- 1Cooperative Agricultural Research Center, College of Agriculture, Food and Natural Resources, Prairie View A&M University, Prairie View, TX, United States
- 2Zachry Department of Civil and Environmental Engineering, Texas A&M University, College Station, TX, United States
Germination is a complex physiological and biochemical process influenced by various factors, including metabolic activation and antioxidant defense mechanisms. This study investigated the effects of zinc oxide nanoparticles (ZnO NPs) of different sizes (ZnO10 and ZnO35) as seed priming agents on the germination, biochemical traits, and antioxidative systems of Amaranthus tricolor seeds. ZnO NPs were characterized by UV-Vis maximum peaks at 352 nm and 364 nm and average sizes of 10.0 nm and 35.2 nm for ZnO10 and ZnO35, respectively. Additionally, zeta potential indicated high stability, while transmission electron microscopy confirmed spherical morphology, energy dispersive X-ray showed high purity, and X-ray diffraction peaks indicated crystallinity. Germination percentage (GP) and germination rate (GR) were significantly improved by ZnO NP treatments, particularly at 400 mg/L, with ZnO10-primed seeds achieving 100% GP compared to 91.5% in ZnO35-primed seeds. Additionally, seedling vigor indices followed a similar trend, with ZnO10-primed seeds showing the highest vigor (2380) compared to ZnO35-primed seeds (1793.4). ZnO NPs significantly enhanced water uptake, with ZnO10 NPs demonstrating superior absorption at increasing concentrations, reaching a maximum of 93.6% at 400 mg/L. The α-amylase activity was also significantly higher in ZnO10-primed seeds (1.9 mg/g) than ZnO35-primed seeds (0.81 mg/g) at 400 mg/L suggesting enhanced enzymatic activation and metabolic efficiency. Antioxidant enzyme activities, including superoxide dismutase, catalase, peroxidase, ascorbate peroxidase, and glutathione peroxidase, were significantly enhanced in ZnO NP-primed seedlings, indicating improved oxidative stress management. Furthermore, lipid peroxidation, measured as malondialdehyde content, was significantly reduced, with ZnO10 NPs demonstrating an 89.3% reduction at 400 mg/L. The non-enzymatic antioxidant response was also enhanced, with total phenolic content and total flavonoid content significantly increased in ZnO NP-treated seedlings. The findings show that smaller-sized ZnO10 NPs enhance seed germination, biochemical activation, and antioxidative defense, improving seedling establishment. The high surface area of NPs enhances seed interaction and water uptake, and stimulates enzymatic activities, ultimately improving metabolic activation and protection against oxidative stress. ZnO NPs demonstrate strong potential as effective priming agents for A. tricolor.
1 Introduction
Sustainable agricultural practices aim to meet the rising global demand for crop production by improving seed germination and emergence—critical stages for successful crop development. Seeds play a vital role in agriculture, and effective management of seed inputs can significantly enhance food security. Nonetheless, stored seeds often encounter issues such as deterioration and oxidative damage, which can compromise their viability and, as a result, affect vigor and seedling establishment, ultimately impacting overall productivity (Butler et al., 2009; Alahakoon et al., 2021; Adhikary et al., 2022). In contrast, fast and consistent seed germination, along with uniform seedling development is essential for successful crop establishment to ensure economic viability and efficient use of production resources (Itroutwar et al., 2020; El-Badri et al., 2021; Sharma et al., 2021; García-Locascio et al., 2024). To address this demand, the advancement and utilization of seed treatment methods and agents that trigger biochemical and metabolic processes in seeds to enhance germination are crucial. Techniques aimed at increasing seed coat permeability to water and oxygen—such as scarification, seed coat removal, and seed nicking—have been explored with varying success in promoting germination and seedling growth (Acharya et al., 2020). However, these methods have shown limited effectiveness for smaller seeds (Fenner and Thompson, 2005). Notably, seed priming presents a promising alternative to overcome these challenges (Chatterjee et al., 2018; Waqas et al., 2019).
Seed priming is a widely applicable and efficient technique that improves key seed quality attributes, including germination speed, vigor, uniform emergence, and strong seedling growth. These enhancements contribute to increased crop productivity and greater resilience to environmental stresses (Srivastava et al., 2014; Chatterjee et al., 2018; Waqas et al., 2019; El-Badri et al., 2021; Zhou et al., 2022; Mazhar et al., 2022). Moreover, seed priming increases the activity of key enzymes such as amylases, proteases, and lipases, which are crucial for embryo growth and development (Acharya et al., 2020). Various natural and synthetic priming methods have been explored, including hydropriming (water), osmopriming (polyethylene glycol and inorganic salts), hormonal priming, nutrient priming and nanopriming (Paparella et al., 2015; Mahakham et al., 2017; Itroutwar et al., 2020; do Espirito Santo Pereira et al., 2021; Shah et al., 2021; Sharma et al., 2021; Mazhar et al., 2022). However, since each priming method has distinct characteristics and varying effectiveness depending on the crop species, careful optimization is required (Horii et al., 2007; Sytar et al., 2019).
More recently, nanopriming has emerged as a promising and effective approach to enhance seed pre-germination metabolic activities and strengthen plant resistance to various stresses (Rai-Kalal and Jajoo, 2021; Naseer et al., 2023; Khan et al., 2022). Nanoparticles (NPs), known for their small size, large surface area, and controlled release properties, have been utilized as priming agents. These unique characteristics facilitate rapid absorption, activating seed metabolism, accelerating germination, and promoting plant growth, crop protection, and overall yield improvement (Mahakham et al., 2017; Itroutwar et al., 2020; Shah et al., 2021; Mazhar et al., 2022). Several metal-based NPs such as silver nanoparticles, zinc oxide and iron oxide have been used as nanopriming agents in many crops to improve antioxidant system, increase seed vigor, enhance expression of aquaporin genes and stress mitigation (Shelar et al., 2024; do Espirito Santo Pereira et al., 2021; Nile et al., 2022).
Zinc oxide nanoparticles (ZnO NPs) have emerged as effective alternatives to conventional zinc fertilizers, enhancing zinc bioavailability in plants while also serving as efficient seed priming agents (Awan et al., 2021; Adhikary et al., 2022). Zinc is a vital micronutrient that functions as a cofactor for numerous enzymes, playing essential roles in physiological and metabolic activities such as chlorophyll and protein synthesis, growth, photosynthesis, cell elongation, pollen function, fertilization, germination, water use efficiency, membrane integrity, antioxidant defense, and disease resistance (Cakmak, 2000; Takahashi et al., 2009; Marreiro et al., 2017; Olechnowicz et al., 2018; Khanm et al., 2018; Cabot et al., 2019; Neto et al., 2020; Noohpisheh et al., 2021). Due to zinc’s vital role in human health, nanomaterial-based biofortification of crops has emerged as a promising strategy for enhancing essential nutrient content in leaves and seeds (Hussain et al., 2013; Iziy et al., 2019; Salama et al., 2019), thus, address the hidden hunger for micronutrients worldwide (Ofori et al., 2022). However, studies indicate that ZnO NPs can have both beneficial and adverse effects on germination rate, antioxidant systems, zinc accumulation, and plant growth, depending on the plant genotype, concentration, and nanoparticle size (Faizan et al., 2020; Estrada-Urbina et al., 2018; Sharma et al., 2021).
Priming small seeds is challenging due to their size, sensitivity, and susceptibility to damage during handling (Taylor et al., 1998). Uneven water absorption complicates hydration, increasing the risk of overhydration, premature germination, and viability loss (Bradford, 2002). Limited storage reserves hinder recovery from priming stress, reducing shelf life (Fenner and Thompson, 2005). Additionally, small seeds are highly sensitive to drying and storage conditions, affecting germination and vigor (Rajjou et al., 2012). Optimizing priming protocols is essential, as smaller seeds are more vulnerable to stress (Fenner and Thompson, 2005), while larger seeds benefit from greater reserves for stronger seedlings (Westoby et al., 2002).
Amaranth (Amaranthus tricolor L.) is a small seeded, highly nutritious leafy vegetable widely cultivated for its edible leaves and seeds. It is rich in proteins, vitamins (A, C, and folate), minerals (iron, calcium, and zinc), and bioactive compounds such as flavonoids and betalains, which contribute to its antioxidant properties (Sarker and Oba, 2020). The plant exhibits high adaptability to various environmental conditions, including drought and heat stress, making it a resilient crop for food security in arid and semi-arid regions (Rastogi and Shukla, 2013; Achigan-Dako et al., 2014). Due to its rapid growth, high yield, and nutritional benefits, amaranth is increasingly promoted as a functional food to combat micronutrient deficiencies and improve dietary diversity (Niveyro et al., 2021; Gupta and Gudu, 2022).
Considering the nutritional value of A. tricolor and the health benefits of zinc, this study aimed to evaluate the impact of ZnO NPs of various sizes and concentrations as a seed priming agent on the germination traits and antioxidant system of A. tricolor seedlings. Specifically, this research investigates how priming A. tricolor seeds with ZnO NPs affects their germination traits, enhances the antioxidant systems of amaranth seedlings, and increases the zinc content in amaranth seedlings. The findings from this study will provide valuable insights into the potential benefits of using ZnO NPs in agricultural practices to improve crop health and growth.
2 Materials and methods
2.1 Characterization of ZnO NPs
Two sizes of ZnO NPs were purchased from Skyspring Nanomaterials, Inc. (Houston, USA) and subjected for characterization. The UV-Vis absorption spectra of ZnO NPS were measured using a Molecular Devices ABS spectrometer over a 200–750 nm range. Particle size and the zeta potential of the samples were determined with a Litesizer 500 (Anton Paar, Austria). Scanning electron microscopy (SEM) integrated with energy-dispersive X-ray spectroscopy (EDX) (JOEL JSM-6010LA, Japan) was used to analyze the morphology of ZnO NPs and determine their elemental composition. Transmission electron microscope (TEM, JEOL-2100, Peabody, MA, USA) was employed to examine the detailed morphological characteristics of ZnO NPs at an accelerating voltage of 200 kV. The crystalline structure of ZnO NPs was analyzed using an X-ray diffractometer (XRD-7000, Shimadzu, Japan). The diffraction pattern was captured using Cu Kα radiation (λ = 1.541 Å) over a 2θ range of 10° to 80°.
2.2 Seed priming experiment
Different concentrations (50, 100, 200 and 400 mg L-1) of ZnO NPs and ZnSO4 were freshly prepared by dispersing in deionized water using ultrasonic vibration (100 w, 40 kHz) for 10 min. Distilled water was used for hydropriming. Commercial A. tricolor seeds (Lot #, 101294) were procured from Johnny’s Selected Seeds (Winslow, ME, USA). The seeds were sterilized by flashing with 0.1% sodium hypochlorite for 5 min and then immediately washed twice with MilliQ water. Then 1000 seeds were submersed in the corresponding treatment of 50, 100, 200 and 400 mg L-1 of the nanosuspensions and ZnSO4 of 50 mL each and constantly agitated by shaking at 160 rpm for 12 h at room temperature (Rai-Kalal and Jajoo, 2021). The seeds were then dried to restore their original moisture content following Rawat et al. (2018). After drying, the seeds were placed in polyethylene bags and stored at room temperature pending germination test and further evaluations.
2.3 Seed germination experiment
The ZnO NPs primed seeds were used for further germination tests and impact on seedlings enzymatic and non-enzymatic antioxidant activities. Healthy dried A. tricolor primed seeds were placed in Petri dishes (30 seeds per dish) bottomed by filter paper and re-hydrated with 5 mL of distilled water. Each priming condition for the respective priming materials (ZnO10 and ZnO35 NPs and distilled water) contained 4 replicates in a completely randomized design. Subsequently, Petri dishes were kept in an incubator under dark condition at 27 °C for 48 h and later transferred to light and temperature regulated growing bench. The germinated seeds were monitored daily for 6 days.
The germination traits of A. tricolor seeds such as germination percentage (El-Beltagi et al., 2022), mean germination time, MGT (Ellis and Roberts, 1981), germination energy, GE (Ullah et al., 2022) and mean germination rate, MGR (Alam et al., 2021) were determined. On the seventh day, 10 seedlings were randomly selected from each Petri dish to measure shoot and root length (Abou-Zeid and Mohamed, 2018). Using shoot and root lengths, vigor index was computed following Kataria et al. (2015).
2.4 Seed water uptake
The water uptake (WU) by A. tricolor seeds through the imbibition process was measured using 1000 seeds in triplicate for each treatment as described by Mazhar et al. (2022). The seeds were weighed and placed on water-saturated cotton in a Petri dish and incubated at 25°C, in 12 h intervals. All seeds were collected, blotted to eliminate excess moisture, and then weighed. Changes in weight resulting from imbibition process were computed as the water absorbed per unit of seed dry weight (Equation 1) as follow:
2.5 α-Amylase activity and total soluble sugar content
To assess starch metabolism in germinated A. tricolor seeds, α-amylase activity was measured using a modified 3,5-dinitrosalicylic acid method (Kishorekumar et al., 2007). α-amylase was extracted from minced germinated seeds using ice-cold distilled water. Its absorbance at 540 nm was measured with a spectrophotometer, and then the α-amylase activity was calculated using a glucose standard curve. For total soluble sugar (TSS) quantification, 0.2 g of pulverized seeds were extracted with 95% ethanol, centrifuged at 5000 x g for 10 min, and the supernatant was further processed with 70% ethanol. The supernatant was then reacted with Antron reagent and heated at 100°C for 10 minutes, with absorbance recorded at 625 nm following Irigoyen et al. (1992) by using glucose for calibration.
2.6 Determination of antioxidant enzymes activity
A total of 1.0 g of fresh leaf samples from each treatment group was homogenized in 2.0 mL of phosphate buffer (PB) with a pH of 7.2. The resulting homogenate was then centrifuged at 10,000 rpm for 10 min. The supernatant obtained from this process was utilized to evaluate various stress-responsive enzymatic activities. Superoxide dismutase (SOD) activity was measured using the Cayman SOD Assay Kit (706002, Cayman Chemical, Ann Arbor, Michigan, USA). The absorbance was read at 450 nm using Spectra Max® PLUS 384 plate reader. Catalase (CAT) activity in units per gram of total proteins (U TP−1) was assayed by measuring the reduction of H2O2 at 240 nm (Dhindsa and Matowe, 1981). Peroxidase (POD) activity was analyzed by monitoring guaiacol oxidation at 470 nm (Yue et al., 2022). Ascorbate peroxidase (APX) activity was measured by the decrease in ascorbate absorbance at 290 nm following Nakano and Asada, 1981. Furthermore, glutathione peroxidase (GPX) activity was determined using the method developed by Sattar et al. (2024), with absorbance readings at 412 nm.
2.7 Malondialdehyde content
MDA content in fresh seedlings was determined using a slight modification of 2-thiobarbituric acid (TBA) colorimetry method detailed by Fathi et al. (2023). Briefly, 0.1 g fresh samples were homogenized with 2 mL of phosphate-buffered saline (PBS; 50 mM, pH 7.8) and then centrifuged at 400 (r/min) for 10 min. Subsequently, 1 mL aliquot of the supernatant was combined with 1 mL of 0.5% solution of TBA dissolved in a 5% trichloroacetic acid solution. The solution was incubated for 10 min a boiling water bath and centrifuged at 10,000 x g for 10 min at 4°C. The absorbance of the resultant solution was measured at 532 nm and 600 nm and then MDA content was quantified in each sample.
2.8 Determination of non-enzymatic antioxidants
The total phenolic and flavonoid contents were determined using the methanol extract from the A. tricolor seedlings. For this, 500 mg of powdered plant material was mixed with 10 mL of 80% (v/v) aqueous methanol and shaken for 24 h at room temperature. The mixtures were then centrifuged at 10,000 rpm for 15 min. The supernatant was collected and stored at −70°C until analysis. To determine total phenolic content, the Folin–Ciocâlteu reagent was used, following Makkar et al. (1993). In this method, 250 μL of the methanol extract was combined with 1750 μL of distilled water and 100 μL of the Folin–Ciocâlteu reagent. After a 10-min incubation, 20 mL of 20% Na2CO3 solution was added. The samples were kept in the dark at room temperature for 2 h, after which the absorbance was measured at 720 nm using a UV-Vis spectrophotometer (SpectraMax® PLUS 384). A standard curve was created using gallic acid at concentrations of 50, 100, 200, 300, 400, 500, 600 and 700 μg/mL.
The total flavonoid content (TFC) in A. tricolor seedling samples was quantified using the AlCl3 colorimetric technique as described by Chang et al. (2002). Specifically, 100 μL of CH3CO2K, 100 μL of AlCl3, and 2.8 mL of distilled water were mixed with 0.5 mL of the methanol extract. The mixtures were allowed to sit at room temperature for 30 min. Absorbance was then measured at 415 nm using a UV-Vis spectrophotometer (SpectraMax® PLUS 384). Quercetin was diluted in methanol at concentrations ranging from 10 to 140 μg/mL to create the standard curve and determine the total TFC as quercetin equivalents (mg QE g-1 dry sample).
2.9 Zinc profiling of amaranth seedlings
Seedlings of A. tricolor were dried at 70°C for 24 h in an oven, and ground into fine powder in triplicate using mortar and pestle. About 250 mg of powdered samples from each treatment were mixed in 2 mL H2O2 (30% v/v) and 7.0 mL HNO3 (65% v/v) in microwave vessel and digested using a high-pressure microwave system (Milestone Ethos UP 1600, Sorisole, Italy). After the samples cooled to room temperature, the digested solutions were filtered through a 0.2-μm nylon membrane pending analyses. The concentration of Zn in each sample was analyzed using Inductive Coupled Plasma Optical Emission Spectrometer (ICP-OES, Agilent ICP-5100) integrated with Agilent SP4 autosampler at high spectral signals of wavelength of 213.86 nm.
2.10 Statistical analysis
The data obtained on germination traits and biochemical parameters were subjected to statistical analysis using one-way analysis of variance (ANOVA) using the different concentrations and the size of NPS (treatments) as the independent variable using JMP software (JMP pro14) and the mean values were compared using Tukey’s test (significance level 5%) for the different concentration levels of ZnO NPs. On the other hand, mean comparisons for the two sizes of NPs at a given concentration were performed using Student’s t-test at 5% probability level (p ≤ 0.05). The results are expressed as means ± standard error of the mean. The experiment was designed in a complete randomized design with triplicates per treatment.
3 Results and discussion
3.1 ZnO NPs characteristics
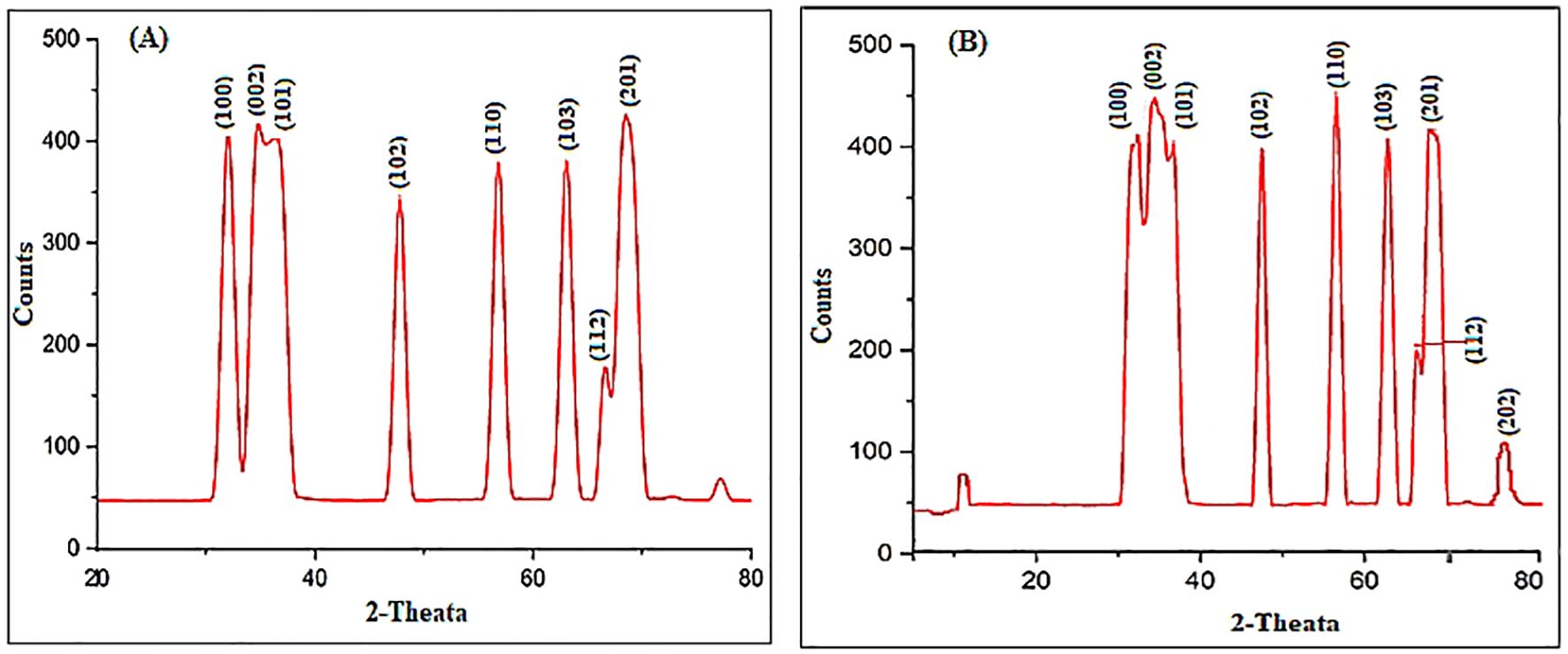
The UV-Vis spectral analysis displayed characteristic peaks at 352 and 364 nm, indicating the presence of ZnO NPs (Figure 1A). A particle size analyzer was employed to determine the average sizes of the synthesized ZnO NPs, revealing measurements of 10.0 nm and 35.2 nm (Figures 1B, C, F). Zeta potential analysis revealed that ZnO10 and ZnO35 NPs had values of -16.8 ± 3.2 mV and -19.3 ± 4.1 mV (Figures 1D, E), respectively, indicating their high stability. The high stability of the ZnO NPs in colloidal suspension is supported by zeta potential values between +30 and −30 mV, which indicate stability and high charge (Afzal et al., 2021; Khepar et al., 2023a). SEM micrographs of ZnO10 and ZnO35 revealed an aggregated particle pattern (Figures 2A, B). Elemental composition analysis using EDX indicated 84.4% Zn and 15.6% O for ZnO10, and 79.9% Zn and 20.1% O for ZnO35 (Figures 2C, D), confirming the nanoparticles’ purity (Geremew et al., 2023; Khepar et al., 2023b). Furthermore, TEM analysis confirmed the ZnO10 and ZnO35 nanoparticles’ spherical morphology with slight difference to DLS analysis with an average size of 10.9 nm and 36.2 nm, respectively (Figures 2E, F). Sharp peaks in XRD reflected the crystallinity of ZnO10 NPs, with Bragg’s reflection peaks at 2θ of 32.03°, 34.8°, 36.45°, 47.77°, 56.82°, 63.06°, 66.27° and 68.5°, corresponding to the planes (100), (002), (101), (102), (110), (103), (112) and (201) (Figure 3A). The peaks were matched with the ICDD card number 01-079–0207 as reported by Jayachandran et al. (2021). For ZnO35, all diffraction peaks at 2θ of 31.6°, 33.92°, 36.7°, 47.47°, 56.56°, 62.83°, 66.36°, 68.03°, and 72.05° are indexed according to the hexagonal phase of the ZnO wurtzite crystal structure with main (100), (002), (101), (102), (110), (103), (112), and smaller (201) and (202) crystal planes (Figure 3B). These values for ZnO35 align well with the standard JCPDS card number 04-003-2106, confirming the particle purity phase (Babayevska et al., 2022).
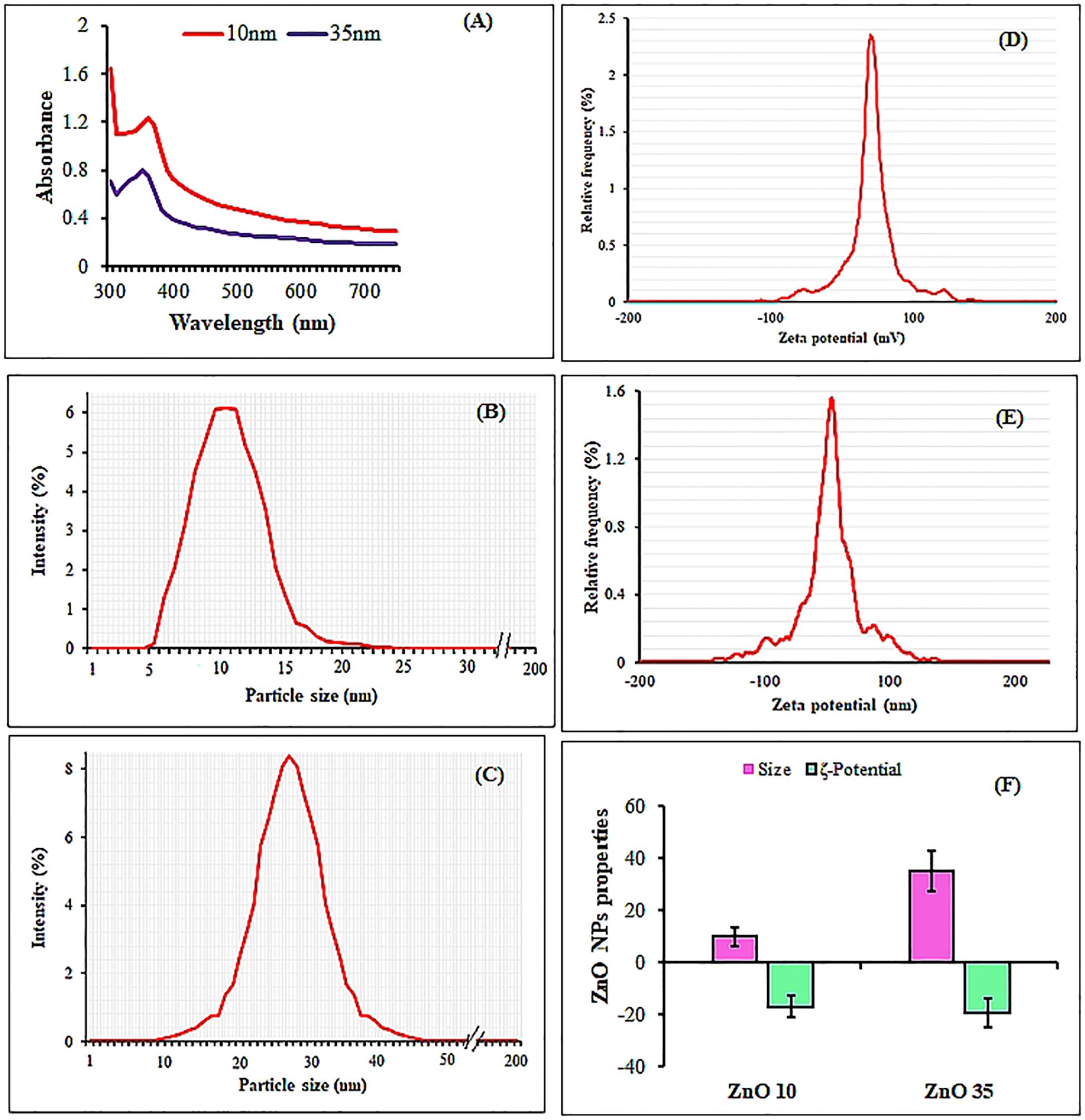
Figure 1. UV-Vis spectra of (A), particle size distribution (B, C) and zeta potential (D, E) from DLS analysis of ZnO10 and ZnO35 nanoparticles, respectively. Also, (F) summarizes the average particle size and zeta potentials of ZnO10 and ZnO35 nanoparticles.
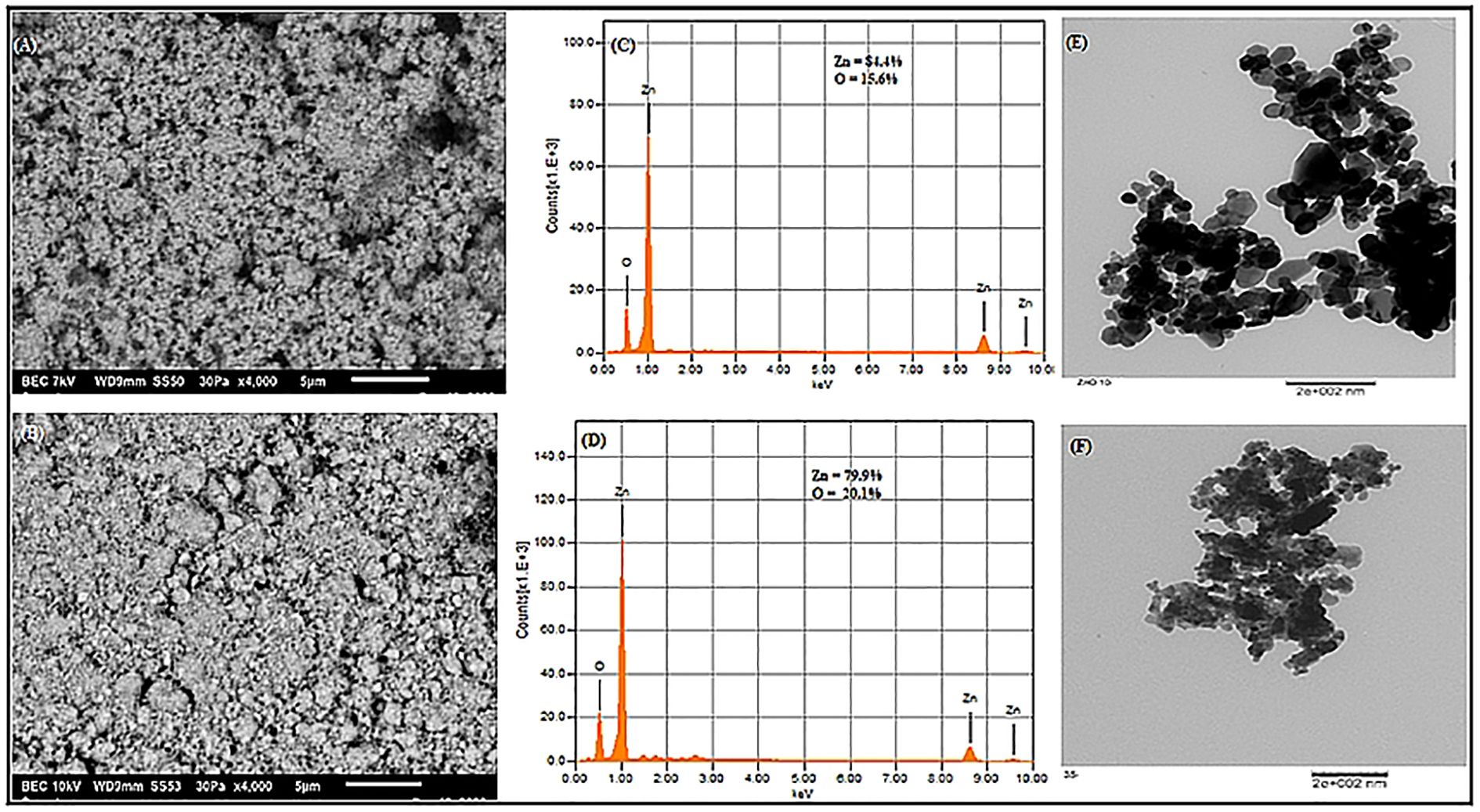
Figure 2. SEM images (A, B), elemental composition from EDS analysis (C, D) and TEM images (E, F) of ZnO10 and ZnO35 nanoparticles.
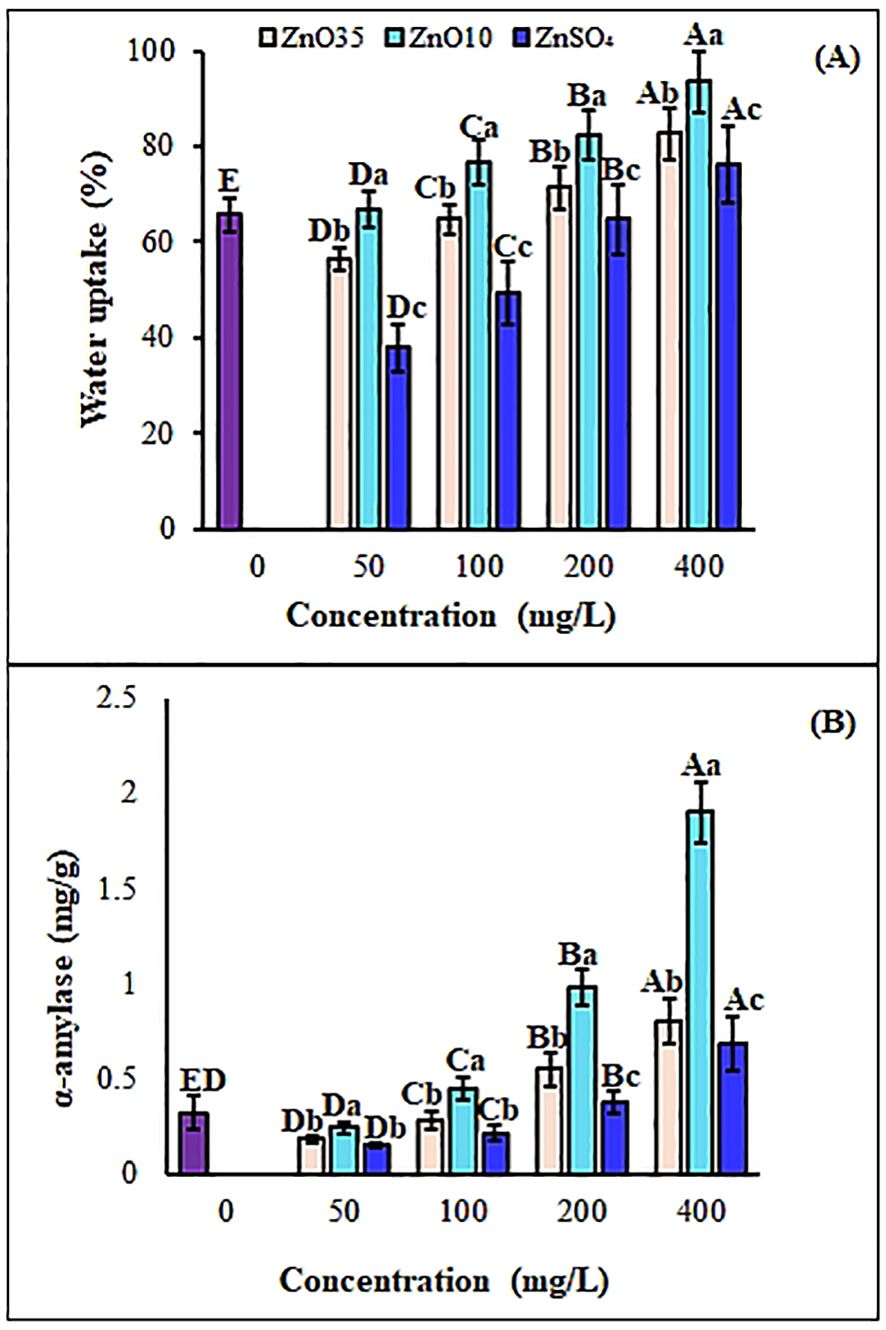
Figure 4. Water uptake (A) and alpha-amylase (B) of A. tricolor seeds treated with different concentrations and sizes of ZnO NPs and ZnSO4. Different lower-case letters denote significant differences (p< 0.05) between ZnO NPs size and ZnSO4 at a particular concentration. Whereas uppercase letters denote significant differences across different concentrations for a particular NP size. The purple bar shows control.
3.2 Effects of ZnO NPs on amaranths seed germination traits
Germination percentage (GP) of A. tricolor seeds primed with ZnO10 NPs significantly increased with dosage, reaching 72.6%, 76.3%, 87.6%, and 100% at 50, 100, 200, and 400 mg/L, respectively, compared to the control (71.3%) and ZnO35 NPs (50 mg/L–65.2%, 100 mg/L–72.4%, 200 mg/L–82.8%, 400 mg/L–91.5%) (p< 0.05, Table 1). A significant difference in GP was observed between ZnO10 and ZnO35 nano-primed seeds at 200 and 400 mg/L with the highest GP of 100% and 91.5%, correspondingly. However, the GP in seeds primed with ZnO35 NPs at lower concentration (50 mg/L) showed a lower efficacy in improving seed germination than that of the control significantly. Additionally, nanoprimed seeds showed a significantly higher GP than the bulk treated (ZnSO4) seeds with increasing concentration. At higher concentration priming amaranths seeds using ZnSO4 demonstrated lower percent germination and germination rate than the nanoprimed and unprimed seeds. The germination rate (GR) of A. tricolor seeds increased significantly with ZnO NP concentrations (50–400 mg/L) compared to the control (p< 0.05), while mean germination time showed the opposite trend (Table 1). The GR of ZnO10 and ZnO35 NPs treated A. tricolor seeds were significantly higher than unprimed seeds at 200 and 400 mg/L in the sixth days, compared to the lowest GR at 50 mg/L. Germination energy was found significantly increased in a dose dependent manner. ANOVA revealed significant differences in shoot and root lengths among seedlings from ZnO10 and ZnO35-NPs-primed seeds at 200 and 400 mg/L compared to unprimed seeds and ZnSO4 primed seeds (Table 1).
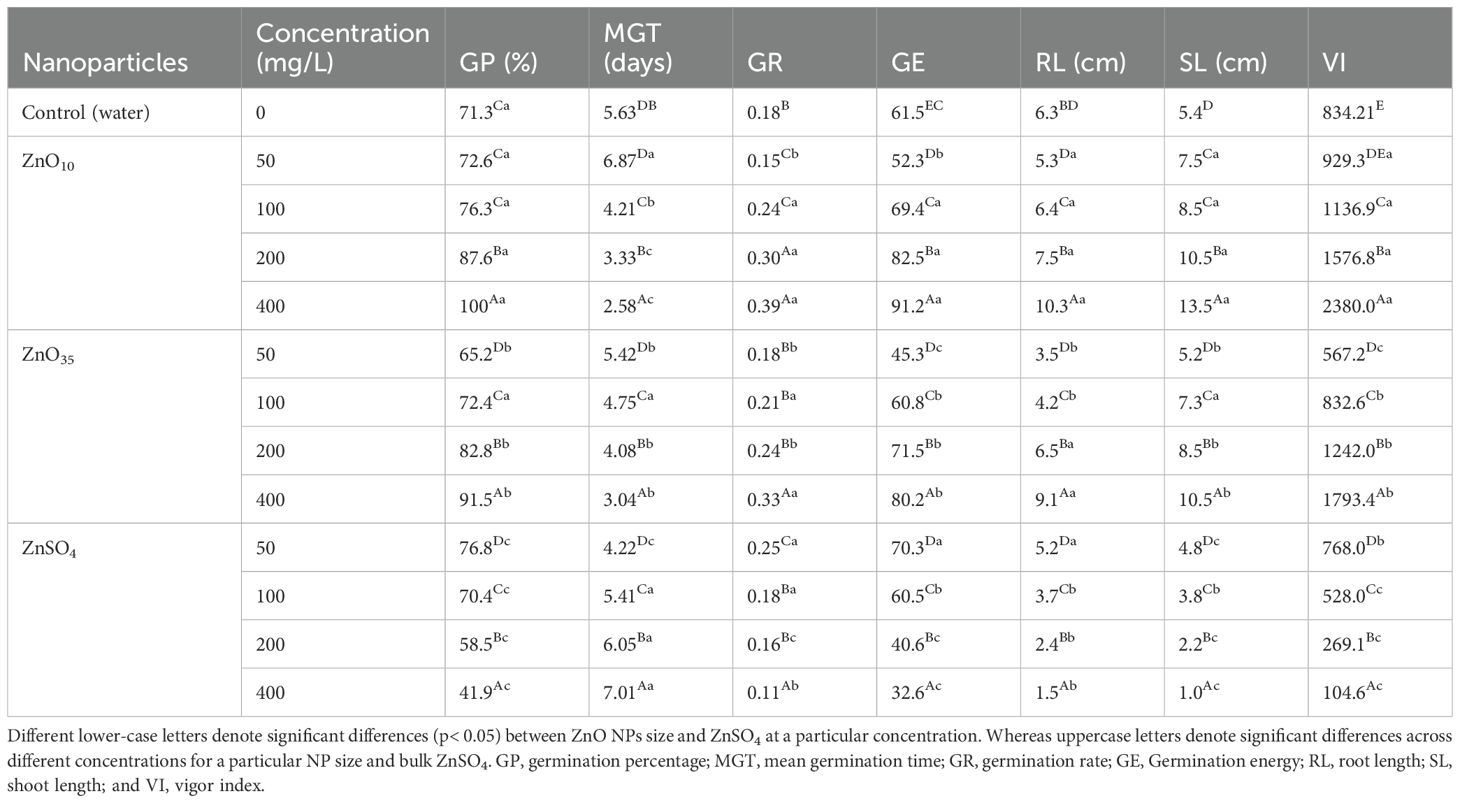
Table 1. Effect of ZnO NPs and ZnSO4 priming treatments on germination and growth parameters of A. tricolor.
Interestingly, priming A. tricolor seeds with ZnO10 and ZnO35 NPs (50–400 mg/L) significantly enhanced seedling vigor, ranging from 929.3 to 2380 and 567.2 to 1793.4, respectively (Table 1). In contrast a significant decrease in seedling vigor was observed in ZnSO4 primed seeds with augmented concentrations. The highest seedling vigor indices were recorded for ZnO10 (2380) and ZnO35 (1793.4) NPs at 400 mg/L. Overall, nanopriming with ZnO NPs enhanced vigor for all priming treatments (P< 0.05). In agreement with our findings, recently, ZnO NPs based priming increased seed germination characteristics such as GP, GR, and vigor (Khalaki et al., 2021). Similarly, Khepar et al. (2024) have reported improved germination traits in rice seeds primed with ZnS nanoparticles. Seeds primed with ZnO NPs exhibited enhanced germination and vigorous seedling growth owing to zinc’s essential role in inducing protein and carbohydrate metabolism, breaking of dormancy, imbibition and enzyme activation, which are critical for early coleoptile and radicle development (Samad et al., 2013; Farooq et al., 2013; Shah et al., 2019; Neto et al., 2020; Rai-Kalal and Jajoo, 2021; Ozturk et al., 2006; do Espirito Santo Pereira et al., 2021; Del Buono et al., 2022). The increased GR and seedling vigor of nanoprimed seeds in this study may be attributed to enhanced α-amylase activity (discussed in the section follows), which accelerates starch hydrolysis during germination (Sharifan et al., 2020; Tondey et al., 2021; Gupta et al., 2022; Kathiravan et al., 2024).
In addition, the enhanced radicle length may be attributed to zinc’s role in modulating hormone metabolism, particularly its influence on auxin levels through the regulation of tryptophan biosynthesis as well as its essential role in biosynthesis of gibberellins (Prasad et al., 2012; Kathiravan et al., 2024). Zinc’s hormonal modulation effect is known to control the early stages of seed germination and radicle development (El-Badri et al., 2021). Also, nanopriming with ZnO NPs often improves germination traits, shoot and root lengths, and seedling vigor by facilitating seed coat penetration and increasing pore formation (Hatami, 2017; Hatami et al., 2019). Thus, these phenomena promoted oxygen transfer to seeds and water uptake potential (Afzal et al., 2021). However, it has been found that ZnO NPs can result in different effects on radicle elongation, also causing severe toxic effects (Stałanowska et al., 2023). In all measured germination traits, significant differences were observed between ZnO10 and ZnO35 NPs. Smaller NPs create more seed coat pores, enhancing water uptake and upregulating aquaporin gene expression compared to larger NPs (Tondey et al., 2021; Gupta et al., 2022; Qian et al., 2013; Kathiravan et al., 2024) and as a consequence enhance seed germination and seedling growth more effectively (Hussain et al., 2017; Afzal et al., 2021). Moreover, during early germination, NPs generate ROS (shown in next sections) as signaling molecules, facilitating reserve mobilization, cell wall loosening, endosperm weakening, improved water absorption, and cell extension, ultimately enhancing germination (Oracz and Karpinski, 2016; Mahakham et al., 2017; Del Buono et al., 2022; Itroutwar et al., 2020).
3.3 Biochemical parameters
To initiate germination and growth, seeds must absorb an adequate amount of water. In the present study, the percentage of water uptake was found higher in A. tricolor seeds when primed with ZnO10 and ZnO35 NPs as compared to the control and ZnSO4 (Figure 4A). Also, the water absorption capacity of the seeds increased with the increased concentration of ZnO NPs and ZnSO4 priming solution. The seed water uptake percentage of primed A. tricolor seeds at 50, 100, 200 and 400 mg/L of ZnO10 NPs dosage was 1.7%, 16.7%, 25.3%, and 42.5%, respectively which was significantly higher (p ≤ 0.05) as compared to distilled water (Figure 2A). The maximum water uptake achieved was 93.6% at 400 mg/L of ZnO10 NPs primed A. tricolor seeds. While the seed water uptake percentage of ZnO35 NP-primed seeds at 50 and 100 mg/L did not increase significantly, seeds primed with 200 and 400 mg/L showed significant increases of 8.7% and 25.7%, respectively, compared to the control. The maximum water uptake was 82.6% at 400 mg/L priming concentrations of ZnO35 NPs for A. tricolor seeds. Water uptake by ZnSO4 primed seeds did not show significant water uptake at 50, 100 and 200 mg/L relative to the control (water). NPs may interact with cell walls to create micropores in the seed coat, thereby enhancing water uptake and upregulating the expression of aquaporin genes (Qian et al., 2013; Kathiravan et al., 2024) and ultimately accelerating seed germination (Soni et al., 2023).
Alpha-amylase content in the seeds plays a vital role in the hydrolysis of endosperm starch to sugars for metabolism. The α-amylase activity in ZnO35 NPs-primed A. tricolor seeds at 50, 100, 200, and 400 mg/L was 0.18, 0.28, 0.55, and 0.81 mg/g, respectively, while ZnO10 NPs-primed seeds showed activities of 0.24, 0.44, 0.98, and 1.9 mg/g at the same concentrations (Figure 4B). A significant difference (p< 0.05) in α-amylase activity was observed between ZnO35 and ZnO10 NPs-primed seeds and ZnSO4 primed seeds at 100, 200 and 400 mg/L, as well as compared to the control (0.32 mg/g). However, no significant enhancement was noted at 50 mg/L for either nanoparticle or ZnSO4 treatment relative to the control. The enhanced α-amylase activity in ZnO NPs-primed seeds may result from increased water uptake during imbibition (Rai-Kalal and Jajoo, 2021), as the case in this study. Our findings align with previous studies highlighting the role of nanopriming in enhancing starch metabolism during germination, where α-amylase facilitates nutrient mobilization and carbohydrate conversion to soluble sugars, supporting germination and seedling growth (Zheng et al., 2016; Sharma et al., 2021). In this regard, zinc ions released from ZnO NPs might activate α-amylase (Rai-Kalal and Jajoo, 2021) and then augment starch hydrolysis that increase soluble sugars to fuel seedling growth (Kathiravan et al., 2024 (Choudhary et al., 2019; Veˇceˇrová et al., 2016; Del Buono et al., 2022). Additionally, it has also been confirmed that some metal-based NPs can cross the seed coat and stimulate the embryonic differentiation by inducing the enzymes that interrupt seed dormancy (García-Locascio et al., 2024).
This study also showed significant stimulation (p< 0.05) in total soluble sugar (TSS) in ZnO NPs-primed A. tricolor seeds compared to the control and the bulk ZnSO4, varying with concentration (Figure 5). Compared to the control, both ZnO35 and ZnO10 NPs at 400 mg/L resulted in the highest TSS content of A. tricolor seeds by 63.6% and 72.3%, respectively. The increase in TSS could be attributed to the higher water absorption and α-amylase activity (Sharma et al., 2021). In agreement with our findings Acharya et al. (2020) and Sharma et al. (2021) found that watermelon and rice seeds treated with Ag NPs and ZnO NPs had higher soluble sugar content during germination compared to untreated seeds.
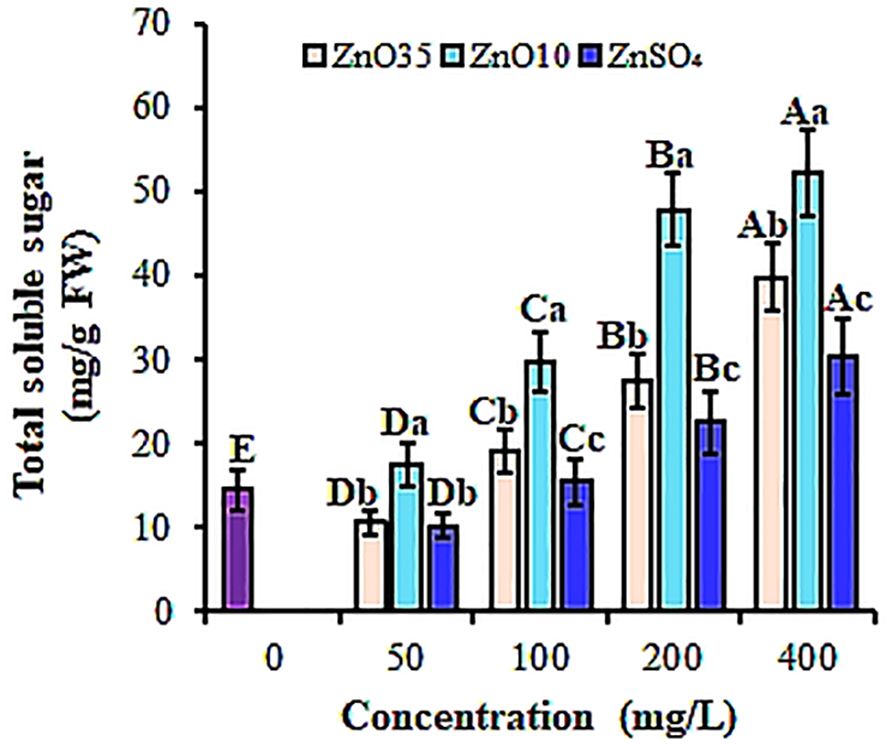
Figure 5. Total soluble sugar content of A. tricolor seeds treated with different concentrations and sizes of ZnO NPs and ZnSO4. Different lower-case letters denote significant differences (p< 0.05) between ZnO NPs size and ZnSO4 at a particular concentration. Whereas uppercase letters denote significant differences across different concentrations for a particular NP size. The purple bar shows control.
3.4 Seed priming with ZnO NPs modulates antioxidative systems
Antioxidative systems, including enzymatic and non-enzymatic components, are essential for neutralizing ROS and maintaining cellular homeostasis in plants (Ahammed et al., 2020; Soni et al., 2023). To counteract ROS, plants activate non-enzymatic antioxidants like phenols and flavonoids, along with enzymatic antioxidants such as catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD). Antioxidant enzymes serve as positive regulators by controlling ROS production and maintaining the balance between its generation and elimination (Bailly et al., 2008; Sharma et al., 2021; Soni et al., 2023).
A. tricolor seedlings oxidative status was investigated by exploring the effect of different size and concentrations of ZnO NPs and ZnSO4 as priming agents on antioxidant enzymes and non-enzymatic antioxidants during early seedling growth. Priming of A. tricolor seeds with ZnO NPs significantly (p< 0.05) enhanced antioxidant enzyme activities compared to the control and the bulk ZnSO4 (Figures 6A-D, Figure 7A). SOD activity rose under all ZnO10 and ZnO35 NPs treatments, with increases of 40.3% to 67.1% and 17.3% to 33.5%, respectively, at concentrations of 50–400 mg/L relative to the control (Figure 6A). CAT activity was also increased by about 10%, 15%, 23%, and 44% in seedlings treated with ZnO35 NPs and 16%, 24%, 46%, and 60.8% in ZnO10 NPs under the same concentration range (Figure 6B). Also, ZnO10 and ZnO35 NP priming significantly enhanced enzyme activities (P< 0.05), with POD increasing by 203.5% and 190.5% (Figure 6C), APX by 207.6% and 149.2% (Figure 6D) and, GPX by 513.7% and 248% (Figure 7A), respectively, in A. tricolor seedlings primed at a concentration of 400 mg/L compared to unprimed seedlings. While priming with 50 mg/L ZnSO4 did not show a significant difference in the stimulation of SOD, CAT, and POD compared to the control, it resulted in notably lower levels of SOD, CAT, POD, APX, and GPX when compared to ZnO10 and ZnO35 NPs across various concentrations. This reflects the minimal effect of the bulk ZnSO4 on antioxidant enzyme enhancement (Khepar et al., 2024). In most instances, the highest antioxidant enzyme activities were noted at maximum ZnO NPs doses, indicating their effectiveness in overcoming oxidative stress. The high SOD activity in ZnO NPs primed A. tricolor seedlings could be linked to augmented binding of Zn2+ to thiols, which induced its synthesis (Li et al., 2021). Additionally, the interconnectedness of the antioxidant enzymes activities like the SOD and its isoenzyme (Zn-SOD) is an important factor for the consistent pattern of their increase (Hajiboland, 2014; López-Vargas et al., 2020). For instance, SOD converts superoxide radicals into H2O2 and O2, while CAT, POD, APX, and GPX further break down H2O2 into H2O and O2, with APX specifically involved in H2O2 scavenging via the glutathione-ascorbate cycle (Soni et al., 2023; Kibinza et al., 2011; Sharma et al., 2021; Afzal et al., 2021). In agreement with our results, increase of antioxidant enzymes have also been observed with the application of ZnO NPs as priming agents in seedlings of several crops such as green gram (Kathiravan et al., 2024), rice (Mazhar et al., 2022; Sharma et al., 2021), pearl millet (Kumar et al., 2024), black gram (Banerjee et al., 2023) and wheat (Rai-Kalal and Jajoo, 2021; Wang et al., 2020) as well as different vegetables (Younes et al., 2020; Sharma et al., 2021; Salam et al., 2022; Selim et al., 2020; Ruszkiewicz et al., 2017). Studies have also shown nano-primed seeds trigger oxidative bursts during germination, fortifying antioxidant defense mechanisms and promoting enhanced seedling vigor and plant growth throughout post-priming germination stages (Chen and Arora, 2013; Shinde et al., 2020; Afzal et al., 2021; Kathiravan et al., 2024; Khepar et al., 2024). As reported for ZnS, FeS and MnS NPs primed seedlings of rice and Brassica the enhancement of antioxidant enzymes in ZnO NPs primed amaranths could linked with the upregulation of target antioxidant genes (Khepar et al., 2024) and elevated CAT and APX transcript levels (Shaw and Hossain, 2013; Sharma et al., 2012). Additionally, nanopriming facilitates the formation of nanopores in shoots, aiding water absorption and activating antioxidant mechanisms, thus enhancing seed germination and growth (Chen and Arora, 2013; Nile et al., 2022; Khepar et al., 2023a). However, priming treatments do not always boost the activity and expression of antioxidant enzymes (Goswami et al., 2013; Farooq et al., 2022) as a result of phytotoxicity, genotype difference and NPs size and concentration discrepancy.
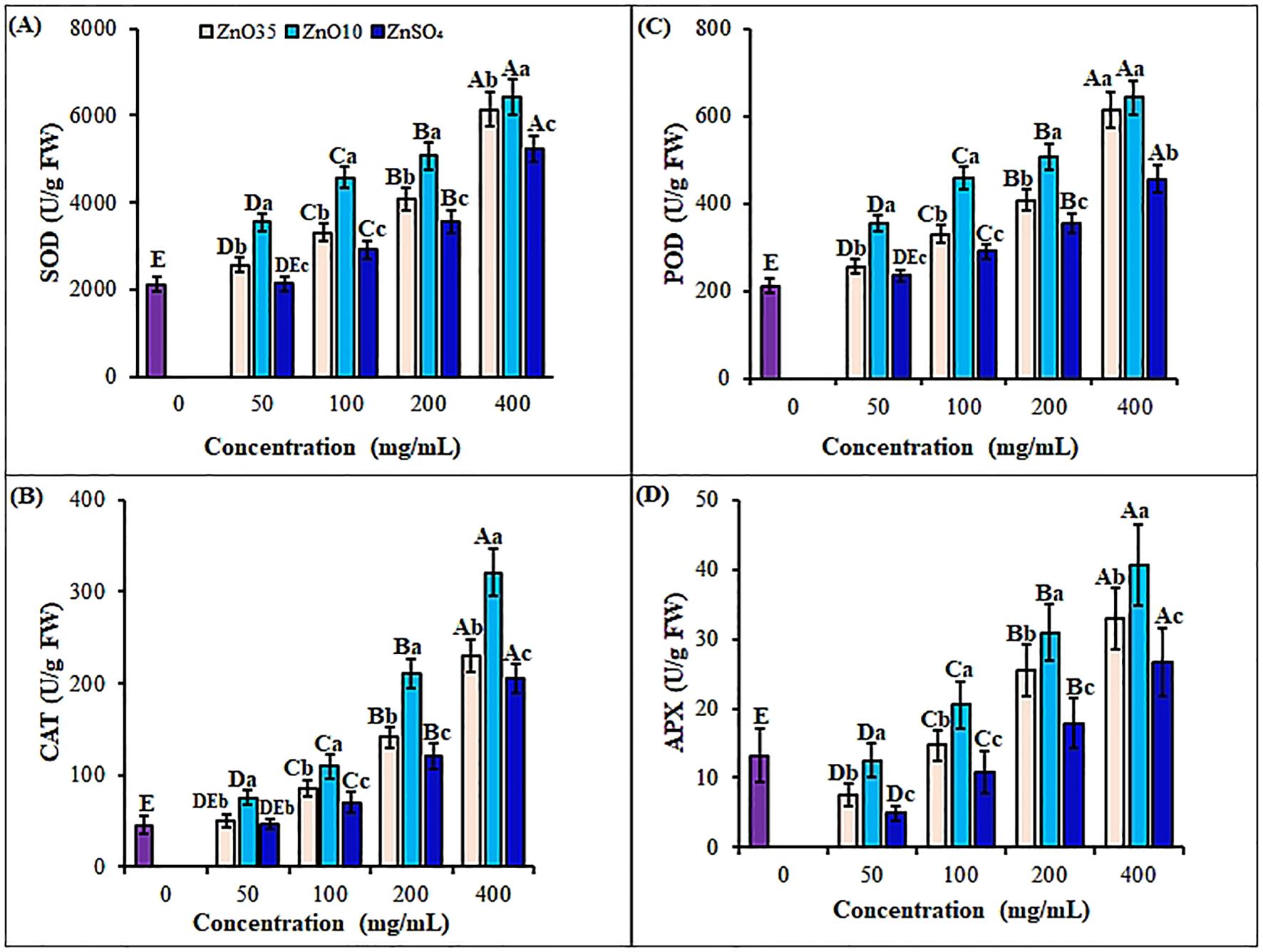
Figure 6. Antioxidant enzymes: SOD (A), CAT (B), POD (C) and APX (D) contents of A. tricolor seedlings from seeds primed with different concentrations and sizes of ZnO NPs and ZnSO4. Different lower-case letters denote significant differences (p< 0.05) between ZnO NPs and ZnSO4 size at a particular concentration. Whereas uppercase letters denote significant differences across different concentrations for a particular NP size. The purple bar shows control.
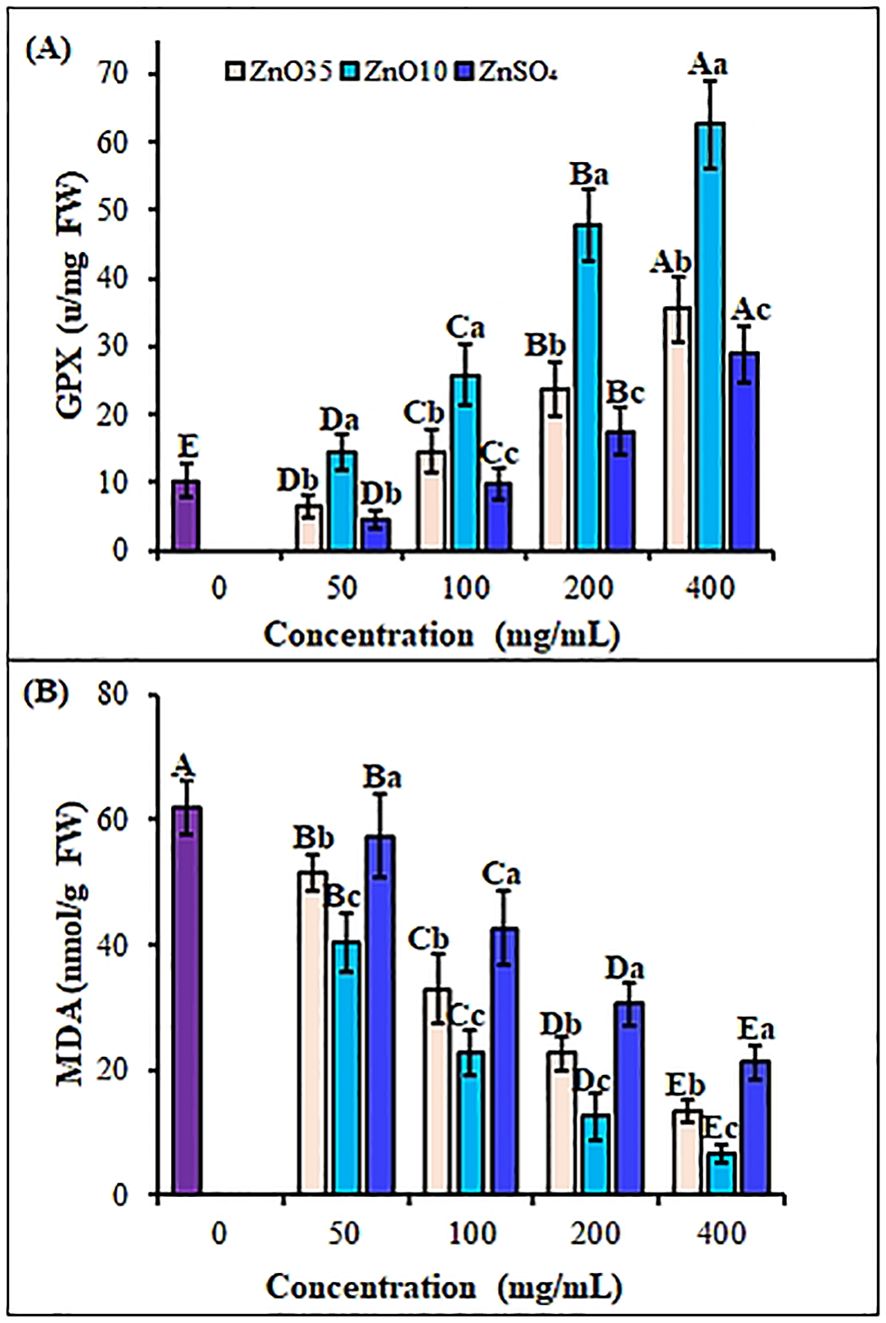
Figure 7. Antioxidant and reactive oxygen response enzymes GPX (A) and, MDA (B) contents, respectively of A. tricolor seedlings from seeds primed with different concentrations and sizes of ZnO NPs and ZnSO4. Different lower-case letters denote significant differences (p< 0.05) between ZnO NPs and ZnSO4 size at a particular concentration. Whereas uppercase letters denote significant differences across different concentrations for a particular NP size. The purple bar shows control.
A balance between ROS production and elimination is essential for successful seed germination and seedling development (Naseer et al., 2023). The MDA content in A. tricolor seedlings was significantly affected by ZnO NPs treatments. The MDA content was reduced significantly by 16.9%, 46.8%, 63.5% and 78.2% at ZnO35 NPs at 50, 100, 200 and 400 mg/L, respectively as compared to untreated control (Figure 7B). A similar pattern of MDA content reduction—34.9%, 63.4%, 79.7%, and 89.3%—was observed for the corresponding ZnO10 NP concentrations relative to the control. ZnO10 NPs and ZnO35 NPs exhibited a significant difference in MDA content (p< 0.05). Despite the decrease in MDA level ranging from 7.4% to 65.6% with increase in ZnSO4 concentration, the MDA level was significantly higher than the ZnO10 and ZnO35 NPs (p< 0.05). The reduction in MDA content and the enhanced activity of antioxidant enzymes in A. tricolor seedlings primed with ZnO NPs suggest a significant decrease in ROS activity. Align with these findings, numerous studies have demonstrated that NPs bolster plant antioxidant systems, mitigating oxidative damage by scavenging ROS, as evidenced by reduced MDA levels (Mishra et al., 2014; Sharma et al., 2021; Kathiravan et al., 2024).
To overcome oxidative stress, together with enzymatic antioxidant system, plants detoxify ROS by upregulating production of nonenzymatic antioxidant phytomolecules including phenols and flavonoids (Singh et al., 2019; Rizk et al., 2025). Seedlings from both ZnO10 and ZnO35 primed A. tricolor seeds revealed a significant (p< 0.05) increase in total phenolic content (TPC) by 49.7% and 31% at 100 mg/L, and 79.9% and 73.2% at 400 mg/L, respectively (Figure 8A) compared with the control. While the ZnSO4 primed seedlings showed an increased TPC, overall, the magnitude was significantly lower than ZnO10 and ZnO35 NPs (P< 0.05). Similarly, studies indicate that plants produce phenolic compounds in response to nanoparticle exposure as a defense against oxidative stress and ROS (Acharya et al., 2020; Rai-Kalal and Jajoo, 2021; Sofo et al., 2017; Del Buono et al., 2022). Although the mechanisms underlying nanoparticle-induced phenol synthesis are largely unknown, studies indicate that ZnO NPs may influence this process through transcriptional regulation (Abbasi et al., 2019). Additionally, the result shown in Figure 8B depicts that with ZnO10 and ZnO35 NPs treatment, TFC increased by about 14.3% and 6.4% in 50 mg/L, 36.2% and 18.4 in 100 mg/L, 48.7% and 36.1% in 200 mg/L, 54.7% and 45.7% in 400 mg/L, correspondingly. Though the ZnSO4 primed seedlings showed stimulating effect on the TFC, their effect is significantly lower than ZnO10 or ZnO35 NPs. NPs have been reported to boost flavonoid content that can reduce lipid peroxidation and mitigate photo-oxidative damage in seedlings (Del Buono et al., 2022; López-Vargas et al., 2020; Banerjee et al., 2021). Higher phenol and flavonoid levels may be linked to their metal-chelating properties, which help limit toxic metal accumulation to optimal levels (Gulcin and Alwasel, 2022). The biostimulatory effects of ZnO NPs in plants depend on their physical properties, including size, shape, roughness, and composition (Juárez-Maldonado et al., 2021), as highlighted by the significant impact of size on flavonoid and phenolic content in this study.
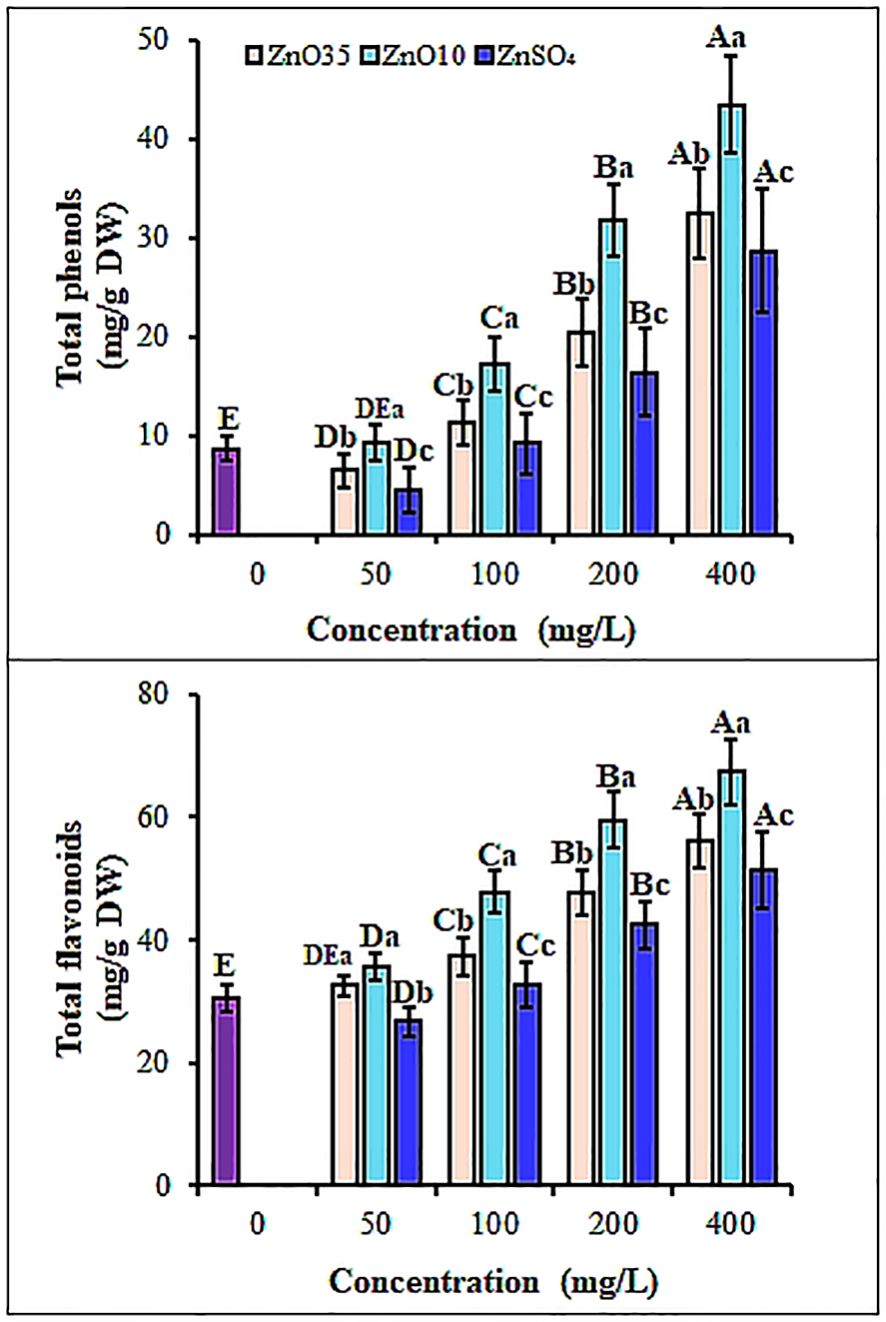
Figure 8. Total phenolic (A) and total flavonoids (B) content of A. tricolor seedlings from seeds primed with different concentrations and sizes of ZnO NPs and ZnSO4. Different lower-case letters denote significant differences (p< 0.05) between ZnO NPs and ZnSO4 size at a particular concentration. Whereas uppercase letters denote significant differences across different concentrations for a particular NP size. The purple bar shows control.
3.5 Effect on zinc profile of A. tricolor seedlings
Zinc is an essential element necessary for growth and development of plants (Pathak et al., 2012). In this study, nanopriming with ZnO10 and ZnO35 NPs led to a significant enhancement in zinc content in A. tricolor seedlings, as verified by ICP-OES measurements. The zinc accumulation displayed a dose-dependent trend (P< 0.05), with the highest efficacy observed at 400 mg/L (Figure 9). ZnO NPs significantly differ in increasing Zinc content compared to the ZnSO4 despite concentration. At this concentration, ZnO10 and ZnO35 NPs increased zinc content by 85% and 80%, respectively, compared to the control, demonstrating effective biofortification through nanoparticle priming. Consistent with these findings, earlier studies have reported that priming with ZnO NPs improves morphometric traits and elevates zinc levels in crops such as maize (Naseer et al., 2023) and wheat (Pandya et al., 2024) at concentrations of 250 and 450 mg/L, respectively. The effect could be partly explained by their small size and high surface area which facilitates better absorption and distribution of zinc within the plant (Sharma et al., 2021; Itroutwar et al., 2020). Furthermore, studies highlight significant zinc content partitioning between the shoots and roots of these crop species (Rameshraddy et al., 2018; Srivastav et al., 2021; Pandya et al., 2024). Similarly, Francisco et al. (2024) demonstrated that priming lettuce with smaller ZnO NPs resulted in a 3.2- to 12.6-fold increase in zinc concentration in leaves, further emphasizing the potential of ZnO NPs for effective biofortification. Studies have also shown that nanopriming with FeS NPs and MnS NPs demonstrated nutritional modulation by enhanced uptake of nanoforms of iron and manganese in rice (Khepar et al., 2023a). ZnO-NP priming significantly increases zinc content in edible plant parts, addressing widespread zinc deficiency in human diets and offering a sustainable, efficient approach to enhance crop nutritional quality, thereby contributing to better health outcomes (Ashwini et al., 2024; Itroutwar et al., 2020).
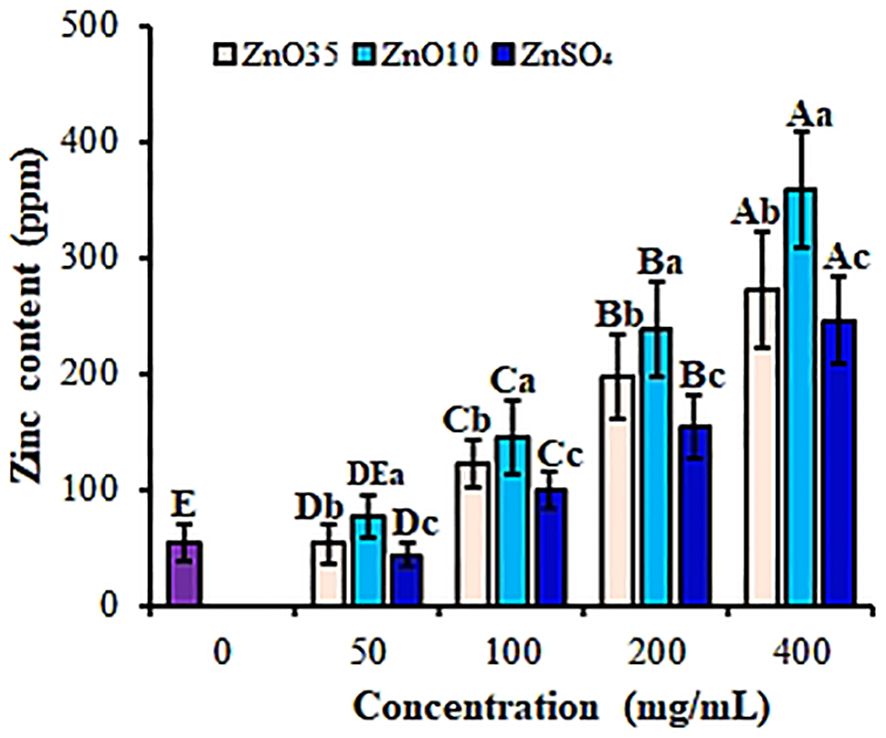
Figure 9. Zinc content of A. tricolor seedlings from seeds primed with different concentrations and sizes of ZnO NPs and ZnSO4. Different lower-case letters denote significant differences (p< 0.05) between ZnO NPs and ZnSO4 size at a particular concentration. Whereas uppercase letters denote significant differences across different concentrations for a particular NP size. The purple bar shows control.
4 Conclusions
The study demonstrates that ZnO NP) are effective seed priming agents for Amaranthus tricolor, significantly enhancing germination traits, seedling vigor, and antioxidant enzyme activities. Improved water uptake, α-amylase activity, and total soluble sugar content are critical for early seedling growth, while increased antioxidant enzyme activities and reduced malondialdehyde content indicate enhanced oxidative stress resistance. ZnO NPs also boost zinc content in seedlings, highlighting their potential for biofortification. These findings suggest that ZnO nanopriming is a promising and sustainable technique to enhance seed germination, seedling growth, and overall crop vigor, offering a practical approach to improve agricultural productivity and resilience to environmental stress, thereby contributing to sustainable agriculture and food security.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AG: Writing – review & editing, Data curation, Writing – original draft, Validation, Methodology, Funding acquisition, Formal analysis. LS: Investigation, Methodology, Writing – review & editing. SW: Investigation, Writing – review & editing, Methodology, Validation. XM: Funding acquisition, Methodology, Data curation, Writing – review & editing. LC: Funding acquisition, Supervision, Resources, Writing – review & editing, Data curation, Project administration, Conceptualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the United States Department of Agriculture, National Institute of Food and Agriculture (USDA-NIFA) Capacity Building Grant, Project #2023-38821-39982.
Acknowledgments
The authors appreciate the Cooperative Agricultural Center, College of Agriculture, Food, and Natural Resources, Prairie View A&M University, for providing space and facility support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1599192/full#supplementary-material
Supplementary Figure 1 | A. tricolor seedlings from ZnO NPs and ZnSO4 primed seeds using different concentrations.
Supplementary Figure 2 | Calibration curve used to determine total phenolic content of A. tricolor seedlings.
Supplementary Figure 3 | Calibration curve used to determine total flavonoids content of A. tricolor seedlings.
References
Abbasi, K. M., Ghorbani, A., and Dadjou, F. (2019). Influence of nanopriming on Festuca ovina seed germination and early seedling traits under drought stress, in Laboratory condition. Ecopersia 7, 133–139.
Abou-Zeid, H. H. and Mohamed, G. S. (2018). The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L. @ to salt stress. Egypt. J. Bot. 58, 73–85. doi: 10.21608/ejbo.2017.1873.1128
Acharya, P., Jayaprakasha, G. K., Crosby, K. M., Jifon, J. L., and Patil, B. S. (2020). Nanoparticle mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 10, 1–16. doi: 10.1038/s41598-020-61696-7
Achigan-Dako, E. G., Sogbohossou, O. E., and Maundu, P. (2014). Current knowledge on Amaranthus spp.: Research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 197, 303–317. doi: 10.1007/s10681-014-1081-9
Adhikary, S., Biswas, B., Chakraborty, D., Timsina, J., Pal, S., Tarafdar, J. C., et al. (2022). Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct−seeded rice (Oryza sativa L.). Sci. Rep. 12, 7103. doi: 10.1038/s41598-022-11307-4
Afzal, S., Sharma, D., and Singh, N. K. (2021). Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L.). Environ. Sci. pollut. Res. 28, 40275–40287. doi: 10.1007/s11356-020-12056-5
Ahammed, G. J., Li, Y., Cheng, Y., Liu, A., Chen, S., and Li, X. (2020). Abscisic acid and gibberellins act antagonistically to mediate epigallocatechin-3-gallate-retarded seed germination and early seedling growth in tomato. J. Plant Growth. Regul. 39, 1414–1424. doi: 10.1007/s00344-020-10089-1
Alahakoon, A. A. C. B., Abeysiriwardena, D. Z., and Gama-Arachchige, N. S. (2021). Low seed moisture and polythene packaging improve storability of seed paddy. J. Stored Prod. Res. 94, 101884. doi: 10.1016/j.jspr.2021.101884
Alam, H., Khattak, J. Z., Ksiksi, T. S., Saleem, M. H., Fahad, S., Sohail, H., et al. (2021). Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant 172, 1336–1351. doi: 10.1111/ppl.13273
Ashwini, M. N., Gajera, H. P., Hirpara, D. G., Savaliya, D. D., and Kandoliya, U. K. (2024). Comparative impact of seed priming with zinc oxide nanoparticles and zinc sulphate on biocompatibility, zinc uptake, germination, seedling vitality, and antioxidant modulation in groundnut. J. Nanopart. Res. 26, 235. doi: 10.1007/s11051-024-06141-w
Awan, S., Shahzadi, K., Javad, S., Tariq, A., and Ilyas, S. (2021). A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleraceae var italica. J. Saudi Soc Agric. Sci. 20, 18–24. doi: 10.1016/j.jssas.2020.10.003
Babayevska, N., Przysiecka, Ł., Iatsunskyi, I., Nowaczyk, G., Jarek, M., Janiszewska, E., et al. (2022). ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 12, 8148. doi: 10.1038/s41598-022-12134-3
Bailly, C., El-Maarouf-Bouteau, H., and Corbineau, F. (2008). From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C. R. Biol. 331, 806–814. doi: 10.1016/j.crvi.2008.07.022
Banerjee, S., Islam, J., Mondal, S., Saha, A., Saha, B., and Sen, A. (2023). Proactive attenuation of arsenic-stress by nano-priming: Zinc Oxide nanoparticles in Vigna mungo (L.) Hepper trigger antioxidant defense response and reduce root-shoot arsenic translocation. J. Hazardous Materials 446, 130735. doi: 10.1016/j.jhazmat.2023.130735
Banerjee, A., Singh, A., Sudarshan, M., and Roychoudhury, A. (2021). Silicon nanoparticle-pulsing mitigates fluoride stress in rice by fine-tuning the ionomic and metabolomic balance and refining agronomic traits. Chemosphere 262, 127826. doi: 10.1016/j.chemosphere.2020.127826
Bradford, K. J. (2002). Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci. 50, 248–260. doi: 10.1614/0043-1745(2002)050[0248:AOHTTQ]2.0.CO2
Butler, L. H., Hay, F. R., Ellis, R. H., Smith, R. D., and Murray, T. B. (2009). Priming and re-drying improve the survival of mature seeds of Digitalis purpurea during storage. Ann. Bot. 103, 1261–1270. doi: 10.1093/aob/mcp059
Cabot, C., Martos, S., Llugany, M., Gallego, B., Tolr`a, R., and Poschenrieder, C. (2019). A role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01171
Cakmak, I. (2000). Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 146, 185–205. doi: 10.1046/j.1469-8137.2000.00630.x
Chang, C. C., Yang, M. H., Wen, H. M., and Chern, J. C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10, 178–182. doi: 10.38212/2224-6614.2748
Chatterjee, N., Sarkar, D., Sankar, A., Sumita, P. A. L., Singh, H. B., Singh, R. K., et al. (2018). On-farm seed priming interventions in agronomic crops. Acta Agric. Slov. 111, 715–735. doi: 10.14720/aas.2018.111.3.19
Chen, K. and Arora, R. (2013). Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 94, 33–45. doi: 10.1016/j.envexpbot.2012.03.005
Choudhary, R. C., Kumaraswamy, R. V., Kumari, S., Sharma, S. S., Pal, A., Raliya, R., et al. (2019). Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 127, 126–135. doi: 10.1016/j.ijbiomac.2018.12.274
Del Buono, D., Luzi, F., Tolisano, C., Puglia, D., and Di Michele, A. (2022). Synthesis of a lignin/zinc oxide hybrid nanoparticles system and its application by nano-priming in maize. Nanomaterials 12, 568. doi: 10.3390/nano12030568
Dhindsa, R. S. and Matowe, W. (1981). Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 32, 79–91. doi: 10.1093/jxb/32.1.79
do Espirito Santo Pereira, A., Caixeta Oliveira, H., Fernandes Fraceto, L., and Santaella, C. (2021). Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 11, 267. doi: 10.3390/nano11020267
El-Badri, A. M., Batool, M., Wang, C., Hashem, A. M., Tabl, K. M., Nishawy, E., et al. (2021). Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 225, 112695–112707. doi: 10.1016/j.ecoenv.2021.112695
El-Beltagi, H. S., Shah, S., Ullah, S., Mansour, A. T., and Shalaby, T. A. (2022). Impacts of ascorbic acid and alpha-tocopherol on chickpea (Cicer arietinum L.) grown in water deficit regimes for sustainable production. Sustainability 14, 8861. doi: 10.3390/su14148861
Ellis, R.A. and Roberts, E.H. (1981). The quantification of ageing and survival in orthodox seeds. Seed Sci Technol. 9, 373–409.
Estrada-Urbina, J., Cruz-alonso, A., Santander-Gonzalez, M., Mendez-Albores, A., and Vazquez-Duran, A. (2018). Nanoscale zinc oxide particles for improving the physiological and sanitary quality of a mexican landrace of red maize. Nanomaterials 8, 247. doi: 10.3390/nano8040247
Faizan, M., Faraz, A., and Hayat, S. (2020). Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J. Plant Biochem. Biotechnol. 29, 553–567. doi: 10.1007/s13562-019-00525-z
Farooq, M., Irfan, M., Aziz, T., Ahmad, I., and Cheema, S. A. (2013). Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 199, 12–22. doi: 10.1111/j.1439-037X.2012.00521.x
Farooq, T., Nisa, Z. U., Hameed, A., Ahmed, T., and Hameed, A. (2022). Priming with copper-chitosan nanoparticles elicit tolerance against PEG-induced hyperosmotic stress and salinity in wheat. BMC Chem. 16, 23. doi: 10.1186/s13065-022-00813-1
Fathi, N., Kazemeini, S. A., Alinia, M., and Mastinu, A. (2023). The effect of seed priming with melatonin on improving the tolerance of Zea mays L. var saccharata to paraquat- induced oxidative stress through photosynthetic systems and enzymatic antioxidant activities. Physiol. Mol. Plant Pathol. 124, 101967. doi: 10.1016/j.pmpp.2023.101967
Fenner, M. and Thompson, K. (2005). The Ecology of Seeds (Cambridge, UK: Cambridge University Press). doi: 10.1017/CBO9780511614101
Francisco, M.d., Fernandes-Silva, P., Durães, L., Romeiro, A., Álvarez-Torrellas, S., and Almendros, P. (2024). Influence of the application of different zinc oxide nanoparticles on a lettuce crop grown in an acidic mediterranean soil. Horticulturae 10, 681. doi: 10.3390/horticulturae10070681
García-Locascio, E., Valenzuela, E. I., and Cervantes-Avilés, P. (2024). Impact of seed priming with Selenium nanoparticles on germination and seedlings growth of tomato. Sci. Rep. 14(1), 6726. doi: 10.1038/s41598-024-57049-3
Geremew, A., Carson, L., Woldesenbet, S., Wang, H., Reeves, S., Brooks, N., et al. (2023). Effect of zinc oxide nanoparticles synthesized from Carya illinoinensis leaf extract on growth and antioxidant properties of mustard (Brassica juncea). Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1108186
Goswami, A., Banerjee, R., and Raha, S. (2013). Drought resistance in rice seedlings conferred by seed priming. Protoplasma 250, 1115–1129. doi: 10.1007/s00709-013-0487-x
Gulcin, İ. and Alwasel, S. H. (2022). Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 10, 132. doi: 10.3390/pr10010132
Gupta, R. and Gudu, S. (2022). Amaranth: A climate-resilient crop for nutritional security. In Proceedings of the International Conference on Climate-Smart Agriculture (pp. 115–123). Nairobi, Kenya: African Agricultural Research Forum.
Gupta, N., Singh, P. M., Sagar, V., Pandya, A., Chinnappa, M., Kumar, R., et al. (2022). Seed priming with ZnO and Fe3O4 nanoparticles alleviate the lead toxicity in Basella alba L. through reduced lead uptake and regulation of ROS. Plants 11, 2227. doi: 10.3390/plants11172227
Hajiboland, R. (2014). “Reactive oxygen species and photosynthesis,” in Oxidative damage to plants (Elsevier, Cambridge, MA, USA), 1–63.
Hatami, M. (2017). “Stimulatory and inhibitory effects of nanoparticulates on seed germination and seedling vigor indices,” in Nanoscience and Plant–Soil Systems, vol. 48 . Eds. Ghorbanpour, M., Manika, K., and Varma., A. (Springer, Cham). doi: 10.1007/978-3-319-46835-8_13
Hatami, M., Hosseini, S. M., Ghorbanpour, M., and Kariman, K. (2019). Physiological and antioxidative responses to GO/PANI nanocomposite in intact and demucilaged seeds and young seedlings of. Salvia mirzayanii. Chemosphere 233, 920–935. doi: 10.1016/j.chemosphere.2019.05.268
Horii, A., McCue, P., and Shetty, K. (2007). Seed vigour studies in corn, soybean and tomato in response to fish protein hydrolysates and consequences on phenolic-linked responses. Bioresour. Technol. 98, 2170–2177. doi: 10.1016/j.biortech.2006.08.030
Hussain, S., Maqsood, M. A., Rengel, Z., Aziz, T., and Abid, M. (2013). Estimated zinc bioavailability in milling fractions of biofortified wheat grains and in flours of different extraction rates. Int. J. Agr. Biol. 15, 921–926.
Hussain, I., Singh, N. B., Singh, A., Singh, H., Singh, S. C., and Yadav, V. (2017). Exogenous application of phytosynthesized nanoceria to alleviate ferulic acid stress in Solanum lycopersicum. Sci. Hortic. 214, 158–164. doi: 10.1016/j.scienta.2016.11.032
Irigoyen, J. J., Emerich, D. W., and Sanchez-Dıaz, M. (1992). Water stress induced changes in concentrations of proline and total soluble sugar in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant 84, 55–60. doi: 10.1111/j.1399-3054.1992.tb08764.x
Itroutwar, P. D., Govindaraju, K., Tamilselvan, S., Kannan, M., Raja, K., and Subramanian, K. S. (2020). Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J. Plant Growth Regul. 39, 717–728. doi: 10.1007/s00344-019-10012-3
Iziy, E., Majd, A., Vaezi-Kakhki, M. R., Nejadsattari, T., and Noureini, S. K. (2019). Effects of zinc oxide nanoparticles on enzymatic and nonenzymatic antioxidant content, germination, and biochemical and ultrastructural cell characteristics of Portulaca oleracea L. Acta Soc Bot. Pol. 88, 3639. doi: 10.5586/asbp.3639
Jayachandran, A., Aswathy, T. R., and Nair, A. S. (2021). Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophysics Rep. 26, 100995. doi: 10.1016/j.bbrep.2021.100995
Juárez-Maldonado, A., Tortella, G., Rubilar, O., Fincheira, P., and Benavides-Mendoza, A. (2021). Biostimulation and toxicity: The magnitude of the impact of nanomaterials in microorganisms and plants. J. Advan. Res. 31, 113–126. doi: 10.1016/j.jare.2020.12.011
Kataria, S., Baghel, L., and Guruprasad, K. N. (2015). Acceleration of germination and early growth characteristics of soybean and maize after pretreatment of seeds with static magnetic field. Int. J. Trop. Agric. 33, 985–992.
Kathiravan, M., Vanitha, C., Umarani, R., Marimuthu, S., Ayyadurai, P., Sathiya, K., et al. (2024). Seed priming with biosynthesized zinc oxide nanoparticles for enhancing seed germination and vigour through promoting antioxidant and hydrolytic enzyme activity in green gram (Vigna radiata). Agric. Res. doi: 10.1007/s40003-024-00792-w
Khalaki, M. A., Moameri, M., AsgariLajayer, B., and Astatkie, T. (2021). Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 93, 13–28. doi: 10.1007/s10725-020-00670-9
Khan, S., Ullah, A., Ullah, S., Saleem, M. H., Okla, M. K., Al-Hashimi, A., et al. (2022). Quantifying temperature and osmotic stress impact on seed germination rate and seedling growth of Eruca sativa Mill. via hydrothermal time model. Life 12, 400. doi: 10.3390/life12030400
Khanm, H., Vaishnavi, B. A., and Shankar, A. G. (2018). Raise of nano-fertilizer Era: effect of nano scale zinc oxide particles on the germination, growth and yield of tomato (Solanum lycopersicum). Int. J. Curr. Microbiol. Appl. Sci. 7, 1861–1871. doi: 10.20546/ijcmas.2018.705.219
Khepar, V., Ahuja, R., Sidhu, A., and Samota, M. K. (2023a). Nano-sulfides of Fe and Mn efficiently augmented the growth, antioxidant defense system, and metal assimilation in rice seedlings. ACS Omega 8, 30231–30238. doi: 10.1021/acsomega.3c03012
Khepar, V., Sidhu, A., Mankoo, R., Manchanda, P., and Sharma, A. B. (2024). Nanobiostimulant action of trigolic formulated zinc sulfide nanoparticles (ZnS-T NPs) on rice seeds by triggering antioxidant defense network and plant growth specific transcription factors. Plant Physiol. Biochem. 210, 108605. doi: 10.1016/j.plaphy.2024.108605
Khepar, V., Sidhu, A., and Sharma, A. B. (2023b). Topologically Zn2+ hybridized ZnS nanospheres (Zn2+/nZnS) efficiently restrained the infection of Fusarium verticillioides in rice seeds by hyphal disorganization and nutritional modulation. Environ. Sci.: Nano 10, 1138. doi: 10.1039/d2en00914e
Kibinza, S., Bazin, J., Bailly, C., Farrant, J. M., Corbineau, F., and El-Maarouf-Bouteau, H. (2011). Catalase is a key enzyme in seed recovery from ageing during priming. Plant Sci. 181, 309–315. doi: 10.1016/j.plantsci.2011.06.003
Kishorekumar, A., Jaleel, C. A., Manivannan, P., Sankar, B., Sridharan, R., and Panneerselvam, R. (2007). Comparative effects of different triazole compounds on growth, photosynthetic pigments and carbohydrate metabolism of Solenostemon rotundifolius. Colloids Surf. B: Biointerfaces 60, 207–212. doi: 10.1016/j.colsurfb.2007.06.008
Kumar, A., Jain, G., Dutta, P., Singh, P., Alam, N., Narayan, S., et al. (2024). Nanopriming with phytofabricated selenium nanoparticles alleviates arsenite−induced oxidative stress in Spinacia oleracea L. Environ. Sci. pollut. Res. 12, 1–15. doi: 10.1007/s11356-024-35183-9
Li, Y., Liang, L., Li, W., Ashraf, U., Ma, L., Tang, X., et al. (2021). ZnO nanoparticle−based seed priming modulates early growth and enhances physio−biochemical and metabolic profiles of fragrant rice against cadmium toxicity. Nanobiotechnol 19, 75. doi: 10.1186/s12951-021-00820-9
López-Vargas, E. R., González-García, Y., Pérez-Álvarez, M., Cadenas-Pliego, G., González-Morales, S., Benavides-Mendoza, A., et al. (2020). Seed priming with carbon nanomaterials to modify the germination, growth, and antioxidant status of tomato seedlings. Agronomy 10, 639. doi: 10.3390/agronomy10050639
Mahakham, W., Sarmah, A. K., Maensiri, S., and Theerakulpisut, P. (2017). Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 7, 8263. doi: 10.1038/s41598-017-08669-5
Makkar, H. P., Blummel, M., Borowy, N. K., and Becker, K. (1993). Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agri 61, 161–165. doi: 10.1002/jsfa.2740610205
Marreiro, D. D. N., Cruz, K. J. C., Morais, J. B. S., Beserra, J. B., Severo, J. S., and De Oliveira, A. R. S. (2017). Zinc and oxidative stress: current mechanisms. Antioxidants 6, 24. doi: 10.3390/antiox6020024
Mazhar, W. M., Ishtiaq, M., Hussain, I., Parveen, A., Bhatt, H. K., Azeem, M., et al. (2022). Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PloS One 17, e0264967. doi: 10.1371/journal.pone.0264967
Mishra, V., Mishra, R. K., Dikshit, A., and Pandey, A. C. (2014). “Interactions of nanoparticles with plants,” in Emerging Technologies and Management of Crop Stress Tolerance (Amsterdam, Netherlands: Elsevier), 159–180.
Nakano, Y. and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880. doi: 10.1093/oxfordjournals.pcp.a076232
Naseer, I., Javad, S., Iqbal, S., Shah, A. A., Alwutayd, K., and Abd Elgawad, H. (2023). Deciphering the role of zinc oxide nanoparticles on physiochemical attributes of Zea mays exposed to saline conditions through modulation in antioxidant enzyme defensive system. South Afr. J. Bot. 160, 469–482. doi: 10.1016/j.sajb.2023.07.035
Neto, M. E., Britt, D. W., Lara., L. M., Cartwright, A., dos Santos, R. F., Inoue, T. T., et al. (2020). Initial development of corn seedlings after seed priming with nanoscale synthetic zinc oxide. Agronomy 10, 307. doi: 10.3390/agronomy10020307
Nile, S. H., Thiruvengadam, M., Wang, Y., Samynathan, R., Shariati, M. A., Rebezov, M., et al. (2022). Nano-priming as emerging seed priming technology for sustainable agriculture recent developments and future perspectives. J. Nanobiotechnol 20, 254. doi: 10.1186/s12951-022-01423-8
Niveyro, S. L., Palmucci, H. E., and Quiroga, M. (2021). Bioactive compounds and nutritional value of Amaranthus species: A promising functional food. Food Chem. 357, 129746.
Noohpisheh, Z., Amiri, H., Mohammadi, A., and Farhadi, S. (2021). Effect of the foliar application of zinc oxide nanoparticles on some biochemical and physiological parameters of Trigonella foenum-graecum under salinity stress. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 155, 267–280. doi: 10.1080/11263504.2020.1739160
Ofori, K. F., Antoniello, S., English, M. M., and Aryee, A. N. A. (2022). Improving nutrition through biofortification: A systematic review. Front. Nutt. 9. doi: 10.3389/fnut.2022.1043655
Olechnowicz, J., Tinkov, A., Skalny, A., and Suliburska, J. (2018). Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 68, 19–31. doi: 10.1007/s12576-017-0571-7
Oracz, K. and Karpinski, S. (2016). Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00864
Ozturk, L., Yazicia, M. A., Yucelb, C., Torunb, A., Cekicc, C., Bagcid, A., et al. (2006). Concentration and localization of zinc during seed development and germination in wheat. Physiol. Plantarum. 128, 144–152. doi: 10.1111/j.1399-3054.2006.00737.x
Pandya, P., Kumar, S., Patil, G., Mankad, M., and Shah, Z. (2024). Impact of ZnO nanopriming on physiological and biochemical traits of wheat (Triticum aestivum L.) seedling. CABI Agric. Bioscience 5, 27. doi: 10.1186/s43170-024-00228-z
Paparella, S., Araujo, S. S., Rossi, G., Wijayasinghe, M., Carbonera, D., and Balestrazzi, A. (2015). Seed priming: state of the art and new perspectives. Plant Cell Rep. 34, 1281–1293. doi: 10.1007/s00299-015-1784-y
Pathak, G. C., Gupta, B., and Pandey, N. (2012). Improving reproductive efficiency of chickpea by foliar application of zinc. Braz. J. Plant Physiol. 24, 173–180. doi: 10.1590/S1677-04202012000300004
Prasad, T.N.V.K.V., Sudhakar, P., Sreenivasulu, Y., Latha, P., Munaswamy, V., Reddy, K. R., et al. (2012). Effect of nanoscale zinc oxide particles on the germination, growth, and yield of peanut. J. Plant Nutr. 35, 905–927. doi: 10.1080/01904167.2012.663443
Qian, H., Peng, X., Han, X., Ren, J., Sun, L., and Fu, Z. (2013). Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 25, 1947–1956. doi: 10.1016/S1001-0742(12)60301-5
Rai-Kalal, P. and Jajoo, A. (2021). Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 160, 341–351. doi: 10.1016/j.plaphy.2021.01.032
Rajjou, L., Duval, M., Gallardo, K., Catusse, J., Bally, J., Job, C., et al. (2012). Seed germination and vigor. Annu. Rev. Plant Biol. 63, 507–533. doi: 10.1146/annurev-arplant-042811-105550
Rameshraddy, S. M., Geetha, K. N., and Shankar, A. G. (2018). ZnO nanoparticle improves maize growth, yield and seed zinc under high soil pH condition. Int. J. Curr. Microbiol. Appl. Sci. 7, 1593–1601. doi: 10.20546/ijcmas.2018.712.187
Rastogi, A. and Shukla, S. (2013). Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr. 53, 109–125. doi: 10.1080/10408398.2010.517876
Rawat, P., Singh, R. K., Pradeep, R., and Pandey, P. (2018). Effect of nanoparticles on wheat seed germination and seedling growth. Int. J. Agric. Biol. Eng. 12, 13–16. doi: 10.5281/zenodo.1315657
Rizk, R., Ahmed, M., Abdul-Hamid, D., Zedan, M., Tóth, Z., and Decsi, K. (2025). Resulting key physiological changes in Triticum aestivum L. plants under drought conditions after priming the seeds with conventional fertilizer and greenly synthesized zinc oxide nanoparticles from corn wastes. Agronomy 15, 211. doi: 10.3390/agronomy15010211
Ruszkiewicz, J. A., Pinkas, A., Ferrer, B., Peres, T. V., Tsatsakis, A., and Aschner, M. (2017). Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 4, 245–259. doi: 10.1016/j.toxrep.2017.05.006
Salam, A., Afridi, M. S., Javed, M. A., Saleem, A., Hafeez, A., Khan, A. R., et al. (2022). Nano-priming against abiotic stress: A way forward towards sustainable agriculture. Sustainability 14, 14880. doi: 10.3390/su142214880
Salama, D. M., Osman, S. A., Abd El-Aziz, M., Abd Elwahed, M. S., and Shaaban, E. (2019). Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 18, 101083. doi: 10.1016/j.bcab.2019.101083
Samad, A., Khan, M. J., Shah, Z., and Tariq, J. A. N. (2013). Determination of optimal duration and concentration of zinc and phosphorus for priming wheat seed. Sarhad J. Agric. 30, 1.
Sarker, U. and Oba, S. (2020). Nutritional and bioactive compounds in selected red and green leafy Amaranthus vegetables. Sci. Rep. 10, 1336. doi: 10.1038/s41598-020-71714-3
Sattar, A. A., Matin, A. A., Hadwan, M. H., Hadwan, A. H., and Mohammed, R. M. (2024). Rapid and effective protocol to measure glutathione peroxidase activity. Bull. Natl. Res. Centre 48, 100. doi: 10.1186/s42269-024-01250-x
Selim, Y. A., Azb, M. A., Ragab, I., and Abd El-Azim, M. H. (2020). Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-60541-1
Shah, T., Latif, S., Khan, H., Munsif, F., and Nie, L. (2019). Ascorbic acid priming enhances seed germination and seedling growth of winter wheat under Low Temperature Due to Late Sowing in Pakistan. Agronomy 9, 757. doi: 10.3390/agronomy911075
Shah, T., Latif, S., Saeed, F., Ali, I., Ullah, S., Alsahli, A. A., et al. (2021). Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ. - Sci. 33, 101207. doi: 10.1016/j.jksus.2020.10.004
Sharifan, H., Moore, J., and Ma, X. (2020). Zinc oxide (ZnO) nanoparticles elevated iron and copper contents and mitigated the bioavailability of lead and cadmium in different leafy greens. Ecotoxicol. Environ. Saf. 191, 110177. doi: 10.1016/j.ecoenv.2020.110177
Sharma, D., Afzal, S., and Singh, N. K. (2021). Nanopriming with phytosynthesized zinc oxide nanoparticles for promoting germination and starch metabolism in rice seeds. J. Biotechnol. 336, 64–75. doi: 10.1016/j.jbiotec.2021.06.014
Sharma, P., Bhatt, D., Zaidi, M. G. H., Saradhi, P., Khanna, P. K., and Arora, S. (2012). Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 167, 2225–2233. doi: 10.1007/s12010-012-9759-8
Shaw, A.K. and Hossain, Z. (2013). Impact of Nano-CuO Stress on Rice (Oryza sativa L.) Seedlings. Chemosphere, 93, 906–915. doi: 10.1016/j.chemosphere.2013.05.044
Shelar, A., Singh, A. V., Chaure, N., Jagtap, P., Chaudhari, P., Shinde, M., et al. (2024). Nanoprimers in sustainable seed treatment: Molecular insights into abiotic-biotic stress tolerance mechanisms for enhancing germination and improved crop productivity. Sci. Tot. Environ. 951, 175118. doi: 10.1016/j.scitotenv.2024.175118
Shinde, S., Paralikar, P., Ingle, A. P., and Rai, M. (2020). Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from Aspergillus Niger. Arab. J. Chem. 13, 3172–3182. doi: 10.1016/j.arabjc.2018.10.001
Singh, J., Kumar, S., Alok, A., Upadhyay, S. K., Rawat, M., Tsang, D. C. W., et al. (2019). The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 214, 1061–1070. doi: 10.1016/j.jclepro.2019.01.018
Sofo, A., Bochicchio, R., Amato, M., Rendina, N., Vitti, A., Nuzzaci, M., et al. (2017). Plant architecture, auxin homeostasis and phenol content in Arabidopsis Thaliana grown in cadmium- and zinc-enriched media. J. Plant Physiol. 216, 174–180. doi: 10.1016/j.jplph.2017.06.008
Soni, A. T., Rookes, J. E., and Arya, S. S. (2023). Chitosan Nanoparticles as seed priming agents to alleviate salinity stress in rice (Oryza sativa L.) seedlings. Polysaccharides 4, 129–141. doi: 10.3390/polysaccharides4020010
Srivastav, A., Ganjewala, D., Singhal, R. K., Rajput, V. D., Minkina, T., Voloshina, M., et al. (2021). Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants 10, 2556. doi: 10.3390/plants10122556
Srivastava, R. K., Pandey, P., Rajpoot, R., Rani, A., and Dubey, R. S. (2014). Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251, 1047–1065. doi: 10.1007/s00709-014-0614-3
Stałanowska, K., Szablińska-Piernik, J., Okorski, A., and Lahuta, L. B. (2023). Zinc oxide nanoparticles affect early seedlings’ growth and polar metabolite profiles of pea (Pisum sativum L.) and wheat (Triticum aestivum L.). Int. J. Mol. Sci. 24, 14992. doi: 10.3390/ijms241914992
Sytar, O., Kumari, P., Yadav, S., Brestic, M., and Rastogi, A. (2019). Phytohormone priming: regulator for heavy metal stress in plants. J. Plant Growth Regul. 38, 739–752. doi: 10.1007/s00344-018-9886-8
Takahashi, M., Nozoye, T., Kitajima, N., Fukuda, N., Hokura, A., Terada, Y., et al. (2009). In vivo analysis of metal distribution and expression of metal transporters in rice seed during germination process by microarray and X-ray fluorescence imaging of Fe, Zn, Mn, and Cu. Plant Soil 325, 39. doi: 10.1007/s11104-009-0045-7
Taylor, A. G., Allen, P. S., Bennett, M. A., Bradford, K. J., Burris, J. S., and Misra, M. K. (1998). Seed enhancements. Seed Sci. Res. 8, 245–256. doi: 10.1017/S0960258500004141
Tondey, M., Kalia, A., Singh, A., Dheri, G. S., Taggar, M. S., Nepovimova, E., et al. (2021). Seed priming and coating by nano-scale zinc oxide particles improved vegetative growth, yield and quality of fodder maize (Zea mays). Agronomy 11, 729. doi: 10.3390/agronomy11040729
Ullah, A., Sadaf, S., Ullah, S., Alshaya, H., Okla, M. K., Alwasel, Y. A., et al. (2022). Using halothermal time model to describe barley (Hordeum vulgare L). Seed germination response to water potential and temperature. Life 12, 209. doi: 10.3390/life12020209
Veˇceˇrová, K., Veˇceˇra, Z., Doˇcekal, B., Oravec, M., Pompeiano, A., Tˇríska, J., et al. (2016). Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ. pollut. 218, 207–218. doi: 10.1016/j.envpol.2016.05.013
Wang, Z., Li, H., Li, X., Xin, C., Si, J., Li, S., et al. (2020). Nano-ZnO priming induces salt tolerance by promoting photosynthetic carbon assimilation in wheat. Arch. Agron. Soil Sci. 66, 1259–1273. doi: 10.1080/03650340.2019.1663508
Waqas, M., Korres, N. E., Khan, M. D., Nizami, A. S., Deeba, F., Ali, I., et al. (2019). “Advances in the concept and methods of seed priming,” in Priming and Pretreatment of Seeds and Seedlings (Springer, Singapore), 11–41.
Westoby, M., Leishman, M., and Lord, J. (2002). Comparative ecology of seed size and dispersal. Am. Nat. 150, 280–298. doi: 10.1098/rstb.1996.0114
Younes, N. A., Hassan, H. S., Elkady, M. F., Hamed, A. M., and Dawood, M. F. A. (2020). Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon 6, e03188. doi: 10.1016/j.heliyon.2020.e03188
Yue, J., Tang, M., Zhang, H., Luo, D., Cao, S., Hu, Y., et al. (2022). The transcription factor HcERF4 confers salt and drought tolerance in kenaf (Hibiscus cannabinus L.). Plant Cell Tissue Organ Culture (PCTOC) 150, 207–221. doi: 10.1007/s11240-022-02260-1
Zheng, J. L., Zhao, L. Y., Shen, B., Jiang, L. H., and Zhu, A. Y. (2016). Effects of salinity on activity and expression of enzymes involved in ionic, osmotic, and antioxidant responses in Eurya emarginata. Acta Physiol. Plant 38, 1–9. doi: 10.1007/s11738-015-2040-3
Keywords: amaranth, germination, antioxidant, zinc oxide nanoparticles, phenols, flavonoid
Citation: Geremew A, Stovall L, Woldesenbet S, Ma X and Carson L (2025) Nanopriming with zinc oxide: a novel approach to enhance germination and antioxidant systems in amaranth. Front. Plant Sci. 16:1599192. doi: 10.3389/fpls.2025.1599192
Received: 24 March 2025; Accepted: 09 June 2025;
Published: 25 June 2025.
Edited by:
Boon Chin Tan, University of Malaya, MalaysiaReviewed by:
Sumera Javad, Lahore College for Women University, PakistanVarinder Khepar, Punjab Agricultural University, India
Copyright © 2025 Geremew, Stovall, Woldesenbet, Ma and Carson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Carson, bGVjYXJzb25AcHZhbXUuZWR1
 Addisie Geremew
Addisie Geremew Leandrea Stovall1
Leandrea Stovall1 Xingmao Ma
Xingmao Ma Laura Carson
Laura Carson