- 1College of Agriculture, Xinjiang Agricultural University, Urumqi, China
- 2Research Institute of Food Crops, Xinjiang Academy of Agricultural Sciences, Urumqi, China
Introduction: Although the mulched drip irrigation system combined with high nitrogen input (240∼310 kg ha-1) in Xinjiang, China, frequently achieves record-high soybean yields (6855 kg ha-1), this practice is not conducive to symbiotic nitrogen fixation and compromises agricultural sustainability.
Methods: Under the mulched drip irrigation, this study evaluation four nitrogen application treatments (N0: 0 kg ha-1, N120: 120 kg ha-1, N180: 180 kg ha-1, and N240: 240 kg ha-1) were evaluated over two consecutive growing seasons to investigate their effects on nodule morphological and physiological traits, stem ureide content, and the percentage of nitrogen derived from the atmosphere (%Ndfa) during the reproductive growth stage.
Results: The application of 180 kg ha-1 nitrogen significantly increased nodule number, nodule dry weight, nodule sucrose content, and nodule starch content, while improving soybean yield and nitrogen agronomic use efficiency. Conversely, the application of nitrogen exceeding 180 kg ha-1 inhibited nitrogenase activity, suppressed leghemoglobin synthesis, disrupted the glutamine synthetase/glutamate synthase metabolic pathway, and reduced ureide translocation from nodules to stems, leading to significant accumulation of ureides in nodules. Correlation and path analyses indicated that nitrogenase activity, leghemoglobin content, urate oxidase activity, and stem ureide content were significantly positively correlated with %Ndfa, whereas nodule ureide content showed a significant negative correlation with %Ndfa. Stem ureide content exhibited a strong direct positive effect on %Ndfa (path coefficient = 0.95), confirming its validity as a robust indicator for assessing SNF capacity.
Discussion: In conclusion, mulched drip irrigation, applying 180 kg ha-1 nitrogen at the beginning pod stage (R3) effectively enhances root nodulation, promotes carbohydrate allocation to nodules, sustains symbiotic nitrogen fixation activity, and ultimately increases soybean yield and nitrogen use efficiency. Thus, under mulched drip irrigation system, applying the correct rate of nitrogen fertilizer is beneficial for enhancing soybean yield and mitigating environmental risks, which holds significant importance for promoting sustainable agricultural development.
Introduction
Crop yields heavily depend on nitrogen (N) fertilizer supply. Owing to the low cost and high N content of urea, farmers often apply N fertilizers excessively and indiscriminately in pursuit of higher yields, leading to yield stagnation, significant declines in nitrogen use efficiency (NUE), and exacerbated environmental pollution through energy-intensive production and application (Liu et al., 2021; Bodirsky et al., 2014; Camargo et al., 2005). Consequently, enhancing NUE is of crucial importance for sustainable agriculture. In Xinjiang, under mulched drip irrigation combined with integrated water-fertilizer management has repeatedly achieved record-high soybean yields in China. However, the total N supply during the growing season remains excessively high (240∼310 kg ha-1), resulting in high yields but low NUE. This inefficiency stems from farmers adopting cotton field management practices for soybeans, ignoring the unique role of symbiotic nitrogen fixation (SNF) in soybean nitrogen acquisition, which differs fundamentally from graminaceous crops like cotton and rice. While soybean nitrogen sources include soil N, fertilizer N, and SNF, relying solely on SNF cannot achieve high yields; thus, targeted exogenous N supplementation based on soil fertility remains essential (Thorburn et al., 2024). Coordinating SNF, N fertilization, and yield by optimizing exogenous N supply to maximize SNF potential, establish an efficient soybean SNF system, and achieve high yield with resource efficiency holds significant practical importance (Goyal et al., 2021; Coba de la Peña et al., 2018).
Soybean plants acquire N through root uptake and SNF in root nodules. However, excessive N supply is well-documented to suppress root nodulation. When soil N availability increases significantly, plants prioritize direct root N absorption over SNF due to its lower energy cost, leading to reduced nodule number, nodule biomass, nitrogenase activity, leghemoglobin content, impaired glutamine synthetase (GS)/glutamate synthase (GOGAT) and glutamate dehydrogenase(GDH) assimilation pathways, and accelerated nodule senescence (Lyu et al., 2020; Albornoz, 2016; Wang et al., 2015). It is suggested by most studies that high N inputs inhibit root hair infection and nodule initiation (Mendes et al., 2018; Santachiara et al., 2018). Conversely, some researchers argue that low-level N fertilization does not compromise nodulation, as nodules may outcompete roots for resources; moderate N application during reproductive growth slightly reduces nodule weight but maintains nitrogenase activity (Ke et al., 2023; Pampana et al., 2018; Carter and Tegeder, 2016). Notably, Nguyen et al. (2020) demonstrated that novel rhizobial strains exhibit robust nodulation and SNF capacity even under high nitrate conditions. Proposed mechanisms for N-mediated suppression of SNF include carbon starvation (Lu et al., 2022; Parvin et al., 2020), nitrogen metabolite feedback regulation (Nazari et al., 2019; Castañeda et al., 2018), and internal oxygen limitation (Larrainzar et al., 2020; Gourion et al., 2015). Studies indicate that 50∼60% of soybean N demand is met via SNF (Salvagiotti et al., 2008), with some cases exceeding 90% (Mastrodomenico and Purcell, 2012). Strong positive correlations exist between nodule number, nodule dry weight, SNF efficiency, and seed yield, underscoring the critical role of nodule health in N fixation and productivity (Ferguson et al., 2019; Burias and Planchon, 1990). To optimize high-yield soybean N demand while minimizing exogenous N input, strategies such as deep placement of slow-release N fertilizers in nodulation zones (Yaseen et al., 2021), early sowing (Kurdali, 1996; Sprent and Thomas, 1984), and split N application after the beginning pod stage (R3) (Zapata et al., 1987) can sustain nodule activity and enhance yield efficiency.
Ureides (allantoin and allantoic acid), the primary SNF products exported from soybean root nodules to stems, are temporarily stored in stems (Atkins and Smith, 2007; Smith and Atkins, 2002). Enhancing ureide export from nodules positively impacts soybean reproductive growth by increasing SNF capacity and nodule number (Carter and Tegeder, 2016). In the enzymatic synthesis of ureides, urate oxidase (UO) activity catalyzes the oxidation of uric acid to allantoate, playing a critical role in nodule development and SNF. Ureide synthesis helps plants avoid ammonia toxicity by reducing glutamine and asparagine accumulation in nodules (Britto and Kronzucker, 2002; Engvild, 1987). Excessive N fertilization suppresses nodule uricase activity, elevates ammonia toxicity risks, and reduces stem ureide content (Wang et al., 2024). Under low N conditions, ureide export from nodules to stems is enhanced, whereas high N supply inhibits ureide translocation. Accumulated ureides in nodules directly suppress nitrogenase activity and indirectly impair SNF via feedback regulation mediated by stem ureide levels (Gil-Quintana et al., 2013; Serraj et al., 1999).
Previous studies have demonstrated that excessive exogenous N supply significantly suppresses soybean root nodulation and SNF (Thorburn et al., 2024). Under the mulched drip irrigation, this study systematically investigates the response mechanisms of root nodule developmental dynamics and nitrogen fixation metabolism in high-yield soybean to varying N levels. We evaluate the adaptability of soybean nodules under different N regimes from the perspectives of N fixation, assimilation, and translocation. We hypothesize that under mulched drip irrigation, excessive N application at the beginning of pod formation will impede nodule formation, downregulate nitrogenase activity, inhibit carbohydrate allocation to nodules, and reduce long-distance transport of ureides (allantoin and allantoic acid) to aboveground tissues.
Materials and methods
Experimental field and meteorological conditions
The experiment was conducted from April 2022 to October 2023 at the Sanping Experimental Farm of Xinjiang Agricultural University in Urumqi, Xinjiang (43°56′N, 87°20′E). This region is characterized by a temperate continental arid climate. Daily maximum and minimum temperatures and precipitation during the soybean growing seasons are shown in Figure 1. The experimental field soil was sandy loam. Before sowing in 2022 and 2023, key soil nutrient parameters in the 0—20 cm layer were: organic matter (13.5 and 14.6 g kg-1), total nitrogen (0.91 and 0.73 g kg-1), alkali-hydrolyzable nitrogen (60.2 and 53.1 mg kg-1), available phosphorus (16.2 and 17.4 mg kg-1), and available potassium (185.2 and 216.0 mg kg-1), respectively.
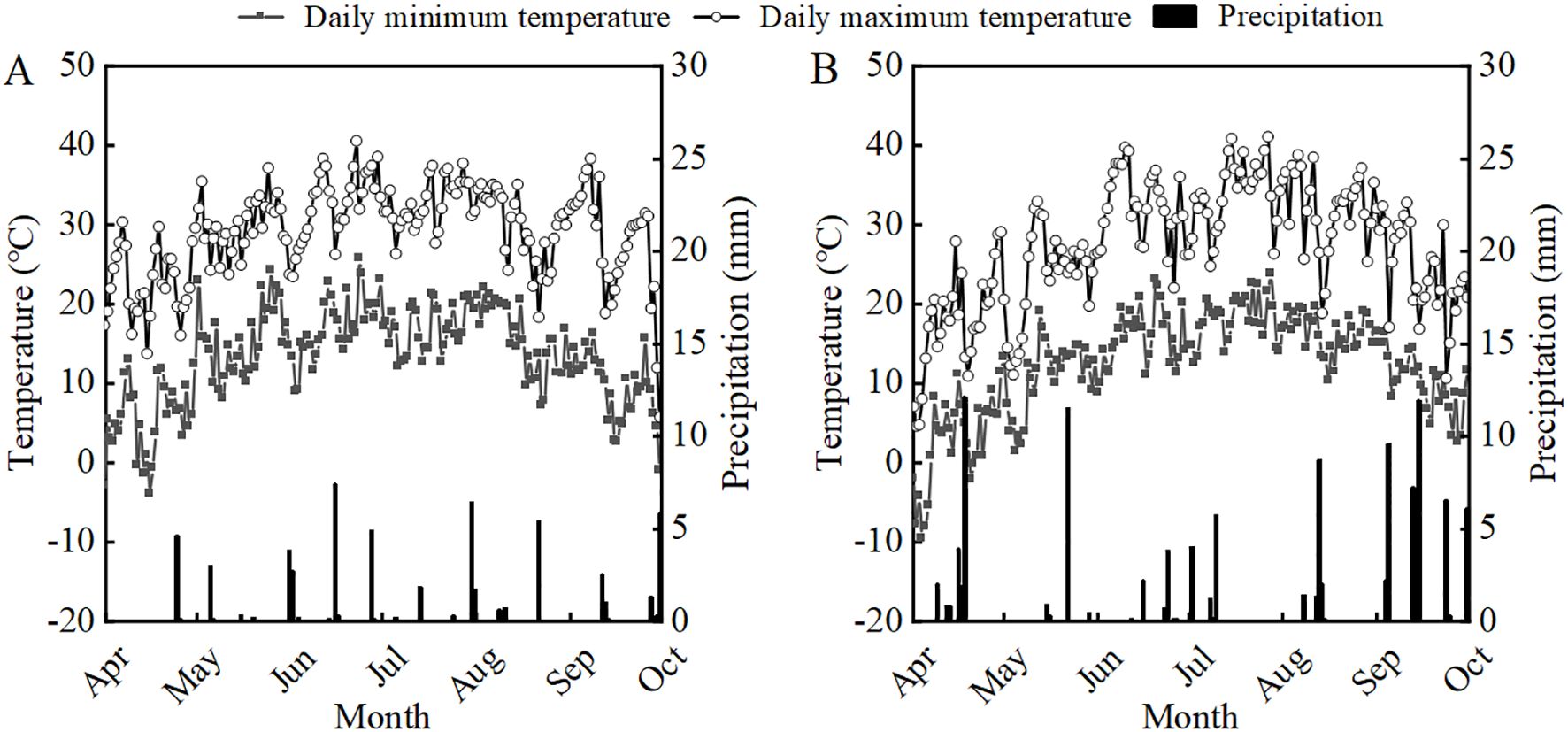
Figure 1. Daily minimum temperature, maximum temperature and rainfall from April to October in 2022 (A) and 2023 (B).
Experimental design and crop husbandry
The experiment followed a randomized complete block design. Four N treatments were implemented at the R3 stage: 0 (N0), 120 (N120), 180 (N180), and 240 kg ha-¹ (N240). Each plot measured 48 m² (4.8 m×10 m) with three replications, separated by 2 m buffer zones. Prior to tillage, calcium superphosphate (containing 19% P2O5) was applied at Soybeans were planted on April 27 and harvested from September 6 to 26 over two consecutive years. The planting used an equal row spacing of 40 cm, with a density 33×104 plants ha-1. The field was mulched with 140 cm wide black plastic film, under which two labyrinth-type drip tapes (57 kg P2O5 ha-1. Φ16 mm with a discharge rate of 2.5–3.5 L h-1) were laid with 40 cm spacing between them. Irrigation was conducted every 10–13 days from June 17–27 to August 5–15 each year, with a total of 5 irrigations and a total water volume of 3300 m3 ha-1. Fertigation was implemented using monopotassium phosphate (containing 34% K2O and 52% P2O5), with total nutrient applications of 51 kg K2O ha-1 and 78 kg P2O5 ha-1, applied in a 1:2 ratio during the initial flowering and pod-setting stages. Two manual weeding were performed during the soybean growing season, with other management practices consistent with conventional field cultivation.
Sample collection
At the full flowering stage (R2), full pod stage (R4), beginning pod stage (R5), full seed stage (R6), and physiological maturity stage (R8), 10 representative plants were selected from each treatment. Soybean roots were carefully excavated, washed with tap water, and nodules with diameter>1 mm were excised with a scalpel and counted. Five plants per treatment were separated into organs, dried to constant weight at 60°C in an oven, ground through a 1 mm sieve, and stored dry. Nodules from the remaining five plants were immediately stored at -80°C until analysis.
Analytic determinations
Metabolite concentration
To determine sucrose, starch, ureides, and nitrate concentrations, dried nodule and stem samples were homogenized with 10 mL of distilled water and incubated in a 95°C water bath for 1 hour to obtain extracts. Supernatants were collected, and sucrose and starch contents in nodules were determined following the method of Xu et al (Xu et al., 2015). Ureide concentrations in nodules and stems were measured using the colorimetric assay described by Van Der Drift et al. (1970). Nitrate content in the main stem was analyzed via the salicylic acid method as outlined by Cataldo et al (Cataldo et al., 1975). Fresh nodules were used to quantify leghemoglobin content according to the protocol of Smagghe et al. (2009).
Enzymatic activities
Nitrogenase activity was determined using the acetylene reduction assay (David et al., 1980). Fresh nodules (1 g) were placed in 8 mL sealed reaction vials. After withdrawing 1 mL of air with a sterile syringe, an equal volume of acetylene was injected. The vials were incubated at 25°C for 30 min, and ethylene production was measured via gas chromatography. Fresh nodules were homogenized in an appropriate extraction buffer, and the supernatant was collected for enzymatic assays: glutamine synthetase (GS), glutamate synthase (GOGAT), glutamate dehydrogenase (GDH), and urate oxidase (UO). GS activity was assayed following the method of O’Neal and Joy (1974). The reaction mixture contained 50 mM Tris-HCl (pH 7.5), 4 mM ATP, 80 mM sodium glutamate, 30 mM MgSO4, 10 mM NH2OH, and 30 mM cysteine. A standard curve was generated using γ-glutamyl hydroxamate. GOGAT activity was measured according to Groat and Vance (1981). The reaction medium consisted of 100 mM potassium phosphate buffer (pH 7.6), 0.1% (v/v) 2-mercaptoethanol, 100 μM NADH, 2.5 mM 2-oxoglutarate, and 100 mM glutamine. GDH activity was determined using the method of Bulen (1956). The reaction system included 0.1 M Tris-HCl (pH 7.5), 0.33 M 2-oxoglutarate, 3 M NH4Cl, and 0.2 mL NADH. UO activity was analyzed following Pineda et al. (1984). The reaction mixture contained 0.1 mM uric acid and 100 mM Tris-HCl (pH 8.5).
Estimation of relative symbiotic nitrogen fixation (%Ndfa)
The relative abundance of ureides (RAU), a parameter characterizing SNF, was estimated using the concentrations of ureides and nitrate in soybean main stems, following Equation 1 as described by Herridge and Peoples (1990).
Where:
RAU: relative abundance of ureide (%).
a and b: concentrations of ureide and nitrate (μmol g-1), respectively.
Ureide contains four N atoms, NO3-contains one N atom.
The percentage of nitrogen derived from the atmosphere (%Ndfa) during soybean growth was estimated using the relative ureide content in main stems via a calibration equation (Herridge and Peoples, 1990). The calibration equation was established based on the ¹5N isotopic dilution technique and cross-validated against two independent methods: the ¹5N isotope dilution approach for multiple crops (Peoples et al., 1996) and the ureide-based %Ndfa estimation method (Alves et al., 2000). Both methods demonstrated a strong correlation. Ndfa was calculated using calibration Equation 2.
Yield and NUE
At maturity stage (R8), 10 representative soybean plants were selected per treatment with 3 replicates, and the number of pods per plant, number of seeds per plant, and 100-seed weight were recorded. For yield determination, a 6.4 m2 (1.6 m×4 m) area was harvested with three replications. Harvested seeds were air-dried, weighed, and plot yield was converted to standard moisture content of 13%. NUE was calculated based on yield data as follows:
Data analysis
An analysis of variance (ANOVA) was employed to assess the effects of N treatments across different years and growth stages on enzyme activities, ureide content, sucrose, and starch. A two-way ANOVA was conducted to evaluate the interactive effects of year and N application rate on nodule number, nodule dry weight, nitrogenase activity, leghemoglobin content, and yield. Data are presented as mean. Post hoc comparisons were carried out with LSD. Duncan’s multiple range test and Pearson correlation analyses were implemented in SPSS 26.0 (SPSS Inc., Chicago, IL, USA). Structural equation modeling (SEM) was fitted via the graphical interface of IBM SPSS AMOS 26 to quantify the influence of SNF indicators on %Ndfa.
Results
Nodules parameters
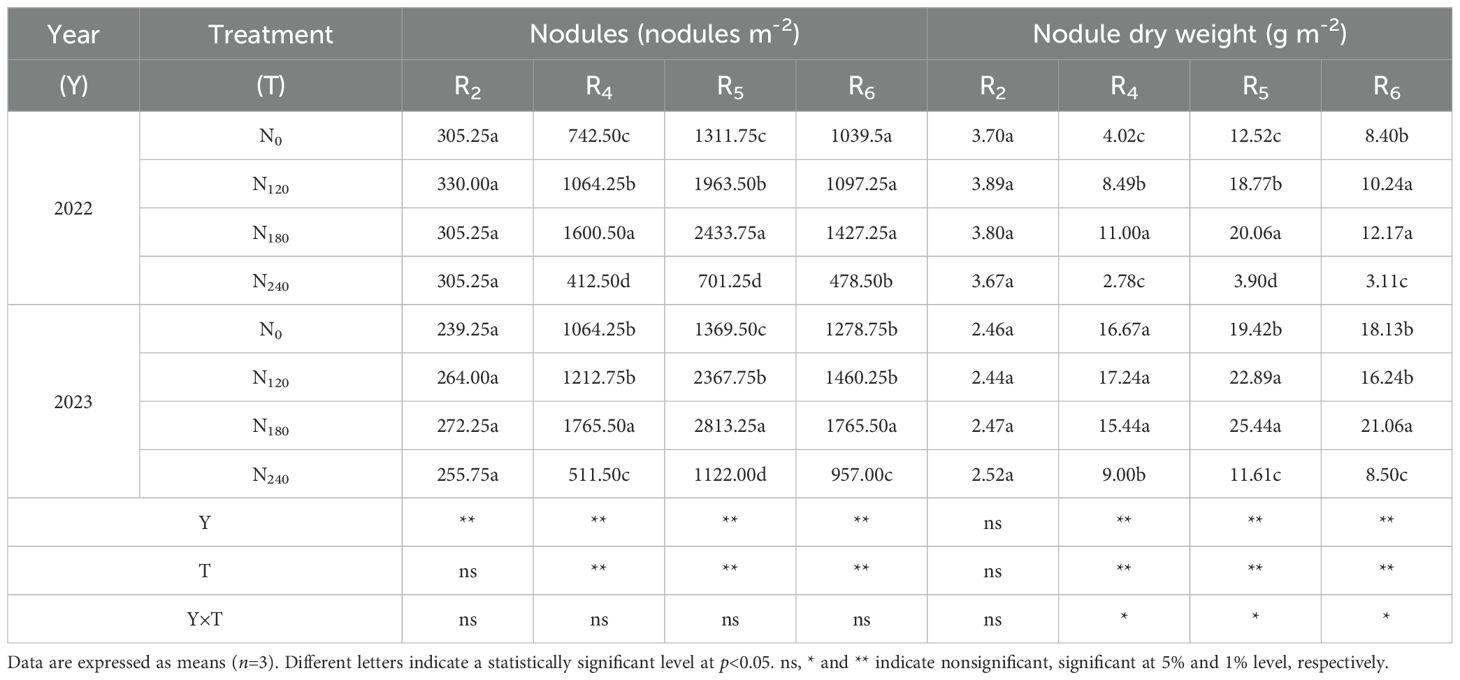
The analysis of variance (Table 1) revealed that, from the R4 to R6 stages, both year and N application rate significantly affected nodule number and nodule dry weight (The significance level for the following data was set at p<0.05), and their interaction significantly influenced nodule dry weight. Across the 2022 and 2023 growing seasons, nodule number and dry weight initially increased and then decreased with advancing growth stages, peaking at the R5 stage before declining. The N240 treatment exhibited the most pronounced reduction, with decreases of 41.23% and 37.25% in nodule number and dry weight, respectively. From R4 to R6, nodule number and dry weight displayed a unimodal response to increasing N rates, reaching maximum values under the N180 treatment. Compared to N180, the N240 treatment reduced nodule number by 72.57%, 65.66%, and 56.14% at R4, R5, and R6 stages, respectively, and decreased nodule dry weight by 58.22%, 67.46%, and 67.05%.
Nodule nitrogenase activity and leghemoglobin
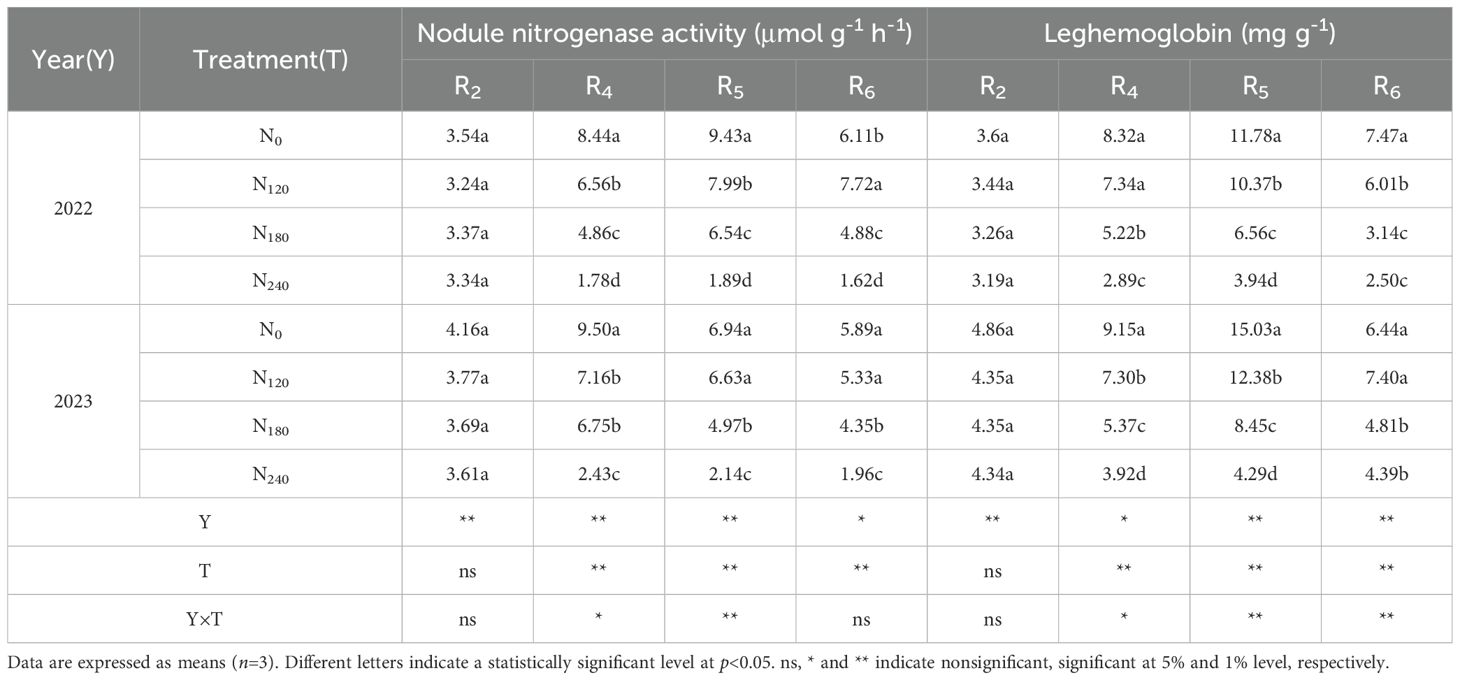
The ANOVA results (Table 2) indicated that, from the R4 to R6 stages, year, N application rate, and their interaction significantly influenced nodule nitrogenase activity and leghemoglobin content. Across the 2022 and 2023 growing seasons, nitrogenase activity and leghemoglobin content in all treatments initially increased and then decreased with advancing growth stages, peaking at the R5 stage. At the R4 and R5 stages, nitrogenase activity and leghemoglobin content gradually decreased with increasing N application. Compared to other treatments, the N240 treatment reduced nitrogenase activity by 63.37%∼78.91% and 56.94%∼9.96%, respectively, and leghemoglobin content by 35.82%∼61.21% and 44.74%∼63.69%. At the R6 stage, nitrogenase activity and leghemoglobin content in the N240 treatment decreased by 66.72%∼376.54% and 9.57%∼140.40%, respectively.
Enzymes involved in nitrogen metabolism of nodules
As shown in Figure 2, GS, GOGAT, and GDH activities in nodules exhibited a unimodal trend during both the 2022 and 2023 growing seasons, peaking at the R5 stage before declining. Increased N application at the R4 and R5 stages significantly influenced GS and GOGAT activities, whereas GDH activity remained unaffected. Similar trends were observed between the two growing seasons.
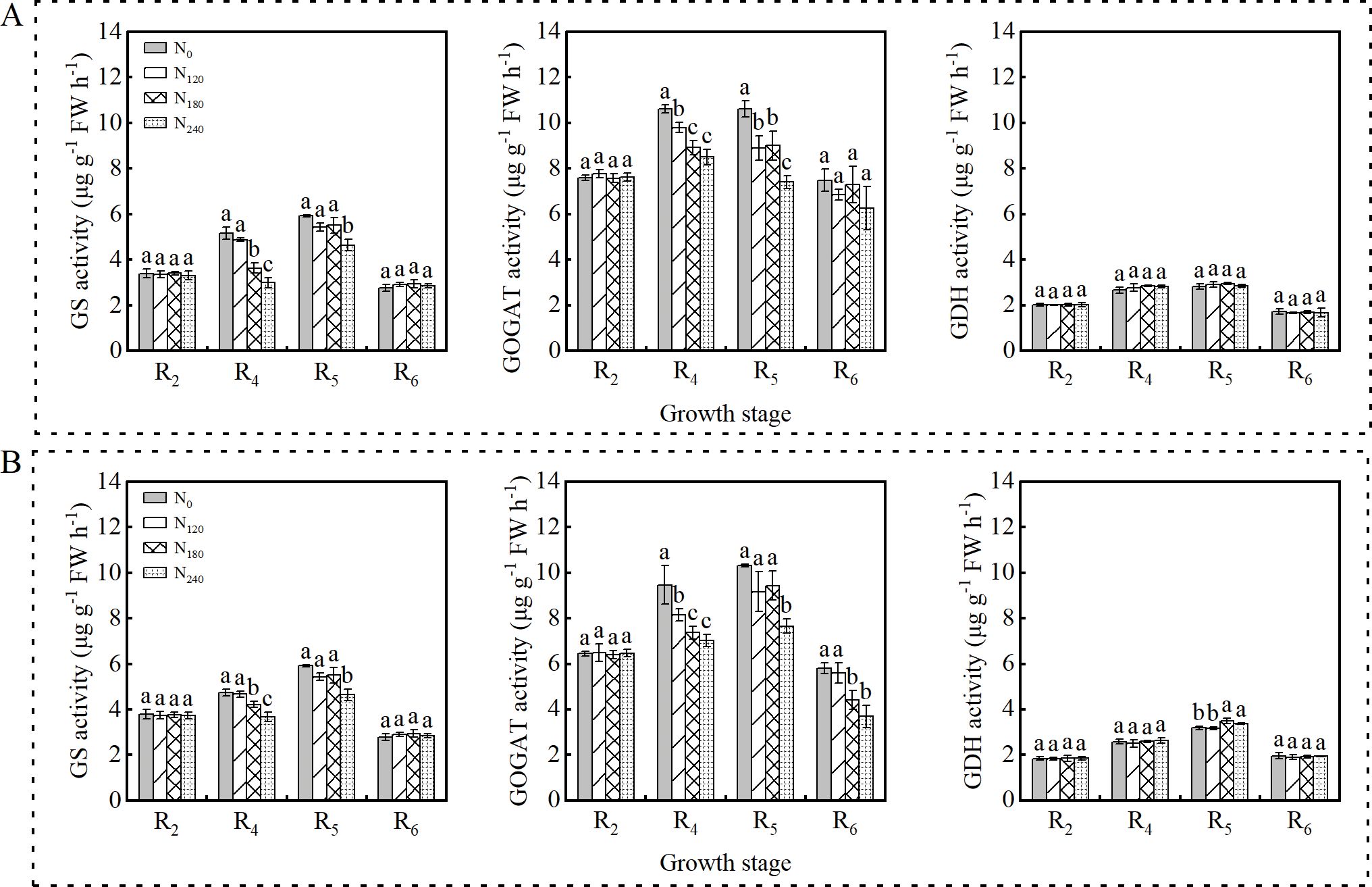
Figure 2. Effects of nitrogen application on nitrogen metabolism enzyme activities in nodules in 2022 (A) and 2023 (B). Bars represent means and error bars standard error (n=3). Different letters represent significant differences (p<0.05) between treatments at the same growth stage.
Urate oxidase activity of nodule
As shown in Figure 3, during both 2022 and 2023 growing seasons, UO activity in nodules first increased and then decreased with advancing growth stages, peaking at the R5 stage. UO activity in the N240 treatment was significantly reduced by 5.68%∼27.14% and 8.01%∼25.68% compared to other treatments in 2022 and 2023, respectively. Increasing N supply significantly decreased UO activity, following the order N0>N120>N180>N240.
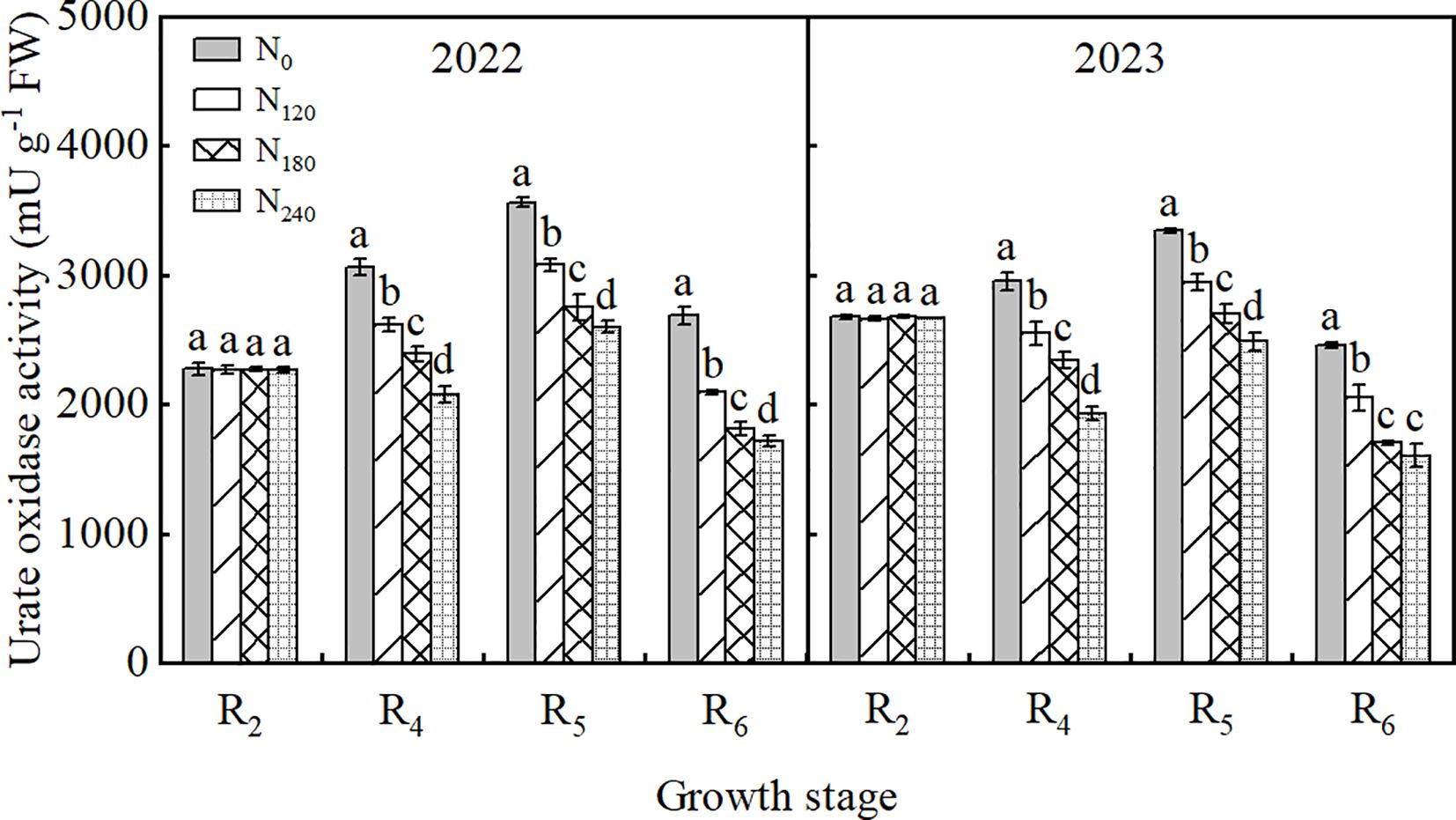
Figure 3. Effect of nitrogen application on the activities of urate oxidase in soybean nodules in 2022 and 2023. Bars represent means and error bars standard error (n=3). Different letters represent significant differences (p<0.05) between treatments at the same growth stage.
Ureide content
As shown in Figure 4A, during both 2022 and 2023 growing seasons, ureide contents in nodules and stems first increased and then decreased with advancing growth stages, peaking at R5 and R6 stages, respectively. Nodule ureide content in the N240 treatment was significantly higher by 3.00%∼24.77% compared to other treatments, while stem ureide content was reduced by 29.15%∼72.31%. Increasing N application promoted ureide accumulation in nodules but showed the opposite trend in stems.
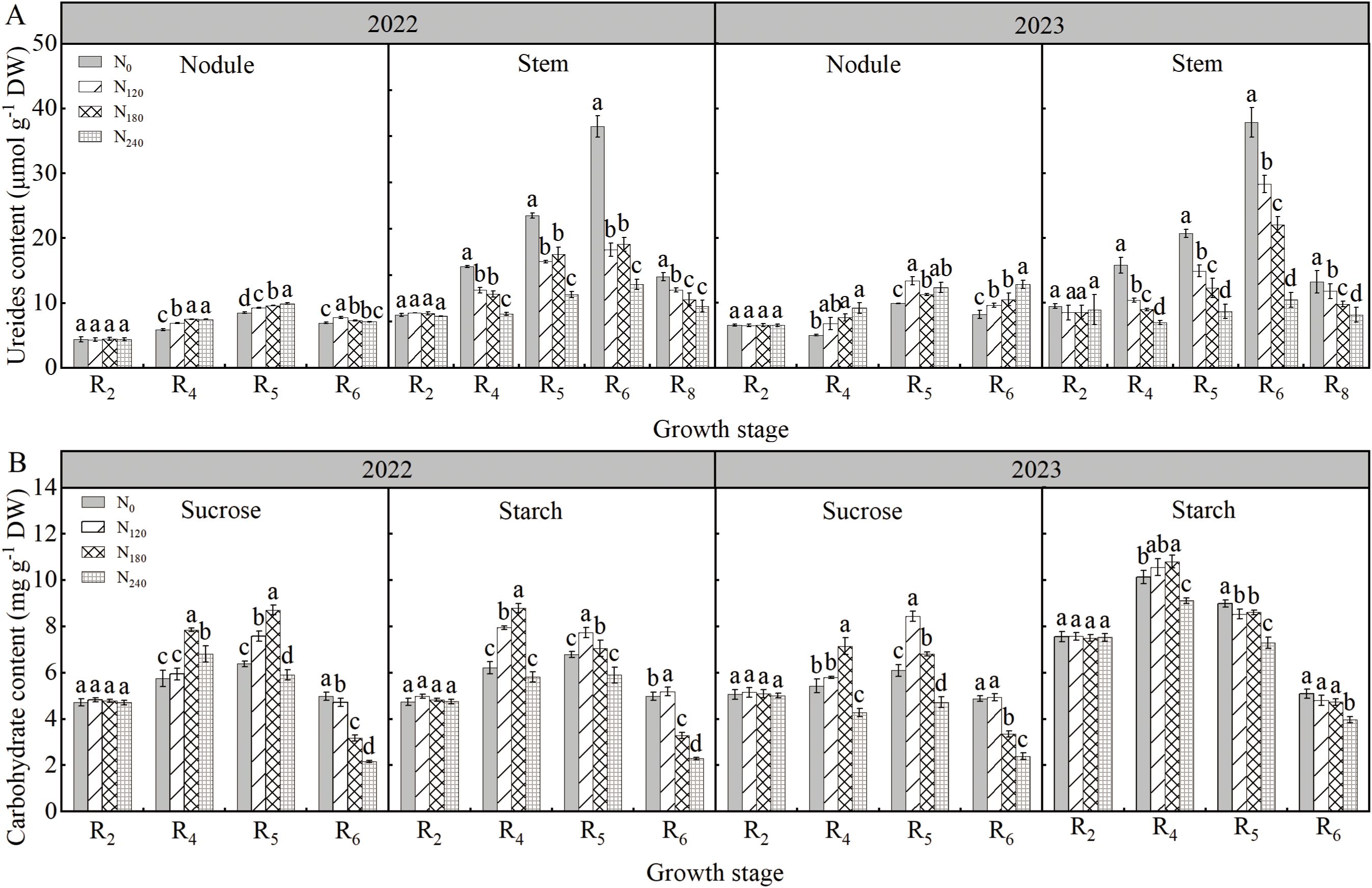
Figure 4. Effects of nitrogen application on ureide content (A) and nodule carbohydrate content (B) in 2022 and 2023. Bars represent means and error bars standard error (n=3). Different letters represent significant differences (p<0.05) between treatments at the same growth stage.
Sucrose and starch content of nodules
As shown in Figure 4B, during both 2022 and 2023 growing seasons, sucrose and starch contents in nodules first increased and then decreased with advancing growth stages, peaking at the R5 and R4 stages, respectively. At the R4 stage across both years, nodule sucrose content was highest under the N180 treatment, increasing by 15.26%∼36.30% and 23.17%∼31.62% compared to other N treatments, respectively. Nodule starch content under N180 increased by 10.59%∼41.39% and 2.30%∼18.59% compared to other N treatments in the two years.
Grain yield and NUE
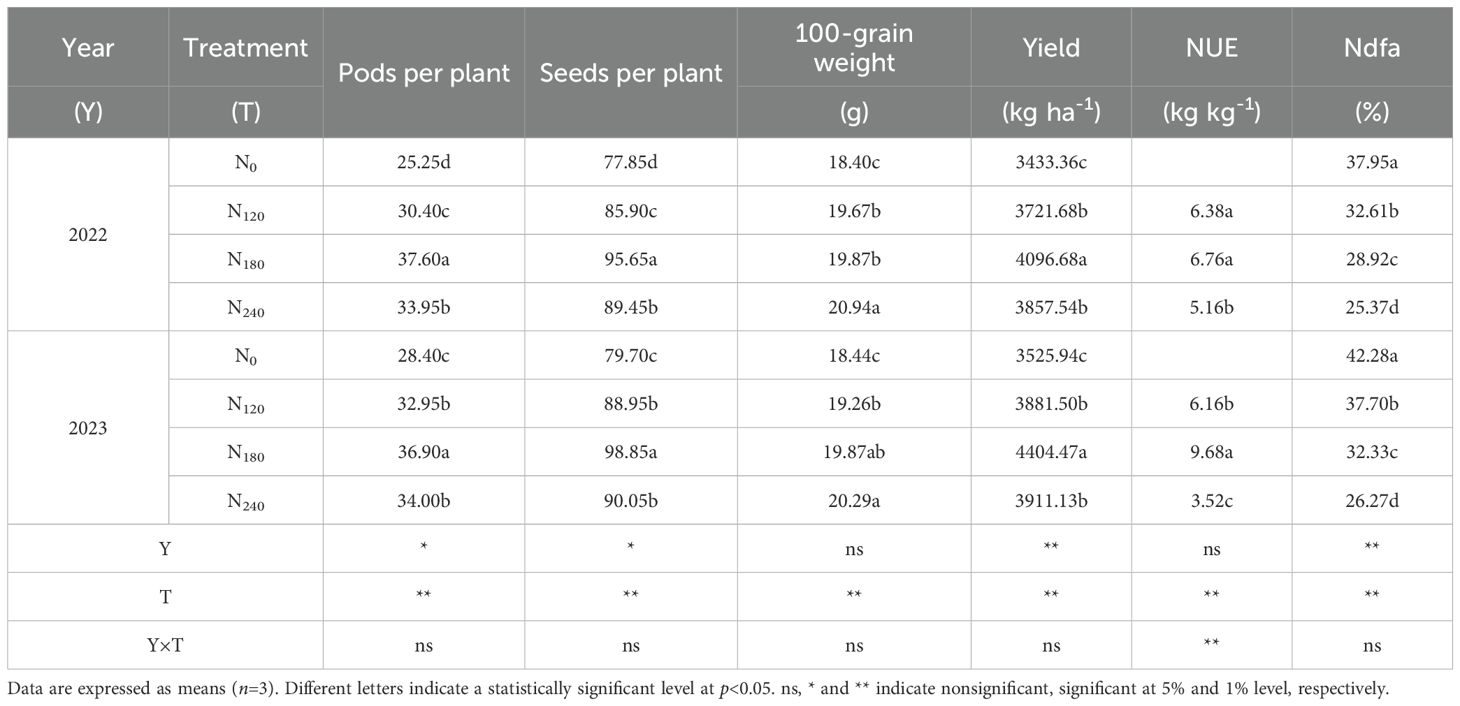
ANOVA results (Table 3) indicated that year and N application rate significantly affected pod number per plant, seed number per plant, yield, and %Ndfa. In 2022, N treatments increased pod number per plant by 20.40%∼34.46%, seed number per plant by 10.34%∼22.86%, and 100-seed weight by 6.90%∼13.80% compared to N0, with consistent trends observed in 2023. The N180 achieved the highest yield across both years, increasing by 6.20%∼19.23% and 12.61%∼24.92% compared to other treatments. N180 significantly improved NUE, with NUE increases of 5.96% and 57.14% over N120 and N240 in 2022, and 31.26% and 175% in 2023. %Ndfa gradually decreased with increasing N application, with reductions of 14.07%∼33.16% and 10.83%∼37.87% in 2022 and 2023, respectively, compared to N0.
The relationship between the parameters of SNF in soybean and %Ndfa
As shown in Figure 5, nodule nitrogenase activity, leghemoglobin content, UO activity, and stem ureide content all exhibited significant positive correlations with %Ndfa. Conversely, nodule ureide content showed a significant negative correlation with %Ndfa. Structural equation modeling (SEM) was used to evaluate the effects of SNF indicators on %Ndfa. UO activity significantly negatively influenced nodule ureide content, with a path coefficient of -0.93 (Figure 6). Nodule ureide content significantly negatively affected stem ureide content. Stem ureide content exerted a highly significant positive effect on %Ndfa, with a path coefficient of 0.95.
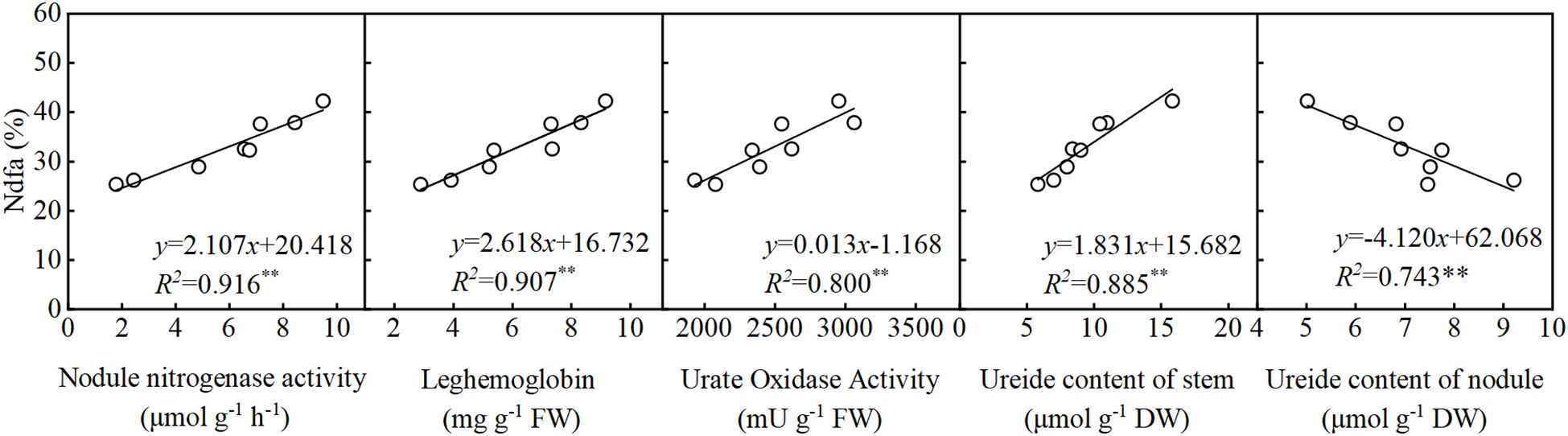
Figure 5. The relationship between the parameters of symbiotic nitrogen fixation in soybean and %Ndfa at the full pod stage (R4).
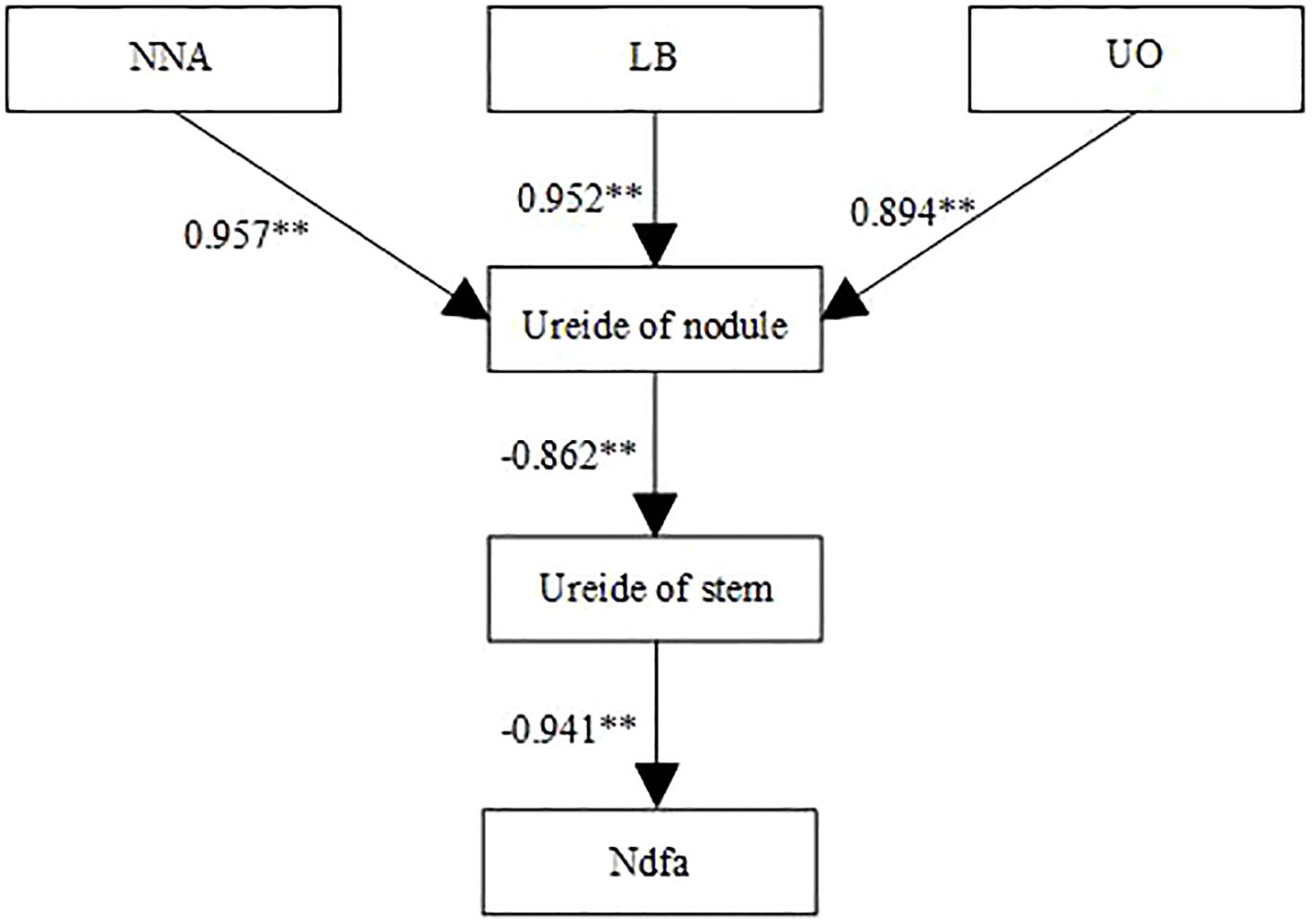
Figure 6. Structural equation modeling (SEM) of the relationships between symbiotic nitrogen fixation traits and nitrogen derived from atmosphere (%Ndfa) at R4 stage in 2022 and 2023. Solid and dashed arrows indicate significant and nonsignificant path coefficients, respectively. NNA, Nodule nitrogenase activity, LB, Leghemoglobin content, UO: Urate oxidase activity. Values above arrows represent standardized path coefficients. ** denote significance at the 0.01 probability levels.
Discussions
In well-managed soybean fields, SNF can supply 70∼85% of the crop’s N demand (Alves et al., 2003). Accumulating evidence from numerous studies indicates that high levels of N in the soil strongly inhibit rhizobial infection efficiency, nodule organogenesis, and nitrogen fixation efficiency, manifesting as reduced nodule number, decreased nodule biomass, inhibited nitrogenase activity, and accelerated nodule senescence or degradation (Pampana et al., 2018; Carter and Tegeder, 2016; Albornoz, 2016; Wang et al., 2015). Conversely, low levels of N enhance nodulation and nitrogen fixation activity (Ke et al., 2023). Given evidence that R3 stage N application significantly impacts grain yield (Mourtzinis et al., 2018; Zapata et al., 1987), this study applied N treatments at R3 stage, resulting in no significant differences in nodule morpho-physiological traits across treatments at the R2 stage. In our experiment, the application of 180 kg ha-1 nitrogen increased nodule biomass and number, while the application of N at rates exceeding 180 kg ha-1 caused significant declines in both parameters. These findings partially contrast with those of Gan et al. (2002) and Nanjareddy et al. (2014), likely due to the lower initial soil N levels in our experimental plots, where N was not the primary limiting factor for nodulation. While nodulation is foundational to SNF, high nodule numbers do not necessarily correlate with high nitrogen fixation capacity (Herridge and Rose, 2000; Song et al., 1995). Extensive research demonstrates that nitrogenase activity strongly links to SNF efficiency and yield, with elevated soil mineral suppressing nitrogenase activity and leghemoglobin content (Liu et al., 2022; Lyu et al., 2020). Our results corroborate this: higher soil N concentrations reduced nodule nitrogenase activity, likely due to diminished leghemoglobin content, which compromises oxygen regulation in infected nodule cells. Since nitrogenase is highly oxygen-sensitive, this dysregulation leads to marked declines in enzymatic activity (Larrainzar et al., 2020; Kawashima et al., 2001). Consistently, our data confirm that N fertilization reduces nodule leghemoglobin content.
Nitrogen availability significantly impacts ammonium assimilation in soybean nodules: elevated soil N enhances GS and GOGAT activities in roots but suppresses these enzymes in nodules, particularly GOGAT (Sawhney et al., 1985). This study also reached the same conclusion, increased N application reduced both GS and GOGAT activities, with GOGAT showing greater sensitivity, consistent with Do Amarante et al. (2022).To prevent excessive ammonium accumulation from inhibiting plant growth, the GDH pathway is activated to promote ammonium assimilation. In this study, nodule GDH activity increased with higher N application. Although GDH activity did not differ significantly among treatments in 2022, these trends still highlight the critical role of GDH in reducing NH3 toxicity in nodules, suggesting a potential shift in ammonium assimilation from the GS/GOGAT pathway to the GDH pathway. While high soil N concentrations transiently affect the nodule GDH pathway, this effect is not persistent. As plants absorb soil N, the inhibitory effect on the GDH pathway is alleviated, likely due to nonsignificant differences in GDH activity among treatments at R6 stage. This phenomenon may also be attributed to accelerated root and nodule senescence under low N conditions during the late growth stages (Nishida and Suzaki, 2018).
Besides ammonium assimilation, the synthesis and transport of ureide compounds in nodules also play crucial roles in the nitrogen metabolism of soybean. High N application elevates ureide content in nodules (Yamashita et al., 2019). In this study, under N-free conditions, nodule ureide export to stems and their allocation were significantly increased compared to other treatments, supported by reduced nodule ureide content and elevated stem ureide content. When N was applied, we observed significant reductions in nodule ureide synthase activities (e.g., UO), yet nodule ureide content remained higher than in N-free treatments, with the opposite trend in stems. This indicates that N application impairs ureide synthesis and transport in nodules, leading to ureide accumulation. Accumulated ureides likely exert feedback inhibition on nitrogenase activity, as evidenced by significantly reduced nitrogenase activity under high N conditions, consistent with findings by Serraj et al. (1999) and Gil-Quintana et al. (2013). Nodules act as strong carbon sinks, requiring energy for ureide synthesis. Thus, insufficient carbon transport to nodules limits SNF and related processes (Lu et al., 2022; Parvin et al., 2020). As the primary form of carbon transport, sucrose accumulation in nodules is closely linked to nitrogen fixation and assimilation. In this study, nodule sucrose and starch contents were lower in N-free and high-N treatments compared to other treatments, particularly in high-N plots. Reduced nodule carbon availability may be associated with decreased ureide export (Rawsthorne et al., 1980), resulting in elevated nodule ureide content, suppressed nitrogen fixation, accelerated nodule senescence, and reduced nitrogen use efficiency.
Although low N levels restrict soybean growth, high N application only marginally enhances yield, while excessive N supply reduces both yield and NUE (Thorburn et al., 2024).In this study, soybean yield reached its maximum at a N application rate of 180 kg ha-1, primarily attributed to increased pod and seed numbers. During the reproductive growth stage, reliance solely on SNF cannot fully meet plant N demand, necessitating supplemental N fertilization. Moderate N application compensates for N deficits, balances vegetative and reproductive growth, and provides energy for pod formation and grain filling, while simultaneously maximizing NUE. To evaluate relationships among nodule growth, stem ureide content, and %Ndfa at the R4 stage, correlation and structural equation modeling revealed significant positive correlations between nitrogenase activity, leghemoglobin content, UO activity, stem ureide content, and %Ndfa, consistent with van Berkum et al. (1985). Conversely, nodule ureide content showed a significant negative correlation with %Ndfa, further confirming that high N inhibits ureide translocation from nodules. Nitrogenase activity and leghemoglobin content positively regulated nodule ureide levels, whereas UO activity exhibited negative regulation, suggesting feedback control of UO activity by ureide accumulation. Nodule ureide content inversely influenced stem ureide content, while stem ureide content strongly and positively affected %Ndfa (path coefficient = 0.95). This explains why stem ureide content serves as a reliable proxy for nitrogenase activity, given that ureides account for approximately 80% of total stem N, aligning with findings by Mcclure and Israel (1979).
Conclusion
Achieving simultaneous improvements in NUE and yield stability in future soybean production represents a core challenge for sustainable agriculture. Across the 2022 and 2023 growing seasons, our data demonstrate that under mulched drip irrigation, the application of 180 kg ha-1 nitrogen significantly enhanced root nodulation capacity (95.5% increase in nodule number and 45.6% increase in nodule dry weight at the R5 stage) and optimized nodule nitrogenase activity (5.8 µmol g-1 h-1 at the R5 stage) and leghemoglobin synthesis (7.5 mg g-1 at the R5 stage). These improvements elevated NUE to 8.2 kg kg-1 and increased yield by 22.1%. However, the application of N exceeding 180 kg ha-1 suppressed SNF (35.5% reduction in SNF) by impairing root nodulation capacity, inhibiting the GS/GOGAT metabolic pathway, and reducing ureide compound translocation from nodules to stems, leading to significant ureide accumulation in nodules. SEM revealed the stem ureide content exhibited a strong direct positive effect on %Ndfa (path coefficient=0.95), establishing stem ureide content as an effective physiological indicator for evaluating SNF capacity. Given the strong dependence of soybean nodule nitrogen fixation capacity on climatic conditions and agronomic practices, future research should focus on understanding interactions between SNF and environmental conditions to optimize adaptability, thereby achieving synergistic improvements in yield and NUE for future cultivars.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YX: Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. QG: Investigation, Methodology, Writing – review & editing. JZ: Supervision, Writing – review & editing. LX: Resources, Writing – review & editing. CW: Methodology, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Major Science and Technology Special Project of Xinjiang Uygur Autonomous Region (2022A02008-2); National Natural Science Funds of China (grant number 32160520); Xinjiang Tianchi Talent Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1604251/full#supplementary-material
References
Albornoz, F. (2016). Crop responses to nitrogen overfertilization: A review. Sci. Horticult. 205, 79–83. doi: 10.1016/j.scienta.2016.04.026
Alves, B. J. R., Boddey, R. M., and Urquiaga, S. (2003). The success of BNF in soybean in Brazil. Plant Soil 252, 1–9. doi: 10.1023/A:1024191913296
Alves, B. J. R., Rezende, C., de, P., Resende, A. S., Macedo, R., Tarre, M., et al. (2000). Estimation of N2 fixation in Desmodium ovalifolium from the relative ureide abundance of stem solutes: Comparison with the 15N-dilution and an in situ soil core technique. Nutr. Cycl. Agroecosyst. 56, 177–193. doi: 10.1023/A:1009831705819
Atkins, C. A. and Smith, P. M. C. (2007). Translocation in legumes: assimilates, nutrients, and signaling molecules1. Plant Physiol. 144, 550–561. doi: 10.1104/pp.107.098046
Bodirsky, B. L., Popp, A., Lotze-Campen, H., Dietrich, J. P., Rolinski, S., Weindl, I., et al. (2014). Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nat. Commun. 5, 3858. doi: 10.1038/ncomms4858
Britto, D. T. and Kronzucker, H. J. (2002). NH4+ toxicity in higher plants: a critical review. J. Plant Physiol. 159, 567–584. doi: 10.1078/0176-1617-0774
Bulen, W. A. (1956). The isolation and characterization of glutamic dehydrogenase from corn leaves. Arch. Biochem. Biophys. 62, 173–183. doi: 10.1016/0003-9861(56)90100-X
Burias, N. and Planchon, C. (1990). Increasing soybean productivity through selection for nitrogen fixation. Agron. J. 82, 1031–1034. doi: 10.2134/agronj1990.00021962008200060001x
Camargo, J. A., Alonso, A., and Salamanca, A. (2005). Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates. Chemosphere 58, 1255–1267. doi: 10.1016/j.chemosphere.2004.10.044
Carter, A. M. and Tegeder, M. (2016). Increasing nitrogen fixation and seed development in soybean requires complex adjustments of nodule nitrogen metabolism and partitioning processes. Curr. Biol. 26, 2044–2051. doi: 10.1016/j.cub.2016.06.003
Castañeda, V., Gil-Quintana, E., Echeverria, A., and González, E. (2018). “Legume nitrogen utilization under drought stress,” in Engineering Nitrogen Utilization in Crop Plants. Eds. Shrawat, A., Zayed, A., and Lightfoot, D. A. (Springer International Publishing, Cham), 173–184. doi: 10.1007/978-3-319-92958-3_10
Cataldo, D. A., Maroon, M., Schrader, L. E., and Youngs, V. L. (1975). Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 6, 71–80. doi: 10.1080/00103627509366547
Coba de la Peña, T., Fedorova, E., Pueyo, J. J., and Lucas, M. M. (2018). The symbiosome: legume and rhizobia co-evolution toward a nitrogen-fixing organelle? Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02229
David, K. A. V., Apte, S. K., Banerji, A., and Thomas, J. (1980). Acetylene reduction assay for nitrogenase activity: gas chromatographic determination of ethylene per sample in less than one minute. Appl. Environ. Microbiol. 39, 1078–1080. doi: 10.1128/aem.39.5.1078-1080.1980
Do Amarante, L., Lima, J. D., and Sodek, L. (2022). Alterations of xylem transport of key metabolic products of assimilatory activity in soybean: do similar alterations occur in roots and nodules? Acta Physiol. Plant 44, 11. doi: 10.1007/s11738-021-03345-8
Engvild, K. C. (1987). Nodulation and nitrogen fixation mutants of pea, Pisum sativum. Theor. Appl. Genet. 74, 711–713. doi: 10.1007/BF00247546
Ferguson, B. J., Mens, C., Hastwell, A. H., Zhang, M., Su, H., Jones, C. H., et al. (2019). Legume nodulation: The host controls the party. Plant Cell Environ. 42, 41–51. doi: 10.1111/pce.13348
Gan, Y., Stulen, I., Posthumus, F., and van Keulen, H. (2002). Effects of N management on growth, N2 fixation and yield of soybean. Nutr. Cycl. Agroecosyst. 62, 163–174. doi: 10.1023/A:1015528132642
Gil-Quintana, E., Larrainzar, E., Seminario, A., Díaz-Leal, J. L., Alamillo, J. M., Pineda, M., et al. (2013). Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. J. Exp. Bot. 64, 2171–2182. doi: 10.1093/jxb/ert074
Gourion, B., Berrabah, F., Ratet, P., and Stacey, G. (2015). Rhizobium–legume symbioses: the crucial role of plant immunity. Trends Plant Sci. 20, 186–194. doi: 10.1016/j.tplants.2014.11.008
Goyal, R. K., Mattoo, A. K., and Schmidt, M. A. (2021). Rhizobial–host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.669404
Groat, R. G. and Vance, C. P. (1981). Root Nodule Enzymes of Ammonia Assimilation in Alfalfa (Medicago sativa L.): Developmental patterns and response to applied nitrogen. Plant Physiol. 67, 1198–1203. doi: 10.1104/pp.67.6.1198
Herridge, D. F. and Peoples, M. B. (1990). Ureide assay for measuring nitrogen fixation by nodulated soybean calibrated by 15N methods. Plant Physiol. 93, 495–503. doi: 10.1104/pp.93.2.495
Herridge, D. and Rose, I. (2000). Breeding for enhanced nitrogen fixation in crop legumes. Field Crops Res. 65, 229–248. doi: 10.1016/S0378-4290(99)00089-1
Kawashima, K., Suganuma, N., Tamaoki, M., and Kouchi, H. (2001). Two types of pea leghemoglobin genes showing different O2-binding affinities and distinct patterns of spatial expression in nodules. Plant Physiol. 125, 641–651. doi: 10.1104/pp.125.2.641
Ke, X., Xiao, H., Peng, Y., Xia, X., and Wang, X. (2023). Nitrogen deficiency modulates carbon allocation to promote nodule nitrogen fixation capacity in soybean. Exploration 4, 20230104. doi: 10.1002/EXP.20230104
Kurdali, F. (1996). Nitrogen and phosphorus assimilation, mobilization and partitioning in rainfed chickpea (Cicer arietinum L.). Field Crops Res. 47, 81–92. doi: 10.1016/0378-4290(96)00034-2
Larrainzar, E., Villar, I., Rubio, M. C., Pérez-Rontomé, C., Huertas, R., Sato, S., et al. (2020). Hemoglobins in the legume–Rhizobium symbiosis. New Phytol. 228, 472–484. doi: 10.1111/nph.16673
Liu, X., Hu, B., and Chu, C. (2022). Nitrogen assimilation in plants: current status and future prospects. J. Genet. Genom. 49, 394–404. doi: 10.1016/j.jgg.2021.12.006
Liu, Y., Wang, H., Jiang, Z., Wang, W., Xu, R., Wang, Q., et al. (2021). Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 590, 600–605. doi: 10.1038/s41586-020-03091-w
Lu, M. Z., Carter, A. M., and Tegeder, M. (2022). Altering ureide transport in nodulated soybean results in whole-plant adjustments of metabolism, assimilate partitioning, and sink strength. J. Plant Physiol. 269, 1–12. doi: 10.1016/j.jplph.2021.153613
Lyu, X., Li, M., Li, X., Li, S., Yan, C., Ma, C., et al. (2020). Assessing the systematic effects of the concentration of nitrogen supplied to dual-root systems of soybean plants on nodulation and nitrogen fixation. Agronomy 10, 763. doi: 10.3390/agronomy10060763
Mastrodomenico, A. T. and Purcell, L. C. (2012). Soybean nitrogen fixation and nitrogen remobilization during reproductive development. Crop Sci. 52, 1281–1289. doi: 10.2135/cropsci2011.08.0414
Mcclure, P. R. and Israel, D. W. (1979). Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 64, 411–416. doi: 10.1104/pp.64.3.411
Mendes, L. W., Raaijmakers, J. M., De Hollander, M., Mendes, R., and Tsai, S. M. (2018). Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J. 12, 212–224. doi: 10.1038/ismej.2017.158
Mourtzinis, S., Kaur, G., Orlowski, J. M., Shapiro, C. A., Lee, C. D., Wortmann, C., et al. (2018). Soybean response to nitrogen application across the United States: A synthesis-analysis. Field Crops Res. 215, 74–82. doi: 10.1016/j.fcr.2017.09.035
Nanjareddy, K., Blanco, L., Arthikala, M., Affantrange, X. A., Sánchez, F., and Lara, M. (2014). Nitrate regulates rhizobial and mycorrhizal symbiosis in common bean (Phaseolus vulgaris L.). JIPB 56, 281–298. doi: 10.1111/jipb.12156
Nazari, M., Mostajeran, A., and Zarinkamar, F. (2019). Strong effect of recovery period between hypoxia events on roots of chickpea (Cicer arietinum L). Rhizosphere 11, 100163. doi: 10.1016/j.rhisph.2019.100163
Nguyen, H. P., Miwa, H., Obirih-Opareh, J., Suzaki, T., Yasuda, M., and Okazaki, S. (2020). Novel rhizobia exhibit superior nodulation and biological nitrogen fixation even under high nitrate concentrations. FEMS Microbiol. Ecol. 96, fiz184. doi: 10.1093/femsec/fiz184
Nishida, H. and Suzaki, T. (2018). Nitrate-mediated control of root nodule symbiosis. Curr. Opin. Plant Biol. 44, 129–136. doi: 10.1016/j.pbi.2018.04.006
O’Neal, D. and Joy, K. W. (1974). Glutamine synthetase of pea leaves. Plant Physiol. 54, 773–779. doi: 10.1104/pp.54.5.773
Pampana, S., Masoni, A., Mariotti, M., Ercoli, L., and Arduini, I. (2018). Nitrogen fixation of grain legumes differs in response to nitrogen fertilization. Ex. Agric. 54, 66–82. doi: 10.1017/S0014479716000685
Parvin, S., Uddin, S., Tausz-Posch, S., Armstrong, R., and Tausz, M. (2020). Carbon sink strength of nodules but no other organs modulates photosynthesis of faba bean (Vicia faba) grown under elevated [CO2] and different water supply. New Phytol. 227, 132–145. doi: 10.1111/nph.16520
Peoples, M. B., Palmer, B., Lilley, D. M., Duc, L. M., and Herridge, D. F. (1996). Application of 15N and xylem ureide methods for assessing N2 fixation of three shrub legumes periodically pruned for forage. Plant Soil 182, 125–137. doi: 10.1007/BF00011001
Pineda, M., Fernández, E., and Cárdenas, J. (1984). Urate oxidase of Chlamydomonas reinhardii. Physiol. Plant 62, 453–457. doi: 10.1111/j.1399-3054.1984.tb04602.x
Rawsthorne, S., Minchin, F. R., Summerfield, R. J., Cookson, C., and Coombs, J. (1980). Carbon and nitrogen metabolism in legume root nodules. Phytochemistry 19, 341–355. doi: 10.1016/0031-9422(80)83181-5
Salvagiotti, F., Cassman, K. G., Specht, J. E., Walters, D. T., Weiss, A., and Dobermann, A. (2008). Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 108, 1–13. doi: 10.1016/j.fcr.2008.03.001
Santachiara, G., Salvagiotti, F., Gerde, J. A., and Rotundo, J. L. (2018). Does biological nitrogen fixation modify soybean nitrogen dilution curves? Field Crops Res. 223, 171–178. doi: 10.1016/j.fcr.2018.04.001
Sawhney, V., Amarjit, and Singh, R. (1985). Effect of applied nitrate on enzymes of ammonia assimilation in nodules of Cicer arietinum L. Plant Soil 86, 241–248. doi: 10.1007/BF02182899
Serraj, R., Vadez, V., Denison, R. F., and Sinclair, T. R. (1999). Involvement of ureides in nitrogen fixation inhibition in soybean1. Plant Physiol. 119, 289–296. doi: 10.1104/pp.119.1.289
Smagghe, B. J., Hoy, J. A., Percifield, R., Kundu, S., Hargrove, M. S., Sarath, G., et al. (2009). Review: Correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers 91, 1083–1096. doi: 10.1002/bip.21256
Smith, P. M. and Atkins, C. A. (2002). Purine biosynthesis. Big in cell division, even bigger in nitrogen assimilation. Plant Physiol. 128, 793–802. doi: 10.1104/pp.010912
Song, L., Carroll, B. J., Gresshoff, P. M., Herridge, D. F., and Box, P. O. (1995). Field assessment of supernodulating genotypes of soybean for yield, N2 fixation and benefit to subsequent crops. Soil Biol. Biochem. 27, 563–569. doi: 10.1016/0038-0717(95)98632-X
Sprent, J. I. and Thomas, R. J. (1984). Nitrogen nutrition of seedling grain legumes: some taxonomic, morphological and physiological constraints. Plant Cell Environ. 7, 637–645. doi: 10.1111/1365-3040.ep11571523
Thorburn, P. J., Biggs, J. S., Puntel, L. A., Sawyer, J. E., Everingham, Y. L., and Archontoulis, S. V. (2024). The nitrogen fertilizer conundrum: why is yield a poor determinant of crops’ nitrogen fertilizer requirements? Agron. Sustain. Dev. 44, 18. doi: 10.1007/s13593-024-00955-7
van Berkum, P., Sloger, C., Weber, D. F., Cregan, P. B., and Keyser, H. H. (1985). Relationship between ureide N and N 2 fixation, aboveground n accumulation, acetylene reduction, and nodule mass in greenhouse and field studies with Glycine max L. (Merr). Plant Physiol. 77, 53–58. doi: 10.1104/pp.77.1.53
Van Der Drift, C., De Windt, F. E., and Vogels, G. D. (1970). Allantoate hydrolysis by allantoate amidohydrolase. Arch. Biochem. Biophys. 136, 273–279. doi: 10.1016/0003-9861(70)90351-6
Wang, L., Jiang, L., Nomura, M., Tajima, S., and Chen, X. (2015). Excessive ammonia inhibited transcription of MsU2 gene and furthermore affected accumulation distribution of allantoin and amino acids in alfalfa Medicago sativa. J. Integr. Agric. 14, 1269–1282. doi: 10.1016/S2095-3119(14)60908-4
Wang, X., Zhang, Y., Lian, Z., Lyu, X., Yan, C., Yan, S., et al. (2024). Nitrate inhibits nodule nitrogen fixation by accumulating ureide in soybean plants. Plants 13, 2045. doi: 10.3390/plants13152045
Xu, W., Cui, K., Xu, A., Nie, L., Huang, J., and Peng, S. (2015). Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant 37, 9. doi: 10.1007/s11738-014-1760-0
Yamashita, N., Tanabata, S., Ohtake, N., Sueyoshi, K., Sato, T., Higuchi, K., et al. (2019). Effects of different chemical forms of nitrogen on the quick and reversible inhibition of soybean nodule growth and nitrogen fixation activity. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00131
Yaseen, M., Ahmad, A., Naveed, M., Ali, M. A., Shah, S. S. H., Hasnain, M., et al. (2021). Subsurface-applied coated nitrogen fertilizer enhanced wheat production by improving nutrient-use efficiency with less ammonia volatilization. Agronomy 11, 2396. doi: 10.3390/agronomy11122396
Keywords: soybean, nitrogen fertilizer, symbiotic nitrogen fixation, nodulation, high yield
Citation: Xu Y, Gao Q, Xue L, Zhang J and Wang C (2025) Optimized nitrogen fertilizer management enhances soybean (Glycine max (L.) Merril.) yield and nitrogen use efficiency by promoting symbiotic nitrogen fixation capacity. Front. Plant Sci. 16:1604251. doi: 10.3389/fpls.2025.1604251
Received: 01 April 2025; Accepted: 16 June 2025;
Published: 02 July 2025.
Edited by:
Asif Ameen, Ayub Agricultural Research Institute (AARI), PakistanReviewed by:
Shahbaz Khan, Colorado State University, United StatesMuhammad Abrar, Lanzhou University, China
Copyright © 2025 Xu, Gao, Xue, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianxin Zhang, emp4aW40MDFAMTI2LmNvbQ==; Cong Wang, c295YmVhbjIwMjBAMTI2LmNvbQ==
 Yaxin Xu
Yaxin Xu Quantong Gao1
Quantong Gao1 Cong Wang
Cong Wang