- 1College of Life Science, China West Normal University, Nanchong, China
- 2Engineering Research Center of Chuanxibei Rural Human Settlement Construction, Mianyang Teachers’ College, Mianyang, China
Introduction: Essential oils from plants contain various volatile compounds with antifungal and antioxidant properties. The synthesis and accumulation of these volatile compounds are closely related to factors such as the plant's geographical origin and harvest period. Investigating the insect-repellent and antimicrobial effects of Zanthoxylum armatum DC. essential oils (EOs) at different harvest stages can optimize its harvest and utilization while also promoting the development of eco-friendly agents.
Methods: This study analyzed the changes in the composition and content of volatile compounds in Z. armatum EOs at different growth stages in Nanchong City using GC-MS.
Results: The results indicate that the accumulation period of volatile compounds occurs before the t5 stage (August 4). Linalool, D-Limonene, and Sabinene were the three most abundant volatile components in the essential oil of Z. armatum pericarp. Many monoterpenes, such as α-Pinene, Sabinene, and β-Myrcene, were found in higher concentrations during the early stages of fruit maturation. Principal component analysis (PCA) revealed a significant difference in volatile composition between the t3, t4, and t5 (t3: July 3, t4: July 18, t5: August 4) stages and the t1, t2, t6, (t1: May 26, t2: June 16, t6: September 9) and t7 (September 28) stages of Z. armatum. Volatile compounds were relatively higher in samples collected in July and August, making these months the optimal harvest period for processing and manufacturing related products. As the fruit of Z. armatum matures, the content of structurally more complex compounds, such as alcohols and esters, increases. The insect-repellent and antifungal experiments demonstrated that Z. armatum EOs exhibited a strong repellent effect against T. castaneum, although the EO’s toxicity was not lethal. The antifungal effect was most pronounced in the EO collected during the t4 stage, where the relative content of various antifungal compounds was higher.
Discussion: This suggests that the antifungal activity of the EOs may result from synergistic or antagonistic interactions among its compounds. By exploring the composition, content, and bioactivities (insect-repellent and antifungal) of Z. armatum EOs at different harvest periods, this study provides theoretical support for developing market-oriented commercial products.
1 Introduction
Essential oils (EOs) are typically extracted from various parts of plant materials, such as flowers, buds, seeds, leaves, branches, bark, herbs, wood, fruits, and roots. They are aromatic, oil-like liquids that are volatile in nature (Burt, 2004). EOs contain a variety of low molecular weight volatile components, such as terpenes and terpenoid compounds, phenolic-derived aromatic components, and fatty components (Bakkali et al., 2008). At different maturity stages, the components of EOs change with the plant’s physiological metabolism. Research by Wu et al. demonstrated that the contents of 3-carene, α-pinene, and β-pinene in Citrus medica EO vary significantly across different stages (Zhen et al., 2013). EOs contain a variety of bioactive plant secondary metabolites that can inhibit or slow the growth of harmful microorganisms (Shenglan et al., 2022). They play a crucial role in plant defense against pathogens and insects. Plant essential oils, as a natural product, are commonly used as natural antimicrobial agents. The essential oils of certain aromatic plants are widely used as food preservatives, helping to extend shelf life and improve the quality of stored foods (Gutierrez et al., 2009). Bhanu et al (Prakash et al., 2012). investigated the antifungal, anti-aflatoxin, and antioxidant properties of Zanthoxylum alatum Roxb. essential oil. They found that the main component of the essential oil, methyl cinnamate, exhibited antifungal and anti-aflatoxin effects at low concentrations (0.6 μl/ml), with fungicidal toxicity. Additionally, the essential oil showed strong antioxidant activity.
Zanthoxylum armatum DC. is a common shrub or small tree in the Rutaceae family, with approximately 250 species of Zanthoxylum plants found worldwide. These aromatic plants are native to subtropical and temperate regions globally and are particularly cultivated extensively in China, Korea, and Japan (Di et al., 2021). Its fruit is green in color and features slightly raised oil gland dots. Due to its enticing aromatic properties, the fruits and extracts of Z. armatum are among the key flavoring agents in the food industry (Hong et al., 2017; Shun et al., 2019). Additionally, as a traditional medicinal plant, Z. armatum is commonly used to treat wounds, toothaches, stomachaches, nausea, diarrhea, and ascariasis. Studies have indicated that Zanthoxylum plants contain various bioactive compounds, such as essential oils, alkaloids, lignans, coumarins, flavonoids, amides, polyphenols, and sterols. In the pericarp of Z. armatum, EOs are abundant and exhibit antioxidant, antibacterial, and anti-inflammatory effects (Gao and Hanbin, 2014). Currently, there is extensive research on the antimicrobial and insecticidal properties of Z. armatum EO, but studies focusing on its specific application in agricultural production are relatively scarce. This may be partly due to the inherent lag in practical agricultural activities and partly due to cost-related challenges associated with the research and application of such green agents.
Tribolium castaneum (Coleoptera: Tenebrionidae) (Campbell et al., 2022) is a significant cosmopolitan storage pest with a wide range of hosts. Due to its high reproductive rate and long reproductive lifespan, it exhibits one of the highest population growth rates among all stored-product beetles. Consequently, it is distributed in nearly 156 countries worldwide (Campbell et al., 2022). The eggs of this pest are scattered on the surface of grain kernels, within grain crevices, or among debris and fine particles. The eggs are often coated with a sticky substance, causing powder and debris to adhere to them, making them difficult to detect. This insect secretes odorous fluids from its scent glands, imparting a musty odor to its host. Furthermore, its secretions contain the carcinogenic compound benzoquinone (J. D. and Roth, 1953). In the search for pest control agents with limited adverse effects on the environment and non-target organisms, researchers have continually explored plant resources in the hope of discovering new plant-based insecticides with high insecticidal activity to address pesticide challenges. The EOs of Cymbopogon spp., Ocimum spp., and Eucalyptus spp. show great promise as repellents against insects and arthropods (Ravi Kant and Gayatri, 2007). Corynespora cassiicola is one of the pathogens causing target spot disease in mulberry trees. This fungal disease significantly impacts the yield and quality of mulberry trees, posing serious threats to the economy and ecological environment (Xinqi, 2023). While chemical control is relatively effective against this pathogen, it still presents certain challenges. Consequently, the development of environmentally friendly agents is expected to become a key focus of future research.
The composition and content of essential oils are influenced by various factors, including the variety, origin, harvest time, and storage year (Qianqian et al., 2024). Therefore, the composition and content of EOs extracted from Z. armatum in different regions may vary, and their inhibitory effects on different pests and fungi may also differ. In this study, samples collected from Nanchong City, Sichuan Province, were analyzed using GC-MS to determine the composition and content of volatile oils, and the insect repellent and antimicrobial activities of the essential oil were subsequently validated. The insecticidal and antimicrobial activities of the EOs were then investigated. This study provides the first systematic time-course analysis of volatile compound profiles in Z. armatum EOs across seven distinct growth stages (from May to September), revealing previously unreported temporal accumulation patterns. By integrating GC-MS metabolomics, PCA-based chemometrics, and bioactivity assays, we developed an innovative framework that correlates harvest timing with optimal functional properties, thereby overcoming the limitations of traditional compositional studies. This research will provide data support for the development of natural plant-based insecticidal/antimicrobial agents.
2 Materials and methods
2.1 Plant materials
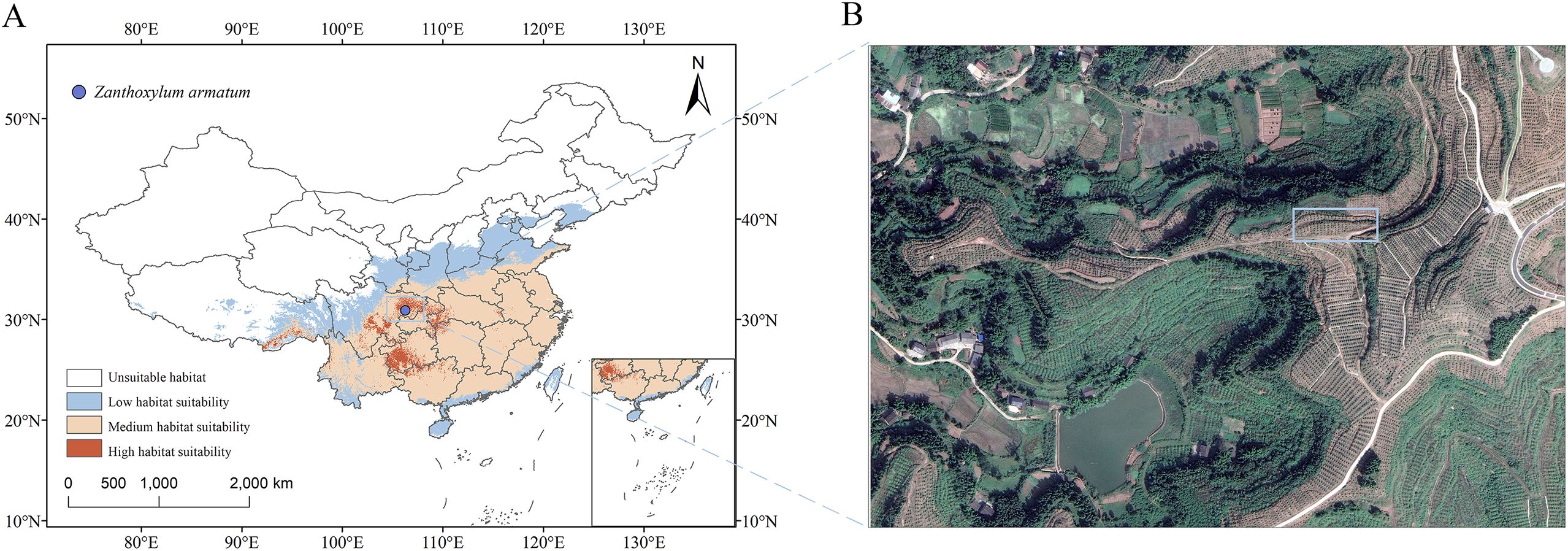
The sample collection site is located in the Z. armatum plantation in Ca’er Town, Gaoping District, Nanchong City, Sichuan Province (N 30.91438, E 106.28455, Altitude 426 m) (Figure 1). Z. armatum samples were collected through local forestry bureaus or zanthoxylum planting companies. In the plantation, 20 healthy Z. armatum trees aged 2–3 years were randomly selected, and fruit was collected from each tree seven times between May and September. The collection dates were as follows: (t1: May 26, t2: June 16, t3: July 3, t4: July 18, t5: August 4, t6: September 9, t7: September 28). The fresh Z. armatum fruit collected during each sampling was dehydrated in an oven at 60 °C for 48 hours. After dehydration, the seeds and pericarps of the Z. armatum were separated, placed into sealed bags, and stored at -20 °C for later use. All the specimens were authenticated by Professor Danping Xu of China West Normal University and stored in the School of Life Sciences (NC 20230526), China West Normal University.
2.2 Biological materials
T. castaneum samples were sourced from the Forest Protection Research Group at the School of Life Sciences, China West Normal University. Healthy adult beetles, 4–7 days post-emergence, were selected for the experiment. The C. cassiicola strain, which is responsible for the target spot disease, was obtained from the Sichuan Academy of Agricultural Sciences. The mycelium was inoculated onto PDA agar medium with an inoculation needle and incubated at a constant temperature in an incubator for 3 days before use. All the specimens were authenticated by Professor Zhihang Zhuo of China West Normal University.
2.3 Chemical reagents and equipment
Reagents: Purified water, anhydrous ethanol (purity >99.8%, Sigma-Aldrich, Germany); sodium carbonate, aluminum nitrate, sodium nitrite, sodium hydroxide, Folin-Ciocalteu reagent (analytical grade, Shanghai Yuanye Biotechnology Co., Ltd.), n-hexane, n-tridecane (HPLC grade, Sigma-Aldrich, Germany), C7-C40 n-alkane series (HJ894-2017, Anpel-Trace, China), anhydrous sodium sulfate (purity >98%, Shanghai Yisheng Biotechnology Co., Ltd.), dimethyl sulfoxide (analytical grade, Arabidopsis Biotechnology Co., Ltd., Chongqing), agar (Xiqiong Biotechnology, Beijing), glucose (purity >98%, Sichuan Baiochurui Biotechnology Co., Ltd., Chengdu), potatoes.
Instruments and Equipment: Balance (Suzhou Science Instrument Co., Ltd.); essential oil extractor, moisture analyzer (Sichuan Shubo Group Co., Ltd.), stereomicroscope (Leica Microsystems), grinder (XICHU Equipment Co., Ltd.), C18 preparative chromatography column [Agilent HC-C18 (4.6×250 mm, 10 μm)], gas chromatography-mass spectrometry (GC-MS) system (Agilent GC-MS 7890A-5975C), HP-5MS chromatography column (30 m×0.25 mm, 0.25 μm), laminar flow cabinet, constant temperature incubator, autoclave, induction cooker, microwave.
2.4 Extraction of essential oil
The oil content of fresh Z. armatum was determined according to the national standard GB/T 17527-2009, with the experiment repeated three times (ACFSMC, 2009). The oil content of dried Z. armatum: A certain amount of dried Z. armatum was ground using a grinder (XICHU Equipment Co., Ltd.) and passed through a 40-mesh sieve. Subsequently, 40 g of the resulting powder and 400 mL of pure water (1:10, v/v) were placed into a round-bottom flask of a volatile oil determination apparatus. The sample was heated with an electric heating mantle for distillation, which lasted for 3 hours at a rate of approximately 5 drops per minute. The distillate was collected in a graduated receiver tube, and anhydrous Na2SO4 was added to achieve water-oil separation. The volume of the oil was recorded, and the oil yield was calculated. The experiment was repeated three times (Qianqian et al., 2024). The extracted volatile oil was stored in amber glass bottles at -20°C, protected from light. The formula for calculating the essential oil extraction yield is as follows:
V (mL) represents the volume of essential oil measured through hydrodistillation during the experiment, and m (g) represents the dry or wet weight of the sample used for extraction in the experiment.
2.5 Analysis of volatile components
GC-MS Analysis: Gas chromatography-mass spectrometry (GC-MS, Agilent GC-MS 7890A-5975C) was used to identify the components of the essential oil. The obtained Z. armatum essential oil was first dried with anhydrous sodium sulfate, then filtered through a 0.22 µm filter membrane and diluted 40 times with n-hexane before being injected. The internal standard used was n-tridecane (HPLC grade, Sigma-Aldrich, Germany). The GC-MS conditions were as follows: 1 mL of the sample solution was placed in an automatic injection vial, with an injection volume set to 10 µL. The chromatography column used was an HP-5MS (30 m×0.25 mm, 0.25 μm) elastic quartz capillary column (30 m×0.25 mm, 0.25 µm). The temperature program was as follows: column temperature set to 50°C (hold for 1 min), then ramped at 1°C/min to 75°C, held for 1 min, ramped at 6°C/min to 120°C, hold for 1 min, ramped at 1°C/min to 135°C, held for 1 min, then ramped at 15°C/min to 200°C and held for 5 min. Helium was used as the carrier gas, with a flow rate of 1.0 mL/min, and a split flow rate of 3 mL/min. The pressure was set to 7.6522 psi, and the injection port temperature was 250°C. The ion source used was electron impact (EI), with an ion source temperature of 230°C and a quadrupole temperature of 150°C (maximum 200°C), electron energy set at 70 eV, interface temperature at 280°C, and the mass scan range was 50–550 amu (Jingxuan et al., 2018). In this study, three methods were employed for the qualitative analysis of the components, including the calculation of retention index (RI) values, comparison with RI values reported in the literature, and the retrieval and matching of collected mass spectra against the NIST library to identify volatile components in the samples. Additionally, the content of each component was analyzed using the internal standard method.
tR(x) represents the retention time of the analyte, tR(n) is the retention time of the n-alkane with n carbon atoms, and tR(n+m) is the retention time of the n-alkane with (n+1) carbon atoms.
2.6 Establishment of fingerprint map
Fingerprint map technology refers to the chromatographic or spectroscopic maps obtained through appropriate processing of complex substances, followed by analysis using certain techniques (chromatography, spectroscopy). These maps reflect the chemical characteristics of the substances and are primarily used to evaluate the authenticity, quality, and stability of samples. Establishing a fingerprint map for the volatile components of Z. armatum is of significant importance for quality control, authenticity identification, and other aspects. The GC-MS mass spectra were analyzed using Agilent OpenLab CDS software. A fingerprint map was established for seven batches of Z. armatum samples using the “Traditional Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System (2012 edition).” A reference map was generated using the median method, with a time window width of 0.1. After multi-point calibration and automatic matching, the volatile component fingerprint map of Z. armatum was obtained.
2.7 The attraction-avoidance experiment of Z. armatum essential oil on T. castaneum
The attraction-avoidance experiment on T. castaneum (male and female) was conducted using the Z. armatum essential oil obtained from previous experiments. The experiment followed the method described by Jilani et al (Jilani and Saxena, 1990), with some modifications. The specific procedure is as follows: First, preliminary experiments were conducted using four solvents: DMSO, acetone, methanol, and ethanol, to determine ethanol as the solvent for the essential oil. Then, several 4–7 day-old adult beetles were selected and kept in a wheat flour incubator for 24 hours. Filter paper (9 cm in diameter) was placed at the bottom of a 9 cm diameter petri dish, and the paper was divided into two halves, completely covering the bottom of the dish with a clear cutting line in the middle.
The Z. armatum essential oil was diluted with 75% ethanol to prepare solutions with volume fractions of 1%, 3%, and 5%. Using a pipette, 300 μL of the Z. armatum essential oil solution was evenly spread on the filter paper on the right side of the petri dish, and left to stand for thirty minutes to allow the solvent to evaporate. Ten healthy male and ten healthy female T. castaneum adults (7 days post-eclosion) were selected and placed in a petri dish. The chosen beetles displayed robust body condition, high mobility, and quick responses to stimuli (e.g., light and vibration). The petri dish was then placed in an incubator at room temperature and away from light. Every 12 hours, the number of T. castaneum beetles on the left and right sides of the petri dish was recorded, and the avoidance rate was calculated. The negative control was an ethanol solution with the same volume. All experiments were repeated three times. The avoidance rate was calculated using the following formula:
Where n1 is the number of insects placed in the petri dish, and n2 is the number of insects observed on the filter paper coated with the essential oil solution.
2.8 The antifungal experiment of Z. armatum essential oil against C. cassiicola
The growth inhibition experiment on C. cassiicola was conducted using the Z. armatum essential oil obtained in previous experiments. The Z. armatum essential oil was diluted with a 2.5% dimethyl sulfoxide (DMSO, analytical grade, Arabidopsis Biotechnology Co., Ltd., Chongqing) solution to concentrations of 0.5%, 0.1%, and 0.05%. Using a pipette, 5 mL of the solution was added to 25 mL of PDA medium, thoroughly mixed to ensure uniformity. The 30 mL mixture was evenly poured into three Petri dishes. After the medium solidified, the C. cassiicola strain, obtained through isolation and purification, was inoculated. The colony diameter was observed and recorded every 24 hours (Na et al., 2017). The negative control was a 2.5% DMSO solution, and the blank control was distilled water.
2.9 Data processing
The data were analyzed using one-way ANOVA in SPSS 22 software. The volatile component fingerprint of Z. armatum was established using the “Traditional Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System (2012 version)”. Principal component analysis (PCA) was performed based on the content of each component in the essential oil. Correlation heatmaps were generated using the OmicShare tool (https://www.omicshare.com/), and all other figures were created with Origin Pro 2022.
3 Results and analysis
3.1 Morphological changes in the fruits of Z. armatum at different harvesting periods
Photographs of Z. armatum fruits at different harvesting periods were taken under a stereomicroscope, clearly revealing the morphological changes in the fruits (as shown in Figure 2). In the early stages, the fruits exhibit a bright green exterior with slightly raised oil glands. As the growth and development process progresses, the color gradually deepens, and the peel turns yellowish-green with more prominent oil glands. By the t7 period, the fruits have transitioned from yellowish-green to red, displaying a more oily sheen on the surface, indicating that the fruits are fully mature at this stage.

Figure 2. Morphology of Z. armatum fruits at different harvest periods [(A-G), t1-t7 periods respectively].
3.2 Analysis of volatile components
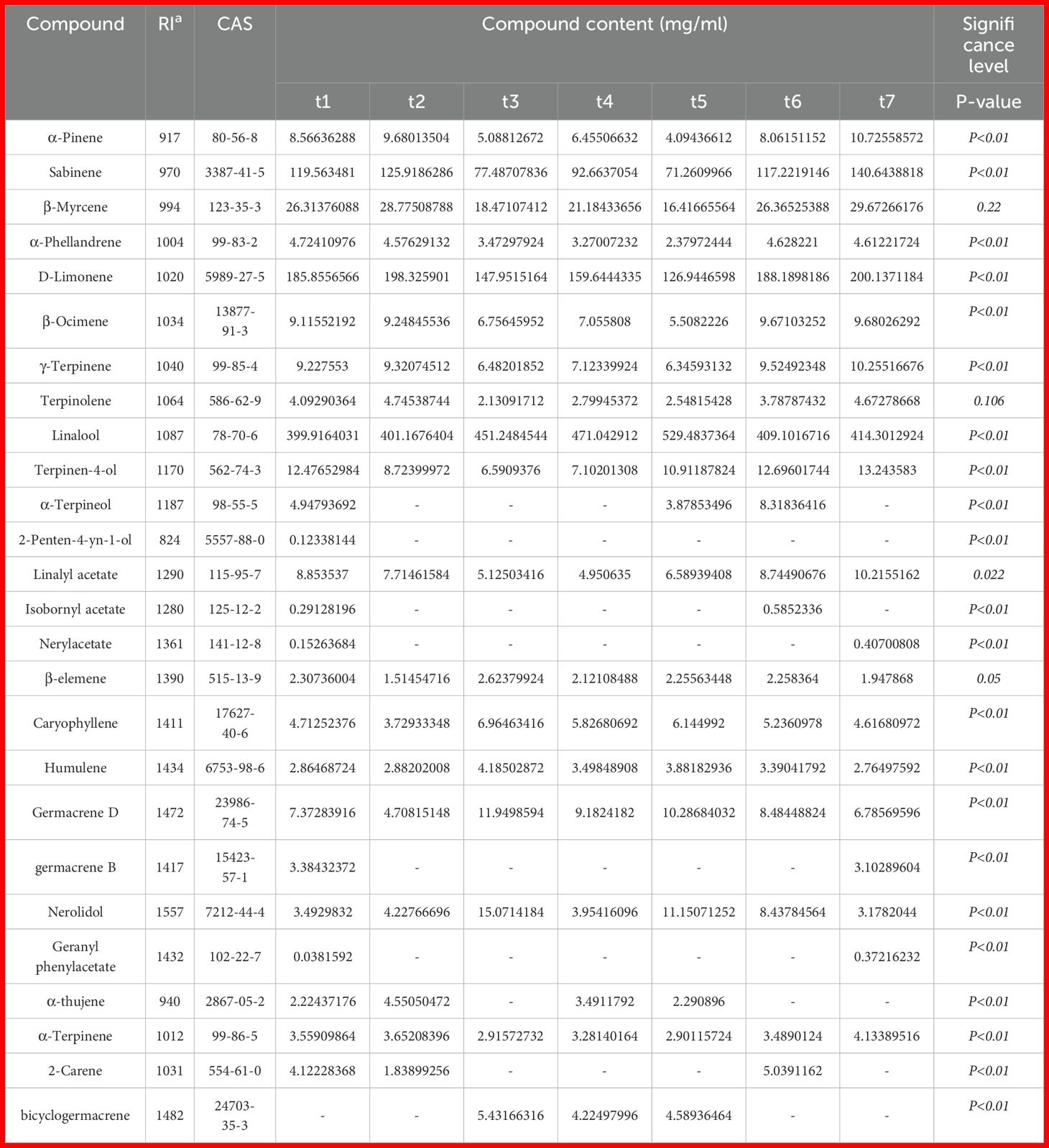
The volatile components in Z. armatum essential oil were analyzed by GC-MS. For statistical analysis, we selected volatile compounds meeting the following criteria: (1) concentration ≥2 mg/mL and (2) match confidence >95% against the NIST database. Through this approach, we initially identified 26 volatile constituents. The compounds are arranged in order of their elution sequence. As shown in Table 1, the relative content of various components varied across different growth stages. Linalool (399.92–529.48 mg/mL), D-Limonene (126.94–200.14 mg/mL), and Sabinene (71.26–140.64 mg/mL) were the three most abundant volatile components in the essential oil of Z. armatum pericarp, followed by β-Myrcene (16.42–29.67 mg/mL), Nerolidol (3.18–15.07 mg/mL), and Terpinen-4-ol (6.59–13.24 mg/mL). These volatile compounds are the primary components of Z. armatum essential oil and contribute to its rich herbal, piney, and fresh aroma (Xiya et al., 2022). Among the three main volatile components, the relative content variations of Sabinene and D-Limonene closely mirrored the overall changes in the essential oil yield. Sabinene reached its minimum relative content of 71.26 mg/mL at the t5 stage and its maximum of 140.64 mg/mL at the t7 (September 28) stage. Similarly, D-Limonene showed a minimum relative content of 126.94 mg/mL at the t5 stage and a maximum of 200.14 mg/mL at the t7 stage, though there was no significant difference compared to the t2 stage. In contrast, Linalool had the lowest relative content of 399.92 mg/mL at the t1 stage and the highest of 529.48 mg/mL at the t5 stage.
Other volatile components with lower concentrations generally show a pattern similar to D-Limonene and Sabinene, decreasing and then increasing as the developmental stages progress, exhibiting a “concave” shape. These include α-Pinene, β-Myrcene, Terpinen-4-ol, Linalyl acetate, etc. Some volatile components, such as α-Phellandrene, β-Ocimene, and γ-Terpinene, show little variation throughout the entire growth period. Overall, the content of volatile oil and the main aromatic compounds in the fruit peel of Z. armatum accumulate most abundantly in August, making it the optimal time for harvesting for further processing and utilization.
3.3 Establishment of the volatile component fingerprint
The “Chinese Medicine Chromatographic Fingerprint Characteristic Map Similarity Evaluation System (2012 version)” was used to establish fingerprint maps for Z. armatum at seven different maturity stages. The shared fingerprint map for the seven stages was matched, and the relative peak areas of the shared fingerprint map were analyzed.
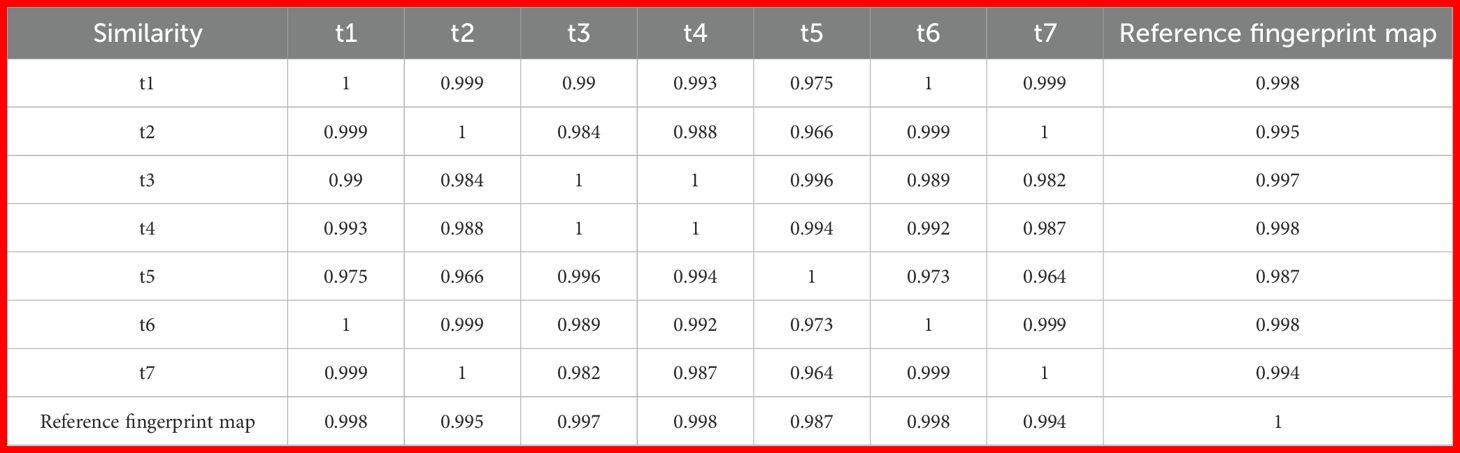
The similarity between the samples from different periods is relatively low between t5 and t2, and between t5 and t7, with similarities of 0.966 and 0.964, respectively (sample similarity comparisons are shown in Table 2). This indicates that the volatile components of t5 differ slightly from those of the other two periods. Overall, the similarity between the seven samples is above 0.96, suggesting that the volatile components of Z. armatum from different periods exhibit good consistency.
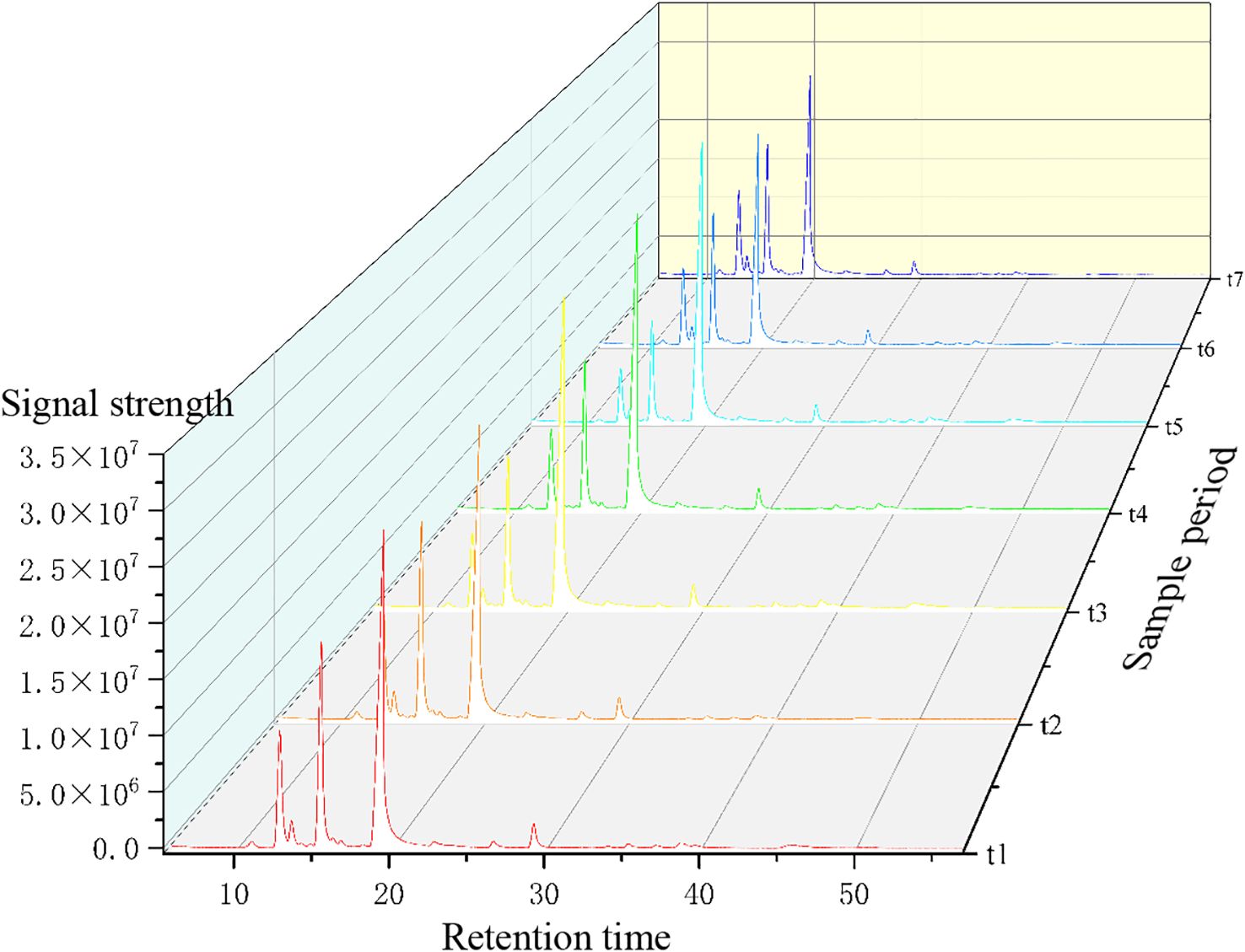
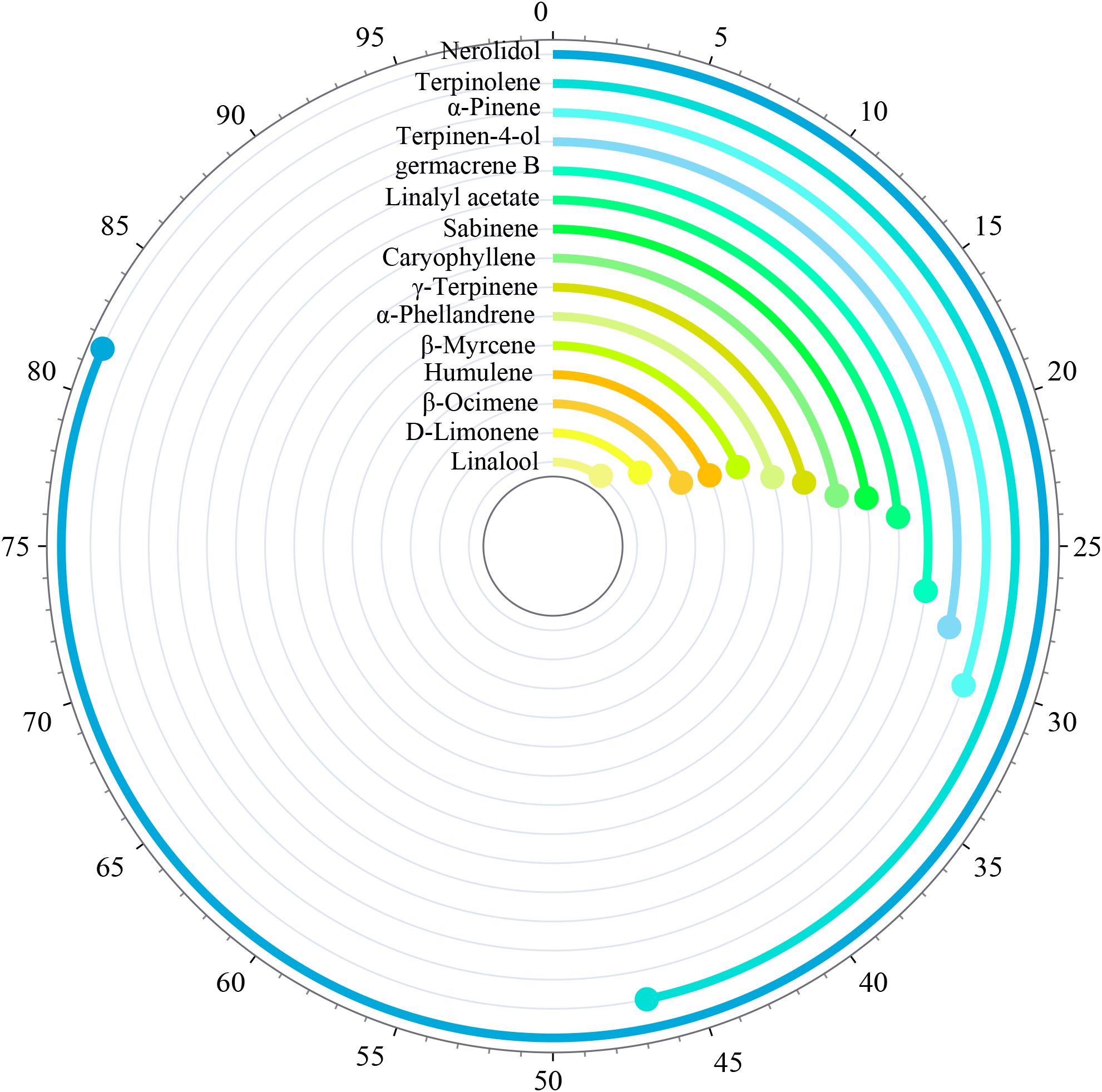
The fingerprint spectrum is shown in Figure 3. The relative peak area of the fingerprint spectrum represents the variation of target substances from t1 to t7. By comparing with the mass spectrum, 15 common mass spectrum peaks with relative peak areas were identified. As shown in Figure 4, the relative peak area of Linalool is 11.79%, indicating that its variation is the smallest during the fruit’s growth and development process. D-Limonene and β-Ocimene also show relatively small changes. In contrast, the relative peak area of Nerolidol is 81.97%, showing the largest variation during the fruit’s growth and development. Following Nerolidol is Terpinolene. The significant changes in these two components, which have strong citrus aromas, may be one of the reasons for the large flavor differences observed in Z. armatum at different maturity stages.
3.4 Principal component analysis
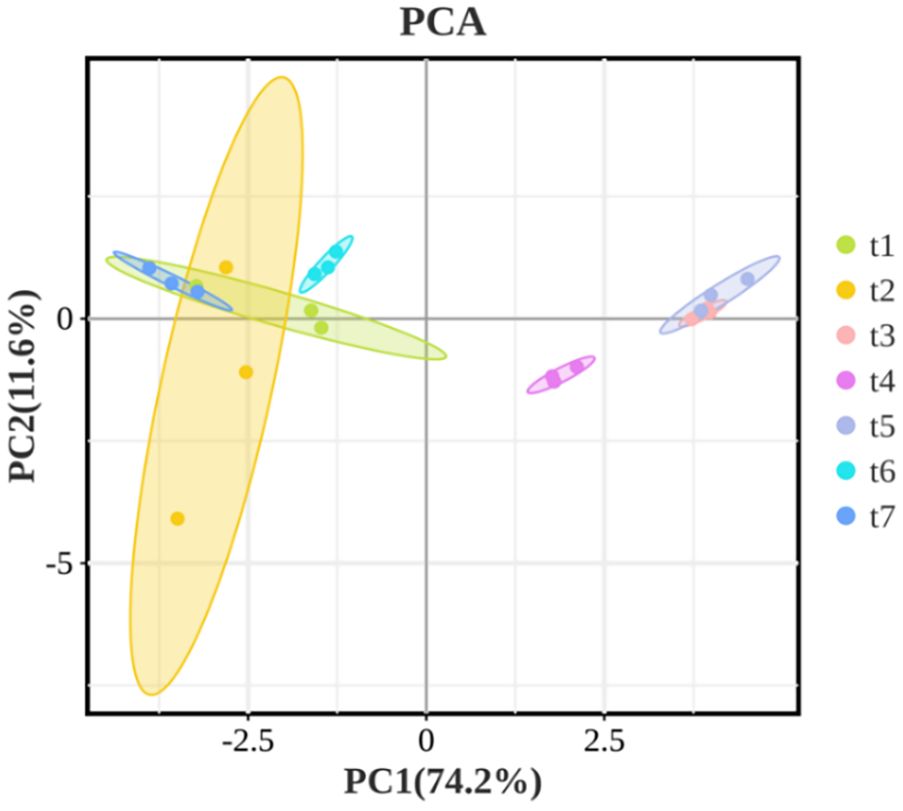
To preliminarily understand the differences and similarities of volatile compounds in Z. armatum at different harvest stages, the PCA analysis of the volatile components is shown in Figure 5. The first principal component (PC1) explains 74.2% of the variance, while the second principal component (PC2) explains 11.6%. In the Figures 5, samples t1, t2, t6, and t7 cluster together and are distributed on the negative half of PC1, while samples t3, t4, and t5 cluster on the positive half of PC1. This suggests that the volatile oil components of Z. armatum during t1 (May 26), t2 (June 16), t6 (September 9), and t7 (September 28) are similar, with significant differences compared to those at t3 (July 3), t4 (July 18), and t5 (August 4). This indicates that the volatile components of the pepper peel continuously change in the early stages of maturity and significantly differ from other growth stages. As the fruit matures, the accumulation of volatile components gradually slows down and tends to stabilize.
3.5 Analysis of insect repellent experiment results
The results of the repellent experiment with Z. armatum EO against T. castaneum are presented in Figure 6 and Table 3. As the treatment time increased, the repellent rate of the essential oil at different concentrations significantly decreased. The highest repellent rate of 100% at 12 hours post-treatment dropped to a maximum of 88% at 36 hours post-treatment, with a significant difference compared to the control group. At 36 hours post-treatment, the 5% concentration of Z. armatum EO still maintained a repellent rate of nearly 80% against T. castaneum. The 3% concentration of Z. armatum EO showed relatively high repellent activity (65%-100%) at 12 hours and 24 hours post-treatment, but the activity was lost after 36 hours. The 1% concentration of Z. armatum EO showed significant repellent effects against T. castaneum only within the first 12 hours post-treatment.
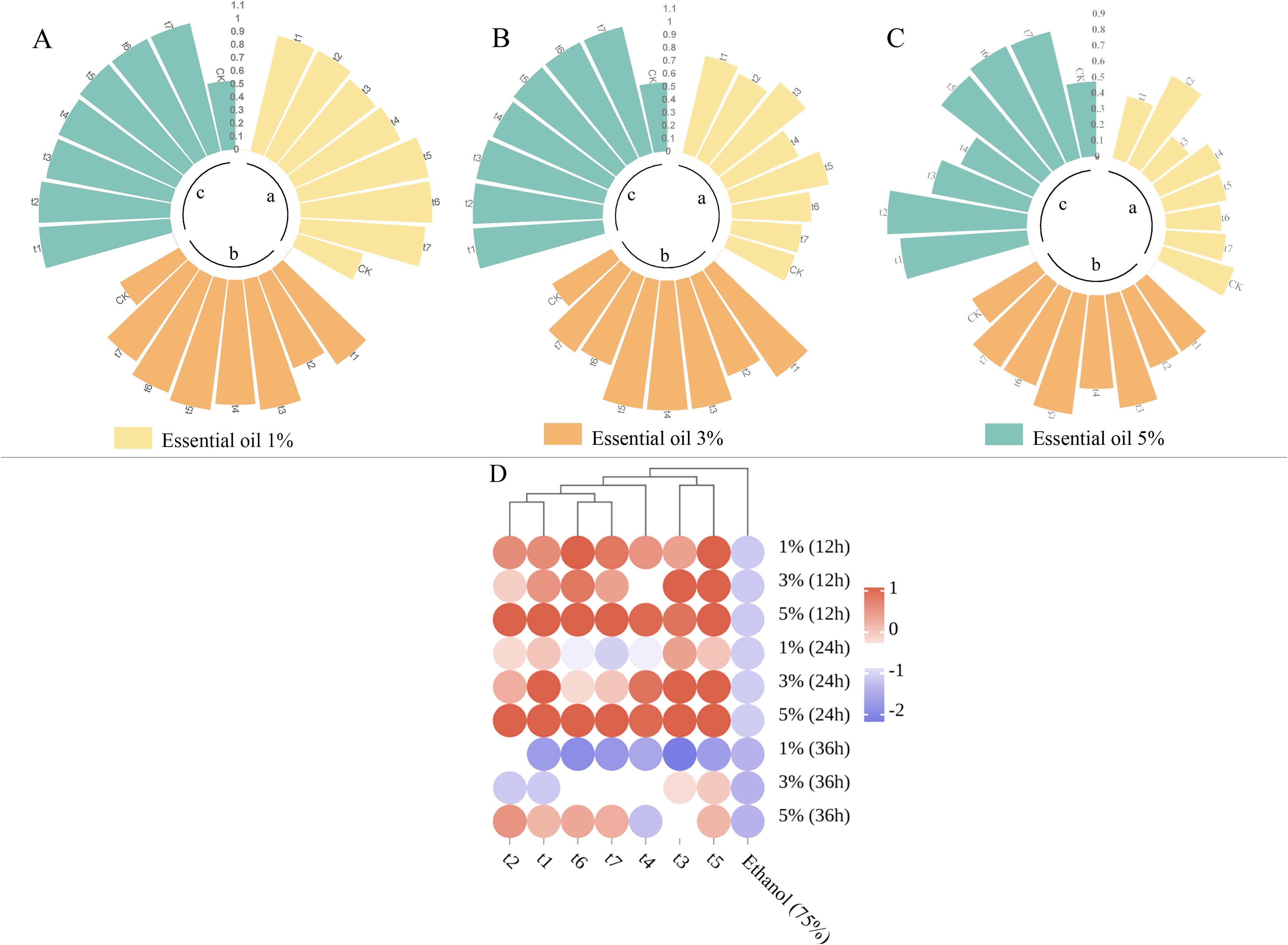
Figure 6. Repellent rates and clustering heatmap of Z. armatum essential oil at different concentrations against T. castaneum after 12, 24, and 36 hours of application. [(A) Repellent rates at 12 hours; (B) Repellent rates at 24 hours; (C) Repellent rates at 36 hours; (D) Clustering heatmap].
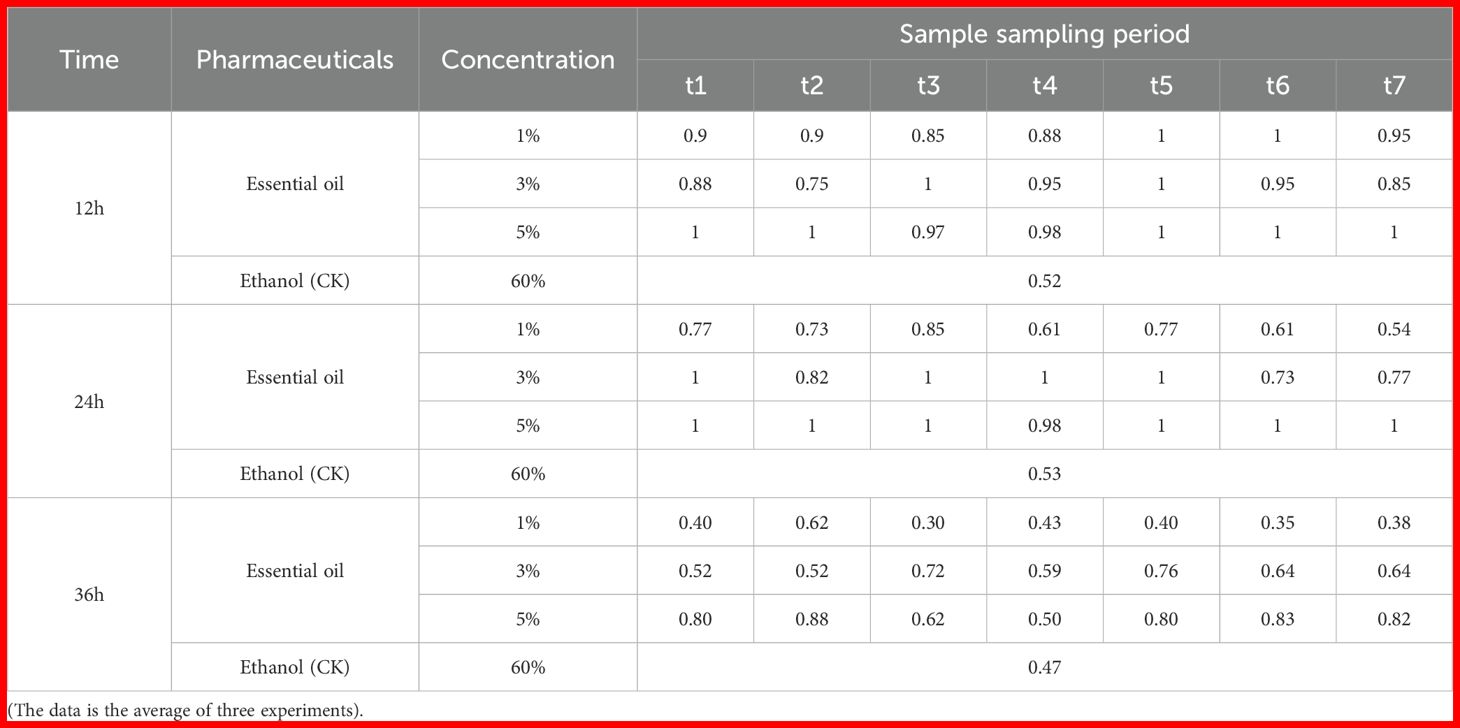
Table 3. The repellent rate of different concentrations of Z. armatum essential oil to Tribolium castaneum after 12, 24 and 36 hours of application.
The EO repellent experiment data were normalized and a clustering heatmap was generated, as shown in Figure 6. The Z. armatum EOs from different maturation stages were grouped into three categories: t1 (May 26), t2 (June 16), t6 (September 9) and t7 (September 28)were clustered into one group; t3 (July 3) and t5 (August 4), into another group; and t4 (July 18) was grouped separately. The clustering results are consistent with the previous analysis of volatile components. This indicates that the primary repellent mechanism of the essential oil relies on fumigant toxicity rather than contact toxicity, providing a reference for the development of insect repellent formulations based on Z. armatum essential oil (Table 4).
3.6 Analysis of antifungal experiment results
The essential oil of Z. armatum from different maturity stages exhibited significant inhibitory effects on C. cassiicola, with the inhibitory effect varying significantly at different concentrations of the essential oil solution. The 0.5% concentration showed the strongest antifungal effect. After 3 days of culture, the colony diameter in the negative control group was 64.1%-70.51% larger compared to the 0.5% group, 55.13%-62.82% larger compared to the 0.1% group, and 8.97%-19.23% larger compared to the 0.05% group (Figure 7). The increase in essential oil concentration significantly enhanced the antifungal effect against C. cassiicola. At 0.5% and 0.1% concentrations, only the essential oil from the t4 stage showed significant differences in antifungal effect compared to the other stages.
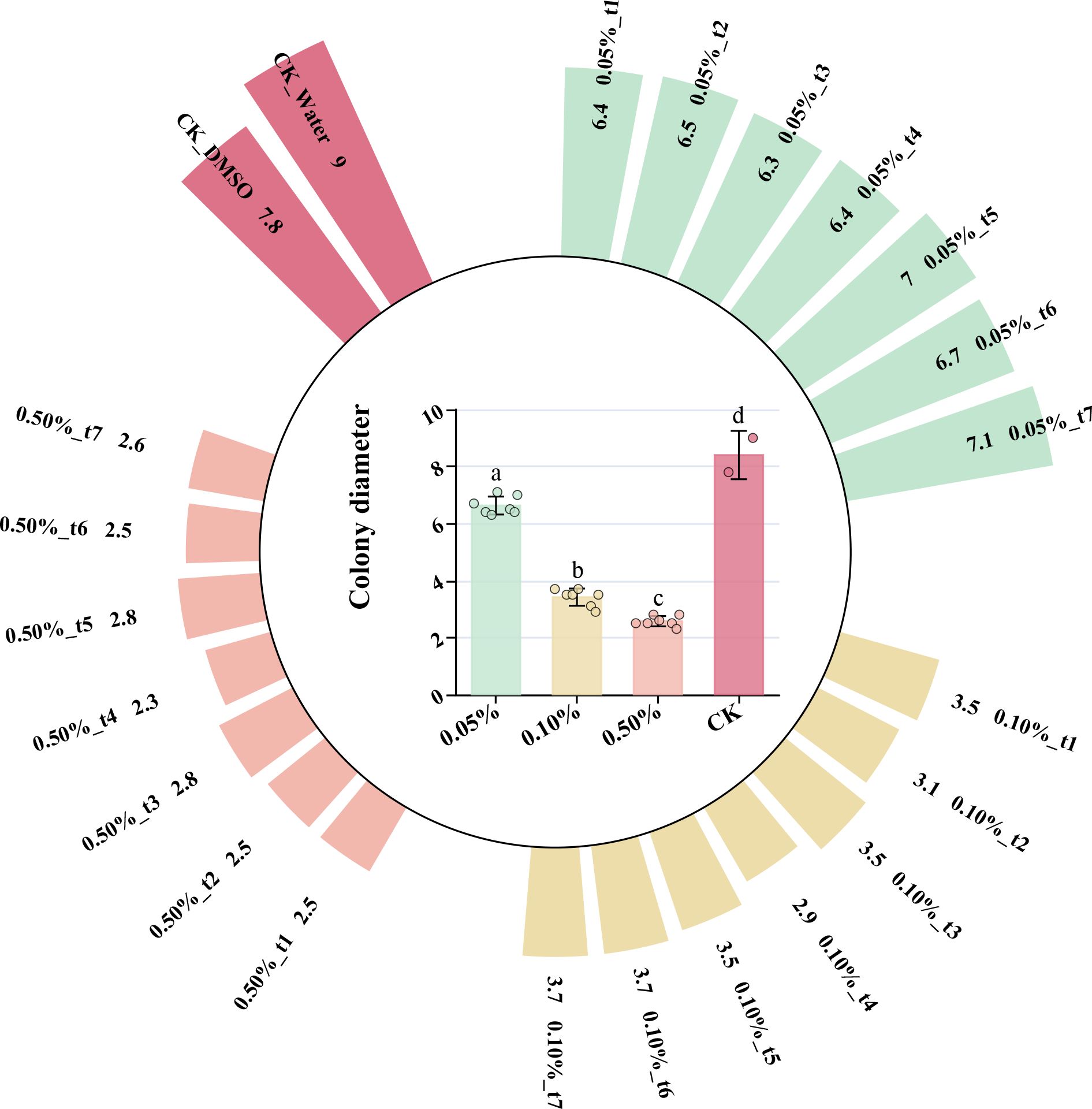
Figure 7. After three days of culture, the colony diameter of C. cassiicola was affected by different concentrations of Z. armatum essential oil solution.
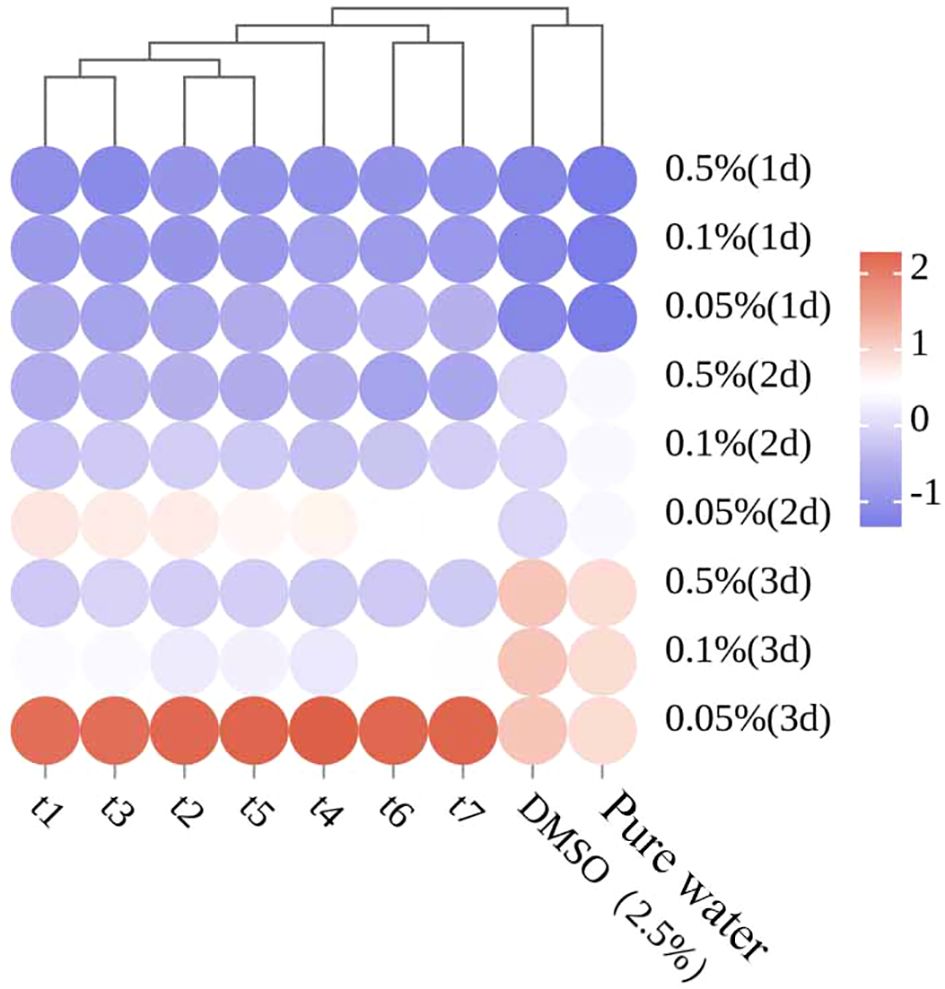
After normalizing the antifungal data of the EOs, a cluster analysis was performed, and the results are shown in Figure 8. The samples from t1 (May 26), t2 (June 16), t3 (July 3), and t5 (August 4) were grouped together, while t4 (July 18) was grouped separately, and t6 (September 9) and t7 (September 28) were grouped together. These results differ from the previous clustering analysis, further indicating that the antifungal properties of the essential oil are not solely influenced by the volatile components or any single component, but rather by the combined effect of multiple components.
4 Discussion
The production, composition, and content of secondary metabolites in plants are influenced by various conditions. Based on market data of volatile oil compounds from Chinese Z. armatum, the projected demand for 2025–2030 is estimated at 40,000-47,500 thousand metric tons, with China’s market share accounting for 15%-18% of the global market. The primary growth drivers stem from expanding applications in: Food and Flavoring Industry, Cosmetics and Personal Care, Pharmaceuticals and Traditional Chinese Medicine, Green Pesticides and so on (Research Report on Monitoring and Analysis of Chinese Zanthoxylum Essential Oil Market (2025 -2030), 2025). For economically important plants with commercial value, optimizing planting time and harvest periods can help obtain commercial products that better meet market demands (A. Cristina et al., 2008). The volatile components of Z. armatum are primarily stored in the fruit peel, with its linalool and d-limonene content being influenced by precipitation-related climatic factors (Qianqian et al., 2024). Therefore, we analyzed the volatile oil components and their content in the fruit peel of Z. armatum from different periods in Nanchong, Sichuan Province. This study aimed to explore the accumulation pattern of volatile oil substances in Z. armatum at different stages of growth and development by measuring the volatile components and their content. We conducted GC-MS analysis of the volatile components in Z. armatum essential oil using an internal standard method, preliminarily identifying and quantifying 28 volatile constituents in relative content. However, since reference standards were not employed for verification, the experimental results have certain limitations. The results show that the accumulation of volatile components occurs before the t5 stage. Studies have shown that in the early developmental stages, the stimulating and aromatic substances produced by Zanthoxylum bungeanum are mainly stored in the leaves and gradually transfer to the fruit peel in the later developmental stages (Lei et al., 2019). Therefore, the volatile components in the fruit may accumulate in the later stages of development. Z. armatum harvested in July and August has a higher content of volatile components compared to other periods, making it the preferred choice for processing and making other products. The principal component analysis results show that there are significant differences in the volatile components of the pepper fruits between the t3 (July 3), t4 (July 18), t5 (August 4) stages and the t1 (May 26), t2 (June 16), t6 (September 9), t7 (September 28) stages. In the early stages of fruit maturation, plants exhibit vigorous metabolic activity, producing a significant amount of monoterpenes (Telci et al., 2009) such as α-Pinene, Sabinene, β-Myrcene, α-Phellandrene, and D-Limonene. As the fruits of Z. armatum mature, the content of more structurally complex compounds, such as alcohols (Kamel et al., 2007) and esters (Moghaddam et al., 2015), increases. For instance, compounds like Linalyl acetate, Terpinen-4-ol, and α-Terpineol are relatively abundant during the fruit’s maturation period. The significantly lower oil yield from dried Z. armatum pericarps during the mid-maturity stage may also be related to the physiological metabolism of the plant. In the early stage, low-molecular-weight volatile compounds accumulate rapidly. During the mid-stage, high-molecular-weight lipid compounds are generated. By the fully mature stage, the content of volatile alcohols and esters in the fruits increases.
Z. armatum EO has a strong repellent effect on T. castaneum, and the effect changes in a gradient with concentration, indicating that the repellent effect is positively correlated with the essential oil content to some extent. Low concentrations of Z. armatum EO solution can also produce a strong repellent effect on T. castaneum at the early stage of application. The volatile compounds in the EO, such as monoterpenes, usually have a strong odor, which may stimulate T. castaneum, causing it to exhibit avoidance behavior. Furthermore, because the odor threshold of various volatile components is relatively low, even at low concentrations, these compounds can induce a repellent response in T. castaneum (Daokui, 2023). Studies have shown that substances like α-pinene and laurene can induce a strong repellent effect on T. castaneum (Kim et al., 2010; Maurya et al., 2018). This all demonstrates that the volatile components of Z. armatum EO have a good repellent effect on T. castaneum. However, the effect decreases as the active ingredients volatilize. Preventing the evaporation of the essential oil could significantly extend its repellent effectiveness. Unlike previous studies, no mortality of T. castaneum adults was observed in this experiment. In previous experiments, Z. armatum essential oil solutions applied to the abdomen of T. castaneum also did not result in insect death within 48 hours. This suggests that the low content of Terpinen-4-ol and the absence of decanal, a compound with strong insecticidal activity, may be the reasons for the lack of mortality in the tested insects (Yang et al., 2019). Another possible reason is that T. castaneum perceives chemical signals in the surrounding environment through sensory organs such as its antennae (Feng et al., 2014). The volatile chemicals in the EO may only interact with the receptors on the insect’s sensory organs (Thomas S. et al., 2012), triggering a repellent response, but not enough to cause death. The specific activity and mechanism need to be determined through more detailed experiments and studies.
In the antimicrobial experiment, the baseline antifungal activity of Z. armatum EOs was determined by comparing the antifungal activities of EOs extracted at different sampling periods and against the negative control. However, since no positive control was included, the practical application value of the EOs could not be fully validated. The results showed that Z. armatum EOs from different maturity stages exhibited significant inhibitory effects on C. cassiicola, and the antimicrobial activity increased with higher EO concentrations. A study by Nirmala et al. showed that methanol extracts of the peel, seeds, and bark of Z. armatum also exhibited antifungal activity against microorganisms such as Bacillus subtilis, Enterococcus faecalis, Staphylococcus aureus, and Staphylococcus epidermidis (Phuyal et al., 2020). Since the best antifungal effect was observed during the t4 period, the EO extracted from Z. armatum harvested in mid-July showed more prominent antifungal activity. Previous studies have shown that the antifungal effect is positively correlated with the content of linalool (Wei et al., 2023). In this study, the t4 period, which exhibited the best antifungal effect, was not the period with the highest linalool content. However, by comparing samples from other periods, it was found that although the relative contents of various compounds in the t4 period were not the highest, they still ranked among the top. Examples include Linalool, Germacrene D, Nerolidol, (+)-Citronellal, and Bicyclogermacrene. These compounds exhibit varying degrees of antibacterial and antifungal activity (M J. et al., 2009; Ricardo et al., 2011; Xiuming et al., 2023). We hypothesize that the specific compositional ratio of components at t4 may lead to synergistic enhancement (e.g., through multi-target disruption of cell membranes), whereas the isolated increase in linalool at t5, accompanied by a decline in other constituents, could result in reduced overall bioactivity. However, this conclusion requires further experimental validation. Studies on the essential oil of Tetraclinis articulata have shown that the combination of antibiotics with extracts from this species is more effective, exhibiting 100% synergistic or partial synergistic effects against the tested bacteria (Djouahri et al., 2014). Therefore, the antifungal effects of different chemical components are inconsistent. Investigating the synergistic and antagonistic interactions between different chemical components in terms of antifungal activity can serve as a new direction for future research.
In the insect repellent and antifungal experiments, different concentrations of Z. armatum essential oil exhibited insecticidal/antifungal activity against T. castaneum and C. cassiicola, with a gradient relationship as the concentration increased. Z. armatum essential oil is characterized by high levels of oxygenated monoterpenes and monoterpene hydrocarbons. These monoterpenes are common components of essential oils and are used as fragrances and flavorings in cosmetics, perfumes, pharmaceuticals, and food products (Domenico et al., 2005). The EO used in the in vitro antifungal tests contains a significant number of monoterpenes. The main components in Z. armatum EO, such as Linalool, D-Limonene, and Sabinene, have been proven to exhibit strong insecticidal/antifungal properties (Djenane, 2015; Xue et al., 2020). This may be the basis for the strong insecticidal and antifungal activities of Z. armatum EO. Regarding the mechanism of action of the EO, it may involve interactions between enzymes and membrane proteins. In some cases, the active compounds in the EO can penetrate the lipid bilayer of the cell membrane, increasing the cell’s permeability and leading to the leakage of important cellular contents (Alvarez-Martinez et al., 2021; Maurya et al., 2022). The antimicrobial activity data herein provide a foundation for future mechanistic studies, though specific targets (e.g., membrane disruption/enzyme inhibition) require validation via component-specific assays. Z. armatum EO, rich in these components, can become a key part of natural plant-based insecticidal and antifungal agents. Additionally, the Z. armatum EO from the t4 period shows superior antifungal effects. For the development of antifungal drugs based on Z. armatum EO, it is recommended to harvest the fruits in mid-July.
5 Conclusion
The different growth and development stages are important factors influencing the accumulation of secondary metabolites in Z. armatum fruits. This study performed GC-MS analysis on the Z. armatum EOs from Nanchong City, Sichuan Province, and conducted insect-repellent and antifungal experiments using EOs from different stages. The GC-MS results showed that the accumulation of volatile compounds occurred before the t5 stage, as low-molecular-weight monoterpenes were rapidly generated in the early fruit ripening stages, while alcohols and esters accumulated in later stages. The EO content and its major fragrance components accumulated richly in July and August. Both insect-repellent and antimicrobial experimental results indicated that the EO from the t4 stage had higher insect-repellent and antifungal activity. Therefore, the insect-repellent and antimicrobial effects of the EOs may result from the synergistic or antagonistic interactions among various compounds. This study provides a reference for utilizing Z. armatum’s different commercial values based on harvest periods and offers a research direction for using plant EOs as natural insect-repellent/antimicrobial agents. Future research can delve into analyzing the interaction mechanisms between different compounds in the essential oil and the underlying mechanisms of its insect-repellent and antifungal activities.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
YP: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft. DX: Conceptualization, Methodology, Supervision, Writing – review & editing. WL: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. QQ: Data curation, Investigation, Software, Writing – review & editing. JW: Investigation, Software, Writing – review & editing. TG: Investigation, Resources, Writing – review & editing. ZZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Fundamental Research Funds of China West Normal University (20A007, 20E051, 21E040 and 22kA011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
ACFSMC (2009). ““Determination of pepper essential oils content”,” in GB/T 17527-2009 (China Standards Press, Beijing).
A. Cristina, F., Jose, B., Luis, P., and Johannes, J. C. (2008). Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Frag. J. 23, 213–226. doi: 10.1002/ffj.1875
Alvarez-Martinez, F. J., Barrajon-Catalan, E., Herranz-Lopez, M., and Micol, V. (2021). Antibacterial plant compounds, extracts and essential oils: an updated review on their effects and putative mechanisms of action. Phytomedicine 90, 153626. doi: 10.1016/j.phymed.2021.153626
Bakkali, F., Averbeck, S., Averbeck, D., and Idaomar, M. (2008). Biological effects of essential oils – a review. Food. Chem. Toxicol. 46, 446–475. doi: 10.1016/j.fct.2007.09.106
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 94, 223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022
Campbell, J. F., Athanassiou, C. G., Hagstrum, D. W., and Zhu, K. Y. (2022). Tribolium castaneum: a model insect for fundamental and applied research. Annu. Rev. Entomol. 67, 347–365. doi: 10.1146/annurev-ento-080921-075157
Daokui, W. (2023). Study on the insecticidal activity of two kinds of plant essential oils against tribolium castaneum herbst. [Master's thesis] (Anhui Province: Anhui Agricultural University).
Di, Z., Xiaoxia, S., Maurizio, B., Xiaoou, W., Jiyong, S., Lei, Z., et al. (2021). A comparative overview on chili pepper (capsicum genus) and sichuan pepper (zanthoxylum genus): from pungent spices to pharma-foods. Trends Food Sci. Technol. 117, 148–162. doi: 10.1016/j.tifs.2021.03.004
Djenane, D. (2015). Chemical profile, antibacterial and antioxidant activity of Algerian citrus essential oils and their application in sardina pilchardus. Foods 4, 208–228. doi: 10.3390/foods4020208
Djouahri, A., Saka, B., Boudarene, L., Benseradj, F., Aberrane, S., Aitmoussa, S., et al. (2014). In vitro synergistic/antagonistic antibacterial and anti-inflammatory effect of various extracts/essential oil from cones of tetraclinis articulata (vahl) masters with antibiotic and anti-inflammatory agents. Ind. Crop Prod. 56, 60–66. doi: 10.1016/j.indcrop.2014.02.035
Domenico, T., Francesco, C., Maria, G. S., Vincenza, V., Mariateresa, C., et al. (2005). Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents. Chemother. 49, 2474–2478. doi: 10.1128/AAC.49.6.2474
Feng, L., Kenneth F., H., Arthur G., A., and Nannan, L. (2014). Antennal olfactory sensilla responses to insect chemical repellents in the common bed bug. J. Chem. Ecol. 40, 522–533. doi: 10.1007/s10886-014-0435-z
Gao, W. and Hanbin, W. (2014). Analgesia synergism of essential oil from pericarp of zanthoxylum schinifolium and verapamil. Evid.-Based Complement Altern. Med. 2014, 505876. doi: 10.1155/2014/505876
Gutierrez, J., Barry-Ryan, C., and Bourke, P. (2009). Antimicrobial activity of plant essential oils using food model media: efficacy, synergistic potential and interactions with food components. Food Microbiol. 26, 142–150. doi: 10.1016/j.fm.2008.10.008
Hong, L., Jing, W., Qing, W., Anxiang, S., Mei, X., Qin, L., et al. (2017). Inhibitory effect of zanthoxylum bungeanum essential oil (zbeo) on escherichia coli and intestinal dysfunction. Food Funct. 8, 1569–1576. doi: 10.1039/c6fo01739h
J. D., L. and Roth, L. (1953). Composition of the odorous secretion of tribolium castaneum. Biology 46, 282–288. doi: 10.1093/AESA/46.2.281
Jilani, G. and Saxena, R. C. (1990). Repellent and feeding deterrent effects of turmeric oil, sweetflag oil, neem oil, and a neem-based insecticide against lesser grain borer (coleoptera: bostrychidae. J. Economic Entomology 83, 629–634. doi: 10.1093/jee/83.2.629
Jingxuan, K., Yuan, Q., Shanshan, L., Guanghui, S., Qingying, L., Hejun, W., et al. (2018). Establishment of gc-ms fingerprint based on essential oil components in zanthoxylum and application on hanyuan zanthoxylum bungeanum. J. Chin. Cereals Oils Assoc. 33, 116–126. doi: 10.3969/j.issn.1003-0174.2018.11.020
Kamel, M., Karim, H., Mouna Ben, T., Thouraya, C., Mohamed Elyes, K., and Brahim, M. (2007). Changes on essential oil composition of coriander (coriandrum sativum l.) Fruits during three stages of maturity. Food Chem. 102, 1131–1134. doi: 10.1016/j.foodchem.2006.06.046
Kim, S., Yoon, J., Jung, J., Ki-Bae, H., Young-Joon, A., and Hyung Wook, K. (2010). Toxicity and repellency of origanum essential oil and its components against tribolium castaneum (coleoptera: tenebrionidae) adults. J. Asia-Pac. Entomol. 13, 369–373. doi: 10.1016/j.aspen.2010.06.011
Lei, Z., Li, W., Xi, C., Wei, P., Yujie, L., Linying, Y., et al. (2019). Comparative studies on flavor substances of leaves and pericarps of zanthoxylum bungeanum maxim. At different harvest periods. Trop. J. Pharm. Res. 18, 279–286. doi: 10.4314/tjpr.v18i2.9
Maurya, A. K., Devi, R., Kumar, A., Koundal, R., Thakur, S., Sharma, A., et al. (2018). Chemical composition, cytotoxic and antibacterial activities of essential oils of cultivated clones ofjuniperus communis and wildjuniperus species. Chem. Biodivers. 15, e1800183. doi: 10.1002/cbdv.201800183
Maurya, A. K., Sharma, A., Mukhia, S., Rani, D., Kumar, A., Kumar, D., et al. (2022). Essential oil composition, in vitro biological activities and safety evaluation of cultivated hedychium spicatum seeds. Indian J. Pharm. Sci. 84, 783–790. doi: 10.36468/pharmaceutical-sciences.973
Moghaddam, M., Miran, S. N. K., Pirbalouti, A. G., Mehdizadeh, L., and Ghaderi, Y. (2015). Variation in essential oil composition and antioxidant activity of cumin (cuminum cyminum l.) Fruits during stages of maturity. Ind. Crop Prod. 70, 163–169. doi: 10.1016/j.indcrop.2015.03.031
Na, G., Yuping, Z., Qi, C., Qingyan, G., Jiao, J., Wei, W., et al. (2017). The preservative potential of amomum tsaoko essential oil against e. Coil, its antibaterial property and mode of action. Food Control 75, 236–245. doi: 10.1016/j.foodcont.2016.12.013
Phuyal, N., Jha, P. K., Raturi, P. P., and Rajbhandary, S. (2020). In vitro antibacterial activities of methanolic extracts of fruits, seeds, and bark ofzanthoxylum armatum dc. J. Trop. Med. 2020, 1–7. doi: 10.1155/2020/2803063
Prakash, B., Singh, P., Mishra, P. K., and Dubey, N. K. (2012). Safety assessment of zanthoxylum alatum roxb. Essential oil, its antifungal, antiaflatoxin, antioxidant activity and efficacy as antimicrobial in preservation of piper nigrum l. Fruits. Int. J. Food Microbiol. 153, 183–191. doi: 10.1016/j.ijfoodmicro.2011.11.007
Qianqian, Q., Zhihang, Z., Yaqin, P., and Danping, X. (2024). Chemical composition variation in essential oil and their correlation with climate factors in chinese prickly ash peels (zanthoxylum armatum dc.) From different habitats. Molecules 29, 1343. doi: 10.3390/molecules29061343
Ravi Kant, U. and Gayatri, J. (2007). Evaluation of biological activities of piper nigrumoil against tribolium castaneum. Bull. Insectol. 60, 57–61.
Research report on monitoring and analysis of chinese zanthoxylum essential oil market, (2025-2030) (2025). Research report on monitoring and analysis of chinese zanthoxylum essential oil market, (2025-2030). Available online at: https://max.book118.com/html/2025/0404/8002034041007050.shtm.
Ricardo, M. M., Luiz, C. A. B., Antonio, J. D., Cleber, J. S., Larissa, S. C., Nélio, J. A., et al. (2011). Chemical composition and antibacterial activity of essential oils from verbenaceae species: alternative sources of (e)-caryophyllene and germacrene-d. Química Nova 34, 1550–1555. doi: 10.1590/S0100-40422011000900013
Shenglan, L., Gang, Y., Shan, H., Bin, L., Aijun, L., and Jianquan, K. (2022). Chemical composition of zanthoxylum schinifolium siebold & zucc. Essential oil and evaluation of its antifungal activity and potential modes of action on malassezia restricta. Ind. Crop Prod. 180, 114698. doi: 10.1016/j.indcrop.2022.114698
Shun, D., Haibo, R., He, T., Bingxin, Z., Xiaoyuan, M., Liyang, Z., et al. (2019). Molecular basis of neurophysiological and antioxidant roles of sichuan pepper. Biomed. Pharmacother. 112, 108696. doi: 10.1016/j.biopha.2019.108696
Telci, I., Demirtas, I., and Sahin, A. (2009). Variation in plant properties and essential oil composition of sweet fennel (foeniculum vulgare mill.) Fruits during stages of maturity. Ind. Crop Prod. 30, 126–130. doi: 10.1016/j.indcrop.2009.02.010
Thomas S., M., Alban, M., Erika, P., Yves Le, C., and Wolfgang, R. (2012). Sensory reception of the primer pheromone ethyl oleate. Naturwissenschaften. 99, 421–425. doi: 10.1007/s00114-012-0909-1
Wei, L., Kongfa, Z., Fenglun, Z., Shikun, G., Yinjiang, H., Wei, N., et al. (2023). Chemical composition analysis and in vitro inhibition of helicobacter pylori in essential oil of zanthoxylum schinifolium from different producing areas. Chin. Wild Plant Resour. 42, 68–74. doi: 10.3969/j.issn.1006-9690.2023.10.012
Xinqi, D. (2023). Integrated transcriptomic and un-targetedmetabolomics analysis the defenseresponse of mulberry against thepathogen fungi of mulberry ring rotdisease. [Master's thesis] (Sichuan Province: West Normal University).
Xiuming, L., Qifang, W., Haosen, L., Xiaoyun, W., Ruimin, Z., Xiaoyu, Y., et al. (2023). Revealing the mechanisms for linalool antifungal activity against fusarium oxysporum and its efficient control of fusarium wilt in tomato plants. Int. J. Mol. Sci. 24, 458. doi: 10.3390/ijms24010458
Xiya, F., Hongwei, W., Zhirong, W., Pimiao, H., and Jianquan, K. (2022). Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, hs-gc-ims and hs-spme-gc–ms. Food Chem. 375, 131671. doi: 10.1016/j.foodchem.2021.131671
Xue, L., Jiaxin, C., Haiming, C., Qiuping, Z., Yaqi, H., Weijun, C., et al. (2020). Antibacterial activity and mechanism of linalool against pseudomonas aeruginosa. Microb. Pathog. 141, 103980. doi: 10.1016/j.micpath.2020.103980
Yang, W., Liting, Z., Yixi, F., Shanshan, G., Xue, P., Di, Z., et al. (2019). Insecticidal and repellent efficacy against stored-product insects of oxygenated monoterpenes and 2-dodecanone of the essential oil from zanthoxylum planispinum var. Dintanensis. Environ. Sci. pollut. Res. 26, 24988–24997. doi: 10.1007/s11356-019-05765-z
Keywords: Z. armatum DC., GC-MS, essential oil, T. castaneum, C. cassiicola
Citation: Peng Y, Xu D, Liao W, Qian Q, Wu J, Gan T and Zhuo Z (2025) Dynamic analysis of composition, insecticidal, and antifungal activities of Zanthoxylum armatum DC. at different harvesting periods. Front. Plant Sci. 16:1603963. doi: 10.3389/fpls.2025.1603963
Received: 01 April 2025; Accepted: 27 June 2025;
Published: 18 July 2025.
Edited by:
Robin Joshi, University of Pennsylvania, United StatesReviewed by:
Ashok Gehlot, Institute of Himalayan Bioresource Technology (CSIR), IndiaTahir Ali, University of Mississippi, United States
Copyright © 2025 Peng, Xu, Liao, Qian, Wu, Gan and Zhuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihang Zhuo, emh1b3poaWhhbmdAZm94bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Yaqin Peng
Yaqin Peng Danping Xu
Danping Xu Wenkai Liao1
Wenkai Liao1 Qianqian Qian
Qianqian Qian Tingjiang Gan
Tingjiang Gan Zhihang Zhuo
Zhihang Zhuo