- 1School of Ecological and Environmental Science, East China Normal University, Shanghai, China
- 2National Marine Environmental Monitoring Center, Dalian, China
- 3Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration, Shanghai, China
- 4Institute of Eco-Chongming (IEC), Shanghai, China
- 5Technology Innovation Center for Land Spatial Eco-restoration in Metropolitan Area, Ministry of Natural Resources, Shanghai, China
- 6Department of Ecology and Resources Engineering, Hetao College, Bayannur, China
Phragmites australis, capable of both sexual and asexual reproduction, widely distribute along the tidal flat gradient in the Chongming Dongtan wetlands, eastern China. This study investigates whether P. australis exhibits maternal effects by examining how seed germination strategies are influenced by habitat origin, soil properties, storage temperature, and salinity conditions. Seeds were collected from different tidal flat habitats, and their germination responses were tested under varying salinity levels (0–2.0%), storage temperatures (4 °C and room temperature), and soil conditions. Germination rates, speeds, index and potentials were measured, and structural equation modeling (SEM) was used to analyze the effects of environmental factors. These results showed that when the salinity was up to 1.5%, the seed germination rate was 1.3% to 7.7%. When treated with a salinity level of 2.0%, the seed germination rates of different tidal flat populations decreased by 94.3% (L), 96.5% (IN), 100% (M) and 99.2% (H), respectively, compared to the control group. With the increase in salinity, the germination speeds of different tidal flats slowed down, and the germination index decreased. 4°C storage significantly enhanced the seed germination rate and germination potential of the low-tidal flat population (L) relative to room-temperature storage. Soil salinity and water content were major factors influenced germination rates after storing seeds at 4°C. However, germination rates and potentials were positively correlated with the soil phosphorus. Meanwhile, the seed germination indexes and speeds were more significantly affected by the room temperature. The SEM model explained 74% and 50% of the seed germination parameters under the seed storage condition at 4°C and room temperature, respectively. And the tide level of its directly decreasing effect for the seed germination parameters was higher at 4°C with -0.97 than at room temperature with -0.16. The results indicated that after ripening (low-temperature seed storage) differentially promoted the P. australis seed germination collected from the tidal flat gradient in a subtropical marine monsoon climate salt marsh and demonstrated maternal effect adaptation. Consequently, using seeds of P. australis along the tidal flat gradient to restore the original population could be considered an effective and economical method.
Introduction
Loss and degradation, as well as the structural and functional damage of the coastal salt marsh wetlands, have become serious problems worldwide for centuries, directly or indirectly due to various human activities (e.g., dam construction) and/or global climate change (Kirwan and Megonigal, 2013; Mariotti and Fagherazzi, 2013). The coastal wetland destruction negatively impacts the environment, causing a decline in plant diversity and soil water content (Feng et al., 2022; Portnoy, 1999; Tan et al., 2020). This could potentially reduce plant resource utilization efficiency, soil organic matter, and the distribution of some native species while increasing the invasion of alien species (Warren et al., 2002; Portnoy, 1999; Portnoy and Giblin, 1997).
Some native plant species serve as foundation species in various ecosystems, maintaining their stability and functionality (Osland et al., 2014; Angelini et al., 2011). The loss of these native species could lead to damage or even collapse of the ecosystem. When restoring the degraded ecosystem, native foundation species often receive significant attention (Dupin et al., 2022). Therefore, it is essential to examine the ecological characteristics of native foundation plant species in various ecosystems. It may provide a crucial theoretical foundation and technical support for implementing effective ecological restoration practices in damaged ecosystems.
P. australis is one of the important native foundation plant species in the coastal wetland in eastern China (Suir et al., 2022; Wang et al., 2010), acting as a primary producer and the carbon sink (Stankovic et al., 2021). P. australis dominates in terms of biomass in numerous wetlands (Eller et al., 2020), particularly in China, and has been employed in various ecological restoration initiatives in damaged wetland areas (Sun et al., 2015; Wang et al., 2012; Xu et al., 1999). P. australis efficiently colonizes bare areas via vegetative propagation, and its seeds are effectively dispersed by water and wind, resulting in a more rapid expansion of the population area compared to growth solely through rhizomes (Eller et al., 2017; Kühl and Zemlin, 2000). In Europe, it was found that both sexual and asexual reproduction contributed to the fast dispersal of P. australis population, and during the colonization stage, it primarily began from seed germination (Koppitz and Kuehl, 2000). Although seed restoration is a low-cost and high-efficiency method for rehabilitating degraded salt marsh wetland vegetation, the seed germination process is often affected by various factors, including hydrological conditions, soil salinity, temperature and so on (Haraguchi, 2014). Seed germination is highly sensitive to the immediate environment of the seed as well as the environment experienced by the mother plant during seed development (Baskin et al., 2003). The maternal environment can affect the dormancy intensity of seeds, and thus affect the germination traits (such as germination rate and germination speed, etc.) (Song et al., 2016; Auge et al., 2017; Bhatt et al., 2020). It is well known that different populations can exhibit local adaptation in response to spatial variability in environmental conditions such as climate, hydrology, and soil parameters (Elnaggar et al., 2019; Bhatt et al., 2020; Leimu et al., 2008). The salinity of coastal salt marshes is considered to be one of the important factors affecting seed germination (Fernandez-Torquemada and Sanchez-Lizaso, 2013; Greenwood and MacFarlane, 2006; Xiao et al., 2016), so a better understanding of the effect of salinity on the mother plant could result in more saline-tolerant seeds, thereby accelerating population colonization and helping to identify suitable habitats for future restoration projects. Therefore, reversing the global salt marsh decline through restoration requires a comprehensive understanding of how different environmental factors impact the colonization and expansion of tidal marsh species, especially the important native foundation plant species (He and Silliman, 2019; Silliman et al., 2015).
Dongtan, as an internationally important wetland for bird migration in Eurasia, provides abundant food, shelter and habitats (Yuan et al., 2020). In Chongming Island, a large scale of P. australis populations were distributed in the middle and high tidal flats of Dongtan wetland, but they can also be found at low tidal flats with high soil salinity near the coastline, in the form of scattered patches, showing its adaptation to various coastal environmental conditions. Based on the wide ecological adaptability of P. australis in coastal wetlands, this study proposed the following hypotheses: (i) seeds of P. australis populations along the tidal flat gradient of the Dongtan salt marsh wetland will show divergent germination strategies, which will be influenced by the long term adaptation of the original populations to the local habitats, especially the soil physicochemical properties; (ii) soil salinity as a main environmental factor along the tidal flat gradient will seriously influence the germination of seeds of P. australis populations, and the germination strategies of seeds collected from different tidal flats will correspond to the soil salinity of the original habitats; (iii) in the subtropical marine monsoon climate area, P. australis seed germination will also be improved by the lower seed storage temperature as an often necessary condition for seed break dormancy.
Materials and methods
Study area
Dongtan as a coastal salt marsh wetland locates in the Chongming Island at the estuary of Yangtze River (121°50′E-122°5′E, 31°25′N-31°38′N). The area locates in the northern edge of the mid-subtropical zone with the marine monsoon climate. The average annual temperature is 15.3°C and the annual precipitation is 1100 mm (Jia et al., 2022).
The seeds of P. australis populations were collected from the Dongtan wetland at a tidal estuary with an irregular semi-diurnal tide type, and the daily tidal flat characterized by two tidal changes of day and night. According to the observations in recent decades, the mean sea level is 2.17 m, the mean low water level is 1.03 m, and the mean high water level is 3.5 m (Yuan et al., 2020). The area is densely covered with tidal creeks, and the zones of high, middle and low tidal flats are very obvious. Along the elevational gradient of the transect, three tide sampling locations were established including the high tidal flat (2.7–3.5 m), the middle tidal flat (2.4–2.7 m), and the low tidal flat (2.2–2.4 m). The P. australis populations at the middle and high tidal flats in the Dongtan marsh wetland were distributed in continuous bands, while at the low tidal flat, it was distributed in patches.
Experimental design
Seed collection and storage
Given that P. australis is polyploid, with ploidy levels ranging from 4x to 12x, non-coding chloroplast DNA (cpDNA) has been the marker of choice in many studies due to its typical maternal inheritance (Saltonstall, 2001, 2016). Researchers have shown that haplotype P, representing an octoploid lineage, is now extensively dispersed in eastern China, with a concentration in coastal salt marshes (Liu et al., 2023, 2022). In late October 2022, seeds of P. australis were collected from the populations along the high, middle, and low tidal flats of the Dongtan salt marsh. Seed maturity and size were designed completely randomly in this study, and collected seeds were stored at 4°C and 20 ± 1 °C (room temperature) until the experiment began (the storage period is from early December 2022 to mid-March 2023). The seed storage temperatures simulated the typical average temperatures in the natural habitats. There was no significant difference in 100-grain weight among different habitats (Supplementary Figure S1).
Seed germination experiments
Seeds were taken out after being stored in the incubator, and then the palea and lemma were peeled from the seeds separately. Firstly, seeds were soaked in 2% sodium hypochlorite solution which was used to disinfect for 10 min, constantly stirring to ensure full contact between the seeds and the solution Secondly, seeds were washed repeatedly with pure water for 4 times to remove the residual disinfectant on them. Finally, they were soaked in the distilled water for 24 h. The seeds were cleaned and disinfected and placed evenly in Petri dishes (Φ= 9 cm), containing 2 mL of NaCl solution with salinity of 0%, 0.5%, 1%, 1.5% and 2%, respectively. Such salinities simulated the typical soil salinities (Biemond et al., 2023; Cai et al., 2015), and the extreme salty tides invading the natural habitats of P. australis populations. The evaporated water from the Petri dishes was replenished every day according to the calibrations.
Each salinity treatment consisted of 3 replicates; and each replicate contained 50 seeds. Seed germination experiments were carried out in an incubator with alternating light and dark conditions at 25/16°C for 14/10 h light/dark regime for 21 d. The standard for identifying seed germination was that the radicle penetrating the episperm and the bud length reaching to 1 mm (Yu et al., 2012). The situations and the numbers of germinated seeds were observed and recorded daily for 21 days, and the parameters (such as germination rate (Gt (Equation 1)) (%), germination index (GI (Equation 2)), germination speed (Gs (Equation 3)) and germination potential (Equation 4) (%)) were calculated.
Where the Gt is the number of germinated seeds at day t (7 d); Dt is the number of days. Gs is the speed of germination; Nn is the total number of days for observing the germinated seeds.
Germination pattern of seeds, which were collected from the populations in the original habitats and then they were treated under different seed storage temperatures (4°C as lower temperature and 20 ± 1°C as room temperature, respectively), were investigated, including a total of 24 Petri dishes (4 types of habitats × 3 replicates for each type of habitat × 2 storage temperatures). The numbers of germinated seeds were recorded daily for 21 days, and the above germination traits were calculated.
Collection of soil samples and measurement of physicochemical properties
Sampling plots were set up in P. australis populations at different tidal flats in the Dongtan salt marsh wetland. Taking the dykes built in 1998 and 2002 as the boundary, respectively, the plots near the coast were named as L group and those within the dyke were named as IN group. At the low tidal flat outside the dyke, 5 patches with a distance of 500 m were randomly set and was named as A, B, C, D and E, respectively. A 15 m × 15 m plot was set in each patch, and then, three 1 m × 1 m sampling quadrats were randomly set in the plot. In the sampling plots within the dyke (IN), five 1 m × 1 m sampling quadrats were randomly set with a distance of 50 m. At the middle (M) and high tidal flats (H), where P. australis populations distributed in large areas, ten 1 m × 1 m quadrats were set with 20 m apart at each tidal flat (Figure 1). Three soil column samples (Φ = 5 cm, H = 20 cm) were randomly taken from each plot; each soil column sample was divided into two depths (0–10 cm, 10–20 cm). Soil samples at each depth were mixed, and then they were put into different cotton cloth bags brought back to laboratory for measuring the physicochemical properties, such as salinity, pH, water content, total nitrogen (TN), total phosphorus (TP), available phosphorus (AVIP), nitrate nitrogen (NO3–N), ammonium nitrogen (NH4+-N) and organic carbon (SOC) were detected according to the other method (Feng et al., 2022; Jia et al., 2022). Soil microbial biomass C (MBC) and microbial biomass N (MBN) were measured by an improved fumigation method (Feng et al., 2022).
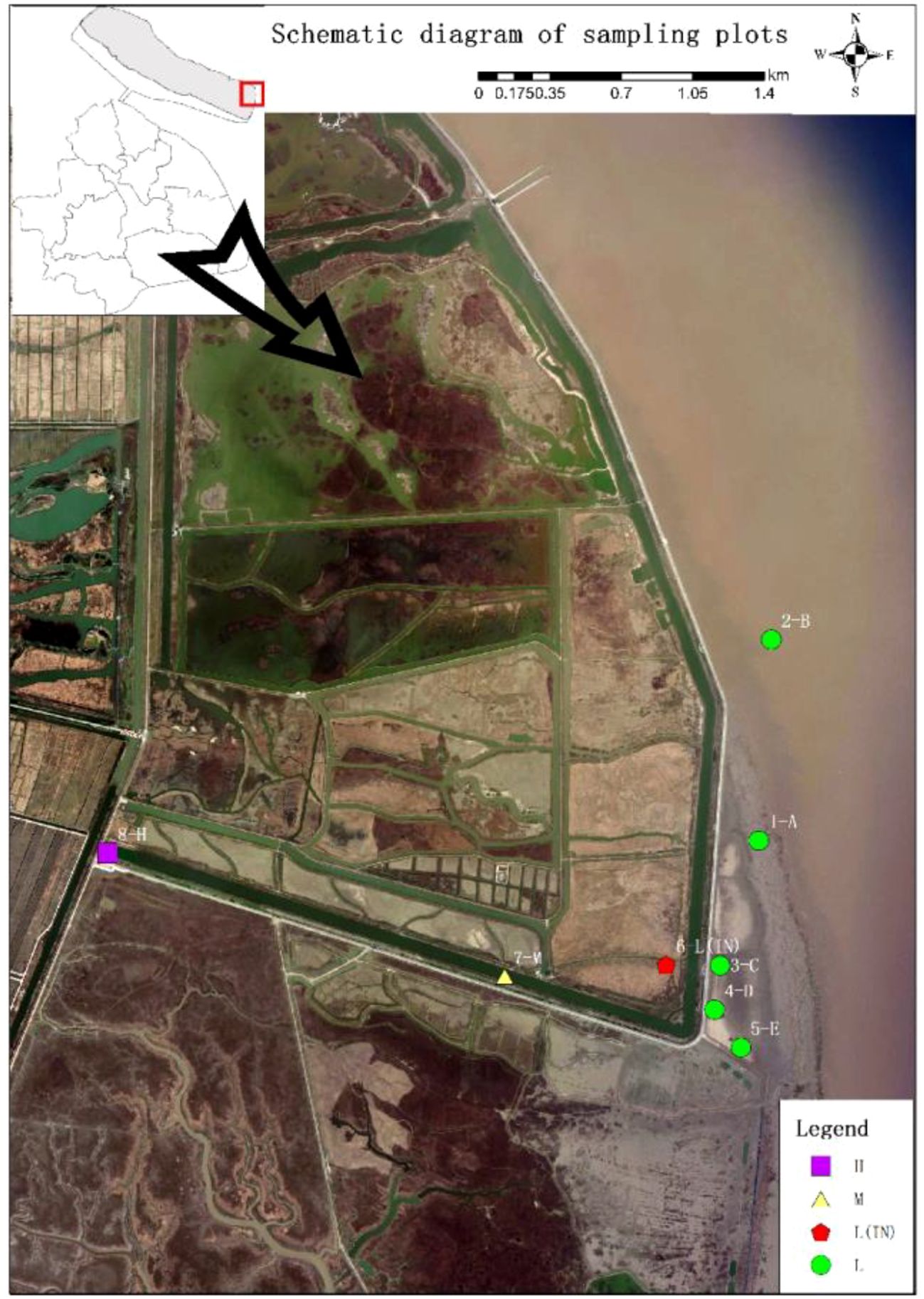
Figure 1. Study area and sample site location (L: low tidal flat outside the dyke; L(IN), low tidal flat inside the dyke; M, middle tidal flat; H, high tidal flat).
Data analysis
SPSS 22.0 was used for analyzing and comparing the data (mean ± standard error) and the significance test. In the absence of interaction, data from the germinated seeds was pooled, and the effects of salinity on the germination parameters were analyzed using one-way ANOVA and the Tukey test for the least significant difference (p=0.05). Two-way ANOVA was used to analyze the interaction of tidal level and treatment salinity on seed germination. Redundancy analysis (RDA) was used to analyze the differences in the correlation between the germination parameters of seeds stored under different temperatures and the soil physicochemical factors (pH, water content, salinity, TP, TN and SOC). Structural equation modeling (SEM) was used to evaluate and quantify the effects of soil physicochemical factors on the differences of germination parameters of seeds collected from the populations at different tidal flats and stored under different temperatures. RDA analysis and SEM used the “vegan” package (Dixon, 2003) and “lavvan” package (Rosseel, 2012) in R 4.1.2 version, respectively.
Results
Effects of soil salinity on seed germination
With the increase of soil salinity, the germination rates, germination indexes and germination speeds of P. australis seeds collected from populations at different tidal flats decreased compared with the control group at 0% salinity (Figures 2a–e; Supplementary Tables S1, S2). When the treatment salinity was 0.5%, the seed germination rate of the P. australis population in the L tidal flat (91.1% ± 0.38) was significantly higher than that in the other several tidal flats(L(IN): 62.2% ± 1.5; M: 55.3% ± 0.5 and H: 66% ± 1.2). At 1% and 1.5% salinity, P. australis seeds collected from the populations at all tidal flats germinated, and the seed germination rates among the tidal flats showed no significant difference, but the germination rate of seeds collected from the populations at the L tidal flat outside the dike was higher than those of seeds collected from populations at the other sites (Figures 2c, d). At 2% salinity, only the seeds collected from the populations at the low tidal flat germinated, and the germination rates decreased by 90% compared with the control group (Figure 2b). The mixed linear model showed that soil salinity, tidal flat and their interactions affected the seeds germination, and the salinity was the main factor (Table 1).
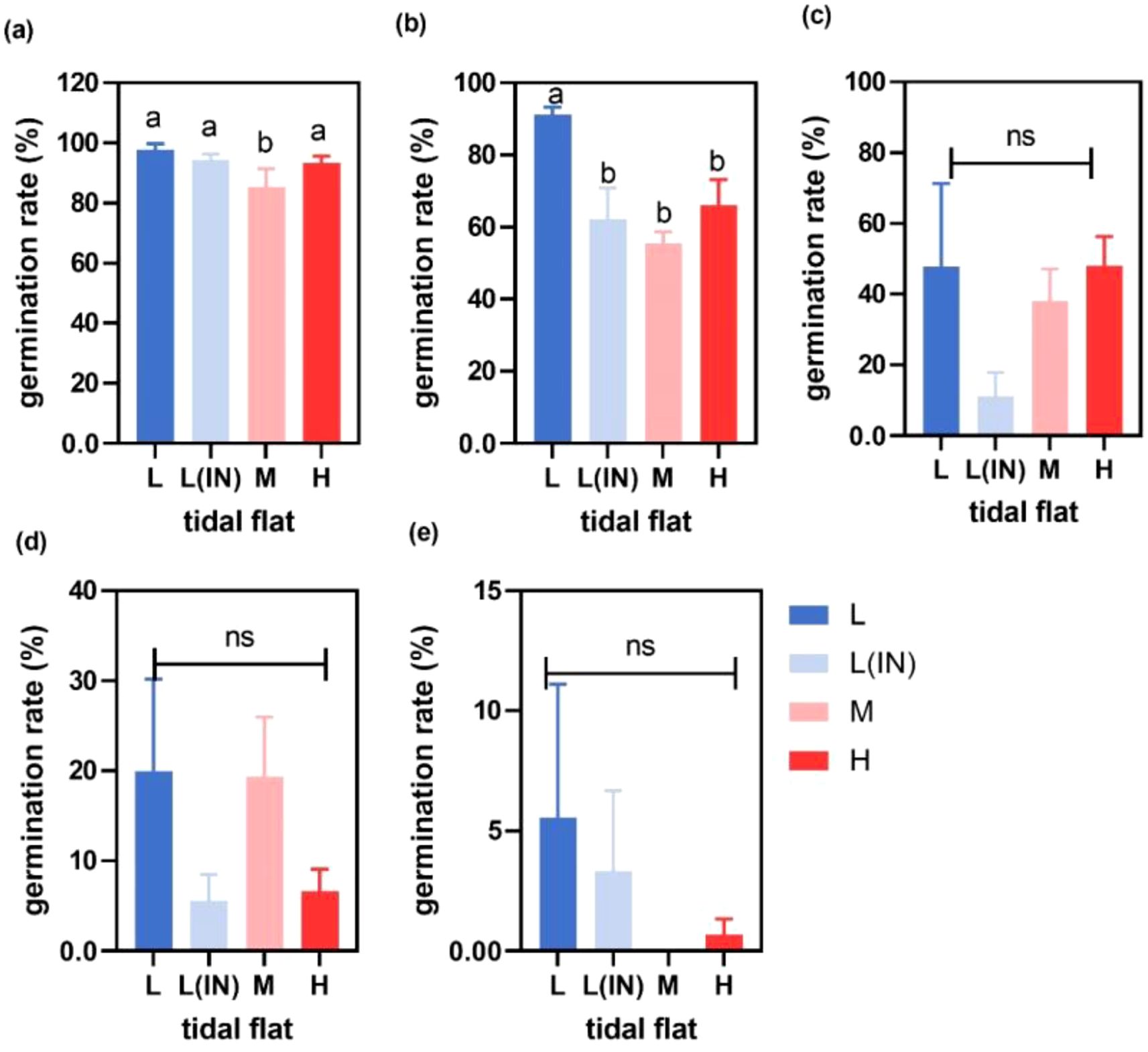
Figure 2. Germination of P. australis seeds collected from the populations at different tidal flats and treated with different salinities conditions (a) water treatment, (b) 0.5% salinity treatment, (c) 1% salinity treatment, (d) 1.5% salinity treatment, (e) 2% salinity treatment. (L, low tidal flat outside the dyke; L(IN), low tidal flat inside the dyke; M, middle tidal flat; H, high tidal flat. Different letters indicated significant differences between treatments and germination parameter, p<0.05, Error bars represented ± SD.).
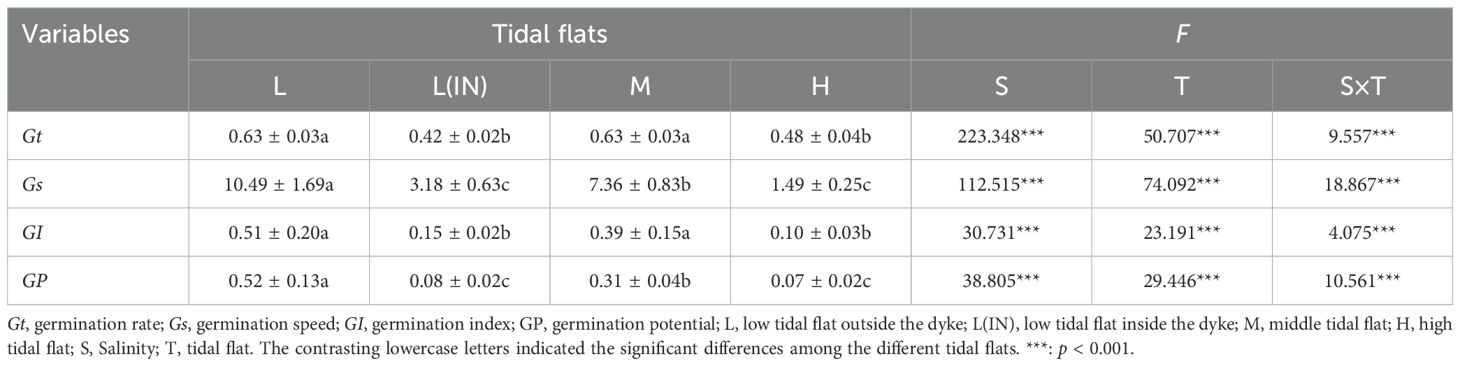
Table 1. Results of two-way ANOVAs testing the effects of salinity, the tidal flats and their interactions on the germination indexes of P. australis seeds collected from the populations at the low, middle and high tidal flats in the Dongtan wetland.
Effects of storage temperatures on seed germination
The germination rates of P. australis seeds collected from populations at the high, middle and low tidal flats inside the dike, and stored at the room temperature (20 ± 1°C) decreased compared to those stored at the low temperature (4°C). The germination rate of seeds collected from the populations at the L tidal flat and stored at the room temperature decreased by 8%-12% compared to those stored at 4°C condition (Figures 3a–d), but the germination rates of seeds were basically kept the same when the treatment salinity was 2% (Figure 3). When the treatment salinity was 0.5%, the seed germination rates of the P. australis populations in the L, L(IN), M and H tidal flats under room temperature storage were 43.0% ± 12.23, 28.0% ± 14.42, 44.4% ± 6.36 and 49.2% ± 5.02, respectively. However, under the same treatment salinity, the seed germination rates of the P. australis populations in the L, L(IN), M and H tidal flats under low-temperature storage were 62.6% ± 6.63, 12.7% ± 3.05, 30.0% ± 2.0 and 35.05% ± 5.47, respectively. When the salinity was treated at 1%, 1.5% and 2% respectively, the seed germination rates of L(IN), M and H tidal flats were increased under room temperature storage conditions compared with low-temperature storage conditions (Figures 3b–e). Regardless of the treatment salinity conditions, the germination rate of L tidal flat seeds after low-temperature storage was higher than that of seeds under room temperature storage conditions (Supplementary Tables S1, S2).
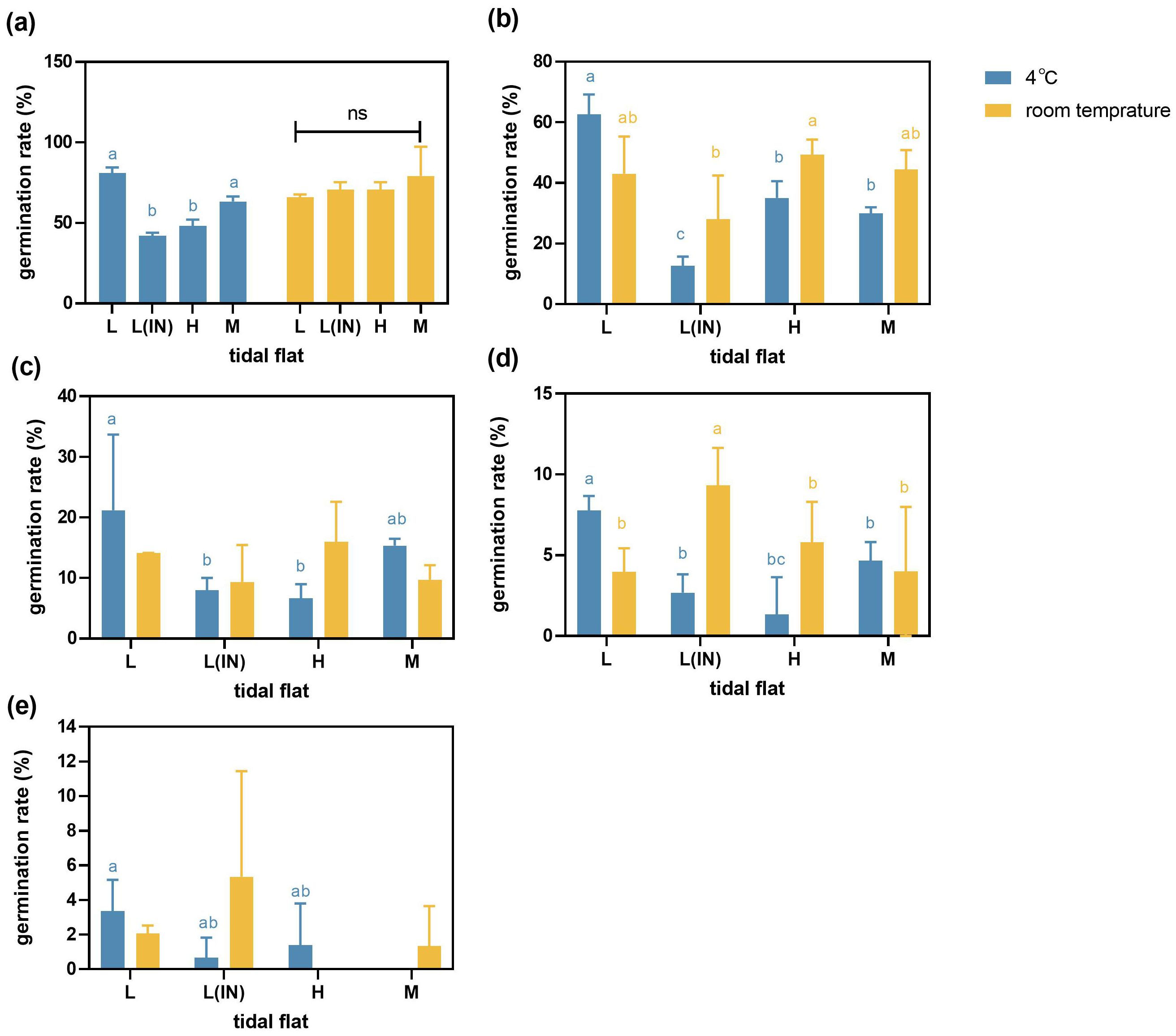
Figure 3. Germination of P. australis seeds collected from the populations at different tidal flats and treated with different salinities under the two temperature storage conditions. (L, low tidal flat outside the dyke; L(IN), low tidal flat inside the dyke; M, middle tidal flat; H, high tidal flat. (a) water treatment, (b) 0.5% salinity treatment, (c) 1% salinity treatment, (d) 1.5% salinity treatment, (e) 2% salinity treatment. Error bars represented ± SD.). The contrasting lowercase letters (a, b, c) indicated the significant differences among the different tidal flats.
Soil physicochemical properties in the original habitats of P. australis populations at different tidal flats
Total nitrogen (TN), organic carbon content (Organic C), pH and available phosphorus (AVIP) of different soil layers in the original habitats of P. australis populations were significantly different. The contents of NO3–N (0.18 ± 0.07 mg/kg), NH4+-N (1.02 ± 0.17 mg/kg) and TN (0.1 ± 0.05g/kg) show a trend that they were higher at high tidal flat than at middle (NO3–N (0.20 ± 0.07 mg/kg), NH4+-N (0.91 ± 0.04 mg/kg) and TN (0.05 ± 0.02g/kg)) and low tidal flats (NO3–N (0.17 ± 0.04 mg/kg), NH4+-N (0.96 ± 0.17 mg/kg) and TN (0.09 ± 0.02g/kg)). Soil physicochemical indexes of different tidal flats were all significantly different, except for total phosphorus (TP). 0–10 cm soil layer salinity at the low tidal flat (0.5 ± 0.18 PSU) was higher than that at the middle (0.33 ± 0.08 PSU) and high tidal flats (0.29 ± 0.14 PSU), respectively, and soil salinity at 0–10 cm layer was higher than that at 10–20 cm layer. The soil pH of the low-tidal flat (8.12 ± 0.24) and the middle-tidal flat (8.31 ± 0.39) were higher than that of the high-tidal flat (7.92 ± 0.38), while the soil salinity of the 0–10 cm layer was lower than that of the 10–20 cm layer. This phenomenon indicates that the soil gradually tends to become salinized from the high tidal flat to the low tidal flat. The soil available phosphorus (AVIP)content in the tidal flat was the highest, being 21.15 ± 3.55 mg/kg. The variation trends of soil water content and AVIP were consistent with those of soil salinity at the tidal flats, but were opposite with those of soil salinity between soil layers. There was no interaction between soil physicochemical properties of soil layers and tidal flats (Table 2).
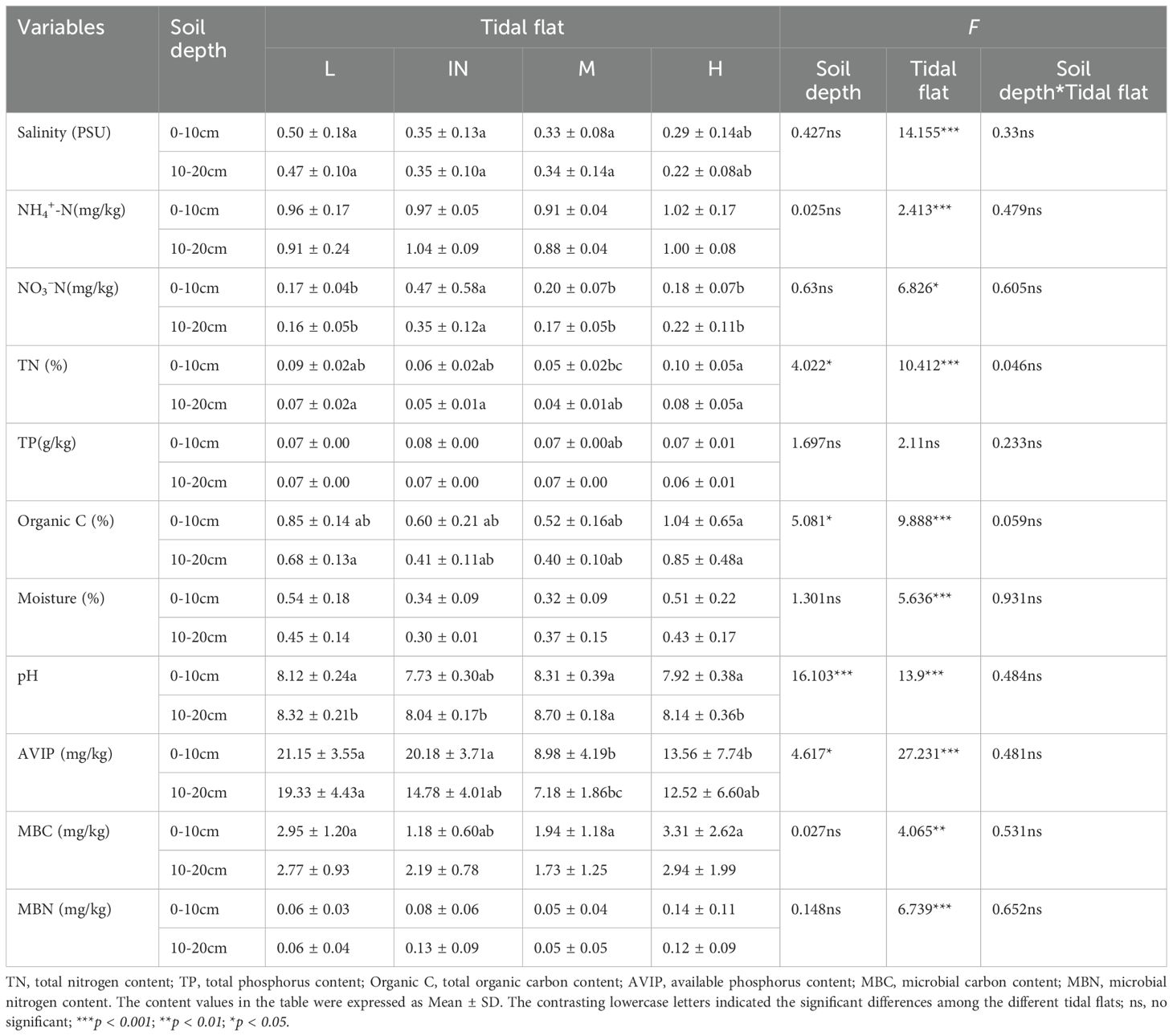
Table 2. Results of one-way ANOVAs testing the effects of soil depth at different tidal flats on the soil physicochemical properties of the habitats of P. australis populations, and two-way ANOVAs testing the effects of soil depth and different tidal flats on the soil physicochemical properties of the habitats of P. australis populations.
Correlation between seed germination parameters under different seed storage conditions and soil physicochemical factors of original habitats of P. australis populations
The correlation analysis between seed germination parameters under 4 °C seed storage condition and soil physicochemical factors of original habitats of P. australis populations showed that germination rates, germination potentials, germination indexes and germination speeds were significant positively correlated with soil salinity, AVIP and water content of original habitats of P. australis populations, while, they were negatively correlated with soil NO3–N in the same soil layer. Among them, seed germination rates and germination speeds had the greatest correlation with soil salinity of original habitats of P. australis populations, which was consistent with the results of seed germination experiment with salt addition treatment (Figure 4a). When seeds stored under the room temperature, seed germination rates were positively correlated with soil N/P and soil microbial biomass carbon in 10–20 cm layer, and negatively correlated with soil TP content of original habitats of P. australis populations. Seed germination potentials were positively correlated with soil pH value, microbial biomass carbon content in 0–10 cm layer, and AVIP in 10–20 cm layer soil, and negatively correlated with soil NH4+-N in 10–20 cm layer of original habitats of P. australis populations. Seed germination indexes and germination rates were positively correlated with soil AVIP, salinity and soil moisture in 10–20 cm layer, and negatively correlated with soil NO3–N of original habitats of P. australis populations (Figure 4b).
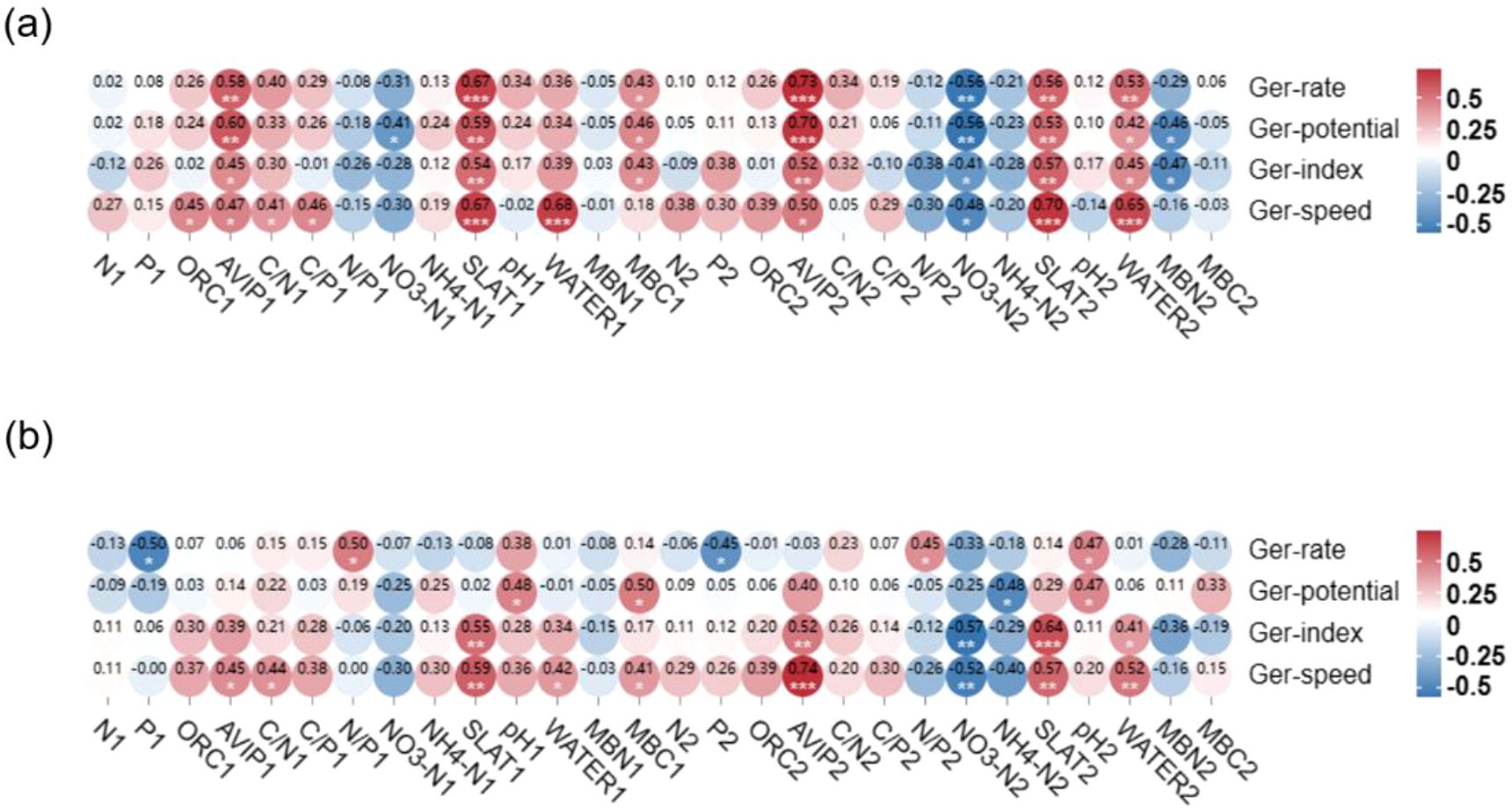
Figure 4. Correlation between the seed germination parameters of untreated seeds subjected to two storage temperatures and the soil physicochemical properties of their original habitat populations. (a) 4°C storage condition, (b) room temperature storage condition. (Ger-rate, germination rate; Ger-potential, germination potential; Ger-index, germination index; Ger-speed, germination speed; TN, total nitrogen content; TP, total phosphorus content; ORC, total organic carbon content; AVIP, available phosphorus content; MBC, microbial carbon content; MBN, microbial nitrogen content; 1 and 2 represent different soil layers).
In order to further explore the effects of interaction between germination parameters of seeds under different seed storage temperatures and the key soil physicochemical factors of original habitats of P. australis populations, RDA was conducted, which showed that germination rates of seeds collected from the populations at different tidal flats and stored under 4 °C were significantly different. Taking the germination rates of seeds stored at 4°C as response variables, and soil salinity, NO3–N, NH4+-N, TN, TP, organic carbon content, moisture content, pH, AVIP, MBC and MBN of original habitats of P. australis populations as the explanatory variables, RDA was further conducted, which showed that the first and second ranking axes of RDA explained 65.77% of the impact of 0–10 cm layer soil physicochemical properties on the variation of seed germination performance. In the RDA ranking diagram, AVIP, C/N of 0–10 cm soil layer, C/P of 0–10 cm layer, and NO3–N of 10–20 cm layer of original habitats of P. australis populations were most closely related to the first axis. In addition, soil salinity, pH at 0–10 cm soil layer, and soil water content in 0–10 cm layer, MBN and NO3–N were also strongly correlated with the first principal component. In the soil physicochemical properties, soil TN had the greatest correlation with the second principal component, which followed by soil C/N. Cluster analysis showed that the germination rates of seeds collected from the populations at the middle and high tide flats had a strong correlation with NO3–N, C/N, N/P and NH4+-N of 0–10 cm and 10–20 cm soil layers, and the germination rates of seeds collected from the populations at the low tidal flat were correlated closely with other soil physicochemical properties of original habitats of P. australis populations (Figure 5a).
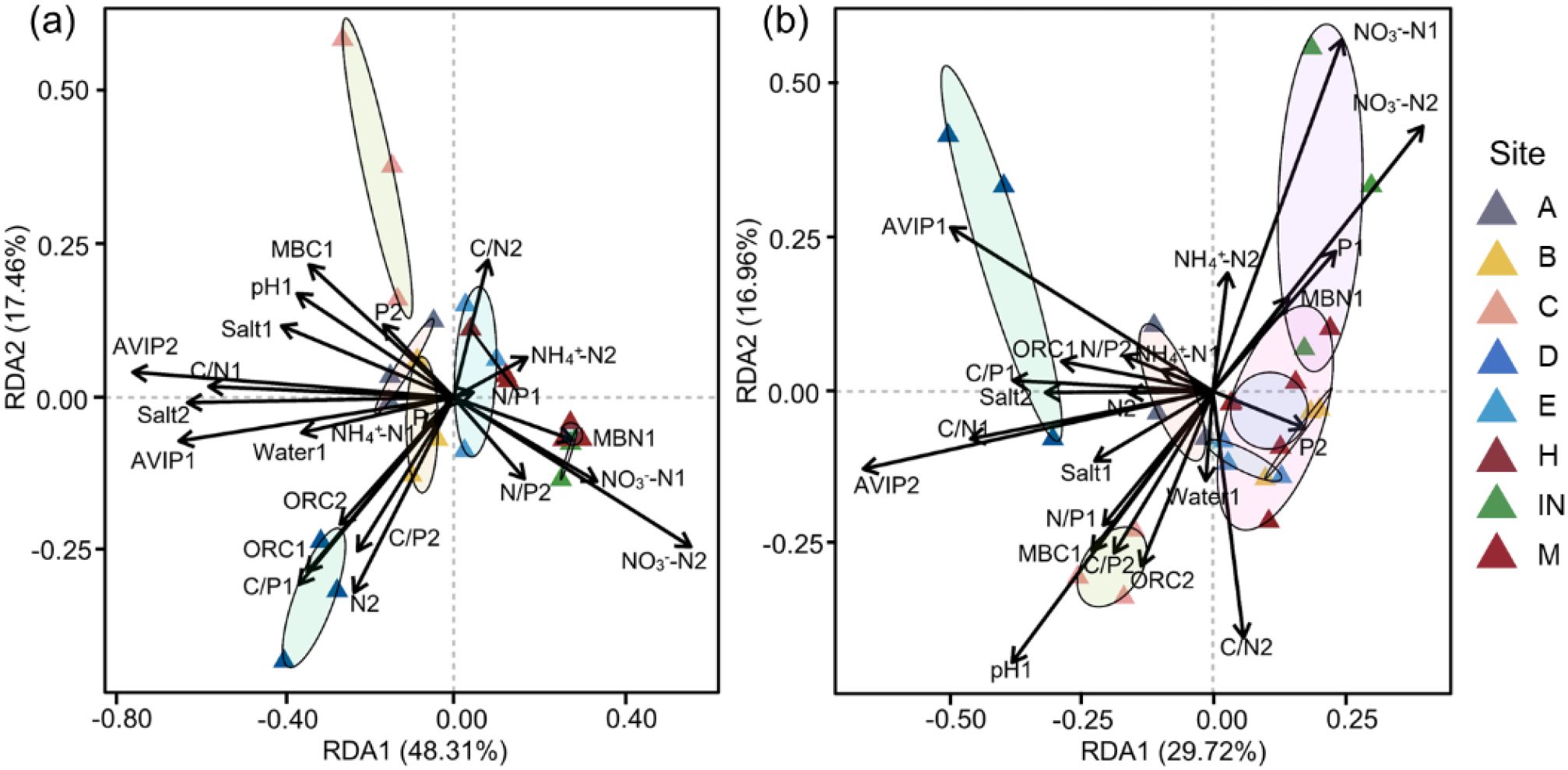
Figure 5. Redundancy analysis of soil physicochemical properties of the original population habitats and germination rates of seeds collected from the populations at different tidal flats (a) stored at 4°C and (b) stored at room temperature.
The relationship between soil physicochemical properties of original habitats of P. australis populations and the germination rates of seeds collected from the populations at different tidal flats and stored at the room temperature was also analyzed through RDA, which showed that total RDA results explained 46.68% of the impact of soil physicochemical properties on the seed germination performance, and the first axis included most of the effective results (Figure 5b). Result showed that AVIP, C/N, C/P and NO3–N content of 0–10 cm soil layer, were most closely related to the first axis. In addition, soil pH, TP in 0–10 cm soil layer, and soil C/N, NH4+-N in 10–20 cm soil layer were also strongly correlated with the second axis. Cluster analysis showed that germination parameters of seeds collected from the populations at the middle and high tidal flats, respectively, had strong correlation with soil NO3–N, TP, C/N and NH4+-N in 10–20 cm soil layer. The germination rates of seeds collected from the populations at the low tidal flat were significantly correlated with other soil physicochemical properties (Figure 5b). The RDA analysis results on the relationships between germination rates of seeds collected from the populations at different tidal flats and stored at different temperatures and the soil physicochemical properties showed that seed germination rates had the same trend of correlation with soil NO3–N content, AVIP, pH and C/N of the soil layers of original habitats of P. australis populations (Figure 5).
The effects of soil physicochemical factors of original habitats of P. australis populations on the differences in seed germination parameters
The germination rates, germination potentials, germination indexes and germination speeds measured in this study were represented as a germination parameter of the germination strategy to construct a structural equation model (SEM). The SEM results showed that under the seed storage condition at 4°C, soil water, N/P1, N/P2 and soil microbial biomass carbon content of original habitats of P. australis populations affected seed germination. The path coefficient between soil moisture, soil N/P1, N/P2, MBC and seed germination was 0.14, 0.33, 0.2 and 0.14, respectively (Figure 6a). The tide level directly reduced the soil organic carbon content of original habitats of P. australis populations, and then indirectly led to the decline of the germination rates of seeds stored at 4°C (Figure 6a). The tidal level promoted the increase of soil moisture content, nitrogen and phosphorus ratio of original habitats of P. australis populations, which indirectly led to the increase of seed germination rates under the same seed storage conditions (Figure 6a). Soil pH of original habitats of P. australis populations was the most important factor affecting seed germination, when seeds were stored at room temperature, and the path coefficients between soil water content, C/P1, pH2 and seed germination was 0.14, 0.17 and 0.3, respectively (Figure 6b). The path coefficient between tidal flats of original habitats of P. australis populations and germination of seeds stored at room temperature was -0.16, while the path coefficient between tidal flats and germination of seeds stored at 4°C was -0.97. The tide level directly increased soil pH, which indirectly led to increase in germination rates of seeds collected from the populations at different tidal flats and stored at room temperature, while, the decrease of soil moisture content and phosphorus content among different tidal flats indirectly lead to the decrease of germination rates of seeds under the same storage conditions (Figure 6b).
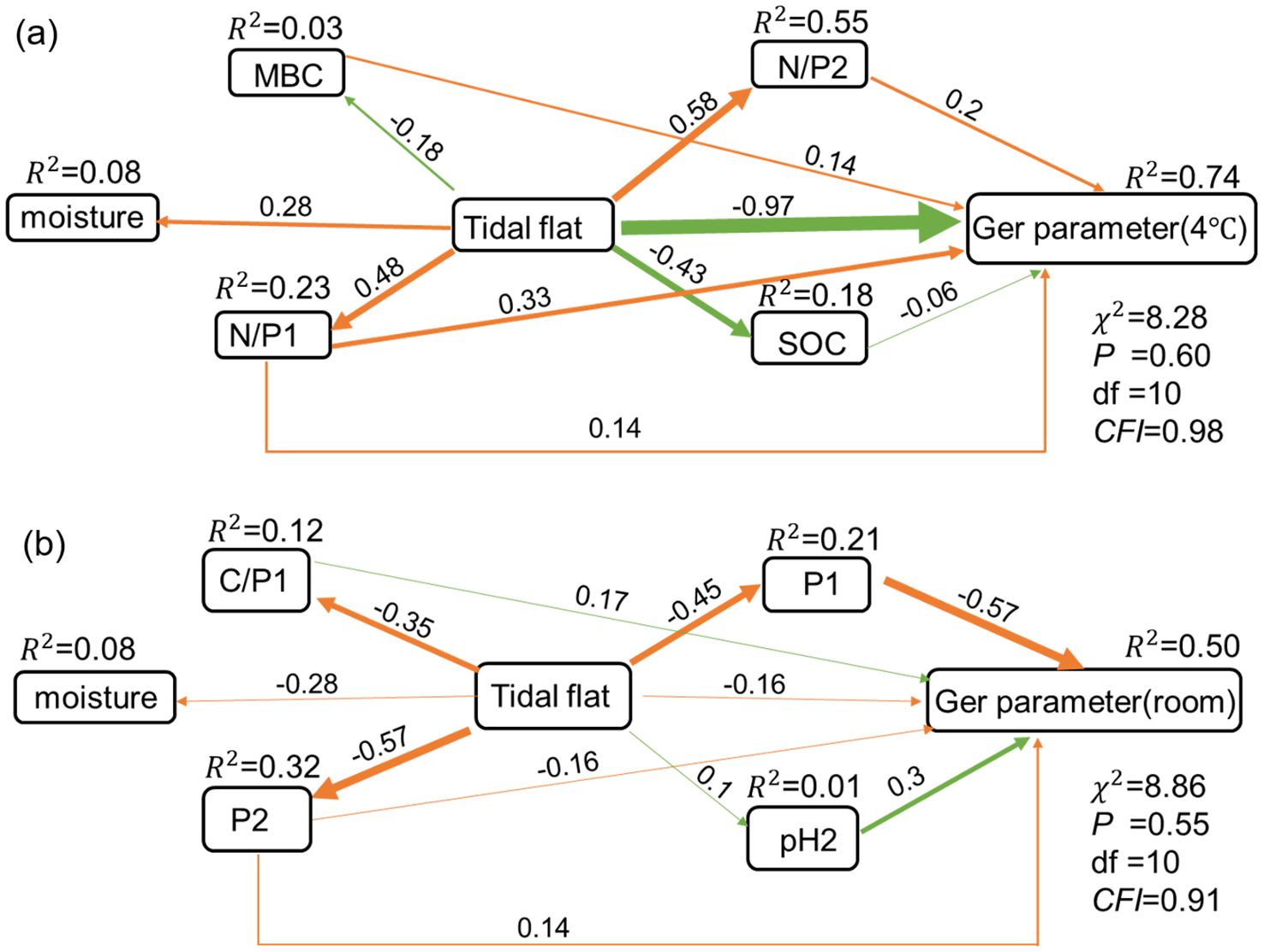
Figure 6. Structural equation model (SEM) for the germination parameters of seeds collected from the populations at different tidal flats and stored under different storage temperatures, and the soil physicochemical properties of the original population habitats. (a) 4°C storge condition, (b) room temperature storge condition.
Discussion
Response of seed germination to salinity in gradient tidal flat P. australis population
The two essential and critical developmental stages in the life cycle of an annual plant were regarded as flowering and seed germination (Ehrlén et al., 2015). Seed germination is usually the first step in the establishment of a plant population. Numerous studies demonstrated the effects of environmental factors such as temperature, salinity, soil moisture and other soil factors on seed germination (Gul et al., 2013; Fernandez-Torquemada and Sanchez-Lizaso, 2013; Gorai et al., 2006; Kettenring and Whigham, 2009). Soil salinity was found to be one of the vital factors directly or indirectly affecting the germination of seeds of P. australis populations along the Dongtan salt marsh wetland (Figure 2). Generally, the germination rates and germination potentials of P. australis seeds decreased with the increase of soil salinity (Wijte and Gallagher, 1996; Zhao et al., 2021). Seed germination rates of P. australis populations in the Yellow River Delta were also found to be inhibited seriously under the condition of high salt (2% - 3%) (Yu et al., 2012). Our research revealed that P. australis seed germination rates topped 80% under 0 salinity conditions (Figure 2). However, when exposed to 2% salinity, the germination rates of P. australis seeds from diverse tidal flats decreased to varying degrees, and some even failed to germinate. In addition, seeds at 2% salinity from middle tidal flats failed to germinate compared to those from the low tidal flat (80.9% ± 0.06%). These suggested that P. australis seeds from the low tidal flat possessed a higher tolerance and adaptability to salinity. Adaptation to a saline habitat that is the low-tidal zone is a likely explanation for our observation (Guan et al., 2016; Haraguchi, 2014). Under the two storage temperature conditions, the germination rate, germination index, germination potential and germination speed of L and L(IN) were all different. This might be due to the construction of the dike, which led to the reduction of hydrological connectivity, thus resulting in the differentiation of germination strategies between these two populations. Consequently, P. australis population could potentially establish and expand gradually based on the seeds of pioneer P. australis genets in low tidal habitats with relatively high soil salinity.
The germination rates of P. australis seeds in the Yangtze River Delta were slightly affected when salinity reached 1%. In contrast, the same salinity level led to a decreased germination rate for P. australis seeds collected from inland regions (Yu et al., 2012). Researchers studied the salt tolerance of P. australis seeds in salt marsh wetlands and inland estuaries, revealing the same pattern that the germination rates of P. australis seeds collected from the salt marsh wetland population were higher than those of seeds collected from the estuarine population (Haraguchi, 2014). The two-way ANOVAs testing the effects of salinity, the tidal flats and their interactions on the germination indexes of P. australis, results showed that salinity was the main factor affecting germination rate (Gt) and germination speed (Gs), while the tidal flat was the main factor affecting germination index (GI) and germination potential (GP) (Table 1). It is well known that salinity is negatively correlated with Gt and GI, low concentrations of NaCl induce seed dormancy, and high concentrations of NaCl induce osmotic stress and ionic toxicity (accumulation of Na+ and Cl-) and inhibit seed germination (Rajabi Dehnavi et al., 2020). In fact, for seeds in a salinity environment, due to the low osmotic potential of the germination medium, salinity can change the imbibition process of seeds to water, and water diffused from low salinity to high salinity, delaying the absorption of water by seeds, thus reducing germination (Farooq et al., 2015). Under salt stress, Na+ and Cl− may be absorbed by seeds, and the toxic effect of NaCl may occur, thus slowing down the germination index and germination speed (Ashrafi and Razmjoo, 2015). The high germination rates, fast germination speeds and high germination vitality were found in P. australis seeds collected from the low tidal flat population growing in habitats with high soil salinity and high tide disturbance frequency (Baldwin et al., 2010; Briea, 2006). Our study also has similar results, low tidal flat P. australis seed Gt is higher, Gs is faster and GP is better than other tidal flats. Table 1 shows that there are significant differences in germination parameters (Gt, Gs, GP, and GI) between different tide levels. As shown in Figure 4, different germination parameters are significantly correlated with some factors in soil physicochemical properties, which indicates that soil sediments and nutrient availability directly or indirectly affect seed germination traits of the reed populations. Such seed germination strategy may help accelerate the recruitment and establishment of P. australis populations at the low tidal flat of the Dongtan salt marsh wetland. Greenberg et al. (2001) revealed that most of the time plant seeds tended to adopt a “sit or wait” germination strategy, i.e., seeds sitting in their habitats and waiting for favorable opportunities for germination caused by environmental disturbances. However, the germination rates, speeds, and vigor of P. australis seeds from middle and high tide levels were inferior to those from low tidal flat populations. This suggested that the underground seeds of P. australis populations in middle and high tide levels might employ a “sit or wait” germination strategy, i.e., they could germinate when favorable opportunities arose. Along the Dongtan salt marsh wetland, the local adaptation of P. australis populations to the edaphic environmental conditions (especially the soil salinities) in the habitats shaped the sexual reproduction and seed germination strategies of P. australis populations as well as the seedling growth, showing the maternal effect in the local populations. In other words, the sexual progeny of P. australis populations at different tide levels in the Dongtan salt marsh wetland, apparently inherited the adaptive characteristics of the original ancestral populations, suggesting that when the sexual offspring of P. australis populations colonize the same habitats of their parental populations at different tide levels, they might perform well and maintain the long term structural and dynamic stability of the local populations.
Effect of chilling environment on seed germination
70% of plant seeds have different types and degrees of dormancy, and plants need specific mechanisms to avoid adverse environmental conditions in nature, such as low temperature and dryness, and try to ensure that plants germinate in an environment suitable for seedling establishment (Afroze and O’Reilly, 2017; Baskin et al., 2003). Dormancy of gramineous seeds is a common phenomenon, and low temperatures in winter could efficiently break the dormancy of seeds of P. australis, suggesting the rejuvenation of seed germination vigor needs a short and proper cold treatment (Milberg, 1999; Li et al., 2013). The germination rates of P. australis seeds collected from the low tidal flat populations under 4°C storage condition for a while was significantly higher than those under the storage condition at room temperature (20 ± 1°C) for the same duration (Figures 2, 3), indicating that different storage temperatures affected the germination vigor of P. australis seeds. The low-temperature storage condition (4°C) promoted the germination rates. Whereas, when P. australis seeds collected from the populations at the middle and high tidal flats were stored in the room temperature condition, the germination rates of seeds were improved, compared with those stored in 4°C condition (Figure 3), which implied that low temperature, as one of the environmental seize factors for seed germination, regulated the after ripening of seeds collected from the populations at the low tidal flats. Low temperature mainly showed slow germination speed and low germination rate (Akinnuoye and Modi, 2015). As a major environmental stress factor, low temperature can break the original metabolic balance of seeds, destroy the enzyme system, accumulate toxic substances, and thus affect the growth and development of plants (Greenwood and MacFarlane, 2006). Temperature is critical to plant growth and development, and many plants in temperate regions can remember past winter seasonal temperature drops and undergo changes in after ripening patterns to ensure the survival and/or reproductive success of offspring (Gao et al., 2022; He and Li, 2018). However, our findings demonstrated that low-temperature treatment enhanced the germination of P. australis seeds collected from low tidal flat populations in the Dongtan coastal salt marsh wetland in a subtropical climate zone. This improvement was notable despite the lack of the same effect on seeds from middle and high tide flats.
The Dongtan wetland (especially the frontier low tidal flat) exposes to frequent wind and sea tidal impact, whose standing water often freezes in winter whenever there is cold weather; thus, after long-term adaptation, the germination of seeds from the low tidal flat populations required moist chilling condition was quite understandable. The chilling environment near the coastal wetland, the metabolism of stored protein was changed by inducing ROS production and reducing the activity of protease in seed cells, thus affecting the seed germination rate (Shutov et al., 2003). Therefore, the seeds produced by the low tide flat P. australis population need after-ripening, breaking dormancy after low temperature environment, and successfully germinating in suitable condition. As for the fact that the germination of seeds collected from the middle and high tidal flats with less ecological stresses in the habitats no longer required after ripening might be attributed to the comprehensive impacts of multiple physiological and environmental factors. Our results showed that the contents of NH4-N and NO3-N in the soil were higher than those at the low tidal flat. The contents of Organic C, MBC and MBN in high tidal flat were higher than those in low tidal flat (Table 2). This means that the middle and high tide zone environment can provide a relatively stable soil sediment and availability of plant nutrients compared to the low tide zone environment with high frequency tidal disturbance. Meanwhile, previous studies have found that the soil structure of the middle and high tide zone of Dongtan salt marsh was sandy or silty soil, while the soil quality of the low tide zone was sandy clay (Liu et al., 2021; Jia et al., 2022). Sandy soil and sandy loam with large pores and low organic matter content are suitable substrates for the germination of Jatropha curcas seeds. The germination rate and germination speed of seeds with clay texture were lower (Valdés-Rodríguez et al., 2013). The soil texture of P. australis population at different tide levels affected the proportion of soil pore water and the content of organic matter, which would change the germination of seeds and the growth and development of plants. The seeds collected from low tidal gradients germinate better when stored at low temperatures, while seeds from middle and high tidal gradients germinate better when stored at room temperature. This phenomenon is one of the ecological drivers for seed germination in this species, as at a low tidal gradient, seeds may experience low and fluctuating temperatures due to a low water table. In contrast, at a middle and high tidal gradient, seeds may experience warmer and constant temperatures due to higher water table (Xiao et al., 2009).
Response of early life history of seeds to habitat
Seed germination is the first stage of plant life, but the abiotic stress environment during seed germination can reduce seed germination by increasing seed quality degradation and reducing seed germination potential and vigor (Wang et al., 2024). The low temperature was often the key factor stimulating the seed germination vigor, while other conditions, such as suitable water, oxygen and nutrients, are still necessary (Lombardi et al., 2019). SEM results showed that germination of P. australis seeds stored at 4°C was affected by soil MBC, N/P ratio and soil water content in the original population habitats from which seeds were collected (Figure 6). Significant differences in soil pH, TN and organic carbon content in soil layers (p<0.05) were found among habitats from which P. australis seeds were collected, and the soil salinity, soil available nitrogen decreased with the increase of tidal flat, while other soil indexes showed no significant differences among the habitats (Table 2). The availability of certain elements is related to soil pH (Al-Wabel et al., 2018), thus, indirectly influencing the plant growing in the habitat. Soil pH is closely related to soil microbial diversity and indirectly affects soil MBC (Naz et al., 2022; Wan et al., 2021), while soil N/P affects the mineralization rate of soil, and further affect soil salinity synergistically with soil moisture (Miller and Geisseler, 2018), and indirectly affects seed germination rate (Feng et al., 2021).
As the mixed results of SEM analysis suggested, the relationship between soil properties, nutrients, and germination may be more complex. Former results also revealed the coupling effects of water, nitrogen and phosphorus in regulating the germination of wetland plant seeds (Zhao et al., 2019). Nitrate plays a role as a signaling molecule to mediate seed germination (Zhang et al., 2023; Li and Wang, 2015). Both endogenous and exogenous nitrate and phosphorus may affect seed germination (Song et al., 2016; Plassmann et al., 2008; Sims et al., 2012). The genetic diversity of P. australis patches in the Rhodes River Basin in the United States shows that almost all population patches are composed of multiple genotypes, and many patches are genetically different from other patches, which indicates that cross-fertilization is one of the sources of genetic diversity (McCormick et al., 2010). Therefore, the possibility of cross-fertilization and gene exchange between different populations may also affect the seed traits such as salt tolerance and germination rates across tidal zones (Lambertini et al., 2020). Except that, such as the water pollution, sediment and soil texture of tidal flats, structure, and availability of nutrients for plants also have direct or indirect influences on the seed germination strategy (Antonio Alburquerque et al., 2014; Bocchese et al., 2008; Peng et al., 2022; Satyanti et al., 2019; Wang et al., 1994). Chongming Dongtan Wetland is located in the lower reaches of the Yangtze River Delta, bearing the pressure of water pollution caused by population activities in the upper reaches, and the pollutants such as nitrogen, phosphorus and COD in the water can be removed by P. australis (Ho et al., 2024). Water pollution may cause significant differences in soil sediments between different tidal levels, and then affect the seed germination rate of different tidal flat populations. Wetland soil is the source, sink and transfer source of chemical pollutants. Anthropogenic activities (such as wetland reclamation, fertilization, and sewage discharge) have exacerbated changes in soil properties (such as SOM, pH, and Eh) of wetland soils/sediments (Bai et al., 2011). Absorption by the roots of P. australis can increase the residence time of these pollutants in the wetland, and then effectively reduce the diffusion of pollutants to the surrounding environment (Wang et al., 2015). In this study, P. australis seeds in coastal wetlands with high salinity showed a higher germination rate, which may be a trade-off in the face of tidal frequency and harsher environment.
Conclusion
To consolidate the existing populations and facilitate their further expansion, as well as to prevent habitat fragmentation, it is recommended to more conservation of P. australis populations which is adaptive to the high salinity environment in the low-tide zone with high physical and biological pressure in Dongtan salt marsh. As for the P. australis populations in the middle and high tidal flats, proper cultivation and maintenance should be carried out in the early stages and the external interference and damage should be avoided, so as to help the populations successfully cross the environmental sieve in the early growth stages and complete life history. Furthermore, P. australis seeds derived from the different tidal flat populations should be used actively in restoring or reestablishing the original populations at the same habitat, respectively. However, it should be noted that due to the frequent erosion of tides, the dynamic changes of water salinity, environmental temperature and soil physicochemistry have complex effects on the germination of P. australis seeds in soil seed bank, and the germination strategy is also dynamically changing. We need to be very careful about extrapolating our laboratory results to natural ecosystems.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JJ: Resources, Visualization, Formal Analysis, Data curation, Writing – review & editing, Conceptualization, Software, Methodology, Investigation, Writing – original draft. PJ: Writing – original draft, Writing – review & editing, Formal Analysis, Visualization, Supervision, Methodology, Software, Investigation, Data curation, Conceptualization. CY: Investigation, Conceptualization, Funding acquisition, Software, Writing – review & editing. DL: Writing – original draft, Investigation, Supervision, Conceptualization, Methodology, Writing – review & editing, Funding acquisition, Resources, Formal Analysis, Project administration. JW: Investigation, Writing – review & editing. YW: Investigation, Writing – review & editing. JC: Writing – review & editing, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Key Laboratory for Environmental Protection of Coastal Marine Ecological Environment Fund Project (20240105), the Project of Young Scientist Exchange for One Belt and One Road Strategy in the International Science and Technology Cooperation of Shanghai Science and Technology Commission (19230742600), Open Research Project for the Technology Innovation Center for Land Spatial Eco-restoration in Metropolitan Area, Ministry of Natural Resources (CXZX2021B01, CXZX2022B01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1598379/full#supplementary-material
References
Afroze, F. and O’Reilly, C. (2017). Effects of seed moisture content, warm, chilling, and exogenous hormone treatments and germination temperature on the germination of blackthorn seeds. Plant Biosyst. 151, 474–483. doi: 10.1080/11263504.2016.1179693
Akinnuoye, D. B. and Modi, A. T. (2015). Germination characteristics of SC701 maize hybrid according to size and shape at different temperature regimes. Plant Product. Sci. 18, 514–521. doi: 10.1626/pps.18.514
Al-Wabel, M. I., Hussain, Q., Usman, A. R. A., Ahmad, M., Abduljabbar, A., Sallam, A. S., et al. (2018). Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land Degrad. Dev. 29, 2124–2161. doi: 10.1002/ldr.2829
Alburquerque, J. A., Calero, J. M., Barrón, V., Torrent, J., del Campillo, M. C., Gallardo, A., et al. (2015). Seed treatment to overcome salt and drought stresses during germination in safflower (Carthamus tinctorius L.). J. Plant Nutr. 38, 2151–2158. doi: 10.1080/01904167.2015.1069331
Angelini, C., Altieri, A. H., Silliman, B. R., and Bertness, M. D. (2011). Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. BioScience 61, 782–789. doi: 10.1525/bio.2011.61.10.8
Antonio Alburquerque, J., Calero, J. M., Barrón, V., Torrent, J., del Campillo, M. C., Gallardo, A., et al (2014). Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 177, 16–25. doi: 10.1002/jpln.201200652
Auge, G. A., Blair, L. K., Neville, H., and Donohue, K. (2017). Maternal vernalization and vernalization-pathway genes influence progeny seed germination. New Phytol. 216, 388–400. doi: 10.1111/nph.14520
Bai, J., Huang, L., Yan, D., Wang, Q., Gao, H., Xiao, R., et al. (2011). Contamination characteristics of heavy metals in wetland soils along a tidal ditch of the Yellow River Estuary, China. Stochastic Environ. Res. Risk Assess. 25, 671–676. doi: 10.1007/s00477-011-0475-7
Baldwin, A. H., Kettenring, K. M., and Whigham, D. F. (2010). Seed banks of Phragmites australis-dominated brackish wetlands: Relationships to seed viability, inundation, and land cover. Aquat. Bot. 93, 163–169. doi: 10.1016/j.aquabot.2010.06.001
Baskin, C. C., Baskin, J. M., and Chester, E. W. (2003). Ecological aspects of seed dormancy-break and germination in Heteranthera limosa (Pontederiaceae), a summer annual weed of rice fields. Weed Res. 43, 103–107. doi: 10.1046/j.1365-3180.2003.00321.x
Bhatt, A., Gairola, S., Carón, M. M., Santo, A., Murru, V., El-Keblawy, A., et al. (2020). Effects of light, temperature, salinity, and maternal habitat on seed germination ofAeluropus lagopoides (Poaceae): an economically important halophyte of arid Arabian deserts. Botany 98, 117–125. doi: 10.1139/cjb-2019-0096
Biemond, B., de Swart, H., and Dijkstra, H. A. A. (2023). Mechanisms of salt overspill at estuarine network junctions explained with an idealized model. J. Geophys. Res. Oceans 128, 1–20. doi: 10.1029/2023JC019630
Bocchese, R. A., Morbeck De Oliveira, A. K., Melotto, A. M., Fernandes, V., and Laura, V. A. (2008). Effects of soil structure on germination of Tabebuia heptaphylla seeds. CERNE 14, 62–67. doi: 10.1080/16483840.2003.10414082
Briea, P. (2006). A study of Phragmites australis along an elevational gradient and seed germination response at different salinity levels. University of Massachusetts.
Cai, H., Savenije, H. H. G., Zuo, S., Jiang, C., and Chua, V. P. (2015). A predictive model for salt intrusion in estuaries applied to the Yangtze estuary. J. Hydrol. 529, 1336–1349. doi: 10.1016/j.jhydrol.2015.08.050
Dixon, P. (2003). VEGAN, a package of R functions for community ecology. J. Veget. Sci. 14, 927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Dupin, B., Durand, B., Cambecèdes, J., and Fromin, N. (2022). Plants playing at home: Advantage of native plant seeds for ski slope revegetation in the French Pyrenees. Ecol. Eng. 174, 106463. doi: 10.1016/j.ecoleng.2021.106463
Ehrlén, J., Raabova, J., and Dahlgren, J. P. (2015). Flowering schedule in a perennial plant; life-history trade-offs, seed predation, and total offspring fitness. Ecology 96, 2280–2288. doi: 10.1890/14-1860.1
Eller, F., Skálová, H., Caplan, J. S., Bhattarai, G. P., Burger, M. K., Cronin, J. T., et al. (2017). Cosmopolitan species as models for ecophysiological responses to global change: the common reed Phragmites australis. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01833
Eller, F., Guo, X., Ye, S., Mozdzer, T. J., and Brix, H. (2020). Suitability of wild Phragmites australis as bio-resource: tissue quality and morphology of populations from three continents. Resources 9, 143. doi: 10.3390/resources9120143
Elnaggar, A., El-Keblawy, A., Mosa, K. A., and Navarro, T. (2019). Adaptive drought tolerance during germination of Salsola drummondii seeds from saline and nonsaline habitats of the arid Arabian deserts. Botany 97, 123–133. doi: 10.1139/cjb-2018-0174
Farooq, M., Hussain, M., Wakeel, A., and Siddique, K. H. M. (2015). Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 35, 461–481. doi: 10.1007/s13593-015-0287-0
Feng, H., Zhao, H., Xia, L., Yang, W., Zhao, Y., Jeelani, N., et al. (2022). Nitrogen cycling in plant and soil subsystems is driven by changes in soil salinity following coastal embankment in typical coastal saltmarsh ecosystems of Eastern China. Ecol. Eng. 174, 106467. doi: 10.1016/j.ecoleng.2021.106467
Feng, L., Xia, J., Liu, J., Song, A., Chen, Y., and Zhao, X. (2021). Effects of mosaic biological soil crusts on vascular plant establishment in a coastal saline land of the Yellow River Delta, China. J. Plant Ecol. 14, 781–792. doi: 10.1093/jpe/rtab031
Fernandez-Torquemada, Y. and Sanchez-Lizaso, J. L. (2013). Effects of salinity on seed germination and early seedling growth of the Mediterranean seagrass Posidonia oceanica (L.) Delile. Estuar. Coast. Shelf Sci. 119, 64–70. doi: 10.1016/j.ecss.2012.12.013
Gao, Z., Zhou, Y., and He, Y. (2022). Molecular epigenetic mechanisms for the memory of temperature stresses in plants. J. Genet. Genomics 49, 991–1001. doi: 10.1016/j.jgg.2022.07.004
Gorai, M., Vadel, A. M., and Neffati, M. (2006). Seed germination characteristics of Phragmites communis: Effects of temperature and salinity. Belgian J. Bot. 139, 78–86. doi: 10.2307/20794596
Greenberg, C. H., Smith, L. M., and Levey, D. J. (2001). Fruit fate, seed germination and growth of an invasive vine - an experimental test of ‘sit and wait’ Strategy. Biol. Invasions 3, 363–372. doi: 10.1023/A:1015857721486
Greenwood, M. E. and MacFarlane, G. R. (2006). Effects of salinity and temperature on the germination of Phragmites australis, Juncus kraussii, and Juncus acutus: Implications for estuarine restoration initiatives. Wetlands 26, 854–861. doi: 10.1672/0277-5212(2006)26[854:EOSATO]2.0.CO;2
Guan, B., Yu, J., Hou, A., Han, G., Wang, G., Qu, F, et al. (2016). The ecological adaptability of Phragmites australis to interactive effects of water level and salt stress in the Yellow River Delta. Aquat Ecol. 51, 107–116. doi: 10.1007/s10452-016-9602-3
Gul, B., Ansari, R., Flowers, T. J., and Khan, M. A. (2013). Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 92, 4–18. doi: 10.1016/j.envexpbot.2012.11.006
Haraguchi, A. (2014). Effects of salinity on germination, seedling growth and ecological properties of Phragmites australis communities in the Estuary of the Chikugogawa River, Southwestern Japan. Am. J. Plant Sci. 05, 584–595. doi: 10.4236/ajps.2014.55073
He, Y. and Li, Z. (2018). Epigenetic environmental memories in plants: establishment, maintenance, and reprogramming. Trends Genet. 34, 856–866. doi: 10.1016/j.tig.2018.07.006
He, Q. and Silliman, B. R. (2019). Climate change, human impacts, and coastal ecosystems in the anthropocene. Curr. Biol. 29, R1021–R1035. doi: 10.1016/j.cub.2019.08.042
Ho, L., Barthel, M., Pham, K., Bodé, S., Van Colen, C., Moens, T., et al. (2024). Regulating greenhouse gas dynamics in tidal wetlands: Impacts of salinity gradients and water pollution. J. Environ. Manage. 364, 121427. doi: 10.1016/j.jenvman.2024.121427
Jia, P., Qu, G., Jia, J., Dezhi, L., and Sun, Y. (2022). Long-term Spartina alterniflora invasion simplified soil seed bank and regenerated community in a coastal marsh wetland. Ecol. Appl. 34, e2754. doi: 10.1002/eap.2754
Kettenring, K. M. and Whigham, D. F. (2009). Seed viability and seed dormancy of non-native Phragmites australis in suburbanized and forested watersheds of the Chesapeake Bay, USA. Aquat. Bot. 91, 199–204. doi: 10.1016/j.aquabot.2009.06.002
Kirwan, M. L. and Megonigal, J. P. (2013). Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504, 53–60. doi: 10.1038/nature12856
Koppitz, H. and Kuehl, H. (2000). To the importance of genetic diversity of Phragmites australis in the development of reed stands. Wetlands Ecol. Manage. 8, 403–414. doi: 10.1023/A:1026557901479
Kühl, H. and Zemlin, R. (2000). Increasing the efficiency of reed plantations on stressed lake and river shores by using special clones of Phragmites australis. Wetlands Ecol. Manage. 8, 415–424. doi: 10.1023/A:1026510018318
Lambertini, C., Guo, W.-Y., Ye, S., Eller, F., Guo, X., Li, X.-Z., et al. (2020). Phylogenetic diversity shapes salt tolerance in Phragmites australis estuarine populations in East China. Sci. Rep. 10, 17645. doi: 10.1038/s41598-020-74727-0
Leimu, R., Fischer, M., and Buckling, A. (2008). A meta-analysis of local adaptation in plants. PloS One 3, e4010. doi: 10.1371/journal.pone.0004010
Li, S., Shi, T., Kong, F., Ma, Q., Mao, Y., and Yin, T.(2013). Methods for breaking the dormancy of eastern redbud (Cercis canadensis) seeds. Seed Science and Technology 41, 27–35. doi: 10.15258/sst.2013.41.1.03
Li, Z. and Wang, J. (2015). Advances in research of physiological and molecular mechanism in seed vigor and germination. Scientia Agricultura Sin. 48, 646–660. doi: 10.3864/j.issn.0578-1752.2015.04.03
Liu, L., Li, D., Sun, Y., Zhu, Y., Li, L., Ren, Z., et al. (2021). Pattern of soil extracellular enzyme activities along a tidal wetland with mosaic vegetation distributions in Chongming Island, China. J. Clean. Product. 315, 127991. doi: 10.1016/j.jclepro.2021.127991
Liu, L. L., Yin, M.-Q., Guo, X., Wang, J.-W., Cai, Y.-F., Wang, C., et al. (2022). Cryptic lineages and potential introgression in a mixed-ploidy species (Phragmites australis) across temperate China. J. Syst. Evol. 60, 398–410. doi: 10.1111/jse.12672
Liu, L., Guo, Y., Wu, Y., Yin, M., Guo, X., Eller, F., et al. (2023). Revealing biogeographic patterns in genetic diversity of native and invasive plants and their association with soil community diversity in the Chinese coast. Oikos 2024, e10116. 10.1111/oik.10116
Lombardi, T., Bedini, S., and Bertacchi, A. (2019). Germination ecology of the aromatic halophyte Artemisia caerulescens L.: influence of abiotic factors and seed after-ripening time. Folia Geobotanica 54, 115–124. doi: 10.1007/s12224-019-09345-4
Mariotti, G. and Fagherazzi, S. (2013). Critical width of tidal flats triggers marsh collapse in the absence of sea-level rise. Proc. Natl. Acad. Sci. 110, 5353–5356. doi: 10.1073/pnas.1219600110
McCormick, M. K., Kettenring, K. M., Baron, H. M., and Whigham, D. F. (2010). Extent and reproductive mechanisms of Phragmites australis spread in Brackish Wetlands in Chesapeake Bay, Maryland (USA). Wetlands (Wilmington N.C.) 30, 67–74. doi: 10.1007/s13157-009-0007-0
Miller, K. S. and Geisseler, D. (2018). Temperature sensitivity of nitrogen mineralization in agricultural soils. Biol. Fertil. Soils 54, 853–860. doi: 10.1007/s00374-018-1309-2
Milberg, P. (1999). The effect of light and number of diurnal temperature fluctuations on germination of Phragmites australis. Seed Science Research 9, 165–170. doi: 10.1017/S0960258599000185
Naz, M., Dai, Z., Hussain, S., Tariq, M., Danish, S., Khan, I. U., et al. (2022). The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manage. 321, 115770. doi: 10.1016/j.jenvman.2022.115770
Osland, M. J., Enwright, N., and Stagg, C. L. (2014). Freshwater availability and coastal wetland foundation species: ecological transitions along a rainfall gradient. Ecology 95, 2789–2802. doi: 10.1890/13-1269.1
Peng, G., Lan, W., and Pan, K. (2022). Mechanisms of metal tolerance in halophytes: A mini review. Bull. Environ. Contam. Toxicol. 109, 671–683. doi: 10.1007/s00128-022-03487-6
Plassmann, K., Brown, N., Jones, M. L. M., and Edwards-Jones, G. (2008). Can atmospheric input of nitrogen affect seed bank dynamics in habitats of conservation interest? The case of dune slacks. Appl. Veget. Sci. 11, 413–420. doi: 10.3170/2008-7-18498
Portnoy, J. W. (1999). Salt marsh diking and restoration; biogeochemical implications of altered wetland hydrology. Environ. Manage. (New York) 24, 111–120. doi: 10.1007/s002679900219
Portnoy, J. W. and Giblin, A. E. (1997). Effects of historic tidal restrictions on salt marsh sediment chemistry. Biogeochemistry 36, 275–303. doi: 10.1023/A:1005715520988
Rajabi Dehnavi, A., Zahedi, M., Ludwiczak, A., Cardenas Perez, S., and Piernik, A. (2020). Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 10, 859–867. doi: 10.3390/agronomy10060859
Rosseel, Y. (2012). lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36. doi: 10.18637/jss.v048.i02
Saltonstall, K. (2001). A set of primers for amplification of noncoding regions of chloroplast DNA in the grasses. Mol. Ecol. Notes 1, 76–78. doi: 10.1046/j.1471-8278.2001.00031.x
Saltonstall, K. (2016). The naming of Phragmites haplotypes. Biol. Invasions 18, 2433–2441. doi: 10.1007/s10530-016-1192-4
Satyanti, A., Guja, L. K., and Nicotra, A. B. (2019). Temperature variability drives within-species variation in germination strategy and establishment characteristics of an alpine herb. Oecologia 189, 407–419. doi: 10.1007/s00442-018-04328-2
Shutov, A. D., Bäumlein, H., Blattner, F. R., and Müntz, K. (2003). Storage and mobilization as antagonistic functional constraints on seed storage globulin evolution. J. Exp. Bot. 54, 1645–1654. doi: 10.1093/jxb/erg165
Silliman, B. R., Schrack, E., He, Q., Cope, R., Santoni, A., van der Heide, T., et al. (2015). Facilitation shifts paradigms and can amplify coastal restoration efforts. Proc. Natl. Acad. Sci. United States America 112, 14295–14300. doi: 10.1073/pnas.1515297112
Sims, L., Pastor, J., Lee, T., and Dewey, B. (2012). Nitrogen, phosphorus, and light effects on reproduction and fitness of wild rice. Botany 90, 876–883. doi: 10.1139/b2012-057
Song, J., Jiachao, Z., Zhao, W., Xu, H., Wang, F., Xu, Y., et al. (2016). Effects of salinity and nitrate on production andgermination of dimorphic seeds applied both through the mother plant and exogenously during germination in Suaedasalsa. Plant Species Biol. 31, 19–28. doi: 10.1111/1442-1984.12071
Stankovic, M., Ambo-Rappe, R., Carly, F., Dangan-Galon, F., Fortes, M. D., Hossain, M. S., et al. (2021). Quantification of blue carbon in seagrass ecosystems of Southeast Asia and their potential for climate change mitigation. Sci. Total Environ. 783, 146858. doi: 10.1016/j.scitotenv.2021.146858
Suir, G. M., Saltus, C. L., and Reif, M. K. (2022). Remote sensing-based structural and functional assessments of Phragmites australis diebacks in the Mississippi River Delta. Ecol. Indic. 135, 108549. doi: 10.1016/j.ecolind.2022.108549
Sun, W., Sun, Z., Sun, J., and Sun, W. (2015). Ecological traits of Phragmites australis community in different restoration phases of the Yellow River estuary, China. Acta Ecol. Sin. 35, 5804–5812. doi: 10.5846/stxb201311212788
Tan, L., Ge, Z., Zhou, X., Li, S., Li, X., and Tang, J. (2020). Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta-analysis. Global Change Biol. 26, 1638–1653. doi: 10.1111/gcb.14933
Valdés-Rodríguez, O. A., Sánchez-Sánchez, O., and Pérez-Vázquez, A. (2013). Effects of soil texture on germination and survival of non-toxic Jatropha curcas seeds. Biomass Bioenergy 48, 167–170. doi: 10.1016/j.biombioe.2012.10.025
Wan, W. J., Hao, X., Xing, Y., Liu, S., Zhang, X., Li, X., et al. (2021). Spatial differences in soil microbial diversity caused bypH-driven organic phosphorus mineralization. Land Degrad. Dev. 32, 766–776. doi: 10.1002/ldr.3734
Wang, J., Bai, J., Gao, Z., Lu, Q., and Zhao, Q. (2015). Soil As Levels and Bioaccumulation inSuaeda salsa and Phragmites australis Wetlands of the Yellow River Estuary, China. BioMed. Res. Int. 2015, 1–7. doi: 10.1155/2015/301898
Wang, C. H., Lu, M., Yang, B., Yang, Q., Zhang, X. D., Hara, T., et al. (2010). Effects of environmental gradients on the performances of four dominant plants in a Chinese saltmarsh: implications for plant zonation. Ecol. Res. 25, 347–358. doi: 10.1007/s11284-009-0662-x
Wang, X., Yu, J., Zhou, D., Dong, H., Li, Y., Lin, Q., et al. (2012). Vegetative ecological characteristics of restored reed (Phragmites australis) wetlands in the Yellow River Delta, China. Environ. Manage. 49, 325–333. doi: 10.1007/s00267-011-9757-6
Wang, S., Jurik, T. W., and van der Valk, A. G. (1994). Effects of sediment load on various stages in the life and death of cattail (Typha × glauca). Wetlands 14, 166–173. doi: 10.1007/BF03160653
Wang, L., Tanveer, M., Wang, H., and Arnao, M. B. (2024). Melatonin as a key regulator in seed germination under abiotic stress. J. Pineal Res. 76, e12937. doi: 10.1111/jpi.12937
Warren, R. S., Fell, P. E., Rozsa, R., Brawley, A. H., Orsted, A. C., Olson, E. T., et al. (2002). Salt marsh restoration in Connecticut :20 years of science and management. Restor. Ecol. 10, 497–513. doi: 10.1046/j.1526-100X.2002.01031.x
Wijte, A. and Gallagher, J. L. (1996). Effect of oxygen availability and salinity on early life history stages of salt marsh plants.1. Different germination strategies of Spartina alterniflora and Phragmites australis (Poaceaei). Am. J. Bot. 83, 1337–1342. doi: 10.1002/j.1537-2197.1996.tb13919.x
Xiao, Y., Sun, J., Liu, F., and Xu, T. (2016). Effects of salinity and sulphide on seed germination of three coastal plants. Flora Morphol. Distribution Funct. Ecol. Plants 218, 86–91. doi: 10.1016/j.flora.2015.12.002
Xiao, D., Zhang, L., and Zhu, Z. (2009). A study on seed characteristics and seed bank of Spartina alterniflora at saltmarshes in the Yangtze Estuary, China. Estuar. Coast. Shelf Sci. 83, 105–110. doi: 10.1016/j.ecss.2009.03.024
Xu, F. L., Tao, S., and Xu, Z. R. (1999). The restoration of riparian wetlands and macrophytes in Lake Chao, an eutrophic Chinese lake: possibilities and effects. Hydrobiologia 405, 169–178. doi: 10.1023/A:1003867309767
Yu, J. B., Wang, X., Ning, K., Li, Y., Wu, H., Fu, Y., et al. (2012). Effects of salinity and water depth on germination of Phragmites australis in coastal wetland of the Yellow River Delta. Clean. Soil Air Water 40, 1154–1158. doi: 10.1002/clen.201100743
Yuan, L., Chen, Y.-H., Wang, H., Cao, H.-B., Zhao, Z.-Y., Tang, C.-D., et al. (2020). Windows of opportunity for salt marsh establishment: the importance for salt marsh restoration in the Yangtze Estuary. Ecosphere 11, e03180. doi: 10.1002/ecs2.3180
Zhang, Y., Wang, R., Wang, X., Zhao, C., Shen, H., and Yang, L. (2023). Nitric oxide regulates seed germination by integrating multiple signalling pathways. Int. J. Mol. Sci. 24, 9052. doi: 10.3390/ijms24109052
Zhao, Y., Wang, G., Zhao, M., Wang, M., Xue, Z., Liu, B., et al. (2021). Seed limitation and saline-alkaline stress restrict wetland restoration potential in the Songnen Plain, northeastern China. Ecol. Indic. 129, 107998. doi: 10.1016/j.ecolind.2021.107998
Keywords: germination strategy, soil properties, salinity, seed storage temperature, ecological restoration
Citation: Jia P, Li D, Yu C, Jia J, Wang J, Wang Y and Chen J (2025) Divergent germination strategies of Phragmites australis seeds for tidal flat gradient adaptation and the implications for coastal wetland restoration. Front. Plant Sci. 16:1598379. doi: 10.3389/fpls.2025.1598379
Received: 23 March 2025; Accepted: 30 June 2025;
Published: 29 July 2025.
Edited by:
Ana Juan, University of Alicante, SpainReviewed by:
Stefanos Hatzilazarou, Aristotle University of Thessaloniki, GreeceJoaquín Moreno, Miguel Hernández University of Elche, Spain
Copyright © 2025 Jia, Li, Yu, Jia, Wang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dezhi Li, ZHpsaUBkZXMuZWNudS5lZHUuY24=; Caifen Yu, eXVjYWlmZW4yNUAxNjMuY29t; Jing Jia, NTIyMDM5MDMwMDdAc3R1LmVjbnUuZWR1LmNu
 Peng Jia
Peng Jia Dezhi Li
Dezhi Li Caifen Yu
Caifen Yu Jing Jia
Jing Jia Jiangtao Wang
Jiangtao Wang Ying Wang
Ying Wang Jing Chen
Jing Chen