- Collaborative Innovation Center for Efficient and Green Production of Agriculture in Mountainous Areas of Zhejiang Province, College of Horticulture Science, Zhejiang A&F University, Hangzhou, Zhejiang, China
Bayberry (Myrica rubra) is a significant subtropical fruit tree, renowned for its distinctive flavor and high nutritional value. WRKY transcription factors are a class of plant-specific zinc-finger proteins that play critical roles in plant growth and development, secondary metabolism, and responses to abiotic stress. However, there is currently limited information about the WRKY gene family in bayberry. This study conducted a systematic bioinformatics analysis of 55 WRKY genes in bayberry, elucidating their phylogenetic relationships, gene structures, conserved motifs, and syntenic characteristics. The results demonstrated that these WRKY family members could be classified into five subfamilies, with each gene containing at least one WRKY domain. The bayberry WRKY genes exhibited significant variations in gene length and intron-exon numbers, while maintaining relatively conserved gene structures within each subfamily. The promoters of WRKY gene members contained multiple regulatory elements, including hormone-responsive elements, light-responsive elements, and abiotic stress-responsive elements. Collinearity analysis revealed that the WRKY family in bayberry experienced six segmental duplication events. Inter-species synteny analysis demonstrated high collinearity between bayberry and Actinidia spp., indicating evolutionary conservation of WRKY genes across different plant species. It was observed that bayberry WRKY genes exhibited significant differential expression across different cultivars and developmental stages of fruits through expression pattern analysis. Further research indicated that MrWRKY14, a member of the bayberry WRKY family, significantly enhanced the promoter activity of MrSWEET1, thereby influencing the process of sugar accumulation. These findings not only provide an important reference for the genome-wide identification of WRKY gene families in plants but also lay a solid foundation for future in-depth functional analysis of bayberry WRKY genes.
1 Introduction
WRKY transcription factor family is one of the largest transcription factor families in higher plants and has been found throughout the green plant lineage (Ulker and Somssich, 2004). The name of “WRKY family” derived from its highly conserved 60-amino acid, four-stranded β-sheet WRKY DNA-binding domain (DBD), which contained the highly conserved N-terminal WRKYGQK motif and a C-terminal zinc finger motif with novel metal-chelating properties (Zhang and Wang, 2005). Methionine and histidine residues coordinated a zinc ion to form a finger-like structure motif. Both the WRKY domain and zinc finger motif were essential for DNA binding activity of the protein (Yang et al., 2009). Based on these features, the WRKY family members were classified into three groups: Group I (with two WRKY DBDs), Group II (with a single DBD and diverse C2H2 zinc finger structures), and Group III (with a single DBD and C2HC zinc finger structures) (Rushton et al., 2010). Group I WRKY genes were characterized by dual WRKY domains, whereas Group II and III WRKY genes possessed only a single domain (Maeo et al., 2001). Structurally, both Group I and II contained a C2H2-type zinc finger motif (C-X4-5-C-X22-23-H-X1-H), with DNA-binding activity exclusively mediated by the C-terminal domain. Group II WRKY proteins were further subdivided into five subgroups (a-e) based on variations in additional amino acid motifs outside the WRKY domain. Notably, Group III exhibited significant divergence in zinc finger configuration compared to Groups I and II, featuring a distinctive C2-HC zinc finger structure with a unique C-X7-C-X23-H-X-C arrangement pattern (Bakshi and Oelmüller, 2014).
WRKY protein family has been extensively identified across diverse plant species, exhibiting significant interspecies variation in gene family size. Notable examples included the identification of 65 members in Arabidopsis thaliana (Wang et al., 2011), 101 members in rice (Oryza sativa) (Abdullah-Zawawi et al., 2021), and 72 members in tomato (Solanum lycopersicum) (Liu et al., 2022). In fruit tree species, the distribution of WRKY family members exhibited a more pronounced diversity and abundance. For instance, 113 members have been identified in apples (Qin et al., 2022), 116 members in kiwifruit (Jing and Liu, 2018), 47 members in sweet orange (Xi et al., 2023), and 59 in grape (Wang et al., 2014).
Extensive research has confirmed that WRKY transcription factors play crucial biological roles in plants, primarily in regulating plant dwarfism, leaf senescence, fruit ripening, and responses to abiotic stress. For instance, studies in apple have revealed that the transcription factor MdWRKY9 promoted dwarfism by directly suppressing the transcriptional activity of MdDWF4, a key enzyme involved in brassinosteroid (BR) biosynthesis, thereby reducing BR production (Zheng et al., 2018). In strawberry, FaWRKY transcription factors FaWRKY48, FaWRKY53, FaWRKY24 and FaWRKY17 were involved in the abscisic acid signaling pathway, promoting fruit ripening by regulating ABA biosynthesis (Garrido-Gala et al., 2022). Additionally, the expression of WRKY gene were induced by thyme chitosan nanoparticles, thereby enhancing tomato resistance to root rot pathogens. The expression of WRKY genes has been demonstrated to enhance plant drought tolerance while simultaneously promoting plant growth and biomass accumulation under drought conditions. For instance, TaWRKY133 functioned as a negative regulator of drought stress responses in wheat. Overexpression of TaWRKY133 reduced the drought tolerance of transgenic plants, highlighting its critical role in regulating abiotic stress responses (Lv et al., 2022). Overexpression of AtWRKY30 has been demonstrated to improve drought tolerance in transgenic wheat by upregulating the expression of genes associated with growth, osmoregulatory substance biosynthesis, gas exchange parameters, and antioxidant enzyme activity (Yang et al., 2025).
Multiple studies have demonstrated that WRKY transcription factors play crucial roles in fruit development, ripening, and quality formation. In bananas, approximately 50% of the MaWRKY gene family members exhibited high expression during fruit ripening, indicating their significant regulatory roles in fruit development and post-harvest ripening processes (Jia et al., 2022). Research on strawberries has shown that FaWRKY71 expression was induced by exogenous abscisic acid, but it does not affect endogenous ABA synthesis. Instead, FaWRKY71 promoted strawberry fruit ripening through auxin regulation rather than the ABA pathway (Yue et al., 2022). In pitaya, HpWRKY3 activated the expression of HpINV2 and HpSuSy1, indicating that this gene may participate in sugar accumulation in dragon fruits by transcriptionally regulating sucrose metabolism-related genes (Wei et al., 2019). In grapes, overexpression of VvWRKY22 reduced the content of sucrose, glucose, and fructose, modulated the expression of genes related to sugars and ABA, and interacted with VvSnRK1.1 or VvSnRK1.2 proteins (sucrose non-fermenting-1 related protein kinases), which were important kinases involved in sugar metabolism (Huang et al., 2021).
Bayberry (Myrica rubra) is an important subtropical fruit tree native to China (Sun et al., 2013). WRKY transcription factors played crucial regulatory roles in plant growth and development, secondary metabolism, and responses to abiotic stress (Rushton et al., 2010). However, current research on the WRKY gene family in bayberry remained relatively limited. In this study, we conducted a comprehensive analysis of the WRKY gene family in bayberry fruits through genome-wide identification and expression pattern analysis, aiming to establish a solid theoretical foundation for elucidating their biological functions. These findings were expected to provide valuable references for elucidating the potential applications and molecular mechanisms of WRKY gene family in plants, thereby supporting their further development and utilization.
2 Materials and methods
2.1 Plant materials
Fruits of two bayberry cultivars (‘Dongkui’ and ‘Shuijing’) were selected as experimental materials, collected from LinGe Family Ecological Farm in Lin’an District, Hangzhou City, Zhejiang Province. Fruit samples were collected at five different developmental stages (designated S1-S5), corresponding to 51, 58, 65, 72, and 80 days after flowering, respectively. The experiment was conducted with three biological replicates, each containing at least five fruits. All collected samples were immediately frozen in liquid nitrogen and stored at -80°C for subsequent analysis.
2.2 Identification of WRKY gene family members in bayberry
To screen the WRKY genes of bayberry, the genome sequences and annotation information of bayberry was downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/datasets/genome/GCA_003952965.2/). The WRKY sequences of Arabidopsis were obtained from The Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/). The hidden Markov model (HMM) for the WRKY conserved domain (PF03106) was retrieved from the Pfam protein family database. Using this HMM model and the Arabidopsis WRKY protein sequences, candidate WRKY genes in bayberry was identified through HMMER and BLASTP tools. Initially, the Arabidopsis sequences were used as query sequences to perform a BLASTP search against the bayberry proteome (e-value < 1e-10, identity >= 40). The WRKY proteins in the bayberry genome was identified using the HMM search function in TBtools, and redundant sequences were removed from the results. Molecular weight (Da), isoelectric points, and other physicochemical properties of WRKY proteins were determined using the online ExPASy tool (http://web.expasy.org/protparam/).
2.3 Phylogenetic tree analysis of WRKY genes
A Clustal X2 sequence alignment was performed on the full-length protein sequences of Arabidopsis and bayberry WRKY proteins. The phylogenetic tree was constructed using the MEGA X software with the Neighbor-Joining method, and bootstrap resampling was set to 1000 repetitions. The phylogenetic tree was subsequently polished using the ITOL online tool (https://itol.embl.de/).
2.4 Gene structure and conserved motifs analysis
The exon and intron information of bayberry WRKY genes was retrieved from the bayberry genome annotation data. The gene structure diagram was generated using the Visualiza Gene Structure function in TBtools. For motif analysis, the MEME suite (http://meme-suite.org/tools/meme) was employed to identify six conserved motifs within the bayberry WRKY protein sequences. The distribution of these motifs was visualized using the Gene Structure View function in TBtools.
2.5 Chromosome location and collinearity analysis
The chromosome positions and tandem repeats of MrWRKY genes were visualized through the BLAST and MCScanX functions in TBtools software based on the MrWRKY protein sequences. Based on the bayberry genome and annotation files, whole-genome sequence alignment was conducted through the MCScanX and gene position extract functions in TBtools, yielding syntenic relationship files of MrWRKYs within the bayberry species. The chromosome length file was obtained using the fasta stats function, while the gene density file was generated via the fasta stats table function. Finally, by integrating the syntenic relationship file, chromosome length file, and gene density file, the homologous gene diagram for MrWRKYs were constructed using the Advanced Circos function zone of TBtools software.
2.6 Cis-acting elements analysis
The cis-acting elements in the 2000 bp promoter regions of each bayberry WRKY gene family members were analyzed using the PlantCARE website (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/). And the analysis results were visualized using the TBtools software.
2.7 Gene expression profile analysis
Total RNA was extracted from various cultivars of bayberry during different fruit development stages using the CTAB method (Liu et al., 2024). RNA quantity and quality were determined using a NanoDrop One spectrophotometer, and RNA integrity was assessed by 1% agarose gel electrophoresis. High-quality cDNA libraries were constructed, and gene expression analysis was performed. A transcriptome-wide expression profile of bayberry WRKY genes was generated using strand-specific RNA-seq, with transcript abundance calculated as reads per kilobase of transcript per million mapped reads (RPKM). Sequencing data used in the current study are available in the NCBI Sequence Read Archive database (project number: PRJNA1105392). Expression patterns of bayberry WRKY genes were visualized as a heatmap using the Heatmap illustrator tool in TBtools. The relative expression levels of genes were calculated using the 2−ΔΔCt method, with Mr-actin serving as the reference gene for normalization. All experiments were conducted with three biological and technical replicates to ensure reproducibility.
2.8 Dual luciferase analysis
The promoter sequence was inserted upstream of the firefly luciferase (LUC) reporter gene vector, while the Renilla luciferase (REN) gene, driven by the 35S promoter, served as an internal control on the same vector. The coding sequence (CDS) of MrWRKY was cloned into the pGreenII 0029 62-SK vector as the effector gene, with the empty vector serving as the negative control. For transient expression assays, Agrobacterium tumefaciens strains harboring either the effector or reporter constructs were co-infiltrated into the abaxial side of Nicotiana benthamiana leaves using Agrobacterium-mediated transformation. After 48 hours of incubation, luciferase activity was measured using a dual-luciferase reporter assay system. The relative LUC/REN ratio was calculated according to the instructions of the dual luciferase reporter gene assay kit.
2.9 Statistical analysis
Data were processed, statistically analyzed and calculated using Microsoft Excel software. Data visualization was performed with OriginPro 2023. Independent two-sample t-tests were performed using IBM SPSS Statistics 27.0, with P<0.05 considered statistically significant. Gene expression profiles were compared using Pearson correlation analysis.
3 Results
3.1 Identification and phylogenetic analysis of bayberry WRKY genes
By integrating the hidden Markov model (HMM) and BLAST search, a total of 55 WRKY gene family members were identified from the bayberry genome data, and were systematically designated as MrWRKY01 to MrWRKY55 based on their chromosomal locations. The MrWRKY proteins exhibited significant variations in their physicochemical properties, including amino acid length and theoretical isoelectric point values (Supplementary Table 1). Specifically, the amino acid numbers of MrWRKYs varied from 131aa (MrWRKY02) to 990 aa (MrWRKY45), the molecular weights ranged from 14879.22 Da (MrWRKY02) to 108143.45 Da (MrWRKY02). And the pI values fluctuated between 4.94 (MrWRKY30) and 9.89 (MrWRKY48). In addition, 42 (76.36%) members of the MrWRKYs were localized in the nuclear, 8 (14.54%) members were localized in the cytoplasmic, 3 (5.45%) members were localized in the chloroplast.
To comprehensively understand the evolutionary relationships and functional conservation of the WRKY genes, multiple sequence alignments were performed on WRKY protein sequences from bayberry and Arabidopsis. As shown in Figure 1, a phylogenetic tree was constructed using the Neighbor-Joining (NJ) method. The WRKY genes of bayberry were systematically classified following the established classification scheme of Arabidopsis WRKY genes. The results showed that bayberry WRKY genes could be divided into five subfamilies: WRKY-Ia, WRKY-Ib, WRKY-IIa, WRKY-IIb, and WRKY-III. Among them, WRKY-Ia subfamily comprised 14 genes, representing the largest subgroup within the MrWRKY gene family, suggesting its highest level of diversity and complexity. In contrast, the WRKY-IIa and WRKY-IIb subfamilies each contained 11 genes, reflecting distinct evolutionary trajectories and adaptive capabilities among WRKY subfamilies. The WRKY-Ib and WRKY-III subfamilies, each consisting of 10 genes, constitute the smallest subgroups, and these quantitative variations likely reflect differential selection pressures and retention mechanisms during evolutionary processes.
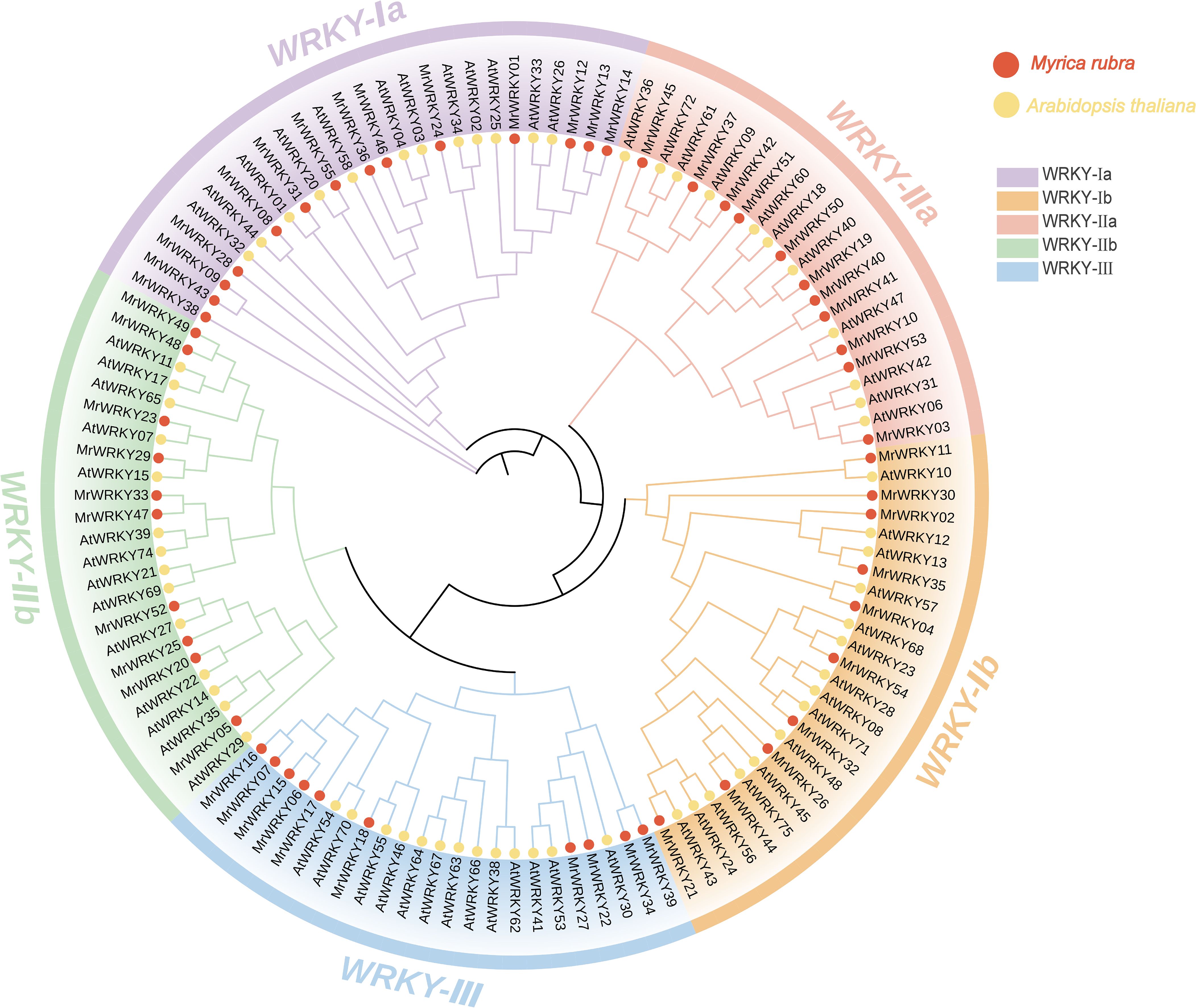
Figure 1. Phylogenetic analysis of WRKY proteins in Myrica rubra and Arabidopsis thaliana. Red dots correspond to MrWRKY genes from bayberry, while yellow dots represent AtWRKY genes from Arabidopsis. The different subfamilies are represented by distinct colored blocks for visual differentiation.
3.2 Chromosome location analysis of bayberry WRKY genes
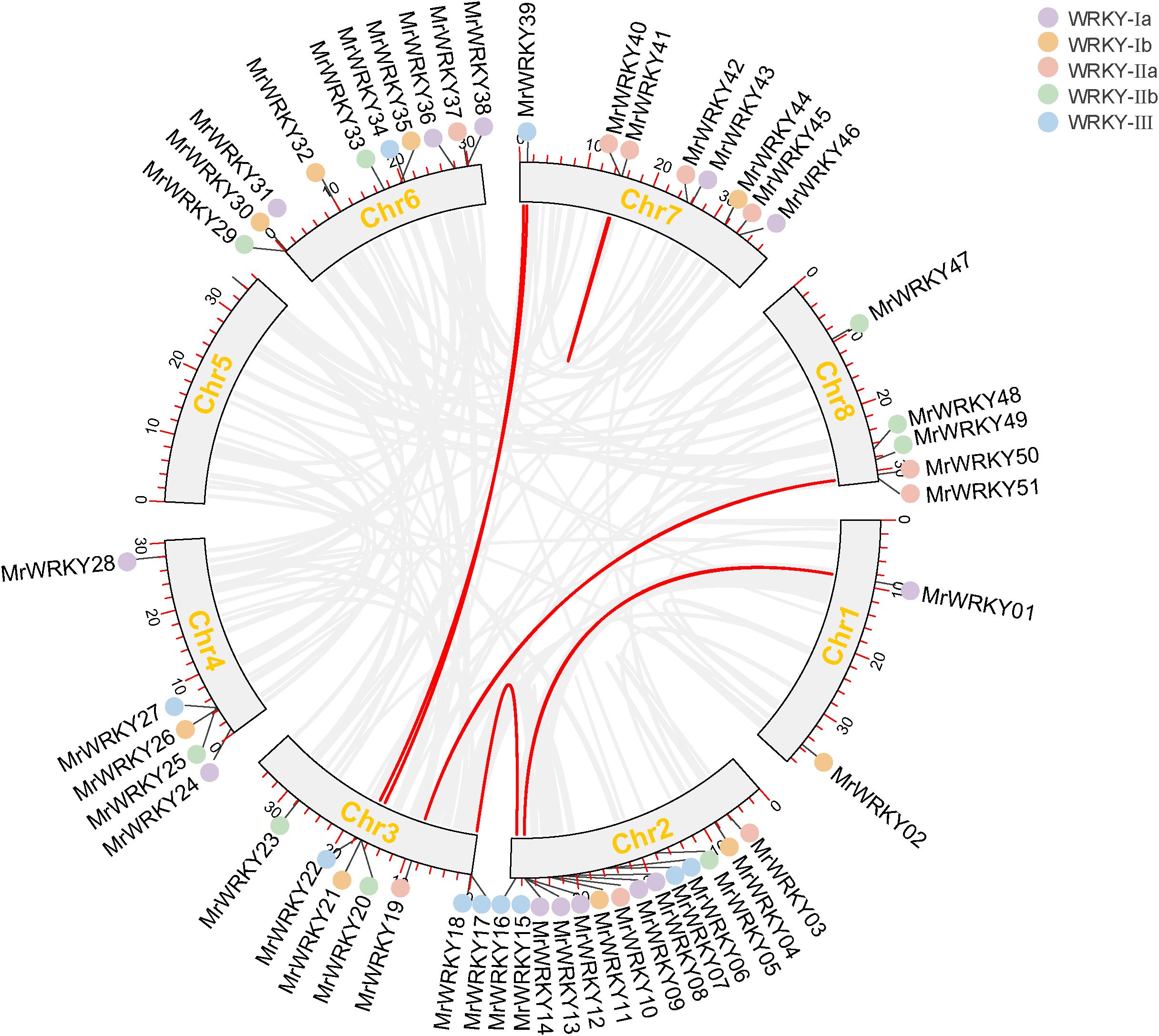
The chromosome location analysis of bayberry WRKY genes were conducted by the Tbtools tool. As shown in Figure 2, 51 MrWRKY genes were mapped across the eight chromosomes of bayberry, while four MrWRKY genes (MrWRKY52, MrWRKY53, MrWRKY54, and MrWRKY55) were localized on the scaffold. These 51 MrWRKY genes were unevenly distributed across eight chromosomes. Notably, Chromosome 2 contained the highest number of MrWRKY genes (14 members), while chromosomes 1, 3, 4, 6, 7, and 8 harbored 2, 7, 5, 9, 8, and 5 genes, respectively. Strikingly, only a single MrWRKY gene was found on chromosome 5. This uneven genomic distribution pattern suggested that the chromosomal allocation of WRKY genes may be associated with structural and functional characteristics of the respective chromosomes.
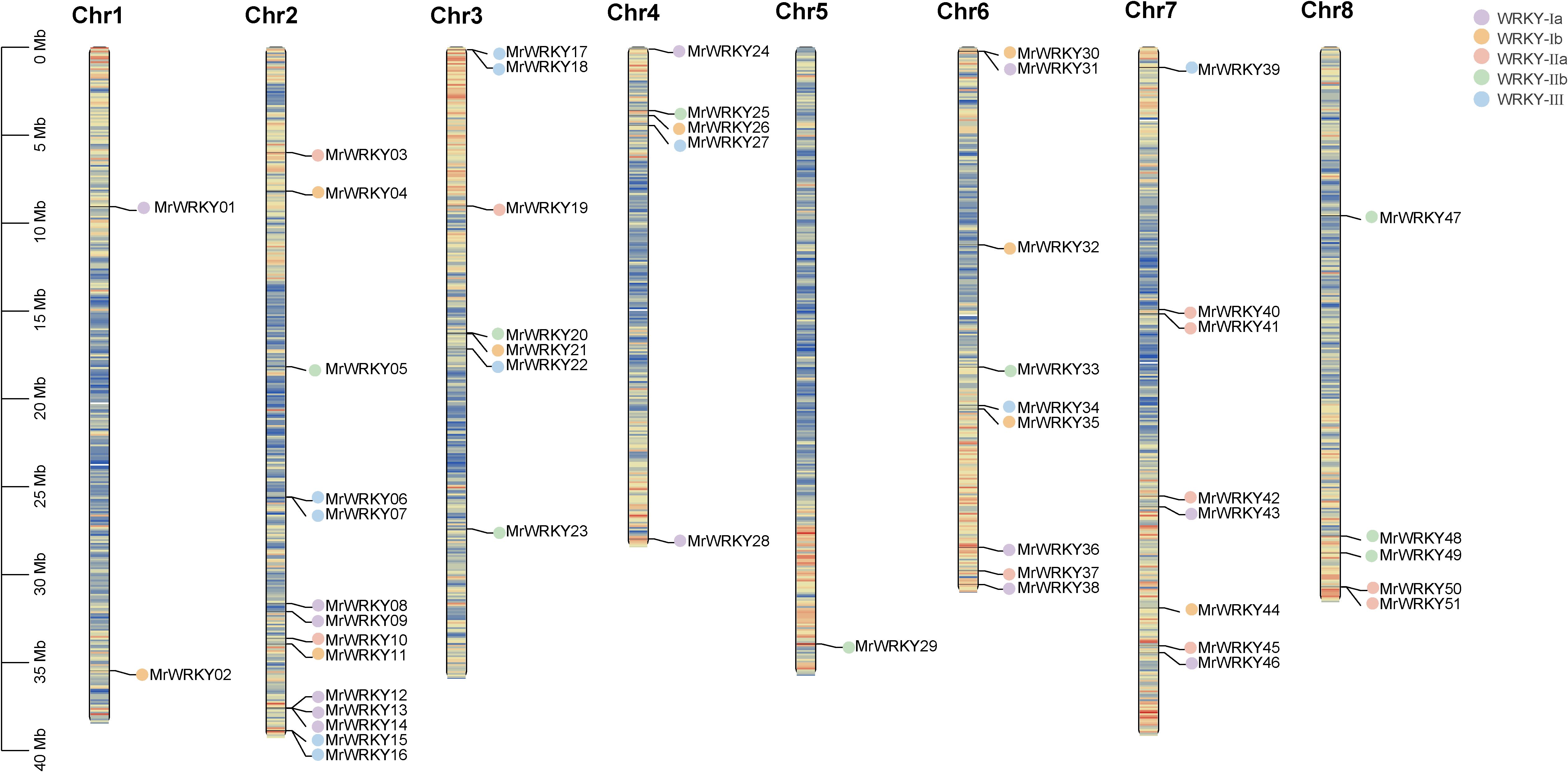
Figure 2. Chromosomal distribution of WRKY genes in bayberry. Genes from different subfamilies are represented by distinct colored dots.
Interestingly, chromosomes 2 and 5 exhibited the most abundant distribution of WRKY gene subfamilies, encompassing WRKY-Ia, WRKY-Ib, WRKY-IIa, WRKY-IIb, and WRKY-III subfamilies. Considering the pivotal role of chromosome scaffolds in gene expression and regulation, these scaffold-associated WRKY genes may confer distinctive biological functions.
3.3 Gene structure analysis of bayberry WRKY genes
To gain deeper insights into the gene structure and phylogenetic relationships of bayberry WRKY genes, we conducted a detailed analysis of the structural features and conserved motifs of the MrWRKY family members. The evolutionary relationship of the bayberry WRKY genes was presented in Figure 3A. As shown in Figure 3B, six motifs were identified among the 55 bayberry WRKY protein sequences. Specifically, 71.43% of the WRKY-Ia subfamily contained four motifs, while approximately 60.00% of the WRKY-Ib subfamily had three motifs. Similarly, 6 (54.54%) members of the WRKY-IIa subfamily possessed five motifs, whereas 17 (80.95%) members of the WRKY-IIb and WRKY-III subfamilies (excluding MrWRKY15, MrWRKY27, and MrWRKY34) had only two motifs.
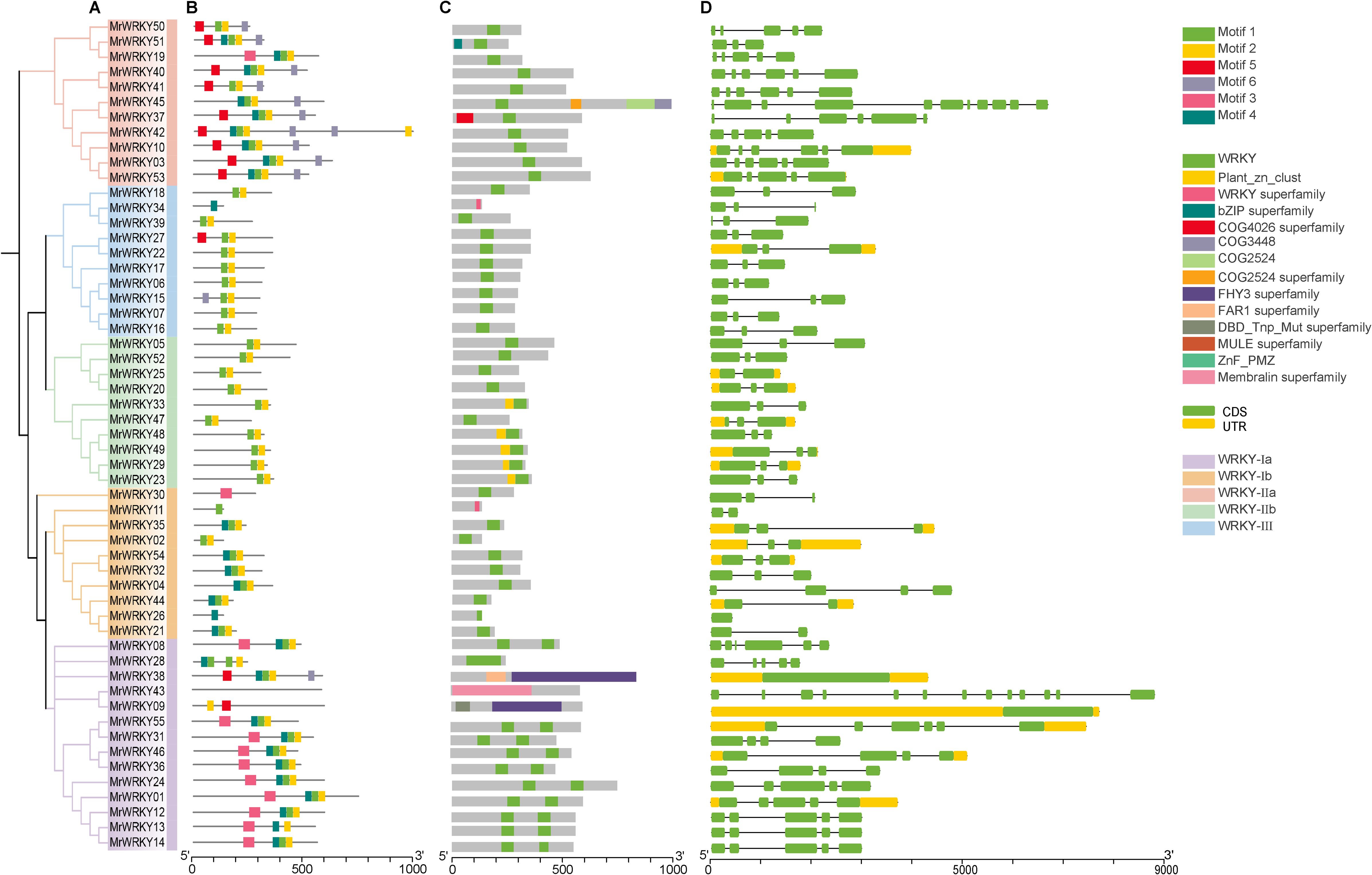
Figure 3. Structural composition of WRKY genes in bayberry. (A) Phylogenetic clustering of bayberry WRKY genes. (B) Distribution patterns of conserved motifs in bayberry WRKY genes. (C) Structural domain configuration of bayberry WRKY proteins. (D) Exon-intron structure of bayberry WRKY genes.
Domain analysis clearly revealed that each WRKY gene contained at least one WRKY domain (Figure 3C). As a hallmark feature of the WRKY gene family, this domain played a pivotal role in regulating various biological processes and was essential for gene expression and functional activity. By comparing structural differences in domains among different genes, we could further elucidate their evolutionary relationships and functional divergence.
Analysis of the MrWRKY gene family structure revealed that 17 (30.90%) MrWRKY members contained untranslated regions (UTRs) (Figure 3D). Specifically, 17 members possessed both 5’ and 3’ UTR exons, with exon numbers ranging from 1 to 13. Notably, MrWRKY43 contained the highest number of exons (13), while MrWRKY26 had only one exon and lacks introns. Within the WRKY-III subfamily, members with three exons accounted for 90.0% of the total. The MrWRKY gene family exhibited significant variation in gene length and intron-exon numbers, while maintaining relatively conserved gene structures within each subfamily. The similarity in gene structures and motif features among subfamilies further validated the reliability of the classification.
3.4 Cis-acting element analysis of bayberry WRKY genes
To further investigate the potential functions and regulatory mechanisms of bayberry WRKY genes, sequences from the upstream 2000 bp promoter regions of WRKY genes were extracted, and an extensive cis-acting element analysis was conducted using the PlantCARE online tool (Figure 4). Analyzing the promoters of bayberry WRKY genes could help identify cis-regulatory elements and understand their regulatory networks, while also uncovering how WRKY genes interact with other signaling pathways in the plant. The analysis revealed that the promoter regions of two WRKY genes, MrWRKY52 (a member of the WRKY-IIa subfamily) and MrWRKY53 (a member of the WRKY-IIb subfamily), showed no detectable cis-acting elements.
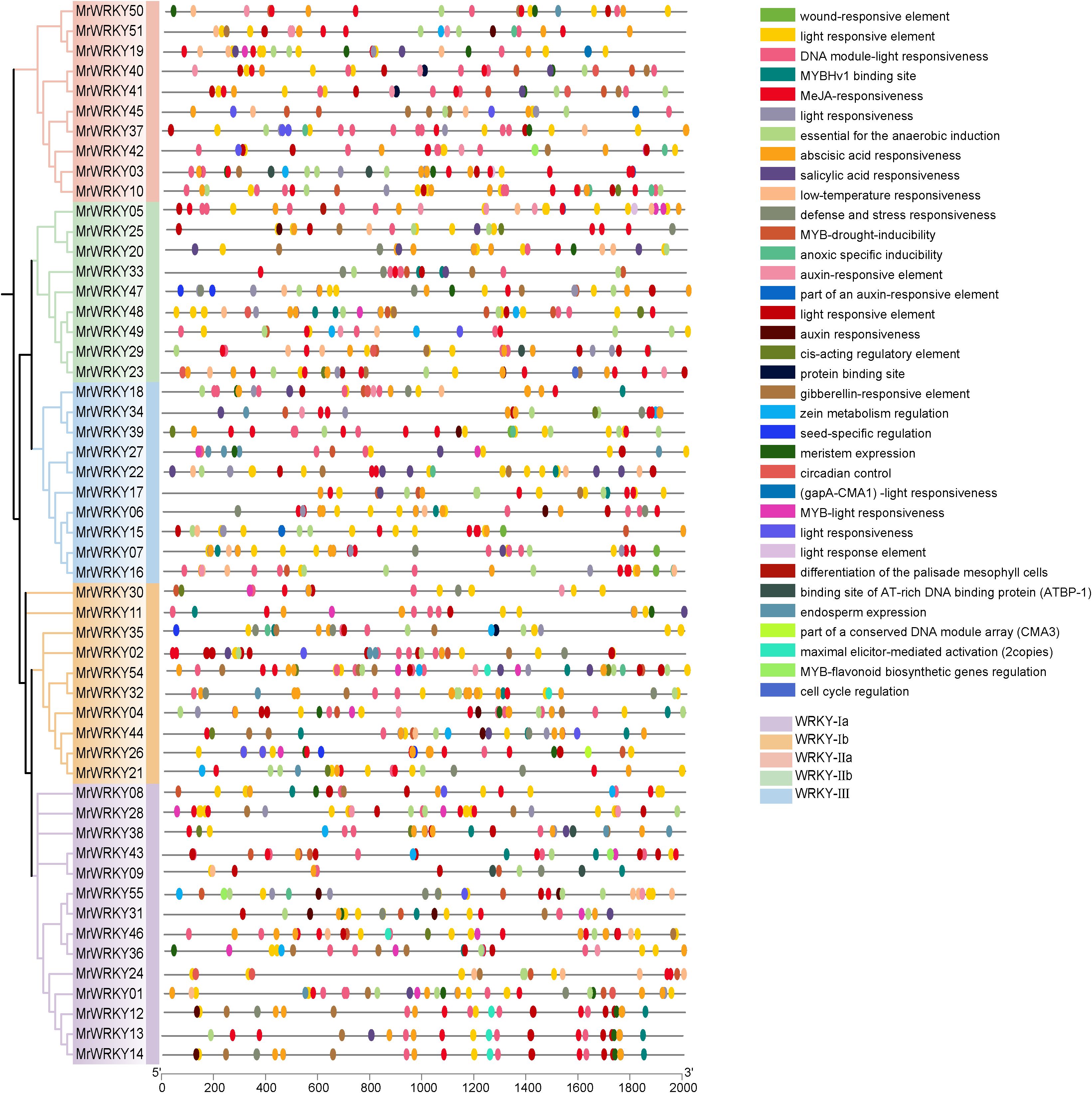
Figure 4. Cis-acting elements analysis of WRKY genes in bayberry. Differently colored blocks represent distinct cis-acting elements.
Through a systematic analysis of the bayberry WRKY promoters, this study identified and classified various cis-regulatory elements into three major categories: hormone response elements, light response elements, and abiotic stress response elements. Notably, light response elements were widely distributed across the WRKY gene family. Specifically, 186 light response elements were detected in the WRKY-Ia subfamily, accounting for 44.39% of the total cis-acting elements in this subfamily. Similarly, 139 light response elements were found in the WRKY-Ib subfamily, representing 44.13% of its total cis-acting elements. Additionally, the WRKY-IIa, WRKY-IIb, and WRKY-III subfamilies contained 127, 103, and 116 light response elements, respectively, which accounted for 44.41%, 39.77%, and 39.73% of their respective total cis-acting elements.
In terms of hormone response elements, the WRKY-Ia subfamily exhibited the highest abundance, containing 62 methyl jasmonate (MeJA) response elements (14.79%) and 50 abscisic acid (ABA) response elements (11.93%). Analysis of stress-responsive elements revealed that the WRKY-III subfamily possessed the highest quantity, containing 60 elements in total, accounting for 20.55% of its total cis-acting elements repertoire. The investigation further demonstrated that the majority of WRKY promoters contained multiple cis-acting elements, with MrWRKY54 exhibiting the maximum count of 49 cis-acting elements. The distribution characteristics of these cis- regulatory elements suggested that WRKY genes likely participate in responding to multiple hormone signals and environmental stresses, indicating that their expression undergoes sophisticated multi-tiered regulation during plant growth and development.
3.5 Collinearity analysis of bayberry WRKY genes
To investigate the evolutionary history of bayberry WRKY genes, collinearity analyses were conducted within and across species. As shown in Figure 5, the results revealed six segmental duplication events involving 12 WRKY genes, accounting for 21.81% of the entire WRKY gene family. Notably, five pairs of duplicated genes were distributed across different chromosomes, indicating that the MrWRKY gene family members experienced chromosomal segment duplication during evolution. Furthermore, a comparative collinearity analysis was performed between bayberry and other species, including Arabidopsis, apple, mandarin orange, peach, grape, and kiwifruit. As shown in Figure 6, 30 orthologous gene pairs were identified between bayberry and Arabidopsis, while apple, mandarin orange, peach, grape, and kiwifruit shared 81, 54, 52, 50, and 88 orthologous gene pairs, respectively. These findings suggested a closer evolutionary relationship between the WRKY gene families of bayberry and kiwifruit.

Figure 6. Comparative synteny analysis of WRKY genes among different species. Gray lines represent syntenic blocks between bayberry and other species, while red lines highlighting homologous WRKY gene pairs.
3.6 Expression profiling analysis of bayberry WRKY genes
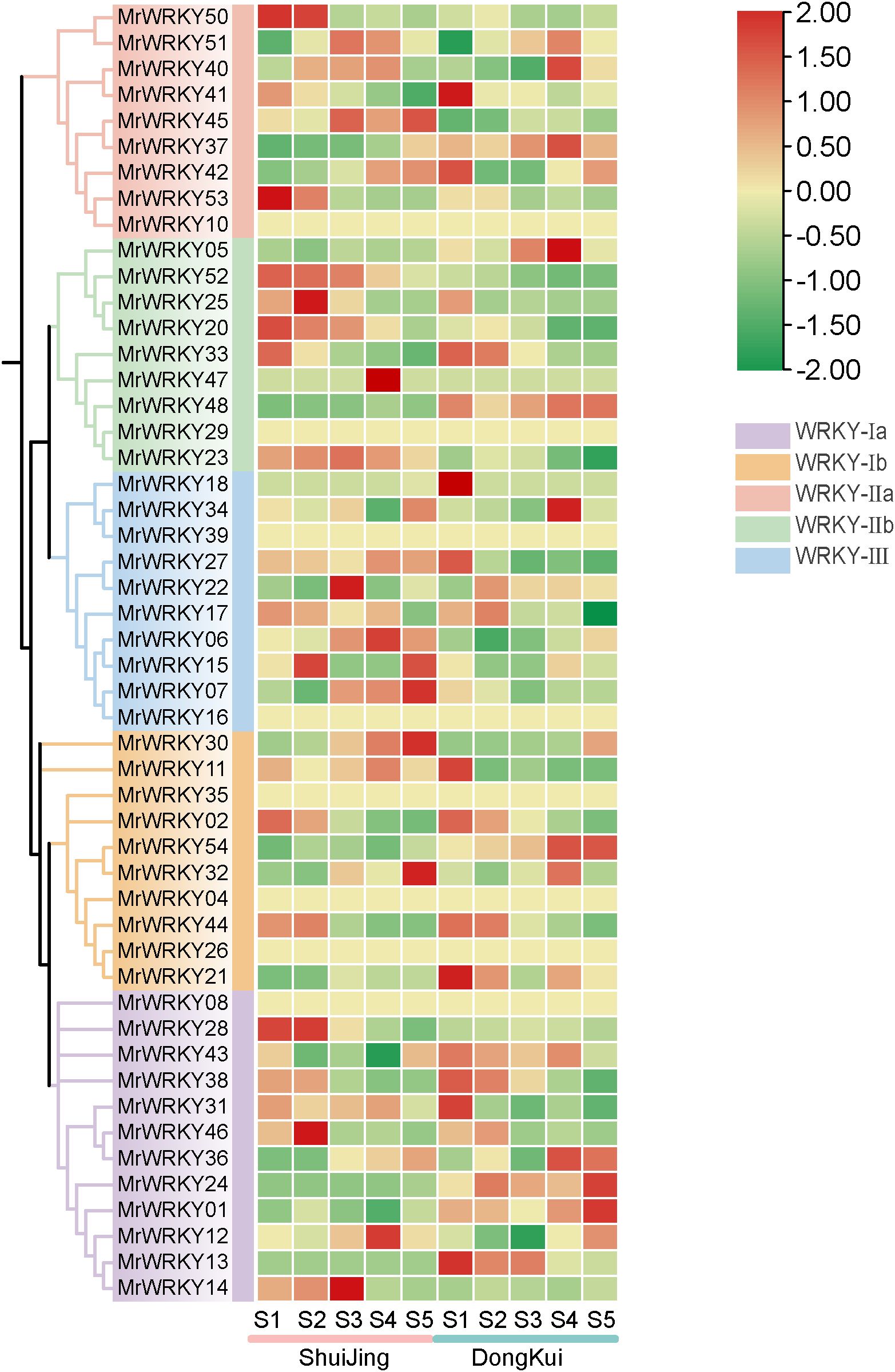
To investigate the expression patterns and biological functions of WRKY genes in bayberry fruits, two cultivars of bayberry fruits at different developmental stages were used as experimental materials (Figure 7). The results revealed diverse expression patterns among the bayberry WRKY subfamilies. In the WRKY-Ia subfamily, MrWRKY28 was specifically highly expressed in ‘Shuijing’ at stages S1 and S2, while MrWRKY13 was predominantly expressed during the early fruit development stage in ‘Dongkui’. MrWRKY01 and MrWRKY24 exhibited peak expression levels in ‘Dongkui’ during the fruit ripening stage. MrWRKY14 exhibited higher expression levels in ‘Shuijing’ fruits compared to ‘Dongkui’. Specifically, it showed markedly elevated expression during early developmental stages (S1-S3) in ‘Shuijing’, with expression abundance progressively increasing, followed by a decline during fruit maturation (S4-S5). In contrast, the expression level in ‘Dongkui’ displayed a rapid decrease throughout the entire fruit development process.
In the WRKY-Ib subfamily, four genes showed high expression during the early fruit development stage. Among these, MrWRKY30 and MrWRKY32 were highly expressed in ‘Shuijing’ during fruit ripening, whereas MrWRKY54 displayed the highest expression levels in ‘Dongkui’ at this stage. For the WRKY-IIa subfamily, MrWRKY50 and MrWRKY53 were both highly expressed in ‘Shuijing’ at stages S1 and S2. In the WRKY-IIb subfamily, four genes exhibited relatively high expression levels in ‘Shuijing’ during the early fruit development stage. Notably, MrWRKY47 reached its highest expression at stage S4 in ‘Shuijing’, while the expression levels of MrWRKY23 gradually decreased as the fruit developed.
Within the WRKY II subfamily, MrWRKY18 and MrWRKY27 showed high expression in ‘Dongkui’ at stage S1. In ‘Shuijing’, MrWRKY06 and MrWRKY07 exhibited increasing expression levels as the fruit developed. These findings demonstrate that the bayberry WRKY gene family exhibits a wide variety of expression patterns during fruit development and ripening.
3.7 Identification and functional analysis of MrWRKY14
To further investigate the biological functions of the WRKY gene family members during bayberry fruit development, molecular regulatory mechanisms were studied. Based on transcriptome sequencing data, a key WRKY transcription factor member, MrWRKY14, which negatively regulated sugar accumulation in the ‘Dongkui’ fruit, was identified. Bioinformatics analysis revealed multiple potential WRKY transcription factor binding sites in the SWEET1 promoter region, suggesting a possible regulatory relationship between them (Figure 8A). Further analysis of the expression patterns of MrWRKY14 and MrSWEET1 during bayberry fruit development showed that both genes exhibited significantly downregulated expression as the fruit matured (Figures 8B, C).
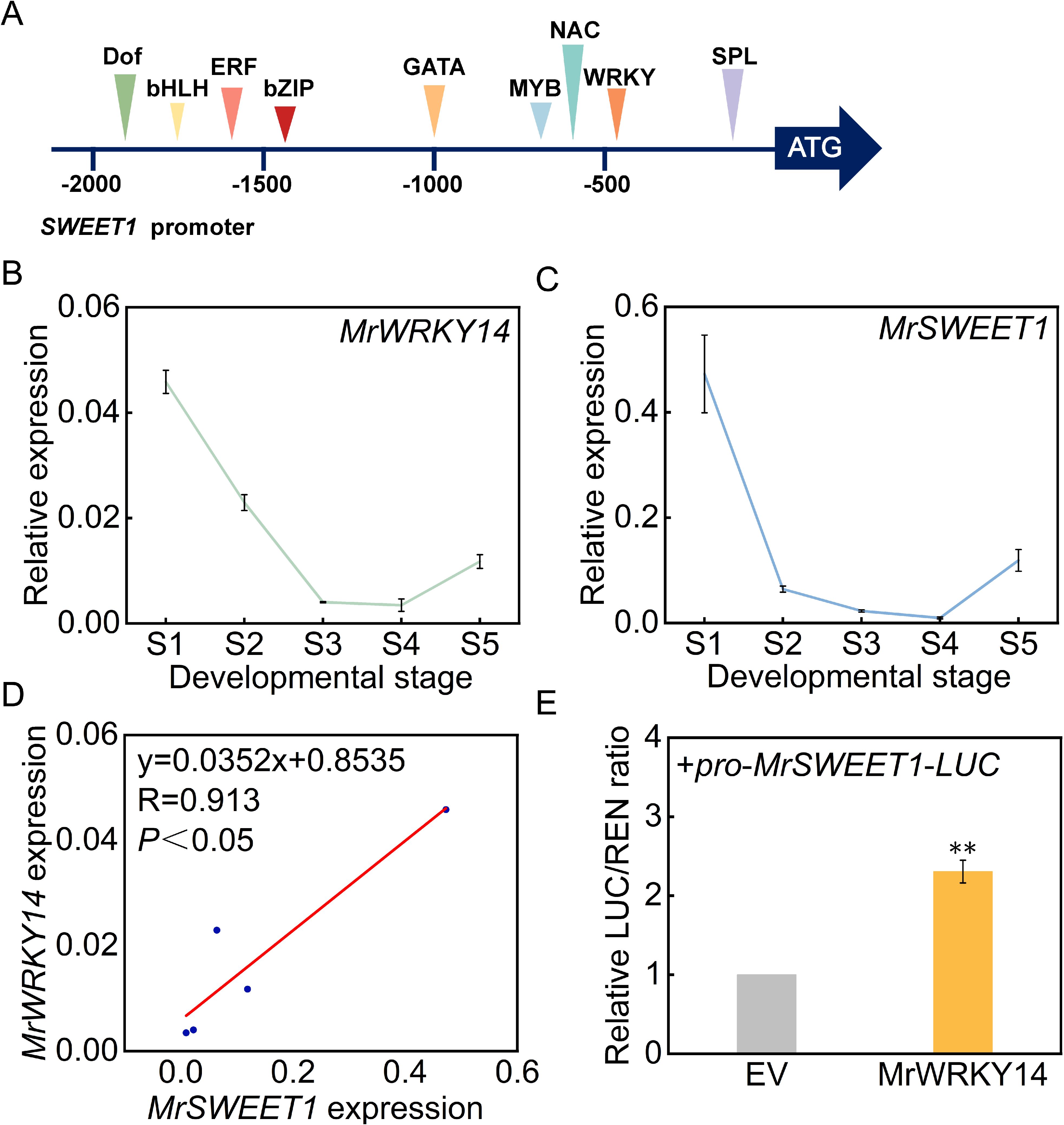
Figure 8. Expression pattern of MrWRKY14 and its transcriptional regulation of MrSWEET1 promoter. (A) Prediction of putative transcription factor binding sites within the MrSWEET1 promoter region; (B) Expression patterns of MrSWEET1 during bayberry fruit development; (C) Expression profiling of MrWRKY14 during bayberry fruit development; (D) Correlation analysis between MrWRKY14 and MrSWEET1 expression; (E) Transcriptional regulation of MrSWEET1 promoter by MrWRKY14 using dual-luciferase reporter system. Error bars represent standard deviations calculated from three biological replicates (n=3). ** indicates significance at p < 0.01.
Pearson correlation analysis demonstrated a highly significant positive correlation between the expression levels of MrSWEET1 and MrWRKY14 (R = 0.913, P < 0.05) (Figure 8D). To validate their regulatory relationship, dual-luciferase reporter assays confirmed that MrWRKY14 significantly enhanced the transcriptional activity of the SWEET1 promoter, with a statistically significant difference compared to the empty vector control (P < 0.05) (Figure 8E). Therefore, this study demonstrated that MrWRKY14 negatively regulated fruit sugar accumulation by activating the transcriptional activity of MrSWEET1.
4 Discussion
Bayberry, as a precious fruit tree resource unique to China, has significant planting potential in the subtropical regions. However, the sustainable development of the bayberry industry faces numerous constraints and urgently requires breakthroughs in breeding and technological innovation (Zhang et al., 2022). WRKY transcription factors, as plant-specific regulatory proteins, play key roles in various aspects such as growth and development, stress response, and secondary metabolism (Cao et al., 2024). Systematically exploring the bayberry WRKY gene family and elucidating its functions not only helps to clarify the molecular regulatory mechanisms of important agronomic traits in bayberry but also accelerates the excavation of excellent genetic resources and the breeding of new varieties, which is of great strategic significance for promoting the innovative development of the bayberry industry.
This study represented the first bioinformatics-based identification of 55 WRKY transcription factor family members from bayberry (Myrica rubra) transcriptome data.
The number of these genes was relatively fewer compared to model plants such as Arabidopsis (72 genes) (Chen et al., 2017) and tomato (88 genes) (Liu et al., 2022), but it was comparable to that of grape (59 genes) (Guo et al., 2014) and peach (58 genes) (Zhong et al., 2021), reflecting the evolutionary conservation of WRKY gene families in woody plants. Phylogenetic analysis revealed that although the WRKY genes of bayberry originated from a common ancestor, they underwent significant differentiation and functional specialization during evolution, resulting in the identification of five major evolutionary clades. Within the WRKY subfamilies of bayberry, the Ia subfamily contained the largest number of members (14 genes), followed by the IIa and IIb subfamilies, each with 11 members. In contrast, the Ib and III subfamilies had the fewest members (10 genes each). Chromosomal distribution analysis of WRKY members in bayberry was uneven across 8 chromosomes, with distinct distribution patterns among different subfamilies. Notably, the highest number of members (14) was found on chromosome 2. Structural characterization demonstrated significant variations in exon-intron organization and conserved motif composition among different subfamilies of bayberry WRKY genes, potentially associated with their functional specificity and diversity.
Previous studies have demonstrated that WRKY genes in plants such as Arabidopsis and tomato play a crucial role in fruit ripening, regulating various fruit traits including size, color, flavor, and nutritional quality. Additionally, these genes have significant impacts on responses to both biotic and abiotic stresses (Liu et al., 2022). As a class of important transcription factors, WRKY genes were widely present in plants. They regulated the expression of downstream genes and participate in multiple processes of plant growth and development. During fruit development, WRKY genes could respond to environmental signals and changes in internal hormones, thereby controlling the expression of genes related to fruit maturation and influencing fruit characteristics (Yang et al., 2025). To further elucidate the functional and regulatory patterns of the WRKY gene family, a cis-element prediction of the bayberry WRKY gene promoters was conducted. The results revealed that bayberry WRKY genes exhibited responsiveness to various environmental stresses, and different subfamilies were subject to distinct regulatory mechanisms during plant growth and development. This discovery provided a new perspective for understanding the molecular mechanisms underlying fruit development and offered new insights for optimizing fruit quality.
Gene expression patterns were crucial for predicting gene functions (Jiang et al., 2025). Through comparative analysis of different developmental stages of bayberry fruit, we found that the genes MrWRKY02, MrWRKY14, MrWRKY33, and MrWRKY41 exhibited high expression levels during the early fruit development stage of both cultivars. These findings suggested that these genes may play essential roles as functional genes in the development of bayberry fruit, exerting significant regulatory effects on fruit development.
Furthermore, it has been observed that MrWRKY14 in bayberry influenced sugar accumulation through the regulation of SWEET. Functionally WRKY transcription factors associated with fruit sugar accumulation have also been identified in other fruits. For instance, in pear (Pyrus spp.), PuWRKY31 has been demonstrated to interact with the PuSWEET15 promoter to activate its transcription, thereby modulating sucrose accumulation (Li et al., 2020). Similarly, RsWRKY40 in radish (Raphanus sativus) regulated sugar accumulation by controlling RsSPS1 expression, thereby enhancing its stress tolerance (Chen et al., 2025).
In this study, a total of 55 WRKY family members were identified in bayberry, which were classified into five subfamilies and randomly distributed across eight chromosomes. Gene structure analysis revealed that each gene contained at least one WRKY domain, with relatively conserved gene structures maintained within each subfamily. The promoter regions of WRKY genes harbored multiple regulatory elements. Collinearity analysis indicated that the bayberry WRKY family underwent six segmental duplication events and exhibited high homology with kiwifruit. Expression pattern analysis demonstrated significant differential expression of bayberry WRKY genes across different cultivars and fruit developmental stages. Further investigation revealed that the WRKY family member MrWRKY14 significantly enhanced the promoter activity of MrSWEET1, thereby influencing sugar accumulation. These findings provided an important theoretical foundation for genome-wide identification of plant WRKY gene families as well as screening and characterization of functional genes.
This study represented the first systematic identification of the bayberry WRKY gene family, unveiling their evolutionary characteristics, structural divergence, and expression patterns. These findings not only lay a solid foundation for deeply understanding the molecular basis of bayberry’s growth and development as well as its adaptation to abiotic stresses but also provide valuable candidate genes for the exploration of excellent genetic resources and molecular design breeding in bayberry. Future research should focus on elucidating the interaction networks and regulatory mechanisms of bayberry WRKY genes to accelerate the advancement of bayberry functional genomics. Furthermore, cross-species comparative genomics and transcriptomic analyses would be instrumental in elucidating the evolutionary history and functional divergence of the WRKY gene family in flowering plants, offering new perspectives for the structural and functional studies of plant WRKY genes. By integrating molecular biology and bioinformatics approaches, our understanding of the functions of WRKY genes in bayberry and other plants can be significantly enhanced in the future. This comprehensive understanding will provide a robust theoretical foundation and technological support for bayberry variety improvement and industrial upgrading.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
XF: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization. MC: Investigation, Methodology, Software, Visualization, Formal analysis, Validation, Writing – review & editing. HZ: Data curation, Formal analysis, Investigation, Software, Validation, Methodology, Visualization, Writing – review & editing. YL: Data curation, Formal analysis, Validation, Visualization, Writing – review & editing. MY: Data curation, Investigation, Methodology, Writing – review & editing. CY: Data curation, Formal analysis, Investigation, Writing – review & editing. HG: Data curation, Investigation, Methodology, Writing – review & editing. KX: Conceptualization, Data curation, Investigation, Project administration, Supervision, Visualization, Writing – review & editing, Formal analysis, Writing – original draft. BW: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the National Natural Science Foundation of China (32402477), the Natural Science Foundation of Zhejiang province (LQ21C150001), and the Scientific Research and Development Foundation of Zhejiang A & F University (2019FR046).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1602750/full#supplementary-material.
Supplementary Table 1 | Physicochemical properties analysis of WRKY proteins in bayberry.
References
Abdullah-Zawawi, M. R., Ahmad-Nizammuddin, N. F., Govender, N., Harun, S., Mohd-Assaad, N., and Mohamed-Hussein, Z. A. (2021). Comparative genome-wide analysis of WRKY, MADS-box and MYB transcription factor families in Arabidopsis and rice. Sci. Rep. 11, 19678. doi: 10.1038/s41598-021-99206-y
Bakshi, M. and Oelmüller, R. (2014). WRKY transcription factors. Plant Signal. Behav. 9, 247–258. doi: 10.4161/psb.27700
Cao, Y. P., Hong, J. Y., Zhao, Y., Li, X. X., Feng, X. F., Wang, H., et al. (2024). De novo gene integration into regulatory networks via interaction with conserved genes in peach. Hortic. Res-England. 11, 2662–6810. doi: 10.1093/hr/uhae252
Chen, F., Hu, Y., Vannozzi, A., Wu, K. C., Cai, H. Y., Qin, Y., et al. (2017). The WRKY transcription factor family in model plants and crops. Crit. Rev. Plant Sci. 36, 311–335. doi: 10.1080/07352689.2018.1441103
Chen, S., Xu, L., Wang, Y., Mao, B., Zhang, X., Song, Q., et al. (2025). RsWRKY40 coordinates the cold stress response by integrating RsSPS1-mediated sucrose accumulation and the CBF-dependent pathway in radish (Raphanus sativus L.). Mol. Hortic. 5, 14. doi: 10.1186/s43897-024-00135-x
Garrido-Gala, J., Higuera, J. J., Rodriguez-Franco, A., Munoz-Blanco, J., Amil-Ruiz, F., and Caballero, J. L. (2022). A comprehensive atudy of the WRKY transcription factor family in strawberry. Plants (Basel) 11, 1585. doi: 10.3390/plants11121585
Guo, C., Guo, R., Xu, X., Gao, M., Li, X., Song, J., et al. (2014). Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J. Exp. Bot. 65, 1513–1528. doi: 10.1093/jxb/eru007
Huang, T., Yu, D., and Wang, X. Q. (2021). VvWRKY22 transcription factor interacts with VvSnRK1.1/VvSnRK1.2 and regulates sugar accumulation in grape. Biochem. Bioph. Res. Co. 554, 193–198. doi: 10.1016/j.bbrc.2021.03.092
Jia, C. H., Wang, Z., Wang, J. Y., Miao, H. X., Zhang, J. B., Xu, B. Y., et al. (2022). Genome-Wide analysis of the banana WRKY transcription factor gene family closely related to fruit ripening and stress. Plants-Basel 11, 2223–7747. doi: 10.3390/plants11050662
Jiang, L., Li, X. X., Lyu, K., Wang, H., Li, Z. Y., Qi, W., et al. (2025). Rosaceae phylogenomic studies provide insights into the evolution of new genes. Hortic. Plant J. 11, 389–405. doi: 10.1016/j.hpj.2024.02.002
Jing, Z. and Liu, Z. (2018). Genome-wide identification of WRKY transcription factors in kiwifruit (Actinidia spp.) and analysis of WRKY expression in responses to biotic and abiotic stresses. Genes Genom. 40, 429–446. doi: 10.1007/s13258-017-0645-1
Li, X., Guo, W., Li, J., Yue, P., Bu, H., Jiang, J., et al. (2020). Histone acetylation at the promoter for the transcription factor PuWRKY31 affects sucrose accumulation in pear fruit. Plant Physiol. 182, 2035–2046. doi: 10.1104/pp.20.00002
Liu, Y. M., Cai, L. Q., Zhu, J. L., Lin, Y., Chen, M. H., Zhang, H. L., et al. (2024). Genome-wide identification, structural characterization and expression profiling of gene family in bayberry (Myrica rubra). BMC Plant Biol. 24, 1139. doi: 10.1186/s12870-024-05945-1
Liu, G., Zhang, D., Zhao, T., Yang, H., Jiang, J., Li, J., et al. (2022). Genome-wide analysis of the WRKY gene family unveil evolutionary history and expression characteristics in tomato and its wild relatives. Front. Genet. 13. doi: 10.3389/fgene.2022.962975
Lv, M., Luo, W., Ge, M., Guan, Y., Tang, Y., Chen, W., et al. (2022). A group I WRKY gene, TaWRKY133, negatively regulates drought resistance in transgenic plants. Int. J. Mol. Sci. 23, 12026. doi: 10.3390/ijms231912026
Maeo, K., Hayashi, S., Kojima-Suzuki, H., Morikami, A., and Nakamura, K. (2001). Role of conserved residues of the WRKY domain in the DNA-binding of tobacco WRKY family proteins. Biosci. Biotechnol. Biochem. 65, 2428–2436. doi: 10.1271/bbb.65.2428
Qin, Y., Yu, H., Cheng, S., Liu, Z., Yu, C., Zhang, X., et al. (2022). Genome-wide analysis of the WRKY gene family in Malus domestica and the role of MdWRKY70L in response to drought and salt stresses. Genes (Basel) 13, 1068. doi: 10.3390/genes13061068
Rushton, P. J., Somssich, I. E., Ringler, P., and Shen, Q. J. (2010). WRKY transcription factors. Trends. Plant Sci. 15, 247–258. doi: 10.1016/j.tplants.2010.02.006
Sun, C., Huang, H., Xu, C., Li, X., and Chen, K. (2013). Biological activities of extracts from chinese bayberry (Myrica rubra Sieb. et Zucc.): a review. Plant Foods Hum. Nutr. 68, 97–106. doi: 10.1007/s11130-013-0349-x
Ulker, B. and Somssich, I. E. (2004). WRKY transcription factors: from DNA binding towards biological function. Curr. Opin. Plant Biol. 7, 491–498. doi: 10.1016/j.pbi.2004.07.012
Wang, M., Vannozzi, A., Wang, G., Liang, Y. H., Tornielli, G. B., Zenoni, S., et al. (2014). Genome and transcriptome analysis of the grapevine (Vitis vinifera L.) WRKY gene family. Hortic. Res. 1, 14016. doi: 10.1038/hortres.2014.16
Wang, Q., Wang, M., Zhang, X., Hao, B., Kaushik, S. K., and Pan, Y. (2011). WRKY gene family evolution in Arabidopsis thaliana. Genetica 139, 973–983. doi: 10.1007/s10709-011-9599-4
Wei, W., Cheng, M. N., Ba, L. J., Zeng, R. X., Luo, D. L., Qin, Y. H., et al. (2019). Pitaya HpWRKY3 is associated with fruit sugar accumulation by transcriptionally modulating sucrose metabolic genes HpINV2 and HpSuSy1. Int. J. Mol. Sci. 20, 1422–0067. doi: 10.3390/ijms20081890
Xi, D., Yin, T., Han, P., Yang, X., Zhang, M., Du, C., et al. (2023). Genome-wide identification of sweet orange WRKY transcription factors and analysis of their expression in response to infection by Penicillium digitatum. Curr. Issues Mol. Biol. 45, 1250–1271. doi: 10.3390/cimb45020082
Yang, L., Fang, S., Liu, L., Zhao, L., Chen, W., Li, X., et al. (2025). WRKY transcription factors: hubs for regulating plant growth and stress responses. J. Integr. Plant Biol. 67, 488–509. doi: 10.1111/jipb.13828
Yang, B., Jiang, Y., Rahman, M. H., Deyholos, M. K., and Kav, N. N. (2009). Identification and expression analysis of WRKY transcription factor genes in canola (Brassica napus L.) in response to fungal pathogens and hormone treatments. BMC Plant Biol. 9, 68. doi: 10.1186/1471-2229-9-68
Yue, M. L., Jiang, L. Y., Zhang, N. T., Zhang, L. X., Liu, Y. Q., Wang, Y., et al. (2022). Importance of FaWRKY71 in strawberry (Fragaria × ananassa) fruit ripening. Int. J. Mol. Sci. 23, 1422–0067. doi: 10.3390/ijms232012483
Zhang, Y. and Wang, L. (2005). The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 5, 1. doi: 10.1186/1471-2148-5-1
Zhang, S. W., Yu, Z. P., Sun, L., Ren, H. Y., Zheng, X. L., Liang, S. M., et al. (2022). An overview of the nutritional value, health properties, and future challenges of Chinese bayberry. Peerj 10, 13070. doi: 10.7717/peerj.13070
Zheng, X., Zhao, Y., Shan, D., Shi, K., Wang, L., Li, Q., et al. (2018). MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. New Phytol. 217, 1086–1098. doi: 10.1111/nph.14891
Keywords: bayberry, WRKY gene family, phylogenetic tree, collinearity, expression pattern analysis
Citation: Fan X, Chen M, Zhang H, Liu Y, Yang M, Ye C, Gu H, Xu K and Wu B (2025) Systematic identification and analysis of WRKY transcription factors reveals the role of MrWRKY14 in Myrica rubra. Front. Plant Sci. 16:1602750. doi: 10.3389/fpls.2025.1602750
Received: 30 March 2025; Accepted: 07 May 2025;
Published: 30 May 2025.
Edited by:
Cheng Song, West Anhui University, ChinaReviewed by:
Yang Yanqing, Ludong University, ChinaLifang Sun, Zhejiang Academy of Agricultural Sciences, China
Copyright © 2025 Fan, Chen, Zhang, Liu, Yang, Ye, Gu, Xu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Xu, eHVrYWlAemFmdS5lZHUuY24=; Boping Wu, Ym9waW5nd3VAemFmdS5lZHUuY24=
†These authors have contributed equally to this work
 Xiurun Fan†
Xiurun Fan† Boping Wu
Boping Wu