- 1Research Center of Agricultural Economy, College of Economics, Sichuan University of Science and Engineering, Yibin, China
- 2Key Laboratory of Alpine Vegetation Ecological Security in Tibet, Institute of Tibet Plateau Ecology, Tibet Agricultural and Animal Husbandry University, Nyingchi, China
Introduction: Selenium (Se)-fortified foods have demonstrated efficacy in augmenting dietary Se intake and ameliorating human Se nutritional status. To mitigate Se deficiency-related health risks in Tibetan populations, systematic biofortification trials targeting highland barley, the primary staple crop in Tibet, are imperative.
Methods: Highland barley was subjected to soil-applied selenate (SeVI) and selenite (SeIV) at seven rates (0, 5, 15, 25, 50, 75, and 100 g·ha-1), followed by comprehensive evaluation of Se transfer dynamics within the soil-crop-diet continuum across Tibet’s agricultural regions.
Results and discussion: Exogenous Se application significantly increased the Se content in highland barley grains (p < 0.05), with SeVI proving to be more effective than SeIV. Selenomethionine (SeMet) accounted for 78-85% of total Se species in grains, and SeIV applications yielding 1.7 times higher organic Se conversion rates compared to SeVI treatments. Se application not only elevated the total Se concentration but also concurrently increased the bioavailable Se fractions in the soil, thereby enhancing Se translocation within the plant. Dietary exposure assessment indicated that application rates of 75 g·ha-1 SeIV and 50 g·ha-1SeVI were optimal, as they satisfied the recommended daily intake (55 μg·day-1) for Tibetan adults while keeping soil Se below 3.0 mg·kg-1. This study demonstrated that soil application of 75 g·ha-1 SeIV or 50 g·ha-1 SeIV achieved effective biofortification without ecological risk, providing a sustainable solution for mitigating Se deficiency in Tibetan agroecosystems.
1 Introduction
As an indispensable micronutrient, selenium (Se) plays pivotal biochemical roles through its incorporation into selenoproteins, including glutathione peroxidase (GPx), iodothyronine deiodinases (DIO), and thioredoxin reductase (TXNRD) systems, which mediate redox homeostasis and thyroid hormone metabolism (Wang et al., 2020b). Epidemiological evidence has established Se deficiency as a risk factor for >40 human pathologies, including Keshan cardiomyopathy and Kashin-Beck osteoarthropathy (Navarro-Alarcon and Cabrera-Vique, 2008). Dietary biofortification through Se supplementation, while preserving cultural dietary patterns, represents the most viable approach to achieve optimal serum Se concentrations (58~150 μg·L-1) across populations, as demonstrated by longitudinal cohort studies (Lemire et al., 2011; Krishnakumar et al., 2018).
Soil application of exogenous Se is an agronomy practice aimed at enhancing both the total and bioavailable Se content in the soil, thereby increasing the Se concentration in the edible parts of crops (Broadley et al., 2010).The Finnish National Se Program (1984~2001) pioneered large-scale Se fertilization, establishing the first government-mandated Se agronomic fortification protocol. Systematic SeIV fertilization increased population-level dietary Se intake from 0.04 mg·day-1·10 MJ-1 in 1985 to 0.08 mg·day-1·10 MJ-1 in 2001, corresponding with elevated plasma Se concentrations (0.89~1.4 µmol·L-1, p < 0.001) (Alfthan et al., 2015). Se bioavailability demonstrates species-specific responses modulated by: crop Se assimilation genetics, Se speciation and application rates. In maize (Zea mays L.) cultivation trials on Loess Plateau in China, SeVI application (1 kg·ha-1) enhanced grain Se content from 0.12 ± 0.03 to 0.33 ± 0.05 µg·kg-1 dry weight (DW) across two phenological cycles (Wang et al., 2013a). Optimal Se dosage (50 g·ha-1) maximized rice yield outputs (8163.5 kg·ha-1; +11.5%), while supra-optimal applications (>75 g·ha-1) induced yield penalties through selenocysteine misincorporation in proteins (Zhang et al., 2014). Contrastingly, buckwheat (Fagopyrum esculentum Moench) exhibited 2.3-fold Se accumulation in seeds under selenite treatment (25 g·ha-1), yet demonstrated phytotoxicity symptoms at application rates exceeding 50 g·ha-1 (Jiang et al., 2015). A potential explanation for these observations is that Se application can alter the transformation and proportions of Se fractions in the soil. Controlled pot experiments demonstrated Se supplementation (10~100 mg·kg-1) increased bioavailable fractions: water-soluble Se (SOL-Se, +120%), exchangeable Se (EXC-Se, +85%), while reducing residual forms (RES-Se, -40%) through enhanced microbial selenate reductase activity (Ali et al., 2017). Soil Se application has been widely utilized to enhance Se concentrations in staple crops like rice (Zhang et al., 2014; Wang et al., 2013b) and wheat (Ali et al., 2017), there remains a notable gap in the research on Se biofortification across different crops in Se-deficient regions.
An estimated 1.1 billion people globally inhabit Se-deficient regions (soil Se content < 0.125 mg·kg-1), predisposing populations to compromised selenoprotein biosynthesis (Paikaray, 2016; Jones et al., 2017). 68% of county-level administrative units was in Se-deficient zones (0.08~0.15 mg·kg-1), where 53% of adults exhibit suboptimal dietary Se intake associated with elevated hypothyroidism risk in China (Dinh et al., 2018). The Tibetan Autonomous Region demonstrates particularly high Se-deficiency comorbidities, with Kashin-Beck disease (KBD) prevalence reaching 12.8% in agricultural counties versus 2.1% nationally, and Keshan cardiomyopathy incidence 6-fold higher than lowland regions (Winkel et al., 2012; Zhang et al., 2018). While government interventions (2006~2015) including Se-fortified salt distribution (coverage: 58% target population) temporarily reduced KBD incidence by 37%, cost-benefit analysis revealed unsustainable implementation costs and logistical barriers in remote highland villages (elevation: > 3800 m ASL) (Li et al., 2012, 2003; Yu et al., 2019). Highland barley (Hordeum vulgare L. var. nudum Hook. f.) dominates Tibetan agriculture, occupying 65000 km2 (82% of arable land) at elevations 3500~4500 m, providing 72% of daily caloric intake and 58% of dietary protein for rural communities (Guo et al., 2017). Our previous research has shown that highland barley is the primary source of dietary Se for rural Tibetan residents, contributing 34.2% of their Se intake (Zhou et al., 2022). Meanwhile, pre-biofortification analyses revealed inadequate Se contribution of highland barley (10.3% of RDA) with mean grain concentrations 0.08 mg·kg-1, resulting in 43 μg·day-1 intake deficit (Zhou et al., 2022). Given the wide-spread consumption of highland barley in Tibet, the production of Se-enriched highland barley grain could be a promising strategy to improve dietary Se intake among Tibetan populations.
Overall, this study systematically addresses three objectives: (i) quantify Se allocation patterns in highland barley tissues under SeVI and SeIV fertilization; (ii) evaluate Se fractionation dynamics in rhizosphere soils; and (iii) establish safe and optimal Se application thresholds through dietary exposure assessment.
2 Materials and methods
2.1 Overview of the experimental site
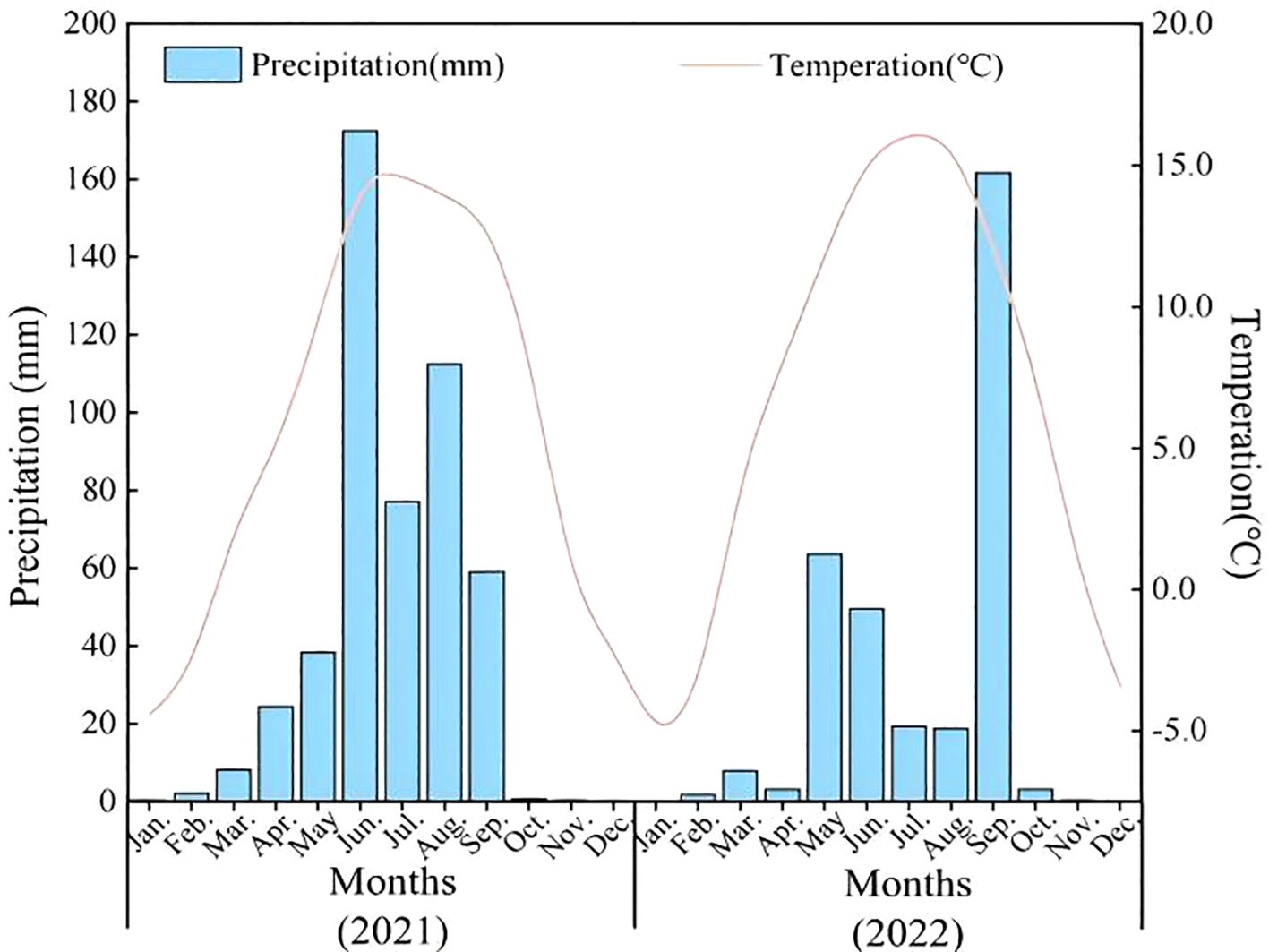
Field trials were implemented during consecutive growing seasons (2021~2022) at 29°53’N 91°07’E in Bairang Village, Linzhou County, Tibet Autonomous Region (elevation: 3890 m ASL), representing a typical high-altitude agricultural system. The site exhibits a typical plateau monsoon climate with mean annual precipitation of 491 mm (80% occurring June-September), mean temperature of 5.2 °C, and diurnal thermal amplitude exceeding 15 °C. Annual solar radiation reaches 7892 MJ·m-2, with 3000 sunshine hours and 122 frost-free days annually. USDA classified the soil as sandy clay loam (clay: 15%, sand: 64%, silt: 21%) with pH 7.2. Key properties included: organic matter content 20.08 g·kg-1, total N content 1.16 g·kg-1, total P content 0.84 g·kg-1, total S 0.28 g·kg-1, total Se content 0.05 mg·kg-1, CaCO3 content 18.79 g·kg-1, CEC 7 cmol(+)/kg; Fe 8.4 mg·kg-1; Mn 9.5 mg·kg-1; Zn 0.66 mg·kg-1; Cu 0.78 mg·kg-1; B 0.47 mg·kg-1. Total soil Se content (0.05 ± 0.01 mg·kg-1, <0.125 mg·kg-1) classified the site as Se-deficient per the Chinese Soil Se Threshold System (Tan et al., 2002). The meteorological conditions of the experimental site from 2021 to 2022 were shown in Figure 1.
2.2 Field trial setup
The highland barley (Hordeum vulgare L. var. nudum Hook. f.) cultivar ‘Zangqing 2000’ was cultivated during the 2021–2022 growing seasons (sowing: 18 Apr 2021/25 Apr 2022; harvesting: 28 Aug 2021/5 Sep 2022) under standard agronomic practices. Sodium selenite (Na2SeO3, CAS 10102-18-8, SeIV) and sodium selenate (Na2SeO4, CAS 13410-01-0, SeVI) were procured from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). A complete randomized block design was implemented with seven Se rates (0, 5, 15, 25, 50, 75, 100 g·ha-1) applied as SeIV or SeVI, generating 14 experimental treatments (n=56 plots). Treatments were randomly allocated to experimental units (4 m×5 m plots) in quadruplicate using R v4.2.1 randomization algorithms. Individual plots were separated by 1 m wide buffer zones filled with inert quartz sand to prevent cross-contamination, maintaining a 2:1 plot-to-buffer area ratio. Based on the specified Se application rates and plot size, the required amounts of SeIV or SeVI were carefully calculated and weighed using an analytical balance. The compounds were then fully dissolved in 2 liters of deionized water to prepare a homogeneous solution, which was uniformly applied to the soil surface of each plot using a manual sprayer after sowing. Standard cultivation and management practices were followed throughout the experiment.
2.3 Sample collection and preparation
At the maturity stage, highland barley plants within a 1 m2 area in the center of each experimental plot were collected, along with corresponding soil samples from the 0–20 cm layer. The plant samples were thoroughly washed with distilled water and subsequently divided into three parts: roots, straws, and grains. After surface moisture was allowed to drain, the samples were placed in an oven at 65 °C and dried to a constant weight, which generally required approximately 72 hours. The dry weight of each part was meticulously recorded. The dried plant samples were then ground into a fine powder using a stainless steel pulverizer and sieved through a 0.5 mm nylon mesh for further analysis. The soil samples were naturally air-dried, and following the removal of impurities such as plant residues and gravel, they were sieved in preparation for subsequent analysis.
2.4 Chemical analysis
2.4.1 Determination of Se concentration in soil and highland barley plants
0.250~0.251 g of plant samples were digested with 8 mL of HNO3. The procedure was as follows: 80 °C for 1.5 h, 120 °C for 1.5 h, 150 °C for 3 h, and 160 °C for 0.5 ~ 1.5 h. Soil samples were digested with a 3:2 (v/v) mixture of HNO3 and HClO4, and the remaining acid digestion steps were the same as those for plant samples. The concentration of Se in the digested solution were measured by Inductively coupled plasma mass spectrometry (ICP-MS, iCAP Q, Thermo Scientic USA.). A wheat grain standard sample (GBW08503c, Academy of State Administration of Grain, SAAG) and a blank were simultaneously digested with the test samples for the QA/QC protocol. The average recovery rate of Se was 85% ~ 105% (Wang et al., 2021).
2.4.2 Determination of Se speciation in highland barley grain
0.40 g of barley grain samples was hydrolyzed by adding 5 mL of 8 mg·mL-1 protease XIV (Sigma Chemical Co., St. Louis, MO, USA). The mixture was shaken at 125 rpm for 24 h at 37 °C. Following centrifugation at 12000 rpm for 15 min, the mixture was filtered through a 0.22 µm cellulose nitrate filters and analyzed using high performance liquid chromatography-ultraviolet treatment-hydride generation-atomic fluorescence spectrometry (HPLC-UV-HG-AFS). Then, Se speciation in barley grain was analyzed by the HG-AFS detection system. The HPLC system was equipped with a Hamilton PRP-X100 (internal diameter=4.1 mm, length=250 mm, particle size=10 µm; Hamilton, Switzerland). The Se standards were Se-methyl-selenocysteine (MeSeCys) (Sigma-Aldrich, St. Louis, MO, USA), selenocystine (SeCys2) (Sigma-Aldrich, St. Louis, MO, USA), seleno-methionine (SeMet) (Sigma-Aldrich, St. Louis, MI, USA), selenite (SeIV), and selenate (SeVI). To evaluate the effect of protease XIV enzymatic hydrolysis on the extraction of Se speciation in barley grain, the total Se concentration in the supernatant was determined after centrifugation (Wang et al., 2020a). The extraction rate was the ratio of the total Se in the supernatant to the total Se in sample.
2.4.3 Sequential extraction of soil Se fractions
(i) Extraction of soluble Se (SOL-Se): 10 mL 0.25 M KCl was added to 1.0 g soil in a 100 mL polypropylene centrifuge tube. Then, the tube was shaken at 200 rpm at 25°C for 1 h. The mixture was centrifuged for 10 min at 4000 rpm, and filtered through a 0.45 μm filter. The remaining precipitate was used for the next step of extraction. The same centrifugation and filtration steps were conducted after each of the following extraction procedure. (ii) Extraction of exchangeable and carbonate-bound Se (EXC-Se): 10 mL 0.7 M KH2PO4 (pH = 5.0) was added to the tubes and shaken at 200 rpm for 4 h at 25°C. (iii) Extraction of Se bound to iron and manganese oxides (FMO-Se): 10 mL 2.5 M HCl was added to the remaining soil. The capped vials were then heated in a water bath at 90°C for 50 min. The centrifuge vials were also shaken intermittently. (iv) Extraction of organic matter bound Se (OM-Se): 8 mL 5% K2S2O8 and 2 mL concentrated HNO3 were added to each remaining soil sample, and the vial was heated for 3 h in a water bath at 95°C. The tube was intermittently shaken from time to time. (v) Extraction of residual Se (RES-Se): The soil residuals were transferred into a Teflon crucible with 8 mL concentrated HNO3 and 2 mL concentrated HClO4. The crucibles were covered and heated at 170°C in a sand bath until the soil appeared white or gray. After acid digestion, the solution was transferred to a 25 mL volumetric flask with deionized water (Wang et al., 2012).
2.5 Data analysis
2.5.1 Parameters characterizing Se behavior in soil
Soil Se redistribution index (Uts), relative binding intensity of Se with soil (IR), and the mobility factor (MF) were used to represent the impact of Se application on soil Se and Se fractions (Ali et al., 2017). The calculation formula of Uts, IR, MF were as follows:
In which, Fi represents the proportion of a specific Se fraction in the Se-treated soil, Fci represents the proportion of a specific Se component in the control soil, i represents the extraction step, F1 represents the proportion of SOL-Se in the soil, and F2 represents the proportion of EXC-Se in the soil.
2.5.2 Estimated dietary Se intake analysis
The estimate of dietary Se intake (DI, µg·adult-1·day-1) was calculated according to this equation: DI=Chighland barley × Whighland barley, where DI (µg·adult-1·day-1) is daily Se intake estimation per adult, Chighland barley (µg·g-1) is the Se concentration in highland barley grain in this study, Whighland barley(g·day-1·adult-1)is the mean daily consumption of highland barley per adult (0.4 kg·adult-1·day-1) (Tashi et al., 2013; Di et al., 2023).
2.5.3 Statistical analysis
All experimental data were expressed as the mean ± standard deviation (mean ± SD) from four replicates. The effects of exogenous Se types, Se application rates, and years on Se concentration and content in different parts of highland barley (grain, straw, and root) were analyzed using a multi-factor analysis of variance (ANOVA). When significant differences were found (P < 0.05), pairwise comparisons among different Se application treatments were performed using Tukey’s LSD test. Statistical analyses were conducted using SPSS version 23.0 (IBM Corp., Armonk, NY, USA), with a significance level of P<0.05. Graphs were generated using Origin 2024b (OriginLab, Northampton, MA, USA) and R version 4.2.3 (R Development Core Team, 2023).
3 Results
3.1 Se concentration and transformation in highland barley
Soil application of exogenous Se significantly elevated Se concentrations in all barley tissues (p < 0.01), with both SeIV and SeVI treatments showing dose-dependent accumulation patterns (Supplementary Figure S1, Figure 2). Dose-response analysis revealed significant linear correlations between application rates and tissue Se accumulation (R2 = 0.83~0.94, p < 0.001), particularly evident in grains (Figure 2). In the 2021 trial, SeIV application (5~100 g·ha-1) induced 2.44~122.73-fold, 2.75~71.02-fold, and 1.57~8.34-fold increases in grain, straw, and root Se concentrations, respectively, compared to non-treated controls (Supplementary Figure S1). The 2022 trial demonstrated greater bioaccumulation with SeVI, showing 4.43~235.27-fold increases in grains, 3.35~113.09-fold increases in straw, and 1.57~20.66-fold increases in roots (Supplementary Figure S1). Obviously, the overall efficacy of soil-applied SeVI was superior to SeIV. Optimal differentiation occurred at moderate application levels (25 g·ha-1), where SeVI produced 4.98-fold and 3.87-fold greater Se accumulation in grains and straw compared to SeIV (p < 0.01; Supplementary Figure S1, Figure 2). At maximum application (100 g·ha-1), root Se concentrations under SeVI treatment remained 2.45 fold higher than SeIV equivalents, suggesting partial rhizospheric conversion of SeVI to immobile forms (p < 0.01; Supplementary Figure S1, Figure 2).
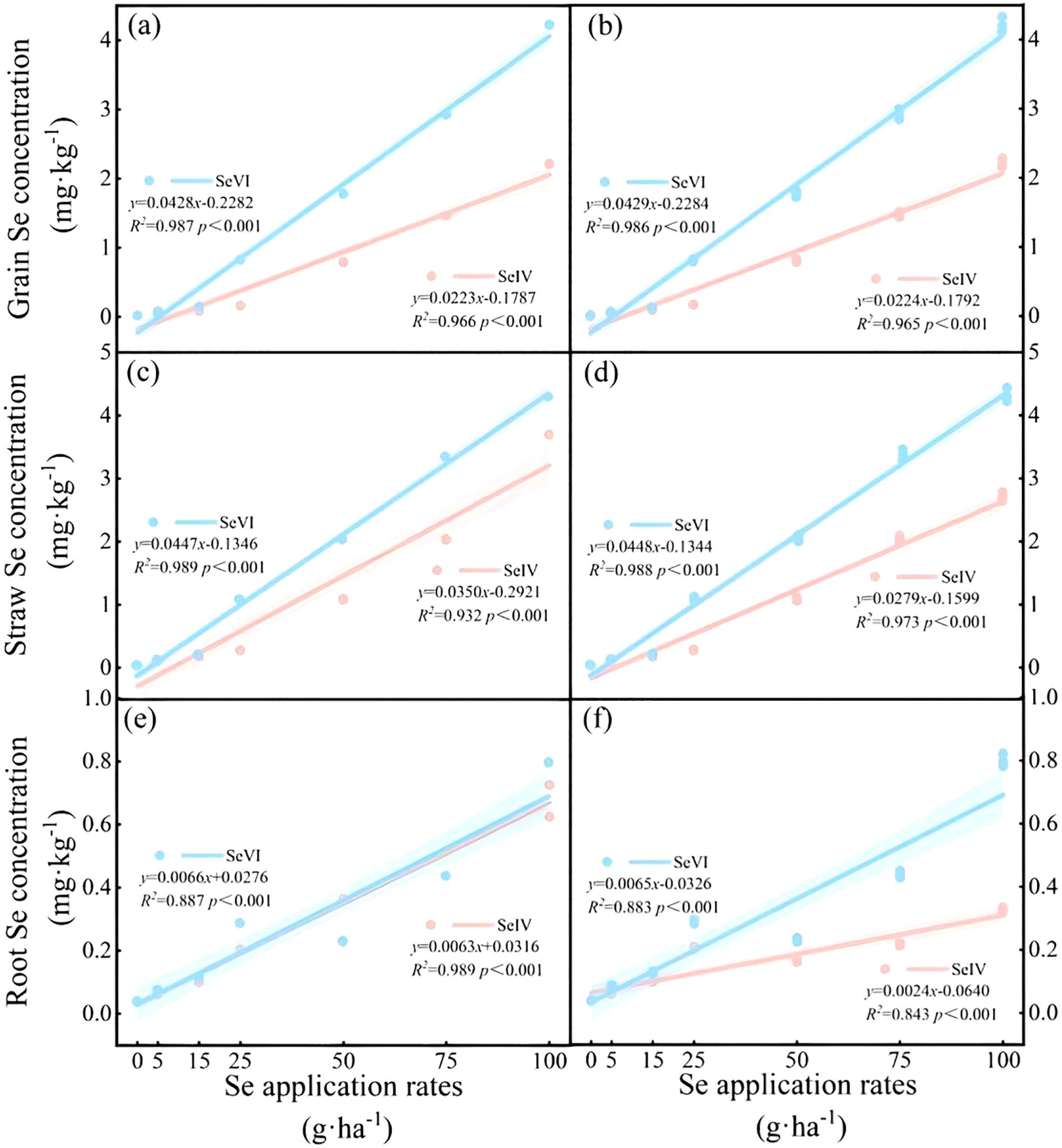
Figure 2. Relationships between total Se concentrations in grains, straws and roots of highland barley and Se application rates (a, c, e ~ Year 2021; b, d, f ~ Year 2022).
Organic Se constituted 78~85% of total Se species in highland barley grains across treatments (Figure 3). A dose-dependent decline in organic Se proportion (80.35%→71.84%) and a speciation hierarchy (SeMet > SeCys2 > MeSeCys > selenite) was observed under SeVI treatments with corresponding SeMet reduction (65.77%→57.14%). An increasing trends of total organic Se (92.33%→95.98%) and SeMet proportion (60.09%→62.22%) under SeIV application was also observed. The application of SeIV produced a 1.2-fold higher SeCys2 accumulation under SeIV versus SeVI treatment (p < 0.01), while no significant difference was observed in the proportion of MeSeCys between two treatments (p < 0.05).
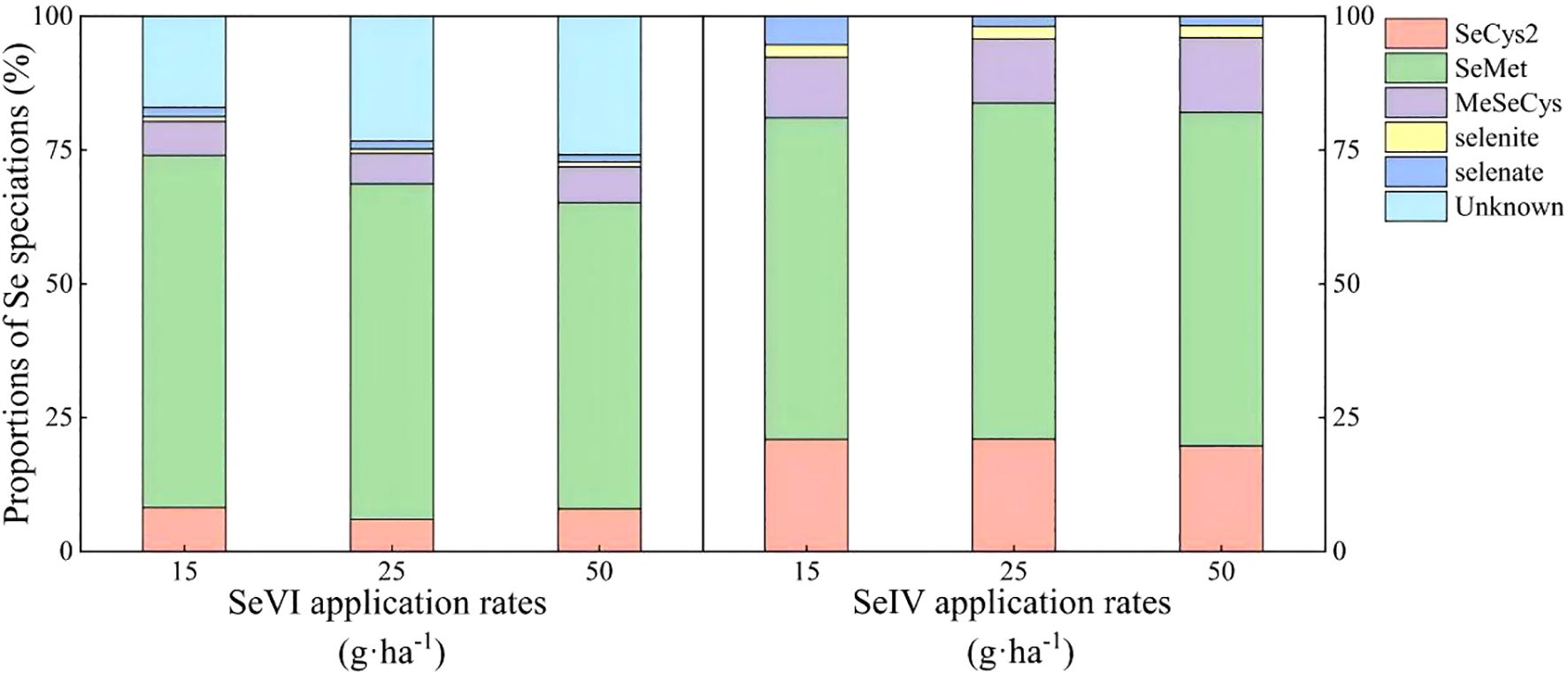
Figure 3. Se speciation distribution in highland barley grains under soil application of exogenous Se (2021).
3.2 Total Se concentration and Se fractions in soil
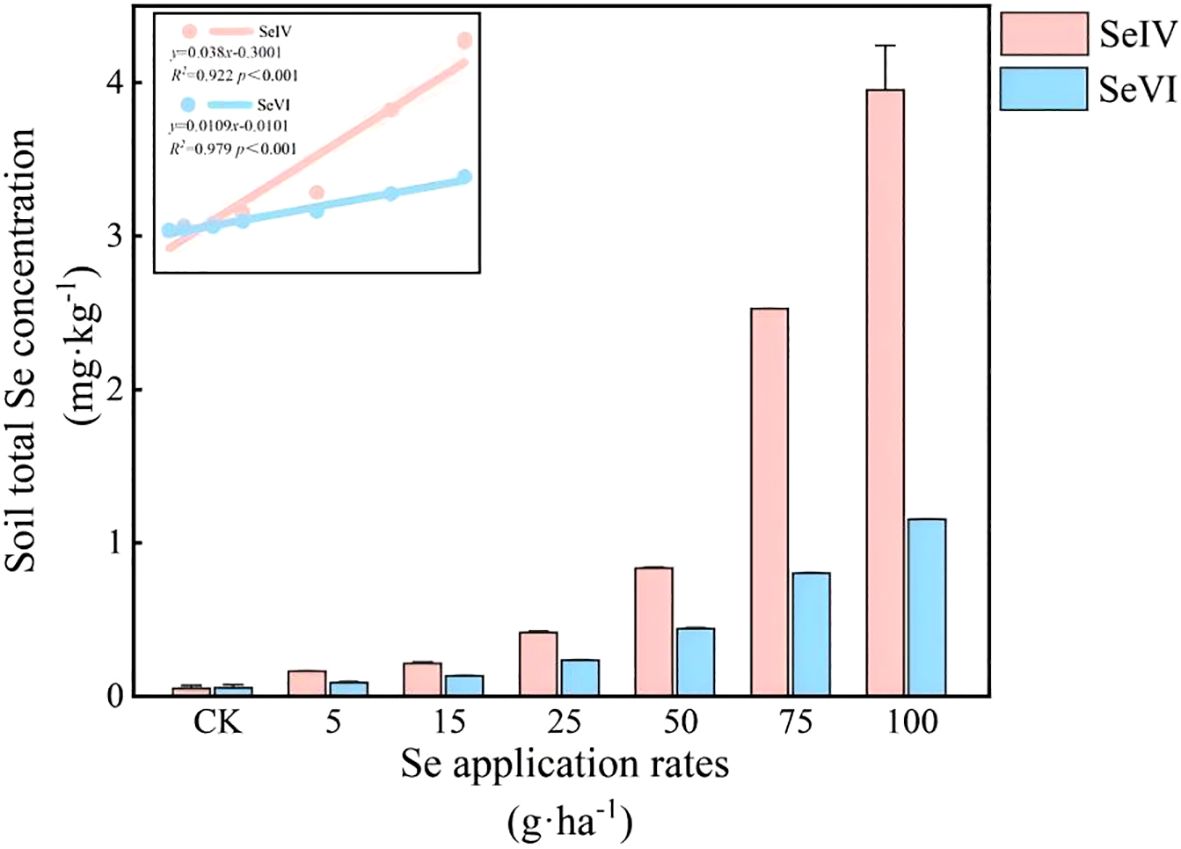
SeIV and SeVI soil amendments (5~100 g·ha-1) increased total soil Se (STSe) by factors of 3.16~76.80 and 1.65~15.47 respectively relative to control plots (p < 0.001), demonstrating dose-dependent accumulation patterns (Figure 4). Linear regression analysis revealed strong positive correlations between application rates and STSe for both exogenous Se species (R2 = 0.96~0.98, p < 0.001). SeIV-amended soils retained 42~68% more STSe than SeVI-treated counterparts at equivalent application rates (p < 0.001), indicating stronger soil binding capacity of SeIV (Figure 4). Exogenous Se application enhanced bioavailable fractions (SOL+EXC-Se) from 26.07% in controls to 37.03~65.45% (p < 0.001), with SeVI showing 12~18% greater bioavailability than SeIV at equivalent doses.
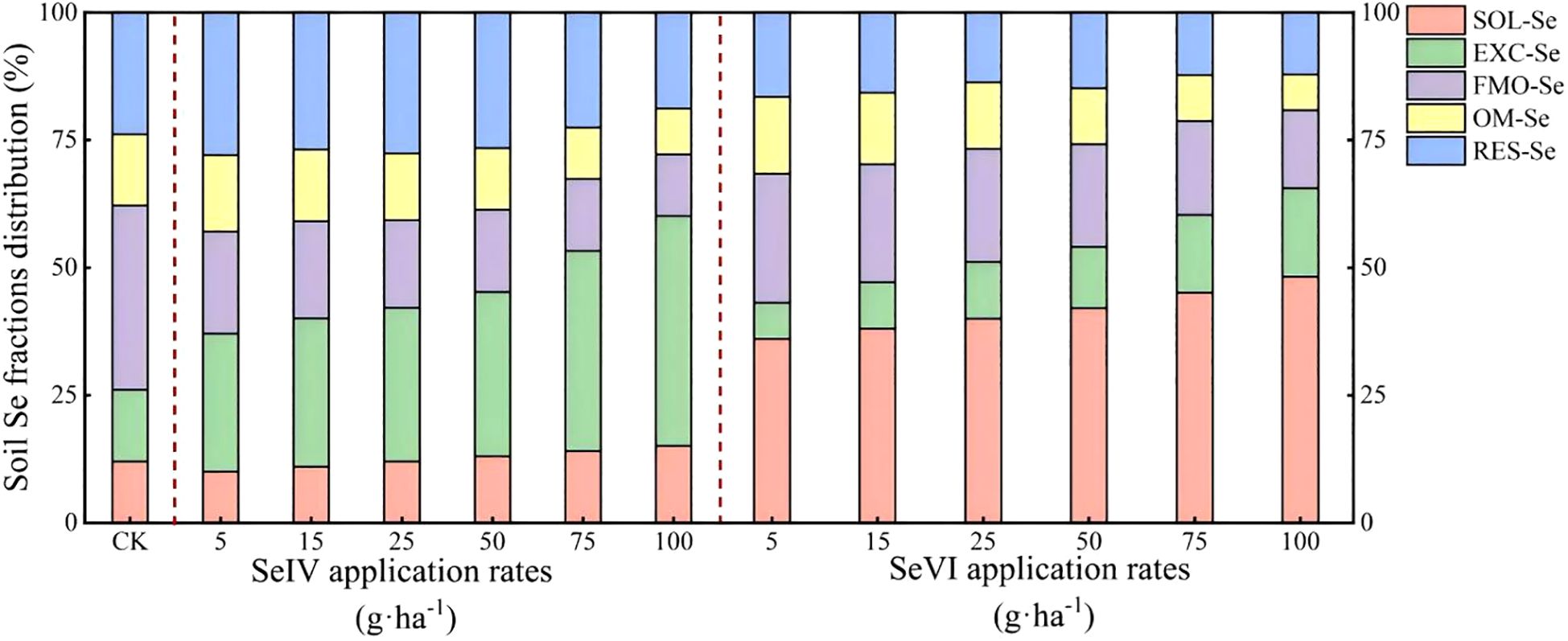
SeIV application induced a marked reduction in FMO-Se proportions (36.06% to 15.08%) concurrent with EXC-Se increases from 27.01% to 45.05% (p < 0.01). The resultant speciation hierarchy under SeIV treatment established EXC-Se (45.05%) as the dominant fraction, followed by RES-Se (23.82%), FMO-Se (15.08%), OM-Se (14.04%), and SOL-Se (12.03%) (Figure 5). Following SeVI application, the proportion of SOL-Se markedly increased from 36.03% to 48.15%, wheras FMO-Se and OM-Se notably decreased with increasing Se application rates. Compared to the control (23.82%), SeVI application reduced the proportion of RES-Se from 16.59% to 11.62%. Following SeVI application, the distribution of Se fractions in the soil followed the order: SOL-Se > FMO-Se > RES-Se > OM-Se > EXC-Se (Figure 5).
3.3 Correlation analysis between Se concentrations of highland barley and parameters characterizing Se behavior in soil
Soil Se dynamics revealed significant treatment effects, with mobility factors (MF) increasing from 0.26 (CK) to 0.58 (100 g·ha-1SeVI) (Supplementary Table S1), reflecting enhanced bioavailability of Se-amended soil. Strong correlations emerged between grain Se and SOL-Se (r=0.82, P<0.01), EXC-Se (r = 0.76, p < 0.01), and MF (r = 0.69, p < 0.01) (Figure 6). SOL-Se explained 14.7% of grain Se variance (%IncMSE = 14.7), surpassing STSe (9.3%), indicating the critical role of dissolved Se in root uptake processes. Root Se showed independence from STSe (p = 0.12) but strong MF dependence (%IncMSE = 18.3), and the machine learning feature importance analysis (Figure 7) identified SOL-Se and FMO-Se as primary drivers (combined %IncMSE > 30%).
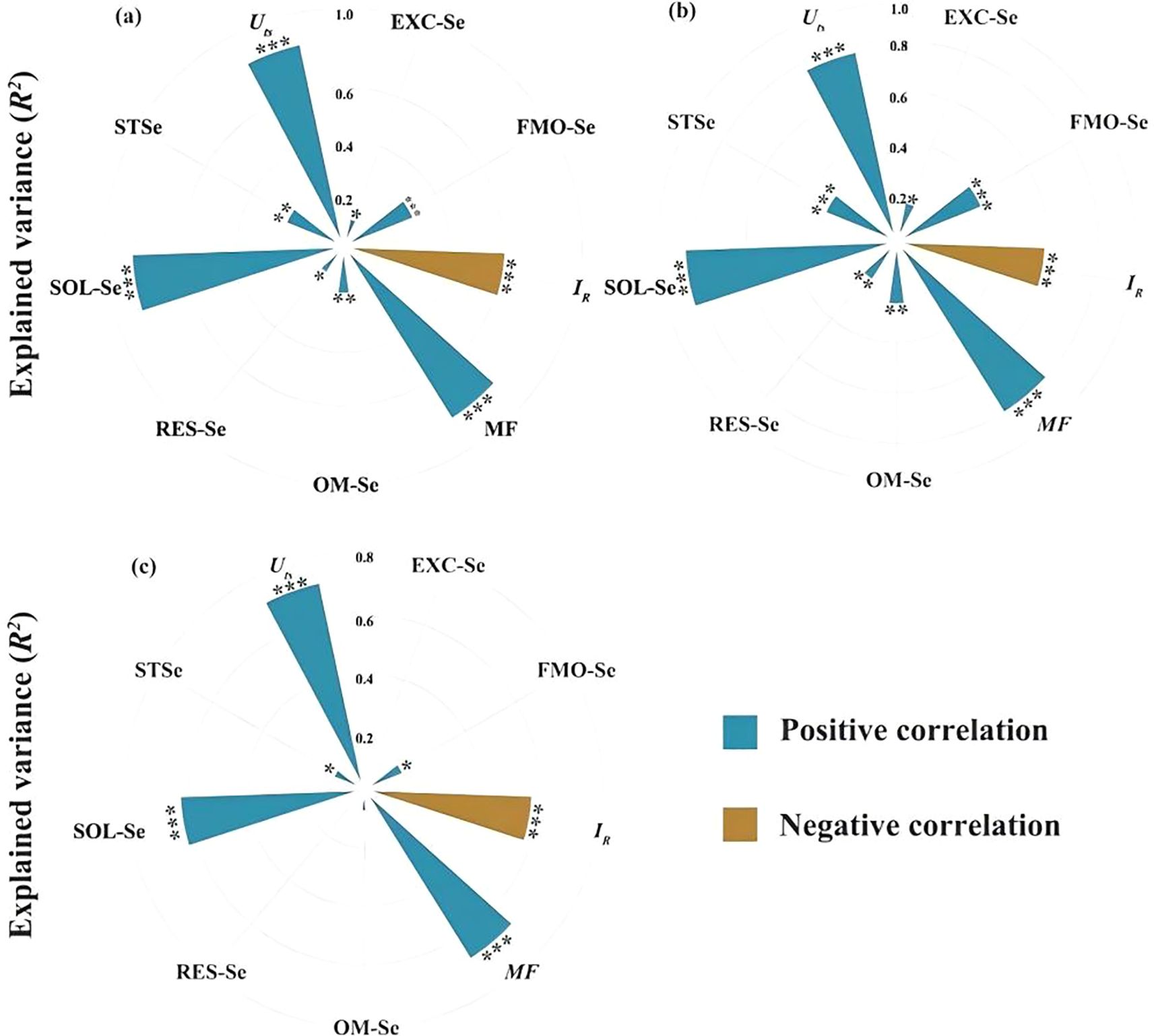
Figure 6. Relationships between Se concentrations in grains (a), straws (b), roots (c) of highland barley and total Se concentration in soil, Se fractions, Se redistribution index (Uts), relative binding intensity of Se with soil (IR), mobility factor (MF). Blue indicates positive correlation, while brown indicates negative correlation.*** indicates p < 0.001,** indicates p < 0.01; * indicates p < 0.05.
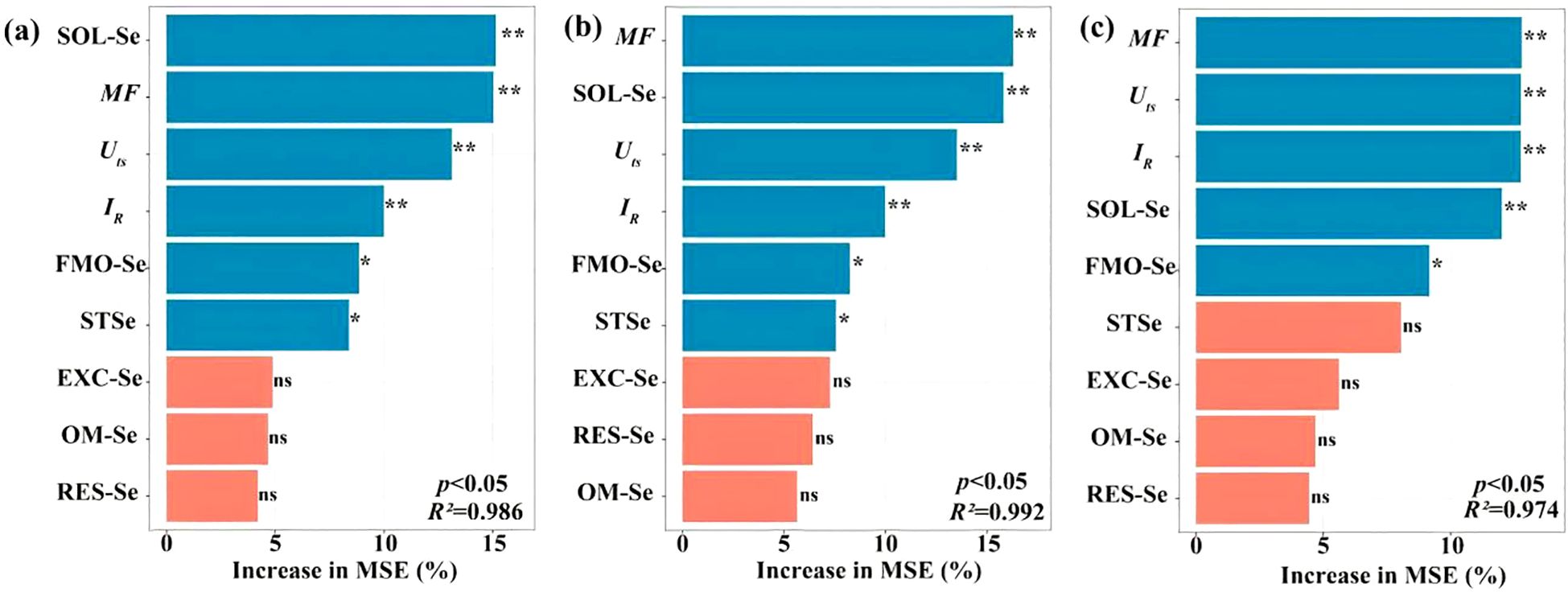
Figure 7. Relative importance of explanatory variables affecting Se concentration in grains (a), straws (b), and roots (c) of highland barley. ** indicates p < 0.01; * indicates p < 0.05; ns indicates p > 0.05.
3.4 Assessment of dietary Se intakes from Se-enriched highland barley grain
According to the Chinese Nutrition Society’s recommended dietary Se intake for adults (60 ~ 400 µg·adult-1·day-1) (Chinese Nutrition Society, 2013), and in light of our previous survey results showing that the current dietary Se intake among adult residents in the study area is only 17 µg·adult-1·day-1 (Zhou et al., 2022), there is a significant deficit of 43 µg·adult-1·day-1 in dietary Se intake for adult residents in agricultural areas of Tibet. As illustrated in Figure 8, the soil application of 75 and 100 g·ha-1 SeIV and 50, 75, and 100 g·ha-1 SeVI resulted in Se-enriched highland barley grains capable of effectively bridging the dietary Se intake gap of 43 µg·adult-1·day-1. The Se-enriched highland barley grains produced under these treatments could elevate the total dietary Se intake to the recommended level of 60 µg·adult-1·day-1, while ensuring that the dietary Se intake remained below the upper threshold of 400 µg·adult-1·day-1 (Table 1). Notably, the dietary Se intake derived from highland barley treated with 75 and 100 g·ha-1 of SeIV accounted for 107.52% and 147.53% of the recommended intake value, respectively, while constituting only 14.64% and 22.13% of the upper intake threshold (Table 1). For highland barley treated with 50, 75, and 100 g·ha-1 SeVI, the dietary Se intake corresponded to 118.38%, 194.63%, and 281.19% of the recommended intake value, while accounting for only 17.75%, 29.20%, and 42.18% of the upper intake thresholds, respectively (Table 1). Therefore, we conclude that the soil application of 50 g·ha-1 SeVI and 75 g·ha-1 SeIV represents the optimal treatments for producing Se-enriched highland barley in Tibet, as these levels ensure adequate Se enrichment without exceeding safe dietary limits.
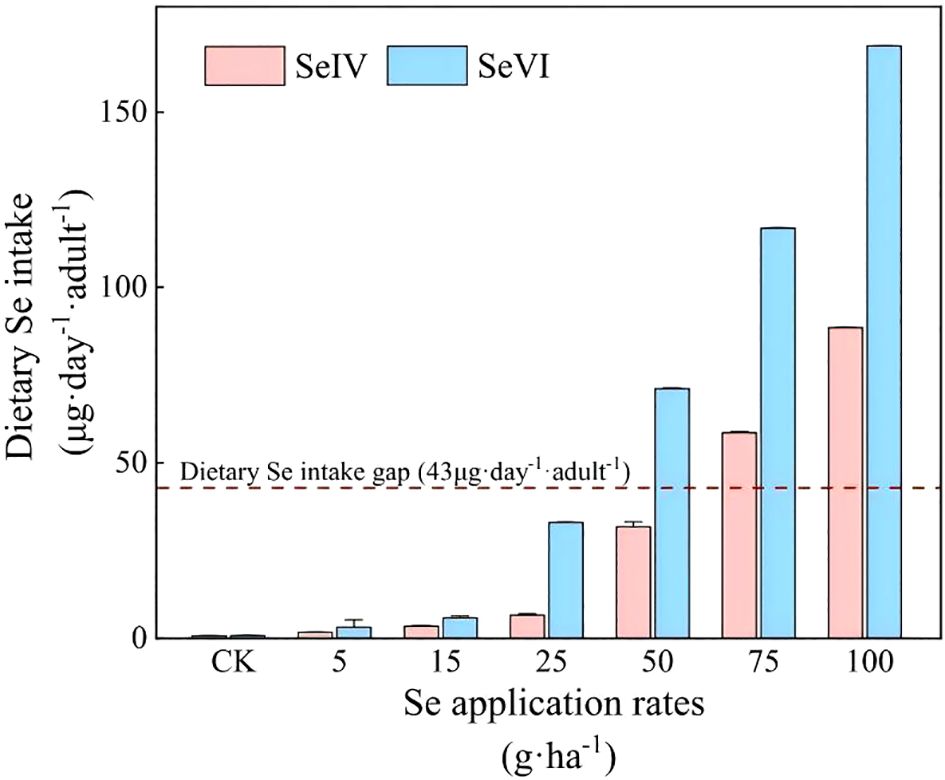
Figure 8. Contribution of Se-enriched highland barley to dietary Se intake of residents (The dashed line shows the gap of dietary Se intake: 43 µg·adult-1·day-1).
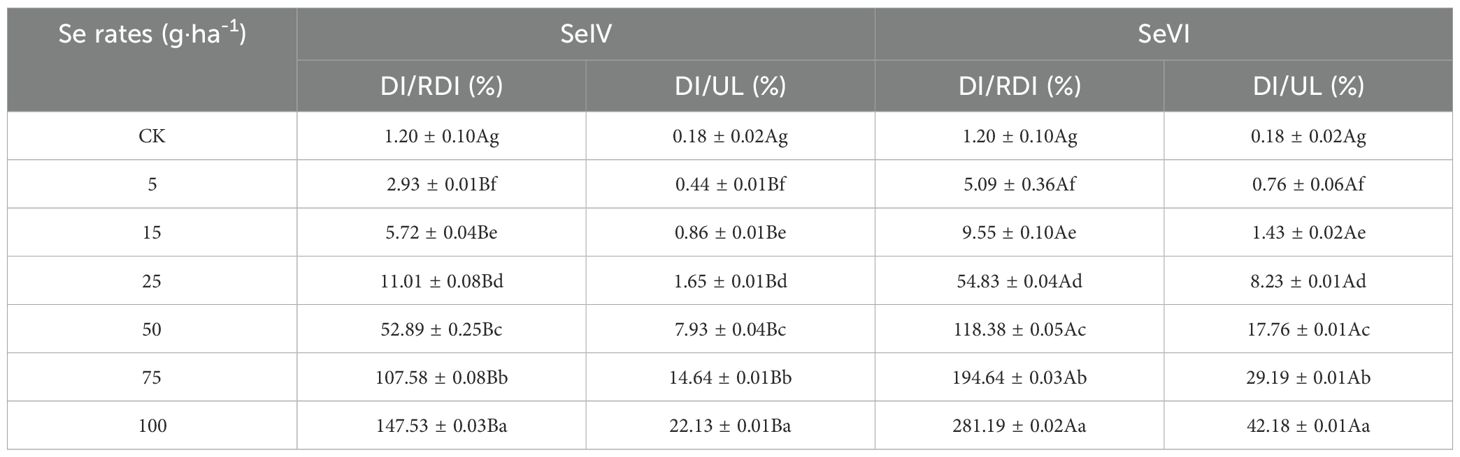
Table 1. Proportion of dietary Se intake of residents (DI) to the recommended amount (RDI) and upper intake threshold (UL).
4 Discussion
A strong linear dose-response relationship (R² = 0.83~0.94, p < 0.001) was observed between Se application rates and tissue-specific accumulation patterns in highland barley (Figure 2), validating soil-applied Se as a reliable biofortification strategy for Tibetan plateau agroecosystems. These results corroborate established Se biofortification mechanisms in wheat (R² = 0.79~0.91) and rice, confirming conserved Se assimilation pathways in Poaceae species (Ramkissoon et al., 2019; Radawiec et al., 2021; Lessa et al., 2019). Our results further showed that SeIV demonstrated 89% greater grain enrichment efficiency than SeVI (p < 0.001), attributable to its rapid conversion to SeMet in rhizospheric redox conditions (Liu et al., 2021). At maximum application (100 g·ha-1), SeIV-applied soils produced 1.9 fold higher grain Se concentrations than SeVI equivalents (95% CI: 1.82~2.01; Supplementary Figure S1), consistent with phytoavailability patterns in alkaline soils (Liu et al., 2021). The difference mechanism between the efficient enrichment of SeIV in barley grains (68% organic Se) and the wheat system might stem from the synergistic effect of the high pH value (7.2) and calcium carbonate content (18.79 g·kg-1) in the soil of the Qinghai-Tibet Plateau. These physicochemical properties enhance the conversion efficiency of SeIV to the organic form by influencing the adsorption-desorption equilibrium of SeIV.
Field trials with wheat demonstrated 590% and 260% Se accumulation enhancement under SeVI and SeIV treatments respectively at 14 g·ha-1, highlighting species-dependent assimilation efficiency (Ros et al., 2016). This divergence originates from differential Se speciation: SeVI primarily exists as mobile SeO42- anions, while SeIV forms surface-adsorbed SeO32- complexes with iron oxides (Poblaciones et al., 2024). SeVI utilizes sulfate transporters (Sultr1;1 and Sultr1;2) for xylem loading (Wang et al., 2022), achieving 82% translocation efficiency to aerial tissues versus 37% for SeIV, as quantified by stable isotope tracing (Sors et al., 2005; Poblaciones et al., 2024). SeIV undergoes rapid enzymatic reduction to Se0 via glutathione reductase (GR), subsequently incorporating into SeCys2 and SeMet through cysteine synthase pathways, with 68% retention in root tissues as quantified by μ-XANES analysis (Li et al., 2008; Liu et al., 2021).
This study demonstrated that both the application rate and the form of exogenous Se significantly influenced the content and speciation of organic Se in highland barley grains. Notably, organic Se fractions exhibited a dose-dependent decline with increasing SeVI application, inversely correlating with SeIV-treated systems (Figure 3). These contrasted with SeVI-fertilized wheat systems where organic Se increased 18~32% with application rates up to 50 g·ha-1, attributable to enhanced xylem transport efficiency (Zhang et al., 2018). Meanwhile, In an experimental study by Gong et al. (2018), SeIV-amended soils induced sequential depletion of gluten-bound Se (23~15%) and SeMet (65~48%) in developing caryopses, concomitant with SeCys2 accumulation (8~22%) during grain filling stages. The reasons for the differences may be related to crop types and the specificity of genotypes. Wheat is a moderately Se-accumulating crop, and its sulfur metabolism pathway has a strong ability to integrate Se; while highland barley may have a lower absorption and transformation efficiency of SeVI due to genotype limitations (such as lower activity of sulfur transport proteins), resulting in a decrease in organic Se content with increasing application rate. In wheat grains, glutenin may preferentially bind to SeMet, while in highland barley, due to structural differences in glutenin or fewer Se binding sites, Se is more likely to exist in a free state, such as SeCys2 (Poblaciones et al., 2024). This study also revealed that, following the application of equal concentrations of SeIV and SeVI to the soil, the content of organic Se speciations in highland barley grains was significantly higher in the SeIV treatment compared to the SeVI treatment. Lavu et al. (2012) demonstrated that soil application of SeIV resulted in 79% of organic Se speciation in Allium tuberosum, contrasting with 45% observed under SeVI treatment. Isomolar application demonstrated 89% greater grain Se biofortification efficiency under SeIV versus SeVI treatment (p < 0.001), with SeMet/SeCys2 ratios diverging significantly. Synchrotron-based μ-XANES analysis confirmed 68 ± 5% selenite(IV) conversion to SeMet/SeCys2 in rhizospheric microzones within 24 h, mediated by glutathione reductase (GR) and cysteine synthase complexes (Nothstein et al., 2016). Our findings corroborate these mechanisms, revealing that SeIV amendment significantly enhanced SeCys2 biosynthesis, with subsequent phloem-mediated allocation to highland barley grains.
Our findings demonstrate that SeIV and SeVI soil applications differentially modified total Se content (STSe) and speciation hierarchy, resulting in distinct bioavailability patterns in highland barley cultivation systems (p < 0.001). SeIV application (5~100 g·ha-1) induced 3.16-76.80-fold STSe enhancement, exhibiting significantly stronger soil retention than SeVI (1.65~15.47-fold), due to phosphate-mediated ligand exchange in alkaline soils. However, plant uptake and accumulation of Se depended more on the abundance of available Se than on STSe (Wang et al., 2012; Qin et al., 2012). Machine learning feature importance analysis (%IncMSE) revealed bioavailable Se (SOL-Se) accounted for 14.7% of grain accumulation variance, surpassing STSe (9.3%)(Figure 7), consistent with phytoavailability models in cereal crops (Wang et al., 2013b). In this study, while STSe exhibited a positive correlation with the Se concentration in grains and straws, the relative importance ranked lower (Figures 6, 7). Furthermore, Se concentration in both grains and straws were all positively correlated with the content of SOL-Se (Figures 6, 7). This suggests that available Se in the soil was more conducive to plant uptake and accumulation of Se by plants compared to STSe. Untreated controls exhibited 83.7% of total Se sequestered in stable oxidized forms (FMO-Se: 58.2 ± 3.1%; OM-Se: 25.5 ± 2.4%) (Figure 5), aligning with Se speciation patterns in alkaline soils reported by Chen et al. (2010). SeIV application induced EXC-Se dominance (42.7~58.3%), reflecting phosphate-mediated ligand exchange, while SeVI treatment enhanced SOL-Se proportions (36.1~48.2%) through competitive anion adsorption (Figure 5). The treatments generated 2.1~3.8-fold increases in bioavailable Se (EXC+SOL-Se), confirming enhanced mobility (MF=0.58) through redox-driven speciation shifts, as documented in paddy systems (Kamei-Ishikawa et al., 2007; Su and Suarez, 2000). SeVI-amended soils exhibited 15.8~28.4% higher available Se than SeIV-treated counterparts (10.5~18.6%, P<0.01), attributable to weaker iron oxide adsorption of SeO42- vs SeO32- (Peng et al., 2017; Li et al., 2015; Longchamp et al., 2015). SeVI application induced dose-dependent RES-Se accumulation (R²= 0.82, p < 0.001), with RES-Se proportions increasing from 13.2% to 28.4% across 5~100 g·ha-1 SeVI treatments (Figure 5). This aligns with Chen et al. (2010) who documented 13~22% RES-Se enrichment in paddy soils under SeVI treatment, particularly noting 18.7% higher RES-Se in rhizosphere versus bulk soil (p < 0.05). Parallel studies in Nicotiana tabacum systems demonstrated 15~28% RES-Se accumulation under SeIV treatment (50~200 g·ha-1) (Fan et al., 2015). The observed increase in RES-Se content could potentially be attributed to the incorporation of Se into the soil lattice (Lu et al., 2005) or its adsorption via electrostatic interactions with layered double hydroxides or anionic clays (Rovira et al., 2007). Meanwhile, this study also revealed that the strong correlation between the application amounts of SeVI and RES-Se was highly consistent with the adsorption mechanism reported in rice system studies (Fan et al., 2014). This consistency may be attributed to the high cation exchange capacity (28.4 cmol·kg-1) and free iron content (12.7 g·kg-1) of the tested soil.
Epidemiological studies demonstrate plasma selenium levels of 70~106 µg·L-1 correlate with 58~63% reduction in prostate and lung cancer incidence through enhanced glutathione peroxidase activity (Ullah et al., 2019). Se deficiency can lead to a range of health issues, including growth retardation, thyroid dysfunction, and infertility (Yin et al., 2019). Chronic Se overdose (>400 µg·day-1) triggers selenosis characterized by hair loss, dermatitis, and, in extreme cases, fatalities (Fairweather-Tait et al., 2011). Therefore, maintaining an appropriate daily Se intake is essential for health. In the control treatment of this study, the dietary Se intake provided by highland barley grains accounted for only 1.2% of the recommended value of 60 µg·adult-1·day-1, which was insufficient to meet the nutritional needs of the local Tibetan adults. However, following soil application of exogenous Se, highland barley grains enriched with low Se application rate (<50 g·ha-1) were insufficient to provide adequate dietary Se (Figure 8). Optimal biofortification achieved at 75 g·ha-1 SeIV and 50 g·ha-1 SeVI, filling the dietary Se intake gap of 43 µg·adult-1·day-1 for local residents, without exceeding the upper threshold of dietary Se intake (400 µg·adult-1·day-1) (Table 1).
5 Conclusion
Exogenous Se application through soil amendment significantly enhanced Se accumulation in all highland barley tissues. SeVI demonstrated superior translocation efficiency across all application rates (5~100 g·ha-1), achieving 1.7~2.5 times higher tissue Se concentrations than SeIV (p < 0.05). Conversely, SeIV treatments yielded higher organic Se conversion rates, with SeMet constituting 60~62% of speciated Se in grains. Soil Se pools showed dose-dependent enrichment, both STSe and bioavailable Se fractions (SOL+EXC-Se) rose after Se soil application. The distribution and behavior of available Se fractions in soil had a substantial impact on the Se concentration in highland barley. Based on the assessment of dietary Se intake, soil application with 75 g·ha-1 of SeIV or 50 g·ha-1 of SeVI on highland barley was proved to be an effective and safe measure to fill the dietary Se intake gap of local Tibetan adults in rural agricultural areas in Tibet.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CZ: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. FD: Data curation, Formal Analysis, Investigation, Visualization, Writing – review & editing. JW: Data curation, Formal Analysis, Investigation, Software, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the open funding of National Engineering and Technology Research Center for Development & Utilization of Phosphate Resources[grand number:NECP2025-011]; and 2024 Second Batch of Talent Introduction Project of Sichuan University of Science and Engineering[grand number: 2024RC108].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1589810/full#supplementary-material
References
Alfthan, G., Eurola, M., Ekholm, P., Venäläinen, E. R., Root, T., Korkalainen, K., et al. (2015). Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 31, 142–147. doi: 10.1016/j.jtemb.2014.04.009
Ali, F., Peng, Q., Wang, D., Cui, Z. W., Huang, J., Fu, D. D., et al. (2017). Effects of selenite and selenate application on distribution and transformation of selenium fractions in soil and its bioavailability for wheat (Triticum aestivum L.). Environ. Sci. pollut. Res. 24, 8315–8325. doi: 10.1007/s11356-017-8512-9
Broadley, M. R., Alcock, J., Alford, J., Cartwright, P., Foot, I., Fairweather-Tait, S. J., et al. (2010). Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 332, 5–18. doi: 10.1007/s11104-009-0234-4
Chen, Q. X., Shi, W. M., and Wang, X. C. (2010). Selenium speciation and distribution characteristics in the rhizosphere soil of rice (Oryza sativa L.) seedlings. Commun. Soil Sci. Plant Anal. 41, 1411–1425. doi: 10.1080/00103624.2010.482164
Di, X. R., Qin, X., Zhao, L. J., Liang, X. F., Xu, Y. M., Sun, Y. B., et al. (2023). Selenium distribution, translocation and speciation in wheat (Triticum aestivum L.) after foliar spraying selenite and selenate. Food Chem. 400, 134077. doi: 10.1016/j.foodchem.2022.134077
Dinh, Q. T., Cui, Z. W., Huang, J., Tran, T. A. T., Wang, D., Yang, W. X., et al. (2018). Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 112, 294–309. doi: 10.1016/j.envint.2017.12.035
Fairweather-Tait, S. J., Bao, Y., Broadley, M. R., Collings, R., Ford, D., Hesketh, J. E., et al. (2011). Selenium in human health and disease. Antioxid. Redox Signal. 14, 1337–1383. doi: 10.1089/ars.2010.3275
Fan, J., Wang, R., Hu, H. Q., Huo, G., Fu, Q. L., and Zhu, J. (2015). Transformation and bioavailability of selenate and selenite added to a Nicotiana tabacum L. planting soil. Commun. Soil Sci. Plant Anal. 46, 1362–1375. doi: 10.1080/00103624.2015.1033544
Fan, Q., Yamaguchi, N., Tanaka, M., Tsukada, H., and Takahashi, Y. (2014). Relationship between the adsorption species of cesium and radiocesium interception potential in soils and minerals: an EXAFS study. J. Environ. Radioact. 138, 92–100. doi: 10.1016/j.jenvrad.2014.08.009
Gong, R. Y., Ai, C. Y., Zhang, B. J., and Cheng, X. L. (2018). Effect of selenite on organic selenium speciation and selenium bioaccessibility in rice grains of two Se-enriched rice cultivars. Food Chem. 264, 443–448. doi: 10.1016/j.foodchem.2018.05.066
Guo, Y., Li, H., Yang, L., Guo, M., Wei, B., Li, Y., et al. (2017). Environmental selenium distribution characteristics and its relationship with the onset of kyphosis in the Yarlung Zangbo River basin. Chin. J. Endem. Dis. 36, 494–497. (in Chinese).
Jiang, Y., Zeng, Z. H., Bu, Y., Ren, C. Z., Li, J. Z., Han, J. J., et al. (2015). Effects of selenium fertilizer on grain yield, Se uptake and distribution in common buckwheat (Fagopyrum esculentum Moench). Plant Soil Environ. 61, 371–377. doi: 10.17221/284/2015-PSE
Jones, G. D., Droz, B., Greve, P., Gottschalk, P., Poffet, D., McGrath, S. P., et al. (2017). Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. U.S.A. 114, 2848–2853. doi: 10.1073/pnas.1611576114
Kamei-Ishikawa, N., Tagami, K., and Uchida, S. (2007). Sorption kinetics of selenium on humic acid. J. Radioanal. Nucl. Chem. 274, 555–561. doi: 10.1007/s10967-006-6951-8
Krishnakumar, S., Iyer, S., Sinkar, P., and Sengupta, C. (2018). Selenium levels in whole blood – The borderline low analysis. Clin. Chim. Acta 487, 309–310. doi: 10.1016/j.cca.2018.10.023
Lavu, R. V. S., Du Laing, G., Van De Wiele, T., Pratti, V. L., Willekens, K., Vandecasteele, B., et al. (2012). Fertilizing soil with selenium fertilizers: Impact on concentration, speciation, and bioaccessibility of selenium in leek (Allium ampeloprasum). J. Agric. Food Chem. 60, 10930–10935. doi: 10.1021/jf302931z
Lemire, M., Fillion, M., Frenette, B., Passos, C. J. S., Guimarães, J. R. D., Barbosa, F., et al. (2011). Selenium from dietary sources and motor functions in the Brazilian Amazon. Neurotoxicology 32, 944–953. doi: 10.1016/j.neuro.2011.04.005
Lessa, J. H. L., Araujo, A. M., Ferreira, L. A., Silva Júnior, E. C., Oliveira, C., Corguinha, A. P. B., et al. (2019). Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant Soil 444, 331–342. doi: 10.1007/s11104-019-04275-8
Li, Q., Li, J., Zha, X., Ding, Z., and Ran, L. (2003). Strategies and measures for the prevention and control of osteoarthropathy in the Qinghai-Tibetan Plateau. Chin. J. Public Health 11, 87. (in Chinese).
Li, Z., Man, N., Wang, S. S., Liang, D. L., and Liu, J. J. (2015). Selenite adsorption and desorption in main Chinese soils with their characteristics and physicochemical properties. J. Soils Sediments 15, 1150–1158. doi: 10.1007/s11368-015-1085-7
Li, H. F., McGrath, S. P., and Zhao, F. J. (2008). Selenium uptake, translocation, and speciation in wheat supplied with selenate or selenite. New Phytol. 178, 92–102. doi: 10.1111/j.1469-8137.2007.02343.x
Li, S., Xiao, T., and Zheng, B. (2012). Medical geology of arsenic, selenium and thallium in China. Sci. Total Environ. 421-422, 31–40. doi: 10.1016/j.scitotenv.2011.02.040
Liu, Y., Huang, S., Jiang, Z., Wang, Y., and Zhang, Z. (2021). Selenium biofortification modulates plant growth, microelement and heavy metal concentrations, selenium uptake, and accumulation in black-grained wheat. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.748523
Longchamp, M., Castrec-Rouelle, M., Biron, P., and Bariac, T. (2015). Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem. 182, 128–135. doi: 10.1016/j.foodchem.2015.02.137
Lu, A. X., Zhang, S. Z., and Shan, X. Q. (2005). Time effect on the fractionation of heavy metals in soils. Geoderma 125, 225–234. doi: 10.1016/j.geoderma.2004.08.002
Navarro-Alarcon, M. and Cabrera-Vique, C. (2008). Selenium in food and the human body: A review. Sci. Total Environ. 400, 115–141. doi: 10.1016/j.scitotenv.2008.06.024
Nothstein, A. K., Eiche, E., Riemann, M., Nick, P., Winkel, L. H. E., Göttlicher, J., et al. (2016). Tracking Se assimilation and speciation through the rice plant-Nutrient competition, toxicity and distribution. PloS One 11, e0152081. doi: 10.1371/journal.pone.0152081
Paikaray, S. (2016). Origin, mobilization and distribution of selenium in a soil/water/air system: A global perspective with special reference to the Indian scenario. Clean 44, 474–487. doi: 10.1002/clen.201300454
Peng, Q., Wang, M. K., Cui, Z. W., Huang, J., Chen, C. E., Guo, L., et al. (2017). Assessment of bioavailability of selenium in different plant-soil systems by diffusive gradients in thin-films (DGT). Environ. pollut. 225, 637–643. doi: 10.1016/j.envpol.2017.03.036
Poblaciones, M. J., García-Latorre, C., Velazquez, R., and Broadley, M. R. (2024). Effects of Selenate application on growth, nutrient bioaccumulation, and bioactive compounds in Broccoli (Brassica oleracea var. italica L.). Horticulturae 10, 808. doi: 10.3390/horticulturae10080808
Qin, H. B., Zhu, J. M., and Su, H. (2012). Selenium fractions in organic matter from Se-rich soils and weathered stone coal in selenosis areas of China. Chemosphere 86, 626–633. doi: 10.1016/j.chemosphere.2011.10.055
Radawiec, A., Rutkowska, B., Tidaback, J. A., Gozdowski, D., Knapowski, T., and Szulc, W. (2021). The impact of selenium fertilization on the quality characteristics of spring wheat grain. Agronomy 11, 2100. doi: 10.3390/agronomy11112100
Ramkissoon, C., Degryse, F., Silva, R. C., Baird, R., Young, S. D., Bailey, E. H., et al. (2019). Improving the efficacy of selenium fertilizers for wheat biofortification. Sci. Rep. 9, 19552. doi: 10.1038/s41598-019-55914-0
Ros, G. H., Van Rotterdam, A. M. D., Bussink, D. W., and Bindraban, P. S. (2016). Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 404, 99–112. doi: 10.1007/s11104-016-2830-4
Rovira, M., Giménez, J., Martínez, M., Martínez-Lladó, X., Pablo, J., Martí, V., et al. (2007). Sorption of selenium(IV) and selenium(VI) onto natural iron oxides: Goethite and hematite. J. Hazard. Mater. 150, 279–284. doi: 10.1016/j.jhazmat.2007.04.098
Sors, T. G., Ellis, D. R., and Salt, D. E. (2005). Selenium uptake, translocation, assimilation, and metabolic fate in plants. Photosynth. Res. 86, 373–389. doi: 10.1007/s11120-005-5222-9
Su, C. M. and Suarez, D. L. (2000). Selenate and selenite sorption on iron oxides: An infrared and electrophoretic study. Soil Sci. Soc Am. J. 64, 101–111. doi: 10.2136/sssaj2000.641101x
Tan, J. A., Zhu, W. Y., Wang, W. Y., Li, R. B., Hou, S. F., Wang, D. C., et al. (2002). Selenium in soil and endemic diseases in China. Sci. Total Environ. 284, 227–235. doi: 10.1016/s0048-9697(01)00889-0
Tashi, N., Tang, Y., and Zeng, X. Q. (2013). “Food preparation from hulless barley in Tibet” in Advance in Barley Sciences, eds. Zhang, G., Li, C., and Liu, X.. (Dordrecht: Springer). doi: 10.1007/978-94-007-4682-4_13
Ullah, H., Liu, G. J., Yousaf, B., Ali, M. U., Irshad, S., Abbas, Q., et al. (2019). A comprehensive review on environmental transformation of selenium: Recent advances and research perspectives. Environ. Geochem. Health 41, 1003–1035. doi: 10.1007/s10653-018-0195-8
Wang, M., Ali, F., Wang, M., Dinh, Q. T., Zhou, F., Banuelos, G. S., et al. (2020a). Understanding boosting selenium accumulation in wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. pollut. Res. 27, 717–728. doi: 10.1007/s11356-019-06914-0
Wang, S., Liang, D., Wang, D., Wei, W., Fu, D. D., and Lin, Z. Q. (2012). Selenium fractionation and speciation in agricultural soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci. Total Environ. 427-428, 159–164. doi: 10.1016/j.scitotenv.2012.03.091
Wang, K., Wang, Y., Li, K., Wan, Y., Wang, Q., Zhuang, Z., et al. (2020b). Uptake, translocation, and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnol. 18, 103. doi: 10.1186/s12951-020-00659-6
Wang, J. W., Wang, Z. H., Mao, H., Zhao, H. B., and Huang, D. L. (2013a). Increasing Se concentration in maize grain with soil- or foliar-applied selenite on the Loess Plateau in China. Field Crops Res. 150, 83–90. doi: 10.1016/j.fcr.2013.06.010
Wang, Y. D., Wang, X., and Wong, Y. S. (2013b). Generation of selenium-enriched rice with enhanced grain yield, selenium content, and bioavailability through fertilisation with selenite. Food Chem. 141, 2385–2393. doi: 10.1016/j.foodchem.2013.05.095
Wang, Y., Xu, Y., Liang, X., Wang, L., Sun, Y., Huang, Q., et al. (2021). Soil application of manganese sulfate could reduce wheat Cd accumulation in Cd-contaminated soil by the modulation of the key parts and ionomics of wheat. Sci. Total Environ. 770, 145328. doi: 10.1016/j.scitotenv.2021.145328
Wang, M., Zhou, F., Cheng, N., Chen, P., Ma, Y., Zhai, H., et al. (2022). Soil and foliar selenium application: Impact on accumulation, speciation, and bioaccessibility of selenium in wheat (Triticum aestivum L.). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.988627
Winkel, L. H. E., Johnson, C. A., Lenz, M., Grundl, T., Leupin, O. X., Amini, M., et al. (2012). Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 46, 571–579. doi: 10.1021/es203434d
Yin, H. Q., Qi, Z. Y., Li, M. Q., Ahammed, G. J., Chu, X. Y., and Zhou, J. (2019). Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content, and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 169, 911–917. doi: 10.1016/j.ecoenv.2018.11.080
Yu, F. F., Qi, X., Shang, Y. N., Ping, Z. G., and Guo, X. (2019). Prevention and control strategies for children Kashin–Beck disease in China. Medicine 98, e17036. doi: 10.1097/MD.0000000000017036
Zhang, M., Tang, S. H., Huang, X., Zhang, F. B., Pang, Y. W., Huang, Q. Y., et al. (2014). Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 107, 39–45. doi: 10.1016/j.envexpbot.2014.05.005
Zhang, D., Zhang, N., Hou, Z. A., Lou, D. X., and Ye, J. (2018). Effects of different application methods of selenite and selenate on selenium distribution within wheat. J. Plant Nutr. 41, 2729–2740. doi: 10.1080/01904167.2018.1544254
Keywords: highland barley, Se spices, soil Se fraction, Se intake, biofortification
Citation: Zhou C, Duan F and Wang J (2025) Impact of soil application with selenite and selenate on ‘soil-highland barley-dietary’ system in Tibet. Front. Plant Sci. 16:1589810. doi: 10.3389/fpls.2025.1589810
Received: 08 March 2025; Accepted: 30 May 2025;
Published: 18 June 2025.
Edited by:
Anoop Kumar Srivastava, Central Citrus Research Institute (ICAR), IndiaCopyright © 2025 Zhou, Duan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenni Zhou, Y2hlbm5pMjAxOEAxMjYuY29t
 Chenni Zhou
Chenni Zhou Fei Duan
Fei Duan Jiangke Wang
Jiangke Wang