- Julius Kühn Institute (JKI) – Federal Research Centre for Cultivated Plants, Genome Editing and Synthetic Biology Group, Institute for Biosafety in Plant Biotechnology, Quedlinburg, Saxony-Anhalt, Germany
The global trend towards plant-based protein sources as an alternative to animal-derived protein has surged due to health benefits, rising adoption of vegan and vegetarian lifestyles. This shift promotes sustainable agriculture by mitigating greenhouse gas emissions and safeguarding biodiversity. Among various plant-based protein sources, legumes have received considerable attention due to their high-protein content, gluten-free nature and nitrogen-fixing capacity, making them indispensable in crop rotation systems. Within the legume family, lupins are gaining global attention for their exceptional nutritional profile and bioactive compounds with promising health benefits. Although lupins offer significant nutritional benefits, challenges such as biotic and abiotic stresses and anti-nutritional factors persist. Addressing these challenges demands advanced breeding techniques capable of mitigating these issues without compromising desirable traits. Genome editing holds promise for enhancing crop traits, including improved nutritional value and resistance to environmental stresses. The availability of complete genome sequences for lupin species provides a foundation for genome editing and accelerated breeding. However, genome editing requires reproducible plant cell culture and transformation protocols. Nonetheless, legumes exhibit a high degree of recalcitrance to in vitro regeneration and genetic transformation, the underlying mechanisms of which remain largely unknown. This review provides a comprehensive examination of the current advancements, challenges and future prospects associated with plant cell culture, genetic transformation, genome editing and double haploid (DH) technologies in the context of lupin improvement. Additionally, this review briefly discusses major obstacles in conventional lupin breeding.
1 Introduction
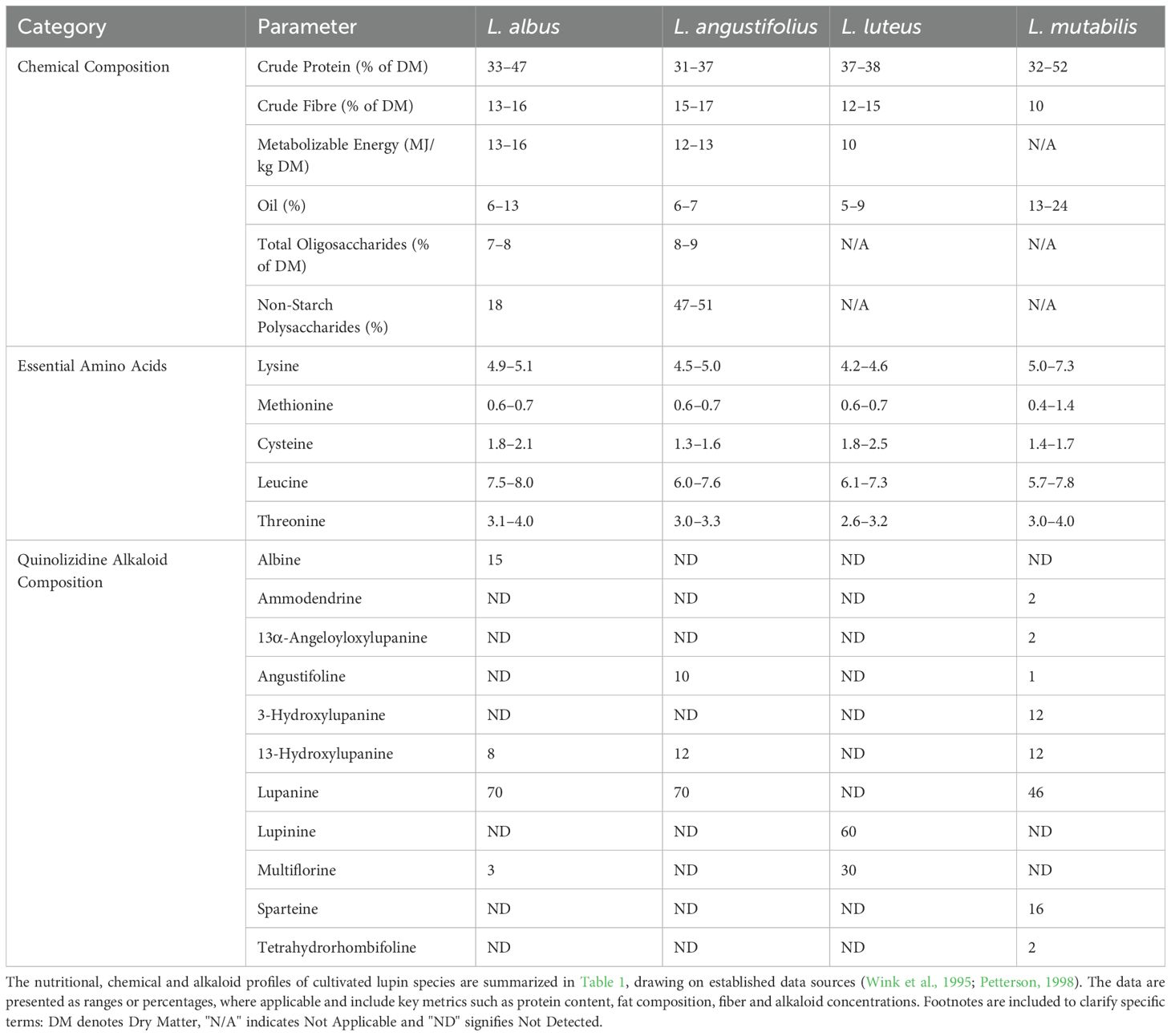
Lupins (members of the Fabaceae family) are distinguished by their ornamental value, characterized by the production of vividly colored inflorescences (Kotecki, 2015). These plants are part of the Lupinus genus, which is highly diverse, encompassing over 300 species (Hughes and Eastwood, 2006; Drummond et al., 2012). Lupins are particularly noteworthy for their high-protein content, abundance of dietary fiber and low fat levels (Table 1). They serve as promising alternatives to processed flours in food formulations and can be used in dairy analogs such as cheese, yogurt and ice cream (Duarte et al., 2022; Pereira et al., 2022). The primary lupin species cultivated as contemporary grain crops include white lupin, yellow lupin, narrow-leafed lupin and Andean lupin (Hondelmann, 1984; Yorgancilar et al., 2009). These species exhibit distinct morphological, physiological and agronomic traits, making them valuable for various agricultural and ecological applications (Pszczółkowski et al., 2025).
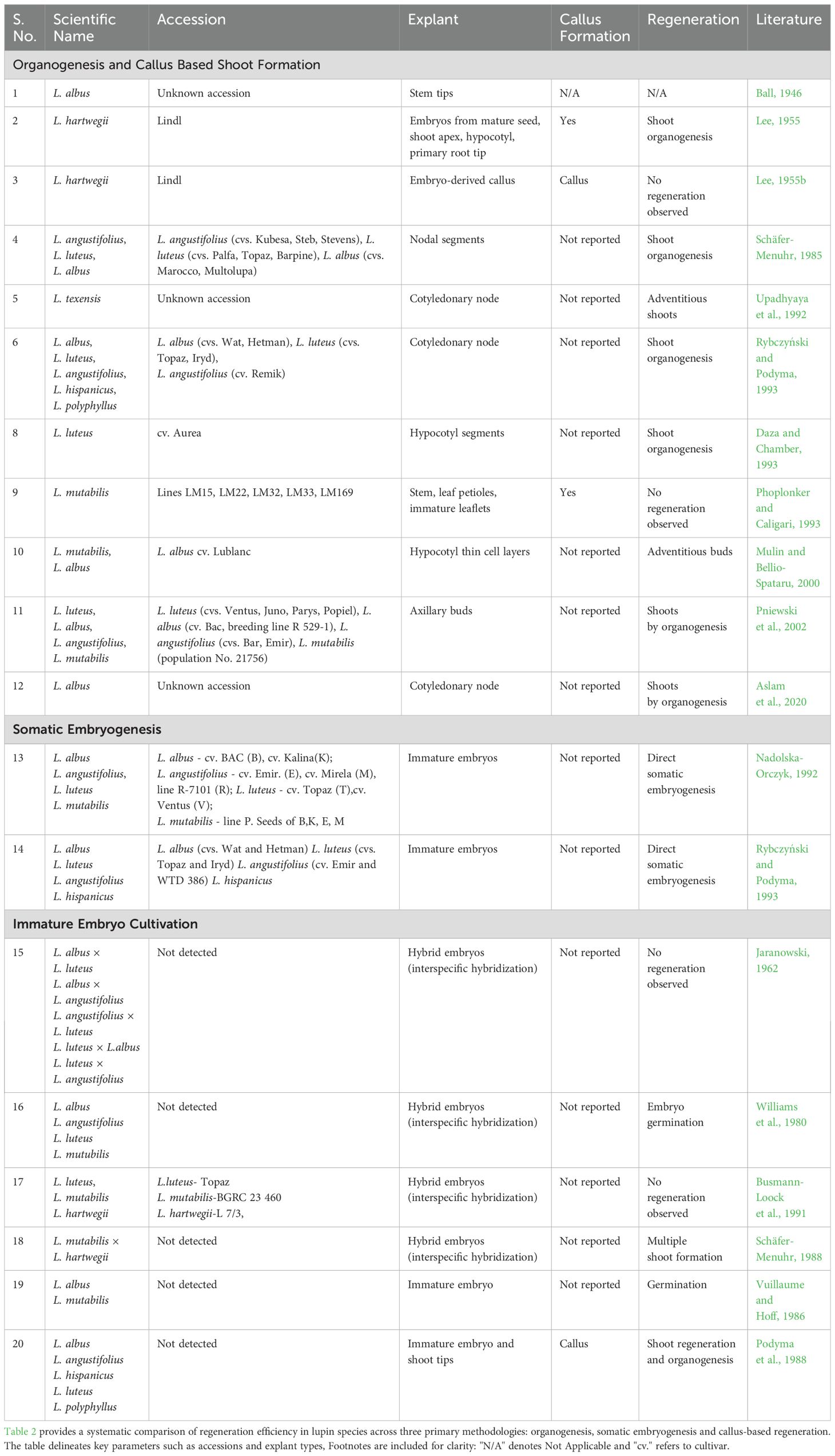
Table 2. Regeneration in lupin species: a comparative analysis of organogenesis, somatic embryogenesis and callus-based regeneration methods.
White lupin (Lupinus albus, 2n = 50, genome size: 653 Mb (Hufnagel et al., 2020)) is characterized by its high-protein content, palmate leaves and spirally arranged dorsal flowers, which are predominantly white but may also appear in blue or pink hues. A defining physiological characteristic of this species is its capacity to form cluster roots specialized structures that exude carboxylates, facilitating phosphate mobilization and improving soil nutrient availability (Petterson, 2016; Abraham et al., 2019; Alkemade et al., 2021). The cultivation of L. albus began approximately 4,000 years ago (Wolko et al., 2011). Yellow lupin (L. luteus, 2n = 52, estimated genome size: 1,024.49 Mb (Lichtin et al., 2020)) is an annual species notable for its nitrogen-fixing ability and dense clusters of bright yellow flowers. Its palmate leaves and adaptability contribute to its attractiveness for pollinators and ornamental use (Kotecki et al., 2020; Martinez-Hernandez et al., 2024). Narrow-leafed lupin (L. angustifolius, 2n = 40, genome size: 975 Mb (Garg et al., 2022)), commonly known as blue lupin, this species has narrow leaves and blue-violet flowers. Breeding efforts have successfully reduced its alkaloid content, resulting in the development of “sweet” cultivars that are now extensively cultivated for human and animal consumption (Pszczółkowski et al., 2025). Andean lupin (L. mutabilis, 2n = 48, genome size: 620 Mb (Pancaldi et al., 2024)), commonly known as tarwi, is a perennial species with multi-colored flowers that transition from white with yellow wings to dark purple. Its nutritional value and adaptability are promising, though it requires careful pest and disease management (Guilengue et al., 2020; Pszczółkowski et al., 2025).
Lupins are unique among protein crops, with seed protein content reaching up to 44%, comparable to soybeans, making them one of the highest-protein plant species (Lucas et al., 2015). Moreover, lupins are generally more tolerant to various abiotic stresses compared to other legumes and they hold significant potential for the restoration of degraded or nutrient-poor soils (Lucas et al., 2015).
Lupins offers health benefits such as improved bowel function, cholesterol reduction and blood glucose regulation (Van De Noort, 2017). Despite their historical use dating back to ancient Egypt and pre-Incan South America, lupins remain an underutilized legume in modern diets (Lucas et al., 2015). Europe’s heavy reliance on soybean imports, governed by trade agreements and quality standards, does not satisfy expectations of European citizens (Lucas et al., 2015). Thereby, Native European lupins, such as L. albus, L. luteus and L. angustifolius offer promising alternatives to soybeans due to their high-quality protein, potential health benefits and suitability for sustainable production (Lucas et al., 2015). However, lupin cultivation in Europe is still insufficient to ensure a consistent supply to the food industry, which must innovate to create appealing lupin-based protein-rich products (Lucas et al., 2015). Despite their agronomic and nutritional advantages, lupins remain underutilized due to significant breeding challenges (Pszczółkowski et al., 2025). Some of these constraints are discussed under the subheading Challenges in lupin breeding in this review.
Lupins exhibit two distinct phenotypes: bitter and sweet defined primarily by their alkaloid composition, which influences both edibility and sensory attributes. These alkaloids render the seeds unpalatable and pose neurotoxic risks to humans and animals (Maknickienė and Asakaviciute, 2008). To address these safety concerns, regulatory authorities in New Zealand, Australia, the United Kingdom and France have set a maximum allowable alkaloid limit of 200 mg/kg in lupin-based food products (Resta et al., 2008).
Bitter lupins synthesize a diverse array (Table 1) of nitrogen-containing secondary metabolites known as quinolizidine alkaloids (QAs) (Wink et al., 1995; Petterson, 1998). These alkaloids serve as chemical defenses against herbivores and exhibiting antimicrobial activity (Erdemoglu et al., 2007; Mancinotti et al., 2023). QAs are biosynthesized from the amino acid L-lysine through a series of enzymatic steps involving decarboxylation, oxidation and cyclization (Mancinotti et al., 2025). Recent studies have proposed the involvement of six to nine enzymes in this pathway, although the complete sequence of reactions and all participating enzymes have yet to be fully elucidated (Mancinotti et al., 2022).
Advancements in metabolic engineering have enabled the manipulation of QA biosynthesis in lupins (Ramírez-Betancourt et al., 2021). For instance, L. angustifolius has been engineered to accumulate elevated levels of sparteine, a QA of industrial relevance due to its role in asymmetric synthesis (Mancinotti et al., 2025). Manipulation of QA biosynthesis remains a key objective in lupin biotechnology, aiming to further reduce anti-nutritional compounds in edible seeds, enhance plant defense mechanisms and facilitate the production of valuable alkaloids for pharmaceutical applications (Osorio and Till, 2021; Mancinotti et al., 2022, 2023, 2025). Current strategies include CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) -based genome editing, RNA interference for targeted gene suppression and heterologous pathway reconstruction. However, the effectiveness of these approaches is constrained by the limited amenability of lupins to tissue culture and genetic transformation.
1.1 Challenges in lupin breeding
The primary objective of contemporary lupin breeding programs is to develop cultivars with enhanced agronomic traits, such as reduced alkaloid content, enhanced disease resistance, greater adaptability to climate change and increased productivity (Pszczółkowski et al., 2025). Fungal pathogens, including anthracnose (Colletotrichum lupini), fusarium wilt (Fusarium oxysporum ssp. lupini) and lupin leaf fall (Pleospora herbarum), pose significant threats to lupin cultivation. These phytopathogens adversely affect both agricultural productivity through yield reduction and seed safety via mycotoxin accumulation (Horoszkiewicz-Janka et al., 2011; Sawicka and Pszczółkowski, 2014; Kotecki et al., 2020). Consequently, breeding programs must prioritize the development of lupin cultivars exhibiting improved resistance against both major pathogens and additional biotic constraints, including gray mold, viral diseases, aphid infestations and competitive weed pressure (Sawicka and Pszczółkowski, 2014).
Yield improvement remains another critical area of research in lupin breeding. Recent efforts have leveraged genomic tools to identify yield-associated genes and integrate them into elite lupin varieties (Mavromatis et al., 2023). Climate change further complicates breeding, necessitating resilient varieties adaptable to shifting environmental conditions. Interspecific hybridization within lupins is challenging, as evidenced by limited success in crossing species such as L. albus and L. mutabilis (Sawicka-Sienkiewicz et al., 2006).
Lupin breeding faces significant challenges in disease resistance, yield enhancement and climate resilience (Pszczółkowski et al., 2025). Scientists are exploring various methods to develop improved lupin varieties, with new genomic techniques revolutionizing plant biotechnology. These advanced methodologies provide numerous advantages, notably their precision and efficiency in the targeted introduction of specific traits into the plant genome. Genome editing, a targeted approach, allows for precise alterations in the plant’s DNA, enabling the enhancement of desirable traits (Abdul Aziz et al., 2022; Yıldırım et al., 2023). The availability of complete genome sequences for lupin species provides a solid foundation for genome-editing research and accelerated breeding efforts (Hufnagel et al., 2020; Wang et al., 2021; Garg et al., 2022).
The limited application of genome editing in certain legume crops underscores the need for further research and innovation to fully leverage the potential of lupins and other legumes for sustainable protein production (Nivya and Shah, 2023). The development of new lupin varieties is crucial to ensuring adaptability and nutritional quality, taking into consideration factors such as cultivation conditions and climate variability. Plant cell culture is integral to both conventional and contemporary breeding methodologies, serving a pivotal function in the advancement of crop improvement strategies (Pathi and Sprink, 2023). In classical breeding, it facilitates the rapid production of double haploid plants, speeding up the breeding process by enabling the identification of desirable traits and more efficient selection of improved plant varieties (Murovec and Bohanec, 2012). In modern breeding, plant cell culture serves as a powerful tool for genetic manipulation and the propagation of desired traits. Within the framework of genome editing, it allows for precise modifications at the cellular level, leading to targeted improvements in plant DNA (Loyola-Vargas and Avilez-Montalvo, 2018). Additionally, plant cell culture supports the mass production of genetically modified plants, ensuring a sufficient number of plants with enhanced genetic traits is being generated (Kowalczyk et al., 2022).
To the best of our knowledge, this is the most comprehensive review to date on the in vitro and biotechnological aspects of lupins. It provides an in-depth examination of key areas including plant tissue culture, genetic transformation, protoplast technology, double haploid production and genetic engineering, while also highlighting existing challenges and future prospects.
2 Lupin cell/tissue culture
Biotechnological approaches such as in vitro mutagenesis, protoplast culture-mediated somatic hybridization and genetic transformation support advances in lupin breeding. These methods rely on optimized protocols for plant cell/tissue culture. However, lupin explants exhibit poor in vitro response.
Despite these challenges, the totipotency of plant cells enables several promising biotechnological applications. While a few successful reports exist, comprehensive efforts in lupin in vitro propagation remain limited (Figure 1). The following sections review the current achievements, challenges and future prospects in this area.
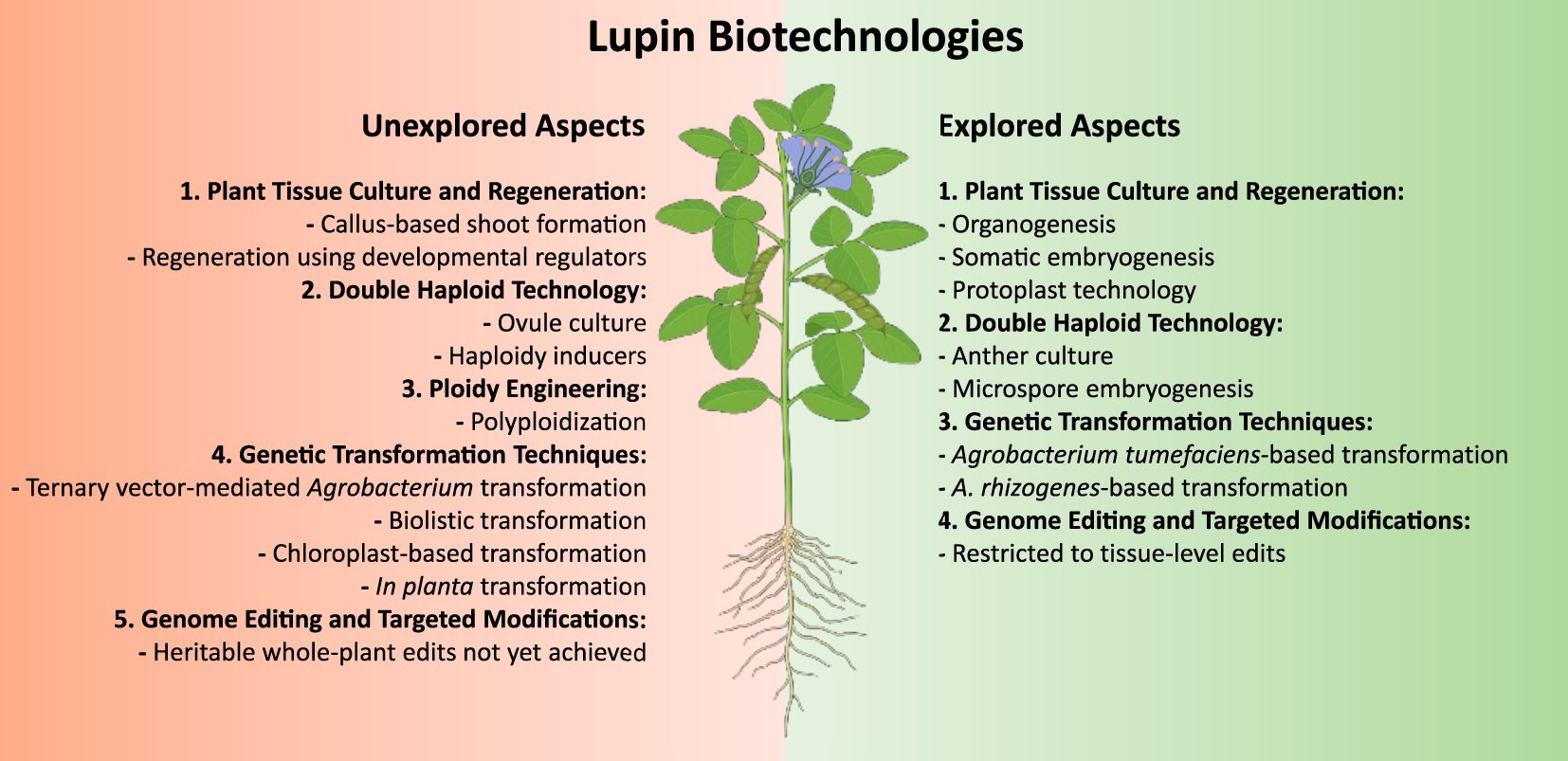
Figure 1. Schematic representation of explored and unexplored aspects in lupin biotechnology. Figure 1 presents a detailed schematic representation of the current research landscape in lupin biotechnology, systematically categorizing both explored and unexplored domains. Explored areas are depicted in green, while unexplored aspects are highlighted in red, providing a visually intuitive distinction between established knowledge and potential avenues for future investigation. This scheme was generated using BioRender.
2.1 Organogenesis
Numerous studies have investigated in vitro organogenesis in lupins, consistently identifying explant source as a critical determinant of morphogenic competence. A diverse range of explants has been evaluated, such as apical meristems (Ball, 1946; Lee, 1955a), shoot tips (Rybczynski and Podyma, 1993; Pigeaire et al., 1997), axillary buds (Schäfer-Menuhr, 1985; Pniewski et al., 2002), nodal regions (Mulin and Bellio-Spataru, 2000), cotyledonary nodes (Upadhyaya et al., 1992; Aslam et al., 2020) and hypocotyl-derived explants (Daza and Chamber, 1993). Among these, apical meristems, axillary buds, hypocotyl-derived explants and cotyledonary nodes consistently exhibit the highest capacity for multiple shoot induction. High shoot multiplication rates have been reported in L. hispanicus (Rybczynski and Podyma, 1993), L. mutabilis (Pniewski et al., 2002), L. texensis (Upadhyaya et al., 1992), L. albus (Aslam et al., 2020) and L. luteus (Daza and Chamber, 1993).
Explant age further modulates regenerative outcomes. Younger tissues, particularly cotyledonary nodes, generally exhibit superior morphogenic responses, as demonstrated in L. albus (Aslam et al., 2020). However, exceptions to this trend highlight the complexity of regeneration biology. For instance, L. albus explants derived from 5-day-old seedlings showed no response under in vitro conditions (Rybczynski and Podyma, 1993). In contrast, the regenerative potential of apical meristems appears independent of age, with successful organogenesis observed from 30-day-old seedlings of L. albus (Ball, 1946) and water-imbibed mature embryos of L. hartwegii (Lee, 1955a). These observations underscore the role of genotype-specific physiological status in determining morphogenic competence.
Most regeneration protocols in lupins utilize Murashige and Skoog (MS) or Gamborg B5 media, whereas early studies employed Robins formulation (Ball, 1946; Lee, 1955a). In L. albus, high-frequency regeneration from half cotyledonary node explants was achieved using a low-nutrient MS medium, with the inclusion of activated charcoal significantly enhancing shoot elongation and reducing tissue browning. Among the carbohydrate sources tested, sucrose led to the highest shoot regeneration frequency, particularly in half cotyledonary node explants (Aslam et al., 2020).
Plant growth regulator (PGR) combinations have a decisive impact on regeneration outcomes. Among cytokinins, benzyladenine (BA), kinetin and 2-isopentenyladenine (2iP) are commonly utilized, with BA and kinetin being particularly effective for multiple shoot induction (Upadhyaya et al., 1992; Daza and Chamber, 1993; Mulin and Bellio-Spataru, 2000; Aslam et al., 2020). Notably, BA in combination with Naphthaleneacetic Acid (NAA) significantly enhanced shoot regeneration in L. angustifolius (Pigeaire et al., 1997).
Considerable progress has been made in shoot induction. However, rooting remains a major bottleneck. Root formation often requires a reduction in basal medium strength and the application of auxins (Schäfer-Menuhr, 1985; Pniewski et al., 2002; Aslam et al., 2020). In L. albus, a rooting frequency of up to 80% was achieved within 28 days on low-strength MS medium supplemented with B5 vitamins and auxins (Aslam et al., 2020). However, prolonged in vitro cultivation was found to reduce rooting efficiency (Pniewski et al., 2002). In cases where shoots do not produce roots, plant establishment has been successfully achieved by grafting regenerated shoots onto decapitated seedlings (Pniewski et al., 2002), underscoring the ongoing challenges in developing robust root systems for regenerated plants.
2.2 Callus based shoot formation
Callus-mediated shoot regeneration in lupins remains largely unsuccessful. Earlier studies were instrumental in identifying responsive explants and showed callus formation from L. hartwegii shoot apices (Lee, 1955b) and various explants of L. mutabilis (Phoplonker and Caligari, 1993), but none of these studies achieved shoot regeneration, highlighting persistent challenges in callus-based shoot formation in lupins
2.3 Somatic embryogenesis
Somatic embryogenesis offers significant advantages for plant regeneration (Pathi et al., 2013; Martínez and Corredoira, 2024), yet its application in lupins remains largely underexplored. Only a limited number of studies have reported species-specific responses, consistently identifying immature cotyledons as the most responsive explant and Gamborg B5 as the optimal basal medium (Nadolska-Orczyk, 1992; Rybczyński and Podyma, 1993). Early work by Nadolska-Orczyk (1992) demonstrated successful somatic embryo induction in L. angustifolius, L. albus and L. mutabilis, whereas L. luteus failed to respond. However, despite successful induction, this protocol did not yield fully regenerated plants, highlighting the problem of poor shoot conversion. Building on this foundation, Rybczyński and Podyma (1993) achieved complete plant regeneration in L. albus by optimizing the hormonal regime and incorporating coconut water into the culture medium. While both protocols proved effective for L. albus, their limited success in other Lupinus species underscores the challenge of developing broadly applicable somatic embryogenesis protocols. Further research is needed to refine these approaches, overcome species-specific response and enhance shoot-to-plant conversion efficiency.
2.4 Immature embryo culture
Interspecific hybridization in lupins has long been constrained by pronounced cross-incompatibilities, limiting its utility in expanding genetic diversity within breeding programs (Jaranowski, 1962; Williams et al., 1980). In this context, embryo rescue techniques have emerged as a pivotal strategy to overcome post-zygotic barriers and facilitate the recovery of viable hybrid progeny (Vuillaume and Hoff, 1986; Bridgen, 1994). Several embryo rescue systems have been established, including agar medium culture (Schafer-Menuhr et al., 1988), paper bridges over liquid medium (Vuillaume and Hoff, 1986) and liquid-over-agar methods (Podyma et al., 1988). These technical advancements have identified critical developmental thresholds such as a minimum embryo size of ≥1 millimeter for successful culture and demonstrated the efficacy of coconut milk supplementation in supporting the development of early heart-stage embryos, enabling successful embryo rescue in L. albus and L. mutabilis (Podyma et al., 1988).
2.5 Double haploid technology
Double haploid technology is highly valuable for fundamental research and plant breeding, accelerating genetic improvement and trait selection. The advantages of DH technology in classical and new breeding methods were reviewed in a previous article (Pathi and Sprink, 2023). However, the advancement of double haploid protocols for plant improvement in Fabaceae has progressed slowly compared to other plant families (Croser et al., 2006; Skrzypkowski and Kiełkowska, 2024).
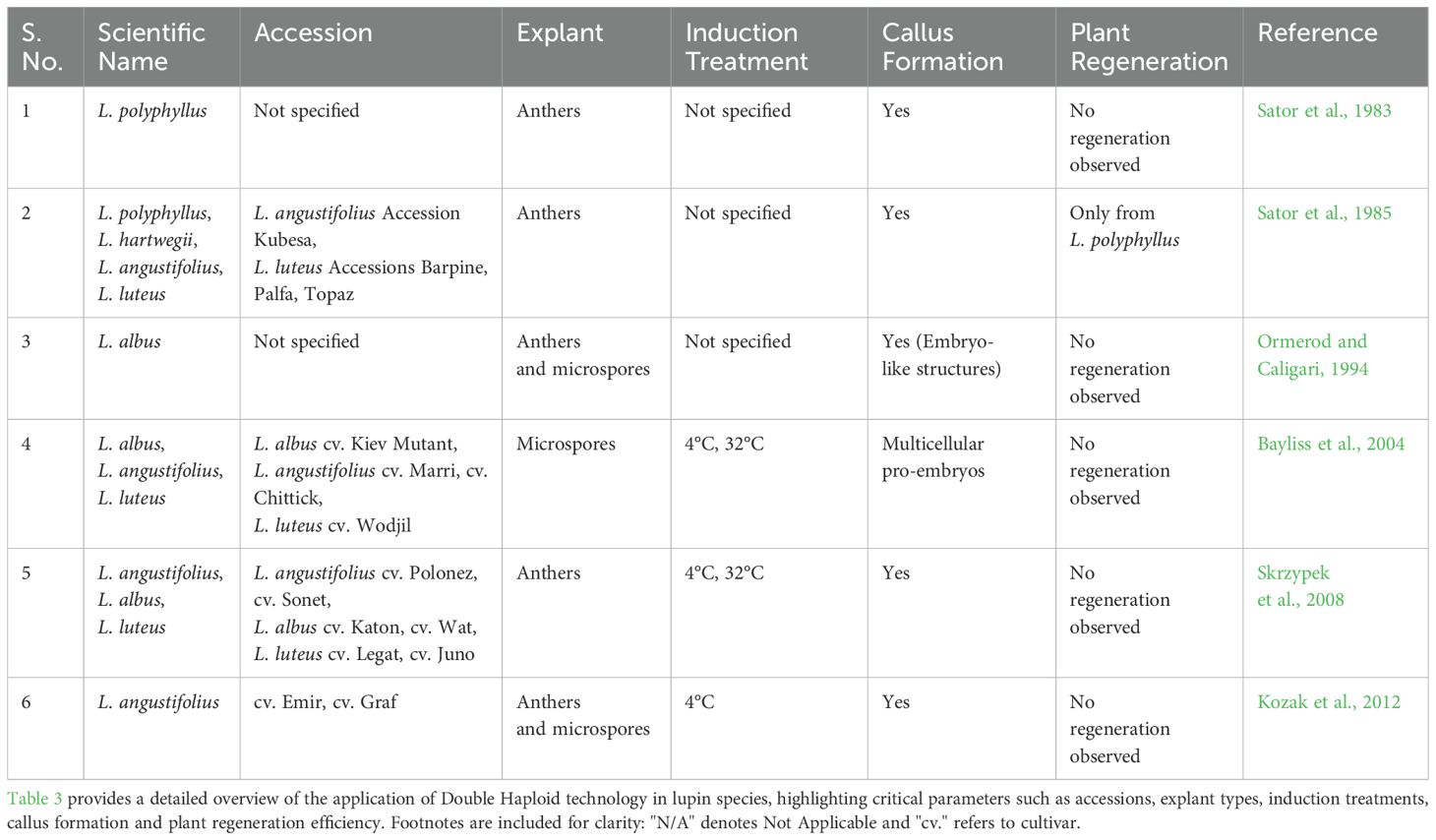
Research on lupin haploid plants production has demonstrated the potential for microspore-derived embryogenesis, with key studies confirming that isolated microspores of L. albus, L. angustifolius and L. luteus can form multicellular pro-embryos under optimized culture conditions (Ormerod and Caligari, 1994; Bayliss et al., 2004). Refinements in donor plant selection such as bud size (5–6 millimeter) and anther color have improved embryogenic responses (Skrzypek et al., 2008; Kozak et al., 2012). Skrzypek et al. (2008) reported an embryogenic response in lupin anther cultures without the need for inflorescence pre-treatment, a finding that is atypical among legume species. Early work by Sator et al. (1983); Sator (1985) demonstrated the feasibility of anther culture in L. polyphyllus, achieving diploid regeneration, although not true double haploids.
Despite these advancements, lupin androgenesis faces persistent challenges. A major bottleneck is the exine barrier, which restricts pro-embryo development (Bayliss et al., 2004). Unlike in model species where exine rupture occurs naturally (Daghma, 2011; Siemons et al., 2025), lupin microspores may require mechanical or enzymatic assistance, a factor that remains underexplored in current protocols. Additionally, species-specific response is evident: while L. polyphyllus regenerates diploid plants (Sator, 1985), other species such as L. luteus and L. angustifolius produce callus but fail to regenerate shoots. Overcoming species-specific barriers, optimizing stress pre-treatments, employing haploidy inducers and integrating insights from model systems will be critical for advancing double haploid (DH) technology in lupins.
2.6 Protoplast technology
Protoplasts, defined as plant cells devoid of a cell wall. The isolation and cultivation of protoplasts present numerous benefits, including opportunities for genetic manipulation, studies on hybridization, investigation into cell physiology, regeneration from a single cell and manipulations at the single-cell level. These advantages have been extensively reviewed in our previous report (Pathi and Sprink, 2023).
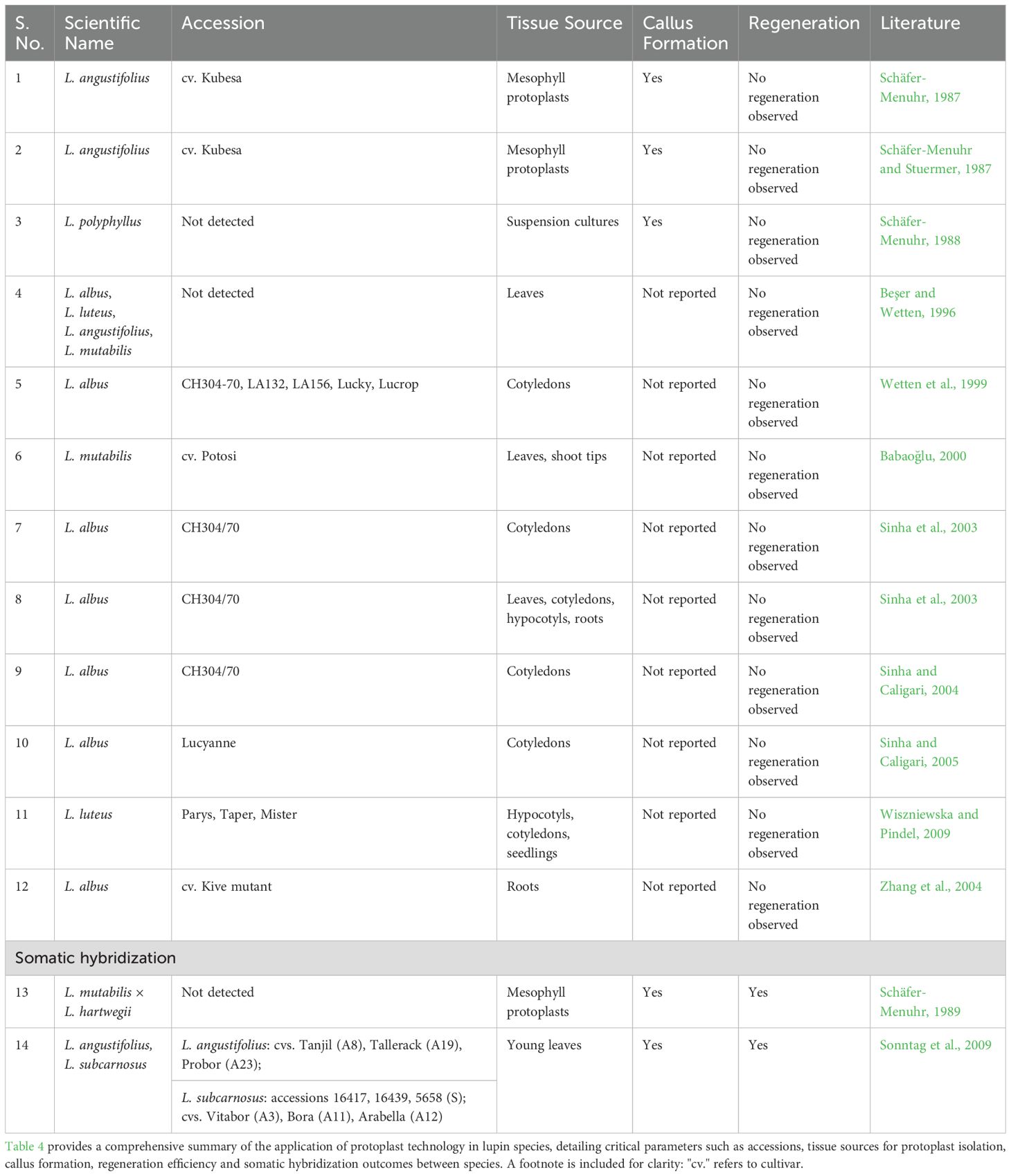
In recent decades, significant progress has been made in protoplast isolation and culture systems in Lupinus species, establishing a foundation for their use in developmental biology and biotechnology. Early work by Schäfer-Menuhr (1987; 1988; 1989) developed high-yield, high-purity protocols for mesophyll protoplast isolation in L. angustifolius, L. polyphyllus and their hybrids. Subsequent studies further identified optimal explant sources for reliable protoplast isolation. Cotyledons from in vitro-grown seedlings of L. albus and mesophyll tissues from mature leaves of L. angustifolius and L. polyphyllus have consistently produced the highest yields of viable protoplasts, reflecting the favorable cellular architecture and developmental plasticity of these tissues (Schäfer-Menuhr, 1987; Schäfer-Menuhr and Stuermer, 1987; Schäfer-Menuhr, 1988; 1989; Babaoğlu, 2000; Sinha et al., 2003; Sinha and Caligari, 2004). Despite these advances, species- and genotype-specific variability continues to hinder protocol standardization, reflecting the biological heterogeneity within the genus (Beşer and Wetten, 1996; Sinha and Caligari, 2004).
In parallel, the development of synthetic culture media initially AS 19 and later K8p (Kao and Michayluk medium) was instrumental in promoting protoplast-derived callus formation and initiating cell division and morphogenesis (Schäfer-Menuhr, 1987; 1988; 1989). Yet, despite the early formation of morphogenic structures, the regeneration of complete plants from lupin protoplasts remains elusive. Most studies terminate at the callus stage, reflecting incomplete organogenic or embryogenic competence and an ongoing inability to fully exploit the totipotent potential of protoplasts (Babaoğlu, 2000; Sinha and Caligari, 2004, 2005).
To enhance culture responsiveness, Sinha et al. (2003); Sinha and Caligari (2004); Sinha and Caligari (2005) refined multiple parameters in L. albus cultures, including enzyme composition, osmotic potential and pH, substantially improving protoplast viability and division rates. Despite these refinements, the developmental trajectory remains unstable. Lupin protoplasts display hypersensitivity to minor fluctuations in culture conditions, such as osmolarity and pH, which can severely compromise both cell viability and morphogenic progression (Babaoğlu, 2000; Sinha and Caligari, 2005).
Furthermore, technical innovations most notably droplet plating on Nunclon surfaces have improved protoplast elongation and mitotic activity, offering enhanced physical environments for single-cell culture (Sinha and Caligari, 2005). Nevertheless, other approaches intended to improve morphogenesis, including the use of embedding matrices such as alginate and filter paper, as well as nurse and suspension cultures, have often yielded poor outcomes (Babaoğlu, 2000; Sinha and Caligari, 2005; Wiszniewska and Pindel, 2009). This suggests that while physical handling techniques have advanced, the cellular microenvironment remains suboptimal for consistent regeneration.
In addition to their regenerative potential, lupin protoplasts have served as valuable models for physiological studies. For instance, Zhang et al. (2004) utilized root-derived protoplasts from L. albus to explore citrate efflux mechanisms under phosphorus deficiency, demonstrating the versatility of protoplast systems in stress physiology.
Despite notable advances in protoplast isolation and culture optimization, efficient whole-plant regeneration from isolated protoplasts remains a pivotal challenge. Addressing this limitation is essential for fully harnessing protoplast technologies in next-generation lupin breeding and genome editing initiatives. Although approaches based on somatic hybridization, discussed in the following section, have explored protoplast fusion strategies, success in achieving complete shoot regeneration has remained limited.
2.6.1 Somatic hybridization
Early studies in lupins have highlighted the potential of protoplast fusion as a strategy for interspecific genetic manipulation and plant regeneration. A seminal contribution by Schäfer-Menuhr (1989) demonstrated, for the first time, that mesophyll protoplasts derived from L. mutabilis × L. hartwegii hybrids could be induced to form calli and regenerate shoots under optimized culture conditions. The regenerated shoots displayed morphological similarity to the parental genotype. These findings established a foundational proof-of-concept for protoplast-based regeneration in lupins. However, the absence of molecular characterization in this study left the hybrid nature and genetic stability of the regenerants unconfirmed.
Building on this work, Sonntag et al. (2009) successfully employed electrofusion of protoplasts from L. angustifolius and L. subcarnosus to generate somatic hybrid calli capable of shoot regeneration. Notably, no shoot development was observed in colonies derived from the parental protoplasts alone. Molecular marker analyses confirmed the hybrid identity of the regenerants, implicating heterotic or synergistic genetic interactions as key drivers of morphogenesis. While this study marked a significant advancement in lupin protoplast technology and genetic improvement, the reproducibility of these outcomes across other Lupinus species remains to be established.
3 Genetic transformation of lupins
Plant transformation involves identifying a target gene, introducing it into plant cells and regenerating a whole plant with the expressed transgene (Chen et al., 2022). Particle bombardment and Agrobacterium-mediated transformation are predominantly used methods for gene transfer, though the latter has become more widely favored due to its accessibility, cost-effectiveness and ability to introduce single or low-copy transgene insertions, making it a preferred approach for plant transformation (Chen et al., 2022; Rahman et al., 2024). Stable transformation, which enables the heritable transmission of integrated genes to subsequent generations, is essential for both functional genomics and transgenic breeding applications (Rahman et al., 2024).
3.1 Agrobacterium-mediated transformation
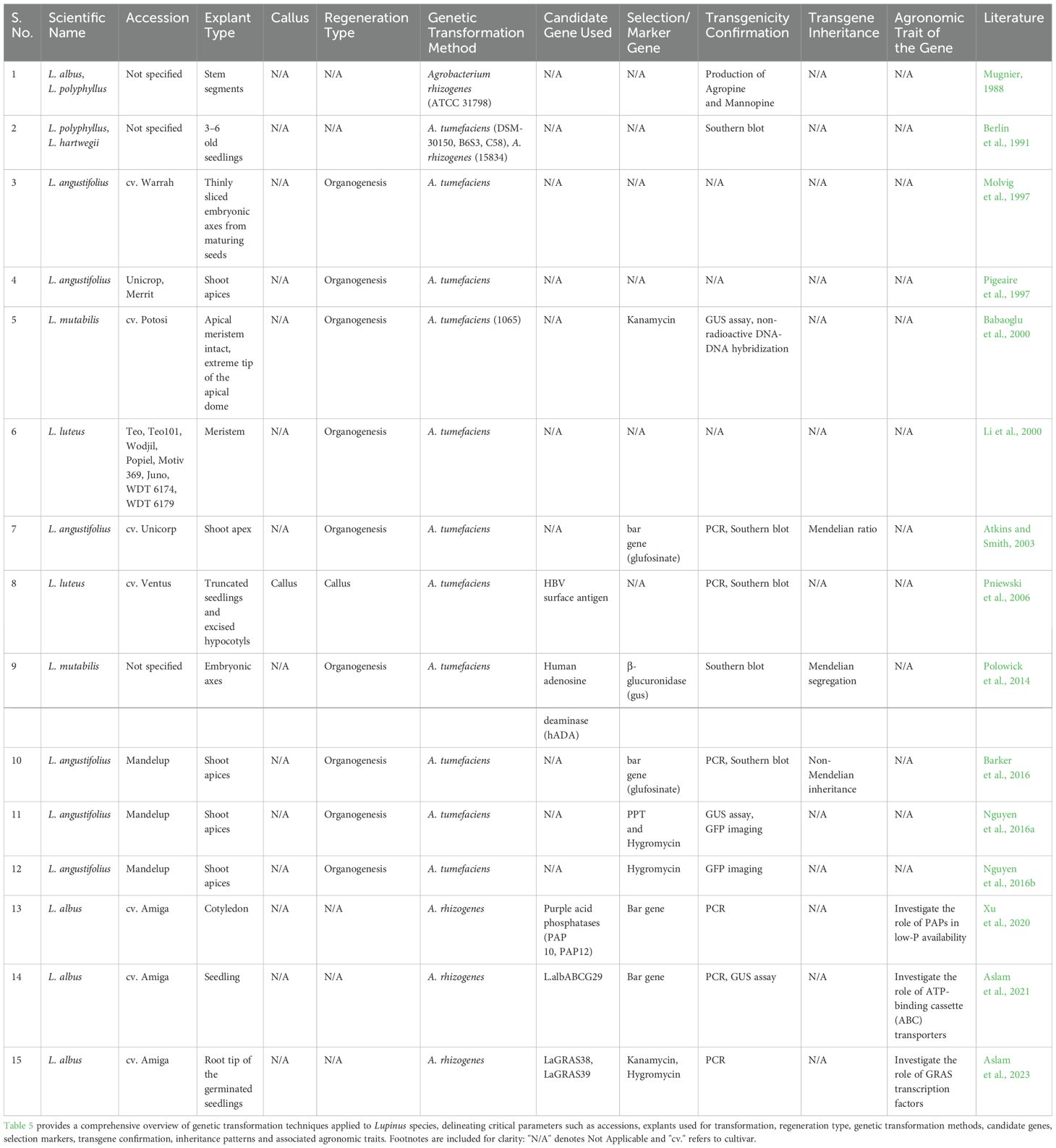
Extensive efforts have been undertaken to establish Agrobacterium-mediated genetic transformation systems in lupins, progressing from early proof-of-concept experiments to more refined protocols capable of generating transgenic lines with agronomically beneficial traits. Despite these advancements, progress has been uneven across species and various technical and biological limitations continue to hinder widespread implementation. Transformation studies have predominantly relied on meristematic tissues (embryonic axes, shoot apices and Leaf primordia), owing to their higher competence for regeneration (Molvig et al., 1997; Pigeaire et al., 1997; Babaoglu et al., 2000; Atkins and Smith, 2003; Polowick et al., 2014; Nguyen et al., 2016b).
A major breakthrough was achieved by Molvig et al. (1997) and Pigeaire et al. (1997), who developed Agrobacterium tumefaciens-mediated transformation protocols for L. angustifolius, using embryonic axes and shoot apices to recover transgenic plants with stable gene integration. However, transformation efficiencies remained low (≤2.8%) and highly genotype-dependent. The approach was subsequently extended to L. mutabilis and L. luteus (Babaoglu et al., 2000; Li et al., 2000). In L. luteus, Li et al. (2000) employed meristem co-cultivation followed by grafting of transformed shoots onto non-transgenic L. angustifolius rootstocks, achieving efficiencies of 0.05–0.75% in the T1 generation. Efforts to engineer agronomic traits, including herbicide resistance, demonstrated the practical potential of transformation. Atkins and Smith (2003) generated stable, herbicide-tolerant L. angustifolius lines with transformation efficiency of ~0.4%. Transgene inheritance in T1 seeds followed a Mendelian 3:1 segregation ratio, affirming stable integration. In contrast, Barker et al. (2016) reported deviations from Mendelian patterns, suggesting persistent chimerism reflecting challenges in achieving uniform transgene integration.
Selection strategies and marker genes have played a critical role in the success of plant transformation. While phosphinothricin (PPT) selection was widely used, Nguyen et al. (2016b) demonstrated that hygromycin selection significantly outperformed standard PPT/bar systems in generating transgenic shoots.
Reporter genes such as uidA (GUS), eGFP (enhanced Green Fluorescent Protein) and nptII (neomycin phosphotransferase II) have been effectively employed across studies to confirm transformation events. Notably, Pniewski et al. (2006) reported a 44% transformation efficiency in L. luteus callus using nptII and uidA and successfully expressed the Hepatitis B surface antigen (S-HBsAg), illustrating the platform’s utility for recombinant protein production.
Substantial improvements were reported by Pniewski et al. (2006) and Polowick et al. (2014), who optimized culture conditions to raise transformation efficiency significantly. However, these gains were largely restricted to callus induction, with limited whole-plant regeneration and poor reproducibility across species. The most notable advancement came from Nguyen et al. (2016a); Nguyen et al. (2016b), who achieved up to 75% transformation efficiency in L. angustifolius through strategic tissue targeting and delayed selection, significantly reducing chimerism. Despite this, the regenerative capacity of transformed tissues remained limited, requiring further subculturing to obtain uniform, heritable transgenic lines.
In parallel to these efforts, A. rhizogenes-mediated transformation systems have emerged as powerful tools for functional studies in root biology and nutrient stress adaptation. The initial demonstration by Mugnier (1988), followed by Berlin et al. (1991), confirmed the feasibility of Agrobacterium rhizogenes-mediated gene delivery in lupins, resulting in the formation of hairy roots. These studies laid the foundation for gene functional analysis in this genus. However, the inability to regenerate whole plants from transformed tissues remains a key limitation. More recently, the application of hairy root transformation has enabled efficient gene validation. Xu et al. (2020); Aslam et al. (2021) and Aslam et al. (2023) used hairy root systems to investigate gene functions related to phosphorus uptake and root development. These studies enabled rapid gene validation through overexpression of candidate genes such as PAP10, PAP12 (Purple acid phosphatase), LalbABCG29 (L. albus ATP-Binding Cassette G family transporter 29) and LaGRAS (L. albus GRAS = named after GAI, RGA and SCR) family members, thereby contributing to a mechanistic understanding of abiotic stress resilience in lupins.
3.2 Biolistic based transformation
The development of particle bombardment has emerged as an effective alternative for delivering DNA into plant cells, particularly in species resistant to Agrobacterium-mediated transformation (Ozyigit and Yucebilgili Kurtoglu, 2020). This biolistic method enables the direct transfer of nucleic acids or ribonucleoprotein (RNP) complexes by coating them onto gold or tungsten particles, which are then accelerated via high-pressure helium discharge to penetrate physical barriers and deliver genetic material into key organelles such as the nucleus and chloroplast. Notably, biolistic transformation facilitates genome editing without T-DNA integration while delivering high gene dosages (Eudes et al., 2014; Ozyigit and Yucebilgili Kurtoglu, 2020).
Particle bombardment-based transformation has been successfully applied in various legume species, demonstrating its versatility across explant types. In soybean, embryonic axes were effectively transformed (Aragão et al., 2000; Rech et al., 2008), while cowpea studies utilized embryonic axes (Ivo et al., 2008; Cruz and Aragão, 2014; Grazziotin et al., 2020). Similarly, chickpea transformation was achieved using epicotyls and embryonal axes (Indurker et al., 2007), whereas pigeon pea relied on cotyledonary nodes (Thu et al., 2007) and leaf explants (Dayal et al., 2003). In black gram, cotyledonary nodes were targeted for transformation (Das, 2018) and in alfalfa, calli derived from petioles and stem sections proved amenable to biolistic delivery (Pereira and Erickson, 1995).
Despite these successes in related legumes, biolistic transformation has not yet been reported in lupins. While the shoot apical meristem remains the primary target for Agrobacterium-mediated transformation in lupins, the low amenability of this genus to transformation necessitates the exploration of alternative methods, including particle bombardment
3.3 Protoplast-based transformation and regeneration
The intrinsic properties of protoplasts, particularly the absence of a rigid cell wall, facilitate high transformation efficiencies. In L. albus, Wetten et al. (1999) demonstrated the use of polyethylene glycol (PEG)-mediated transfection for direct gene delivery into protoplasts, establishing a critical proof-of-concept for genetic manipulation in this transformation-challenged legume. Their investigations identified critical factors affecting transformation success, including the molecular weight and concentration of PEG, plasmid DNA levels and magnesium ion concentration. While these findings laid the foundation for optimizing gene delivery protocols, stable transformation and subsequent plant regeneration were not achieved, reflecting a broader limitation across many crop species.
4 Genome editing in lupins
Genome editing represents a contemporary and increasingly prevalent application in the domain of crop breeding (Zhang et al., 2018; Chen et al., 2024). Although it has been applied in crop improvement for over a decade, recent advancements have led to the continuous emergence of more refined and versatile editing tools (Capdeville et al., 2023; Chen et al., 2024). These include base editors (Molla et al., 2021), prime editors (Vu et al., 2024), homology-directed repair (Schreiber et al., 2024), micro-homology-mediated end joining (Van Vu et al., 2021) and chromosome engineering (Puchta and Houben, 2024). The deployment of these tools has enabled the development of crops with enhanced nutritional quality (Kumar et al., 2022), increased pathogen resistance (Pathi et al., 2020; Schenke and Cai, 2020; Pathi, 2021) and enhanced adaptability to changing environments (Chennakesavulu et al., 2021).
In legumes, genome editing offers a versatile approach to improving traits beyond stress resistance, including the removal of allergenic or anti-nutritional compounds (e.g., in peanut and grass pea) (Xu et al., 2018; Biswas et al., 2022; Verma et al., 2023) and the functional dissection of genes related to symbiotic nitrogen fixation (Wang et al., 2017, 2019). Despite these advantages, genome editing in legumes remains significantly hindered by low transformation efficiency, which restricts the production of edited events necessary for downstream selection.
To circumvent this challenge, transient protoplast assays have emerged as a valuable alternative in species where stable transformation is inefficient. These assays allow for the rapid pre-screening of guide RNAs (gRNAs) by testing their cleavage efficiency in isolated cells, thereby enabling the selection of high-performing gRNAs prior to stable transformation. Such strategies have been successfully applied in Vigna unguiculata (Bridgeland et al., 2023), Arachis hypogaea (Yuan et al., 2019; Biswas et al., 2022), and Cicer arietinum (Badhan et al., 2021), facilitating targeted mutagenesis in these species.
Among legumes, soybean remains the most advanced model for genome editing due to the availability of reliable transformation systems (Li et al., 2015). Genome editing has been used to improve yield-related traits such as node length and pod number (Chen et al., 2020), alter flowering time (Cai et al., 2018, 2020) and enhance amino acid content (Do et al., 2019). Notably, high-oleic acid soybeans became the first gene-edited crop to reach commercial markets in the United States (Ledford, 2016). Although editing in other legumes has been attempted with good editing efficiencies in the T₀ generation, these are tissue-specific rather than regenerated genome-edited plants (Juranić et al., 2020; Gupta et al., 2023). Nonetheless, examples of regenerated, edited plants include yellow pea lines with improved flavor and fatty acid profiles (Bhowmik et al., 2023) and alfalfa lines with enhanced yield through altered leaf-to-stem ratios (Zhao et al., 2024).
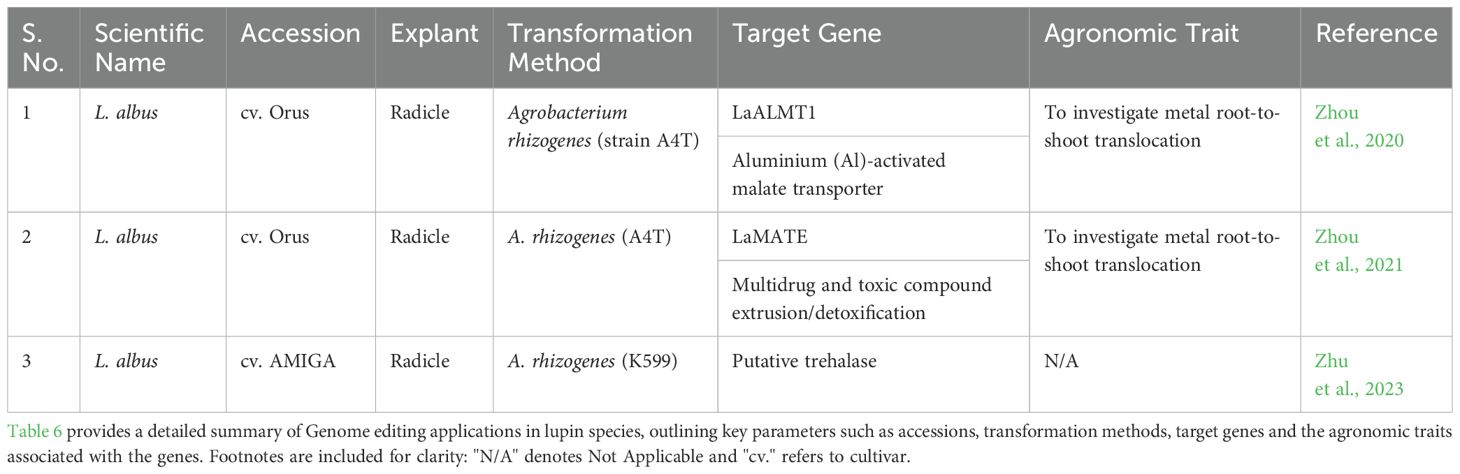
Recent advances in CRISPR-based genome editing have substantially enhanced functional genomics in L. albus, particularly for traits related to nutrient uptake and abiotic stress tolerance. The successful editing of the MATE (Multidrug and Toxic Compound Extrusion) and ALMT (Aluminum-Activated Malate Transporter) genes, critical for aluminum toxicity tolerance (Zhou et al., 2020, 2021), highlights the potential of genome editing for improving environmental resilience in lupin. The optimization of multiplex genome editing through A. rhizogenes-mediated transformation further broadened this toolkit, enabling simultaneous targeting of multiple genes, as demonstrated with the Lalb_Chr05g0223881 trehalase gene (Zhu et al., 2023). However, despite these advances, A. rhizogenes-mediated transformation remains restricted to root tissues full-plant regeneration remains the bottleneck, limiting the evaluation of whole-plant traits essential for comprehensive crop improvement.
5 Potential challenges
Multiple interdependent factors govern the efficiency of in vitro plant regeneration and genetic transformation in Lupinus species, with genotype dependency, explant selection, culture medium composition, Agrobacterium strain and selection marker systems representing key determinants. Despite significant research efforts, the low responsiveness of lupins to in vitro regeneration and stable genetic transformation remains a major bottleneck, impeding progress in genetic improvement programs. Systematic exploration of various regeneration strategies has yielded limited success, underscoring the need for more efficient and reproducible protocols.
Early attempts to achieve lupin regeneration through somatic embryogenesis, particularly from immature cotyledons, have been largely unsuccessful, with low regeneration efficiency reported primarily in L. albus (Nadolska-Orczyk, 1992; Rybczyitski and Podyma, 1993). Organogenesis-based regeneration from meristematic tissues has also been investigated; however, transformation attempts using these tissues often result in chimeric plants, with transformation efficiencies remaining exceptionally low. Similarly, biolistic gene gun transformation, a promising alternative, has been scarcely explored in lupins, highlighting a critical gap in the development of robust transformation methodologies.
Protoplast isolation has been successfully achieved in lupins, but progress in subsequent callus formation and whole-plant regeneration has been minimal, representing a significant technical challenge. Double haploid (DH) technology through microspore and anther culture has achieved limited success in lupins, primarily due to low in vitro responsiveness, inefficient exine rupture, limited callus formation and poor regeneration rates. These challenges have constrained the effective application of DH technology in lupin breeding programs.
The implementation of precision breeding technologies, including targeted genome editing approaches, remains in the early stages of development for lupins. While a few studies have reported the generation of mutated alleles via A. rhizogenes-mediated transformation, these applications remain tissue-specific and have not yet achieved full-plant regeneration. Establishing efficient and reproducible genome editing protocols is essential to unlock the full potential of lupin genetic improvement.
6 Future perspectives
Addressing key biological constraints, such as genotype dependence and tissue-specific regeneration limitations in lupins, is critical for advancing automated transformation systems and enhancing their efficiency and scalability. While conventional approaches involving plant growth regulators and nutrient optimization have shown limited success. The challenges posed by genotype dependency can be partially mitigated through fundamental research aimed at elucidating the underlying biological processes and genetic mechanisms. For instance, identifying genes and pathways associated with genotype dependency is crucial. A notable example is the knockout of SAUR15, an early auxin-responsive gene in maize, which significantly enhanced regeneration efficiency (Wang et al., 2022). Such insights highlight the potential of targeted genetic modifications to overcome regeneration barriers.
Recent studies demonstrate that the expression of developmental regulators (DRs) can significantly enhance regeneration capacity and transformation efficiency in recalcitrant species (Gordon-Kamm et al., 2019). For instance, the GRF4–GIF1 chimera (GROWTH-REGULATING FACTOR4–GRF-INTERACTING FACTOR1) successfully overcame regeneration and transformation recalcitrance in durum wheat, bread wheat and triticale (Debernardi et al., 2020). Similarly, constitutive expression of GRF5 in sugar beet accelerated shoot organogenesis and improved transformation efficiency in hard-to-transform varieties (Kong et al., 2020). In addition to GRFs, several other developmental regulators such as SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE (SERK), WOUND-INDUCED DEDIFFERENTIATION1 (WIND1), LEAFY COTYLEDON1 and 2 (LEC1/2), WUSCHEL (WUS) and BABY BOOM (BBM) have demonstrated significant potential in enhancing regeneration across a variety of plant species by helping to bypass recalcitrance-related barriers (Braybrook and Harada, 2008; Jha and Kumar, 2018; Lian et al., 2022; Yarra and Krysan, 2023; Xu et al., 2024). Targeted expression of DRs offers a potential solution to lupin recalcitrance in regeneration and transformation. Moreover, integrating DR genes into protoplast-based systems could enhance regeneration efficiency, paving the way for improved protoplast fusion and transformation. Successful protoplast regeneration would further enable the use of RNP complexes for precise genome editing, thereby expanding the genetic engineering toolkit for lupin improvement.
Agrobacterium-mediated transformation is progressing in multiple directions, with various strategies being developed to address transformation challenges. These include the utilization of mutated versions of virulence genes, such as VirGN54D (Mortensen et al., 2019) and the adoption of ternary vector systems incorporating helper plasmids containing additional virulence genes, such as pSB1, pHP71539, pVS1-VIR2 and pKL2299 (Komari et al., 1996; Kumlehn et al., 2006; Anand et al., 2018; Zhang et al., 2019; Kang et al., 2022). Additionally, engineered Agrobacterium strains utilizing a type III secretion system to deliver Pseudomonas effectors effectively suppressing host defense responses have markedly increased transformation efficiency in crops like wheat, alfalfa and switchgrass (Raman et al., 2022). Further, the use of auxotrophic strains, such as LBA4404 Thy- and EHA105 Met-, minimizes the need for antibiotics to prevent Agrobacterium overgrowth on tissues, thereby aiding in the optimization and streamlining of transformation protocols (Lowe et al., 2018; Prías-Blanco et al., 2022; Zhong et al., 2025b).
Alongside these advancements, the progress in the development of tissue culture-free transformation (TCFT) systems represents a significant breakthrough in plant biotechnology, offering a promising solution to the persistent challenge of genotype dependency and bypass the need for lengthy in vitro regeneration (Zhong et al., 2025a). Beyond conventional double haploid production methods, emerging approaches utilizing haploidy inducers are gaining traction as alternative strategies for DH generation (Lv and Kelliher, 2023). These methodologies hold great promise for accelerating breeding programs and facilitating the rapid development of superior lupin cultivars.
Collectively, these biotechnological advancements hold transformative potential for overcoming existing limitations and unlocking novel opportunities for the genetic enhancement of lupins.
Author contributions
KMP: Validation, Formal Analysis, Project administration, Data curation, Supervision, Methodology, Writing – review & editing, Conceptualization, Visualization, Software, Funding acquisition, Writing – original draft, Investigation, Resources. TS: Writing – review & editing, Funding acquisition.
Author’s Note
Author KMP has senior authorship for this article.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the German Federal Ministry for Food and Agriculture (Bundesministerium für Ernährung und Landwirtschaft) as part of the Forschungsinitiative Klimaschutz-Sofortprogramm 2022 (RessortForschtKlima) Project name: TRIP.
Acknowledgments
The authors express their sincere gratitude to Hannah Ziesche and Max Wenzel for their exceptional technical assistance in the TRIP project. Additionally, the authors extend their appreciation to Dr. Anja Hühnlein, Grit Lautenbach and the staff members of the JKI library for their dedicated efforts in procuring literature from multiple sources. Furthermore, the authors are thankful to Helge Flüß (JKI, ZL) for providing essential information regarding ploidy. The authors thank Dr. Frank Hartung and Dr. Ralf Wilhelm for their valuable inputs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdul Aziz, M., Brini, F., Rouached, H., and Masmoudi, K. (2022). Genetically engineered crops for sustainably enhanced food production systems. Front. Plant Sci. 13, 2022. doi: 10.3389/fpls.2022.1027828
Abraham, E. M., Ganopoulos, I., Madesis, P., Mavromatis, A., Mylona, P., Nianiou-Obeidat, I., et al. (2019). The use of lupin as a source of protein in animal feeding: genomic tools and breeding approaches. ntenational J. Mol. Sci. 20, 851. doi: 10.3390/ijms20040851
Alkemade, J. A., Messmer, M. M., Arncken, C., Leska, A., Annicchiarico, P., Nazzicari, N., et al. (2021). A high-throughput phenotyping tool to identify field-relevant anthracnose resistance in white lupin. Plant Dis. 105, 1719–1727. doi: 10.1094/PDIS-07-20-1531-RE
Anand, A., Bass, S. H., Wu, E., Wang, N., Mcbride, K. E., Annaluru, N., et al. (2018). An improved ternary vector system for Agrobacterium-mediated rapid maize transformation. Plant Mol. Biol. 97, 187–200. doi: 10.1007/s11103-018-0732-y
Aragão, F. J. L., Sarokin, L., Vianna, G. R., and Rech, E. L. (2000). Selection of transgenic meristematic cells utilizing a herbicidal molecule results in the recovery of fertile transgenic soybean [Glycine max (L.) Merril] plants at a high frequency. Theor. Appl. Genet. 101, 1–6. doi: 10.1007/s001220051441
Aslam, M. M., Fritschi, F. B., Di, Z., Wang, G., Li, H., Lam, H. M., et al. (2023). Overexpression of LaGRAS enhances phosphorus acquisition via increased root growth of phosphorus-deficient white lupin. Physiol. Plant 175, e13962. doi: 10.1111/ppl.v175.4
Aslam, M. M., Karanja, J. K., Zhang, Q., Lin, H., Xia, T., Akhtar, K., et al. (2020). In vitro regeneration potential of white lupin (Lupinus albus) from cotyledonary nodes. Plants (Basel) 9, 318. doi: 10.3390/plants9030318
Aslam, M. M., Waseem, M., Zhang, Q., Ke, W., Zhang, J., and Xu, W. (2021). Identification of ABC transporter G subfamily in white lupin and functional characterization of L.albABGC29 in phosphorus use. BMC Genomics 22, 723. doi: 10.1186/s12864-021-08015-0
Atkins, C. A. and Smith, P. M. C. (2003). ““Transformation of lupins,”,” in Applied genetics of leguminosae biotechnology. Eds. Jaiwal, P. K. and Singh, &R. P. (Springer Netherlands, Dordrecht), 205–211.
Babaoğlu, M. (2000). Protoplast isolation in lupin (Lupinus mutabilis Sweet): determination of optimum explant sources and isolation conditions. Turkish J. Bot. 24, 177–186.
Babaoglu, M., Mccabe, M., Power, J., and Davey, M. (2000). Agrobacterium-mediated transformation of Lupinus mutabilis L. using shoot apical explants. Acta Physiologiae Plantarum 22, 111–119. doi: 10.1007/s11738-000-0064-8
Badhan, S., Ball, A. S., and Mantri, N. (2021). First report of CRISPR/cas9 mediated DNA-free editing of 4CL and RVE7 genes in chickpea protoplasts. Int. J. Mol. Sci. 22, 396. doi: 10.3390/ijms22010396
Ball, E. (1946). Development in sterile culture of stem tips and subjacent regions of Tropaeolum majus L. and Lupinus albus L. Am. J. Bot. 33, 301–318. doi: 10.1002/j.1537-2197.1946.tb10379.x
Barker, S. J., Si, P., Hodgson, L., Ferguson-Hunt, M., Khentry, Y., Krishnamurthy, P., et al. (2016). Regeneration selection improves transformation efficiency in narrow-leaf lupin. Plant Cell Tissue Organ Culture (PCTOC) 126, 219–228. doi: 10.1007/s11240-016-0992-7
Bayliss, K., Wroth, J., and Cowling, W. (2004). Pro-embryos of Lupinus spp. produced from isolated microspore culture. Aust. J. Agric. Res. 55, 589–593. doi: 10.1071/AR03226
Berlin, J., Fecker, L., Rügenhagen, C., Sator, C., Strack, D., Witte, L., et al. (1991). Isoflavone glycoside formation in transformed and non-transformed suspension and hairy root cultures of Lupinus polyphyllus and Lupinus hartwegii. Z. für Naturforschung C 46, 725–734. doi: 10.1515/znc-1991-9-1003
Beşer, N. and Wetten, A. (1996). Protoplast isolation in lupin. Lupinus J. Agric. Sci. 02, 73–79. doi: 10.1501/Tarimbil_0000000674
Bhowmik, P., Yan, W., Hodgins, C., Polley, B., Warkentin, T., Nickerson, M., et al. (2023). CRISPR/Cas9-mediated lipoxygenase gene-editing in yellow pea leads to major changes in fatty acid and flavor profiles. Front. Plant Sci. 14, 2023. doi: 10.3389/fpls.2023.1246905
Biswas, S., Wahl, N. J., Thomson, M. J., Cason, J. M., Mccutchen, B. F., and Septiningsih, E. M. (2022). Optimization of protoplast isolation and transformation for a pilot study of genome editing in peanut by targeting the allergen gene ara h 2. Int. J. Mol. Sci. 23, 837. doi: 10.3390/ijms23020837
Braybrook, S. A. and Harada, J. J. (2008). LECs go crazy in embryo development. Trends Plant Sci. 13, 624–630. doi: 10.1016/j.tplants.2008.09.008
Bridgeland, A., Biswas, S., Tsakirpaloglou, N., Thomson, M. J., and Septiningsih, E. M. (2023). Optimization of gene editing in cowpea through protoplast transformation and agroinfiltration by targeting the phytoene desaturase gene. PloS One 18, e0283837. doi: 10.1371/journal.pone.0283837
Bridgen, M. P. (1994). A review of plant embryo culture. In: In vitro breeding techniques. Proceedings of a workshop held at the 88th ASHS annual meeting, (University Park, PA, USA: ASHS annual meeting).
Busmann-Loock, A., Dambroth, M., and Menge-Hartmann, U. (1991). Histologische Untersuchungen zur Embryo- und Endospermentwicklung von Lupinus luteus, L. mutabilis und L. bartwegii. Landbauforschung Völkenrode 41, 123–132. Institut für Pflanzenbau und Pflanzenzüchtung.
Cai, Y., Chen, L., Sun, S., Wu, C., Yao, W., Jiang, B., et al. (2018). CRISPR/cas9-mediated deletion of large genomic fragments in soybean. Int. J. Mol. Sci. 19, 3835. doi: 10.3390/ijms19123835
Cai, Y., Wang, L., Chen, L., Wu, T., Liu, L., Sun, S., et al. (2020). Mutagenesis of GmFT2a and GmFT5a mediated by CRISPR/Cas9 contributes for expanding the regional adaptability of soybean. Plant Biotechnol. J. 18, 298–309. doi: 10.1111/pbi.13199
Capdeville, N., Schindele, P., and Puchta, H. (2023). Getting better all the time - recent progress in the development of CRISPR/Cas-based tools for plant genome engineering. Curr. Opin. Biotechnol. 79, 102854. doi: 10.1016/j.copbio.2022.102854
Chen, F., Chen, L., Yan, Z., Xu, J., Feng, L., He, N., et al. (2024). Recent advances of CRISPR-based genome editing for enhancing staple crops. Front. Plant Sci. Volume 15, 2024. doi: 10.3389/fpls.2024.1478398
Chen, Z., Debernardi, J. M., Dubcovsky, J., and Gallavotti, A. (2022). Recent advances in crop transformation technologies. Nat. Plants 8, 1343–1351. doi: 10.1038/s41477-022-01295-8
Chen, L., Nan, H., Kong, L., Yue, L., Yang, H., Zhao, Q., et al. (2020). Soybean AP1 homologs control flowering time and plant height. J. Integr. Plant Biol. 62, 1868–1879. doi: 10.1111/jipb.v62.12
Chennakesavulu, K., Singh, H., Trivedi, P. K., Jain, M., and Yadav, S. R. (2021). State-of-the-art in CRISPR technology and engineering drought, salinity, and thermo-tolerant crop plants. Plant Cell Rep. 41, 815–831. doi: 10.1007/s00299-021-02681-w
Croser, J. S., M., L. M., A., D. P., J., C. H., L., B. K., N., M., et al. (2006). Toward doubled haploid production in the fabaceae: progress, constraints, and opportunities. Crit. Rev. Plant Sci. 25, 139–157. doi: 10.1080/07352680600563850
Cruz, A. and Aragão, F. (2014). RNA i-based enhanced resistance to C owpea severe mosaic virus and C owpea aphid-borne mosaic virus in transgenic cowpea. Plant Pathol. 63, 831–837. doi: 10.1111/ppa.2014.63.issue-4
Daghma, D. E. S. (2011). Structural changes during the initiation of pollen embryogenesis in barley [Doctoral dissertation]. (Martin-Luther-Universität Halle-Wittenberg. Universitäts- und Landesbibliothek Sachsen-Anhalt). doi: 10.25673/1233
Das, D. (2018). Expression of a bacterial chitinase (ChiB) gene enhances resistance against Erysiphae polygoni induced powdery mildew disease in the transgenic black gram (Vigna mungo L.)(cv. T9). Am. J. Plant Sci. 9, 1759–1770. doi: 10.4236/ajps.2018.98128
Dayal, S., Lavanya, M., Devi, P., and Sharma, K. (2003). An efficient protocol for shoot regeneration and genetic transformation of pigeonpea [Cajanus cajan (L.) Millsp.] using leaf explants. Plant Cell Rep. 21, 1072–1079. doi: 10.1007/s00299-003-0620-y
Daza, A. and Chamber, M. A. (1993). Plant regeneration from hypocotyl segments of Lupinus luteus cv. L. Aurea. Plant Cell Tissue Organ Culture 34, 303–305. doi: 10.1007/BF00029721
Debernardi, J. M., Tricoli, D. M., Ercoli, M. F., Hayta, S., Ronald, P., Palatnik, J. F., et al. (2020). A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 38, 1274–1279. doi: 10.1038/s41587-020-0703-0
Do, P. T., Nguyen, C. X., Bui, H. T., Tran, L. T. N., Stacey, G., Gillman, J. D., et al. (2019). Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2–1A and GmFAD2–1B genes to yield a high oleic, low linoleic and α-linolenic acid phenotype in soybean. BMC Plant Biol. 19, 311. doi: 10.1186/s12870-019-1906-8
Drummond, C. S., Eastwood, R. J., Miotto, S. T., and Hughes, C. E. (2012). Multiple continental radiations and correlates of diversification in Lupinus (Leguminosae): testing for key innovation with incomplete taxon sampling. Syst. Biol. 61, 443–460. doi: 10.1093/sysbio/syr126
Duarte, C. M., Mota, J., Assunção, R., Martins, C., Ribeiro, A. C., Lima, A., et al. (2022). New alternatives to milk from pulses: chickpea and lupin beverages with improved digestibility and potential bioactivities for human health. Front. Nutr. Volume 9, 2022. doi: 10.3389/fnut.2022.852907
Erdemoglu, N., Ozkan, S., and Tosun, F. (2007). Alkaloid profile and antimicrobial activity of Lupinus angustifolius L. alkaloid extract. Phytochem. Rev. 6, 197–201. doi: 10.1007/s11101-006-9055-8
Eudes, F., Shim, Y.-S., and Jiang, F. (2014). Engineering the haploid genome of microspores. Biocatalysis Agric. Biotechnol. 3, 20–23. doi: 10.1016/j.bcab.2013.11.002
Garg, G., Kamphuis, L. G., Bayer, P. E., Kaur, P., Dudchenko, O., Taylor, C. M., et al. (2022). A pan-genome and chromosome-length reference genome of narrow-leafed lupin (Lupinus angustifolius) reveals genomic diversity and insights into key industry and biological traits. Plant J. 111, 1252–1266. doi: 10.1111/tpj.v111.5
Gordon-Kamm, B., Sardesai, N., Arling, M., Lowe, K., Hoerster, G., Betts, S., et al. (2019). Using morphogenic genes to improve recovery and regeneration of transgenic plants. Plants 8, 38. doi: 10.3390/plants8020038
Grazziotin, M. A., Cabral, G. B., Ibrahim, A. B., MaChado, R. B., and Aragão, F. J. (2020). Expression of the Arcelin 1 gene from Phaseolus vulgaris L. @ in cowpea seeds (Vigna unguiculata L.) confers bruchid resistance. Ann. Appl. Biol. 176, 268–274. doi: 10.1111/aab.v176.3
Guilengue, N., Alves, S., Talhinhas, P., and Neves-Martins, J. (2020). Genetic and genomic diversity in a tarwi (Lupinus mutabilis Sweet) germplasm collection and adaptability to Mediterranean climate conditions. Agronomy 10, 21. doi: 10.3390/agronomy10010021
Gupta, S. K., Vishwakarma, N. K., Malakar, P., Vanspati, P., Sharma, N. K., and Chattopadhyay, D. (2023). Development of an Agrobacterium-delivered codon-optimized CRISPR/Cas9 system for chickpea genome editing. Protoplasma 260, 1437–1451. doi: 10.1007/s00709-023-01856-4
Hondelmann, W. (1984). The lupin - ancient and modern crop plant. Theor. Appl. Genet. 68, 1–9. doi: 10.1007/BF00252301
Horoszkiewicz-Janka, J., Korbas, M., Jajor, E., and Krawczyk, R. (2011). Health of narrow-leaved lupin (Lupinus angustifolius L.) cultivated in the conventional farming system and in transition period to the ecological system. J. Res. Appl. Agric. Eng. 55, 143–146.
Hufnagel, B., Marques, A., Soriano, A., Marquès, L., Divol, F., Doumas, P., et al. (2020). High-quality genome sequence of white lupin provides insight into soil exploration and seed quality. Nat. Commun. 11, 492. doi: 10.1038/s41467-019-14197-9
Hughes, C. and Eastwood, R. (2006). Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl. Acad. Sci. U.S.A. 103, 10334–10339. doi: 10.1073/pnas.0601928103
Indurker, S., Misra, H. S., and Eapen, S. (2007). Genetic transformation of chickpea (Cicer arietinum L.) with insecticidal crystal protein gene using particle gun bombardment. Plant Cell Rep. 26, 755–763. doi: 10.1007/s00299-006-0283-6
Ivo, N. L., Nascimento, C. P., Vieira, L. S., Campos, F. A., and Aragao, F. J. (2008). Biolistic-mediated genetic transformation of cowpea (Vigna unguiculata) and stable Mendelian inheritance of transgenes. Plant Cell Rep. 27, 1475–1483. doi: 10.1007/s00299-008-0573-2
Jaranowski, J. (1962). Fertilization and embryo development in the genus Lupinus Tourn. Part II Fertilization embryo Dev. following reciprocal species hybridization. 3, 19–25.
Jha, P. and Kumar, V. (2018). BABY BOOM (BBM): a candidate transcription factor gene in plant biotechnology. Biotechnol. Lett. 40, 1467–1475. doi: 10.1007/s10529-018-2613-5
Juranić, M., Nagahatenna, D. S. K., Salinas-Gamboa, R., Hand, M. L., Sánchez-León, N., Leong, W. H., et al. (2020). A detached leaf assay for testing transient gene expression and gene editing in cowpea (Vigna unguiculata [L.] Walp.). Plant Methods 16, 88. doi: 10.1186/s13007-020-00630-4
Kang, M., Lee, K., Finley, T., Chappell, H., Veena, V., and Wang, K. (2022). An improved agrobacterium-mediated transformation and genome-editing method for maize inbred B104 using a ternary vector system and immature embryos. Front. Plant Sci. 13, 2022. doi: 10.3389/fpls.2022.860971
Komari, T., Hiei, Y., Saito, Y., Murai, N., and Kumashiro, T. (1996). Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 10, 165–174. doi: 10.1046/j.1365-313X.1996.10010165.x
Kong, J., Martin-Ortigosa, S., Finer, J., Orchard, N., Gunadi, A., Batts, L. A., et al. (2020). Overexpression of the transcription factor GROWTH-REGULATING FACTOR5 improves transformation of dicot and monocot species. Front. Plant Sci. 11, 572319. doi: 10.3389/fpls.2020.572319
Kotecki, A.. (2015). Adaptation of the Adean lupin (Lupinus mutabilis Sweet) to natural conditions of south-western Poland. Edition No. 195. (Wrocław: Uniwersytet Przyrodniczy we Wrocławiu).
Kotecki, A., Bobrecka-Jamro, D., Fordoński, G., Kozak, M., Prusiński, J., Pszczółkowska, A., et al. (2020). Leguminous plants with coarse seeds. Crop cultivation 3, 21–231.
Kowalczyk, T., Merecz-Sadowska, A., Picot, L., Brčić Karačonji, I., Wieczfinska, J., Śliwiński, T., et al. (2022). Genetic manipulation and bioreactor culture of plants as a tool for industry and its applications. Molecules 27, 795. doi: 10.3390/molecules27030795
Kozak, K., Galek, R., Waheed, M. T., and Sawicka-Sienkiewicz, E. (2012). Anther culture of Lupinus angustifolius: callus formation and the development of multicellular and embryo-like structures. Plant Growth Regul. 66, 145–153. doi: 10.1007/s10725-011-9638-2
Kumar, D., Yadav, A., Ahmad, R., Dwivedi, U. N., and Yadav, K. (2022). CRISPR-based genome editing for nutrient enrichment in crops: A promising approach toward global food security. Front. Genet. 13, 932859. doi: 10.3389/fgene.2022.932859
Kumlehn, J., Serazetdinova, L., Hensel, G., Becker, D., and Loerz, H. (2006). Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with Agrobacterium tumefaciens. Plant Biotechnol. J. 4, 251–261. doi: 10.1111/j.1467-7652.2005.00178.x
Ledford, H. (2016). Gene-editing surges as US rethinks regulations. Nature 532, 158–159. doi: 10.1038/532158a
Lee, A. E. (1955a). Growth in culture of excised portions of lupine embryos. Botanical Gazette 116, 359–364. doi: 10.1086/335879
Lee, A. E. (1955b). Potentially unlimited growth of lupine callus. Botanical Gazette 116, 364–368. doi: 10.1086/335880
Li, Z., Liu, Z.-B., Xing, A., Moon, B. P., Koellhoffer, J. P., Huang, L., et al. (2015). Cas9-guide RNA directed genome editing in soybean. Plant Physiol. 169, 960–970. doi: 10.1104/pp.15.00783
Li, H., Wylie, S. J., and Jones, M. G. K. (2000). Transgenic yellow lupin (Lupinus luteus). Plant Cell Rep. 19, 634–637. doi: 10.1007/s002990050785
Lian, Z., Nguyen, C. D., Liu, L., Wang, G., Chen, J., Wang, S., et al. (2022). Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol. J. 20, 1622–1635. doi: 10.1111/pbi.13837
Lichtin, N., Salvo-Garrido, H., Till, B., Caligari, P. D. S., Rupayan, A., Westermeyer, F., et al. (2020). Genetic and comparative mapping of Lupinus luteus L. highlight syntenic regions with major orthologous genes controlling anthracnose resistance and flowering time. Sci. Rep. 10, 19174. doi: 10.1038/s41598-020-76197-w
Lowe, K., La Rota, M., Hoerster, G., Hastings, C., Wang, N., Chamberlin, M., et al. (2018). Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell Dev. Biol. Plant 54, 240–252. doi: 10.1007/s11627-018-9905-2
Loyola-Vargas, V. M. and Avilez-Montalvo, R. N. (2018). Plant tissue culture: A battle horse in the genome editing using CRISPR/cas9. Methods Mol. Biol. 1815, 131–148. doi: 10.1007/978-1-4939-8594-4
Lucas, M. M., Stoddard, F. L., Annicchiarico, P., Frias, J., Martinez-Villaluenga, C., Sussmann, D., et al. (2015). The future of lupin as a protein crop in Europe. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00705
Lv, J. and Kelliher, T. (2023). Recent advances in engineering of in vivo haploid induction systems. Methods Mol. Biol. 2653, 365–383. doi: 10.1007/978-1-0716-3131-7_22
Maknickienė, Z. and Asakaviciute, R. (2008). Alkaloid content variations in lupin (Lupinus L.) genotypes and vegetation periods. Biologija 54, 112–115. doi: https://doi.org/10.2478/v10054-008-0023-7
Mancinotti, D., Czepiel, K., Taylor, J. L., Golshadi Galehshahi, H., Møller, L. A., Jensen, M. K., et al. (2023). The causal mutation leading to sweetness in modern white lupin cultivars. Sci. Adv. 9, eadg8866. doi: 10.1126/sciadv.adg8866
Mancinotti, D., Frick, K. M., and Geu-Flores, F. (2022). Biosynthesis of quinolizidine alkaloids in lupins: mechanistic considerations and prospects for pathway elucidation. Natural Product Rep. 39, 1423–1437. doi: 10.1039/D1NP00069A
Mancinotti, D., Yang, T., and Geu-Flores, F. (2025). Metabolic engineering of narrow-leafed lupin for the production of enantiomerically pure (–)-sparteine. Plant Biotechnol. J. 23, 467–476. doi: 10.1111/pbi.14509
Martínez, M. T. and Corredoira, E. (2024). Recent advances in plant somatic embryogenesis: where we stand and where to go? Int. J. Mol. Sci. 25, 8912. doi: 10.3390/ijms25168912
Martinez-Hernandez, J. E., Salvo-Garrido, H., Levicoy, D., Caligari, P. D., Rupayán, A., Moyano, T., et al. (2024). Chromosome-level genome assembly of yellow lupin (Lupinus luteus) provides novel insights into genome evolution, crop adaptation and seed protein in the three most cultivated lupins. Preprint, Research Square. doi: 10.21203/rs.3.rs-4171664/v1
Mavromatis, A., Nianiou-Obeidat, I., Polidoros, A., Parissi, Z., Tani, E., Irakli, M., et al. (2023). Characterization of lupin cultivars based on phenotypical, molecular and metabolomic analyses. Agronomy 13, 370. doi: 10.3390/agronomy13020370
Molla, K. A., Sretenovic, S., Bansal, K. C., and Qi, Y. (2021). Precise plant genome editing using base editors and prime editors. Nat. Plants 7, 1166–1187. doi: 10.1038/s41477-021-00991-1
Molvig, L., Tabe, L. M., Eggum, B. O., Moore, A. E., Craig, S., Spencer, D., et al. (1997). Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius L.) expressing a sunflower seed albumin gene. Proc. Natl. Acad. Sci. U.S.A. 94, 8393–8398. doi: 10.1073/pnas.94.16.8393
Mortensen, S., Bernal-Franco, D., Cole, L. F., Sathitloetsakun, S., Cram, E. J., and Lee-Parsons, C. W. T. (2019). EASI transformation: an efficient transient expression method for analyzing gene function in catharanthus roseus seedlings. Front. Plant Sci. 10, 755. doi: 10.3389/fpls.2019.00755
Mugnier, J. (1988). Establishment of new axenic hairy root lines by inoculation with Agrobacterium rhizogenes. Plant Cell Rep. 7, 9–12. doi: 10.1007/BF00272966
Mulin, M. and Bellio-Spataru, A. (2000). Organogenesis from hypocotyl thin cell layers of Lupinus mutabilis and Lupinus albus. Plant Growth Regul. 30, 177–183. doi: 10.1023/A:1006345401325
Murovec, J. and Bohanec, B. (2012). Haploids and doubled haploids in plant breeding. In: Plant Breeding, ed. Abdurakhmonov, I. IntechOpen. doi: 10.5772/29982
Nadolska-Orczyk, A. (1992). Somatic embryogenesis of agriculturally important lupin species (Lupinus angustifolius, L. albus, L. mutabilis). Plant Cell Tissue Organ Culture 28, 19–25. doi: 10.1007/BF00039911
Nguyen, A., Hodgson, L., Erskine, W., and Barker, S. (2016a). An approach to overcoming regeneration recalcitrance in genetic transformation of lupins and other legumes. Plant Cell Tissue Organ Culture (PCTOC) 127, 623–635. doi: 10.1007/s11240-016-1087-1
Nguyen, A. H., Wijayanto, T., Erskine, W., and Barker, S. J. (2016b). Using green fluorescent protein sheds light on Lupinus angustifolius L. transgenic shoot development. Plant Cell Tissue Organ Culture (PCTOC) 127, 665–674. doi: 10.1007/s11240-016-1079-1
Nivya, V. M. and Shah, J. M. (2023). Recalcitrance to transformation, a hindrance for genome editing of legumes. Front. Genome Editing 5. doi: 10.3389/fgeed.2023.1247815
Ormerod, A. and Caligari, P. (1994). Anther and microspore culture of Lupinus albus in liquid culture medium. Plant Cell Tissue Organ Culture 36, 227–236. doi: 10.1007/BF00037724
Osorio, C. E. and Till, B. J. (2021). A bitter-sweet story: unraveling the genes involved in quinolizidine alkaloid synthesis in lupinus albus. Front. Plant Sci. 12, 795091. doi: 10.3389/fpls.2021.795091
Ozyigit, I. and Yucebilgili Kurtoglu, K. (2020). Particle bombardment technology and its applications in plants. Mol. Biol. Rep. 47, 9831–9847. doi: 10.1007/s11033-020-06001-5
Pancaldi, F., Gulisano, A., Severing, E. I., Van Kaauwen, M., Finkers, R., Kodde, L., et al. (2024). The genome of Lupinus mutabilis: Evolution and genetics of an emerging bio-based crop. Plant J. 120, 881–900. doi: 10.1111/tpj.v120.3
Pathi, K. (2021). Establishment of maize resistance to fungal diseases by host-induced gene silencing and site-directed mutagenesis [Doctoral dissertation]. (Hannover: Institutionelles Repositorium der Leibniz Universität Hannover).
Pathi, K. M., Rink, P., Budhagatapalli, N., Betz, R., Saado, I., Hiekel, S., et al. (2020). Engineering smut resistance in maize by site-directed mutagenesis of LIPOXYGENASE 3. Front. Plant Sci. 11, 543895. doi: 10.3389/fpls.2020.543895
Pathi, K. M. and Sprink, T. (2023). From petri dish to field: plant tissue culture and genetic engineering of oats for improved agricultural outcomes. Plants 12, 3782. doi: 10.3390/plants12213782
Pathi, K. M., Tula, S., and Tuteja, N. (2013). High frequency regeneration via direct somatic embryogenesis and efficient Agrobacterium-mediated genetic transformation of tobacco. Plant Signal Behav. 8, e24354. doi: 10.4161/psb.24354
Pereira, L. F. and Erickson, L. (1995). Stable transformation of alfalfa (Medicago sativa L.) by particle bombardment. Plant Cell Rep. 14, 290–293. doi: 10.1007/BF00232030
Pereira, A., Ramos, F., and Sanches Silva, A. (2022). Lupin (Lupinus albus L.) seeds: balancing the good and the bad and addressing future challenges. Molecules 27. doi: 10.3390/molecules27238557
Petterson, D. (1998). ““Composition and food uses of lupins,”,” in Lupins as crop plants: biology, production, and utilization. Ed. Gladstones, J. S. (CAB INTERNATIONAL, Wallingford), 353–384.
Petterson, D. S. (2016). ““Lupin: overview,”,” in Encyclopedia of food grains (Second edition). Eds. Wrigley, C., Corke, H., Seetharaman, K., and Faubion, &J. (Academic Press, Oxford), 280–286.
Phoplonker, M. A. and Caligari, P. D. S. (1993). Cultural manipulations affecting callus formation from seedling explants of the pearl lupin (Lupinus mutabilis Sweet). Ann. Appl. Biol. 123, 419–432. doi: 10.1111/j.1744-7348.1993.tb04104.x
Pigeaire, A., Abernethy, D., Smith, P. M., Simpson, K., Fletcher, N., Lu, C.-Y., et al. (1997). Transformation of a grain legume (Lupinus angustifolius L.) via Agrobacterium tumefaciens-mediated gene transfer to shoot apices. Mol. Breed. 3, 341–349. doi: 10.1023/A:1009642620907
Pniewski, T., Kapusta, J., and Legocki, A. B. (2002). In vitro micropropagation of four lupin species. Acta Physiologiae Plantarum 24, 417–424. doi: 10.1007/s11738-002-0038-0
Pniewski, T., Kapusta, J., and Płucienniczak, A. (2006). Agrobacterium-mediated transformation of yellow lupin to generate callus tissue producing HBV surface antigen in a long-term culture. J. Appl. Genet. 47, 309–318. doi: 10.1007/BF03194640
Podyma, E., Turzynski, D., and Rybczyitski, J. J. (1988). An immature embryo culture, vegetative propagation and somatic cell genetic manipulation of Lupinus taxa. Proc. Vth Int. Lupin Conf., 439–443.
Polowick, P., Loukanina, N., and Doshi, K. (2014). Agrobacterium-mediated transformation of tarwi (Lupinus mutabilis Sweet), a potential platform for the production of plant-made proteins. In Vitro Cell. Dev. Biol. - Plant 50, 401–411. doi: 10.1007/s11627-014-9601-9
Prías-Blanco, M., Chappell, T. M., Freed, E. F., Illa-Berenguer, E., Eckert, C. A., and Parrott, W. A. (2022). An Agrobacterium strain auxotrophic for methionine is useful for switchgrass transformation. Transgenic Res. 31, 661–676. doi: 10.1007/s11248-022-00328-4
Pszczółkowski, P., Barbara, S., Barbaś, P., and Krochmal-Marczak, B. (2025). ““Lupin (Lupinus spp.) breeding and biotechnology: new perspectives and methods,”,” in Breeding of ornamental crops: annuals and cut flowers. Eds. Al-Khayri, M., Jain, S. M., and Wani, &M. A. (Springer Nature Switzerland, Cham), 165–220.
Puchta, H. and Houben, A. (2024). Plant chromosome engineering – past, present and future. New Phytol. 241, 541–552. doi: 10.1111/nph.v241.2
Rahman, S. U., Khan, M. O., Ullah, R., Ahmad, F., and Raza, G. (2024). Agrobacterium-mediated transformation for the development of transgenic crops; present and future prospects. Mol. Biotechnol. 66, 1836–1852. doi: 10.1007/s12033-023-00826-8
Raman, V., Rojas, C. M., Vasudevan, B., Dunning, K., Kolape, J., Oh, S., et al. (20222581). Agrobacterium expressing a type III secretion system delivers Pseudomonas effectors into plant cells to enhance transformation. Nat. Commun. 13, 2581. doi: 10.1038/s41467-022-30180-3
Ramírez-Betancourt, A., Hernández-Sánchez, A. M., Salcedo-Morales, G., Ventura-Zapata, E., Robledo, N., Wink, M., et al. (2021). Unraveling the biosynthesis of quinolizidine alkaloids using the genetic and chemical diversity of mexican lupins. Diversity 13, 375. doi: 10.3390/d13080375
Rech, E. L., Vianna, G. R., and Aragão, F. J. L. (2008). High-efficiency transformation by biolistics of soybean, common bean and cotton transgenic plants. Nat. Protoc. 3, 410–418. doi: 10.1038/nprot.2008.9
Resta, D., Boschin, G., D’agostina, A., and Arnoldi, A. (2008). Quantification of quinolizidine alkaloids in lupin seeds, lupin-based ingredients and foods. In: Palta, J. A. and Berger, J. B. (eds) Lupins for Health and Wealth. Proceedings of the 12th International Lupin Conference, 14–18 September 2008, Fremantle, Western Australia. Canterbury, New Zealand: International Lupin Association, pp 533–535.
Rybczyitski, J. J. and Podyma, E. (1993). Preliminary studies of plant regeneration via somatic embryogenesis induced on immature cotyledons of white lupin (Lupinus albus L.). Genetica Polonica 34, 249–257.
Rybczyński, J. and Podyma, E. (1993). Preliminary studies of plant regeneration via somatic embryogenesis induced on immature cotyledons of white lupin (Lupinus albus L.). Genetica Polonica 34, 249–257.
Rybczynski, J. J. and Podyma, E. (1993). Micropropagation of some lupinus species from seedling explants. Genetica Polonica 34, 237–247.
Sator, C. (1985). Plant regeneration from anthers of lupins. Landbauforschung Voelkenrode (Germany FR) 35, 5–7.
Sator, C., Mix, G., and Menge, U. (1983). Investigations of anther culture of. Lupinus polyphyllus. 18, 37–46.
Sawicka, B. and Pszczółkowski, P. (2014). Resistance cultivars yellow lupine to Fusarium. Fragm. Agron. 31, 83–94.
Sawicka-Sienkiewicz, E., Galek, R., Zalewski, D., and Augiewicz, J. (2006). Comparison of interspecific hybrids Lupinus albus (sensu lato)× Lupinus mutabilis in respect of some quantitative characters. Biul. Inst. Hod. Aklim. Roślin 240/241, 253–260.
Schäfer-Menuhr, A. (1985). In vitro techniques: propagation and long term storage (Dordrecht, Netherlands: Springer Netherlands) 210 pp.
Schäfer-Menuhr, A. (1987). Isolation und Kultur von Lupinenprotoplasten: I. Protoplasten aus Blättern von Lupinus angustifolius Sorte Kubesa. Landbauforschung Völkenrode: FAL Agric. Res. 37, 117–120.
Schäfer-Menuhr, A. (1988). Isolation und Kultur von Lupinenprotoplasten: III. Protoplasten aus Zellsuspensionskulturen von Lupinus polyphyllus. Vol. 38 (Landbauforschung Völkenrode: FAL agricultural research) 38, 99–102.
Schäfer-Menuhr, A. (1989). Isolation und Kultur von Lupinenprotoplasten: IV. Regeneration von Pflanzen aus Protoplasten. Landbauforschung Völkenrode: FAL Agric. Res. 39, 133–136.
Schafer-Menuhr, A., Busmann, A., and Czerwinski, T. (1988). Embryo rescue of interspecific hybrids. Proc. 5th Int. Lupin Conf., 424–428.
Schäfer-Menuhr, A. and Stuermer, S. (1987). Isolation und Kultur von Lupinenprotoplasten: II. Modifikation von Nährmedien zur beschleunigten Teilung von Protoplasten aus Blättern von Lupinus angustifolius Sorte Kubesa Vol. 37 (Landbauforschung Völkenrode: FAL agricultural research), 231–234.
Schenke, D. and Cai, D. (2020). Applications of CRISPR/cas to improve crop disease resistance: beyond inactivation of susceptibility factors. iScience 23, 101478. doi: 10.1016/j.isci.2020.101478
Schreiber, T., Prange, A., Schäfer, P., Iwen, T., Grützner, R., Marillonnet, S., et al. (2024). Efficient scar-free knock-ins of several kilobases in plants by engineered CRISPR-Cas endonucleases. Mol. Plant 17, 824–837. doi: 10.1016/j.molp.2024.03.013
Siemons, C., Jonkers, S., Vlieg, R. C., Corral-Martínez, P., Van Noort, J., and Boutilier, K. (2025). Establishment and maintenance of embryogenic cell fate during microspore embryogenesis. Plant J. 121, e17243. doi: 10.1111/tpj.v121.4
Sinha, A. and Caligari, P. D. S. (2004). Aspects of isolation underpinning mitotic behaviour in lupin protoplasts. Aust. J. Bot. 52, 669–676. doi: 10.1071/BT03153
Sinha, A. and Caligari, P. D. S. (2005). Enhanced protoplast division by encapsulation in droplets: An advance towards somatic hybridisation in recalcitrant white lupin. Ann. Appl. Biol. 146, 441–448. doi: 10.1111/j.1744-7348.2005.040097.x
Sinha, A., Wetten, A., and Caligari, P. (2003). Effect of biotic factors on the isolation of Lupinus albus protoplasts. Aust. J. Bot. - Aust. J. Bot. 51, 103–109. doi: 10.1071/BT01104
Skrzypek, E., Czyczyło-Mysza, I., Marcińska, I., and Wędzony, M. (2008). Prospects of androgenetic induction in. Lupinus Plant cell Tissue Organ culture 94, 131–137. doi: 10.1007/s11240-008-9396-7
Skrzypkowski, W. and Kiełkowska, A. (20241031). Current status of haploidization in cool-season grain legume crop species. Agriculture 14, 1031. doi: 10.3390/agriculture14071031
Sonntag, K., Ruge-Wehling, B., and Wehling, P. (2009). Protoplast isolation and culture for somatic hybridization of Lupinus angustifolius and L. subcarnosus. Plant Cell Tissue Organ Culture (PCTOC) 96, 297–305. doi: 10.1007/s11240-008-9487-5
Thu, T. T., Dewaele, E., Trung, L. Q., Claeys, M., Jacobs, M., and Angenon, G. (2007). Increasing lysine levels in pigeonpea (Cajanus cajan (L.) Millsp) seeds through genetic engineering. Plant Cell Tissue Organ Culture 91, 135–143. doi: 10.1007/s11240-007-9227-2
Upadhyaya, A., Davis, T. D., Sankhla, D., and Sankhla, N. (1992). Micropropagation of Lupinus texensis from cotyledonary node explants. HortScience HortSci 27, 1222–1223. doi: 10.21273/HORTSCI.27.11.1222
Van De Noort, M. (2017). ““Chapter 10 - lupin: an important protein and nutrient source,”,” in Sustainable protein sources. Eds. Nadathur, S. R., Wanasundara, J. P. D., and Scanlin, &L. (Academic Press, San Diego), 165–183.
Van Vu, T., Thi Hai Doan, D., Kim, J., Sung, Y. W., Thi Tran, M., Song, Y. J., et al. (2021). CRISPR/Cas-based precision genome editing via microhomology-mediated end joining. Plant Biotechnol. J. 19, 230–239. doi: 10.1111/pbi.13490
Verma, A., Kaur, L., Kaur, N., Bhardwaj, A., Pandey, A. K., and Kandoth, P. K. (2023). An efficient hairy root system for genome editing of a β-ODAP pathway gene in Lathyrus sativus. bioRxiv. 2023.2004. 2003.535460. doi: 10.1101/2023.04.03.535460
Vu, T., Nguyen, N., Kim, J., Song, Y., Nguyen, T., and Kim, J.-Y. (2024). Breakthrough in dicot prime editing: enabling heritable desired edits in tomato and arabidopsis. Nat. Plants 10, 1502–1513. doi: 10.1038/s41477-024-01786-w
Vuillaume, E. and Hoff, T. (1986). Développement in vitro d’embryons immatures de Lupinus albus L. et de Lupinus mutabilis Sweet par culture de gousses, d’ovules ou d’embryons isolés. Agronomie 6, 925–930. doi: 10.1051/agro:19861008
Wang, Y., He, S., Long, Y., Zhang, X., Zhang, X., Hu, H., et al. (2022). Genetic variations in ZmSAUR15 contribute to the formation of immature embryo-derived embryonic calluses in maize. Plant J. 109, 980–991. doi: 10.1111/tpj.v109.4
Wang, L., Rubio, M. C., Xin, X., Zhang, B., Fan, Q., Wang, Q., et al. (2019). CRISPR/Cas9 knockout of leghemoglobin genes in Lotus japonicus uncovers their synergistic roles in symbiotic nitrogen fixation. New Phytol. 224, 818–832. doi: 10.1111/nph.v224.2
Wang, L., Wang, L., Zhou, Y., and Duanmu, D. (2017). Use of CRISPR/cas9 for symbiotic nitrogen fixation research in legumes. Prog. Mol. Biol. Transl. Sci. 149, 187–213. doi: 10.1016/bs.pmbts.2017.03.010
Wang, P., Zhou, G., Jian, J., Yang, H., Renshaw, D., Aubert, M. K., et al. (2021). Whole-genome assembly and resequencing reveal genomic imprint and key genes of rapid domestication in narrow-leafed lupin. Plant J. 105, 1192–1210. doi: 10.1111/tpj.v105.5
Wetten, A., Suso, H.-P., and Caligari, P. D. S. (1999). “Direct gene transfer to Lupinus albus protoplasts,” in Lupin, an Ancient Crop for the New Millenium. Proceedings of the 9th International Lupin Conference (Canterbury, New Zealand).
Williams, W., Akhtar, M., and Faluyi, M. (1980). Cross compatibility between European and American Lupinus species. Botanical J. Linn. Soc. 81, 225–232. doi: 10.1111/j.1095-8339.1980.tb01675.x
Wink, M., Meißner, C., and Witte, L. (1995). Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry 38, 139–153. doi: 10.1016/0031-9422(95)91890-D
Wiszniewska, A. and Pindel, A. (2009). Improvement in Lupinus luteus (Fabaceae) protoplast culture – stimulatory effect of agarose embedding and chemical nursing on protoplast divisions. Aust. J. Bot. 57, 502–511. doi: 10.1071/BT09068
Wolko, B., Clements, J. C., Naganowska, B., Nelson, M. N., and Yang, H. A. (2011). ““Lupinus,”,” in Wild crop relatives: genomic and breeding resources: legume crops and forages. Ed. Kole, C. (Springer Berlin Heidelberg, Berlin, Heidelberg), 153–206.
Xu, Q., Song, B., Liu, F., Song, Y., Chen, P., Liu, S., et al. (2018). Identification and characterization of β-lathyrin, an abundant glycoprotein of grass pea (Lathyrus sativus L.), as a potential allergen. J. Agric. Food Chem. 66, 8496–8503. doi: 10.1021/acs.jafc.8b02314
Xu, W., Zhang, Q., Yuan, W., Xu, F., Muhammad Aslam, M., Miao, R., et al. (2020). The genome evolution and low-phosphorus adaptation in white lupin. Nat. Commun. 11, 1069. doi: 10.1038/s41467-020-14891-z
Xu, P., Zhong, Y., Xu, A., Liu, B., Zhang, Y., Zhao, A., et al. (2024). Application of developmental regulators for enhancing plant regeneration and genetic transformation. Plants (Basel) 13, 1272. doi: 10.3390/plants13091272
Yarra, R. and Krysan, P. J. (2023). GRF-GIF duo and GRF-GIF-BBM: novel transformation methodologies for enhancing regeneration efficiency of genome-edited recalcitrant crops. Planta 257, 60. doi: 10.1007/s00425-023-04096-1
Yıldırım, K., Miladinović, D., Sweet, J., Akin, M., Galović, V., Kavas, M., et al. (2023). Genome editing for healthy crops: traits, tools and impacts. Front. Plant Sci. 14, 2023. doi: 10.3389/fpls.2023.1231013
Yorgancilar, M., Babaoglu, M., Hakki, E., and Atalay, E. (2009). Determination of the relationship among Old World Lupin (Lupinus sp.) species using RAPD and ISSR markers. Afr. J. Biotechnol. 8, 3524–3530.
Yuan, M., Zhu, J., Gong, L., He, L., Lee, C., Han, S., et al. (2019). Mutagenesis of FAD2 genes in peanut with CRISPR/Cas9 based gene editing. BMC Biotechnol. 19, 24. doi: 10.1186/s12896-019-0516-8
Zhang, Y., Massel, K., Godwin, I. D., and Gao, C. (2018). Applications and potential of genome editing in crop improvement. Genome Biol. 19, 210. doi: 10.1186/s13059-018-1586-y
Zhang, W. H., Ryan, P. R., and Tyerman, S. D. (2004). Citrate-permeable channels in the plasma membrane of cluster roots from white lupin. Plant Physiol. 136, 3771–3783. doi: 10.1104/pp.104.046201
Zhang, Q., Zhang, Y., Lu, M. H., Chai, Y. P., Jiang, Y. Y., Zhou, Y., et al. (2019). A novel ternary vector system united with morphogenic genes enhances CRISPR/cas delivery in maize. Plant Physiol. 181, 1441–1448. doi: 10.1104/pp.19.00767
Zhao, H., Zhao, S., Cao, Y., Jiang, X., Zhao, L., Li, Z., et al. (2024). Development of a single transcript CRISPR/Cas9 toolkit for efficient genome editing in autotetraploid alfalfa. Crop J. 12, 788–795. doi: 10.1016/j.cj.2024.04.001
Zhong, H., Elumalai, S., Li, C., Liu, W., Dong, S., and Que, Q. (2025a). Development of high-throughput tissue culture-free plant transformation systems. Plant J. 121, e17163. doi: 10.1111/tpj.v121.1
Zhong, L., Guo, H., Wu, L., and Cheng, Q. (2025b). Effective use of novel auxotrophic agrobacterium tumefaciens strains for transformation and biocontainment. Plants 14, 925. doi: 10.3390/plants14060925
Zhou, Y., Neuhäuser, B., Neumann, G., and Ludewig, U. (2020). LaALMT1 mediates malate release from phosphorus-deficient white lupin root tips and metal root to shoot translocation. Plant Cell Environ. 43, 1691–1706. doi: 10.1111/pce.13762
Zhou, Y., Olt, P., Neuhäuser, B., Moradtalab, N., Bautista, W., Uhde-Stone, C., et al. (2021). Loss of LaMATE impairs isoflavonoid release from cluster roots of phosphorus-deficient white lupin. Physiol. Plant 173, 1207–1220. doi: 10.1111/ppl.v173.3
Keywords: alkaloids, alternative plant proteins, genetic engineering, micropropagation, protoplast culture, recalcitrant species
Citation: Pathi KM and Sprink T (2025) Lupins in the genome editing era: advances in plant cell culture, double haploid technology and genetic transformation for crop improvement. Front. Plant Sci. 16:1601216. doi: 10.3389/fpls.2025.1601216
Received: 27 March 2025; Accepted: 30 May 2025;
Published: 24 June 2025.
Edited by:
Brigitte Mauch-Mani, Retired, Fribourg, SwitzerlandReviewed by:
Cheng Yuan, Yunnan Academy of Tobacco Agricultural Sciences, ChinaBeimi Cui, University of Edinburgh, United Kingdom
Copyright © 2025 Pathi and Sprink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krishna Mohan Pathi, a3Jpc2huYS5wYXRoaUBqdWxpdXMta3VlaG4uZGU=
† ORCID: Krishna Mohan Pathi, orcid.org/0000-0003-4693-6747
Thorben Sprink, orcid.org/0000-0002-0423-5128
 Krishna Mohan Pathi
Krishna Mohan Pathi Thorben Sprink
Thorben Sprink