- 1Institute for Breeding Research on Agricultural Crops, Julius Kühn Institute (JKI), Federal Research Centre for Cultivated Plants, Sanitz, Germany
- 2Plant Pathology and Crop Protection Division, Georg-August University Goettingen, Goettingen, Germany
- 3KWS LOCHOW GmbH, Bergen, Germany
- 4Nordsaat Saatzucht GmbH, Zuchtstation Granskevitz, Schaprode, Germany
- 5Saatzucht Bauer GmbH & Co. KG, Obertraubling, Germany
Introduction: Fusarium head blight (FHB), caused by various Fusarium species, poses a significant threat to oat grain quality and yield. The presence of multiple Fusarium species raises the question whether FHB resistance in oats can be broadly effective and species non-specific (cross-resistance), or whether it is rather species-specific. While several morphological and biochemical factors are known to influence FHB resistance, the role of hull trichomes in oat resistance remains unclear.
Methods: In this study, 25 oat genotypes were evaluated for resistance to Fusarium graminearum (FG), F. sporotrichioides (FS), and F. poae (FP) in multiple field trials across Germany. Infection severity was quantified using Fusarium species-specific qPCR. Microscopic analyses were conducted to characterize trichome size and density on the lemma and palea.
Results and discussion: Species-specific qPCR showed the highest fungal biomass for FP, followed by FS and FG. Variability due to environmental factors was very high, resulting in rather low heritabilities for FG (0.50) and FS (0.36), and no significant genotype effect for FP. A significant positive correlation was found between FP and FS infection, whereas FG infection was not correlated with either FP or FS. Trichome size and density showed significant genotype-specific variation with high heritability (0.97). FG biomass was positively correlated with trichome size and density, and FG hyphae were observed in close interaction with trichomes and stomata. Our results indicate the presence of partial cross-resistance for FS and FP in addition to mostly species-specific resistance and suggest a role for trichomes in susceptibility to FG. These findings provide important insights for the development of Fusarium-resistant oat varieties while underscoring the complexity of breeding for broad FHB resistance in oats.
1 Introduction
Oats (Avena sativa L.) are well known for their beneficial nutritional properties and positive effects on soil health, but like other small grain cereals such as wheat and barley, they are susceptible to Fusarium head blight disease (FHB) caused by several Fusarium species (Placinta et al., 1999; Bottalico and Perrone, 2002). FHB in oats can lead to yield losses, reduced seed germination and the accumulation of mycotoxins such as deoxynivalenol (DON), nivalenol (NIV), T-2 and HT-2 which are harmful to human and animal health (Scott, 1989; Bjørnstad and Skinnes, 2008; Tekle et al., 2013). The spectrum of Fusarium species on oats and the severity of infection are subject to strong regional and annual variation, depending on the previous crop, tillage practices, the proportion of cereals and maize in the rotation and weather conditions (Bottalico and Perrone, 2002; Hofgaard et al., 2016a; Parikka et al., 2012).
A recent survey of the occurrence of Fusarium species in oats in Germany identified the presence of F. poae, F. tricinctum, F. avenaceum, F. culmorum, F. equiseti, F. graminearum, F. sporotrichioides, F. langsethiae and F. cerealis. The most prevalent species was F. poae, followed by F. tricinctum and F. avenaceum (Rodemann et al., 2023). Similar species occurrences and a dominance of F. poae, F. graminearum, F. avenaceum and F. langsethiae were observed in other studies conducted across Central Europe (Nielsen et al., 2011; Kiecana et al., 2012; Dal Prá et al., 2014; Vanheule et al., 2014; Georgieva et al., 2018; Schöneberg et al., 2018). In Scandinavia, Finland and Canada, F. avenaceum, F. culmorum and F. sporotrichioides were also very common (Fredlund et al., 2013; Hietaniemi et al., 2016; Hofgaard et al., 2016b; Tekauz et al., 2008).
The co-occurrence of several Fusarium species raises the question of whether it is necessary to breed for resistance to each important species individually, or, ideally, whether generally effective, species non-specific FHB resistances are present in oats (i.e. cross-resistance). In wheat and barley, several studies have shown the broad effectiveness of different sources of resistance for different Fusarium species (Akinsanmi et al., 2006; Holzknecht et al., 2009; Mesterházy et al., 1999, 2005; Šíp et al., 2011; Tóth et al., 2008; van Eeuwijk et al., 1995). Nevertheless, there is still a lack of knowledge about the numerous other toxins that occur in addition to DON and that are relevant for consumer and animal protection (Mesterhazy, 2024). Further QTL analyses with segregating populations and inoculation with additional Fusarium species are required to validate the species-non-specific resistance observed in wheat (Mesterhazy, 2024). In oats, the situation regarding the species-specificity of resistance to FHB is not well understood. Some studies indicate that resistance to FHB is rather species-specific in oats (Aamot et al., 2022; Hofgaard et al., 2022; Tekauz et al., 2004). The clearest example is the variety Odal, which is moderately resistant to DON but is very susceptible to T-2/HT-2 accumulation (Hofgaard et al., 2022). However, Herrmann et al. (2020) found a modest but significant correlation between the ranking for T-2/HT-2 and DON contamination and a rather weak correlation between DON and T-2 levels in another unpublished series of experiments on modern variety material. Therefore, it cannot be ruled out that both species-unspecific (cross-resistance) and species-specific resistance factors exist in oats. Further research is therefore required to clarify this issue.
Resistance of small grain cereals against FHB is a complex phenomenon with several passive and active components (Mesterházy, 1995). Active defense responses include reinforcement of cell walls, counteracting oxidative stress or detoxifying mycotoxins, which can be virulence factors in wheat or barley (Lemmens et al., 2005; Spanic et al., 2017; Kheiri et al., 2019; Soni et al., 2021; Khairullina et al., 2022; Bethke et al., 2023). A few passive, morphology-based FHB resistance components have been identified in oats so far, such as plant height, epicuticular wax layer or anther extrusion (He et al., 2013; Loskutov et al., 2017; Hautsalo et al., 2020; Herrmann et al., 2020; Tekle et al., 2020; Willforss et al., 2020). As the primary interface for FHB infection is usually the outer parts of the flower where the Fusarium spores germinate, anatomical features of the hulls, such as trichomes, could also play an important role in infection in oats (Tekle et al., 2012). Trichomes are hair-like structures found on the aerial organs of most terrestrial plants and play different roles in plant development and stress response (Zhang et al., 2021; Han et al., 2022). It has been demonstrated that trichomes represent a preferred entry site for Fusarium hyphae in barley, maize, wheat and Brachypodium (Imboden et al., 2018; Linkmeyer et al., 2013; Peraldi et al., 2011; Sun et al., 2024). Similarly, in several wild Avena species, trichome abundance was positively correlated with DON and Fusarium biomass (Gagkaeva et al., 2017). However, also the opposite, where a higher trichome density was associated with FHB resistance, has been observed (Duba et al., 2019). In oats, the genetic variability of trichome abundance on the hulls and its relationship to FHB resistance has yet to be investigated.
The objectives of this study were (I) to assess resistance to three Fusarium species (F. graminearum, F. sporotrichioides and F. poae) to gain further insight into species-specific and cross-resistance mechanisms and (II) to characterize trichome variations on the surface of the hulls and to investigate their role during Fusarium infection in a panel of mostly modern German oat varieties.
2 Materials and methods
2.1 Plant material
The studied oat panel consisted of 25 spring oat genotypes with similar plant heights and panicle emergence dates to limit the effect of these traits on potential differences in Fusarium resistance. Most genotypes were modern German varieties. Supplementary Table S1 provides a detailed description of the panel.
2.2 Fungal isolates and inoculum production
The fungal isolates used in this study comprised four F. poae, four F. graminearum and two F. sporotrichioides isolates derived from field samples of oats and maize from different sites in Germany and Austria. We selected these three Fusarium species because they were found to be highly prevalent in German oat fields in a previous monitoring project (Georgieva et al., 2018) and because they belong to the main producers of the three important mycotoxins DON (F. graminearum), T-2/HT-2 (F. sporotrichioides) and NIV (F. poae). A detailed description of the fungal isolates is given in Supplementary Table S2. Spawn inoculum was prepared following a protocol adapted from Dr. Bernd Rodemann (Julius Kühn-Institut, Germany) and Tekle et al. (2018). Fungal isolates were grown on potato dextrose agar (PDA) plates at room temperature for approximately 7 days. SacO2 Autoclave Bags (MycoGenetics, Münster, Germany) filled with approximately 1.1 kg of oat kernels and 1 L ddH2O were autoclaved twice. After cooling, the bags were inoculated with small pieces of PDA containing mycelium of each isolate for each Fusarium species separately. The bags were incubated at room temperature for 1–2 weeks until all kernels were colonized. Then, the colonized kernels were spread in trays to allow further fungal development and to dry for 2–3 weeks. After that, the inoculum was collected and stored at – 20°C until inoculation of the field trials. For spray-inoculation, liquid inoculum was prepared from the colonized kernels as described in Linkmeyer et al. (2013). In brief, colonized kernels were incubated in 0.02% Tween20 in tap water for 15 min. The conidia suspension was filtered through filter paper, sedimented overnight and the supernatant was stored at 4°C until usage.
2.3 Field trials
The oat panel was evaluated in three locations across Germany (Groß Lüsewitz (GL; coordinates 54.0714, 12.3238), Wohlde (WL; coordinates 52.8074, 10.0003), Böhnshausen (BH, coordinates 51.8596, 10.9611) in the years 2021 and 2022. The genotypes were sown in plots of 1.8 sqm with a density of 350 kernels/sqm in a randomized complete block design with three replications for each Fusarium species. The trials for the different Fusarium species were planted with at least 9 m distance to avoid cross-contamination. Each plot was spawn inoculated with 50 ml colonized kernels at stem elongation in 2021 (May 28 to June 8) and panicle emergence in 2022 (June 10 and 16). Inoculation was performed at different time points at the sites to account for differences in growth stages. Plots were harvested 134 to 139 days after sowing either by a combine harvester or by hand, cleaned after threshing using the same wind sifter and after cleaning, seeds were analyzed for Fusarium biomass.
2.4 DNA extraction and quantification of fungal biomass
Fungal DNA of F. graminearum, F. sporotrichioides and F. poae was quantified using quantitative real-time PCR (qPCR). Kernels were lyophilized and finely ground to a size of approx. 1 mm using a swing mill (Retsch MM 400, Retsch, Haan, Germany). DNA extraction was performed by using a CTAB-based extraction protocol described previously by Guerra et al. (2020). Quality and quantity of DNA extracts were assessed on agarose gels (0.8% (w/v) in 1x TAE (Tris-acetate-EDTA buffer) stained with Midori Green (Nippon Genetics Europe, Düren, Germany). Before qPCR analysis samples were diluted 1:50 in double-distilled H2O (ddH2O). DNA standards for qPCR assays were obtained from F. poae DSM62376, F. graminearum IFA66 und F. sporotrichioides DSM62423. DNA for quantification standards were extracted using a CTAB-based protocol (Brandfass and Karlovsky, 2008) and quantified by densitometry (Nutz et al., 2011). PCR analysis was carried out using a CFX 384 Thermocycler (Biorad, Rüdigheim, Germany) in 384-well microplates. The reaction was carried out with 1 µL diluted DNA extracts in 4 µL reaction volume. Previously published species-specific primers were used ( Supplementary Table S3, Nicholson et al., 1998; Parry and Nicholson, 1996; Wilson et al., 2004). All final concentrations of the used reagents are given in Supplementary Table S4. All standards and negative controls (ddH2O) were amplified in duplicates. Standard curves were generated from 1:3 serial dilutions of 100 pg/µL fungal DNA. PCR conditions and quantification limits (LOQ) are given in Supplementary Table S5. After amplification, melting curves were generated by increasing temperature from 55°C to 95°C with 0.5°C increase per step and continuous fluorescence measurement (Beule et al., 2019). Samples with concentrations of fungal DNA below the LOQ but with positive melting curves were set to half of the LOQ (Clarke, 1998).
2.5 Analysis of trichome variation
To assess variation in hull trichome size and density, the lemma and palea of the 25 oat genotypes studied for Fusarium cross-resistance were examined. For this, samples from the field in Groß Lüsewitz in 2021 and 2022, as well as samples from plants grown in the greenhouse in 2021 and 2022 were used (= four environments). The lemma and palea from six individual plants per environment were collected at anthesis and fixed in 96 % Ethanol (EtOH). EtOH was replaced by fresh EtOH several times until all chlorophyll had been removed from the tissue. Three images per hull were captured using a Nikon Eclipse 90i fluorescence microscope with the standard series Nikon DAPI filter block (Excitation 375/28 nm, Barrier 460/60 nm). The cell counter plug-in in ImageJ (Vos, 2001; Schneider et al., 2012) was used to count the number of small (< 25 µm), medium (25 – 100 µm) and big (> 100 µm) trichomes on the exterior of the hulls (see Supplementary Figure S2 for example picture). A trichome index was calculated using the following formula: with n.ts: number of small trichomes, n.tm: number of medium trichomes and n.tb: number of big trichomes. Weighing factors were chosen to take trichome size into account.
2.6 Confocal laser-scanning microscopy
To visualize fungal growth on the lemma and palea, oat plants grown in the greenhouse were spray-inoculated with F. graminearum (5x104 conidia per ml) at anthesis as this represents the most susceptible stage for infection (Siou et al., 2014). The oat varieties used were ‘Gabriel’, ‘Contender’ and ‘Max’. Inoculated panicles were covered with clear plastic bags for 48 h. Samples were collected at 24 hpi, 48 hpi and 96 hpi and fixed in 96% EtOH. EtOH was replaced by fresh EtOH several times until all chlorophyll had been removed from the tissue. The samples were stained with Wheat Germ Agglutinin, AF488 Labelled (WGA-AF488, AAT Bioquest, CA, USA), trypan blue (TB, Merck) and aniline blue diammonium salt (AB, Sigma-Aldrich Chemie, Traufkirchen, Germany) as described by Becker et al. (2018) with the following modifications: Before staining, lemma samples were incubated for 3 h and palea samples were incubated for 1 h in 1 M KOH at room temperature. Staining solution contained 0.02% AB, 0.02% TB, 0.0005% WGA-AF488 and 0.02% Tween20 in PBS (pH 7.4). Confocal laser scanning microscopy (CLSM) was done using a Leica TCS SP8 as described by Becker et al. (2018) with the following changes: AB, TB and WGA-AF488 were excited simultaneously with the 405 nm diode, 488 nm argon ion and 561 nm diode-pumped solid-state (DPSS) lasers. Four detectors were used in sequential acquisition mode to capture emission fluorescence from the dyes and plant autofluorescence: HyD1 (380 nm – 414 nm, grey pseudocolor, plant autofluorescence), PMT2 (447 nm – 481 nm, blue pseudocolor, emission from WGA-AF488), HyD3 (593 nm – 626 nm, green pseudocolor, plant autofluorescence and emission from AB) and PMT4 (674 nm – 749 nm, red pseudocolor, plant autofluorescence and emission from AB and TB).
2.7 Data analysis
All statistical data analyses were performed in R (version 4.1.0; R Core Team, 2021). Fusarium DNA contents were square root transformed for statistical analyses. The data curation resulted in an unbalanced data set in terms of the number of replicates and years for the three inocula and genotypes. The homogeneity of variances was tested with the Levenetest function in the library car for factors genotype (GEN) and inoculum (INOC) with original as well as square root transformed data. A REML-based mixed model was then run with replicates and interaction as random effects and other factors as fixed effects using the lmer function in the ‘lme4’ package (version 1.1.35.1; Bates et al., 2015) using the formula
including fixed factors genotype (GEN), environment (ENV = year-site combination), inoculum (INOC), und random factors replicate (REP) and error (e). The resulting mixed model was used for an ANOVA for the fixed effects.
For separated Fusarium species data BLUEs were estimated by fitting a mixed linear model with the ‘lmer’ function of the ‘lme4’ package (version 1.1.35.1; Bates et al., 2015). Genotype effects were set as fixed and environment, environment-genotype interaction and replication effects nested in the environment were set as random. For estimation of variance components, the same model was applied, but with setting genotype effects as random as well. Significance of genotype effects was calculated using the ‘anova’ function (version 4.1.0; R Core Team, 2021). Broad-sense heritability was estimated with the formula according to Becker (2019) with var = variance and “:” denoting the interaction of the terms. Pearson correlation coefficients and respective p values were calculated using the ‘rcorr’ function in the ‘Hmisc’ package (version 4.7.2; Harrell, 2025). Post-hoc testing was done using the ‘dunn_test’ function in the ‘rstatix’ package_with Bonferroni p-value adjustment (Kassambara, 2021). Significance groups were defined using the function ‘multcompLetters’ in the ‘multcompView’ package with p.adj threshold=0.05 (doi: 10.32614/CRAN.package.multcompView).
3 Results
3.1 Oat resistance to different Fusarium species
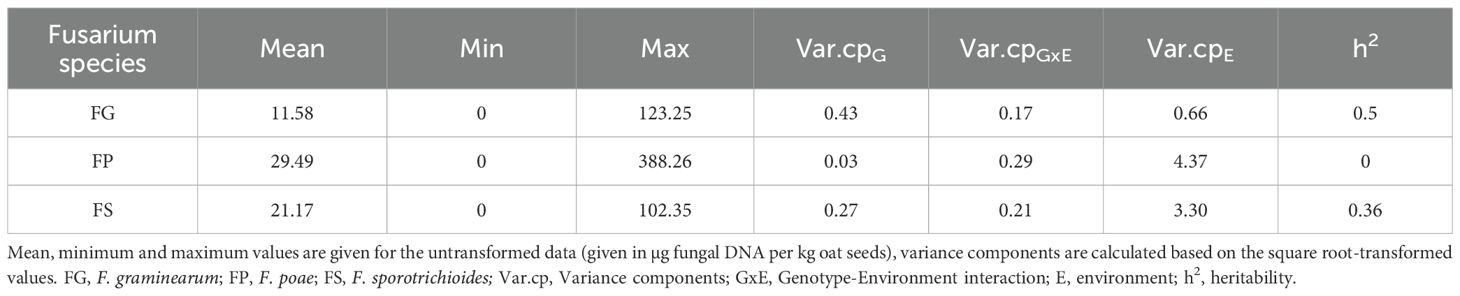
To assess resistance levels against different Fusarium species, field trials using 25 oat genotypes were separately inoculated with F. graminearum (FG), F. sporotrichioides (FS) and F. poae (FP) field isolate mixtures via spawn-inoculation. The trials were performed with three replicates per oat genotype and Fusarium species in three locations across Germany (BH: Böhnshausen, GL: Groß Lüsewitz, WL: Wohlde) in two years (2021, 2022). In oats, macroscopic FHB symptoms are only visible under extremely high infection conditions as in Tekle et al. (2018), which did not occur in any of our experiments. Therefore, we didn’t observe any visual symptoms in our experiments. Infection severity was assessed via quantification of fungal DNA in the harvested oat seeds by species-specific qPCR assays. This showed very high variations across replicates, locations and years and the observed values ranged from 0 to 388 µg fungal DNA per kg of harvested seeds (Figure 1A; Table 1). In 2022, infection levels for FG and FS were extremely low across all locations, and only for FP notable DNA quantities were detected. This might be due to the very dry weather conditions prevalent during infection in 2022 (ufz drought monitor, https://www.ufz.de/index.php?de=47252). Hence, for correlation among Fusarium species and heritability calculation, we only used the data from 2021.
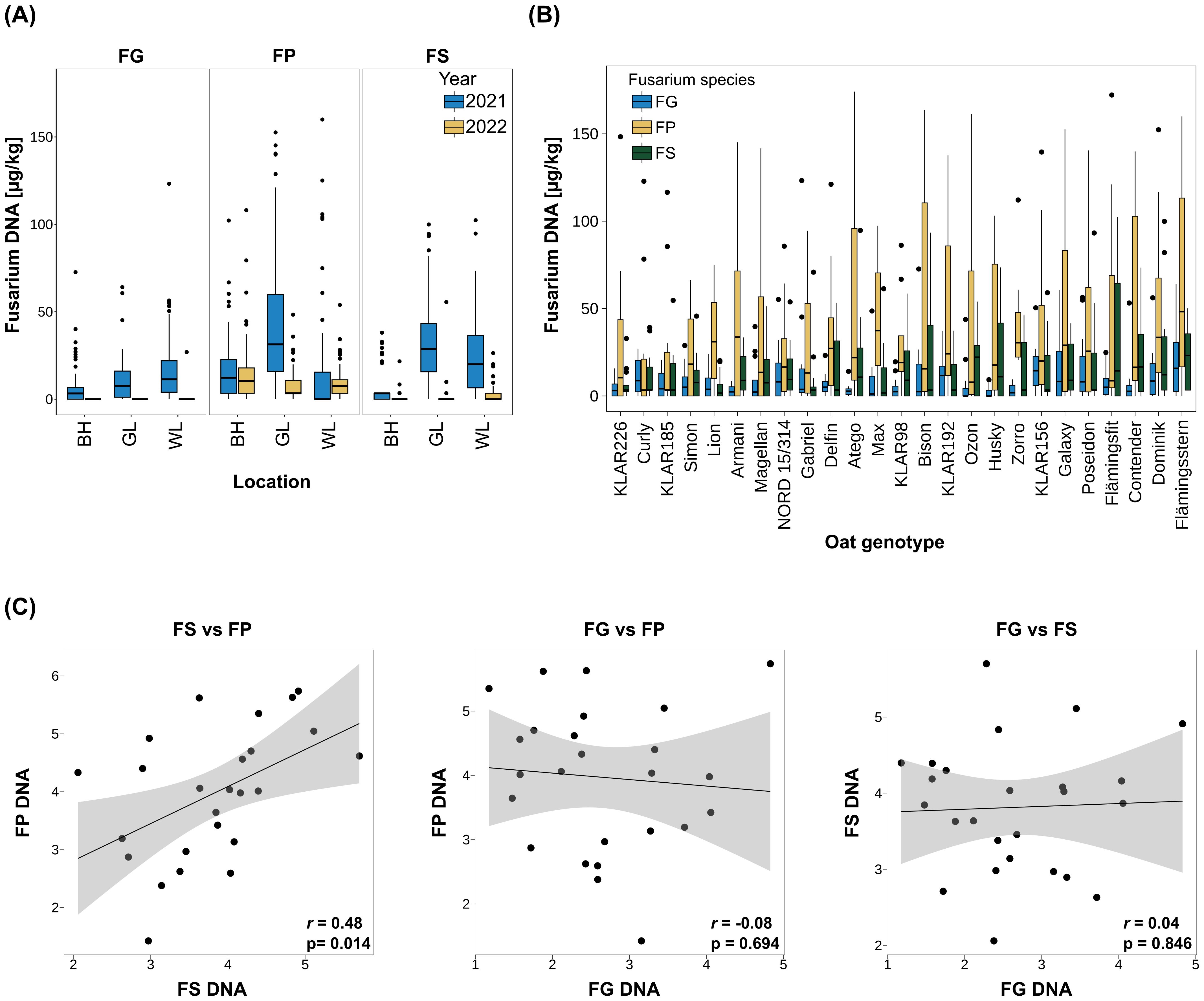
Figure 1. (A) Fusarium biomass in oat seeds across locations and years for different Fusarium species. Fungal biomass was assessed via quantification of fungal DNA per kg oat seeds. n=3 replicate plots per Fusarium species, oat genotype, study location and year. BH: Böhnshausen; GL: Groß Lüsewitz; WL: Wohlde. For visualization purposes, the y-axis limit was set to 180 µg/kg. (B) Fusarium biomass across 25 oat genotypes for different Fusarium species. Genotypes are ordered from low (left) to high (right) sum of fungal DNA of all three Fusarium species. Only 2021 data used. For visualization purposes, the y-axis limit was set to 180 µg/kg. (C) Correlation of biomass of the different Fusarium species across oat genotypes. BLUEs were used for calculation of Pearson rank correlation coefficients. Values were square root transformed before BLUE calculation. r: Pearson correlation coefficient; p: p-value. FG, F. graminearum; FP, F. poae; FS, F sporotrichioides.
Across locations and genotypes, fungal DNA content was highest for FP (29.49 µg/kg), followed by FS (21.17 µg/kg), and lowest for FG (11.58 µg/kg; Table 1, see Supplementary Table S11 for significance groups). For all three fungal species, the environment explained most of the observed variance (Table 1). Accordingly, heritability was rather low for FS and FG (0.36 and 0.50 respectively, Table 1). For FP there was no significant genotype effect, hence heritability was zero (Table 1).
Across genotypes, mean FP infection was by far the highest in GL 2021 (56.04 µg/kg vs. 17.34 µg/kg in BH and 15.10 µg/kg in WL; Figure 1A, Supplementary Table S6, see Supplementary Table S12 for significance groups). For FS, highest mean infection was as well observed in GL (33.3 µg/kg), while lowest mean infection was observed in BH (8.9 µg/kg; Figure 1A). FG mean infection was the highest in WL (17.47 µg/kg), followed by GL (10.62 µg/kg) and BH (6.64 µg/kg; Figure 1A, Supplementary Table S6, see Supplementary Table S12 for significance groups).
The fungal DNA content in the harvested seeds of the tested oat genotypes for the different Fusarium species is shown in Figure 1B, significance groups are given in Supplementary Table S13. The genotypes are ordered from lowest (left) to highest (right) total fungal DNA (sum of FG, FP and FS DNA of each genotype). In line with the overall highest mean, in most genotypes, DNA contents were highest for FP (12 genotypes), closely followed by FS (10 genotypes). Accordingly, in the majority of oat genotypes, FG DNA contents were the lowest compared to FS and FP (17 genotypes, Figure 1B).
To clarify whether species-specific resistance or species-unspecific resistance (i.e. cross-resistance) mechanisms were present, Pearson correlations between the different Fusarium species were calculated (Figure 1C). BLUEs were used for calculation of Pearson correlation coefficients and values were square root transformed before BLUE estimation. This identified a significant positive correlation of 0.48 between FP and FS infection severity. No significant correlations were detected between FG and FS or FP.
The ANOVA based on the mixed model with curated data of both years displayed significant effects for factors environment and inoculum but not for genotype ( Supplementary Table S10).
Despite the low correlation between the three Fusarium species, oat genotypes with good resistances to all three Fusarium species could be identified in the analyzed oat panel (see genotypes on the left of Figure 1B, e.g. KLAR226, Curly, KLAR185, and NORD 15/314). Significant genotype effects were found in the ANOVAs with species separated data sets for FG and FS but not for FP.
3.2 Variation of trichome size and density on oat hulls
To evaluate variations in surface morphology that could affect oat – Fusarium interactions, we examined differences in trichome size and density on the paleas and lemmas of the 25 oat genotypes in the cross-resistance panel. For this, lemma and palea samples were taken at anthesis from plants grown in the field and in the greenhouse in 2021 and 2022 (= four environments). Trichomes were visualized by autofluorescence using a fluorescence microscope and counted classified by size. All trichomes observed were of the prickle type, no macro hairs were noted. Trichome density generally decreased from tip to base of both organs. To summarize trichome size and density, a trichome index considering both values was calculated. Heritability of the trichome index was very high (0.97) and trichome density and size profiles differed strongly between the analyzed oat genotypes (Figure 2A, Supplementary Table S14). Figure 2B shows exemplary paleas of the extreme genotypes Atego (lowest trichome index) and KLAR156 (highest trichome index). In general, trichome density and index was higher on the lemma than the palea, and most trichomes fell into the ‘medium’ category. Furthermore, trichome indices as well as trichome densities of lemma and palea showed a strong significant positive correlation (Pearson correlation coefficient 0.83 and 0.99, respectively, Supplementary Figure S1).
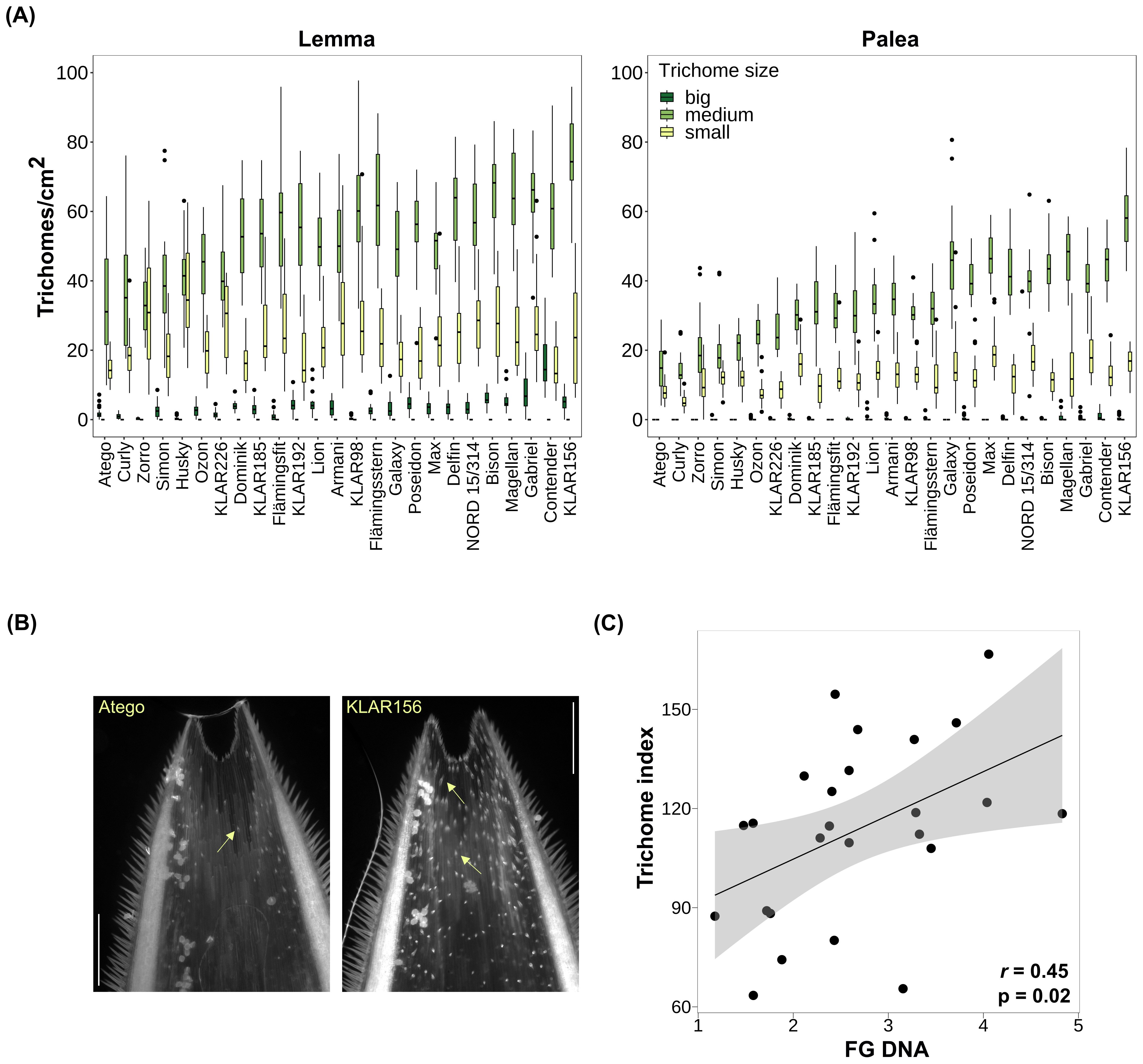
Figure 2. (A) Trichome density on lemma and palea across oat genotypes. Genotypes are ordered by trichome index from low (left) to high (right). Small trichomes: < 25 µm; medium trichomes: 25 – 100 µm; big trichomes > 100 µm. (B) Exemplary paleas of oat genotypes with lowest (Atego) and highest (KLAR156) trichome index. Trichomes were visualized through autofluorescence using the DAPI filter on a fluorescence microscope. Arrows indicate trichomes. Scale bar: 500 µm. (C) Correlation of lemma and palea trichome index and F. graminearum biomass across oat genotypes. BLUEs were used for calculation of Pearson rank correlation coefficients. FG, F. graminearum; rs, Pearson-rank correlation coefficient; p, p-value.
3.3 Relationship of trichome size and density and Fusarium infection
To understand whether the observed differences in trichome density and size profiles could play a role during Fusarium infection, we calculated their correlation with Fusarium biomass. For FG, a significant positive correlation with both trichome index and trichome density was found (r=0.45 and 0.43, respectively; Figure 2C, Supplementary Figure S1). Furthermore, the individual values of trichome index and density of palea and lemma were also significantly positively correlated with FG biomass and showed similar correlation coefficients (r= 0. 43 and 0.45, respectively, Supplementary Figure S1). The strongest correlation with FG biomass was observed with the density of medium-sized trichomes on lemmas (r=0.53, Supplementary Figure S1). Furthermore, the trichome density of the five oat genotypes most susceptible to FG was about 1.6 times higher than that of the five oat genotypes most resistant to FG. In contrast, there was no significant correlation of any of the trichome measures with FS and FP ( Supplementary Figure S1).
To gain further insight into the possible role of trichomes during infection, the interaction of FG hyphae with trichomes on the surface of paleas was investigated using confocal laser-scanning microscopy (Figure 3). Florets of the oat varieties ‘Gabriel’, ‘Contender’ and ‘Max’ were spray inoculated with FG conidia at anthesis and samples were taken at 24, 48 and 96 hpi. In several cases, hyphae grew in close contact with trichomes, often with accumulation of hyphae at the trichome base below the spike-like structure (Figure 3A-F). Occasionally, extensive hyphal growth was observed inside a broken trichome (Figure 3D). Growth on the palea surface without close interaction with trichomes (Supplementary Figure S3) and on/inside stomata was also common (Figure 3D-F).
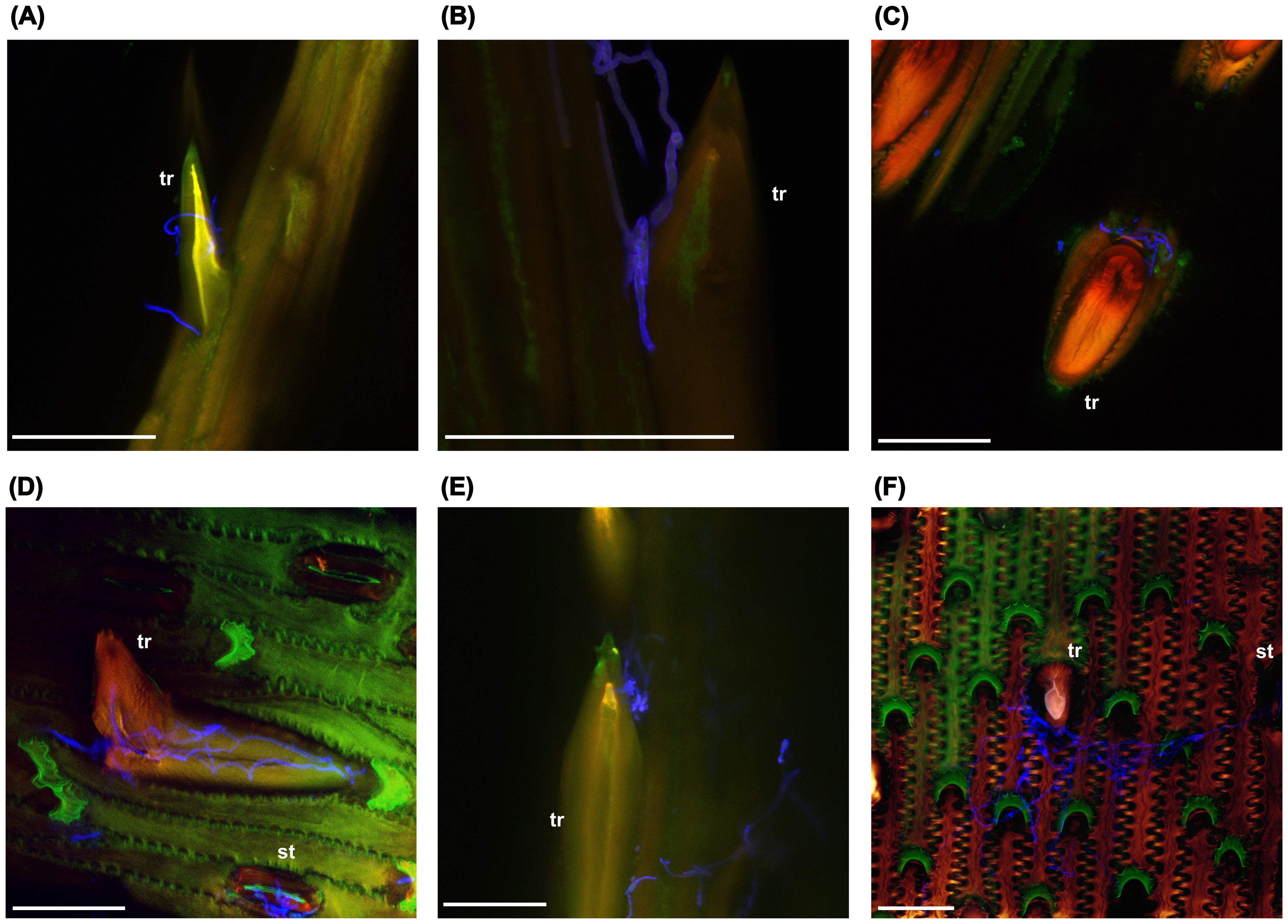
Figure 3. F. graminearum growth on trichomes (tr) and stomata (st) of oat paleas. (A) 24 hpi. (B–D) 48 hpi. (E, F) 96 hpi. Fungal hyphae were stained using WGA-AF488 and are shown in blue. Maximum projections of CLSM overlay images. hpi: hours post inoculation. Scale bar: 50 µm.
4 Discussion
4.1 Environmental effects on FHB
In our study, the severity of Fusarium infection varied significantly depending on the fungal species and environment. Notably, most of the observed variance was explained by the environment, reflecting a strong influence of environmental conditions on Fusarium infection dynamics. These results align with other observations showing that Fusarium infection and mycotoxin contamination are strongly affected by temperature and moisture-related weather variables such as rainfall or relative air humidity especially during and after flowering (Hooker et al., 2002; Kriss et al., 2012; Parry et al., 1995; Xu et al., 2008). The production and distribution of conidia and ascospores from the spawn inoculum is also known to be strongly influenced by wind, temperature, rainfall and humidity, hence environmental conditions prior to infection are also important (Champeil et al., 2004; Crane and Bergstrom, 2014). In 2021, FP had the highest overall biomass, followed by FS, while FG had the lowest biomass. Environmental conditions may have favored the other Fusarium species over FG. Similar to our observations across Germany, in Switzerland, the UK and Spain, FP was also found to be most prevalent among all Fusarium species, suggesting a general dominance of FP in oats across Europe (Schöneberg et al., 2018; Gil-Serna et al., 2022). Furthermore, site-specific analysis showed that FG was highest in WL, which was in a region with higher rainfall than the other sites ( Supplementary Table S9). The 2022 growing season was characterized by exceptionally dry conditions (ufz drought monitor, https://www.ufz.de/index.php?de=47252), which was accompanied by almost no infection by FS and FG. Only FP still showed considerable amounts of fungal biomass, suggesting that FP is more adapted to dry conditions than the other Fusarium species. This is consistent with the different environmental preferences reported previously for these Fusarium species. FP generally shows higher incidence after dry and warm weather conditions, whereas FG thrives in wetter and warmer environments (Doohan et al., 2003; Henriksen, 1999; Kriss et al., 2012; Meyer et al., 2021; Parikka et al., 2012; Turner and Jennings, 1997; Xu et al., 2008). Furthermore, even within one field, considerable variability in Fusarium disease severity has often been observed, suggesting that very specific microclimatic conditions also play an important role (Oerke et al., 2010; Xu et al., 2008). In our study, weather data were not directly recorded at the field sites, but data from nearby regional stations were used to get a rough estimate of the environmental conditions. To gain a more detailed understanding of the specific preferences of the different Fusarium species in oats, measurements directly derived from the field that would better characterize the local microenvironment should be taken. In addition to environmental conditions, other factors such as tillage, previous crop and soil type can also affect the incidence of FHB, but to a lesser extent (Schaafsma and Hooker, 2007). In our study, these factors were the same for the three Fusarium species, but differed between the experimental sites and could also partly explain some of the observed site-specific differences. Taken together, our observations underline the necessity of evaluating Fusarium resistance across a large number of environments and replicates to ensure robust results.
4.2 Fusarium species-specificity and cross-resistance
FHB is caused by various Fusarium species. A generally effective, species-unspecific resistance would greatly help in breeding oat varieties with a reliably low risk of contamination with Fusarium mycotoxins. In our study, FP showed a significant positive correlation with FS biomass across host genotypes, whereas FG biomass was not correlated with either FS or FP. The positive correlation between FP and FS infection suggests partial cross-resistance, while the lack of correlation of FS and FP with FG rather suggests species-specific resistance mechanisms. Furthermore, the ANOVA across all three Fusarium species did not reveal any significant genotype effects, which also points towards a lack of general resistance in oats. In wheat, also a positive association between FP and FS was found and it was hypothesized that this could be due to the preferred production of microconidia in both species, and their relatively low competitiveness and aggressiveness (Oerke et al., 2010). In oats, mostly species-specific resistances and some general resistances have been reported. For example, Edwards (2009) found no correlation between HT-2 and DON levels in UK oat grain and therefore speculated that sources of resistance to one mycotoxin do not confer increased resistance to other mycotoxins. Similarly, no correlation of FHB resistance between FG- and FP-infested oat kernels was observed in a Canadian study (Tekauz et al., 2004). In a study by Hofgaard et al. (2022), the overall ranking of oat varieties based on HT-2/T-2 was not correlated with the ranking based on DON, although many varieties showed a similar ranking for both mycotoxins, suggesting some general resistance in addition to species-specific resistance. Comparably, Herrmann et al. (2020) observed significant differences for DON and HT-2/T-2 levels, but also found a correlation between the ranking for both mycotoxins in some cases. Interestingly, the same oat UDP-glycosyltransferases are able to detoxify and confer resistance towards DON, NIV and HT-2 by heterologous expression in yeast, which would suggest that they could provide general resistance to different Fusarium species (Khairullina et al., 2022). However, the role of the different mycotoxins as virulence factors and the respective resistance mechanisms are still largely unknown in oats. A possible reason for species-specificity of resistance could be the different epidemiology of the Fusarium species. In addition to distinct preferences for environmental conditions, the timing of the influence of weather conditions on infection may also differ between Fusarium species (Hjelkrem et al., 2017, 2018). Some morphological or phenological characteristics, such as plant height, earliness or anther retention, may also explain some of the differences in resistance, but were not or only partly assessed in our study.
Heritability was markedly low for FP, where no significant genotype effect was found. This indicates a lack of significant genetic variability or extreme dependence on environmental conditions particularly for this Fusarium species. Another study also found no significant differences in FP DNA levels between oat genotypes but rather location-specific effects on FP abundance (Martin et al., 2018). The reason for the difference in genotypic effect sizes between the Fusarium species is unclear, but could be related to species-specific resistance mechanisms as well. In our study, oat genotypes had small differences in plant height and earliness, which are factors known to influence Fusarium resistance ranking. The possibility that greater differences in these factors would result in significant differences in resistance to FP should be investigated. The lack of genetic effect for resistance to FP is concerning as this makes breeding for FP resistance particularly difficult. This is especially problematic because, although FP is considered a rather weak pathogen, its prevalence has been increasing and it is often found to be one of the most abundant Fusarium species in oats (Karlsson et al., 2023; Kuchynková and Kalinová, 2021; Martin et al., 2018; Parikka et al., 2012; Parry et al., 1995; Stenglein, 2009; Valverde-Bogantes et al., 2020; Xue et al., 2019). Also in our study, FP biomass was overall much higher than FG and FS biomass.
Although some correlation could be found for resistance to different Fusarium species, it is questionable whether there is sufficiently strong cross-resistance in oats. Therefore, separate tests are most likely required to reliably determine resistance to different Fusarium species. This makes breeding for durable Fusarium resistance even more challenging, as the occurrence of Fusarium species is expected to constantly shift and previously marginal species may become more important due to climate change (Parikka et al., 2012; Hietaniemi et al., 2016; Moretti et al., 2019; Valverde-Bogantes et al., 2020; Karlsson et al., 2022). However, there are some genotypes in our study showing consistent resistance across Fusarium species and could be useful candidates for breeding programs. Their resistance phenotypes should however be validated in additional environments due to the high variability. Whether their resistance is based on broadly effective mechanisms or on a combination of different resistance sources for each Fusarium species is not clear and should be investigated. Our results demonstrate the possibility of developing cultivars with good resistance to several Fusarium species, which is further confirmed by recent breeding progress (Hofgaard et al., 2022).
4.3 Variation of trichome size and density
This study revealed a significant variation in hull trichome density and size among oat genotypes, and the genotypic differences were stable across environments. This is reflected by a very high heritability, suggesting that this trait is strongly influenced by genetic factors.
Furthermore, the trichome traits were highly correlated between lemma and palea, suggesting a coordinated regulation of trichome development across these organs. This also means that the laborious trichome phenotyping process could be simplified by focusing on only one of these organs and using less environments in future studies.
The observation that only prickle trichomes were present on the lemma and palea is consistent with findings in wheat, where prickle trichomes were also the only type on the florets (Sun et al., 2024). In contrast, florets of two-row barley display distinct, dome-shaped trichomes, while prickle-like trichomes are present mostly on six-row barley (Imboden et al., 2018).
In wheat and rice, several loci that control trichomes on florets and/or leaves have been mapped (Angeles-Shim et al., 2012; Chen et al., 2021; Wu et al., 2021; Luo et al., 2022; Fan et al., 2023). In oats however, the genetic basis of hull trichome formation has not been investigated until now. It would be interesting to use contrasting genotypes identified in this study to develop a segregating population to map genetic loci for trichome development in oats as well.
4.4 Trichomes in Fusarium-host interactions
We found a significant positive correlation between FG biomass and trichome density, meaning that oat genotypes with fewer trichomes on the hulls were generally more resistant, suggesting that a higher number of trichomes may facilitate colonization by FG. This is consistent with results from wild Avena species, where trichome density and Fusarium infection were positively correlated as well (Gagkaeva et al., 2017). Interestingly, in wheat, genetic loci controlling trichome length and density overlap with QTLs for FHB resistance, which also points towards a close relationship between trichomes and Fusarium infection (Häberle et al., 2009; Zheng et al., 2021). In contrast, Duba et al. (2019) found that leaf trichome density was higher in resistant than susceptible wheat lines, rather suggesting a protective role of trichomes. It should however be noted that the observed correlation with FG is based on infection data from a relatively small number of environments and should therefore be interpreted with caution. Nevertheless, the involvement of trichomes in FG infection in oats is further supported by our microscopic observations, where FG hyphae frequently accumulated in close contact with trichomes. FG hyphae were also growing on stomata, indicating multiple colonization routes. This is in line with findings from other Fusarium-host systems. In maize, FG hyphae penetrate through the tip of prickle trichomes (Nguyen et al., 2016), while in wheat, the base is penetrated (Sun et al., 2024). In barley, also the base of prickle trichomes is invaded, while trichomes remain intact (Imboden et al., 2018; Linkmeyer et al., 2013). In wheat, invasion is more aggressive and trichomes are destroyed by secretion of cell wall-degrading enzymes during the infection process (Sun et al., 2024). Furthermore, entry through stomata is a common infection route for Fusarium in various hosts as well (Beccari et al., 2011; Kang and Buchenauer, 2000; Linkmeyer et al., 2013; Pritsch et al., 2000). We also observed growth of FG hyphae on broken trichomes, which may indicate that the fungus is actively destroying trichomes for penetration, similar to what happens in wheat. A more detailed study of the microscopic interaction of hyphae and trichomes as infection progresses is required to clarify this and to determine whether the hyphae specifically penetrate the trichome base in oats as well.
There are several possible reasons for the important role of trichomes for Fusarium infection. Firstly, trichomes could serve as attachment points for spores, preventing them from being easily carried away by wind or rain. Alternatively, trichomes could provide a microclimate favorable to fungal growth, such as increased humidity due to water droplets adhering to them (Calo et al., 2006; Imboden et al., 2018). In grasses, trichomes accumulate silica, similar to other cell types that are also preferred entry sites for Fusarium hyphae such as vascular bundles, stomata and silica cells. This suggests that the fungus may be specifically attracted to high silica levels (Rittenour and Harris, 2010; Boenisch and Schäfer, 2011; Imboden et al., 2018). The trichome base also appears to be a particularly vulnerable site for Fusarium infection (Sun et al., 2024). Differences in cell wall composition between trichomes and other epidermal cells may therefore provide another explanation.
In contrast to FG, no significant correlation was identified between trichomes and FS or FP biomass in our study. This may be due to the lower heritabilities of FS and FP infection severity, but may also indicate species-specific interactions with trichomes in oats. Depending on the actual function of trichomes in the infection process, different explanations are possible. Fusarium species have different climatic preferences, so the microclimate provided by the trichomes may only be conducive to the growth of FG, but not to the other Fusarium species tested. Furthermore, the conidia of FG, FS and FP differ considerably in size and shape. The large lunate-shaped macroconidia of FG might be more efficiently trapped by the prickle trichomes, whereas the smaller and more rounded micro- and mesoconidia of FP and FS are not as efficiently attached (Palicova et al., 2025). It is also conceivable that not all Fusarium species possess the enzymes necessary to degrade the oat trichome cell walls. However, in contrast to our results, in barley, penetration of trichomes was found to be an important infection route for FS as well (Linkmeyer, 2012), and to our knowledge, no Fusarium species-specificity for the role trichomes has been described previously. Whether there are species-specific interactions with trichomes in oats or whether the lack of correlation is rather due to the low heritability in the field trials needs to be clarified in future studies. For this, infection results from controlled experiments will be helpful, as the involvement of trichomes may be easier to assess in the absence of highly variable environmental conditions.
In conclusion, it might appear promising that by the use of oat genotypes with fewer trichomes, FG infection could be reduced. However, it should be taken into account that trichomes also serve important positive functions such as reducing insect feeding and increasing resistance to UV radiation (Mauricio and Rausher, 1997; Peeters, 2002; Yan et al., 2012; Zhang et al., 2021). Consequently, the reduction of trichome density might not be an advisable breeding target. As the trichome base seems to be the most vulnerable part, Sun et al. (2024) suggest that breeding efforts should be directed towards strengthening the trichome base to make penetration more difficult. Another potential approach might be the targeted expression of antifungal compounds in trichomes to inhibit fungal infection. In Arabidopsis, resistance to Botrytis cinerea could be increased by trichome-specific expression of a Trichoderma α-1,3-glucanase, an enzyme that hydrolyses the cell wall of various fungi (Calo et al., 2006). However, how useful these approaches would be in practical applications is uncertain and remains to be explored.
Data availability statement
The original contributions presented in the study are included in the article/ material. Further inquiries can be directed to the corresponding author.
Author contributions
SS: Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. CR: Investigation, Methodology, Writing – review & editing. KO: Resources, Writing – review & editing. StB: Resources, Writing – review & editing. SoB: Resources, Writing – review & editing. AV: Resources, Writing – review & editing. MH: Conceptualization, Formal Analysis, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the project ‘Monitoring of Fusarium species and development of genomic tools for more effective breeding of oats (FUGE)’, grant no. 28AINO2A20, funded by the Federal Office for Agriculture and Food in the Federal Ministry of Agriculture, Food and Regional Identity of Germany and the project ‘Climate Resilient Orphan croPs for increased DIVersity in Agriculture (CROPDIVA)’, grant 364 agreement no. 101000847, funded by the EU’s Horizon 2020 Research and Innovation Program.
Acknowledgments
We would like to acknowledge the imaging facility of the Julius Kühn Institute for Epidemiology and Pathogen Diagnostics for use of the Leica TCS SP8 confocal laser-scanning microscope and are grateful to Yvonne Becker for helpful support on sample preparation and image acquisition.
Conflict of interest
Author KO was employed by the company KWS LOCHOW GmbH. Author StB was employed by the company Nordsaat Saatzucht GmbH. Author SoB was employed by the company Saatzucht Bauer GmbH & Co. KG.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. DeepL Write (beta) was used to support language editing.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1608030/full#supplementary-material
References
Aamot, H. U., Mousavi, H., Razzaghian, J., Brodal, G., Sulyok, M., Krska, R., et al. (2022). Fusarium langsethiae and mycotoxin contamination in oat grain differed with growth stage at inoculation. Eur. J. Plant Pathol. 164, 59–78. doi: 10.1007/s10658-022-02539-1
Akinsanmi, O. A., Backhouse, D., Simpfendorfer, S., and Chakraborty, S. (2006). Pathogenic variation of Fusarium isolates associated with head blight of wheat in Australia. J. Phytopathol. 154, 513–521. doi: 10.1111/j.1439-0434.2006.01137.x
Angeles-Shim, R. B., Asano, K., Takashi, T., Shim, J., Kuroha, T., Ayano, M., et al. (2012). A WUSCHEL-related homeobox 3B gene, depilous (dep), confers glabrousness of rice leaves and glumes. Rice 5, 28. doi: 10.1186/1939-8433-5-28
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Beccari, G., Covarelli, L., and Nicholson, P. (2011). Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant Pathol. 60, 671–684. doi: 10.1111/j.1365-3059.2011.02425.x
Becker, Y., Green, K., Scott, B., and Becker, M. (2018). Artificial inoculation of Epichloë festucae into Lolium perenne, and visualisation of endophytic and epiphyllous fungal growth. BIO-PROTOCOL 8, 1–14. doi: 10.21769/BioProtoc.2990
Becker, H. (2019). Pflanzenzüchtung. (Stuttgart, Deutschland: utb GmbH). doi: 10.36198/9783838549507
Bethke, G., Huang, Y., Hensel, G., Heinen, S., Liu, C., Wyant, S. R., et al. (2023). UDP-glucosyltransferase HvUGT13248 confers type II resistance to Fusarium graminearum in barley. Plant Physiol. 193, 2691–2710. doi: 10.1093/plphys/kiad467
Beule, L., Lehtsaar, E., Rathgeb, A., and Karlovsky, P. (2019). Crop diseases and mycotoxin accumulation in temperate agroforestry systems. Sustain. 11, 1–21. doi: 10.3390/su11102925
Bjørnstad, Å. and Skinnes, H. (2008). Resistance to Fusarium infection in oats (Avena sativa L.). Cereal Res. Commun. 36, 57–62. doi: 10.1556/CRC.36.2008.Suppl.B.9
Boenisch, M. J. and Schäfer, W. (2011). Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 11, 110. doi: 10.1186/1471-2229-11-110
Bottalico, A. and Perrone, G. (2002). Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 108, 611–624. doi: 10.1023/A:1020635214971
Brandfass, C. and Karlovsky, P. (2008). Upscaled CTAB-based dna extraction and real-time pcr assays for Fusarium culmorum and F. graminearum dna in plant material with reduced sampling error. Int. J. Mol. Sci. 9, 2306–2321. doi: 10.3390/ijms9112306
Calo, L., Garcia, I., Gotor, C., and Romero, L. C. (2006). Leaf hairs influence phytopathogenic fungus infection and confer an increased resistance when expressing a Trichoderma -1,3-glucanase. J. Exp. Bot. 57, 3911–3920. doi: 10.1093/jxb/erl155
Champeil, A., Doré, T., and Fourbet, J. F. (2004). Fusarium head blight: Epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Sci. 166, 1389–1415. doi: 10.1016/j.plantsci.2004.02.004
Chen, Z., Zheng, Z., Luo, W., Zhou, H., Ying, Z., and Liu, C. (2021). Detection of a major QTL conditioning trichome length and density on chromosome arm 4BL and development of near isogenic lines targeting this locus in bread wheat. Mol. Breed. 41, 10. doi: 10.1007/s11032-021-01201-8
Clarke, J. U. (1998). Evaluation of censored data methods to allow statistical comparisons among very small samples with below detection limit observations. Environ. Sci. Technol. 32, 177–183. doi: 10.1021/es970521v
Crane, J. M. and Bergstrom, G. C. (2014). Spatial distribution and antifungal interactions of a Bacillus biological control agent on wheat surfaces. Biol. Control 78, 23–32. doi: 10.1016/j.biocontrol.2014.07.002
Dal Prá, M., Tonti, S., Montanari, M., Stefani, E., and Alberti, I. (2014). Occurrence of Fusarium langsethiae on oat kernels in Italy. 7th ISTA Seed Health Symposium, 32–33.
Doohan, F. M., Brennan, J., and Cooke, B. M. (2003). Influence of climatic factors on Fusarium species pathogenic to cereals. Eur. J. Plant Pathol. 109, 755–768. doi: 10.1023/A:1026090626994
Duba, A., Goriewa-Duba, K., Wachowska, U., Głowacka, K., and Wiwart, M. (2019). The associations between leaf morphology, phenylalanine ammonia lyase activity, reactive oxygen species, and Fusarium resistance in selected species of wheat with different ploidy levels. Plants 8, 360. doi: 10.3390/plants8100360
Edwards, S. G. (2009). Fusarium mycotoxin content of UK organic and conventional oats. Food Addit. Contam. - Part A 26, 1063–1069. doi: 10.1080/02652030902788953
Fan, H., Xu, J., Ao, D., Jia, T., Shi, Y., Li, N., et al. (2023). QTL mapping of trichome traits and analysis of candidate genes in leaves of wheat (Triticum aestivum L.). Genes. 15, 42. doi: 10.3390/genes15010042
Fredlund, E., Gidlund, A., Sulyok, M., Börjesson, T., Krska, R., Olsen, M., et al. (2013). Deoxynivalenol and other selected Fusarium toxins in Swedish oats — Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 167, 276–283. doi: 10.1016/j.ijfoodmicro.2013.06.026
Gagkaeva, T. Y., Gavrilova, O. P., Orina, A. S., Blinova, E. V., and Loskutov, I. G. (2017). Response of wild Avena species to fungal infection of grain. Crop J. 5, 499–508. doi: 10.1016/j.cj.2017.04.005
Georgieva, P., Tiedemann, A., Rathgeb, A., Karlovsky, P., Sulyok, M., and Winter, M. (2018). Fusarium head blight in German oats - genetic, toxigenic and phenotypic diversity of Fusarium langsethiae, F. sporotrichioides and F. poae. 14th Eur. Fusarium Semin.
Gil-Serna, J., Patiño, B., Verheecke-Vaessen, C., Vázquez, C., and Medina, Á. (2022). Searching for the Fusarium spp. Which Are Responsible for Trichothecene Contamination in Oats Using Metataxonomy to Compare the Distribution of Toxigenic Species in Fields from Spain and the UK. Toxins (Basel). 14, 592. doi: 10.3390/toxins14090592
Guerra, V., Beule, L., Lehtsaar, E., Liao, H.-L., and Karlovsky, P. (2020). Improved protocol for DNA extraction from subsoils using phosphate lysis buffer. Microorganisms 8, 532. doi: 10.3390/microorganisms8040532
Häberle, J., Schweizer, G., Schondelmaier, J., Zimmermann, G., and Hartl, L. (2009). Mapping of QTL for resistance against Fusarium head blight in the winter wheat population Pelikan//Bussard/Ning8026. Plant Breed. 128, 27–35. doi: 10.1111/j.1439-0523.2008.01540.x
Han, G., Li, Y., Yang, Z., Wang, C., Zhang, Y., and Wang, B. (2022). Molecular mechanisms of plant trichome development. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.910228
Harrell, F., Jr. (2025). Hmisc: Harrell Miscellaneous. R package version 5.2-4. Available online at: https://github.com/harrelfe/hmisc (Accessed April 07, 2025).
Hautsalo, J., Jauhiainen, L., Hannukkala, A., Manninen, O., Veteläinen, M., Pietilä, L., et al. (2020). Resistance to Fusarium head blight in oats based on analyses of multiple field and greenhouse studies. Eur. J. Plant Pathol. 158, 15–33. doi: 10.1007/s10658-020-02039-0
He, X., Skinnes, H., Oliver, R. E., Jackson, E. W., and Bjørnstad, A. (2013). Linkage mapping and identification of QTL affecting deoxynivalenol (DON) content (Fusarium resistance) in oats (Avena sativa L.). Theor. Appl. Genet. 126, 2655–2670. doi: 10.1007/s00122-013-2163-0
Henriksen, B. (1999). Factors Affecting Fusarium Infection and Mycotoxin Content in Cereal Grains. Agricultural University of Norway, Doctor Scientiarum thesis.
Herrmann, M. H., Hautsalo, J., Georgieva, P., Bund, A., Winter, M., and Beuch, S. (2020). Relationship between genetic variability of flowering traits and Fusarium mycotoxin contamination in oats. Crop Sci. 60, 852–862. doi: 10.1002/csc2.20125
Hietaniemi, V., Rämö, S., Yli-Mattila, T., Jestoi, M., Peltonen, S., Kartio, M., et al. (2016). Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. Part A 33, 831–848. doi: 10.1080/19440049.2016.1162112
Hjelkrem, A. G. R., Aamot, H. U., Brodal, G., Strand, E. C., Torp, T., Edwards, S. G., et al. (2018). HT-2 and T-2 toxins in Norwegian oat grains related to weather conditions at different growth stages. Eur. J. Plant Pathol. 151, 501–514. doi: 10.1007/s10658-017-1394-3
Hjelkrem, A.-G. R., Torp, T., Brodal, G., Aamot, H. U., Strand, E., Nordskog, B., et al. (2017). DON content in oat grains in Norway related to weather conditions at different growth stages. Eur. J. Plant Pathol. 148, 577–594. doi: 10.1007/s10658-016-1113-5
Hofgaard, I. S., Aamot, H. U., Torp, T., Jestoi, M., Lattanzio, V. M. T., Klemsdal, S. S., et al. (2016a). Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. World Mycotoxin J. 9, 365–378. doi: 10.3920/WMJ2015.2003
Hofgaard, I. S., Brodal, G., Almvik, M., Lillemo, M., Russenes, A. L., Edwards, S. G., et al. (2022). Different resistance to DON versus HT2 + T2 producers in nordic oat varieties. Toxins. 14, 313. doi: 10.3390/toxins14050313
Hofgaard, I. S., Seehusen, T., Aamot, H. U., Riley, H., Razzaghian, J., Le, V. H., et al. (2016b). Inoculum potential of Fusarium spp. relates to tillage and straw management in norwegian fields of spring oats. Front. Microbiol. 7, 1–15. doi: 10.3389/fmicb.2016.00556
Holzknecht, P., Bury, P., Lemmens, M., and Buerstmayr, H. (2009). Fusarium head blight in barley: identification of the causal Fusarium species in Europe and testing of resistance using artificial inoculation. 60. Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs, 177–178.
Hooker, D. C., Schaafsma, A. W., and Tamburic-Ilincic, L. (2002). Using weather variables pre- and post-heading to predict deoxynivalenol content in winter wheat. Plant Dis. 86, 611–619. doi: 10.1094/PDIS.2002.86.6.611
Imboden, L., Afton, D., and Trail, F. (2018). Surface interactions of Fusarium graminearum on barley. Mol. Plant Pathol. 19, 1332–1342. doi: 10.1111/mpp.12616
Kang, Z. and Buchenauer, H. (2000). Ultrastructural and cytochemical studies on cellulose, xylan and pectin degradation in wheat spikes infected by Fusarium culmorum. J. Phytopathol. 148, 263–275. doi: 10.1046/j.1439-0434.2000.00489.x
Karlsson, I., Mellqvist, E., and Persson, P. (2023). Temporal and spatial dynamics of Fusarium spp. and mycotoxins in Swedish cereals during 16 years. Mycotoxin Res. 39, 3–18. doi: 10.1007/s12550-022-00469-9
Kassambara, A. (2021). Pipe-friendly framework for basic statistical tests [R Package “rstatix” version 0.7. 0]. R Found (Vienna: Stat. Comput).
Khairullina, A., Tsardakas Renhuldt, N., Wiesenberger, G., Bentzer, J., Collinge, D. B., Adam, G., et al. (2022). Identification and functional characterisation of two oat UDP-glucosyltransferases involved in deoxynivalenol detoxification. Toxins. 14, 446. doi: 10.3390/toxins14070446
Kheiri, A., Moosawi Jorf, S. A., and Malihipour, A. (2019). Infection process and wheat response to Fusarium head blight caused by Fusarium graminearum. Eur. J. Plant Pathol. 153, 489–502. doi: 10.1007/s10658-018-1576-7
Kiecana, I., Cegiełko, M., Mielniczuk, E., and Perkowski, J. (2012). The occurrence of Fusarium poae (Peck) Wollenw. on oat (Avena sativa L.) panicles and its harmfulness. Acta Agrobot. 65, 169–178. doi: 10.5586/aa.2012.035
Kriss, A. B., Paul, P. A., Xu, X., Nicholson, P., Doohan, F. M., Hornok, L., et al. (2012). Quantification of the relationship between the environment and Fusarium head blight, Fusarium pathogen density, and mycotoxins in winter wheat in Europe. Eur. J. Plant Pathol. 133, 975–993. doi: 10.1007/s10658-012-9968-6
Kuchynková, H. and Pexová Kalinová, J. (2021). Influence of variety and growing conditions on Fusarium occurrence, mycotoxicological quality, and yield parameters of hulled oats. Cereal Res. Commun. 49, 577–585. doi: 10.1007/s42976-021-00133-5
Lemmens, M., Scholz, U., Berthiller, F., Dall’Asta, C., Koutnik, A., Schuhmacher, R., et al. (2005). The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant-Microbe Interact. 18, 1318–1324. doi: 10.1094/MPMI-18-1318
Linkmeyer, A. (2012). Fusarium Head Blight of Barley: Epidemiology and host-pathogen interaction [dissertation. Tech. Univ. Munich.
Linkmeyer, A., Götz, M., Hu, L., Asam, S., Rychlik, M., Hausladen, H., et al. (2013). Assessment and introduction of quantitative resistance to Fusarium head blight in elite spring barley. Phytopathology 103, 1252–1259. doi: 10.1094/PHYTO-02-13-0056-R
Loskutov, I. G., Blinova, E. V., Gavrilova, O. P., and Gagkaeva, T. Y. (2017). The valuable characteristics and resistance to Fusarium disease of oat genotypes. Russ. J. Genet. Appl. Res. 7, 290–298. doi: 10.1134/S2079059717030108
Luo, W., Zhou, J., Liu, J., Liu, Y., Mu, Y., Tang, H., et al. (2022). Fine mapping of the Hairy glume (Hg) gene in a chromosome variation region at the distal terminus of 1AS. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1006510
Martin, C., Schöneberg, T., Vogelgsang, S., Mendes Ferreira, C. S., Morisoli, R., Bertossa, M., et al. (2018). Responses of oat grains to Fusarium poae and F. langsethiae infections and mycotoxin contaminations. Toxins. 10, 1–18. doi: 10.3390/toxins10010047
Mauricio, R. and Rausher, M. D. (1997). Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution. 51, 1435–1444. doi: 10.1111/j.1558-5646.1997.tb01467.x
Mesterházy, A. (1995). Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 114, 377–386. doi: 10.1111/j.1439-0523.1995.tb00816.x
Mesterhazy, A. (2024). What is Fusarium head blight (FHB) resistance and what are its food safety risks in wheat? Problems and solutions—a review. Toxins. 16, 31. doi: 10.3390/toxins16010031
Mesterházy, Á., Bartók, T., Kászonyi, G., Varga, M., Tóth, B., and Varga, J. (2005). Common resistance to different Fusarium spp. causing Fusarium head blight in wheat. Eur. J. Plant Pathol. 112, 267–281. doi: 10.1007/s10658-005-2853-9
Mesterházy, Á., Bartók, T., Mirocha, C. G., and Komoróczy, R. (1999). Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed. 118, 97–110. doi: 10.1046/j.1439-0523.1999.118002097.x
Meyer, J. C., Hennies, I., Wessels, D., Schwarz, K., Chr, J., Hennies, I., et al. (2021). Survey of mycotoxins in milling oats dedicated for food purposes between 2013 and 2019 by LC – MS/MS. Food Addit. Contam. Part A 00 38 (11), 1934–1947. doi: 10.1080/19440049.2021.1950931
Moretti, A., Pascale, M., and Logrieco, A. F. (2019). Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 84, 38–40. doi: 10.1016/j.tifs.2018.03.008
Nguyen, T. T. X., Dehne, H.-W., and Steiner, U. (2016). Maize leaf trichomes represent an entry point of infection for Fusarium species. Fungal Biol. 120, 895–903. doi: 10.1016/j.funbio.2016.05.014
Nicholson, P., Simpson, D. R., Weston, G., Rezanoor, H. N., Lees, A. K., Parry, D. W., et al. (1998). Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant Pathol. 53, 17–37. doi: 10.1006/pmpp.1998.0170
Nielsen, L. K., Jensen, J. D., Nielsen, G. C., Jensen, J. E., Spliid, N. H., Thomsen, I. K., et al. (2011). Fusarium head blight of cereals in Denmark: Species complex and related mycotoxins. Phytopathology® 101, 960–969. doi: 10.1094/PHYTO-07-10-0188
Nutz, S., Döll, K., and Karlovsky, P. (2011). Determination of the LOQ in real-time PCR by receiver operating characteristic curve analysis: application to qPCR assays for Fusarium verticillioides and F. proliferatum. Anal. Bioanal. Chem. 401, 717–726. doi: 10.1007/s00216-011-5089-x
Oerke, E. C., Meier, A., Dehne, H.-W., Sulyok, M., Krska, R., and Steiner, U. (2010). Spatial variability of Fusarium head blight pathogens and associated mycotoxins in wheat crops. Plant Pathol. 59, 671–682. doi: 10.1111/j.1365-3059.2010.02286.x
Palicova, J., Chrpova, J., Tobolkova, A., Ovesna, J., and Stranska, M. (2025). Effect of pulsed electric field on viability of Fusarium micromycetes. Cereal Res. Commun. 53, 913–919. doi: 10.1007/s42976-024-00561-z
Parikka, P., Hakala, K., and Tiilikkala, K. (2012). Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climatic change. Food Addit. Contam. Part A 29, 1543–1555. doi: 10.1080/19440049.2012.680613
Parry, D. W., Jenkinson, P., and McLeod, L. (1995). Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol. 44, 207–238. doi: 10.1111/j.1365-3059.1995.tb02773.x
Parry, D. W. and Nicholson, P. (1996). Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol. 45, 383–391. doi: 10.1046/j.1365-3059.1996.d01-133.x
Peeters, P. J. (2002). Correlations between leaf constituent levels and the densities of herbivorous insect guilds in an Australian forest. Austral Ecol. 27, 658–671. doi: 10.1046/j.1442-9993.2002.01227.x
Peraldi, A., Beccari, G., Steed, A., and Nicholson, P. (2011). Brachypodium distachyon: a new pathosystem to study Fusarium head blight and other Fusarium diseases of wheat. BMC Plant Biol. 11, 100. doi: 10.1186/1471-2229-11-100
Placinta, C., D’Mello, J. P., and Macdonald, A. M. (1999). A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 78, 21–37. doi: 10.1016/S0377-8401(98)00278-8
Pritsch, C., Muehlbauer, G. J., Bushnell, W. R., Somers, D. A., and Vance, C. P. (2000). Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Mol. Plant-Microbe Interact. 13, 159–169. doi: 10.1094/MPMI.2000.13.2.159
R Core Team (2021). R: A language and environment for statistical computing. Available online at: https://www.r-project.org/ (Accessed January 03, 2021).
Rittenour, W. R. and Harris, S. D. (2010). An in vitro method for the analysis of infection-related morphogenesis in Fusarium graminearum. Mol. Plant Pathol. 11, 361–369. doi: 10.1111/j.1364-3703.2010.00609.x
Rodemann, C., Alhussein, M., and Von Tiedemann, A. (2023). “Auftreten von Fusarium spp. im deutschen Haferanbau - Ergebnisse aus einem dreijährigen Monitoring.” in Deutsche Pflanzenschutztagung: Pflanzenschutz morgen - Transformation durch Wissenschaft; 26. bis 29. September 2023, - Kurzfassungen der Vorträge und Poster -. eds. Kühn-Institut, J, 63. doi: 10.5073/20230803-074309-0
Schaafsma, A. W. and Hooker, D. C. (2007). Climatic models to predict occurrence of Fusarium toxins in wheat and maize. Int. J. Food Microbiol. 119, 116–125. doi: 10.1016/j.ijfoodmicro.2007.08.006
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Schöneberg, T., Jenny, E., Wettstein, F. E., Bucheli, T. D., Mascher, F., Bertossa, M., et al. (2018). Occurrence of Fusarium species and mycotoxins in Swiss oats—Impact of cropping factors. Eur. J. Agron. 92, 123–132. doi: 10.1016/j.eja.2017.09.004
Scott, P. M. (1989). “The natural occurrence of trichothecenes,” in Trichothecene mycotoxicosis: pathophysiologic effects. Ed. Beasley, V. R. (Boca Raton: CRC Press, Taylor & Francis Group), 1–26. doi: 10.1201/9781315121284
Siou, D., Gélisse, S., Laval, V., Repinçay, C., Canalès, R., Suffert, F., et al. (2014). Effect of wheat spike infection timing on fusarium head blight development and mycotoxin accumulation. Plant Pathol. 63, 390–399. doi: 10.1111/ppa.12106
Šip, V., Chrpova, J., and Štockova, L. (2011). Evaluation of resistance to Fusarium head blight in wheat using different sources of inoculum. Czech J. Genet. Plant Breed. 47, 131–139. doi: 10.17221/112/2011-CJGPB
Soni, N., Altartouri, B., Hegde, N., Duggavathi, R., Nazarian-Firouzabadi, F., and Kushalappa, A. C. (2021). TaNAC032 transcription factor regulates lignin-biosynthetic genes to combat Fusarium head blight in wheat. Plant Sci. 304, 110820. doi: 10.1016/j.plantsci.2021.110820
Spanic, V., Viljevac Vuletic, M., Abicic, I., and Marcek, T. (2017). Early response of wheat antioxidant system with special reference to Fusarium head blight stress. Plant Physiol. Biochem. 115, 34–43. doi: 10.1016/j.plaphy.2017.03.010
Stenglein, S. A. (2009). Fusarium poae: A pathogen that needs more attention. J. Plant Pathol. 91, 25–36. doi: 10.4454/jpp.v91i1.621
Sun, Z., Zhu, F., Chen, X., and Li, T. (2024). The pivotal role of trichomes in wheat susceptibility to Fusarium head blight. Plant Pathol. 73, 1615–1618. doi: 10.1111/ppa.13933
Tekauz, A., Fetch, J. W. M., Rossnagel, B. G., and Savard, M. E. (2008). Progress in assessing the impact of Fusarium head blight on oat in western Canada and screening of Avena germplasm for resistance. Cereal Res. Commun. 36, 49–56. doi: 10.1556/CRC.36.2008.Suppl.B.8
Tekauz, A., McCallum, B., Ames, N., and Fetch, J. M. (2004). Fusarium head blight of oat — current status in western Canada. Can. J. Plant Pathol. 26, 473–479. doi: 10.1080/07060660409507167
Tekle, S., Lillemo, M., Skinnes, H., Reitan, L., Buraas, T., and Bjørnstad, Å. (2018a). Screening of oat accessions for fusarium head blight resistance using spawn-inoculated field experiments. Crop Sci. 58, 143–151. doi: 10.2135/cropsci2017.04.0264
Tekle, S., Lillemo, M., Skinnes, H., Reitan, L., Buraas, T., and Bjørnstad, Å. (2018b). Screening of oat accessions for fusarium head blight resistance using spawn-inoculated field experiments. Crop Sci. 58, 143–151. doi: 10.1007/s10658-011-9888-x
Tekle, S., Schofer, S. S., He, X., Dong, Y., and Bjørnstad, Å. (2020). Variation in anther extrusion and its impact on Fusarium head blight and deoxynivalenol content in oat (Avena sativa L.). Agronomy 10 (3), 354. doi: 10.3390/agronomy10030354
Tekle, S., Skinnes, H., and Bjørnstad, Å. (2013). The germination problem of oat seed lots affected by Fusarium head blight. Eur. J. Plant Pathol. 135, 147–158. doi: 10.1007/s10658-012-0074-6
Tekle, S., Dill-Macky, R., Skinnes, H., Tronsmo, A. M., and Bjørnstad, Å. (2012). Infection process of Fusarium graminearum in oats (Avena sativa L.). Agronomy 132, 431–442. doi: 10.3390/agronomy10030354
Tóth, B., Kászonyi, G., Bartók, T., Varga, J., and Mesterházy, Á. (2008). Common resistance of wheat to members of the Fusarium graminearum species complex and F. culmorum. Plant Breed. 127, 1–8. doi: 10.1111/j.1439-0523.2008.01412.x
Turner, J. A. and Jennings, P. (1997). The effect of increasing humidity on Fusarium ear blight and grain quality. Cereal Res. Commun. 25, 825–826. doi: 10.1007/BF03543864
Valverde-Bogantes, E., Bianchini, A., Herr, J. R., Rose, D. J., Wegulo, S. N., and Hallen-Adams, H. E. (2020). Recent population changes of Fusarium head blight pathogens: drivers and implications. Can. J. Plant Pathol. 42, 315–329. doi: 10.1080/07060661.2019.1680442
van Eeuwijk, F. A., Mesterhazy, A., Kling, C. I., Ruckenbauer, P., Saur, L., Bürstmayr, H., et al. (1995). Assessing non-specificity of resistance in wheat to head blight caused by inoculation with European strains of Fusarium culmorum, F. graminearum and F. nivale using a multiplicative model for interaction. Theor. Appl. Genet. 90, 221–228. doi: 10.1007/BF00222205
Vanheule, A., Audenaert, K., De Boevre, M., Landschoot, S., Bekaert, B., Munaut, F., et al. (2014). The compositional mosaic of Fusarium species and their mycotoxins in unprocessed cereals, food and feed products in Belgium. Int. J. Food Microbiol. 181, 28–36. doi: 10.1016/j.ijfoodmicro.2014.04.012
Vos, K. (2001). Cell counter. Available online at: https://imagej.nih.gov/ij/plugins/cell-counter.html (Accessed December 16, 2021).
Willforss, J., Leonova, S., Tillander, J., Andreasson, E., Marttila, S., Olsson, O., et al. (2020). Interactive proteogenomic exploration of response to Fusarium head blight in oat varieties with different resistance. J. Proteomics 218. doi: 10.1016/j.jprot.2020.103688
Wilson, A., Simpson, D., Chandler, E., Jennings, P., and Nicholson, P. (2004). Development of PCR assays for the detection and differentiation of Fusarium sporotrichioides and Fusarium langsethiae. FEMS Microbiol. Lett. 233, 69–76. doi: 10.1016/j.femsle.2004.01.040
Wu, P., Yang, L., Guo, G., Hu, J., Qiu, D., Li, Y., et al. (2021). Molecular mapping and identification of a candidate gene for new locus Hg2 conferring hairy glume in wheat. Plant Sci. 307, 110879. doi: 10.1016/j.plantsci.2021.110879
Xu, X.-M., Nicholson, P., Thomsett, M. A., Simpson, D., Cooke, B. M., Doohan, F. M., et al. (2008). relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology® 98, 69–78. doi: 10.1094/PHYTO-98-1-0069
Xue, A. G., Chen, Y., Seifert, K., Guo, W., Blackwell, B. A., Harris, L. J., et al. (2019). Prevalence of Fusarium species causing head blight of spring wheat, barley and oat in Ontario during 2001–2017. Can. J. Plant Pathol. 41, 392–402. doi: 10.1080/07060661.2019.1582560
Yan, A., Pan, J., An, L., Gan, Y., and Feng, H. (2012). The responses of trichome mutants to enhanced ultraviolet-B radiation in Arabidopsis thaliana. J. Photochem. Photobiol. B Biol. 113, 29–35. doi: 10.1016/j.jphotobiol.2012.04.011
Zhang, H., Liu, P., Wang, B., and Yuan, F. (2021). The roles of trichome development genes in stress resistance. Plant Growth Regul. 95, 137–148. doi: 10.1007/s10725-021-00733-5
Keywords: Avena sativa, Fusarium graminearum, Fusarium poae, Fusarium sporotrichioides, qPCR, resistance
Citation: Schurack S, Rodemann C, Oldach K, Beuch S, Brodführer S, von Tiedemann A and Herrmann MH (2025) Evaluation of oat genotypes for species-specific and cross-resistance to Fusarium species and the role of trichomes in susceptibility. Front. Plant Sci. 16:1608030. doi: 10.3389/fpls.2025.1608030
Received: 08 April 2025; Accepted: 18 July 2025;
Published: 12 August 2025.
Edited by:
Rebecca Grumet, Michigan State University, United StatesReviewed by:
Soumya Moonjely, Michigan State University, United StatesAdamu Masari Abubakar, Ahmadu Bello University, Nigeria
Copyright © 2025 Schurack, Rodemann, Oldach, Beuch, Brodführer, von Tiedemann and Herrmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Heinrich Herrmann, bWF0dGhpYXMuaGVycm1hbm5AanVsaXVzLWt1ZWhuLmRl
 Selma Schurack
Selma Schurack Charlotte Rodemann2
Charlotte Rodemann2 Sophie Brodführer
Sophie Brodführer Andreas von Tiedemann
Andreas von Tiedemann Matthias Heinrich Herrmann
Matthias Heinrich Herrmann