2016; Kamineni and Shaw, 2020) and biofuel-relevant industrial fungi (Masi et al., 2021). The coordination between glycolysis, the OxPPP, and lipid biosynthesis represents a critical metabolic nexus in high-lipid plants, fundamentally governed by ATP and NADPH requirements. Glycolytic flux begins with glucose-6-phosphate (Glc-6P) from sucrose breakdown, which enters both glycolysis and OxPPP, yielding pyruvate (a precursor for plastidic Ac-CoA), ATP (energy for FA elongation), and NADH (fed into the mitochondrial electron transport chain (ETC)), with key regulatory points including hexokinase activation in high-lipid ryegrass (Winichayakul et al., 2022) and plastidic PDC upregulation (Chu et al., 2022). Meanwhile, the OxPPP plays a dual role as the primary NADPH generator (producing four molecules per Glc-6P) and maintains redox balance during lipid synthesis, supplying NADPH (Figure 1, Li-Beisson et al., 2013). This intricate interplay ensures efficient C allocation and energy provision for lipid biosynthesis.
2.3 Malate and pyruvate reactions in high-lipid plants
Cytosolic pyruvate supports mitochondrial tricarboxylic acid cycle activity, producing C4 compounds like malate (Figure 1). Malate facilitates the transfer of reducing equivalents between the cytosol and mitochondria via the malate-aspartate shuttle and can be exported to the chloroplast through the “malate valve” (Selinski and Scheibe, 2019). NADP-malic enzyme (NADP-ME) activity, through malate decarboxylation, maintains metabolic flexibility and redox balance by producing pyruvate and NADPH (Figure 2). Recent studies demonstrate that NADP-ME activity not only supplies NADPH for lipid biosynthesis but also mitigates oxidative stress by regenerating NADP+, essential for sustaining photosynthetic electron flow (Chu et al., 2022; Winichayakul et al., 2025). This is particularly relevant in high-lipid plant leaves, where manipulation of NADP-ME activity in tobacco and Arabidopsis increased leaf lipid content by up to 30%, demonstrating its potential as a target for metabolic engineering (Wheeler et al., 2005; Chu et al., 2022). In these studies, ¹³CO2 metabolic flux modelling and acyl-acyl carrier protein analysis confirmed that starch production, sucrose cycling, and NADP-ME contribute significantly to lipid synthesis. Enzyme activity assays further support this role. Additionally, NADP-ME activity helps maintain redox balance by regenerating NADP+, which is essential for sustaining photosynthetic electron flow and preventing ROS overproduction (Figure 2). Additionally, fluxes to Ac-CoA and FA production increased independently of lipid pool size data and without the need to impose constraints on lipid fluxes. These findings highlight the plant’s inherent capacity to establish a uniquely developmentally regulated carbon sink. Thus, manipulating NADP-ME activity could serve as a promising target for future efforts aimed at engineering high-lipid crops.
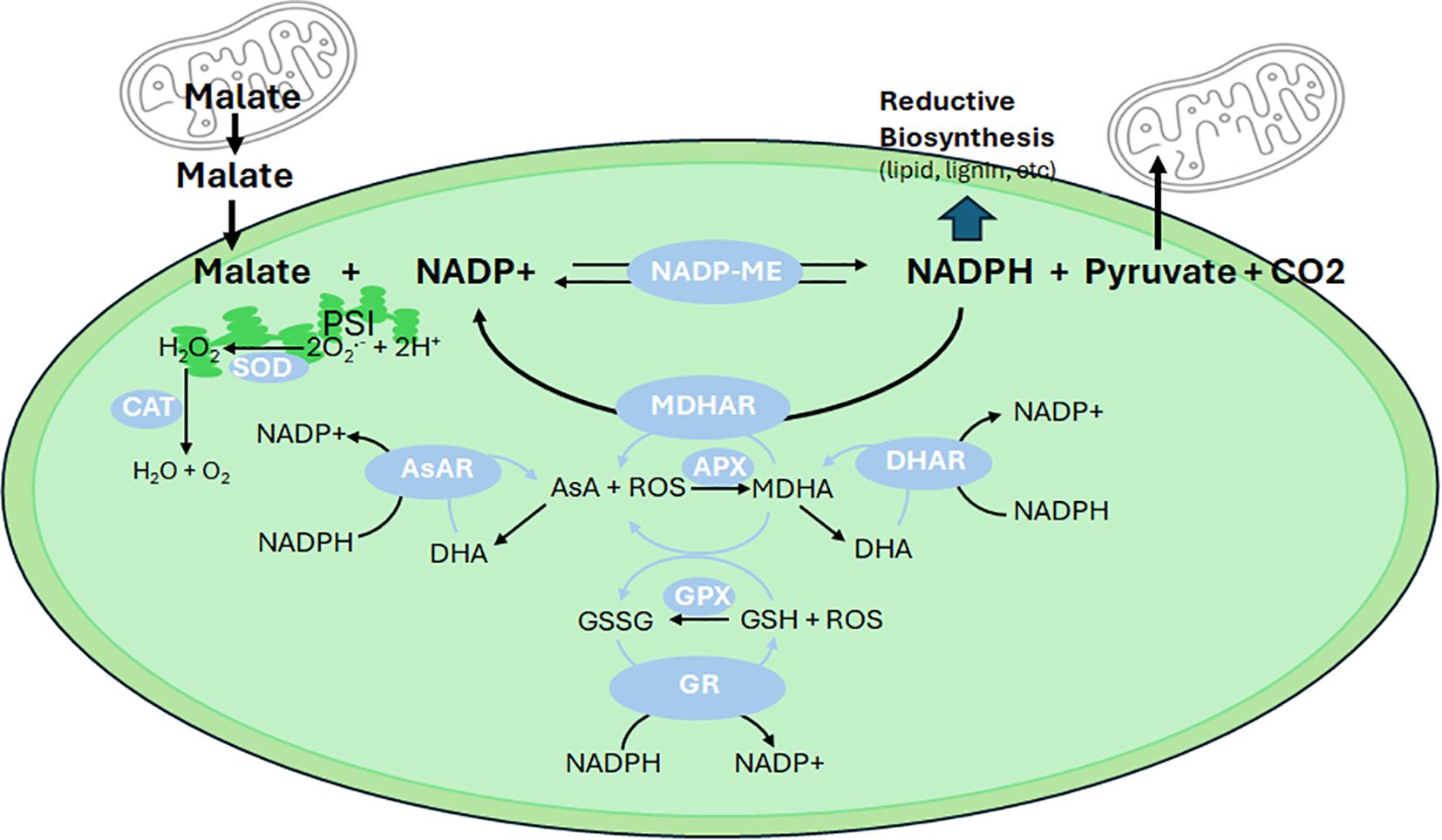
Figure 2. Role of NADP-malic enzyme and the SOD-AsA-GSH cycle in redox balance. Malate exported from the mitochondria to the cytosol is subsequently transported into chloroplasts, where it undergoes decarboxylation by NADP-malic enzyme (NADP-ME). This reaction generates NADPH, pyruvate, and CO2. NADPH plays a vital role in biosynthetic processes, such as the production of fatty acids, flavonoids, and lignin, while pyruvate can be transported back to the mitochondria for further metabolic functions as shown in Figure 1. NADPH also directly supports the ascorbate (AsA)-glutathione (GSH) cycle, which is crucial for detoxifying reactive oxygen species (ROS) and maintaining redox homeostasis. In this cycle, monodehydroascorbate (MDHA) is reduced to AsA by MDHA reductase (MDHAR) using NADPH as the reducing power. AsA, through the action of ascorbate peroxidase (APX), scavenges ROS and is subsequently oxidized to form MDHA and dehydroascorbate (DHA). DHA is then rapidly reduced back to MDHA by DHA reductase (DHAR) or to AsA by ascorbate reductase (AsAR), both utilizing NADPH as an electron donor. Furthermore, glutathione (GSH) plays a role in ROS detoxification by glutathione peroxidase (GPX), which converts GSH to its oxidized form, GSSG. Glutathione reductase (GR) then regenerates GSH from GSSG using NADPH. GSH can also directly reduce MDHA to AsA, highlighting its integral role in the AsA-GSH cycle. Photochemically generated ROS, such as superoxide (O2-·), is converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). The resulting H2O2 is either reduced to water (H2O) via the AsA-GSH cycle or decomposed by catalase (CAT).
2.4 Regulatory role of WRINKLED1 and related factors
Transcription factors such as WRINKLED1 (WRI1) directly stimulate the expression of key enzymes in both the glycolytic and lipid biosynthesis pathways. WRI1 upregulates glycolytic enzymes to supply substrates for lipid production, with overexpression enhancing TAG accumulation and FA turnover (Sanjaya et al., 2011; Kuczynski et al., 2022). Recent discovered targets of WRI1 include enzymes in glycolysis and the pentose phosphate pathway, such as plastidic isoforms of fructokinase 3 and phosphoglucose isomerase 1 (Kuczynski et al., 2022). However, in combined with WRI1 expression, blocking starch synthesis or FA turnover negatively impacts FA accumulation (Fan et al., 2017). KIN10 is a catalytic subunit of the sucrose non-fermented 1 related-kinase 1 (SnRK1) complex, a central regulator of energy homeostasis in plants. Notably, WRI1 stability is modulated by trehalose-6-phosphate (T6P), which inhibited KIN10-mediated phosphorylation of WRI1, enhancing FA biosynthesis (Zhai et al., 2018). Synergistic interactions between WRI1 and TAG-synthesizing enzymes like Ac-CoA: diacylglycerol acyltransferase 1 (DGAT1) and phosphatidylcholine: diacylglycerol acyltransferase 1 (PDAT1) (Figure 1) have been leveraged to engineer oil-rich vegetative tissues and seeds (reviewed in Vanhercke et al., 2019). WRI1 synergizes with DGAT1 and PDAT1 by co-upregulating glycolytic genes (e.g., plastidic fructokinase) while DGAT1 channels fatty acids into TAG, avoiding cytotoxic free FA accumulation (Vanhercke et al., 2019). For example, co-expression of WRI1 and DGAT1 in tobacco increased leaf TAG by 15-fold compared to WRI1 alone (Zhou et al., 2020).
2.5 Impacts of carbon sink manipulation
Diverting C allocation from sugars to lipids impacts various metabolic pathways and subsequent growth, with outcomes depending on the species and lipid storage extent. In some plants, enhanced lipid storage improves water-use efficiency (WUE), photosynthetic N-use efficiency (NUE), and stress tolerance without growth penalties, although these improvements may not lead to more robust plants (Vanhercke et al., 2014; Beechey-Gradwell et al., 2018 & 2020). These benefits may arise from lipid C sinks mitigating photosynthetic feedback inhibition (Paul and Eastmond, 2020), with T6P and SnRK1 signaling pathways playing a crucial role in balancing sink limitation (Nunes et al., 2013). Elevated T6P levels signal sufficient sucrose availability, inhibiting SnRK1 activity and reducing photosynthetic C fixation to prevent overload (Tsai and Gazzarrini, 2014). In contrast, low energy levels activate SnRK1 to conserve resources, suppressing anabolic processes like photosynthesis (Baena-González and Lunn, 2020). Altered T6P/SnRK1 signaling complexes have been observed in high-lipid ryegrass, supporting this hypothesis (Winichayakul et al., 2022). Like high-lipid ryegrass, other high-lipid plants (Alameldin et al., 2017; Vanhercke et al., 2019; Kannan et al., 2022; Luo et al., 2022; Cao et al., 2023; Morales et al., 2024) exemplify the potential of manipulating C sinks to enhance agricultural energy density. However, excessive lipid biosynthesis may divert resources from essential processes such as membrane lipid and cell wall synthesis or protein production, potentially imposing growth penalties (Kelly et al., 2013; Beechey-Gradwell et al., 2020; Mitchell et al., 2020).
Independent of N availability, increased lipid content in non-photosynthetic tissues influences plant growth through multiple mechanisms. Lipids serve as energy-dense C reserves, providing ATP and C skeletons via β-oxidation during germination or stress recovery, supporting metabolic demands (Yang and Benning, 2018; Yang et al., 2022). In storage organs (e.g., seeds), high lipid content is adaptive and may not hinder growth if C supply is sufficient (Baud and Lepiniec, 2010). Lipids also play critical roles in membrane integrity and stress resilience; increased unsaturated FAs, for example, enhance membrane fluidity, sustaining cellular processes under abiotic stress (Dutta et al., 2025). Additionally, lipid-derived signaling molecules (e.g., jasmonates) modulate growth-defense trade-offs, potentially prioritizing stress responses over rapid growth (Wang et al., 2021). The overall impact on growth depends on C partitioning—non-photosynthetic tissues with high lipid content may act as strong C sinks, either supporting later growth phases (e.g., perennials) or limiting immediate structural growth if lipid synthesis outweighs carbohydrate allocation (Troncoso-Ponce et al., 2011; Schwender et al., 2015). Additionally, tobacco with >15% leaf TAG shows stunted growth due to resource competition with cell wall biosynthesis (Zhou et al., 2020). However, ryegrass engineered for moderate lipid accumulation (up to 6.5% DW) maintains biomass yield, suggesting species-specific adaptations further shape this relationship (Beechey-Gradwell et al., 2022; Theodoulou and Eastmond, 2012). Thus, the balance between lipid storage and growth hinges on C allocation strategies, tissue type, and environmental conditions, with trade-offs often observed in vegetative tissues but benefits in storage or stress-adapted organs (Cai and Shanklin, 2022).
To mitigate these trade-offs in vegetative tissues, strategies that integrate transcription factors and metabolic feedback and signaling controls are essential. For examples, engineering plants to enhance the expression of metabolite and regulatory proteins such as T6P, SnRK1, and TPT could optimize C partitioning, particularly when photosynthetic C supply is limited (Nunes et al., 2013; Tsai and Gazzarrini, 2014; Baena-González and Lunn, 2020). In addition, comparative studies using lipidomics, metabolic flux analyses, and multi-omics integration can reveal metabolic flexibility and subsequent regulatory differences and evaluate plant performance under different stresses across a range of high-lipid species (Peng et al., 2023; Liu et al., 2024). These approaches, coupled with field trial data and computational models, could offer insights into predicting plant productivity across diverse climatic conditions, informing field applications.
3 Nitrogen partitioning in high-lipid crops: a key aspect of sustainable crop improvement
N partitioning in high-lipid crops is critical for achieving high yields and sustainability goals. Optimizing photosynthetic NUE and understanding the interplay between N metabolism and lipid biosynthesis can reduce reliance on synthetic fertilizers, mitigate environmental impacts, and enhance economic viability. This section explores the role of N partitioning in photosynthetic efficiency, lipid biosynthesis, and stress responses, with a focus on how N availability and form influence plant performance.
3.1 Nitrogen demand in high-lipid crops
Leaf N levels are essential for maintaining photosynthetic efficiency, providing C precursors and energy, particularly during vegetative growth and under stress (Mengesha, 2021). Redistributing N toward vegetative lipid storage tissues may alter sink strength, affecting resource allocation between source and sink organs. For example, in high-lipid ryegrass, increased C allocation to vegetative oil enhances N uptake for growth and energy requirements, particularly under non-limiting N conditions (Beechey-Gradwell et al., 2018). This suggests that high-lipid crops may achieve higher photosynthetic NUE by optimizing N allocation between photosynthesis and lipid biosynthesis.
Assessing the dynamic C/N ratio and Rubisco’s maximum carboxylation capacity can help evaluate plant responses to N limitation (Cooney et al., 2021). Under N-deficient conditions, lipid accumulation often increases at the expense of growth, as seen in microalgae (Mata et al., 2010; Breuer et al., 2012). This trade-off highlights the need to balance N availability with lipid production to avoid growth penalties. For instance, in Arabidopsis, phospholipase Dϵ promotes growth and N signaling under severe N deprivation by increasing phosphatidic acid (PA) content, linking lipid metabolism to N stress responses (Hong et al., 2009).
3.2 Nitrogen partitioning in lipid storage vegetative tissues
Approximately 75% of leaf N is allocated to photosynthesis machinery, including Rubisco carboxylation (NRubisco), bioenergetics (electron transport and ATP synthesis, NE), and light-harvesting pigment-protein complexes (NP) (Hikosaka and Terashima, 1995). N is also distributed in leaves in other forms (No), such as soluble components (NO3-, NH4+, amino acids) and insoluble components (e.g., cell walls, membranes, and other structures) (Feng et al., 2009; Wei et al., 2022). Small changes in photosynthetic N allocation can significantly affect carboxylation efficiency and photosynthetic NUE (Feng et al., 2009; Onoda et al., 2017). For example, in high-lipid ryegrass, increased N allocation to NE at the expense of NO was observed under NO3- treatment, without compromising NRubisco or NP (Cooney et al., 2021). This optimal N allocation correlated with higher photosynthetic NUE, suggesting that high-lipid plants can adaptively balance nutrient allocation to achieve functional efficiency.
In high-lipid ryegrass, elevated leaf NO3- under NO3- supply suggests limited NO3- ammonification or extensive nitrification of NH4+ in shoots, potentially mitigating oxidative stress from FA biosynthesis. Elevated shoot NO3- has also been noted in other species as a local and systemic signal, regulating genome-wide gene expression and phytohormone signaling pathways through Ca²+ mediation (Alvarez et al., 2012).
3.3 Nitrogen form: the balance between growth and lipid biosynthesis
The form of N availability (NH4+ vs. NO3-) significantly influences growth, lipid accumulation, and N partitioning. Elevated atmospheric CO2 levels can inhibit NO3- assimilation in C3 plants and algae, potentially reducing NO3- use efficiency in future climates (Bloom, 2015 & 2020). However, this relationship is complex and remains a subject of debate. Andrews et al. (2019) discuss the variability of this response, noting that inhibition of N assimilation under elevated CO2 is not universal and depends on species-specific traits and N sources. While NH4+, is theoretically preferred due to its lower energy cost for assimilation, it can be toxic at high concentrations, necessitating strategies to improve NH4+ use efficiency (Di, 2023; Xiao et al., 2023).
In high-lipid ryegrass, shoot dry weight and FA content increased across N supply ranges, with no difference in response to N form at the T0 stage (Beechey-Gradwell et al., 2018). However, at the T2 stage, plants exhibited higher shoot growth rates under NH4+ than NO3- at 20 mM concentrations, possibly due to genotypic background or non-limiting N conditions. In oleaginous fungi, NH4+ boosts lipid production due to its lower energy cost, redirecting resources toward FA synthesis (Wang et al., 2024b). Conversely, NO3- prolongs N uptake by stimulating cytokinin production, delaying leaf senescence and maintaining root N uptake activity (Heuermann et al., 2021).
Crop-specific responses to N forms vary. For examples, Camelina prefers NO3- as its N source, which has been associated with increased seed oil content and improved drought tolerance, while specific lipid percentages may vary depending on environmental conditions and cultivation practices (Li-Beisson et al., 2013). Duckweed responds optimally to NH4+ over NO3-, with up to a 35% increase in TAG content and strong adaptation to NH4+ -rich wastewater environments (Tian et al., 2021; Liang et al., 2022). These findings highlight the importance of optimizing N fertilization strategies to balance lipid yield, growth, and N agronomic efficiency.
3.4 Interactions with other nutrients
N partitioning in high-lipid crops is often intertwined with interactions with other nutrients, such as sulfur (S) and phosphorus (P). Sulphur regulates N metabolism and lipid biosynthesis, with S deficiency leading to reduced protein synthesis and altered lipid composition (Anjum et al., 2012). Similarly, synergistic effects of N and P enhance lipid production and crop resilience, as seen in microalgae and oilseed crops (Huang et al., 2019). For example, in rapeseed, combined N and P application increased lipid content by 20% compared to N application alone, highlighting the importance of integrated nutrient management for optimizing lipid biosynthesis (Peng et al., 2023).
4 Redox regulation in the photosynthetic tissues and its influence in non-seed lipid storage
The shift from conventional lipid storage in seeds to vegetative tissues requires a deeper understanding of the interplay between redox potential and metabolic regulation. Redox regulation is central to managing cellular energy and oxidative stress, particularly in photosynthetic tissues where light-driven electron transport generates ROS. This section explores the mechanisms of redox regulation, its impact on lipid biosynthesis, and its role in plant stress responses, with a focus on non-seed lipid storage.
4.1 Redox homeostasis in photosynthesis
During photosynthesis, ROS are produced at multiple sites (Figure 1). PSII generates singlet oxygen through the excitation of chlorophyll. PSI produces superoxide radicals (O2-·) via the Mehler reaction. Photorespiration generates hydrogen peroxide (H2O2) in peroxisomes through glycolate oxidase activity.
These ROS molecules act as signaling intermediates, modulating metabolic pathways and stress responses. Under optimal conditions, ROS production is balanced by antioxidant systems, maintaining redox homeostasis. However, excessive ROS accumulation can damage lipids, proteins, and DNA, impairing cellular function (Apel and Hirt, 2004).
4.2 Redox regulation of lipid biosynthesis
The chloroplast ETC supplies much of the reducing power, with ferredoxin (Fdx) playing a key role in transferring electrons to NADP+, forming NADPH (Shikanai, 2007). This NADPH is utilized by ACCase and the FAS complex for FA biosynthesis (Li-Beisson et al., 2013).
Redox regulation also influences FA desaturation, a critical step in lipid biosynthesis. FA desaturases require Fdx as an electron donor, linking redox status to membrane fluidity and stress tolerance (Shanklin and Cahoon, 1998).
4.3 ROS scavenging and lipid protection
To mitigate ROS-induced damage, plants employ a suite of antioxidant systems, including the superoxide dismutase-ascorbate-glutathione (SOD-AsA-GSH) cycle (Foyer-Halliwell-Asada pathway). This cycle involves (Figure 2): SOD converts O2-· to H2O2; ascorbate peroxidase reduces H2O2 to water using AsA as an electron donor; monodehydroascorbate reductase and dehydroascorbate reductase regenerate AsA from its oxidized forms; and glutathione reductase maintains GSH levels, essential for ROS scavenging and redox signaling (Polle, 2001).
In high-lipid plants, ROS scavenging is particularly important due to the increased metabolic activity associated with lipid biosynthesis. For example, in high-lipid ryegrass, elevated SOD activity and AsA levels, alongside reduced expression of cytosolic L-AsA oxidase and APX, reflect the need to regulate ROS and conserve reducing equivalents for FA biosynthesis (Winichayakul et al., 2022). These adaptations help maintain redox balance while supporting lipid accumulation.
4.4 Redox regulation of TAG turnover
Leaf TAG is used as a short-term storage intermediate of thylakoid lipid during ongoing membrane turnover, remodeling, and senescence (Slocombe et al., 2009; James et al., 2010). TAG turnover in leaves is dynamic, particularly during vegetative growth and senescence, with rates of ~1.2 mol% per minute in Arabidopsis under 22°C conditions (Bao et al., 2000; Troncoso-Ponce et al., 2013). TAG recycling during high energy demand can increase β-oxidation activity in peroxisomes, potentially elevating ROS production (Figure 1; Yu et al., 2019). In Arabidopsis, SDP1 lipase-mediated TAG degradation supplies C for dark survival (Fan et al., 2017), while lipid peroxidation products (e.g., jasmonates) regulate senescence timing (Yu et al., 2020a). Protecting the TAG β-oxidation may improve vegetative oil yield and reduce ROS-induced damage. Strategies include: engineering cysteine oleosin to enhance TAG-associated protein cross-linking, stabilizing lipid droplets (Winichayakul et al., 2013); RNAi suppression of SDP1 to reduce TAG lipase activity, minimizing TAG turnover (Kelly et al., 2013; Fan et al., 2014), and disrupting CGI-58 and PXA1 to inhibit peroxisomal TAG breakdown, reducing ROS production (Park et al., 2013).
5 Environmental impacts and field evaluation
High-lipid crops offer transformative potential for sustainable agriculture and bioenergy production. However, their successful deployment depends on adaptability to diverse environmental conditions, efficient agronomic management, and minimal ecological trade-offs. This section reviews the performance of high-lipid crops under key abiotic stresses—temperature, light, and water availability—and discusses their ecological and agronomic implications.
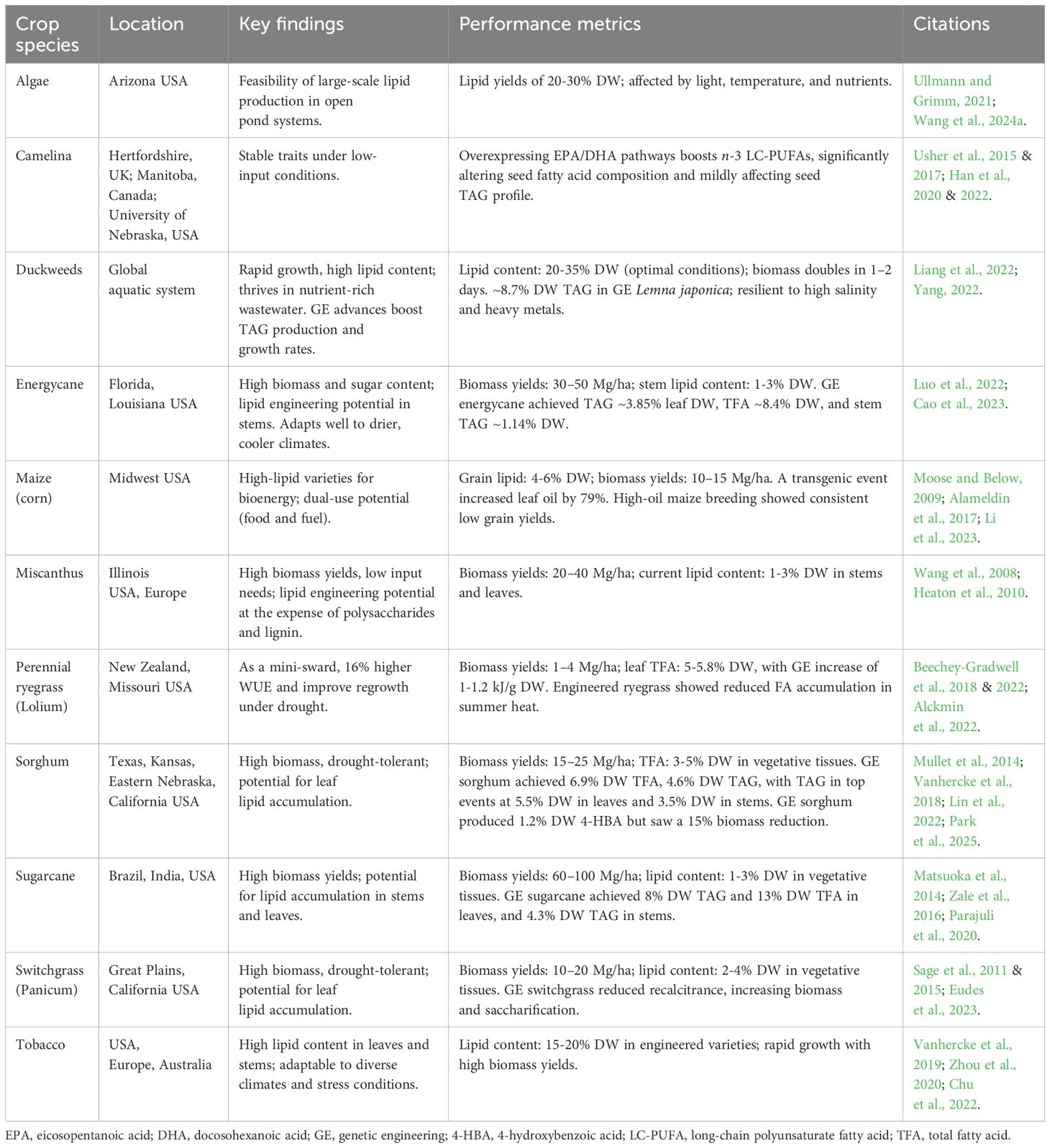
5.1 Temperature effects on lipid synthesis
Temperature fluctuations significantly influence lipid synthesis and plant performance (Table 2). Cooler temperatures promote polyunsaturated FA synthesis, such as increased 18:3 at the expense of 18:2, maintaining plasma membrane fluidity (Zheng et al., 2011). However, chilling temperatures can impair photosynthesis and induce oxidative damage, particularly in warm-climate species (Allen and Ort, 2001).
Recent advances in cold acclimation research have identified strategies to enhance cold resilience. For example, plasma membrane-localized proteins reduce lipid peroxidation and ROS generation in cold-resilient crops (Juurakko et al., 2021). Antifreeze proteins from perennial ryegrass, featuring leucine-rich repeat domains and a β-helical carboxyl ice-binding domain, improve freeze protection (Lauersen et al., 2011). Symbiotic relationships with plant-associated microbiomes also promote growth and reduce lipid peroxidation under cold stress (Zhu et al., 2010; Osman et al., 2013).
In contrast, warm climates stimulate faster growth and heightened metabolic activity, enhancing the biosynthesis of specific lipids like TAGs (Mueller et al., 2017). TAG accumulation in vegetative tissues may help plants cope with abiotic stress through lipase-mediated lipid remodeling (Lu et al., 2020). However, high leaf-oil Arabidopsis lines exhibit increased susceptibility to heat stress (Yurchenko et al., 2018), highlighting the complexity of lipid metabolism under thermal stress.
Extreme heat impairs membrane stability and lipid functionality through oxidative stress and lipid peroxidation (Shiva et al., 2020). Field trials of high-lipid cool-season perennial ryegrass in Missouri, USA, revealed a decline in foliar FA accumulation under summer conditions (Beechey-Gradwell et al., 2022), potentially linked to rapid TAG turnover to support heat-induced stomatal opening (Korte et al., 2023). These findings underscore the need for multi-site validation of high-lipid technologies and exploration of their application in warm-climate forage species, which often produce heat-shock proteins and lipid antioxidants to mitigate heat-induced damage (Oksala et al., 2014; Rahman et al., 2022).
5.2 Light impacts on lipid biosynthesis
Light is a critical regulator of lipid biosynthesis, particularly in photosynthetic tissues where C and energy derived from photosynthesis play a central role (Fan et al., 2017; Lu et al., 2020). Seasonal variations in light intensity, day length, and spectral composition significantly influence lipid metabolism. Low light intensity, shorter day lengths, and a higher proportion of red and infrared light in winter can limit photosynthesis and growth. Conversely, high light intensity, longer day lengths, and a higher proportion of blue and ultraviolet wavelengths in summer support robust photosynthesis but may induce stress under excessive light.
Yu et al. (2020a) demonstrated how plants fine-tune FA and glycerolipid biosynthesis in response to long-term changes in light conditions (Table 2). Arabidopsis mutants defective in glycerolipid biosynthesis (tdg1) exhibited altered growth patterns and impaired thylakoid membrane remodeling under high light. Excess light stress can lead to ROS production, with phospholipids and galactolipids degraded to provide substrates for TAG synthesis as a protective mechanism (Olzmann and Carvalho, 2019; Obaseki et al., 2024). TAG accumulation in chloroplast-associated lipid droplets may prevent lipotoxicity from oxidative damage to FAs.
Low-light conditions reduce ROS-mediated FA breakdown, allowing greater accumulation of free FAs in leaf tissues. However, low light can disrupt the balance between photosynthate production and utilization. Plastidic ACCase is regulated at transcriptional and posttranslational levels, underscoring its role in plant acclimation to changing irradiance (Miller et al., 2017; Yu et al., 2020a). WRI1, a key transcriptional regulator of lipid biosynthesis, integrates environmental signals and developmental cues to synchronize FA production with plant energy status (Kuczynski et al., 2022; Han et al., 2025). Transcriptomic analyses further reveal the interconnected regulation of genes involved in C metabolism, sugar signaling, mitochondrial respiration, and redox potential, all of which influence lipid metabolism under low light conditions (Winichayakul et al., 2022).
5.3 Water availability impact on lipid biosynthesis
Water stress, whether from drought, salinity, or flooding, significantly alters lipid composition and biosynthesis. While moderate water stress can trigger adaptive lipid responses, severe or prolonged stress typically suppresses overall lipid production, impairing growth, seed development, and stress tolerance.
Water deficit induces ROS production, leading to lipid peroxidation (Table 2). This triggers signaling pathways that modulate lipid biosynthesis (Sharma et al., 2023). Water scarcity limits the availability of energy and NADPH required for FA biosynthesis in plastids, reducing overall lipid production. In Arabidopsis, total leaf lipid content decreased progressively under drought, although plants exhibited cellular adaptations, such as increased galactolipid ratios and FA unsaturation (Gigon et al., 2004). While key enzymes like ACCase and FAS are often downregulated under drought, phospholipase Dϵ activity increases, generating phosphatidic acid, which participates in osmotic stress responses and cellular signaling (Wang, 2005; Sharma et al., 2023).
Recent studies highlight the role of altered FA composition in drought acclimation. For example, Yin et al. (2024) reported that high 18:3 levels contributed to drought tolerance in maize, while 16:2 and 16:3 were more critical for drought recovery. Beechey-Gradwell et al. (2018) found that high-lipid perennial ryegrass exhibited greater regrowth and 16% higher WUE under limited water supply compared to controls. These findings suggest that high vegetative-lipid technology may enhance drought tolerance, although further field testing is needed to confirm these effects (Blum, 2005).
Salinity stress similarly disrupts water uptake and generally leads to reduced transpiration due to stomatal closure, leading to osmotic stress and enhanced photorespiration (Munns, 2005). Photorespiration generates significant ROS, which can damage lipids and other cellular components (Apel and Hirt, 2004). The balance between ROS production and scavenging enzyme activity determines whether signaling or damage occurs (Zhang et al., 2014). For instance, increased production of ascorbate peroxidase and glutathione reductase protects plants from oxidative stress under drought and salinity (Table 2, Ratnayaka et al., 2003; Chaki et al., 2020; Qamer et al., 2021).
5.4 Crop selection and field evaluation
The development and deployment of high-lipid crops require a dual focus on crop selection and field evaluation. Crop selection depends on the intended application, with ongoing debate about the impact of using food crops as biofuel feedstocks (Singh et al., 2023). Agricultural residues and non-food feedstocks like algae and duckweed offer competitive alternatives (Ullmann and Grimm, 2021; Yang, 2022). Algae and duckweeds exhibit high lipid content under controlled conditions, but their performance in open pond systems or natural aquatic environments requires validation to ensure consistent yields (Wang et al., 2024a). Table 3 summarizes key findings from laboratory to agricultural and bioenergy applications, including performance metrics for scaling cultivation.
High-lipid crops must demonstrate adaptability to a wide range of environmental conditions, including variations in temperature, light, water availability, and soil quality. For example, perennial ryegrass has shown promise in cool climates but exhibits reduced lipid accumulation under summer heat, highlighting the need for climate-specific optimization (Beechey-Gradwell et al., 2022). Camelina have demonstrated resilience to drought and marginal soils, making them suitable for cultivation in water-limited regions (Usher et al., 2015 & 2017; Han et al., 2020). Tobacco, traditionally grown for nicotine production, has emerged as a promising high-lipid crop due to its rapid growth and adaptability to diverse climates including drought and high salinity, making it a robust option for cultivation in challenging environments (Zhou et al., 2020). Field trials have shown that tobacco can accumulate significant lipid content, reaching up to 15-20% DW in its leaves and stems, particularly under stress conditions, making it a viable candidate for bioenergy production (Vanhercke et al., 2019). However, at these levels of vegetative lipid accumulation, tobacco typically experiences severe growth penalties, limiting its potential for commercialization.
Several genetic and biotechnological strategies have been explored to enhance vegetative oil production in crops such as maize, sorghum, and sugarcane, due to their adaptability to diverse environmental conditions, including drought and high temperatures (Table 3) (Dida, 2024). Maize engineered for increased lipid content in seeds and vegetative tissues often shows reduced grain yield (Moose and Below, 2009; Alameldin et al., 2017; Li et al., 2023). The ‘push, pull, protect’ (3P) GM strategy (Xu and Shanklin, 2016) achieved TAG concentrations of up to 8.4% DW in sorghum leaves, though trait stability and growth effects in subsequent generations were not assessed due to multiple T-DNA insertions (Vanhercke et al., 2018). Recently, Park et al. (2025) reported successful field trials of sorghum engineered for high vegetative oil content, achieving 5.5% DW in leaves and 3.5% DW in stems without growth penalties. This approach combined the 3P strategy with medium-chain FA generation, contrasting with growth inhibition observed in engineered sugarcane and energycane (Matsuoka et al., 2014; Zale et al., 2016; Parajuli et al., 2020; Luo et al., 2022; Cao et al., 2023). Notably, engineered sorghum lines exhibited higher DGAT1 expression than WRI1, suggesting sufficient DGAT1 activity to enhance FA biosynthesis flux through the ER glycerolipid pathway. Imbalances between WRI1 induction and DGAT activity can lead to toxic free FA accumulation, a key bottleneck in high-lipid crop development (Yang et al., 2015; Parajuli et al., 2020; Kannan et al., 2022).
C4 perennial species such as Miscanthus spp., switchgrass, and prairie cordgrass are also gaining recognition as bioenergy feedstocks in cool climates. These crops benefit from the abundance of arable land in the Northern Hemisphere and the growing demand for biofuels (Sage et al., 2011 & 2015). These grasses hold potential for lipid engineering at the expense of polysaccharides (Eudes et al., 2023). Breeding efforts must prioritize cold-tolerant traits to ensure robust growth in cooler climates (Sage et al., 2015). For instance, pyruvate phosphate dikinase, a key enzyme in C4 photosynthesis, exhibits higher protein content and activity in Miscanthus x giganteus under cooler conditions, enhancing photosynthetic capacity (Wang et al., 2008; Heaton et al., 2010). Transgenic maize overexpressing this enzyme showed improved photosynthetic rates at low temperatures, highlighting its potential for expanding bioenergy crop suitability (Thomashow et al., 2001).
6 Summary and future directions
Integrating TAG storage into non-seed tissues holds great promise for enhancing crop energy density. The degree of vegetative TAG accumulation varies across species, influenced by the biotechnological approach employed. While some plants experience growth penalties, others exhibit enhanced growth at different lipid accumulation levels. Although improved photosynthetic efficiency in high-lipid plants is thought to result from mitigating feedback inhibition through the dynamic redirection of carbohydrate flux, this metabolic shift is far more complex, involving changes in carbon and nitrogen partitioning, as well as redox homeostasis, affecting plant responses to diverse environmental conditions.
To fully realize the agricultural potential of lipid biosynthesis in crop biomass, several key research directions should be prioritized:
1. Integrative multi-omics approaches
This integrative approach could identify novel targets for enhancing crop resilience and productivity in diverse field conditions.
2. Environmental stress adaptation
Investigating how lipid accumulation influences stress signaling and energy metabolism will be crucial for developing cultivars with improved tolerance to environmental stresses.
3. Carbon-nitrogen interactions
Understanding the interplay between C and N metabolism, particularly how N availability affects lipid biosynthesis and redox balance, will inform breeding strategies aimed at optimizing NUE while maintaining high lipid yields.
4. Field trials and real-world applications
Conducting extensive field trials under varying environmental conditions is essential for validating the benefits observed in controlled settings. These trials will refine cultivation practices and assess the feasibility of scaling up lipid biosynthesis technologies for large-scale agricultural deployment.
5. Alternative crop species and systems
Expanding research to include other crop species, such as C4 plants, duckweeds, and microalgae, could broaden the applications of these technologies. Duckweed and microalgae, for examples, have demonstrated promising lipid accumulation under stress conditions, making them potential candidates for biofuel production and carbon sequestration.
By addressing these key areas, future research can bridge the gap between laboratory breakthroughs and field implementation, paving the way for sustainable agricultural innovations that meet global food and energy demands.
Author contributions
SW: Conceptualization, Writing – original draft. NR: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The publication of this manuscript was financially supported by AgResearch Strategic Science Investment Fund programme PRJ0771996.
Acknowledgments
The authors sincerely thank Prof. Richard Macknight (University of Otago, Dunedin, New Zealand) for his critical review and insightful comments on this manuscript. His expertise and feedback have significantly contributed to improving the quality of this work.
Conflict of interest
Authors SW and NR were employed by AgResearch Ltd.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alameldin, H., Izadi-Darbandi, A., Smith, S. A., Balan, V., Jones, A. D., and Sticklen, M. (2017). Production of seed-like storage lipids and increase in oil bodies in corn (Maize; Zea mays L.) vegetative biomass. Ind. Crops Prod. 108, 526–534. doi: 10.1016/j.indcrop.2017.07.021
Alckmin, G. T., Lucieer, A., Rawnsley, R., and Kooistra, L. (2022). Perennial ryegrass biomass retrieval through multispectral UAV data. Comp. Electron. Agric. 193, 106574. doi: 10.1016/j.compag.2021.106574
Allen, D. J. and Ort, D. R. (2001). Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 6, 36–42. doi: 10.1016/S1360-1385(00)01808-2
Alvarez, J. M., Vidal, E., and Gutierrez, R. A. (2012). Integration of local and systemic signaling pathways for plant N responses. Curr. Opin. Plant Biol. 15, 185–191. doi: 10.1016/j.pbi.2012.03.009
Andrews, M., Condron, L. M., Kemp, P. D., Topping, J. F., Lindsey, K., Hodge, S., et al. (2019). Elevated CO2 effects on nitrogen assimilation and growth of C3 vascular plants are similar regardless of N-form assimilated. J. Exp. Bot. 70, 683–690. doi: 10.1093/jxb/ery371
Anjum, N. A., Gill, S. S., Umar, S., Ahmad, I., Duarte, A. C., and Pereira, E. (2012). Improving growth and productivity of oleiferous Brassica under changing environment: significant of nitrogen and sulphur nutrition, and underlying mechanisms. Sci. World J. 2012, 657808. doi: 10.1100/2012/657808
Apel, K. and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Baena-González, E. and Lunn, J. E. (2020). SnRK1 and trehalose 6-phosphate —two ancient pathways converge to regulate plant metabolism and growth. Curr. Opin. Plant Biol. 55, 52–59. doi: 10.1016/j.pbi.2020.01.010
Bao, X., Focke, M., Pollard, M., and Ohlrogge, J. (2000). Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J. 22, 39–50. doi: 10.1046/j.1365-313x.2000.00712.x
Baud, S. and Lepiniec, L. (2010). Physiological and developmental regulation of seed oil production. Prog. Lipid Res. 49, 235–249. doi: 10.1016/j.plipres.2010.01.001
Bayat, A. R., Tapio, I., Vilkki, J., Shingfield, K. J., and Leskinen, H. (2018). Plant oil supplements reduce methane emissions and improve milk fatty acid composition in dairy cows fed grass silage-based diets without affecting milk yield. J. Dairy Sci. 101, 1136–1151. doi: 10.3168/jds.2017-13545
Beechey-Gradwell, Z., Cooney, L., Winichayakul, S., Andrews, M., Hea, S. Y., Crowther, T., et al. (2020). Storing carbon in leaf lipid sinks enhances perennial ryegrass carbon capture especially under high N and elevated CO2. J. Exp. Bot. 71, 2351–2361. doi: 10.1093/jxb/erz494
Beechey-Gradwell, Z., Kadam, S., Bryan, G., Cooney, L., Nelson, K., Richardson, K., et al. (2022). Lolium perenne engineered for elevated leaf lipids exhibit greater energy density in filed canopies under defoliation. Field Crops Res. 275, 108340. doi: 10.1016/j.fcr.2021.108340
Beechey-Gradwell, Z., Winichayakul, S., and Roberts, N. (2018). High lipid perennial ryegrass growth under variable nitrogen, water and carbon dioxide supply. J. New Zeal. Grasslands 80, 219–224. doi: 10.33584/jnzg.2018.80.349
Bloom, A. J. (2015). The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant Biol. 25, 10–16. doi: 10.1016/j.pbi.2015.03.002
Bloom, A. J., Chapin, F. S., and Mooney, H. A. (1985). Resource limitation in plants—an economic analogy. Annu. Rev. Ecol. Evol. Syst. 16, 363–392. doi: 10.1146/annurev.es.16.110185.002051
Bloom, A. J., Kasemsap, P., and Rubio-Asensio, J. S. (2020). Rising atmospheric CO2 concentration inhibits nitrate assimilation in shoots but enhances it in roots of C3 plants. Physiol. Plant 168, 963–972. doi: 10.1111/ppl.v168.4
Blum, A. (2005). Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aus. J. Agric. Res. 56, 1159–1168. doi: 10.1071/AR05069
Breuer, G., Lamers, P. P., Martens, D. E., Draaisma, R. B., and Wijffels, R. H. (2012). The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgal strains. Bioresour. Technol. 124, 217–226. doi: 10.1016/j.biortech.2012.08.003
Cai, Y. and Shanklin, J. (2022). A toolkit for plant lipid engineering: surveying the efficacies of lipogenic factors for accumulating specialty lipids. Front. Plant Sci. 13, 1064176. doi: 10.3389/fpls.2022.1064176
Cao, V. D., Luo, G., Korynta, S., Liu, H., Liang, Y., Shanklin, J., et al. (2023). Intron-mediated enhancement of DIACYLGLYCEROL ACYLTRANSFERASE1 expression in energycane promotes a step change for lipid accumulation in vegetative tissues. Biotechnol. Biofuels Bioprod. 16, 153. doi: 10.1186/s13068-023-02393-1
Chaki, M., Begara-Morales, J. C., and Barroso, J. B. (2020). Oxidative stress in plants. Antioxid. 9, 481. doi: 10.3390/antiox9060481
Chaput, V., Martin, A., and Lejay, L. (2020). Redox metabolism: the hidden player in carbon and nitrogen signalling? J. Exp. Bot. 71, 3816–3826. doi: 10.1093/jxb/eraa078
Cho, M.-H., Jang, A., Bhoo, S. H., Jeon, J.-S., and Hahn, T.-R. (2012). Manipulation of triose phosphate/phosphate translocator and cytosolic fructose-1,6-bisphosphatase, the key components in photosynthetic sucrose synthesis, enhances the source capacity of transgenic Arabidopsis plants. Photosynth. Res. 111, 261–268. doi: 10.1007/s11120-012-9720-2
Chu, K. L., Koley, S., Jenkins, L. M., Bailey, S. R., Kambhampati, S., Foley, K., et al. (2022). Metabolic flux analysis of the non-transitory starch tradeoff for lipid production in mature tobacco leaves. Metab. Eng. 69, 231–248. doi: 10.1016/j.ymben.2021.12.003
Cooney, L., Beechey-Gradwell, Z., Winichayakul, S., Richardson, R., Crowther, T., Anderson, P., et al. (2021). Changes in leaf-level nitrogen partitioning and mesophyll conductance deliver increased photosynthesis for Lolium perenne leaves engineered to accumulate lipid carbon sinks. Front. Plant Sci. 12, 641822. doi: 10.3389/fpls.2021.641822
Di, D.-W. (2023). New molecular mechanisms of plant response to ammonium nutrition. Appl. Sci. 13, 11570. doi: 10.3390/app132011570
Dida, G. (2024). Biotechnology towards energy crops. CABI Agric. Biosci. 5, 45. doi: 10.1186/s43170-024-00245-y
Durrett, T. P., Benning, C., and Ohlrogge, J. (2008). Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 54, 593–607. doi: 10.1111/j.1365-313X.2008.03442.x
Dutta, S., Islam, Z., Das, S., Barman, A., Chowdhury, M., and Prasad., B. (2025). Harmonizing plant resilience: unveiling the symphony of membrane lipid dynamics in response to abiotic stresses: a review. Discov. Plant 2, 61. doi: 10.1007/s44372-025-00152-0
Eudes, A., Lin, C.-Y., Ben, C. D., Ortega, J., Lee, M. Y., Chen, Y.-C., et al. (2023). Field performance of switchgrass plants engineered for reduced recalcitrance. Front. Plant Sci. 14, 1181035. doi: 10.3389/fpls.2023.1181035
Fan, J., Yan, C., Roston, R., Shanklin, J., and Xu, C. (2014). Arabidopsis lipins, PDAT1 acyltransferase, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward β-oxidation, thereby maintaining membrane lipid homeostasis. Plant Cell. 26, 4119–4134. doi: 10.1105/tpc.114.130377
Fan, J., Yu, L., and Xu, C. (2017). A central role for triacylglycerol in membrane lipid breakdown, fatty acid β-oxidation, and plant survival under extended darkness. Plant Physiol. 174, 1517–1530. doi: 10.1104/pp.17.00653
Feng, Y.-L., Lei, Y.-B., Wang, R.-F., Callaway, R. M., Valiente-Banuet, A., Inderjit, et al. (2009). Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. U.S.A. 106, 1853–1856. doi: 10.1073/pnas.0808434106
Fliege, R., Flügge, U. I., Werdan, K., and Heldt, H. W. (1978). Specific transport of inorganic phosphate, 3-phosphoglycerate and triphosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim. Biophys. Acta 502, 232–247. doi: 10.1016/0005-2728(78)90045-2
Gigon, A., Matos, A.-R., Laffray, D., Zuily-Fodil, Y., and Pham-Thi, A.-T. (2004). Effect of drought stress on lipid metabolism in the laves of Arabidopsis thaliana (ecotype Columbia). Ann. Bot. 94, 345–351. doi: 10.1093/aob/mch150
Han, X., Peng, Y., Yin, S., Zhao, H., Zong, Z., Tan, Z., et al. (2025). Transcriptional regulation of transcription factor genes WRI1 and LAFL during Brassica napus seed development. Plant Physiol. 197 (2), kiae378. doi: 10.1093/plphys/kiae378
Han, L., Silvestre, S., Sayanova, O., Haslam, R. P., and Napier, J. A. (2022). Using field evaluation and systematic iteration to rationalize the accumulation of omega-3 long-chain polyunsaturated fatty acids in transgenic Camelina sativa. Plant Biotechnol. J. 20, 1833–1852. doi: 10.1111/pbi.13867
Han, L., Usher, S., Sandgrind, S., Hassall, K., Sayanova, O., Michaelson, L. V., et al. (2020). High level accumulation of EPA and DHA in field-grown transgenic camelina–a multi-territory evaluation of TAG accumulation and heterogeneity. Plant Biotechnol. J. 18, 2280–2291. doi: 10.1111/pbi.v18.11
Hashida, Y., Hirose, T., Okamura, M., Hibara, K.-I., Ohsugi, R., and Aoki, N. (2016). A reduction of sucrose phosphate synthase (SPS) activity affects sucrose/starch ratio in leaves but does not inhibit normal plant growth in rice. Plant Sci. 253, 40–49. doi: 10.1016/j.plantsci.2016.08.017
Heaton, E. A., Dohleman, F. G., Miguez, A. F., Juvik, J. A., Lozovaya, V., Widholm, J., et al. (2010). Chapter 3 - Miscanthus: a promising biomass crop. Adv. Bot. Res. 56, 75–137. doi: 10.1016/B978-0-12-381518-7.00003-0
Heaton, E. A., Flavell, R. B., Mascia, P. N., Thomas, S. R., Dohleman, F. G., and Long, S. P. (2008). Herbaceous energy crop development: recent progress and future prospects. Cur. Opin. Biotechnol. 19, 202–209. doi: 10.1016/j.copbio.2008.05.001
Heuermann, D., Hahn, H., and von Wirén, N. (2021). Seed yield and nitrogen efficiency in oilseed rape after ammonium nitrate or urea fertilization. Front. Plant Sci. 11, 608785. doi: 10.3389/fpls.2020.608785
Hikosaka, K. and Terashima, I. (1995). A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 18, 605–618. doi: 10.1111/j.1365-3040.1995.tb00562.x
Hong, Y., Devaiah, S. P., Bahn, S. C., Thamasandra, B. N., Li, M., Welti, R., et al. (2009). Phospholipase D epsilon and phosphatidic acid enhance Arabidopsis nitrogen signaling and growth. Plant J. 58, 376–387. doi: 10.1111/j.1365-313X.2009.03788.x
Huang, B., Marchand, J., Blanckaert, V., Lukomska, E., Ulmann, L., and Wielgosz-Collin, G. (2019). Nitrogen and phosphorus limitations induce carbon partitioning and membrane lipid remodelling in the marine diatom Phaeodactylum tricornutum. Euro. J. Phycol. 54, 342–358. doi: 10.1080/09670262.2019.1567823
Huber, S. C. and Huber, J. L. (1992). Role of sucrose-phosphate synthase in sucrose metabolism in leaves. Plant Physiol. 99, 1275–1278. doi: 10.1104/pp.99.4.1275
James, C. N., Horn, P. J., Case, C. R., Gidda, S. K., Zhang, D., Mullen, R. T., et al. (2010). Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants. PNAS. 107, 17833–17838. doi: 10.1073/pnas.0911359107
Juurakko, C. L., DiCenzo, G. C., and Walker, V. K. (2021). Cold acclimation and prospects for cold-resilient crops. Plant Stress. 2, 100028. doi: 10.1016/j.stress.2021.100028
Kamineni, A. and Shaw, J. (2020). Engineering triacylglycerol production from sugars in oleaginous yeasts. Curr. Opin. Biotechnol. 62, 239–247. doi: 10.1016/j.copbio.2019.12.022
Kannan, B., Liu, H., Shanklin, J., and Altpeter, F. (2022). Towards oilcane: preliminary field evaluation of metabolically engineered sugarcane with hyper-accumulation of triacylglycerol in vegetative tissues. Mol. Breed. 42, 64. doi: 10.1007/s11032-022-01333-5
Kelly, A. A., van Erp, H., Quettier, A.-L., Shaw, E., Menard, G., Kurup, S., et al. (2013). The SUGAR-DEPENDENT1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol. 162, 1282–1289. doi: 10.1104/pp.113.219840
Korte, P., Unzner, A., Damm, T., Berger, S., Krischke, M., and Mueller, M. J. (2023). High triacylglycerol turnover is required for efficient opening of stomata during heat stress in Arabidopsis. Plant J. 115, 81–96. doi: 10.1111/tpj.v115.1
Kuczynski, C., McCorkle, S., Keereetaweep, J., Shanklin, J., and Schwender, J. (2022). An expanded role for the transcription factor WRINKLED1 in the biosynthesis of triacylglycerol during seed development. Front. Plant Sci. 13, 955589. doi: 10.3389/fpls.2022.955589
Lauersen, K. J., Brown, A., Middleton, A., Davies, P. L., and Walker, V. K. (2011). Expression and characterization of an antifreeze protein from the perennial rye grass, Lolium perenne. Cryobiol. 62, 194–201. doi: 10.1016/j.cryobiol.2011.03.003
Laura, B., Silvia, P., Francesca, F., Benedetta, S., and Carla, C. (2018). Epigenetic control of defense genes following MeJA-induced priming in rice (O. sativa). J. Plant Physiol. 228, 166–177. doi: 10.1016/j.jplph.2018.06.007
Li, H., Fernie, A. R., and Yang, X. (2023). Using systems metabolic engineering strategies for high-oil maize breeding. Curr. Opin. Biotechnol. 79, 102847. doi: 10.1016/j.copbio.2022.102847
Liang, Y., Yu, X.-H., Anaokar, S., Shi, H., Dahi, W. B., Cai, Y., et al. (2022). Engineering triacylglycerol accumulation in duckweed (Lemna japonica). Plant Biotechnol. J. 21, 317–330.
Li-Beisson, Y., Shorrosh, B., Beisson, F., Andersson, M. X., Arondel, V., Bates, P. D., et al. (2013). Acyl-lipid metabolism. Arabidopsis Book 11, e0161. doi: 10.1199/tab.0161
Lin, C.-Y., Tian, Y., Nelson-Vasilchik, K., Hague, J., Kakumanu, R., Lee, M. Y., et al. (2022). Engineering sorghum for higher 4-hydroxybenzoic acid content. Metab. Eng. Commun. 15, e00207. doi: 10.1016/j.mec.2022.e00207
Liu, Q., Guo, Q., Akbar, S., Zhi, Y., El Tahchy, A., Mitchell, M., et al. (2016). Genetic enhancement of oil content in potato tuber (Solanum tuberosum L.) through an integrated metabolic engineering strategy. Plant Biotechnol. J. 15, 56–67. doi: 10.1111/pbi.12590
Liu, H.-Z., Li, Y.-K., Chen, Y.-L. -., Zhou, Y., Sahu, S. K., Liu, N., et al. (2024). Exploring the plant lipidome: techniques, challenges, and prospects. Adv. Biotechnol. 2, 11. doi: 10.1007/s44307-024-00017-9
Lu, J., Xu, Y., Wang, J., Singer, S. D., and Chen, G. (2020). The role of triacylglycerol in plant stress response. Planta (Basel) 9, 472. doi: 10.3390/plants9040472
Luo, G., Cao, V. D., Kannan, B., Liu, H., Shanklin, J., and Altpeter, F. (2022). Metabolic engineering of energycane to hyperaccumulate lipids in vegetative biomass. BMC Biotechnol. 22, 24. doi: 10.1186/s12896-022-00753-7
Masi, A., Mach, R. L., and Mach-Aigner, A. R. (2021). The pentose phosphate pathway in industrially relevant fungi: crucial insights for bioprocessing. Appl. Microbiol. Biotechnol. 105, 4017–4031. doi: 10.1007/s00253-021-11314-x
Mata, T. M., Martins, A. A., and Caetano, N. S. (2010). Microalgae for biodiesel production and other applications: a review. Renew. Sust. Energ. Rev. 1, 217–232. doi: 10.1016/j.rser.2009.07.020
Matsuoka, S., Kennedy, A. J., Gustavo D. dos Santos, E., and Tomazela, A. L. (2014). Energy cane: Its concept, development, characteristics, and prospects. Adv. Bot. 2014, 597275. doi: 10.1155/2014/597275
Mengesha, M. (2021). Effect and roles of nitrogen supply on photosynthesis. Int. J. Photochem. Photobiol. 5, 19–27. doi: 10.11648/j.ijpp.20210502.12
Miller, M. A. E., O’Cualain, R., Selley, J., Knight, D., Karim, M. F., Hubbard, S. J., et al. (2017). Dynamic acclimation to high light in Arabidopsis thaliana involves widespread reengineering of the leaf proteome. Front. Plant Sci. 8, 1239. doi: 10.3389/fpls.2017.01239
Mitchell, M. C., Prichard, J., Okada, S., Zhang, J., Vebables, I., Vanhercke, T., et al. (2020). Increasing growth and yield by altering carbon metabolism in a transgenic leaf oil crop. Plant Biotechnol. J. 18, 2042–2052. doi: 10.1111/pbi.v18.10
Moose, S. and Below, F. E. (2009). Biotechnology approaches to improving maize nitrogen use efficiency. Biotechnol. Agric. Forest (Berlin, Heidelberg: Springer Nature Link) 63, 65–77. doi: 10.1007/978-3-540-68922-5_6
Morales, M. M., Hoshide, A. K., Carvalho, L. M. P., and Tardin, F. D. (2024). Sorghum biomass as an alternative source for bioenergy. Biomass. 4, 1017–1030. doi: 10.3390/biomass4030057
Mueller, S. P., Unger, M., Guender, L., Fekete, A., and Mueller, M. J. (2017). Phospholipid: diacylglycerol acyltransferase-mediated triacylglycerol synthesis augments basal thermotolerance. Plant Physiol. 175, 486–497. doi: 10.1104/pp.17.00861
Mullet, J., Morishige, D., McCormick, R., Truong, S., Hilley, J., McKinley, B., et al. (2014). Energy sorghum —A genetic model for the design of C4 grass bioenergy crops. J. Exp. Bot. 65, 3479–3489. doi: 10.1093/jxb/eru229
Munns, R. (2005). Genes and salt tolerance: bringing them together. New Phytol. 167, 645–663. doi: 10.1111/j.1469-8137.2005.01487.x
Nunes, C., O’Hara, L. E., Primavesi, L. F., Delatte, T. L., Schluepmann, H., Somsen, G. W., et al. (2013). The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol. 162, 1720–1732. doi: 10.1104/pp.113.220657
Nunes-Nesi, A., Fernie, A. R., and Stitt, M. (2010). Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 3, 973–996. doi: 10.1093/mp/ssq049
Obaseki, E., Adebayo, D., Bandyopadhyuy, S., and Hariri, H. (2024). Lipid droplets and fatty acid-induced lipotoxicity: in a nutshell. FEBS Lett. 598, 1207–1214. doi: 10.1002/1873-3468.14808
Oksala, N. K. J., Ekmekçi, F. G., Özsoy, E., Kirankaya, Ş., Kokkola, T., and Emecen, G. (2014). Natural thermal adaptation increases heat shock protein levels and decreases oxidative stress. Redox Biol. 3, 25–28. doi: 10.1016/j.redox.2014.10.003
Olzmann, J. A. and Carvalho, P. (2019). Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155. doi: 10.1038/s41580-018-0085-z
Onoda, Y., Wright, I. J., Evans, J. R., Hikosaka, K., Kitajima, K., Niinemets, U., et al. (2017). Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 214, 1447–1463. doi: 10.1111/nph.2017.214.issue-4
Osman, M. E., Kasim, W. A., Omar, M. N., El-Daim, A., Bejai, A. I. S., and Meijer, J. (2013). Impact of bacterial priming on some stress tolerance mechanisms and growth of cold stressed wheat seedlings. Int. J. Plant Biol. 4, e8. doi: 10.4081/pb.2013.e8
Parajuli, S., Kannan, B., Karan, R., Sanahuja, G., Liu, H., Garcia-Ruiz, E., et al. (2020). Towards oilcane: engineering hyperaccumulation of triacylglycerol into sugarcane stems. GCB Bioenerg. 12, 476–490. doi: 10.1111/gcbb.12684
Park, S., Gidda, S. K., James, C. N., Horn, P. J., Khuu, N., Seay, D. C., et al. (2013). The α/β hydrolase CGI-58 and peroxisomal transport protein PXA1 coregulate lipid homeostasis and signaling in Arabidopsis. Plant Cell. 25, 1726–1739. doi: 10.1105/tpc.113.111898
Park, K., Quach, T., Clark, T. J., Kim, H., Zhang, T., Wang, M., et al. (2025). Development of vegetative oil sorghum: From lab-to-field. Plant Biotechnol. J. 23, 660–673. doi: 10.1111/pbi.14527
Paul, M. J. and Eastmond, P. J. (2020). Turning sugars into oil —making photosynthesis blind to feedback inhibition. J. Exp. Bot. 71, 2216–2218. doi: 10.1093/jxb/erz504
Peng, Y., Lou, H., Tan, Z., Ouyang, Z., Zhang, Y., Lu, S., et al. (2024). Lipidomic and metabolomic analyses reveal changes of lipid and metabolite profiles in rapeseed during nitrogen deficiency. Plant Cell Physiol. 65, 904–915. doi: 10.1093/pcp/pcad128
Perlikowski, D., Lechowicz, K., Skirycz, A., Michaelis, A., Pawlowicz, I., and Kosmala, A. (2022). The role of triacylglycerol in the protection of cells against lipotoxicity under drought in Lolium multiforum/Festucarrundinacea introgression forms. Plant Cell Physiol. 63, 353–368. doi: 10.1093/pcp/pcac003
Polle, A. (2001). Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modelling. Computer simulations as a step towards flux analysis. Plant Physiol. 126, 445–462. doi: 10.1104/pp.126.1.445
Qamer, Z., Chaudhary, M. T., Du, X., Hinze, L., and Azhar, M. T. (2021). Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. @ in response to extreme abiotic conditions. J. Cotton Res. 4, 9. doi: 10.1186/s42397-021-00086-4
Rahman, M., Woo, J. H., Song, Y., Lee, S.-H., Hasan, M., Azad, Md., A. K., et al. (2022). Heat shock proteins and antioxidant genes involved in heat combined with drought stress responses in perennial ryegrass. Life (Basel) 12, 1426. doi: 10.3390/life12091426
Ratnayaka, H. H., Molin, W. T., and Sterling, T. M. (2003). Physiological and antioxidant responses of cotton and spurred anoda under interference and mild drought. J. Exp. Bot. 54, 2293–2305. doi: 10.1093/jxb/erg251
Sage, R. F., de Melo Peixoto, M., Friesen, P., and Deen, B. (2015). C4 bioenergy crops for cool climates, with special emphasis on perennial C4 grasses. J. Exp. Bot. 66, 4195–4212. doi: 10.1093/jxb/erv123
Sage, R. F., Kocacinar, F., and Kubien, D. S. (2011). “C4 photosynthesis and temperature,” in C4 photosynthesis and related CO2 concentrating mechanisms. Eds. Raghavendra, A. S. and Sage, R. F. (Springer, Dordrecht, The Netherlands), 161–195.
Sanjaya, T. P., Durrett, S. E., and Weise, C. B. (2011). Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnol. J. 9, 874–883. doi: 10.1111/j.1467-7652.2011.00599.x
Schneider, A., Häusler, R. E., Kolukisaoglu, Ü., Kunze, R., van der Graaff, E., Schwacke, R., et al. (2002). An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilization is abolished. Plant J. 32, 685–699. doi: 10.1046/j.1365-313X.2002.01460.x
Schwender, J., Hebbelmann, I., Heinzel, N., Hildebrandt, T., Rogers, A., and Naik, D. (2015). Quantitative multilevel analysis of central metabolism in developing oilseeds of oilseed rape during in vitro culture. Plant Physiol. 168, 828–848. doi: 10.1104/pp.15.00385
Selinski, J. and Scheibe, R. (2019). Malate valves: old shuttles with new perspectives. Plant Biol. 21, 21–30. doi: 10.1111/plb.2019.21.issue-S1
Shanklin, J. and Cahoon, E. B. (1998). Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 611–641. doi: 10.1146/annurev.arplant.49.1.611
Sharma, P., Lakra, N., Goyal, A., Ahlawat, Y., Zaid, A., and Siddique, K. H. M. (2023). Drought and heat stress mediated activation of lipid signaling in plants: a critical review. Front. Plant Sci. 14, 1216835. doi: 10.3389/fpls.2023.1216835
Shikanai, T. (2007). Cyclic electron transport around photosystem I: genetic approaches. Annu. Rev. Plant Biol. 58, 199–217. doi: 10.1146/annurev.arplant.58.091406.110525
Shiva, S., Samarakoon, T., Lowe, K. A., Roach, C., Vu, H. S., Colter, M., et al. (2020). Leaf lipid alterations in response to heat stress of Arabidopsis thaliana. Plants (Basel) 9, 845. doi: 10.3390/plants9070845
Singh, A., Prajapati, P., Vyas, S., Gaur, V. K., Sindhu, R., Binod, P., et al. (2023). A comprehensive review of feedstocks as sustainable substrates for next-generation biofuels. Bioenergy Res. 16, 105–122. doi: 10.1007/s12155-022-10440-2
Slocombe, S. P., Cornah, J., Pinfield-Wells, H., Soady, K., Zhang, Q., Gilday, A., et al. (2009). Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol. J. 7, 694–703. doi: 10.1111/j.1467-7652.2009.00435.x
Theodoulou, F. L. and Eastmond, P. J. (2012). Seed storage oil catabolism: a story of give and take. Curr. Opin. Plant Biol. 15, 322–328. doi: 10.1016/j.pbi.2012.03.017
Thomashow, M. F., Gilmour, S. J., Stockinger, E. J., Jaglo-Ottosen, K. R., and Zarka, D. G. (2001). Role of the Arabidopsis CBF transcriptional activators in cold acclimation. Physiol. Plant 112, 171–175. doi: 10.1034/j.1399-3054.2001.1120204.x
Tian, X., Fang, Y., Jin, Y., Yi, Z., Li, J., Du, A., et al. (2021). Ammonium detoxification mechanism of ammonium-tolerant Duckweed (Landoltia punctata) revealed by carbon and nitrogen metabolism under ammonium stress. Environ. Pollut. 277, 116834. doi: 10.1016/j.envpol.2021.116834
Troncoso-Ponce, M. A., Cao, X., Yang, Z., and Ohlrogge, J. B. (2013). Lipid turnover during senescence. Plant Sci. 205-206, 13–19. doi: 10.1016/j.plantsci.2013.01.004
Troncoso-Ponce, M. A., Kilaru, A., Cao, X., Durrett, T. P., Fan, J., Jensen, J. K., et al. (2011). Comparative deep transcriptional profiling of four developing oilseeds. Plant J. 68, 1014–1027. doi: 10.1111/j.1365-313X.2011.04751.x
Tsai, A. Y.-L. and Gazzarrini, S. (2014). Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: the emerging picture. Front. Plant Sci. 5, 119. doi: 10.3389/fpls.2014.00119
Ullmann, J. and Grimm, D. (2021). Algae and their potential for a future bioeconomy, landless food production, and the socio-economic impact of an algae industry. Org. Agric. 11, 261–267. doi: 10.1007/s13165-020-00337-9
Usher, S., Han, L., Haslam, R. P., Michaelson, L. V., Sturtevant, D., Aziz, M., et al. (2017). Tailoring seed oil composition in the real world: optimising omega-3 long chain polyunsaturated fatty acid accumulation in transgenic Camelina sativa. Sci. Rep. 7, 6570. doi: 10.1038/s41598-017-06838-0
Usher, S., Haslam, R. P., Ruiz-Lopez, N., Sayanova, O., and Napier, J. A. (2015). Field trial evaluation of the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Camelina sativa: making fish oil substitutes in plants. Metab. Eng. Commun. 9, 93–98. doi: 10.1016/j.meteno.2015.04.002
Vanhercke, T., Belide, S., Taylor, M. C., El Tahchy, A., Okada, S., Rolland, V., et al. (2018). Up-regulation of lipid biosynthesis increases the oil content in leaves of Sorghum bicolor. Plant Biotechnol. J. 17, 220–232. doi: 10.1111/pbi.12959
Vanhercke, T., Divi, U. K., El Tahchy, A., Liu, Q., Mitchell, M., Taylor, M. C., et al. (2017). Step changes in leaf oil accumulation via iterative metabolic engineering. Metab. Eng 39, 237–246. doi: 10.1016/j.ymben.2016.12.007
Vanhercke, T., Dyer, J. M., Mullen, R. T., Kilaru, A., Rahman, M., Petrie, J. R., et al. (2019). Metabolic engineering for enhanced oil in biomass. Prog. Lipid Res. 74, 103–129. doi: 10.1016/j.plipres.2019.02.002
Vanhercke, T., El Tahchy, A., Liu, Q., Zhou, X. R., Shrestha, P., Divi, U. K., et al. (2014). Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol. J. 12, 231–239. doi: 10.1111/pbi.2014.12.issue-2
Wang, D., Portis, A. R., Jr., Moose, S. P., and Long, S. P. (2008). Cool C4 photosynthesis: pyruvate Pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus×giganteus. Plant Physiol. 148, 557–567. doi: 10.1104/pp.108.120709
Wang, M., Ye, X., Bi, H., and Shen, Z. (2024a). Microalgae biofuels: illuminating the path to a sustainable future amidst challenges and opportunities. BMC Biotechnol. Biofuel. Bioprod. 17, 10. doi: 10.1186/s13068-024-02461-0
Wang, X. (2005). Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 139, 566–573. doi: 10.1104/pp.105.068809
Wang, X., Li, S., Pang, S., Liu, Q., and Song, Y. (2024b). Regulation of AreA on lipid biosynthesis under different nitrogen sources and C/N ratios in the model oleaginous fungus Mucor circinelloides. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids. 1869, 159537. doi: 10.1016/j.bbalip.2024.159537
Wang, Y., Gao, C., Wang, S., and He, X. (2020). Changes in photoinhibition and fatty acid composition in the thylakoid membrane of kidney bean leaves under low temperature and weak light stress. Acta Prataculturae Sinca. 29, 116–125.
Wang, Y., Mostafa, S., Zeng, W., and Jin, B. (2021). Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 22, 8568. doi: 10.3390/ijms22168568
Wei, X., Yang, Y., Yao, J., Han, J., Yan, M., Zhang, J., et al. (2022). Improve utilization of nitrate nitrogen through within-leaf nitrogen allocation trade-off in Leymus chinensis. Front. Plant Sci. 13, 870681. doi: 10.3389/fpls.2022.870681
Wheeler, M. C. G., Tronconi, M. A., Drincovich, M. F., Andreo, C. S., Flügge, U.-F., and Maurino, V. G. (2005). A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol. 139, 39–51. doi: 10.1104/pp.105.065953
Winichayakul, S., Beechy-Gradwell, Z., Muetzel, S., Molano, G., Crowther, T., Lewis, S., et al. (2020). In vitro gas production and rumen fermentation profile of fresh and ensiled genetically modified high-metabolizable energy ryegrass. J. Dairy Sci. 103, 2405–2418. doi: 10.3168/jds.2019-16781
Winichayakul, S., Macknight, R., Le Lievre, L., Beechey-Gradwell, Z., Lee, R., Cooney, L., et al. (2022). Insight into the regulatory networks underlying the high lipid perennial ryegrass growth under different irradiances. PloS One 17, e0275503. doi: 10.1371/journal.pone.0275503
Winichayakul, S., Scott, W. R., Roldan, M., Hatier, J. H. B., Livingston, S., Cookson, R., et al. (2013). In vivo packaging of triacylglycerols enhances Arabidopsis leaf biomass and energy density. Plant Physiol. 162, 626–639. doi: 10.1104/pp.113.216820
Xiao, C., Fang, Y., Wang, S., and He, K. (2023). The alleviation of ammonium toxicity in plants. J. Integr. Plant Biol. 65, 1362–1368. doi: 10.1111/jipb.13467
Xu, C. and Shanklin, J. (2016). Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu. Rev. Plant Biol. 67, 179–206. doi: 10.1146/annurev-arplant-043015-111641
Xu, X., Vanhercke, T., Shrestha, P., Luo, J., Akbar, S., Konik-Rose, C., et al. (2019). Upregulated lipid biosynthesis at the expense of starch production in potato (Solanum tuberosum) vegetative tissues via simultaneous downregulation of ADP-glucose pyrophosphorylase and sugar dependent 1 expressions. Front. Plant Sci. 10, 103389. doi: 10.3389/fpls.2019.01444
Yang, G.-L. (2022). Duckweed is a promising feedstock of biofuels: advantages and approaches. Int. J. Mol. Sci. 23, 15231. doi: 10.3390/ijms232315231
Yang, Y. and Benning, C. (2018). Functions of triacylglycerols during plant development and stress. Curr. Opin. Biotecnol. 49, 191–198. doi: 10.1016/j.copbio.2017.09.003
Yang, Y., Kong, Q., Lim, A. R. Q., Lu, S., Zhao, H., Guo, L., et al. (2022). Transcriptional regulation of oil biosynthesis in seed plants: Current understanding, applications, and perspectives. Plant Commun. 3, 100328. doi: 10.1016/j.xplc.2022.100328
Yang, Y., Munz, J., Cass, C., Zienkiewicz, A., Kong, Q., Ma, W., et al. (2015). Ectopic expression of WRINKLED1 affects fatty acid homeostasis in Brachypodium distachyon vegetative tissues. Plant Physiol. 169, 1836–1847. doi: 10.1104/pp.15.01236
Yin, L., Xu, J., Zhang, L., Liu, D., Zhang, C., Liu, T., et al. (2024). Altered fatty acid composition confers improved drought acclimation in maize. Plant Physiol. Biochem. 206, 108274. doi: 10.1016/j.plaphy.2023.108274
Yu, Z., Duan, X., Luo, L., Dai, S., Ding, Z., and Xia, G. (2020b). How plant hormones mediate salt stress responses. Trends Plant Sci. 25, 1117–1130. doi: 10.1016/j.tplants.2020.06.008
Yu, L., Fan, J., and Xu, C. (2019). Peroxisomal fatty acid β-oxidation negatively impact plant survival under salt stress. Plant Signal. Behav. 14, 1561121. doi: 10.1080/15592324.2018.1561121
Yu, L., Fan, J., Zhou, C., and Xu, C. (2020a). Chloroplast lipid biosynthesis is fine-tuned to thylakoid membrane remodeling during light acclimation. Plant Physiol. 185, 94–107. doi: 10.1093/plphys/kiaa013
Yurchenko, O., Kimberlin, A., Mehling, M., Koo, A. J., Chapman, K. D., Mullen, R. T., et al. (2018). Response of high leaf-oil Arabidopsis thaliana plant lines to biotic or abiotic stress. Plant Signal. Behav. 13, 1–5. doi: 10.1080/15592324.2018.1464361
Zale, J., Jung, J. H., Kim, J. Y., Pathak, B., Karan, R., Liu, H., et al. (2016). Metabolic engineering of sugarcane to accumulate energy-dense triacylglycerols in vegetative biomass. Plant Biotechnol. J. 14, 661–669. doi: 10.1111/pbi.2016.14.issue-2
Zeeman, S. C., Tiessen, A., Pilling, E., Kato, L., Donald, A. M., and Smith, A. M. (2002). Starch synthesis in Arabidopsis. Granule synthesis, composition, and structure. Plant Physiol. 129, 516–529. doi: 10.1104/pp.003756
Zhai, Z., Keereetaweep, J., Liu, H., Feil, R., Lunn, J. E., and Shanklin, J. (2018). Trehalose 6-phosphate positively regulates fatty acid synthesis by stabilizing WRINKLED1. Plant Cell. 30, 2616–2627. doi: 10.1105/tpc.18.00521
Zhai, Z., Liu, H., Xu, C., and Shanklin, J. (2017). Sugar potentiation of fatty acid and triacylglycerol accumulation. Plant Physiol. 175, 696–707. doi: 10.1104/pp.17.00828
Zhang, L., Ma, H., Chen, T., Pen, J., Yu, S., and Zhao, X. (2014). Morphological and physiological responses of cotton (Gossypium hirsutum L.) plants to salinity. PloS One 9, e112807. doi: 10.1371/journal.pone.0112807
Zhang, H., Wu, C., Wu, Q., Dai, J., and Song, Y. (2016). Metabolic flux analysis of lipid biosynthesis in the yeast Yarrowia lipolytica using 13C-labled glucose and gas chromatography-mass spectrometry. PloS One 11, e0159187. doi: 10.1371/journal.pone.0159187
Zheng, G., Tian, B., Zhang, F., Tao, F., and Li, W. (2011). Plant adaptation to frequent alterations between high and low temperatures: remodeling of membrane lipids and maintenance of unsaturation levels. Plant Cell Environ. 34, 1431–1442. doi: 10.1111/j.1365-3040.2011.02341.x
Zhou, X.-R., Bhandari, S., Johnson, B. S., Kotapati, H. K., Allen, D. K., Vanhercke, T., et al. (2020). Reorganization of acyl flux through the lipid metabolic network in oil-accumulating tobacco leaves. Plant Physiol. 182, 739–755. doi: 10.1104/pp.19.00667
Keywords: C/N partitioning, non-seed lipid storage, redox regulation, sustainable agriculture, bioenergy, abiotic stress
Citation: Winichayakul S and Roberts N (2025) Toward sustainable crops: integrating vegetative (non-seed) lipid storage, carbon-nitrogen dynamics, and redox regulation. Front. Plant Sci. 16:1589127. doi: 10.3389/fpls.2025.1589127
Received: 06 March 2025; Accepted: 14 May 2025;
Published: 03 June 2025.
Edited by:
Marianela Soledad Rodriguez, National Institute of Agricultural Technology (INTA), ArgentinaReviewed by:
Chinedu Charles Nwafor, University of Nebraska-Lincoln, United StatesPaula Pereira, Universidade Federal de Viçosa, Brazil
Copyright © 2025 Winichayakul and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Somrutai Winichayakul, c29tcnV0YWkud2luaWNoYXlha3VsQGFncmVzZWFyY2guY28ubno=
†ORCID: Somrutai Winichayakul, orcid.org/0000-0003-2721-5071
Nick Roberts, orcid.org/0000-0003-3862-1833
 Somrutai Winichayakul
Somrutai Winichayakul Nick Roberts
Nick Roberts