- 1Tobacco Research Institute, Chinese Academy of Agricultural Sciences, Qingdao, China
- 2The Graduate School, Chinese Academy of Agricultural Sciences, Beijing, China
- 3Technology Center, Heilongjiang Tobacco Industry Limited Liability Company, Harbin, China
- 4Hunan Tobacco Corporation, Changsha, China
Coumarins, a class of metabolites derived from the phenylpropanoid pathway, play critical roles in plant development and interactions with environmental factors. In recent years, numerous studies have revealed that catalytic enzymes, physiological conditions, and environmental stimuli collectively regulate coumarin metabolism in plants. This regulation is not only essential for normal growth and development, but also enhances plant resistance to environmental stresses. In this review, we summarize recent advances in understanding the roles of coumarins in plant development, the key enzymes and genes involved in their biosynthesis, and the genetic regulatory mechanisms that mediate plant responses to both biotic and abiotic stresses, including drought, salinity, UV radiation, and attacks by pathogenic bacteria and insects. The strategic implementation of multi-gene regulatory approaches holds great promise for enhancing plant stress tolerance and has significant potential applications in agriculture.
1 Introduction
Coumarins constitute a special family of secondary metabolites in plants. To date, a total of 574 coumarins, reported from plant sources, have been enumerated. These coumarins are distributed over nearly 30 families and 150 species, such as Umbelliferae, Apiaceae, Asteraceae, Fabaceae, Moraceae, Oleaceae, Rutaceae, Thymelaeaceae, and so on (Leal et al., 2024; Nan et al., 2025). The discovery of natural products of coumarin dates back two centuries, with the nomenclature originating from the plant Coumarouna odorata (Dipteryx odorata) (Barot et al., 2015; Pereira et al., 2018; Annunziata et al., 2020). Subsequent investigations have isolated and characterized structurally diverse coumarins and their derivatives from various plant tissues, such as roots, leaves, fruits, and flowers, and discovered their broad-spectrum bioactivities and multifaceted application prospects in the fields including pharmaceutical and healthcare industries, fragrance production, and sustainable agricultural practices (Wu et al., 2009; Hussain et al., 2019; Nan et al., 2025).
So far, the main biosynthetic pathways of coumarins in plants have been well-characterized, encompassing an upstream phenylpropanoid pathway and a downstream coumarin-specific branch pathway. These processes involve a suite of catalytic enzymes, including phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H) and 4-coumarate-CoA ligase (4CL), which catalyzed the initial three steps of the pathway and provide the basis for coumarins branch, as well as coumarate 2’-hydroxylase (C2’H), p-coumaroyl shikimate 3’-hydroxylase (C3H), and feruloyl-CoA 6’-hydroxylase (F6’H), prenyltransferases (PTs) and cytochrome P450 monooxygenases, which were responsible for the formation of simple and complex coumarin derivatives. Up to now, Apiaceae is the only lineage in which the complete biosynthetic pathway has been decoded (Thomas, 2010; Huang et al., 2024; Liang et al., 2024; Goldenberg et al., 2025). Notably, the homeostasis of coumarins in plants is dynamically regulated by biotic and abiotic stresses, reflecting an adaptive mechanism evolved by plants to cope with intricate and fluctuating environments. The biosynthesis and accumulation of coumarins in plants are tightly regulated by a combination of abiotic factors (e.g., nutrient availability, water status, temperature, and light conditions) and biotic stressors (e.g., pathogenic microorganisms and herbivorous pests) (Duan et al., 2019; Robe et al., 2020; Huang et al., 2022; Bai et al., 2024).
An in-depth understanding of the biosynthesis of coumarins and the molecular regulatory mechanisms under diverse stress conditions will extend our knowledge of plant physiological and ecological processes. It will offer solid theoretical and practical guidance for crop breeding, agricultural production, and biomedical research. This review outlines the regulatory roles of enzymes in coumarin metabolism, their contributions to coordinating plant development and plant-environment interactions, and the molecular mechanisms driving coumarin accumulation in response to biotic and abiotic stresses. We also highlight the current research gaps in applying these findings to breeding and agriculture, proposing future research directions for improving plant stress resistance through genetic engineering methods.
2 The structure and functions of coumarins
2.1 Structural composition of coumarins
Coumarin constitutes an important class of phenolic compounds and a mid-size family of secondary metabolites in plants. Common examples include scopoletin (7-hydroxy-6-methoxy-coumarin), umbelliferone (7-hydroxy-coumarin), and esculetin (6,7-dihydroxy-coumarin), etc (Leal et al., 2024). Structurally, coumarins consist of a benzene ring that is fused with an α-pyrone ring, forming a benzopyrone structure. Based on their structural characteristics, coumarins are classified into simple and composite coumarins (Sierra et al., 2021; Rohman et al., 2024). Simple coumarins are characterized by a 1,2-benzopyrone core, substituted on the benzene ring with hydroxyl, methoxy, methylenedioxy, or isopentenyl groups, and lacking fused heterocyclic systems (e.g., furan or pyran rings) formed via linkages between the C-7 hydroxyl group and C-6/C-8 positions. For instance, esculetin, which exists in C. fraxini, and the scoparone isolated from A. capillaris (Zhu and Jiang, 2018; Annunziata et al., 2020). The specific arrangements of tetrahydropyran (THP) and tetrahydrofuran (THF) rings with lactone structures give rise to at least four types of composite coumarins: linear furan coumarins, angular furan coumarins, linear pyranocoumarins, and angular pyranocoumarins. Through dehydration condensation between the C-7 hydroxyl group on the benzene ring and adjacent C-6 or C-8 positions, simple coumarins are converted into furanocoumarins, forming linear (e.g., psoralen) or angular (e.g., isopsoralen) subtypes fused with a furan ring. Pyranocoumarins are formed through cyclization between a hydroxyl or methoxy group at positions such as C-5 or C-6 and adjacent carbons (e.g., C-4 or C-7) on the benzene ring, generating a fused pyran ring, and are categorized into linear (e.g., xanthyletin) or angular (e.g., seselin) subtypes based on the spatial orientation of the pyran ring (Zhu and Jiang, 2018; Annunziata et al., 2020) (Figure 1).
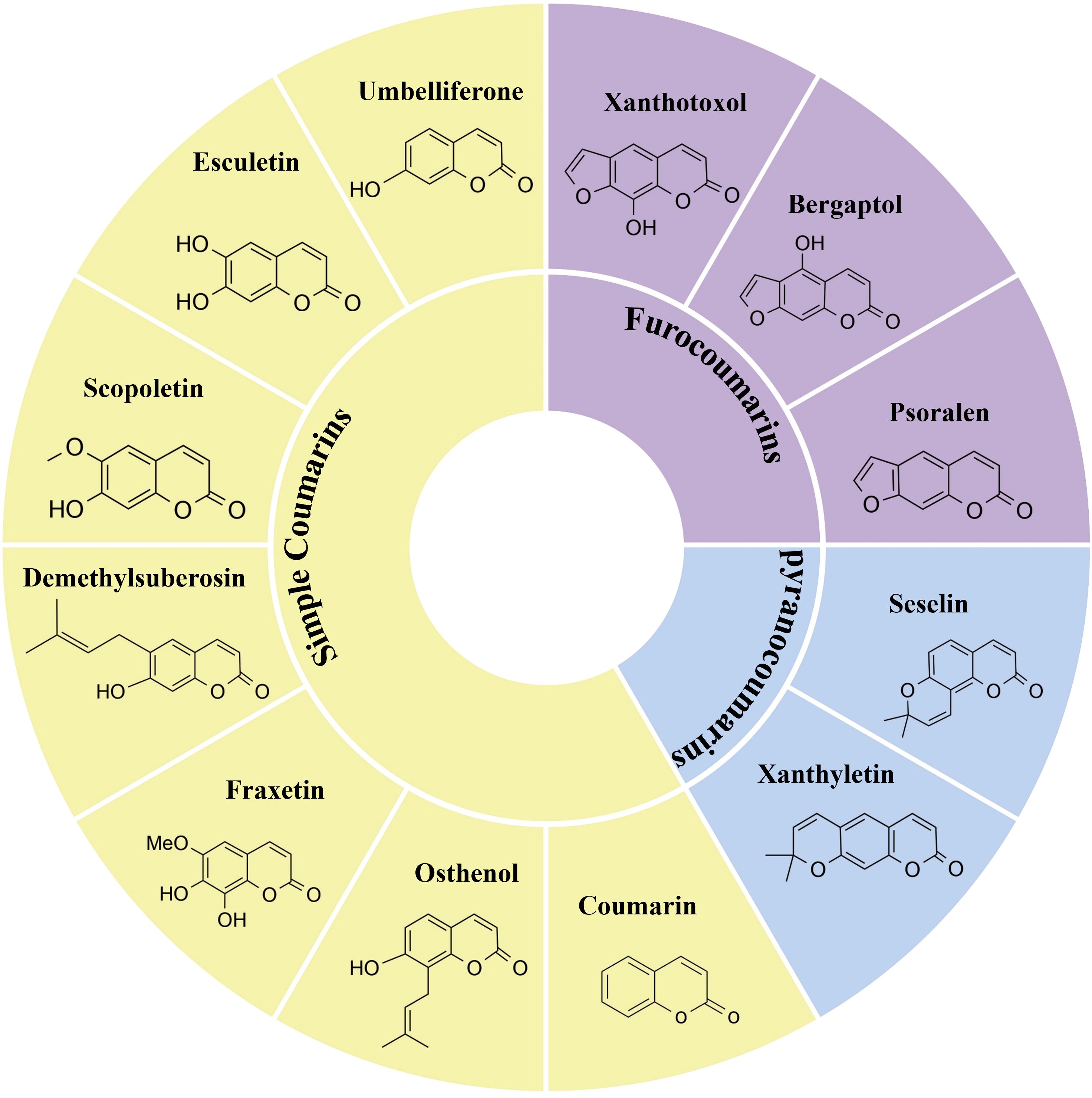
Figure 1. Examples of simple and composite coumarins. Coumarin, osthenol, fraxetin, demethylsuberosin, scopoletin, esculetin, umbelliferone are Simple coumarins. Psoralen, bergaptol and xanthotoxol are furocoumarins. Seselin and xanthyletin are pyranocoumarins.
2.2 Functions of coumarins
The structural diversity of coumarins endows them with a wide range of biological activities, thus making them important lead compounds in drug discovery and development (Nan et al., 2025). As a kind of natural product of plants, coumarin and its derivatives exhibit diverse therapeutic applications such as anti-human immunodeficiency virus (HIV) therapy, antitumor, antioxidant, antimicrobial, anti-inflammatory analgesic, anti-coagulant activities (Zhu and Jiang, 2018; Sierra et al., 2021; Zeng et al., 2024). In the 1990s, the tetracyclic pyranocoumarin compounds calanolide A and calanolide B, isolated from Calophyllum lanigerum, were first discovered to exhibit potent inhibitory activity against HIV replication, becoming the first anti-HIV plant extract to enter clinical trials (Kashman et al., 1992). Subsequently, a large number of coumarin structurally modified derivatives with increased anti-HIV activity gradually emerged, such as psoralen, bergapten, imperatorin, suksdorfin, byakangelicin, and 3-phenylcoumarins. The widely used warfarin (4-hydroxy-3-(3-oxo-1-phenylbutyl) coumarin, acenocoumarol, and phenprocoumon exert anticoagulant properties by inhibiting the vitamin K epoxide reductase complex (Zhu and Jiang, 2018; Hussain et al., 2019). New coumarin-thiazolidinone and/or thiazole conjugates can treat tuberculosis (TB) by inhibiting the InhA enzyme (Ebaid et al., 2025). Osthole can trigger cAMP/PKA-dependent relaxation of airways to induce bronchodilation without desensitizing receptors or increasing the risk of death (Wang et al., 2020).
Coumarin not only in terms of human health, but also shows great application prospects in green agricultural production, including regulating plant growth, serving as phytoalexin, insecticide, and bacteriostatic agent. For example, furanocoumarins (FCs), a subgroup of coumarins, are considered as natural insecticides and fungicides because they can prevent the invasion of plant-pathogenic microorganisms and the predation of herbivorous insects (Shao et al., 2022). Bituminaria bituminosa (L.) accumulates high concentrations of furanocoumarins, such as angelicin and psoralen, which help the plant resist infection and herbivore attack (Walker et al., 2012). Coumarins can delay seed germination by reducing endogenous GA4 levels, thereby decreasing the accumulation of Reactive Oxygen Species (ROS) (Chen et al., 2021). Most 7-vinylcoumarin derivatives exhibit good inhibitory activity against Colletotrichum gloeosporioides (Zhang et al., 2025). Some of coumarin derivatives containing the 1,3,4-oxadiazole/thiadiazole moiety exhibited good in vivo antiviral efficacy against tobacco mosaic virus (TMV) (Hu et al., 2025). Phakopsora pachyrhizi (Pp) can cause Asian soybean rust (SBR) disease. Scopoletin has action of antibacterial activity against Pp, which is related to the reduction of the accumulation of reactive oxygen species (ROS) in the fungal pre-infection structure (Beyer et al., 2019).
Due to its sweet aroma, coumarin has been extensively utilized in perfumery since its discovery in the 19th century. In violet-type scents, coumarin imparts the quintessential “hay-like” and “sweet” olfactory profile, thereby becoming a defining component in modern perfumery. As a fragrance enhancer, coumarin can be used in products such as soaps, detergents, and cosmetics to mask unpleasant odors and endow them with a soft and sustained fragrance (Camillo et al., 2023).
3 Coumarin synthesis pathway and its transcriptional regulation
3.1 General pathway
The phenylpropanoid pathway in plants is one of the most important and highly conserved biosynthetic pathways of secondary metabolites. It starts with phenylalanine, which is synthesized via the glycolysis and the shikimate pathway. In a three-step process catalyzed by phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumarate-CoA ligase (4CL), cinnamic acid, p-coumaric acid, and p-coumarate-CoA are produced sequentially (Thomas, 2010) (Figure 2). These metabolites serve as common precursors for the downstream metabolic processes, generating a diverse array of secondary metabolites, including lignin, flavonoids, coumarins, phenolics, and organic acids, forming a complex and multi-branched phenylpropanoid biosynthetic pathway. Among these, the lignin and flavonoid synthesis pathways are the most prominent ones, whereas the coumarin biosynthetic pathway is another essential branch of the phenylpropanoid pathway (Dong and Lin, 2021). The transcriptional activation of the upstream phenylpropanoid pathway often triggers a metabolic reprogramming cascade in the downstream networks (Chen et al., 2023). For instance, overexpression of the C4H gene exhibited corresponding increases in C4H enzymatic activity, which enhanced the biosynthesis of pyranocoumarin decursinol angelate in Angelica gigas hairy roots (Park et al., 2012).
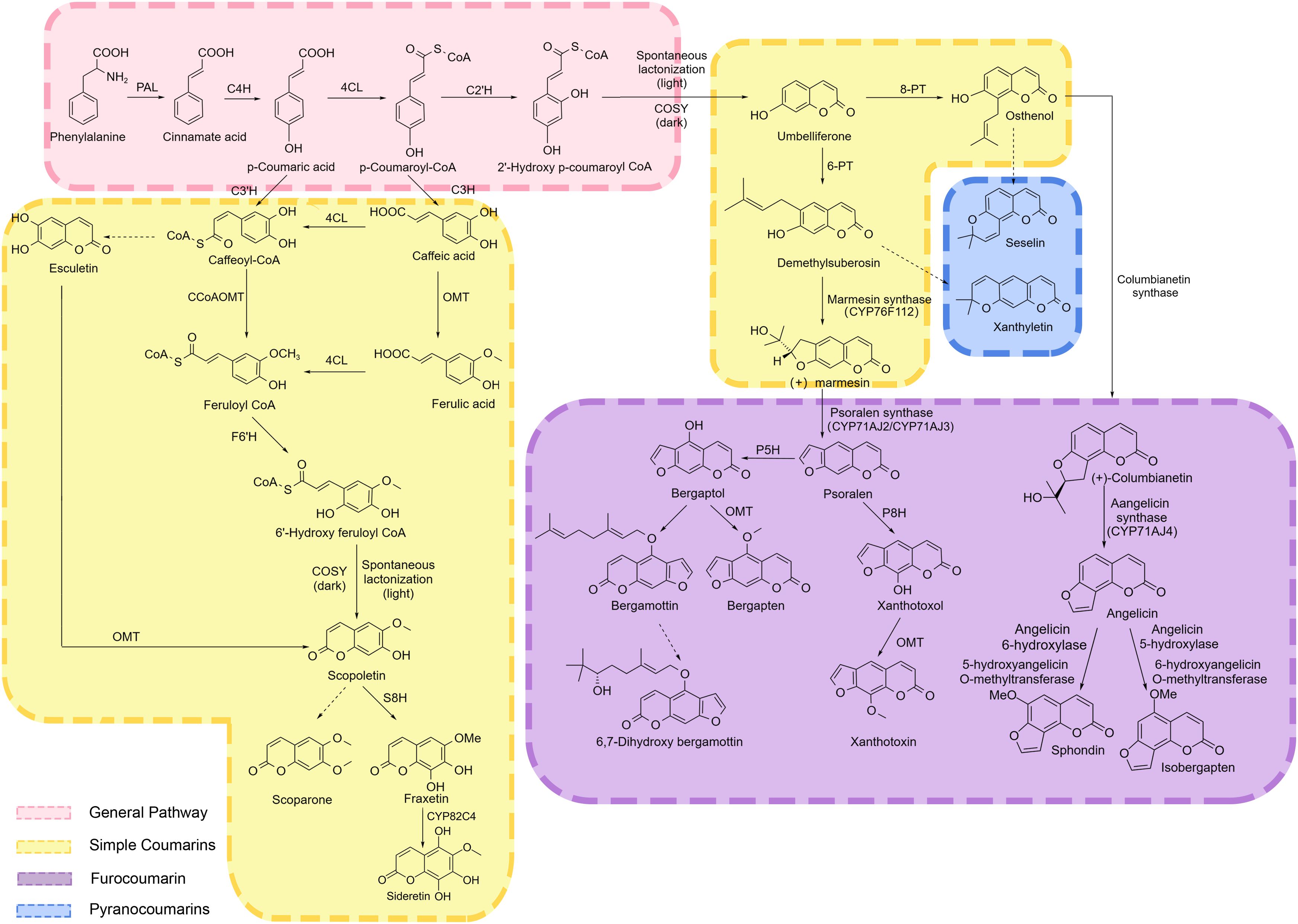
Figure 2. Coumarin synthesis pathway. PAL, phenylalanine ammonia-lyase; C4H, cinnamic acid 4-hydroxylase; 4CL, 4-coumarate-CoA ligase; C2’H, Cinnamate-2-hydroxylase; C3’H, Coumarate-3-hydroxylase; CCoAOMT, Caffeoyl-CoA-O-methyltransferase; F6’H, Feruloyl-CoA 6‘-hydroxylase; COSY, Coumarin synthase; S8H, Scopoletin 8-hydroxylase; PT, prenyltransferase; P5H, psoralen 5-hydroxylase; P8H, psoralen 8-hydroxylase; OMT, O-Methyltransferases. Black arrows indicate single direct reaction, dashed black arrows represent unauthenticated steps. General pathway metabolites are indicated by pink background. Simple Coumarins biosynthesis pathway metabolites are indicated by yellow background. Furococoumarin biosynthesis pathway metabolites are indicated by purple background. Pyranocoumarins biosynthesis pathway metabolites are indicated by blue background.
3.2 Coumarin branching pathway
3.2.1 Simple coumarin synthesis
The ortho-hydroxylation of cinnamic acid derivatives, leading to the formation of the coumarin lactone ring, is recognized as a critical step in coumarin biosynthesis. Ruolan et al. isolated a putative ortho-hydroxylase, p-coumaroyl CoA 2’-hydroxylase (C2’H), from P. praeruptorum Dunn, which exhibited the highest transcriptional activity in roots. Prokaryotic expression assays confirmed that PpC2’H catalyzes the conversion of p-coumaroyl-CoA to a hydroxylated intermediate, followed by spontaneous lactonization under light conditions or COSY-catalyzed lactonization in darkness to generate umbelliferone (Ruolan et al., 2017). In Arabidopsis thaliana, the biosynthesis of umbelliferone proceeds through the following sequential steps. CCoAOMT1, exhibiting 3’-O-methyltransferase activity, catalyzes the conversion of caffeoyl-CoA to feruloyl-CoA. The T-DNA insertion mutants of CCoAOMT1 displayed a significant reduction in scopoletin and scopolin levels in the roots. Feruloyl-CoA 6’-hydroxylase 1 (F6’H1) subsequently hydroxylates feruloyl-CoA to generate 6’-hydroxyferuloyl-CoA, which is partially spontaneously converted to scopoletin. This spontaneous process is significantly enhanced by the enzymatic activity of coumarin synthase (COSY), thereby optimizing scopoletin biosynthesis efficiency (Kai et al., 2008). Functional analysis of T-DNA mutants identified CYP98A3 as the key enzyme catalyzing the 3’-hydroxylation of p-coumarate in A. thaliana. A 97% reduction in scopoletin and scopolin content was observed in the mutant roots, suggesting the critical role of CYP98A3 in scopoletin and its derivatives biosynthesis (Kai et al., 2006). COSY catalyzes the biosynthesis of umbelliferone, esculetin, and scopoletin from their respective ortho-hydroxycinnamoyl-CoA thioester precursors through two sequential reaction steps: trans-cis isomerization followed by lactonization (Vanholme et al., 2019). C2’H and F6’H are two types of 2-oxoglutarate-dependent (2-OGD) dioxygenase family. Goldenberg et al. demonstrated that single dual specificity C2’H/F6’H directs FCs production in citrus leaves and fruit. F6’H only catalyzes the synthesis of scopoletin products, while C2’H catalyzes the synthesis of umbelliferone products (Goldenberg et al., 2025). Scopoletin 8-hydroxylase (S8H), a 2-oxoglutarate-dependent dioxygenases (2-ODDs), catalyzes the hydroxylation of scopoletin at the C-8 position, mediating its conversion to fraxetin. Fraxetin is further oxidized by the CYP enzyme (CYP82C4) to produce sideretin (Rajniak et al., 2018; Siwinska et al., 2018).
Glycosylation, a key modification in coumarin biosynthesis, is mediated by UDP-glycosyltransferases (UGTs) in the cytoplasm, thereby enhancing the solubility, stability, and bioactivity of coumarins. Among the 189 MaUGT genes identified in the Melilotus albus genome, 16 exhibited differential expression between the low-coumarin-content genotype (Ma44) and high-coumarin-content genotype (Ma49), suggesting their potential involvement in coumarin biosynthesis. Further heterologous expression assays confirmed that MaUGT186 is responsible for the glycosylation of scopoletin (Duan et al., 2021). The gene cluster composed of six β-glucosidase (BGLU) genes contributes to the evolution of the coumarin biosynthesis pathway in Melilotus albus. MaBGLU1 was confirmed to be involved in scopoletin (coumarin derivative) synthesis (Wu et al., 2022). The tobacco glucosyltransferase TOGT has been identified as a highly active enzyme capable of catalyzing the glucosylation of p-hydroxycoumarin and hydroxycinnamic acids. TOGT-depleted tobacco plants exhibited diminished UGT activity toward scopoletin, and a significant reduction in the accumulation of glucoside form of scopoletin (scopolin). Taguchi et al. cloned the cDNA encoding GTase (NtGT2) in Nicotiana tabacum L. and found that the recombinant enzyme (rNTGT2) expressed in Escherichia coli exhibited glucosylation activity toward 3-hydroxycoumarin (Fraissinet-Tachet et al., 1998; Chong et al., 2002; Taguchi et al., 2003). The glycosyltransferase AdCGT isolated from Angelica sinensis uses 5,7-dihydroxycoumarin as a substrate to produce a C-glycosylated product at C-8 position (Wang et al., 2022).
3.2.2 Synthesis of composite coumarins
Umbelliferone serves as a precursor for the biosynthesis of other coumarins and as an entry point for furanocoumarins biosynthesis. Downstream of umbelliferone, multiple prenyltransferases (PTs) and cytochrome P450 family members are predicted to participate in the biosynthesis of furanocoumarins (Goldenberg et al., 2025). PTs are the main determinants of the structural diversity of composite coumarins (CCs). Umbelliferone 6/8-isopentenyltransferase promotes the substitution of umbelliferone by an isopentenyl group at the 6 or 8 position to form demethylsuberosin (DMS) or osthenol, respectively. This step is the starting point for the synthesis of composite coumarins and determines whether angular or linear coumarins are formed (Huang et al., 2024; Li et al., 2024). In Pastinaca sativa, PsPT1 and PsPT2 use umbelliferone and dimethylallylpyrophosphate (DMAPP) as substrates to target plastids and synthesize osthenol and demethylsuberosin (DMS) (Munakata et al., 2016). In Petroselinum crispum, PcPT exhibits strict substrate specificity toward umbelliferone and dimethylallyl diphosphate, shows a preference for acting on the C-6 position of the prenylated product (demethylsuberosin) to generate linear furanocoumarins. Only a small amount of the C8-prenylated derivative (osthenol) is produced (Karamat et al., 2014) (Figure 2).
Cytochrome P450s play key roles in the biosynthesis and hydroxylation of furanocoumarins, allowing the utilization of psoralen as a new substrate for the synthesis of bergamot alcohol. CYP76F112 acts as marmesin synthase (MS) that converts DMS to (+)-marmesin. Psoralen synthase (PS) converts (+)-marmesin to psoralen (Romain et al., 2007; Villard et al., 2021; Rodrigues et al., 2022). Psoralen is hydroxylated at the 5- and 8-positions by psoralen 5-hydroxylase (P5H) and psoralen 8-hydroxylase (P8H) to produce bergaptol and xanthotoxol. These products are then further converted to bergapten lactone and xanthotoxin by the corresponding O-methyltransferases (Figure 2) (Zhao et al., 2016, 2019; Zhang et al., 2022; Bouillé et al., 2025). CYP71AJ2 and CYP71AJ3 exhibited psoralen synthase activity and were responsible for converting marmesin into psoralen. They promote the synthesis of linear furanocoumarins by catalyzing this reaction. CYP71AJ4 has angelicin synthase activity and can catalyze the conversion of columbianetin into angelicin. Angelicin is converted into sphondin under the action of angelicin 6-hydroxylase and 5-hydroxyangelicin O-methyltransferase. It is transformed into isobergapten under the effect of angelicin 5-hydroxylase and 6-hydroxyangelicin O-methyltransferase. CYP71AJ4 enables the smooth synthesis of angular furanocoumarins (Larbat et al., 2009; Roselli et al., 2017).
Seselin and xanthyletin are two pyranocoumarins whose biosynthetic precursors are osthenol and demethylsuberosin, respectively, although their exact biosynthetic pathways have not yet been experimentally validated (Goldenberg et al., 2025).
3.3 Transcriptional regulation in the coumarin synthesis pathway
The co-expression network and cis-element analysis of the promoters revealed that differentially expressed MYB, bHLH, AP2, and WRKY transcription factors may play crucial roles in regulating the expression of coumarin biosynthetic genes (Chen et al., 2023). The MYB superfamily plays a critical role in plant growth, development, defense against environmental stress, and the biosynthesis of secondary metabolites. In Peucedanum praeruptorum Dunn, the expression patterns of R2R3-MYB transcriptional factors PpMYB3 and PpMYB103 in roots exhibited a strong spatiotemporal correlation with coumarins (including praeruptorin A, praeruptorin B, praeruptorin E, scopoletin, and isoscopoletin) accumulation profiles, suggesting their potential roles as positive transcriptional regulators of coumarins biosynthesis (Liao et al., 2024). Furanocoumarins serve as the primary bioactive constituents in Angelica dahurica var. formosana (ADF), which belongs to the umbelliferae family. These compounds are predominantly localized within the oil tubes of the root phloem of ADF. Functional studies revealed that overexpression of the AdNAC20 transcription factor in ADF resulted in a 9.28% reduction in total coumarin content, accompanied by a significant 12.28% increase in lignin accumulation. Conversely, AdNAC20-knockout mutants exhibited a 16.3% elevation in total coumarins and a substantial 33.48% decrease in lignin levels. Confirming the dual-pathway regulatory effect of AdNAC20, that is, positively enhancing lignin formation but negatively controlling coumarin formation (Qu et al., 2024). The MYB72 transcription factor has been found to be involved in regulating the biosynthesis of scopoletin (including β-glucosidase BGLU42), whereas BGLU42 mediates scopoletin secretion into the rhizosphere by modifying its precursor (Lundberg and Teixeira, 2018). In Gossypium hirsutum(cotton), GhF6’H1, which is regulated by GhWRKY33, regulates the accumulation of scopoletin (Gao et al., 2024). Moreover, in Nicotiana tabacum, the ERF transcription factor WAX INDUCER1 (NtWIN1) inhibits the accumulation of scopoletin through the activity of NtF6’H1 (He et al., 2024).
4 Molecular regulations of coumarin metabolic pathways in response to abiotic stress
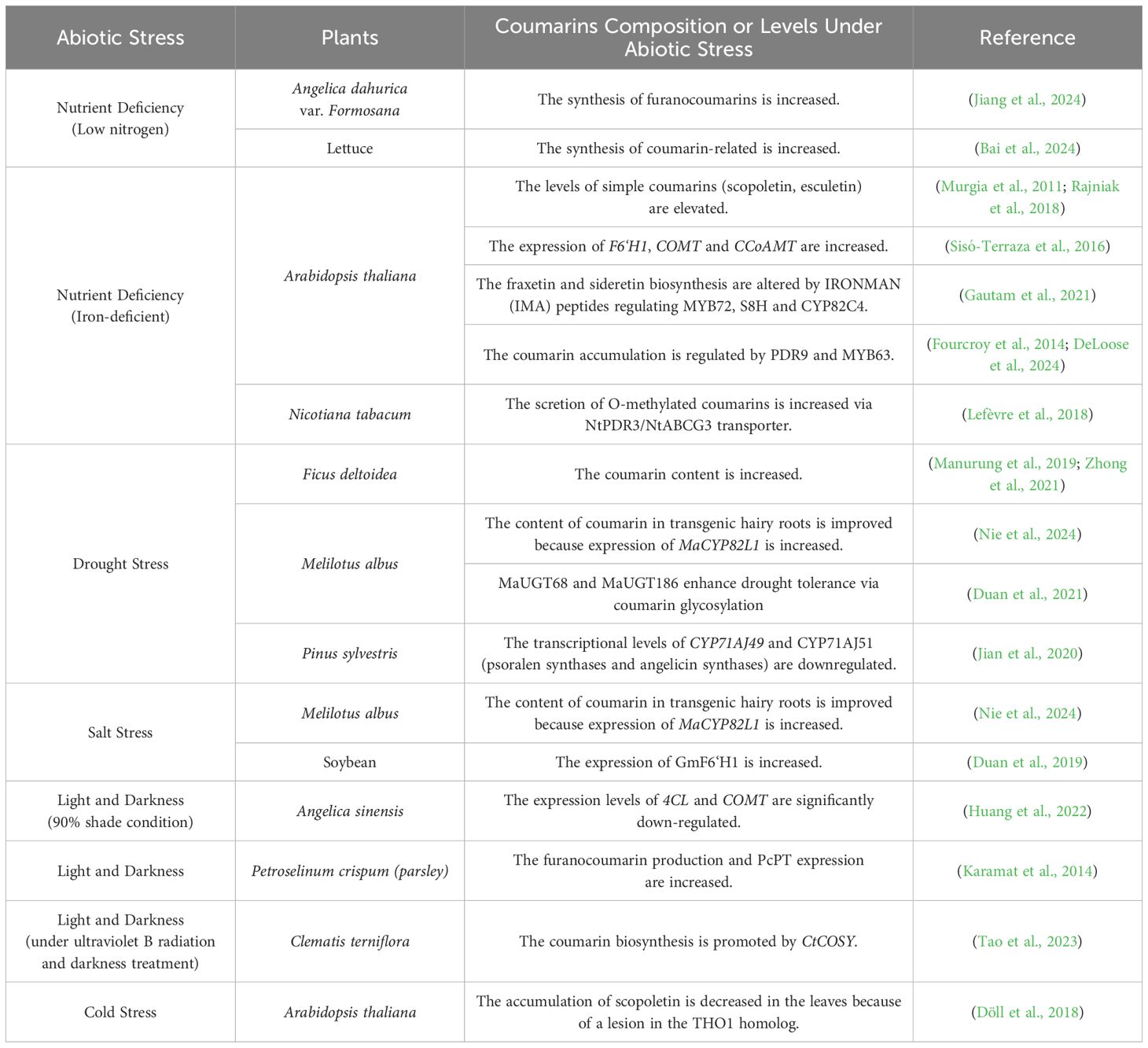
4.1 Molecular regulation in response to nutrient deficiency
The interaction of soil microorganisms with coumarins participates in plant nutrient metabolism, which represents a crucial step in enhancing plant resistance to nutrient deficiencies. Low nitrogen promotes the secretion of carbon-containing compounds into the rhizosphere, substantially altering the composition of the rhizosphere bacterial community and stimulating the growth of Angelica dahurica and the synthesis of furanocoumarins (Jiang et al., 2024). Under nutrient-deprived conditions, inoculation with the plant growth-promoting rhizosphere bacteria (PGPR) strain Bacillus velezensis SAAS-63 reshaped rhizosphere metabolism by reprogramming phenylpropanoid pathway flux. The inoculant suppressed flavone and isoflavone biosynthesis while redirecting phenylalanine toward the synthesis of lignin precursors and coumarin-derived metabolites, which collectively alleviated nutrient deficiencies and enhanced plant resistance in lettuce (Bai et al., 2024).
Under iron-deficiency conditions, the expressions of S8H and CYP82C4 were upregulated, accompanied by a large accumulation of coumarins in root secretions, including scopoletin, rehmanin, and astragalus in A. thaliana (Murgia et al., 2011; Rajniak et al., 2018). During A. thaliana growth and development, the secretion of esculetin, scopoletin, isoephedrine, and methoxycoumarins can be selectively enhanced by buffered nutrient solutions at pH 5.5 or 7.5. At the same time, the expression of F6’H1 increased 4-fold and 8-fold at pH 5.5 and pH 7.5, respectively. The expression of COMT and CCoAMT genes in roots increased only at pH 7.5 under iron-deficient conditions (Sisó-Terraza et al., 2016). The peptides IRONMAN (IMA) work in concert with the environmental pH. By regulating the expression of key genes such as MYB72, S8H and CYP82C4, it alters the biosynthesis and secretion of coumarins (such as fraxetin and sideretin), optimizes the iron absorption process, and helps plants adapt to the iron nutritional status in different acidic and alkaline environments (Gautam et al., 2021). Recently, the ABC transporter G family member PDR9 has been reported to affect the coumarin accumulation and homeostasis under combined Fe and P deficiency. MYB63 transcription factor controls dedicated coumarin production by regulating both COSY and F6’H1 expression while orchestrating secretion through PDR9 genes under Fe and P combined deficiency (Fourcroy et al., 2014; DeLoose et al., 2024). Nicotiana tabacum secretes coumarins in response to iron deficiency. NtPDR3/NtABCG3, which is a plasma-membrane ABC transporter belonging to the pleiotropic drug resistance (PDR) family in Nicotiana tabacum, plays a crucial role in mediating secretion of O-methylated coumarins to the rhizosphere (Lefèvre et al., 2018) (Table 1).
4.2 Molecular regulation in response to drought stress
Drought stress has been demonstrated to induce marked accumulation of coumarins in plants. Ficus deltoidea leaf extracts exhibit a 2.3-fold increase in total coumarin content under water deficit conditions (Manurung et al., 2019; Zhong et al., 2021). In Melilotus albus, MaCYP82L1 is an important regulator of drought and salt resistance. Yeast cells transformed with MaCYP82L1 exhibited significant resistance to drought and salt stress. Moreover, overexpression of MaCYP82L1 led to an increase in the content of coumarin in transgenic hairy roots, laying a foundation for exploring the connection between coumarin metabolism and abiotic stresses (Nie et al., 2024). Two CYP71AJ enzymes (psoralen synthase and angelicin synthase) are involved in the biosynthesis of furanocoumarins in Pinus sylvestris. Under drought conditions, the transcriptional levels of both CYP71AJ49 and CYP71AJ51 are significantly downregulated (Jian et al., 2020). UDP-glycosyltransferases (UGTs) are responsible for the glycosylation modification of coumarin (Nie et al., 2024). UGT expression is significantly upregulated under drought stress, increasing coumarin biosynthesis and promoting adaptation to abiotic stress. MaUGT68 and MaUGT186 in the genome of Melilotus albus have effects on the growth and stress (drought and salt) resistance of yeast cells. The transformed yeast cells carrying pYES2-MaUGT68 and pYES2-MaUGT186 constructs showed remarkable resistance to 30% PEG-6000 treatment, especially at a 105-fold dilution, indicating that MaUGT68 and MaUGT186 enhance drought tolerance in yeast (Duan et al., 2021) (Table 1).
4.3 Molecular regulation in response to salt stress
Salt stress plays an important role in the regulation of coumarin biosynthesis. In Melilotus albus, expression of MaCYP82L1 was also increased in response to salt treatment, and its heterologous expression in yeast led to significantly increased resistance to drought and salt stresses (Nie et al., 2024). Salt stress significantly induced the expression of GmF6’H1 in soybean. When GmF6’H1 was constitutively expressed in A. thaliana under the control of the 35S promoter, a significantly increased salt tolerance was observed (Duan et al., 2019) (Table 1).
4.4 Molecular regulation in response to light and darkness
The influence of light on the biosynthetic accumulation of coumarins might be mediated through the modulation of primary metabolic processes associated with photosynthesis, particularly carbon and nitrogen metabolism, which serve as fundamental sources of carbon skeletons and nitrogen precursors for coumarins synthesis (Ruan et al., 2010; Wu et al., 2023). By analyzing the differentially expressed genes (DEGs) related to coumarin biosynthesis in Angelica sinensis under 50%, 70%, and 90% shade conditions, researchers found that the expression levels of 4CL and COMT were significantly down-regulated at 90% shade conditions (Huang et al., 2022). Ultraviolet (UV) irradiation will increase the expression of PcPT in various tissues of parsley (Petroselinum crispum), and at the same time, the production of furanocoumarins also increases (Karamat et al., 2014). The content of coumarins in Clematis terniflora leaves increased under ultraviolet B radiation and darkness treatment (UV-D). Under UV-D stress, CtCOSY influenced the biosynthesis of phenylpropanoid compounds in C. terniflora, promoting coumarin biosynthesis and affecting purine metabolism (Tao et al., 2023) (Table 1).
4.5 Molecular regulation in response to cold stress
Scopoletin accumulation was also detected in cells subjected to cold stress. The THO/TREX complex is a critical multi-protein complex involved in coupling transcription, mRNA processing, and nuclear export. In Arabidopsis thaliana, the Tho1 mutant resulted from a lesion in the THO1 homolog encoding a THO/TREX complex subunit. This mutation led to a cold-induced decrease in the accumulation of scopoletin in the leaves. Mutations in genes encoding putative Arabidopsis THO/TREX complex subunits, aside from THO1, partially reduced the scopoletin content in roots under osmotic/high-carbon stress and in leaves under cold stress. Mutations in AGO1, RDR6, and SGS3, which are involved in the RNA silencing pathway, also affected the cold-induced accumulation of scopoletin in leaves (Döll et al., 2018) (Table 1).
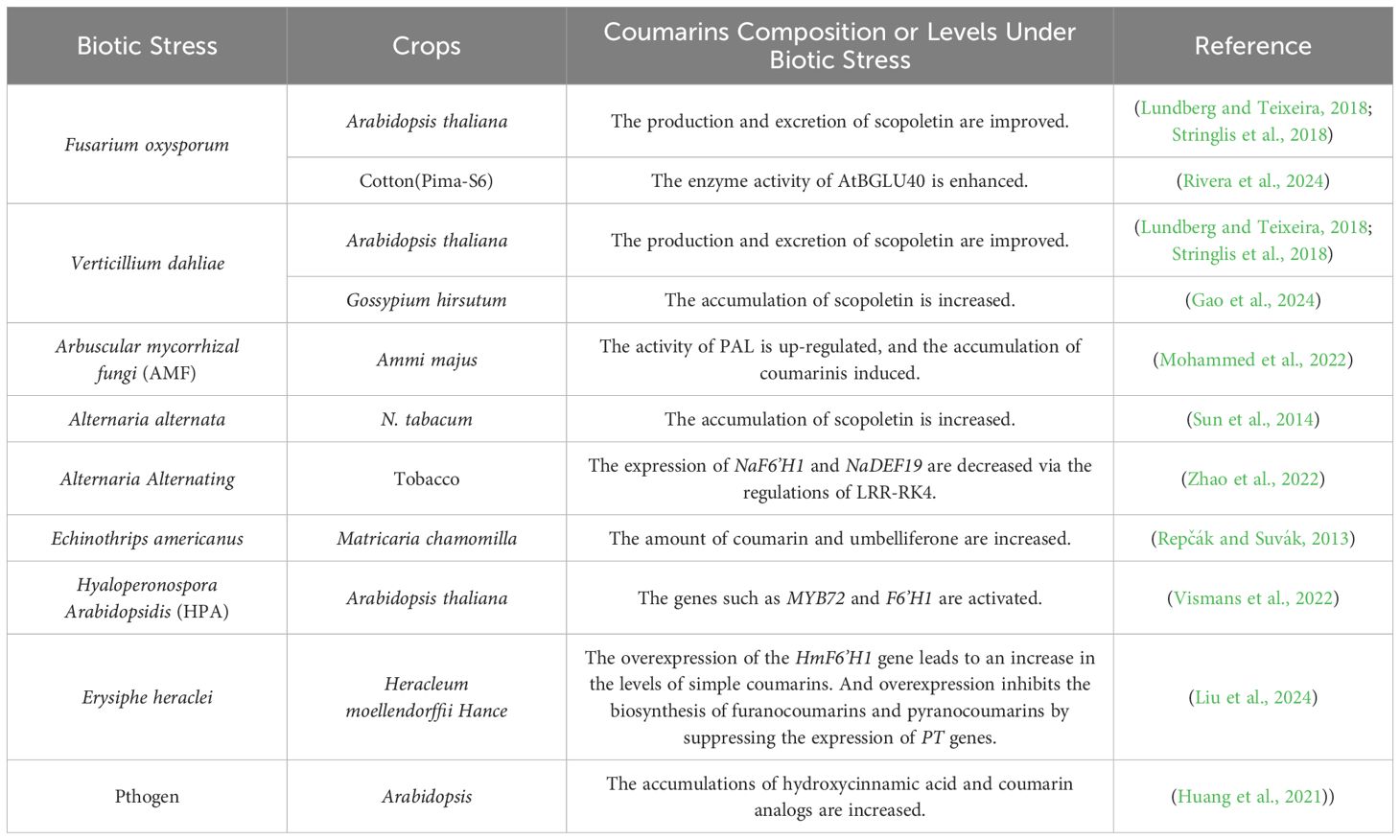
5 Molecular regulations of coumarin metabolic pathways in response to biotic stresses
Coumarins and their derivatives play a crucial role in plant responses to biotic stress. They are involved in plant defense reactions against various pathogens, and their synthesis and accumulation are finely regulated by multiple factors. Numerous studies have shown that when plants face different biotic stresses, coumarin-related metabolic pathways undergo significant changes, thereby affecting plant disease resistance and growth and development (Dixon, 2001; Wu et al., 2023).
Scopoletin specifically inhibits soil-borne fungal pathogens such as Fusarium oxysporum and Verticillium dahliae. The plant and beneficial rhizobacteria work together to trigger MYB72/BGLU4-dependent scopoletin production and excretion, which in turn selectively inhibits these soilborne fungal pathogens (Stringlis et al., 2018). As a transcription factor, MYB72 may be a key regulator of the genes involved in scopoletin biosynthesis (including BGLU42). BGLU42 modifies the precursors of scopoletin and mediates its secretion into the rhizosphere (Lundberg and Teixeira, 2018). In Gossypium hirsutum(cotton), GhF6’H1, regulated by GhWRKY33-like factors, plays a crucial role in enhancing cotton resistance to Verticillium dahliae by regulating scopoletin accumulation (Gao et al., 2024). AtBGLU40 belongs to a family of β-glucosidases with coumarin-hydrolyzing activity, and its enzyme activity is enhanced by Fusarium oxysporum infection (Rivera et al., 2024). The combined action of arbuscular mycorrhizal fungi (AMF) and elevated CO2 (eCO2) led to the accumulation of sucrose in the stems and roots of Ammi majus. This up-regulated the activity of PAL, induced the accumulation of coumarin, and enhanced the medicinal and pharmacological value of Ammi majus (Mohammed et al., 2022). After infection with Alternaria alternata, the jasmonate (JA) signaling pathway was activated in the leaves of N. attenuata. Subsequently, part of the regulation of scopoletin biosynthesis occurred through MYC2 to defend against A. alternata. The higher levels of scopoletin accumulated in young leaves contributed to their stronger resistance (Sun et al., 2014). LRR-RK4 was identified as the first leucine-rich repeat receptor-like kinase involved in the resistance to Alternaria in tobacco species, and it regulates NaERF109 and NaDEF19. In virus-induced gene silencing (VIGS) plants with silenced NaERF109, the expression of A. alternata-induced NaF6’H1 and NaDEF19 was significantly reduced, which enhanced the susceptibility of NaLRR-RK4-RNAi lines to A. alternata (Zhao et al., 2022). After Hyaloperonospora arabidopsidis (Hpa) infection, Arabidopsis thaliana activates coumarin biosynthesis-related genes such as MYB72 and F6’H1, inducing the generation of soil-borne legacy (SBL). Subsequently, this signal is transmitted through the salicylic acid (SA) pathway, enabling the plant to develop resistance against subsequent foliar downy mildew infections (Vismans et al., 2022). After being infected by Erysiphe heraclei, the overexpression of the HmF6’H1 gene leads to an increase in the levels of simple coumarins. This overexpression inhibits the biosynthesis of furanocoumarins and pyranocoumarins by suppressing the expression of PT genes, thereby enhancing the resistance of Heracleum moellendorffii Hance to powdery mildew (Liu et al., 2024). The attack of thrips and Echinothrips americanus not only led to an increase in the content of GMCAs and herniarin, but also increased the amount of coumarin and umbelliferone (Repčák and Suvák, 2013). During pathogen invasion, the glycosyltransferase UGT73C7 catalyzes the formation of glucosinolates from coumaric acid and ferulic acid. This redirects the phenylpropane metabolism, leading to the accumulation of hydroxycinnamic acid and coumarin analogs, which stimulate the expression of the disease-resistance gene snc1 and regulate plant immunity (Huang et al., 2021) (Table 2).
6 Conclusions
Several genes, including PAL, COMT, CCoAOMT, 4CL, C3H, CSE, and C4H, are involved in coumarin biosynthesis, and they exhibit tissue-specific expression patterns. Differentially expressed MYB, bHLH, AP2, and WRKY transcription factors have been found to play critical roles in regulating the expression of coumarin biosynthetic genes. However, compared to MYB transcription factors, there are relatively few studies on other transcription factors in the coumarin synthesis pathway. Moreover, compared to coumarins, the regulation of these transcription factors in the synthesis pathways of other phenylpropanoids (such as flavonoids and lignans) has been more extensively studied. Since these pathways belong to the same phenylpropanoid metabolism network as coumarin synthesis, they can provide valuable clues for studying the transcriptional regulation of the coumarin synthesis pathway.
Recent studies have shown that nutrient deficiency, drought, salinity, and light-dark changes can influence the expression of coumarin biosynthetic genes and induce coumarin accumulation. Plants rely on the antioxidant capacity of coumarin to help them cope with stress and adapt to the environment. Certain pathogenic microorganisms and insect infestations also induce coumarin accumulation, which is utilized by plants to defend against pests and diseases. Although numerous genes and genetic regulatory mechanisms related to coumarin synthesis and metabolism have been reported, most of these studies are applied in biomedical research. There are very few investigations into the genes that are practically relevant in breeding and agricultural production.
Coumarins often interact with other phenylpropanoids in response to biotic and abiotic stresses in plants, and the molecular regulation of these substances is connected. Therefore, the relationship between coumarin and other phenylpropanoid interactions and plant responses to biotic and abiotic stresses warrants further investigation. As the related enzyme genes in the coumarin anabolic pathway also affect the biosynthesis of other phenylpropanoids, genetic engineering may be used to conduct multi-gene combination regulation in the future. This approach aims to enhance plant stress tolerance and achieve practical applications in production.
Author contributions
YW: Writing – original draft. TG: Writing – original draft. XY: Writing – original draft. JY: Writing – original draft. XZ: Writing – original draft. AC: Writing – original draft. CY: Writing – review & editing. ZF: Writing – review & editing. KL: Writing – review & editing. YL: Writing – review & editing.
Funding
This work was supported by the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (ASTIP-TRIC01), the Key Science and Technology Program of China National Tobacco Corporation, Heilongjiang Tobacco Industry Co., Ltd. and Hunan Tobacco Corporation (110202101007(JY-07), 202300000034002 and HM2024KJ01). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
Authors TG, XY, and KL were employed by Heilongjiang Tobacco Industry Co., Ltd. Author JY was employed by Hunan Tobacco Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JS declared a shared affiliation with the authors YW, YS, XZ, AC, CY, ZF, and YL to the handling editor at time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Annunziata, F., Pinna, C., Dallavalle, S., Tamborini, L., and Pinto, A. (2020). An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 21, 4618. doi: 10.3390/ijms21134618
Bai, Y., Song, K., Gao, M., Ma, J., Zhou, Y., Liu, H., et al. (2024). Using multi-omics to explore the effect of Bacillus velezensis SAAS-63 on resisting nutrient stress in lettuce. Appl. Microbiol. Biotechnol. 108, 313–313. doi: 10.1007/S00253-024-13153-Y
Barot, K. P., Jain, S. V., Kremer, L., Singh, S., and Ghate, M. D. (2015). Recent advances and therapeutic journey of coumarins: current status and perspectives. Medicinal Chem. Res. 24, 2771–2798. doi: 10.1007/s00044-015-1350-8
Beyer, S. F., Beesley, A., Rohmann, P. F. W., Schultheiss, H., Conrath, U., and Langenbach, C. J. G. (2019). The Arabidopsis non-host defence-associated coumarin scopoletin protects soybean from Asian soybean rust. Plant J. 99, 397–413. doi: 10.1111/tpj.14426
Bouillé, A., Larbat, R., Kumari, R., Olry, A., Charles, C., Nelson, D. R., et al. (2025). Lineage-specific patterns in the Moraceae family allow identification of convergent P450 enzymes involved in furanocoumarin biosynthesis. New Phytol. 245, 2085–2102. doi: 10.1111/NPH.20381
Camillo, M. R. T., Russo, M., Trozzi, A., Mondello, L., and Dugo, P. (2023). Quantification of coumarins, furocoumarins and polymethoxyflavones in hydroalcoholic fragrances by supercritical fluid chromatography-tandem mass spectrometry. J. Essential Oil Res. 35, 461–470. doi: 10.1080/10412905.2023.2236626
Chen, B.-X., Peng, Y.-X., Yang, X.-Q., and Liu, J. (2021). Delayed germination of Brassica parachinensis seeds by coumarin involves decreased GA4 production and a consequent reduction of ROS accumulation. Seed Sci. Res. 31, 224–235. doi: 10.1017/S0960258521000167
Chen, C., Xu, L., Xia, W., Qi, C., Liu, J., Hua, B., et al. (2023). Metabolomics and transcription profiling of pumpkin fruit reveals enhanced bioactive flavonoids and coumarins in giant pumpkin (Cucurbita maxima). J. Agric. Food Chem. 71, 10459–10469. doi: 10.1021/ACS.JAFC.3C01883
Chong, J., Baltz, R., Schmitt, C., Beffa, R., Fritig, B., and Saindrenan, P. (2002). Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 14, 1093–1107. doi: 10.1105/tpc.010436
DeLoose, M., Cho, H., Bouain, N., Choi, I., Prom-U-Thai, C., Shahzad, Z., et al. (2024). PDR9 allelic variation and MYB63 modulate nutrient-dependent coumarin homeostasis in Arabidopsis. Plant J. 117, 1716–1727. doi: 10.1111/tpj.16678
Dixon, R. A. (2001). Natural products and plant disease resistance. Nature 411, 843–847. doi: 10.1038/35081178
Döll, S., Kuhlmann, M., Twan Rutten, M. F. M., Scharfenberg, S., Petridis, A., Berreth, D.-C., et al. (2018). Accumulation of the coumarin scopolin under abiotic stress conditions is mediated by the Arabidopsis thaliana THO/TREX complex. Plant journal: Cell Mol. Biol. 93, 431–444. doi: 10.1111/tpj.13797
Dong, N.-Q. and Lin, H.-X. (2021). Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 63, 180–209. doi: 10.1111/jipb.13054
Duan, C., Mao, T., Sun, S., Guo, X., Guo, L., Huang, L., et al. (2019). Constitutive expression of GmF6’H1 from soybean improves salt tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 141, 446–455. doi: 10.1016/j.plaphy.2019.06.027
Duan, Z., Yan, Q., Wu, F., Wang, Y., Wang, S., Zong, X., et al. (2021). Genome-wide analysis of the UDP-glycosyltransferase family reveals its roles in coumarin biosynthesis and abiotic stress in melilotus albus. Int. J. Mol. Sci. 22, 10826–10826. doi: 10.3390/IJMS221910826
Ebaid, M. S., Machala, M. K., Shaldam, M. A., Thabit, M. G., Kawka, M., Dziadek, B., et al. (2025). Exploring antitubercular activity of new coumarin derivatives targeting enoyl acyl carrier protein reductase (InhA): Synthesis, biological evaluation and computational studies. J. Mol. Structure 1336, 142074–142074. doi: 10.1016/J.MOLSTRUC.2025.142074
Fourcroy, P., Sisó-Terraza, P., Sudre, D., Savirón, M., Reyt, G., Gaymard, F., et al. (2014). Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol. 201, 155–167. doi: 10.1111/nph.12471
Fraissinet-Tachet, L., Baltz, R., Chong, J., Kauffmann, S., Fritig, B., and Saindrenan, P. (1998). Two tobacco genes induced by infection, elicitor and salicylic acid encode glucosyltransferases acting on phenylpropanoids and benzoic acid derivatives, including salicylic acid. FEBS Lett. 437, 319–323. doi: 10.1016/s0014-5793(98)01257-5
Gao, L., Wang, P., Yan, X., Li, J., Ma, L., Hu, M., et al. (2024). Feruloyl-CoA 6’-hydroxylase-mediated scopoletin accumulation enhances cotton resistance to Verticillium dahliae. Plant Physiol. 196, 3007–3022 doi: 10.1093/PLPHYS/KIAE508
Gautam, C. K., Tsai, H.-H., and Schmidt, W. (2021). IRONMAN tunes responses to iron deficiency in concert with environmental pH. Plant Physiol. 187, 1728–1745. doi: 10.1093/plphys/kiab329
Goldenberg, L., Ghuge, S. A., Faigenboim, A. D., Weissberg, M. C., Shaya, F., Rozen, A., et al. (2025). A 2OGD multi-gene cluster encompasses functional and tissue specificity that direct furanocoumarin and pyranocoumarin biosynthesis in citrus. New Phytol. 245, 1547–1562. doi: 10.1111/NPH.20322
He, S., Gao, J., Li, B., Luo, Z., Liu, P., Xu, X., et al. (2024). NtWIN1 regulates the biosynthesis of scopoletin and chlorogenic acid by targeting NtF6’H1 and NtCCoAMT genes in Nicotiana tabacum. Plant Physiol. Biochem. 214, 108937. doi: 10.1016/j.plaphy.2024.108937
Hu, Y., Sun, Z., Zeng, W., Qiu, Y., Xu, Z., Zhang, J., et al. (2025). Coumarin derivatives containing the 1,3,4 oxadiazole/thiadiazole moiety discovered as potential anti-tobacco mosaic virus agents. Mol. Diversity, 1–14. doi: 10.1007/S11030-024-11098-Y
Huang, X. C., Tang, H., Wei, X., He, Y., Hu, S., Wu, J. Y., et al. (2024). The gradual establishment of complex coumarin biosynthetic pathway in Apiaceae. Nat. Commun. 15, 6864–6864. doi: 10.1038/S41467-024-51285-X
Huang, X.-X., Wang, Y., Lin, J.-S., Chen, L., Li, Y.-J., Liu, Q., et al. (2021). A novel pathogen-responsive glycosyltransferase UGT73C7 mediates the redirection of phenylpropanoid metabolism and promotes SNC1-dependent Arabidopsis immunity. Plant journal: Cell Mol. Biol. 107, 149–165. doi: 10.1111/TPJ.15280
Huang, Y., Zhai, Y., Huang, Y., Huang, Y., Liu, K., Zhang, J., et al. (2022). Effects of light intensity on physiological characteristics and expression of genes in coumarin biosynthetic pathway of angelica dahurica. Int. J. Mol. Sci. 23, 15912–15912. doi: 10.3390/IJMS232415912
Hussain, M. I., Syed, Q. A., Khattak, M. N. K., Hafez, B., Reigosa, M. J., and El-Keblawy, A. (2019). Natural product coumarins: biological and pharmacological perspectives. Biologia 74, 863–888. doi: 10.2478/s11756-019-00242-x
Jian, X., Zhao, Y., Wang, Z., Li, S., Li, L., Luo, J., et al. (2020). Two CYP71AJ enzymes function as psoralen synthase and angelicin synthase in the biosynthesis of furanocoumarins in Peucedanum praeruptorum Dunn. Plant Mol. Biol. 104, 327–337. doi: 10.1007/s11103-020-01045-4
Jiang, Y., Zhang, Y., Liu, Y., Zhang, J., Jiang, M., Nong, C., et al. (2024). Plant Growth-Promoting Rhizobacteria Are Key to Promoting the Growth and Furanocoumarin Synthesis of Angelica dahurica var. formosana under Low-Nitrogen Conditions. J. Agric. Food Chem. 72, 6964–6978. doi: 10.1021/ACS.JAFC.3C09655
Kai, K., Mizutani, M., Kawamura, N., Yamamoto, R., Tamai, M., Yamaguchi, H., et al. (2008). Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. 55, 989–999. doi: 10.1111/j.1365-313X.2008.03568.x
Kai, K., Shimizu, B.-i., Mizutani, M., Watanabe, K., and Sakata, K. (2006). Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 67, 379–386. doi: 10.1016/j.phytochem.2005.11.006
Karamat, F., Olry, A., Munakata, R., Koeduka, T., Sugiyama, A., Paris, C., et al. (2014). A coumarin-specific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley. Plant J. 77, 627–638. doi: 10.1111/tpj.12409
Kashman, Y., Gustafson, K. R., Fuller, R. W., Cardellina, J. H., McMahon, I. B., Currens, M. J., et al. (1992). The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J. Med. Chem. 35, 2735–2743. doi: 10.1021/jm00093a004
Larbat, R., Hehn, A., Hans, J., Schneider, S., Jugdé, H., Schneider, B., et al. (2009). Isolation and functional characterization of CYP71AJ4 encoding for the first P450 monooxygenase of angular furanocoumarin biosynthesis. J. Biol. Chem. 284, 4776–4785. doi: 10.1074/jbc.M807351200
Leal, L. E., Moreira, E. S., Correia, B. L., Bueno, P. S. A., Comar, J. F., Sá-Nakanishi, A., et al. (2024). Comparative study of the antioxidant and anti-inflammatory effects of the natural coumarins 1,2-benzopyrone, umbelliferone and esculetin: in silico, in vitro and in vivo analyses. Naunyn Schmiedebergs Arch. Pharmacol. 397, 173–187. doi: 10.1007/s00210-023-02606-2
Lefèvre, F., Fourmeau, J., Pottier, M., Baijot, A., Cornet, T., Abadía, J., et al. (2018). The Nicotiana tabacum ABC transporter NtPDR3 secretes O-methylated coumarins in response to iron deficiency. J. Exp. Bot. 69, 4419–4431. doi: 10.1093/jxb/ery221
Li, Q., Dai, Y., Huang, X. C., Sun, L., Wang, K., Guo, X., et al. (2024). The chromosome-scale assembly of the Notopterygium incisum genome provides insight into the structural diversity of coumarins. Acta Pharm. Sin. B 14, 3760–3773. doi: 10.1016/J.APSB.2024.04.005
Liang, X., Wang, Y., Shen, W., Liao, B., Liu, X., Yang, Z., et al. (2024). Genomic and metabolomic insights into the selection and differentiation of bioactive compounds in citrus. Mol. Plant 17, 1753–1772. doi: 10.1016/J.MOLP.2024.10.009
Liao, R., Yao, J., Zhang, Y., Liu, Y., Pan, H., Han, B., et al. (2024). MYB transcription factors in Peucedanum Praeruptorum Dunn: the diverse roles of the R2R3-MYB subfamily in mediating coumarin biosynthesis. BMC Plant Biol. 24, 1135–1135. doi: 10.1186/S12870-024-05864-1
Liu, H., Wang, Y., Chang, Q., Li, Q., Fang, J., Cao, N., et al. (2024). Combined metabolome and transcriptome reveal HmF6’H1 regulating simple coumarin accumulation against powdery mildew infection in Heracleum moellendorffii Hance. BMC Plant Biol. 24, 507. doi: 10.1186/s12870-024-05185-3
Lundberg, D. S. and Teixeira, P. J. P. L. (2018). Root-exuded coumarin shapes the root microbiome. Proc. Natl. Acad. Sci. United States America 115, 5629–5631. doi: 10.1073/pnas.1805944115
Manurung, H., Kustiawan, W., Kusuma, I. W., Marjenah, M., and Nugroho, R. A. (2019). Growth, phytochemical profile, and antioxidant activity of cultivated tabat barito (Ficus deltoidea Jack) under drought stress. 14, 366–378 doi: 10.12692/ijb/14.1.366-378
Mohammed, A. E., Alotaibi, M. O., and Elobeid, M. (2022). Interactive influence of elevated CO2 and arbuscular mycorrhizal fungi on sucrose and coumarin metabolism in Ammi majus. Plant Physiol. Biochem. 185, 45–54. doi: 10.1016/J.PLAPHY.2022.05.029
Munakata, R., Olry, A., Karamat, F., Courdavault, V., Sugiyama, A., Date, Y., et al. (2016). Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis. New Phytol. 211, 332–344. doi: 10.1111/nph.13899
Murgia, I., Tarantino, D., Soave, C., and Morandini, P. (2011). Arabidopsis CYP82C4 expression is dependent on Fe availability and circadian rhythm, and correlates with genes involved in the early Fe deficiency response. J. Plant Physiol. 168, 894–902. doi: 10.1016/j.jplph.2010.11.020
Nan, Z. D., Shang, Y., Zhu, Y. D., Zhang, H., Sun, R. R., Tian, J. J., et al. (2025). Systematic review of natural coumarins in plants, (2019-2024): chemical structures and pharmacological activities. Phytochemistry 235, 114480. doi: 10.1016/J.PHYTOCHEM.2025.114480
Nie, G., Wu, F., Duan, Z., Wang, S., Ao, B., Zhou, P., et al. (2024). Genome-wide analysis of the cytochrome P450 superfamily suggests its roles in coumarin biosynthesis and salt stress response in Melilotus albus. Environ. Exp. Bot. 220, 105718. doi: 10.1016/J.ENVEXPBOT.2024.105718
Park, N. I., Park, J. H., and Park, S. U. (2012). Overexpression of cinnamate 4-hydroxylase gene enhances biosynthesis of decursinol angelate in Angelica gigas hairy roots. Mol. Biotechnol. 50, 114–120. doi: 10.1007/s12033-011-9420-8
Pereira, T. M., Franco, D. P., Vitorio, F., and Kummerle, A. E. (2018). Coumarin compounds in medicinal chemistry: some important examples from the last years. Curr. Top. Med. Chem. 18, 124–148. doi: 10.2174/1568026618666180329115523
Qu, W., Huang, W., Chen, C., Chen, J., Zhao, L., Jiang, Y., et al. (2024). AdNAC20 Regulates Lignin and Coumarin Biosynthesis in the Roots of Angelica dahurica var. Formosana. Int. J. Mol. Sci. 25, 7998–7998. doi: 10.3390/IJMS25147998
Rajniak, J., Giehl, R. F. H., Chang, E., Murgia, I., Wirén, N.v., and Sattely, E. S. (2018). Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nat. Chem. Biol. 14, 442–450. doi: 10.1038/s41589-018-0019-2
Repčák, M. and Suvák, M. (2013). Methyl jasmonate and Echinothrips americanus regulate coumarin accumulation in leaves of Matricaria chamomilla. Biochem. Systematics Ecol. 47, 38–41. doi: 10.1016/j.bse.2012.10.009
Rivera, J. O. O., Ulloa, M., Zavala, F. G. P., González, H. R. N., Roberts, P. A., Villalobos, L. Y., et al. (2024). Enhanced phenylpropanoid metabolism underlies resistance to Fusarium oxysporum f. sp. vasinfectum race 4 infection in the cotton cultivar Pima-S6 (Gossypium barbadense L.). Front. Genet. 14. doi: 10.3389/FGENE.2023.1271200
Robe, K., Izquierdo, E., Vignols, F., Rouached, H., and Dubos, C. (2020). The coumarins: secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 26, 248–259. doi: 10.1016/J.TPLANTS.2020.10.008
Rodrigues, J. L., Gomes, D., and Rodrigues, L. R. (2022). Challenges in the heterologous production of furanocoumarins in escherichia coli. Molecules 27, 7230–7230. doi: 10.3390/MOLECULES27217230
Rohman, N., Ardiansah, B., Wukirsari, T., and Judeh, Z. (2024). Recent trends in the synthesis and bioactivity of coumarin, coumarin-chalcone, and coumarin-triazole molecular hybrids. Molecules 29, 1026. doi: 10.3390/molecules29051026
Romain, L., Sandra, K., Silvia, S., Alain, H., Eric, G., Joachim, H., et al. (2007). Molecular cloning and functional characterization of psoralen synthase, the first committed monooxygenase of furanocoumarin biosynthesis. J. Biol. Chem. 282, 542–554. doi: 10.1074/jbc.M604762200
Roselli, S., Olry, A., Vautrin, S., Coriton, O., Ritchie, D., Galati, G., et al. (2017). A bacterial artificial chromosome (BAC) genomic approach reveals partial clustering of the furanocoumarin pathway genes in parsnip. Plant J. 89, 1119–1132. doi: 10.1111/tpj.13450
Ruan, J., Haerdter, R., and Gerendás, J. (2010). Impact of nitrogen supply on carbon/nitrogen allocation: a case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant Biol. (Stuttg) 12, 724–734. doi: 10.1111/j.1438-8677.2009.00288.x
Ruolan, Y., Yucheng, Z., Tingting, L., Chuanlong, H., Sheng, X., Ziwei, S., et al. (2017). Identification and functional characterization of a p-coumaroyl CoA 2’-hydroxylase involved in the biosynthesis of coumarin skeleton from Peucedanum praeruptorum Dunn. Plant Mol. Biol. 95, 199–213. doi: 10.1007/s11103-017-0650-4
Shao, X., Zhang, Z., Qian, X., Wang, L., Zhang, Y., and Luo, Y. (2022). Potential Biochemical Pesticide—Synthesis of Neofuranocoumarin and Inhibition the Proliferation of Spodoptera frugiperda Cells through Activating the Mitochondrial Pathway. Toxins 14, 677–677. doi: 10.3390/TOXINS14100677
Sierra, E. J. T., Cordeiro, C. F., Diniz, L., Caldas, I. S., Hawkes, J. A., and Carvalho, D. T. (2021). Coumarins as potential antiprotozoal agents: biological activities and mechanism of action. Rev. Bras. Farmacognosia 31 (5), 592–611. doi: 10.1007/s43450-021-00169-y
Sisó-Terraza, P., Luis-Villarroya, A., Fourcroy, P., BRIAT, J.-F., Abadía, A., Gaymard, F., et al. (2016). Accumulation and Secretion of Coumarinolignans and other Coumarins in Arabidopsis thaliana Roots in Response to Iron Deficiency at High pH. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01711
Siwinska, J., Siatkowska, K., Olry, A., Grosjean, J., Hehn, A., Bourgaud, F., et al. (2018). Scopoletin 8-hydroxylase: a novel enzyme involved in coumarin biosynthesis and iron-deficiency responses in Arabidopsis. J. Exp. Bot. 69, 1735–1748. doi: 10.1093/jxb/ery005
Stringlis, I. A., Yu, K., Feussner, K., Jonge, R.d., Bentum, S. V., Verk, M. C. V., et al. (2018). MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. United States America 115, E5213–E5222. doi: 10.1073/pnas.1722335115
Sun, H., Wang, L., Zhang, B., Ma, J., Hettenhausen, C., Cao, G., et al. (2014). Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot. 65, 4305–4315. doi: 10.1093/jxb/eru203
Taguchi, G., Ubukata, T., Hayashida, N., Yamamoto, H., and Okazaki, M. (2003). Cloning and characterization of a glucosyltransferase that reacts on 7-hydroxyl group of flavonol and 3-hydroxyl group of coumarin from tobacco cells. Arch. Biochem. Biophys. 420, 95–102. doi: 10.1016/j.abb.2003.09.027
Tao, M., Liu, S., Li, Y., Liu, A., Sun, Z., Maharaj, V., et al. (2023). Comprehensive multi-omics analysis reveals the importance of CtCOSY in the energy metabolism and coumarin biosynthesis in Clematis terniflora DC. Ind. Crops Products 195, 116444. doi: 10.1016/J.INDCROP.2023.116444
Vanholme, R., Sundin, L., Seetso, K. C., Kim, H., Xinyu Liu, J. L., Meester, B. D., et al. (2019). COSY catalyses trans-cis isomerization and lactonization in the biosynthesis of coumarins. Nat. Plants 5, 1066–1075. doi: 10.1038/s41477-019-0510-0
Villard, C., Munakata, R., Kitajima, S., Velzen, R.v., Schranz, M. E., Larbat, R., et al. (2021). A new P450 involved in the furanocoumarin pathway underlies a recent case of convergent evolution. New Phytol. 231, 1923–1939. doi: 10.1111/NPH.17458
Vismans, G., Bentum, S.v., Spooren, J., Song, Y., Goossens, P., Valls, J., et al. (2022). Coumarin biosynthesis genes are required after foliar pathogen infection for the creation of a microbial soil-borne legacy that primes plants for SA-dependent defenses. Sci. Rep. 12, 22473. doi: 10.1038/s41598-022-26551-x
Walker, D. J., Martínez-Fernández, D., Correal, E., Romero-Espinar, P., and Río, J. (2012). Accumulation of furanocoumarins by Bituminaria bituminosa in relation to plant development and environmental stress. Plant Physiol. Biochem. 54, 133–139. doi: 10.1016/j.plaphy.2012.03.001
Wang, Z., He, Y., Liao, L., Zhang, Y., Zhao, Y., Xiao, Y., et al. (2022). Forming coumarin C-glycosides via biocatalysis: Characterization of a C-glycosyltransferase from Angelica decursiva. Biochem. Biophys. Res. Commun. 614, 85–91. doi: 10.1016/j.bbrc.2022.05.008
Wang, S., Xie, Y., Huo, Y.-W., Li, Y., Abel, P. W., Jiang, H., et al. (2020). Airway relaxation mechanisms and structural basis of osthole for improving lung function in asthma. Sci. Signaling 13, eaax0273. doi: 10.1126/SCISIGNAL.AAX0273
Wu, F., Duan, Z., Xu, P., Yan, Q., Meng, M., Cao, M., et al. (2022). Genome and systems biology of Melilotus albus provides insights into coumarins biosynthesis. Plant Biotechnol. J. 20, 592–609. doi: 10.1111/pbi.13742
Wu, B., Shi, S., Zhang, H., Lu, B., Nan, P., and A, Y. (2023). Anabolic metabolism of autotoxic substance coumarins in plants. PeerJ 11, e16508. doi: 10.7717/peerj.16508
Wu, L., Wang, X., Xu, W., Farzaneh, F., and Xu, R. (2009). The structure and pharmacological functions of coumarins and their derivatives. Curr. medicinal Chem. 16, 4236–4260. doi: 10.2174/092986709789578187
Zeng, Y., Ahmed, H.G.M.-D., Li, X., Yang, L.e., Pu, X., Yang, X., et al. (2024). Physiological mechanisms by which the functional ingredients in beer impact human health. Molecules 29, 3110. doi: 10.3390/molecules29133110
Zhang, Y., Bai, P., Zhuang, Y., and Liu, T. (2022). Two O-methyltransferases mediate multiple methylation steps in the biosynthesis of coumarins in cnidium monnieri. J. Natural products 85, 2116–2121. doi: 10.1021/ACS.JNATPROD.2C00410
Zhang, J., Gao, L., Lei, Z., Liu, H., Li, J., Luo, Y., et al. (2025). Design, synthesis, and evaluation of 7-vinylcoumarin derivatives as agrochemicals: biological activities and molecular docking insights. Chem. biodiversity, e202403118. doi: 10.1002/CBDV.202403118
Zhao, M., Ma, L., Song, N., Cheng, J., Zhao, Z., and Wu, J. (2022). The regulation of Alternaria alternata resistance by LRR-RK4 through ERF109, defensin19 and phytoalexin scopoletin in Nicotiana attenuata. Plant Sci. 323, 111414. doi: 10.1016/j.plantsci.2022.111414
Zhao, Y., Wang, N., Sui, Z., Huang, C., Zeng, Z., and Kong, L. (2019). The molecular and structural basis of O-methylation reaction in coumarin biosynthesis in peucedanum praeruptorum dunn. Int. J. Mol. Sci. 20, 1533–1533. doi: 10.3390/ijms20071533
Zhao, Y., Wang, N., Zeng, Z., Xu, S., Huang, C., Wang, W., et al. (2016). Cloning, functional characterization and catalytic mechanism of a bergaptol O-methyltransferase from Peucedanum praeruptorum Dunn. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00722
Zhong, L., Liao, P.-R., Liu, C.-Z., Qian, J.-P., He, W.-C., Luo, B., et al. (2021). Effects of drought stress on physiological and biochemical and chemical components of Cinnamomum cassia seedlings. Zhongguo Zhong Yao Za Zhi 46, 2158–2166. doi: 10.19540/j.cnki.cjcmm.20210224.101
Keywords: coumarin, biosynthetic pathway, phenylpropanoids, environmental stress, genetic regulation
Citation: Wang Y, Guan T, Yue X, Yang J, Zhao X, Chang A, Yang C, Fan Z, Liu K and Li Y (2025) The biosynthetic pathway of coumarin and its genetic regulation in response to biotic and abiotic stresses. Front. Plant Sci. 16:1599591. doi: 10.3389/fpls.2025.1599591
Received: 25 March 2025; Accepted: 27 May 2025;
Published: 19 June 2025.
Edited by:
Chang-Jun Liu, Brookhaven National Laboratory (DOE), United StatesReviewed by:
Jiang Shi, Tea Research Institute, Chinese Academy of Agricultural Sciences, ChinaYawen Zeng, Yunnan Academy of Agricultural Sciences, China
Copyright © 2025 Wang, Guan, Yue, Yang, Zhao, Chang, Yang, Fan, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiting Li, bGl5aXRpbmdAY2Fhcy5jbg==; Keqiang Liu, NzUxNTY3M0BxcS5jb20=
†These authors have contributed equally to this work
 Yixue Wang
Yixue Wang Tiqing Guan3†
Tiqing Guan3† Jiashuo Yang
Jiashuo Yang Changqing Yang
Changqing Yang Yiting Li
Yiting Li