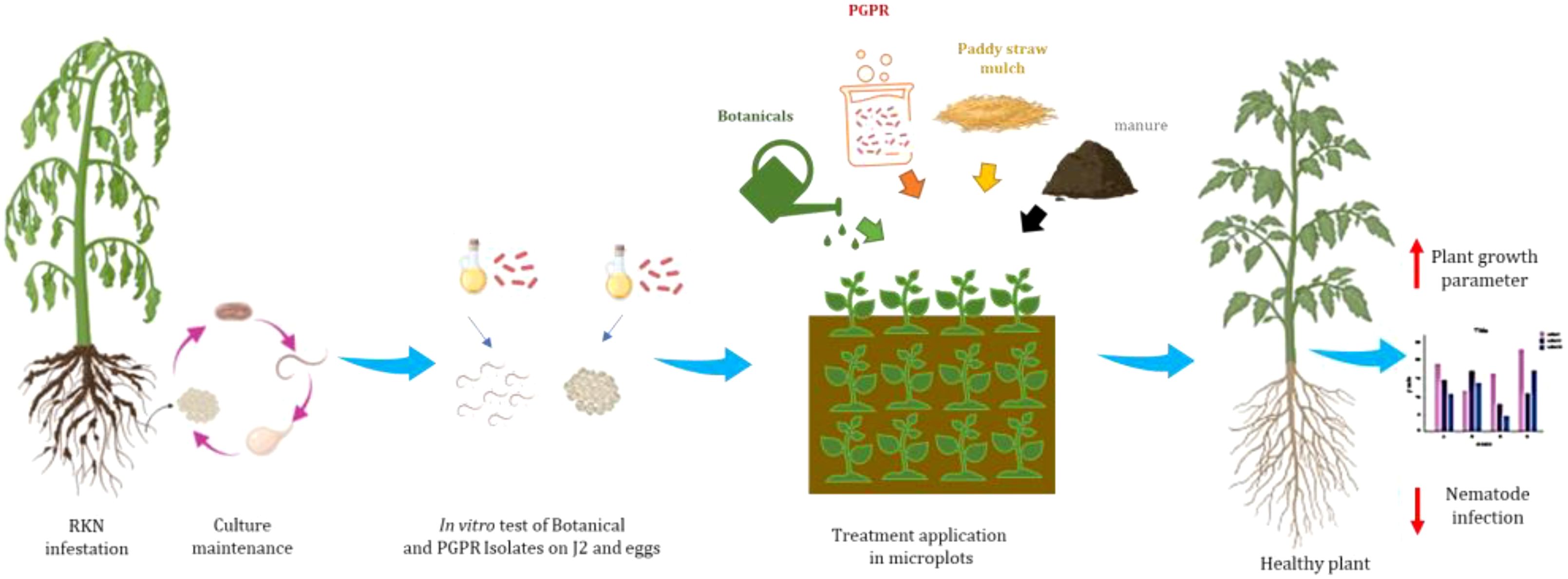
- 1Division of Nematology, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 2Division of Agricultural Chemicals, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 3Division of Plant Pathology, ICAR-Indian Agricultural Research Institute, New Delhi, India
Root-knot nematode (Meloidogyne incognita) causes up to 30% yield loss in tomato (Solanum lycopersicum) worldwide, and reliance on synthetic nematicides poses serious environmental and health risks. This study explores sustainable alternatives by evaluating the nematicidal potential of Mentha spicata and Piper longum essential oils and extracts, along with Bacillus amyloliquefaciens and B. subtilis, under both In vitro and microplot conditions. Essential oils exhibited significant juvenile mortality and egg hatching inhibition at low concentrations, outperforming solvent extracts. In microplots, all treatments—including combinations with organic amendments (farm yard manure, vermicompost, and paddy straw)—significantly reduced nematode populations, improved tomato growth, and enhanced soil fertility. The combined biocontrol treatments performed comparably to the chemical nematicide Velum Prime 400 SC, while also increasing soil organic carbon and NPK content (P < 0.05). These findings demonstrate that integrating botanicals, plant growth promoting rhizobacteria and organic amendments provides an effective, eco-friendly alternative for managing root-knot nematodes, contributing to resilient and sustainable tomato production systems.
Introduction
Tomato is the most widely commercially cultivated vegetable crop belongs to Solanaceae family. India ranks 2nd worldwide in both area and production of tomatoes, following China (Gupta et al., 2021). Tomato cultivation is hindered by several biotic and abiotic stresses, with root-knot nematodes (RKNs) as one of the most significant biotic threats. Solanum lycopersicum L. (Tomato), one of the most important crops is highly susceptible to root-knot nematodes, with infestations leading to yield losses of up to 30% (Sikora and Fernandez, 2005). In India the production of tomato suffers an estimated yield reduction of 23% translating annual financial loss of approximately ₹6,035.2 million due to RKNs suggesting the urgent need for sustainable and effective nematode management strategies (Kumar et al., 2020).
The management of root-knot nematode has traditionally relied on synthetic nematicides (Meher et al., 2010; Radwan et al., 2012; Pankaj et al., 2015). These nematicides effectively suppress nematode populations but their extensive use poses significant risks to human health, non-target organisms, and soil microbial diversity (Chen et al., 2020; Poveda et al., 2020; Sasanelli et al., 2021). In response, many countries have imposed stringent regulations on the use of synthetic nematicides (Jones, 2017), leading to a growing demand for sustainable, eco-friendly alternatives that ensure effective nematode control without compromising environmental and agricultural sustainability. “Green pesticides,” including botanicals, biocontrol agents, and organic amendments, present an eco-friendly and effective solution of nematode management.
Botanical extracts and essential oils have gained attention as eco-friendly alternatives for managing plant-parasitic nematodes due to their bioactive compounds with nematicidal properties (Saroj et al., 2020; Sarri et al., 2024). Mentha spicata is well known for its natural insect-repelling abilities, effectively deterring and eliminating insect pests and mites through its essential oils and secondary metabolites (Mondal et al., 2024; Cai et al., 2025),. Similarly, the essential oil of Piper longum (long pepper) have been identified as safer alternatives for controlling of nematode and plant pathogens (Kumar et al., 2023; Páez and Leite, 2025), offering its nematicidal potential.
Biocontrol agents are preferred due to their specificity, environmental safety, cost-effectiveness, and non-resistance-inducing nature (Bhat et al., 2023; Chamkhi et al., 2022). It is reported that plant growth-promoting rhizobacteria (PGPR) suppress nematodes through multiple mechanisms, including competition, antibiosis, and induced systemic resistance in plants (Grewal et al., 2005; Pires et al., 2022; Antil et al., 2023). Bacillus species are widely studied and commercially utilized as biocontrol agents against plant-parasitic nematodes (Ramezani et al., 2014; Moreira et al., 2025; Ali et al., 2025). A combination of Bacillus species has shown to enhance nematode management more effectively than individual strains (Vasantha-Srinivasan et al., 2025).
The incorporation of organic nutrient sources, such as farmyard manure (El-Ashry, 2021), vermicompost (Gebrehana et al., 2025), and paddy straw (Wang et al., 2019), has been widely recognized for their role in enhancing soil fertility and improving plant resilience against nematode infestations (Khanna et al., 2022; Edussuriya et al., 2023).
A comprehensive review of the literature highlights the potential of eco-friendly alternatives such as botanicals, bio-control agents, and organic nutrient amendments in managing RKNs by improving soil health, enhancing plant resilience, and disrupting nematode life cycles. However, their individual efficacy in nematode suppression requires further validation. This study, therefore, evaluates the effectiveness of oils and extracts from M. spicata and P. longum and two biocontrol agents, Bacillus amyloliquefaciens and B. subtilis, and organic amendments (farmyard manure, vermicompost, and paddy straw) against M. incognita in tomato. The findings provide valuable insights into sustainable nematode management strategies, offering environmentally safe alternatives to synthetic nematicides while supporting long-term agricultural productivity.
Materials and methods
Nematode culturing and inoculum preparation
Populations of M. incognita were collected from severely infected brinjal (Solanum melongena) plants maintained in microplots at the Division of Nematology, ICAR-IARI, New Delhi. The nematodes were identified based on perineal pattern, cultured and used to evaluate the bio-efficacy of organic nutrient sources and bio-control agents. Egg masses were carefully removed from infected roots using sterile forceps. The collected galls were placed in a modified Baermann funnel setup to facilitate nematode hatching. After 24 hours, freshly hatched second-stage juveniles (J2s) were collected in Petri plates containing sterile distilled water. To assess the effect of treatments on egg hatching, egg masses were extracted from infected roots and immersed in 0.1% sodium hypochlorite (NaOCl) for three minutes to release eggs. The suspension was then sieved through a 500 µm mesh screen. The eggs retained on the sieve were thoroughly washed with sterile distilled water to remove residual NaOCl (Hussey and Barker, 1973). The nematode suspension was transferred to a measuring cylinder, thoroughly mixed using a pipette, and concentrated to a specified volume to standardize the inoculum density. The total number of nematodes was estimated using a counting chamber, and the mean of three replicates was used for accuracy.
Nematicidal activity of oils and extracts and biocontrol agents
In this study, different oils and extracts from M. spicata and P. longum and plant growth-promoting rhizobacteria (PGPR) were evaluated for their efficacy against Meloidogyne incognita through in vitro and field conditions. The stock solution (1.0%) of the essential oils from the leaves of M. spicata and fruits of P. longum and their hexane and methanolic extracts were prepared by dissolving them separately in distilled water and Tween-80 (3.0%) as surfactant. The solution was stirred continuously for 30 minutes to ensure homogeneity and was then diluted to obtain test of 2000, 1000, 500, 250, 125 and 62.5ppm. Distilled water with Tween-80 (3.0%) and Velum Prime were used as negative and positive controls. The prepared solutions were stored at room temperature (25 ± 2 °C) until further use.
Preparation of biocontrol agents
The PGPR treatments consisted of Bacillus amyloliquefaciens DSBA 11, B. subtilis DTBS 5, and their combination and nutrient broth and sterile distilled water as controls. B. amyloliquefaciens DSBA-11 (ITCC BJ-0013) and B. subtilis DTBS-5 (ITCC BJ-0011) were obtained from the Bacteriology Lab, Indian Type Culture Collection (ITCC), Division of Plant Pathology, ICAR-IARI, New Delhi, India. A single colony from a 24-hour-old pure culture plate was inoculated into 50 mL of sterile King’s B broth in 100 mL Erlenmeyer flasks and incubated at 37°C with constant shaking at 150 RPM for 24 hours to promote bacterial growth. After incubation, the bacterial culture was centrifuged at 10,000 RPM for 15 minutes at 4°C to separate the supernatant. The cell-free culture filtrate was obtained by passing the supernatant through a 0.22 µm syringe filter and was subsequently used for bioassays after confirming the absence of viable bacterial cells.
Egg hatching inhibition bioassay
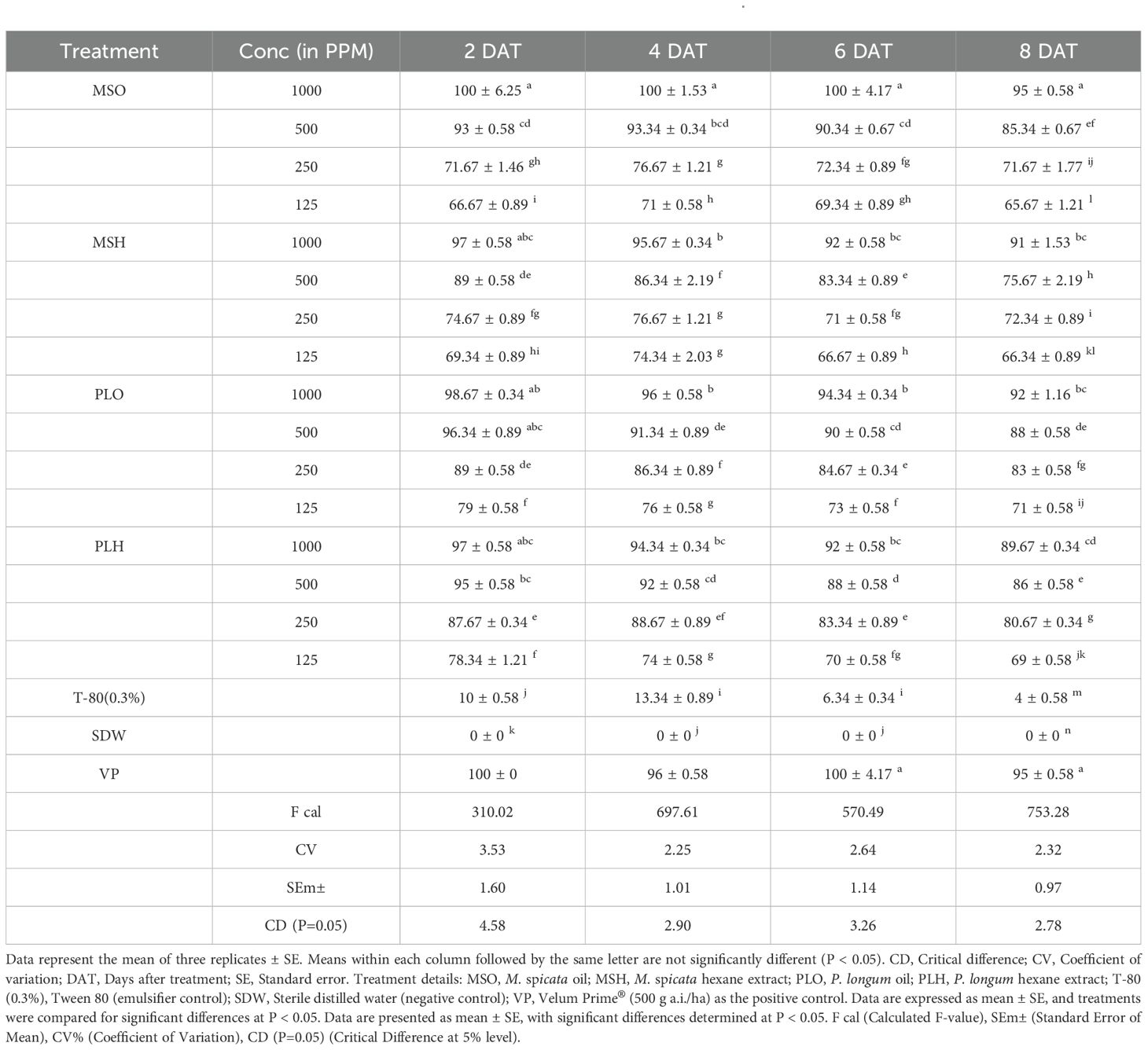
The egg hatching inhibition bioassay was conducted using a 48-well culture plate, with each well containing Meloidogyne incognita egg suspension (100 eggs/10 µL). To each well, 1 mL solution of different concentration of oils and extracts (1000, 500, 325, 250, 125, and 62.5 ppm) and biocontrol agents (100%, 50%, and 25%) were added. The plates were incubated at 28 ± 2°C. Velum Prime® (Fluopyram 400 SC) was used as the positive control, while Tween-80 (3.0%), nutrient broth, and sterile distilled water served as negative controls. Egg hatching was monitored at 2, 4, 6, and 8 days post-treatment using a stereoscopic binocular microscope. The percentage of egg hatching was calculated based on the ratio of hatched to unhatched eggs. Hatching inhibition was determined by comparing the number of unhatched eggs in treated samples to the control groups.
Juvenile mortality bioassay
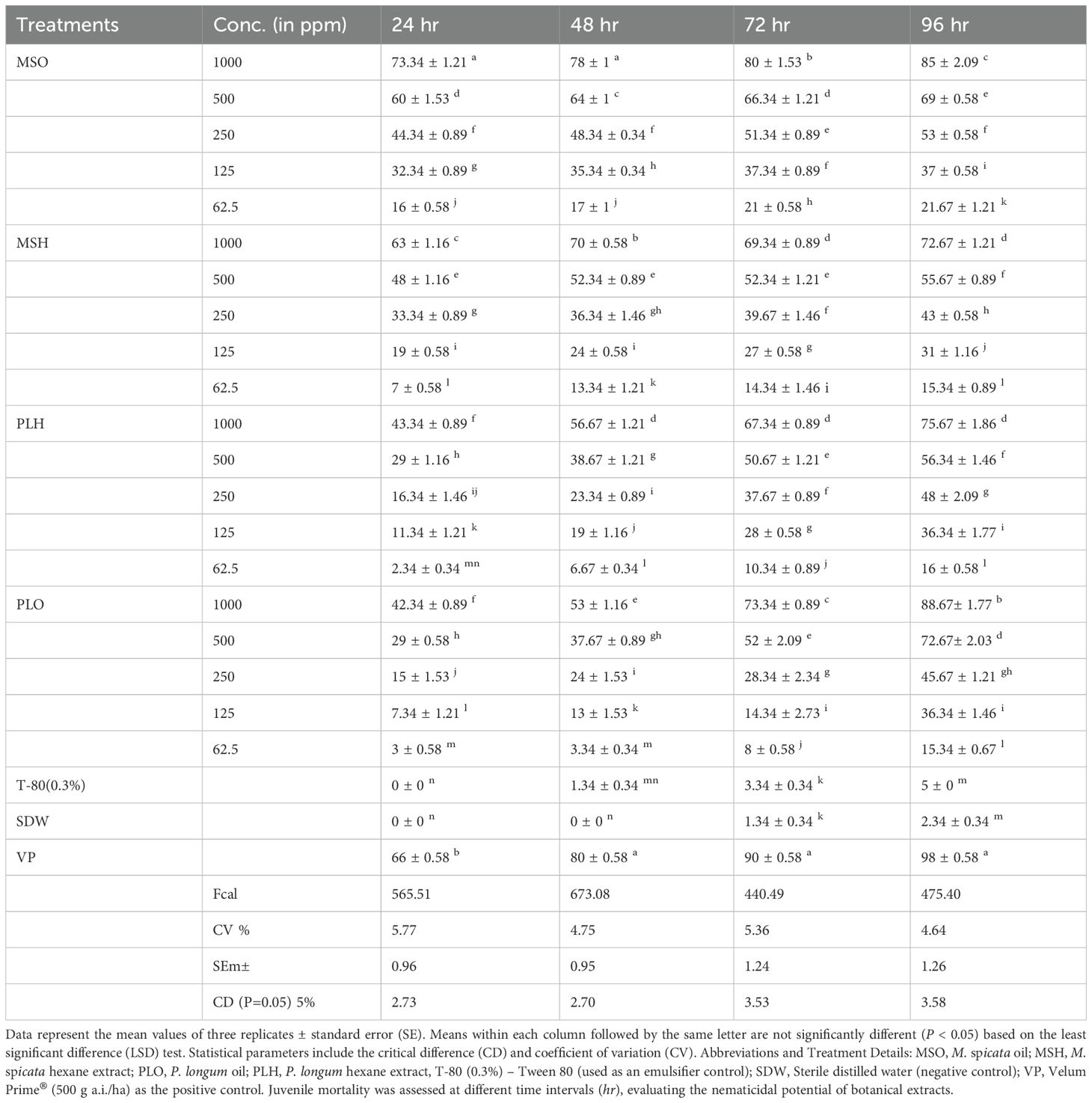
A 48-well culture plate was prepared by adding M. incognita suspension (100 second-stage juveniles (J2s)/10 µL) to each well. Subsequently, 1 mL of different concentrations of oils and extracts (1000, 500, 325, 250, 125 and 62.5 ppm) and biocontrol agents (100%, 50%, and 25%) were added, thoroughly mixed, and incubated at 28 ± 2°C. Velum Prime® (Fluopyram 400 SC) was used as the positive control, while Tween-80 (3.0%), nutrient broth, and sterile distilled water served as negative controls. Nematode mortality was assessed at 24, 48, 72, and 96 hours post-treatment, with three replicates per treatment. The percentage of juvenile mortality was calculated based on the ratio of dead juveniles to the total juveniles in each well, following Abbott’s formula (Culver et al., 1925).
Soil sample analysis for NPK and organic carbon
Soil samples were collected from micro plots at the Division of Nematology, ICAR-IARI, New Delhi, using a hand hoe, ensuring minimal disturbance to the soil structure. The collected samples were air-dried at room temperature, cleared of debris and roots, and sieved through a 2 mm mesh for uniformity. The processed samples were stored in labeled containers until further analysis. Soil nitrogen content was determined using the Kjeldahl method, which involved digestion, distillation, and titration (Bremner, 1960). Available phosphorus was extracted using the Olsen method and quantified calorimetrically using a spectrophotometer (Sims, 2000). Soil potassium was extracted with ammonium acetate and measured using flame photometry (Jackson, 1958). Soil organic carbon content was estimated using the Walkley-Black chromic acid wet oxidation method. All analyses were performed in triplicate to ensure accuracy and reproducibility. Standard reference materials and blanks were incorporated for instrument calibration and validation of results.
Application of botanicals and PGPR as soil drenching
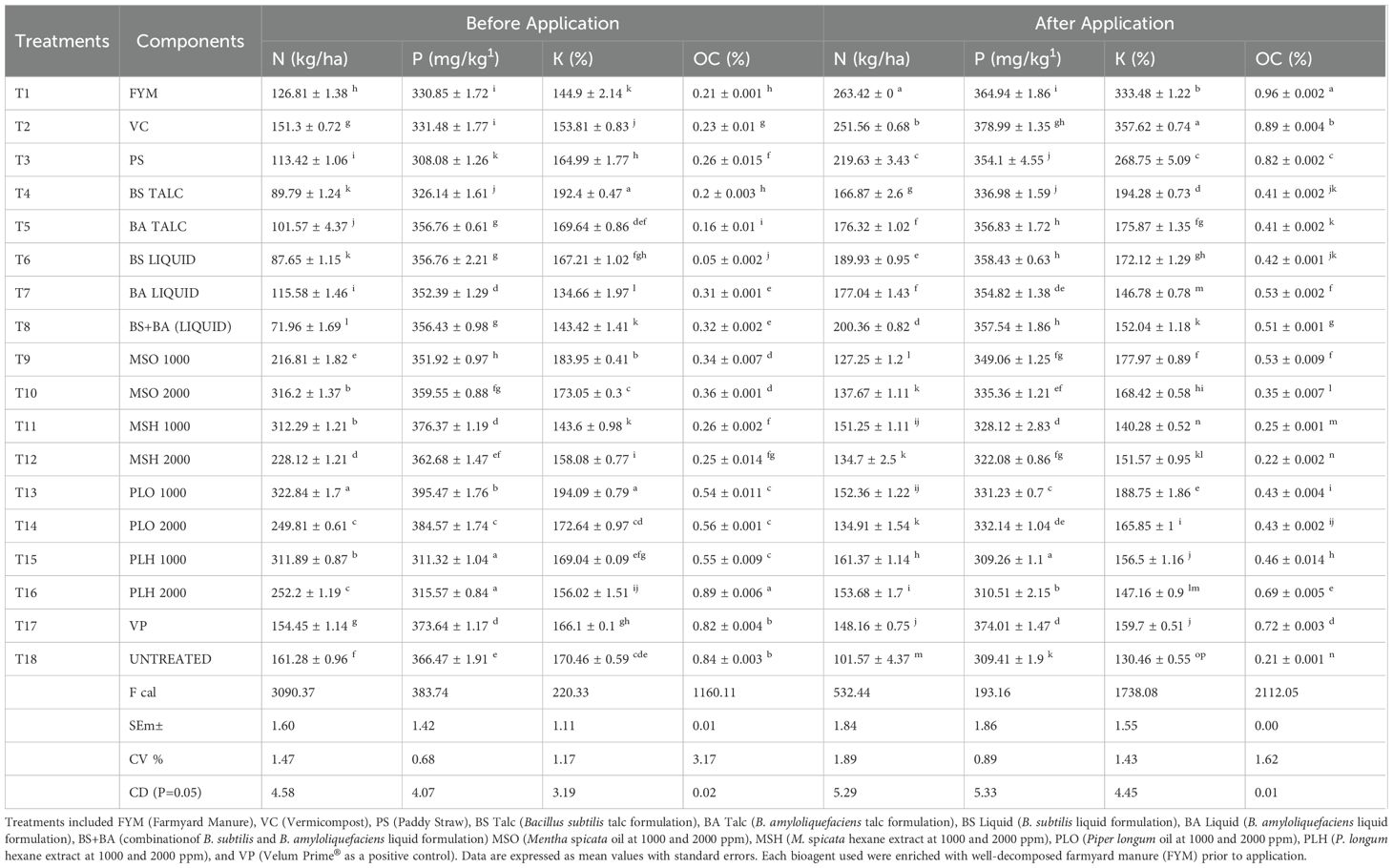
Bio-control agent isolates were applied to the soil by drenching at a rate of 100 mL/plot or 500 g/plot (108 CFU/mL) two weeks before transplanting. After this period, 21-day-old Pusa Ruby tomato seedlings were carefully uprooted from nursery pro-trays and transplanted into each plot at a density of five seedlings per plot. Each treatment was replicated three times. Velum Prime® (500 g a.i./ha) served as the positive control, while untreated soil was used as the negative control. Observations were recorded at 30, 60, and 90 days post-transplantation. At the end of the experiment (90 days), plants were carefully uprooted to assess growth parameters, including shoot length (cm), root length (cm), fresh shoot weight (g), and fresh root weight (g). Nematode infestation was evaluated by recording the number of galls per root system, number of egg masses per root system, and number of eggs per egg mass. Additionally, soil nutrient status, including nitrogen (N), phosphorus (P), potassium (K), and organic carbon content, was analyzed to assess the impact of treatments on soil fertility. The other treatment details for in vivo experiment under micro plot have been given in Table 1. Formulations of B. amyloliquefaciens and B. subtilis in both talc- and liquid-based forms were used either individually or in combination. These bioagents were applied after enrichment in well-decomposed farmyard manure (FYM). For enrichment, each bioagent formulation was thoroughly mixed with FYM and incubated for 15 days under moist conditions to promote microbial multiplication. The enriched FYM was then applied to the soil as per the treatment schedule.
Experimental design and statistical analysis
Both in vitro and in vivo experiments were conducted using a completely randomized block design (CRBD). The recorded data were subjected to statistical analysis using the Web Agri Stat Package (WASP) version 2.0 at a 5% level of significance. The median lethal concentration (LC50) was determined using Probit analysis in the SPSS statistical package (version 16.0).
Results
Nematicidal activity of biocontrol agents
The efficacy of biocontrol agents against M. incognita juveniles (J2s) was evaluated under in vitro laboratory conditions. The data presented in Figure 1 and Supplementary Table 1 indicate that cell-free culture filtrates of two bio-control agents and a combination formulation significantly increased J2 mortality (P < 0.05). A clear dose- and time-dependent trend was observed, where higher concentrations and prolonged exposure times resulted in greater nematode mortality. After 96 hours of exposure, the highest juvenile mortality (92.67%) was recorded in the combination treatment (B. subtilis + B. amyloliquefaciens) at 100% concentration, which was significantly higher than the individual isolates B. amyloliquefaciens (74.67%) and B. subtilis (72.34%). The effectiveness of both individual isolates and the combination remained evident even at lower concentrations (50% and 25%), where juvenile mortality ranged between 50% and 75% after 96 hours. These findings confirm that the combined application of bio-control agents has a synergistic effect, enhancing nematicidal activity against M. incognita. Notably, no juvenile mortality was observed in the control treatments (nutrient broth and distilled water), reaffirming that the observed nematicidal effects were attributed to the bio-control agents.
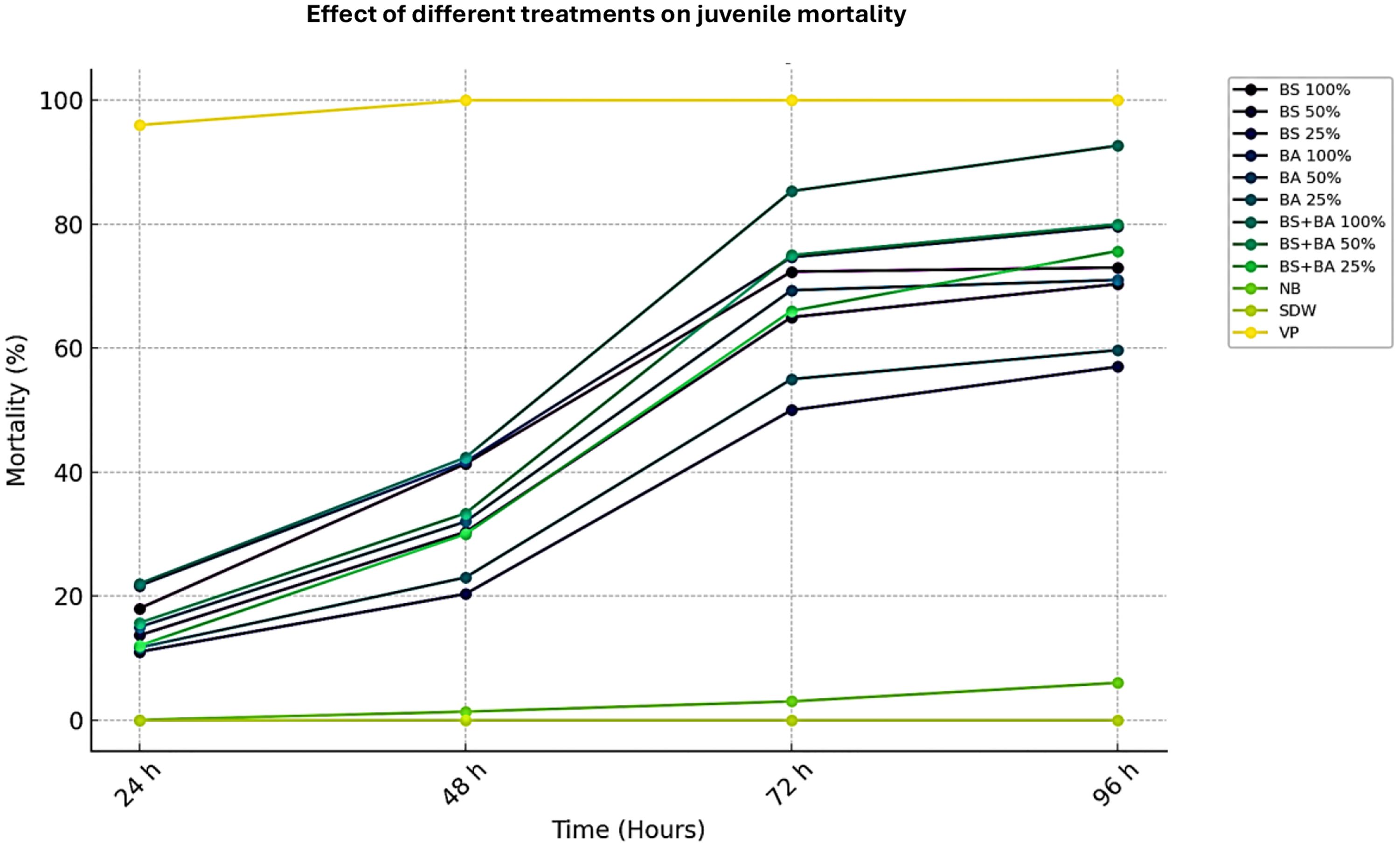
Figure 1. Bio-efficacy of different bio-control agents on juvenile (J2) mortality of Meloidogyne incognita. Abbreviations and Treatment Details: Ba, Bacillus amyloliquefaciens (strain DSBA 11); Bs, Bacillus subtilis (strain DTBS 5) Ba + Bs (combination): Combined application of B. amyloliquefaciens and B. subtilis; NB, Nutrient broth (negative control); SDW, Sterile distilled water (negative control); VP, Velum Prime® (500 g a.i./ha) as the positive control. All treatments were assessed at various exposure times (in hours), and their efficacy was evaluated based on the mortality rate of M. incognita second-stage juveniles (J2s).
Egg hatching inhibition activity of biocontrol agents
The efficacy of bio-control agents in inhibiting M. incognita egg hatching was assessed under laboratory conditions. The data presented in Figure 2 and Supplementary Table 2 indicate that both individual bio-control agents and their combination exhibited significant inhibition of egg hatching (P < 0.05), with effectiveness varying based on concentration and exposure duration. After 8 days of incubation, the highest hatching inhibition (85.34%) was observed in the combinationtreatment (B. subtilis + B. amyloliquefaciens) at 100% concentration, which was slightly higher than the inhibition rates of B. amyloliquefaciens (84%) and B. subtilis (77%) at the same concentration. A concentration-dependent trend was evident, as all treatments, including those at 50% and 25% concentrations, significantly reduced egg hatching. The inhibition effect increased with both concentration and incubation period, highlighting the potency of bio-control agents in disrupting nematode reproduction. Among all the tested treatments, the combination (B. subtilis + B. amyloliquefaciens) consistently exhibited the highest efficacy across all concentrations. In contrast, no egg hatching inhibition was observed in the distilled water control, while a minimal inhibition rate of 8% was recorded in the nutrient broth after 8 days.
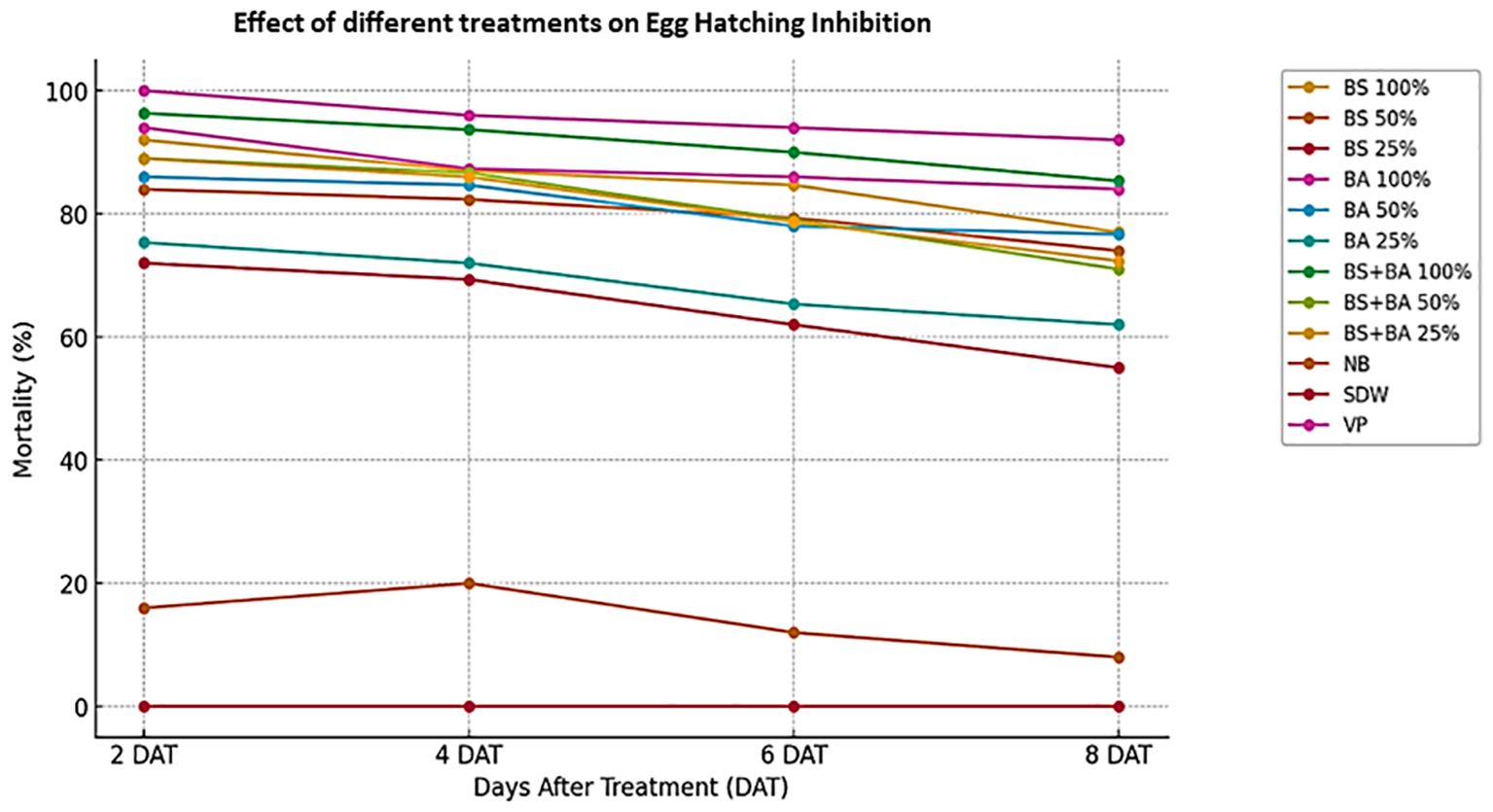
Figure 2. Bio-efficacy of bio-control agents on egg hatching inhibition of Meloidogyne incognita. Abbreviations and Treatment Details: Ba, Bacillus amyloliquefaciens (strain DSBA 11); Bs, Bacillus subtilis (strain DTBS 5); Ba + Bs (combination), Combined application of B. amyloliquefaciens and B. subtilis; NB, Nutrient broth (negative control); SDW, Sterile distilled water (negative control); VP, Velum Prime® (500 g a.i./ha) as the positive control. Egg hatching inhibition was evaluated at different time intervals (days after treatment—DAT).
Nematicidal activity of oils and extracts
The nematicidal activity of essential oils and hexane extract from M. spicata and P. longum was carried out against M. incognita juveniles (J2s), and the results are presented in Table 2. Results indicated that M. spicata and P. longum oil exhibited significantly higher nematicidal activity compared to the hexane extracts of both M. spicata and P. longum (P < 0.05). A dose and time-dependent trend was observed, where higher concentrations (500 and 1000 ppm) and prolonged exposure durations (72 and 96 hours) resulted in greater J2 mortality compared to lower concentrations (250 and 500 ppm) with shorter exposure times (24 and 48 hours). After 96 hours of exposure, the highest mortality was recorded in P. longum oil (88.67%) at 1000 ppm, followed by M. spicata oil (85.0%). In contrast, the hexane extract of P. longum demonstrated relatively lower efficacy, causing 75.67% mortality and that of M. spicata causing 72.67% mortality. The results confirm that essential oils were more effective than their respective hexane extracts, emphasizing their potential as potent natural nematicides. No juvenile mortality was observed in the negative controls, confirming that the recorded nematicidal effects were attributed solely to the botanical treatments.
Egg hatching inhibition activity of oils and extracts
The data presented in Table 3 illustrates the effect of different essential oils and extracts on the egg hatching inhibition of M. incognita. Overall, botanicals and essential oils exhibited greater efficacy in inhibiting egg hatching compared to hexane extracts. The inhibitory effect increased with higher concentrations, demonstrating a dose-dependent response. After 8 days, the highest hatching inhibition (95%) was recorded with M. spicata oil at 1000 ppm, followed by P. longum oil (88%) at the same concentration. The lowest inhibition (75.67%) was observed in P. longum hexane extract at 1000 ppm. All botanical treatments exhibited significant differences (P<0.05) and were effective across all tested concentrations (1000, 500, 250, and 125 ppm). The results indicate that egg hatching inhibition is directly proportional to both concentration and exposure duration. In contrast, no hatching inhibition was recorded in control treatments with Tween 80 (3%) and distilled water, further confirming the nematicidal potential of the tested botanicals.
In-vitro comparative toxicity of botanicals on egg hatching and juvenile mortality of M. incognita
Table 4 summarizes the in vitro toxicity (LC50) of various botanicals, including M. spicata oil, M. spicata hexane extract, P. longum oil, P. longum hexane extract, and Velum Prime, against M. incognita. The toxicity pattern observed after 96 hours follows the order: M. spicata hexane extract < P. longum hexane extract < P. longum oil < M. spicata oil. A strong correlation was observed between juvenile mortality and both exposure duration and concentration, with a maximum mortality rate of 80% recorded at higher concentrations (1000 and 500 ppm). The LC50 values decreased with increasing concentrations, demonstrating the efficacy of the tested oils and extracts. After 96 hours, the recorded LC50 values were 214.63 ppm (M. spicata oil), 231.15 ppm (P. longum oil), 306.11 ppm (P. longum hexane extract), and 348.11 ppm (M. spicata hexane extract), indicating that juvenile mortality increased as LC50 decreased.
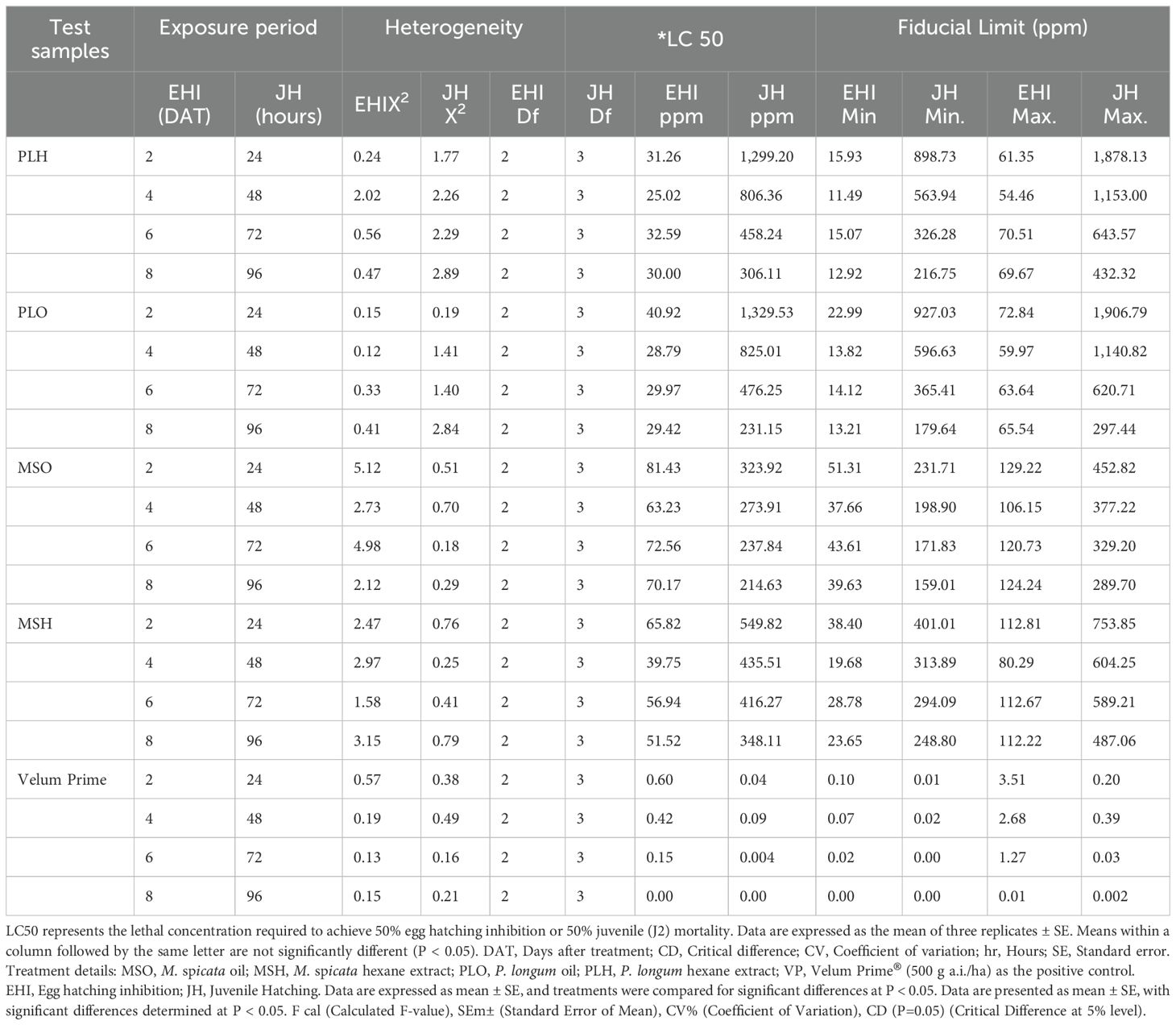
Table 4. Comparative toxicity (LC 50) of different botanicals against Meloidogyne incognita in comparison with velum prime (VP) as a positive control.
Similarly, Table 4 also presents the in vitro toxicity (LC50) of these botanicals on egg hatching inhibition after eight days. The toxicity pattern followed the order: M. spicata oil < M. spicata hexane extract < P. longum hexane extract < P. longum oil. Maximum hatching inhibition was recorded at higher concentrations (1000 and 500 ppm). The LC50 values after eight days were 29.42 ppm (M. spicata oil), 30.00 ppm (M. spicata hexane extract), 51.52 ppm (P. longum hexane extract), and 70.17 ppm (P. longum oil), demonstrating that a lower LC50 value corresponds to a higher rate of egg hatching inhibition.
In vivo experiments
Soil nutrient analysis (N, P, K)
Soil samples were collected from all microplots before and after treatment application to analyze nitrogen (N) content using the Kjeldahl method, phosphorus (P) using the Olsen method, and potassium (K) using a flame photometer. After 90 days of treatment, a significant increase in N, P, and K content was observed in plots treated with farmyard manure (FYM), vermicompost (VC), paddy straw (PS), and a combination of B. subtilis (BS) and B. amyloliquefaciens (BA). Among the organic amendments, FYM exhibited the highest increase in nutrient content, following the trend: FYM > VC > PS. The highest recorded values were nitrogen (263.42 kg/ha), phosphorus (378.99 kg/ha), and potassium (357.62 kg/ha) in plots treated with FYM (5–10 kg/plot). In contrast, plots without organic amendments or bio-agents showed a slight decline in NPK content. A significant difference (P < 0.05) was observed in NPK levels before and after treatment application across all treatments. Notably, the untreated control exhibited a drastic reduction in NPK content, emphasizing the role of organic and biological amendments in improving soil fertility (Table 5).
Organic carbon analysis (Walkley-Black chromic acid wet oxidation method)
Soil organic carbon content was determined using the Walkley-Black chromic acid wet oxidation method. After 90 days, a notable increase in organic carbon was recorded in plots treated with FYM, VC, PS, and Bacillus spp. The highest increase was observed in FYM-treated plots, following the trend: FYM > VC > PS. The highest organic carbon content (0.96%) was recorded in plots treated with FYM (5–10 kg/plot). Conversely, treatments lacking organic amendments exhibited a slight decrease in organic carbon content. A significant difference was observed among all treatments (P < 0.05), as well as between pre- and post-treatment applications. In the untreated control, a drastic reduction in organic carbon content (0.22%) was noted, further supporting the efficacy of organic amendments in enhancing soil health (Table 5).
Effect of treatments on plant growth parameters
Plant growth parameters, including shoot length, shoot weight, root length, and root weight, were significantly influenced by the application of vermicompost (VC), farmyard manure (FYM), paddy straw (PS), and the Bacillus spp. combination (B. subtilis + B. amyloliquefaciens) (Supplementary Table 4). Data presented in Figure 3 indicate a substantial increase in plant growth across all treated plots compared to untreated controls (P < 0.05). After 90 days of treatment application, the maximum shoot length was recorded in vermicompost-treated plots (121.17 cm), followed by FYM (112.7 cm) and paddy straw (80.27 cm). In contrast, the lowest shoot length (ranging from 51.9 cm to 57.2 cm) was observed in plots treated with botanical extracts, including M. spicata oil (MSO) and hexane extract (MSH), as well as P. longum oil (PLO) and hexane extract (PLH), at both 1000 ppm and 2000 ppm concentrations. Notably, plants treated with Velum Prime recorded the highest shoot length of 134.14 cm (Supplementary Table 3).
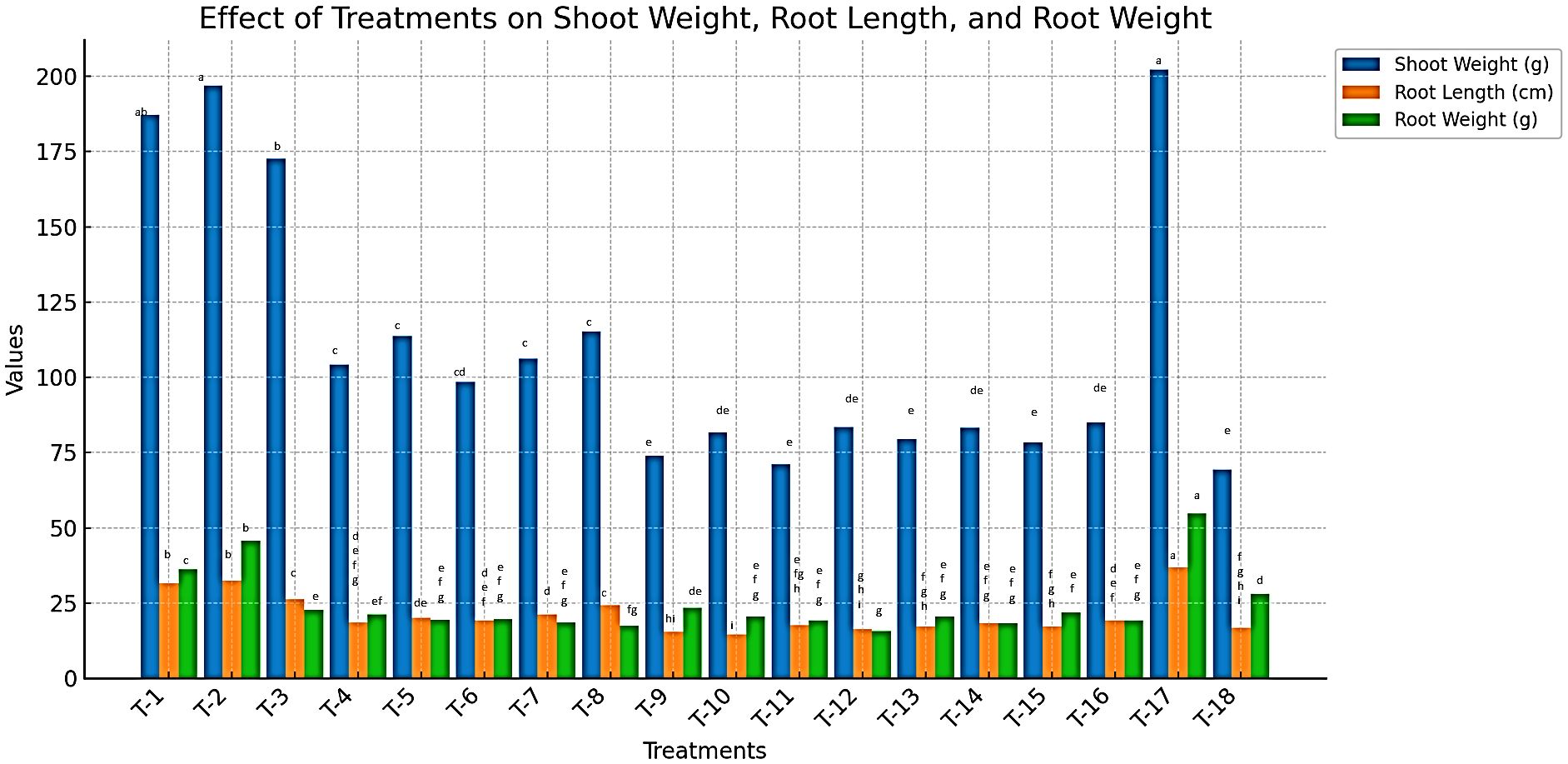
Figure 3. Effect of organic nutrient sources, bio-control agents, and botanicals on plant growth parameters in tomato infected by M. incognita under microplot.
Similarly, shoot weight followed the same trend, with the highest fresh shoot weight recorded in VC-treated plants (196.83 g), followed by FYM (187.24 g) and paddy straw (172.63 g). The lowest shoot weight (71.03 g to 84.96 g) was observed in plots treated with botanical extracts at 1000 and 2000 ppm concentrations, while Velum Prime-treated plants exhibited the highest fresh shoot weight (202.13 g).
Root growth also showed significant improvement in response to organic amendments. The maximum root length was observed in VC-treated plants (32.5 cm), followed by FYM (31.47 cm) and paddy straw (26.2 cm). The shortest root lengths (14.47 cm to 19.24 cm) were recorded in botanical extract treatments, while Velum Prime-treated plants had the highest root length (36.9 cm).
Likewise, root weight was highest in VC-treated plants (45.76 g), followed by FYM (36.2 g) and paddy straw (22.73 g). The lowest root weight (15.73 g to 21.93 g) was recorded in botanical extract treatments, whereas Velum Prime-treated plants exhibited the highest fresh root weight (54.83 g).
Overall, the results revealed that organic amendments significantly improved plant growth, following the trend: VC > FYM > PS. A direct correlation was observed between shoot length, shoot weight, root length, and root weight with the applied treatments. Shoot length measurements were taken at 30, 60, and 90 days, confirming a progressive increase over time. These findings emphasize the role of organic amendments in promoting plant growth and resilience against M. incognita infestation.
Effect of treatments on nematode reproduction parameters
Data presented in Figure 4 indicate a significant reduction in nematode gall formation in plots treated with bio-control agents in various formulations (P < 0.05). The lowest number of root galls (6 galls/root) was recorded in plots treated with the B. amyloliquefaciens + B. subtilis (BA+BS) combination. This was followed by plots treated with B. subtilis (BS) and B. amyloliquefaciens (BA) in liquid formulations applied via soil drenching, which exhibited 6.67 to 8.67 galls/root (Supplementary Table 5). In contrast, the highest number of galls was recorded in plots treated with organic amendments such as farmyard manure (FYM), vermicompost (VC), and paddy straw (PS). Similarly, a reduction in egg mass formation was observed, with the lowest number of egg masses (6.67 egg masses/root) recorded in plots treated with the BA+BS combination. This was followed by plots treated with BS and BA (talc and liquid formulations), applied via soil drenching, which recorded 9.33 to 13.33 egg masses/root. Conversely, the highest number of egg masses (48 to 55.67 egg masses/root) was observed in plots treated with FYM, VC, and PS applied as broadcasting amendments (Supplementary Table 5).
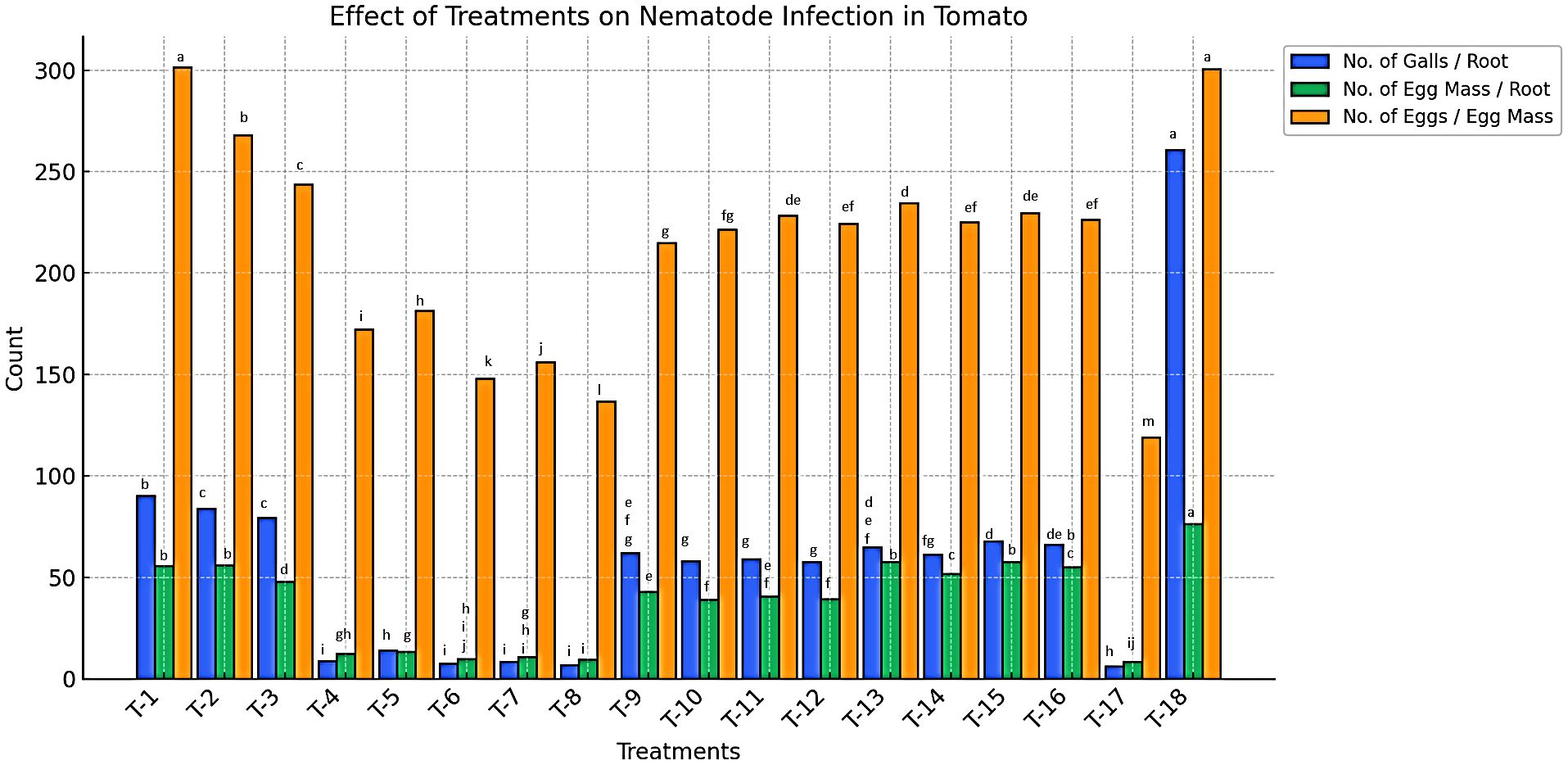
Figure 4. Effect of organic nutrient sources, bio-control agents, and botanicals on nematode infection in tomato infected by M. incognita under microplot.
Botanical treatments also exhibited a notable reduction in nematode reproduction. Plots treated with M. spicata oil (MSO), M. spicata hexane extract (MSH), P. longum oil (PLO), and P. longum hexane extract (PLH) at concentrations of 1000 ppm and 2000 ppm showed reduced gall formation (58 to 68 galls/root) and egg mass production (39 to 57.67 egg masses/root). Egg production per egg mass followed a similar trend. The lowest number of eggs per egg mass (136.67 eggs/egg mass) was recorded in the BA+BS combination-treated plots, followed by plots treated with BS and BA (talc and liquid formulations), which recorded 148 to 172.33 eggs per egg mass. In contrast, the highest number of eggs per egg mass (243.67 to 301.67 eggs/egg mass) was observed in plots treated with FYM, VC, and PS. Botanical treatments also effectively reduced egg production, with plots treated with MSO, MSH, PLO, and PLH (1000 and 2000 ppm) recording 215 to 234.67 eggs per egg mass. All treatments significantly reduced nematode reproduction parameters compared to untreated controls (P < 0.05). The reduction in the number of galls, egg masses, and eggs per egg mass was directly proportional to the type, formulation, and concentration of the treatment applied. Higher concentrations resulted in a greater reduction in nematode infection levels, highlighting the potential of bio-control agents, botanicals, and organic amendments as effective alternatives to synthetic nematicides.
Discussion
This study demonstrates the potential of essential oils, plant extracts, biocontrol agents, and organic amendments as effective alternatives to chemical nematicides for managing Meloidogyne incognita in tomato. An in vitro evaluation of various botanicals—Mentha spicata oil, M. spicata hexane extract, Piper longum oil, and P. longum hexane extract—revealed that nematode mortality and egg hatching inhibition were both concentration- and time-dependent. Among the treatments, the essential oils of P. longum and M. spicata showed significantly higher nematicidal activity than their respective hexane extracts, indicating their superior bioefficacy. Our findings, consistent with Taimoor and Shahina (2018), confirm that nematode mortality and egg hatching inhibition are both dose- and time-dependent. Joyamati et al. (2013) reported that petroleum ether extracts of Melia azedarach were highly effective in inhibiting nematode egg hatching at 1000 ppm, aligning with our findings that nematode mortality and egg hatching inhibition are dose- and time-dependent. The nematicidal activity observed in our study can be attributed to bioactive compounds such as carvone in M. spicata oil and piperine in P. longum oil. Essential oils, comprising both polar and non-polar compounds, are known to disrupt nematode neuromuscular activity by inhibiting acetylcholinesterase (Rhode, 1960). Similar results were reported by Kimbaris et al. (2017), who identified piperitenone epoxide as the most potent compound in M. spicata oil against M. javanica. Additionally, M. longifolia oil and its major component, piperitenone oxide, showed significant activity against M. incognita (Gowda et al., 2023). The microplot experiment revealed significant differences among treatments, suggesting the effectiveness of botanicals in enhancing plant growth and suppressing nematode populations. However, prolonged exposure to higher concentrations of M. spicata and P. longum oils and their hexane extracts adversely affected tomato growth. Treated plots showed marked reductions in shoot and root development compared to the control, indicating potential phytotoxicity at elevated concentrations. These findings highlight the importance of optimizing application rates to achieve effective nematode control while minimizing negative impacts on plant health. Essential oils, particularly carvone from M. spicata and piperine from P. longum, were most effective in inducing juvenile mortality and inhibiting egg hatching of M. incognita, likely due to their neurotoxic and enzymatic inhibition properties (Caboni et al., 2013; Morcia et al., 2016; Rajasekharan et al., 2020; Kumar et al., 2023; Mondal et al., 2024).
In the present study, B. amyloliquefaciens (BA), B. subtilis (BS), and their combination (BS + BA) were evaluated under laboratory conditions for their effects on M. incognita juvenile mortality and egg hatching inhibition. The enhanced nematicidal activity of the B. amyloliquefaciens and B. subtilis combination likely stems from their synergistic secretion of bioactive compounds and improved root colonization. The findings indicate a synergistic interaction between B. amyloliquefaciens and B. subtilis, enhancing their antagonistic efficacy against M. incognita. These results are consistent with those of Manju and Subramanian (2019), who reported increased nematode mortality through the combined action of B. amyloliquefaciens strain B4 and B. subtilis strain BG 31. Similar outcomes were observed by Alfianny et al. (2017) and Ramezani Moghaddam et al. (2014), supporting the potential of PGPR consortia in nematode biocontrol. The synergistic effect is likely due to the combined secretion of nematicidal compounds, enhanced root colonization, and improved nutrient solubilization. PGPR represent a promising biocontrol strategy for PPNs through multiple mechanisms including competition, antibiosis, mycoparasitism, lysis, and the induction of systemic resistance (Siddiqui and Mahmood, 1999; El-Nagdi and Youssef, 2004; Abdel Razek and Yaseen, 2020). Sayre and Starr (1985) further demonstrated that antagonistic PGPR produce nematicidal metabolites such as hydrogen cyanide, 2,4-diacetylphloroglucinol, gluconates, chitinases, proteases, and lipases, which inhibit nematode development and reproduction. Moreover, when used in combination with organic amendments, biocontrol agents often exhibit additive or synergistic effects, further enhancing nematode suppression (Sikora et al., 2005). Organic amendments improve soil health and foster microbial and predator populations that collectively suppress nematodes (Renco, 2013). The present study revealed that the application of FYM at 5 kg/plot resulted in the highest nitrogen content (263.42 kg/ha), while vermicompost significantly increased potassium levels (357.62 kg/ha). Additionally, the highest organic carbon content (0.96%) was observed in FYM-treated plots, suggest the beneficial impact of organic amendments on soil fertility. These findings align with those of Saha et al. (2010), who reported similar enhancements in soil nutrient status following the application of organic manures. Microplot experiments revealed a significant improvement in plant growth parameters across all organic amendment treatments. Vermicompost-treated plots recorded the highest shoot length (121.17 cm) and shoot weight (196.83 g), followed by FYM (112.7 cm, 187.24 g) and paddy straw (80.27 cm, 172.63 g). The superior performance of vermicompost may be attributed to its rich nutrient profile and its ability to enhance beneficial microbial communities that support plant growth and nematode suppression. These results are consistent with Odhiambo and Aguyoh (2019), who observed that farmyard manure reduced root galling and nematode populations in cucumber, leading to improved yields.
Despite nematode infestation, organic amendments significantly enhanced plant vigor, promoting both shoot and root development. Vermicompost-treated plants also exhibited higher concentrations of sugars, proteins, and fats compared to untreated controls. This enhancement is likely due to increased organic matter mineralization, which improves nutrient availability and releases nematicidal secondary metabolites such as ammonia, hydrogen sulfide, phenols, and organic acids (Kesba and Al-Shalaby, 2008). Additionally, organic matter supports soil biodiversity by encouraging the proliferation of nematode predators and parasitoids (Mankau, 1963).
The integration of biocontrol agents further improved nematode management. A significant reduction in nematode incidence was recorded in plots treated with the biocontrol consortium of B. amyloliquefaciens (BA) and B. subtilis (BS), which showed the lowest nematode pathogenicity. In contrast, individual applications of BA and BS were less effective, suggesting a synergistic effect in the combined treatment. Botanical treatments, M. spicata oil (MSO) and P. longum hexane extract (PLH) also reduced gall formation, though to a lesser extent than the biocontrol agents.
These findings align with previous reports that biocontrol agents suppress nematodes via synergistic interactions and by inducing systemic resistance through defense-related enzymes (Meena et al., 2012; Alfianny et al., 2017). The liquid formulation of the BA+BS consortium proved particularly effective, significantly reducing gall formation and enhancing plant defense responses. Similar synergistic effects were reported by Manju and Subramanian (2019), who found that the combination of B. subtilis strain BG 31 and B. amyloliquefaciens strain B4 improved nematode suppression. Additional studies have also demonstrated the efficacy of biocontrol consortia in nematode management (Alfianny et al., 2017; Munif et al., 2013; Majzoob et al., 2012).
The potential of integrating bioagents and botanicals with organic amendments such as FYM offers a promising avenue for enhancing nematode management through synergistic effects. Although the present study primarily evaluated individual treatments and the bioagent + FYM combination, future research should focus on exploring multi-component strategies, including their influence on host resistance mechanisms such as defense-related enzyme activity. This integrated approach may lead to more sustainable and robust nematode suppression under field conditions.
Conclusion
This study provides comprehensive insight into the efficacy of biocontrol agents, essential oils and plant extracts and organic amendments in the management of M. incognita. The findings demonstrate that the combined application of B. subtilis and B. amyloliquefaciens strains exhibits promising effect by enhancing nematode control activity due to egg hatching inhibition and high juvenile mortality of M. incognita. The essential oils from M. spicata and P. longum were also found to have potent nematode control activity than extracts. Organic amendments with farmyard manure, vermicompost, and paddy straw also improved soil fertility (NPK and organic carbon content) and promoted plant growth while reducing nematode infection levels by enhancing soil fertility. Future research should focus on elucidating the molecular mechanisms underlying the nematicidal action of these treatments and evaluating their large-scale field applicability in diverse agroecosystems and crops. These findings contribute to the growing body of knowledge supporting eco-friendly nematode management strategies, paving the way for sustainable agricultural practices that minimize reliance on synthetic pesticides while ensuring crop productivity and soil health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
VG: Data curation, Visualization, Methodology, Investigation, Validation, Formal analysis, Writing – original draft, Software, Writing – review & editing. MM: Writing – review & editing, Data curation, Methodology. VR: Methodology, Conceptualization, Resources, Writing – review & editing. NS: Methodology, Resources, Conceptualization, Writing – review & editing. RP: Resources, Writing – review & editing, Methodology. AS: Data analysis, Writing – original draft, Visualization. RK: Formal analysis, Writing – review & editing. MJ: Formal analysis, Writing – review & editing. P: Project administration, Methodology, Supervision, Writing – original draft, Writing – review & editing, Resources, Conceptualization, Investigation. AG: Methodology, Investigation, Writing – review & editing. VK: Methodology, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are thankful to the Director, Dean and Joint Director (Research & Education), ICAR-Indian Agricultural Research Institute, New Delhi (110012), India, for providing facilities to conduct experiments in the institution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1602326/full#supplementary-material
References
Abdel Razek, G. M. and Yaseen, R. (2020). Effect of some rhizosphere bacteria on root-knot nematodes. Egypt. J. Biol. Pest Control 30, 1–11. doi: 10.1186/s41938-020-00340-y
Alfianny, R., Aryantha, I. N. P., and Syamsudin, T. S. (2017). Role of indigenous rhizosphere bacteria in suppressing root-knot nematode and improve plant growth tomato. P. Path. J. (Faisalabad) 16, 25–32. doi: 10.3923/ppj.2017.25.32
Ali, D. F. I., El-Nahrawy, S., EL-Zawawy, H. A., and Omara, A. E. D. (2025). Effective applications of Bacillus subtilis and B. amyloliquefaciens as biocontrol agents of damping-off disease and biostimulation of tomato plants. Stresses 5, 9. doi: 10.3390/stresses5010009
Antil, S., Kumar, R., Pathak, D. V., and Kumari, A. (2023). Recent advances in utilizing bacteria as biocontrol agents against plant parasitic nematodes emphasizing. Meloidogyne Biol. Control 183, 105244. doi: 10.1016/j.biocontrol.2023.105244
Bhat, A. A., Shakeel, A., Waqar, S., Handoo, Z. A., and Khan, A. A. (2023). Microbes vs. nematodes: Insights into biocontrol through antagonistic organisms to control root-knot nematodes. Plants 12, 451. doi: 10.3390/plants12030451
Bremner, J. M. (1960). Determination of nitrogen in soil by the kjeldahl method. J. Agric. Sci. 55, 11–33. doi: 10.1017/S0021859600021572
Caboni, P., Saba, M., Tocco, G., Casu, L., Murgia, A., Maxia, A., et al. (2013). Nematicidal activity of mint aqueous extracts against the root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 61, 9784–9788. doi: 10.1021/jf403684h
Cai, H., Zhang, X., Lin, Z., Li, S., Lin, H., and Lin, Y. (2025). Mint essential oil: A natural and effective agent for controlling house dust mites. PloS One 20, e0318639. doi: 10.1371/journal.pone.0318639
Chamkhi, I., El Omari, N., Balahbib, A., El Menyiy, N., Benali, T., and Ghoulam, C. (2022). Is——the rhizosphere a source of applicable multi-beneficial microorganisms for plant enhancement? Saudi J. Bio. Sci. 29, 1246–1259. doi: 10.1016/j.sjbs.2021.09.032
Chen, J., Li, Q. X., and Song, B. (2020). Chemical nematicides: Recent research progress and outlook. J. Agric. Food Chem. 68, 12175–12188. doi: 10.1021/acs.jafc.0c02871
Culver, J. J., Vienna, V., and Burgess, P. A. (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267. doi: 10.1093/jee/18.2.265
Edussuriya, R., Rajapaksha, A. U., Jayasinghe, C., Pathirana, C., and Vithanage, M. (2023). Influence of biochar on growth performances, yield of root and tuber crops and controlling plant-parasitic nematodes. Biochar 5, 68. doi: 10.1007/s42773-023-00261-7
El-Ashry, R. M. (2021). Application of animal manure and plant growth-promoting rhizobacteria as effective tools to control soil nematode population and increase crop yield in grapevine orchards. Egypt. J. Agro. 20, 34–52. doi: 10.21608/ejaj.2021.141311
El-Nagdi, W. M. A. and Youssef, M. M. A. (2004). Soaking faba bean seed in some bio-agent as prophylactic treatment for controlling Meloidogyne incognita root-knot nematode infection. J. Pest Sci. 77, 75–78. doi: 10.1007/s10340-003-0029-y
Gebrehana, Z. G., Gebremikael, M. T., Beyene, S., Wesemael, W., and De Neve, S. (2025). Suppression potential of selected vermicomposts against root-knot nematode (Meloidogyne incognita) under in vitro, greenhousepot and field conditions. Front. P. Sci. 16, 1532800. doi: 10.3389/fpls.2025.1532800
Gowda, A. A. P., Pankaj, N. A., Rana, V. S., Singh, A. K., Bhatt, K. C., and Devaraja, K. P. (2023). Chemical composition and nematicidal activity of essential oil and piperitenone oxide of Mentha longifolia L. against Meloidogyne incognita. Allelopathic J. 58, 165–182. doi: 10.26651/allelo.j/2023-58-2-1427
Grewal, P. S., Ehlers, R. U., and Shapiro-Ilan, D. I. (2005). Nematodes as biocontrol agents. (Cambridge, MA & Wallingford, UK: Cabi publishing). doi: 10.1079/9780851990170.0000
Gupta, B. K., Dwivedi, S. V., Mishra, B. P., Mishra, D., Ojha, P. K., Verma, A. P., et al. (2021). Adoption gap analysis in tomato cultivation in banda district of bundelkhand (U.P.). Ind. J. Ext. Edu. 57, 126–130. doi: 10.48165/IJEE.2021.57427
Hussey, R. S. and Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. P. Dis. Rep. 57, 1025–1028.
Jones, R. K. (2017). “Nematode control and nematicides: developments since 1982 and future trends,” in Nematology in South Africa: a view from the 21st century, (Springer, Cham) 129–150.
Joyamati, L., Zenith, N. G., Ronibala, K. H., and Purnima, P. (2013). Efficacy of essential oil extracts of medicinal plants against rice root- knot nematode Meloidogyne graminicola in pots. Ind. J. Nemat. 43, 86–89.
Kesba, H. and Al-Shalaby, M. (2008). Survival and reproduction of Meloidogyne incognita on tomato as affected by humic acid. Nematology 10, 243–249. doi: 10.1163/156854108783476304
Khanna, K., Gautam, V., Kapoor, D., Sharma, N., Sharma, P., Bhardwaj, T., et al. (2022). “Conventional and organic management as divergent drivers for plant parasitic nematodes control,” in Sustainable management of nematodes in agriculture, vol. 1: organic management (Springer International Publishing, Cham), 157–185.
Kimbaris, A. C., Gonzalez-Coloma, A., Andres, M. F., Vidali, V. P., Polissiou, M. G., and Santana-Meridas, O. (2017). Biocidal compounds from Mentha sp. essential oils and their structure-activity relationships. Chem. Biodiversity 14, 160–270. doi: 10.1002/cbdv.201600270
Kumar, V., Khan, M. R., and Walia, R. K. (2020). Crop loss estimations due to plant-parasitic nematodes in major crops in India. Natl. Acad. Sci. Lett. 43, 409–412. doi: 10.1007/s40009-020-00895-2
Kumar, V., Mondal, P. C., Kumar, R., Pankaj, Kaushik, P., Shakil, N. A., Gowda, A. P. A., et al. (2023). Composition and nematicidal activity of the essential oil from Piper longum against root knot nematode. Ind. J. Agric. Sci. 93, 774–779. doi: 10.56093/ijas.v93i7.135197
Majzoob, S., Karegar, A., Taghavi, M., and Hamzehzarghani, H. (2012). Evaluation of rhizobacteria for antagonistic activity against root-knot nematode, Meloidogyne javanica on cucumber, under greenhouse condition. Ira. J. Pl. Path. 48, 27–29.
Manju, P. and Subramanian, S. (2019). “Management of root knot nematode Meloidogyne incognita by plant growth promoting rhizobacteria Bacillus spp. on Gerbera jamesonii,” in III international symposium on underutilized plant species, (Leuven, Belgium: International Society for Horticultural Science (ISHS)) vol. 1241, 487–492. Available at: https://doi.org/10.17660/ActaHortic.2019.1241.72.
Mankau, R. (1963). Effect of organic soil amendments on nematode populations. In. Phytopathology 53, 881–82).
Meena, K. S., Jonathan, E. I., Devrajan, K., and Raguchander, T. (2012). Pseudomonas fluorescens induced systemic resistance in tomato against Meloidogyne incognita. Ind. J. Nemat. 42, 5–10.
Meher, H. C., Gajbhiye, V. T., Singh, G., Kamra, A., and Chawla, G. (2010). Persistence and nematicidal efficacy of carbosulfan, cadusafos, phorate, and triazophos in soil and uptake by chickpea and tomato crops under tropical conditions. J. Agric. Food Chem. 58, 1815–1822. doi: 10.1021/jf903609d
Mondal, P. C., Kumar, V., Kaushik, P., and Shakil, N. A. (2024). New nematicidal compounds from Mentha spicata L. against Meloidogyne incognita. J. Pl. Dis. Prot. 131, 1983–1992. doi: 10.1007/s41348-024-00977-z
Morcia, C., Tumino, G., Ghizzoni, R., and Terzi, V. (2016). “Carvone (Mentha spicata L.) oils,” in Essential oils in food preservation, flavor and safety (London, UK: Academic Press), 309–316.
Moreira, A. C. S., Lopes, E. A., Visôtto, L. E., Soares, M. S., Londe, M. L. A., Ribeiro, L. B., et al. (2025). Bacillus amyloliquefaciens strain BaNCT02: An antagonist with multiple mechanisms of action against Meloidogyne incognita. Plant Pathol. 74 (2), 320–329. doi: 10.1111/ppa.14021
Munif, A., Hallmann, J., and Sikora, R. A. (2013). The influence of endophytic bacteria on Meloidogyne incognita infection and tomato plant growth. J. Int. Soc. Southeast Asian Agric. Sci. 19, 68–74.
Odhiambo, J. A. and Aguyoh, J. N. (2019). Influence of farm yard manure on nematode infestation, yield and quality of cucumber. Asian J. Agric. Food Sci. (ISSN 2321–1571), 7(06).
Páez, A. R. and Leite, J. P. V. (2025). “Antimicrobial activity of essential oils: Uses and application,” (s against plant pathogens. In Nat. Pest. Allelo. (pp. 147-167). (Boca Raton, FL: CRC Press).
Pankaj, H. S., Singh, K., and Lal, J. (2015). Management of rice root-knot nematode, Meloidogyne graminicola in rice (Oryza sativa). Ind. J. Agric. Sci. 85, 701–704. doi: 10.56093/ijas.v85i5.48511
Pires, D., Vicente, C. S., Menéndez, E., Faria, J. M., Rusinque, L., Camacho, M. J., et al. (2022). The fight against plant-parasitic nematodes: Current status of bacterial and fungal biocontrol agents. Pathogens 11, 1178. doi: 10.3390/pathogens11101178
Poveda, J., Abril-Urias, P., and Escobar, C. (2020). Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Frontiers in Microbiology, 11, 992.
Radwan, M. A., Farrag, S. A. A., Abu-Elamayem, M. M., and Ahmed, N. S. (2012). Efficacy of some granular nematicides against root-knot nematode, Meloidogyne incognita associated with tomato. Pak. J. Nemat. 30, 41–47.
Rajasekharan, S. K., Raorane, C. J., and Lee, J. (2020). Nematicidal effects of piperine on the pinewood nematode Bursaphelenchus xylophilus. J. Asia-Pacific Entomology 23, 863–868. doi: 10.1016/j.aspen.2020.07.022
Ramezani Moghaddam, M., Mahdikhani Moghaddam, E., Baghaee Ravari, S., and Rouhani, H. (2014). The nematicidal potential of local Bacillus species against the root-knot nematode infecting greenhouse tomatoes. Biocontrol science and technology, 24 (3), 279–290.
Renco, M. (2013). Organic amendments of soil as useful tools of plant parasitic nematodes control. Helminthologia 50, 3–14. doi: 10.2478/s11687-013-0101-y
Rhode, R. A. (1960). Acetyle cholinesterase in plant parasitic nematodes and an antiacetyle cholinesterase from Asparagus. Proc. Helminthological Soc. Washington 27, 121–123.
Saha, R., Mishra, V. K., Majumdar, B., Laxminarayana, K., and Ghosh, P. K. (2010). Effect of integrated nutrient management on soil physical properties and crop productivity under a maize (Zea mays)–mustard (Brassica campestris) cropping sequence in acidic soils of northeast India. Commun. Soil Sci. Plant Anal. 41, 2187–2200. doi: 10.1080/00103624.2010.504799
Saroj, A., Oriyomi, O. V., Nayak, A. K., and Haider, S. Z. (2020). “Phytochemicals of plant-derived essential oils: A novel green approach against pests,” in Natural remedies for pest, disease and weed control (London, UK: Academic Press), 65–79.
Sarri, K., Mourouzidou, S., Ntalli, N., and Monokrousos, N. (2024). Recent advances and developments in the nematicidal activity of essential oils and their components against root-knot nematodes. Agronomy 14, 213. doi: 10.3390/agronomy14010213
Sasanelli, N., Konrat, A., Migunova, V., Toderas, I., Iurcu-Straistaru, E., Rusu, S., et al. (2021). Review on control methods against plant parasitic nematodes applied in southern member states (C Zone) of the european union. Agriculture 11, 602. doi: 10.3390/agriculture11070602
Sayre, R. M. and Starr, M. P. (1985). Pasteuria penetrans a mycelial and endospore-forming bacterium parasitic in plant-parasitic nematodes. Proc. Helmin. Soc Washington 52, 149–165.
Siddiqui, Z. A. and Mahmood, I. (1999). Role of bacteria in the management of plant parasitic nematodes: a review. BioResource Tech 69, 167–179. doi: 10.1016/S0960-8524(98)00122-9
Sikora, R. A., Bridge., J., and Starr, J. L. (2005). “Management practices: an overview of integrated nematode management technologies,” in Plant parasitic nematodes in subtropical and tropical agriculture (2nd ed.), 793–825. In: Luc, M., Sikora, R. A., and Bridge, J. (Eds.). Wallingford, UK: CAB International.
Sikora, R. A. and Fernandez, E. (2005). “Nematodes parasites of vegetables,” in Plant parasitic nematodes in subtropical and tropical agriculture (CAB International, Wallingford (GBR), 319–392.
Sims, J. T. (2000). “Soil test phosphorus: Olsen P,” in Methods of phosphorus analysis for soils, sediments, residuals, and waters, (Raleigh, NC: North Carolina State University) 20.
Taimoor, K. and Shahina, F. (2018). Management of banana plants against Meloidogyne incognita with indigenous medicinal and aromatic plants. Pak. J. Nemat. 36, 83–88. doi: 10.18681/pjn.v36.i01.p83-110
Vasantha-Srinivasan, P., Park, K. B., Kim, K. Y., Jung, W. J., and Han, Y. S. (2025). The role of Bacillus species in the management of plant-parasitic nematodes. Front. Microbiol. 15, 1510036. doi: 10.3389/fmicb.2024.1510036
Keywords: root-knot nematode, Meloidogyne incognita, tomato (Solanum lycopersicum), biocontrol agents, green pesticides
Citation: G. V, Machal M, Rana VS, Gowda AP A, Kumar V, Shakil NA, Pervez R, Singh AK, Kumar R, Jaiman M and Pankaj (2025) Effect of botanicals, organic nutrient sources, and bio-control agents on root-knot nematode (Meloidogyne incognita) infecting tomato. Front. Plant Sci. 16:1602326. doi: 10.3389/fpls.2025.1602326
Received: 29 March 2025; Accepted: 02 June 2025;
Published: 02 July 2025.
Edited by:
Mahfouz M. M. Abd-Elgawad, National Research Centre, EgyptReviewed by:
Francesca De Luca, National Research Council (CNR), ItalyTarique Hassan Askary, Sher-e-Kashmir University of Agricultural Sciences and Technology, India
Fatma Abdel Mohsen Mostafa, Mansoura University, Egypt
Copyright © 2025 G., Machal, Rana, Gowda AP, Kumar, Shakil, Pervez, Singh, Kumar, Jaiman and Pankaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pankaj, cGFua2FqX25lbWFAeWFob28uY29t; Ashish Kumar Singh, c2FzaGlzaDA4MjVAZ21haWwuY29t
 Vimala G.1
Vimala G.1 Virendra Singh Rana
Virendra Singh Rana Najam Akhtar Shakil
Najam Akhtar Shakil Ashish Kumar Singh
Ashish Kumar Singh Ravinder Kumar
Ravinder Kumar Mukesh Jaiman
Mukesh Jaiman Pankaj
Pankaj