- 1College of Horticulture, Hebei Agricultural University, Baoding, Hebei, China
- 2Research Center of Chinese Jujube, Hebei Agricultural University, Baoding, Hebei, China
- 3College of Food Science and Technology, Hebei Agricultural University, Baoding, Hebei, China
The combination of headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) and sensory evaluation technique was employed to detect and analyze the volatile organic compounds (VOCs) in jujube flowers at different developmental stages across various varieties, and to compare them with other plant flowers known for their characteristic aromas. A total of 65 volatile compounds were identified in jujube flowers at different stages. The aroma fingerprint analysis revealed 24 distinct aromas, with 14 aromas increasing in intensity from the bud stage to full bloom. Varieties such as Fuxiang, Dongzao, and Xingguang exhibited significantly stronger aromas at the flowering stage compared to other varieties. Additionally, sensory evaluation indicated a preference for the fragrance of jujube flowers among men. This study offers new insights into the development of jujube flower-based spices, highlighting their considerable potential and laying a foundation for further research on plant flower spices.
Highlights
● The volatile fingerprints of jujube flowers in different periods were established by HS-GC-IMS.
● HS-GC-IMS combined with PCA can well differentiate the volatile differences of jujube flowers in different periods.
● Sensory evaluation showed men prefer preferred jujube aroma.
● Jujube flowers aroma has the potential to be developed into men ‘s perfume.
1 Introduction
Jujube (Ziziphus jujuba Mill.) belongs to the genus Ziziphus of the family Rhamnaceae (Gao et al., 2013). It is native to China and is cultivated in Asia, Europe and the Americas (Liu et al., 2020). China has the largest the world’s largest jujube cultivation area globally, with a history over 4,000 years (Hernández et al., 2015; Li et al., 2009; Wojdyło et al., 2016). Jujube fruit contains vitamins, carbohydrates, organic acids, cAMP, and proline, making it both a medicinal and edible treasure (Choi et al., 2011; Guo et al., 2015; Li et al., 2007; Liu et al., 2015). The jujube flower is the primary source of fragrance in the jujube tree, and it is highly esteemed for its pleasant aroma and abundance. It also provides valuable nectar, making it important to investigate the fragrance attributes of jujube flowers.
Generally, Jujube flowers bloom in May and can last until July. Jujube flowers are small and numerous, exhibiting a yellow-green color as a whole, with a uniquely warm and inviting fragrance.
Flavor is an important perceptual property of foods, and it that is used in assessing their nutrition-related properties and freshness (Shi et al., 2017). Floral scents are primarily derived from VOCs synthesized within plant organs and released into the air. Generally, Jujube flowers bloom in May and can last until July. Jujube flowers are small and numerous, exhibiting a yellow-green color as a whole, with a uniquely warm and inviting fragrance. The aroma of jujube flowers serves as a signal to attract honey bees, which collect nectar to produce jujube honey after its own transformation process (Ma W. et al., 2020). Jujube flowers contain many volatile compounds, including esters, alkanes, aldehydes, and ketones, which contribute to their pleasant aroma (Xia et al., 2020). Furthermore, these flowers contain essential nutritional components, including calcium (Ca), iron (Fe), magnesium (Mg), as well as bioactive compounds such as mannose and phenols, making them a unique blend of scent and nutrition (Du et al., 2024; Fahim et al., 2014).
Volatile compounds are crucial not only for the fragrance of plants but also for their ecological interactions and potential applications in flavor, fragrance, and health-related industries. As the cultivation area of jujube continues to expand, understanding the aromatic characteristics of jujube flowers becomes increasingly important. The fragrance of jujube flowers, known for its delicate, restrained, and enduring profile, has long been appreciated, particularly in East Asian cultures, where it aligns with traditional preferences. This subtle yet distinctive aroma holds promising potential for both commercial and cultural applications. While previous studies have primarily focused on fruit aroma (Feng et al., 2022), the volatile compounds in flowers, and their dynamic changes throughout development and in different varieties, have not been systematically explored. Moreover, there is a significant gap in research comparing the volatile components of jujube within other flowers.
The study of plant aroma compounds plays a crucial role in understanding plant sensory characteristics, with aroma being a key distinguishing feature of jujube. Jujube aroma consists of a complex mixture of volatile compounds produced through various biotransformations of sugars, amino acids and other metabolites (Wang et al., 2018; Zhu and Xiao, 2018). Due to its ease of operation, enhanced sensitivity, streamlined processing, and efficient analytical speed, gas chromatography-ion mobility spectometry (GC-IMS) has become a widely used method food aroma analysis (Wang et al., 2020). Different plant tissue exhibit unique flavor profile, with flowers often having the most pronounced aromas (Ma et al., 2023). GC-IMS is a novel technique for odors detection, with IMS separating ions based on their mobility under atmospheric pressure (Li et al., 2019). Combining IMS with other analytical instruments enhances its performance and improves the accuracy of results. In recent years, headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) has been widely applied to investigate volatile compounds in food science. For example (Feng et al., 2022), used GC-IMS and sensory evaluation to characterize the volatile organic compounds (VOCs) characteristics in Molixiang grapes from different regions. In another study (Leng et al., 2021), employed GC-IMS to analyze postharvest preservation of peach fruit, finding a significant increase in esters and alcohols levels with prolonged storage. Similarly (Yao et al., 2024), examined the volatile components and aroma profiles of Yijiangzi monofloral honey and its corresponding flowers by GC-IMS, discovering that most of the compounds in honey originated from flowers. PCA is a detection method based on multivariate statistics, which is used to reduce the dimension or transform multiple indicators into several comprehensive indicators for extracting features and revealing the relationship between variables. The converted score information also can be entered into a cluster analysis and further discriminant analysis. PCA can be combined with HS-GC-IMS to detect the volatile flavor substances in plant flower. Based on these previous studies, GC-IMS appears to be a promising technique for analyzing VOCs in jujube flowers.
This study aims to fill these knowledge gaps by systematically investigating the volatile components of jujube flowers. Flowers in three development stages and eleven varieties were studied to reveal the distribution of volatile compounds and identify the characteristic components. Furthermore, jujube flowers were compared with other plant species renowned for their distinctive floral aromas, providing a comprehensive orientation towards understanding the diversity of floral fragrance profiles. The findings from this research will provide valuable insights for the development and application of jujube flower aroma and serve as a reference for future studies on the volatile compound accumulation mechanisms in jujube. This work will contribute to the broader understanding of plant volatiles and their potential in various industries.
2 Materials and methods
2.1 Plant material
All jujube flowers samples used in this study were collected from the Third Farm Branch of Hebei Agricultural University (115°48′E, 38°85′N) in June 2023. Samples were harvested at three developmental stages: flower bud stage, flowering stage, and late flowering stage, and immediately transported to the laboratory for analysis. Jujube flowers with uniform appearance and no physical damage were selected as experimental samples (Figure 1). The study included eleven jujube varieties: Fushuai, Fuxiang, Xingguang, Dongzao, Yueguang, Yuhong, Chenguang, Yushuai, Zanshuo, Jinsixiaozao, and Dajinsiwang. For comparative analysis, flowers of Lily bulb, Rose, Chrysanthemum, and Carnation were obtained from the Baoding Agro-ecological Park in Hebei Province (Figure 1). These four species were selected due to their widespread recognition and distinctive aromatic properties. All samples were healthy, free from pest damage, and exhibited no signs of spoilage.
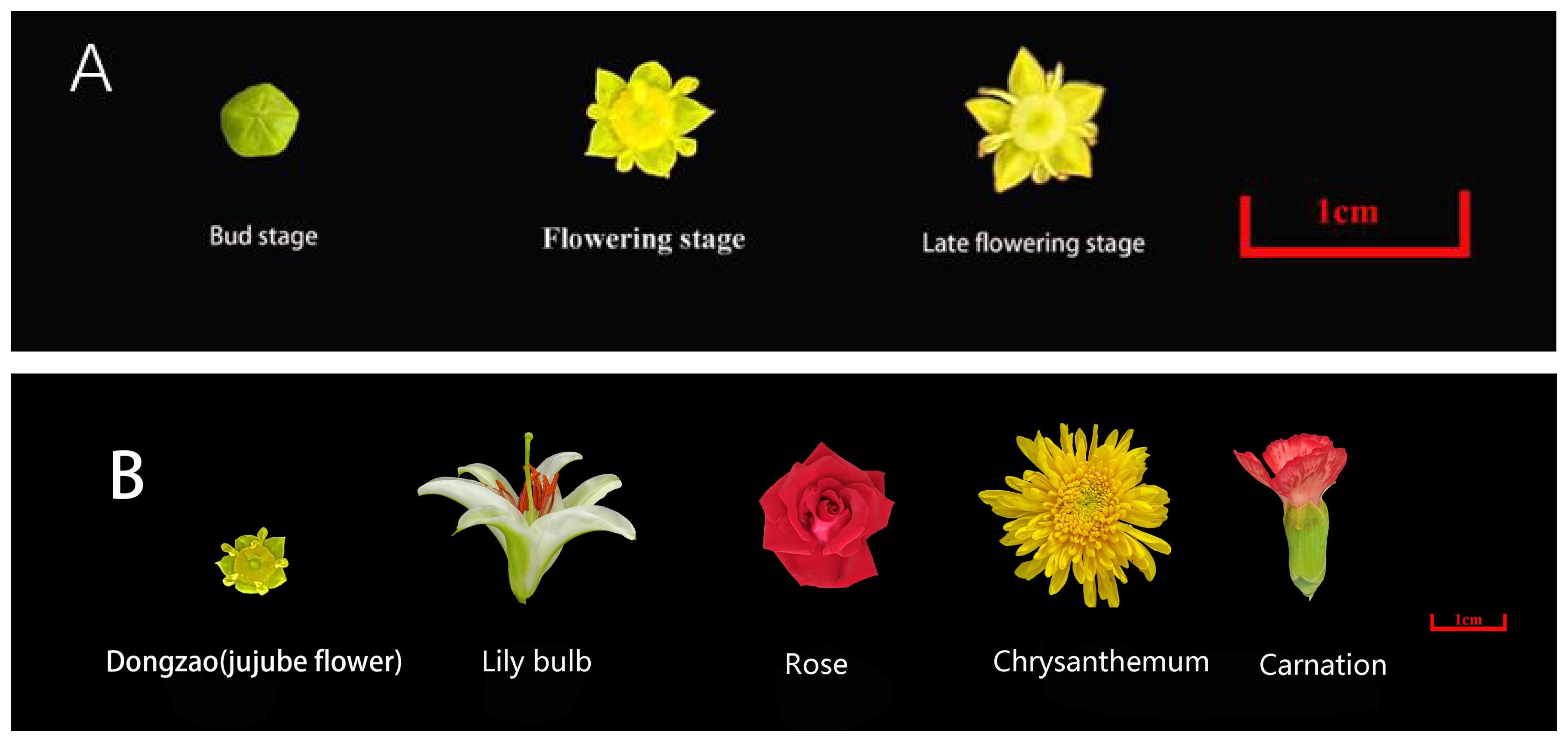
Figure 1. (A) Jujube flowers in three periods. (B) Jujube flowers and four other commonly recognized flowers.
2.2 Sensory evaluation
The sensory evaluation was conducted in accordance with the ethical guidelines outlined in the World Medical Association (Helsinki Declaration). A total of 20 healthy, non-smoking participants (10 men and 10 women, aged 20–42 years) were recruited from students and faculty members of our institution. Prior to the aroma evaluation, all participants underwent a one-week training program, which included more than two hours of daily sessions to familiarize them with the characteristics of plant floral aromas and the requirements for sensory evaluation. During this training, participants were introduced to the descriptive terminology used for plant floral aromas. Written informed consent was obtained from each participant before commencing the sensory evaluation.
2.3 Headspace gas chromatography-ion mobility spectrometry analysis
The volatile compounds of jujube flowers were analyzed using HS-GC-IMS flavor analyzer (Flavour Spec ®) provided by the company für Analytische Sensorysteme mb H (G.A.S., Dortmund, Germany). For the analysis, 1g of flower sample was weighed and placed in a 20ml headspace glass extraction bottle. The samples were incubated at 60°C for 15 min. Following incubation, 0.5 ml of the headspace phase was automatically injected into the sampler in a splitless injection mode with a syringe at 45°C. Volatile components were separated using an MXT-WAX capillary column (30 m × 0.53 mm ID, 1.0 μm df, RESTEK, USA) in the gas chromatography and coupled to IMS at 45°C. Nitrogen (purity of 99.999%) was used as the carrier gas. The program flow rate was as follows: 2 mL/min for the first 2 min, increasing linearly to 10 mL/min over 8 min, then increasing to 100 mL/min over the final 15 minutes, after which the flow was stopped. The analysis was conducted under normal pressure. The analyte was separated on a column maintained at 60°C and ionized in the IMS ionization chamber at 45°C. The drift gas (nitrogen) flow rate was set to 150 mL/min. Each sample was analyzed in triplicate. The retention index (RI) of volatile compounds was calculated using C4-C8 n-ketone (China National Pharmaceutical Chemical Reagents Beijing Co., Ltd.) as an external reference under the same chromatographic conditions. Volatile compounds were identified by comparing the standard drift time and RI with those in the GC-IMS libraries and NIST 2014 (National Institute of Standards and Technology, Gaithersburg, Maryland, United States).
2.4 Statistical analysis
The HS-GC-IMS data is processed using the laboratory analysis instrument Vocal and its three plug-ins, the NIST database and the IMS database. The topographic map and fingerprint of volatile compounds in jujube flowers were established by using the Reporter and Gallery Plot plug-in. Principal Component Analysis (PCA) was performed using the Dynamic PCA plug-in (g.a.s., Dortmund, Germany) to evaluate the regularity and difference between test samples. The analysis was performed using Origin software (version 2021b, USA). OPLS-DA and PLSR analyses were conducted to further evaluate the characteristics of samples using SIMCA 14.1.
3 Results and discussion
3.1 Variation of volatile flavor compounds in jujube flowers at different growth stages
3.1.1 HS-GC-IMS topographic maps of jujube flowers across different growth stage
HS-GC-IMS was employed to analyze the differences in volatile substances in jujube flowers at various growth stages. Taking the Fuxiang variety as an example, Figure 2 showed the topographic map of volatile substances, where X, Y and Z represent ion migration time, GC retention time and ion peak intensity, respectively. As illustrated in Figure 2, jujube flowers at different stages showed similar visualization patterns, with slight variations in ion peak intensity. The red-circled area in Figure 2 clearly highlights the differences in peak intensity between the various stages. Notably, the ion peak intensity during the flowering stage was significantly higher compared to the bud stage and the late flowering stage. GC-IMS separates mixtures into individual compounds through a gas chromatography column, then ionizes these compounds using ion mobility spectrometry, and further analyzes them based on the migration rates of each compound’s ions under the influence of an electric field (Yang et al., 2021). This dual separation allows for the distinct identification of each compound. By analyzing the migration time and ion peak intensity of each volatile compound, the volatile profile of jujube flowers can be qualitatively analyzed.
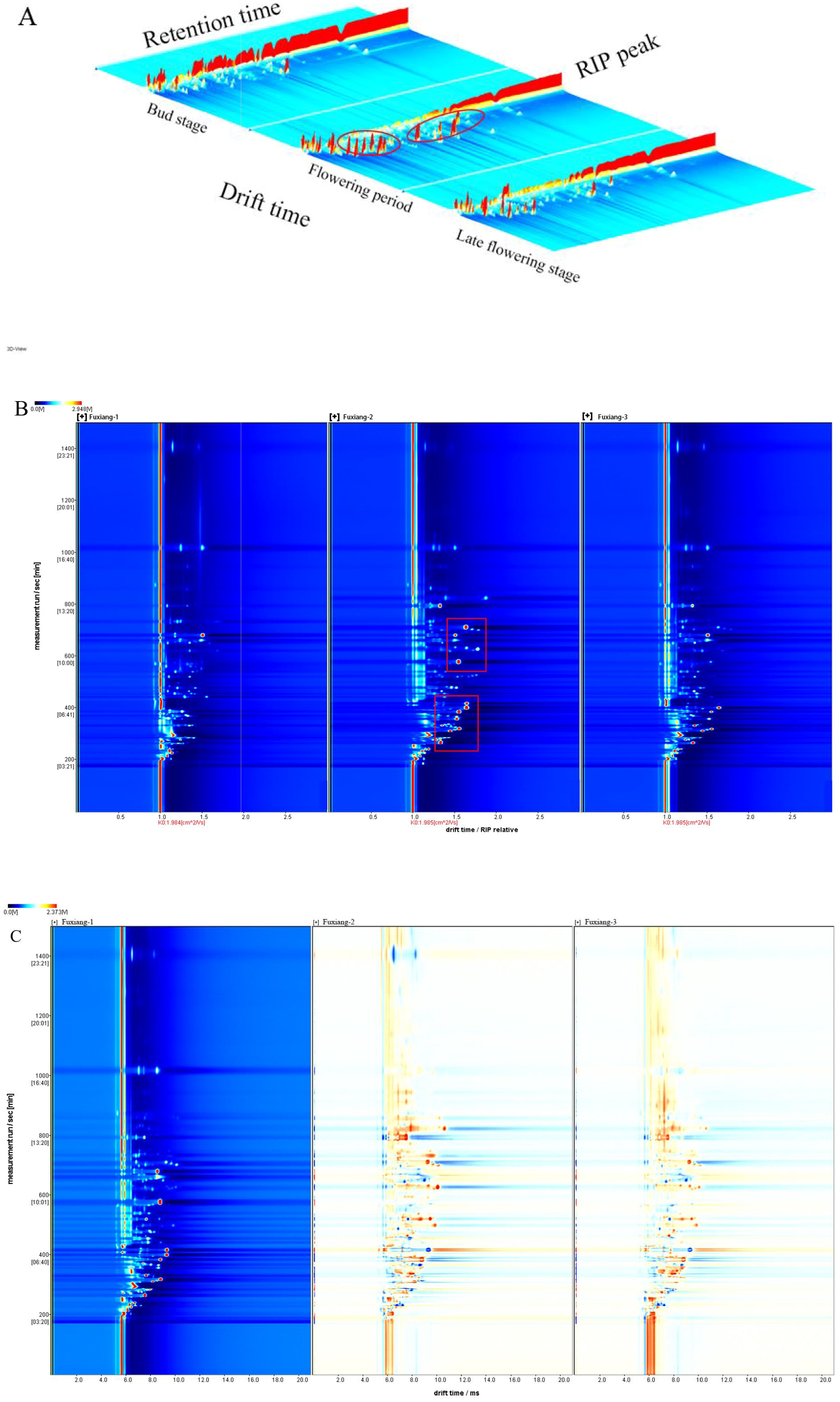
Figure 2. GC-IMS analysis of Fuxiang flowers in different periods. (A) 3D-topographic plots of Fuxiang flowers; (B) 2D-topographic plots of Fuxiang flowers; (C) The difference comparison topographic plots of Fuxiang flowers.
Because the topographic map is relatively rough, the volatile substances in jujube flowers cannot be clearly displayed. To better distinguish the volatile flavor components at different stages of flowering, we normalized the ion migration time and reaction ion peak (RIP). Each point around the RIP represents a volatile substance found in jujube flowers. Most volatile substance signals appeared within a drift time of 1.0-1.75 ms and a retention time of 200-1000 s. In the visualization, the color of each point indicates the signal intensity of a single substance: red indicates a higher intensity, blue signifies lower intensity, and the color depth correlated with the intensity level. Different models were applied to distinguish the volatile components of jujube flowers across various growth stages, as shown in Figure 2. From the two-dimensional topographic plots (Figure 2), it is evident that the signal intensities within the red box are markedly higher in jujube flowers during the flowering stage compared to those at the other two developmental periods. In Figure 2, the bud stage of Fuxiang variety is used as a reference to compare the other two stages. Red indicates a higher intensity than the reference, while blue indicates a lower intensity. When the drift time was 1.0-1.5 ms and the retention time was 400-800 s, the volatile substances in both the flowering stage and the late flowering stage were significantly higher than those in the bud stage, with the flowering stage showing slightly more red than that in the late flowering stage. As jujube flowers continue to bloom, certain aroma components are continuously produced. However, during the late flowering stage, the levels of these aroma components gradually decline as a result of flower ovary pollination and the subsequent formation of fruits. To further explore the volatile substances represented by these red dots, an in-depth statistical analysis of jujube samples across different periods is necessary.
3.1.2 Qualitative analysis volatile flavor components in jujube flowers at different growth stages
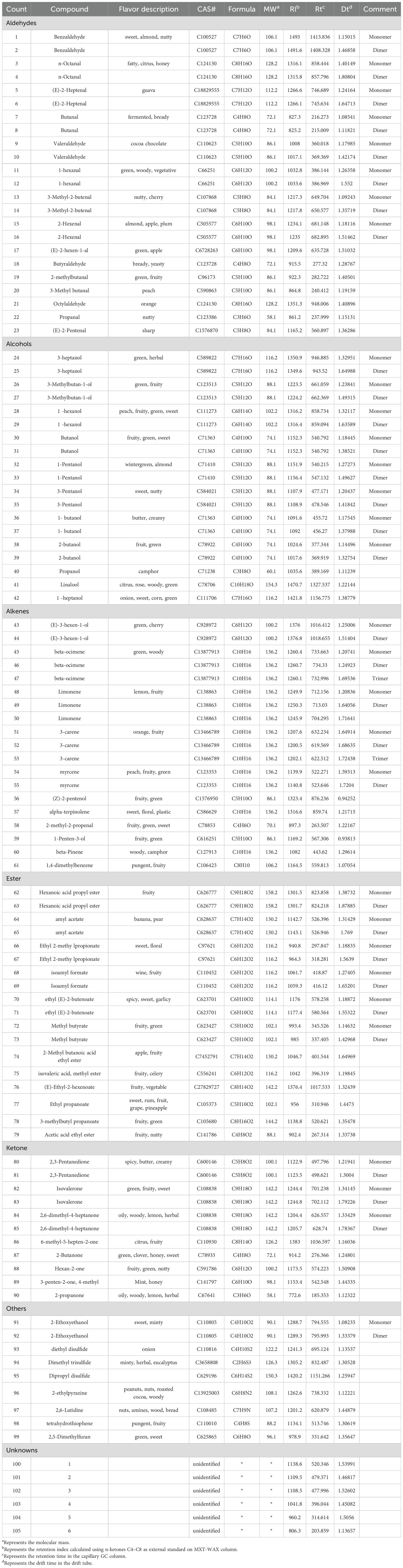
In this study, the two-dimensional cross-qualitative approach, utilizing the GC-IMS Library Search, was employed to further identify the detected compounds. Figure 3 presents the results of the library search-based qualitative analysis of the sample. In Figure 3, the horizontal axis represents the drift time, while the vertical axis corresponds to the retention time. The red numbers displayed in Figure 3, denote the identified organic compounds. Table 1 provides the qualitative results of volatile components detected in jujube flowers across different growth stages. The red numbers in Figure 3, align with the compound numbers listed in Table 1. Notably, due to the enhanced proton affinity and higher concentration of dimers, their drift time is greater than that of monomers (Lantsuzskaya et al., 2015). Some compounds may produce multiple signals due to varying concentrations, such as monomers and dimers (Li et al., 2019). According to our study results, we found that a total of 31 compounds exist in the form of dimers. Ultimately, we detected 105 peaks and identified 65 volatile compounds from our samples, including 15 aldehydes, 12 esters, 11 alcohols, 11 olefins, 8 ketones, 4 ethers, and 4 other substances (1 furan, 1 pyrazine, 1 thiazole and 1 pyridine), as show in Table 1.
3.1.3 Analysis of volatile flavor components of jujube flowers in different periods
To distinguish the differences between jujube flowers in different periods, the Gallery Plot plug-in was used to generate volatile fingerprints (Figure 4). The row represents the detected volatile organic compounds, and the column showed the signal intensity of the same compound presented in different jujube flowers samples. Each point in the figure represents a volatile substance, and the depth of the color is related to the content of the volatile substance. The brighter the color, the higher the content of the substance in the sample.
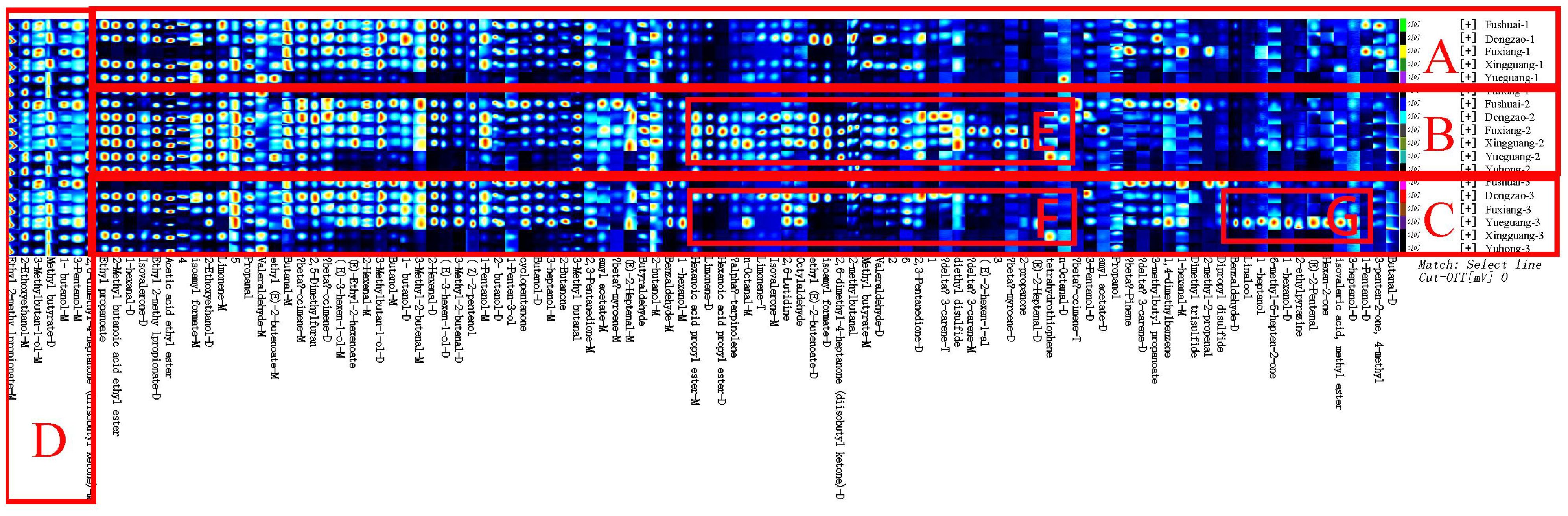
Figure 4. Fingerprint comparison of volatile organic compounds (VOCs) in jujube flowers samples determined by HS-GC-IMS.
As illustrated in Figure 4, the aromatic profiles of jujube flowers were compared across different growth stages. The highest diversity of aroma components was observed during the flowering stage (Figure 4, red box B), while the bud stage exhibited the least variety (Figure 4, red box A). Common to all samples were compounds such as methyl butyrate, 1-butanol, 3-pentanol, 2,6-dimethyl-4-heptanone, ethyl 2-methylpropionate, 2-ethoxyethanol, and 3-methylbutan-1-ol (Figure 4, red box D). During the bud stage, the aroma profile was predominantly characterized by a higher presence of alcohols and aldehydes (Figure 4, red box A). Notably, compounds like 3-heptanol, 3-methylbutan-1-ol, n-octanal, benzaldehyde, 2-butanol, butyraldehyde, 2-methylbutanal, and 3-methylbutanal were found in higher concentrations, imparting grassy, nutty, and jasmine-like scents to the jujube flowers. As the flower buds developed and accumulated nutrients, the aromatic intensity of the flowers gradually increased.
The flowering stage marked a significant divergence in aroma composition compared to the other stages, with an increase in 14 distinct aroma substances, including valeraldehyde, (E)-2-hexen-1-al, octylaldehyde, limonene, 3-carene, myrcene, terpinolene, hexanoic acid propyl ester, isoamyl formate, ethyl (E)-2-butenoate, 2,3-pentanedione, 2,6-dimethyl-4-heptanone, 2-propanone, and diethyl disulfide (Figure 4, red box E). These newly emerged compounds were primarily esters and alkenes. Substances such as myrcene, terpinolene, 2-nonanone, 2,3-pentadione, and phellandrene, which are yellow liquids under standard conditions, contribute to the yellow coloration of the nectar disc and enhance the flowers’ robust fragrance.
During the late flowering stage, some of the aroma components that increased during the flowering stage may have oxidized due to prolonged exposure to air. Consequently, the newly formed aroma components from the flowering period were largely absent in the late flowering stage (Figure 4, red box F). However, as indicated in Figure 4, red box G, eight additional aroma components were identified in the late flowering stage, including dipropyl disulfide, 2-methylbutanal, linalool, 6-methyl-5-hepten-2-one, 1-heptanol, (E)-2-pentenal, isovaleric acid, and methyl ester. Similar compounds, such as Benzaldehyde, (E) -2-Heptenal, and Butanal, have also been found in jujube fruit (Yang et al., 2019).
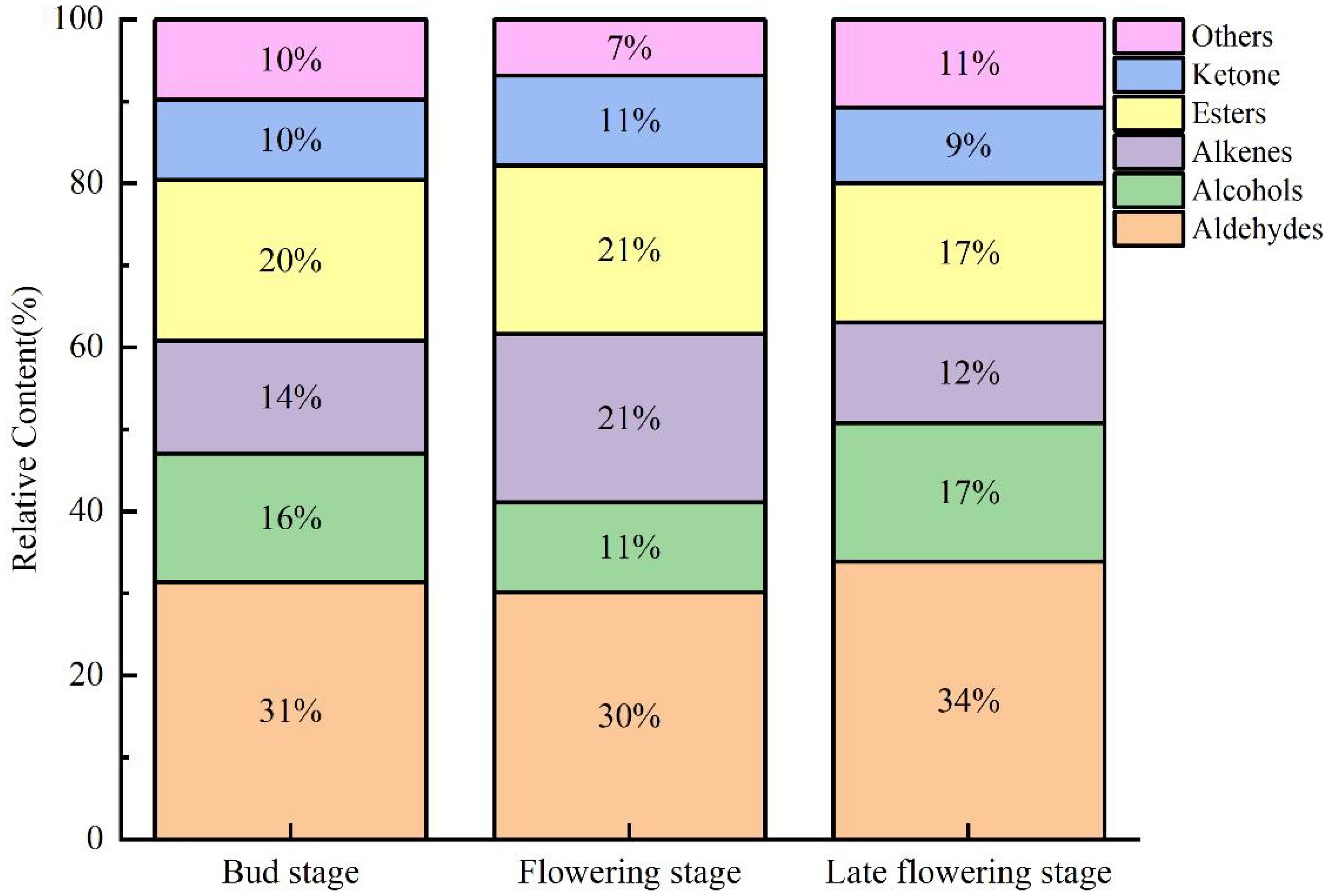
Similarly, aldehydes were found to be the most abundant across all three stages, followed by esters, alkenes, alcohols, and ketones (Figure 5). Notably, the levels of esters and alkenes during the flowering stage were significantly higher compared to the other two stages, serving as the primary factor contributing to the differences observed across the periods. In contrast, ketones and aldehydes exhibited minimal changes during the flowering stage, suggesting that they have a limited influence on the aroma profile of jujube flowers. During the flowering bud stage, a higher concentration of aldehydes and alcohols was formed, which were subsequently transformed into esters and alkenes during the flowering stage, thereby enhancing the aromatic profile of jujube flowers. The disappearance of certain compounds in the late flowering stage may be attributed to factors such as bee pollination, flower ovary fertilization, and oxidation due to prolonged exposure to air. Comparable findings have been reported in studies on truffles, red peppers, green peppers, and tea (Korkmaz et al., 2023; Shao et al., 2023; Yue et al., 2023).
3.1.4 PCA of volatile flavor components in jujube flowers across different developmental stages
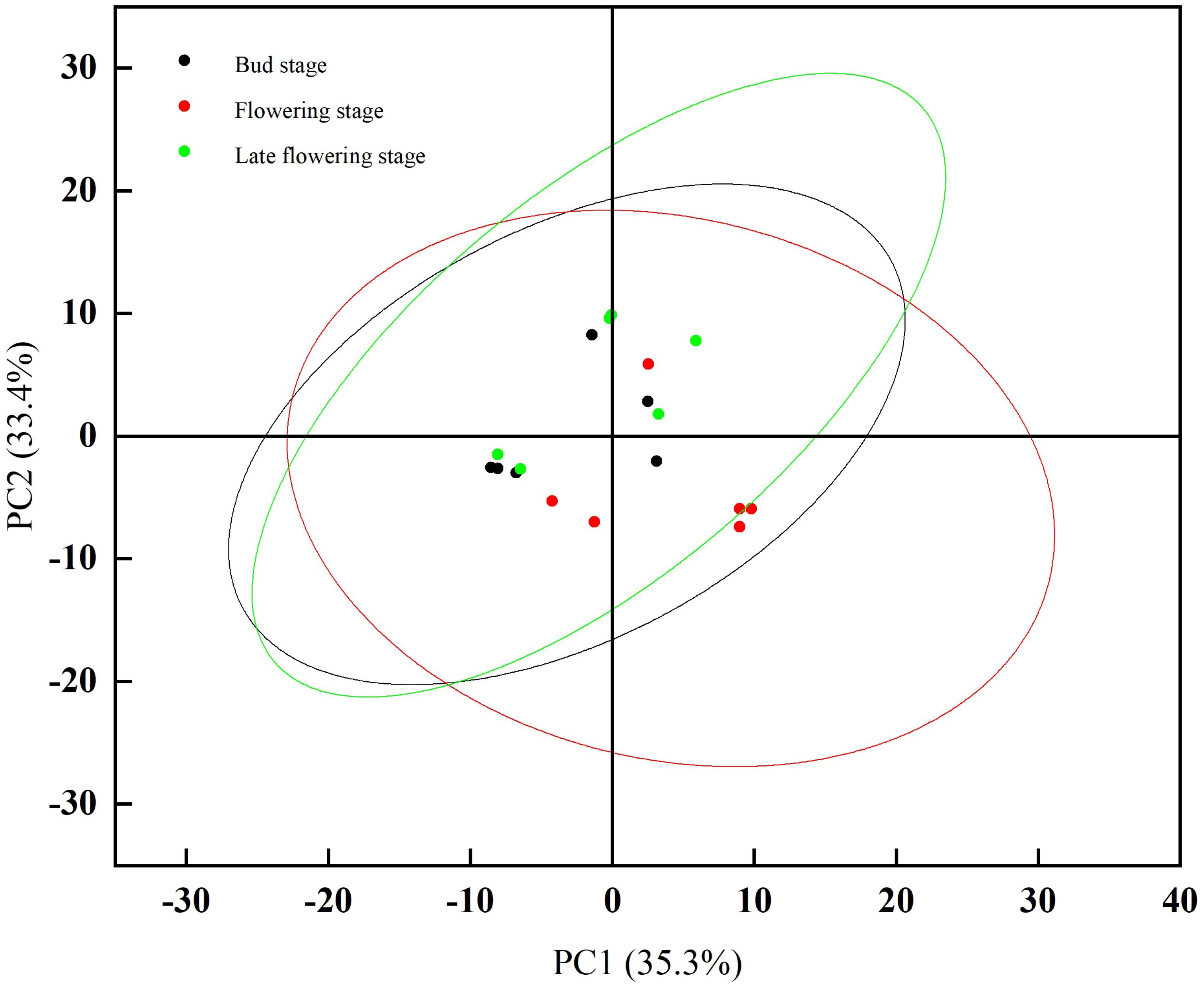
Principal Component Analysis (PCA) is a widely utilized unsupervised analytical tool for the analysis of volatile compounds. In a PCA plot, samples with similar expression profiles exhibit a higher degree of clustering, while those with greater differences are positioned farther apart (Ma Q. et al., 2020). To better distinguish and comprehensively analyze the variations in volatile compounds across different stages of jujube flowers, we applied principal component analysis. As shown in Figure 6, the combined contribution of PC1 and PC2 for jujube flowers across different stages exceeds 60%, which is sufficient to highlight the distinctions among the stages. The aroma profile of jujube flowers at the flowering stage is notably distant from those at the bud stage and the late flowering stage, indicating a significant difference between the flowering stage and the other two periods. This finding is consistent with the results derived from the fingerprint analysis, as previously discussed.
3.2 Volatile flavor components variations among different jujube flowers varieties at the flowering stage
Based on the analysis of aroma profiles in jujube flowers across different growth stages, the flowering period was identified as the phase with the highest concentration of aromatic compounds. To identify cultivars with the most abundant aromatic profiles, we conducted a comparative study of 11 major jujube varieties during their flowering stage, representing nearly all principal cultivars currently cultivated. The analysis revealed that Fuxiang, Dongzao, and Xingguang exhibited the richest composition of aromatic compounds during flowering (Figure 7, red box A), whereas Yushuai, Zanshuo, Jinsixiaozao, and Dajinsiwang showed the lowest concentrations of these compounds (Figure 7, red box B). These findings suggest that Fuxiang, Dongzao, and Xingguang represent the most suitable varieties for the extraction of aromatic compounds from jujube flowers.
3.3 Differences in volatile flavor components between jujube flowers and other plant flowers
3.3.1 Qualitative analysis of jujube flowers and other plant flowers in volatile flavor components
Table 2 presents the qualitative analysis of volatile components in different plant flowers. A total of 102 signal peaks were detected, with 61 compounds successfully identified. These compounds were categorized as follows: 10 alcohols, 12 esters, 9 olefins, 11 aldehydes, 11 ketones, and 8 other substances, including 2 furans, 2 pyrazines, 1 aromatic hydrocarbon, 1 alkane, 1 acid, 1 amine and 8 substances unidentified.
3.3.2 Analysis of volatile flavor components of different plant flowers
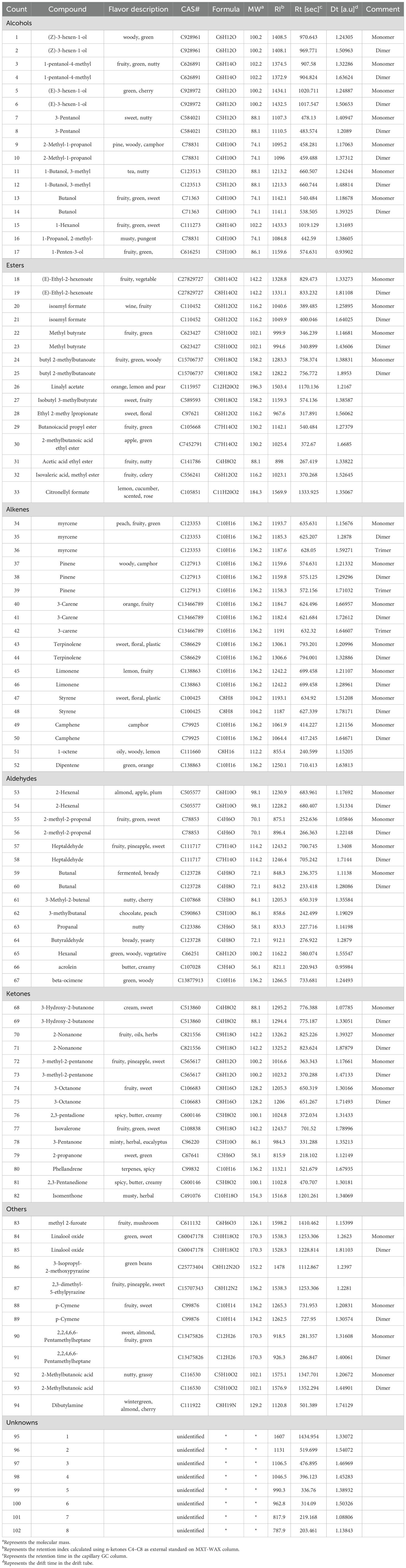
As shown in Figure 8, the aroma profile of jujube flowers exhibits significant differences compared to the other four plant species. Common volatile compounds detected across all plant flower samples include methyl butyrate, 1-octene, butanol, acetic acid ethyl ester, 3-methyl-2-butenal, 3-pentanol, propanal, 2-hexenal, and 1-butanol, 3-methyl (Figure 9, red box B). Notably, jujube flowers contain 24 unique aroma components and 4 unidentified compounds (Figure 8, red box A). The clustering heat map analysis further confirmed that these 24 volatile compounds effectively distinguish jujube flowers from other samples (Figure 9).
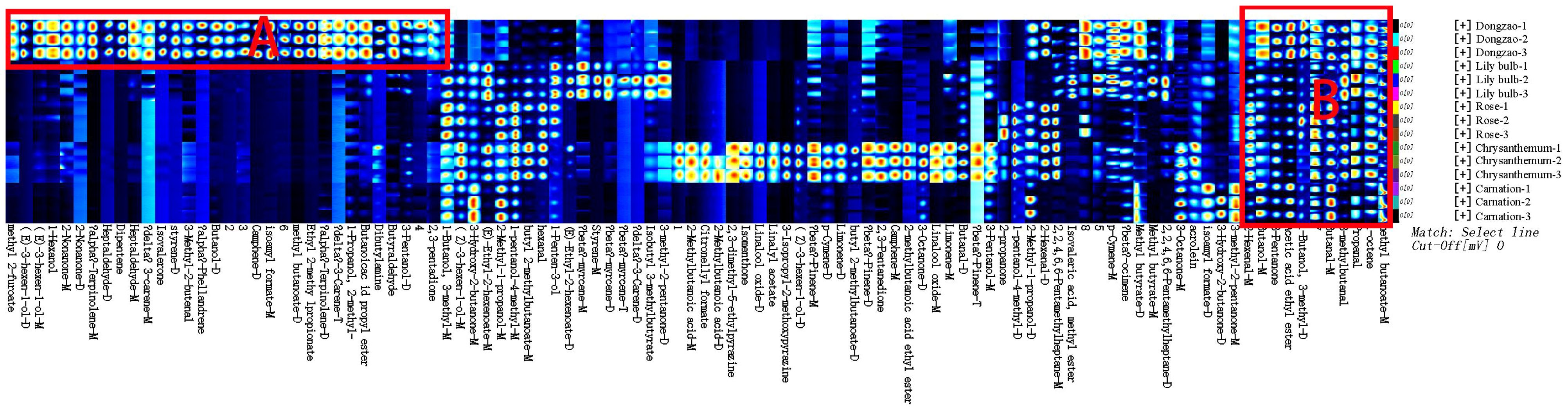
Figure 8. Comparison of HS-GC-IMS fingerprints of volatile organic compounds (VOCs) in jujube flowers and other plant flowers samples.
Several key compounds contribute to the distinctive aroma and functional properties of jujube flowers. For instance, (E)-3-hexen-1-ol, 3-pentanol, 1-hexanol, 1-propanol, 2-methyl-, (E)-ethyl-2-hexenoate, isoamyl formate, isobutyl 3-methylbutyrate, butanoic acid propyl ester, phellandrene, 2,3-pentadione, 2-nonanone, heptaldehyde, and dipentene impart fruity, nutty, and creamy aromas to jujube flowers. Additionally, terpinolene and α-phellandrene exhibit anti-inflammatory and antioxidant activities, which are beneficial for in vitro wound healing (de Christo Scherer et al., 2019). Camphene, commonly found in vegetables and herbs (Tiwari and Kakkar, 2009), is also present in jujube flowers. Isoamyl formate, a colorless oily liquid with a distinctive plum and black currant aroma, possesses antimicrobial properties that inhibit or kill bacteria, yeast, filamentous fungi, and oomycetes, thereby promoting plant health (Hummadi et al., 2022). These compounds collectively protect jujube flowers from pests and diseases during their development. Ketones, which are key products of the Maillard reaction and precursors to many flavor compounds (Smuda and Glomb, 2013),are also prominent in jujube flowers. For example, 2-nonanone, a colorless to light yellow liquid with an herbal aroma, can be utilized in active packaging systems to preserve fruit freshness post-harvest and prevent flavor degradation caused by high volatile content (Almenar et al., 2009). Terpinolene, 2-nonanone, 2,3-pentadione, and phellandrene are major components of the yellow liquid in jujube nectar. Furthermore, 3-carene, 3-pentanol, and isoamyl formate in jujube flowers contribute to pathogen resistance, underscoring the dual medicinal and nutritional value of jujube fruit (Shu et al., 2020; Song et al., 2015).These findings highlight the unique aromatic and functional characteristics of jujube flowers, which are integral to their role in both ecological and agricultural contexts.
3.3.3 Jujube flowers and other plant flowers PCA of volatile flavor components
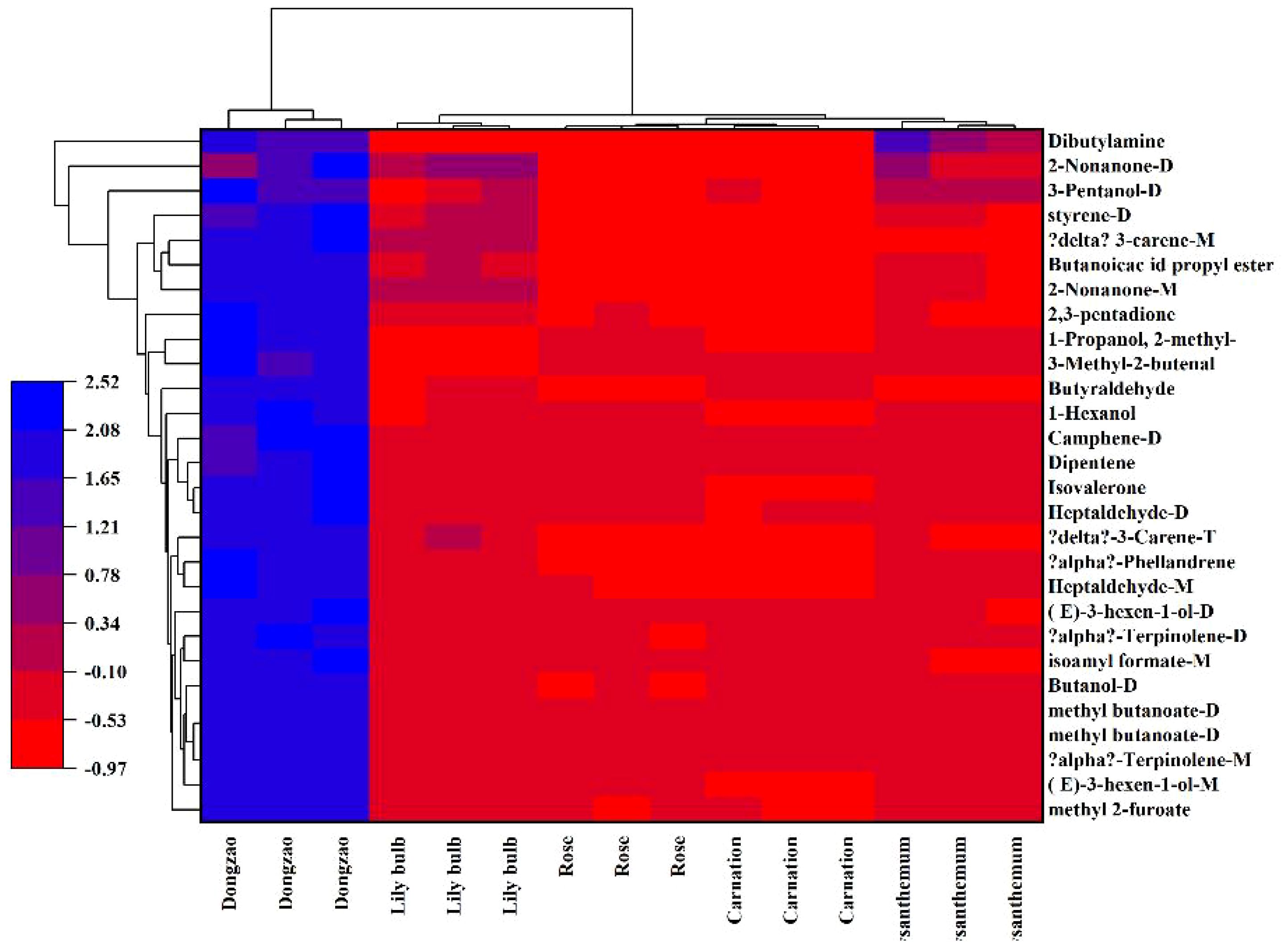
Principal Component Analysis (PCA) of the fragrance profiles of jujube flowers and four other plant species revealed a total contribution rate of 70.5% for the principal components, with PC1 accounting for 43.7% and PC2 for 26.8% of the cumulative variance. This level of contribution is sufficient to effectively capture the differences in fragrance between jujube flowers and the other plant species (Figure 10). The significant spatial separation observed between jujube flowers and the other four plant species in the PCA plot further underscores the distinctiveness of jujube flower fragrance. These findings align well with the results presented in the fingerprint analysis (Figure 8), reinforcing the conclusion that jujube flowers possess a unique aromatic profile compared to the other plants studied.
3.3.4 Similarity analysis of 24 characteristic volatile organic compounds of jujube flowers
A PCA model was constructed to analyze the 24 characteristic aroma compounds of jujube flowers and other plant species. The results revealed that jujube flowers were predominantly located in the first and fourth quadrants, demonstrating a clear distinction from the other four plant species (Figure 11). In this study, the model exhibited excellent fit and predictability, as indicated by R2X, R2Y, and Q2 values approaching 1. Figure 11 further illustrates that the aroma profile of jujube flowers is distributed across the first and fourth quadrants, consistent with the PCA results. To ensure the robustness of the model and avoid overfitting, a permutation test was conducted. As shown in Figure 11, after 200 cross-validations, the Q and abscissa intercepts were negative, and the R2 and Q2 values remained below the original values, confirming that the model did not overfit the data.
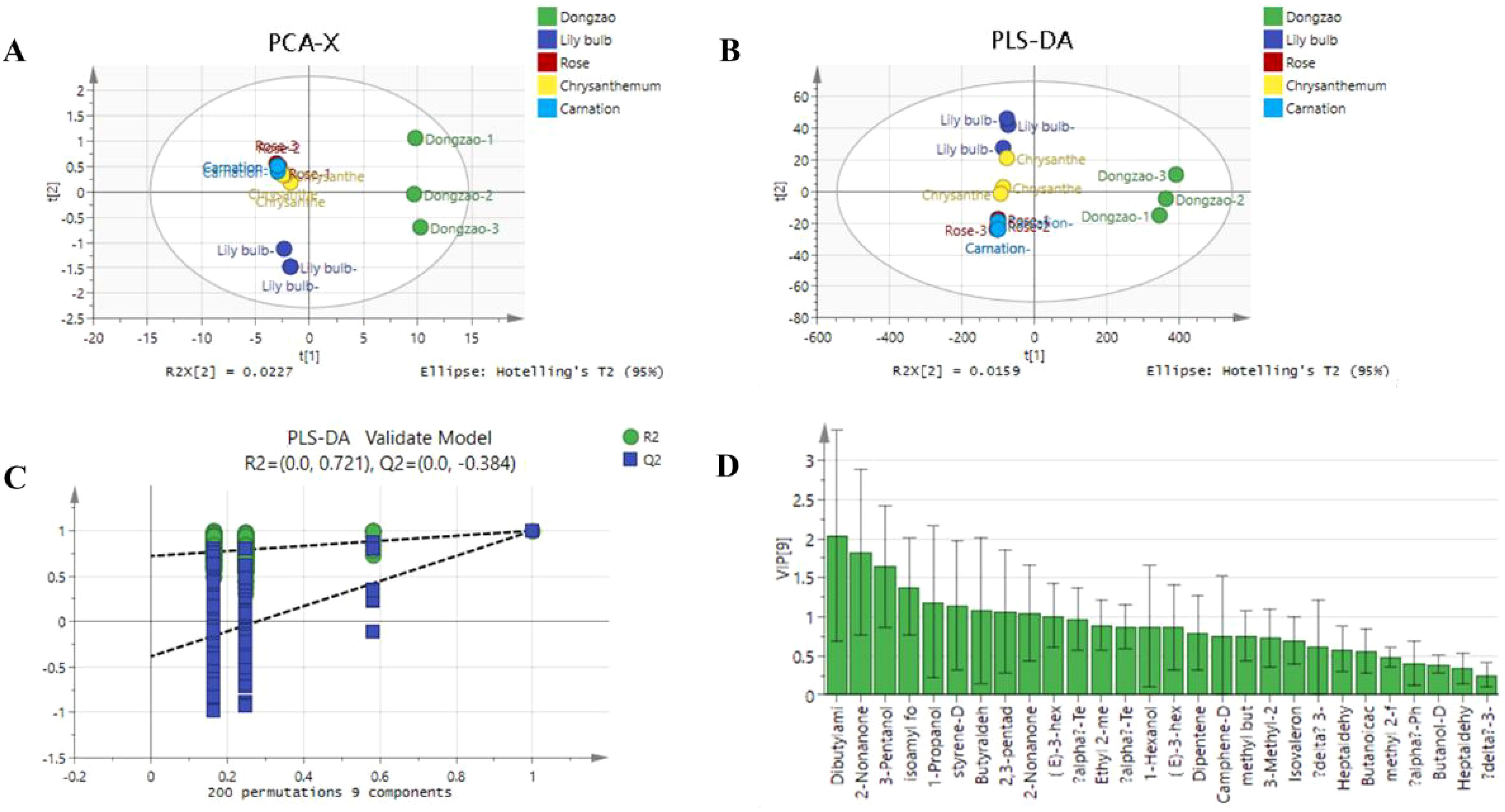
Figure 11. PCA analysis of 24 characteristic aroma compounds of jujube flowers and other plant species. (A) PCA-X score plot showing clustering of Dongzao, Lily bulb, Rose, Chrysanthemum, and Carnation samples. (B) PLS- DA score plot with similar groupings. (C) PLS-DA validation model displaying R2 and Q2values across permutations. (D) Bar chart of variable importance in projection (VIP) scores for different chemical compounds, with Dibutylam and 2-Nonanone as top compounds.
The contribution of individual aroma compounds to the overall profile of jujube flowers was evaluated using VIP (Variable Importance in Projection) values. As depicted in Figure 11, nine aroma components with VIP values greater than 1 were identified as significant contributors: dibutylamine, 2-nonanone, 3-pentanol, isoamyl formate, 1-propanol, 2-methyl-, styrene, butyraldehyde, 2,3-pentadione, and (E)-3-hexen-1-ol. These compounds are considered the primary volatile substances responsible for the distinctive aroma of jujube flowers.
3.4 Sensory evaluation of jujube flowers and other plant flowers volatile flavor components
For sensory evaluation, we recruited a panel of 10 male and 10 female participants, which revealed significant gender-based differences in fragrance preferences. Most male participants expressed a preference for the fragrance of jujube flowers, describing it as pleasant and unique compared to the aromas of the other flowers (Figure 12). In contrast, female participants tended to favor more vibrant and fragrant flowers, with Lily bulb receiving the highest score among them (Figure 12). These results highlight the differing aromatic preferences between men and women, with jujube flowers and Lily bulb representing the favored choices of each group, respectively (Figure 12).
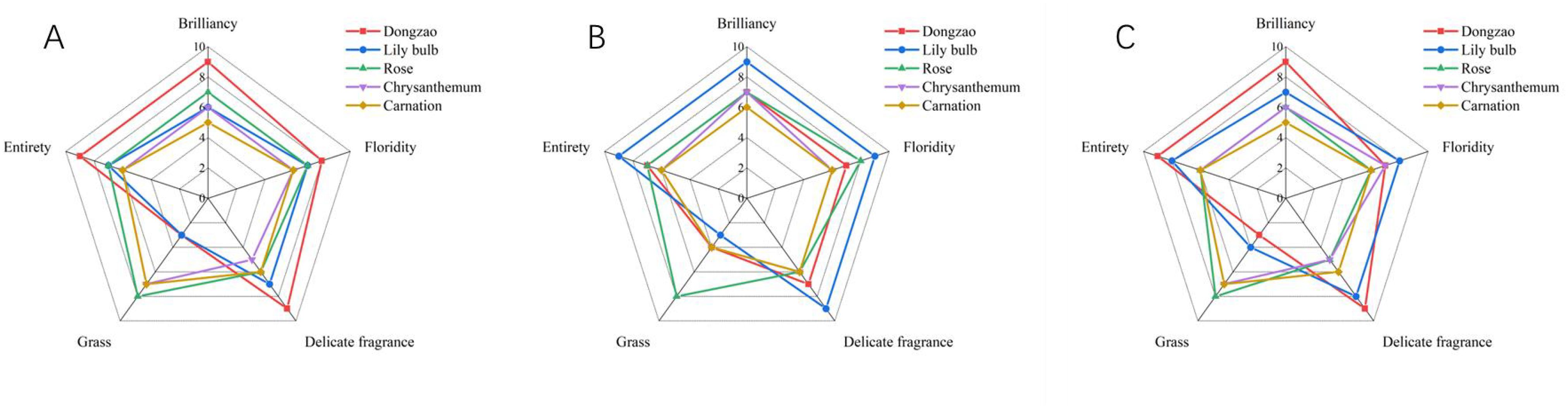
Figure 12. The sensory evaluation map of different plant floral fragrances. (A) Men’s sensory evaluation score. (B) Women’s sensory evaluation score. (C) Comprehensive sensory evaluation score.
Based on the positive feedback from male evaluators regarding the fragrance of jujube flowers (Dongzao), there is potential to extract and utilize its pleasant aroma for the development of male-oriented fragrances or air fresheners in the future. This approach aligns with the sensory preferences identified in the study and offers a promising avenue for product innovation.
4 Conclusion
Aroma is a critical factor in evaluating jujube flowers. In this study, we employed GC-IMS combined with fingerprint analysis and PCA to identify the primary aroma components in jujube flowers. The results revealed that the flowering stage contains the highest concentration of aroma components, with 14 specific compounds showing a significant increase from the bud stage to the flowering stage. Further analysis using clustering heat maps and PLS-DA highlighted 24 characteristic aroma components in jujube flowers, among which 9 were identified as the dominant contributors to their fragrance. Dongzao, Fuxiang, and Xingguang were identified as the three jujube varieties with the most abundant and distinctive aromatic profiles. These findings confirm that GC-IMS combined with fingerprint and PCA analysis is an efficient and reliable method for studying jujube flower aromas. Additionally, sensory evaluation revealed that male participants exhibited a stronger preference for the fragrance of jujube flowers, describing it as pleasant and appealing. This suggests potential applications for jujube flower aroma in the development of men’s perfumes, air fresheners, aromatherapy products, and more. In conclusion, the analytical methods employed in this study provide valuable insights into the flavor substances of jujube flowers and offer a foundation for further research and product development.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The sensory evaluation was conducted in accordance with The Code of Ethics of the World Medical Association (Helsinki Declaration). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PR: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing. RD: Resources, Writing – review & editing. LW: Writing – review & editing. NM: Writing – review & editing. YS: Methodology, Writing – review & editing. ML: Resources, Writing – review & editing. ZZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was financially supported by the Key R&D Projects of Ministry of Science and Technology of the People’s Republic of China (2022YFD1600403).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1590072/full#supplementary-material
References
Almenar, E., Catala, R., Hernandez-Muñoz, P., and Gavara, R. (2009). Optimization of an active package for wild strawberries based on the release of 2-nonanone. LWT-Food Sci. Technol. 42, 587–593. doi: 10.1016/j.lwt.2008.09.009
Choi, S.-H., Ahn, J.-B., Kozukue, N., Levin, C. E., and Friedman, M. (2011). Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 59, 6594–6604. doi: 10.1021/jf200371r
de Christo Scherer, M. M., Marques, F. M., Figueira, M. M., Peisino, M. C. O., Schmitt, E. F. P., Kondratyuk, T. P., et al. (2019). Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue viability 28, 94–99. doi: 10.1016/j.jtv.2019.02.003
Du, Y., Xu, K., Zhao, H., Wu, Y., Jiang, H., He, J., et al. (2024). Preliminary Study on the Pathogenic Mechanism of Jujube Flower Disease in Honeybees (Apis mellifera ligustica) Based on Midgut Transcriptomics. Genes 15, 533. doi: 10.3390/genes15050533
Fahim, H., Dasti, J. I., Ali, I., Ahmed, S., and Nadeem, M. (2014). Physico-chemical analysis and antimicrobial potential of A pis dorsata, A pis mellifera and Z iziphus jujube honey samples from Pakistan. Asian Pacific J. Trop. biomedicine 4, 633–641. doi: 10.12980/APJTB.4.2014APJTB-2014-0095
Feng, T., Sun, J., Song, S., Wang, H., Yao, L., Sun, M., et al. (2022). Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chemistry: X 15, 100423. doi: 10.1016/j.fochx.2022.100423
Gao, Q.-H., Wu, C.-S., and Wang, M. (2013). The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 61, 3351–3363. doi: 10.1021/jf4007032
Guo, S., Duan, J.-A., Zhang, Y., Qian, D., Tang, Y., Zhu, Z., et al. (2015). Contents changes of triterpenic acids, nucleosides, nucleobases, and saccharides in jujube (Ziziphus jujuba) fruit during the drying and steaming process. Molecules 20, 22329–22340. doi: 10.3390/molecules201219852
Hernández, F., Legua, P., Melgarejo, P., Martínez, R., and Martínez, J. (2015). Phenological growth stages of jujube tree (Ziziphus jujube): codification and description according to the BBCH scale. Ann. Appl. Biol. 166, 136–142. doi: 10.1111/aab.12169
Hummadi, E. H., Cetin, Y., Demirbek, M., Kardar, N. M., Khan, S., Coates, C. J., et al. (2022). Antimicrobial volatiles of the insect pathogen Metarhizium brunneum. J. Fungi 8, 326. doi: 10.3390/jof8040326
Korkmaz, C., Hellal, K., Taş Küçükaydın, M., Çayan, F., Küçükaydın, S., and Duru, M. E. (2023). Volatile compound profiling of seven tuber species using HS-SPME-GC-MS and classification by a chemometric approach. ACS omega 8, 34111–34119. doi: 10.1016/j.ijbiomac.2015.01.061
Lantsuzskaya, E., Krisilov, A., and Levina, A. (2015). Structure of the cluster ions of ketones in the gas phase according to ion mobility spectrometry and ab initio calculations. Russian J. Phys. Chem. A 89, 1838–1842. doi: 10.1134/S0036024415100179
Leng, P., Hu, H.-W., Cui, A.-H., Tang, H.-J., and Liu, Y.-G. (2021). HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. Lwt 149, 111963. doi: 10.1016/j.lwt.2021.111963
Li, J.-W., Fan, L.-P., Ding, S.-D., and Ding, X.-L. (2007). Nutritional composition of five cultivars of Chinese jujube. Food Chem. 103, 454–460. doi: 10.1016/j.foodchem.2006.08.016
Li, H., Li, F., Wang, L., Sheng, J., Xin, Z., Zhao, L., et al. (2009). Effect of nano-packing on preservation quality of Chinese jujube (Ziziphus jujuba Mill. var. inermis (Bunge) Rehd). Food Chem. 114, 547–552. doi: 10.1016/j.foodchem.2008.09.085
Li, M., Yang, R., Zhang, H., Wang, S., Chen, D., and Lin, S. (2019). Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 290, 32–39. doi: 10.1016/j.foodchem.2019.03.124
Liu, G., Liu, X., Zhang, Y., Zhang, F., Wei, T., Yang, M., et al. (2015). Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao. Int. J. Biol. macromolecules 76, 169–175. doi: 10.1016/j.ijbiomac.2015.01.061
Liu, M., Wang, J., Wang, L., Liu, P., Zhao, J., Zhao, Z., et al. (2020). The historical and current research progress on jujube–a superfruit for the future. Horticulture Res. 7. doi: 10.1038/s41438-020-00346-5
Ma, Q., Chen, X., Zhang, K., Yao, D., Yang, L., Wang, H., et al. (2020). Chemical fingerprint analysis for discovering markers and identifying Saussurea involucrata by HPLC coupled with OPLS-DA. J. analytical Methods Chem 2020. doi: 10.1155/2020/7560710
Ma, Y., Yin, J., Wang, J., Liu, X., He, J., Zhang, R., et al. (2023). Selenium speciation and volatile flavor compound profiles in the edible flowers, stems, and leaves of selenium-hyperaccumulating vegetable Cardamine violifolia. Food Chem. 427, 136710. doi: 10.1016/j.foodchem.2023.136710
Ma, W., Zheng, X., Li, L., Shen, J., Li, W., and Gao, Y. (2020). Changes in the gut microbiota of honey bees associated with jujube flower disease. Ecotoxicology Environ. Saf. 198, 110616. doi: 10.1016/j.ecoenv.2020.110616
Shao, Y., Liu, X., Zhang, Z., Wang, P., Li, K., and Li, C. (2023). Comparison and discrimination of the terpenoids in 48 species of huajiao according to variety and geographical origin by E-nose coupled with HS-SPME-GC-MS. Food Res. Int. 167, 112629. doi: 10.1016/j.foodres.2023.112629
Shi, X., Lv, Y., and Guo, S. (2017). Effects of heat treatment and β-cyclodextrin addition on soymilk flavor. Trans. Chin. Soc. Agric. Eng 33, 293–300. doi: 10.11975/j.issn.1002-6819.2017.08.039
Shu, H., Zhang, W., Yun, Y., Chen, W., Zhong, Q., Hu, Y., et al. (2020). Metabolomics study on revealing the inhibition and metabolic dysregulation in Pseudomonas fluorescens induced by 3-carene. Food Chem. 329, 127220. doi: 10.1016/j.foodchem.2020.127220
Smuda, M. and Glomb, M. A. (2013). Fragmentation pathways during Maillard-induced carbohydrate degradation. J. Agric. Food Chem. 61, 10198–10208. doi: 10.1021/jf305117s
Song, G. C., Choi, H. K., and Ryu, C.-M. (2015). Gaseous 3-pentanol primes plant immunity against a bacterial speck pathogen, Pseudomonas syringae pv. tomato via salicylic acid and jasmonic acid-dependent signaling pathways in Arabidopsis. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00821
Tiwari, M. and Kakkar, P. (2009). Plant derived antioxidants–geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitro 23, 295–301. doi: 10.1016/j.tiv.2008.12.014. Get rights and content.
Wang, S., Chen, H., and Sun, B. (2020). Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 315, 126158. doi: 10.1016/j.foodchem.2019.126158
Wang, L., Zhu, J., Wang, Y., Wang, X., Chen, F., and Wang, X. (2018). Characterization of aroma-impact compounds in dry jujubes (Ziziphus jujube Mill.) by aroma extract dilution analysis (AEDA) and gas chromatography-mass spectrometer (GC-MS). Int. J. Food properties 21, 1844–1853. doi: 10.1080/10942912.2016.1273234
Wojdyło, A., Carbonell-BarraChina, Á.A., Legua, P., and Hernández, F. (2016). Phenolic composition, ascorbic acid content, and antioxidant capacity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chem. 201, 307–314. doi: 10.1016/j.foodchem.2016.01.090
Xia, Y., Liu, Y., Wang, J., and Shuang, Q. (2020). Assessment of key aroma compounds in fresh jujube brandy by GC-O-MS and odor activity value. J. Food Process. Preservation 44, e14494. doi: 10.1111/jfpp.14494
Yang, L., Liu, J., Wang, X., Wang, R., Ren, F., Zhang, Q., et al. (2019). Characterization of volatile component changes in jujube fruits during cold storage by using headspace-gas chromatography-ion mobility spectrometry. Molecules 24 (21)3904. doi: 10.3390/molecules24213904
Yang, Y., Wang, B., Fu, Y., Shi, Y.-G., Chen, F.-L., Guan, H.-N., et al. (2021). HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 346, 128880. doi: 10.1016/j.foodchem.2020.128880
Yao, L., Cai, R., Wang, H., Yu, C., Tong, C., He, Z., et al. (2024). The volatile composition, aroma profile and antioxidant capacity of Yijiangzi (Aatragalus sinicus L.) monofloral honey and its correlation with the flower. Lwt 205, 116565. doi: 10.1016/j.lwt.2024.116565
Yue, C., Cao, H., Zhang, S., Hao, Z., Wu, Z., Luo, L., et al. (2023). Aroma characteristics of Wuyi rock tea prepared from 16 different tea plant varieties. Food Chemistry: X 17, 100586. doi: 10.1016/j.fochx.2023.100586
Keywords: jujube flowers, different periods, varieties, GC-IMS, PCA
Citation: Ren P, Dao R, Wang L, Muhammad N, Sang Y, Liu M and Zhao Z (2025) HS-GC-IMS with sensory evaluation technique to analyze volatile flavor compounds of jujube flowers. Front. Plant Sci. 16:1590072. doi: 10.3389/fpls.2025.1590072
Received: 11 March 2025; Accepted: 03 June 2025;
Published: 12 September 2025.
Edited by:
Mariam Gaid, Independent researcher, Braunschweig, GermanyReviewed by:
Yaxi Zhou, Beijing Union University, ChinaYanqin Yang, Chinese Academy of Agricultural Sciences, China
Antim Maurya, University of Mississippi, United States
Copyright © 2025 Ren, Dao, Wang, Muhammad, Sang, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihui Zhao, bHl6aGlodWl6aGFvQDEyNi5jb20=
 Peixing Ren1,2
Peixing Ren1,2 Noor Muhammad
Noor Muhammad Mengjun Liu
Mengjun Liu Zhihui Zhao
Zhihui Zhao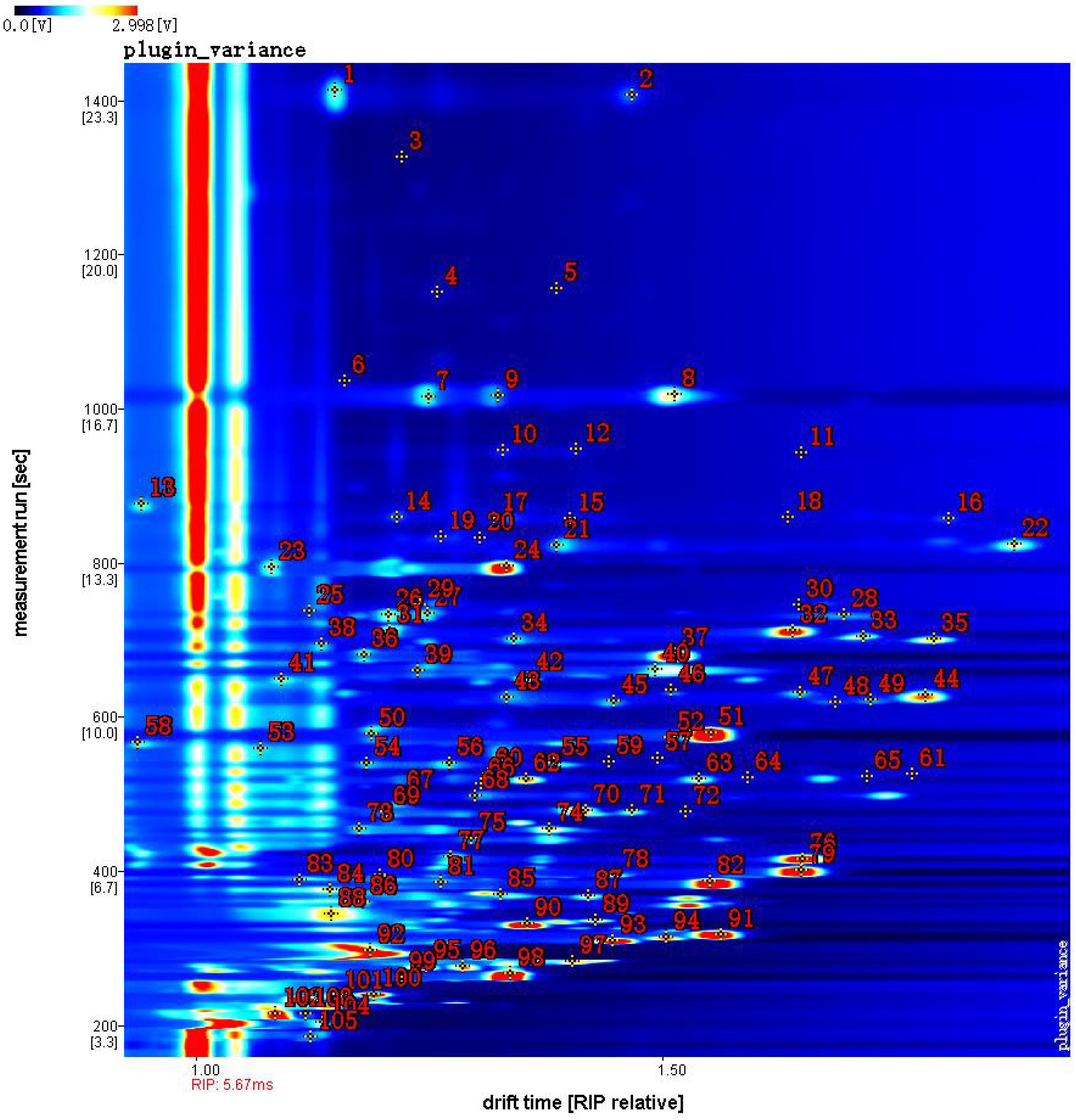
![A multicolored heatmap featuring rows and columns with a variety of chemical names listed vertically. The matrix displays differing intensity levels, highlighted in blue, red, and yellow. Two red rectangles labeled “A” and “B” emphasize specific sections. To the right, a list of names includes “Fushui,” “Dongzao,” and others. The bottom right notes “Select Line” and “Cut-Off [mV] 0.](https://www.frontiersin.org/files/Articles/1590072/fpls-16-1590072-HTML/image_m/fpls-16-1590072-g007.jpg)