- 1State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Xinjiang Uyghur Autonomous Region Academy of Surveying and Mapping, Urumqi, Xinjiang, China
- 3School of Ethnic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Introduction: Angelica dahurica is a traditional medicinal plant known for its high content of bioactive coumarins. With climate change potentially affecting both species distribution and secondary metabolite accumulation, there is a pressing need to integrate ecological and chemical data to guide future cultivation and resource utilization strategies.
Methods: This study combined the Maximum Entropy (MaxEnt) ecological modeling approach with chemometric analysis to (i) predict the suitable habitat distribution of A. dahurica under current and future climate scenarios and (ii) evaluate the correlation between environmental variables and coumarin accumulation.
Results: (1) The key environmental variables influencing the distribution of A. dahurica were identified as BIO_13 (precipitation of the wettest month), BIO_11 (mean temperature of the coldest quarter), and elevation (DEM). (2) Presently, the highly suitable regions for A. dahurica cultivation are mainly in Sichuan, Henan, and Hebei provinces. (3) Under future climate scenarios, the highly suitable habitats are expected to expand and shift geographically, especially toward Henan and Jiaozuo, with parts of Hubei, Shaanxi, and Shandong transitioning into highly suitable zones. (4) Chemometric analyses revealed that A. dahurica samples from highly suitable areas contained significantly higher total coumarin content than those from medium-suitability regions. (5) A strong correlation was observed between key environmental factors (especially BIO_11 and DEM) and the relative content of five major coumarin components.(6) Spatial mapping of chemical composition indicated distinct regional differences in coumarin distribution, suggesting the potential for geoherbalism-based classification.
Discussion: The integration of ecological modeling with chemical analysis provides a powerful framework for understanding the impact of environmental variables on both the distribution and chemical quality of A. dahurica. These findings offer valuable guidance for targeted cultivation and resource management under future climate change conditions.
1 Introduction
Angelica dahurica, a member of the Apiaceae family, has dried roots that serve as key components in several traditional Chinese medicine prescriptions, such as Qingfeng Baidu San and Cang’erzi San State pharmacopoeia committee (2020). Modern pharmacological studies have demonstrated that A. dahurica possesses notable antibacterial, antioxidant, antitumor, and analgesic activities Zhao et al. (2022). Among the secondary metabolites of A. dahurica, coumarin serves as the primary active component. Coumarins are a class of benzopyrone derivatives known for their distinctive planar aromatic structures, which enable interactions with various biological targets. Structurally, they consist of a fused benzene and α-pyrone ring, with various substitutions influencing their pharmacological properties. For example, imperatorin and isoimperatorin possess furan rings, contributing to their lipophilicity and biological activity, including modulation of cytochrome P450 enzymes and anti-inflammatory pathways Dixon and Paiva (1995); Zhou et al. (2015). These compounds have attracted increasing interest due to their multi-target pharmacological activities. Recent reviews have emphasized that natural coumarins and isocoumarins exhibit significant anticancer potential by modulating apoptosis, autophagy, angiogenesis, drug resistance, and immune regulation pathways Aqib et al. (2025). Other studies have also pointed out their neuroprotective roles, especially in Alzheimer’s disease, where they can reduce Aβ aggregation, inhibit tau hyperphosphorylation, and regulate GSK-3 activity, offering a multitargeted therapeutic strategy Hussain et al. (2025). Furthermore, coumarins have been recognized as privileged scaffolds in medicinal chemistry. They demonstrate broad-spectrum activities, including anti-inflammatory, monoamine oxidase inhibition, antithrombotic, antidiabetic, hepatoprotective, and antimicrobial effects, underscoring their structural versatility and drug development potential Hussain et al. (2024). Additionally, the design of synthetic hybrids, such as benzocoumarin-stilbene derivatives, has shown promising antitumor efficacy through mechanisms like DNA ligase I inhibition, providing a potential lead for next-generation anticancer drugs Hussain et al. (2017). The chemical structures of the components detected in this study are shown in Figure 1.
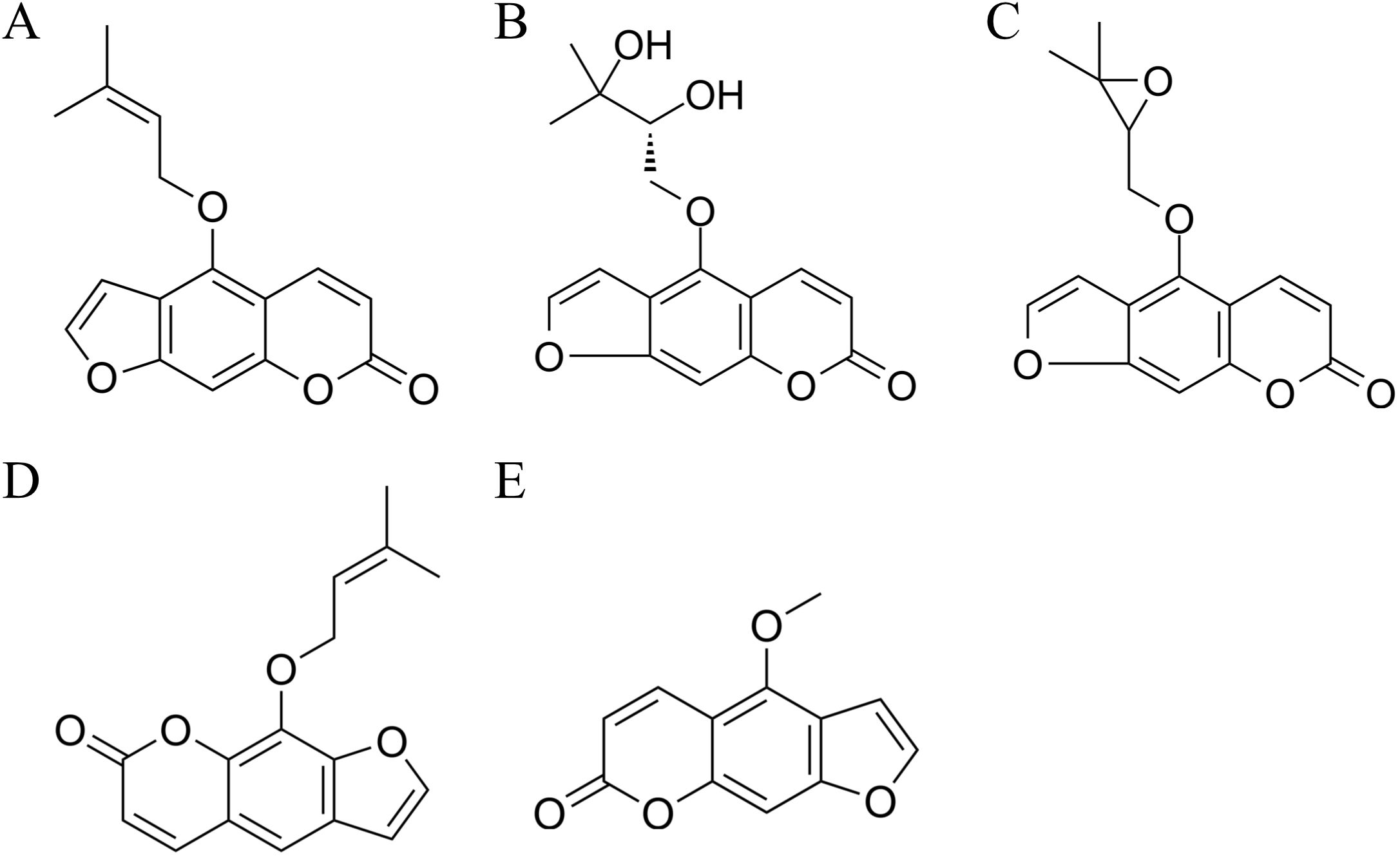
Figure 1. Chemical structures of the components detected in this study. (A) Isoimperatorin; (B) Oxypeucedanin hydrate; (C) Oxypeucedanin; (D) Imperatorin; (E) Bergapten.
A. dahurica plays a pivotal role in traditional Chinese medicine not only due to its rich coumarin content, but also because of its multifaceted therapeutic potential. The root contains bioactive components that exhibit antimicrobial, anti-inflammatory, neuroprotective, and hepatoprotective properties, contributing to its wide usage in treating skin diseases, rhinitis, headaches, and digestive disorders Zhao et al. (2022); Zhou et al. (2015). Additionally, its effects on the central nervous system and vascular systems make it biologically relevant in developing treatments for modern ailments such as migraines, atherosclerosis, and inflammation-related syndromes.
Various variables, including soil conditions, climate, and water sources, can influence the growth of A. dahurica and the accumulation of its medicinal constituents. For instance, the concentration of coumarin compounds in the roots of A. dahurica peaks in mid to late July. Additionally, different processing methods, such as sun drying and sulfur fumigation, impact the concentration of coumarin compounds, with sun drying being more effective in preserving these compounds Mengyue (2003). Since the Last Interglacial (LIG), the climate has undergone several drastic changes that have significantly affected the distribution of A. dahurica Blois et al. (2013). This species has a long history of cultivation in China, with records indicating its cultivation in Jiangsu and Zhejiang provinces as early as the late Ming Dynasty Wang et al. (2001). Currently, A. dahurica is produced in Hebei, Henan, Anhui, Sichuan, and other regions across China Ma et al. (2009), and it is also found in parts of Europe and North America Wang et al. (2020).
Global climate change poses a significant threat to the sustainable utilization of medicinal plants, primarily manifested through substantial reductions or shifts in their survival environments. Robiansyah et al. (2023). Cahyaningsih et al. Cahyaningsih et al. (2021) indicated that climate change has significantly impacted medicinal plants in Indonesia, highlighting the potential threat posed by global climate change to these vital species. The utilization of climate data to develop species distribution models has emerged as a widely adopted methodology among researchers studying the responses of various species to climate change Abdulwahab et al. (2022). The maximum entropy modeling (MaxEnt) is a widely used machine learning algorithm for predicting species distributions based on presence-only data. It estimates the probability distribution of maximum entropy (i.e., the most spread out or uniform distribution) constrained by environmental variables at known occurrence sites, thereby predicting suitable habitats in geographic space. MaxEnt has become a standard tool in ecological niche modeling due to its strong predictive performance, especially under limited or biased occurrence data conditions Baldwin (2009); Phillips et al. (2025). On the other hand, environmental variables significantly influence the accumulation of chemical components in medicinal plants Pant et al. (2021); Li et al. (2020). Recent studies have indicated that the habitat of medicinal plants is a critical factor in the accumulation of effective components Lu et al. (2020). Reports suggest that many plants found in highly suitable habitats exhibit elevated levels of chemical components Xu et al. (2018). Although the mechanism by which climate change affects plant quality remains unclear, the results of these studies may contribute to assessing the geographical distribution of future medicinal plants through habitat suitability analysis.
In addition, high-performance liquid chromatography (HPLC) can effectively evaluate the differences in chemical components of A. dahurica from different regions. Studies indicate that the quality of A. dahurica from Henan and Hebei is significantly superior to that from Sichuan and from Anhui Chen et al. (2019). However, these samples are predominantly concentrated in traditional production areas and their immediate surroundings. The quality of A. dahurica in other environments remains unclear, as does the impact of climate change on the distribution of highquality A. dahurica. In the face of the increasing demand for A. dahurica in the market in recent years, relevant industries and departments need to consider how to delineate suitable planting areas to achieve sustainable supply of A. dahurica. Currently, research on A. dahurica mainly focuses on its pharmacology and medicinal effects, and in the reported studies on the distribution of A. dahurica, although online database occurrence records have been used, the content of chemical components has not been included in the scope of investigation, and the evaluation of the quality of A. dahurica in areas that may be suitable for planting A. dahurica cannot provide comprehensive reference value Zhang et al. (2024a), which is not conducive to the formulation of planting area expansion plans.
This study aims to: (1) Predict changes in A. dahurica’s suitable habitat under climate change; (2) Identify key environmental variables affecting its distribution; (3) Analyze how habitat suitability influences coumarin accumulation.
2 Materials and methods
2.1 Samples and species occurrence records
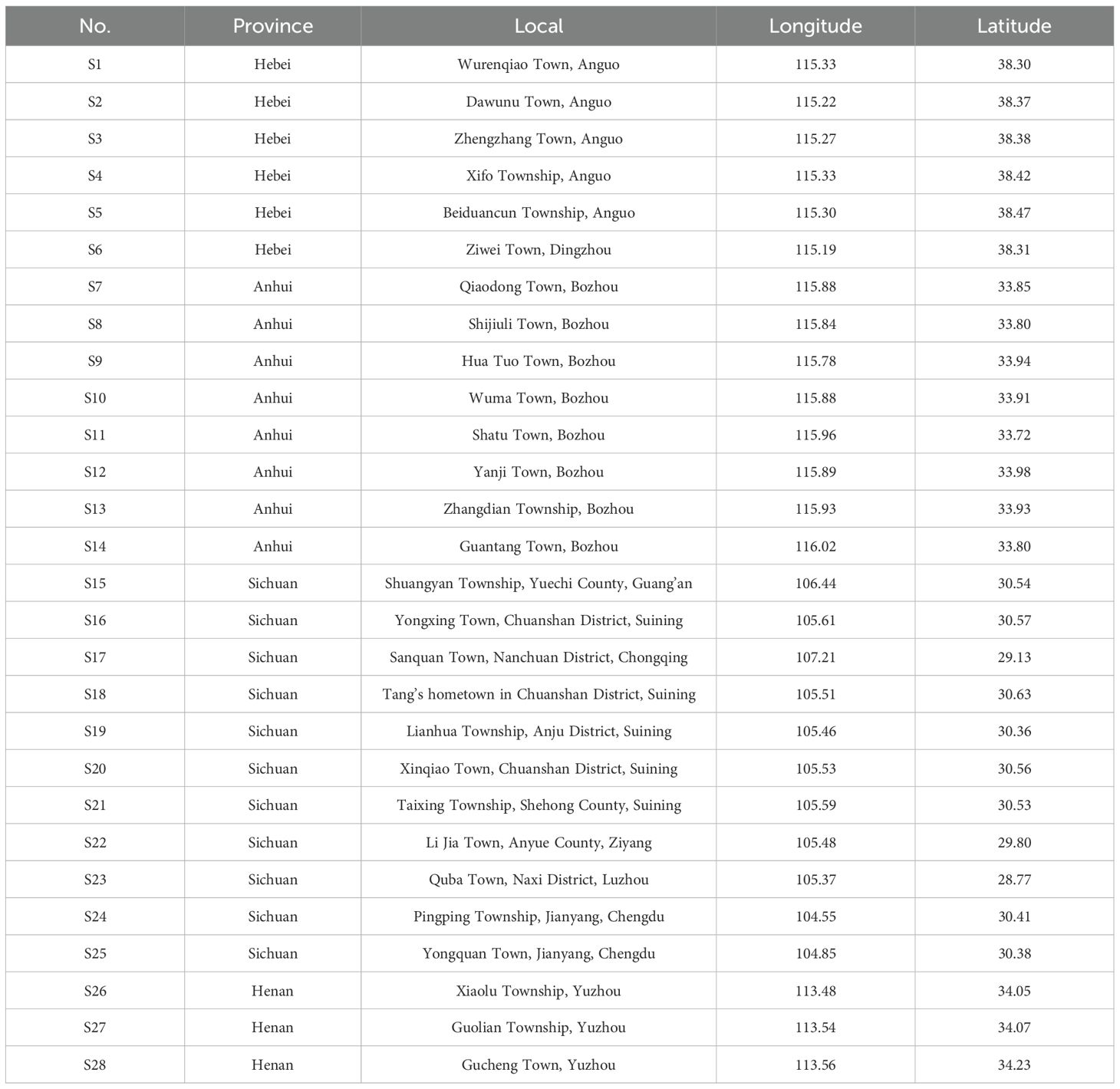
The species occurrence records of this study were obtained from the National Specimen Information Infrastructure(NSII) and the Chinese Virtual Herbarium(CVH). To prevent overfitting, ENMTools in R was used to filter occurrence data, retaining only one record per 1km2 grid Wang et al. (2024b); Zhao et al. (2024). After screening, a total of 246 valid occurrence records were used in this study (Supplementary Table S1 for details), as shown in Figure 2. And this study collected 28 batches of A. dahurica samples in Suining City, Jianyang City, Ziyang City, Luzhou City, and Guang’an City in Sichuan Province, Nanchuan District in Chongqing, Yuzhou City in Henan Province, Anguo City in Hebei Province, and Bozhou City in Anhui Province, with precise geographic coordinates recorded (Table 1). All 28 batches were taxonomically identified as Angelica dahurica (Fisch.ex Hoffm.) Benth.et Hook.f. or Angelica dahurica (Fisch.ex Hoffm.) Benth.et Hook.f.var.formosana(Boiss.) Shan et Yuan of the Apiaceae family by Professor Guihua Jiang of the School of Pharmacy, Chengdu University of Traditional Chinese Medicine.
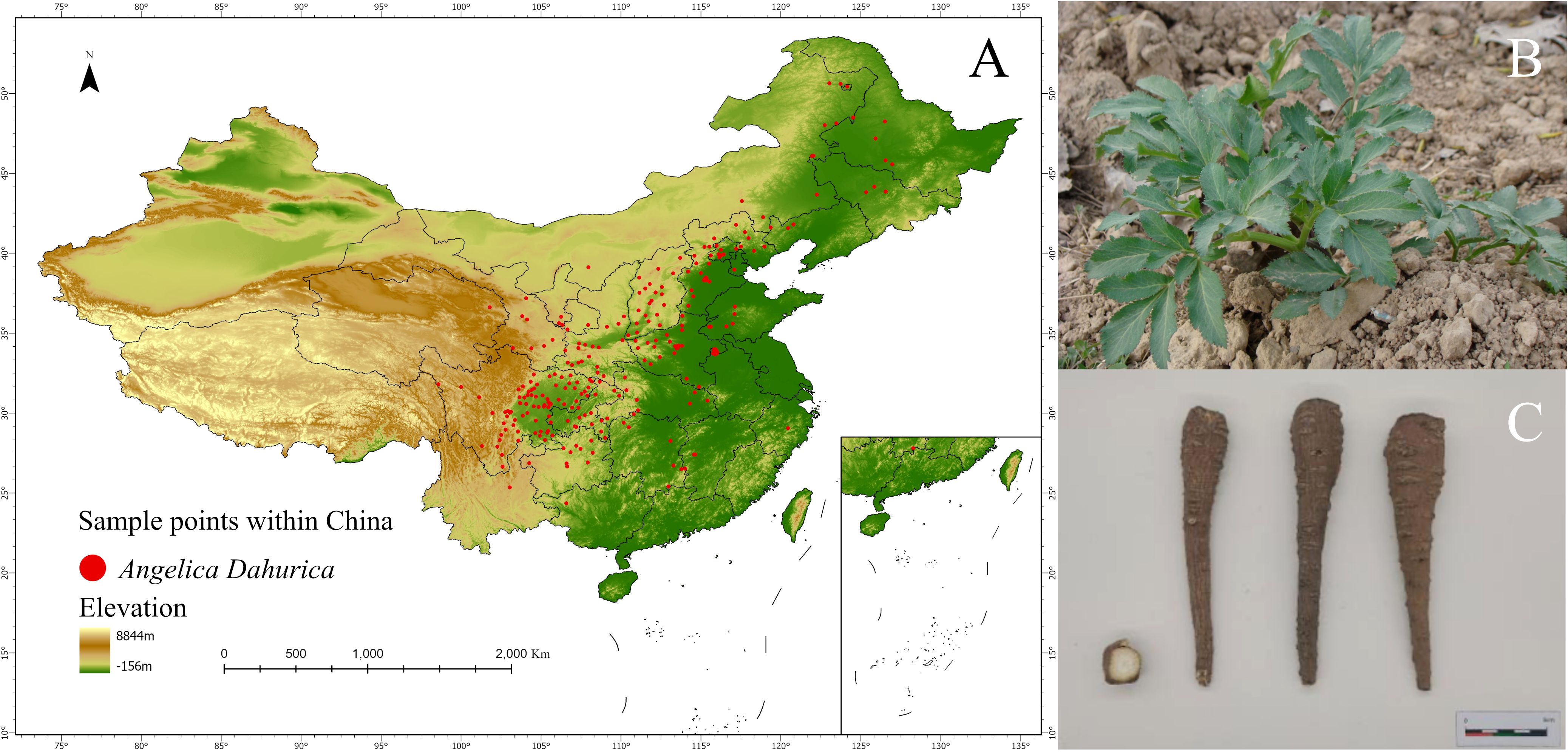
Figure 2. (A) Distribution of A. dahurica; (B) Original plant of A. dahurica; (C) Parts of A. dahurica used for medicine.
2.2 Environmental variables
Species distribution is influenced by both abiotic variables (e.g., climatic conditions, edaphic properties, and topographic features) and biotic components. For modeling the current distribution pattern of A. dahurica, 31 candidate environmental variables were selected, including: Bioclimatic variables for the current period (1970-2000), 2041-2060, and 2061–2080 provided by the World Climate Database (www.worldclim.org) under the assumption of future carbon emissions as SSP126 (the most stringent carbon emission control scenario) Yang et al. (2025); Soil-related variables provided by the Harmonized World Soil Database (HWSD, version 1.1) constructed by the Food and Agriculture Organization (FAO) and the International Institute for Applied Systems Analysis (IIASA) Lu and Liu (2019): ADD_PROP [Other properties (gelic, vertic, petric)], AWC_CLASS (AWC Range), REF_DEPTH (Reference Soil Depth), SU_SYM90 [Soil Unit Symbol (FAO-90)], T_CACO3 (Topsoil Calcium Carbonate), T_CEC_CLAY [Topsoil CEC (clay)], T_CEC_SOIL [Topsoil CEC (soil)], T_GRAVEL (Topsoil Gravel Content), T_OC (Topsoil Organic Carbon), T_PH_H2O [Topsoil pH (H2O)], T_SAND (Topsoil Sand Fraction), T_SILT (Topsoil Silt Fraction), T_TEXTURE (Topsoil Texture), and other soil data; DEM (Elevation) provided by the General Bathymetric Chart of the Oceans (GEBCO, www.gebco.net) GEBCO Bathymetric Compilation Group (2023). The spatial resolution of these data is 30 seconds (≈ 1km).
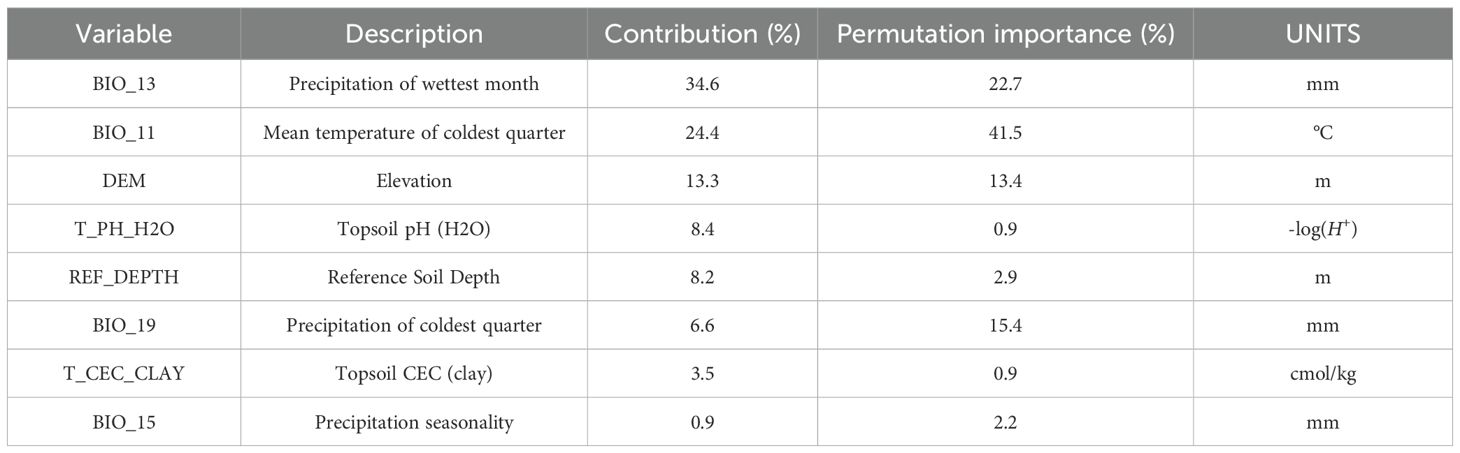
Excessive environmental variables can result in overfitting issues, such as multicollinearity and autocorrelation within the model Yang et al. (2025). This study identified environmental variables with non-zero contribution rates through preliminary experiments. Subsequently, Pearson correlation analysis was conducted using R to evaluate the selected environmental variables, leading to the selection of those with a correlation coefficient |r| ≥ 0.8 and a significant contribution rate (See Supplementary Table S2 for relevant analysis). Ultimately, the model retained four climatic variables (BIO_13, BIO_11, BIO_19, BIO_15), three soil variables (T_PH_H2O, REF_DEPTH, T_CEC_CLAY), and one topographic variable (DEM) for predicting the potential suitable habitat of A. dahurica, as illustrated in Table 2.
2.3 Species distribution modeling process
2.3.1 Model establishment and optimization
MaxEnt is widely used to predict potential species distribution areas. It correlates species occurrence data with environmental variables using a specific algorithm, then applies this relationship across the study area to generate distribution predictions Zhao et al. (2024). The R package ENMeval optimizes MaxEnt models by reducing complexity and overfitting, improving prediction accuracy Kass et al. (2021).
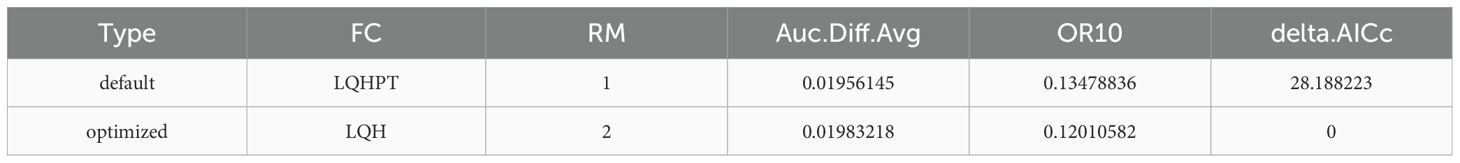
In this study, ENMeval was used to optimize MaxEnt parameters. The Regularization Multiplier (RM) values (0.5–5, step 0.5) were tested with five feature types (L, LQ, H, LQH, LQHP, LQHPT), forming 60 combinations. Using 246 A. dahurica occurrence points and eight environmental variables, ENMeval performed cross-validation and optimization under different RM and feature class (FC) combinations Wang et al. (2023, 2024a). Model selection was based on the Akaike Information Criterion (AICc) for complexity and fit, while the 10% training omission rate (avg.OR10) and the average AUC difference (avg.diff.AUC) assessed model performance. The model with the lowest deltaAICc was chosen as the optimal model Lin et al. (2023).
2.3.2 Predictive results and evaluation of the model
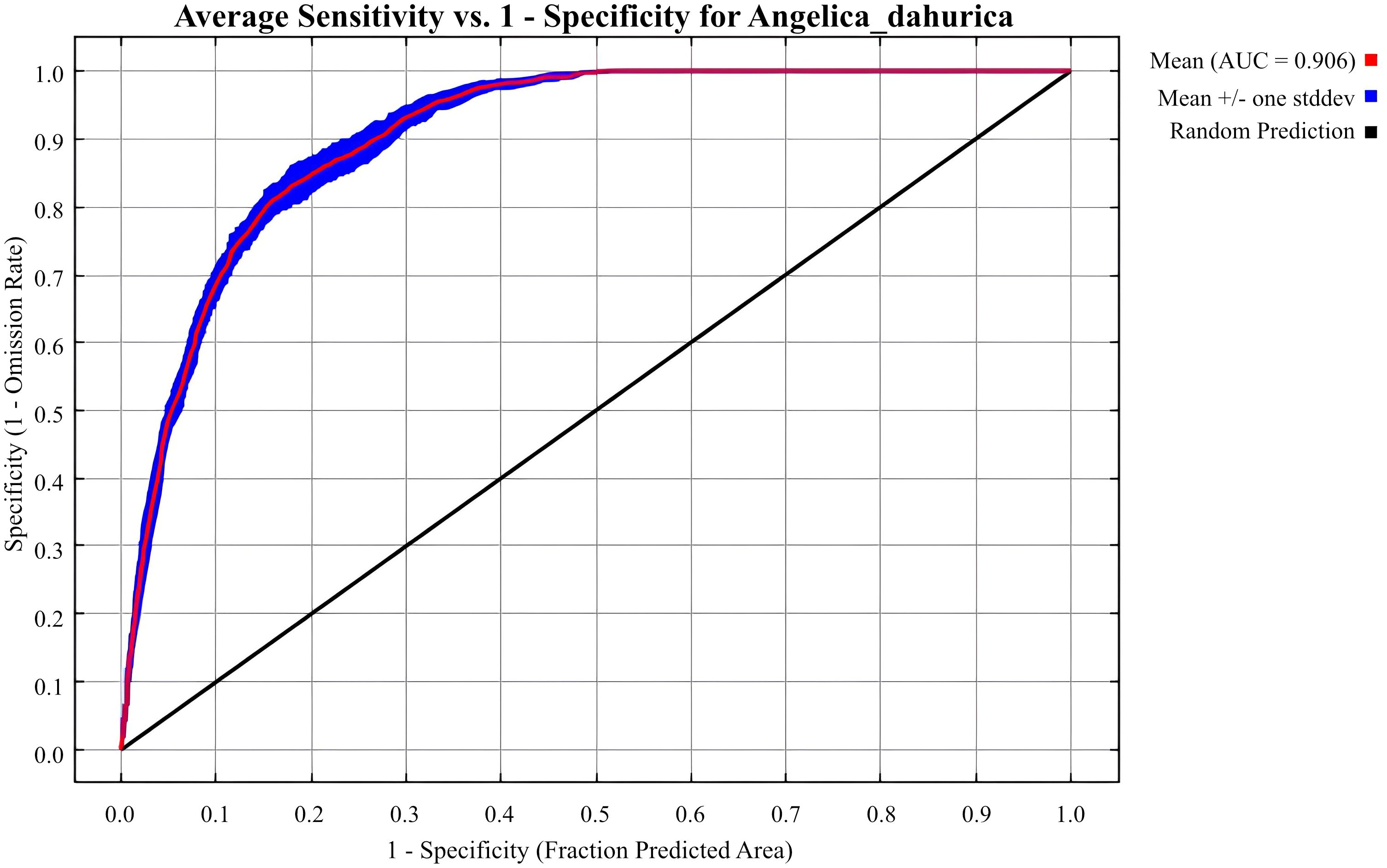
In this study, MaxEnt (Version 3.4.4) Phillips et al. (2025) was used to analyze and predict suitable habitats for A. dahurica, 75% of the occurrence data was used for training and 25% for testing. The model was run with 10 Bootstrap replicates Zhao et al. (2021b). Model performance was evaluated using the Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) curve. AUC values range from 0 to 1, with higher values indicating greater predictive accuracy: <0.7 (poor), 0.7–0.8 (moderate), 0.8–0.9 (good), and >0.9 (excellent) Zheng et al. (2024); Zhan et al. (2022); Wang et al. (2024a); Qiu et al. (2022).
2.3.3 Suitable habitat division of A. dahurica
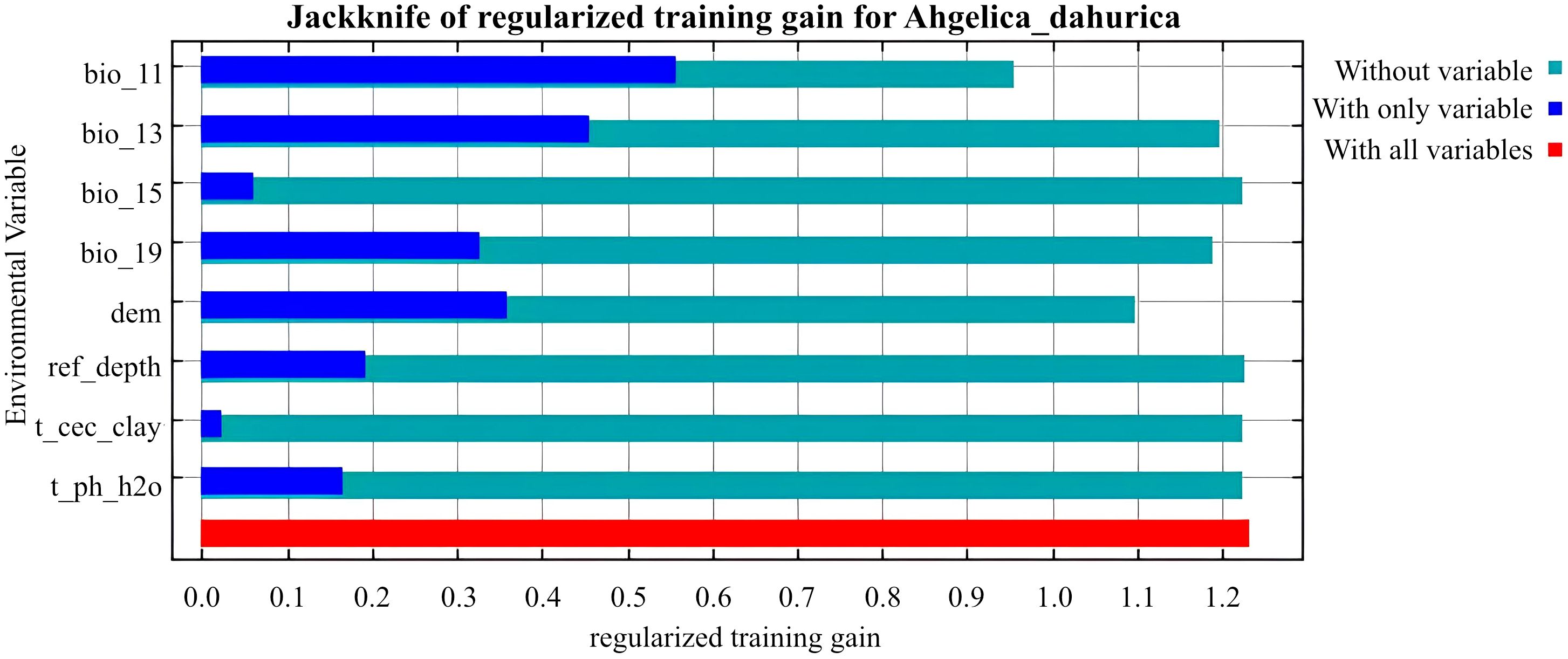
In this study, ArcGIS Pro (Version 3.0.2) was used to classify the habitat suitability of A. dahurica using the natural breakpoint method Zhao et al. (2024); Zheng et al. (2024); Zhao et al. (2021b). Suitability was categorized into four levels: high, medium, low, and unsuitable habitat. The jackknife method was used to assess the relative influence of environmental variables. The key variables affecting A. dahurica distribution and their suitable ranges were identified based on variable contribution rates and permutation importance Wang et al. (2024a).
2.4 Changes in the core distribution
ArcGIS Pro was used to analyze changes in A. dahurica’s suitable habitat over time. Regional data from three periods were simplified into vector cores, and the change trend of the suitable area was calculated. Centroid shifts were used to indicate the migration direction of suitable habitats Qiu et al. (2022); Zhan et al. (2022).
2.5 Materials treatment and HPLC analysis
28 batches of A. dahurica samples were washed, dried, and ground. Approximately 1g of powder was accurately weighed into a 50mL conical flask, followed by the addition of 25mL methanol. After weighing, the sample was sonicated (300W, 40kHz) for 1h, removed and cooled, the weight was adjusted, and the filtrate was collected after shaking and filtering. The resulting filtrate was passed through a 0.22µm microporous membrane and collected for further analysis. Standard solutions of imperatorin, imperatorin oxide, isoimperatorin, hydrated imperatorin oxide, and bergapten were prepared by dissolving accurately weighed amounts in methanol to achieve final concentrations of 0.1028 mg/mL, 0.1239 mg/mL, 0.0519 mg/mL, 0.0258 mg/mL, and 0.00968 mg/mL, respectively.
In this study, the contents of five coumarins were determined using HPLC on an Agilengt 1200 Infinity LC high performance liquid chromatograph (Agilent) and WondaSil C1 8-WR (250×4.6mm) chromatographic column (Shimadzu Jiyou Trading Co., Ltd.). The mobile phase was methanol (A), tetrahydrofuran (B) and pure water (C), gradient elution (0 ∼ 10 min, 32% A, 8% B;10 ∼ 45 min, 32% ∼ 53% A, 8% ∼ 12% B; 45 ∼ 55 min, 53% A, 12% B; 55 ∼ 65 min, 53% ∼ 32% A, 12% ∼ 8% B), flow rate was 1.0 ml/min, detection wavelength 305 nm, column temperature 25°C, injection volume 10µl. By comparing the retention time wit standards, the isolated compounds were identified, and quantitative analysis was performed using peak area measurements.
2.6 HCA analysis
HCA (Hierarchical Cluster Analysis) is a commonly used unsupervised clustering analysis method, which divides samples into different clusters by calculating the similarity between samples. The ward method clusters by minimizing the variance within each cluster. In this study, the content data of the five coumarins measured in HPLC were standardized, and the ward method was used for cluster analysis and the dendrogram of the results was drawn using a Python program.
2.7 Construct a model of the relationship between content and environmental variables
Multiple regression analysis is a statistical method for modeling the relationship between a dependent variable and multiple independent variables. Stepwise regression refines this process by systematically adding or removing variables to identify the most significant predictors and construct the best predictive model. In this study, ArcGIS Pro was used to extract eight environmental variables from the coordinates of 28 A. dahurica samples. R was then used to perform multiple stepwise regression analysis, correlating environmental variables with the measured content of imperatorin, imperatorin oxide, isoimperatorin, hydrated imperatorin oxide, and bergapten in A. dahurica. This analysis established a relationship model between chemical composition and environmental variables. Using raster calculation and mapping functions in ArcGIS Pro, unsuitable areas from the ecological suitability prediction were removed. The spatial distribution of the five coumarins in A. dahurica under current and future climate conditions was then predicted and visualized.
3 Results
3.1 Model evaluation
The default FC in MaxEnt is LQHPT with a RM of 1, yielding a delta.AICc of 28.18 and an Auc.Diff.Avg of 0.01956145. When FC is set to LQH and RM to 2, delta.AICc is 0, and Auc.Diff.Avg is 0.01983218, indicating an optimal model (Table 3). The average training AUC for the replicate runs(10 repetitions) is 0.906, and the standard deviation is 0.006 (Figure 3), confirming the model’s predictive accuracy. Compared to the default model, the response curve of the optimized model, adjusted using the ENMeval package, is significantly smoother and aligns more closely with a normal distribution, consistent with Shelford’s tolerance law Shelford (1931); Zhao et al. (2021a).
3.2 Analysis of environmental variables
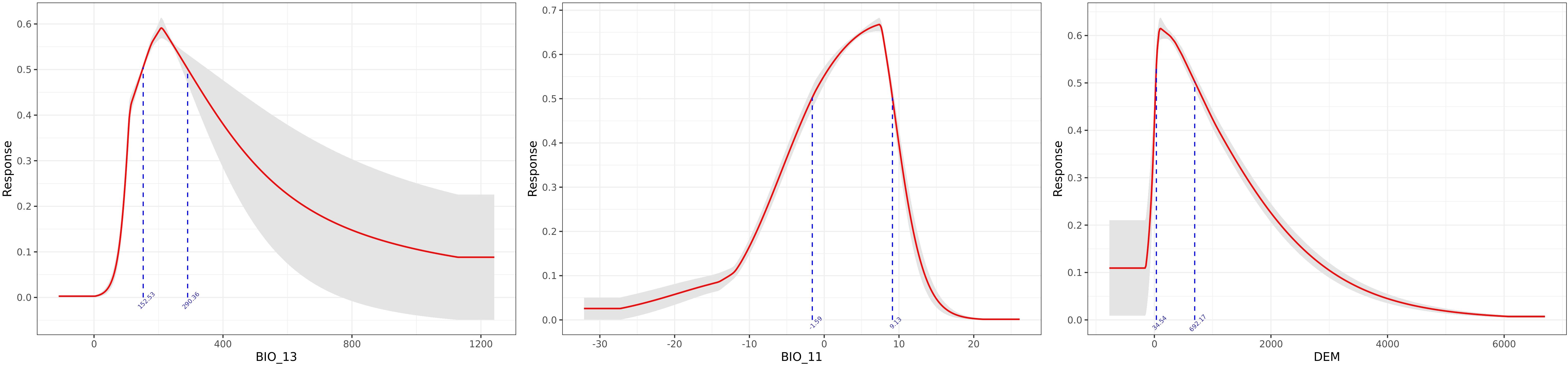
As shown in Table 2, BIO_13, BIO_11, and DEM are the key environmental variables influencing the distribution of A. dahurica. Their cumulative contribution rate and permutation importance reach 72.3% and 77.6%, respectively. BIO_13 has the highest contribution rate, while BIO_11 has the greatest permutation importance. When analyzed individually, temperature and precipitation related variables (such as BIO_13 and BIO_11) carry the highest weights (Figure 4). As shown in Figure 5, the three response curves follow a unimodal distribution.
3.3 Prediction of distribution under current climate situation
The results indicate that the average suitability of the 246 distribution records is 0.530025, with a maximum of 0.857906 (Shehong City, Suining, Sichuan Province) and a minimum of 0.052913 (Hulunbuir, Inner Mongolia). Under the current climate scenario, the total suitable habitat area (highly and mediumly suitable) is 89.11020 × 104 km2 (Figures 6A), with highly suitable areas covering 19.54812 × 104 km2. These are primarily located in Guangyuan, Bazhong, Dazhou, Nanchong, Suining, and Mianyang (Sichuan); Luoyang, Jiaozuo, Zhengzhou, Hebi, and Anyang (Henan); and Handan, Shijiazhuang, and Baoding (Hebei). Mediumly suitable habitats span 69.56208 × 104 km2, surrounding highly suitable regions, including western Chongqing, central and southern Henan, and western Shandong (Figure 6B).
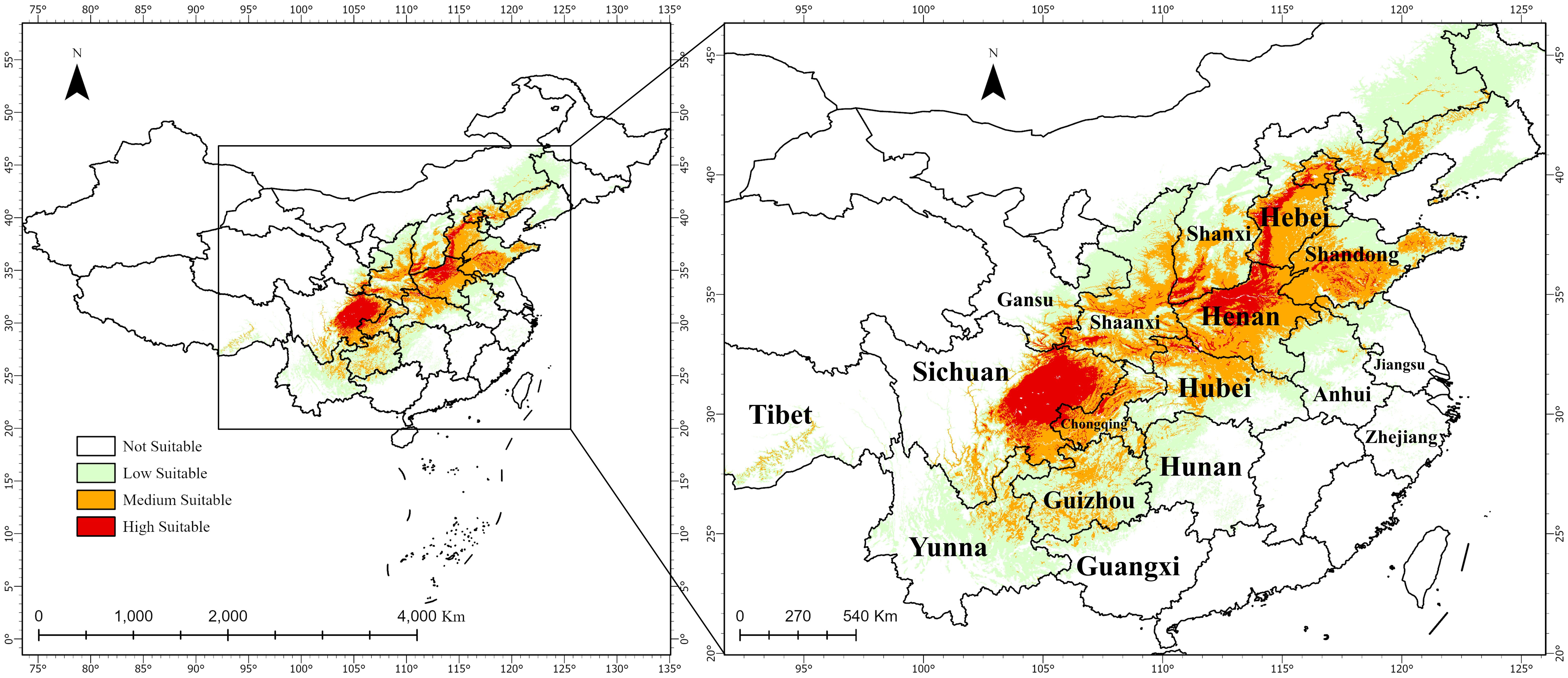
Figure 6. Distribution of suitable habitats for A. dahurica in China under current climate scenarios.
3.4 Predict the future distribution of A. dahurica
3.4.1 Future changes in suitable habitat for A. dahurica
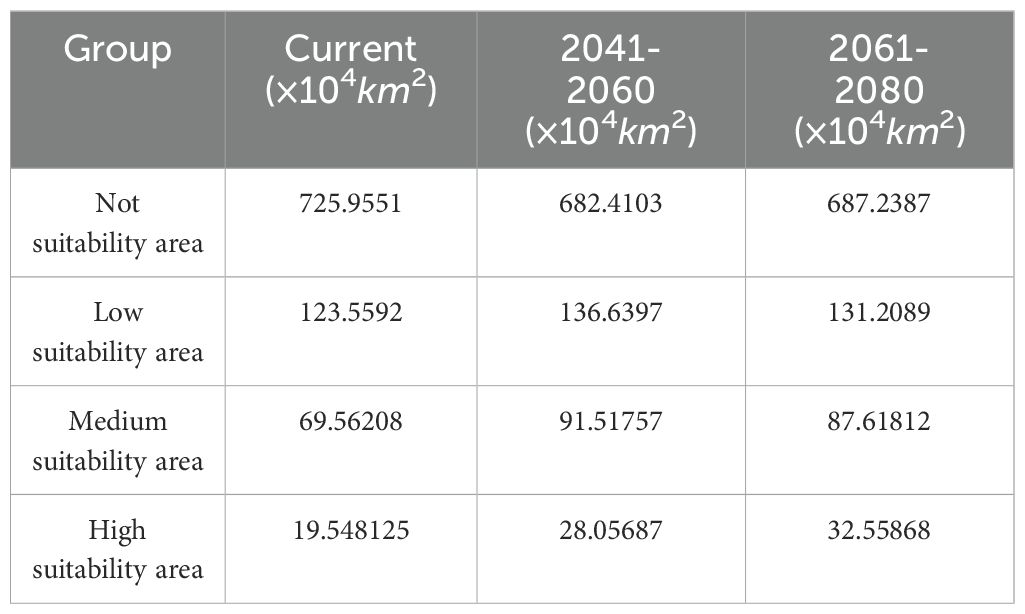
Figure 7 and Table 4 illustrate future changes in A. dahurica’s suitable habitats. Under different climate scenarios, highly, mediumly, and lowly suitable areas will expand or contract to varying degrees. Compared to the current climate: Under the 2041–2060 scenario, highly, mediumly, and lowly suitable habitats will increase by 8.50875 × 104 km2, 21.95549 × 104 km2, and 13.0805 × 104 km2, respectively; Under the 2061–2080 scenario, these areas will increase by 13.01056 × 104 km2, 18.05604×104 km2, and 7.6497×104 km2, respectively (Table 4). Overall, highly suitable habitats are projected to expand over time, allowing A. dahurica to grow and reproduce across a wider range, increasing its population and distribution. While medium and low suitability areas fluctuate, some regions in northern Hubei, central and southern Shaanxi, and Shandong may transition into highly suitable habitats.
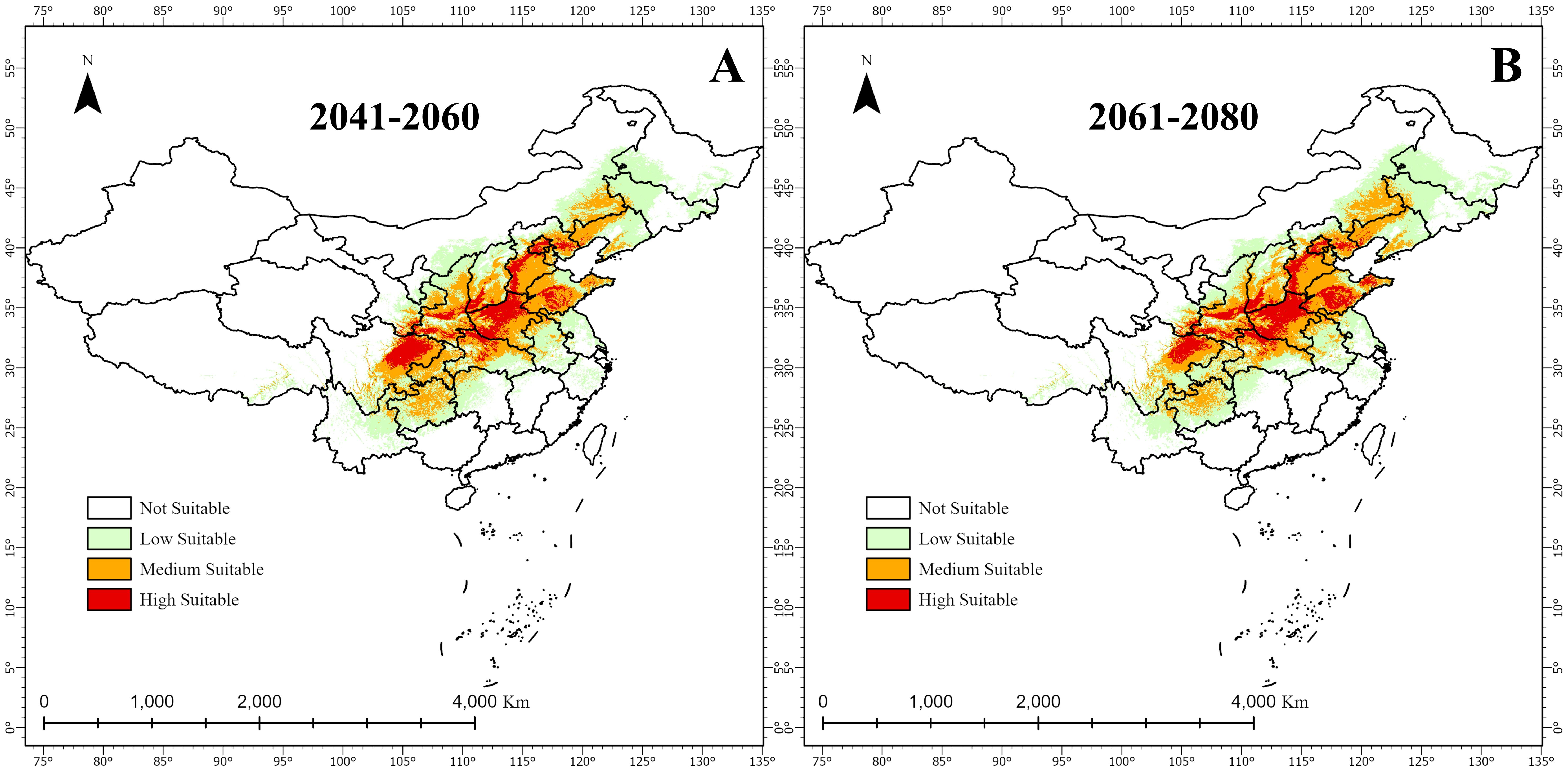
Figure 7. Future changes in suitable habitats for A. dahurica in China; (A) 2041-2060; (B) 20612080.
3.4.2 Changes in core locations of suitable habitat
Currently, the core of the suitable habitat for A. dahurica is located in Yunxi County, Shiyan City, Hubei Province (110.54221, 33.209579). It is predicted that under the 2041–2060 climate scenario, the core of the suitable habitat will migrate to Yichuan County, Luoyang City, Henan Province (112.349646, 34.386318), migration distance is 211.78 km, and under the 2061–2080 climate scenario, it will migrate to Wen County, Jiaozuo City, Henan Province (113.01311, 34.884234), migration distance is 82.32 km, as shown in Supplementary Figure S1.
3.5 Analysis of coumarin content by HPLC
HPLC results show variability in the five coumarin contents across the 28 sample batches (Figure 8A). Except for S3 from the mediumly suitable area, imperatorin content in all other samples (Figure 8A; dark blue) meets the PPRC standard (≥ 0.080%)State pharmacopoeia committee (2020). Apart from isoimpermen (Figure 8B; green), the average content of the other four coumarins is higher in samples from highly suitable areas compared to those from moderate and low suitability regions. This suggests that A. dahurica grown in highly suitable areas has superior quality. Additionally, isoimpermen content does not show a consistent increase with habitat suitability.
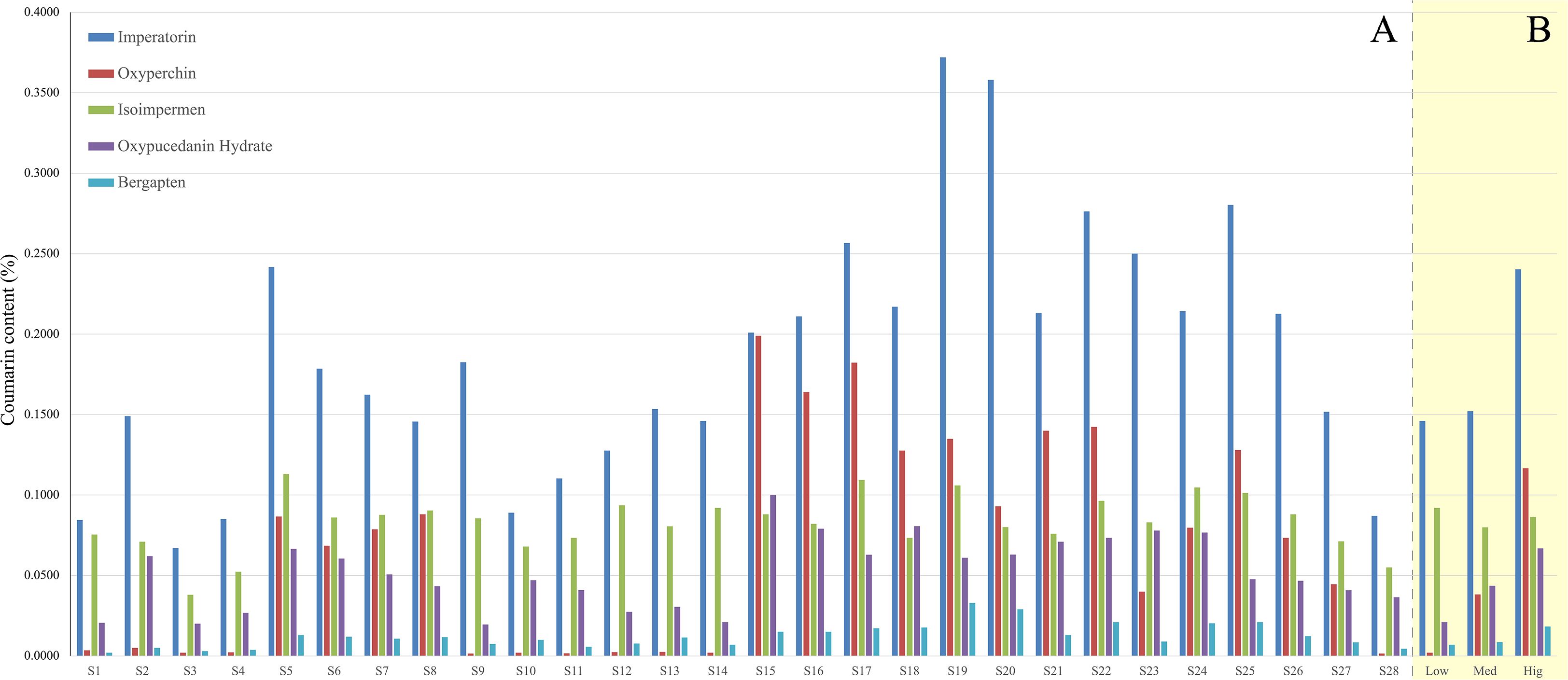
Figure 8. (A) The coumarin content of A. dahurica in each sample; (B) Average coumarin content of A. dahurica in different suitable areas (Low, low suitable area; Med, median suitable area; Hig, high suitable area).
3.6 Analysis of HCA
The HCA results (Figure 9) show that when the critical value approaches 6, the 28 sample batches are divided into two distinct clusters. Samples S5, S6, S7, S8, S17, and S23 from the mediumly suitable area cluster with those from the highly suitable area (Figure 9A; blue), while the remaining samples form a separate cluster. However, Figure 8B (green) reveals that isoimpermen content does not correlate with habitat suitability, suggesting it may influence clustering results. After removing isoimpermen data and reanalyzing, the clustering improved: at a critical value of 6, only three samples (S17, S26, S28) were misclassified (Figure 9B). This revised classification more accurately reflects the actual distribution, with highly suitable area samples forming one cluster and moderately or low suitable area samples forming another.
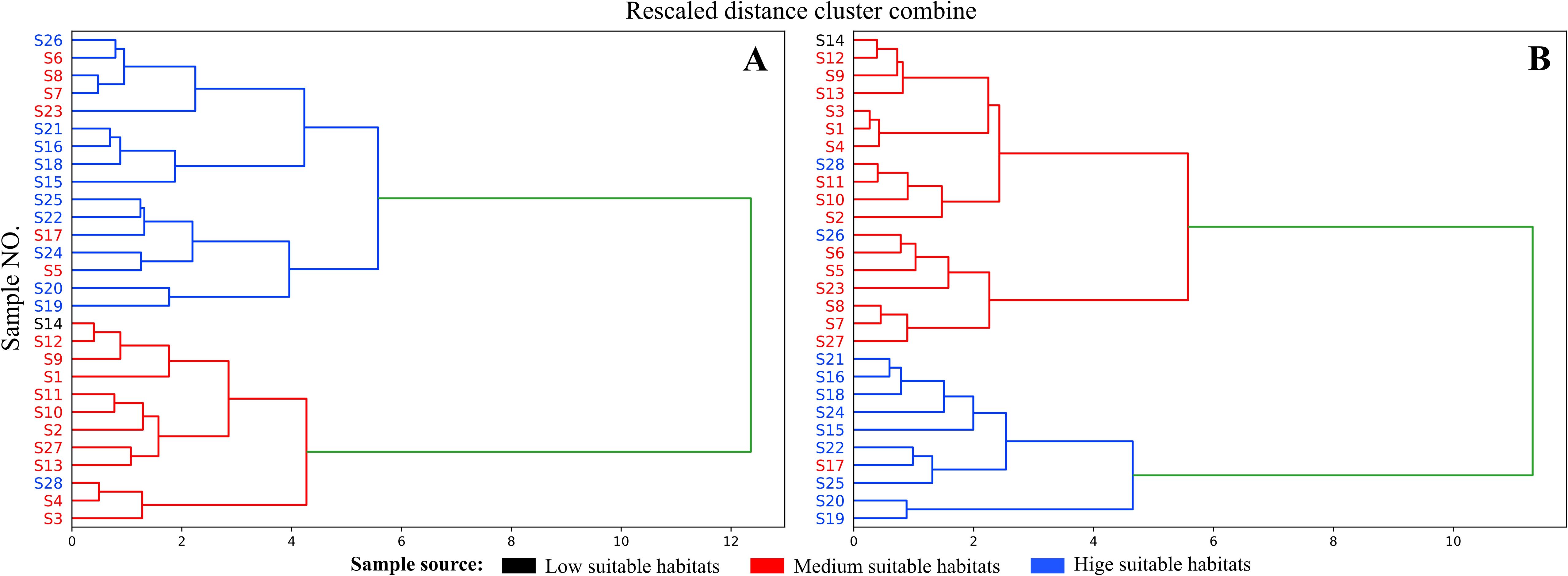
Figure 9. (A) Cluster results obtained using five coumarin contents; (B) Cluster results obtained after removing Isomperatorin.
3.7 Analysis of the relationship between environmental variables and contents of chemical components
HPLC data and the 8 environmental variables used in MaxEnt were analyzed using multiple stepwise regression to establish relationship models between chemical components and environmental variables (Equations 1–5). Each model has a P-value < 0.001, indicating strong statistical significance and suitability for predicting component content. The models show that: Imperatorin (Equation 1) is primarily influenced by BIO_11, BIO_13, and T_PH_H2O; Oxypeucedanin (Equation 2) is affected by BIO_11, BIO_19, DEM, and T_CEC_CLAY; Isoimperatorin (Equation 3) is influenced by BIO_11, BIO_19, DEM, and T_PH_H2O; Oxypeucedanin hydrate (Equation 4) is mainly affected by BIO_11, BIO_13, BIO_15, and T_CEC_CLAY; Bergapten (Equation 5) is influenced by BIO_11, BIO_15, DEM, and T_PH_H2O. Notably, BIO_11 affects all five coumarins, while DEM influences multiple components, highlighting their key roles in A. dahurica metabolite production.
3.8 Spatial distribution of relative content of chemical components
Based on the relationship model between content and environmental variables, obtain spatial distribution maps of the relative content of various coumarin components in suitable habitats areas at different periods. As shown in Figure 10, coumarin content in highly suitable habitats differs significantly from that in other suitable areas. Comparing the distribution maps (Figure 10A) reveals that imperatorin (Figure 10B) and oxypucedanin hydrate (Figure 10E) are more abundant in highly suitable areas, while isoimpermen (Figure 10C), oxyperchin (Figure 10D), and bergapten (Figure 10F) are less concentrated. Additionally, all five coumarins share a common trend: higher relative content in high-altitude regions, particularly at the junction of Yunnan, Guizhou, and Sichuan.
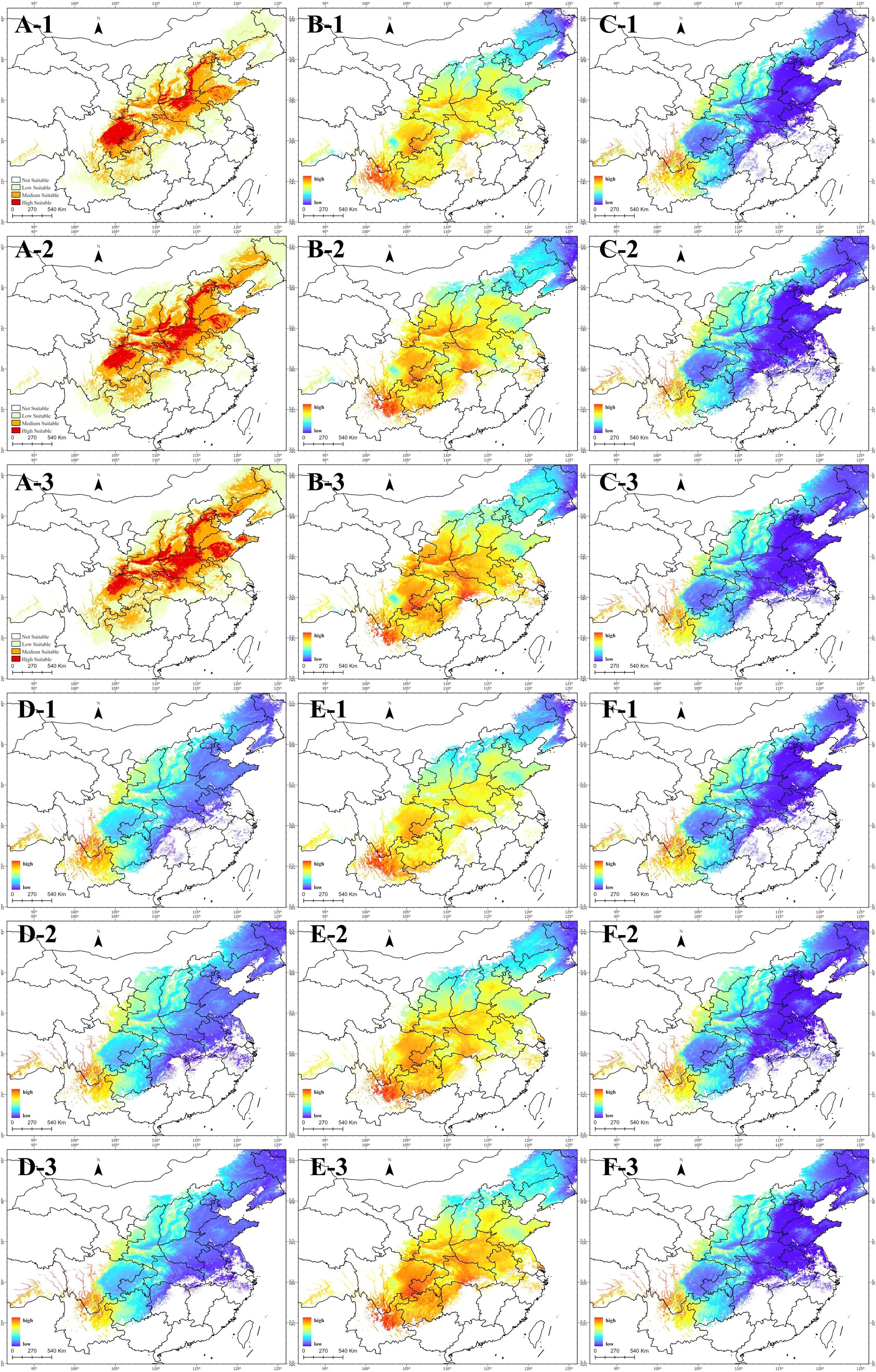
Figure 10. (A) Suitable area; (B) Relative content of Imperatorin; (C) Relative content of Isoimpermen; (D) Relative content of Oxyperchin; (E) Relative content of Oxypucedanin Hydrate; (F) Relative content of Bergapten; 1, current; 2, 2041-2060; 3, 2061-2080.
4 Discussion
4.1 Key environmental variables affecting A. dahurica distribution
A. dahurica is highly adaptable, thriving in mild, humid climates with ample sunlight. It has strong cold resistance but does not tolerate high temperatures. Based on response curves with values exceeding 0.5, several key environmental thresholds were identified: (1) The value range of BIO_13 is 152.53 ∼ 290.36mm, indicating that A. dahurica more suitable for growth in environments with concentrated seasonal precipitation, which is in line with A. dahurica’s growth characteristics of liking humid environments. The range of values shows that the precipitation does not exceed 300mm, indicating that extreme heavy rainfall may need to be taken into account when planting A. dahurica. In fact, in the traditional planting area of A. dahurica in the Sichuan Basin, the accumulation of rainy water in summer may pose a risk of waterlogging, requiring well drained soil and flood prevention measures; (2) The range of BIO_11 is −1.59 ∼ 9.13°C, which is consistent with the good cold resistance and preference for mild temperatures of A. dahurica, and indicates that it may not be suitable for climates that are too warm or too cold in winter; (3) The range of DEM values is 34.54 ∼ 692.17m, indicating that A. dahurica suitable for growth in low altitude (such as plains) to medium altitude (such as hills and mountains) areas. The increase in altitude in a region often has an impact on temperature (for every 100m increase in altitude, the temperature drops by 0.6°C), so there is an important connection between DEM and BIO_11. Within the range of values, when the altitude is high, A. dahurica will be in a lower temperature environment, and the average temperature in the coldest quarter may be close to the lower limit of the range, while at low altitudes it may be close to the upper limit. In addition, different altitudes can also form different terrains, such as windward slopes, leeward slopes, basins, etc., resulting in different climates, which is the main reason affecting precipitation. Considering three key variables, the ideal climate type for the growth environment of A. dahurica should be the monsoon climate zone, which includes the actual planting areas of A. dahurica such as Suining (subtropical humid monsoon climate), Yuzhou (warm temperate semi humid continental monsoon climate), Anguo (temperate monsoon climate), etc. The key variables selected by the model results of this study, although different from previous studies, are consistent with the actual situation and have high reliability.
4.2 Changes of suitable habitat for A. dahurica
In recent years, annual consumption has risen significantly, with sales exceeding 1000 tons in cities like Zhengzhou (Henan), Tengzhou (Shandong), Wuhan (Hubei), Chengdu (Sichuan), and Yulin (Guangxi). The demand for A. dahurica as a spice alone exceeds 8000 tons annually (data from www.zyctd.com). With the stimulation of the market, the planting area of A. dahurica in various regions is also increasing. For example, as of 2024, the city of Suining, known as the “hometown of Chinese white atractylodes”, will have a planting area of 25000 mu of white atractylodes, and is expected to increase to 30000 mu by 2025. But The planting area of traditional Chinese medicinal materials cannot determine the annual production capacity, and changes in environmental factors can lead to a decrease in yield per mu or even complete crop failure. For example, in low-lying areas such as Shangqiu in Henan and Bozhou in Anhui, due to abnormal weather, the growth period of A. dahurica shows a pattern of drought followed by flooding. These regions are on the edge of moderate suitability zones and adjacent to low suitability zones under the current climate background in this study. With future climate change, these areas will still be in moderate suitability zones, but adjacent areas may transition from low suitability zones to moderate suitability zones, which will have a positive impact on the cultivation of A. dahurica. At present, the emerging production area of Anhui, relying on China’s largest traditional Chinese medicine trading market, has become the largest main production area and distribution center of A. dahurica in the country, and is actively expanding its cultivation, which is consistent with the suitable area expansion direction predicted in this study, verifying the reliability of this study.
4.3 Environmental influence on the chemical composition of A. dahurica
Quality control is vital for ensuring both the therapeutic efficacy and economic value of medicinal plants. Studies indicate that coumarin compounds, particularly imperatorin and isoimperatorin, are key indicators of A. dahurica quality. Environmental variables influence the volatile composition and coumarin content of A. dahurica from different regions, affecting its pharmacological properties Sun et al. (2024); Zhang et al. (2019); Zhou et al. (2015). HPLC analysis based on the samples collected in the field, and the results showed that in the high suitable habitats, A. dahurica accumulated a relatively high content of coumarin overall. This indicates that under optimal growth conditions, plants can obtain more sufficient resources for metabolic synthesis, thereby improving the overall level of effective components. This makes A. dahurica from the high suitable habitats have better quality.
The constructed regression relationship model integrates the environmental data of the entire suitable area, revealing that different coumarins respond differently to environmental variables. Notably, BIO_11 is shown to influence the content of all coumarins examined in this research. Previous studies report that A. dahurica from southern Sichuan (low suitability area in this study) contains higher total coumarin, imperatorin, and isoimperatorin levels than samples from Yuzhou, Henan (high suitability area in this study) Zhang et al. (2024b). These findings align with predicted spatial distribution of coumarin content in this study. That is, although the total coumarin content in highly suitable areas is generally higher, the relative content of specific single components may not always be positively correlated with growth suitability. This may be due to: (1) Differences in the biosynthetic pathways and regulatory mechanisms of different coumarins; (2) In low suitable areas, some stress pressures may induce the activation of specific secondary metabolic pathways, thereby relatively increasing the content of some coumarins. For example, in plants such as tomatoes and tea trees, stress pressures often promote the accumulation of antioxidants such as flavonoids and phenols, which are considered to be adaptive strategies for plant self-protection Nakabayashi et al. (2014); Dixon and Paiva (1995). The combination of the two reveals the multi-level impact of environmental variables on the accumulation of chemical components in A. dahurica, that is, not only affecting the absolute content of the overall effective components, but also regulating the proportional distribution of each component. This finding provides a reference for the actual expansion of the planting area: when selecting the planting area, it is necessary to pay attention not only to the overall growth suitability, but also to consider the specific performance of each component under different environments, so as to achieve refined management of medicinal material quality. These results suggest that different environmental pressures may induce plants to initiate specific secondary metabolic responses, which have implications for further research on the ecological adaptation and metabolic regulation mechanisms of A. dahurica and other medicinal plants.
5 Conclusion
This study is the first to integrate MaxEnt with chemometrics to systematically explore the suitable habitat distribution of A. dahurica and its relationship with coumarin content and environmental variables. The key findings are as follows:
1. Environmental Influences: BIO_13, BIO_11, and DEM are the primary factors determining the distribution of A. dahurica.
2. Current Habitat Distribution: The most suitable habitats are concentrated in Sichuan, Henan, and Hebei.
3. Future Habitat Shifts: Under climate change scenarios, suitable regions will expand, with core distribution areas shifting toward Henan and Jiaozuo. Northern Hubei, central and southern Shaanxi, and parts of Shandong may transition from medium or low suitability to high suitability.
4. Chemical Composition Differences: HPLC and HCA analysis indicate that A. dahurica from highly suitable habitats contains higher total coumarin content than those from medium suitability regions. In addition, different coumarin compounds exhibit different content distributions, which can be used to classify A. dahurica, and this classification is consistent with habitat suitability.
5. Environmental Correlation: Multiple stepwise regression analysis reveals a significant correlation between five coumarin compounds and environmental variables, particularly BIO_11 and DEM, highlighting their impact on secondary metabolite accumulation.
6. Spatial Distribution of Coumarins: Different coumarin compounds exhibit varying spatial patterns, suggesting that habitat differences influence chemical composition.
Despite the promising insights provided by this study, several limitations should be acknowledged. First, the sample collection was primarily concentrated in moderately to highly suitable habitats, while low-suitability areas were underrepresented. This may limit the robustness of the regression models and the generalizability of chemical-environment relationships across the full ecological gradient of A. dahurica. Future studies should include more diverse sampling from marginal or low-suitability regions to enhance the comprehensiveness of spatial predictions.
Second, while the MaxEnt model offers high predictive performance based on presence-only data, it does not account for biotic interactions, land use changes, or anthropogenic disturbances, which may significantly influence the actual distribution of A. dahurica. Integrating land use data, socio-economic factors, and climate-resilient agricultural modeling would enhance future habitat suitability assessments.
Moreover, the current study focused primarily on five coumarin compounds. However, A. dahurica contains a wide spectrum of secondary metabolites, including volatile oils and polysaccharides, which also contribute to its pharmacological activity. Expanding the chemical spectrum and integrating metabolomics or transcriptomics approaches could provide a more holistic understanding of the plant’s adaptive responses.
Looking forward, this integrative framework combining species distribution modeling with chemometric analysis offers a valuable tool for ecological zoning, medicinal resource conservation, and precision cultivation. With the increasing impact of climate change, this approach could support dynamic planning of cultivation areas, ensuring both the quality and sustainability of A. dahurica resources. Additionally, the methodology can be extended to other traditional Chinese medicinal plants, paving the way for eco-pharmacological zoning and quality control strategies in herbal medicine production.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZG: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. JM: Data curation, Investigation, Writing – review & editing. XL: Writing – original draft. JXL: Writing – original draft. JKL: Writing – original draft. LP: Supervision, Writing – review & editing. GJ: Supervision, Writing – review & editing. YL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No: 82173928), Sichuan Provincial Natural Science Foundation Project (No: 2024NSFSC0704) and Chengdu University of Traditional Chinese Medicine ‘Xinglin scholar’ subject talent research promotion plan (No: QJJJ2022025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the authors used ChatGPT and Deepseek to enhance the readability of this article. After using these tools, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1600491/full#supplementary-material
References
Abdulwahab, U. A., Hammill, E., and Hawkins, C. P. (2022). Choice of climate data affects the performance and interpretation of species distribution models. Ecol. Model. 471, 110042. doi: 10.1016/j.ecolmodel.2022.110042
Aqib, M., Khatoon, S., Ali, M., Sajid, S., Assiri, M. A., Ahamad, S., et al. (2025). Exploring the anticancer potential and mechanisms of action of natural coumarins and isocoumarins. Eur. J. Med. Chem. 282, 117088. doi: 10.1016/j.ejmech.2024.117088
Baldwin, R. A. (2009). Use of maximum entropy modeling in wildlife research. Entropy 11, 854–866. doi: 10.3390/E11040854
Blois, J. L., Zarnetske, P. L., Fitzpatrick, M. C., and Finnegan, S. (2013). Climate change and the past, present, and future of biotic interactions. Science 341, 499–504. doi: 10.1126/science.1237184
Cahyaningsih, R., Phillips, J., Brehm, J. M., Gaisberger, H., and Maxted, N. (2021). Climate change impact on medicinal plants in Indonesia. Global Ecol. Conserv. 30. doi: 10.3389/fphar.2024.1496792
Chen, L., Tang, Z.-S., Song, Z.-X., Liu, Y.-R., Hu, J.-H., Shi, X.-B., et al. (2019). Quantitative determination of nine furanocoumarins for quality evaluation of Angelica dahurica from different habitats. Zhongguo. Zhong. Yao. Za. Zhi. 44, 3002–3009. doi: 10.19540/j.cnki.cjcmm.20190505.102
Dixon, R. A. and Paiva, N. L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell 8, 1085–1097. doi: 10.1105/tpc.7.7.1085
GEBCO Bathymetric Compilation Group (2023). The GEBCO_2023 grid - a continuous terrain model of the global oceans and land. doi: 10.5285/f98b053b-0cbc-6c23-e053-6c86abc0af7b
Hussain, M. K., Ahmad, M., Khatoon, S., Khan, M. V., Azmi, S., Arshad, M., et al. (2025). Phytomolecules as alzheimer’s therapeutics: A comprehensive review. Eur. J. Med. Chem. 288, 117401. doi: 10.1016/j.ejmech.2025.117401
Hussain, M. K., Khatoon, S., Khan, M. F., Akhtar, M. S., Ahamad, S., and Saquib, M. (2024). Coumarins as versatile therapeutic phytomolecules: A systematic review. Phytomedicine 134, 155972. doi: 10.1016/j.phymed.2024.155972
Hussain, M. K., Singh, D. K., Singh, A., Asad, M., Ansari, M. I., Shameem, M., et al. (2017). A novel Benzocoumarin-Stilbene hybrid as a DNA ligase I inhibitor with in vitro and in vivo anti-tumor activity in breast cancer models. Sci. Rep. 7, 10715. doi: 10.1038/s41598-017-10864-3
Kass, J. M., Muscarella, R., Galante, P. J., Bohl, C. L., Pinilla-Buitrago, G. E., Boria, R. A., et al. (2021). Enmeval 2.0: Redesigned for customizable and reproducible modeling of species’niches and distributions. Methods Ecol. Evol. 12, 1602–1608. doi: 10.1111/2041-210X.13628
Li, Y., Kong, D., Fu, Y., Sussman, M. R., and Wu, H. (2020). The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 148, 80–89. doi: 10.1016/j.plaphy.2020.01.006
Lin, S., Yao, D., Jiang, H., and Feng, Z. (2023). Prediction and analysis of potential suitable habitats of bandicota indica in China based on optimized maxent model. Plant Prot. 49, 92–100. doi: 10.16688/j.zwbh.2022400
Lu, Y., Guo, S., Yan, H., Zhang, F., Wang, H., Jin, L., et al. (2020). Regionalization of production of medicinal and edible fruit of lycium barbarum associated with ecological factors and chemical constituents. Acta Pharm. Sin. 55, 2466–2477. doi: 10.16438/j.0513-4870.2020-0572
Lu, L. and Liu, C. (2019). Chinese soil dataset based on the World Soil Database (HWSD). doi: 10.12072/ncdc.westdc.db3647.2023
Ma, Y., Guo, D., Jiang, G., Xiao, D., Gao, Y., Tang, S., et al. (2009). Investigation report on the germplasm resources of Angelica dahurica. West. China J. Pharm. Sci. 24, 457–460. doi: 10.13375/j.cnki.wcjps.2009.05.021
Mengyue, W. (2003). Study on the ancient and modern medicinal overview of Angelica dahurica and coumarin components (Chengdu University of Traditional Chinese Medicine). doi: 10.7666/d.y569296
Nakabayashi, R., Mori, T., and Saito, K. (2014). Alternation of flavonoid accumulation under drought stress inarabidopsis thaliana. Plant Signaling Behav. 9, e29518. doi: 10.4161/psb.29518
Pant, P., Pandey, S., and Dall’Acqua, S. (2021). The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 18, e2100345. doi: 10.1002/cbdv.202100345
Phillips, S. J., Dudík, M., and Schapire, R. E. (2025). Maxent software for modeling species niches and distributions. http://biodiversityinformatics.amnh.org/open_source/maxent/
Qiu, X., Zhang, Y., and Zhang, Y. (2022). Effects of global climate change on the potential suitable habitats of salvia miltiorrhiza bunge. Chin. J. Inf. Tradit. Chin. Med. 29, 1–10. doi: 10.19879/j.cnki.1005-5304.202201398
Robiansyah, I., Primananda, E., Zulkarnaen, R. N., Helmanto, H., Kusuma, Y. W. C., and Yudaputra, A. (2023). “Climate change impact on medicinal plants: An insight from the iucn red list of threatened species,” in Medicinal Plants: Biodiversity, Biotechnology and Conservation (Singapore: Springer), 115–131. doi: 10.1007/978-981-19-9936-9_4
State pharmacopoeia committee (2020). Chinese pharmacopoeia(I) (Beijing: China Pharmaceutical Science and Technology Press).
Sun, J., Gan, Z., Kang, D., Wang, Y., Lian, Y., and Jiang, G. (2024). Establishment of fingerprints and spectrum-effect(relieving migraine)relationship of baizhi(白芷)produced in different regions. Pharmacol. Clinics Chin. Mater. Med. 40, 65–70. doi: 10.13412/j.cnki.zyyl.20240712.001
Wang, X., Dua, n., Y., Jin, L., Wang, C., Peng, M., Li, Y., et al. (2023). Prediction of historical,present and future distribution of quercus sect.heterobalanus based on the optimized maxent model in China. Acta Ecol. Sin. 43, 6590–6604. doi: 10.5846/stxb202205141353
Wang, N., Huang, L., Yang, B., Makiba, K., Yokouchi, M., Yuan, C., et al. (2001). Determination of content of nodakenin in notopterygium incisum ting from different source by hplc. China J. Chin. Mater. Med. 26, 733–736. doi: 10.3321/j.issn:1001-5302.2001.11.004
Wang, E., You, L., Yi, Z., and Du, W. (2024a). Prediction of potential distribution of phragmites australis in China based on maxent. Hunan. Agric. Sci. 2024, 66–72. doi: 10.16498/j.cnki.hnnykx.2024.008.014
Wang, L., Zhao, J., Wang, Y., Mi, Y., Zhang, N., Zhao, M., et al. (2024b). Spatial distribution of cultivation suitable area for panax notoginseng and its response to climate change. Acta Agronom. Sin. 50, 2860–2869. doi: 10.3724/SP.J.1006.2024.44005
Wang, Y., Zhao, J., Weng, Q., Jin, Y., Zhang, W., Peng, H., et al. (2020). Textual research on classical prescriptions of Angelicae dahuricae radix. Modern. Chin. Med. 22, 1320–1330. doi: 10.13313/j.issn.1673-4890.20200528006
Xu, R., Wu, J., Dong, L., Xu, J., Chen, P., Liu, M., et al. (2018). Analysis of global ecology of acanthopanax senticosus in suitability and quality. Acta Pharm. Sin. 53, 313–320. doi: 10.16438/j.0513-4870.2017-1062
Yang, Z., Wei, Y., Guo, L., and Cheng, L. (2025). Prediction of the distribution of potential habitat areas of ageratina adenophora in China based on an optimized maxent model. J. Inner. Mongolia. Agric. University(Natural. Sci. Edition). 46, 49–56. doi: 10.16853/j.cnki.1009-3575.2025.01.007
Zhan, P., Wang, F., Xia, P., Zhao, G., Wei, M., Wei, F., et al. (2022). Assessment of suitable cultivation region for panax notoginseng under different climatic conditions using maxent model and high-performance liquid chromatography in China. Ind. Crops Products. 176, 114416. doi: 10.1016/j.indcrop.2021.114416
Zhang, F.-G., Liang, F., Wu, K., Xie, L., Zhao, G., and Wang, Y. (2024a). The potential habitat of Angelica dahurica in China under climate change scenario predicted by maxent model. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1388099
Zhang, S., Wang, J., Sheng, Y., Li, S., Pan, Y., and Ao, H. (2019). Comparative study on the volatile constituents of baizhi from six different producing regions. Storage. Process. 19, 176–183. doi: 10.3969/j.issn.1009-6221.2019.04.028
Zhang, J., Yan, Y., Wu, Q., Huang, C., Du, J., Huang, X., et al. (2024b). Quality evaluation and suitable harvest period of different germplasm of Angelicae dahuricae radix. Chin. Tradit. Herbal. Drugs 55, 5245–5255. doi: 10.7501/j.issn.0253-2670.2024.15.024
Zhao, G., Cui, X., Sun, J., Li, T., Wang, Q., Ye, X., et al. (2021a). Analysis of the distribution pattern of chinese ziziphus jujuba under climate change based on optimized biomod2 and maxent models. Ecol. Indic. 132, 108256. doi: 10.1016/j.ecolind.2021.108256
Zhao, G., Cui, X., Wang, Z., Jing, H., and Fan, B. (2021b). Prediction of potential distribution of ziziphus jujuba var. spinosa in China under context of climate change. Sci. Silvae Sin. 57, 158–168. doi: 10.11707/j.1001-7488.20210618
Zhao, H., Feng, Y.-L., Wang, M., Wang, J.-J., Liu, T., and Yu, J. (2022). The Angelica dahurica: A review of traditional uses, phytochemistry and pharmacology. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.896637
Zhao, Q., Mou, D., Laba, C., Cai, J., Lamao, Y., Wu, X., et al. (2024). Distribution and prediction of hazard area of Aconitum gymnandrum in Qinghai Province. Grassland and Turf. 45 (1), 161–172. doi: 10.13817/j.cnki.cyycp.2025.01.019
Zheng, M., Song, Y., Li, C., Na, M., Wu, Y., Ma, J., et al. (2024). Analysis of potential geographic distribution of solanum rostratum based on optimized maxent model in agro-pastoral ecotone of northern China. Acta Agrestia. Sin. 32, 3905–3914. doi: 10.11733/j.issn.1007-0435.2024.12.026
Keywords: Angelica dahurica, chemometrics, MAXENT model, HPLC, environmental variables, coumarin, multiple stepwise regression
Citation: Gan Z, Ma J, Liu X, Luo J, Li J, Pu L, Jiang G and Lian Y (2025) Integrating MaxEnt with chemometrics to evaluate the impact of environmental variables on the coumarin content and the distribution of Angelica dahurica. Front. Plant Sci. 16:1600491. doi: 10.3389/fpls.2025.1600491
Received: 26 March 2025; Accepted: 12 June 2025;
Published: 07 July 2025.
Edited by:
Tao Zhou, Xi’an Jiaotong University, ChinaReviewed by:
Alejandro Bugarin, Florida Gulf Coast University, United StatesPratik Yadav, University of Notre Dame, United States
Copyright © 2025 Gan, Ma, Liu, Luo, Li, Pu, Jiang and Lian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Lian, bGlhbnlhbkBjZHV0Y20uZWR1LmNu; Guihua Jiang, MTE0Njk0MTNAcXEuY29t; Lili Pu, eGpfcGxsQGZveG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Zhengkun Gan
Zhengkun Gan Jun Ma1†
Jun Ma1† Xinyu Liu
Xinyu Liu Guihua Jiang
Guihua Jiang Yan Lian
Yan Lian